Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - AMAG PHARMACEUTICALS, INC. | a17-1656_1ex99d3.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a17-1656_1ex99d1.htm |
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a17-1656_18k.htm |
Exhibit 99.2
AMAG Pharmaceuticals J.P. Morgan Healthcare Conference January 10, 2017

Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including, among others, expectations for AMAG’s growth strategy; AMAG’s anticipated expansion in women’s health through the licensing transaction with Palatin Technologies, Inc. and plans regarding the development activity of Rekynda; the structure of planned and pending clinical trials for Rekynda; the expected timeline, indications and expenditures for clinical development and commercialization of Rekynda, including the timing for the new drug application (NDA), subsequent FDA action and commercial launch; Rekynda’s strategic fit for AMAG’s women’s health business; the competitive landscape and breadth of the female sexual dysfunction (FSD) and hypoactive sexual desire disorder (HSDD) markets and Rekynda’s market potential; expectations for future next-generation formulations of Rekynda; AMAG’s and Palatin’s anticipated intellectual property rights associated with Rekynda; the expected timing for the closing of the Rekynda transaction; the timing and value of payments by AMAG under the Rekynda licensing transaction; the impact of the Rekynda product on AMAG’s financial results; expectations regarding the safety, efficacy and benefits of Rekynda, including that no boxed warning or restrictive REMS programs are anticipated; Makena’s position in the market and future growth drivers for Makena, including the potential market opportunity, progress for the next-generation development programs and customer engagement and outreach; beliefs that the current subcutaneous autoinjector (SC) formulation for Makena offers potential for more convenient and alternative administration; plans to explore alternative injection sites and formulations for Makena; expected timing for AMAG’s supplemental new drug application (sNDA) for Makena (including expected timing for an FDA decision on the sNDA) and collection and analysis of data, and results, from the definitive pharmacokinetic (PK) study; growth drivers for Cord Blood Registry (CBR), including plans to differentiate CBR offerings and increase engagement and communications in the industry; expectations for Feraheme, including growth for the intravenous (IV) iron market and the position of Feraheme within the IV iron market; anticipated growth drivers for Feraheme, including plans to optimize net revenue per gram and grow in key segments; expected timing for reporting clinical trial results and submitting the sNDA for the expanded Feraheme label and expectations that the size of the addressable market, if the broader indication is approved, would double; preliminary and unaudited financial results and cash and debt position for 2016 and AMAG’s 2017 finan cial guidance, including forecasted GAAP and non-GAAP revenues, GAAP and non-GAAP net income, GAAP operating income and adjusted EBITDA; expectations for AMAG’s key priorities in 2017, including plans to drive net product sales growth, increase non-GAAP adjusted EBITDA and undertake portfolio expansion activities, including by licensing and acquisition of products or companies; plans to continue to diversify the AMAG portfolio and achieve a mix of commercial assets and development pipeline for long-term growth; and plans to continue to enhance and scale AMAG’s internal capabilities are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others, (1) the possibility that the closing conditions set forth in the license agreement for Rekynda will not be met and that the parties will be unable to consummate the proposed transactions, (2) uncertainties regarding AMAG’s and Palatin’s ability to successfully and timely complete clinical development programs and obtain regulatory approval for Rekynda in North America, (3) the possibility that significant safety or drug interaction problems could arise with respect to Rekynda, (4) the ability of AMAG to raise awareness and understanding of HSDD and the potential benefits of Rekynda, (5) uncertainties regarding the manufacture of Rekynda, (6) uncertainties relating to our and Palatin’s patents and proprietary rights associated with Rekynda in North America, (7) that the cost of the Rekynda transaction to AMAG will be more than planned and/or will not provide the intended positive financial results, (8) that AMAG or Palatin will fail to fully perform under the Rekynda license agreement, (9) uncertainty regarding AMAG’s ability to compete in the FSD market in North America, and (10) as well as those risks identified in AMAG’s filings with the U.S. Securities and Exchange Commission (SEC), including its Annual Report on Form 10-K for the year ended December 31, 2015, its Quarterly Reports on Form 10-Q for the quarters ended March 31, 2016, June 30, 2016 and September 30, 2016 and subsequent filings with the SEC. Any such risks and uncertainties could materially and adversely affect AMAG’s results of operations, its profitability and its cash flows, which would, in turn, have a significant and adverse impact on AMAG’s stock price. AMAG cautions you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. AMAG Pharmaceuticals® and Feraheme® are registered trademark of AMAG Pharmaceuticals, Inc. MuGard® is a registered trademark of Abeona Therapeutics, Inc. Makena® is a registered trademark of AMAG Pharmaceuticals IP, Ltd. Cord Blood Registry® and CBR® are registered trademarks of CBR Systems, Inc. Rekynda is a trademark of Palatin Technologies, Inc. 2
AMAG Portfolio Feraheme® • Used for the treatment of iron deficiency anemia (IDA) in adult patients with chronic kidney disease (CKD) Makena® • The only FDA-approved therapy to reduce recurrent preterm birth in certain at-risk women Cord Blood Registry® • World’s largest umbilical cord stem cell collection and storage company MuGard® • Prescriptive mucoadhesive for the management of oral mucositis, a common side effect of radiation or chemotherapy, affecting ~400,000 U.S. cancer patients with oral mucositis in U.S. annually1 Velo Option • At completion of a Phase 2b/3a clinical trial, AMAG has the option to acquire an orphan drug candidate for the treatment of severe preeclampsia 1 Sonis ST. Oral Oncol. 2009 Dex;45(12):1015-20. 3 New Addition • Recent successful completion of two Phase 3 clinical trials for on-demand treatment of hypoactive sexual desire disorder (HSDD). NDA filing planned for early 2018, potential approval early 2019.

