Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - Tempest Therapeutics, Inc. | a16-12101_3ex99d3.htm |
| EX-99.1 - EX-99.1 - Tempest Therapeutics, Inc. | a16-12101_3ex99d1.htm |
| 8-K - 8-K - Tempest Therapeutics, Inc. | a16-12101_38k.htm |
Exhibit 99.2
Prospectus Supplement Summary
Overview
OvaScience is a global fertility company focused on the discovery, development and commercialization of new fertility treatment options for women. The current standard of treatment for infertility is in vitro fertilization, or IVF. IVF, however, fails approximately 70% of the time. The discovery of egg precursor, or EggPCSM cells, which are immature egg cells found in the protective outer layer of a woman’s own ovaries, countered a long-held medical belief that women are born with a set number of eggs, and this discovery enabled our new fertility treatment options. Our portfolio of fertility treatment options uses our patented technology, including proprietary methods to identify and isolate EggPC cells from a patient’s own ovarian tissue.
More women around the world are waiting until later in life to start families and are in need of new fertility treatment options. Fertility decreases with age, and approximately 72 million women worldwide have been diagnosed with infertility. The main cause of age related infertility is poor egg health, which is linked to a reduction in the number of functioning mitochondria. Other causes of poor egg health relating to mitochondria deficiency include Type 2 diabetes. Accordingly, approximately 40 million women throughout the world seek treatment options for infertility and up to 800,000 women per year undergo some form of IVF treatment. According to a 2014 report from Allied Market Research, the global IVF market in 2012 was valued at $9.3 billion, and projected to grow to $21.6 billion by 2020. Based on this, we estimate that our fertility treatments could address a growing global $2 to $3 billion market opportunity.
Our first commercial treatment, the AUGMENTSM treatment, is specifically designed to improve egg health by supplementing a mitochondrial deficiency which may, in turn, offer the potential for enhanced IVF success rates. With the AUGMENT treatment, energy-producing mitochondria from a woman’s own EggPC cells are added to the woman’s mature eggs during the IVF process to supplement the existing mitochondria. The AUGMENT treatment has been introduced in our partner IVF clinics outside of the United States, which we refer to as our partner clinics. We plan to expand the network of clinics and clinicians offering the AUGMENT treatment, focusing our efforts initially in Canada and Japan. The AUGMENT treatment is not currently available in the United States.
Last year, the AUGMENT treatment was offered in two clinics in Canada. In 2016, we entered into agreements with two additional partner clinics in Canada, for a total of four partner clinics, who will be offering the AUGMENT treatment. Further, enrollment of the non-commercial preceptorship training program at IVF Japan is complete. In May 2016, we entered into a letter of intent with IVF Japan, and are in the process of negotiating a final commercial agreement. The letter of intent enables IVF Japan to treat commercial patients on the same pricing terms set forth in the letter of intent while we are negotiating the final commercial agreement. IVF Japan has started treating a small number of commercial patients under such pricing terms. We plan to continue to build the infrastructure to support our potential commercialization in Japan while we negotiate the commercial agreement with IVF Japan. There can be no assurance that we will successfully complete negotiation of a commercial agreement in Japan or that IVF Japan will continue to offer the treatment to patients.
The following table sets forth certain of our estimates relating to the annual IVF volume at current and potential near term partner clinics in Canada and Japan.
|
Clinics |
|
Province/City |
|
# of Locations |
|
Annual IVF |
|
Canadian Clinics |
|
|
|
|
|
|
|
Current Clinic Networks(4) |
|
Ontario |
|
7 |
|
~5,000 |
|
Additional agreements expected in 2016 |
|
Quebec, British Columbia and Alberta |
|
TBD |
|
3,000 - 5,000 |
|
|
|
Canada Total |
|
7+ |
|
8,000 - 10,000 |
|
Japanese Clinics |
|
|
|
|
|
|
|
Current Clinic Network—Preceptorship(1) |
|
Osaka |
|
3 |
|
~10,000 |
|
|
|
Total |
|
10+ |
|
18,000 - 20,000 |
* All cycle data are estimates and are based on conversations with clinics; no public clinic data is available.
There can be no assurance that we will enter into the expected additional agreements in Canada in 2016, that any new clinical partners will have the cycle volume that we anticipate or that our partner clinics will continue to provide the AUGMENT treatment to patients.
