Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ZOGENIX, INC. | d325965d8k.htm |

Company Presentation January 2017 NASDAQ: ZGNX Exhibit 99.1

Forward Looking Statement Zogenix cautions you that statements included in this press release that are not a description of historical facts are forward-looking statements. Words such as "believes," "anticipates," "plans," "expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended to identify forward-looking statements. These statements are based on the company's current beliefs and expectations. These forward-looking statements include statements regarding the potential commercialization of ZX008; the timing of top-line results from the Phase 3 clinical trial of ZX008 in Dravet syndrome and other 2017 milestones; and the timing of any submission of a new drug application to the U.S. Food and Drug Administration or comparable market authorization filing in Europe. The inclusion of forward-looking statements should not be regarded as a representation by Zogenix that any of its plans will be achieved. Actual results may differ from those set forth in this release due to the risks and uncertainties inherent in Zogenix's business, including, without limitation: the uncertainties associated with the clinical development and regulatory approval of product candidates such as ZX008, including potential delays in the commencement, enrollment and completion of clinical trials; the potential that earlier clinical trials and studies may not be predictive of future results; Zogenix's reliance on third parties to conduct its clinical trials, enroll patients, manufacture its preclinical and clinical drug supplies and manufacture commercial supplies of its drug products, if approved; unexpected adverse side effects or inadequate therapeutic efficacy of ZX008 that could limit approval and/or commercialization, or that could result in recalls or product liability claims; Zogenix's ability to fully comply with numerous federal, state and local laws and regulatory requirements, as well as rules and regulations outside the United