Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Juno Therapeutics, Inc. | d250473d8k.htm |

Exhibit 99.1

Forward-looking Statements This presentation and the accompanying oral commentary contain forward-looking statements that involve risks, uncertainties, and assumptions. If the risks or uncertainties ever materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward-looking statements. All statements other than statements of historical fact could be deemed forward-looking, including, but not limited to, any expectations regarding investment returns; any projections of financial information; any statements about historical results that may suggest trends for our business; any statements of the plans, strategies, and objectives of management for future operations, including our manufacturing and process development; any statements of expectation or belief regarding future events, potential markets or market size, technology developments, our product pipeline, clinical data, enforceability of our intellectual property rights, competitive strengths or our position within the industry; any statements regarding the anticipated benefits of our Celgene collaboration or other strategic transactions; and any statements of assumptions underlying any of the items mentioned. These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance. Actual results could differ materially from our current expectations as a result of many risks and uncertainties, including but not limited to, risks associated with: the success, cost, and timing of our product development activities and clinical trials; our ability to obtain regulatory approval for and to commercialize our product candidates; our ability to establish a commercially-viable manufacturing process and manufacturing infrastructure; regulatory requirements and regulatory developments; the effects of competition and technological advances; our dependence on third-party collaborators and other contractors in our research and development activities, including for the conduct of clinical trials and the manufacture of our product