Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Aimmune Therapeutics, Inc. | d286257d8k.htm |

J.P. Morgan Healthcare Conference January 2017 Less risk. More life. Stephen Dilly, CEO Exhibit 99.1

Forward-Looking Statements This presentation contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, clinical development plans, anticipated milestones, product candidate benefits, potential market size, product adoption, market positioning, competitive strengths, product development, and other clinical, business and financial matters. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially. Risks and uncertainties include, but are not limited to, our limited operating history, our need for additional financing to achieve our goals, our dependence on our lead product AR101, the need for additional clinical testing of AR101, uncertainties relating to the regulatory process, uncertainties relating to the timing and operation of clinical trials, potential safety issues, possible lack of market acceptance of our product candidates, the intense competition in the biopharmaceutical industry, our dependence on exclusive third-party suppliers and manufacturers, and limitations on intellectual property protection. A further list and description of these risks, uncertainties and other factors can be found in our report on Form 10-Q filed on November 14, 2016. Copies of this filing are available online at www.sec.gov or www.aimmune.com. Any forward-looking statements made in this presentation speak only as of the date of the presentation. We do not undertake to update any forward-looking statements as a result of new information or future events or developments.

Aimmune Therapeutics Today Addressing a Critical Unmet Need Peanut Allergy: No approved therapies for a life-threatening disease Planning for Success Building for 2018 regulatory filings and launch readiness in U.S. and EU Broad Platform Aimmune has full global development and commercial rights for lead asset and pipeline Encouraging Clinical Profile 80% Phase 2 intent-to-treat efficacy; largely mild safety profile Leading the Field Multiple cutting-edge scientific collaborations; recent $145 million equity investment Lead Asset in Phase 3 N>550 patients (U.S., Canada, and Europe) in PALISADE Phase 3 Trial; results expected around YE 2017
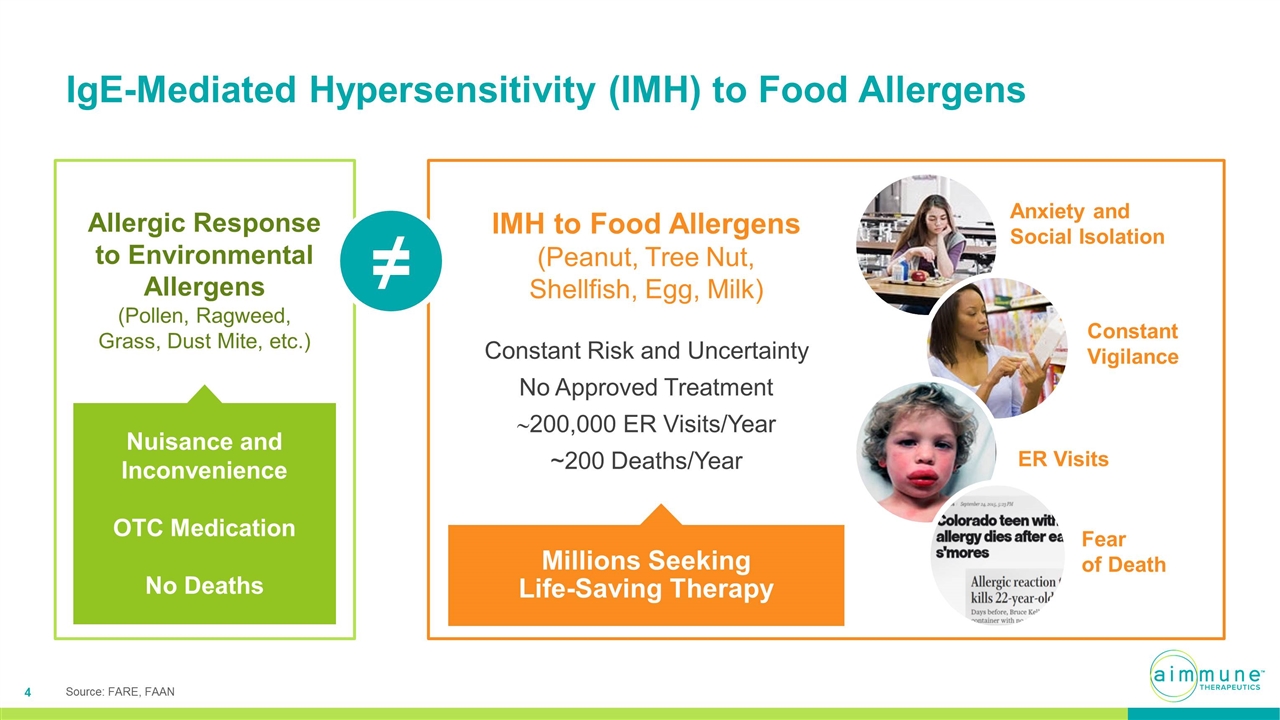
IgE-Mediated Hypersensitivity (IMH) to Food Allergens IMH to Food Allergens (Peanut, Tree Nut, Shellfish, Egg, Milk) Constant Risk and Uncertainty No Approved Treatment ~200,000 ER Visits/Year ~200 Deaths/Year Millions Seeking Life-Saving Therapy Anxiety and Social Isolation Constant Vigilance ER Visits Fear of Death Allergic Response to Environmental Allergens (Pollen, Ragweed, Grass, Dust Mite, etc.) Nuisance and Inconvenience OTC Medication No Deaths ≠ Source: FARE, FAAN
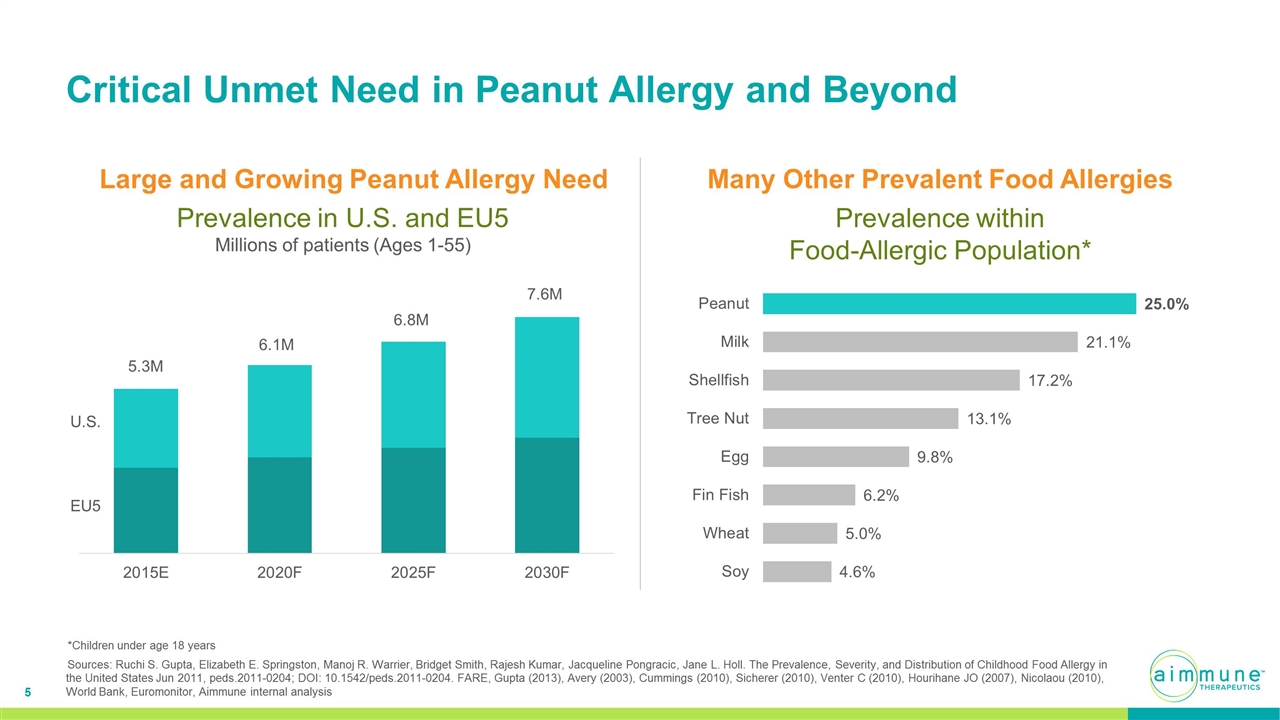
Prevalence in U.S. and EU5 Millions of patients (Ages 1-55) Large and Growing Peanut Allergy Need U.S. EU5 5.3M 6.1M 6.8M 7.6M Critical Unmet Need in Peanut Allergy and Beyond Prevalence within Food-Allergic Population* Many Other Prevalent Food Allergies *Children under age 18 years Sources: Ruchi S. Gupta, Elizabeth E. Springston, Manoj R. Warrier, Bridget Smith, Rajesh Kumar, Jacqueline Pongracic, Jane L. Holl. The Prevalence, Severity, and Distribution