Attached files
| file | filename |
|---|---|
| 8-K - 8-K - EXACT SCIENCES CORP | a17-1646_18k.htm |
| EX-99.1 - EX-99.1 - EXACT SCIENCES CORP | a17-1646_1ex99d1.htm |
Exhibit 99.2
35th Annual J.P. Morgan Healthcare Conference Kevin Conroy, Chairman and CEO January 12, 2017

Safe harbor statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended that are intended to be covered by the "safe harbor" created by those section s. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as "believe," "expect," "may," "will," "should," "could," "seek," "intend," "plan," "estimate," "anticipate" or other comparable terms. All statements other than statements of historical facts included in this presentation regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding expected future operating results, anticipated results of our sales and marketing efforts, expectations concerning payor reimbursement and the anticipated results of our product development efforts. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward -looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward -looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to successfully and profitably market our products and services; the acceptance of our products and services by patients and healthcare providers; the willingness of health insurance companies and other payors to cover Cologuard and reimburse us for our performance of the Cologuard test; the amount and nature of competition from othe r cancer screening products and services; the effects of any healthcare reforms, including the Affordable Care Act, or changes in healthcare pricing, coverage and reimbursement; recommendations, guidelines and/or quality metrics issued by various organizations such as the U.S. Preventive Services Task Force, the American Cancer Society and the National Committee for Quality Assurance regarding cancer screening or our products and services; our ability to successfully develop new products and services; our success establishing and maintaining collaborative licensing and supplier arrangements; our ability to maintain regulatory approvals and comply with applicable regulations; and the other risks and uncertainties described in t he Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our most recently filed Annual Report on Form 10-K and our subsequently filed Quarterly Report(s) on Form 10-Q. We undertake no obligation to publicly update any forward-looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise.

Overview of presentation Exact Sciences’ mission Opportunity for Cologuard Strong performance in 2016 Becoming the standard of care Pipeline built on Cologuard platform 3
