Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zynerba Pharmaceuticals, Inc. | a16-14354_18k.htm |
Exhibit 99.1
July 2016 A clinical-stage pharmaceutical company dedicated to the development of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs

Disclaimer The statements in this presentation may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements, among other things relate to the future operations, opportunities or financial performance of Zynerba Pharmaceuticals, Inc. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “proposed,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the Company’s current expectations. These and other risks are described in our filings with the Securities and Exchange Commission, available at www.sec.gov. Any forward-looking statements that the Company makes in this presentation speak only as of the date of this PRESENTATION. The Company assumes no obligation to update forward-looking statements whether as a result of new information, future events or otherwise, after the date of this presentation. 2 © 2016 Zynerba Pharmaceuticals, Inc. All rights reserved. Zynerba is a registered trademark of Zynerba Pharmaceuticals, Inc. All other trademarks and registered trademarks are property of their respective owners.
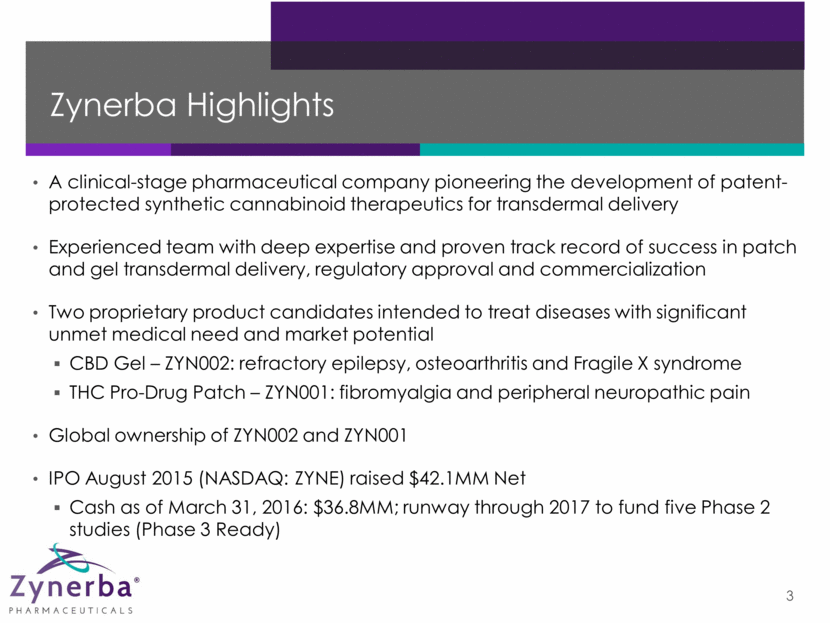
Zynerba Highlights 3 A clinical-stage pharmaceutical company pioneering the development of patent-protected synthetic cannabinoid therapeutics for transdermal delivery Experienced team with deep expertise and proven track record of success in patch and gel transdermal delivery, regulatory approval and commercialization Two proprietary product candidates intended to treat diseases with significant unmet medical need and market potential CBD Gel – ZYN002: refractory epilepsy, osteoarthritis and Fragile X syndrome THC Pro-Drug Patch – ZYN001: fibromyalgia and peripheral neuropathic pain Global ownership of ZYN002 and ZYN001 IPO August 2015 (NASDAQ: ZYNE) raised $42.1MM Net Cash as of March 31, 2016: $36.8MM; runway through 2017 to fund five Phase 2 studies (Phase 3 Ready)
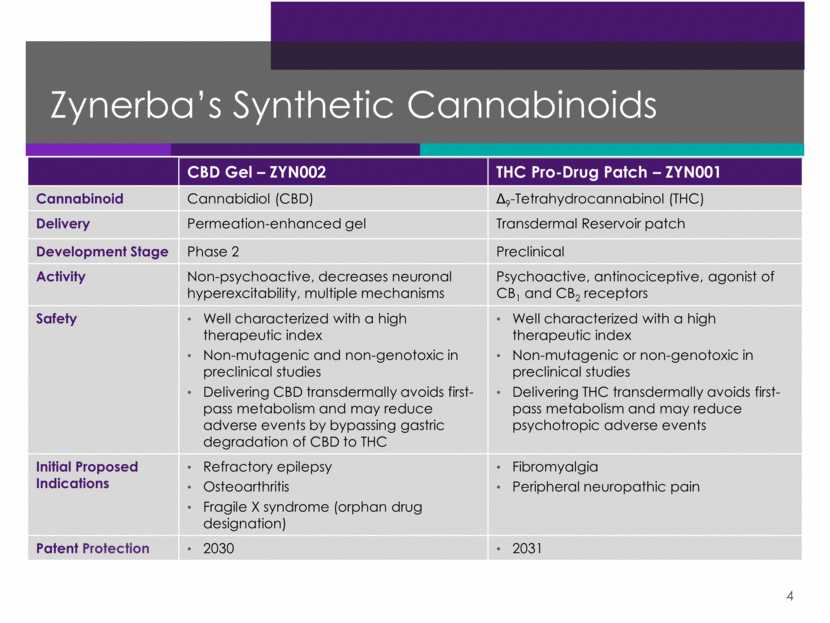
CBD Gel – ZYN002 THC Pro-Drug Patch – ZYN001 Cannabinoid Cannabidiol (CBD) Δ9-Tetrahydrocannabinol (THC) Delivery Permeation-enhanced gel Transdermal Reservoir patch Development Stage Phase 2 Preclinical Activity Non-psychoactive, decreases neuronal hyperexcitability, multiple mechanisms Psychoactive, antinociceptive, agonist of CB1 and CB2 receptors Safety Well characterized with