Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE - ClearPoint Neuro, Inc. | ex99-1.htm |
| 8-K - 8-K FILING - ClearPoint Neuro, Inc. | f16-0890.htm |
Exhibit 99.1
Ticker: MRIC Investor Presentation November, 2016 Transforming minimally invasive neurosurgery by enabling real-time visualization with MRI © 2016 MRI INTERVENTIONS, INC. | 1

Forward Looking Statements Certain statements in this presentation may constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Forward-looking statements often can be identified by words such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would,” or the negative of these words or other words of similar meaning. Forward-looking statements by their nature address matters that, to different degrees, are uncertain and involve risk. Uncertainties and risks may cause MRI Interventions’ actual results and the timing of events to differ materially from those expressed in or implied by MRI Interventions’ forward-looking statements. Particular uncertainties and risks include, among others: demand and market acceptance of our products; our ability to successfully expand, and achieve full productivity from, our sales, clinical support and marketing capabilities; availability and adequacy of reimbursement from third party payors for procedures utilizing our products; the sufficiency of our cash resources to maintain planned commercialization efforts and research and development programs; future actions of the FDA or any other regulatory body that could impact product development, manufacturing or sale; our ability to protect and enforce our intellectual property rights; our dependence on collaboration partners; the impact of competitive products and pricing; the impact of the commercial and credit environment on us and our customers and suppliers; and our ability to successfully complete the development of, and to obtain regulatory clearance or approval for, our ClearTrace system. More detailed information on these and additional factors that could affect MRI Interventions’ actual results and the timing of events are described in its filings with the Securities and Exchange Commission. Except as required by law, we undertake no obligation to publicly update or revise any forward-looking statements made in this presentation to reflect any change in our expectations or any change in events, conditions or circumstances on which any such statements are based. © 2016 MRI INTERVENTIONS, INC. | 2
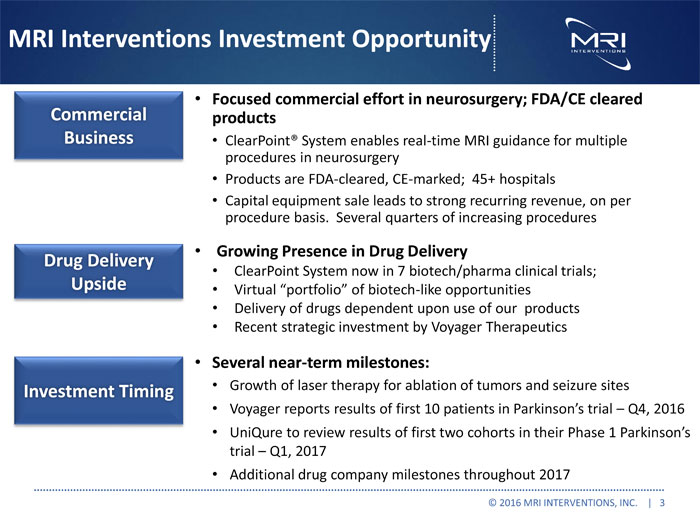
MRI Interventions Investment Opportunity Focused commercial effort in neurosurgery; FDA/CE cleared Commercial products Business ClearPoint® System enables real-time MRI guidance for multiple procedures in neurosurgery Products are FDA-cleared, CE-marked; 45+ hospitals Capital equipment sale leads to strong recurring revenue, on per procedure basis. Several quarters of increasing procedures Growing Presence in Drug Delivery Drug Delivery ClearPoint System now in 7 biotech/pharma clinical trials; Upside Virtual “portfolio” of biotech-like opportunities Delivery of drugs dependent upon use of our products Recent strategic investment by Voyager Therapeutics Several near-term milestones: Investment Timing Growth of laser therapy for ablation of tumors and seizure sites Voyager reports results of first 10 patients in Parkinson’s trial Q4, 2016 UniQure to review results of first two cohorts in their Phase 1 Parkinson’s trial Q1, 2017 Additional drug company milestones throughout 2017 © 2016 MRI INTERVENTIONS, INC. | 3
