Attached files
| file | filename |
|---|---|
| EX-21.1 - EX-21.1 - NAVIDEA BIOPHARMACEUTICALS, INC. | navb-ex211_8.htm |
| EX-23.1 - EX-23.1 - NAVIDEA BIOPHARMACEUTICALS, INC. | navb-ex231_9.htm |
| EX-32.2 - EX-32.2 - NAVIDEA BIOPHARMACEUTICALS, INC. | navb-ex322_14.htm |
| EX-32.1 - EX-32.1 - NAVIDEA BIOPHARMACEUTICALS, INC. | navb-ex321_13.htm |
| EX-31.1 - EX-31.1 - NAVIDEA BIOPHARMACEUTICALS, INC. | navb-ex311_15.htm |
| EX-31.2 - EX-31.2 - NAVIDEA BIOPHARMACEUTICALS, INC. | navb-ex312_16.htm |
| EX-24.1 - EX-24.1 - NAVIDEA BIOPHARMACEUTICALS, INC. | navb-ex241_10.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2015
or
|
¨ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE EXCHANGE ACT |
For the transition period from to to
Commission file number 001-35076
NAVIDEA BIOPHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware |
|
31-1080091 |
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
5600 Blazer Parkway, Suite 200, Dublin, Ohio |
|
43017-7550 |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant's telephone number, including area code (614) 793-7500
Securities registered pursuant to Section 12(b) of the Act:
|
Common Stock, par value $.001 per share |
|
NYSE MKT |
|
(Title of Class) |
|
(Name of Each Exchange on Which Registered) |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
o |
|
Accelerated filer |
x |
|
Non-accelerated filer |
o |
|
Smaller reporting company |
o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12-b-2 of the Act.) Yes o No x
The aggregate market value of shares of common stock held by non-affiliates of the registrant on June 30, 2015 was $239,805,940.
The number of shares of common stock outstanding on March 1, 2016 was 155,566,583.
DOCUMENTS INCORPORATED BY REFERENCE
None.
The Private Securities Litigation Reform Act of 1995 (the Act) provides a safe harbor for forward-looking statements made by or on behalf of the Company. Statements in this document which relate to other than strictly historical facts, such as statements about the Company’s plans and strategies, expectations for future financial performance, new and existing products and technologies, anticipated clinical and regulatory pathways, the ability to obtain, and timing of, regulatory approvals of the Company’s products, the timing and anticipated results of commercialization efforts, and anticipated markets for the Company’s products, are forward-looking statements within the meaning of the Act. The words “believe,” “expect,” “anticipate,” “estimate,” “project,” and similar expressions identify forward-looking statements that speak only as of the date hereof. Investors are cautioned that such statements involve risks and uncertainties that could cause actual results to differ materially from historical or anticipated results due to many factors including, but not limited to, the Company’s continuing operating losses, uncertainty of regulatory approvals for and market acceptance of its products, reliance on third party manufacturers, accumulated deficit, future capital needs, uncertainty of capital funding, dependence on limited product line and distribution channels, competition, limited marketing and manufacturing experience, risks of development of new products, and other risks set forth below under Item 1A, “Risk Factors.” The Company undertakes no obligation to publicly update or revise any forward-looking statements.
PART I
Item 1. Business
Development of the Business
Navidea Biopharmaceuticals, Inc. (Navidea, the Company, or we), a Delaware corporation (NYSE MKT: NAVB), is a biopharmaceutical company focused on the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. Navidea is developing multiple precision-targeted products based on our Manocept™ platform to help identify the sites and pathways of undetected disease and enable better diagnostic accuracy, clinical decision-making, targeted treatment and, ultimately, patient care.
Navidea’s Manocept platform is predicated on the ability to specifically target the CD206 mannose receptor expressed on activated macrophages. The Manocept platform serves as the molecular backbone of Lymphoseek® (technetium Tc 99m tilmanocept) injection, the first product developed by Navidea based on the platform. Lymphoseek is a novel, state-of-the-art, receptor-targeted, small-molecule radiopharmaceutical used in the evaluation of lymphatic basins that may have cancer involvement in patients. Lymphoseek is designed for the precise identification of lymph nodes that drain from a primary tumor, which have the highest probability of harboring cancer. Lymphoseek is approved by the U.S. Food and Drug Administration (FDA) for use in solid tumor cancers where lymphatic mapping is a component of surgical management and for guiding sentinel lymph node biopsy in patients with clinically node negative breast cancer, melanoma or squamous cell carcinoma of the oral cavity. Lymphoseek has also received European approval in imaging and intraoperative detection of sentinel lymph nodes in patients with melanoma, breast cancer or localized squamous cell carcinoma of the oral cavity.
Building on the success of Lymphoseek, the flexible and versatile Manocept platform acts as an engine for the design of purpose-built molecules offering the potential to be utilized across a range of diagnostic modalities, including single photon emission computed tomography (SPECT), positron emission tomography (PET), intra-operative and/or optical-fluorescence detection in a variety of disease states.
Recent preclinical data generated by the Company in studies using tilmanocept linked to a therapeutic agent also suggest that tilmanocept’s binding affinity to CD206 receptors demonstrates the potential for this technology to be useful in treating diseases linked to the over-activation of macrophages. This includes various cancers as well as autoimmune, infectious, cardiovascular, and central nervous system diseases. Our efforts in this area were further supported by the January 2015 formation of Macrophage Therapeutics, Inc., a majority-owned subsidiary that was formed specifically to further explore therapeutic applications for the Manocept platform.
Our focus on development of our proprietary Manocept platform technology further supports the 2014 decision by the Company’s Board of Directors to reduce our support for, while seeking to partner or out-license, our two neurological development programs, NAV4694 and NAV5001. The NAV5001 sublicense was terminated in April 2015.
Other than Lymphoseek, none of the Company’s drug product candidates have been approved for sale in any market.
A Brief Look at Our History
We were originally incorporated in Ohio in 1983 and reincorporated in Delaware in 1988. From inception until January 2012, we operated under the name Neoprobe Corporation. In January 2012, we changed our name to Navidea Biopharmaceuticals, Inc. in connection with both the sale of our medical device business and our strategic repositioning as a precision medicines company focused on “NAVigating IDEAs” that result in the development and commercialization of precision diagnostic and therapeutic pharmaceuticals.
2
Since our inception, the majority of our efforts and resources have been devoted to the research and clinical development of radiopharmaceutical technologies primarily related to the intraoperative diagnosis and treatment of cancers. From the late 1990’s through 2011, we also devoted substantial effort towards the development and commercialization of medical devices, including a line of handheld gamma detection devices which was sold in 2011 and a line of blood flow measurement devices which we operated from 2001 through 2009.
From our inception through August 2011, we manufactured a line of gamma radiation detection medical devices called the neoprobe® GDS system (the GDS Business). We sold the GDS Business to Devicor Medical Products, Inc. (Devicor) in August 2011. In exchange for the assets of the GDS Business, Devicor made net cash payments to us totaling $30.3 million, assumed certain liabilities of the Company associated with the GDS Business, and agreed to make royalty payments to us of up to an aggregate maximum amount of $20 million based on the net revenue attributable to the GDS Business through 2017. Based on the 2015 GDS Business revenue, we earned royalty payments of $1.2 million which we expect to receive in March 2016. We did not earn or receive any such royalty payments prior to 2015.
Following the sale of the GDS business and the subsequent strategic repositioning as a precision medicines company, the Company in-licensed the two neuro-tracer product candidates, NAV4694 and NAV5001. The Company progressed the development of both product candidates over the course of 2012 through 2014, moving both into Phase 3 clinical trials. However, in May 2014, the Navidea Board announced that, based on its belief that the public markets were not giving appropriate value to its Phase 3 pipeline products and were likely penalizing the Company for allocating resources to these programs, the Company would be restructuring its development efforts to focus on cost effective development of the Manocept platform while it sought development partners for NAV4694 and NAV5001. In April 2015, the Company entered into an agreement with Alseres Pharmaceuticals, Inc. (Alseres) to terminate the NAV5001 sub-license agreement. The Company is currently engaged in discussions related to the potential partnering or divestiture of NAV4694.
In December 2014, we announced the formation of a new business unit, Macrophage Therapeutics, to further explore therapeutic applications for the Manocept platform, which was incorporated as Macrophage Therapeutics, Inc. in January 2015.
Our Technology and Product Candidates
Our primary development efforts over the last few years have been focused on diagnostic products including our now-approved Lymphoseek product, as well as other diagnostic and therapeutic line extensions based on our Manocept platform, while we have sought to partner or divest our two neuro-imaging product candidates. Efforts to partner or divest NAV4694 are still active, while the in-license of NAV5001 we had with Alseres was terminated in April 2015.
Navidea remains committed to realizing the full potential of Lymphoseek. In mid-2015, we deployed our own field sales force and began implementing a new strategy to accelerate the strong year-over-year growth of this product. The Company believes that the resources being devoted to drive Lymphoseek sales will lead to positive cash flows and profitability. We are focused on expanding the market for Lymphoseek in all relevant markets.
The Company also continues working to establish new sources of non-dilutive funding, including collaborations and grant funding that can augment the balance sheet as the Company works to reduce spending to levels that can be increasingly offset by growing Lymphoseek revenue. In particular, substantial progress on the Manocept platform has resulted in several promising opportunities, including our R-NAV, LLC venture which began in July 2014, the formation of Macrophage Therapeutics, Inc. in January 2015, and Macrophage Therapeutics' research collaboration agreement with BIND Therapeutics, Inc. executed in June 2015.
Lymphoseek – Regulatory Background
Lymphoseek is a lymph node targeting radiopharmaceutical agent intended for use in intraoperative lymphatic mapping procedures and lymphoscintigraphy employed in the overall diagnostic assessment of certain solid tumor cancers. Lymphoseek has the potential to provide oncology surgeons with information to identify key predictive lymph nodes that may harbor cancer and to help avoid the unnecessary removal of non-cancerous lymph nodes and the surrounding tissue in patients with a variety of solid tumor cancers. Lymphoseek was approved and indicated for use in lymphatic mapping for breast cancer and melanoma by the FDA in March 2013. In June 2014, the FDA approved a supplemental New Drug Application (sNDA) for the expanded use of Lymphoseek indicated for guiding sentinel lymph node biopsy in head and neck cancer patients with squamous cell carcinoma of the oral cavity. In September 2014, the FDA granted Orphan Drug Designation for use in sentinel lymph node detection in patients with cancer of the head and neck. This designation provides for a seven-year market exclusivity period in this indication as well as certain incentives, including federal grants, tax credits and a waiver of filing fees. In October 2014, the FDA approved a second sNDA for lymphatic mapping in solid tumors and added sentinel lymph node detection for breast cancer and melanoma to the approved indications. The FDA also allowed expanded utilization of Lymphoseek with or without scintigraphic imaging, known as lymphoscintigraphy, to enable pre-operative imaging and mapping of lymph nodes to facilitate node localization during surgical procedures. Lymphoseek is now the first and only FDA-approved radiopharmaceutical agent for sentinel lymph node detection and is the only FDA-approved agent for
3
lymphatic mapping of solid tumors. Additional trials, including trials in anal/rectal, endometrial, and cervical cancers, and others in various stages of execution, planning or consideration, are anticipated to provide additional data to potentially support expansion of the Lymphoseek opportunity.
We submitted our Marketing Authorization Application (MAA) for Lymphoseek to the European Medicines Agency (EMA) in December 2012. In September 2014, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion recommending marketing authorization for Lymphoseek for use in the EU in imaging and intraoperative detection of sentinel lymph nodes draining a primary tumor in adult patients with breast cancer, melanoma, or localized squamous cell carcinoma of the oral cavity. The CHMP's positive opinion was reviewed by the European Commission (EC), which has the authority to approve medicinal products for use in the 28 countries of the EU and generally follows the recommendations of the CHMP. The EC granted marketing authorization for Lymphoseek in the EU in November 2014. Our partner, SpePharm AG, is currently performing the customary pre-launch market access activities to support commercial launch in the EU later in 2016. Concurrently, we are completing manufacturing validation activities on a finished drug product contract manufacturing facility to support the Company’s supply chain, primarily in Europe.
Lymphoseek - Clinical Data and Licensing Background
In January 2015, we announced that an analysis comparing sentinel lymph node (SLN) biopsy procedures using Lymphoseek (TcTM) + vital blue dye (VBD) to filtered [99mTc] sulfur colloid (fTcSC) + VBD in breast cancer patients was published in the Annals of Surgical Oncology. Results demonstrated that (i) Lymphoseek patients had significantly fewer SLNs removed per procedure (mean TcTM: 1.85 vs. fTcSC: 3.24, p < 0.0001); (ii) proportionally fewer nodes were necessary to detect cancer spread; and (iii) nodes removed using Lymphoseek held greater predictive value for diagnosing the spread of breast cancer to lymph nodes. The study, “Comparison of [99mTc]Tilmanocept and Filtered [99mTc]Sulfur Colloid for Identification of SLNs in Breast Cancer Patients,” authored by Anne Wallace, M.D., et. al., at the UC San Diego School of Medicine was published in the January print issue of the journal Annals of Surgical Oncology.
In February 2015, we announced the peer-reviewed publication of results from a Phase 3 clinical trial of Lymphoseek in patients with certain head and neck cancer in the journal Annals of Surgical Oncology. The trial assessed the performance of Lymphoseek-guided sentinel node biopsy against the standard of care, nodal pathology, in planned elective neck dissection. Results demonstrated that Lymphoseek met the primary efficacy endpoint of accurately identifying sentinel lymph nodes in subjects with node-negative squamous cell carcinoma of the oral cavity, as compared to the removal of all lymph nodes during multiple level nodal dissection surgery of the head and neck. Pathology assessment of lymph nodes from the multiple-level nodal dissection surgery is considered the “gold standard” to determine the presence and extent of cancer spread. The study, “[99mTc]Tilmanocept Accurately Detects Sentinel Lymph Nodes and Predicts Pathology Status in Patients with Oral Squamous Cell Carcinoma of the Head and Neck: Results of a Phase III Multi-Institutional Trial” was published as an Online First article in the journal Annals of Surgical Oncology. Data from this study were previously presented in part at the 2013 Society of Nuclear Medicine and Molecular Imaging Annual Meeting (Vancouver, British Columbia), at the 2013 American College of Surgeons Clinical Congress (Washington, DC), and at the 6th European Congress on Head and Neck Oncology-2014 (Liverpool, UK).
In June 2015, results of an investigator-initiated, comparative study of Lymphoseek versus fTcSC measuring injection site pain in patients with breast cancer undergoing lymphoscintigraphy were presented at the 2015 Society of Nuclear Medicine and Molecular Imaging conference. The results of the trial, led by Anne Wallace, M.D., professor of surgery at University of California, San Diego (UCSD) School of Medicine, highlighted that fTcSC caused statistically significant greater levels of pain after injection compared to Lymphoseek. The randomized, double-blind clinical trial compared post-injection site pain using fTcSC versus Lymphoseek in 52 [(27) fTcSC and (25) Lymphoseek] breast cancer patients undergoing lymphoscintigraphy. Pain was evaluated with a visual analogue scale and short form McGill Pain Questionnaire at 1, 2, 3, 4, 5, 15 and 30 minutes post-injection. Analysis of the data indicates baseline pain scores were similar between groups. At one minute post-injection, patients receiving fTcSC experienced a mean change in pain of 16.8mm (standard deviation (SD) 19.5) compared to 0.2mm (SD 7.3) in the Lymphoseek group (p =0.0002). Overall, patients receiving Lymphoseek experienced statistically significant less change in pain scores compared to patients receiving fTcSC at 1-3 minutes post-injection.
In July 2015, we announced the peer-reviewed publication of data verifying the Lymphoseek CD206-binding mechanism of action in the Journal of Immunology. Strong evidence-based studies demonstrate macrophages are the major target cell and identify CD206, the mannose receptor, as the tilmanocept-binding receptor. CD206 is highly expressed on the surface of tissue macrophages that are known to reside in the sentinel lymph nodes (SLNs) draining a primary tumor.
In August 2015, we announced the publication of the results from an investigator-initiated, comparative study of Lymphoseek versus filtered Tc-99m Sulfur Colloid (fTcSC) measuring injection site pain in patients with breast cancer undergoing lymphoscintigraphy. The paper, titled “Comparison of Post-injection Site Pain Between Technetium Sulfur Colloid and Technetium Tilmanocept in Breast Cancer Patients Undergoing Sentinel Lymph Node Biopsy,” was published online in the Annals of Surgical Oncology and indicated, with patient-reported data, a statistically significant reduction in the level of post-injection associated pain using Lymphoseek
4
compared with use of an fTcSC tracer. The publication included results of the randomized, double-blind clinical trial comparing post-injection site pain using fTcSC versus Lymphoseek in 52 [(27) fTcSC and (25) Lymphoseek] breast cancer patients undergoing lymphoscintigraphy. Pain was evaluated with a visual analogue scale and short form McGill Pain Questionnaire at 1, 2, 3, 4, 5, 15 and 30 minutes post-injection. Analysis of the data indicated baseline pain scores were similar between groups. At one minute post-injection, patients receiving fTcSC experienced a mean change in pain of 16.8mm (standard deviation (SD) 19.5) compared to 0.2mm (SD 7.3) in the Lymphoseek group (p =0.0002). Overall, patients receiving Lymphoseek experienced statistically significant less change in pain scores compared to patients receiving fTcSC at 1-3 minutes post-injection.
In December 2015, we announced that results from an investigator-initiated imaging study demonstrated Lymphoseek reduced imaging time by more than 50% in SLN biopsy procedures in malignant melanoma compared to Tc99m sulfur colloid (SC). This finding suggests hospitals and oncology treatment teams can achieve greater patient throughput and workflow efficiencies utilizing Lymphoseek. Results of the study, conducted at the Thomas Jefferson University Hospitals and led by Charles M. Intenzo, M.D., Professor of Radiology, Director, Nuclear Medicine and Molecular Imaging in the Department of Radiology, were presented at the Radiological Society of North America Annual Meeting (RSNA 2015) in Chicago, Illinois. A total of 34 consecutive patients with malignant melanoma underwent SLN mapping with Lymphoseek. Patients received the Lymphoseek dose in 4 intradermal administrations around the tumor site. Images were acquired at intervals up to 40 minutes after injection, which is the department’s standard-of-care protocol used for SC procedures. This site’s previous experience showed that SC injections required 40 to 45 minutes after injection for visualization of all lymph nodes in patients with malignant melanoma. Using Lymphoseek, the results show that in all 34 patients, all lymph nodes seen in the final 40-minute image were identified in the 20-minute image, providing rapid and stable localization and identification of the sentinel nodes. The study concludes that in malignant melanoma, SLN mapping with Lymphoseek involves a total imaging time of 20 minutes which is one-half of the time required for SC. From a clinical perspective, the authors conclude that utilizing Lymphoseek is more time-efficient than SC by facilitating patient throughput and expediting subsequent transport to the operating room.
Also in December 2015, we announced that results from an investigator-initiated retrospective analysis demonstrated Lymphoseek was successful in lymph node identification rate, node-positivity rate, and number of total nodes evaluated in SLN biopsy procedures in clinically node-negative breast cancer patients undergoing neoadjuvant chemotherapy (NAT) compared to patients undergoing initial surgical treatment. These findings suggest that Lymphoseek offers breast surgeons the confidence to specifically identify and remove sentinel lymph nodes in this patient population. Results of the study conducted at the University of California, San Diego, School of Medicine, led by Anne Wallace M.D., Professor of Surgery, and Jonathan Unkart, M.D., Department of Surgery, UC San Diego Health, were presented at the San Antonio Breast Cancer Conference in San Antonio, Texas. The aim of the study was to compare identification rate, node-positivity rate and total number of nodes evaluated during SLN biopsy with Lymphoseek and VBD in clinically node-negative patients receiving neoadjuvant endocrine or chemotherapy versus initial surgical treatment. A retrospective review of patients undergoing SLN biopsy with Lymphoseek plus VBD from May 2013-2015 at UCSD was conducted. Of the 417 total SLN cases identified, 72 (17.2%) cases were in patients who had received NAT (61- chemo, 11- endocrine). The SLN identification rate was 100% in both groups (p=1.0). Overall, there were 68 (16.3%) cases of SLN-positivity, 14 (19.4%) in the NAT group versus 54 (15.7%) in the non-NAT group (p= 0.54). The median number of identified nodes was 3 in both groups. In the a zero-truncated negative binomial count model, age, surgeon and evaluating pathologist were significant predictors of the total number of SLNs evaluated. The use of NAT did not significantly affect the number of SLNs evaluated. These findings show Lymphoseek’s usefulness in the complicated NAT population and that the outcomes are not different from the standard breast cancer population. This analysis provides compelling evidence that Lymphoseek was successfully used for SLN biopsy in the breast cancer NAT population and could potentially reduce the necessity for unnecessary and morbid axillary dissections, and improve the quality of life for patients.
In January 2016, we announced that the first pediatric patient was enrolled in a clinical study comparing Lymphoseek and VBD in a pediatric population of patients with melanoma, rhabdomyosarcoma, or other solid tumors. The study is designed to investigate how Lymphoseek compares with VBD in identifying lymph nodes as well as evaluate safety and tolerability in the pediatric population. Lymphoseek is currently approved for adult use only. Enrollment is currently planned at approximately six sites throughout the U.S. The first patient was enrolled by Jennifer Aldrink, M.D., Assistant Professor of Clinical Surgery at The Ohio State University College of Medicine and Director of Surgical Oncology, Division of Pediatric Surgery at Nationwide Children's Hospital in Columbus, Ohio. Primary goals of this prospective, open-label, multicenter study are to evaluate safety and tolerability of Lymphoseek in this subject population and determine the concordance of in vivo detection rates of Lymphoseek and of VBD in tissue excised and histologically confirmed as lymph nodes. In addition, the study will measure other efficacy signals including assessment of the identified lymph node(s) to confirm: the presence/absence of tumor metastases; agent localization per tumor type; degree of localization (nodes per subject both intraoperatively and with preoperative SPECT/CT); reverse concordance parameters; change of subject stage based on histopathology and descriptive assessment on change in treatment plan; and number of lymph nodes detected with Lymphoseek intraoperatively compared with preoperative SPECT/CT imaging.
