Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AGILE THERAPEUTICS INC | a17-1209_1ex99d1.htm |
| 8-K - 8-K - AGILE THERAPEUTICS INC | a17-1209_18k.htm |
Exhibit 99.2
Agile Announces Positive Top-line Phase 3 Results for Twirla® SECURE Conference Call and Webcast January 3, 2017

Certain information contained in this press release includes "forward-looking statements" related to the Company's clinical trials, regulatory submissions and potential market opportunity for its product candidates. We may, in some cases use terms such as "predicts," "believes," "potential," "continue," "anticipates", "estimates," "expects," "plans," "intends," "may," "could," 'might," "will," "should" or other words that convey uncertainty of the future events or outcomes to identify these forward-looking statements. Our forward-looking statements are based on current beliefs and expectations of our management team that involve risks, potential changes in circumstances, assumptions and uncertainties. Any or all of the forward-looking statements may turn out to be wrong, or be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. Our statements about the results and conduct of our clinical trial could be affected by the potential that there are changes in the data or interpretation of the data by the FDA (for example, the FDA may include additional pregnancies in its calculation of the Pearl Index, which would increase the Pearl Index), whether the results will be deemed satisfactory by the FDA (for example, we describe the results of the SECURE trial as positive, the FDA may disagree with that characterization), and whether additional studies will be required or other issues will arise that will delay resubmission of our NDA or negatively impact acceptance, review and approval of Twirla by the FDA; our statements about the potential commercial opportunity could be affected by the potential that our product does not receive regulatory approval, does not receive reimbursement by third party payors, or a commercial market for the product does not develop because of any of the risks inherent in the commercialization of contraceptive products. For all these reasons, actual results and developments could be materially different from those expressed in or implied by our forward-looking statements. All forward looking statements are subject to risks detailed in our filings with the U.S. Securities and Exchange Commission, including the Company's Annual Report on Form 10-K and our Quarterly Reports on Form 10-Q. You are cautioned not to place undue reliance on these forward-looking statements, which are made only as of the date of this press release. We undertake no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstances. Forward-Looking Statement

Agenda Introduction Presented by Agile Chief Executive Officer, Al Altomari Top-line Results Presented by Agile Chief Medical Officer, Elizabeth Garner, M.D., M.P.H. Study Design and Top-line Results Basis for resubmission Concluding Remarks

