Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Juno Therapeutics, Inc. | d201119dex991.htm |
| 8-K - 8-K - Juno Therapeutics, Inc. | d201119d8k.htm |

Exhibit 99.2

Forward-looking statements This presentation and the accompanying oral commentary contain forward-looking statements that involve risks, uncertainties, and assumptions. If the risks or uncertainties ever materialize or the assumptions prove incorrect, our results may differ materially from those expressed or implied by such forward-looking statements. All statements other than statements of historical fact could be deemed forward-looking, including, but not limited to, any expectations regarding investment returns; any projections of financial information; any statements about historical results that may suggest trends for our business; any statements of the plans, strategies, and objectives of management for future operations, including our manufacturing and process development; any statements of expectation or belief regarding future events, potential markets or market size, technology developments, our product pipeline, clinical data, enforceability of our intellectual property rights, competitive strengths or our position within the industry; any statements regarding the anticipated benefits of our Celgene collaboration or other strategic transactions; and any statements of assumptions underlying any of the items mentioned. These statements are based on estimates and information available to us at the time of this presentation and are not guarantees of future performance. Actual results could differ materially from our current expectations as a result of many risks and uncertainties, including but not limited to, risks associated with: the success, cost, and timing of our product development activities and clinical trials; our ability to obtain regulatory approval for and to commercialize our product candidates; our ability to establish a commercially-viable manufacturing process and manufacturing infrastructure; regulatory requirements and regulatory developments; the effects of competition and technological advances; our dependence on third-party collaborators and other contractors in our research and development activities, including for the conduct of clinical trials and the manufacture of our product candidates; our dependence on Celgene for the development and commercialization outside of North America and China of our CD19 product candidates and any other product candidates for which Celgene exercises an option; Juno’s dependence on JW Therapeutics (Shanghai) Co., Ltd, over which Juno does not exercise complete control, for the development and commercialization of product candidates in China; our ability to obtain, maintain, or protect intellectual property rights related to our product candidates; among others. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to our business in general, see our Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission on May 10, 2016 and our other periodic reports filed from time to time with the Securities and Exchange Commission. Except as required by law, we assume no obligation and do not intend to update these forward-looking statements or to conform these statements to actual results or to changes in our expectations.

Building the leading T cell company Significant advances in CD19-directed product candidates with a go-to-market strategy defined in ALL, NHL, and CLL Moving beyond CD19 target with encouraging data in CD22 and WT-1; more targets and approaches in the clinic Progress with potential best-in-class product candidates and platform; JCAR017 on the market as early as 2018 with multiple indications by the end of 2019 Broad program in solid organ tumors in significant areas of unmet need Building platform and self-sustaining research capability to support leadership in the engineered T cell space With Celgene, global manufacturing, development, and commercial capabilities to bring engineered T cells to market globally
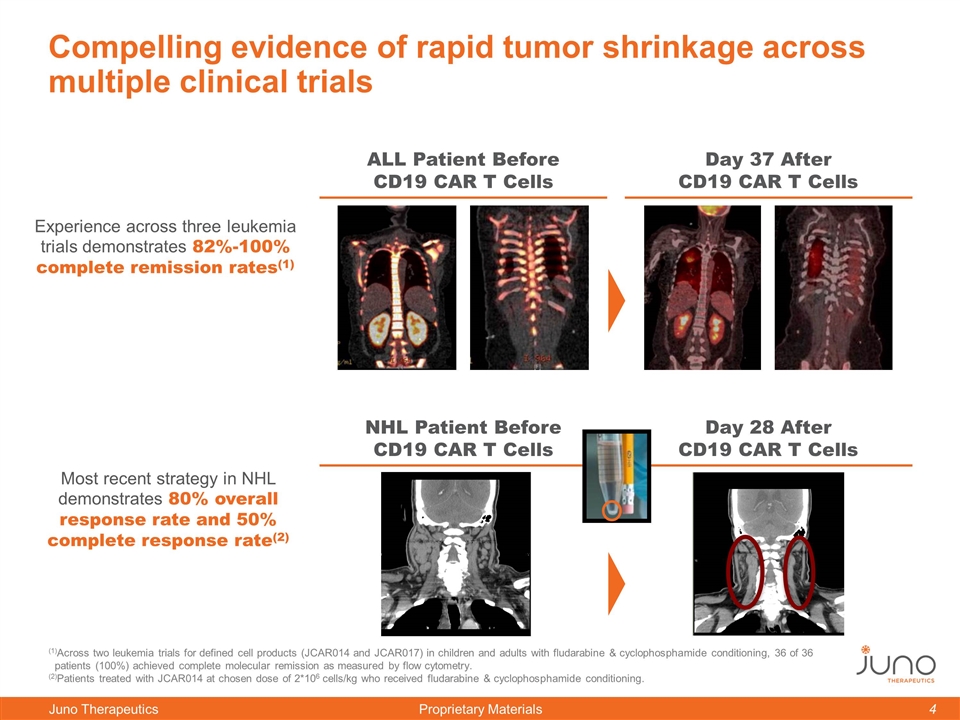
