Attached files
| file | filename |
|---|---|
| 8-K - MICROBOT FORM 8-K - Microbot Medical Inc. | form8k1219.htm |
TRANSFORMING MEDICAL TREATMENTS THROUGH MICRO-INVASIVE ROBOTICS Investor Presentation December 2016 NASDAQ:MBOT

FORWARD LOOKING STATEMENTS THIS DOCUMENT IS PRIVATE AND CONFIDENTIAL, CONTAINS SENSITIVE BUSINESS INFORMATION OF MICROBOT MEDICAL INC. (“MICROBOT”) AND IS NOT FOR PUBLIC DISTRIBUTION. This document has been prepared for informational purposes only and is provided personally to you. By accepting this document you agree to keep its contents strictly confidential, not to copy any portion of this document and to return it to Microbot promptly upon its request. This document contains summary information about Microbot, does not purport to be complete, and no representations or warranties about such information are made by Microbot or its representatives. This document does not constitute an offer to sell or a solicitation of an offer to purchase any securities of Microbot. Any such offer will be made only pursuant to an effective registration statement or an exemption from registration. This document contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended, relating to future events or the future financial performance and operations of Microbot. Forward-looking statements, which involve assumptions and describe Microbot’s intent, belief or current expectations about its business opportunities, prospects, performance and results, are generally identifiable by use of the words “may,” “could,” “should,” “will,” “would,” “expect,” “anticipate,” “plan,” “potential,” “estimate,” “believe,” “intend,” “project,” “forecast,” the negative of such words and other variations on such words or similar terminology. These forward-looking statements are not guarantees of future performance and by their nature involve known and unknown risks and uncertainties that may cause actual opportunities, prospects, performance and results to vary from those presented in this document, and those variances may be material. In evaluating such statements, prospective investors should carefully consider the various risks and uncertainties identified in Microbot’s public filings with the Securities and Exchange Commission, such as market risk, liquidity risk, competitive risk, regulatory risk and other commonly recognized forms of risk relating to Microbot and its securities. In light of these risks, uncertainties and assumptions, the forward-looking events discussed in this document might not occur. Microbot is not obligated to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. *

TRANSFORMING MEDICAL TREATMENTS THROUGH MICRO-INVASIVE ROBOTICS HAREL GADOT CEO, President & Chairman NASDAQ:MBOT

THE INITIAL SPARK… *
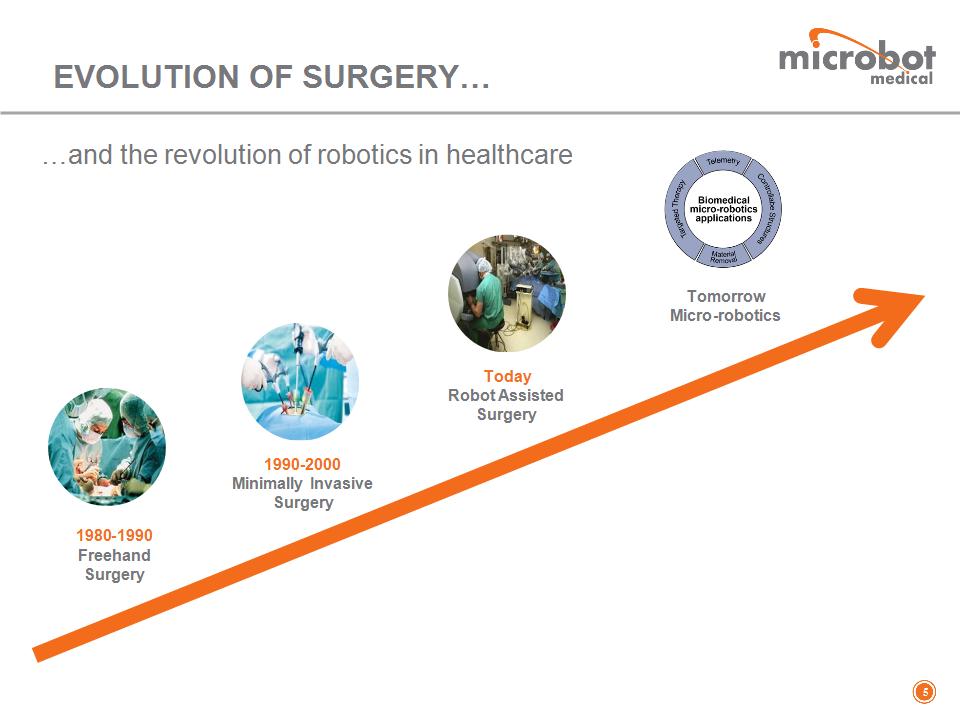
…and the revolution of robotics in healthcare EVOLUTION OF SURGERY… * 1980-1990 Freehand Surgery 1990-2000 Minimally Invasive Surgery Today Robot Assisted Surgery Tomorrow Micro-robotics
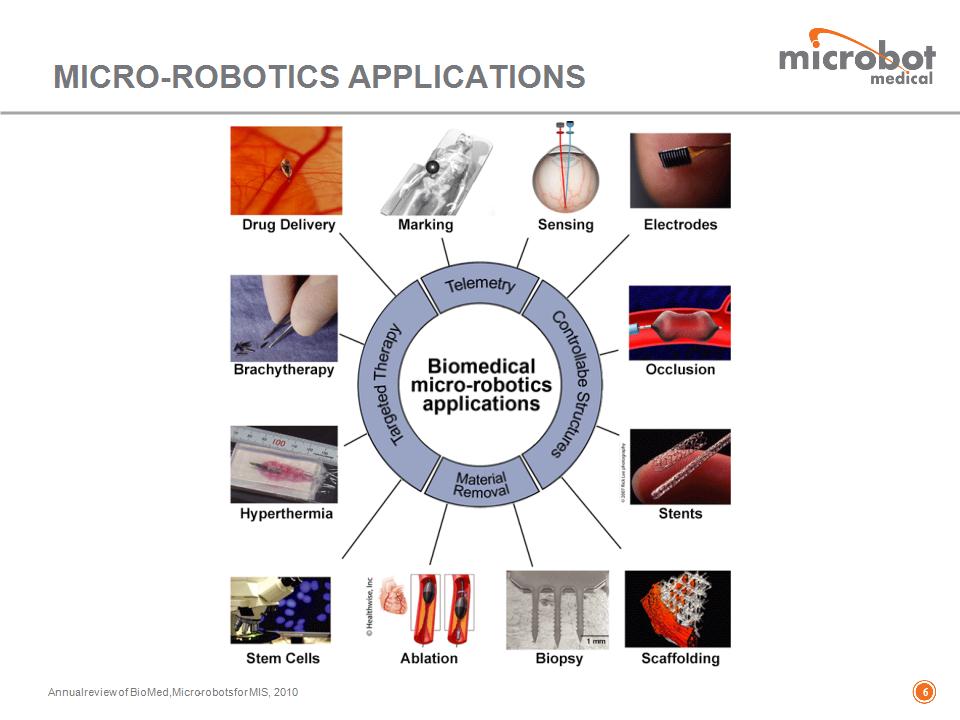
Annual review of BioMed, Micro-robots for MIS, 2010 MICRO-ROBOTICS APPLICATIONS *

