Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE - Protara Therapeutics, Inc. | exh_991.htm |
| 8-K - FORM 8-K - Protara Therapeutics, Inc. | f8k_121316.htm |
EXHIBIT 99.2

©2016 Proteon Therapeutics, Inc. PATENCY - 1 Top - Line Results December 13, 2016

This presentation contains statements that are, or may be deemed to be, "forward - looking statements." In some cases these forwa rd - looking statements can be identified by the use of forward - looking terminology, including the terms “believes,” “estimates,” “anticipates,” "expects,” “plans,” "intends,” “ may,” “could,” “might,” “will,” “should,” “approximately,” “potential,” or, in each case, their negatives or other variations thereon or comparable terminology, although not all forwar d - l ooking statements contain these words. These statements, including the number of patients to be enrolled in and the timing of enrollment in the PATENCY - 2 Phase 3 clinical study of vonapanitase, when the Company expects to report top - line data from the PATENCY - 2, the potential treatment of renal and vascular diseases with vonapanitase, the effect of vonapanitase in patients with chronic kidney disease, whether vonapanitase improves fistula patency or use, whether vonapanitase may inhibit cell migration, the potential surgical and endovascular applications for vonapanitase, the sufficiency of the Company’s cash, cash - equivalents and available - for - sale investments to fund the Company’s operations into the third quarter of 2018, timing and likelihood of success, plans and objectives of management for future operations, and future results of anticipated produc t candidates, involve substantial known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achiev eme nts to differ materially from those expressed or implied by these forward - looking statements. These risks, uncertainties and other factors, including whether our cash resources will be sufficient to fund the our operating expenses and capital expenditure requirements for the period anticipated; whether data from early clinical trials will be indicative of th e d ata that will be obtained from future clinical trials; whether vonapanitase will advance through the clinical trial process on the anticipated timeline and warrant submission for regulatory approval; whethe r s uch a submission would receive approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies on a timely basis or at all; an d w hether we can successfully commercialize and market our product candidates, are described more fully in our Annual Report on Form 10 - K for the year ended December 31, 2015, as filed with the Securities and Exchange Commission (“SEC”) on March 14, 2016, and our subsequent Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K, as file d with the SEC, particularly in the sections titled “Risk Factors” and “Management's Discussion and Analysis of Financial Condition and Results of Operations.” In light of the significant uncertainties in our forward - looking statements, you should not place undue reliance on these statements or regard these statements as a representation or warrant y b y us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. The forward - looking statements contained in this presentation repre sent our estimates and assumptions only as of the date of this presentation and, except as required by law, we undertake no obligation to update or revise publicly any forward - lo oking statements, whether as a result of new information, future events or otherwise after the date of this presentation . This presentation also contains estimates, projections and other information concerning our industry, our business, and the m ark ets for our drug candidates, as well as data regarding market research, estimates and forecasts prepared by our management. Information that is based on estimates, foreca sts , projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and ci rcumstances reflected in this information. New risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and un certainties. Cautionary Note Regarding Forward - Looking Statements 2
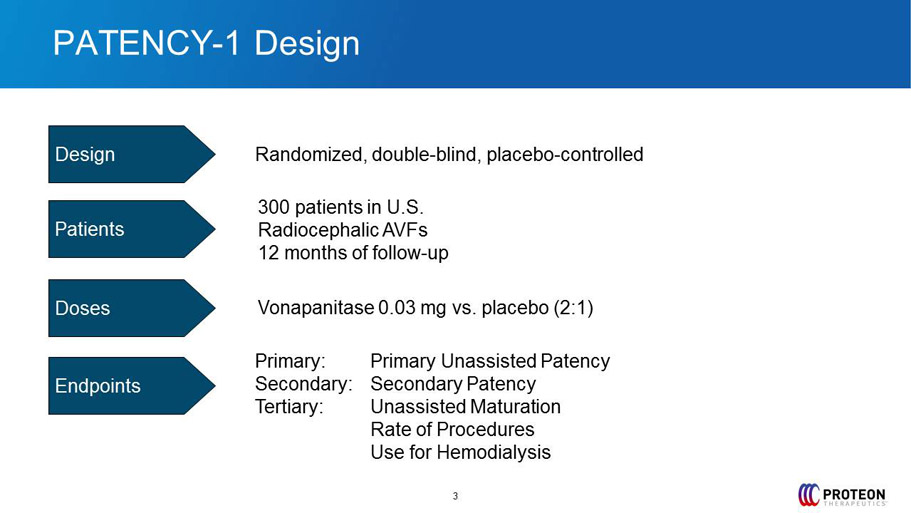
PATENCY - 1 Design Randomized, double - blind , placebo - controlled 300 patients in U.S. Radiocephalic AVFs 12 months of follow - up Vonapanitase 0.03 mg vs. placebo (2:1) Primary: Primary Unassisted Patency Secondary: Secondary Patency Tertiary: Unassisted Maturation Rate of Procedures Use for Hemodialysis Design Patients Doses Endpoints 3
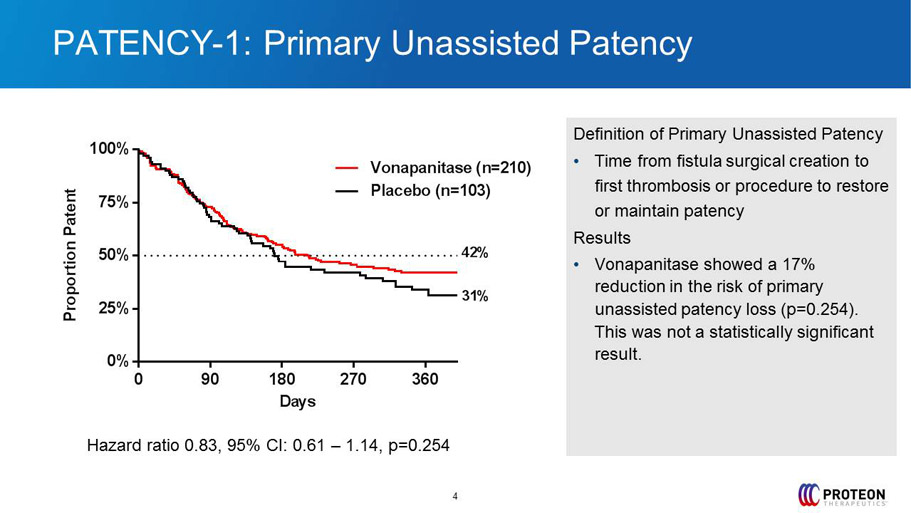
PATENCY - 1: Primary Unassisted Patency 4 Definition of Primary Unassisted Patency • Time from fistula surgical creation to first thrombosis or procedure to restore or maintain patency Results • Vonapanitase showed a 17% reduction in the risk of primary unassisted patency loss ( p=0.254). This was not a statistically significant result. 0 90 180 270 360 0% 25% 50% 75% 100% Days P r o p o r t i o n P a t e n t Placebo (n=103) Vonapanitase (n=210) 31% 42% Hazard ratio 0.83, 95% CI: 0.61 – 1.14, p=0.254

