UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of report (Date of earliest event reported): September 8, 2016
COLUCID PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-37358 | 20-3419541 | ||
| (State or other jurisdiction of incorporation or organization) |
(Commission File Number) |
(I.R.S. Employer Identification No.) | ||
| 222 Third Street, Cambridge, Massachusetts | 02142 | |||
| (Address of principal executive offices) | (Zip Code) | |||
(857) 285-6495
(Registrant’s telephone number, include area code)
N/A
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Item 8.01. Other Events.
CoLucid Pharmaceuticals, Inc. hereby provides the following information relating to its pivotal Phase 3 randomized, double-blind, placebo-controlled clinical trial of lasmiditan, or SAMURAI, conducted under a special protocol assessment, or SPA, agreement, with the U.S. Food and Drug Administration, or FDA:
In September 2016, we released top-line data results and announced that SAMURAI achieved both primary and key secondary efficacy endpoints with statistical significance (p < 0.001). Lasmiditan was also well tolerated. However, top-line data of a clinical trial do not necessarily predict final results. Top-line data are based on a preliminary analysis of currently available efficacy and safety data, and therefore the reported results, findings and conclusions related to SAMURAI are subject to change following a comprehensive review of the more extensive data that we expect to receive related to SAMURAI. We expect to present the final detailed results from SAMURAI in mid-September 2016 at a symposium during the 5th European Headache and Migraine Trust International Congress taking place in Glasgow, Scotland. SAMURAI was a randomized, double-blind, placebo-controlled parallel group study designed to evaluate the efficacy and safety of lasmiditan (100 mg and 200 mg) in comparison to placebo.
Efficacy. The primary endpoint of SAMURAI was the efficacy of lasmiditan (100 mg and 200 mg) in comparison to placebo based on freedom from migraine headache pain two hours after dosing. The key secondary endpoint was the efficacy of lasmiditan based on freedom from the most bothersome associated symptom, or MBS, of migraine (nausea, phonophobia or photophobia) two hours after dosing. Data from the study were collected using electronic diaries during the treated attack. Beginning pre-dose, patients indicated their degree of headache pain on a 4-point scale: 0 or no pain; 1 or mild pain; 2 or moderate pain; or 3 or severe pain. Patients also indicated the presence or absence of nausea, phonophobia or photophobia, and at the pre-dose time point identified the associated symptom present that was “most bothersome.” At each time point assessment, patients were asked to indicate the degree of headache pain and the presence or absence of each associated symptom. The MBS endpoint was patient-centric and measured treatment effect of study drug on associated symptoms. Both the primary and key secondary endpoints of SAMURAI conform to the FDA’s Draft Guidance for Industry, Migraine: Developing Drugs for Acute Treatment, issued in October 2014. The primary and key secondary endpoints of SAMURAI were powered at greater than 90% and greater than 80%, respectively.
| Primary Endpoint |
Lasmiditan (100mg) |
Lasmiditan (200mg) |
Placebo | |||||||||
| % of patients migraine headache pain free at two hours |
28.2% | 32.2% | 15.3% | |||||||||
| Odds Ratio (95% confidence interval) |
2.2 (1.6 – 3.0) | 2.6 (2.0 – 3.6) | ||||||||||
| p-value |
p < 0.001 | p < 0.001 | ||||||||||
| Key Secondary Endpoint |
||||||||||||
| % of patients MBS free at two hours |
40.9% | 40.7% | 29.5% | |||||||||
| Odds Ratio (95% confidence interval) |
1.7 (1.3 – 2.2) | 1.6 (1.3 – 2.1) | ||||||||||
| p-value |
p < 0.001 | p < 0.001 | ||||||||||
The following table sets forth data regarding the selection of the MBS in SAMURAI.
