Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AERIE PHARMACEUTICALS INC | d437092d8k.htm |
 Company Overview September 21, 2016 Exhibit 99.1 |
 2 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our product candidates
being approved by regulatory authorities. In addition, any discussion of clinical trial
results for Rhopressa™ (netarsudil ophthalmic solution) 0.02% relate
to the results in its first Phase 3 registration trials, Rocket 1 and Rocket 2, and for Roclatan™ (netarsudil/latanoprost ophthalmic solution) 0.02%/0.005% relate to the results in its Phase 3 registration trial.
The information in this presentation is current only as of its date and may have
changed or may change in the future. We undertake no obligation to update
this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete.
Certain statements in this presentation, including the updated guidance presented
herein, are “forward-looking statements” within the meaning
of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,”
“projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and
expectations. Known and unknown risks, uncertainties and other factors could cause
actual results to differ materially from those contemplated by the
statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. In particular, the topline Mercury 1
data presented herein is preliminary and based solely on information available to us as
of the date of this document and additional information about the results
may be disclosed at any time, including at our Investor Day on October 5, 2016. In addition, the preclinical research discussed in this presentation is preliminary and the outcome of such preclinical studies
may not be predictive of the outcome of later trials. Any future clinical trial results
may not demonstrate safety and efficacy sufficient to obtain regulatory
approval related to the preclinical research findings discussed in this presentation. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in
the sections titled “Risk Factors” and “Management’s Discussion and
Analysis of Financial Condition and Results of Operations.” Such
forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise,
except as otherwise required by law. |
 3 Aerie Late Stage IOP–Lowering Products Pre-Clinical Research • Rhopressa™ • Potential for disease modification and neuroprotection
• AR-13154 • Significant lesion size reduction in wet AMD • Drug Delivery
- Front and back of the eye • Rhopressa™ (netarsudil ophthalmic solution) 0.02%
• Inhibits ROCK, NET, lowers EVP, targets diseased tissue • NDA filed Q3 2016 • Roclatan™ (netarsudil/latanoprost ophthalmic solution)
0.02%/0.005% •
Fixed combination of Rhopressa™ and latanoprost
• Two P3’s in process; first P3 achieved primary efficacy endpoint Aerie – Building a Major Ophthalmic Pharmaceutical Company Data on file |
 FY 2015
U.S. Glaucoma Market = $2.5B; 34M TRx Market Share in TRx
PGA Market Non-PGA Market 2015 US GLAUCOMA MARKET Once Daily 2-3 Times Daily Bimatoprost Travoprost Latanoprost BB Fixed Combo AA CAI PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor
Source: IMS MIDAS. IMS NPA
13% 15% 8% 10% 9% 35% 4 8% |
 5 Rhopressa™ and Roclatan™ Expected Aerie Market
Positioning Commercialization
Strategy – Rhopressa TM Now Entering Launch Mode North America : ~100 sales reps targeting ~10,000 high prescribers Europe and Japan: Currently exploring opportunities Rhopressa™ Roclatan™ Potential drug of choice as adjunctive therapy to PGAs when additional IOP lowering is desired Potential drug of choice for patients requiring maximal IOP lowering |