Business Confidential 1: ‘Global Minimally Invasive Surgery Market is Expected to Reach USD 50.60 Billion in 2019’, Transparency Market Research. http://www.transparencymarketresearch.com/minimally-invasive-surgery-market.html THE NEXT BIG-SMALL THING Minimally Invasive Surgery (MIS) is one of the largest trends in surgical healthcare Global market for MIS devices in 2012 was valued at USD $25.03 Billion and is expected to reach a market value of USD $50.60 Billion by 2019. The MIS market is expected to grow at a CAGR of 10.5% during the forecast period 2013 to 20191 Minimally invasive techniques have been applied to most of the surgical specialties (including cardiothoracic, orthopedic, urological, vascular and neurological procedures) Minimally invasive procedures are undergoing a transformation by becoming smaller, automated, more precise instruments *

OUR MISSION Enable physicians to transform medical treatments and improve patient efficacy through our micro-invasive robotic platforms
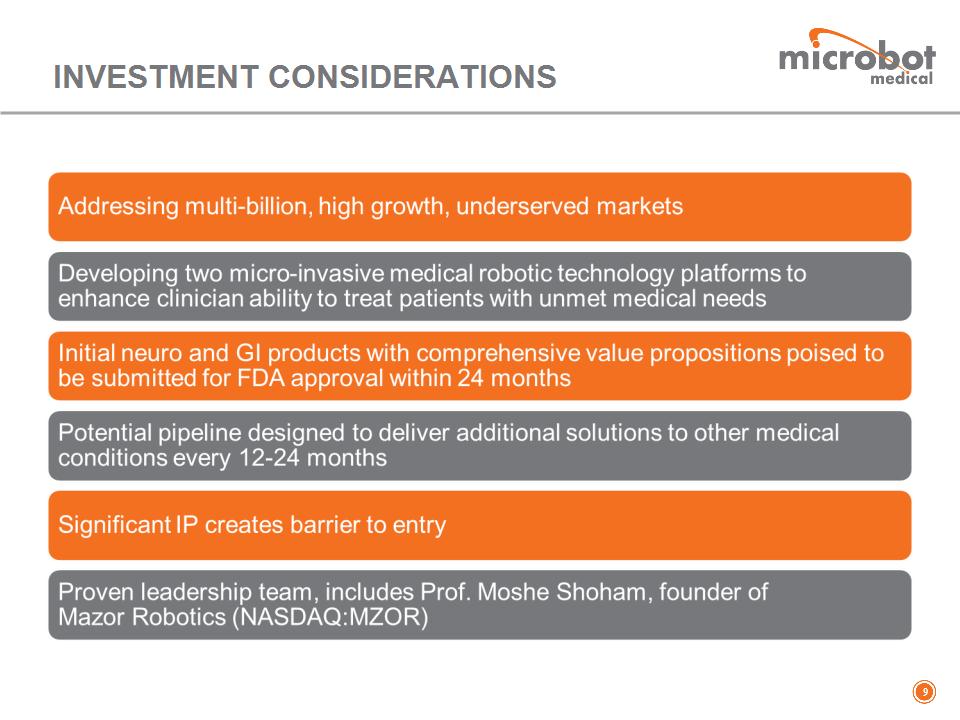
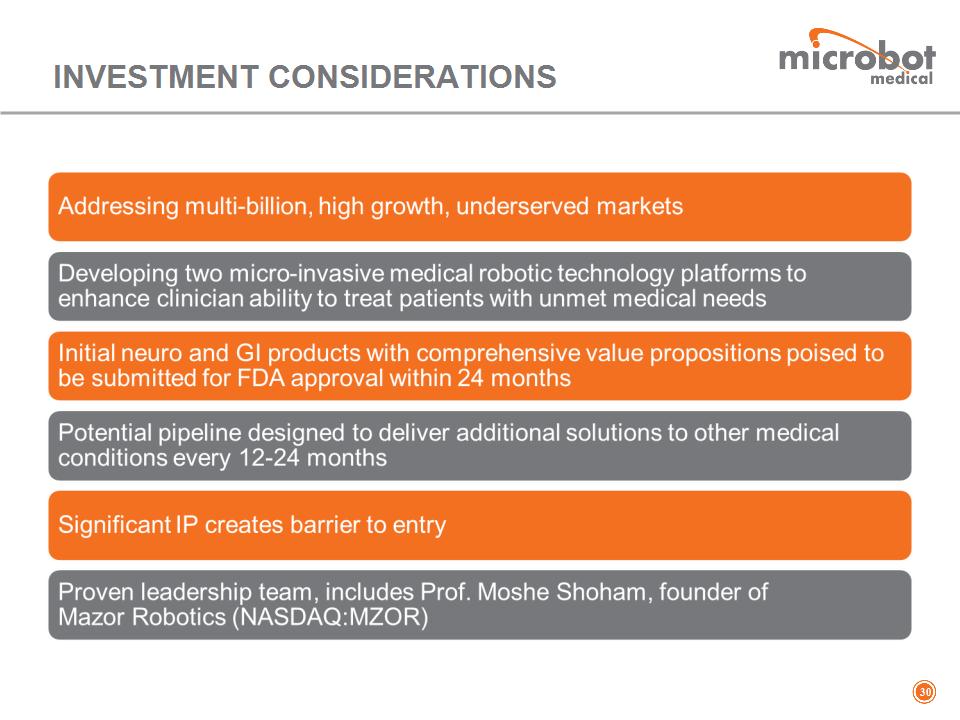
INVESTMENT CONSIDERATIONS *

TECHNOLOGY PLATFORMS ViRob Autonomous Advancing Micro-Robot (AAMR) ViRob demonstrates the ability to advance within cavities similar to the typical human body's lumens * TipCAT TipCAT is a disposable, flexible, self-propelled, see & treat endoscope/catheter
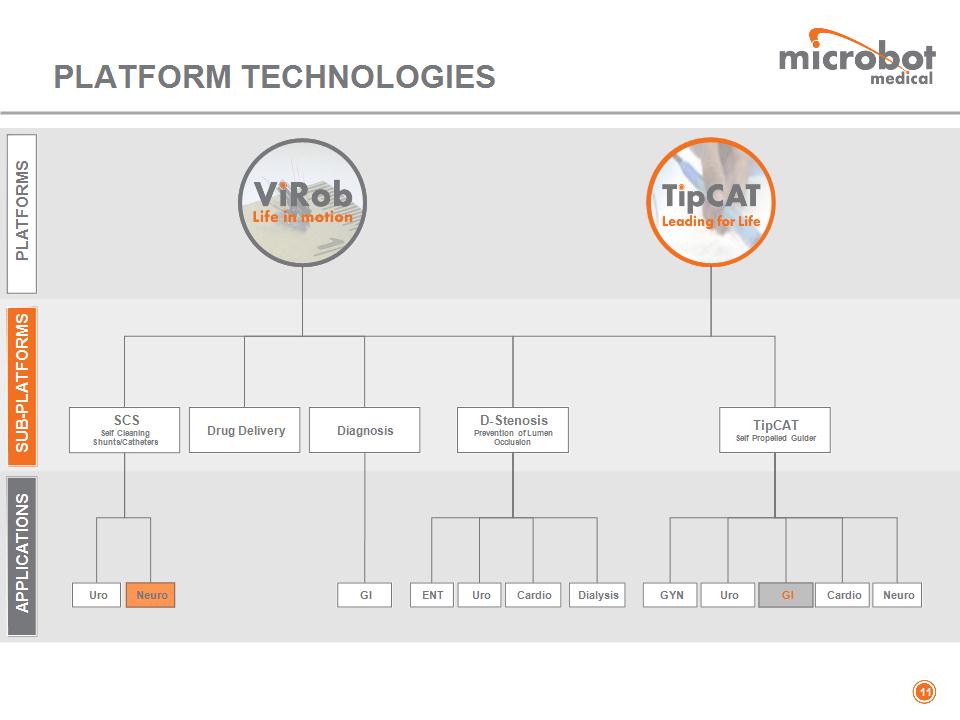
SCS Self Cleaning Shunts/Catheters PLATFORMS SUB-PLATFORMS D-Stenosis Prevention of Lumen Occlusion TipCAT Self Propelled Guider APPLICATIONS Diagnosis Drug Delivery Cardio Dialysis Neuro Cardio GI Uro GYN Uro Neuro Uro GI ENT PLATFORM TECHNOLOGIES *

