Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - KERYX BIOPHARMACEUTICALS INC | d245471d8k.htm |

Keryx Biopharmaceuticals, Inc. Keryx Biopharmaceuticals, Inc. September 7, 2016 Exhibit 99.1

Safe Harbor Statement Various remarks that we make about our future expectations, plans and prospects constitute forward-looking statements for purposes of the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. Important factors may cause our actual results to differ materially, including: those regarding the commercialization and ongoing clinical development of Auryxia as well as the expected impact of the supply interruption of Auryxia; the expected timing of when we will have a sufficient supply of Auryxia to make it available to patients again; our ability to quickly and successfully identify and resolve the production-related issue; our ability to quickly and successfully identify and engage secondary suppliers of finished drug product; our ability to receive FDA approval of any secondary suppliers of finished drug product; our ability to return our business to the level of sales that we had achieved prior to the supply interruption; whether we can increase adoption of Auryxia® in patients with CKD on dialysis; the risk that the FDA may not concur with our interpretation of our Phase 3 study results in non-dialysis dependent CKD, supportive data, conduct of the studies, or any other part of our regulatory submission and could ultimately deny approval of ferric citrate for the treatment of IDA in adults with stage 3-5 non-dialysis dependent (NDD) CKD; the risk that if approved for use in NDD-CKD that we may not be able to successfully market Auryxia; and other risk factors identified from time to time in our reports filed with the Securities and Exchange Commission. These and other important factors that may affect our results are discussed under the heading “Risk Factors” in public filings including our 2015 Annual Report on Form 10-K, Form 10-Qs, as well as other filings we periodically make with the SEC. In addition, any forward-looking statements made during this presentation speak only as of the date of this presentation. While we may update these forward-looking statements to reflect events or circumstances that occur after this date, we specifically disclaim any obligation to do so, even if our estimates and expectations change.
