Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OPIANT PHARMACEUTICALS, INC. | v448756_8-k.htm |
Exhibit 99.1

TREATMENTS FOR SUBSTANCE ABUSE, ADDICTIVE AND EATING DISORDERS Corporate Presentation September 2016 OPNT

This presentation contains forward - looking statements. These statements relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our or our industry's actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed, implied or inferred by these forward - looking statements. In some cases, you can identify forward - looking statements by terminology such as "may," "will," "should," "could," "would," "expects," "plans," "intends," "anticipates," "believes," estimates," "predicts," "projects," "potential" or "continue" or the negative of such terms and other comparable terminology. These statements are only predictions based on available information and our current expectations and projections about future events. You should not place undue reliance on these statements. Actual events or results may differ materially. In evaluating these statements, you should specifically consider various risks and factors affecting us and our industry. These and other factors may cause our actual results to differ materially from any forward - looking statement. We undertake no obligation to update any of the forward - looking statements after the date of this presentation to conform those statements to reflect the occurrence of unanticipated events, except as required by applicable law. 2 Forward looking s tatement

3 Opiant is a specialty pharma company focused on substance use, addictive and eating disorders » Highly capable management team, pioneering the development of NARCAN ® Nasal Spray , that went from concept to approval in under 3 years. » Only FDA approved naloxone nasal spray for opioid licensed to Adapt Pharma » Based in Santa Monica, CA » Issued and expanding IP portfolio » Ticker: OPNT » Now developing pipeline of treatments in substance use, addictive and eating disorders Core area is development of opioid antagonists delivered nasally
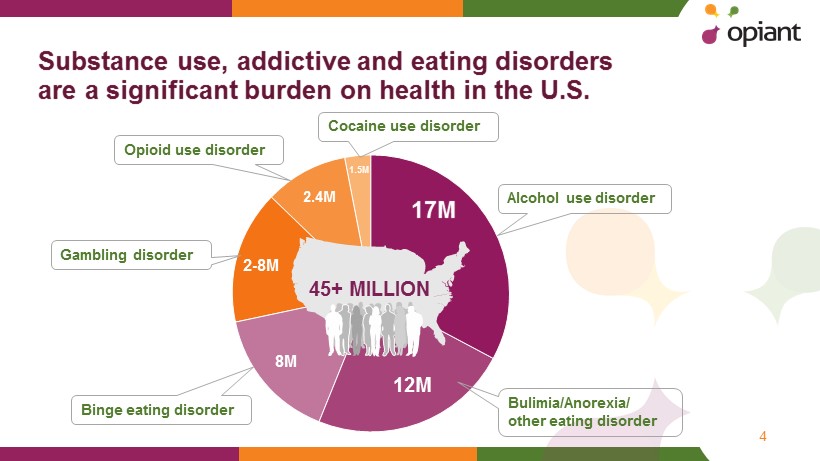
Substance u se , addictive and eating disorders are a s ignificant b urden on health in the U.S. 4 Alcohol use disorder Bulimia/Anorexia/ other eating disorder Binge eating disorder Gambling disorder Opioid use disorder Cocaine use disorder 45+ MILLION 17M 12M 8M 2 - 8M 2.4M 1.5M

5 For patients with substance u se , a ddictive and eating d isorders there is a need for effective t reatments Significant burden to the economy Need for new safe and effective treatments Compelling cost savings with effective treatments Few recently approved drugs Annual cost of substance use in the U.S. >$600B annually For every $1 spent on treatment up to $12 is saved in criminal and healthcare costs

6 A common neural circuitry is responsible for substance use, addictive and eating disorders NAc = Nucleus Accumbens GABA = Aminobutyric Acid VTA = Ventral Tegmental Area DA = Dopamine Opioids enhance dopamine release directly and indirectly Reward Circuitry

O pioid antagonists block the reward circuitry in the brain 7 The Addicted Brain

8 Opioid antagonists have been validated as therapeutics with an excellent safety profile

Opiant aims to become a leading p harma c ompany focused on addiction, substance u se and eating d isorders 9 Replicate recent success Enter Larger Market opportunities Invest long term Deliver on first program • More programs expected, with the potential for filing NDAs within 3 years at relatively low cost, primarily developing nasal opioid antagonists • Programs with larger addressable patient populations and greater revenue potential • Identify early stage opportunities with novel modes of action x NARCAN® Nasal Spray FDA approved, licensed and commercialized, from concept to market in under 3 years

