Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - REGENERX BIOPHARMACEUTICALS INC | v448619_8k.htm |
Exhibit 99.1

Rodman & Renshaw 18 th Annual Global Investment Conference September 2016

2 OTCQB: RGRX Forward - Looking Statements This investor presentation contains certain forward - looking statements that involve risks and uncertainties that could cause actual results to be materially different from historical results or from any future results expressed or implied by such forward - looking statements . Examples of such forward - looking statements include statements concerning the target dates for completing the company’s or its partners’ ongoing clinical trials for ophthalmic and orphan indications, the potential size of addressable markets, including the market for eye drops and parenteral delivery products, the company’s ability to enter into any collaborations with respect to the development or commercialization of its product candidates, and the therapeutic potential of Tβ 4 for ophthalmic, cardiovascular and neurovascular disorders . Factors that may cause actual results to differ materially from any future results expressed or implied by any forward - looking statements include the risk that although Tβ 4 has demonstrated potential therapeutic benefit for dermal, ophthalmic, cardiovascular and neurovascular disorders, the company’s product candidates may not demonstrate safety and/or efficacy in clinical trials, the risk that encouraging results from early research, preclinical studies, compassionate use or clinical trials may not be confirmed upon further analysis of the detailed results, the risk that additional information relating to the safety, efficacy or tolerability of our product candidates may be required by regulatory agencies, the risk that the company or its licensees will not obtain approval to market the company’s product candidates in the U . S . or abroad, the risks associated with reliance on outside financing to meet capital requirements, the risks associated with reliance on licensees for the funding or conduct of further development and commercialization activities relating to the company’s product candidates, the risks that the company’s patents will not be enforceable or expire prior to commercial marketing, and such other risks described in the company’s latest Annual Report on Form 10 - K, and other filings the company makes with the SEC . Any forward - looking statements are made pursuant to Section 27 A of the Securities Act of 1933 , as amended, and Section 21 E of the Securities Exchange Act of 1934 , as amended, and, as such, speak only as of the date made . The Company undertakes no obligation to publicly update any forward - looking statements, whether as a result of new information, future events or otherwise .
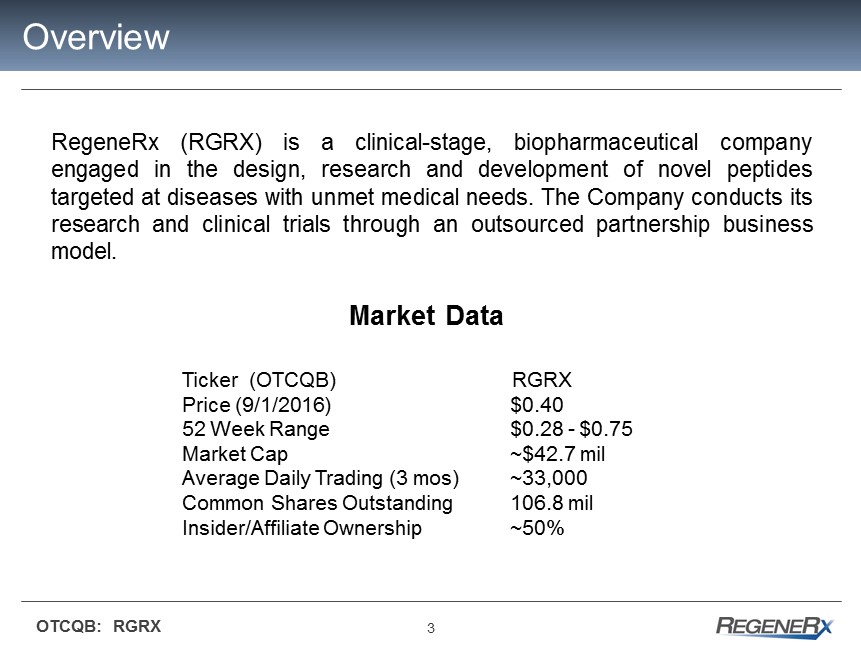
