Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Eiger BioPharmaceuticals, Inc. | d181171d8k.htm |
| EX-99.2 - EX-99.2 - Eiger BioPharmaceuticals, Inc. | d181171dex992.htm |

Building a Franchise in HDV Sarasar® (lonafarnib) Pegylated Interferon Lambda-1a Exhibit 99.1

This presentation and the oral commentary contain “forward-looking” statements that involve substantial risks and uncertainties. All statements other than statements of historical facts, including statements regarding our future financial condition, timing for and outcomes of clinical results, business strategy and plans and objectives for future operations, are forward looking statements. These forward-looking statements include terminology such as “believe,” “will,” “may,” “estimate,” “continue,” “anticipate,” “contemplate,” “intend,” “target,” “project,” “should,” “plan,” “expect,” “predict,” “could,” “potentially” or the negative of these terms Forward looking statements are our current statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned clinical development, the timing of and our ability to initiate or enroll clinical trials, and our ability to make regulatory filings and obtain and maintain regulatory approvals for Sarasar, PEG IFN Lambda and our other product candidates, our intellectual property position, the potential safety, efficacy, reimbursement, convenience clinical and pharmaco-economic benefits of our product candidates, commercial opportunities, including potential market sizes and segments, our ability to commercialize, expectations regarding clinical trial data and FDA outcomes, our results of operations, cash needs, spending of the proceeds from this offering, financial condition, liquidity, prospects, growth and strategies, the industry in which we operate and the trends that may affect the industry or us. Forward-looking statements involve known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Forward-looking statements represent our beliefs and assumptions only as of the date of this presentation. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. Forward-Looking Statements © 2016 Eiger Biopharmaceuticals, Inc., all rights reserved. Sarasar is a registered trademark of Merck Sharp & Dohme Corp. All other trademarks belong to their respective owners.

PEG IFN Lambda The Next Frontier in Interferon Therapy Donnelly R et al, J Interferon Cytokine Res, 2010, 30(8): 555. Journal of Interferon & Cytokine Research
PEG IFN Lambda Background First developed by Zymogenetics Clinical Development into Phase 2 in early 2000’s Proposed benefit: improved safety and tolerability vs PEG IFN α Target indication: HCV Acquired by Bristol-Myers Squibb in 2010 Greater than 3,000 patients in 17 clinical trials Phase 2 and Phase 3 studies in HCV and HBV Discontinued following advent of all oral HCV combinations ZymoGenetics Bristol-Myers Squibb
PEG IFN Lambda A targeted interferon for HDV A novel, first in class Type III interferon - Native Lambda is generated by human immune system in viral infections Binds to a unique receptor versus Type I interferons - Highly expressed on hepatocytes - Limited expression on hematopoietic cells and CNS cells Uses similar downstream signaling pathway as Type I interferons Anti HCV / Anti HBV activity demonstrated in clinical studies Antiviral activity with less of the typical IFN α related side effects Anti HDV activity demonstrated in humanized liver mouse model
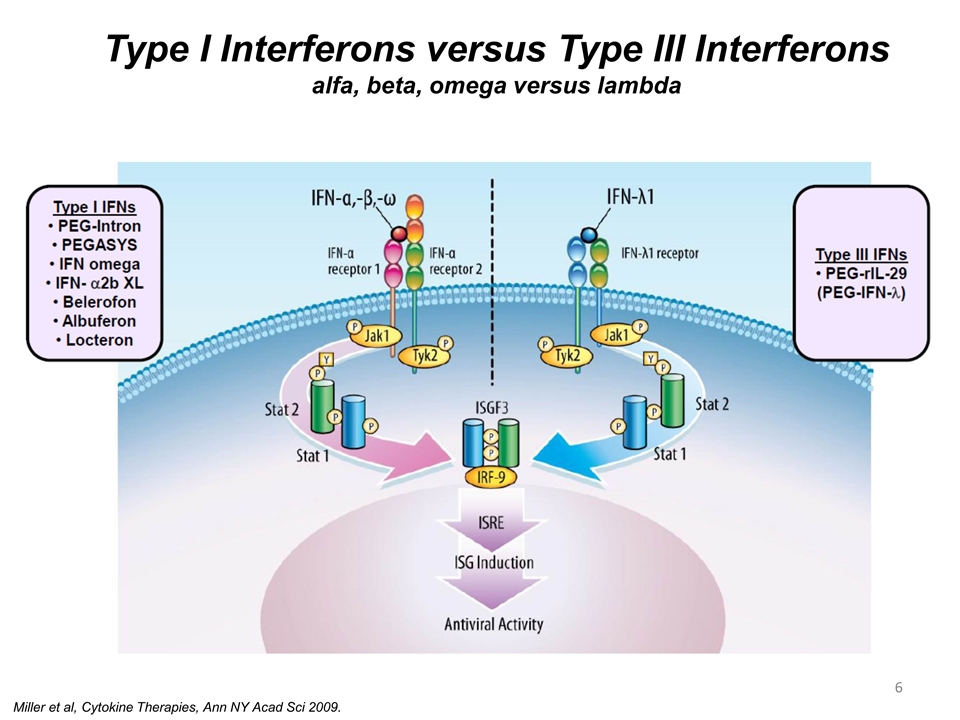
PEG IFN λ Negotiation Type I Interferons versus Type III Interferons alfa, beta, omega versus lambda Miller et al, Cytokine Therapies, Ann NY Acad Sci 2009.
