Attached files
| file | filename |
|---|---|
| EX-99.1 - PRESS RELEASE OF IMMUDYNE, INC., DATED OCTOBER 11, 2016, WITH RESPECT TO THE COMPANY'S PRELIMINARY FINANCIAL RESULTS FOR PERIOD ENDED SEPTEMBER 30, 2016 (FURNISHED ONLY). - LifeMD, Inc. | f8k101116ex99i_immudyne.htm |
| 8-K - CURRENT REPORT - LifeMD, Inc. | f8k101116_immudyne.htm |
Exhibit 99.2

Investor Presentation Q4 2016 (OTCQB: IMMD) www.immudyne.com

Statements in this presentation that are not descriptions of historical facts are forward - looking statements relating to future events, and as such all forward - looking statements are made pursuant to the Securities Litigation Reform Act of 1995 . Statements may contain certain forward - looking statements pertaining to future anticipated or projected plans, performance and developments, as well as other statements relating to future operations and results . Any statements in this presentation that are not statements of historical fact may be considered to be forward - looking statements . Words such as "may," "will," "expect," "believe," "anticipate," "estimate," "intends," "goal," "objective," "seek," "attempt," or variations of these or similar words, identify forward - looking statements . Market statistics contained herein are derived from management estimates based on publicly available information . These forward - looking statements by their nature are estimates of future results only and involve substantial risks and uncertainties, including but not limited to risks associated with the uncertainty of future financial results, additional financing requirements, development of new products, successful completion of the Company’s proposed restructuring, the impact of competitive products or pricing, technological changes, the effect of economic conditions and other uncertainties detailed from time to time in our reports filed with the Securities and Exchange Commission . There can be no assurance that our actual results will not differ materially from expectations and other factors more fully described in our public filings with the U . S . Securities and Exchange Commission, which can be reviewed at www . sec . gov . Safe Harbor 2

Overview ▪ ImmuDyne is a leader in the development and marketing of OTC heath and wellness products addressing large and unmet needs ▪ The company is focused on marketing its safe and efficacious products based on its proprietary immune - modulator, Beta - 1/3,1/6 - glucan ▪ Clinically shown to protect against pathogens, amplify cytotoxic agents treating cancer, and naturally improve age or immune - related epidermal conditions ▪ Validated by growing purchase orders from top tier global cosmetic companies ▪ ImmuDyne has recently in - licensed a proprietary hair loss product line, with a beta launch planned for Q4 ▪ $5M+ 2016 run - rate in direct - to - consumer sales for 2 Cosmetic SKUs (launched in December, 2015) ▪ other follow - on products coming soon 3
Corporate Highlights ▪ 391 % increase in revenue through Q3 2016 ▪ Profitable ▪ Strong cash position and conservative monthly overhead ▪ Large unmet need; $18B U.S. skincare market lacks products that ‘work’ ▪ Fast path to commercialization with new SKUs addressing cumulative $50+ billion opportunity; GRAS - status with FDA supports fast and inexpensive commercialization ▪ New data coming from two separate studies (topical, oral) could open up new niche applications, 2 new patents expected to be filed by the end of October, 2016) ▪ Beta - 1/3,1/6 - glucan has demonstrated anti - tumor activity as an adjuvant therapy with chemotherapy ($30B in sales) or monoclonal antibodies ($15B in sales) ▪ Potential applications in antibiotic resistance ($40B) advanced wound healing ($9.4B) and inflammation or auto - immune disorders ($30B) 4