 6 Rhopressa™ Trials for Q3 2016 NDA Submission “Rocket 1” 90-Day Efficacy Registration Trial U.S. Rhopressa™ 0.02% QD ~200 patients timolol BID ~200 patients (Total enrollment: 411 patients) “Rocket 2” One Year Safety (3 Mo. Interim Efficacy) Registration Trial U.S. Rhopressa™ 0.02% QD ~230 patients Rhopressa™ 0.02% BID ~230 patients timolol BID ~230 patients (Total enrollment: 756 patients) ClinicalTrials.gov Identifier: NCT02207491, NCT02207621 |
 7 Rhopressa™ Trials for EU Filing; Not Needed for NDA Filing ClinicalTrials.gov Identifier: NCT02246764, NCT02558374 “Rocket 4” 3 Month Efficacy 6 Month Safety Rhopressa™ 0.02% QD ~350 patients timolol BID ~350 patients “Rocket 3” One Year Safety Trial Canada Rhopressa™ 0.02% QD ~90 patients Rhopressa™ 0.02% BID ~90 patients timolol BID ~60 patients |
 8 Rocket 2 and Rocket 1 Performance Maximum Baseline IOP <25 mmHg Rocket 2 Rocket 1 Data on file |
 9 Rocket 2: 12 Month 8am IOP Efficacy Safety Population, Completed Patients (<27 mmHg) Difference in 8am mean IOP from Day 90 (Month 3) to Month 12: Rhopressa TM QD: Full population: +0.1 mmHg Subset analyses: -0.3 to +0.3 mmHg Timolol BID: Full population: +0.5 mmHg Subset analyses: +0.1 to +1.4 mmHg Day 90 Month 12 AR-13324 0.02% q.d. (n=118) BL W2 W6 M3 M6 M9 M12 Data on file: AR-13324 – CS302 |
 10 Rocket 2: Safety/Tolerability Overview of Rhopressa TM QD (Interim 12-Month) * Incidence of conjunctival hyperemia ~50% including baseline at ~20% ** The updated term for these deposits based on recent MedDRA coding revisions There were no drug-related serious adverse events (SAEs) No new adverse events introduced over the twelve-month period The most common adverse event was conjunctival hyperemia with ~50% incidence*, the majority mild Other ocular AEs AEs occurring in ~5-23% of patients included: conjunctival hemorrhage, corneal deposits (verticillata**), blurry vision and reduced visual acuity |
 11 Rhopressa Adverse Events Summary Data on File: AR-13324-CS302 * The updated term for these deposits based on MedDRA coding revisions Hyperemia – absent or sporadic for 90% of
patients
- only 10% of patients had hyperemia AE at all 6 study Conjunctival Hemorrhage – sporadic subconjunctival petechiae - none noted at month 12 visit Corneal Deposits (verticillata*) – asymptomatic non-toxic lipid deposits - high resolution rate Visual Acuity Reduced – sporadic, mostly single visit, only one eye - incidence reduced over time Vision Blurred – sporadic and significantly reduced over 12 months TM visits |
 12 When Present, 80% of Rhopressa QD Hyperemia Graded as Mild Grade Image Description 0 None/Normal 1 Mild 2 Moderate 3 Severe Data on File: AR-13324 – CS302 For illustrative purposes only TM |
 13 Conjunctival Hemorrhage Using Biomicroscopy Evaluation • Subconjunctival petechiae seen sporadically in Rhopressa Rocket studies • MedDRA coded to conjunctival hemorrhage • No conjunctival hemorrhages noted at month 12 visit Data on File: An example of conjunctival hemorrhage from AR-13324 – CS302 TM |
 14 Aerie Perspective on Potential Rhopressa Advantages *Data on file: AR-13324 – CS301 and AR-13324 – CS302 **Anti-fibrotic Effects of AR-13324 in a 3D Bioengineered Human Trabecular Meshwork Model.
Cheng-Wen Lin, Feryan
Ahmed, Dhruba Bharali, Karen Torrejon, Casey Kopczynski; The Mechanisms of Increasing Outflow Facility by AR-13324M in Human
Eyes. Haiyan Gong, Ruiyi
Ren, Guorong Li, Casey Kopczynski, Daniel Stamer; and Visualization of conventional outflow tissue responses to netarsudil
in living mouse eyes. Li G, Mukherjee D, Navarro I, Ashpole NE, Sherwood JM, Chang J, Overby DR, Yuan F, Gonzalez P, Kopczynski CC, Farsiu S, Stamer WD. Eur J Pharmacol. 2016. 787:20-31. ***Topical administration of a Rock/Net inhibitor promotes retinal ganglion cell survival and axon regeneration
after optic nerve injury. Shaw PX, Sang A, Wang Y, Ho D, Douglas C,
Dia L, Goldberg JL.