a high therapeutic index Non-mutagenic and non-genotoxic in preclinical studies Delivering CBD transdermally avoids first-pass metabolism and may reduce adverse events by bypassing gastric degradation of CBD to THC Well characterized with a high therapeutic index Non-mutagenic or non-genotoxic in preclinical studies Delivering THC transdermally avoids first-pass metabolism and may reduce psychotropic adverse events Initial Proposed Indications Refractory epilepsy Osteoarthritis Fragile X syndrome (orphan drug designation) Fibromyalgia Peripheral neuropathic pain Patent Protection 2030 2031 Zynerba’s Synthetic Cannabinoids 4
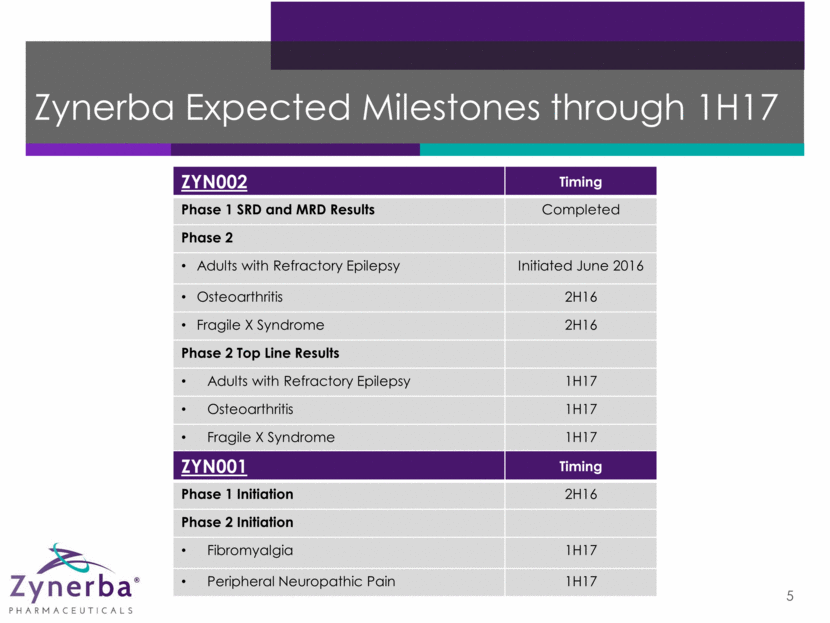
Zynerba Expected Milestones through 1H17 5 ZYN002 Timing Phase 1 SRD and MRD Results Completed Phase 2 Adults with Refractory Epilepsy Initiated June 2016 Osteoarthritis 2H16 Fragile X Syndrome 2H16 Phase 2 Top Line Results Adults with Refractory Epilepsy 1H17 Osteoarthritis 1H17 Fragile X Syndrome 1H17 ZYN001 Timing Phase 1 Initiation 2H16 Phase 2 Initiation Fibromyalgia 1H17 Peripheral Neuropathic Pain 1H17
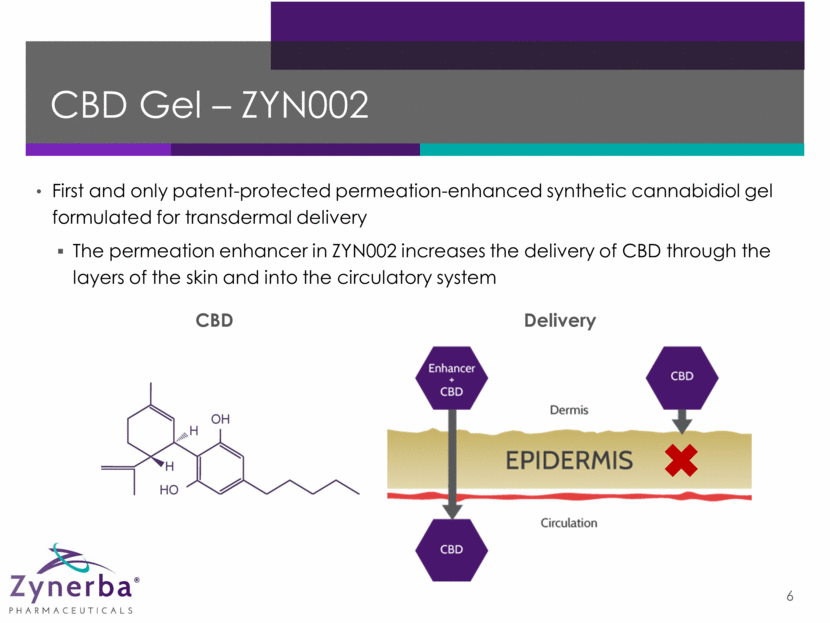
CBD Gel – ZYN002 First and only patent-protected permeation-enhanced synthetic cannabidiol gel formulated for transdermal delivery The permeation enhancer in ZYN002 increases the delivery of CBD through the layers of the skin and into the circulatory system 6 CBD Delivery
CBD Gel – ZYN002 vs. Oral CBD 7 Potential Benefits of Transdermal ZYN002 vs. Oral CBD Avoids first-pass metabolism in the liver More consistent, controlled and sustained plasma level Avoids the GI tract Very low incidence of gastrointestinal event Fewer drug-drug interactions Lower incidence of negative psychoactive effects; Avoids degradation of CBD to THC
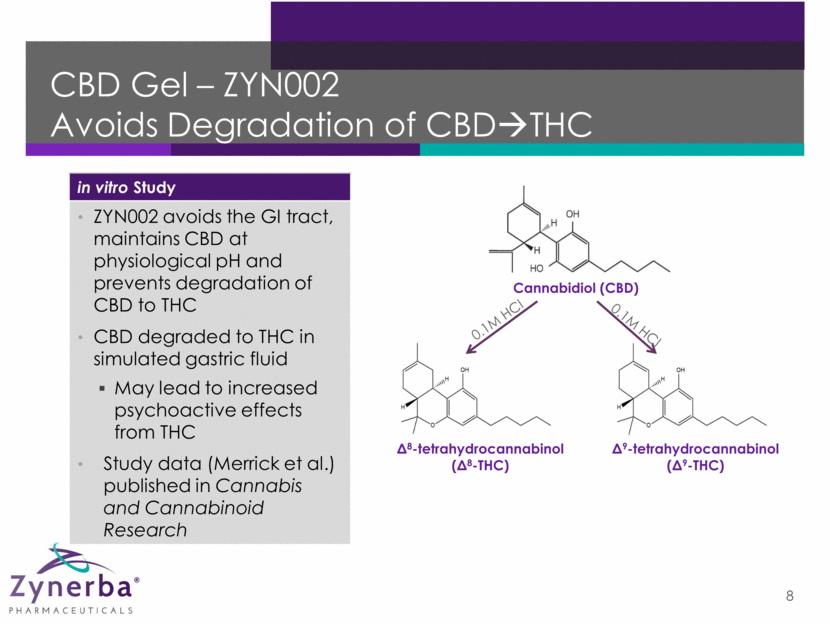
CBD Gel – ZYN002 Avoids Degradation of CBD THC 8 Δ8-tetrahydrocannabinol (Δ8-THC) Δ9-tetrahydrocannabinol (Δ9-THC) Cannabidiol (CBD) 0.1M HCl 0.1M HCl in vitro Study ZYN002 avoids the GI tract, maintains CBD at physiological pH and prevents degradation of CBD to THC CBD degraded to THC in simulated gastric fluid May lead to increased psychoactive effects from THC Study data (Merrick et al.) published in Cannabis and Cannabinoid Research