In February 2016, we announced enrollment of the first patient in a clinical study evaluating Lymphoseek in women with known cervical cancer. The study, funded by a Fast Track grant from the National Institutes of Health (NIH), will assess the use of Lymphoseek in SLN biopsy during cervical cancer surgery in support of the existing Lymphoseek label in lymphatic mapping.
5
Enrollment is currently planned in up to six sites throughout the U.S. The first patient was enrolled by Michael M. Frumovitz, M.D., M.P.H., Associate Professor, Department of Gynecologic Oncology and Reproductive Medicine, principal investigator at The University of Texas MD Anderson Cancer Center. This multi-center, prospective, open-label study intends to enroll up to 40 women with International Federation of Gynecology and Obstetrics IA2-IIA1 staging. Subjects will receive a single dose of Lymphoseek administered peritumorally approximately 1-2 hours before surgery. The results will report per-patient false negative rates and compare the pathology status of Lymphoseek-identified sentinel lymph nodes relative to the pathology status of non-sentinel lymph nodes in nodal staging of patients. Additionally, the study will report sensitivity, negative predictive value, and accuracy.
Manocept Platform - Diagnostics and Therapeutics Background
Navidea’s Manocept platform is predicated on the ability to specifically target the CD206 mannose receptor expressed on activated macrophages. Activated macrophages play important roles in many disease states and are an emerging target in many diseases where diagnostic uncertainty exists. This flexible and versatile platform serves as an engine for purpose-built molecules that may significantly impact patient care by providing enhanced diagnostic accuracy, clinical decision-making, and target-specific treatment. This disease-targeted drug platform provides the capability to utilize a breadth of diagnostic modalities, including SPECT, PET, intra-operative and/or optical-fluorescence detection, as well as delivery of therapeutic compounds that target macrophages, and their role in a variety of immune- and inflammation-based disorders. The Company’s FDA-approved sentinel node/lymphatic mapping agent, Lymphoseek, is representative of the ability to successfully exploit this mechanism to develop powerful new products.
Impairment of the macrophage-driven disease mechanisms is an area of increasing focus in medicine. The number of people affected by all the inflammatory diseases combined is estimated at more than 40 million in the United States and perhaps 700 million worldwide, making macrophage-mediated diseases an area of remarkable clinical importance. There are many recognized disorders having macrophage involvement, including rheumatoid arthritis (RA), atherosclerosis/vulnerable plaque, Crohn’s disease, systemic lupus erythematosis, Kaposi’s sarcoma (KS), and others that span clinical areas in oncology, autoimmunity, infectious diseases, cardiology, central nervous system (CNS) diseases, and inflammation. Data from studies using agents from the Manocept platform in RA, KS and tuberculosis (TB) were published in a special supplement, Nature Outlook: Medical Imaging, in Nature’s October 31, 2013 issue. The supplement included a White Paper by Navidea entitled “Innovations in receptor-targeted precision imaging at Navidea: Diagnosis up close and personal,” focused on the Manocept platform.
Manocept Platform - Diagnostics Clinical Data
In February 2014, data utilizing compounds from our Manocept platform in models of RA were presented by representatives from The Ohio State University at a Keystone Symposia on Molecular Cell Biology of Macrophages in Human Disease. The studies demonstrate the ability of fluorescent Cy3-tilmanocept to identify and localize to disease-state macrophages when administered intravenously, enabling detection of immune-mediated arthritis in affected joints in vivo in mice. Results were confirmed using histopathology. The data highlighted the identification of immune-mediated inflammation seen in arthritic joints of arthritis-affected mice but not in control mice or un-affected joints within arthritis-affected mice. The imaging results in this study showed preferential localization of macrophages by Cy3-tilmanocept in affected joints with little to no localization in unaffected joints.
In April 2014, collaborators from the University of California, San Francisco presented results at the 2014 American Association for Cancer Research conference, highlighting the potential utility of imaging agents derived from the Manocept platform in identifying affected tissues and lymph nodes in patients with KS. The investigators concluded that, based on the results obtained, labeled imaging agents from the CD206-targeting Manocept platform provide potential avenues to enhance diagnosis and staging in this disorder.
In July 2014, Navidea announced that it formed a joint enterprise with Essex Woodlands-backed Rheumco, LLC, to develop and commercialize radiolabeled diagnostic and therapeutic products for rheumatologic and arthritic diseases. The joint enterprise, called R-NAV, LLC (R-NAV), will combine Navidea’s proprietary Manocept CD206 macrophage targeting platform and Rheumco’s proprietary Tin-117m radioisotope technology to focus on leveraging the platforms across several indications with high unmet medical need.
Also in July 2014, the Company completed a license agreement with UCSD for the exclusive world-wide rights in all diagnostic and therapeutic uses of tilmanocept. The license agreement is effective until the third anniversary of the expiration date of the longest-lived underlying patent. Under the terms of the license agreement, UCSD has granted Navidea the exclusive rights to make, use, sell, offer for sale and import licensed products for all diagnostic and therapeutic uses as defined in the agreement and to practice the defined licensed methods during the term of the agreement. Navidea may also sublicense the patent rights, subject to certain sublicense terms as defined in the agreement. In consideration for the license rights, Navidea agreed to pay UCSD a license issue fee of $25,000 and license maintenance fees of $25,000 per year. We also agreed to make payments to UCSD upon successfully reaching certain clinical, regulatory and cumulative sales milestones, and a royalty on net sales of licensed products subject to a $25,000 minimum annual royalty. Navidea also agreed to reimburse UCSD for all patent-related costs and to meet certain diligence targets.
6
In June 2015, results from several pre-clinical Manocept studies in RA were presented at the EULAR 2015 European Congress of Rheumatology. The results of the studies, led by Wael Jarjour, M.D. and Thomas J. Rosol, D.V.M., Ph.D., of The Ohio State University Wexner Medical Center and College of Veterinary Medicine, respectively, highlighted the potential of CD206-targeting Manocept constructs to detect immune-mediated inflammation in RA which could be used diagnostically, to monitor therapeutic efficacy, or as a potential therapeutic platform. The presentation showed results from synovial fluid and tissue acquired from RA patients for comparison to normal frozen archival tissue and synovial tissue procured from patients with osteoarthritis (OA). Tissues were probed with Manocept-Cy3, DAPI nuclear stain, and anti CD206-cyanine. Mononuclear cells were isolated from RA synovial fluid and analyzed by flow cytometry. Results demonstrated that archival synovial tissue and synovial fluid obtained from patients diagnosed with RA contain a significant population of macrophages that express high levels of the CD206 receptor. It was shown that these macrophages strongly co-localize Manocept-Cy3 and CD206 receptors. The degree of macrophage infiltration in tissue from healthy or osteoarthritic patients was significantly lower than in RA tissues. Additionally, in an in-vivo animal study, arthritis was induced in mice and was followed with intravenous injection of Manocept-Cy3 and epi-fluorescent imaging. Imaging results indicated that Manocept can be detected in inflamed joints in an in vivo animal model of RA.
In July 2015, imaging results from the Manocept clinical trial in KS and other preclinical studies were presented at the 18th International Workshop on Kaposi's Sarcoma Herpesvirus (KSHV) and Related Agents. The clinical imaging study, using Tc 99m tilmanocept in both HIV+ and HIV- patients suggests that KS tumor lesions, both cutaneous and suspected extra-cutaneous sites, can be easily visualized and mapped, demonstrating that this technique may potentially provide a means for routine patient assessment. The results also demonstrate that use of Manocept represents a potential therapeutic pathway for targeting tumor-associated macrophages (TAMs). Manocept agents are designed to target CD206, which is highly expressed on TAMs and the KS tumor itself. As a potential therapeutic, Manocept could be used as a precision vehicle to deliver payloads to tumor sites throughout the body. Five Human Herpes Virus8 positive (HHV8+) patients (4 HIV+, 1HIV-) were enrolled in the NAV3-12 study. Patients received a single subcutaneous injection of Tc 99m tilmanocept in the region of a cutaneous KS lesion and imaging was performed at 1, 4 and 24 hours post-injection to visualize localization of tilmanocept. Results represented by whole body SPECT/CT imaging scans from study patients were presented. Collectively, the scans show localization of tilmanocept specifically in KS and detected multiple cutaneous lesions in the extremities, as well as extra-cutaneous localization found in the nasopharynx, lymph nodes and brain. Results also indicate that KS lesions are anatomically linked in chains by and within the lymph ducts. The study concludes that both HIV+ and HIV- patients have pan-tumor expression of CD206, strongly suggests tilmanocept crosses the blood-brain barrier and that a Manocept-drug conjugate may have the potential as a therapeutic with high target effect and low off-target concerns. The data from these studies also suggest a novel theory on the genesis of KS in which KS arises from an HHV8 infected macrophage type cell and its interaction with the lymphatic system. This interaction provides the means for access of the KS through CD206 receptor for diagnosis, evaluation, and potential therapy using the Manocept platform.
In July 2015, we received a notice of award for a Phase 1 Small Business Innovation Research (SBIR) grant providing $322,000 from the National Heart Lung and Blood Institute, NIH. The study, currently ongoing in collaboration with Massachusetts General Hospital and Harvard Medical School, will examine the ability of Tc 99m tilmanocept to localize in high-risk atherosclerotic plaques. These specific plaques are rich in CD206 expressing macrophages and are at high risk for near term rupture resulting in myocardial infarctions, sudden cardiac death and strokes. The consequences of atherosclerosis and the cardiovascular disease that atherosclerosis causes, while severe in all populations of people, are particularly concentrated in HIV+ patients. Recently, it has been observed that CD206 expressing macrophages densely populate vulnerable plaques or thin cap fibroatheromas but not other kinds (i.e., calcified plaques) of atherosclerotic plaques. A primary goal for this grant involves an approved clinical investigation of up to 18 individuals with and without aortic and high risk coronary atherosclerotic plaques and with and without HIV infection to determine the feasibility of Tc 99m tilmanocept to image high risk plaque by SPECT/CT. Contrast with NaF18 is a parallel evaluation. Results have the potential to provide evidence of the potential of Tc 99m tilmanocept to accumulate in high risk morphology plaques, the ability to make preliminary comparisons of aortic Tc 99m tilmanocept uptake by SPECT/CT in each group, and to evaluate the ability of Tc 99m tilmanocept to identify the same aortic atherosclerotic plaques that are identified by contrast enhanced coronary computed tomography angiography and/or PET/CT.
Also in July 2015, we received an initial notice of award for a Fast Track SBIR grant providing for up to $1.7 million from the NIH's National Institute of Arthritis and Musculoskeletal and Skin Diseases, to fund preclinical animal studies and a Phase 1/2 human clinical study examining the ability of Tc 99m tilmanocept to identify skeletal joints that are inflamed due to RA. RA is a chronic, progressive, systemic autoimmune disease characterized by inflammation of numerous skeletal joints. If not treated successfully, RA can lead to disability, disfigurement and premature death. The funds for this Fast Track grant will be released in two parts, which together have the potential to provide a total of $1.7 million in resources over two and a half years to achieve the specific aims and objectives of the grant. The first part will provide $225,000 to support preclinical animal studies and to support activities needed to prepare for the Phase 1/2 clinical study. The second part of the award will support the Phase 1/2 study, the results from which are expected to confirm the safety and effectiveness of Tc 99m tilmanocept to identify skeletal joint inflammation due to RA.
In September 2015, we received an initial notice of award for a Fast Track SBIR grant providing for up to $1.8 million from the NIH’s National Cancer Institute to fund preclinical studies examining the safety of intravenous (IV) injection of Tc99m tilmanocept, a Manocept platform product, followed by a clinical study providing the initial evaluation of the safety and efficacy of SPECT imaging
7
studies with IV Tc99m tilmanocept to identify and quantify both skin- and organ-associated KS lesions in human patients. The grant is awarded in two parts with the potential for total grant money of up to $1.8 million over two and a half years. The first six-month funding segment of $300,000, which has already been awarded, is expected to enable Navidea to secure necessary collaborations and Institutional Review Board approvals. The second funding segment could provide for up to an additional $1.5 million to be used to accrue participants, perform the Phase 1/2 study and perform data analyses to confirm the safety and effectiveness of intravenously administered Tc99m tilmanocept.
Over the course of the last few years, management has provided periodic updates regarding the status of the NAV1800 development program we previously referred to as the RIGS® (radioimmunguided surgery) program. NAV1800 was originally intended to use a monoclonal antibody as an aid in identifying TAG-72, a specific factor associated with a primary tumor, ascertaining tumor margins, or determining the extent and location of occult and metastatic tumor in patients with solid tumor cancers, such as colorectal cancer, ovarian cancer, or endometrial cancer. The detection of clinically occult tumor was originally intended to provide the surgeon with a more accurate assessment of the extent and location of disease, and therefore impact the surgical and therapeutic management of the patient.
Over the last few years, our commercial evaluation of new clinical data has caused us to question the viability of the monoclonal antibody initiative as it was originally envisioned. During the same time period, we learned significantly more about tilmanocept, the underlying Manocept backbone, and the potential utility of tilmanocept in identifying TAMs, and their consequent potential utility in identifying multifocal tumor disease itself. To that end, we petitioned the NIH to repurpose the $1.5 million grant we were previously awarded towards the study of TAMs in colorectal cancer, and subsequently received confirmation of the acceptance of this repurposing. This repurposed grant now supports a Manocept-based diagnostic approach in patients with anal/rectal cancer and possibly colon cancer. We recognize this repurposing represents a major refocusing of the original NAV1800 initiative, but we are confident that this change represents the best course of action at this time towards benefiting patients afflicted with colorectal cancer and is one which is consistent with the excitement we are seeing on many fronts related to our work on the Manocept platform. However, we cannot assure you that if further clinical trials for this product proceed, that they will be successful, that the product will achieve regulatory approval, or if approved, that it will achieve market acceptance. See Risk Factors.
Macrophage Therapeutics Background
In December 2014, the Company formed a new business unit, Macrophage Therapeutics, to further explore therapeutic applications for the Manocept platform. In January 2015, Navidea incorporated the business unit as Macrophage Therapeutics, Inc. (MT), initially a wholly-owned subsidiary of Navidea.
In December 2014, MT hosted a conference where data were presented using the Manocept platform compound, tilmanocept, that was generated by Navidea and independent academic collaborators with expertise in the HIV/AIDS, cancer, TB, RA and cardiovascular disease therapeutic areas. The technical presentations highlighted tilmanocept’s ability to target activated macrophages implicated in the natural history of numerous diseases.
In February 2015, Navidea announced the appointment of leading experts to a newly formed scientific advisory board (SAB) to serve as a strategic resource to MT as MT looks to develop therapeutic applications for Navidea’s Manocept platform. The inaugural SAB consortium is comprised of world-renowned scientists and clinicians in the areas of oncology, immunology, autoimmune diseases and macrophage biology. The SAB will serve as an ongoing resource to provide counsel and guidance pertaining to the research, development, and clinical use of our Manocept technology in therapeutic applications.
In March 2015, MT entered into a Securities Purchase Agreement to sell up to 50 shares of its Series A Convertible Preferred Stock (MT Preferred Stock) and warrants to purchase up to an additional 1,500 common shares of Macrophage Therapeutics, Inc. (MT Common Stock) to Platinum-Montaur Life Sciences LLC, an affiliate of Platinum Management (NY) LLC, Platinum Partners Value Arbitrage Fund L.P., Platinum Partners Liquid Opportunity Master Fund L.P., Platinum Liquid Opportunity Management (NY) LLC, and Montsant Partners LLC (collectively, Platinum) and Dr. Michael Goldberg, one of our directors and CEO of Macrophage Therapeutics, Inc. (collectively, the Investors); the agreed purchase price was $50,000 per unit. On March 13, 2015, Navidea announced that definitive agreements with the Investors had been signed for the sale of the first 10 shares of MT Preferred Stock and warrants to purchase 300 shares of MT Common Stock to the Investors, with gross proceeds to MT of $500,000. Under the agreement, 40% of the MT Preferred Stock and warrants are committed to be purchased by Dr. Goldberg, and the balance by Platinum. The full 50 shares of MT Preferred Stock and warrants that may be sold under the agreement are convertible into and exercisable for MT Common Stock representing an aggregate 1% interest on a fully converted and exercised basis. Navidea retains ownership of the remainder of MT Common Stock.
In addition, Navidea entered into a Securities Exchange Agreement with the Investors providing them an option to exchange their MT Preferred Stock for our common stock in the event that MT has not completed a public offering with gross proceeds to MT of at least $50 million by the second anniversary of the closing of the initial sale of MT Preferred Stock, at an exchange rate per share obtained by dividing $50,000 by the greater of (i) 80% of the twenty-day volume weighted average price per share of our common stock on the second anniversary of the initial closing or (ii) $3.00. To the extent that the Investors do not timely exercise their exchange right, MT
8
has the right to redeem their MT Preferred Stock for a price equal to $58,320 per share. Navidea also granted MT an exclusive license for certain therapeutic applications of the Manocept technology.
In June 2015, BIND Therapeutics, Inc. (BIND), an early clinical-stage nanomedicine company developing targeted and programmable therapeutics called Accurins™, and MT entered into a research collaboration to engineer Accurins with the Manocept targeting platform. This agreement was renewed in February 2016 to extend the agreement through February 2017. Disease-associated macrophages generally play a pro-tumoral role and are immunosuppressive, preventing the immune system from mounting an attack on tumor cells. Based on the expression of CD206 mannose receptors on disease-associated macrophages, BIND and MT plan to consider joint research programs that may be capable of concentrating various therapeutic payloads to the tumor microenvironment.
In September 2015, MT announced that it had developed preliminary processes for producing the first two therapeutic Manocept immunoconstructs, MT-1001, designed to specifically target and kill activated CD206+ macrophages and MT-2001, designed to inhibit the inflammatory activity of activated CD206+ macrophages. These constructs are the result of the activities of Navidea’s clinical development and research group. MT-1001 and MT-2001 were developed from the Manocept platform technology and the efforts of Navidea’s development team and contain a similar chemical scaffold and targeting moieties designed to selectively target CD206+ macrophages. A payload of a therapeutic molecule is conjugated to each immunoconstruct through a linkage that will release the molecule within the targeted tissue: MT-1001 has doxorubicin, an anthracycline antitumor antibiotic, conjugated to the Manocept backbone and MT-2001 has a potent anti-inflammatory agent conjugated to it. MT has contracted with an independent facility to produce sufficient quantities of MT-1001 and MT-2001 along with the concomitant analytical standards, to provide material for planned preclinical animal studies.
Macrophage Therapeutics Clinical Data
In March 2015, Navidea and MT announced that data from an ongoing human study indicated that the Manocept technology platform has the ability to safely cross the blood brain barrier without losing its ability to deliver its payload to the intended target. Based on these data and on the advice of the Company’s SAB, MT hopes to expand the SAB to include members with specific expertise in CNS diseases. The blood brain barrier has proven to be a significant obstacle to treating many diseases of the central nervous system. In an imaging study using the Manocept targeted delivery system, foci on the other side of the blood brain barrier were observed that strongly and specifically localized tilmanocept. Many of the leading diseases of the central nervous system such as Alzheimer’s and Parkinson’s diseases as well as autoimmune CNS diseases such as multiple sclerosis and ALS have pathologies that can in part be attributed to over-active macrophages, the target for Manocept delivery technology.
In July 2015, Navidea and MT announced that preclinical results in KS demonstrated that a cytotoxic drug, doxorubicin, linked to Manocept was targeted to and dose-dependently taken up in CD206+ KS tumor cells and TAMs and caused apoptotic death of the KS tumor cells and TAMs. The results were presented at the 18th International Workshop on KSHV and Related Agents by Michael S. McGrath, M.D., Ph.D., Professor, Departments of Laboratory Medicine, Pathology, and Medicine at the University of California, San Francisco (UCSF). The study also shows that Cy3-Manocept and a Cy3-Manocept-doxorubicin conjugate quantitatively permitted the evaluation of tumor burden, tissue uptake of Manocept and tumor response to therapy in vitro and ex vivo, supporting the potential for the Manocept platform to be used not only diagnostically but as a precision targeted molecule to deliver payloads to tumor sites throughout the body. In summary, the data presented include evidence that:
|
|
· |
KS tissue based cells take up Cy3-Manocept or Cy3-Manocept-doxorubicin into both KS tumor cells and TAMs. |
|
|
· |
Manocept conjugate uptake is dose and time dependent in CD206+ macrophages. |
|
|
· |
Cy3-Manocept and Cy3-Manocept-doxorubicin bind to CD206 positive macrophages equivalently indicating that the linkage of a drug conjugate did not lessen the CD206 binding ability. |
|
|
· |
Manocept-doxorubicin killed CD206 expressing macrophages. After 24 hours, Cy3-Manocept-doxorubicin killed 70% of CD206 positive macrophages in tissue cultures. Doxorubicin alone showed no toxicity. |
|
|
· |
KS organ culture treated with Manocept-doxorubicin resulted in the loss of macrophages and induced programmed tumor cell death and apoptosis in KS HHV8+ spindle cells, and showed anti-HIV activity in HIV infected macrophage cultures. |
Navidea and MT continue to evaluate emerging data in other disease states to define areas of focus, development pathways and partnering options to capitalize on the Manocept platform, including ongoing studies in KS and RA. The immune-inflammatory process is remarkably complex and tightly regulated with indicators that initiate, maintain and shut down the process. Macrophages are immune cells that play a critical role in the initiation, maintenance, and resolution of inflammation. They are activated and deactivated in the inflammatory process. Because macrophages may promote dysregulation that accelerates or enhances disease progression, diagnostic and therapeutic interventions that target macrophages may open new avenues for controlling inflammatory diseases. We cannot assure you that further evaluation or development will be successful, that any Manocept platform product candidate will ultimately achieve regulatory approval, or if approved, the extent to which it will achieve market acceptance. See Risk Factors.
9
NAV4694 (Candidate for Out-License)
NAV4694 is a fluorine-18 (F-18) labeled PET imaging agent being developed as an aid in the imaging and evaluation of patients with signs or symptoms of Alzheimer’s disease (AD) and mild cognitive impairment (MCI). NAV4694 binds to beta-amyloid deposits in the brain that can then be imaged in PET scans. Amyloid plaque pathology is a required feature of AD and the presence of amyloid pathology is a supportive feature for diagnosis of probable AD. Patients who are negative for amyloid pathology do not have AD. NAV4694 has been studied in rigorous pre-clinical studies and clinical trials in humans. Clinical studies through Phase 3 have included subjects with MCI, suspected AD patients, and healthy volunteers. Results suggest that NAV4694 has the potential ability to image patients quickly and safely with high sensitivity and specificity.