Exp Eye Res. 2016 Jul 18. pii: S0014-4835(16)30181-6. [Epub ahead of print] Clinical*: Demonstrated once-daily IOP lowering in Phase 3 trials Three mechanisms of action Potential PGA synergy More consistent IOP-lowering across baselines than PGAs and timolol No systemic side effects Research: Targets diseased trabecular meshwork in glaucoma Potential to preserve health of trabecular outflow pathway** Potential to promote retinal ganglion cell survival and axon regeneration*** TM |
 15 Roclatan™ Registration Trial Design “Mercury 1”* One Year Safety (3 Mo. Interim Efficacy) Registration Trial U.S. “Mercury 2”** 90-Day Efficacy Registration Trial U.S. and Canada “Mercury 3” 6 Mo. Efficacy and Safety Registration Trial Europe Roclatan™ QD ~230 patients Rhopressa™ 0.02% QD ~230 patients latanoprost QD ~230 patients Roclatan™ QD ~230 patients Comparator (TBD) ~230 patients * ClinicalTrials.gov Identifier: NCT02558400 ** ClinicalTrials.gov Identifier: NCT02674854 Roclatan™ QD ~230 patients Rhopressa™ 0.02% QD ~230 patients latanoprost QD ~230 patients |
 16 • Roclatan TM met the criteria for demonstrating superiority over both latanoprost and Rhopressa TM for the primary efficacy analysis – statistical superiority of Roclatan™ was demonstrated at all 9 time points versus latanoprost and versus Rhopressa™ (p<0.0001) • IOP-lowering effect of Roclatan™ was greater (1-3 mmHg) than monotherapy with either latanoprost or Rhopressa™ throughout the duration of the study (i.e., Week 2, Week 6, Month 3) • Roclatan TM reduced mean diurnal IOPs to 16 mmHg or lower in 61 percent of patients, a significantly higher percentage than observed in the comparator arms • The main adverse event for Roclatan TM was conjunctival hyperemia, which was reported in ~50% of patients and was scored as mild for ~80% of these patients • There were no drug-related serious adverse events and no evidence of treatment-related systemic effects Mercury 1: Roclatan TM Achieves Primary Clinical Endpoint Data on file and PG324 - CS301 Top Line Data |
 17 Mercury 1: Study Design And Analysis • Trial design follows FDA requirement for fixed dose combination – Superiority of combination over each individual component – Statistically significant difference at each measured time point – Higher combo efficacy vs. components at ~1-3 mmHg, as previously accepted by FDA for product approval (Simbrinza ® ) • The primary statistical modeling of the primary efficacy analysis was agreed with the FDA using the intent to treat (ITT) population with imputation for any missing data Data on file |