2016 Highlights Makena Launched single-dose, preservative free formulation, driving 45% growth over 4Q-2015 Initiated and fully enrolled pharmacokinetic (PK) study –Comparing bioavailability between subcutaneous (SC) and intramuscular (IM) injections Feraheme Completed enrollment in 2,000 patient broad iron deficiency anemia (IDA) study Accelerated potential approval timeline for IDA label expansion by ~6 months Cord Blood Registry (CBR) Enhanced messaging to capitalize on generational shift Increased stored units by ~40k in 2016 Financial - Drove significant top-and bottom-line growth Achieved record sales of Makena and Feraheme Realized strong operating income/adjusted EBITDA results Ended 2016 with $579M of cash 4
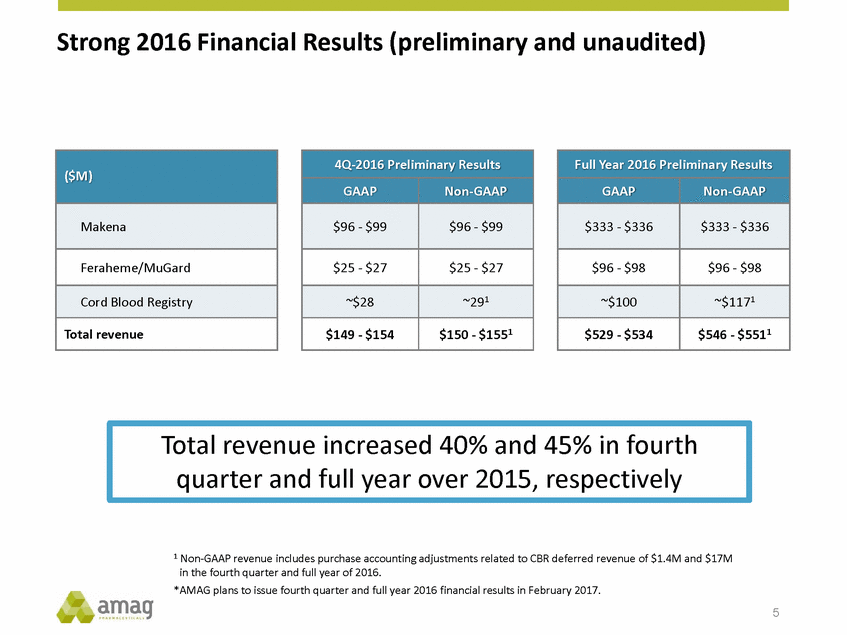
Strong 2016 Financial Results (preliminary and unaudited) 1 Non-GAAP revenue includes purchase accounting adjustments related to CBR deferred revenue of $1.4M and $17M in the fourth quarter and full year of 2016. *AMAG plans to issue fourth quarter and full year 2016 financial results in February 2017. 5 Total revenue increased 40% and 45% in fourth quarter and full year over 2015, respectively Full Year 2016 Preliminary Results GAAP Non-GAAP $333 - $336 $333 - $336 $96 - $98 $96 - $98 ~$100 ~$1171 $529 - $534 $546 - $5511 4Q-2016 Preliminary Results GAAP Non-GAAP $96 - $99 $96 - $99 $25 - $27 $25 - $27 ~$28 ~291 $149 - $154 $150 - $1551 ($M) Makena Feraheme/MuGard Cord Blood Registry Total revenue
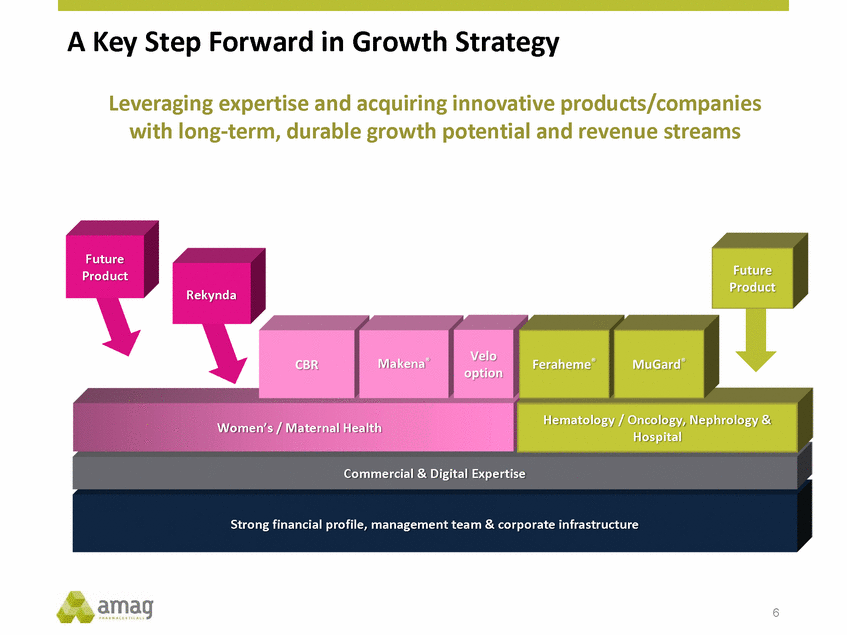
A Key Step Forward in Growth Strategy Leveraging expertise and acquiring innovative products/companies with long-term, durable growth potential and revenue streams Future Product Rekynda Makena® Hematology / Oncology, Nephrology & Hospital Women’s / Maternal Health Commercial & Digital Expertise Strong financial profile, management team & corporate infrastructure 6
Rekynda (bremelanotide)

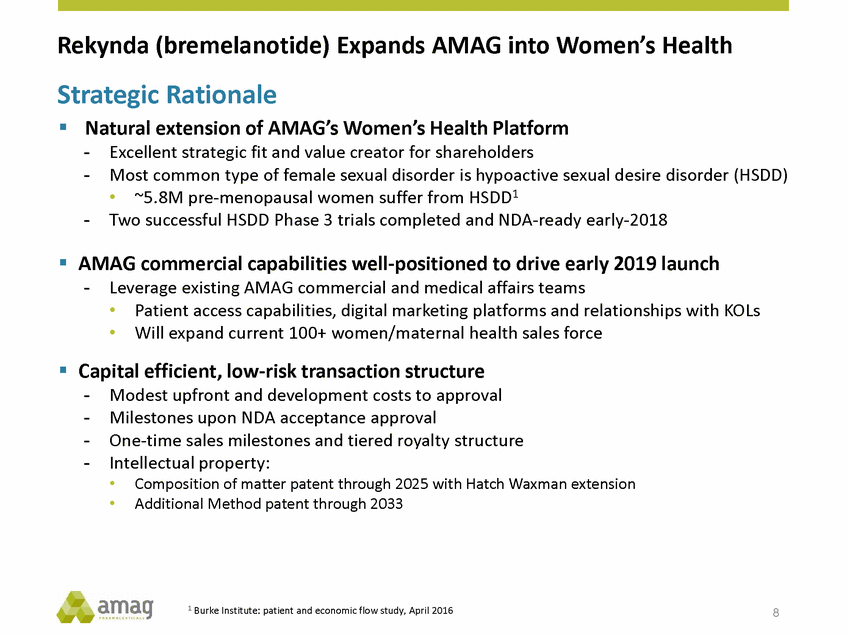
Rekynda (bremelanotide) Expands AMAG into Women’s Health Strategic Rationale Natural extension of AMAG’s Women’s Health Platform - - Excellent strategic fit and value creator for shareholders Most common type of female sexual disorder is hypoactive sexual desire disorder (HSDD) • ~5.8M pre-menopausal women suffer from HSDD1 - Two successful HSDD Phase 3 trials completed and NDA-ready early-2018 AMAG commercial capabilities well-positioned to drive early 2019 launch - Leverage existing AMAG commercial and medical affairs teams • • Patient access capabilities, digital marketing platforms and relationships with KOLs