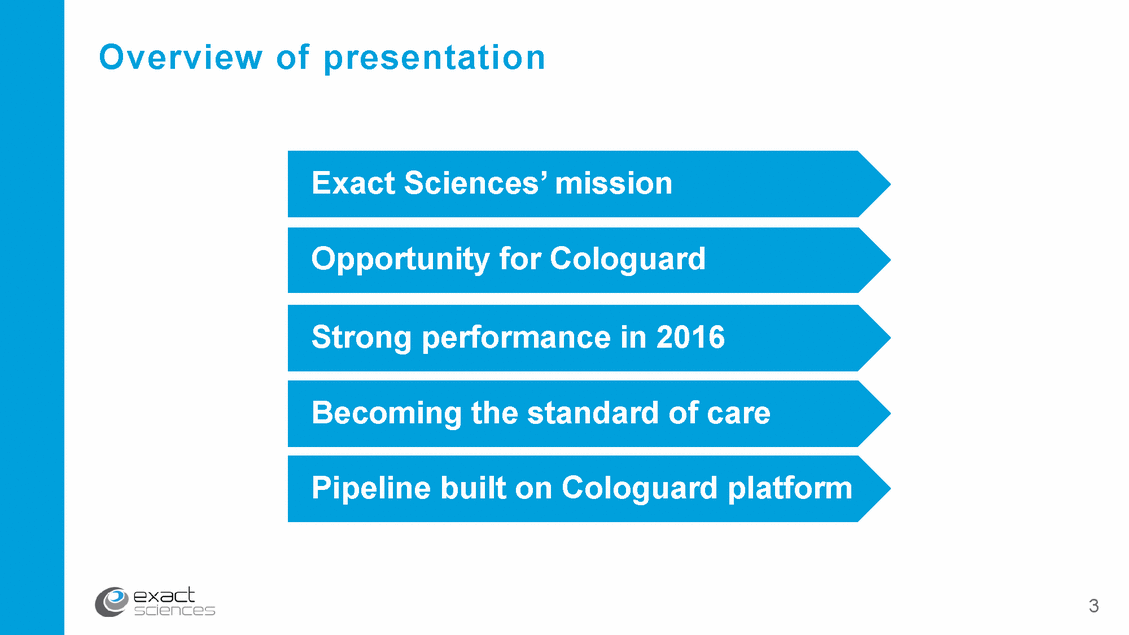
Becoming the leader in advanced cancer diagnostics Unique Use deep regulatory, guideline & reimbursement experience Regulat expoerryiene Extend Cologuard platform to next generation of liquid biopsy cancer diagnostics Product breadth Proven leader in technology & business model Innovation Mission & vision Clinical knowledge Leverage Mayo Clinic collaboration & internal knowledge Commercial expertise Build on commercial capabilities to reach physicians & patients 4
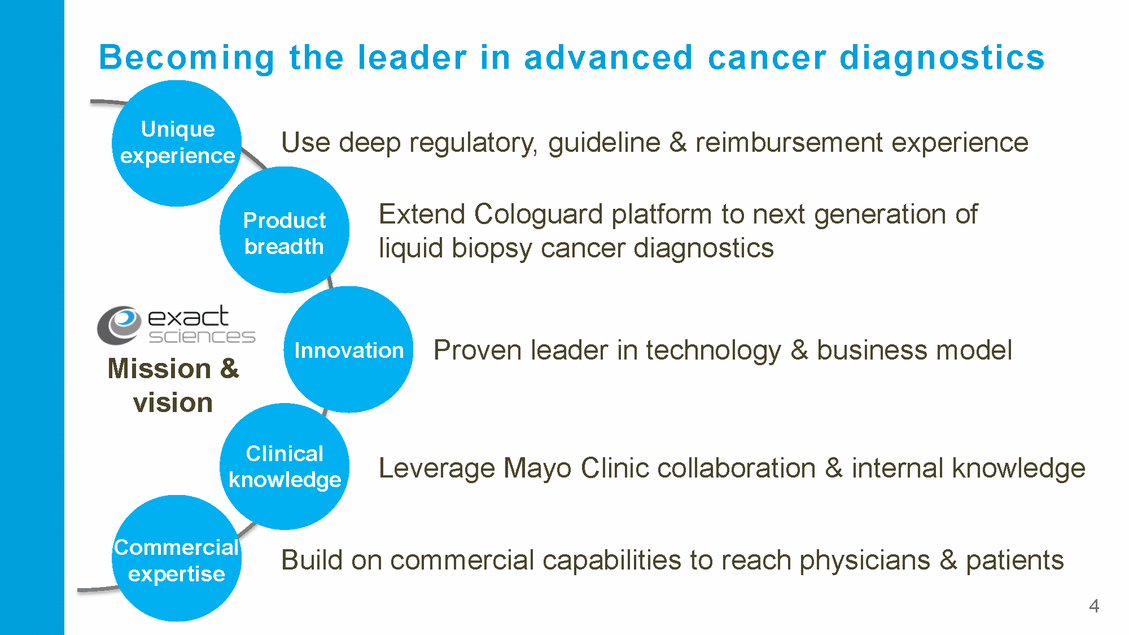
Colon cancer: America’s second deadliest cancer 155,870 50,260 43,090 41,070 26,730 15,690 Esophageal Prostate Breast Pancreas Colorectal Lung Annual cancer deaths 5 Source: American Cancer Society, Cancer Facts & Figures 2017; all figures annual 135,430 new diagnoses 50,260 deaths
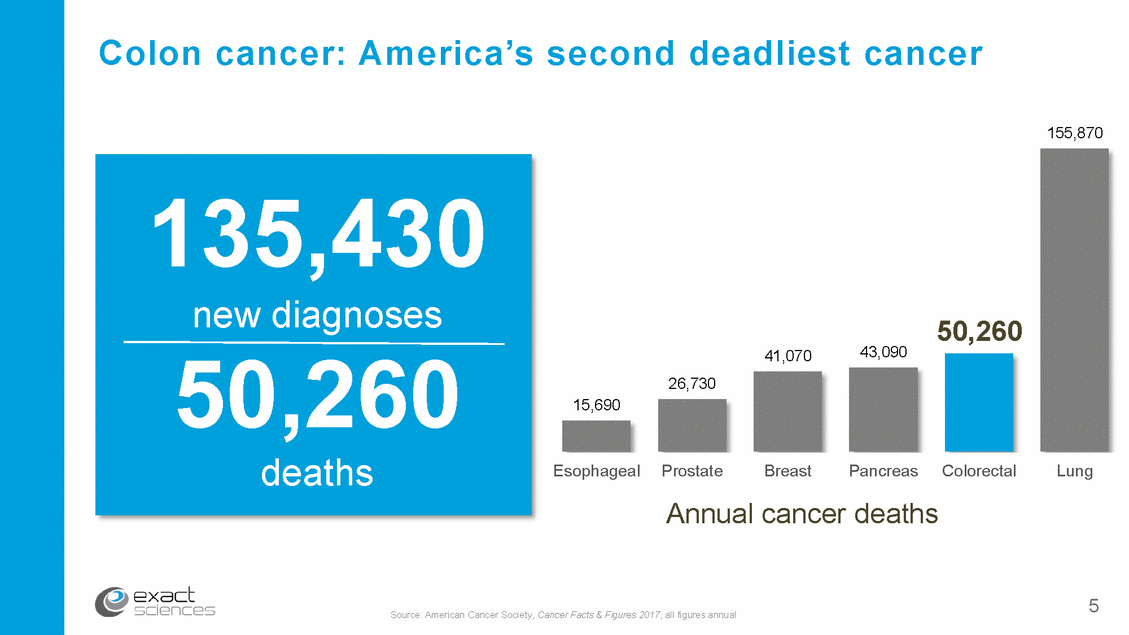
“The most preventable, yet cancer” least prevented – Journal of the National Cancer Institute form of Pre-cancerous polyp Cancer 10+ years 6 Sources: J Natl Cancer Inst. 2009; 101:1225-1227 (Itzkowitz) Gastro 1997;112:594-692 (Winawer)
Detecting colorectal cancer early is critical Majority of patients diagnosed in stages III-IV Diagnosed in Stages I or II Diagnosed in Stage IV 9 out of 10 survive 5 years 1 out of 10 survive 5 years 7 Sources: SEER 18 2004-2010 American Cancer Society, Cancer Facts & Figures 2016; all figures annual
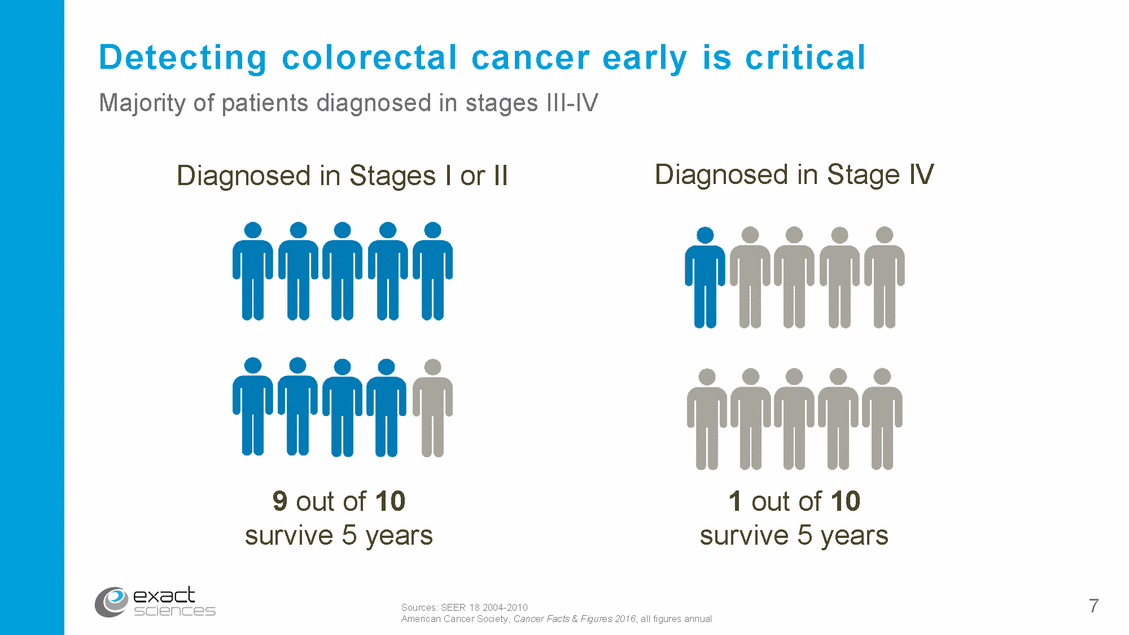
America’s stagnant colon cancer screening rate 80% goal 59% 58% 52% 50% 2005 2008 2010 2013 2018 Actual colon cancer screening rates Sources: CDC NHIS survey results as published in the CDC’s MMWR between 2006 and 2015 CDC BFRSS survey as published in MMWR (2013) 8 Rx Only
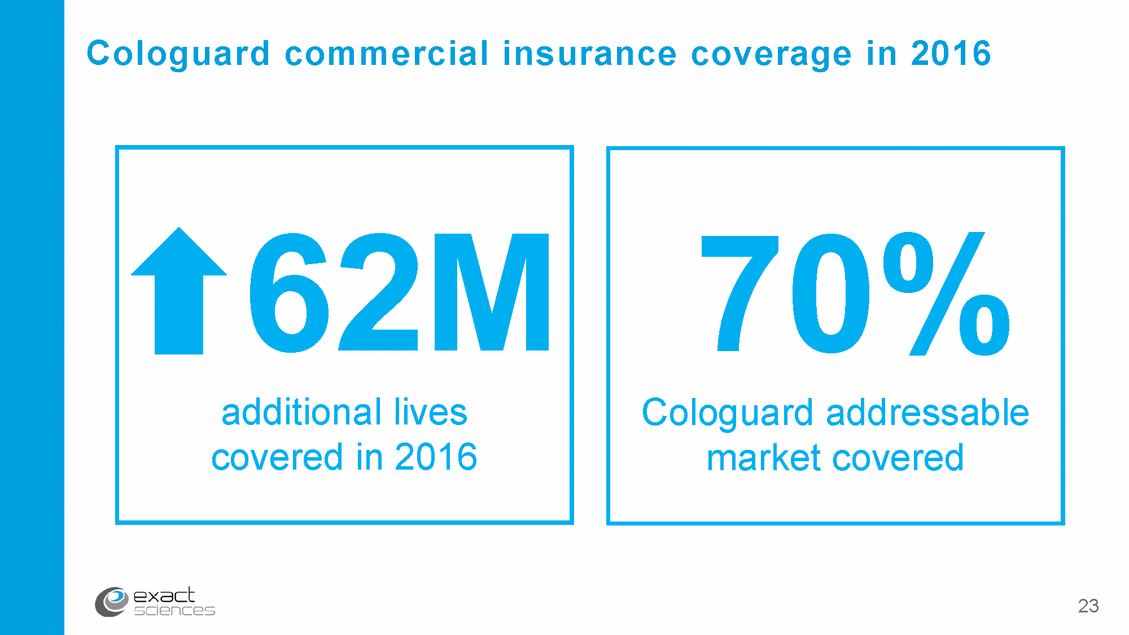
Cologuard: Addressing the colon cancer challenge 94% early-stage cancer sensitivity Powered by advanced DNA technology Easy-to-use & non-invasive while requiring no preparation, sedation, or time-off FDA approved & included in major guidelines Insurance coverage for 70% of addressable population (80M people), including Medicare developed with 9 Rx Only Source: Imperiale TF et al., N Engl J Med (2014)

Driving patient compliance with colon cancer screening Reminder call Cologuard delivered to home Reminder letter 67% Welcome call 24/7 patient support line Patient compliance 10 Cologuard’s compliance rate is derived from the number of completed tests reported divided by the number of collection kits shipped to patients during the 12-month period ending 60 days prior to Dec. 31, 2016, excluding program orders.