 18 Roclatan TM Achieved Statistical Superiority Over Individual Components at All Time Points Mean IOP at Each Time Point (ITT) Data on file and PG324 - CS301 Top Line Data ***p<0.0001 vs Latanoprost and Rhopressa TM |
  19 Mercury 1: Roclatan TM Phase 3, ITT Roclatan TM superior to latanoprost by 1.3–2.5 mmHg (p<0.0001) Roclatan TM superior to Rhopressa TM by 1.8-3.0 mmHg (p<0.0001) 8:00 AM 24.8 24.8 24.6 10:00 AM 23.7 23.5 23.4 4:00 PM 22.6 22.6 22.4 Mean Diurnal 23.7 23.6 23.5 8:00 AM 15.6 18.6 17.8 -3.0 (-3.6, -2.5) -2.2 (-2.8, -1.7) 10:00 AM 14.9 17.8 17.4 -2.9 (-3.5, -2.3) -2.5 (-3.1, -1.9) 4:00 PM 14.8 17.2 17.2 -2.4 (-2.9, -1.9) -2.3 (-2.9, -1.8) Mean Diurnal 15.1 17.9 17.5 -2.8 (-3.3, -2.3) -2.4 (-2.9, -1.9) 8:00 AM 16.0 19.0 17.7 -3.0 (-3.6, -2.4) -1.7 (-2.3, -1.1) 10:00 AM 15.3 18.0 17.1 -2.7 (-3.3, -2.2) -1.9 (-2.5, -1.3) 4:00 PM 15.3 17.5 17.0 -2.2 (-2.7, -1.6) -1.7 (-2.2, -1.1) Mean Diurnal 15.5 18.2 17.3 -2.7 (-3.1, -2.2) -1.8 (-2.3, -1.3) 8:00 AM 16.2 18.9 17.6 -2.7 (-3.4, -2.1) -1.5 (-2.1, -0.9) 10:00 AM 15.3 18.2 16.9 -2.9 (-3.5, -2.3) -1.6 (-2.2, -1.0) 4:00 PM 15.4 17.2 16.7 -1.8 (-2.4, -1.2) -1.3 (-2.0, -0.7) Mean Diurnal 15.6 18.1 17.1 -2.5 (-3.0, -2.0) -1.5 (-2.0, -1.0) Day 90 Mean IOP mmHg Rhopressa™ Day 43 Day 15 Latanoprost Difference from Roclatan™ (95% CI) Roclatan™ N=238 Rhopressa™ N=244 Latanoprost N=236 Baseline Data on file and PG324 - CS301 Top Line Data |
 20 Mercury 1: Roclatan TM Phase 3 Responder Analysis Day 90: % of Patients with IOP Reduced to 18 mmHg or Lower ***p<0.0001 vs Latanoprost and Rhopressa TM ### p<0.0001 vs Rhopressa TM , p<0.05 vs Latanoprost Data on file and PG324 - CS301 Top Line Data 14% 23% 32% 42% 54% 15% 25% 39% 54% 69% 33% 44% 61% 71% 82% Rhopressa TM (n=198) Latanoprost (n=223) Roclatan TM (n=200) IOP on Treatment |
 21 Mercury 1: Safety/Tolerability Overview of Roclatan TM • There were no drug-related serious adverse events (SAEs) • There was no evidence of treatment-related systemic effects • The most common adverse event was conjunctival hyperemia with ~50% incidence*, ~80% mild on biomicroscopy • Other ocular AEs – AEs occurring in ~5-11% of subjects receiving Roclatan included: conjunctival hemorrhage, eye pruritus, lacrimation increased and cornea verticillata. * Incidence of conjunctival hyperemia ~50% including baseline at ~20% Data on file and PG324 - CS301 Top Line Data TM |
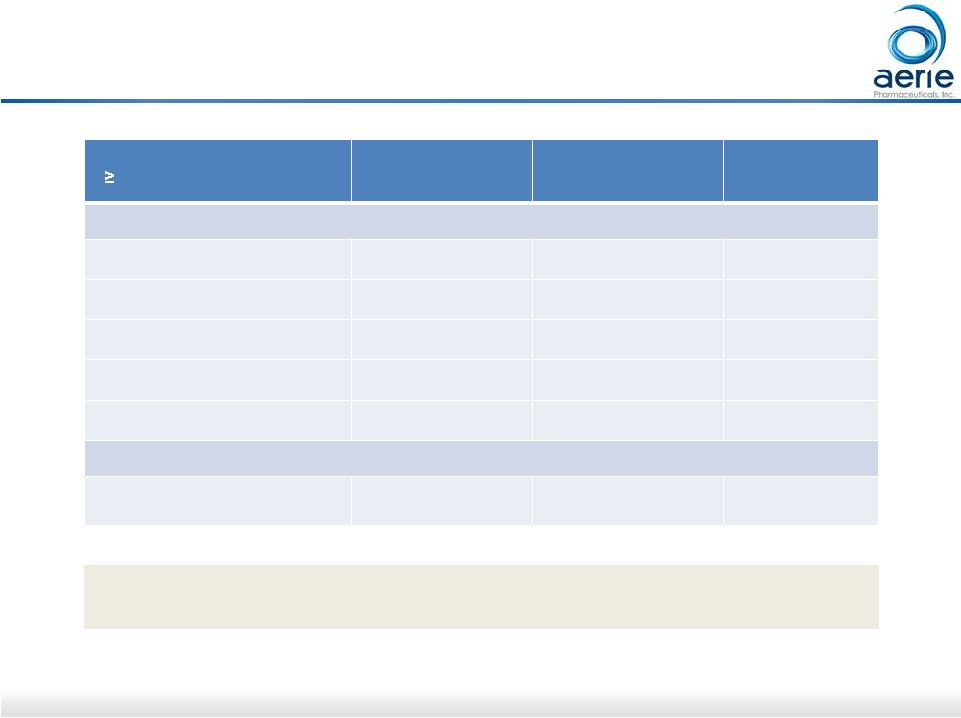 22 Mercury 1: Roclatan TM Phase 3 Safety Profile Adverse Events