States, that apply to its product development activities; Fast Track designation may not result in an expedited regulatory review process; the potential for distraction of management related to the transition of management responsibilities; and other risks described in Zogenix's prior press releases as well as in public periodic filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and Zogenix undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof. All forward-looking statements are qualified in their entirety by this cautionary statement.

ZX008 (Low Dose Fenfluramine) for the Treatment of Uncontrolled Seizures DRAVET SYNDROME Phase 3 Studies Underway with First Top Line Results Q2 2017 Highly Focused on Developing and Commercializing Therapies for Orphan CNS Disorders LENNOX GASTAUT SYNDROME Targeting Phase 3 Study Initiation Mid-2017

Exclusive World-Wide Rights Orphan Drug Designation in U.S. and E.U. in Dravet Syndrome Fast Track Designation for Development in Dravet Syndrome On-Going Phase 3 Program in Dravet Syndrome; Multiple Data Readouts in 2017 Seeking IND Acceptance to Commence Phase 3 Clinical Development in Lennox Gastaut Syndrome Preparing to Commercialize Directly in U.S. and E.U. Future Asia-Pacific Partnering Opportunity ZX008 Overview

Dravet Syndrome Genetic epilepsy syndrome (SCN1A) => developmental encephalopathy Pharmaco-resistant epilepsy Intellectual disability Other comorbidities – ADHD, behavior, crouch gait Recent incidence study 1/15,700 births May be higher due to undiagnosed or mis-diagnosed cases Higher incidence of status epilepticus and SUDEP compared to other childhood epilepsies Polypharmacy is standard of care but no effective, long-term treatment exists Valproate, topiramate, levetiracetam, clobazam, clonazepam, sitirpentol (E.U.) Effective rescue therapy Sodium channel blockers (e.g., carbamazepine, oxcarbazepine, lamotrigine) make seizures worse Intractable, Severe Epilepsy Which Begins in Infancy