Compelling evidence of rapid tumor shrinkage across multiple clinical trials Day 37 After CD19 CAR T Cells ALL Patient Before CD19 CAR T Cells Day 28 After CD19 CAR T Cells NHL Patient Before CD19 CAR T Cells (1)Across two leukemia trials for defined cell products (JCAR014 and JCAR017) in children and adults with fludarabine & cyclophosphamide conditioning, 36 of 36 patients (100%) achieved complete molecular remission as measured by flow cytometry. (2)Patients treated with JCAR014 at chosen dose of 2*106 cells/kg who received fludarabine & cyclophosphamide conditioning. Experience across three leukemia trials demonstrates 82%-100% complete remission rates(1) Most recent strategy in NHL demonstrates 80% overall response rate and 50% complete response rate(2)
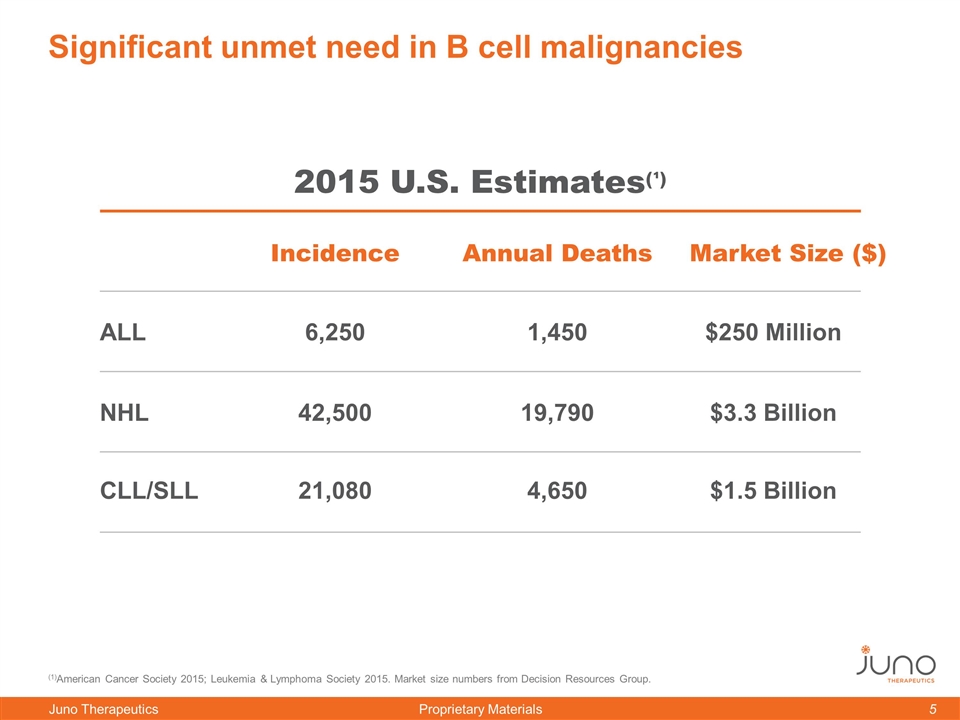
Significant unmet need in B cell malignancies (1)American Cancer Society 2015; Leukemia & Lymphoma Society 2015. Market size numbers from Decision Resources Group. Incidence Annual Deaths ALL NHL CLL/SLL 6,250 42,500 21,080 1,450 19,790 4,650 2015 U.S. Estimates(¹) Market Size ($) $250 Million $3.3 Billion $1.5 Billion
JCAR015 Phase II ROCKET Trial Update FDA = Food and Drug Administration Two patient deaths within a week due to severe neurotoxicity, following the recent addition of fludarabine (flu) to the pre-conditioning regimen, resulted in a clinical hold. A systematic review of multiple possible factors that could have caused or contributed to the increased neurotoxicity has been conducted, including, but not limited to: Based on our review of the data, including prior experience in 129 patients in ALL with both cyclophosphamide (cy) only and flu/cy, we believe that patient characteristics, conditioning, and cell dose/activity could have potentially influenced JCAR015 neurotoxicity. While potentially multifactorial, we believe fludarabine is the most likely and most appropriately modifiable factor contributing to the increased neurotoxicity. Juno proposes to return to Cy only conditioning in order to preserve appropriate risk/benefit for relapsed/refractory ALL patients in the trial and further characterize the safety and efficacy of JCAR015. The FDA has requested that Juno submit, as a Complete Response to the Clinical Hold: a revised patient informed consent form, a revised investigator brochure, a revised trial protocol, and a copy of the presentation made to the agency on July 6. Juno’s intention is to submit the requested information to the FDA by July 8. Conditioning regimen Patient characteristics Toxicity management Cell Dose Product/CMC attributes
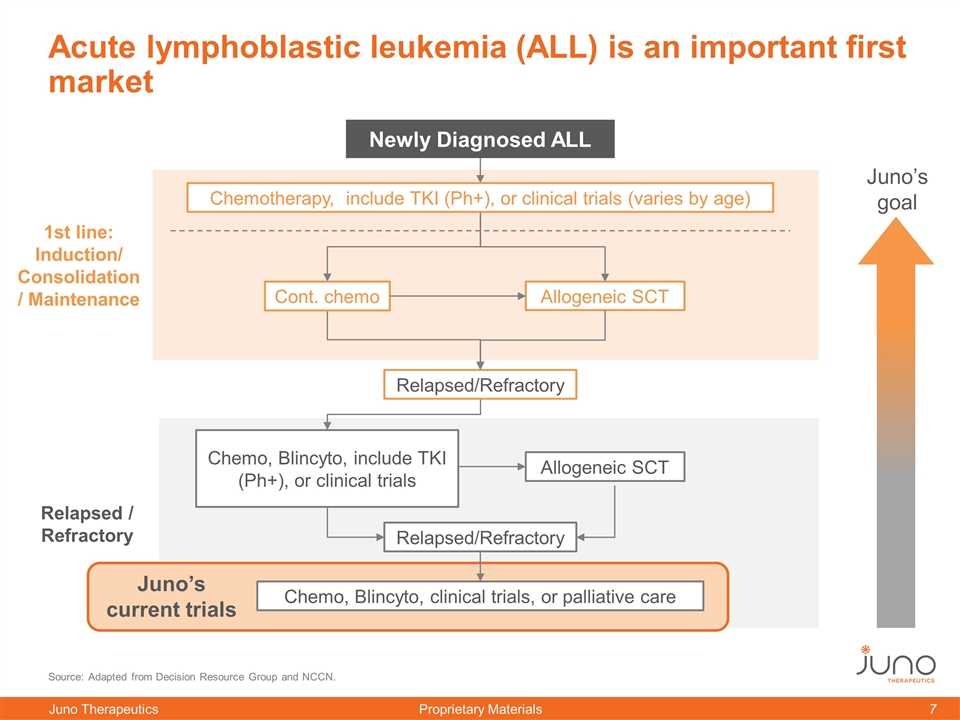
Source: Adapted from Decision Resource Group and NCCN. Relapsed/Refractory Chemo, Blincyto, include TKI (Ph+), or clinical trials Relapsed/Refractory Chemo, Blincyto, clinical trials, or palliative care 1st line: Induction/ Consolidation/ Maintenance Relapsed / Refractory Acute lymphoblastic leukemia (ALL) is an important first market Juno’s goal Chemotherapy, include TKI (Ph+), or clinical trials (varies by age) Newly Diagnosed ALL Allogeneic SCT Juno’s current trials Cont. chemo Allogeneic SCT
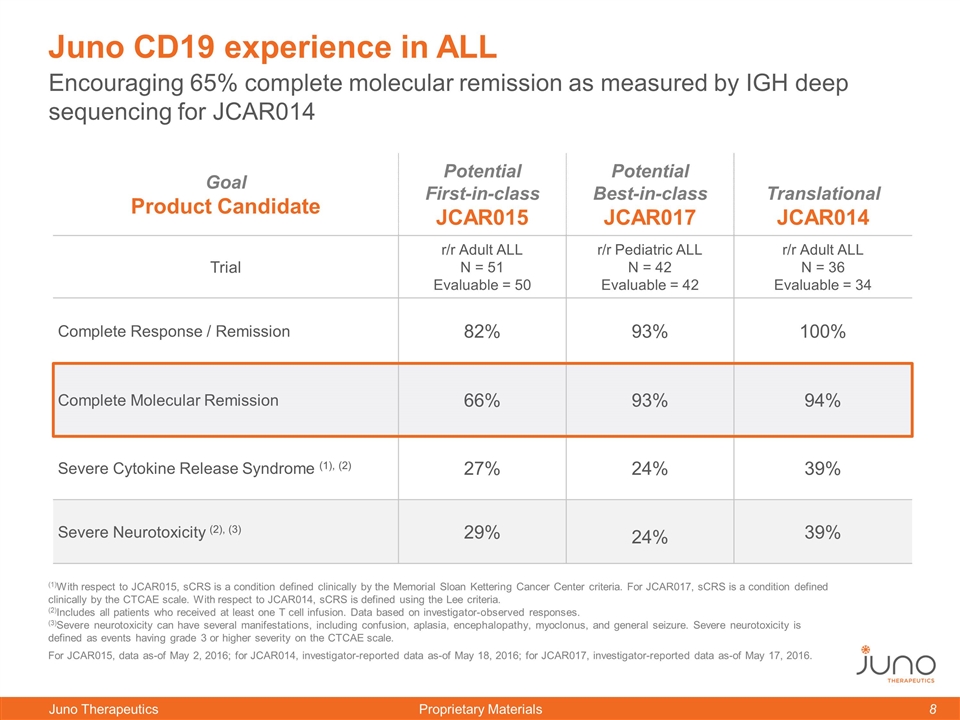
Encouraging 65% complete molecular remission as measured by IGH deep sequencing for JCAR014 Juno CD19 experience in ALL Goal Product Candidate Potential First-in-class JCAR015 Potential Best-in-class JCAR017 Translational JCAR014 Trial r/r Adult ALL N = 51 Evaluable = 50 r/r Pediatric ALL N = 42 Evaluable = 42 r/r Adult ALL N = 36 Evaluable = 34 Complete Response / Remission 82% 93% 100% Complete Molecular Remission 66% 93% 94% Severe Cytokine Release Syndrome (1), (2) 27% 24% 39% Severe Neurotoxicity (2), (3) 29% 24% 39% (1)With respect to JCAR015, sCRS is a condition defined clinically by the Memorial Sloan Kettering Cancer Center criteria. For JCAR017, sCRS is a condition defined clinically by the CTCAE scale. With respect to JCAR014, sCRS is defined using the Lee criteria. (2)Includes all patients who received at least one T cell infusion. Data based on investigator-observed responses. (3)Severe neurotoxicity can have several manifestations, including confusion, aplasia, encephalopathy, myoclonus, and general seizure. Severe neurotoxicity is defined as events having grade 3 or higher severity on the CTCAE scale. For JCAR015, data as-of May 2, 2016; for JCAR014, investigator-reported data as-of May 18, 2016; for JCAR017, investigator-reported data as-of May 17, 2016.
