Attached files
| file | filename |
|---|---|
| EX-23.1 - EXHIBIT 23.1 - HANSEN MEDICAL INC | exhibit231-1231201510k.htm |
| EX-31.1 - EXHIBIT 31.1 - HANSEN MEDICAL INC | exhibit311-1231201510k.htm |
| EX-32.1 - EXHIBIT 32.1 - HANSEN MEDICAL INC | exhibit321-1231201510k.htm |
| EX-23.2 - EXHIBIT 23.2 - HANSEN MEDICAL INC | exhibit232-1231201510k.htm |
| EX-32.2 - EXHIBIT 32.2 - HANSEN MEDICAL INC | exhibit322-1231201510k.htm |
| EX-31.2 - EXHIBIT 31.2 - HANSEN MEDICAL INC | exhibit312-1231201510k.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
to
Commission file number: 001-33151
HANSEN MEDICAL, INC.
(Exact name of registrant as specified in its charter)
Delaware | 14-1850535 | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
800 East Middlefield Road, Mountain View, CA 94043
(Address of principal executive offices) (Zip Code)
Registrant’s telephone number, including area code: (650) 404-5800
Securities registered under Section 12(b) of the Act:
Title of each class | Name of each exchange on which registered | |
Common stock, $0.0001 par value per share | The NASDAQ Global Market | |
Securities registered under Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined by Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ¨ | Accelerated filer | x |
Non-accelerated filer | o (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No x
As of June 30, 2015, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant, based upon the closing price of a share of the registrant’s common stock as reported by the Nasdaq Global Market on that date,was $75.4 million. This calculation does not reflect a determination that certain persons are affiliates of the registrant for any other purpose.
As of April 15, 2016, the registrant had outstanding 18,989,280 shares of common stock.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement for its 2016 Annual Meeting of Stockholders, to be filed with the Securities and Exchange Commission no later than 120 days after the end of the fiscal year, are incorporated by reference to Part III of this Annual Report on Form 10-K to the extent stated herein.
HANSEN MEDICAL, INC.
ANNUAL REPORT ON FORM 10-K
For the Year Ended December 31, 2015
Table of Contents
Part I | ||
Item 1 | ||
Item 1A | ||
Item 1B | ||
Item 2 | ||
Item 3 | ||
Item 4 | ||
Part II | ||
Item 5 | ||
Item 6 | ||
Item 7 | ||
Item 7A | ||
Item 8 | ||
Item 9 | ||
Item 9A | ||
Item 9B | ||
Part III | ||
Item 10 | ||
Item 11 | ||
Item 12 | ||
Item 13 | ||
Item 14 | ||
Part IV | ||
Item 15 | ||
2
PART I
ITEM 1. BUSINESS
This Annual Report on Form 10-K contains forward-looking statements. These forward-looking statements are based on our current expectations about our business and industry and include statements regarding our strategies, products and product capabilities. In some cases, these statements may be identified by terminology such as “may,” “will”, “should,” “expects,” “could,” “intends,” “might,” “plans,” “anticipates,” “targets,” “believes,” “estimates,” “predicts,” “projects,” “potential,” or “continue,” or the negative of such terms and other comparable terminology. Examples of such statements include our expectations for future operating and financial trends and results. Our forward-looking statements involve known and unknown risks and uncertainties that may cause our results, levels of activity, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. Factors that may cause or contribute to such differences include, among others, those discussed in this report in Item 1A “Risk Factors.” Furthermore, such forward-looking statements speak only as of the date of this report. Except as may be required by law, we undertake no obligation to update any forward-looking statement to reflect events after the date of this report. Given these risks and uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements.
We have applied for trademark registration of, and claim trademark rights in, “Magellan” and “Hansen Medical Magellan.” We have obtained trademark registration for, and claim trademark rights in “Artisan Extend,” “Hansen Medical,” “Hansen Medical (with Heart Design),” “Heart Design (Logo),” “Sensei,” “Artisan,” “Instinctive Motion,” "CoHesion™" and "IntelliSense Technology™" This report also includes other trademarks, service marks and trade names of other companies.
Overview
We develop, manufacture and sell a new generation of medical robotics designed for accurate positioning, manipulation and stable control of catheters and catheter-based technologies. While earlier generations of medical robotics were designed primarily for manipulating rigid surgical instruments, our technology is designed to enable and improve medical procedures that are reliant upon flexible tools like guide wires, catheters, sheaths, balloons and stents.
Over the past thirty years, one of the most significant medical trends has been the development of less traumatic or minimally invasive methods of diagnosing and treating disease. These less traumatic methods have largely fallen into two groups:
• | Minimally invasive surgery, which reduces the size of incisions in body walls, generally results in fewer complications, shorter hospitalization and recovery times and substantially reduced pain and suffering. These procedures generally use rigid instruments. |
• | Interventional procedures, which minimize trauma by using blood vessels and other tubular anatomic structures such as the nose, mouth, urethra, rectum and cervix as “highways” to guide flexible instruments such as catheters to the area of treatment. |
Minimally invasive surgery reduces the trauma of open surgery, and interventional procedures cause even less trauma and can reach many areas of the body that rigid-instrument robotic surgery cannot. Each year, catheter-based technologies are used for millions of interventional diagnostic and therapeutic medical procedures worldwide. However, manually-controlled hand-held catheter delivery devices, even in the hands of the most skilled specialists, have inherent instrument control limitations. In traditional interventional procedures, devices are manually manipulated by physicians, who twist and push the external ends of the instrument in an iterative process that attempts to thread the internal end of the instrument through tubular anatomic structures to a specific treatment site. Manual control of the working tip of the catheter becomes increasingly difficult as more turns are required to navigate the instrument to the treatment site. These control problems are significant in constrained tubular spaces such as blood vessels and become even more difficult in unconstrained spaces such as the atria and ventricles of the heart and large diameter blood vessels such as the aorta and tortuous vessels commonly seen in patients with vascular disease. In addition, while sophisticated imaging, mapping and location-sensing systems have provided visualization for interventional procedures and allowed physicians to treat more complex conditions using flexible instruments, the substantial lack of integration of these information systems requires the physician to mentally integrate and process large quantities of information from different sources in real time during an interventional procedure. These systems display data differently, requiring physicians to continuously reorient themselves to the different formats and displays as they shift their focus from one data source to the next while at the same time manually controlling an inherently difficult-to-control catheter.
Electrophysiology
3
Electrophysiology, or EP, is the study of electrical impulses through the heart. EP is focused primarily on diagnosing and treating cardiac arrhythmias, which are conditions in which electrical impulses within the heart vary from the normal rate or rhythm of a heartbeat. Such conditions may be associated with significant risks to patients.
Our Sensei® Robotic Catheter System, or Sensei System, is designed to allow physicians to instinctively navigate flexible catheters with solid stability and control in interventional procedures within the atrial heart chambers. Instinctive navigation refers to the ability of our Sensei System to enable physicians to direct the movements of a robotic catheter like our currently marketed Artisan Extend® Control Catheter. We believe our Sensei System and its corresponding disposable catheters enable physicians to perform procedures that historically have been difficult or time consuming to accomplish routinely with manually-controlled, hand-held catheters and catheter-based technologies, or that we believe could be accomplished only by the most skilled physicians. We believe that our Sensei System has the potential to benefit patients, physicians, hospitals and third-party payors by improving outcomes and permitting complex procedures to be performed interventionally.
Our products are currently labeled and marketed in the United States for manipulation, positioning and control of certain mapping catheters in the atria of the heart connected with these procedures and in the European Union ("EU") and certain other countries for manipulation, positioning and control of diagnostic and therapeutic catheters within the atria of the heart. When the U.S. Food and Drug Administration ("FDA") cleared our technology for promotion in the U.S., the FDA required that we label our products in the United States with language spelling out that the safety and effectiveness of our products for use with cardiac ablation catheters, in the treatment of cardiac arrhythmias including atrial fibrillation, have not been established. We do not promote any off-label use of our Sensei System, including the use for ablation procedures for treating atrial fibrillation. Physicians may, however, choose to use our Sensei System in procedures involving the mapping and ablation of cardiac tissue to treat atrial fibrillation.
Atrial fibrillation, which is the most common form of arrhythmia, results from abnormal electrical impulses that cause a rapid, irregular heartbeat within the upper chambers of the heart, leading to ineffective pumping of the blood through the heart, as well as complications that include a significantly increased risk of stroke.
We have received approval for an Investigational Device Exemption, or IDE, application to investigate the use of our Sensei System and Artisan Extend Control Catheter in the treatment of atrial fibrillation in a clinical study designed to support a submission to the FDA to obtain clearance or approval for the expansion of our current labeling in the United States beyond mapping. The study has paused enrollment at 150 patients (125 in the initial phase and 25 additional patients) and involves the ablative treatment of atrial fibrillation. The Bayesian design study includes a seven-day follow-up for safety and a one-year follow-up for efficacy at intervals of 90, 180 and 365 days. Enrollment of the first patient occurred in May 2010. If successful, we intend to use the data from this study to support a submission to the FDA to obtain clearance or approval for use in atrial fibrillation procedures.
Vascular
An additional and more recent focus of our robotic catheter technology has been the endovascular treatment of vascular disease, an area that we expect to be a key growth driver for the business. Endovascular procedures are catheter-based procedures done in the arterial and venous vasculatures and include interventions involving the abdominal and thoracic aorta repair, embolization, percutaneous transluminal angioplasty, or PTA, atherectomy and stenting of branches of the aorta and arterial system including the coronary and carotid arteries and the iliac, femoral, popliteal, infra popliteal renal and mesenteric vessels. To address this market, we have developed our Magellan™ Robotic System, or Magellan System, and associated Magellan™ Robotic Catheters which is based on our Sensei System and preserves the open architecture featured in the Sensei System to allow for the subsequent delivery of therapeutic devices on the market today. The Magellan System with the Magellan Robotic Catheter, or “Vascular System”, cannulates peripheral vessels with a proprietary technology that delivers simultaneous and independent distal tip control of a catheter and a sheath as well as robotic manipulation of standard guide wires, from a centralized, remote workstation. This technology is designed to provide a robotically stabilized conduit for the manual placement and delivery of other companies’ therapeutic devices. Additionally, the Magellan System is designed to allow for sufficient extension inside the body to access hard to reach, distal peripheral anatomy.
We believe this platform and its clinical capability has the potential to open new markets for Hansen by providing vascular surgeons, interventional cardiologists, and interventional radiologists with accurate control of the catheter tip to enable vessel navigation, helping them to perform more complex catheter based procedures in a systematic way.
We believe that robotic control of flexible instruments has potential application to a broad range of interventional procedures. For the most part, catheters and catheter-based technologies have used blood vessels and other tubular anatomic structures as “highways” to constrain and guide their movement to specific parts of the body. However, we believe that physicians have limited ability to accurately control the distal working tips of these manually-controlled, hand-held instruments, which may hinder the physician’s ability to perform procedures that require precise navigation and stability of
4
catheters in tortuous vessels. These issues are magnified in larger open spaces such as the atria and ventricles of the heart and large diameter blood vessels of the body such as the aorta where the navigation of the tip of the catheter is no longer aided by vessel walls.
We believe our technology has additional applications outside of the fields of EP and peripheral vascular intervention. We plan to investigate the advancement of our technology into these markets so that the benefits of flexible robotics and instinctive control can be experienced by a larger group of physicians and patients.
The Hansen Medical Solution
Our systems principally consist of two modules: a physician control console and a patient-side module that can be easily connected to most procedure tables and do not require any specialty room designs. Physicians sit at the control console outside the radiation field and use their hands to instinctively control the motion of and navigate our disposable catheters, which are attached to the patient-side module. Our catheters are designed to accurately deliver third-party catheters and catheter-based technologies to specific sites in a broad range of procedures.
We believe our Sensei System and Magellan System, combined with our disposable catheters, overcome the limitations of hand-held navigation catheters. We designed our systems to have the following attributes:
• | Instinctive control. Our systems utilize computer-controlled robotics to directly translate the motions of the physician’s hands from our control console into corresponding accurate manipulations of the catheters and catheter-based technologies inside the body. We believe the instinctive robotic control of the catheters may be easier to use than manual catheter approaches and therefore has the potential to reduce procedure times, improve efficacy and enable newer or additional procedures to be performed through catheter navigation. In addition, we believe this instinctive control enables physicians to be trained in the use of our system in a relatively short period of time which can increase the number of physicians who are capable of performing these catheter-based procedures. |
• | Variable force at the catheter tip. To effectively perform a broad range of catheter-based procedures, physicians must have the ability to apply variable force at the working tip of the catheters and other catheter-based technologies. We designed our disposable electrophysiology catheter products to provide variable support while maintaining the flexibility required to navigate the catheter. In addition, we have developed our proprietary IntelliSense® force-sensing technology to measure and display the amount of force being applied by a catheter throughout the procedure. |
• | Stability. We believe our systems provide the accuracy and control required while treating a number of conditions in which the stable and repeated placement of a catheter is necessary for an effective outcome. |
• | Compatibility with third-party devices. Our catheters are based on an open architecture design, compatible with a broad range of therapeutic catheters made by other manufacturers. With the Artisan Extend catheter and the family of Magellan catheters, we can deliver therapeutic devices ranging from 3Fr up to 8Fr in size. |
• | Adaptability. Our systems primarily consist of two modules: a physician control console and a patient-side module that can be connected to most procedure tables and do not require any specialty room designs. In addition, the system includes a portable electronics rack. These modules can be wheeled between procedure rooms and reinstalled by a Hansen service technician and do not require a dedicated space, any special facility modification or magnetic shielding to prevent interference for their use. |
We believe that our robotic solution may offer the potential for substantial benefits to patients, physicians, hospitals and third-party payors including:
• | improving control, consistency and stability in catheter-based procedures; |
• | permitting access to complex existing cases and enabling broad use of catheter-based treatments for diseases where catheters are rarely used today, if at all; |
• | enabling more physicians to perform complex interventional procedures through ease of use and reduced training time; |
• | reducing X-ray radiation exposure by allowing the primary physician to conduct procedures away from the radiation source or in an adjacent room which greatly reduces their radiation exposure; and |
• | increasing hospital efficiency by allowing for additional cases to be performed in one day as a result of reduced physician fatigue and reduced risk of physician back and neck problems from heavy lead protective clothing. Our robotic solutions allow physicians to sit comfortably at our control console during a procedure instead of standing at table-side. |
5
Since the 2007 commercial launch of our Sensei System, clinical studies have been performed outside the United States comparing robotic versus manual procedures and describe differences in efficacy and treatment outcomes, procedure times, power use and power settings, complication rates, and fluoroscopy time (exposure to radiation). A growing body of evidence from these clinical studies, in which an estimated 19,000 atrial fibrillation patients have been treated, demonstrates:
• | Technical success in delivering therapeutic modality, and |
• | Similar or lower complication rate as compared to manual cases. |
Presently, there is one active EP study to assess and to further describe the use of flexible robotic navigation for atrial arrhythmia. This study has sites both in the United States and in Europe. This study is being conducted prospectively and is a multi center multi operator study. This study is being performed to evaluate the safety and effectiveness of the Sensei System. As this study concludes, we believe there will be greater empirical data for the acceptance and adoption of our products and technology.
Our Products
Sensei System
The Sensei System describes our Sensei X and Sensei X2 generations of systems and is principally comprised of two modules: a physician control console and a patient-side module that can be connected to most procedure tables. The control console can be located inside the Electro-Physiology Laboratory, or EP lab, and close to the patient or outside the EP lab in a separate location shielded from radiation. The control console features an instinctive motion controller, which robotically controls the patient-side module to move the catheter within the patient anatomy. Our robotics technology uses sophisticated software and system control algorithms to command the motion of our catheters. Having navigated the catheter to the targeted area, the physician uses instinctive controls to place the working tip of the control catheters at the desired location.
Our patient-side module is a robotic manipulator actuated by motors that control pull-wires in our catheters. The manipulator is mounted on an articulating, or pivoting, arm that is in turn mounted to the procedure table in the EP lab or other treatment room. The manipulator may be directed over the patient during a procedure and thus positioned optimally for that procedure.
We received CE Mark approval for our Sensei System in the fourth quarter of 2006 and made our first commercial shipments to the European Union in the first quarter of 2007. In May 2007, we received CE Mark approval for our Artisan Control Catheter and also received FDA clearance to market and promote our Sensei System and Artisan Extend Control Catheter in the United States for manipulation, positioning and control of certain mapping catheters during EP procedures.
To assist physicians in applying the appropriate force with the catheter tip, we have developed our proprietary IntelliSense® Technology to measure and display the amount of force in grams transmitted along the shaft of the catheter as a result of catheter tissue contact. We obtained premarket notification, or 510(k) clearance, from the FDA for this feature in 2008. In the third quarter of 2009, we introduced Sensei X, our next generation Sensei System. We received FDA clearance for the latest-generation system, the Sensei X2, in August 2014 and a CE mark in October 2014. Our end customers for these systems range from large university medical centers to community hospitals and many systems were shipped to distributors outside the United States.
Artisan Extend catheter
Our disposable Artisan Extend catheter assembly consists of a telescoping set of control catheters that are integrated to provide the desired motion of the tip of a diagnostic or therapeutic catheter that is inserted through the center lumen of the Artisan Extend catheter. In this manner, the Artisan Extend assembly is designed to accurately control the movement of a third-party catheter chosen by the physician. As a result, physicians are not limited to using particular proprietary catheters. In addition, the center lumen of the Artisan Extend catheter is designed to allow physicians outside the United States to adapt and expand the procedures they can perform as other manufacturers invent new therapeutic or diagnostic catheters. Each Artisan Extend catheter is designed to be used only once and then discarded.
Our disposable Artisan Extend catheters are designed to move together or independently, and can move with multiple degrees of freedom when attached to the robotically-controlled motors of our Sensei System. In addition, our Artisan Extend catheter has a programmable chip that prevents use of an Artisan Extend catheter that has been previously used and that restricts other control catheters from being plugged into our Sensei System patient-side module. In May 2007, we received CE Mark approval for our Artisan Extend Control Catheter and also received FDA clearance for the marketing of our Artisan Extend catheter for manipulation, positioning and control of certain mapping catheters during EP procedures. In the fall of 2009, we introduced our Artisan Extend catheter, which provides improved flexibility, longer reach and ease of use. A newly-
6
designed Artisan Extend Control Catheter with a new easier to use flush assembly was cleared by the FDA and the CE marked and introduced into the market in 2012.
CoHesion™ 3D Visualization Module
Our CoHesion 3D Visualization Module, or CoHesion Module, is a software interface between our Sensei System and the St. Jude Medical EnSite™ System for EP procedures. It is designed to provide physicians with 3D visualization to augment their ability to move a catheter throughout the atria, as well as increase control over placement of the catheter in specific locations. The CoHesion Module expands the utility of the EnSite and Sensei Systems to provide physicians with a comprehensive and easy-to-use remote navigation and mapping system for EP procedures. Key features of the CoHesion Module include:
• | importation of EnSite 3D geometry into the Sensei System’s main navigation window; |
• | localization of the percutaneous catheter tip within the EnSite 3D geometry; and |
• | instinctive navigation of the localized catheter tip. |
Published clinical studies have shown that the use of the CoHesion Module results in the reduction of radiation exposure to the patient compared to conventional procedures. The CoHesion Module became commercially available in 2008.
The EnSite System is a computer-based technology marketed worldwide which facilitates EP procedures by creating real-time 3D graphical displays or maps of cardiac structures and arrhythmias. These maps are designed to provide the visual guidance necessary to navigate catheters used during EP procedures. Two-dimensional technologies such as fluoroscopy or ultrasound can also be used to assist physicians with guiding catheters inside the heart, but provide limited information regarding the three-dimensional space inside the heart. Combining the Sensei and EnSite technologies provides physicians with 3D visualization that augments their ability to confidently move a catheter throughout the heart, as well as increase control over placement of the catheter in specific locations. The EnSite System is used by EP clinicians during EP procedures to create 3D models of their patients’ cardiac anatomy and then to visualize catheters used in those procedures as they are navigated to critical anatomical targets. The system collects and organizes activation and voltage data from the inner surface of the heart, which allows physicians to visualize arrhythmias on the 3D model and more easily determine a treatment strategy. Localization of our Artisan Extend catheter within the EnSite System’s 3D map gives physicians the ability to move the catheter deliberately and accurately while seeing specifically, in three dimensions, the location of the catheter inside the heart. We believe this integrated functionality enables clinicians of varying skill levels to effectively treat complex cardiac arrhythmias.
Magellan System
Our Magellan System is similarly comprised of two modules: a physician control console and a patient-side robotic arm that can be connected to most procedure tables. The control console can be located inside the lab and close to the patient or outside in a separate location shielded from radiation. The control console features an instinctive motion controller as well as a pendant interface, which can both be used to robotically control the patient-side module to accurately move the catheter within the patient anatomy. Our robotics technology uses sophisticated software and system control algorithms to command the motion of our catheters. The Magellan System controls the proprietary Magellan Robotic Catheter, which is a telescoping, robotically steerable catheter designed to facilitate remote catheter navigation and vessel cannulation, and to provide a robotically stabilized catheter for manual delivery of therapeutic devices.
Our patient-side module is a robotic manipulator actuated by motors that can control the remote insertion, rotation and bend of the Magellan Robotic Catheter, as well as the remote insertion and rotation of a standard-sized guide wire. The manipulator is mounted on an articulating, or pivoting, arm that is in turn mounted to the procedure table in the interventional lab or hybrid operating room. The manipulator may be adjusted for optimal positioning over the patient.
In July 2011, we received a CE Mark for our Magellan System and in October 2011 received a CE Mark for the Magellan 9Fr Robotic Catheter and related accessories designed for use with the Magellan System. We received FDA clearance for the marketing of our Magellan System including the catheter and accessories in June 2012. We received FDA clearance for the marketing of our Magellan System including the catheter and accessories in June 2012 and we received FDA clearance for the marketing of our Magellan 6Fr Robotic Catheter in February 2014. In November 2014, we received a CE Mark for the marketing of our Magellan 6Fr Robotic Catheter. In July 2015, we received FDA clearance to market our Magellan 10Fr Robotic Catheter in the U.S. In April 2015, we received CE Mark clearance to market our Magellan 10Fr Robotic Catheter for use in the peripheral vasculature. We recognized revenue on our first Magellan System in the first quarter of 2012.
The Magellan System employs the use of three types of catheters: the Magellan 9Fr, the Magellan 6Fr and the Magellan 10Fr Catheters. Each catheter allows for features such as distal tip shaping of the catheter, navigation that does not require vessel wall touches and strong catheter stability when locked in place in preparation for therapy delivery. The Magellan 9Fr Catheter received FDA clearance in 2012 and a CE Mark in 2011. The Magellan 6Fr Catheter received FDA clearance and a
7
CE Mark in 2014. The Magellan 10Fr Catheter received FDA clearance and a CE Mark in 2015. In 2014, we also launched the Magellan Transport System or MTS which allows for the Magellan robotic arm to be easily deployed or removed from a patient table. The ability to remove the arm frees up potential table space for non-robotic procedures and allows for Magellan to be easily transported out of the room.
Our Strategy
Our goal is to establish our technology as the leading robotic platform for complex interventional catheter-based procedures for cardiovascular and peripheral vascular diseases. We believe our Sensei System and Magellan System will accomplish this objective by potentially improving outcomes, reducing physician fatigue which may allow for additional cases to be performed in one day, reducing radiation exposure for operating physicians and reducing overall procedure costs and hospital expenditures. We also believe that we will be able to increase the number of procedures treated with catheter-based approaches and enable more doctors to perform such procedures. We market our products in the United States through a direct sales force of regional sales employees, supported by clinical sales representatives who provide training, clinical support and other services to our customers. Outside the United States, primarily in the European Union, Saudi Arabia, Australia and Singapore, we use a combination of a direct sales force and distributors to market, sell and support our products.
Elements of our strategy include:
• | Focus commercial, clinical, and engineering activities to drive adoption and utilization of the Magellan platform in vascular applications and the Sensei technology in electrophysiology. We have focused our efforts on projects with the highest level of clinical value and which we believe will have attractive returns on investment. We have also oriented our investments in marketing and clinical resources to better educate the physician community as to the benefits of our technology. We believe that through both simultaneous broad and focused physician interaction, publications of our clinical experiences, dissemination of those experiences, and engagement of current and new users, we can positively affect system utilization. We also believe that committing to utilization and physician interaction we will also drive improvements in adoption of the technology. |
• | Target key institutions and thought leaders to encourage adoption of our systems. We are currently focusing our marketing efforts on the academic and community hospitals where the majority of procedures are performed. We believe these efforts will benefit those hospitals which adopt our technology by reinforcing their reputations as centers of excellence in their local markets in the specialties that benefit from procedures performed with our systems. In late 2013, we began to place increased commercial emphasis on communicating the clinical and economic value of our Magellan System to physicians and administrators in the U.S. community hospital setting. |
• | Continue ongoing research and development efforts to broaden our technology platform and extend our leadership. We intend to enhance and maintain our technology leadership with focused research and development efforts. Currently, we sell systems for EP procedures and for the treatment of vascular diseases. We believe that these platforms and their clinical capability could provide us access to significant markets. We believe that a robotic system with flexibility, in terms of tools and disposables, has the potential to allow us to broadly address market needs for both complex interventions as well as simpler cases. |
• | Increase use of our systems. Following the initial placement within a given hospital, we endeavor to expand the number of physicians who use our systems. Our goal is to increase usage per system, leading to higher volume sales of our disposable catheters and sales of additional systems at each hospital. Through December 31, 2015, we estimate approximately 20,000 procedures have been performed using our systems. |
• | Expand potential applications for our systems. We have commenced post-marketing studies to provide evidence of the benefits which we believe our technology brings to the clinician, which should help to drive adoption of our technology. We have received FDA approval to investigate the use of our Artisan Extend Control Catheter in the treatment of atrial fibrillation in an IDE clinical study designed to support a submission to the FDA to obtain clearance or approval for the expansion of our current labeling in the U.S. beyond mapping. |
• | Leverage the open architecture of our platforms. We believe that our broad compatibility with 3rd party devices and technologies will facilitate adoption of our systems in the marketplace. We also believe that adoption of both of our systems will be enhanced because physicians will be able to use existing approved catheters in the lumen of our robotic catheters. We plan to collaborate with manufacturers of disposable interventional products and imaging equipment to optimize compatibility with future enhancements of our systems. Further, our open architecture allows us to benefit from third-party development efforts that advance current catheter and imaging technologies. For example, we are encouraged with the recent movement within imaging technology companies to create a convergence of surgical operating rooms and interventional suites into hybrid interventional operating labs. We believe this convergence of imaging for intervention and operative intervention is consistent with our strategy to create increased efficacy, safety and efficiency through the pairing of visualization and imaging technology with our flexible robotics. |
8
Our Sensei System and Artisan Extend catheter were cleared by the FDA for manipulation and control of certain mapping catheters in EP procedures. This robotic catheter system is compatible with fluoroscopy, ultrasound, 3D surface map and patient electrocardiogram data. In the United States, the Sensei System is not approved for use in guiding ablation procedures; this use remains investigational. The U.S. product labeling therefore provides that the safety and effectiveness of the Sensei System and Artisan Extend catheter for use with cardiac ablation catheters in the treatment of cardiac arrhythmias, including atrial fibrillation, have not been established. Our future business prospects, however, will depend on the use of our Sensei System in the treatment of atrial fibrillation and other cardiovascular procedures. Depending upon regulatory requirements and our understanding of the needs of the physician community, we plan to seek future clearances or approvals for labeling that includes certain ablation procedures. Without such clearance or approval, each of these uses is considered an off-label use of our Sensei System, and we are prohibited from labeling or promoting our Sensei System, or training physicians, for such off-label use. Due to these legal constraints, our sales and marketing efforts focus on the general technical attributes and benefits of our Sensei System and its use to map the atrial anatomy. As a result of promotional limits based on our labeling and the competitive nature of the market, some hospitals or physicians may not adopt our Sensei System or use our products unless and until we are able to broaden the scope of FDA clearance for our products. In addition, if the FDA determines that we have engaged in off-label promotion, we could be subject to significant liability. See Item 1A — Risk Factors — “We may incur significant liability if it is determined that we are promoting off-label use of our products in violation of federal, state and countries regulations in the United States or elsewhere” and “If we fail to comply with healthcare laws and regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.”
Clinical Focus
Electrophysiology
Electrophysiology, or EP, is the study of electrical impulses through the heart. EP is focused primarily on diagnosing and treating cardiac arrhythmias, which are conditions in which electrical impulses within the heart vary from the normal rate or rhythm of a heartbeat. Such conditions may be associated with significant risks to patients. Drug therapies have traditionally been used as initial treatments but they often fail to adequately control the arrhythmia and may have significant side effects. As a result, a significant unmet medical need for long-term solutions persists.
Severe heart rhythm disturbances were historically treated by highly invasive open chest heart surgery and are therefore typically only performed in conjunction with other procedures unrelated to the arrhythmia such as coronary artery bypass surgery or valve replacement and, as such, the total procedure can be very expensive. While generally very effective, these procedures are extremely traumatic for the patient, and usually require long hospital stays followed by a significant period of convalescence. Minimally invasive surgical procedures for the treatment of severe heart rhythm disturbances, including some which are robotically controlled, were devised to add visualization and instrument control using an endoscope in order to reduce the trauma for the patient. While these minimally invasive surgical techniques have been used for a number of anti-arrhythmic procedures, the results have been mixed and the trauma to the patient and procedure cost remain high.
Interventional EP further advanced these surgical procedures in EP labs through visualization provided by real-time X-ray imaging, often enhanced by electro-anatomic mapping and intracardiac ultrasound. These advances enable physicians to insert and navigate catheters into the vasculature, then into the open chambers of the heart to deliver diagnostic and therapeutic technologies.
In EP mapping and ablation procedures, physicians have traditionally used specialized hand-held catheters. These catheters are manually navigated using a system of mechanical control cables to first map the electrical signals within the patient’s heart and then to ablate the heart tissue to eliminate arrhythmias. Generally, ablation is accomplished by applying radiofrequency energy or electrical energy, or freezing the diseased tissue giving rise to the arrhythmia, usually through a catheter which creates a small scar that is incapable of generating or conducting heart arrhythmias. EP procedures have proven highly effective at treating arrhythmias at sites accessible through the vasculature.
Control of the hand-held devices used in these EP procedures requires significant skill, because navigation in the blood vessels and the chambers of the heart can be difficult. The path that the interventional device must follow to arrive at the treatment site can be complex and tortuous and can include crossing the septum of the heart. Existing hand-held devices are limited in their ability to accurately navigate the tip of the mapping and ablation catheter to the treatment site on the heart wall, to keep the catheter in the targeted place and maintain adequate tissue contact within a beating heart to effect treatment and perform complex ablations within the left atrium of the heart. Physicians using manually-controlled, hand-held devices often utilize a range of different catheters and sheaths in an attempt to find the right device or combination of devices for the procedure being performed. Our Sensei System has been designed to address the challenges associated with the use of current hand-held devices in performing many EP procedures.
9
We believe the instinctive robotic control of our Sensei System may provide greater accuracy, tip stability and control, reduce the variability of procedure times and improve the efficacy of EP procedures, including:
• | General mapping and ablation. A physician typically performs a diagnostic procedure in which the electrical signal patterns of the heart wall are mapped to identify the heart tissue generating the aberrant electrical signals. Mapping allows the physician to measure the timing and strength of the electrical activity. Following the mapping procedure, the physician may then use an ablation catheter to disable the aberrant electrical signal or signal path, restoring the heart to its normal rhythm. |
• | Atrial fibrillation. The most common arrhythmia is atrial fibrillation, which is characterized by rapid, disorganized contractions of the heart’s upper chambers, the atria. Atrial fibrillation leads to ineffective pumping of the blood through the heart and significantly increases the risk of stroke. According to GlobalData in 2014, over 3.3 million people in the United States currently suffer from atrial fibrillation. We believe that the number of atrial fibrillation procedures has the potential to grow if quicker, more effective and easier to accomplish interventional treatments are available. We believe that due primarily to the difficulties of accurately controlling the manual catheter, the efficacy of ablation to treat atrial fibrillation is reported to be only approximately 50% to 75% according to GlobalData studies in 2014, and the procedure has significant risks, including stroke. As a result, atrial fibrillation ablations are generally only performed by very experienced physicians. We believe that many of the electrophysiologists in the United States do not regularly perform these catheter-based procedures because of their complexity and time-consuming nature and evolving clinical data. These procedures often last three to seven hours because of their complexity. The length of these procedures exposes the physician and staff to extensive radiation, requiring them to wear heavy lead vests for many hours at a time. As noted above, our Sensei System has not been cleared in the United States for use with ablation catheters or for the treatment of atrial fibrillation. We are required by the FDA to label our products with language specifying that the safety and effectiveness of our products for use with cardiac ablation catheters, in the treatment of cardiac arrhythmias including atrial fibrillation, have not been established. |
Vascular Market
Peripheral vascular disease presents a significant market opportunity for us. Patients with peripheral vascular disease can suffer from decreased blood flow which can lead to disability and amputation, stroke or blood clots, and death. The rates of diabetes, hypertension, atherosclerosis and obesity are also on the rise worldwide and are contributors to the growth rate of vascular disease in an aging population. Endovascular procedures are catheter-based procedures done in the arterial and venous vasculatures and include interventions involving abdominal and thoracic aorta repair, embolization as well as access PTA, atherectomy and stenting of branches of the aorta and arterial system including the carotid arteries and the iliac, femoral, popliteal, renal and mesenteric vessels. In 2013, Millennium Research Group forecasted that approximately 1.1 million of these procedures would be performed in the United States alone in 2013.
We believe that our technology has the potential to improve efficacy, safety and efficiency for a number of procedures that can generally be grouped into the category of endovascular therapies. From simple, straightforward procedures, to complex, challenging endovascular procedures, we believe robotic catheter control has the potential to (1) allow endovascular physicians to perform procedures less invasively, (2) reduce radiation exposure to physician and reduce procedural fatigue, and (3) reduce the likelihood of prolonged procedure times.
Complex vascular procedures, which we estimate range from 10% to 20% of endovascular interventions, have limited technical success, longer procedure times and fluoroscopy use, and expose the patient to significantly increased risks of adverse events and extended hospitalizations. We believe that the individual robotic control of the distal tips of both the outer and inner catheters as well as robotic manipulation of standard guide wires, can enable navigation to the anatomical area of interest inside an artery and can facilitate placement of a stent, graft or other therapeutic or implantable device. In particularly diseased or tortuous arteries, facilitation of steering of the catheter may assist in avoiding contact with atheromatous (and calcification) disease present in the vessel wall, may facilitate procedures that are now difficult to do and could impact outcomes. For some patients with particularly challenging anatomy or lesions, robotic catheter control may enable endovascular procedures in situations that might conventionally have been treated surgically. In these circumstances, we believe there may be significant benefits to the physician and hospital. In addition, similar to EP applications, we believe the use of robotic catheter control can lead to material reductions in radiation exposure to the physician, and potentially the patient, in the treatment of vascular disease. Remote navigation using the Sensei System has already demonstrated reductions in radiation exposure to the physician in electrophysiology applications. Finally, robotic catheter control has the potential to provide reduction in radiation exposure reduction and improved control and stability for more straightforward endovascular or interventional cases.
We also believe there is the potential to convert surgical cases to endovascular cases using robotic catheter control, an innovation that could, if achieved, materially affect the course of endovascular disease management.
10
Our Magellan System has been designed and engineered to meet the needs of vascular surgeons, interventional cardiologists, interventional radiologists and interventional neuro-radiologists, and extends the current Hansen Sensei architectural achievements in robotic catheter manipulation, catheter design, robotic capabilities, instinctive control, and data visualization. The architecture is intended to constitute a platform that we believe will extend our robotic capabilities significantly, and therefore depart from the Sensei architecture in the following ways:
1. | Our Magellan Robotic Catheter is smaller in diameter, has thinner walls, is more flexible, and is able to accommodate standard therapeutic device delivery; it is also longer, enabling it to reach distal anatomical targets. |
2. | We believe novel robotic technology with substantially more control mechanisms and visualization technology will provide a broader base of capabilities to selectively employ in a suite of tools for endovascular intervention and, in the future, structural heart interventions. The bedside robotic system, which includes the bedside robot, setup joint and bedside box, has been designed to allow for independent, individual robotic control of the distal tip, manipulating more delicate catheters with more degrees of freedom over longer travel distances as well as enabling the manipulation of guide wires. |
We anticipate that our Magellan System will be used in a variety of endovascular interventions, which may include some of the vascular applications described below. To date, The Magellan System has been used in over 500 endovascular procedures. We have initially focused on addressing the lower extremity and thoraco-abdominal markets, which include endovascular treatment of the ilio-femoro-popliteal disease, and renal, celiac and mesenteric artery disease. We also intend to pursue robotic catheters for the diagnosis and treatment of disease in the coronary, carotid and neurovascular areas with future development.
Potential endovascular interventions
Lower Extremity Artery Interventions. Critical limb ischemia, or CLI, occurs when the patient’s lower extremity arteries, such as femoral, popliteal and below-the-knee arteries, cannot carry sufficient oxygen to ensure proper metabolism. This may limit the patient’s ability to perform common activities such as walking or climbing stairs. If untreated, CLI often results in limb amputation. According to Millennium Research Group, the number of angioplasty and stenting procedures of the ilio-femoro-popliteal and infrapopliteal segment in the U.S. and in portions of Europe (the U.K., France, Germany, Italy and Spain) in 2015 was forecasted to be approximately 633,800 and 364,400, respectively. The lower extremity market continues to be a fast-growing market showing device and technique innovation. While a broad range of therapeutic tools is available to the endovascular specialist, acute iliac bifurcations, tortuous iliac arteries, and chronic total occlusions, or CTOs, appear to remain key contributors to procedure complexity and unpredictability. We believe that the control at the catheter tip and the increased stability provided by the Vascular System may simplify the navigation of complex iliac bifurcation and other anatomic branch points and areas of tortuosity, minimize time from femoral stick to angioplasty or stenting, and result in shorter, more predictable procedure times. In addition, the Vascular System has the potential to provide a systematic approach for navigation of the iliac bifurcations and access to the contralateral leg, which may reduce the physician’s dependency on proper guide wire and catheter selection, and may reduce delays or technical failures. We also believe that by providing user-friendly and individual control of a guide wire and the catheter tip and through the provision of increased catheter support, the Vascular System may allow the crossing of CTOs, reduce the volume of conversions to bypass surgery, reduce procedure time and radiation exposure and enable more endovascular specialists to expand their practice to include the treatment of complex vascular disease.
Visceral Artery Interventions. According to Millennium Research Group, the number of renal artery stenting procedures forecasted in 2015 in the U.S. and in Europe was approximately 48,600 and 15,400, respectively. There has been an ongoing controversy on the superiority of renal artery stenting over medical therapy, and one of the concerns associated with renal artery stenting has been the risk of embolization caused by the dislodgement of embolic debris during manipulation of conventional catheters. We believe that providing instinctive steering of the catheter and accurate manipulation of the catheter tip to avoid point contact with the vessel wall may positively impact outcomes of renal, mesenteric and celiac artery stenting.
Abdominal and Thoracic Aortic Aneurysm Repair. According to a Millennium Research Group study, in 2014, the number of abdominal and thoracic aneurysm endovascular repairs performed in the U.S. was forecasted to be approximately 46,000 and 11,000, respectively. Endovascular aneurysm repair, or EVAR, has been growing rapidly and cannibalizing surgical repair, because it has proven to be a minimally invasive procedure with similar effectiveness to surgical grafting and fast recovery times, which reduces costs of convalescence and perioperative complications and makes EVAR a cost-effective solution for medical institutions.
EVAR, however, is in part technically challenging in situations where cannulation of portions of the graft must be achieved under fluoroscopy, or vessel embolization must be achieved prior to deployment of the endograft. The complex
11
anatomy and the difficulty of controlling guide wires and catheters in an enlarged, diseased aorta may produce significant variability in procedure times, fluoroscopy use and complication rates in patients needing pant leg grafts, or fenestrated or branched grafts. We believe that our Vascular System may be able to provide a more systematic approach to vessel and graft cannulation, which may result in shorter, more consistent procedure times, and reduce radiation exposure and use of contrast.
Embolization Procedures: According to the Millennium Research group, the U.S. market for peripheral vascular embolization will experience a 6.3% procedure growth through 2016 (CAGR 2012-2022), with cancer treatment embolization procedures as the highest growth factor. The Magellan System is currently being utilized in a variety of embolization procedures in which the robotic catheter navigates to a desired target location and then occlusive agents are deployed in the blood vessel to block blood flow to a tumor or other target. Embolization may also involve the deployment of chemotherapy or radioactive seeds for cancer treatment. Further embolization procedures where the Magellan System is currently being utilized are in the treatment of uterine fibroids in women and benign prostatic hyperplasia (enlargement of the prostate) in men. Uterine fibroids are the most common solid pelvic tumor in women, causing symptoms in roughly 25% of all women of child-bearing age. By age 35, 25% of Caucasian women, 40% of Hispanic women, and 50% of African American women have clinically significant fibroids. Surgery is typically how large fibroids are ultimately treated. In the U.S., there are approximately 40,000 myomectomies performed annually as stand-alone treatments for fibroids and it is estimated that 33-50% of the approximately 600,000 annual hysterectomies in the U.S. are a result of uterine fibroids. A uterine fibroid embolization procedure (UFE) facilitated by the Magellan System addresses the fibroid by cutting off blood supply to the key vessels by employing an embolic blocking agent. When the fibroid is denied its blood supply, it will ultimately shrink and be absorbed by the body. Robotic UFE can be an inpatient or outpatient procedure, and studies suggest that fibroids are not likely to grow back after a UFE procedure. Currently there are only approximately 25,000 UFE procedures performed annually in the U.S., which may be related to a variety of factors including challenges such as access to patients and skills required to perform the procedure manually. Benign Prostatic Hyperplasia (BPH) is a condition that affects approximately 50% of the male population over 50 and up to 90% from 70 to 89 years old. BPH is highlighted by frequent urination, a weak urine stream and/or an urge to urinate. The current gold standard of treatment is either medication or a surgical procedure, TURP (Transurethral Resection of the Prostate). TURP is commonly performed by urologists, but studies suggest it is only effective 70% of time and can result in a series of post-operative complications such as pain, bleeding, incontinence or impotence. Prostatic Artery Embolization (PAE) has been performed since 2009 and early studies show it to be a promising procedure which avoids many of the open surgical complications that result with TURP. We believe the introduction of the Magellan System for this procedure may prove to overcome vessel navigation challenges and other difficulties inherent with this procedure.
Carotid Artery Stenting. Carotid artery stenting, or CAS, is an alternate therapy to surgical carotid endarterectomy, and may be performed by the interventional cardiologist, the interventional radiologist, or the vascular surgeon. According to Millennium Research Group, the number of CAS procedures forecasted in 2015 in the U.S. and in Europe was approximately 24,200 and 19,400, respectively. The growth of CAS has been hindered by controversial clinical results. CAS is a procedure that requires, in many cases, navigation of tortuous anatomy, placement of multiple devices in sequence to achieve procedural success, and manipulation of these devices at a distance to achieve appropriate placement and long-term efficacy. We believe that, by enabling fine control of the catheter tip and increased catheter stability, our Vascular System may provide a systematic approach for navigation of the aortic arch, improve access consistency for physicians treating complex arches and tortuous carotid anatomy, potentially resulting in more consistent procedure times, and possibly reducing risk of embolization caused by the lack of control of the distal end of conventional catheters.
Coronary Artery Interventions and Chronic Total Occlusion Crossing. According to Millennium Research Group in 2008, the number of percutaneous coronary interventions forecasted in 2010 in the U.S. and in Europe was approximately 1,000,000 and 740,000, respectively. Based on physician interviews we have conducted, technical difficulties associated with coronary artery stenting seem to originate from the lack of stability of the distal section of the guiding catheter and from limited ability of catheters and guide wires to cannulate acutely angulated coronary branches. We believe that by providing stability and steering at the tip of the catheter, as well as instinctive driving of the catheter, the Vascular System may facilitate branch cannulation. We also believe that active catheter control through tip steering and catheter pull wire manipulation can provide increased stability at the site of intervention and may decrease the risk of having the guiding catheter be dislodged out of the coronary artery during guide wire manipulation and therapy delivery.
Transcatheter Aortic Valve Replacement (TAVR). Transcatheter aortic valve replacement represents an emerging alternative therapy for high-risk and inoperable patients with severe valve disease, and may offer advantages over open heart surgery. For example, non-surgical heart valve replacement may minimize complications associated with general anesthesia, opening the chest wall and the use of heart-lung bypass machines. Percutaneous aortic valve replacement using a catheter-based approach may enable surgeons to perform procedures under local anesthesia in a cardiac catheterization lab. This may be a preferred alternative for high-risk valve disease patients who otherwise have no choice but open heart surgery, and more importantly, for those patients with life-threatening valve disease who cannot undergo surgery. We believe that the controlled
12
and precise access and deployment that robotics can provide in an endovascular aortic valve replacement has the potential for enabling current surgical valve candidates to be treated less invasively.
Research and Development
As of December 31, 2015, our research and development team consisted of 20 people and our regulatory, clinical and quality team consisted of 17 people. We have assembled an experienced team with recognized expertise in robotics, mechanical and electrical engineering, software, control algorithms, systems integration and disposable device design, as well as significant clinical knowledge and expertise.
Our research and development efforts are focused in the following major areas:
• | continuing to enhance the capabilities of our Magellan System and Magellan Robotic Catheters and related accessories; |
• | continuing to enhance the capabilities of our existing Sensei System and our Artisan Extend catheters through ongoing product and software development; |
• | developing new capabilities for our robotic technology; |
• | designing new proprietary disposable interventional devices for use with our Sensei System and our Magellan System; and |
• | developing new applications for our technology and related additions to our Sensei System and our Magellan System, new control catheters, or integration with other imaging technologies or other modalities. |
Our research and development team works independently and with other manufacturers of lab equipment to integrate our open architecture platform with key imaging, location sensing and information systems in the labs. We also collaborate with a number of highly regarded electrophysiologists, vascular surgeons, interventional radiologists and cardiologists in key clinical areas in search of new applications for our technology.
We have historically spent a significant portion of our capital resources on research and development. Our research and development expenses were $13.8 million, $18.0 million and $16.1 million in 2015, 2014 and 2013, respectively. See Item 1A, “Risk Factors — We may not be able to further develop our Magellan System as planned, which could significantly harm our ability to achieve future regulatory approvals and market acceptance” and “— Our products and related technologies can be applied in different applications, and we may fail to focus on the most profitable areas or we may be unable to address successfully financial and technology risks associated with new applications, including applications for the vascular market.”
Sales and Marketing
We market, sell and support our products in the United States through a direct sales force of regional sales employees, supported by clinical account managers who provide training, clinical support and other services to our customers. Outside the United States, primarily in the European Union, Saudi Arabia, Australia and Singapore, we use a combination of a direct sales force and distributors to market, sell and support our products. We have established sales subsidiaries in the U.K. and Germany and have hired sales representatives in the U.K. We currently have distribution agreements in several other countries outside of the United States, U.K. and Germany. A summary of our financial information by geographic location is found in Note 14, “Segments Information,” in the Notes to Consolidated Financial Statements. Our international operations and sales subject us to a variety of risks; see Item 1A, “Risk Factors,” for further discussion.
As of December 31, 2015, we had a direct sales force, clinical support team and marketing team of 29 employees. We use the same sales and marketing force to drive sales of both our Sensei System and our Magellan System.
Our sales and marketing process consists of two important steps: selling systems directly to the customer; and leveraging our installed base of systems to drive recurring sales of disposable interventional devices, software and services.
Our end users fall into three broad categories:
• | leading academic institutions with physician thought leaders who are interested in performing complex new procedures enabled or simplified by our system; |
• | high-volume non-academic regional centers interested in the benefits of our system; and |
• | medium and low volume community hospitals that are competing for patients, attempting to minimize referrals of complex cases to other centers and focusing on gaining market share in their regional markets. |
Many hospitals are part of an integrated delivery network, or IDN, and/or a group purchasing organization, or GPO. When possible, we focus on leveraging opportunities in which we believe a sale to one member of an IDN or GPO creates interest and drives competition within that group. We also focus sales and marketing development activities where strategic synergies or competition exists between our current installed base and other area hospitals.
13
Following the initial sale of a system to a hospital, we endeavor to expand the number of physicians who use our systems at that hospital. We believe these efforts will benefit early-adopting hospitals by increasing their market share in the procedures and specialties that benefit from procedures performed with our systems. We expect these efforts to increase demand for our disposable products among hospitals, physicians and referring physicians.
Sales of medical capital equipment generally follow a staged sales process that includes the following:
• | generating initial customer interest; |
• | gaining commitment from the customer, which often involves a formal written proposal; |
• | helping the customer secure formal budget approval for the system purchase; |
• | receiving a formal purchase order from the customer after its approval process is complete and after sales terms have been agreed upon; and |
• | installing the system at the customer’s site and providing physician and staff training so it is used properly. |
Our systems utilize proprietary control catheters, as well as software tailored to specific clinical applications. After a system is installed and initial training has been completed, we provide ongoing support in order to increase customers’ familiarity with system features and benefits, with the goal of increasing usage of the system. More frequent usage will result in increased consumption of our disposable catheters. One year of customer support is generally included with each system. Thereafter, we market and sell extended service plans to continue to provide service and support, and we anticipate that service beyond the basic warranty will increasingly be an important additional source of revenue.
We expect that our relationships with physician thought leaders in the fields of EP and vascular disease will continue to be an important element of our selling efforts. These relationships are often built around obtaining feedback from such physicians with respect to our products that enable us to better understand and articulate the most useful features and benefits of our system as well as to develop new solutions to long-standing challenges in interventional electrophysiology and peripheral vascular disease. We plan to continue to provide support for and consult with highly regarded physicians in order to accelerate market awareness and adoption of our systems.
We have developed a comprehensive solution to assist our customers in marketing their Sensei System and robotic EP program. The flexible robotics program is a broad based and robust toolkit designed to assist our customer hospitals in using the development of a robotic EP program as a tool to market the hospital’s quality, commitment to patient care and innovation. The program is a virtual toolbox that contains both the programmatic and content elements that are designed to plan, initiate, and execute public relations and outreach campaigns, influence and change referral patterns to improve market share in the hospital’s catchment area, enliven hospital personnel and patients around the benefits of our innovative robotic technology, and develop substantial awareness of the technology and the physicians employing it. We have experienced a number of hospital level examples of the benefits that this program brings to all of our constituents and believe it will be an important component of the drive to adoption and utilization of the technology. We have also developed these tools for use in our distributor channels. Leveraging off our experience with the Sensei System, we have developed a program to similarly market our Magellan System. See Item 1A — “Risk Factors — If we fail to comply with healthcare laws and regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.”