CBD Gel ZYN002 – Refractory Epilepsy in Adults 9 * Data provided by Decision Resources ~ 700,000 adult epilepsy patients are refractory to two or more anti-epileptic drugs in the US* Third-party Phase 3 clinical trial (n=120) with oral botanical CBD to assess safety and efficacy in children with Dravet syndrome, a severe and poorly controlled type of epilepsy Median reduction in monthly convulsive seizures was 39% compared with a reduction of 13 % on placebo (p=0.01) Third-party Phase 3 clinical trial (n=171) with oral botanical CBD to assess safety and efficacy in patients with Lennox-Gastaut syndrome (LGS), a rare and severe childhood-onset epilepsy Median reduction in monthly drop seizures was 44% compared with a reduction of 22% on placebo (p=0.01) Third-party placebo controlled clinical trial of oral CBD showed significant response 88% of the CBD-treated group had a response to treatment; only 13% of the placebo treated group responded to treatment Third-party open-label trial with oral botanical CBD to assess safety and efficacy in treatment-resistant epilepsy (motor seizures, Dravet and Lennox-Gastaut syndrome) Median reduction of 49.8% for Dravet; median reduction in monthly motor seizures was 36.5%, and 36.8% reduction in motor seizures for LGS Post-hoc analysis shows patients with focal seizures had a 55% reduction in seizures
ZYN002 – Osteoarthritis 10 ~31 million Osteoarthritis sufferers in the US Several pre-clinical studies demonstrate the efficacy of cannabidiol for inflammation and pain in animal models for osteoarthritis/rheumatoid arthritis (OA/RA) In a third-party placebo-controlled study of 58 subjects with rheumatoid arthritis, botanical CBD/THC combination showed significant improvement at 5 weeks in: Pain on movement Pain at rest Sleep quality Disease Activity Score (DAS- 28) and Short Form McGill Pain Questionnaire * Data provided by Decision Resources. Data for osteoarthritis represents market for osteoarthritis patients not currently served by over-the-counter medications.
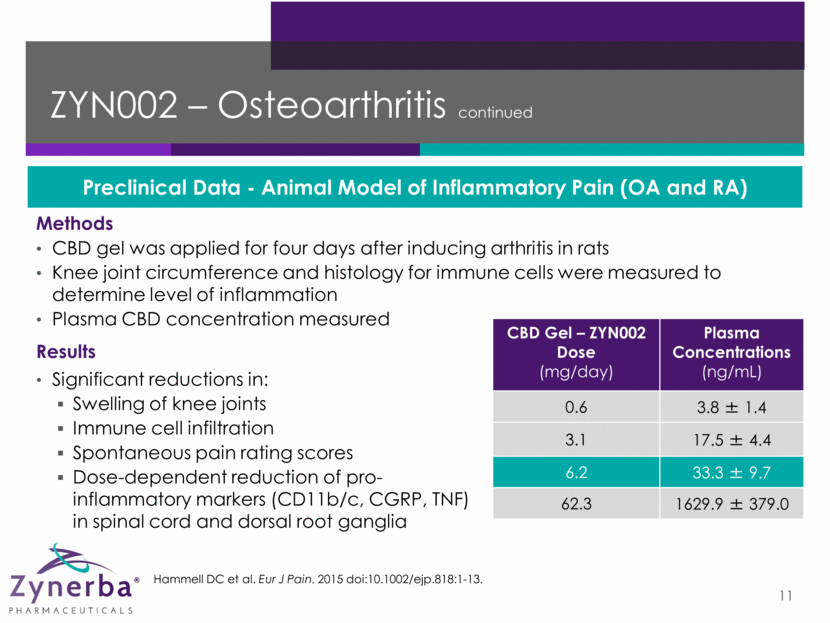
ZYN002 – Osteoarthritis continued 11 Results Significant reductions in: Swelling of knee joints Immune cell infiltration Spontaneous pain rating scores Dose-dependent reduction of pro-inflammatory markers (CD11b/c, CGRP, TNF) in spinal cord and dorsal root ganglia Methods CBD gel was applied for four days after inducing arthritis in rats Knee joint circumference and histology for immune cells were measured to determine level of inflammation Plasma CBD concentration measured Preclinical Data - Animal Model of Inflammatory Pain (OA and RA) CBD Gel – ZYN002 Dose (mg/day) Plasma Concentrations (ng/mL) 0.6 3.8 ± 1.4 3.1 17.5 ± 4.4 6.2 33.3 ± 9.7 62.3 1629.9 ± 379.0 Hammell DC et al. Eur J Pain. 2015 doi:10.1002/ejp.818:1-13.