candidates; our dependence on Celgene for the development and commercialization outside of North America and China of our CD19 product candidates and any other product candidates for which Celgene exercises an option; Juno’s dependence on JW Therapeutics (Shanghai) Co., Ltd, over which Juno does not exercise complete control, for the development and commercialization of product candidates in China; our ability to obtain, maintain, or protect intellectual property rights related to our product candidates; among others. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on August 5, 2016 and our other periodic reports filed from time to time with the Securities and Exchange Commission. Except as required by law, we assume no obligation and do not intend to update these forward-looking statements or to conform these statements to actual results or to changes in our expectations.

Building the Leading T Cell Company Significant advances in CD19-directed product candidates with a go-to-market strategy defined in ALL, NHL, and CLL Moving beyond CD19 target with encouraging data in CD22 and WT-1; more targets and approaches in the clinic Progress with potential best-in-class product candidates and platform; JCAR017 on the market as early as 2018 Broad program in solid organ tumors in significant areas of unmet need Building platform and self-sustaining research capability to support leadership in the engineered T cell space With Celgene, global manufacturing, development, and commercial capabilities to bring engineered T cells to market globally
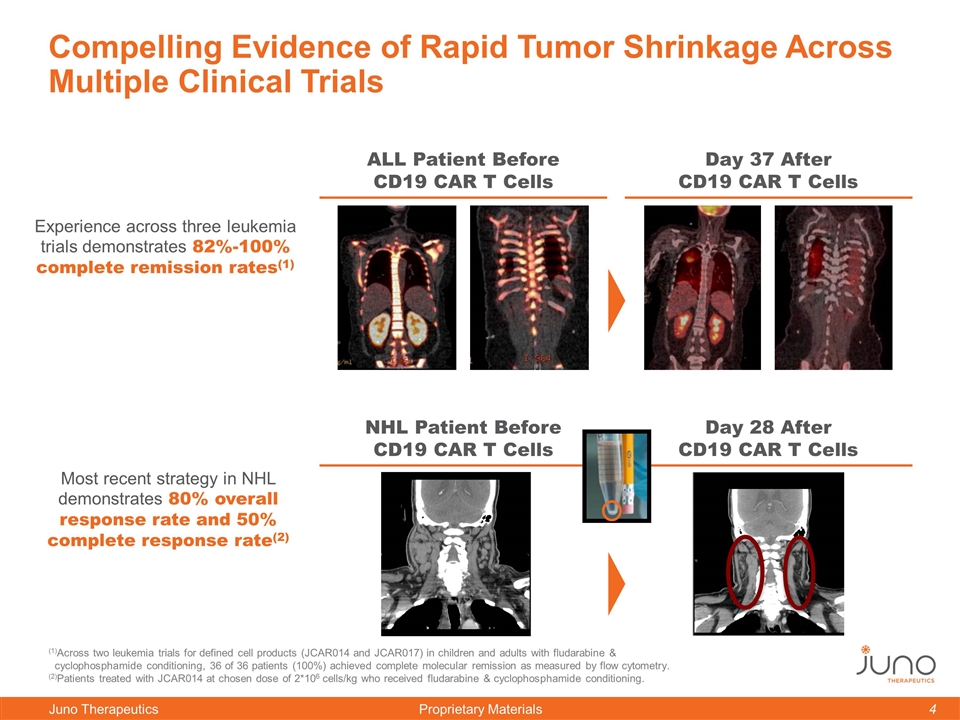
Compelling Evidence of Rapid Tumor Shrinkage Across Multiple Clinical Trials Day 37 After CD19 CAR T Cells ALL Patient Before CD19 CAR T Cells Day 28 After CD19 CAR T Cells NHL Patient Before CD19 CAR T Cells (1)Across two leukemia trials for defined cell products (JCAR014 and JCAR017) in children and adults with fludarabine & cyclophosphamide conditioning, 36 of 36 patients (100%) achieved complete molecular remission as measured by flow cytometry. (2)Patients treated with JCAR014 at chosen dose of 2*106 cells/kg who received fludarabine & cyclophosphamide conditioning. Experience across three leukemia trials demonstrates 82%-100% complete remission rates(1) Most recent strategy in NHL demonstrates 80% overall response rate and 50% complete response rate(2)
