Attached files
Exhibit
10.6
LICENSE
AGREEMENT
THIS
LICENSE AGREEMENT is made as of this 1st Day of August 2013
(“Execution Date”) by and between the GREEN MOLECULAR
S.L., a Spanish corporation with a principal address at Parc
Cientific Universidad de Valencia, Polígono La Coma s/n, 46980
Paterna, Valencia, Spain (“GM”) and Chromadex, Inc. , a
corporation organized and existing under the laws of California
with a principal address 10005 Muirlands Bvld Suite G, Irvine ,
California 92618 (“CHROMADEX”)
RECITALS
WHEREAS, GM has developed inventions and
desire to commercialize such inventions related to
Pterostilbene.
WHEREAS, CHROMADEX
wishes to acquire certain rights and licenses with respect to the
Patent Rights in accordance with the terms and conditions
hereinafter set forth.
NOW,
THEREFORE, in consideration of the premises and mutual covenants
contained herein, and intending to be legally bound herby, the
parties hereto agree as follows:
ARTICLE
1
DEFINITIONS
1.1
Unless otherwise
provided in this Agreement, the following terms when used with
initial capital letters shall have the meanings set forth
below:
"Affiliate" means, when used
with reference to CHROMADEX, any Person directly or indirectly
controlling, controlled by or under common control with
CHROMADEX.
"Bankruptcy Event" means the
person in question becomes insolvent, or voluntary or involuntary
proceedings by or against such person are instituted in bankruptcy
or under any insolvency law, or a receiver or custodian is
appointed for such person, or proceedings are instituted by or
against such person for corporate dissolution of such person, which
proceedings, if involuntary, shall not have been dismissed within
sixty (60) days after the date of filing, or such person makes an
assignment for the benefit of creditors, or substantially all of
the assets of such person are seized or attached and not released
within sixty (60) days thereafter.
"Calendar Quarter" means each
three-month period, or any portion thereof, beginning on January 1,
April 1, July 1 and October 1.
"Confidential Information" means
all technical information, developments, discoveries, methods,
techniques, formulae, processes and other information relating to
Pterostilbene that GM or CHROMADEX owns or controls on the date
hereof or owns or controls during the term of this Agreement,
including by way of illustration and not limitation, designs, data,
drawings, documents, models, business practices, financial data and
other similar information.
"Effective Date" shall mean the
date that is the earlier of (i) the date of completion of the
formulation feasibility study or (ii) six (6) months from the
Execution Date of this Agreement.
-1-
"Field" means the use of
Pterostilbene and Pterostiblene combinations as listed in the
PATENTS for topical over the counter products, Topical cosmetics,
physician dispensed topical products and topical Rx treatments with
structure and or function claims related to the Valid Claims as
defined below.
“Expanded
Field” means the use of Pterostilbene and Pterostiblene
combinations as listed in the PATENTS for topical and oral over the
counter products, Topical cosmetics, physician dispensed topical or
oral products and topical or oral Rx treatments with structure and
or function claims related to the Valid Claims as defined
below.
"Net Sales Price" means the
gross amount charged by CHROMADEX for a Licensed Product less any
(a) trade, quantity and cash discounts on Licensed Products
actually provided to third parties in connection with arms length
transactions, (b) credits, allowances or refunds, not to exceed the
original invoice amount, for actual claims, damaged goods,
rejections or returns of Licensed Products, and (c) excise, sale,
use, value added or other taxes, other than income taxes, paid by
Licensee due to the Sale of Licensed Products. If a Licensed
Product is sold for consideration other than solely cash, the fair
market value of such other consideration shall be included in the
Net Sales Price. If a Licensed Product is sold in a package, kit,
or blended with other products or services which is not a Licensed
Product, the Net Sales Price for purposes of calculating the
royalty under Article 3 hereof shall be calculated by multiplying
the Net Sales Price of the combination product or service by the
fraction of A/A+B, where "A" is the Net Sales Price of the Licensed
Product or Service when sold separately and "B" is the Net Sales
Price of the other product or service or products or services when
sold.
"Patent(s)" means the any
patents or applications which claim the invention(s) summarized in
Appendix A which relate to the compound known as Pterostilbene,
including without limitation any United States Letters Patent, and
all continuations, continuations-in-part, additions, divisions,
renewals, extensions, reexaminations and reissues of any of the
foregoing, all foreign counterparts of any of the foregoing, and
any other patents which relate to the Pterostilbene and
Pterostilbene combinations in the Field of the present agreement
owned or controlled by GM during the term of this
Agreement.
“Patent Right(s)” means all legal rights belonging to
GM which are provided by the Patents.
“Patent
Expenses” means all out-of-pocket fees, expenses, and
charges related to the Patent Rights incurred by GM in connection
with the preparation, filing, prosecution, issuance, re-issuance,
re-examination, interference, and/or maintenance of applications
for patent or equivalent protection for the Patent
Rights.
"Person"
means an individual, partnership, corporation, joint venture,
unincorporated association, or other entity, or a government or
department of agency thereof.
"Licensed Products" means any
article or portion thereof which is made, produced, sold or used in
whole or in part, by or with the use of the licensed Patent Rights.
Licensed Products include Pterostilbene sold to 3rd parties for use in
dietary supplement products and used in dietary supplement products
sold directly by CHROMADEX. Licensed Products does not include
Pterostilbene sold by CHROMADEX as an analytical reference
standard.
“Sunk Patent
Expenses” means Patent Expenses incurred by GM prior
to the Effective Date of the Agreement.
"Valid Claim" means a claim of
an unexpired issued Patent that has not been withdrawn, canceled or
disclaimed or held invalid by a court or governmental authority of
competent jurisdiction in an unappealed or unappealable
decision.
"Sabinsa Application" means U.S.
Patent Application No. 12/408,808 and any divisional, continuation,
continuation-in-part, reissue, or foreign application(s) related to
same.