Building a Leading Renal Company Today Near Term Future One medicine, multiple indications Multi-product company focused on improving the lives of people with renal disease One medicine, one indication Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; Ferric citrate is being evaluated for use in non-dialysis dependent CKD patients as a treatment for iron deficiency anemia.
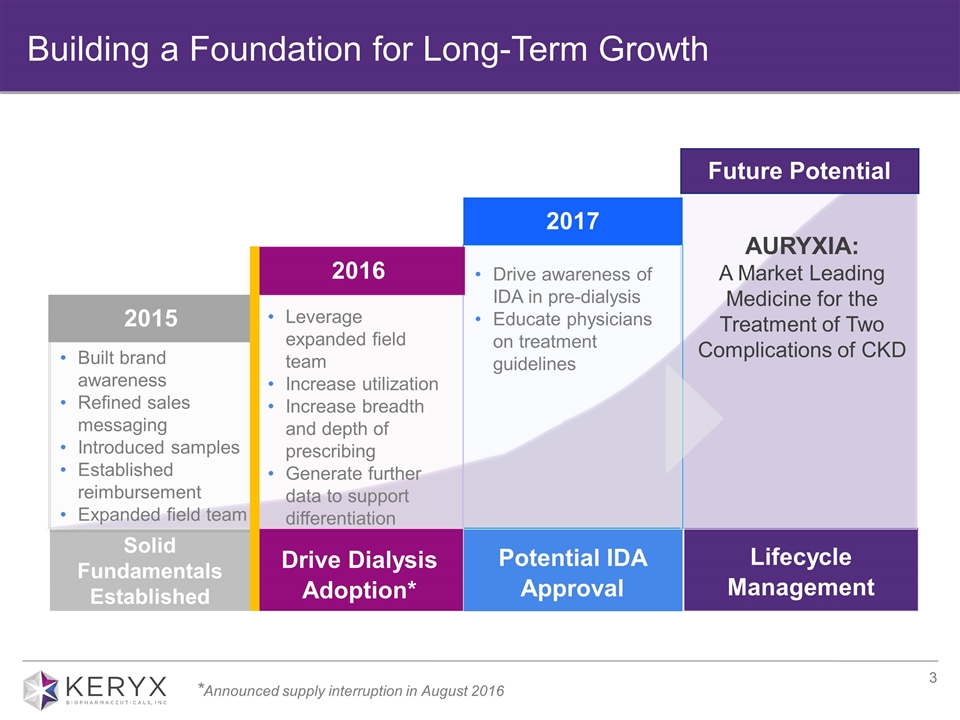
2016 2017 Future Potential Leverage expanded field team Increase utilization Increase breadth and depth of prescribing Generate further data to support differentiation Built brand awareness Refined sales messaging Introduced samples Established reimbursement Expanded field team Drive awareness of IDA in pre-dialysis Educate physicians on treatment guidelines AURYXIA: A Market Leading Medicine for the Treatment of Two Complications of CKD 2015 Drive Dialysis Adoption* Lifecycle Management Potential IDA Approval Solid Fundamentals Established Building a Foundation for Long-Term Growth *Announced supply interruption in August 2016
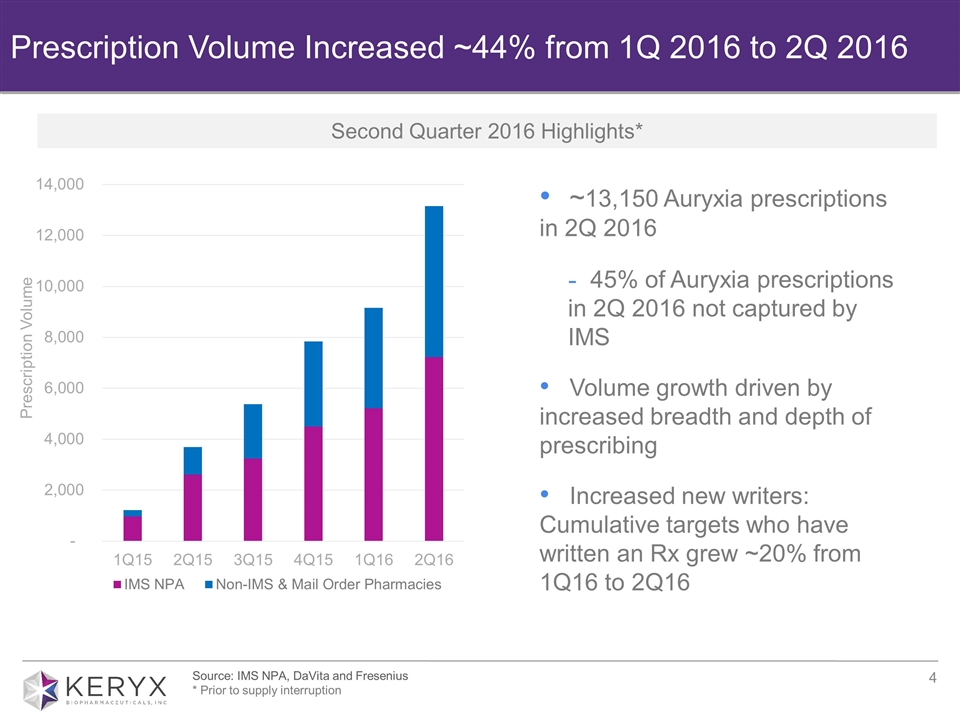
Source: IMS NPA, DaVita and Fresenius * Prior to supply interruption Prescription Volume Increased ~44% from 1Q 2016 to 2Q 2016 ~13,150 Auryxia prescriptions in 2Q 2016 45% of Auryxia prescriptions in 2Q 2016 not captured by IMS Volume growth driven by increased breadth and depth of prescribing Increased new writers: Cumulative targets who have written an Rx grew ~20% from 1Q16 to 2Q16 Second Quarter 2016 Highlights*
Production-related issue converting API to finished drug product Does not affect safety profile of currently available Auryxia Working with two contract manufacturers to restore supply CMO1 Identified step in process where issue is occurring Working together to resolve issue and resume production CMO2 Commercial drug being produced without same issue Two manufacturing sites Submitted for approval with FDA; PDUFA date November 13, 2016 Plan to have adequate supply restored and Auryxia available to patients during 4Q Overview of Auryxia Supply Interruption
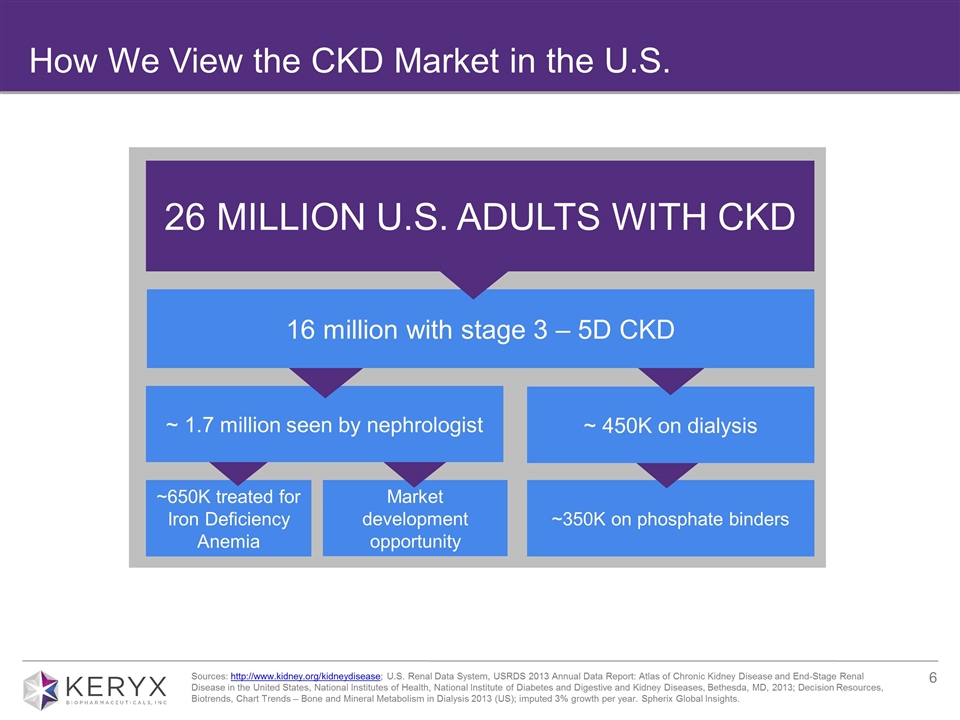