We have introduced the AUGMENT treatment in clinics in different countries outside of the United States through non-commercial preceptorship training programs that transition to commercial operations as well as in studies conducted by partner clinics. In some cases, we and our partner clinics have had discussions with local regulatory bodies, such as the Ministry of Health and La Comisión Nacional de Reproducción Humana Asistida, or CNRHA in Spain, and the Ministry of Health and Japan Society of Obstetrics and Gynecology (JSOG), prior to introducing the AUGMENT treatment. In connection with the recent approval of the AUGMENT treatment by JSOG in Japan, we plan to build the infrastructure to support expansion there. Japan is one of the largest IVF markets in the world. We are also working with the IVI Group, the largest IVF clinic network in the world, to obtain prospective patient experience data in a pilot study in Valencia, Spain and we plan to work with other partner clinics on additional studies of the AUGMENT treatment. In the first quarter of 2016, the IVI Group continued enrollment of patients under the age of 43 and with a history of at least one previous cycle of IVF with embryo transfer and no pregnancy due to low embryo quality in its controlled, double-blind, prospective and randomized egg allocation study of the AUGMENT treatment. This adaptive study, which compares standard IVF to the AUGMENT treatment, allows for an interim analysis after completion and evaluation of data from 60 patients. At that point, based on the interim results, we may continue the study, adapt the study or cease enrolling new patients, which could occur as a result of positive or negative interim results. Absent any modification or cessation of the study, following completion of the interim analysis we expect that up to an additional 130 patients would be enrolled for a total of up to 190 patients in the study. We expect that final data from this study will be announced in the second half of 2017.
The OvaPrimeSM treatment is a potential fertility treatment that could enable a woman to increase her egg reserve. Approximately 25% of women who start an IVF cycle fail to produce a sufficient number of eggs, or the eggs are too immature. The OvaPrime treatment is designed to replenish a woman’s egg reserve by transferring a patient’s EggPC cells from the protective ovarian lining back into the patient’s own ovaries where they may mature into fertilizable eggs during the IVF process. We reported large animal toxicology and small animal proof-of-concept studies relating to the OvaPrime treatment in 2014. In December 2015, we commenced a clinical study with the OvaPrime treatment in an international region outside the United States to gain insight into the clinical efficacy and feasibility
of the treatment. The study is designed to look at hormone level changes, including FSH and AMH, and follicular development, as evaluated by ultrasound. We plan to provide an update on the path forward for OvaPrime by the end of 2016.
The OvaTureSM treatment is a potential next-generation fertility treatment that could help a woman produce healthy, young, fertilizable eggs without the need for hormone injections. The OvaTure treatment seeks to mature a woman’s own EggPC cells into eggs outside her body. This potential treatment may be an option for women with compromised eggs, who are unable to make eggs, or who may be unwilling or unable to undergo hormone hyperstimulation. We established human preclinical proof-of-concept and continue to optimize the maturation process by demonstrating that human EggPC cells can be matured into eggs outside of the body. The aim is to characterize EggPC cells and mature them into oocytes to determine their ability to mature into fertilizable eggs. We are continuing development of the OvaTure procedure with a goal of further understanding and defining the clinical path forward in 2016. We are seeking permission from regulatory authorities outside the United States for certain steps necessary to complete testing of EggPC-derived eggs.
The AUGMENT treatment
We have introduced the AUGMENT treatment in select international IVF clinics and we anticipate that we will introduce the AUGMENT treatment into new international regions. The AUGMENT treatment is not available in the United States.
An AUGMENT treatment cycle begins upon our receipt of the patient’s ovarian tissue after biopsy, which is obtained through a biopsy performed by the patient’s doctor prior to hormone stimulation. Our proprietary process identifies and isolates the patient’s own EggPC cells, and then the patient’s own mitochondria from these EggPC cells are isolated. The patient’s own mitochondria are then injected into her egg at the time of intracytoplasmic sperm injection, or ICSI. We expect to receive payment before processing tissue and defer revenue until we deliver the mitochondria to the clinic, which is timed with the patient’s standard IVF cycle and can stretch from 30 to 120 days or more. Within certain of our programs, revenue recognition may be further deferred. In the future, however, as we change our operational model and introduce further efficiencies, we anticipate that we will be able to recognize revenue on a shorter time frame.
We have established our international headquarters in the United Kingdom to coordinate our international commercial efforts. We plan to focus our initial efforts on the regions of Canada and Japan. Within these regions we plan to target customers that combine elements of the following key criteria:
· High quality patient clinical care
· High volume IVF clinics
· High quality IVF labs
· Out-of-pocket pay and high average cost per cycle
· Potential for reimbursement by healthcare providers
· Donor egg restrictions
We continue to explore the optimal business model for the AUGMENT treatment based on our initial commercial experience. We plan to:
· pursue broader use, including first line treatment for various egg health and male factor indications;
· review the optimal manufacturing model(s) in certain regions, while continuing to utilize onsite manufacturing, to handle demand resulting from expanded indications, ongoing publication of patient experience and broad geographic expansion; and
· continue to optimize commercial operations and logistics.