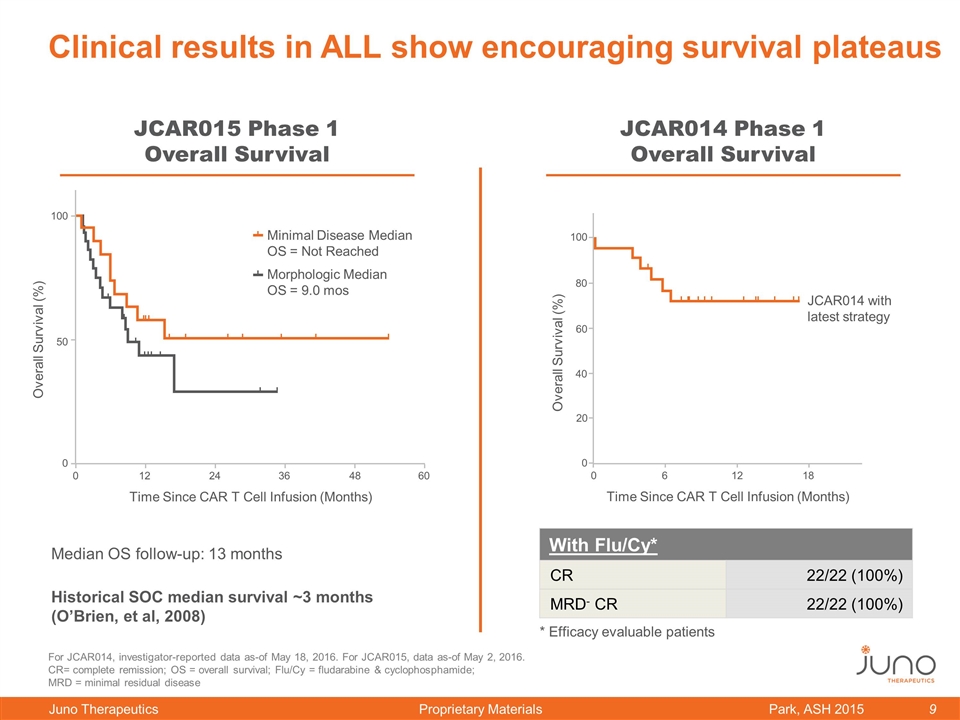
Clinical results in ALL show encouraging survival plateaus Park, ASH 2015 Median OS follow-up: 13 months Historical SOC median survival ~3 months (O’Brien, et al, 2008) JCAR015 Phase 1 Overall Survival With Flu/Cy* CR 22/22 (100%) MRD- CR 22/22 (100%) JCAR014 Phase 1 Overall Survival For JCAR014, investigator-reported data as-of May 18, 2016. For JCAR015, data as-of May 2, 2016. CR= complete remission; OS = overall survival; Flu/Cy = fludarabine & cyclophosphamide; MRD = minimal residual disease * Efficacy evaluable patients 0 50 100 0 12 24 36 48 60 Time Since CAR T Cell Infusion (Months) Overall Survival (%) Minimal Disease Median OS = Not Reached Morphologic Median OS = 9.0 mos 0 60 100 80 40 20 0 6 12 18 Time Since CAR T Cell Infusion (Months) Overall Survival (%) JCAR014 with latest strategy
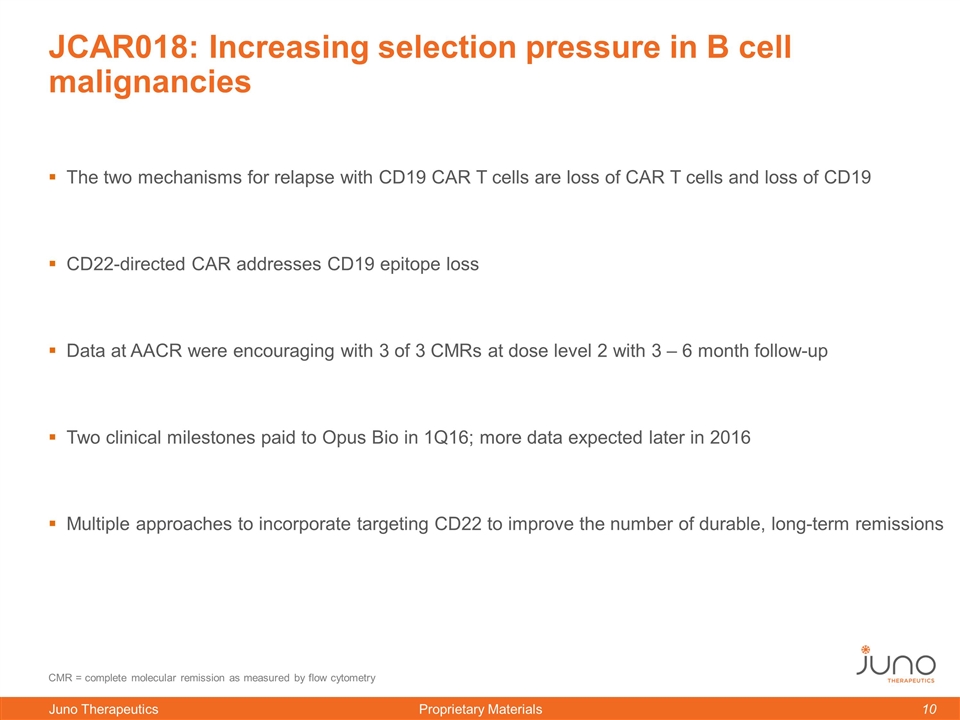
JCAR018: Increasing selection pressure in B cell malignancies The two mechanisms for relapse with CD19 CAR T cells are loss of CAR T cells and loss of CD19 CD22-directed CAR addresses CD19 epitope loss Data at AACR were encouraging with 3 of 3 CMRs at dose level 2 with 3 – 6 month follow-up Two clinical milestones paid to Opus Bio in 1Q16; more data expected later in 2016 Multiple approaches to incorporate targeting CD22 to improve the number of durable, long-term remissions CMR = complete molecular remission as measured by flow cytometry
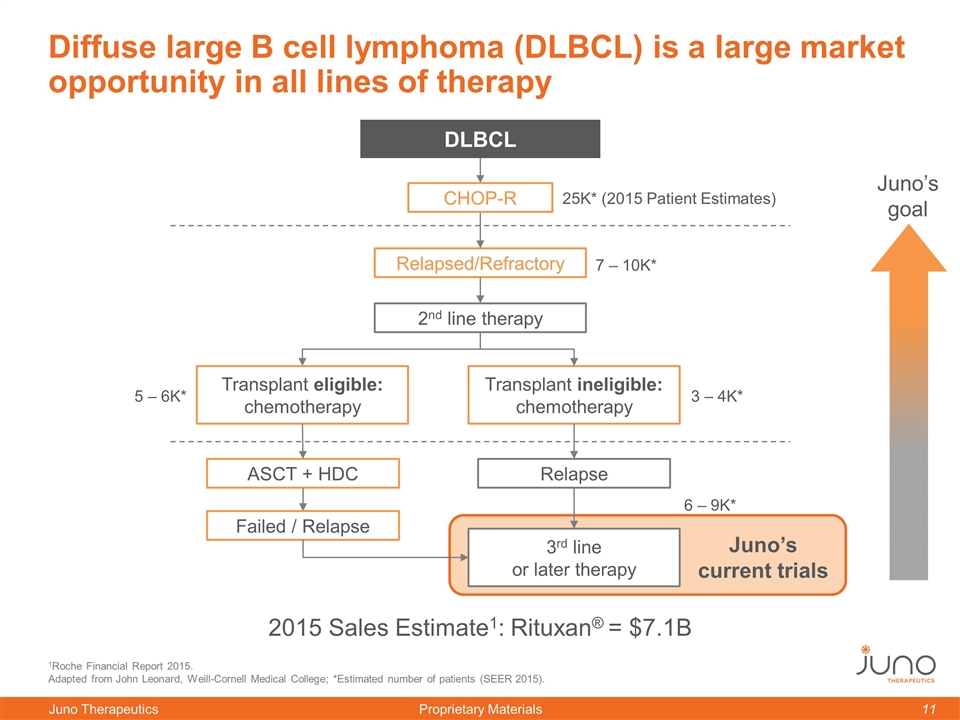
1Roche Financial Report 2015. Adapted from John Leonard, Weill-Cornell Medical College; *Estimated number of patients (SEER 2015). Relapsed/Refractory Transplant ineligible: chemotherapy Diffuse large B cell lymphoma (DLBCL) is a large market opportunity in all lines of therapy Juno’s goal CHOP-R DLBCL 2015 Sales Estimate1: Rituxan® = $7.1B 3rd line or later therapy 25K* (2015 Patient Estimates) Transplant eligible: chemotherapy 7 – 10K* 5 – 6K* 3 – 4K* 6 – 9K* Juno’s current trials ASCT + HDC Failed / Relapse 2nd line therapy Relapse
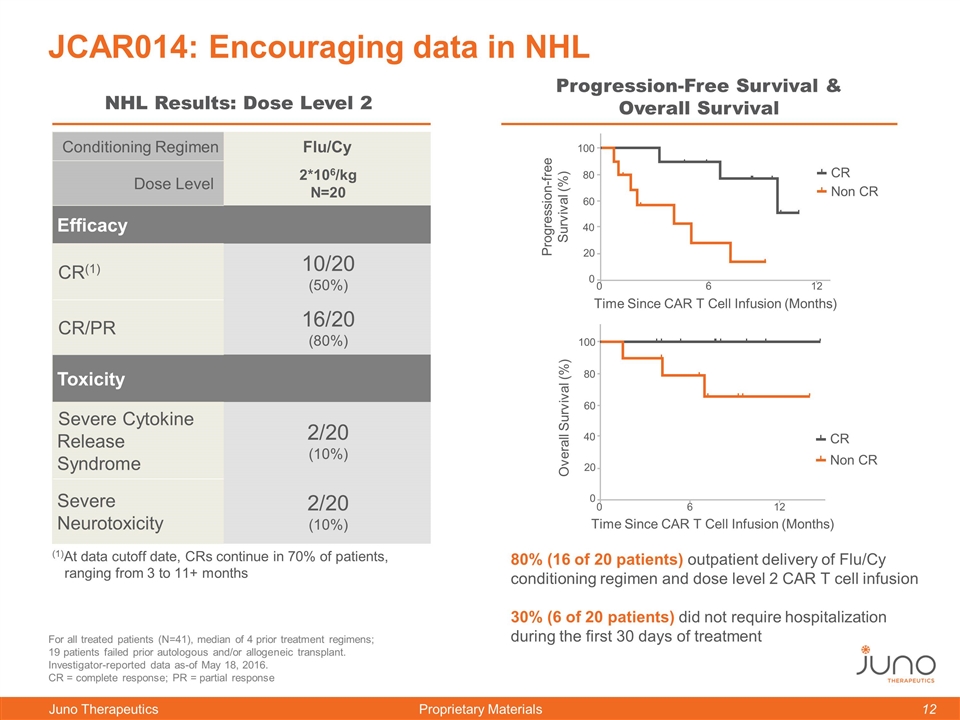
