Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - ACCELERON PHARMA INC | xlrn-2017x1x9ex991.htm |
| 8-K - 8-K - ACCELERON PHARMA INC | xlrn-2017x1x9form8xk.htm |

Transforming Patient Care with
Breakthrough Science
35TH ANNUAL J.P. MORGAN HEALTHCARE CONFERENCE JANUARY 9, 2017
Exhibit 99.2

Acceleron Forward-Looking Statements
This presentation contains forward-looking statements about the Company's strategy, future plans and
prospects, including statements regarding the development of the Company's compounds, including
sotatercept, luspatercept, dalantercept, ACE-083, ACE-2494, the Company’s IntelliTrap™ drug discovery
platform, and the Company's TGF-beta superfamily program generally, the timeline for clinical development
and regulatory approval of the Company's compounds, the expected timing for the reporting of data from
ongoing trials, and the structure of the Company's planned or pending clinical trials. The words “anticipate,”
“appear,” “believe,” “continue,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “may,” “plan,”
“potential,” “predict,” “project,” “should,” “target,” “will,” “would,” and similar expressions are intended to
identify forward-looking statements, although not all forward-looking statements contain these identifying
words.
Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ
materially from those expressed or implied in such statement. Applicable risks and uncertainties include the
risks that the Company's cash, cash equivalents and investments will be insufficient to fund operations into
the second half of 2019, that preclinical testing of the Company's compounds and data from clinical trials
may not be predictive of the results or success of ongoing or later clinical trials, that data may not be
available when the Company expects it to be, that the Company or its collaboration partner, Celgene, will be
unable to successfully complete the clinical development of the Company’s compounds, that the
development of the Company's compounds will take longer or cost more than planned, that the Company or
Celgene may be delayed in initiating or completing any clinical trials, and that the Company's compounds will
not receive regulatory approval or become commercially successful products.
