Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ACCURAY INC | a17-1702_18k.htm |
Exhibit 99.1
35th Annual JP Morgan Healthcare Conference January 11, 2017 Josh Levine President and Chief Executive Officer

Safe Harbor Statement Statements in this presentation (including the oral commentary that accompanies it) that are not statements of historical fact are forward-looking statements and are subject to the "safe harbor” provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements in this presentation relate, but are not limited to: our future results of operations and financial position, including the sufficiency of cash resources and expected cash flows to fund future operations, including the next 12 months; our backlog and expectations regarding age-outs, cancellations of contracts and foreign currency impacts, the effects of our process improvements on age-outs, backlog and revenue; expected uses of cash during fiscal 2017; the anticipated drivers of our future capital requirements; the success of our products, their impact on our business; our expectations regarding the factors that will impact long-term success, sales, competitive positioning of our products; our belief that our products offer clinicians and patients significant benefits over other radiation therapy systems in the market; the anticipated risks associated with our foreign operations and fluctuations in the U.S. dollar and foreign currencies as well as our ability to mitigate such risks; the sufficiency of our cash, cash flow equivalents and investments to meet our anticipated cash needs for working capital and capital expenditures and our business strategy, plans and objectives. Forward-looking statements generally can be identified by words such as “anticipates,” “believes,” “estimates,” “expects,” “intends,” “plans,” “predicts,” “projects,” “may,” “will be,” “will continue,” “will likely result,” and similar expressions. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from expectations, including those risks detailed in Part I, Item 1A of the Company’s annual report on Form 10-K for fiscal year 2016, in Part II Item 1A of the Company’s quarterly reports on Form 10-Q for the quarter ended September 30, 2016, and other filings we make with the Securities and Exchange Commission. Forward-looking statements speak only as of the date the statements are made and are based on information available to the Company at the time those statements are made and/or management’s good faith belief as of that time with respect to future events. The Company assumes no obligation to update forward-looking statements to reflect actual performance or results, changes in assumptions or changes in other factors affecting forward-looking information, except to the extent required by applicable securities laws. Accordingly, investors should not place undue reliance on any forward-looking statements. This presentation also contains non-GAAP financial information. Management believes that this non-GAAP financial measure provides useful supplemental information to management and investors regarding the performance of the company and facilitates a more meaningful comparison of results for current periods with previous operating results. Additionally, it will assist management in analyzing future trends, making strategic and business decisions and establishing internal budgets and forecasts. A reconciliation is available in the Appendix. Forward Looking Statement 2
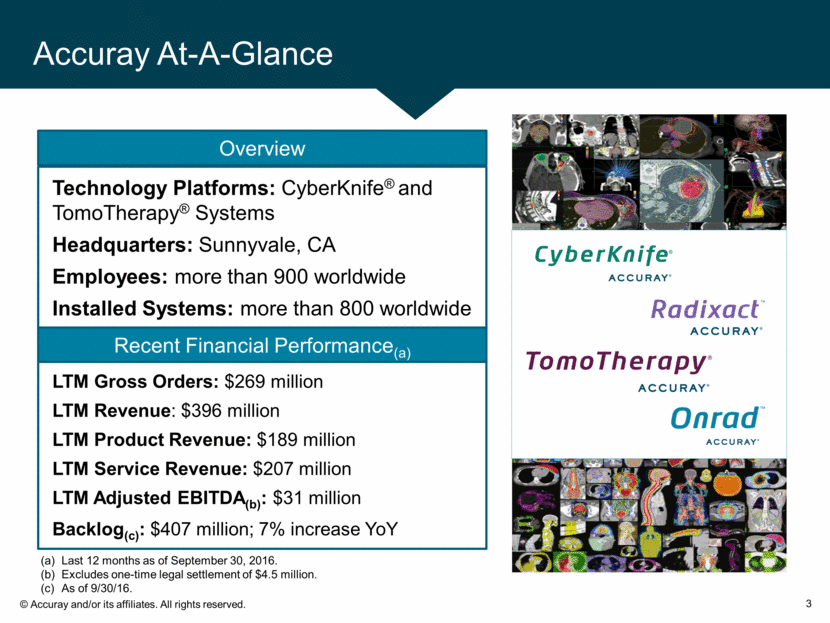
LTM Gross Orders: $269 million LTM Revenue: $396 million LTM Product Revenue: $189 million LTM Service Revenue: $207 million LTM Adjusted EBITDA(b): $31 million Backlog(c): $407 million; 7% increase YoY Recent Financial Performance(a) Technology Platforms: CyberKnife® and TomoTherapy® Systems Headquarters: Sunnyvale, CA Employees: more than 900 worldwide Installed Systems: more than 800 worldwide Accuray At-A-Glance 3 Last 12 months as of September 30, 2016. Excludes one-time legal settlement of $4.5 million. As of 9/30/16. Overview
Accuray Poised for Growth in 2H FY 2017 and Beyond 4 CyberKnife® SBRT growth, Radixact™ and Onrad™ full commercial launch Growth outside the U.S.: Europe, China, Japan Improving financial performance Growing global demand for radiation therapy Customer focus and enhanced commercial execution Strategic partnerships
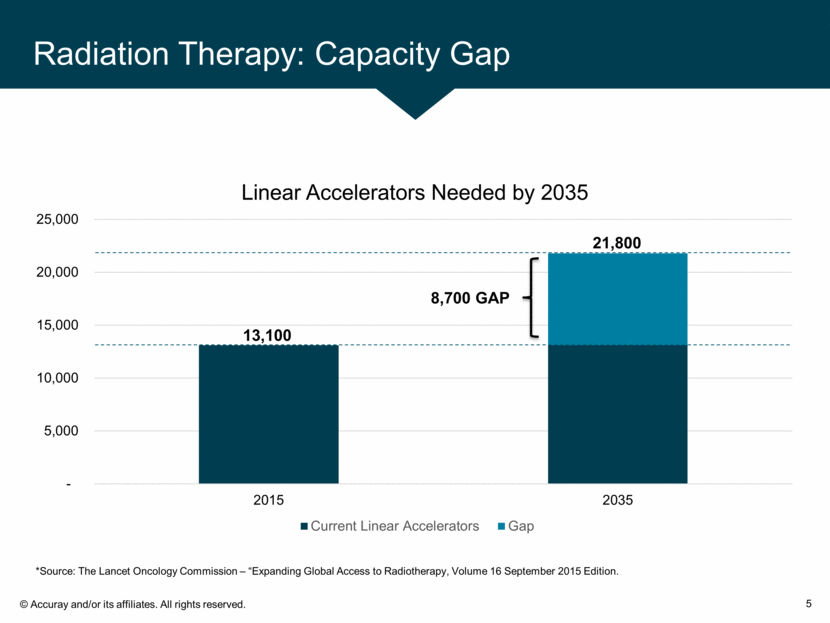
Radiation Therapy: Capacity Gap 5 21,800 13,100 *Source: The Lancet Oncology Commission – “Expanding Global Access to Radiotherapy, Volume 16 September 2015 Edition.