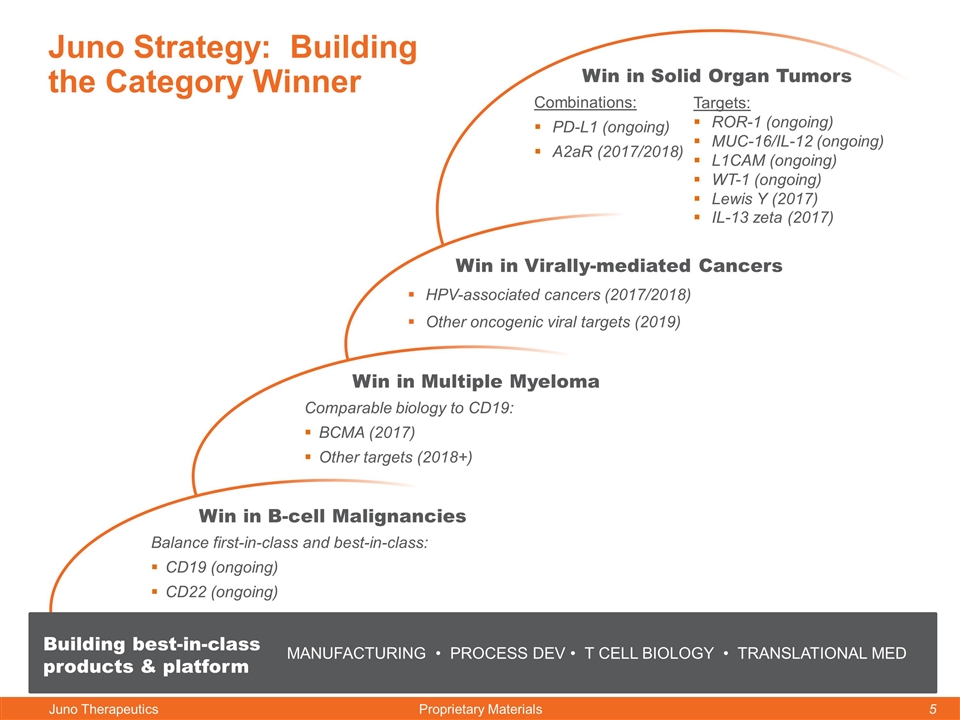
Juno Strategy: Building the Category Winner Win in B-cell Malignancies Balance first-in-class and best-in-class: CD19 (ongoing) CD22 (ongoing) Win in Multiple Myeloma Comparable biology to CD19: BCMA (2017) Other targets (2018+) Win in Virally-mediated Cancers HPV-associated cancers (2017/2018) Other oncogenic viral targets (2019) Win in Solid Organ Tumors Combinations: PD-L1 (ongoing) A2aR (2017/2018) MANUFACTURING • PROCESS DEV • T CELL BIOLOGY • TRANSLATIONAL MED Building best-in-class products & platform Targets: ROR-1 (ongoing) MUC-16/IL-12 (ongoing) L1CAM (ongoing) WT-1 (ongoing) Lewis Y (2017) IL-13 zeta (2017)
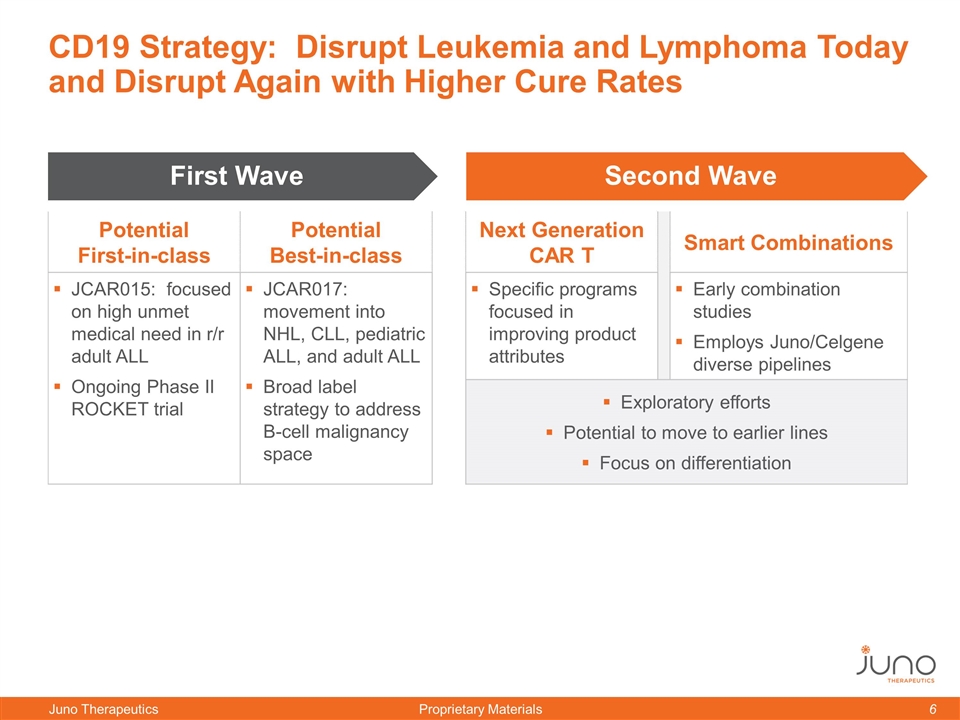
CD19 Strategy: Disrupt Leukemia and Lymphoma Today and Disrupt Again with Higher Cure Rates Potential First-in-class Potential Best-in-class Next Generation CAR T Smart Combinations JCAR015: focused on high unmet medical need in r/r adult ALL Ongoing Phase II ROCKET trial JCAR017: movement into NHL, CLL, pediatric ALL, and adult ALL Broad label strategy to address B-cell malignancy space Specific programs focused in improving product attributes Early combination studies Employs Juno/Celgene diverse pipelines Exploratory efforts Potential to move to earlier lines Focus on differentiation Second Wave First Wave
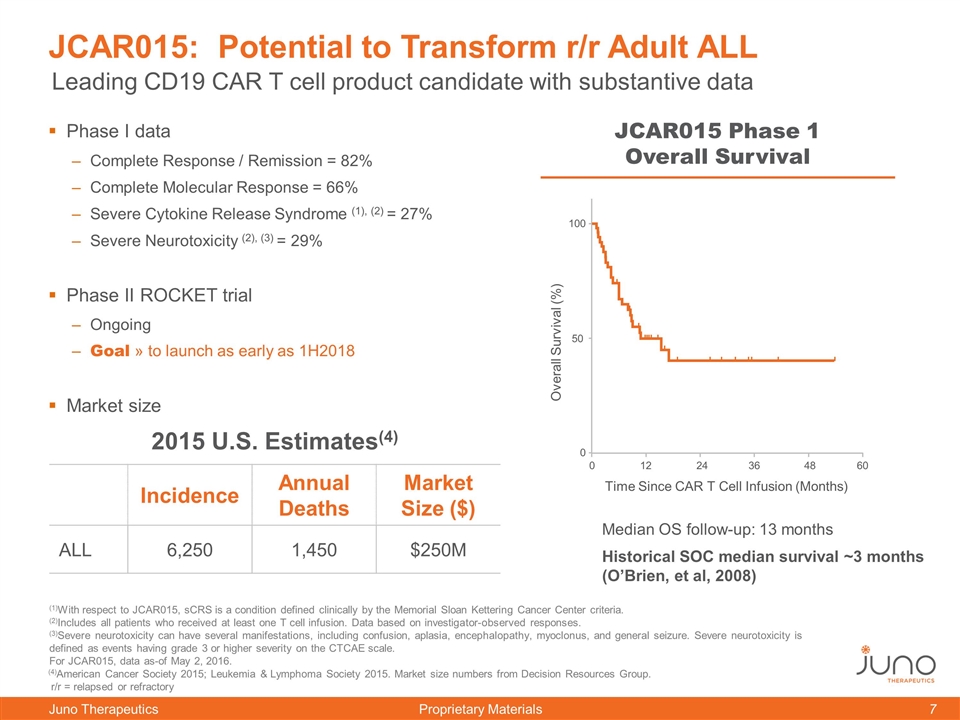
JCAR015: Potential to Transform r/r Adult ALL Phase I data Complete Response / Remission = 82% Complete Molecular Response = 66% Severe Cytokine Release Syndrome (1), (2) = 27% Severe Neurotoxicity (2), (3) = 29% Phase II ROCKET trial Ongoing Goal » to launch as early as 1H2018 Market size Median OS follow-up: 13 months Historical SOC median survival ~3 months (O’Brien, et al, 2008) JCAR015 Phase 1 Overall Survival Leading CD19 CAR T cell product candidate with substantive