3 OTCQB: RGRX Overview RegeneRx (RGRX) is a clinical - stage, biopharmaceutical company engaged in the design, research and development of novel peptides targeted at diseases with unmet medical needs . The Company conducts its research and clinical trials through an outsourced partnership business model . Market Data Ticker (OTCQB) RGRX Price (9/1/2016 ) $ 0.40 52 Week Range $0.28 - $0.75 Market Cap ~$ 42.7 mil Average Daily Trading (3 mos) ~33,000 Common Shares Outstanding 106.8 mil Insider/Affiliate Ownership ~ 50% 3

4 OTCQB: RGRX Investment Highlights ▪ Developing Thymosin beta 4 (T β4) , a novel therapeutic peptide for tissue and organ protection, repair, and regeneration. − Potential new paradigm for treatment of ophthalmic, cardiac, central nervous system (CNS), peripheral nervous system (PNS), and dermal disorders − Supported by deep intellectual property portfolio ▪ Focused on RGN - 259 preservative - free eye drops in late stage clinical development for dry eye syndrome and neurotrophic keratitis (NK) − $4.4 billion global annual dry eye market; $500 million global annual NK market ▪ Approaching commercialization with completed and ongoing Phase 3 clinical trials ▪ Low - cost outsourced business model − 3 active global strategic partnerships via out - licensing and JV agreements − Royalties, regulatory and commercial milestone payments − Equity in U.S. joint venture + royalty − Cash runway into Q4 2017 and Phase 3 data
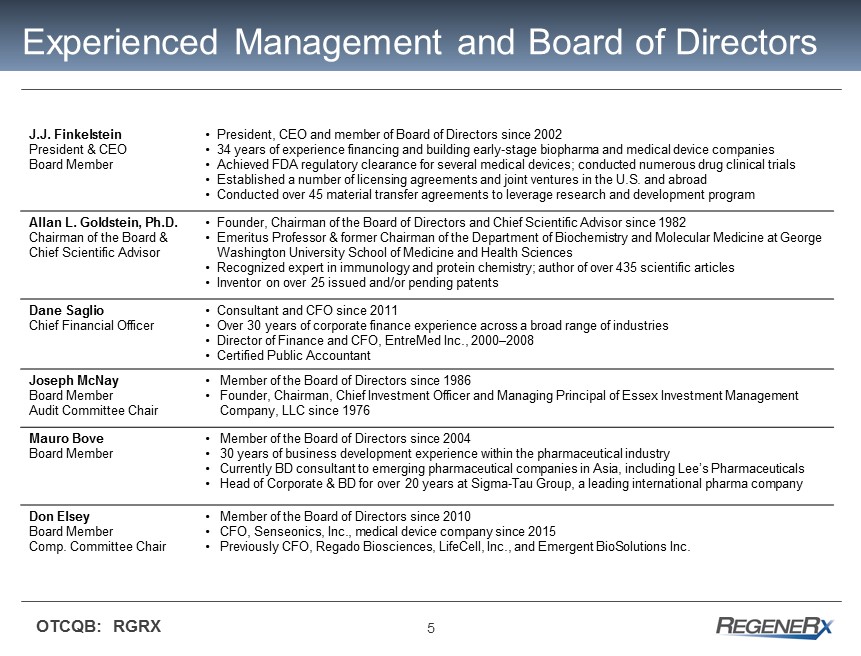
5 OTCQB: RGRX J.J. Finkelstein President & CEO Board Member • President, CEO and member of Board of Directors since 2002 • 34 years of experience financing and building early - stage biopharma and medical device companies • Achieved F DA regulatory clearance for several medical devices; conducted numerous drug clinical trials • Established a number of licensing agreements and joint ventures in the U.S. and abroad • Conducted over 45 material transfer agreements to leverage research and development program Allan L. Goldstein, Ph.D. Chairman of the Board & Chief Scientific Advisor • Founder, Chairman of the Board of Directors and Chief Scientific Advisor since 1982 • Emeritus Professor & former Chairman of the Department of Biochemistry and Molecular Medicine at George Washington University School of Medicine and Health Sciences • Recognized expert in immunology and protein chemistry; author of over 435 scientific articles • Inventor on over 25 issued and/or pending patents Dane Saglio Chief Financial Officer • Consultant and CFO since 2011 • Over 30 years of corporate finance experience across a broad range of industries • Director of Finance and CFO, EntreMed Inc., 2000 – 2008 • Certified Public Accountant Joseph McNay Board Member Audit Committee Chair • Member of the Board of Directors since 1986 • Founder, Chairman, Chief Investment Officer and Managing Principal of Essex Investment Management Company, LLC since 1976 Mauro Bove Board Member • Member of the Board of Directors since 2004 • 30 years of business development experience within the pharmaceutical industry • Currently BD consultant to emerging pharmaceutical companies in Asia, including Lee’s Pharmaceuticals • Head of Corporate & BD for over 20 years at Sigma - Tau Group, a leading international pharma company Don Elsey Board Member Comp. Committee Chair • Member of the Board of Directors since 2010 • CFO, Senseonics, Inc., medical device company since 2015 • Previously CFO, Regado Biosciences, LifeCell, Inc., and Emergent BioSolutions Inc. Experienced Management and Board of Directors