of Childhood Food Allergy in the United States Jun 2011, peds.2011-0204; DOI: 10.1542/peds.2011-0204. FARE, Gupta (2013), Avery (2003), Cummings (2010), Sicherer (2010), Venter C (2010), Hourihane JO (2007), Nicolaou (2010), World Bank, Euromonitor, Aimmune internal analysis
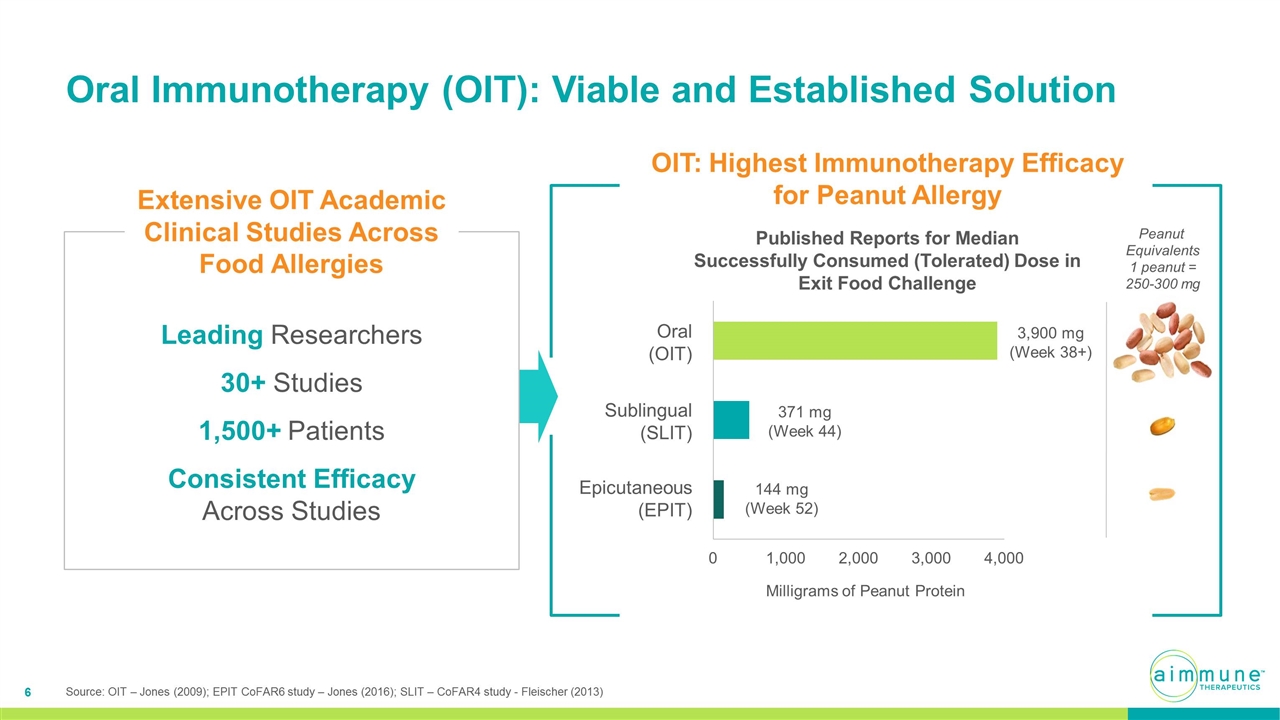
OIT: Highest Immunotherapy Efficacy for Peanut Allergy Published Reports for Median Successfully Consumed (Tolerated) Dose in Exit Food Challenge 144 mg (Week 52) 371 mg (Week 44) 3,900 mg (Week 38+) Epicutaneous (EPIT) Sublingual (SLIT) Oral (OIT) Milligrams of Peanut Protein Oral Immunotherapy (OIT): Viable and Established Solution Leading Researchers 30+ Studies 1,500+ Patients Consistent Efficacy Across Studies Extensive OIT Academic Clinical Studies Across Food Allergies Peanut Equivalents 1 peanut = 250-300 mg Source: OIT – Jones (2009); EPIT CoFAR6 study – Jones (2016); SLIT – CoFAR4 study - Fleischer (2013)
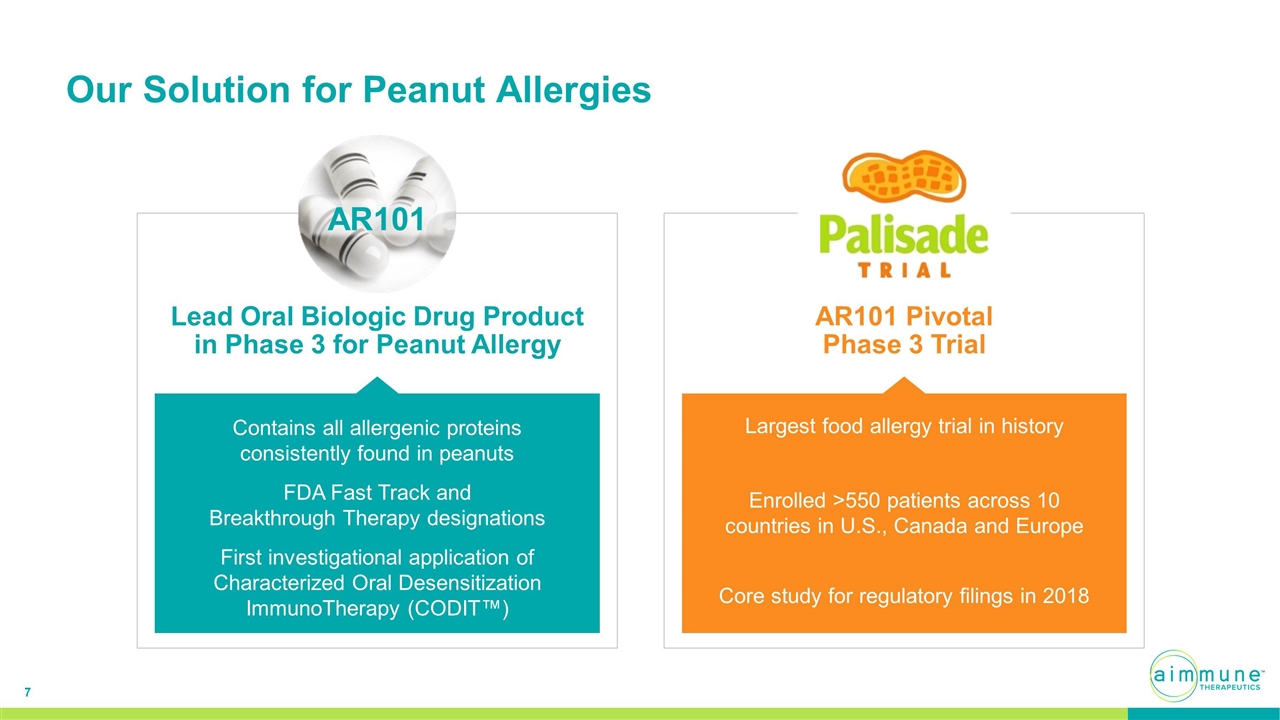
Our Solution for Peanut Allergies AR101 Pivotal Phase 3 Trial Largest food allergy trial in history Enrolled >550 patients across 10 countries in U.S., Canada and Europe Core study for regulatory filings in 2018 Lead Oral Biologic Drug Product in Phase 3 for Peanut Allergy Contains all allergenic proteins consistently found in peanuts FDA Fast Track and Breakthrough Therapy designations AR101 First investigational application of Characterized Oral Desensitization ImmunoTherapy (CODIT™)
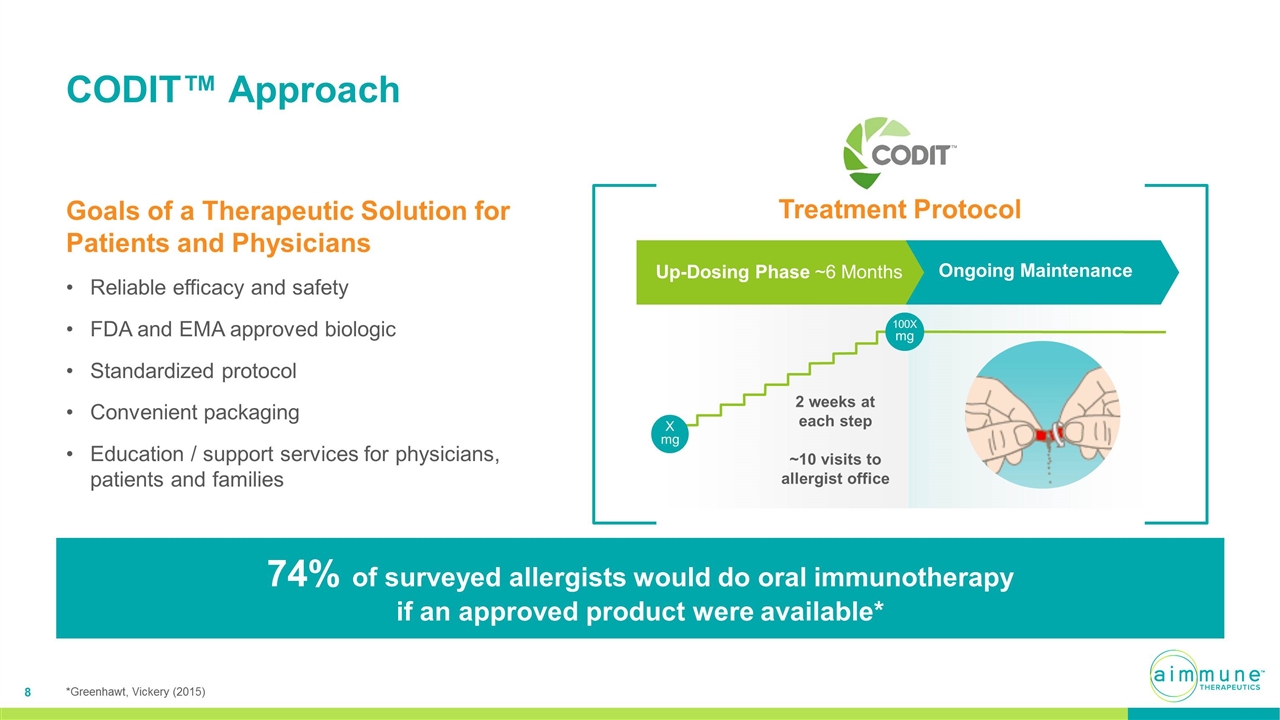
