Attached files
| file | filename |
|---|---|
| EX-4.1 - EX-4.1 - TESARO, Inc. | tsro-20160630ex41a212a80.htm |
| 10-Q - 10-Q - TESARO, Inc. | tsro-20160630x10q.htm |
| EX-32.2 - EX-32.2 - TESARO, Inc. | tsro-20160630ex322af6edd.htm |
| EX-32.1 - EX-32.1 - TESARO, Inc. | tsro-20160630ex321017805.htm |
| EX-31.2 - EX-31.2 - TESARO, Inc. | tsro-20160630ex3124750dd.htm |
| EX-31.1 - EX-31.1 - TESARO, Inc. | tsro-20160630ex3110d60f3.htm |
| EX-10.3 - EX-10.3 - TESARO, Inc. | tsro-20160630ex103eb9aec.htm |
| EX-10.2 - EX-10.2 - TESARO, Inc. | tsro-20160630ex102442380.htm |
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
COLLABORATION AND LICENSE AGREEMENT
BY AND BETWEEN
TESARO, INC.
AND
JANSSEN BIOTECH, INC.
TABLE OF CONTENTS
|
|
Page |
|
Article 1 DEFINITIONS |
1 |
|
|
|
|
Article 2 GOVERNANCE |
22 |
|
|
|
|
2.1 Joint Steering Committee |
22 |
|
2.2 Joint Development Committee |
25 |
|
2.3 Joint Commercialization Committee |
28 |
|
2.4 Joint Manufacturing Committee |
30 |
|
2.5 Joint Finance Committee |
31 |
|
2.6 Committee Membership and Operations |
33 |
|
2.7 Additional Subcommittees |
35 |
|
2.8 Authority |
35 |
|
2.9 Alliance Managers |
35 |
|
2.10 Compliance with Merger Control Laws |
35 |
|
|
|
|
Article 3 LICENSES |
36 |
|
|
|
|
3.1 Licenses to Company |
36 |
|
3.2 License to TESARO |
39 |
|
3.3 [***] |
40 |
|
3.4 Retained Rights |
41 |
|
3.5 No Implied Licenses |
41 |
|
3.6 [***] |
41 |
|
|
|
|
Article 4 DEVELOPMENT |
43 |
|
|
|
|
4.1 General |
43 |
|
4.2 Development Activities |
43 |
|
4.3 Development Diligence Obligations |
47 |
|
4.4 Compliance with Law |
48 |
|
4.5 Records |
48 |
|
4.6 Subcontracting |
49 |
|
4.7 Audits |
49 |
|
|
|
|
Article 5 REGULATORY RESPONSIBILITIES |
49 |
|
|
|
|
5.1 General |
49 |
|
5.2 Regulatory Activities – General |
50 |
|
5.3 INDs and Marketing Approvals |
51 |
|
5.4 Interactions with Regulatory Authorities |
57 |
|
5.5 Sharing of Regulatory Documentation |
58 |
|
5.6 Rights of Reference |
58 |
|
5.7 Adverse Event Reporting and Safety Data Exchange |
60 |
|
5.8 Medical Affairs |
60 |
|
5.9 Recalls and Voluntary Withdrawals |
62 |
|
5.10 Subcontracting |
63 |
|
|
|
|
Article 6 COMMERCIALIZATION |
64 |
|
|
|
|
6.1 General |
64 |
|
6.2 Commercialization Activities |
64 |
|
6.3 Commercialization Diligence Obligations |
67 |
|
6.4 Registration & Distribution Option |
67 |
|
6.5 Trademarks; Product Packaging |
68 |
|
6.6 Transparency Reporting |
68 |
|
6.7 Compliance with Law |
69 |
|
6.8 Advertising and Promotional Materials |
69 |
|
6.9 Subcontracting |
69 |
|
6.10 [***] |
69 |
|
|
|
|
Article 7 MANUFACTURING |
70 |
|
|
|
|
7.1 Single Agent Products |
70 |
|
7.2 Fixed Dose Combination Products |
71 |
|
7.3 API Supply |
71 |
|
7.4 Joint Manufacturing Committee |
73 |
|
7.5 Compliance with Law |
74 |
|
7.6 Subcontracting; Audit Rights |
74 |
|
7.7 Supply Shortages |
75 |
|
|
|
|
Article 8 CONSIDERATION |
75 |
|
|
|
|
8.1 Upfront Payment |
75 |
|
8.2 Milestone Payments |
76 |
|
8.3 Single Agent Alliance Payments |
80 |
|
8.4 Company Royalty Obligations |
82 |
|
8.5 Royalty Term |
86 |
|
8.6 Royalty Rate Reduction |
87 |
|
8.7 Manner of Royalty Payment |
87 |
|
8.8 Currency |
88 |
|
8.9 Third Party Financial Obligations |
89 |
|
8.10 Taxes |
92 |
|
8.11 Audit |
93 |
|
8.12 [***] |
94 |
|
Confidential |
ii |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
8.13 Other TESARO Invoices to Company |
95 |
|
8.14 Invoices |
95 |
|
8.15 Late Payment |
95 |
|
|
|
|
Article 9 INTELLECTUAL PROPERTY MATTERS |
95 |
|
|
|
|
9.1 Ownership of Inventions |
95 |
|
9.2 Disclosure of Inventions |
96 |
|
9.3 Prosecution of Patents |
96 |
|
9.4 Patent Term Extensions in the Territory |
98 |
|
9.5 Infringement of Patents by Third Parties |
99 |
|
9.6 Infringement of Third Party Rights in the Territory |
101 |
|
9.7 Patent Oppositions and Other Proceedings |
103 |
|
|
|
|
Article 10 REPRESENTATIONS, WARRANTIES AND COVENANTS |
104 |
|
|
|
|
10.1 Mutual Representations, Warranties and Covenants |
104 |
|
10.2 Additional Representations and Warranties of TESARO |
105 |
|
10.3 Additional Representations and Warranties of Company |
109 |
|
10.4 Merck License Agreement |
109 |
|
10.5 Certain Compliance Matters |
109 |
|
10.6 No Other Representations or Warranties |
110 |
|
|
|
|
Article 11 CONFIDENTIALITY |
110 |
|
|
|
|
11.1 Nondisclosure and Nonuse |
110 |
|
11.2 Exceptions |
111 |
|
11.3 Authorized Disclosure |
112 |
|
11.4 Terms of this Agreement |
112 |
|
11.5 Publicity |
113 |
|
11.6 Securities Filings |
113 |
|
11.7 Relationship to Confidentiality Agreement and Merck License Agreement |
113 |
|
11.8 Equitable Relief |
114 |
|
11.9 Publications |
114 |
|
11.10 Return of Confidential Information |
114 |
|
|
|
|
Article 12 TERM AND TERMINATION |
114 |
|
|
|
|
12.1 Term |
114 |
|
12.2 Termination upon Termination of the Merck License Agreement or Either AstraZeneca License Agreement |
114 |
|
12.3 Unilateral Termination by Company |
114 |
|
12.4 Termination for Material Breach |
114 |
|
12.5 Termination by Company for Safety Reasons |
115 |
|
Confidential |
iii |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
12.6 [Reserved.] |
115 |
|
12.7 Termination for Bankruptcy |
115 |
|
12.8 [***] |
117 |
|
12.9 Effects of Termination |
117 |
|
12.10 Remedies |
121 |
|
12.11 Survival |
121 |
|
|
|
|
Article 13 DISPUTE RESOLUTION |
121 |
|
|
|
|
13.1 Exclusive Dispute Resolution Mechanism |
121 |
|
13.2 Resolution by Executive Officers |
121 |
|
13.3 Arbitration |
122 |
|
13.4 Provisional Remedies |
123 |
|
13.5 Confidentiality |
123 |
|
|
|
|
Article 14 INDEMNIFICATION |
123 |
|
|
|
|
14.1 Indemnification by Company |
123 |
|
14.2 Indemnification by TESARO |
125 |
|
14.3 Indemnification Procedures |
126 |
|
14.4 Insurance |
129 |
|
14.5 Limitation of Liability |
129 |
|
|
|
|
Article 15 MISCELLANEOUS |
129 |
|
|
|
|
15.1 Notices |
129 |
|
15.2 Governing Law |
130 |
|
15.3 [***] |
131 |
|
15.4 Assignment |
132 |
|
15.5 Designation of Affiliates |
132 |
|
15.6 Relationship of the Parties |
133 |
|
15.7 Force Majeure |
133 |
|
15.8 Entire Agreement; Amendments |
133 |
|
15.9 Severability |
133 |
|
15.10 English Language |
134 |
|
15.11 Waiver and Non-Exclusion of Remedies |
134 |
|
15.12 Further Assurance |
134 |
|
15.13 Headings |
134 |
|
15.14 Construction |
134 |
|
15.15 Counterparts |
135 |
|
Confidential |
iv |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
LIST OF EXHIBITS
|
Exhibit 1.63 |
Initial Compound |
|
Exhibit 1.69 |
2016 Johnson & Johnson Universal Calendar |
|
Exhibit 1.117 |
TESARO Patents |
|
Exhibit 4.2(c) |
TESARO Development Plan |
|
Exhibit 4.2(f) |
TESARO INDs |
|
Exhibit 4.2(h) |
Example of Third Party Development Report |
|
Exhibit 5.5(a) |
Regulatory Documentation relating to Initial Compound and Initial Product |
|
Exhibit 7.1(a) |
Company Supply Requirements and Timeline |
|
Exhibit 8.2(d) |
Merck Milestone Payments |
|
Exhibit 8.3 |
Alliance Payments |
|
Exhibit 8.4(b)(i)(1) |
Pass-through Merck Royalty |
|
Attachment 1 to Exhibit 8.4(b)(i)(1) |
Merck Compound Patent Rights |
|
Exhibit 8.4(b)(i)(2) |
Pass-through AstraZeneca Royalties |
|
[***] |
[***] |
|
[***] |
[***] |
|
Summary of Economic Transfers between the Parties |
|
|
Exhibit 10.2(j) |
TESARO Disclosure of Certain Development Activities |
|
Exhibit 10.4 |
Merck License Agreement Provisions |
|
Exhibit 10.5 |
Compliance with Laws |
|
Exhibit 11.5 |
Press Release |
|
Confidential |
v |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
COLLABORATION AND LICENSE AGREEMENT
THIS COLLABORATION AND LICENSE AGREEMENT (“Agreement”) is entered into as of April 5, 2016 (the “Effective Date”), by and between JANSSEN BIOTECH, INC., a Pennsylvania corporation, having its principal place of business at 800/850 Ridgeview Drive, Horsham, PA 19044 (hereinafter “Company”) and TESARO, INC., a Delaware corporation having its principal place of business at 1000 Winter Street, Suite 3300, Waltham, MA 02451 (“TESARO”). Company and TESARO are sometimes referred to herein individually as a “Party” and collectively as the “Parties”.
WHEREAS, TESARO is currently developing a small molecule poly ADP ribose polymerase (PARP) inhibitor, Niraparib, for certain cancer indications (including breast cancer and ovarian cancer);
WHEREAS, Company desires to obtain, and TESARO desires to grant to Company, a worldwide (excluding Japan), exclusive license to develop and commercialize Niraparib for prostate cancer indications, subject to the terms and conditions of this Agreement; and
WHEREAS, TESARO and Company desire to collaborate with respect to the development, manufacture and commercialization of Niraparib as set forth in this Agreement.
NOW, THEREFORE, in consideration of the foregoing and the premises and conditions set forth herein, the Parties agree as follows:
1.1“Acquirer” means any Third Party that is an acquirer in any Change of Control transaction and any of such Third Party’s Affiliates.
1.2“Affiliate” means, with respect to a particular Person and a particular time, another Person that controls, is controlled by or is under common control with such first Person at any such time during the Term. For the purposes of this definition, the word “control” (including, with correlative meaning, the terms “controlled by” or “under the common control with”) means the actual power, either directly or indirectly through one or more intermediaries, to direct or cause the direction of the management and policies of a Person, whether by the ownership of fifty percent (50%) or more of the voting stock of such Person, by contract, or otherwise.
1.3“Affordable Basis” means making a product available to patients at the lowest cost possible. For clarification, Affordable Basis is satisfied if a Party sells such product for no more than [***]. In determining Affordable Basis, the Parties recognize that, to the extent that a Party engages a Third Party in the commercialization of a product on an Affordable Basis, such Third Party shall be entitled to [***]; provided that the applicable Party uses Commercially Reasonable Efforts to minimize such Third Party profits.
1.4“Alliance Manager” means the person appointed by each Party from within its respective organization to coordinate and facilitate the communication, interaction and cooperation of the Parties pursuant to this Agreement.
1.5“ANDA” means an abbreviated new drug application submitted to the FDA pursuant to 21 U.S.C. § 355(j), and all amendments and supplements thereto
1.6“Applicable Law” means all applicable statutes, ordinances, regulations, rules or orders of any kind whatsoever of any Governmental Authority, including the FFDCA, 21 U.S.C. §301 et seq., U.S. Patent Act (35 U.S.C. §1 et seq.), Federal False Claims Act (31 U.S.C. §3729 et seq.), the Anti-Kickback Statute (42 U.S.C. §1320a-7b et seq.), Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use, Directive 2001/20/EC on the approximation of the laws, regulations and administrative provisions of the EU member states relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use, Commission Directive 2005/28/EC of 8 April 2005 laying down principles and detailed guidelines for good clinical practice as regards investigational medicinal products for human use and related guidance, Directive 95/46/EC on the protection of individuals with regard to the processing of personal data and on the free movement of such data, the related national implementing laws and regulations of the EU member states, provisions of the national laws and industry and professionals codes of EU member states governing anti-bribery and anti-kickback practices, all as amended from time to time, together with any rules, regulations, and compliance guidance promulgated thereunder.
1.7“AstraZeneca” means AstraZeneca UK Limited or any assignee of such entity under the AstraZeneca License Agreements.
1.8“AstraZeneca License Agreements” means, collectively, (a) the Patent License Agreement, dated October 4, 2012, between TESARO and AstraZeneca, relating to certain intellectual property licensed to AstraZeneca by the Institute of Cancer Research (the “AstraZeneca-ICR License Agreement”), and (b) the Patent License Agreement, dated October 4, 2012, between TESARO and AstraZeneca, relating to certain intellectual property licensed to AstraZeneca by the University of Sheffield (the “AstraZeneca-Sheffield License Agreement”), in each case ((a) and (b)), including all amendments thereto.
1.9“AstraZeneca Patents” means the “Licensed Patents” as such term is defined in each of the AstraZeneca License Agreements.
1.10“Business Day” means a day other than Saturday, Sunday or any other day on which banking institutions in New York, New York are closed for business.
1.11“Calendar Quarter” means a financial quarter based on the Johnson & Johnson Universal Calendar; provided, however, that the first Calendar Quarter for the first Calendar Year extends from the Effective Date to the end of the then-current Calendar Quarter and the last
|
Confidential |
2 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Calendar Quarter extends from the first day of such Calendar Quarter until the effective date of the termination or expiration of this Agreement.
1.12“Calendar Year” means a year based on the Johnson & Johnson Universal Calendar for that year. The last Calendar Year of the Term begins on the first day of the Johnson & Johnson Universal Calendar Year for the year during which termination or expiration of this Agreement will occur, and the last day of such Calendar Year will be the effective date of such termination or expiration.
1.13“Centralized Approval Procedure” means, to the extent compulsory or permitted for the Marketing Approval of a pharmaceutical product in Iceland, Liechtenstein, Norway or any country in the European Union, the procedure administrated by the EMA which results in a single Marketing Approval granted by the European Commission (excluding any pricing or reimbursement approval) that is valid in all countries in the European Union and following recognition, Iceland, Liechtenstein and Norway.
1.14“Change of Control” shall occur if: (a) any Third Party acquires directly or indirectly the beneficial ownership of any voting security of a Party, or if the percentage ownership of such person or entity in the voting securities of a Party is increased through stock redemption, cancellation or other recapitalization, and immediately after such acquisition or increase such Third Party is, directly or indirectly, the beneficial owner of voting securities representing more than fifty percent (50%) of the total voting power of all of the then outstanding voting securities of a Party; (b) a merger, consolidation, recapitalization or reorganization of a Party is consummated, other than any such transaction, which would result in stockholders or equity holders of such Party immediately prior to such transaction, owning, directly or indirectly, at least fifty percent (50%) of the outstanding securities of the surviving entity (or its parent entity) immediately following such transaction; (c) the stockholders or equity holders of a Party approve a plan of complete liquidation of such Party, or an agreement for the sale or disposition by such Party of all or substantially all of such Party’s assets, other than pursuant to a transaction described above or to an Affiliate; (d) individuals who, as of the date hereof, constitute the Board of Directors of a Party (the “Incumbent Board”) cease for any reason to constitute at least a majority of the Board of Directors of such Party (provided, however, that any individual becoming a director subsequent to the date hereof whose election, or nomination for election by such Party’s shareholders, was recommended or approved by a vote of at least a majority of the directors then comprising the Incumbent Board shall be considered as though such individual were a member of the Incumbent Board); or (e) the sale or transfer to a Third Party of all or substantially all of such Party’s assets is effected.
1.15“Clinical Data” means any and all data (anonymized, to the extent required by Applicable Law or relevant contracts) generated from any Clinical Trial, or from testing or analysis of subjects or samples from a Clinical Trial (e.g., in vitro testing of tissue samples from subjects enrolled in a Clinical Trial), including raw data, any top-line results reports, and the final clinical study report from such Clinical Trial.
|
Confidential |
3 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.16“Clinical Trials” means a Phase 1 Trial, Phase 2 Trial, Phase 2/3 Trial, Phase 3 Trial or Phase 4 Trial, as applicable.
1.17“CMC Development” means the Development activities related to the composition, manufacture, and specification of the drug substance and the drug product intended to assure the proper identification, quality, purity and strength of the drug, including, but not limited to: test method development and stability testing, process development, drug substance development, process validation, process scale-up, formulation development, delivery system development, quality assurance and quality control development.
1.18“Co-Packaged Product” means any product containing, in a single package or container and intended for coordinated sale or use, two (2) or more separate products as components, one (1) of which is a Product and the other one (1) or more are for preventative, therapeutic, or diagnostic use.
1.19“Combination Product” means any Product containing as a single formulation, two or more active pharmaceutical ingredients (each, an “API”) as components, including (i) a Compound and (ii) one or more other APIs.
1.20“Combination Use” means the use of a Single Agent Product in combination with at least one other product that is not a Product for the diagnosis, treatment or prevention of any indication, where such Single Agent Product and other product are packaged and sold separately.
1.21“Commercialization” means any activities directed to marketing, promoting and/or Distributing a pharmaceutical product. When used as a verb, “Commercialize” means to engage in Commercialization activities.
1.22“Commercially Reasonable Efforts” means, with respect to the efforts and resources to be expended, or activities to be undertaken, by a Party or its Affiliate with respect to any objective or activity to be undertaken hereunder, reasonable efforts and resources intended in good faith to accomplish such objective or activity as such Party would normally use to accomplish a similar objective or activity under similar circumstances, it being understood and agreed that, with respect to the Development, Manufacture, seeking and obtaining Marketing Approval, or Commercialization of a Compound or Product, such efforts and resources shall be consistent with those efforts and resources normally used by a Party under similar circumstances for similar compounds or products to which it has similar rights, which compound or product, as applicable, is at a similar stage in its development or product life and is of similar market potential, taking into account all commercial, scientific, economic and other factors (not including payments due to a Party hereunder), including: (a) issues of efficacy, safety, and expected and actual approved labeling; (b) the expected and actual competitiveness of alternative products sold by Third Parties in the marketplace; (c) the expected and actual product profile of the Compound or Product; (d) the expected and actual patent and other proprietary position of the Compound or Product; (e) the likelihood of Marketing Approval of the Product given the regulatory structure involved, including the likelihood of obtaining regulatory exclusivity; (f) the ability to formulate and Develop Combination Products; and (g) the market penetration, actual
|
Confidential |
4 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
profitability and the return on investment potential of the Product, including pricing and reimbursement status achieved or likely to be achieved and expected and actual Third Party costs. Additionally, neither Party shall take into account the Alliance Payments, royalties or milestone payments due to the other Party under this Agreement in connection with its use of Commercially Reasonable Efforts. To the extent that the performance of a Party’s obligations hereunder is adversely affected by the other Party’s failure to perform its obligations hereunder, the impact of such performance failure will be taken into account in determining whether such first Party has used Commercially Reasonable Efforts to perform its affected obligations.
1.23“Company Field” means the diagnosis, treatment or prevention of prostate cancer in humans.
1.24“Company Know-How” means any Know-How Controlled by Company or its Affiliates during the Term that is used by Company or any of its Affiliates or (sub)licensees to Exploit the Compounds or Single Agent Products in the Company Field in the Company Territory, but excluding any such Know-How to the extent that it (a) is used to Exploit a Compound due to its incorporation or use as part of a Combination Product, and (b) is not necessary or useful for the Exploitation of Compounds as Single Agent Products.
1.25“Company Net Sales” means, with respect to any Product sold by Company or any of its Affiliates, licensees or sublicensees, the gross amounts invoiced on sales of such Product by Company or any of its Affiliates, licensees or sublicensees to a Third Party purchaser in an arms-length transaction, less the following customary deductions, determined in accordance with GAAP and internal policies and actually taken, paid, accrued or allocated based on good faith estimates:
(a) trade, cash and/or quantity discounts, allowances, and credits, excluding commissions for commercialization;
(b) excise taxes, use taxes, tariffs, sales taxes and customs duties, and/or other government taxes imposed on the sale of Product (including VAT, but only to the extent that such VAT taxes are not reimbursable or refundable), specifically excluding, for clarity, any income taxes assessed against the income arising from such sale;
(c) outbound freight, shipment and insurance costs to the extent included in the price and separately itemized on the invoice price;
(d) [***], cash rebates payable to, or the impact of reimbursement caps established by, a governmental authority (or agent thereof) pursuant to governmental regulations by reason of any national or local health insurance program or similar program, including government levied fees as a result of healthcare reform policies;
|
Confidential |
5 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(e) retroactive price reductions, credits or allowances actually granted upon rejections or returns of Product, including for recalls or damaged goods, or billing errors and reserves for returns;
(f) rebates, chargebacks, administrative fees and discounts (or equivalent thereof) payable to managed health care organizations, group purchasing organizations, pharmacy benefit managers (or equivalent thereof), specialty pharmacy providers, federal, state/provincial, local or other governments, or their agencies or purchasers, reimbursers, or trade customers;
(g) amounts payable to patients through co-pay assistance cards or similar forms of rebate directly related to the prescribing of the Product; and
(h) any invoiced amounts which are not collected by Company or its Affiliates, including bad debts.
All aforementioned deductions shall only be allowable to the extent they are commercially reasonable and shall be determined, on a country-by-country basis, as incurred in the ordinary course of business in type and amount consistent with Company’s, its Affiliate’s, or Third Party licensee’s or sublicensee’s (as the case may be) business practices consistently applied across its product lines and accounting standards and verifiable based on Company’s and its Affiliates’ sales reporting system.
Sales of a Product by and between Company and its Affiliates, licensees or sublicensees are not sales to Third Parties and shall be excluded from Net Sales calculations for all purposes so long as such Product is subsequently resold to a Third Party end user. Sales of a Product on an Affordable Basis in Developing Countries shall be excluded from Net Sales calculations for purposes of royalty calculations under ARTICLE 8. Any disposition of a Product as free samples, donations, patient assistance, test marketing programs or other similar programs or studies where such Product is supplied free of charge shall not be subject to royalties.
Notwithstanding the foregoing, but subject to Section 3.1(e), with respect to any Combination Product that is sold by Company or any of its Affiliates, licensees or sublicensees, the Net Sales of such Combination Product for the determination of royalties of Combination Products will be calculated by multiplying the Net Sales of such Combination Product (calculated as set forth above) by the fraction 1/A, where A is the total number of separate active pharmaceutical ingredients included in such Combination Product.
1.26“Company Parent” means Johnson & Johnson, a New Jersey corporation.
1.27“Company Patents” means all Patents Controlled by Company or its Affiliates during the Term that are necessary to Exploit Compounds or the Single Agent Products in the Company Field in the Company Territory, but excluding any such Patent to the extent that it (a) Covers such Compound due to its incorporation or use as part of a Combination Product and (b) is not necessary or useful for the Exploitation of Compounds as Single Agent Products.
|
Confidential |
6 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.28“Company Technology” means, collectively, the Company Patents and the Company Know-How.
1.29“Company Territory” means worldwide, excluding Japan.
1.30“Compound” means (a) the Initial Compound; (b) the compound licensed to TESARO under the Merck License Agreement and known as MK-2512; or (c) with respect to both (a) and (b), any pharmaceutically acceptable salt, polymorph, crystal form, prodrug or solvate thereof.
1.31“Conducting Party” means the Party that is (or the Party whose Affiliate, licensee or sublicensee is) conducting a Clinical Trial of a Product.
1.32“Confidential Information” means, subject to ARTICLE 11, (a) all non-public or proprietary information disclosed by a Party to the other Party under this Agreement, which may include Know-How; and (b) all other non-public or proprietary information (including Know-How) that is expressly deemed in this Agreement to be Confidential Information of such Party whether or not disclosed by such Party or any of its Affiliates to the other Party or any of its Affiliates; in each case ((a) and (b)) without regard as to whether any of the foregoing is marked “confidential” or “proprietary,” or disclosed in oral, written, graphic, or electronic form. Confidential Information shall include: (a) the terms and conditions of this Agreement; and (b) Confidential Information disclosed by either Party pursuant to the Mutual Non-Disclosure Agreement dated December 29, 2015, between TESARO and Janssen Research & Development LLC, an Affiliate of Company (the “Prior CDA”).
1.33“Control” or “Controlled” means, with respect to any Know-How, Patent, Trademark or other intellectual property right, possession by a Party or[***] any of its Affiliates, of the ability (without taking into account any rights granted by one Party to the other Party under the terms of this Agreement) to grant access, a license or a sublicense to such Know-How, Patent, Trademark or other intellectual property right without violating the terms of any agreement or other arrangement with, or necessitating the consent of, any Third Party, but with respect to TESARO excluding any Know-How, Patent, Trademark or other intellectual property right that comes into the Control of TESARO pursuant to a Change of Control of TESARO, except to the extent and only to the extent that, such Know-How, Patent, Trademark or other intellectual property right is (a) made, conceived or reduced to practice by the Acquirer following a Change of Control through the use of, or reference to, any Know-How, Patent, Trademark or other intellectual property right Controlled by TESARO prior to the Change of Control and the failure of Company to have access to such intellectual property right would prevent Company from exercising its rights under Section 3.1 to Exploit Compounds and Products, as they existed immediately prior to the Change of Control; or (b) actually used by TESARO or its Affiliates, or the Acquirer, to Exploit the Compounds or Products following the consummation of such Change of Control. For clarity, “Control” of a compound by a Party shall mean that such Party Controls Know-How, Patents or other intellectual property rights with respect to such compound.
1.34“Cover” or “Covering” means, with respect to a product, technology, process or method, that, in the absence of ownership of or a license granted under a Valid Claim, the practice or
|
Confidential |
7 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exploitation of such product, technology, process or method would infringe such Valid Claim (or, in the case of a Valid Claim that has not yet issued, would infringe such Valid Claim if it were to issue).
1.35“CPR” means the International Institute for Conflict Prevention & Resolution or any successor organization having substantially the same function.
1.36“Critical Observation” means a significant and meaningful GMP deviation or deficiency that requires immediate remediation.
1.37“Currency Hedge Rate” means the Johnson & Johnson currency hedge rate[***]. The Johnson & Johnson currency hedge rate is calculated [***].
1.38“Developing Countries” means those countries defined by the World Bank as either “low income countries” or “lower middle income economies” as of the Effective Date. If, during the Term, countries reported by the World Bank are moved in or out of these designations, the Parties will discuss in good faith the royalty reduction treatment for such countries.
1.39“Development” means all research and non-clinical and clinical drug development activities and processes, including toxicology, pharmacology, project management and other non-clinical efforts, statistical analysis, formulation development, delivery system development, statistical analysis, CMC Development, the performance of Clinical Trials (including the Manufacturing of Compound or Product for use in Clinical Trials) and other activities, reasonably necessary to obtain or maintain Marketing Approval of a pharmaceutical product. When used as a verb, “Develop” means to engage in Development activities.
1.40“Distribution” means offering to sell, selling, distributing, exporting, transporting and other activities associated with the foregoing activities with respect to the distribution of a pharmaceutical product, including inventory management and control, storage, warehousing and distribution, invoicing, collection of sales proceeds, booking of sales, preparation of sales records and reports, customer relations and services and handling of returns. When used as a verb, “Distribute” means to engage in Distribution activities.
1.41“DMF Equivalent” means (a) a Drug Master File (as defined in 21 CFR 314.420) with respect to the U.S.; (b) Module 3 of the Common Technical Document with respect to countries in the EU; or (c) any equivalent of any of the foregoing with respect to any country outside the U.S. or EU.
1.42“Drug Approval Application” means an MAA or NDA.
1.43“EMA” means the European Medicines Agency or any successor agency(ies) or authority having substantially the same function.
|
Confidential |
8 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.44“European Commission” means the executive body of the European Union that is responsible, among other things, for granting marketing authorization for medicinal products through the Centralized Approval Procedure.
1.45“European Union” or “EU” means the European Union member states as then-currently constituted; provided, however, that the EU shall always be deemed to include the Major EU Countries. As of the Effective Date, the European Union member states are Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and the United Kingdom.
1.46“Executive Officers” means (a) with respect to Company, either (i) prior to the first Marketing Approval of any Product in the Company Field in the Company Territory, the Global Head of Research and Development (or his or her designee), and (ii) following the first Marketing Approval of any Product in the Company Field in the Company Territory, the President of U.S. oncology commercial operations (or his or her designee), and (b) with respect to TESARO, the Chief Executive Officer (or his or her designee).
1.47“Exploitation” means research, use, practice, Development, Manufacture or Commercialization. When used as a verb, to “Exploit” means to engage in Exploitation activities.
1.48“FDA” means the U.S. Food and Drug Administration and any successor agency(ies) or authority having substantially the same function.
1.49“FFDCA” means the U.S. Federal Food, Drug, and Cosmetic Act (21 U.S.C. §301 et seq.), as amended from time to time.
1.50“Field” means, (a) with respect to Company and its Affiliates and (sub)licensees, the Company Field, and (b) with respect to TESARO and its Affiliates and (sub)licensees, the TESARO Field. References to a “Party’s Field” shall be interpreted accordingly.
1.51“First Commercial Sale” means, on a Product-by-Product and country-by-country basis, the first sale for monetary value of such Product under this Agreement by a Party, its Affiliates, licensees or sublicensees to an end user for use, consumption or resale of such Product in such country in the Company Field after Marketing Approval of such Product in the Company Field has been obtained in such country, where such sale results in a Net Sale. Sale of a Product under this Agreement by a Party to an Affiliate of such Party or a licensee or sublicensee of such Party shall not constitute a First Commercial Sale unless such Affiliate, licensee or sublicensee is the end user of such Product. For the avoidance of doubt, the sale of Product for clinical study purposes, early access programs (such as to provide patients with a Product prior to Marketing Approval pursuant to treatment INDs or protocols, named patient programs or compassionate use programs) or any similar uses shall not constitute a First Commercial Sale.
|
Confidential |
9 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.52“Fixed Dose Combination Product” or “FDC” means any Product containing, as a single formulation, two or more APIs as components, including (a) a Compound and (ii) one or more other APIs. For clarity, an FDC is a “Combination Product” hereunder.
1.53“Force Majeure” means any event beyond the reasonable control of the affected Party, which may include embargoes; war or acts of war, including terrorism; insurrections, riots, or civil unrest; strikes, lockouts or other labor disturbances; epidemics, fire, floods, earthquakes or other acts of nature; acts, omissions or delays in acting by any Governmental Authority (other than delays incident to the ordinary course of drug development); and failure of plant or machinery.
1.54“GAAP” means generally accepted accounting principles in the U.S., consistently applied.
1.55“Generic Approval” means, with respect to a pharmaceutical product for which Marketing Approval has been granted in any country or jurisdiction, a subsequent Marketing Approval for another pharmaceutical product in such country or any other country that is granted based upon: (a) reference to an existing Marketing Approval for such pharmaceutical product (or any of its APIs), or to any Regulatory Documentation or Clinical Data underlying such existing Marketing Approval but not including pursuant to a Right of Reference granted under this Agreement; and (b) in the U.S., that pharmaceutical product is approved with an “A” rating under an ANDA, or outside of the U.S., that pharmaceutical product is similarly approved.
1.56“Generic Version” means, with respect to a given pharmaceutical product and a given country or jurisdiction, a version of such pharmaceutical product that: (a) contains the same active pharmaceutical ingredient(s) as such pharmaceutical product (and no other active pharmaceutical ingredient(s)); (b) has the same indication; (c) has the same dosage form and strength; (d) is approved for marketing or sale in such country or jurisdiction pursuant to a Generic Approval; and (e) is sold by a Third Party in such country pursuant to such Generic Approval.
1.57“Good Clinical Practices” or “GCP” means the then-current standards, practices and procedures promulgated or endorsed by the FDA as set forth in 21 C.F.R. Parts 50, 54 and 56 (or any successor statute or regulation), and in the guideline adopted by the International Conference on Harmonization (“ICH”), titled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance” (or any successor document), including related regulatory requirements imposed by the FDA and comparable regulatory standards, practices and procedures promulgated by the European Commission or other Regulatory Authority, as they may be updated from time to time.
1.58“Good Laboratory Practices” or “GLP” means the then-current standards, practices and procedures promulgated or endorsed by the FDA as set forth in 21 C.F.R. Part 58 (or any successor statute or regulation), including related regulatory requirements imposed by the FDA and comparable regulatory standards, practices and procedures promulgated by the European
|
Confidential |
10 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Commission or other Regulatory Authority, as they may be updated from time to time, including applicable guidelines promulgated under the ICH.
1.59“Good Manufacturing Practices” or “GMP” means the then-current good manufacturing practices required by the FFDCA, as amended, and the regulations promulgated thereunder by the FDA at 21 C.F.R. Parts 210 and 211, for the manufacture and testing of pharmaceutical materials, and comparable Applicable Law related to the manufacture and testing of pharmaceutical materials in jurisdictions outside the U.S., including the quality guideline promulgated by the ICH designated ICH Q7A, titled “Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients” and the regulations promulgated thereunder, in each case as they may be updated from time to time.
1.60“Governmental Authority” means any multi-national, supra-national, federal, state, local, municipal or other government authority of any nature (including any governmental division, subdivision, department, agency, bureau, branch, office, commission, council, court or other tribunal).
1.61“IND” means (a) an Investigational New Drug application as defined in the FFDCA and applicable regulations promulgated thereunder by the FDA, including 21 C.F.R. Part 312; or (b) a Clinical Trial authorization application or other documentation filed with a Regulatory Authority in any other country or jurisdiction outside the U.S., the filing or becoming effective of which (in the case of (a) or (b)) is necessary to commence or conduct clinical testing of a pharmaceutical product in humans in such country or jurisdiction.
1.62“Indication” means a discrete clinically recognized form of a disease in the Company Field. For purposes of this Agreement, treatment of different subpopulations within a population of patients treated for a particular cancer type/indication or different lines of treatment for the same cancer type/indication shall also be treated as separate Indications (e.g., front-line treatment, relapsed refractory treatment and maintenance treatment of the same disease shall be considered different Indications).
1.63“Initial Compound” means the compound known as Niraparib, as further described in Exhibit 1.63.
1.64“Initial Indications” means [***].
1.65“Initial Product” means the Single Agent Product Developed by TESARO prior to the Effective Date that contains the Initial Compound as its sole active pharmaceutical ingredient.
1.66“International Public Organization” means any of the organizations listed in 8 C.F.R. § 316.20, as amended from time to time.
1.67“Invention” means any invention, discovery or development, whether or not patentable, made, conceived or reduced to practice under this Agreement, whether made, conceived or
|
Confidential |
11 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
reduced to practice solely by, or on behalf of, TESARO, Company, the Parties jointly, or any Affiliate of the same.
1.68“JAA” means any anti-androgen compound Controlled by Company or any of its Affiliates during the Term or a product containing such compound as a sole active pharmaceutical ingredient, as the context requires. For clarity, the term “JAA” does not include any FDC or other Combination Product containing a JAA as a component.
1.69“Johnson & Johnson Universal Calendar” means the universal calendar system used by Company Parent and its Affiliates for internal and external reporting purposes (a copy of which for the year 2016 is attached hereto as Exhibit 1.69 and a copy of which prior to the beginning of each such year for succeeding years shall be provided to TESARO).
1.70“Know-How” means information, inventions, discoveries, formulations, formulas, practices, procedures, processes, methods, knowledge, know-how, trade secrets, technology, techniques, designs, drawings, correspondence, computer programs, documents, apparatus, results, strategies, Regulatory Documentation, information and submissions pertaining to, or made in association with, filings with any Governmental Authority, data (including pharmacological, toxicological, non-clinical data, Clinical Data, analytical and quality control data, manufacturing data and descriptions, market data, financial data or descriptions), devices, assays, chemical formulations, specifications, material, product samples and other samples, physical, chemical and biological materials, and the like, in written, electronic, oral or other tangible or intangible form, whether or not patentable.
1.71“Knowledge” means, as applied to a Party, that such Party shall be deemed to have knowledge of a particular fact or other matter to the extent that a reasonably prudent person with primary responsibility for the applicable subject matter (whether an officer or employee of such Party) knew or should have known of such fact or other matter.
1.72“[***]” means [***].
1.73“[***]” means [***].
1.74“Major EU Countries” means, collectively, France, Germany, Italy, Spain, and the United Kingdom (whether or not such countries are EU member states).
1.75“Major Markets” mean the U.S., Canada and the Major EU Countries.
1.76“[***]” means [***].
1.77“Manufacture” means all activities and processes related to the manufacturing of a pharmaceutical product, or any ingredient thereof, including labeling, packaging, in-process and finished product testing, release of such pharmaceutical product or any component or ingredient thereof, performance of quality assurance activities related to manufacturing and release of such pharmaceutical product, and the performance of ongoing stability tests and regulatory activities
|
Confidential |
12 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
related to any of the foregoing. Where the context so requires, Manufacture shall also include obtaining product from contract manufacturers subject to the terms and conditions of this Agreement. When used as a verb, to “Manufacture” means to engage in Manufacturing activities.
1.78“Manufacturing Costs” means [***]. Costs included will be consistent with the activities outlined in Section 1.77 (the “Manufacture” definition).
1.79“Marketing Approval” means, with respect to a pharmaceutical product and any country or jurisdiction, any and all: (a) Regulatory Approvals that are necessary to market or sell such pharmaceutical product in such country or jurisdiction, including approval of a Drug Approval Application; and (b) pricing and reimbursement approvals that are necessary to obtain before initiating Commercialization of such pharmaceutical product in such country or jurisdiction. For purposes of illustration with respect to the Major EU Countries, the following pricing and reimbursement approvals are examples of those described in clause (b): in France, publication of the reimbursed price level in the official journal and registration on a reimbursement list by or on behalf of Comité Economique des Produits de Santé or Haute Autorité de Santé (or a successor agency); in Italy, publication of reimbursement in the Government’s Official Gazette (by Agenzia Italiana del Farmaco or a successor agency); in Germany, execution of contract with the head association of sick funds (GKV-Spitzenverband, Gesetzlichen Krankenversicherung, or a successor agency); in Spain, authorization by La Comisión Interministerial de Precios de los Medicamentos or La Comisión Nacional para el Uso Racional de los Medicamentos (or a successor agency) for national patient access to reimbursement by or on behalf of a Governmental Authority; and in the United Kingdom, a recommendation by the National Institute for Health and Care Excellence (or a successor agency) to obtain mandatory funding to enable broad market access.
1.80“Marketing Authorization Application” or “MAA” means an application for authorization to market and/or sell a drug product submitted to a Regulatory Authority in any country or jurisdiction other than the U.S., and amendments and supplements thereto, including, with respect to the European Union, a marketing authorization application filed with the EMA pursuant to the Centralized Approval Procedure or with the applicable Regulatory Authority of a country in the European Economic Area with respect to the decentralized procedure, mutual recognition or any national approval procedure.
1.81“Merck” means Merck Sharp & Dohme Corp. or any assignee of such entity under the Merck License Agreement.
1.82“Merck Know-How” has the meaning set forth in the Merck License Agreement.
1.83“Merck License Agreement” means the License Agreement, dated May 22, 2012, between Merck and TESARO, including all amendments thereto.
1.84“Merck Patents” means the “Compound Patent Rights” as such term is defined in the Merck License Agreement.
|
Confidential |
13 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.85“Merck Term” means the “Term” of the Merck License Agreement as such term is defined in the Merck License Agreement.
1.86“Merger Control Laws” means all U.S. federal and state, and any foreign, statutes, rules, regulations, orders, administrative and judicial doctrines, and other Applicable Law, relating to antitrust or competition matters, or that are designed or intended to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade or lessening of competition.
1.87“Monotherapy Use” means the use of one Single Agent Product alone for the diagnosis, treatment or prevention of any indication.
1.88“NDA” means a new drug application submitted to the FDA pursuant to Section 505(b) of the FFDCA, 21 U.S.C. § 355(b), and all amendments and supplements thereto.
1.89“Net Sales” means (a) with respect to any Product sold by Company or any of its Affiliates, licensees or sublicensees, Company Net Sales and (b) with respect to any Product sold by TESARO or any of its Affiliates, licensees or sublicensees, TESARO Net Sales.
1.90“Orange Book” means, as applicable, the most recent and electronic editions of the Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations.
1.91“Other TESARO Patents” means TESARO Patents other than the Merck Patents and the AstraZeneca Patents.
1.92“Pass-through Third Party Royalties” means the Pass-through Merck Royalty, the Pass-through AstraZeneca-ICR Royalty and the Pass-through AstraZeneca-Sheffield Royalty.
1.93“Patents” means all: (a) patents, including any utility or design patent; (b) patent applications, including provisionals, substitutions, divisionals, continuations, continuations in-part or renewals; (c) patents of addition, restorations, extensions, supplementary protection certificates, registration or confirmation patents, patents resulting from post-grant proceedings, re-issues and re-examinations; (d) other patents or patent applications claiming priority directly or indirectly to (i) any such specified patent or patent application specified in (a) through (c), or (ii) any patent or patent application from which a patent or patent application specified in (a) through (c) claim direct or indirect priority; (e) inventor’s certificates; and (f) other rights issued from a Governmental Authority similar to any of the foregoing; in each case of (a) through (f), irrespective of whether such patent, patent application or other right arises in the U.S. or any other country or jurisdiction.
1.94“Person” means an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, incorporated association, joint venture or similar entity or organization, including a government or political subdivision, department or agency of a government.
|
Confidential |
14 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.95“Phase 1 Trial” means a clinical trial of a pharmaceutical product that (a) (i) is a first-in-humans trial on subjects who are patients, (ii) is for the purposes of establishing initial safety, tolerability, pharmacokinetic and pharmacodynamic data for such product, (iii) exposes subjects to such product and (iv) is designed to provide the sponsor of the clinical trial with sufficient data about such product to initiate a Phase 2 Trial; or (b) meets the definition in 21 C.F.R. §312.21(a) or any of its foreign equivalents.
1.96“Phase 2 Trial” means a clinical trial of a pharmaceutical product (a) with the endpoint of evaluating its effectiveness for a particular indication or indications, its short term tolerance and safety, as well as its pharmacokinetic and pharmacodynamic data in patients with the indications under study and is not intended to be pivotal to support Marketing Approval for such product; or (b) that meets the definition in 21 C.F.R. §312.21(b) or any of its foreign equivalents.
1.97“Phase 2/3 Trial” means a Phase 2 Trial involving a sufficient number of subjects that, prior to commencement of the trial or at any other defined point in the trial, satisfies both of the following ((a) and (b)):
(a) such trial is designed to (i) establish that the applicable pharmaceutical product is safe and efficacious for its intended use, and (ii) define and determine warnings, precautions, and adverse reactions that are associated with the pharmaceutical product in the dosage range to be prescribed, which trial is intended to support Marketing Approval for such Product or a similar clinical study prescribed by the FDA; and
(b) such trial is or becomes a registration trial sufficient for filing an application for a Marketing Approval the applicable pharmaceutical product in the U.S., as evidenced by (i) an agreement with or statement from the FDA on a Special Protocol Assessment or equivalent, or (ii) other guidance or minutes issued by the FDA, for such registration trial.