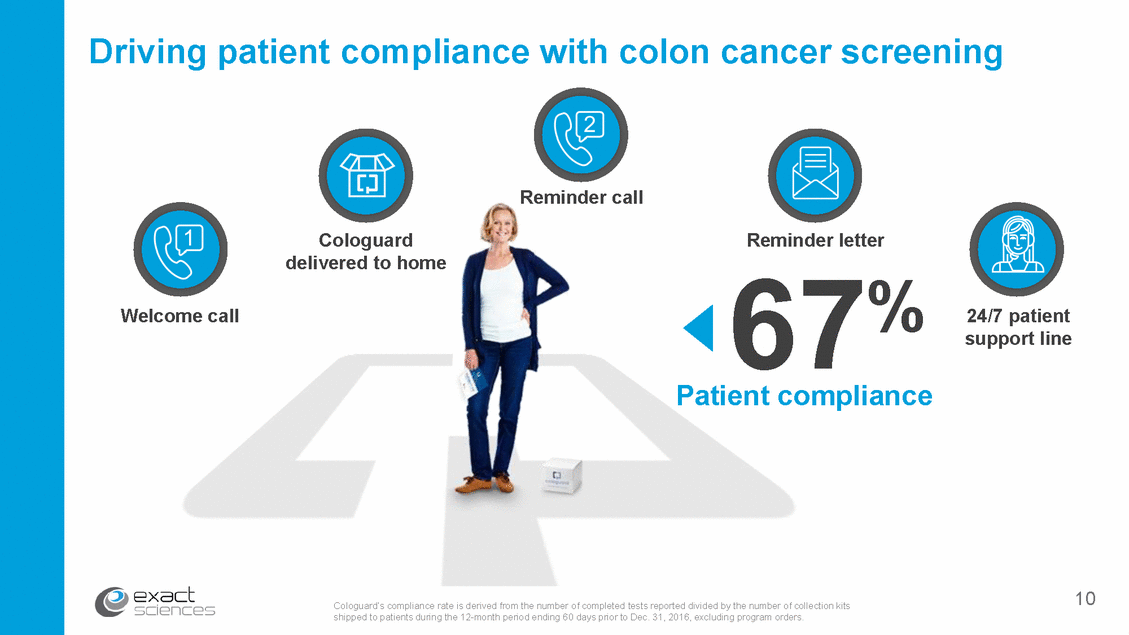
Impact of patient navigation program on compliance 67% FOBT* colonoscopy** *** Sources: *Patient adherence over 3 years’ Liang PS., et al., Am J Gastroenterol. 2016 **Patient compliance within 1 year; Arch Intern Med 2012; 172(7):575-582 (Inadomi) ***Cologuard’s compliance rate is derived from the number of completed tests reported divided by the number of collection kits shipped to patients during the 12-month period ending 60 days prior to Dec. 31, 2016, excluding program orders. During the past 12 months, the patient compliance rate for Cologuard held stable at approximately 70-75% for Medicare and approximately 55-60% for commercially insured. Medicare beneficiaries account for approximately 2/3 of Cologuard orders. 11 38% 14%
Strong customer satisfaction with Cologuard Physicians’ expectations % Patients rated Cologuard experience % 12 Sources: ZS survey conducted for Exact Sciences, n=300 Exact Sciences Laboratories patient satisfaction survey data is cumulative; n = 2,799 90very positive met or exceeded 98
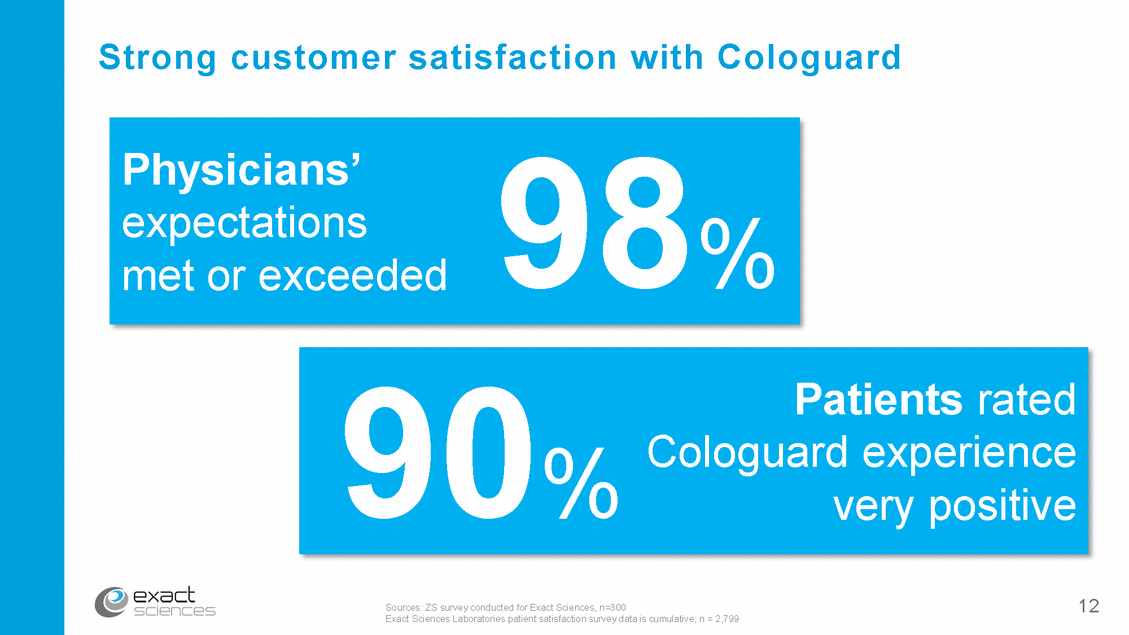
Impact of Cologuard during 2016 * Early-stage colorectal cancer detected in ~3 patients a day 244,000 completed ~1100 early-stage cancers Cologuard tests ~1500 cancers *Exact Sciences internal estimates based upon prevalence and detection rates from DeeP-C study (Imperiale TF et al., N Engl J Med (2014) 13
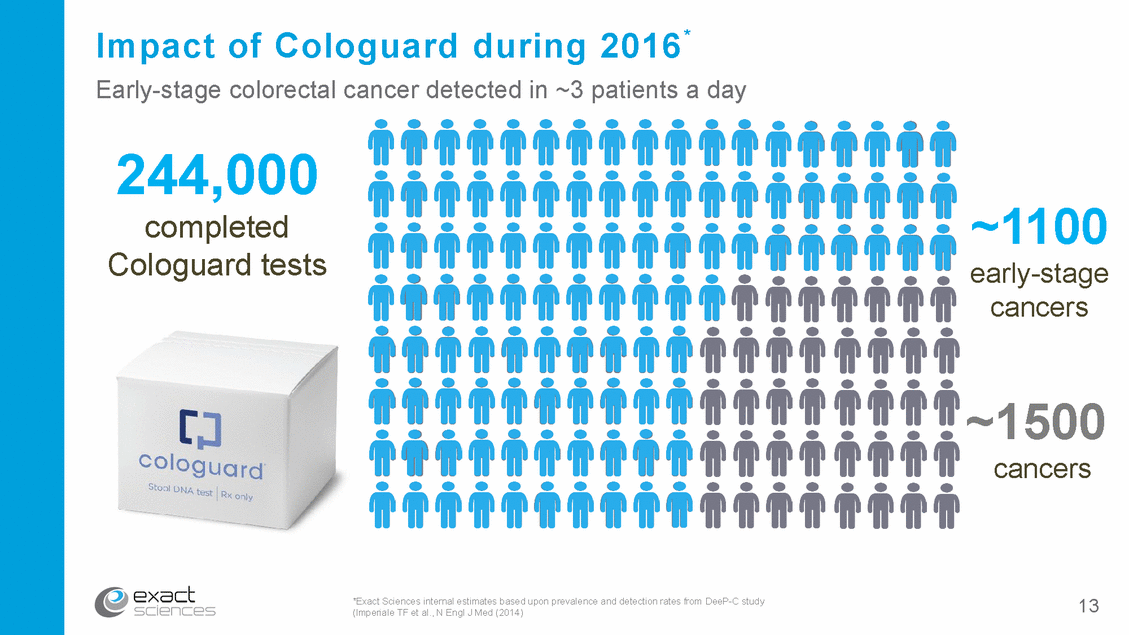
A multi-billion-dollar U.S. market opportunity Potential 80M-patient U.S. screening market* Target U.S. market opportunity for Cologuard Unscreened** $4B*** 24M pat 30% s Colog 24M ard**** ients Colonoscopy & FOBT***** 32M patients 40% *80 million average-risk, asymptomatic people ages 50-85 **Assumes unscreened decreases from 42% to 30% ***Assumes 24M people screened with Cologuard every three years with ASP of $500 ****Assumes 30% market share for Cologuard *****Assumes 40% market share for colonoscopy & FOBT 14