Other risks and uncertainties include those identified under the heading "Risk Factors" included in the
Company's Annual Report on Form 10-K filed with the Securities and Exchange Commission (SEC) on February
25, 2016, and other filings that the Company has made and may make with the SEC in the future. The
forward-looking statements contained in this presentation reflect the Company's current views with respect
to future events, and the Company does not undertake and specifically disclaims any obligation to update any
forward-looking statements.
2
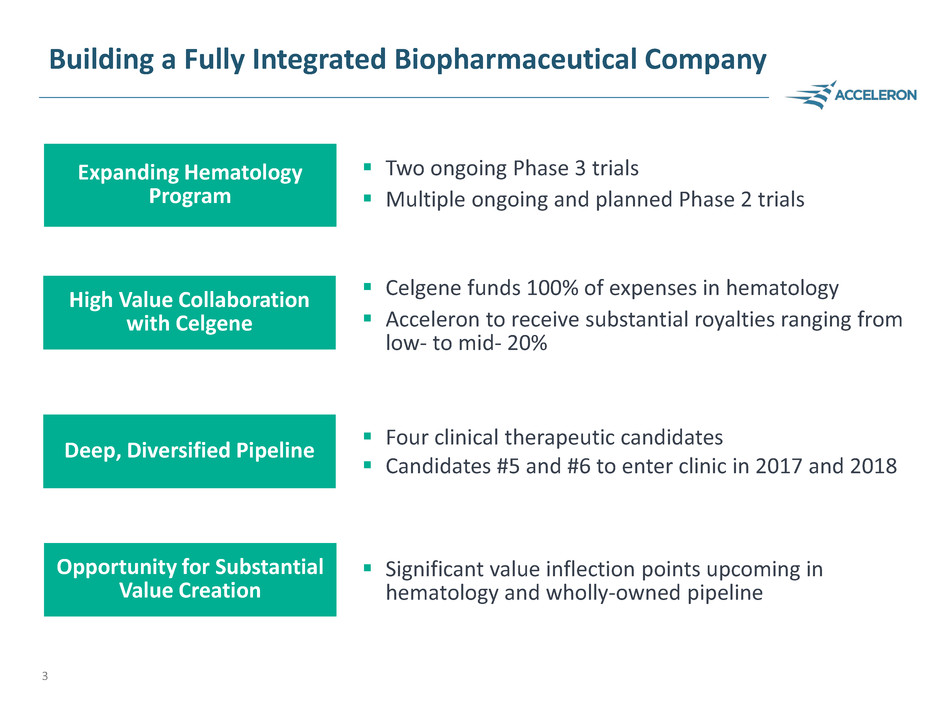
Building a Fully Integrated Biopharmaceutical Company
Two ongoing Phase 3 trials
Multiple ongoing and planned Phase 2 trials
Expanding Hematology
Program
High Value Collaboration
with Celgene
Deep, Diversified Pipeline
Four clinical therapeutic candidates
Candidates #5 and #6 to enter clinic in 2017 and 2018
Celgene funds 100% of expenses in hematology
Acceleron to receive substantial royalties ranging from
low- to mid- 20%
Significant value inflection points upcoming in
hematology and wholly-owned pipeline
Opportunity for Substantial
Value Creation
3
Acceleron’s Therapeutic Candidates Engage the Body’s Ability
to Rebuild and Repair Itself
Human cells and tissues have a remarkable ability to regenerate, rebuild and
repair themselves
The TGF-beta superfamily of proteins can profoundly influence many of these
biological processes
Leveraging >12 years of TGF-β biology R&D expertise, Acceleron has pioneered a
new approach to engage these targets for therapeutic benefit
Fibrosis Erythropoiesis Skeletal Muscle Vasculature
4
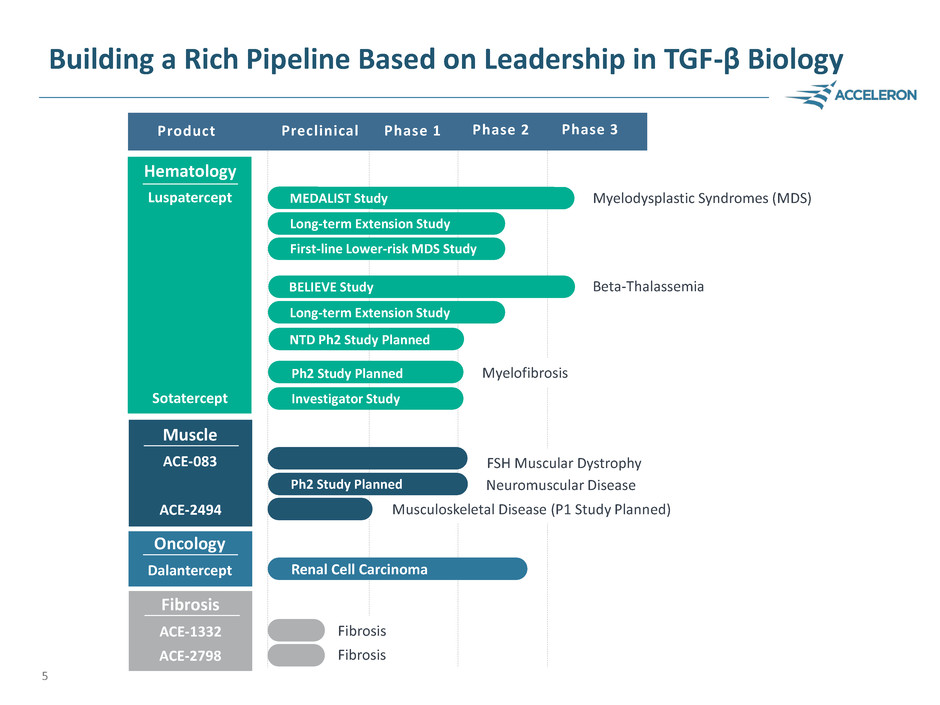
Building a Rich Pipeline Based on Leadership in TGF-β Biology
Phase 1
Myelodysplastic Syndromes (MDS)
Beta-Thalassemia
FSH Muscular Dystrophy
Musculoskeletal Disease (P1 Study Planned)
Fibrosis
Preclinical Phase 2 Phase 3 Product
Hematology
Luspatercept
Sotatercept
Dalantercept
Oncology
ACE-2494
Muscle
ACE-083
Fibrosis ACE-1332
ACE-2798
Fibrosis
MEDALIST Study
Long-term Extension Study
First-line Lower-risk MDS Study
BELIEVE Study
Long-term Extension Study
Myelofibrosis
Investigator Study
Ph2 Study Planned
NTD Ph2 Study Planned
5
Ph2 Study Planned Neuromuscular Disease
Renal Cell Carcinoma