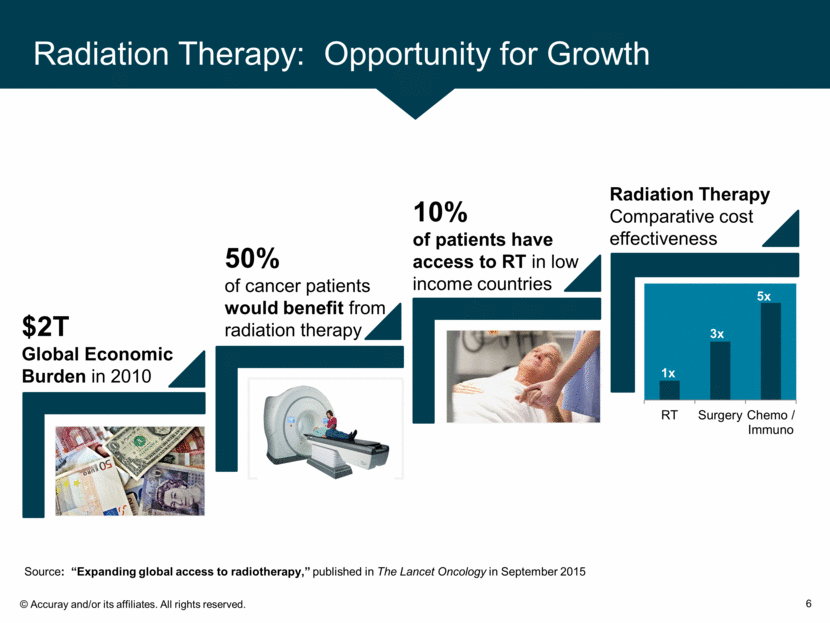
Radiation Therapy: Opportunity for Growth 6 Source: “Expanding global access to radiotherapy,” published in The Lancet Oncology in September 2015 $2T Global Economic Burden in 2010 10% of patients have access to RT in low income countries 50% of cancer patients would benefit from radiation therapy Radiation Therapy Comparative cost effectiveness 3x 5x RT Surgery Chemo / Immuno 1x
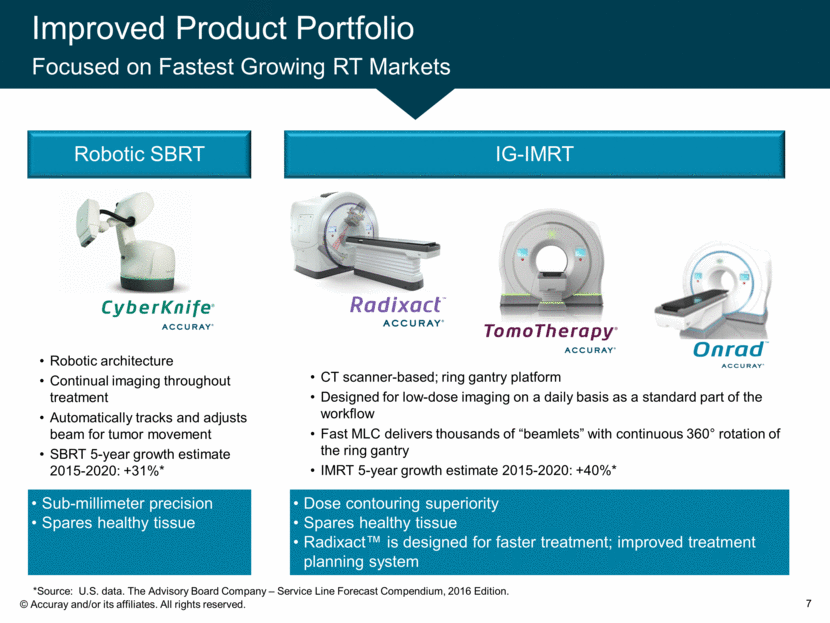
Improved Product Portfolio Focused on Fastest Growing RT Markets 7 IG-IMRT CT scanner-based; ring gantry platform Designed for low-dose imaging on a daily basis as a standard part of the workflow Fast MLC delivers thousands of “beamlets” with continuous 360° rotation of the ring gantry IMRT 5-year growth estimate 2015-2020: +40%* Dose contouring superiority Spares healthy tissue Radixact™ is designed for faster treatment; improved treatment planning system Robotic architecture Continual imaging throughout treatment Automatically tracks and adjusts beam for tumor movement SBRT 5-year growth estimate 2015-2020: +31%* Robotic SBRT Sub-millimeter precision Spares healthy tissue *Source: U.S. data. The Advisory Board Company – Service Line Forecast Compendium, 2016 Edition.