6 OTCQB: RGRX Strategic Outsourced/Partnership Business Model ▪ Objectives: − Partner with companies with clinical development and/or commercialization strengths • Strategy enables multiple paths to commercialization • Mitigates risks and facilitates efficiency − Obtain royalties and development and commercial milestone payments from licensees − Retain significant equity position and royalties through joint ventures − Leverage R&D through Material Transfer Agreements (MTAs) with major research institutions − M inimum fixed costs and funding obligations will provide strong operating leverage No Financial Obligations Required in Any Strategic Partnership or MTA
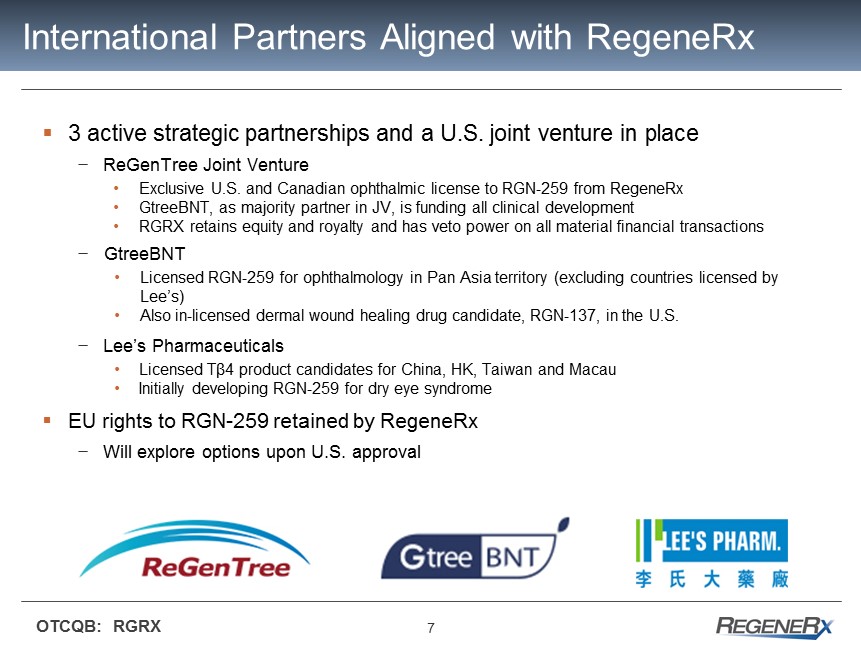
7 OTCQB: RGRX International Partners Aligned with RegeneRx ▪ 3 active strategic partnerships and a U.S. joint venture in place − ReGenTree Joint Venture • Exclusive U.S. and Canadian ophthalmic license to RGN - 259 from RegeneRx • GtreeBNT, as majority partner in JV, is funding all clinical development • RGRX retains equity and royalty and has veto power on all material financial transactions − GtreeBNT • Licensed RGN - 259 for ophthalmology in Pan Asia territory (excluding countries licensed by Lee’s) • Also in - licensed dermal wound healing drug candidate, RGN - 137, in the U.S. − Lee’s Pharmaceuticals • L icensed T β 4 product candidates for China, HK, Taiwan and Macau • Initially developing RGN - 259 for dry eye syndrome ▪ EU rights to RGN - 259 retained by RegeneRx − Will explore options upon U . S . approval
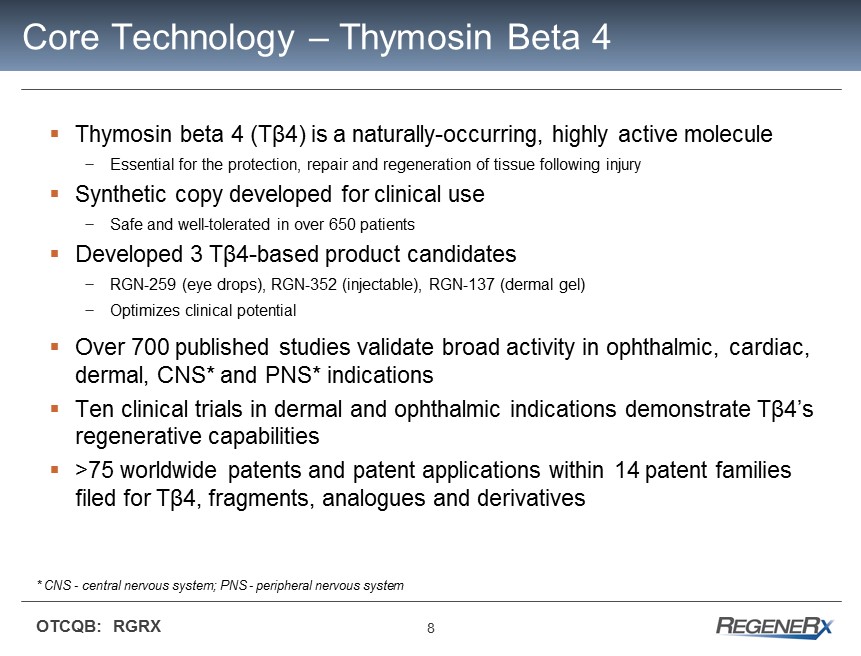
8 OTCQB: RGRX Core Technology – Thymosin Beta 4 ▪ Thymosin beta 4 (T β 4) is a naturally - occurring, highly active molecule − Essential for the protection , repair and regeneration of tissue following injury ▪ Synthetic copy developed for clinical use − Safe and well - tolerated in over 650 patients ▪ Developed 3 T β 4 - based product candidates − RGN - 259 (eye drops), RGN - 352 (injectable), RGN - 137 (dermal gel) − Optimizes clinical potential ▪ Over 700 published studies validate broad activity in ophthalmic, cardiac, dermal, CNS* and PNS * indications ▪ Ten clinical trials in dermal and ophthalmic indications demonstrate T β 4’s regenerative capabilities ▪ > 75 worldwide patents and patent applications within 14 patent families filed for T β4 , fragments, analogues and derivatives * CNS - central nervous system; PNS - peripheral nervous system
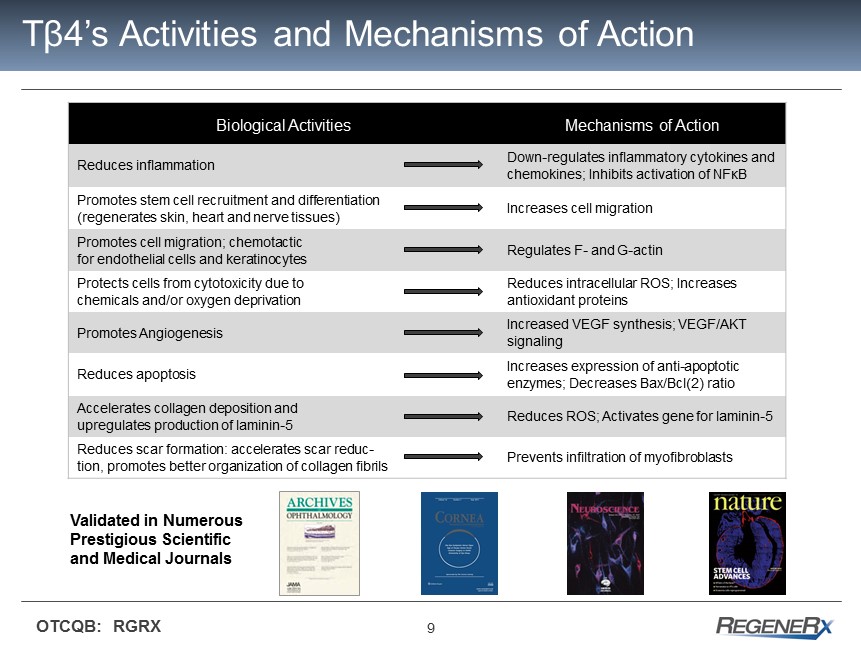