Luspatercept: Building a Blockbuster Brand
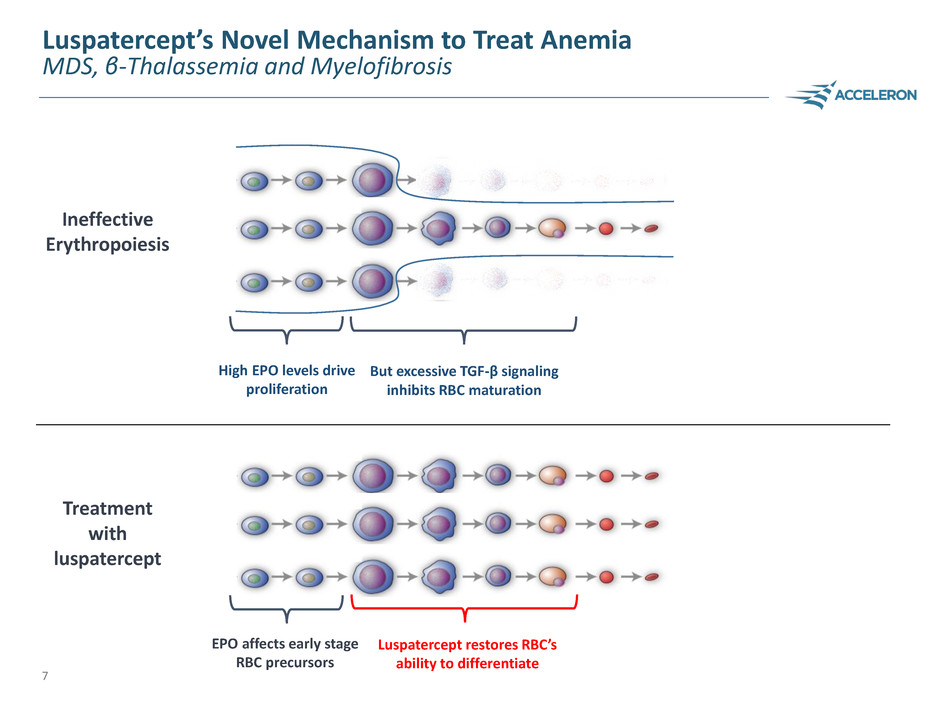
Luspatercept’s Novel Mechanism to Treat Anemia
MDS, β-Thalassemia and Myelofibrosis
EPO affects early stage
RBC precursors
Luspatercept restores RBC’s
ability to differentiate
Ineffective
Erythropoiesis
Treatment
with
luspatercept
High EPO levels drive
proliferation
But excessive TGF-β signaling
inhibits RBC maturation
7

Luspatercept in MDS
Opportunity to improve patient care in multiple population segments
ESA Refractory (RS+)
Lower-Risk
ESA Naïve
(RS+ and/or RS-)
ESA Ineligible (RS+)
First-line
Second-line
Ongoing
Phase 3
MDS Trial
Phase 3
Lower-Risk MDS
Populations
First-line
Expanded MDS Populations
+
40,000 to 50,000 patients in
US/EU
20,000 to 25,000 patients
in US/EU
+
8

