Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aldeyra Therapeutics, Inc. | d267860dex992.htm |
| 8-K - 8-K - Aldeyra Therapeutics, Inc. | d267860d8k.htm |

A Novel Pharmaceutical Platform Focused on Inflammation and Rare Inborn Errors of Metabolism Research and Development Day September 26, 2016 Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation and various remarks which may be made during this presentation contain forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations , expenses and financing needs, business strategies and plans, research and development plans or expectations, trends, the structure , timing and success of Aldeyra’s planned or pending clinical trials, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development and clinical plans for Aldeyra’s product candidates. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Aldeyra’s Annual Report on Form 10-K for the year ended December 31, 2015 and Quarterly Report on Form 10-Q for the quarter ended June 30, 2016, which are on file with the Securities and Exchange Commission (SEC) and available on the SEC's website at www.sec.gov. Additional factors may also be set forth in Aldeyra’s Quarterly Report on Form 10-Q for the quarter ending September 30, 2016, to be filed with the SEC in the fourth quarter of 2016. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this presentation is provided only as of September 26, 2016, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.

Agenda Aldeyra R&D Highlights Todd Brady, M.D., Ph.D., CEO David Clark, M.D., CMO Aldehydes: A Novel Target in Human Disease Brian Day, Ph.D., Professor, Department of Medicine, Colorado School of Public Health Ocular Inflammation: A Steroid-Sparing Approach John Sheppard, M.D., Professor of Ophthalmology, Eastern Virginia Medical School Sjögren-Larsson Syndrome: An Inborn Error of Aldehyde Metabolism William Rizzo, M.D., Professor, Division of Inherited Metabolic Diseases, Nebraska Medical Center Summary

R&D Highlights Todd Brady, M.D., Ph.D. Chief Executive Officer, President & Director Aldeyra Therapeutics
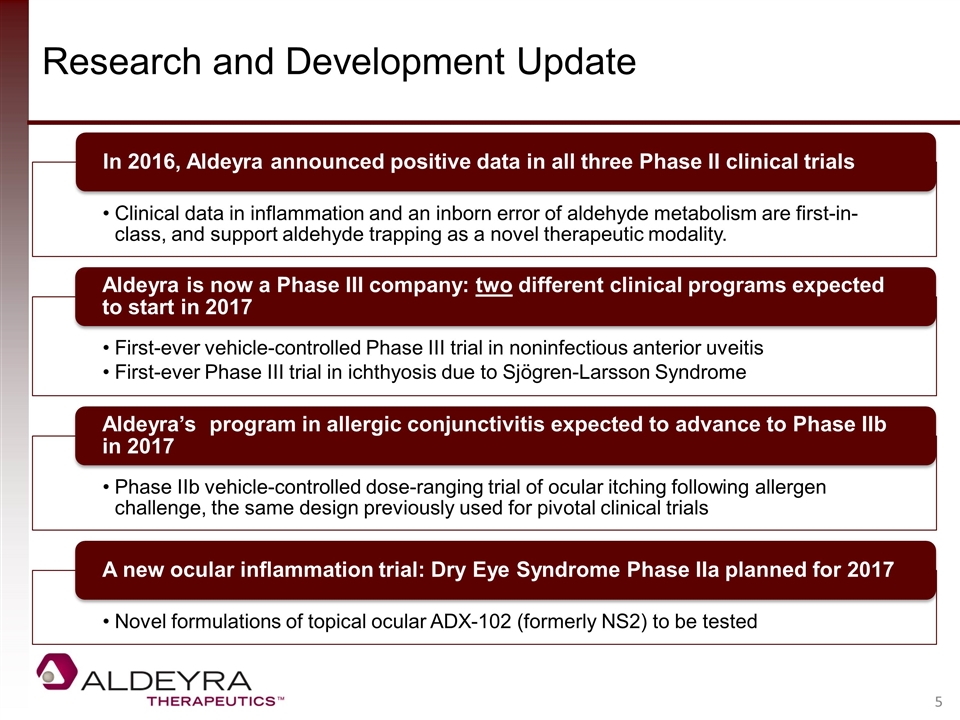
Research and Development Update Aldeyra is now a Phase III company: two different clinical programs expected to start in 2017 Aldeyra’s program in allergic conjunctivitis expected to advance to Phase IIb in 2017 Clinical data in inflammation and an inborn error of aldehyde metabolism are first-in-class, and support aldehyde trapping as a novel therapeutic modality. In 2016, Aldeyra announced positive data in all three Phase II clinical trials Phase IIb vehicle-controlled dose-ranging trial of ocular itching following allergen challenge, the same design previously used for pivotal clinical trials Novel formulations of topical ocular ADX-102 (formerly NS2) to be tested A new