The CRL Expressed a Clear Rationale for a New Study Focused on two key elements Improved study conduct Reduced loss to follow-up rate compared to previous Phase 3 trials Support subject compliance and overall retention Demonstration of acceptable efficacy in a representative population “An acceptable Pearl Index and upper bound of the 95% confidence interval” “A representative sample of women in the U.S. who are seeking hormonal contraception” “A sufficiently large and diverse population so that efficacy can be assessed in subgroups” Quotes sourced from FDA correspondence CRL = Complete Response Letter FDA = Food & Drug Administration
Rigorous trial design was focused on key elements of the CRL The SECURE Trial Was Designed to Assess the Efficacy and Safety of Twirla® in a Real-World Population Multicenter, single-arm, open-label 13-cycle trial at 102 experienced U.S. clinical sites ~ 2,000 healthy subjects aged > 18 treated with laser-etched patches Representative sample of women seeking hormonal contraception No exclusions for BMI/weight Stringent Trial Design Frequent pregnancy testing Exclusion of cycles for BOTH use of back-up contraception and lack of sexual activity Analysis Efficacy measure was Pearl Index in an ITT population of subjects 35 years of age and under Prespecified analysis related to BMI and body weight CRL = Complete Response Letter ITT = Intent to Treat
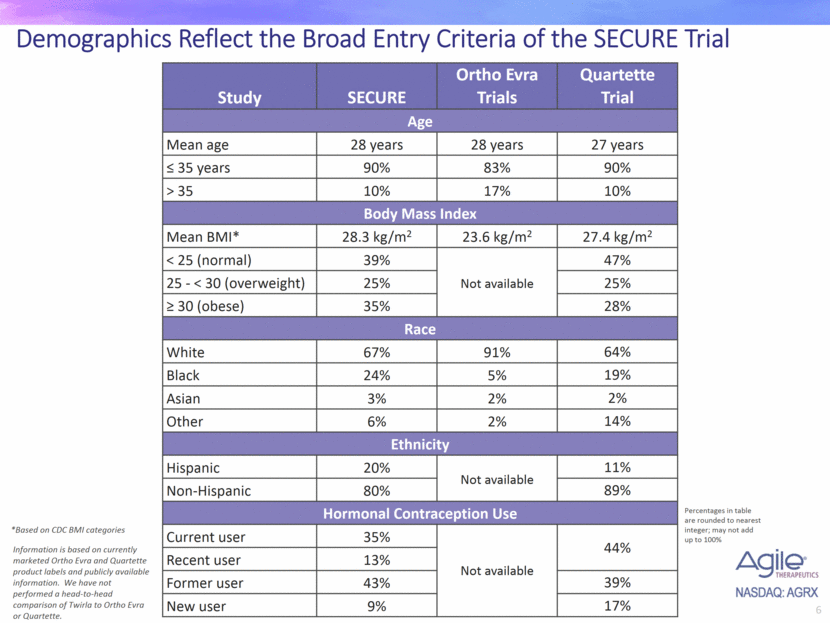
Demographics Reflect the Broad Entry Criteria of the SECURE Trial Study SECURE Ortho Evra Trials Quartette Trial Age Mean age 28 years 28 years 27 years < 35 years 90% 83% 90% > 35 10% 17% 10% Body Mass Index Mean BMI* 28.3 kg/m2 23.6 kg/m2 27.4 kg/m2 < 25 (normal) 39% Not available 47% 25 - < 30 (overweight) 25% 25% > 30 (obese) 35% 28% Race White 67% 91% 64% Black 24% 5% 19% Asian 3% 2% 2% Other 6% 2% 14% Ethnicity Hispanic 20% Not available 11% Non-Hispanic 80% 89% Hormonal Contraception Use Current user 35% Not available 44% Recent user 13% Former user 43% 39% New user 9% 17% *Based on CDC BMI categories Information is based on currently marketed Ortho Evra and Quartette product labels and publicly available information. We have not performed a head-to-head comparison of Twirla to Ortho Evra or Quartette. Percentages in table are rounded to nearest integer; may not add up to 100%
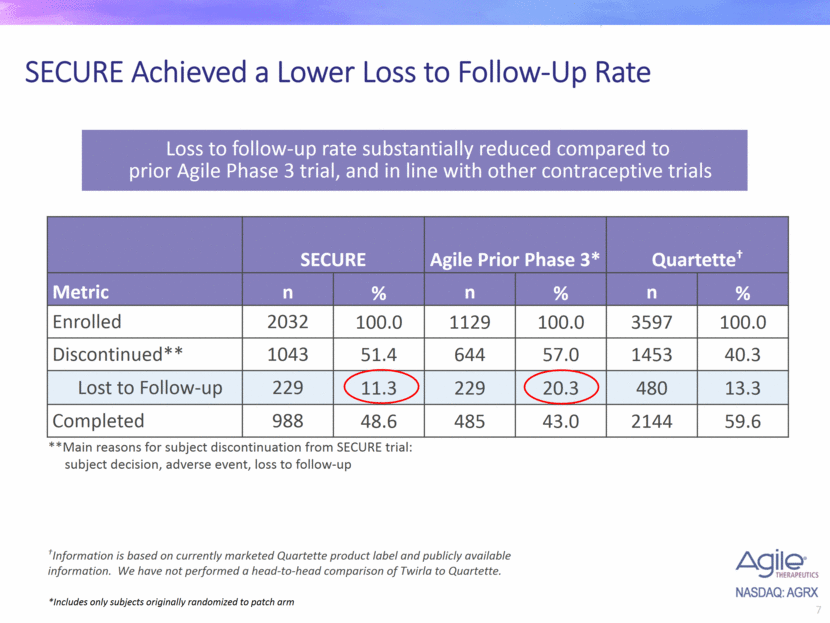
SECURE Achieved a Lower Loss to Follow-Up Rate *Includes only subjects originally randomized to patch arm Loss to follow-up rate substantially reduced compared to prior Agile Phase 3 trial, and in line with other contraceptive trials SECURE Agile Prior Phase 3* Quartette† Metric n % n % n % Enrolled 2032 100.0 1129 100.0 3597 100.0 Discontinued** 1043 51.4 644 57.0 1453 40.3 Lost to Follow-up 229 11.3 229 20.3 480 13.3 Completed 988 48.6 485 43.0 2144 59.6 **Main reasons for subject discontinuation from SECURE trial: subject decision, adverse event, loss to follow-up †Information is based on currently marketed Quartette product label and publicly available information. We have not performed a head-to-head comparison of Twirla to Quartette.