*

NIH, National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/hydrocephalus/detail_hydrocephalus.htm National Hydrocephalus Foundation. http://nhfonline.org/facts-about-hydrocephalus.htm VP SHUNT MARKET OPPORTUNITY Hydrocephalus and Normal Pressure Hydrocephalus (NPH), are medical conditions in which there is an abnormal accumulation of cerebrospinal fluid (CSF) in the ventricles of the brain. Hydrocephalus occurs in about 1 in every 500 births in the U.S. alone1,2 Over 1,000,000 people in the United States currently live with hydrocephalus1 It is estimated that more than 700,000 Americans have NPH, but less than 20% receive an appropriate diagnosis1 The problem is often misdiagnosed as Dementia, Alzheimer’s, or Parkinson’s2 NPH can cause dementia, difficulty in walking and urinary incontinence2 *
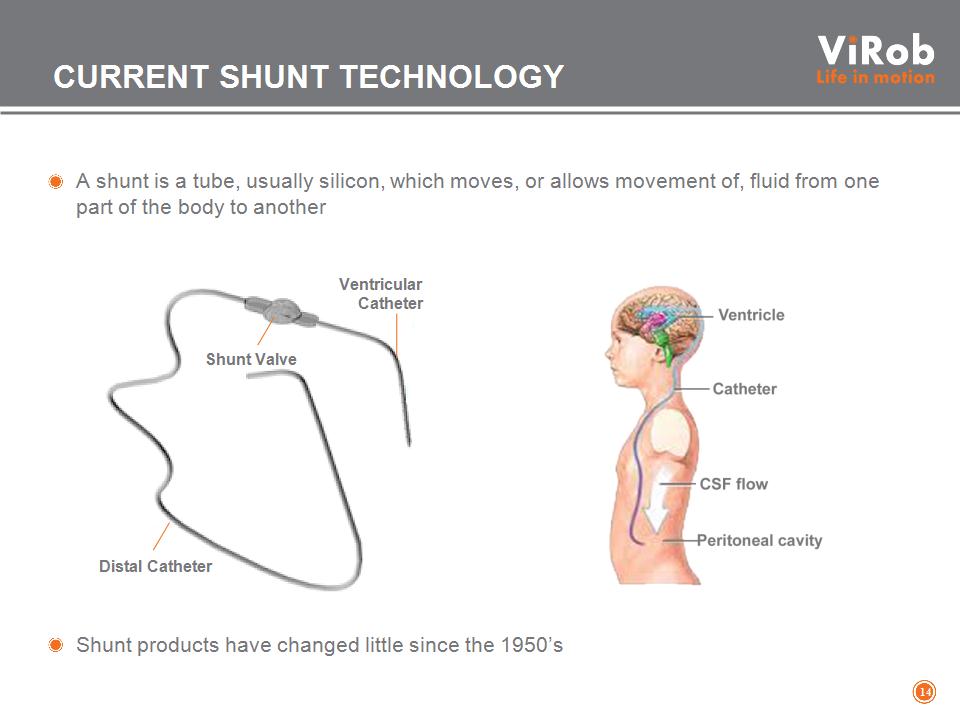
CURRENT SHUNT TECHNOLOGY A shunt is a tube, usually silicon, which moves, or allows movement of, fluid from one part of the body to another Shunt products have changed little since the 1950’s * Shunt Valve Ventricular Catheter Distal Catheter

Hydrocephalus Association. http://www.hydroassoc.org/complications-of-shunt-systems/ World Federation of Neurological Societies. http://www.wfns.org/pages/read_the_reviews/97.php?rid=5 CURRENT SHUNT FAILURE Approximately 50% of shunts in the pediatric population fail within two years of placement and repeated neurosurgical operations are often required1 Ventricular catheter blockage is by far the most frequent event2 *