data 2015 U.S. Estimates(4) Incidence Annual Deaths Market Size ($) ALL 6,250 1,450 $250M 0 100 50 0 12 24 36 Time Since CAR T Cell Infusion (Months) Overall Survival (%) 48 60 (4)American Cancer Society 2015; Leukemia & Lymphoma Society 2015. Market size numbers from Decision Resources Group. r/r = relapsed or refractory (1)With respect to JCAR015, sCRS is a condition defined clinically by the Memorial Sloan Kettering Cancer Center criteria. (2)Includes all patients who received at least one T cell infusion. Data based on investigator-observed responses. (3)Severe neurotoxicity can have several manifestations, including confusion, aplasia, encephalopathy, myoclonus, and general seizure. Severe neurotoxicity is defined as events having grade 3 or higher severity on the CTCAE scale. For JCAR015, data as-of May 2, 2016.
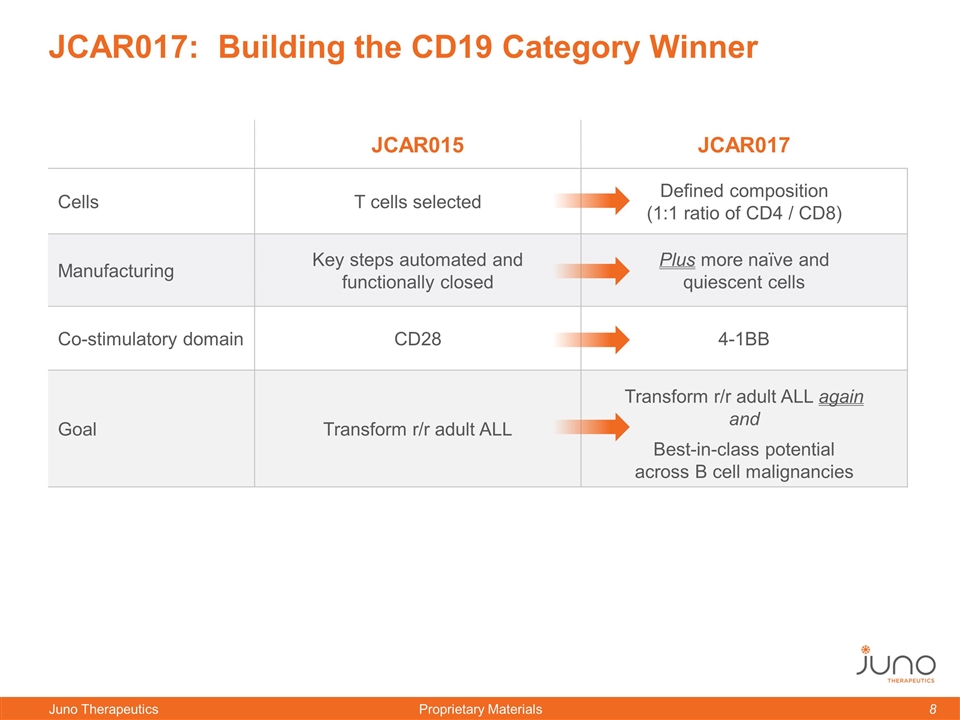
JCAR017: Building the CD19 Category Winner JCAR015 JCAR017 Cells T cells selected Defined composition (1:1 ratio of CD4 / CD8) Manufacturing Key steps automated and functionally closed Plus more naïve and quiescent cells Co-stimulatory domain CD28 4-1BB Goal Transform r/r adult ALL Transform r/r adult ALL again and Best-in-class potential across B cell malignancies
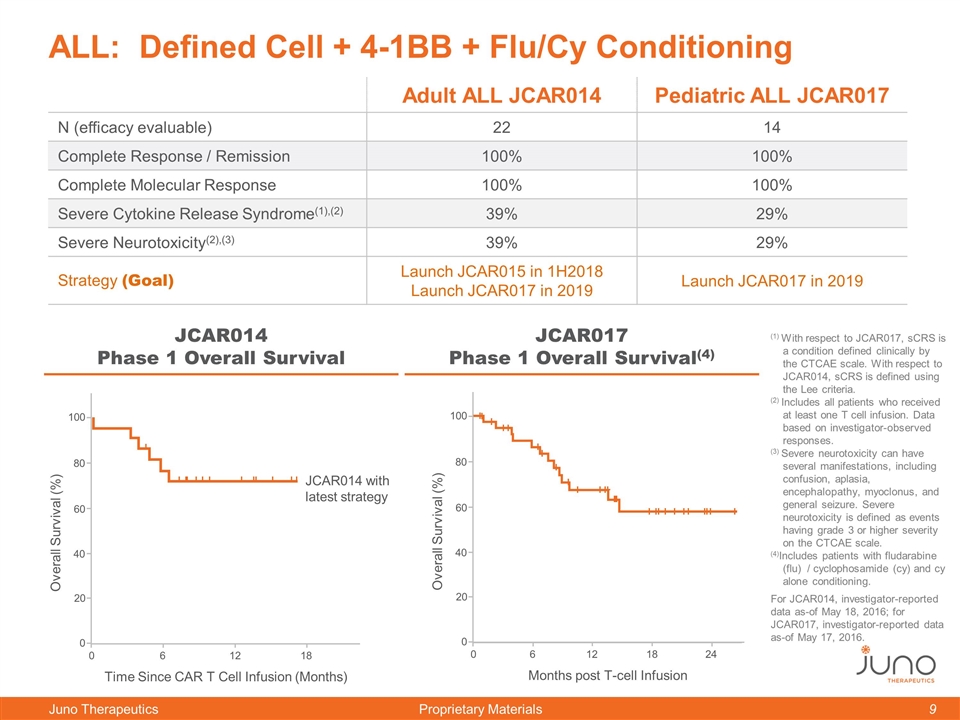
ALL: Defined Cell + 4-1BB + Flu/Cy Conditioning JCAR017 Phase 1 Overall Survival(4) 0 60 100 80 40 20 0 6 18 24 Months post T-cell Infusion Overall Survival (%) 12 JCAR014 Phase 1 Overall Survival 0 60 100 80 40 20 0 6 12 18 Time Since CAR T Cell Infusion (Months) Overall Survival (%) JCAR014 with latest strategy (1) With respect to JCAR017, sCRS is a condition defined clinically by the CTCAE scale. With respect to JCAR014, sCRS is defined using the Lee criteria. (2) Includes all patients who received at least one T cell infusion. Data based on investigator-observed responses. (3) Severe neurotoxicity can have several manifestations, including confusion, aplasia, encephalopathy, myoclonus, and general seizure. Severe neurotoxicity is defined as events having grade 3 or higher severity on the CTCAE scale. (4)Includes