-2-
ARTICLE 2
GRANT OF LICENSE
2.1
Grant of License. Subject to
the terms and conditions contained in this Agreement, GM hereby
grants to CHROMADEX an exclusive, non-transferrable (except
otherwise allowed in this Agreement), worldwide, royalty-bearing
right and license to use and practice the Patent Rights to make,
have made, use, and sell Licensed Products in the
Field.
2.2.1
Right to
Sub-license.. Subject
to the further provisions of this Section 2.2.2, Licensee may grant
sublicenses of the licenses granted to Licensee in Section 2.1
above to third parties by entering into a written agreement with
any such third party (each such agreement shall be referred to
herein as a “Sublicense” and each such third party
shall be referred to herein as a “Sublicensee”). Only
Licensee (and not any Sublicensee) may enter into a Sublicense, and
each Sublicense shall expressly prohibit the Sublicensee from
granting further sublicenses.
2.2.2
Requirements of each Sublicense
Agreement. Licensee agrees that it will require all
Sublicensees to comply with the terms and conditions set forth in
this Agreement and applicable to Licensee. In furtherance of the
foregoing but without limiting the generality thereof, each
Sublicense shall, for the express benefit of GM, bind the
Sublicensee to terms and conditions no less favorable to GM than
those between GM and Licensee contained in this Agreement. To the
extent that any term, condition, or limitation of any Sublicense is
inconsistent with the terms, conditions and limitations contained
in this Agreement, such term, condition, and/or limitation shall be
null and void against GM. . Within thirty (30) days after the
effective date of any Sublicense, Licensee shall provide GM a
complete copy of the Sublicense including, without limitation, any
and all exhibits and/or attachments thereto. If the Sublicense is
written in a language other than English, the copy of the
Sublicense shall be accompanied by a complete translation written
in English. Upon delivery of such translation to GM, Licensee shall
be deemed to represent and warrant to GM that such translation is a
true and accurate translation of the Sublicense.
2.2.3
Sublicensing Royalty Rate. Licensee shall
pay GM two and one half percent (2.5%) for all over the counter
sublicensing revenues for those received beyond the supply of the
product and five percent (5.0%) for all RX sublicensing revenues
received beyond the supply of the product.
2.2.4
Right of First Refusal:
(a)
'308 Application Right of First
Refusal: Application: GM shall notify Licensee of any
decision by a patent office to allow claims to United States Patent
Application No. 11/631,912 or to any continuation, divisional,
reissue or foreign counterpart application to same. Licensee shall
have sixty (60) days from receipt of each notice to elect to expand
the Field to include non-topical cosmetics. Licensee shall notify
GM of any such election. An expansion of the Field pursuant to this
section shall only apply to the nation of the particular patent
office which is the subject of the notification of allowed claims.
For the avoidance of doubt, all patent aplications described in
this paragraph shall be considered to be and treated as
Patents.
-3-
(b)
Right of First Refusal
Payments: Upon the first election by Licensee to expand the
Field pursuant to paragraph (a) of this section in the United
States, a one-time payment of $ ___20,000______ shall be owed
to GM. Upon the first election by Licensee to expand the Field
pursuant to paragraph (a) of this section in any nation other than
the United States, a one-time payment of $ 20,000____ shall be owed
to GM. Any payment owed by Licensee pursuant to this paragraph for
expansion of the Field in the United States shall become due and
payable upon sales of _$100,000___ (QUANTITY/DOLLARS) of Licensed
Products intended for use in the United States. Any payment owed by
Licensee pursuant to this paragraph for expansion of the Field in
any nation other than the United States shall become due and
payable upon sales of $100,000______(QUANTITY/DOLLARS) of Licensed
Products intended for use outside of the United States. For the
avoidance of doubt, it is understood that subsequent elections by
Licensee to expand the Field of Use do not incur additional
payments pursuant to this section.
2.3
No Rights by Implication. No
rights or licenses with respect to the Patent Rights are granted or
deemed granted hereunder or in connection herewith, other than
those rights or licenses expressly granted in this
Agreement.
ARTICLE 3
LICENSING FEES AND EQUITY
3.1
Upfront and Milestone Payments.
In consideration of the license granted hereunder, CHROMADEX shall
pay GM the following non-refundable payments:
(a)
Pterostilbene-Quercetin Combination
Cosmetic Claims Milestone : A one-time Payment of
$____10,000_____ will be due upon the allowance and issuance of the
first United States patent included in the Patent Rights whose
scope, provided by either composition or method claims, includes
exclusive rights to the use of a formulation comprised of
pterostilbene and quercetin (optionally with additional active
ingredients) for either the prevention or treatment of cosmetic
skin damage, prevention or treatment of wrinkles, or for the
prevention of the effects of radiation from the sun (i.e., as a
sunscreen).
(b)
Pterostilbene Cosmetic Claims
Milestone: A one-time Payment of $__25,000______ will be due
upon the allowance and issuance of the first United States patent
included in the Patent Rights whose scope, provided by either
composition or method claims, includes exclusive rights to the use
of a formulation comprised of pterostilbene with or without
additional active ingredients for either the prevention or
treatment of cosmetic skin damage, prevention or treatment of
wrinkles, or for the prevention of the effects of radiation from
the sun (i.e., as a sunscreen).
(c)
Pterostilbene-Quercetin Combination
Therapeutic Claims Milestone: A Payment of $__15,000_______
will be due upon the allowance and issuance of the first United
States patent included in the Patent Rights whose scope, provided
by either composition or method claims, includes exclusive rights
to the use of a formulation comprised of pterostilbene and
quercetin (optionally with additional active ingredients) for the
treatment of skin diseases.
(d)
Pterostilbene Therapeutic Claims
Milestone: A one-time Payment of $_____25,000_____ will be
due upon the allowance and issuance of the first United States
patent included in the Patent Rights whose scope, provided by
either composition or method claims, includes exclusive rights to
the use of a formulation comprised of pterostilbene with or without
additional active ingredients for the treatment of skin
diseases.
-4-
(e)
$15,000
within thirty (30) day of the Execution Date, and a second Payment
of $15,000 will be due upon completion of formulation feasibility
studies or 18 months from the Execution date whichever is
soon.