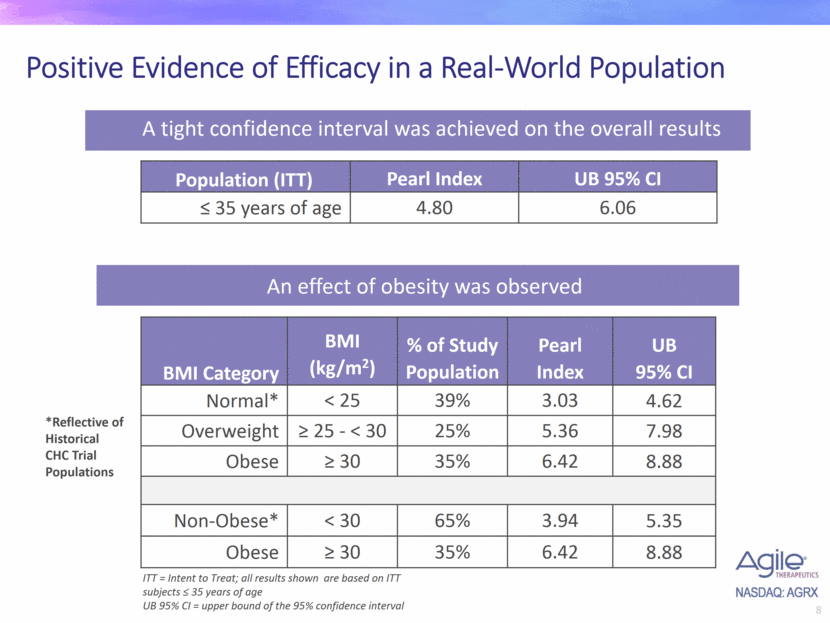
Positive Evidence of Efficacy in a Real-World Population BMI Category BMI (kg/m2) % of Study Population Pearl Index UB 95% CI Normal* < 25 39% 3.03 4.62 Overweight > 25 - < 30 25% 5.36 7.98 Obese > 30 35% 6.42 8.88 Non-Obese* < 30 65% 3.94 5.35 Obese > 30 35% 6.42 8.88 A tight confidence interval was achieved on the overall results ITT = Intent to Treat; all results shown are based on ITT subjects < 35 years of age UB 95% CI = upper bound of the 95% confidence interval An effect of obesity was observed Population (ITT) Pearl Index UB 95% CI < 35 years of age 4.80 6.06 *Reflective of Historical CHC Trial Populations
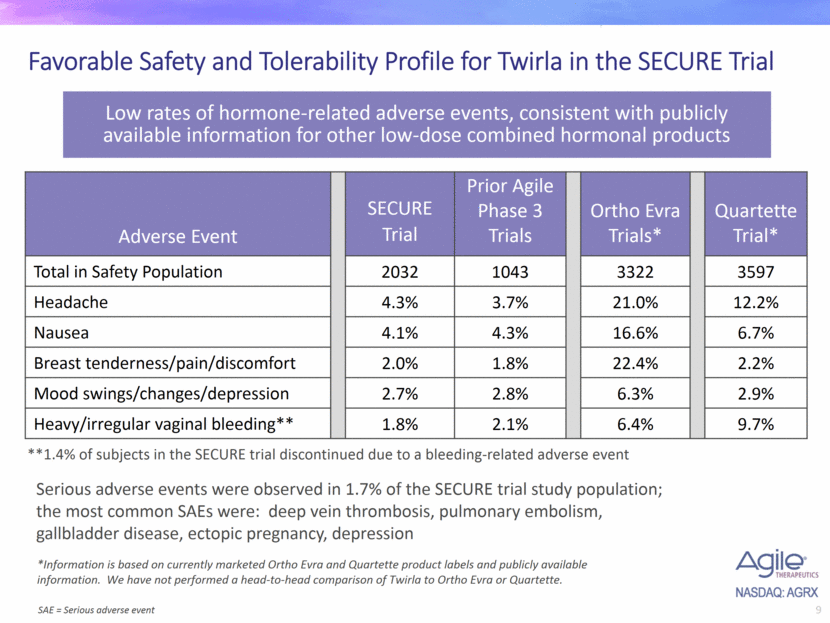
Favorable Safety and Tolerability Profile for Twirla in the SECURE Trial Low rates of hormone-related adverse events, consistent with publicly available information for other low-dose combined hormonal products Serious adverse events were observed in 1.7% of the SECURE trial study population; the most common SAEs were: deep vein thrombosis, pulmonary embolism, gallbladder disease, ectopic pregnancy, depression *Information is based on currently marketed Ortho Evra and Quartette product labels and publicly available information. We have not performed a head-to-head comparison of Twirla to Ortho Evra or