Customer Service and Support
As of December, 31, 2015, we had a customer service and support team of 8 employees. These employees form a call center, a network of field service engineers and a service parts logistics repair and delivery system. We also outsource the after-hours portion of our call center, including weekends and holidays, to an answering service. This infrastructure provides a single point of contact for our customers via telephone and email and enables us to provide online, telephone and on-site technical support services 24 hours a day, seven days a week. In addition, we offer post-warranty maintenance plans for our customers to manage their on-going support needs.
Manufacturing
As of December 31, 2015, we had a manufacturing team of 40 employees. We manufacture our systems and catheters using a combination of in-house manufacturing and third-party contract manufacturers. Some of the components for our systems are single sourced. We may not be able to quickly establish additional or replacement suppliers for our single-source components, in part because of FDA requirements and because of the custom nature of the parts we utilize. Any supply interruption for any of these components or interruptions at our contract manufacturers could limit our ability to manufacture our products, which could have a material adverse effect on our business.
14
We face technical challenges in our manufacturing processes, including manufacturing cost reductions, equipment design and automation, material procurement, problems with production yields and quality control and assurance. Developing our current manufacturing capacity has required the investment of substantial funds, and additional changes in the future to reduce manufacturing costs or to adapt our capacity to market fluctuations may require the investment of substantial additional funds and the hiring and retaining of management and technical personnel who have the necessary manufacturing experience. We may not successfully complete any future required change in manufacturing ability on a timely basis or at all.
Lead times for materials and components ordered by us and our contract manufacturers vary and depend on factors such as the specific supplier, contract terms and demand for a component at a given time. We and our contract manufacturers acquire materials, complete standard subassemblies and assemble fully configured systems based on sales forecasts. If orders do not match forecasts, we and our contract manufacturers may have excess or inadequate inventory of materials and components. See Item 1A, “Risk Factors — If we are unable to manufacture our systems and catheters in a manner that yields sufficient gross margins, we will be unable on a commercial scale to achieve profitable commercialization” and “— We are continuing to develop our experience in manufacturing and assembling our products and may encounter problems at our manufacturing facilities or otherwise experience manufacturing delays that could result in lost revenue or diminishing margins.”
The Sensei System and Magellan System
Our systems incorporate a number of custom parts and components that we have designed and which are manufactured to our specifications by third parties. Our manufacturing strategy for our systems is to assemble some critical subsystems in-house while outsourcing less critical subsystems, and to complete the final assembly and testing of those components in-house in order to control quality.
Artisan Extend and Magellan catheters and guide catheter assembly
Our catheters consist almost entirely of custom parts which we have designed and are manufactured either by us or by contract manufacturers to our specifications. We currently assemble catheters in-house. We outsource the manufacture of certain other disposable products, including sterile drapes used with our system. We also manufacture prototype disposables to facilitate future product development.
Software
We develop the software components of our systems, including control and application software, both internally and with integrated modules which we purchase or license from third parties. We perform final testing of software products in-house prior to commercial release.
Regulatory Framework
Our manufacturing facilities operate under processes designed to meet the FDA’s requirements under the Quality System Regulation. We underwent an FDA inspection, which employed the Quality System Inspection Technique, or QSIT, in 2014 and received one inspectional observation. We received the establishment inspection report (EIR) from the FDA on January 26, 2015 after the agency closed the inspection per CFR 20.64. If the FDA were to find that we are not operating in compliance with applicable regulations, we could be subject to enforcement action by the FDA and/or the U.S. Department of Justice, which may include one or more of the following administrative or judicial sanctions:
• | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
• | unanticipated expenditures to address or defend such actions; |
• | customer notifications or mandatory plans for product repair, product replacement or refunds; |
• | recall, detention or seizure of our products; |
• | operating restrictions or partial suspension or total shutdown of production; |
• | refusing or delaying our submission seeking 510(k) clearance, investigational device exemptions (IDE) to perform clinical studies or premarket approval of new products or modified products; |
• | operating restrictions; |
• | withdrawing 510(k) clearances or IDE/premarket approvals that have already been granted; |
• | refusal to grant export approval or issue export documentation for our products; |
• | import holds; or |
• | criminal prosecution. |
15
Our existing quality management system passed a European Notified Body audit in 2015, and it was determined that we are in compliance with the requirements of the ISO 13485 standard. Through such compliance with the ISO 13485 standard, we benefit from a presumption of conformity with the relevant quality system requirements laid down in the Annexes to Council Directive 93/42/EEC concerning medical devices (Medical Devices Directive). If we fail to maintain compliance with the FDA requirements or maintain compliance with the ISO 13485 standard and the relevant quality system requirements laid down in the Annexes to the Medical Devices Directive in the future, we may be subject to enforcement action by the FDA, the U.S. Department of Justice and/or the competent authorities of the European Economic Area ("EEA"), the suspension or withdrawal of our CE Certificate of Conformity by our Notified Body or we may be required to cease all or part of our manufacturing operations for some period of time until we can demonstrate that appropriate steps have been taken to comply with such standards.
Our facility and our clinical investigational sites operate under procedures that govern the conduct and management of FDA-regulated clinical studies. The FDA conducted a Bioresearch Monitoring or BiMo inspection in September 2015 at the Hansen Medical facility and no findings were reported. A BiMo inspection assesses compliance with applicable regulatory requirements, including those at 21 CFR Parts 50, 54, 56 and 812, our procedures and the applicable clinical protocol. If the FDA were to find that we or our clinical investigators are not operating in compliance with applicable regulations, we could be subject to enforcement action as well as FDA refusal to accept all or part of our data in support of a 510(k) notification or PMA and/or we may need to conduct additional studies.
Our manufacturing facility also has been inspected and licensed by the California Department of Health Services, or CDHS, and remains subject to re-inspection at any time. Failure to maintain a license from the CDHS or to meet the inspection criteria of the CDHS would disrupt our manufacturing processes. If an inspection by the CDHS were to indicate that there are deficiencies in our manufacturing process, we could be required to take remedial actions at potentially significant expense, and our facility may be temporarily or permanently closed.
Force Dimension Development and Supply Agreement
On November 9, 2004, we entered into a Development and Supply Agreement with Force Dimension Sàrl, a Swiss limited liability company. Pursuant to the terms of the agreement, Force Dimension manufactures and supplies to us specially-configured motion controllers in accordance with a predefined pricing matrix. We may terminate the agreement for any reason upon 30 days’ notice to Force Dimension, provided that we will remain obligated to purchase all delivered and ordered master input devices at the time of such termination. Either party may terminate the agreement for a material breach by the other party if the material breach is not cured within 90 days of notice of the material breach. Force Dimension is a single-source supplier for the motion controllers in our Sensei System and our Magellan System.
Reimbursement
We expect that healthcare facilities and physicians in the United States will continue to bill various third-party payers, such as Medicare, Medicaid, other governmental programs and private insurers, for services performed using our products. We believe that procedures performed with our products are generally already reimbursable under governmental programs and most private plans and claims for services using our products are generally reimbursed under existing billing codes. We cannot be certain, however, that current coverage, coding and reimbursement policies of third-party payers will continue or the extent to which future changes to coding, coverage and reimbursement policies will affect some or all of the procedures that would use our robotic systems.
Future legislation, regulation or coverage and reimbursement policies of third-party payers may adversely affect the demand for our products (currently marketed and under development) and limit our ability to profitably sell our products. For example, the Budget Control Act of 2011, enacted on August 2, 2011, established a process to reduce federal budget deficits through an automatic “sequestration” process beginning in January 2013 if deficit reductions targets were not otherwise reached. Under the terms of the Budget Control Act, sequestration imposes across-the-board cuts to a wide range of federal programs, including but not limited to Medicare payments to plans and providers (subject to a 2% cap), in the years 2013 to 2021. Medicare payments to medical providers and health plans were reduced by 2% beginning in April 2013. The Bipartisan Budget Act of 2013, enacted on December 26, 2013, extends these cuts to 2023. Such changes to the reimbursement rates for procedures in which our products are used, or alternative budget proposals that include reductions in health care spending, could adversely impact our business. In addition, the Medicare payment systems for hospitals (inpatient and outpatient) and physicians are updated annually and reimbursement rates can vary from year to year based on a number of legislative, regulatory, and/or other policy changes. For example, there is a statutory formula known as the sustainable growth rate, or SGR, formula for determining annual payment updates for physicians’ professional services. For several years, this statutory formula has resulted in significant decreases to physician reimbursement, but Congress has repeatedly enacted legislation to
16
temporarily block the cuts. Most recently, Congress included a provision in the Pathway for SGR Reform Act of 2013, enacted on December 26, 2013, to prevent a scheduled 20.1% cut from going into effect; instead, Medicare physician payment rates generally increased by 0.5% through March 31, 2014. If Congress fails to intervene in the future to block schedule physician reimbursement reductions under the SGR formula, or if future legislation decreases Medicare payments to physicians, it could adversely impact our business.
Reimbursement for the treatment of patients with our products in EEA countries is governed by complex mechanisms established on a national level in each country. These mechanisms vary widely between the EEA countries. Moreover, these mechanisms evolve constantly, reflecting the efforts of these countries to reduce public spending on healthcare. As a result, obtaining reimbursement for the treatment of patients with medical devices has become more and more challenging. We cannot, therefore, guarantee that the treatment of patients with our products would be reimbursed in any of these countries.
We are aware that physicians may elect to use products we sell for off-label indications, including, for example, the use of our Sensei System for procedures to treat atrial fibrillation in the United States. We believe that both physicians and hospitals are currently reimbursed for these and certain other procedures even when the procedures are performed off-label using other manufacturers’ products. We cannot be certain, however, that third-party payors will continue to provide coverage and/or reimbursement to physicians and hospitals for off-label use of products to treat atrial fibrillation or any other procedures. In addition, we cannot be certain that third-party payors will not require extensive clinical support showing the efficacy and cost effectiveness of off-label uses of our products before providing coverage and reimbursement for such procedures. If such support is required, we may not be able to satisfy such requests within the limitations of our FDA cleared labeling or the intended purposes for which our devices are CE marked.
Intellectual Property
Since our inception, our strategy has been to patent the technology, inventions and improvements that we consider important to the development of our business and technology. Our intellectual property portfolio, including patents and patent applications that we own or license, covers key aspects of our Sensei and Magellan Systems and catheter products, as well as other technology that we have under development. As a result, we believe that we are building an extensive intellectual property portfolio to protect the fundamental scope of our technology, including our robotic technology, navigational methods, procedures, systems, disposable interventional devices and our three dimensional integration technology. As of December 31, 2015, we licensed or owned 111 issued U.S. patents and 52 pending U.S. patent applications, 37 granted foreign patents and over 14 pending foreign applications. We also share the rights to a number of patents and patent applications under the September 2005 cross license agreement and the January 2010 cross license agreement with Intuitive Surgical, Inc. ("Intuitive"), which in turn shares rights to certain of our patents and pending patent applications pursuant to the cross license agreements. In addition, we received a license to certain patents and patent applications licensed or owned by Luna Innovations Incorporated, or Luna, as part of the litigation settlement entered in January 2010. Some of our rights in these patents licensed from Luna have in turn been sublicensed to Philips in connection with our February 2011 agreements. We also have a number of invention disclosures under consideration and several new patent applications that are being prepared for filing, and we continue to gain the benefit of certain new patent applications and patents by virtue of the cross license agreement with Intuitive. Accordingly, we anticipate that the number of pending patent applications and patents in our portfolio will increase.
In addition to our existing patent coverage that we expect to build upon, we believe it would be technically difficult and costly to reverse engineer our products and technology. Further, we have developed substantial know-how in robotic design, catheter design and manufacturing and robotic instrument control which we maintain as trade secrets or copyrighted software.
Further successful commercializing of our Sensei System, our Magellan System and any other products we may develop, will depend in part on our not infringing patents held by third parties. It is possible that one or more of our products, including those that we have developed in conjunction with third parties, infringes existing patents. From time to time, we receive letters from one or more third parties alleging that certain aspects of our systems infringe issued patent(s) or asking us to consider licensing their patent rights. While we do not believe that our systems infringe any valid and enforceable patent of any third party, there can be no assurance that any third party will not take further action, such as filing a patent infringement lawsuit, including a request for injunctive relief, to bar the manufacture and sale of our systems in the United States or elsewhere. There also can be no assurance that we will not seek to take the initiative in defending ourselves by instituting litigation against such third party challenges.
We have applied for trademark registration of, and claim trademark rights in, “Magellan” and “Hansen Medical Magellan.” We have obtained trademark registration for, and claim trademark rights in “Artisan Extend,” “Hansen Medical,” “Hansen Medical (with Heart Design),” “Heart Design (Logo),” “Sensei,” “Artisan,” “Instinctive Motion,” "CoHesion™" and "IntelliSense Technology™" . This report also includes other trademarks, service marks and trade names of other companies.
17
Cross License Agreement with Intuitive Surgical
On September 1, 2005, we entered into a cross license agreement with Intuitive. Pursuant to this agreement, Intuitive granted us a co-exclusive, worldwide license in the field of intravascular approaches for the diagnosis and treatment of cardiovascular, neurovascular and peripheral vascular diseases. In return, we granted Intuitive a co-exclusive, worldwide license in the fields of endoscopic, laparoscopic, thoracoscopic or open diagnosis and/or surgical procedures, including endoluminal applications in gastrointestinal, respiratory, ear, nose and throat, urologic and gynecologic surgery. These licenses cover our and Intuitive’s patents and patent applications that were filed on or prior to the date of the agreement, as well as later filed divisionals, continuations and continuations in part with respect to the matters that were part of the original patents and patent applications as of the date of the agreement, but not any other later-filed patents and patent applications. In addition, these licenses cover all trade secrets and other know-how that we and Intuitive disclosed to each other prior to the date of the agreement. Each party retained full rights to practice its own technology for all purposes. As consideration for the licenses granted by Intuitive, we issued 125,000 shares of our Series B preferred stock to Intuitive in 2005 (which converted into 12,500 shares of our common stock at the time of our initial public offering) and owe royalties to Intuitive on certain product sales, including annual minimum royalties of $200,000. We will not receive any royalties or other compensation from Intuitive under the agreement.
Each party has agreed not to engage in activities outside its licensed field that, to its knowledge, would infringe the other party’s licensed patents. Although we believe that there are opportunities for us to operate outside the licensed field of use without the use of the Intuitive intellectual property, Intuitive, from time to time has told us that it believes certain of our past activities that have fallen outside the licensed field have infringed its intellectual property rights. Although we disagree with Intuitive’s position, we presently remain focused within our licensed field and so have agreed to inform Intuitive before commencing any further outside clinical investigations for endoluminal applications or engaging in external technology exhibitions at non-intravascular conferences. There can be no assurance that Intuitive will not challenge any activities we engage in outside the intravascular space and we cannot be sure that in the event of such a challenge we would be able to reach agreement with Intuitive on whether activities outside our licensed field may be conducted without the use of Intuitive’s intellectual property. Any disputes regarding a party’s potential infringement of the other party’s licensed patents that cannot be resolved through discussions between the parties will be settled by litigation. If such litigation results in a judgment of infringement that cannot be appealed and the infringing party fails to cease such infringement within a specified cure period, the non-infringing party will have the right to terminate the agreement. The parties have also agreed on a procedure under which either party may, but is not obligated to, ask an arbitration panel to make a binding determination as to whether or not a new product being developed by such party would, if commercialized outside such party’s licensed field, infringe any issued patents of the other party.
The agreement may be terminated by either party for bankruptcy of the other party. We also have the right to terminate the agreement at any time on or after March 1, 2018, and if we exercise this termination right, the licenses granted to us by Intuitive will terminate, but the licenses granted by us to Intuitive will survive. Neither party is permitted to terminate the agreement based on a breach by the other party, except in the event of the other party’s failure to cease infringing activity as described above or to remedy a significant payment default that has been established through a court judgment that cannot be appealed. If a party terminates the agreement for one of these types of breaches, the licenses granted by this party will terminate, but the licenses granted to this party will survive. In the absence of any early termination, the agreement will expire upon the expiration of the last to expire of the patents licensed under the agreement.
On January 12, 2010, in association with the agreements signed as a result of our settlement with Luna, we entered into a cross license agreement with Intuitive, under which we and Intuitive granted each other royalty-free, non-exclusive licenses within the medical robotics field to certain fiber optic shape sensing/localization technology owned or in-licensed by each party as of the effective date of the agreement or within the five-year period after the effective date of the agreement. The non-exclusive licenses can only be sublicensed in connection with each party’s respective products, except for our right to sublicense for single degree of freedom medical devices.
In addition, we and Intuitive granted each other royalty-free, co-exclusive licenses within the medical robotics field to certain fiber optic shape-sensing/localization technology developed in whole or in part by Luna for each party. The co-exclusive licenses can only be sublicensed in connection with each party’s respective products, except that we may freely sublicense single degree of freedom medical devices. We have the right, along with Intuitive, to enforce the co-exclusively licensed intellectual property. The term of this agreement is until the expiration of the last to expire of the patents licensed under the agreement, unless extended or shortened by mutual agreement.
In October 2012, we signed an amendment to our license agreement with Intuitive under which Intuitive paid us a $20 million licensing fee. The amendment of the license agreement is an update to the co-exclusive cross license agreement signed by the companies in 2005. Under the terms of the amended agreement, Intuitive's existing co-exclusive rights to our patent
18
portfolio to certain non-vascular procedures have been extended to include patents filed or conceived by us subsequent to the original 2005 agreement up to and including the period three years subsequent to the amendment though we have no obligations to conduct any research activities under the amendment. In addition, the first sentence of the definition of “Hansen Field of Use” was modified by the Amended License Agreement to read: “Hansen Field of Use” means the research, development, manufacture, use, sale, promotion, distribution and importation of medical devices and systems for intravascular approaches for the diagnosis and/or treatment of cardiovascular, neurovascular, and peripheral vascular diseases, wherein the distal end of the medical device or system, after entering a blood vessel, remains within the blood vessel or branches of the blood vessel for the delivery of the diagnostic or therapeutic modality for which the device or system is being used.” We retain the right to use our intellectual property for all clinical applications, both vascular and non-vascular. Under the terms of the amended agreement, Intuitive’s co-exclusive rights extended to include intellectual property rights filed or conceived by the Company in the non-vascular space subsequent to the original 2005 agreement through October 26, 2015. The term of the intellectual property capture period expired on October 26, 2015. The cross-licenses and royalty obligations remain in effect.
License Agreement with Mitsubishi Electric Research Laboratories
On March 7, 2003, we entered into a License Agreement with Mitsubishi Electric Research Laboratories, Inc. ("Mitsubishi"). Pursuant to this agreement, we obtained an exclusive, worldwide license to certain Mitsubishi patents and related know-how for use in the field of therapeutic or diagnostic vascular or endoluminal intervention involving robotics, automation or telemanipulation. In consideration for such license, we issued 938 shares of our common stock to Mitsubishi and paid commercialization milestones. Additionally, we owe minimum annual royalties of $100,000 which reduces to $55,000 if the license becomes non-exclusive and royalties on certain product sales, subject to an annual royalty cap. Under the agreement, we are obligated to use reasonable commercial efforts to commercialize royalty-bearing products. The agreement may be terminated by Mitsubishi in the event of an uncured material breach by us. In addition, we can terminate the agreement for any reason with advanced written notice to Mitsubishi.
License Agreement with Luna Innovations Incorporated
On January 12, 2010, we entered into license agreement with Luna. Under the license agreement, Luna granted us a co-exclusive (with Intuitive), royalty-free, fully paid, perpetual and irrevocable license to Luna’s fiber optic shape sensing/localization technology within the medical robotics field. The license can only be sublicensed in connection with our products, except that we can grant full sublicenses to third parties for single degree of freedom medical devices. Intuitive received a corresponding co-exclusive license within the medical robotics field from Luna under a separate license agreement between Intuitive and Luna. Under the license agreement, Luna also granted to us a royalty-free, fully paid, perpetual and irrevocable license to Luna’s fiber optic shape-sensing/localization technology for non-robotic medical devices, which license is exclusive (and fully sublicenseable) within the orthopedics, vascular and endoluminal fields and otherwise co-exclusive with Luna. We have the right along with Intuitive to enforce the intellectual property licensed by Luna within the medical robotics field.
The license agreement provides for us to grant to Luna a nonexclusive, sublicenseable, royalty-free, fully paid, perpetual and irrevocable license under certain of our fiber optic shape sensing/localization technology outside of the medical robotics field and outside the orthopedics, vascular and endoluminal fields. In this agreement, Luna confirmed our ownership of certain intellectual property developed in whole or in part by Luna under the parties’ prior development agreement as well as ownership of certain Hansen patents in the event it is determined that any Luna personnel are inventors with respect to such patents.
In January 2014, Luna sold to Intuitive substantially all of its assets related to its medical shape sensing business, including all of the patents and patent applications used or useful for its fiber optical shape sensing and localization technology. Luna’s transfer of technology to Intuitive was made subject to their existing licenses and related obligations with us and Philips and we have not assigned or transferred any of our agreements with Luna to Intuitive.
Agreements with Philips
In the fourth quarter of 2009, we entered into an extended joint development agreement with Philips Electronics, N.V. , or Philips. Under the terms of the agreement, we have, with support and collaboration from Philips, developed a vascular robotics platform and associated catheters, or Vascular System. The Vascular System does not include our Sensei Robotic Catheter System or any system used for endoluminal, cardiac or other non-vascular procedures. Pursuant to the agreement, Philips partially funded our development costs based upon our achievement of development milestones for the Vascular System and will receive royalties based on sales of the Vascular System subject to caps. In February 2011, we amended the extended joint development agreement, which increased the amount of funding provided by Philips for the development of the Vascular System and extended and increased the royalties to be paid to Philips. We reached the final milestone under the agreement and
19
received our final payment from Philips in October 2011. We will pay Philips royalties based on the number of Magellan Systems and Magellan Robotic Catheters that are sold, subject to caps, through October 2017.
In February 2011, we entered, directly and through a wholly-owned subsidiary, into patent and technology license, sublicense and purchase agreements with Philips to allow them to develop and commercialize the non-robotic applications of our Fiber Optic Shape Sensing and Localization, or FOSSL, technology. Under the terms of the FOSSL agreements, Philips has the exclusive right to develop and commercialize the FOSSL technology in the non-robotic vascular, endoluminal and orthopedic fields. Philips also receives non-exclusive rights in other non-robotic medical device fields, but not to any multi-degree of freedom robotic applications. If Philips does not meet certain specified commercialization obligations, we have the right to re-acquire the licenses granted to Philips for pre-determined payments, which payments in the aggregate would be greater than the upfront payment amounts we received from Philips in connection with the agreements related to the FOSSL technology. The FOSSL agreements also contains customary representations, warranties and indemnification provisions by each party.
Each party may terminate the FOSSL agreements for material breach by the other party. Philips also has the right to terminate the FOSSL agreements and its rights under the agreement if we are acquired by a competitor of the relevant business unit of Philips. In connection with the FOSSL agreements, we received upfront payments of $23.0 million and will be eligible to receive up to an additional $78.0 million in future payments associated with the successful commercialization by Philips or its collaborators of products containing FOSSL technology. Approximately two-thirds of these potential future payments could arise from Philips’ sublicensing the FOSSL technology and approximately one-third of the potential future payments are based on Philips’ royalty obligations on its sales of products containing the FOSSL technology. We would receive less than half of Philips’ proceeds for its sublicensing FOSSL technology, if and following Philips entering into an applicable sublicensing transaction. Philips’ FOSSL-related royalty obligations are calculated on a consistent annual basis between 2014 and 2020 and arise in any year only to the extent that Philips achieves a substantial number of commercial placements of FOSSL-enabled products in the calendar year.
In August 2015, we entered into an amendment with Philips to amend the FOSSL Agreement and the JDA (the “Amended Agreements”), extinguishing the Company’s rights to re-acquire the licenses in the non-robotic vascular, endoluminal and orthopedic fields in FOSSL technology in exchange for a reduction of all royalties owed or due between the parties of fifty percent (50%). Under the Amended Agreements, the Company’s royalty obligations to Philips based on sales of the Vascular Systems were reduced by fifty percent (50%). The Company’s royalty obligations continue through October 2017. Similarly, Philips royalty obligations were also all reduced by fifty percent (50%), including but not limited to all royalty obligations due under the Amended Agreements that arise from Philips’ sublicensing the FOSSL technology. Under the Amended Agreements, Philips’ FOSSL-related royalty obligations will be calculated on a consistent basis for the royalty period starting with the first calendar year after the first sale by Philips of a FOSSL system and ending with the sixth calendar year after the first sale, but only to the extent that Philips achieves certain number of commercial placements of FOSSLenabled products during the royalty period. The Amended Agreements contain customary representations, warranties and indemnification provisions by each party. Each party may terminate the agreements for material breach by the other party. Philips also has the right to terminate the agreement and its rights under the agreement if the Company is acquired by a competitor of the relevant business unit of Philips.
Under the terms of the Amended Agreements, we continue to have no minimum obligation with Philips, and the royalty obligation is based on per unit sales of the Magellan Systems and Vascular Catheters.
Competition
The markets for medical devices are intensely competitive and are characterized by rapid technological advances, frequent new product introductions, evolving industry standards and price erosion.
We believe that the principal competitive factors in our market include:
• | safety, efficacy and high-quality performance of products; |
• | integration with a three-dimensional visualization methodology; |
• | ease of use and comfort for the physician; |
• | cost of capital equipment and disposable products, including the cost of installation and maintenance; |
• | eligibility for coverage and reimbursement; |
• | procedure times and improved clinical outcomes for patients; |
• | effective sales, marketing and distribution; |
• | brand awareness and strong acceptance by healthcare professionals, hospital administration and patients; |
20
• | training, service and support and comprehensive education for patients and physicians; and |
• | intellectual property leadership and superiority. |
We consider our primary competition for our robotic systems to be in the following areas:
• | Drug therapies. Drug therapy is currently considered the first line treatment for electrophysiological conditions such as atrial fibrillation. As a result, physicians typically attempt to treat these conditions with drugs designed to control heart rate and heart rhythm before indicating interventional procedures. Among atrial fibrillation patients, long-term efficacy of medications is poor in 60% to 80% of patients, per GlobalData, Electrophysiology – Global Analysis and Market Forecasts report in March 2014. Atrial fibrillation patients who do respond well to medication are not considered candidates for interventional treatment. Therefore, we face competition with the companies who currently market or are developing drugs or gene therapies to treat electrophysiological conditions such as atrial fibrillation. To the extent that more effective drug therapies are developed and approved for use in treating EP conditions such as atrial fibrillation, we will face increased competition. |
• | Manual catheter-based interventional techniques. The vast majority of interventional EP and peripheral vascular procedures performed today are performed with several types of hand-held catheters including Medtronic, Inc.’s Cryoablation products for EP and manual sheaths and guiding catheters for peripheral vascular procedures. These products evolve rapidly, and their manufacturers are constantly attempting to make them more efficacious in performing the broad range of endovascular procedures conducted by endovascular physicians. |
• | Magnetic guidance systems for steering catheters. Stereotaxis, Inc. markets a system that has been on the market in the United States and in Europe since 2003 and that uses magnets to control the working tip of catheters and other control catheters during interventional EP and other procedures. Because the system was introduced prior to our Sensei System and has a significant installed base, we believe it currently leads the market for guidance systems for controlling the working tip of catheters and catheter-based technologies. |
• | New approaches. We expect to face competition from companies that are developing new approaches and products for use in interventional procedures. Some of these companies may attempt to use robotic techniques to compete directly with us, such as Corindus, Inc., Catheter Robotics, Inc. and Verb Surgical, Inc. We believe, however, that several of these solutions merely involve robotic control of typical manual devices like off the shelf wires and catheters. We believe that, solely from a clinical perspective, the inability to more effectively control these devices robotically at their tip is a significant departure from the value we have realized through the application of flexible robotics. Many potential competitors also have an established presence in the field of interventional cardiology, including the major imaging, capital equipment and disposables companies that are currently selling products in the EP and vascular lab. |
The use of catheters and catheter-based technologies is common for a broad range of interventional procedures in cardiology, vascular and in other medical specialties. Other companies may market guidance systems for use in complex vascular procedures as well as other uses. In addition, we believe that Intuitive is developing a system to guide flexible medical devices in fields such as urology, gynecology, gastrointestinal disease, and other medical fields outside of cardiology. While they may not use our patents in EP, cardiology or vascular procedures, Intuitive may attempt to compete directly with us in EP and cardiology, and will likely compete with us if we decide to offer products outside of our licensed field of treating cardiovascular, neurovascular and peripheral vascular diseases. We also face competition from large medical device companies that have significantly greater financial and human resources for product development, sales and marketing, and patent litigation. Large medical device companies such as Johnson & Johnson, St. Jude Medical, Boston Scientific and others, as well as a variety of smaller innovative companies, such as Stereotaxis, Inc., are also expected to be targeting the EP and cardiology markets for guiding flexible medical devices. Increased competition could result in price reductions, reduced net revenue and profit margins and loss of market share, any of which could harm our business. See Item 1A, “Risk Factors,” for further discussion of risks regarding competition.
Government Regulation
The healthcare industry, and thus our business, is subject to extensive federal, state, local and foreign regulation. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. In addition, these laws and their interpretations are subject to change.
Both federal and state governmental agencies continue to subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. As indicated by work plans and reports issued by these agencies, the federal government will continue to scrutinize, among other things, the billing practices of healthcare providers and the marketing of healthcare products. The federal government also has increased funding in recent years to fight healthcare fraud, and various agencies, such as the U.S. Department of Justice, the Office of Inspector General of the Department of Health and Human Services, or OIG, and state Medicaid Fraud Control Units, are coordinating their enforcement efforts.
21
We believe that we have structured our business operations and relationships with our customers to comply with all applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise. In addition, because there is a risk that our products are used off label, we believe we are subject to increased risk of prosecution under these laws and by these entities even if we believe we are acting appropriately. We discuss below the statutes and regulations that are most relevant to our business and most frequently cited in enforcement actions.
U.S. Food and Drug Administration Regulation
The FDA strictly regulates medical devices under the authority of the Federal Food, Drug and Cosmetic Act, or FFDCA, and the regulations promulgated under the FFDCA. The FFDCA and the implementing regulations govern, among other things, the following activities relating to our medical devices: preclinical and clinical testing, design, manufacture, safety, efficacy, premarket clearance and approval, labeling, storage, record keeping, sales and distribution, product import and export, postmarket adverse event reporting, postmarket studies and surveillance, recalls, and advertising and promotion.
Our medical devices are categorized under the statutory framework described in the FFDCA. This framework is a risk-based system that classifies medical devices into three classes from lowest risk (Class I) to highest risk (Class III). In general, Class I devices are subject to only general controls (e.g., establishment registration and listing products with the FDA, recordkeeping, labeling, reporting adverse events, good manufacturing practice requirements, and prohibitions against adulteration and misbranding). In some cases, a Class I device may not be marketed until the FDA clears a 510(k) notification via the 510(k) clearance process described below. Class II devices are also subject to general control, but also may be subject to special controls such as performance standards and the FDA guidelines that are not applied to Class I devices. Most Class II devices may not be marketed until the FDA clears a 510(k) notification via the 510(k) clearance process described below. Finally most Class III devices may not be marketed until FDA approves a premarket approval application, or PMA, via the PMA approval process described below. Class III devices are those deemed by the FDA to pose the greatest risk, such as a life-sustaining, life-supporting or implantable device, or a device deemed not substantially equivalent to any previously cleared 510(k) device. Both 510(k) notifications and PMA require the payment of user fees at the time of submission for FDA review. The currently-cleared uses of our Sensei/Artisan and Magellan products are classified in Class II, but future applications such as ablation in treatment of specific arrhythmias, could be classified in Class III.
The 510(k) Clearance Process. In the 510(k) process, the FDA reviews a premarket notification and determines whether or not a proposed device is “substantially equivalent” to a previously cleared 510(k) device, a device that has been reclassified from Class III to Class I or Class II, or a device that was in commercial distribution before May 28, 1976 for which the FDA has not yet called for the submission of premarket approval applications, any of which is referred to as a “predicate device.” In making this determination, the FDA compares the proposed device to the predicate device. If the new device is substantially equivalent in intended use and safety and effectiveness to the predicate device, the new device may be cleared for marketing. FDA has committed to review most premarket notifications within 90 days, but the review clock may be stopped due to requests for additional information. A decision may take significantly longer and clearance is never guaranteed. In reviewing a premarket notification, the FDA may request additional information, including clinical data. Recent changes to FDA’s expectations for data support for 510(k) clearance may require the submission of more clinical or pre-clinical data than has previously been required. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require PMA approval. The FDA requires each manufacturer to make this determination in the first instance, but the agency can review any such decision. If the FDA disagrees with a manufacturer’s decision not to seek a new 510(k) clearance, the agency may retroactively require the manufacturer to seek 510(k) clearance or PMA approval. The FDA also can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or PMA approval is obtained. Also, the manufacturer may be subject to significant administrative or judicial sanctions, as described below.
In May 2007, the FDA cleared our Sensei System and Artisan Extend Control Catheter for promotion for use in mapping the heart anatomy with two specified mapping catheters. When the FDA cleared our technology for promotion in the U.S., it concluded that there is a reasonable likelihood that our products will be used for an intended use not identified in the proposed labeling and that such statutory uses could cause harm. The FDA therefore required that we label our products in the United States with language that the safety and effectiveness of our products for use with cardiac ablation catheters, in the treatment of cardiac arrhythmias including atrial fibrillation, have not been established. Although the FDA did not require contraindication of this use in the labeling, the FDA, with supporting data (for example, based on observed trends in postmarket adverse event reports) could later choose to require a specific contraindication for use in cardiac ablation procedures. We plan to seek FDA clearance or approval for labeling that includes ablation procedures but we cannot assure you that the FDA will grant such clearance or approval for our Sensei System and related catheters for use in such procedures.
22
In 2012, the FDA cleared our Magellan System for use to facilitate navigation to anatomical targets in the peripheral vasculature. Our FDA cleared labeling does not further specify the scope of the targets within the peripheral vasculature that are encompassed in the 510(k) clearance. FDA could disagree with our interpretation of the scope of this clearance. If the FDA concludes that our promotional materials exceed the scope of this clearance, the agency may retroactively require us to seek 510(k) clearance or PMA approval. The FDA also can require us to cease marketing for any claims beyond the scope of the clearance and we are subject to the administrative or judicial sanctions described below.
In February 2014, the FDA cleared our Magellan 6Fr Robotic Catheter for use to facilitate navigation to anatomical targets in the peripheral vasculature. In August 2014, the FDA cleared our Sensei X2 System and Magellan Transport System for sale in the U.S. In July 2015, the FDA cleared our Magellan 10Fr Robotic Catheter for use in the peripheral vasculature.
The PMA Approval Process. A PMA must be submitted if a device cannot be cleared through the 510(k) process. The PMA process is generally more costly and time consuming than the 510(k) process. A PMA must be supported by extensive data (including, but not limited to, technical, preclinical, and clinical data), manufacturing information and proposed labeling to demonstrate to the FDA’s satisfaction the safety and effectiveness of the device for its intended use. After a premarket approval application is sufficiently complete, the FDA will accept the application and begin an in-depth review of the submitted information. During this review period, the FDA may request additional information or clarification of information already provided. FDA may also convene an advisory panel of experts from outside the FDA to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The panel’s recommendation is given great weight, but is not dispositive of the agency’s decision. Prior to approving the PMA, the FDA will conduct an inspection of the manufacturing facilities and a number of the clinical sites where the supporting study was conducted. The facility inspection evaluates compliance with the Quality System Regulation, or QSR, which impose elaborate testing, control, documentation and other quality assurance procedures in the manufacturing process. FDA has committed to review most PMAs within 180 days where an advisory panel is not required and within 320 days where an advisory panel is required, but the review clock may be stopped due to requests for additional information. A decision may take significantly longer and approval is never assured. The FDA may approve the PMA with post approval conditions intended to ensure the safety and effectiveness of the device including, among other things, restrictions on labeling, promotion, sale and distribution. Failure to comply with the conditions of approval can result in material adverse enforcement action, including the loss or withdrawal of the approval. Even after approval of a PMA, a new PMA or PMA supplement is required in the event of a modification to the device, its labeling or its manufacturing process. Supplements to a PMA often require the submission of the same type of information required for an original PMA, except that the supplement is generally limited to that information needed to support the proposed change from the product covered by the original PMA.
Clinical Trials. Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) clearance. Such trials, if conducted in the United States, generally require an investigational device exemption application, or IDE, approved in advance by the FDA for a specified number of patients and study sites, unless the product is deemed an non-significant risk device eligible for more abbreviated IDE requirements. Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an institutional review board, or IRB, for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. To conduct a clinical trial, we also are required to obtain the patients’ informed consent that complies with FDA requirements, state and federal privacy regulations and human subject protection regulations. We, the FDA or the IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Additionally, we may decide at any time, for business or other reasons, to terminate a study. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device, may be equivocal, or may otherwise not be sufficient to obtain FDA clearance or approval to market the product in the U.S. Following completion of a study, we would need to collect, analyze and present the data in an appropriate submission to the FDA, either a 510(k) premarket notification or a PMA. In addition, FDA may perform a BiMo inspection of a study and if it finds deficiencies, we will need to expand resources to correct those deficiencies, which may delay clearance or approval or the deficiencies may be so great that FDA could refuse to accept all or part of our data or trigger enforcement action.
We have received approval of an IDE application to investigate the use of our Sensei System and Artisan Extend family of catheters in the treatment of atrial fibrillation in a clinical study designed to support the expansion of our current labeling in the U.S. beyond mapping. We enrolled our first patient in May 20150 and the study, which involved the treatment of atrial fibrillation has enrolled 150 patients. The study includes a seven-day follow-up for safety and a one-year follow-up for efficacy at intervals of 90, 180 and 365 days. If successful, we intend to use the data from this study to support a submission to the FDA to obtain clearance or approval for use in atrial fibrillation procedures.
Pervasive and Continuing Regulation. After a device is placed on the market, numerous FDA regulatory requirements apply. These include:
23
• | product listing and establishment registration (with the payment of associated user fees), which helps facilitate FDA inspections and other regulatory action; |
• | Quality System Regulation, or QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the development and manufacturing process; |
• | labeling regulations and various statutory provisions, which prohibit false or misleading labeling as well as the promotion of products for uncleared, unapproved or off-label uses or indications; |
• | clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of cleared devices; |
• | approval of product modifications that affect the safety or effectiveness of approved devices; |
• | medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
• | post-approval restrictions or conditions, including post-approval study commitments; |
• | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
• | the FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
• | regulations pertaining to voluntary recalls; and |
• | reporting to the FDA of corrections or removals. |
Advertising and promotion of medical devices, in addition to being regulated by the FDA and U.S. Department of Justice, are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. Accordingly, we may not market or promote our Magellan and Sensei Systems for any off-label use. For example, the FDA has taken the position that we are not permitted to promote our Sensei System in the United States for use with any other mapping catheter other than the two specified in our 510(k) clearance, use with any ablation catheters, or in any other procedure such as ablation procedures. The FDA has specifically indicated that the commercial distribution of these devices for use in ablation procedures will require us to obtain a new 510(k) clearance or, more likely, PMA approval with significant clinical data. If the FDA determines that our promotional materials or training constitutes promotion of an unapproved use, it could request that we modify our training or promotional materials or subject us to administrative or judicial sanctions, as described below. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and adoption of the products would be impaired.
Furthermore, our products could be subject to voluntary recall if we or the FDA determine, for any reason, that our products pose a risk of injury or are otherwise defective. Moreover, the FDA can order a mandatory recall if there is a reasonable probability that our device would cause serious adverse health consequences or death.
The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations, and these inspections may include the manufacturing facilities of some of our subcontractors. In particular, we and our suppliers are required to comply with the FDA’s QSR, and International Standards Organization, or ISO, regulations for the manufacture of our products and other regulations which cover the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval. The failure by us or one of our suppliers to comply with applicable statutes and regulations administered by the FDA and other regulatory bodies, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, enforcement actions. We underwent an FDA inspection, which employed the Quality System Inspection Technique, or QSIT, in 2014 and received one inspectional observation. We received the establishment inspect report (EIR) from the FDA on January 26, 2015 after the agency closed the inspection per CFR 20.64.
In addition, later discovery of previously unknown problems with our robotic systems, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its
24
clearance or off-label by a physician in the practice of medicine, or observations found during a future inspection, could result in enforcement action by the FDA or other regulatory authorities.
If the FDA were to find that we are not operating in compliance with applicable regulations, we could be subject to enforcement action by the FDA and/or the U.S. Department of Justice, which may include administrative or judicial sanctions:
Foreign Regulation
In order for us to market our products in other countries, we must comply with extensive safety and quality regulations in other countries. These regulations, including the requirements for approvals, clearance, or grant of Conformité Européene ("CE") Certificates of Conformity and the time required for regulatory review, vary from country to country. Failure to obtain regulatory approval, clearance, or CE Certificates of Conformity (or equivalent) in any foreign country in which we plan to market our products may harm our ability to generate revenue and harm our business.
The primary regulatory environment in Europe is that of the European Economic Area, or EEA, which is comprised of the 28 Member States of the European Union, or EU, Iceland, Liechtenstein and Norway. In the EEA, our devices are required to comply with the Essential Requirements laid down in Annex I to the EU Medical Devices Directive (applicable in the non-EU EEA Member States via the Agreement on the European Economic Area). We are also required to ensure compliance with the relevant quality system requirements laid down in the Annexes to the Medical Devices Directive. Compliance with these requirements entitles us to affix the CE Mark to our medical devices, without which they cannot be commercialized. To demonstrate compliance with the Essential Requirements laid down in Annex I of the Medical Devices Directive obtain the right to affix the CE Mark to our medical devices, and thus be permitted to place our medical devices, and/or put them into service, on the EEA market, we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. With the exception of low risk medical devices (Class I devices with no measuring function and which are not sterile), in relation to which the manufacturer may issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the Essential Requirements laid down in the Medical Devices Directive, a conformity assessment procedure requires the intervention of a Notified Body. This is an organization designated by the competent authorities of an EEA country to conduct conformity assessments. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine products’ Technical File and the quality system for the manufacture, design and final inspection of our devices before issuing a CE Certificate of Conformity. This Certificate demonstrates substantive compliance with the relevant Essential Requirements laid down in Annex I of the Medical Devices Directive or the relevant quality system requirements laid down in the Annexes to the Directive and constitutes the basis for manufacturers to issue their mandatory Declaration of Conformity. Companies compliant with ISO requirements such as “ISO 13485: Medical devices — Quality management systems — Requirements for regulatory purposes” benefit from a presumption of conformity with the relevant quality system requirements laid down in the Annexes to the Medical Devices Directive. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the Essential Requirements and quality system requirements. This Certificate entitles the manufacturer to affix the CE Mark to its medical devices after having prepared and signed a related EC Declaration of Conformity. In September 2006, we received CE Certificate of Conformity from our Notified Body permitting us issue a Declaration of Conformity, affix the CE Mark and market our Sensei System in the EEA. In May 2007, we received a CE Certificate of Conformity for our Artisan Extend Control Catheter which allows us to issue a Declaration of Conformity, affix the CE Mark to market our system for ablation procedures and to market it in the EEA. In February 2013, our Notified Body issued a further CE Certificate of Conformity for our Artisan Extend Control Catheter. In July 2011, following the drawing up of our Declaration of Conformity we affixed the CE Mark for sale of our Magellan System after having received a CE Certificate of Conformity from our Notified Body and, in October 2011, we received another CE Certificate of Conformity permitting us to draw up a Declaration of Conformity and affix the CE Mark to our Magellan Robotic Catheter and related accessories. In November 2014, we received a CE Mark for the marketing of our Magellan 6Fr Robotic Catheter. In July 2015, we received FDA clearance to market our Magellan 10Fr Robotic Catheter in the U.S. In April 2015, we received CE Mark clearance to market our Magellan 10Fr Robotic Catheter for use in the peripheral vasculature.
If we modify existing products or develop new products in the future, including new devices, we will need to notify our Notified Body and go through a conformity assessment procedure before having the right to affix the CE Mark to such products. We will be subject to regulatory audits, currently conducted biannually, in order to maintain any CE Certificates of Conformity that have been issued by our Notified Body. We cannot be certain that we will be able to obtain CE Certificates of Conformity for new or modified products or that we will continue to meet the quality, safety and efficacy requirements necessary to maintain the CE Certificates of Conformity that we have already received. If we are unable to maintain our existing CE Certificates of Conformity for our products, we will no longer be able to affix the CE Mark to them and sell our products in EEA countries. We will evaluate regulatory approval in other foreign countries on an opportunistic basis.
Anti-Kickback Statutes and False Claims Acts
25
In the United States, there are federal and state anti-kickback laws that generally prohibit the offer, payment or receipt of kickbacks, bribes or other remuneration in exchange for the referral of patients or other health-related business or the prescribing or recommendation of certain products. For example, the federal healthcare program Anti-Kickback Statute prohibits persons from knowingly and willfully offering, paying soliciting or receiving remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual, or the purchasing, leasing, ordering, or arranging for or recommending the purchase, lease or order of any healthcare item or service, for which payment may be made, in whole or in part, under federal healthcare program such as the Medicare and Medicaid programs. The statute has been interpreted to apply to arrangements between medical device manufacturers on the one hand and prescribers and purchasers on the other. The definition of “remuneration” has been broadly interpreted to include anything of value, including, among other things, gifts, discounts, the furnishing of supplies or equipment, credit arrangements, payments of cash and waivers of payments. Because the federal Anti-Kickback Statute’s broad language prohibits many arrangements and practices that are permissible in businesses outside of the healthcare industry, Congress and the OIG have established statutory exceptions and regulatory safe harbors to protect arrangements that satisfy all of the relevant elements from prosecution or regulatory sanctions.
In addition, the Patient Protection and Affordable Care Act clarified that a person or entity need not have actual knowledge of the federal Anti-kickback Statute or specific intent to violate it. Penalties for violations of the federal Anti-Kickback Statute include imprisonment, criminal fines, civil monetary penalties and exclusion from participation in Medicare, Medicaid and other federal healthcare programs. In addition, under the Patient Protection and Affordable Care Act, any claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act, discussed in more detail below.
Many states have adopted laws similar to the Federal Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any payer, not only federal healthcare programs like Medicare and Medicaid. We are unable to predict how individual states will enforce their kickback statutes and increased enforcement may result in substantial fines, reputational risk, or administrative penalties. We are unable to predict whether we could be subject to actions under the federal Anti-Kickback Statute or any comparable state laws, or the impact of such actions.
Another trend affecting the healthcare industry is the increased use of the federal False Claims Act which imposes penalties against individuals or entities for, among other things, knowingly presenting, or causing to be presented, claims for payment of government funds that are false or fraudulent, or knowingly making, using or causing to be made or used a false record or statement material to an obligation to pay money to the government, or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. The False Claims Act has been used to assert liability based on allegations including off-label promotion and violations of the Anti-Kickback Statute, including but not limited to improper grants to clinical sites or hospitals, improper use of advisory boards, inappropriate relationships with physicians, improper loans or the inappropriate provision of free equipment The False Claims Act also includes “whistleblower” or “qui tam” provisions that allow a private individual, often former employees, to bring actions for violations on behalf of the government. In recent years, the number of suits brought against manufacturers and others by private individuals has increased dramatically and resulted in settlements with the government and/or private individuals worth billions of dollars. In addition, various states have enacted laws modeled after the federal False Claims Act and some states’ laws may apply more broadly to claims for items or services reimbursed regardless of payer.
Federal civil False Claims Act violations may result in treble monetary damages, civil penalties of between $5,500 to $11,000 for each separate false or fraudulent claim and exclusion from participation in federal healthcare programs. There are also criminal penalties, including imprisonment and criminal fines, for making or presenting a false or fictitious or fraudulent claim to the federal government. We are unable to predict whether we could be subject to actions under the federal civil False Claims Act or any comparable state laws, or the impact of such actions. However, the costs of defending claims under the False Claims Act or any comparable state laws, as well as sanctions imposed under the False Claims Act, could significantly affect our financial performance.
The OIG has the authority to exclude companies from federal healthcare program participation for numerous violations of civil and criminal statutes and must exclude for certain felony convictions. If the company is found liable under the federal Anti-Kickback Statute, the False Claims Act, or a number of other healthcare statutes, such as the federal criminal false statements statute, the OIG has discretion to exclude the company.
U.S. Attorneys’ offices, state attorney general offices, and other government agencies have focused their enforcement efforts a wide range of activates, including the discounting of medical devices, the provision of free equipment, the promotion of healthcare services and products and interactions with physicians, hospitals and other healthcare organizations. We are unable to predict how third parties will interpret these laws and apply the OIG guidance and third parties may challenge our practices and promotional activities under one or more of these laws. If our past or present operations are found to be in
26
violation of any of these laws, we could be subject to civil and criminal penalties, which could hurt our business, our operations and financial condition.
Interactions between medical device manufacturers and physicians are also governed by strict laws, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct in the individual EEA countries. The provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medical devices is prohibited in the EEA. The provision of benefits or advantages to physicians is also governed by the national anti-bribery laws of the EEA countries. One example is the U.K. Bribery Act 2010. This Act applies to any company incorporated in or “carrying on business” in the U.K., irrespective of where in the world the alleged bribery activity occurs. This Act could have implications for our interactions with physicians both in and outside the U.K. Violation of these laws could result in substantial fines and imprisonment.
Payments made to physicians in certain EU Member States must be publically disclosed. Moreover, agreements with physicians must often be the subject of prior notification and approval by the physician’s employer, his/her competent professional organization, and/or the competent authorities of the individual EEA country. These requirements are provided in the national laws, industry codes, or professional codes of conduct, applicable in the EEA countries. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
Federal Physician Payment Sunshine Act and state marketing and disclosure laws
As of August 1, 2013, the federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires certain medical device manufacturers to engage in extensive tracking of payments and other transfers of value to physicians and teaching hospitals, maintenance of a database containing such data, and public reporting of such data. Medical device manufacturers with products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program are required to track and report such payments. We began tracking applicable payments and transfers of value on August 1, 2013 and began reporting payment data to the Centers for Medicare & Medicaid Services in March 2014 and will continue to do so annually thereafter. Failure to comply with the reporting obligations may result in civil monetary penalties.