3.2
Royalties. In further
consideration of the rights and licenses granted hereunder, during
the Term of the Agreement CHROMADEX shall pay GM the following
non-refundable royalty payments:
(a)
For the
period in which no United States patent has issued in connection to
the Sabinsa Application, CHROMADEX shall pay GM TWO percent (2.00%)
of Net Sales of all Licensed Products sold to third parties for
ingredients intended for retail products covered in the field and
intended for treatment of conditions listed in VALID CLAIMS in the
nation(s) where sales of the retail products occur and two and one
percent (1.0%) of all Net sales of CHROMADEX retail products
containing pterostilbene ,covered in the field and intended for
treatment of conditions listed in VALID CLAIMS in the nation(s)
where sales of the retail products occur . CHROMADEX agrees to pay
GM at least the following minimum royalties during the term of this
Agreement and the time in which no United States patent has issued
in connection to the Sabinsa Application:
Calender Year 1:
$25,000
Year 2:
$35,000
Year 3
and beyond. $45,000 per year
(b) For the period
in which one or more United States patents has issued in connection
the Sabinsa Application, CHROMADEX shall pay GM _4.0___% of Net
Sales of all Licensed Products sold to third parties for
ingredients intended for retail products covered in the field and
intended for treatment of conditions listed in VALID CLAIMS where
sales of the retail products occur in the United State,
______2.5____% of all Net Sales of CHROMADEX retail products
containing pterostilbene ,covered in the field and intended for
treatment of conditions listed in VALID CLAIMS in the United
States, four percent (4.00%) of Net Sales of all Licensed Products
sold to third parties for ingredients intended for retail products
covered in the field and intended for treatment of conditions
listed in VALID CLAIMS in the nation(s) where sales of the retail
products occur other than in the United States and two and one half
percent (2.5%) of all Net sales of CHROMADEX retail products
containing pterostilbene ,covered in the field and intended for
treatment of conditions listed in VALID CLAIMS in the nation(s)
where sales of the retail products occur other than in the United
States. CHROMADEX agrees to pay GM at least the following minimum
royalties (in addition to those included in 3.2.a) during the term
of this Agreement and the time in which one or more United States
patents has issued in connection to the Sabinsa
Application:
Year 1:
10,000
Year 2:
$20,000
Year 3
and beyond: $30,000
In the
event an United States patent issues in connection to the Sabinsa
Application, the minimum royalty for the year in which the first
such patent issues shall be adjusted on a pro-rata
basis.
-5-
3.3
Payments. Royalties and other
amounts payable under this Agreement shall be paid within thirty
(30) days following the last day of the Calendar Quarter in which
royalties and other amounts accrue. The last such payment shall be
made within thirty (30) days after termination of this Agreement.
Payments shall be deemed paid as of the day on which they are
received by GM.
3.4
Reports. CHROMADEX shall
deliver to GM within thirty (30) days after the end of each
Calendar Quarter following commercial sale of a Licensed Product a
report setting forth in reasonable detail the calculation of the
royalties and other amounts payable to GM for such Calendar Quarter
pursuant to this Article 4, including, without limitation, the
Licensed Products sold in each country during such Calendar
Quarter, and the Net Sales Price.
3.5
Currency, Place of Payment,
Interest.
(a)
All dollar amounts
referred to in this Agreement are expressed in United States
dollars. All payments to GM under this Agreement shall be made in
United States dollars (or other legal currency of the United
States), as directed by GM, by check payable or by wire transfer to
an account as GM may designate from time to time.
(b)
If CHROMADEX
receives revenues from sales of Licensed Products in a currency
other than United States dollars, royalties shall be converted into
United States dollars at the applicable conversion rate for the
foreign currency as published in the “Exchange Rates”
table in the eastern edition of The Wall Street Journal as of the last
date of the applicable Calendar Quarter.
(c)
Amounts that are
not paid when due shall accrue interest from the due date until
paid, at an annual rate equal to the “Prime Rate” plus
5% as published in the “Money Rates” table in the
eastern edition of The Wall Street
Journal as of the due date.
3.6
Records. CHROMADEX will
maintain complete and accurate books and records that enable the
royalties payable hereunder to be verified. The records for each
Calendar Quarter shall be maintained for two years after the
submission of each report under Article 3.5 hereof. Upon reasonable
prior notice to CHROMADEX, GM and its accountants shall have access
to the books and records of CHROMADEX to conduct a review or audit
thereof. Such access shall be available during normal business
hours. Upon reasonable prior notice to CHROMADEX, GM and its
accountants shall have access to the books and records of CHROMADEX
to conduct a review or audit thereof no more than two (2) times per
year. Such access shall be available during normal business hours.
In the event such audit reveals any error in the computation of Net
Sales which results in an underpayment of royalties in excess of 5%
of the amount owed during the applicable period, then CHROMADEX
shall promptly reimburse GM for all reasonable expenses and costs
incurred in the conduct of such review or audit.
3.7.
CHROMADEX will
reimburse GM for future Patent Expenses incurred during the term of
this Agreement within thirty (30) days of receipt of an invoice
from GM.
ARTICLE 4
CERTAIN OBLIGATIONS OF CHROMADEX
4.1
CHROMADEX Efforts; Reporting.
CHROMADEX shall use its reasonable efforts to develop for
commercial use and to market a Licensed Product as soon as
practicable, and to continue to market a Licensed Product as long
as commercially viable, all as is consistent with sound and
reasonable business practice.
4.2
Compliance with Laws. CHROMADEX
shall use its best efforts to comply with all prevailing laws,
rules and regulations pertaining to the development, testing,
manufacture, marketing and import or export of Licensed Products.
Without limiting the foregoing, CHROMADEX acknowledges that the
transfer of certain commodities and technical data is subject to
United States laws and regulations controlling the export of such
commodities and technical data, including all Export Administration
Regulations of the United States Department of Commerce. These laws
and regulations, among other things, prohibit or require a license
for the export of certain types of technical data to specified
countries. CHROMADEX will comply with all United States laws and
regulations controlling the export of commodities and technical
data.