9 OTCQB: RGRX T β 4’s Activities and Mechanisms of Action Biological Activities Mechanisms of Action Reduces inflammation Down - regulates inflammatory cytokines and chemokines; Inhibits activation of NF κ B Promotes stem cell recruitment and differentiation (regenerates skin, heart and nerve tissues) Increases cell migration Promotes cell migration; chemotactic for endothelial cells and keratinocytes Regulates F - and G - actin Protects cells from cytotoxicity due to chemicals and/or oxygen deprivation Reduces intracellular ROS; Increases antioxidant proteins Promotes Angiogenesis Increased VEGF synthesis; VEGF/AKT signaling Reduces apoptosis Increases expression of anti - apoptotic enzymes; Decreases Bax/Bcl(2) ratio Accelerates collagen deposition and upregulates production of laminin - 5 Reduces ROS; Activates gene for laminin - 5 Reduces scar formation: accelerates scar reduc - tion, promotes better organization of collagen fibrils Prevents infiltration of myofibroblasts Validated in Numerous Prestigious Scientific and Medical Journals
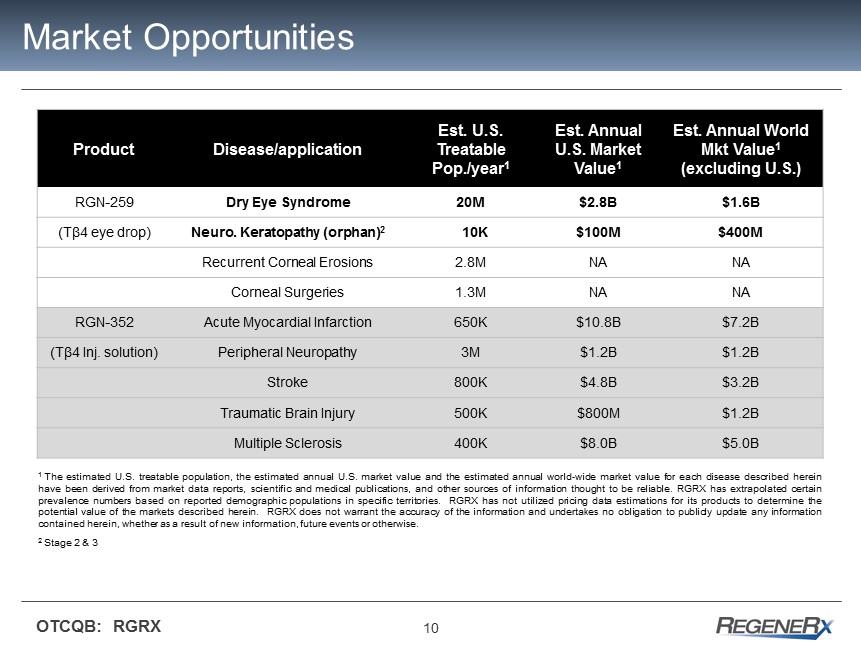
10 OTCQB: RGRX Market Opportunities Product Disease/application Est. U.S. Treatable Pop./year 1 Est. Annual U.S. Market Value 1 Est. Annual World Mkt Value 1 (excluding U.S.) RGN - 259 Dry Eye Syndrome 20M $2.8B $1.6B (T β4 eye drop) Neuro. Keratopathy (orphan) 2 10K $100 M $400M Recurrent Corneal Erosions 2.8M NA NA Corneal Surgeries 1.3M NA NA RGN - 352 Acute Myocardial Infarction 650K $10.8B $7.2B (T β4 Inj. s olution) Peripheral Neuropathy 3M $1.2B $1.2B Stroke 800K $4.8B $3.2B Traumatic Brain Injury 500K $800M $1.2B Multiple Sclerosis 400K $8.0B $5.0B 1 The estimated U . S . treatable population, the estimated annual U . S . market value and the estimated annual world - wide market value for each disease described herein have been derived from market data reports, scientific and medical publications, and other sources of information thought to be reliable . RGRX has extrapolated certain prevalence numbers based on reported demographic populations in specific territories . RGRX has not utilized pricing data estimations for its products to determine the potential value of the markets described herein . RGRX does not warrant the accuracy of the information and undertakes no obligation to publicly update any information contained herein, whether as a result of new information, future events or otherwise . 2 Stage 2 & 3
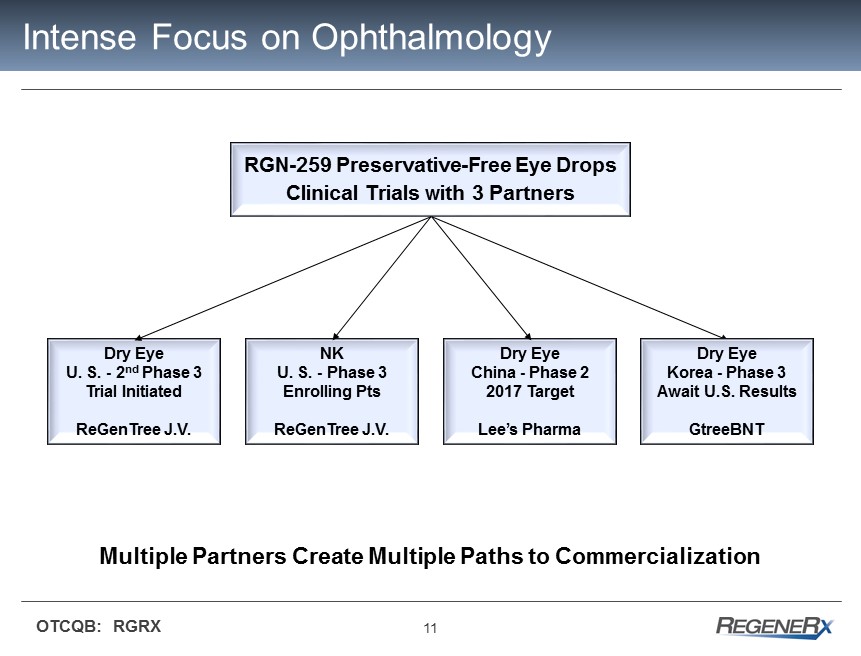
11 OTCQB: RGRX Intense Focus on Ophthalmology RGN - 259 Preservative - Free Eye Drops Clinical Trials with 3 Partners Dry Eye U. S. - 2 nd Phase 3 Trial Initiated ReGenTree J.V. Dry Eye China - Phase 2 2017 Target Lee’s Pharma NK U. S. - Phase 3 Enrolling Pts ReGenTree J.V. Multiple Partners Create Multiple Paths to Commercialization Dry Eye Korea - Phase 3 Await U.S. Results GtreeBNT

12 OTCQB: RGRX Efficacy Results from Phase 2b/3 Dry Eye Trial ▪ RGN - 259 demonstrated statistically significant improvement in both signs and symptoms of dry eye − 317 patient, double - masked, placebo - controlled U.S. dry eye trial in controlled adverse environment (CAE) model − Patients had a statistically significant reduction in ocular discomfort at end of treatment • observed in previous Phase 2 trial and an approvable endpoint − Improved ocular staining at end of treatment in patients with low or compromised tear film break - up time with statistically