Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ra Pharmaceuticals, Inc. | a16-22521_18k.htm |
Exhibit 99.1
International PNH Interest Group Annual Meeting American Society of Hematology Scientific Sessions Manchester Grand Hyatt, San Diego CA Friday 2nd December 2016

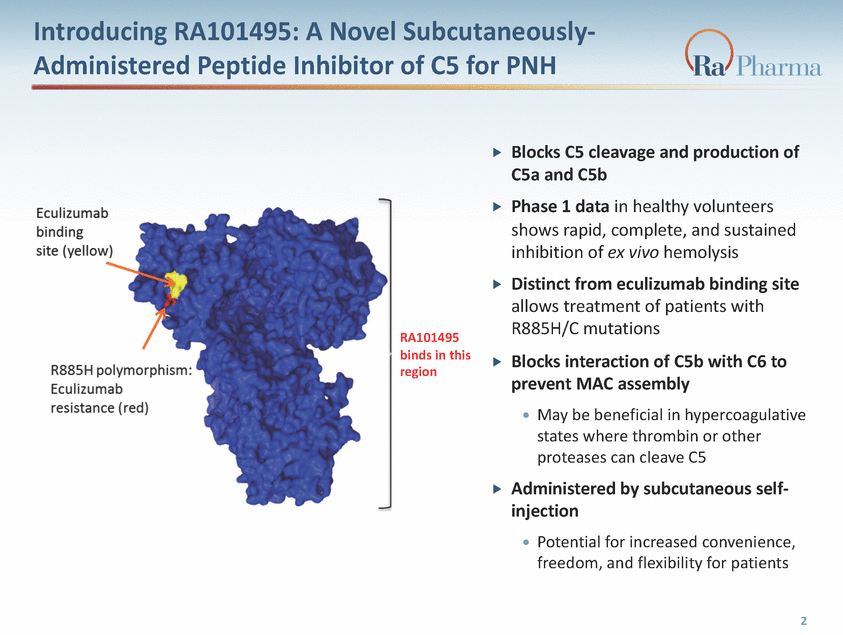
Introducing RA101495: A Novel Subcutaneously-Administered Peptide Inhibitor of C5 for PNH Blocks C5 cleavage and production of C5a and C5b Phase 1 data in healthy volunteers shows rapid, complete, and sustained inhibition of ex vivo hemolysis Distinct from eculizumab binding site allows treatment of patients with R885H/C mutations Blocks interaction of C5b with C6 to prevent MAC assembly May be beneficial in hypercoagulative states where thrombin or other proteases can cleave C5 Administered by subcutaneous self-injection Potential for increased convenience, freedom, and flexibility for patients RA101495 binds in this region Eculizumab binding (yellow) R885H polymorphism: Eculizumab resistance (red) 2
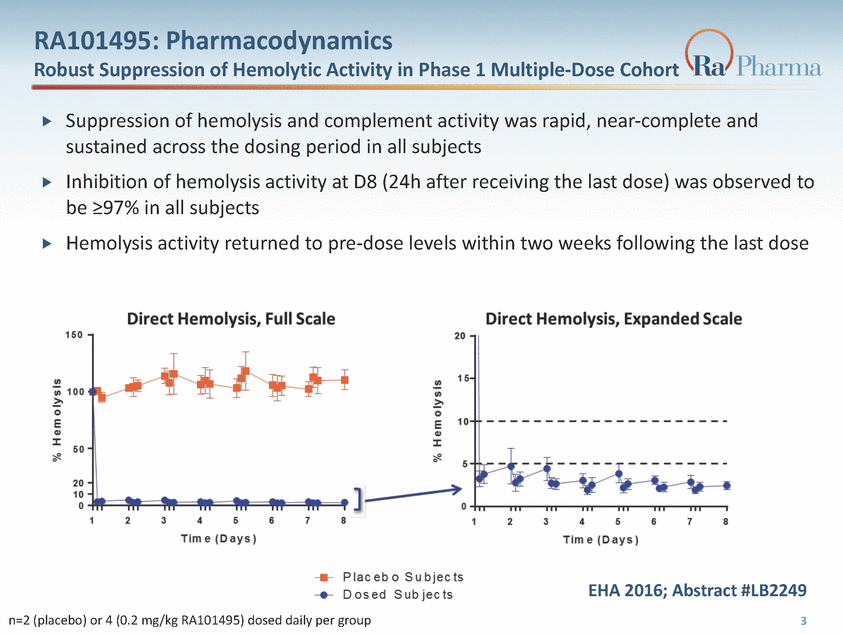