VIROB FIRST PRODUCT Self Cleaning Shunt (SCS) A novel shunt technology for the continuous prevention of shunt blockage in hydrocephalus and NPH patients *
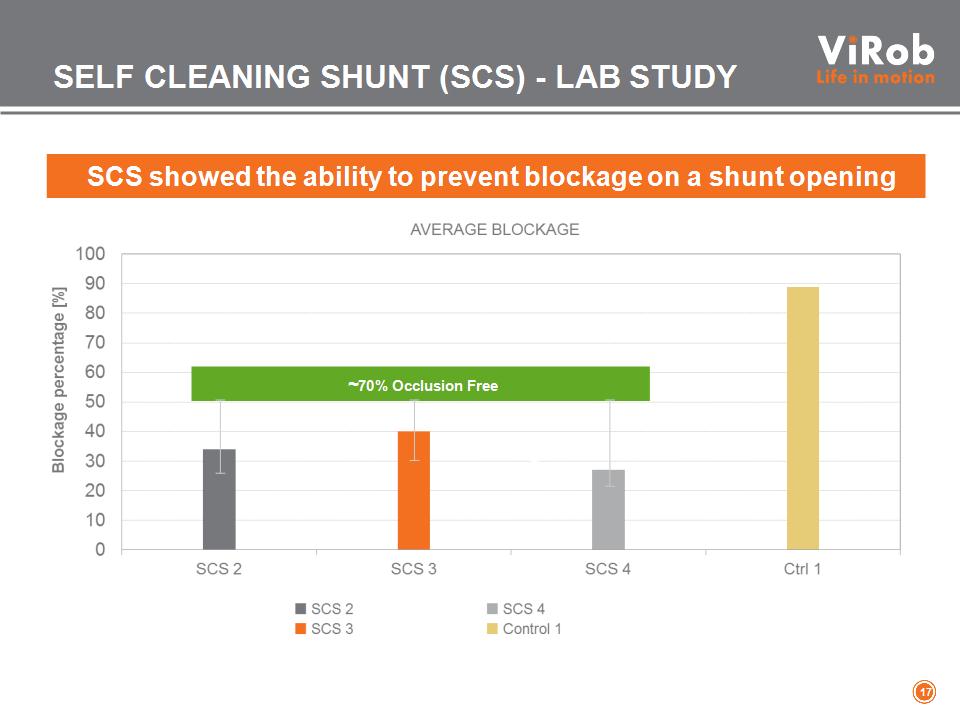
SCS showed the ability to prevent blockage on a shunt opening ~70% Occlusion Free SELF CLEANING SHUNT (SCS) – LAB STUDY *

SELF CLEANING SHUNT (SCS) – LAB STUDY “Microbot Medical SCS presents low hydrodynamic resistance. The SCS behaves as a standard ventricular catheter and does not change the hydrodynamic performance of adjustable hydrocephalus valves.” UK Shunt Testing Lab, Cambridge University, UK CONCLUSIONS: *

*

GI ENDOSCOPE MARKET The North American colonoscope and upper GI endoscope segments are valued at about $600 million.1 Annually, between 15 and 20 million endoscopy procedures are conducted with reusable endoscope devices to screen various components of a patient’s GI tract in the USA.2 North American Flexible Endoscopes Market Research Report. http://www.micromarketmonitor.com/market/ north-america-flexible-endoscopes-1829607717.html American Journal of Infection Control ,V(6)41, June 2013, Pages S24, 40th Annual Conference Abstracts, APIC 2013, Ft Lauderdale, FL APIC 40th Annual Conference *
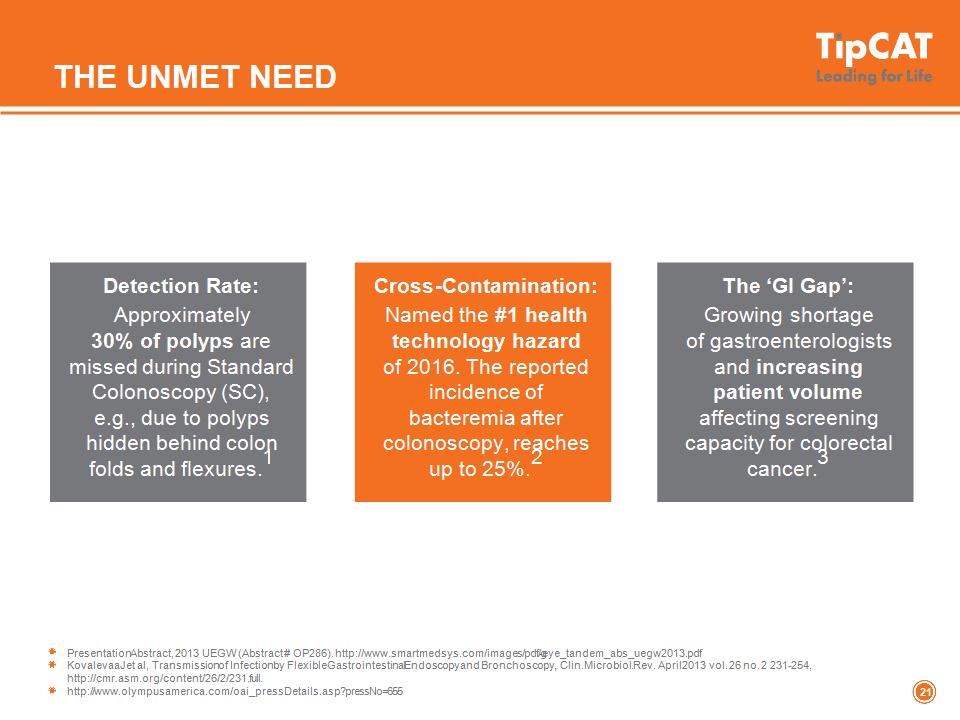
THE UNMET NEED * Detection Rate: Approximately 30% of polyps are missed during Standard Colonoscopy (SC), e.g., due to polyps hidden behind colon folds and flexures.1 Presentation Abstract, 2013 UEGW (Abstract # OP286). http://www.smartmedsys.com/images/pdf/g-eye_tandem_abs_uegw2013.pdf Kovalevaa Jet al, Transmission of Infection by Flexible Gastrointestinal Endoscopy and Bronchoscopy, , Clin. Microbiol. Rev. April 2013 vol. 26 no. 2 231-254, http://cmr.asm.org/content/26/2/231.full. http://www.olympusamerica.com/oai_pressDetails.asp?pressNo=655 Cross-Contamination: Named the #1 health technology hazard of 2016. The reported incidence of bacteremia after colonoscopy, reaches up to 25%.2 The ‘GI Gap’: Growing shortage of gastroenterologists and increasing patient volume affecting screening capacity for colorectal cancer.3