1.98“Phase 3 Trial” means a clinical trial of a pharmaceutical product (a) on a sufficient number of patients, which trial (i) is designed to establish that such product is safe and efficacious for its intended use, (ii) is designed to define warnings, precautions and adverse reactions that are associated with such product in the dosage range to be prescribed, and (iii) is pivotal to support Marketing Approval for such product; or (b) that meets the definition in 21 C.F.R. §312.21(c) or any of its foreign equivalents.
1.99“Phase 4 Trial” means a clinical trial of a pharmaceutical product, possibly including pharmacokinetic studies, which trial (a) is not required to be completed prior to obtaining Marketing Approval for an indication; and (b) either (i) is required by the applicable Regulatory Authority as mandatory to be conducted on or after the Marketing Approval of an indication, or (ii) is conducted voluntarily to enhance scientific knowledge of such product (e.g., providing additional drug profile, safety data or marketing support information, or supporting expansion of product labeling).
|
Confidential |
15 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.100“Product” means any pharmaceutical product, including all forms, presentations, strengths, doses and formulations (including any method of delivery), containing a Compound alone or in combination with other APIs. For the sake of clarity, all forms, presentations, doses and formulations of a pharmaceutical product containing a Compound shall be considered the same Product for purposes of this Agreement, so long as each form, presentation, dose and formulation contains the same Compound and same other APIs (and no other Compounds or other APIs).
1.101“Regulatory Approval” means any and all approvals, licenses (including import licenses), registrations or authorizations from any Regulatory Authority that are required under Applicable Law or reasonably necessary to Exploit a pharmaceutical product in any country or jurisdiction for one or more uses, and all amendments and supplements thereto.
1.102“Regulatory Authority” means any applicable Governmental Authority with authority over the Exploitation of a pharmaceutical product in a country or jurisdiction, including (a) in the U.S., the FDA; and (b) in the European Union, the EMA and the European Commission.
1.103“Regulatory Documentation” means, with respect to the Compounds and Products, (a) all applications for Regulatory Approval of any Compound or Product, including Drug Approval Applications; (b) all Regulatory Approvals for any Compound or Product, including INDs and Marketing Approvals; (c) all supporting documents created for, referenced in, submitted to or received from an applicable Regulatory Authority relating to any of the applications or Regulatory Approvals described in clauses (a) or (b), including DMF Equivalents, annual reports, advertising and promotion documents required to be submitted to Regulatory Authorities, adverse event files, complaint files and Manufacturing records; (d) all correspondence made to, made with, or received from any Regulatory Authority (including written correspondence and minutes from meetings, discussions or conferences) relating to any of the foregoing applications, Regulatory Approvals or documents described in clauses (a), (b) or (c), in each case ((a), (b), (c) and (d)), including all Know-How included therein.
1.104“Right of Reference” shall have the meaning set forth in 21 C.F.R. §314.3(b) or equivalents thereto under Applicable Law in countries or jurisdictions outside the U.S.
1.105“Royalty Term” means, on a Product-by-Product and country-by-country basis, the time period beginning with the First Commercial Sale of such Product in such country (or, if earlier with respect to a Prostate-Approved Single Agent Product, the first sale or other transfer of such Product in such country for a use described in clause (B) or (C) of the definition of “Prostate-Approved Single Agent Product” where such sale or transfer results in a Net Sale) and continuing until the later of: (a) the expiration of the last Valid Claim of a TESARO Patent that Covers such Product in such country; or (b) ten (10) years from the First Commercial Sale of such Product in such country.
|
Confidential |
16 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.106“Single Agent Product” means (a) any Product that contains a Compound as its sole API and (b) any Product that is deemed to be a Single Agent Product pursuant to Section 3.1(e).
1.107“Supplemental Application” means, with respect to any pharmaceutical product for which a Drug Approval Application has been approved by the applicable Regulatory Authority, an application to supplement or amend such Drug Approval Application to expand the approved label for such pharmaceutical product to include use of such pharmaceutical product for an additional indication.
1.108“Tax” or “Taxes” means any taxes, levies, imposts, duties, charges, assessments or fees of any nature (including any interest thereon).
1.109“Technology Transfer Costs” means internal and external costs incurred by TESARO for the transfer of copies of all tangible embodiments of Know-How necessary for Company’s establishment of Manufacturing capabilities for Compound API as described in the API Supply Agreement. With respect to internal costs, FTEs will be invoiced at an annual rate of $[***] per FTE (i.e., an hourly rate of $[***] per FTE, assuming [***] working days per year and an 8 hour workday). For the avoidance of doubt, Technology Transfer Costs exclude expenses for travel and time spent in general planning meetings. A quarterly budget for TESARO consulting services related to technology transfer will be established and agreed to by the Parties in accordance with the API Supply Agreement. All Technology Transfer Costs will be invoiced according to Section 8.13.
1.110“Territory” means, (i) with respect to Company and its Affiliates and (sub)licensees, the Company Territory, and (ii) with respect to TESARO and its Affiliates and (sub)licensees, the TESARO Territory. References to a “Party’s Territory” shall be interpreted accordingly.
1.111“TESARO Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31, for so long as this Agreement is in effect; provided, however, that the first TESARO Calendar Quarter for the first TESARO Calendar Year extends from the Effective Date to the end of the then-current TESARO Calendar Quarter and the last TESARO Calendar Quarter extends from the first day of such TESARO Calendar Quarter until the effective date of the termination or expiration of this Agreement.
1.112“TESARO Calendar Year” means each successive period of twelve (12) months commencing on January 1 and ending on December 31, for so long as this Agreement is in effect. The last TESARO Calendar Year of the Term begins on January 1 for the year during which termination or expiration of this Agreement will occur, and the last day of such TESARO Calendar Year will be the effective date of such termination or expiration.
1.113“TESARO Field” means (a) with respect to Japan, all uses, including the diagnosis, treatment or prevention of any disease in humans, and (b) with respect to any country or jurisdiction outside Japan, all uses outside of the Company Field.
1.114“TESARO Know-How” means all Know-How Controlled by TESARO during the Term that is necessary or useful to Exploit the Compounds or Products in the Company Field in the Company Territory.
|
Confidential |
17 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.115“TESARO License Agreements” means (a) the Merck License Agreement, and (b) the AstraZeneca License Agreements.
1.116“TESARO Net Sales” means with respect to any Product, the gross amounts invoiced and included as current period sales of such Product by TESARO or any of its Affiliates or (sub)licensees to a Third Party purchaser in an arm’s-length transaction, less the following customary deductions, to the extent they are actually taken, paid, accrued or allocated based on good faith estimates, all determined in accordance with GAAP and internal policies, consistently applied:
(a) trade and quantity discounts other than early payment cash discounts;
(b) returns, rebates, chargebacks and other allowances;
(c) retroactive price reductions that are actually allowed or granted;
(d) sales commissions paid to Third Party distributors and/or selling agents;
(e) deductions to gross invoice price of Product imposed by Regulatory Authorities or other governmental entities;
(f) the standard inventory cost of devices or delivery systems used for dispensing or administering Product; and
(g) a fixed amount equal to three percent (3%) of the amount invoiced to cover bad debt, early payment cash discounts, transportation and insurance and custom duties.
1.117“TESARO Patents” means all Patents Controlled by TESARO during the Term that are necessary or useful to Exploit the Compounds or Products in the Company Field in the Company Territory. TESARO Patents include, as of the Effective Date, those Patents set forth in Exhibit 1.117, the Merck Patents and AstraZeneca Patents.
1.118“TESARO Technology” means, collectively, the TESARO Patents and the TESARO Know-How.
1.119“TESARO Territory” means worldwide.
1.120“Third Party” means any Person other than (a) Company, (b) TESARO or (c) an Affiliate of either of Company or TESARO.
1.121“Third Party Royalties” means royalties required to be paid by TESARO or its Affiliates on Product sales to Third Parties.
1.122“Trademark” means any word, name, symbol, colour, designation, slogan, catch phrase or device or any combination thereof used or intended to be used in commerce and indicating the
|
Confidential |
18 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
source for a product or service, including any domain name, trademark, trade dress, brand mark, trade name, brand name, logo or business symbol, any registrations of any of the foregoing or any pending applications therefor.
1.123“U.S.” means the United States of America, including its territories and possessions.
1.124“Valid Claim” means either: (a) a claim of an issued and unexpired Patent, to the extent such claim has not been revoked, held invalid or unenforceable by a patent office, court or other Governmental Authority of competent jurisdiction in a final order, from which no further appeal can be taken, and which claim has not been disclaimed, denied or admitted to be invalid or unenforceable through reissue, re-examination or disclaimer or otherwise; or (b) a claim of a patent application that has not been pending for more than seven (7) years from the date of its first priority patent application filing anywhere in the world and that has not been revoked, cancelled, withdrawn, held invalid or abandoned.
Additional Definitions. Each of the following definitions is set forth in the Section of this Agreement indicated below:
|
Section |
|
|
[***] |
[***] |
|
Agreement |
Preamble |
|
Alliance Payments |
8.3(a) |
|
Alliance Payments Report |
Exhibit 8.3 |
|
Annual Alliance Payment Forecast |
8.3(e) |
|
Annual Royalty Payment Forecast |
8.4(c) |
|
API |
1.19 |
|
API Supply Agreement |
7.3(b) |
|
[***] |
[***] |
|
AstraZeneca-ICR License Agreement |
1.8 |
|
AstraZeneca-Sheffield License Agreement |
1.8 |
|
Bankruptcy Laws |
12.7(b) |
|
Blocking Intellectual Property |
8.9(b) |
|
Breaching Party |
12.4(a) |
|
Clinical Supply Agreement |
7.1(a)(ii) |
|
Company |
Preamble |
|
Company Combination Product Net Sales |
8.4(a)(iii) |
|
Company Development Plan |
4.2(c) |
|
Company Field License Agreement |
8.9(b)(i) |
|
Company Indemnitee |
14.2 |
|
Company Infringement Proceeding |
14.1(h) |
|
Company Product Liability Proceeding |
14.1(g) |
|
Company Prostate-Approved Single Agent Product Net Sales |
8.4(a)(ii) |
|
Confidential |
19 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Section |
|
|
Combined Worldwide Prostate-Approved Single Agent Product Net Sales |
8.4(a)(i) |
|
Company Combination Product Net Sales |
8.4(a)(ii) |
|
Company Prostate-Approved Single Agent Product Net Sales |
8.4(a)(iii) |
|
Company Registration Plan |
5.2(c) |
|
Company Regulatory Documentation |
5.6(b) |
|
Company Trademarks |
6.5(a) |
|
[***] |
[***] |
|
[***] |
[***] |
|
Compound API |
7.3(a) |
|
CPR Rules |
13.3 |
|
Cross-Indication License Agreement |
8.9(b)(iii) |
|
Cure Period |
12.4(a) |
|
Deadlocked Committee Matter |
2.1(d) |
|
Defending Party |
14.3(d) |
|
Development Milestone |
8.2(a) |
|
Development Milestone Payment |
8.2(a) |
|
Development Plans |
4.2(c) |
|
Disclosing Party |
11.1(a) |
|
Dispute(s) |
13.1 |
|
[***] |
[***] |
|
Equitable Exceptions |
10.1(b) |
|
Escalation Procedures |
2.1(d)(i) |
|
Effective Date |
Preamble |
|
FATCA |
8.10(a) |
|
First Prostate Use |
Exhibit 8.3 |
|
Good Faith Dispute |
9.4 |
|
ICH |
1.57 |
|
Incremental Tax Cost |
8.10(d) |
|
Indemnification Notice |
14.3(a) |
|
Indemnifying Party |
14.3(a) |
|
Indemnitee |
14.3(a) |
|
Infringement Claim |
9.6(a) |
|
Infringement Recovery |
9.5(c) |
|
Insolvency Event |
12.7(a) |
|
Insolvent Party |
12.7(b) |
|
Joint Committee |
2.6(a) |
|
Joint Inventions |
9.1 |
|
Confidential |
20 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Section |
|
|
Joint Patents |
9.3(e) |
|
JCC |
2.3(a) |
|
JDC |
2.2(a) |
|
JFC |
2.5(a) |
|
JMC |
2.4 |
|
JSC |
2.1(a) |
|
Licensed Patents |
10.2(b) |
|
Losses |
14.1 |
|
Merck Milestone |
8.2(d) |
|
Merck Milestone Payment |
8.2(d) |
|
[***] |
[***] |
|
Milestone Events |
8.2 |
|
Milestone Payments |
8.2 |
|
Monotherapy Registration & Distribution Notice |
5.3(b)(iii) |
|
[***] |
[***] |
|
Non-Insolvent Party |
12.7(b) |
|
Non-Paying Party |
8.10(d) |
|
Notice Indication |
4.3(b) |
|
Objection |
4.2(d)(iii) |
|
Officials |
10.5(b) |
|
Other Included Countries |
Exhibit 8.3 |
|
Party/Parties |
Preamble |
|
Pass-through AstraZeneca-ICR Royalty |
8.4(b)(i)(2) |
|
Pass-through AstraZeneca-Sheffield Royalty |
8.4(b)(i)(2) |
|
Pass-through Merck Royalty |
8.4(b)(i)(1) |
|
Patent Extension(s) |
9.4 |
|
Paying Party |
8.10(d) |
|
Payment |
10.5(b) |
|
[***] |
[***] |
|
Potential Recall Notice |
5.9(a) |
|
Pricing Terms |
6.2(d)(i) |
|
Prior CDA |
1.32 |
|
Proceeding |
14.1 |
|
[***] |
[***] |
|
[***] |
[***] |
|
[***] |
[***] |
|
Promotional Materials |
6.8 |
|
8.4(a)(iv) |
|
|
8.4(a)(v) |
|
Confidential |
21 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Section |
|
|
Prostate Sales Allocation Factor |
Exhibit 8.3 |
|
Protocol |
13.3(b)(iii) |
|
PVA |
5.7(c) |
|
Receiving Party |
11.1(a) |
|
Redactable Information |
4.2(f)(ii) |
|
Related Indemnitees |
14.3(a) |
|
Registration Plans |
5.2(c) |
|
Regulatory Milestone |
8.2(b) |
|
Regulatory Milestone Payment |
8.2(b) |
|
Residual Allocation Methodology |
Exhibit 8.3 |
|
Residual Prostate Sales Allocation Factor |
Exhibit 8.3 |
|
Reverted Single Agent Product |
12.9(a)(ii) |
|
Reviewing Party |
4.2(d)(i) |
|
[***] |
[***] |
|
[***] |
[***] |
|
Royalty Payment Report |
8.7 |
|
Royalty Tier |
8.4(a)(i) |
|
Sales Milestone |
8.2(c) |
|
Sales Milestone Payment |
8.2(c) |
|
[***] |
[***] |
|
Shared Third Party Obligations |
8.9(e) |
|
Single Agent Agreement |
12.9(a)(vi) |
|
Single Agent Regulatory Approval |
12.9(a)(iii) |
|
Single Agent Technology |
12.9(a)(ii) |
|
Sole Inventions |
9.1 |
|
[***] |
[***] |
|
[***] |
[***] |
|
Term |
12.1 |
|
Terminating Party |
12.4(a) |
|
Termination Effective Date |
12.9 |
|
Termination Notice Date |
12.9 |
|
Territory-Wide Company Combination Product Net Sales |
8.4(a)(vi) |
|
8.4(a) |
|
|
TESARO |
Preamble |
|
TESARO Development Plan |
4.2(c) |
|
TESARO Field License Agreement |
8.9(b)(ii) |
|
TESARO Incremental Royalty |
8.4(b)(ii) |
|
TESARO Indemnitee |
14.1 |
|
TESARO Infringement Proceeding |
14.2(h) |
|
Confidential |
22 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Section |
|
|
TESARO Prostate-Approved Single Agent Product Net Sales |
8.4(a)(viii) |
|
TESARO Registration Plan |
5.2(c) |
|
TESARO Regulatory Documentation |
5.6(a) |
|
TESARO Trademarks |
6.5(b) |
|
Third Party Development Report |
4.2(h) |
|
Third Party Patent Challenge |
9.7(b) |
|
[***] |
[***] |
|
[***] |
[***] |
GOVERNANCE
(a) Formation and Purpose. The Parties agree to establish and convene a joint steering committee (the “JSC”) within ninety (90) days after the Effective Date. The JSC shall consist of representatives from each Party as further described in Section 2.6(a) and operate in accordance with this Section 2.1 and Section 2.6. The purpose of the JSC shall be to serve as a forum for discussing, monitoring and sharing information with respect to the Parties’ Exploitation of the Compounds and Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory, and to resolve any Deadlocked Committee Matter pursuant to Section 2.1(d). Notwithstanding the foregoing or anything else contained herein to the contrary, Exploitation of the Compounds or Products by or on behalf of TESARO, in each case, (i) for Combination Products, Co-Packaged Products or Combination Use in the TESARO Field in the TESARO Territory or (ii) in Japan, shall not be subject to the oversight or authority of the JSC or any other Joint Committee; provided, that the foregoing shall not limit the provisions of ARTICLE 7 with respect to the allocation of Single Agent Products or Compound API in the event of an anticipated or actual shortage or any provisions of this Agreement expressly requiring TESARO to provide to a Joint Committee or Company any reports, plans or other information regarding Exploitation of Compounds or Products by or on behalf of TESARO, except to the extent Combination Products, Co-Packaged Products or Combination Use in the TESARO Field are expressly excluded from such provisions.
(b) Responsibilities of the JSC. The JSC shall:
(i)discuss and monitor Development, regulatory, Manufacturing, medical affairs and Commercialization activities with respect to the Compounds
|
Confidential |
23 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
and Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory consistent with this Agreement;
(ii)review summaries of results and progress of the Development under the TESARO Development Plan and Company Development Plan presented or submitted by the Parties in accordance with Section 4.2(h), including periodic reviews of data, results and analyses from the Development activities conducted under the TESARO Development Plan or Company Development Plan (including clinical, regulatory and safety data, results, reports and analyses);
(iii)establish working groups or committees as necessary or appropriate and agreed to by the JSC, and delegate tasks within its authority to further discuss and share information with respect to the Development and Commercialization of the Compounds and Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory;
(iv)resolve Deadlocked Committee Matters to the extent authorized by Section 2.1(d); and
(v)perform other obligations specifically delegated to it and permitted under this Agreement.
(c)JSC Decisions and Actions. The JSC shall not have any formal decision-making authority, except with respect to the resolution of Deadlocked Committee Matters as described in Section 2.1(d). Actions to be taken and decisions to be made by the JSC with respect to such matters shall be taken or made by unanimous agreement, with each Party’s JSC representatives collectively having one (1) vote.
(d)Escalation Procedures for Deadlocked Committee Matters. Either Party may refer a matter that is expressly designated in Section 2.2(c), 2.3(c), 2.5(c), 7.4(c) or 7.7 as a “Deadlocked Committee Matter” to the JSC for attempted resolution. For clarity, no matter, dispute or failure of a Joint Committee to reach unanimous agreement with respect to any matter, other than those expressly designated as Deadlocked Committee Matters in the foregoing Sections shall be subject to the Escalation Procedures or resolution as a Deadlocked Committee Matter hereunder. If the JSC does not reach unanimous agreement on any Deadlocked Committee Matter within thirty (30) Business Days from the date that the matter is first presented to the JSC, such matter shall be resolved as follows, which shall be the exclusive mechanism for resolving Deadlocked Committee Matters:
(i)Either Party may, by written notice to the other, have such Deadlocked Committee Matter referred to the Executive Officers for resolution. The Executive Officers shall meet promptly to discuss such Deadlocked
|
Confidential |
24 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Committee Matter and seek in good faith to determine a resolution. If the Executive Officers do not agree upon a resolution in a timely manner, which shall in no case be more than thirty (30) calendar days after the Deadlocked Committee Matter was referred to them, then such Deadlocked Committee Matter shall be resolved in accordance with Section 2.1(d)(ii) and the relevant provision of this Agreement pursuant to which such Deadlocked Committee Matter was referred to the JSC. The procedures set forth in this Section 2.1(d)(i) are referred to as the “Escalation Procedures.”
(ii)If another provision of this Agreement expressly designates a matter as a Deadlocked Committee Matter and provides: (A) for a resolution methodology or a specific final resolution with respect to such Deadlocked Committee Matter in the event mutual agreement is not reached other than final decision-making authority of a Party (e.g., resolution by an independent Third Party accountant, or the status quo prevails), then such resolution methodology or specific final resolution shall be binding on the Parties; or (B) that one of the Parties has final decision-making authority with respect to such Deadlocked Committee Matter in the event mutual agreement is not reached, then the decision of the Party with final decision-making authority on such matter shall be final and binding on the Parties subject to the limitations set forth in Section 2.1(d)(iii).
(iii)Notwithstanding anything to the contrary in this Agreement, neither Party may exercise its final decision-making authority with respect to a Deadlocked Committee Matter to: (1) amend this Agreement or a Party’s rights or obligations under this Agreement; (2) determine that either Party has fulfilled or breached a Party’s obligations under this Agreement; (3) make any decision that expressly requires TESARO’s or Company’s approval or agreement or the approval or agreement of both Parties under this Agreement; (4) resolve any dispute regarding whether a Milestone Event has been achieved, the amount of any royalties or other payments owed by one Party to the other Party, or the Net Sales of a Product allocable to an Indication; (5) resolve any dispute regarding whether a matter is a Deadlocked Committee Matter or subject to TESARO or Company final decision-making under this Agreement, or whether a matter is a Dispute hereunder; (6) cause either Party to violate Applicable Law or regulatory requirements; or (7) cause the other Party to violate or not be in compliance with its own health care compliance policies and procedures of general application, consistently applied by such Party and its Affiliates, or any corporate integrity agreement to which such Party is subject.
|
Confidential |
25 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(iv)Except as expressly provided in this Agreement with respect to the Deadlocked Committee Matters, no matter, decision, or action of the Parties shall require the agreement, approval or consent of a Joint Committee.
(v)If another provision of this Agreement expressly provides for the Parties to engage a Third Party accounting firm to resolve a Deadlocked Committee Matter, the following terms shall apply:
(1) The Parties shall mutually select and engage an independent Third Party accounting firm that has no auditing or other financial relationship with either Party or any of its Affiliates.
(2) Such accounting firm shall, as soon as reasonably practicable after such firm is engaged, deliver a report to each Party with its analysis and determination of such Deadlocked Committee Matter.
(3) The costs of such firm’s services shall be shared equally by the Parties (for clarity, subject to Section 8.11 in the event of any audit conducted thereunder).
(e)General Performance. If either Party reasonably believes that the other Party intends to take any action relating to any Compound or Product that would reasonably be expected to materially and adversely affect such Party’s Development or Commercialization of the Compounds or Products in its Field in its Territory, then such Party may so notify the other Party; provided, however, that this Section 2.1(e) shall not apply with respect to any Deadlocked Committee Matter or any matter that a Joint Committee is expressly authorized to determine, approve or resolve under this Agreement. If a Party receives a notice pursuant to this Section 2.1(e) and it has not yet taken the action identified therein, such Party shall use commercially reasonable efforts to refrain from taking such action until the Parties have attempted to resolve the notifying Party’s concerns in accordance with this Section 2.1(e). The Parties shall meet promptly thereafter (and, in any event, within ten (10) Business Days thereafter) to discuss the concerns raised in such notice and shall use good faith efforts to agree upon a mutually acceptable resolution to such concerns within thirty (30) calendar days after receipt of the notice. If the Parties do not agree upon a resolution within such thirty (30) day period, either Party may, by written notice to the other, have the matter referred to the Executive Officers for resolution. The Executive Officers shall meet promptly to discuss such matter and to seek in good faith to determine a mutually acceptable resolution. If the Executive Officers do not agree upon a resolution in a timely manner, which shall in no case be more than ten (10) calendar days after the matter was referred to them, then the final decision regarding the taking, or refraining from taking, the applicable action will be made by the Party proposing
|
Confidential |
26 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
to take such action, or, if either Party has the sole right under this Agreement to take such action, the Party with the sole right to take the action. For the avoidance of doubt, if a Party has the right to take an action under this Agreement, it shall not be a breach of this Agreement if such Party takes such action prior to the conclusion of the process contemplated by this Section 2.1(e); provided, that (i) it is reasonably necessary for such Party to take such action, and (ii) such Party sought in good faith to reach mutual agreement on the matter.
2.2Joint Development Committee.
(a)Formation and Purpose. The Parties agree to establish and convene a joint development committee (the “JDC”) within ninety (90) days after the Effective Date. The JDC shall consist of representatives from each Party as further described in Section 2.6(a) and operate in accordance with this Section 2.2 and Section 2.6. The purpose of the JDC shall be to serve as a forum for discussing, monitoring and sharing information with respect to the Parties’ Development of, and regulatory activities related to, the Compounds and Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory, and to determine, approve or resolve matters with respect to such Development and regulatory activities that the JDC is expressly authorized under Section 2.2(c) to determine, approve or resolve under this Agreement.
(b)Responsibilities of the JDC. The JDC shall:
(i)discuss and monitor Development and regulatory activities with respect to the Compounds and Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory;
(ii)review and discuss the Development Plans submitted by the Parties in accordance with Section 4.2(c), and discuss any concerns raised by either Party with respect to such Development Plans in accordance with Section 4.2(c);
(iii)discuss any Objection with respect to a Clinical Trial that is referred to the JDC pursuant to Section 4.2(d);
(iv)discuss the logistics of sharing Know-How pursuant to Section 4.2(f) and sharing safety data and Regulatory Documentation pursuant to Section 5.5;
(v)to the extent requested by a Party pursuant to Section 4.2(f), discuss issues related to the Development of the Compounds or Products;
(vi)review summaries of results and progress of the Development under the TESARO Development Plan and Company Development Plan, including
|
Confidential |
27 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
periodic reviews of data, results and analyses from the Development activities conducted pursuant under the TESARO Development Plan or Company Development Plan (including clinical, regulatory and safety data, results, reports and analyses) provided by the Parties pursuant to Section 4.2(h);
(vii)review and discuss the Registration Plans submitted by the Parties in accordance with Section 5.2(c), and discuss any concerns raised by either Party with respect to the Registration Plans in accordance with Section 5.2(c);
(viii)review and discuss the Parties’ medical affairs plans with respect to the Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory (e.g. field-based medical education activities and grant-based medical education programs) in accordance with Section 5.8;
(ix)discuss any Phase 4 Trials of the Products;
(x)consult with the Joint Manufacturing Committee with respect to clinical supply manufacturing in accordance with Section 7.4(b);
(xi)discuss a publication strategy with respect to the Compounds and Products and the review of such publications, in accordance with Section 11.9;
(xii)establish working groups or committees as necessary or appropriate and agreed to by the JDC, and delegate tasks within its authority to further discuss and share information with respect to the Development of the Compounds and Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory, such as a quality subcommittee and separate clinical quality subcommittees for purposes of overseeing the conduct of Clinical Trials in accordance with GLP, GCP and the PVA; and
(xiii)perform other obligations specifically delegated to it and permitted under Section 2.2(c) or another provision of this Agreement.
(c)JDC Decisions and Actions. The JDC shall not have any formal decision-making authority, except with respect to the following matters:
(i)The JDC shall determine whether any Drug Approval Application for a Monotherapy Use in the Company Field submitted by Company to the JDC in accordance with Section 5.3(b)(iv) satisfies the appropriate efficacy and safety criteria outlined in the applicable protocol and notify Company of the results of such review. If the JDC does not reach unanimous agreement
|
Confidential |
28 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
on such matter within thirty (30) Business Days from the date that the matter is first presented to the JDC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, TESARO shall have final decision-making authority with respect to such Deadlocked Committee Matter.
(ii)The JDC shall determine which portions of a Drug Approval Application for Combination Use or a Combination Product, in each case, in the Company Field in the Company Territory are subject to review and comment by the Parties pursuant to Sections 5.3(c)(iii) and 5.3(d)(iii). If the JDC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JDC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, the Party submitting such Drug Approval Application shall have final decision-making authority with respect to such Deadlocked Committee Matter. For clarity, this Section 2.2(c)(ii) does not apply to Combination Use or Combination Products in the TESARO Field in the TESARO Territory.
Actions to be taken and decisions to be made by the JDC with respect to such matters shall be taken or made by unanimous agreement, with each Party’s JDC representatives collectively having one (1) vote. If the JDC does not reach unanimous agreement on an action or decision with respect to any Deadlocked Committee Matter within thirty (30) Business Days from the date that the matter is first presented to the JDC, such Deadlocked Committee Matter shall be referred to the JSC or otherwise subject to resolution in accordance with Section 2.1(d).
2.3Joint Commercialization Committee.
(a)Formation and Purpose. The Parties agree to establish and convene a joint commercialization committee (the “JCC”) within ninety (90) days after the Effective Date. The JCC shall consist of representatives from each Party as further described in Section 2.6(a) and operate in accordance with this Section 2.3 and Section 2.6. The purpose of the JCC shall be to serve as a forum for discussing, monitoring and sharing information with respect to the Parties’ Commercialization of the Compounds and Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory, to seek alignment between the Parties with respect to certain aspects of such Commercialization and to determine, approve or resolve matters with respect to
|
Confidential |
29 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
such Commercialization that the JCC is expressly authorized under Section 2.3(c) to determine, approve or resolve under this Agreement.
(b)Responsibilities of the JCC. The JCC shall:
(i)discuss and monitor the Commercialization of the Products in the Company Field in the Company Territory and in the TESARO Field in the TESARO Territory, including promotional activities with respect thereto, to the extent permissible under Applicable Law;
(ii)discuss and seek to reach alignment with respect to key aspects of the Distribution of Single Agent Products in accordance with Section 6.2(c)(i);
(iii)review and discuss Pricing Terms with respect to the Products to the extent provided under Section 6.2(d);
(iv)review and discuss the commercialization plans submitted by the Parties, and seek to reach alignment on key Commercialization issues relating to the Products, in accordance with Section 6.2(e);
(v)discuss and seek to reach alignment with respect to recommendations of the JFC on certain matters as described in Exhibit 8.3, or in the event the JFC fails to reach unanimous agreement with respect to any such matters; and
(vi)perform other obligations specifically delegated to it and permitted under Section 2.3(c) or another provision of this Agreement.
(c)JCC Decisions and Actions. The JCC shall not have any formal decision-making authority, except with respect to the following matters:
(i)The JCC shall determine procedures for TESARO’s regulatory compliance review and approval of Company’s Promotional Materials in accordance with Section 6.8. If the JCC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JCC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, TESARO shall have final decision-making authority with respect to such Deadlocked Committee Matter.
(ii)The JCC shall approve a Major Market Net Sales Allocation Methodology and Residual Allocation Methodologies based on recommendations from
|
Confidential |
30 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
the JFC in accordance with Exhibit 8.3. If the JCC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JCC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, the Parties shall mutually select and engage an independent Third Party accounting firm to resolve such Deadlocked Committee Matter pursuant to Section 2.1(d)(v).
(iii)The JCC shall determine which countries outside the Major Markets will be included as Other Included Countries in accordance with Exhibit 8.3. If the JCC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JCC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, the proposed change to the list of Other Included Countries will not be made.
(iv)The JCC shall reassess the Major Market Net Sales Allocation Methodology and its data sources on an annual basis, and approve required changes to such methodology, in accordance with Exhibit 8.3. If the JCC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JCC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, [***].
(v)The JCC shall reassess the use of the Major Market Net Sales Allocation Methodology in the Other Included Countries on an annual basis, and determine whether additional countries should be included as Other Included Countries, in accordance with Exhibit 8.3. If the JCC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JCC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, no change will be made to such use or list of Other Included Countries for such year.
(vi)The JCC shall determine the Distribution costs and prostate commercialization and other related costs incurred by TESARO to be
|
Confidential |
31 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
deducted from TESARO Prostate-Approved Single Agent Product Net Sales for purposes of calculating Alliance Payments pursuant to Exhibit 8.3. If the JCC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JCC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, the Parties shall mutually select and engage an independent Third Party accounting firm to resolve such Deadlocked Committee Matter pursuant to Section 2.1(d)(v).
Actions to be taken and decisions to be made by the JCC with respect to such matters shall be taken or made by unanimous agreement, with each Party’s JCC representatives collectively having one (1) vote. If the JCC does not reach unanimous agreement on a Deadlocked Committee Matter within thirty (30) Business Days from the date that the matter is first presented to the JCC, such Deadlocked Committee Matter shall be referred to the JSC or otherwise subject to resolution in accordance with Section 2.1(d).
2.4Joint Manufacturing Committee. The Parties shall establish a joint manufacturing and supply chain committee (the “JMC”) in accordance with Section 7.4.
(a)Formation and Purpose. The Parties agree to establish and convene a joint finance committee (the “JFC”) within ninety (90) days after the Effective Date. The JFC shall consist of individuals from each Party with reasonable expertise in the areas of accounting, cost allocation, budgeting and financial reporting as further described in Section 2.6(a) and operate in accordance with this Section 2.5 and Section 2.6. The JFC shall be a forum for discussing, monitoring, sharing information and seeking to reach alignment regarding the conduct of the accounting, reporting, reconciliation and other related finance and accounting activities set forth in this Agreement, and to determine, approve or resolve matters with respect to such finance and accounting activities that the JFC is expressly authorized under Section 2.5(c) to determine, approve or resolve under this Agreement.
(b)Responsibilities of the JFC. The JFC shall:
(i)discuss and seek to make unanimous recommendations to the JCC on certain matters as described in Exhibit 8.3;
(ii)if the Major Market Net Sales Allocation Methodology, Residual Allocation Methodology, list of Other Included Countries or any other decision described in Exhibit 8.3 is not resolved prior to launch of a
|
Confidential |
32 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Prostate-Approved Single Agent Product in a particular country, develop and seek to agree upon an interim methodology to allow each Party to appropriately record and report its financial position and contributions as necessary at quarter or year-end with respect to sales of such Prostate-Approved Single Agent Product in such country in accordance with Exhibit8.3;
(iii)upon the request of either Party, review and discuss the allocation of rebates payable to federal, state/provincial, local or other governments, or their agencies, with respect to sales of a Prostate-Approved Single Agent Product in a country in accordance with Section 3.2 of Exhibit 8.3; and
(iv)perform other obligations specifically delegated to it and permitted under Section 2.5(c) or another provision of this Agreement.
(c)JFC Decisions and Actions. The JFC shall not have any formal decision-making authority, except with respect to the following matters:
(i)The JFC shall develop and approve procedures for quarterly reporting of actual results, and review and discussion of potential discrepancies, quarterly reconciliation, reasonable forecasting, and other finance and accounting matters in accordance with Section 8.3(b). If the JFC does not reach unanimous agreement on any such matter within thirty (30) Business Days from the date that the matter is first presented to the JFC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, each Party may, in good faith, select its own reasonable procedures for such reporting not otherwise inconsistent with this Agreement or applicable Third Party license agreements.
(ii)The JFC shall determine the timing for delivery of the Annual Alliance Payment Forecast in accordance with Section 8.3(e). If the JFC does not reach unanimous agreement on such determination within thirty (30) Business Days from the date that the matter is first presented to the JFC, such forecast shall be delivered by November 1 of each year.
(iii)The JFC shall determine whether any additional information should be included in the Annual Alliance Payment Forecast in accordance with Section 8.3(e). If the JFC does not reach unanimous agreement on any such matter within thirty (30) Business Days from the date that the matter is first presented to the JFC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, TESARO shall not be required to provide such additional information.
(iv)The JFC shall develop and approve a process for the Parties to agree upon the estimated Alliance Payments for a TESARO Calendar Quarter before the close of such TESARO Calendar Quarter in accordance with Section 8.3(e). If the JFC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JFC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not
|
Confidential |
33 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
resolved pursuant to the Escalation Procedures, TESARO shall have final decision-making authority with respect to such Deadlocked Committee Matter.
(v)The JFC shall determine the timing for delivery of the Annual Royalty Payment Forecast in accordance with Section 8.4(c). If the JFC does not reach unanimous agreement on such determination within thirty (30) Business Days from the date that the matter is first presented to the JFC, such forecast shall be delivered by November 1 of the year prior to the year covered by such forecast.
(vi)The JFC shall determine whether any additional information should be included in the Annual Royalty Payment Forecast in accordance with Section 8.4(c). If the JFC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JFC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, neither Party shall be required to provide any such additional information; provided, that the foregoing shall not limit the Parties express obligations with respect to the Annual Royalty Payment Forecast under Section 8.4(c).
(vii)The JFC shall review and approve any proposed reallocations and adjustments of Net Sales of Prostate-Approved Single Agent Products in accordance with Section 3.3 of Exhibit 8.3. If the JCC does not reach unanimous agreement on such matter within thirty (30) Business Days from the date that the matter is first presented to the JCC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, the Parties shall mutually select and engage an independent Third Party accounting firm to resolve such Deadlocked Committee Matter pursuant to Section 2.1(d)(v).
|
Confidential |
34 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Actions to be taken and decisions to be made by the JFC shall be taken or made by unanimous agreement, with each Party’s JFC representatives collectively having one (1) vote. If the JFC does not reach unanimous agreement on an action or decision with respect to any such matter to be recommended to the JCC within thirty (30) Business Days from the date that the matter is first presented to the JFC or within the time period otherwise expressly provided in this Agreement, such action or decision shall be referred to the JCC for its consideration. If the JFC does not reach unanimous agreement on a Deadlocked Committee Matter within thirty (30) Business Days from the date that the matter is first presented to the JFC, such Deadlocked Committee Matter shall be referred to the JSC or otherwise subject to resolution in accordance with Section 2.1(d).
2.6Committee Membership and Operations.
(a)Membership. Each of the JSC, JDC, JCC, JMC and JFC (each, a “Joint Committee”) shall consist of up to six (6) representatives, one-half of which shall be appointed by Company and one-half of which shall be appointed by TESARO. Each Joint Committee may elect to vary the number of representatives from time to time, provided that each Joint Committee shall maintain an equal number of representatives from each Party. Each representative shall have the appropriate level of experience in the subject area of the applicable Joint Committee, and at least one (1) representative shall have sufficient seniority within the applicable Party’s organization to have the necessary decision-making authority in order for such Joint Committee to fulfill its responsibilities. Either Party may designate substitutes for its Joint Committee representatives if one (1) or more of such Party’s designated representatives is unable to be present at a meeting. A Party may replace any of its Joint Committee representatives by written notice to the other Party specifying the prior representative(s) and their replacement(s). Each Joint Committee representative shall be bound by confidentiality and non-use obligations at least as restrictive as those set forth in this Agreement to, and shall be an employee of, the Party that it represents or one of such Party’s Affiliates.
(b)Joint Committee Chairperson. Each Joint Committee will have a chairperson, to be designated by Company. The chairperson shall be responsible for calling and convening meetings of its Joint Committee (provided, the chairperson shall call meetings at the reasonable request of TESARO or any of its representatives in accordance with Section 2.6(c)), but shall have no special authority over the other members of its Joint Committee. The chairperson of each Joint Committee (or its designee) shall: (i) prepare and circulate an agenda reasonably in advance of each upcoming meeting of such Joint Committee (provided that any agenda shall be mutually agreed by the committee members); and (ii) prepare and issue minutes of each meeting of such Joint Committee within thirty (30) days thereafter. Each representative on each Joint Committee may, prior to any meeting, submit to the chairperson materials to be circulated to each member of the Joint Committee
|
Confidential |
35 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
together with the meeting agenda or otherwise. The minutes for each Joint Committee meeting shall not be finalized until each representative on such Joint Committee reviews and approves such minutes, provided that any minutes from a Joint Committee meeting shall be deemed approved unless a representative on such Joint Committee objects to the accuracy of such minutes within fifteen (15) days after circulation by the chairperson of such Joint Committee.
(i)Timing and Frequency. Promptly following its formation, each Joint Committee will hold an in-person meeting to establish its operating procedures consistent with this Agreement. After its initial meeting, the JSC shall meet at least twice per year during the Term and each other Joint Committee shall meet at least once every Calendar Quarter during the Term or as frequently as agreed by the Parties. Additional meetings of a Joint Committee may be held with the consent of each Party (such consent not to be unreasonably withheld, delayed or conditioned), as required under this Agreement or to discuss any Deadlocked Committee Matter referred to such Joint Committee in accordance with this Agreement. In the case of any matter or deadlock referred to a Joint Committee, such meeting shall be held within ten (10) Business Days following referral to such Joint Committee, or as soon as reasonably possible thereafter.
(ii)Meeting Procedures. Meetings of a Joint Committee shall be effective only if a majority of representatives on such Joint Committee of each Party are present or participating, including at least one (1) representative of each Party. Other than the initial meeting, each Joint Committee may meet either (x) in person at either Party’s facilities (alternating between facilities of each Party unless otherwise agreed) or at such locations as the Parties may otherwise agree, at least twice every Calendar Year; or (y) by audio or video teleconference. Each Party shall be responsible for all of its own expenses incurred in connection with its representatives’ participation in each Joint Committee meeting, including all travel and lodging. All other Third Party expenses incurred by a Joint Committee for one of its meeting, such as expenses associated with off-site meetings, shall be shared equally by the Parties.
(iii)Non-Member Participation. Additional non-members of a Joint Committee having relevant experience may be invited to participate in a meeting of such Joint Committee, provided that such participants shall have no voting rights or powers. Non-member participants of a Party or its Affiliates shall be allowed to attend a meeting of a Joint Committee only if: (x) the other Party’s representatives on such Joint Committee have consented to the attendance (such consent not to be unreasonably
|
Confidential |
36 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
withheld, delayed or conditioned); and (y) such non-member participant is subject to confidentiality and non-use obligations at least as restrictive as those set forth in this Agreement.
2.7Additional Subcommittees. Each Joint Committee may establish subcommittees to perform any responsibilities assigned to such Joint Committee under this Agreement; provided, that no subcommittee shall (a) have any decision-making authority, or (b) be delegated any matter that is or could become a Deadlocked Committee Matter. The purpose, scope and procedures of any such subcommittee shall be agreed in writing by the Joint Committee that establishes such subcommittee. Unless otherwise agreed by the Parties, any such subcommittee shall consist of an equal number of representatives appointed by Company and TESARO, or their respective representatives on the applicable Joint Committee, and the number of representatives on any such subcommittee may be varied from time to time by the applicable Joint Committee or by mutual agreement of the Parties, subject to the foregoing.
2.8Authority. The Parties agree that it shall be conclusively presumed that, unless otherwise explicitly stated, each voting member of each Joint Committee and subcommittee has the authority and approval of such member’s respective senior management in casting his or her vote. Each Joint Committee and subcommittee shall each have only the powers expressly assigned to it in this ARTICLE 2 or elsewhere in this Agreement, and shall not have any power to amend, modify or waive compliance with this Agreement. Notwithstanding anything else contained herein to the contrary, except as expressly provided in this Agreement with respect to the Deadlocked Committee Matters, no matter, decision, or action of the Parties shall require the agreement, approval or consent of a Joint Committee or any subcommittee thereof.
2.9Alliance Managers. Promptly following the Effective Date, each Party shall designate in writing an Alliance Manager to serve as the primary point of contact for the Parties regarding all collaboration activities contemplated under this Agreement. Each Alliance Manager shall facilitate communication and coordination of the Parties’ activities under this Agreement relating to the Compounds and the Products. The Alliance Managers shall be allowed to attend, as a non-voting observer, meetings of each Joint Committee and subcommittee of which the Alliance Manager is not a member.
2.10Compliance with Merger Control Laws. It is the intent of the Parties to at all times during the Term comply with applicable Merger Control Laws. If at any time during the Term, either Party in good faith reasonably determines upon the advice of counsel that the performance by either Party of its obligations under this Agreement or this Article 2, including with respect to the sharing of information, would violate applicable Merger Control Laws, then the Parties shall negotiate in good faith to agree upon mutually acceptable alternative arrangements that comply with applicable Merger Control Laws, including, if reasonably requested by a Party, arranging for information deliverable to a Party hereunder to be delivered to a mutually agreed Third Party.
|
Confidential |
37 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
ARTICLE 3
LICENSES
(a)License to Company. Subject to the terms and conditions of this Agreement, TESARO hereby grants to Company a royalty-bearing, non-transferable (except to the extent permitted under Section 15.4) license, with the right to grant sublicenses as provided in Section 3.1(c), under the TESARO Technology and TESARO Trademarks, which license shall be non-exclusive with respect to the Merck Know-How and exclusive (even as to TESARO, except to the extent necessary for TESARO to perform its obligations under this Agreement, including Sections 5.2(d), 6.2(c) and 6.2(d)) with respect to any TESARO Technology (other than the Merck Know-How) and the TESARO Trademarks, to Exploit the Compounds and Products in the Company Field in the Company Territory. The foregoing license grant by TESARO does not include any grant of rights under the TESARO Technology or TESARO Trademarks to Exploit any active pharmaceutical compound that is not a Compound, other than, to the extent applicable, any API(s) Controlled by TESARO (if any) that are included in a Product that is deemed to be a Single Agent Product pursuant to Section 3.1(e).