Clinical experience with the AUGMENT treatment
To date, 30 babies have been born using the AUGMENT treatment, and in total more than 270 women have gone through or are currently in treatment.
We first introduced the AUGMENT treatment in select partner IVF clinics outside of the United States in 2014 and thus our patient experience with this treatment is limited. Some of the physicians with whom we work at our partner IVF clinics have presented data about their own experiences with the AUGMENT treatment in their clinics. We believe this data is encouraging and gives us reason to believe that the AUGMENT treatment offers a potential new treatment option for women undergoing an IVF procedure, in particular women who have failed to become pregnant using traditional IVF techniques or who otherwise have poor prognoses due to poor egg health or embryo health due to age or other causes. In some cases, data has been presented at scientific conferences and, in some cases, has been published in a peer-review journal.
However, it should be noted that none of these observations were made as part of a controlled clinical trial, nor were patients treated under a regulator-approved clinical trial protocol. Controlled clinical trials are often designed and conducted to evaluate the safety and efficacy of a new therapy, and are typically designed to take into account variables such as patient age, physiological condition and other factors and to compare results observed using the study therapy versus results observed using either active or placebo controls. In contrast, the AUGMENT observations presented to date represent patient experiences at a small number of clinics and from a small number of patients with different diagnoses, ages and prior IVF histories. Accordingly, caution should be applied in interpreting and relying on these prior results. For example, in some of these clinical experiences it was observed that the AUGMENT treatment appeared to result in pregnancy rates for certain cohorts of patients that were higher than pregnancy rates achieved by these same patients in prior IVF cycles using traditional IVF techniques. In certain cohorts of patients that underwent an egg allocation study, eggs subjected to an AUGMENT procedure were more likely to result in a pregnancy versus eggs subjected to a traditional IVF technique. However, because these observations were not made as part of a controlled clinical trial with pre-defined success criteria or with a sufficient sample size, caution should be applied in interpreting these results, and these results may not be indicative of future results we experience in our partner IVF clinics, or in future clinical studies.
Canada and UAE clinical experiences
In August 2015, the peer-reviewed, Journal of Fertilization: In Vitro—IVF-Worldwide Reproductive Medicine, Genetic & Stem Cell Biology, reported clinical results based on observations of an aggregate of 93 patients managed in AUGMENT treatment centers in Canada (TCART; 34 patients) and the United Arab Emirates, or UAE (FAKIH IVF; 59 patients). The patient experiences were reported by Michael Fakih, M.D., Co-Founder and Medical Director of Fakih IVF, and Robert F. Casper, M.D., F.R.C.S.(C), Medical Director of TCART Fertility Partners (now TRIO Fertility) in Canada. Drs. Fakih and Casper have used the AUGMENT treatment in clinical practice since 2014, each initially focused on poor prognostic women with histories of failed IVF cycles.
The article reported that the AUGMENT treatment was used in a population of difficult-to-treat patients with a poor prognosis for success with standard IVF where the next potential step was seeking
a donor egg. The following tables set forth patient characteristics and results across the select AUGMENT treatment centers:
|
|
|
PRIOR TO AUGMENT TREATMENT | ||
|
|
|
TCART |
|
FAKIH IVF |
|
PATIENT HISTORY |
|
|
|
|
|
Number of patients |
|
34 |
|
59 |
|
Average current age |
|
36.0 (Range: 26 - 44) |
|
37.3 (Range: 20 - 48) |
|
Background/Diagnoses |
|
Poor oocyte and embryo quality |
|
Poor oocyte and embryo quality: |
|
PRIOR IVF HISTORY |
|
|
|
|
|
Total previous IVF cycles initiated |
|
71 |
|
257 |
|
Average cycles per patient |
|
2 (Range: 1 - 5) |
|
4.3 (Range: 1 - 16) |
|
Total previous embryo transfers |
|
79 |
|
Unknown due to international patient base |
|
Historical clinical pregnancy rate: |
|
|
|
|
|
Per cycle initiated |
|
8/71 (11)% |
|
9/257 (4)% |
|
Per embryo transfer |
|
8/79 (10)% |
|
|
|
Historical live birth rate: |
|
|
|
|
|
Per cycle initiated |
|
1/71 (1.4)% |
|
4/257 (2)% |
|
Per embryo transfer |
|
1/79 (1.3)% |
|
|
|
|
|
WITH THE AUGMENT | ||
|
|
|
TCART |
|
FAKIH IVF |
|
Total AUGMENT cycles initiated |
|
34 |
|
60 |
|
Average cycles per patient |
|
1 |
|
1 |
|
Total embryo transfers |
|
26 |
|
34 |
|
Clinical pregnancy rate: |
|
|
|
|
|
Per cycle initiated |
|
12/34 (35)% |
|
13/60 (22)% |
|
Per embryo transfer |
|
12/26 (46)% |
|
13/34 (38)% |
|
Ongoing clinical pregnancy and live birth rate: |
|
|
|
|
|
Per cycle initiated |
|
9/34 (26)%* |
|
11/60 (18)%** |
|
Per embryo transfer |
|
9/26 (35)%* |
|
11/34 (32)%** |
* includes 1 live birth
** includes 2 live births (two sets of twins)
Note: All on-going clinical pregnancies reported were continuously on-going pregnancies at the time the publication was submitted.