How We View the CKD Market in the U.S. Sources: http://www.kidney.org/kidneydisease; U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013; Decision Resources, Biotrends, Chart Trends – Bone and Mineral Metabolism in Dialysis 2013 (US); imputed 3% growth per year. Spherix Global Insights. 26 MILLION U.S. ADULTS WITH CKD ~650K treated for Iron Deficiency Anemia ~350K on phosphate binders ~ 450K on dialysis Market development opportunity ~ 1.7 million seen by nephrologist 16 million with stage 3 – 5D CKD
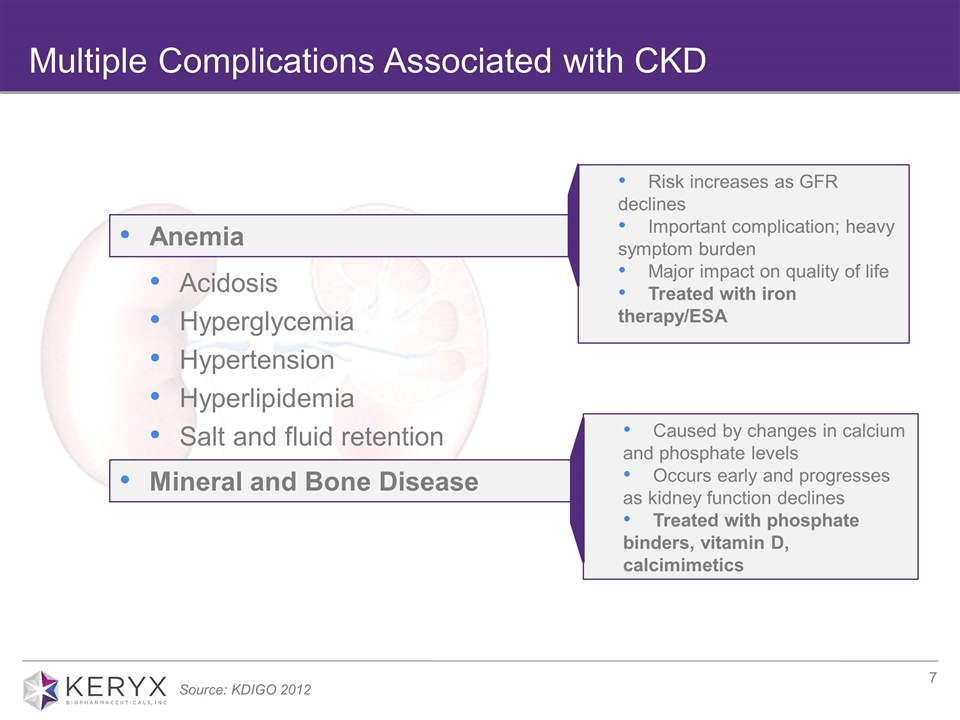
Multiple Complications Associated with CKD Caused by changes in calcium and phosphate levels Occurs early and progresses as kidney function declines Treated with phosphate binders, vitamin D, calcimimetics Acidosis Hyperglycemia Hypertension Hyperlipidemia Salt and fluid retention Risk increases as GFR declines Important complication; heavy symptom burden Major impact on quality of life Treated with iron therapy/ESA Mineral and Bone Disease Anemia Source: KDIGO 2012
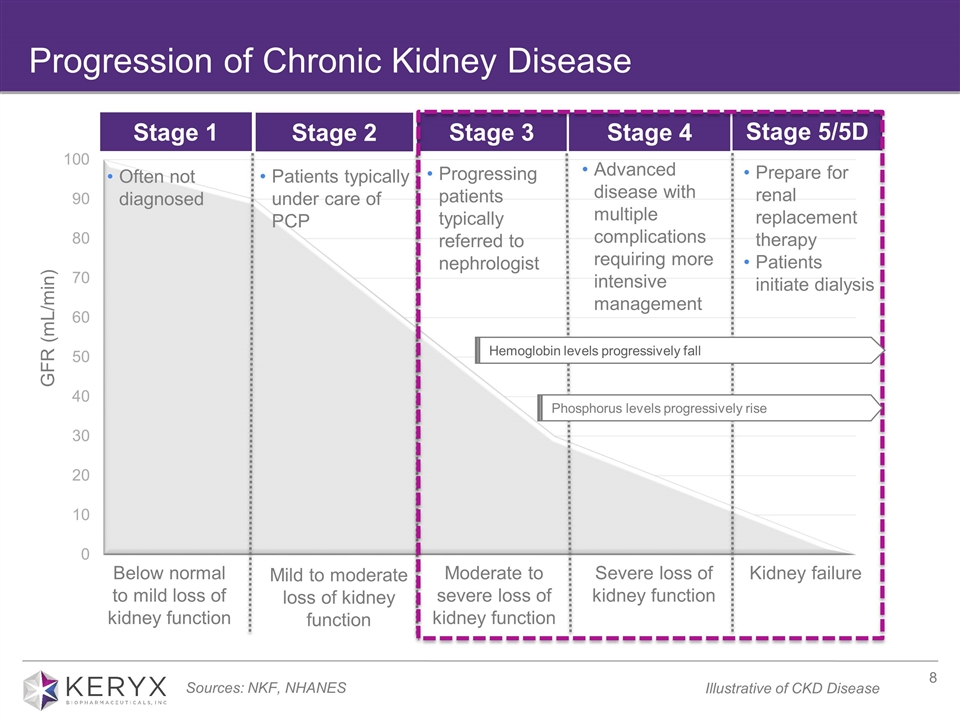
Progression of Chronic Kidney Disease Progressing patients typically referred to nephrologist Prepare for renal replacement therapy Patients initiate dialysis GFR (mL/min) Often not diagnosed Patients typically under care of PCP Moderate to severe loss of kidney function Severe loss of kidney function Kidney failure Below normal to mild loss of kidney function Mild to moderate loss of kidney function Stage 3 Stage 4 Stage 5/5D Stage 1 Stage 2 Hemoglobin levels progressively fall Sources: NKF, NHANES Advanced disease with multiple complications requiring more intensive management Phosphorus levels progressively rise Illustrative of CKD Disease
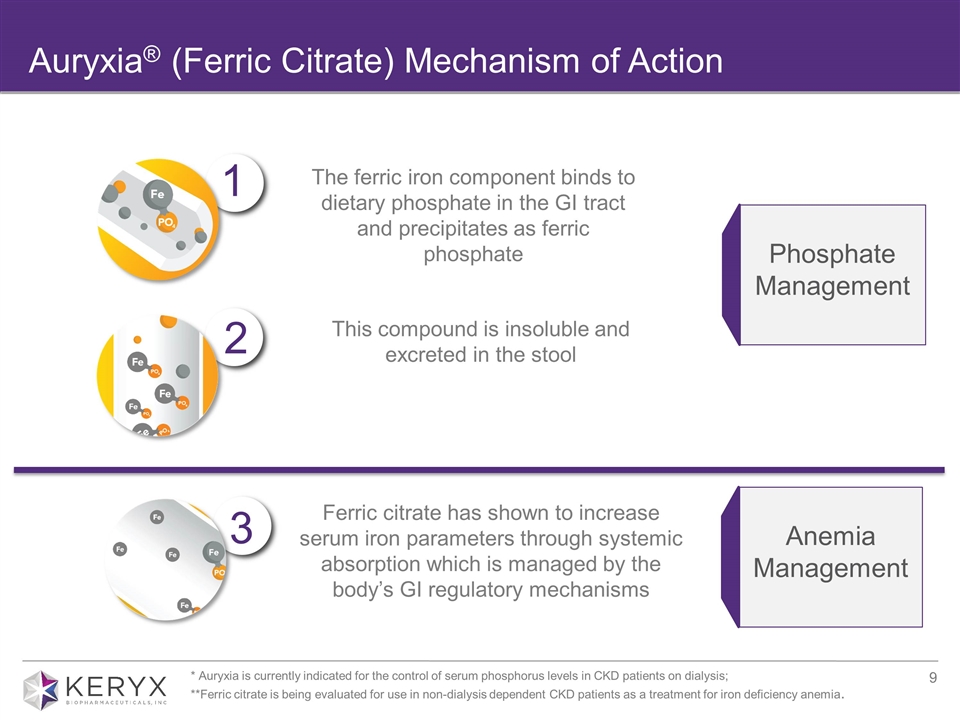
Auryxia® (Ferric Citrate) Mechanism of Action Phosphate Management Anemia Management 2 1 3 The ferric iron component binds to dietary phosphate in the GI tract and precipitates as ferric phosphate This compound is insoluble and excreted in the stool Ferric citrate has shown to increase serum iron parameters through systemic absorption which is managed by the body’s GI regulatory mechanisms * Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; **Ferric citrate is being evaluated for use in non-dialysis dependent CKD patients as a treatment for iron deficiency anemia.