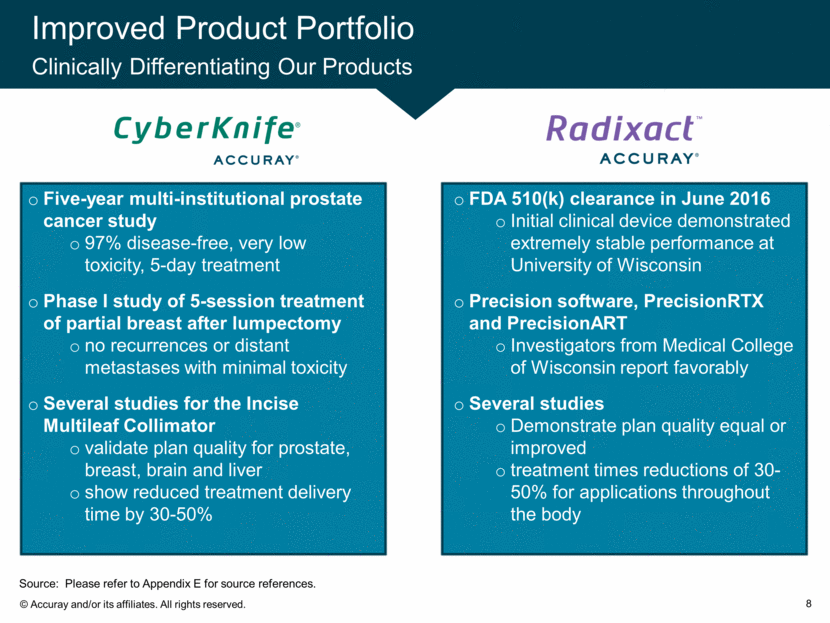
Improved Product Portfolio Clinically Differentiating Our Products 8 Five-year multi-institutional prostate cancer study 97% disease-free, very low toxicity, 5-day treatment Phase I study of 5-session treatment of partial breast after lumpectomy no recurrences or distant metastases with minimal toxicity Several studies for the Incise Multileaf Collimator validate plan quality for prostate, breast, brain and liver show reduced treatment delivery time by 30-50% FDA 510(k) clearance in June 2016 Initial clinical device demonstrated extremely stable performance at University of Wisconsin Precision software, PrecisionRTX and PrecisionART Investigators from Medical College of Wisconsin report favorably Several studies Demonstrate plan quality equal or improved treatment times reductions of 30-50% for applications throughout the body Source: Please refer to Appendix E for source references.
CyberKnife® Positioned for SBRT Growth Multi-Institutional Prostate SBRT Study - Long-term treatment results 9 Only CyberKnife continually tracks and automatically corrects for movement of the prostate in real-time with sub-millimeter precision 97% of low- and intermediate-risk patients had excellent cancer control five years after treatment Shorter course of treatment cost-effective for healthcare providers and payers Serious side effects were rare after more than five years of follow-up Very low rate of recurrence, even in intermediate-risk patients Source: Meier et al. Five-Year Outcomes From a Multicenter Trial of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2016 Oct 1;96(2S):S33-S34; abstract 74
High Treatment Precision for Broader Case Mix Treatment of simple and complex cases Adaptive treatment and retreatment planning Unique helical delivery and integrated imaging Radixact™ System: Faster, Leading-Edge 10 Faster, More Efficient, Reliable Delivery Increased reliability and serviceability Viable solution for single and dual vault facilities Faster linear accelerator and improved imaging Precision Treatment Planning & iDMS Pathway for Leading-Edge Innovation Upgrade pathway for motion management and real-time adaptive therapy
Onrad™ System A new TomoTherapy® configuration for the value market segment 11 Goal Meet growing demand in a market segment new to Accuray Our Solution: fast, easy to use; treats extensive range of cases Based upon TomoH™ platform IG-IMRT and 3DCRT system using fixed beam angles Streamlined and productive workflows Fast planning Compact footprint and on-board beam shielding Integrated QA tools Onrad is a trademark of Accuray Incorporated in China, Japan and the EU.