Low Dose Fenfluramine: Long Term Efficacy and Safety in Treating Dravet Syndrome – Original Cohort First Report – Epilepsia (2012) Long-term open-label study of fenfluramine adjunctive anticonvulsant therapy 12 Dravet syndrome patients, age 9 months to 13 years (11 with confirmatory genetic mutation) 67% of patients seizure-free, >1 year minimum 9/12 patients had overall ≥75% seizure reduction 5-year Follow-up – Epilepsia (2016) 10 patients from 2012 publication still on fenfluramine therapy treated between 2010-2014 90% had average seizure frequency < 1/month over entire observation period 3/10 patients seizure free for entire 5 years; 4/10 patients seizure free for at least 2 years Overall Safety Well tolerated with no signs of clinically important cardiac valve disease (prospective echocardiography) Clinical Data / IP Licensed by Zogenix
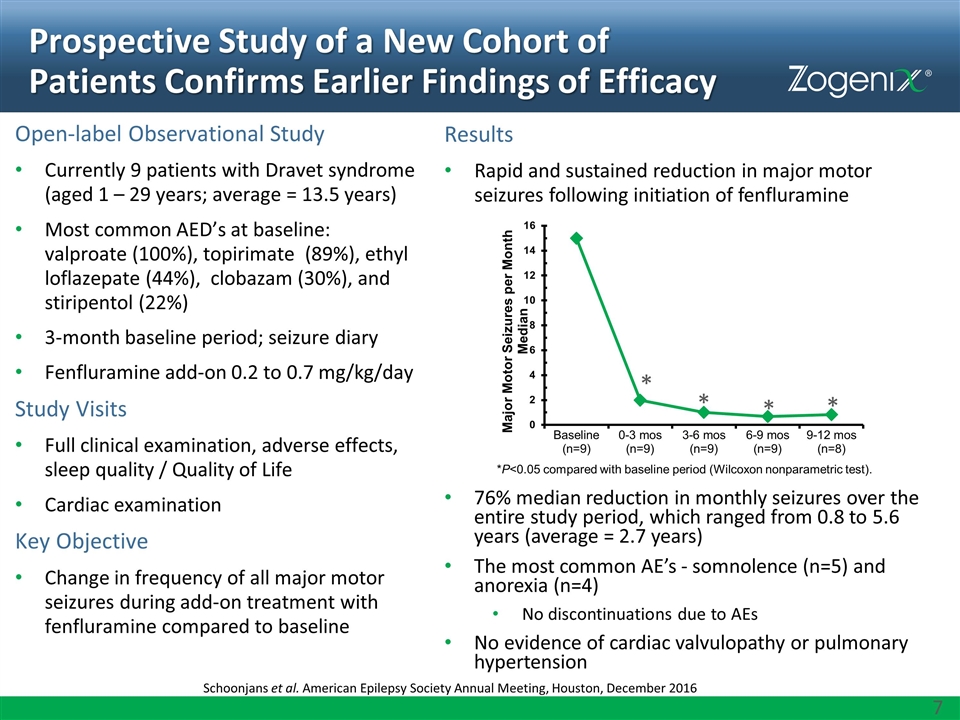
Results Rapid and sustained reduction in major motor seizures following initiation of fenfluramine 76% median reduction in monthly seizures over the entire study period, which ranged from 0.8 to 5.6 years (average = 2.7 years) The most common AE’s - somnolence (n=5) and anorexia (n=4) No discontinuations due to AEs No evidence of cardiac valvulopathy or pulmonary hypertension Prospective Study of a New Cohort of Patients Confirms Earlier Findings of Efficacy Schoonjans et al. American Epilepsy Society Annual Meeting, Houston, December 2016 Open-label Observational Study Currently 9 patients with Dravet syndrome (aged 1 – 29 years; average = 13.5 years) Most common AED’s at baseline: valproate (100%), topirimate (89%), ethyl loflazepate (44%), clobazam (30%), and stiripentol (22%) 3-month baseline period; seizure diary Fenfluramine add-on 0.2 to 0.7 mg/kg/day Study Visits Full clinical examination, adverse effects, sleep quality / Quality of Life Cardiac examination Key Objective Change in frequency of all major motor seizures during add-on treatment with fenfluramine compared to baseline *P<0.05 compared with baseline period (Wilcoxon nonparametric test). * * * *
