Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Aptevo Therapeutics Inc. | apvo-8k_20160808.htm |
Exhibit 99.1
 August 2016 Aptevo Therapeutics Investor Presentation
August 2016 Aptevo Therapeutics Investor Presentation
Forward-Looking Statements This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact, including our financial guidance, and any other statements containing the words “believes”, “expects”, “anticipates”, “intends”, “plans”, “forecasts”, “estimates” and similar expressions in conjunction with, among other things, discussions of financial performance or financial condition, growth strategy, product sales, manufacturing capabilities, product development, regulatory approvals or expenditures are forward-looking statements. These forward-looking statements are based on our current intentions, beliefs and expectations regarding future events. We cannot guarantee that any forward-looking statement will be accurate. Investors should realize that if underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could differ materially from our expectations. Investors are, therefore, cautioned not to place undue reliance on any forward-looking statement. Any forward-looking statement speaks only as of the date of this presentation, and, except as required by law, we do not undertake to update any forward-looking statement to reflect new information, events or circumstances. There are a number of important factors that could cause Aptevo's actual results to differ materially from those indicated by such forward-looking statements, including possible negative effects on Aptevo’s business operations, assets or financial results as a result of the separation; a deterioration in the business or prospects of Aptevo; adverse developments in Aptevo’s customer-base or markets; our ability to enter into and maintain selective collaboration and partnership arrangements; the timing of and our ability to achieve milestones in collaboration and partnership contracts; our ability and the ability of our contractors and suppliers to maintain compliance with cGMP and other regulatory obligations; the results of regulatory inspections; the rate and degree of market acceptance and clinical utility of our products; the success of our ongoing and planned development programs; the timing of and our ability to obtain and maintain regulatory approvals for our product candidates; and our commercialization, marketing and manufacturing capabilities and strategy and changes in regulatory, social and political conditions. Additional risks and factors that may affect results are set forth in Aptevo’s filings with the Securities and Exchange Commission, including Aptevo’s Registration Statement on Form 10, as amended. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from our expectations in any forward-looking statement. Investors should consider this cautionary statement, as well as the risk factors identified in our periodic reports filed with the SEC, when evaluating our forward-looking statements. Aptevo™, ADAPTIR™ and any and all Aptevo Therapeutics Inc. brand, product, service, and feature names, logos, and slogans are trademarks or registered trademarks of Aptevo Therapeutics Inc. or its subsidiaries in the United States or other countries. All rights reserved.
AGENDA Leadership in Bispecific Antibody Development • Aptevo today • What sets us apart • Summary
Aptevo: At a Glance Focus Oncology/Hematology Commercial Products 4 Product Pipeline Platform Technologies Employees Clinical: 2 Preclinical: Multiple ADAPTIR™ ~130 Headquarters Seattle, WA Q1 2016 Revenue $8M 2015 Revenue $33.6M Cash Runway $100M $65M cash (as of 8/4/16) $20M EBS future contribution $15M 2nd tranche (term loan) Leading Oncology Platform • Innovative ADAPTIR platform technology utilizing a promising approach in the highly attractive immuno-oncology field Leveraging Technology • Targeted investments in bispecific ADAPTIR therapeutics • Increased awareness of the RTCC Mechanism of Action Strong IP Estate • Will own or exclusively license patent rights for entire product portfolio • Will seek exclusive licenses for supporting technologies
Executive Leadership Senior Management Board of Directors Marvin White – President & CEO Former Emergent Director; Former CFO, St. Vincent’s Health; Former Exec. Director & CFO, Lilly USA Jeff Lamothe – SVP, CFO Former Emergent VP, Finance; Former CFO, Cangene Corporation Randy Maddux – SVP, Operations Former VP, Global Mfg & Supply, GSK; Former VP, Mfg Ops & Quality, Human Genome Sciences Dr. Scott Stromatt – SVP, CMO Former Emergent SVP, CMO; Former CMO, Trubion Dr. Jane Gross – VP, Res/Non-Clin. Dev. Emergent VP, Research/Non-Clinical Development; Former VP Immunology Research ZymoGenetics Inc. Mike Adelman – VP, Commercial Ops. Former Emergent VP, Commercial Operations; Former, VP Commercial Operations, Cangene Corporation Shawnte Mitchell – VP, Gen’l Counsel Former Emergent VP, Associate General Counsel Marvin White Former Emergent Director; Former CFO, St. Vincent’s