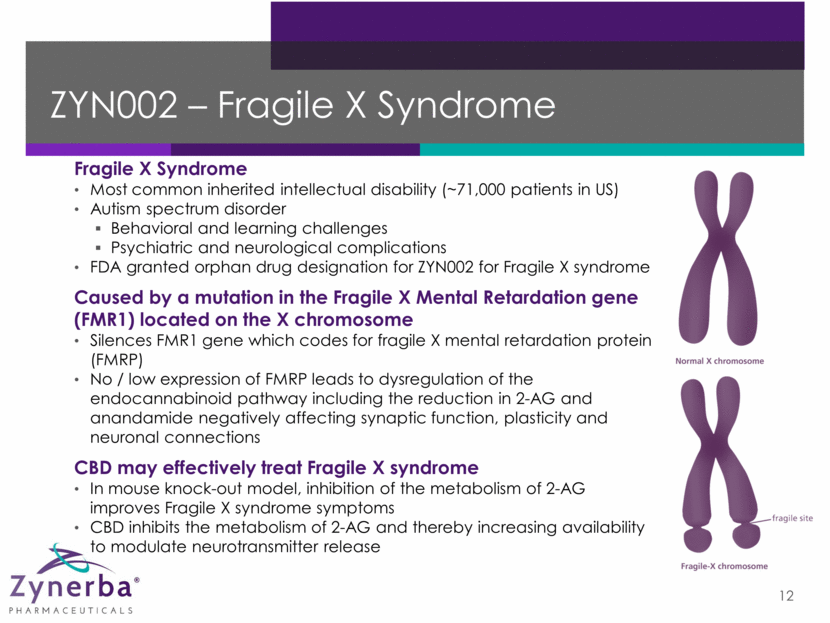
ZYN002 – Fragile X Syndrome 12 Fragile X Syndrome Most common inherited intellectual disability (~71,000 patients in US) Autism spectrum disorder Behavioral and learning challenges Psychiatric and neurological complications FDA granted orphan drug designation for ZYN002 for Fragile X syndrome Caused by a mutation in the Fragile X Mental Retardation gene (FMR1) located on the X chromosome Silences FMR1 gene which codes for fragile X mental retardation protein (FMRP) No / low expression of FMRP leads to dysregulation of the endocannabinoid pathway including the reduction in 2-AG and anandamide negatively affecting synaptic function, plasticity and neuronal connections CBD may effectively treat Fragile X syndrome In mouse knock-out model, inhibition of the metabolism of 2-AG improves Fragile X syndrome symptoms CBD inhibits the metabolism of 2-AG and thereby increasing availability to modulate neurotransmitter release
CBD Gel – ZYN002 Status Initiated STAR 1 trial in refractory epilepsy in June 2016 Initiating Phase 2 Studies in OA and FXS in 2H2016 Multiple Rising Dose Phase I Study initiated January 2016 24 healthy volunteers; 12 epilepsy patients Randomized, double-blind, placebo controlled Top line results announced June 2016 Single Rising Dose Phase 1 Study initiated October 2015 32 healthy volunteers; 10 epilepsy patients Randomized, double-blind, placebo controlled Final results announced June 2016 In vivo studies in guinea pig, rat, porcine and primate Findings: Sustained plasma levels obtained in all species; CBD plasma concentration linear in relationship to dose 13
Title Multiple Rising Dose Phase 1 Study Subjects Results from 24 healthy volunteers (n= 18 ZYN002; n= 6 placebo) and 12 epilepsy patients (n=9 ZYN002; n=3 placebo) Dose Three dose levels (200, 250, and 500 mg CBD) Safety & Tolerability ZYN002 was safe and well tolerated at all dose levels Transdermal application well tolerated with minimal erythema Skin dryness at application site No somnolence or fatigue reported Very low incidence of GI events observed Incidence of adverse events associated with ZYN002 in healthy volunteers and epilepsy patients was similar to placebo Cognition/Mood Demonstrated no impairment in cognitive function based on Trail Making, Divided Attention, & PASAT No changes in mood as accessed by IDAS and PANAS demonstrating that ZYN002 is not associated with declines in psychological health CBD Gel – ZYN002 Phase I MRD Study 14
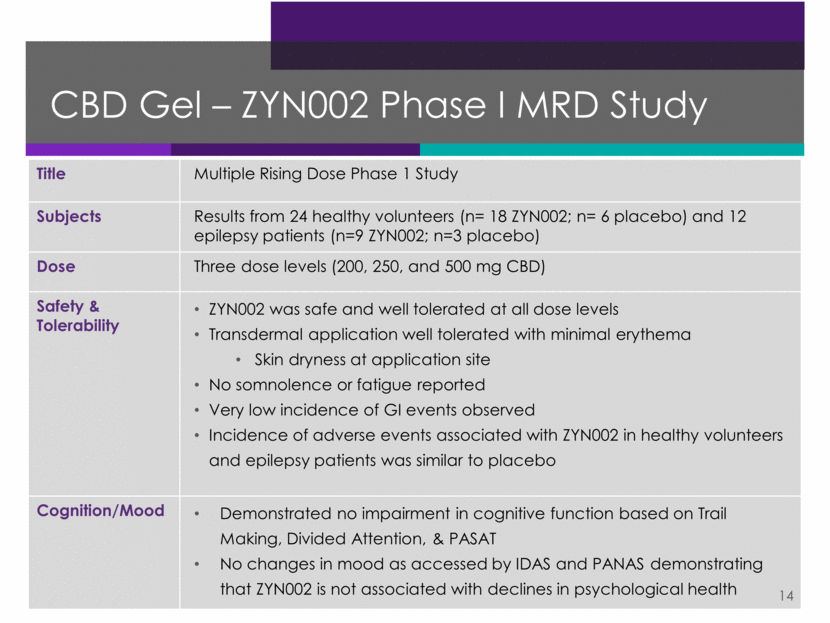
CBD Gel – ZYN002 Phase 1 Clinical Plan 15 Note: Subject to change due to further regulatory, clinical and other considerations. Study Patients Dosing PD Evaluations Timing Single Rising Dose Study in Normal Subjects and Patients with Epilepsy Pharmacokinetic profile and tolerability evaluation 32 healthy volunteers and 12 epilepsy patients (focal seizures) Single dose Cognition and visual attention (Trail Making) Completed Multiple Rising Dose Study in Normal Subjects and Patients with Epilepsy Multi-dose pharmacokinetic/ pharmacodynamics profile and tolerability evaluation 24 healthy volunteers and 12 epilepsy patients (focal seizures) Dosing for seven days Cognition and attention (Trail Making, PASAT and Divided Attention); mood (PANAS, IDAS) Completed Bioequivalence Study Evaluation of different applications sites – upper arm vs. thigh vs. back 24 healthy volunteers Single dose N/A 2016