ocular inflammation trial: Dry Eye Syndrome Phase IIa planned for 2017 First-ever vehicle-controlled Phase III trial in noninfectious anterior uveitis First-ever Phase III trial in ichthyosis due to Sjögren-Larsson Syndrome
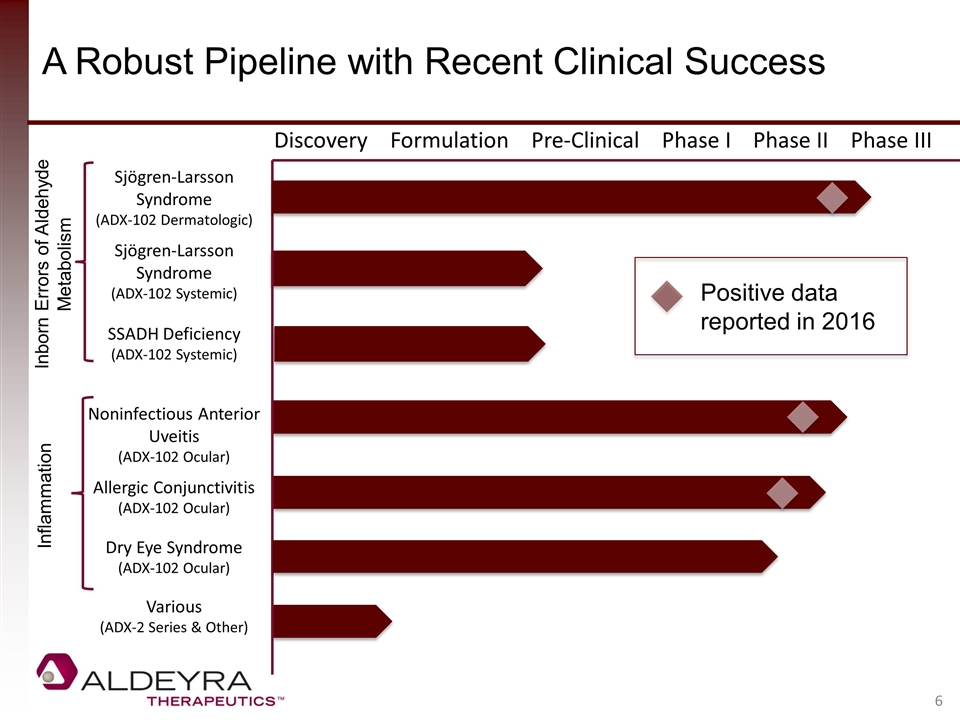
A Robust Pipeline with Recent Clinical Success Discovery Formulation Pre-Clinical Phase I Phase II Phase III Sjögren-Larsson Syndrome (ADX-102 Dermatologic) Sjögren-Larsson Syndrome (ADX-102 Systemic) SSADH Deficiency (ADX-102 Systemic) Noninfectious Anterior Uveitis (ADX-102 Ocular) Allergic Conjunctivitis (ADX-102 Ocular) Dry Eye Syndrome (ADX-102 Ocular) Various (ADX-2 Series & Other) Inflammation Inborn Errors of Aldehyde Metabolism Positive data reported in 2016
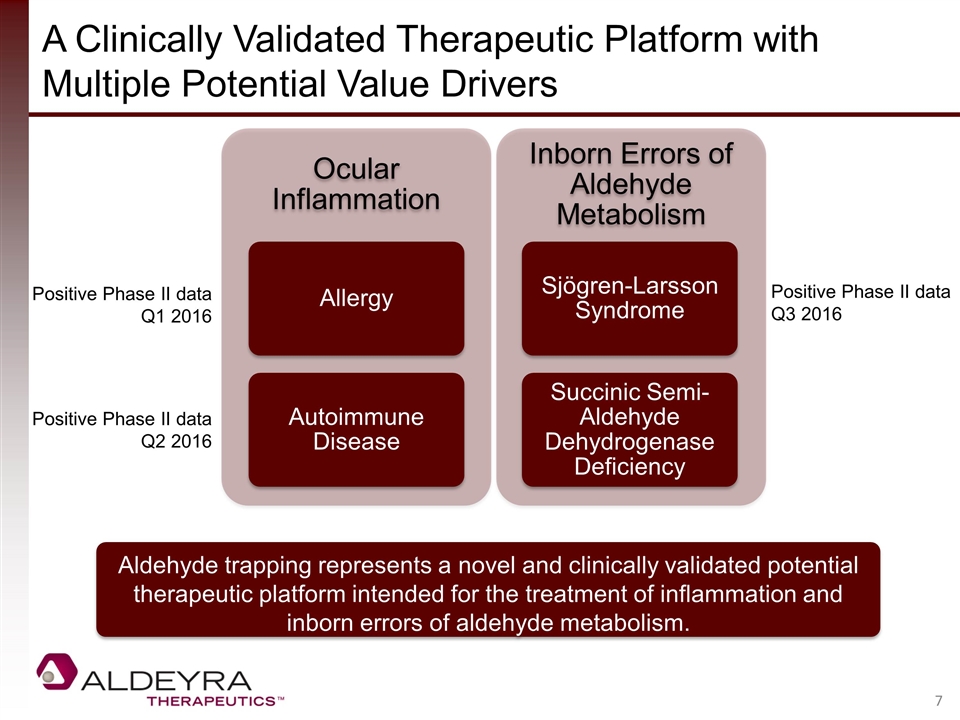
A Clinically Validated Therapeutic Platform with Multiple Potential Value Drivers Aldehyde trapping represents a novel and clinically validated potential therapeutic platform intended for the treatment of inflammation and inborn errors of aldehyde metabolism. Positive Phase II data Q1 2016 Positive Phase II data Q2 2016 Positive Phase II data Q3 2016 Ocular Inflammation Allergy Autoimmune Disease Inborn Errors of Aldehyde Metabolism Sjögren-Larsson Syndrome Succinic Semi-Aldehyde Dehydrogenase Deficiency
Review of ADX-102 Data in Ocular Inflammation David J. Clark, M.D., M.R.C.P., A.F.P.M. Chief Medical Officer Aldeyra Therapeutics