patients with fludarabine (flu) / cyclophosamide (cy) and cy alone conditioning. For JCAR014, investigator-reported data as-of May 18, 2016; for JCAR017, investigator-reported data as-of May 17, 2016. Adult ALL JCAR014 Pediatric ALL JCAR017 N (efficacy evaluable) 22 14 Complete Response / Remission 100% 100% Complete Molecular Response 100% 100% Severe Cytokine Release Syndrome(1),(2) 39% 29% Severe Neurotoxicity(2),(3) 39% 29% Strategy (Goal) Launch JCAR015 in 1H2018 Launch JCAR017 in 2019 Launch JCAR017 in 2019
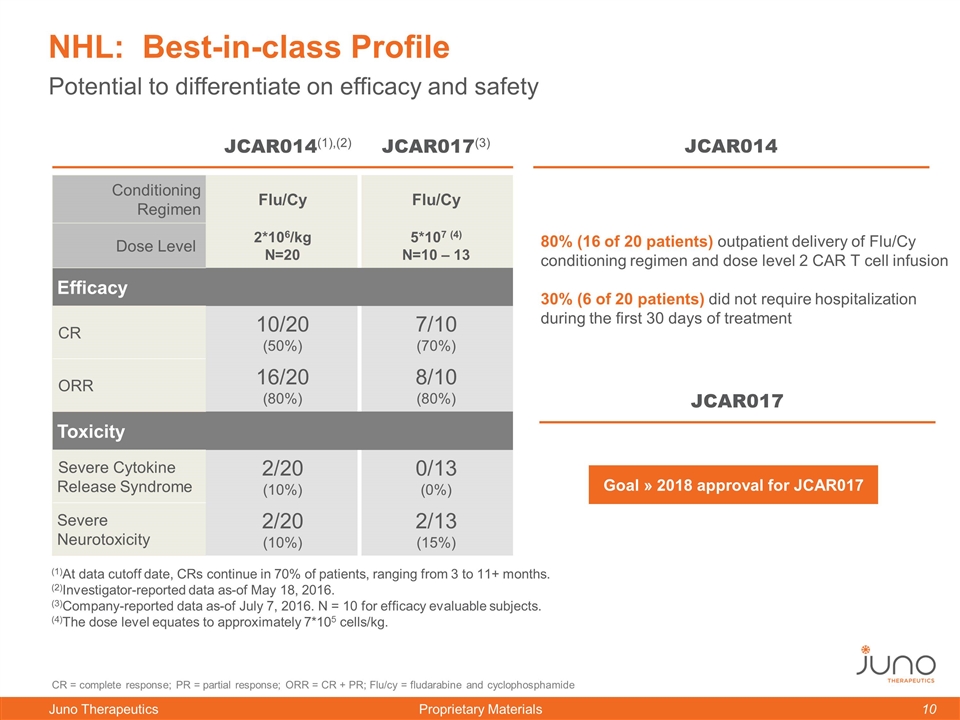
Potential to differentiate on efficacy and safety NHL: Best-in-class Profile JCAR014(1),(2) JCAR014 CR = complete response; PR = partial response; ORR = CR + PR; Flu/cy = fludarabine and cyclophosphamide Goal » 2018 approval for JCAR017 (1)At data cutoff date, CRs continue in 70% of patients, ranging from 3 to 11+ months. (2)Investigator-reported data as-of May 18, 2016. (3)Company-reported data as-of July 7, 2016. N = 10 for efficacy evaluable subjects. (4)The dose level equates to approximately 7*105 cells/kg. Conditioning Regimen Flu/Cy Flu/Cy Dose Level 2*106/kg N=20 5*107 (4) N=10 – 13 Efficacy CR 10/20 (50%) 7/10 (70%) ORR 16/20 (80%) 8/10 (80%) Toxicity Severe Cytokine Release Syndrome 2/20 (10%) 0/13 (0%) Severe Neurotoxicity 2/20 (10%) 2/13 (15%) JCAR017(3) 80% (16 of 20 patients) outpatient delivery of Flu/Cy conditioning regimen and dose level 2 CAR T cell infusion 30% (6 of 20 patients) did not require hospitalization during the first 30 days of treatment JCAR017
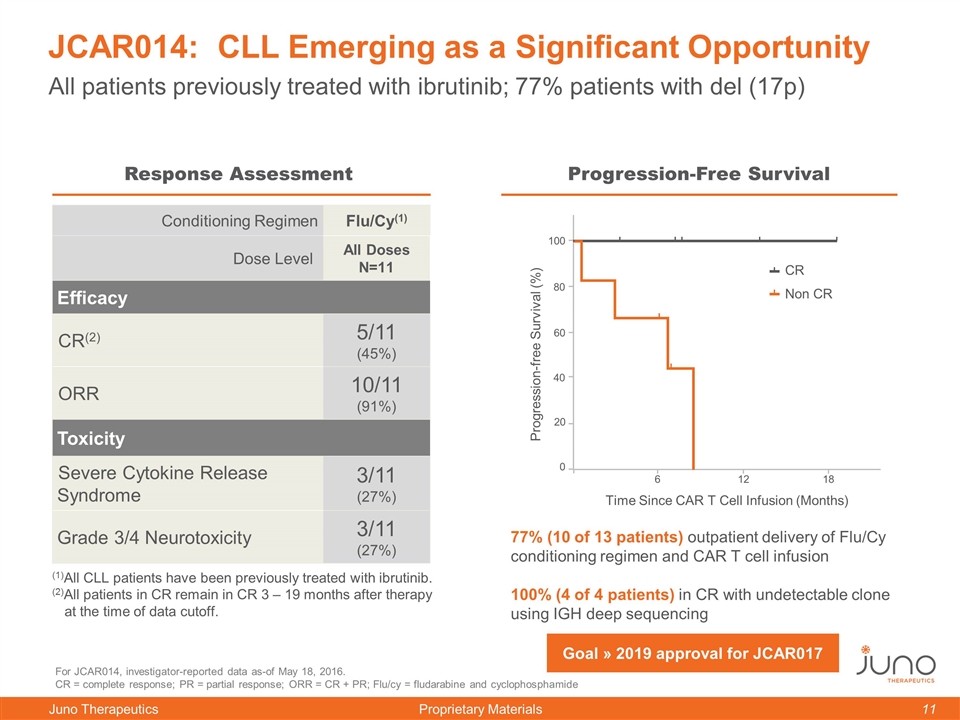
JCAR014: CLL Emerging as a Significant Opportunity For JCAR014, investigator-reported data as-of May 18, 2016. CR = complete response; PR = partial response; ORR = CR + PR; Flu/cy = fludarabine and cyclophosphamide Conditioning Regimen Flu/Cy(1) Dose Level All Doses N=11 Efficacy CR(2) 5/11 (45%) ORR 10/11 (91%) Toxicity Severe Cytokine Release Syndrome 3/11 (27%) Grade 3/4 Neurotoxicity 3/11 (27%) (1)All CLL patients have been previously treated with ibrutinib. (2)All patients in CR remain in CR 3 – 19 months after