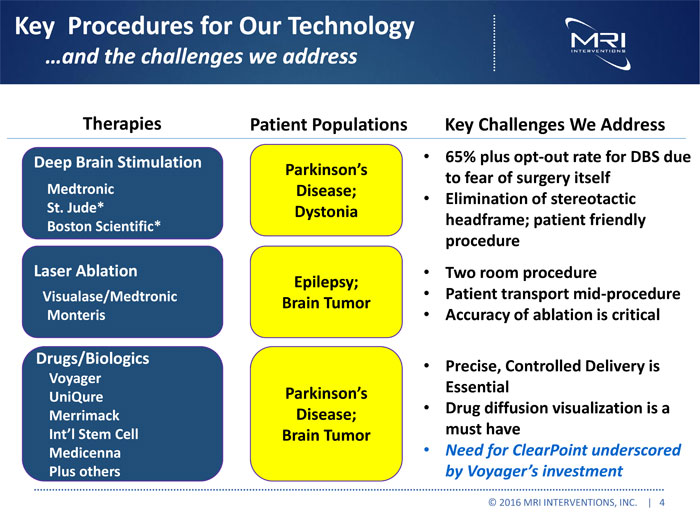
Key Procedures for Our Technology …and the challenges we address Therapies Patient Populations Key Challenges We Address Deep Brain Stimulation 65% plus opt-out rate for DBS due Parkinson’s to fear of surgery itself Medtronic Disease; Elimination of stereotactic St. Jude* Dystonia headframe; patient friendly Boston Scientific* procedure Laser Ablation Two room procedure Epilepsy; Visualase/Medtronic Patient transport mid-procedure Brain Tumor Monteris Accuracy of ablation is critical Drugs/Biologics Precise, Controlled Delivery is Voyager Parkinson’s Essential UniQure Disease; Drug diffusion visualization is a Merrimack Int’l Stem Cell Brain Tumor must have Medicenna Need for ClearPoint underscored Plus others by Voyager’s investment © 2016 MRI INTERVENTIONS, INC. | 4
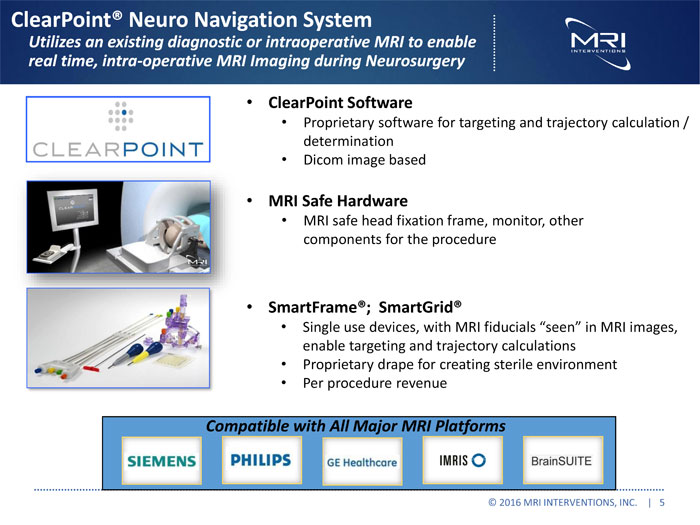
ClearPoint® Neuro Navigation System Utilizes an existing diagnostic or intraoperative MRI to enable real time, intra-operative MRI Imaging during Neurosurgery ClearPoint Software Proprietary software for targeting and trajectory calculation / determination Dicom image based MRI Safe Hardware MRI safe head fixation frame, monitor, other components for the procedure SmartFrame®; SmartGrid® Single use devices, with MRI fiducials “seen” in MRI images, enable targeting and trajectory calculations Proprietary drape for creating sterile environment Per procedure revenue Compatible with All Major MRI Platforms © 2016 MRI INTERVENTIONS, INC. | 5