In May 2014, the Board of Directors made the decision to refocus the Company's resources to better align the funding of our pipeline programs with the expected growth in Lymphoseek revenue. This realignment primarily involved reducing our near-term support for our neurological product candidates, including NAV4694, as we sought a development partner or partners for these programs. The Company is currently engaged in discussions related to the potential partnering or divestiture of NAV4694. We continue to have active interest from potential partners or acquirers; however, our negotiations have experienced delays due in large part to litigation brought by one of the potential partners (see Item 3 – Legal Proceedings). The Company believes the suit is without merit and has filed a motion to dismiss the action. While it is not possible to determine with any degree of certainty the ultimate outcome of this legal proceeding, including making a determination of liability, we believe that we have meritorious defenses with respect to the claims asserted against us and intend to vigorously defend our position.
NAV5001 (In-License Terminated)
NAV5001 is an iodine-123 (I-123) labeled SPECT imaging agent being developed as an aid in the diagnosis of Parkinson’s disease (PD) and other movement disorders, with potential use as a diagnostic aid in dementia. The agent binds to the dopamine transporter (DAT) on the cell surface of dopaminergic neurons in the striatum and substantia nigra regions of the brain. Loss of these neurons is a hallmark of PD. In addition to its potential use as an aid in the differential diagnosis of PD and movement disorders, NAV5001 may also be useful in the diagnosis of Dementia with Lewy Bodies, one of the most common forms of dementia after AD.
In May 2014, the Board of Directors made the decision to refocus the Company's resources to better align the funding of our pipeline programs with the expected growth in Lymphoseek revenue. This realignment primarily involved reducing our near-term support for our neurological product candidates, including NAV5001.
In April 2015, the Company entered into an agreement with Alseres to terminate the sub-license agreement dated July 31, 2012 for research, development and commercialization of NAV5001. Under the terms of this agreement, Navidea transferred all regulatory, clinical and manufacturing-related data related to NAV5001 to Alseres. Alseres agreed to reimburse Navidea for any incurred maintenance costs of the contract manufacturer retroactive to March 1, 2015. In addition, Navidea has supplied clinical support services for NAV5001 on a cost-plus reimbursement basis. However, to this point, Alseres has been unsuccessful in raising the funds necessary to restart the program and reimburse Navidea. As a result, we have taken steps to end our obligations under the agreement and notified Alseres that we consider them in breach of the agreement. We are in the process of trying to recover the funds we expended complying with our obligations under the termination agreement.
Market Overviews
Lymphoseek - Cancer Market Overview
Cancer is the second leading cause of death in the U.S. and Western Europe. The American Cancer Society (ACS) estimates that cancer will cause over 596,000 deaths in 2016 in the U.S. alone. The Agency for Healthcare Research and Quality (AHRQ) has estimated that the direct medical costs for cancer in the U.S. for 2013 were $74.8 billion. Additionally, the ACS estimates that approximately 1.7 million new cancer cases will be diagnosed in the U.S. during 2016. For the types of cancer to which our oncology agents may be applicable (breast, melanoma, head and neck, prostate, lung, colorectal, gastrointestinal and gynecologic), the ACS has estimated that over 1.1 million new cases will occur in the U.S. in 2016.
Currently, the application of intraoperative lymphatic mapping (ILM) is most established in breast cancer. Breast cancer is the second leading cause of death from cancer among all women in the U.S. The probability of developing breast cancer generally increases with age, rising from about 1.9% in women under age 49 to 6.7% in women age 70 or older. According to the ACS, over 247,000 new cases of breast cancer are expected to be diagnosed during 2016 in the U.S. alone. The incidence rate for breast cancer appears to be stable. Thus, we believe that the aging of the population, combined with improved education and awareness of breast cancer and diagnostic methods, will continue to lead to an increased number of breast cancer surgical diagnostic procedures.
10
The use of ILM is also common in melanoma. The ACS estimates that approximately 76,000 new cases of melanoma will be diagnosed in the U.S. during 2016. In addition to breast cancer and melanoma, we believe that our oncology products may have utility in other cancer types with another 809,000 new cases expected during 2016 in the U.S.
If the potential of Lymphoseek as a radioactive tracing agent is ultimately realized, it may address not only the breast and melanoma markets on a procedural basis, but also assist in the clinical evaluation and staging of solid tumor cancers and expanding lymph node mapping to other solid tumor cancers such as prostate, gastric, colon, head and neck, gynecologic, and non-small cell lung. Lymphoseek is approved by the U.S. FDA for use in solid tumor cancers where lymphatic mapping is a component of surgical management and for guiding sentinel lymph node biopsy in patients with clinically node negative breast cancer, melanoma or squamous cell carcinoma of the oral cavity. Lymphoseek has also received European approval in imaging and intraoperative detection of sentinel lymph nodes in patients with melanoma, breast cancer or localized squamous cell carcinoma of the oral cavity.
Manocept Diagnostics and Macrophage Therapeutics Market Overview
Impairment of the macrophage-driven disease mechanism is an area of increasing focus in medicine. The number of people affected by all the inflammatory diseases combined is estimated at more than 40 million in the United States and perhaps 700 million worldwide, making these macrophage-mediated diseases an area of remarkable clinical importance. There are many recognized disorders having macrophage involvement, including RA, atherosclerosis/vulnerable plaque, Crohn’s disease, TB, systemic lupus erythematosis, KS, and others that span clinical areas in oncology, autoimmunity, infectious diseases, cardiology, and inflammation. Data from studies using agents from the Manocept platform in RA, KS and TB were published in a special supplement, Nature Outlook: Medical Imaging, in Nature’s October 31, 2013 issue. The supplement included a White Paper by Navidea entitled “Innovations in receptor-targeted precision imaging at Navidea: Diagnosis up close and personal,” focused on the Manocept platform. While Navidea’s development of the Manocept platform still in relatively early stage, below is a table summarizing potential target markets in which Manocept may have potential diagnostic or therapeutic applications:
|
Macrophage-Associated Diseases for CD206 Targeting (thousands) |
|
||||||||||||||||||||||||||
|
|
Incidence |
|
Prevalence |
|
|||||||||||||||||||||||
|
Disease |
Kaposi's Sarcoma |
|
Tuberculosis |
|
Multiple Sclerosis |
|
Crohn's Disease |
|
Systemic Lupus Erythematosus |
|
Rheumatoid Arthritis |
|
Neuro-degenerative Diseases |
|
Diabetes |
|
Athero-sclerosis |
|
|||||||||
|
Worldwide |
|
50 |
|
|
8,700 |
|
|
1,800 |
|
|
3,800 |
|
|
27,100 |
|
|
60,000 |
|
|
33,000 |
|
|
122,000 |
|
|
480,000 |
|
NAV4694 - Alzheimer’s Disease Market Overview
The Alzheimer’s Association (AA) estimates that more than 5.3 million Americans had AD in 2015. On a global basis, Alzheimer’s Disease International estimated in 2015 that there were 46.8 million people living with dementia. AA estimates that total costs for AD care was approximately $226 billion in 2015 and is expected to rise to more than $1 trillion by 2050. AA also estimates that there are over 15 million AD and dementia caregivers providing 17.9 billion hours of unpaid care valued at $217.7 billion. AD is the sixth-leading cause of death in the country and the only cause of death among the top 10 in the U.S. that cannot be prevented, cured or even slowed. Based on mortality data from 2000-2013, deaths from AD have risen 71 percent while deaths attributed to the number one cause of death, heart disease, decreased 14 percent during the same period. In February 2013, the American Academy of Neurology reported in the online issue of Neurology that the number of people with AD may triple by 2050.
Marketing and Distribution
We believe the most preferable and likely distribution partners for Lymphoseek would be entities with established radiopharmaceutical distribution channels, although it is possible that other entities with more traditional oncology or neurological pharmaceutical portfolios may also have interest.
During the fourth quarter of 2007, we executed an agreement with Cardinal Health Inc.’s (Cardinal) Nuclear Pharmacy Services division for the exclusive distribution of Lymphoseek in the U.S. The agreement is for a term of five years from the date of FDA marketing clearance, March 13, 2013. Under the terms of this agreement, Navidea receives a significant share of the revenue from each patient dose of Lymphoseek sold. In addition, Navidea will receive up to $3 million in payments upon the achievement of certain sales milestones by Cardinal Health. We cannot assure you that we will be able to maintain a successful relationship with Cardinal Health, on terms acceptable to the Company, or at all.
In May 2013 the Company announced the commercial launch of Lymphoseek in the U.S. through a distribution agreement with Cardinal. In addition to distributing Lymphoseek to hospitals, Cardinal augments product promotion to nuclear medicine professionals. The Navidea commercial team is responsible for designing and driving Lymphoseek promotional activities and conducting medical education programs tailored to the oncology treatment team, including surgeons and nuclear medicine physicians. Although it is early after the most recent FDA label expansion, we believe we are seeing positive signs in measures of success we believe are critical to the success of our new commercial strategy and deployment.
11
With respect to Lymphoseek commercialization in Europe, we are aiming to deploy a specialty pharmaceutical strategy to commercialization that would be supportive of premium product positioning and reinforce Lymphoseek's clinical value proposition, as opposed to a commodity or a generics positioning approach. Unlike the U.S., where institutions typically rely on radiopharmaceutical products which are compounded and delivered by specialized radiopharmacy distributors such as Cardinal, institutions in Europe predominantly purchase non-radiolabeled material and compound the radioactive product on-site. In March 2015, we entered into an exclusive sublicense agreement for the commercialization and distribution of Lymphoseek 250 microgram kit for radiopharmaceutical preparation (tilmanocept) in the European Union with SpePharm AG (an affiliate of Norgine BV), a European specialist pharmaceutical company with an extensive pan-European presence. Under the terms of the exclusive license agreement, Navidea will supply Lymphoseek product to SpePharm; however, Navidea will transfer responsibility for regulatory maintenance of the Lymphoseek Marketing Authorization to SpePharm. SpePharm will also be responsible for pricing, reimbursement, sales, marketing, medical affairs, and regulatory activities. In connection with entering into the agreement, Navidea is entitled to an upfront payment of $2 million, milestones totaling up to an additional $5 million, and royalties on European net sales. The initial territory covered by the agreement includes all 28 member states of the European Economic Community with the option to expand into additional geographical areas. SpePharm is currently performing the customary pre-launch market access activities to support commercial launch in the EU later in 2016. Concurrently, we are completing manufacturing validation activities on a finished drug product contract manufacturing facility to support the Company’s supply chain, primarily in Europe.
In August 2014, Navidea entered into an exclusive agreement with a wholly-owned subsidiary of Hainan Sinotau Pharmaceutical Co., Ltd. (Sinotau), a pharmaceutical organization with a broad China focus in oncology and other therapeutic areas, who will develop and commercialize Lymphoseek in China. In exchange, Navidea will earn revenue based on unit sales to Sinotau, a royalty based on Sinotau’s sales of Lymphoseek and up to $2.5 million in milestone payments from Sinotau, including a $300,000 non-refundable upfront payment. As part of the agreement, Sinotau is responsible for costs and conduct of clinical studies and regulatory applications to obtain Lymphoseek approval by the China Food and Drug Administration (CFDA). Upon approval, Sinotau will be responsible for all Lymphoseek sales, marketing, market access and medical affairs activities in China and excluding Hong Kong, Macau and Taiwan. Navidea and Sinotau will jointly support certain pre-market planning activities with a joint commitment on clinical and market development programs pending CFDA approval. In addition to the $300,000 upfront payment, Navidea is eligible for $700,000 in milestone payments up to and through product approval, and an additional $1.5 million in sales milestones.
The Company also has distribution arrangements covering other global markets such as Canada, Taiwan, Puerto Rico, and select medical centers in the Middle East. Lymphoseek is in various stages of approval in these territories and sales to this point in these markets, if any, have not been material. However, we believe that with international partnerships to complement our position in the U.S. and EU, we will help establish Lymphoseek as a global leader in lymphatic mapping, as we are aware of no other company which has this global geographic range. We cannot assure you that Lymphoseek will achieve regulatory approval in any market outside the U.S. or EU, or if approved, that it will achieve market acceptance in any market. We also cannot assure you that we will be successful in securing collaborative partners for other global markets or radiopharmaceutical products, or that we will be able to negotiate acceptable terms for such arrangements. See Risk Factors.
Manufacturing
We currently use and expect to continue to be dependent upon contract manufacturers to manufacture each of our product candidates. We have established a quality control and quality assurance program, including a set of standard operating procedures and specifications with the goal that our products and product candidates are manufactured in accordance with current good manufacturing practices (cGMP) and other applicable domestic and international regulations. We will need to invest in additional manufacturing and supply chain resources, and may seek to enter into additional collaborative arrangements with other parties that have established manufacturing capabilities. It is likely that we will continue to rely on third-party manufacturers for our development and commercial products on a contract basis.
Lymphoseek Manufacturing
Reliable Biopharmaceutical Corporation (Reliable) produces the drug substance and OSO BioPharmaceuticals Manufacturing, LLC (OsoBio) performs final product manufacturing including final drug formulation, lyophilization (freeze-drying) and packaging processes for Lymphoseek to be sold in the U.S. Gipharma S.r.l. (Gipharma) will perform final product manufacturing for reduced-mass vials to be sold in the EU. Once packaged, the vialed drug can then be shipped to a hospital or regional commercial radiopharmacy where it will be made radioactive (radiolabeled) with 99m Tc to become the final form of Lymphoseek to be administered to a patient. All three organizations have assisted Navidea in the preparation of the chemistry, manufacturing and control (CMC) sections of our submissions to the FDA and the EMA. Reliable, OsoBio and Gipharma are registered manufacturers with the FDA and the EMA.
In November 2009, we completed a Manufacture and Supply Agreement with Reliable for the manufacture of the bulk drug substance with an initial term of 10 years. In September 2013, we entered into a Manufacturing Services Agreement with OsoBio for contract
12
pharmaceutical development, manufacturing, packaging and analytical services for Lymphoseek. The current agreement with OsoBio runs through December 2016, and automatically renews for additional two-year periods. Also in September 2013, we completed a Service and Supply Master Agreement with Gipharma for process development, manufacturing and packaging of reduced-mass vials for sale in the EU. The agreement with Gipharma has an initial term of three years and automatically renews for additional one-year periods. We cannot assure you that we will be successful in completing future agreements for the supply of Lymphoseek on terms acceptable to the Company, or at all.
NAV4694 Manufacturing
Supplies of NAV4694 used in clinical development through Phase 2b were manufactured by AstraZeneca through various arrangements. In May 2012, we executed an agreement with Molecular NeuroImaging, LLC (MNI) to produce and distribute NAV4694 to imaging centers within a specified geographic region. In October 2012, we completed an agreement with Spectron mrc, LLC (Spectron) to produce NAV4694 for use at certain clinical trial sites. In August 2013, we entered into a Manufacturing Services Agreement with PETNET Solutions, Inc. (PETNET) for the manufacture and distribution of NAV4694 with an initial term of 3 years. Under the terms of the agreement, PETNET will manufacture NAV4694 clinical trial material at select U.S. radiopharmacies, with the possibility of expanding into additional PETNET locations in the future. Navidea has continued to incur costs related to maintaining our NAV4694 manufacturing sites while seeking to partner or out-license the product, and we expect to continue to incur such costs while we complete our partnering/divestiture activities.
Summary
We cannot assure you that we will be successful in securing and/or maintaining the necessary manufacturing, supply and/or radiolabeling capabilities for our product candidates in clinical development. If and when established, we also cannot assure you that we will be able to maintain agreements or other purchasing arrangements with our subcontractors on terms acceptable to us, or that our subcontractors will be able to meet our production requirements on a timely basis, at the required levels of performance and quality, including compliance with FDA cGMP requirements. In the event that any of our subcontractors are unable or unwilling to meet our production requirements, we cannot assure you that an alternate source of supply could be established without significant interruption in product supply or without significant adverse impact to product availability or cost. Any significant supply interruption or yield problems that we or our subcontractors experience would have a material adverse effect on our ability to manufacture our products and, therefore, a material adverse effect on our business, financial condition, and results of operations until a new source of supply is qualified. See Risk Factors.
Competition
Competition in the pharmaceutical and biotechnology industries is intense. We face competition from a variety of companies focused on developing oncology and neurology diagnostic drugs. We compete with large pharmaceutical and other specialized biotechnology companies. We also face competition from universities and other non-profit research organizations. Many emerging medical product companies have corporate partnership arrangements with large, established companies to support the research, development, and commercialization of products that may be competitive with our products. In addition, a number of large established companies are developing proprietary technologies or have enhanced their capabilities by entering into arrangements with or acquiring companies with technologies applicable to the detection or treatment of cancer and other diseases targeted by our product candidates. Smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large pharmaceutical and established biotechnology companies. Many of these competitors have products that have been approved or are in development and operate large, well-funded research and development programs. Many of our existing or potential competitors have substantially greater financial, research and development, regulatory, marketing, and production resources than we have. Other companies may develop and introduce products and processes competitive with or superior to ours.
We expect to encounter significant competition for our pharmaceutical products. Companies that complete clinical trials, obtain required regulatory approvals and commence commercial sales of their products before us may achieve a significant competitive advantage if their products work through a similar mechanism as our products and if the approved indications are similar. A number of biotechnology and pharmaceutical companies are developing new products for the treatment of the same diseases being targeted by us. In some instances, such products have already entered late-stage clinical trials or received FDA approval and may be marketed for some period prior to the approval of our products.
We believe that our ability to compete successfully will be based on our ability to create and maintain scientifically advanced “best-in-class” technology, develop proprietary products, attract and retain scientific personnel, obtain patent or other protection for our products, obtain required regulatory approvals and manufacture and successfully market our products, either alone or through third parties. We expect that competition among products cleared for marketing will be based on, among other things, product efficacy, safety, reliability, availability, price, and patent position. See Risk Factors.
13
Surgeons who practice the lymphatic mapping procedure for which Lymphoseek is intended currently use other radiopharmaceuticals such as a sulfur colloid compound in the U.S., and other colloidal compounds in other markets. In addition, some surgeons still use vital blue dyes to assist in the visual identification of the draining lymphatic tissue around a primary tumor. In the U.S., sulfur colloid is manufactured by Pharmalucence, a subsidiary of Sun Pharmaceutical Industries Ltd. Sulfur colloid had been used “off-label” in the U.S. for ILM until July 2011, when it was approved by the FDA for use in lymphatic mapping in breast cancer patients based on a statistical meta-analysis of published literature that compared the use of sulfur colloid with that of the vital blue dyes. The product label for sulfur colloid was expanded to cover lymphatic mapping in melanoma in August 2012, again on the basis of a meta-analysis of published literature. In the EU and certain Pacific Rim markets, there are other colloidal-based compounds with various levels of approved labeling for use in lymphatic mapping, although a number of countries still employ the use of products used “off-label.”
NAV4694 Competition
Several potential competitive [18F] products have been approved for use as biomarkers to aid in detection of AD. Developed through Eli Lilly’s wholly-owned Avid Radiopharmaceuticals (Avid), florbetapir, now known as Amyvid, received FDA approval to market in April 2012. Florbetapir also received marketing authorization in the EU in January 2013. In addition to fluorbetapir, there are two other beta-amyloid imaging agents available: florbetaben from Piramal Enterprises, Imaging Division, and flutemetamol from GE Healthcare. In October 2013, the FDA approved flutemetamol, under the name VizamylTM, for adults being evaluated for AD and dementia with PET brain imaging. Florbetaben, now called NeuraceqTM, received EMA approval for use in PET imaging of the brain to estimate beta-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for AD and other causes of cognitive decline from the EMA in February 2014 and from the FDA in March 2014.
Patents and Proprietary Rights
The patent position of biotechnology, including our company, generally is highly uncertain and may involve complex legal and factual questions. Potential competitors may have filed applications, or may have been issued patents, or may obtain additional patents and proprietary rights relating to products or processes in the same area of technology as that used by the Company. The scope and validity of these patents and applications, the extent to which we may be required to obtain licenses thereunder or under other proprietary rights, and the cost and availability of licenses are uncertain. We cannot assure you that our patent applications or those licensed to us will result in additional patents being issued or that any of our patents or those licensed to us will afford protection against competitors with similar technology; nor can we assure you that any of these patents will not be designed around by others or that others will not obtain patents that we would need to license or design around.
We also rely upon unpatented trade secrets. We cannot assure you that others will not independently develop substantially equivalent proprietary information and techniques, or otherwise gain access to our trade secrets, or disclose such technology, or that we can meaningfully protect our rights to our unpatented trade secrets.
We require our employees, consultants, advisers, and suppliers to execute a confidentiality agreement upon the commencement of an employment, consulting or manufacturing relationship with us. The agreement provides that all confidential information developed by or made known to the individual during the course of the relationship will be kept confidential and not disclosed to third parties except in specified circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual will be the exclusive property of our company. We cannot assure you, however, that these agreements will provide meaningful protection for our trade secrets in the event of an unauthorized use or disclosure of such information. We also employ a variety of security measures to preserve the confidentiality of our trade secrets and to limit access by unauthorized persons. We cannot assure you, however, that these measures will be adequate to protect our trade secrets from unauthorized access or disclosure. See Risk Factors.
Manocept/Lymphoseek Intellectual Property
Lymphoseek, as well as certain aspects of intellectual property underlying the Manocept platform, is under exclusive worldwide license from the Regents of the University of California. The license grants Navidea the commercialization rights to Lymphoseek for diagnostic imaging and intraoperative detection applications.
Lymphoseek, including the Manocept backbone composition and methods of use, is the subject of multiple patent families totaling 26 patents and patent applications in the United States and certain major foreign markets. The patents and patent applications held by the Regents of the University of California have been licensed exclusively to Navidea for all diagnostic and therapeutic uses worldwide.
The first composition of matter patent covering Manocept was issued in the United States in June 2002. This patent will expire in May 2020, but a request for patent term extension has been filed to further extend the life of this patent. The claims of the composition of matter patent covering Manocept have been allowed in the EU and issued in the majority of major-market EU
14
countries in 2005. These patents will expire in 2020, but a request for supplemental protection certificates are in process to further extend the life of these patents. The composition of matter patent has also been issued in Japan. This patent will expire in 2020.
