Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ZIOPHARM ONCOLOGY INC | d221546d8k.htm |
The "ins" and "outs" of Genetic Engineering of T cells for Human Applications Laurence Cooper M.D., Ph.D. ljncooper@ziopharm.com ASH workshop 3:55 to 4:15 pm July 14, 2016 Exhibit 99.1

This presentation contains certain forward-looking information about ZIOPHARM Oncology, Inc. that is intended to be covered by the safe harbor for "forward-looking statements" provided by the Private Securities Litigation Reform Act of 1995, as amended. Forward-looking statements are statements that are not historical facts, and in some cases can be identified by terms such as "may," "will," "could," "expects," "plans," "anticipates," and "believes." These statements include, but are not limited to, statements regarding the progress, timing and results of preclinical and clinical trials involving the Company's drug candidates, and the progress of the Company's research and development programs. All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied by, the forward-looking statements. These risks and uncertainties include, but are not limited to: whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK cell-based therapies, or any of our other therapeutic candidates will advance further in the pre-clinical or clinical trials process and whether and when, if at all, they will receive final approval from the U.S. Food and Drug Administration or equivalent foreign regulatory agencies and for which indications; whether chimeric antigen receptor T cell (CAR T) approaches, Ad-RTS-IL-12, TCR and NK cell-based therapies, and our other therapeutic products will be successfully marketed if approved; the strength and enforceability of our intellectual property rights; competition from other pharmaceutical and biotechnology companies; and the other risk factors contained in our periodic and interim SEC reports filed from time to time with the Securities and Exchange Commission, including but not limited to, our Annual Report on Form 10-K for the fiscal year ended December 31, 2015, and our Quarterly Report on Form 10-Q for the quarter ended March 31, 2016. Readers are cautioned not to place undue reliance on these