Research on Potential Anti-Seizure Mechanisms of Fenfluramine Serotonin Zebrafish (-/-) scn1Lab mutant model of Dravet syndrome demonstrates anti-seizure activity of fenfluramine via 5HT1D, 2A and 2C receptor stimulation 1 Sigma-1 Fenfluramine binds to sigma-1 receptors and alters activity of the sigma receptor (Zogenix, manuscript in preparation) Allosteric modulation of sigma-1 receptors has been shown to have anti-seizure activity 2 Ongoing work in 2017 to better understand this potential MOA (1) Sourbron, Schneider et al. ACS Chem Neurosci Feb 2016 (2) Guo et al. Br. J. Pharmacol. 2015;172(16):4052-65. Anti-seizure Activity of Fenfluramine in Epileptic Encephalopathies May be Mediated via Specific Serotonergic Receptors and Sigma Receptor-related Mechanisms
ZX008 Clinical Program in Dravet Syndrome Clinical Program includes three double-blind, randomized, controlled 12-week efficacy and safety trials (RCTs) of ZX008 as adjunctive treatment for seizures in children with Dravet syndrome Studies 1501, 1502: identical trials running in North America (1501) and E.U./AUS (1502) Two dose levels of ZX008 versus placebo, n=35/treatment arm; N=105 Stiripentol and cannabinoids not allowed as background medications Study 1504, Clinical Cohort 2: “Stiripentol Incomplete Responder” trial Single dose level of ZX008 versus placebo, n=35/treatment arm; N=70 All subjects are on stiripentol regimen as background medication (stiripentol, valproate and clobazam) Study 1503: Long-term, open-label, flexible- dose extension Adult and pediatric PK investigations, food effect study Program meets anticipated regulatory requirements 1 RCT plus available safety data for E.U. 2 RCT plus available safety data for U.S. Study 1504 in stiripentol incomplete responders meets registration requirements; also maintains E.U. orphan exclusivity and supports pricing and reimbursement
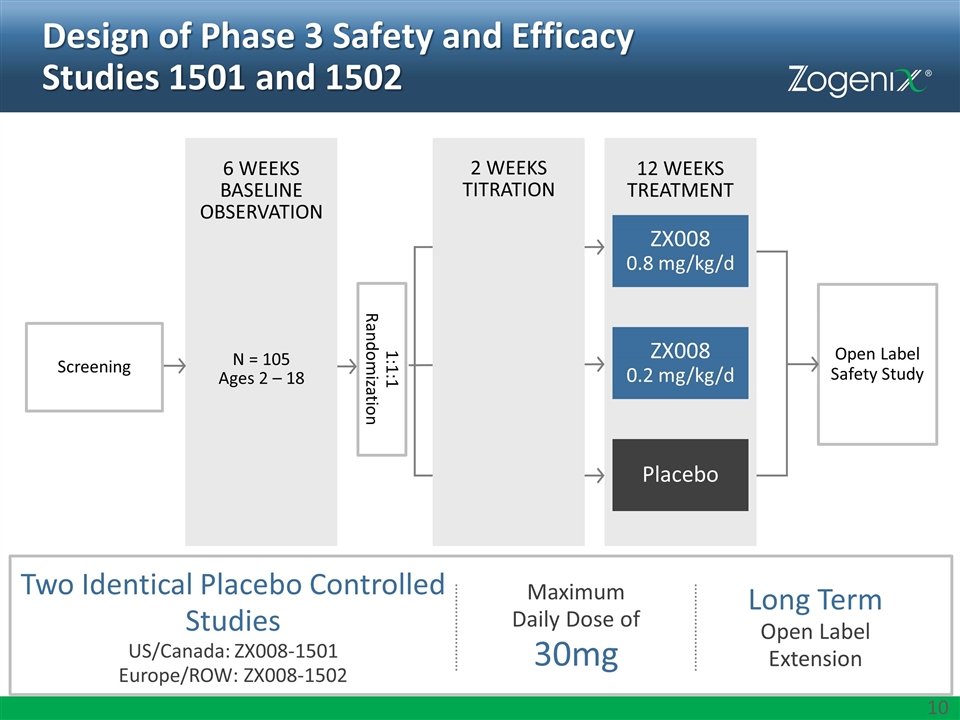
Design of Phase 3 Safety and Efficacy Studies 1501 and 1502 Two Identical Placebo Controlled Studies US/Canada: ZX008-1501 Europe/ROW: ZX008-1502 Maximum Daily Dose of 30mg Long Term Open Label Extension 6 WEEKS BASELINE OBSERVATION Screening Open Label Safety Study N = 105 Ages 2 – 18 1:1:1 Randomization 12 WEEKS TREATMENT Placebo ZX008 0.8 mg/kg/d ZX008 0.2 mg/kg/d 2 WEEKS TITRATION