Several states now require medical device manufacturers to report expenses relating to the marketing and promotion or require them to implement compliance programs or marketing codes. For example, California, Connecticut and Nevada mandate implementation of corporate compliance programs, while Massachusetts and Vermont impose more detailed restrictions on device manufacturer marketing practices and tracking and reporting of gifts, compensation and other remuneration to health care providers.
Health Insurance Portability and Accountability Act of 1996
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payers. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from government sponsored programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry in connection with the delivery of or payment for healthcare benefits, items or services.
In addition to creating the two new federal healthcare crimes, HIPAA, and the regulations promulgated thereunder, also establish uniform standards for certain covered entities governing the conduct of certain electronic healthcare transactions and protecting the security and privacy of protected health information, or PHI, maintained or created by healthcare providers, health plans and healthcare clearinghouses.
Although we believe we are not a covered entity under HIPAA, we expect that our customers generally will be covered entities and they may obligate us to contractually comply with certain aspects of HIPAA. While the government intended this legislation to reduce administrative expenses and burdens for the healthcare industry, to the extent we contractually agree to comply with certain provisions of HIPAA we may experience significant costs. If we fail to comply with the standards that we have agreed to contractually, we may be subject to liability for violation of related contractual obligations we have with our customers. Pursuant to the HITECH Act and its implementing regulations, to the extent we are a business associate of our covered entity customers, we will be obligated to enter into certain agreements-business associate agreements- and directly subject to the HIPAA Security Rule, Breach Notification Rule and portions of the Privacy Rule and can be directly liable to HHS for noncompliance. Our compliance with these standards may entail significant costs for us and we cannot predict the effect of increased enforcement efforts in this area. To the extent we are subject to HIPAA, noncompliance can result in civil or
27
criminal enforcement. Even if we are not directly subject to HIPAA, we could be subject to criminal penalties if we knowingly obtain or disclose individually identifiable health information maintained by a HIPAA-covered entity in a manner that is not authorized or permitted by HIPAA.
In addition to federal regulations issued under HIPAA, many states have enacted privacy and security statutes or regulations that regulate the use and disclosure of health information, including state medical privacy laws, state breach notification laws, and federal and state consumer protection laws. In some cases, these laws are more stringent than those issued under HIPAA and are not preempted. It may be necessary to modify our planned operations and procedures to comply with applicable state laws. If we fail to comply with applicable state laws and regulations, we could be subject to additional sanctions.
Foreign Corrupt Practices Act
The federal Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the U.S. Securities and Exchange Commission. A determination that our operations or activities are not, or were not, in compliance with United States or foreign laws or regulations could result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence. Violations of these laws may result in criminal or civil sanctions, which could disrupt our business and result in a material adverse effect on our reputation, business, and result of operations or financial condition.
Certificate of Need Laws
In approximately two-thirds of the states, a certificate of need or similar regulatory approval is required prior to the acquisition of high-cost capital items or various types of advanced medical equipment, such as our system. At present, many of the states in which we expect to sell our system have laws that require institutions located in those states to obtain a certificate of need in connection with the purchase of our system, and we anticipate that some of our purchase orders may be conditioned upon our customer’s receipt of necessary certificate of need approval. Certificate of need laws were enacted to contain rising healthcare costs, prevent the unnecessary duplication of health resources, and increase patient access for health services. In practice, certificate of need laws have prevented hospitals and other providers who have been unable to obtain a certificate of need from acquiring new equipment or offering new services. A further increase in the number of states regulating our business through certificate of need or similar programs could adversely affect us. Moreover, some states may have additional requirements. For example, we understand that California’s certificate of need law also incorporates seismic safety requirements which must be met before a hospital can acquire our system.
Employees
As of December 31, 2015, we had 130 employees, 20 of whom were engaged directly in research and development, 17 in regulatory, clinical affairs and quality activities, 29 in sales and marketing activities, 8 in customer service and support, 40 in manufacturing and 16 in general administrative and accounting activities. None of our employees are covered by a collective bargaining agreement, and we consider our relationship with our employees to be good.
Additional Information
Hansen Medical, Inc. was incorporated in Delaware in 2002 under the name AutoCath, Inc. We file reports and other information with the Securities and Exchange Commission, or SEC, including annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and proxy or information statements. Those reports and statements as well as all amendments to those documents filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, (1) are available at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, DC 20549, (2) are available at the SEC’s internet site (www.sec.gov), which contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC and (3) are available free of charge through our website as soon as reasonably practicable after electronic filing with, or furnishing to, the SEC. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330.
Our website address is www.hansenmedical.com. Information on our website is not incorporated by reference nor otherwise included in this report. Our principal executive offices are located at 800 East Middlefield Road, Mountain View, California 94043 and our telephone number is (650) 404-5800.
28
ITEM 1A. RISK FACTORS
Risks Related to Our Business
We have incurred substantial losses since inception and anticipate that we will incur continued losses through at least the next year, we may not be able to raise additional financing to fund future losses and we may not be able to continue to operate as a going concern. We have experienced an event of default under our credit agreement which gives the lender the right to request immediate acceleration of repayment.
We have experienced substantial net losses since our inception in late 2002 and expect such losses to continue through at least the year ending December 31, 2016 as we continue to commercialize our technologies and develop new applications and technologies. As of December 31, 2015, we had an accumulated deficit of $453.9 million. We have funded our operations to date principally from the sale of our securities, the issuance of debt and through partnering and the licensing of intellectual property. As of December 31, 2015, our cash, cash equivalents, short-term investments and restricted cash total was $ 28.0 million. We anticipate that our existing available capital resources as of December 31, 2015 and the estimated amounts received through the sale of our products and services will not be sufficient to meet our anticipated cash requirements for the next twelve months. We incurred an operating loss of $46.2 million and had negative cash flows from operations of $37.7 million for the fiscal year ended December 31, 2015. In addition, we are also subject to minimum liquidity requirements under our existing borrowing arrangement with White Oak Global Advisors, LLC ("White Oak") that requires us to maintain $15.0 million in liquidity, consisting of at least $13.0 million in cash, cash equivalents and investments, of which $5.0 million is required to be restricted subject to lenders’ control, and the lesser of $2.0 million or 65% of eligible accounts receivable. Additionally, we are required to obtain an audit opinion from our independent certified public accountants on the annual financial statements that does not include an explanatory uncertainty paragraph raising substantial doubt about its ability to continue as a going concern or any qualification or exception as to the scope of such audit. As of December 31, 2015, we were in compliance with all covenants; however, there was an anticipated default of the covenants listed in Section 6.2(a)(i) and Section 8.2(a), with respect to Section 6.2(a)(i), as of December 31, 2015, which was cured by the Forbearance Agreement as discussed below. As of March 31, 2016, we were no longer in compliance with certain covenants and events of default listed in Sections 6.1(c), 6.2(a)(i), 6.2(a)(iv), 6.10, 8.2(a) and 8.2(b) of the loan and security agreement with White Oak (the “Specified Events of Default”). On April 19, 2016, we entered into a Forbearance Agreement with White Oak (“Forbearance Agreement”) whereby White Oak granted forbearance of the Specified Events of Default until August 17, 2016 in exchange for a $150,000 non-refundable transaction fee. Pursuant to the Forbearance Agreement, on April 21, 2016, we made a prepayment to White Oak, with respect to our obligations under the loan and security agreement with White Oak, in the amount of $5.0 million from our restricted account. Additionally, the outstanding obligations under the White Oak loan will become due on or before August 17, 2016. As of December 31, 2015, the debt was classified as a short-term liability.
Based on our current operating projections, we do not have sufficient liquidity to meet our anticipated cash requirements through the next twelve months. These factors raise substantial doubt about our ability to continue as a going concern. In order to meet our current and long-term anticipated cash requirements, we need to obtain additional financing or continue to adopt additional cost-cutting measures. There can be no assurance, however, that such a financing will be successfully completed on terms acceptable to us or that we can implement cost cutting measures sufficient to extend our cash and liquidity. We may seek additional financing at any time by selling additional equity or debt securities, licensing core or non-core intellectual property assets, entering into future research and development funding arrangements, refinancing or restructuring existing debt arrangements, or entering into a credit facility. If we seek additional funding in the future by selling additional equity or debt securities or entering into debt or credit facilities, such additional funding may result in substantial dilution to existing stockholders, may contain unfavorable terms or may not be available on any terms.
Conditions in the global financial and credit markets may limit our ability to raise additional funds. We cannot guarantee that future equity or debt financing will be available in amounts or on terms acceptable to us, if at all. Further, even if financing is available, the cost to us may be significantly higher than in the past. Our ability to access the capital markets and raise funds required for our operations may be severely restricted by general market conditions at a time when we would like, or need, to do so, which could have an adverse effect on our ability to meet our current and future funding requirements and on our flexibility to react to changing economic and business conditions. This could leave us without adequate financial resources to fund our operations as presently conducted or as we plan to conduct them in the future. If adequate funds are not available, we may be required to adopt additional cost-cutting measures, including additional reductions in our work force, reducing the scope of, delaying or eliminating some or all of our planned research, development and commercialization activities and/or reducing marketing, customer support or other resources devoted to our products. If we seek additional funding through partnering and licensing transactions, we could be required to license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize ourselves or on terms that are less attractive than they might otherwise be. Any of these factors could materially harm our business and may negatively impact our ability to continue to operate as a going concern.
29
Because we may not be successful in significantly increasing sales of our products, the extent of our future losses and the timing of achieving sustained profitability are highly uncertain, and we may never achieve sustained profitable operations. If we require more time than we expect to generate significant revenue and achieve sustained profitability, we may not be able to continue our operations. Even if we achieve significant revenues, we may never become profitable on a sustained basis.
On April 19, 2016, we entered into an Agreement and Plan of Merger (the “Merger Agreement”), with Auris Surgical Robotics, Inc. (“Auris”) and Pineco Acquisition Corp. (“Sub”), a wholly owned subsidiary of Auris, providing for the merger of Sub with and into the Company, with the Company surviving the Merger as a wholly owned subsidiary of Auris (“the Merger”). Completion of the Merger remains subject to the satisfaction of various conditions, including the approval of the Company’s stockholders and customary closing conditions, many of which are outside of our control. There is no assurance that all of the various conditions will be satisfied, or that the Merger will be completed on the proposed terms, within the expected time frame, or at all. In addition, if the Merger is not completed by October 20, 2016, subject to certain limitations, either the Company or Auris may choose not to proceed with the Merger.
Our efforts to continue to scale the manufacturing, assembling, testing, marketing and selling of our Sensei System and Magellan System may encounter obstacles and delays which could significantly harm our ability to generate revenue.
Our ability to generate revenues depends upon the successful scaling of the manufacturing, assembling, testing, marketing and selling of our Sensei System and Magellan System. These commercialization efforts may not succeed for a number of reasons, including those set forth in this Item 1A and the following:
• | our systems may not be accepted by physicians or hospitals; |
• | we may not be able to sell our systems and associated catheters in volumes and at prices that allow us to meet the revenue targets necessary to generate revenue necessary to achieve profitability; |
• | the use of our systems by customers may not achieve more predictable procedure times, enable more complex cases or result in other physician or clinical benefits that we believe will drive adoption of our products in sufficient volume; |
• | we, or the investigators of our products, may not be able to generate sufficient information regarding outcomes with our systems to satisfy potential purchasers; |
• | the availability and perceived advantages of alternative treatments may hinder acceptance of our systems in sufficient volume; |
• | our assumptions regarding the economic value proposition of our systems for hospitals, including the reimbursement rates that hospitals may achieve for procedures using our systems, may not be sufficiently accurate to drive adoption in sufficient volume; |
• | any rapid technological changes may make our products obsolete; |
• | we may not be able to manufacture our systems or catheters in commercial quantities or at an acceptable cost; |
• | we may not have adequate financial or other resources to complete the manufacture, assembly, testing, marketing and sale of our systems on a commercial scale or the development of new products; and |
• | we may not obtain regulatory clearance for the applications for which many physicians wish to use our systems and, accordingly, our label may hinder our ability to successfully market and sell our EP products in the United States to a broader group of potential customers. |
If we are not successful in the commercialization of our Sensei System for uses other than for mapping in EP procedures or our continued efforts to scale the manufacturing, assembling, testing, marketing and selling of our Magellan System, we may never achieve sustained profitability and may be forced to cease operations.
Successful commercialization of our Magellan System is subject to manufacturing, marketing, sales and customer service risks which could significantly harm our ability to generate revenue.
We may encounter unexpected manufacturing problems when scaling up the production of our Magellan System, Magellan Robotic Catheters and related accessories in commercial quantities. While we have experience marketing and selling the Sensei System following its initial regulatory approvals in 2007 and the Magellan System following its regulatory approvals in July 2012, the marketing and sales effort to sell our systems on a larger scale involves different customers, value propositions and purchasing processes, and we are only beginning to gain experience in marketing and selling our Magellan System on a larger scale. Our Magellan System is a novel device, and hospitals are traditionally slow to adopt new products and treatment practices. Our Magellan System is an expensive capital equipment purchase which slows the sales process. We are also still growing our Magellan System's product and brand recognition. Furthermore, we do not believe hospitals will purchase our products unless the physicians at those hospitals express a strong desire to use our products and we cannot predict
30
whether or not they will do so in sufficient numbers for us to achieve profitability and realize long-term success. The ability to obtain market acceptance of a new product such as the Magellan System is highly variable and subject to many risks. As a result, our commercialization plans may be delayed, incomplete or unsuccessful. In addition, larger-scale commercial introduction of products sometimes results in the identification of latent or new product defects or quality issues that were not evident in the testing of the products. Similarly, as greater numbers of physicians gain experience with our Magellan System, we may identify areas where new or further training is required. If we encounter any of these issues as we endeavor to continue to commercialize our Magellan System on a larger scale, our financial condition and results of operations and business could be adversely impacted, and we may never achieve sustained profitability or realize long-term success.
We may not be able to further develop our Magellan System as planned, which could significantly harm our ability to achieve future regulatory approvals and market acceptance.
We intend to further develop our Magellan System, including our Magellan Robotic Catheters and related accessories. Due to the advanced electrical, mechanical, and software capabilities of this new robotic platform, we may encounter challenges in designing, engineering and manufacturing future enhancements to the platform, which may lead to compatibility obstacles with operating room and catheter laboratory layouts, equipment quality or performance issues, unmet customer expectations regarding features or functionality or other defects in future versions of the platform. Any such difficulties could result in delays in our submissions to regulatory agencies, delays in achieving or the failure to achieve additional regulatory approvals or clearances for enhancements to the system, lack of physician adoption of our system, higher than expected service claims, litigation and negative press coverage which may damage our business.
If we are unable to manufacture our systems and catheters in a manner that yields sufficient gross margins, we will be unable to achieve profitable commercialization.
We may encounter unexpected problems in manufacturing, assembling or testing our current products on a commercial scale. Our products contain expensive materials and are expensive to manufacture, particularly in limited quantities. In addition to increasing sales to increase manufacturing overhead absorption, we need to reduce the variable manufacturing costs of our catheters in order to achieve our operational and financial goals. We face challenges in order to produce disposable catheters effectively, to appropriately phase in new products and designs, to efficiently utilize our manufacturing facility and to achieve planned manufacturing cost reductions. If we are unable to effectively manage these issues, our costs of producing our products will negatively affect our gross margins which will negatively impact our business.
We have a debt facility with White Oak Global Advisors, LLC that requires us to meet certain restrictive covenants that may limit our operating flexibility.
In August 2013, we entered into an amended and restated $33.0 million loan and security agreement with White Oak, as a lender and as agent for the lenders under the loan and security agreement. We are obligated to pay only interest on the loan until the loan’s maturity date, which is December 30, 2017. At our option, we may prepay all or a portion of the outstanding principal balance, subject to paying a prepayment fee of 3.5% of the principal amount of the loan prepaid if our prepayment is made on or before the third anniversary of the funding of the loan or 1.0% of the principal amount of the loan prepaid if our prepayment is made after the third anniversary and on or before the fourth anniversary of the funding of the loan. We are also required to make mandatory prepayments upon certain events of loss and certain dispositions of our assets as described in the loan and security agreement.
The loan and security agreement contains customary events of default, including if we fail to make a payment on its due date, fail to perform specified obligations, fail to comply with certain covenants in the loan and security agreement, experience a material adverse change, or become insolvent. We have granted the lenders a first priority security interest in substantially all of our assets, excluding any of our intellectual property, now owned or hereafter acquired, and all proceeds and products thereof. Two of our wholly-owned subsidiaries, AorTx, Inc. and Hansen Medical International, Inc., have guaranteed our obligations under the loan and security agreement and have granted first priority security interests in their assets, excluding any of their intellectual property, to secure their guarantee obligations. Under the loan and security agreement, neither we nor AorTx, Inc. and Hansen Medical International, Inc. may grant a lien on any intellectual property to third parties. We have also pledged to the lenders shares of each of our direct and indirect subsidiaries as collateral for the loan. We are also subject to certain affirmative and negative covenants, and also to minimum liquidity requirements which require us to maintain $15.0 million in liquidity at all times, consisting of at least $13.0 million in cash, cash equivalents and investments of which $5.0 million of which shall be funds subject to lenders’ control, and the lesser of $2.0 million or 65% of eligible accounts receivable. Additionally, we are required to obtain an audit opinion from our independent certified public accountants on the annual financial statements that does not include an explanatory uncertainty paragraph raising substantial doubt about its ability to continue as a going concern or any qualification or exception as to the scope of such audit. We are subject to limitations on our ability to: undergo certain change of control events; convey, sell, lease, transfer, assign or otherwise dispose of our assets; create, incur, assume, or be liable with respect to certain indebtedness not including, among other items, subordinated debt;
31
grant liens; pay dividends and make certain other restricted payments; make loans, acquisitions, or certain investments; create subsidiaries or enter into joint ventures; repurchase certain equity interest; make payments on any subordinated debt; make material changes to our core business of any of our subsidiaries; enter into transactions with any of our affiliates outside of the ordinary course of business; or permit our subsidiaries to do the same. We are also required to make mandatory prepayments upon certain events of loss and certain dispositions of our assets described in the loan and security agreement.
As of December 31, 2015, we were in compliance with all covenants; however, there was an anticipated default of the covenants listed in Section 6.2(a)(i) and Section 8.2(a), with respect to Section 6.2(a)(i), as of December 31, 2015, which was cured by the Forbearance Agreement as discussed below. As of March 31, 2016, we were no longer in compliance with certain covenants and events of default listed in Sections 6.1(c), 6.2(a)(i), 6.2(a)(iv), 6.10, 8.2(a) and 8.2(b) of the loan and security agreement with White Oak (the “Specified Events of Default”). On April 19, 2016, we entered into a Forbearance Agreement with White Oak (“Forbearance Agreement”) whereby White Oak granted forbearance of the Specified Events of Default until August 17, 2016 in exchange for a $150,000 non-refundable transaction fee. Pursuant to the Forbearance Agreement, on April 21, 2016, we made a prepayment to White Oak, with respect to our obligations under the loan and security agreement with White Oak, in the amount of $5.0 million from our restricted account. Additionally, the outstanding obligations under the White Oak loan will become due on or before August 17, 2016. As of December 31, 2015, the debt was classified as a short-term liability.
In the event we were to further violate any covenants or if White Oak believes that we have violated any covenants, and such violations are not cured pursuant to the terms of the loan and security agreement, we would be in default under the loan and security agreement, which would entitle the lenders to exercise their remedies, including the right to accelerate the debt, upon which we may be required to repay all amounts then outstanding under the loan and security agreement. Complying with these covenants may make it more difficult for us to successfully execute our business strategy.
If Philips is unable to develop or license new products or applications for the FOSSL technology, or such products are not commercially viable, we may not realize the full benefits of our agreements with Philips which would harm our results of operations and could delay and or impair our ability to successfully commercialize that technology.
The realization of the full potential benefits of our agreements with Philips, including the receipt of any of the up to $78.0 million in future payments associated with the successful commercialization by Philips or its collaborators of products containing the FOSSL technology, requires the development of new products and applications of technology that are subject to design, engineering and manufacturing challenges, and potential safety and regulatory issues that could delay, suspend or terminate clinical studies, regulatory approvals or sales, and our reliance on third parties to develop, obtain regulatory approval for, manufacture, market and sell products containing FOSSL technology. Approximately two-thirds of the potential future payments could arise from Philips’ sublicensing the FOSSL technology, but Philips has no obligations to do so. Under the amended terms of our agreements with Philips, we no longer have the right to reacquire certain of the rights licensed to Philips. In addition, Philips’ sales of products containing the FOSSL technology could be too low to result in any royalty payments to us. If any of these events occurred, we would be unable to realize the full financial benefits of our agreements with Philips and may be unable to monetize the FOSSL technology in other areas, harming our research and development efforts and adversely affecting our business.
We have completed enrollment in the initial reportable cohort of the ongoing IDE clinical trial and are working closely with FDA on the initial outline of the PMA. It is possible that we may need to enroll additional patients in the clinical trial to meet the statistical requirements and as such we may be unable to complete the trial for the treatment of atrial fibrillation or other future trials, or we may experience significant delays in completing the clinical trials, which could prevent or delay regulatory approval of our Sensei System for expanded uses and impair our financial position.
We have received Investigational Device Exemption, or IDE, approval to investigate the use of our Sensei System and Artisan Extend catheters in the treatment of atrial fibrillation in a clinical study designed to support the expansion of our current labeling in the U.S. beyond mapping. Initially the study was designed to enroll 300 patients, and the first patient was enrolled in May 2010. In January 2013, we proposed a modification to the study protocol to change the study design and reduce the required sample size. The modified Bayesian design study which requires a minimum enrollment of 125 subjects, was approved by the FDA in August 2013. The study has enrolled 150 patients to date. The study enrollment is now paused and the necessary patient follow up is ongoing. The study includes a seven-day follow-up for safety and a one-year follow-up for efficacy at intervals of 90, 180 and 365 days.
In addition, the completion of the trial, and any future clinical trials, could be delayed, suspended or terminated for several reasons, including:
• | ongoing discussions with regulatory authorities regarding the scope or design of our preclinical results or clinical trial or requests for supplemental information with respect to our preclinical results or clinical trial results; |
32
• | our failure or inability to conduct the clinical trials in accordance with regulatory requirements; |
• | sites participating in the trial may drop out of the trial, which may require us to engage new sites or petition the FDA for an expansion of the number of sites that are permitted to be involved in the trial; |
• | patients may not remain in or complete the clinical trial at the rates we expect; |
• | patients may experience serious adverse events or side effects during the trial, which, whether or not related to our products, could cause the FDA or other regulatory authorities to place the clinical trial on hold; and |
• | clinical investigators may not perform our clinical trials on our anticipated schedule or consistent with the clinical trial protocol and good clinical practices. |
If our clinical trials are delayed it will take us longer to commercialize a product for the treatment of atrial fibrillation and generate revenues from such product. Moreover, our development costs will increase if we have material delays in our clinical trials or if we need to perform more or larger clinical trials than planned.
Even if we complete our trial for the treatment of atrial fibrillation or other clinical trials, these trials may not produce results that are sufficient to support approval of a PMA or 510(k) application.
We will consider our Sensei System to be effective if the trial for the treatment of atrial fibrillation meets target performance goals based upon the manual control of specified ablation catheters, but there is a risk that, even if we achieve our trial endpoints, the FDA may not approve our Sensei System for use in the treatment of atrial fibrillation. In addition, there is a risk that the FDA may require us to conduct a larger or longer clinical trial, submit additional follow-up data, or engage in other costly and time consuming activities that may delay the FDA’s clearance or approval of the Sensei System for use in atrial fibrillation. We plan to file a premarket approval, or PMA, based on data from our trial for the use of Sensei System in the treatment of atrial fibrillation, which is time consuming and costly. If our clinical trial fails to produce sufficient data to support a PMA, it will take us longer to ultimately commercialize a product for the treatment of atrial fibrillation, or any other intended treatment, and generate revenue or the delay could result in our being unable to do so. Moreover, our development costs will increase if we need to perform more or larger clinical trials than planned.
We have incurred substantial management and employee turnover and we may lose additional key personnel or fail to attract and retain additional personnel needed for us to operate our business effectively.
Our management team includes several members hired since January 2014, including our Chief Executive Officer who joined us in May 2014. In addition, the position of Chief Financial Officer is currently held on an interim basis. If we are unable to recruit and retain qualified individuals, including retaining our Chief Executive Officer and/or hiring a permanent Chief Financial Officer, our product development and commercialization efforts could be materially delayed or be unsuccessful. We have periodically reduced our work force and we may undertake additional actions to reduce our work force in the future. These reductions in force may make it more difficult to retain and attract the qualified personnel required, placing a significant strain on our management. Retaining such personnel and recruiting necessary new employees in the future will be critical to our success. There is intense competition from other companies and research and academic institutions for qualified personnel in the areas of our activities. If we fail to identify, attract, retain and motivate these highly skilled management and personnel, we may be unable to continue our development and commercialization activities and our business will be harmed.
We are highly dependent on the principal members of our management and scientific staff. We do not carry “key person” insurance covering any members of our senior management. Each of our officers and key employees may terminate his or her employment at any time without notice and without cause or good reason. The loss of any of these persons could prevent the implementation and completion of our objectives, including the development and introduction of our products, and could require the remaining management members to direct immediate and substantial attention to seeking a replacement.
Credit, financial market and general economic conditions could delay or prevent potential customers from purchasing our products, which would adversely affect our sales, financial condition and results of operation.
The sale of our systems often represents a significant capital purchase for our customers and many customers finance their purchase of our systems through a credit facility or other financing. If prospective customers that need to finance their capital purchases are not able to access the credit or capital markets on terms that they consider acceptable, they may decide to postpone or cancel a potential purchase of our system. Potential customers with limited capital budgets may decide to spend those dollars on other technologies rather than on our products. Also, even customers with sufficient financial resources to make such purchases without resorting to the credit and capital markets may be less likely to make capital purchases during periods when they view the overall economic conditions unfavorably or with uncertainty. Many potential customers have delayed making a decision to purchase a Sensei or Magellan System, which has significantly impacted our sales, financial condition and results of operations. If we are unable to obtain market acceptance for our products’ value proposition, potential
33
customers may not make these significant capital purchases and our sales, financial condition and results of operations would be harmed.
We are continuing to develop our capabilities and experience with the sales, marketing and distribution of our products on a commercial scale, which could impair our ability to achieve sustained profitability.
While we have experience marketing and selling the Sensei System following its initial regulatory approvals in 2007 and the Magellan System following its regulatory approvals in July 2012 in the United States, we are still continuing to develop our capabilities and experience with the sales, marketing and distribution of our products on a larger commercial scale. We market our systems and catheters through a direct sales force of regional sales employees, supported by clinical sales representatives who provide training, clinical support and other services to our customers. Our direct sales force competes against the experienced and well-funded sales organizations of our competitors, some of which have more experience and greater capabilities with the sales, marketing and distribution of their products on large, commercial scales. Our revenues depend largely on the effectiveness of our sales force and, if we fail to effectively manage any of the risks identified in this Item 1A, we may never achieve sustained profitability. We face significant challenges and risks related to our direct sales force and the marketing of our current and future products, including, among others:
• | the ability of sales personnel to obtain access to or persuade hospitals to purchase our system and catheters and physicians to use our system and catheters in sufficient volume; |
• | our ability to retain, properly motivate, recruit and train adequate numbers of qualified sales and marketing personnel in sufficient volume; |
• | our ability to successfully integrate new management, including our future Chief Financial Officer; |
• | the costs associated with an independent sales and marketing organization; |
• | hiring, maintaining and expanding an independent sales and marketing organization; and |
• | our ability to promote our products effectively while maintaining compliance with government regulations and labeling restrictions with respect to the healthcare industry. |
Outside the United States, primarily in the EU, we are establishing a combination of a direct sales force and distributors to market, sell and support our current and future products. If we fail to select and maintain appropriate distributors, appropriately disengage from unsuccessful distributors or effectively use our distributors or sales personnel and coordinate our efforts for distribution of our systems and catheters in the EU or if their and our sales and marketing strategies are not effective in generating sales of our system, our revenues would be adversely affected and we may never become profitable on a sustained basis.
We are continuing to develop our experience in manufacturing and assembling our products on a commercial scale and may encounter problems at our manufacturing facilities or otherwise experience manufacturing delays that could result in lost revenue or diminishing margins.
We may encounter unexpected problems in manufacturing, assembling or testing our current products on a commercial scale. In addition, for our Sensei System and Magellan System, we subcontract the manufacturing of major components and complete the final assembly and testing of those components in-house. We face challenges in order to produce our products effectively, to appropriately phase in new products and product designs, to efficiently utilize our manufacturing facility and to achieve manufacturing cost reductions. These challenges include equipment design and automation, material procurement, low or variable production yields on catheters and quality control and assurance. The costs resulting from these challenges have had and will continue to have a significant impact on our gross margins and may result in significant fluctuations of gross margins from quarter to quarter. As we continue to scale our operations, these risks increase. Additionally, we may not successfully complete required manufacturing changes or planned improvements in manufacturing efficiency on a timely basis or at all. The Company has in the past experienced recalls associated with its manufacturing processes and such recalls may occur again. Any future manufacturing issues may result in our being unable to meet the expected demand for our products, maintain control over our expenses or otherwise successfully manage our manufacturing capabilities. If we are unable to satisfy demand for our systems or catheters, our ability to generate revenue could be impaired and hospitals may instead purchase, or physicians may use, our competitors’ products. Since our Sensei System and Magellan System require the use of disposable Artisan Extend catheters and Magellan Robotic Catheters, respectively, our failure to meet demand for catheters from hospitals that have purchased our systems could adversely affect the market acceptance of our products and damage our commercial reputation.
In addition, all of our manufacturing operations are conducted at our facilities leased in Mountain View, California. We could encounter problems at these facilities, which could delay or prevent us from manufacturing, assembling or testing our products or maintaining our manufacturing capabilities or otherwise conducting operations.
34
Our reliance on third-party manufacturers and on suppliers, and in certain cases, a single-source supplier, could harm our ability to meet demand for our products in a timely manner or within budget, and could cause harm to our business and financial condition.
We depend on third-party manufacturers to produce most of the components of our systems and other current products, and have not entered into formal agreements with several of these third parties. We also depend on various third-party suppliers for various components we use in our systems and for our catheters. For example, Force Dimension Sàrl, a single-source supplier, manufactures customized motion controllers that are a part of our Sensei System and Magellan System. We also obtain the motors for our Sensei System and Magellan System from a single supplier, Maxon Motor AG, from whom we purchase on a purchase order basis, and we generally do not maintain large volumes of inventory.
Our reliance on third parties involves a number of risks, including, among other things, the risk that:
• | suppliers may fail to comply with regulatory requirements or make errors in manufacturing components that could negatively affect the efficacy or safety of our products or cause delays in or prevent shipments of our products; |
• | we may not be able to respond to unanticipated changes and increases in customer orders; |
• | we may be subject to price fluctuations due to a lack of long-term supply arrangements for key components with our suppliers; |
• | we may lose access to critical services and components, resulting in an interruption in the manufacture, assembly and shipment of our systems and other products; |
• | our suppliers manufacture products for a range of customers, and fluctuations in demand for products these suppliers manufacture for others may affect their ability to deliver components to us in a timely manner; |
• | our suppliers may wish to discontinue supplying goods or services to us; |
• | if the components necessary for our system become unavailable we may not be able to find new or alternative components or reconfigure our system and manufacturing processes in a timely manner; and |
• | our suppliers may encounter financial hardships unrelated to our demand for components, which could inhibit their ability to fulfill our orders and meet our requirements. |
If any of these risks materialize, it could significantly increase our costs and impact our ability to meet demand for our products.
In addition, if these manufacturers or suppliers stop providing us with the components or services necessary for the operation of our business, we may not be able to identify alternative sources in a timely fashion. Any transition to alternative manufacturers or suppliers or a decision to discontinue our relationship with a current manufacturer or supplier could result in operational problems, increased expenses or write-down of capitalized assets that would adversely affect operating results and could delay the shipment of, or limit our ability to provide, our products. We cannot assure you that we would be able to enter into agreements with new manufacturers or suppliers on commercially reasonable terms on a timely basis or at all. Additionally, obtaining components from a new supplier may require qualification of a new supplier in the form of a new or supplemental filing with applicable regulatory authorities and clearance or approval of the filing before we could resume purchasing components for inclusion in our products. Any disruptions in product supply may harm our ability to generate revenues, lead to customer dissatisfaction, damage our reputation and result in additional costs or cancellation of orders by our customers. We currently purchase a number of the components for our systems in foreign jurisdictions. Any event causing a disruption of imports, including the imposition of import restrictions, could adversely affect our business and our financial condition.
If we fail to maintain necessary FDA clearances/approvals and the CE Certificates of Conformity for our medical device products or are seen to violate any FDA or European Economic Area ("EEA") regulations or guidance, or if future clearances, approvals or the delivery of CE Certificates of Conformity are delayed, we will be unable to commercially distribute and market our products.
The process of seeking regulatory clearance, or approval (in the United States) or CE Certificates of Conformity (in the EEA) to market a medical device is expensive and time-consuming and clearance, approval and grant of CE Certificates of Conformity is never guaranteed and, even if granted or obtained, clearance, approval or CE Certificates of Conformity may be suspended, withdrawn or revoked. Staying in compliance with all of the complex FDA and EEA regulations and guidance is a time-consuming and difficult endeavor, and the government may disagree with our compliance efforts or interpretations of FDA regulations and guidance. If the FDA or the competent authorities of the EEA countries determine that our promotional materials or training constitutes promotion of a use which has not been cleared or approved or does not fall within the scope of the current CE mark, they could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, injunction, seizure, civil fine and criminal penalties.
35
In May 2007, we received FDA clearance in the United States to commercialize our Sensei System and Artisan Extend catheters only to facilitate manipulation, positioning and control, for collecting electrophysiological data within the heart atria with electro-anatomic mapping and recording systems using two specified mapping catheters. Because the FDA has determined that there is a reasonable likelihood that our Sensei System and Artisan Extend catheters could be used by physicians for uses not encompassed by the scope of the present label and that such uses may cause harm, we are required to label these products to state that their safety and effectiveness for use with cardiac ablation catheters in the treatment of cardiac arrhythmias including atrial fibrillation have not been established. Accordingly, the scope of the current label may hinder our ability to successfully market and sell our EP products in the United States to a broader group of potential customers.
We received FDA clearance for marketing our Magellan System, including the Magellan 9Fr Robotic Catheter and accessories in June 2012, the Magellan 6Fr Robotic Catheter in February 2014, and the Magellan 10Fr Robotic Catheter in July 2015. Our FDA cleared labeling does not further specify the scope of the targets within the peripheral vasculature that are encompassed in the 510(k) clearance. The FDA could disagree with our interpretation of the scope of this clearance. If the FDA concludes that our promotional materials exceed the scope of this clearance, the agency may retroactively require us to seek 510(k) clearance or PMA approval and such uses could be subject to the same restrictions as the use of the Sensei System in cardiac ablation procedures.
Our promotional materials and training methods regarding physicians must comply with FDA requirements and other applicable laws and regulations. Both our Magellan and Sensei Systems are cleared by the FDA and CE marked in the EEA for defined uses. We believe that the specific procedures for which our products are marketed fall within the scope of the FDA clearances in the United States and CE Marks in the EEA. The FDA and other competent authorities and agencies actively enforce regulations prohibiting promotion of off-label uses and the promotion of products for which marketing clearance, approval or CE Certificates of Conformity has not been obtained. Moreover, scrutiny of such practices by the FDA, the U.S. Department of Justice, and other competent authorities and agencies has recently increased. In the United States, promotional activities for FDA regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. A company that is found to have improperly promoted off-label uses may be subject to significant liability, including civil and administrative remedies under the Federal False Claims Act and various other federal and state laws, as well as criminal sanctions. Administrative, civil and criminal sanctions can also be imposed in foreign countries.
We will be required to seek a separate 510(k) clearance or PMA approval to market our Sensei System for uses other than those in the current label. We cannot assure that FDA would not impose a more burdensome level of premarket review on other intended uses or modifications to approved products. We plan to seek future approval of our Sensei System for other indications, including atrial fibrillation and other cardiac ablation procedures. We cannot assure the timing or potential for success of those efforts. We cannot assure the study will be completed at all or in a timely manner, nor that the study will be executed in a manner consistent with FDA requirements or yield sufficient data to support approval. Clinical studies are subject to FDA audits under the Bioresearch Monitoring program, and if our study execution or that of our participating sites and investigators is found to be deficient, this may result in delays in approval or could prevent approval from being obtained. Any significant violations can also result in further enforcement action, as outlined above.
With regard to our Sensei System, our Magellan System, or other products, the FDA can delay, limit or deny clearance of a 510(k), or PMA approval, for many reasons, including:
• | our inability to demonstrate safety or effectiveness to the FDA’s satisfaction; |
• | the data from our preclinical studies and clinical trials may be insufficient to support approval; |
• | the facilities of our third-party manufacturers or suppliers may not meet applicable requirements; |
• | our failure to comply with preclinical, clinical or other regulations; |
• | our inability to meet the FDA’s statistical requirements or changes in statistical tests or significance levels the FDA requires for approval of a medical device, including ours; and |
• | changes in the FDA approval policies, expectations with regard to the type or amount of scientific data required or adoption of new regulations that may require additional data or additional clinical studies. |
Furthermore, in order to market our products outside of the United States, we will need to establish and comply with the numerous and varying regulatory requirements of other countries regarding quality, safety and efficacy. We received a CE Certificate of Conformity in the EEA for our Sensei System in September 2006, for our Artisan Extend catheters in May 2007, for our Magellan System in July 2011, for our Magellan 9Fr Robotic Catheter and related accessories designed for use with the Magellan System in October 2011, for our Artisan Extend catheters in February 2013, for our Magellan 6Fr Robotic Catheter in October 2014, and for our Magellan 10Fr Robotic Catheter in April 2015. However, we may be required to go through new conformity assessment procedures with our Notified Body in the EEA in order to market our products for any additional uses.
36
Regulatory approvals or CE Certificates of Conformity may be difficult and costly to obtain, or may not be granted or obtained at all.
If we are unable to maintain our regulatory clearances and CE Certificates of Conformity and obtain future clearances and CE Certificates of Conformity for our products and be seen to be in full compliance with the relevant FDA regulations and guidance, our financial condition and cash flow may be adversely affected, and our ability to grow domestically and internationally may be limited.
If the FDA or U.S. Department of Justice takes the position that we are not marketing or training physicians in a manner consistent with FDA regulations, the FDA and the competent authorities in the EEA countries could require us to stop promoting our products for certain procedures until we obtain FDA clearance or approval or a specific CE Certificate of Conformity for them and/or could require us to initiate corrective actions that could include issuing corrective advertising. In addition, the FDA and the competent authorities in the EEA countries could require us to generate and submit significant quality, safety and efficacy data to support use in those procedures for which the agency or the competent authorities of the EEA countries require clearance, approval or a specific CE Certificate of Conformity. If we are perceived not to be in compliance with all of the governmental restrictions, we could be subject to various enforcement measures, including investigations, administrative proceedings and country, federal and state court litigation, which would likely be costly to defend and harmful to our business. If the FDA, the U.S. Department of Justice, or another competent authority ultimately concludes we are not in compliance with such restrictions, we could be subject to significant liability, including civil and administrative remedies, exclusion, injunctions, significant monetary and punitive penalties and criminal sanctions, any or all of which would be harmful to our business and in certain instances may cause us to have to cease operations.
If physicians and hospitals do not believe that our Sensei System and Artisan Extend catheters are a viable alternative to existing mapping technologies used in atrial fibrillation and other cardiac ablation procedures, or if they do not believe that our Magellan System and Magellan Robotic Catheters are a viable alternative for vascular diseases, they may choose not to use our products.
We believe that physicians will not use, and hospitals will not purchase, our systems unless they determine that they provide a safe and effective alternative to existing treatments. Since we have received FDA clearance to market our Sensei System and disposable Artisan Extend catheters only for guiding catheters to map the heart anatomy, we will not be able to label or promote these products, or train physicians, for use in guiding catheters for cardiac ablation until such clearance or approval is obtained. Currently, there is only limited clinical data on our Sensei System with which to assess its safety and efficacy in any procedure, including atrial fibrillation and other cardiac ablation procedures. A number of studies have been published since the commercial launch of our Sensei System in 2007 on the efficacy, safety and efficiency of our products, especially by comparison to manual techniques. While we believe many of those studies have demonstrated the benefits of our products, some of these studies have been cited by our competitors to portray our products in an unfavorable light. A number of additional studies are underway both in the United States and Europe assessing the clinical experience with our products and continuing to compare usability and success of treatment between procedures performed with our Sensei System and manual technique. If these studies, or other clinical studies performed by us or others, or clinical experience indicate that procedures with our Sensei System or the type of procedures that can be performed with the Sensei System are not effective or safe for such uses, physicians may choose not to use our Sensei System. Reluctance by physicians to use our Sensei System or to perform procedures enabled by the Sensei System would harm sales. Furthermore, we commenced the commercialization of our Magellan System and Magellan Robotic Catheters for use during the treatment of peripheral vascular diseases, but there is very little clinical data for the system’s safety and efficacy. Reluctance by physicians to use our Magellan System or to perform procedures enabled by the Magellan System would harm these sales. Further, unsatisfactory patient outcomes or patient injury in either of our major products could cause negative publicity for our products, particularly in the early phases of product introduction. In addition, physicians may be slow to adopt our products if they perceive liability risks arising from the use of these new products. It is also possible that as our products become more widely used, latent or other defects could be identified, creating negative publicity and liability problems for us, thereby adversely affecting demand for our products. If physicians do not adopt the use of our products in their practices, we likely will not become profitable on a sustained basis and our business will be harmed.
In addition, our research and development efforts and our marketing strategy depend heavily on obtaining support and collaboration from highly regarded physicians at leading hospitals. If we are unable to gain or maintain such support and collaboration, our ability to market our Sensei System and Magellan System and, as a result, our business and results of operations, could be harmed.
We expect to derive substantially all of our revenues from sales of our Magellan System and the associated catheters and accessories. If hospitals do not purchase our systems, we may not generate sufficient revenues to continue our operations.
37
We continue our focus on our Magellan products. We received the CE Mark in Europe for our Magellan System in July 2011 and for the Magellan Robotic Catheter and related accessories designed for use with the Magellan System in October 2011, for our Magellan 6Fr Robotic Catheter in October 2014, and for our Magellan 10Fr Robotic Catheter in April 2015. We received FDA clearance to commercialize our Magellan System including the Magellan 9Fr Robotic Catheter and accessories in June 2012, we received FDA clearance for the marketing of our Magellan 6Fr Robotic Catheter in February 2014 and our Magellan 10Fr Robotic Catheter in July 2015. If hospitals do not widely adopt our Sensei or Magellan products, or if they decide that our systems are too expensive to purchase or operate, we may never achieve significant revenue, become profitable or sustain profitability.
The training required for physicians to use our Sensei System and Magellan System could reduce the market acceptance of our system and reduce our revenue.
It is critical to the success of our sales efforts to ensure that there are a sufficient number of physicians familiar with, trained on and proficient in the use of our Sensei System and Magellan System. Convincing physicians to dedicate the time and energy necessary for adequate training in the use of our systems is challenging, and we cannot assure you that we will be successful in these efforts.
It is our policy to train U.S. physicians to only insert, navigate, map and remove catheters using our Sensei System. Physicians must obtain training elsewhere to learn how to ablate cardiac tissue to treat atrial fibrillation, which is an off-label procedure with our Sensei System. This training may be provided in the U.S. by third parties, such as hospitals and universities and through independent peer-to-peer training among doctors. We cannot assure you that a sufficient number of U.S. physicians will become aware of training programs or that physicians will dedicate the time, funds and energy necessary for adequate training in the use of our system for these off-label procedures. Additionally, we will have no control over the quality of these training programs. If physicians are not properly trained, they may misuse or ineffectively use our products. This may result in unsatisfactory outcomes, patient injury, negative publicity or lawsuits against us, any of which could negatively affect our reputation and sales of our products. Furthermore, our inability to educate and train U.S. physicians to use our Sensei System for cardiac ablation procedures may lead to inadequate demand for our products and have a material adverse impact on our business, financial condition and results of operation.
We monitor our training to ensure that off-label use is not promoted or enabled. However, from time to time, we may sponsor third party training. There is a risk that independent peer-to-peer interaction between physicians and other third party training may include discussion or observation of off-label procedures because most procedures performed to date using the Sensei System involve both mapping and cardiac ablation. If any such activities are attributed to us, the FDA or other governmental entities could conclude that we have engaged in off-label promotion of our products, which could subject us to significant liability.
We expect to continue to experience extended and variable sales cycles, which could cause significant variability in our results of operations for any given quarter.
Our systems have a lengthy sales cycle because they involve a relatively expensive capital equipment purchase, which generally requires the approval of senior management at hospitals, inclusion in the hospitals’ budget process for capital expenditures and, in some instances, a certificate of need from the state or other regulatory clearance. We continue to estimate that this sales cycle may take between six and 18 months, though we have seen sales cycles trend towards the longer end of this range as many potential customers have postponed purchase decisions. Additionally, the majority of our revenue is often shipped in the last weeks of a given quarter. Any disruption in our supply chain during those critical weeks or an inability to fulfill our deliverables during that compressed time frame could significantly impact the timing of our ability to recognize revenue on those items. These factors have contributed in the past and may contribute in the future to substantial fluctuations in our quarterly operating results, particularly in the near term and during any other periods in which our sales volume is relatively low. As a result, in future quarters our operating results could differ from our announcements of guidance regarding future operating or financial results or may fail to meet the expectations of securities analysts or investors, in which event our stock price would likely decrease. These fluctuations also mean that you will not be able to rely upon our operating results in any particular period as an indication of future performance. In addition, the introduction of new products such as our Magellan System and Magellan Robotic Catheters could adversely impact our sales cycle, as customers take additional time to assess the benefits of new investments in capital products.
The use of our products could result in product liability claims that could be expensive, divert management’s attention and harm our reputation and business.
Our business exposes us to significant risks of product liability claims that are inherent in the testing, manufacturing and marketing of medical devices. Moreover, the FDA has expressed concerns regarding the safety and efficacy of our Sensei
38
System for ablation and other therapeutic indications, including for the treatment of atrial fibrillation and has specifically instructed that our products be labeled to inform our customers that the safety and effectiveness of our technology for use with cardiac ablation catheters in the treatment of cardiac arrhythmias, including for atrial fibrillation, have not been established. We presently believe that to date, all of the procedures in which our Sensei System has been used in the United States have included off-label uses such as cardiac ablation, for which our Sensei System and Artisan Extend catheters have not been cleared by the FDA and which therefore could increase the risk of product liability claims. The medical device industry has historically been subject to extensive litigation over product liability claims. We may be subject to claims by consumers, healthcare providers, third-party payers or others selling our products if the use of our products were to cause, or merely appear to cause, injury or death. Any weakness in training and services associated with our products may also result in product liability lawsuits. Although we maintain clinical trial liability and product liability insurance, the coverage is subject to deductibles and limitations, and may not be adequate to cover future claims. Additionally, we may be unable to maintain our existing product liability insurance in the future at satisfactory rates or adequate amounts. A product liability claim, regardless of its merit or eventual outcome could result in:
• | decreased demand for our products; |
• | injury to our reputation; |
• | diversion of management’s attention; |
• | withdrawal of clinical trial participants; |
• | significant costs of related litigation; |
• | payment of substantial monetary awards to patients; |
• | product recalls or market withdrawals; |
• | loss of revenue; and |
• | the inability to commercialize our products under development. |
Our products and related technologies can be applied in different applications, and we may fail to focus on the most profitable areas or we may be unable to address successfully financial and technology risks associated with new applications, including applications for the vascular market.
We may be unable to develop or commercialize our technology for additional applications. The technology underlying our systems is designed to have the potential for applications beyond EP and vascular disease which require a control catheter to approach diseased tissue. We further believe that the technology underlying our system can provide multiple opportunities to improve the speed and capability of many diagnostic and therapeutic procedures. However, we may be unable, due to limited financial or managerial resources, to develop these applications and seek a separate 510(k) clearance or PMA approval from the FDA for these applications of our technology. Also, due to our limited financial and managerial resources, we may be required to focus on products in selected applications and to forego efforts with regard to other products and industries including expansion of our EP and vascular applications as well as the development of other applications. Failure to capitalize on other applications for our technology may limit the addressable market for our products and our ability to grow our revenues and expand our operations.
We are dedicating significant resources to the development and commercialization of our Magellan System, Magellan Robotic Catheters and associated accessories. These efforts may not produce viable commercial products and may divert our limited resources from more profitable market opportunities. Moreover, we may devote resources to developing products in additional areas but may be unable to justify the value proposition or otherwise develop a commercial market for products we develop in these areas, if any. In that case, the return on investment in these additional areas may be limited, which could negatively affect our results of operations.
If we fail to maintain collaborative relationships with providers of imaging and visualization technology on terms favorable to us, or at all, our Sensei System may not be able to gain market acceptance and our business may be harmed.
Our success depends on our ability to continually enhance and broaden our product offerings in response to changing technologies, customer demands and competitive pressures. We believe that integrating our Sensei System with key imaging and visualization technologies using an open architecture approach is a key element in establishing our Sensei System as important for complex interventional procedures. Our Sensei System currently utilizes a variety of imaging means to visualize and assist in navigating our catheters. These imaging systems include fluoroscopy, intravascular ultrasound and electro-anatomic mapping systems, as well as pre-operatively acquired three-dimensional computed tomography and magnetic resonance imaging. We believe that in the future, as imaging companies develop increasingly sophisticated three-dimensional imaging systems, we will need to integrate advanced imaging into our Sensei System in order to compete effectively. There can be no assurance that we can timely and effectively integrate these systems or components into our Sensei System in order to
39
remain competitive. We expect to face competition from companies that are developing new approaches and products for use in interventional procedures and that have an established presence in the field of interventional cardiology, including the major imaging, capital equipment and disposables companies that are currently selling products in the electrophysiology laboratory. We may not be able to acquire or develop three- dimensional imaging and visualization technology for use with our Sensei System. In addition, developing or acquiring key imaging and visualization technologies could be expensive and time-consuming and may not integrate well with our Sensei System. If we are unable to timely acquire, develop or integrate imaging and visualization technologies, or any other changing technologies, effectively, our revenue may decline and our business will suffer.
Indemnification obligations to our current and former directors and officers and contractual indemnification obligations to underwriters of our securities offerings could adversely affect our ability to defend claims for which we may be liable, our results of operations, our financial condition and our cash flows.