Will expand current 100+ women/maternal health sales force Capital efficient, low-risk transaction structure - - - - Modest upfront and development costs to approval Milestones upon NDA acceptance approval One-time sales milestones and tiered royalty structure Intellectual property: • • Composition of matter patent through 2025 with Hatch Waxman extension Additional Method patent through 2033 1 Burke Institute: patient and economic flow study, April 2016 8
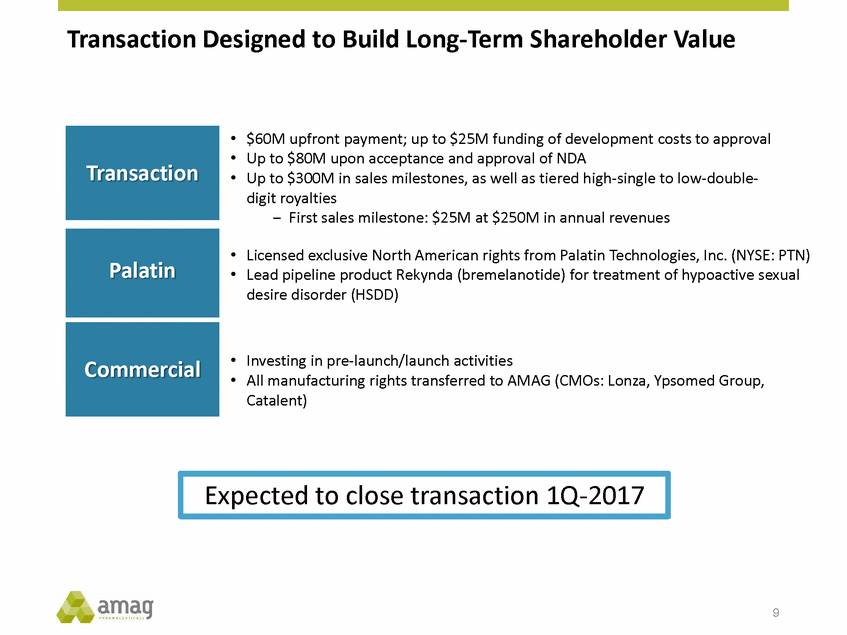
Transaction Designed to Build Long-Term Shareholder Value • • • $60M upfront payment; up to $25M funding of development costs to approval Up to $80M upon acceptance and approval of NDA Up to $300M in sales milestones, as well as tiered high-single to low-double-digit royalties – First sales milestone: $25M at $250M in annual revenues • • Licensed exclusive North American rights from Palatin Technologies, Inc. (NYSE: PTN) Lead pipeline product Rekynda (bremelanotide) for treatment of hypoactive sexual desire disorder (HSDD) • • Investing in pre-launch/launch activities All manufacturing rights transferred to AMAG (CMOs: Lonza, Ypsomed Group, Catalent) 9 Expected to close transaction 1Q-2017 Commercial Palatin Transaction
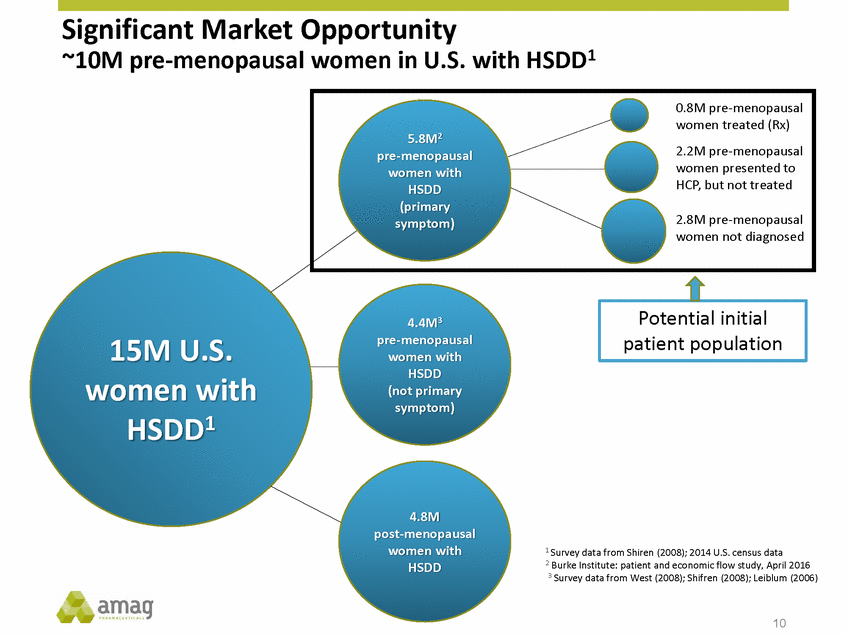
Significant Market Opportunity with HSDD1 ~10M pre-menopausal women in U.S. 5.8M2 women presented to women with 2.8M pre-menopausal symptom) 4.4M3 pre-menopausal women with 15M U.S. women with HSDD1 HSDD (not primary symptom) 4.8M post-menopausal women with HSDD 1 Survey data from Shiren (2008); 2014 U.S. census data 2 Burke Institute: patient and economic flow study, April 2016 3 Survey data from West (2008); Shifren (2008); Leiblum (2006) 10 Potential initial patient population 0.8M pre-menopausal women treated (Rx) pre-menopausal2.2M pre-menopausal HSDDHCP, but not treated (primary women not diagnosed
Rekynda (bremelanotide) Profile & Phase 3 Results Taken on-demand with a self-administered auto-injector pen in anticipation of sexual activity Novel mechanism of action: melancortin receptor agonist (MCR4) – Targets pathways involved in sexual desire and arousal response Two successful Phase 3 studies completed - - Randomized 1,267 pre-menopausal women with primary HSDD Co-primary efficacy endpoints met (top-line Phase 3 results released 4Q-2016) • Improvement in sexual desire and decrease in distress associated with low sexual desire Generally well tolerated; most common adverse events were nausea, headache and flushing (generally mild/moderate) • – ~80% of patients that completed the Phase 3 trials elected to participate in the open label roll-over safety study 11