Additionally, the article presented results of a Fakih IVF retrospective analysis of the eggs in a sub-set of 25 women with a poor prognosis for IVF success and no prior live births following the failure of an average of four IVF cycles. Eggs from the same woman were prospectively allocated to two treatment groups: one group of eggs received the AUGMENT treatment during the ICSI step of IVF and the other group of eggs received only ICSI during IVF (ICSI-Only Treatment), as identified in the tables below. With certain patients who produce an unequal number of eggs, their eggs were not allocated evenly. In addition, embryologists were not blinded to the treatment groups and embryo transfer was limited to the treatment group with the highest quality embryo(s) as determined by euploidy and morphokinetics. A retrospective analysis of the 25 intent-to-treat (ITT) patients was performed to compare the results of the AUGMENT treatment to IVF. Statistically significant higher rates of embryo selection (based on morphogenetic analysis) and embryo transfers were observed among the AUGMENT-treated group of eggs compared to the ICSI-only treated group of eggs. Further, the AUGMENT-treated eggs had statistically significant higher pregnancy rates, including ongoing clinical pregnancy rates.
|
Embryo assessment* and embryo transfer by treatment groups based on morphogenetic criteria | ||||
|
Embryo Transfer |
|
ICSI-Only Treatment |
|
AUGMENT Treatment |
|
+ |
|
2 |
|
14 |
|
- |
|
23** |
|
11** |
|
Total |
|
2 of 25 |
|
14 of 25 |
* Based on Society for Assisted Reproductive Technology (SART) Criteria
** In nine patients embryos from both treatment groups failed to meet the morphogenetic criteria for embryo transfer.
Increased Pregnancy Rates with the AUGMENT treatment
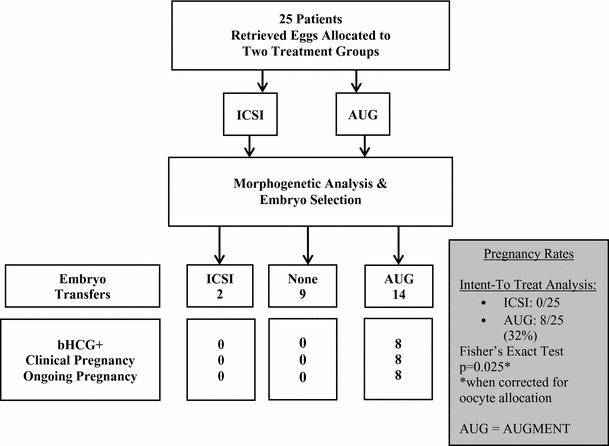
* The conventional method for measuring the statistical significance of a result is known as the “p-value,” which represents the probability that random chance caused the result (e.g., a p-value = 0.001 means that there is a 0.1% or less probability that the difference between the control group and the treatment group is purely due to random chance). A p-value <= 0.05 is a commonly used criterion for statistical significance.