RA101495: Pharmacodynamics Robust Suppression of Hemolytic Activity in Phase 1 Multiple-Dose Cohort Suppression of hemolysis and complement activity was rapid, near-complete and sustained across the dosing period in all subjects Inhibition of hemolysis activity at D8 (24h after receiving the last dose) was observed to be >97% in all subjects Hemolysis activity returned to pre-dose levels within two weeks following the last dose EHA 2016; Abstract #LB2249 3 n=2 (placebo) or 4 (0.2 mg/kg RA101495) dosed daily per group
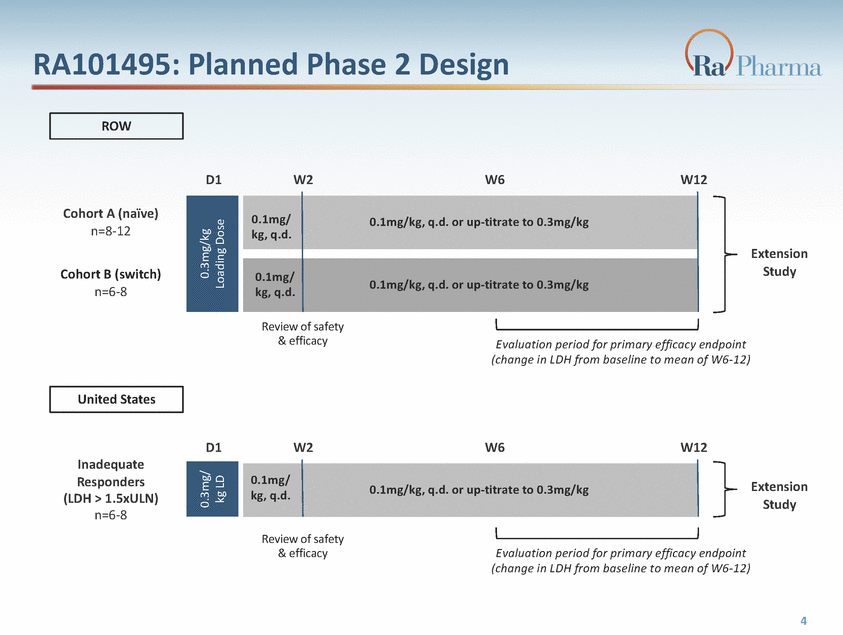
RA101495: Planned Phase 2 Design ROW D1 W2 W6 W12 Cohort A (naïve) n=8-12 Extension Study Cohort B (switch) n=6-8 Review of safety & efficacy Evaluation period for primary efficacy endpoint (change in LDH from baseline to mean of W6-12) United States D1 W2 W6 W12 Inadequate Responders (LDH > 1.5xULN) n=6-8 Extension Study kg, q.d. Review of safety & efficacy Evaluation period for primary efficacy endpoint (change in LDH from baseline to mean of W6-12) 4 0.3mg/ kg LD 0.3mg/kg Loading Dose 0.1mg/0.1mg/kg, q.d. or up-titrate to 0.3mg/kg 0.1mg/ kg, q.d.0.1mg/kg, q.d. or up-titrate to 0.3mg/kg 0.1mg/0.1mg/kg, q.d. or up-titrate to 0.3mg/kg kg, q.d.