Innovation Through Partnerships 12 Accuray - RaySearch Partnership: Developing innovative and user-friendly OIS and treatment planning software solutions for CyberKnife® and TomoTherapy® System customers Leveraging partnerships to bring critical solutions to market faster Accuray - medPhoton Partnership: Developing advanced imaging capabilities for CyberKnife® System customers
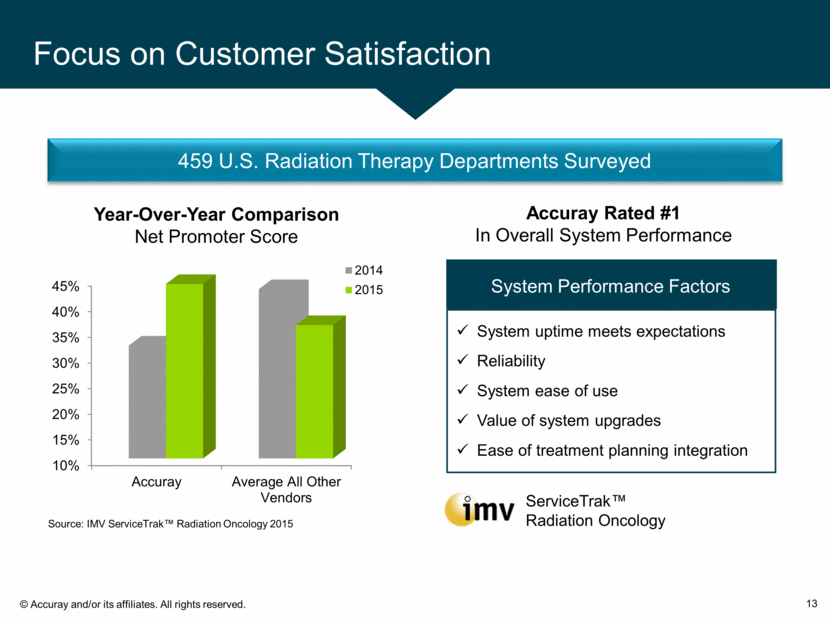
Focus on Customer Satisfaction 13 System Performance Factors System uptime meets expectations Reliability System ease of use Value of system upgrades Ease of treatment planning integration Accuray Rated #1 In Overall System Performance Year-Over-Year Comparison Net Promoter Score ServiceTrak™ Radiation Oncology Source: IMV ServiceTrak™ Radiation Oncology 2015 459 U.S. Radiation Therapy Departments Surveyed 10% 15% 20% 25% 30% 35% 40% 45% Accuray Average All Other Vendors 2014 2015
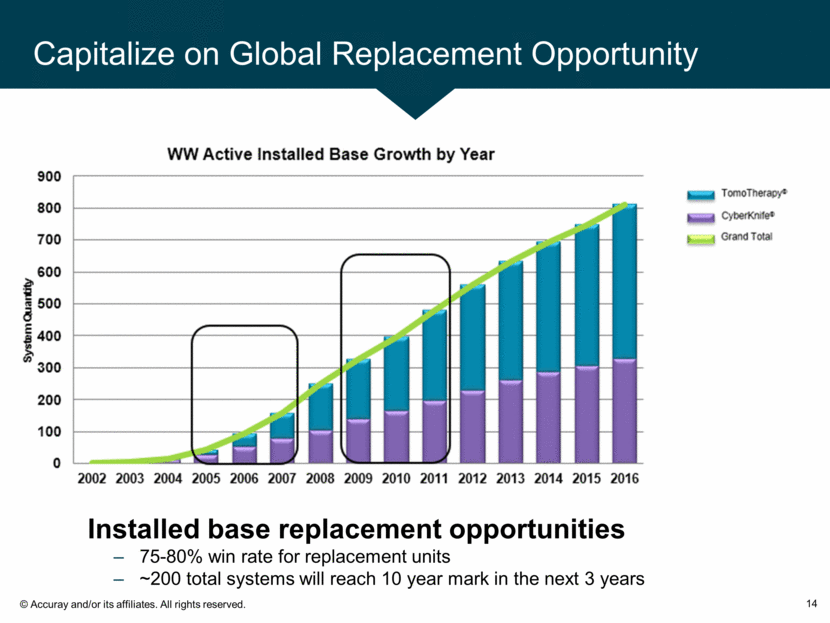
Capitalize on Global Replacement Opportunity 14 Installed base replacement opportunities 75-80% win rate for replacement units ~200 total systems will reach 10 year mark in the next 3 years
Hong Kong Sanatorium & Hospital order for 3 Radixact™ Systems Strong mix of CyberKnife® orders >50%(a) of TomoTherapy® / Radixact™ System orders were in single and dual vault settings >80%(a) of CyberKnife M6 System orders included MLC Multiple sites in the US and Europe now equipped and treating patients with the Radixact™ System driving Q2 FY 2017 orders Forecasted Q2 FY 2017 gross orders of $78 million Recent Commercial Execution 15 (a) Percentages based on trailing 12 months data from September 30, 2016.
Growth Outside the U.S. 16 Europe Largest growth region in FY 2016 gross orders Multi-system orders continue to drive growth- NHS order in Q4 2016, largest in Accuray history Japan Accuray’s largest market share region Radixact launch expected in 2H FY 2017 China Significant unmet radiotherapy gap Accuray to participate in the value segment with Onrad
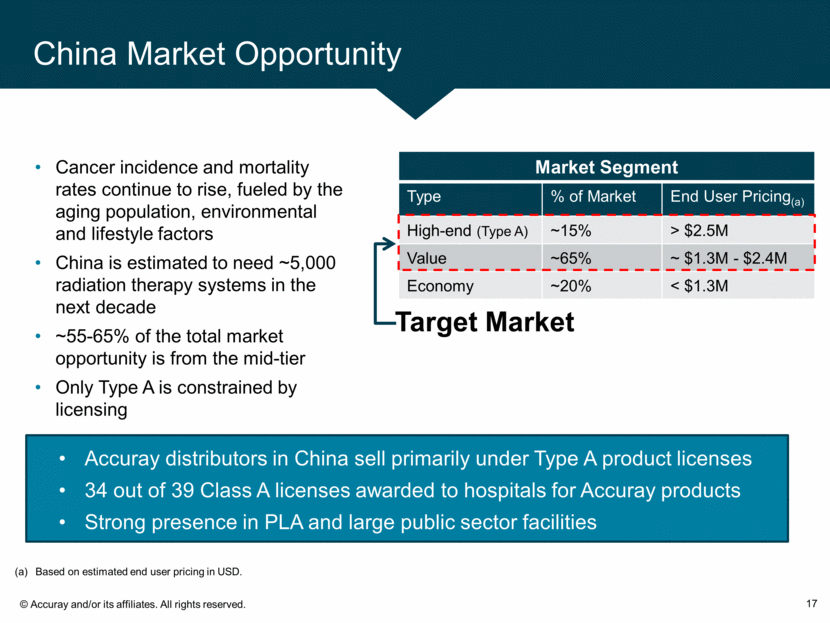
China Market Opportunity 17 Cancer incidence and mortality rates continue to rise, fueled by the aging population, environmental and lifestyle factors China is estimated to need ~5,000 radiation therapy systems in the next decade ~55-65% of the total market opportunity is from the mid-tier Only Type A is constrained by licensing Market Segment Type % of Market End User Pricing(a) High-end (Type A) ~15% > $2.5M Value ~65% ~ $1.3M - $2.4M Economy ~20% < $1.3M Target Market Accuray distributors in China sell primarily under Type A product licenses 34 out of 39 Class A licenses awarded to hospitals for Accuray products Strong presence in PLA and large public sector facilities Based on estimated end user pricing in USD.