Under Delaware law, our charter documents and certain indemnification agreements, we may have an obligation to indemnify our current and former officers, employees and directors under certain circumstances. In addition, we have contractual indemnification obligations to the underwriters and placement agents in our prior public and private offerings, as applicable, of our equity securities. Some of these advancement and indemnification obligations may not be covered by our directors’ and officers’ insurance policies or may exceed the coverage limits of those policies. If we incur significant uninsured advancement or indemnity obligations, it could have a material adverse effect on our ability to defend claims for which we may be liable, our results of operations, our financial condition and our cash flows.
Future acquisitions could disrupt our business and harm our financial condition and operating results.
Our success will depend, in part, on our ability to expand our offerings and markets and grow our business in response to changing technologies, customer demands and competitive pressures. In some circumstances, we may determine to do so through the acquisition of complementary businesses, solutions or technologies rather than through internal development. The identification of suitable acquisition candidates can be difficult, time-consuming and costly, and we may not be able to successfully complete identified acquisitions. Furthermore, even if we successfully complete an acquisition, we may not be able to successfully assimilate and integrate the business, technologies, solutions, personnel or operations of the company that we acquired, particularly if key personnel of an acquired company decide not to work for us. In addition, we may issue equity securities to complete an acquisition, which would dilute our stockholders’ ownership and could adversely affect the price of our common stock. Acquisitions may also involve the entry into geographic or business markets in which we have little or no prior experience. Consequently, we may not achieve anticipated benefits of the acquisitions which could harm our operating results.
Software defects may be discovered in our products which would damage our ability to sell our products and our results of operations, financial conditions and cash flows.
Our systems incorporate sophisticated computer software. Complex software frequently contains errors, especially when first introduced. Because our products are designed to be used to perform complex interventional procedures, we expect that physicians and hospitals will have an increased sensitivity to the potential for software and other defects. We cannot assure you that our software will not experience errors or performance problems in the future. If we experience software errors or performance problems, we would likely also experience:
• | loss of revenue; |
• | an increase in reportable adverse events to applicable authorities such as the FDA; |
• | delay in market acceptance of our products; |
• | damage to our reputation; |
• | additional regulatory filings; |
• | product recalls; |
• | increased service or warranty costs; and/or |
• | product liability claims relating to the software defects. |
Our costs could substantially increase if we receive a significant number of service claims which would harm our results of operations, financial condition and cash flows.
We typically provide post-contract customer service for each of our products against defects in materials and workmanship for a period of approximately 12 months from the delivery or acceptance of our product by a customer which is normally when the system is shipped. The associated expenses are charged to cost of revenues as incurred. We have a limited history of commercial placements of our Sensei Systems and a very limited history of commercial placements of our Magellan
40
Systems from which to judge our rate of claims against our service contracts. Our obligation under these service contracts may be impacted by product failure rates, material usage and service costs. Unforeseen exposure under these post-contract customer service contracts could negatively impact our business, financial condition and results of operations.
Hospitals or physicians may be unable to obtain coverage or reimbursement from third-party payors for procedures using our Sensei System and Magellan System, which could affect the adoption or use of our systems and may cause our revenues to decline.
While we anticipate that third-party payors will continue to reimburse hospitals and physicians under existing billing codes for the vast majority of the procedures involving our products, there is increased pressure for our customers to reduce costs for their services and operations; similarly, the federal healthcare programs are under increasing pressure to reduce overall costs for items and services. While we expect that healthcare facilities and physicians in the United States will continue to bill various third-party payors, such as Medicare, Medicaid, other governmental programs and private insurers, for services performed using our products, we are unable to predict how government healthcare programs or private insurers will cover or reimburse these procedures in the future. We believe that procedures associated for use with our products are generally already reimbursable under government programs and most private plans. Accordingly, while we believe providers in the United States will generally not be required to obtain new billing authorizations or codes in order to be compensated for performing medically necessary procedures using our products on insured patients, we are unable to predict how future statutes or regulatory guidance will impact coverage reimbursement or the use of specific codes.
There can be no assurance, however, that coverage, coding and reimbursement policies of third-party payors will not change in the future with respect to some or all of the procedures that would use our systems. Additionally, in the event that a physician uses our Sensei System or Magellan System for indications not approved by the FDA, there can be no assurance that the coverage or reimbursement policies of third-party payors will be comparable to FDA-approved uses. Future legislation, regulation or coverage, coding and reimbursement policies of third-party payors may adversely affect the demand for our products currently under development and limit our ability to profitably sell our products. For example, in prior years, certain regulatory changes were made to the methodology for calculating payments for inpatient procedures in certain hospitals, resulting in a decrease to Medicare payment rates for surgical and cardiac procedures, including those procedures for which our products are targeted. The majority of the procedures performed with our Sensei System and Artisan Extend catheter are done on an in-patient basis and thus are paid under the Medicare severity diagnosis related group, or MS-DRG system.
We believe that the majority of procedures performed using our Sensei technology fall under MS-DRG 251, percutaneous cardiovascular procedures without coronary artery stent or acute myocardial infarction without major cardiovascular complication. The Centers for Medicare & Medicaid Services update the MS-DRG payment rates annually effective October 1 through September 30 of the following year. Because hospital inpatient reimbursement is largely dependent on geographical location and other hospital-specific factors, an individual hospital’s revenues from using our technology can vary significantly. At this time, although payments for these cardiac procedures have not undergone further reductions, we cannot predict the full impact any future rate changes, including rate reductions, will have on our revenues or business. We do not currently know which MS-DRGs will apply to procedures performed with our Magellan System or whether reimbursement amounts will be considered favorable by hospitals.
Our success in international markets also depends upon the eligibility of our products for coverage and reimbursement by government-sponsored healthcare payment systems and third-party payors. Recent legislative initiatives in the United States to reform healthcare and government insurance programs have included a focus on healthcare costs which could limit the coverage and reimbursement for procedures utilizing our products. In both the United States and foreign markets, healthcare cost-containment efforts are prevalent and are expected to continue and may increase. The failure of our customers to obtain sufficient reimbursement could have a material adverse impact on our financial condition and harm our business.
Legislative reforms to the United States healthcare system may adversely affect our revenues and business.
From time to time, legislative reform measures are proposed or adopted that would impact healthcare expenditures for medical services, including the medical devices used to provide those services. For example, in March 2010, U.S. President Barack Obama signed the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the Affordable Care Act. The Affordable Care Act made a number of substantial changes in the way health care is financed by both governmental and private insurers and the way that Medicare providers are reimbursed. Among other things, the Affordable Care Act requires certain medical device manufacturers and importers to pay an excise tax equal to 2.3% of the price for which such medical devices are sold, beginning January 1, 2013. As of December 2015, the medical device tax has been suspended for two years.
41
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. On August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2.0% per fiscal year. On January 2, 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or the ATRA, which delayed for another two months the budget cuts mandated by these sequestration provisions of the Budget Control Act of 2011. On March 1, 2013, the President signed an executive order implementing sequestration, and on April 1, 2013, the 2% Medicare payment reductions went into effect. The Bipartisan Budget Act of 2013, enacted on December 26, 2013, extends these cuts to 2023. The ATRA also, among other things, reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. In December 2014, Congress passed an omnibus funding bill and a tax extenders bill, both of which may negatively impact coverage and reimbursement of healthcare items and services. We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for our products or additional pricing pressure.
Government and private sector initiatives to limit the growth of health care costs, including price regulation, competitive pricing, coverage and payment policies, comparative effectiveness reviews of therapies, technology assessments, and managed-care arrangements, are continuing. Government programs, including Medicare and Medicaid, private health care insurance and managed-care plans have attempted to control costs by limiting the amount of reimbursement they will pay for particular procedures or treatments, tying reimbursement to outcomes, and other mechanisms designed to constrain utilization and contain costs, including delivery reforms such as expanded bundling of services. Hospitals are also seeking to reduce costs through a variety of mechanisms, which may increase price sensitivity among customers for our products, and adversely affect sales, pricing, and utilization of our products. Some third-party payors must also approve coverage for new or innovative devices or therapies before they will reimburse health care providers who use the medical devices or therapies. We cannot predict the potential impact of cost-containment trends on future operating results.
New regulations related to conflict minerals could adversely impact our business.
The Dodd-Frank Wall Street Reform and Consumer Protection Act contains provisions to improve transparency and accountability concerning the supply of certain minerals, known as conflict minerals, originating from the Democratic Republic of Congo (DRC) and adjoining countries. As a result, in August 2012 the SEC adopted annual disclosure and reporting requirements for those companies who use conflict minerals mined from the DRC and adjoining countries in their products. These new requirements required due diligence efforts in 2013 and 2014, with initial disclosure requirements beginning in May 2014. In May 2014 and June 2015, we filed a Specialized Disclosure Report on Form SD with the SEC disclosing our on-going diligence efforts and our determination that our products were conflict undeterminable at that time. We expect to file our next specialized Disclosure Report on Form SD with SEC in May 2016.
There have been and will be costs associated with complying with these disclosure requirements, including for diligence to determine the sources of conflict minerals used in our products and other potential changes to products, processes or sources of supply as a consequence of such verification activities. The implementation of these rules could adversely affect the sourcing, supply and pricing of materials used in our products. As there may be only a limited number of suppliers offering “conflict free” conflict minerals, we cannot be sure that we will be able to obtain necessary conflict minerals from such suppliers in sufficient quantities or at competitive prices. Also, we may face reputational challenges if we determine that certain of our products contain minerals not determined to be conflict free or if we are unable to sufficiently verify the origins for all conflict minerals used in our products through the procedures we may implement.
Environmental laws and regulations such as the RoHS directives, could cause a disruption in our business and operations.
We are subject to various state, federal and international laws and regulations governing the environment, including those restricting the presence of certain substances in electronic products and making manufacturers of those products financially responsible for the collection, treatment and recycling and disposal of certain products. Such laws and regulations have been passed in several jurisdictions in which we operate, including various EU member countries. For example, the EU has enacted the WEEE directives. The WEEE directive obligates parties that place electrical and electronic equipment on the market in the EU to put a clearly identifiable mark on the equipment, register with and report to EU member countries regarding distribution of the equipment and provide a mechanism to take back and properly dispose of the equipment. Another example is the RoHS2 directives. On July 1, 2011, the Official Journal of the EU published the revised Directive 2011/65/EU (RoHS 2) on the restriction on the use of certain hazardous substances (six materials specifically identified) in electrical and electronic
42
equipment. This revised Directive (RoHS2) became effective July 21, 2011. The EU used a time-phased approach and identified 10 categories of products that needed to meet these new standards at various start dates.
Category 8 includes medical devices and applies to our products. Compliance with this revised directive for category 8 was required by July 22, 2014. All new products held for commerce in the EU must be in compliance with RoHS 2 as of that date. Any remaining inventory that is not in compliance with RoHS2 will be used in products sold in non-EU countries. RoHS2 compliant products may be sold in both EU and non-EU countries. However, only RoHS2 compliant products may be sold in EU countries. In order to minimize duplicate inventory, we plan to produce all future products in compliance with RoHS2 in order to sell them in both EU and non-EU countries. We recently undertook the lengthy process of changing the materials and processes used, where necessary, to bring our products into compliance with RoHS2. As of the date of filing of this Annual Report on Form10-K, all of our products sold in the EU are RoHS2 compliant. There can be no assurance that similar programs will not be implemented in other jurisdictions resulting in additional costs, possible delays in delivering products, and even the discontinuance of existing and planned future product replacements if the cost were to become prohibitive.
We sell our systems internationally and are subject to various risks relating to such international activities which could adversely affect our international sales and operating performance.
A portion of our current and future revenues will come from international sales. To expand internationally, we will need to hire, train and retain additional qualified personnel. Engaging in international business inherently involves a number of difficulties and risks, including:
• | required compliance with existing and changing foreign regulatory requirements and laws; |
• | export or import restrictions and controls relating to technology; |
• | pricing pressure; |
• | laws and business practices favoring local companies; |
• | longer payment cycles; |
• | shipping delays; |
• | difficulties in enforcing agreements and collecting receivables through certain foreign legal systems; |
• | political and economic instability; |
• | potentially adverse tax consequences, tariffs and other trade barriers; |
• | international terrorism and anti-American sentiment; |
• | difficulties in penetrating markets in which our competitors’ products are more established; |
• | difficulties and costs of staffing and managing foreign operations; and |
• | difficulties in enforcing intellectual property rights. |
If one or more of these risks are realized, it could require us to dedicate significant resources to remedy the situation, and if we are unsuccessful at finding a solution, our revenue may decline.
Our financial results are subject to currency fluctuations as a result of our international operations which could decrease our revenues.
In the fiscal year ended December 31, 2015, approximately 56% of our total revenues were generated outside the United States. While some of these revenues were denominated in U.S. dollars, approximately 8% of our total revenues for the fiscal year ended December 31, 2015 were generated in other currencies. We translate results of transactions denominated in local currencies into U.S. dollars using market conversion rates applicable to the period in which the transaction is reported. As a result, changes in exchange rates during a period can unpredictably and adversely affect our consolidated operating financial results and our asset and liability balances, even if the underlying value of the item in its original currency has not changed. A hypothetical 10% increase in the United States dollar exchange rate used would have resulted in an immaterial decrease in revenues for the fiscal year ended December 31, 2015.
We may be liable for contamination or other harm caused by materials that we handle, and changes in environmental regulations could cause us to incur additional expense.
Our research and development, manufacturing and clinical processes involve the handling of potentially harmful biological materials as well as other hazardous materials. We are subject to federal, state and local laws and regulations governing the use, handling, storage and disposal of hazardous and biological materials and we incur expenses relating to compliance with these laws and regulations. If violations of environmental, health and safety laws occur, we could be held liable for damages, penalties and costs of remedial actions. These expenses or this liability could have a significant negative
43
impact on our financial condition. We may violate environmental, health and safety laws in the future as a result of human error, equipment failure or other causes. Environmental laws could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations. We are subject to potentially conflicting and changing regulatory agendas of political, business and environmental groups. Changes to or restrictions on permitting requirements or processes, hazardous or biological material storage or handling might require an unplanned capital investment or relocation. Failure to comply with new or existing laws or regulations could harm our business, financial condition and results of operations.
The pendency of our agreement to be acquired by Auris Surgical Robotics, Inc. or our failure to complete the merger or successfully integrate with Auris Surgical Robotics, Inc. could have an adverse effect on our business.
On April 19, 2016, we entered into an Agreement and Plan of Merger (the “Merger Agreement”), with Auris Surgical Robotics, Inc. (“Auris”) and Pineco Acquisition Corp. (“Sub”), a wholly owned subsidiary of Auris, providing for the merger of Sub with and into the Company, with the Company surviving the Merger as a wholly owned subsidiary of Auris (“the Merger”). Completion of the Merger remains subject to the satisfaction of various conditions, including the approval of the Company’s stockholders and customary closing conditions, many of which are outside of our control. There is no assurance that all of the various conditions will be satisfied, or that the Merger will be completed on the proposed terms, within the expected time frame, or at all. In addition, if the Merger is not completed by October 20, 2016, subject to certain limitations, either the Company or Auris may choose not to proceed with the Merger. The Merger gives rise to risks that include:
• | the inability to complete the Merger due to the failure to satisfy conditions to the completion of the Merger; |
• | potential stockholder litigation and the costs thereto that could prevent or delay the Merger or otherwise negatively impact our business and operations; |
• | if the Merger is not completed, the price of our common stock will change to the extent that the current market price of our stock reflects an assumption that the Merger will be completed; |
• | the pendency of the Merger, even if ultimately completed, may create uncertainty in the marketplace and could lead customers and prospective customers to purchase products from other vendors or delay purchasing products from the Company; |
• | the amount of cash to be paid under the agreement governing the Merger is fixed and will not be adjusted for changes in our business, assets, liabilities, prospects, outlook, financial condition or results of operations, including any potential long-term value of the successful execution of our current strategy as an independent company or in the event of any change in the market price of, analyst estimates of, or projections relating to, our common stock; |
• | the possibility of disruption to our business, including increased costs and diversion of management time and resources; |
• | difficulties maintaining and renewing business and operational relationships, including relationships with vendors and other business partners; |
• | the fact that under the terms of the Merger Agreement, we are unable, subject to certain exceptions, to solicit or enter into discussions concerning other acquisition proposals during the pendency of the Merger; |
• | the amount of the costs, fees, expenses and charges related to the Merger Agreement or the Merger; |
• | the possibility that our employees could lose productivity as a result of uncertainty regarding their employment post-Merger; |
• | developments beyond our control including, but not limited to, changes in domestic or global economic conditions that may affect the timing or success of the Merger; |
• | relationships with customers, vendors and other business partners may be adversely impacted; |
• | the risk that the Merger is not completed by August 17, 2016 when our obligations under the loan and security agreement with White Oak will become due, and if such amounts were to become due on such date, we would not have sufficient cash to repay such indebtedness, as further described below; and |
• | financial results may be adversely impacted due to costs incurred in connection with the proposed Merger. |
If the Merger Agreement is terminated, we may, under certain circumstances, be obligated to pay a termination fee to Auris and these costs could require us to use cash that we are otherwise required to retain under our arrangement with White Oak or may have been available for general corporate purposes.
If the Merger Agreement is terminated, in certain circumstances, we would be required to pay Auris a termination fee of $3,325,000. If the Merger Agreement is terminated, the termination fee we may be required to pay, if any, under the Merger
44
Agreement may require us to use cash that we are otherwise required to retain under our arrangement with White Oak or may have been available for general corporate purposes. For these and other reasons, a failed Merger could materially and adversely affect our business, results of operations or financial condition, which in turn would materially and adversely affect our business or financial condition, the price per share of our common stock or our perceived acquisition value.
The Company is in default under the loan and security agreement with White Oak and White Oak may exercise its rights and remedies thereunder if the Merger is not completed by August 17, 2016.
The Company has entered into the Forbearance Agreement with White Oak, whereby White Oak granted forbearance of the Specified Events of Default until August 17, 2016 or the date that the Forbearance Agreement is terminated in exchange for a $150,000 non-refundable transaction fee and a prepayment of $5.0 million of the Company’s obligations under the loan and security agreement with White Oak. Under the Merger Agreement, Auris has agreed, promptly following the effectiveness of the Merger, to pay or cause to be paid in full all of the Company’s obligations under the loan and security agreement with White Oak. However, if the Merger is not completed before August 17, 2016, we would experience an event of default under the Forbearance Agreement. In the event of a default under the Forbearance Agreement, the Forbearance Agreement will automatically terminate and White Oak is entitled to exercise its rights and remedies under the Forbearance Agreement and loan and security agreement with White Oak, including acceleration of the loan payment and foreclosing on our assets. Failure to comply with the terms of the indebtedness under the loan and security agreement with White Oak or the Forbearance Agreement could result in a material adverse effect to our business, including our financial condition and liquidity.
While the Merger is pending, we are subject to business uncertainties and contractual restrictions that could harm our operations and the future of our business or result in a loss of customers and employees.
The Merger Agreement includes restrictions on the conduct of our business prior to the completion of the Merger, generally requiring us to conduct our businesses and operations in the ordinary course and in accordance with past practices and to refrain from engaging in certain kinds of transactions, and subjecting us to a variety of specified limitations absent Auris’ prior written consent. We may find that these and other contractual arrangements in the Merger Agreement may delay or prevent us from or limit our ability to respond effectively to competitive pressures, industry developments and future business opportunities that may arise during such period, even if our management and board of directors think they may be advisable. The pendency of the Merger may also divert management’s attention and our resources from ongoing business and operations. Our employees, customers or potential customers, and vendors may have uncertainties about the effects of the Merger. In connection with the pending Merger, it is possible that some customers, vendors and other persons with whom we have a business relationship may delay or defer certain business decisions or might decide to seek to terminate, change or renegotiate their relationship with us as a result of the Merger. Similarly, current and prospective employees may experience uncertainty about their future roles with us following completion of the Merger, which may harm our ability to attract and retain key employees. If any of these effects were to occur, it could materially and adversely impact our business results and financial condition, as well as the market price of our common stock and our perceived acquisition value, regardless of whether the Merger is completed. In addition, whether or not the Merger is completed, while it is pending we will continue to incur costs, fees, expenses and charges related to the proposed Merger, which may materially and adversely affect our business results and financial condition.
Risks Related to Our Intellectual Property
If we are unable to protect the intellectual property contained in our products from use by third parties, our ability to compete in the market will be harmed.
Our commercial success will depend in part on obtaining patent and other intellectual property protection for the technologies contained in our products, and on successfully defending our patents and other intellectual property against third party challenges. We expect to incur substantial costs in obtaining patents and, if necessary, defending our proprietary rights. The patent positions of medical device companies, including ours, can be highly uncertain and involve complex and evolving legal and factual questions. We do not know whether we will be able to obtain the patent protection we seek, or whether the protection we do obtain will be found valid and enforceable if challenged or that such patent protection will confer any significant commercial advantage. We also do not know whether we will be able to develop additional patentable proprietary technologies. If we fail to obtain adequate protection of our intellectual property, or if any protection we obtain is reduced or eliminated, others could use our intellectual property without compensating us, resulting in harm to our business. We may also determine that it is in our best interests to voluntarily challenge a third party’s products or patents in litigation or administrative proceedings, including patent interferences or reexaminations. In the event that we seek to enforce any of our owned or exclusively licensed patents against an infringing party, it is likely that the party defending the claim will seek to invalidate the patents we assert, which, if successful could result in the loss of the entire patent or the relevant portion of our patent, which would not be limited to any particular party. Any litigation to enforce or defend our patent rights, even if we were to prevail, could be costly and time-consuming and could divert the attention of our management and key personnel from our business operations. Our competitors may independently develop similar or alternative technologies or products without infringing any of our patent or other intellectual property rights, or may design around our proprietary technologies.
45
United States patents and patent applications may also be subject to interference proceedings and United States patents may be subject to reexamination proceedings and, starting in 2012, post grant and inter partes review in the United States Patent and Trademark Office, and foreign patents may be subject to opposition or comparable proceedings in the corresponding foreign patent offices, which proceedings could result in either loss of the patent or denial of the patent application, or loss or reduction in the scope of one or more of the claims of, the patent or patent application. In addition, such interference, reexamination, post grant review, inter partes review, and opposition proceedings may be costly. Some of our technology was, and continues to be, developed in conjunction with third parties, and thus there is a risk that such third parties may claim rights in our intellectual property. Thus, any patents that we own or license from others may provide limited or no protection against competitors. Our pending patent applications, those we may file in the future, or those we may license from third parties, may not result in patents being issued. If issued, they may not provide us with proprietary protection or competitive advantages against competitors with similar technology.
Non-payment or delay in payment of patent fees or annuities, whether intentional or unintentional, may result in loss of patents or patent rights important to our business. Many countries, including certain countries in Europe, have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of the patent. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States, particularly in the field of medical products and procedures.
Our trade secrets, nondisclosure agreements and other contractual provisions to protect unpatented technology provide only limited and possibly inadequate protection of our rights. As a result, third parties may be able to use our unpatented technology, and our ability to compete in the market would be reduced. In addition, employees, consultants and others who participate in developing our products or in commercial relationships with us may breach their agreements with us regarding our intellectual property, and we may not have adequate remedies for the breach.
Third parties may assert that we are infringing their intellectual property rights which may result in litigation.
Successfully commercializing our Sensei System, our Magellan System and any other products we may develop, will depend in part on our not infringing patents held by third parties. It is possible that one or more of our products, including those that we have developed in conjunction with third parties, infringes existing patents. From time to time, we have received, and likely will continue to receive, communications from third parties inviting us to license their patents or accusing us of infringement. There can be no assurance that a third party will not take further action, such as filing a patent infringement lawsuit, including a request for injunctive relief, to bar the manufacture and sale of our Sensei System in the United States or elsewhere or the sale of our Magellan System in the United States or elsewhere. We may also choose to defend ourselves by initiating litigation or administrative proceedings to clarify or seek a declaration of our rights. As competition in our market grows, the possibility of a patent infringement claim against us or litigation we will initiate increases.
There may be existing patents which may be broad enough to cover aspects of our future technology. In addition, because patent applications in many countries such as the United States are maintained under conditions of confidentiality and can take many years to issue, there may be applications now pending of which we are unaware and which may later result in issued patents that our products infringe. We do not know whether any of these patents, if challenged, would be upheld as valid, enforceable and infringed by our products or technology. We may be sued by, or become involved in an administrative proceeding with, one or more of these or other third parties. We cannot assure you that a court or administrative body would agree with any arguments or defenses we may present concerning the invalidity, unenforceability or non-infringement of any third-party patent. In addition to the issued patents of which we are aware, other parties may have filed, and in the future are likely to file, patent applications covering products that are similar or identical to ours. We cannot assure you that any patents issuing from applications will not cover our products or will not have priority over our own products and patent applications.
We may not be able to maintain or obtain all the licenses from third parties necessary or advisable for promoting, manufacturing and selling our Sensei System and our Magellan System, which may cause harm to our business, operations and financial condition.
We rely on technology that we license from others, including technology that is integral to our Sensei System and our Magellan System, such as patents and other intellectual property that we have co-exclusively licensed from Intuitive. Under our agreement with Intuitive, we received the right to apply Intuitive’s patent portfolio in the field of intravascular approaches for the diagnosis or treatment of cardiovascular, neurovascular and peripheral vascular diseases. To the extent that we develop or commercialize robotic capability outside the field of use covered by our license with Intuitive, which we may choose to do at some time in the future, we may not have the patent protection and the freedom to operate outside the field which is afforded by the license inside the field. Although we believe that there are opportunities for us to operate outside the licensed field of use
46
without using Intuitive’s intellectual property, Intuitive from time to time has told us that it believes certain of our past activities that have fallen outside the licensed field have infringed its intellectual property rights. Although we disagree with Intuitive’s position, we presently remain focused within our licensed field and so have agreed to inform Intuitive before commencing any further outside clinical investigations for endoluminal applications or engaging in external technology exhibitions at non-intravascular conferences. There can be no assurance that Intuitive will not challenge any activities we engage in outside the intravascular space, and we cannot assure you that in the event of such a challenge we would be able to reach agreement with Intuitive on whether activities outside our licensed field may be conducted without the use of the Intuitive’s intellectual property. If Intuitive asserts that any of our activities outside the licensed field are infringing their patent or other intellectual property rights or commences litigation against us, we will incur significant costs defending against such claims or seeking an additional license from Intuitive, and we may be required to limit use of our systems or future products and technologies within our licensed intravascular field if any of our activities outside the licensed field are judged to infringe Intuitive’s intellectual property, any of which could cause substantial harm our business, operations and financial condition. Although Intuitive is restricted in how it can terminate our license, if Intuitive were ever to successfully do so, and if we are unable to obtain another license from Intuitive, we could be required to abandon use of our existing product technology completely and could have to undergo a substantial redesign and design-around effort, which we cannot assure you would be successful. In October 2012, we signed an updated license agreement with Intuitive. Under the terms of the agreement, Intuitive’s existing co-exclusive rights to our patent portfolio to certain non-vascular procedures were extended to include patents filed or conceived by us subsequent to the original 2005 agreement through October 26, 2015. We retain the right to use our intellectual property for all clinical applications, both vascular and non-vascular.
The medical device industry is characterized by patent litigation and we could become subject to litigation that could be costly, result in the diversion of management’s attention, require us to pay damages and discontinue selling our products.
The medical device industry is characterized by frequent and extensive litigation and administrative proceedings over patent and other intellectual property rights. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often difficult to predict, and the outcome may be uncertain until the relevant court has entered final non-appealable judgement. Our competitors may assert, and have asserted in the past, that our products or the use of our products are covered by United States or foreign patents held by them. This risk is heightened due to the numerous issued and pending patents relating to the use of robotic and catheter-based procedures in the medical technology field. For example, we have received correspondence from a third party indicating it believes it holds a patent that our Sensei System may infringe. While we do not believe that the Sensei System infringes this patent, there can be no assurance that the third party will not take further action, such as filing a patent infringement lawsuit, including a request for injunctive relief, to bar the manufacture and sale of our Sensei System in the United States.
If relevant patents are upheld as valid and enforceable and we are found to infringe, we could be prevented from selling our system unless we can obtain a license to use technology or ideas covered by such patent or are able to redesign our products to avoid infringement. A license may not be available at all or on commercially reasonable terms, and we may not be able to redesign our products to avoid infringement. Modification of our products or development of new products could require us to conduct additional clinical trials and to revise our filings with the FDA and other regulatory bodies, which would be time-consuming and expensive. If we are not successful in obtaining a license or redesigning our products, we may be unable to sell our products and our business could suffer. In addition, our patents may be subject to various invalidity attacks, such as those based upon earlier filed patent applications, patents, publications, products or processes, which might invalidate or limit the scope of the protection that our patents afford.
Infringement actions, validity challenges and other intellectual property claims and proceedings, whether with or without merit, may cause us to incur substantial costs and could place a significant strain on our financial resources, divert the attention of management from our business and harm our reputation. We have incurred, and expect to continue to incur, substantial costs in obtaining patents and expect to incur substantial costs defending our proprietary rights. Incurring such costs could have a material adverse effect on our financial condition, results of operations and cash flow.
We cannot be certain that we will successfully defend our patents from infringement or claims of invalidity or unenforceability, or that we will successfully defend against allegations of infringement of third-party patents. In addition, any public announcements related to litigation or administrative proceedings initiated or threatened by us, or initiated or threatened against us, could cause our stock price to decline.
We may be subject to damages resulting from claims that our employees or we have wrongfully used or disclosed alleged trade secrets of their former employers.
Many of our employees were previously employed at universities or other medical device companies, including our competitors or potential competitors. We could in the future be subject to claims that these employees, or we, have
47
inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending against such claims, a court could order us to pay substantial damages and prohibit us from using technologies or features that are essential to our products, if such technologies or features are found to incorporate or be derived from the trade secrets or other proprietary information of the former employers. An inability to incorporate technologies or features that are important or essential to our products would have a material adverse effect on our business, and may prevent us from selling our products. In addition, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize certain potential products, which could severely harm our business. Even if we are successful in defending against these claims, such litigation could result in substantial costs and be a distraction to management. Incurring such costs could have a material adverse effect on our financial condition, results of operations and cash flow.
Additional Risks Related to Regulatory Matters
If we fail to obtain regulatory clearances in other countries for existing products or products under development, we will not be able to commercialize these products in those countries.
In order to market our products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding quality, safety and efficacy of our products. Approval and CE marking procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval or CE Certificate of Conformity in other countries might differ from that required to obtain FDA clearance. The regulatory approval or CE marking process in other countries may include all of the risks detailed above regarding FDA clearance in the United States. Regulatory approval or the CE marking of a product in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval or a CE Certificate of Conformity in one country may negatively impact the regulatory process in others. Failure to obtain regulatory approval or a CE Certificate of Conformity in other countries or any delay or setback in obtaining such approval could have the same adverse effects described above regarding FDA clearance in the United States.
For example, in the EEA, our devices are required to comply with the Essential Requirements laid down in Annex I to the Medical Devices Directive (applicable in the non-EU EEA member states via the Agreement on the EEA). We are also required to ensure compliance with the relevant quality system requirements laid down in the Annexes to the Medical Devices Directive. Companies compliant with ISO requirements such as “EN ISO 13485: 2003 Medical devices — Quality management systems — Requirements for regulatory purposes” benefit from a presumption of conformity with the relevant Essential Requirements or the quality system requirements laid down in the Annexes to the Medical Devices Directive. Following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the Essential Requirements and quality system requirements, the Notified Body issues a CE Certificate of Conformity. This Certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity. We received a CE Certificate of Conformity for our Sensei System in September 2006, our Artisan Extend catheters in May 2007, our Magellan System in July 2011, our Magellan Robotic Catheter and related accessories designed for use with the Magellan System in October 2011, our Artisan Extend catheters in February 2013, our Magellan 6Fr Robotic Catheter in October 2014 and our Magellan 10Fr Robotic Catheter in April 2015. We cannot be certain that we will be successful in meeting and continuing to meet the requirements of the Medical Devices Directive in the EEA.
We may fail to comply with continuing postmarket regulatory requirements of the FDA and other authorities and become subject to substantial penalties, or marketing experience may show that our device is unsafe, forcing us to recall or withdraw it permanently from the market.
We must comply with continuing regulation by the FDA and other authorities, including the FDA’s Quality System Regulation ("QSR"), requirements, labeling and promotional requirements and medical device adverse event and other reporting requirements. If the adverse event reports we file with the FDA regarding death, serious injuries or malfunctions indicate or suggest that the device presents an unacceptable risk to patients, including when used off-label by physicians, we may be forced to recall the device and/or modify the device or its labeling, or withdraw it permanently from the market. The FDA has expressed concerns regarding the safety of the Sensei System when used with catheters and in procedures not specified in the current label, such as ablation catheters and ablation procedures, and we have already filed Medical Device Reports reporting adverse events during procedures utilizing our technology. Physicians may be using our device off-label with ablation catheters in ablation procedures, as well as in other EP procedures for which we have not collected safety data, and we therefore cannot assure you that clinical experience will demonstrate that the device is safe for these uses.
Any failure to comply, or any perception that we are not complying, with continuing regulation by the FDA or other authorities, including restrictions regarding off-label promotion, could result in enforcement action that may include suspension or withdrawal of regulatory clearances approvals, or CE Certificates of Conformity, recalling products, ceasing product
48
marketing, seizure and detention of products, paying significant fines and penalties, criminal prosecution and similar actions that could limit product sales, delay product shipment and harm our profitability and reputation.
In many foreign countries in which we market our products, we are subject to regulations affecting, among other things, product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. Many of these regulations are similar to those of the FDA. In addition, in many countries the national health or social security organizations require our products to be qualified before procedures performed using our products become eligible for coverage and reimbursement. Failure to receive, or delays in the receipt of, relevant foreign qualifications could have a material adverse effect on our business, financial condition and results of operations. If we fail to comply with applicable foreign regulatory requirements, we may be subject to fines, suspension or withdrawal of regulatory clearances and CE Certificates of Conformity, product recalls, seizure of products, operating restrictions and criminal prosecution.
If we or our contract manufacturers fail to comply with the FDA’s Quality System Regulations, California Department of Health Services requirements or EEA quality system requirements, our manufacturing operations could be interrupted and our product sales and operating results could suffer.
Our manufacturing processes, and those of some of our contract manufacturers, are required to comply with the FDA’s QSR which cover the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our devices. The FDA enforces the QSR through periodic inspections of manufacturing facilities. We and our contract manufacturers are subject to such inspections. Similar quality system requirements also apply in the EEA. If our manufacturing facilities or those of any of our contract manufacturers fail to take satisfactory corrective action in response to an adverse quality system inspection, the FDA, the U.S. Department of Justice, the Notified Body or the competent authorities in the EEA could take enforcement action, including any of the following administrative or judicial sanctions, which could have a material impact on our operations:
• | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
• | unanticipated expenditures to address or defend such actions; |
• | customer notifications or mandatory plan products for repair, replacement or refunds; |
• | recall, detention or seizure of our products; |
• | operating restrictions or partial suspension or total shutdown of production; |
• | refusing or delaying our requests for submissions seeking 510(k) clearance, IDE to perform clinical studies or premarket approval of new products or modified products; |
• | operating restrictions; |
• | withdrawing 510(k) clearances or IDE/PMA approvals that have already been granted; |
• | suspension or withdrawal of our CE Certificates of Conformity; |
• | refusal to grant export approval or issue export documentation for our products; |
• | import holds; or |
• | criminal prosecution. |
We underwent an FDA inspection, which employed the Quality System Inspection Technique, or QSIT, in 2014 and received one inspectional observation. We received the establishment inspection report (EIR) from the FDA on January 26, 2015 after the agency closed the inspection per CFT 20.64.We are subject to the licensing requirements of the California Department of Health Services, or CDHS. We have been inspected and licensed by the CDHS and remain subject to re-inspection at any time. Failure to maintain a license from the CDHS or to meet the inspection criteria of the CDHS would disrupt our manufacturing processes. If an inspection by the CDHS indicates that there are deficiencies in our manufacturing process, we could be required to take remedial actions at potentially significant expense, and our facility may be temporarily or permanently closed.
If our products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions. An increased frequency of filing Medical Device Reports, or MDRs, or Manufacturers’ Incident Reports in the EEA concerning adverse events occurring during procedures performed with our technology could result in increased regulatory scrutiny of our products and could delay or prevent the adoption of our products.
Under the FDA’s medical device reporting regulations, medical device manufacturers are required to report to the FDA when the manufacturer becomes aware of information from any source that alleges that a device marketed by the manufacturer has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or one of our similar devices were to recur. A manufacturer
49
may determine that an event may not meet the FDA’s reporting criteria so that an MDR is not necessary. However, the FDA can review a manufacturer’s decision and may disagree. We have made decisions that certain types of events are not MDR reportable. In the EEA, similar reporting requirements are imposed on medical device manufacturers. When a medical device is suspected to be a contributory cause of an event that led or might have led to death of or the serious deterioration of the health of a patient, or user or of other person, its manufacturer or authorized representative in the EU must report the event to the competent authority of the EEA country where the incident occurred. There can be no assurance that the FDA or the competent authorities in the EEA country will agree with our decisions. If we fail to report MDRs to the FDA within the required timeframes, or at all, or if the FDA or the competent authorities of the EEA countries disagree with any of our determinations that events are not reportable, the FDA or the competent authorities of the EEA countries could take enforcement action against us. Any such adverse event involving our products also could result in future voluntary corrective actions, such as products withdrawals and recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
We have filed MDRs and Manufacturer’s Incident Reports reporting adverse events during procedures utilizing our technology and have developed internal systems and processes that are designed to evaluate future events that may require adverse event reporting to the FDA or the competent authorities in the EEA countries. As the frequency of use of our technology in EP and vascular procedures increases, we are experiencing, and anticipate continuing to experience, it being necessary to file an increased number of MDRs and Manufacturer’s Incident Reports resulting from the increased frequency of use of our technology. An increased frequency of filing MDRs and Manufacturer’s Incident Reports or a failure to timely file MDRs may result in requests for further information from the FDA or the competent authorities of the EEA countries, which could delay other matters that we may have pending before the FDA, the competent authorities of the EEA or our Notified Body or result in additional regulatory action. An increased frequency of MDRs and Manufacturer’s Incident Reports could also reduce confidence in the safety of our products and delay or prevent the acceptance of our products by physicians and hospitals, which would harm our business and cause our stock price to decline.
Our products may in the future be subject to product recalls that could harm our reputation, business and financial results. As a manufacturer we are sometimes required to make decisions about whether to take corrective action in the field and whether to report that activity to the FDA or the competent authorities of the EEA countries. If the FDA or the competent authorities of the EEA countries disagrees with those decisions, we may be subject to enforcement action and our product sales and operating results could suffer.
The FDA and similar foreign competent authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, foreign competent authorities have the authority to require the recall of our products in the event of material deficiencies or defects in design, manufacture or performance of the products and inadequacy in the labeling or Instruction for Use. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. We have conducted voluntary recalls in the past. Recalls of any of our products would likely divert managerial and financial resources and could have an adverse effect on our financial condition and results of operations.
The FDA requires that certain classifications of recalls be reported to the FDA within 10 working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA or other competent authorities. We have in the past initiated voluntary actions involving our products that we determined did not require notification of the FDA, and we may in the future initiate additional voluntary actions that we determine do not require notification of the FDA. If the FDA or the competent authorities of the EEA countries disagree with our determinations, they could require us to report those actions as recalls. Additionally, we have, and may again in the future, take actions in the field that we do not consider to be recalls. If the FDA or other competent authorities disagree with our determinations, they could require us to treat these actions as recalls, issue communications, or report those actions to FDA. The agency may also initiate other enforcement action if they disagree with our recall decisions, including but not limited to issuing warning letters, or more serious actions such as civil or criminal penalties. A future recall announcement or enforcement action could harm our reputation with customers and negatively affect our sales. In addition, the FDA or other competent authorities could take enforcement action for failing to treat certain actions as recalls and report the recalls when they were conducted.
Modifications to our products may, and in some instances, will, require new regulatory clearances, approvals or CE Certificates of Conformity and may require us to recall or cease marketing our products until clearances, approvals or CE Certificates of Conformity are obtained.
50
Modifications to our products may require new regulatory approvals or clearances, including 510(k) clearances or PMAs, and may require us to recall or cease marketing the modified devices until these clearances or approvals are obtained. The FDA requires device manufacturers to initially make and document a determination of whether or not modifications require a new approval, supplement or clearance. A manufacturer of a 510(k) cleared product is required to obtain 510(k) clearance for device modifications that could significantly affect the safety or effectiveness of the device, or constitute a major change in the intended use of the subject device. Accordingly, a manufacturer may determine that a modification could not significantly affect safety or efficacy and does not represent a major change in its intended use so that no new 510(k) clearance is necessary. However, the FDA can review a manufacturer’s decision and may disagree. The FDA may also on its own initiative determine that a new clearance or approval is required.
For those products sold in the EEA, we must notify and await completion of the review of our Notified Body before introducing substantial changes to the products or to our quality system. Following its review, our Notified Body will decide whether our existing CE Certificates of Conformity can be maintained or varied, or whether new certificate are required.
We have made modifications to our products in the past and may make additional modifications in the future that we believe do not or will not require additional review, clearances or approvals. There can be no assurance that the FDA, our Notified Body or the competent authorities of the EEA countries will agree with our approach in such matters or that, if required, subsequent requests for 510(k) clearance, PMA approval or CE Certificates of Conformity will be received in a timely fashion, if at all. The FDA, our Notified Body or the competent authorities of the EEA countries may require us to cease supply, recall and to stop marketing our products as modified or to disable features pending clearance or approval or the granting of a CE Certificate of Conformity which would significantly harm our ability to sell our products and cause harm to our existing customer relationships and business. Even if we are not required to take such action, delays in obtaining clearances, approvals or CE Certificate of Conformity for features would adversely affect our ability to introduce enhanced products in a timely manner and would harm our revenue and operating results. The FDA our Notified Body or the competent authorities of the EEA countries could also take other enforcement action, including but not limited to, issuing a warning letter relating to our decision to implement features and other product modifications without submission of a new 510(k) notice or PMA and suspension or withdrawal of our existing CE Certificates of Conformity.
Clinical trials and clinical investigations necessary to support any future 510(k), PMA application or CE marking of our products will be expensive and may require the enrollment of large numbers of clinical sites and patients, and suitable patients may be difficult to identify and recruit. Delays or failures in our clinical trials may prevent us from commercializing any modified or new products and will adversely affect our business, operating results and prospects.
Initiating and completing clinical trials and clinical investigations necessary to support a 510(k), PMA application or CE marking for expanded indications for use of our existing products, will be time consuming and expensive and the outcome uncertain. Moreover, the results of early clinical trials are not necessarily predictive of future results, and any product we advance into clinical trials may not have favorable results in later clinical trials and clinical investigations.
Conducting successful clinical studies and clinical investigations may require the enrollment of large numbers of clinical sites and patients, and suitable patients may be difficult to identify and recruit. Patient enrollment in clinical trials and clinical investigations and completion of patient participation and follow-up depends on many factors, including the size of the patient population, the nature of the clinical trial investigation/protocol, the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects, the availability of appropriate clinical trial investigators, support staff, and proximity of patients to clinical sites and ability to comply with the eligibility and exclusion criteria for participation in the clinical trial and patient compliance. For example, patients may be discouraged from enrolling in our clinical trials if the protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and effectiveness of our products or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials/investigations if they choose to participate in contemporaneous clinical trials/investigations of competitive products or they can obtain the treatment without participating in our trial/investigations through physicians who use the product off-label. Development of sufficient and appropriate clinical protocols to demonstrate quality, safety and efficacy may be required and we may not adequately develop such protocols to support clearance or approval. Delays in patient enrollment or failure of patients to consent or continue to participate in a clinical trial/investigation may cause an increase in costs and delays in the approval and attempted commercialization of our products or result in the failure of the clinical trial/investigation. In addition, despite considerable time and expense invested in our clinical trials, FDA, our Notified Body or the competent authorities of the EEA countries may not consider our data adequate to demonstrate quality, safety and efficacy. Such increased costs and delays or failures could adversely affect our business, operating results and prospects.
If we fail to comply with healthcare laws and regulations, we could face substantial penalties and our business, operations and financial condition could be adversely affected.
51
Our activities, and the activities of our agents, including some contracted third parties, are subject to extensive government regulation and oversight both in the U.S. and in foreign jurisdictions. Our interactions in the U.S. or abroad with physicians and other potential referral sources who prescribe or purchase our products are subject to government regulation designed to prevent health care fraud and abuse. Relevant U.S. laws include:
• | the federal healthcare program Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, paying or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual, for an item or service or the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any item or service, for which payment may be made, in whole or in part, by federal healthcare programs such as the Medicare and Medicaid; |
• | federal civil False Claims Act which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment of government funds that are false or fraudulent; |
• | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters and which also imposes certain obligations relating to safeguarding the privacy, security and transmission of individually identifiable health information; |
• | the federal Foreign Corrupt Practices Act of 1997, which makes it illegal to offer or provide money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage; and |
• | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, state breach notification laws, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians. Some states, such as California, Massachusetts and Nevada, mandate implementation of commercial compliance programs and/or impose restrictions on device manufacturer marketing practices and tracking and reporting of gifts, compensation and other remuneration to physicians. |
The FDA, the Office of Inspector General for the Department of Health and Human Services ("OIG"), the U.S. Department of Justice, states’ Attorneys General and other governmental authorities actively enforce the laws and regulations discussed above. In the U.S., pharmaceutical and device manufacturers have been the target of numerous government prosecutions and investigations alleging violations of law, including claims asserting impermissible off-label promotion of pharmaceutical and medical device products, payments intended to influence the referral of federal or state health care business, and submission of false or fraudulent claims for government payments. The Affordable Care Act also clarified that a person or entity need not have actual knowledge of the Anti-Kickback Statute or specific intent in order to violate it. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act or federal civil money penalties statute. As part of our compliance program, we have reviewed our sales contracts and marketing materials and practices to assure compliance with these federal and state laws, and inform employees and marketing. Although compliance programs can mitigate the risk of investigation and prosecution for violations of these laws, the risks cannot be entirely eliminated. We cannot rule out the possibility that the government or other third parties could interpret these laws differently and challenge our practices under one or more of these laws.
The Affordable Care Act also imposes new tracking and disclosure requirements on device manufacturers for any “transfer of value” made or distributed to physicians and teaching hospitals. Device manufacturers with products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program were required to begin tracking such payments on August 1, 2013 and submit reports to the Center for Medicare and Medicaid Services, or CMS, by March 31, 2014, and by the 90th day of each subsequent calendar year. We began tracking applicable payments and transfers of value on August 2013 and began reporting payment data to the CMS in March 2014 and will continue to do so annually thereafter.
If our past or present operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion of our products from reimbursement under federal healthcare programs like Medicare and Medicaid and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws or regulations is increased by the fact that many of these laws or regulations have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal
52
expenses and divert our management’s attention from the operation of our business. Moreover, to achieve compliance with applicable federal and state privacy, security, and electronic transaction laws, we may be required to modify our operations with respect to the handling of patient information. Implementing these modifications may prove costly. At this time, we are not able to determine the full consequences to us, including the total cost of compliance, of these various federal and state laws.
Our international operations expose us to liability under global anticorruption laws.
We are also subject to the U.S. Foreign Corrupt Practices Act ("FCPA") and similar worldwide anti-bribery laws in non-U.S. jurisdictions, such as the U.K. Bribery Act 2010, which generally prohibit companies and their intermediaries from making improper payments to government officials and/or other persons for the purpose of obtaining or retaining business. Because of the predominance of government-sponsored healthcare systems around the world, many of our customer relationships outside of the United States involve governmental entities and are therefore subject to such anti-bribery laws. Our policies mandate compliance with these anti-bribery laws. Despite our training and compliance programs, our internal control policies and procedures may not protect us from negligent, reckless or criminal acts committed by our employees or agents. Moreover, even a perceived or alleged violation could result in costly investigations or proceedings that could harm our financial position and reputation.
The application of state certificate of need regulations and compliance by providers with federal and state licensing requirements, as well as accreditation requirements, could substantially limit our ability to sell our products and grow our business.
Some states require healthcare providers to obtain a certificate of need or similar regulatory approval prior to the acquisition of high-cost capital items such as our Sensei and Magellan Systems. In many cases, a limited number of these certificates are available and, as a result, hospitals and other healthcare providers may be unable to obtain a certificate of need for the purchase of our Sensei and Magellan Systems. Further, our sales cycle for our system is typically longer in certificate of need states due to the time it takes our customers to obtain the required approvals. In addition, our customers must meet various federal and state regulatory and/or accreditation requirements in order to receive reimbursement from government-sponsored healthcare programs such as Medicare and Medicaid and other third-party payors. Any lapse by our customers in maintaining appropriate licensure, certification or accreditation, or the failure of our customers to satisfy the other necessary requirements under government-sponsored healthcare programs, could cause our sales to decline.
Risks Related to Ownership of Our Common Stock
The trading price of our common stock has been volatile and is likely to be volatile in the future.
The trading price of our common stock has been highly volatile. From October 1, 2012 through December 31, 2015, our closing stock price has fluctuated from a low of $2.33 to a high of $27.50 (adjusted to reflect the Reverse Split). The market price for our common stock may be affected by a number of factors, including those set forth in this Item 1A as well as:
• | the announcement of our operating results, including the number of systems sold during a period and our revenue for the period, and the comparison of these results to the expectations of analysts and investors; |
• | the receipt, denial or timing of regulatory clearances, approvals or actions of our products or competing products; |
• | sales of common stock or other debt or equity securities by us or our stockholders in the future; |
• | the success of any collaborations we may undertake with other companies; |
• | our ability to develop, introduce and market new or enhanced versions of our products on a timely basis; |
• | additions or departures of key scientific or management personnel; |
• | the pace of enrollment or results of our currently planned clinical trial of at least 125 patients or any other clinical trials; |
• | changes in policies affecting third-party coverage and reimbursement in the United States and other countries; |
• | ability of our products to achieve market success; |
• | the performance of third-party contract manufacturers and component suppliers; |
• | our ability to develop sales and marketing capabilities; |
• | our ability to manufacture our products to meet commercial and regulatory standards; |
• | our ability to manage costs and improve margins; |
• | actual or anticipated volatility in our results of operations or those of our competitors; |
• | announcements of new products, technological innovations or product advancements by us or our competitors; |
• | announcements of acquisitions or dispositions by us or our competitors; |
• | developments with respect to patents and other intellectual property rights; |
53
• | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
• | trading volume of our common stock; |
• | our announcements of guidance regarding future operating or financial results which fails to meet investor or analyst expectations or which differs from our previously-announced guidance; |
• | changes in earnings estimates or recommendations by securities analysts, failure to obtain analyst coverage of our common stock or our failure to achieve analyst earnings estimates; |
• | public statements by analysts or clinicians regarding their perceptions of the effectiveness of our products; |
• | developments in our industry; |
• | general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors; and |
• | the impact of shareholder lawsuits and governmental investigations both on us and on our public perception. |
The stock prices of many companies in the medical device industry have experienced wide fluctuations that have often been unrelated to the operating performance of these companies. Following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against those companies. Additional class action securities litigation, if instituted against us, could result in substantial costs and a diversion of our management resources, which could significantly harm our business.