Conditioning Regimen Flu/Cy Dose Level 2*106/kg N=20 Efficacy CR(1) 10/20 (50%) CR/PR 16/20 (80%) Toxicity Severe Cytokine Release Syndrome 2/20 (10%) Severe Neurotoxicity 2/20 (10%) JCAR014: Encouraging data in NHL NHL Results: Dose Level 2 Progression-Free Survival & Overall Survival For all treated patients (N=41), median of 4 prior treatment regimens; 19 patients failed prior autologous and/or allogeneic transplant. Investigator-reported data as-of May 18, 2016. CR = complete response; PR = partial response 80% (16 of 20 patients) outpatient delivery of Flu/Cy conditioning regimen and dose level 2 CAR T cell infusion 30% (6 of 20 patients) did not require hospitalization during the first 30 days of treatment (1)At data cutoff date, CRs continue in 70% of patients, ranging from 3 to 11+ months Progression-free Survival (%) 0 60 100 80 40 20 0 6 12 Time Since CAR T Cell Infusion (Months) Non CR CR Overall Survival (%) 0 60 100 80 40 20 0 6 12 Time Since CAR T Cell Infusion (Months) Non CR CR
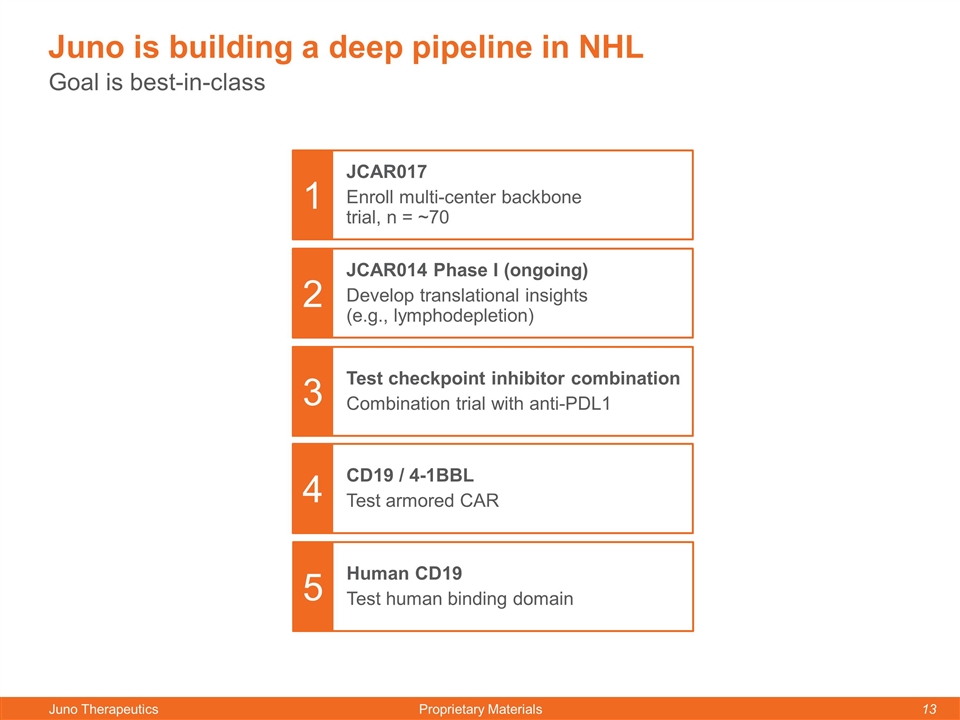
Goal is best-in-class Juno is building a deep pipeline in NHL JCAR017 Enroll multi-center backbone trial, n = ~70 JCAR014 Phase I (ongoing) Develop translational insights (e.g., lymphodepletion) Test checkpoint inhibitor combination Combination trial with anti-PDL1 CD19 / 4-1BBL Test armored CAR 1 2 3 4 Human CD19 Test human binding domain 5
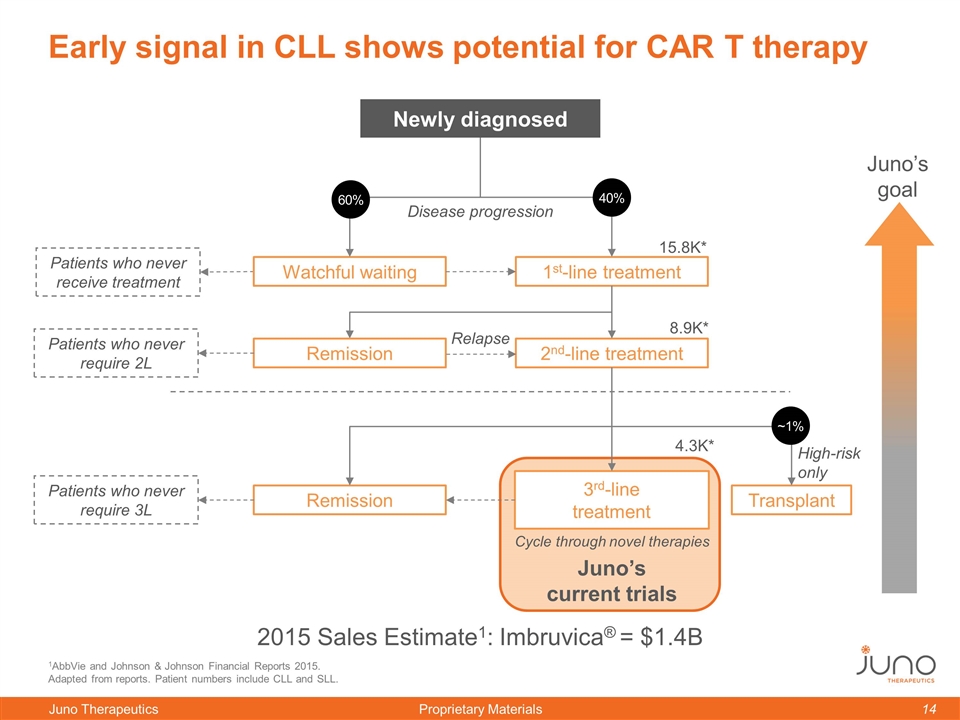
1AbbVie and Johnson & Johnson Financial Reports 2015. Adapted from reports. Patient numbers include CLL and SLL. Watchful waiting 1st-line treatment 2nd-line treatment Early signal in CLL shows potential for CAR T therapy Juno’s goal Newly diagnosed 2015 Sales Estimate1: Imbruvica® = $1.4B 3rd-line treatment Disease progression Remission 15.8K* 4.3K* Patients who never receive treatment 60% 40% Relapse 8.9K* Patients who never require 2L Cycle through novel therapies Remission Patients who never require 3L Transplant ~1% High-risk only Juno’s current trials
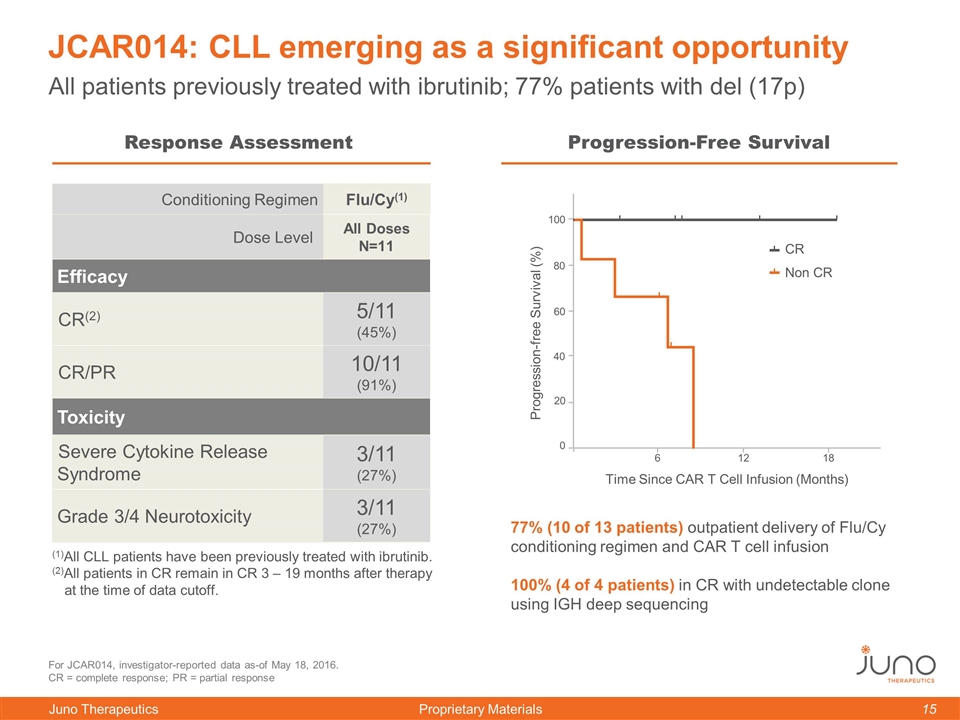