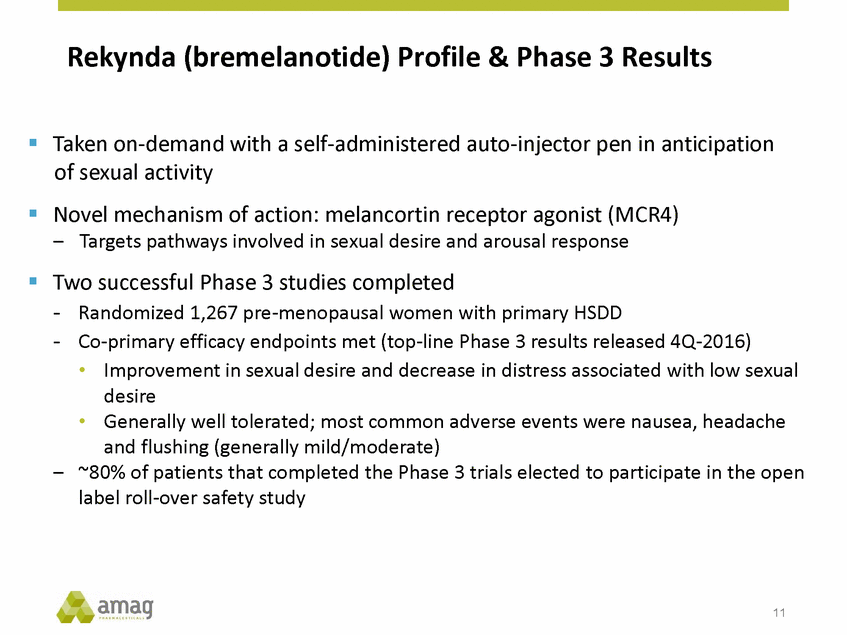
Rekynda (bremelanotide) Highly Differentiated Target Product Profile • Patient administered with subcutaneous auto-injector pen hypoactive sexual desire disorder (HSDD) 12 Rekynda (bremelanotide) Dosing & Administration • Taken on-demand in anticipation of sexual activity • Rapid onset of activity ~30 minutes • Treatment effective up to 8-10 hours Indication • The treatment of pre-menopausal women with acquired generalized Safety • Does not interact with alcohol (alcohol interaction study completed) • Generally well tolerated; most frequent adverse event was nausea (generally mild/moderate) • No Boxed Warning anticipated • Not anticipated to have a restrictive REMS program
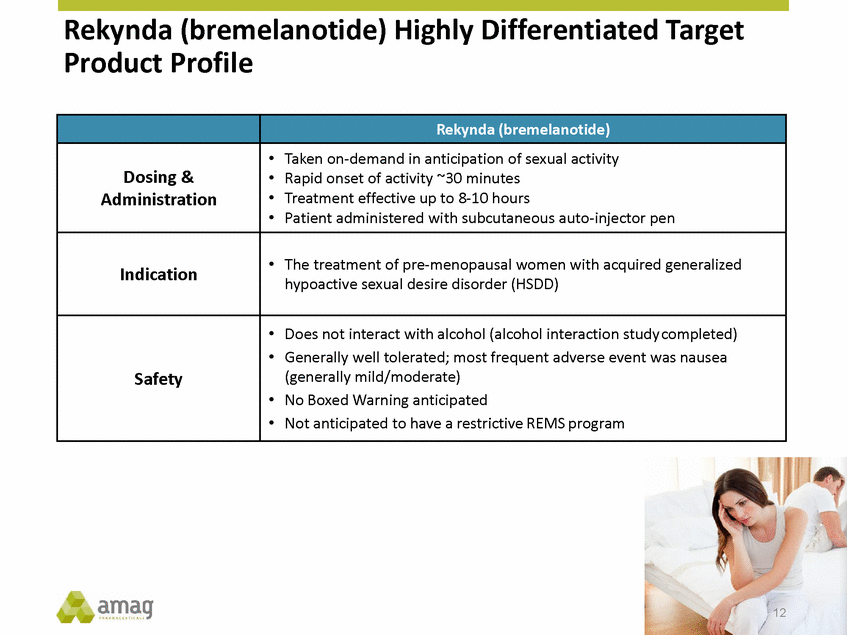
Rekynda (bremelanotide) Timeline Phase 3 trials initiated Completed Phase 3 enrollment Phase 3 studies completed Phase 3 top-line results / Co-primary endpoints met Phase 1 standard safety pharmacology and drug-drug interaction studies NDA submission Expected FDA action 4Q-2014 2H-2015 3Q-2016 4Q-2016 2017 early 2018 early 2019 13
Maternal Health: Makena

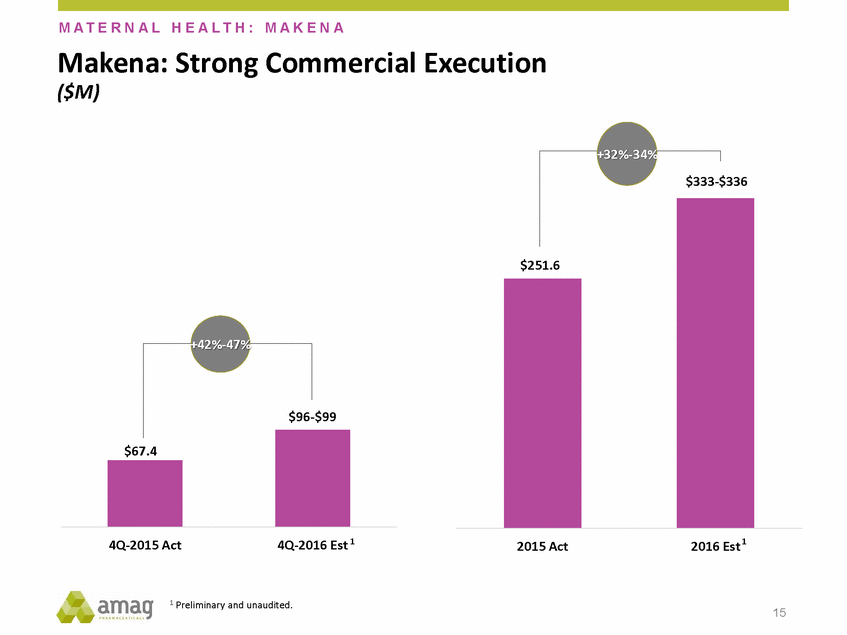
M A T E R N A L H E A L T H : M A K E N A Commercial Makena: ($M) Strong Execution $336 +42%-47% -$99 4Q-2016 Est 1 2016 Est 1 4Q-2015 Act 2015 Act 1 Preliminary and unaudited. 15 $67.4 $96 $251.6 +32%-34% $333-
M A T E R N A L H E A L T H : M A K E N A Makena gained 12 Percentage Share Points in 2016 $1B Market Opportunity1 Market Share2 Estimated December 31, 20164 December 31, 2015 Off Guidance3 30% Off Guidance3 30% Makena 30% Makena 42% Compounded Hydroxyprogesterone Caproate 40% Compounded Hydroxyprogesterone Caproate 28% 1 Based on 140,000 patients, >16 injections/patient and net revenue of ~$425-$450/injection. 2 Company estimates Makena market share based on distributor dispensing data and all other market share based on physician market research data conducted by AMAG. 3 Off guidance represents patients treated outside guidance of Society for Maternal Fetal Medicine, including patients treated with unapproved therapies and untreated patients. 4 Actual market share based on Company estimates will be disclosed in February 2017 with the release of actual 2016 results. 16