4.3
Government Approvals. CHROMADEX
will be responsible for obtaining, at its cost and expense, all
governmental approvals required to commercially market Licensed
Products.
4.4
Patent Notices. CHROMADEX shall
mark or cause to be marked all Licensed Products made or sold in
the United States with all applicable patent numbers for the
Patents. If it is not practical for a Licensed Product to be so
marked, then CHROMADEX shall mark or cause to be marked the package
for each Licensed Product with all applicable patent numbers for
the Patents. GM shall provide CHROMADEX with assistance in
performing such marking upon request.
4.5
Bankruptcy or Equivalent.
CHROMADEX will provide written notice to GM prior to the filing of
a petition in bankruptcy or equivalent if CHROMADEX intends to file
a voluntary petition, or, if known by CHROMADEX through statements
or letters from a creditor or otherwise, if a Third Party intends
to file an involuntary petition in bankruptcy against CHROMADEX.
Notice will be given at least 75 days before the planned filing or,
if such notice is not feasible, as soon as CHROMADEX is aware of
the planned filing where any such notice is allowable under
bankruptcy laws. CHROMADEX's failure to perform this obligation is
deemed to be a material pre-petition incurable breach under this
Agreement not subject to the 60-day notice requirement of Article
9.2, and GM is deemed to have terminated this Agreement forty-five
(45) days prior to the filing of the bankruptcy unless such notice
is not allowable under bankruptcy laws.
ARTICLE 5
REPRESENTATIONS
5.1
Representations of GM. GM
represents to CHROMADEX as follows:
(a)
this Agreement,
when executed and delivered by GM, will be the legal, valid and
binding obligation of GM, enforceable against GM in accordance with
its terms;
(b)
GM, and to
GM’s knowledge, has not granted rights in the Patent Rights
to any Person other than CHROMADEX;
(c)
GM has not received
any written notice that the Patent Rights infringe the proprietary
rights of any third party;
(d)
the inventions
claimed in the Patents to the knowledge of GM have not been
publicly used, offered for sale, or disclosed in a printed
publication by employees of more than one year prior to the filing
of the U.S. application for the Patents.
5.2
Representations and Warranties of
CHROMADEX. CHROMADEX represents and warrants to GM as
follows:
(a)
CHROMADEX is a
corporation duly organized, validly existing and in good standing
under the laws of California and has all requisite corporate power
and authority to execute, deliver and perform this
Agreement;
(b)
This Agreement,
when executed and delivered by CHROMADEX, will be the legal, valid
and binding obligation of CHROMADEX, enforceable against CHROMADEX
in accordance with its terms;
(c)
the execution,
delivery and performance of this Agreement by CHROMADEX does not
conflict with, or constitute a breach or default
under,
(i)
the charter
documents of CHROMADEX,
(ii)
any law, order,
judgment or governmental rule or regulation applicable to
CHROMADEX, or
(iii)
any provision of
any agreement, contract, commitment or instrument to which
CHROMADEX is a party; and the execution, delivery and performance
of this Agreement by CHROMADEX does not require the consent,
approval or authorization of, or notice, declaration, filing or
registration with, any governmental or regulatory
authority.
ARTICLE 6
LIABILITY AND INDEMNIFICATION
6.1
No warranties; Limitation on
Liability. Except as explicitly set forth in this agreement,
GM makes no representations or warranties, express or implied, with
respect to: (i) commercial utility; or (ii) merchantability or
fitness for a particular purpose; or (iii) that the use of the
patent rights will not infringe any patent, copyright or trademark
or other proprietary or property rights of others. GM shall not be
liable to CHROMADEX, CHROMADEX’s successors or assigns or any
third party with respect to any claim on account of, or arising
from, the use of information in connection with the patent rights
supplied hereunder or the manufacture, use or sale of licensed
products or any other material or item derived there
from.
-6-
6.2
CHROMADEX Indemnification.
CHROMADEX will indemnify and hold harmless GM, its trustees,
officers, agents and employees (collectively, the
“Indemnified Parties”), from and against any and all
liability, loss, damage, action, claim or expense suffered or
incurred by the Indemnified Parties which results from or arises
out of (individually, a “Liability” and collectively,
the “Liabilities”):
(a)
breach
by CHROMADEX of any covenant or agreement contained in this
Agreement;
(b)
the development,
use, manufacture, promotion, sale, distribution or other
disposition of any Licensed Products by CHROMADEX, its Affiliates,
assignees, vendors or other third parties, for personal injury,
including death, or property damage arising from any of the
foregoing. The indemnification obligation under Article 6.3 shall
not apply to any contributory negligence or product liability of
the Indemnified Party which may have occurred prior to the
execution of this Agreement. CHROMADEX will indemnify and hold
harmless the Indemnified Parties from and against any Liabilities
resulting from:
(i)
any product
liability or other claim of any kind related to the use by a third
party of a Licensed Product that was manufactured, sold,
distributed or otherwise disposed by CHROMADEX, its Affiliates,
assignees, vendors or other third parties;
(ii)
clinical trials or
studies conducted by or on behalf of CHROMADEX relating to any
Licensed Product and the Patent Rights, including, without
limitation, any claim by or on behalf of a human subject of any
such clinical trial or study, any claim arising from the procedures
specified in any protocol used in any such clinical trial or study,
any claim of deviation, authorized or unauthorized, from the
protocols of any such clinical trial or study, any claim resulting
from or arising out of the manufacture or quality control by a
third party of any substance administered in any clinical trial or
study;
(iii)
CHROMADEX’s
failure to comply with all prevailing laws, rules and regulations
pertaining to the development, testing, manufacture, marketing and
import or export of a Licensed Product.
6.3
Procedures. The Indemnified
Party shall promptly notify CHROMADEX of any claim or action giving
rise to a Liability subject to the provisions of Article 6.3.