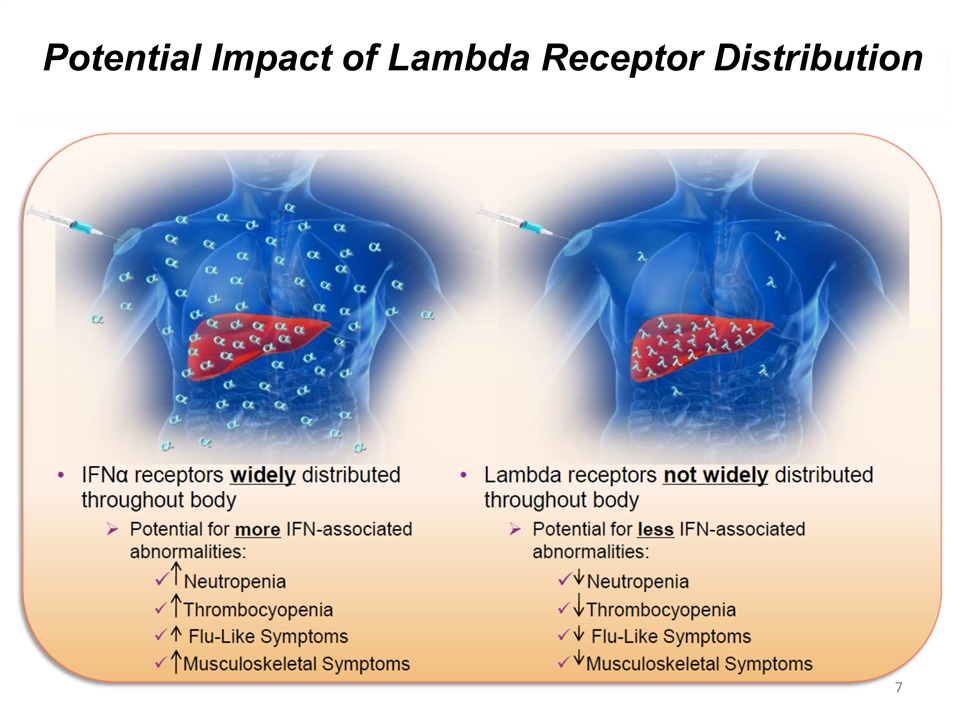
Potential Impact of Lambda Receptor Distribution
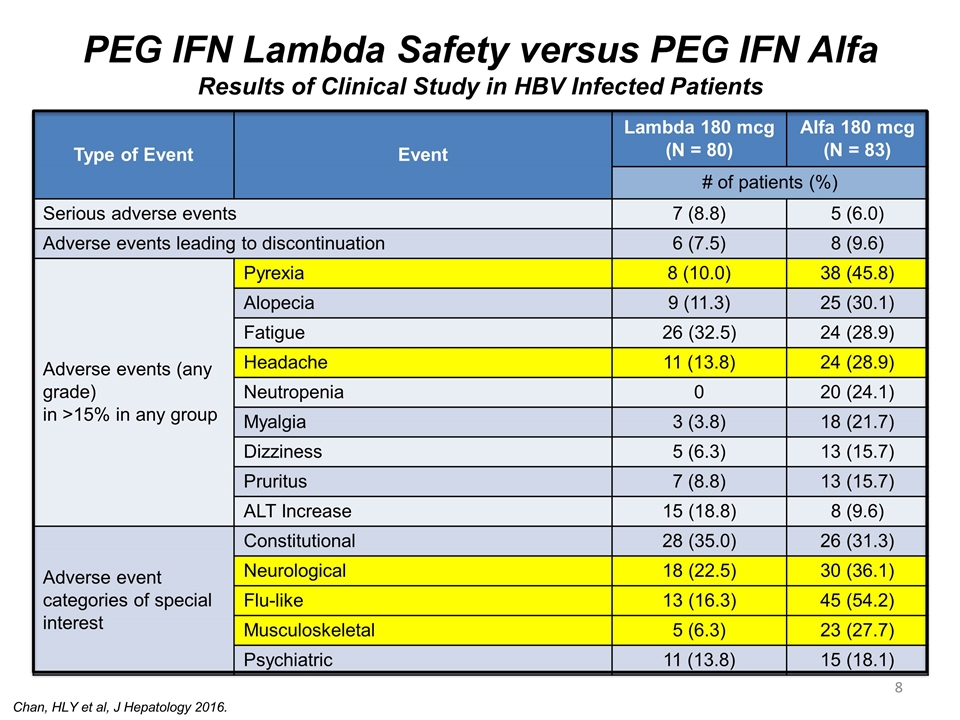
PEG IFN Lambda Safety versus PEG IFN Alfa Results of Clinical Study in HBV Infected Patients Type of Event Event Lambda 180 mcg (N = 80) Alfa 180 mcg (N = 83) # of patients (%) Serious adverse events 7 (8.8) 5 (6.0) Adverse events leading to discontinuation 6 (7.5) 8 (9.6) Adverse events (any grade) in >15% in any group Pyrexia 8 (10.0) 38 (45.8) Alopecia 9 (11.3) 25 (30.1) Fatigue 26 (32.5) 24 (28.9) Headache 11 (13.8) 24 (28.9) Neutropenia 0 20 (24.1) Myalgia 3 (3.8) 18 (21.7) Dizziness 5 (6.3) 13 (15.7) Pruritus 7 (8.8) 13 (15.7) ALT Increase 15 (18.8) 8 (9.6) Adverse event categories of