M A T E R N A L H E A L T H :M A K E N A Accomplishments & Growth Drivers 2016 Accomplishments Successfully launched single-dose, preservative free formulation Increased market share by 40% over 2015 Enhanced patient access to Makena Expanded Optum Home Health Services relationship Initiated and fully enrolled PK study 2017 Growth Drivers Continue to gain share Grow Makena @Home administration Expand share of voice with target customers Penetrate late pre-term birth segment Progress next-generation development programs 17
M A T E R N A L H E A L T H : M A K E N A Makena Next-Generation Program Update Makena IM orphan drug exclusivity (ODE) through February 2018 Makena SC auto-injector program - Definitive PK study and comparative pain study • Initiated October 2016 - Collection and analysis of PK data ongoing, with results expected 1Q-2017 • Preliminary data suggest transient injection site burning/stinging sensation • • ~25% subjects in SC arm <5% subjects in IM arm • Pain study discontinued (similar observations as in PK study); will not request ODE - - - sNDA filing anticipated in 2Q-2017, assuming comparable bioavailability Expect 6 month review (vs. 10 month review) SC auto-injector covered by multiple issued Antares patents extending to 2026 • Additional patent applications pending Makena SC plans for 2017 and beyond - Current SC formulation offers the potential for: • • Greater convenience for HCPs Alternative to IM injection for patients - As part of broader next-generation program, exploring alternative injection sites and formulations 18
Maternal Health: Cord Blood Registry (CBR)
M A T E R N A L H E A L T H : C O R D B L O O D R E G I S T R Y Attractive Recurring Revenue Stream ($M) Non-GAAP, Pro Forma CBR Fourth Quarter Revenue3 GAAP CBR Fourth Quarter Revenue $28.8 ~$28.0 ~$29 $17.0 4Q-2016 Est 1 4Q-2016 Est 1 4Q-2015 Act 4Q-2015 Act Non-GAAP, Pro Forma CBR Full Y ear Revenue3,4 GAAP CBR Full Year Revenue $118.6 ~$117 ~$100.0 $24.1 2015 Act 2 2016 Est 1 2016 Est 1 2015 Act 1 Preliminary and unaudited. 2 Represents revenues from August 17, 2015 to December 31, 2015. CBR was acquired by AMAG on August 17, 2015. 3 Non-GAAP CBR revenue includes purchase accounting adjustments related to CBR deferred revenue of $1.4M and $17M for the fourth quarter and full year of 2016, respectively. 4 Represents pro forma revenue for 2015. AMAG acquired CBR on August 17, 2015. 20
M A T E R N A L H E A L T H : C O R D B L O O D R E G I S T R Y Accomplishments & Growth Drivers 2016 Accomplishments Drove ~$117M in 2016 revenues (non-GAAP) Grew revenue per consumer 8.5% year over year Increased stored units by ~40k in 2016 Enhanced messaging to capitalize on generational shift Emphasized competitive advantages and market position 2017 Growth Drivers Differentiate CBR’s offerings - - Highlight cord tissue storage offering Enhance product offerings Build value proposition on storing newborn stem cells Increase engagement with the HCP community Strengthen and expand communication channels 21