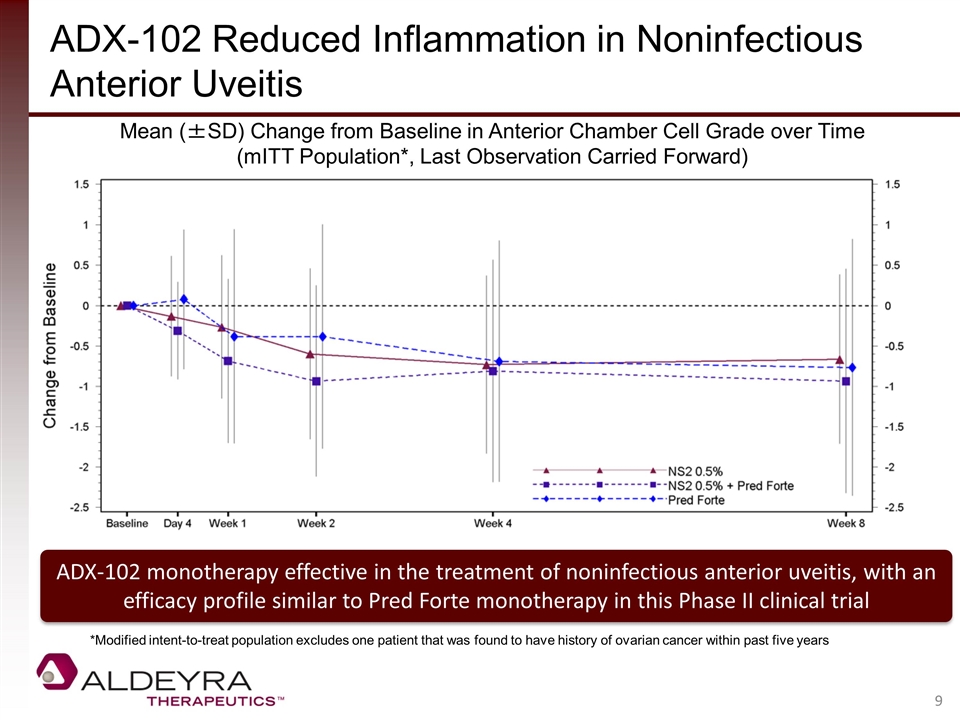
ADX-102 Reduced Inflammation in Noninfectious Anterior Uveitis Mean (±SD) Change from Baseline in Anterior Chamber Cell Grade over Time (mITT Population*, Last Observation Carried Forward) *Modified intent-to-treat population excludes one patient that was found to have history of ovarian cancer within past five years ADX-102 monotherapy effective in the treatment of noninfectious anterior uveitis, with an efficacy profile similar to Pred Forte monotherapy in this Phase II clinical trial
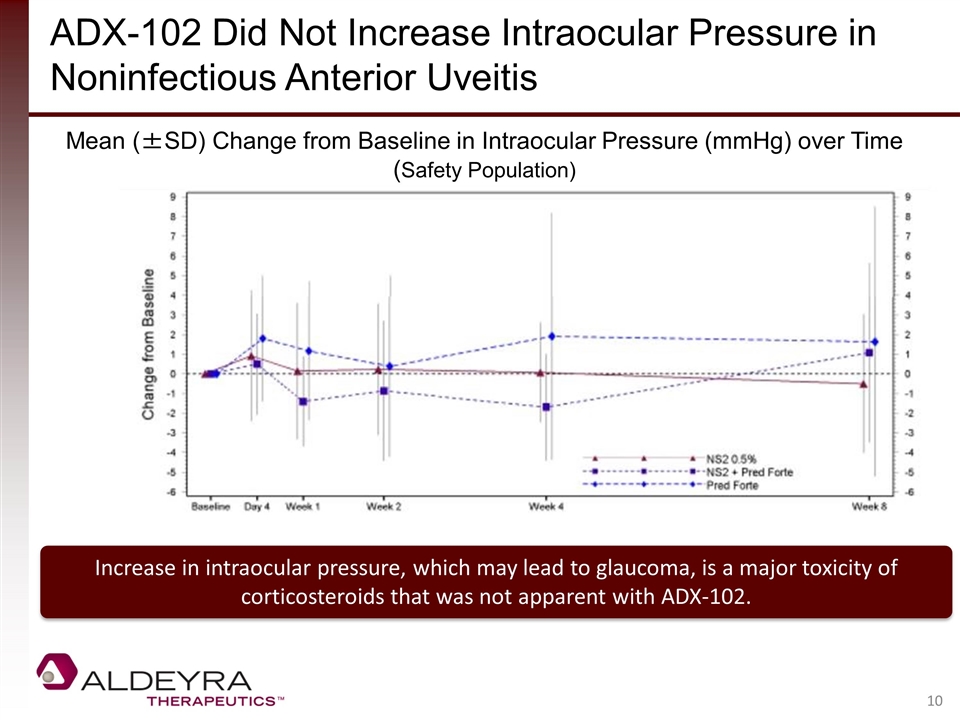
ADX-102 Did Not Increase Intraocular Pressure in Noninfectious Anterior Uveitis Increase in intraocular pressure, which may lead to glaucoma, is a major toxicity of corticosteroids that was not apparent with ADX-102. Mean (±SD) Change from Baseline in Intraocular Pressure (mmHg) over Time (Safety Population)
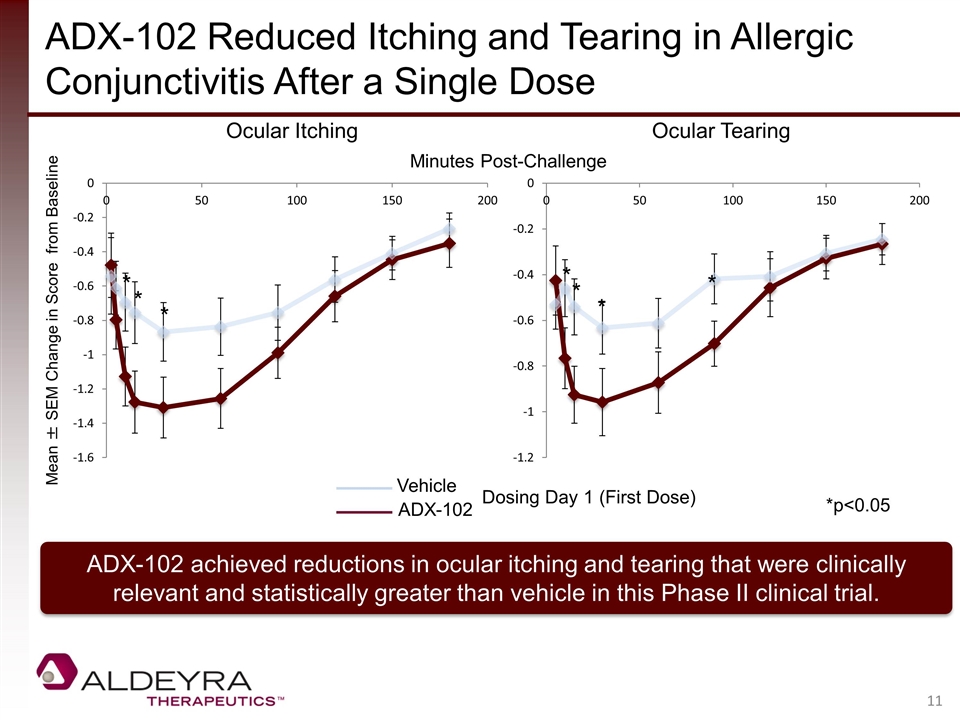
ADX-102 Reduced Itching and Tearing in Allergic Conjunctivitis After a Single Dose ADX-102 achieved reductions in ocular itching and tearing that were clinically relevant and statistically greater than vehicle in this Phase II clinical trial. Mean ± SEM Change in Score from Baseline Ocular Itching Ocular Tearing Vehicle ADX-102 Dosing Day 1 (First Dose) Minutes Post-Challenge * * * * * * * *p<0.05
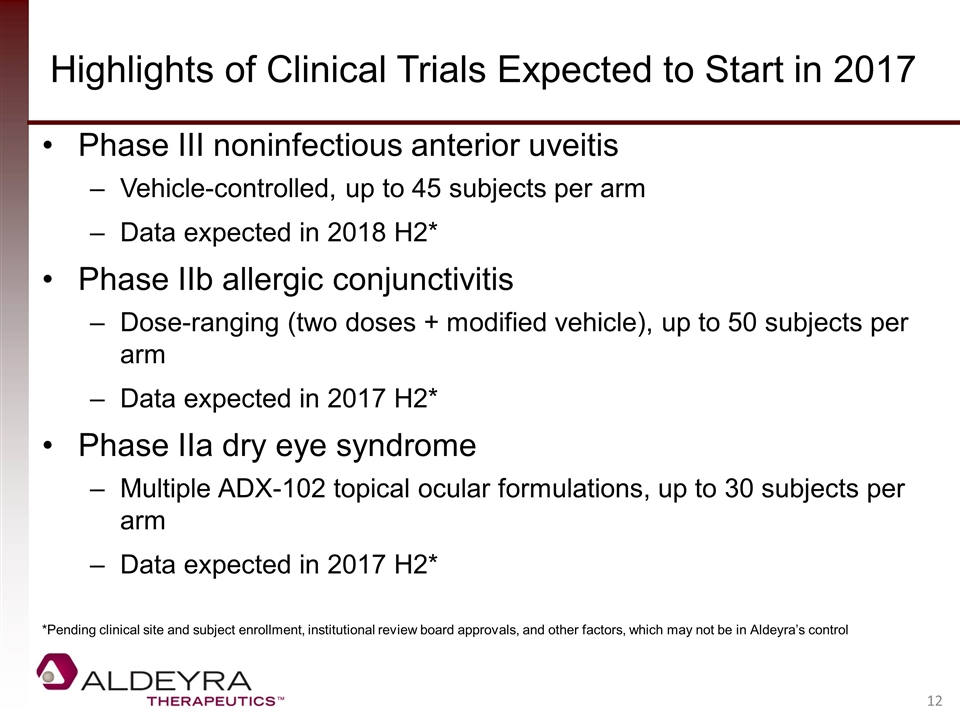