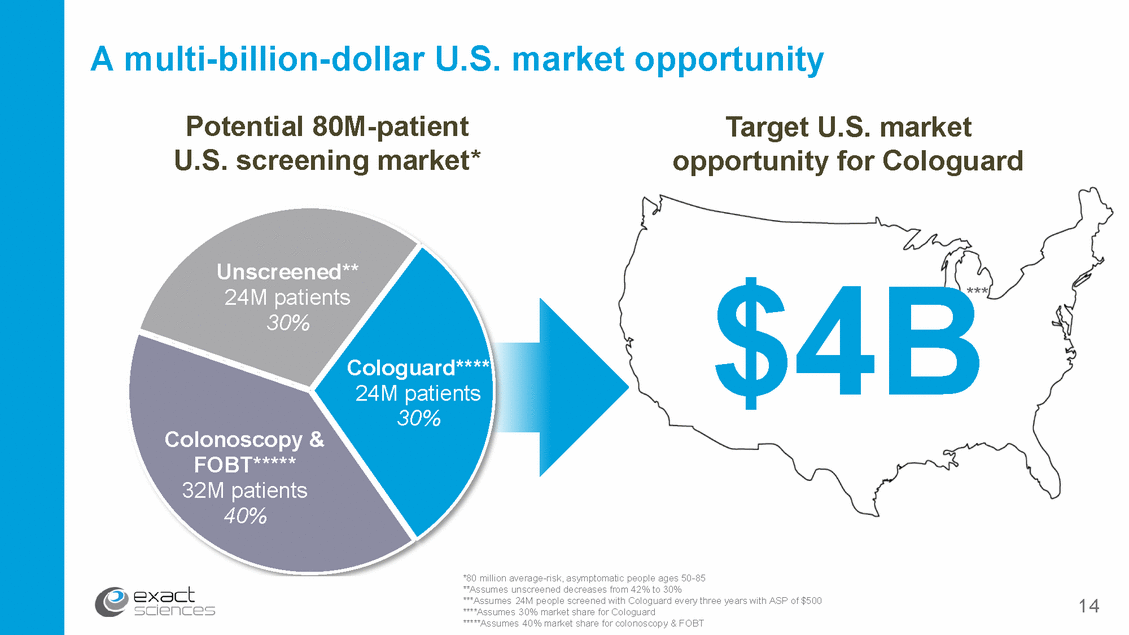
Commercial strategy engages key audiences National TV campaign Public relations Digital marketing Primary care sales force Major screening guidelines Market access team Clinical & health publications Publications Collateral materials 15 Physicians Patients
National TV campaign increasing ordering & adoption I Qt exact 16 sciences
Exact Sciences nationwide sales force: Establishing a new standard for colon cancer screening 17 Inside sales force •Extend reach of sales force coverage •Rapidly fulfill new business opportunities •Support physician office & lab clients Primary care sales force •Educate physicians & office staff •Focus on top 2 to 3 deciles of primary care physician offices •Create repeat ordering of Cologuard
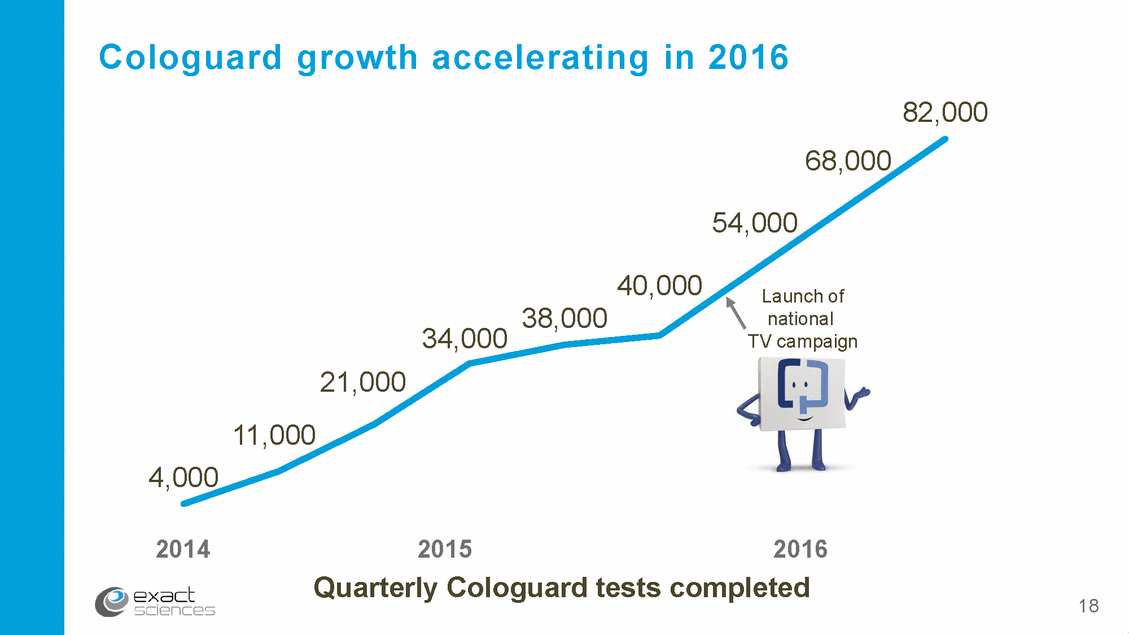
Cologuard growth accelerating in 2016 82,000 68,000 54,000 40,000 Launch of national TV campaign 38,000 34,000 21,000 11,000 4,000 2014 2015 Quarterly Cologuard tests 2016 completed 18
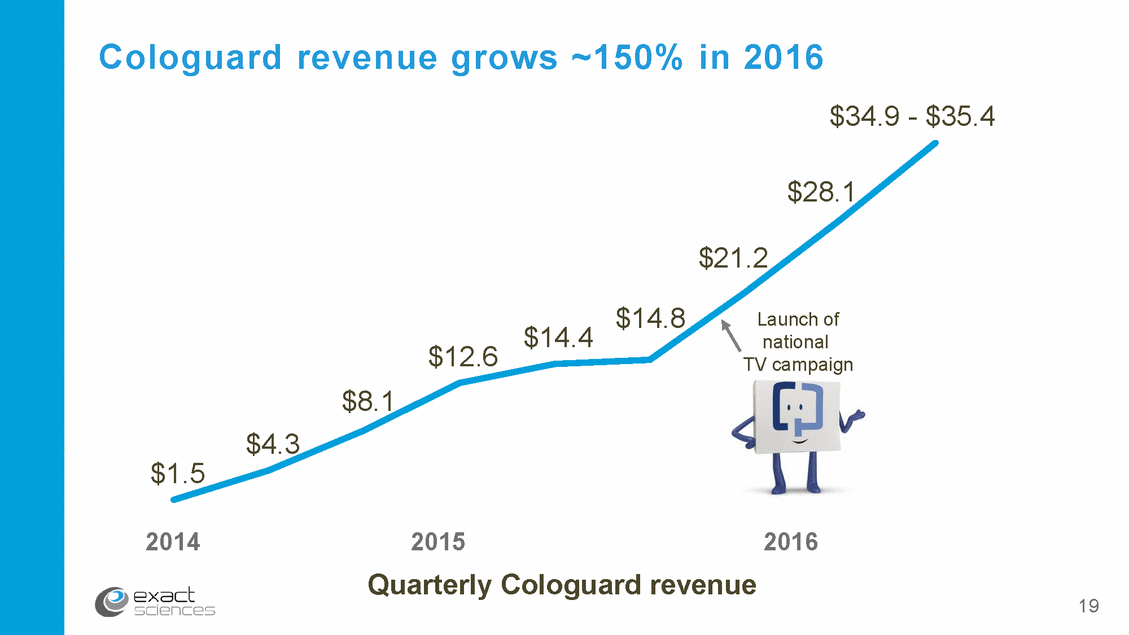
Cologuard revenue grows ~150% in 2016 $34.9 - $35.4 $28.1 $21.2 $14.8 Launch of national TV campaign $14.4 $12.6 $8.1 $4.3 $1.5 2014 2015 Quarterly Cologuard revenue 2016 19
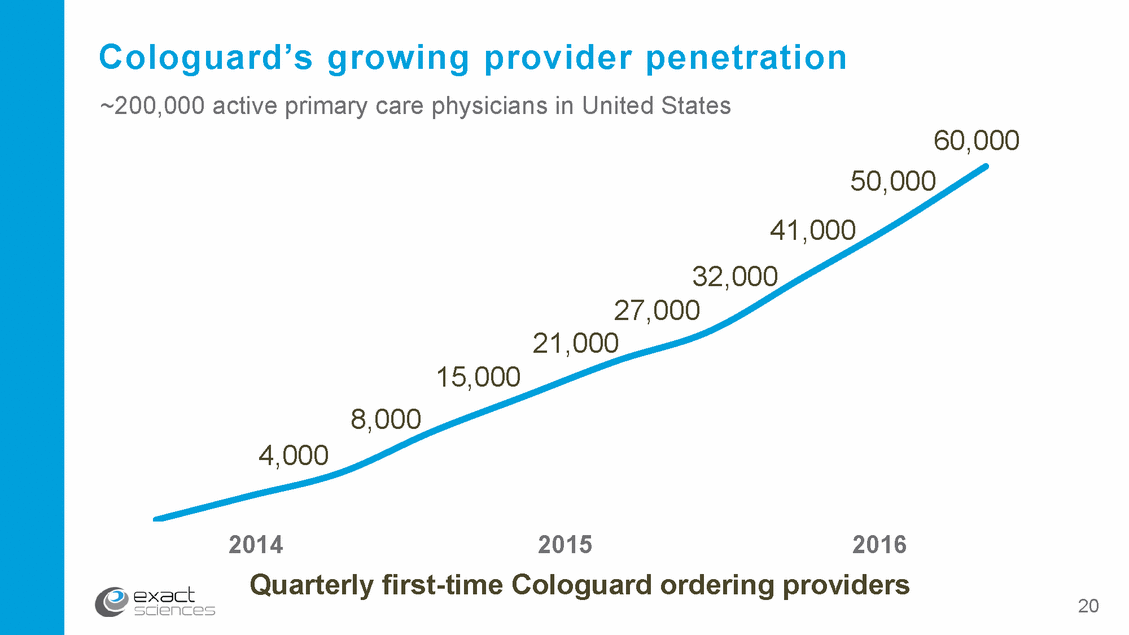
Cologuard’s growing provider penetration ~200,000 active primary care physicians in United States 60,000 50,000 41,000 32,000 27,000 21,000 15,000 8,000 4,000 2014 2015 2016 providers Quarterly first-time Cologuard ordering 20
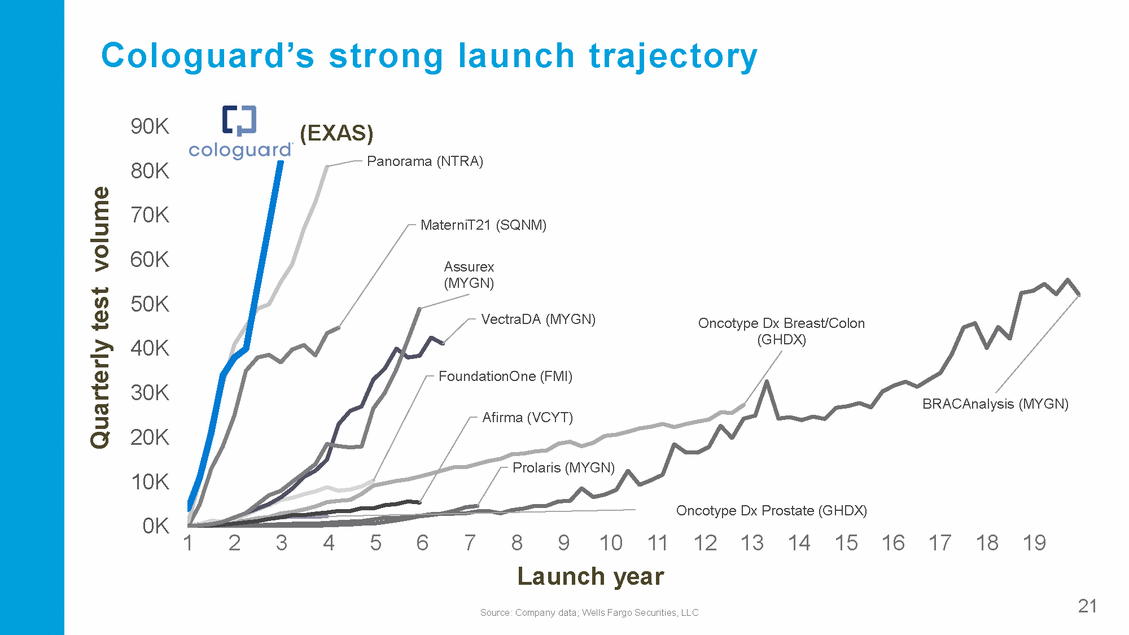