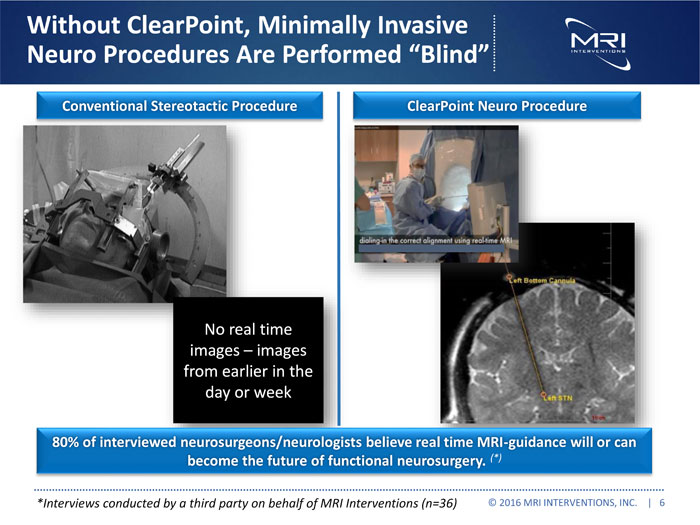
Without ClearPoint, Minimally Invasive Neuro Procedures Are Performed “Blind” Conventional Stereotactic Procedure ClearPoint Neuro Procedure No real time images – images from earlier in the day or week 80% of interviewed neurosurgeons/neurologists believe real time MRI-guidance will or can become the future of functional neurosurgery. (*) *Interviews conducted by a third party on behalf of MRI Interventions (n=36) © 2016 MRI INTERVENTIONS, INC. | 6
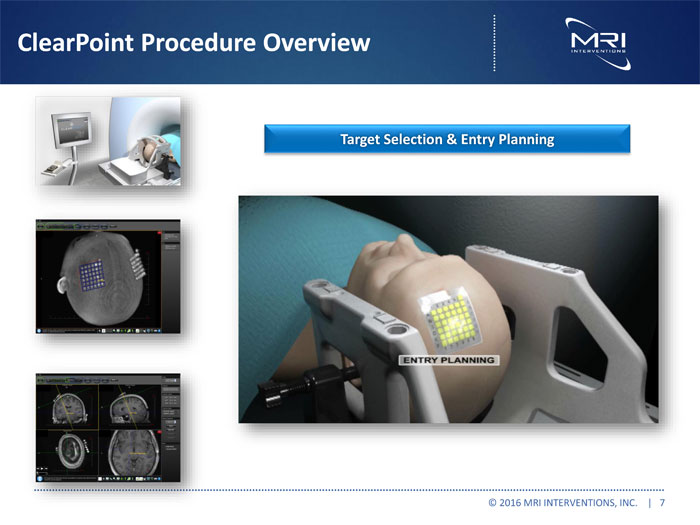
ClearPoint Procedure Overview Target Selection & Entry Planning © 2016 MRI INTERVENTIONS, INC. | 7
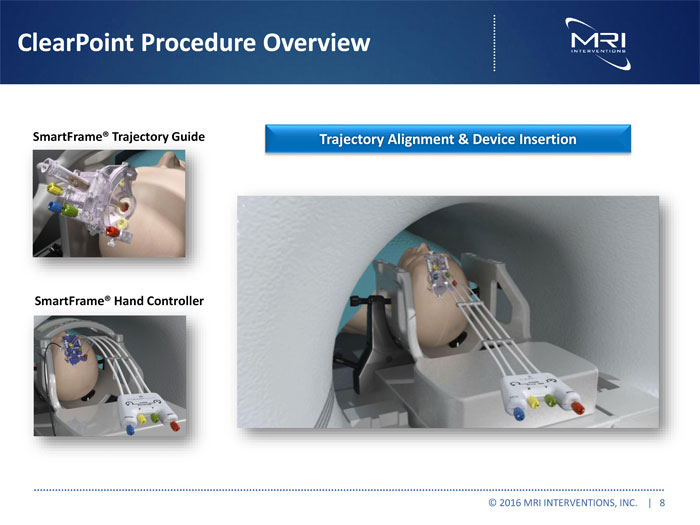
ClearPoint Procedure Overview SmartFrame® Trajectory Guide Trajectory Alignment & Device Insertion SmartFrame® Hand Controller © 2016 MRI INTERVENTIONS, INC. | 8
Clinical Support, Validation Notable Neurosurgeon Supporters Dr. Philip Starr ASSFN Past President Dr. Paul Larson UCSF & VA Dr. Robert Gross Emory University Dr. Robert Wharen, Jr. Mayo Clinic -Jacksonville Dr. Krys Bankiewicz Bankiewicz Lab, UCSF Dr. Russ Lonser OSU - NUH Published Peer-Reviewed Journal Support © 2016 MRI INTERVENTIONS, INC. | 9
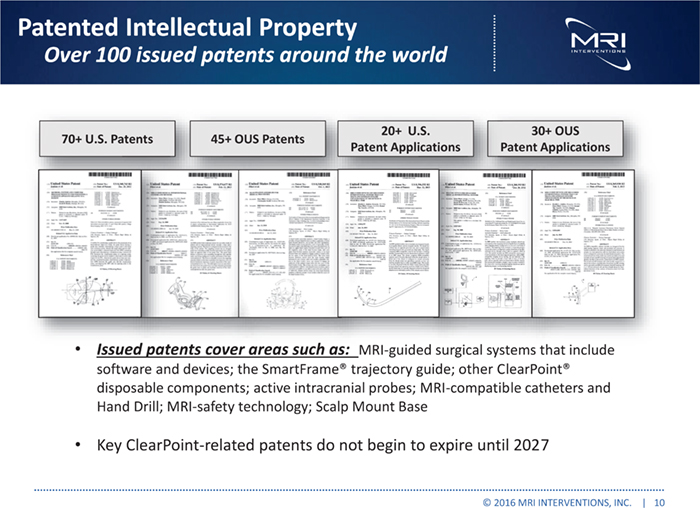
Patented Intellectual Property Over 100 issued patents around the world 20+ U.S. 30+ OUS 70+ U.S. Patents 45+ OUS Patents Patent Applications Patent Applications Issued patents cover areas such as: MRI-guided surgical systems that include software and devices; the SmartFrame® trajectory guide; other ClearPoint® disposable components; active intracranial probes; MRI-compatible catheters and Hand Drill; MRI-safety technology; Scalp Mount Base Key ClearPoint-related patents do not begin to expire until 2027 © 2016 MRI INTERVENTIONS, INC. | 10