forward-looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward-looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non-occurrence of any events. Forward-looking statements Some of technology described was advanced through research conducted at the MD Anderson Cancer Center by Laurence J.N. Cooper, M.D., Ph.D. On May 7, 2015, Dr. Cooper was appointed as the Chief Executive Officer at ZIOPHARM. Dr. Cooper is now a Visiting Scientist at MD Anderson.
Objectives NK cells ab T cells TH17 cells NKT cells Treg cells gd T cells T cells can be genetically modified to introduce desired transgenes and edited to remove undesired endogenous genes. The combination of insertion and elimination can be harnessed to develop T cells with desired specificity, improve potency, and widen bioavailability.
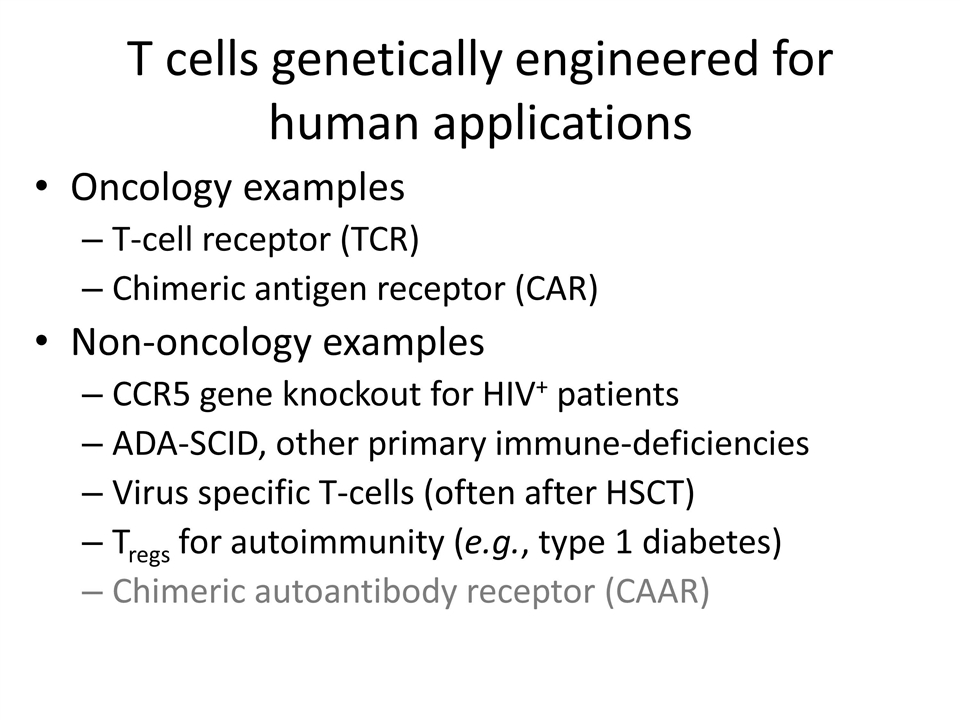
T cells genetically engineered for human applications Oncology examples T-cell receptor (TCR) Chimeric antigen receptor (CAR) Non-oncology examples CCR5 gene knockout for HIV+ patients ADA-SCID, other primary immune-deficiencies Virus specific T-cells (often after HSCT) Tregs for autoimmunity (e.g., type 1 diabetes) Chimeric autoantibody receptor (CAAR)
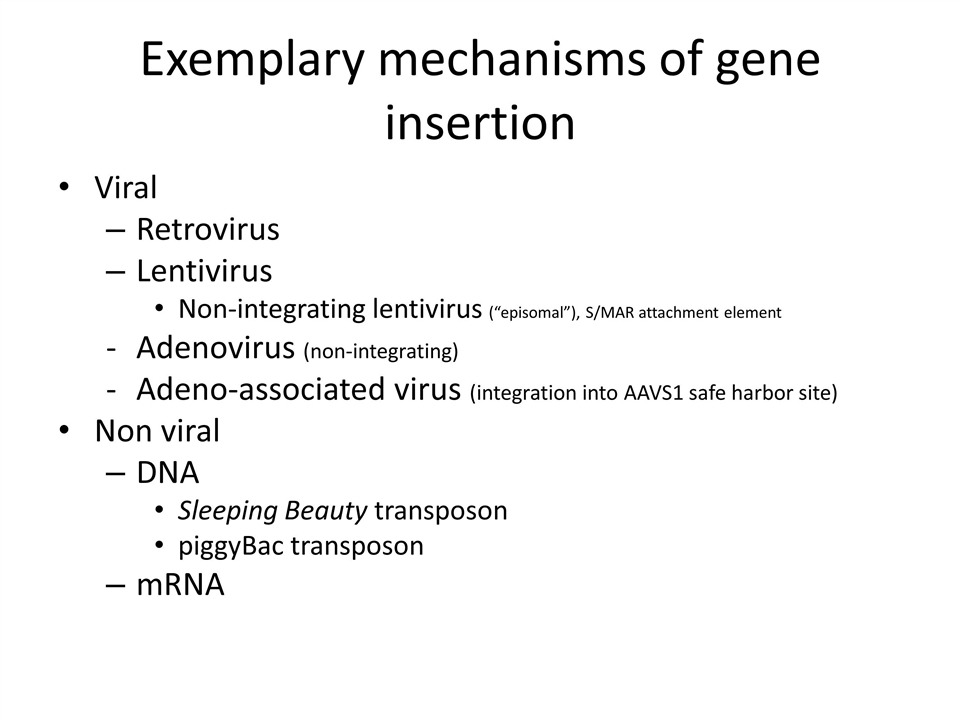
Exemplary mechanisms of gene insertion Viral Retrovirus Lentivirus Non-integrating lentivirus (“episomal”), S/MAR attachment element Adenovirus (non-integrating) Adeno-associated virus (integration into AAVS1 safe harbor site) Non viral DNA Sleeping Beauty transposon piggyBac transposon mRNA
Targeted integration Examples: Meganucleases Zinc Finger Nucleases TAL Effector Nucleases CRISPR/Cas9 nucleases Homologous recombination (and selection) Note: “Nucleases” are often used as paired nickases
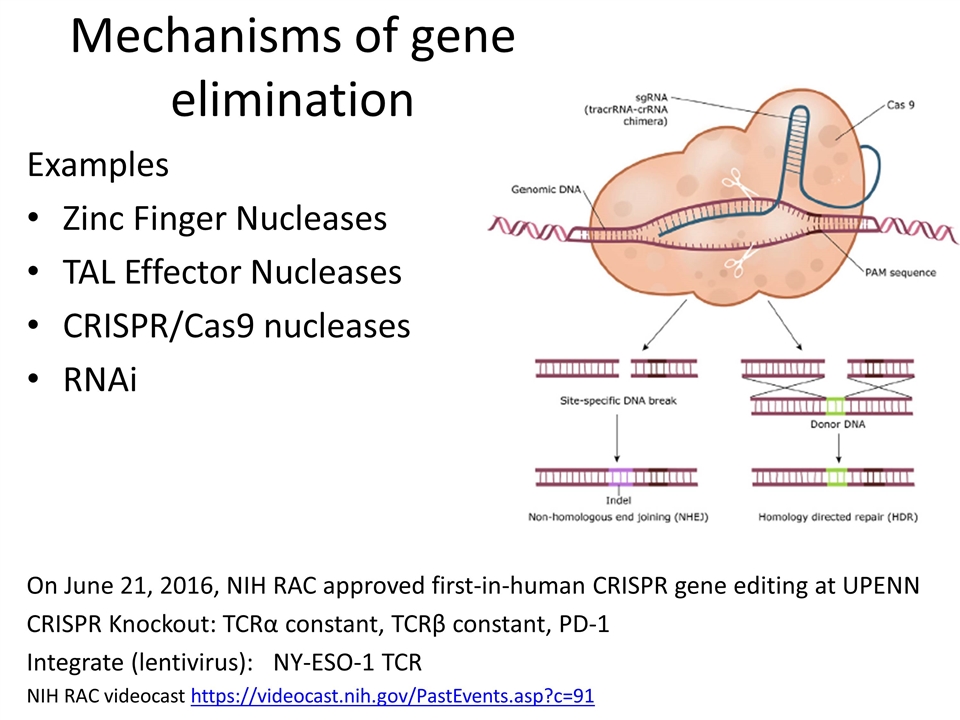
Mechanisms of gene elimination Examples Zinc Finger Nucleases TAL Effector Nucleases CRISPR/Cas9 nucleases RNAi On June 21, 2016, NIH RAC approved first-in-human CRISPR gene editing at UPENN CRISPR Knockout: TCRα constant, TCRβ constant, PD-1 Integrate (lentivirus): NY-ESO-1 TCR NIH RAC videocast https://videocast.nih.gov/PastEvents.asp?c=91
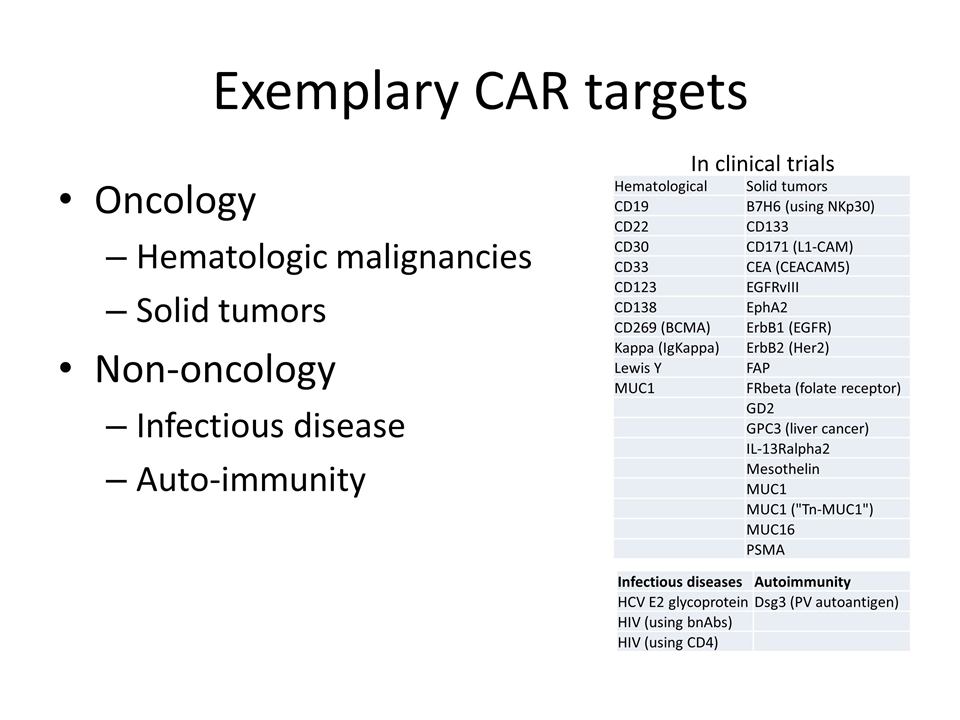