In March 2016, at the 2016 Society for Reproductive Investigation (SRI) conference, Robert F. Casper, M.D., F.R.C.S.(C) provided an update on AUGMENT patient experiences for 48 patients (inclusive of the 34 patients identified above) in AUGMENT treatment centers in Canada, and presented the results in two age categories based on whether the patient was under forty years old. The
results of this study showed that patients under the age of 40 had better clinical pregnancy rates and live birth rates than those patients over the age of 40, as set forth below.
|
Metric |
|
Under 40 Years Old |
|
40 Years Old and Over |
|
Number of patients |
|
26 |
|
22 |
|
Average current age |
|
35 |
|
42.4 |
|
Previous IVF cycles |
|
51 |
|
72 |
|
Total AUGMENT cycles initiated |
|
26 |
|
22 |
|
Total embryo transfers (fresh and frozen) |
|
18 fresh + 18 frozen* |
|
15 fresh + 13 frozen |
|
Ongoing pregnancy per fresh embryo transfer |
|
3/18 (17%) |
|
0/15 (0%) (3 miscarriages) |
|
Ongoing pregnancy per frozen embryo transfer |
|
11/18 (61%) |
|
1/13 (7.6%) (2 miscarriages) |
|
Ongoing clinical pregnancy and live birth rate: |
|
|
|
|
|
Per cycle initiated |
|
14/26 (54%) (includes 10 live births) |
|
1/22 (4.5%) |
|
Per embryo transfer |
|
14/36 (39%) |
|
1/28 (3.6%) |
* In some cases, patients received more than one embryo transfer in their AUGMENT cycle.
Turkey clinical experiences
At a scientific symposium held during the European Society for Human Reproduction and Embryology (ESHRE) annual meeting, Kutluk Oktay, M.D., F.A.C.O.G., of Gen-Art IVF in Ankara, Turkey, reported clinical experiences based on observations of 11 patients, the results of which are included in the tables below.
|
|
|
PRIOR IVF PREGNANCY RATES FOR AUGMENT |
|
PATIENT HISTORY |
|
|
|
Number of patients |
|
11 |
|
Average current age |
|
35.2 (Range: 27 - 46) |
|
Background/Diagnoses |
|
Diminished reserve and/or gamete/embryo quality |
|
Total previous IVF cycles initiated |
|
41 |
|
Average cycles per patient |
|
3.7 (Range: 2 - 7) |
|
Total previous embryo transfers |
|
40 |
|
Historical clinical pregnancy rate: |
|
|
|
Per cycle initiated |
|
0/41 (0%) |
|
Per embryo transfer |
|
0/40 (0%) |
|
Historical live birth rate: |
|
|
|
Per cycle initiated |
|
0/41 (0%) |
|
Per embryo transfer |
|
0/40 (0%) |
|
|
|
PATIENT EXPERIENCE WITH |
|
Total AUGMENT cycles initiated |
|
11 |
|
Average cycles per patient |
|
1 |
|
Total embryo transfers |
|
9 |
|
Clinical pregnancy rate: |
|
|
|
Per cycle initiated |
|
3/11 (27%) |
|
Per embryo transfer |
|
3/9 (33%) |
|
Live birth rate: |
|
|
|
Per cycle initiated |
|
1/11 (9%) |
|
Per embryo transfer |
|
1/9 (11%) |
In December 2015, Reproductive Sciences published Oktay, K. et al.’s updated clinical experience with the AUGMENT treatment. In total 17 patients were evaluated for eligibility, which required at least 2 IVF failures and poor oocyte/embryo quality. Two patients were excluded due to unavailability of prior records. Of the remaining 15 patients, 12 received the AUGMENT treatment and ten underwent embryo transfer, as shown in the figure below. Of the ten embryo transfers, four resulted in pregnancies; two pregnancies were lost; one resulted in a livebirth and there was one ongoing pregnancy reported at the time of publication.
Figure 1. Study Design.
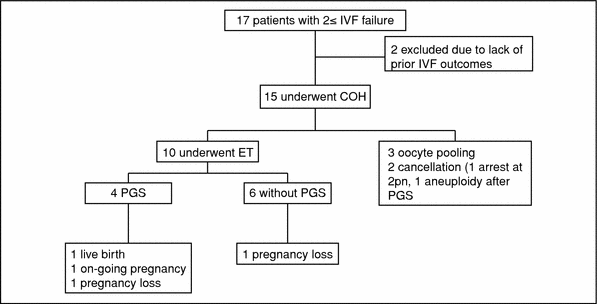
COH= Controlled Ovarian Hyperstimulation
Table 1. Characteristics and Cycle Outcomes of Patients Undergoing AMI Treatment.