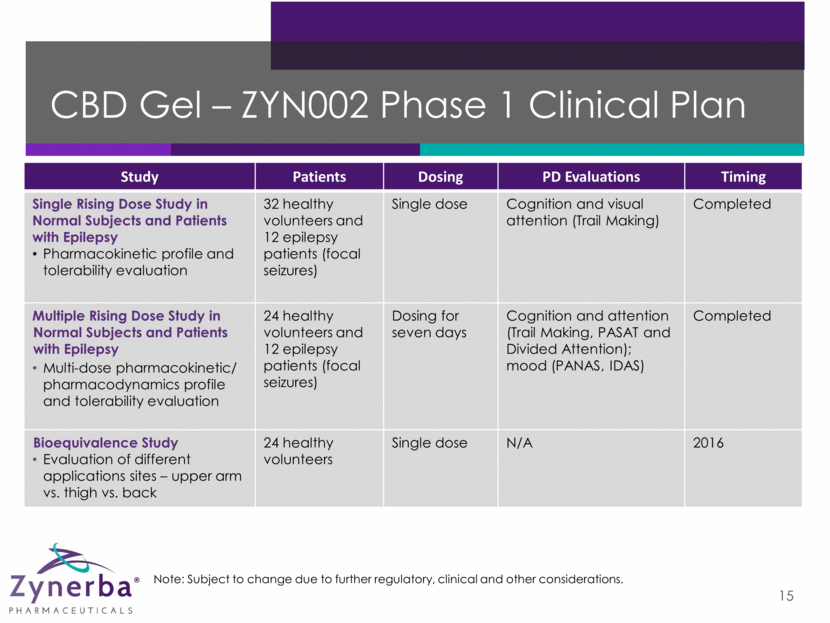
CBD Gel – ZYN002 Phase 2 Clinical Plan 16 Note: Subject to change due to further regulatory, clinical and other considerations. Study Patients Dosing Primary Endpoint Timing Efficacy and Tolerability in Patients with Refractory Epilepsy Adjunctive therapy, double-blind, placebo-controlled 180 patients to be randomized 12 weeks Median percentage change in seizure frequency over the treatment period Trial initiated June 2016 Efficacy and Tolerability in Patients with Osteoarthritis Double-blind, placebo-controlled 300 patients to be randomized 12 weeks Change from baseline in weekly mean 24-hour pain score Trial initiation expected in 2H2016 Efficacy and Tolerability in Patients with Fragile X Syndrome Open label 10- 20 patients 12 weeks Change in Clinical Global Impression-Improvement (CGI-I) Trial initiation expected in 2H2016
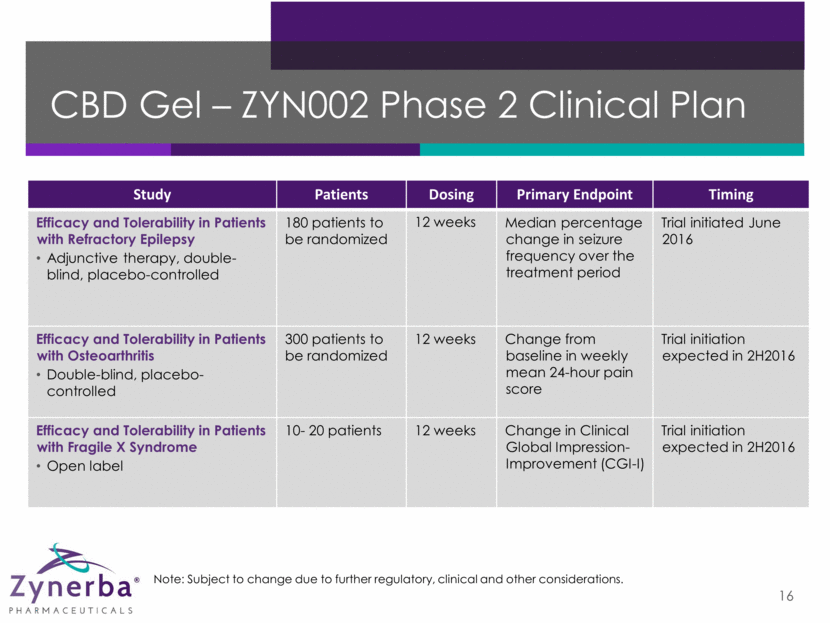
ZYN002 – STAR1 Phase 2a Study Initiated June 2016 17 Treatment A: ZYN002 – high dose for 12 weeks Treatment B: ZYN002 – low dose for 12 weeks Placebo for 12 weeks 8-week baseline period to assess seizure frequency and type STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy) is a study of ZYN002 (transdermal gel) in patients with focal seizures Randomized (1:1:1), double-blind, placebo-controlled, multi-center, multi-dose study of up to 180 patients Primary endpoint: Median percentage change in seizure frequency over the treatment period Patients assessed at baseline, randomization, and 4, 8, and 12 weeks post-randomization Secondary endpoints: safety and tolerability
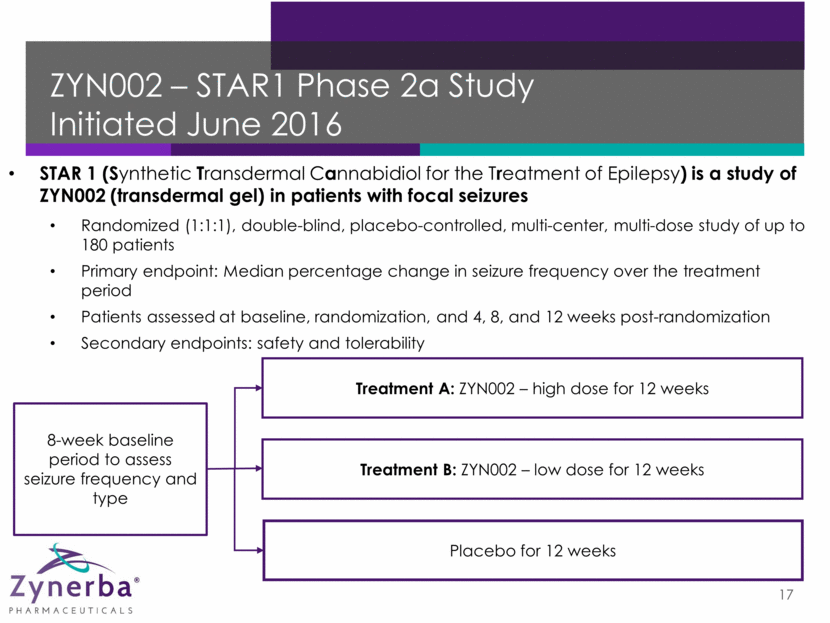
CBD Gel – ZYN002 Initial Market Opportunity 18 Refractory Epilepsy Osteoarthritis Fragile X Syndrome 2012 US Market Size* (# of Patients) 2.2 million 31 million 71,000 Orphan drug designation granted** 2012 EU and Japan* Market Size (# of Patients) 3.1 million 53 million 88,542 * Except for Fragile X syndrome data, based on data provided by Decision Resources. Data for epilepsy represents market for all types of epilepsy. Data for osteoarthritis represents market for osteoarthritis patients not currently served by over-the-counter medications. Fragile X syndrome data based on 2012 U.S. Census data, The World Bank data, and data provided by the National Fragile X Foundation. ** In February 2016, the FDA granted orphan-drug designation of ZYN002 for the treatment of patients with Fragile X syndrome in the US. An orphan drug is a drug or biologic intended to treat a rare disease or condition affecting fewer than 200,000 persons in the US.