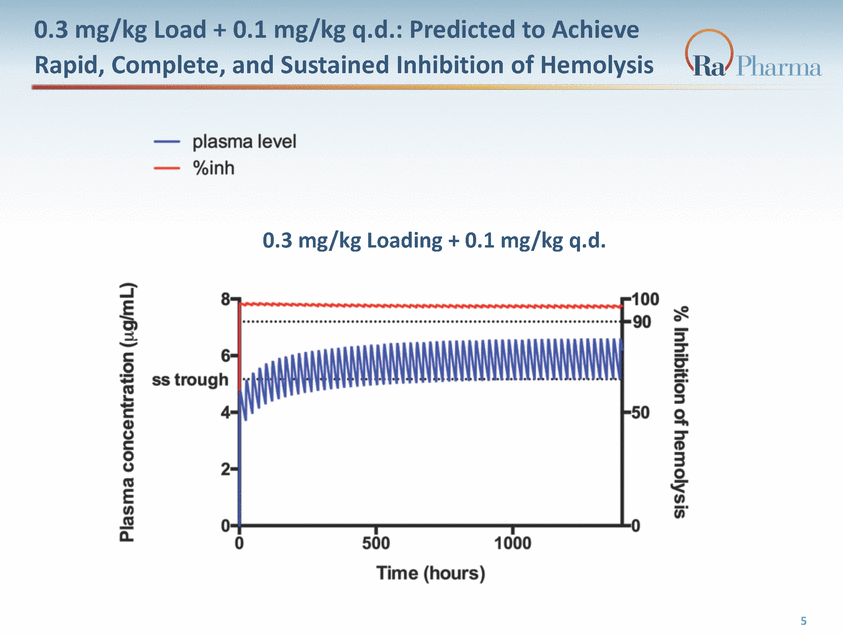
0.3 mg/kg Load + 0.1 mg/kg q.d.: Predicted to Achieve Rapid, Complete, and Sustained Inhibition of Hemolysis 0.3 mg/kg Loading + 0.1 mg/kg q.d. 5
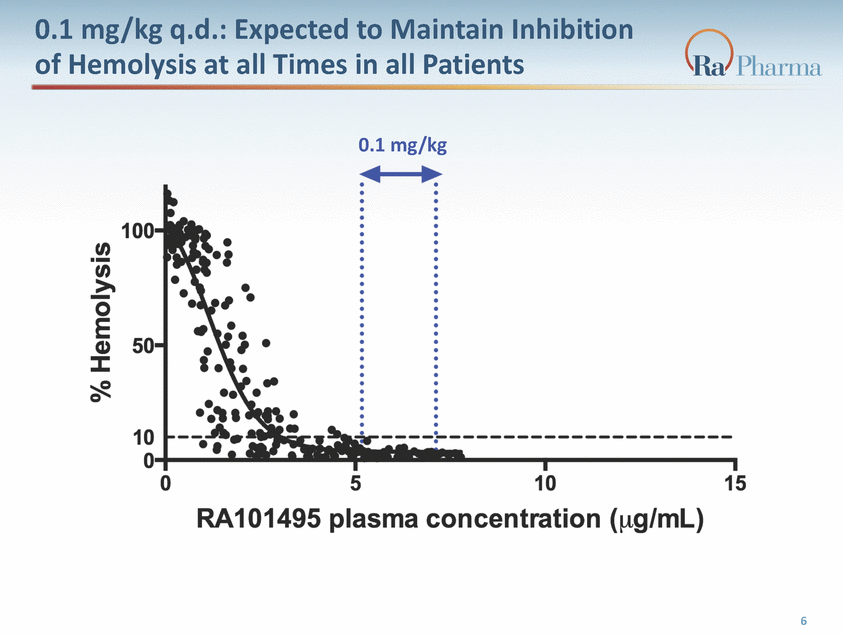
0.1 mg/kg q.d.: Expected to Maintain Inhibition of Hemolysis at all Times in all Patients 0.1 mg/kg 6
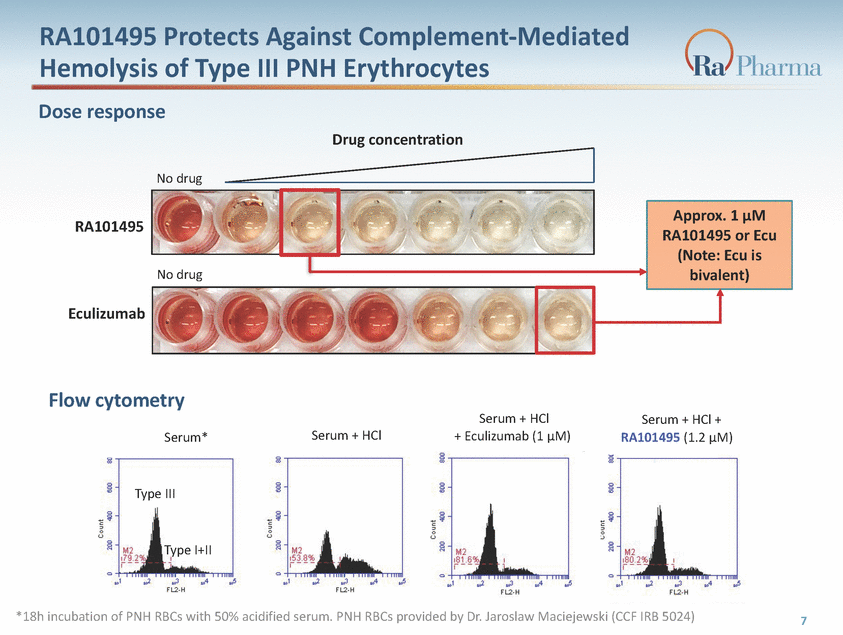
RA101495 Protects Against Complement-Mediated Hemolysis of Type III PNH Erythrocytes Dose response Drug concentration No drug Approx. 1 μM RA101495 RA101495 or Ecu (Note: bivalent) No drug Eculizumab Flow cytometry Serum + HCl Serum + HCl + Serum + HCl + Eculizumab (1 μM) Serum* RA101495 (1.2 μM) Type III Type I+II *18h incubation of PNH RBCs with 50% acidified serum. PNH RBCs provided by Dr. Jaroslaw Maciejewski (CCF IRB 5024) 7 Ecu is