Luspatercept Phase 3 Trial Design in Lower-Risk MDS
Primary Endpoint
Proportion of patients that become RBC-transfusion independent (≥ 8 weeks) during the
first 24 weeks
Key Secondary Endpoints
Proportion of patients that become RBC-transfusion independent (≥ 8 weeks) during the
first 48 weeks
International Working Group hematologic improvement-erythroid (IWG HI-E) or erythroid
response
Duration of transfusion independence (RBC-TI)
Trial ID: NCT02631070
Lower-Risk
MDS
RS+
luspatercept
(n = 140)
Placebo
(n = 70)
2:1
Randomized Treated SC
Every 3
Weeks
Primary
Endpoint
Secondary
Endpoint
Week 24 Week 48
Double-blind
9

Reduction in Transfusion Burden in Patients with > 3 Mo. of Treatment
Phase 2 results presented at ASH 2016
61% (17/28) patients achieved RBC transfusion independence ≥ 8 weeks
85% (24/28) of patients had a clinically meaningful erythroid response (IWG HI-E)
%
C
h
a
n
g
e
i
n
R
B
C
T
ra
n
sf
u
s
io
n
s
-100
-80
-60
-40
-20
0
4 4 12 14 6 10 9 6 6 14 8 10 8 6 6 6 4 4 4 4 2 2 2 2 2 2 2 2
Baseline RBC Transfusion Units (8 Weeks)
HI-E Non-Responder HI-E Responder Transfusion Independence (RBC-TI)
Data as of 09 Sep 2016
10
IWG HI-E Criteria:
LTB (< 4 Units/8 wk, Hb <10 g/dL) = Hb ↑ ≥1.5 g/dL /≥8 weeks
HTB (≥ 4 Units/8 wk) = ≥ 4 unit ↓ /8 wk

Luspatercept in β-Thalassemia
Opportunity to improve patient care in multiple population segments
One of the most common genetic diseases in the world
Transfusion Dependent
Phase 3
β-Thalassemia
Population
Non-Transfusion Dependent /
Occasionally Transfused
Additional Phase 2 Study Planned
Expanded β-Thalassemia
Populations
Ongoing
Phase 3
β-Thal Trial
Transfusion
Dependent
20,000 patients
in EU/N.A.
>250,000 ROW
20,000 patients in EU/N.A.
>250,000 ROW
+
11

Luspatercept Phase 3 Trial Design in β-Thalassemia
Primary Endpoint
Proportion of patients with ≥ 33% reduction in transfusion burden from weeks 13-24
compared to the 12 weeks preceding treatment
Key Secondary Endpoints
Proportion of patients with ≥ 33% reduction in transfusion burden from weeks 37-48
compared to the 12 weeks preceding treatment
Proportion of patients with ≥ 50% reduction in transfusion burden from weeks 13-24 and
weeks 37-48 compared to the 12 weeks preceding treatment
Trial ID: NCT02604433
luspatercept
(n = 200)
Placebo
(n = 100)
2:1
Randomized
Treated
SC
Every 3
Weeks
Primary
Endpoint
Secondary
Endpoint
Weeks
13 - 24
Weeks
37 - 48
12-week
prospective
pre-
treatment
period
Double-blind
β-Thalassemia
Transfusion
Dependent
12