|
Patients |
|
Age, |
|
D2-3 |
|
D2-3 |
|
AMH, |
|
N of |
|
Fresh/ |
|
N of |
|
N of |
|
Fertil. |
|
N of |
|
PGS |
|
N of |
|
Pregnancy |
|
|
#1 |
|
35 |
|
7.4 |
|
47 |
|
1.8 |
|
5 |
|
Fresh |
|
6 |
|
5 |
|
100 |
% |
5 |
|
NP |
|
2 |
|
— |
|
|
#2 |
|
34 |
|
15.2 |
|
100 |
|
1.4 |
|
7 |
|
Frozen-thawed |
|
6 |
|
4 |
|
75 |
% |
3 |
|
1 normal of 3 |
|
1 |
|
Live birth |
|
|
#3 |
|
27 |
|
6.1 |
|
57 |
|
2.5 |
|
6 |
|
Fresh |
|
8 |
|
7 |
|
59 |
% |
4 |
|
NP |
|
2 |
|
— |
|
|
#4 |
|
31 |
|
7.2 |
|
39 |
|
3.5 |
|
3 |
|
Frozen-thawed |
|
10 |
|
10 |
|
70 |
% |
7 |
|
2 normal of 7 |
|
2 |
|
— |
|
|
#5 |
|
36 |
|
5.5 |
|
134 |
|
1.1 |
|
3 |
|
Frozen-thawed |
|
8 |
|
7 |
|
43 |
% |
3 |
|
NP |
|
2 |
|
— |
|
|
#6 |
|
35 |
|
6.9 |
|
NA |
|
NA |
|
4 |
|
Fresh |
|
8 |
|
6 |
|
83 |
% |
5 |
|
NP |
|
2 |
|
— |
|
|
#7 |
|
41 |
|
5.6 |
|
39 |
|
0.7 |
|
3 |
|
Frozen-thawed |
|
6 |
|
3 |
|
100 |
% |
3 |
|
NP |
|
1 |
|
Pregnancy loss |
|
|
#8 |
|
40 |
|
9 |
|
NA |
|
NA |
|
7 |
|
Fresh |
|
14 |
|
11 |
|
73 |
% |
7 |
|
NP |
|
2 |
|
— |
|
|
#9 |
|
32 |
|
6.3 |
|
NA |
|
NA |
|
2 |
|
Frozen-thawed |
|
16 |
|
16 |
|
88 |
% |
14 |
|
4 normal of 8 |
|
1 |
|
Pregnancy loss |
|
|
#10 |
|
36 |
|
7.2 |
|
NA |
|
NA |
|
2 |
|
Frozen-thawed |
|
10 |
|
7 |
|
100 |
% |
7 |
|
1 normal of 7 |
|
1 |
|
Ongoing pregnancy |
|
Abbreviations: AMH, anti-Mullerian hormone; E2, estradiol; FSH, follicle-stimulating hormone; IVF, in vitro fertilization; PGS, preimplantation genetic screening; NP, not performed; NA, not available.
Figure 1 and Table 1 above are from Oktay K, Baltaci V, Sonmezer M et al. Oogonial Precursor Cell-Derived Autologous Mitochondria Injection to Improve Outcomes in Women With Multiple IVF Failures Due to Low Oocyte Quality: A Clinical Translation. Reproductive Sciences 2015. 22 (12) 1612-1617.
See “Risk factors—Risks related to the company—The science underlying the AUGMENT treatment is based on recent scientific research. We have limited clinical experience with the AUGMENT treatment and our approach has not yet been evaluated in a controlled clinical trial or long-term studies. The clinical outcomes experienced in IVF clinics to date may not be representative of future results.”
Our strategy
We are focused on three primary objectives, or pillars, that we hope will serve as the foundation to grow our business. First, we aim to expand our operational platform and execute a targeted expansion of the AUGMENT treatment, with a narrow and deep focus on Canada and Japan as our primary commercial markets. Second, we aim to increase our patient support services by building a patient-centered operating model though local, on-the-ground teams to support patients and clinics. Third, we plan to continue to generate clinical data to further validate our portfolio of novel fertility treatments and demonstrate their clinical benefit.
Regarding our first objective, we are currently working to expand access to the AUGMENT treatment in key IVF markets by taking a narrow and deep approach to the commercial launch. We have already made significant progress in building our ongoing commercial operations and infrastructure, particularly in Canada, where, in addition to existing partnerships, we recently entered into agreements with two new partner clinics, who we expect to begin offering the AUGMENT treatment commercially following the completion of a preceptorship training program. From our experience in that market, we expect to draw meaningful conclusions to inform our strategy in Japan and other future regions.
In addition, we plan to enhance our patient services, in part by shifting to a patient-centered marketing approach in our targeted regions. With this type of commercial model, we plan to be more proactive in raising awareness and educating patients directly about the AUGMENT treatment. In the case of fertility treatment, we believe patients are sophisticated consumers who are informed and will pursue breakthrough treatments.
With respect to the U.S., we are determining our FDA strategy with respect to the AUGMENT treatment.