Quartette. **1.4% of subjects in the SECURE trial discontinued due to a bleeding-related adverse event Adverse Event SECURE Trial Prior Agile Phase 3 Trials Ortho Evra Trials* Quartette Trial* Total in Safety Population 2032 1043 3322 3597 Headache 4.3% 3.7% 21.0% 12.2% Nausea 4.1% 4.3% 16.6% 6.7% Breast tenderness/pain/discomfort 2.0% 1.8% 22.4% 2.2% Mood swings/changes/depression 2.7% 2.8% 6.3% 2.9% Heavy/irregular vaginal bleeding** 1.8% 2.1% 6.4% 9.7% SAE = Serious adverse event
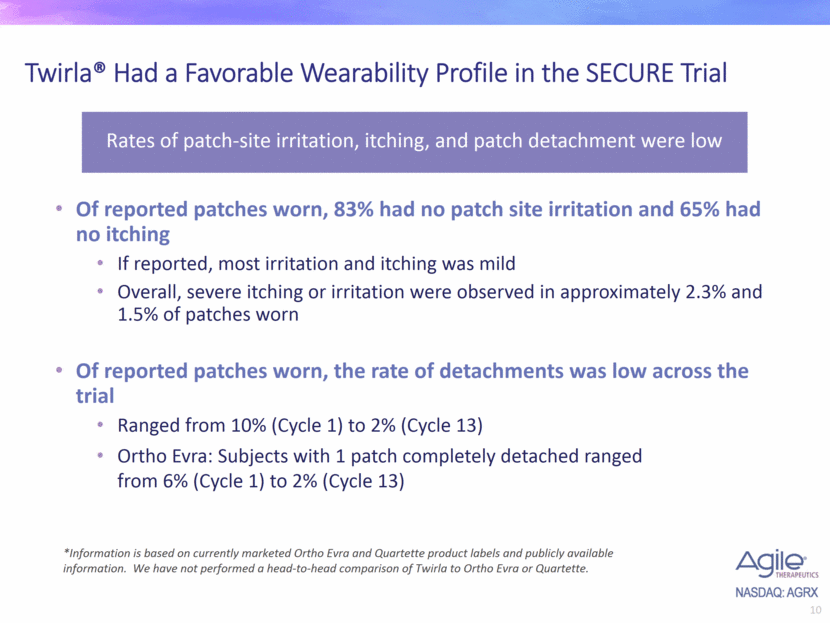
Twirla® Had a Favorable Wearability Profile in the SECURE Trial Of reported patches worn, 83% had no patch site irritation and 65% had no itching If reported, most irritation and itching was mild Overall, severe itching or irritation were observed in approximately 2.3% and 1.5% of patches worn Of reported patches worn, the rate of detachments was low across the trial Ranged from 10% (Cycle 1) to 2% (Cycle 13) Ortho Evra: Subjects with 1 patch completely detached ranged from 6% (Cycle 1) to 2% (Cycle 13) Rates of patch-site irritation, itching, and patch detachment were low *Information is based on currently marketed Ortho Evra and Quartette product labels and publicly available information. We have not performed a head-to-head comparison of Twirla to Ortho Evra or Quartette.