Today – Auryxia Controls Elevated Serum Phosphorus Levels in People on Dialysis
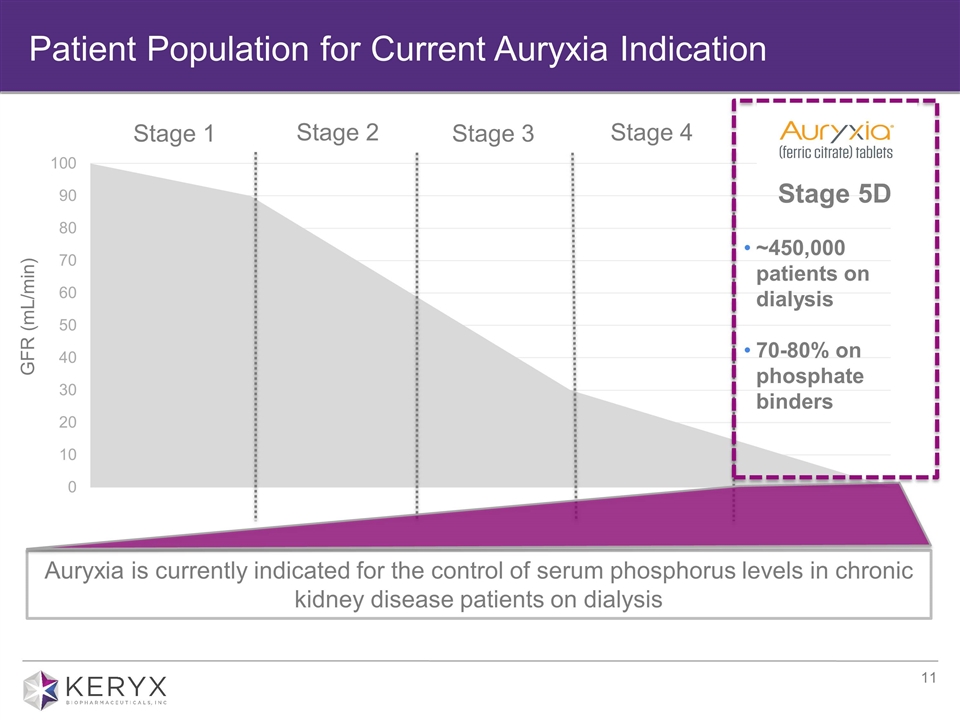
Patient Population for Current Auryxia Indication Stage 1 Stage 2 Stage 3 Stage 4 GFR (mL/min) Auryxia is currently indicated for the control of serum phosphorus levels in chronic kidney disease patients on dialysis Stage 5D ~450,000 patients on dialysis 70-80% on phosphate binders
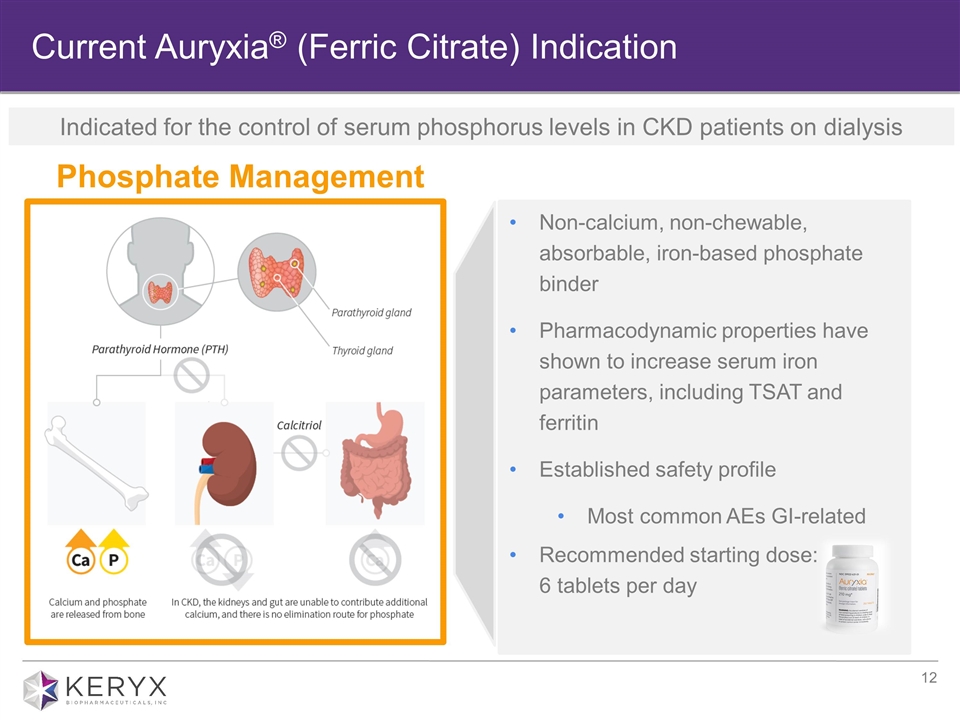
Current Auryxia® (Ferric Citrate) Indication Phosphate Management Non-calcium, non-chewable, absorbable, iron-based phosphate binder Pharmacodynamic properties have shown to increase serum iron parameters, including TSAT and ferritin Established safety profile Most common AEs GI-related Recommended starting dose: 6 tablets per day Indicated for the control of serum phosphorus levels in CKD patients on dialysis
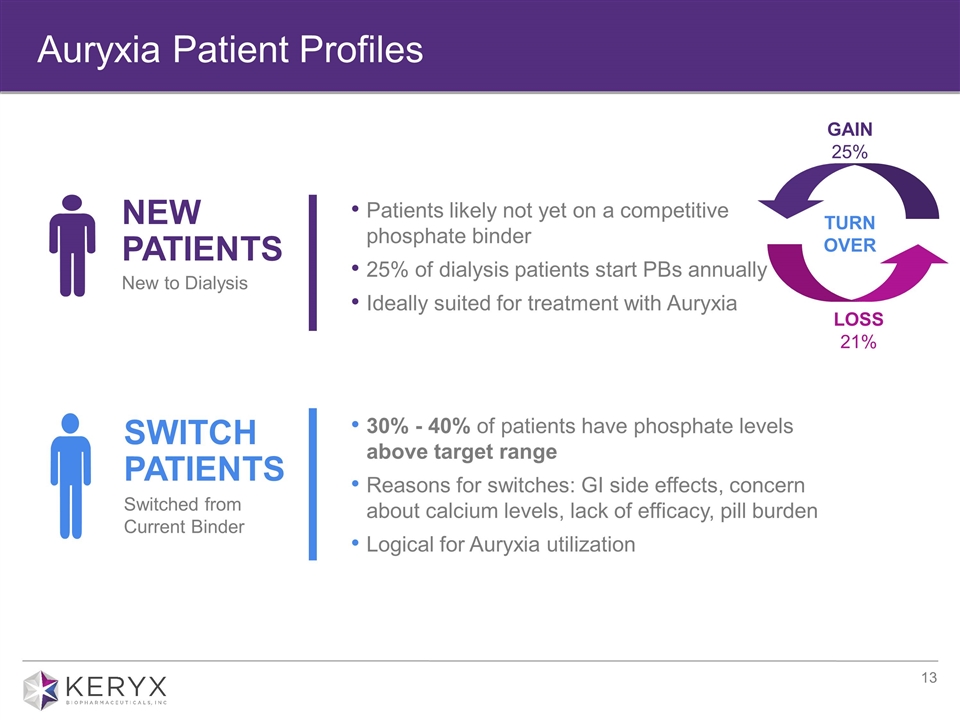
Auryxia Patient Profiles SWITCH PATIENTS Switched from Current Binder 30% - 40% of patients have phosphate levels above target range Reasons for switches: GI side effects, concern about calcium levels, lack of efficacy, pill burden Logical for Auryxia utilization NEW PATIENTS New to Dialysis Patients likely not yet on a competitive phosphate binder 25% of dialysis patients start PBs annually Ideally suited for treatment with Auryxia GAIN 25% LOSS 21% TURN OVER