What is Beta - 1/3,1/6 - glucan? ▪ Beta - 1/3,1/6 - glucan (“ β - glucan ”) is a safe and potent immune modulator derived from the cell wall of Baker’s yeast ▪ Natural polysaccharide that b inds to the dectin - 1, CR3, and TLR receptors found on immune cells, including natural killer (NK) cells, dendritic cells (DC) et al. ▪ Binding to immune cells activates the innate and adaptive immune system ▪ Over 6000 studies show β - glucan to have anti - inflammatory, antimicrobial, wound healing, weight loss, antidiabetic, cholesterol lowering and anti - cancer benefits ▪ β - glucan is regulated as a Generally Regarded As Safe (GRAS) product by the FDA 5
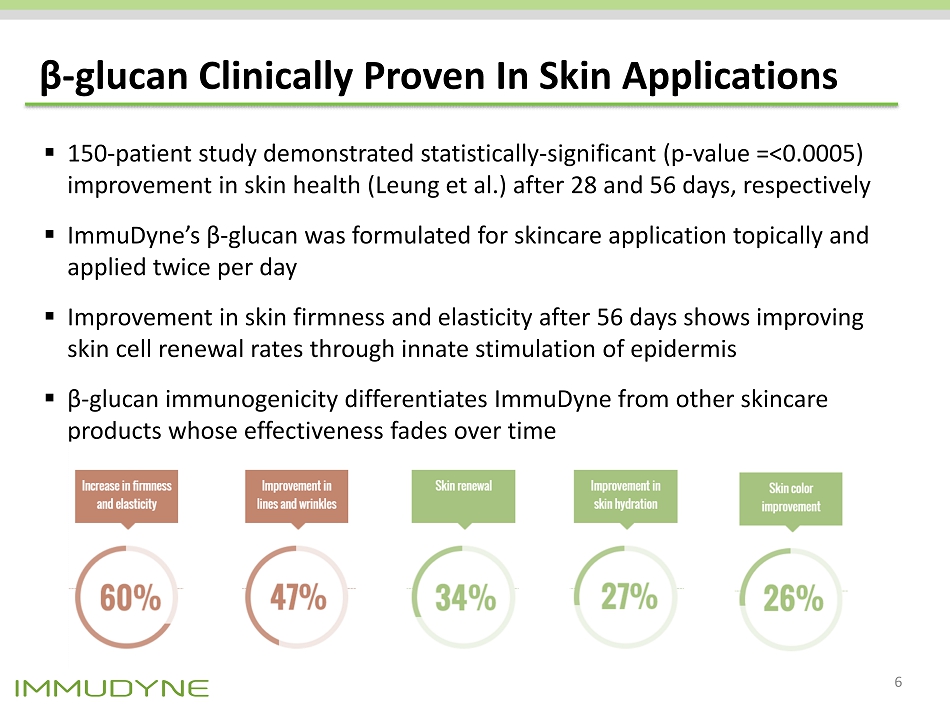
β - glucan Clinically Proven In Skin Applications ▪ 150 - patient study demonstrated statistically - significant (p - value =<0.0005) improvement in skin health (Leung et al.) after 28 and 56 days, respectively ▪ ImmuDyne’s β - glucan was formulated for skincare application topically and applied twice per day ▪ Improvement in skin firmness and elasticity after 56 days shows improving skin cell renewal rates through innate stimulation of epidermis ▪ β - glucan immunogenicity differentiates ImmuDyne from other skincare products whose effectiveness fades over time 6

β - glucan vs. Competition in $18B Skin Market ▪ Popular anti - aging creams rely on synthetic peptides to stimulate skin cells to produce collagen; the clinical benefit is usually not proven. ▪ Botulinum toxin, or Botox ($2.2B, FY’14 sales) injection made by Allergan enhances the appearance of skin by paralyzing facial muscles; considering extremely toxic and can lead to life - threatening infection. ImmuDyne stimulates the epidermis by delivering β - glucan beneath the surface of the skin with the help of a proprietary natural hydrocolloid agent (“SGM”). β - glucan stimulates the immune system to generate new skin cells, naturally, without any adverse effect. 7