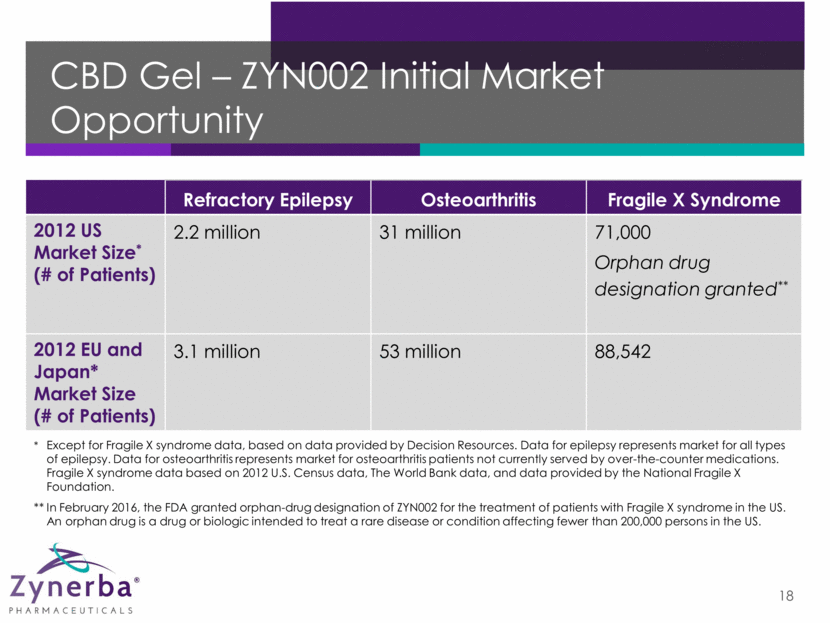
THC Pro-Drug Patch – ZYN001 19 Patent-protected synthetic D-glyceric acid ester-Δ9-tetrahydrocannabinol in a transdermal patch ZYN001 is a pro-drug A drug administered in an inactive or less active form, designed to enable more effective delivery, and then converted into a different form through a normal metabolic process Unlike THC, ZYN001 is able to be efficiently absorbed into the skin through transdermal delivery After crossing the stratum corneum, ZYN001 is hydrolyzed to THC and glyceric acid under physiological conditions Structure & Conversion Delivery ZYN001
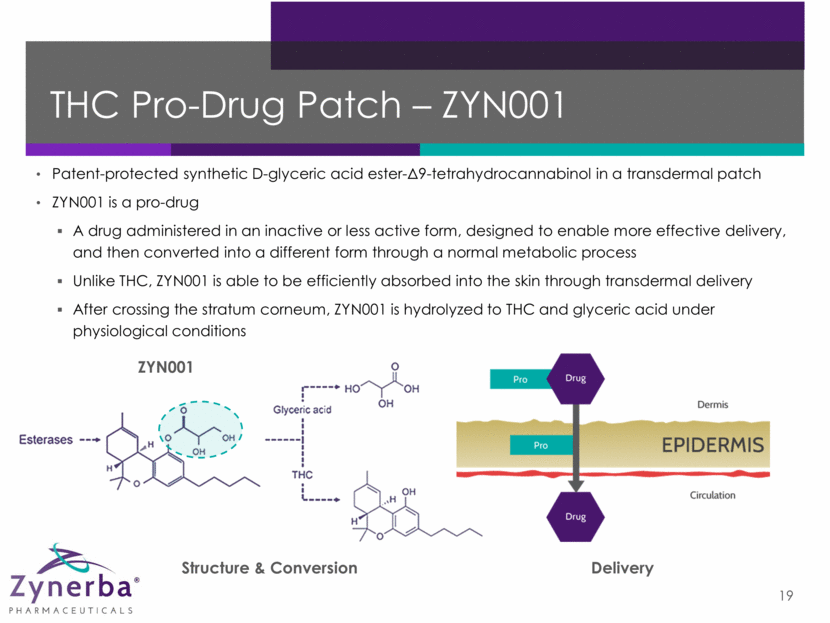
Note: Based upon FDA approved patch products. These results are not indicative of any preclinical or clinical data for ZYN001. Why a Patch? 20 Requirement Purpose Non-oral Avoids first-pass metabolism with increased bioavailability and more consistent plasma levels Controlled More consistent, controlled and sustained delivery No “peaks and valleys” as seen with oral Safe Improved safety profile with lower peak plasma levels Non-invasive Blood Level Time Max. Effective Level Min. Effective Level Oral Dosage Form Transdermal System Illustrative Controlled Delivery
~ 5.6 million patients suffer from Fibromyalgia in the US* CB1 agonists Have an analgesic effect in chronic pain models Act at many sites along pain transmission pathways Nabilone (THC analogue) in patients with fibromyalgia Third-party 4-week, randomized, double-blind, placebo-controlled trial (N=40) — significant decreases in pain scores on multiple instruments (e.g., VAS, FIQ, FIQ anxiety) Third-party randomized, double-blind, active-controlled cross-over study in patients with coexisting chronic insomnia (N=29) Nabilone and amitriptyline x 2 weeks with a 2-week washout Nabilone was superior to amitriptyline in sleep quality ZYN001 – Fibromyalgia 21 VAS, Visual analog scale FIQ, Fibromyalgia Impact Questionnaire * Data provided by Decision Resources
~ 14 million patients suffer from Peripheral Neuropathic Pain in the US* Third-party randomized, double-blind, placebo-controlled crossover trial in diabetic peripheral neuropathy (DPN) demonstrated that THC is effective in reducing neuropathic pain 16 patients with DPN received low (1% THC), medium (4% THC) or high (7% THC) dose of vaporized Cannabis and placebo in randomized crossover design Pain scores, cognitive effects and tolerability were assessed All THC doses were significantly better than placebo in reducing spontaneous pain The high dose THC (7%) impaired performance on 2 of 3 neuropsychological tests Analysis of THC plasma concentration showed that levels < 15 ng /ml correlated with pain relief (evoked pain –vf) but levels > 15ng/ml showed no correlation. Several third-party placebo controlled trials of vaporized cannabis, smoked cannabis and cannabis via an inhaler have demonstrated efficacy in peripheral neuropathic pain ZYN001 – Peripheral Neuropathic Pain 22 * Data provided by Decision Resources
THC Pro-Drug Patch – ZYN001 Status FDA pre-IND meeting completed August 2013 in vitro and in vivo studies Effective skin permeation with sustained delivery Non-mutagenic and non-genotoxic Safety pharmacology GLP studies Profile similar to THC GLP toxicology studies ongoing Expected Phase 1 to be initiated 2H2016 23 Note: Subject to change due to further regulatory, clinical and other considerations.