(b)Rights under Merck Patents [***]. Notwithstanding the definition of “Company Field” or anything to the contrary contained herein, Company acknowledges and agrees that (i) the license granted to TESARO under the Merck License Agreement does not include the right or license under the Merck Patents or the Merck Know-How to Exploit the Compounds or Products for the diagnosis of any indication in humans, and therefore the foregoing license grant by TESARO to Company hereunder does not include a sublicense under the Merck Patents or the Merck Know-How to Exploit the Compounds or Products for the diagnosis of any indication in humans[***].
(c)Sublicensing. Company shall have the right to grant sublicenses of the rights granted to Company under this Section 3.1 to its Affiliates and Third Parties through multiple tiers provided that:
(i)Company has provided at least five (5) Business Days’ prior written notice to TESARO of any sublicense; provided, that sublicenses granted solely in connection with Development (but not Commercialization), such as licenses to contract manufacturers (other than any contract manufacturer engaged by TESARO or its Affiliates), contract research organizations or clinical study sites, shall not require any notice to TESARO; and
(ii)each such sublicense shall (1) refer to and be subordinate to this Agreement, (2) be consistent in all material respects with the terms and conditions of this Agreement and the TESARO License Agreements, and
|
Confidential |
38 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(3) include Section 10.4 and the provisions of the Merck License Agreement set forth on Exhibit 10.4.
Company shall use reasonable efforts to procure the prompt performance by any sublicensee of the terms of each such sublicense agreement and ensure that any sublicensee will comply with the applicable terms and conditions of this Agreement. Company shall be liable for all acts and omissions of any sublicensee and shall indemnify TESARO against all Losses incurred or suffered by TESARO, or for which TESARO may become liable, arising out of any act or omission of any sublicensee, pursuant to Section 14.1. The grant of any sublicense will not relieve Company of its obligations under this Agreement, except to the extent such obligations are satisfactorily performed by such sublicensee. Notwithstanding Section 3.1(c)(i) above, Company shall provide to TESARO copies of each sublicense with a Third Party that grants such Third Party the right to directly Develop or Commercialize the Compounds or Products in the Company Field in any country or jurisdiction in the Company Territory within five (5) days after execution thereof; provided that Company shall have the right to reasonably redact commercially sensitive information from such copies. Information regarding the scope of the license grants, territory or term of each such sublicense and the identity of the Third Party shall not be considered commercially sensitive.
(d)Exclusivity. During the period commencing on the Effective Date and ending on the [***] anniversary of the Effective Date (the “Exclusivity Period”), neither TESARO nor any of its Affiliates shall, directly or indirectly: (i) [***], except to the extent necessary to perform TESARO’s obligations under this Agreement or the TESARO License Agreements; (ii) [***], except to the extent necessary to perform TESARO’s obligations under this Agreement; (iii) [***]; or (iv) collaborate with, license, enable or otherwise authorize or grant any rights to any Third Party to conduct any of the activities described in clause (i), (ii) or (iii) of this Section 3.1(d), or enter into any agreement, amendment to an existing agreement or option to do any of the same; or (v) [***]. Notwithstanding anything to the contrary herein, the restrictions in this Section 3.1(d) are subject to the terms of [***].
(e)Products containing Non-Cancer APIs. If either Party or its Affiliates desires to Develop or Commercialize, directly or indirectly, any Product that contains a Compound and at least one other API that is not intended to treat cancer (e.g., [***]) it shall notify the other Party in writing. If such Product includes another API intended to treat cancer (e.g., a JAA), such Product shall be deemed to be a “Combination Product” for purposes of this Agreement. If such Product does not include another API intended to treat cancer, such Product shall be deemed to be a “Single Agent Product” for purposes of this Agreement. Following receipt of such notice, the Parties shall negotiate in good faith and attempt to agree upon an
|
Confidential |
39 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
adjustment to the calculation of Net Sales of any such Product in the Company Field, in each case, that fairly reflects the value of the Compound included in such Product and the other API(s) included in such Product. If the Parties fail to reach Agreement on such an adjustment prior to the First Commercial Sale of any such Product anywhere in the Company Territory, then such Net Sales shall be calculated in accordance with Section 1.116 or Section 1.25, as applicable; provided, that such failure may be referred to the dispute resolution process under ARTICLE 13 as a Dispute hereunder, and the resolution of such Dispute shall include a retroactive reconciliation back to the date of such First Commercial Sale, as appropriate. For the avoidance of doubt, this Section 3.1(e) does not apply to any Combination Product Developed or Commercialized, directly or indirectly, by TESARO in the TESARO Field or to any Product Developed or Commercialized, directly or indirectly, by TESARO solely in Japan.
(f)Co-Packaged Products. Company shall not Exploit any Compound or Product as a component of any Co-Packaged Product unless and until the Parties amend this Agreement to set forth (i) terms and conditions applicable to pricing of Co-Packaged Products and (ii) any other terms and conditions applicable to Commercialization of Co-Packaged Products that the Parties agree should be different from the terms and conditions relating to Commercialization of other Products. [***] If Company desires to Exploit any Compound or Product as a component of Co-Packaged Products in the Company Field and the Company Territory, then the Parties will negotiate in good faith to execute such amendment as soon as reasonably practicable. For the avoidance of doubt, [***].
3.2License to TESARO.
(a)License to TESARO. Subject to the terms and conditions of this Agreement, Company hereby grants to TESARO a non-exclusive, fully-paid, royalty-free, non-transferable (except in accordance with Section[***] 15.4), license, with the right to grant sublicenses as provided in Section 3.2(b), under the Company Technology to Exploit the Compounds and Single Agent Products in the TESARO Field (but not including prostate cancer in humans) in the Company Territory. The foregoing license grant by Company does not include (x) any grant of rights under the Company Technology to Exploit any active pharmaceutical compound that is not a Compound other than, to the extent applicable, any API(s) Controlled by Company (if any) that are included in a Product that is deemed to be a Single Agent Product pursuant to Section 3.1(e), (y) any grant of rights under the Company Technology to Exploit any Compound or Product in Japan in the Company Field or (z) any grant of rights under the Company Technology to Exploit any Compound or Product in Japan in the TESARO Field. If requested by TESARO, Company shall grant to TESARO a non-exclusive, royalty-bearing, non-transferable (except in accordance with Section[***] 15.4), license, with the right to grant sublicenses as provided in Section 3.2(b), under the Company
|
Confidential |
40 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Technology to Exploit Products in Japan on commercially reasonable terms to be negotiated in good faith and mutually agreed upon by the Parties (such agreement not to be unreasonably withheld, conditioned or delayed). If the Parties do not reach agreement on such terms within ninety (90) days after TESARO’s request for such a license, the Parties shall select and agree upon a mutually acceptable independent Third Party expert who is neutral, disinterested and impartial, and has experience relevant to such licenses. Each Party may make written and oral submissions of its position to such expert. Such expert shall, no later than ten (10) Business Days after the last submission, select one of the Party’s positions as his or her final decision, and shall not have the authority to modify either Party’s position or render any substantive decision other than to select the position of either TESARO or Company. A basis of such selection would be fair compensation to Company for the value of Company Technology as applied to any or all Products in Japan. The Parties agree that the decision of such expert shall be binding on each of them.
(b)Sublicensing. TESARO shall have the right to grant sublicenses of the rights granted by Company under Section 3.2(a) to its Affiliates and Third Parties through multiple tiers. Each such sublicense shall refer to and be subordinate to this Agreement, and shall be consistent in all material respects with the terms and conditions of this Agreement. TESARO shall remain responsible for the performance of this Agreement and the performance of its sublicensees hereunder. TESARO shall provide to Company copies of each sublicense with a Third Party that grants such Third Party the right to directly Develop or Commercialize a Compound or Single Agent Product in the TESARO Field in any country or jurisdiction in the TESARO Territory, provided that TESARO shall have the right to redact commercially sensitive information from such copies. Information regarding the scope of the license grants, territory or term of each such sublicense and the identity of the Third Party shall not be considered commercially sensitive.
3.4Retained Rights. Except as exclusively licensed to Company under this Agreement and otherwise subject to Section 3.1(d), TESARO retains all rights under the TESARO Technology and TESARO Trademarks: (a) to Exploit any Compound alone as the sole active pharmaceutical ingredient or in combination with other active pharmaceutical ingredients, including in a co-packaged or co-formulated product, in the TESARO Field and throughout the TESARO Territory, whether or not expressly stated herein; and (b) to Exploit any Compound in combination with one or more other active pharmaceutical ingredients in the TESARO Field, in each case ((a) and (b)), without the consent of Company or any Joint Committee.
3.5No Implied Licenses. All licenses and rights are granted only as expressly provided in this
|
Confidential |
41 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Agreement and no license or other right is or shall be created or granted under this Agreement by implication, estoppel, or otherwise. All rights not expressly granted by a Party under this Agreement are reserved by such Party and may not be used by the other Party for any purpose.
DEVELOPMENT
4.1General. Prior to the Effective Date, TESARO commenced Development of the Initial Product for certain indications in the TESARO Field, including breast cancer and ovarian cancer. During the Term, Company shall have the right to Develop the Compounds, Single Agent Products and Combination Products in the Company Field in the Company Territory, and TESARO shall retain the right to Develop the Compounds, Single Agent Products and Combination Products in the TESARO Field in the TESARO Territory, in each case subject to the terms and conditions of this Agreement. Certain aspects of the Parties’ Development of the Compounds and Products shall be communicated through the JDC, as further described in ARTICLE 2 and this ARTICLE 4, but neither Party’s Development activities and decisions require the approval, consent or agreement of the JDC, JSC, any other Joint Committee, subcommittee, or the other Party except as expressly provided in this Agreement.
4.2Development Activities
(a)Company Development Activities. Except as expressly provided in this Agreement, Company shall be solely responsible for and have sole authority with respect to, at its own expense, all Development of the Compounds, Single Agent Products and Combination Products in the Company Field in the Company Territory, including Development for Monotherapy Use or Combination Use in the Company Field in the Company Territory. Company shall conduct such Development in accordance with the Company Development Plan, which may be updated from time to time by Company in accordance with Section 4.2(c).
(b)TESARO Development Activities. Except as expressly provided in this Agreement, TESARO shall be solely responsible for and have sole authority with respect to, at its own expense, all Development of the Compounds, Single Agent Products and Combination Products in the TESARO Field in the TESARO Territory, including Development for Monotherapy Use or Combination Use in the TESARO Field in the TESARO Territory.
(c)Communication of Development Activities. A summary of all ongoing and planned Phase 2 Trials and Phase 3 Trials of Products in the TESARO Field in the TESARO Territory is attached hereto as Exhibit 4.2(c) (the “TESARO Development Plan”), and a summary of Company’s planned Clinical Trials of the Products in the Company Field in the Company Territory will be delivered to TESARO within [***] after the Effective Date (the “Company Development
|
Confidential |
42 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Plan” and, together with the TESARO Development Plan, the “Development Plans”). Each Party shall update its Development Plan twice per year for so long as such Party (or any of its Affiliates or (sub)licensees) is conducting Development of the Products, and shall deliver such updated Development Plan to the JDC for review and discussion; provided, that one of such updated Development Plans shall be delivered no later than [***] after the start of each Calendar Year. In addition to such updates, each Party may amend its Development Plan at any time, and shall deliver such amended Development Plan to the JDC for review and discussion at its next regularly scheduled meeting. For clarity, approval of a Party’s Development Plan by the JDC, JSC or other Party shall not be required. Each updated and amended Development Plan shall contain sufficient details regarding the Development activities described therein for (a) the other Party to assess potential adverse effects of such Development activities on the other Party’s ongoing or planned Development activities with respect to the Products, to the extent that such information is available and (b) TESARO to prepare the Third Party Development Reports in accordance with Section 4.2(h). If either Party reasonably believes that any activity set forth in the other Party’s Development Plan could adversely affect any of its ongoing or planned Development activities with respect to the Products, such Party shall so notify the other Party, stating the basis for its belief, and the JDC shall meet to discuss such concerns.
(d)Right to Object to Clinical Trials. Neither Party, nor any of its Affiliates, licensees or sublicensees, shall conduct or have conducted on its behalf a Clinical Trial of a Product unless such Party first provides the other Party with an opportunity to review and, if permitted below, to object to such Clinical Trial as follows:
(i)The Conducting Party shall provide the draft protocol (or, if not available, a detailed description of the proposed protocol and trial design) and investigator’s brochure for such Clinical Trial to the other Party (the “Reviewing Party”) at least [***] before the first submission of the protocol to an institutional review board or ethics committee.
(ii)The Reviewing Party may, but is not obligated to, provide comments on the draft protocol and investigator’s brochure for such Clinical Trial to the Conducting Party no later than [***] after receipt of such draft protocol and investigator’s brochure. The Conducting Party shall reasonably consider such comments in good faith.
(iii)In addition, the Reviewing Party may notify the Conducting Party that it objects to the design for such Clinical Trial (such notice, an “Objection”) no later than [***] after receipt of the draft protocol and investigator’s brochure on one or more of the following grounds:
|
Confidential |
43 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(1)if Company is the Reviewing Party, Company reasonably determines in good faith that the proposed Clinical Trial would result in a safety or tolerability finding that would raise material concerns regarding the clinical benefit of any Product in the Company Field; or
(2)if TESARO is the Reviewing Party, TESARO reasonably determines in good faith that the proposed Clinical Trial would result in a safety or tolerability finding that would raise material concerns regarding the clinical benefit of any Product in the TESARO Field.
(iv)Any notice of an Objection shall include specific proposals relating to the design of the Clinical Trial in order to address and overcome the ground(s) for Objection, and the Parties shall promptly discuss such proposals in good faith. If the Parties do not agree on the proposals raised in an Objection within [***] of the date of such notice, the Conducting Party may conduct the proposed Clinical Trial in accordance with such draft protocol and investigator’s brochure.
(v)If the Conducting Party intends to materially change a protocol or investigator’s brochure for a Clinical Trial of a Product after providing a draft of such protocol or investigator’s brochure to the Reviewing Party, such Clinical Trial shall be subject again to the procedures set forth above in this Section 4.2(d), unless such material change is required or recommended by a Regulatory Authority, institutional review board or ethics committee, in which case, the Conducting Party shall only be required to provide as much notice as is reasonably practicable prior to implementing such change and the Reviewing Party shall have no right to raise an Objection thereto; provided, that the review of material changes contemplated by this Section 4.2(d)(v) shall not require the Conducting Party to terminate or suspend any ongoing Clinical Trial.
(e)Clinical Trial Registries. Each Party shall be responsible, in accordance with Applicable Law, for registering each Clinical Trial of a Product that such Party conducts in the appropriate clinical trial registry and posting the results of such Clinical Trial.
(f)Sharing of Development Know-How.
(i)Within [***] after the Effective Date, TESARO shall provide Company with electronic copies of the INDs relating to Development of the Initial Compound and Initial Product set forth on Exhibit 4.2(f).
|
Confidential |
44 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(ii)Following the Effective Date: (A) within thirty (30) days after Company’s reasonable request (provided, that such requests may not be more frequent than once per Calendar Quarter with respect to any given Product after the first [***] of Development of such Product by Company), TESARO shall provide to Company any TESARO Know-How relating to the Development of the Compounds and Products that is necessary or useful for Company to Develop the Compounds or Products in the Company Field in the Company Territory, including any such Know-How relating to the Development of diagnostics with respect to the Compounds or Products (it being understood and acknowledged by Company that TESARO is not granting rights to Company that it does not Control under the Merck License Agreement or AstraZeneca License Agreements, including any rights relating to the Development of diagnostics with respect to the Compounds or Products)); (B) the Conducting Party, with respect to any Clinical Trial, shall provide the other Party with any safety data resulting from such Clinical Trial in accordance with the PVA or as otherwise determined by the Parties; and (C) the Conducting Party with respect to any Clinical Trial shall provide the other Party with a copy of the final clinical study report from such Clinical Trial promptly following completion thereof. The Parties shall discuss the logistics of sharing Know-How pursuant to this Section 4.2(f)(ii) through the JDC. In the event Company reasonably determines that any TESARO Know-How that is Controlled by TESARO pursuant to the Merck License Agreement or any AstraZeneca License Agreement is necessary to seek Marketing Approval of a Product in the Company Field, TESARO will use reasonable efforts to obtain such TESARO Know-How from Merck or AstraZeneca, as the case may be, if TESARO is not in possession of such TESARO Know-How. Notwithstanding anything to the contrary in this Agreement, a Party that is required to provide any Know-How pursuant to this Section 4.2(f)(ii) may, prior to providing such Know-How to the other Party, redact any [***] “Redactable Information”).
(iii)Each Party shall promptly disclose to the other Party in writing any other issue related to the Development of the Compounds or Products that could reasonably impact the other Party’s ongoing or planned Development activities with respect to the Compounds or Products. Such disclosure shall contain sufficient details regarding the issue described therein for the other Party to assess the impact of such issue on the other Party’s ongoing or planned Development activities with respect to the Compounds or Products. If requested by a Party following such disclosure, the JDC shall meet to discuss the issue.
(g)Ownership of Development Information and Results. Company shall own all Clinical Data and other Know-How generated under Clinical Trials of and other
|
Confidential |
45 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Development of the Compounds and Products in the Company Field in the Company Territory. TESARO shall own all Clinical Data and other Know-How generated under Clinical Trials of and other Development of the Compounds and Products in the TESARO Field in the TESARO Territory.
(h)Development Reports. Twice each Calendar Year, each Party shall provide to the other Party in writing (and verbally to the JDC) a report detailing such Party’s efforts and progress during the six (6) months prior to such date, as applicable, to Develop the Compounds and Products in such Party’s Field and Territory. Each such report shall describe material Development activities completed since the last report, including the object and parameters of the Development, when initiated, when completed and a summary of all material results; provided, however, that neither Party shall be required to include any Redactable Information in such report. TESARO may use the information contained in the reports provided by Company pursuant to this Section 4.2(h) to prepare a development report with respect to the Compounds and Products in the format, and with the same level of detail, as the report attached hereto as Exhibit 4.2(h) (a “Third Party Development Report”). TESARO may disclose such Third Party Development Report to Merck and AstraZeneca to the extent necessary for TESARO to satisfy its obligations under Sections 3.02(b) and 3.03 of the Merck License Agreement and Section 4 of the AstraZeneca License Agreements. Prior to disclosing a Third Party Development Report to Merck or AstraZeneca, TESARO shall provide a draft of such Third Party Development Report to Company for review and, upon Company’s request, TESARO shall redact from such Third Party Development Report any Redactable Information. TESARO acknowledges and agrees that the Third Party Development Report shall be sufficient to satisfy its obligations to provide reports with respect to the Development of the Compounds and Products by Company under Sections 3.02(b) and 3.03 of the Merck License Agreement and Section 4 of the AstraZeneca License Agreements.
4.3Development Diligence Obligations.
(a)Company shall use Commercially Reasonable Efforts to Develop, and to seek Marketing Approval for, [***] and shall continue to use such efforts until Marketing Approval is obtained for [***]. For clarity, but without limiting the foregoing, in no event shall Company be obligated to Develop or seek Regulatory Approval for [***].
(b)Without limitation of Section 4.3(a), if in connection with its exercise of Commercially Reasonable Efforts, Company determines to cease Development of any Product(s) for any Initial Indication prior to the achievement of the Development Milestone(s) or any Regulatory Milestone(s) associated with such ceased Development, then Company shall promptly notify TESARO in writing
|
Confidential |
46 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
and present such decision to the JDC at its next regular planned meeting for discussion and explanation. If Company determines, in its sole discretion and consistent with its obligation of Commercially Reasonable Efforts, to recommence the ceased Development or commence new Development for the Initial Indication or commence Development of any Product(s) for an Indication that is not an Initial Indication (in each case, such Indication, a “Notice Indication”), then Company shall promptly notify TESARO of such decision in writing. Upon delivery of such notice, if the Development Milestone(s) or any Regulatory Milestone(s) associated with the ceased Development of the Initial Indication have not yet been achieved, then such Development Milestone(s) and Regulatory Milestone(s) shall be deemed to be amended to continue or replace the ceased Development of the Initial Indication with the Development of the Notice Indication.
4.4Compliance with Law. The Parties will, and will cause their Affiliates and Third Party subcontractors to, comply with Applicable Law in performing Development activities with respect to the Compounds and Products, including those pertaining to the use of laboratory animals, GLP, GMP or GCP, as appropriate.
4.5Records. The Parties will, and will cause their Affiliates and Third Party subcontractors to, maintain complete and accurate records of all work conducted in performing Development activities with respect to the Compounds and Products, and all results, data, Inventions and Know-How made during the performance of such Development. Subject to a Party’s agreements with Third Parties (provided that such agreements are entered into in accordance with this Agreement and such Party has notified the other Party of the existence of such agreements), each Party shall have unrestricted access to all results, data, Inventions and Know-How Controlled by such Party or its Affiliates related to any Compound or Product (not including Combination Products in the TESARO Field) to ensure data integrity, traceability, acceptability, quality and operational compliance with all Applicable Law; provided, however, that neither Party shall have access to the other Party’s Redactable Information. Such records shall contain sufficient detail and be in good scientific and, as appropriate, GLP, GCP, and GMP manner suitable for patent preparation and filing and regulatory purposes. The Parties will maintain or cause to be maintained such records with respect to the Compounds and each such Product for a period at least five (5) years after receipt of Marketing Approval of any Product in an ICH region and until there are no pending or contemplated Drug Approval Applications or Regulatory Approvals in an ICH region or at least five years have elapsed since the formal discontinuation of clinical Development of such Product; provided, however, that such records shall be retained for a longer period if required by the applicable Regulatory Authority. If either Party plans to destroy or discard any of such records after the retention period has expired, such Party will notify the other Party in writing. The Parties will then discuss and decide whether to send the records to an offsite storage facility, to the other Party, or another alternative as agreed to by the Parties.
4.6Subcontracting. Each Party may subcontract the performance of any Development activities conducted in accordance with this Agreement to any of its Affiliates or any Third
|
Confidential |
47 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Party, provided that such Party shall oversee the performance of any subcontracted activities in a manner that would be reasonably expected to result in their successful and timely completion and shall remain responsible for the performance of such subcontracted activities in accordance with this Agreement. Each Party agrees to use reasonable efforts to include in any contract or other written arrangement with each of its subcontractors a clause permitting the other Party to exercise its right to inspect any facilities at which the subcontractor conducts Development activities with respect to the Compounds or Products (not including Combination Products in the TESARO Field) under Section 4.7.
4.7Audits. With respect to any facility or site at which either Party conducts Development with respect to the Compounds or Products (including, where commercially reasonable and permitted under the applicable agreement between such Party and its subcontractor, such subcontractor’s facilities or sites, but not including Combination Products in the TESARO Field), the other Party will have the right, exercisable no more than once per Calendar Year (or more often, but not more than quarterly, if there are material compliance issues), upon reasonable written notice and during normal business hours, to inspect such site and facility and any records relating thereto, at its expense, in each case only to the extent reasonably necessary to verify such Party’s or its subcontractors compliance with the terms of this Agreement relating to GMP, GLP and GCP that could reasonably affect the inspecting Party’s Development or Commercialization of Compounds or Products in its Field. Such inspection shall be subject to the confidentiality provisions of this Agreement. In the event that, during such an inspection visit, the inspected facilities are found to be non-compliant with one or more GMP, GLP, GCP standards, the inspecting Party shall so notify the inspected Party of such non-compliance and the inspected Party shall submit a proposed recovery/corrective action plan to the other Party within sixty (60) days after the other Party’s notification to Conducting Party of such non-compliance. The foregoing inspection rights shall only be exercised by a Party to the extent necessary for that Party to Exploit its rights hereunder.
REGULATORY RESPONSIBILITIES
5.1General. During the Term, Company shall have the right to obtain and maintain Regulatory Approvals for the Single Agent Products and Combination Products in the Company Field in the Company Territory and shall otherwise have the regulatory responsibilities for Products in the Company Field in the Company Territory as set forth in this ARTICLE 5; provided, however, that TESARO shall have the right to obtain and maintain Marketing Approval for Monotherapy Use of the Single Agent Products in the Company Field in the Company Territory on the terms set forth in this ARTICLE 5. TESARO shall retain the right to obtain and maintain Regulatory Approvals for the Single Agent Products and Combination Products in the TESARO Field in the TESARO Territory, subject to the terms and conditions of this Agreement. Certain aspects of the Parties’ regulatory activities with respect to the Products shall be communicated through the JDC as expressly described in ARTICLE 2 and this ARTICLE 5, but neither Party’s regulatory activities and decisions require the approval, consent
|
Confidential |
48 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
or agreement of the JDC, JSC, any other Joint Committee, subcommittee, or the other Party except as expressly provided in this Agreement.
5.2Regulatory Activities – General.
(a)Company Regulatory Activities. Company shall have the sole right and authority, at its own expense but subject to TESARO’s rights under Section 5.3(b) to file for, obtain and maintain Marketing Approval of the Single Agent Products for Monotherapy Use in the Company Field, to: (i) develop and implement the overall regulatory strategy with respect to obtaining Marketing Approval of the Products in the Company Field in the Company Territory, including Marketing Approval of the Single Agent Products for Combination Use in the Company Field in the Company Territory; (ii) prepare, obtain and maintain all Regulatory Documentation with respect to Products in the Company Field in the Company Territory; and (iii) subject to Sections 5.7 and 5.9, conduct communications with the relevant Regulatory Authorities with respect to the Compounds and Products in the Company Field in the Company Territory. Regulatory Documentation shall include, for the purposes of this ARTICLE 5, all INDs, Drug Approval Applications and Marketing Approvals.
(b)TESARO Regulatory Activities. TESARO shall have the sole right and authority, at its own expense, to: (i) develop and implement the overall regulatory strategy with respect to obtaining Marketing Approval of the Products in the TESARO Field in the TESARO Territory; (ii) prepare, obtain, and maintain all Regulatory Documentation to the Products in the TESARO Field in the TESARO Territory; (iii) subject to Sections 5.7 and 5.9, conduct communications with the relevant Regulatory Authorities with respect to those Products in the TESARO Field in the TESARO Territory; and (iv) subject to Section 5.2(a)(ii) and 5.3(b), file for, obtain and maintain Marketing Approval for the Single Agent Product for Monotherapy Use in the Company Field in the Company Territory.
(c)Communication of Regulatory Activities. At least [***] prior to filing a first Drug Approval Application for a Single Agent Product in the TESARO Field in any country or jurisdiction in the TESARO Territory, TESARO shall use reasonable efforts to deliver to the JDC a summary of its planned activities with respect to obtaining Marketing Approval for the Single Agent Products in the TESARO Field throughout the TESARO Territory (the “TESARO Registration Plan”). At least [***] prior to filing a first Drug Approval Application for a Product in the Company Field in any country or jurisdiction in the Company Territory, Company shall use reasonable efforts to deliver to the JDC a summary of its planned activities with respect to obtaining Marketing Approval for the Products in the Company Field throughout the Company Territory (the “Company Registration Plan” and, together with the TESARO Registration Plan, the “Registration Plans”). Each Party shall update its Registration Plan
|
Confidential |
49 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
once per Calendar Quarter for so long as such Party (or any of its Affiliates or (sub)licensees) is seeking Marketing Approval for the Products, and shall deliver such updated Registration Plan to the JDC for review and discussion (but not approval). For clarity, approval of a Party’s Registration Plan by the JDC or other Party shall not be required; provided that the Registration Plan shall be consistent with the rights of that Party. Each Registration Plan shall contain: (i) a list of each country and jurisdiction in which the applicable Party plans to file a Drug Approval Application for each Product; (ii) for each country and jurisdiction described in clause (i), (A) the expected timing of submission of the Drug Approval Application, (B) the expected approval date of the Drug Approval Application, (C) the proposed indication(s) for which approval will be sought, and (D) the anticipated date of First Commercial Sale of such Product in such country and jurisdiction (or first commercial sale with respect to Single Agent Products in the TESARO Field). If, following delivery of any Registration Plan by a Party, the other Party reasonably believes that any activity set forth in such Registration Plan could adversely affect any of its ongoing or planned Development or regulatory activities with respect to the Products, such Party shall so notify the other Party and the JDC shall meet to discuss such concerns.
(d)Regulatory Updates. Company shall promptly provide TESARO with notice of the granting of any Marketing Approval of a Product obtained by Company, and TESARO shall promptly provide Company with notice of the granting of any Marketing Approval of a Single Agent Product obtained by TESARO. TESARO may disclose information to Merck and AstraZeneca with respect to Company’s regulatory activities with respect to the Products in the Company Field in the Company Territory consistent with the level of detail set forth in the Third Party Development Reports, to the extent necessary for TESARO to comply with its reporting requirements under Section 4.01(f) of the Merck License Agreement and Section 4 of each of the AstraZeneca License Agreements. Notwithstanding the foregoing, prior to disclosing such information to Merck or AstraZeneca, TESARO shall provide a draft of such disclosure to Company for review and, upon Company’s request, TESARO shall redact from such disclosure any Redactable Information. TESARO acknowledges and agrees that the information that TESARO is permitted to disclose to Merck and AstraZeneca pursuant to this Section 5.2(d) shall be sufficient to satisfy its obligations to provide information under Section 4.01(f) of the Merck License Agreement and Section 4 of each of the AstraZeneca License Agreements.
5.3INDs and Marketing Approvals.
(i)Prior to the Effective Date, TESARO filed the following INDs with respect to the Initial Product: IND 100,996 (Ovarian) and IND 117,580
|
Confidential |
50 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(Breast). Following the Effective Date, TESARO shall continue to maintain such INDs to the extent required for Company to initiate and conduct the Clinical Trials set forth in the Company Development Plan.
(ii)Following the Effective Date, Company shall prepare, file and maintain all INDs for the Products that are required for Company to conduct the Clinical Trials set forth in the Company Development Plan, including any Clinical Trials of a Single Agent Product in combination with any other any pharmaceutical product and any Clinical Trials of a Combination Product.
(b)Marketing Approvals – Single Agent Products for Monotherapy Use.
(i) TESARO shall have the sole right and authority to prepare, obtain and maintain all Drug Approval Applications and Marketing Approvals for any Monotherapy Use in the TESARO Field in the TESARO Territory.
(ii) Company shall have the sole right and authority to prepare all Drug Approval Applications for any Monotherapy Use in the Company Field in the Company Territory. TESARO shall have the first right, and Company shall have the second right, to submit any such Drug Approval Application to a Regulatory Authority in any country or jurisdiction in the Company Field, and to obtain and maintain the resulting Marketing Approval, in accordance with the terms set forth in the remainder of this Section 5.3(b).
(iii)If Company desires to submit a Drug Approval Application for a Monotherapy Use in the Company Field to a Regulatory Authority in any country or jurisdiction in the Company Territory, Company shall so notify TESARO no later than [***] prior to the date on which Company desires to submit such Drug Approval Application to such Regulatory Authority. Within [***] after receipt of such notice, TESARO shall deliver a written notice to Company (such notice, a “Monotherapy Registration & Distribution Notice”) stating: (x) whether or not TESARO will submit such Drug Approval Application, and obtain and maintain the resulting Marketing Approval on behalf of the Company in accordance with Section 5.3(b)(iii); (y) whether or not TESARO has submitted a Drug Approval Application for, or obtained Marketing Approval of, the corresponding Monotherapy Use in the TESARO Field in the applicable country or jurisdiction; and (z) whether or not TESARO will Distribute the applicable Single Agent Product in the applicable country or jurisdiction in accordance with Section 6.2(c).
(iv) In the event that TESARO delivers a Monotherapy Registration & Distribution Notice stating that TESARO will submit the applicable Drug Approval Application for a Monotherapy Use in the Company Field, and
|
Confidential |
51 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
obtain and maintain the resulting Marketing Approval, in the applicable country or jurisdiction in the Company Territory, then the provisions of this Section 5.3(b)(iv) shall apply with respect to such Drug Approval Application and Marketing Approval.
(1)No later than [***] prior to the date on which Company desires to submit such Drug Approval Application to the applicable Regulatory Authority, Company shall submit such Drug Approval Application to the JDC for review. Within [***] following submission of a such Drug Approval Application to the JDC the JDC shall review such Drug Approval Application to reasonably determine whether it satisfies the appropriate efficacy and safety criteria outlined in the applicable protocol and notify Company of the results of such review.
(2)If a notice given by the JDC in accordance with clause (1) above indicates that the applicable Drug Approval Application does not satisfy the appropriate efficacy and safety criteria outlined in the applicable protocol, then TESARO shall have no obligation to submit such Drug Approval Application to the applicable Regulatory Authority unless and until Company revises such Drug Approval Application to satisfy the appropriate efficacy and safety criteria outlined in the applicable protocol.
(3)If a notice given by the JDC in accordance with clause (1) above indicates that the applicable Drug Approval Application satisfies the appropriate efficacy and safety criteria outlined in the applicable protocol, then TESARO shall submit such Drug Approval Application to the applicable Regulatory Authority in the applicable country or jurisdiction in the Company Field upon the request of Company; provided, however, that (x) if TESARO previously submitted a Drug Approval Application for the corresponding Monotherapy Use in the TESARO Field in such country or jurisdiction, then TESARO shall not be obligated to submit Company’s Drug Approval Application until TESARO’s Drug Approval Application is approved or finally rejected by the applicable Regulatory Authority and (y) if Marketing Approval for the corresponding Monotherapy Use in the TESARO Field has been previously granted in such country or jurisdiction, such Drug Approval Application in the Company Field shall be submitted as a Supplemental Application to such Marketing Approval. TESARO shall pay any filing fees required to submit any Drug Approval Application or Supplemental Application, as applicable, or to
|
Confidential |
52 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
obtain or maintain the resulting Marketing Approval, to such Regulatory Authority and shall either seek reimbursement of such filing fees from Company pursuant to Section 8.13 or deduct such filing fees from the TESARO Prostate-Approved Single Agent Product Net Sales as part of calculating Alliance Payments in accordance with Exhibit 8.3.
(4)Upon TESARO’s submission of a Drug Approval Application or Supplemental Application to a Regulatory Authority in accordance with clause (3) above, TESARO shall, and hereby does, appoint Company as its authorized agent to assume primary responsibility with respect to such Drug Approval Application or Supplemental Application, as applicable (and the resulting Marketing Approval) and all associated communications with such Regulatory Authority; provided that Company complies with all Applicable Laws in connection with such communications and keeps TESARO informed of all associated communications in accordance with Section 5.4(b). In furtherance of the foregoing, at Company’s request prior to the filing of such Drug Approval Application or Supplemental Application, as applicable, TESARO shall execute a letter, in a mutually agreed form, appointing Company as authorized agent to assume primary responsibility with respect to such Drug Approval Application or Supplemental Application, as applicable, and the Parties shall submit such letter to the applicable Regulatory Authority.
(5)If the applicable Regulatory Authority approves a Drug Approval Application or Supplemental Application submitted by TESARO in accordance with clause (3) above, then, subject to Company’s rights under clause (4) above, TESARO shall be the holder of the resulting Marketing Approval in the Company Field and, if applicable, shall remain as the holder of the corresponding Marketing Approval in the TESARO Field. Except with respect to a Change of Control, TESARO shall not, sell, transfer or assign any such Drug Approval Application, Supplemental Application or Marketing Approval with respect to the Company Field (or grant any Third Party any rights relating to such Marketing Approval) without Company’s prior written consent, such consent not to be unreasonably withheld. If TESARO desires to withdraw or to cease to maintain any such Marketing Approval, TESARO shall so notify Company at least [***] prior to the date upon which TESARO intends to withdraw or cease to maintain such Marketing Approval and, upon Company’s request prior to the expiration of such [***], TESARO shall assign
|
Confidential |
53 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
such Marketing Approval to Company and shall take all actions necessary to effectuate such assignment, and the Parties shall negotiate in good faith and agree upon any modifications to this Agreement necessary to enable Company to maintain such Marketing Approval.
(v) In the event that (A) TESARO delivers a Monotherapy Registration & Distribution Notice stating that it will not submit the applicable Drug Approval Application for a Monotherapy Use in the Company Field in a country or jurisdiction in the Company Territory, and obtain and maintain the resulting Marketing Approval or (B) TESARO fails to deliver a Monotherapy Registration & Distribution Notice within [***] after Company notifies TESARO of its desire to submit a Drug Approval Application for a Monotherapy Use in the Company Field in a country or jurisdiction in the Company Territory, then, in each case ((A) and (B)), the provisions of this Section 5.3(b)(v) shall apply with respect to such Drug Approval Application and Marketing Approval.
(1)Prior to submitting such Drug Approval Application to the applicable Regulatory Authority in such country or jurisdiction, Company shall provide TESARO with a draft of such Drug Approval Application, and TESARO may provide comments on such Drug Approval Application, which Company shall consider in good faith; provided, however, that Company may redact any Redactable Information prior to providing such draft to TESARO.
(2)Following TESARO’s review of a Drug Approval Application in accordance with clause (1) above, Company may submit such Drug Approval Application to the applicable Regulatory Authority at any time and shall promptly notify TESARO after such submission. Company shall pay any filing fees required to submit such Drug Approval Application to such Regulatory Authority or to obtain or maintain the resulting Marketing Approval. Company shall be solely responsible for all communications with such Regulatory Authority with respect to such Drug Approval Application; provided that Company shall keep TESARO informed of all associated communications in accordance with Section 5.4(b).
(3)If the applicable Regulatory Authority approves a Drug Approval Application submitted by Company in accordance with clause (2) above, Company shall be the holder of the resulting Marketing Approval.
|
Confidential |
54 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(vi) If at any time Company is the holder of any Marketing Approval for Monotherapy Use in the Company Field, upon TESARO’s request at any time after the grant of such Marketing Approval, Company shall assign and transfer such Marketing Approval to TESARO and shall take all actions necessary to effectuate such assignment and transfer, and the provisions of clauses (3) (with respect to fees), (4) and (5) of Section 5.3(b)(iv) shall thereafter apply to such Marketing Approval.
(c)Marketing Approvals – Single Agent Products for Combination Use.
(i)TESARO shall have the sole right and authority to prepare, obtain and maintain all Drug Approval Applications and Marketing Approvals for any Combination Use in the TESARO Field in the TESARO Territory.
(ii)Company shall have the sole right and authority to prepare, obtain and maintain all Drug Approval Applications and Marketing Approvals for any Combination Use in the Company Field in the Company Territory; provided, however, Company may, in its sole discretion, seek Marketing Approval for such Combination Use using a Supplemental Application to a Marketing Approval for Monotherapy Use in the Company Field, in which case the corresponding provisions of Section 5.3(b) shall apply (substituting “Combination Use in the Company Field” for “Monotherapy Use in the Company Field” in the provisions thereof) with respect to the preparation, submission and maintenance of such Supplemental Application and the resulting Marketing Approval. For clarity, Company is not granting any rights to TESARO under this Agreement with respect to any such Combination Use.
(iii)Prior to submitting any Drug Approval Application for a Combination Use in the Company Field in the Company Territory (and, in any event, at least [***] in advance of submission) in accordance with this Section 5.3(c), a Party shall provide the other Party with a draft of relevant portions of the Drug Approval Application as determined by the JDC, and the other Party may provide comments on any portions of such Drug Approval Application that relate to a Compound, which the submitting Party shall consider in good faith; provided, however, that, prior to submitting such draft to the other Party, the submitting Party may redact any Redactable Information from such Drug Approval Application.
(d)Marketing Approvals – Combination Products.
(i)Subject to Sections 3.1(d) and 3.4, TESARO shall have the sole right and authority to prepare, obtain and maintain all Drug Approval Applications and Marketing Approvals for any Combination Product in the TESARO Field in the TESARO Territory.
|
Confidential |
55 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(ii)Company shall have the sole right and authority to prepare, obtain and maintain all Drug Approval Applications and Marketing Approvals for the Combination Products in the Company Field in the Company Territory. For clarity, Company is not granting any rights to TESARO under this Agreement with respect to any such Combination Product.
(iii)Prior to submitting any Drug Approval Application for a Combination Product in the Company Field in the Company Territory (and, in any event, at least [***] in advance of submission) in accordance with this Section 5.3(d)(iii), Company shall provide TESARO with a draft of relevant portions of the Drug Approval Application as determined by the JDC, and TESARO may provide comments on any portions of such Drug Approval Application that relate to a Compound, which Company shall consider in good faith; provided, however, that, prior to providing such drafts to TESARO, Company may redact any Redactable Information.
5.4Interactions with Regulatory Authorities.
(a)Regulatory Meetings. Where Applicable Law so permits, each Party shall have the right to have two (2) representatives participate in all material meetings, conferences and discussions between the other Party and the applicable Regulatory Authority with respect to any Compound or Product (provided that such representatives shall not be actively involved in seeking Regulatory Approval for a product other than a Product in any indication that will be discussed at such meeting, conference or discussion); provided, however, that [***]. Each Party shall provide the other Party with reasonable advance notice (and, in any event, at least five (5) Business Days advance notice) of all such meetings, conferences and discussions and all copies of all relevant information with respect to such meetings, conferences and discussions; provided, however, that, prior to providing such information to the other Party, the providing Party may redact any Redactable Information.
(b)Regulatory Correspondence. Each Party shall provide the other Party with drafts of any material Regulatory Documentation related to a Party’s Development or Commercialization of a Product (excluding Drug Approval Applications and Supplemental Applications, which are governed by Sections 5.3(b), 5.3(c) and 5.3(d)) that such Party plans to submit to any Regulatory Authority reasonably in advance of submission (and, in any event, at least five (5) Business Days in advance of submission, if practicable, and otherwise, with as much notice as practicable), and the other Party may provide comments on such Regulatory Documentation, which the submitting Party shall consider in good faith; provided, however, that the submitting Party may, prior to providing such Regulatory Documentation to the other Party, redact any Redactable Information. Notwithstanding anything to the contrary in this Agreement, in no event shall
|
Confidential |
56 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Company be obligated to provide TESARO with any DMF Equivalents with respect to any Combination Product Developed by Company. For clarity, this Section 5.4(b) shall apply with respect to any proposed changes to the label for a Product (other than proposed label changes set forth in a Supplemental Application submitted in accordance with Section 5.3(b)(i) or 5.3(c)(ii)).
(c)Post-Marketing Commitments. Each Party shall perform all activities necessary to satisfy any post-marketing requirements imposed by a Regulatory Authority with respect to a Single Agent Product in such Party’s Field, in accordance with the timelines established by such Regulatory Authority.
5.5Sharing of Regulatory Documentation.
(a)Initial Transfer of Regulatory Documentation. Within [***] after the Effective Date, TESARO shall deliver to Company electronic copies (unless otherwise required by Applicable Law) of all Regulatory Documentation relating to the Initial Compound and Initial Product set forth on Exhibit 5.5(a).
(b)Sharing of Safety Data. Upon a Party’s request or as required under the PVA, each Party shall provide the other Party with any safety data relating to the Compounds or Products that is necessary for such other Party to prepare, obtain or maintain any Regulatory Approval that such Party has a right or obligation to prepare, obtain or maintain pursuant to this ARTICLE 5. The Parties shall discuss the logistics of sharing safety data pursuant to this Section 5.5(b) through the JDC.
(c)Ongoing Sharing of Regulatory Documentation. Upon a Party’s reasonable request, each Party shall provide the other Party with copies of any Regulatory Documentation or Know-How Controlled by such Party that relates to a Compound or Product, to the extent that such Regulatory Documentation or Know-How is required or reasonably expected to be required for the other Party to prepare, obtain or maintain any Regulatory Approval for a Compound or Product that such other Party has a right or obligation to prepare, obtain or maintain under this ARTICLE 5. The Parties shall discuss the logistics of sharing Regulatory Documentation pursuant to this Section 5.5(c) through the JDC. Notwithstanding anything to the contrary in this Agreement, a Party that is required to provide Regulatory Documentation or Know-How pursuant to this Section 5.5(c) may, prior to providing such Regulatory Documentation or Know-How to the other Party, redact any Redactable Information. Notwithstanding anything to the contrary in this Agreement, in no event shall Company be obligated to provide TESARO with any DMF Equivalents with respect to any Combination Product Developed by Company.
|
Confidential |
57 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
5.6Rights of Reference.