CROSS-CONTAMINATION IN THE NEWS *
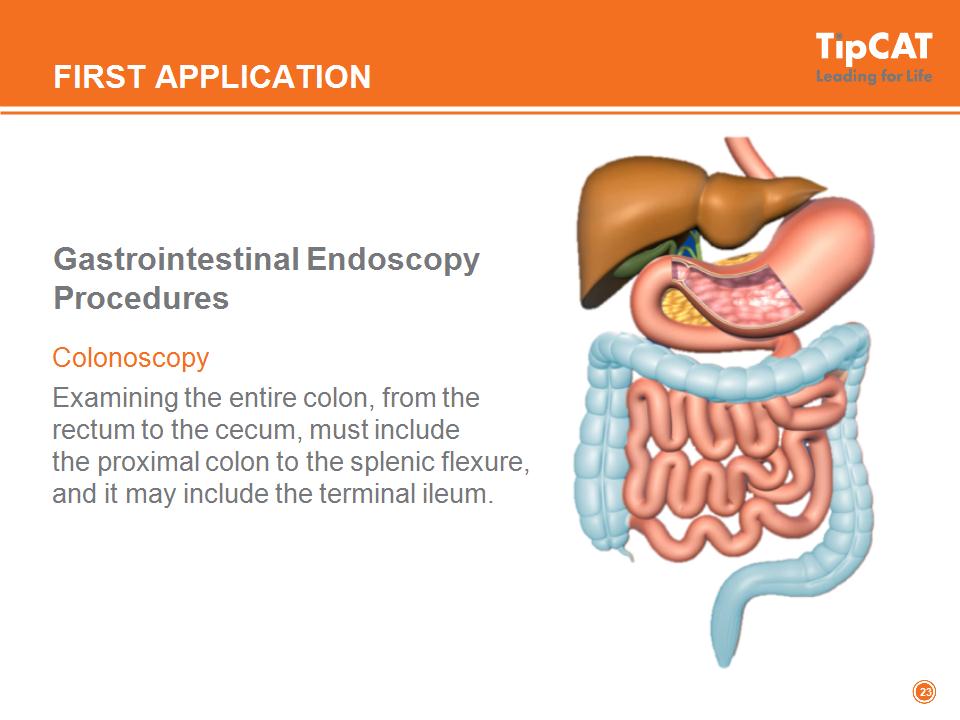
FIRST APPLICATION Gastrointestinal Endoscopy Procedures Colonoscopy Examining the entire colon, from the rectum to the cecum, must include the proximal colon to the splenic flexure, and it may include the terminal ileum. *
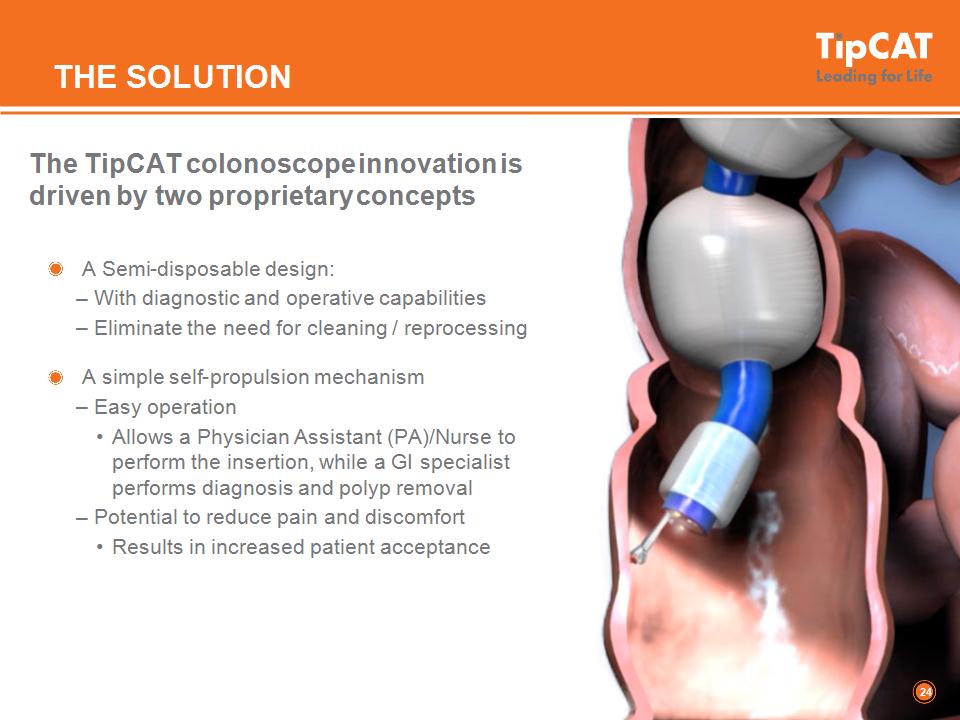
THE SOLUTION The TipCAT colonoscope innovation is driven by two proprietary concepts A Semi-disposable design: With diagnostic and operative capabilities Eliminate the need for cleaning / reprocessing A simple self-propulsion mechanism Easy operation Allows a Physician Assistant (PA)/Nurse to perform the insertion, while a GI specialist performs diagnosis and polyp removal Potential to reduce pain and discomfort Results in increased patient acceptance *
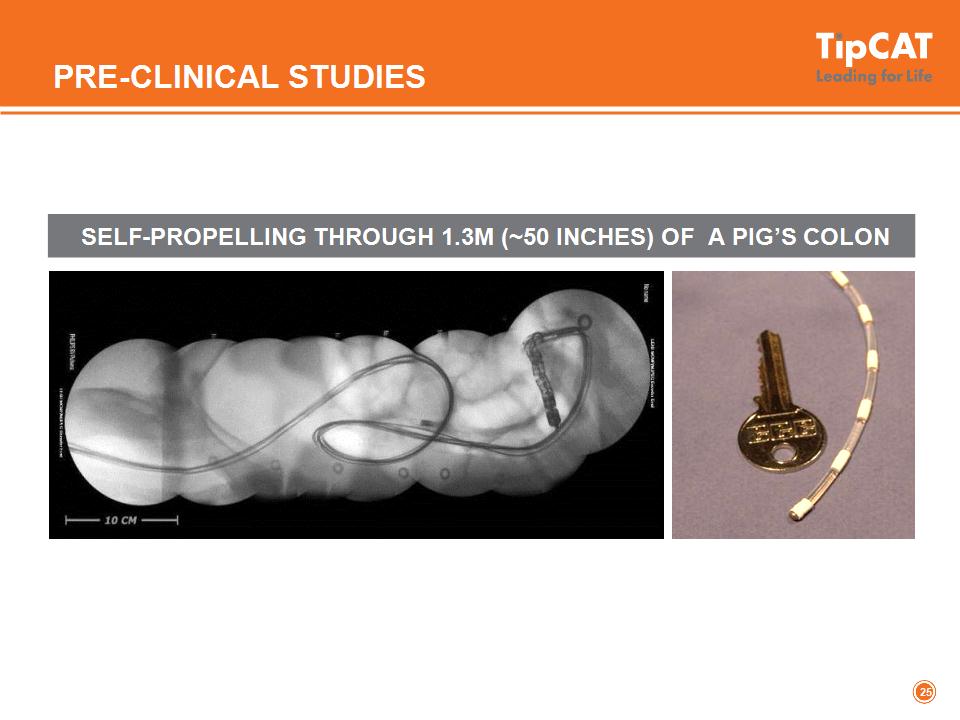
PRE-CLINICAL STUDIES SELF-PROPELLING THROUGH 1.3M (~50 INCHES) OF A PIG’S COLON