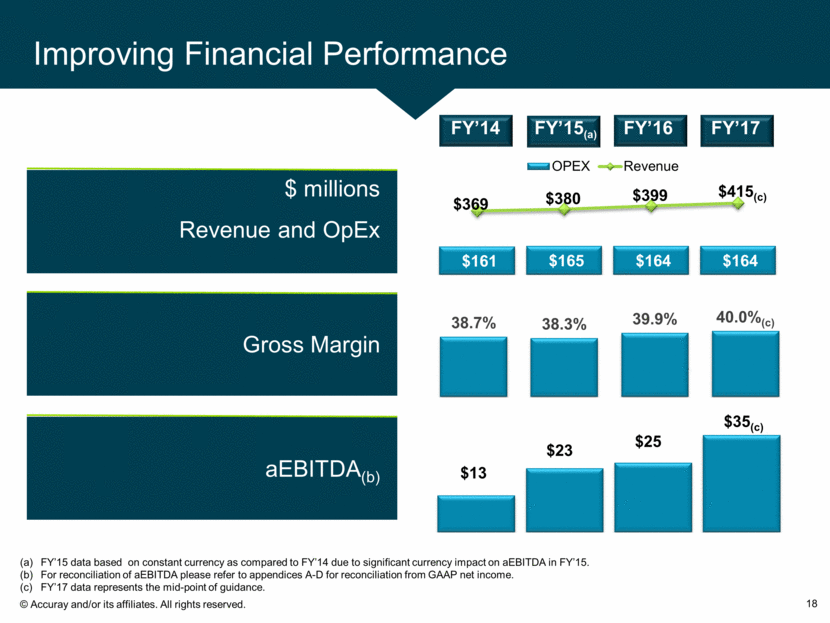
Improving Financial Performance 18 aEBITDA FY’13 aEBITDA(b) $ millions Revenue and OpEx Gross Margin $13 $23 $25 $35(c) FY’15 data based on constant currency as compared to FY’14 due to significant currency impact on aEBITDA in FY’15. For reconciliation of aEBITDA please refer to appendices A-D for reconciliation from GAAP net income. FY’17 data represents the mid-point of guidance. FY’14 FY’17 FY’15(a) FY’16 $161 $165 $164 $164 $369 $380 $399 $ 415 (c) OPEX Revenue 38.7% 38.3% 39.9% 40.0% (c)
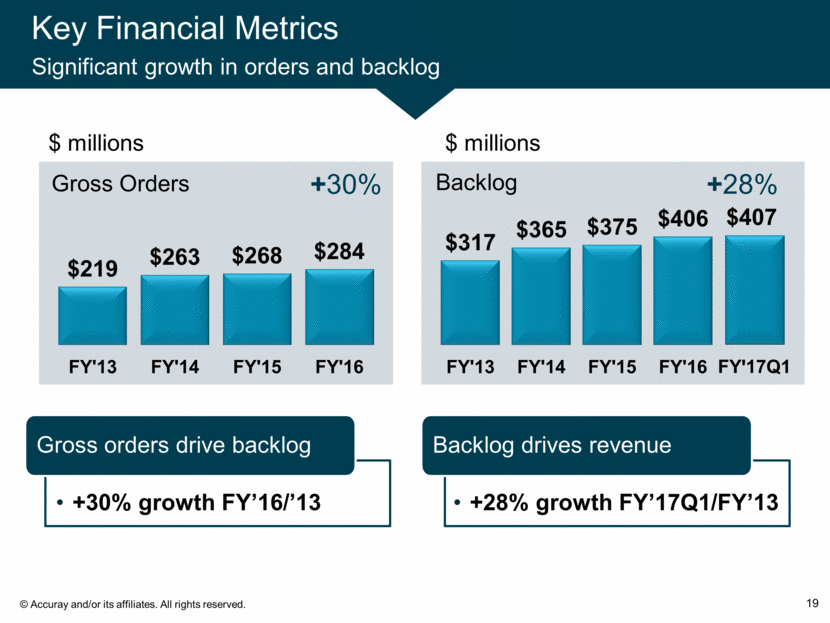
Key Financial Metrics Significant growth in orders and backlog 19 $ millions +30% +28% Gross orders drive backlog Backlog drives revenue $ millions +30% growth FY’16/’13 +28% growth FY’17Q1/FY’13 $219 $263 $268 $284 FY'13 FY'14 FY'15 FY'16 Gross Orders $317 $365 $375 $406 FY'13 FY'14 FY'15 FY'16 Backlog $407 FY ? 17Q1
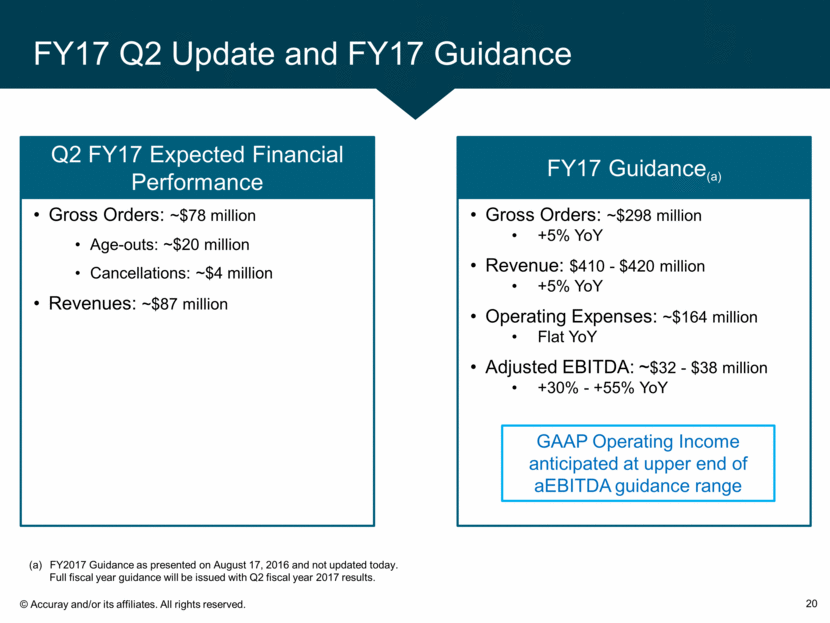
FY17 Q2 Update and FY17 Guidance 20 FY2017 Guidance as presented on August 17, 2016 and not updated today. Full fiscal year guidance will be issued with Q2 fiscal year 2017 results. Gross Orders: ~$298 million +5% YoY Revenue: $410 - $420 million +5% YoY Operating Expenses: ~$164 million Flat YoY Adjusted EBITDA: ~$32 - $38 million +30% - +55% YoY FY17 Guidance(a) Gross Orders: ~$78 million Age-outs: ~$20 million Cancellations: ~$4 million Revenues: ~$87 million Q2 FY17 Expected Financial Performance GAAP Operating Income anticipated at upper end of aEBITDA guidance range
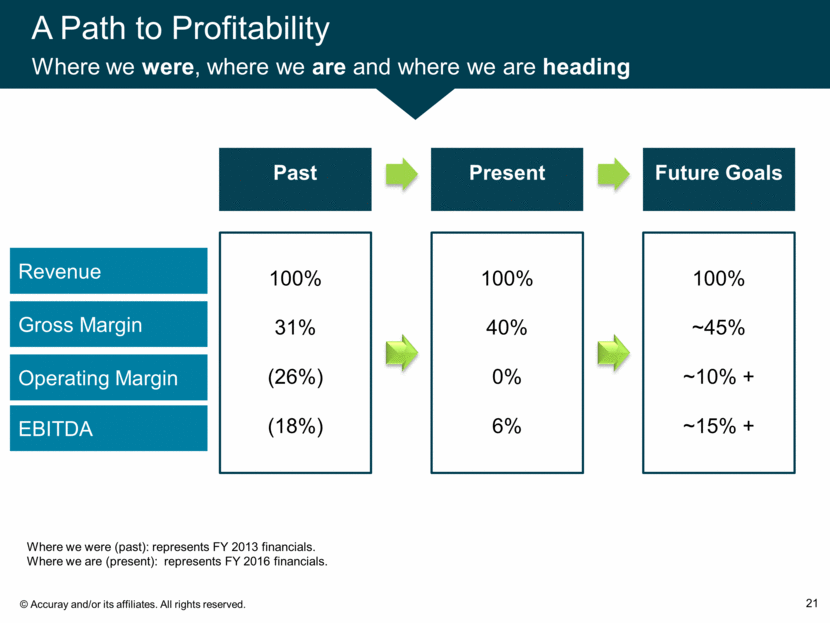