Key Assessments Adverse events Laboratory safety parameters (hematology, chemistry, urinalysis) Vital signs Cardiac examination (baseline, midway, and endpoint) Doppler echocardiograms 12-lead electrocardiogram SAFETY Analyses for ZX008 0.8 mg/kg/day vs. Placebo, and ZX008 0.2 mg/kg/day vs. Placebo: Change in frequency of convulsive seizures* Patients who achieve ≥50% reduction in convulsive seizure frequency Longest convulsive seizure-free interval Clinical global impression (as assessed by parent / caregiver and investigator) * Change in frequency of convulsive seizures in ZX008 0.8 mg/kg/day vs. Placebo is the primary outcome measure EFFICACY
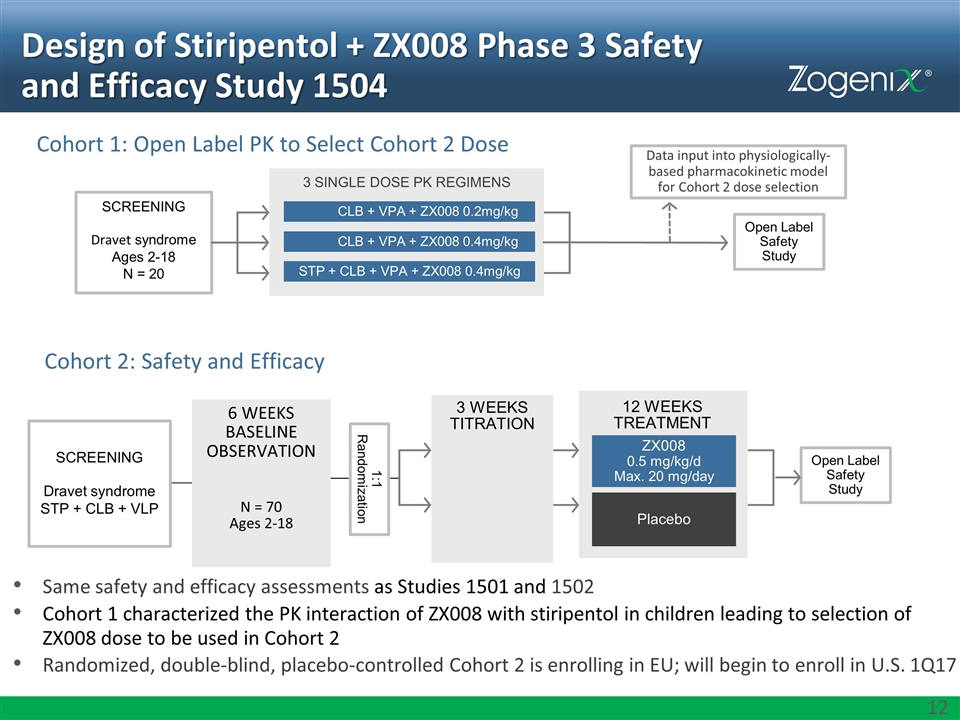
Design of Stiripentol + ZX008 Phase 3 Safety and Efficacy Study 1504 Same safety and efficacy assessments as Studies 1501 and 1502 Cohort 1 characterized the PK interaction of ZX008 with stiripentol in children leading to selection of ZX008 dose to be used in Cohort 2 Randomized, double-blind, placebo-controlled Cohort 2 is enrolling in EU; will begin to enroll in U.S. 1Q17 Screening Dravet syndrome STP + CLB + VLP 12 WEEKS TREATMENT Placebo ZX008 0.5 mg/kg/d Max. 20 mg/day 1:1 Randomization Open Label Safety Study Cohort 2: Safety and Efficacy Cohort 1: Open Label PK to Select Cohort 2 Dose 3 Single Dose PK Regimens CLB + VPA + ZX008 0.2mg/kg CLB + VPA + ZX008 0.4mg/kg STP + CLB + VPA + ZX008 0.4mg/kg Screening Dravet syndrome Ages 2-18 N = 20 Data input into physiologically-based pharmacokinetic model for Cohort 2 dose selection Open Label Safety Study 6 WEEKS BASELINE OBSERVATION N = 70 Ages 2-18 3 WEEKS TITRATION
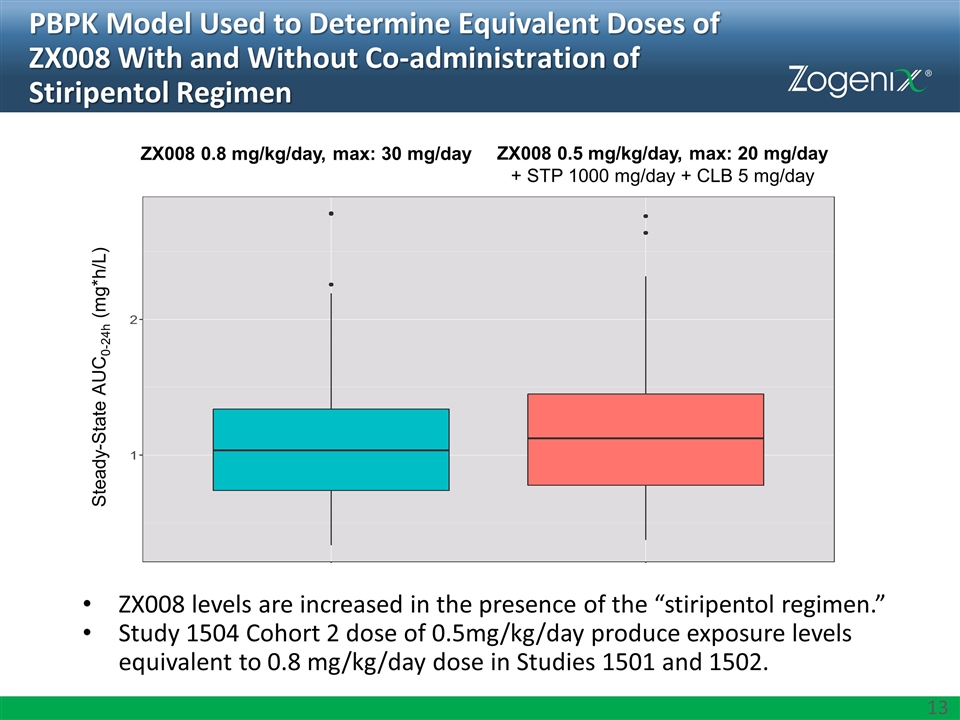
PBPK Model Used to Determine Equivalent Doses of ZX008 With and Without Co-administration of Stiripentol Regimen ZX008 levels are increased in the presence of the “stiripentol regimen.” Study 1504 Cohort 2 dose of 0.5mg/kg/day produce exposure levels equivalent to 0.8 mg/kg/day dose in Studies 1501 and 1502. ZX008 0.8 mg/kg/day, max: 30 mg/day ZX008 0.5 mg/kg/day, max: 20 mg/day + STP 1000 mg/day + CLB 5 mg/day Steady-State AUC0-24h (mg*h/L)