significant reduction of fluorescein staining • observed in previous Phase 2 trial and an approvable endpoint − More severe dry eye patients showed better results − Trial showed a dose response with higher dose providing better response − Exhibited fast - acting treatment effect in adverse environment and natural environment • Efficacy in a natural environmental was demonstrated in more symptomatic patients at baseline with statistically significant improvements in ocular discomfort at Day 28 prior to CAE exposure − Safe and well - tolerated Results Provide Clear Direction for Clinical Regulatory Pathway
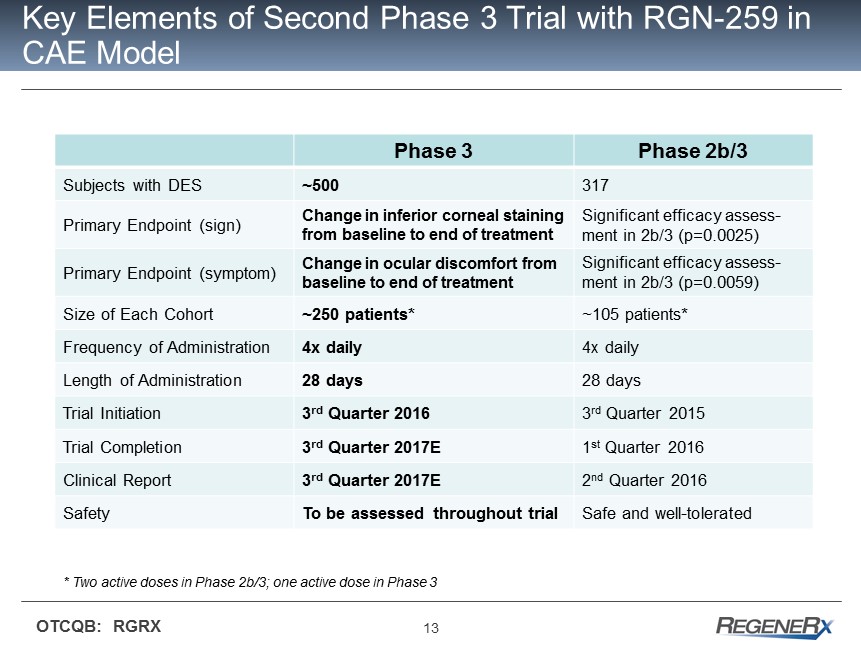
13 OTCQB: RGRX Key Elements of Second Phase 3 Trial with RGN - 259 in CAE Model Phase 3 Phase 2b/3 Subjects with DES ~500 317 Primary Endpoint (sign) Change in inferior corneal staining from baseline to end of treatment Significant efficacy assess - ment in 2b/3 (p=0.0025) Primary Endpoint (symptom) Change in ocular discomfort from baseline to end of treatment Significant efficacy assess - ment in 2b/3 (p=0.0059) Size of Each Cohort ~250 patients* ~105 patients* Frequency of Administration 4x daily 4x daily Length of Administration 28 days 28 days Trial Initiation 3 rd Quarter 2016 3 rd Quarter 2015 Trial Completion 3 rd Quarter 2017E 1 st Quarter 2016 Clinical Report 3 rd Quarter 2017E 2 nd Quarter 2016 Safety To be assessed throughout trial Safe and well - tolerated * Two active doses in Phase 2b/3; one active dose in Phase 3

14 OTCQB: RGRX U.S. Dry Eye Market ▪ Restasis ® and Lifitegrast (Xiidra™) are only Rx products in U.S. for dry eye − >$1 billion annual Restasis ® sales (1) − Patients experience burning & stinging with Restasis ® − Efficacy typically not seen for 6 months − Package insert states Restasis ® is only 15% effective vs. 5% for placebo ▪ Xiidra ™ to be launched in U.S. shortly − Patients report irritation, reduced visual acuity, and dysgeusia (foul taste in mouth) ▪ Clinical data for RGN - 259 shows effects are within days, with no burning or stinging or dysgeusia reported ▪ RGN - 259 demonstrated higher efficacy than Restasis ® when compared side by side in animal model of dry eye RGN - 259 Compares Favorably to Approved Drugs for Dry Eye (1) Source: Drugs.com Statistics, 2013 ( http://www.drugs.com/stats/top100/sales ).

15 OTCQB: RGRX U.S. Neurotrophic Keratopathy Clinical Trial ▪ Neurotrophic Keratopathy (NK) − Chronic degenerative disease characterized by decreased corneal sensitivity and limited or no corneal healing − Approximately 50,000 cases of NK in EU and U.S. (1) • Estimated 10,000 stage 2 and 3 cases − Estimated $500 million annual market worldwide − No effective drugs currently available ▪ Orphan drug designation received on December 31, 2013 − Accelerated clinical development timeline − 7 years of market exclusivity in the U.S., 10 years in the E.U. − Certain tax credits upon marketing approval ▪ ReGenTree Phase 3 trial completion expected in 2017 − RGRX will have access for use in other territories, i.e., EU (1) Clinical Ophthalmology 2014; 8: 571 – 579 .