We believe our EggPC technology has the potential to make significant advances in the field of fertility because it is designed to address poor egg health and embryo quality due to age and other causes. We believe our EggPC technology could improve IVF by:
· Increasing live birth rates and reducing the number of IVF cycles. By improving egg health, we believe we may increase the percentage of live births and reduce the number of IVF cycles required.
· Reducing the incidence of multiple births. By generating higher quality eggs, we believe our EggPC technology may allow for the transfer of fewer embryos per IVF cycle and, as a result, lower the incidence of multiple births and the associated complications.
· Lowering the overall cost of the IVF process. If we reduce the number of IVF cycles required for a live birth and the incidence of multiple births, we believe our fertility treatment options may also lower the overall costs associated with the IVF process.
· Replenishing the ovary for women who make too few or no eggs. Our OvaPrime treatment is designed to replenish a woman’s egg reserve by transferring a patient’s EggPC cells from the protective ovarian lining back into the patient’s own ovaries where they may mature into fertilizable eggs.
· Reducing the need for hormonal hyperstimulation. We are designing our OvaTure treatment to mature EggPC cells into fertilizable eggs in vitro, or outside the body. If successful, the OvaTure treatment could reduce, or possibly eliminate, the need for hormonal hyperstimulation for the maturation of multiple oocytes prior to egg retrieval in the IVF process.
· Developing new treatments for diseases. OvaXonSM is a joint venture with Intrexon Corporation, or Intrexon, which is focused on developing significant improvements in human and animal health using our EggPC cell technology and Intrexon’s synthetic biology and high throughput platform for applications.
Risks associated with our business
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk factors” section of this prospectus supplement immediately following this prospectus supplement summary. These risks include, but are not limited to, the following:
· We have incurred significant losses since our inception and expect to incur losses for the foreseeable future and may never achieve or maintain profitability. As of March 31, 2016, we had an accumulated deficit of $189.7 million.
· We will need substantial additional funding. If we are unable to raise capital when needed, we would be forced to delay, reduce or eliminate our fertility treatment development programs or commercialization efforts.
· We have limited patient experience with the AUGMENT treatment. We have not evaluated the AUGMENT treatment in any controlled clinical trials, nor have we evaluated the long-term safety and efficacy of this procedure in clinical trial or pre-clinical studies. No assurance can be given as to the efficacy or the safety of this approach, nor as to the long-term safety of this approach for the patient or her offspring. Caution should be applied in interpreting the clinical observations presented in this prospectus, which represent patient experiences at a small number of clinics and from a small number of patients with different diagnoses, ages and prior IVF histories. These results may not be indicative of future results we experience in our partner IVF clinics, or in future clinical studies.
· The science underlying the AUGMENT treatment, the OvaPrime treatment and the OvaTure treatment is based on recent discoveries. We have only recently started treatment of human patients with the OvaPrime treatment, and the OvaTure treatment has not been used in humans. The preclinical data we have generated for the OvaPrime treatment and the OvaTure treatment is under development and may not be replicated in humans. Additionally, we may determine not to proceed with the OvaPrime treatment based on data that we receive from the non-commercial preceptorship training program that we commenced in December 2015. Accordingly, we may not be able to successfully develop or commercialize our fertility treatments.
· The pilot study being conducted by the IVI Group in Spain is based on an adaptive design and allows for an interim analysis after completion and evaluation of data from 60 patients. At that point, based on the interim results, we may continue the study, adapt the study or cease enrolling new patients, which could occur as a result of positive or negative interim results.
· International IVF clinics that we work with may determine not to provide or continue providing or studying the AUGMENT treatment or the OvaPrime treatment, or to delay providing such treatments, based on clinical efficacy, safety or commercial, logistic, regulatory, enrollment, inability to agree on commercial terms or other reasons.
· Further preclinical testing and development, including any necessary clinical trials of the OvaPrime treatment and the OvaTure treatment and any of our other current or potential fertility treatments may not be successful. If we are unable to develop and commercialize our potential fertility treatments, or if we experience significant delays in doing so, our business will be materially harmed.
· If serious adverse or inappropriate side effects or other safety issues are identified during the development or commercialization of the AUGMENT treatment, the OvaPrime treatment or the OvaTure treatment, or with any procedures with which these fertility treatments are used, we may need to abandon or limit our development or commercialization of those current or potential fertility treatments, which could have a material adverse effect on our business, results of operations or financial condition.