CODIT™ Approach Goals of a Therapeutic Solution for Patients and Physicians Reliable efficacy and safety FDA and EMA approved biologic Standardized protocol Convenient packaging Education / support services for physicians, patients and families 74% of surveyed allergists would do oral immunotherapy if an approved product were available* Treatment Protocol Ongoing Maintenance 2 weeks at each step ~10 visits to allergist office Up-Dosing Phase ~6 Months X mg 100X mg *Greenhawt, Vickery (2015)
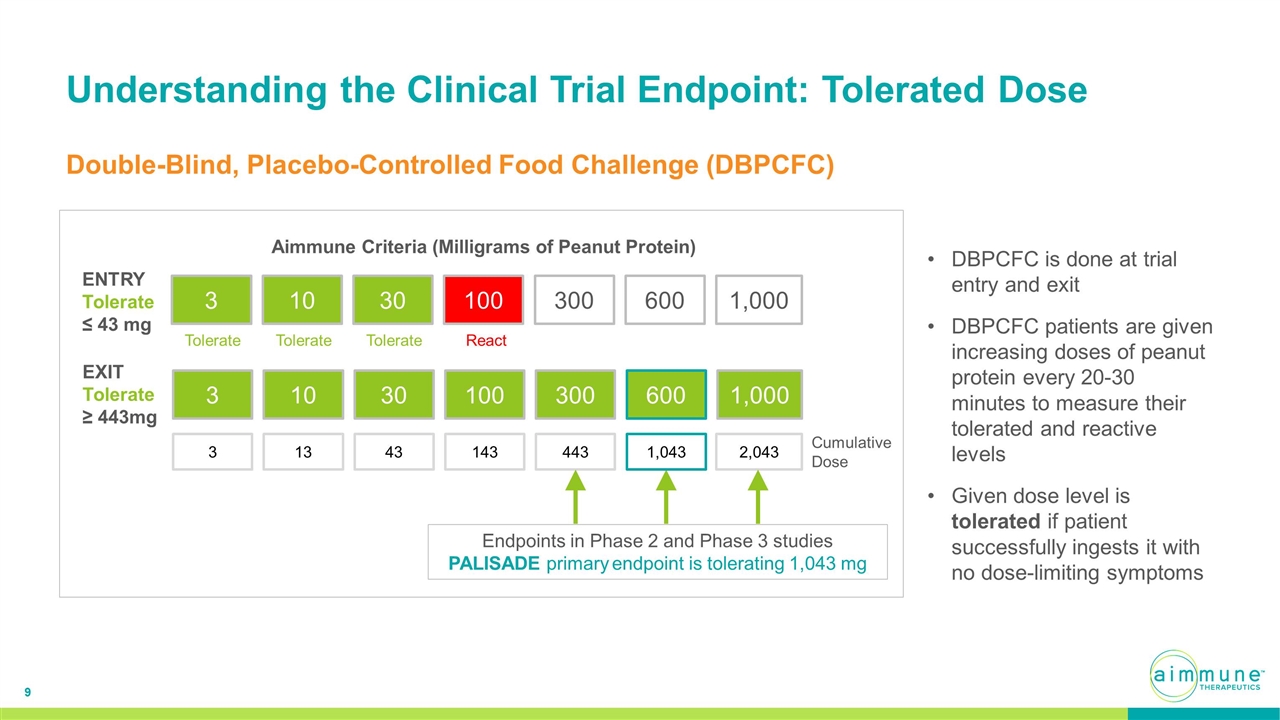
Understanding the Clinical Trial Endpoint: Tolerated Dose Aimmune Criteria (Milligrams of Peanut Protein) ENTRY Tolerate ≤ 43 mg EXIT Tolerate ≥ 443mg 3 3 10 13 30 43 100 143 300 443 600 1,043 3 10 30 100 300 600 1,000 1,000 2,043 Cumulative Dose Endpoints in Phase 2 and Phase 3 studies PALISADE primary endpoint is tolerating 1,043 mg React Tolerate Tolerate Tolerate Double-Blind, Placebo-Controlled Food Challenge (DBPCFC) DBPCFC is done at trial entry and exit DBPCFC patients are given increasing doses of peanut protein every 20-30 minutes to measure their tolerated and reactive levels Given dose level is tolerated if patient successfully ingests it with no dose-limiting symptoms
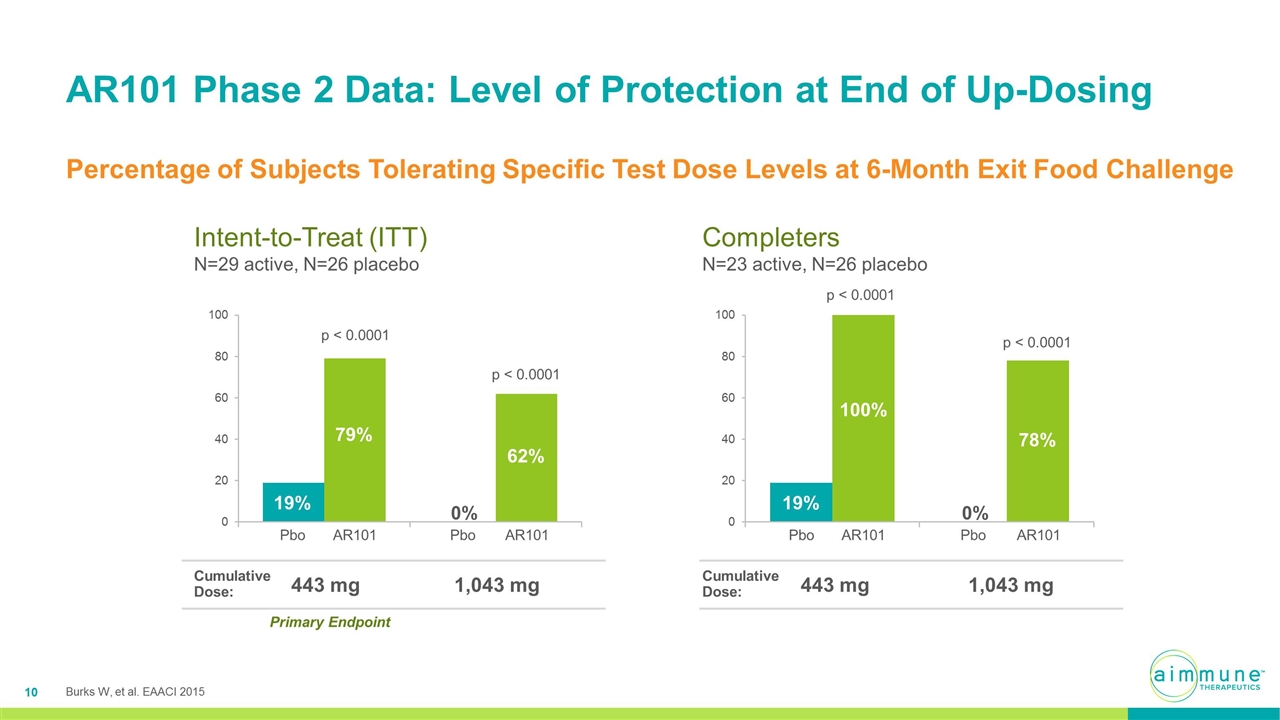
AR101 Phase 2 Data: Level of Protection at End of Up-Dosing Percentage of Subjects Tolerating Specific Test Dose Levels at 6-Month Exit Food Challenge Pbo AR101 Pbo AR101 Pbo AR101 Pbo AR101 p < 0.0001 p < 0.0001 p < 0.0001 p < 0.0001 Intent-to-Treat (ITT) N=29 active, N=26 placebo Completers N=23 active, N=26 placebo 443 mg 1,043 mg Cumulative Dose: 443 mg 1,043 mg Cumulative Dose: Primary Endpoint Burks W, et al. EAACI 2015
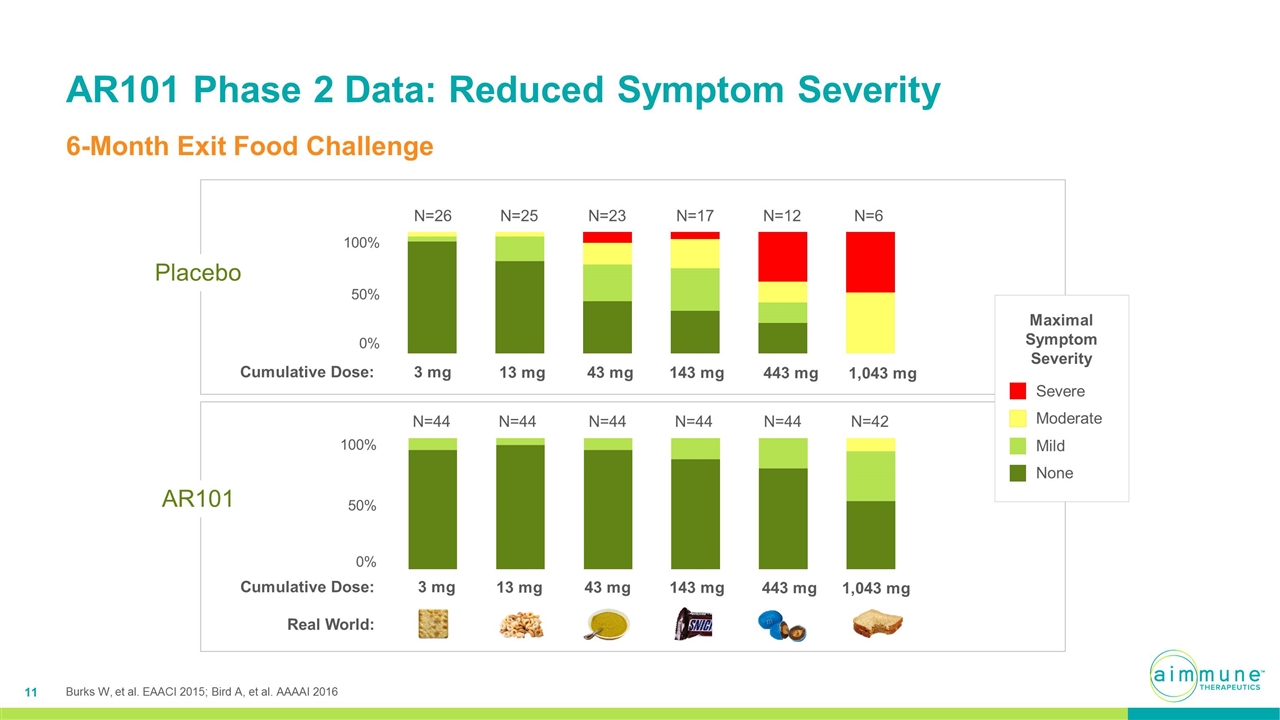