Solid Fundamentals to Support Return to Market Physician Payer Patient 44% increased prescription volume over 1Q Increased breadth and depth of adoption Expanded field base team fully trained Sound commercial strategy Majority of phosphate binder patients have unrestricted access ~70% of co-pays <$10 Patient persistency at ~70% Auryxia source of business: 70% switch / 30% new patients Source: IMS NPA, Spherix * Fundamentals prior to supply interruption Second Quarter 2016 Highlights*
Near Term: Potential to Treat Iron Deficiency Anemia in People with NDD-Chronic Kidney Disease
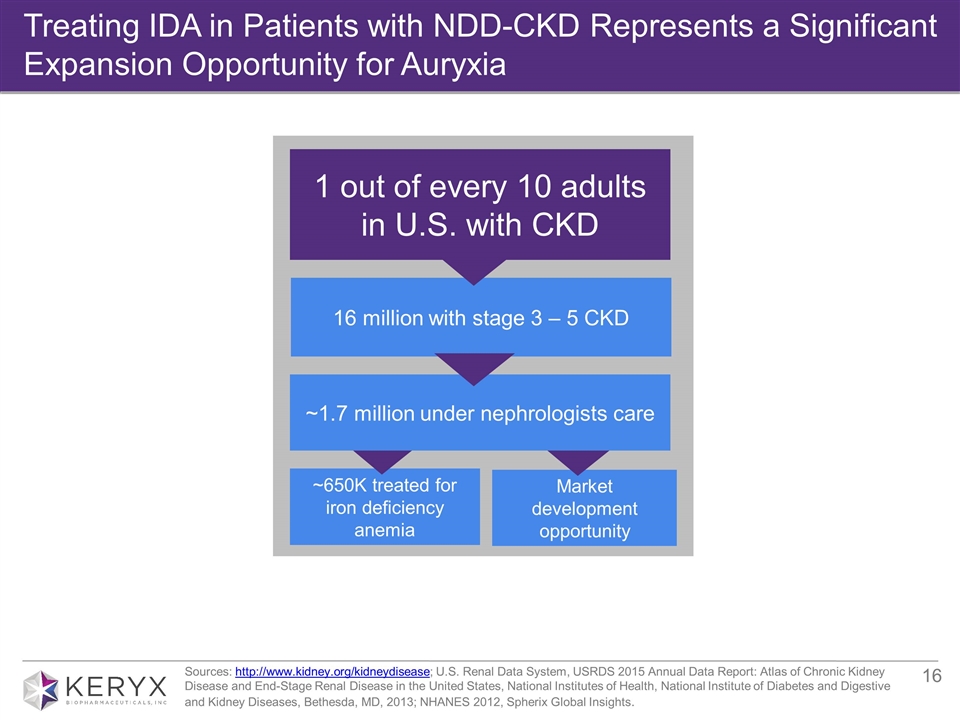
Treating IDA in Patients with NDD-CKD Represents a Significant Expansion Opportunity for Auryxia Sources: http://www.kidney.org/kidneydisease; U.S. Renal Data System, USRDS 2015 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2013; NHANES 2012, Spherix Global Insights. 1 out of every 10 adults in U.S. with CKD 16 million with stage 3 – 5 CKD ~650K treated for iron deficiency anemia Market development opportunity ~1.7 million under nephrologists care
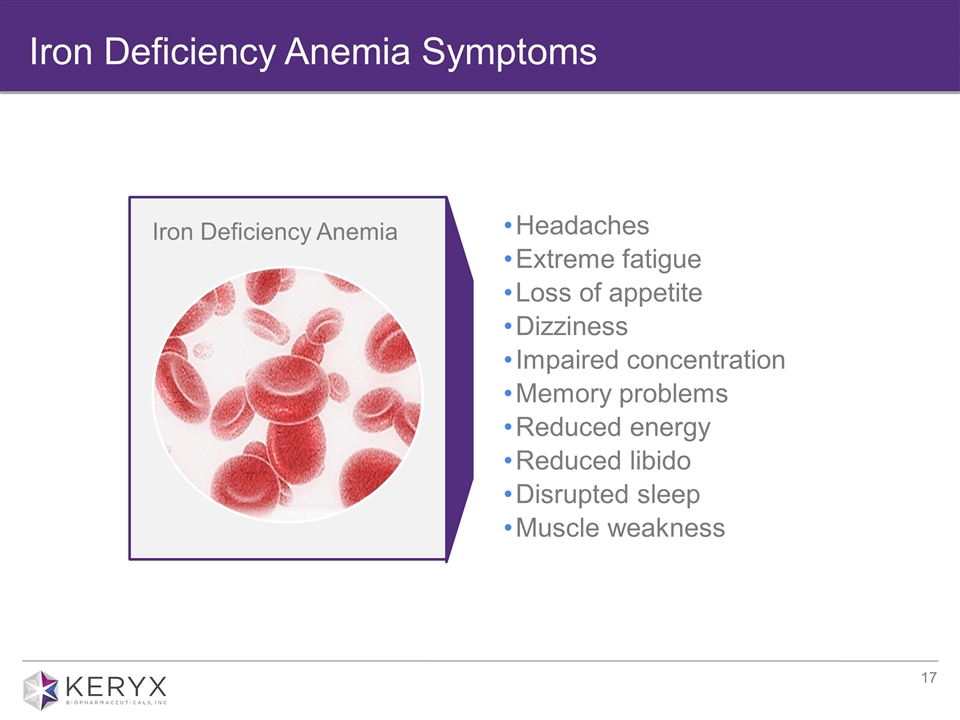