Cologuard’s strong launch trajectory 90K 80K (EXAS) Panorama (NTRA) 70K 60K 50K MaterniT21 (SQNM) Assurex (MYGN) VectraDA (MYGN) Oncotype Dx Breast/Colon (GHDX) 40K 30K 20K FoundationOne (FMI) BRACAnalysis (MYGN) Afirma (VCYT) Prolaris (MYGN) 10K 0K Oncotype Dx Prostate (GHDX) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Launch year Source: Company data; Wells Fargo Securities, LLC 21 Quarterly test volume
Cologuard commercial insurance coverage in 2016 Blue Cross and Blue Shield of Montana Highmark Blue Cross Blue Shield West Virginia Regence BlueCross BlueShield of Utah Pacific Source Health Plans Rocky Mountain Health Plans (RMHP) Arise Health Plan Regence BlueShield of Idaho Group Health Cooperative of Eau Claire New Mexico Health Connections Health Tradition Health Plan, Inc. Asuris Northwest Health FirstCarolinaCare Insurance Company BridgeSpan Health Company Cigna Corporation Humana, Inc. Blue Cross and Blue Shield of Illinois Health Net, Inc. Blue Cross and Blue Shield of Texas Centene Corporation Highmark Blue Cross Blue Shield Blue Cross and Blue Shield of Florida, Inc. Blue Cross and Blue Shield of Minnesota Medica Health Plans Blue Cross and Blue Shield of Louisiana Medical Mutual Wellmark Blue Cross and Blue Shield of Iowa Harvard Pilgrim Health Care, Inc. Blue Cross and Blue Shield of Oklahoma Blue Cross Blue Shield of Arizona Blue Cross and Blue Shield of Kansas Regence BlueShield MVP Health Care BlueCross BlueShield of Western New York and BlueShield of Northeastern New York Blue Cross of Idaho Health Service, Inc. Geisinger Health Plan Blue Cross and Blue Shield of New Mexico Regence BlueCross BlueShield of Oregon Highmark Blue Cross Blue Shield Delaware 1199SEIU United Healthcare Workers East Gateway Health Plan AvMed Wellmark Blue Cross and Blue Shield of South Dakota 22
Cologuard commercial insurance coverage in 2016 23 70% Cologuard addressable market covered 62M additional lives covered in 2016