Highlights of Clinical Trials Expected to Start in 2017 Phase III noninfectious anterior uveitis Vehicle-controlled, up to 45 subjects per arm Data expected in 2018 H2* Phase IIb allergic conjunctivitis Dose-ranging (two doses + modified vehicle), up to 50 subjects per arm Data expected in 2017 H2* Phase IIa dry eye syndrome Multiple ADX-102 topical ocular formulations, up to 30 subjects per arm Data expected in 2017 H2* *Pending clinical site and subject enrollment, institutional review board approvals, and other factors, which may not be in Aldeyra’s control
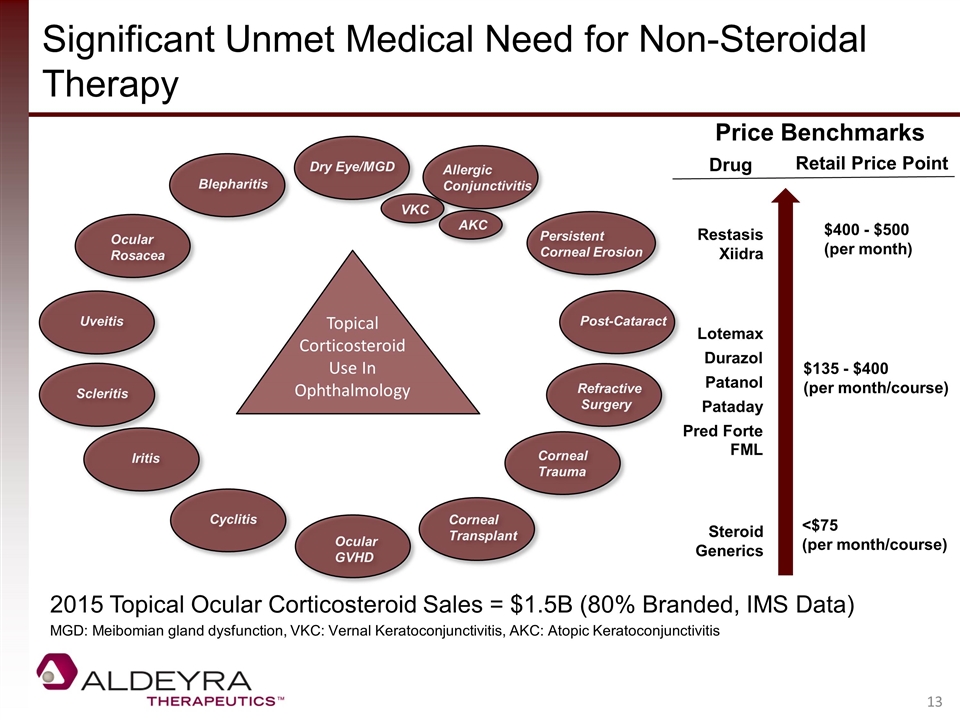
Significant Unmet Medical Need for Non-Steroidal Therapy VKC AKC Topical Corticosteroid Use In Ophthalmology Dry Eye/MGD Allergic Conjunctivitis Refractive Surgery Post-Cataract Ocular Rosacea Blepharitis Iritis Cyclitis Corneal Trauma Ocular GVHD Persistent Corneal Erosion Uveitis Scleritis Corneal Transplant Price Benchmarks Restasis Xiidra Lotemax Patanol Durazol Pred Forte FML Steroid Generics Drug Retail Price Point $400 - $500 (per month) $135 - $400 (per month/course) <$75 (per month/course) Pataday 2015 Topical Ocular Corticosteroid Sales = $1.5B (80% Branded, IMS Data) MGD: Meibomian gland dysfunction, VKC: Vernal Keratoconjunctivitis, AKC: Atopic Keratoconjunctivitis
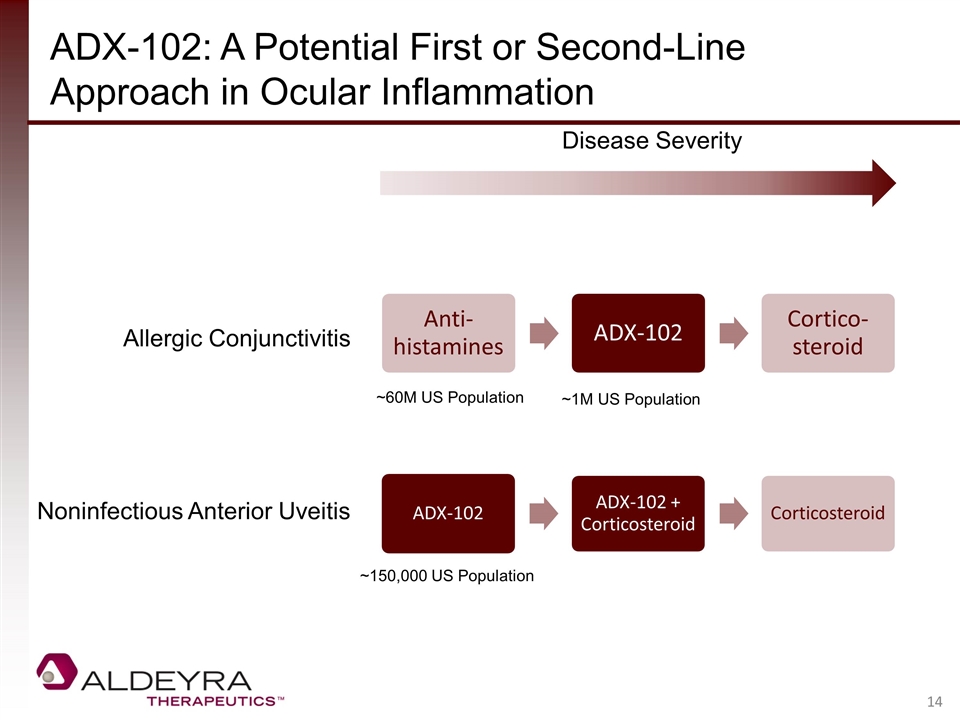