AR101 Phase 2 Data: Reduced Symptom Severity 6-Month Exit Food Challenge N=23 N=12 N=17 N=26 N=6 N=25 N=44 N=44 N=44 N=44 N=42 N=44 3 mg 13 mg 1,043 mg 43 mg 143 mg 443 mg 50% 50% 0% 0% 3 mg 13 mg 1,043 mg 43 mg 143 mg 443 mg Real World: Cumulative Dose: Cumulative Dose: Placebo AR101 Severe Moderate Mild None Maximal Symptom Severity Burks W, et al. EAACI 2015; Bird A, et al. AAAAI 2016
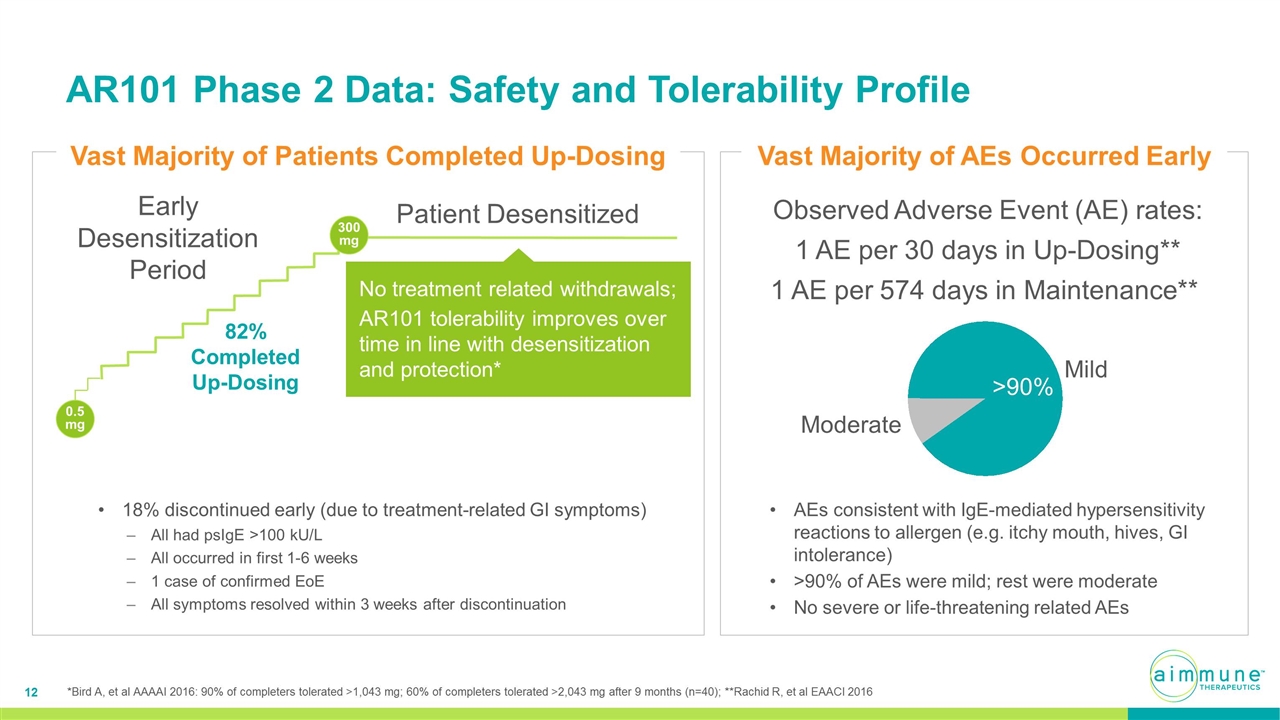
AR101 Phase 2 Data: Safety and Tolerability Profile Observed Adverse Event (AE) rates: 1 AE per 30 days in Up-Dosing** 1 AE per 574 days in Maintenance** AEs consistent with IgE-mediated hypersensitivity reactions to allergen (e.g. itchy mouth, hives, GI intolerance) >90% of AEs were mild; rest were moderate No severe or life-threatening related AEs Mild Moderate > 18% discontinued early (due to treatment-related GI symptoms) All had psIgE >100 kU/L All occurred in first 1-6 weeks 1 case of confirmed EoE All symptoms resolved within 3 weeks after discontinuation Vast Majority of Patients Completed Up-Dosing 300 mg Early Desensitization Period Patient Desensitized 0.5mg 82% Completed Up-Dosing No treatment related withdrawals; AR101 tolerability improves over time in line with desensitization and protection* Vast Majority of AEs Occurred Early *Bird A, et al AAAAI 2016: 90% of completers tolerated >1,043 mg; 60% of completers tolerated >2,043 mg after 9 months (n=40); **Rachid R, et al EAACI 2016
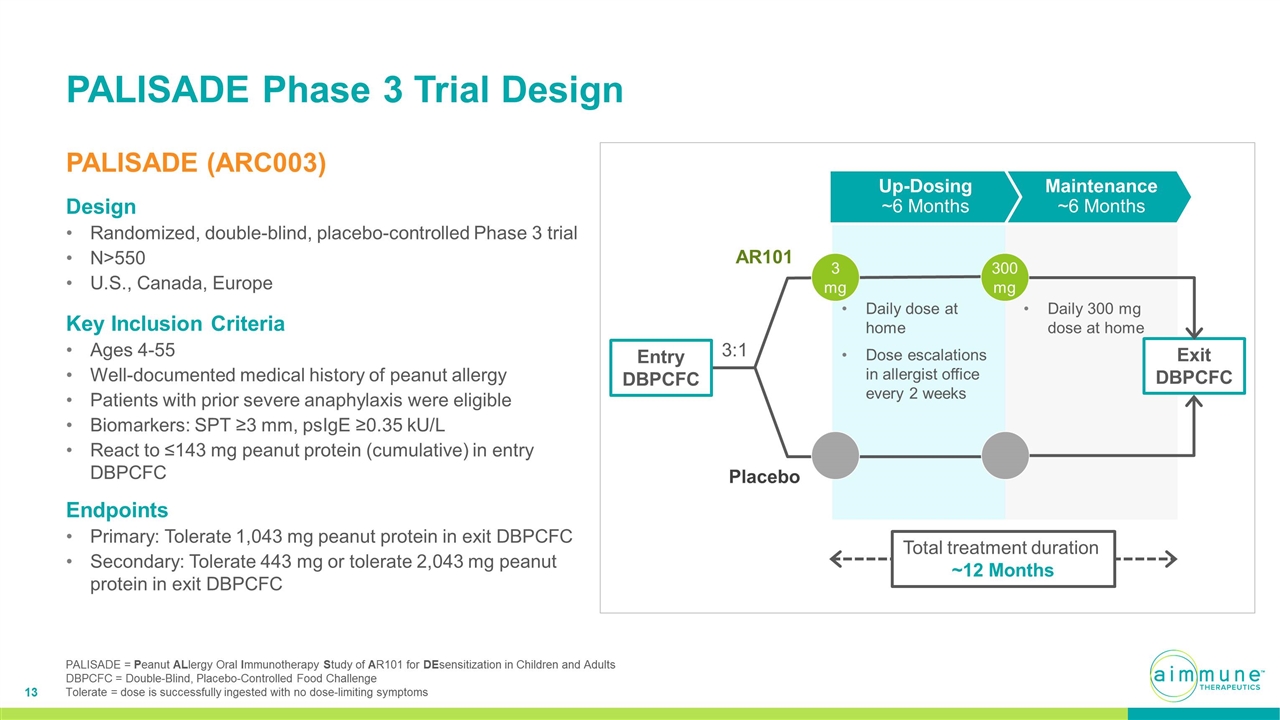
PALISADE Phase 3 Trial Design PALISADE (ARC003) Design Randomized, double-blind, placebo-controlled Phase 3 trial N>550 U.S., Canada, Europe AR101 Placebo 3:1 3 mg 300 mg Daily dose at home Dose escalations in allergist office every 2 weeks Maintenance ~6 Months Up-Dosing ~6 Months