| Selected MBS |
Lasmiditan (100mg) (n=469) |
Lasmiditan (200mg) (n=481) |
Placebo (n=488) | |||
| Nausea |
115 (24.5%) | 118 (24.5%) | 115 (23.6%) | |||
| Phonophobia |
117 (24.9%) | 96 (20.0%) | 104 (21.3%) | |||
| Photophobia |
237 (50.5%) | 267 (55.5%) | 269 (55.1%) |
Adverse Events. Lasmiditan was well tolerated, with the majority of treatment emergent adverse events, or TEAE, being nervous system-related, and 91% of TEAE in lasmiditan-treated patients being described as mild or moderate in nature. Importantly, there was not a significant increase in cardiovascular adverse events in patients
2
who dosed with lasmiditan versus placebo. There were no serious adverse events in SAMURAI that were considered to be related to treatment. The following table sets forth the number and percentage of patients who reported each particular TEAE within the safety population for each dose.
| TEAE |
Lasmiditan (100mg) (n=630) |
Lasmiditan (200mg) (n=609) |
Placebo (n=617) | |||
| Dizziness |
75 (11.9%) | 94 (15.4%) | 19 (3.1%) | |||
| Paresthesia |
36 (5.7%) | 46 (7.6%) | 13 (2.1%) | |||
| Somnolence |
33 (5.2%) | 32 (5.3%) | 14 (2.3%) | |||
| Nausea |
16 (2.5%) | 29 (4.8%) | 9 (1.5%) | |||
| Fatigue |
24 (3.8%) | 18 (3.0%) | 1 (0.2%) | |||
| Lethargy |
12 (1.9%) | 14 (2.3%) | 1 (0.2%) | |||
| Vertigo |
6 (1.0%) | 2 (0.3%) | 0 (0.0%) |
The following table sets forth information regarding the occurrence of the cardiovascular TEAEs, all of which were coded as mild or moderate.
| TEAE |
Lasmiditan (100mg) (n=630) |
Lasmiditan (200mg) (n=609) |
Placebo (n=617) |
All Patients (n=1,856) | ||||
| Palpitations |
2 (0.3%) | 4 (0.7%) | 0 (0.0%) | 6 (0.3%) | ||||
| Reasonably or possibly related |
2 (0.3%) | 3 (0.5%) | 0 (0.0%) | 5 (0.3%) | ||||
| Bradycardia |
1 (0.2%) | 0 (0.0%) | 1 (0.2%) | 2 (0.1%) | ||||
| Reasonably or possibly related |
1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | ||||
| LV Hypertrophy |
0 (0.0%) | 0 (0.0%) | 1 (0.2%) | 1 (0.1%) | ||||
| Reasonably or possibly related |
0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| Sinus bradycardia |
0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 1 (0.1%) | ||||
| Reasonably or possibly related |
0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||||
| Tachycardia |
1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 1 (0.1%) | ||||
| Reasonably or possibly related |
0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Demographics. 2,231 patients were randomized at approximately 80 U.S. sites to participate in SAMURAI to treat a single migraine. Patients randomized had a mean age of 41.6 years, 83% were females, and 74% were Caucasian, with a mean migraine history of over 19 years. Patients randomized experienced an average of over five migraines per month and suffered severe disability from migraine, with an average MIDAS score of 31. Approximately 31% of patients randomized had a history of experiencing aura in connection with migraine. Over 25% of patients randomized used prophylactic medication to reduce the frequency of migraine. 82% of patients randomized had multiple cardiovascular risk factors, or CVRF, or cardiovascular conditions. The most prevalent CVRF were obesity, family history of coronary artery disease, or CAD, smoking, hypertension, post-menopausal women, men over 40 years of age, hyperlipidemia and type 2 diabetes. The most prevalent cardiovascular conditions were arrhythmias, mitral valve disease, angina, atrial fibrillation, congestive heart failure, prior myocardial infarction, Raynaud’s disease, deep vein thrombosis, ischemic stroke, and cerebral infarction. The following table sets forth the cardiovascular risk factors present in SAMURAI.