Exemplary CAR targets Oncology Hematologic malignancies Solid tumors Non-oncology Infectious disease Auto-immunity Infectious diseases Autoimmunity HCV E2 glycoprotein Dsg3 (PV autoantigen) HIV (using bnAbs) HIV (using CD4) Hematological Solid tumors CD19 B7H6 (using NKp30) CD22 CD133 CD30 CD171 (L1-CAM) CD33 CEA (CEACAM5) CD123 EGFRvIII CD138 EphA2 CD269 (BCMA) ErbB1 (EGFR) Kappa (IgKappa) ErbB2 (Her2) Lewis Y FAP MUC1 FRbeta (folate receptor) GD2 GPC3 (liver cancer) IL-13Ralpha2 Mesothelin MUC1 MUC1 ("Tn-MUC1") MUC16 PSMA In clinical trials
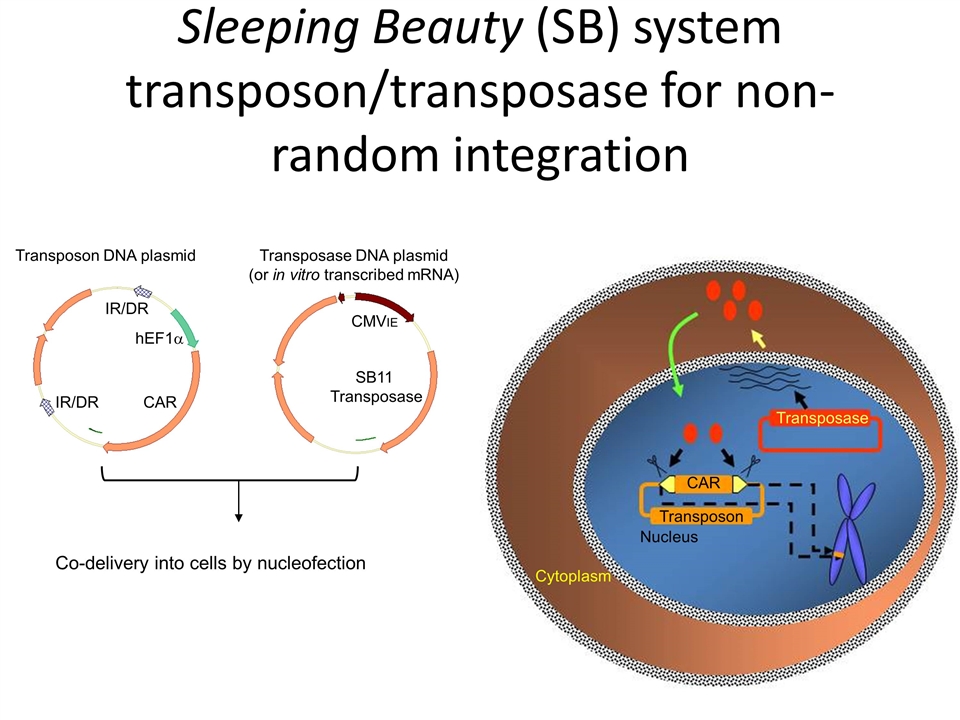
IR/DR IR/DR hEF1a CAR CMVIE SB11 Transposase Co-delivery into cells by nucleofection Transposon DNA plasmid Transposase DNA plasmid (or in vitro transcribed mRNA) Sleeping Beauty (SB) system transposon/transposase for non-random integration CAR Transposon Nucleus Transposase Cytoplasm
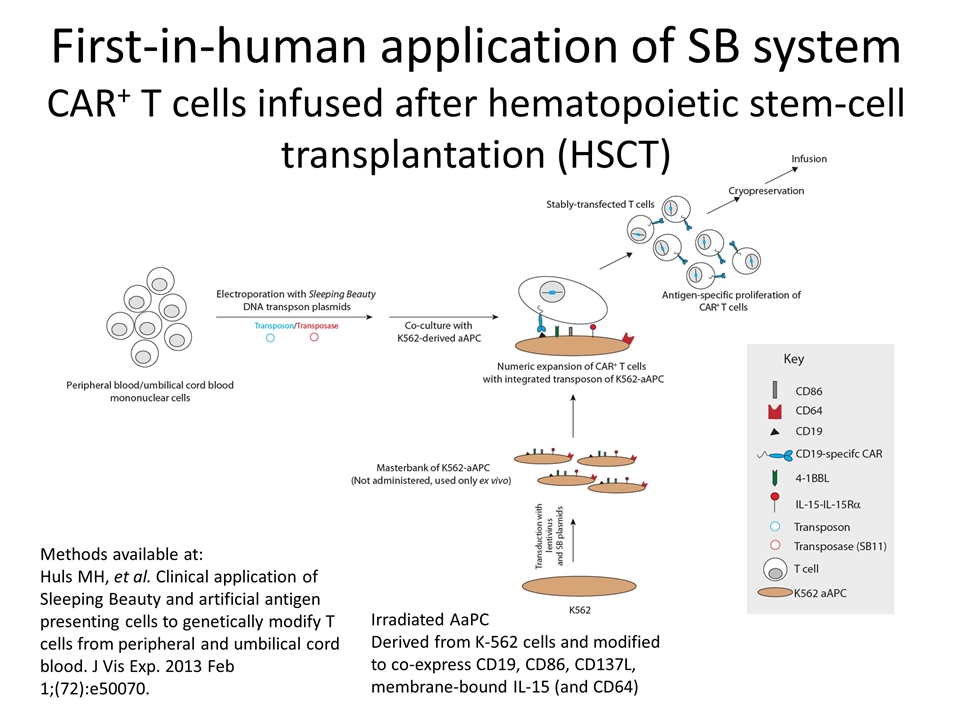
First-in-human application of SB system CAR+ T cells infused after hematopoietic stem-cell transplantation (HSCT) Methods available at: Huls MH, et al. Clinical application of Sleeping Beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood. J Vis Exp. 2013 Feb 1;(72):e50070. Irradiated AaPC Derived from K-562 cells and modified to co-express CD19, CD86, CD137L, membrane-bound IL-15 (and CD64)
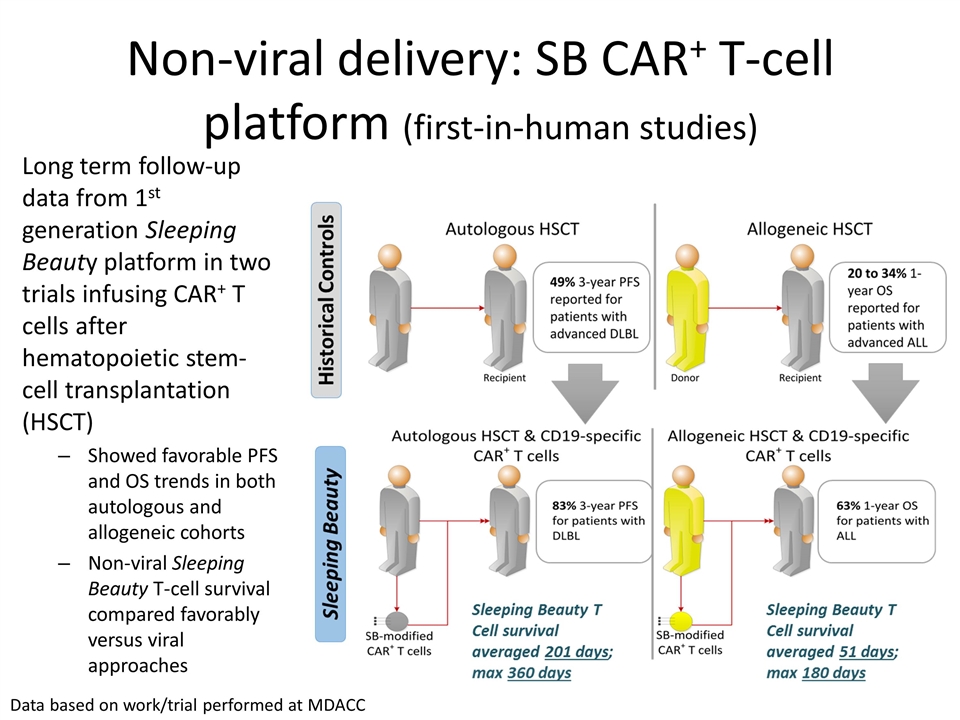
Non-viral delivery: SB CAR+ T-cell platform (first-in-human studies) Long term follow-up data from 1st generation Sleeping Beauty platform in two trials infusing CAR+ T cells after hematopoietic stem-cell transplantation (HSCT) Showed favorable PFS and OS trends in both autologous and allogeneic cohorts Non-viral Sleeping Beauty T-cell survival compared favorably versus viral approaches Data based on work/trial performed at MDACC
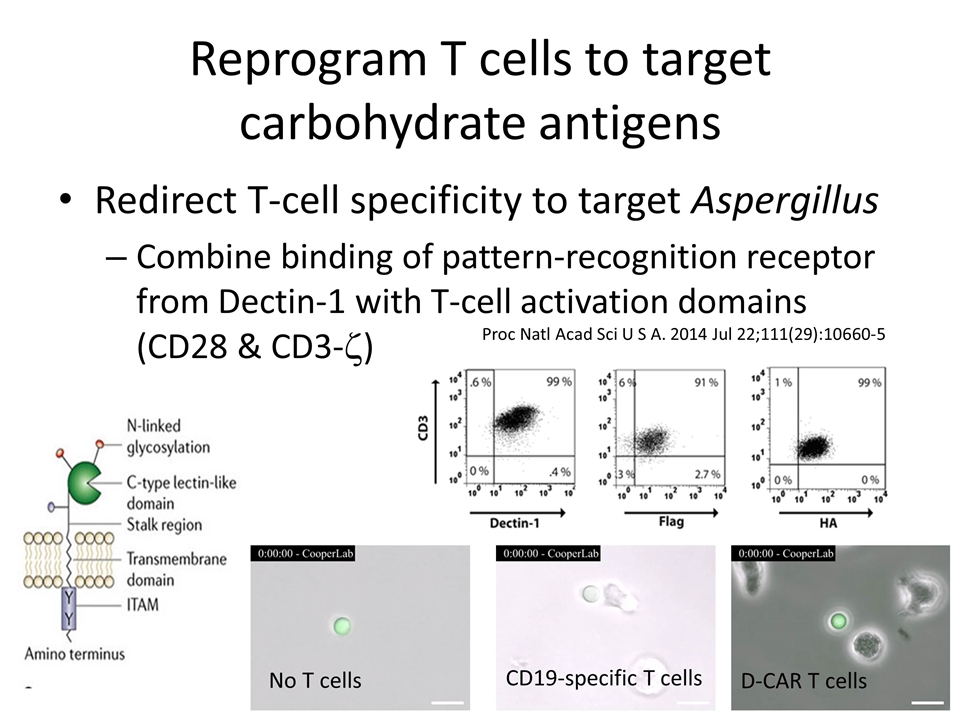
Reprogram T cells to target carbohydrate antigens Redirect T-cell specificity to target Aspergillus Combine binding of pattern-recognition receptor from Dectin-1 with T-cell activation domains (CD28 & CD3-z) Proc Natl Acad Sci U S A. 2014 Jul 22;111(29):10660-5 No T cells CD19-specific T cells D-CAR T cells
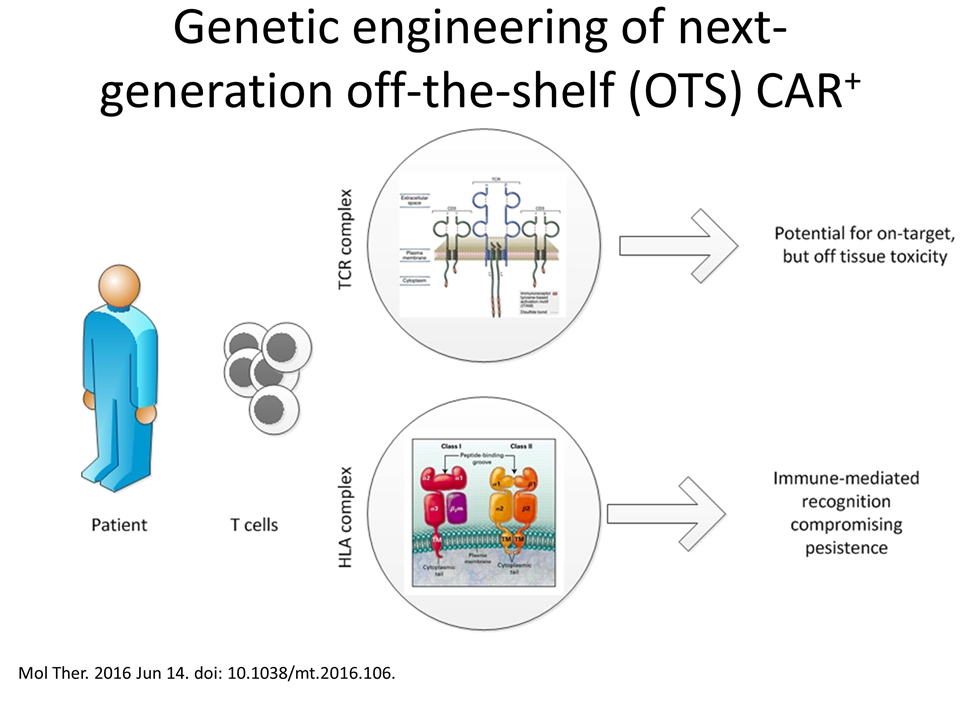
Genetic engineering of next-generation off-the-shelf (OTS) CAR+ cells Mol Ther. 2016 Jun 14. doi: 10.1038/mt.2016.106.