* 1 subject discontinued before completing 12 weeks, not shown
Baseline units/12 weeks
% C
h
an
ge
in
R
B
C
Units
T
ran
sf
u
se
d
Transfusion reduction from 12 wks pre-treatment to any 12-wk period on treatment
83% (20/24) of TD patients experienced ≥ 33% reduction in transfusion burden
71% (17/24) of TD patients experienced ≥ 50% reduction in transfusion burden
Data as of 02 Sep 2016
8 14 7 12 12 7 8 9 7 5 6 6 7 8 8 8 8 15 6 8 8 7 4
-33
-40
-60
-80
-100
-20
0
Reduction in Transfusion Burden in Patients in Extension Study
Phase 2 results presented at ASH 2016
13
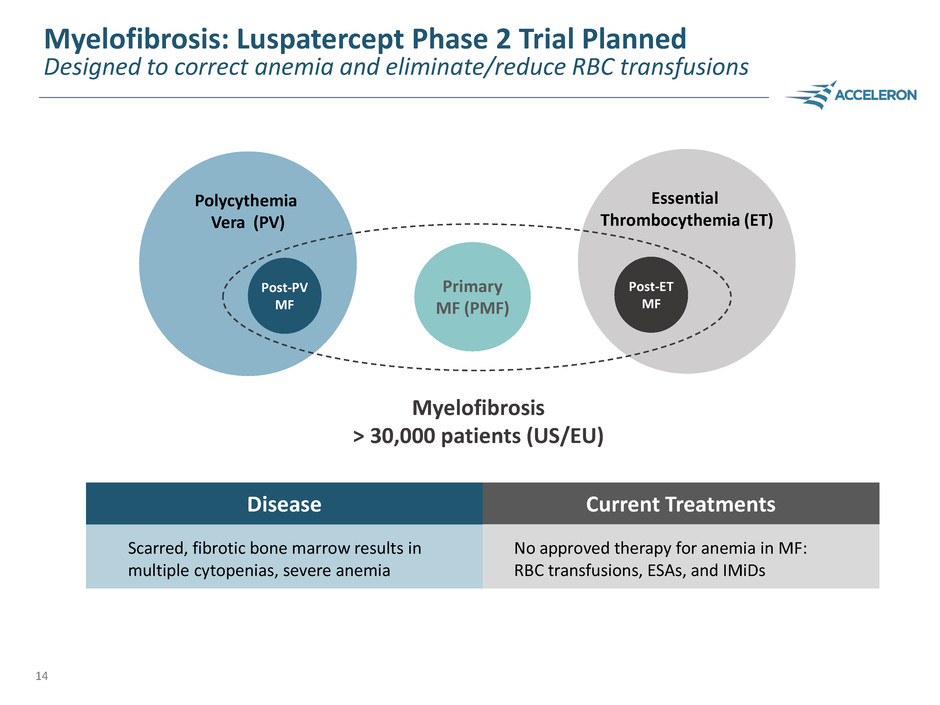
Myelofibrosis: Luspatercept Phase 2 Trial Planned
Designed to correct anemia and eliminate/reduce RBC transfusions
Disease Current Treatments
Scarred, fibrotic bone marrow results in
multiple cytopenias, severe anemia
No approved therapy for anemia in MF:
RBC transfusions, ESAs, and IMiDs
Essential
Thrombocythemia (ET)
Polycythemia
Vera (PV)
Post-PV
MF
Primary
MF (PMF)
Post-ET
MF
Myelofibrosis
> 30,000 patients (US/EU)
14