ADX-102: A Potential First or Second-Line Approach in Ocular Inflammation Disease Severity Allergic Conjunctivitis Noninfectious Anterior Uveitis ~60M US Population ~1M US Population ~150,000 US Population ADX-102 ADX-102 + Corticosteroid Corticosteroid Anti-histamines ADX-102 Cortico -steroid
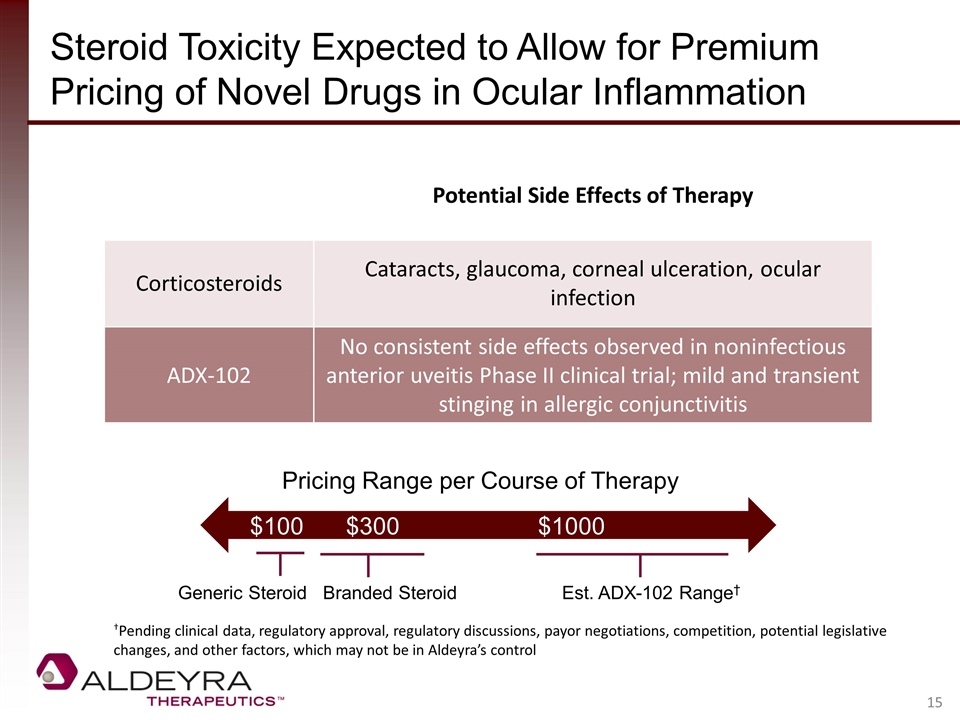
Steroid Toxicity Expected to Allow for Premium Pricing of Novel Drugs in Ocular Inflammation Generic Steroid Branded Steroid Est. ADX-102 Range† Pricing Range per Course of Therapy $100$300$1000 Potential Side Effects of Therapy Corticosteroids Cataracts, glaucoma, corneal ulceration, ocular infection ADX-102 No consistent side effects observed in noninfectious anterior uveitis Phase II clinical trial; mild and transient stinging in allergic conjunctivitis †Pending clinical data, regulatory approval, regulatory discussions, payor negotiations, competition, potential legislative changes, and other factors, which may not be in Aldeyra’s control

Review of ADX-102 Data in SLS David J. Clark, M.D., M.R.C.P., A.F.P.M. Chief Medical Officer Aldeyra Therapeutics
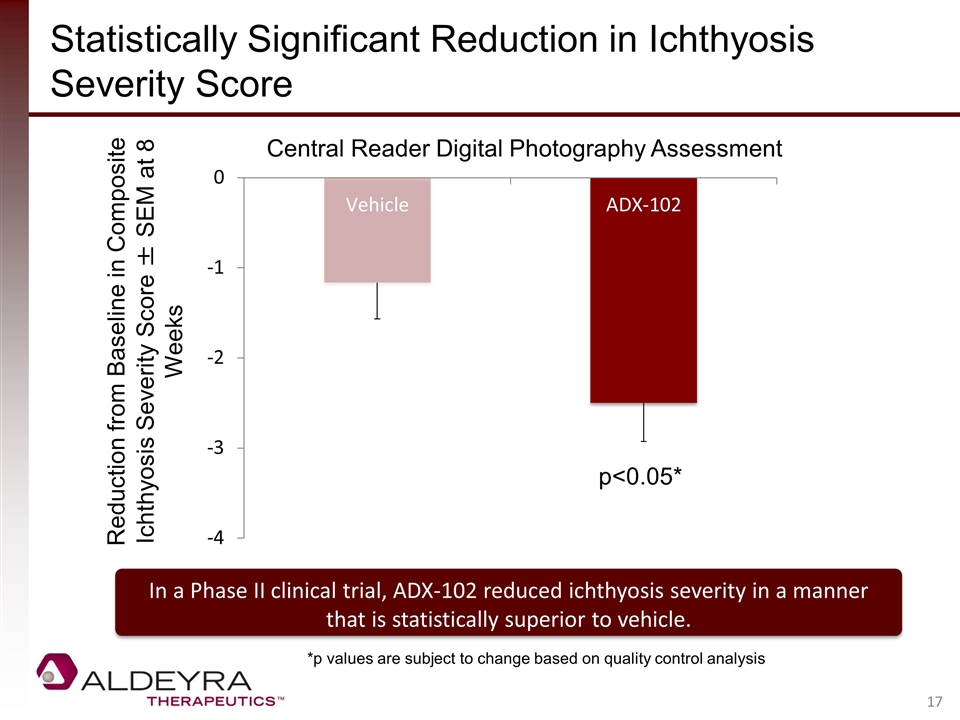
Statistically Significant Reduction in Ichthyosis Severity Score Central Reader Digital Photography Assessment Reduction from Baseline in Composite Ichthyosis Severity Score ± SEM at 8 Weeks p<0.05* *p values are subject to change based on quality control analysis In a Phase II clinical trial, ADX-102 reduced ichthyosis severity in a manner that is statistically superior to vehicle.