Health; Former Exec. Director & CFO, Lilly USA Fuad El-Hibri Founder, Executive Chairman, Emergent BioSolutions Daniel Abdun-Nabi President & CEO, Emergent BioSolutions Grady Grant, III Mead Johnson Nutrition; Eli Lilly & Co. Zsolt Harsanyi, Ph.D. N-Gene Research Labs; Exponential Biotherapies; Porton Int’l Barbara Lopez Kunz DIA; Battelle; Thermo Fisher Scientific; ICI/Uniqema John Niederhuber, M.D. Inova Translational Medicine Institute; NCI; Johns Hopkins Univ. Deep R&D, Commercial Financial Expertise and Experience
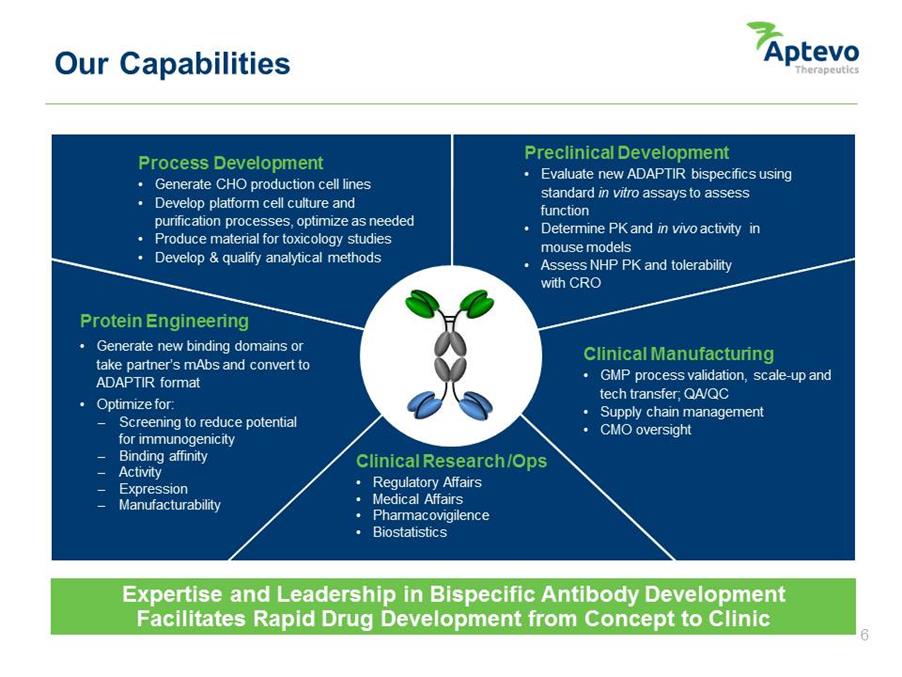
Our Capabilities Protein Engineering • Generate new binding domains or take partner’s mAbs and convert to ADAPTIR format • Optimize for: Screening to reduce potential for immunogenicity Binding affinity Activity Expression Manufacturability Preclinical Development • Evaluate new ADAPTIR bispecifics using standard in vitro assays to assess function • Determine PK and in vivo activity in mouse models • Assess NHP PK and tolerability with CRO Process Development • Generate CHO production cell lines • Develop platform cell culture and purification processes, optimize as needed • Produce material for toxicology studies • Develop & qualify analytical methods Clinical Research /Ops • Regulatory Affairs • Medical Affairs • Pharmacovigilence • Biostatistics Clinical Manufacturing • GMP process validation, scale-up and tech transfer; QA/QC • Supply chain management • CMO oversight Expertise and Leadership Antibody Development Facilitates Rapid Drug development from concept to clinic
Our Strategy 1 2 3 Advance novel ADAPTIR™ product candidates, primarily in I/O Expand collaborations and partnerships Maximize cash flow from commercial portfolio to support R&D funding
AGENDA Leadership in Bispecific Antibody Development • Aptevo today • What sets us apart • Summary
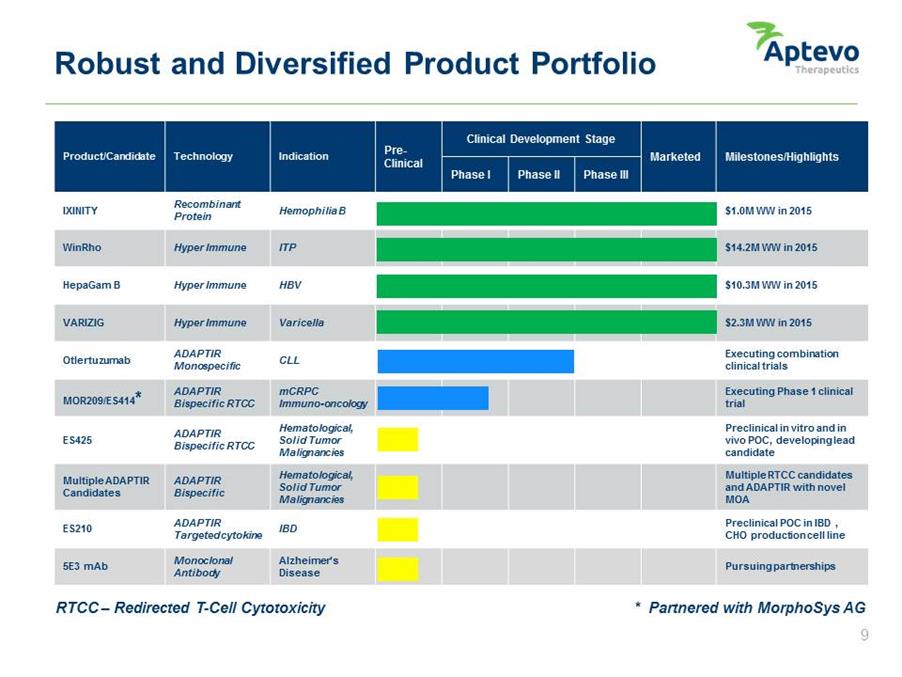
Robust and Diversified Product Portfolio Product/Candidate Technology Indication Pre-Clinical Clinical Development Stage Marketed Milestones/Highlights Phase I Phase II Phase III $1.0M WW in 2015 $14.2M WW in 2015 $10.3M WW in 2015 $2.3M WW in 2015 Executing combination clinical trials Executing Phase 1 clinical trial Preclinical in vitro and in vivo POC, developing lead candidate Multiple RTCC candidates and ADAPTIR with novel MOA Preclinical POC in IBD , CHO production cell line Pursuing partnerships IXINITY WinRho HepaGam B VARIZIG Otlertuzumab MOR209/ES414* ES425 Multiple ADAPTIR Candidates ES210 5E3 mAb Recombinant Protein Hyper Immune Hyper