(a)Rights of Reference Granted to Company. Subject to the terms and conditions of this Agreement, TESARO (on behalf of itself and its Affiliates) hereby grants to Company a non-exclusive Right of Reference (with the right to grant further Rights of Reference to any of Company’s Affiliates, sublicensees or Third Party distributors that will hold the Marketing Approval for any Product in the Company Field in any country or jurisdiction in the Company Territory) to any Regulatory Documentation Controlled by TESARO (including the IND for the Single Agent Product filed by Merck prior to the Effective Date and the corresponding Phase 1 Clinical Trial data) that is required or reasonably expected to be required to obtain or maintain any Regulatory Approval of a Compound or Product in the Company Field in the Company Territory that Company is permitted to obtain or maintain under Section 5.2(a) or Section 5.3 (such Regulatory Documentation, the “TESARO Regulatory Documentation”), for the sole purpose of preparing, obtaining and maintaining such Regulatory Approval. Company shall notify TESARO of any additional right of reference granted by Company in accordance with this Section 5.6(a), and shall obtain from the referee an undertaking that it shall comply with applicable provisions of this Agreement and Applicable Law with respect thereto.
(b)Rights of Reference Granted to TESARO. Subject to the terms and conditions of this Agreement, Company (on behalf of itself and its Affiliates) hereby grants to TESARO a non-exclusive Right of Reference (with the right to grant further Rights of Reference to any of TESARO’s Affiliates, (sub)licensees or Third Party distributors that will hold the Marketing Approval for any Single Agent Product in the TESARO Field in any country or jurisdiction in the TESARO Territory) to any Regulatory Documentation Controlled by Company that is required or reasonably expected to be required to obtain or maintain any Regulatory Approval of a Compound or Product that TESARO is permitted to obtain or maintain under Section 5.2(b) or Section 5.3 (the “Company Regulatory Documentation”), for the sole purpose of preparing, obtaining and maintaining such Regulatory Approval; provided, however, that such Right of Reference shall not apply to the following, (i) any DMF equivalent for a Combination Product, (ii) any portion of any Company Regulatory Documentation that includes CMC information with respect to a Combination Product or (iii) any portion of any Company Regulatory Documentation that relates to any compound that is not a Compound or any product that is not a Product. TESARO shall notify Company of any additional right of reference granted by TESARO in accordance with this Section 5.6(b), and shall obtain from the referee an undertaking that it shall comply with applicable provisions of this Agreement and Applicable Law with respect thereto.
(c)Documentation. If requested by a Party, the other Party shall provide any signed statement that authorizes any Right of Reference granted to such Party under this
|
Confidential |
58 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Section 5.6 that is required by Applicable Law or the Regulatory Authority in the applicable country or jurisdiction. In the event that any Affiliate, licensee, sublicensee or Third Party distributor of a Party holds any Regulatory Documentation to which the other Party is granted a Right of Reference under this Section 5.6, such Party will cause such Affiliate, licensee, sublicensee or Third Party distributor to grant a Right of Reference to the other Party to the same extent that such Party is required to grant such Right of Reference under this Section 5.6.
(d)Access. The Rights of Reference granted pursuant to this Section 5.6 shall include the right to access all Know-How included or referenced in the applicable Regulatory Documentation; provided, however, that such right of access shall not apply to any Know-How that is Redactable Information.
5.7Adverse Event Reporting and Safety Data Exchange.
(a)Company Responsibilities. Company will maintain the global safety database, safety monitoring, pharmacovigilance surveillance, compliance and filing of all required safety reports to all Regulatory Authorities in the Company Territory with respect to any Combination Product (including any FDC) Developed by Company in the Company Field and, to the extent required by Applicable Law, any Combination Use of a Single Agent Product solely in the Company Field, including annual safety reports, throughout the Development and Commercialization of such Combination Product or combination regimen, as applicable.
(b)TESARO Responsibilities. TESARO will maintain the global safety database, safety monitoring, pharmacovigilance surveillance, compliance and filing of all required safety reports to all Regulatory Authorities in the Company Territory and TESARO Territory with respect to each Single Agent Product (other than with respect to any Combination Use in the Company Field for which Company is responsible pursuant to Section 5.7(a)) and with respect to any Combination Product Developed by TESARO in the TESARO Field, including annual safety reports, throughout the Development and Commercialization of such Single Agent Product or Combination Product, as applicable.
(c)Safety Information Exchange; Pharmacovigilance Agreement. The Parties shall cooperate to develop methods or procedures for sharing information relating to the clinical experiences with respect to the Products in accordance with safety reporting requirements of the respective Regulatory Authorities and as necessary to comply with Applicable Law. Specific details regarding the management of safety data (including adverse events reports) related to the Development and Commercialization of the Products will be delineated in a separate global pharmacovigilance agreement (the “PVA”). The Parties shall agree upon the
|
Confidential |
59 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
terms of the PVA no later than the anticipated date of the First Commercial Sale or clinical activity with respect to any Product in the Company Field in the Company Territory. In the event of any conflicts or inconsistencies between the PVA and this Agreement, the terms of the PVA shall take precedence for matters relating to pharmacovigilance.
5.8Medical Affairs. The Parties shall review and discuss medical affairs activities with respect to the Products (including the matters described in this Section 5.8 and investigator initiated studies, cooperative group studies, early access programs, HECOR studies, RWE and company-sponsored medical affairs studies) through the JDC (or, if created by the JDC, a joint medical affairs subcommittee).
(a)Medical Science Liaisons. Each Party shall determine independently how and if to utilize and deploy its medical science liaisons for activities relating to the Products in the Company Territory, provided that any arrangements a Party makes with its medical science liaisons are subject to the provisions of Sections 5.8(b) and 5.8(c).
(b)Responses to Medical Inquiries.
(i)Each Party shall apply its standard guidelines and procedures for determining and providing appropriate responses to medical inquiries for which it is responsible according to this Section 5.8(b). Each Party shall ensure that any such response procedures and standard response documents comply with Applicable Law.
(ii)Company shall be responsible for dealing with medical inquiries about the Products in the Company Field in the Company Territory. TESARO shall refer any medical inquiries that it receives about the Products in the Company Field in the Company Territory to Company or such Persons as Company may designate from time to time.
(iii)TESARO shall be responsible for dealing with medical inquiries about the Products in the TESARO Field in the TESARO Territory. Company shall refer any medical inquiries that it receives about the Products in the TESARO Field in the TESARO Territory or such Persons as TESARO may designate from time to time.
(iv)Each Party shall provide the other Party, on a recurring basis, with access to current medical affairs communications and training materials, including anticipated questions and answers, relating to the Products and as used by such Party’s medical affairs personnel in the Company Territory. Each Party will conduct training of its medical affairs personnel on the use of such materials and on following the procedures of such training materials. Company may respond to any medical inquiries about the Products in the TESARO Field in the Company Territory strictly in accordance with the information contained in the medical affairs communications materials provided by TESARO. TESARO may respond to any medical inquiries about
|
Confidential |
60 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
the Products in the Company Field in the Company Territory strictly in accordance with the information contained in the medical affairs communications materials provided by Company. Any medical inquiry seeking or requiring information not contained in such materials shall be referred by the receiving Party to the other Party or such Persons as the other Party may designate from time to time.
(c)Conferences and Symposia. Except for any Third Party medical education activities (e.g., accredited CME programs) over which a Party has no control or influence beyond providing funding or as otherwise agreed by the Parties: (i) Company shall not, and shall ensure that its Affiliates do not, participate in or sponsor any formal presentation or panel or medical booths with respect to any Product in the TESARO Field at any conference or symposia, or provide any information with respect to any Product in the TESARO Field at any conference or symposia, without the prior consent of TESARO; and (ii) TESARO shall not, and shall ensure that its Affiliates do not, participate in or sponsor any formal presentation or panel or medical booths with respect to any Product in the Company Field at any conference or symposia, or provide any information with respect to any Product in the Company Field at any conference or symposia, without the prior consent of Company.
5.9Recalls and Voluntary Withdrawals.
(a)Notification. Each Party shall notify the other Party in writing promptly following its determination that any event, incident or circumstance related to safety issues or regulatory concerns has occurred that is reasonably likely to result in the need for a recall, market suspension or market withdrawal of a Product, and shall include in such notice (a “Potential Recall Notice”) the reasoning behind such determination and any supporting facts. Such Potential Recall Notice shall be given no later than five (5) Business Days after such determination is made; provided, however, that if any Regulatory Authority (i) threatens or initiates any action to remove a Product from the market in any country or (ii) requires a Party, or any of its Affiliates or (sub)licensees, to distribute a “Dear Doctor” letter or its equivalent regarding the use of a Product in such Party’s Field, then, in either case ((i) or (ii)), the Potential Recall Notice shall be given within one (1) Business Day after such Party becomes aware of the action, threat or requirement (as applicable). TESARO shall be permitted to disclose any Potential Recall Notice delivered by Company to Merck to extent necessary to satisfy its obligations under Section 4.01(g) of the Merck License Agreement.
|
Confidential |
61 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(b)Decision-Making. If the Party that receives a Potential Recall Notice does not agree with the determination or recommendation of the other Party that the recommended recall, market suspension or market withdrawal of the applicable Product is advisable or necessary, then the Parties shall promptly meet to discuss whether to conduct such recall, market suspension or market withdrawal. If the Parties do not reach agreement on whether to conduct such recall, market suspension or market withdrawal within five (5) Business Days after a Potential Recall Notice is given, then: (i) if such Product is a Single Agent Product or a Combination Product Developed by TESARO in the TESARO Field, TESARO shall have the final decision-making authority with respect to such recall, market suspension or market withdrawal; and (ii) if such Product is a Combination Product Developed by Company in the Company Field, Company shall have the final decision-making authority with respect to such recall, market suspension or market withdrawal. Notwithstanding the foregoing, the Parties shall abide by any decision of the applicable Regulatory Authority with respect to any recall, market suspension or market withdrawal of a Product and, in the event TESARO notifies Company that it believes a recall, market suspension or market withdrawal of a Combination Product Developed by Company in the Company Field should occur due to safety concerns regarding the Compound included therein, then TESARO shall have the final decision-making authority with respect to such recall, market suspension or market withdrawal.
(c)Recall Procedure and Recall Expenses. TESARO shall be responsible for implementing, directing and administering (including communicating with Regulatory Authorities with respect to) any recall, market suspension or market withdrawal of a Single Agent Product or a Combination Product Developed by TESARO in the TESARO Field that is determined to be advisable or necessary in accordance with Section 5.9(b) at its own expense, provided that Company shall, upon the request of TESARO, provide any reasonable assistance in implementing or administering such recall, market suspension or market withdrawal. Company shall be responsible for implementing, directing and administering (including communicating with Regulatory Authorities with respect to) any recall, market suspension or market withdrawal of a Combination Product Developed by Company in the Company Field that is determined to be advisable or necessary in accordance with Section 5.9(b) at its own expense, provided that TESARO shall, upon the request of Company, provide any reasonable assistance in implementing or administering such recall, market suspension or market withdrawal.
(d)Recall Records. Each Party shall maintain records of all sales of the Products to direct accounts sufficient to administer a recall until the later of: (a) the third (3rd) anniversary of the last day of the Term; (b) the third (3rd) anniversary of the last sale of any Product; (c) the last expiration date of the last Product sold by or on behalf of such Party; or (d) the expiration of any period as may be required under any Applicable Law.
|
Confidential |
62 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(e)Public Statements regarding Recalls. TESARO shall be solely responsible for making all public statements in respect of any recall, market suspension or market withdrawal of any Single Agent Product or any Combination Product Developed by TESARO in the TESARO Field. Before issuing any such public statement that contains the name of or that could reasonably be construed to refer to Company or any of its Affiliates, TESARO shall provide Company with a copy of such public statement and reasonably consider the comments of Company. TESARO shall comply with any request by Company to remove any of its Confidential Information (except to the extent such information is required by Applicable Law to be disclosed). Company shall be solely responsible for making all public statements in respect of any recall, market suspension or market withdrawal of any Combination Product Developed by Company in the Company Field. Before issuing any such public statement that contains the name of or that could reasonably be construed to refer to TESARO or any of its Affiliates, Company shall provide TESARO with a copy of such public statement and reasonably consider the comments of TESARO. Company shall comply with any request by Company to remove any of its Confidential Information (except to the extent such information is required by Applicable Law to be disclosed). The provisions of Section 11.5 shall not apply to a public statement made under this Section 5.9(e).
5.10Subcontracting. Each Party may subcontract the performance of any activities conducted in accordance with this ARTICLE 5 to any of its Affiliates or any Third Party, provided that such Party shall oversee the performance of any subcontracted activities in a manner that would be reasonably expected to result in their successful and timely completion and shall remain responsible for the performance of such subcontracted activities in accordance with this Agreement.
COMMERCIALIZATION
6.1General. During the Term, Company shall have the right to Commercialize the Products in the Company Field in the Company Territory, and TESARO shall retain the right to Commercialize the Products in the TESARO Field in the TESARO Territory, in each case subject to the term and conditions of this ARTICLE 6. Certain aspects of the Parties’ Commercialization of the Products shall be subject be communicated through the JCC, as expressly described in ARTICLE 2 and this ARTICLE 6, to the extent permitted by Applicable Law, but neither Party’s Commercialization activities and decisions require the approval, consent or agreement of the JCC, JSC, any other Joint Committee, subcommittee, or the other Party except as expressly provided in this Agreement.
6.2Commercialization Activities.
(a)Company Commercialization Activities – In General. Subject to Sections 6.2(c) and 6.2(d), Company shall be solely responsible for and have sole authority
|
Confidential |
63 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
with respect to, at its own expense, all aspects of the Commercialization of the Products in the Company Field in the Company Territory, including all Fixed Dose Combination Products Developed by Company in the Company Field.
(b)TESARO Commercialization Activities – In General. TESARO shall be solely responsible for and have sole authority with respect to, at its own expense, all aspects of the Commercialization of the Products in the TESARO Field in the TESARO Territory.
(i)Notwithstanding anything to the contrary in this Agreement, TESARO shall be solely responsible for and have sole authority with respect to all aspects of the Distribution of Single Agent Products in the TESARO Field in the TESARO Territory and in the Company Field in the Company Territory. Company hereby grants TESARO, and TESARO hereby accepts, the exclusive authority and responsibility to Distribute each Single Agent Product in the Company Field in each country or jurisdiction where such Single Agent Product has received Marketing Approval for Monotherapy Use in the Company Field during the Term. Notwithstanding the foregoing, Company shall have the right and authority to Distribute a Single Agent Product in the Company Field in a country or jurisdiction to the extent permitted under Section 6.4. The JCC shall discuss and seek to reach alignment with respect to key aspects of Distribution of a Single Agent Product in countries or jurisdictions where such Single Agent Product has received Marketing Approval for Monotherapy Use in both the TESARO Field and Company Field.
(ii)Company shall be solely responsible for and have sole authority with respect to, at its own expense, all aspects of the Distribution of the Combination Products in the Company Field in the Company Territory.
(iii)In the event that a Party receives any orders for a Product in a country or jurisdiction where the other Party is responsible for Distributing, or that the other Party is responsible for Distributing, such Party shall refer such orders to the distributing Party.
(iv)TESARO shall prepare and maintain complete and accurate written records and accounts with respect to Distribution activities conducted pursuant to this Section 6.2(c) in conformity with Applicable Law and TESARO’s standard practices, provided that in no case shall such records be maintained for less than three (3) years following the Calendar Year to which such records pertain (or any longer period required by Applicable Law).
|
Confidential |
64 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(d)Pricing Matters.
(i)Subject to Section 6.2(d)(iv), TESARO shall be solely responsible for and shall have final decision-making authority with respect to all decisions regarding the prices charged and discounts, rebates and other sale and reimbursement terms and conditions (collectively, “Pricing Terms”) for the Single Agent Products in the Company Field in the Company Territory.
(ii)TESARO shall be solely responsible for and shall have final decision-making authority with respect to all decisions regarding Pricing Terms for the Products in the TESARO Field in the TESARO Territory.
(iii)Company shall be solely responsible for and shall have final decision-making authority with respect to all decisions regarding Pricing Terms for the Combination Products in the Company Field in the Company Territory.
(e)Commercialization Plans. Each Party shall submit to the JCC a high-level summary of its commercialization plans for Products on an annual basis. Each such summary shall include the following topics: Product labeling, contracted services, brand vision and lifecycle management, package design and configuration, positioning and messaging, branding and Trademarks. The JCC shall review and discuss such commercialization plans, and seek to reach alignment with respect to key Commercialization issues related to the Products reflected in such plans, such as Product labeling, strategic branding elements, brand security, brand integrity, customer targeting, product positioning and promotional programs (e.g. speaker programs and payer activities). For clarity, approval of a Party’s commercialization plan by the other Party, the JCC or JSC shall not be required.
6.3Commercialization Diligence Obligations.
(a)Following receipt of Marketing Approval for a Product in the Company Field in any country in the Company Territory, Company shall use Commercially Reasonable Efforts to Commercialize such Product in the Company Field in the Company Territory; provided, however, that in no event shall Company be obligated to use Commercially Reasonable Efforts to Commercialize more than one Product in any given country in the Company Territory.
(b)Following Company’s receipt of Marketing Approval for a Single Agent Product for Monotherapy Use in any Indication in the Company Field in a country or jurisdiction where such Single Agent Product has received Marketing Approval
|
Confidential |
65 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
for Monotherapy Use in the TESARO Field during the Term, except for any country or jurisdiction for which TESARO delivers a Monotherapy Registration & Distribution Notice stating that TESARO will not Distribute the Single Agent Product in the applicable country or jurisdiction, TESARO shall use Commercially Reasonable Efforts to Distribute such Single Agent Product for such Monotherapy Use in such Indication in the Company Field in such country or jurisdiction.
6.4Registration & Distribution Option. In the event that TESARO delivers a Monotherapy Registration & Distribution Notice stating that TESARO will not Distribute the Single Agent Product in the applicable country or jurisdiction, then Company shall have the right to Distribute the applicable Single Agent Product in the Company Field in such country or jurisdiction on the terms set forth in this Section 6.4. Company may exercise such right by providing written notice of such exercise to TESARO within thirty (30) days following receipt of such Monotherapy Registration & Distribution Notice and, upon exercise of such right, the following terms shall apply with respect to the applicable Single Agent Product and applicable country or jurisdiction:
(a)Company shall be solely responsible for and have sole authority with respect to all aspects of the Distribution of such Single Agent Product in the Company Field in such country or jurisdiction. Company shall prepare and maintain complete and accurate written records and accounts with respect to such Distribution activities conducted in conformity with Applicable Law and Company’s standard practices, provided that in no case shall such records be maintained for less than three (3) years following the Calendar Year to which such records pertain (or any longer period required by Applicable Law).
(b)Company shall be solely responsible for all decisions regarding the Pricing Terms for such Single Agent Product in the Company Field in such country or jurisdiction, including negotiating and obtaining pricing and reimbursement approvals with respect to such Single Agent Product in such country or jurisdiction. To the extent permitted by Applicable Law, the JCC shall review and discuss decisions relating such Pricing Terms.
(c)Company shall purchase from TESARO, and TESARO will Manufacture and supply to Company, Company’s requirements of such Single Agent Product for commercial sale in the Company Field in such country or jurisdiction at Manufacturing Cost [***]. The Parties shall negotiate in good faith and attempt to agree upon an amendment to the API Supply Agreement or a separate supply agreement for the supply of such Single Agent Product by TESARO to Company (and, if necessary, a related quality agreement).
(d)Upon TESARO’s written request at any time after Company’s exercise of its right to Distribute such Single Agent Product in the Company Field in such country or jurisdiction, Company shall transfer the responsibility for the Distribution of such
|
Confidential |
66 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Single Agent Product in such country or jurisdiction to TESARO. Such transfer shall be conducted as soon as reasonably practicable following TESARO’s request and shall be conducted in accordance with a transition plan to be mutually agreed upon by the Parties.
6.5Trademarks; Product Packaging.
(a)Company shall have sole responsibility, at its own expense, for all matters relating to the use of, and shall own, all Trademarks used in the sale of Combination Products in the Company Field in the Company Territory (the “Company Trademarks”) (but excluding the TESARO Trademarks and any trademark that is confusingly similar to a TESARO Trademark), including the selection, filing, prosecution, maintenance, defense and enforcement thereof.
(b)TESARO shall have sole responsibility, at its own expense, for all matters relating to the use of, and shall own, all trademarks used in the sale of Single Agent Products in any field in any territory and in the sale of Combination Products in the sale of Combination Products in the TESARO Field in the TESARO Territory (the “TESARO Trademarks”) (but excluding the Company Trademarks and any trademark that is confusingly similar to a Company Trademark), including the selection, filing, prosecution, maintenance, defense and enforcement thereof.
(c)To the extent permitted by Applicable Law: (i) on and after the first Marketing Approval is obtained in a country in the Company Territory for a Product in the Company Field, TESARO shall include Company’s name (or its local Affiliate’s name) with equal prominence, or as close thereto as permitted by Applicable Law, on all packaging and other materials related to such Product in such country; and (ii) Company shall include TESARO’s name (or its local Affiliate’s name) with equal prominence, or as close thereto as permitted by Applicable Law, on all packaging and other materials related to any Combination Product in each country in the Company Territory.
6.6Transparency Reporting. Company and TESARO shall each be responsible for tracking and reporting transfers of value initiated and controlled by its and its Affiliates’ employees, contractors, and agents pursuant to the requirements of the marketing reporting laws or research expense reporting laws of any Governmental Authority in the Company Territory, including Section 6002 of the U.S. Patient Protection and Affordable Care Act of 2010, commonly referred to as the “Sunshine Act.”
6.7Compliance with Law. In performing Commercialization activities with respect to the Compounds and Products, each Party will, and will cause its Affiliates and Third Party subcontractors to, comply with Applicable Law and, to the extent applicable to such Party’s Commercialization activities under this Agreement, the other Party’s health care compliance policies and procedures and any corporate integrity agreement to which the other Party is
|
Confidential |
67 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
subject. Each Party will provide the other Party with copies of any such health care compliance policies and procedures and corporate integrity agreements.
6.8Advertising and Promotional Materials. Each Party shall develop and approve its own written sales, promotion and advertising materials relating to Products (“Promotional Materials”) consistent with its standard operating procedures, for use in the Company Territory by such Party. All such Promotional Materials shall be compliant with all Applicable Law and the provisions of the applicable Regulatory Approvals. Company shall provide to TESARO, for TESARO’s regulatory compliance review and prior written approval according to procedures to be agreed upon by the Parties through the JCC (including a reasonable period of time in which such review must be completed, not to exceed five (5) days), copies of all Promotional Materials relating to a Product that Company plans to use in a country in which TESARO holds the Regulatory Approval for such Product. Company agrees that it shall not make use of such Promotional Materials until it has received the prior related written approval of TESARO in accordance with such procedures.
6.9Subcontracting. Each Party may subcontract the performance of any Commercialization activities conducted by such Party in accordance with this Agreement to any of its Affiliates or any Third Party, provided that (a) such Party shall oversee the performance of any subcontracted activities in a manner that would be reasonably expected to result in their successful and timely completion and shall remain responsible for the performance of such subcontracted activities in accordance with this Agreement[***].
6.10[***].
MANUFACTURING
(i)TESARO shall have the sole responsibility for and sole authority with respect to Manufacturing all Single Agent Products for use in Clinical Trials and other Development activities to be conducted by Company in the Company Field in the Company Territory. TESARO shall bear all costs of such Manufacturing, subject to the terms of this ARTICLE 7 and ARTICLE 8.
(ii)Promptly after the Effective Date, the Parties shall negotiate in good faith and attempt to agree within [***] after the Effective Date upon a clinical supply agreement (the “Clinical Supply Agreement”) for the supply of Single Agent Products by TESARO to Company and a related quality agreement. The Clinical Supply Agreement shall include provisions regarding allocation of Single Agent Products between Company and
|
Confidential |
68 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
TESARO in the event of shortages. Pursuant to the Clinical Supply Agreement, TESARO shall supply the Single Agent Product labeled in final packaging unless Company requests that Company applies the final packaging and labeling, at TESARO’s Manufacturing Cost [***]. Pursuant to the Clinical Supply Agreement or API Supply Agreement, TESARO shall also provide Company with Compound API for Company’s CMC Development needs, such as for FDC development, at TESARO’s Manufacturing Cost [***]. The related quality agreement with respect to the Clinical Supply Agreement shall include provisions allowing Company to audit the manufacturing sites (provided, that Company personnel are accompanied by TESARO personnel), to request remediation of deficiencies that are required to be remediated and to allow Company’s representatives to observe critical manufacturing observations with respect to the Single Agent Products.
(iii)Between the Effective Date and the effective date of the Clinical Supply Agreement, TESARO shall use reasonable efforts to Manufacture and supply to Company Products for use in Clinical Trials and other Development activities to be conducted by Company during that period as set forth on Exhibit 7.1(a).
(b)Commercial Supply. TESARO shall have the sole responsibility for and sole authority with respect to Manufacturing all Single Agent Products for use and sale by TESARO pursuant to Section 6.2(c) and by Company pursuant to Section 6.4 in the Company Field in the Company Territory. TESARO shall bear all costs of such Manufacturing, subject to the terms of this ARTICLE 7 and ARTICLE 8.
(c)Manufacturing Site Audits. Promptly after the Effective Date (and in no event more than thirty (30) days after the Effective Date), Company shall have the right to request a prompt audit of all manufacturing sites where Products and Compound API are manufactured by or for TESARO. TESARO shall facilitate the accommodation of such request; provided, that TESARO personnel shall accompany any Company personnel conducting such audit. Following the completion of such audit, Company may request the remediation of deficiencies that are not in compliance with GMP and identified by Company during such audit. In the case of any Critical Observation that TESARO or its contract manufacturer cannot or will not remediate, Company will have the right to request the use of different manufacturers for the supply of Product or Compound API for the Company’s Clinical Trials.
(d)CMC Development. TESARO shall have the sole responsibility for and sole authority with respect to CMC Development of all Single Agent Products at its own cost.
|
Confidential |
69 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
7.2Fixed Dose Combination Products.
(a)Clinical and Commercial Supply. Company shall have the sole responsibility for and sole authority with respect to Manufacturing all Fixed Dose Combination Products (i) for use in Clinical Trials and other Development activities to be conducted by Company in the Company Field in the Company Territory and (ii) for use and sale by Company in the Company Field in the Company Territory. Company shall bear all costs of such Manufacturing, subject to the terms of ARTICLE 8. TESARO shall have the right to request and conduct an audit of all manufacturing sites where all Fixed Dose Combination Products are manufactured by or for Company only if, and to the extent that, TESARO has the right under this Agreement to use such Fixed Dose Combination Products in a Clinical Trial sponsored by TESARO or to sell such Fixed Dose Combination Products under its trade name. Company shall facilitate the accommodation of such request; provided, that Company personnel shall accompany any TESARO personnel conducting such audit. Following the completion of such audit, TESARO may request the remediation of deficiencies that are not in compliance with GMP and identified by TESARO during such audit. In the case of any Critical Observation that Company or its contract manufacturer cannot or will not remediate, TESARO will have the right to request the use of different manufacturers for the supply of Fixed Dose Combination Products.
(b)CMC Development. Company shall have the sole responsibility for and sole authority with respect to CMC Development of all Fixed Dose Combination Products at its own cost.
7.3API Supply.
(a)General. Except as provided in this ARTICLE 7 or the API Supply Agreement, TESARO shall have the sole responsibility for and sole authority with respect to Manufacturing all Compounds for use as active pharmaceutical ingredients (“Compound API”) in any Products to be Manufactured by either Party for use in the Company Field in the Company Territory; provided, however, that Company shall have the right to Manufacture Compound API, or through its Affiliates or Third Party contractors, for Company’s use in Manufacturing any FDCs Developed by Company in the Company Field.
(b)API Supply Agreement. The Parties shall negotiate in good faith and attempt to agree within [***] after the Effective Date upon a supply agreement for the supply of Compound API by TESARO to Company at TESARO’s Manufacturing Cost [***] (except API supplied for Company’s CMC or clinical Development and technology transfer needs will be at TESARO’s Manufacturing Cost [***]) (the “API Supply Agreement”) and a related quality agreement. The API Supply Agreement shall include: (i) technology transfer obligations of TESARO and
|
Confidential |
70 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Technology Transfer Costs to be paid by Company, its Affiliates and Third Party contractors; and (ii) provisions regarding allocation of Compound API between Company and TESARO in the event of shortages, which shall be consistent with Section 7.7 unless otherwise agreed by the Parties. Additionally, the Parties may agree that Company or its Affiliates (directly or indirectly through a Third Party) shall serve as a second source supplier of Single Agent Products or Compound API for use in the Manufacture of Single Agent Products, in which case TESARO would have audit rights with respect to all manufacturing sites where Compound API is manufactured for use in Single Agent Products used in Clinical Trials sponsored by TESARO or sold by TESARO under its trade name.
(c)Interim API Supply. Between the Effective Date and the effective date of the API Supply Agreement, TESARO shall use reasonable efforts to Manufacture and supply to Company all of Company’s requirements of Compound API for use (i) in CMC Development activities of Company conducted pursuant to this ARTICLE 7 and (ii) in Manufacturing by Company of Fixed Dose Combination Products for use in Clinical Trials conducted by Company pursuant to this Agreement, as set forth on Exhibit 7.1(a).
(d)Guaranteed Volume of Compound API and Clinical Supply. TESARO shall, at a minimum, Manufacture and supply to Company [***] of its requirements of Single Agent Product for Company’s planned Phase 1 Clinical Trial, [***] of its requirements of Single Agent Product for the run-in phase of Company’s planned Phase 2 Clinical Trial, [***] of its requirements of Single Agent Product for the Registration Phase Trial for Monotherapy Use and [***] of its requirements of Single Agent Product for Company’s planned Phase 2 and Phase 3 Clinical Trials for Combination Use, as such requirements are set forth on Exhibit 7.1(a). TESARO shall use reasonable efforts to provide all such supplies no later than [***] prior to the anticipated date of dosing of the first patient in the applicable Clinical Trial.
7.4Joint Manufacturing Committee.
(a)Formation and Purpose. The Parties agree to establish and convene the JMC within thirty (30) days after the Effective Date. The JMC shall consist of representatives from each Party as further described in Section 2.6(a) and operate in accordance with this Section 7.4 and Section 2.6. The purpose of the JMC shall be to serve as a forum for the Parties to discuss and share information regarding the Manufacture and CMC Development of the Products and Compound API, and to determine, approve or resolve only those matters with respect to such Manufacture and CMC Development that the JMC is expressly authorized to determine, approve or resolve, pursuant to this ARTICLE 7. Neither Party’s Manufacturing and CMC Development activities and decisions require the approval, consent or agreement of the JMC, JSC, any other Joint
|
Confidential |
71 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Committee, subcommittee, or the other Party except as expressly provided in this Agreement, the Clinical Supply Agreement or the API Supply Agreement.
(b)Responsibilities of the JMC. The JMC shall:
(i)review and discuss annual capacity planning and supply continuity plans for the Single Agent Products and Compound API, including allocation of Single Agent Products and Compound API in the event of shortages and the possibility of Company or its Affiliates (directly or indirectly through a Third Party) serving as a second supplier of Single Agent Products or Compound API for us in the Manufacture of Single Agent Products;
(ii)consult with the JDC regarding clinical supply manufacturing;
(iii)review and discuss potential changes in Third Party suppliers and subcontractors, including the potential benefits to the Parties of Company’s participation in TESARO’s contract negotiations with such Third Parties; and
(iv)perform other obligations specifically delegated to it and permitted under this Agreement.
(c)JMC Decisions and Actions. The JMC shall not have any formal decision-making authority, except with respect to the following matters:
(i)The JMC shall discuss and approve an allocation mechanism in the event of anticipated or actual shortages of Single Agent Products or Compound API in accordance with Section 7.7.
(ii)If the JMC does not reach unanimous agreement with respect to the existence of an anticipated or actual shortage of Single Agent Products or Compound API within thirty (30) Business Days from the date that the matter is first presented to the JMC, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, TESARO shall have final decision-making authority with respect to such Deadlocked Committee Matter.
(iii)If the JMC reaches unanimous agreement with respect to the existence of an anticipated or actual shortage of Single Agent Products or Compound API but does not reach unanimous agreement with respect to such allocation mechanism within thirty (30) Business Days from the date that the matter is first presented to the JMC, such matter shall be resolved in accordance with Section 7.7.
|
Confidential |
72 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Actions to be taken and decisions to be made by the JMC with respect to such matters shall be taken or made by unanimous agreement, with each Party’s JMC representatives collectively having one (1) vote.
7.5Compliance with Law. The Parties will, and will cause their Affiliates and Third Party subcontractors and (sub)licensees to, comply with Applicable Law in performing Manufacturing activities with respect to the Compounds and Products, including GMP.
7.6Subcontracting; Audit Rights. Each Party may subcontract the performance of any Manufacturing or CMC Development activities conducted in accordance with this Agreement to any of its Affiliates or any Third Party, provided that such Party shall oversee the performance of any subcontracted activities in a manner that would be reasonably expected to result in their successful and timely completion and shall remain responsible for the performance of such subcontracted activities in accordance with this Agreement. Company shall have the right to audit the performance of any Third Party suppliers, contractors and subcontractors used by TESARO in the Manufacturing of Single Agent Products, Compound API or Fixed Dose Combination Products, as applicable, for use by either Party in the Company Field in accordance with this Agreement. TESARO shall have the right to audit the performance of any Third Party suppliers, contractors and subcontractors used by Company in the Manufacturing of Single Agent Products, Compound API or Fixed Dose Combination Products, as applicable, for use by TESARO only if, and to the extent that, TESARO has the right under this Agreement to use such Single Agent Products or Fixed Dose Combination Products in a Clinical Trial sponsored by TESARO, to sell such Single Agent Products or Fixed Dose Combination Products under its trade name or to use such Compound API in the Manufacturing of Single Agent Products for such use or sale. The terms of such audit rights will be negotiated by the Parties in good faith and set forth in the quality agreements described in this ARTICLE 7 (with respect to Single Agent Product clinical supply and Compound API supply) and in separate quality agreements, but in any event such terms shall include the following: (a) following the completion of an audit, a Party may request the remediation of deficiencies identified by the other Party during such audit and any Critical Observations shall be remediated by the applicable Third Party supplier, contractor or subcontractor; (b) in the event that the applicable Third Party supplier, contractor or subcontractor cannot or will not agree to remediate a Critical Observation (as determined under Applicable Law) identified during an audit, the auditing Party will have the right to request that the other Party engage a different Third Party supplier, contractor or subcontractor with respect to any Manufacturing or CMC Development activities conducted in accordance with this Agreement; and (c) the auditing Party shall be permitted to observe key manufacturing events during the Manufacturing process.
7.7Supply Shortages. In the event TESARO becomes aware of anticipated or actual shortages of Single Agent Products or Compound API, TESARO shall [***] notify Company of such shortage. In the event Company reasonably believes that there is an anticipated or actual shortage of Single Agent Products or Compound API, Company shall notify TESARO of such belief. Subject to Section 7.4(c), after either Party gives such notice to the other Party, the JMC shall meet promptly thereafter to (x) determine whether an anticipated or actual shortage exists
|
Confidential |
73 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
and (y) if so, agree upon a mechanism for allocating between the Parties the available quantities of Single Agent Product or Compound API, as applicable (including any Compound API being Manufactured by Company). If the JMC does not agree upon an allocation mechanism within thirty (30) Business Days from the date that Company is notified of such shortage, then the available quantities of Single Agent Product or Compound API, as applicable, shall be allocated in accordance with the following principles: [***]. If the JMC does not reach unanimous agreement on an allocation mechanism within thirty (30) Business Days from the date that Company is notified of such shortage and the JMC does not reach unanimous agreement on a specific allocation mechanism consistent with the foregoing allocation principles within ten (10) Business Days of such thirtieth (30th) Business Day, such matter will be a Deadlocked Committee Matter. Either Party may refer such matter to the JSC for attempted resolution pursuant to the Escalation Procedures. If the matter is not resolved pursuant to the Escalation Procedures, [***]. Notwithstanding the foregoing or anything else in this Agreement to the contrary, in the event of an actual shortage of Single Agent Products or Compound API, during the pendency of any Escalation Procedure initiated under this Section 7.7, to the extent necessary, TESARO may implement an allocation procedure consistent with the allocation principles set forth in this Section 7.7.
CONSIDERATION
8.1Upfront Payment. On or after the Effective Date, TESARO shall submit an invoice to Company for Thirty-Five Million Dollars ($35,000,000) as a one-time, non-refundable, non-creditable upfront payment. Such invoice shall be due and payable within [***] after receipt by Company.
8.2Milestone Payments. Company will notify TESARO within [***] following the achievement by Company, its Affiliate or sublicensee of each Development Milestone, each Regulatory Milestone and each Sales Milestone as applicable (collectively, the “Milestone Events”). Within [***] after such achievement of each such Milestone Event, Company shall remit the applicable Development Milestone Payment, Regulatory Milestone Payment or Sales Milestone Payment (collectively, the “Milestone Payments”) to TESARO. Each Milestone Payment by Company pursuant to this Section 8.2 shall be payable only once.
(a)Development Milestones. The following payments (each, a “Development Milestone Payment”) shall be made on a one-time basis with respect to the first Product to achieve the corresponding milestone event (each, a “Development Milestone”):
|
Confidential |
74 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Development Milestone |
Development |
|
1.[***] |
$[***] |
|
2.[***] |
$[***] |
|
3.[***] |
$[***] |
|
4.[***] |
$[***] |
[***]. Additionally, each Development Milestone Payment may be earned upon the achievement of an alternative Development Milestone in accordance with Section 4.3(b).
(b)Regulatory Milestones. The following payments (each, a “Regulatory Milestone Payment”) shall be made on a one-time basis with respect to the first Product to achieve the corresponding milestone event (each, a “Regulatory Milestone”):
|
Regulatory Milestone |
Regulatory Milestone Payment (USD) |
|
|
|
FDA |
EMA |
|
1.[***] |
$[***] |
$[***] |
|
2.[***] |
$[***] |
$[***] |
|
3.[***] |
$[***] |
$[***] |
|
4.[***] |
$[***] |
$[***] |
|
5.[***] |
$[***] |
$[***] |
|
6.[***] |
$[***] |
$[***] |
(c)Sales Milestones. The following payments (each, a “Sales Milestone Payment”) shall each be payable to TESARO one-time only, upon the first time during the Term that the annual Territory-Wide Prostate Net Sales of Products in the Company Territory in any Calendar Year during the applicable Royalty Terms for such Products exceed the amounts set forth in the following table (each, a “Sales Milestone”):
|
Annual Territory-Wide Prostate Net Sales Milestones |
|
|
Sales Milestone |
Sales Milestone |
|
Upon the first occasion that annual Territory-Wide Prostate Net Sales of Products in the Company Territory in a Calendar Year exceeds $[***] |
$[***] |
|
Upon the first occasion that annual Territory-Wide Prostate Net Sales of Products in the Company Territory in a Calendar Year exceeds $[***] |
$[***] |
|
Upon the first occasion that annual Territory-Wide Prostate Net Sales of Products in the Company Territory in a Calendar Year exceeds $[***] |
$[***] |
|
Confidential |
75 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
If more than one Sales Milestone described in this Section 8.2(c) is achieved during the same Calendar Year, Company shall pay TESARO each Sales Milestone Payment that corresponds to such Sales Milestones. For purposes of clarity, only one Sales Milestone Payment shall be owed on the first occasion that annual Territory-Wide Prostate Net Sales of Products in the Company Territory in a Calendar Year exceeds $[***], $[***] or $[***].
(d)Merck Milestones. The Parties acknowledge that TESARO shall be responsible for making any milestone payments that become payable under the Merck License Agreement with respect to Company’s activities hereunder. Notwithstanding the foregoing, if [***] any of the development milestones set forth on Exhibit 8.2(d) (each, a “Merck Milestone”) are attained [***] and (2) the corresponding milestone payment set forth on Exhibit 8.2(d) becomes payable by TESARO to Merck pursuant to Section 7.02 of the Merck Agreement (each, a “Merck Milestone Payment”), then [***].
8.3Single Agent Alliance Payments.
(a)General. TESARO shall pay to Company certain other amounts (“Alliance Payments”) for all Prostate-Approved Single Agent Products that are sold at any time during the Term by or on behalf of TESARO, its Affiliates, licensees (other than Company) or sublicensees in the Company Territory calculated in accordance with Exhibit 8.3 and paid quarterly as set forth below in this Section 8.3.
(i)Within [***] after the end of each TESARO Calendar Quarter, TESARO shall perform the calculations described in Exhibit 8.3, and prepare and deliver to Company the Alliance Payments Report described in Exhibit 8.3. Each Alliance Payments Report shall be the Confidential Information of each Party. Procedures for quarterly reporting of actual results, and review and discussion of potential discrepancies, quarterly reconciliation, reasonable forecasting, and other finance and accounting matters, to the extent not set forth in Exhibit 8.3, will be established by the JFC. Such procedures will provide the ability to comply with financial reporting requirements of each Party. Such report shall also include the
|
Confidential |
76 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
exchange rates and other methodology used in converting Net Sales into U.S. Dollars from the currencies in which such sales were made. In addition, the report shall provide a reasonable allocation of the information contained therein between U.S. and non-U.S. activity (including any TESARO Incremental Royalties or Pass-through Third Party Royalty amounts due with respect to TESARO Prostate-Approved Single Agent Product Net Sales).
(ii)Within [***] after the end of each Calendar Quarter (except, with respect to the fourth Calendar Quarter of a Calendar Year, within [***] after the end of such Calendar Quarter) (or, if Company Parent delivers a public earnings report within any such [***] period, within three (3) calendar days following the delivery of such earnings report, if later), the Company shall provide to TESARO a report of total Company Prostate-Approved Single Agent Product Net Sales and Company Combination Product Net Sales for the Calendar Quarter. In the event that Company Parent determines that it will deliver its public earnings report later than [***] after the end of a Calendar Quarter, Company shall promptly notify TESARO and the Parties shall mutually agree upon a reasonable extension to the deadline for the delivery of the report described in this Section 8.3(b)(ii), provided that such extension shall take into account TESARO’s obligation to deliver royalty reports to Merck within forty-five (45) days following the end of each TESARO Calendar Quarter.
(c)Payment of Alliance Payments. Following receipt of an Alliance Payments Report, (i) if the Alliance Payments set forth therein are greater than [***], Company shall invoice TESARO for the Alliance Payments set forth therein, and TESARO shall pay such invoice, within [***] after receipt in accordance with Section 8.14 and (ii) if the Alliance Payments are less than [***], TESARO shall invoice Company for the Alliance Payments set forth therein, and Company shall pay such invoice, within [***] after receipt in accordance with Section 8.14. Notwithstanding the foregoing, if Company initiates an investigation following receipt of an Alliance Payments Report in accordance with Section 8.3(d), then the due date for paying such invoice shall be extended by the longer of (i) [***] and (ii) the number of days it takes to complete the investigation.
(d)Errors in Alliance Payments Reports. If Company reasonably believes that there is an error in an Alliance Payments Report (or the calculations underlying such Alliance Payments Report), Company shall so notify TESARO within [***of receipt of such Alliance Payments Report. The Parties shall meet promptly after such notice is given to investigate the potential error. If such investigation confirms or reveals an error, TESARO shall correct and re-issue the Alliance Payments Report within [***]. If Company has already issued an invoice to TESARO with respect to the applicable TESARO Calendar Quarter, then
|
Confidential |
77 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
promptly following receipt of such re-issued Alliance Payments Report, Company shall issue an invoice or a credit note, as applicable, equal to the amount of the difference between the Alliance Payments set forth in the re-issued report and the Alliance Payments set forth in Company’s original invoice for such TESARO Calendar Quarter and, if applicable, TESARO shall pay such invoice in accordance with Section 8.3(c). If Company has not yet issued an invoice to TESARO with respect to the applicable TESARO Calendar Quarter, then Company shall use the re-issued Alliance Payments Report to prepare the invoice for such Calendar Quarter in accordance with Section 8.3(c).
(e)Alliance Payments Forecasts. For each TESARO Calendar Year, TESARO shall prepare and deliver to Company a forecast for the next TESARO Calendar Year containing a good faith estimate of the Alliance Payments, including all deductions, for each TESARO Calendar Quarter (the “Annual Alliance Payment Forecast”). The timing of such Annual Alliance Payment Forecast shall be agreed to by the JFC. Each Annual Alliance Payment Forecast shall be the Confidential Information of each Party. The JFC may agree upon additional information to be included in each Annual Alliance Payment Forecast. The Parties shall also develop, through the JFC, a process to agree upon the estimated Alliance Payments for a TESARO Calendar Quarter before the close of such TESARO Calendar Quarter.
8.4Company Royalty Obligations.