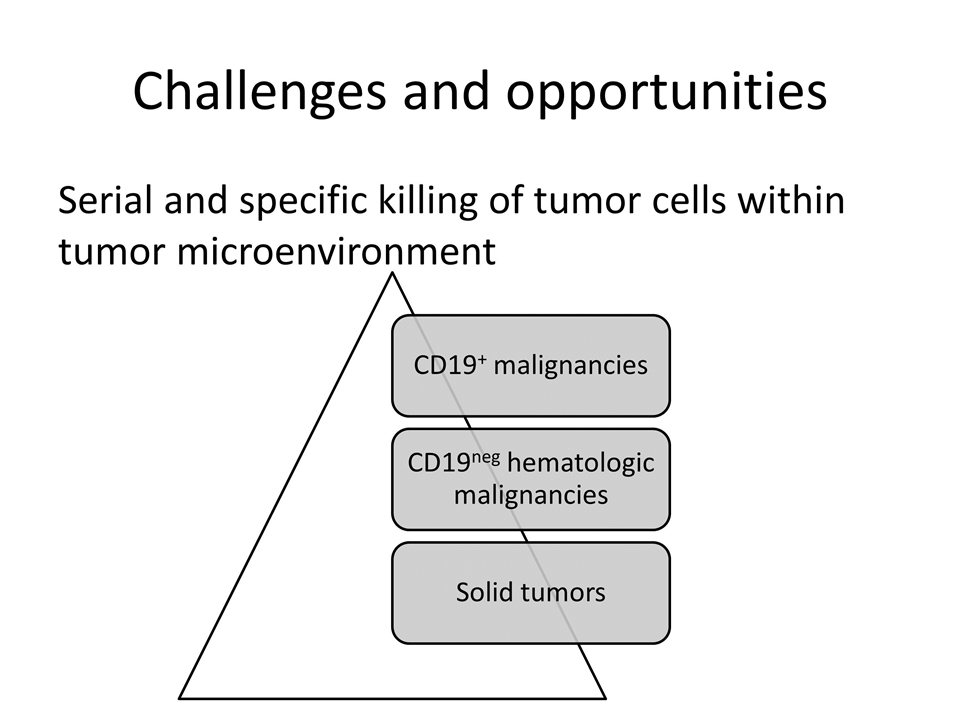
Challenges and opportunities Serial and specific killing of tumor cells within tumor microenvironment CD19 + malignancies CD19 neg hematologic malignancies Solid tumors
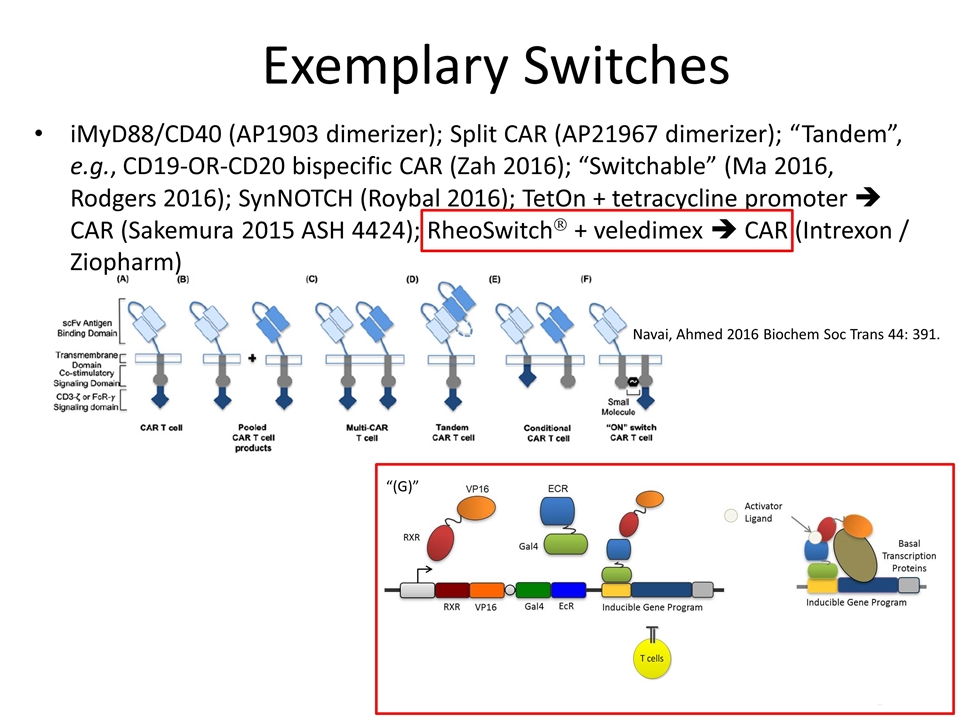
Exemplary Switches iMyD88/CD40 (AP1903 dimerizer); Split CAR (AP21967 dimerizer); “Tandem”, e.g., CD19-OR-CD20 bispecific CAR (Zah 2016); “Switchable” (Ma 2016, Rodgers 2016); SynNOTCH (Roybal 2016); TetOn + tetracycline promoter è CAR (Sakemura 2015 ASH 4424); RheoSwitchÒ + veledimex è CAR (Intrexon / Ziopharm) Navai, Ahmed 2016 Biochem Soc Trans 44: 391. CAR Activator “(G)”
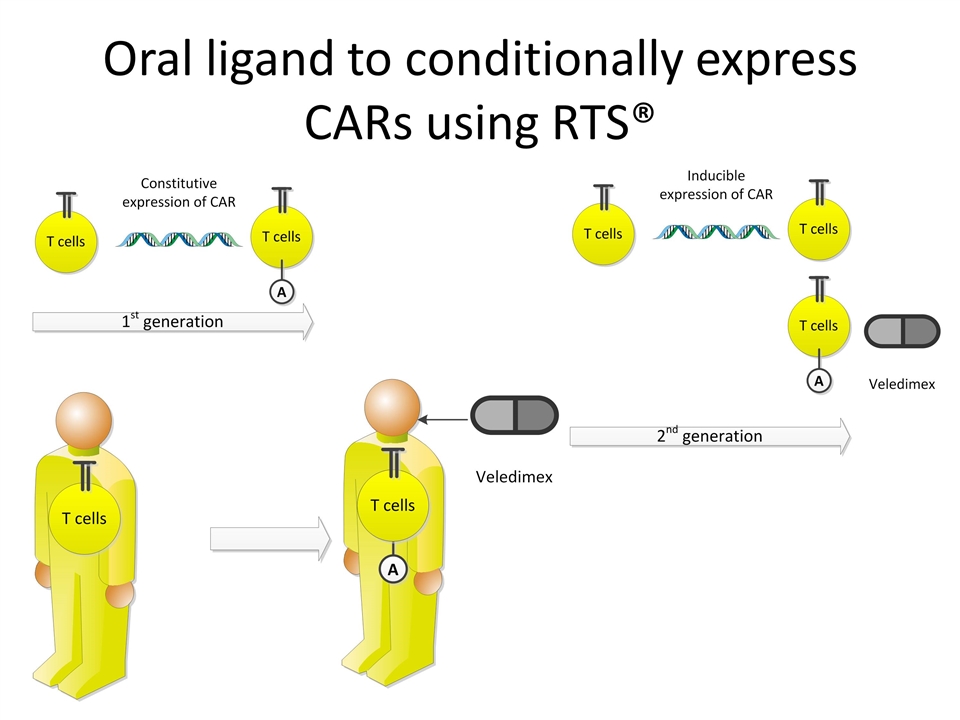
Oral ligand to conditionally express CARs using RTS®
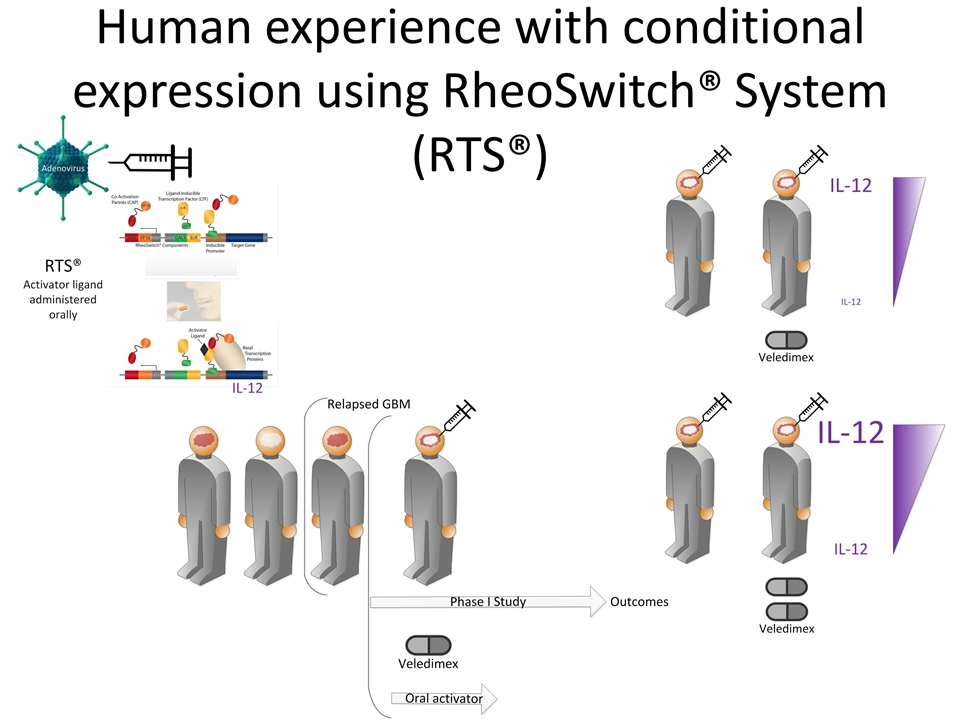
Human experience with conditional expression using RheoSwitch® System (RTS®)
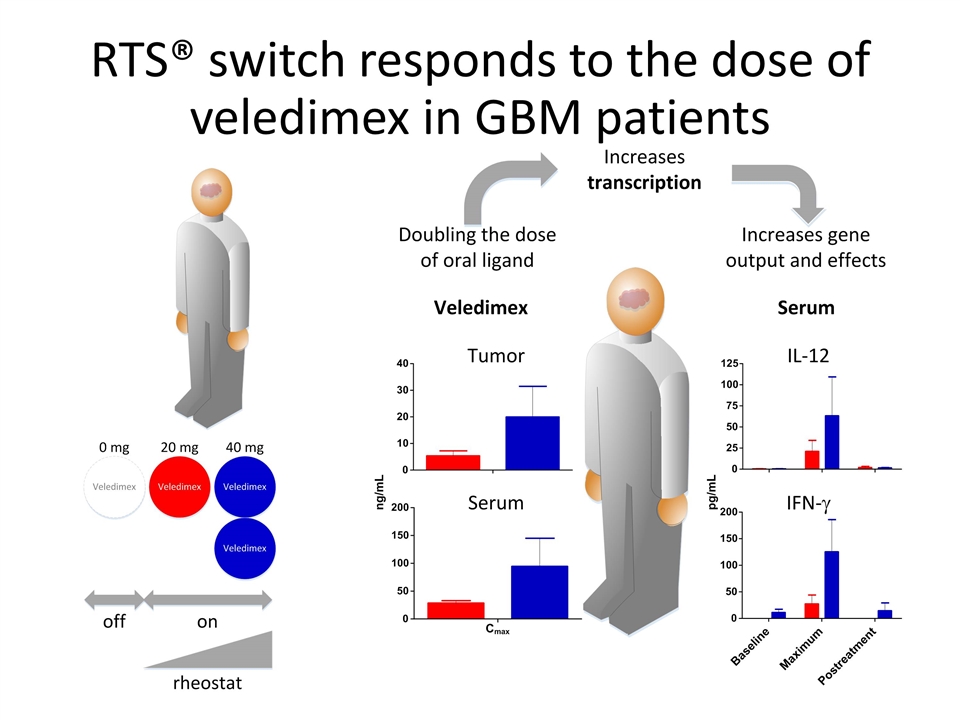
RTS® switch responds to the dose of veledimex in GBM patients
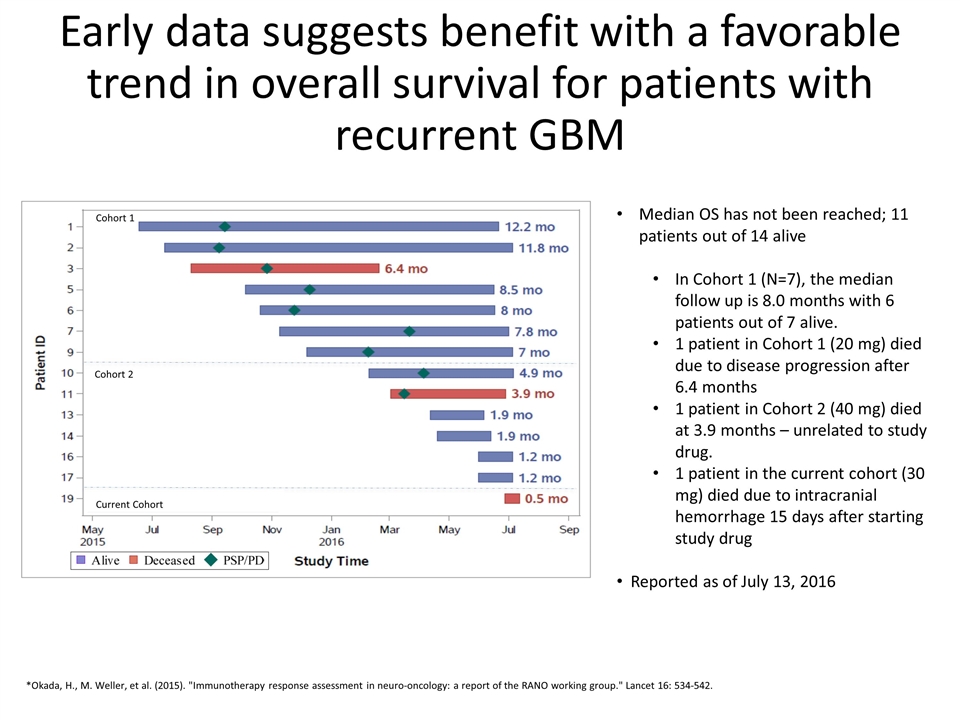
Early data suggests benefit with a favorable trend in overall survival for patients with recurrent GBM Median OS has not been reached; 11 patients out of 14 alive In Cohort 1 (N=7), the median follow up is 8.0 months with 6 patients out of 7 alive. 