CHROMADEX shall have the right to defend any such claim or action,
at its cost and expense. Indemnified Party must have the right to
approve counsel to represent it and such approval will not be
unreasonably withheld. In the event CHROMADEX or any of its
parents, affiliates or subsidiaries is also named in a particular
claim, CHROMADEX may choose the same attorneys who defend the
Indemnified Parties to defend CHROMADEX unless there arises a
conflict of interest between the CHROMADEX and one or more of the
Indemnified Parties or among the Indemnified Parties. The
indemnification rights of GM or other Indemnified Party contained
herein are in addition to all other rights which such Indemnified
Party may have at law or in equity or otherwise.
6.4
Product Liability Insurance.
CHROMADEX shall maintain general liability and product liability
insurance that is reasonable based upon industry standards, but not
less than two million dollars ($2,000,000) per incident and two
million dollars ($2,000,000) in the aggregate. The insurance
amounts specified herein shall not be deemed a limitation on
CHROMADEX’s indemnification liability under this Agreement.
CHROMADEX shall provide GM with copies of such policies, upon
request of GM. CHROMADEX shall notify GM at least ten (10) days
prior to cancellation of any such coverage.
-7-
ARTICLE 7
PATENTS AND INFRINGEMENT
7.1
Prosecution of
Patents.
(a)
Responsibilities for Patent Rights.
(i)
GM through its
patent attorneys is responsible for preparing, filing, and
prosecuting any patent applications, maintaining any issued
patents, and prosecuting and maintaining any and all continuations,
continuations-in-part, divisional, substitutions, reissues, or
re-examinations (or the foreign equivalent of these) related to the
Patent Rights. CHROMADEX will reimburse GM for patent expenses as
detailed in Article 3.7.
(ii)
GM will prepare,
file, and prosecute patent applications for the Patent Rights in
the United States. GM will also prepare, file, and prosecute
international applications for the Patent Rights under the Patent
Cooperation Treaty.
(a)
Such international
applications shall designate the European Patent Office as the
International Searching Authority, and shall designate at a minimum
the European States (defined as “EP” on the
international application form of the Patent Cooperation Treaty),
and additional countries specified by CHROMADEX.
(b)
CHROMADEX will
specify in writing to GM the additional foreign countries in which
patent applications are to be filed and prosecuted. GM when
possible will notify CHROMADEX ninety (90) days in advance of a
national stage filing deadline for all Patent Rights, and CHROMADEX
will specify such additional countries no later than thirty (30)
days before the national stage filing deadline for the pertinent
patent application.
(iii)
GM is solely responsible for making decisions regarding the content
of U.S. and foreign applications to be filed under Patent Rights
and prosecution of the applications, continuations,
continuations-in-part, divisional, substitutions, reissues, or
re-examinations (or the foreign equivalent of these) related
thereto. GM will not seek to narrow the scope of a pending
application without obtaining CHROMADEX’s consent, which
consent shall not be unreasonably withheld or delayed. GM shall use
its good faith efforts to provide CHROMADEX with a copy of all
materials to be filed with the U.S. Patent and Trademark Office and
its foreign equivalents at least thirty (30) business days prior to
the planned filing and afford CHROMADEX the right to
comment.
(iv)
CHROMADEX will
cooperate with GM in the filing, prosecution, and maintenance of
any Patent Rights. GM will advise CHROMADEX promptly as to all
material developments with respect to the applications. Copies of
all papers received and filed in connection with prosecution of
applications in all countries will be provided promptly after
receipt or filing to CHROMADEX to enable it to advise GM concerning
the applications.
(v)
No party shall be
liable for any loss, as a whole or in part, of a patent term
extension granted by the U.S. Patent and Trademark Office (or its
foreign equivalents) on a patent issuing under the Patent Rights,
even if such loss results from acts or omissions of the prosecuting
party or its personnel.
-8-
(vi)
Each party agrees
to promptly forward all written communications from the other party
regarding prosecution of Patent Rights to its patent counsel as
appropriate, with a written confirmation to the other party that
the communications have been forwarded.
7.2
Infringement by Third Party. In
the event that CHROMADEX or GM become aware of suspected
infringement of the Patent Rights, they shall promptly notify the
other parties of such suspected infringement. CHROMADEX and GM
directly or together, may bring suit to abate infringement of the
Patent Rights, or communicate with a potential infringer, with
prior approval from the other parties. In the event that one party
intends to bring suit relating to suspected infringement, it shall
promptly notify the other parties of its intention to sue so that
the other parties may have the opportunity to approve and
participate in and share costs and recoveries from said suit. If
only one party brings suit and the other parties choose not to
participate in said suit, the party that brings the suit shall be
liable for all litigation costs and shall be entitled to retain all
recoveries therefrom. In such an event, the other parties shall
provide reasonable cooperation, at the expense of the party
bringing suit, in the maintenance of such a suit. In the event
CHROMADEX chooses to bring suit and GM declines to participate, GM
agrees to join such suit should GM be deemed a necessary party to
such suit. In the event neither party elects to brings suit within
90 days of notice of the suspected infringement, GM and CHROMADEX
shall make commercially reasonable efforts to end the
infringement.
ARTICLE 8
CONFIDENTIALITY AND PUBLICATIONS
8.1
Confidentiality. To the extent
allowed by law, both parties shall maintain in confidence and shall
not disclose to any third party the Confidential Information
received pursuant to this Agreement, without the prior written
consent of the disclosing party except that the Confidential
Information may be disclosed by either party only to those third
parties (x) who have a need to know the information in connection
with the exercise by either party of its rights under this
Agreement and who agreed in writing to keep the information
confidential to the same extent as is required of the parties under
this Article 8.1, or (y) to whom either party is legally obligated
to disclose the information. The foregoing obligation shall not
apply to information which:
(a)
is, at the time of
disclosure, publicly known or available to the public, provided
that Information will not be deemed to be within the public domain
merely because individual parts of such Information are found
separately within the public domain, but only if all the material
features comprising such Information are found in combination in
the public domain;
(b)
is known to
recipient at the time of disclosure of such Confidential
Information provided that recipient promptly notifies disclosing
party in writing of this prior knowledge within thirty (30) days of
receipt;
(c)
is hereafter
furnished to recipient by a third party, as a matter of right and
without restriction on disclosure, provided that recipient promptly
notifies disclosing party in writing of this third party disclosure
after receipt thereof;
(d)
is made public by
disclosing party;
(e)
is disclosed with
the written approval of either party;
(f)
is the subject of a
legally binding court order compelling disclosure, or is otherwise
subject to any law or regulation or regulatory body compelling
disclosure, provided that recipient must give disclosing party
reasonable advance notice of such required disclosure, and
recipient must cooperate with disclosing party in attempting to
prevent or limit such disclosure.