(a)Definitions. For purposes of this Agreement, the following terms shall have the following meanings:
(i)“Combined Worldwide Prostate-Approved Single Agent Product Net Sales” means, with respect to any Prostate-Approved Single Agent Product sold by either Party or any of its Affiliates or (sub)licensees in the Company Territory in a particular TESARO Calendar Quarter or Calendar Quarter, respectively, the sum of: (A) the total TESARO Prostate-Approved Single Agent Product Net Sales for such Prostate-Approved Single Agent Product in all countries in the Company Territory during the corresponding TESARO Calendar Quarter; (B) the total TESARO Net Sales of such Prostate-Approved Single Agent Product in the TESARO Field in all countries in the TESARO Territory during the corresponding TESARO Calendar Quarter; and (C) the total Company Prostate-Approved Single Agent Product Net Sales for such Prostate-Approved Single Agent Product in the Company Field all countries in the Company Territory during such Calendar Quarter.
(ii)“Company Combination Product Net Sales” means, with respect to any Combination Product sold by Company or any of its Affiliates or
|
Confidential |
78 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(sub)licensees in any country in the Company Territory in a particular Calendar Quarter, the total Net Sales of such Combination Product by Company or any of its Affiliates or sublicensees in such country during such Calendar Quarter.
(iii)“Company Prostate-Approved Single Agent Product Net Sales” means, with respect to a Prostate-Approved Single Agent Product sold by Company or any of its Affiliates or (sub)licensees in a country in the Company Territory in a particular Calendar Quarter pursuant to Section 6.4, the total Net Sales of such Prostate-Approved Single Agent Product by Company or its Affiliates or (sub)licensees in such country during such Calendar Quarter.
(iv)“Prostate-Approved Single Agent Product” means, with respect to a country in the Company Territory, any Single Agent Product: (A) that has received Marketing Approval (which, for purposes of this definition, means Marketing Approval as defined in Section 1.79 but without respect to the pricing and reimbursement approvals referred to in clause (b) of Section1.79); (B) if such country is the U.S., the use of which for an Indication in the Company Field is included, or approved for inclusion, in a compendium recognized by the Centers for Medicare & Medicaid Services; or (C) that has been distributed for an Indication in the Company Field as part of an early access program operated by either Party in such country on a named patient basis or early access clinical trial basis.
(v)“Prostate-Approved Single Agent Product Net Sales” means, with respect to any Prostate-Approved Single Agent Product in a country in the Company Territory in a particular TESARO Calendar Quarter or Calendar Quarter, respectively, the amount equal to both: (A) if TESARO or its Affiliates, licensees (other than Company) or sublicensees sold such Prostate-Approved Single Agent Product in such country during such TESARO Calendar Quarter, the TESARO Prostate-Approved Single Agent Product Net Sales with respect to such Prostate-Approved Single Agent Product in such country during the corresponding TESARO Calendar Quarter; and (B) if Company or any of its Affiliates or sublicensees sold such Prostate-Approved Single Agent Product in such country during such Calendar Quarter in accordance with Section 6.4, the Company Prostate-Approved Single Agent Product Net Sales in such country during such Calendar Quarter.
(vi)“Territory-Wide Company Combination Product Net Sales” means, with respect to any Combination Product sold by Company or any of its Affiliates or (sub)licensees in the Company Territory in a particular Calendar Quarter, the total Company Combination Product Net Sales for
|
Confidential |
79 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
such Combination Product in all countries in the Company Territory during such Calendar Quarter.
(vii)“Territory-Wide Prostate Net Sales” means, with respect to a particular Calendar Quarter, the amount equal to the sum of (A) the TESARO Prostate-Approved Single Agent Product Net Sales for all Prostate-Approved Single Agent Products sold by TESARO or any of its Affiliates or (sub)licensees in the Company Territory during such TESARO Calendar Quarter; (B) the Company Prostate-Approved Single Agent Product Net Sales for all Prostate-Approved Single Agent Products sold by Company or any of its Affiliates or (sub)licensees in the Company Territory during such Calendar Quarter pursuant to Section 6.4; and (C) the Territory-Wide Company Combination Product Net Sales for all Combination Products sold by Company or any of its Affiliates or (sub)licensees in the Company Territory during such Calendar Quarter.
(viii)“TESARO Prostate-Approved Single Agent Product Net Sales” means, with respect to a Prostate-Approved Single Agent Product sold by TESARO or any of its Affiliates or (sub)licensees (other than Company) in a country in the Company Territory in a particular TESARO Calendar Quarter, the total Net Sales of such Prostate-Approved Single Agent Product attributable to use in the Company Field, as determined under and further defined in Exhibit 8.3 (including any prior Calendar Quarter adjustments made pursuant to Exhibit 8.3).
(b)Royalty Rates.
(i)Pass-through Third Party Royalties.
(1)Merck. In the event that royalties are payable by TESARO to Merck with respect to any Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales under the Merck License Agreement, Company shall pay to TESARO royalties on a Product-by-Product basis at the rates set forth in the following table (the “Pass-through Merck Royalty”) with respect to (A) Prostate-Approved Single Agent Product Net Sales of each Prostate-Approved Single Agent Product in each country in the Company Territory during the Merck Royalty Term for such Single Agent Product and (B) Company Combination Product Net Sales of each Combination Product in each country in the Company Territory during the Merck Royalty Term for such Combination Product in such country, in each case ((A) and (B)), calculated in accordance with the terms set forth on Exhibit
|
Confidential |
80 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
8.4(b)(i)(1) and either deducted or paid as set forth in Section 8.7(a):
|
Name of Merck |
Combined Worldwide Prostate-Approved |
Pass-through Merck |
|
Tier 1 |
On the portion of Combined Worldwide Prostate-Approved Single Agent Product Net Sales of the relevant Product or Territory-Wide Company Combination Product Net Sales of the relevant Product, as applicable, in a Calendar Year less than or equal to $[***] |
[***]% |
|
Tier 2 |
On the portion of Combined Worldwide Prostate-Approved Single Agent Product Net Sales of the relevant Product or Territory-Wide Company Combination Product Net Sales of the relevant Product, as applicable, in a Calendar Year greater than $[***] and less than or equal to $[***] |
[***]% |
|
Tier 3 |
On the portion of Combined Worldwide Prostate-Approved Single Agent Product Net Sales of the relevant Product or Territory-Wide Company Combination Product Net Sales of the relevant Product, as applicable, in a Calendar Year greater than $[***] |
[***]% |
For clarity, the amount of the Pass-through Merck Royalty for a given Prostate-Approved Single Agent Product is calculated by multiplying the applicable Pass-through Merck Royalty Rate by the Prostate-Approved Single Agent Product Net Sales of such Product, not by the Combined Worldwide Prostate-Approved Single Agent Product Net Sales of such Product.
(2)AstraZeneca. In the event that royalties are payable by TESARO to AstraZeneca with respect to any Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales under either of the AstraZeneca License Agreements, Company shall pay to TESARO, on a Product-by-Product basis: (A) a royalty of [***] (the “Pass-through AstraZeneca-ICR Royalty”) with respect to (i) Prostate-Approved Single Agent Product Net Sales for each Prostate-Approved Single Agent Product in each country in the Company Territory during the AstraZeneca-ICR Royalty Term for such Single Agent Product in such country and (ii) Company Combination Product Net Sales of
|
Confidential |
81 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
each Combination Product in each country in the Company Territory during the AstraZeneca-ICR Royalty Term for such Combination Product in such country; and (B) a royalty of [***] (the “Pass-through AstraZeneca-Sheffield Royalty”) with respect to (i) Prostate-Approved Single Agent Product Net Sales for each Prostate-Approved Single Agent Product in each country in the Company Territory during the AstraZeneca-Sheffield Royalty Term for such Single Agent Product in such country and (ii) Company Combination Product Net Sales of each Combination Product in each country in the Company Territory during the AstraZeneca-Sheffield Royalty Term for such Combination Product in such country, in each case ((A) and (B)), in accordance with the terms set forth on Exhibit 8.4(b)(i)(2) and either deducted or paid as set forth in Section 8.7(a).
(ii)TESARO Incremental Royalties. Company shall pay to TESARO royalties at the rates set forth in the following table (the “TESARO Incremental Royalty”) with respect to (A) Prostate-Approved Single Agent Product Net Sales of each Single Agent Product in each country in the Company Territory during the Royalty Term for such Single Agent Product and (B) Company Combination Product Net Sales of each Combination Product in each country in the Company Territory during the Royalty Term for such Combination Product in such country, in each case ((A) and (B)), calculated in accordance with the terms set forth in Sections 8.4(d), 8.5 and 8.6 and either deducted or paid as set forth in Section 8.7(a):
|
Territory-Wide Prostate Net Sales |
TESARO |
|
|
Tier 1 |
On the portion of Territory-Wide Prostate Net Sales, in a Calendar Year less than or equal to $[***] |
[***]% |
|
Tier 2 |
On the portion of Territory-Wide Prostate Net Sales, in a Calendar Year greater than $[***] and less than or equal to $[***] |
[***]% |
|
Tier 3 |
On the portion of Territory-Wide Prostate Net Sales, in a Calendar Year greater than $[***] |
[***]% |
(c)Annual Royalty Payment Forecasts. For each Calendar Year, each Party shall prepare and deliver to the other Party a forecast for the next Calendar Year containing: (i) in Company’s forecast, a good faith estimate of the total Prostate-Approved Single Agent Product Net Sales in all countries in the Company Territory during such Calendar Year; and (ii) in TESARO’s forecast, the forecasted Pass-through Merck Royalty Rate(s) (and, if applicable, the forecasted
|
Confidential |
82 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Effective Merck Royalty Rate(s) determined in accordance with in Exhibit 8.4(b)(i)(1)) for each TESARO Calendar Quarter of the TESARO Calendar Year that corresponds to such Calendar Year (the “Annual Royalty Payment Forecast”). The timing of such Annual Royalty Payment Forecast shall be agreed to by the JFC. Each Annual Royalty Payment Forecast shall be the Confidential Information of each Party. The JFC may agree upon additional information to be included in each Annual Royalty Payment Forecast.
(d)No Multiple Royalties. For each Net Sale of a Prostate-Approved Single Agent Product or Combination Product, the Company shall be obligated to pay no more than one Pass-through Merck Royalty, one Pass-through AstraZeneca-ICR Royalty, one Pass-through AstraZeneca-Sheffield Royalty and one TESARO Incremental Royalty under Section 8.3 and this Section 8.4.
8.5Royalty Term. The TESARO Incremental Royalty under Section 8.4(b)(ii) with respect to Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales, as applicable, shall be payable on a country-by-country basis during the applicable Royalty Term with respect to such Product in such country. Following the expiration of the Royalty Term with respect to a Product in a country of the Company Territory, subject to the terms and conditions of this Agreement, Company shall have a perpetual, irrevocable, non-exclusive, fully paid-up and royalty-free right and license, with the right to grant sublicenses without restriction, under the TESARO Technology and TESARO Trademarks to Exploit such Product in the Company Field in such country of the Company Territory.
8.6Royalty Rate Reduction. The TESARO Incremental Royalty Rates shall be reduced (a) by [***], on a country-by-country basis and Product-by-Product basis, in each country in which, at any given time, no Valid Claim of a TESARO Patent Covers the applicable Product in such country and (b) by [***], on a country-by-country basis and Product-by-Product basis, in each country in which, at any given time, there is competition for the applicable Product in such country from a Generic Version of such Product. With respect to the Fixed Dose Combination Product or another Combination Product, the reduction to the royalty set forth in Section 8.6(b) shall apply only when there is a Generic Version of the entire Product (i.e., all active pharmaceutical ingredients within such Product are approved as a Generic Version and a Generic Version of the Product itself has been approved).
(a)Pass-through Merck Royalty, Pass-through AstraZeneca-ICR Royalty, Pass-through AstraZeneca-Sheffield Royalty and TESARO Incremental Royalty amounts due with respect to TESARO Prostate-Approved Single Agent Product Net Sales will be deducted by TESARO when calculating the Alliance Payments due to Company, as set forth on Exhibit 8.3, and will not be payable by Company to TESARO pursuant to this Section 8.7. Pass-through Merck Royalty, Pass-through AstraZeneca-ICR Royalty, Pass-through AstraZeneca-Sheffield Royalty
|
Confidential |
83 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
and TESARO Incremental Royalty amounts due with respect to Company Prostate-Approved Single Agent Product Net Sales and Company Combination Product Net Sales will be payable by Company to TESARO pursuant to this Section 8.7.
(b)Within [***] following the end of each Calendar Quarter after the commencement of the Royalty Term for a Product in the Company Territory (or, if such Calendar Quarter is the fourth Calendar Quarter of a Calendar Year, the later of (1) [***] following the end of such Calendar Quarter or (2) [***] after the Merck Royalty Rate or Effective Merck Royalty Rate is reported by TESARO to Company pursuant to Exhibit 8.4(b)(i)(1)), Company shall provide TESARO with a report (a “Royalty Payment Report”) setting forth, on a Product-by-Product and country-by-country basis: (i) Company Prostate-Approved Single Agent Product Net Sales for each Prostate-Approved Single Agent Product and Company Combination Product Net Sales for each Combination Product in such country; (ii) the applicable Pass-through Merck Royalty, Pass-through AstraZeneca-ICR Royalty, Pass-through AstraZeneca-Sheffield Royalty and TESARO Incremental Royalty with respect to such Net Sales; and (iii) the total royalty amount due with respect to such Net Sales. Such report shall also include: (A) the gross sales of each Product on a country-by-country basis, (B) with respect to the U.S., to the extent available within the Company’s Records a reasonably detailed statement of any deductions taken from Company Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales taken from such gross sales to arrive at Company Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales, as applicable, for the applicable Calendar Quarter, (C) with respect to countries other than the U.S., the aggregate amount of deductions taken from Company Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales taken from such gross sales to arrive at Company Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales, as applicable, in such country for the applicable Calendar Quarter, (iv) the exchange rates and other methodology used in converting Net Sales into U.S. Dollars from the currencies in which such sales were made for purposes of calculating the appropriate royalty rate and the royalty payment due, and (v) the application of the reductions, if any, made in accordance with the terms of Section 8.6 or Section 8.9. Company shall pay all amounts due to TESARO pursuant to Section 8.4 with respect to Company Prostate-Approved Single Agent Product Net Sales and Company Combination Product Net Sales for such Calendar Quarter in U.S. Dollars at the time the submission of such quarterly report is due.
(c)If TESARO reasonably believes that there is an error in a Royalty Payment Report (or the calculations underlying such Royalty Payment Report), TESARO shall so notify Company within [***] of receipt of such Royalty Payment Report. The Parties shall meet promptly after such notice is given to investigate the
|
Confidential |
84 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
potential error. If such investigation confirms or reveals an error, Company shall correct and re-issue the Royalty Payment Report within [***]. If TESARO has already issued an invoice to Company with respect to the applicable Calendar Quarter, then promptly following receipt of such re-issued Royalty Payment Report, TESARO shall issue an invoice or a credit note, as applicable, equal to the amount of the difference between the royalty payment due as set forth in the re-issued report and the royalty payment due as set forth in TESARO’s original invoice for such Calendar Quarter and, if applicable, Company shall pay such invoice in accordance within [***] after receipt. If TESARO has not yet issued an invoice to Company with respect to the applicable Calendar Quarter, then TESARO shall use the re-issued Royalty Payment Report to prepare the invoice for such Calendar Quarter in accordance with this Section 8.7.
(a)All payments under this Agreement shall be payable in U.S. Dollars.
(b)For the Company, with respect to sales of a Product invoiced in a currency other than U.S. Dollars, such amounts and the amounts payable hereunder shall be expressed in their U.S. Dollars equivalent calculated using the Currency Hedge Rates. For each Calendar Year during which royalties become due under Section 8.4, Company shall provide TESARO the Currency Hedge Rate to be used for the local currency of each country in the Company Territory in which any royalty-bearing Net Sales are expected to occur, in writing no later than [***] after the Currency Hedge Rate(s) are available [***], which is customarily [***]. Each Currency Hedge Rate will remain constant throughout the upcoming Calendar Year. Company shall use the Currency Hedge Rate(s) to convert Net Sales to U.S. Dollars for the purpose of calculating royalty payments hereunder.
(c)For TESARO, in the case of sales outside the U.S., payments received and payments to be made by TESARO will be expressed in the U.S. Dollar equivalent calculated on a TESARO Calendar Quarterly basis in the currency of the country of sale and converted to their U.S. Dollar equivalent using the average rate of exchange over the applicable TESARO Calendar Quarter to which the sales relate, in accordance with GAAP and the then current standard methods of TESARO, to the extent reasonable and consistently applied; provided, however, that if, at such time, TESARO does not use a rate for converting into U.S. Dollar equivalents that is maintained in accordance with GAAP, then TESARO shall use a rate of exchange which corresponds to the official market closing rate of exchange for such currency reported by the Wall Street Journal as of the last day of the applicable reporting period (or, if unavailable on such date, the first date thereafter on which such rate is available). TESARO will inform Company as to the specific exchange rate translation methodology used for a particular country or countries.
|
Confidential |
85 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
8.9Third Party Financial Obligations.
(a)Obligations under TESARO License Agreements. TESARO shall be solely responsible for the payment of any royalties, sublicense revenues, milestones or other payments that become due under the TESARO License Agreements as a result of the Exploitation of the Compounds and Products in the Company Field in the Company Territory or in the TESARO Field in the TESARO Territory. Company shall notify TESARO in writing within [***] after the achievement of any of the milestone events set forth on Exhibit 8.2(d).
(b)Future License Agreements. If, during the Term, either Party determines, in its reasonable judgment, that it is necessary to obtain a license or other rights under any Patent or other intellectual property owned or controlled by a Third Party in order to Exploit (A) a Single Agent Product or (B) a Compound, to the extent included in a Combination Product, in each case ((A) or (B)) in the Company Field in the Company Territory or in the TESARO Field in the TESARO Territory (such Patent or other intellectual property to the extent relating to the Compound, “Blocking Intellectual Property”), such Party shall so notify the other Party and the following provisions of this Section 8.9(b) shall apply.
(i)If such Blocking Intellectual Property relates solely to the Exploitation of the Single Agent Products in the Company Field in the Company Territory or to the Exploitation of a Combination Product Developed by Company in the Company Field, then (x) Company shall have the sole right to negotiate and enter into an agreement with the applicable Third Party to obtain a license or other rights to such Blocking Intellectual Property (such agreement, a “Company Field License Agreement”) or (y) at Company’s expense, TESARO shall use Commercially Reasonable Efforts to take any actions reasonably requested by Company to modify the Single Agent Product or the Exploitation thereof in the Company Field to avoid the need to obtain a license or other rights to such Blocking Intellectual Property.
(ii)If such Blocking Intellectual Property relates solely to the Exploitation of the Single Agent Products in the TESARO Field in the TESARO Territory or to the Exploitation of a Combination Product Developed by TESARO in the TESARO Field, then TESARO shall have the sole right to negotiate and enter into an agreement with the applicable Third Party to obtain a license or other rights to such Blocking Intellectual Property (such agreement, a “TESARO Field License Agreement”).
(iii)If such Blocking Intellectual Property relates to the Exploitation of the Products in both the Company Field in the Company Territory and the TESARO Field in the TESARO Territory, then the Parties shall discuss in
|
Confidential |
86 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
good faith and attempt to agree upon a course of action, which may include (1) modifying the Products or the Exploitation thereof to avoid the need to obtain a license or other rights to such Blocking Intellectual Property or (2) entering into an agreement with the applicable Third Party to obtain a license or other rights to such Blocking Intellectual Property (such agreement, a “Cross-Indication License Agreement”). If the Parties do not reach agreement within thirty (30) days, then TESARO shall have the right to determine the course of action. TESARO shall have the right to negotiate and enter into any Cross-Indication License Agreement; provided, however, that Company shall have the right to consent (such consent not to be unreasonably withheld) to the terms of such Cross-Indication License Agreement prior to TESARO’s execution thereof if: (A) such Cross-Indication License Agreement includes a grant of rights with respect to any product that is not a Product; or (B) any royalties, sublicense revenues, milestones or other payments payable to the applicable Third Party under such Cross-Indication License Agreement are different for the Company Field than the TESARO Field.
(c)Obligations under Company Field License Agreements. Company shall be solely responsible for the payment of any royalties, sublicense revenues, milestones or other payments that become due under the Company Field License Agreements as a result of the Exploitation of the Products in the Company Field in the Company Territory. Company may credit [***] of any such payments that are attributable and allocable to the Exploitation of a Combination Product or Prostate-Approved Single Agent Product in the Company Field in the Company Territory against any TESARO Incremental Royalty amounts payable to TESARO under Section 8.4(b)(ii). Company shall take such credit during any Calendar Quarter for which royalties are payable hereunder, provided that in no event will such credit, together with any reductions under Section 8.6, reduce such royalties payable to TESARO under Section 8.4(b)(ii) for such Calendar Quarter by more than [***]. Any share of such payments that cannot be credited during a Calendar Quarter as a result of the immediately preceding sentence may be carried forward and credited to subsequent Calendar Quarters. For clarity, payments due under a Company Field License Agreement that are creditable against TESARO Incremental Royalty amounts under this Section 8.9(c) may also be creditable against the Pass-through Merck Royalty amounts under Exhibit 8.4(b)(i)(1), and Company shall credit [***] of such payments against the TESARO Incremental Royalty amounts (to the extent permitted under this Section 8.9(c)) and [***] of such payments against Pass-through Merck Royalty amounts (to the extent permitted under Exhibit 8.4(b)(i)(1)).
(d)Obligations under TESARO Field License Agreements. TESARO shall be solely responsible for the payment of any royalties, sublicense revenues, milestones or other payments that become due under the TESARO Field License
|
Confidential |
87 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Agreements as a result of the Exploitation of the Products in the TESARO Field in the TESARO Territory.
(e)Obligations under Cross-Indication License Agreements. TESARO shall be solely responsible for the payment of any royalties, sublicense revenues, milestones or other payments that become due under the Cross-Indication License Agreements as a result of the Exploitation of the Products in the TESARO Field in the TESARO Territory or the Company Field in the Company Territory (such payments, the “Shared Third Party Obligations”). TESARO may deduct the portion of any Shared Third Party Obligation that is allocable to the Company Field from the Alliance Payments as further described in Exhibit 8.3; provided that, in each relevant Alliance Payments Report, TESARO provides Company with a reasonable allocation of such proposed deduction as between the U.S. and non-U.S. markets.
(f)If TESARO fails to pay any amount due under the Merck License Agreement, AstraZeneca License Agreements or any Cross-Indication License Agreement and such payment is not reasonably disputed by TESARO or the failure to make such payment has placed TESARO in breach of an agreement with the applicable Third Party, Company may elect, in its sole discretion upon [***] prior notice, to make such payment to such Third Party on behalf of TESARO. If Company makes such payment to such Third Party, Company may deduct the amount of such payment from any payments that are owed or that become owed to TESARO under this Agreement or, if such deduction is not applicable, TESARO shall reimburse Company the amount of such payment within [***] after Company makes such payment.
(a)The Parties agree to make all payments under this Agreement without deduction or withholding for Taxes, including Taxes pursuant to the Foreign Account Tax Compliance Act (“FATCA”), except to the extent that any such deduction or withholding is required by Applicable Law in effect at the time of payment.
(b)Any Tax required to be withheld on amounts payable under this Agreement, regardless of whether such amounts are actually withheld from any amounts payable, shall be paid by payor on behalf of payee to the appropriate Governmental Authority, and payor shall furnish payee with proof of payment of such Tax. Any Tax required to be withheld shall be an expense of and borne by payee. If any such Tax is assessed against and paid by payor, then payee will indemnify and hold harmless payor from and against such Tax.
(c)Company and TESARO shall cooperate with respect to (i) minimizing any and all deductions or withholding on account of Taxes, including by taking reasonable actions necessary for the purpose of claiming treaty benefits that would reduce
|
Confidential |
88 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Taxes owed in connection with any payments, such actions to include, but not be limited to, the provision of documentation required by any Governmental Authority or reasonably requested by a Party to secure a reduction in the rate of applicable withholding Taxes, and (ii) obtaining any refunds in respect of Taxes deducted or withheld. Notwithstanding the foregoing, if the payee is entitled under any applicable Tax treaty or otherwise to a reduction of the rate of, or the elimination of, the applicable withholding Tax, including FATCA withholding Tax, it may deliver to the payor or the appropriate Governmental Authority (with the assistance of the payor to the extent reasonably required) the prescribed forms necessary to reduce the applicable rate of withholding or to relieve the payor of its obligation to withhold Tax, and the payor shall apply the reduced rate of withholding, or, as the case may be, dispense with withholding of Tax.
(d)Notwithstanding anything to the contrary in this Agreement, if, as a result of (i) a permitted assignment of this Agreement (in whole or in part) by a Party owing payments under this Agreement (a “Paying Party”) to an Affiliate or a Third Party outside of the U.S. or (ii) the exercise by a Paying Party of its rights under this Agreement (in whole or in part) through an Affiliate or Third Party outside of the U.S. (or the direct exercise of such rights by an Affiliate of such Party outside of the U.S.), foreign withholding Tax in excess of the withholding Tax amount that would have been payable by such Paying Party in the absence of such assignment or exercise of rights becomes payable with respect to amounts due to the other Party hereunder or thereunder, such amounts owed by the Paying Party to the other Party (the “Non-Paying Party”) shall be increased for the Incremental Tax Cost (as defined below) so that the amount actually paid by such Paying Party to such Non-Paying Party equals the amount that would have been payable to such Non-Paying Party in the absence of the assignment or exercise of rights that caused the excess withholding (after withholding of the excess withholding Tax and any additional withholding Tax on such increased amount). However, if a similar assignment or exercise of rights described in (i) or (ii) of the preceding sentence by a Non-Paying Party or the triggering of a change of control by a Non-Paying Party results in foreign withholding tax in excess of the withholding Tax amount that would have been payable in the absence of such assignment or exercise of rights to such Non-Paying Party, any amount due to such Non-Paying Party shall not be increased for such excess withholding and, subject to the terms of this Agreement, the required amount shall be withheld and submitted to the appropriate Governmental Authority. For the purposes of this Section 8.10, the “Incremental Tax Cost” shall equal any tax amount withheld under this Section 8.10 in each year in which such tax is withheld from the Non-Paying Party. To the extent the Non-Paying Party utilizes a foreign tax credit or claims a deduction on its US Federal income tax returns related to any such Incremental Tax Cost that has the effect of reducing cash payments currently owed to the U.S. Federal government in any year, the Non-Paying Party will refund to the Paying Party an amount equal to (i) one hundred percent (100%) of
|
Confidential |
89 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
the foreign tax credit utilized or (ii) the cash tax benefit realized by the Non-Paying Party resulting from any related foreign tax deductions, which benefit will be calculated as the amount claimed as a deduction multiplied by the effective federal corporate tax rate applicable to the Non-Paying Party. To assist the Paying Party in determining when a refund is due from the Non-Paying Party pursuant to the foregoing sentence, beginning with the first Annual tax return for the year in which the Paying Party pays the Non-Paying Party an increased payment under this Section 8.10, and each year thereafter (including, for clarity, all years in which the Non-Paying Party utilizes a tax credit or claims a deduction for any foreign tax that is withheld and ending when either no related foreign tax credit or previously claimed deduction remains to be utilized), the Non-Paying Party will provide the Paying Party with a certification by its third party tax return preparer as to the amount of credits utilized or deductions taken against taxes owed by the Non-Paying party in the current period and the cash benefit realized.
(e)The Parties hereby agree that each Party shall be entitled to any deductions for Tax purposes attributable to such Party pursuant to or permitted by Applicable Law and all Tax returns reflecting any such amounts shall be filed consistent with the foregoing. For the avoidance of doubt, one hundred percent (100%) of amounts paid or incurred by TESARO pursuant to this Agreement shall be deductible or amortizable solely by TESARO, and one hundred percent (100%) of amounts paid or incurred by Company pursuant to this Agreement shall be deductible or amortizable solely by Company.
8.11Audit. Each Party and its Affiliates shall keep complete and accurate records of the items underlying Net Sales, Alliance Payments and the other elements required to prepare the reports or calculate payments required by Sections 8.2(d) and 8.4, Exhibit 8.3, Exhibit 8.4(b) and any other payments under this Agreement. Each Party will have the right, not more frequently than every [***] or more frequently as required by the Merck License Agreement, at its own expense to have an independent, certified public accountant, selected by such Party and reasonably acceptable to the other Party, review any such records of the other Party and its Affiliates in the location(s) where such records are maintained by the other Party or its Affiliates upon thirty (30) days prior written notice and during regular business hours and under obligations of confidence, for the sole purpose of verifying the basis and accuracy of payments made and the reconciliation procedures, and any other payments due under this Agreement, within the prior 36-month period. Each period may be subject to an audit only one time (except as otherwise required by the Merck License Agreement). The report of the independent public accountant shall be shared with the audited Party at least fifteen (15) days prior to distribution of the final report to the auditing Party, such that the audited Party can provide the independent public accountant with justifying remarks for inclusion in the report prior to sharing the conclusions of such independent public audit with the auditing Party. The final audit report will be shared with the auditing Party and audited Party at the same time and specify whether the amounts paid to the auditing Party pursuant thereto were correct or, if incorrect, the amount of any underpayment or overpayment. For royalties, the audit report shall only contain the
|
Confidential |
90 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
information relevant to support the statement as to whether the royalties were calculated and paid accurately and shall not include any Confidential Information of either Party (or additional information that is ordinarily not included in the royalty reports) disclosed to the auditor during the course of the audit. If the review of such records reveals that the other Party has failed to accurately report information, or make any payment (or portion thereof) required under this Agreement, then the other Party shall promptly pay to the auditing Party any underpaid amounts due, or otherwise due under this Agreement, together with interest calculated in the manner provided in Section 8.15. If any such discrepancies are an underpayment of amounts due under this Agreement greater than [***] of the amounts actually due for any Calendar Year or TESARO Calendar Year, as applicable, the other Party shall pay all reasonable costs incurred in conducting such review. Once a Party has conducted a review and audit of the other Party in respect of any given period, it may not subsequently re-inspect the other Party’s or its Affiliates’ records in respect of such period, unless a subsequent audit of a separate reporting period uncovers fraud on the part of the audited Party that is reasonably expected to have been occurring during the prior audited period. For clarity, however, if a discrepancy is identified by the accountant during the course of an audit and the Parties do not agree upon a resolution of such discrepancy, then the auditing Party’s accountant may re-inspect the books and records to the extent reasonably relevant to resolving such discrepancy. Additionally, Company shall (a) keep and maintain records of sales of Products until the later of (i) five (5) years after the end of the period to which such records pertain, and (ii) the expiration of the applicable tax statute of limitations (or any extensions thereof), or for such longer period as may be required by Applicable Law, and (b) if any audit conducted by Merck under the Merck License Agreement or by AstraZeneca under the AstraZeneca License Agreements reveals an underpayment of royalties, then Company shall be responsible for a proportional percentage of any audit fees due to Merck and/or AstraZeneca to the extent that such underpayment is attributable to Company Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales.
8.12[***]
8.13Other TESARO Invoices to Company. Costs incurred by TESARO that are reimbursable by Company under this Agreement but not deducted under Section 8.3 shall be invoiced by TESARO individually to Company, including prostate-specific regulatory filing fees reimbursable pursuant to Section 3.5(iii) of Exhibit 8.3, Technology Transfer Costs reimbursable pursuant to Section 7.3, and certain costs of clinical supply and Compound API reimbursable pursuant to ARTICLE 7. Company shall pay such invoices in accordance with Section 8.14.
8.14Invoices. Except to the extent otherwise provided in this Agreement, for all payments due under this Agreement, except royalty payments, (a) the receiving Party shall submit an invoice for such payment to the paying Party and (b) such invoice shall be due and payable within [***] after receipt by the paying Party, unless the paying Party disputes a portion thereof in good faith (in which event the paying Party shall pay the undisputed portion thereof within [***] after receipt).
|
Confidential |
91 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
8.15Late Payment. All payments due to a Party hereunder, other than payments disputed in good faith by a Party (but only until such dispute is resolved), shall be made in U.S. Dollars by wire transfer of immediately available funds into an account designated by the receiving Party. Any sums owing but unpaid shall bear interest calculated on a daily basis from the date due (taking into account any extension of the applicable due date pursuant to another provision of this Agreement) until the date paid at a rate equal to [***] in excess of overnight U.S. LIBOR or a comparable reference interbank rate per currency.
INTELLECTUAL PROPERTY MATTERS
9.1Ownership of Inventions. Each Party shall own any Inventions made solely by its (or its Affiliates’) own employees, agents, or independent contractors in the course of conducting its activities under this Agreement, together with all intellectual property rights therein (“Sole Inventions”). The Parties shall jointly own any Inventions for which the inventors include at least one employee, agent, or independent contractor of each Party (or its respective Affiliates) engaged in performing activities under this Agreement, together with all intellectual property rights therein (“Joint Inventions”). Ownership of Inventions shall be determined in accordance with U.S. patent laws and consistent throughout the TESARO Territory. Subject to any licenses granted under this Agreement, each Party will have the right to practice and Exploit any Joint Inventions without the duty of accounting to any other Party or seeking consent (for licensing, assigning or otherwise exploiting Joint Inventions) from the other Party by reason of the joint ownership thereof; and each Party hereby waives any right such Party may have under the laws of any jurisdiction to require any such approval or accounting and, to the extent there are any Applicable Laws that prohibit such a waiver, each Party will be deemed to have so consented. In furtherance thereof, at the reasonable written request of a Party, the other Party will in writing grant such consents and confirm that no such accounting is required to effect the foregoing regarding Joint Inventions.
9.2Disclosure of Inventions. Each Party shall promptly disclose to the other Party any Invention that is necessary or useful to Exploit Compounds or Products in the other Party’s Field during the Term. With respect to any Joint Invention, each Party shall promptly disclose to the other Party any invention disclosures, or other similar documents, submitted to it by its employees, agents or independent contractors describing the Joint Invention, and all Know-How relating to such Invention to the extent necessary for the use of such Invention in the Exploitation of the Compounds and Products in such other Party’s Field and, to the extent patentable, for the preparation, filing and maintenance of any Patent with respect to such Invention. No licenses to Know-How are granted by this Section 9.2, and nothing under this Section 9.2 shall require a Party to disclose to the other Party any Know-How related to an active pharmaceutical ingredient that is not a Compound or to a proprietary formulation technology.
|
Confidential |
92 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
9.3Prosecution of Patents.
(a)Merck Patents. Company acknowledges and agrees that Merck has the first right to prosecute and maintain the Merck Patents under the Merck License Agreement. TESARO shall promptly provide to Company any information received from Merck relating to the prosecution and maintenance of the Merck Patents. TESARO shall consult with Company, to the extent practicable, and shall consider Company’s comments in good faith, prior to exercising its rights under Sections 8.01 or 8.02 of the Merck License Agreement. If TESARO exercises its right under Section 8.02 of the Merck License Agreement to prosecute and maintain a Merck Patent, such Merck Patent shall be deemed to be an Other TESARO Patent and TESARO shall have the right to prepare, file, prosecute and maintain such Patent as set forth in Section 9.3(c).
(b)AstraZeneca Patents. Company acknowledges and agrees that AstraZeneca has the sole right to prosecute and maintain the AstraZeneca Patents. TESARO shall promptly notify Company upon receiving notice from AstraZeneca that AstraZeneca has declined to continue to prosecute or maintain any AstraZeneca Patent in any jurisdiction.
(c)Other TESARO Patents. TESARO shall have the sole right and authority to prepare, file, prosecute and maintain the Other TESARO Patents [***]. TESARO shall provide Company a reasonable opportunity to review and comment on its efforts to prepare, file, prosecute and maintain Other TESARO Patents in the Company Territory, including by providing Company with a copy of material communications from any patent authority in the Company Territory regarding any Other TESARO Patent, and by providing drafts of any material filings or responses to be made to such patent authorities in advance of submitting such filings or responses. TESARO shall consider Company’s comments regarding such communications and drafts in good faith.
(d)Company Patents. Company shall have the sole right and authority to prepare, file, prosecute and maintain the Company Patents [***]. Company shall provide TESARO a reasonable opportunity to review and comment on its efforts to prepare, file, prosecute and maintain Company Patents in the TESARO Territory, including by providing TESARO with a copy of material communications from any patent authority regarding any Company Patent in the TESARO Territory, and by providing drafts of any material filings or responses to be made to such patent authorities in advance of submitting such filings or responses. Company shall consider TESARO’s comments regarding such communications and drafts in good faith.
(e)Joint Patents. Except as otherwise provided in this Section 9.3(e), Company shall have the primary right and authority to prepare, file, prosecute and maintain the Patents included in the Joint Inventions (“Joint Patents”) [***]. Company shall provide TESARO with a reasonable opportunity to review and comment on
|
Confidential |
93 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
its efforts to prepare, file, prosecute and maintain Joint Patents, including by providing TESARO with a copy of material communications from any patent authority regarding any Joint Patent, and by providing drafts of any material filings or responses to be made in advance of submitting such filings or responses. Company shall consider TESARO’s comments regarding such communications and drafts in good faith. If Company determines, in its discretion, to abandon or not maintain any Joint Patent(s) in any country(ies) of the world that Cover a Compound or Single Agent Product, then Company shall provide TESARO with written notice of such determination within a period of time reasonably necessary to allow TESARO to determine its interest in such Joint Patent(s) (which notice from Company shall be given no later than sixty (60) days prior to any final deadline for any pending action or response that may be due with respect to such Joint Patent(s) with the applicable patent authority). If TESARO provides written notice expressing its interest in obtaining such Joint Patent(s), Company shall[***] assign and transfer to TESARO the ownership of, and interest in, such Joint Patent(s) in such country(ies), at TESARO’s own expense, and Company shall cooperate with TESARO for assignment and transfer of such Joint Patent(s) in such country. Thereafter, all such assigned and transferred Patents will be deemed Other TESARO Patents and TESARO shall have the right to prepare, file, prosecute and maintain such Patents as set forth in Section 9.3(c).
(f)Cooperation in Prosecution. Each Party shall provide the other Party all reasonable assistance and cooperation in the Patent prosecution efforts provided above in this Section 9.3, including providing any necessary powers of attorney and executing any other required documents or instruments for such prosecution, as well as further actions as set forth below. Such assistance and cooperation shall include making a Party’s inventors and other scientific advisors reasonably available to assist the other Party’s Patent prosecution efforts.
(i)The Parties shall respectively prepare, file, maintain and prosecute the Other TESARO Patents, the Company Patents and the Joint Patents as set forth in this Section 9.3. As used herein, “prosecution” of such Patents shall include all communication and other interaction with any patent office or patent authority having jurisdiction over a patent application in connection with pre-grant proceedings.
(ii)All communications between the Parties relating to the preparation, filing, prosecution or maintenance of the Other TESARO Patents, the Company Patents and the Joint Patents, including copies of any draft or final documents or any communications received from or sent to patent offices or patenting authorities with respect to such Patents, shall be considered Confidential Information of the Party Controlling the relevant Patent and subject to the confidentiality provisions of ARTICLE 11.
|
Confidential |
94 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(iii)Assignments in the Other TESARO Patents, Joint Patents and Company Patents shall be effected as follows: (i) employees or agents of TESARO (or its Affiliates) that are named as inventors on the Other TESARO Patents shall assign their interest in such Patents to TESARO or its Affiliate; (ii) employees or agents of Company or TESARO (or their respective Affiliates) that are named as inventors on the Joint Patents shall assign their interest in such Patents to their respective employer; and (iii) employees or agents of Company (or its Affiliates) that are named as inventors on the Company Patents shall assign their interests in such Patents to Company or its Affiliate.
9.4Patent Term Extensions in the Territory. For each Product in the Company Field as to which a Party owns a Marketing Approval, such Party shall decide for which, if any, of the Patents Controlled by such Party, its Affiliates or designees the Parties should seek patent term extensions, supplemental protection certificates or their equivalents (each a “Patent Extension” and collectively “Patent Extensions”) in the Company Territory and such Party shall have the right to seek such Patent Extensions. Each Party may request of the other Party that a TESARO Patent, Joint Patent or Company Patent Controlled by such Party, its Affiliates or designees be made available to the requesting Party for Patent Extension for a Product in the Company Field as to which it owns the Marketing Approval and such other Party will not unreasonably deny such request. Neither Party shall seek any Patent Extension that is reasonably likely to have a material adverse effect on the Commercialization of a Product or if there is a good faith dispute between the Parties as to whether a Patent Extension is being sought for a Patent that does not Cover the applicable Product (a “Good Faith Dispute”). The Party that does not apply for a Patent Extension hereunder will cooperate fully with the other Party in making such filings or actions, including making available all required regulatory data and information and executing any required authorizations to apply for such Patent Extension. [***] Notwithstanding any provision of this Section 9.4 to the contrary, TESARO shall have the first right to select TESARO Patents for Patent Extensions and may object to the selection by Company of a TESARO Patent for a Patent Extension and may, by written notice to Company prohibit such selection.
9.5Infringement of Patents by Third Parties.
(a)Notification. Each Party shall promptly notify the other Party in writing of any existing, alleged or threatened infringement of any TESARO Patent, Joint Patent or Company Patent of which it becomes aware, and shall provide all information in such Party’s possession or control demonstrating such infringement.
(b)Infringement of TESARO Patents, Company Patents or Joint Patents.
(i)TESARO, subject to Sections 9.5(b)(ii) through 9.5(b)(vii) and to the extent TESARO has such right under the TESARO License Agreements, shall have the first right, but not the obligation, to elect to bring an
|
Confidential |
95 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
appropriate suit or other action against any Third Party engaged in any existing, alleged or threatened infringement of any TESARO Patent, Company Patent or Joint Patent in each case Covering a Compound or Single Agent Product.
(ii)TESARO shall notify Company of its election to take any action or not to take any action in accordance with Section 9.5(b)(i) within the earlier of: (i) [***] after the first notice under Section 9.5(a); or (ii) [***] before any time limit set forth in Applicable Law or regulation, including the time limits set forth under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly referred to as the “Hatch-Waxman Act.” If TESARO elects to initiate a lawsuit or take other reasonable action with respect to an infringement described in Section 9.5(b)(i), TESARO shall not initiate any such suit or take such other action with respect to any TESARO Patent, Company Patent or Joint Patent without first consulting with Company and giving good faith consideration to any reasonable objection from Company regarding TESARO’s proposed course of action, and TESARO shall not initiate any such suit or take such other action with respect to a Company Patent without the prior written consent of Company, such consent not to be unreasonably withheld, delayed or conditioned. Company shall cooperate in the prosecution of any suit under this Section 9.5 as may be reasonably requested by TESARO. If TESARO elects not to initiate a lawsuit or take other reasonable action with respect to an infringement described in Section 9.5(b)(i), Company shall have the right, but not the obligation, to initiate such suit or take such other action, after providing [***] (or [***] in the event there is a time limit) notice to TESARO and giving good faith consideration to TESARO’s reason(s) for not initiating a suit or taking other action.
(iii)If one Party elects to bring suit or take action under this Section 9.5(b) against an infringement, then the other Party shall have the right, prior to commencement of the suit or action, to join any such suit or action.
(iv)Each Party shall provide to the Party enforcing any such rights under this Section 9.5(b) reasonable assistance in such enforcement, at such enforcing Party’s request and expense, including joining such action as a party plaintiff if required by Applicable Law to pursue such action. The enforcing Party shall keep the other Party regularly informed of the status and progress of such enforcement efforts, shall reasonably consider the other Party’s comments on any such efforts, and shall consult the other Party in any important aspects of such enforcement, including determination of material litigation strategy and filing of important papers to the competent court.
|
Confidential |
96 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(v)Each Party shall bear all of its own internal costs incurred in connection with its activities under this Section 9.5(b). In the event that the Parties are joined in suit or action against the infringement or the non-enforcing Party elects to join such suit or action and, in either case, the non-enforcing Party elects to be represented by the same outside counsel as the enforcing Party, then the enforcing Party shall be responsible for all expenses arising from such outside counsel, provided that the enforcing Party consents to such joint representation by outside counsel, such consent not to be unreasonably withheld, delayed or conditioned.
(vi)The Party not bringing an action with respect to infringement under this Section 9.5(b) shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, but such Party shall at all times cooperate fully with the Party bringing such action.
(vii)Neither Party shall settle any claim, suit or action that it brought under this Section 9.5 involving TESARO Patents, Company Patents or Joint Patents without the prior written consent of the other Party, such consent not to be unreasonably withheld, delayed or conditioned.