PATENCY - 1: Secondary Patency 5 Definition of Secondary Patency • Time from fistula surgical creation to abandonment of the fistula Results • Vonapanitase showed a 33% reduction in the risk of secondary patency loss ( p=0.048). 0 90 180 270 360 0% 25% 50% 75% 100% Days P r o p o r t i o n P a t e n t Placebo (n=103) Vonapanitase (n=210) 61% 74% Hazard ratio 0.66, 95% CI: 0.43 – 1.00, p=0.048
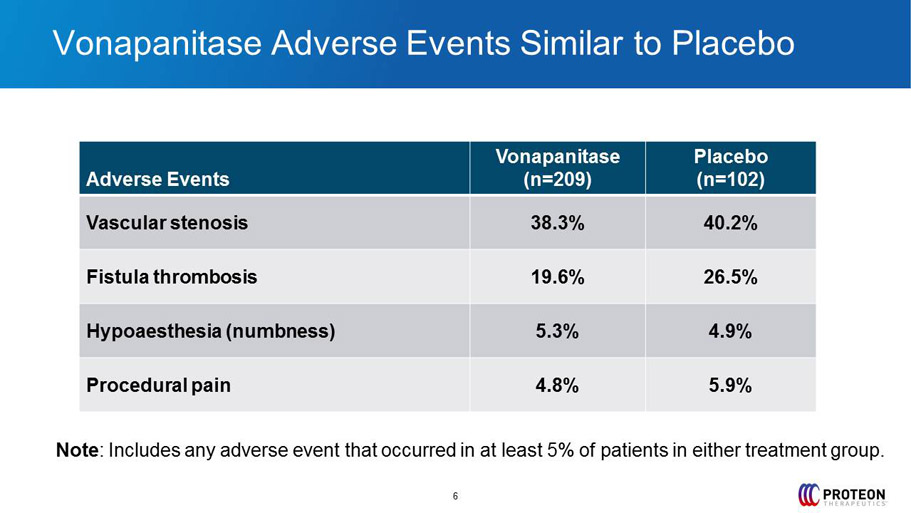
6 Vonapanitase Adverse Events Similar to Placebo Adverse Events Vonapanitase (n=209) Placebo (n=102) Vascular stenosis 38.3% 40.2% Fistula thrombosis 19.6% 26.5% Hypoaesthesia (numbness ) 5.3% 4.9% Procedural pain 4.8% 5.9% Note : Includes any adverse event that occurred in at least 5% of patients in either treatment group.

©2016 Proteon Therapeutics, Inc. PATENCY - 1 Top - Line Results December 13, 2016