therapy at the time of data cutoff. All patients previously treated with ibrutinib; 77% patients with del (17p) 77% (10 of 13 patients) outpatient delivery of Flu/Cy conditioning regimen and CAR T cell infusion 100% (4 of 4 patients) in CR with undetectable clone using IGH deep sequencing 0 12 Response Assessment Progression-Free Survival 0 60 100 80 40 20 18 Time Since CAR T Cell Infusion (Months) Progression-free Survival (%) CR Non CR 12 6 Goal » 2019 approval for JCAR017
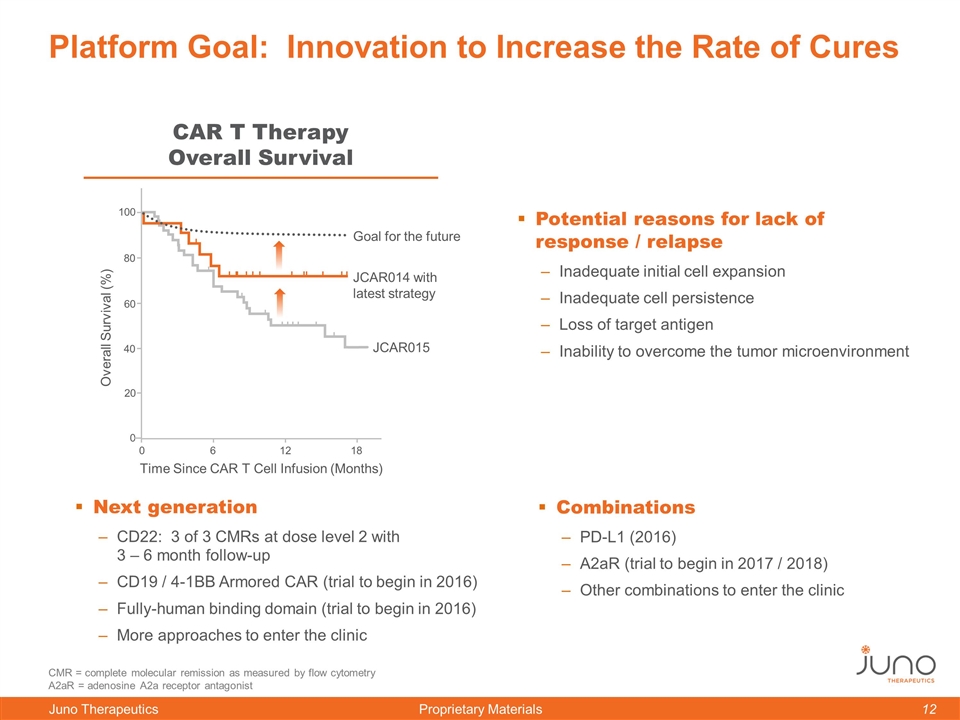
Platform Goal: Innovation to Increase the Rate of Cures Next generation CD22: 3 of 3 CMRs at dose level 2 with 3 – 6 month follow-up CD19 / 4-1BB Armored CAR (trial to begin in 2016) Fully-human binding domain (trial to begin in 2016) More approaches to enter the clinic Combinations PD-L1 (2016) A2aR (trial to begin in 2017 / 2018) Other combinations to enter the clinic Time Since CAR T Cell Infusion (Months) CMR = complete molecular remission as measured by flow cytometry A2aR = adenosine A2a receptor antagonist CAR T Therapy Overall Survival 0 60 100 80 40 20 0 6 12 18 Overall Survival (%) JCAR014 with latest strategy Goal for the future JCAR015 Time Since CAR T Cell Infusion (Months) Potential reasons for lack of response / relapse Inadequate initial cell expansion Inadequate cell persistence Loss of target antigen Inability to overcome the tumor microenvironment
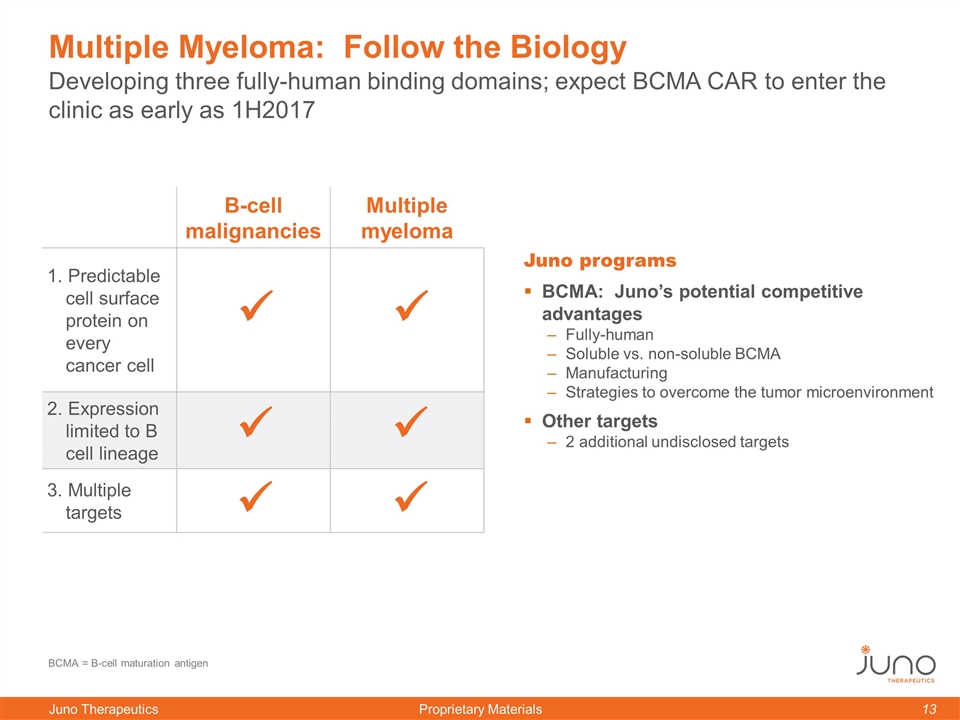
Multiple Myeloma: Follow the Biology Juno programs BCMA: Juno’s potential competitive advantages Fully-human Soluble vs. non-soluble BCMA Manufacturing Strategies to overcome the tumor microenvironment Other targets 2 additional undisclosed targets B-cell malignancies Multiple myeloma 1. Predictable cell surface protein on every cancer cell 2. Expression limited to B cell lineage 3. Multiple targets ü ü ü ü ü ü Developing three fully-human binding domains; expect BCMA CAR to enter the clinic as early as 1H2017 BCMA = B-cell maturation antigen