Iron Deficiency Anemia Symptoms Headaches Extreme fatigue Loss of appetite Dizziness Impaired concentration Memory problems Reduced energy Reduced libido Disrupted sleep Muscle weakness Iron Deficiency Anemia
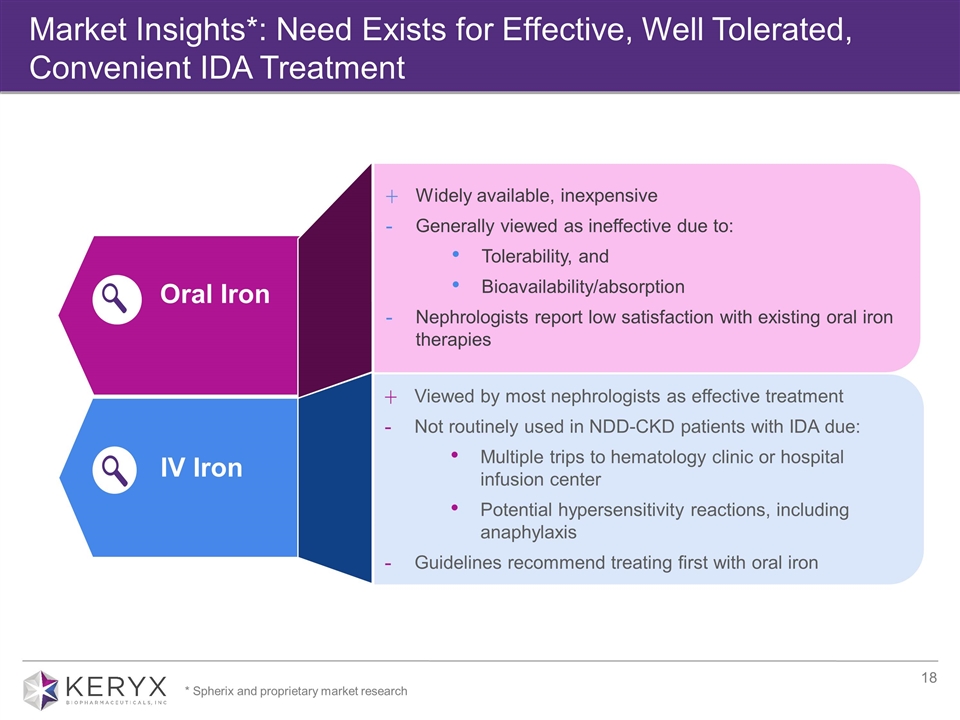
Oral Iron Widely available, inexpensive Generally viewed as ineffective due to: Tolerability, and Bioavailability/absorption Nephrologists report low satisfaction with existing oral iron therapies Viewed by most nephrologists as effective treatment Not routinely used in NDD-CKD patients with IDA due: Multiple trips to hematology clinic or hospital infusion center Potential hypersensitivity reactions, including anaphylaxis Guidelines recommend treating first with oral iron IV Iron Market Insights*: Need Exists for Effective, Well Tolerated, Convenient IDA Treatment * Spherix and proprietary market research
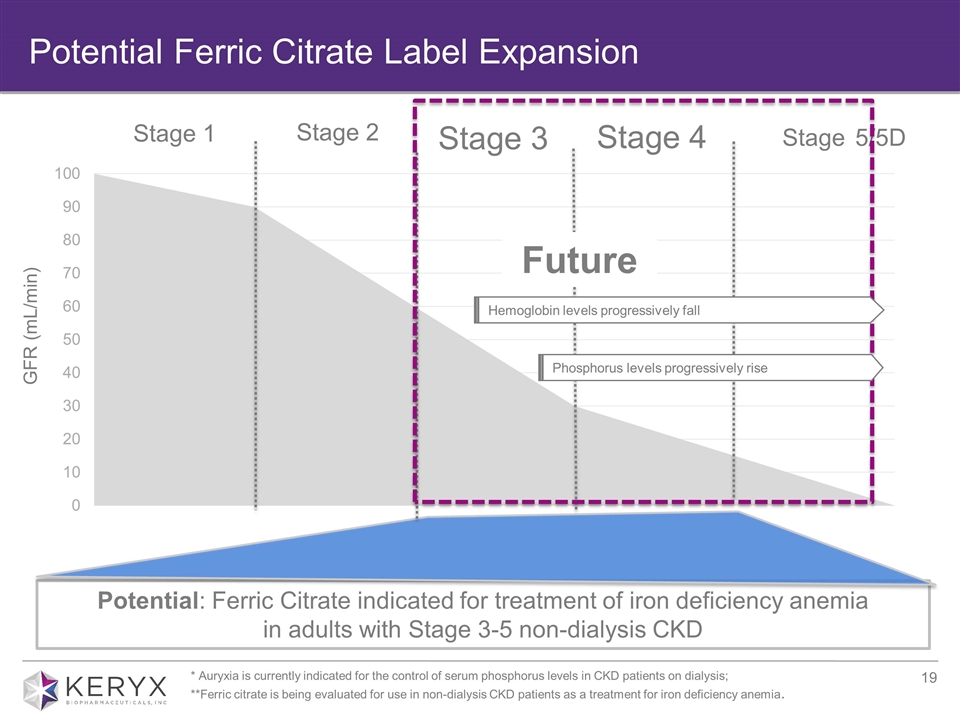
Potential Ferric Citrate Label Expansion Stage 1 Stage 2 Stage 3 Stage 4 Stage 5/5D GFR (mL/min) Potential: Ferric Citrate indicated for treatment of iron deficiency anemia in adults with Stage 3-5 non-dialysis CKD Hemoglobin levels progressively fall Phosphorus levels progressively rise Future * Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; **Ferric citrate is being evaluated for use in non-dialysis CKD patients as a treatment for iron deficiency anemia.