A Path to Profitability Where we were, where we are and where we are heading 21 Revenue Gross Margin Operating Margin EBITDA Where we were (past): represents FY 2013 financials. Where we are (present): represents FY 2016 financials. Past Present Future Goals 100% 31% (26%) (18%) 100% 40% 0% 6% 100% ~45% ~10% + ~15% +
Recent system launches create potential for double digit growth in 2H Fiscal 2017 order growth Expanded product portfolio addresses long-term opportunities Industry-leading precision and reliability are keys to replacement wins Significant operating expense leverage Cash generation and debt retirement Compelling current valuation Drivers of Future Value 22
Thank You 23

APPENDICES 24

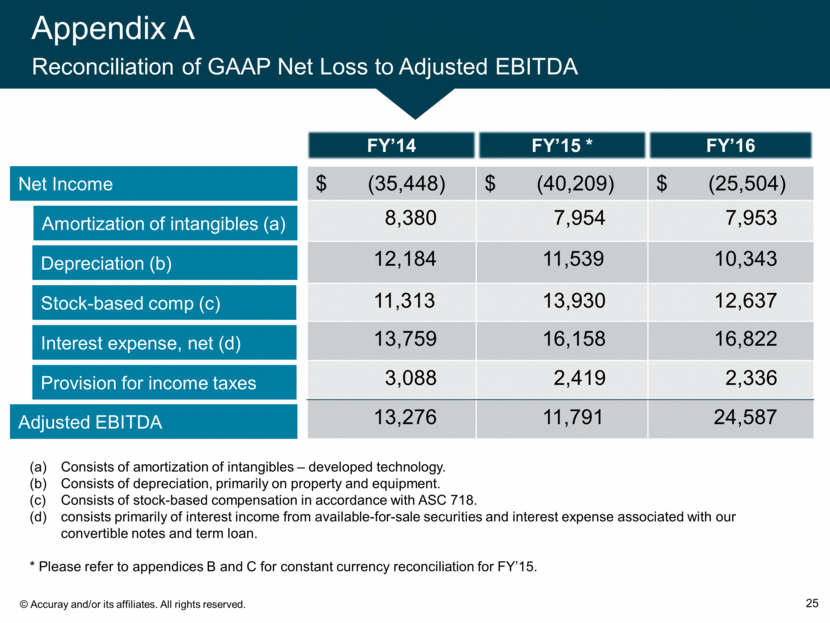
Appendix A Reconciliation of GAAP Net Loss to Adjusted EBITDA 25 Net Income Amortization of intangibles (a) Depreciation (b) Adjusted EBITDA Stock-based comp (c) Interest expense, net (d) Provision for income taxes $ (35,448) $ (40,209) $ (25,504) 8,380 7,954 7,953 12,184 11,539 10,343 11,313 13,930 12,637 13,759 16,158 16,822 3,088 2,419 2,336 13,276 11,791 24,587 FY’15 * FY’14 FY’16 Consists of amortization of intangibles – developed technology. Consists of depreciation, primarily on property and equipment. Consists of stock-based compensation in accordance with ASC 718. consists primarily of interest income from available-for-sale securities and interest expense associated with our convertible notes and term loan. * Please refer to appendices B and C for constant currency reconciliation for FY’15.