JCAR014: CLL emerging as a significant opportunity For JCAR014, investigator-reported data as-of May 18, 2016. CR = complete response; PR = partial response Conditioning Regimen Flu/Cy(1) Dose Level All Doses N=11 Efficacy CR(2) 5/11 (45%) CR/PR 10/11 (91%) Toxicity Severe Cytokine Release Syndrome 3/11 (27%) Grade 3/4 Neurotoxicity 3/11 (27%) (1)All CLL patients have been previously treated with ibrutinib. (2)All patients in CR remain in CR 3 – 19 months after therapy at the time of data cutoff. All patients previously treated with ibrutinib; 77% patients with del (17p) 77% (10 of 13 patients) outpatient delivery of Flu/Cy conditioning regimen and CAR T cell infusion 100% (4 of 4 patients) in CR with undetectable clone using IGH deep sequencing 0 12 Response Assessment Progression-Free Survival 0 60 100 80 40 20 18 Time Since CAR T Cell Infusion (Months) Progression-free Survival (%) CR Non CR 12 6
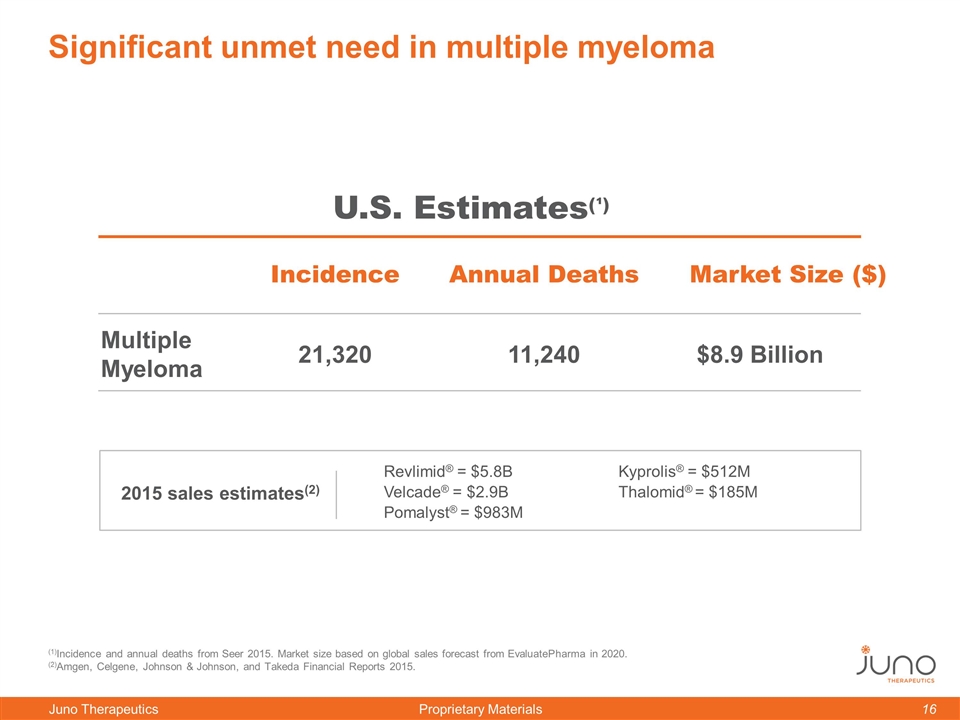
Significant unmet need in multiple myeloma Incidence Annual Deaths Multiple Myeloma 21,320 11,240 U.S. Estimates(¹) $8.9 Billion (1)Incidence and annual deaths from Seer 2015. Market size based on global sales forecast from EvaluatePharma in 2020. (2)Amgen, Celgene, Johnson & Johnson, and Takeda Financial Reports 2015. Revlimid® = $5.8B Velcade® = $2.9B Pomalyst® = $983M 2015 sales estimates(2) Kyprolis® = $512M Thalomid® = $185M Market Size ($)
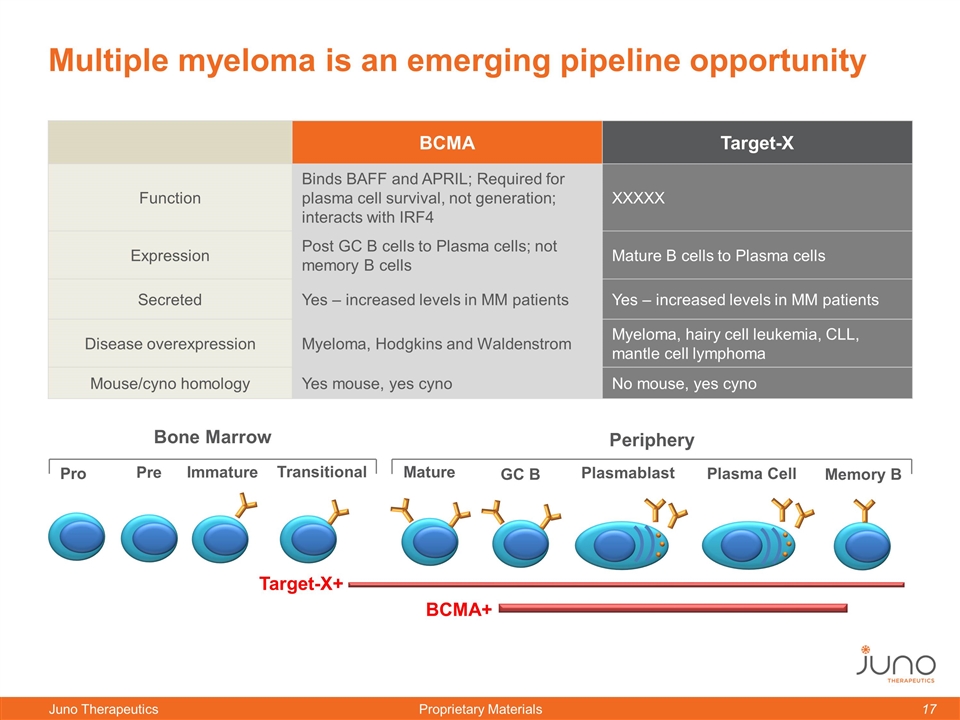
Multiple myeloma is an emerging pipeline opportunity BCMA Target-X Function Binds BAFF and APRIL; Required for plasma cell survival, not generation; interacts with IRF4 XXXXX Expression Post GC B cells to Plasma cells; not memory B cells Mature B cells to Plasma cells Secreted Yes – increased levels in MM patients Yes – increased levels in MM patients Disease overexpression Myeloma, Hodgkins and Waldenstrom Myeloma, hairy cell leukemia, CLL, mantle cell lymphoma Mouse/cyno homology Yes mouse, yes cyno No mouse, yes cyno Bone Marrow Periphery Pro Immature Pre Transitional Mature GC B Plasmablast Plasma Cell Memory B BCMA+ Target-X+
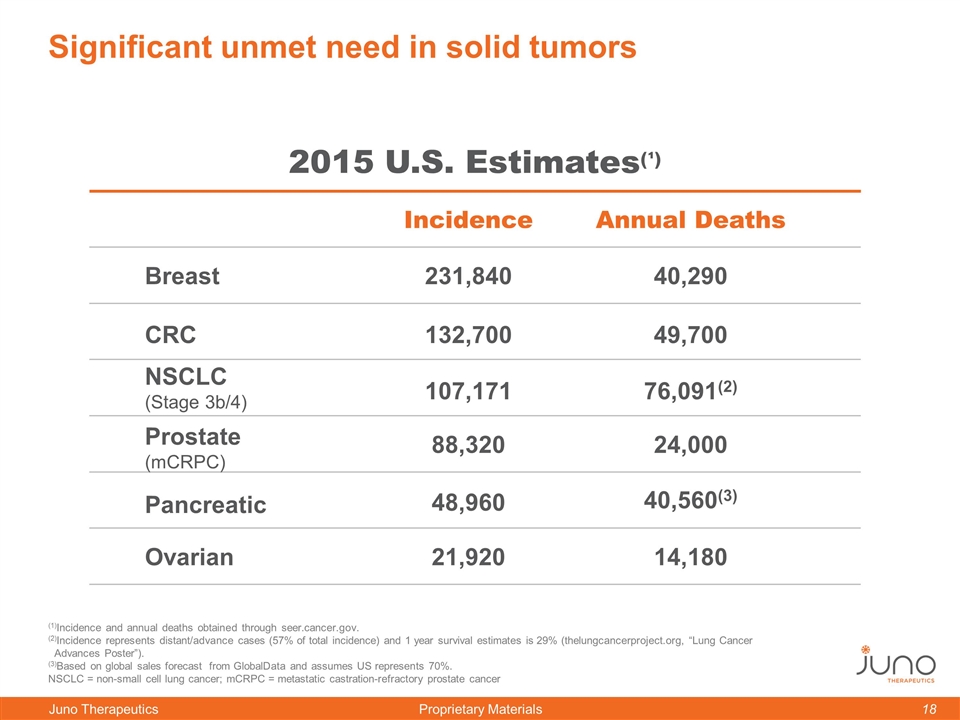