( 5.0% in any group) Roclatan n=238 Rhopressa n=244 Latanoprost n=236 Eye Disorders Conjunctival Hyperemia 126 (52.9%) 99 (40.6%) 33 (14.0%) Conjunctival Hemorrhage 25 (10.5%) 34 (13.9%) 1 ( 0.4%) Eye Pruritus 18 ( 7.6%) 17 ( 7.0%) 3 ( 1.3%) Lacrimation Increased 14 ( 5.9%) 15 ( 6.1%) 1 ( 0.4%) Cornea Verticillata 12 ( 5.0%) 9 ( 3.7%) 0 (0.0%) Administration Site Conditions Instillation site pain 45 (18.9%) 51 ( 20.9%) 15 ( 6.4%) Patients with known contraindications or hypersensitivity to latanoprost were excluded Data on file and PG324 - CS301 Top Line Data TM TM |
 23 Mercury 1: Roclatan™ Summary • Demonstrated superiority over both latanoprost and Rhopressa™ for the primary efficacy analysis at all 9 time points (p<0.0001) • IOP-lowering effect was greater (1-3 mmHg) than monotherapy with either latanoprost or Rhopressa™ throughout the duration of the study • There were no drug-related serious adverse events and was no evidence of treatment-related systemic effects • The main adverse event was conjunctival hyperemia, ~50% of patients and was scored as mild for ~80% of these patients Next Steps: • Mercury 2: Topline 3-month expected H1 2017 • Mercury 3 (Europe): Efficacy study, comparing to a leading combo product marketed in Europe, expected to commence in H1 2017 • Roclatan NDA filing expected near year-end 2017 Aerie will present additional details at Investor Day in NYC, Oct 5 Data on file and PG324 - CS301 Top Line Data TM |
 24 Mercury 1: Rhopressa™ Arm Performance Summary • Demonstrated non-inferiority to latanoprost in patients with baseline IOP < 25 mmHg • Efficacy relative to latanoprost improves as baseline IOP declines • Rhopressa maintained consistent IOP lowering across all baseline IOPs including > 25 mmHg • Stable efficacy from week 2 to month 3 • Adverse event profile consistent with previous studies Data on file and PG324 - CS301 Top Line Data TM |
 25 Expanding Aerie Franchise to Europe and Japan Europe Current clinical plan expected to satisfy EU regulatory requirements (including Rocket 4 for Rhopressa and Mercury 3 for Roclatan ) Establishing KOL relationships in top 5 countries Targeting completion of EU commercialization strategy by YE16 Expect to file EU MAA for Rhopressa™ by approximately mid-2017 Japan Discussions regarding potential Rhopressa™ out-licensing are ongoing Also preparing to advance clinical development on our own Conferring with PMDA to confirm path to approval Prepared to secure a CRO relationship in 2016 to conduct trials TM TM |
 26 Enhancing the Aerie Pipeline Rhopressa preclinical findings* Disease modification potential – anti-fibrotic and increased perfusion Neuroprotection potential From our proprietary molecule library – preclinical AR-13154 for wet AMD and diabetic retinopathy Drug Delivery opportunities – front of the eye (Glaucoma) and back of the eye (e.g., AR-13154 for wet AMD) * Data on file TM |