1 patient in Cohort 1 (20 mg) died due to disease progression after 6.4 months 1 patient in Cohort 2 (40 mg) died at 3.9 months – unrelated to study drug. 1 patient in the current cohort (30 mg) died due to intracranial hemorrhage 15 days after starting study drug Reported as of July 13, 2016 *Okada, H., M. Weller, et al. (2015). "Immunotherapy response assessment in neuro-oncology: a report of the RANO working group." Lancet 16: 534-542. Cohort 1 Cohort 2 Current Cohort
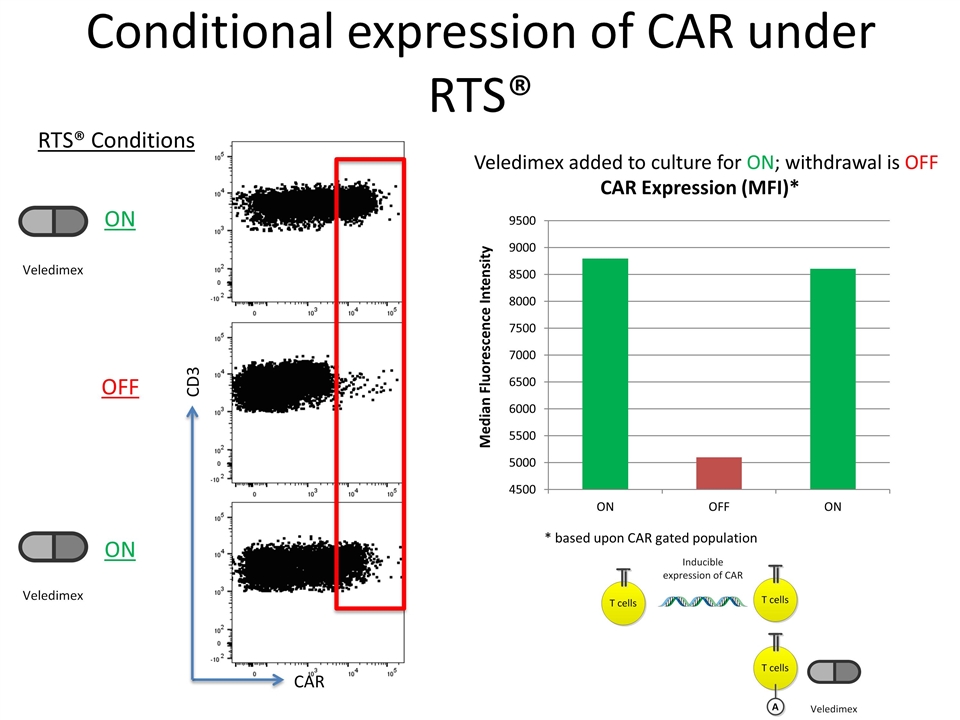
Conditional expression of CAR under RTS® CD3 Veledimex added to culture for ON; withdrawal is OFF CAR RTS® Conditions ON OFF ON
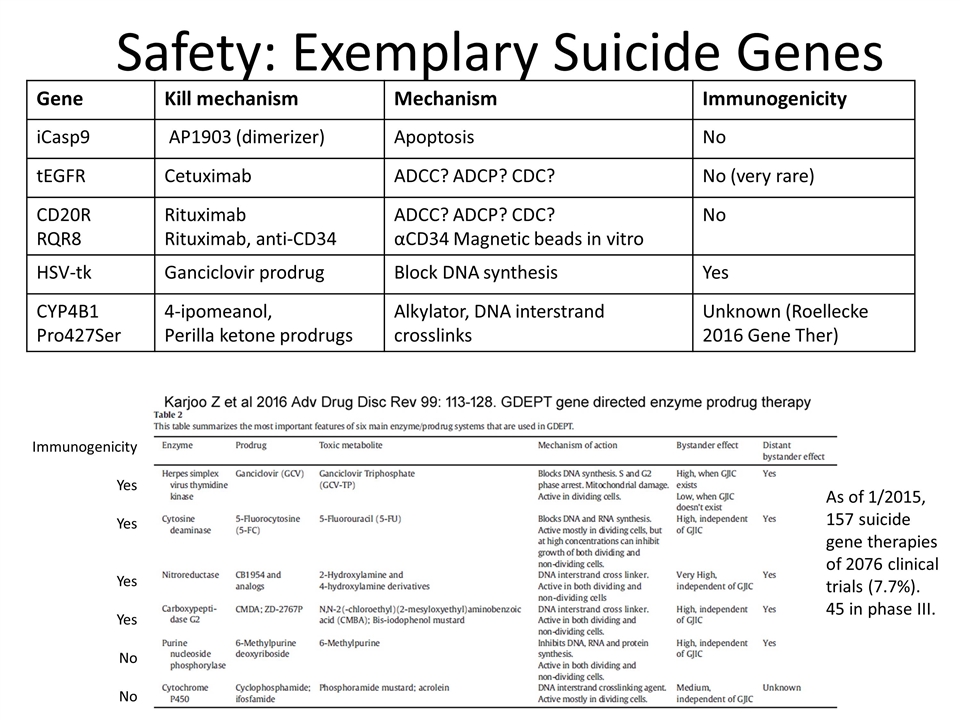
Safety: Exemplary Suicide Genes Gene Kill mechanism Mechanism Immunogenicity iCasp9 AP1903 (dimerizer) Apoptosis No tEGFR Cetuximab ADCC? ADCP? CDC? No (very rare) CD20R RQR8 Rituximab Rituximab, anti-CD34 ADCC? ADCP? CDC? αCD34 Magnetic beads in vitro No HSV-tk Ganciclovir prodrug Block DNA synthesis Yes CYP4B1 Pro427Ser 4-ipomeanol, Perilla ketone prodrugs Alkylator, DNA interstrand crosslinks Unknown (Roellecke 2016 Gene Ther) Immunogenicity Yes Yes Yes Yes No No As of 1/2015, 157 suicide gene therapies of 2076 clinical trials (7.7%). 45 in phase III.