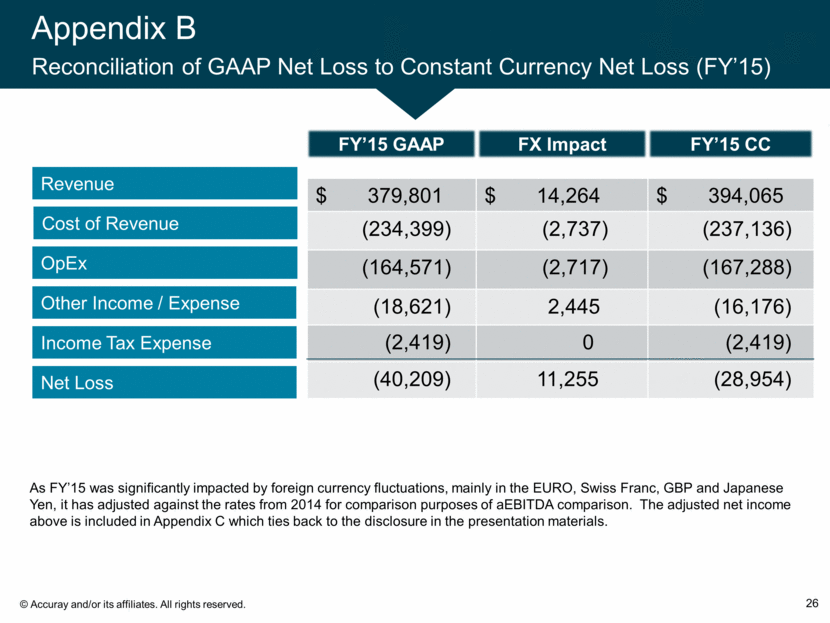
Appendix B Reconciliation of GAAP Net Loss to Constant Currency Net Loss (FY’15) 26 Revenue Cost of Revenue OpEx Other Income / Expense Income Tax Expense Net Loss $ 379,801 $ 14,264 $ 394,065 (234,399) (2,737) (237,136) (164,571) (2,717) (167,288) (18,621) 2,445 (16,176) (2,419) 0 (2,419) (40,209) 11,255 (28,954) FX Impact FY’15 GAAP FY’15 CC As FY’15 was significantly impacted by foreign currency fluctuations, mainly in the EURO, Swiss Franc, GBP and Japanese Yen, it has adjusted against the rates from 2014 for comparison purposes of aEBITDA comparison. The adjusted net income above is included in Appendix C which ties back to the disclosure in the presentation materials.
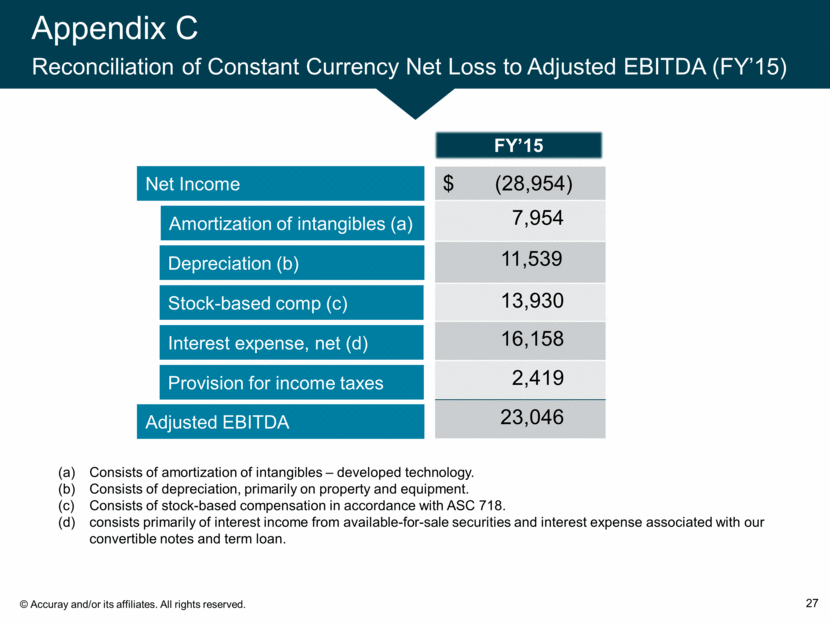
Appendix C Reconciliation of Constant Currency Net Loss to Adjusted EBITDA (FY’15) 27 Net Income Amortization of intangibles (a) Depreciation (b) Adjusted EBITDA Stock-based comp (c) Interest expense, net (d) Provision for income taxes $ (28,954) 7,954 11,539 13,930 16,158 2,419 23,046 FY’15 Consists of amortization of intangibles – developed technology. Consists of depreciation, primarily on property and equipment. Consists of stock-based compensation in accordance with ASC 718. consists primarily of interest income from available-for-sale securities and interest expense associated with our convertible notes and term loan.
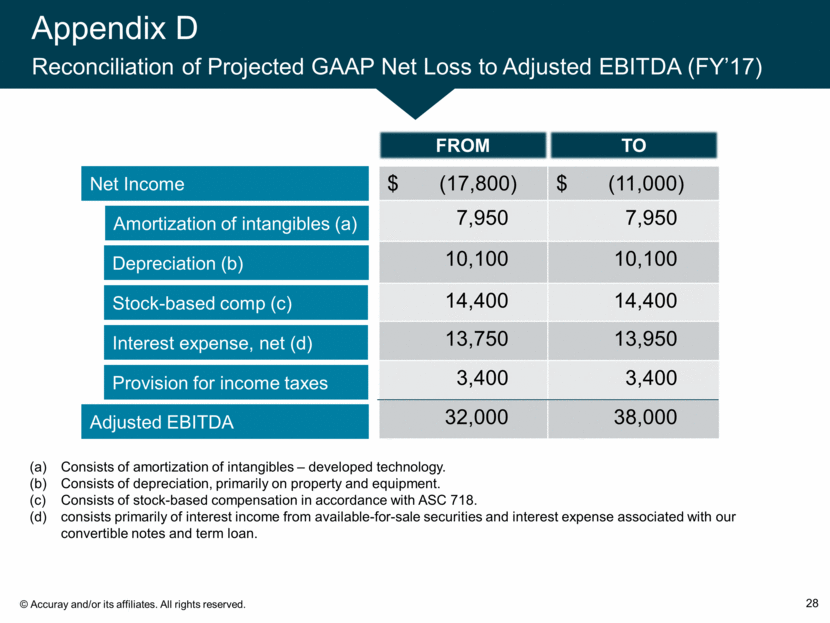
Appendix D Reconciliation of Projected GAAP Net Loss to Adjusted EBITDA (FY’17) 28 Net Income Amortization of intangibles (a) Depreciation (b) Adjusted EBITDA Stock-based comp (c) Interest expense, net (d) Provision for income taxes $ (17,800) $ (11,000) 7,950 7,950 10,100 10,100 14,400 14,400 13,750 13,950 3,400 3,400 32,000 38,000 TO FROM Consists of amortization of intangibles – developed technology. Consists of depreciation, primarily on property and equipment. Consists of stock-based compensation in accordance with ASC 718. consists primarily of interest income from available-for-sale securities and interest expense associated with our convertible notes and term loan.