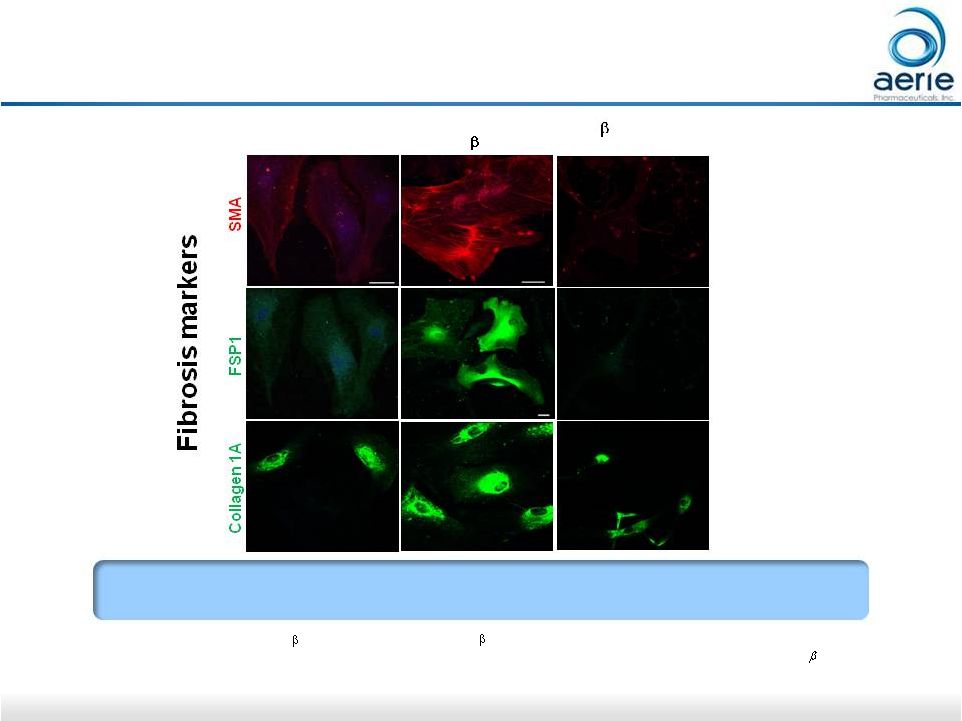 27 * Active ingredient of Rhopressa™; TGF 2: Transforming growth factor 2; SMA: Smooth muscle actin; FSP1: Fibroblast-specific protein 1 • Pattabiraman, Padmanabhan P., et. Al., (2015 March) “Effects of Rho Kinase inhibitor AR-13324 on actin cytoskeleton and TGF 2 and CTGF- induced fibrogenic activity in Human Trabecular Meshwork Cells”. AR-13324* May Have Anti-Fibrotic Activity in Human Trabecular Meshwork Cells P. Vasantha Rao, Duke University Control TGF 2 (8ng/ml) TGF 2 (8 ng/ml) + AR-13324 (500nM) AR-13324 Blocks TGF-beta-Induced Expression of Fibrosis Proteins in Human TM Cells |
 28 Netarsudil* Causes Expansion of TM Tissue, Opening Spaces for Increased Outflow Dan Stamer (Duke), Haiyan Gong (Boston University) Control + Netarsudil TM: Trabecular Meshwork SC: Schlemm’s Canal Control = buffered saline solution Increasing Trabecular Outflow, Reducing Fibrosis Could Stop Degeneration of Outflow Tissues in Glaucoma *Active ingredient of Rhopressa™ Source: The Mechanisms of Increasing Outflow Facility by AR-13324M in Human Eyes. Haiyan Gong, Ruiyi Ren, Guorong Li, Casey Kopczynski, Daniel Stamer |
 29 Rhopressa TM Promotes RGC Survival and Axon Regeneration Following Optic Nerve Injury Jeffrey Goldberg (Stanford University)* RGC Survival Retina Location Rat optic nerve crush model *Topical administration of a Rock/Net inhibitor promotes retinal ganglion cell survival and axon regeneration after optic nerve injury.
Shaw PX, Sang A, Wang Y, Ho D, Douglas C, Dia L, Goldberg JL. Exp Eye Res. 2016 Jul 18. pii: S0014-4835(16)30181-6. [Epub ahead of print] AR-13324 (n=3) Placebo (n=3) Axon Regeneration Distance from crush site ( m) AR-13324 (n=3) Placebo (n=3) |
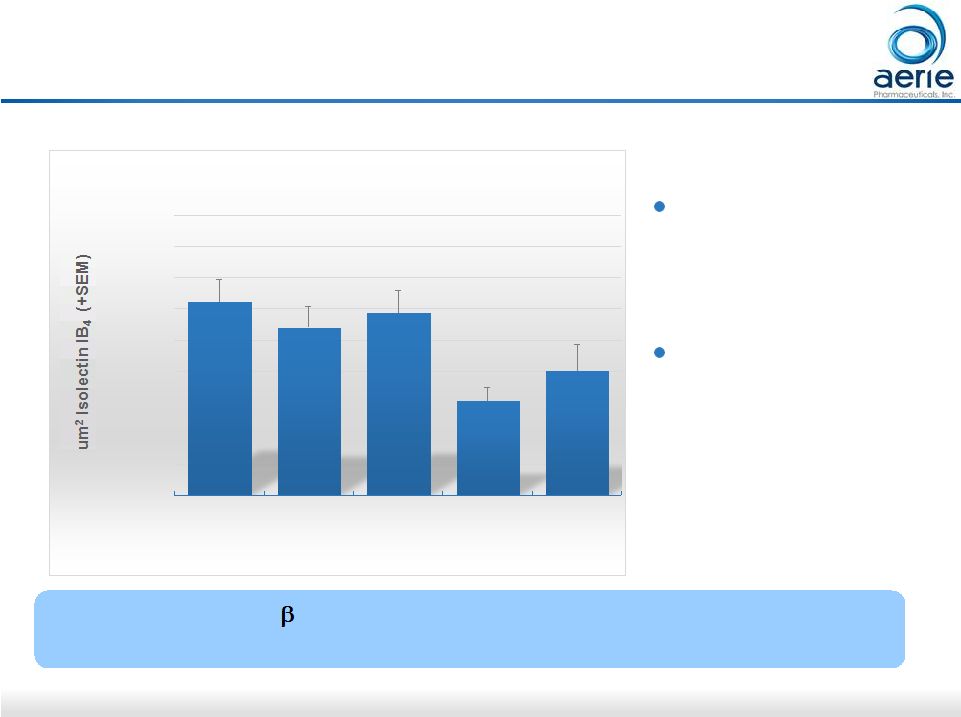 30 AR-13154 vs. Eylea in Preclinical AMD Model 20000 30000 40000 50000 60000 70000 80000 90000 100000 110000 Saline n=49 0.06 ug/mL AR-13154 n=28 0.6 ug/mL AR-13154 n=25 6 ug/mL AR-13154 n=25 800 ug/mL Eylea n=20 Total CNV Lesion Area (Day 21) ** * * p<0.05 vs. Saline ** p<0.001 Laser-induced choroidal neovascularization (CNV) in rats Compounds delivered by intravitreal injection ROCK/JAK2/PDGFR Inhibitor AR-13154 Numerically More Effective than Eylea ® in Rat Model of AMD Data on file; Effectiveness of AR-13154 Monotherapy and Combination Therapy in Animal Models of Wet Age-related Macular
Degeneration and Proliferative Diabetic Retinopathy. Cheng-Wen Lin, Jill M.