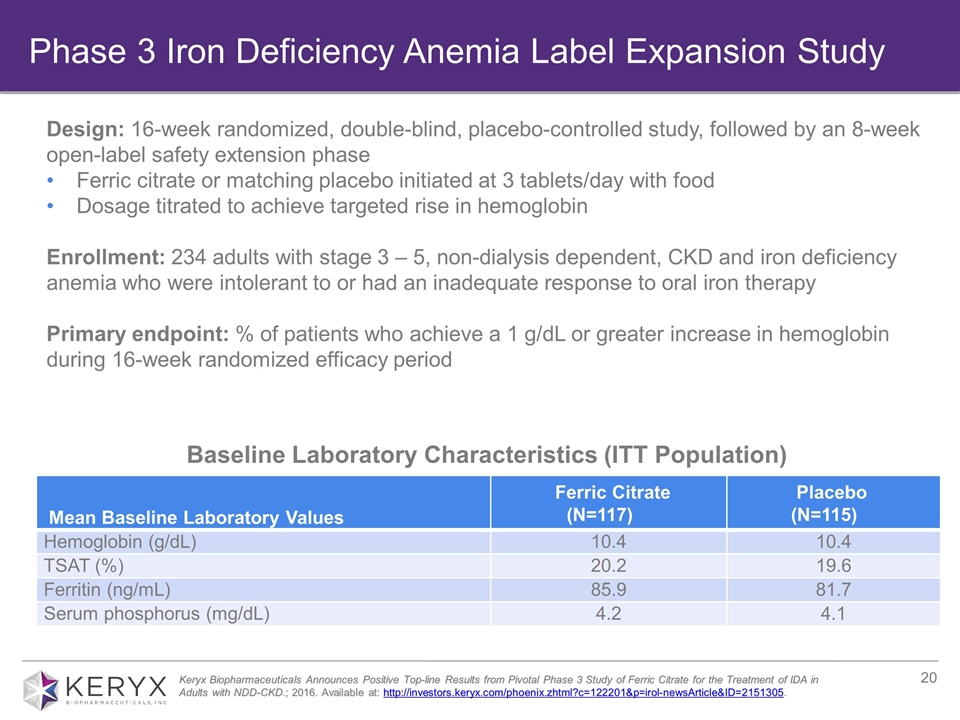
Baseline Laboratory Characteristics (ITT Population) Mean Baseline Laboratory Values Ferric Citrate (N=117) Placebo (N=115) Hemoglobin (g/dL) 10.4 10.4 TSAT (%) 20.2 19.6 Ferritin (ng/mL) 85.9 81.7 Serum phosphorus (mg/dL) 4.2 4.1 Keryx Biopharmaceuticals Announces Positive Top-line Results from Pivotal Phase 3 Study of Ferric Citrate for the Treatment of IDA in Adults with NDD-CKD.; 2016. Available at: http://investors.keryx.com/phoenix.zhtml?c=122201&p=irol-newsArticle&ID=2151305. Design: 16-week randomized, double-blind, placebo-controlled study, followed by an 8-week open-label safety extension phase Ferric citrate or matching placebo initiated at 3 tablets/day with food Dosage titrated to achieve targeted rise in hemoglobin Enrollment: 234 adults with stage 3 – 5, non-dialysis dependent, CKD and iron deficiency anemia who were intolerant to or had an inadequate response to oral iron therapy Primary endpoint: % of patients who achieve a 1 g/dL or greater increase in hemoglobin during 16-week randomized efficacy period Phase 3 Iron Deficiency Anemia Label Expansion Study
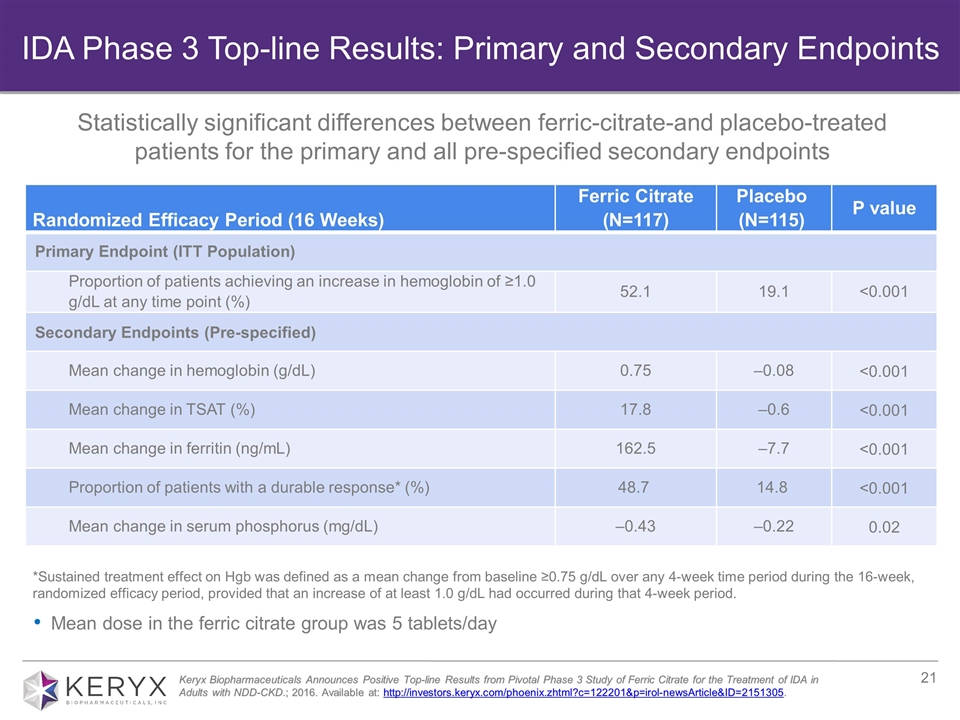
*Sustained treatment effect on Hgb was defined as a mean change from baseline ≥0.75 g/dL over any 4-week time period during the 16-week, randomized efficacy period, provided that an increase of at least 1.0 g/dL had occurred during that 4-week period. Randomized Efficacy Period (16 Weeks) Ferric Citrate (N=117) Placebo (N=115) P value Primary Endpoint (ITT Population) Proportion of patients achieving an increase in hemoglobin of ≥1.0 g/dL at any time point (%) 52.1 19.1 <0.001 Secondary Endpoints (Pre-specified) Mean change in hemoglobin (g/dL) 0.75 ‒0.08 <0.001 Mean change in TSAT (%) 17.8 ‒0.6 <0.001 Mean change in ferritin (ng/mL) 162.5 ‒7.7 <0.001 Proportion of patients with a durable response* (%) 48.7 14.8 <0.001 Mean change in serum phosphorus (mg/dL) ‒0.43 ‒0.22 0.02 Mean dose in the ferric citrate group was 5 tablets/day Keryx Biopharmaceuticals Announces Positive Top-line Results from Pivotal Phase 3 Study of Ferric Citrate for the Treatment of IDA in Adults with NDD-CKD.; 2016. Available at: http://investors.keryx.com/phoenix.zhtml?c=122201&p=irol-newsArticle&ID=2151305. IDA Phase 3 Top-line Results: Primary and Secondary Endpoints Statistically significant differences between ferric-citrate-and placebo-treated patients for the primary and all pre-specified secondary endpoints