* ORGANIZATION & INFRASTRUCTURE TO EXECUTE PLAN
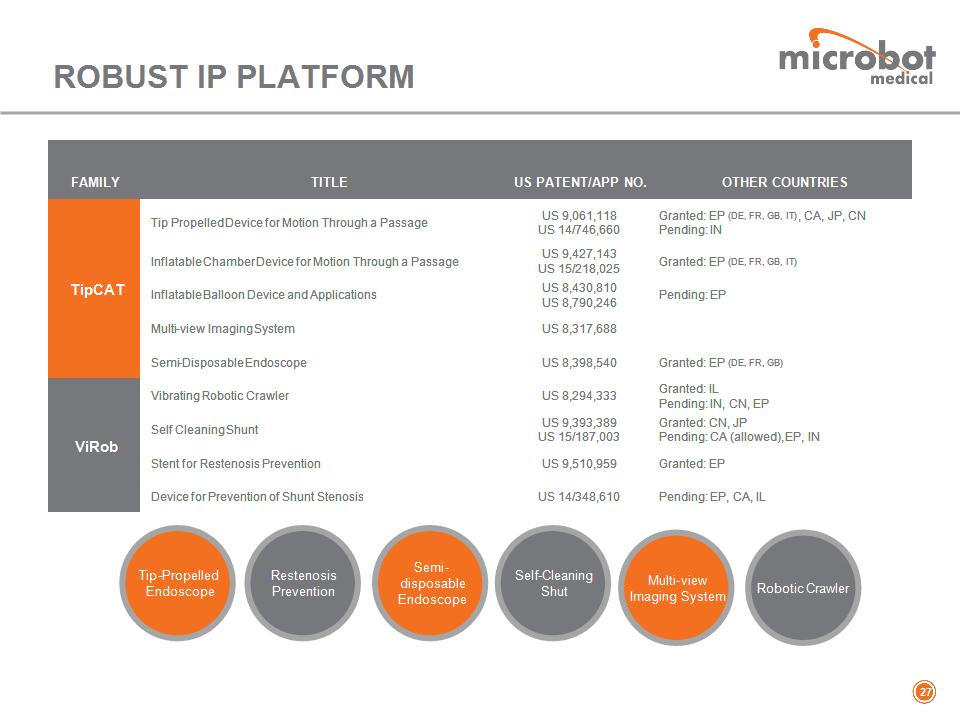
FAMILY TITLE US PATENT/APP NO. OTHER COUNTRIES TipCAT Tip Propelled Device for Motion Through a Passage US 9,061,118 US 14/746,660 Granted: EP (DE, FR, GB, IT), CA, JP, CN Pending: IN TipCAT Inflatable Chamber Device for Motion Through a Passage US 9,427,143 US 15/218,025 Granted: EP (DE, FR, GB, IT) TipCAT Inflatable Balloon Device and Applications US 8,430,810 US 8,790,246 Pending: EP TipCAT Multi-view Imaging System US 8,317,688 TipCAT Semi-Disposable Endoscope US 8,398,540 Granted: EP (DE, FR, GB) ViRob Vibrating Robotic Crawler US 8,294,333 Granted: IL Pending: IN, CN, EP ViRob Self Cleaning Shunt US 9,393,389 US 15/187,003 Granted: CN, JP Pending: CA (allowed), EP, IN ViRob Stent for Restenosis Prevention US 9,510,959 Granted: EP ViRob Device for Prevention of Shunt Stenosis US 14/348,610 Pending: EP, CA, IL ROBUST IP PLATFORM * Tip-Propelled Endoscope Restenosis Prevention Semi- disposable Endoscope Self-Cleaning Shut Multi-view Imaging System Robotic Crawler