16 OTCQB: RGRX Key Milestones Over Next 15 Months x Q3 2016 – Positive FDA communication on Phase 3 Dry Eye Protocol x Q3 2016 - Begin 2 nd U.S. Phase 3 Dry Eye Trial ▪ Mid - 2017E - Lee’s Pharma to Start Phase 2b Dry Eye Trial in China ▪ H2 2017E - Complete Enrollment of Phase 3 Dry Eye Trial ▪ H2 2017E - Data Analysis on Phase 3 Dry Eye Trial ▪ H2 2017E - Report Data from Phase 3 Dry Eye Trial ▪ H2 2017 - Meet with FDA on U.S. Phase 3 Trial/NDA ▪ H2 2017 - Complete U.S. Phase 3 NK study

17 OTCQB: RGRX RGN - 352 Multiple Opportunities in Cardiac, CNS and PNS Disorders
18 OTCQB: RGRX Potential New Paradigm in the Treatment of MI* ▪ Designed to protect myocardium and repair damaged heart tissue from STEMI, the deadliest form of heart attack ▪ Promotes cardiac repair and regeneration by multiple mechanisms in various in vitro and in vivo models − In vivo and human safety data support large market indications such as Acute Myocardial Infarction and Heart Failure − In vivo and human safety data also support CNS and PNS indications such as Traumatic Brain Injury, Peripheral Neuropathy, Multiple Sclerosis, and Stroke ▪ RGN - 352 is safe and well - tolerated − 27 preclinical toxicology and pharmacology studies − Successful 80 - subject Phase 1 trial with no drug related adverse events at 4 dose levels − Supports acute systemic use ▪ Currently holding discussions with potential strategic partners while applying for government grants to fund initial clinical trials Phase 2 - Ready * Myocardial infarction or heart attack
19 OTCQB: RGRX Similar Regenerative Effects in CNS & PNS Models ▪ Preclinical data support potent regenerative and protective effects of Tβ4 on the central nervous and peripheral nervous systems − Data show statistical significance and dose responses in all injury models ▪ Tβ4 acts to increase stem cell recruitment, decrease inflammation, promote cell survival, increase blood supply, decrease scar formation ▪ Peer - reviewed scientific publications indicate significant potential for the treatment of various neurological injuries* − Traumatic Brain Injury − Stroke − Peripheral Neuropathy − Multiple Sclerosis − Spinal Cord Injury Multiple Potential CNS and PNS Applications * See Appendix for detailed Potential CNS Treatment Opportunities
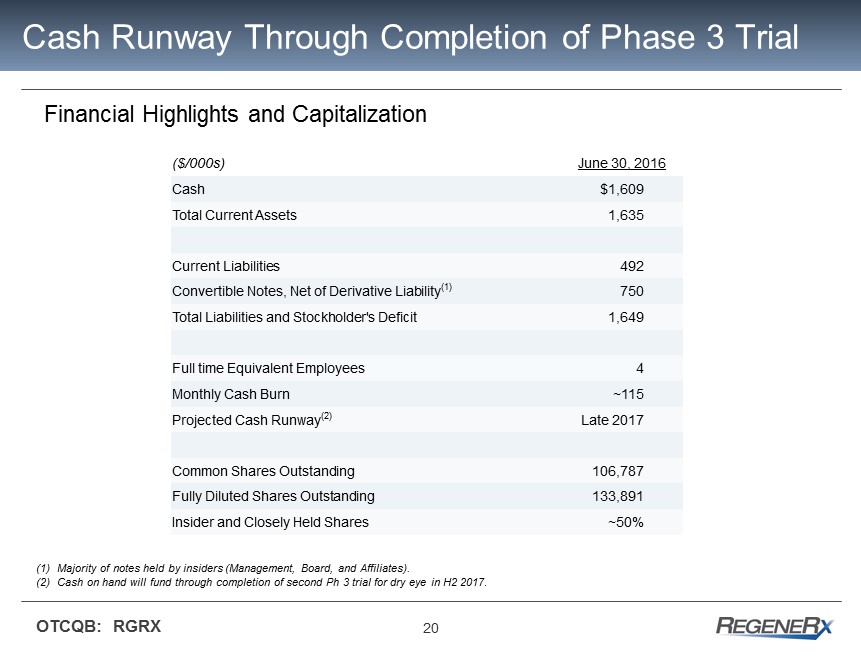
20 OTCQB: RGRX Cash Runway Through Completion of Phase 3 Trial Financial Highlights and Capitalization (1) Majority of notes held by insiders (Management, Board, and Affiliates). (2) Cash on hand will fund through completion of second Ph 3 trial for dry eye in H2 2017 . ($/000s) June 30, 2016 Cash $1,609 Total Current Assets 1,635 Current Liabilities 492 Convertible Notes, Net of Derivative Liability (1 ) 750 Total Liabilities and Stockholder's Deficit 1,649 Full time Equivalent Employees 4 Monthly Cash Burn ~115 Projected Cash Runway (2) Late 2017 Common Shares Outstanding 106,787 Fully Diluted Shares Outstanding 133,891 Insider and Closely Held Shares ~50%
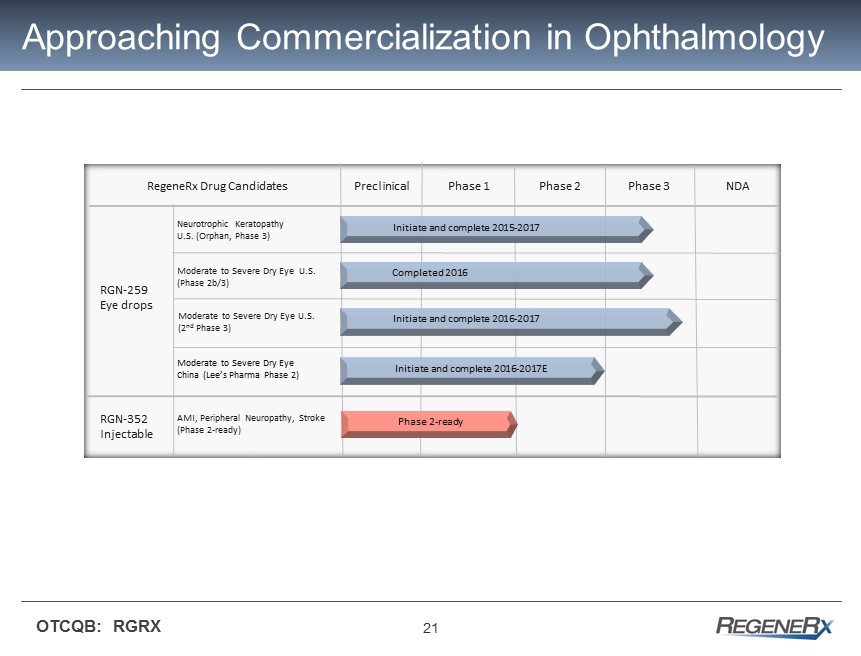