Hematology/Oncology: Feraheme
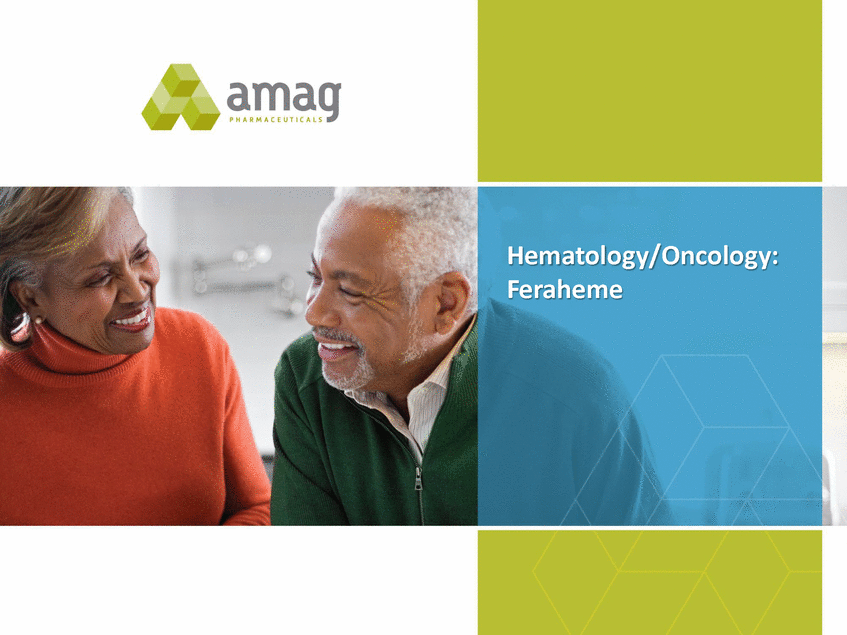
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E / M U G A R D Differentiated ($M) Product, Expect Future Growth +6-9% $96-$98 +6%-15% $25-$27 $23.5 3 4Q-2015 Act 2 4Q-2016 Est 2015 Act2 2016 Est 3 1 Based on IMS volume data through December 23, 2016. 2 Feraheme and MuGard. 3 Preliminary and unaudited numbers for Feraheme and MuGard. 23 $90.2 2016 IV iron market grew ~10% vs. 20151 4Q-2016 IV iron market grew ~10% vs. 4Q-20151
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E Accomplishments & Growth Drivers 2016 Accomplishments Achieved an estimated $96-$98M in revenues (includes Mugard) 6% growth in grams over 2015 Expanded presence in integrated delivery networks and group purchasing organizations Continued broad patient access strategy, creating reimbursement predictability Completed enrollment in head-to-head Phase 3 trial for broader indication well ahead of schedule - Accelerates sNDA filing to mid-2017 2017 Growth Drivers Continued growth in key segments Optimize net revenue per gram Prepare for expanded label to include IDA patients with all causes – Would double addressable market, if approved 24

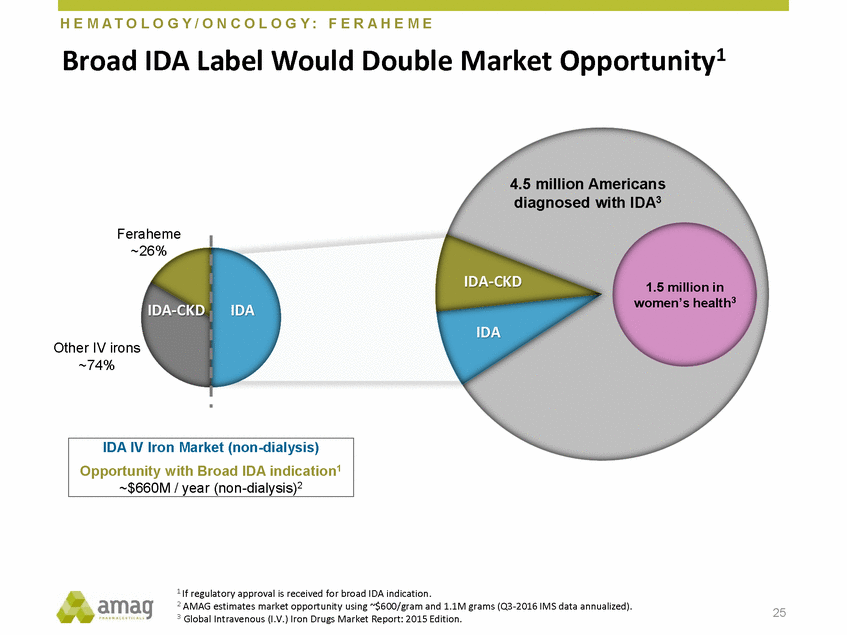
H E M A T O L O G Y / O N C O L O G Y : F E R A H E M E Market Opportunity1 Broad IDA Label Would Double 4.5 million Americans diagnosed with IDA3 Feraheme ~26% IDA-CKD 1.5 million in women’s health3 IDA-CKD IDA IDA Other IV irons ~74% 1 If regulatory approval is received for broad IDA indication. 2 AMAG estimates market opportunity using ~$600/gram and 1.1M grams (Q3-2016 IMS data annualized). 3 Global Intravenous (I.V.) Iron Drugs Market Report: 2015 Edition. 25 IDA IV Iron Market (non-dialysis) Opportunity with Broad IDA indication1 ~$660M / year (non-dialysis)2
Financial Overview

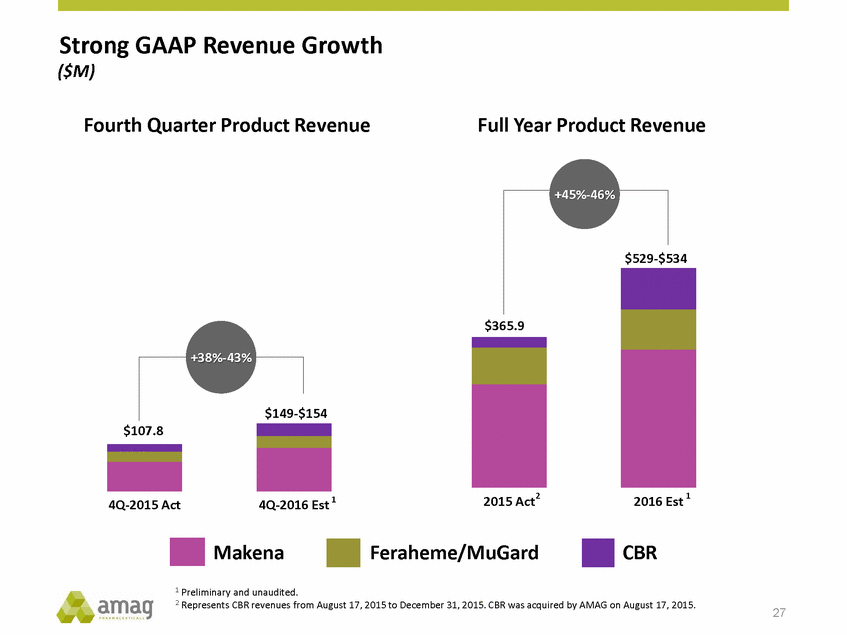
Strong GAAP Revenue Growth ($M) Fourth Quarter Product Revenue Full Year Product Revenue $534 $365.9 +38%-43% $154 $107.8 2 1 4Q-2016 Est 1 2015 Act 2016 Est 4Q-2015 Act Makena Feraheme/MuGard CBR 1 Preliminary and unaudited. 2 Represents CBR revenues from August 17, 2015 to December 31, 2015. CBR was acquired by AMAG on August 17, 2015. 27 $149-+45%-46% $529-
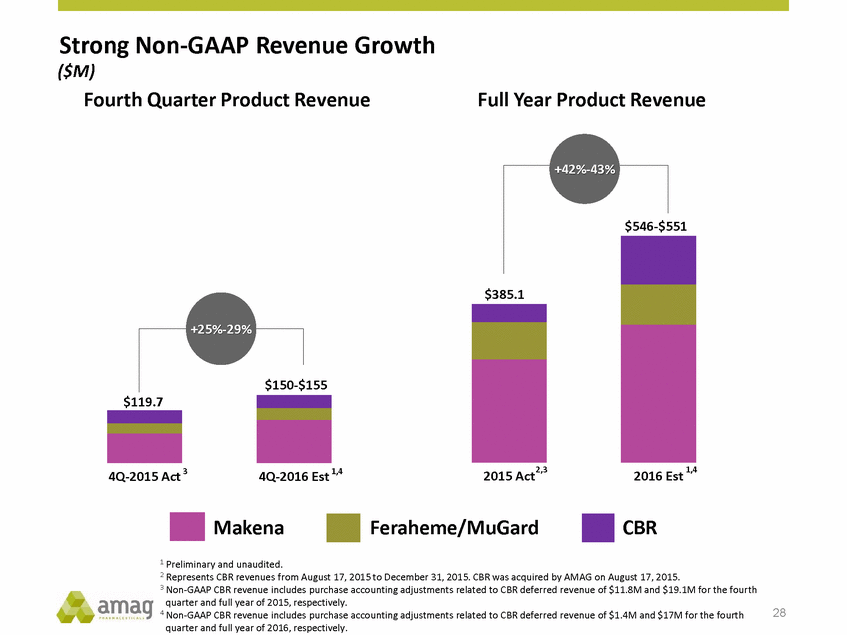