Significant unmet need in solid tumors Incidence Annual Deaths NSCLC (Stage 3b/4) 107,171 132,700 21,920 76,091(2) 49,700 14,180 2015 U.S. Estimates(¹) 88,320 24,000 (1)Incidence and annual deaths obtained through seer.cancer.gov. (2)Incidence represents distant/advance cases (57% of total incidence) and 1 year survival estimates is 29% (thelungcancerproject.org, “Lung Cancer Advances Poster”). (3)Based on global sales forecast from GlobalData and assumes US represents 70%. NSCLC = non-small cell lung cancer; mCRPC = metastatic castration-refractory prostate cancer Ovarian CRC Breast Prostate (mCRPC) Pancreatic 231,840 40,290 48,960 40,560(3)
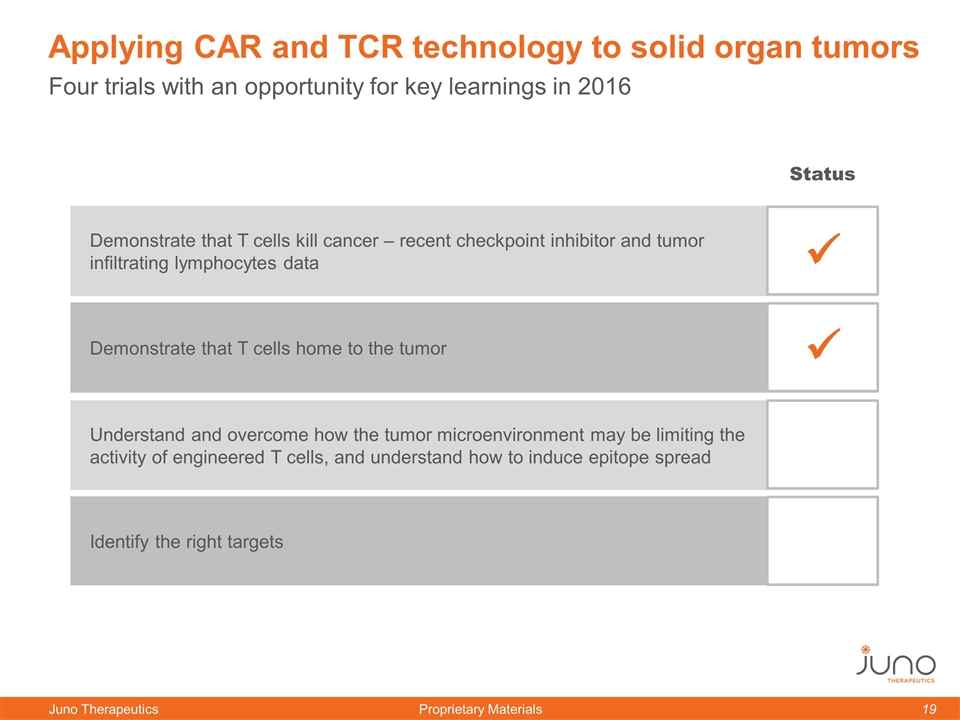
Four trials with an opportunity for key learnings in 2016 Applying CAR and TCR technology to solid organ tumors Demonstrate that T cells kill cancer – recent checkpoint inhibitor and tumor infiltrating lymphocytes data Demonstrate that T cells home to the tumor Understand and overcome how the tumor microenvironment may be limiting the activity of engineered T cells, and understand how to induce epitope spread Identify the right targets ü ü Status ü
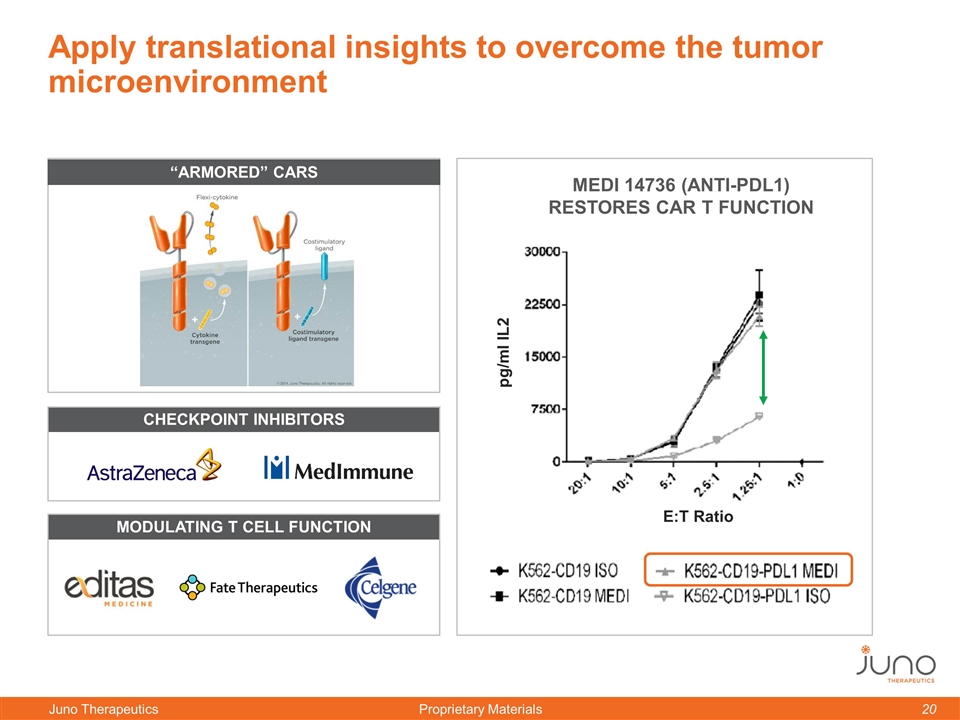
Apply translational insights to overcome the tumor microenvironment Checkpoint Inhibitors Modulating T Cell Function pg/ml IL2 Medi 14736 (anti-PDL1) restores CAR T Function “Armored” CARs E:T Ratio
Active programs in clinical development The first set of solid tumor targets WT-1 (TCR) AML / NSCLC / Mesothelioma First ongoing partial response reported in refractory mesothelioma at AACR 2016; response correlated to cell expansion and persistence Generally well tolerated and demonstrated early encouraging clinical activity Ongoing studies in AML, mesothelioma and NSCLC L1CAM (CAR) Neuroblastoma Overexpressed in neuroblastoma and a variety of solid tumors Preliminary pre-clinical data show encouraging safety profile ROR-1 (CAR) ROR-1 Expressed Tumors Overexpressed on a wide variety of cancers including NSCLC and B cell CLL Preliminary pre-clinical data show encouraging safety profile MUC-16 / IL-12 “Armored CAR” Ovarian First “Armored” CAR to advance into clinical testing MUC-16 is a protein cleaved to make CA125; overexpressed in the majority of ovarian cancers IL-12 is a pluripotent immune stimulator Multiple in-house and partner pre-clinical programs