The NDA Resubmission is Expected to Address the Clinical CRL Questions Substantially improved study conduct Lower loss to follow up rate compared to previous Phase 3 trial Greater confidence in the reliability of the results based on improved loss to follow-up rate and focus on data quality Study population reflects the broad entry criteria for the trial Allowed for efficacy to be assessed across different groups No restrictions on BMI (unlike historical contraceptive trials) Evidence of efficacy and safety Positive evidence of efficacy observed in a real-world study population Favorable safety profile; rates of adverse events consistent with publicly available information for other low-dose combined hormonal products We expect to submit a robust data package that more clearly defines the risk/benefit profile for Twirla

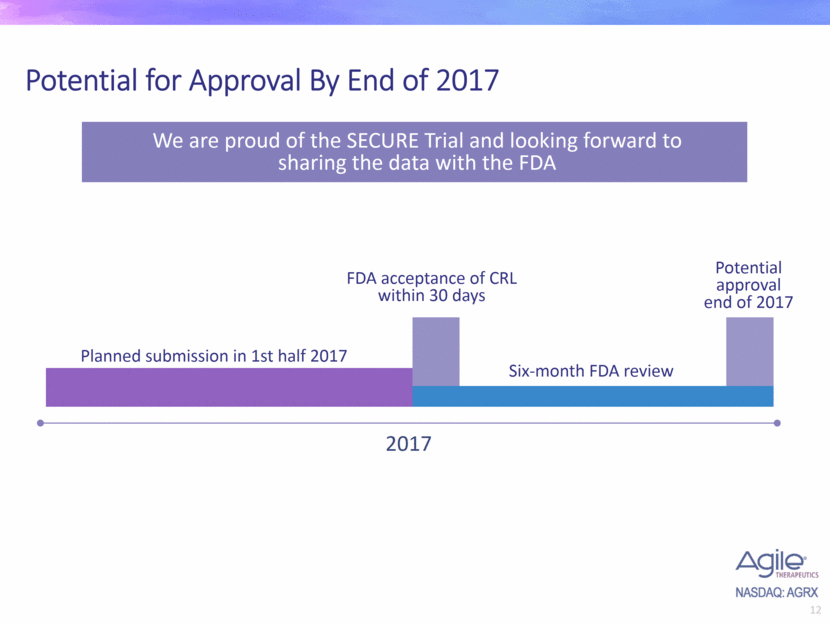
Potential for Approval By End of 2017 2017 Planned submission in 1st half 2017 FDA acceptance of CRL within 30 days Potential approval end of 2017 Six-month FDA review We are proud of the SECURE Trial and looking forward to sharing the data with the FDA
APPENDIX FDA/Regulatory Perspective on BMI and CHC Effectiveness 2007 Advisory Panel Recommendations Evolution of Study Populations Around BMI/Weight 2015 FDA Meta-Analysis on Obesity Datamonitor Study on CRL Statistics Contraceptive Method Effectiveness Chart