Product Portfolio ImmuDyne is developing β - glucan as a natural alternative or adjuvant agent for serious, unmet medical needs in oncology, infectious disease and wound care. Our SGM delivery technology offers the potential for rapid onset action, extended - release and more potent drug activity. Name Development Phase Marketing Rights/Partner R&D Pre - Commercial Marketed Oral β - glucan for Immune Support Topical β - glucan + SGM for Skincare Oral β - glucan in Prostate Cancer Topical β - glucan in Oral Wound Care β - glucan Raw Ingredient SGM Raw Ingredient 8

ImmuDyne subsidiary Inate Scientific ▪ 39,648 trial orders as of 9/30/16 ▪ Anticipate launch of straight sale product line in Q4 of 2016 with improved margins and better customer retention ▪ 3 new products recently added to this SKU We believe that our clinically activity β - glucan products, if marketed properly, could capture significant market share in the $18B U.S. skin care market. 9

Key Scientific Publications Of β - glucan ▪ Immune - Regulating Properties. Immune - modulatory effects of dietary Yeast Beta - 1,3/1,6 - D - glucan (Nutritional Journal, Stier et al) ▪ Adjuvant For Cancer Therapy. Therapeutic potential of various beta - glucan sources in conjunction with anti - tumor monoclonal antibody in cancer therapy (Cancer Biology & Therapy, Driscoll et al.) ▪ Anti - Infective Agent. Yeast (1,3) - (1,6) - beta - glucan helps to maintain the body's defence against pathogens: a double - blind, randomized, placebo - controlled, multicentric study in healthy subjects (European Journal of Nutrition, Auinger et al.) ▪ Anti - Inflammatory & Wound Healing Activity. β(1 - 3) - D - glucan affects adipogenesis , wound healing and inflammation (Journal of Oriental Pharmacy and Experimental Medicine, Vetvicka et al.) 10

Strong Management & BOD ▪ Mark McLaughlin , President & CEO, former SVP at Oppenheimer & Co., Lehman Brothers. Led ImmuDyne's commercial build - out, and turn - around to profitable biotech company. ▪ Dr. Anthony Bruzzese , Chairman, Radiologist certified by the American Board of Internal Medicine and the American Board of Radiology. Graduated from Brown University. ▪ John Strawn, Jr. , Director, partner at Strawn Pickens LLP focused on complex commercial litigation and patent law. Graduate of University of Texas and Dartmouth College. ▪ Dr. Joseph DiTrolio , Director, Clinical Professor of Surgery, Division of Urology at New Jersey Medical School. Graduate of the University of Richmond, University of Paris, Sorbonne and New Jersey Medical School. 11

Upcoming Catalysts IMMD will see multiple value - creating milestones: ▪ Profitability expected in Fiscal 2016 ▪ Results of BIO - EC study expected to open new market in Q4 (topical application, anticipate new patent to be filed in late October, 2016 and seek path to commercialization with strategic partners ) ▪ Results of study at prominent Medical Center expected to open new market in Q4 (oral application, anticipate new patent to be filed in late October, 2016 and seek path to commercialization with strategic partners) ▪ Q4 expected launch of proprietary, patented hair loss products for men and women (direct - to - consumer) ▪ Uplisting to senior exchange planned for late 2017 12

Investment Summary ▪ Profitable & Growing. Immudyne is profitable and is anticipating triple digit sales growth in FY2016. ▪ Proprietary & Effective Product. We manufacture, sell and out - license the purest, all natural yeast beta glucan on the market. β - glucan has been proven in thousands of clinical studies to regulate the human immune system. ▪ Large Addressable Market. Skincare is a $50B annual business. We have a product that we believe can capture meaningful market share. ▪ Inate Scientific Could Be Transformational. Our JV achieved a $10M run - rate within 6 weeks of launch and we are optimistic about future revenue potential. ▪ Strong Management Team. We are led by a responsible, shareholder minded CEO with approximately 30% ownership in the Company. ▪ Fully Financed. Cash and short - term receivables of approximately $550k ; expected to be sufficient to execute on 2016 growth plan. 13
www.immudyne.com OTCQB: IMMD Investor Contact: Mark McLaughlin, CEO markmcl@immudyne.com 14