(viii)Company acknowledges and agrees that, under the terms of the AstraZeneca License Agreements, AstraZeneca has the sole right to enforce the AstraZeneca Patents and to defend any claim pertaining to the validity or enforceability of the AstraZeneca Patents. [***]
(c)Allocation of Proceeds. If either Party recovers monetary damages from any Third Party in a suit or action brought under Section 9.5(b), 9.7(a) or 9.7(b) or any royalties, milestones or other payments from a license agreement with a Third Party related to any alleged infringement related to a Product, whether such damages or royalties result from the infringement of TESARO Patents, Joint Patents or Company Patents, such recovery (“Infringement Recovery”) shall be allocated first to the reimbursement of any expenses incurred by the Parties in such litigation, action or license negotiations, and any remaining amounts shall be allocated as follows:
(i)If the Infringement Recovery is based upon lost profits of a Product, then the Parties will divide revenue or pay royalties as provided in this Agreement, calculated on the Net Sales which formed the basis of the lost profit claim for the remaining recovery amount as if a Party had made such Net Sales; and
(ii)If the Infringement Recovery is based on the allocation of a reasonable royalty as to a competing product, then, relative to a Compound, the Parties will allocate the remaining recovery amount based on the ratio that Net Sales of a Product in an Indication in the country in which such suit or
|
Confidential |
97 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
action was brought bears to net sales of all Products outside of an Indication in such country, with Company receiving that portion of the Infringement Recovery attributable to Net Sales of a Product in an Indication based on this ratio.
The allocation described above is subject to any allocation provided by the Merck License Agreement or the AstraZeneca License Agreements.
9.6Infringement of Third Party Rights in the Territory.
(a)Notice. If any Product used or sold by either Party, its Affiliates, or (sub)licensees becomes the subject of a Third Party’s claim or assertion of infringement of a Patent granted by a jurisdiction within the Company Territory (each, an “Infringement Claim”), the Party first having notice of the claim or assertion shall promptly notify the other Party. In the event that such Infringement Claim relates to the use or sale of the Single Agent Product in the Company Field, TESARO shall use Commercially Reasonable Efforts to take any actions reasonably requested by Company to mitigate damages or modify the infringing activity to render it non-infringing with respect to the Company Field.
(i)With respect to any Infringement Claim that solely relates to the use or sale of the Single Agent Product in the Company Field, Company shall have the first right, but not the obligation, to defend, settle or otherwise dispose of such Infringement Claim [***]. If Company does not commence actions to defend, settle or otherwise dispose of such Infringement Claim within [***] after it receives notice thereof (or within [***] after it should have given notice thereof to TESARO as required by Section 9.6(a)), then, to the extent allowed by Applicable Law, TESARO shall have the right, but not the obligation, to defend, settle or otherwise dispose of such Infringement Claim using counsel of its choice[***].
(ii)With respect to any Infringement Claim that relates to the use or sale of the Single Agent Product other than an Infringement Claim described in Section 9.6(b)(i), TESARO shall have the first right, but not the obligation, to defend, settle or otherwise dispose of such Infringement Claim [***]. If TESARO does not commence actions to defend settle or otherwise dispose of such Infringement Claim within [***] after it receives notice thereof, then, to the extent allowed by Applicable Law, Company shall have the right, but not the obligation, to defend, settle or otherwise dispose of such Infringement Claim using counsel of its choice[***].
(iii)With respect to any Infringement Claim that relates to use or sale of a Combination Product Developed by Company in the Company Field,
|
Confidential |
98 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Company shall have the sole right, but not the obligation, to defend, settle or otherwise dispose of such Infringement Claim [***].
(iv)With respect to any Infringement Claim that relates to use or sale of a Combination Product Developed by TESARO in the TESARO Field, TESARO shall have the sole right, but not the obligation, to defend, settle or otherwise dispose of respond to such Infringement Claim [***].
(v)With respect to any Infringement Claim, the non-defending Party shall reasonably cooperate with the Party that defends or otherwise responds to such Infringement Claim in accordance with this Section 9.6(b), including if required to conduct such defense, furnishing a power of attorney.
(c)Settlement; Licenses. A Party shall not enter into any settlement of any Infringement Claim that affects the other Party’s rights or interests without the other Party’s prior written consent, such consent not to be unreasonably withheld, delayed or conditioned. Each Party shall have the right to decline to defend or to tender defense of any Infringement Claim upon reasonable notice to the other Party, including if the other Party fails to agree to a settlement that the declining Party proposes. In the event that it is determined by any court of competent jurisdiction in response to an Infringement Claim that the Exploitation of a Compound or the Products, conducted in accordance with the terms and conditions of this Agreement, infringes any Patent, copyright, Trademark, data exclusivity right or trade secret right arising under Applicable Law of any Third Party, then notwithstanding anything to the contrary in Section 8.9(b), the Party defending such Infringement Claim shall use Commercially Reasonable Efforts to: (1) negotiate and procure a license from such Third Party authorizing itself and the other Party to continue to conduct such activities (and the costs of such license shall be borne by the Parties as set forth in Section 8.9); or (2) in good faith with the other Party modify such activities so as to render the activities non-infringing.
(d)Applicability to Merck Patents. Notwithstanding anything to the contrary set forth in this Section 9.6, if the notice delivered under Section 9.6(a) relates to a Merck Patent that is subject to Section 8.04 of the Merck License Agreement, TESARO’s obligations under this Section 9.6 shall be subject to compliance with Section 8.04 of the Merck License Agreement.
9.7Patent Oppositions and Other Proceedings.
(a)Third Party Patent Rights. If either Party desires to bring an opposition, action for declaratory judgment, nullity action, interference, declaration for non-infringement, reexamination or other attack upon the validity, title or enforceability of a Patent owned or controlled by a Third Party and having one or more claims that Cover a Compound or Single Agent Product, or the use, sale, offer for sale or importation thereof (except insofar as such action is a
|
Confidential |
99 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
counterclaim to or defense of, or accompanies a defense of, an Infringement Claim under Section 9.6, in which case the provisions of Section 9.6 shall govern), such Party shall so notify the other Party and the Parties shall promptly confer to determine whether to bring such action or the manner in which to settle such action. For each Product in the Company Field as to which a Party owns the Marketing Approval, such Party shall have the first right, but not the obligation, to bring[***] in its sole control, such action [***]. The Party not bringing an action under this Section 9.7(a) shall be entitled to separate representation in such proceeding by counsel of its own choice and at its own expense, and shall cooperate fully with the Party bringing such action. Any awards or amounts received in bringing any such action shall be first allocated to reimburse the initiating Party’s expenses in such action[***].
(b)Parties’ Patent Rights. If any TESARO Patent, Company Patent or Joint Patent becomes the subject of any proceeding commenced by a Third Party in connection with an opposition, reexamination request, action for declaratory judgment, nullity action, interference or other attack upon the validity, title or enforceability thereof (a “Third Party Patent Challenge”) (except insofar as such action is a counterclaim to or defense of, or accompanies a defense of, an action for infringement against a Third Party under Section 9.6, in which case the provisions of Section 9.6 shall govern), then the Party responsible for filing, preparing, prosecuting and maintaining such Patent as set forth in Section 9.3 hereof shall control such defense [***]. The controlling Party shall permit the non-controlling Party to participate in the proceeding to the extent permissible under Applicable Law, and to be represented by its own counsel in such proceeding, at the non-controlling Party’s expense. If either Party decides that it does not wish to defend against such action, then the other Party shall have a backup right to assume defense of such Third Party action [***]. Any awards or amounts received in defending any such Third Party action shall be [***].
REPRESENTATIONS, WARRANTIES AND COVENANTS
10.1Mutual Representations, Warranties and Covenants. Each of the Parties hereby represents and warrants to the other Party as of the Effective Date and, as applicable, covenants as of the Effective Date that:
(a)Organization. It is a corporation duly organized, validly existing, and in good standing under the laws of the jurisdiction of its organization, and has all requisite corporate power and authority to execute, deliver, and perform this Agreement.
(b)Binding Agreement. This Agreement is a legal and valid obligation binding upon such Party and enforceable in accordance with its terms, subject to the effects of bankruptcy, insolvency, or other laws of general application affecting
|
Confidential |
100 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
the enforcement of creditor rights, judicial principles affecting the availability of specific performance, and general principles of equity (whether enforceability is considered a proceeding at law or equity) (the “Equitable Exceptions”).
(c)Authorization. The execution, delivery, and performance of this Agreement by such Party have been duly authorized by all necessary corporate action and do not conflict with any agreement, obligation, instrument, or understanding, oral or written, to which it is a party or by which it is bound, nor violate any Applicable Law or any order, writ, judgment, injunction, decree, determination, or award of any Governmental Authority presently in effect applicable to such Party.
(d)No Further Approval. It is not aware of any government authorization, consent, approval, license, exemption of or filing or registration with any Governmental Authority under any Applicable Law, currently in effect, necessary for the execution and delivery of this Agreement or any other agreement or instrument executed in connection herewith, or for the performance by it of its obligations under this Agreement and such other agreements (save for Regulatory Approvals, Marketing Approvals and other authorizations, consents, approvals, licenses, exemptions, filings, registrations or permits from Governmental Authorities necessary for the Exploitation of the Compounds and Products as contemplated hereunder).
(e)TESARO License Agreements. Each Party shall, and shall cause their respective Affiliates and sublicensees, to comply with the terms and conditions of the TESARO License Agreements that apply to sublicensees of the rights granted to TESARO under such TESARO License Agreements.
(f)No Inconsistent Obligations. It is not under any obligation, contractual or otherwise, to any Person that conflicts with or is inconsistent in any material respect with the terms of this Agreement, or that would impede the diligent and complete fulfillment of its obligations hereunder.
10.2Additional Representations and Warranties of TESARO. TESARO represents and warrants as of the Effective Date and, as applicable, covenants as of the Effective Date to Company that:
(a)TESARO (or its Affiliates) is the sole and exclusive owner of, or otherwise Controls pursuant to an existing Third Party agreement, the TESARO Technology and the TESARO Regulatory Documentation. TESARO has all rights necessary to grant the licenses under the TESARO Technology and Rights of Reference under the TESARO Regulatory Documentation that it grants to Company in this Agreement. Neither TESARO nor any of its Affiliates has granted a license to any Third Party under the TESARO Technology or TESARO Regulatory Documentation to Exploit the Compounds or Products in the TESARO Field or Company Field, other than those licenses granted by TESARO in the ordinary
|
Confidential |
101 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
course of its business to Third Parties working under TESARO’s direction and control, such as licenses to contract research organizations, clinical trial sites, contract manufacturing organizations and licenses in connection with the development of companion diagnostic products. During the Term, TESARO shall not, and shall cause its Affiliates not to, grant to any Third Party rights that conflict with the rights granted to Company hereunder with respect to the TESARO Technology or TESARO Regulatory Materials.
(b)The Patents set forth in Exhibit 1.117 (“Licensed Patents”) represent all Patents that TESARO or any of its Affiliates owns or Controls that Cover or disclose any invention necessary or used by TESARO for the Exploitation of the Compounds or Products by TESARO as of the Effective Date. The Licensed Patents are free and clear of liens, charges or encumbrances other than licenses granted to or by Third Parties that are not inconsistent with the rights and licenses granted to Company hereunder. To TESARO’s knowledge, the Licensed Patents are not invalid or unenforceable in whole or part. No Third Party has challenged or threatened in writing to challenge the scope, validity or enforceability of any Licensed Patent (including, by way of example, through opposition or the institution or written threat of institution of interference, nullity or similar invalidity proceedings before the U.S. Patent and Trademark Office or any analogous foreign Governmental Authorities). During the period TESARO has Controlled the Licensed Patents, TESARO or its Affiliates have timely paid or caused to be paid all filing and renewal fees payable with respect to any Licensed Patents for which TESARO controls prosecution and maintenance. The development of the Licensed Patents has not been funded, in whole or in part, by the U.S. government.
(c)TESARO or its Affiliates own or Control all Know-How necessary or used by TESARO for the Exploitation of the Compounds or Products by TESARO as of the Effective Date. The TESARO Know-How is free and clear of liens, charges or encumbrances other than licenses granted by TESARO to Third Parties that are not inconsistent with the rights and licenses granted to Company hereunder. TESARO and its Affiliates have taken commercially reasonable measures consistent with industry practices to protect the secrecy, confidentiality and value of all TESARO Know-How that constitutes trade secrets under Applicable Law (including requiring all TESARO and its Affiliates’ employees, consultants and independent contractors to execute binding and enforceable agreements, except as enforceability may be limited or affected by the Equitable Exceptions, requiring all such employees, consultants and independent contractors to maintain the confidentiality of such TESARO Know-How). The development of the TESARO Know-How has not been funded, in whole or in part, by the U.S. government.
(d)There is no actual or, to TESARO’s Knowledge, threatened infringement or misappropriation of the TESARO Technology by any Person in the Company
|
Confidential |
102 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Field in the Company Territory. TESARO has not received any written notice or threat of any material suit, legal claim, action, proceeding or investigation against TESARO or any of its Affiliates that relates to the TESARO Technology, and no judgment or settlement is owed by TESARO or any of its Affiliates in connection with the TESARO Technology.
(e)The TESARO Technology collectively constitutes all intellectual property that is necessary or used by TESARO for the Exploitation of the Compounds and the Products by TESARO as of the Effective Date. To TESARO’s Knowledge, except as otherwise disclosed by TESARO to Company, the Exploitation of the Compounds or Products in the Company Territory does not and will not infringe or misappropriate the Patents (including any Third Party patent application published as of the Effective Date, when and if the claims thereunder issue in their current form) or other intellectual property or proprietary rights of any Third Party in the Company Territory.
(f)All current and former officers, employees, agents, advisors, consultants, contractors or other representatives of TESARO or any of its Affiliates who are inventors of or have otherwise contributed in a material manner to the creation or development of any TESARO Technology have executed and delivered to TESARO or any such Affiliate a valid and enforceable assignment or other agreement, except as enforceability may be limited or affected by the Equitable Exceptions, regarding the protection of proprietary information and the assignment to TESARO or any such Affiliate of such person’s entire right, title and interest in and to any TESARO Technology. To TESARO’s Knowledge, no current officer, employee, agent, advisor, consultant or other representative of TESARO or any of its Affiliates is in violation of any term of any assignment or other agreement regarding the protection of TESARO Patents or other TESARO Technology or of any employment contract or any other contractual obligation relating to the relationship of any such Person with TESARO or any such Affiliate. Except for consideration expressly contemplated by this Agreement, Company shall have no obligation to contribute to any remuneration of any inventor employed or previously employed by TESARO or any of its Affiliates in respect of any Know-How and intellectual property rights therein that are so assigned to TESARO or its Affiliate(s). TESARO will pay all such remuneration due to such inventors with respect to such Know-How and intellectual property rights therein.
(g)TESARO has prepared, maintained and retained all Regulatory Documentation pursuant to and in accordance in all material respects with all Applicable Law, including, as applicable, GLP, and such Regulatory Documentation does not contain any materially false and misleading statements.
|
Confidential |
103 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(h)TESARO has conducted, and has used reasonable efforts to cause its contractors and consultants to conduct, all studies, tests and pre-clinical trials of the Compounds and the Products conducted prior to, or being conducted on, the Effective Date in accordance in all material respects with the applicable experimental protocols, procedures and controls pursuant to accepted professional scientific standards, accepted ethical standards and all Applicable Law, including, as applicable, GLP. Except as disclosed in the adverse event reports disclosed or made available to Company prior to the Effective Date (or otherwise disclosed in writing by TESARO to Company prior to the Effective Date), no adverse event involving human subjects has occurred in connection with any study, test or pre-clinical trial of the Compounds or the Products.
(i)TESARO has disclosed or made available to Company all material information in its possession and control relating to the Compounds and Products, and the Development, Manufacture and Commercialization of the Initial Compound and Initial Product as conducted prior to the Effective Date, including by providing or making available complete and correct copies of the following: (i) adverse event reports; (ii) clinical study reports and material study data; and (iii) FDA inspection reports, notices of adverse findings, warning letters, Regulatory Approval filings and other material Regulatory Documentation.
(j)Except for (i) Development activities (other than Clinical Trials) conducted on behalf of TESARO in the ordinary course of business and (ii) the Development activities described on Exhibit 10.2(j), neither TESARO nor any of its Affiliates has conducted, nor granted any Third Party a license or otherwise enabled a Third Party to conduct, any Development of the Compounds or Products in the Company Field in the Company Territory.
(k)Neither TESARO nor any of its Affiliates has been debarred by the FDA, is the subject of a conviction described in Section 306 of the FFDCA or is subject to any similar sanction of other Governmental Authorities in the Company Territory. Neither TESARO nor any of its Affiliates has used, in any capacity, any Person who either has been debarred by the FDA, is the subject of a conviction described in Section 306 of the FFDCA or is subject to any such similar sanction in the Development, Manufacture or Commercialization of the Initial Compound or Initial Product as conducted prior to the Effective Date. TESARO shall not engage, in any capacity in connection with this Agreement or any ancillary agreements, any Person who either has been debarred by the FDA, is the subject of a conviction described in Section 306 of the FFDCA or is subject to any such similar sanction. TESARO shall inform Company in writing promptly if it or any Person engaged by TESARO or any of its Affiliates who is performing services under this Agreement or any ancillary agreements is debarred or is the subject of a conviction described in Section 306 of the FFDCA, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to TESARO’s
|
Confidential |
104 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Knowledge, is threatened, relating to the debarment or conviction of TESARO, any of its Affiliates or any such Person performing services hereunder or thereunder.
(l)TESARO has provided Company with complete and correct copies of each TESARO License Agreement (including all amendments and supplements thereto). Each TESARO License Agreement is in full force and effect and TESARO and its Affiliates are in compliance in all material respects with the terms of each TESARO License Agreement. During the Term, TESARO shall continue to comply in all material respects with the terms of each TESARO License Agreement, and shall not terminate, modify, waive any rights under or otherwise take any action with respect to any TESARO License Agreement that would reasonably be expected to materially impact the rights granted to Company hereunder without Company’s prior written consent.
(m)None of TESARO or any of its Affiliates has, nor, to the Knowledge of TESARO, has any Third Party that is a party to a TESARO License Agreement, materially breached or materially defaulted under, or is in material breach of or material default under, its obligations under such TESARO License Agreement.
(n)Neither TESARO nor any of its Affiliates has received written notice from any Third Party that is a party to a TESARO License Agreement claiming that TESARO or any of its Affiliates has breached or defaulted under, or is in breach of or default under, its obligations under such TESARO License Agreement. If TESARO or any of its Affiliates receives any such written notice during the Term, TESARO shall provide a copy thereof to Company promptly after receipt and, following consultation with Company, TESARO shall (and shall cause its Affiliates to) consider Company’s input in good faith and shall take such actions as may be reasonably necessary to cure any breach or default.
(o)Neither TESARO nor any of its Affiliates has waived any material right under any TESARO License Agreement.
(p)Subject to the terms of the Merck License Agreement, TESARO has the right to grant sublicenses under each TESARO License Agreement in accordance with the terms and conditions thereof and to the extent such sublicenses are granted to Company hereunder without the consent of any Third Party that is a party to such TESARO License Agreement.
10.3Additional Representations and Warranties of Company. Company represents and warrants as of the Effective Date and covenants as of the Effective Date to TESARO that:
(a)Neither Company nor any of its Affiliates has been debarred by the FDA, is the subject of a conviction described in Section 306 of the FFDCA or is subject to any similar sanction of other Governmental Authorities in the Company Territory,
|
Confidential |
105 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
and neither Company nor any of its Affiliates has used, in any capacity, any Person who either has been debarred by the FDA, is the subject of a conviction described in Section 306 of the FFDCA or is subject to any such similar sanction. Company shall not engage, in any capacity in connection with this Agreement or any ancillary agreements, any Person who either has been debarred by the FDA, is the subject of a conviction described in Section 306 of the FFDCA or is subject to any such similar sanction. Company shall inform TESARO in writing promptly if it or any Person engaged by Company or any of its Affiliates who is performing services under this Agreement or any ancillary agreements is debarred or is the subject of a conviction described in Section 306 of the FFDCA, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to Company’s Knowledge, is threatened, relating to the debarment or conviction of Company, any of its Affiliates or any such Person performing services hereunder or thereunder.
(b)Company is not subject to any agreement with any Third Party which would limit or restrict its ability to perform its obligations under this Agreement in any material respect.
10.4Merck License Agreement. The provisions of the Merck License Agreement set forth on Exhibit 10.4 attached hereto are specifically incorporated herein, with the terms used therein that are defined in the Merck License Agreement having the same meanings as ascribed to such terms in the Merck License Agreement, and Company shall comply with such provisions during the Merck Term as if Company were TESARO thereunder. Company hereby covenants that it shall not, nor shall it cause, permit or enable any Affiliate or (sub)licensee to knowingly use or practice, directly or indirectly, any Merck Know-How or Merck Patents for any purposes other than those expressly permitted by this Agreement.
10.5Certain Compliance Matters.
(a)Notwithstanding any other provision of this Agreement, neither Party shall be required to undertake any activity or obligation under this Agreement which it has reason to believe may violate any Applicable Laws; provided, however, a Party which so believes shall promptly inform the other Party of such belief.
(b)Neither Party nor its Affiliates will make any payment, either directly or indirectly, of money or other assets, including the compensation such Party derives from this Agreement (collectively, a “Payment”), to government or political party officials, officials of International Public Organizations, candidates for public office, or representatives of other businesses or persons acting on behalf of any of the foregoing (collectively, “Officials”) or other individuals where such Payment would constitute violation of any Applicable Law, including the Foreign Corrupt Practices Act of 1977, 15 U.S.C. §§ 78dd-1, et seq., and the United Kingdom Bribery Act. In addition regardless of legality, neither Party nor
|
Confidential |
106 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
its Affiliates will make any Payment either directly or indirectly to Officials or other individuals if such Payment is for the purpose of improperly influencing decisions or actions to secure a business advantage, including with respect to the subject matter of this Agreement. Each Party shall have necessary procedures in place to prevent bribery and corrupt conduct by itself and each of its Affiliates and subcontractors. All activities will be conducted in compliance with the U.S. False Claims Act and the U.S. Anti-Kickback Statute. Each Party and each of its Affiliates and subcontractors shall conduct its activities hereunder in accordance with the provisions of Exhibit 10.5 to this Agreement.
10.6No Other Representations or Warranties. EXCEPT AS EXPRESSLY SET FORTH IN THIS ARTICLE 10, THE PARTIES MAKE NO REPRESENTATIONS OR WARRANTIES OF ANY KIND WHATSOEVER, EITHER EXPRESS OR IMPLIED, WRITTEN OR ORAL, IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE, AND EACH PARTY SPECIFICALLY DISCLAIMS ANY OTHER WARRANTIES, INCLUDING ANY EXPRESS OR IMPLIED WARRANTY OF QUALITY, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR WARRANTY OF NON-INFRINGEMENT OR AS TO THE VALIDITY OF ANY PATENTS. EACH PARTY HEREBY DISCLAIMS ANY REPRESENTATION OR WARRANTY THAT THE RESEARCH, DEVELOPMENT, MANUFACTURE OR COMMERCIALIZATION OF ANY COMPOUND OR PRODUCT, INCLUDING PURSUANT TO THIS AGREEMENT, WILL BE SUCCESSFUL, THAT ANY REGULATORY APPROVAL OR MARKETING APPROVAL WILL BE OBTAINED, OR THAT ANY PARTICULAR SALES LEVEL WITH RESPECT TO ANY COMPOUND OR PRODUCT WILL BE ACHIEVED. EXCEPT AS EXPRESSLY SET FORTH IN THIS AGREEMENT, TESARO MAKES NO REPRESENTATION OR WARRANTY WITH RESPECT TO ANY PRODUCT, COMPONENT OR ACTIVE PHARMACEUTICAL INGREDIENT CONTROLLED BY COMPANY OR A THIRD PARTY (FOR CLARITY, EXCLUDING A COMPOUND (AS DEFINED IN SECTION 1.30)) THAT COMPANY INCORPORATES INTO A COMBINATION PRODUCT.
CONFIDENTIALITY
(a)Each Party (the “Receiving Party”) shall (and shall cause its Affiliates to), during the Term and for a period of five (5) years thereafter, (i) maintain all Confidential Information of the other Party (the “Disclosing Party”) in trust and confidence, (ii) not disclose any such Confidential Information to any Third Party without the prior written consent of the Disclosing Party, except as expressly provided hereunder, and (iii) not use any such Confidential Information for any purpose except and only to the extent required to perform its obligations under, and exercise its rights under, this Agreement. Notwithstanding anything to the contrary in the foregoing, the obligations of confidentiality and non-use with
|
Confidential |
107 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
respect to any trade secret within such Confidential Information shall survive such five (5) year period for so long as such Confidential Information remains protected as a trade secret under Applicable Law.
(b)Each Party will (and will cause its Affiliates to) use at least the same standard of care as it uses to protect its own Confidential Information of a similar nature to prevent unauthorized disclosures or uses of Confidential Information of the other Party, but no less than reasonable care.
(c)If any information is expressly deemed to be Confidential Information of each Party under this Agreement: (i) Company’s maintenance, use and disclosure of such information shall be subject to the provisions of this ARTICLE 11 applicable to Confidential Information of TESARO; and (ii) TESARO’s maintenance, use and disclosure of such information shall be subject to the provisions of this ARTICLE 11 applicable to Confidential Information of Company. If any information is both expressly deemed to be Confidential Information of TESARO only under this Agreement and described in clause (a) of the definition of “Confidential Information,” it shall be treated as Confidential Information of TESARO only. If any information is both expressly deemed to be Confidential Information of Company only under this Agreement and described in clause (a) of the definition of “Confidential Information,” it shall be treated as Confidential Information of Company only.
11.2Exceptions. The obligations in Section 11.1 shall not apply with respect to any portion of the Confidential Information that the Receiving Party can show by competent evidence:
(a)is publicly disclosed by the Disclosing Party, either before or after it is disclosed to the Receiving Party hereunder;
(b)is known to the Receiving Party or any of its Affiliates, without any obligation to keep it confidential or any restriction on its use, in each case, to the Disclosing Party, prior to disclosure to the Receiving Party or any of its Affiliates by the Disclosing Party;
(c)is subsequently disclosed to the Receiving Party or any of its Affiliates on a non-confidential basis by a Third Party that, to the Receiving Party’s Knowledge, is not bound by a similar duty of confidentiality or restriction on its use, in each case, to the Disclosing Party;
(d)is now, or hereafter becomes, through no act or failure to act on the part of the Receiving Party or any of its Affiliates in violation of this Agreement, generally known or available, either before or after it is disclosed to the Receiving Party by the Disclosing Party;
|
Confidential |
108 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(e)is independently discovered or developed by or on behalf of the Receiving Party or any of its Affiliates without the use of or reference to the Confidential Information belonging to the Disclosing Party; or
(f)is the subject of written permission to disclose provided by the Disclosing Party.
11.3Authorized Disclosure. The Receiving Party may disclose Confidential Information belonging to the Disclosing Party only to the extent such disclosure is reasonably necessary in the following instances:
(a)filing, prosecuting, maintaining, enforcing or defending Patents as permitted by this Agreement;
(b)as reasonably required in generating Regulatory Documentation, and obtaining Regulatory Approvals, with respect to the Compounds and Products;
(c)prosecuting or defending litigation, including responding to a subpoena in a Third Party litigation;
(d)complying with Applicable Law or court or administrative orders, including as contemplated by Section 4.2(e);
(e)complying with any obligation under this Agreement; provided, however, that TESARO shall not disclose any Confidential Information of Company pursuant to a TESARO License Agreement except to the extent expressly permitted by another provision of this Agreement; or
(f)to its Affiliates, sublicensees or prospective sublicensees, subcontractors or prospective subcontractors, consultants, agents and advisors on a “need-to-know” basis in order for the Receiving Party to exercise its rights or fulfill its obligations under this Agreement, each of whom prior to disclosure must be bound by obligations of confidentiality and restrictions on use of such Confidential Information that are no less restrictive than those set forth in this ARTICLE 11; provided, however, that, in each of the above situations, the Receiving Party shall remain responsible for any failure by any Person who receives Confidential Information pursuant to this Section 11.3(f) to treat such Confidential Information as required under this ARTICLE 11.
If and whenever any Confidential Information is disclosed in accordance with this Section 11.3, such disclosure shall not cause any such information to cease to be Confidential Information except to the extent that such disclosure results in a public disclosure of such information (other than by breach of this Agreement). Notwithstanding the foregoing, in the event a Party is required to make a disclosure of the other Party’s Confidential Information pursuant to Section 11.3(a) through Section 11.3(e), it will, except where impracticable or not legally permitted, give reasonable
|
Confidential |
109 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
advance notice to the other Party of such disclosure and use not less than the same efforts to secure confidential treatment of such information as it would to protect its own confidential information from disclosure.
11.4Terms of this Agreement. The Parties acknowledge that this Agreement and all of the respective terms of this Agreement shall be treated as Confidential Information of both Parties, subject to the provisions of Sections 11.3(f) and 11.6.
11.5Publicity. Each Party may, but is not obligated to, make a public announcement of the execution of this Agreement, which shall be issued at a time and in a form to be mutually agreed by the Parties, but no later than four (4) days after the Effective Date. Except as required to comply with Applicable Law, each Party agrees not to issue any other press release or other public statement disclosing other information relating to this Agreement or the transactions contemplated hereby without the prior written consent of the other Party, such consent not to be unreasonably withheld, delayed or conditioned.
11.6Securities Filings. Notwithstanding anything to the contrary in this ARTICLE 11, in the event either Party proposes to file with the Securities and Exchange Commission or the securities regulators of any state or other jurisdiction a registration statement or any other disclosure document that describes or refers to the terms and conditions of this Agreement or any related agreements between the Parties, or requires the filing of this Agreement as an exhibit to such registration, statement or disclosure document, such Party shall notify the other Party of such intention and shall provide the other Party with a copy of relevant portions of the proposed filing at least five (5) Business Days prior to such filing (and any revisions to such portions of the proposed filing a reasonable time prior to the filing thereof), including any exhibits thereto that refer to the other Party or the terms and conditions of this Agreement or any related Agreements between the Parties. The Party making such filing shall cooperate in good faith with the other Party to obtain confidential treatment of the terms and conditions of this Agreement or any related Agreements between the Parties that the other Party reasonably requests be kept confidential or otherwise afforded confidential treatment, and shall only disclose Confidential Information that it is advised by outside counsel is legally required to be disclosed. Each Party acknowledges that the other Party may be required by securities regulators, including the Securities and Exchange Commission, or advised by such other Party’s outside counsel that the financial terms, including the milestone amounts or royalty rates must be included in such filings. No notice shall be required under this Section 11.6 if the description of or reference to this Agreement or a related agreement between the Parties contained in the proposed filing has been included in any previous filing made by either Party in accordance with this Section 11.6 or otherwise approved by the other Party.
11.7Relationship to Confidentiality Agreement and Merck License Agreement. This Agreement supersedes the Prior CDA; provided, however, that (a) all “Confidential Information” disclosed or received by the Parties and their Affiliates thereunder shall be deemed Confidential Information hereunder and shall be subject to the terms and conditions of this Agreement, and (b) notwithstanding anything in this Agreement to the contrary, Section 10 of the Prior CDA
|
Confidential |
110 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
shall remain in effect in accordance with its terms unless a later signed agreement between the Parties containing a standstill provision specifically supersedes Section 10 of the Prior CDA by specific reference to it. If any terms of Article 9 of the Merck License Agreement are in conflict with, or more restrictive than, the terms set forth in this ARTICLE 11, the terms of Article 9 of the Merck License Agreement shall prevail with respect to any of Merck’s Proprietary Information (as such term is defined in the Merck License Agreement) that is disclosed by TESARO or Merck to Company and marked as Merck’s “confidential” or “proprietary” information (or, if communicated to Company orally, if such information is designated as Merck’s “confidential” or “proprietary” information at the time of disclosure and reduced to writing, marked as “confidential” or “proprietary” to Merck and delivered to Company within ten (10) Business Days from such disclosure).
11.8Equitable Relief. Given the nature of the Confidential Information and the competitive damage that could result to a Party upon unauthorized disclosure, use or transfer of its Confidential Information to any Third Party, the Parties agree that monetary damages may not be a sufficient remedy for any breach of this ARTICLE 11. In addition to all other remedies, a Party shall be entitled to seek specific performance and injunctive and other equitable relief as a remedy for any breach or threatened breach of this ARTICLE 11.
11.9Publications. Each Party shall have the right to publish results of all Clinical Trials that it conducts with respect to a Compound or a Product pursuant to this Agreement, subject to ten (10) days prior review by the other Party for issues of patentability and protection of its Confidential Information, in a manner consistent with Applicable Laws and industry practices. The JDC shall establish a publication strategy with respect to other publications relating to the Compounds and Products, and shall discuss and oversee the review of such publications.
11.10Return of Confidential Information. Upon expiration or termination of this Agreement, each Party will use reasonably diligent efforts (including a reasonably diligent search of files and computer storage devices) to return or destroy all Confidential Information of the other Party except to the extent such Confidential Information is necessary to exercise any license or other right surviving termination of this Agreement. Additionally, each Party will be allowed to keep one archival copy of any Confidential Information of the other Party for record keeping purposes only.
TERM AND TERMINATION
12.1Term. This Agreement shall become effective as of the Effective Date and, unless earlier terminated pursuant to this ARTICLE 12, shall continue in full force and effect as long as Company continues to Exploit the Compounds or the Products in accordance with the terms and conditions of this Agreement (the “Term”).
12.2Termination upon Termination of the Merck License Agreement or Either AstraZeneca License Agreement. Company shall have the right to immediately terminate this
|
Confidential |
111 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Agreement upon written notice to TESARO upon the termination of the Merck License Agreement or either of the AstraZeneca License Agreements.
12.3Unilateral Termination by Company. Company shall have the right to terminate this Agreement in its entirety at any time after the first anniversary of the Effective Date, for any or no reason, upon providing ninety (90) days’ prior written notice to TESARO.
12.4Termination for Material Breach.
(a)Either Party (the “Terminating Party”) may terminate this Agreement in its entirety, or on a country-by-country basis, in the event the other Party (the “Breaching Party”) has materially breached this Agreement, and such material breach has not been cured within one hundred eighty (180) days after receipt of written notice of such breach by the Breaching Party from the Terminating Party (the “Cure Period”). The written notice describing the alleged material breach shall provide sufficient detail to put the Breaching Party on notice of such material breach. Any termination of this Agreement pursuant to this Section 12.4(a) shall become effective at the end of the Cure Period, unless the Breaching Party has cured any such material breach prior to the expiration of such Cure Period (or, if such material breach is reasonably able to be cured within the Cure Period, the Breaching Party has notified the Terminating Party of its plan for curing such and has commenced and sustained its efforts to cure such material breach during the Cure Period). The right of either Party to terminate this Agreement as provided in this Section 12.4(a) shall not be affected in any way by such Party’s waiver of or failure to take action with respect to any previous breach under this Agreement.
(b)If the Parties reasonably and in good faith disagree as to whether there has been a material breach or a cure thereof, the Party that disputes whether there has been a material breach or a cure may contest the allegation in accordance with ARTICLE 13. Notwithstanding anything to the contrary contained in Section 12.4(a), the Cure Period for any material breach that is the subject of a Dispute will run from the date that written notice was first provided to the Breaching Party by the Terminating Party through the resolution of such Dispute pursuant to ARTICLE 13 and for ten (10) days thereafter, and it is understood and acknowledged that, during the pendency of a Dispute pursuant to this Section 12.4(b), all of the terms and conditions of this Agreement shall remain in effect, and the Parties shall continue to perform all of their respective obligations under this Agreement, except that all payment obligations from one Party to the other Party under this Agreement which are subject to the Dispute shall be tolled until the resolution of such Dispute in accordance with ARTICLE 13.
12.5Termination by Company for Safety Reasons. Company shall have the right to terminate this Agreement, at any time after the Effective Date, at any time upon providing thirty
|
Confidential |
112 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(30) days’ prior written notice to TESARO: (a) if senior executives responsible for Company’s pharmacovigilance and clinical science functions determine in good faith that the risk/benefit profile of any Product is such that the Product cannot continue to be Developed or administered to patients safely; or (b) upon the occurrence of serious adverse events related to the use of a Product that cause Company to conclude that the continued use of such Product by patients will result in patients being exposed to a product in which the risks outweigh the benefits.
12.7Termination for Bankruptcy.
(a)Either Party may terminate this Agreement in its entirety upon providing written notice to the other Party on or after the time that such other Party makes a general assignment for the benefit of creditors, files an insolvency petition in bankruptcy, petitions for or acquiesces in the appointment of any receiver, trustee or similar officer to liquidate or conserve its business or any substantial part of its assets, commences under the laws of any jurisdiction any proceeding involving its insolvency, bankruptcy, reorganization, adjustment of debt, dissolution, liquidation or any other similar proceeding for the release of financially distressed debtors, or becomes a party to any proceeding or action of the type described above (each, an “Insolvency Event”), and such proceeding or action remains un-dismissed or un-stayed for a period of more than ninety (90) days.
(b)All rights and licenses granted under or pursuant to this Agreement, including, for the avoidance of doubt, the licenses granted to Company pursuant to Section 3.1, are, and shall otherwise be deemed to be, for purposes of Section 365(n) of Title 11 of the U.S. Code and other similar laws in any jurisdiction outside the U.S. (collectively, the “Bankruptcy Laws”), licenses of rights to “intellectual property” as defined under the Bankruptcy Laws. Upon the occurrence of any Insolvency Event with respect to a Party (the “Insolvent Party”), the Insolvent Party agrees that the other Party (the “Non-Insolvent Party”), as licensee of such rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the Bankruptcy Laws. Further, each Party agrees and acknowledges that all payments hereunder, other than the payments pursuant to Section 8.4, do not constitute royalties within the meaning of Section 365(n) of the Bankruptcy Code or relate to licenses of intellectual property hereunder. Each Party shall, during the Term, create and maintain current copies or, if not amenable to copying, detailed descriptions or other appropriate embodiments, to the extent feasible, of all such intellectual property (TESARO Technology in the case of TESARO and Company Technology in the case of Company). Each Party agrees and acknowledges that “embodiments” of intellectual property within the meaning of Section 365(n) include, without limitation, laboratory notebooks, cell lines, product samples and inventory, research studies and data, and Regulatory Documentation in each case to the extent related to the Compounds and Products.
|
Confidential |
113 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
If (i) a case is commenced during the Term by or against a Party under the Bankruptcy Laws, (ii) this Agreement is rejected as provided for under the Bankruptcy Laws, and (iii) the Non-Insolvent Party elects to retain its rights hereunder as provided for under the Bankruptcy Laws, then the Insolvent Party (in any capacity, including debtor-in-possession) and its successors and assigns (including a Title 11 trustee), shall (x) provide to the Non-Insolvent Party immediately upon the Non-Insolvent Party’s written request copies of all such intellectual property (including embodiments thereof) held by the Insolvent Party and such successors and assigns, or otherwise available to them, and (y) not interfere with the Non-Insolvent Party’s rights under this Agreement, or any related agreements between the Parties, to such intellectual property (including such embodiments), including any right to obtain such intellectual property (or such embodiments) from another entity, to the extent provided in the Bankruptcy Laws. Whenever the Insolvent Party or any of its successors or assigns provides to the Non-Insolvent Party any of the intellectual property licensed hereunder (or any embodiment thereof) pursuant to this Section 12.7(b), the Non-Insolvent Party shall have the right to perform the Insolvent Party’s obligations hereunder with respect to such intellectual property, but neither such provision nor such performance by the Non-Insolvent Party shall release the Insolvent Party from liability resulting from rejection of the license or the failure to perform such obligations. All rights, powers and remedies of the Non-Insolvent Party as provided herein are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity (including the Bankruptcy Laws) in the event of the commencement of a case by or against a Party under the Bankruptcy Laws. In particular, it is the intention and understanding of the Parties to this Agreement that the rights granted to the Parties under this Section 12.7 are essential to the Parties’ respective businesses and the Parties acknowledge that damages are not an adequate remedy. The Parties agree that they intend the following rights to extend to the maximum extent permitted by Applicable Law, and to be enforceable under Section 365(n) of Title 11 of the U.S. Code: (A) the right of access to any intellectual property (including embodiments thereof) of the Insolvent Party, or any Third Party with whom the Insolvent Party contracts to perform an obligation of the Insolvent Party under this Agreement, and, in the case of the Third Party, which is necessary for the Exploitation of Compounds or Products; and (B) the right to contract directly with any Third Party to complete the contracted work upon failure of the Insolvent Party to comply with its applicable obligations.
12.9Effects of Termination. All of the following effects of termination are in addition to the other rights and remedies that may be available to either of the Parties under this Agreement and shall not be construed to limit any such rights or remedies. For purposes of this Section 12.8, the “Termination Notice Date” shall be the date on which notice of termination is given in
|
Confidential |
114 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
accordance with this ARTICLE 12 and the “Termination Effective Date” shall be the date on which termination becomes effective pursuant to this ARTICLE 12 (or, if later, the effective date of termination specified in the termination notice).
(a)Consequences of Termination by TESARO or Company. Upon termination of this Agreement for any reason, the provisions of this Section 12.9(a) shall apply.
(i)From and after the Termination Effective Date, all rights and licenses granted by TESARO to Company will terminate, and Company and its Affiliates and sublicensees will cease all use of TESARO Technology and TESARO Trademarks and all Exploitation of Products, except to the extent necessary for Company to perform its obligations under this Section 12.9(a). Any sublicense granted by Company or its Affiliates pursuant to Section 3.1(c) shall terminate on the Termination Effective Date; provided, however, any such sublicense shall, at the request of TESARO prior to the Termination Effective Date, be assigned to TESARO to the extent possible under the terms of the applicable sublicense and to the extent that the applicable sublicense solely relates to Single Agent Products, subject to the applicable sublicensee’s prior written consent. In the event that (A) TESARO does not request assignment of any such sublicense prior to the Termination Effective Date, (B) the applicable sublicensee does not consent to assignment or (C) any such sublicense does not solely relate to Single Agent Products, then such sublicense shall terminate concurrently with termination of Company’s rights under this Agreement.
(ii)From and after the Termination Effective Date, Company hereby grants to TESARO a non-exclusive, worldwide, fully-paid, royalty-free, non-transferable license, with the right to freely sublicense, under the Single Agent Technology for the sole purpose of Exploiting the Reverted Single Agent Products; provided, however, that if any such Single Agent Technology is in-licensed from a Third Party and subject to payment obligations to such Third Party, Company shall disclose such obligations to TESARO in writing promptly following the Termination Effective Date and such Single Agent Technology shall be subject to the license granted in this Section 12.9(a)(ii) only if TESARO agrees in writing to reimburse all amounts owed to such Third Party as a result of TESARO’s exercise of such license. For clarity, the foregoing license grant by Company does not include any grant of rights under the Company Technology to Exploit any active pharmaceutical compound that is not a Compound. For purposes of this Section 12.9(a): (A) “Single Agent Technology” means any Company Know-How that solely relates to a Reverted Single
|
Confidential |
115 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Agent Product, any safety data Controlled by Company as of the Termination Effective Date that is necessary for TESARO to Exploit a Reverted Single Agent Product and any Company Patent that Covers a Reverted Single Agent Product; and (B) “Reverted Single Agent Product” means a Single Agent Product that was in active Development or being Commercialized in the Company Field prior to the Termination Effective Date.
(iii)Promptly following the Termination Effective Date, Company shall promptly assign and transfer (or cause to be assigned and transferred) to TESARO any Regulatory Approval (or application therefor) held by or on behalf of Company that solely relates to a Reverted Single Agent Product in the Company Field (a “Single Agent Regulatory Approval”), and shall take such actions and execute such other instruments, assignments and documents as may be necessary to effect the transfer of all Single Agent Regulatory Approvals to TESARO; provided, however, that Company shall be permitted to retain any Single Agent Regulatory Approval to the extent, and for so long after the Termination Effective Date as, necessary for Company to perform its obligations under this Section 12.9(a). If Applicable Law prevents or delays the transfer of ownership of any Single Agent Regulatory Approval to TESARO, or Company retains any Single Agent Regulatory Approval after the Termination Effective Date to perform its obligations under this Section 12.9(a), Company hereby grants to TESARO an exclusive and irrevocable right of access and reference to such Single Agent Regulatory Approval, and shall cooperate fully to make the benefits of such Single Agent Regulatory Approval available to TESARO.
(iv)Promptly following the Termination Effective Date, Company shall withdraw any Regulatory Approval (or application therefor) that solely relates to a Combination Product or Combination Use in the Company Field.