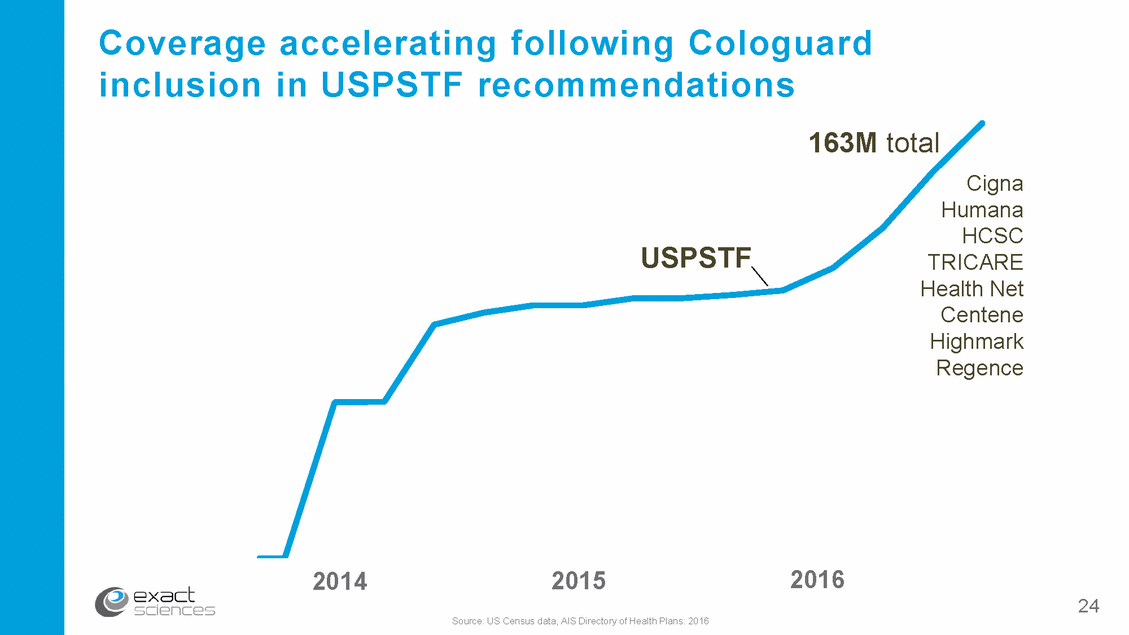
Coverage inclusion accelerating following Cologuard in USPSTF recommendations 163M total Cigna Humana HCSC TRICARE Health Net Centene Highmark Regence USPSTF 2016 2014 2015 Source: US Census data, AIS Directory of Health Plans: 2016 24
Cologuard becoming the standard of Demand increasing with guideline inclusion & quality credit care Quality measures Regulatory Guidelines 25

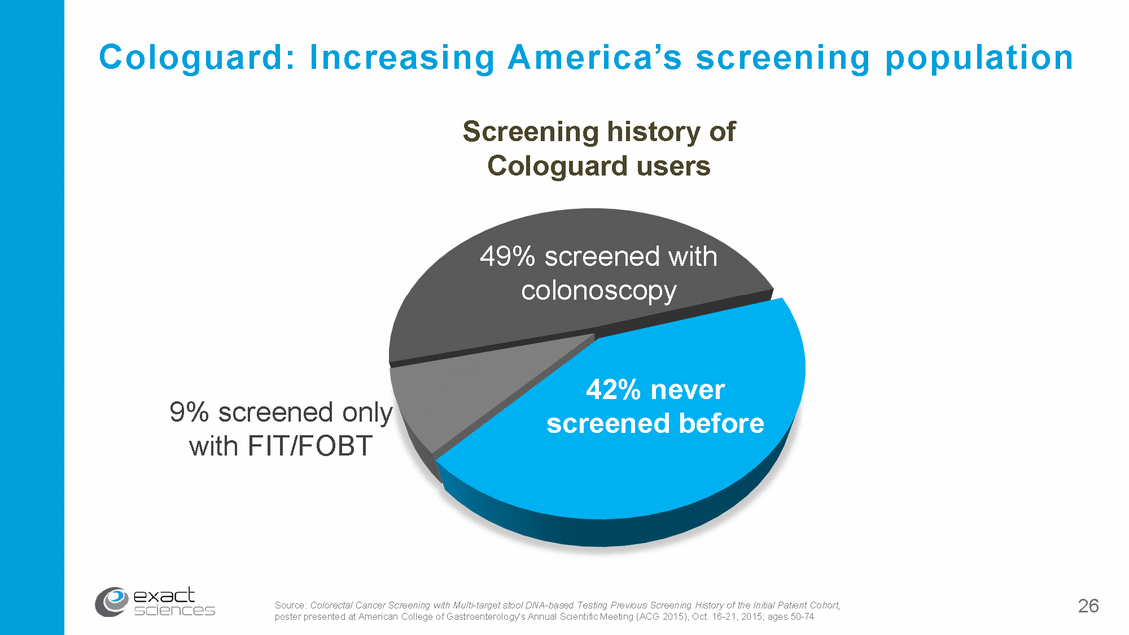
Cologuard: Increasing America’s screening population Screening history of Cologuard users 49% screened with colonoscopy 42% never screened before 9% screened only with FIT/FOBT 26 Source: Colorectal Cancer Screening with Multi-target stool DNA-based Testing Previous Screening History of the Initial Patient Cohort, poster presented at American College of Gastroenterology's Annual Scientific Meeting (ACG 2015), Oct. 16-21, 2015; ages 50-74
Building a pipeline on Cologuard platform

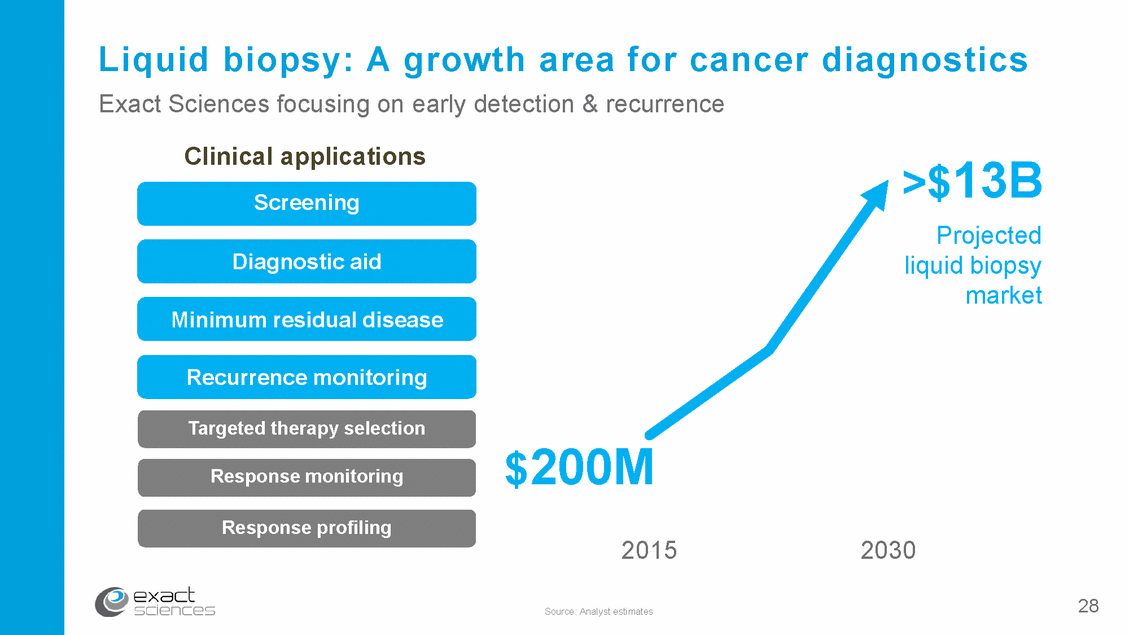
Liquid biopsy: A growth area for cancer Exact Sciences focusing on early detection & recurrence diagnostics Clinical applications Screening >$13B Projected liquid biopsy market Diagnostic aid Minimum residual disease Recurrence monitoring Targeted therapy selection $200M 2015 Response monitoring Response profiling 2030 28 Source: Analyst estimates
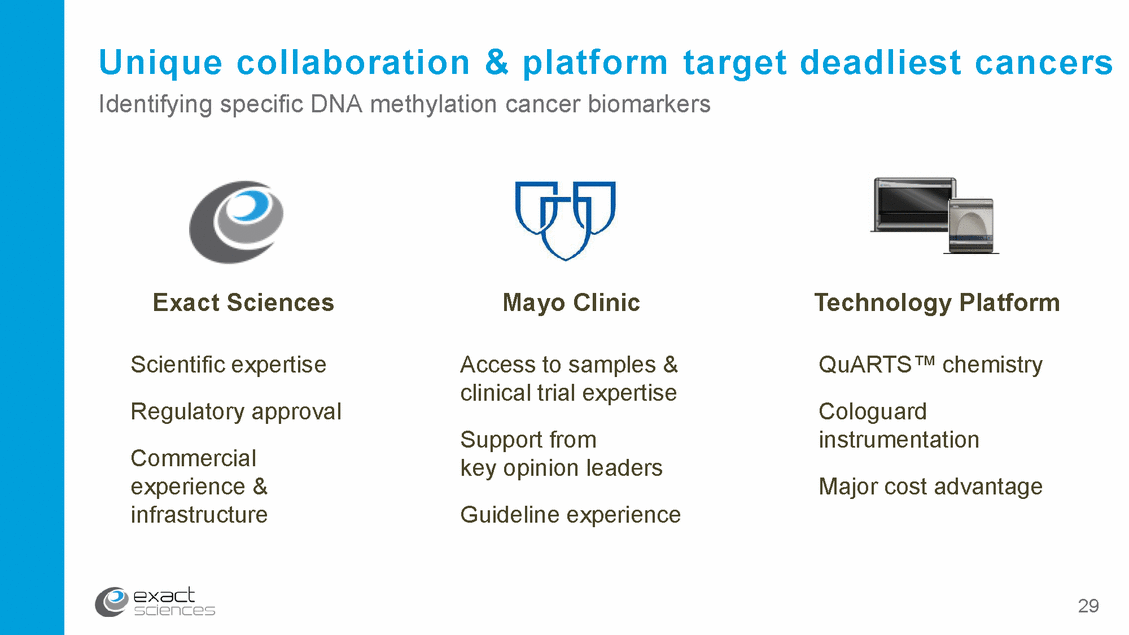
Unique collaboration & platform target Identifying specific DNA methylation cancer biomarkers deadliest cancers Exact Sciences Mayo Clinic Technology Platform Scientific expertise Regulatory approval Commercial experience & infrastructure Access to samples & clinical trial expertise Support from key opinion leaders Guideline experience QuARTS™ chemistry Cologuard instrumentation Major cost advantage 29