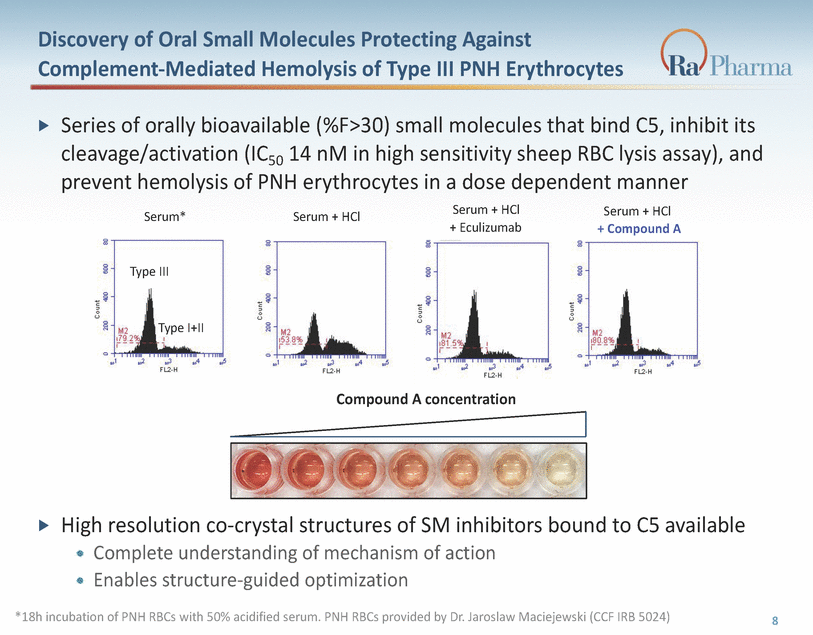
Discovery of Oral Small Molecules Protecting Against Complement-Mediated Hemolysis of Type III PNH Erythrocytes Series of orally bioavailable (%F>30) small molecules that bind C5, inhibit its cleavage/activation (IC50 14 nM in high sensitivity sheep RBC lysis assay), and prevent hemolysis of PNH erythrocytes in a dose dependent manner Serum + HCl + Eculizumab Serum + HCl + Compound A Serum* Serum + HCl Type III Type I+II Compound A concentration High resolution co-crystal structures of SM inhibitors bound to C5 available Complete understanding of mechanism of action Enables structure-guided optimization *18h incubation of PNH RBCs with 50% acidified serum. PNH RBCs provided by Dr. Jaroslaw Maciejewski (CCF IRB 5024) „ 8