Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Moleculin Biotech, Inc. | v448561_8k.htm |
Exhibit 99.1

August 2016

Disclaimer All statements contained herein other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in the “Risk Factors” section of the registration statement on Form S-1 we filed on April 27, 2016. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward looking statements.

We built Moleculin around a diverse portfolio of disruptive cancer technologies, each with high revenue potential. Our lead leukemia drug candidate has already shown life-saving potential in clinical trials. We have a unique compound that appears to both attack the most difficult cancers directly while also stimulating the patient’s natural immune response and we have a promising new approach to reaching and treating brain tumors. - Wally Klemp, Chairman

Moleculin Team

Veteran Management Team Cisplatin Carboplatin COO Harvard, Tufts, UVA Dana Farber, FDA #1 INC 500 List $400 million in revenue CMO CEO

Advisors and Board Members Reata Pharmaceuticals Big 4 audit partners Multi-billion dollar exit Cancer experts

All of our technologies were developed at MD Anderson Cancer Center, the world’s largest cancer research facility headquartered within the world’s largest medical center With over 1,500 cancer researchers, our sponsored research has been supported by truly state of the art resources

Technology Portfolio
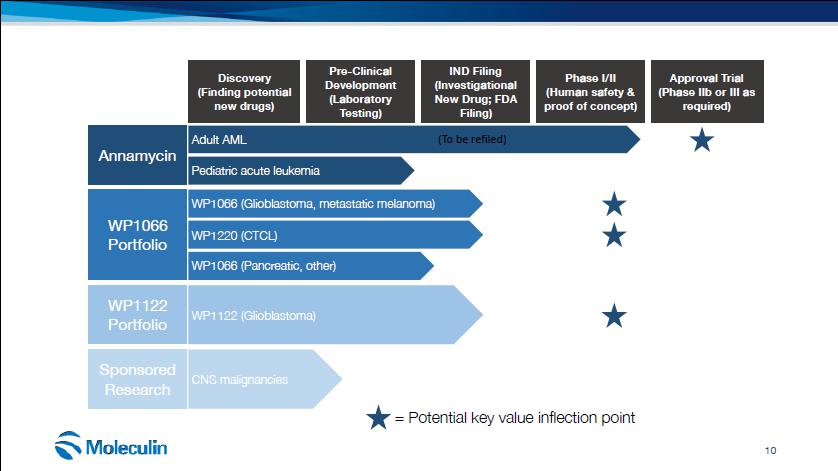
Discovery (Finding potential new drugs) Pre-Clinical Development (Laboratory Testing) IND Filing (Investigational New Drug; FDA Filing) Phase I/II (Human safety & proof of concept) Approval Trial (Phase IIb or III as required) Adult AML WP1066 (Glioblastoma, metastatic melanoma) WP1122 (Glioblastoma) Pediatric acute leukemia WP1220 (CTCL) WP1066 (Pancreatic, other) (To be refiled) Annamycin WP1066 Portfolio WP1122 Portfolio = Potential key value inflection point Sponsored CNS malignancies Research

Annamycin


BMT (Results in Cure) 20% 80% Success Failure Induc4on Therapy (Qualify for BMT) 80% 20% Success Failure

Non-cardiotoxic Avoids multidrug resistance More potent Cardiotoxic Defeated by multidrug resistance Current Drugs Annamycin
Doxorubicin Example

Annamycin Example

Annamycin Delivers Relapsed/Refractory Acute Leukemia Patients 50% Efficacy Signal 30% Cleared Bone Marrow Blasts Annamycin gives new hope to patients who have run out of options Patients failed an average of 5 previous Induction Therapy attempts with standard of care

Annamycin (based on last trial) What is possible if we get to patients sooner?

WP1066 Directly attacks the worst cancers Recruits natural immune system IRB approved for human trial

WP1122 Cuts off fuel supply to brain tumors Outperforms billion-dollar drug Human trials in 1-2 years

We Are Excited

Investment Highlights Multiple shots Worldclass collaboration Game-changing breakthroughs Solid protection Veteran management