If we fail to comply with the continued listing requirements of the NASDAQ Capital Market, our common stock may be delisted and the price of our common stock and our ability to access the capital markets could be negatively impacted.
Our common stock is listed for trading on the NASDAQ Capital Market (“NASDAQ”). We must satisfy NASDAQ’s continued listing requirements, including, among other things, a minimum closing bid price requirement of $1.00 per share for 30 consecutive business days. If a company trades for 30 consecutive business days below the $1.00 minimum closing bid price requirement, NASDAQ will send a deficiency notice to the company, advising that it has been afforded a “compliance period” of 180 calendar days to regain compliance with the applicable requirements. Thereafter, if such a company does not regain compliance with the bid price requirement, a second 180-day compliance period may be available.
A delisting of our common stock from NASDAQ could materially reduce the liquidity of our common stock and result in a corresponding material reduction in the price of our common stock. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the potential loss of confidence by investors, employees and fewer business development opportunities.
On April 13, 2015, we received a letter from the Listing Qualifications Staff of NASDAQ notifying us that because the closing bid price of our common stock had been below $1.00 for 30 consecutive business days, our stock no longer complied with the requirements for continued listing on the NASDAQ Capital Market. In accordance with NASDAQ rules, we were provided a period of 180 calendar days, or until October 12, 2015, in which to regain compliance. In order to regain compliance with the minimum bid price requirement, the closing bid price of our common stock must be at least $1.00 per share for a minimum of 10 consecutive business days during this 180 day period.
On September 22, 2015, we effected a reverse stock split of our common stock by a ratio of 1-for-10 (the “Reverse Split”). As a result of the Reverse Split every ten outstanding shares of our common stock became one share of common stock. During the 10 consecutive business days following the Reverse Split and through October 12, 2015, the closing bid price of our common stock was at least $1.00 per share. Therefore, we are as of the date hereof in compliance with the NASDAQ requirements for continued listing. However, there can be no assurance that we will continue to comply with the minimum bid price requirement or maintain compliance with other listing requirements.
Securities analysts may not continue, or additional securities analysts may not initiate, coverage for our common stock or may issue negative reports, and this may have a negative impact on the market price of our common stock.
Currently, several securities analysts provide research coverage of our common stock. Several analysts have already published statements that do not portray our technology, products or procedures using our products in a positive light and others may do so in the future. If we are unable to educate those who publicize such reports about the benefits we believe our technology provides, or if one or more of the analysts who elects to cover us downgrades our stock, our stock price would likely decline rapidly.
If one or more of these analysts ceases coverage of our company, we could lose visibility in the market, which in turn could cause our stock price to decline. The trading market for our common stock may be affected in part by the research and reports that industry or financial analysts publish about us or our business. If sufficient securities analysts do not cover our common stock, the lack of research coverage may adversely affect the market price of our common stock. It may be difficult
54
for companies such as ours, with smaller market capitalizations, to attract and maintain sufficient independent financial analysts that will cover our common stock. This could have a negative effect on the market price of our stock.
Our principal stockholders, directors and management own a large percentage of our voting stock, which allows them to exercise significant influence over matters subject to stockholder approval.
Based on our review of publicly available filings as of April 15, 2016, all those known by the Company to be beneficial owners of more than five percent of our common stock together with our executive officers and directors, beneficially own or control approximately 62.4 percent of our outstanding common stock. Accordingly, our principal stockholders and our executive officers and directors have substantial influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transaction. These stockholders may also delay or prevent a change of control or otherwise discourage a potential acquirer from attempting to obtain control of us, even if such a change of control would benefit our other stockholders. This significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
We have not paid dividends in the past and do not expect to pay dividends in the future, and any return on investment may be limited to the value of our common stock.
We have never paid dividends on our common stock and do not anticipate paying dividends on our common stock in the foreseeable future. The payment of dividends on our common stock will depend on our earnings, financial condition and other business and economic factors affecting us at such time as our Board of Directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates. In addition, pursuant to our loan and security agreement, we must obtain the lenders’ prior written consent in order to pay any dividends on our common stock.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders.
Provisions in our amended and restated certificate of incorporation and amended and restated bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would benefit our stockholders. These provisions:
• | permit our Board of Directors to issue up to 10,000,000 shares of preferred stock, with any rights, preferences and privileges as they may designate, including the right to approve an acquisition or other change in our control; |
• | provide that the authorized number of directors may be changed only by resolution of the Board of Directors; |
• | provide that all vacancies, including newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum; |
• | divide our Board of Directors into three classes; |
• | require that any action to be taken by our stockholders must be effected at a duly called annual or special meeting of stockholders and not be taken by written consent; |
• | provide that stockholders seeking to present proposals before a meeting of stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide notice in writing in a timely manner, and also specify requirements as to the form and content of a stockholder’s notice; |
• | do not provide for cumulative voting rights, therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose; |
• | provide that special meetings of our stockholders may be called only by the chairman of the Board, our chief executive officer or by the Board of Directors pursuant to a resolution adopted by a majority of the total number of authorized directors; and |
• | provide that stockholders will be permitted to amend our amended and restated bylaws only upon receiving at least 66 2/3 percent of the votes entitled to be cast by holders of all outstanding shares then entitled to vote generally in the election of directors, voting together as a single class. |
In addition, we are subject to Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any broad range of business combinations with any stockholder who owns, or at any time in the last three years owned, 15 percent or more of our outstanding voting stock for a period of three years following the date on which the stockholder became an interested stockholder. This provision could have the effect of delaying or preventing a change of control, whether or not it is desired by or beneficial to our stockholders.
55
Future sales or issuances of shares of our common stock, the announcement to undertake such sales or issuances, or the perception that such sales or issuances may occur, may dilute our existing stockholders and depress the market price of our common stock.
Sales of our common stock or securities convertible into or exercisable for our common stock by us or by our stockholders, announcements of the proposed sales of our common stock or securities convertible into or exercisable for our common stock or the perception that sales may be made, could cause the market price of our common stock to decline. We may issue additional shares of our common stock or securities convertible into or exercisable for our common stock in follow-on offerings to raise additional capital or in connection with acquisitions, corporate alliances or settlements with third parties and we plan to issue additional shares to our employees, directors or consultants in connection with their services to us. For example, in March 2015, we sold 53,846 shares of our Series A convertible preferred stock which converted into 5,509,492 shares of our common stock, as well as Series E Warrants to purchase an aggregate of 5,384,600 shares of our common stock in a private placement. The issuance of the shares of common stock resulted in immediate dilution to our stockholders and the on-going exercises of outstanding warrants has caused, and may continue to cause, further dilution to our stockholders in the future.
Our financial results may vary significantly from period to period, which may reduce our stock price.
Our financial results may fluctuate as a result of a number of factors, many of which are outside of our control, which may cause the market price of our common stock to fall. For these reasons, comparing our operating results on a period-to-period basis may not be meaningful, and you should not rely on our past results as an indication of our future performance. Our financial results may be negatively affected by any of the risk factors listed in this “Risk Factors” section.
We incur significant costs as a result of operating as a public company, and our management is required to devote substantial time to new compliance initiatives.
As a public company, we incur significant legal, accounting and other expenses. In addition, the Sarbanes-Oxley Act, as well as rules subsequently implemented by the SEC and the Nasdaq Global Market, have imposed various new requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Our management and other personnel devote a substantial amount of time to these requirements. Moreover, these rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
We lease approximately 63,000 square feet of manufacturing, laboratory and office space in Mountain View, California. We extended the term of this lease until January 2020, with an option to extend the lease for another five years. As of December 31, 2015, the Company has exercised the option to exit the Mountain View lease in 2016. As of December 31, 2015, the Company has signed a new lease to relocate to a smaller manufacturing, laboratory and office space in San Jose, California.
ITEM 3. LEGAL PROCEEDINGS
From time to time, we are involved in litigation that we believe is of the type common to companies engaged in our line of business, including commercial disputes and employment issues. As of the date of this Annual Report on Form 10-K, we are not involved in any pending legal proceedings that we believe could have a material adverse effect on our financial condition, results of operations or cash flows. From time to time, we may pursue litigation to assert our legal rights and such litigation may be costly and divert the efforts and attention of our management and technical personnel which could adversely affect our business.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
56
PART II.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market For Our Common Stock
Our common stock is traded on The Nasdaq Global Market under the symbol “HNSN.”
As of April 15, 2016, there were approximately 139 holders of record of our common stock and 18,989,280 shares of common stock outstanding. Because many of our shares of common stock are held by brokers or other institutions on behalf of stockholders, we are unable to estimate the total number of stockholders represented by the record holders. No dividends have been paid on our common stock to date, and we do not anticipate paying any dividends in the foreseeable future. Our $33 million loan and security agreement with White Oak limits our ability to pay dividends.
The following table sets forth the high and low closing price of our common stock as reported on the NASDAQ Global Market for each period indicated:
2015 | 2014 | ||||||||||||||
High * | Low * | High | Low | ||||||||||||
First quarter | $ | 11.20 | $ | 5.70 | $ | 2.60 | $ | 1.65 | |||||||
Second quarter | $ | 10.70 | $ | 7.80 | $ | 2.75 | $ | 1.19 | |||||||
Third quarter | $ | 9.20 | $ | 3.71 | $ | 1.32 | $ | 1.12 | |||||||
Fourth quarter | $ | 4.24 | $ | 2.33 | $ | 1.18 | $ | 0.55 | |||||||
*The closing price of the Company’s common stock has been retroactively adjusted to reflect the reverse stock split of its outstanding shares of common stock at a ratio of one-for-ten effective on September 22, 2015.
The closing price for our common stock as reported by the Nasdaq Global Market on April 15, 2016 was $3.07 per share.
Securities Authorized for Issuance Under Equity Compensation Plan
See Item 12, “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” for information regarding securities authorized for issuance.
Recent Sales of Unregistered Securities
The Company did not sell or issue unregistered securities during the year ended December 31, 2015 other than as previously disclosed in a Form 8-K or Form 10-Q filed with the SEC.
Uses of Proceeds from Sale of Registered Securities
Not applicable.
Issuer Purchases of Equity Securities
None.
Performance Graph1
The following graph shows a comparison of cumulative total return for our common stock, the Nasdaq Composite Index, and the Nasdaq Medical Equipment Index. Such returns are based on historical results and are not intended to suggest future performance. The graph assumes $100 was invested in our common stock and in each of the indexes on December 31, 2010.
Data for the Nasdaq Composite Index and the Nasdaq Medical Equipment Index assume reinvestment of dividends. We have never paid dividends on our common stock and we do not anticipate paying any dividends in the foreseeable future.
The stockholder return shown on the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
57
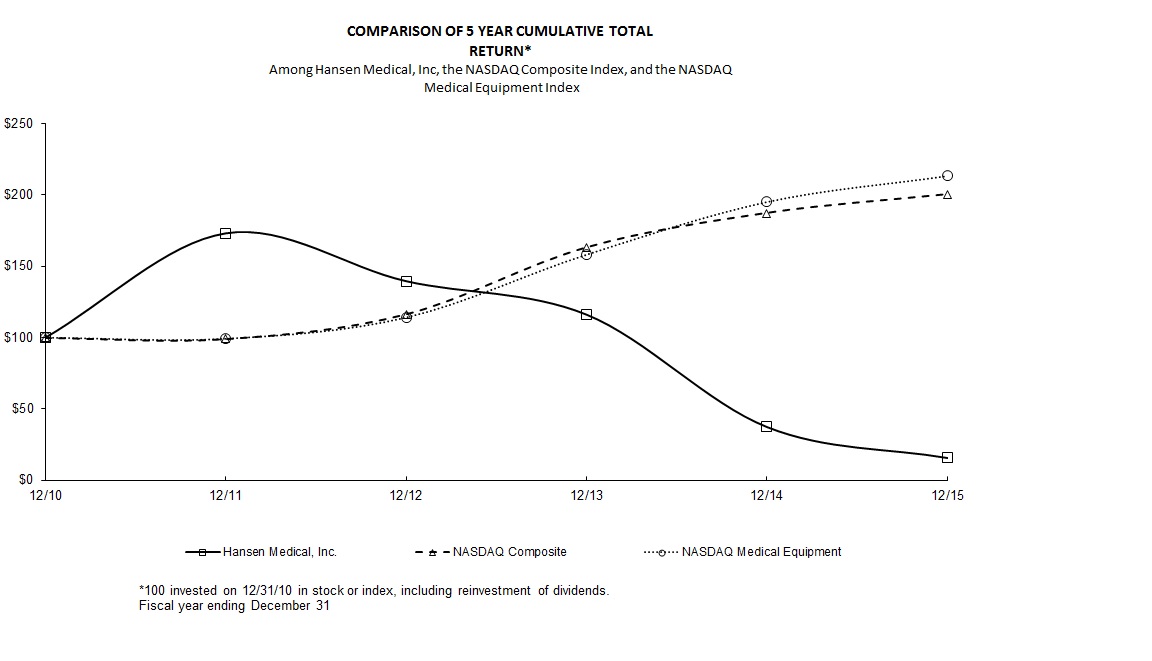
December 31, 2010 | December 31, 2011 | December 31, 2012 | December 31, 2013 | December 31, 2014 | December 31, 2015 | ||||||||||||||||||
Hansen Medical, Inc. | $ | 100.00 | $ | 173.15 | $ | 139.60 | $ | 116.11 | $ | 37.32 | $ | 15.64 | |||||||||||
Nasdaq Composite | $ | 100.00 | $ | 99.17 | $ | 116.48 | $ | 163.21 | $ | 187.27 | $ | 200.31 | |||||||||||
Nasdaq Medical Equipment | $ | 100.00 | $ | 99.18 | $ | 114.05 | $ | 158.19 | $ | 195.19 | $ | 213.43 | |||||||||||
1This Section is not “soliciting material,” is not deemed “filed” with the Securities and Exchange Commission and is not to be incorporated by reference into any filing of Hansen Medical under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
58
ITEM 6. SELECTED FINANCIAL DATA
The following table sets forth certain financial data with respect to our business. The information set forth below is not necessarily indicative of results of future operations and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 and the financial statements and related notes thereto in Item 8. The operations data for the years ended December 31, 2015, 2014 and 2013 and the financial position data for December 31, 2015 and 2014 are derived from, and are qualified by reference to, the audited consolidated financial statements that are included in this Form 10-K. The remaining financial data are derived from audited, consolidated financial statements which are not included in this Form 10-K.
2015(1) | 2014(2) | 2013(3) | 2012(4) | 2011(5) | |||||||||||
(In thousands, except per share data) | |||||||||||||||
Operations: | |||||||||||||||
Revenues | $ | 16,068 | $ | 19,495 | $ | 16,982 | $ | 17,636 | $ | 22,129 | |||||
Loss from operations | (37,843 | ) | (46,284 | ) | (48,903 | ) | (18,503 | ) | (15,666 | ) | |||||
Net loss | (46,207 | ) | (54,246 | ) | (55,722 | ) | (22,145 | ) | (16,712 | ) | |||||
Net loss attributable to common stockholders | (82,565 | ) | (54,246 | ) | (55,722 | ) | (22,145 | ) | (16,712 | ) | |||||
Basic and diluted net loss per common share * | (4.89 | ) | (4.63 | ) | (7.05 | ) | (3.54 | ) | (3.02 | ) | |||||
Shares used to compute basic and diluted net loss per common share * | 16,871 | 11,723 | 7,905 | 6,247 | 5,536 | ||||||||||
Financial Position: | |||||||||||||||
Cash and cash equivalents | $ | 20,375 | $ | 24,528 | $ | 27,995 | $ | 32,749 | $ | 36,520 | |||||
Short-term investments | 1,501 | 1,973 | 1,945 | 8,424 | 15,690 | ||||||||||
Working capital | (6,941 | ) | 33,894 | 38,988 | 43,238 | 54,349 | |||||||||
Total assets | 45,718 | 53,675 | 61,159 | 64,092 | 76,759 | ||||||||||
Debt | 35,141 | 34,385 | 33,358 | 29,417 | 29,147 | ||||||||||
Accumulated deficit | (453,929 | ) | (406,910 | ) | (352,664 | ) | (296,942 | ) | (274,797 | ) | |||||
Stockholders’ equity | 1,448 | 7,769 | 16,876 | 24,739 | 33,481 | ||||||||||
________________
*The Company’s financial statements have been retroactively adjusted to reflect the reverse stock split of its outstanding shares of common stock at a ratio of one-for-ten effective on September 22, 2015.
(1) | Net loss and basic and diluted net loss per common share for 2015 include the impact of $2.9 million of change in fair value of warrant liability. Loss from operations, net loss and basic and diluted net loss per share for 2015 include the impact of $1.7 million in restructuring expense. Net loss attributable to common stockholders and basic and diluted net loss per common share for the second quarter of 2015 includes the impact of non-cash charges of $35.5 million and $0.8 million for the deemed dividend related to beneficial conversion feature of Series A convertible preferred stock and accretion of discount, and cumulative dividend on Series A convertible preferred stock, respectively. |
(2) | Net loss and basic and diluted net loss per common share for 2014 include the impact of $2.9 million of warrant exchange, $0.7 million write-off of discontinued Lynx catheter product line and $1.0 million executive transition and employee separation. |
(3) | Loss from operations, net loss and basic and diluted net loss per common share for 2013 include the impact of the loss on settlement of the derivative litigation of $4.5 million. |
(4) | Loss from operations, net loss and basic and diluted net loss per common share for 2012 include the impact of the gain on the licensing of intellectual property of $20.0 million. |
(5) | Loss from operations, net loss and basic and diluted net loss per common share for 2011 include the impact of the gain on sale of intellectual property of $23.0 million. |
59
ITEM 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Except for the historical information contained herein, the matters discussed in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and elsewhere in this Form 10-K are forward-looking statements that involve risks and uncertainties. In some cases, these statements may be identified by terminology such as “may,” “will,” “should,” “expects,” “could,” “intends,” “might,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” or “continue,” or the negative of such terms and other comparable terminology. These statements involve known and unknown risks and uncertainties that may cause our business results, levels of activity, performance or achievements to be materially different from those expressed or implied by the forward-looking statements. These forward-looking statements include, among others, statements regarding our strategies and expectations regarding, our future revenues, cost of revenues and other expenses and losses. The factors listed in Item 1A “Risk Factors,” as well as any cautionary language in this Annual Report on Form 10-K, provide examples of risks, uncertainties and events that may cause our actual results to differ materially from those projected. Except as may be required by law, we undertake no obligation to update any forward-looking statement to reflect events after the date of this report.
Overview
The following discussion of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes thereto included elsewhere in this Annual Report on Form 10-K.
We develop, manufacture and sell a new generation of medical robotics designed for accurate positioning, manipulation and stable control of catheters and catheter-based technologies. Our Sensei® Robotic Catheter System, or Sensei System, is designed to allow physicians to instinctively navigate flexible catheters with solid stability and control in electrophysiology procedures. Our Magellan™ Robotic System, or Magellan System, is designed to allow physicians to instinctively navigate flexible catheters in the vasculature. We believe our systems and the corresponding disposable catheters will enable physicians to perform procedures that historically have been too difficult or time consuming to accomplish routinely with manually-controlled, hand-held catheters and catheter-based technologies, or that we believe could be accomplished only by the most skilled physicians. We believe that our systems have the potential to benefit patients, physicians, hospitals and third-party payors by improving outcomes and permitting complex procedures to be performed interventionally.
From inception to December 31, 2015, we have incurred losses totaling approximately $453.9 million and have not generated positive cash flows from operations. We expect such losses to continue through at least the year ended December 31, 2016 as we continue to commercialize our technologies and develop new applications and products. We have financed our operations primarily through the sale of public and private equity securities, the issuance of debt, partnering and the licensing of intellectual property.
We market our products in the United States primarily through a direct sales force of regional sales employees, supported by clinical account managers who provide training, clinical support and other services to our customers. Outside the United States, we use a combination of a direct sales force and distributors to market, sell and support our products.
Critical Accounting Policies, Estimates and Judgments
We prepare our consolidated financial statements in accordance with accounting principles generally accepted in the United States. In doing so, we have to make estimates and assumptions that affect our reported amounts of assets, liabilities, revenues and expenses, as well as related disclosures of contingent assets and liabilities. In many cases, we could reasonably have used different accounting policies and estimates. In some cases, changes in the accounting estimates are reasonably likely to occur from period to period. Accordingly, actual results could differ materially from our estimates. We base our estimates on our past experience and on other assumptions that we believe are reasonable under the circumstances, and we evaluate these estimates on an ongoing basis. To the extent that there are material differences between these estimates and actual results, our financial condition or results of operations will be affected.
While our significant accounting policies are fully described in Note 2 to our Consolidated Financial Statements included in Part II, Item 8 of this Annual Report on Form 10-K, we believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results.
Revenue Recognition
Our revenues are primarily derived from the sale of the Sensei System and the Magellan System and the associated catheters as well as the sale of customer service contracts, which includes post-contract customer support, or PCS. We sell our products directly to customers as well as through distributors. Under our revenue recognition policy, revenues are recognized when persuasive evidence of an arrangement exists, title and risk of loss has passed, delivery to the customer has occurred or
60
the services have been fully rendered, the sales price is fixed or determinable and collectability is reasonably assured. If any of these criteria are not met, we defer recognizing the revenue until such time as all criteria are met.
It is common for the sale of Sensei and Magellan Systems to include multiple elements which have standalone value and qualify as separate units of accounting. These elements commonly include the sale of the system and PCS, in addition to installation of the system and initial training in the first year. For multiple-element arrangements revenue is allocated to each element based on their relative selling prices. Relative selling prices are based first on vendor specified objective evidence, or VSOE, then on third-party evidence of selling price, or TPE, when VSOE does not exist, and then on management’s best estimate of the selling price, or ESP, when VSOE or TPE do not exist. Because we have neither VSOE nor TPE for our system, the allocation of revenue is based on ESP for the systems sold. We determine ESP by considering multiple factors, including, but not limited to, features and functionality of the system, geographies, type of customer, pricing practices and market conditions. We regularly review ESP and maintain internal controls over the establishment and updates of these estimates.
In situations where we shipped a system under a limited commercial evaluation program to allow certain strategic accounts to install and utilize our systems for a limited trial period of three to six months, we do not recognize revenue and the system under this program remains our property and are recorded in inventory.
Significant management judgments and estimates are made in connection with the determination of revenue to be recognized and the period in which it is recognized. If different judgments and estimates were utilized, the amount of revenue to be recognized and the period in which it is recognized could differ materially from the amounts reported.
Investments
Fair Value
We determine the appropriate classification of investments at the time of purchase and evaluate such designation as of each balance sheet date. We classify all investments with maturities greater than three months at the time of purchase as short-term investments as they are subject to use within one year in current operations. We make investments based on specific guidelines approved by our board of directors with a view to liquidity and capital preservation and regularly review our investments for performance. As of December, 31, 2015, all our investments have been classified as available-for-sale and are carried on the balance sheet at fair value with unrealized gains and losses, if any, included in other comprehensive income within stockholders’ equity. Any unrealized losses which are determined to be other than temporary are included in earnings.
Other-than-temporary impairment
We periodically evaluate our investments for impairment. In the event that the carrying value of an investment exceeds its fair value and the decline in fair value is determined to be other than temporary, an impairment charge is recorded and a new cost basis for the investment is established. The primarily differentiating factors we considered to determine whether a decline in value is other than temporary are our intent and ability to retain our investment in the issuer for a period of time sufficient to allow for any anticipated recovery in market value, the length of the time and the extent to which the market value of the investment has been less than cost, the financial condition and near-term prospects of the issuer. Given the current market conditions, these judgments could prove to be wrong, and companies with relatively high credit ratings and solid financial conditions may not be able to fulfill their obligations.
No impairment losses were recorded during the years ended December 31, 2015, and 2014. As of December 31, 2015, and 2014, net unrealized gains (losses) on investments of $0.5 million and $0.02 million, net of tax, respectively, were included in accumulated other comprehensive income (loss). During the year ended December 31, 2013, we recorded pre-tax losses of $0.6 million related to a decline in the value of our investment in Luna Innovations Incorporated common stock that we concluded were other-than-temporary.
Inventories
Inventories, which includes material, labor and overhead costs, are stated at standard cost, which approximates actual cost, determined on a first-in, first-out basis, and not in excess of net realizable value. We record reserves, when necessary, to reduce the carrying value of excess or obsolete inventories to their net realizable value. These reserves are based on our best estimates after considering projected future demand. In the event that actual demand for our inventory differs from our best estimates or we fail to receive the necessary regulatory approvals, increases to inventory reserves may become necessary.
Impairment of Long-Lived Assets
We evaluate the recoverability of our long-lived assets in accordance with authoritative accounting guidance. When events or changes in circumstances indicate that the carrying value of long-lived assets may not be recoverable, we recognize such impairment if the net book value of such assets exceeds the future undiscounted cash flows attributable to such assets. Should an impairment exist, the impairment loss would be measured based on the excess carrying value of the asset over the
61
asset’s fair value or discounted estimates of future cash flows attributable to the assets. As of December 31, 2015, we had $2.3 million of property and equipment, net. If estimates or the related assumptions change in the future, we may record impairment charges to reduce the carrying value of certain groups of these assets. Changes in the valuation of long-lived assets could materially impact our operating results and financial position.
Stock-Based Compensation
We account for stock-based compensation under the fair value recognition provisions of authoritative guidance related to stock-based compensation. Stock-based payment expense is estimated at the grant date based on the fair value of the award and is recognized as expense ratably over the requisite service period of the award. The recording of compensation expense related to stock-based awards is significant to our financial statements but does not result in the payment of cash by us. Determining the appropriate fair value model used to calculate the fair value of stock-based awards requires significant management judgment. Additionally, the calculation of the fair value of stock-based awards requires us to make significant estimates and judgments, including the expected volatility, the expected term of the award, risk-free interest rate and dividend yield.
We use the Black-Scholes option valuation model as the most appropriate method for determining the estimated fair value for our stock-based awards. The Black-Scholes model requires the use of highly subjective and complex assumptions in determining the fair value of stock-based awards, including the expected volatility of the underlying stock, expected term, risk-free interest rate and the dividend yield. The two assumptions that can significantly impact the recording of our stock-based compensation expense are expected volatility and expected term.
• | Expected Volatility. Our estimate of volatility is based on the historical volatilities of our stock price. |
• | Expected Term. We estimate the expected term based on our historical settlement experience related to vesting and contractual terms while giving consideration to awards that have life cycles less than the contractual terms and vesting schedules in accordance with authoritative guidance. |
• | Risk-Free Interest Rate. The risk-free interest rate that we use in the Black-Scholes option valuation model is the implied yield in effect at the time of option grant based on U.S. Treasury zero-coupon issues with a remaining term equivalent to the expected term of our option grants. For Employee Stock Purchase Plan, or ESPP, grants, we use the 6-month Constant Maturity Treasury, or CMT, rate. |
• | Dividend Yield. We have never paid any cash dividends on our common stock and we do not anticipate paying any cash dividends in the foreseeable future. |
In addition to the Black-Scholes assumptions noted above, we also estimate a forfeiture rate for our stock-based awards. We estimate our forfeiture rate based on historical data on actual forfeitures and future expectations related to employee turnover and other factors. The impact from any forfeiture rate adjustment would be recognized in full in the period of adjustment and if the actual number of future forfeitures differs from our estimates, we might be required to record adjustments to stock-based compensation in future periods.
To the extent that future evidence regarding these variables is available and provides estimates that we determine are more indicative of actual trends, we may refine or change our approach to deriving these input estimates. These changes could significantly impact the stock-based compensation expense recorded in the future.
Net Operating Loss Carryforwards
We make certain judgments and estimates in determining and valuing deferred tax assets. These judgments arise from differences in timing of recognizing certain expenses for tax purposes and in the calculation of credit and net operating loss carryforwards.
At December 31, 2015, we had federal and state net operating loss carryforwards of approximately $117.1 million and $125.3 million, respectively. At December 31, 2014, we had federal and state net operating loss carryforwards of approximately $285.7 million and $179.5 million, respectively. At December 31, 2013, we had federal and state net operating loss carryforwards of approximately $242.0 million and $205.3 million, respectively. These net operating loss carryforwards will expire in varying amounts from 2016 through 2035 if not utilized. We maintained a full valuation allowance against our deferred tax asset totaling $78.0 million and $147.5 million at December 31, 2015 and 2014, respectively. The determination to maintain an allowance is highly subjective. The factors we considered in making this determination include, but are not limited to (i) our historical cumulative net losses, after adjustment for permanent tax differences, over the previous three years through 2015; (ii) our dependence on continued high growth rates in achieving forecasted profitability; (iii) operation in an industry subject to rapid technological changes; and (iv) the unknown impact of current negative macroeconomic factors on our forecasted results of operations. Based on our consideration of these factors, we believe there is sufficient uncertainty regarding our ability to generate future taxable income. We will retain a full valuation allowance until such time that we determine it is more likely than not that we will recognize the benefit of the deferred tax assets. Throughout 2016, we will continually evaluate
62
these, and other, factors, and the impact any changes in these factors has on our judgment regarding the realization of the deferred tax assets.
Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, provide for annual limitations on the utilization of net operating loss and research and experimentation credit carryforwards if we were to undergo an ownership change, as defined in Section 382. Based on an analysis under Section 382 of the Internal Revenue Code, we experienced various ownership changes through 2015 which substantially limit the future use of our pre-change NOLs and certain other pre-change tax attributes per year. We have excluded the NOLs and R&D credits that will expire as a result of the annual limitations in the deferred tax assets as of December 31, 2015. To the extent that we do not utilize our carry-forwards within the applicable statutory carry-forward periods, either because of Section 382 limitations or the lack of sufficient taxable income, the carry-forwards will expire unused.
We have evaluated and will continue to evaluate alternative analyses permitted under Section 382 and IRS notices to determine whether or not any ownership changes have occurred and may occur (and if so, when they occurred) that would result in limitations on our net operating losses, or NOLs, or certain other tax attributes.
Restructuring expense
In November 2015, we initiated a cost-saving restructuring plan in order to align our headcount to reduce operating expenses, consolidate its back-office operations by closing our office in United Kingdom and relocate our headquarter. We recorded restructuring charges of approximately $1.7 million consisting of employee severance and termination benefits, lease cancellation fees and expenses related to the closing down of our United Kingdom office. We have classified approximately $1.5 million of these charges as restructuring expense in operating expenses and $188,000 as a component of cost of sales. We recorded employee severance costs provided under an ongoing benefit arrangement once they are both probable and estimable in accordance with the provisions of ASC 712. We accounted for lease cancellation fees and expenses related to the closing down our United Kingdom office in accordance with ASC 420. Under ASC 420, we establish a liability for a cost associated with an exit or disposal activity, when the liability is incurred, rather than at the date that we commit to an exit plan. We reassess the expected cost to complete the exit or disposal activities at the end of each reporting period and adjust our remaining estimated liabilities, if necessary.
Results of Operations
Comparison of the year ended December 31, 2015 to the year ended December 31, 2014 and the year ended December 31, 2014 to the year ended December 31, 2013:
2013:
Revenues
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Product | $ | 10,719 | $ | 13,812 | $ | 11,790 | (22 | )% | 17 | % | |||||||
Service | 5,349 | 5,683 | 5,192 | (6 | )% | 9 | % | ||||||||||
Total revenues | $ | 16,068 | $ | 19,495 | $ | 16,982 | (8 | )% | 15 | % | |||||||
Product revenues were $10.7 million in 2015 compared to $13.8 million in 2014, a decrease of $3.1 million or 22%, compared to 2014. Product revenue in 2015 included the shipment of nine Magellan Systems and two Sensei Systems compared to nine Magellan Systems and three Sensei Systems in 2014. The decrease in product revenue was primarily due to fewer system sold and two systems shipped with extended payment terms. or contingent payment terms. For sale with extended payment terms or contingent payment terms, we defer recognition of revenue until payment is due and payable.
Service revenue decreased 6% in 2015 compared to 2014 driven by lower new system sales. We generally sell our system bundled with first year maintenance, lower system sale will affect the allocation of revenue to service maintenance for new systems. We sold ten systems with PCS bundled in the sale of new systems in 2015 compared to 2014 with 12 new systems sale bundled with PCS.
Our product revenues were $13.8 million in 2014 compared to $11.8 million in 2013, an increase of $2.0 million or 17%, compared to 2013. Product revenue in 2014 included the sale of nine Magellan Systems and three Sensei Systems compared to seven Magellan Systems and five Sensei Systems in 2013. The increase in product revenue was driven by mix of product sold, our Magellan Systems which typically have a higher selling price than our Sensei Systems; an 11% increase in volume of
63
catheters sold, as well as higher average sales price on the systems sold in the EMEA market. The growth in catheter sales was driven by an increase in the number of installed systems, which require the use of our catheters.
Service revenue increased 9% in 2014 compared to 2013 driven by an increase in the installed base of systems.
We have experienced significant fluctuations in quarterly revenues, primarily attributable to being in the early stages of our commercial launch of our Magellan products and difficult general economic and capital market conditions. We expect these fluctuations to continue through 2016.
Cost of Revenues and Gross Profit
Years Ended December 31, | ||||||||||||
2015 | 2014 | 2013 | ||||||||||
(Dollars in thousands) | ||||||||||||
Product | $ | 12,016 | $ | 13,123 | $ | 11,150 | ||||||
Service | 3,049 | 3,368 | 2,331 | |||||||||
Total cost of revenues | $ | 15,065 | $ | 16,491 | $ | 13,481 | ||||||
As a percentage of revenues | 94 | % | 85 | % | 79 | % | ||||||
Gross profit | $ | 1,003 | $ | 3,004 | $ | 3,501 | ||||||
Gross margin (as a percentage of total revenues) | 6 | % | 15 | % | 21 | % | ||||||
Product margin (as a percentage of product revenues) | (12 | )% | 5 | % | 5 | % | ||||||
Service margin (as a percentage of service revenues) | 43 | % | 41 | % | 55 | % | ||||||
Gross profit for 2015 was $1.0 million, or 6% of revenues in 2015, compared to $3.0 million, or 15% of revenues in 2014. Lower product gross margin in 2015 was primarily due to two systems sold that were deferred because of extended payment terms or contingent payment terms. For sales with extended payment terms, we recognize revenue when due and payable but record the full cost for the system at the time of sale. We also recorded a $1.1 million inventory reserve for the Sensei System product line in the fourth quarter of fiscal 2015 for excess and obsolescence. Service gross margin increased slightly due to lower warranty repairs volume in 2015, reflecting efficiency improvements in the field.
Gross profit for 2014 was $3.0 million, or 15% of revenues in 2014, compared to $3.5 million, or 21% of revenues in 2013. Gross margin decreased 6 percentage point to 15% in 2014 from 21% in 2013. Product gross margin in 2014 was negatively impact by a $0.7 million write-off due to discontinued Lynx catheter product line in the fourth quarter. Service gross margin was impacted by an increase in service and repair calls volume in 2014.
We expect that cost of revenues and gross profit, both as a percentage of revenues and on a dollar basis, may continue to vary from quarter to quarter in 2016 due, among other things, to the mix of products sold, average selling prices on newly launched products, manufacturing levels and manufacturing yields.
Operating Expenses
Research and Development
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Research and development | $ | 13,812 | $ | 18,034 | $ | 16,139 | (23 | )% | 12 | % | |||||||
Research and development expenses in 2015 decreased $4.2 million or 23% compared to 2014 primarily attributable to a decrease in salaries and wages of $1.8 million associated with decrease in headcount, $0.8 million decrease in consultant expense, $0.6 million decrease in prototype materials and supplies due to project timing, and $0.6 million decrease in clinical trial costs.
Research and development expenses in 2014 increased $1.9 million, or 12%, compared to 2013 primarily attributable to an increase of $1.0 million in expenses associated with clinical trial and $0.9 million in development of prototype materials for new product initiatives in 2014.
64
We expect research and development expenses will increase in the future as we continue to make substantial investments in our product development on the next generation products and higher retention and key incentives for our key employee talents.
Selling, General and Administrative
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Selling, general and administrative | $ | 23,495 | $ | 31,254 | $ | 31,765 | (25 | )% | (2 | )% | |||||||
Selling, general and administrative expenses in 2015 decreased $7.8 million, or 25%, compared to 2014. The decrease was due primarily to a $3.7 million decrease in employee related salaries and wages associated with a reduction in force and a leaner workforce, $1.7 million decrease in consultants and outside services, $0.7 million in lower sales commissions, $0.6 million decrease in legal costs associated with general legal fees, patent and exploring strategic alternatives matters, and $0.5 million lower convention and marketing costs.
Selling, general and administrative expenses in 2014 decreased $0.5 million, or 2%, compared to 2013. The decrease was due primarily to a $2.6 million decrease in legal costs associated with litigation, patent and financing-related matter partially offset by a $1.5 million increase in sales and marketing activities associated with the development of our global sales organization and a $0.6 million increase in costs associated with executive transitions.
Stock-Based Compensation Expense
Cost of revenues, research and development and selling, general and administrative expense included stock-based compensation expense for stock-based awards as follows (in thousands):
Years ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Cost of revenues | $ | 233 | $ | 230 | $ | 402 | |||||
Research and development | 632 | 704 | 1,320 | ||||||||
Selling, general and administrative | 1,893 | 1,898 | 3,167 | ||||||||
Total | $ | 2,758 | $ | 2,832 | $ | 4,889 | |||||
Total stock-based compensation decreased slightly in 2015 compared to 2014. The decrease is primarily due to a lower closing price of our common stock and a fewer number of employees in 2015 as compared to 2014,which was partially offset by Performance Stock Units (“PSU”) granted to executives and employees in lieu of cash bonuses in 2015.
Total stock-based compensation decreased $2.1 million in 2014 compared to 2013. The decrease is primarily due to our incentives program for employees has changed to cash-based payment to employees in 2014 from a traditionally granting performance-based restricted stock units to employees in 2013.
Restructuring expense
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Restructuring expense | $ | 1,539 | $ | — | $ | — | 100 | % | — | % | |||||||
In November 2015, we initiated a cost-saving restructuring plan in order to align our headcount to reduce operating expenses, consolidate its back-office operations by closing our office in United Kingdom and relocate our headquarter. We recorded charges of approximately $1.7 million consisting of termination benefits, lease cancellation fees and expenses related to the closing down of our United Kingdom office. We have classified approximately $1.5 million of these charges as restructuring expense and $188,000 as a component of cost of sales.
65
Loss on Settlement of Litigation
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Loss on settlement of litigation | $ | — | $ | — | $ | 4,500 | — | % | (100 | )% | |||||||
On May 9, 2013, we and the plaintiff parties entered into a stipulation of settlement in the matter Curry v. Hansen Medical, Inc. et al., Case No. 09-05094 and consolidated actions, pursuant to which the plaintiffs received an aggregate of $8.5 million, $4.0 million of which was funded in cash by our insurer and other sources. We recorded a loss on litigation settlement of $4.5 million during the quarter ended March 31, 2013. On December 5, 2013, the Court granted final approval of the settlement. We settled the remaining $4.5 million by issuing $4.25 million worth of our common stock, the number of shares was determined based on the average closing price of the common stock for the 10 trading days preceding final Court approval of the settlement of the class action, which average was $1.8490 per share, and by paying $250,000 in cash.
Warrant exchange and Change in Fair Value of Warrant Liability
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Warrant exchange and change in fair value of warrant liability | $ | 2,993 | $ | 2,914 | $ | — | 3 | % | 100 | % | |||||||
On March 11, 2015, we raised $35 million in gross proceeds from the sale of 53,846 shares of convertible preferred stock at a per share price of $650. In connection with the issuance of Series A convertible preferred stock on March 11, 2015, we also issued Series E Warrants to the participating investors to purchase an aggregate of 5,384,600 shares of common stock with an exercise period of two years from the date of issuance. However, certain of the Series E Warrants could not be exercised until the Requisite Stockholder Approval was obtained. As a result of this contingency which was deemed outside our control as well as the variable number of underlying shares due to the floating exercise price, such Series E Warrants did not meet the criteria for classification as equity under ASC 815. We used a third party valuation that utilized the Monte Carlo simulation model to estimate the fair value of the Series A convertible preferred stock and Series E Warrants. The valuation used a simulation of our periodic stock price, expected volatility of the price, adjusted for conversion price, and the remaining contractual term of the Series E Warrants. The fair value of the Series E Warrants was determined at $14.8 million upon issuance and we classified the Series E Warrants as current liabilities. Once the Requisite Stockholder Approval was obtained on May 12, 2015, the exercised price of the Series E warrants was fixed at $9.75. We remeasured the fair value of our warrant liability to be $17.8 million. The change in fair value of $3.0 million was recorded on the consolidated statements of operations for the year ended December 31, 2015.
On July 30, 2014, we entered into a definitive agreement with certain warrantholders to cancel and exchange an aggregate of 2,044,234 of our outstanding warrants to purchase shares of our common stock. In exchange, we issued warrants to purchase an aggregate of 2,672,837 shares of our common stock with a lower exercise price than the exchanged warrants. The exchange was completed in August 2014. As a result of the warrant exchange, we incurred a one-time $2.9 million expense in 2014 based upon the difference between the fair value of the exchanged warrants immediately prior to the exchange and the fair value of the newly issued warrants.
Interest Income
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Interest income | $ | 21 | $ | 23 | $ | 25 | (9 | )% | (8 | )% | |||||||
Interest income from cash, cash equivalents and short-term investments decreased for the year ended December 31, 2015 to $21 million, compared with $23 million for 2014 and $25 million for 2013 primarily due to lower average invested balances.
Interest Expense
66
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Interest expense | $ | 5,287 | $ | 4,789 | $ | 3,952 | 10 | % | 21 | % | |||||||
Interest expense increased for the year ended December 2015 to $5.2 million, compared with $4.8 million for 2014 and $3.9 million for 2013 primarily due to the payment in kind interest added to the debt principal associated with the interest only credit facility with White Oak entered in July 2013. We expect interest expense in 2016 slightly increase due to the payment in kind interest added to the principal.
Other Expense, net
Years Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Other expense, net | $ | 58 | $ | 254 | $ | 842 | (77 | )% | (70 | )% | |||||||
Other expense, net decreased in 2015 compared to 2014 was primarily due to lower loss on realized foreign exchange partially offset by gain on asset disposal in 2015.
Other expense, net decreased in 2014 compared to 2013 included a $0.6 million write down of our equity investment in Luna.
Income Tax Expense
Year Ended December 31, | Years Ended December 31 Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 vs 2014 (%) | 2014 vs 2013 (%) | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Income tax expense | $ | 47 | $ | 28 | $ | 115 | 68 | % | 76 | % | |||||||
Income tax expense consisted of provision for income taxes related to foreign taxes, the increase in 2015 compared to 2014 was primarily due to increase in taxable income in foreign jurisdictions.
Income tax expense consisted of provision for income taxes related to foreign taxes, the decrease in 2014 compared to 2013 was primarily due to decrease in taxable income in foreign jurisdictions.
Loss on Extinguishment of Debt
Year Ended December 31, | Change | ||||||||||||||||
2015 | 2014 | 2013 | % | % | |||||||||||||
(Dollars in thousands) | |||||||||||||||||
Loss on extinguishment of debt | $ | — | $ | — | $ | 1,935 | — | % | (100 | )% | |||||||
In the third quarter of 2013, we fully settled and extinguished our previous loan obligation to Oxford Finance LLC and Silicon Valley Bank. The $1.9 million loss on debt extinguishment included a $0.9 million prepayment penalty, an additional $0.5 million end-of-term payment and a $0.5 million of the unamortized discount from warrants and issuance costs.
Liquidity and Capital Resources
67
2015 | 2014 | 2013 | |||||||||
(Dollars in thousands) | |||||||||||
Cash and cash equivalents | $ | 20,375 | $ | 24,528 | $ | 27,995 | |||||
Short-term investments | 1,501 | 1,973 | 1,945 | ||||||||
Restricted cash | 6,113 | 5,376 | 5,394 | ||||||||
Total cash, cash equivalents, short-term investments and restricted cash | $ | 27,989 | $ | 31,877 | $ | 35,334 | |||||
Cash used in operating activities | $ | (37,694 | ) | $ | (42,398 | ) | $ | (42,755 | ) | ||
Cash (used) provided by investing activities | (1,058 | ) | (443 | ) | 760 | ||||||
Cash provided by financing activities | 34,650 | 39,448 | 37,241 | ||||||||
Net decrease in cash and cash equivalents | $ | (4,102 | ) | $ | (3,393 | ) | $ | (4,754 | ) | ||
Cash, Cash Equivalents, Short-Term Investments and Restricted Cash
Our cash, cash equivalents, short-term investment balances and restricted cash are held primarily in money market funds in addition to corporate equity securities. Cash in excess of immediate requirements is invested in accordance with our investment policy primarily with a view towards liquidity and capital preservation. Pursuant to our loan and security agreement executed in 2013, we are required to maintain $5.0 million of restricted cash subject to lenders’ control. We also maintains $750,000 in restricted cash as collateral to obtain a letter of credit used as a lease security for our new office in San Jose, California.
Net Cash Used in Operating Activities
Net cash used in operating activities in 2015 of $37.7 million primarily reflects the net loss of $46.2 million, which included substantial non-cash charges totaled $8.9 million in 2015, primarily in the form of change in fair value of warrant liability, stock-based compensation, depreciation and amortization, write-off of discontinued product line and reserve for excess and obsolescence in inventory, payment-in-kind interest on a loan, and write-off of a discontinued catheter product line. In addition there was a $1.0 million decrease in inventories, $1.1 million related to the reserve for excess and obsolescence of our Sensei product line and other net changes in operating assets and liabilities which provided cash to offset the net loss.
Net cash used in operating activities in 2014 of $42.4 million primarily reflects the net loss of $54.2 million, which included substantial non-cash charges totaled $10.3 million in 2014, primarily in the form of warrant exchange expense, depreciation and amortization, stock-based compensation, payment-in-kind interest on a loan, and write-off of a discontinued catheter product line. In addition there was a $1.4 million decrease in other assets from the sale of long-term receivables and other net changes in operating assets and liabilities which provided cash to offset the net loss.
Net cash used in operating activities in 2013 of $42.8 million primarily reflects the net loss of $55.7 million, which included substantial non-cash charges totaled $15.9 million in 2013, primarily in the form of loss on settlement of litigation, loss on extinguishment of debt, net realized loss on investments, depreciation and amortization, payment-in-kind interest on loan, and stock-based compensation. Additionally, net cash used in operations in 2013 was negatively impacted by an increase of $3.1 million in inventory due to timing of purchases to support projected future sales, and an increase of $1.4 million in long-term accounts receivable arising from system sales, included in other assets, offset by an increase in accounts payable and accrued liabilities.
Net Cash (Used in) Provided by Investing Activities
Net cash used in investing activities in 2015 primarily relates to capital expenditures and $750,000 increased in restricted cash as collateral to obtain a letter of credit used as lease security for our new office in San Jose, California.
Net cash used in investing activities in 2014 primarily relates to capital expenditures.
Net cash provided by investing activities in 2013 primarily relates to the proceeds from the net maturities of short-term investments as we manage our portfolio to provide liquidity and interest income for our operations.
Net Cash Provided by Financing Activities
Net cash provided by financing activities in 2015 of $34.7 million primarily relates to $35.0 million proceeds from sale of Series A convertible preferred stock and $0.2 million of proceeds from purchases under our employee stock purchase plan, partially offset by $0.5 million payment for Series A convertible preferred stock issuance costs.
Net cash provided by financing activities in 2014 of $39.5 million primarily relates to $37.1 million in proceeds from the exercise of Series A Warrants and Series B/C Exchange Warrants to purchase an aggregate of 31,824,463 shares of our common
68
stock as well as $3.1 million in proceeds from the exercise of stock options and purchases under our employee stock purchase plan offset by $0.6 million in withholding taxes paid on vested restricted stock units.
Net cash provided by financing activities in 2013 of $31.8 million primarily relates to gross proceeds of $39.3 million received upon completion of the private placement equity financing less $2.1 million of related costs, gross proceeds of $33.0 million related to the White Oak debt financing less debt issuance costs of $1.5 million and $0.7 million proceeds from the exercise of stock options and purchases under our employee stock purchase plan, partially offset by $32.1 million of principal payments and final repayment fees related to the extinguishment of the Oxford Loan and a provision for restricted cash of $5.0 million as required under the White Oak loan agreement.
Historical Financing Activities
We have incurred significant losses since our inception in September 2002 and, as of December 31, 2015, we had an accumulated deficit of $453.9 million. We have financed our operations to date principally through the sale of capital stock, exercise of warrants, debt financing and the sale of our products and, beginning in 2009, through partnering and licensing of intellectual property. In November 2011, we sold approximately 478,500 shares of our common stock, resulting in approximately $10.0 million of net proceeds. In October 2012, we sold 529,101 shares of our common stock, resulting in approximately $10.0 million of net proceeds. In July 2013, we sold 2,845,528 shares of our common stock, resulting in approximately $37.2 million of net proceeds. Between August 2013 and August 2014, we issued warrants exercisable for up to 4,043,236 shares of our common stock and through December 31, 2015, a total of 3,182,446 warrants have been exercised, resulting in gross proceeds of approximately $37.1 million. In March 2015, we sold 53,846 shares of Series A convertible preferred stock, all of which were converted into 5,509,492 shares of common stock on May 12, 2015, resulting in approximately $35.0 million of proceeds. In connection with the March 2015 financing, we issued Series E Warrants to purchase an aggregate of 5,384,600 shares, all of which remain outstanding as of September 30, 2015 at an exercise price of $9.75 per share.
Oxford Loan
In December 2011, we entered into a $30.0 million loan and security agreement with Oxford Finance LLC and Silicon Valley Bank (the “Oxford Loan”). Under the loan agreement, we were obligated to pay interest only payments on the Oxford Loan through June 30, 2013, following which time the Oxford Loan required interest and principal payments through January 1, 2016. The Oxford Loan accrued interest at a stated rate of 9.45% and included an additional final interest payment of 3.95% of the original principal amount. The Oxford Loan provided for a prepayment option that allowed us to prepay all of the outstanding principal balance, subject to a pre-payment fee. In connection with the Oxford Loan, we issued warrants to purchase 66,079 shares of common stock. The warrants have an exercise price of $22.70 per share and expire in December 2018.