Good acceleration of enrollment during Q4 2016; positive momentum anticipated to continue through Q1 2017 Data from identical trials (1501 and 1502) pooled into a single analysis dataset and reported as two trials, sequentially Enables earliest evaluation of benefit/risk Consistent with original development program strategy First study (“Study 1”) has met enrollment target to allow Q2 2017 results Second study (“Study 2”) top line data anticipated in Q3 2017 Study 1504 (stiripentol study) clinical cohort top line data anticipated Q4 2017 No safety concerns have arisen in the Phase 3 program ZX008 Dravet Syndrome Phase 3 Progress
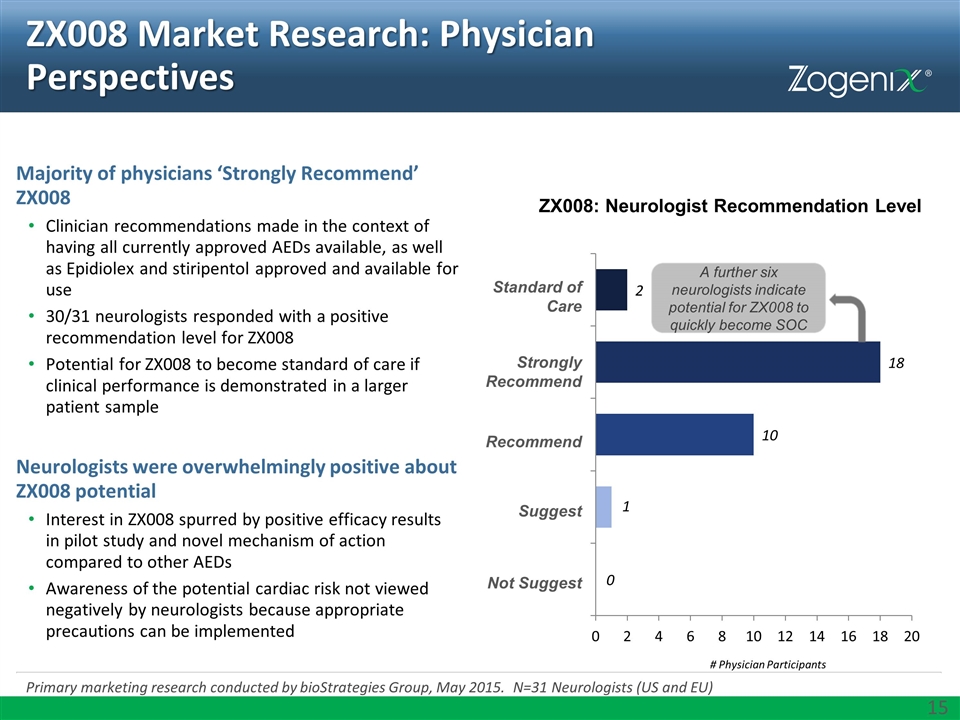
ZX008 Market Research: Physician Perspectives Primary marketing research conducted by bioStrategies Group, May 2015. N=31 Neurologists (US and EU) Majority of physicians ‘Strongly Recommend’ ZX008 Clinician recommendations made in the context of having all currently approved AEDs available, as well as Epidiolex and stiripentol approved and available for use 30/31 neurologists responded with a positive recommendation level for ZX008 Potential for ZX008 to become standard of care if clinical performance is demonstrated in a larger patient sample Neurologists were overwhelmingly positive about ZX008 potential Interest in ZX008 spurred by positive efficacy results in pilot study and novel mechanism of action compared to other AEDs Awareness of the potential cardiac risk not viewed negatively by neurologists because appropriate precautions can be implemented ZX008: Neurologist Recommendation Level # Physician Participants Suggest Recommend Strongly Recommend Standard of Care Not Suggest A further six neurologists indicate potential for ZX008 to quickly become SOC