Immune Hyper Immune ADAPTIR Monospecific ADAPTIR Bispecific RTCC ADAPTIR Bispecific RTCC ADAPTIR Bispecific ADAPTIR Targeted cytokine Monoclonal Antibody Hemophilia B ITP HBV Varicella CLL mCRPC Immuno-oncology Hematological, Solid Tumor Malignancies Hematological, Solid Tumor Malignancies IBD Alzheimer’s Disease RTCC – Redirected T-Cell Cytotoxicity * Partnered with MorphoSys AG
Commercial Products to Support R&D Funding [coagulation factor IX (recombinant)] An intravenous recombinant human coagulation factor IX therapeutic for use in patients with Hemophilia B US: [Rho (D) Immune Globulin Intravenous (Human)] Canada: (Rho(D) Immune Globulin (Human) for injection) Immune Thrombocytopenic Purpura (ITP) and suppression of Rhesus (Rh) isoimmunization US: [Hepatitis B Immune Globulin Intravenous (Human)] Canada: (Hepatitis B Immune Globulin (Human) Injection) Prevention of hepatitis B recurrence following liver transplantation in HBsAgpositive patients and post exposure prophylaxis after acute hepatitis B exposure US: VARIZIG® [Varicella Zoster Immune Globulin (Human)] Canada: VariZIG® (Varicella Zoster Immune Globulin (Human)) Post-exposure prophylaxis of varicella zoster in high risk individuals
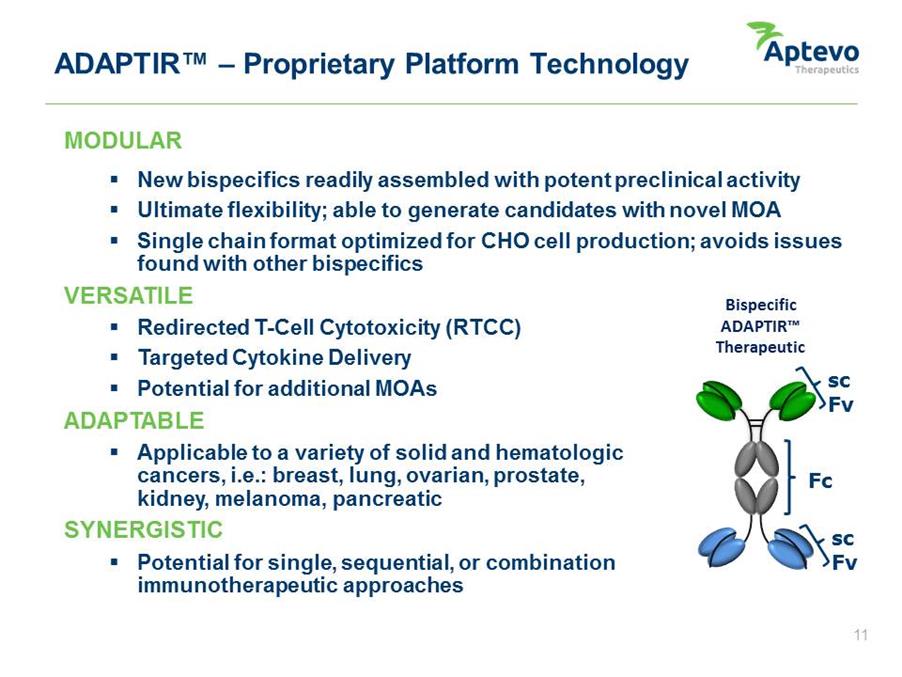
ADAPTIR™ – Proprietary Platform Technology MODULAR • New bispecifics readily assembled with potent preclinical activity • Ultimate flexibility; able to generate candidates with novel MOA • Single chain format optimized for CHO cell production; avoids issues found with other bispecifics VERSATILE Bispecific • Redirected T-Cell Cytotoxicity (RTCC) ADAPTIR™ Therapeutic • Targeted Cytokine Delivery • Potential for additional MOAs ADAPTABLE • Applicable to a variety of solid and hematologic cancers, i.e.: breast, lung, ovarian, prostate, kidney, melanoma, pancreatic SYNERGISTIC • Potential for single, sequential, or combination immunotherapeutic Fc sc Fv sc Fv
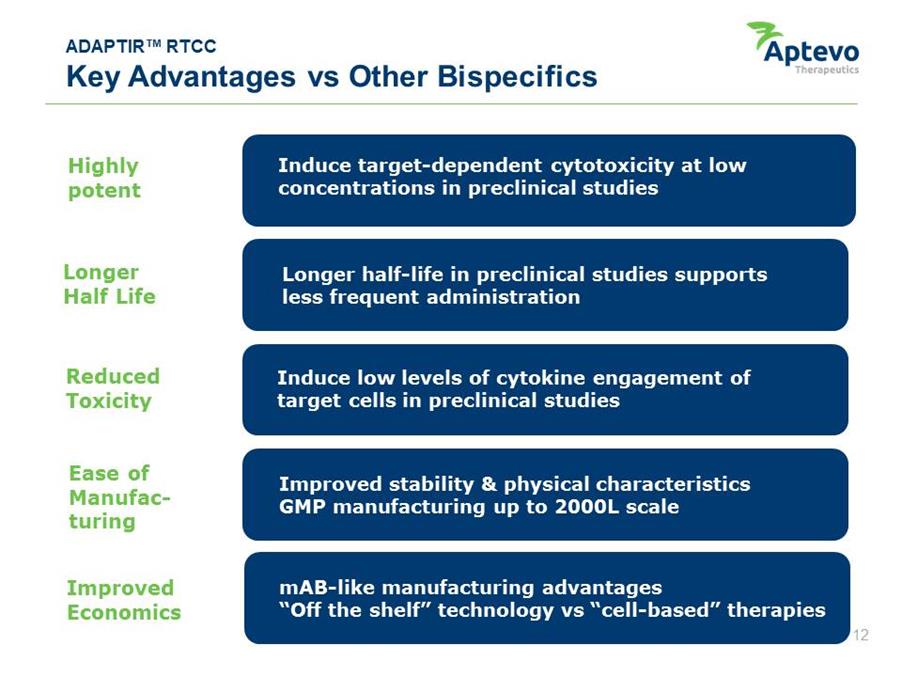
ADAPTIR™ RTCC Key Advantages vs Other Bispecifics Highly potent Longer Half Life Reduced Toxicity Ease of Manufacturing Improved Economics Longer half-life in preclinical studies supports less frequent administration Induce target-dependent cytotoxicity at low concentrations in preclinical studies Induce low levels of cytokine engagement of target cells in preclinical studies Improved stability & physical characteristics GMP manufacturing up scale mAB-like manufacturing advantages “Off the shelf” technology vs “cell-based” therapies
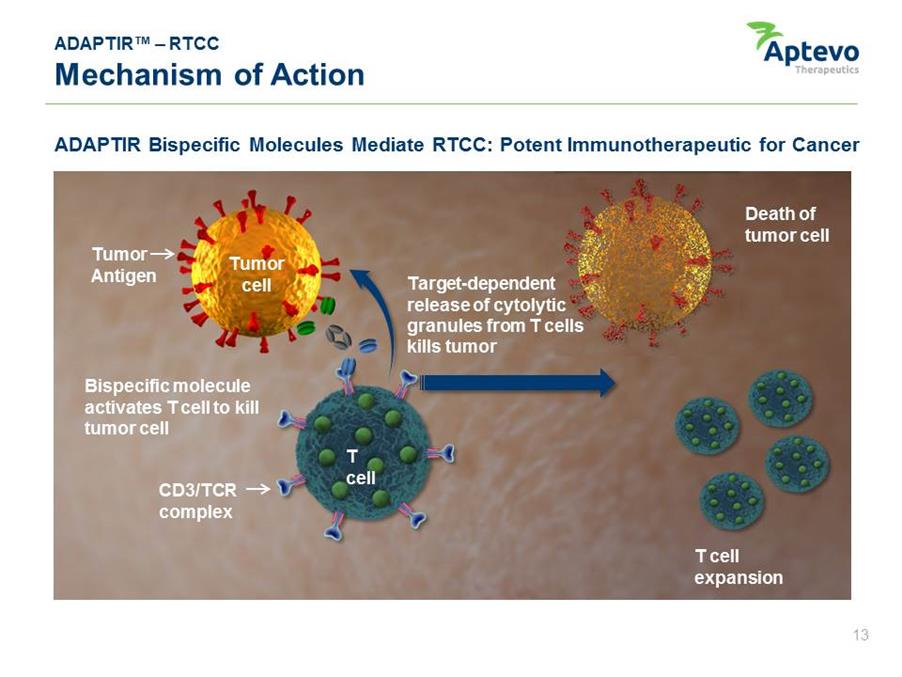