We have filed additional patent applications in the U.S. and certain major foreign markets related to manufacturing processes for Lymphoseek and Manocept, the first of which was issued in the U.S. in 2013. These patents and/or applications will expire between 2029 and 2032. We have filed further patent applications jointly with The Ohio State Innovation Foundation related to CD206 expressing cell-related disorders. These patents and/or applications will expire between 2034 and 2035. We have filed further patent applications related to 2-heteroaryl substituted benzofurans. These patents and/or applications will expire between 2036 and 2037.
We will also rely on trademark protection for products that we expect to commercialize and have registered or are in the process of registering the marks Lymphoseek®, Manocept™, and the Lymphoseek logo™ in the U.S. and other markets.
NAV4694 Intellectual Property
NAV4694 is being developed under an exclusive worldwide license from AstraZeneca. The NAV4694 license grants Navidea commercialization rights to the fluorine-18 labeled biomarker for use as an aid in the diagnosis of AD. NAV4694 is the subject of 3 issued patents in the U.S. and 29 patents issued or pending in 13 foreign jurisdictions covering the [18F]NAV4694 drug substance and the NAV4694 precursor. These patents and/or applications will expire between 2028 and 2029.
Government Regulation
The research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of our products are extensively regulated by governmental authorities in the United States and other countries. In the United States, the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, Public Health Service Act, and their implementing regulations. Failure to comply with applicable U.S. requirements may subject us to administrative or judicial sanctions, such as FDA refusal to approve pending new drug applications or supplemental applications, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions and/or criminal prosecution. We also may be subject to regulation under the Occupational Safety and Health Act, the Atomic Energy Act, the Toxic Substances Control Act, the Export Control Act and other present and future laws of general application as well as those specifically related to radiopharmaceuticals.
Most aspects of our business are subject to some degree of government regulation in the countries in which we conduct our operations. As a developer, manufacturer and marketer of medical products, we are subject to extensive regulation by, among other governmental entities, the FDA and the corresponding state, local and foreign regulatory bodies in jurisdictions in which our products are intended to be sold. These regulations govern the introduction of new products, the observance of certain standards with respect to the manufacture, quality, safety, efficacy and labeling of such products, the maintenance of certain records, the tracking of such products, performance surveillance and other matters.
Failure to comply with applicable federal, state, local or foreign laws or regulations could subject us to enforcement action, including product seizures, recalls, withdrawal of marketing clearances, and civil and criminal penalties, any one or more of which could have a material adverse effect on our business. We believe that we are in substantial compliance with such governmental regulations. However, federal, state, local and foreign laws and regulations regarding the manufacture and sale of radiopharmaceuticals are subject to future changes. We cannot assure you that such changes will not have a material adverse effect on our company.
For some products, and in some countries, government regulation is significant and, in general, there is a trend toward more stringent regulation. In recent years, the FDA and certain foreign regulatory bodies have pursued a more rigorous enforcement program to ensure that regulated businesses like ours comply with applicable laws and regulations. We devote significant time, effort and expense addressing the extensive governmental regulatory requirements applicable to our business. To date, we have not received a noncompliance notification or warning letter from the FDA or any other regulatory bodies of alleged deficiencies in our compliance with the relevant requirements, nor have we recalled or issued safety alerts on any of our products. However, we cannot assure you that a warning letter, recall or safety alert, if it occurred, would not have a material adverse effect on our company. See Risk Factors.
In the early- to mid-1990s, the review time by the FDA to clear medical products for commercial release lengthened and the number of marketing clearances decreased. In response to public and congressional concern, the FDA Modernization Act of 1997 (the 1997 Act) was adopted with the intent of bringing better definition to the clearance process for new medical products. While the FDA review times have improved since passage of the 1997 Act, we cannot assure you that the FDA review processes will not delay our Company's introduction of new products in the U.S. in the future. In addition, many foreign countries have adopted more stringent regulatory requirements that also have added to the delays and uncertainties associated with the development and release of new products, as well as the clinical and regulatory costs of supporting such releases. It is possible that delays in receipt of, or failure to receive, any necessary clearance for our new product offerings could have a material adverse effect on our business, financial condition or results of operations. See Risk Factors.
15
The U.S. Drug Approval Process
None of our drugs may be marketed in the U.S. until such drug has received FDA approval. The steps required before a drug may be marketed in the U.S. include:
|
|
· |
preclinical laboratory tests, animal studies and formulation studies; |
|
|
· |
submission to the FDA of an Investigational New Drug (IND) application for human clinical testing, which must become effective before human clinical trials may begin; |
|
|
· |
adequate and well-controlled human clinical trials to establish the safety and efficacy of the investigational product for each indication; |
|
|
· |
submission to the FDA of a New Drug Application (NDA); |
|
|
· |
satisfactory completion of FDA inspections of the manufacturing and clinical facilities at which the drug is produced, tested, and/or distributed to assess compliance with cGMPs and current good clinical practices (cGCP) standards; and |
|
|
· |
FDA review and approval of the NDA. |
Preclinical tests include laboratory evaluation of product chemistry, toxicity and formulation, as well as animal studies. The conduct of the preclinical tests and formulation of the compounds for testing must comply with federal regulations and requirements. The results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND, which must become effective before human clinical trials may begin. An IND will automatically become effective 30 days after receipt by the FDA unless, before that time, the FDA raises concerns or questions about issues such as the conduct of the trials as outlined in the IND. In such a case, the IND sponsor and the FDA must resolve any outstanding FDA concerns or questions before clinical trials can proceed. We cannot be sure that submission of an IND will result in the FDA allowing clinical trials to begin.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND.
Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. The study protocol and informed consent information for study subjects in clinical trials must also be approved by an institutional review board at each institution where the trials will be conducted. Study subjects must sign an informed consent form before participating in a clinical trial. Phase 1 usually involves the initial introduction of the investigational product into people to evaluate its short-term safety, dosage tolerance, metabolism, pharmacokinetics and pharmacologic actions, and, if possible, to gain an early indication of its effectiveness. Phase 2 usually involves trials in a limited subject population to (i) evaluate dosage tolerance and appropriate dosage, (ii) identify possible adverse effects and safety risks, and (iii) evaluate preliminarily the efficacy of the product candidate for specific indications. Phase 3 trials usually further evaluate clinical efficacy and further test its safety by using the product candidate in its final form in an expanded subject population. There can be no assurance that Phase 1, Phase 2 or Phase 3 testing will be completed successfully within any specified period of time, if at all. Furthermore, we or the FDA may suspend clinical trials at any time on various grounds, including a finding that the subjects or patients are being exposed to an unacceptable health risk.
The FDA and the IND sponsor may agree in writing on the design and size of clinical studies intended to form the primary basis of an effectiveness claim in an NDA application. This process is known as a Special Protocol Assessment (SPA). These agreements may not be changed after the clinical studies begin, except in limited circumstances. The existence of a SPA, however, does not assure approval of a product candidate.
Assuming successful completion of the required clinical testing, the results of the preclinical studies and of the clinical studies, together with other detailed information, including information on the manufacturing quality and composition of the investigational product, are submitted to the FDA in the form of an NDA requesting approval to market the product for one or more indications. The testing and approval process requires substantial time, effort and financial resources. Submission of an NDA requires payment of a substantial review user fee to the FDA. Before approving a NDA, the FDA usually will inspect the facility or the facilities where the product is manufactured, tested and distributed and will not approve the product unless cGMP compliance is satisfactory. If the FDA evaluates the NDA and the manufacturing facilities as acceptable, the FDA may issue an approval letter or a complete response letter. A complete response letter outlines conditions that must be met in order to secure final approval of the NDA. When and if those conditions have been met to the FDA’s satisfaction, the FDA will issue an approval letter. The approval letter authorizes commercial marketing of the drug for specific indications. As a condition of approval, the FDA may require post-marketing testing and surveillance to monitor the product’s safety or efficacy, or impose other post-approval commitment conditions.
The NDA for Lymphoseek was submitted with the intention for use in intraoperative lymphatic mapping across a broad range of cancers. As a part of their review, the FDA examined the pre-clinical, clinical and CMC data supporting our application, and, as is also typical of such reviews, conducted site audits of our facilities and those of the sites where the referenced clinical trials were performed, as well as of contract suppliers and third party vendors being used in the manufacturing and quality assessment processes
16
for Lymphoseek. Lymphoseek was approved and indicated for use in lymphatic mapping for breast cancer and melanoma by the FDA in March 2013. In June 2014, the FDA approved a sNDA for the expanded use of Lymphoseek indicated for guiding sentinel lymph node biopsy in head and neck cancer patients with squamous cell carcinoma of the oral cavity. In October 2014, the FDA approved a second sNDA for lymphatic mapping in solid tumors and added sentinel lymph node detection for breast cancer and melanoma to the approved indications. The FDA also allowed expanded utilization of Lymphoseek with or without scintigraphic imaging, known as lymphoscintigraphy, to enable pre-operative imaging and mapping of lymph nodes to facilitate node localization during surgical procedures. Additional trials, including an ongoing trial in colorectal cancer, and others in various stages of execution, planning or consideration, are anticipated to provide additional data to potentially support expansion of the Lymphoseek opportunity. We cannot assure you that Lymphoseek will achieve regulatory approval in any market outside the U.S. or EU, or if approved, that it will achieve market acceptance in any market. See Risk Factors.
The FDA has various programs, including fast track, priority review and accelerated approval, which are intended to expedite or simplify the process of reviewing drugs and/or provide for approval on the basis of surrogate endpoints. Generally, drugs that may be eligible for one or more of these programs are those for serious or life threatening conditions, those with the potential to address unmet medical needs and those that provide meaningful benefit over existing treatments. We cannot assure you that any of our drug candidates will qualify for any of these programs, or that, if a drug candidate does qualify, the review time will be reduced or the product will be approved.
After approval, certain changes to the approved product, such as adding new indications, making certain manufacturing changes or making certain additional labeling claims, are subject to further FDA review and approval. Obtaining approval for a new indication generally requires that additional clinical studies be conducted.
U.S. Post-Approval Requirements
Holders of an approved NDA are required to: (i) conduct pharmacovigilance and report certain adverse reactions to the FDA, (ii) comply with certain requirements concerning advertising and promotional labeling for their products, and (iii) continue to have quality control and manufacturing procedures conform to cGMP. The FDA periodically inspects the sponsor’s records related to safety reporting and/or manufacturing and distribution facilities; this latter effort includes assessment of compliance with cGMP. Accordingly, manufacturers must continue to expend time, money and effort in the area of production, quality control and distribution to maintain cGMP compliance. We use and will continue to use third-party manufacturers to produce our products in clinical and commercial quantities, and future FDA inspections may identify compliance issues at our facilities or at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct. In addition, discovery of problems with a product after approval may result in restrictions on a product, manufacturer or holder of an approved NDA, including withdrawal of the product from the market.
Marketing of prescription drugs is also subject to significant regulation through federal and state agencies tasked with consumer protection and prevention of medical fraud, waste and abuse. We must comply with restrictions on off-label use promotion, anti-kickback, ongoing clinical trial registration, and limitations on gifts and payments to physicians.
Non-U.S. Regulation
Before our products can be marketed outside of the United States, they are subject to regulatory approval similar to that required in the U.S., although the requirements governing the conduct of clinical trials, including additional clinical trials that may be required, product licensing, pricing and reimbursement vary widely from country to country. No action can be taken to market any product in a country until an appropriate application has been approved by the regulatory authorities in that country. The current approval process varies from country to country, and the time spent in gaining approval varies from that required for FDA approval. In certain countries, the sales price of a product must also be approved. The pricing review period often begins after market approval is granted. Even if a product is approved by a regulatory authority, satisfactory prices may not be approved for such product.
In Europe, marketing authorizations may be submitted at a centralized, a decentralized or national level. The centralized procedure is mandatory for the approval of biotechnology products and provides for the grant of a single marketing authorization that is valid in all EU member states. As of January 1995, a mutual recognition procedure is available at the request of the applicant for all medicinal products that are not subject to the centralized procedure.
We submitted our MAA for Lymphoseek to the EMA in December 2012. In September 2014, the CHMP adopted a positive opinion recommending marketing authorization for Lymphoseek for use in the EU in imaging and intraoperative detection of sentinel lymph nodes draining a primary tumor in adult patients with breast cancer, melanoma, or localized squamous cell carcinoma of the oral cavity. The CHMP's positive opinion was reviewed by the EC, which has the authority to approve medicinal products for use in the 28 countries of the EU and generally follows the recommendations of the CHMP. The EC granted marketing authorization for Lymphoseek in the EU in November 2014.
17
While we are unable to predict the extent to which our business may be affected by future regulatory developments, we believe that our substantial experience dealing with governmental regulatory requirements and restrictions on our operations throughout the world, and our development of new and improved products, should enable us to compete effectively within this environment.
Regulation Specific to Radiopharmaceuticals
Our radiolabeled targeting agents and biologic products, if developed, would require a regulatory license to market from the FDA and from comparable agencies in foreign countries. The process of obtaining regulatory licenses and approvals is costly and time consuming, and we have encountered significant impediments and delays related to our previously proposed biologic products.
The process of completing pre-clinical and clinical testing, manufacturing validation and submission of a marketing application to the appropriate regulatory bodies usually takes a number of years and requires the expenditure of substantial resources, and we cannot assure you that any approval will be granted on a timely basis, if at all. Additionally, the length of time it takes for the various regulatory bodies to evaluate an application for marketing approval varies considerably, as does the amount of preclinical and clinical data required to demonstrate the safety and efficacy of a specific product. The regulatory bodies may require additional clinical studies that may take several years to perform. The length of the review period may vary widely depending upon the nature and indications of the proposed product and whether the regulatory body has any further questions or requests any additional data. Also, the regulatory bodies require post-marketing reporting and surveillance programs (pharmacovigilance) to monitor the side effects of the products. We cannot assure you that any of our potential drug or biologic products will be approved by the regulatory bodies or approved on a timely or accelerated basis, or that any approvals received will not subsequently be revoked or modified.
The Nuclear Regulatory Commission (NRC) oversees medical uses of nuclear material through licensing, inspection, and enforcement programs. The NRC issues medical use licenses to medical facilities and authorized physician users, develops guidance and regulations for use by licensees, and maintains a committee of medical experts to obtain advice about the use of byproduct materials in medicine. The NRC (or the responsible Agreement State) also regulates the manufacture and distribution of these products. The FDA oversees the good practices in the manufacturing of radiopharmaceuticals, medical devices, and radiation-producing x-ray machines and accelerators. The states regulate the practices of medicine and pharmacy and administer programs associated with radiation-producing x-ray machines and accelerators. We cannot assure you that we will be able to obtain all necessary licenses and permits and be able to comply with all applicable laws. The failure to obtain such licenses and permits or to comply with applicable laws would have a materially adverse effect on our business, financial condition, and results of operations.
Corporate Information
Our executive offices are located at 5600 Blazer Parkway, Suite 200, Dublin, OH 43017. Our telephone number is (614) 793-7500. “Navidea”, the Navidea logo, “Lymphoseek” and “RIGS” are trademarks of Navidea Biopharmaceuticals, Inc. or its subsidiaries in the U.S. and/or other countries. Other trademarks or service marks appearing in this report may be trademarks or service marks of other owners.
The address for our website is http://www.navidea.com. We make available free of charge on our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings pursuant to Section 13(a) or 15(d) of the Exchange Act, and amendments to such filings, as soon as reasonably practicable after each is electronically filed with, or furnished to, the SEC.
Financial Statements
Our consolidated financial statements and the related notes, including revenues, income (loss), total assets and other financial measures are set forth at pages F-1 through F-31 of this Form 10-K.
Research and Development
We spent approximately $12.8 million, $16.8 million and $23.7 million on research and development activities in the years ended December 31, 2015, 2014 and 2013, respectively.
Employees
As of February 29, 2016, we had 51 full-time and 6 part-time employees. We consider our relations with our employees to be good.
18
An investment in our common stock is highly speculative, involves a high degree of risk, and should be made only by investors who can afford a complete loss. You should carefully consider the following risk factors, together with the other information in this Form 10-K, including our financial statements and the related notes, before you decide to buy our common stock. Our most significant risks and uncertainties are described below; however, they are not the only risks we face. If any of the following risks actually occur, our business, financial condition, or results of operations could be materially adversely affected, the trading of our common stock could decline, and you may lose all or part of your investment therein.
If we do not achieve commercial success with our approved product, Lymphoseek, or if we do not successfully develop any additional product candidates into marketable products, we may be unable to generate significant revenue or become profitable.
Our near-term financial success depends in large part on Lymphoseek achieving commercial success in the U.S. and, pending approval in other markets, on achievement of commercial success in those markets as well. Lymphoseek was approved and indicated for use in lymphatic mapping for breast cancer and melanoma by the FDA in March 2013. In June 2014, the FDA approved a sNDA for the expanded use of Lymphoseek indicated for guiding sentinel lymph node biopsy in head and neck cancer patients with squamous cell carcinoma of the oral cavity. In October 2014, the FDA approved a second sNDA for lymphatic mapping in solid tumors and added sentinel lymph node detection for breast cancer and melanoma to the approved indications. The FDA also allowed expanded utilization of Lymphoseek with or without scintigraphic imaging, known as lymphoscintigraphy, to enable pre-operative imaging and mapping of lymph nodes to facilitate node localization during surgical procedures. Additional trials, including an ongoing trial in colorectal cancer, and others in various stages of execution, planning or consideration, are anticipated to provide additional data to potentially support expansion of the Lymphoseek opportunity. We began generating revenues from product sales of Lymphoseek in the second quarter of 2013.
We submitted our MAA for Lymphoseek to the EMA in December 2012. In September 2014, the CHMP adopted a positive opinion recommending marketing authorizations for Lymphoseek for use in the EU in imaging and intraoperative detection of sentinel lymph nodes draining a primary tumor in adult patients with breast cancer, melanoma, or localized squamous cell carcinoma of the oral cavity. The CHMP’s positive opinion was reviewed by the EC, which granted marketing authorization for Lymphoseek in the EU in November 2014. We announced an exclusive EU distribution partnership for Lymphoseek with SpePharm AG, a subsidiary of Norgine B.V., in March 2015, and we expect to commence marketing of Lymphoseek in the EU in late 2016.
Notwithstanding these marketing approvals, we cannot assure you that Lymphoseek will achieve commercial success in the U.S. or any other global market, that we will realize sales at levels necessary for us to achieve sales milestone payments, or that revenue from Lymphoseek will lead to us becoming profitable.
Additional diagnostic and therapeutic applications of the Manocept platform, including diagnosis of other solid cancers, rheumatoid arthritis and cardiovascular disease, are in various stages of pre-clinical and clinical development. Regulatory approval of label expansions for Lymphoseek or additional Manocept-based product candidates may not be successful, or if successful, may not result in increased sales. Additional clinical testing for products based on our Manocept platform or other product candidates may not be successful and, even if they are, we may not be successful in developing any of them into a commercial product which will provide sufficient revenue to make us profitable.
We have announced that we are seeking to partner or sub-license our NAV4694 candidate, which is designed to enable PET imaging of beta-amyloid deposits in the brain, believed to correlate with the presence of AD. While discussions with a potential licensee have progressed, our pending litigation with Sinotau Pharmaceuticals has prevented completion of a licensing transaction. See Item 3 – Legal Proceedings. Pending resolution of the Sinotau litigation, we are incurring significant costs to continue clinical evaluation of this product candidate to preserve it for eventual sub-licensing.
Many companies in the pharmaceutical industry suffer significant setbacks in advanced clinical trials even after reporting promising results in earlier trials. Even if our Lymphoseek and Manocept trials are viewed as successful, we may not get regulatory approval for expansion of the label for Lymphoseek or marketing of any Manocept product candidate. Our Manocept product candidates will be successful only if:
|
|
· |
they are developed to a stage that will enable us to commercialize them or sell related marketing rights to pharmaceutical companies; |
|
|
· |
we are able to commercialize them in clinical development or sell the marketing rights to third parties; and |
|
|
· |
upon being developed, they are approved by the regulatory authorities. |
We are dependent on the achievement of a number of these goals in order to generate future revenues. The failure to generate such revenues may preclude us from continuing our research and development of these and other product candidates.
19
We cannot guarantee that we will obtain regulatory approval to manufacture or market our unapproved drug candidates and our approval to market our products or anticipated commercial launch may be delayed as a result of the regulatory review process.
Obtaining regulatory approval to market drugs to diagnose or treat cancer and other diseases is expensive, difficult and risky. Preclinical and clinical data as well as information related to the CMC processes of drug production can be interpreted in different ways which could delay, limit or preclude regulatory approval. Negative or inconclusive results, adverse medical events during a clinical trial, or issues related to CMC processes could also delay, limit or prevent regulatory approval. Even if we receive regulatory clearance to market a particular product candidate, the approval could be conditioned on us conducting additional costly post-approval studies or could limit the indicated uses included in our labeling.
We may be unable to complete partnering or divestiture activities related to NAV4694 at a reasonable price, on a timely basis, or at all.
We have announced that we are seeking to partner or sub-license our NAV4694 candidate, which is designed to enable PET imaging of beta-amyloid deposits in the brain, believed to correlate with the presence of AD. While discussions with a potential licensee have progressed, our pending litigation with Sinotau Pharmaceuticals has prevented completion of a licensing transaction. See Item 3 – Legal Proceedings. Pending resolution of the Sinotau litigation, we continue to incur costs to maintain our ability to support future clinical evaluation of this product candidate to preserve it for eventual sub-licensing.
Our pharmaceutical products will remain subject to ongoing regulatory review following the receipt of marketing approval. If we fail to comply with continuing regulations, we could lose these approvals and the sale of our products could be suspended.
Approved products may later cause adverse effects that limit or prevent their widespread use, force us to withdraw it from the market or impede or delay our ability to obtain regulatory approvals in additional countries. In addition, any contract manufacturer we use in the process of producing a product and its facilities will continue to be subject to FDA review and periodic inspections to ensure adherence to applicable regulations. After receiving marketing clearance, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion and record-keeping related to the product will remain subject to extensive regulatory requirements. We may be slow to adapt, or we may never adapt, to changes in existing regulatory requirements or adoption of new regulatory requirements.