Building a category leader Manufacturing capabilities JuMP facility online Defined cell product Global reach Celgene collaboration - CD19 opt-in JW Therapeutics - investment in China Research capabilities Internal and BD opportunities Strong balance sheet $1.13 billion as of 1Q16
Manufacturing infrastructure to support patient supply Facility online to supply clinical material for ongoing Phase II ROCKET trial Capacity for thousands of patients with a scalable and modular design Flexible capacity and able to process multiple products, currently JCAR015 and JCAR017 Functionally closed process with extensive use of automation Processes predictably produce CAR T cells without patient pre-selection to date Defined cell process positions Juno to exploit biologic insights today and in the future Medium term goal to implement two day manufacturing process
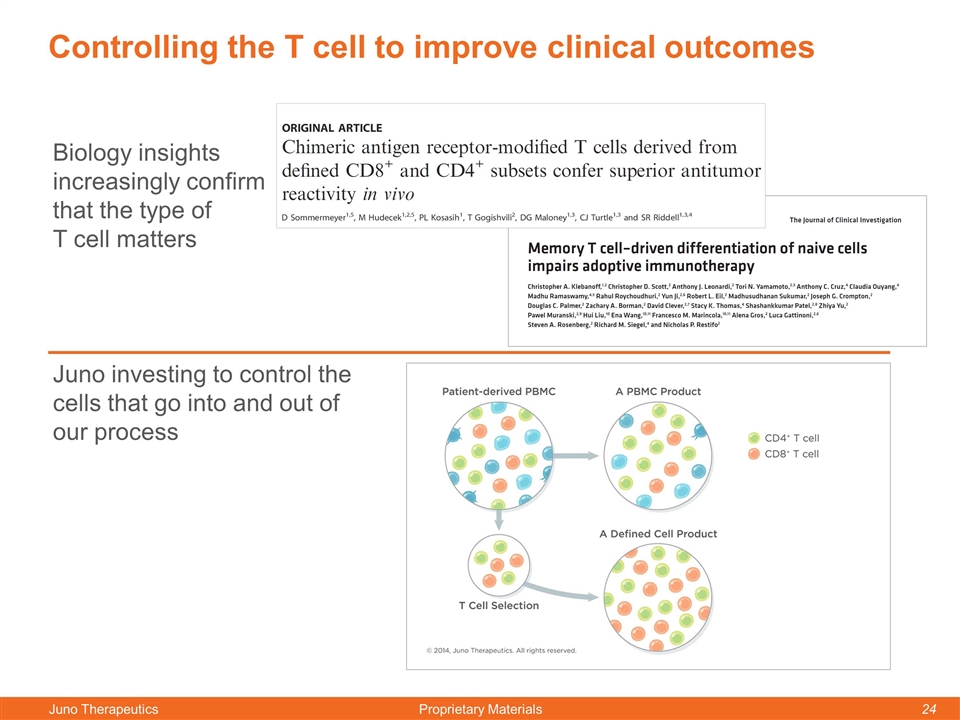
Controlling the T cell to improve clinical outcomes Juno investing to control the cells that go into and out of our process Biology insights increasingly confirm that the type of T cell matters
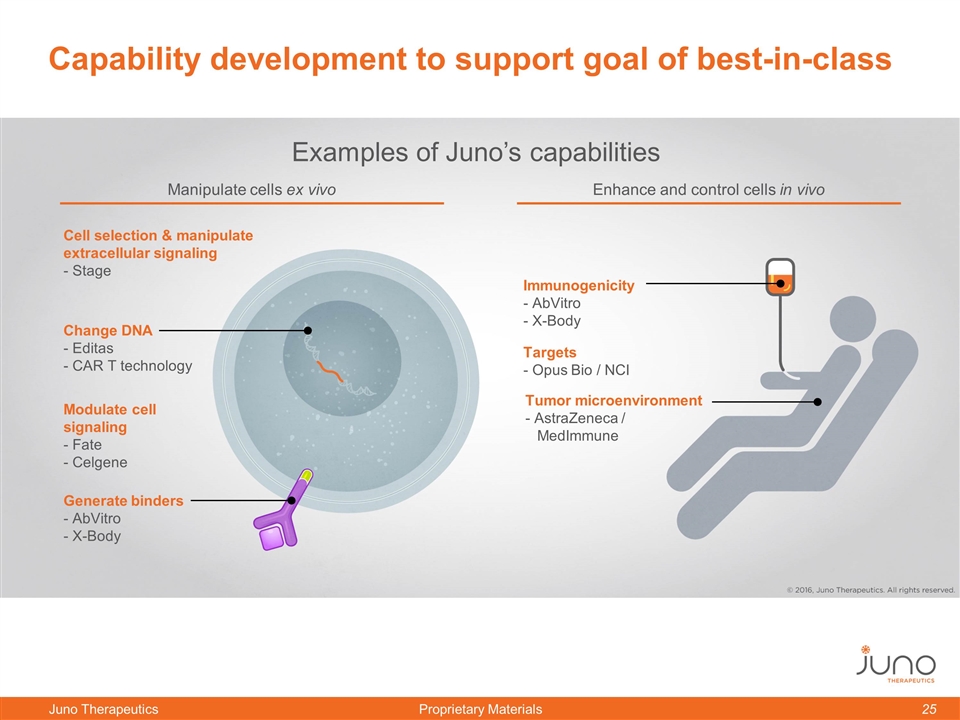
Cell selection & manipulate extracellular signaling - Stage Targets - Opus Bio / NCI Tumor microenvironment - AstraZeneca / MedImmune Generate binders - AbVitro - X-Body Examples of Juno’s capabilities Capability development to support goal of best-in-class Change DNA - Editas - CAR T technology Modulate cell signaling - Fate - Celgene Immunogenicity - AbVitro - X-Body Manipulate cells ex vivo Enhance and control cells in vivo
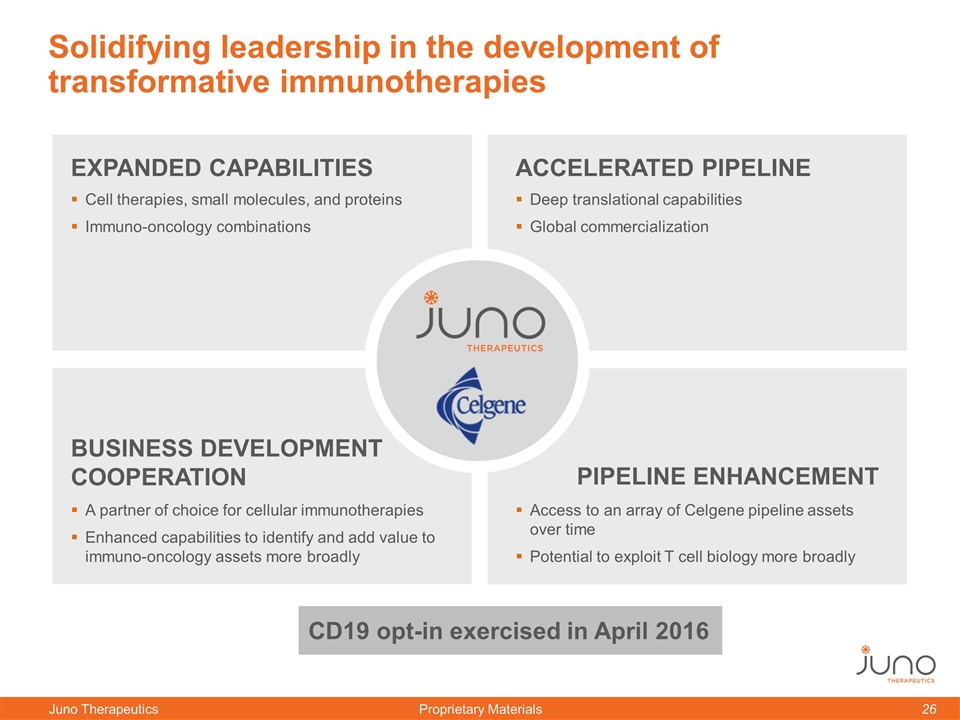
Solidifying leadership in the development of transformative immunotherapies BUSINESS DEVELOPMENT COOPERATION EXPANDED CAPABILITIES ACCELERATED PIPELINE PIPELINE ENHANCEMENT Cell therapies, small molecules, and proteins Immuno-oncology combinations Deep translational capabilities Global commercialization A partner of choice for cellular immunotherapies Enhanced capabilities to identify and add value to immuno-oncology assets more broadly Access to an array of Celgene pipeline assets over time Potential to exploit T cell biology more broadly CD19 opt-in exercised in April 2016