Summary of Recommendations from the 2007 FDA Advisory Committee Meeting on Contraceptive Trial Design Entry criteria should be more reflective of real-world prescribing regarding BMI, smoking, VTE family history Subgroup analyses could be performed to assess efficacy Arbitrary limits for the UB of the 95% CI should be avoided in order to promote the widest range of new contraceptive products being developed and brought to market Substantial flexibility should be exercised in accepting given point estimates and UB of CI Provide all the information to the clinician and patient in an easily understandable format in labeling and let them make the final decision on which product is most appropriate Phase 4 trials may be used to obtain better estimates of true “actual use” effectiveness Product labeling should be modified to include pregnancy rates or safety data for subgroups when available Source: 2007 FDA Advisory Committee for Reproductive Health Drugs, Summary of Recommendations http://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4274m1.pdf
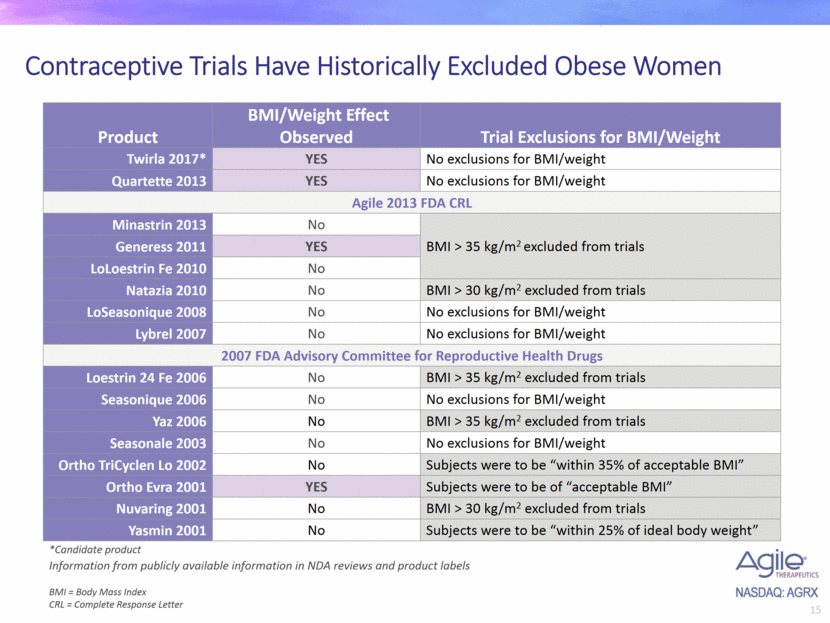
Contraceptive Trials Have Historically Excluded Obese Women Product BMI/Weight Effect Observed Trial Exclusions for BMI/Weight Twirla 2017* YES No exclusions for BMI/weight Quartette 2013 YES No exclusions for BMI/weight Agile 2013 FDA CRL Minastrin 2013 No BMI > 35 kg/m2 excluded from trials Generess 2011 YES LoLoestrin Fe 2010 No Natazia 2010 No BMI > 30 kg/m2 excluded from trials LoSeasonique 2008 No No exclusions for BMI/weight Lybrel 2007 No No exclusions for BMI/weight 2007 FDA Advisory Committee for Reproductive Health Drugs Loestrin 24 Fe 2006 No BMI > 35 kg/m2 excluded from trials Seasonique 2006 No No exclusions for BMI/weight Yaz 2006 No BMI > 35 kg/m2 excluded from trials Seasonale 2003 No No