If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities or previously unknown problems with our products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including:
|
|
· |
restrictions on the products, manufacturers or manufacturing processes; |
|
|
· |
warning letters; |
|
|
· |
civil or criminal penalties; |
|
|
· |
fines; |
|
|
· |
injunctions; |
|
|
· |
product seizures or detentions; |
|
|
· |
import bans; |
|
|
· |
voluntary or mandatory product recalls and publicity requirements; |
|
|
· |
suspension or withdrawal of regulatory approvals; |
|
|
· |
total or partial suspension of production; and |
|
|
· |
refusal to approve pending applications for marketing approval of new drugs or supplements to approved applications. |
Even if our drug candidates are successful in clinical trials, we may not be able to successfully commercialize them.
We have dedicated and will continue to dedicate substantially all of our resources to the research and development of our Lymphoseek and Manocept technologies and related compounds. Lymphoseek is now approved for use in lymphatic mapping in solid tumors and in sentinel lymph node detection for breast cancer and melanoma in the U.S. Lymphoseek has also been approved for use in imaging and intraoperative detection of sentinel lymph nodes draining a primary tumor in adult patients with breast cancer, melanoma, or localized squamous cell carcinoma of the oral cavity in the EU. However, our other compounds currently are in research or development and have not received marketing approval.
20
There are many difficulties and uncertainties inherent in pharmaceutical research and development (R&D) and the introduction of new products. A high rate of failure is inherent in new drug discovery and development. The process to bring a drug from the discovery phase to regulatory approval can take 12 to 15 years or longer and cost more than $1 billion. Failure can occur at any point in the process, including late in the process after substantial investment. As a result, most research programs will not generate financial returns. New product candidates that appear promising in development may fail to reach the market or may have only limited commercial success. Delays and uncertainties in the regulatory approval processes in the US and in other countries can result in delays in product launches and lost market opportunities. Consequently, it is very difficult to predict which products will ultimately be approved. Due to the risks and uncertainties involved in the R&D process, we cannot reliably estimate the nature, timing, completion dates, and costs of the efforts necessary to complete the development of our R&D projects, nor can we reliably estimate the future potential revenue that will be generated from a successful R&D project.
Prior to commercialization, each product candidate requires significant research, development and preclinical testing and extensive clinical investigation before submission of any regulatory application for marketing approval. The development of radiopharmaceutical technologies and compounds, including those we are currently developing, is unpredictable and subject to numerous risks. Potential products that appear to be promising at early stages of development may not reach the market for a number of reasons including that they may:
|
|
· |
be found ineffective or cause harmful side effects during preclinical testing or clinical trials; |
|
|
· |
fail to receive necessary regulatory approvals; |
|
|
· |
be difficult to manufacture on a scale necessary for commercialization; |
|
|
· |
be uneconomical to produce; |
|
|
· |
fail to achieve market acceptance; or |
|
|
· |
be precluded from commercialization by proprietary rights of third parties. |
The occurrence of any of these events could adversely affect the commercialization of our product candidates. Products, if introduced, may not be successfully marketed and/or may not achieve customer acceptance. If we fail to commercialize products or if our future products do not achieve significant market acceptance, we will not likely generate significant revenues or become profitable.
Clinical trials for our product candidates will be lengthy and expensive and their outcome is uncertain.
Before obtaining regulatory approval for the commercial sale of any product candidates, we must demonstrate through preclinical testing and clinical trials that our product candidates are safe and effective for use in humans. Conducting clinical trials is a time consuming, expensive and uncertain process and may take years to complete.
With respect to Lymphoseek, we expect to support a number of efforts in various indications, whether internally sponsored or under investigator-sponsored studies, to provide additional data to support expansion of the Lymphoseek opportunity. We also expect to sponsor efforts to explore the Manocept platform, whether in potential diagnostic uses or investigation of uses related to Macrophage Therapeutics.
We continually assess our clinical trial plans and may, from time to time, initiate additional clinical trials to support our overall strategic development objectives. Historically, the results from preclinical testing and early clinical trials often do not predict the results obtained in later clinical trials. Frequently, drugs that have shown promising results in preclinical or early clinical trials subsequently fail to establish sufficient safety and efficacy data necessary to obtain regulatory approval. At any time during the clinical trials, we, the participating institutions, the FDA or the EMA might delay or halt any clinical trials for our product candidates for various reasons, including:
|
|
· |
ineffectiveness of the product candidate; |
|
|
· |
discovery of unacceptable toxicities or side effects; |
|
|
· |
development of disease resistance or other physiological factors; |
|
|
· |
delays in patient enrollment; or |
|
|
· |
other reasons that are internal to the businesses of our potential collaborative partners, which reasons they may not share with us. |
While we have achieved some level of success in our clinical trials for Lymphoseek as indicated by the FDA and EMA approvals, the results of some of these clinical trials that have not been yet reviewed by the FDA or other regulatory bodies, as well as pending and future trials for these and other product candidates that we may develop or acquire, are subject to review and interpretation by various regulatory bodies during the regulatory review process and may ultimately fail to demonstrate the safety or effectiveness of our
21
product candidates to the extent necessary to obtain regulatory approval, or that commercialization of our product candidates is worthwhile. Any failure or substantial delay in successfully completing clinical trials and obtaining regulatory approval for our product candidates could materially harm our business.
We extensively outsource our clinical trial activities and usually perform only a small portion of the start-up activities in-house. We rely on independent third-party contract research organizations (CROs) to perform most of our clinical studies, including document preparation, site identification, screening and preparation, pre-study visits, training, post-study audits and statistical analysis. Many important aspects of the services performed for us by the CROs are out of our direct control. If there is any dispute or disruption in our relationship with our CROs, our clinical trials may be delayed. Moreover, in our regulatory submissions, we rely on the quality and validity of the clinical work performed by third-party CROs. If any of our CROs’ processes, methodologies or results were determined to be invalid or inadequate, our own clinical data and results and related regulatory approvals could be adversely impacted.
If we fail to establish and maintain collaborations or if our partners do not perform, we may be unable to develop and commercialize our product candidates.
We have entered into collaborative arrangements with third parties to develop and/or commercialize product candidates and are currently seeking additional collaborations. Such collaborations might be necessary in order for us to fund our research and development activities and third-party manufacturing arrangements, seek and obtain regulatory approvals and successfully commercialize our existing and future product candidates. If we fail to enter into collaborative arrangements or fail to maintain our existing collaborative arrangements, the number of product candidates from which we could receive future revenues would decline.
Our dependence on collaborative arrangements with third parties will subject us to a number of risks that could harm our ability to develop and commercialize products including that:
|
|
· |
collaborative arrangements may not be on terms favorable to us; |
|
|
· |
disagreements with partners or regulatory compliance issues may result in delays in the development and marketing of products, termination of our collaboration agreements or time consuming and expensive legal action; |
|
|
· |
we cannot control the amount and timing of resources partners devote to product candidates or their prioritization of product candidates and partners may not allocate sufficient funds or resources to the development, promotion or marketing of our products, or may not perform their obligations as expected; |
|
|
· |
partners may choose to develop, independently or with other companies, alternative products or treatments. including products or treatments which compete with ours; |
|
|
· |
agreements with partners may expire or be terminated without renewal, or partners may breach collaboration agreements with us; |
|
|
· |
business combinations or significant changes in a partner's business strategy might adversely affect that partner's willingness or ability to complete its obligations to us; and |
|
|
· |
the terms and conditions of the relevant agreements may no longer be suitable. |
The occurrence of any of these events could adversely affect the development or commercialization of our products.
If users of our products are unable to obtain adequate reimbursement from third-party payers, or if new restrictive legislation is adopted, market acceptance of our products may be limited and we may not achieve anticipated revenues.
Our ability to commercialize our products will depend in part on the extent to which appropriate reimbursement levels for the cost of our products and related treatment are obtained by governmental authorities, private health insurers and other organizations such as health maintenance organizations (HMOs). Generally, in Europe and other countries outside the U.S., the government-sponsored healthcare system is the primary payer of patients’ healthcare costs. Third-party payers are increasingly challenging the prices charged for medical care. Also, the trend toward managed health care in the United States and the concurrent growth of organizations such as HMOs which could control or significantly influence the purchase of health care services and products, as well as legislative proposals to further reform health care or reduce government insurance programs, may all result in lower prices for our products if approved for commercialization. The cost containment measures that health care payers and providers are instituting and the effect of any health care reform could materially harm our ability to sell our products at a profit.
In August 2013, we announced that the CMS had issued a HCPCS “C Code” for Lymphoseek. The cost pass-through provisions supporting this C Code extended through December 31, 2015. Lymphoseek has also been granted a permanent “A Code” effective January 1, 2014. Following the expiration of the pass-through C Code, the cost of Lymphoseek must be absorbed by the institution as a part of the bundled procedural code for the surgical procedure in which Lymphoseek is used. Although we have not as of the date of
22
this report seen a negative impact of this change in reimbursement on Lymphoseek sales, we cannot assure you that the change in reimbursement will not adversely affect the rate of grown in sales of the product.
We may be unable to establish or contract for the pharmaceutical manufacturing capabilities necessary to develop and commercialize our potential products.
We are in the process of establishing third-party clinical manufacturing capabilities for our compounds under development. We intend to rely on third-party contract manufacturers to produce sufficiently large quantities of drug materials that are and will be needed for clinical trials and commercialization of our potential products. Third-party manufacturers may not be able to meet our needs with respect to timing, quantity or quality of materials.
We have a supply agreement with Reliable to manufacture the drug substance for our Lymphoseek product and a manufacturing agreement with OsoBio for the finishing and vialing of our Lymphoseek product. These are single-source relationships, and we are actively looking to add other supply and manufacturing relationships. However, if we are unable to contract for a sufficient supply of needed materials on acceptable terms, or if we should encounter delays or difficulties in our relationships with manufacturers, revenues from Lymphoseek may be adversely impacted. In addition, clinical trials for our other product candidates may be delayed, thereby delaying the submission of product candidates for regulatory approval and the market introduction and subsequent commercialization of our potential products, and for approved products, any such delays, interruptions or other difficulties may render us unable to supply sufficient quantities to meet demand. Any such delays or interruptions may lower our revenues and potential profitability.
We and any third-party manufacturers that we may use must continually adhere to cGMPs and regulations enforced by the FDA through its facilities inspection program and/or foreign regulatory authorities where our products will be tested and/or marketed. If our facilities or the facilities of third-party manufacturers cannot pass a pre-approval plant inspection, the FDA and/or foreign regulatory authorities will not grant approval to market our product candidates. In complying with these regulations and foreign regulatory requirements, we and any of our third-party manufacturers will be obligated to expend time, money and effort on production, record-keeping and quality control to assure that our potential products meet applicable specifications and other requirements. The FDA and other regulatory authorities may take action against a contract manufacturer who violates cGMPs.
Our product supply and related patient access could be negatively impacted by, among other things: (i) product seizures or recalls or forced closings of manufacturing plants; (ii) disruption in supply chain continuity including from natural or man-made disasters at a critical supplier, as well as our failure or the failure of any of our suppliers to comply with cGMPs and other applicable regulations or quality assurance guidelines that could lead to manufacturing shutdowns, product shortages or delays in product manufacturing; (iii) manufacturing, quality assurance/quality control, supply problems or governmental approval delays; (iv) the failure of a sole source or single source supplier to provide us with the necessary raw materials, supplies or finished goods within a reasonable timeframe; (v) the failure of a third-party manufacturer to supply us with bulk active or finished product on time; and (vi) other manufacturing or distribution issues, including limits to manufacturing capacity due to regulatory requirements, and changes in the types of products produced, physical limitations or other business interruptions.
We may lose out to larger or better-established competitors.
The biotech and pharmaceutical industries are intensely competitive. Many of our competitors have significantly greater financial, technical, manufacturing, marketing and distribution resources as well as greater experience in the industry than we have. The particular medical conditions our product lines address can also be addressed by other medical procedures or drugs. Many of these alternatives are widely accepted by physicians and have a long history of use.
To remain competitive, we must continue to launch new products and technologies. To accomplish this, we commit substantial efforts, funds, and other resources to research and development. A high rate of failure is inherent in the research and development of new products and technologies. We must make ongoing substantial expenditures without any assurance that our efforts will be commercially successful. Failure can occur at any point in the process, including after significant funds have been invested. Promising new product candidates may fail to reach the market or may only have limited commercial success because of efficacy or safety concerns, failure to achieve positive clinical outcomes, inability to obtain necessary regulatory approvals, limited scope of approved uses, excessive costs to manufacture, the failure to establish or maintain intellectual property rights, or infringement of the intellectual property rights of others. Even if we successfully develop new products or enhancements or new generations of our existing products, they may be quickly rendered obsolete by changing customer preferences, changing industry standards, or competitors' innovations. Innovations may not be accepted quickly in the marketplace because of, among other things, entrenched patterns of clinical practice or uncertainty over third-party reimbursement. We cannot state with certainty when or whether any of our products under development will be launched, whether we will be able to develop, license, or otherwise acquire compounds or products, or whether any products will be commercially successful. Failure to launch successful new products or new indications for existing products may cause our products to become obsolete, causing our revenues and operating results to suffer.
23
Physicians may use our competitors’ products and/or our products may not be competitive with other technologies. Lymphoseek is expected to continue to compete against sulfur colloid in the U.S. and other colloidal agents in other global markets.
If our competitors are successful in establishing and maintaining market share for their products, our sales and revenues may not occur at the rate we anticipate. In addition, our current and potential competitors may establish cooperative relationships with larger companies to gain access to greater research and development or marketing resources. Competition may result in price reductions, reduced gross margins and loss of market share.
Several pharmaceutical companies currently have product candidates in development that they expect to have a significant impact on the diagnosis and treatment of AD in coming years. The prospects for these product candidates could have a significant impact, either positive or negative, on our ability to sub-license our NAV4694 product candidate.
We may be exposed to product liability claims for our product candidates and products that we are able to commercialize.
The testing, manufacturing, marketing and use of our commercial products, as well as product candidates in development, involve substantial risk of product liability claims. These claims may be made directly by consumers, healthcare providers, pharmaceutical companies or others. In recent years, coverage and availability of cost-effective product liability insurance has decreased, so we may be unable to maintain sufficient coverage for product liabilities that may arise. In addition, the cost to defend lawsuits or pay damages for product liability claims may exceed our coverage. If we are unable to maintain adequate coverage or if claims exceed our coverage, our financial condition and our ability to clinically test our product candidates and market our products will be adversely impacted. In addition, negative publicity associated with any claims, regardless of their merit, may decrease the future demand for our products and impair our financial condition.
The administration of drugs in humans, whether in clinical studies or commercially, carries the inherent risk of product liability claims whether or not the drugs are actually the cause of an injury. Our products or product candidates may cause, or may appear to have caused, injury or dangerous drug interactions, and we may not learn about or understand those effects until the product or product candidate has been administered to patients for a prolonged period of time. We may be subject from time to time to lawsuits based on product liability and related claims, and we cannot predict the eventual outcome of any future litigation. We may not be successful in defending ourselves in the litigation and, as a result, our business could be materially harmed. These lawsuits may result in large judgments or settlements against us, any of which could have a negative effect on our financial condition and business if in excess of our insurance coverage. Additionally, lawsuits can be expensive to defend, whether or not they have merit, and the defense of these actions may divert the attention of our management and other resources that would otherwise be engaged in managing our business.
As a result of a number of factors, product liability insurance has become less available while the cost has increased significantly. We currently carry product liability insurance that our management believes is appropriate given the risks that we face. We will continually assess the cost and availability of insurance; however, there can be no guarantee that insurance coverage will be obtained or, if obtained, will be sufficient to fully cover product liabilities that may arise.
If any of our license agreements for intellectual property underlying Lymphoseek, our Manocept platform, or any other products or potential products are terminated, we may lose the right to develop or market that product.
We have licensed intellectual property, including patents and patent applications relating to the underlying intellectual property for Lymphoseek and our Manocept platform. We may also enter into other license agreements or acquire other product candidates. The potential success of our product development programs depend on our ability to maintain rights under these licenses, including our ability to achieve development or commercialization milestones contained in the licenses. Under certain circumstances, the licensors have the power to terminate their agreements with us if we fail to meet our obligations under these licenses. We may not be able to meet our obligations under these licenses. If we default under any license agreement, we may lose our right to market and sell any products based on the licensed technology.
We may not have sufficient legal protection against infringement or loss of our intellectual property, and we may lose rights or protection related to our intellectual property if diligence requirements are not met, or at the expiry of underlying patents.
Our success depends, in part, on our ability to secure and maintain patent protection for our products and product candidates, to preserve our trade secrets, and to operate without infringing on the proprietary rights of third parties. While we seek to protect our proprietary positions by filing United States and foreign patent applications for our important inventions and improvements, domestic and foreign patent offices may not issue these patents. Third parties may challenge, invalidate, or circumvent our patents or patent applications in the future. Competitors, many of which have significantly more resources than we have and have made substantial investments in competing technologies, may apply for and obtain patents that will prevent, limit, or interfere with our ability to make, use, or sell our products either in the United States or abroad.
24
Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are or may be developing products. As the biotechnology and pharmaceutical industry expands and more patents are issued, the risk increases that we will be subject to claims that our products or product candidates, or their use, infringe the rights of others. In the United States, most patent applications are secret for a period of 18 months after filing, and in foreign countries, patent applications are secret for varying periods of time after filing. Publications of discoveries tend to significantly lag the actual discoveries and the filing of related patent applications. Third parties may have already filed applications for patents for products or processes that will make our products obsolete, limit our patents, invalidate our patent applications or create a risk of infringement claims.
Under recent changes to U.S. patent law, the U.S. has moved to a “first to file” system of patent approval, as opposed to the former “first to invent” system. As a consequence, delays in filing patent applications for new product candidates or discoveries could result in the loss of patentability if there is an intervening patent application with similar claims filed by a third party, even if we or our collaborators were the first to invent.
We or our suppliers may be exposed to, or threatened with, future litigation by third parties having patent or other intellectual property rights alleging that our products, product candidates and/or technologies infringe their intellectual property rights or that the process of manufacturing our products or any of their respective component materials, or the component materials themselves, or the use of our products, product candidates or technologies, infringe their intellectual property rights. If one of these patents was found to cover our products, product candidates, technologies or their uses, or any of the underlying manufacturing processes or components, we could be required to pay damages and could be unable to commercialize our products or use our technologies or methods unless we are able to obtain a license to the patent or intellectual property right. A license may not be available to us in a timely manner or on acceptable terms, if at all. In addition, during litigation, a patent holder could obtain a preliminary injunction or other equitable remedy that could prohibit us from making, using or selling our products, technologies or methods.
Our currently held and licensed patents expire over the next one to sixteen years. Expiration of the patents underlying our technology, in the absence of extensions or other trade secret or intellectual property protection, may have a material and adverse effect on us.
In addition, it may be necessary for us to enforce patents under which we have rights, or to determine the scope, validity and unenforceability of other parties’ proprietary rights, which may affect our rights. There can be no assurance that our patents would be held valid by a court or administrative body or that an alleged infringer would be found to be infringing. The uncertainty resulting from the mere institution and continuation of any patent related litigation or interference proceeding could have a material and adverse effect on us.
We typically require our employees, consultants, advisers and suppliers to execute confidentiality and assignment of invention agreements in connection with their employment, consulting, advisory, or supply relationships with us. They may breach these agreements and we may not obtain an adequate remedy for breach. Further, third parties may gain unauthorized access to our trade secrets or independently develop or acquire the same or equivalent information.
Agencies of the United States government conducted some of the research activities that led to the development of antibody technology that some of our proposed antibody-based surgical cancer detection products use. When the United States government participates in research activities, it retains rights that include the right to use the technology for governmental purposes under a royalty-free license, as well as rights to use and disclose technical data that could preclude us from asserting trade secret rights in that data and software.
We and our collaborators may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our product candidates and products, when and if we have any, in every jurisdiction would be prohibitively expensive. Competitors may use our technologies in jurisdictions where we or our licensors have not obtained patent protection to develop their own products. These products may compete with our products, when and if we have any, and may not be covered by any of our or our licensors' patent claims or other intellectual property rights.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and many companies have encountered significant problems in protecting and defending such rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology and/or pharmaceuticals, which could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
The intellectual property protection for our product candidates depends on third parties.
With respect to Lymphoseek and Manocept, and NAV4694, we have exclusively licensed certain issued patents and pending patent applications covering the respective technologies underlying these product candidates and their commercialization and use and we
25
have licensed certain issued patents and pending patent applications directed to product compositions and chemical modifications used in product candidates for commercialization, and the use and the manufacturing thereof.
The patents and pending patent applications underlying our licenses do not cover all potential product candidates, modifications and uses. In the case of patents and patent applications licensed from UCSD, we did not have any control over the filing of the patents and patent applications before the effective date of the Lymphoseek license, and have had limited control over the filing and prosecution of these patents and patent applications after the effective date of the Lymphoseek license. In the case of patents and patent applications licensed from AstraZeneca, we have limited control over the filing, prosecution or enforcement of these patents or patent applications. We cannot be certain that such prosecution efforts have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents. We also cannot be assured that our licensors or their respective licensing partners will agree to enforce any such patent rights at our request or devote sufficient efforts to attain a desirable result. Any failure by our licensors or any of their respective licensing partners to properly protect the intellectual property rights relating to our product candidates could have a material adverse effect on our financial condition and results of operation.
We may become involved in disputes with licensors or potential future collaborators over intellectual property ownership, and publications by our research collaborators and scientific advisors could impair our ability to obtain patent protection or protect our proprietary information, which, in either case, could have a significant effect on our business.
Inventions discovered under research, material transfer or other such collaborative agreements may become jointly owned by us and the other party to such agreements in some cases and the exclusive property of either party in other cases. Under some circumstances, it may be difficult to determine who owns a particular invention, or whether it is jointly owned, and disputes could arise regarding ownership of those inventions. These disputes could be costly and time consuming and an unfavorable outcome could have a significant adverse effect on our business if we were not able to protect our license rights to these inventions. In addition, our research collaborators and scientific advisors generally have contractual rights to publish our data and other proprietary information, subject to our prior review. Publications by our research collaborators and scientific advisors containing such information, either with our permission or in contravention of the terms of their agreements with us, may impair our ability to obtain patent protection or protect our proprietary information, which could significantly harm our business.
Security breaches and other disruptions could compromise our information and expose us to liability, which would cause our business and reputation to suffer.
In the ordinary course of our business, we collect and store sensitive data, including intellectual property, our proprietary business information and that of our suppliers and business partners, and personally identifiable information of employees and clinical trial subjects, in our data centers and on our networks. The secure maintenance and transmission of this information is critical to our operations and business strategy. Despite our security measures, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise our networks and the information stored there could be accessed, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, and regulatory penalties, disrupt our operations, and damage our reputation, which could adversely affect our business, revenues and competitive position.