Collaborating with Celgene, the Leader in Hematology
Luspatercept Collaboration Highlights
Celgene funds 100% of development costs
Acceleron will receive tiered royalties in the low-to mid- 20% range
$185M of milestones still outstanding for regulatory and commercial
achievements
Companies will co-promote in North America, Celgene promotes ROW
Celgene funds 100% of Acceleron’s commercialization costs for
North American co-promote
15
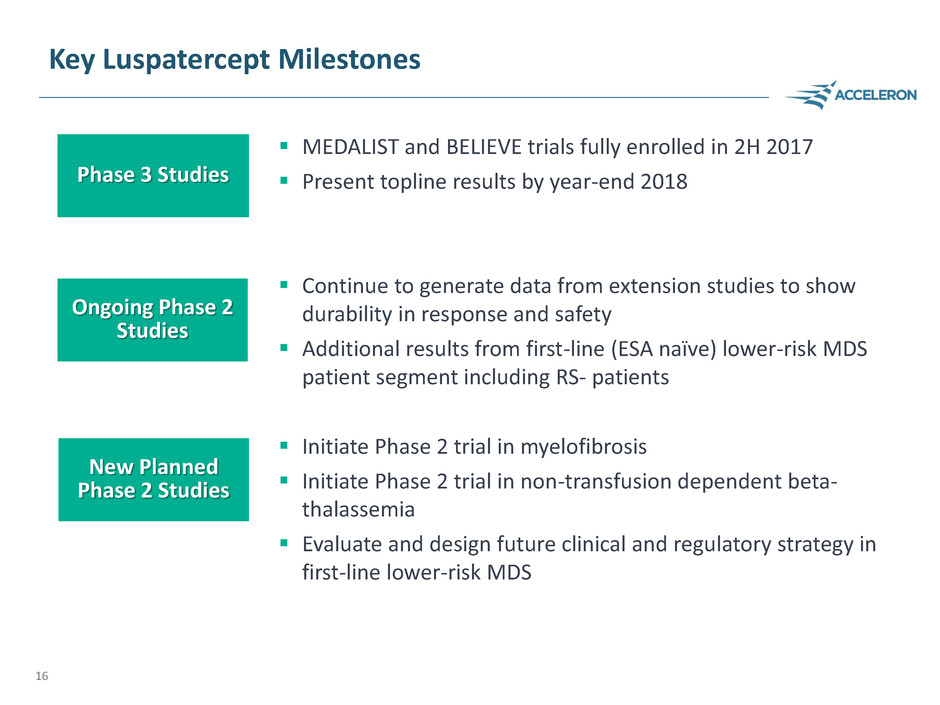
Key Luspatercept Milestones
MEDALIST and BELIEVE trials fully enrolled in 2H 2017
Present topline results by year-end 2018
Continue to generate data from extension studies to show
durability in response and safety
Additional results from first-line (ESA naïve) lower-risk MDS
patient segment including RS- patients
Initiate Phase 2 trial in myelofibrosis
Initiate Phase 2 trial in non-transfusion dependent beta-
thalassemia
Evaluate and design future clinical and regulatory strategy in
first-line lower-risk MDS
16
Phase 3 Studies
Ongoing Phase 2
Studies
New Planned
Phase 2 Studies

Neuromuscular Diseases: Unprecedented
Results in Early Clinical Studies

ACE-083
Designed to increase muscle mass and strength selectively
in the muscles in which the drug is administered
ACE-083: Targeted Intervention for Muscle Loss
18
Phase 1
Produced substantial dose-dependent increases in muscle
volume in a Phase 1 study
‒ 9% to 15% muscle volume increases with two doses
Phase 2
FSHD trial initiated and underway
Plan to initiate trial in another neuromuscular disease

ACE-083 Targeted Muscle Therapy in FSHD
19
Weakness
limits the ability to:
Feed oneself
Lift objects
Maintain hygiene
Weakness
Causes foot drop
Impairs ambulation
Increases risk of falls
FSHD Epidemiology
• Approx. 20,000 patients in the U.S.
• Disabling focal muscle loss that can be
asymmetric
Biceps
Strengthening
the bicep muscle
should allow
independent activities
of daily living
Tibialis Anterior
Strengthening the
TA muscle should
alleviate foot drop;
improving ambulation
and stair-climbing
ability