MRIC’s Unique Opportunity in Drug Delivery Major Challenges in Delivering Drugs to the Brain - Blood brain barrier blocks systemic delivery (pills, shots, IV) of almost all drugs 98% of small molecules - Direct injection without ClearPoint is blind, so target is frequently missed - Neopharm Trial-51% of 572 catheters failed to meet all positioning criteria Major Benefits of Drug Delivery with ClearPoint - Eliminates the blood brain barrier issue - Neurosurgeon sees that target is reached - Surgeon can watch drug infusion real time, is able to see appropriate coverage - Reduces/eliminates unwanted systemic side effects - Reduces dosage levels (as little as 1/300th of systemic volume) Business Model MRIC Partners with Drug Companies and Researchers - MRIC provides ClearPoint; Drug company provides drug candidate - Drug company/sponsor pays for trial - If drug is approved, MRIC gets device revs (~$7,000 -$14,000/case); Drug co gets drug revenues Provides MRIC with “biotech-like upside” without “all or nothing downside” © 2016 MRI INTERVENTIONS, INC. | 11
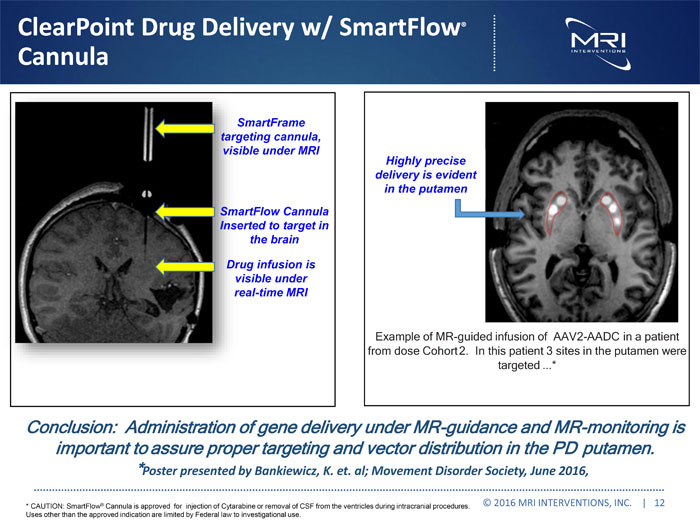
ClearPoint Drug Delivery w/ SmartFlow® Cannula SmartFrame targeting cannula, visible under MRI SmartFlow Cannula Inserted to target in the brain Drug infusion is visible under real-time MRI Highly precise delivery is evident in the putamen Example of MR-guided infusion of AAV2-AADC in a patient from dose Cohort2. In this patient 3 sites in the putamen were targeted...