Daily 300 mg dose at home Exit DBPCFC Entry DBPCFC Total treatment duration ~12 Months Key Inclusion Criteria Ages 4-55 Well-documented medical history of peanut allergy Patients with prior severe anaphylaxis were eligible Biomarkers: SPT ≥3 mm, psIgE ≥0.35 kU/L React to ≤143 mg peanut protein (cumulative) in entry DBPCFC Endpoints Primary: Tolerate 1,043 mg peanut protein in exit DBPCFC Secondary: Tolerate 443 mg or tolerate 2,043 mg peanut protein in exit DBPCFC PALISADE = Peanut ALlergy Oral Immunotherapy Study of AR101 for DEsensitization in Children and Adults DBPCFC = Double-Blind, Placebo-Controlled Food Challenge Tolerate = dose is successfully ingested with no dose-limiting symptoms
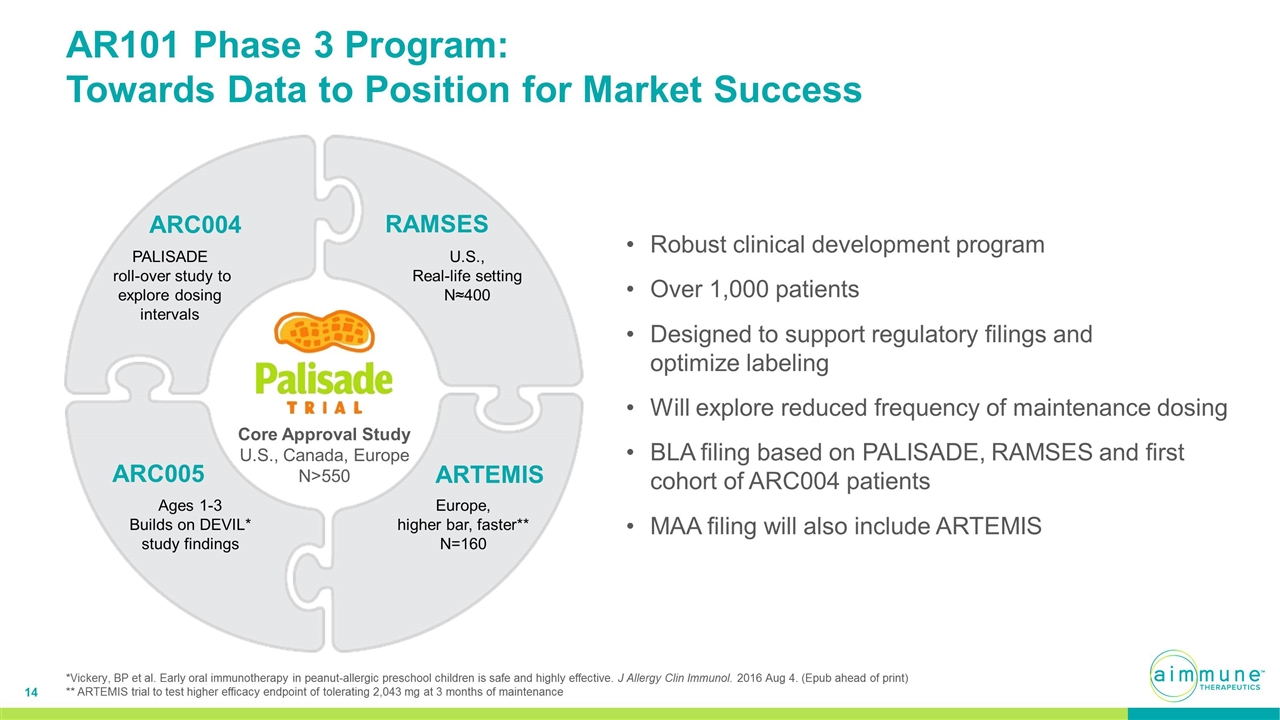
AR101 Phase 3 Program: Towards Data to Position for Market Success Robust clinical development program Over 1,000 patients Designed to support regulatory filings and optimize labeling Will explore reduced frequency of maintenance dosing BLA filing based on PALISADE, RAMSES and first cohort of ARC004 patients MAA filing will also include ARTEMIS U.S., Real-life setting N≈400 RAMSES Europe, higher bar, faster** N=160 ARTEMIS PALISADE roll-over study to explore dosing intervals ARC004 Ages 1-3 Builds on DEVIL* study findings ARC005 Core Approval Study U.S., Canada, Europe N>550 *Vickery, BP et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. 2016 Aug 4. (Epub ahead of print) ** ARTEMIS trial to test higher efficacy endpoint of tolerating 2,043 mg at 3 months of maintenance
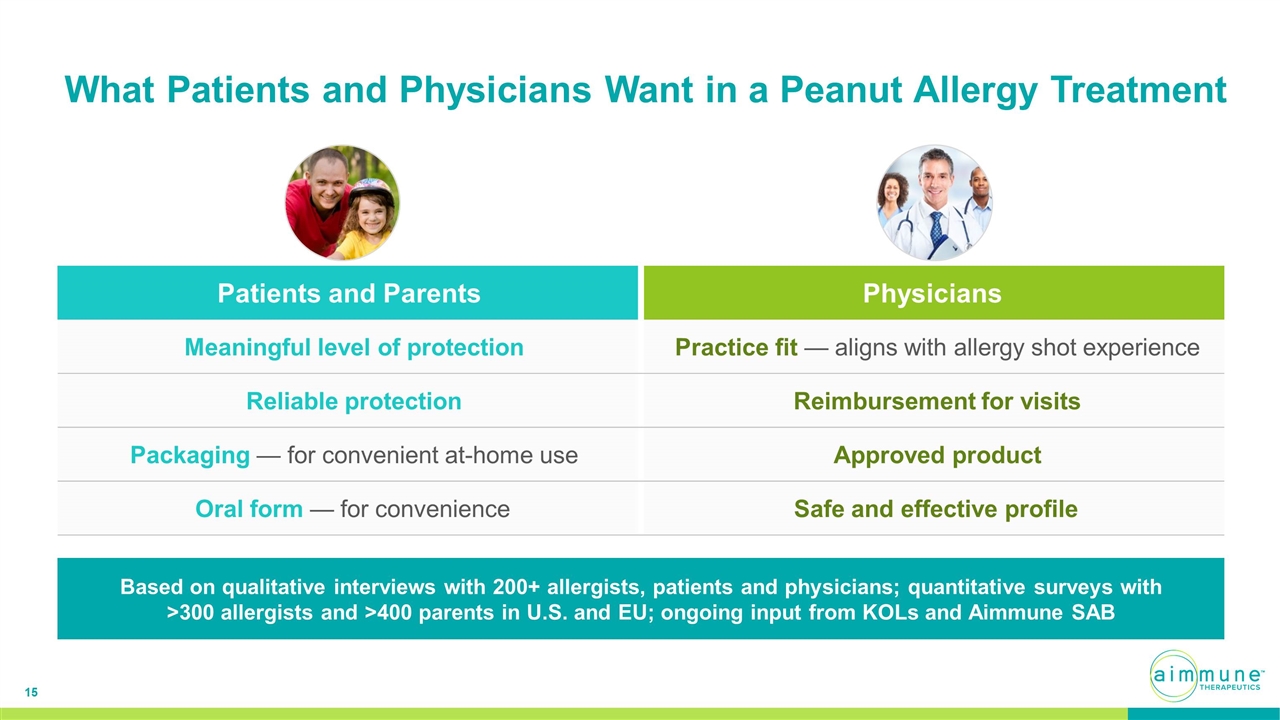