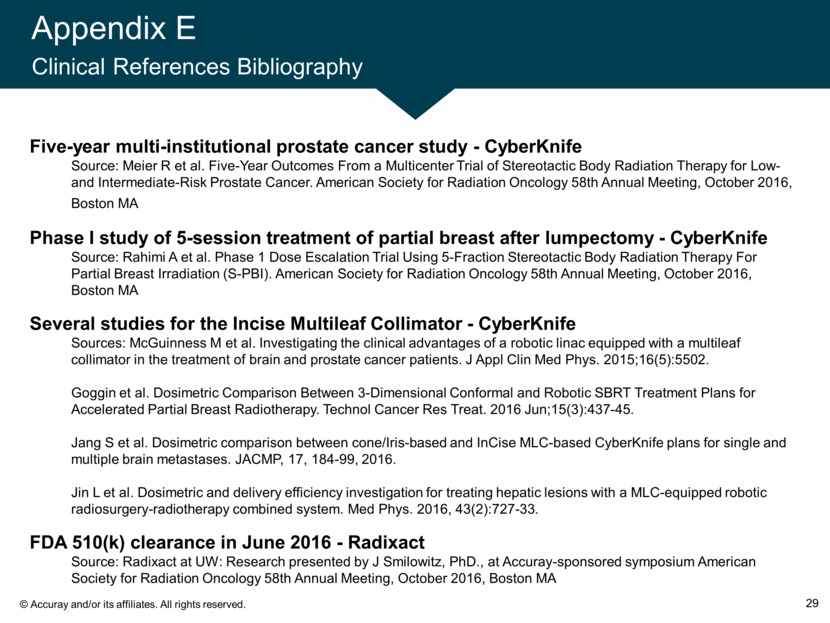
Appendix E Clinical References Bibliography 29 Five-year multi-institutional prostate cancer study - CyberKnife Source: Meier R et al. Five-Year Outcomes From a Multicenter Trial of Stereotactic Body Radiation Therapy for Low- and Intermediate-Risk Prostate Cancer. American Society for Radiation Oncology 58th Annual Meeting, October 2016, Boston MA Phase I study of 5-session treatment of partial breast after lumpectomy - CyberKnife Source: Rahimi A et al. Phase 1 Dose Escalation Trial Using 5-Fraction Stereotactic Body Radiation Therapy For Partial Breast Irradiation (S-PBI). American Society for Radiation Oncology 58th Annual Meeting, October 2016, Boston MA Several studies for the Incise Multileaf Collimator - CyberKnife Sources: McGuinness M et al. Investigating the clinical advantages of a robotic linac equipped with a multileaf collimator in the treatment of brain and prostate cancer patients. J Appl Clin Med Phys. 2015;16(5):5502. Goggin et al. Dosimetric Comparison Between 3-Dimensional Conformal and Robotic SBRT Treatment Plans for Accelerated Partial Breast Radiotherapy. Technol Cancer Res Treat. 2016 Jun;15(3):437-45. Jang S et al. Dosimetric comparison between cone/Iris-based and InCise MLC-based CyberKnife plans for single and multiple brain metastases. JACMP, 17, 184-99, 2016. Jin L et al. Dosimetric and delivery efficiency investigation for treating hepatic lesions with a MLC-equipped robotic radiosurgery-radiotherapy combined system. Med Phys. 2016, 43(2):727-33. FDA 510(k) clearance in June 2016 - Radixact Source: Radixact at UW: Research presented by J Smilowitz, PhD., at Accuray-sponsored symposium American Society for Radiation Oncology 58th Annual Meeting, October 2016, Boston MA
Appendix E Clinical References Bibliography (cont.) 30 Precision software, PrecisionRTX and PrecisionART - Radixact Sources: Lim SN et al. Automated Dose Deformation for Re-Irradiation. American Association of Medical Physics 58th Annual Meeting, July 31-Aug 4, 2016, Washington DC. Kainz . et al. Automated Tracking of Fractional and Accumulated Doses for Triggering Adaptive Replanning. American Society for Radiation Oncology 58th Annual Meeting, October 2016, Boston MA Several studies - Radixact Sources: Rudovsky L et al. Lung and liver sbrt using helical tomotherapy--a dosimetric comparison of fixed jaw and dynamic jaw delivery. Journal of applied clinical medical physics 2014;15:4664. Murai et al. Efficacy of stereotactic radiotherapy for brain metastases using dynamic jaws technology in the helical tomotherapy system. Br J Radiol. 2016 Oct;89(1066). Van Gestel et al. Fast Helical Tomotherapy in a head and neck cancer planning study: is time priceless? Radiation Oncology (2015) 10:261 Katayama et al. Accelerated tomotherapy delivery with TomoEdge technique. J Appl Clin Med Phys. 2015 Mar 8;16(2):4964. Sugie C et al. Efficacy of the Dynamic Jaw Mode in Helical Tomotherapy With Static Ports for Breast Cancer. Technol Cancer Res Treat. 2015;14(4):459-65. Katayama et al. Accelerated tomotherapy delivery with TomoEdge technique. J Appl Clin Med Phys. 2015 Mar 8;16(2):4964.