* Conclusion: Administration of gene delivery under MR-guidance and MR-monitoring is important to assure proper targeting and vector distribution in the PD putamen. *Poster presented by Bankiewicz, K. et. al; Movement Disorder Society, June 2016, * CAUTION: SmartFlow® Cannula is approved for injection of Cytarabine or removal of CSF from the ventricles during intracranial procedures. Uses other than the approved indication are limited by Federal law to investigational use. © 2016 MRI INTERVENTIONS, INC. | 12

ClearPoint Drug Delivery w/ SmartFlow Cannula MR visualization of neuro target MR-guided placement of catheter Therapeutic agent delivered under MR-guidance* Specialized, FDA-cleared drug delivery cannula’s / catheters Conclusion: The ClearPoint system allows Real-time Convection-enhanced Delivery to be performed with a high level of precision, predictability, and safety. Growing Set of Peer-reviewed Publications… * CAUTION: SmartFlow® Cannula is approved for injection of Cytarabine or removal of CSF from the ventricles during intracranial procedures. Uses other than the approved indication are limited by Federal law to investigational use. © 2016 MRI INTERVENTIONS, INC. | 13
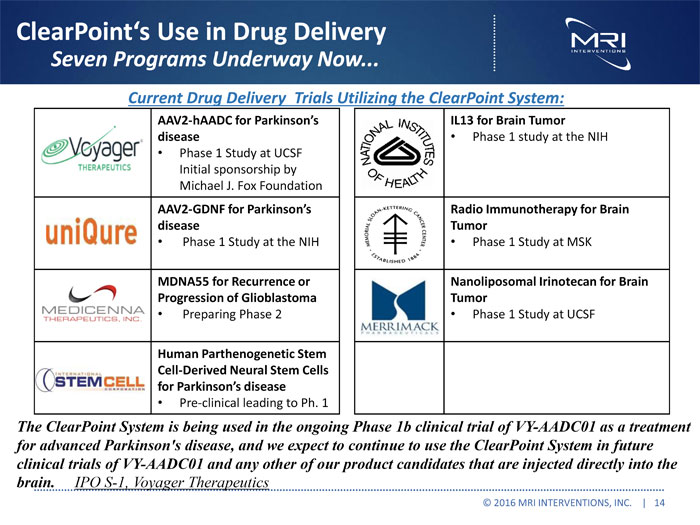
ClearPoint’s Use in Drug Delivery Seven Programs Underway Now... Current Drug Delivery Trials Utilizing the ClearPoint System: AAV2-hAADC for Parkinson’s disease Phase 1 Study at UCSF Initial sponsorship by Michael J. Fox Foundation AAV2-GDNF for Parkinson’s disease Phase 1 Study at the NIH MDNA55for Recurrence or Progression of Glioblastoma Preparing Phase 2 Human Parthenogenetic Stem Cell-Derived Neural Stem Cells for Parkinson’s disease