ADAPTIR™ – RTCC Mechanism of Action ADAPTIR Bispecific Molecules Mediate RTCC: Potent Immunotherapeutic for Cancer Tumor Antigen Bispecific molecule activates T cell to kill tumor cell CD3/TCR complex Target-dependent release of cytolytic granules from T cells kills tumor T cell expansion Death of tumor cell T cell Tumor cell
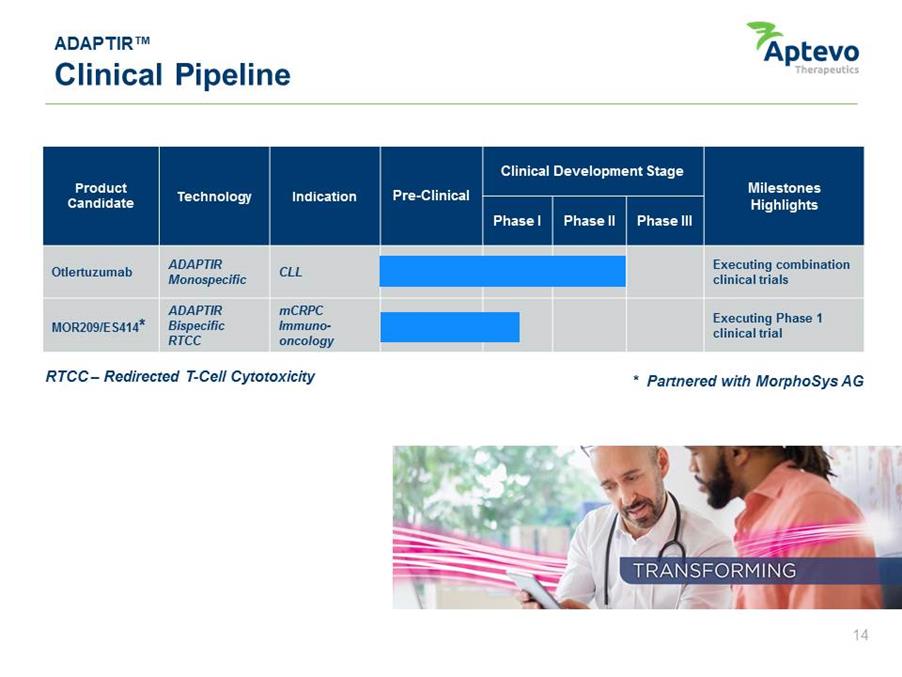
ADAPTIR™ Clinical Pipeline Product Candidate Technology Indication Pre-Clinical Clinical Development Stage Milestones Highlights Phase I Phase II Phase III ADAPTIROtlertuzumab Monospecific ADAPTIR Bispecific RTCCMOR209/ES414* CLL mCRPC Immunooncology RTCC – Redirected T-Cell Cytotoxicity Executing combination clinical trials Executing Phase 1 clinical trial * Partnered with MorphoSys AG
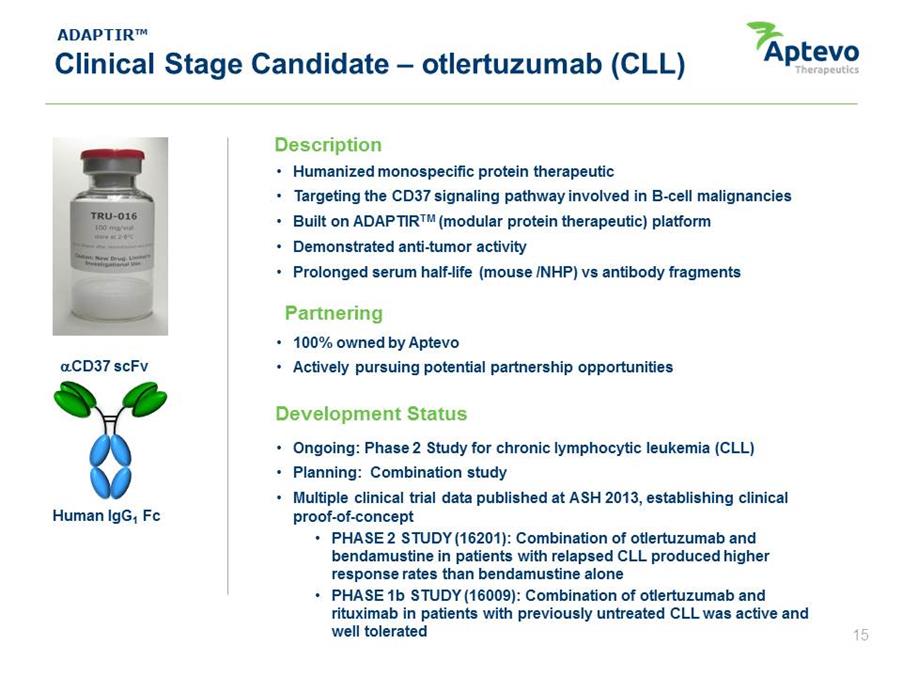
ADAPTIR™ Clinical Stage Candidate – otlertuzumab (CLL) .CD37 scFv Human IgG1 Fc Description • Humanized monospecific protein therapeutic • Targeting the CD37 signaling pathway involved in B-cell malignancies • Built on ADAPTIRTM (modular protein therapeutic) platform • Demonstrated anti-tumor activity • Prolonged serum half-life (mouse /NHP) vs antibody fragments Partnering • 100% owned by Aptevo • Actively pursuing potential partnership opportunities Development Status • Ongoing: Phase 2 Study for chronic lymphocytic leukemia (CLL) • Planning: Combination study • Multiple clinical trial data published at ASH 2013, establishing clinical proof-of-concept • PHASE 2 STUDY (16201): Combination of otlertuzumab and bendamustine in patients with relapsed CLL produced higher response rates than bendamustine alone • PHASE 1b STUDY (16009): Combination of otlertuzumab and rituximab in patients with previously untreated CLL was active and well tolerated
ADAPTIR™ Clinical Stage Candidate – MOR209/ES414 (mCRPC) Anti-PSMA Anti-CD3 Description • Humanized bispecific protein therapeutic • Targeting PSMA and CD3, a component of the T-cell receptor • Demonstrated redirection of T-cells to kill tumor cells expressing PSMA in vitro and in vivo • Prolonged serum half-life (mouse/NHP) vs antibody fragments Partnering • Co-development/Co-commercialization partnership with MorphoSys AG established August 2014 Development Status • Open-label Phase 1 Study underway (U.S. & Australia) • Safety, tolerability, and clinical activity in patients with metastatic castration-resistant prostate cancer (mCRPC) to be conducted in 2 stages • Stage 1: Primary Objective --identify MTD administered intravenously. Secondary Objectives --evaluate tolerability, PK, PD, immunogenicity, cytokine response, and clinical activity • Stage 2: Primary Objective --evaluate clinical activity in patients that have have not received chemotherapy