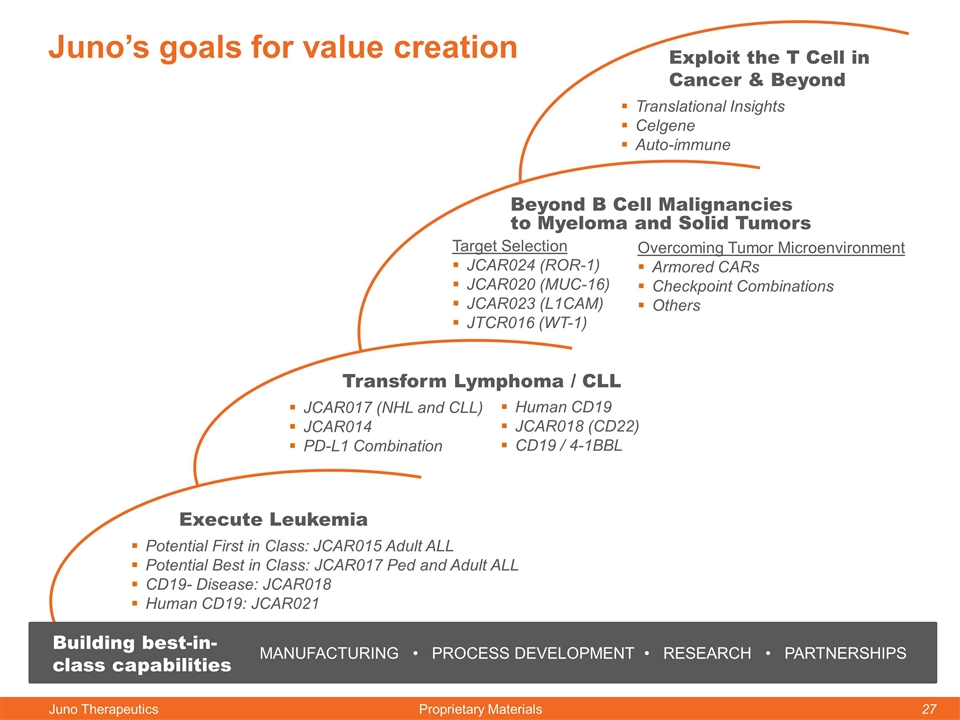
Juno’s goals for value creation Execute Leukemia Potential First in Class: JCAR015 Adult ALL Potential Best in Class: JCAR017 Ped and Adult ALL CD19- Disease: JCAR018 Human CD19: JCAR021 Transform Lymphoma / CLL JCAR017 (NHL and CLL) JCAR014 PD-L1 Combination Beyond B Cell Malignancies to Myeloma and Solid Tumors Target Selection JCAR024 (ROR-1) JCAR020 (MUC-16) JCAR023 (L1CAM) JTCR016 (WT-1) Exploit the T Cell in Cancer & Beyond Translational Insights Celgene Auto-immune MANUFACTURING • PROCESS DEVELOPMENT • RESEARCH • PARTNERSHIPS Building best-in-class capabilities Human CD19 JCAR018 (CD22) CD19 / 4-1BBL Overcoming Tumor Microenvironment Armored CARs Checkpoint Combinations Others
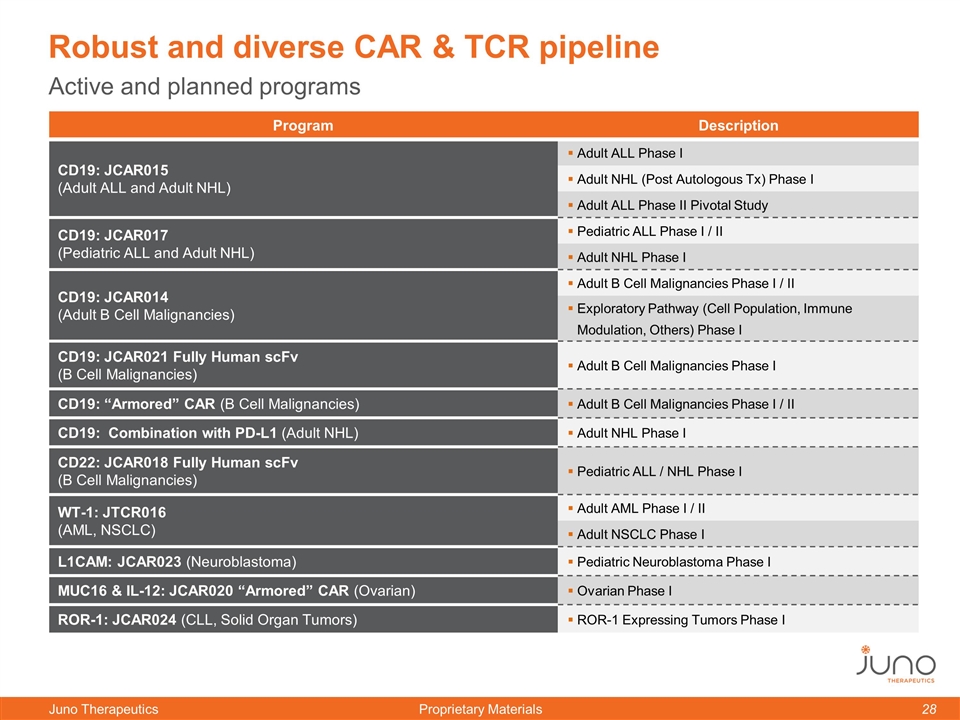
Program Description CD19: JCAR015 (Adult ALL and Adult NHL) Adult ALL Phase I Adult NHL (Post Autologous Tx) Phase I Adult ALL Phase II Pivotal Study CD19: JCAR017 (Pediatric ALL and Adult NHL) Pediatric ALL Phase I / II Adult NHL Phase I CD19: JCAR014 (Adult B Cell Malignancies) Adult B Cell Malignancies Phase I / II Exploratory Pathway (Cell Population, Immune Modulation, Others) Phase I CD19: JCAR021 Fully Human scFv (B Cell Malignancies) Adult B Cell Malignancies Phase I CD19: “Armored” CAR (B Cell Malignancies) Adult B Cell Malignancies Phase I / II CD19: Combination with PD-L1 (Adult NHL) Adult NHL Phase I CD22: JCAR018 Fully Human scFv (B Cell Malignancies) Pediatric ALL / NHL Phase I WT-1: JTCR016 (AML, NSCLC) Adult AML Phase I / II Adult NSCLC Phase I L1CAM: JCAR023 (Neuroblastoma) Pediatric Neuroblastoma Phase I MUC16 & IL-12: JCAR020 “Armored” CAR (Ovarian) Ovarian Phase I ROR-1: JCAR024 (CLL, Solid Organ Tumors) ROR-1 Expressing Tumors Phase I Active and planned programs Robust and diverse CAR & TCR pipeline