exclusions for BMI/weight Ortho TriCyclen Lo 2002 No Subjects were to be “within 35% of acceptable BMI” Ortho Evra 2001 YES Subjects were to be of “acceptable BMI” Nuvaring 2001 No BMI > 30 kg/m2 excluded from trials Yasmin 2001 No Subjects were to be “within 25% of ideal body weight” *Candidate product BMI = Body Mass Index CRL = Complete Response Letter Information from publicly available information in NDA reviews and product labels
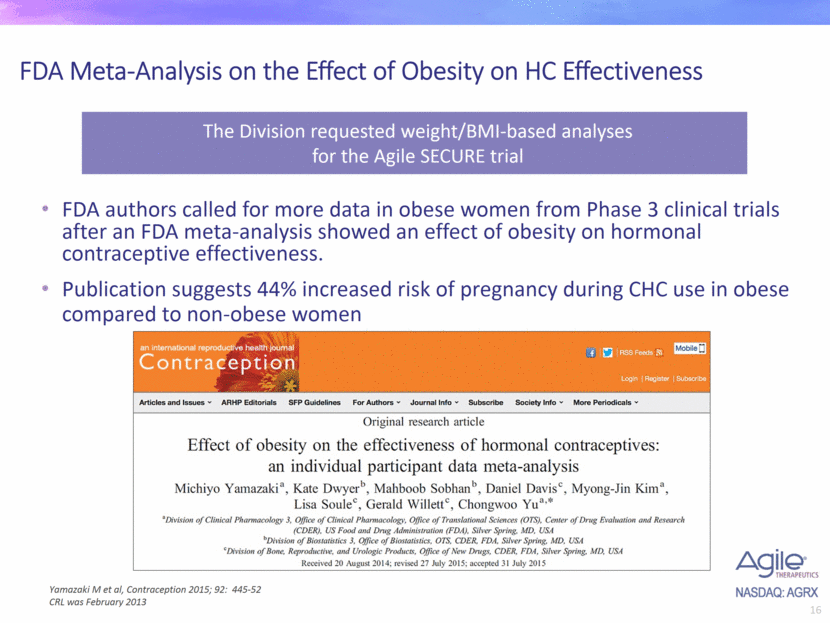
FDA Meta-Analysis on the Effect of Obesity on HC Effectiveness FDA authors called for more data in obese women from Phase 3 clinical trials after an FDA meta-analysis showed an effect of obesity on hormonal contraceptive effectiveness. Publication suggests 44% increased risk of pregnancy during CHC use in obese compared to non-obese women Yamazaki M et al, Contraception 2015; 92: 445-52 CRL was February 2013 The Division requested weight/BMI-based analyses for the Agile SECURE trial
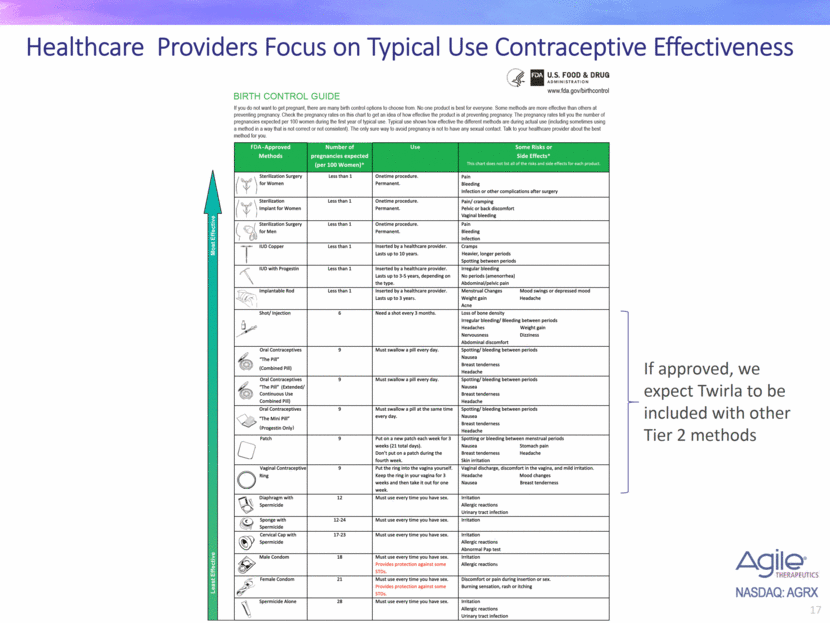
Healthcare Providers Focus on Typical Use Contraceptive Effectiveness If approved, we expect Twirla to be included with other Tier 2 methods
Datamonitor Analyst Study on FDA CRLs CRLs were received by 42% of NDAs/BLAs 29% of NDAs receiving CRLs were withdrawn 71% were resubmitted or open at time of analysis; of resubmitted NDAs with a documented FDA decision, 80 of 81 were approved Most failures to gain approval were because the applicant chose not to resubmit CRL = Complete Response Letter Source: Datamonitor Analysis-Analyst Opinion; study of 356 NDAs/BLAs from Oct 2008 to Sept 2012; Jan 2013.