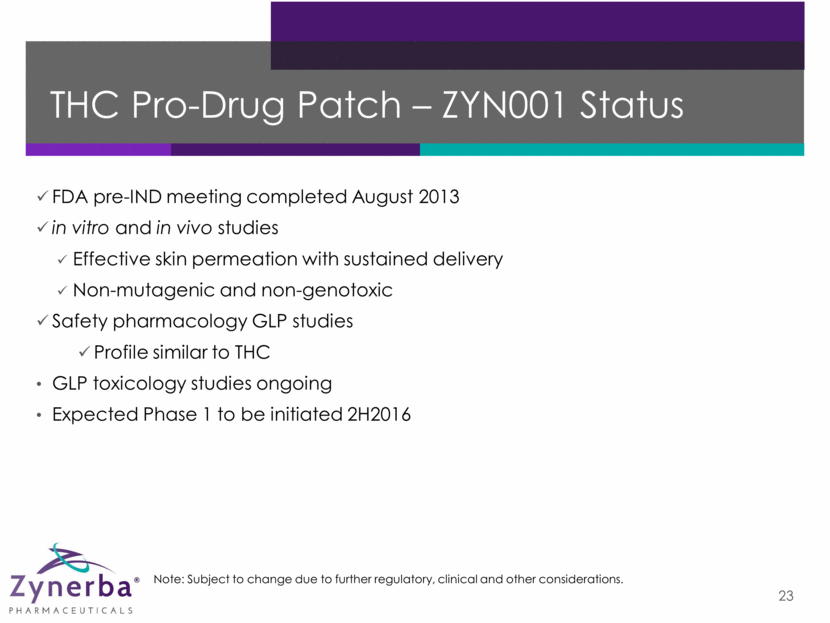
THC Pro-Drug Patch – ZYN001 Preliminary Phase 1 Clinical Plan 24 Study Patients Dosing PD Evaluations Timing Single Rising Dose Study in Normal Subjects Pharmacokinetic profile and tolerability evaluation 32 healthy volunteers and 12 patients with fibromyalgia Single dose Cognition and attention (Trail Making, PASAT and Divided Attention); mood (PANAS); subjective drug effects (ACRI) Trial initiation expected 2H2016 Multiple Rising Dose Study in Normal Subjects and Patients with Fibromyalgia Multi-dose pharmacokinetic/ pharmacodynamic profile and tolerability evaluation 16 healthy volunteers and 12 patients with fibromyalgia Dosing for seven days Capsaicin, UV-B and Cold Pressor pain models; cognition and attention (Trail Making, PASAT and Divided Attention); mood (PANAS); subjective drug effects (ACRI) Trial initiation expected 1H2017 Bioequivalence Study Evaluation of different application sites – upper arm vs. thigh vs. back 24 healthy volunteers Single dose Bioequivalence at arm, thigh and back Trial initiation expected in 2017 Note: Subject to change due to further regulatory, clinical and other considerations.
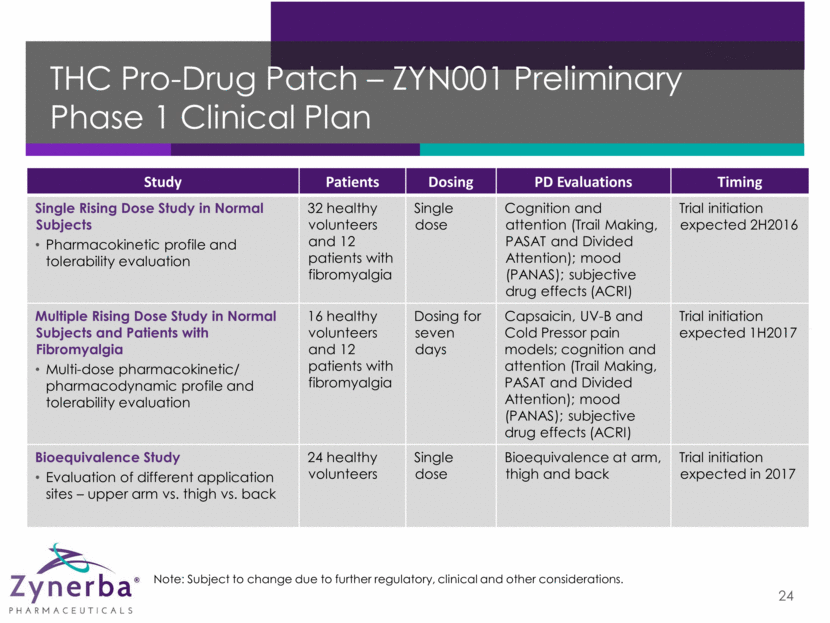
THC Pro-Drug Patch – ZYN001 Preliminary Phase 2 Clinical Plan 25 Note: Subject to change due to further regulatory, clinical and other considerations. Study Patients Dosing Primary Endpoint Timing Efficacy and Tolerability in Patients with Fibromyalgia Double-blind, placebo-controlled 120 patients 12 weeks Change from baseline in Fibromyalgia Impact Questionnaire (FIQ) total score Trial initiation expected 1H2017 Efficacy and Tolerability in Patients with Peripheral Neuropathic Pain Double-blind, placebo-controlled 120 patients 12 weeks Visual Analog Scale for pain intensity Trial initiation expected 1H2017
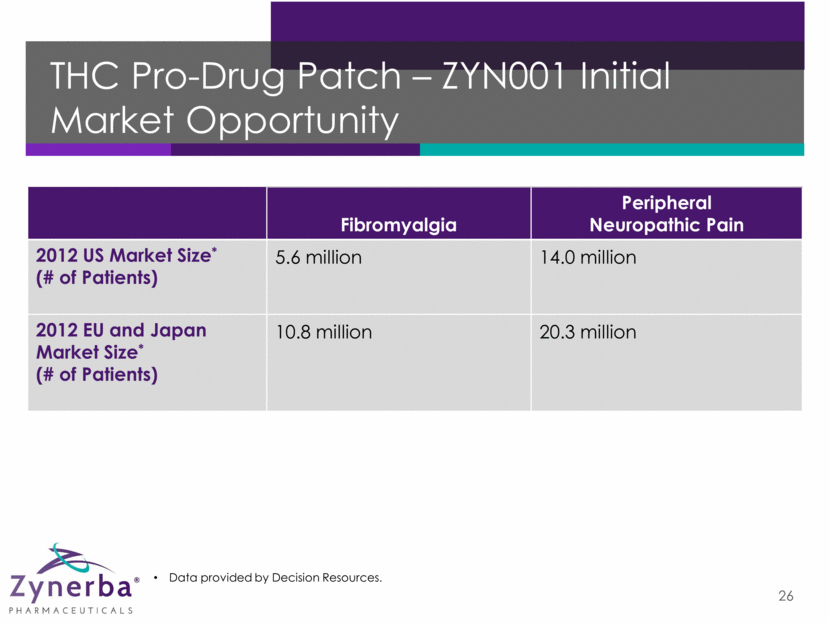
THC Pro-Drug Patch – ZYN001 Initial Market Opportunity 26 Fibromyalgia Peripheral Neuropathic Pain 2012 US Market Size* (# of Patients) 5.6 million 14.0 million 2012 EU and Japan Market Size* (# of Patients) 10.8 million 20.3 million Data provided by Decision Resources.