What Patients and Physicians Want in a Peanut Allergy Treatment Based on qualitative interviews with 200+ allergists, patients and physicians; quantitative surveys with >300 allergists and >400 parents in U.S. and EU; ongoing input from KOLs and Aimmune SAB Patients and Parents Physicians Meaningful level of protection Practice fit — aligns with allergy shot experience Reliable protection Reimbursement for visits Packaging — for convenient at-home use Approved product Oral form — for convenience Safe and effective profile

Preparing for Successful Commercial Launch of AR101 Initial Investments in U.S. and EU Pricing and Reimbursement Medical Affairs and Education Community and Advocacy Relationships CODIT™ Branding and Packaging Specialty Allergist Call Point Private clinics, community hospitals, academic centers Focused field (i.e., 50-100 reps in U.S.) starting with current practitioners Favorable pricing and market access insight in U.S. and EU – expected pricing in line with other chronic therapies that reduce acute events ~5,000 in U.S. ~10,000 in EU5* * Note: Includes physician with sub-specialty in allergy. Based on market research survey, interviews and analysis

Practice Capacity Can Meet Large Demand for AR101 Over Time 50 patients up-dosed per allergist per year 2,000 allergists adopting AR101 (top 40%) 100,000 patients up-dosed annually at scale: new AR101 patients, most converting to ongoing maintenance treatment with AR101 U.S. Allergists Annual Capacity at Scale U.S. Peanut Allergy Market Annual up-dosing at scale 100,000 Prevalent number of peanut-allergic patients in the U.S., likely modest decrease to 2030 3,000,000
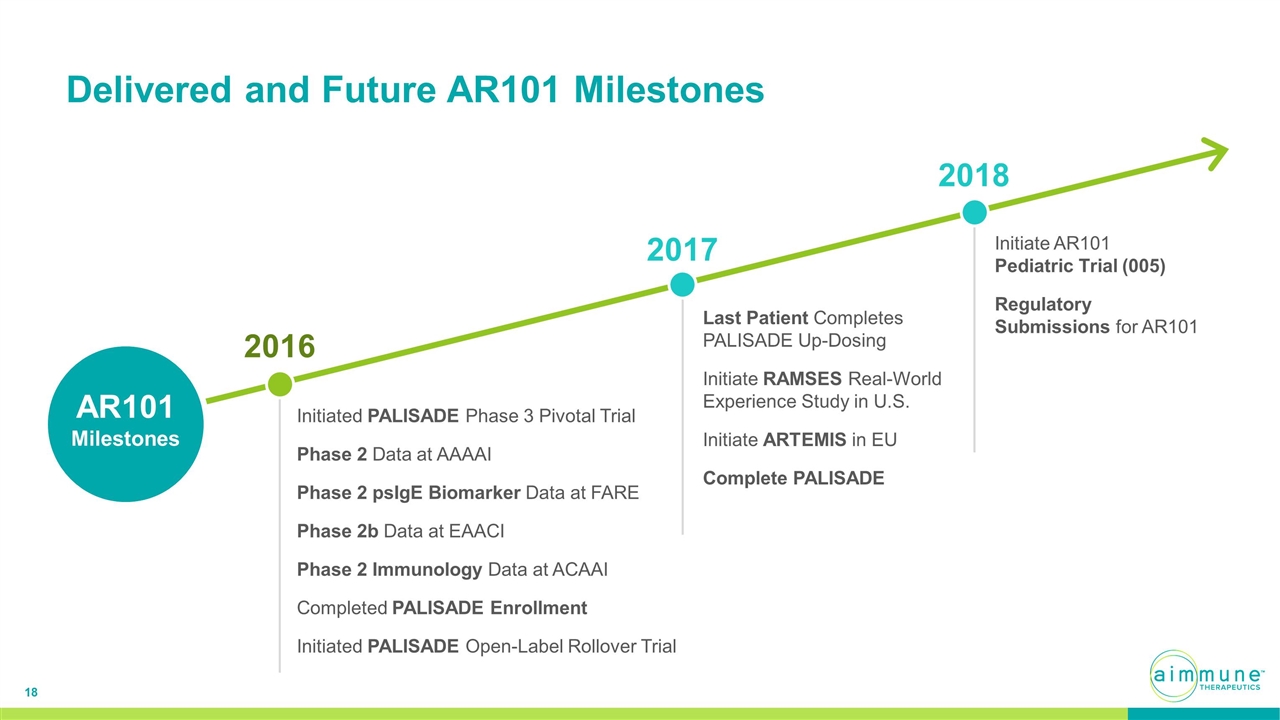