· Our plans to continue and expand the commercialization of the AUGMENT treatment outside of the United States and introduce the OvaPrime treatment in selected regions outside of the United States depend upon the AUGMENT treatment and the OvaPrime treatment meeting the requirements of a class of products or a type of practice or treatment exempt from pre-market review and approval of applications for marketing authorizations in such regions. There can be no assurance that this will be the case in any particular jurisdiction. Determinations by regulators in the markets we target that the AUGMENT treatment and the OvaPrime treatment do not meet the requirements for a class of products exempt from pre-market review and approval could significantly delay or prevent commercialization of those products. Failure to obtain marketing approval in international regions, to the extent required, would prevent our potential fertility treatments from being marketed in such regions. Additionally, our plans to define a clinical pathway for the OvaTure treatment will depend upon the regulatory pathway applicable in regions of the world that we target.
· We have limited patient experience with the AUGMENT treatment and our experience with the AUGMENT treatment to date may not be representative of what women will experience in the future. Our ability to gain market acceptance of the AUGMENT treatment will depend on the experience women have with this treatment option over time.
· We have limited experience in marketing and selling our fertility treatments, and accordingly it is difficult for us to predict how quickly we will be able to grow our commercial sales, if at all. If
we are unable to successfully commercialize our fertility treatments, our business and operating results will be adversely affected. If the continued and expanded commercialization of our AUGMENT treatment to new regions or the commercial launch of the OvaPrime treatment or other potential fertility treatments is delayed or does not occur for any reason, it could have a material adverse effect our business, results of operations or financial condition.
· To manage our anticipated commercial expansion, we must continue to implement and improve our managerial, operational and financial systems, and continue to recruit and train additional qualified personnel. Also, our management may need to divert a disproportionate amount of its attention away from its day-to-day activities and devote a substantial amount of time to managing development and commercialization activities. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. This may result in weaknesses in our infrastructure, give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. The physical expansion of our operations may lead to significant costs and may divert financial resources from other projects, such as the development of our fertility treatments. If our management is unable to effectively manage our expected development and expansion, our expenses may increase more than expected, our ability to generate or increase our revenue could be reduced and we may not be able to implement our business strategy.
· We currently rely and will in the future rely on selected international IVF clinics to continue to commercialize, gain experience and generate data on the AUGMENT treatment and the OvaPrime treatment. We will also rely on third parties to conduct any necessary clinical trials for other potential fertility treatments. Such third parties may not perform satisfactorily, including failing to meet volume expectations, quality standards or deadlines for the completion of such studies or trials.
· If we are unable to obtain and maintain patent protection for our technology and current and potential fertility treatments, or if our licensors are unable to obtain and maintain patent protection for the technology or potential fertility treatments that we license from them, our competitors could develop and commercialize technology and potential fertility treatments similar or identical to ours, and our ability to successfully commercialize our technology and potential fertility treatments may be adversely affected.
· It is unclear what approval pathway FDA will ultimately require us to follow with the AUGMENT treatment, the OvaPrime treatment or our potential fertility treatments prior to commercialization in the United States. FDA may conclude that the AUGMENT treatment and/or our potential fertility treatments constitute drugs, biologics or medical devices. If FDA makes such a determination for any of our potential fertility treatments, we would be required to conduct clinical trials under an IND (or IDE for a medical device) and seek FDA pre-market review and approval pursuant to an NDA or BLA (or PMA for a medical device). In that event, we may abandon pursuing that potential fertility treatment in the United States, or suffer significant delays and expense seeking to approve any necessary approval. In September 2013, we received an “untitled” letter from the FDA advising us to file an Investigational New Drug, or IND, application for the AUGMENT treatment. As a result, we chose to suspend enrollment in our AUGMENT study in the United States.
· We are currently subject to a securities class action lawsuit, the unfavorable outcome of which may have a material adverse effect on our financial condition, results of operations and cash flows.
Our corporate information
We were incorporated under the laws of the State of Delaware in April 2011 under the name Ovastem, Inc. and changed our name to OvaScience, Inc. in May 2011. Our principal executive offices are located at 9 4th Avenue, Waltham, Massachusetts 02451 and our telephone number is (617) 500-2802. Our website address is www.ovascience.com. The information on our website is not incorporated by reference into this prospectus supplement or the accompanying prospectus and should not be considered to be part of this prospectus supplement or the accompanying prospectus.
As used in this prospectus supplement, unless the context otherwise requires, references to “OvaScience,” “we,” “us,” “our” and similar references refer to OvaScience, Inc. and, where appropriate, our consolidated subsidiaries. The trademarks, trade names and service marks appearing in this prospectus supplement are the property of their respective owners.