Failure to comply with domestic and international privacy and security laws can result in the imposition of significant civil and criminal penalties. The costs of compliance with these laws, including protecting electronically stored information from cyber-attacks, and potential liability associated with failure to do so could adversely affect our business, financial condition and results of operations. We are subject to various domestic and international privacy and security regulations, including but not limited to The Health Insurance Portability and Accountability Act of 1996 (HIPAA). HIPAA mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. In addition, many states have enacted comparable laws addressing the privacy and security of health information, some of which are more stringent than HIPAA.
We do not currently carry cyber risk insurance.
We are subject to domestic and foreign anticorruption laws, the violation of which could expose us to liability, and cause our business and reputation to suffer.
We are subject to the U.S. Foreign Corrupt Practices Act and similar anti-corruption laws in other jurisdictions. These laws generally prohibit companies and their intermediaries from engaging in bribery or making other prohibited payments to government officials for the purpose of obtaining or retaining business, and some have record keeping requirements. The failure to comply with these laws could result in substantial criminal and/or monetary penalties. We operate in jurisdictions that have experienced corruption, bribery,
26
pay-offs and other similar practices from time-to-time and, in certain circumstances, such practices may be local custom. We have implemented internal control policies and procedures that mandate compliance with these anti-corruption laws. However, we cannot be certain that these policies and procedures will protect us against liability. There can be no assurance that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or agents are found to have engaged in such practices, we could suffer severe criminal or civil penalties and other consequences that could have a material adverse effect on our business, financial position, results of operations and/or cash flow, and the market value of our common stock could decline.
Our international operations expose us to economic, legal, regulatory and currency risks.
Our operations extend to countries outside the United States, and are subject to the risks inherent in conducting business globally and under the laws, regulations, and customs of various jurisdictions. These risks include, but are not limited to: (i) compliance with a variety of national and local laws of countries in which we do business, including but not limited to restrictions on the import and export of certain intermediates, drugs, and technologies, (ii) compliance with a variety of US laws including, but not limited to, the Iran Threat Reduction and Syria Human Rights Act of 2012; and rules relating to the use of certain “conflict minerals” under Section 1502 of the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) changes in laws, regulations, and practices affecting the pharmaceutical industry and the health care system, including but not limited to imports, exports, manufacturing, quality, cost, pricing, reimbursement, approval, inspection, and delivery of health care, (iv) fluctuations in exchange rates for transactions conducted in currencies other than the functional currency, (v) adverse changes in the economies in which we or our partners and suppliers operate as a result of a slowdown in overall growth, a change in government or economic policies, or financial, political, or social change or instability in such countries that affects the markets in which we operate, particularly emerging markets, (vi) differing local product preferences and product requirements, (vii) changes in employment laws, wage increases, or rising inflation in the countries in which we or our partners and suppliers operate, (viii) supply disruptions, and increases in energy and transportation costs, (ix) natural disasters, including droughts, floods, and earthquakes in the countries in which we operate, (x) local disturbances, terrorist attacks, riots, social disruption, or regional hostilities in the countries in which we or our partners and suppliers operate and (xi) government uncertainty, including as a result of new or changed laws and regulations. We also face the risk that some of our competitors have more experience with operations in such countries or with international operations generally and may be able to manage unexpected crises more easily. Furthermore, whether due to language, cultural or other differences, public and other statements that we make may be misinterpreted, misconstrued, or taken out of context in different jurisdictions. Moreover, the internal political stability of, or the relationship between, any country or countries where we conduct business operations may deteriorate. Changes in a country’s political stability or the state of relations between any such countries are difficult to predict and could adversely affect our operations, profitability and/or adversely impact our ability to do business there. The occurrence of any of the above risks could have a material adverse effect on our business, financial position, results of operations and/or cash flow, and could cause the market value of our common stock to decline.
We may have difficulty raising additional capital, which could deprive us of necessary resources to pursue our business plans.
We expect to devote significant capital resources to fund research and development, to maintain existing and secure new manufacturing resources, and to acquire new product candidates. In order to support the initiatives envisioned in our business plan, we will likely need to raise additional funds through the sale of assets, public or private debt or equity financing, collaborative relationships or other arrangements. Our ability to raise additional financing depends on many factors beyond our control, including the state of capital markets, the market price of our common stock and the development or prospects for development of competitive technology by others. Sufficient additional financing may not be available to us or may be available only on terms that would result in further dilution to the current owners of our common stock.
Our future expenditures on our programs are subject to many uncertainties, including whether our product candidates will be developed or commercialized with a partner or independently. Our future capital requirements will depend on, and could increase significantly as a result of, many factors, including:
|
|
· |
the costs of seeking regulatory approval for our product candidates, including any nonclinical testing or bioequivalence or clinical studies, process development, scale-up and other manufacturing and stability activities, or other work required to achieve such approval, as well as the timing of such activities and approval; |
|
|
· |
the extent to which we invest in or acquire new technologies, product candidates, products or businesses and the development requirements with respect to any acquired programs; |
|
|
· |
the scope, prioritization and number of development and/or commercialization programs we pursue and the rate of progress and costs with respect to such programs; |
|
|
· |
the costs related to developing, acquiring and/or contracting for sales, marketing and distribution capabilities and regulatory compliance capabilities, if we commercialize any of our product candidates for which we obtain regulatory approval without a partner; |
27
|
|
· |
the timing and terms of any collaborative, licensing and other strategic arrangements that we may establish; |
|
|
· |
the extent to which we will need to expand our workforce to pursue our business plan, and the costs involved in recruiting, training and incentivizing new employees; |
|
|
· |
the effect of competing technological and market developments; and |
|
|
· |
the cost involved in establishing, enforcing or defending patent claims and other intellectual property rights. |
We believe that we currently have access to limited financial resources with which to fund our operations and those of our subsidiaries for the foreseeable future, including the proceeds of grants, cash flow generated from sales of Lymphoseek, and the Platinum line of credit. Given our recent low stock price and covenants in the Securities Exchange Agreement under which we issued our Series LL warrants, we may have only limited ability to raise equity financing, and our access to debt capital is limited by the terms of our indebtedness to Capital Royalty Partners II L.P. These constraints may adversely affect our ability to develop our product candidates or expand the labeling for Lymphoseek, as well as the timing of those efforts. If our current funds become inadequate, we may not be able to obtain sufficient additional funding for such activities, on satisfactory terms, if at all. If we are unsuccessful in raising additional capital, or the terms of raising such capital are unacceptable, we may have to modify our business plan and/or significantly curtail our planned development activities, acquisition of new product candidates and other operations.
There may be future sales or other dilution of our equity, which may adversely affect the market price of shares of our common stock.
Our existing warrants or other securities convertible into or exchangeable for our common stock, or securities we may issue in the future, may contain adjustment provisions that could increase the number of shares issuable upon exercise, conversion or exchange, as the case may be, and decrease the exercise, conversion or exchange price. The market price of our shares of common stock could decline as a result of sales of a large number of shares of our common stock or other securities in the market, the triggering of any such adjustment provisions or the perception that such sales could occur in the future.
Our indebtedness imposes significant restrictions on us, and a default could materially adversely affect our operations and financial condition.
All of our material assets, except our intellectual property, have been pledged as collateral for our borrowings under the Term Loan Agreement (the CRG Loan Agreement) with Capital Royalty Partners II L.P. (CRG).
In addition to the security interest in our assets, the CRG Loan Agreement carries covenants that impose significant requirements on us, including, among others, requirements that we:
|
|
· |
pay all principal, interest and other charges on the outstanding balance of the borrowed funds when due; |
|
|
· |
meet certain annual EBITDA or revenue targets ($22.5 million of revenue from Lymphoseek sales in 2016) as defined in the CRG Loan Agreement; |
|
|
· |
maintain liquidity of at least $5 million during the term of the CRG Loan Agreement; |
|
|
· |
provide certain financial information and reports to CRG in a timely manner; and |
|
|
· |
indemnify CRG against certain liabilities. |
Additionally, with certain exceptions, the CRG Loan Agreement prohibits us from:
|
|
· |
making any material dispositions of our assets, except for permitted dispositions; |
|
|
· |
making any changes in our business or business locations; |
|
|
· |
entering into any merger or consolidation without CRG’s consent; |
|
|
· |
acquiring or making investments in any other person other than permitted investments; |
|
|
· |
incurring any indebtedness, other than permitted indebtedness; |
|
|
· |
granting or permitting liens against our assets, other than permitted liens; |
|
|
· |
declaring or paying any dividends or making any other distributions; or |
|
|
· |
entering into any material transaction with any affiliate, other than in the ordinary course of business. |
The events of default under the CRG Loan Agreement also include a failure of Platinum to perform its funding obligations under the Platinum Loan Agreement (as defined below) at any time as to which the Company had negative EBITDA for the most recent fiscal
28
quarter, as a result either of Platinum’s repudiation of its obligations under the Platinum Loan Agreement, or the occurrence of an insolvency event with respect to Platinum. The events of default also include a change of control with respect to the Company. Our ability to comply with these provisions may be affected by changes in our business condition or results of our operations, or other events beyond our control. The breach of any of these covenants would result in a default under the CRG Loan Agreement.
We call to your attention that we did not achieve the 2015 annual Lymphoseek sales revenue target of $11 million initially established under the CRG Loan Agreement, but in December 2015 CRG agreed to a reduction of that target to $10 million and we were able to meet that reduced target with Lymphoseek sales revenue of $10.3 million, thereby complying with the covenant. We also call to your attention that as of the time of filing this report, it appears likely that we will need to draw on the Platinum line of credit in order to maintain compliance with the $5 million liquidity covenant of the CRG Loan Agreement beginning in the second quarter of 2016. In the event Platinum does not honor the draw request, our inability to meet the liquidity covenant would be an event of default under the CRG Loan Agreement. In addition, if we are unable to reach the 2016 annual Lymphoseek sales revenue target of $22.5 million, this would also be an event of default under the CRG Loan Agreement; however, potential shortfalls to this revenue covenant are curable by the Company depositing 2.5 times the amount of the shortfall in a bank account controlled by CRG. Our ability to comply with these covenants may be affected by changes in our business condition or results of operations, or other events beyond our control. An event of default would entitle CRG to accelerate the maturity of our indebtedness, increase the interest rate to the default rate of 18% per annum, and invoke other remedies available to it under the loan agreement and the related security agreement, including sale of the assets securing the debt, which could raise substantial doubt about the Company’s ability to continue as a going concern. See Management’s Discussion and Analysis of Financial Conditions and Results of Operations – Liquidity and Capital Resources.
In addition, our Loan Agreement with Platinum (the Platinum Loan Agreement) carries standard non-financial covenants typical for commercial loan agreements, many of which are similar to those contained in the CRG Loan Agreement, that impose significant requirements on us. Our ability to comply with these provisions may be affected by changes in our business condition or results of our operations, or other events beyond our control. The breach of any of these covenants would result in a default under the Platinum Loan Agreement, permitting Platinum to terminate our ability to obtain additional draws under the Platinum Loan Agreement and accelerate the maturity of the debt, subject to the limitations of the Subordination Agreement with CRG. Such actions by Platinum could materially adversely affect our operations, results of operations and financial condition, including causing us to substantially curtail our product development activities.
Platinum may exercise its conversion right, and that could dilute your ownership and the net tangible book value per share of our common stock.
Platinum may exercise the right to convert all or any portion of the unpaid principal or unpaid interest (the Conversion Amount) accrued on any draw advanced by Platinum under the Platinum Loan Agreement into shares of Navidea’s common stock, provided that our common stock is trading above $2.53 per share. Platinum may also exercise a conversion right on the amount of any mandatory repayment due following the Company achieving $2,000,000 in cumulative revenues from sales or licensing of Lymphoseek, provided that our common stock is trading above $2.53 per share, if the Company is prohibited from making such repayment under the terms of the Subordination Agreement between Platinum, CRG and the Company. If Platinum exercises any or all of its conversion rights, the percentage ownership of our current stockholders will be reduced. The issuance of additional common stock may also result in dilution in the net tangible book value per share of our common stock.
Shares of common stock are equity securities and are subordinate to our existing and future indebtedness and preferred stock.
Shares of our common stock are common equity interests. This means that our common stock ranks junior to any preferred stock that we may issue in the future, to our indebtedness and to all creditor claims and other non-equity claims against us and our assets available to satisfy claims on us, including claims in a bankruptcy or similar proceeding. Our existing indebtedness restricts payment of dividends on our common stock, and future indebtedness and preferred stock may restrict payments of dividends on our common stock.
Additionally, unlike indebtedness, where principal and interest customarily are payable on specified due dates, in the case of our common stock, (i) dividends are payable only when and if declared by our Board of Directors or a duly authorized committee of our Board of Directors, and (ii) as a corporation, we are restricted to making dividend payments and redemption payments out of legally available assets. We have never paid a dividend on our common stock and have no current intention to pay dividends in the future. Furthermore, our common stock places no restrictions on our business or operations or on our ability to incur indebtedness or engage in any transactions, subject only to the voting rights available to shareholders generally.
29
The continuing contentious federal budget negotiations may have an impact on our business and financial condition in ways that we currently cannot predict, and may further limit our ability to raise additional funds.
The continuing federal budget disputes not only may adversely affect financial markets, but could also delay or reduce research grant funding and adversely affect operations of government agencies that regulate us, including the FDA, potentially causing delays in obtaining key regulatory approvals.
Our failure to maintain continued compliance with the listing requirements of the NYSE MKT exchange could result in the delisting of our common stock.
Our common stock has been listed on the NYSE MKT since February 2011. The rules of NYSE MKT provide that shares be delisted from trading in the event the financial condition and/or operating results of the Company appear to be unsatisfactory, the extent of public distribution or the aggregate market value of the common stock has become so reduced as to make further dealings on the NYSE MKT inadvisable, the Company has sold or otherwise disposed of its principal operating assets, or has ceased to be an operating company, or the Company has failed to comply with its listing agreements with the Exchange. For example, the NYSE MKT may consider suspending trading in, or removing the listing of, securities of an issuer that has stockholders’ equity of less than $6.0 million if such issuer has sustained losses from continuing operations and/or net losses in its five most recent fiscal years. As of December 31, 2015, the Company had a stockholders’ deficit of approximately $53.8 million. Even if an issuer has a stockholders’ deficit, the NYSE MKT will not normally consider removing from the list securities of an issuer that fails to meet these requirements if the issuer has (1) total value of market capitalization of at least $50,000,000; or total assets and revenue of $50,000,000 each in its last fiscal year, or in two of its last three fiscal years; and (2) the issuer has at least 1,100,000 shares publicly held, a market value of publicly held shares of at least $15,000,000 and 400 round lot shareholders. Based on the number of outstanding shares of our common stock, recent trading price of that stock, and number of round lot holders, we believe that we meet these exception criteria and that our common stock will not be delisted as a result of our failure to meet the minimum stockholders' equity requirement for continued listing. We cannot assure you that the Company will continue to meet these and other requirements necessary to maintain the listing of our common stock on the NYSE MKT. For example, we may determine to grow our organization or product pipeline or pursue development or other activities at levels or on timelines that reduces our stockholders’ equity below the level required to maintain compliance with NYSE MKT continued listing standards.
The NYSE MKT Company Guide also provides that the Exchange may suspend or remove from listing any common stock selling for a substantial period of time at a low price per share, if the issuer shall fail to effect a reverse split of such shares within a reasonable time after being notified that the Exchange deems such action to be appropriate under all the circumstances. The Company’s common stock has recently traded for a price as low as $0.75 per share, and if the low trading price persists, there is a risk that the Exchange may require the Company to effect a reverse split of its common stock in order to maintain its NYSE MKT listing, and that the shares will be delisted if such action is not taken to the satisfaction of the NYSE MKT.
The delisting of our common stock from the NYSE MKT likely would reduce the trading volume and liquidity in our common stock and may lead to decreases in the trading price of our common stock. The delisting of our common stock may also materially impair our stockholders’ ability to buy and sell shares of our common stock. In addition, the delisting of our common stock could significantly impair our ability to raise capital, which is critical to the execution of our current business strategy.
The price of our common stock has been highly volatile due to several factors that will continue to affect the price of our stock.
Our common stock traded as low as $0.75 per share and as high as $2.50 per share during the 12-month period ended February 29, 2016. The market price of our common stock has been and is expected to continue to be highly volatile. Factors, including announcements of technological innovations by us or other companies, regulatory matters, new or existing products or procedures, concerns about our financial position, operating results, litigation, government regulation, developments or disputes relating to agreements, patents or proprietary rights, may have a significant impact on the market price of our stock. In addition, potential dilutive effects of future sales of shares of common stock by the Company and by stockholders, and subsequent sale of common stock by the holders of warrants and options could have an adverse effect on the market price of our shares.
Some additional factors which could lead to the volatility of our common stock include:
|
|
· |
market perceptions of our ability to meet the revenue and liquidity covenants contained in the CRG Loan Agreement; |
|
|
· |
price and volume fluctuations in the stock market at large or of companies in our industry which do not relate to our operating performance; |
|
|
· |
changes in securities analysts’ estimates of our financial performance or deviations in our business and the trading price of our common stock from the estimates of securities analysts; |
|
|
· |
FDA or international regulatory actions and regulatory developments in the U.S. and foreign countries; |
30
|
|
· |
financing arrangements we may enter that require the issuance of a significant number of shares in relation to the number of shares currently outstanding; |
|
|
· |
public concern as to the safety of products that we or others develop; |
|
|
· |
activities of short sellers in our stock; and |
|
|
· |
fluctuations in market demand for and supply of our products. |
The realization of any of the foregoing could have a dramatic and adverse impact on the market price of our common stock. In addition, class action litigation has often been instituted against companies whose securities have experienced substantial decline in market price. Moreover, regulatory entities often undertake investigations of investor transactions in securities that experience volatility following an announcement of a significant event or condition. Any such litigation brought against us or any such investigation involving our investors could result in substantial costs and a diversion of management’s attention and resources, which could hurt our business, operating results and financial condition.
An investor’s ability to trade our common stock may be limited by trading volume.
During the 12-month period beginning on March 1, 2015 and ending on February 29, 2016, the average daily trading volume for our common stock on the NYSE MKT was approximately 600,000 shares. We cannot assure you that this trading volume will be consistently maintained in the future.
The market price of our common stock may be adversely affected by market conditions affecting the stock markets in general, including price and trading fluctuations on the NYSE MKT exchange.
The market price of our common stock may be adversely affected by market conditions affecting the stock markets in general, including price and trading fluctuations on the NYSE MKT. These conditions may result in (i) volatility in the level of, and fluctuations in, the market prices of stocks generally and, in turn, our shares of common stock, and (ii) sales of substantial amounts of our common stock in the market, in each case that could be unrelated or disproportionate to changes in our operating performance.
Because we do not expect to pay dividends on our common stock in the foreseeable future, stockholders will only benefit from owning common stock if it appreciates.
We have paid no cash dividends on any of our common stock to date, and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. As a result, with respect to our common stock, we do not expect to pay any cash dividends in the foreseeable future, and payment of cash dividends, if any, will also depend on our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our Board of Directors. Furthermore, we are subject to various laws and regulations that may restrict our ability to pay dividends and we may in the future become subject to contractual restrictions on, or prohibitions against, the payment of dividends. Due to our intent to retain any future earnings rather than pay cash dividends on our common stock and applicable laws, regulations and contractual obligations that may restrict our ability to pay dividends on our common stock, the success of your investment in our common stock will likely depend entirely upon any future appreciation and there is no guarantee that our common stock will appreciate in value.
We may have difficulty attracting and retaining qualified personnel and our business may suffer if we do not.
Our business has experienced a number of successes and faced several challenges in recent years that have resulted in several significant changes in our strategy and business plan, including the shifting of resources to support our current development initiatives. Our management will need to remain flexible to support our business model over the next few years. However, losing members of the Navidea management team could have an adverse effect on our operations. Our success depends on our ability to attract and retain technical and management personnel with expertise and experience in the pharmaceutical industry, and the acquisition of additional product candidates may require us to acquire additional highly qualified personnel. The competition for qualified personnel in the biotechnology industry is intense and we may not be successful in hiring or retaining the requisite personnel. If we are unable to attract and retain qualified technical and management personnel, we will suffer diminished chances of future success.
We may be adversely affected if our controls over external financial reporting fail or are circumvented.
We regularly review and update our internal controls, disclosure controls and procedures, and corporate governance policies. In addition, we are required under the Sarbanes Oxley Act of 2002 to report annually on our internal control over financial reporting. If it were to be determined that our internal control over financial reporting is not effective, such shortcoming could have an adverse effect on our business and financial results and the price of our common stock could be negatively affected. This reporting
31
requirement could also make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. Any system of internal controls, however well designed and operated, is based in part on certain assumptions and can provide only reasonable, not absolute, assurances that the objectives of the system are met. Any failure or circumvention of the controls and procedures or failure to comply with regulation concerning control and procedures could have a material effect on our business, results of operation and financial condition. Any of these events could result in an adverse reaction in the financial marketplace due to a loss of investor confidence in the reliability of our financial statements, which ultimately could negatively affect the market price of our shares, increase the volatility of our stock price and adversely affect our ability to raise additional funding. The effect of these events could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors and our Board committees and as executive officers.
We call to your attention that our management’s evaluation of the effectiveness of the Company’s internal controls over financial reporting as of December 31, 2015 concluded that our controls were not effective, due to a material weakness resulting from the failure of officers of our subsidiary, Macrophage Therapeutics, Inc., to provide accurate and timely information to the Company. However, notwithstanding this material weakness, management has concluded that the consolidated financial statements included in this Report fairly present, in all material respects, the Company’s consolidated financial position, results of operations and cash flows for the periods presented therein, in conformity with accounting principles generally accepted in the United States of America. Although the Company is taking steps to remediate the material weakness, there can be no assurance that similar incidents can be prevented in the future if the internal controls are not followed by officers of the Company or our subsidiaries. See Controls and Procedures—Disclosure Controls and Procedures.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We currently lease approximately 25,000 square feet of office space at 5600 Blazer Parkway, Dublin, Ohio, as our principal offices. The current lease term expires in October 2022, at a monthly base rent of approximately $25,000 during 2016. We must also pay a pro-rata portion of the operating expenses and real estate taxes of the building. We believe this facility is in good condition.