Pre-clinical leading to Ph. 1 IL13 for Brain Tumor Phase 1 study at the NIH Radio Immunotherapy for Brain Tumor Phase 1 Study at MSK Nanoliposomal Irinotecan for Brain Tumor Phase 1 Study at UCSF The ClearPoint System is being used in the ongoing Phase 1b clinical trial of VY-AADC01 as a treatment for advanced Parkinson’s disease, and we expect to continue to use the ClearPoint System in future clinical trials of VY-AADC01 and any other of our product candidates that are injected directly into the brain. IPO S-1, Voyager Therapeutics © 2016 MRI INTERVENTIONS, INC. | 14
ClearPoint Revenue Model BUSINESS MODEL - RAZOR / RAZORBLADE ClearPoint Hardware/Software: $100,000 - $150,000 ASP ClearPoint Disposables: $7,500 (average) ASP per procedure with potentially strong margins Recurring revenue from the sale of disposables Procedures covered by existing inpatient DRG reimbursement codes © 2016 MRI INTERVENTIONS, INC. | 15
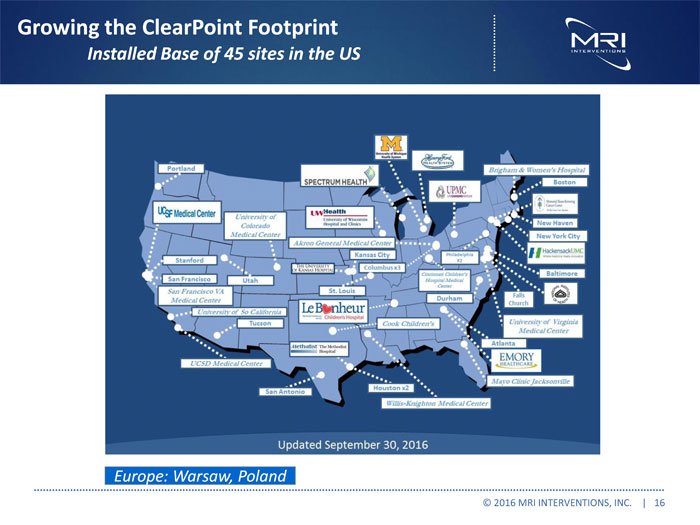
Growing the ClearPoint Footprint Installed Base of 45 sites in the US Europe: Warsaw, Poland © 2016 MRI INTERVENTIONS, INC. | 16
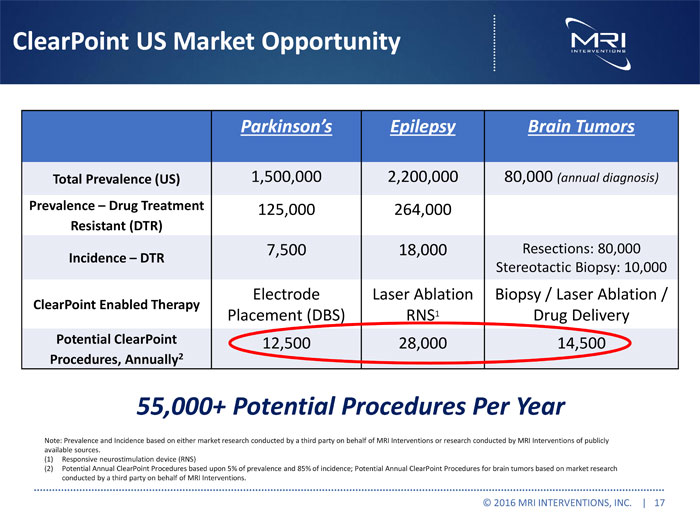
ClearPoint US Market Opportunity Parkinson’s Epilepsy Brain Tumors Total Prevalence (US) 1,500,000 2,200,000 80,000 (annual diagnosis) Prevalence Drug Treatment 125,000 264,000 Resistant (DTR) 7,500 18,000 Resections: 80,000 Incidence DTR Stereotactic Biopsy: 10,000 Electrode Laser Ablation Biopsy / Laser Ablation / ClearPoint Enabled Therapy Placement (DBS) RNS1 Drug Delivery Potential ClearPoint 12,500 28,000 14,500 Procedures, Annually2 55,000+ Potential Procedures Per Year Note: Prevalence and Incidence based on either