Sturdivant, Mitchell A. deLong and Casey C. Kopczynski
® |
 31 Topical AR-13154(S) Provides Added Efficacy to Eylea in Proliferative Diabetic Retinopathy Model -37% -34% -57% ** *** *** *** Oxygen-induced retinopathy model of PDR (mouse) 0.06% AR-13154(S) delivered topically from P12 to P17 Eylea delivered IP Confirms AR-13154(S) potential as effective adjunct to anti-VEGF therapies *** p < 0.0001 vs. vehicle control ** p < 0.001 vs. monotherapy Data on file; Effectiveness of AR-13154 Monotherapy and Combination Therapy in Animal Models of Wet Age-related Macular
Degeneration and Proliferative Diabetic Retinopathy. Cheng-Wen Lin, Jill M.
Sturdivant, Mitchell A. deLong and Casey C. Kopczynski 0%
20% 40% 60% 80% 100% 120% Vehicle Control (n=55) AR -13154(S) topical (n=28) Eylea 1mg/kg IP (n=26) AR -13154(S) + Eylea (n=18) Total Neovascular Area |
 32 Financial Summary • Cash Balance as of June 30, 2016 = $112M • 26.6M shares outstanding • Expected Cash Balance as of September 30, 2016 of ~$250M • ~33M shares outstanding • Expected Cash Balance as of December 31, 2016 of ~$230M • Updated Full-Year 2016 Cash Burn Guidance of ~$85M • Use of Proceeds from Recent Financings • General Corporate Purposes and Working Capital Requirements • Complete Funding of Rhopressa™ Commercialization Costs • Execution of Clinical Trials in Japan • Commencement of Construction of a Manufacturing Plant in Ireland |
 33 Summary • Key Clinical Priorities • Rhopressa™: NDA filing submitted Q3
2016
Rocket 4 in process (not required for NDA filing)
• Roclatan™: Mercury 1 and Mercury 2 completion Mercury 3 commencement 1H 2017 (EU) • Research Initiatives • Rhopressa™ disease modification, neuroprotection, sustained release
• AR-13154 potential in wet AMD, etc. • Evaluating Aerie’s 3,000+ proprietary molecules • Business Development and Expansion Opportunities • Drug delivery opportunities for front and back of the eye • EU/JP clinical path and commercialization strategy • Ireland Manufacturing Facility |
 34 Rhopressa™ and Roclatan™ Key Milestones Q3-2017: Roclatan™ P3 Mercury 1 Topline safety (12 mos) Near YE 2017: Roclatan™ NDA filing
expected
1H-2017: Roclatan™
P3 Mercury 3 (EU) to be initiated Q1-2016: Rhopressa™ Rocket 2 Topline safety (12 mos) Q3-2016: Rhopressa™ NDA filed Q4-2016: Rhopressa™ Rocket 4 Topline efficacy (3 mos) Q3-2016: Roclatan™ P3 Mercury 1 Topline efficacy (3 mos) Q2-2017: Roclatan™ P3 Mercury 2 Topline efficacy (3 mos) Q1-2016: Roclatan™ P3 Mercury 2 initiated 2016 2017 |