special interest Constitutional 28 (35.0) 26 (31.3) Neurological 18 (22.5) 30 (36.1) Flu-like 13 (16.3) 45 (54.2) Musculoskeletal 5 (6.3) 23 (27.7) Psychiatric 11 (13.8) 15 (18.1) Chan, HLY et al, J Hepatology 2016.
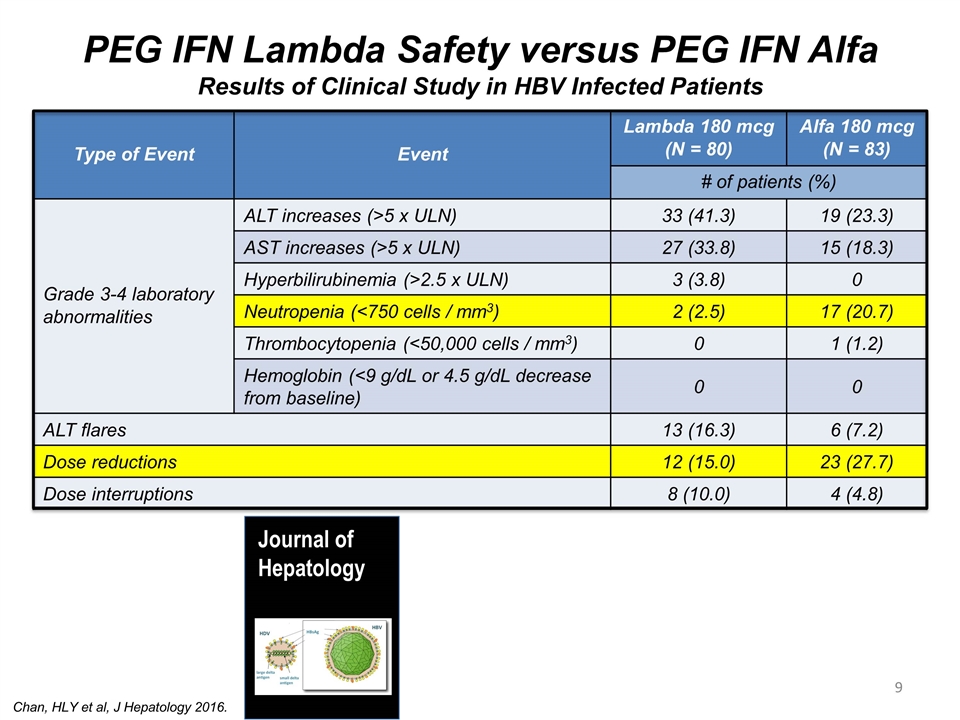
Type of Event Event Lambda 180 mcg (N = 80) Alfa 180 mcg (N = 83) # of patients (%) Grade 3-4 laboratory abnormalities ALT increases (>5 x ULN) 33 (41.3) 19 (23.3) AST increases (>5 x ULN) 27 (33.8) 15 (18.3) Hyperbilirubinemia (>2.5 x ULN) 3 (3.8) 0 Neutropenia (<750 cells / mm3) 2 (2.5) 17 (20.7) Thrombocytopenia (<50,000 cells / mm3) 0 1 (1.2) Hemoglobin (<9 g/dL or 4.5 g/dL decrease from baseline) 0 0 ALT flares 13 (16.3) 6 (7.2) Dose reductions 12 (15.0) 23 (27.7) Dose interruptions 8 (10.0) 4 (4.8) PEG IFN Lambda Safety versus PEG IFN Alfa Results of Clinical Study in HBV Infected Patients Chan, HLY et al, J Hepatology 2016. Journal of Hepatology
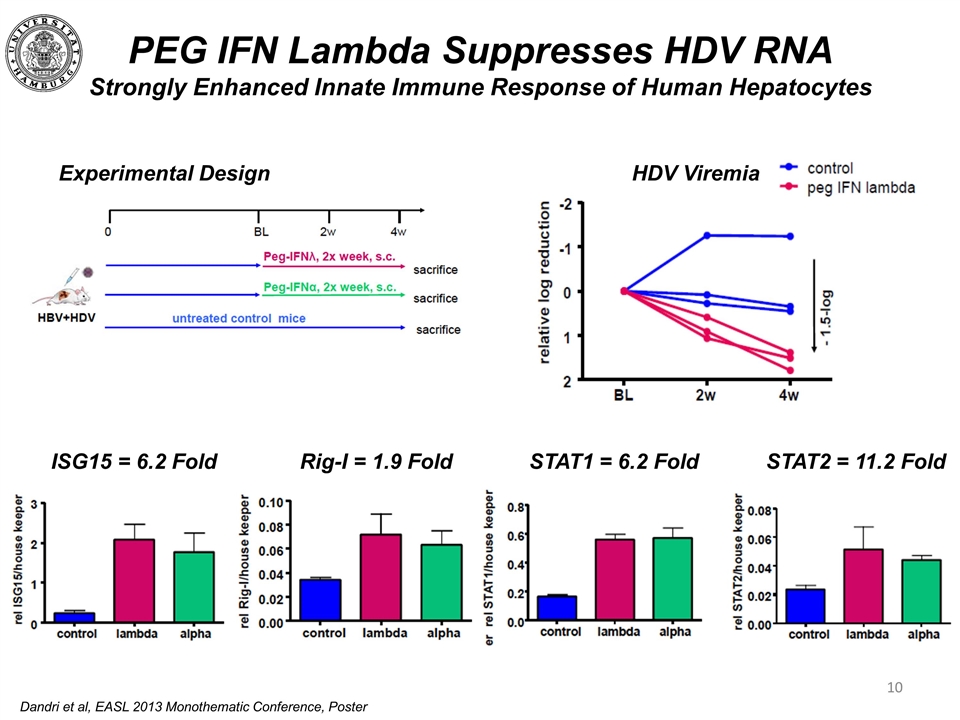
PEG IFN Lambda Suppresses HDV RNA Strongly Enhanced Innate Immune Response of Human Hepatocytes HDV Viremia Experimental Design ISG15 = 6.2 Fold STAT2 = 11.2 Fold Dandri et al, EASL 2013 Monothematic Conference, Poster Rig-I = 1.9 Fold STAT1 = 6.2 Fold
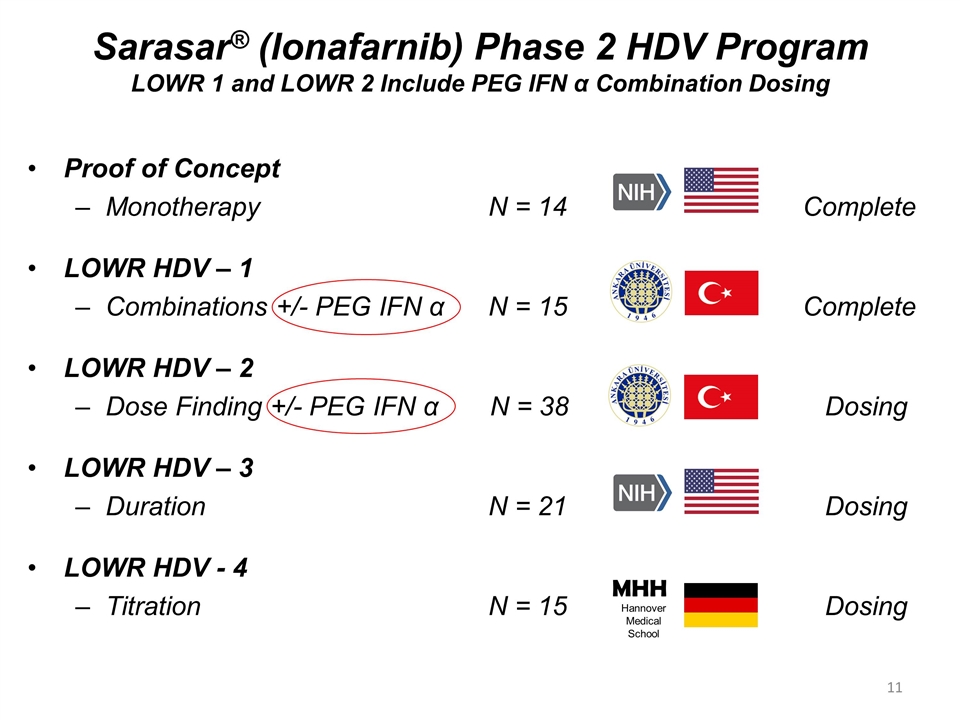
Sarasar® (lonafarnib) Phase 2 HDV Program LOWR 1 and LOWR 2 Include PEG IFN α Combination Dosing Proof of Concept Monotherapy N = 14 Complete LOWR HDV – 1 Combinations +/- PEG IFN α N = 15 Complete LOWR