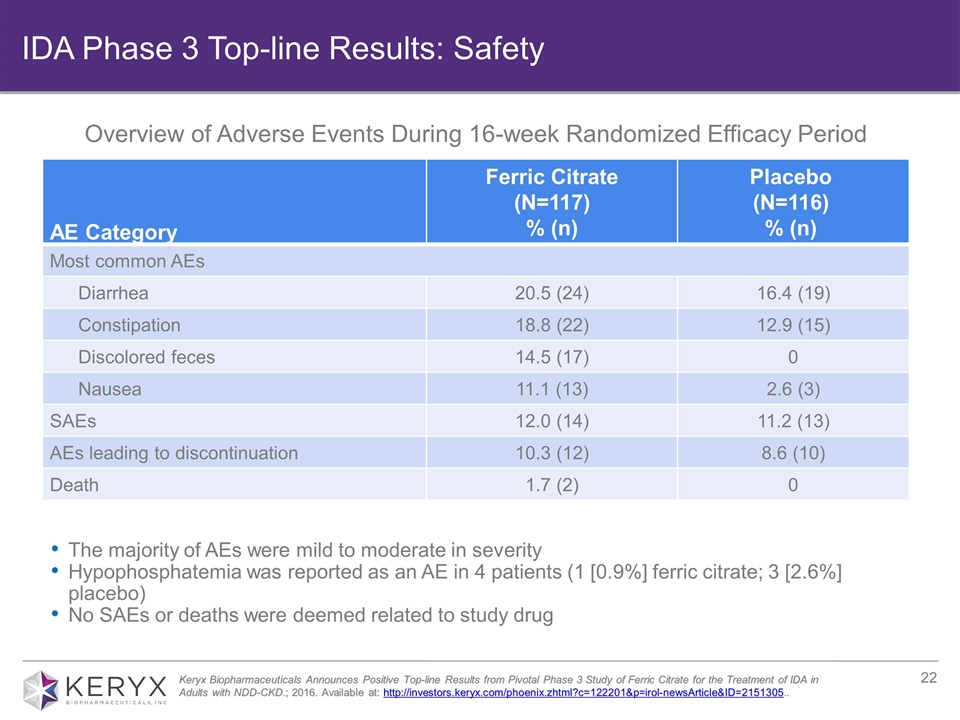
AE Category Ferric Citrate (N=117) % (n) Placebo (N=116) % (n) Most common AEs Diarrhea 20.5 (24) 16.4 (19) Constipation 18.8 (22) 12.9 (15) Discolored feces 14.5 (17) 0 Nausea 11.1 (13) 2.6 (3) SAEs 12.0 (14) 11.2 (13) AEs leading to discontinuation 10.3 (12) 8.6 (10) Death 1.7 (2) 0 The majority of AEs were mild to moderate in severity Hypophosphatemia was reported as an AE in 4 patients (1 [0.9%] ferric citrate; 3 [2.6%] placebo) No SAEs or deaths were deemed related to study drug Keryx Biopharmaceuticals Announces Positive Top-line Results from Pivotal Phase 3 Study of Ferric Citrate for the Treatment of IDA in Adults with NDD-CKD.; 2016. Available at: http://investors.keryx.com/phoenix.zhtml?c=122201&p=irol-newsArticle&ID=2151305.. Overview of Adverse Events During 16-week Randomized Efficacy Period IDA Phase 3 Top-line Results: Safety
Ferric Citrate has Potential to Benefit Many More Patients with Chronic Kidney Disease Anemia Management Phase 3 study for IDA, NDD-CKD demonstrated statistically significant differences between the ferric citrate and placebo groups for the primary and all pre-specified secondary endpoints Expect to submit sNDA in early 4Q 2016 Four IDA-related abstracts accepted for poster presentations at 2016 ASN Plan to submit data for publication in a peer reviewed journal Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; Ferric citrate is being evaluated for use in non-dialysis dependent CKD patients as a treatment for iron deficiency anemia.
Potential to Manage Two Complications of CKD Phosphate Management Anemia Management Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; Ferric citrate is being evaluated for use in non-dialysis dependent CKD patients as a treatment for iron deficiency anemia.

Key Takeaways Expect to make Auryxia available to patients on dialysis during 4Q 2016 Submit sNDA for potential label expansion of Auryxia Present IDA/CKD phase 3 data to medical community Developing go-to-market strategy for IDA/NDD-CKD opportunity Auryxia is currently indicated for the control of serum phosphorus levels in CKD patients on dialysis; Ferric citrate is being evaluated for use in non-dialysis dependent CKD patients as a treatment for iron deficiency anemia.

Keryx Biopharmaceuticals, Inc. Keryx Biopharmaceuticals, Inc. September 7, 2016