The DNA methylation advantage DNA methylation (epigenetics) changes genetic function without changing DNA sequence High specificity & sensitivity achieved with a small number of markers Methylation markers enable cancer site specificity Exact Sciences’ technology platform, coupled with a small number of markers, is capable of driving major cost advantages over Next-Generation Sequencing (NGS) 30 Our collaboration with Mayo Clinic has identified proprietary methylation markers for most major cancers
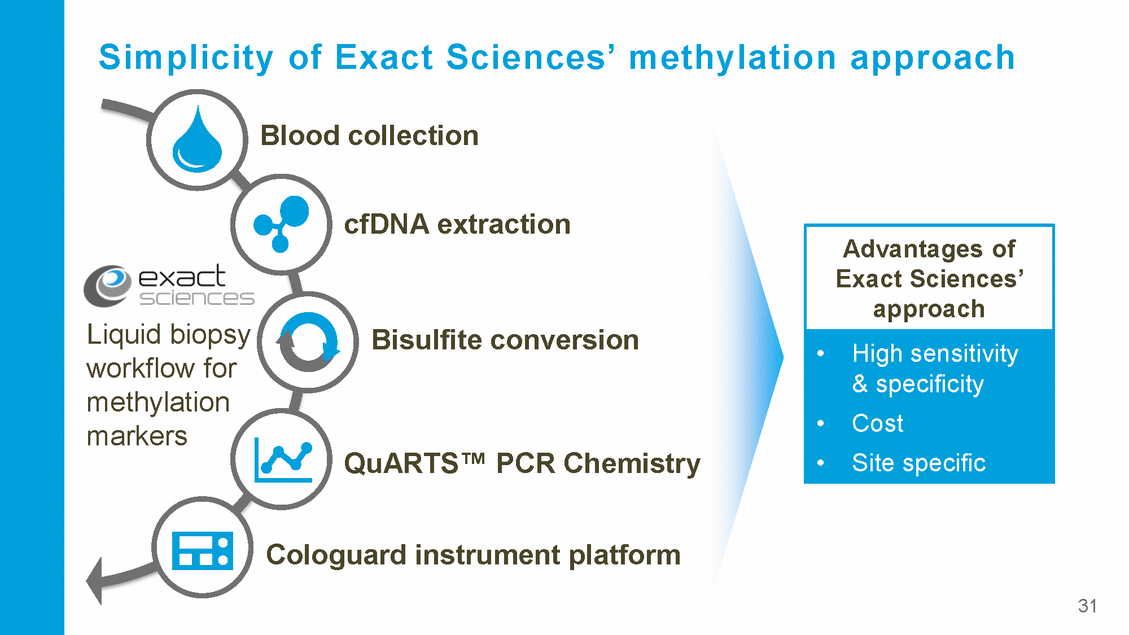
Simplicity of Exact Sciences’ methylation approach Blood collection cfDNA extraction Liquid biopsy workflow for methylation markers Bisulfite conversion QuARTS™ PCR Chemistry Cologuard instrument platform 31 Advantages of Exact Sciences’ approach •High sensitivity & specificity •Cost •Site specific
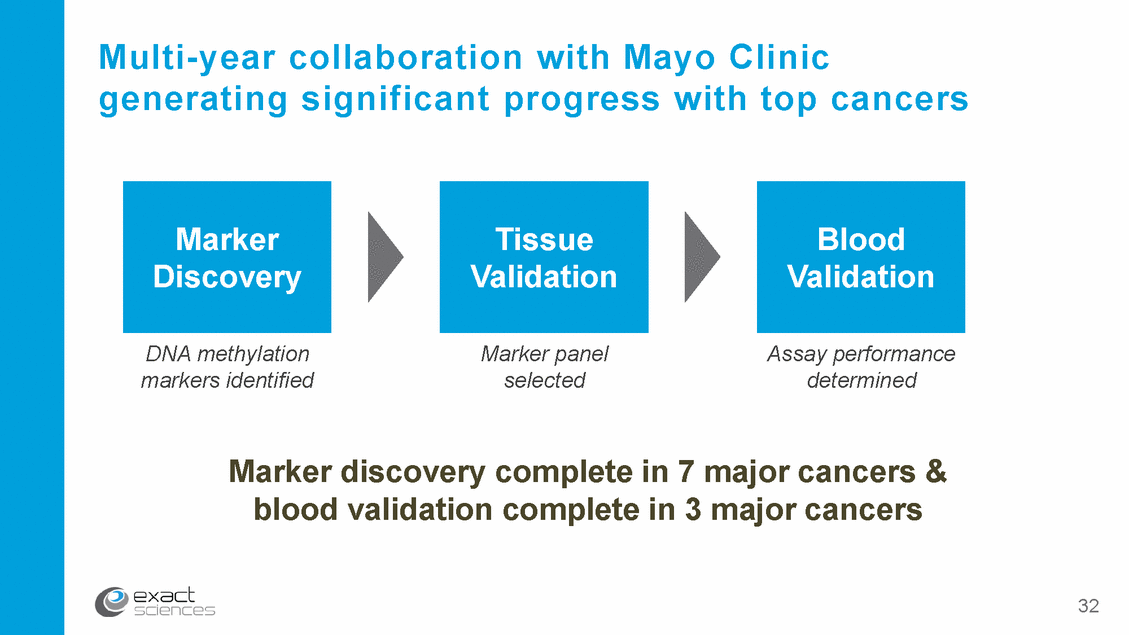
Multi-year collaboration with Mayo Clinic generating significant progress with top cancers DNA methylation markers identified Marker panel selected Assay performance determined Marker discovery complete in 7 major cancers & blood validation complete in 3 major cancers 32 Blood Validation Tissue Validation Marker Discovery
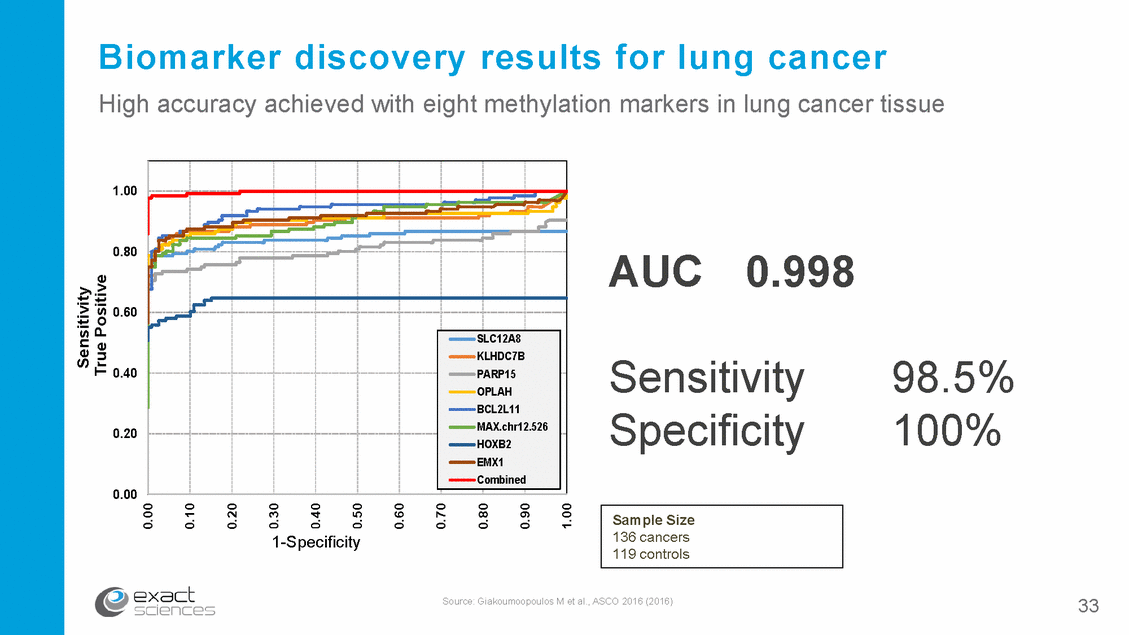
Biomarker discovery results for lung cancer High accuracy achieved with eight methylation markers in lung cancer tissue 1.00 0.80 AUC 0.998 0.60 Sensitivity Specificity 98.5% 100% 0.40 0.20 0.00 1-S1p-eScpifei ctifyicity Source: Giakoumoopoulos M et al., ASCO 2016 (2016) 33 Sensitivity True Positive 0.00 0.10 0.20 0.30 0.40 0.50 0.60 0.70 0.80 0.90 1.00 Sample Size 136 cancers 119 controls SLC12A8 KLHDC7B PARP15 OPLAH BCL2L11 MAX.chr12.526 HOXB2 EMX1 Combined
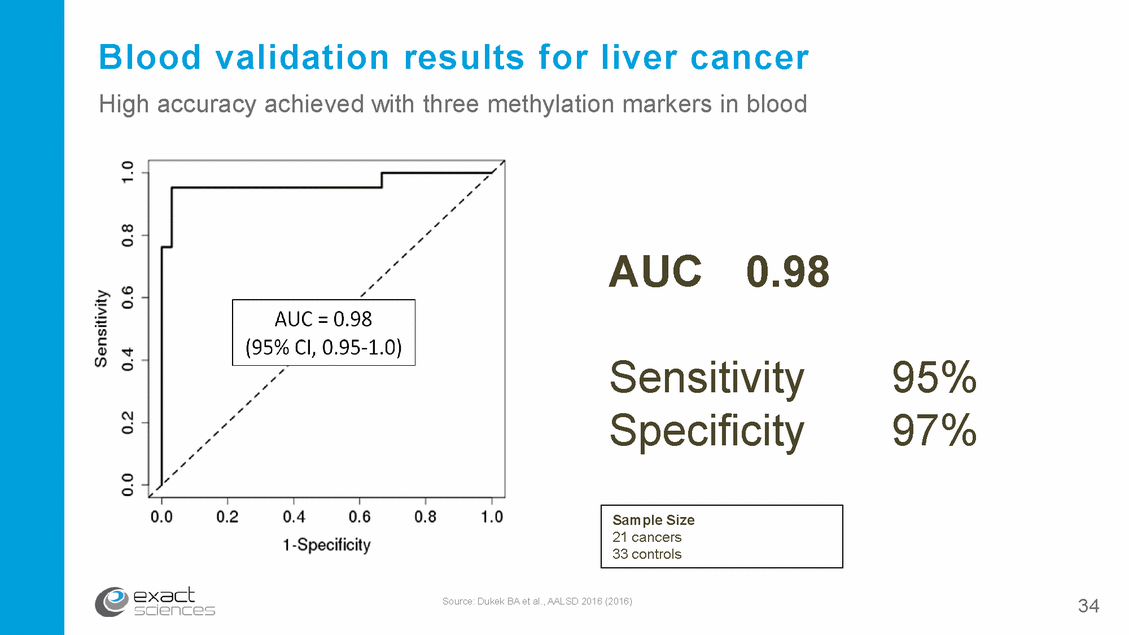
Blood validation results for liver cancer High accuracy achieved with three methylation markers in blood AUC 0.98 Sensitivity Specificity 95% 97% Source: Dukek BA et al., AALSD 2016 (2016) 34 Sample Size 21 cancers 33 controls