HDV – 2 Dose Finding +/- PEG IFN α N = 38 Dosing LOWR HDV – 3 Duration N = 21 Dosing LOWR HDV - 4 Titration N = 15 Dosing MHH Hannover Medical School
PEG IFN Lambda Plans Replace PEG IFN α in next Eiger HDV studies Efficiently study potential use as: An effective monotherapy in HDV An effective combination therapy with Lonafarnib in HDV Identify potential for better tolerability versus PEG IFN α in HDV Offer a proprietary interferon with more optimal efficacy / tolerability Apply for Orphan Designation & Fast Track status Create an HDV franchise opportunity at Eiger
PEG IFN Lambda Expected Timelines Drug Product on hand sufficient for Phase 2 Quantities may supply development through registration Monotherapy study in HDV to begin in 2H2016 Lambda alone dose ranging study Combination study in HDV to begin in 2H2016 Lonafarnib + Ritonavir + Lambda Efficient generation of Phase 2 POC data in 4Q2017 Multiple, international sites
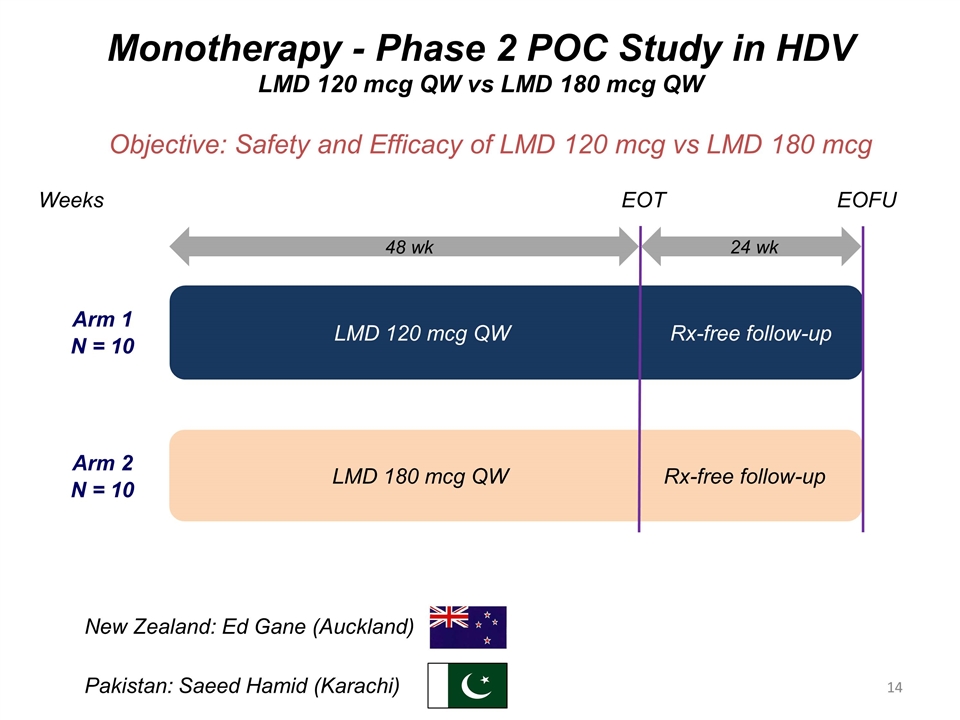
Monotherapy - Phase 2 POC Study in HDV LMD 120 mcg QW vs LMD 180 mcg QW Objective: Safety and Efficacy of LMD 120 mcg vs LMD 180 mcg LMD 180 mcg QW Rx-free follow-up Arm 1 N = 10 Arm 2 N = 10 LMD 120 mcg QW Rx-free follow-up EOT EOFU Weeks 48 wk 24 wk New Zealand: Ed Gane (Auckland) Pakistan: Saeed Hamid (Karachi)
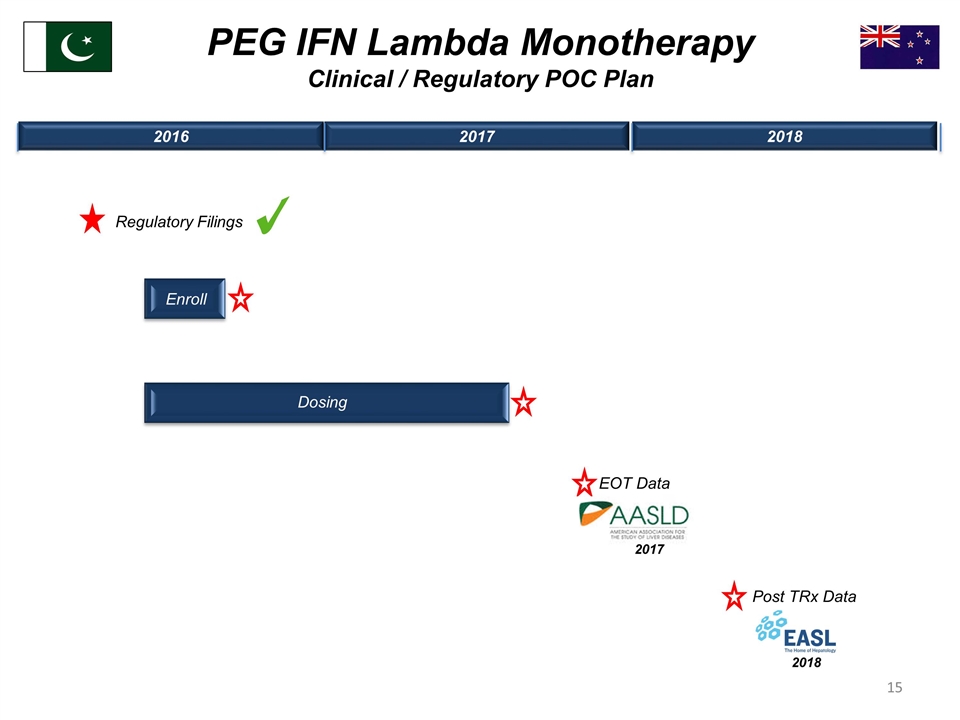
Enroll 2016 2017 2018 PEG IFN Lambda Monotherapy Clinical / Regulatory POC Plan Regulatory Filings EOT Data 2017 Post TRx Data 2018 Dosing