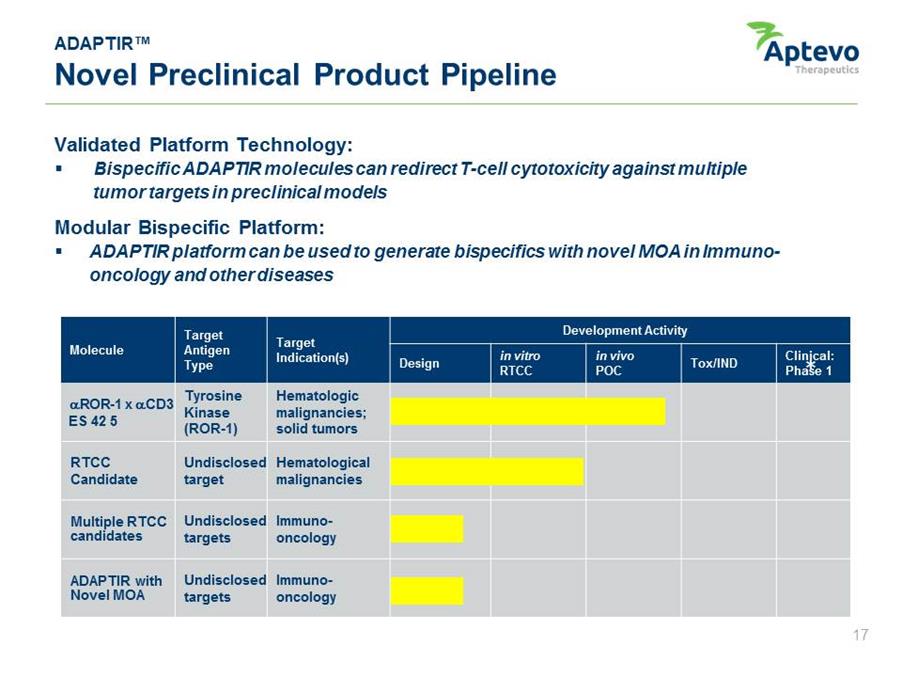
ADAPTIR™ Novel Preclinical Product Pipeline Validated Platform Technology: • Bispecific ADAPTIR molecules can redirect T-cell cytotoxicity against multiple tumor targets in preclinical models Modular Bispecific Platform: • ADAPTIR platform can be used to generate bispecifics with novel MOA in Immunooncology and other diseases Molecule Target Antigen Type Target Indication(s) Development Activity Design in vitro RTCC in vivo POC Tox/IND Clinical: Phase 1 * .ROR-1 x .CD3 ES 42 5 Tyrosine Kinase (ROR-1) Hematologic malignancies; solid tumors RTCC Undisclosed Hematological Candidate target malignancies Multiple RTCCcandidates Undisclosed targets Immunooncology ADAPTIR with Undisclosed Immuno-Novel MOA targets oncology
ADAPTIR™ RTCC Preclinical Candidate ROR-1/ES425 • Targets hematologic malignancies and solid tumors – Triple-negative breast cancer, ovarian cancer, NSCL, prostate kidney cancers • Preclinical in vitro and in vivo proof of concept established • Improved preclinical PK • Developing lead candidate
AGENDA Leadership in Bispecific Antibody Development • Aptevo today • What sets us apart • Summary
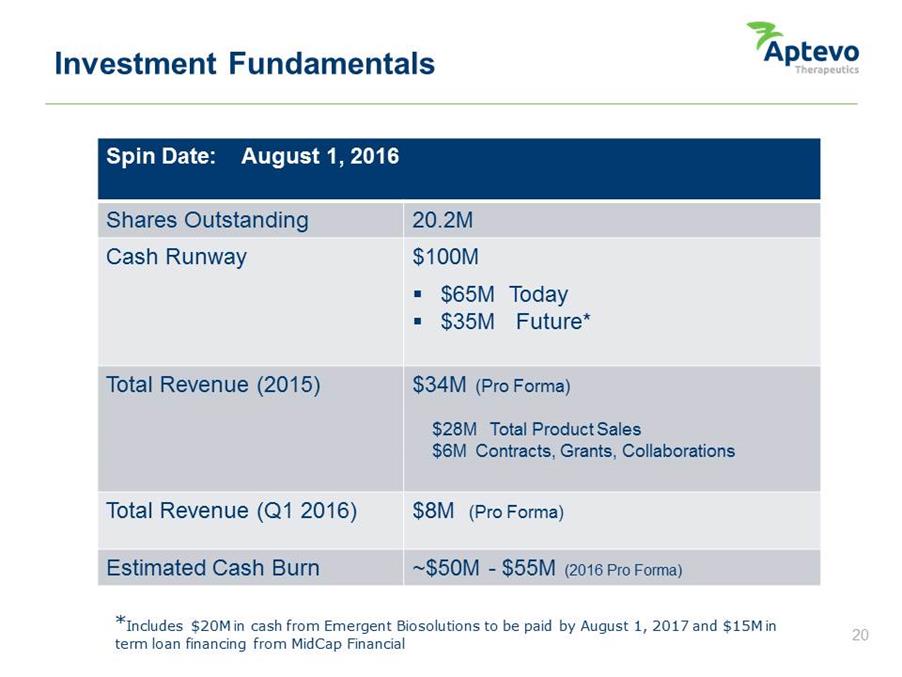
Investment Fundamentals Spin Date: August 1, 2016 Shares Outstanding 20.2M Cash Runway $100M • $65M Today • $35M Future* Total Revenue (2015) $34M (Pro Forma) $28M Total Product Sales $6M Contracts, Grants, Collaborations Total Revenue (Q1 2016) $8M (Pro Forma) Estimated Cash Burn ~$50M -$55M (2016 Pro Forma) *Includes $20M in cash from Emergent Biosolutions to be paid by August 1, 2017 and $15M in term loan financing from MidCap Financial
Key Milestones – 18-24 Months Development • Complete Phase 1 study of MOR209/ES414 and advance into Phase 2 development in partnership with MorphoSys • Generate new ADAPTIR-based RTCC candidates • Expand application of ADAPTIR-based candidates into new MOA • Advance new preclinical ADAPTIR-based candidates into the clinic Operational/Financial • Capture incremental market share of Hemophilia B market with expanded sales of IXINITY • Expand ex-US commercial market opportunities through new regulatory filings in select foreign jurisdictions • Continue current and initiate future partnering discussions around product candidates 21 Multiple Upcoming Valuation Catalysts