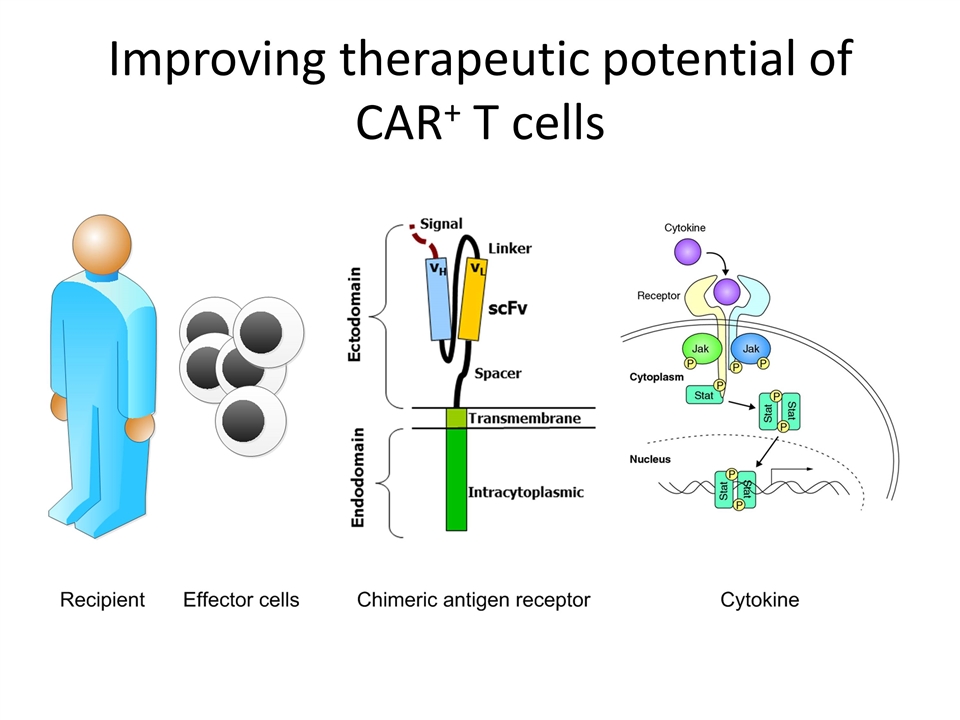
Improving therapeutic potential of CAR+ T cells
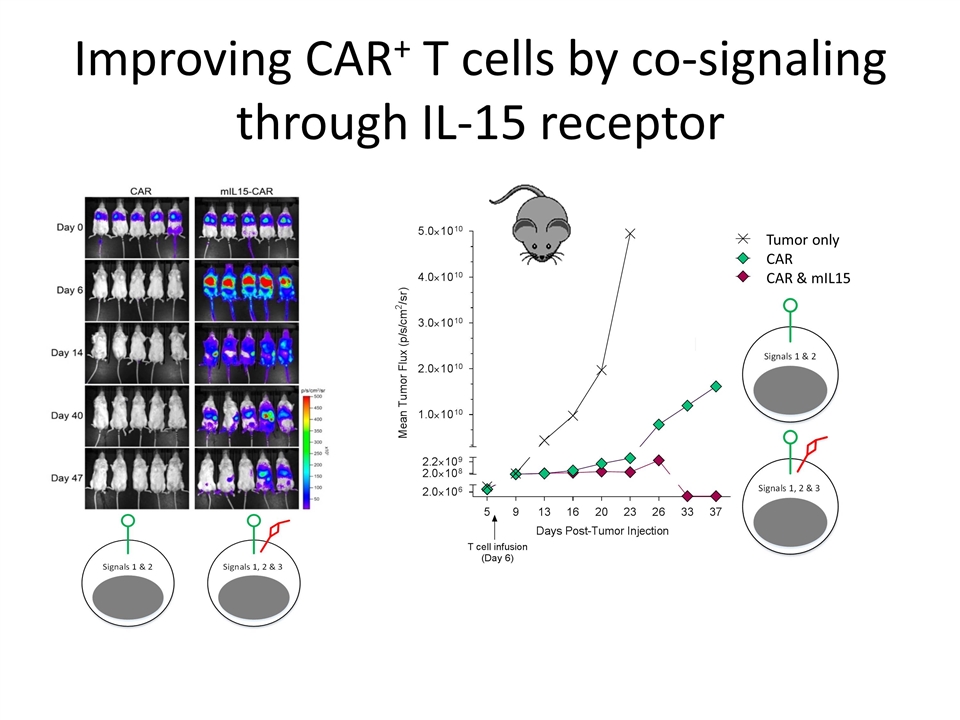
Improving CAR+ T cells by co-signaling through IL-15 receptor T-cell survival Tumor only CAR CAR & mIL15
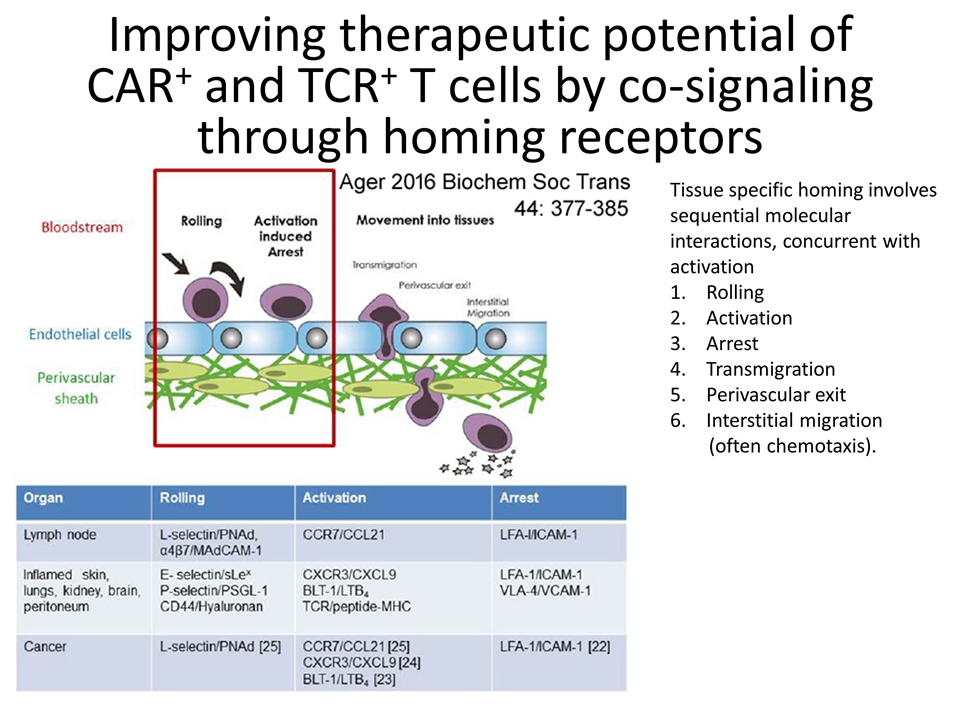
Improving therapeutic potential of CAR+ and TCR+ T cells by co-signaling through homing receptors Tissue specific homing involves sequential molecular interactions, concurrent with activation Rolling Activation Arrest Transmigration Perivascular exit Interstitial migration (often chemotaxis).