21 OTCQB: RGRX Approaching Commercialization in Ophthalmology Preclinical Phase 1 Phase 3 RegeneRx Drug Candidates RGN - 259 Eye drops RGN - 352 Injectable AMI, Peripheral Neuropathy, Stroke (Phase 2 - ready) Neurotrophic Keratopathy U.S. (Orphan, Phase 3) Moderate to Severe Dry Eye U.S. (2 nd Phase 3) Phase 2 - ready Moderate to Severe Dry Eye U.S. (Phase 2b/3) Completed 2016 Moderate to Severe Dry Eye China (Lee’s Pharma Phase 2) Initiate and complete 2016 - 2017E Initiate and complete 2016 - 2017 Initiate and complete 2015 - 2017 Phase 2 NDA
22 OTCQB: RGRX Appendix
23 OTCQB: RGRX Prestigious Scientific Advisory Board Allan L. Goldstein, Ph.D. Chairman of the Board & Chief Scientific Advisor • Founder, Chairman of the Board of Directors and Chief Scientific Advisor since 1982 • Emeritus Professor & former Chairman of the Department of Biochemistry and Molecular Medicine at George Washington University School of Medicine and Health Sciences • Recognized expert in immunology and protein chemistry; author of over 435 scientific articles • Inventor on over 25 issued and/or pending patents Hynda K. Kleinman, Ph.D. • Former Chief of the Cell Biology Section at the National Institute of Dental and Craniofacial Research, Bethesda, Maryland • Expert in mechanisms of action of T β 4 • Oversees Material Transfer Agreements with research institutions around the world; PI on T β 4 grants Gabriel Sosne, M.D. • Ophthalmologist, Associate Professor, Wayne State University and Kresge Eye Institute • Expert on mechanisms of action and clinical effects of T β 4 • Conducted first human ophthalmic clinical studies with T β 4 Deepak Srivastava, M.D. • Director, Gladstone Institute of Cardiovascular Disease • Professor, Pediatrics and Biochemistry & Biophysics, Wilma and Adeline Pirag Distinguished Professor in Pediatric Development Cardiology, University of California, San Francisco, California • Discovered regenerative effects of T β 4 in the heart Ewald Hannappel, Ph.D. • Professor of Biochemistry, University of Erlangen, Nuremberg, Germany • Expert in the chemical structure and of T β 4 Barrett Katz, M.D., Ph.D. • Clinical Professor, Department of Ophthalmology, Cornell University Medical School, New York, New York • Expert in Neuro - ophthalmology Jo - David Fine, M.D., M.P.H. • Professor of Medicine, Division of Dermatology, Vanderbilt University Medical Center, Nashville, Tennessee • Pediatric Dermatologist; Expert in Epidermolysis Bullosa Brian Schreiber, M.D. • Assistant Professor, Department of Medicine, Division of Nephrology, Medical College of Wisconsin • Vice President, Medical Affairs, Sigma - Tau Pharmaceuticals, Gaithersburg, MD

24 OTCQB: RGRX ReGenTree U.S. Joint Venture ▪ ReGenTree − Joint Venture with GtreeBNT, a Korean biopharmaceutical co. − Exclusive U.S. and Canadian ophthalmic license to RGN - 259 from RegeneRx − Currently RGRX owns 42% and GtreeBNT owns 58% of equity − GtreeBNT, as operating partner in JV, is obligated to fund all clinical development through commercialization − RGRX ownership: • 42% - 25% equity ownership depending on clinical and regulatory development • 5% - 10% royalty depending on commercialization track • Minimum total ownership = 40% in the event of sale to 3 rd party • Veto power on all material financial transactions