Strong Non-GAAP Revenue Growth ($M) Fourth Quarter Product Revenue Full Year Product Revenue $551 $385.1 +25%-29% $155 $119.7 2015 Act2,3 2016 Est 1,4 4Q-2015 Act 3 4Q-2016 Est 1,4 Makena Feraheme/MuGard CBR 1 Preliminary and unaudited. 2 Represents CBR revenues from August 17, 2015 to December 31, 2015. CBR was acquired by AMAG on August 17, 2015. 3 Non-GAAP CBR revenue includes purchase accounting adjustments related to CBR deferred revenue of $11.8M and $19.1M for the fourth quarter and full year of 2015, respectively. 4 Non-GAAP CBR revenue includes purchase accounting adjustments related to CBR deferred revenue of $1.4M and $17M for the fourth quarter and full year of 2016, respectively. 28 $150-+42%-43% $546-
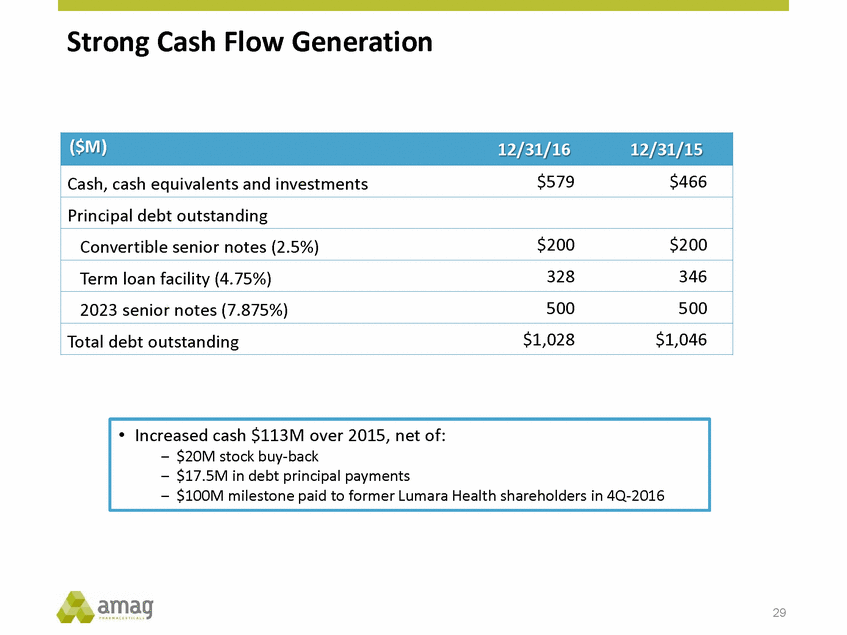
Strong Cash Flow Generation 29 • Increased cash $113M over 2015, net of: – $20M stock buy-back – $17.5M in debt principal payments – $100M milestone paid to former Lumara Health shareholders in 4Q-2016 ($M)12/31/1612/31/15 Cash, cash equivalents and investments$579$466 Principal debt outstanding Convertible senior notes (2.5%)$200$200 Term loan facility (4.75%)328346 2023 senior notes (7.875%)500500 Total debt outstanding$1,028$1,046
AMAG: Growth and Diversification Today Profitable company with 4 marketed products and services Multiple therapeutic areas in attractive segments Maturing next generation development pipeline - Feraheme IDA label expansion1 - Makena SC auto-injector1 Velo option agreement for the treatment of severe preeclampsia Tomorrow Well-diversified portfolio of products and services Mix of commercial assets and development pipeline for long-term growth Enhanced internal capabilities that come with scaled organization Yesterday Dependent on single product Significant cash burn Limited opportunities for organic growth Makena Feraheme CBR MuGard Rekynda Product 6 1 If regulatory approval is received. 30
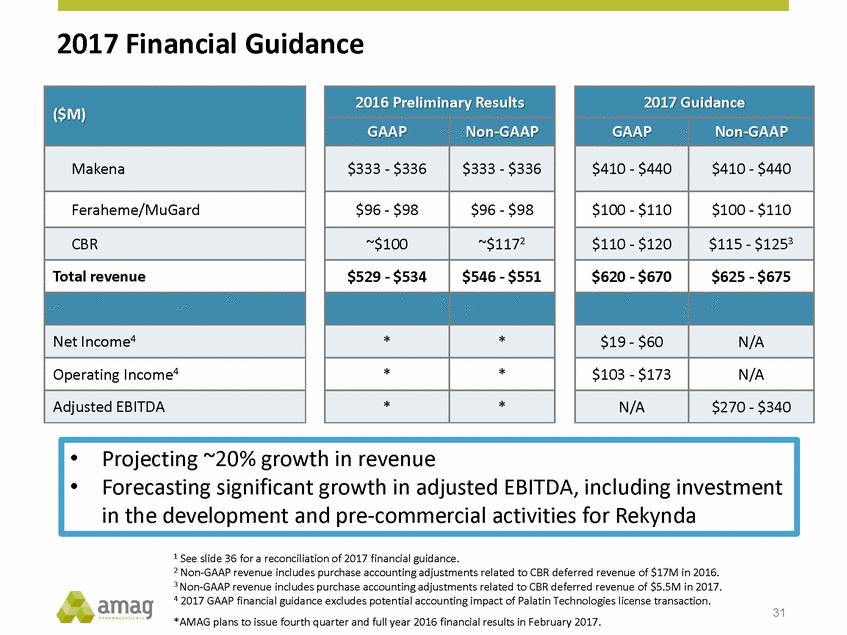
2017 Financial Guidance 1 See slide 36 for a reconciliation of 2017 financial guidance. 2 Non-GAAP revenue includes purchase accounting adjustments related to CBR deferred revenue of $17M in 2016. 3 Non-GAAP revenue includes purchase accounting adjustments related to CBR deferred revenue of $5.5M in 2017. 4 2017 GAAP financial guidance excludes potential accounting impact of Palatin Technologies license transaction. *AMAG plans to issue fourth quarter and full year 2016 financial results in February 2017. 