8.2
Publications. Should GM desire
to disclose publicly, in writing or by oral presentation,
Confidential Information related to the Patent Rights, GM shall
notify CHROMADEX in writing of its intention at least ninety (90)
days before such disclosure. GM shall include with such notice a
description of the oral presentation or, in the case of a
manuscript or other proposed written disclosure, a current draft of
such written disclosure.
If the
content of such disclosure represents in the eyes of CHROMADEX a
new Invention or significant improvement to the state of the art
that may result in a new patent, CHROMADEX may request GM, no later
than ninety (90) days following the receipt of GM’s notice,
to file a patent application, copyright or other filing related to
such Invention. All such filings shall be subject to the provisions
of Article 7.1 of this Agreement. Upon receipt of such request, GM
shall arrange for a delay in publication, to permit filing of a
patent or other application . Should the parties reasonably
determine that more than ninety (90) days is required in order to
file any such patent information (including additional time
required to perform additional research required for adequate
patent disclosure), or, if CHROMADEX reasonably determines that
such Confidential Information cannot be adequately protected
through patenting and such Confidential Information has commercial
value as a trade secret, then publication or disclosure shall be
postponed until the parties can mutually agree upon a reasonable
way to proceed.
8.3
Use of Name. Neither CHROMADEX
nor GM shall directly or indirectly use the other party’s
name, or the name of any trustee, officer or employee thereof,
without that party’s prior written consent, or disclose the
terms of this Agreement to third parties except that GM or
CHROMADEX may disclose this Agreement to an Affiliate and may
disclose an accurate description of the terms of this Agreement to
the extent required under federal or state securities, tax, grant
administration, or other disclosure laws. GM shall take steps to
preserve the confidentiality of such information to the extent
allowed by law.
ARTICLE 9
TERM AND TERMINATION
9.1
Term. This Agreement and the
licenses granted herein shall commence on the Effective Date and
shall continue, subject to earlier termination under Articles 9.2
or 9.3 hereof, until the expiration of the last to expire of the
Patents.
9.2
Termination by GM. Upon the
occurrence of any of the events set forth below (“Events of
Default”), GM shall have the right to terminate this
Agreement by giving writtennotice of termination, such termination
effective with the giving of such notice:
(a)
nonpayment of any amount payable to GM that is continuing sixty
(60) calendar days after GM gives CHROMADEX written notice of such
nonpayment;
-9-
(b) any
breach by CHROMADEX of any covenant (other than a payment breach
referred to in clause (a) above or a Commercialization Plan breach
referred to in Article 9.3 below) or any representation or warranty
contained in this Agreement that is continuing sixty (60) calendar
days after GM gives CHROMADEX written notice of such
breach;
(c)
CHROMADEX fails to comply with the terms of the license granted
under Article 2 hereof and such noncompliance is continuing sixty
(60) calendar days after GM gives CHROMADEX notice of such
noncompliance;
(d)
CHROMADEX becomes subject to a Bankruptcy Event;
(e) the
dissolution or cessation of operations by CHROMADEX;
(f) If
after the first commercial sale of a Licensed Product and during
the term of this Agreement, CHROMADEX fails to make reasonable
efforts to commercialize at least one (1) Licensed Product or fails
to keep at least one (1) Licensed Product on the market after the
first commercial sale for a continuous period of one (1) year,
where such noncompliance is continuing sixty( 60) calendar days
after GM gives CHROMADEX written notice of such noncompliance. The
inclusion of at least one (1) Licensed Product in an available
catalog of products containing Pterostilbene or in an available
catalog of products directed for treatment of the conditions in the
Patent shall be deemed a reasonable effort to commercialize under
this section.
9.3
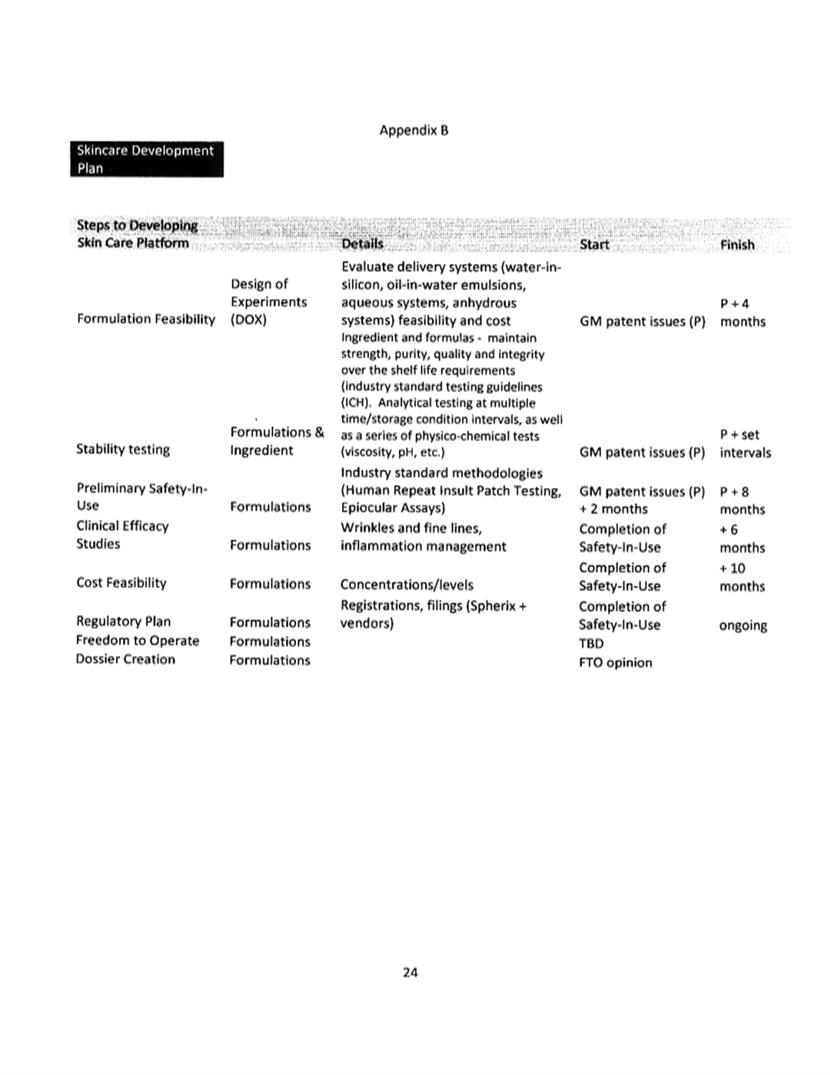
Commercialization Plan.