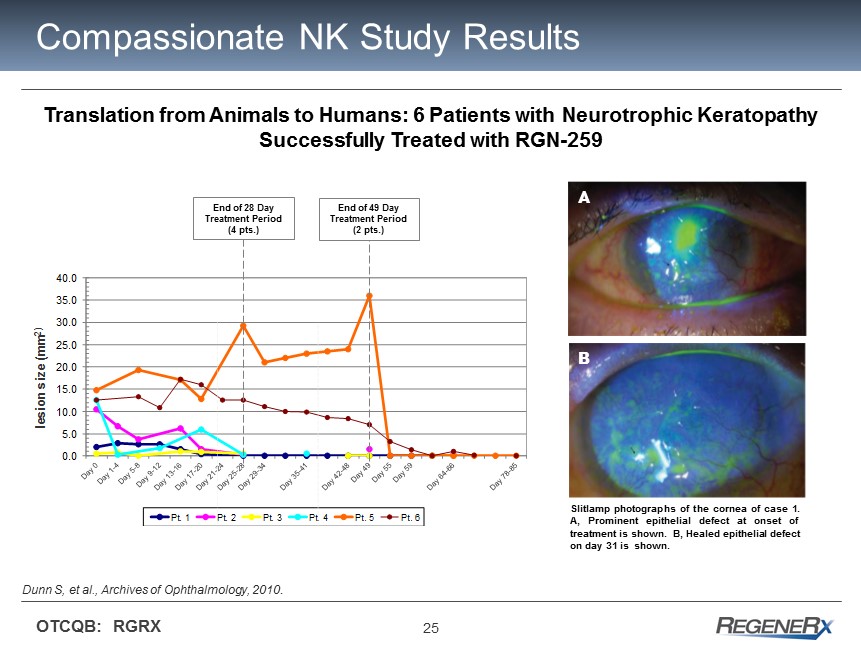
25 OTCQB: RGRX Compassionate NK Study Results 0.0 5.0 10.0 15.0 20.0 25.0 30.0 35.0 40.0 lesion size (mm 2) Pt. 1 Pt. 2 Pt. 3 Pt. 4 Pt. 5 Pt. 6 Translation from Animals to Humans: 6 Patients with Neurotrophic Keratopathy Successfully Treated with RGN - 259 End of 49 Day Treatment Period (2 pts.) End of 28 Day Treatment Period (4 pts.) Dunn S, et al., Archives of Ophthalmology, 2010 . Slitlamp photographs of the cornea of case 1 . A, Prominent epithelial defect at onset of treatment is shown . B, Healed epithelial defect on day 31 is shown . A B
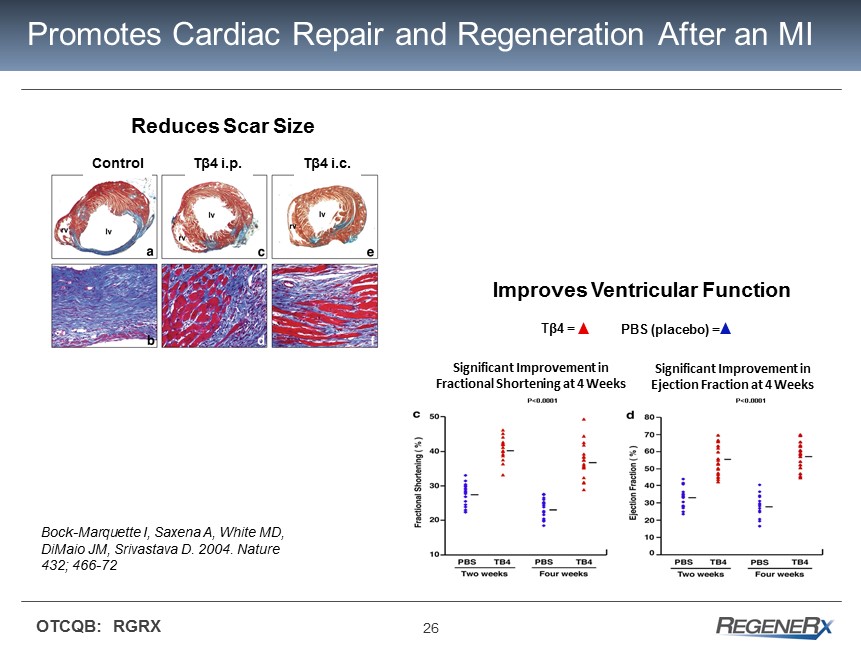
26 OTCQB: RGRX Promotes Cardiac Repair and Regeneration After an MI Control Tβ4 i.p. Tβ4 i.c. Bock - Marquette I, Saxena A, White MD, DiMaio JM, Srivastava D. 2004. Nature 432; 466 - 72 Significant Improvement in Fractional Shortening at 4 Weeks Significant Improvement in Ejection Fraction at 4 Weeks T β 4 = PBS (placebo) = Reduces Scar Size Improves Ventricular Function
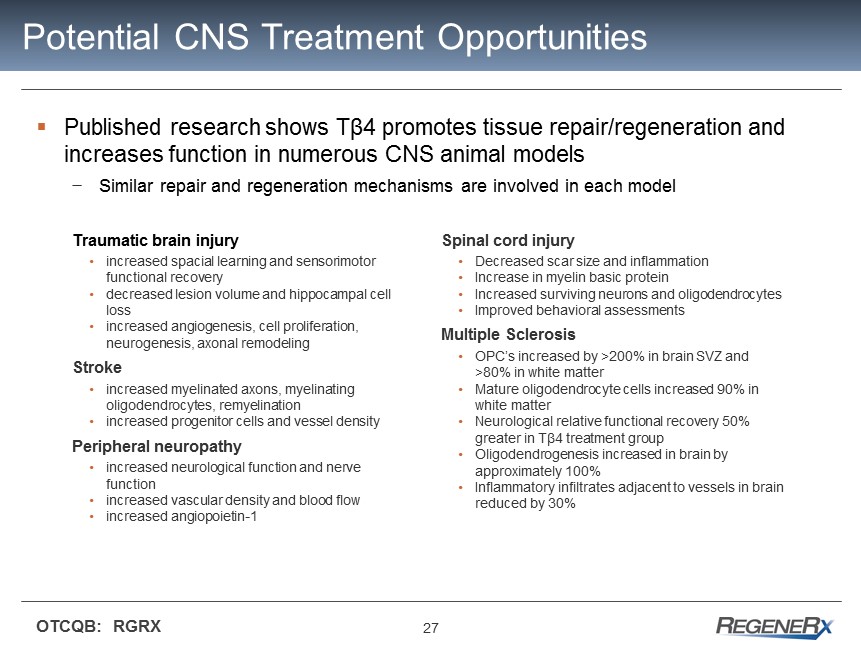
27 OTCQB: RGRX Potential CNS Treatment Opportunities ▪ Published research shows Tβ4 promotes tissue repair/regeneration and increases function in numerous CNS animal models − Similar repair and regeneration mechanisms are involved in each model Spinal cord injury • Decreased scar size and inflammation • Increase in myelin basic protein • Increased surviving neurons and oligodendrocytes • Improved behavioral assessments Multiple Sclerosis • OPC’s increased by >200% in brain SVZ and >80% in white matter • Mature oligodendrocyte cells increased 90% in white matter • Neurological relative functional recovery 50% greater in T β4 treatment group • Oligodendrogenesis increased in brain by approximately 100% • Inflammatory infiltrates adjacent to vessels in brain reduced by 30% Traumatic brain injury • increased spacial learning and sensorimotor functional recovery • decreased lesion volume and hippocampal cell loss • increased angiogenesis, cell proliferation, neurogenesis, axonal remodeling Stroke • increased myelinated axons, myelinating oligodendrocytes, remyelination • increased progenitor cells and vessel density Peripheral neuropathy • increased neurological function and nerve function • increased vascular density and blood flow • increased angiopoietin - 1
28 OTCQB: RGRX Extensive IP Portfolio ▪ Key U.S. patent recently granted for maintaining blood concentrations of T β 4 through injection or infusion that has broad range of coverage − Expires in late 2031, or longer, depending on USPTO extensions ▪ >75 total patents and patent applications within 14 patent families worldwide for T β4 , fragments, analogues and derivatives, including: ▪ Expiries range from 2019 - 2033