31 •Projecting ~20% growth in revenue •Forecasting significant growth in adjusted EBITDA, including investment in the development and pre-commercial activities for Rekynda 2017 Guidance GAAP Non-GAAP $410 - $440 $410 - $440 $100 - $110 $100 - $110 $110 - $120 $115 - $1253 $620 - $670 $625 - $675 $19 - $60 N/A $103 - $173 N/A N/A $270 - $340 2016 Preliminary Results GAAP Non-GAAP $333 - $336 $333 - $336 $96 - $98 $96 - $98 ~$100 ~$1172 $529 - $534 $546 - $551 * * * * * * ($M) Makena Feraheme/MuGard CBR Total revenue Net Income4 Operating Income4 Adjusted EBITDA
2017 Key Priorities

2017 Key Priorities 33 2017 Financial Drive net product sales of $650M Grow adjusted EBITDA to +$300M Makena Report results from subcutaneous auto-injector PK study File supplemental New Drug Application Expect FDA decision 4Q-2017 Feraheme Report results from head-to-head Phase 3 clinical trial evaluating the safety of Feraheme compared to Injectafer in adults with IDA File supplemental New Drug Application mid-2017 Portfolio Execute pre-launch and launch activities for Makena and Feraheme line extensions Expansion Filing and launch preparation for Rekynda

AMAG Pharmaceuticals J.P. Morgan Healthcare Conference January 10, 2017

Appendix

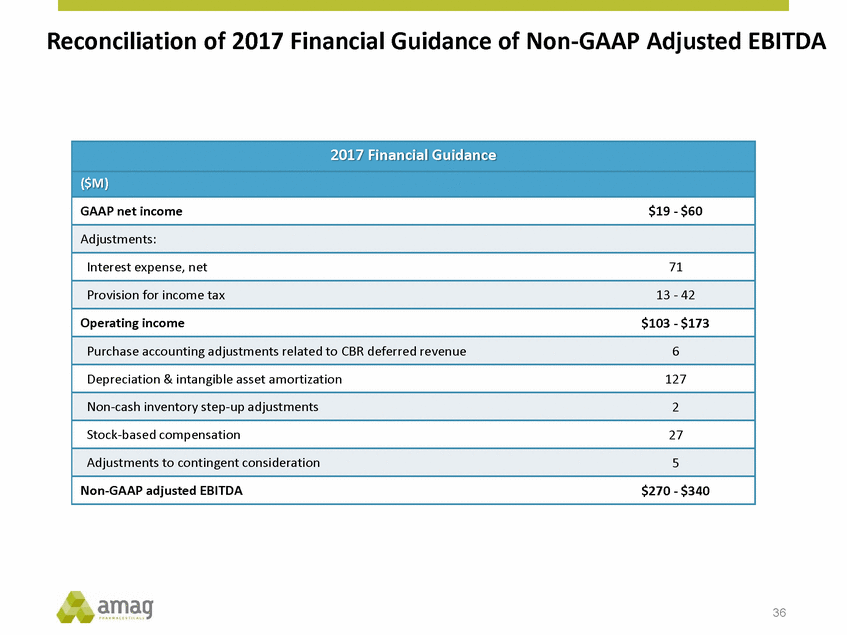
Reconciliation of 2017 Financial Guidance of Non-GAAP Adjusted EBITDA 36 2017 Financial Guidance ($M) GAAP net income$19 - $60 Adjustments: Interest expense, net71 Provision for income tax13 - 42 Operating income$103 - $173 Purchase accounting adjustments related to CBR deferred revenue6 Depreciation & intangible asset amortization127 Non-cash inventory step-up adjustments2 Stock-based compensation27 Adjustments to contingent consideration5 Non-GAAP adjusted EBITDA$270 - $340
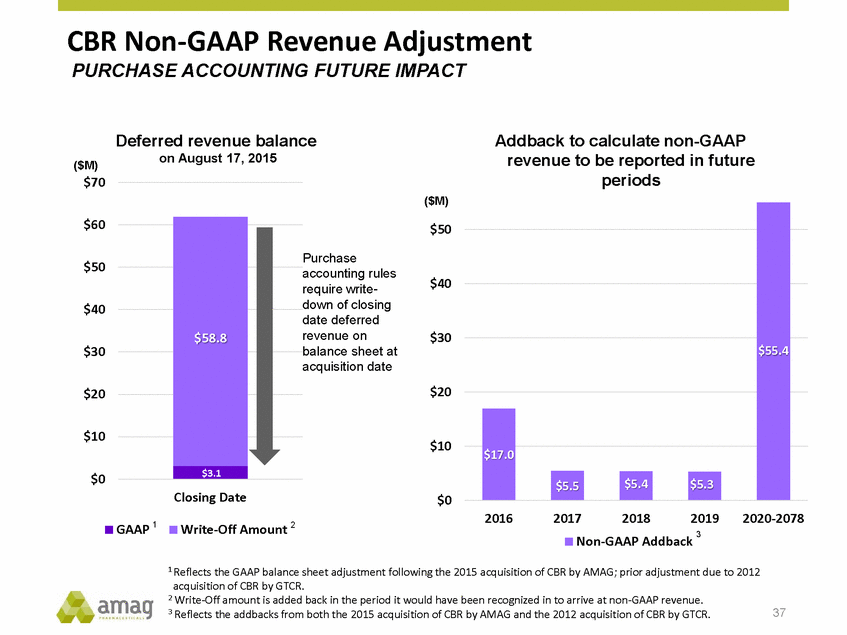
CBR Non-GAAP Revenue Adjustment PURCHASE ACCOUNTING FUTURE IMPACT Deferred revenue balance on August 17, 2015 Addback to calculate non-GAAP revenue to be reported in future periods ($M) $70 ($M) $60 $50 Purchase accounting rules $50 $40 require write-down of closing $40 date deferred revenue on balance sheet at $30 $30 acquisition date $20 $20 $10 $10 $0 Closing Date $0 2016 2017 2018 2019 2020-2078 1 2 GAAP Write-Off Amount 3 Non-GAAP Addback 1 Reflects the GAAP balance sheet adjustment following the 2015 acquisition of CBR by AMAG; prior adjustment due to 2012 acquisition of CBR by GTCR. 2 Write-Off amount is added back in the period it would have been recognized in to arrive at non-GAAP revenue. 3 Reflects the addbacks from both the 2015 acquisition of CBR by AMAG and the 2012 acquisition of CBR by GTCR. 37 $58.8 $3.1 $55.4 $17.0 $5.5$5.4 $5.3
AMAG Pharmaceuticals J.P. Morgan Healthcare Conference January 10, 2017