In August 2013, the Oxford Loan was fully repaid and extinguished under the loan agreement’s prepayment option. We paid a final interest balloon of $1.2 million plus accrued interest and recognized a prepayment penalty of $0.9 million. In 2013, we recognized a loss on extinguishment of debt of $1.9 million which included a $0.9 million prepayment penalty, a $0.5 million additional end-of-term payment and a $0.5 million unamortized discount from warrants and issuance costs.
White Oak Loan
In July 2013, we executed a loan and security agreement with White Oak as a lender and agent for several lenders. On August 23, 2013, the loan and security agreement was amended and restated and the loan was funded. The amended loan and security agreement provides for term loan debt financing of $33.0 million with a single principal balloon payment due at maturity on December 30, 2017. Cash interest accrues at an 11.0% per annum rate and is payable quarterly. Additionally, a 3.0% per annum payment-in-kind accrues quarterly and is accretive to the principal amount owed under the agreement. Substantially all of the proceeds from the loan were used to fully repay and extinguish previous indebtedness. In connection with the loan, we incurred costs of approximately $1.5 million, including payments to the lender agent totaling $0.7 million and the placement agent totaling $0.3 million, which in aggregate are accounted for as debt issuance costs and amortized to interest expense over the life of the loan. Under the loan and security agreement, we are obligated to pay White Oak certain servicing, administration and monitoring fees of $32,000 annually. We may prepay all or a portion of the outstanding principal balance, subject to paying a prepayment fee of 3.5% of the principal amount of the loan prepaid if the prepayment is made on or before the third anniversary of the funding of the loan or 1.0% of the principal amount of the loan prepaid if the prepayment is made after the third anniversary and on or before the fourth anniversary of the funding of the loan. We are also required to make mandatory prepayments upon certain events of loss and certain dispositions of our assets as described in the loan and security agreement. We recognized expense of $0.4 million, $0.4 million and $0.1 million in 2015, 2014 and 2013, respectively, for the amortization of debt issuance costs related to the White Oak loan.
69
The loan is collateralized by substantially all of our assets then owned or thereafter acquired, other than our intellectual property, and all proceeds and products thereof. Two of our wholly-owned subsidiaries, AorTx, Inc. and Hansen Medical International, Inc., have entered into agreements to guarantee our obligations under the loan and security agreement and have granted first priority security interests in their assets, excluding any of their intellectual property, to secure their guarantee obligations. Under the loan and security agreement, neither we nor AorTx, Inc. or Hansen Medical International, Inc. may grant a lien on any intellectual property to third parties. We additionally agreed to pledge to the lenders shares of each of our direct and indirect subsidiaries as collateral for the loan. Pursuant to the loan and security agreement, we are subject to certain affirmative and negative covenants and also to minimum liquidity requirements which require us to maintain $15.0 million in liquidity at all times, consisting of at least $13.0 million in cash, cash equivalents and investments, of which $5.0 million is required to be restricted subject to lenders’ control, and the lesser of $2.0 million or 65% of eligible accounts receivable. Additionally, we are required to obtain an audit opinion from our independent certified public accountants on the annual financial statements that does not include an explanatory uncertainty paragraph raising substantial doubt about its ability to continue as a going concern or any qualification or exception as to the scope of such audit. The loan also limits our ability to (a) undergo certain change of control events; (b) convey, sell, lease, transfer, assign or otherwise dispose of any of our assets; (c) create, incur, assume, or be liable with respect to certain indebtedness, not including, among other items, subordinated debt; (d) grant liens; (e) pay dividends and make certain other restricted payments; (f) make certain investments; (g) make payments on any subordinated debt; or (h) enter into transactions with any of our affiliates outside of the ordinary course of business, or permit our subsidiaries to do the same. We are also required to make mandatory prepayments upon certain events of loss and certain dispositions of our assets as described in the loan and security agreement. In the event we were to violate any covenants or if White Oak has reason to believe that we have violated any covenants, including a significant adverse event clause, and such violations are not cured pursuant to the terms of the loan and security agreement, we would be in default under the loan and security agreement, which would entitle lenders to exercise their remedies, including the right to accelerate the debt, upon which we may be required to repay all amounts then outstanding under the loan and security agreement.
As of December 31, 2015, we were in compliance with all covenants; however, there was an anticipated default of the covenants listed in Section 6.2(a)(i) and Section 8.2(a), with respect to Section 6.2(a)(i), as of December 31, 2015, which was cured by the Forbearance Agreement as discussed below. As of March 31, 2016, we were no longer in compliance with certain covenants and events of default listed in Sections 6.1(c), 6.2(a)(i), 6.2(a)(iv), 6.10, 8.2(a) and 8.2(b) of the loan and security agreement with White Oak (the “Specified Events of Default”). On April 19, 2016, we entered into a Forbearance Agreement with White Oak (“Forbearance Agreement”) whereby White Oak granted forbearance of the Specified Events of Default until August 17, 2016 in exchange for a $150,000 non-refundable transaction fee. Pursuant to the Forbearance Agreement, on April 21, 2016, we made a prepayment to White Oak, with respect to our obligations under the loan and security agreement with White Oak, in the amount of $5.0 million from our restricted account. Additionally, the outstanding obligations under the White Oak loan will become due on or before August 17, 2016. As of December 31, 2015, the debt was reclassified as a short-term liability.
Equity Transactions
On July 30, 2013, we entered into a securities purchase agreement to sell an aggregate of 2,845,528 shares of our common stock at a per share price of $12.30 and warrants to purchase an aggregate of 3,414,634 shares of common stock at a per warrant price of $1.25 in a private placement transaction. The warrants were comprised of the following three series: Series A warrants exercisable for an aggregate 1,138,211 shares of common stock, with an exercise price equal to $12.30 per share (the “Series A Warrants”); Series B warrants exercisable for an aggregate 1,138,211 shares of common stock, with an exercise price equal to $15.0 per share (the “Series B Warrants”); and Series C warrants exercisable for an aggregate 1,138,211 shares of common stock, with an exercise price equal to $20.0 per share (the “Series C Warrants”). The Series A Warrants were subject to mandatory exercise subsequent to the receipt of regulatory approval for the new Magellan 6Fr Robotic Catheter in the U.S., which occurred in February 2014. The financing resulted in gross proceeds to us of approximately $39.3 million prior to placement fees and offering costs of approximately $2.1 million. At the closing of the private placement financing, we entered into an investor rights agreement with the purchasers of the shares and warrants in which we agreed to file a registration statement covering resales of the shares (which occurred in November 2013) and the purchasers agreed not to transact in any shares of our common stock for a one-year period following the closing, subject to certain exceptions. In the first quarter of 2014, subsequent to the receipt of regulatory approval for the new Magellan 6Fr Robotic Catheter in the U.S., Series A Warrants for 1.1 million shares of our common stock were exercised for total proceeds of $14.0 million in accordance with the terms and conditions of a securities purchase agreement dated July 30, 2013. All of the Series A Warrants were mandatorily exercised in the first quarter of 2014 pursuant to our achievement of a regulatory milestone as set forth in the Series A Warrants.
On July 30, 2014, we entered into a definitive agreement (the “Exchange Agreement”) with certain warrant holders to cancel and exchange (the “Exchange”) an aggregate of 1,022,117 of our outstanding Series B Warrants and an aggregate of 1,022,117 of our outstanding Series C Warrants. In exchange, we issued warrants (the “Exchange Warrants”) to purchase an aggregate of 2,672,837 shares of common stock. The Exchange was completed in August 2014.
70
The Exchange Warrants are comprised of the following two tranches: (a) Series B/C exchange warrants (“Series B/C Exchange Warrants”) exercisable for an aggregate of 2,044,235 shares of common stock, with an exercise price equal to $11.30 per share, the NASDAQ consolidated closing bid price for the common stock on July 29, 2014, the last completed trading day before the Exchange Agreement was executed (the “Closing Bid Price”); and (b) Series D warrants (“Series D Warrants”) exercisable for an aggregate of 628,602 shares of common stock, with an exercise price per share equal to the Closing Bid Price. The Series B/C Exchange Warrants were subject to mandatory exercise within 14 days of issuance and were exercised in August 2014, resulting in gross proceeds to us of approximately $23.1 million. The Series D Warrants have an exercise period of five years, and if fully exercised, would result in additional gross proceeds to us of approximately $7.1 million. The Series B Warrants and Series C Warrants previously carried an expiration date of August 2015. The remaining Series B Warrants and Series C Warrants not included in the Exchange will remain outstanding until their exercise or expiration.
On March 11, 2015, we raised $35.0 million in gross proceeds from the sale of 53,846 shares of Series A convertible preferred stock at a per share price of $650. The Series A convertible preferred stock was converted into 5,509,492 shares of common stock. The number of shares of common stock issued were equal to the number obtained by dividing (i) the sum of $650 and the amount of any accrued but unpaid dividends by (ii) $0.65. The conversion of the Series A convertible preferred stock occurred automatically upon receipt of Requisite Stockholder Approval on May 12, 2015 and the dividends included in the conversion formula were recorded as an increase to the carrying value of Series A convertible preferred stock when declared on May 12, 2015. The proceeds from this transaction were used to support our commercialization efforts with the Magellan System and strengthen our operations.
Future Capital Requirements
We recognized our first revenues in 2007 and we have not achieved profitability or generated net income to date. We have experienced significant fluctuations in quarterly shipments and revenues and, beginning in the fourth quarter of 2008, we saw many potential customers lengthen their sales cycles and postpone purchase decisions.
We have incurred cumulative net losses of approximately $453.9 million and negative working capital of $6.9 million as of December 31, 2015. We expect such losses to continue through at least the year ending December 31, 2016 as we continue to commercialize our products, maintain and develop the infrastructure required to manufacture and sell our products, pursue additional applications for our technology platform, develop new products and operate as a publicly traded company. As of December 31, 2015, our cash, cash equivalents, short-term investments and restricted cash balances were $28.0 million. We incurred a net loss of $46.2 million and negative cash flows from operations of $37.7 million for the year ended December 31, 2015. In addition, we are also subject to minimum liquidity requirements under our existing borrowing arrangements with White Oak which require us to maintain $15.0 million in liquidity at all times, consisting of at least $13.0 million in cash, cash equivalents and investments, and the lesser of $2.0 million or 65% of eligible accounts receivable. In addition, $5.0 million investment in Certificate of Deposit along with investments in Luna Innovations, Inc., is required to be restricted subject to lenders’ control. Additionally, we are required to obtain required to obtain an audit opinion from our independent certified public accountants on the annual financial statements that does not include an explanatory uncertainty paragraph raising substantial doubt about the Company’s ability to continue as a going concern or any qualification or exception as to the scope of such audit. As of December 31, 2015, we were in compliance with all covenants; however, there was an anticipated default of the covenants listed in Section 6.2(a)(i) and Section 8.2(a), with respect to Section 6.2(a)(i), as of December 31, 2015, which was cured by the Forbearance Agreement as discussed below. We are currently in default of this requirement and certain other covenants and events of default listed in Sections 6.1(c), 6.2(a)(i), 6.2(a)(iv), 6.10, 8.2(a) and 8.2(b) of the loan and security agreement with White Oak (the “Specified Events of Default”). On April 19, 2016, we entered into a Forbearance Agreement with White Oak (“Forbearance Agreement”) whereby White Oak granted forbearance of the Specified Events of Default until August 17, 2016 in exchange for a $150,000 non-refundable transaction fee. Pursuant to the Forbearance Agreement, on April 21, 2016, we made a prepayment to White Oak, with respect to our obligations under the loan and security agreement with White Oak, in the amount of $5.0 million from our restricted account. Additionally, the outstanding obligations under the White Oak loan will become due on or before August 17, 2016. As of December 31, 2015, the debt was reclassified as a short-term liability.
We also maintain $750,000 in restricted cash as collateral to obtain a letter of credit used as lease security for our new office in San Jose, California.
Based on our current operating projections, we do not have sufficient liquidity to meet our anticipated cash requirements through the next twelve months. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
71
In order to continue our operations, we will need to obtain sufficient additional funding to satisfy our current and longer term liquidity requirements and may attempt to do so at any time by, for example, selling equity or debt securities, licensing core or non-core intellectual property assets, entering into future research and development funding arrangements, refinancing or restructuring existing debt arrangements or entering into a credit facility in order to meet our continuing cash needs. If such financing, licensing, funding or credit arrangements do not meet our longer term needs, we may be required to extend our existing cash and liquidity by adopting additional cost-cutting measures, including reductions in our work force, reducing the scope of, delaying or eliminating some or all of our planned research, development and commercialization activities and/or reducing marketing, customer support or other resources devoted to our products. Any of these factors could harm our financial condition. There can be no assurance, however, that such a funding alternative will be successfully completed on terms acceptable to us or that the Company can implement cost cutting measures sufficient to extend our cash and liquidity. Management is currently considering various financing alternatives. Failure to raise additional funding or manage our spending may adversely impact our ability to achieve our long term intended business objectives. We will continue to evaluate the extent of our implemented cost-saving measures based upon changing future economic conditions and will consider the implementation of additional cost reductions if and as circumstances warrant.
If we seek additional funding in the future by selling additional equity or debt securities, refinancing or restructuring existing debt arrangements or entering into a credit facility, such additional funding may result in substantial dilution to existing stockholders, may contain unfavorable terms or may not be available on any terms. If we are unable to obtain any needed additional funding, we may be required to reduce the scope of, delay, or eliminate some or all of, our planned research, development and commercialization activities or to license to third parties the rights to commercialize products or technologies that we would otherwise seek to commercialize ourselves or on terms that are less attractive than they might otherwise be, any of which could materially harm our business. The timing and exact amounts of our capital requirements will depend on many factors, including but not limited to the following:
• | our ability to achieve and maintain compliance with debt covenants; |
• | the cash collected from and the revenue and margins generated by sales of our current and future products; |
• | the terms and timing of any collaborative, licensing or other arrangements that we may establish; |
• | the success of our research and development efforts; |
• | our ability to generate revenue in a time of overall economic uncertainty; |
• | the expenses we incur in manufacturing, marketing and selling our products, developing new products and operating our company; |
• | our ability to achieve and maintain manufacturing cost reductions; |
• | our ability to achieve and maintain operating cost reductions; |
• | the rate of progress and cost of our clinical trials and other development activities; |
• | the cost and timing of future regulatory actions; |
• | the costs of filing, prosecuting, defending and enforcing any patent claims and other intellectual property or other legal rights, or participating in litigation-related activities; |
• | the costs of defending against lawsuits brought against us or individuals indemnified by us; |
• | the emergence of competing or complementary technological developments; and |
• | the acquisition of businesses, products and technologies. |
We cannot guarantee that future equity or debt financing or credit facilities will be available in amounts or on terms acceptable to us, if at all, or that we can achieve and maintain compliance with debt covenants. This could leave us without adequate financial resources to fund our operations as we plan to conduct them in the future.
Contractual Obligations
The following table summarizes our outstanding contractual obligations as of December 31, 2015 and the effect those obligations are expected to have on our liquidity and cash flows in future periods (in thousands):
Contractual Obligations | Total | Less than 1 Year | 1-3 Years | 3-5 years | Thereafter | ||||||||||||||
Operating leases—real estate | $ | 8,353 | $ | 1,728 | $ | 907 | $ | 1,275 | $ | 4,443 | |||||||||
Debt, including interest | 45,790 | 45,790 | — | — | — | ||||||||||||||
Total | $ | 54,143 | $ | 47,518 | $ | 907 | $ | 1,275 | $ | 4,443 | |||||||||
72
The table above reflects only payment obligations that are fixed and determinable. Our commitments for operating leases relate principally to the lease for our corporate headquarters in Mountain View, California which will terminate in August 2016 and the new lease in San Jose, California for the period of August 2016 to February 2027. Future debt payments relate to principal and interest payments related to the $35.1 million we have borrowed under our loan agreement with White Oak as of December 31, 2015.
Additionally, we have minimum royalty obligations of $100,000 per year under a license agreement with Mitsubishi Electric Research Laboratories, Inc. which reduces to $55,000 per year if the license becomes non-exclusive. The royalty obligation expires in 2018. We also have minimum royalty obligations of $200,000 per year under the terms of our cross license agreement with Intuitive Surgical. We also have royalty obligations under the amended joint development agreement with Philips which provides for the payment of royalties to Philips through October 2017. As of December 31, 2015, we had $1.4 million in uncertain tax positions. If it is determined in some future period that these amounts are not allowed to be deducted for tax purposes and if we do not have credits or carryforwards to cover these amounts, they could result in payments by us to taxing authorities.
Recent Accounting Pronouncements
See Note 2 to our consolidated financial statements under the caption “Recent Accounting Pronouncements” for a discussion of new accounting pronouncements. We are currently assessing whether the impact of these new pronouncements or interpretations upon adoption will have a material impact on our results of operations, financial position or cash flows.
Off-balance Sheet Arrangements
As of December 31, 2015, we had no off-balance sheet arrangements as defined in Item 303(a)(4) of Regulation S-K.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
In the normal course of business, our financial position is subject to a variety of risks, including market risk associated with interest rate movements and foreign currency exchange risk. We regularly assess these risks and have established policies and business practices to protect against these and other exposures. As a result, we do not anticipate material potential losses in these areas.
The primary objective for our investment activities is to preserve principal while maximizing yields without significantly increasing risk. This is accomplished by investing in widely diversified short-term investments, consisting primarily of investment grade securities. As of December 31, 2015, the fair value of our cash, cash equivalents, short-term investments and restricted cash was approximately $28.0 million. A hypothetical 100 basis point increase in interest rates would not result in a material decrease or increase in our interest income nor in the fair value of our available-for-sale securities. We have no investments denominated in foreign country currencies and therefore our investments are not subject to foreign currency exchange risk.
A portion of our operations consist of sales activities outside of the United States and, as such, we have foreign currency exposure to non-United States dollar revenues and accounts receivable. Currently, we sell our products mainly in United States dollars, Euros and Great Britain Pounds although we may in the future transact business in other currencies. Future fluctuations in the exchange rates of these currencies may impact our revenues. In the past, we have not hedged our exposures to foreign currencies or entered into any other derivative instruments and we have no current plans to do so. For the year ended December 31, 2015, sales denominated in foreign currencies were approximately 8% of total revenue. A hypothetical 5% increase in the United States dollar exchange rate used would have resulted in an immaterial decrease to revenues for 2015
73
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index to Financial Statements
Page | |
74
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Hansen Medical, Inc.
Mountain View, California
We have audited the accompanying consolidated balance sheets of Hansen Medical, Inc. as of December 31, 2015 and 2014 and the related consolidated statements of operations, comprehensive loss, convertible preferred stock and stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2015. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Hansen Medical, Inc. at December 31, 2015 and 2014, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations, negative working capital and a net capital deficiency that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Hansen Medical, Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated April 22, 2016 expressed an adverse opinion thereon.
/s/ BDO USA, LLP
San Jose, California
April 22, 2016
75
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Hansen Medical, Inc.:
We have audited the accompanying consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows of Hansen Medical, Inc. and subsidiaries (the “Company”) for the year ended December 31, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the financial statements based on our audits.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the results of operations of Hansen Medical, Inc. and subsidiaries as of December 31, 2013 and their cash flows for the year ended December 31, 2013, in conformity with accounting principles generally accepted in the United States of America.
/s/ Deloitte & Touche LLP
San Francisco, California
March 13, 2014
76
HANSEN MEDICAL, INC.
Consolidated Balance Sheets
(In thousands, except per share data)
December 31, | |||||||
2015 | 2014 | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 20,375 | $ | 24,528 | |||
Short-term investments | 1,501 | 1,973 | |||||
Accounts receivable, net of allowance of $23 and $0 at December 31, 2015 and 2014 | 4,551 | 5,121 | |||||
Inventories | 8,659 | 11,492 | |||||
Prepaids and other current assets | 1,814 | 1,678 | |||||
Total current assets | 36,900 | 44,792 | |||||
Property and equipment, net | 2,325 | 2,328 | |||||
Restricted cash | 6,113 | 5,376 | |||||
Other assets | 380 | 1,179 | |||||
Total assets | $ | 45,718 | $ | 53,675 | |||
LIABILITIES AND STOCKHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 664 | $ | 2,534 | |||
Accrued liabilities | 4,113 | 4,942 | |||||
Current portion of deferred revenue | 3,923 | 3,422 | |||||
Current portion of long-term debt | 35,141 | — | |||||
Total current liabilities | 43,841 | 10,898 | |||||
Deferred rent, net of current portion | 262 | 366 | |||||
Deferred revenue, net of current portion | 91 | 105 | |||||
Long-term debt, net of current portion | — | 34,385 | |||||
Other long-term liabilities | 76 | 152 | |||||
Total liabilities | 44,270 | 45,906 | |||||
Commitments and contingencies (Note 7) | |||||||
Stockholders’ equity: | |||||||
Preferred stock, par value $0.0001: Authorized: 10,000 shares Issued and outstanding: none | — | — | |||||
Common stock, par value $0.0001: Authorized: 30,000 shares Issued and outstanding: 18,897 and 13,326 at December 31, 2015 and 2014 * | 2 | 13 | |||||
Additional paid-in capital | 455,585 | 414,361 | |||||
Accumulated other comprehensive income | (210 | ) | 305 | ||||
Accumulated deficit | (453,929 | ) | (406,910 | ) | |||
Total stockholders’ equity | 1,448 | 7,769 | |||||
Total liabilities and stockholders’ equity | $ | 45,718 | $ | 53,675 | |||
*The Company’s financial statements have been retroactively adjusted to reflect the reverse stock split of its outstanding shares of common stock at a ratio of one-for-ten effective on September 22, 2015.
The accompanying notes are an integral part of these consolidated financial statements.
77
HANSEN MEDICAL, INC.
Consolidated Statements of Operations
(In thousands, except per share data)
Years ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Revenues: | |||||||||||
Product | $ | 10,719 | $ | 13,812 | $ | 11,790 | |||||
Service | 5,349 | 5,683 | 5,192 | ||||||||
Total revenues | 16,068 | 19,495 | 16,982 | ||||||||
Cost of revenues: | |||||||||||
Product | 12,016 | 13,123 | 11,150 | ||||||||
Service | 3,049 | 3,368 | 2,331 | ||||||||
Total cost of revenues | 15,065 | 16,491 | 13,481 | ||||||||
Gross profit | 1,003 | 3,004 | 3,501 | ||||||||
Operating expenses: | |||||||||||
Research and development | 13,812 | 18,034 | 16,139 | ||||||||
Selling, general and administrative | 23,495 | 31,254 | 31,765 | ||||||||
Restructuring expense | 1,539 | — | — | ||||||||
Loss on settlement of litigation (Note 7) | — | — | 4,500 | ||||||||
Total operating expenses | 38,846 | 49,288 | 52,404 | ||||||||
Loss from operations | (37,843 | ) | (46,284 | ) | (48,903 | ) | |||||
Interest income | 21 | 23 | 25 | ||||||||
Loss on extinguishment of debt | — | — | (1,935 | ) | |||||||
Interest expense | (5,287 | ) | (4,789 | ) | (3,952 | ) | |||||
Warrant exchange and change in fair value of warrant liability (Note 10) | (2,993 | ) | (2,914 | ) | — | ||||||
Other expense, net | (58 | ) | (254 | ) | (842 | ) | |||||
Loss before income taxes | (46,160 | ) | (54,218 | ) | (55,607 | ) | |||||
Income tax expense | (47 | ) | (28 | ) | (115 | ) | |||||
Net loss | $ | (46,207 | ) | $ | (54,246 | ) | $ | (55,722 | ) | ||
Deemed dividend related to beneficial conversion feature of Series A convertible preferred stock and accretion of discount | (35,546 | ) | — | — | |||||||
Cumulative dividend on Series A convertible preferred stock | (812 | ) | — | — | |||||||
Net loss attributable to common stockholders | $ | (82,565 | ) | (54,246 | ) | (55,722 | ) | ||||
Basic and diluted net loss per common share * | $ | (4.89 | ) | $ | (4.63 | ) | $ | (7.05 | ) | ||
Shares used to compute basic and diluted net loss per common share * | 16,871 | 11,723 | 7,905 | ||||||||
*The Company’s financial statements have been retroactively adjusted to reflect the reverse stock split of its outstanding shares of common stock at a ratio of one-for-ten effective on September 22, 2015.
The accompanying notes are an integral part of these consolidated financial statements.
78
HANSEN MEDICAL, INC.
Consolidated Statements of Comprehensive Loss
(In thousands)
Years ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Net loss | $ | (46,207 | ) | $ | (54,246 | ) | $ | (55,722 | ) | ||
Other comprehensive income (loss), net: | |||||||||||
Change in unrealized gains (losses) on investments | (464 | ) | 19 | 276 | |||||||
Amounts reclassified to other income | — | — | 627 | ||||||||
Foreign currency translation adjustment | (51 | ) | (74 | ) | 35 | ||||||
Change in other comprehensive income (loss) | (515 | ) | (55 | ) | 938 | ||||||
Comprehensive loss | $ | (46,722 | ) | $ | (54,301 | ) | $ | (54,784 | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
79
HANSEN MEDICAL, INC.
Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity
(In thousands)
Convertible Preferred Stock | Common Stock | Additional Paid- In Capital | Accumulated Other Comprehensive Income (Loss) | Accumulated Deficit | Total Stockholders’ Equity | |||||||||||||||||||||||||
Shares | Amount | Shares * | Amount | |||||||||||||||||||||||||||
Balances, December 31, 2012 | — | $ | — | 6,708 | $ | 7 | $ | 322,252 | $ | (578 | ) | $ | (296,942 | ) | $ | 24,739 | ||||||||||||||
Issuance of common stock under equity incentive plan | — | — | 79 | — | 114 | — | — | 114 | ||||||||||||||||||||||
Withholding taxes paid on vested restricted stock units | — | — | — | — | (18 | ) | — | — | (18 | ) | ||||||||||||||||||||
Issuance of common stock under employee stock purchase plan | — | — | 39 | — | 560 | — | — | 560 | ||||||||||||||||||||||
Issuance of common stock and warrants pursuant to private placement | — | — | 2,846 | 3 | 37,123 | — | — | 37,126 | ||||||||||||||||||||||
Issuance of common shares in connection with the litigation settlement | — | — | 230 | — | 4,250 | — | — | 4,250 | ||||||||||||||||||||||
Employee share-based compensation expense | — | — | — | — | 4,889 | — | — | 4,889 | ||||||||||||||||||||||
Net loss | — | — | — | — | — | — | (55,722 | ) | (55,722 | ) | ||||||||||||||||||||
Change in unrealized gain on investments | — | — | — | — | — | 276 | — | 276 | ||||||||||||||||||||||
Amounts reclassified to other income (expense) | — | — | — | — | — | 627 | — | 627 | ||||||||||||||||||||||
Translation adjustment | — | — | — | — | — | 35 | — | 35 | ||||||||||||||||||||||
Balances, December 31, 2013 | — | — | 9,902 | 10 | 369,170 | 360 | (352,664 | ) | 16,876 | |||||||||||||||||||||
Issuance of common stock under equity incentive plan | — | — | 187 | — | 2,586 | — | — | 2,586 | ||||||||||||||||||||||
Withholding taxes paid on vested restricted stock units | — | — | — | — | (620 | ) | — | — | (620 | ) | ||||||||||||||||||||
Issuance of common stock under employee stock purchase plan | — | — | 55 | — | 519 | — | — | 519 | ||||||||||||||||||||||
Issuance of common stock upon exercise of Series A warrants | — | — | 1,138 | 1 | 13,999 | — | — | 14,000 | ||||||||||||||||||||||
Issuance of common stock upon Series B/C warrant exchange | — | — | 2,044 | 2 | 25,875 | — | — | 25,877 | ||||||||||||||||||||||
Employee share-based compensation expense | — | — | — | — | 2,832 | — | — | 2,832 | ||||||||||||||||||||||
Net loss | — | — | — | — | — | — | (54,246 | ) | (54,246 | ) | ||||||||||||||||||||
Change in unrealized gain on investments | — | — | — | — | — | 19 | — | 19 | ||||||||||||||||||||||
Translation adjustment | — | — | — | — | — | (74 | ) | — | (74 | ) | ||||||||||||||||||||
Balances, December 31, 2014 | — | — | 13,326 | 13 | 414,361 | 305 | (406,910 | ) | 7,769 | |||||||||||||||||||||
Issuance of common stock under equity incentive plan | — | — | — | — | 3 | — | — | 3 | ||||||||||||||||||||||
Issuances of common stock from restricted stock units | — | — | 29 | — | — | — | — | — | ||||||||||||||||||||||
Withholding taxes paid on vested restricted stock units | — | — | — | — | (26 | ) | — | — | (26 | ) | ||||||||||||||||||||
Issuance of common stock under employee stock purchase plan | — | — | 44 | — | 214 | — | — | 214 | ||||||||||||||||||||||
Issuance of Series A convertible preferred stock,net of discount related to warrants of $14,776 and issuance cost of $546 | 54 | 19,678 | — | — | — | — | — | — | ||||||||||||||||||||||
Beneficial conversion feature of Series A convertible preferred stock | — | (20,224 | ) | — | — | 20,224 | — | — | 20,224 | |||||||||||||||||||||
Deemed dividend related to beneficial conversion feature of Series A Convertible preferred stock and accretion of discount | — | 35,546 | — | — | (35,546 | ) | — | — | (35,546 | ) | ||||||||||||||||||||
Reclassification of Series E warrant liability to equity | — | — | — | — | 17,774 | — | — | 17,774 | ||||||||||||||||||||||
Cumulative dividend on Series A convertible preferred stock | — | 812 | — | — | — | (812 | ) | (812 | ) | |||||||||||||||||||||
Issuance of common stock upon conversion of Series A convertible preferred stock | (54 | ) | (35,000 | ) | 5,510 | 5 | 34,995 | — | — | 35,000 | ||||||||||||||||||||
Issuance of common stock related to Series A convertible preferred stock dividend | — | (812 | ) | — | 1 | 811 | — | — | 812 | |||||||||||||||||||||
Retire Treasury shares | — | — | (12 | ) | — | — | — | — | — | |||||||||||||||||||||
Retire fraction shares due to reverse stock split | — | — | — | (17 | ) | 17 | — | |||||||||||||||||||||||
Employee share-based compensation expense | — | — | — | — | 2,758 | — | — | 2,758 | ||||||||||||||||||||||
Net loss | — | — | — | — | — | — | (46,207 | ) | (46,207 | ) | ||||||||||||||||||||
Change in unrealized loss on investments | — | — | — | — | — | (464 | ) | — | (464 | ) | ||||||||||||||||||||
Translation adjustments | — | — | — | — | — | (51 | ) | — | (51 | ) | ||||||||||||||||||||
Balances, December 31, 2015 | — | $ | — | 18,897 | $ | 2 | $ | 455,585 | $ | (210 | ) | $ | (453,929 | ) | $ | 1,448 | ||||||||||||||
*The Company's financial statements have been retroactively adjusted to reflect the reverse stock split of its outstanding shares of common stock at a ratio of one-for-ten effective on September 22, 2015.
The accompanying notes are an integral part of these consolidated financial statements.
80
HANSEN MEDICAL, INC.
Consolidated Statements of Cash Flows
(In thousands)
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Cash flows from operating activities | |||||||||||
Net loss | $ | (46,207 | ) | $ | (54,246 | ) | $ | (55,722 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | |||||||||||
Depreciation | 1,027 | 2,555 | 2,949 | ||||||||
Stock-based compensation | 2,758 | 2,832 | 4,889 | ||||||||
Warrant exchange and change in fair value of warrant liability | 2,993 | 2,914 | — | ||||||||
Payment-in-kind interest on loan | 1,058 | 1,026 | 597 | ||||||||
Reserve for excess and obsolescence in inventory | 1,083 | 706 | — | ||||||||
Loss on settlement of litigation | — | — | 4,500 | ||||||||
Amortization of deferred financing costs and discount on debt | (67 | ) | 387 | 184 | |||||||
Amortization of common stock warrants | — | — | 195 | ||||||||
Gain on disposal of property and equipment | (36 | ) | — | — | |||||||
Net realized gains and losses on investments | — | — | 677 | ||||||||
Loss on extinguishment of debt | — | — | 1,935 | ||||||||
Provision (recovery) of doubtful accounts | 49 | (100 | ) | — | |||||||
Changes in operating assets and liabilities: | |||||||||||
Accounts receivable | 558 | 93 | 120 | ||||||||
Inventories | 1,044 | (775 | ) | (3,069 | ) | ||||||
Prepaids and other current assets | (127 | ) | 227 | 315 | |||||||
Other assets | 564 | 1,387 | (1,464 | ) | |||||||
Accounts payable | (1,870 | ) | (803 | ) | 226 | ||||||
Accrued liabilities | (828 | ) | 1,034 | 568 | |||||||
Deferred revenue | 486 | 412 | 345 | ||||||||
Other liabilities | (179 | ) | (47 | ) | — | ||||||
Net cash used in operating activities | (37,694 | ) | (42,398 | ) | (42,755 | ) | |||||
Cash flows from investing activities | |||||||||||
Purchase of property and equipment | (357 | ) | (462 | ) | (550 | ) | |||||
Proceeds from sales and maturities of short-term investments | 36 | — | 6,704 | ||||||||
Changes in restricted cash | (737 | ) | 19 | (5,394 | ) | ||||||
Net cash (used) provided by investing activities | (1,058 | ) | (443 | ) | 760 | ||||||
Cash flows from financing activities | |||||||||||
Proceeds from issuance of common stock and warrants | 35,000 | — | 39,268 | ||||||||
Proceeds from exercise of Series A, B and C warrants | — | 37,100 | — | ||||||||
Costs related to issuance of preferred stock, common stock and warrants | (546 | ) | (137 | ) | (2,087 | ) | |||||
Proceeds from loans | — | — | 33,000 | ||||||||
Costs related to issuance of loans | — | — | (1,538 | ) | |||||||
Repayments of loan principal | — | — | (30,000 | ) | |||||||
Final loan repayment fees | — | — | (2,058 | ) | |||||||
Proceeds from exercise of common stock options | 3 | 2,586 | 114 | ||||||||
Proceeds from employee stock purchase plan | 214 | 519 | 560 | ||||||||
Withholding taxes paid on vested restricted stock units | (21 | ) | (620 | ) | (18 | ) | |||||
Net cash provided by financing activities | 34,650 | 39,448 | 37,241 | ||||||||
Effect of exchange rate changes on cash and cash equivalents | (51 | ) | (74 | ) | — | ||||||
Net (decrease) in cash and cash equivalents | (4,153 | ) | (3,467 | ) | (4,754 | ) | |||||
Cash and cash equivalents at beginning of year | 24,528 | 27,995 | 32,749 | ||||||||
Cash and cash equivalents at end of year | $ | 20,375 | $ | 24,528 | $ | 27,995 | |||||
Supplemental disclosures of cash flow information | |||||||||||
Cash paid during the period for interest | $ | 3,878 | $ | 3,763 | $ | 5,207 | |||||
Supplemental schedule of non-cash investing and financing activities | |||||||||||
Equity issuance costs not yet paid | $ | — | $ | — | $ | 55 | |||||
Equipment transfers from inventories to property and equipment | $ | 705 | $ | 779 | $ | — | |||||
Cumulative dividend on Series A preferred stock | $ | 812 | $ | — | $ | — | |||||
Deemed dividend related to beneficial conversion feature of Series A convertible preferred stock and accretion of discount | $ | 35,546 | $ | — | $ | — | |||||
The accompanying notes are an integral part of these consolidated financial statements.
81
HANSEN MEDICAL, INC.
Notes to Consolidated Financial Statements
1. The Company
Nature of Operations
Hansen Medical, Inc. (the “Company”) develops, manufactures and markets a new generation of medical robotics designed for accurate positioning, manipulation and stable control of catheters and catheter-based technologies. The Company was incorporated in the state of Delaware in September 2002 and is headquartered in Mountain View, California. The Company has wholly-owned subsidiaries located in the United Kingdom and Germany. Both subsidiaries are engaged in marketing the Company’s products in the Europe, Middle East and Africa (“EMEA”) region.
Going Concern
These consolidated financial statements are prepared on a going concern basis that contemplates the realization of assets and discharge of liabilities in the normal course of business. Since inception, the Company has incurred cumulative net losses of approximately $453.9 million. The Company expects such losses to continue through at least the year ending December 31, 2016 as it continues to commercialize its technologies and develop new applications and products. The Company also has a negative working capital of $6.9 million.
The Company continues to face significant uncertainties and challenges. The Company faces uncertainty related to the commercialization of its MagellanTM Robotic System ("Magellan System") and its projected revenue is heavily dependent on a successful commercialization of this system. In addition, the Company is also subject to minimum liquidity requirements under its existing borrowing arrangements with White Oak Global Advisors, LLC ("White Oak"), which require the Company to maintain $15.0 million in liquidity at all times, consisting of at least $13.0 million in cash, cash equivalents, restricted cash and investments and the lesser of $2.0 million or 65% in eligible accounts receivable. Additionally, the Company is required by White Oak to obtain an audit opinion from its independent certified public accountants on the annual financial statements that does not include an explanatory uncertainty paragraph raising substantial doubt about its ability to continue as a going concern or any qualification or exception as to the scope of such audit. As of December 31, 2015, the Company was in compliance with all covenants; however, there was an anticipated default of the covenants listed in Section 6.2(a)(i) and Section 8.2(a), with respect to Section 6.2(a)(i), as of December 31, 2015, which was cured by the Forbearance Agreement as discussed below. As of March 31, 2016, the Company was no longer in compliance with certain covenants and events of default listed in Sections 6.1(c), 6.2(a)(i), 6.2(a)(iv), 6.10, 8.2(a) and 8.2(b) of the loan and security agreement with White Oak (the “Specified Events of Default”). On April 19, 2016, the Company entered into a Forbearance Agreement with White Oak (“Forbearance Agreement”) whereby White Oak granted forbearance of the Specified Events of Default until August 17, 2016 in exchange for a $150,000 non-refundable transaction fee. Pursuant to the Forbearance Agreement, on April 21, 2016, the Company made a prepayment to White Oak, with respect to our obligations under the loan and security agreement with White Oak, in the amount of $5.0 million from the Company’s restricted account. Additionally, the outstanding obligations under the White Oak loan will become due on or before August 17, 2016. As of December 31, 2015, the debt was classified as a short-term liability.
In connection with this arrangement, White Oak also requires that the Company’s investment in a Certificate of Deposit of $5.0 million, along with investments in Luna Innovations, Inc., to be restricted subject to lenders’ control. As of December 31, 2015, the Company’s cash, cash equivalents, short-term investments and restricted cash balances were $28.0 million.
The Company anticipates that its existing available capital resources as of December 31, 2015 and the estimated amounts received through the sale of its products and services will not be sufficient to meet its anticipated cash requirements for the next twelve months. These factors raise substantial doubt about the Company’s ability to continue as a going concern. The Company will need to obtain sufficient additional funding to satisfy its current and longer term liquidity requirements and may attempt to do so at any time by, for example, selling equity or debt securities, licensing core or non-core intellectual property assets, entering into future research and development funding arrangements, refinancing or restructuring existing debt arrangements or entering into a credit facility in order to meet its continuing cash needs. The Company cannot guarantee that future equity or debt financing or credit facilities will be available in amounts or on terms acceptable to it, if at all, that it will achieve or maintain compliance with debt covenants, that it will be able to license core or non-core intellectual property assets or enter into future research and development funding arrangements. If such financing, licensing, funding or credit arrangements do not meet the Company’s current and longer term needs, the Company may be required to extend its existing cash and liquidity by adopting additional cost-cutting measures, including reductions in its work force, reducing the scope of, delaying or eliminating some or all of its planned research, development and commercialization activities and/or reducing marketing, customer support or other resources devoted to the Company’s products. Any of these factors could harm the Company’s financial condition. Failure to raise additional funding or manage spending may adversely impact the Company’s ability to achieve its long term
82
intended business objectives. The Company will continue to evaluate its financial condition based upon changing future economic conditions, and will consider the implementation of additional cost reductions if and as circumstances warrant. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Reclassification of Prior Period Balances
Certain reclassifications have been made to prior period amounts to conform to the current-year presentation.Such reclassifications did not have a material impact on the Company’s results of operations or financial position.
2. Summary of Significant Accounting Policies
Basis of Presentation
The Company has prepared the accompanying consolidated financial statements in accordance with generally accepted accounting principles in the United States of America (“GAAP”). The Company’s fiscal year ends on December 31. The consolidated financial statements include the accounts of the Company and its subsidiaries. All significant intercompany accounts and transactions have been eliminated.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Significant estimates relate to the recognition of revenue, the evaluation of customer credit risk, the valuation of investments, inventory valuations, warrant valuations, the determination of impairment of assets, stock-based compensation, loss contingencies and the valuation of our deferred tax assets, among others. Actual results could differ from those estimates.
Reverse Split of Common Stock
On May 12, 2015, at the Annual Meeting of Stockholders of the Company, the stockholders approved a series of alternate amendments to the Company’s Certificate of Incorporation (the "Certificate") to effect, at the discretion of the Company’s Board of Directors (the "Board"), a reverse stock split of the Company’s common stock whereby each outstanding four, six, eight or ten shares would be combined into one share of common stock and a proportional reduction in the number of authorized shares of common stock. On September 18, 2015, the Board approved a reverse stock split of the Company’s outstanding shares of common stock at a ratio of one-for-ten and the related amendment to the Certificate providing for the combination of each outstanding ten shares of Company common stock into one share of Company common stock. The amendment to the Certificate was filed with the Secretary of State of the State of Delaware on September 22, 2015, decreasing the Company’s authorized common stock from 300,000,000 to 30,000,000. Historical share information presented in the accompanying financial statements has been retroactively adjusted to reflect this reverse stock split.
Revenue Recognition
The Company’s revenues are primarily derived from the sale of the Sensei System and the Magellan System and the associated catheters as well as the sale of customer service contracts, which includes post-contract customer support (“PCS”). The Company sells its products directly to customers as well as through distributors. Under the Company’s revenue recognition policy, revenues are recognized when persuasive evidence of an arrangement exists, title and risk of loss has passed, delivery to the customer has occurred or the services have been fully rendered, the sales price is fixed or determinable and collectability is reasonably assured.
• | Persuasive Evidence of an Arrangement. Persuasive evidence of an arrangement for sales of systems is generally determined by a sales contract signed and dated by both the customer and the Company, including approved terms and conditions or the receipt of an approved purchase order. Evidence of an arrangement for the sale of disposable products is determined through an approved purchase order from the customer. Evidence of an arrangement for the sale of customer service is determined through either a signed sales contract or an approved purchase order from the customer. Sales are generally not subject to any performance, cancellation, termination or return rights. |
• | Delivery. |
• | Systems and Disposable Products. Typically, ownership of systems, catheters and other disposable products passes to customers upon shipment, at which time delivery is deemed to be complete. |
• | Customer Service Revenue. The Company recognizes customer service revenue from the sale of its PCS contracts which includes planned and corrective maintenance services, software updates, bug fixes, and warranty. Revenue |
83
from customer services, whether sold individually or as a separate unit of accounting in a multi-element arrangement, is deferred and amortized over the service period, which is typically one year.
• | Multiple-element Arrangements. It is common for the sale of Sensei and Magellan Systems to include multiple elements which have standalone value and qualify as separate units of accounting. These elements commonly include the sale of the system and a product maintenance plan, in addition to installation of the system and initial training. Less commonly, these elements may include the sale of certain disposable products or other elements. For multiple-element arrangements revenue is allocated to each element based on their relative selling prices. Relative selling prices are based first on vendor specified objective evidence (“VSOE”), then on third-party evidence of selling price (“TPE”) when VSOE does not exist, and then on management’s best estimate of the selling price (“ESP”) when VSOE and TPE do not exist. Because the Company has neither VSOE nor TPE for its system, the allocation of revenue is based on ESP for the systems sold. The objective of ESP is to determine the price at which the Company would transact a sale, had the product been sold on a stand-alone basis. The Company determines ESP for its systems by considering multiple factors, including, but not limited to, features and functionality of the system, geographies, type of customer, and market conditions. The Company regularly reviews ESP and maintains internal controls over the establishment and updates of these estimates. |
• | Sales Price Fixed or Determinable. The Company assesses whether the sales price is fixed or determinable at the time of the transaction. Sales prices are documented in the executed sales contract or purchase order received prior to shipment The Company’s standard terms do not allow for contingencies, such as trial or evaluation periods, refundable orders, or extended payment terms payments contingent upon the customer obtaining financing or other contingencies which would impact the customer’s obligation. In situations where these or other contingencies are included, all related revenue is deferred until the contingency is resolved. In the third quarter of 2012, the Company began shipping systems under a limited commercial evaluation program to allow certain strategic accounts to install and utilize systems for a limited trial period of three to six months while the purchase opportunity is being evaluated by the hospital. Systems under this program remain the property of the Company and are recorded in inventory and a sale only occurs upon the issuance of a purchase order from the customer. |
• | Collectability. The Company assesses whether collection is probable based on a number of factors, including the customer’s past transaction history and credit worthiness. If collection of the sales price is not deemed probable, the revenue is deferred and recognized at the time collection becomes probable, which is usually upon receipt of cash. The Company’s sales contracts generally do not allow the customer the right of cancellation, refund or return, except as provided under the Company’s standard warranty. If such rights were allowed, all related revenues would be deferred until such rights expired. |
Significant management judgments and estimates are made in connection with the determination of revenue to be recognized and the period in which it is recognized. If different judgments and estimates were utilized, the amount of revenue to be recognized and the period in which it is recognized could differ materially from the amounts reported.
Concentration of Credit Risk and Other Risks and Uncertainties
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and cash equivalents, short-term investments and accounts receivable. Cash and cash equivalents are deposited in demand and money market accounts at one financial institution. At times, such deposits may be in excess of insured limits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
The Company had four customers who constituted 19%, 18%, 12% and 12%, respectively of the Company’s net accounts receivable at December 31, 2015. The Company had four customers who constituted 26%, 14%, 12%, and 12%, respectively of the Company’s net accounts receivable at December 31, 2014. The Company carefully monitors the creditworthiness of potential customers. As of December 31, 2015, the Company has not experienced any significant losses on its accounts receivable.
Two customers accounted for 11% and 11%, respectively of total revenues for fiscal year 2015. Two customers accounted for 13% and 10%, respectively of total revenues for fiscal year 2014. One customer accounted for 10% of total revenues for fiscal year 2013.
Most of the products developed by the Company require clearance from the U.S. Food and Drug Administration (“FDA”) or corresponding approval by foreign regulatory agencies prior to commercial sales. These clearances and approvals are required for both the Sensei and Magellan Systems and all related catheters and accessories:
Sensei: The Company received CE Mark approval to market its Sensei System in Europe in the fourth quarter of 2006 and received CE Mark approval to market its Artisan® Control Catheter in Europe in May 2007. The Company received FDA
84
clearance for the marketing of its Sensei System and Artisan Extend catheters for manipulation, positioning and control of certain mapping catheters during electrophysiology (“EP”) procedures in the United States in May 2007.
Magellan: At the current time the Company has received clearance and approval for the Magellan System and the Magellan 6Fr, 9Fr and 10Fr Robotic Catheters and related accessories in various territories. The Company received CE Mark approval for its Magellan System in July 2011 and received CE Mark approval for the Magellan 9Fr Robotic Catheter and related accessories in October 2011. The Magellan 6Fr Robotic Catheter received CE Mark approval in October 2014, while the Magellan 10Fr Robotic Catheter received CE Mark approval in April 2015. The Company received FDA clearance for marketing its Magellan System, including the Magellan 9Fr Robotic Catheter and accessories in June 2012, the Magellan 6Fr Robotic Catheter in February 2014, and the Magellan 10Fr Robotic Catheter in July 2015. The FDA clearances and CE Mark approvals enable the company to initiate use of all Magellan Systems and catheters with its customers in the United States, the European Union and certain other geographies. However, there can be no assurance that current products or any new products the Company develops in the future will receive the clearances or approvals necessary to allow the Company to market those products in certain desirable markets. If the Company is denied clearance or approvals or clearance or approvals are delayed, it could have a material adverse impact on the Company.
The medical device industry is characterized by frequent and extensive litigation and administrative proceedings over patent and other intellectual property rights. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often difficult to predict, and the outcome may be uncertain until the court has entered final judgment and all appeals are exhausted. The Company’s competitors may assert, and have asserted in the past, that the Company’s products or the use of the Company’s products are covered by United States or foreign patents held by them. This risk is heightened due to the numerous issued and pending patents relating to the use of catheter-based procedures in the medical technology field.
Loss Contingencies
The Company evaluates potential loss contingencies as circumstances dictate. Should a specific loss contingency meet the definition of a liability under authoritative accounting guidance, the Company would record a loss and a liability. As of December 31, 2015, the Company had not recorded any loss contingencies as liabilities. However, if estimates and assumptions change in the future, the Company may record charges to its financial statements. This could materially impact its operating results and financial position.
Foreign Currency
Assets and liabilities of non-U.S. subsidiaries that operate in a local currency environment, where that local currency is the functional currency, are translated at current exchange rates as of the end of the accounting period. The related revenues and expenses are translated at average exchange rates in effect during the period. Net exchange gains and losses resulting from translation are excluded from income and are recorded as part of accumulated other comprehensive income. Transactions denominated in a foreign currency are revalued at the current exchange rate at the transaction date and any related gains and losses are reflected in investment and other income, net in the consolidated statements of income.
Fair Value Measurements
GAAP defines fair value as an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. As a basis for considering such assumptions, authoritative guidance establishes a three-tier value hierarchy, which prioritizes the inputs used in measuring fair value as follows:
• Level 1 Inputs | Quoted prices (unadjusted) in active markets for identical assets or liabilities. | |
• Level 2 Inputs | Inputs other than quoted prices in active markets that are observable either directly or indirectly. | |
• Level 3 Inputs | Unobservable inputs in which there is little or no market data, which require the Company to develop its own assumptions. | |
This hierarchy requires the use of observable market data when available and to minimize the use of unobservable inputs when determining fair value.
Fair Value of Financial Instruments
85
Carrying amounts of certain of the Company’s financial instruments, including cash and cash equivalents, accounts receivable, and accounts payable approximate fair value due to their short maturities.