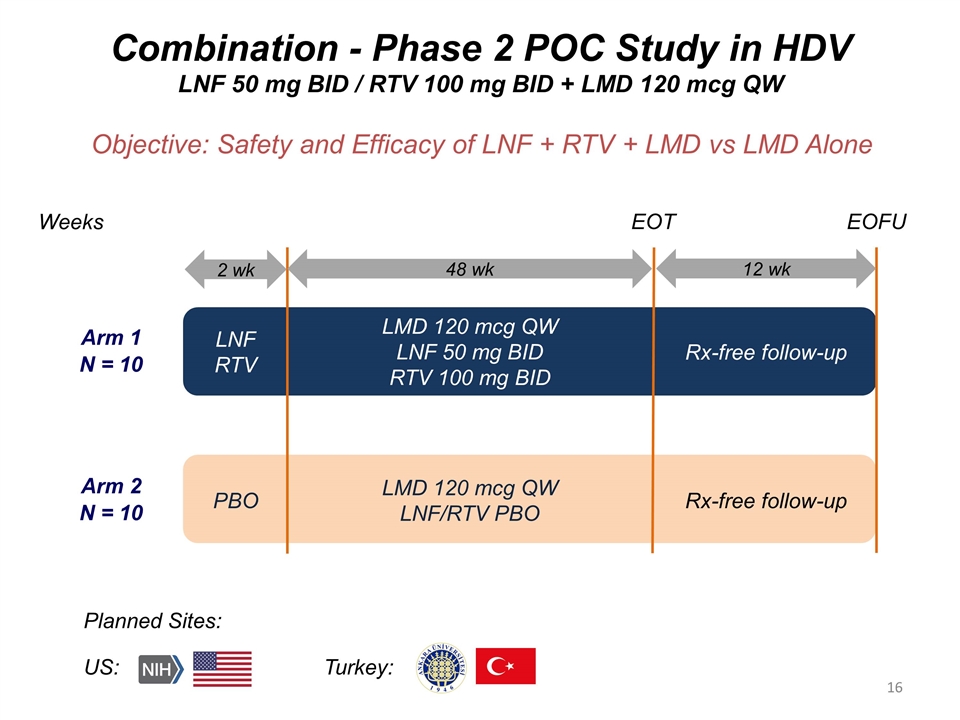
Combination - Phase 2 POC Study in HDV LNF 50 mg BID / RTV 100 mg BID + LMD 120 mcg QW Objective: Safety and Efficacy of LNF + RTV + LMD vs LMD Alone Arm 1 N = 10 Arm 2 N = 10 Weeks Planned Sites: US: Turkey: EOT EOFU 2 wk 48 wk 12 wk LNF RTV LMD 120 mcg QW LNF 50 mg BID RTV 100 mg BID Rx-free follow-up PBO LMD 120 mcg QW LNF/RTV PBO Rx-free follow-up
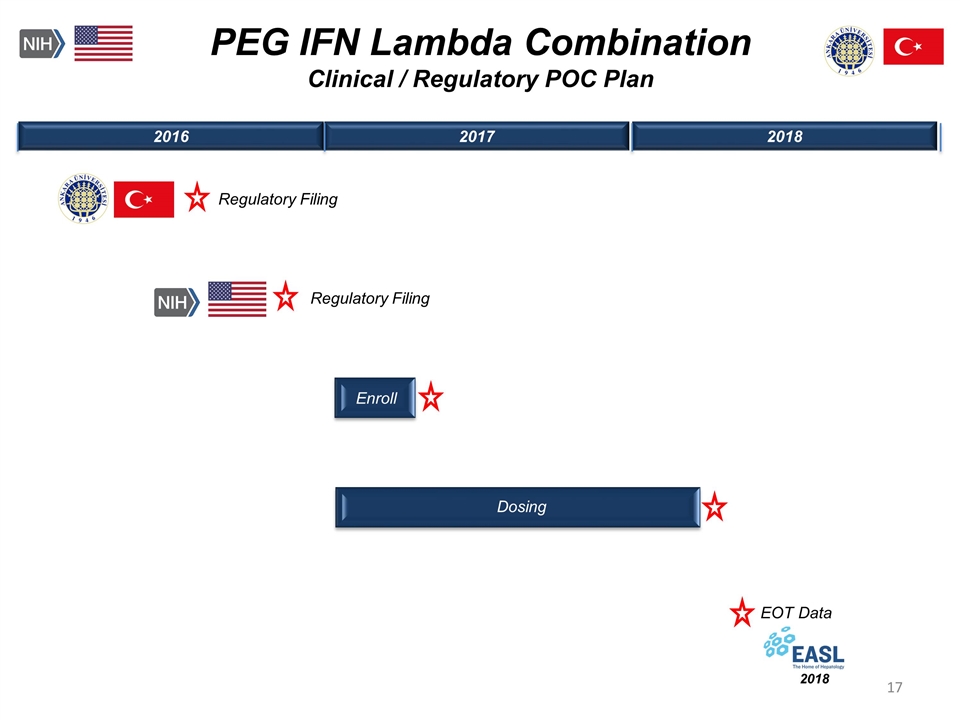
Enroll 2016 2017 2018 PEG IFN Lambda Combination Clinical / Regulatory POC Plan Regulatory Filing EOT Data 2018 Dosing Regulatory Filing
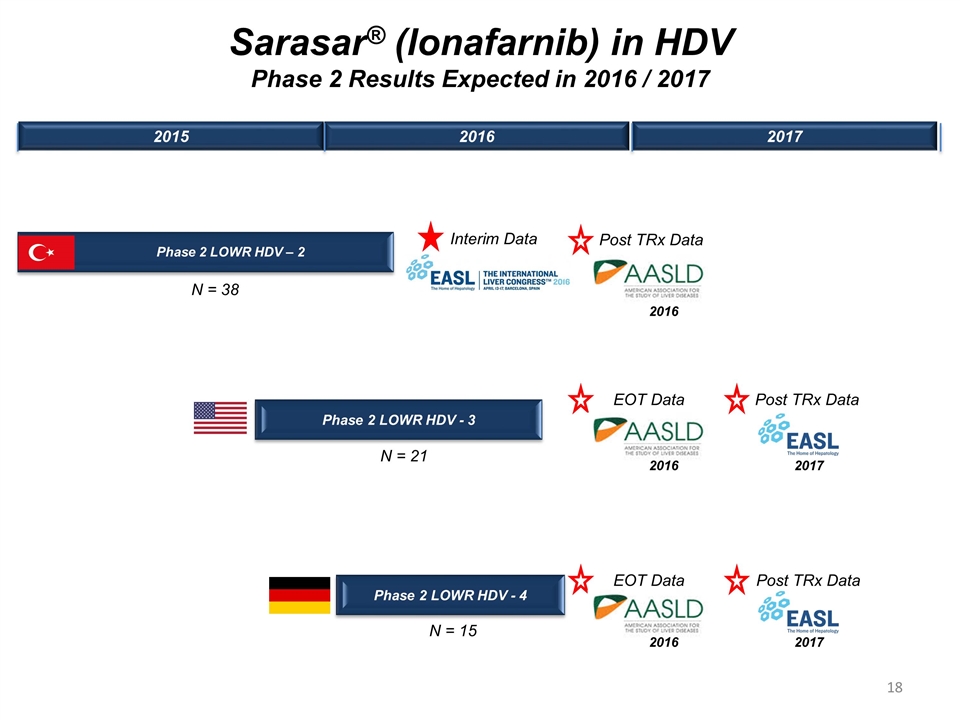