| Cardiovascular Risk Factors |
% of SAMURAI | |
| Randomized population |
100% | |
| Migraine + 1 additional CVRF |
82% | |
| BMI > 30 kg/m2 |
45% | |
| Significant family history CAD |
34% |
3
| Cardiovascular Risk Factors |
% of SAMURAI | |
| Former smokers |
16% | |
| Current smokers |
16% | |
| Hypertension |
13% | |
| Post-menopausal women |
12% | |
| Male > 40 years old |
10% | |
| Hyperlipidemia |
5% | |
| Hypercholesterolemia |
5% | |
| Type 2 diabetes |
1% |
Analysis. In accordance with the SPA agreement for SAMURAI, the protocol pre-specified the analysis population as the modified intent to treat, or mITT, population. The mITT population was defined in the protocol as all randomized patients who used at least one dose of study drug to treat a qualifying migraine attack and had any post-dose assessments. Patients were evaluated by the study medication to which they were randomized and a qualifying migraine attack was defined as a migraine treated with study drug within four hours of onset. Similar to other migraine clinical trials, approximately 30% of patients randomized in SAMURAI were not included in the mITT population due to either a failure to dose a qualifying migraine during the trial, failure to use the electronic diaries for any time point assessment, or post-randomization ineligibility (clinical lab values). In the trial, the safety population included 1,239 patients who took at least one dose of lasmiditan versus 617 who took at least one dose of placebo. The mITT population consisted of 1,021 patients who took lasmiditan and 524 who took placebo to treat a qualifying migraine. Analysis was conducted using a one-sided test from a logistic regression model with treatment group and background use of medication to reduce the frequency of migraines as covariates. The following table sets forth the SAMURAI analysis populations.
| Analysis Population |
Number | Notes | ||||
| Randomized |
2,231 | |||||
| Randomization failures |
(120 | ) | ||||
| Never dosed |
(255 | ) | ||||
| Safety Population |
1,856 | Dosed with study drug | ||||
| Did not record a single time point |
(185 | ) | ||||
| ITT |
1,671 | Dosed and recorded > 1 time point | ||||
| Dosed > 4 hours after start |
(126 | ) | ||||
| mITT |
1,545 | Dosed a qualifying migraine | ||||
The table below summarizes the current clinical phase of development for each of our product candidates:
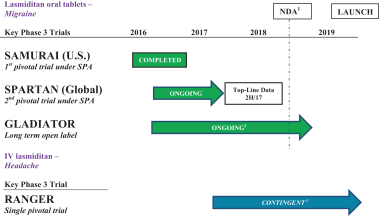
| 1 | If we complete SPARTAN when we anticipate and the trial is successful, we expect to submit an NDA to the FDA for lasmiditan in the first half of 2018. |
| 2 | GLADIATOR is ongoing and we currently expect to complete the clinical trial by the end of 2018, subject to raising additional capital to fund the completion of the trial. |
| 3 | Currently, the timeline for RANGER is contingent upon us securing additional capital. |
4
Risks Related to the Reported Results of SAMURAI
The reported results of SAMURAI are based on top-line data and may ultimately differ from actual results once additional data are received and fully evaluated.
The reported results of SAMURAI that we have publicly disclosed, and that are discussed herein, consist of top-line data. Top-line data are based on a preliminary analysis of currently-available efficacy and safety data, and therefore the reported results, findings and conclusions related to SAMURAI are subject to change following a comprehensive review of the more extensive data that we expect to receive related to SAMURAI. Top-line data are based on important assumptions, estimations, calculations and information currently available to us, and we have not received or had an opportunity to fully and carefully evaluate all of the data related to SAMURAI. As a result, the top-line results of SAMURAI that we have reported may differ from future results, or different conclusions or considerations may qualify such results, once additional data have been received and fully evaluated. In addition, third parties, including regulatory agencies, may not accept or agree with our assumptions, estimations, calculations or analyses or may interpret or weigh the importance of data differently, which could impact the value of lasmiditan, the approvability or commercialization of lasmiditan and our business in general. If the top-line data that we have reported related to SAMURAI differ from actual results, our ability to obtain approval for, and commercialize, our products may be harmed, which could harm our business, financial condition, operating results or prospects.
5
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| COLUCID PHARMACEUTICALS, INC. | ||||||
| Date: September 8, 2016 | By: | /s/ Thomas P. Mathers | ||||
| Thomas P. Mathers | ||||||
| Chief Executive Officer | ||||||