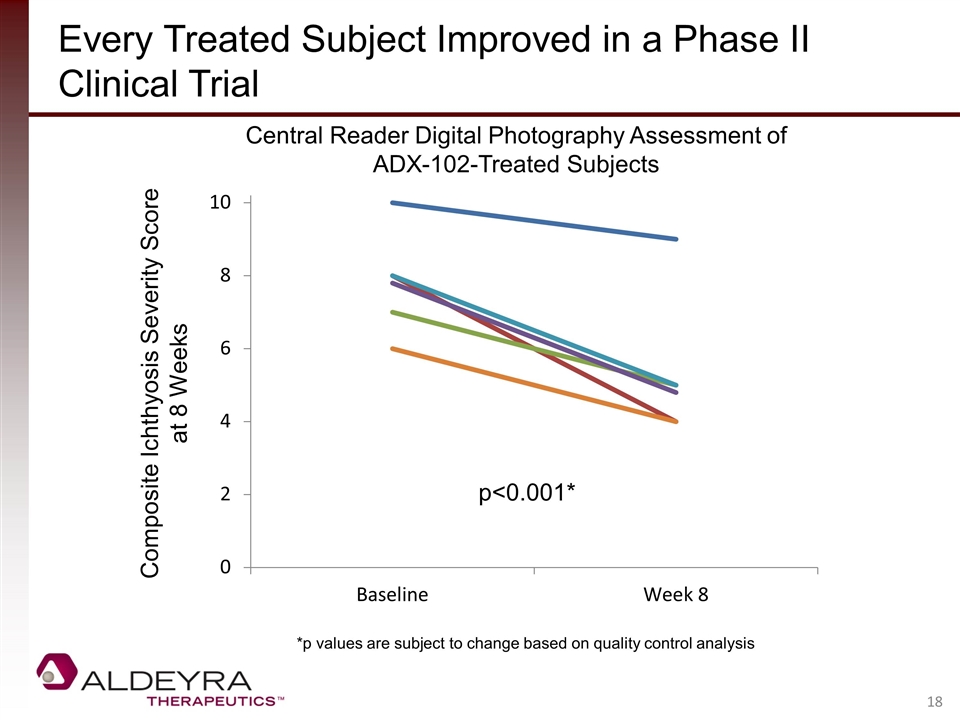
Every Treated Subject Improved in a Phase II Clinical Trial Central Reader Digital Photography Assessment of ADX-102-Treated Subjects Composite Ichthyosis Severity Score at 8 Weeks p<0.001* *p values are subject to change based on quality control analysis

Clinical Development Plans* Phase III Sjögren-Larsson Syndrome Dermatologic, Expected initiation 2017 H1 Pivotal design pending FDA/EMA feedback, estimated 20-30 subjects ADX-102 systemic lead oral formulation selected; IND filing planned for 2017 H2 Inborn errors of aldehyde metabolism: Sjögren-Larsson Syndrome and Succinic Semi-aldehyde Dehydrogenase Deficiency Biomarker-based Phase IIa clinical trials Estimated 5-10 subjects per trial Initiation expected in 2018 H1 *Pending regulatory agency discussions, additional pre-clinical data, funding, and other factors, which may not be in Aldeyra’s control
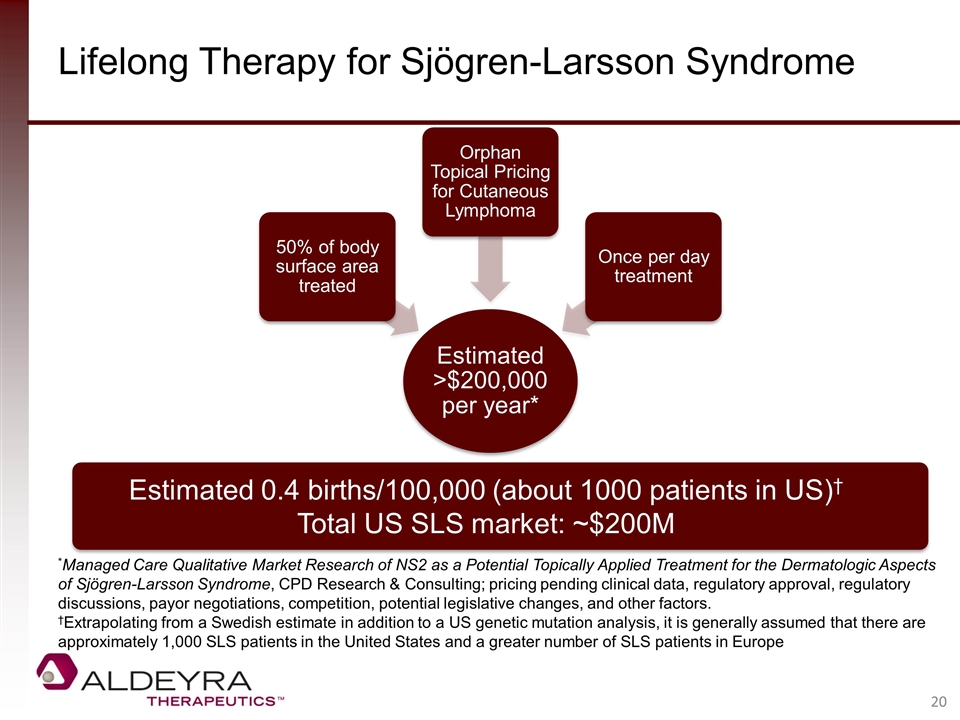
Lifelong Therapy for Sjögren-Larsson Syndrome Estimated 0.4 births/100,000 (about 1000 patients in US)† Total US SLS market: ~$200M *Managed Care Qualitative Market Research of NS2 as a Potential Topically Applied Treatment for the Dermatologic Aspects of Sjögren-Larsson Syndrome, CPD Research & Consulting; pricing pending clinical data, regulatory approval, regulatory discussions, payor negotiations, competition, potential legislative changes, and other factors. †Extrapolating from a Swedish estimate in addition to a US genetic mutation analysis, it is generally assumed that there are approximately 1,000 SLS patients in the United States and a greater number of SLS patients in Europe Estimated >$200,000 per year* 50% of body surface area treated Orphan Topical Pricing for Cutaneous Lymphoma Once per day treatment

Summary Todd Brady, M.D., Ph.D. Chief Executive Officer, President & Director Aldeyra Therapeutics
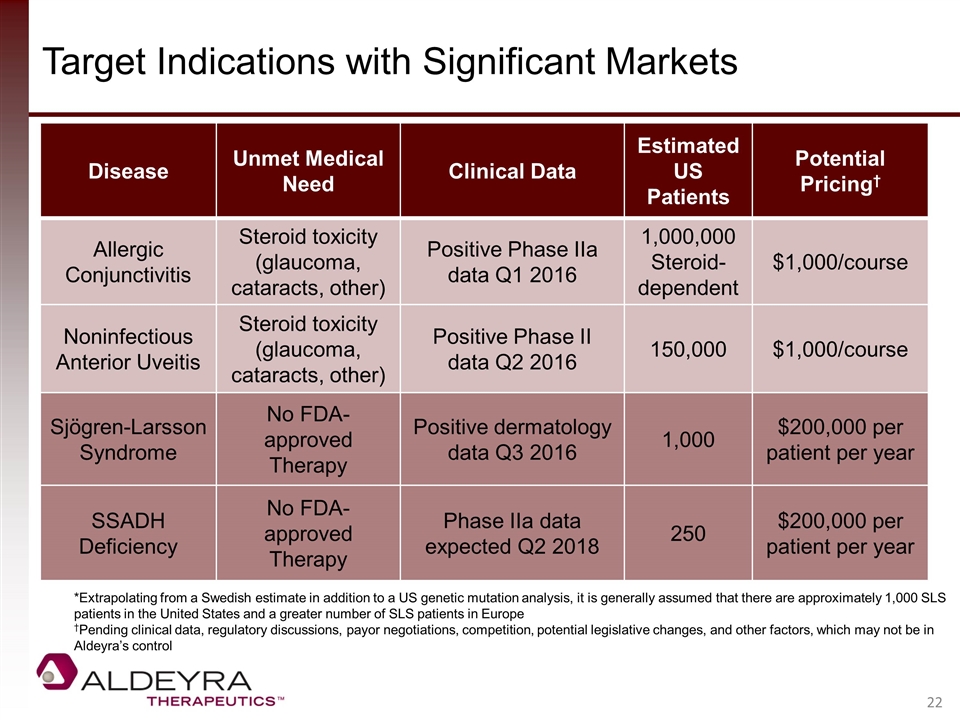