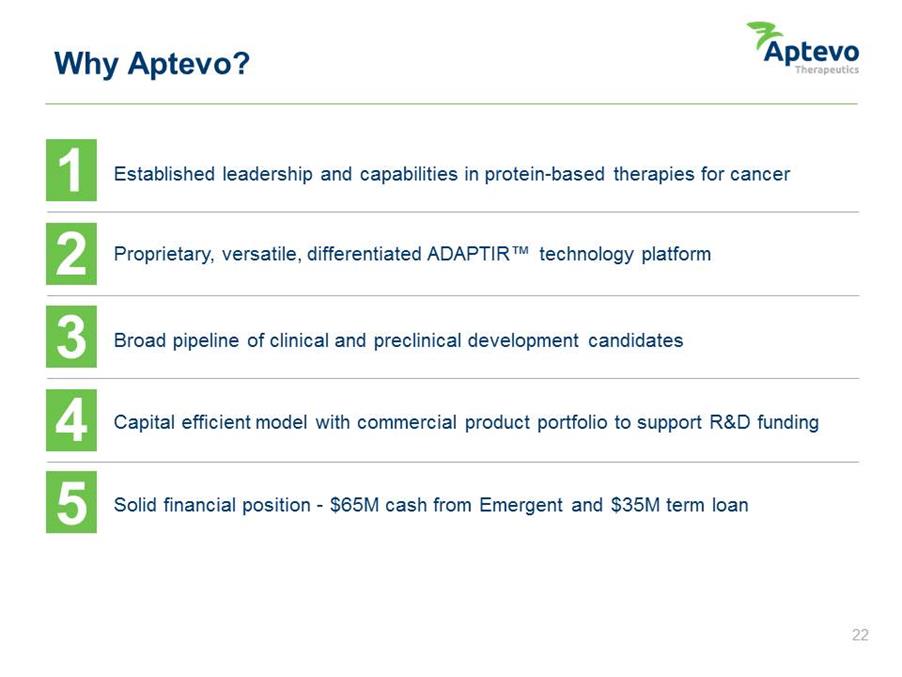
Why Aptevo? Established leadership and capabilities in protein-based therapies for cancer Proprietary, versatile, differentiated ADAPTIR™ technology platform Broad pipeline of clinical and preclinical development candidates Capital efficient model with commercial product portfolio to support R&D funding Solid financial position -$65M cash from Emergent and $35M term loan
Appendix
Intellectual Property Estate APTEVO owns or exclusively licenses patent rights protecting •IXINITY • ADAPTIR • otlertuzumab • MOR209/ES414 • ES210 • ES425 •5E3mAb APTEVO’S General Patent Filing • Will seek patent protection on all – Exception – existing hyperimmune products • Will practice life cycle management – File new patent applications as products and related methods evolve • Will seek broad geographic • Will seek exclusive for supporting technologies
Portfolio of Marketed Products IXINITY® [coagulation factor IX (recombinant)] IXINITY® is an intravenous recombinant human coagulation factor IX therapeutic for the control and prevention of bleeding episodes and for perioperative management in adults and children, =12 years of age, with Hemophilia B. What is Hemophilia B? Hemophilia B is a bleeding disorder caused by a mutation on the factor IX gene resulting in a deficiency of clotting factor IX in the blood, which controls bleeding. The primary aim of care is to prevent and treat bleeding by replacement with the deficient clotting factor. How does IXINITY work? IXINITY contains recombinant coagulation factor IX (trenonacog alfa) which replaces the deficient clotting factor. IXINITY was approved by the FDA in April 2015 and launched into the market in June 2015.
Portfolio of Marketed Products US: [Rho (D) Immune Globulin Intravenous (Human)] Canada: (Rho (D) Immune Globulin (Human) for injection) WinRho® SDF is a Rho(D) Immune Globulin Intravenous (Human) product indicated for use in clinical situations requiring an increase in platelet count to prevent excessive hemorrhage in the treatment of non-splenectomized, Rho(D)-positive: • Children with chronic or acute Immune Thrombocytopenic Purpura (ITP) • Adults with chronic ITP • Children and adults with ITP secondary to HIV infection What is ITP? Immune Thrombocytopenic Purpura (ITP) is a type of autoimmune bleeding disorder. It occurs because of a reduction in cells (platelets) that normally cause blood to clot. Sometimes, ITP occurs after an infection, especially in children. How does WinRho SDF work? WinRho is a sterile, liquid gamma globulin (IgG) fraction containing antibodies to the Rho(D) antigen (D antigen). WinRho has been shown to increase platelet counts through the formation of red blood cell complexes which spare antibody coated platelets from removal. WinRho SDF has been used to treat ITP in the U.S. since 1995.
