Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ATOSSA THERAPEUTICS, INC. | v454654_8k.htm |
Exhibit 99.1
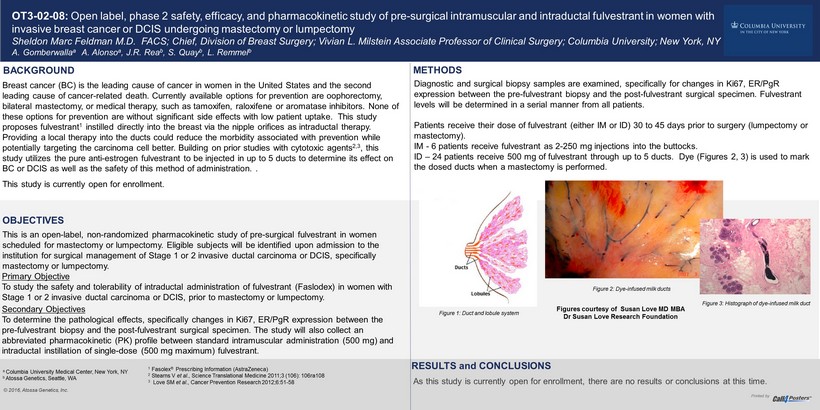
OT3 - 02 - 08: Open label, phase 2 safety, efficacy, and pharmacokinetic study of pre - surgical intramuscular and intraductal fulvestrant in wom en with invasive breast cancer or DCIS undergoing mastectomy or lumpectomy Sheldon Marc Feldman M.D. FACS; Chief, Division of Breast Surgery; Vivian L. Milstein Associate Professor of Clinical Surger y; Columbia University; New York, NY A. Gomberwalla a A. Alonso a , J.R. Rea b , S. Quay b , L. Remmel b , METHODS BACKGROUND Breast cancer (BC) is the leading cause of cancer in women in the United States and the second leading cause of cancer - related death. Currently available options for prevention are oophorectomy, bilateral mastectomy, or medical therapy, such as tamoxifen, raloxifene or aromatase inhibitors. None of these options for prevention are without significant side effects with low patient uptake. This study proposes fulvestrant 1 instilled directly into the breast via the nipple orifices as intraductal therapy. Providing a local therapy into the ducts could reduce the morbidity associated with prevention while potentially targeting the carcinoma cell better. Building on prior studies with cytotoxic agents 2,3 , this study utilizes the pure anti - estrogen fulvestrant to be injected in up to 5 ducts to determine its effect on BC or DCIS as well as the safety of this method of administration. . This study is currently open for enrollment. © 2016, Atossa Genetics, Inc. Primary Objective To study the safety and tolerability of intraductal administration of fulvestrant (Faslodex) in women with Stage 1 or 2 invasive ductal carcinoma or DCIS, prior to mastectomy or lumpectomy. Secondary Objectives To determine the pathological effects, specifically changes in Ki67, ER/PgR expression between the pre - fulvestrant biopsy and the post - fulvestrant surgical specimen. The study will also collect an abbreviated pharmacokinetic (PK) profile between standard intramuscular administration (500 mg) and intraductal instillation of single - dose (500 mg maximum) fulvestrant. OBJECTIVES RESULTS and CONCLUSIONS As this study is currently open for enrollment, there are no results or conclusions at this time. Printed by This is an open - label, non - randomized pharmacokinetic study of pre - surgical fulvestrant in women scheduled for mastectomy or lumpectomy. Eligible subjects will be identified upon admission to the institution for surgical management of Stage 1 or 2 invasive ductal carcinoma or DCIS, specifically mastectomy or lumpectomy. Diagnostic and surgical biopsy samples are examined, specifically for changes in Ki67, ER/PgR expression between the pre - fulvestrant biopsy and the post - fulvestrant surgical specimen. Fulvestrant levels will be determined in a serial manner from all patients. Patients receive their dose of fulvestrant (either IM or ID) 30 to 45 days prior to surgery (lumpectomy or mastectomy). IM - 6 patients receive fulvestrant as 2 - 250 mg injections into the buttocks. ID – 24 patients receive 500 mg of fulvestrant through up to 5 ducts. Dye (Figures 2, 3) is used to mark the dosed ducts when a mastectomy is performed. Figure 1: Duct and lobule system Figure 2: Dye - infused milk ducts Figure 3: Histograph of dye - infused milk duct Figures courtesy of Susan Love MD MBA Dr Susan Love Research Foundation a Columbia University Medical Center, New York, NY b Atossa Genetics, Seattle, WA 1 Fasolex ® Prescribing Information (AstraZeneca) 2 Stearns V et al ., Science Translational Medicine 2011;3 (106): 106ra108 3 Love SM et al ., Cancer Prevention Research 2012;6:51 - 58