Opiant’s pipeline well positioned to replicate NARCAN® Nasal Spray success 10 Opioid Overdose OPNT001 Binge Eating Disorder OPNT001 Cocaine Use Disorder Phase I Phase II Phase III NDA Partner FDA Approved Commercialized OPNT001 Bulimia Nervosa OPNT002 Undisclosed Disorder ✔ *as of September 2016

11 Opioid Overdose

People can stop breathing if they overdose from any opioid, including heroin, fentanyl and opioid painkillers 12 Opioid Overdose • Inappropriate prescribing • Abuse of prescription opioids • Use of illicit opioids Overdose of opioids removes the drive for breathing More deaths occur in the US from opioid overdoses than from road traffic accidents Death Ensues Widespread Impact
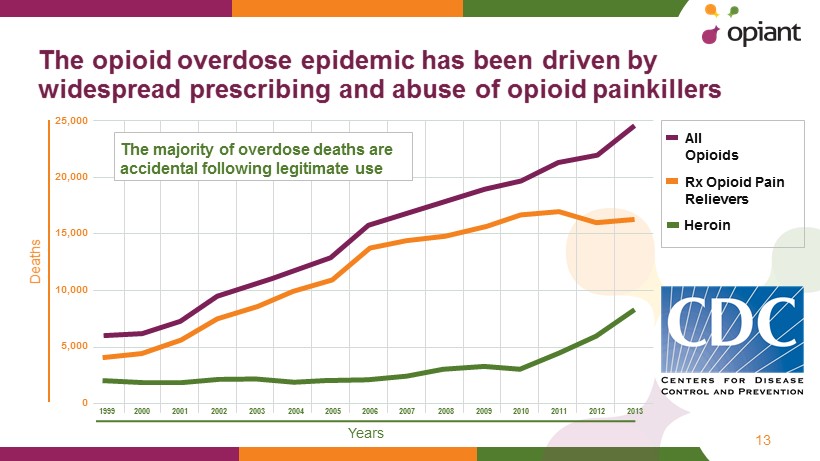
The opioid o verdose e pidemic h as been d riven by widespread prescribing and abuse of opioid p ainkillers 13 All Opioids 25,000 20,000 15,000 10,000 5,000 0 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 Rx Opioid Pain Relievers Heroin Y ears D eaths The majority of overdose deaths are accidental following legitimate use

14 Naloxone saves lives if given quickly However: only trained personnel able to deliver injectable form, which delays lifesaving treatment Timing is Everything: Understanding the Urgency to Respond Quickly Dial 911 Await ambulance Patient already stopped breathing Help often arrives too late Opioid pain killers or heroin taken & unresponsive More lives saved if naloxone is given sooner

15 Opiant’s NARCAN ® Nasal Spray has several a dvantages over i njectable n aloxone and nasal s pray k its Improvised Nasal Kits Injectable Naloxone Autoinjectors • O nly trained personnel • Prep time • N eedles • Very expensive • Needles • Assembly • Not FDA approved • No data • Training • Excellent data • M ulti - directional • Ease of use NARCAN® Nasal Spray

16 Opiant’s NARCAN® Nasal Spray achieves equal treatment levels compared to injectable and is easier to administer Time Post Administration (hour) Naloxone Concentration (ng/ml) Dose 3 – 0.4mg Intramuscular Dose 1 – 4mg Intranasal Dose 2 – 2mg Intranasal 6.0 5 .0 4.0 3.0 2.0 1.0 0 .0 0 .0 0.5 1.0 1.5 2.0 2.5 3.0