Item 3. Legal Proceedings
Section 16(b) Action
On August 12, 2015, a shareholder of the Company filed an action in the United States District Court for the Southern District of New York against two funds managed by Platinum alleging violations of Section 16(b) of the Securities Exchange Act in connection with purchases and sales of the Company’s common stock by the Platinum funds, and seeking disgorgement of the short-swing profits realized by the funds. The Company is a nominal defendant in the action, no relief is sought against the Company, and a portion of any amount awarded to the plaintiff by judgment or settlement of the action will likely accrue to the benefit of the Company. Platinum has moved to dismiss the action, and a decision on the motion to dismiss is expected shortly following oral argument, currently scheduled for March 30, 2016.
Sinotau Litigation
On August 31, 2015, Sinotau Pharmaceutical Group (Sinotau) filed a suit for damages, specific performance and injunctive relief against the Company in the United States District Court for the District of Massachusetts alleging breach of a letter of intent for licensing to Sinotau of the Company’s NAV4694 product candidate and technology. The Company believes the suit is without merit and has filed a motion to dismiss the action. While it is not possible to determine with any degree of certainty the ultimate outcome of this legal proceeding, including making a determination of liability, we believe that we have meritorious defenses with respect to the claims asserted against us and intend to vigorously defend our position.
Item 4. Mine Safety Disclosure
Not applicable.
32
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock trades on the NYSE MKT exchange under the trading symbol NAVB. Prior to our name change from Neoprobe Corporation to Navidea Biopharmaceuticals, Inc. on January 5, 2012, our common stock was traded on the NYSE MKT under the trading symbol NEOP. The prices set forth below reflect the quarterly high and low sales prices for shares of our common stock during the last two fiscal years.
|
|
|
High |
|
|
Low |
|
||
|
Fiscal Year 2015: |
|
|
|
|
|
|
|
|
|
First Quarter |
|
$ |
1.96 |
|
|
$ |
1.55 |
|
|
Second Quarter |
|
|
1.67 |
|
|
|
1.22 |
|
|
Third Quarter |
|
|
2.50 |
|
|
|
1.28 |
|
|
Fourth Quarter |
|
|
2.40 |
|
|
|
1.32 |
|
|
|
|
|
|
|
|
|
|
|
|
Fiscal Year 2014: |
|
|
|
|
|
|
|
|
|
First Quarter |
|
$ |
2.12 |
|
|
$ |
1.62 |
|
|
Second Quarter |
|
|
1.99 |
|
|
|
1.29 |
|
|
Third Quarter |
|
|
1.53 |
|
|
|
1.20 |
|
|
Fourth Quarter |
|
|
2.02 |
|
|
|
0.97 |
|
As of March 1, 2016, we had approximately 650 holders of common stock of record.
We have not paid any dividends on our common stock and do not anticipate paying cash dividends on our common stock in the foreseeable future. We intend to retain any earnings to finance the growth of our business. We cannot assure you that we will ever pay cash dividends. Whether we pay cash dividends in the future will be at the discretion of our Board of Directors and will depend upon our financial condition, results of operations, capital requirements and any other factors that the Board of Directors decides are relevant. See Management’s Discussion and Analysis of Financial Condition and Results of Operations.
During the three-month period ended December 31, 2015, we issued 18,501 shares of our common stock to our Board of Directors as payment of their second and third quarter 2015 retainers. The issuance of these securities was exempt from registration under Section 4(2) of the Securities Act and Regulation D promulgated thereunder.
There were no repurchases of our common stock during the three-month period ended December 31, 2015.
33
The following graph compares the cumulative total return on a $100 investment in each of the common stock of the Company, the Russell 3000, and the NASDAQ Biotechnology Index for the period from December 31, 2010 through December 31, 2015. This graph assumes an investment in the Company’s common stock and the indices of $100 on December 31, 2010 and that any dividends were reinvested.
COMPARISON OF 5 YEAR CUMULATIVE TOTAL RETURN*
Among Navidea Biopharmaceuticals, the Russell 3000 Index, and the NASDAQ Biotechnology Index
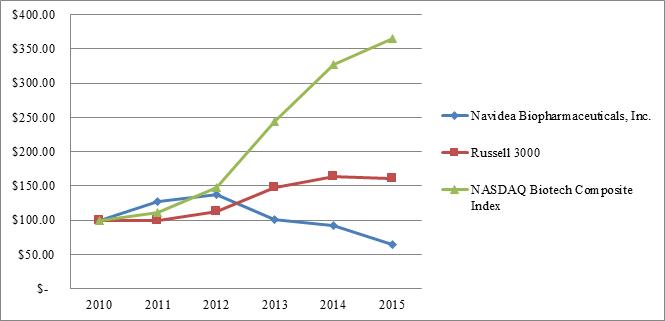
|
* |
$100 invested on 12/31/2010 in stock or index, including reinvestment of dividends. |
|
|
|
Cumulative Total Return as of December 31, |
|
|||||||||||||||||||||
|
|
|
2010 |
|
|
2011 |
|
|
2012 |
|
|
2013 |
|
|
2014 |
|
|
2015 |
|
||||||
|
Navidea Biopharmaceuticals |
|
|
100.00 |
|
|
|
127.18 |
|
|
|
137.38 |
|
|
|
100.49 |
|
|
|
91.75 |
|
|
|
64.56 |
|
|
Russell 3000 |
|
|
100.00 |
|
|
|
99.08 |
|
|
|
112.93 |
|
|
|
147.87 |
|
|
|
163.33 |
|
|
|
160.92 |
|
|
NASDAQ Biotechnology |
|
|
100.00 |
|
|
|
111.81 |
|
|
|
147.48 |
|
|
|
244.24 |
|
|
|
327.52 |
|
|
|
364.93 |
|
34
Item 6. Selected Financial Data
The following summary financial data are derived from our consolidated financial statements that have been audited by our independent registered public accounting firm. These data are qualified in their entirety by, and should be read in conjunction with, our Consolidated Financial Statements and Notes thereto included elsewhere in this Form 10-K as well as Management’s Discussion and Analysis of Financial Condition and Results of Operations. Summary financial data for 2015 and 2011 reflect the disposition of our gamma detection device business in August 2011 and the reclassification of certain related items to discontinued operations.
|
(Amounts in thousands, except per share data) |
Years Ended December 31, |
|
|||||||||||||||||
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
2012 |
|
|
2011 |
|
|||||
|
Statement of Operations Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenue |
$ |
13,249 |
|
|
$ |
6,275 |
|
|
$ |
1,131 |
|
|
$ |
79 |
|
|
$ |
598 |
|
|
Cost of goods sold |
|
1,755 |
|
|
|
1,586 |
|
|
|
333 |
|
|
|
— |
|
|
|
— |
|
|
Research and development expenses |
|
12,788 |
|
|
|
16,780 |
|
|
|
23,710 |
|
|
|
16,890 |
|
|
|
15,154 |
|
|
Selling, general and administrative expenses |
|
17,257 |
|
|
|
15,542 |
|
|
|
15,526 |
|
|
|
11,178 |
|
|
|
9,548 |
|
|
Loss from operations |
|
(18,551 |
) |
|
|
(27,633 |
) |
|
|
(38,438 |
) |
|
|
(27,989 |
) |
|
|
(24,104 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other expenses, net |
|
(10,208 |
) |
|
|
(8,094 |
) |
|
|
(4,261 |
) |
|
|
(1,168 |
) |
|
|
(943 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Benefit from income taxes |
|
436 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
7,880 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from continuing operations |
|
(28,323 |
) |
|
|
(35,727 |
) |
|
|
(42,699 |
) |
|
|
(29,157 |
) |
|
|
(17,167 |
) |
|
Discontinued operations, net of tax effect |
|
759 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
22,780 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net (loss) income |
|
(27,564 |
) |
|
|
(35,727 |
) |
|
|
(42,699 |
) |
|
|
(29,157 |
) |
|
|
5,613 |
|
|
Less loss attributable to noncontrolling interest |
|
(1 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Deemed dividend |
|
(46 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Preferred stock dividends |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(43 |
) |
|
|
(100 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Loss) income attributable to common stockholders |
$ |
(27,609 |
) |
|
$ |
(35,727 |
) |
|
$ |
(42,699 |
) |
|
$ |
(29,200 |
) |
|
$ |
5,513 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Loss) income per common share (basic and diluted): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Continuing operations |
$ |
(0.19 |
) |
|
$ |
(0.24 |
) |
|
$ |
(0.35 |
) |
|
$ |
(0.29 |
) |
|
$ |
(0.17 |
) |
|
Discontinued operations |
$ |
0.01 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
0.23 |
|
|
(Loss) income attributable to common stockholders |
$ |
(0.18 |
) |
|
$ |
(0.24 |
) |
|
$ |
(0.35 |
) |
|
$ |
(0.29 |
) |
|
$ |
0.06 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Shares used in computing (loss) income per common share: (1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
151,180 |
|
|
|
148,748 |
|
|
|
121,809 |
|
|
|
99,060 |
|
|
|
90,509 |
|
|
|
As of December 31, |
|
|||||||||||||||||
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|
2012 |
|
|
2011 |
|
|||||
|
Balance Sheet Data: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets |
$ |
14,965 |
|
|
$ |
11,830 |
|
|
$ |
39,626 |
|
|
$ |
11,677 |
|
|
$ |
30,603 |
|
|
Long-term liabilities |
|
62,616 |
|
|
|
32,573 |
|
|
|
32,703 |
|
|
|
7,107 |
|
|
|
6,122 |
|
|
Accumulated deficit |
|
(380,547 |
) |
|
|
(352,984 |
) |
|
|
(317,257 |
) |
|
|
(274,558 |
) |
|
|
(245,357 |
) |
|
(1) |
Basic earnings (loss) per share is calculated by dividing net income (loss) attributable to common stockholders by the weighted-average number of common shares and, except for periods with a loss from operations, participating securities outstanding during the period. Diluted earnings (loss) per share reflects additional common shares that would have been outstanding if dilutive potential common shares had been issued. Potential common shares that may be issued by the Company include convertible securities, options and warrants. |
35
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion should be read together with our Consolidated Financial Statements and the Notes related to those statements, as well as the other financial information included in this Form 10-K. Some of our discussion is forward-looking and involves risks and uncertainties. For information regarding risk factors that could have a material adverse effect on our business, refer to Item 1A of this Form 10-K, Risk Factors.
The Company
Navidea Biopharmaceuticals, Inc. is a biopharmaceutical company focused on the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. Navidea is developing multiple precision-targeted products based on our Manocept platform to help identify the sites and pathways of undetected disease and enable better diagnostic accuracy, clinical decision-making, targeted treatment and, ultimately, patient care.
Navidea’s Manocept platform is predicated on the ability to specifically target the CD206 mannose receptor expressed on activated macrophages. The Manocept platform serves as the molecular backbone of Lymphoseek, the first product developed by Navidea based on the platform. Lymphoseek is a novel, state-of-the-art, receptor-targeted, small-molecule radiopharmaceutical used in the evaluation of lymphatic basins that may have cancer involvement in patients. Lymphoseek is designed for the precise identification of lymph nodes that drain from a primary tumor, which have the highest probability of harboring cancer. Lymphoseek is approved by the U.S. FDA for use in solid tumor cancers where lymphatic mapping is a component of surgical management and for guiding sentinel lymph node biopsy in patients with clinically node negative breast cancer, melanoma or squamous cell carcinoma of the oral cavity. Lymphoseek has also received European approval in imaging and intraoperative detection of sentinel lymph nodes in patients with melanoma, breast cancer or localized squamous cell carcinoma of the oral cavity.
Building on the success of Lymphoseek, the flexible and versatile Manocept platform acts as an engine for the design of purpose-built molecules offering the potential to be utilized across a range of diagnostic modalities, including SPECT, PET, intra-operative and/or optical-fluorescence detection in a variety of disease states.
Preclinical data being developed by the Company using tilmanocept linked to a therapeutic agent also suggest that tilmanocept’s binding affinity to CD206 receptors demonstrates the potential for this technology to be useful in treating diseases linked to the over-activation of macrophages. This includes various cancers as well as autoimmune, infectious, cardiovascular, and central nervous system diseases. Thus, in January 2015, the Company formed a subsidiary, Macrophage Therapeutics, Inc., to further explore therapeutic applications for the Manocept platform.
Our increasing focus on development of our proprietary Manocept platform technology further supports the 2014 decision by the Company’s Board of Directors to reduce our support for, while seeking to partner or out-license, our two neurological development programs, NAV4694 and NAV5001. The NAV5001 sublicense was terminated in April 2015.
Other than Lymphoseek, none of the Company’s drug product candidates have been approved for sale in any market.
Executive Summary
In 2015, the Company created and executed a new commercialization strategy for Lymphoseek to better leverage the label expansion approved by the FDA in October 2014 and, over time, accelerate its market penetration. This included a complete refresh of the brand, moving away from a feature-based selling approach to a customer-centric one focused on the benefits to the surgeon and patient.
In concert with the rebranding efforts, the Company also recruited, hired and trained a new salesforce in the latter part of the second quarter of 2015. The new direct sales team focused on targeting the highest priority territories. The Company anticipated the sales team’s contributions would be most significant starting the fourth quarter of 2015 and onward based on the four- to six-month sales cycle of Lymphoseek.
The initial launch of the new commercialization strategy resulted in the following during 2015:
|
|
· |
Grew Lymphoseek sales by 142% year over year, based on Lymphoseek sales to Navidea of $10.3 million for 2015; |
|
|
· |
Ended the year at an annualized sales run rate for Lymphoseek of over $15 million in revenue to Navidea, which does not reflect the opportunity for additional growth in existing and expansion sales territories throughout 2016; and |
|
|
· |
Achieved an increase of approximately 60% in “average daily doses sold” from the end of 2014 through the end of 2015. |
We enter 2016 with positive momentum behind our new commercialization strategy. We expect our sales team’s strong contributions to continue to accelerate product revenues throughout the year. In addition, European sales revenues are expected to be generated from commercialization efforts with SpePharm AG, an affiliate of Norgine BV, to begin in the fourth quarter of 2016.
36
In parallel, our R&D team is aggressively advancing our immunodiagnostics pipeline focused on significantly larger market opportunities including RA, which has a prevalence of approximately 3.8 million patients in the U.S. and Europe.
The Company continues to work toward a more focused development program using its Manocept platform in immunodiagnostics, including the FDA label expansion for Lymphoseek into RA and KS. Importantly, the costs of these development programs will be defrayed by NIH grants awarded to the Company in 2015 totaling over $3.8 million.
In the near term, the Company intends to:
|
|
· |
Meet with the FDA before the end of March 2016 to share preclinical results on the intravenous route of administration (IVROA) and discuss and agree on the Phase 1 and 2 clinical plan for our RA immunodiagnostic program. This is a follow-up meeting to the one that took place in May 2015, where the Company and the FDA confirmed requirements for a preclinical submission package for the use of Lymphoseek in IVROA; |
|
|
· |
Finalize preparations to initiate a Phase 1 pilot trial evaluating subcutaneous injection of Lymphoseek in active RA subjects in the first half of 2016. Initiate a subsequent Phase 1/2 registrational trial of IV-administered Lymphoseek for the RA immunodiagnostic application during the second half of 2016; |
|
|
· |
Expand patient enrollment in the FDA/EMA mandated pediatric trial for children with melanoma, rhabdomyosarcoma, or other solid tumors who are undergoing lymph node mapping and who meet special criteria for pediatric sentinel node biopsy. Since the first patient was dosed in December 2015, two new sites have been added including Cincinnati Children’s Hospital Medical Center; |
|
|
· |
Enroll first patient at the MD Anderson Cancer Center in the Company’s multi–center cervical cancer study; |
|
|
· |
Initiate patient enrollment in an investigator-initiated endometrial cancer study directed by Dr. Michael McHale at UC San Diego Health System; and |
|
|
· |
Expand patient enrollment in the cardiovascular immunodiagnostic study, an investigator-initiated study in collaboration with Massachusetts General Hospital. Based on encouraging findings in the first patients dosed, we are very excited by the potential and are seeking to accelerate and expand this funded program using a protocol for IVROA. |
Substantial progress on the Manocept platform has resulted in several promising opportunities, including our R-NAV venture which began in July 2014, the formation of Macrophage Therapeutics, Inc. in January 2015, and Macrophage Therapeutics' research collaboration agreement with BIND Therapeutics, Inc. executed in June 2015, which we believe may further expand the Company's pipeline. Our progress in moving forward the Manocept pipeline was made possible in large part by the May 2014 decision to refocus the Company’s resources to better align the funding of our pipeline programs with the expected growth in Lymphoseek revenue. This realignment has resulted in the near-term reduction of expenditures on the two neurological product candidates, NAV4694 and NAV5001. In April 2015, the Company entered into an agreement with Alseres to terminate the NAV5001 sub-license agreement. The Company is currently engaged in discussions related to the potential partnering or divestiture of NAV4694.
Our Outlook
Following the U.S. approval of Lymphoseek in March 2013, the Company undertook the initial stages of product launch in the U.S. with our commercialization partner, Cardinal Health, in May 2013. In October 2014, we received approval from FDA for a significantly expanded product label for Lymphoseek. During the second quarter of 2015, we successfully deployed Navidea's direct sales personnel as part of our effort to accelerate Lymphoseek revenue growth in the remainder of 2015 and beyond. Our strategy for increasing Lymphoseek revenue focuses on a new brand strategy reflective of the expanded product label that allows the delivery of a compelling clinical value proposition message targeting the oncology treatment team including surgical oncologists and nuclear medicine physicians, focusing on areas where the concentration of cancer diagnosis occurs to increase the total number of hospitals using Lymphoseek, and increasing the number of doses utilized per account, while continuing to evolve the brand.
37
Our operating expenses in recent years have been focused primarily on support of Lymphoseek, our Manocept platform, and NAV4694 and NAV5001 product development. We incurred approximately $12.8 million, $16.8 million and $23.7 million in total on research and development activities during the years ended December 31, 2015, 2014 and 2013, respectively. Of the total amounts we spent on research and development during those periods, excluding costs related to our internal research and development headcount and our general and administrative staff which we do not currently allocate among the various development programs that we have underway, we incurred out-of-pocket charges by program as follows:
|
Development Program * |
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Lymphoseek |
|
$ |
2,365,128 |
|
|
$ |
995,511 |
|
|
$ |
4,702,829 |
|
|
Manocept platform |
|
|
767,431 |
|
|
|
503,587 |
|
|
|
503,338 |
|
|
Macrophage Therapeutics |
|
|
538,813 |
|
|
|
— |
|
|
|
— |
|
|
NAV4694 |
|
|
3,448,724 |
|
|
|
6,788,286 |
|
|
|
7,812,602 |
|
|
NAV5001 |
|
|
385,344 |
|
|
|
1,441,442 |
|
|
|
2,602,461 |
|
* Certain development program expenditures were offset by grant reimbursement revenues totaling $1.7 million, $1.7 million, and $490,000 during the years ended December 31, 2015, 2014 and 2013, respectively.
We expect to continue the advancement of our efforts with Lymphoseek and our Manocept platform during 2016. The divestiture of NAV5001 and the suspension of active patient accrual in our NAV4694 trials have decreased our development costs over the past year, however, we continue to incur costs to maintain the trials and drug production while we complete our partnering/divestiture activities. We expect that our total research and development expenses, including both out-of-pocket charges as well as internal headcount and support costs, to be lower in 2016 than in 2015, perhaps by as much as 30%. This estimate excludes charges related to our subsidiary, Macrophage Therapeutics, Inc., which are currently expected to be funded separately. In addition, these estimates include carrying costs for NAV4694 only through mid-year.
Lymphoseek was approved and indicated for use in lymphatic mapping in patients with breast cancer and melanoma by the FDA in March 2013, with expanded use of Lymphoseek indicated for guiding sentinel lymph node biopsy in head and neck cancer patients with squamous cell carcinoma of the oral cavity approval in June 2014, and for lymphatic mapping in solid tumors and sentinel lymph node detection for breast cancer and melanoma as well as with or without scintigraphic imaging, known as lymphoscintigraphy, in October 2014. Lymphoseek was also approved by the EMA for use in imaging and intraoperative detection of sentinel lymph nodes draining a primary tumor in adult patients with breast cancer, melanoma, or localized squamous cell carcinoma of the oral cavity in the EU in November 2014.
Although our marketing partners share a portion of the direct marketing, sales and distribution costs related to the sale of Lymphoseek, we expect to incur ongoing costs to support product marketing efforts targeting surgical oncologists at the core of the oncology treatment team, as well as medical education-related and market outreach activities associated with Lymphoseek commercialization. Additionally, we anticipate that we will incur costs related to supporting the other product, regulatory, manufacturing and commercial activities related to the potential marketing registration and sale of Lymphoseek in other markets, including a reduced-mass vial intended for sale in the EU. We also expect to incur costs related to ongoing clinical development efforts to support the use of Lymphoseek in additional cancer types. We cannot assure you that Lymphoseek will achieve regulatory approval in any other market outside the U.S. or EU, or if approved in those markets, that it will achieve market acceptance in the U.S., EU or any other market.
We are currently evaluating existing and emerging data on the potential use of Manocept-related agents in the diagnosis and disease-staging of disorders in which macrophages are involved, such as KS, RA, vulnerable plaque/atherosclerosis, TB and other disease states, to define areas of focus, development pathways and partnering options to capitalize on the Manocept platform. In the near-term, our more active development efforts with respect to the Manocept platform will likely be limited to such evaluations. We will also be evaluating potential funding and other resources required for continued development, regulatory approval and commercialization of any Manocept platform product candidates that we identify for further development, and potential options for advancing development. We cannot assure you that further evaluation or development will be successful, that any Manocept platform product candidate will ultimately achieve regulatory approval, or if approved, the extent to which it will achieve market acceptance.
The Company projects that its total revenue for 2016, including Lymphoseek product sales revenue, license revenue, grant and other revenue, will be in the range of $23 million to $25 million. Gross margins on Lymphoseek product sales are expected to continue to remain at over 80% in the coming quarters. Based on our current projections, we expect total operating expenses for 2016 to be between $21.5 million and $23.5 million, excluding charges related to our subsidiary, Macrophage Therapeutics, Inc., which are currently expected to be funded separately. As a result of our revenue and margin expectations for 2016, coupled with our expectations of operating expenses for the year, we also expect to reach cash flow breakeven on an operating or per-share basis in the second half of 2016.