Portfolio of Marketed Products US: [Hepatitis B Immune Globulin Intravenous (Human)] Canada: (Hepatitis B Immune Globulin (Human) Injection) HepaGam B® is the only Hepatitis B Immune Globulin approved by the FDA for the prevention of hepatitis B recurrence following liver transplantation in HBsAg-positive patients. HepaGam B is also approved for post-exposure prophylaxis after acute exposure to the hepatitis B virus (HBV). What is HBV? HBV causes the liver disease Hepatitis B. The virus interferes with liver functioning and causes pathological damage. A small percentage of infected people cannot get rid of the virus and become chronically infected – these people are at higher risk of death from cirrhosis of the liver and liver cancer. How does HepaGam B work? HepaGam B is a sterile solution of purified gamma globulin (IgG) fraction of human plasma containing antibodies to hepatitis B surface antigen. HepaGam B provides passive immunization for individuals exposed to the hepatitis B virus, by binding to the surface antigen of the virus and reducing the rate of hepatitis B infection. HEPAGAM B is the ONLY hepatitis B immune globulin (HBIg) approved by the FDA to both prevent hepatitis B virus (HBV) recurrence following liver transplantation in HBsAg-positive patients and provide post-exposure prophylaxis
Portfolio of Marketed Products US: VARIZIG® [Varicella Zoster Immune Globulin (Human)] Canada: VariZIG® (Varicella Zoster Immune Globulin (Human)) VARIZIG® is intended for use as post-exposure prophylaxis to reduce the severity of chickenpox infections in high risk patient groups (see respective U.S. and Canadian prescribing information for details). What is Varicella? Varicella-zoster virus (VZV) causes an illness commonly known as chickenpox. This easily spread disease can be a serious health issue for high risk patient groups. Chickenpox causes a blister-like rash, itching, tiredness, and fever. How does VARIZIG work? VARIZIG is a sterile lyophilized preparation of purified human immune globulin G (IgG) containing antibodies to VZV that can reduce the severity of varicella infections. VARIZIG was approved by the FDA in 2012 and is the only approved post exposure treatment for VZV.