Lennox Gastaut Syndrome Onset peaks 5-7 years of age Characterized by clinical triad Multiple seizure types, including drop seizures Cognitive impairment Characteristic EEG abnormality Underlying etiologies ~66% - Brain abnormalities (malformation, infection, hypoxia) ~33% - unknown No known genetic abnormality identified US Prevalence: 14,500 - 18,500 children (< 18 y.o.) and at least 30,000 children and adults 1-4% of all childhood epilepsies Polypharmacy with AEDs is standard of care but no effective, long-term treatment exists despite the availability of five US-approved drugs for LGS Felbamate, Topiramate, Rufinamide, Clobazam, Lamotrigine Refractory, Debilitating Childhood-Onset Epilepsy
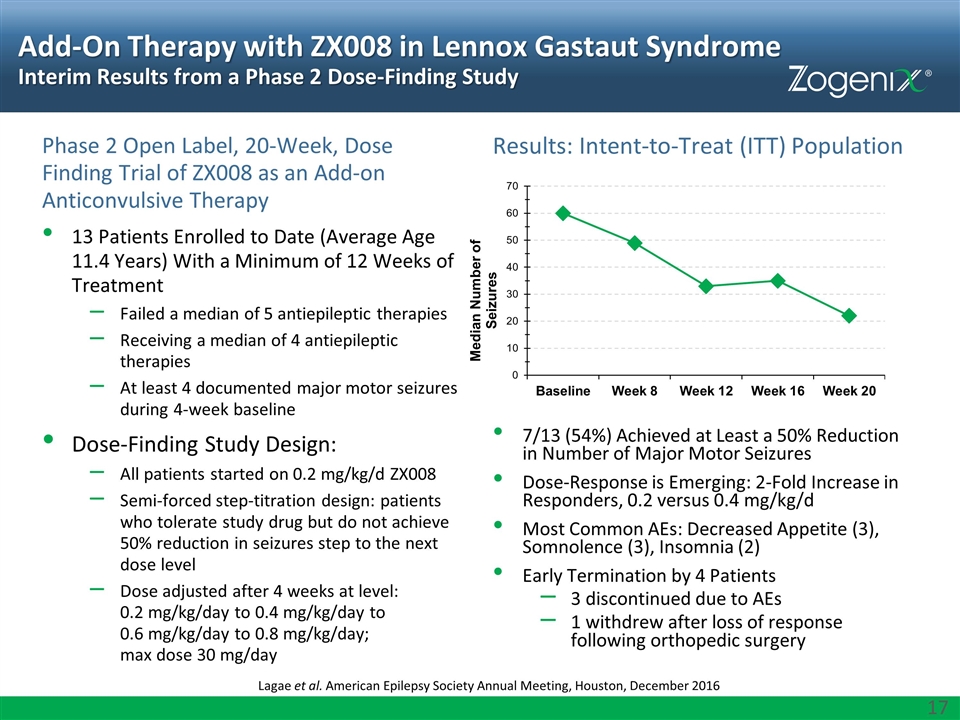
Add-On Therapy with ZX008 in Lennox Gastaut Syndrome Interim Results from a Phase 2 Dose-Finding Study Phase 2 Open Label, 20-Week, Dose Finding Trial of ZX008 as an Add-on Anticonvulsive Therapy 13 Patients Enrolled to Date (Average Age 11.4 Years) With a Minimum of 12 Weeks of Treatment Failed a median of 5 antiepileptic therapies Receiving a median of 4 antiepileptic therapies At least 4 documented major motor seizures during 4-week baseline Dose-Finding Study Design: All patients started on 0.2 mg/kg/d ZX008 Semi-forced step-titration design: patients who tolerate study drug but do not achieve 50% reduction in seizures step to the next dose level Dose adjusted after 4 weeks at level: 0.2 mg/kg/day to 0.4 mg/kg/day to 0.6 mg/kg/day to 0.8 mg/kg/day; max dose 30 mg/day Results: Intent-to-Treat (ITT) Population 7/13 (54%) Achieved at Least a 50% Reduction in Number of Major Motor Seizures Dose-Response is Emerging: 2-Fold Increase in Responders, 0.2 versus 0.4 mg/kg/d Most Common AEs: Decreased Appetite (3), Somnolence (3), Insomnia (2) Early Termination by 4 Patients 3 discontinued due to AEs 1 withdrew after loss of response following orthopedic surgery Lagae et al. American Epilepsy Society Annual Meeting, Houston, December 2016

Clinical and Regulatory Plan for ZX008 Development in Lennox Gastaut Syndrome Propose to conduct one global clinical study in LGS leveraging study sites participating in the current Dravet syndrome studies Two ZX008 doses compared to placebo using a study design similar to the on-going safety and efficacy Phase 3 trials in Dravet syndrome Pre-IND meeting responses from FDA in February with the intent of submitting an IND to enable Phase 3 initiation mid-2017 Primary endpoint: Change from baseline in number of drop seizures during the treatment period Approx. 