CHROMADEX has provided GM with a Commercialization Plan acceptable
to GM. Such Commercialization Plan is contained in Appendix B and
is incorporated herein by reference. GM shall be entitled to
terminate this Agreement if CHROMADEX fails to meet the
pre-established development milestones contained in the
Commercialization Plan. If the event that Sabinsa Patent Issues,
CHROMADEX and GM will work in good faith to establish a new
Commercialization plan.The milestones may be changed as agreed upon
in advance in writing by both parties. GM shall give written notice
of its decision to terminate this Agreement specifying a failure of
the Commercialization Plan milestones. Unless CHROMADEX has
remedied such failure or both parties have agreed, in writing, to a
revised milestone schedule within sixty (60) days after receipt of
such notice, this Agreement will be deemed to terminate as of the
expiration of such sixty (60) day period.
9.4
Termination by CHROMADEX.
CHROMADEX shall have the right to terminate this Agreement, at any
time and with or without cause, upon one hundred and twenty (120)
days’ written notice to GM.
9.5
Rights and Duties Upon
Termination. Within thirty (30) days after termination of
this Agreement, each party shall return to the other party any
Confidential Information of the other party. In the event of an
early termination of this Agreement, CHROMADEX shall have the right
to use or sell all the Licensed Product(s) on hand or in the
process of manufacturing at the time of such early termination,
provided that CHROMADEX shall be obligated to pay to GM a royalty
on such sales as set forth in this Agreement if, at that time there
remains in existence any of Licensor’s Patent Rights covering
the transfer of such Licensed Product(s) and a royalty or other
payment is payable pursuant to the terms of this
Agreement.
-10-
9.6
Provisions Surviving
Termination. CHROMADEX’s obligation to pay any
royalties accrued but unpaid prior to termination of this Agreement
shall survive such termination. In addition, all provisions
required to interpret the rights and obligations of the parties
arising prior to the termination date shall survive expiration or
termination of this Agreement.
ARTICLE 10
OTHER TERMS AND CONDITIONS
10.1
Assignment. This Agreement and the rights and benefits
conferred upon CHROMADEX hereunder may not be transferred or
assigned by CHROMADEX to any party without the prior written
consent of GM, such permission will not be unreasonably withheld,
except for:
(a)
an assignment in
connection with a merger, sale or reorganization of CHROMADEX, or
the sale or transfer of all or substantially all of
CHROMADEX’s assets which relate to the manufacture of a
Licensed Product or use of the Patent Rights provided that
CHROMADEX demonstrates to GM’s reasonable satisfaction that
the buyer or transferee is at least as financially stable as
CHROMADEX and following the sale or transfer would be as capable of
performing its obligations under this Agreement as CHROMADEX would
be; or
(b)
an assignment of a
security interest in this Agreement as a part of a security
interest in all or substantially all of the CHROMADEX’s
assets which relate to the Patent Rights or a Licensed Product. Any
prohibited assignment of this Agreement on the rights hereunder
shall be null and void. No assignment shall relieve CHROMADEX of
responsibility for the performance of any accrued obligations which
it has prior to such assignment. This Agreement shall inure to the
benefit of permitted assigns of CHROMADEX.
For the
avoidance of doubt, the parties agree that any assignment of this
Agreement made in accordance with this Article 10.1 in which GM has
given written consent shall relieve the assignor of all obligations
under this Agreement, whether fixed, accrued, contingent or
otherwise, whereupon the effect shall be the same as if this
Agreement had been executed by the assignee in the first instant
and the assignor had never been a party hereto.
10.2
Assignment. This Agreement and the rights and benefits
conferred upon CHROMADEX hereunder may not be transferred or
assigned in whole or any part by GM to any party without the prior
written consent of CHROMADEX, such permission will not be
unreasonably withheld,
10.3
No Waiver. A waiver by either
party of a breach or violation of any provision of this Agreement
will not constitute or be construed as a waiver of any subsequent
breach or violation of that provision or as a waiver of any breach
or violation of any other provision of this Agreement.
10.4
Independent Contractor. Nothing
herein shall be deemed to establish a relationship of principal and
agent between GM and CHROMADEX, nor any of their agents or
employees for any purpose whatsoever. This Agreement shall not be
construed as constituting GM and CHROMADEX as partners, or as
creating any other form of legal association or arrangement which
could impose liability upon one party for the act or failure to act
of the other party. No employees or staff of GM shall be entitled
to any benefits applicable to employees of CHROMADEX. Neither party
shall be bound by the acts or conduct of the other
party.
10.5
Notices. Any notice under this
Agreement shall be sufficiently given if sent in writing recognized
commercial delivery service with proof of delivery." addressed as
follows:
if to
GM, to: Dr. Jose Mª Estrela
Parc
Cientific Universidad de Valencia,
Polígono La
Coma s/n,
46980
Paterna, Valencia, Spain ______________
if to
CHROMADEX, to:
ChromaDex
Inc,
Chief
Financial Officer
10005
Muirlands Bvld
Suite
G
Irvine,
CA 92618
or to
such other addresses as may be designated from time to time by
notice given in accordance with the terms of this Article
10.4.