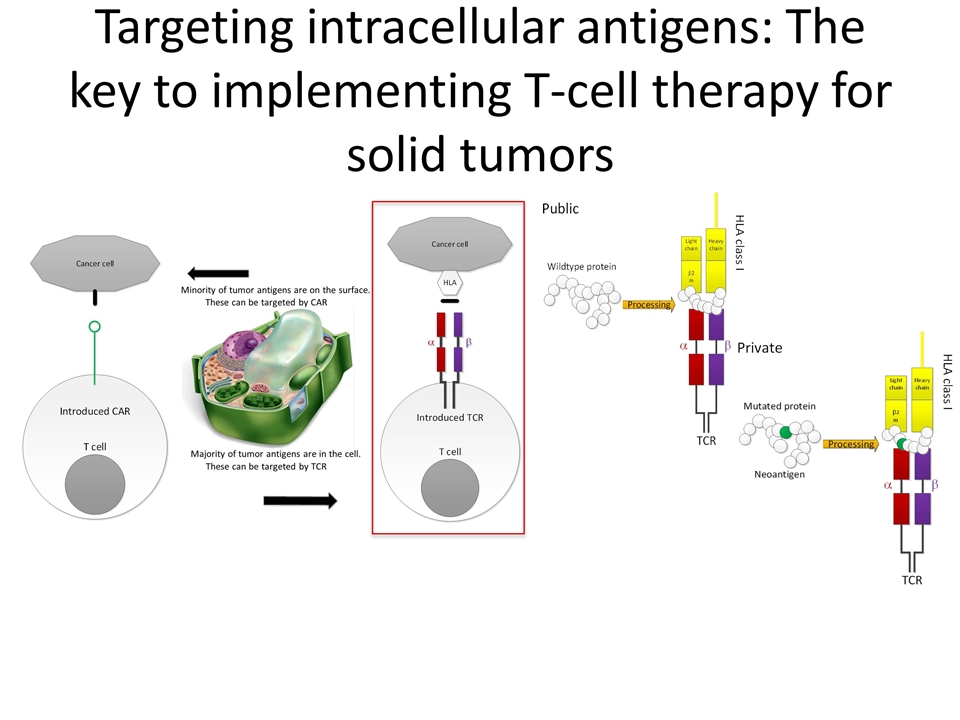
Targeting intracellular antigens: The key to implementing T-cell therapy for solid tumors Minority of tumor antigens are on the surface. These can be targeted by CAR Majority of tumor antigens are in the cell. These can be targeted by TCR
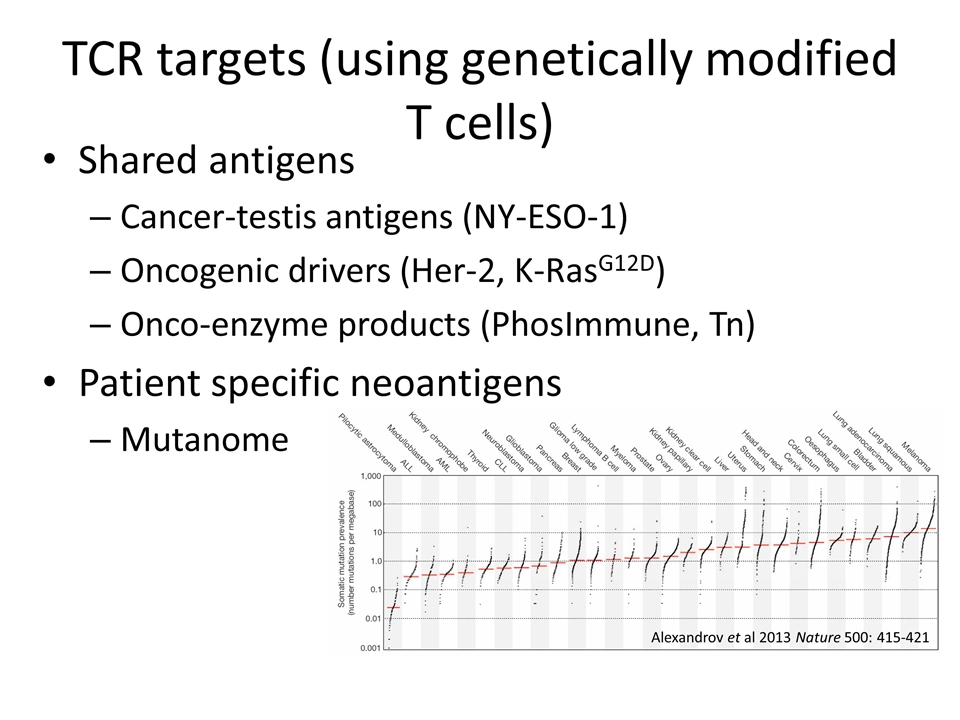
TCR targets (using genetically modified T cells) Shared antigens Cancer-testis antigens (NY-ESO-1) Oncogenic drivers (Her-2, K-RasG12D) Onco-enzyme products (PhosImmune, Tn) Patient specific neoantigens Mutanome Alexandrov et al 2013 Nature 500: 415-421
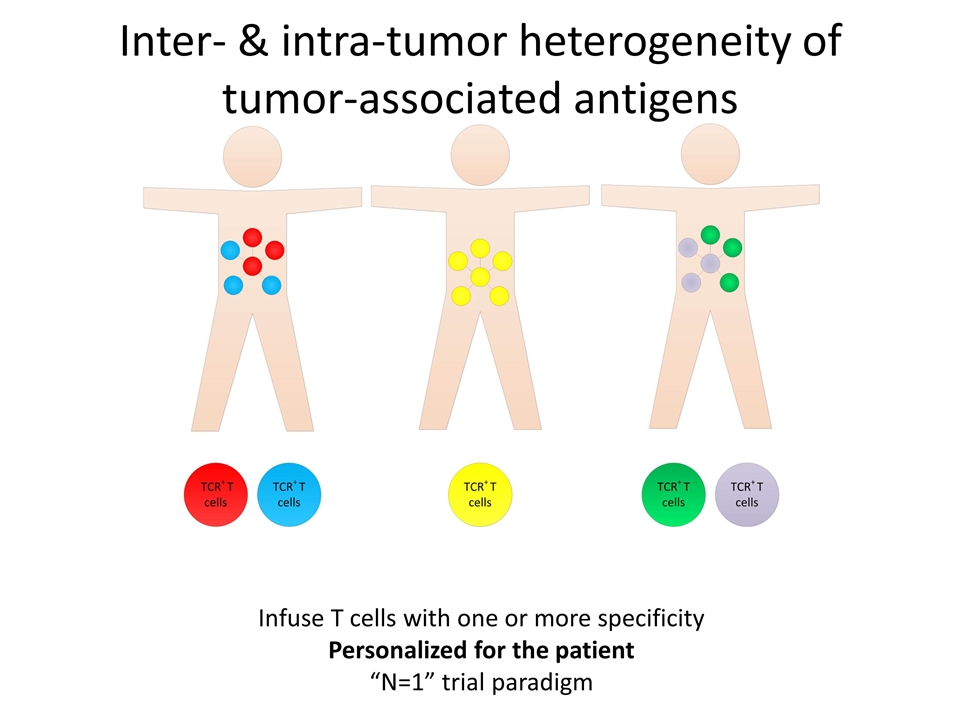