Target Indications with Significant Markets Disease Unmet Medical Need Clinical Data Estimated US Patients Potential Pricing† Allergic Conjunctivitis Steroid toxicity (glaucoma, cataracts, other) Positive Phase IIa data Q1 2016 1,000,000 Steroid-dependent $1,000/course Noninfectious Anterior Uveitis Steroid toxicity (glaucoma, cataracts, other) Positive Phase II data Q2 2016 150,000 $1,000/course Sjögren-Larsson Syndrome No FDA-approved Therapy Positive dermatology data Q3 2016 1,000 $200,000 per patient per year SSADH Deficiency No FDA-approved Therapy Phase IIa data expected Q2 2018 250 $200,000 per patient per year *Extrapolating from a Swedish estimate in addition to a US genetic mutation analysis, it is generally assumed that there are approximately 1,000 SLS patients in the United States and a greater number of SLS patients in Europe †Pending clinical data, regulatory discussions, payor negotiations, competition, potential legislative changes, and other factors, which may not be in Aldeyra’s control
Research and Development Summary Aldeyra is now a Phase III company: two different clinical programs expected to start in 2017 Aldeyra’s program in allergic conjunctivitis expected to advance to Phase IIb in 2017 Clinical data in inflammation and an inborn error of aldehyde metabolism are first-in-class, and support aldehyde trapping as a novel therapeutic modality. In 2016, Aldeyra announced positive data in all three Phase II clinical trials Phase IIb vehicle-controlled dose-ranging trial of ocular itching following allergen challenge, the same design previously used for pivotal clinical trials Novel formulations of topical ocular ADX-102 (formerly NS2) to be tested A new ocular inflammation trial: Dry Eye Syndrome Phase IIa planned for 2017 First-ever vehicle-controlled Phase III trial in noninfectious anterior uveitis First-ever Phase III trial in ichthyosis due to Sjögren -Larsson Syndrome

Questions