Delivered and Future AR101 Milestones AR101 Milestones 2016 Initiated PALISADE Phase 3 Pivotal Trial Phase 2 Data at AAAAI Phase 2 psIgE Biomarker Data at FARE Phase 2b Data at EAACI Phase 2 Immunology Data at ACAAI Completed PALISADE Enrollment Initiated PALISADE Open-Label Rollover Trial 2017 Last Patient Completes PALISADE Up-Dosing Initiate RAMSES Real-World Experience Study in U.S. Initiate ARTEMIS in EU Complete PALISADE 2018 Initiate AR101 Pediatric Trial (005) Regulatory Submissions for AR101

Collaboration with Nestlé Health Science Building AR101 Value Pipeline Expansion Scientific Discovery and Innovation $145M Strategic Equity Investment Two-year Working Collaboration Aimmune Retains Full Global Rights to All CODIT™ Pipeline Assets, Including AR101 &
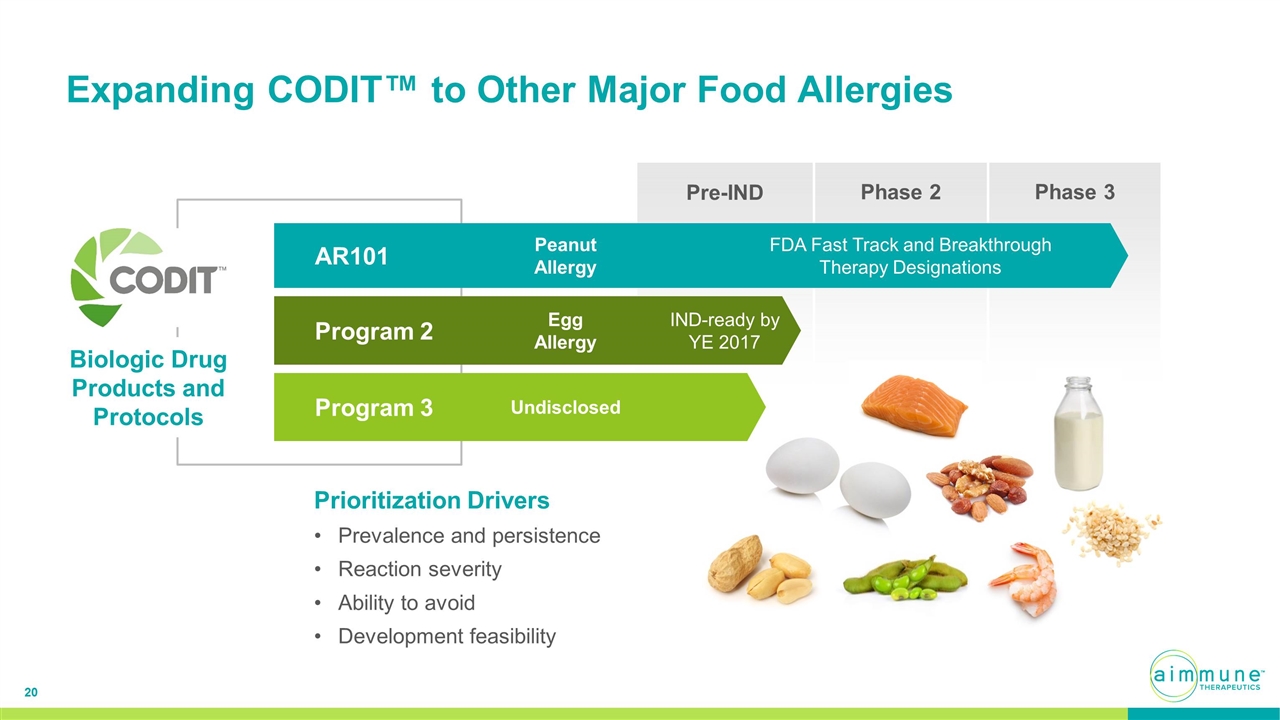
Expanding CODIT™ to Other Major Food Allergies Pre-IND Phase 2 Phase 3 Prioritization Drivers Prevalence and persistence Reaction severity Ability to avoid Development feasibility AR101 Peanut Allergy FDA Fast Track and Breakthrough Therapy Designations Program 3 Undisclosed Program 2 Egg Allergy IND-ready by YE 2017 Biologic Drug Products and Protocols

2017 Will Be an Exciting Year for Aimmune Well capitalized with ~$300M* in cash to support clinical development and regulatory filings Phase 3 PALISADE results expected around year-end Strategic collaborations with Nestlé Health Science and others to advance food allergy field Aimmune has full global development and commercialization rights for AR101 and pipeline Planning for success – building for 2018 regulatory filings and launch readiness in U.S. and EU *$159.3M cash, cash equivalents and investments as of 9/30/16 + $145M from Nestlé Health Science

Less risk. More life.