10.6
Entire Agreement. This
Agreement embodies the entire understanding between the parties
relating to the subject matter hereof and supersedes all prior
understandings and agreements, whether written or oral. This
Agreement may not be modified or varied except by a written
document signed by duly authorized representatives of both
parties.
10.7
Severability. In the event that
any provision of this Agreement shall be held to be unenforceable,
invalid or in contravention of applicable law, such provision shall
be of no effect, the remaining portions of this Agreement shall
continue in full force and effect, and the parties shall negotiate
in good faith to replace such provision with a provision which
effects to the extent possible the original intent of such
provision.
10.8
Force Majure In the event that
either party’s performance of its obligations under this
Agreement shall be prevented by any cause beyond its reasonable
control, including without limitation acts of God, acts of
government, shortage of material, accident, fire, delay or other
disaster, provided that the effected party shall have used its
reasonable best efforts to avoid or remove the cause of such
nonperformance and to minimize the duration and negative affect of
such nonperformance, then such effected party’s performance
shall be excused and the time for performance shall be extended for
the period of delay or inability to perform due to such occurrence.
The affected party shall continue performance under this Agreement
using its best efforts as soon as such cause is
removed.
10.9
Headings. Any headings and
captions used in this Agreement are for convenience of reference
only and shall not affect its construction or
interpretation.
10.10
No Third Party Benefits.
Nothing in this Agreement, express or implied, is intended to
confer on any Person other than the parties hereto or their
permitted assigns, any benefits, rights or remedies.
-11-
10.11
Governing Law. This Agreement
shall be construed in accordance with and governed by the laws of
the State of New York.. The parties hereto hereby irrevocably
submits to the exclusive jurisdiction of the state and federal
courts sitting in the City of New York, Borough of Manhattan for
the adjudication of any dispute hereunder or in connection herewith
or with any transaction contemplated hereby or discussed herein,
and hereby irrevocably waives, and agrees not to assert in any
proceeding, any claim that it is not personally subject to the
jurisdiction of any such court or that such proceeding is improper.
Each party hereto hereby irrevocably waives personal service of
process and consents to process being served in any such proceeding
by mailing a copy thereof via registered or certified mail or
overnight delivery (with evidence of delivery) to such party at the
address in effect for notices to it under this Agreement and agrees
that such service shall constitute good and sufficient service of
process and notice thereof.
10.12
Counterparts. This Agreement
shall become binding when any one or more counterparts hereof,
individually or taken together, shall bear the signatures of each
of the parties hereto. This Agreement may be executed in any
numberr of counterparts, each of which shall be deemed an original
as against the party whose signature appears thereon, but all of
which taken together shall constitute but one and the same
instrument.
10.12
Resolution of Disputes. If the
parties are unable to reach agreement by negotiating in good faith
about any matter under this Agreement, the parties agree to resolve
the dispute themselves, and if failing to do so, they agree to seek
resolution of the dispute through the mediation in New York, New
York.
-12-
IN
WITNESS WHEREOF, the parties hereto have duly executed this License
Agreement as of the date first above written.
Green
Molecular S.L
/s/ Manuel Castellon
Leal
8/1/2013
Manuel
Castellón
Leal
Date
By
Power of Attorney on behalf of Green Molecular
CHROMADEX,
INC.
/s/
Frank L. Jaksch
Jr
8/1/2013
Frank
L. Jaksch Jr.
Chief Executive
Officer
Date
-13-
APPENDIX A
TITLE:
USE
OF PTEROSTILBENE (PTER) AS MEDICAMENT FOR PREVENTION AND/OR
TREATMENT OF SKIN DISEASES, DAMAGES OR INJURIES OR AS
COSMETIC
|
COUNTRY
|
PATENT NUMBER
|
LEGAL STATUS
|
|
AUSTRALIA
|
2010311326
|
Pending
|
|
BRAZIL
|
BR
112012010070-0
|
Pending
|
|
CANADA
|
2,778,151
|
Pending
|
|
CHINE
|
201080048865.5
|
Pending
|
|
EUROPE
|
10775793.2
|
Granted: Mention of
grant will be published on 24.07.2013; Validation in designated
European countries to be decided
|
|
ISRAEL
|
219318
|
Pending
|
|
JAPAN
|
2012-535862
|
Pending
|
|
REPUBLIC OF
KOREA
|
10-2012-7013257
|
Pending
|
|
MEXICO
|
MX/a/2012/005013
|
Pending
|
|
RUSSIA
|
2012122241
|
Pending
|
|
UNITED
STATES
|
13/504,056
|
Pending
|
-14-
TITLE:
COMBINED
USED OF PTEROSTILBENE AND QUERCETIN FOR THE PRODUCTION OF CANCER
TREATMENT MEDICAMENT
|
COUNTRY
|
PATENT NUMBER
|
LEGAL STATUS
|
|
AUSTRIA
|
05774387.4 (E
425747)
|
Granted
|
|
BELGIUM
|
05774387.4
|
Granted
|
|
SWITZERLAND
|
05774387.4
|
Granted
|
|
GERMANY
|
05774387.4 (60 2005
013 390.9-08)
|
Granted
|
|
DENMARK
|
05774387.4
|
Granted
|
|
FRANCE
|
05774387.4
|
Granted
|
|
UNITED
KINGDOM
|
05774387.4
|
Granted
|
|
REPUBLIC OF
KOREA
|
05774387.4
|
Granted
|
|
IRELAND
|
05774387.4
|
Granted
|
|
ITALY
|
05774387.4
|
Granted
|
|
NETHERLANDS
|
05774387.4
|
Granted
|
|
PORTUGAL
|
05774387.4
|
Granted
|
|
SWEDEN
|
05774387.4
|
Granted
|
|
SPAIN
|
05774387.4
|
Granted
|
|
TURKEY
|
05774387.4 (TR 2009
04262)
|
Granted
|
|
UNITED
STATES
|
11/631,912
|
Granted
|
-15-