Cash and Cash Equivalents, and Restricted Cash
The Company considers all highly liquid investments with maturities of three months or less at the time of purchase to be cash equivalents. Cash and cash equivalents, and restricted cash include money market funds and various deposit accounts, which are readily convertible to cash and are stated at cost, which approximates market. Pursuant to the Company’s loan and security agreement executed in 2013, the Company is obligated to maintain $5.0 million of restricted cash subject to lenders’ control. The Company also maintains $750,000 in restricted cash as collateral to obtain a letter of credit used as a lease security for its new office in San Jose, California.
Short-Term Investments
Available-for-sale investments. The Company determines the appropriate classification of investments at the time of purchase and evaluates such classification as of each balance sheet date. The Company classifies all investments with maturities greater than three months at the time of purchase as short-term investments as they are subject to use within one year in current operations. The Company makes investments based upon specific guidelines approved by its board of directors with a view to liquidity and capital preservation and regularly reviews its investments for performance. As of December 31, 2015, all of the Company’s investments have been classified as available-for-sale and are carried on the balance sheet at fair value with the unrealized gains and losses, if any, included in other comprehensive income within stockholders’ equity. Any unrealized losses which are determined to be other than temporary are included in earnings.
Other-than-temporary impairment. The Company periodically evaluates its investments for impairment. In the event that the carrying value of an investment exceeds its fair value and the decline in fair value is determined to be other than temporary, an impairment charge is recorded and a new cost basis for the investment is established. The primarily differentiating factors the Company considered to determine whether a decline in value is other than temporary are our intent and ability to retain its investment in the issuer for a period of time sufficient to allow for any anticipated recovery in market value, the length of the time and the extent to which the market value of the investment has been less than cost, the financial condition and near-term prospects of the issuer. Given the current market conditions, these judgments could prove to be wrong, and companies with relatively high credit ratings and solid financial conditions may not be able to fulfill their obligations.
During the year ended December 31, 2013, the Company recorded pre-tax losses of $0.6 million related to a decline in the value of our investment in Luna Innovations Incorporated common stock that the Company concluded were other-than-temporary. No impairment loss were recorded during the years ended December 31, 2015 and 2014. As of December 31, 2015 and 2014, net unrealized gains (loss) on investments of $(0.4) million and $0.02 million, net of tax, respectively, were included in accumulated other comprehensive income (loss). Significant management judgment is required in determining whether an other-than-temporary decline in the fair value of an investment exists. Changes in the Company’s assessment of the valuation of investments could materially impact future operating results and financial position.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable primarily include amount due from hospitals and distributors. The Company establishes allowances for doubtful accounts based on a review of the credit profiles of customers, contractual terms and conditions, current economic trends and historical collection experience. The allowance for doubtful accounts is reassessed each period based on management’s assessment of historical expected net collections and other collection indicators.
Inventories
Inventories, which includes material, labor and overhead costs, are stated at standard cost, which approximates actual cost, determined on a first-in, first-out basis, and not in excess of net realizable value. The cost basis of the Company’s inventory is reduced for any products that are considered excessive or obsolete based upon assumptions about future demand and market conditions. In the event actual demand for our inventory differs from our best estimates or we fail to receive necessary regulatory approvals, further reduction in our basis of inventory may become necessary.
Property and Equipment, Net
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation of property and equipment is computed using the straight-line method over their estimated useful lives of two to five years. Leasehold improvements are amortized on a straight-line basis over the lesser of their useful life or the term of the lease. Depreciation expense was $1.0 million, $2.6 million and $2.9 million for the years ended December 31, 2015, 2014 and 2013, respectively.
86
Impairment of Long-Lived Assets
The Company evaluates the recoverability of its long-lived assets in accordance with authoritative accounting guidance. When events or changes in circumstances indicate that the carrying value of long-lived assets may not be recoverable, the Company recognizes such impairment if the net book value of such assets exceeds the future undiscounted cash flows attributable to such assets. Should an impairment exist, the impairment loss would be measured based on the excess carrying value of the asset over the asset’s fair value or discounted estimates of future cash flows attributable to the assets. As of December 31, 2015, the Company had $2.3 million of property and equipment, net. If estimates or the related assumptions change in the future, the Company may record impairment charges to reduce the carrying value of certain groups of these assets. Changes in the valuation of long-lived assets could materially impact the Company’s operating results and financial position.
Advertising Expense
The Company expenses advertising costs as incurred. Advertising costs are recorded in general, sales and marketing expenses within the accompanying consolidated statements of operations was $0.6 million and $0.7 million for fiscal year 2015 and 2014, respectively and was immaterial for fiscal year 2013.
Stock-Based Compensation
The Company accounts for share-based compensation plan in accordance with the provisions of the Financial Accounting Standards Board (“FASB”) Accounting Standard Codification ("ASC") No. 718-10-30, Stock Compensation Initial Measurement, which requires the measurement and recognition of compensation expense for all stock-based awards made to employees and directors including employee stock options, restricted stock units (“RSUs”), performance stock units (“PSUs”) and employee stock purchases under our Employee Stock Purchase Plan (“ESPP”) based on estimated fair values and recognizes stock-based compensation expense, net of estimated forfeitures, on a ratable basis over the requisite service period. The Company estimates the fair value of stock options granted using the Black-Scholes-Merton (“BSM”) option valuation model.
The BSM option valuation model is used to determine the fair value of stock-based awards with the following assumptions
• | Expected Volatility. The Company’s estimate of volatility is based on the historical volatilities of its stock price. |
• | Expected Term. The Company estimates the expected term based on its historical settlement experience related to vesting and contractual terms while giving consideration to awards that have life cycles less than the contractual terms and vesting schedules in accordance with authoritative guidance. |
• | Risk-Free Interest Rate. The risk-free interest rate that the Company uses in the BSM option valuation model is the implied yield in effect at the time of option grant based on U.S. Treasury zero-coupon issues with a remaining term equivalent to the expected term of its option grants. For ESPP grants, the Company uses the 6-month Constant Maturity Treasury rate. |
• | Dividend Yield. The Company has never paid any cash dividends on its common stock and it does not anticipate paying any cash dividends in the foreseeable future. Consequently, the Company uses a dividend yield of zero in the BSM option valuation model. |
The Company measures the fair value of RSUs and PSUs using the closing stock price of a share of the Company’s common stock on the grant date. In addition, the Company also estimates a forfeiture rate for its stock options and RSUs. The Company estimates its forfeiture rate based on an analysis of its actual forfeitures based on actual forfeiture experience, analysis of employee turnover behavior and other factors. The impact from any forfeiture rate adjustment would be recognized in full in the period of adjustment and, if the actual number of future forfeitures differs from its estimates, the Company might be required to record adjustments to its stock-based compensation in future periods. For PSUs, the Company recognizes stock-based compensation expense based on the probable outcome that the performance condition will be achieved.
Research and Development
Research and development costs are charged to expense as incurred. Research and development costs include, but are not limited to, payroll and other personnel expenses, prototype materials, laboratory supplies, and consulting costs.
Income Taxes
The Company accounts for income taxes using the liability method whereby deferred tax asset and liability account balances are determined based on differences between financial reporting and tax bases of assets and liabilities and are
87
measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are established to reduce deferred tax assets when management estimates, based on available objective evidence, that it is more likely than not that the benefit will not be realized for the deferred tax assets. The Company accounts for uncertainty in income taxes using a two-step approach to recognizing and measuring uncertain tax positions. The first step is to evaluate the tax position for recognition by determining if the weight of available evidence indicates that it is more likely than not that the position will be sustained on audit, including resolution of related appeals or litigation processes, if any. The second step is to measure the tax benefit as the largest amount that is more than 50% likely of being realized upon settlement. The Company recognizes interest and penalties related to unrecognized tax benefits as a component of income tax expense. As of December 31, 2015, the Company has no accrued interest or penalties related to uncertain tax positions. The Company does not anticipate that total unrecognized tax benefits will significantly change due to the settlement of audits and the expiration of statute of limitations periods in 2015.
Comprehensive Loss
The Company follows the accounting standards for the reporting and presentation of comprehensive income (loss) and its components. Comprehensive loss includes all changes in stockholders’ equity during a period from non-owner sources. Comprehensive loss for each of the years ended December 31, 2015, 2014 and 2013 was equal to net loss adjusted for unrealized gains and losses on investments, reclassifications of realized gains and losses on investments to other income (expense) and foreign currency translation adjustments.
Computation of Net Loss Per Share
Basic net loss per share is computed by dividing net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period. Diluted net loss per share is computed using the weighted-average number of shares and diluted potential shares outstanding during the period. Dilutive potential shares are excluded when the effect would be to reduce a net loss per share. The Company’s dilutive potential shares primarily consists of outstanding common stock options, warrants, estimated shares to be issued under the Company’s employee stock purchase plan and unvested restricted stock, which have not been included in the computation of diluted net loss per share for all periods as the result would be anti-dilutive.
Recent Accounting Pronouncements
In May 2014, the FASB issued Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes the revenue recognition requirements in ASC 605, Revenue Recognition. ASU 2014-09 is based on the principle that revenue is recognized to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. ASU 2014-09 also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from customer contracts, including significant judgments and changes in judgments and assets recognized from costs incurred to obtain or fulfill a contract. This new guidance is effective for annual reporting periods beginning after December 15, 2016, including interim periods within those fiscal years. Early adoption is not permitted. The Company is currently assessing the impact of the adoption of ASU 2014-09 on its consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern. The ASU provides guidance on determining when and how to disclose going-concern uncertainties in the financial statements. The new standard requires management to perform interim and annual assessments of an entity’s ability to continue as a going concern within one year of the date the financial statements are issued. An entity must provide certain disclosures if “conditions or events raise substantial doubt about the entity’s ability to continue as a going concern.” The ASU applies to all entities and is effective for annual periods ending after December 15, 2016, and interim periods thereafter, with early adoption permitted. The Company has been assessing the going concern issue since 2010 on an interim and annual basis and will continue to assess whether the financial conditions based on this new guidance have an impact on the Company’s consolidated financial statements or footnotes.
In January 2015, the FASB issued ASU 2015-01, Simplifying Income Statement Presentation by Eliminating the Concept of Extraordinary Items. This ASU eliminates from GAAP the concept of extraordinary items, and is effective for annual periods beginning after December 15, 2015, with early adoption permitted. The change primarily involves presentation and disclosure and, therefore, is not expected to have a material impact on the Company’s financial condition, results of operations or cash flows.
In April 2015, the FASB issued ASU 2015-03, Interest-Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Cost, which changes the presentation of debt issuance costs in financial statements. Under the ASU, an entity presents costs in the balance sheet as a direct deduction from the related debt liability rather than as an asset.
88
Amortization of the costs is reported as interest expense. Under current guidance, an entity reports debt issuance costs in the balance sheet as deferred charges (i.e. as an asset). For public companies, the ASU is effective for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years, with early adoption permitted. Entities would apply the new guidance retrospectively to all prior periods. The Company is currently assessing the impact of the adoption of ASU 2015-03 on its consolidated financial statements.
In July 2015, the FASB issued ASU 2015-11, Simplifying the Measurement of Inventory. Under this ASU, inventory will be measured at the “lower of cost and net realizable value” and options that currently exist for “market value” will be eliminated. The ASU defines net realizable value as the “estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation.” No other changes were made to the current guidance on inventory measurement. ASU 2015-11 is effective for interim and annual periods beginning after December 15, 2016. Early adoption is permitted and should be applied prospectively. The Company does not expect the adoption of this accounting standard update to impact its consolidated financial statements.
In November 2015, the FASB issued ASU 2015-17, Balance Sheet Classification of Deferred Taxes, which simplifies the presentation of deferred income taxes. This ASU amends the existing guidance to require presentation of deferred tax assets and liabilities as noncurrent within a classified statement of financial position. The Company early adopted ASU 2015-17 effective December 2015 on a prospective basis. The adoption did not have an impact on the financial statements of the Company.
In February 2016, the FASB issued ASU 2016-02, Leases, which require a lessee to recognize lease assets and liabilities on the balance sheet for all arrangements with terms longer than 12 months. Lessor accounting remains largely consistent with existing U.S. GAAP. The new standard takes effect in 2019 for public business entities and 2020 for all other entities. The Company is currently assessing the impact of the adoption of ASU 2016-02 on its consolidated financial statements.
In March 2016, the FASB issued ASU 2016-09, Compensation-Stock Compensation (Topic 718): Improvements to Employee Share-Based Payment Accounting. ASU 2016-09 requires among other things that all excess tax benefits and tax deficiencies (including tax benefits of dividends on share-based payment awards) to be recognized as income tax expense or benefit in the income statement. The tax effects of exercised or vested awards should be treated as discrete items in the reporting period in which they occur. An entity also should recognize excess tax benefits, and assess the need for a valuation allowance, regardless of whether the benefit reduces taxes payable in the current period. The ASU also requires excess tax benefits to be classified along with other income tax cash flows as an operating activity in the statement of cash flows. This new guidance is effective for annual reporting periods beginning after December 15, 2016, including interim periods within those fiscal years. Early adoption is permitted. The Company is currently assessing the impact of the adoption of ASU 2016-09 on its consolidated financial statements.
3. Fair Value of Assets and Liabilities
The Company’s financial instruments consist principally of cash and cash equivalents, short-term investments, accounts receivable, accounts payable and short-term and long-term debt. Cash and cash equivalents, short-term investments and accounts receivable, net of allowance, are reported at their respective fair values on the Consolidated Balance Sheets. Short-term and long-term debt are reported at their amortized cost on our Consolidated Balance Sheets. The remaining financial instruments are reported on the Consolidated Balance Sheets at amounts that approximate current fair values.
The amortized cost and fair value of assets, along with gross unrealized gains and losses, were as follows (in thousands):
Cash, Cash Equivalents, Short-term Investments and Restricted Cash
89
Amortized Cost | Gross Unrealized Gains | Gross Unrealized Losses | Fair Value | Balance Sheet Classification | |||||||||||||||||||||||
Cash and cash Equivalents | Short-term Investments | Restricted Cash | |||||||||||||||||||||||||
December 31, 2015: | |||||||||||||||||||||||||||
Cash | $ | 1,408 | $ | — | $ | — | $ | 1,408 | $ | 1,408 | $ | — | $ | — | |||||||||||||
Money market funds | 25,080 | — | — | 25,080 | 18,967 | — | 6,113 | ||||||||||||||||||||
Corporate equity securities | 1,572 | — | (71 | ) | 1,501 | — | 1,501 | — | |||||||||||||||||||
$ | 28,060 | $ | — | $ | (71 | ) | $ | 27,989 | $ | 20,375 | $ | 1,501 | $ | 6,113 | |||||||||||||
December 31, 2014: | |||||||||||||||||||||||||||
Cash | $ | 3,586 | $ | — | $ | — | $ | 3,586 | $ | 3,586 | $ | — | $ | — | |||||||||||||
Money market funds | 26,318 | — | — | 26,318 | 20,942 | — | 5,376 | ||||||||||||||||||||
Corporate equity securities | 1,572 | 401 | — | 1,973 | — | 1,973 | — | ||||||||||||||||||||
$ | 31,476 | $ | 401 | $ | — | $ | 31,877 | $ | 24,528 | $ | 1,973 | $ | 5,376 | ||||||||||||||
Gross unrealized losses at December 31, 2015 include our investment in Luna Innovations (“Luna”) equity securities which have been in a gross unrealized loss for less than 12 months.
Fair Value Measurements
The fair value hierarchy of the Company’s assets and liabilities that are measured at fair value, by level, is as follows (in thousands):
Fair Value Measurements Using | |||||||||||||||
Quoted Prices in Active Markets for Identical Assets (Level 1 Inputs) | Significant other Observable Inputs (Level 2 Inputs) | Unobservable Inputs (Level 3 Inputs) | Total | ||||||||||||
December 31, 2015: | |||||||||||||||
Assets: | |||||||||||||||
Money market funds | $ | 25,080 | $ | — | $ | — | $ | 25,080 | |||||||
Corporate equity securities | 1,501 | — | — | 1,501 | |||||||||||
$ | 26,581 | $ | — | $ | — | $ | 26,581 | ||||||||
December 31, 2014: | |||||||||||||||
Money market funds | $ | 26,318 | $ | — | $ | — | $ | 26,318 | |||||||
Corporate equity securities | 1,973 | — | — | 1,973 | |||||||||||
$ | 28,291 | $ | — | $ | — | $ | 28,291 | ||||||||
Investment instruments valued using Level 1 inputs include the Company’s money market securities and certain of the corporate equity securities which were obtained by the Company as part of the Luna litigation settlement.
The Company periodically assesses whether significant facts and circumstance have arisen to indicate that an impairment, which is other than temporary, of the fair value of any underlying investment has occurred. In 2013, the Company determined that there was an other than temporary impairment of its investment in Luna Innovations, or Luna, which the Company received as part of a litigation settlement entered into in January 2010 and had recorded as an available for sale corporate equity security. As such, the Company wrote down the value of that investment and recorded a loss of $0.6 million in other expense in the consolidated statement of operations. No other investments have been in an unrealized loss position for longer than twelve months.
90
The changes in Level 3 liabilities measured at fair value on a recurring basis was as follow (in thousands):
Level 3 Input for Year Ended December 31, 2015 | |||
(In thousands) | |||
Balance at December 31, 2014 | $ | — | |
Additions at March 11, 2015 | (14,776 | ) | |
Change in fair value of warrant liability | (2,993 | ) | |
Reclassification to additional paid-in capital | 17,769 | ||
Balance at December 31, 2015 | $ | — | |
Warrant Liability
In connection with the issuance of Series A convertible preferred stock on March 11, 2015, the Company also issued Series E Warrants to the participating investors to purchase an aggregate of 5,384,600 shares of common stock with an exercise period of two years from the date of issuance. On the date of issuance, the exercise price for the Series E Warrants was the lesser of $9.75 per share or a 50% premium on the per share trailing volume weighted average share price of the common stock on NASDAQ for the ten trading days ending on dates specified in the form of Series E Warrants filed with the SEC. The Series E Warrants were not exercisable until receipt of stockholder approval to among other things increase the number of authorized shares of common stock of the Company. The proposal relating to such approval was presented and received the stockholder approval at the 2015 Annual Meeting of Stockholders of the Company held on May 12, 2015 (the “Requisite Stockholder Approval”). As a result of the contingency which was deemed outside the Company’s control, such Series E Warrants did not originally meet the criteria for classification as equity under ASC 815. As such, the Company classified the Series E Warrants as current liabilities at fair value upon issuance of $14.8 million. The Company used a third party valuation that utilized the Monte Carlo simulation model to estimate the fair value of the Series A convertible preferred stock and Series E Warrants. The valuation used a simulation of the Company’s periodic stock price, expected volatility of the price, adjusted for conversion price, and the remaining contractual term of the warrants. The Series E Warrants were subject to re-measurement at each balance sheet date, with any change in fair value recognized as warrant expense, a component of other income (expense) reflected within the statement of operations. The Company recorded the change in fair value of $3.0 million on the consolidated statements of operations for the year ended December 31, 2015.
Upon receipt of Requisite Stockholder Approval, all criteria for the Series E Warrants to be classified as equity were met. Using the BSM model with an exercise price of $9.75 per share for the Series E Warrants, the Company revalued the warrants and reclassified the warrant liability of $17.8 million, representing the fair value of the Company’s warrant liability as of the Requisite Stockholder Approval date, to additional paid-in capital. See Note 10 for further information regarding the Company’s 2015 private placement of Series A convertible preferred stock and Series E Warrants. All of the Series E Warrants are currently issued and outstanding as of December 31, 2015.
Long-term Debt
The fair value of the Company’s long-term debt was estimated to be $35.1 million as of December 31, 2015 based on an internal valuation model that utilized the then-current rates available to the Company for debt of a similar term and remaining maturity, which constitutes Level 2 inputs under the fair value hierarchy. Considerable judgment is required in interpreting market data to develop estimates of fair value. Accordingly, the fair value estimate presented herein is not necessarily indicative of the amount that the Company or holders of the instruments could realize in a current market exchange. The use of different assumptions and/or estimation methodologies may have a material effect on the estimated fair value. See Note 8 for further information regarding the Company’s long-term debt.
4. Balance Sheet Components
Allowance for Doubtful Accounts (in thousands)
91
December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Balance at Beginning of the Year | $ | — | $ | 554 | $ | 554 | |||||
Additions Charges to Cost and Expenses | 49 | 21 | — | ||||||||
Write-offs | (26 | ) | (475 | ) | — | ||||||
Balance Before Recoveries | $ | 23 | $ | 100 | $ | 554 | |||||
Less: Recoveries | — | (100 | ) | — | |||||||
Balance at the End of the Year | $ | 23 | $ | — | $ | 554 | |||||
Inventories, net (in thousands)
December 31, | |||||||
2015 | 2014 | ||||||
Raw materials | $ | 2,613 | $ | 4,996 | |||
Work in process | 3,575 | 4,571 | |||||
Finished goods | 2,471 | 1,925 | |||||
Inventories | $ | 8,659 | $ | 11,492 | |||
Property and equipment, net (in thousands)
December 31, | |||||||
2015 | 2014 | ||||||
Furniture and leasehold improvements | $ | 11,329 | $ | 11,455 | |||
Laboratory equipment | 12,158 | 11,374 | |||||
Computer equipment and software | 2,956 | 2,911 | |||||
26,443 | 25,740 | ||||||
Less: Accumulated depreciation and amortization | (24,118 | ) | (23,412 | ) | |||
Property and equipment, net | $ | 2,325 | $ | 2,328 | |||
Accrued liabilities (in thousands)
December 31, | |||||||
2015 | 2014 | ||||||
Accrued restructuring | $ | 1,581 | $ | — | |||
Accrued salaries, commission, bonus and benefits | 1,094 | 2,835 | |||||
Tax accruals | 333 | 274 | |||||
Accrued royalties | 330 | 594 | |||||
Accrued legal and other professional fees | 228 | 169 | |||||
Clinical related accruals | 60 | 394 | |||||
Other accrued expenses | 487 | 676 | |||||
Total | $ | 4,113 | $ | 4,942 | |||
5. Agreements with Intuitive
In October 2012, the Company signed an updated license agreement with Intuitive Surgical Operations, Inc. and Intuitive Surgical, Inc. (collectively, “Intuitive”), under which Intuitive paid the Company a $20.0 million licensing fee, and entered into a stock purchase agreement to buy 529,101 shares of the Company’s common stock for an aggregate purchase price of $10.0 million. The amendment of the license agreement updated the co-exclusive cross license agreement signed by the companies in 2005. Under the terms of the amended agreement, Intuitive’s co-exclusive rights extended to include intellectual property rights filed or conceived by the Company in the non-vascular space subsequent to the original 2005 agreement through October 26, 2015. The Company retained the right to use its intellectual property for all clinical applications, both vascular and non-vascular. The Company previously concluded that the value associated with patents filed or conceived in the three years subsequent to the amendment was de minimis and therefore the $20.0 million upfront payment for the licensing of intellectual property was recognized in the statement of operations in fiscal year 2012. The $10.0 million associated with the stock
92
purchase agreement was recorded to common stock and additional paid-in capital on the balance sheet in 2012. The term of the intellectual property capture period expired on October 26, 2015. The cross-licenses and royalty obligations remain in effect.
The Company has minimum royalty obligations of $0.2 million per year under the terms of its cross license agreement with Intuitive Surgical. For fiscal years 2015, 2014 and 2013, the Company incurred cost in the amount of $0.2 million, $0.3 million and $0.2 million, respectively related to the royalty obligations to Intuitive Surgical.
6. Agreements with Philips
In February 2011, the Company entered, directly and through a wholly-owned subsidiary, into various agreements with Koninklijke Philips Electronics N.V. (“Philips”) to allow Philips to develop and commercialize the non-robotic applications of the Company’s Fiber Optic Shape Sensing and Localization (“FOSSL”) technology (the “FOSSL Agreement”). Under the terms of the FOSSL Agreement, Philips obtained exclusive right to develop and commercialize the FOSSL technology in the non-robotic vascular, endoluminal and orthopedic fields. Philips also received non-exclusive rights in other non-robotic medical device fields, but not to any multi-degree of freedom robotic applications. Under the terms of the FOSSL Agreement, if Philips did not meet certain specified commercialization obligations, the Company reserved rights to re-acquire the licenses granted to Philips for pre-determined payments, which payments in the aggregate would be greater than the upfront payment amounts received by the Company from Philips in connection with the agreements related to the FOSSL technology. The FOSSL Agreement also contains customary representations, warranties and indemnification provisions by each party. Each party may terminate the agreement for material breach by the other party. Philips also has the right to terminate the agreement and its rights under the agreement if the Company is acquired by a competitor of the relevant business unit of Philips.
In February 2011, the Company amended its extended joint development agreement with Philips (the “JDA”), increasing the amount of funding provided by Philips for the development of the Vascular System and potentially extending and increasing certain royalty fees to be paid to Philips based on sales of the Vascular System, subject to caps based on the amounts Philips contributed to the development of the system. Under the amendment, the Company was eligible to receive up to an additional $78.0 million in future payments associated with the successful commercialization by Philips or its collaborators of products containing FOSSL technology. Approximately two-thirds of these potential future payments could arise from Philips’ sublicensing the FOSSL technology and approximately one-third of the potential future payments were based on Philips’ royalty obligations on its sales of products containing the FOSSL technology. The Company would receive less than half of Philips’ proceeds for its sublicensing FOSSL technology if and following Philips entering into an applicable sublicensing transaction. Philips’ FOSSL-related royalty obligations were to be calculated on a consistent annual basis between 2014 and 2020 in any year only to the extent that Philips achieved a substantial number of commercial placements of FOSSL-enabled products in the calendar year. The Company’s royalty obligations under the JDA provided for the payment of royalties to Philips through October 2017.
In August 2015, the Company and Philips entered into an amendment to the FOSSL Agreement and the JDA (the “Amended Agreements”), extinguishing the Company’s rights to re-acquire the licenses in the non-robotic vascular, endoluminal and orthopedic fields in FOSSL technology in exchange for a reduction of all royalties owed or due between the parties of fifty percent (50%). Under the Amended Agreements, the Company’s royalty obligations to Philips based on sales of the Vascular Systems were reduced by fifty percent (50%). The Company’s royalty obligations continue through October 2017. Similarly, Philips royalty obligations were also all reduced by fifty percent (50%), including but not limited to all royalty obligations due under the Amended Agreements that arise from Philips’ sublicensing the FOSSL technology. Under the Amended Agreements, Philips’ FOSSL-related royalty obligations will be calculated on a consistent basis for the royalty period starting with the first calendar year after the first sale by Philips of a FOSSL system and ending with the sixth calendar year after the first sale, but only to the extent that Philips achieves certain number of commercial placements of FOSSL-enabled products during the royalty period.
The Amended Agreements contain customary representations, warranties and indemnification provisions by each party. Each party may terminate the agreements for material breach by the other party. Philips also has the right to terminate the agreement and its rights under the agreement if the Company is acquired by a competitor of the relevant business unit of Philips.
Under the terms of the Amended Agreements, the Company continues to have no minimum obligation with Philips, and the royalty obligation is based on per unit sales of the Magellan Systems and vascular catheters. For fiscal years 2015, 2014 and 2013, the Company incurred cost in the amount of $0.7 million, $1.2 million and $0.9 million, respectively related to the royalty obligations to Philips.
93
7. Commitments and Contingencies
Operating Leases
The Company rents its office and laboratory facilities in Mountain View, California under an operating lease. On December 15, 2015, the Company entered into a Lease Termination Agreement with its landlord for the termination of the Company's existing lease in Mountain View, California. The Lease Termination is to become effective not earlier than June 1, 2016 but no later than August 31, 2016. Upon the effective date of the Lease Termination, the Company will be released from all of its obligations under the Existing lease, except for certain expenses and indemnification obligations as set forth in the Lease Termination. Concurrently, the Company entered into a lease agreement with LBA Realty Fund11-WBP VII, Inc. to rent 32,552 square feet of office and manufacturing space located in San Jose, California with a commencement date of June 1, 2016 but no later than August 1, 2016, and expiring in February 2027.
The Company also leases approximately 3,300 square feet of office space in London, England under an operating lease of which ends in June 30, 2020. As of December 31, 2015, the Company has exercised the option to exit the 3,300 square feet of office lease space in London, England in 2015 and incurred $0.1 million exit cost recorded on the consolidated statement of operations. On March 17, 2015, the Company signed a rental agreement with Regus Management (UK) limited to rent office space for our London office.
Rent expense on a straight-line basis was as follows (in thousands):
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Rent expense | $ | 2,782 | $ | 2,613 | $ | 2,310 | |||||
At December 31, 2015, future minimum payments under the leases are as follows (in thousands):
Years ended December 31, | Future Minimum Lease Payments | ||
2016 | $ | 1,728 | |
2017 | 297 | ||
2018 | 610 | ||
2019 | 628 | ||
2020 | 647 | ||
Thereafter | 4,443 | ||
Total | $ | 8,353 | |
Warranties
The Company generally provides one year of post-contract customer service on the sale of its systems. Post-contract customer service revenue is recognized ratably over the term of the service period and associated expenses are charged to cost of revenues as incurred. The Company provides a limited warranty on the sale of robotic systems and records a warranty reserve at the time of sale to cover the estimated warranty costs. The Company’s warranty obligation may be impacted by product failure rates, material usage and service costs associated with its warranty obligations. The Company periodically evaluates and adjusts the warranty reserve to the extent actual warranty expense differs from the original estimates. Movement in the warranty liability has not been significant for the years ended December 31, 2015 and 2014, respectively.
Other Royalty Obligations
The Company has minimum royalty obligations of $100,000 per year under a license agreement with Mitsubishi Electric Research Laboratories, Inc. which reduces to $55,000 per year if the license becomes non-exclusive. The royalty obligation expires in 2018 The Company incurred cost in the amount of $0.1 million related to the royalty obligations to Mitsubishi in each of the fiscal years 2015, 2014 and 2013.
Indemnification
The Company has agreements with each member of its Board of Directors, its President and Chief Executive Officer, its former President and its Interim Chief Financial Officer indemnifying them against liabilities arising from actions taken against
94
the Company. To date, the Company has not incurred any material costs as a result of such indemnifications and has not accrued any liabilities related to such obligations in the accompanying financial statements.
The Company has agreements with certain customers indemnifying them against liabilities arising from legal actions relating to the customer’s use of intellectual property owned by the Company. To date, the Company has not incurred any material costs as a result of such indemnifications and has not accrued any liabilities related to such obligations in the accompanying financial statements.
Legal Proceedings
Following the Company’s October 19, 2009 announcement that it would restate certain of its financial statements, a securities class action lawsuit was filed on October 23, 2009 in the United States District Court for the Northern District of California, naming the Company and certain of its now former officers. Curry v. Hansen Medical, Inc. et al., Case No. 9-5094. The complaint asserted claims for violation of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 on behalf of a putative class of purchasers of Hansen stock between May 1, 2008 and October 18, 2009, inclusive, and alleged, among other things, that defendants made false and/or misleading statements and/or failed to make disclosures regarding the Company’s financial results and compliance with GAAP while improperly recognizing revenue; that these misstatements and/or nondisclosures resulted in overstatement of Company revenue and financial results and/or artificially inflated the Company’s stock price; and that following the Company’s October 19, 2009 announcement, the price of the Company’s stock declined. On November 4, 2009 and November 13, 2009, substantively identical complaints were filed in the Northern District of California by other purported Hansen stockholders asserting the same claims on behalf of the same putative class of Hansen stockholders. Livingstone v. Hansen Medical, Inc. et al., Case No. 9-5212 and Prenter v. Hansen Medical, Inc., et al., Case No. 9-5367. All three complaints sought certification as a class action and unspecified compensatory damages plus interest and attorneys’ fees. On December 22, 2009, two purported Hansen stockholders, Mina and Nader Farr, filed a joint application for appointment as lead plaintiffs and for consolidation of the three actions. On February 25, 2010, the Court issued an order granting Mina and Nader Farr’s application for appointment as lead plaintiffs and consolidating the three securities class actions. On July 15, 2010, the Court entered an order granting lead plaintiffs’ motion for leave to file a second amended complaint. Lead plaintiffs’ second amended complaint, in addition to alleging that shareholders suffered damages as a result of the decline in the Company’s stock price following the October 19, 2009 announcement, also alleged that shareholders suffered additional damages as the result of share price declines on July 28, 2009, July 31, 2009, January 8, 2009, July 6, 2009, and August 4, 2009, all of which lead plaintiffs alleged were caused by the disclosure of what they claim was previously misrepresented information. The Defendants filed their motion to dismiss the second amended complaint on October 13, 2010. The Court granted Defendants’ motion to dismiss with leave to amend on August 25, 2011. Plaintiffs’ third amended complaint was filed on October 18, 2011. Defendants filed their motions to dismiss on January 9, 2012. On August 10, 2012, the Court denied in part and granted in part Defendants’ motions to dismiss. On January 4, 2013, lead plaintiffs sought leave to amend their complaint to add certain of Hansen’s current and former directors and Hansen’s former auditor. Hansen filed an opposition to lead plaintiffs’ motion on February 11, 2013.
On May 9, 2013, the parties entered a stipulation of settlement pursuant to which the plaintiffs would receive an aggregate of $8.5 million, $4.0 million of which would be funded in cash by the Company’s insurer and other sources. The Company would fund the remaining portion by issuing $4.25 million worth of the Company’s common stock, the number of shares to be determined based on the average closing price of the common stock for the 10 trading days preceding final Court approval of the settlement of the class action, and paying $250,000 in cash. The Company recorded a loss on litigation settlement of $4.5 million in its first quarter ended March 31, 2013. On December 5, 2013, the U.S. District Court for the Northern District of California granted final approval of a settlement. On December 19, 2013, the Company issued 229,854 shares, as determined by the average closing price of the Company’s common stock for the 10 trading days preceding final court approval of the settlement, which average was $18.49 per share.
8. Short-term and Long-term Debt
Oxford Loan
In December 2011, the Company entered into a $30.0 million loan and security agreement with Oxford Finance LLC and Silicon Valley Bank (the “Oxford Loan”). Under the loan agreement, the Company was obligated to pay interest only payments on the Oxford Loan through June 30, 2013, following which time the Oxford Loan required interest and principal payments through January 1, 2016. The Oxford Loan accrued interest at a stated rate of 9.45% and included an additional final interest payment of 3.95% of the original principal amount. The Oxford Loan provided for a prepayment option that allowed the Company to prepay all of the outstanding principal balance, subject to a pre-payment fee. In connection with the Oxford Loan, the Company issued warrants to purchase 66,079 shares of common stock. The warrants have an exercise price of $22.70 per share and expire in December 2018.
95
In August 2013, the Oxford Loan was fully repaid and extinguished under the loan agreement’s prepayment option. The Company paid a final interest balloon of $1.2 million plus accrued interest and a prepayment penalty. Following the repayment of the Oxford Loan, the Company recognized a loss on extinguishment of debt of $1.9 million, which included $0.9 million prepayment penalty, $0.5 million additional end of term payment and $0.5 million unamortized discount from warrants and issuance costs.
White Oak Loan
In July 2013, the Company executed a loan and security agreement with White Oak, as a lender and agent for several lenders. On August 23, 2013, the loan agreement was amended and restated and the loan was funded. The amended loan and security agreement provides for term loan debt financing of $33.0 million with a single principal balloon payment due at maturity on December 30, 2017. Cash interest accrues at an 11.0% per annum rate and is payable quarterly. Additionally, a 3.0% per annum payment-in-kind accrues quarterly and is accretive to the principal amount owed under the agreement. Substantially all of the proceeds from the loan were used to fully repay and extinguish previous indebtedness. In connection with the loan, the Company incurred costs of approximately $1.5 million including payments to the lender agent totaling $0.7 million and the placement agent totaling $0.3 million which in aggregate are accounted for as debt issuance costs and amortized to interest expense over the life of the loan. Under the loan and security agreement, the Company is obligated to pay White Oak certain servicing, administration and monitoring fees of $32,000 annually. The Company may prepay all or a portion of the outstanding principal balance, subject to paying a prepayment fee of 3.5% of the principal amount of the loan prepaid if the prepayment is made on or before the third anniversary of the funding of the loan or 1.0% of the principal amount of the loan prepaid if the prepayment is made after the third anniversary and on or before the fourth anniversary of the funding of the loan. The Company is also required to make mandatory prepayments upon certain events of loss and certain dispositions of the Company’s assets as described in the loan and security agreement. For fiscal years 2015, 2014 and 2013, the Company recognized expense of $0.4 million, $0.4 million and 0.1 million, respectively, for the amortization of debt issuance costs related to the White Oak loan.
The loan is collateralized by substantially all of the Company’s assets then owned or thereafter acquired, other than its intellectual property, and all proceeds and products thereof. Two of the Company’s wholly-owned subsidiaries, AorTx, Inc. and Hansen Medical International, Inc., have entered into agreements to guarantee the Company’s obligations under the loan and security agreement and have granted first priority security interests in their assets, excluding any of their intellectual property, to secure their guarantee obligations. Under the loan and security agreement, neither the Company nor AorTx, Inc. and Hansen Medical International, Inc. may grant a lien on any intellectual property to third parties. The Company additionally pledged to the lenders shares of each of its direct and indirect subsidiaries as collateral for the loan. Pursuant to the loan and security agreement, the Company is subject to certain affirmative and negative covenants and also to minimum liquidity requirements which require the Company to maintain $15.0 million in liquidity at all times, consisting of at least $13.0 million in cash, cash equivalents and investments, of which $5.0 million is required to be restricted subject to lenders’ control and the lesser of $2.0 million or 65% of eligible accounts receivable. Additionally, the Company is required to obtain an audit opinion from its independent certified public accountants on the annual financial statements that does not include an explanatory uncertainty paragraph raising substantial doubt about its ability to continue as a going concern or any qualification or exception as to the scope of such audit. The loan also limits the Company’s ability to (a) undergo certain change of control events; (b) convey, sell, lease, transfer, assign or otherwise dispose of any of its assets; (c) create, incur, assume, or be liable with respect to certain indebtedness, not including, among other items, subordinated debt; (d) grant liens; (e) pay dividends and make certain other restricted payments; (f) make certain investments; (g) make payments on any subordinated debt; or (h) enter into transactions with any of its affiliates outside of the ordinary course of business, or permit its subsidiaries to do the same. The Company is also required to make mandatory prepayments upon certain events of loss and certain dispositions of its assets described in the loan and security agreement. In the event the Company were to violate any covenants or if White Oak has reason to believe that the Company has violated any covenants, including a significant adverse event clause, and such violations are not cured pursuant to the terms of the loan and security agreement, the Company would be in default under the loan and security agreement, which would entitle lenders to exercise their remedies, including the right to accelerate the debt, upon which the Company may be required to repay all amounts then outstanding under the loan and security agreement. As of December 31, 2015, the Company was in compliance with all covenants except for the covenant relating to obtaining and audit opinion that does not include a going concern explanatory paragraph. As of December 31, 2015, the Company was in compliance with all covenants; however, there was an anticipated default of the covenants listed in Section 6.2(a)(i) and Section 8.2(a), with respect to Section 6.2(a)(i), as of December 31, 2015, which was cured by the Forbearance Agreement as discussed below. The Company is currently in default of this requirement and certain covenants and other events of default listed in Sections 6.1(c), 6.2(a)(i), 6.2(a)(iv), 6.10, 8.2(a) and 8.2(b) of the loan and security agreement with White Oak (the “Specified Events of Default”). On April 19, 2016, the Company entered into a Forbearance Agreement with White Oak (“Forbearance Agreement”) whereby White Oak granted forbearance of the Specified Events of Default until August 17, 2016 in exchange for a $150,000 non-refundable transaction fee. Pursuant to the Forbearance Agreement, on April 21, 2016, the Company made a prepayment to White Oak, with respect to our obligations under the loan and security agreement with White Oak, in the amount of $5.0
96
million from the Company’s restricted account. Additionally, the outstanding obligations under the White Oak loan will become due on or before August 17, 2016. As of December 31, 2015, the debt was classified as a short-term liability.
Future annual payments due on the debt outstanding as of December 31, 2015 are as follows (in thousands):
2016 | 45,790 | ||
Total remaining payments | 45,790 | ||
Less: Amount representing interest | (10,649 | ) | |
35,141 | |||
Less: Current portion of long-term debt | 35,141 | ||
Long-term debt, net of current portion | $ | — | |
9. Restructuring
In November 2015, the Company initiated a cost-saving restructuring plan in order to align its headcount to reduce operating expenses, consolidate its back-office operations by closing its office in United Kingdom and relocate its headquarter. This restructuring plan would impact approximately 35 full-time positions in its Mountain View, California office and 9 full-time employees in its international offices. The Company recorded restructuring charges of approximately $1.7 million consisting of employee severance and termination benefits, operating lease cancellation fees and expenses related to closing down its United Kingdom office. The Company recorded employee severance costs provided under an ongoing benefit arrangement once they are both probable and estimable in accordance with the provisions of ASC 712. The Company accounted for lease cancellation fees and closing down our United Kingdom office in accordance with ASC 420. Under ASC 420, the Company established a liability for a cost associated with an exit or disposal activity, when the liability was incurred, rather than at the date that the Company committed to an exit plan. The Company reassesses the expected cost to complete the exit or disposal activities at the end of each reporting period and adjusts its remaining estimated liabilities, if necessary.
The Company agreed to pay $0.5 million of broker commission to the third party who assisted the Company to negotiate an early termination of its office in Mountain View, California. The Company recorded $0.2 million as exit costs related to the closing off its office in United Kingdom. The Company has classified approximately $1.5 million of these charges as restructuring expense and $188,000 as a component of cost of sales. The Company has completed phase one of its restructuring activities in the fourth quarter of 2015 and expects to complete phase 2 by the end of 2016. The Company's restructuring activities for the year ended December 31, 2014 and 2013 were not material.
The table below presents the restructuring activities for the year ended December 31, 2015 (in thousands):
Employee Severance and Termination Benefits | Operating Lease Cancellation | Exit Cost | Total | ||||||||||||
Accrued restructuring balance as of January 1, 2015 | $ | — | $ | — | $ | — | $ | — | |||||||
Additional accruals | 1,027 | 500 | 200 | 1,727 | |||||||||||
Cash payments | (146 | ) | — | — | (146 | ) | |||||||||
Accrued restructuring balance as of December 31, 2015 | $ | 881 | $ | 500 | $ | 200 | $ | 1,581 | |||||||
10. Stockholders' Equity
2013 Private Placement Transaction
In March 2013, the Company executed an “at the market” agreement pursuant to which the Company may have offered to sell shares of common stock up to an aggregate offering price of up to $25.0 million. In July 2013, the Company exercised its right to terminate the agreement. No shares were offered or sold pursuant to the agreement.
On July 30, 2013, the Company entered into a securities purchase agreement to sell an aggregate of 2,845,528 shares of its common stock at a per share price of $12.30 and warrants to purchase an aggregate of 3,414,634 shares of common stock at a per warrant price of $1.25 in a private placement transaction. The warrants were comprised of the following three series: Series A warrants exercisable for an aggregate 1,138,211 shares of common stock, with an exercise price equal to $12.30;
97
Series B warrants exercisable for an aggregate 1,138,211 shares of common stock, with an exercise price equal to $15.00 per share; and Series C warrants exercisable for an aggregate 1,138,211 shares of common stock, with an exercise price equal to $20.00 per share. Series A warrants were subject to mandatory exercise subsequent to the receipt of regulatory approval for the new 6Fr Magellan catheter in the U.S., which occurred in February 2014. The financing resulted in gross proceeds to the Company of approximately $39.3 million prior to placement fees and offering costs of approximately $2.1 million. At the closing of the private placement financing, the Company entered into an investor rights agreement with the purchasers of the shares and warrants in which the Company agreed to file a registration statement covering resale of the shares and the purchasers agreed not to transact in any shares of the Company’s common stock for a one-year period following the closing, subject to certain exceptions. In the first quarter of 2014, subsequent to the receipt of regulatory approval for the new Magellan 6Fr Robotic Catheter in the U.S., Series A warrants for 1.1 million shares of the Company’s common stock were exercised for total proceeds of $14.0 million in accordance with the terms and conditions of a securities purchase agreement dated July 30, 2013. All of the Series A Warrants were mandatorily exercised in the first quarter of 2014 pursuant to the Company’s achievement of a regulatory milestone as set forth in the Series A Warrants.
2014 Warrant Exchange
On July 30, 2014, the Company entered into a definitive agreement (the “Exchange Agreement”) with certain warrantholders to cancel and exchange (the “Exchange”) an aggregate of 1,022,117 of the Company’s outstanding Series B Warrants and an aggregate of 1,022,117 of the Company’s outstanding Series C Warrants. In exchange, the Company issued warrants (the “Exchange Warrants”) to purchase an aggregate of 2,672,837 shares of common stock. The Exchange was completed in August 2014.
The Exchange Warrants are comprised of the following two tranches: (a) Series B/C Exchange Warrants (“Series B/C Exchange Warrants”) exercisable for an aggregate of 2,044,235 shares of common stock, with an exercise price equal to $11.30 per share, the NASDAQ consolidated closing bid price for the Common Stock on July 29, 2014, the last completed trading day before the Exchange Agreement was executed (the “Closing Bid Price”); and (b) Series D Warrants (“Series D Warrants”) exercisable for an aggregate of 628,602 shares of common stock, with an exercise price equal to the Closing Bid Price. The Series B/C Exchange Warrants were subject to mandatory exercise within 14 days of issuance and were exercised in August 2014, resulting in gross proceeds to the Company of approximately $23.1 million. The Series D Warrants have an exercise period of five years, and if fully exercised, would result in additional gross proceeds to the Company of approximately $7.1 million. The Series B Warrants and Series C Warrants previously carried an expiration date of August 2015. The remaining Series B Warrants and Series C Warrants not included in the Exchange will remain outstanding until their exercise or expiration.
As a result of a change in the terms and conditions of the Series B and C Warrants, the transaction was treated as a modification of the original award using the accounting guidance in ASC 718-20-35-3, this guidance implies that the entity repurchases the original instrument by issuing a new instrument of equal or greater value, incurring additional incremental value. Incremental cost shall be measured as the excess, if any, of the fair value of the modified award over the fair value of the original award immediately before its terms are modified, measured based on the share price, Black-Scholes options pricing model and other pertinent factors at that date. These variables include the Company’s expected stock price volatility over the term of the award, expected term, risk-free interest rate and expected dividend rate. The Company recorded $2.9 million warrant exchange charge in the consolidated statement of operations during the year ended December 31, 2014 based upon the difference between the fair value of the Series B and C Warrants immediately prior to the exchange and the fair value of the newly issued Series B/C Exchange Warrants and Series D Warrants.
2015 Private Placement of Redeemable Convertible Preferred Stock and Warrants
On March 11, 2015, the Company entered into a securities purchase agreement (“Purchase Agreement”) to sell an aggregate of 53,846 shares of Series A convertible preferred stock at a per share price of $650. After receipt of Requisite Stockholder Approval on May 12, 2015, the Series A convertible preferred stock converted into 5,509,492 shares of common stock. The conversion ratio was equal to the number obtained by dividing (i) the sum of $650 and the amount of any accrued but unpaid dividends by (ii) $0.65. The amount of accrued but unpaid dividends included as part of the conversion calculation included an initial rate of 2% (the “first period dividend”) plus an increase of 2% in the second quarter of 2015 (the “second period dividend”) based on the number of days the Series A convertible preferred stock remained outstanding. As a result of the Requisite Stockholder Approval, the first period dividend and second period dividend were the only dividends included in the conversion calculation. On the date of the issuance, the allocated fair value of the common stock was greater than the proceeds received for the Series A convertible preferred stock. As such, the Company accounted for the beneficial conversion features under ASC 470-20, Debt with Conversion and Other Options. The Company recorded a deemed dividend charge of $35.5 million for the accretion of a discount on the Series A convertible preferred stock resulting from an allocation of a portion of the proceeds to the warrants and a beneficial conversion feature embedded within the Series A convertible preferred stock, which equals the amount by which the estimated fair value of the common stock issuable upon conversion of the issued Series
98
A convertible preferred stock exceeded the proceeds from such issuance, and a cumulative Series A convertible preferred stock dividend of $0.8 million. Additionally, upon conversion of the preferred stock, a deemed dividend of $15.3 million was recorded due to the discount on the preferred stock created by allocating a portion of the proceeds to the warrants. The deemed dividend and cumulative dividend were non-cash transactions and reflected below net loss to arrive at net loss allocable to common stockholders.
As part of the Purchase Agreement, the Company also issued Series E Warrants to the participating investors to purchase an aggregate of 5,384,600 shares of common stock with an exercise period of two years from the date of issuance. On the issuance date, the exercise price for the Series E Warrants was the lesser of $9.75 per share or a 50% premium on the per share trailing volume-weighted average share price of the common stock on NASDAQ for the ten trading days ending on dates specified in the form of Series E Warrants filed with the SEC. However, certain of the Series E Warrants could not be exercised until the Requisite Stockholder Approval was obtained. As a result of this contingency which was deemed outside the Company’s control as well as the variable number of underlying shares due to the floating exercise price, such Series E Warrants did not meet the criteria for classification as equity under ASC 815. As such, the Company classified the Series E Warrants as current liabilities at fair value upon issuance. The Series E Warrants were subject to re-measurement at each balance sheet date, with any change in fair value recognized as warrant expense, a component of other income (expense) reflected within the statement of operations. The Company used a third party valuation that utilized the Monte Carlo simulation model to estimate the fair value of the Series A convertible preferred stock and Series E Warrants. The valuation used a simulation of the Company’s periodic stock price, expected volatility of the price, adjusted for conversion price, and the remaining contractual term of the Series E Warrants. The fair value of the Series E Warrants was $14.8 million upon issuance. Once the Requisite Stockholder Approval was obtained on May 12, 2015, there was sufficient authorized shares underlying the warrants and the exercise price was fixed at $9.75 per share such that the variable number of shares that could be issued became fixed. As a result, all criteria for classification of the Series E Warrants as equity were met. The Company reclassified the warrant liability amounting to $17.8 million to additional paid-in capital, which equals to the fair value of the Company’s warrant liability on May 12, 2015. The change in fair value of $3.0 million was recorded on the consolidated statements of operations for the year ended December 31, 2015.
The Company had incurred and capitalized approximately $0.5 million of costs associated with this offering, which were recorded as an offset to additional paid-in capital on the consolidated balance sheets.