market research conducted by a third party on behalf of MRI Interventions or research conducted by MRI Interventions of publicly available sources. (1) Responsive neurostimulation device (RNS) (2) Potential Annual ClearPoint Procedures based upon 5% of prevalence and 85% of incidence; Potential Annual ClearPoint Procedures for brain tumors based on market research conducted by a third party on behalf of MRI Interventions. © 2016 MRI INTERVENTIONS, INC. | 17
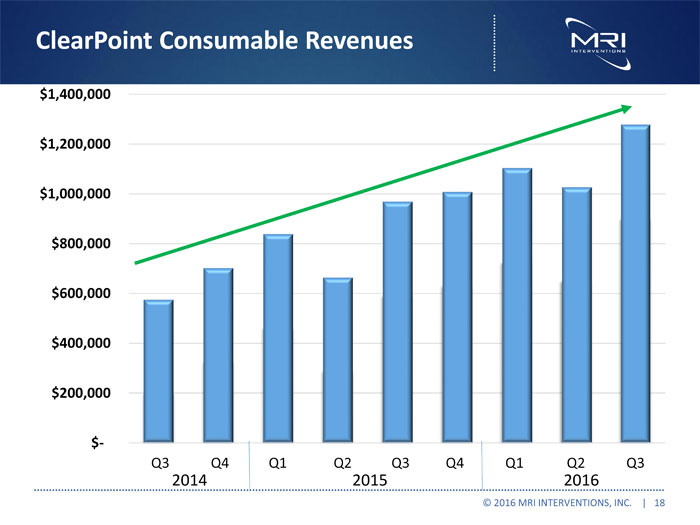
ClearPoint Consumable Revenues © 2016 MRI INTERVENTIONS, INC. | 18
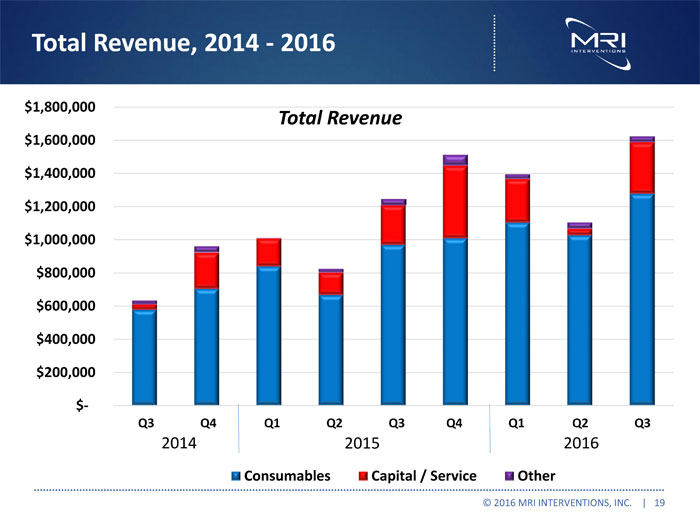
Total Revenue, 2014 - 2016 © 2016 MRI INTERVENTIONS, INC. | 19
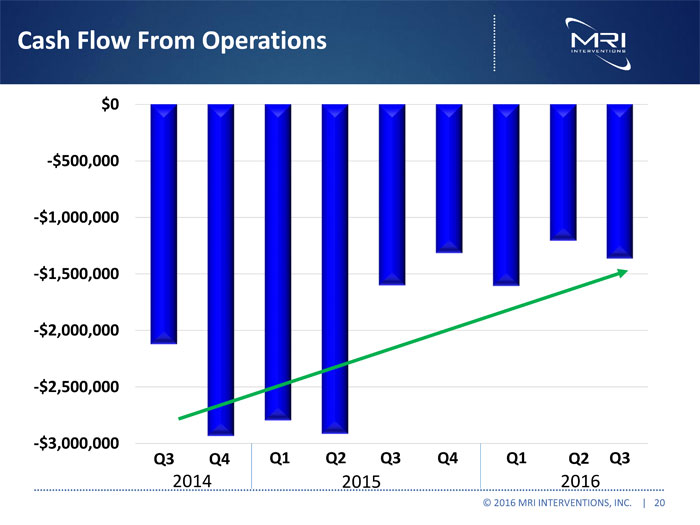
Cash Flow From Operations © 2016 MRI INTERVENTIONS, INC. | 20
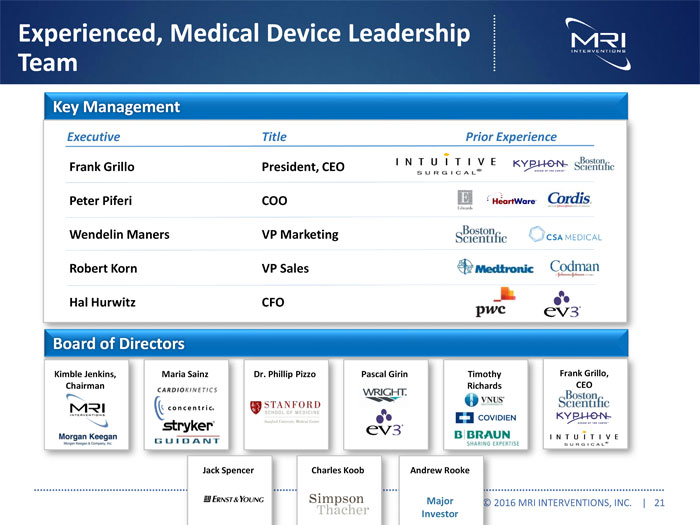
Experienced, Medical Device Leadership Team
| Key Management | ||
| Executive | Title | Prior Experience |
| Frank Grillo | President, CEO | |
| Peter Piferi | COO | |
| Wendelin Maners | VP Marketing | |
| Robert Korn | VP Sales | |
| Hal Hurwitz | CFO |
| Board of Directors | |||||
| Kimble Jenkins, | Maria Sainz | Dr. Phillip Pizzo | Pascal Girin | Timothy | Frank Grillo, |
| Chairman | Richards | CEO | |||
| Spencer | Charles Koob | Andrew Rooke | |||
| Major Investor |
© 2016 MRI INTERVENTIONS, INC. | 21
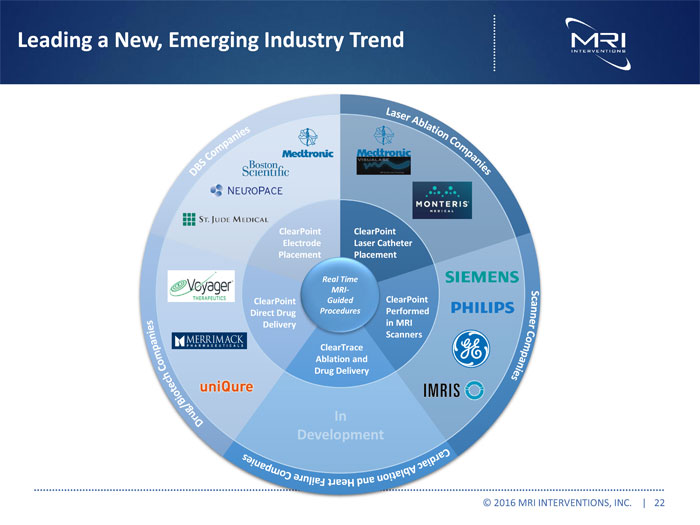
Leading a New, Emerging Industry Trend © 2016 MRI INTERVENTIONS, INC. | 22
Ticker: MRIC MRI Interventions, Inc. Irvine, CA 949.900.6833 www.mriinterventions.com Transforming minimally invasive neurosurgery by enabling real-time visualization with MRI © 2016 MRI INTERVENTIONS, INC. | 23