Inter- & intra-tumor heterogeneity of tumor-associated antigens Infuse T cells with one or more specificity Personalized for the patient “N=1” trial paradigm
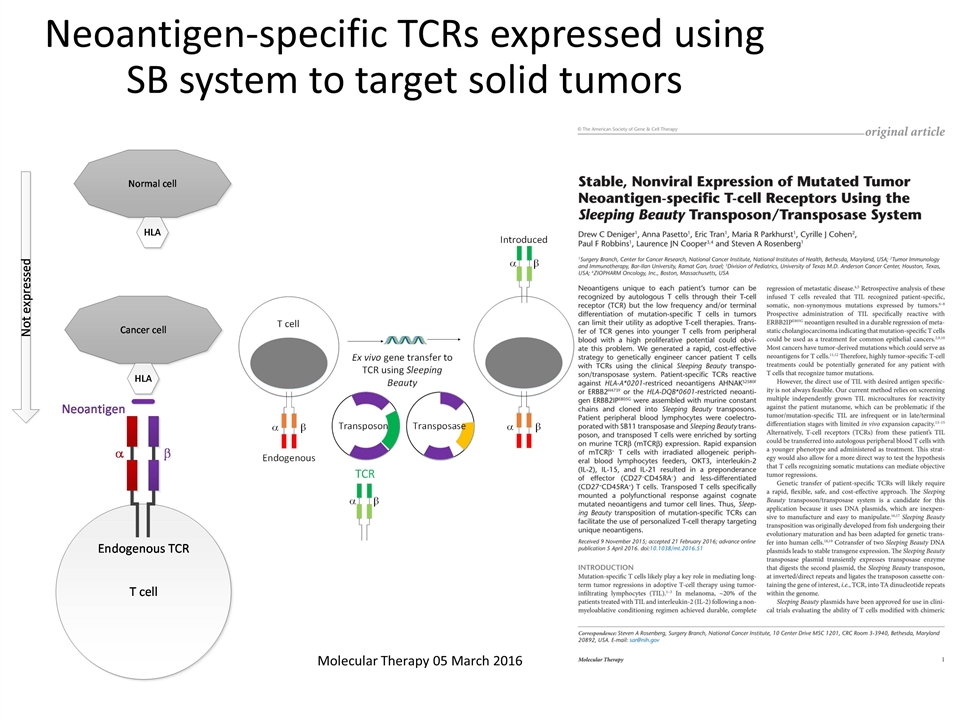
Neoantigen-specific TCRs expressed using SB system to target solid tumors Molecular Therapy 05 March 2016
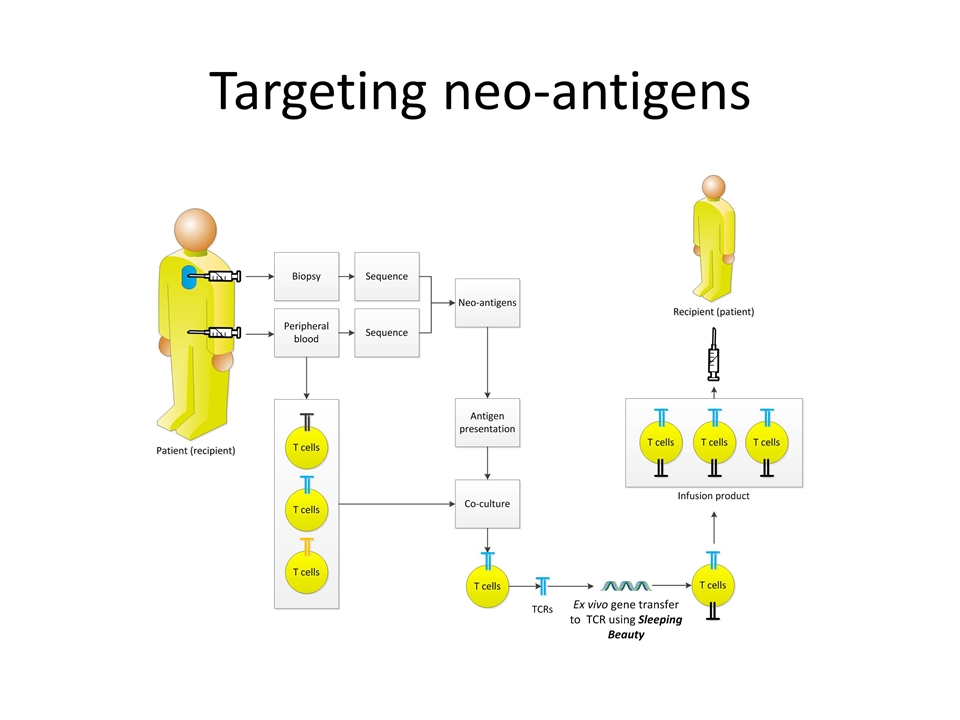
Targeting neo-antigens
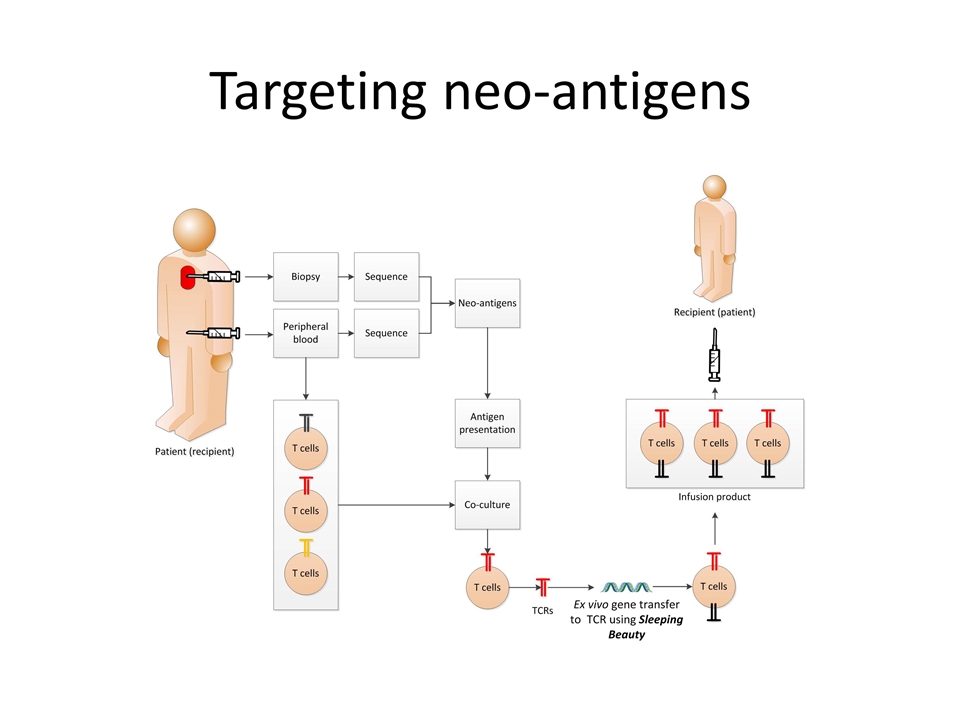
Targeting neo-antigens
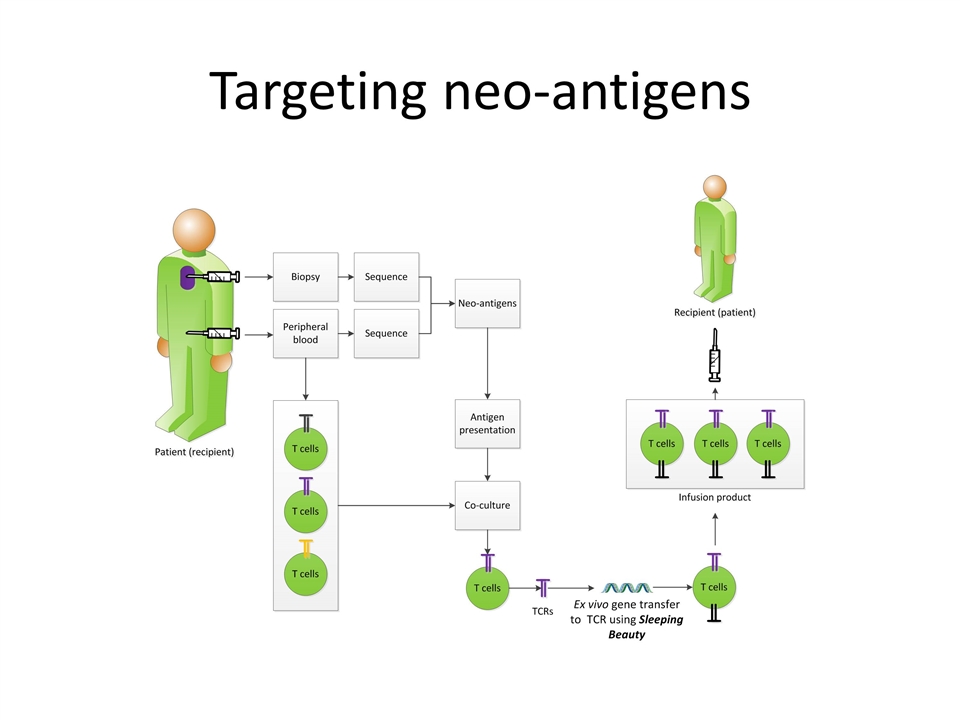
Targeting neo-antigens
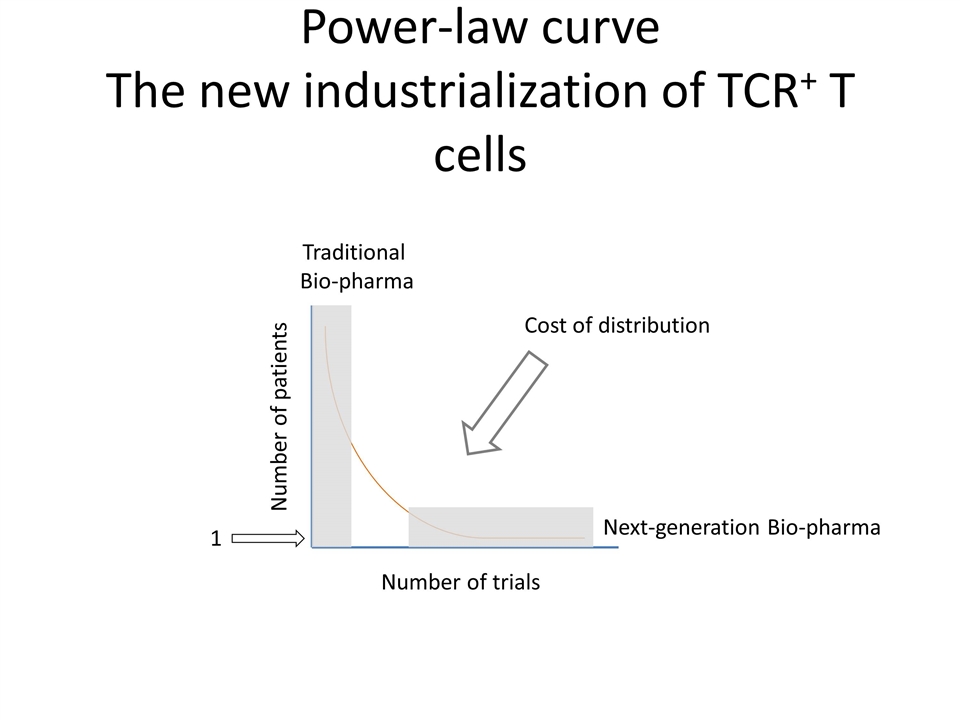
Power-law curve The new industrialization of TCR+ T cells Cost of distribution Number of trials Number of patients Traditional Bio-pharma Next-generation Bio-pharma 1

Thank you