(v)Promptly following the Termination Effective Date, Company shall provide to TESARO copies of: (A) all Single Agent Regulatory Approvals and any other Regulatory Documentation Controlled by Company that solely relates to a Reverted Single Agent Product; (B) any other Company Know-How that solely relates to a Reverted Single Agent Product; and (C) any other Know-How Controlled by Company on the Termination Effective Date that is not solely related to a Reverted Single Agent Product, but that is necessary for TESARO to Exploit a Reverted Single Agent Product in the Company Field in the Company Territory. Notwithstanding anything to the contrary in ARTICLE 11, TESARO shall be free to use and disclose such Single Agent Regulatory Approvals, Regulatory Documentation and Know-How in connection with the
|
Confidential |
116 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exploitation of the Reverted Single Agent Products anywhere in the world.
(vi)At TESARO’s request prior to the Termination Effective Date, Company shall, with respect to any Single Agent Agreement, use reasonable efforts to (A) if assignment is permitted under such Single Agent Agreement, assign such Single Agent Agreement to TESARO; or (B) if assignment is not permitted under such Single Agent Agreement, grant to TESARO other rights to provide to TESARO the benefit of such Single Agent Agreement, at TESARO’s expense (to the extent mutually agreed), to the extent permitted under the terms of such Single Agent Agreement. For purposes of this Section 12.9(a)(vi), “Single Agent Agreement” means any Third Party agreement to which Company or any of its Affiliates are a party that solely relates to the Exploitation of Reverted Single Agent Products in the Company Field in the Company Territory (including agreements for sale, promotion, distribution or use of a Reverted Single Agent Product in the Company Field in the Company Territory).
(vii)At TESARO’s request within [***] after the Termination Effective Date, Company shall transfer to TESARO any inventory of Compound API or Reverted Single Agent Product in the possession of Company or any of its Affiliates, at a cost equal to Manufacturing Cost [***].
(viii)If Company is conducting any Clinical Trials with respect to a Reverted Single Agent Product on the Termination Notice Date, TESARO shall elect to either (A) have Company wind-down or complete such Clinical Trial or (B) have responsibility for such Clinical Trial transferred to TESARO. TESARO shall make such election within [***] following the Termination Notice Date and, if TESARO fails to make such election prior to the expiration of such [***] period, then TESARO shall be deemed to have made the election described in clause (A). If TESARO elects to have Company wind-down or complete a Clinical Trial, then Company shall wind-down or complete such Clinical Trial in its sole discretion [***]. If TESARO elects to have such Clinical Trial transferred to TESARO, then the Parties shall develop a plan to transition such Clinical Trial as soon as practicable following the Termination Notice Date and [***].
(ix)If Company is conducting any Clinical Trials with respect to a Combination Product on the Termination Notice Date, Company shall wind-down or complete such Clinical Trial in accordance with Applicable Laws in its sole discretion [***].
(b)Consequences of Termination for Safety Reasons. Upon termination of this Agreement by Company pursuant to Section 12.5, the provisions of Section
|
Confidential |
117 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
12.9(a) shall apply; provided, however, that Company shall not be obligated to transfer assets to TESARO pursuant to Section 12.9(a) to the extent Company reasonably determines that the use of such assets in the continued Development of the Compounds or Products by TESARO following the Termination Effective Date would expose Company to additional Losses unless TESARO agrees to indemnify Company for Losses arising from TESARO’s use of such assets following the Termination Effective Date on the terms set forth in Section 14.2. Immediately upon such agreement, Company shall transfer those assets that were not previously transferred to TESARO pursuant to this Section 12.9(b).
12.10Remedies. Termination or expiration of this Agreement shall not relieve the Parties of any liability or obligation which accrued hereunder prior to the effective date of such termination or expiration. Each Party shall be free, pursuant to ARTICLE 13, to seek, without restriction as to the number of times it may seek, damages, costs and remedies that may be available to it under Applicable Law or in equity and shall be entitled, following final resolution of a Dispute in accordance with ARTICLE 13, to offset the amount of any damages and costs awarded pursuant to a final determination under Section 13.3 against any amounts due to such other Party under this Agreement.
12.11Survival. In the event of termination or expiration of this Agreement, in addition to the provisions of this Agreement that continue in effect in accordance with their terms, the following provisions of this Agreement shall survive: ARTICLE 1 (Definitions) (as applicable), ARTICLE 11 (Confidentiality), ARTICLE 12 (Term and Termination), ARTICLE 13 (Dispute Resolution), ARTICLE 14 (Indemnification) (solely as to activities arising during the Term or as to any activities conducted in the course of a Party’s exercise of a license surviving the Term), ARTICLE 15 (Miscellaneous); Sections 3.5 (No Implied Licenses), 4.2(g) (Ownership of Development Information and Results), 4.5 (Records), 5.9(d) (Recall Records), 6.2(c)(iv), 6.4(a) (last sentence only), 6.6 (Transparency Reporting), 8.5 (Royalty Term), 8.10 (Taxes), 8.11 (Audit), 8.15 (Late Payment), 9.1 (Ownership of Inventions), Section 9.2 (Disclosure of Inventions) and 10.6 (No Other Representations or Warranties); and any other provisions of this Agreement that are necessary to interpret or effectuate the intent of the foregoing provisions.
13.1Exclusive Dispute Resolution Mechanism. The Parties recognize that a dispute may arise out of or in relation to or in connection with this Agreement, including any alleged failure to perform, or breach, of this Agreement, or any issue relating to the interpretation, application, enforcement, termination or validity of this Agreement (except for any Deadlocked Committee Matters or as determined by a Third Party expert pursuant to Section 3.2(a), each, a “Dispute”). The Parties agree that the procedures set forth in this ARTICLE 13 shall be the exclusive mechanism for resolving Disputes. For the avoidance of doubt, this ARTICLE 13 (including the right to pursue provisional remedies under Section 13.4) shall not apply to any failure of a Joint Committee to reach consensus on any matter within such Joint Committee’s responsibilities or
|
Confidential |
118 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
any Deadlocked Committee Matter; provided, that a dispute regarding any of the following shall constitute a Dispute hereunder: (a) whether a matter is within a Joint Committee’s responsibilities; (b) whether a matter is a Deadlocked Committee Matter; or (c) whether an exercise of final decision-making authority is made in accordance with Section 2.1(d)(iv). Any Dispute, including Disputes that may involve Company Parent or Affiliates of any Party, shall be resolved in accordance with this ARTICLE 13.
13.2Resolution by Executive Officers. Except as otherwise provided in this ARTICLE 13, in the event of any Dispute, the Parties shall first attempt in good faith to resolve such Dispute by negotiation and consultation between themselves. In the event that such Dispute is not resolved on such basis within fifteen (15) Business Days (unless otherwise agreed by the Parties), either Party may, by written notice to the other Party, refer the Dispute to the Executive Officers for attempted resolution by good faith negotiation within thirty (30) Business Days after such notice is received (unless otherwise agreed by the Parties). Each Party may, in its discretion, seek resolution of any and all Disputes that are not resolved under this Section 13.2 in accordance with Section 13.3.
13.3Arbitration. If the Parties fail to resolve the Dispute pursuant to Section 13.2, and a Party desires to pursue resolution of the Dispute, the Dispute shall be submitted by either Party for resolution in arbitration pursuant to the then current CPR Non-Administered Arbitration Rules (“CPR Rules”) (www.cpradr.org), except where they conflict with these provisions, in which case these provisions control. The arbitration will be held in New York, New York. All aspects of the arbitration shall be treated as confidential.
(a)Arbitrators.
(i)The arbitrators will be chosen from the CPR Panel of Distinguished Neutrals, unless a candidate not on such panel is approved by both Parties. Each arbitrator shall be a lawyer with at least fifteen (15) years’ experience with a law firm or corporate law department of over twenty-five (25) lawyers or who was a judge of a court of general jurisdiction. To the extent that the Dispute requires special expertise, the Parties will so inform CPR prior to the beginning of the selection process.
(ii)The arbitration tribunal shall consist of three arbitrators, of whom each Party shall designate one in accordance with the “screened” appointment procedure provided in CPR Rule 5.4. The chair will be chosen in accordance with CPR Rule 6.4. If, however, the aggregate award sought by the Parties is less than [***] dollars ($[***]) and equitable relief is not sought, a single arbitrator shall be chosen in accordance with the CPR Rules.
(iii)The Parties agree to select the arbitrator(s) within forty-five (45) days of initiation of the arbitration.
|
Confidential |
119 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(b)Procedures.
(i)The hearing will be concluded within nine (9) months after selection of the arbitrator(s) and the award will be rendered within sixty (60) days of the conclusion of the hearing, or of any post‑hearing briefing, which briefing will be completed by both sides within forty-five (45) days after the conclusion of the hearing. In the event the Parties cannot agree upon a schedule, then the arbitrator(s) shall set the schedule following the time limits set forth above as closely as practical.
(ii)The hearing will be concluded in ten (10) hearing days or less. Multiple hearing days will be scheduled consecutively to the greatest extent possible. A transcript of the testimony adduced at the hearing shall be made and shall be made available to each Party.
(iii)The arbitrator(s) shall be guided, but not bound, by the CPR Protocol on Disclosure of Documents and Presentation of Witnesses in Commercial Arbitration (www.cpradr.org) (“Protocol”). The Parties will attempt to agree on modes of document disclosure, electronic discovery, witness presentation, etc. within the parameters of the Protocol. If the Parties cannot agree on discovery and presentation issues, the arbitrator(s) shall decide on presentation modes and provide for discovery within the Protocol, understanding that the Parties contemplate reasonable discovery.
(iv)The arbitrator(s) shall decide the merits of any Dispute in accordance with the law governing this Agreement, without application of any principle of conflict of laws that would result in reference to a different law. The arbitrator(s) may not apply principles such as “amiable compositeur” or “natural justice and equity.”
(v)The arbitrator(s) are expressly empowered to decide dispositive motions in advance of any hearing and shall endeavor to decide such motions as would a United States District Court Judge sitting in the jurisdiction whose substantive law governs.
(vi)The arbitrator(s) shall render a written opinion stating the reasons upon which the award is based. The Parties consent to the jurisdiction of the United States District Court for the district in which the arbitration is held for the enforcement of these provisions and the entry of judgment on any award rendered hereunder. Should such court for any reason lack jurisdiction, any court with jurisdiction may act in the same fashion.
13.4Provisional Remedies. Each Party has the right to seek from the appropriate court provisional remedies such as attachment, preliminary injunction, replevin, etc. to avoid irreparable harm, maintain the status quo, or preserve the subject matter of a Dispute pending the
|
Confidential |
120 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
resolution of the Dispute under this ARTICLE 13. Rule 14 of the CPR Rules does not apply to this Agreement.
13.5Confidentiality. Any and all activities conducted under this ARTICLE 13 shall be deemed Confidential Information of each of the Parties, and shall be subject to ARTICLE 11 above.
14.1Indemnification by Company. Company hereby agrees to defend, indemnify and hold harmless TESARO and its Affiliates, and each of their respective directors, officers, employees, agents and representatives (each, a “TESARO Indemnitee”) from and against any and all claims, suits, actions, demands, liabilities, expenses and/or losses, including reasonable legal expenses and attorneys’ fees (collectively, the “Losses”), to which any TESARO Indemnitee may become subject as a result of any claim, demand, action or other proceeding by any Third Party (each, a “Proceeding”), to the extent such Losses arise directly or indirectly out of:
(a)the breach by Company of, or any inaccuracy in, any representation or warranty made by Company in this Agreement;
(b)the breach by Company or any of its Affiliates of, or failure to perform or comply with, any covenant or obligation of Company or any of its Affiliates set forth in this Agreement;
(c)the gross negligence, willful misconduct or violation of Applicable Law by Company or any of its Affiliates or sublicensees in connection with the exercise of any rights granted to Company or any of its Affiliates, or the performance of any obligations imposed on Company or any of its Affiliates, under this Agreement;
(d)the Exploitation by Company or any of its Affiliates or (sub)licensees of the Joint Inventions or Joint Patents in connection with any compound that is not a Compound or any product that is not a Product;
(e)the Exploitation of any Compound or Product by Company or any of its Affiliates or sublicensees in the Company Field in the Company Territory, except to the extent resulting from the reasonable reliance of Company or such Affiliate on safety information or Regulatory Documentation relating to any Compound or Product provided to Company or any of its Affiliates by TESARO or any of its Affiliates;
(f)the Exploitation of any Compound or Product by TESARO or any of its Affiliates or sublicensees in the TESARO Field in the TESARO Territory, to the extent resulting from the reasonable reliance of TESARO or such Affiliate on safety
|
Confidential |
121 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
information, Regulatory Documentation or other information relating to any Compound or Product provided to TESARO or any of its Affiliates by Company or any of its Affiliates;
(g)any Proceeding that arises from (or is alleged to arise from) personal injury or death caused by (i) the use of a Single Agent Product in the Company Field or (ii) the use of a Combination Product Developed by Company in the Company Field, except, in each case ((i) and (ii)), to the extent arising from defective Single Agent Product or Compound API Manufactured by TESARO (a “Company Product Liability Proceeding”); or
(h)any Proceeding that arises out of an Infringement Claim that solely relates to the use or sale of a Single Agent Product in the Company Field or an Infringement Claim that relates to the use or sale of a Combination Product Developed by Company in the Company Field (a “Company Infringement Proceeding”);
except, with respect to each of clauses (a) through (h) above, to the extent such Losses arise directly or indirectly from any of the causes for which TESARO is obligated to indemnify Company under Section 14.2.
14.2Indemnification by TESARO. TESARO hereby agrees to defend, indemnify and hold harmless Company and its Affiliates, and each of their respective directors, officers, employees, agents and representatives (each, a “Company Indemnitee”) from and against any and all Losses to which any Company Indemnitee may become subject as a result of any Proceeding, to the extent such Losses arise directly or indirectly out of:
(a)the breach by TESARO of, or any inaccuracy in, any representation or warranty made by TESARO in this Agreement;
(b)the breach by any TESARO or any of its Affiliates of, or failure to perform or comply with, any covenant or obligation of TESARO or any of its Affiliates set forth in this Agreement;
(c)the gross negligence, willful misconduct or violation of Applicable Law by TESARO or any of its Affiliates or (sub)licensees in connection with the exercise of any rights granted to TESARO or any of its Affiliates, or the performance of any obligations imposed on TESARO or any of its Affiliates, under this Agreement;
(d)the Exploitation by TESARO or any of its Affiliates or (sub)licensees of the Joint Inventions or Joint Patents in connection with any compound that is not a Compound or any product that is not a Product;
(e)the Exploitation of any Compound or Product by TESARO or any of its Affiliates or sublicensees in the TESARO Field in the TESARO Territory (or in any field in
|
Confidential |
122 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
any territory prior to the Effective Date), except to the extent resulting from the reasonable reliance of TESARO or such Affiliate on safety information, Regulatory Documentation or other information relating to any Compound or Product provided to TESARO or any of its Affiliates by Company or any of its Affiliates;
(f)the Exploitation of any Compound or Product by Company or any of its Affiliates or sublicensees in the Company Field in the Company Territory, to the extent resulting from the reasonable reliance of Company or such Affiliate on safety information or Regulatory Documentation relating to any Compound or Product provided to Company or any of its Affiliates by TESARO or any of its Affiliates;
(g)any Proceeding that arises from (or is alleged to arise from) personal injury or death caused by a Product that is not a Company Product Liability Proceeding; or
(h)any Proceeding that arises out of an Infringement Claim that is not a Company Infringement Proceeding (a “TESARO Infringement Proceeding”);
except, with respect to each of clauses (a) through (h) above, to the extent such Losses arise directly or indirectly from any of the causes for which Company is obligated to indemnify TESARO under Section 14.1.
14.3Indemnification Procedures.
(a)Notice. Promptly after a TESARO Indemnitee or a Company Indemnitee (each, an “Indemnitee”) receives notice of a pending or threatened Proceeding, such Indemnitee shall give written notice of such Proceeding (an “Indemnification Notice”) to the Party from whom the Indemnitee is entitled to receive indemnification pursuant to Section 14.1 or 14.2, as applicable (the “Indemnifying Party”). Failure or delay in providing an Indemnification Notice to the Indemnifying Party shall not relieve the Indemnifying Party of any liability it may have to the Indemnitee with respect to the applicable Proceeding, except and only to the extent that such failure or delay causes actual harm to the Indemnifying Party with respect to such Proceeding. The Indemnification Notice shall specify, in reasonable detail, the Proceeding with respect to which such Indemnitee or any other Person that is entitled to receive indemnification from the same Indemnifying Party pursuant to Section 14.1 or 14.2 (collectively, “Related Indemnitees”) intends to base a request for indemnification or reimbursement under Section 14.1 or 14.2, as applicable. Failure to provide such reasonable detail in an Indemnification Notice will not relieve the Indemnifying Party of any liability it may have to the Indemnitee with respect to the applicable Proceeding, except and only to the extent that such failure causes actual harm to the Indemnifying Party with respect to such Proceeding. The Indemnitee shall enclose with the Indemnification Notice a copy of any papers served with respect to such Proceeding. Within thirty (30) days after receipt of an Indemnification
|
Confidential |
123 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Notice, the Indemnifying Party shall notify the Indemnitee whether the Indemnifying Party desires to assume the defense of the applicable Proceeding.
(b)Control of Defense. If, within thirty (30) days after receipt of an Indemnification Notice, the Indemnifying Party (i) notifies the Indemnitee that it desires to assume control of the applicable Proceeding and (ii) acknowledges in writing an obligation to indemnify or reimburse fully the Indemnitee and its Related Indemnitees for all Losses arising from the Proceeding for which it is obligated to indemnify the Indemnitee under Section 14.1 or 14.2 as applicable (which acknowledgment shall not be deemed or construed as an admission of liability, either under this ARTICLE 14 or otherwise), then, subject to Section 9.6 and Section 14.3(d), the Indemnifying Party shall have the right to defend, settle and otherwise dispose of such Proceeding; provided, however, that if the Indemnifying Party does not have the right to defend, settle or otherwise dispose of such Proceeding, then the Parties shall confer and negotiate in good faith to determine whether to enter into a joint defense agreement pursuant to which the Parties shall allocate the respective rights and obligations of the Parties with respect to the control of the Proceeding, including whether to designate one of the Parties as the defending Party. In the event that there are two or more Indemnitees that are subject to the same Proceeding, this Section 14.3(b) shall be construed to apply separately to such Proceeding as it applies to each such Indemnitee, provided that the Parties shall take account of such fact in connection with their negotiations pursuant to this Section 14.3(b) with respect to the defense, control and settlement of such Proceeding as it applies to each such Indemnitee. Any out-of-pocket costs and expenses (including reasonable attorneys’ fees) reasonably incurred by the Indemnifying Party in connection with its control of a Proceeding shall constitute Losses with respect to such Proceeding.
(c)Interim Control. Unless and until the Parties determine which Party shall defend, settle and otherwise dispose of a Proceeding in accordance with Section14.3(b), the Indemnitee shall have the right to defend and control such Proceeding, but shall not have the right to consent to the entry of any judgment or enter into any settlement with respect to the Proceeding for which it would be seeking indemnification without the prior written consent of the Indemnifying Party (which consent shall not be unreasonably withheld, conditioned or delayed).
(d)Control by the Defending Party. If the Parties determine that either Party shall defend, settle and otherwise dispose of a Proceeding (other than a Company Infringement Proceeding or TESARO Infringement Proceeding) in accordance with Section 14.3(b) (such Party, the “Defending Party”), then: (i) the other Party may retain separate co-counsel at its sole cost and expense (from and after the Defending Party’s assumption of control of the Proceeding) and participate in the defense of the Proceeding but the Defending Party shall control the investigation, defense and settlement thereof; (ii) the other Party will not consent
|
Confidential |
124 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
to the entry of any judgment or enter into any settlement with respect to the Proceeding for which it would be seeking indemnification or reimbursement hereunder without the prior written consent of the Defending Party (which consent shall not be unreasonably withheld, conditioned or delayed); and (iii) the Defending Party will not, without the prior written consent of the other Party, consent to the entry of any judgment or enter into any settlement with respect to the Proceeding, to the extent such judgment or settlement (A) provides for equitable relief (or any other relief other than solely for money damages) or liability or obligation that cannot be assumed and performed by the Defending Party in full (without any recourse to the other Party and its Related Indemnitees), or for any monetary relief that will not be fully discharged by the Defending Party (without any recourse to the other Party and its Related Indemnitees) concurrently with the effectiveness of such judgment or settlement, provided that the other Party’s consent shall not be unreasonably withheld, conditioned or delayed to the extent that the sole relief is monetary, (B) does not effect a full and unconditional release of the other Party and its Related Indemnitees with respect to all claims in such Proceeding (or the portion thereof to which the judgment or settlement relates), or (C) contains an admission of wrongdoing on the part of the other Party or its Related Indemnitees. The Parties shall act in good faith in responding to, defending against, settling or otherwise dealing with such Proceeding, provided that (x) an Indemnitee shall not be obligated pursuant to this sentence to enter into or consent to the entry of any judgment or settlement in relation to any Proceeding and (y) in any event, an Indemnifying Party shall not be relieved of its obligations under this sentence as a result of any breach of this sentence (determined after giving effect to clause (x)), except to the extent that the Indemnifying Party is actually prejudiced by such breach. Notwithstanding anything to the contrary in this Agreement, an Indemnifying Party shall not be entitled to assume the defense of any Proceeding that seeks an injunction or other equitable relief (or any other relief other than solely money damages) against the Indemnitee. The Parties shall also cooperate in any such defense and give each other reasonable access to all non-privileged information relevant thereto to the extent permitted by Applicable Law. Notwithstanding the foregoing, the defense, settlement or other disposal of any Company Infringement Proceeding or TESARO Infringement Proceeding shall be governed by the provisions of Section 9.6.
(e)Election Not to Control. If there is no Defending Party with respect to a given Proceeding (other than a Company Infringement Proceeding or TESARO Infringement Proceeding), then the Indemnitee will be entitled to assume control of the Proceeding, at its cost and expense (or, to the extent the Indemnitee is entitled to indemnification or reimbursement with respect thereto pursuant to Section 14.1 or 14.2, at the expense of the other Party to such extent) upon delivery of notice to such effect to the other Party, provided that the Indemnifying Party shall have the right to participate in the Proceeding at its sole cost and
|
Confidential |
125 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
expense, but the Indemnitee shall control the investigation, defense and settlement thereof.
(f)Unauthorized Settlements. Whether or not the Indemnifying Party is the Defending Party with respect to any Proceeding (other than a Company Infringement Proceeding or TESARO Infringement Proceeding), the Indemnifying Party shall not be obligated to indemnify or reimburse the Indemnitee hereunder for any settlement entered into, or any judgment that was consented to, by the Indemnitee with respect to such Proceeding without the Indemnifying Party’s prior written consent (which consent shall not be unreasonably withheld, conditioned or delayed).
(g)Certain Infringement Losses. Notwithstanding Section 14.2(h), any Losses that arise directly or indirectly out of a TESARO Infringement Proceeding that relates to the use or sale of a Single Agent Product in both the Company Field and TESARO Field in a given country shall be allocated between the Parties using the same methodology that Net Sales of such Single Agent Product in such country are allocated between the Parties in accordance with Exhibit 8.3.
(h)Allocations. The allocation between the Parties (whether by indemnification, reimbursement or otherwise) of any Losses, if not otherwise determined in a court of law, shall be considered by the Parties and, if the Parties do not reach agreement in writing on such allocation, either Party shall have the right to refer the Dispute to dispute resolution pursuant to Section 13.1. The Parties, the Executive Officers or the arbitrator, as the case may be, shall make such allocation based on the indemnification principles set forth in this ARTICLE 14. Notwithstanding the foregoing, the Parties shall not be entitled to refer any Dispute with respect to Losses arising under a Proceeding pursuant to this Section 14.3(h) to arbitration to the extent that the liability of either Party for such Losses is being contested in such Proceeding (or any other Proceeding that would be binding with respect to such first Proceeding).
(i)Third Party Rights. The Parties shall attempt in good faith to reach alignment with respect to the rights set forth in this Section 14.3, including any rights with respect to the control of any applicable Proceeding, to take into account the rights of any Third Party (e.g., a subcontractor of a Party) that has an obligation to indemnify any Party (or its Related Indemnitees) with respect to any Losses covered by this ARTICLE 14.
14.4Insurance. At such time as Company or any of its Affiliates or sublicensees begins to sell or distribute Products, Company shall, at its own expense, procure and maintain product liability insurance in the amount of [***] Dollars ($[***]). All such policies shall name Merck as an additional insured, and insurers will waive all rights of subrogation against Merck. Company shall notify TESARO not less than twenty five (25) days in advance of any material change or
|
Confidential |
126 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
cancellation of any policy. Company shall continue to maintain such insurance in effect after the expiration or termination of this Agreement during any period in which Company or its sublicensees continue to make, have made, use, sell, offer to sell or import Products. If any insurance is on a claims made basis, Company will maintain such insurance for a period of not less than five (5) years after it has ceased all commercial sale, distribution or use of any Product. TESARO shall maintain the insurance amounts required by Section 11.06 of the Merck License Agreement. Upon request, each Party shall promptly provide the other Party with a certificate of insurance for itself and, in the case of Company, its sublicensees, evidencing the coverage required under this Section 14.4 or Section 11.06 of the Merck License Agreement, as applicable. Such insurance shall not be construed to create a limit of a Party’s liability with respect to its indemnification obligations under this ARTICLE 14. The Parties acknowledge and agree that Company may meet its obligations under this Section 14.4 through self-insurance.
14.5Limitation of Liability. EXCEPT WITH RESPECT TO BREACHES OF EACH PARTY’S OBLIGATIONS UNDER ARTICLE 11, NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY FOR SPECIAL, INDIRECT, INCIDENTAL, PUNITIVE, MULTIPLIED OR CONSEQUENTIAL DAMAGES (INCLUDING FOR LOST PROFITS), WHETHER IN CONTRACT, WARRANTY, NEGLIGENCE, TORT, STRICT LIABILITY OR OTHERWISE, ARISING UNDER OR IN CONNECTION WITH THIS AGREEMENT OR ANY ANCILLARY AGREEMENT. NOTHING IN THIS SECTION 14.5 IS INTENDED TO OR SHALL OPERATE TO LIMIT A PARTY’S OBLIGATIONS OF INDEMNITY OR REIMBURSEMENT OF LOSSES UNDER THIS ARTICLE 14.
ARTICLE 15
15.1Notices. All notices and other communications given or made pursuant hereto shall be in writing and shall be deemed to have been duly given on the date delivered, if delivered personally, or on the next Business Day after being sent by reputable overnight courier (with delivery tracking provided, signature required and delivery prepaid), in each case, to the Parties at the following addresses, or on the date sent and confirmed by electronic transmission to the telecopier number specified below (or at such other address or telecopier number for a Party as shall be specified by notice given in accordance with this Section 15.1).
(a)If to Company:
Janssen Biotech, Inc.
800/850 Ridgeview Dr.
Horsham, PA 19044
Attention: President
Facsimile: [***]
with copies (which shall not constitute notice) to:
Johnson & Johnson
|
Confidential |
127 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
One Johnson & Johnson Plaza
New Brunswick, NJ 08933
Attention: General Counsel, Pharmaceuticals
Facsimile: [***]
(b)If to TESARO:
TESARO, Inc.
Attention: General Counsel
1000 Winter Street, Suite 3300
Waltham, MA 02451
Facsimile No.: (339) 469-8966
with copies (which shall not constitute notice) to:
Hogan Lovells US LLP
Attention: Asher M. Rubin
100 International Drive, Suite 2000
Baltimore, MD 21202
Facsimile No.: (410) 659-2701
15.2Governing Law. This Agreement and all disputes arising out of or related to this Agreement or any breach hereof shall be governed by and construed under the laws of the State of New York, without giving effect to any choice of law principles that would require the application of the laws of a different jurisdiction.
15.4Assignment. The terms and provisions of this Agreement shall inure to the benefit of, and be binding upon, the Parties and their respective successors and permitted assigns. Except as expressly permitted in this Agreement, neither Party may, without the prior written consent of the other Party, assign or otherwise transfer any of its rights and interests or subcontract or otherwise delegate any of its obligations under this Agreement. Notwithstanding the foregoing, (a) either Party, without such consent, may assign its rights and delegate its duties under this Agreement to an Affiliate that is a directly or indirectly wholly-owned subsidiary of TESARO or Company Parent, as the case may be, provided that such assignment or delegation to an Affiliate shall terminate automatically at such time, if any, as such Affiliate ceases to be wholly-owned, directly or indirectly, by TESARO or Company Parent, as the case may be and (b) either Party, without such consent, may assign this Agreement to an Affiliate that is directly or indirectly controlled by TESARO or Company Parent, as the case may be (as the term “controlled by” is defined in Section 1.2), provided that such assignment to an Affiliate shall terminate automatically at such time, if any, as such Affiliate ceases to be directly or indirectly controlled by TESARO or Company Parent, as the case may be. The Parties acknowledge and agree that a
|
Confidential |
128 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Change of Control transaction of the type described in clause (a), (b), (c) or (d) of the definition of “Change of Control” shall not be deemed to be an assignment of this Agreement for purposes of this Section 15.4. Either Party may, without the consent of the other Party, assign this Agreement in its entirety to an Acquirer pursuant to a Change of Control transaction of the type described in clause (e) of the definition of “Change of Control” if such Acquirer agrees in writing to be bound by the terms and conditions of this Agreement. Any purported assignment, transfer, subcontract or delegation by either Party in violation of the terms of this Section 15.4 shall be null and void and of no legal effect.
15.5Designation of Affiliates. Each Party may discharge any obligation and exercise any right hereunder through delegation of its obligations or rights to any of its Affiliates. Each Party hereby guarantees the performance by its Affiliates of such Party’s obligations under this Agreement, and shall cause its Affiliates to comply with the provisions of this Agreement in connection with such performance. Any breach by a Party’s Affiliate of any of such Party’s obligations under this Agreement shall be deemed a breach by such Party, and the other Party may proceed directly against such Party without any obligation to first proceed against such Party’s Affiliate.
15.6Relationship of the Parties. It is expressly agreed that TESARO, on the one hand, and Company, on the other hand, are independent contractors, and it is further agreed that the Parties fully intend and expect that the relationship between the two Parties shall not constitute a partnership, joint venture or agency. Except as expressly provided herein, neither TESARO nor Company shall have the authority to make any statements, representations or commitments of any kind, or to take any action which shall be binding on the other, without the prior written consent of the other Party to do so. All individuals employed by a Party shall be employees of that Party and not of the other Party and all costs and obligations incurred by reason of such employment shall be for the account and expense of such Party.
15.7Force Majeure. Both Parties shall be excused from the performance of their obligations under this Agreement to the extent that such performance is prevented by Force Majeure and the nonperforming Party promptly provides notice of such Force Majeure circumstances to the other Party. Such excuse shall be continued so long as the condition constituting Force Majeure continues and the nonperforming Party takes reasonable efforts to remove the condition. Notwithstanding the foregoing, a Party shall not be excused from making payments owed hereunder because of a Force Majeure affecting such Party. If a Force Majeure persists for more than ninety (90) days, then the Parties shall discuss in good faith the modification of the Parties’ obligations under this Agreement in order to mitigate the delays caused by such Force Majeure. In the event a Party is prevented from performing its obligations under this Agreement due to Force Majeure for more than six (6) months according to this Section 15.7, the other Party shall have the right to terminate this Agreement upon sixty (60) days’ notice after the expiration of such period. A termination under this Section 15.7 by either Party shall be treated as a termination under Section 12.4 and the corresponding provisions for termination under Section 12.9(a) shall apply.
|
Confidential |
129 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
15.8Entire Agreement; Amendments. This Agreement, including the Exhibits hereto, sets forth the complete, final and exclusive agreement and all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties with respect to the subject matter hereof and supersedes, as of the Effective Date, all prior and contemporaneous agreements and understandings between the Parties with respect to the subject matter hereof, including the Prior CDA. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties with respect to the subject matter hereof other than as are set forth herein and therein. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party. In the event of any inconsistency between the body of this Agreement and any Exhibits to this Agreement or any subsequent agreements ancillary to this Agreement, unless otherwise express stated to the contrary in such Exhibit or ancillary agreement, the terms contained in this Agreement shall control. For the avoidance of doubt, as between this Agreement and any supply agreement, quality agreement or PVA entered into between the Parties following the Effective Date, the terms and conditions of such supply agreement, quality agreement or PVA shall govern and control with respect to the subject matter thereof.
15.9Severability. If any one or more of the provisions of this Agreement is held to be invalid or unenforceable by any court of competent jurisdiction from which no appeal can be or is taken, the provision shall be considered severed from this Agreement and shall not serve to invalidate any remaining provisions hereof. The Parties shall make good faith efforts to replace any invalid or unenforceable provision with a valid and enforceable one such that the objectives contemplated by the Parties when entering this Agreement may be realized.
15.10English Language. This Agreement shall be written in and executed in, and all other communications under or in connection with this Agreement shall be in, the English language. Any translation into any other language shall not be an official version hereof or thereof, and in the event of any conflict in interpretation between the English version and such translation, the English version shall control.
15.11Waiver and Non-Exclusion of Remedies. Any term or condition of this Agreement may be waived at any time by the Party that is entitled to the benefit thereof, but no such waiver shall be effective unless set forth in a written instrument duly executed by or on behalf of the Party waiving such term or condition. The waiver by either Party of any right hereunder or of the failure to perform or of a breach by the other Party shall not be deemed a waiver of any other right hereunder or of any other breach or failure by such other Party whether of a similar nature or otherwise. The rights and remedies provided herein are cumulative and do not exclude any other right or remedy provided by Applicable Law or otherwise available except as expressly set forth herein.
15.12Further Assurance. Each Party shall duly execute and deliver, or cause to be duly executed and delivered, such further instruments and do and cause to be done such further acts and things, including the filing of such assignments, agreements, documents, and instruments, as
|
Confidential |
130 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
may be necessary or as the other Party may reasonably request in connection with this Agreement to carry out more effectively the provisions and purposes hereof.
15.13Headings. The headings of each Article and Section in this Agreement have been inserted for convenience of reference only and are not intended to limit or expand on the meaning of the language contained in the particular Article or Section.
15.14Construction. Whenever this Agreement refers to a number of days without using a term otherwise defined herein, such number refers to calendar days, whether or not “calendar days” is expressly stated. Except where the context otherwise requires, (a) wherever used, the singular shall include the plural, the plural shall include the singular; (b) the use of any gender shall be applicable to all genders; (c) the terms “including,” “include,” “includes” and “for example” shall not limit the generality of any description preceding such term and, as used herein, shall have the same meaning as “including, but not limited to,” and “including, without limitation”; (d) the words “herein”, “hereof” and hereunder”, and words of similar import, refer to this Agreement in its entirety and not to any particular provision hereof; (e) the word “will” means “shall”; (f) if a period of time is specified and dates from a given day or Business Day, or the day or Business Day of an act or event, it is to be calculated exclusive of that day or Business Day; (g) “Dollar”, “USD” or “$” means U.S. Dollars; (h) references to a particular Person include such Person’s successors and assigns to the extent not prohibited by this Agreement; (i) a capitalized term not defined herein but reflecting a different part of speech than a capitalized term which is defined herein shall be interpreted in a correlative manner; and (j) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein). The language of this Agreement shall be deemed to be the language mutually chosen by the Parties and no rule of strict construction shall be applied against either Party. Each Party represents that it has been represented by legal counsel in connection with this Agreement and acknowledges that it has participated in the drafting hereof.
15.15Counterparts. This Agreement may be executed in two (2) or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. This Agreement may be executed by .pdf or other electronically transmitted signatures and such signatures shall be deemed to bind each Party as if they were the original signatures.
SIGNATURE PAGE FOLLOWS
|
Confidential |
131 |
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
IN WITNESS WHEREOF, the Parties have signed this Agreement as of the date first set forth above.
|
JANSSEN BIOTECH, INC. |
||
|
|
|
|
|
By: |
/s/ Scott White |
|
|
|
|
|
|
Name: |
Scott White |
|
|
|
|
|
|
Title: |
VP Janssen Biotech, Inc. |
|
|
|
|
|
|
|
|
|
|
TESARO, INC. |
||
|
|
|
|
|
|
|
|
|
By: |
/s/ Leon O. Moulder, Jr. |
|
|
|
|
|
|
Name: |
Leon O. Moulder, Jr. |
|
|
|
|
|
|
Title: |
CEO |
|
[Signature Page to Collaboration and License Agreement]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 1.63
Initial Compound
Niraparib, formerly known as MK-4827, licensed to TESARO under the Merck License Agreement, a potent and selective poly (ADP-ribose) polymerase (PARP) inhibitor, with the chemical name as follows:
[***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 1.69
2016 Johnson & Johnson Universal Calendar
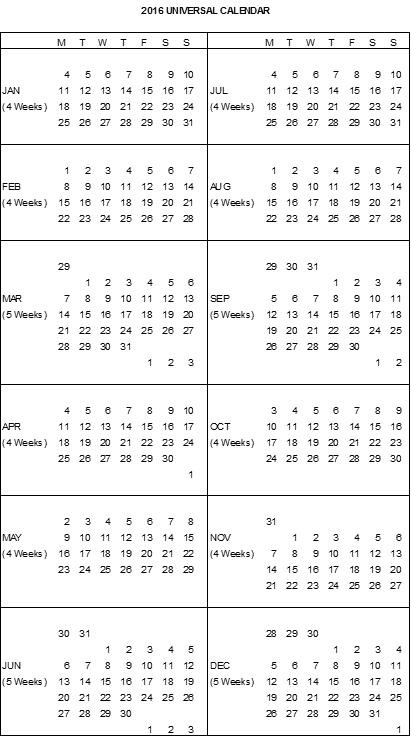
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 1.117
TESARO Patents
Patent schedule for MK-4827 and MK-2512
(1) IRT-ONC-0121Y: Amide substituted indazole and benzotriazole derivatives as poly(ADP-ribose)polymerase(PARP) inhibitors
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Australia |
2007232297 |
02 April 2007 |
Granted |
2007232297 |
03 January 2013 |
|
Canada |
2647545 |
02 April 2007 |
Granted |
2647545 |
23 February 2016 |
|
China P.R. |
200780012001.6 |
02 April 2007 |
Granted |
101415686 |
13 March 2013 |
|
European Patent Convention |
07733600.6 |
02 April 2007 |
Allowed |
||
|
India |
8150/DELNP/08 |
02 April 2007 |
Pending |
||
|
Japan |
2009-503667 |
02 April 2007 |
Granted |
4611441 |
22 October 2010 |
|
United States |
13/091427 |
21 April 2011 |
Abandoned |
(2) IRT-ONC-0121Y2: Amide substituted indazoles as poly(ADP-ribose)polymerase(PARP) inhibitors
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Albania |
AL/P/2011/3701 |
08 January 2008 |
Granted |
3755 |
23 March 2011 |
|
Algeria |
090395 |
08 January 2008 |
Granted |
6603 |
30 May 2011 |
|
Argentina |
P080100061 |
08 January 2008 |
Granted |
AR064777B1 |
23 April 2014 |
|
Armenia |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Australia |
2008204380 |
08 January 2008 |
Granted |
2008204380 |
28 November 2013 |
|
Austria |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Austria |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Azerbaijan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Barbados |
2001/1480 |
08 January 2008 |
Pending |
||
|
Belarus |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Belgium |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Belgium |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Belize |
587.09 |
08 January 2008 |
Granted |
587.09 |
05 March 2010 |
|
Bermuda |
418EP |
08 January 2008 |
Granted |
418P |
26 September 2012 |
|
Bosnia-Herzegovina |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Brazil |
PI0806245-5 |
08 January 2008 |
Pending |
||
|
Bulgaria |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Canada |
2674436 |
08 January 2008 |
Granted |
2674436 |
17 July 2012 |
|
Chile |
00046-2008 |
08 January 2008 |
Pending |
48.598 |
03 May 2013 |
|
China P.R. |
200880001926.5 |
08 January 2008 |
Granted |
101578279 |
17 July 2013 |
|
Colombia |
09068415 |
08 January 2008 |
Granted |
2532 |
24 August 2012 |
|
Costa Rica |
10910 |
08 January 2008 |
Granted |
3180 |
23 March 2015 |
|
Croatia |
08702101.0 |
08 January 2008 |
Granted |
P20110447 |
23 March 2011 |
|
Cyprus |
CY20111100595 |
08 January 2008 |
Granted |
CY1111584 |
23 March 2011 |
|
Czech Republic |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Czech Republic |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Denmark |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Denmark |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Dominican Republic |
P2009-0170 |
08 January 2008 |
Pending |
||
|
Ecuador |
SP-09-9484 |
08 January 2008 |
Pending |
||
|
Egypt |
PCT1065/2009 |
08 January 2008 |
Pending |
||
|
El Salvador |
2009003321 |
08 January 2008 |
Granted |
20090008141 |
14 February 2011 |
|
Estonia |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Eurasian Patent Convention |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
European Patent Convention |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
European Patent Convention |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
European Patent Convention |
14176452.2 |
26 November 2014 |
Pending |
||
|
Finland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Finland |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
France |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
France |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Georgia |
AP2008011413 |
08 January 2008 |
Granted |
P5337 |
25 November 2011 |
|
Germany |
08702101.0 |
08 January 2008 |
Granted |
602008005711.9 |
23 March 2011 |
|
Germany |
11157369.7 |
08 March 2011 |
Granted |
602008033393.0 |
16 July 2014 |
|
Great Britain |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Great Britain |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Greece |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Greece |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Guatemala |
A-2009-00190 |
08 January 2008 |
Granted |
5761 |
05 May 2014 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Gulf Cooperation Council |
2008/9897 |
05 January 2008 |
Pending |
||
|
Honduras |
2009-001260 |
08 January 2008 |
Granted |
5355 |
08 March 2013 |
|
Hong Kong |
09110676.1 |
08 January 2008 |
Granted |
1131137 |
09 December 2011 |
|
Hong Kong |
11108534.3 |
08 January 2008 |
Granted |
1154386 |
06 February 2015 |
|
Hungary |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Iceland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
India |
4674/DELNP/2009 |
08 January 2008 |
Granted |
IN270342 |
14 December 2015 |
|
Indonesia |
W00200901818 |
08 January 2008 |
Granted |
IDP0030184 |
10 February 2012 |
|
Ireland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Ireland |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Israel |
199264 |
08 January 2008 |
Granted |
199264 |
28 September 2013 |
|
Italy |
502011901952934 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Italy |
502014000000867 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Japan |
2009-545240 |
08 January 2008 |
Granted |
4611444 |
22 October 2010 |
|
Kazakhstan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Korea South |
10-2009-7014520 |
08 January 2008 |
Pending |
||
|
Kyrgyzstan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Latvia |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Lebanon |
8156 |
08 January 2008 |
Granted |
8156 |
22 September 2008 |
|
Lithuania |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Luxembourg |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Macao |
J/001224 |
08 January 2008 |
Granted |
J/00124 |
11 December 2013 |
|
Macedonia |
P-2011/142 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Malaysia |
PI 20092876 |
08 January 2008 |
Granted |
147789 |
31 January 2013 |
|
Malta |
EP00300 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Mexico |
MX/a/2009/007200 |
08 January 2008 |
Granted |
277664 |
29 July 2010 |
|
Moldova |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Mongolia |
4314 |
08 January 2008 |
Granted |
3334 |
25 October 2009 |
|
Morocco |
PV32111 |
08 January 2008 |
Granted |
31554 |
02 August 2010 |
|
Netherlands |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Netherlands |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
New Zealand |
578256 |
08 January 2008 |
Granted |
578256 |
02 April 2012 |
|
Nicaragua |
2009-0135 |
08 January 2008 |
Pending |
||
|
Norway |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Norway |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Pakistan |
20/2008 |
08 January 2008 |
Pending |
||
|
Peru |
97 |
08 January 2008 |
Granted |
6371 |
29 February 2012 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Philippines |
1-2009-501286 |
08 January 2008 |
Granted |
1-2009-501286 |
21 September 2012 |
|
Poland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Poland |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Portugal |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Portugal |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Romania |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Russian Federation |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Serbia |
P-236/2011 |
08 January 2008 |
Granted |
51780 |
23 March 2011 |
|
Singapore |
200904280-5 |
08 January 2008 |
Granted |
153520 |
13 July 2012 |
|
Slovak Republic |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Slovak Republic |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Slovenia |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Solvenia |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
South Africa |
2009/03898 |
08 January 2008 |
Granted |
2009/03898 |
28 April 2010 |
|
Spain |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Spain |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Sweden |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Sweden |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Switzerland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Switzerland |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Taiwan |
097100730 |
08 January 2008 |
Pending |
||
|
Tajikistan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Thailand |
0801000088 |
08 January 2008 |
Pending |
||
|
Trinidad |
TT/A/2009/00132 |
08 January 2008 |
Pending |
||
|
Tunisia |
TN2009/0286 |
08 January 2008 |
Granted |
21034 |
20 September 2011 |
|
Turkey |
2011/05543 |
08 January 2008 |
Granted |
2011 05543 |
23 March 2011 |
|
Turkmenistan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Ukraine |
2009 08335 |
08 January 2008 |
Granted |
97658 |
12 March 2012 |
|
United States |
12/006993 |
08 January 2008 |
Granted |
8071623 |
06 December 2011 |
|
Venezuela |
2008-000029 |
08 January 2008 |
Pending |
||
|
Vietnam |
1-2009-01655 |
08 January 2008 |
Pending |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(3) TER-ONC-1729: Pharmaceutically acceptable salts of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Australia |
2009203598 |
08 January 2009 |
Granted |
2009203598 |
09 January 2014 |
|
Brazil |
PI0906020-0 |
08 January 2009 |
Pending |
||
|
Canada |
2711491 |
08 January 2009 |
Pending |
2653529 |
09 February 2016 |
|
China P.R. |
200980101861.6 |
08 January 2009 |
Pending |
||
|
European Patent Convention |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
France |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Germany |
09700579.7 |
08 January 2009 |
Granted |
602009032466.7 |
29 July 2015 |
|
Great Britain |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
India |
4920/DELNP/2010 |
08 January 2009 |
Pending |
||
|
Ireland |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Israel |
206201 |
08 January 2009 |
Granted |
206201 |
01 November 2011 |
|
Italy |
502015000061194 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Japan |
2010-541101 |
08 January 2009 |
Pending |
||
|
Korea South |
10-2010-7015011 |
08 January 2009 |
Pending |
||
|
Mexico |
MX/a/2010/006593 |
08 January 2009 |
Pending |
||
|
Netherlands |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
New Zealand |
586675 |
08 January 2009 |
Granted |
586675 |
06 August 2012 |
|
Russian Federation |
2010133241 |
08 January 2009 |
Granted |
2495035 |
10 October 2013 |
|
South Africa |
2010/03902 |
08 January 2009 |
Granted |
2010/03902 |
23 February 2011 |
|
Spain |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Switzerland |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Turkey |
2015/13219 |
08 January 2009 |
Granted |
2015 13219 |
29 July 2015 |
|
United States |
12/811922 |
08 January 2009 |
Granted |
8436185 |
07 May 2013 |
(4) IRT-ONC-0129: Pyridinone and pyridazinone derivatives as inhibitors of poly(ADP-ribose)polymerase (PARP)
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Australia |
2007266836 |
25 May 2007 |
Granted |
2007266836 |
09 January 2014 |
|
Brazil |
PI0711741-8 |
25 May 2007 |
Pending |
||
|
Canada |
2653529 |
25 May 2007 |
Pending |
||
|
China P.R. |
200780020136.7 |
25 May 2007 |
Pending |
||
|
China P.R. |
201310316726.1 |
25 July 2013 |
Pending |
||
|
European Patent Convention |
07733716.0 |
25 May 2007 |
Pending |
||
|
India |
9794/DELNP/2008 |
25 May 2007 |
Pending |
||
|
Israel |
195113 |
25 May 2007 |
Granted |
195113 |
29 August 2014 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Japan |
2009-512681 |
25 May 2007 |
Granted |
5351755 |
30 August 2013 |
|
Korea South |
10-2008-7029132 |
25 May 2007 |
Granted |
10-1529891 |
12 June 2015 |
|
Mexico |
MX/a/2008/015014 |
25 May 2007 |
Granted |
311801 |
29 July 2013 |
|
New Zealand |
572815 |
25 May 2007 |
Granted |
572815 |
05 March 2012 |
|
Norway |
2008 5397 |
25 May 2007 |
Pending |
||
|
Russian Federation |
2008152824 |
25 May 2007 |
Granted |
2472782 |
20 January 2013 |
|
South Africa |
2008/09238 |
25 May 2007 |
Granted |
2008/09238 |
30 December 2009 |
|
United States |
12/227513 |
25 May 2007 |
Granted |
8188084 |
29 May 2012 |
(5) IRT-ONC-0152: Pyridazinone derivatives as PARP inhibitors
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Australia |
2008322676 |
14 November 2008 |
Granted |
2008322676 |
20 March 2014 |
|
Brazil |
PI0820236-2 |
14 November 2008 |
Pending |
||
|
Canada |
2704714 |
14 November 2008 |
Pending |
||
|
China P.R. |
200880115590.5 |
14 November 2008 |
Granted |
101855221 |
30 October 2013 |
|
European Patent Convention |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
France |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Great Britain |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Germany |
08850429.5 |
14 November 2008 |
Granted |
602008034267.0 |
03 September 2014 |
|
India |
2638/CHENP/2010 |
14 November 2008 |
Pending |
||
|
Israel |
205142 |
14 November 2008 |
Granted |
205142 |
31 December 2014 |
|
Italy |
502014000002462 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Japan |
2010-533666 |
14 November 2008 |
Granted |
5758630 |
12 June 2015 |
|
Korea South |
10-2010-7013113 |
14 November 2008 |
Pending |
||
|
Mexico |
MX/a/2010/005070 |
14 November 2008 |
Granted |
298063 |
12 April 2012 |
|
Netherlands |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
New Zealand |
585395 |
14 November 2008 |
Granted |
585395 |
09 July 2012 |
|
Russian Federation |
2010123874 |
14 November 2008 |
Granted |
2490265 |
20 August 2013 |
|
South Africa |
2010/02466 |
14 November 2008 |
Granted |
2010/02466 |
29 December 2010 |
|
Switzerland |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Spain |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Turkey |
201414390 |
14 November 2008 |
Granted |
2014 14390 |
03 September 2014 |
|
United States |
12/739262 |
14 November 2008 |
Granted |
8268827 |
18 September 2012 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
[***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 4.2(c)
TESARO Development Plan
A summary of ongoing and planned Phase 2 and Phase 3 clinical studies is provided as shown below:
[***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 4.2(f)
TESARO INDs
1.IND 100,996 (Ovarian)
2.IND 117,580 (Breast)
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 4.2(h)
Example of Third Party Development Report
See attached.