Financial Highlights 27 IPO August 2015 (NASDAQ: ZYNE) raised $42.1MM Net Cash and Cash Equivalents as of March 31, 2016, $36.8MM Expected to Fund Company Through 2017 and Five Phase 2 Studies (Phase 3 Ready). Total shares outstanding as of May 11, 2016 was 9,199,919 shares No Debt

Proven Leadership in Transdermal Drug Development 28 Armando Anido Chairman of the Board & Chief Executive Officer Terri Sebree President Richard A. Baron VP & Chief Financial Officer Donna Gutterman, PharmD VP, Medical Brian Boyd VP, Manufacturing Nancy Tich, PhD VP, Clinical

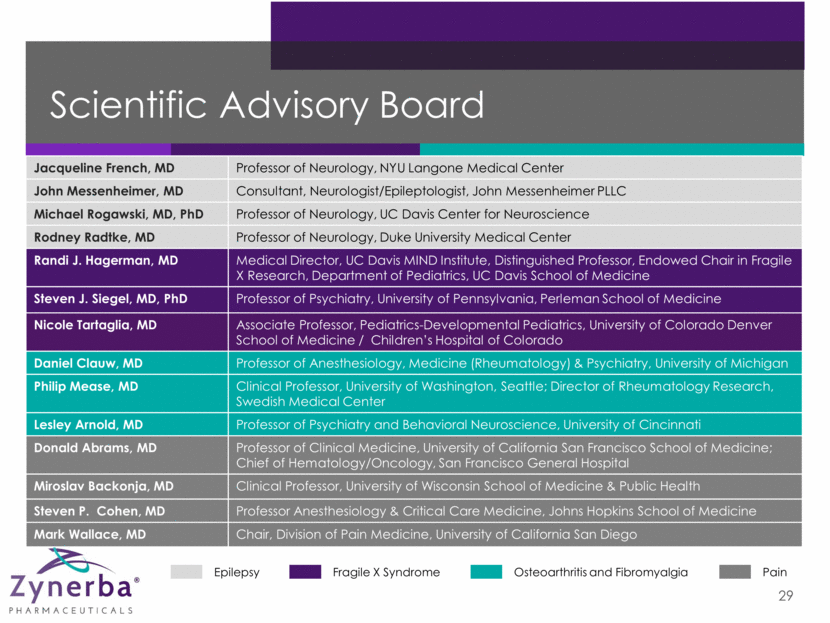
Jacqueline French, MD Professor of Neurology, NYU Langone Medical Center John Messenheimer, MD Consultant, Neurologist/Epileptologist, John Messenheimer PLLC Michael Rogawski, MD, PhD Professor of Neurology, UC Davis Center for Neuroscience Rodney Radtke, MD Professor of Neurology, Duke University Medical Center Randi J. Hagerman, MD Medical Director, UC Davis MIND Institute, Distinguished Professor, Endowed Chair in Fragile X Research, Department of Pediatrics, UC Davis School of Medicine Steven J. Siegel, MD, PhD Professor of Psychiatry, University of Pennsylvania, Perleman School of Medicine Nicole Tartaglia, MD Associate Professor, Pediatrics-Developmental Pediatrics, University of Colorado Denver School of Medicine / Children’s Hospital of Colorado Daniel Clauw, MD Professor of Anesthesiology, Medicine (Rheumatology) & Psychiatry, University of Michigan Philip Mease, MD Clinical Professor, University of Washington, Seattle; Director of Rheumatology Research, Swedish Medical Center Lesley Arnold, MD Professor of Psychiatry and Behavioral Neuroscience, University of Cincinnati Donald Abrams, MD Professor of Clinical Medicine, University of California San Francisco School of Medicine; Chief of Hematology/Oncology, San Francisco General Hospital Miroslav Backonja, MD Clinical Professor, University of Wisconsin School of Medicine & Public Health Steven P. Cohen, MD Professor Anesthesiology & Critical Care Medicine, Johns Hopkins School of Medicine Mark Wallace, MD Chair, Division of Pain Medicine, University of California San Diego Scientific Advisory Board 29 Epilepsy Fragile X Syndrome Osteoarthritis and Fibromyalgia Pain
Zynerba Highlights 30 A clinical-stage pharmaceutical company pioneering the development of patent-protected synthetic cannabinoid therapeutics for transdermal delivery Experienced team with deep expertise and proven track record of success in patch and gel transdermal delivery, regulatory approval and commercialization Two proprietary product candidates intended to treat diseases with significant unmet medical need and market potential CBD Gel – ZYN002: refractory epilepsy, osteoarthritis and Fragile X syndrome THC Pro-Drug Patch – ZYN001: fibromyalgia and peripheral neuropathic pain Global ownership of ZYN002 and ZYN001 IPO August 2015 (NASDAQ: ZYNE) raised $42.1MM Net Cash as of March 31, 2016: $36.8MM; runway through 2017 to fund five Phase 2 studies (Phase 3 Ready)

Investor Relations 31 NASDAQ: ZYNE Analyst Coverage* Jefferies: Biren Amin, PhD Piper Jaffray: Charles C. Duncan, PhD Canaccord Genuity: Arlinda Lee, PhD Oppenheimer: Rohit Vanjani Roth: Michael Higgins * Note: Any opinions, estimates or forecasts regarding Zynerba Pharmaceuticals, Inc.’s performance made by these analysts are theirs alone and do not represent opinions, forecasts or predictions of Zynerba Pharmaceuticals, Inc. or its management. Zynerba Pharmaceuticals, Inc. does not by its reference above imply its endorsement of or concurrence with such information, conclusions or recommendations. 484.581.7505 investorrelations@zynerba.com www.zynerba.com @ZynerbaPharma Zynerba Zynerba Investor Contacts Richard Baron, Vice President and CFO Argot Partners Investor Contacts Kimberly Minarovich 212.600.1902

July 2016 A clinical-stage pharmaceutical company dedicated to the development of innovative transdermal synthetic cannabinoid treatments for patients with high unmet medical needs