Phase 2 LOWR HDV – 2 Phase 2 LOWR HDV - 3 Interim Data 2015 2016 2017 Sarasar® (lonafarnib) in HDV Phase 2 Results Expected in 2016 / 2017 EOT Data Phase 2 LOWR HDV - 4 EOT Data 2016 2016 Post TRx Data 2016 Post TRx Data Post TRx Data 2017 2017 N = 38 N = 21 N = 15
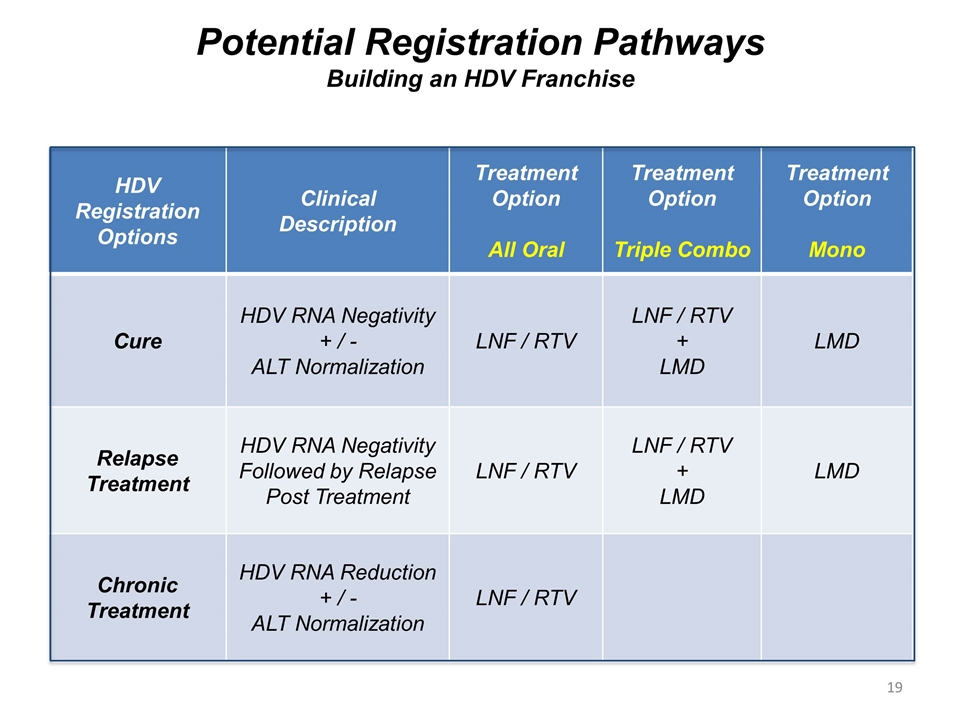
Potential Registration Pathways Building an HDV Franchise HDV Registration Options Clinical Description Treatment Option All Oral Treatment Option Triple Combo Treatment Option Mono Cure HDV RNA Negativity + / - ALT Normalization LNF / RTV LNF / RTV + LMD LMD Relapse Treatment HDV RNA Negativity Followed by Relapse Post Treatment LNF / RTV LNF / RTV + LMD LMD Chronic Treatment HDV RNA Reduction + / - ALT Normalization LNF / RTV

Building a Franchise in HDV Sarasar® (lonafarnib) Pegylated Interferon Lambda-1a