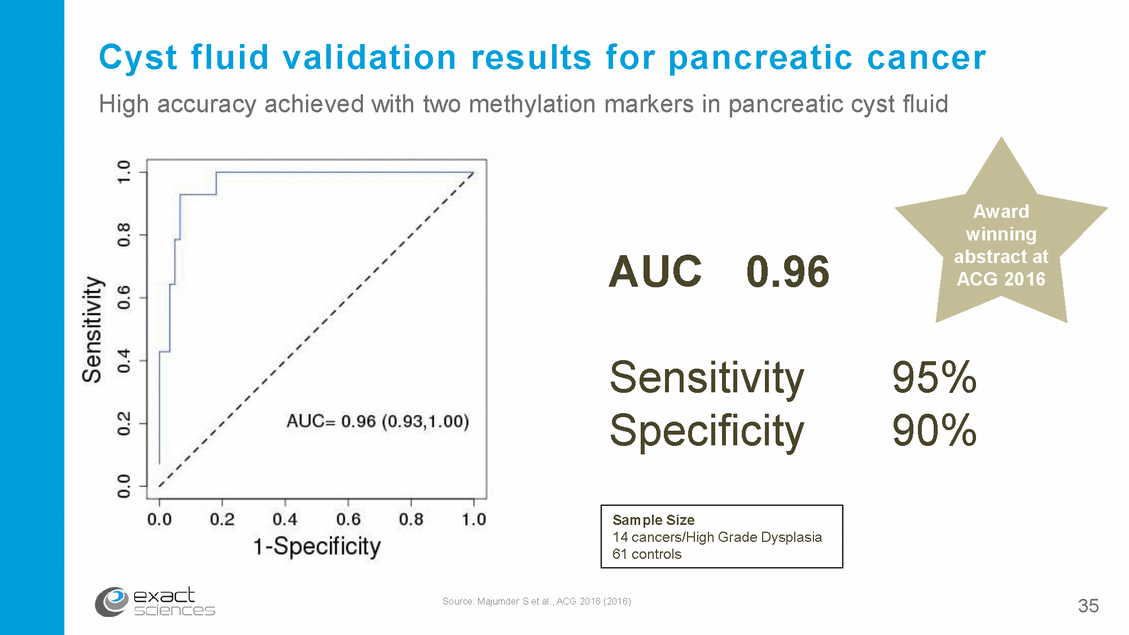
Cyst fluid validation results for pancreatic cancer High accuracy achieved with two methylation markers in pancreatic cyst fluid Award winning abstract at ACG 2016 AUC 0.96 Sensitivity Specificity 95% 90% Source: Majumder S et al., ACG 2016 (2016) 35 Sample Size 14 cancers/High Grade Dysplasia 61 controls
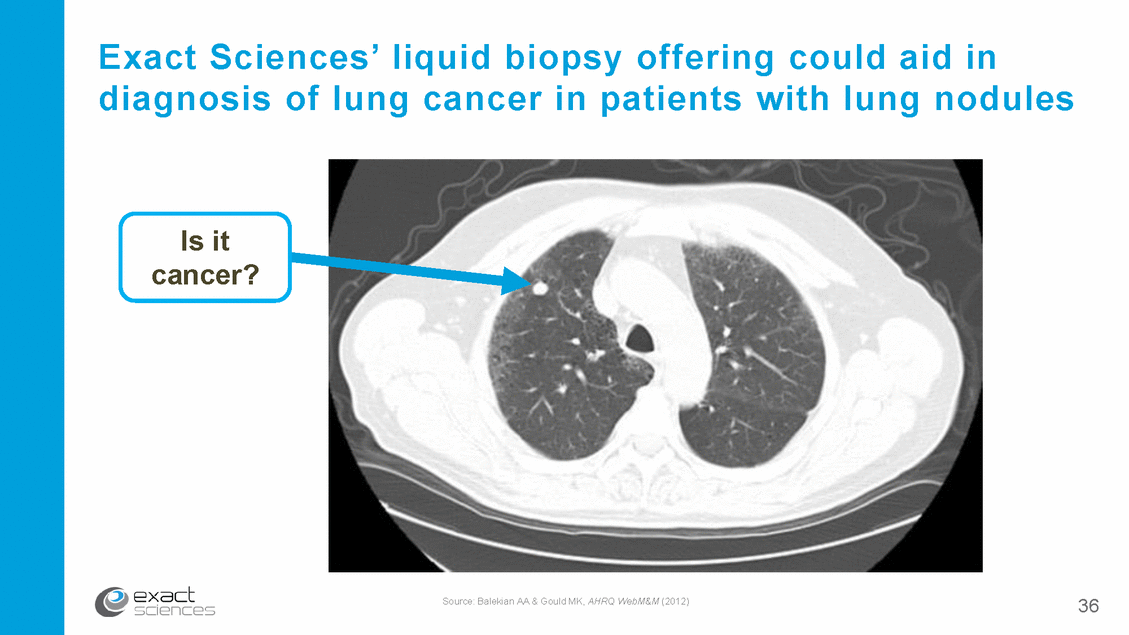
Exact Sciences’ liquid biopsy offering could aid in diagnosis of lung cancer in patients with lung nodules Is it cancer? Source: Balekian AA & Gould MK, AHRQ WebM&M (2012) 36
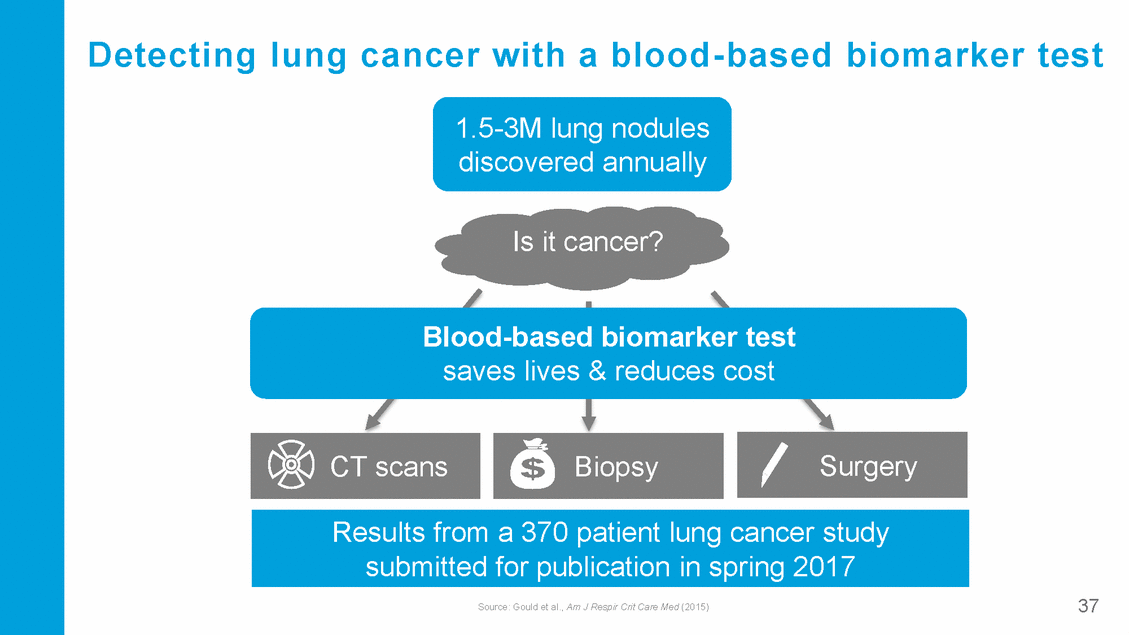
Detecting lung cancer with a blood -based biomarker test 1.5-3M lung nodules discovered annually Is it cancer? Blood-based biomarker test saves lives & reduces cost delayed cancer treatment (under-treatment) and high costs 37 Source: Gould et al., Am J Respir Crit Care Med (2015) Results Ufnronemcesasar3y7co0mpliacatitieo nst(oluvenrtgreactmaennct),er study submitted for publication in spring 2017 Surgery Biopsy CT scans
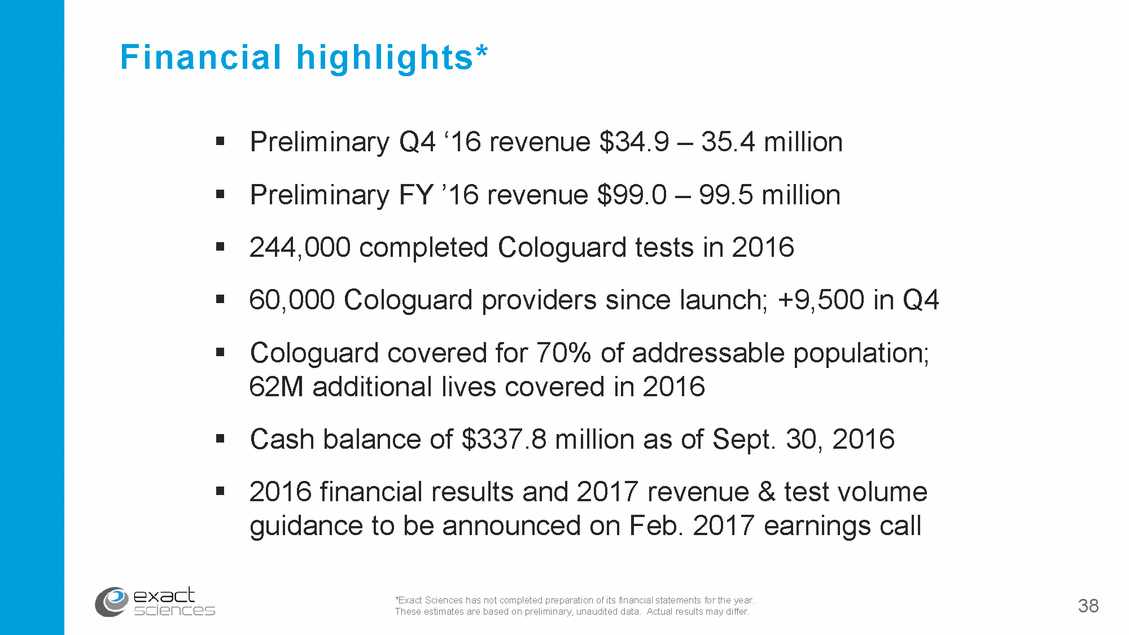
Financial highlights* Preliminary Q4 ‘16 revenue $34.9 – 35.4 million Preliminary FY ’16 revenue $99.0 – 99.5 million 244,000 completed Cologuard tests in 2016 60,000 Cologuard providers since launch; +9,500 in Q4 Cologuard covered for 70% of addressable population; 62M additional lives covered in 2016 Cash balance of $337.8 million as of Sept. 30, 2016 2016 financial results and 2017 revenue & test volume guidance to be announced on Feb. 2017 earnings call *Exact Sciences has not completed preparation of its financial statements for the year. These estimates are based on preliminary, unaudited data. Actual results may differ. 38
exact sciences