Acceleron Newsflow and Catalysts
Luspatercept
– Complete enrollment in MEDALIST and BELIEVE Phase 3 trials in 2H 2017
– Release topline Phase 3 Results by YE 2018
– Develop clinical and regulatory strategy in first-line lower-risk MDS in 2017
– Initiate new Phase 2 trials in myelofibrosis and NTD beta-thalassemia by YE 2017
– Additional Phase 2 extension study results at medical conferences in 2017
ACE-083
– Initial FSHD Phase 2 Part 1 dose-escalation results by late 2017
– Initiate Phase 2 trial in a second neuromuscular disease in 2017
Dalantercept
– Topline PFS results in RCC in 2H 2017
ACE-2494
– Initiate Phase 1 healthy volunteer study in 2017
Next Acceleron compound to enter the clinic in 2018
20
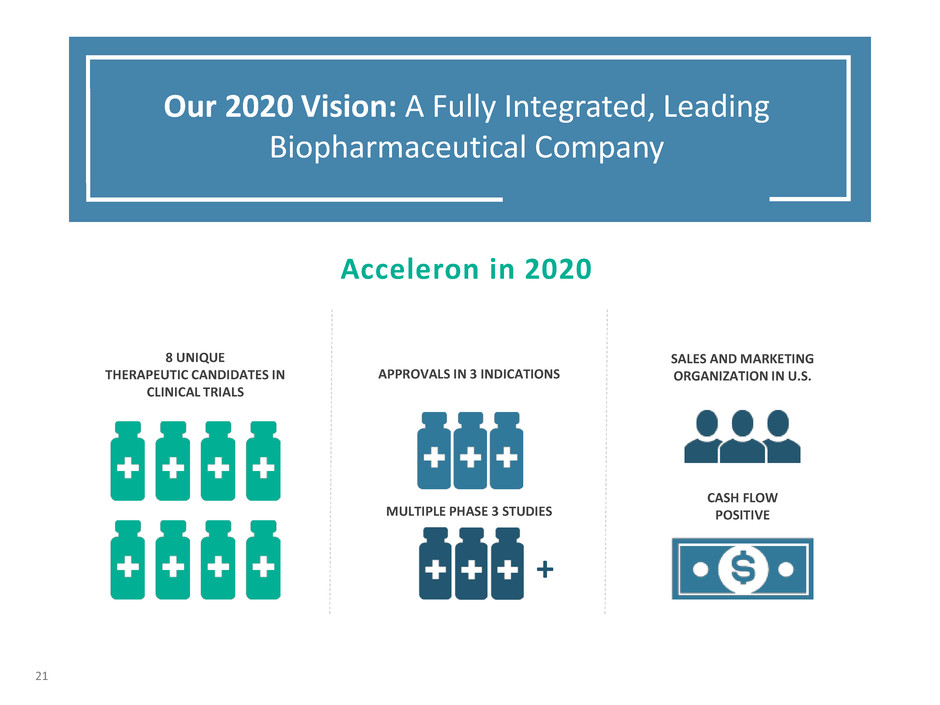
APPROVALS IN 3 INDICATIONS
CASH FLOW
POSITIVE
Acceleron in 2020
8 UNIQUE
THERAPEUTIC CANDIDATES IN
CLINICAL TRIALS
MULTIPLE PHASE 3 STUDIES
SALES AND MARKETING
ORGANIZATION IN U.S.
Our 2020 Vision: A Fully Integrated, Leading
Biopharmaceutical Company
+
21

Key Takeaways
A valuable mix of early, mid- and late-stage assets in clinical
development that will drive value in 2017 and beyond
Global partnership with Celgene in multiple late-stage anemia
indications with blockbuster potential
Powerful discovery engine generating additional new candidates against
novel targets in the TGF-β superfamily of proteins
Rapidly expanding wholly-owned pipeline across multiple serious and
rare diseases in the areas of muscle, fibrosis and vasculature
Well-capitalized
– Hematology programs fully funded by Celgene
– Current capital into 2H 2019
22

Transforming Patient Care with
Breakthrough Science
35TH ANNUAL J.P. MORGAN HEALTHCARE CONFERENCE JANUARY 9, 2017