Virally-mediated Cancers: Straightforward Solid Organ Tumor Targets Identifying and selecting target antigens Viral epitopes that are only on cancer cells, not on healthy tissues Human Papillomavirus (HPV) as an example Targeting tumors caused by HPV 16 and HPV 18 Juno has fully-human, high affinity HPV-specific TCRs Generating receptors to target multiple HLA haplotypes HPV causes a significant percentage of head/neck and cervical cancers Juno’s Research program in other virally-mediated cancers underway
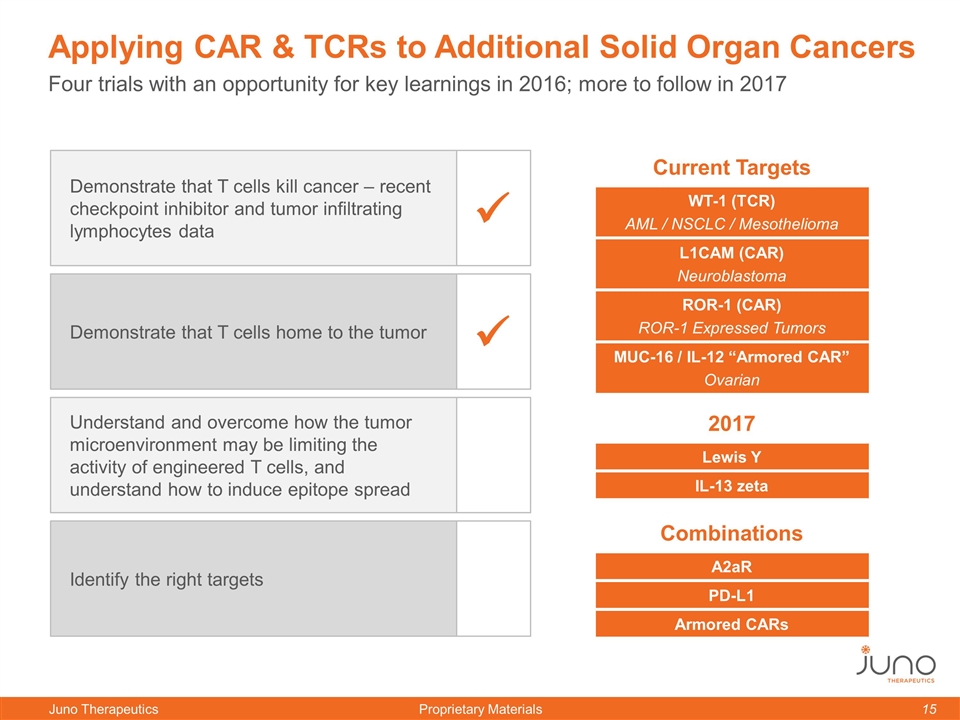
Demonstrate that T cells kill cancer – recent checkpoint inhibitor and tumor infiltrating lymphocytes data Demonstrate that T cells home to the tumor Understand and overcome how the tumor microenvironment may be limiting the activity of engineered T cells, and understand how to induce epitope spread Identify the right targets ü ü Four trials with an opportunity for key learnings in 2016; more to follow in 2017 Applying CAR & TCRs to Additional Solid Organ Cancers Current Targets WT-1 (TCR) AML / NSCLC / Mesothelioma L1CAM (CAR) Neuroblastoma ROR-1 (CAR) ROR-1 Expressed Tumors MUC-16 / IL-12 “Armored CAR” Ovarian 2017 Lewis Y IL-13 zeta Combinations A2aR PD-L1 Armored CARs

Building a Category Leader Manufacturing capabilities JuMP facility online Defined cell product Global reach Celgene collaboration - CD19 opt-in JW Therapeutics - investment in China Research capabilities Internal and BD opportunities Strong balance sheet $1.11 billion as of 2Q16 Strong, nimble biotech can win
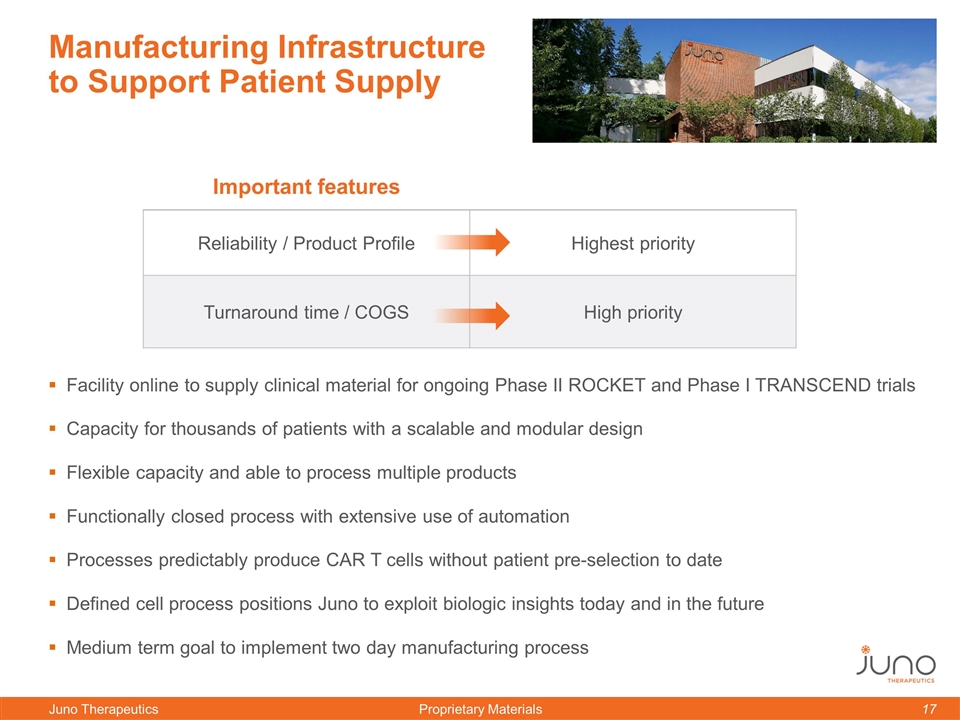
Manufacturing Infrastructure to Support Patient Supply Facility online to supply clinical material for ongoing Phase II ROCKET and Phase I TRANSCEND trials Capacity for thousands of patients with a scalable and modular design Flexible capacity and able to process multiple products Functionally closed process with extensive use of automation Processes predictably produce CAR T cells without patient pre-selection to date Defined cell process positions Juno to exploit biologic insights today and in the future Medium term goal to implement two day manufacturing process Important features Reliability / Product Profile Highest priority Turnaround time / COGS High priority