Prof. Moshe Shoham Member of the Scientific Advisory Board & Co-Founder Prof. Shoham is a worldwide acclaimed authority in the field of robotics, conducting research in the robotic field for over the past 20 years, with a special focus on kinematics and dynamics of robots, sensor integration, multi-finger hands and medical applications. Founder of Mazor Surgical Technologies Ltd. (Nasdaq: MZOR) Foreign Member, US National Academy of Engineering Head of the robotics lab at Israel's Technion's Faculty of Mechanical Engineering. Formerly the director of the robotic laboratory of the Department of Mechanical Engineering, Columbia University, NY. Business Confidential PROVEN LEADERSHIP TEAM Harel Gadot CEO, President & Chairman Mr. Gadot was formerly a Worldwide Group Marketing Director at Ethicon Inc., a multi-billion dollar division of Johnson & Johnson company (NYSE: JNJ). Harel was with J&J for a decade between 2000-2010. Previously held leadership positions for Ethicon Inc. in Europe, Middle East and Africa. Served on the board of directors and led the business development for ConTIPI Ltd., an early stage medical device company, which was acquired by Kimberly Clark Corp (NYSE:KMB) in 2012. Hezi Himelfarb GM & COO Mr. Himelfarb has more than 30 years of management experience in hi-tech and medical device companies. Previously served as CEO of IceCure Medical, a TASE publicly traded company. Hezi was responsible for establishing a U.S subsidiary and leading the company's transition from clinical phase to commercial sales. Previously was Chief Executive Officer of Remon Medical Technologies Ltd., a developer of smart, miniature implants, which was acquired in 2008 by Boston Scientific Corporation. *

Business Confidential Ahava Stein Director, Regulatory Affairs Ms. Stein, is a regulatory affairs, clinical and QA consultant with over 18 years of experience working directly with the FDA. Regulatory experience includes all types of regulatory submissions for a wide variety of innovative medical devices. * Simon Sharon Chairman, Scientific Advisory Board Mr. Sharon brings 23 years of R&D and general management in the medical devices space. Prior to Microbot Medical Mr. Sharon managed the R&D at Icecure Medical, an early stage, public medical device company. Mr. Sharon was the General Manger of Anorad Israel, a subsidiary of Rockwell Automation which manufactures sub-micron precision motion systems. Holds a B.Sc. from the Technion Institute of Technology and an M.Sc in Mechanical engineering from MIT where he specialized in motion control and Robotics PROVEN LEADERSHIP TEAM Mr. Ben Naim is a CPA licensed in the State of Israel. Prior to joining Microbot Medical, Mr. Ben Naim operated DBN Financial. Previously served as CFO of Insuline Medical Ltd, a public company listed on the Tel-Aviv Stock Exchange (TASE:INSL). Prior to that Mr. Ben Naim served as CFO of Crow Technologies 1977 Ltd, a public company listed on the OTCQB (CRWTF), from 2008 – 2011. David Ben Naim CFO
INVESTMENT CONSIDERATIONS *

YOUR QUESTIONS: Michael Polyviou EVC Group mpolyviou@evcgroup.com