NARCAN® Nasal Spray NARCAN® Nasal Spray licensed to Adapt Pharma and FDA approved Nov 2015 17 Key Financial Terms Adapt Pharma Limited » FDA Approved November 18, 2015 » Competitor rejected » Issued IP » $55M potential milestone payments » Up to double - digit royalties » Previous AZUR Pharma founders – $500M sale to Jazz Pharma » U.S. commercial expertise
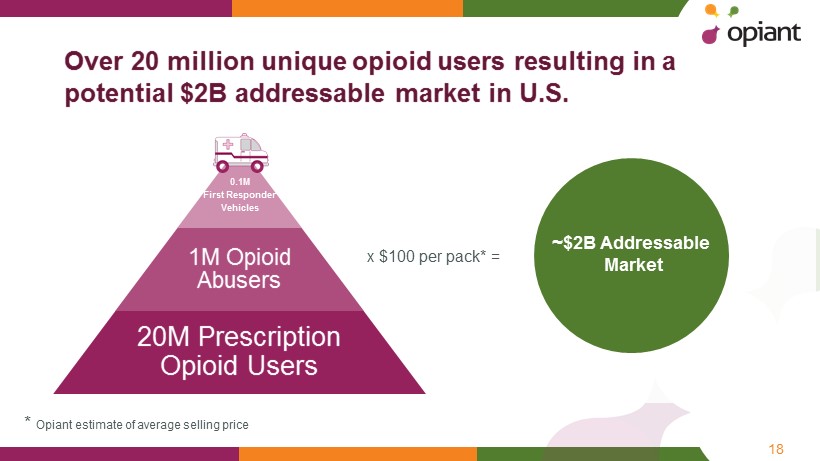
18 Over 20 million unique opioid users resulting in a potential $2B addressable market in U.S. * Opiant estimate of average selling price 0.1M First Responder Vehicles 1M Opioid Abusers 20M Prescription Opioid Users x $100 per pack* = ~$2B Addressable Market

19 Binge Eating Disorder

» Part of DSM - 5 » Associated with significant physical and psychological problems » Food and food “cues” activate brain reward system 20 Binge eating d isorder (BED) is a common m edical p roblem… » Shire’s Vyvanse®: only drug approved (2015) - $300M peak US sales forecast » …new regulatory path for BED » …but unsuitable for many patients (scheduled drug and CV risks) Market appears ready for a new entrant! Most common eating disorder: 8 million diagnosed in U.S.

164.8 171.3 174.8 179.9 188.6 199.9 0 - 40 - 80 - 120 - 160 - 180 Base 1 - 20 - 60 - 100 - 140 2 3 4 5 6 Opiant’s nasal naloxone data s hows p romise for B inge Eating Disorder 21 Phase IIa POC study ( Helsinki) » 127 patients » Randomized , double blind, placebo controlled » Significant reduction in binge eating for naloxone group vs placebo (125 vs 84 mins /week ; p=0.024 ) Placebo Naloxone Months bingeing/minutes American Psychiatry Association Poster Presentation May 2013 Further studies planned ….

22 Cocaine Use Disorder

23 The challenge of cocaine u se d isorder – no a pproved m edication » 1.5M current cocaine abusers in U.S. » Chronic cocaine exposure activates opioid and dopamine reward circuitry » Treatment is limited to behavioral interventions, with limited efficacy and high relapse rates » No FDA approved treatment

24 Novel functional MRI s tudy u nderway in c ocaine p atients » Funded by NIDA » Nasal opioid antagonist » Collaboration with University of Pennsylvania » 12 patients » Record brain response to visual cocaine cues using fMRI +/ - treatment

Prospective key m ilestone e vents Cocaine Addiction Eating Disorders Opioid Overdose 25 4Q15 NARCAN ® Nasal FDA approval 1Q16 First commercial sale NARCAN ® Nasal 4Q16 Formal evaluation of nasal formulation with novel excipient 4Q15 NIDA and UPenn collaboration 4Q16 UPenn interim study results 4Q16 BN study Phase II initiation 1H 17 BED study initiation ✔ ✔ ✔

Arvind Agrawal Executive Vice President, Medical Affairs Management 26 Dr. Roger Crystal Chief Executive Officer Kevin Pollack, Esq. Chief Financial Officer Dr. Michael Sinclair Chairman Dr. Mark Ellison Executive Vice President, Development, Manufacturing and Quality

TREATMENTS FOR SUBSTANCE ABUSE, ADDICTIVE AND EATING DISORDERS 27 September 2016 401 Wilshire Blvd, 12th Floor, Santa Monica CA 90401 O: 1 (424) 252 4756