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
[***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 5.5(a)
Regulatory Documentation Relating to Initial Compound and Initial Product
A.Major Niraparib Interactions with FDA:[***]
B.Major Niraparib Interactions with EMA:[ ***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 7.1(a)
Company Supply Requirements and Timeline
This Exhibit 7.1(a) will be revised upon execution of the Clinical Supply Agreement.
7.1(a)(iii) Clinical Supplies
The following reflects Company’s projected requirements for clinical supply of Single Agent Products for the Clinical Trials listed below:[***]
*For Trial execution about [***] of total supply is required at least [***] before [***]. A [***] resupply is required for each of the clinical trials to be provided [***] (a detailed schedule will be included in the Clinical Supply Agreement)
7.3(c)API Supply for Fixed Dose Formulation Development
The following reflects Company’s projected requirements of Compound API for the periods listed below:
[***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 8.2(d)
Merck Milestone Payments
|
Merck Milestone Payments* |
|
|
Merck Milestone |
Merck Milestone Payment Amount |
|
On a Licensed Product-by-Licensed Product basis |
1st Indication for a given Licensed Product |
|
1. Initiation** of the first Phase III Clinical Trial for a Licensed Product |
$[***] |
|
2. Filing and acceptance of the first NDA for a Licensed Product in the United States |
$[***] |
|
3. Filing of the first application for marketing authorization of a Licensed Product in the EU |
$[***] |
|
4. Granting of Marketing Authorization for a Licensed Product in the U.S. |
$[***] |
|
5. Granting of Marketing Authorization *** for a Licensed Product in the EU |
$[***] |
* All capitalized terms used in this Exhibit 8.2(d) shall have the meaning set forth in Article I of the Merck License Agreement.
** “Initiation” means first dosing of the first properly enrolled patient in the relevant Clinical Trial.
*** This milestone requires granting of Marketing Authorization for a Licensed Product for the relevant indication in any three (3) of the Major European Countries.
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 8.3
Alliance Payments
1. Definitions.
“Commercialization Expenses” means [***].
“Distribution Expenses” means [***].
“First Prostate Use” of a Prostate-Approved Single Agent Product in a country in the Company Territory means the first to occur of the event described in the definition of “Prostate-Approved Single Agent Product” with respect to such Prostate-Approved Single Agent Product in such country.
2.Selection of Reporting Services for Net Sales Allocation
2.1 Major Market Net Sales Allocation Methodology. No later than [***] prior to the anticipated First Prostate Use of a Prostate-Approved Single Agent Product in any Major Market, the JCC, based on a recommendation by the JFC, will develop and approve a methodology that will be used to allocate sales of Prostate-Approved Single Agent Products by indication in each of the Major Markets (the “Major Market Net Sales Allocation Methodology”). The Major Market Net Sales Allocation Methodology will also be used to allocate sales of Prostate-Approved Single Agent Products by indication in any other countries deemed appropriate by the JCC (the “Other Included Countries”). [***] On an [***] basis, the JCC will reassess the Major Market Net Sales Allocation Methodology and its data sources for each of the Major Markets, for prospective use, and will use reasonable efforts to agree to any required changes in such methodology. The JCC will also prospectively reassess the use of such methodology in the Other Included Countries and whether additional countries should be included as Other Included Countries. If two or more distinct Prostate-Approved Single Agent Products are sold in the same country, the same Major Market Net Sales Allocation Methodology will be used for all such products unless otherwise determined by the JCC. The methodology selected can be in the form of Net Sales or volume of units.
2.2 Residual Allocation Methodologies. The JCC will also develop and approve methodologies to be utilized to allocate the sales of Prostate-Approved Single Agent Products by indication in countries that are not Major Markets or Other Included Countries on a region-by-region basis (each, a “Residual Allocation Methodology”) no later than [***] prior to the anticipated First Prostate Use in each such country. The Parties expect that Residual Allocation Methodologies will be used in countries in the following regions: [***]. There will be one Residual Allocation Methodology per region; provided, however, that if either Party reasonably believes that within a region utilization of Prostate-Approved Single Agent Products in any one country is significantly different from the other countries in the region such that applying the regional Residual Allocation Methodology would result in an inappropriate allocation methodology, the Parties shall discuss in good faith and attempt to agree on whether a country-specific allocation
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
methodology should be used in such country for a defined timeframe. If two or more distinct Prostate-Approved Single Agent Products are sold in a region, the same Residual Allocation Methodology will be used for all such products in such region, unless otherwise determined by the JCC. The Residual Allocation Methodologies selected can be in the form of Net Sales or volume of units.
2.3 Deadlocks. If the JCC does not reach unanimous agreement to the Major Market Net Sales Allocation Methodology, a Residual Allocation Methodology, the list of Other Included Countries or any other JCC decision described in this Exhibit 8.3 as provided in Section 2.3(c), such matter will be deemed to be a Deadlocked Committee Matter as provided in Section 2.3(c). If a Deadlocked Committee Matter relating to the Major Market Net Sales Allocation Methodology or a Residual Allocation Methodology for a country is not resolved prior to launch of a Prostate-Approved Single Agent Product in such country, [***].
3.Calculation of the Alliance Payments. For each country in the Company Territory where a Prostate-Approved Single Agent Product is sold by TESARO (or any of its Affiliates, licensees (other than Company), sublicensees or Third Party distributors) during a TESARO Calendar Quarter, TESARO shall calculate the Alliance Payments within [***] after the end of such TESARO Calendar Quarter as follows. For clarity, any reference in this Exhibit 8.3 to “Net Sales” shall mean Net Sales as defined in this Agreement, without regard to the definition of Net Sales or a similar term in any agreement between TESARO and a Third Party.
3.1 Determination of Prostate Attributable Net Sales
For each country that is a Major Market or an Other Included Country, the amount of Net Sales of such Prostate-Approved Single Agent Product in a TESARO Calendar Quarter attributable to use in the Company Field shall be calculated as follows, on a country-by-country basis:
(a) Utilizing the Major Market Net Sales Allocation Methodology, the Parties will calculate a “Prostate Sales Allocation Factor” for such country for such TESARO Calendar Quarter. The Prostate Sales Allocation Factor is a percentage equal to [***]. The JCC shall determine whether to use Net Sales or volume of units to calculate the Prostate Sales Allocation Factor for a country, depending on which data sources will be used in such country.
(b) The Prostate Sales Allocation Factor will then be multiplied by [***].
For each country that is neither a Major Market nor an Other Included Country, the amount of Net Sales of such Prostate-Approved Single Agent Product in a TESARO Calendar Quarter attributable to use in the Company Field shall be calculated as follows, on a region-by-region basis:
(a) Utilizing the Residual Allocation Methodology, the Parties will calculate a “Residual Prostate Sales Allocation Factor” for such region for such TESARO
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Calendar Quarter. The Residual Prostate Sales Allocation Factor is a percentage equal to [***]. The JCC shall determine whether to use Net Sales or volume of units to calculate the Prostate Sales Allocation Factor for a region, depending on which data sources will be used in such region.
(b) The Residual Prostate Sales Allocation Factors will then be multiplied by [***].
3.2 Disproportionate Allocation of Rebates. Upon the request of either Party, the JFC shall review and discuss whether rebates payable to federal, state/provincial, local or other governments, or their agencies, with respect to sales of a Prostate-Approved Single Agent Product in a country should be allocated on a disproportionate basis between the Company Field and TESARO Field when calculating Net Sales of such Prostate-Approved Single Agent Product in such country to reflect the actual payor mix of such Prostate-Approved Single Agent Product in such country.
3.3 Adjustments to Prior Period Single Agent Prostate Net Sales
It is acknowledged by both Parties that [***]. For this reason, the mechanism described below allows for an adjustment in a subsequent TESARO Calendar Quarter.
Within [***] after the end of such TESARO Calendar Quarter and upon [***], TESARO shall determine whether any of the Prostate Sales Allocation Factors and Residual Prostate Sales Allocation Factors applied to the Net Sales of Prostate-Approved Single Agent Products under Section 3.1 of this Exhibit 8.3 for a prior TESARO Calendar Quarter needs to be revised. If a revision is required, TESARO will recalculate on a region-by-region or country-by-country basis, as applicable, the Net Sales of Prostate-Approved Single Agent Products attributable to use in the Company Field under the applicable Major Market Net Sales Allocation Methodology or Residual Allocation Methodology. The revised allocation of Net Sales of Prostate-Approved Single Agent Products for such TESARO Calendar Quarter will be compared to the initial allocation of such Net Sales to determine the required adjustment. After TESARO completes such recalculations, the JFC shall review and approve any proposed reallocations and adjustments before they become effective. [***].
3.4 Total Net Sales of Prostate-Approved Single Agent Products
The total TESARO Net Sales of a Prostate-Approved Single Agent Product attributable to use in the Company Field in any country in the Company Territory during a given TESARO Calendar Quarter shall be the sum of: (a) the amount of TESARO Net Sales of such Prostate-Approved Single Agent Product attributable to use in the Company Field in such country (calculated as described above in Sections 3.1 of this Exhibit 8.3); and (b) any prior-quarter Net Sales adjustments with respect to such Single Agent Product that are required due to updated Prostate Sales Allocation Factors and Residual Prostate Sales Allocation Factors (as described above in Section 3.3 of this Exhibit 8.3) (the “TESARO Prostate-Approved Single Agent Product Net Sales”).
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
3.5 Determination of Alliance Payments Due to Company
TESARO will deduct from the aggregate TESARO Prostate-Approved Single Agent Product Net Sales in all countries in the Company Territory in a given TESARO Calendar Quarter (calculated as described above in Section 3.4 of this Exhibit 8.3) the following costs to calculate the Alliance Payment for such TESARO Calendar Quarter: [***]
For clarity, TESARO shall not deduct any milestone payments due to it as part of this calculation under this Section 3 of this Exhibit 8.3.
If the balance of TESARO Net Sales of Prostate-Approved Single Agent Products attributable to use in the Company Field in a given TESARO Calendar Quarter less the deductions detailed above results in a deficit, such balance shall be reported to Company in the Alliance Payments report and TESARO shall invoice the Company due with terms consistent with Section 8.14 to the extent Company has not reimbursed TESARO for such amounts pursuant to another provision of this Agreement.
4.Alliance Payments Report. TESARO shall deliver a written report (the “Alliance Payments Report”) to Company setting forth its calculations of the Alliance Payments, together with reasonable supporting documentation, within [***] after the end of each TESARO Calendar Quarter. Details to be included in this report shall be defined by the JFC, and will include a sufficient level of detail to provide Company with information to assess the Alliance Payments calculation.
5.Example Calculation. An example of the calculations to be performed by TESARO under Section 3 of this Exhibit 8.3 is included below for illustrative purposes only:
[***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 8.4(b)(i)(1)
Pass-through Merck Royalty
1.Definitions. For purposes of this Exhibit 8.4(b)(i)(1), the following terms shall have the following meanings:
“Effective Merck Royalty Rate” means, with respect to a given Prostate-Approved Single Agent Product and a given TESARO Calendar Quarter, the amount equal to A/B expressed as a percentage, where:
A = the aggregate amount of royalties payable by TESARO to Merck pursuant to the Merck License Agreement (taking into account all applicable royalty reductions) with respect to Combined Worldwide Prostate-Approved Single Agent Product Net Sales for such Prostate-Approved Single Agent Product during such TESARO Calendar Quarter; and
B = the Combined Worldwide Prostate-Approved Single Agent Product Net Sales for such Prostate-Approved Single Agent Product during such Calendar Quarter.
In the event that TESARO effects a reduction in, or elimination of, the applicable royalty rate(s) under the Merck License Agreement through a buy down or other negotiated agreement with Merck, then “A” shall be calculated by applying the Pass-through Merck Royalty Rates set forth in Section 8.4(b)(i)(1) and the royalty reductions set forth in this Exhibit 8.4(b)(i)(1) to the applicable Combined Worldwide Prostate-Approved Single Agent Product Net Sales.
“Merck Compound Patent Rights” means those patents and patent applications that are listed on Attachment 1 to this Exhibit 8.4(b)(i)(1) and claim the composition of matter or use of a Compound, together with all (a) substitutions, divisions, continuations, continuations-in-part, continued prosecution applications, reissues, renewals, registrations, certificates of invention, confirmations, re-examinations, extensions, supplementary protection certificates or the like, or the provisional applications of any such patents and patent applications and (b) foreign equivalents of any of the above.
“Merck First Commercial Sale” means, with respect to a country in the Company Territory, the first sale of commercial quantities of a Product in such country to a Third Party on arm’s length terms by a Party, its Affiliate or (sub)licensee for use in the Field after the receipt of Marketing Approval in such country. Sales for test marketing, sampling and promotional uses, Clinical Trial purposes or compassionate or similar use shall not be considered to constitute a Merck First Commercial Sale.
“Merck Royalty Term” means, with respect to a Product and a given country, the period commencing upon the Merck First Commercial Sale of such Product in such country and continuing until the later of: (i) the expiration of the last to expire Merck Valid Claim of a Merck Compound Patent Right covering or claiming such Product (or the Compounds contained in, or
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
comprising, such Product); or (ii) the tenth (10th) anniversary of the date of the Merck First Commercial Sale of such Product in such country (the “Merck Royalty Term”).
“Merck Valid Claim” means a claim of an issued and unexpired patent included within the Merck Compound Patent Rights, that has not been revoked or held unenforceable or invalid by a decision of a court or other governmental agency of competent jurisdiction, unappealable or unappealed within the time allowed for appeal, and that has not been disclaimed, denied or admitted to be invalid or unenforceable through reissue or disclaimer.
2.Pass-through Merck Royalty Term. The Pass-through Merck Royalty under Section 8.4(b)(i)(1) with respect to Prostate-Approved Single Agent Product Net Sales or Company Combination Product Net Sales, as applicable, shall be payable on a Product-by-Product and country-by-country basis for so long as royalties are payable by TESARO to Merck under the Merck License Agreement with respect to the applicable Prostate-Approved Single Agent Product or Combination Product in such country; provided, however, that if TESARO effects a reduction in, or elimination of, the applicable royalty rate(s) under the Merck License Agreement through a buy down or other negotiated agreement with Merck, then the Pass-through Merck Royalty shall be payable until the expiration of the Merck Royalty Term with respect to the applicable Prostate-Approved Single Agent Product or Combination Product in such country.
3.Pass-through Merck Royalty Adjustments. Notwithstanding Section 8.4(b)(i)(1), in the event that the sale of a Product in a country in the Company Territory during the applicable royalty term pursuant to Section 2 of this Exhibit 8.4(b)(i)(1) with respect to such Product and such country, would not infringe a Merck Valid Claim, then Pass-through Merck Royalty Rates set forth in Section 8.4(b)(i)(1) shall be reduced by [***] with respect to Prostate-Approved Single Agent Product Net Sales or Combination Product Net Sales, as applicable, of such Product in such country. If a Product is covered by Merck Valid Claims of more than one Merck Compound Patent Right, the Pass-through Merck Royalty under Section 8.4(b)(i)(1) shall be payable only once.
4.[***].
5.Calculation of Royalty Tier for Pass-through Merck Royalty. In the event that there are both Company Prostate-Approved Single Agent Product Net Sales and TESARO Prostate-Approved Single Agent Product Net Sales with respect to a Prostate-Approved Single Agent Product during a TESARO Calendar Quarter, then TESARO shall use the report delivered by Company pursuant to Section 8.3(b)(ii) to determine the Combined Worldwide Prostate-Approved Single Agent Product Net Sales during such TESARO Calendar Quarter and the Pass-through Merck Royalty Rate(s) applicable to such Combined Worldwide Prostate-Approved Single Agent Product Net Sales during such TESARO Calendar Quarter. In the event that two (2) or more Pass-through Merck Royalty Rate(s) apply with respect to such Combined Worldwide Prostate-Approved Single Agent Product Net Sales during such TESARO Calendar Quarter, TESARO shall calculate the Effective Merck Royalty Rate with respect to such Combined Worldwide Prostate-Approved Single Agent Product Net Sales. TESARO shall
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
notify Company of the applicable Pass-through Merck Royalty Rate or Effective Merck Royalty Rate with respect to such Calendar Quarter within [***] after receipt of the report delivered by Company pursuant to Section 8.3(b)(ii). Company shall use such Pass-through Merck Royalty Rate or Effective Merck Royalty Rate to calculate the Pass-through Merck Royalty amounts payable by Company to TESARO with respect to the Company Prostate-Approved Single Agent Product Net Sales during such Calendar Quarter, and TESARO shall use such Pass-through Merck Royalty Rate or Effective Merck Royalty Rate to calculate the Pass-through Merck Royalty amounts to be deducted when calculating the Alliance Payments due to Company with respect to TESARO Prostate-Approved Single Agent Product Net Sales.
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Attachment 1 to Exhibit 8.4(b)(i)(1)
Merck Compound Patent Rights
Patent schedule for MK-4827 and MK-2512
(1) IRT-ONC-0121Y: Amide substituted indazole and benzotriazole derivatives as poly(ADP-ribose)polymerase(PARP) inhibitors
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Australia |
2007232297 |
02 April 2007 |
Granted |
2007232297 |
03 January 2013 |
|
Canada |
2647545 |
02 April 2007 |
Granted |
2647545 |
23 February 2016 |
|
China P.R. |
200780012001.6 |
02 April 2007 |
Granted |
101415686 |
13 March 2013 |
|
European Patent Convention |
07733600.6 |
02 April 2007 |
Allowed |
||
|
India |
8150/DELNP/08 |
02 April 2007 |
Pending |
||
|
Japan |
2009-503667 |
02 April 2007 |
Granted |
4611441 |
22 October 2010 |
|
United States |
13/091427 |
21 April 2011 |
Abandoned |
(2) IRT-ONC-0121Y2: Amide substituted indazoles as poly(ADP-ribose)polymerase(PARP) inhibitors
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Albania |
AL/P/2011/3701 |
08 January 2008 |
Granted |
3755 |
23 March 2011 |
|
Algeria |
090395 |
08 January 2008 |
Granted |
6603 |
30 May 2011 |
|
Argentina |
P080100061 |
08 January 2008 |
Granted |
AR064777B1 |
23 April 2014 |
|
Armenia |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Australia |
2008204380 |
08 January 2008 |
Granted |
2008204380 |
28 November 2013 |
|
Austria |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Austria |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Azerbaijan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Barbados |
2001/1480 |
08 January 2008 |
Pending |
||
|
Belarus |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Belgium |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Belgium |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Belize |
587.09 |
08 January 2008 |
Granted |
587.09 |
05 March 2010 |
|
Bermuda |
418EP |
08 January 2008 |
Granted |
418P |
26 September 2012 |
|
Bosnia-Herzegovina |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Brazil |
PI0806245-5 |
08 January 2008 |
Pending |
||
|
Bulgaria |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Canada |
2674436 |
08 January 2008 |
Granted |
2674436 |
17 July 2012 |
|
Chile |
00046-2008 |
08 January 2008 |
Pending |
48.598 |
03 May 2013 |
|
China P.R. |
200880001926.5 |
08 January 2008 |
Granted |
101578279 |
17 July 2013 |
|
Colombia |
09068415 |
08 January 2008 |
Granted |
2532 |
24 August 2012 |
|
Costa Rica |
10910 |
08 January 2008 |
Granted |
3180 |
23 March 2015 |
|
Croatia |
08702101.0 |
08 January 2008 |
Granted |
P20110447 |
23 March 2011 |
|
Cyprus |
CY20111100595 |
08 January 2008 |
Granted |
CY1111584 |
23 March 2011 |
|
Czech Republic |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Czech Republic |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Denmark |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Denmark |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Dominican Republic |
P2009-0170 |
08 January 2008 |
Pending |
||
|
Ecuador |
SP-09-9484 |
08 January 2008 |
Pending |
||
|
Egypt |
PCT1065/2009 |
08 January 2008 |
Pending |
||
|
El Salvador |
2009003321 |
08 January 2008 |
Granted |
20090008141 |
14 February 2011 |
|
Estonia |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Eurasian Patent Convention |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
European Patent Convention |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
European Patent Convention |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
European Patent Convention |
14176452.2 |
26 November 2014 |
Pending |
||
|
Finland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Finland |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
France |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
France |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Georgia |
AP2008011413 |
08 January 2008 |
Granted |
P5337 |
25 November 2011 |
|
Germany |
08702101.0 |
08 January 2008 |
Granted |
602008005711.9 |
23 March 2011 |
|
Germany |
11157369.7 |
08 March 2011 |
Granted |
602008033393.0 |
16 July 2014 |
|
Great Britain |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Great Britain |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Greece |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Greece |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Guatemala |
A-2009-00190 |
08 January 2008 |
Granted |
5761 |
05 May 2014 |
|
Gulf Cooperation Council |
2008/9897 |
05 January 2008 |
Pending |
||
|
Honduras |
2009-001260 |
08 January 2008 |
Granted |
5355 |
08 March 2013 |
|
Hong Kong |
09110676.1 |
08 January 2008 |
Granted |
1131137 |
09 December 2011 |
|
Hong Kong |
11108534.3 |
08 January 2008 |
Granted |
1154386 |
06 February 2015 |
|
Hungary |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Iceland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
India |
4674/DELNP/2009 |
08 January 2008 |
Granted |
IN270342 |
14 December 2015 |
|
Indonesia |
W00200901818 |
08 January 2008 |
Granted |
IDP0030184 |
10 February 2012 |
|
Ireland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Ireland |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Israel |
199264 |
08 January 2008 |
Granted |
199264 |
28 September 2013 |
|
Italy |
502011901952934 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Italy |
502014000000867 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Japan |
2009-545240 |
08 January 2008 |
Granted |
4611444 |
22 October 2010 |
|
Kazakhstan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Korea South |
10-2009-7014520 |
08 January 2008 |
Pending |
||
|
Kyrgyzstan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Latvia |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Lebanon |
8156 |
08 January 2008 |
Granted |
8156 |
22 September 2008 |
|
Lithuania |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Luxembourg |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Macao |
J/001224 |
08 January 2008 |
Granted |
J/00124 |
11 December 2013 |
|
Macedonia |
P-2011/142 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Malaysia |
PI 20092876 |
08 January 2008 |
Granted |
147789 |
31 January 2013 |
|
Malta |
EP00300 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Mexico |
MX/a/2009/007200 |
08 January 2008 |
Granted |
277664 |
29 July 2010 |
|
Moldova |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Mongolia |
4314 |
08 January 2008 |
Granted |
3334 |
25 October 2009 |
|
Morocco |
PV32111 |
08 January 2008 |
Granted |
31554 |
02 August 2010 |
|
Netherlands |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Netherlands |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
New Zealand |
578256 |
08 January 2008 |
Granted |
578256 |
02 April 2012 |
|
Nicaragua |
2009-0135 |
08 January 2008 |
Pending |
||
|
Norway |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Norway |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Pakistan |
20/2008 |
08 January 2008 |
Pending |
||
|
Peru |
97 |
08 January 2008 |
Granted |
6371 |
29 February 2012 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Philippines |
1-2009-501286 |
08 January 2008 |
Granted |
1-2009-501286 |
21 September 2012 |
|
Poland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Poland |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Portugal |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Portugal |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Romania |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Russian Federation |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Serbia |
P-236/2011 |
08 January 2008 |
Granted |
51780 |
23 March 2011 |
|
Singapore |
200904280-5 |
08 January 2008 |
Granted |
153520 |
13 July 2012 |
|
Slovak Republic |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Slovak Republic |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Slovenia |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Solvenia |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
South Africa |
2009/03898 |
08 January 2008 |
Granted |
2009/03898 |
28 April 2010 |
|
Spain |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Spain |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Sweden |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Sweden |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Switzerland |
08702101.0 |
08 January 2008 |
Granted |
2109608 |
23 March 2011 |
|
Switzerland |
11157369.7 |
08 March 2011 |
Granted |
2336120 |
16 July 2014 |
|
Taiwan |
097100730 |
08 January 2008 |
Pending |
||
|
Tajikistan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Thailand |
0801000088 |
08 January 2008 |
Pending |
||
|
Trinidad |
TT/A/2009/00132 |
08 January 2008 |
Pending |
||
|
Tunisia |
TN2009/0286 |
08 January 2008 |
Granted |
21034 |
20 September 2011 |
|
Turkey |
2011/05543 |
08 January 2008 |
Granted |
2011 05543 |
23 March 2011 |
|
Turkmenistan |
200970674 |
08 January 2008 |
Granted |
016079 |
30 January 2012 |
|
Ukraine |
2009 08335 |
08 January 2008 |
Granted |
97658 |
12 March 2012 |
|
United States |
12/006993 |
08 January 2008 |
Granted |
8071623 |
06 December 2011 |
|
Venezuela |
2008-000029 |
08 January 2008 |
Pending |
||
|
Vietnam |
1-2009-01655 |
08 January 2008 |
Pending |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(3) TER-ONC-1729: Pharmaceutically acceptable salts of 2-{4-[(3S)-piperidin-3-yl]phenyl}-2H-indazole-7-carboxamide
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Australia |
2009203598 |
08 January 2009 |
Granted |
2009203598 |
09 January 2014 |
|
Brazil |
PI0906020-0 |
08 January 2009 |
Pending |
||
|
Canada |
2711491 |
08 January 2009 |
Pending |
2653529 |
09 February 2016 |
|
China P.R. |
200980101861.6 |
08 January 2009 |
Pending |
||
|
European Patent Convention |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
France |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Germany |
09700579.7 |
08 January 2009 |
Granted |
602009032466.7 |
29 July 2015 |
|
Great Britain |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
India |
4920/DELNP/2010 |
08 January 2009 |
Pending |
||
|
Ireland |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Israel |
206201 |
08 January 2009 |
Granted |
206201 |
01 November 2011 |
|
Italy |
502015000061194 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Japan |
2010-541101 |
08 January 2009 |
Pending |
||
|
Korea South |
10-2010-7015011 |
08 January 2009 |
Pending |
||
|
Mexico |
MX/a/2010/006593 |
08 January 2009 |
Pending |
||
|
Netherlands |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
New Zealand |
586675 |
08 January 2009 |
Granted |
586675 |
06 August 2012 |
|
Russian Federation |
2010133241 |
08 January 2009 |
Granted |
2495035 |
10 October 2013 |
|
South Africa |
2010/03902 |
08 January 2009 |
Granted |
2010/03902 |
23 February 2011 |
|
Spain |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Switzerland |
09700579.7 |
08 January 2009 |
Granted |
2240466 |
29 July 2015 |
|
Turkey |
2015/13219 |
08 January 2009 |
Granted |
2015 13219 |
29 July 2015 |
|
United States |
12/811922 |
08 January 2009 |
Granted |
8436185 |
07 May 2013 |
(4) IRT-ONC-0129: Pyridinone and pyridazinone derivatives as inhibitors of poly(ADP-ribose)polymerase (PARP)
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Australia |
2007266836 |
25 May 2007 |
Granted |
2007266836 |
09 January 2014 |
|
Brazil |
PI0711741-8 |
25 May 2007 |
Pending |
||
|
Canada |
2653529 |
25 May 2007 |
Pending |
||
|
China P.R. |
200780020136.7 |
25 May 2007 |
Pending |
||
|
China P.R. |
201310316726.1 |
25 July 2013 |
Pending |
||
|
European Patent Convention |
07733716.0 |
25 May 2007 |
Pending |
||
|
India |
9794/DELNP/2008 |
25 May 2007 |
Pending |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Israel |
195113 |
25 May 2007 |
Granted |
195113 |
29 August 2014 |
|
Japan |
2009-512681 |
25 May 2007 |
Granted |
5351755 |
30 August 2013 |
|
Korea South |
10-2008-7029132 |
25 May 2007 |
Granted |
10-1529891 |
12 June 2015 |
|
Mexico |
MX/a/2008/015014 |
25 May 2007 |
Granted |
311801 |
29 July 2013 |
|
New Zealand |
572815 |
25 May 2007 |
Granted |
572815 |
05 March 2012 |
|
Norway |
2008 5397 |
25 May 2007 |
Pending |
||
|
Russian Federation |
2008152824 |
25 May 2007 |
Granted |
2472782 |
20 January 2013 |
|
South Africa |
2008/09238 |
25 May 2007 |
Granted |
2008/09238 |
30 December 2009 |
|
United States |
12/227513 |
25 May 2007 |
Granted |
8188084 |
29 May 2012 |
(5) IRT-ONC-0152: Pyridazinone derivatives as PARP inhibitors
|
Country |
App. No. |
Filing Date |
Status |
Pat. No. |
Grant Date |
|
Australia |
2008322676 |
14 November 2008 |
Granted |
2008322676 |
20 March 2014 |
|
Brazil |
PI0820236-2 |
14 November 2008 |
Pending |
||
|
Canada |
2704714 |
14 November 2008 |
Pending |
||
|
China P.R. |
200880115590.5 |
14 November 2008 |
Granted |
101855221 |
30 October 2013 |
|
European Patent Convention |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
France |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Great Britain |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Germany |
08850429.5 |
14 November 2008 |
Granted |
602008034267.0 |
03 September 2014 |
|
India |
2638/CHENP/2010 |
14 November 2008 |
Pending |
||
|
Israel |
205142 |
14 November 2008 |
Granted |
205142 |
31 December 2014 |
|
Italy |
502014000002462 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Japan |
2010-533666 |
14 November 2008 |
Granted |
5758630 |
12 June 2015 |
|
Korea South |
10-2010-7013113 |
14 November 2008 |
Pending |
||
|
Mexico |
MX/a/2010/005070 |
14 November 2008 |
Granted |
298063 |
12 April 2012 |
|
Netherlands |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
New Zealand |
585395 |
14 November 2008 |
Granted |
585395 |
09 July 2012 |
|
Russian Federation |
2010123874 |
14 November 2008 |
Granted |
2490265 |
20 August 2013 |
|
South Africa |
2010/02466 |
14 November 2008 |
Granted |
2010/02466 |
29 December 2010 |
|
Switzerland |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Spain |
08850429.5 |
14 November 2008 |
Granted |
2220073 |
03 September 2014 |
|
Turkey |
201414390 |
14 November 2008 |
Granted |
2014 14390 |
03 September 2014 |
|
United States |
12/739262 |
14 November 2008 |
Granted |
8268827 |
18 September 2012 |
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 8.4(b)(i)(2)
Pass-through AstraZeneca Royalties
[***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 10.2(j)
TESARO Disclosure of Certain Development Activities
[***]
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 10.4
Merck License Agreement Provisions
2.04Sublicense Agreements. . . . Licensee shall (i) use reasonable efforts to procure the performance by any sublicensee of the terms of each such sublicense Agreement, and (ii) ensure that any sublicensee will comply with the applicable terms and conditions of this Agreement. The grant of any such sublicense will not relieve Licensee of its obligations under this Agreement, except to the extent such obligations are satisfactorily performed by such Affiliate or sublicensee. Licensee shall be liable for the performance or non-performance of its Affiliates and sublicensees hereunder.
9.04No Publicity. A Party may not use the name of the other Party in any publicity or advertising and may not issue a press release or otherwise publicize or disclose any information related to the existence of this Agreement or the terms or conditions herein, except (a) on the advice of its counsel as required by law (e.g., any Securities and Exchange Commission filings and disclosures) and provided the Party who will be disclosing such information has consulted with the other Party to the extent feasible prior to such disclosure with respect to the substance of the disclosure; or (b) as consented to in advance by the other Party in writing. The Parties shall agree on a form of initial press release that may be used by either Party on an ongoing basis to describe this Agreement. Licensee shall provide Merck with reasonable advance written notice of any press release or other public disclosure of the results of any of its work on Licensed Product under this Agreement.
9.05Terms of Agreement. Neither Party nor its Affiliates shall disclose any terms or conditions of this Agreement to any Third Party without the prior consent of the other Party, except as follows: A Party and its Affiliates may disclose the terms or conditions of this Agreement (but not any other Proprietary Information, which may be disclosed only as described elsewhere in this Article IX), (a) on a need-to-know basis to its legal and financial advisors to the extent such disclosure is reasonably necessary, provided that such advisors are subject to confidentiality with regard to such information under an agreement or ethical obligation; (b) to a Third Party in connection with (i) a financing (or proposed financing) or an equity investment (or proposed investment) in such Party or its Affiliates, including to its shareholders and prospective shareholders, (ii) a merger, consolidation or similar transaction by such Party or its Affiliates, (iii) the sale of all or substantially all of the assets of such Party or its Affiliates, or (iv) in connection with a sale of the royalties or other rights of payments contained herein, provided that such Third Party executes a non-use and non-disclosure agreement that provides for substantially the same protection of such information as such disclosing Party owes under this Agreement with respect to Proprietary Information of the other Party; (c) to the United States Securities and Exchange Commission or any other securities exchange or governmental entity, including as required to make an initial or subsequent public offering, or (d) as otherwise required by law or regulation, provided that in the case of (c) and (d) the disclosing Party shall (x) if practicable, provide the other Party with reasonable advance notice of and an opportunity to comment on any such required disclosure, (y) if requested by such other Party, seek, or cooperate with such Party’s efforts to obtain, confidential treatment or a protective order with respect to any such
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
disclosure to the extent available at such other Party’s expense, and (z) use good faith efforts to incorporate the comments of such other Party in any such disclosure or request for confidential treatment or protective order.
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 10.5
Compliance with Laws
1.1.Each Party acknowledges that the other Party aims to perform its activities, and to have other parties (such as the other Party hereunder) with which it enters into business arrangements to perform their activities under such arrangements, in accordance with the highest ethical standards and best industry practices, including any voluntary codes of practice applicable in the industry. The Parties agree to use reasonable efforts to help ensure that the Parties do not fail to meet such aim with respect to activities hereunder through any violation of the U.S. Foreign Corrupt Practices Act (the “FCPA”), the U.S. False Claims Act (the “FCA”), the U.S. Anti-Kickback Law (the “AKA”), the United Kingdom Bribery Act (the “UKBA”), or any laws or regulations regarding governmental research grants (e.g., rules governing grants from the National Institutes of Health, or other governmental agencies.)
1.2.Each Party shall comply with all laws and regulations concerning its efforts in any country or jurisdiction where it is providing work hereunder or otherwise applying to any of its activities under this Agreement. Each Party shall use reasonable efforts to ensure that its personnel performing hereunder become reasonably familiar with the FCPA, the FCA, the UKBA and the AKA, and their prohibitions and purposes, and that they will not undertake any actions that would violate the FCPA, the FCA, the UKBA and the AKA. Accordingly, each Party hereby warrants that:
(i)neither it nor its agents or employees whose duties pertain to this Agreement are excluded from a federal health care program as outlined in Sections 1128 and 1156 of the Social Security Act (see the Office of Inspector General of the Department of Health and Human Services List of Excluded Individuals / Entities at http://oig.hhs.gov/exclusions/exclusions_list.asp);
(ii)neither it nor its agents or employees whose duties pertain to this Agreement are debarred by the FDA under 21 U.S.C. 335a (see the FDA Office of Regulatory Affairs Debarment List at http://www.fda.gov/ICECI/EnforcementActions/FDADebarmentList/default.htm);
(iii)neither it, nor its agents or employees are otherwise excluded from contracting with the federal government (see the System for Award Management at https://www.sam.gov/portal/SAM/#1);
(iv)it, or its agents or employees, if required, are duly licensed and in good standing in accordance with applicable U.S., state or other applicable governmental licensing requirements;
(v)no payment or offer to pay, or the giving or offering to give, anything of value to an official or employee of any government or any department, agency or
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
instrumentality thereof (including any health or medical providers owned or controlled by the government), or to any political party or any candidate for political office, shall be made with the purpose of influencing any decisions favorable to either Party or its Affiliates and the business resulting therefrom in contravention of the FCPA or the laws of the country in which it is providing work;
(vi)it has not paid, nor offered or agreed to pay, nor caused to be paid, directly or indirectly, any political contributions, fees or commissions to any governmental employee or representative (including any employee of any health or medical provider owned or controlled by the government) that would appear to cause a violation of the FCPA;
(vii)it will not directly or indirectly offer, pay, promise to pay, or authorize the giving of money or anything of value to any governmental official or representative, to any political party or official thereof, or to any candidate for political office, or to any other person, for the purpose of:
a.inappropriately influencing any act or decisions of such person, official, political party, party official, or candidate in, if applicable, its official capacity, including a decision to fail to perform official functions; or
b.inducing such person, official, political party, party official, or candidate to use influence with the government, any instrumentality thereof, or any other entity to affect or influence any act or decision of such government or instrumentality, or entity, in order to assist TESARO or Company in obtaining or retaining business for or with, or directing business to, any Affiliate or Third Party.
Each Party further agrees that if subsequent developments cause the certifications and information reported herein to be no longer accurate or complete, it will immediately so advise the other Party in writing.
1.3In the event of a claim or investigation, or an official request for a Party to cooperate with respect to any such claim or investigation, by a Regulatory Authority or other legal authority having jurisdiction over either Party, of an alleged violation of the FCPA arising from any activities conducted by the other Party relating specifically to this Agreement or to the Products, the other Party shall reasonably provide such Regulatory Authority or other legal authority having jurisdiction over the Party with access to the other Party’s facilities, records (financial and otherwise), and supporting documentation, as reasonably requested by the Party or its agents in order to cooperate in connection with such claim or investigation. Each Party acknowledges that the provisions of this Exhibit 10.5 granting the other Party certain audit rights shall in no way relieve the Party of any of its obligations under the Agreement, nor shall such provisions require the other Party to conduct any such audits.
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.4During the Term, each Party shall maintain true, accurate and complete books and records, including those: (i) documenting its interactions with any government or its officials or employees relating to its activities in connection with any Product; (ii) payments made to any officials or employees of any government or any department, agency or instrumentality thereof; and (iii) political contributions. Each Party shall also maintain a reasonable system of internal accounting controls sufficient to satisfy the FCPA.
1.5 Each Party acknowledges and agrees that any breach of its obligations under this Exhibit 10.5 shall be a basis for notification of a material breach of this Agreement.
1.6Notwithstanding anything to the contrary in this Agreement, a Party may disclose its terms and conditions (including any financial terms) to any governmental authorities in the U.S. or those in a country where it performs activities in connection with any Product that such Party determines in good faith has a legitimate need for access to such information for purposes of investigating or determining either Party’s compliance with Applicable Law.
1.7The Parties acknowledge that certain laws, now or in the future, may require pharmaceutical, medical device and other companies to disclose information on compensation, gifts or other remuneration provided to physicians, institutions, and other health care professionals. Either Party may report information about remuneration provided under this Agreement. Once reported, such information may be publicly accessible.
|
Confidential |
|
|
[***] INDICATES MATERIAL THAT WAS OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT WAS REQUESTED. ALL SUCH OMITTED MATERIAL WAS FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24b-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