Accumulated Other Comprehensive Income (Loss)
The component of accumulated other comprehensive income (loss), net of tax for years ended December 31, 2015 and 2014 are as follows (in thousands):
Unrealized Gains (Losses) on Securities | Foreign Currency Translation Losses | Total | |||||||||
December 31, 2015: | |||||||||||
Beginning balance | $ | 392 | $ | (87 | ) | $ | 305 | ||||
Net current-period other comprehensive loss | (464 | ) | (51 | ) | (515 | ) | |||||
Ending Balance | $ | (72 | ) | $ | (138 | ) | $ | (210 | ) | ||
December 31, 2014: | |||||||||||
Beginning balance | $ | 373 | $ | (13 | ) | $ | 360 | ||||
Net current-period other comprehensive loss | 19 | (74 | ) | (55 | ) | ||||||
Ending Balance | $ | 392 | $ | (87 | ) | $ | 305 | ||||
11. Stock-Based Compensation
On September 22, 2015, the Company effected a reverse stock split of its common stock by a ratio of 1-for-10 (the “Reverse Split”). As a result of the Reverse Split every ten outstanding shares of common stock became one share of common stock. No fractional shares were issued in connection with the Reverse Split. A stockholder who would otherwise have been entitled to receive a fractional share of common stock received a cash payment equal to the closing sales price of the Company’s common stock on September 22, 2015 as reported on the NASDAQ times the amount of the fractional share. The Reverse Split also changed the number of shares of common stock that the Company is authorized to issue from 300,000,000 to
99
30,000,000 but did not change the par value of the Company’s common or preferred stock or the number of authorized shares of preferred stock. The Reverse Split resulted in a proportionate adjustment to the per share exercise price and the number of shares of common stock issuable upon the exercise of outstanding warrants and stock options, as well as the number of shares of common stock issuable upon the vesting of restricted stock units. All of the information in these financial statements has been presented to reflect the impact of the 1-for-10 Reverse Split on a retroactive basis.
Stock Option and Equity Incentive Plans
2002 Stock Option Plan
The Company’s 2002 Stock Option Plan (the “2002 Plan”) was created for the purpose of issuing stock options to employees, directors and consultants of the Company. Options granted under the 2002 Plan were either incentive stock options (“ISO”) or nonqualified stock options (“NSO”). ISOs may be granted only to Company employees (including officers and directors), whereas NSOs may be granted to Company employees and consultants. Options expire on terms as determined by the board of directors but not more than ten years after the date of grant. The Company reserved a total of 457,901 shares of its common stock for issuance under its 2002 Plan. Upon effectiveness of the Company’s IPO in November 2006, the Company ceased issuing stock options under the 2002 Plan. At that time, all shares remaining available for grant under the 2002 Plan became available for grant instead under the 2006 Equity Incentive Plan. However, cancelled shares under the 2002 Plan do not become available for grant under the 2006 Equity Incentive Plan. All outstanding options granted under the 2002 Plan continue to be administered under the 2002 Plan.
Stock options granted under the 2002 Plan provided employee option holders the right to elect to exercise unvested options in exchange for restricted common stock. Unvested shares were subject to a repurchase right held by the Company at the original issuance price in the event the optionees’ employment is terminated either voluntarily or involuntarily. For exercises of employee options, this right usually lapsed 25% on the first anniversary of the vesting start date and in 36 equal monthly amounts thereafter. These repurchase terms were considered to be a forfeiture provision and did not result in variable accounting. As of December 31, 2015, there were no unvested shares outstanding.
2006 Equity Incentive Plan
In August 2006, the Company’s board of directors approved the 2006 Equity Incentive Plan (the “2006 Plan”) to be effective on the date of the Company’s IPO. The 2006 Plan provides for the grant of ISOs, non-statutory stock options, restricted stock awards, restricted stock unit awards, stock appreciation rights, performance-based stock awards, and other forms of equity compensation, or collectively, stock awards, and performance-based cash awards, all of which may be granted to employees, including officers, non-employee directors and consultants. Options expire on terms as determined by the board of directors but not more than ten years after the date of grant.
The Company initially reserved a total of 200,000 shares for issuance under the 2006 Plan in addition to those shares which remained available for grant under the 2002 Plan. In addition, the number of shares of common stock reserved for issuance under the 2006 Plan automatically increases on January 1st each year by the lowest of (a) 4% of the total number of shares of our common stock outstanding on December 31st of the preceding calendar year, (b) 350,000 shares, or (c) a number determined by the board of directors that is less than (a) or (b). At December 31, 2015, 336,642 shares were available for grant under the 2006 Plan.
Option activity under both the 2002 Plan and the 2006 Plan for 2015 is as follows:
Shares | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||
(in thousands) | (in years) | (in thousands) | ||||||||||
Balance at January 1, 2015 | 919 | $ | 16.88 | 7.8 | $ | — | ||||||
Granted | 91 | $ | 9.18 | |||||||||
Exercised | (1 | ) | $ | 5.23 | 2 | |||||||
Cancelled | (219 | ) | $ | 24.06 | 23 | |||||||
Balance at December 31, 2015 | 790 | $ | 14.08 | 7.4 | $ | — | ||||||
Options vested and expected to vest at December 31, 2015 | 673 | $ | 14.56 | 6.0 | $ | — | ||||||
Options vested at December 31, 2015 | 361 | $ | 19.49 | 7.2 | $ | — | ||||||
100
The weighted-average grant-date fair value of options granted in 2015, 2014, and 2013 was $5.11, $6.22 and $12.49 per share, respectively. The total fair value of options that vested in 2015, 2014 and 2013 was $1.6 million, $1.9 million and $2.6 million, respectively.
The estimated grant date fair values of the employee stock options were calculated using the following assumptions:
Years Ended December 31, | |||||
2015 | 2014 | 2013 | |||
Expected volatility | 73% | 73% | 77% - 89% | ||
Risk-free interest rate | 1.3% | 1.4% | 0.6% - 1.5% | ||
Expected term (in years) | 4.13 | 4.31 | 4.38 - 4.57 | ||
Dividend yield | —% | —% | —% | ||
As of December 31, 2015, total unamortized stock-based compensation related to unvested stock options was $1.6 million, with a weighted-average remaining recognition period of 2.17 years.
The intrinsic value of exercised stock options is calculated based on the difference between the exercise price and the quoted closing market price of the Company’s common stock on the exercise date. The total intrinsic value of stock options exercised in 2014, 2013 and 2012 was $2,000, $0.6 million, and $19,000, respectively.
The options outstanding, vested and currently exercisable by exercise price under both the 2002 Plan and the 2006 Plan at December 31, 2015 are as follows (share options in thousands):
Options Outstanding | Options Exercisable and Vested | ||||||||||||
Exercise Price | Number of Options | Weighted- Average Remaining Contractual Life | Number of Options | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Life | ||||||||
(in years) | (in years) | ||||||||||||
$6.45-$6.48 | 145 | 8.94 | — | $ | — | 0 | |||||||
$6.49-$9.18 | 214 | 9.03 | 65 | $ | 7.69 | 9.09 | |||||||
$9.19-$20.75 | 206 | 7.45 | 107 | $ | 14.79 | 6.60 | |||||||
$20.76-$77.60 | 225 | 4.83 | 189 | $ | 26.24 | 4.62 | |||||||
790 | 7.41 | 361 | $ | 19.49 | 6.02 | ||||||||
Restricted stock unit activity under the 2006 Plan is as follows:
Restricted Stock Units | Weighted- Average Grant- Date Fair Value | |||||
(in thousands) | ||||||
Balance at January 1, 2015 | 84 | $ | 18.02 | |||
Awarded | 568 | $ | 8.93 | |||
Vested | (29 | ) | $ | 14.18 | ||
Cancelled | (138 | ) | $ | 11.14 | ||
Balance at December 31, 2015 | 485 | $ | 9.57 | |||
The fair value of restricted stock units is the quoted market price of the Company’s common stock as of the close of the grant date. The total fair value of shares vested pursuant to restricted stock units in 2015, 2014, and 2013 was $2.6 million, $2.5 million and $2.6 million, respectively. As of December 31, 2015, total unamortized stock-based compensation related to unvested restricted stock units was $1.0 million, with a weighted-average remaining recognition period of 2.67 years.
2006 Employee Stock Purchase Plan
In August 2006, the Company’s board of directors approved the 2006 Employee Stock Purchase Plan (the “Stock Purchase Plan”) which became effective upon the Company’s IPO. Commencing on January 1, 2007, the Stock Purchase Plan allows participating employees to contribute up to 15% of their earnings, up to a maximum of $25,000, to purchase shares of
101
the Company’s stock at a price per share equal to the lower of (a) 85% of the fair market value of a share of our common stock on the first date of the offering period, or (b) 85% of the fair market value of a share of our common stock on the date of purchase. The Company’s board of directors may specify offerings with durations of not more than 27 months, and may specify shorter purchase periods within each offering. Each offering will have one or more purchase dates on which shares of the Company’s common stock will be purchased for employees participating in the offering.
The Company initially reserved a total of 62,500 shares of common stock for issuance under the Stock Purchase Plan. In addition, the plan provides for automatic increases on January 1st, from January 1, 2007 through January 1, 2016, by the lesser of (a) 2% of the total number of shares of common stock outstanding on December 31st of the preceding calendar year, (b) 75,000 shares of common stock or (c) a number determined by the board of directors that is less than (a) and (b).
The estimated fair values of the shares issued under the Stock Purchase Plan were calculated using the following assumptions:
Years Ended December 31, | |||||
2015 | 2014 | 2013 | |||
Expected volatility | 78% | 111% | 65% | ||
Risk-free interest rate | 0.1% | 0.1% | 0.1% | ||
Expected term (in years) | 0.50 | 0.50 | 0.50 | ||
Dividend yield | —% | —% | —% | ||
Total Stock-based Compensation
Total stock-based compensation expense was allocated to cost of revenues, research and development and selling, general and administrative expense as follows (in thousands):
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Cost of revenues | $ | 233 | $ | 230 | $ | 402 | |||||
Research and development | 632 | 704 | 1,320 | ||||||||
Selling, general and administrative | 1,893 | 1,898 | 3,167 | ||||||||
Total | $ | 2,758 | $ | 2,832 | $ | 4,889 | |||||
Stock-based compensation expense related to stock options granted to non-employees is recognized on an accelerated basis as the stock options are earned. The final measurement occurs at the later of a performance commitment or when performance is complete. The Company believes that the fair value of the stock options is more reliably measurable than the fair value of the services received. The fair value of the stock options granted is calculated at each reporting date using the Black-Scholes option valuation model. Stock-based compensation expense charged to operations for options granted to non-employees for the years ended December 31, 2015 and 2014, 2013 was immaterial.
In fiscal 2015, the Company granted 318,721 PSUs to executives and employees in-lieu of cash bonuses with vesting based on the Company’s 2015 corporate goals and department goals over the vesting period of 1 year. Each PSU represents the right to receive one share of the Company’s common stock upon the vesting of such PSU, and is subject to the terms of the Company’s 2006 Equity Incentive Plan. Any PSUs not vesting on a vesting date due to the Company’s corporate goals and department goals for the fiscal year 2015 not meeting the target for such fiscal year established by the Compensation Committee shall be forfeited. The grant date fair value of these awards was $2.9 million. The Company recognized stock-based compensation expense of $0.8 million related to the PSUs as a result of achieving department goals.
In fiscal 2014, the Company awarded 41,407 restricted stock units to certain executives, which are subject to certain financial performance targets for the year ending December 31, 2015 and their continued employment, before vesting can occur. The Compensation Committee will determine the achievement for these restricted stock units within 30 days after the Company files its annual report on Form 10-K for the year ended December 31, 2015. Of these awards, 22,388 awards were cancelled as a result of employee terminations. No compensation cost related to these awards was recorded in 2015 and 2014 based on the estimated probability of achieving the financial performance targets for the year ending December 31, 2015.
12. Income Taxes
102
The Company’s pre-tax loss consists of the following (in thousands):
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Domestic | $ | (46,382 | ) | $ | (54,457 | ) | $ | (55,877 | ) | ||
Foreign | 222 | 239 | 270 | ||||||||
Pre-tax loss | $ | (46,160 | ) | $ | (54,218 | ) | $ | (55,607 | ) | ||
The Company had significant losses in 2015 and as such the $47,000 tax expense for fiscal year 2015 relates to foreign taxes. The Company’s effective tax rate differs from the U.S. federal statutory rate as follows:
Years Ended December 31, | ||||||||||
2015 | 2014 | 2013 | ||||||||
Federal tax benefit at statutory rate | (34 | )% | (34 | )% | (34 | )% | ||||
Permanent difference due to non-deductible expenses | 3 | % | 2 | % | 1 | % | ||||
State tax benefit, net of federal impact | — | % | — | % | — | % | ||||
Change in deferred tax asset valuation allowance | 31 | % | 33 | % | 33 | % | ||||
General business credits | — | % | (1 | )% | — | % | ||||
Effective tax rate | — | % | — | — | % | — | — | % | ||
The tax effects of temporary differences and carryforwards that give rise to significant portions of the deferred tax assets are as follows (in thousands):
December 31, | |||||||
2015 | 2014 | ||||||
Net operating loss carryforwards | $ | 46,942 | $ | 108,245 | |||
Research and development credits | 4,193 | 10,564 | |||||
Capitalized research and development | 19,803 | 19,926 | |||||
Fixed assets | 2,243 | 2,464 | |||||
Stock-based compensation | 1,442 | 3,214 | |||||
Accruals, reserves and other | 2,637 | 2,259 | |||||
Intangibles | 694 | 857 | |||||
77,954 | 147,529 | ||||||
Less: Valuation allowance | (77,954 | ) | (147,529 | ) | |||
Net deferred tax asset | $ | — | $ | — | |||
At December 31, 2015, the Company has federal and state net operating loss carryforwards of approximately $117.1 million and $125.3 million, respectively, available to offset future taxable income. These net operating loss carryforwards will expire in varying amounts from 2016 through 2035 if not utilized. The net operating loss carryforwards include $1.9 million which relates to stock option deductions that will be recognized through additional paid-in capital when utilized. As such, these deductions are not reflected in our deferred tax assets. The Company also has federal and California research and development tax credit carryforwards of $0.2 million and $8.2 million, respectively, available to offset future taxes payable. The federal credits begin to expire in 2035, while the state credits have no expiration.
Due to uncertainty surrounding realization of the deferred tax assets in future periods, the Company has placed a 100% valuation allowance against its net deferred tax assets. The valuation allowance increased (decreased) by $(69.6) million, $17.1 million and $21.4 million during the years ended December 31, 2015, 2014 and 2013, respectively. At such time as it is determined that it is more likely than not that the deferred tax assets are realizable, the valuation allowance will be reduced. Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, provide for annual limitations on the utilization of net operating loss and research and experimentation credit carryforwards if the Company were to undergo an ownership change, as defined in Section 382. In general, an ownership change occurs whenever the percentage of the shares of a corporation owned, directly or indirectly, by 5-percent shareholders, as defined in Section 382, increases by more than 50% percentage points over the lowest percentage of the shares of such corporation owned, directly or indirectly, by such 5-percent shareholders at any time over the preceding three years.
103
Based on an analysis under Section 382 of the Internal Revenue Code, we experienced various ownership changes through 2015 which substantially limit the future use of our pre-change NOLs and certain other pre-change tax attributes per year. We have excluded the NOLs and R&D credits that will expire as a result of the annual limitations in the deferred tax assets as of December 31, 2015. To the extent that we do not utilize our carry-forwards within the applicable statutory carry-forward periods, either because of Section 382 limitations or the lack of sufficient taxable income, the carry-forwards will expire unused.
The Company has not provided for U.S. federal income and foreign withholding taxes on any undistributed earnings from non-U.S. operations because such earnings are intended to be reinvested indefinitely outside of the United States. If these earnings were distributed, foreign tax credits may become available under current law to reduce or eliminate the resulting U.S. income tax liability. As of December 31, 2015, there is $0.8 million in cumulative foreign earnings upon which U.S. income taxes have not been provided.
The Company files income tax returns in the United States, various state jurisdictions and in the countries of United Kingdom and Germany. As of December 31, 2015, the Company’s federal tax returns for years ended 2012 through the current period and most state returns for the years ended 2011 through the current period are still open to examination. In addition, all of the net operating loss and research and development credit carryforwards that may be used in future years are still subject to adjustment. The Company is also subject to examination in the United Kingdom and Germany beginning in 2010 through the current period. There are no tax examinations currently in progress.
A reconciliation of the beginning and ending balance of unrecognized tax benefits is as follows (in thousands):
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Balance at beginning of period | $ | 4,220 | $ | 3,864 | $ | 3,358 | |||||
Additions based on tax positions related to the current year | 165 | 384 | 301 | ||||||||
Additions (Reduction) based on tax positions related to prior years | (2,287 | ) | (28 | ) | 205 | ||||||
Balance at end of period | $ | 2,098 | $ | 4,220 | $ | 3,864 | |||||
As of December 31, 2015, the company had a total of $2.1 million unrecognized tax benefits, none of which would affect the effective tax rate upon realization. While it is often difficult to predict the final outcome of any particular uncertain tax position, management does not believe that it is reasonably possible that the estimates of unrecognized tax benefits will change significantly in the next twelve months.
We recognize interest and penalties related to uncertain tax positions in income tax expense. As of December 31, 2015, we have no accrued interest or penalties related to uncertain tax positions. The Company does not anticipate that total unrecognized tax benefits will significantly change due to the settlement of audits and the expiration of statute of limitations period to December 31, 2015.
13. Net Loss per Share
The following table sets forth the computation of basic and diluted net loss per share (in thousands, except per share data):
Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Net loss | $ | (46,207 | ) | $ | (54,246 | ) | $ | (55,722 | ) | ||
Deemed dividend related to beneficial conversion feature of Series A convertible preferred stock and accretion of discount | (35,546 | ) | — | — | |||||||
Cumulative dividend on Series A convertible preferred stock | (812 | ) | — | — | |||||||
Net loss attributable to common stockholders | $ | (82,565 | ) | $ | (54,246 | ) | $ | (55,722 | ) | ||
Weighted average shares used to compute basic and diluted net loss per share | 16,871 | 11,723 | 7,905 | ||||||||
Basic and diluted net loss per share | $ | (4.89 | ) | $ | (4.63 | ) | $ | (7.05 | ) | ||
The following securities that could potentially dilute basic net loss per share are not included in the calculation of diluted net loss per share because to do so would be anti-dilutive as of the end of each period presented (in thousands):
104
December 31, | ||||||||
2015 | 2014 | 2013 | ||||||
Stock options outstanding | 790 | 919 | 778 | |||||
Unvested restricted stock units | 485 | 84 | 132 | |||||
Estimated shares to be issued under the employee stock purchase plan | 3 | 13 | 4 | |||||
Warrants | 6,079 | 927 | 3,481 | |||||
14. Segment Information
The Company operates its business in one operating segment: the development and marketing of medical devices. The Company’s chief operating decision maker is its Chief Executive Officer who reviews the financial information presented on a consolidated basis for the purpose of making operating decisions and assessing financial performance.
The Company’s medical robotics systems are developed and marketed to a broad base of hospitals and distributors in the United States and internationally. The Company considers all such sales to be part of a single operating segment. Information regarding total revenue is as follows (in thousands):
Years ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Revenues: | |||||||||||
United States | $ | 7,124 | $ | 10,041 | $ | 8,580 | |||||
International (1) | 8,944 | 9,454 | 8,402 | ||||||||
Total revenues | $ | 16,068 | $ | 19,495 | $ | 16,982 | |||||
The majority of the Company’s long-lived assets are located in the United States. Revenues are attributed to countries based on the location of the customer.
(1) | For fiscal year 2015, Italy and Belgium accounted for 11% and 10% of total revenues, respectively. For fiscal year 2014, only Japan within international accounted for 13% of total revenues. No single location within international accounted for greater than 10% of total revenues in fiscal year 2013. |
15. Quarterly Data (unaudited)
The following table represents certain unaudited quarterly information for the eight quarters ended December 31, 2015. This data has been derived from unaudited consolidated financial statements that, in the opinion of the Company’s management, include all adjustments, consisting only of normal recurring adjustments, necessary for a fair presentation of such information when read in conjunction with the Company’s annual audited consolidated financial statements and notes thereto appearing elsewhere in this report. These operating results are not necessarily indicative of results for any future period (in thousands, except per share data):
First Quarter | Second Quarter As Restated (2)(3) | Third Quarter (5) | Fourth Quarter (4) | ||||||||||||
2015: | |||||||||||||||
Revenues | $ | 5,794 | $ | 3,092 | $ | 3,183 | $ | 3,999 | |||||||
Gross profit | 1,156 | 358 | 204 | (715 | ) | ||||||||||
Net loss | (11,933 | ) | (12,507 | ) | (10,227 | ) | (11,540 | ) | |||||||
Net loss attributable to common stockholders | (11,933 | ) | (48,865 | ) | (10,227 | ) | (11,540 | ) | |||||||
Basic and diluted net loss per common share (1) | (0.90 | ) | (3.00 | ) | (0.54 | ) | (0.61 | ) | |||||||
2014: | |||||||||||||||
Revenues | $ | 3,699 | $ | 6,887 | $ | 3,877 | $ | 5,032 | |||||||
Gross profit | 398 | 1,902 | 702 | 2 | |||||||||||
Net loss attributable to common stockholders | (14,445 | ) | (12,290 | ) | (15,594 | ) | (11,917 | ) | |||||||
Basic and diluted net loss per common share (1) | (1.40 | ) | (1.10 | ) | (1.30 | ) | (0.90 | ) | |||||||
105
(1) | The Company’s financial statements have been retroactively adjusted to reflect the reverse stock split of its outstanding shares of common stock at a ratio of one-for-ten effective on September 22, 2015. |
(2) | Net loss, net loss attributable to common stockholders and basic and diluted net loss per common share for the second quarter of 2015 includes the impact of $2.9 million of change in fair value for warrant liability. |
(3) | Net loss attributable to common stockholders and basic and diluted net loss per common share for the second quarter of 2015 includes the impact of non-cash charges of $35.5 million and $0.8 million for the deemed dividend related to beneficial conversion feature of Series A convertible preferred stock and accretion of discount, and cumulative dividend on Series A convertible preferred stock, respectively. The net loss attributable to common stockholders and basic and diluted net loss per common share during the second quarter of 2015 have been restated to reflect a $15.3 million adjustment for the portion of the deemed dividend relating to the accretion of discount. |
(4) | Net loss, Net loss attributable to common stockholders and basic and diluted net loss per common share for the fourth quarter of 2015 include the impact of $1.7 million in restructuring expense. |
(5) | Net income and basic and diluted net loss per common share for the third quarter of 2014 include the impact of the warrant exchange of $2.9 million. |
16. Subsequent Events
As disclosed in the Company’s Current Report on Form 8-K filed with the SEC on April 20, 2016, the Company, Auris Surgical Robotics, Inc. ("Parent"), and Pineco Acquisition Corp., a wholly owned subsidiary of Parent ("Merger Sub"), entered into an Agreement and Plan of Merger (the "Merger Agreement"), pursuant to which, subject to satisfaction or waiver of the conditions therein, Merger Sub will merge with and into the Company (the "Merger"), with the Company surviving the Merger as a wholly owned subsidiary of Parent.
Subject to the terms of the Merger Agreement, which has been unanimously approved by the board of directors of the Company, at the effective time of the Merger (the "Effective Time"), each share of Company common stock issued and outstanding immediately prior to the Effective Time (other than shares owned by Parent or any of its subsidiaries, shares held by the Company as treasury stock, and shares held by stockholders who have perfected their statutory rights of appraisal under Section 262 of the Delaware General Corporation Law) will be converted into the right to receive $4.00 in cash, without interest (the "Merger Consideration").
Immediately prior to the Effective Time, each outstanding option to purchase shares of Company common stock granted under a Company stock plan will be cancelled for no consideration. Additionally, immediately prior to the Effective Time, each outstanding award of restricted stock units with respect to shares of Company common stock (each, an "RSU Award") granted pursuant to a Company stock plan will be fully vested and cancelled and, in exchange therefor, each holder of any such cancelled RSU Award will be entitled to receive, in consideration of the cancellation of such RSU Award and in settlement therefor, a payment in cash of an amount equal to the product of (i) the Merger Consideration multiplied by (ii) the number of restricted stock units subject to such RSU Award, without interest (less any required tax withholdings). To the extent an RSU Award is subject to performance conditions, the number of restricted stock units that vest will be determined (A) for RSU Awards with a performance period that by its terms has ended prior to the Effective Time, based on actual performance through the end of such performance period, and (B) for RSU Awards with a performance period that by its terms has not ended prior to the Effective Time, by deeming such performance conditions to have been satisfied at 100% of the target levels specified in the applicable equity plans and award agreements.
The Merger Agreement contains customary representations, warranties and covenants of the Company, Parent and Merger Sub, including, among others, covenants by the Company to conduct its business in the ordinary course during the interim period between execution of the Merger Agreement and consummation of the Merger and not to engage in certain kinds of transactions during such period. The Company has also agreed, subject to certain exceptions, not to enter into discussions concerning, or provide confidential information in connection with, any alternative transaction. Parent has agreed, promptly following the Effective Time, to pay or cause to be paid in full all of the Company’s obligations under the loan and security agreement with White Oak. In addition, each of the parties has agreed to use its reasonable best efforts to cause the Merger to be consummated. The board of directors of the Company has agreed to hold a stockholder meeting to consider and vote upon the adoption of the Merger Agreement.
The Merger is subject to satisfaction of the conditions set forth in the Merger Agreement, including the approval by the stockholders of the Company; that none of the lender parties to the loan and security agreement with White Oak shall have
106
exercised any of the rights and remedies available to any of them under either the loan and security agreement with White Oak or the Forbearance Agreement upon the occurrence and during the continuance of an Event of Default (as defined in the loan and security agreement with White Oak) or as a result of the Company breaching or being in default of any of the covenants, agreements or other provisions of the Forbearance Agreement, other than delivering notice of such Event of Default under the loan and security agreement with White Oak or such breach or default under the Forbearance Agreement; that none of the Rollover Stockholders (as defined below) shall have invalidated or terminated the stock purchase agreement with Parent (as described below); and other customary closing conditions. The obligation of each party to consummate the Merger is also conditioned upon the other party’s representations and warranties being true and correct (subject to certain materiality exceptions), and the other party having performed in all material respects its obligations under the Merger Agreement, and the obligation of Parent to consummate the Merger is conditioned upon the Company not having suffered a material adverse effect.
The Merger Agreement contains certain termination rights for both the Company and Parent, and provides that, upon termination of the Merger Agreement under specified circumstances, the Company may be required to pay Parent a termination fee of $3.325 million, including if the Company were to terminate the Merger Agreement in order to accept an unsolicited superior acquisition proposal.
Concurrently with the Company, Parent and Merger Sub entering into the Merger Agreement, holders of approximately 65.4% of the outstanding shares of Company common stock, including all of the directors and executive officers of the Company, Larry Feinberg and certain affiliated entities, Jack Schuler and certain affiliated entities, and an affiliated entity of Lawrence T. Kennedy, Jr., entered into voting agreements in favor of Parent and the Company (collectively, the "Voting Agreements") pursuant to which they agreed, among other things, to vote their shares of Company common stock in favor of the adoption of the Merger Agreement, against any alternative acquisition proposal, and against any reorganization, recapitalization, dissolution, liquidation or winding-up of the Company or any other extraordinary transaction involving the Company other than the Merger. The Voting Agreements terminate upon the termination of the Merger Agreement in accordance with its terms, including if the Company were to terminate the Merger Agreement in order to accept an unsolicited superior acquisition proposal.
Additionally, concurrently with entering into the Merger Agreement, certain Company stockholders owning approximately 64.6% of the outstanding shares of the Company common stock, including Larry Feinberg and certain affiliated entities, Jack Schuler and certain affiliated entities and an affiliated entity of Lawrence T. Kennedy, Jr. (the "Rollover Stockholders"), executed and delivered a stock purchase agreement with Parent whereby each Rollover Stockholder has unconditionally agreed to acquire shares of preferred stock of Parent immediately following the Effective Time on the terms set forth therein, in exchange for an investment of approximately $49 million (representing the aggregate amount of consideration payable to the Rollover Stockholders in the Merger).
Further, concurrently with the Company entering into the Merger Agreement, the Company and the lender parties under the loan and security agreement with White Oak entered into the Forbearance Agreement. The "going concern" qualification in the opinion and report for this report constitutes an Event of Default (as defined in the loan and security agreement with White Oak). Pursuant to the Forbearance Agreement, the lender parties have agreed not to pursue any remedies related to such "going concern" default during the period from the date of the Forbearance Agreement through the earlier of (x) August 17, 2016 and (y) the date when the forbearance period under the Forbearance Agreement is otherwise terminated (such period, the "Forbearance Period").
In addition, the Company expects that it will fail to comply with the minimum liquidity required by Section 6.10(a) of the loan and security agreement with White Oak at some point during the Forbearance Period. The Company’s failure to comply with such minimum liquidity requirement would constitute an Event of Default under the loan and security agreement with White Oak. Under the Forbearance Agreement, the Lender Parties have agreed not to pursue any remedies related to such minimum liquidity default during the Forbearance Period.
The Forbearance Agreement provides that the Company shall, as promptly as reasonably practicable and in any event at or before the expiration of the Forbearance Period, (x) consummate the Merger and (y) pay and satisfy all obligations owed by the Company to the Lender Parties in full in cash. The failure to consummate the Merger during the Forbearance Period would constitute a default under the Forbearance Agreement.
Pursuant to the Forbearance Agreement, the Company has prepaid $5.0 million of the outstanding principal amount of the term loan under the loan and security agreement with White Oak.
For additional details regarding the terms and conditions of the Merger Agreement, refer to the Merger Agreement and other documentation filed as exhibits to the Current Report on Form 8-K filed with the SEC on April 20, 2016. Additional
107
information regarding the proposed transaction, including risks associated with the proposed transaction, will be contained in a definitive proxy statement to be filed by the Company with the SEC. No assurance can be given that the Merger will be completed.
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Effective October 6, 2014, the Audit Committee of the Board of Directors of the Company chose to (i) dismiss Deloitte & Touche LLP (“Deloitte”) as the Company’s independent registered public accounting firm, and (ii) formally engage BDO USA, LLP to be the independent registered public accounting firm for the Company for the fiscal year ending December 31, 2014. We initially reported this change in a Current Report on Form 8-K dated October 6, 2014 and filed with the Securities and Exchange Commission on October 9, 2014.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management is responsible for establishing and maintaining a system of disclosure controls and procedures (as defined in Rule 13a-15e and 15d-15e under the Securities Exchange Act of 1934 (the “Exchange Act”)) designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive officer or officers and principal financial officer or officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
In connection with our decision to restate our financial statements for the quarter ended June 30, 2015 and September 30, 2015, our management have carried out a reevaluation, under the supervision of and with the participation of our CEO and CFO, of the effectiveness of the design and operation of our disclosure controls and procedures and concluded that our disclosure controls and procedures were not effective as of December 31, 2015 as a result of the material weakness in our control over financial reporting as discussed below.
Material Weaknesses
The design and operating effectiveness of our controls were inadequate to ensure that a complex financial instrument transaction was properly accounted for and reviewed. Specifically, we failed to deduct a non-cash deemed dividend of $15.3 million upon the conversion of Series A convertible preferred stock in the calculation of net loss attributable to common stockholders for the purposes of determining basic and diluted net loss per common share for the three and six months ended June 30, 2015 and the nine months ended September 30, 2015.
Remediation Status
We continue to evaluate the effectiveness of our remediation efforts, including demonstrating that the new or improved controls operate effectively for a reasonable period of time. To remedy the material weakness, we are enhancing our procedures to provide additional management and third party oversight and review of our accounting activities over future complex financial instrument transactions.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed by, or under the supervision of, our Chief Executive Officer and Interim Chief Financial Officer, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP and includes those policies and procedures that:
i. | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of assets of the company; |
ii. | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the Company; and |
108
iii. | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Also projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015. In making its assessment of internal control over financial reporting, management used the criteria described in Internal Control — Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on our assessment under this framework, our management concluded that our internal control over financial reporting was ineffective as of December 31, 2015.
The ineffectiveness of our internal control over financial reporting as of December 31, 2015 has been audited by BDO USA, LLP, the independent registered public accounting firm who has also audited our consolidated financial statements. BDO USA, LLP’s report on our internal control over financial reporting is included below.
Changes in Internal Control Over Financial Reporting
In connection with management’s annual assessment of the overall effectiveness of our internal controls over financial reporting included in our Form 10-K for the year ended December 31, 2015, management based its assessment on the framework set forth in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations (“COSO”) of the Treadway Commission. On May 14, 2013, COSO issued an updated framework, referred to as the 2013 COSO framework, which supersedes the 1992 framework after December 15, 2014. We have reviewed the COSO 2013 framework and integrated the changes into the Company’s internal controls over financial reporting. Management’s assessment of the overall effectiveness of our internal controls over financial reporting for the year ended December 31, 2015 is based on the COSO 2013 framework.
Our management, including our Chief Executive Officer and Interim Chief Financial Officer, evaluated our “internal control over financial reporting” as defined in Exchange Act Rule 13a-15(f) to determine whether any changes in our internal control over financial reporting occurred during the fourth quarter of 2014 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. Based on that evaluation, other than the material weakness and remediation noted above, there were no changes in our internal control over financial reporting during the quarter ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect our internal control over financial reporting.
Limitations of the Effectiveness of Controls
We are committed to continuing to improve our internal control processes and will continue to diligently review our financial reporting controls and procedures in order to ensure compliance with the requirements of the Sarbanes-Oxley Act and the related rules promulgated by the Securities and Exchange Commission. However, because of the inherent limitations in all control systems, any control system, regardless of how well designed, operated and evaluated, can provide only reasonable, not absolute, assurance that the control objectives will be met. The design of any system of controls is based, in part, on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
109
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
Hansen Medical, Inc.
Mountain View, California
We have audited Hansen Medical, Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Hansen Medical, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Item 9A, Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. A material weakness regarding management’s failure to design and maintain controls over the accounting for a complex financial instrument has been identified and described in management’s assessment. This material weakness was considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2015 financial statements, and this report does not affect our report dated April 22, 2016 on those financial statements. We do not express an opinion or any other form of assurance on management’s statements referring to any corrective actions taken by the company after the date of management’s assessment.
In our opinion, Hansen Medical, Inc. did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of Hansen Medical, Inc. as of December 31, 2015 and 2014, and the related consolidated statements of operations, comprehensive loss, stockholders’ equity, and cash flows for the year ended December 31, 2015 and 2014, and our report dated April 22, 2016 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
San Jose, California
April 22, 2016
110
ITEM 9B. OTHER INFORMATION
None.
111
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this Item concerning our directors and executive officers is incorporated herein by reference to information contained in the sections of our Proxy Statement for our 2016 Annual Meeting of Stockholders to be filed with the SEC within 120 days after the end of our fiscal year ended December 31, 2015 (the “2016 Proxy Statement”) entitled “Proposal 1 Election of Directors,” “Corporate Governance — Code of Business Conduct and Ethics,” “Corporate Governance — Audit Committee,” “Executive Officers” and “Section 16(a) Beneficial Ownership Reporting Compliance.”
Item 11. Executive Compensation.
The information required by this Item is incorporated herein by reference to the sections of our 2016 Proxy Statement entitled “Executive Compensation,” “Compensation Discussion and Analysis,” “Corporate Governance — Compensation Committee Interlocks and Insider Participation,” “Compensation Committee Report” and “Corporate Governance — 2015 Director Compensation Table.”
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this Item is incorporated herein by reference to the sections of our 2016 Proxy Statement entitled “Executive Compensation-Securities Authorized for Issuance under Equity Compensation Plans,” “Security Ownership of Certain Beneficial Owners and Management” and “Executive Compensation.”
Item 13. Certain Relationships and Related Transactions and Director Independence.
The information required by this Item is incorporated herein by reference to the section of our 2016 Proxy Statement entitled “Certain Relationships and Related Party Transactions.”
Item 14. Principal Accountant Fees and Services
The information required by this Item is incorporated herein by reference to the section of our 2016 Proxy Statement entitled “Proposal 2 Ratification of Appointment of Independent Registered Public Accounting Firm — Principal Accountant Fees and Services.”
112
PART IV.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) | Financial Statements and Schedules: Financial Statements for the three years ended December 31, 2015 are included in Part II, Item 8. All schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto. |
(b) | Exhibits: The list of exhibits on the Exhibit Index on pages 134 through 137 of this report is incorporated herein by reference. |
113
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
By: | /S/ CARY G. VANCE | ||
Dated: April 22, 2016 | President and Chief Executive Officer (Principal Executive Officer) | ||
POWERS OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Cary G. Vance and Christopher P. Lowe, and each of them, as his true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this report, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them or their substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Signature | Title | Date | ||
/S/ CARY G. VANCE | President and Chief Executive Officer and Director | April 22, 2016 | ||
Cary G. Vance | (Principal Executive Officer) | |||
/S/ CHRISTOPHER P. LOWE | Interim Chief Financial Officer and Director | April 22, 2016 | ||
Christopher P. Lowe | (Principal Accounting and Financial Officer) | |||
/S/ MICHAEL L. EAGLE | Chairman of the Board | April 22, 2016 | ||
Michael L. Eagle | ||||
/S/ MARJORIE L. BOWEN | Director | April 22, 2016 | ||
Marjorie L. Bowen | ||||
/S/ KEVIN HYKES | Director | April 22, 2016 | ||
Kevin Hykes | ||||
/S/ STEPHEN L. NEWMAN, M.D. | Director | April 22, 2016 | ||
Stephen L. Newman, M.D. | ||||
/S/ WILLIAM R. ROHN | Director | April 22, 2016 | ||
William R. Rohn | ||||
/S/ NADIM YARED | Director | April 22, 2016 | ||
Nadim Yared | ||||
114
EXHIBIT INDEX
Exhibit Number | Description of Document | |
3.1(1) | Amended and Restated Certificate of Incorporation of the Registrant. | |
3.2(2) | Amended and Restated Bylaws of the Registrant. | |
3.3(3) | Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Registrant. | |
3.4(4) | Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Registrant. | |
3.5(5) | Certificate of Designations, Preferences and Rights of Series A Convertible Participating Preferred Stock of the Registrant. | |
4.1(6) | Specimen Common Stock Certificate. | |
4.2(7) | Stock Purchase Agreement, by and among the Registrant and Oracle Partners, LP, Oracle Institutional Partners, LP and Oracle Ten Fund Master, LP, dated November 7, 2011. | |
4.3(7) | Stock Purchase Agreement, by and between the Registrant and Jack W. Schuler., dated November 7, 2011. | |
4.4(8) | Form of Warrant to Purchase Stock, issued to the Lenders, dated as of December 8, 2011. | |
4.5(9) | Stock Purchase Agreement, by and between Registrant and Intuitive Surgical Operations, Inc., dated as of October 26, 2012. | |
4.6(10) | Form of Series A, B and C Warrant, dated as of August 8, 2013. | |
4.7(11) | Exchange Agreement, by and among the Registrant and each warrant holder identified therein, dated July 30, 2014. | |
4.8(11) | Form of Series B/C Exchange Warrant, dated August 11, 2014. | |
4.9(11) | Form of Series D Warrant, dated August 11, 2014. | |
4.10(11) | Amended and Restated Investor Rights Agreement, by and among the Registrant and each purchaser identified therein, dated August 11, 2014. | |
4.11(5) | Second Amended and Restated Investor Rights Agreement, by and among the Registrant and each purchaser identified therein, dated March 11, 2015 | |
4.12(5) | Securities Purchase Agreement, by and among the Registrant and each purchaser identified therein, dated March 9, 2015. | |
4.13(5) | Form of Series E Warrant, dated as of March 11, 2015. | |
10.1(12)+ | Form of Indemnity Agreement for Executive Officers. | |
10.2(13)+ | Form of Amended and Restated Indemnity Agreement for certain Directors. | |
10.3(12)+ | 2002 Stock Plan. | |
10.4(9)+ | 2006 Equity Incentive Plan, as amended December 11, 2012. | |
10.5.1(12)+ | Form of Option Grant Notice and Form of Option Agreement under 2006 Equity Incentive Plan. | |
10.5.2(9)+ | Form of Option Grant Notice and Form of Option Agreement for Non-Employee Directors under 2006 Equity Incentive Plan, as amended December 11, 2012. | |
10.6(14)+ | 2006 Employee Stock Purchase Plan. | |
10.7(12)+ | Form of Offering Document under 2006 Employee Stock Purchase Plan. | |
10.8(12)* | Cross License Agreement, by and between the Registrant and Intuitive Surgical, Inc., dated September 1, 2005. | |
10.9(12)* | License Agreement, by and between the Registrant and Mitsubishi Electric Research Laboratories, Inc., dated March 7, 2003. | |
10.10(12) | Development and Supply Agreement, by and between the Registrant and Force Dimension, dated November 10, 2004. | |
115
10.11(15) | Office Lease, by and between the Registrant and MTV Research, LLC, dated July 18, 2007. | |
10.12(16) | Lease Termination Agreement, by and between the Registrant and Google Inc, as successor-in-interest to the property owned by BXP Research Park LLC, LLC, dated December 15, 2015. | |
10.13(16) | Multi-Tenant Commercial/Industrial Lease, by and between the Registrant and LBA Realty Fund II-WBP VII, Inc., dated December 15, 2015. | |
10.14(17)+ | Hansen Medical, Inc. Management Cash Incentive Plan, dated April 7, 2008. | |
10.15(18) | First Amendment to Office Lease, dated June 27, 2008, by and between the Registrant and BP MV Research Park LLC. | |
10.16(19)* | License Agreement, by and between the Registrant and Luna Innovations Incorporated, dated January 12, 2010. | |
10.17(19)* | Cross License Agreement, by and between the Registrant and Intuitive Surgical, Inc., dated January 12, 2010. | |
10.18(20)+ | Form of Retention Agreement for executive officers. | |
10.19(21)* | Patent and Technology License and Purchase Agreement, by and among the Registrant, Koninklijke Philips Electronics N.V. and Philips Medical Systems Nederland B.V., dated February 2, 2011. | |
10.20(21)* | Sublicense Agreement, by and among ECL7, LLC, Koninklijke Philips Electronics N.V. and Philips Medical Systems Nederland B.V., dated February 3, 2011. | |
10.21(21)* | Assignment and License Agreement, by and among the Registrant and ECL7, LLC, dated February 3, 2011. | |
10.22(21)* | Security Agreement, by and among ECL7, LLC, Koninklijke Philips Electronics N.V., and Philips Medical Systems Nederland B.V., dated February 3, 2011. | |
10.23(21) | Patent License Security Agreement, by and between ECL7, LLC, Koninklijke Philips Electronics N.V. and Philips Medical Systems Nederland B.V., dated February 3, 2011. | |
10.24(21)* | Amendment No. 1 to Patent and Technology License and Purchase Agreement, by and between the Registrant, Koninklijke Philips Electronics N.V. and Philips Medical Systems Nederland B.V., dated April 7, 2011. | |
10.25(22)* | Amendment No. 2 to Extended joint Development Agreement and Amendment to Patent and Technology License and Purchase Agreement and Sublicense Agreement, by and among the Registrant and Philips Medical Systems Nederland B.V., dated August 5, 2015. | |
10.26(18)+ | Offer Letter, by and between the Registrant and Susan Leonard, dated December 13, 2011. | |
10.27(23)+ | Offer Letter, by and between the Registrant and Joseph Guido, dated as of March 19, 2012. | |
10.28(24)+ | Amendment of Stock Option Agreement for option grants to non-employee directors. | |
10.29(9) | First Amendment to Cross License Agreement, by and between Registrant and Intuitive Surgical Operations, Inc., dated as of October 26, 2012. | |
10.30(9)+ | Offer Letter, by and between Registrant and William Sutton, dated as of November 12, 2012. | |
10.31(9)+ | Amendment of Notice of Restricted Stock Unit Award and Restricted Stock Unit Award Agreement. | |
10.32(9)+ | Form of Amendment to Amended and Restated Indemnity Agreement for certain directors. | |
10.33(9)+ | Form of Indemnity Agreement for Directors Approved November 9, 2012. | |
10.34(9)+ | Non-Employee Director Compensation Arrangements, effective as of January 1, 2013. | |
10.35(9) | Non-Employee Director Compensation Arrangements, effective as of January 1, 2015 | |
10.36(18)+ | Offer Letter, by and between the Registrant and Robert Cathcart, dated January 10, 2013. | |
10.37(25)+ | Form of Retention Agreement for executive officers. | |
10.38(6) | Amended and Restated Loan and Security Agreement, dated August 23, 2013, by and among the Registrant, White Oak Global Advisors, LLC and each lender identified therein. | |
116
10.39(18) | Second Amendment to Office Lease, dated December 13, 2013, by and between the Registrant and BXP Research Park LP. | |
10.40(26)+ | Offer Letter, by and between the Registrant and Cary G. Vance, dated April 25, 2014. | |
10.41(26)+ | Retention Letter Agreement, by and between the Registrant and Robert O. Cathcart, dated May 12, 2014. | |
10.42(26)+ | Retention Letter Agreement, by and between the Registrant and William Sutton, dated May 12, 2014. | |
10.43(26)+ | Revised Offer Letter, by and between the Registrant and Christopher P. Lowe, dated June 11, 2014. | |
10.44(27)+ | Waiver by Cary G. Vance, dated August 12, 2014. | |
10.45(27)+ | Offer Letter, by and between the Registrant and Brian Sheahan, dated September 12, 2014. | |
10.46(28)+ | Letter Agreement, by and between the Registrant and Christopher P. Lowe, dated October 28, 2014. | |
10.47(29)+ | Separation Agreement, dated March 3, 2014, by and between the Registrant and Bruce J Barclay. | |
10.48(30) | Separation Agreement, dated November 6, 2015, by and between the Registrant and William M. Sutton. | |
10.49(26)+ | Separation Agreement, by and between the Registrant and Peter J. Mariani, dated June 18, 2014. | |
10.50(31)+ | Severance and Release Agreement, by and between the Registrant and Joseph Guido, dated March 1, 2016. | |
10.51(28)+ | Non-Plan Option Grant Notice and Non-Plan Option Agreement by and between the Registrant and Cary G. Vance, dated May 23, 2014. | |
10.52(32) | Merger Agreement by and between the Registrant, Auris Surigcal Robotics, Inc. and Pineco Acquisition Corp., dated April 19, 2016. | |
10.53(32) | Forbearance Agreement by and between the Registrant and White Oak Global Advisors, LLC, dated April 19, 2016 | |
10.54(33)+ | Retention Agreement, by and between the Registrant and Christopher P. Lowe, dated April 18, 2016 | |
23.1 | Consent of BDO USA LLP, Independent Registered Public Accounting Firm. | |
23.2 | Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm. | |
24.1 | Powers of Attorney. Reference is made to the signature page to this report. | |
31.1 | Certification of Chief Executive Officer required by Rule 13a-15(e) or Rule 15d-15(e). | |
31.2 | Certification of Chief Financial Officer required by Rule 13a-15(e) or Rule 15d-15(e). | |
32.1** | Certification of Chief Executive Officer required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the Unites States Code (18 U.S.C. §1350). | |
32.2** | Certification of Chief Financial Officer required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the Unites States Code (18 U.S.C. §1350). | |
101.INS | XBRL Instance Document | |
101.SCH | XBRL Taxonomy Extension Schema Document | |
101.CAL | XBRL Taxonomy Calculation Linkbase Document | |
101.DEF | XBRL Taxonomy Definition Linkbase Document | |
101.LAB | XBRL Taxonomy Label Linkbase Document | |
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | |
(1) | Previously filed as an exhibit to Registrant’s Annual Report on Form 10-K, filed on March 28, 2007 and incorporated herein by reference. |
(2) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on February 16, 2007 and incorporated herein by reference. |
(3) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on August 9, 2013 and incorporated herein by reference. |
(4) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on September 23, 2015 and incorporated herein by reference. |
(5) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on March 12, 2015 and incorporated herein by reference. |
(6) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on November 8, 2013 and incorporated herein by reference. |
(7) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on November 7, 2011 and incorporated herein by reference. |
(8) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on December 9, 2011 and incorporated herein by reference. |
117
(9) | Previously filed as an exhibit to Registrant’s Annual Report on Form 10-K, filed on March 18, 2013 and incorporated herein by reference. |
(10) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on July 31, 2013 and incorporated herein by reference. |
(11) | Previously filed as an exhibit to Registrant’s Quarterly Report Form 10-Q, filed on November 6, 2014 and incorporated herein by reference. |
(12) | Previously filed as an exhibit to Registrant’s Registration Statement on Form S-1, as amended, originally filed on August 16, 2006 and incorporated herein by reference. |
(13) | Previously filed as an exhibit to Registrant’s Annual Report on Form 10-K, filed on March 15, 2012 and incorporated herein by reference. |
(14) | Previously filed as an exhibit to Registrant’s Registration Statement on Form S-8, filed on May 8, 2009 and incorporated herein by reference. |
(15) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on November 2, 2007 and incorporated herein by reference. |
(16) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on December 21, 2015 and incorporated herein by reference. |
(17) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on April 9, 2008 and incorporated herein by reference. |
(18) | Previously filed as an exhibit to Registrant’s Annual Report on Form 10-K, filed on March 13, 2014 and incorporated herein by reference. |
(19) | Previously filed as an exhibit to Registrant’s Annual Report on Form 10-K, filed on March 16, 2010 and incorporated herein by reference. |
(20) | Previously filed as an exhibit to Registrant’s Annual Report on Form 10-K, filed on March 16, 2011 and incorporated herein by reference. |
(21) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on May 10, 2011 and incorporated herein by reference. |
(22) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on August 10, 2015 and incorporated herein by reference. |
(23) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on May 7, 2012 and incorporated herein by reference. |
(24) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on November 9, 2012 and incorporated herein by reference. |
(25) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on May 10, 2013 and incorporated herein by reference. |
(26) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on August 8, 2014 and incorporated herein by reference. |
(27) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on November 6, 2014 and incorporated herein by reference. |
(28) | Previously filed as an exhibit to Registrant's Annual Report on Form 10-K filed on March 16, 2015 and incorporated herein by reference. |
(29) | Previously filed as an exhibit to Registrant’s Quarterly Report on Form 10-Q, filed on May 9, 2014 and incorporated herein by reference. |
(30) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K/A, filed on November 19, 2015 and incorporated herein by reference. |
(31) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K/A, filed on March 9, 2016 and incorporated herein by reference. |
(32) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on April 20, 2016 and incorporated herein by reference. |
(33) | Previously filed as an exhibit to Registrant’s Current Report on Form 8-K, filed on April 22, 2016 and incorporated herein by reference. |
+ Indicates management contract or compensatory plan.
* Confidential treatment has been granted with respect to certain portions of this exhibit.
** The certifications attached hereto as Exhibits 32.1 and 32.2 accompany this Annual Report on Form 10-K are not deemed filed with the U.S. Securities and Exchange Commission and are not to be incorporated by reference into any filing of Hansen Medical, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing.
118