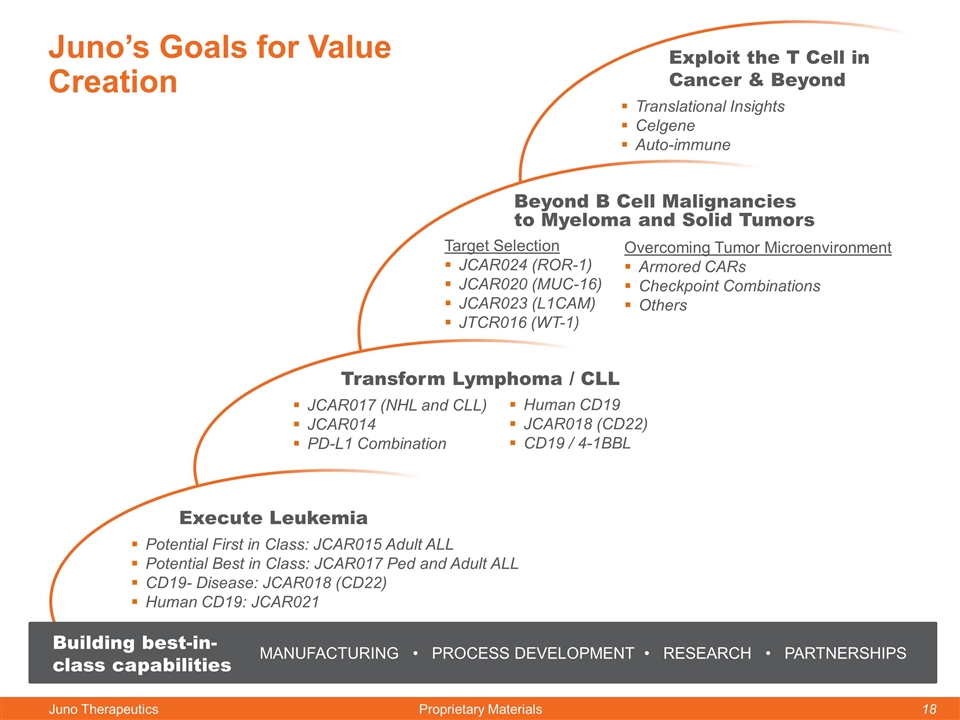
Juno’s Goals for Value Creation Execute Leukemia Potential First in Class: JCAR015 Adult ALL Potential Best in Class: JCAR017 Ped and Adult ALL CD19- Disease: JCAR018 (CD22) Human CD19: JCAR021 Transform Lymphoma / CLL JCAR017 (NHL and CLL) JCAR014 PD-L1 Combination Beyond B Cell Malignancies to Myeloma and Solid Tumors Target Selection JCAR024 (ROR-1) JCAR020 (MUC-16) JCAR023 (L1CAM) JTCR016 (WT-1) Exploit the T Cell in Cancer & Beyond Translational Insights Celgene Auto-immune MANUFACTURING • PROCESS DEVELOPMENT • RESEARCH • PARTNERSHIPS Building best-in-class capabilities Human CD19 JCAR018 (CD22) CD19 / 4-1BBL Overcoming Tumor Microenvironment Armored CARs Checkpoint Combinations Others
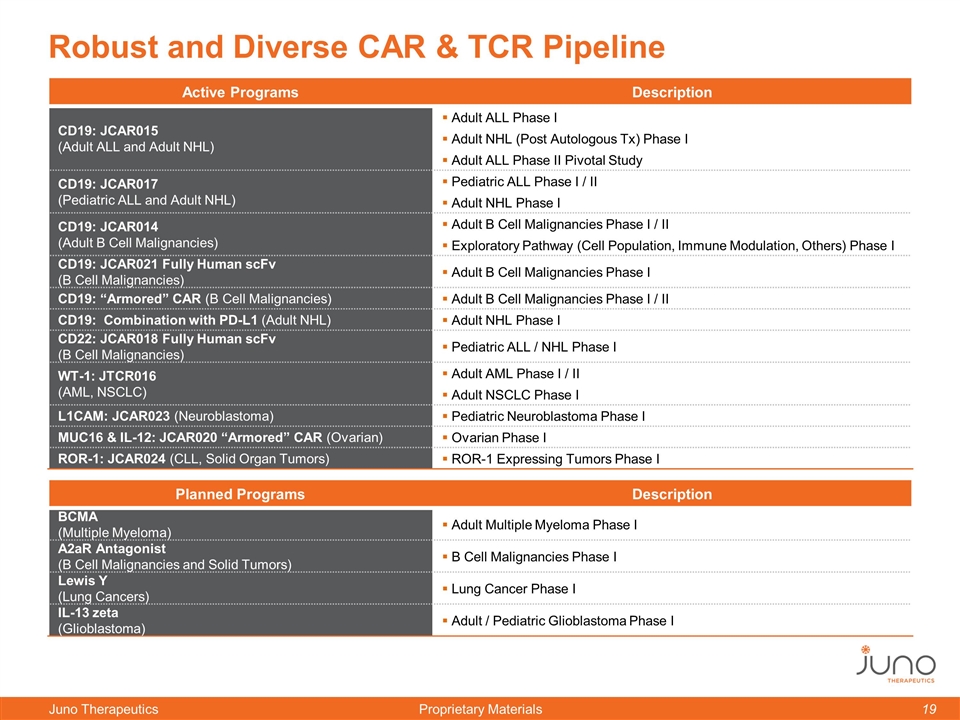
Active Programs Description CD19: JCAR015 (Adult ALL and Adult NHL) Adult ALL Phase I Adult NHL (Post Autologous Tx) Phase I Adult ALL Phase II Pivotal Study CD19: JCAR017 (Pediatric ALL and Adult NHL) Pediatric ALL Phase I / II Adult NHL Phase I CD19: JCAR014 (Adult B Cell Malignancies) Adult B Cell Malignancies Phase I / II Exploratory Pathway (Cell Population, Immune Modulation, Others) Phase I CD19: JCAR021 Fully Human scFv (B Cell Malignancies) Adult B Cell Malignancies Phase I CD19: “Armored” CAR (B Cell Malignancies) Adult B Cell Malignancies Phase I / II CD19: Combination with PD-L1 (Adult NHL) Adult NHL Phase I CD22: JCAR018 Fully Human scFv (B Cell Malignancies) Pediatric ALL / NHL Phase I WT-1: JTCR016 (AML, NSCLC) Adult AML Phase I / II Adult NSCLC Phase I L1CAM: JCAR023 (Neuroblastoma) Pediatric Neuroblastoma Phase I MUC16 & IL-12: JCAR020 “Armored” CAR (Ovarian) Ovarian Phase I ROR-1: JCAR024 (CLL, Solid Organ Tumors) ROR-1 Expressing Tumors Phase I Robust and Diverse CAR & TCR Pipeline Planned Programs Description BCMA (Multiple Myeloma) Adult Multiple Myeloma Phase I A2aR Antagonist (B Cell Malignancies and Solid Tumors) B Cell Malignancies Phase I Lewis Y (Lung Cancers) Lung Cancer Phase I IL-13 zeta (Glioblastoma) Adult / Pediatric Glioblastoma Phase I