200 subjects, 2-35 years Subjects who complete study will be eligible to enter an open-label extension safety study Anticipated regulatory filing strategy is sNDA (U.S.) and MAA variation (E.U.) pending Dravet syndrome approval

Establishing Product Protection ORPHAN DRUG STATUS Provides 7 Years in U.S. and 10 Years of Market Exclusivity in E.U. Further 3 Years in E.U. Due to Clinical Development via a Pediatric Investigational Plan PATENT APPLICATIONS Several Patent Families Being Pursued Globally Including Method(s) of Use, Elements of a Future REMS Program, and Novel Drug Synthesis Process Application 13/887,014 - Method For the Treatment of Dravet Syndrome – Newly Allowed by USPTO (Patent Expiry in 2033) Ongoing Preclinical and Development Work Providing Additional IP Opportunities PRODUCT SPECIFIC REMS Will Include Patient Registry and Cardiac Monitoring Difficult to Circumvent and Expensive to Replicate

Multiple Milestones in 2017 for Creating Value IND Approval to Initiate Phase 3 in LGS (Q1 2017) First Phase 3 Results in Dravet Syndrome (Q2 2017) Results From Remaining Phase 3 Trials in Dravet Syndrome (Q3/4 2017) Potential NDA / MAA Submissions for Dravet Syndrome (Q4 2017) Phase 3 Start in LGS (Mid-2017)
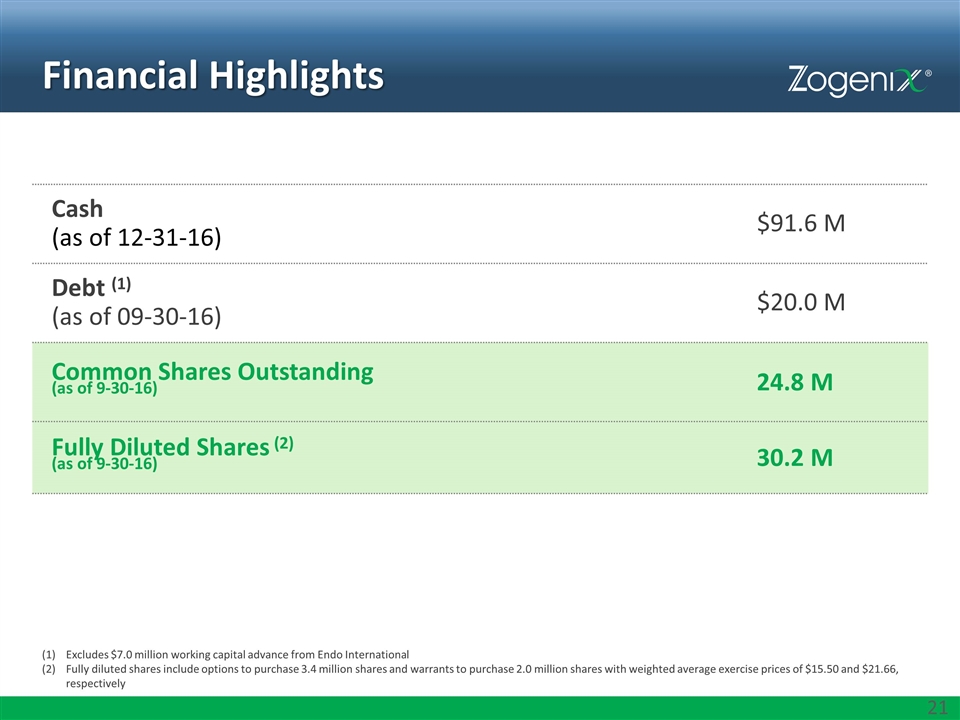
Financial Highlights Cash (as of 12-31-16) $91.6 M Debt (1) (as of 09-30-16) $20.0 M Common Shares Outstanding (as of 9-30-16) 24.8 M Fully Diluted Shares (2) (as of 9-30-16) 30.2 M Excludes $7.0 million working capital advance from Endo International Fully diluted shares include options to purchase 3.4 million shares and warrants to purchase 2.0 million shares with weighted average exercise prices of $15.50 and $21.66, respectively

HIGHLY FOCUSED ON ORPHAN CNS DISORDERS DRAVET SYNDROME PHASE 3 RESULTS ON TRACK FOR Q2 2017 EXPERIENCED TEAM IN PLACE FULLY FUNDED THROUGH 2017 Planning LGS Phase 3 Initiation in miD-2017 NASDAQ: ZGNX