38
Years Ended December 31, 2015 and 2014
Net Sales and Margins. Net sales of Lymphoseek were $10.3 million during 2015, compared to $4.2 million during 2014. The increase was primarily the result of continued efforts to increase sales. Gross margins on net sales were 83% and 63% for 2015 and 2014, respectively. Cost of goods sold in 2015 included a net benefit of $253,000 related to our ability to sell certain previously reserved inventory, partially offset by net inventory losses of $93,000 related to a production matter and reserves for inventory obsolescence totaling $52,000 related to specific lots which expired during the period. Cost of goods sold in 2014 included a reserve for inventory obsolescence of $539,000 related to a specific lot which was originally produced for validation purposes but was nearing its product expiry and therefore was no longer expected to be sold. Cost of goods sold in both periods included post-production testing activities required by regulatory authorities, which are charged as one-time period costs, and a royalty on net sales payable under our license agreement with UCSD.
Lymphoseek License Revenue. During 2015, we recognized $833,000 of the $2.0 million non-refundable upfront payment received by the Company related to the Lymphoseek license and distribution agreement for Europe, which the Company is recognizing on a straight-line basis over two years. During 2015 and 2014, we recognized $300,000 of Lymphoseek license revenue from non-refundable milestone payments received by the Company related to the Lymphoseek distribution agreement for China, for which the Company has no future obligations.
Grant and Other Revenue. During 2015, we recognized $1.9 million of grant and other revenue as compared to $1.7 million in 2014. Grant revenue during 2015 was primarily related to SBIR grants from the NIH supporting NAV4694, Lymphoseek and Manocept platform development. Grant revenue during 2014 was primarily related to SBIR grants from the NIH supporting NAV4694 and NAV1800 development. Grant and other revenue for 2015 and 2014 also included $140,000 and $90,000, respectively, related to services provided to R-NAV for Manocept development.
Research and Development Expenses. Research and development expenses decreased $4.0 million, or 24%, to $12.8 million during 2015 from $16.8 million during 2014. The decrease was primarily due to net decreases in drug project expenses related to (i) decreased NAV4694 development costs of $3.3 million including decreased clinical trial costs and manufacturing-related activities while we continued our efforts to divest the program; and (ii) decreased NAV5001 development costs of $1.1 million including decreased clinical trial costs and manufacturing-related activities; offset by (iii) increased Lymphoseek development costs of $1.4 million due to a refund of sNDA filing fees of $1.1 million reducing costs incurred in 2014, increased manufacturing-related activities, preclinical testing, and clinical trial costs, offset by decreased license fees; (iv) increased therapeutics development costs of $539,000 including increased scientific advisory board fees and manufacturing-related activities; and (v) increased Manocept platform development costs of $264,000 including increased clinical trial costs, license fees and manufacturing-related activities, offset by decreased preclinical testing. The net decrease in research and development expenses also included decreased compensation including incentive-based awards and other expenses related to net decreased headcount of $1.0 million following the first quarter 2015 and second quarter 2014 reductions in force coupled with decreased travel, office and other support costs of $767,000.
Selling, General and Administrative Expenses. Selling, general and administrative expenses increased $1.8 million, or 11%, to $17.3 million during 2015 from $15.5 million during 2014. The net increase was primarily due to increased compensation including incentive-based awards and other expenses related to increased internal commercial and medical education headcount coupled with costs related to the first quarter 2015 reduction in force (discussed in Note 15 to the consolidated financial statements). The net increase in selling, general and administrative expenses also included increased legal and professional services, license fees related to Lymphoseek, investor relations costs, market development expenses related to NAV4694, and travel, office and other support costs, offset by decreased costs for contracted medical science liaisons and decreased professional services and market development expenses related to Lymphoseek.
Other Income (Expense). Other expense, net, was $10.2 million during 2015 as compared to other expense, net of $8.1 million during 2014. Interest expense, net increased $3.2 million to $6.9 million during 2015 from $3.7 million for 2014, primarily due to the higher outstanding balances and higher interest rates related to the CRG Term Loan and Oxford Notes in 2015 versus the Oxford Notes and GECC/MidCap Notes in 2014, coupled with the higher outstanding balances and higher interest rates of the Platinum Note in 2015 compared to 2014. Of this interest expense, $493,000 and $844,000 in 2015 and 2014, respectively, was non-cash in nature related to the amortization of debt discounts related to the CRG Term Loan, Oxford Notes and GECC/MidCap Notes. An additional $2.0 million of this interest expense was compounded and added to the balance of our notes payable during 2015. During 2015 and 2014, we recorded $2.4 million and $2.6 million, respectively, of losses on the extinguishment of the Oxford Notes and GECC/MidCap Notes. For 2015 and 2014, we recorded non-cash expenses of $615,000 and $1.3 million, respectively, related to changes in the estimated fair value of financial instruments. During 2015 and 2014, we recorded non-cash equity in the loss of R-NAV of $305,000 and $524,000, respectively.
Income from Discontinued Operations. In connection with the sale of our GDS Business to Devicor in August 2011, Devicor agreed to make royalty payments to us of up to an aggregate maximum amount of $20 million based on the net revenue attributable to the GDS Business through 2017. During 2015, we recorded net income from the GDS Business of $759,000 related to royalty amounts
39
earned based on 2015 GDS Business revenue. The royalty amount of $1.2 million was offset by $436,000 in estimated taxes which were allocated to discontinued operations, but were fully offset by the tax benefit from our net operating loss for 2015. We did not record any income from discontinued operations in 2014.
Years Ended December 31, 2014 and 2013
Net Sales and Margins. Net sales of Lymphoseek were $4.2 million during 2014, compared to $614,000 during 2013. The increase was primarily due to sales starting in late April of 2013, coupled with an increase in the initial transfer price to Cardinal beginning in the second quarter of 2014. Gross margins on net sales were 63% in 2014 and 46% in 2013. Cost of goods sold in 2014 included a reserve for inventory obsolescence of $539,000 related to a specific lot that was originally produced for commercial validation purposes and that is nearing its product expiry and therefore was no longer expected to be sold. Excluding the one-time inventory obsolescence charge, gross margin on net sales for 2014 would have been 75%. Cost of goods sold in both periods included post-production testing activities required by regulatory authorities, which are charged as one-time period costs, and a royalty on net sales payable under our license agreement with UCSD.
Lymphoseek License Revenue. During 2014, we recognized $300,000 of Lymphoseek license revenue from a non-refundable upfront milestone payment received by the Company related to the Lymphoseek distribution agreement for China for which the Company has no future obligations. No Lymphoseek milestone revenue was recognized during 2013.
Grant and Other Revenue. During 2014, we recognized $1.7 million of grant revenue as compared to $516,000 during 2013, primarily related to SBIR grants from the NIH supporting NAV4694 and Manocept platform development. The net increase was primarily due to higher NAV4694 grants offset by lower Manocept platform and Lymphoseek grants. Grant and other revenue for 2014 also included $90,000 of revenue related to services provided to R-NAV for Manocept development.
Research and Development Expenses. Research and development expenses decreased $6.9 million, or 29%, to $16.8 million during 2014 from $23.7 million in 2013. The decrease was primarily due to net decreases in drug project expenses related to (i) decreased Lymphoseek development costs of $3.7 million including decreased regulatory filing fees and consulting costs coupled with decreased manufacturing-related activities, offset by increased license fees related to EMA approval; (ii) decreased NAV5001 development costs of $1.2 million including decreased manufacturing-related activities and license fees, offset by increased clinical trial costs; and (iii) a net decrease in NAV4694 development costs of $1.0 million including decreased manufacturing-related activities and program management expenses, offset by increased clinical trial costs. The net decrease in research and development expenses also included decreased compensation including incentive-based awards and other expenses related to net decreased headcount resulting from the second quarter 2014 reduction in force of $567,000.
Selling, General and Administrative Expenses. Selling, general and administrative expenses remained steady at $15.5 million for both 2014 and 2013. Decreased investor relations of $880,000, compensation including incentive-based awards and other expenses related to net decreased headcount resulting from the second quarter 2014 reduction in force of $458,000, and out-of-pocket Lymphoseek marketing costs of $138,000 were offset by increased support costs including depreciation, facilities, Board compensation and travel of $808,000, medical education costs to support Lymphoseek of $484,000, and legal and professional services costs of $274,000.
Other Income (Expense). Other expense, net, was $8.1 million during 2014 as compared to $4.3 million during 2013. Interest expense increased $927,000 to $3.7 million during 2014 from $2.8 million in 2013, primarily due to increased interest related to the Oxford Note, offset by decreased interest related to the GECC/MidCap Notes, Hercules Note and Platinum credit facility. Of this interest expense, $844,000 and $765,000 in 2014 and 2013, respectively, was non-cash in nature related to the amortization of debt issuance costs and debt discounts related to the Oxford Note, GECC/MidCap Notes, and Hercules Note. During 2014, we recorded a loss on extinguishment of debt of $2.6 million related to paying off the balance of the GECC/MidCap Notes. During 2013, we recorded losses on extinguishment of debt of $943,000 related to the modification of the Platinum credit facility and $429,000 upon paying off the balance of the Hercules Note. For the years ended December 31, 2014 and 2013, we recorded non-cash expenses of $1.3 million and $112,000, respectively, related to changes in the estimated fair value of financial instruments. During 2014, we recorded non-cash expense from our equity in the loss of R-NAV of $524,000.
Liquidity and Capital Resources
Cash balances increased to $7.2 million at December 31, 2015 from $5.5 million at December 31, 2014. The net increase was primarily due to cash received from the CRG Term Loan of $50.0 million, draws under the Platinum credit facility of $4.5 million and the issuance of MT Preferred Stock of $500,000, offset by principal payments on the Oxford Notes of $30.0 million, cash used to fund our operations of $19.1 million, payment of debt-related costs of $3.9 million, and a principal payment on the R-NAV note of $333,000.
We did not achieve the 2015 annual Lymphoseek sales revenue target of $11 million as initially established under the CRG Loan Agreement, but in December 2015 CRG agreed to a reduction of that target and we were able to meet that reduced target, thereby complying with the covenant. As of the time of filing this report, it appears likely that we will need to draw on the Platinum line of
40
credit on order to maintain compliance with the $5 million liquidity covenant of the CRG Loan Agreement beginning in the second quarter of 2016. Our inability to meet the liquidity covenant would be an event of default under the CRG Loan Agreement. In addition, if we are unable to reach the 2016 annual Lymphoseek sales revenue target of $22.5 million, this would also be an event of default under the CRG Loan Agreement; however, potential shortfalls to this revenue covenant are curable by the Company depositing 2.5 times the amount of the shortfall in a bank account controlled by CRG. The events of default under the CRG Loan Agreement also include a failure of Platinum to perform its funding obligations under the Platinum Loan Agreement at any time as to which the Company had negative EBITDA for the most recent fiscal quarter, as a result either of Platinum’s repudiation of its obligations under the Platinum Loan Agreement, or the occurrence of an insolvency event with respect to Platinum. Our ability to comply with these provisions may be affected by changes in our business condition or results of our operations, or other events beyond our control. The breach of any of these covenants would result in a default under the CRG Loan Agreement, permitting CRG to accelerate the maturity of our indebtedness, increase the interest rate to the default rate of 18% per annum, and invoke other remedies available to it under the loan agreement and the related security agreement, including sale of the assets securing the debt, which could raise substantial doubt about the Company’s ability to continue as a going concern. Such actions by CRG could materially adversely affect our operations, results of operations and financial condition, including causing us to substantially curtail our product development activities. See Risk Factors.
In addition, the Platinum Loan Agreement carries standard non-financial covenants typical for commercial loan agreements, many of which are similar to those contained in the CRG Loan Agreement, that impose significant requirements on us. Our ability to comply with these provisions may be affected by changes in our business condition or results of our operations, or other events beyond our control. The breach of any of these covenants would result in a default under the Platinum Loan Agreement, permitting Platinum to terminate our ability to obtain additional draws under the Platinum Loan Agreement and accelerate the maturity of the debt, subject to the limitations of the Subordination Agreement with CRG. Such actions by Platinum could materially adversely affect our operations, results of operations and financial condition, including causing us to substantially curtail our product development activities. As of the time of filing of this Report, we are in compliance with all covenants under the Platinum Loan Agreement. See Risk Factors.
As of December 31, 2015, $27.3 million was still immediately available under the Platinum credit facility, which was reaffirmed by Platinum in March 2016. We believe that our current cash balance, our credit facility with Platinum, our projected revenue derived from sales of Lymphoseek, our ability to control expenses, the potential for partnership funding, the potential to access debt or royalty instruments, and the potential to access capital markets through our shelf registration (though we have no current intention to raise additional equity capital using the shelf registration), provide us with adequate financial resources to continue to fund our business plan for the foreseeable future and enable us to reach cash flow breakeven from operations in the second half of 2016.
Operating Activities. Cash used in operations decreased $10.0 million to $19.1 million during 2015 compared to $29.1 million used in 2014.
Accounts and other receivables increased to $3.7 million at December 31, 2015 from $894,000 at December 31, 2014, primarily due to increased receivables due from Cardinal Health resulting from the increase in sales of Lymphoseek of $1.7 million coupled with $1.2 million of royalties due from Devicor associated with the 2011 sale of the GDS Business.
Inventory levels decreased to $653,000 at December 31, 2015 from $932,000 at December 31, 2014, primarily due to finished goods inventory sold and materials inventory consumed for process development, offset by materials and finished goods inventory produced. We expect inventory levels to increase during 2016 as we produce additional Lymphoseek inventory to build our safety stock levels and meet increasing demand.
Prepaid expenses and other current assets decreased to $1.1 million at December 31, 2015 from $1.3 million at December 31, 2014, primarily due the application of a prepaid deposit to a new lot of materials inventory, partially offset by incremental insurance premiums related to policy renewals.
Accounts payable increased to $1.8 million at December 31, 2015 from $1.5 million at December 31, 2014, primarily due to net increased payables due to NAV4694, Lymphoseek, investor relations, legal and professional services vendors, offset by net decreased payables due to regulatory, commercial, and medical education vendors. Accrued liabilities and other current liabilities decreased to $3.0 million at December 31, 2015 from $3.2 million at December 31, 2014. Decreased accruals for termination, legal and professional services, interest, and Lymphoseek development were offset by increased accruals for compensation-related costs, strategic investor relations services, NAV4694 development, royalties and Macrophage Therapeutics. Our payable and accrual balances will continue to fluctuate but will likely decrease overall as we continue to decrease our level of development activity related to NAV4694 and NAV5001, offset by planned increases in commercial activity related to Lymphoseek and development activity related to the Manocept platform and Macrophage Therapeutics.
Investing Activities. Investing activities used $28,000 during 2015 compared to using $1.5 million in 2014. Capital expenditures of $39,000 during 2015 were primarily for Lymphoseek production equipment and computers. Proceeds from sales of equipment of $38,000 were offset by patent and trademark costs of $27,000 during 2015. Capital expenditures of $1.1 million during 2014 were primarily for leasehold improvements associated with relocation of our Dublin office, office furniture and NAV4694 production
41
equipment. Investing activities in 2014 also included investment in R-NAV of $333,000 and patent and trademark costs of $77,000. We expect our overall capital expenditures for 2016 will be slightly higher than for 2015 as we maintain our technology infrastructure.
Financing Activities. Financing activities provided $20.8 million during 2015 compared to $3.2 million provided in 2014. The $20.8 million provided by financing activities in 2015 consisted primarily of proceeds from the CRG Term Loan of $50.0 million, draws under the Platinum credit facility of $4.5 million, and proceeds from issuance of MT Preferred Stock of $500,000, offset by principal payments on the Oxford Notes of $30.0 million, payment of debt-related costs of $3.9 million, and a principal payment on the R-NAV note of $333,000. The $3.2 million provided by financing activities in 2014 consisted primarily of proceeds from the Oxford Notes of $30.0 million, offset by principal payments on the GECC/MidCap Notes of $25.0 million and payment of debt-related costs of $1.8 million.
Investment in Macrophage Therapeutics, Inc.
In March 2015, MT, our previously wholly-owned subsidiary, entered into a Securities Purchase Agreement to sell up to 50 shares of its Series A Convertible Preferred Stock (MT Preferred Stock) and warrants to purchase up to 1,500 common shares of MT (MT Common Stock) to Platinum and Dr. Michael Goldberg (collectively, the Investors) for a purchase price of $50,000 per unit. A unit consists of one share of MT Preferred Stock and 30 warrants to purchase MT Common Stock. Under the agreement, 40% of the MT Preferred Stock and warrants are committed to be purchased by Dr. Goldberg, and the balance by Platinum. The full 50 shares of MT Preferred Stock and warrants that may be sold under the agreement are convertible into, and exercisable for, MT Common Stock representing an aggregate 1% interest on a fully converted and exercised basis. Navidea owns the remainder of the MT Common Stock. On March 11, 2015, definitive agreements with the Investors were signed for the sale of the first tranche of 10 shares of MT Preferred Stock and warrants to purchase 300 shares of MT Common Stock to the Investors, with gross proceeds to MT of $500,000.
In addition, we entered into a Securities Exchange Agreement with the Investors providing them an option to exchange their MT Preferred Stock for our common stock in the event that MT has not completed a public offering with gross proceeds to MT of at least $50 million by the second anniversary of the closing of the initial sale of MT Preferred Stock, at an exchange rate per share obtained by dividing $50,000 by the greater of (i) 80% of the twenty-day volume weighted average price per share of our common stock on the second anniversary of the initial closing or (ii) $3.00. To the extent that the Investors do not timely exercise their exchange right, MT has the right to redeem their MT Preferred Stock for a price equal to $58,320 per share. We also granted MT an exclusive license for certain therapeutic applications of the Manocept technology.
Investment in R-NAV, LLC
In July 2014, Navidea formed a joint enterprise with Essex Woodlands-backed Rheumco, LLC, to develop and commercialize radiolabeled diagnostic and therapeutic products for rheumatologic and arthritic diseases. The joint enterprise, called R-NAV, LLC, combines Navidea’s proprietary Manocept CD206 macrophage targeting platform and Rheumco’s proprietary Tin-117m radioisotope technology to focus on leveraging the platforms across several indications with high unmet medical need, including the detection and treatment of RA and veterinary osteoarthritis.
Both Rheumco and Navidea have contributed licenses for intellectual property and technology to R-NAV in exchange for common units in R-NAV. R-NAV was initially capitalized through a $4.0 million investment from third-party private investors, and the technology contributions from Rheumco and Navidea. Navidea committed an additional $1.0 million investment to be paid over three years, with $333,334 in cash contributed at inception and a promissory note in the principal amount of $666,666, payable in two equal installments on the first and second anniversaries of the transaction. In exchange for its capital and in-kind investment, the Company received 1,000,000 Series A preferred units of R-NAV (Series A Units). The Company will receive an additional 500,000 Series A Units for management and technical services associated with the programs described above to be performed by the Company for R-NAV pursuant to a services agreement. The Series A Units are convertible into common units at the option of the holder for a conversion price of $1 per unit, subject to broad-based weighted average anti-dilution rights.
Navidea initially owned approximately 33.7% of the combined entity. At December 31, 2015, Navidea owns approximately 27.3% of R-NAV. Joint oversight over certain aspects of R-NAV is shared between Navidea and the other investors; Navidea does not control the operations of R-NAV. Navidea has three-year call options to acquire, at its sole discretion, all of the equity of R-NAV’s Subsidiary 1A (focused on diagnosis of human RA) for $10.5 million prior to the launch of a Phase 3 clinical trial for its development program, and all of the equity of R-NAV’s Subsidiary 1B (focused on treatment of human RA) at fair value upon completion of radiochemistry and biodistribution studies for its development program.
Navidea's investment in R-NAV is being accounted for using the equity method of accounting. Navidea's equity in the loss of R-NAV was $305,000 for the year ended December 31, 2015. The Company's obligation to provide $500,000 of in-kind services to R-NAV is being recognized as those services are provided. The Company provided $64,000 of in-kind services during the year ended December 31, 2015. As of December 31, 2015, the Company has $398,000 of in-kind services remaining to provide under this obligation. A
42
principal payment of $333,333 was made on the note payable to R-NAV in July 2015. As of December 31, 2015, the outstanding principal balance of the note payable to R-NAV was $333,333. The final payment of $333,333 is due in July 2016.
Platinum Credit Facility
In July 2012, we entered into an agreement with Platinum to provide us with a credit facility of up to $50 million (the Platinum Loan Agreement). Following the approval of Lymphoseek, Platinum was committed under the terms of the agreement to extend up to $35 million in debt financing to the Company. The agreement also provided for Platinum to extend an additional $15 million on terms to be negotiated. Through June 25, 2013, we drew a total of $8.0 million under the original facility.
In June 2013, in connection with entering into the GECC/MidCap Loan Agreement (discussed below), the Company and Platinum entered into an Amendment to the Platinum Loan Agreement (the First Platinum Amendment). Concurrent with the execution of the First Platinum Amendment, the Company delivered an Amended and Restated Promissory Note (the First Amended Platinum Note) to Platinum, which amended and restated the original promissory note issued to Platinum, in the principal amount of up to $35 million. The First Amended Platinum Note also adjusted the interest rate to the greater of (a) the U.S. Prime Rate as reported in the Wall Street Journal plus 6.75%; (b) 10.0%; or (c) the highest rate of interest then payable pursuant to the GECC/MidCap Loan Agreement plus 0.125%. In addition, the First Platinum Amendment granted Platinum the right, at Platinum’s option subject to certain conditions, to convert all or any portion of the unpaid principal or unpaid interest accrued on any future draw (the Conversion Amount), beginning on a date two years from the date the draw is advanced, into the number of shares of Navidea’s common stock computed by dividing the Conversion Amount by a conversion price equal to the lesser of (i) 90% of the lowest VWAP for the 10 trading days preceding the date of such conversion request, or (ii) the average VWAP for the 10 trading days preceding the date of such conversion request. The First Platinum Amendment also provided a conversion right on the same terms with respect to the amount of any mandatory repayment due following the Company achieving $2 million in cumulative revenues from sales or licensing of Lymphoseek.
Also in connection with the First Platinum Amendment, the Company and Platinum entered into a Warrant Exercise Agreement (Exercise Agreement), pursuant to which Platinum exercised its Series X Warrant and Series AA Warrant. The warrants were exercised on a cashless basis by canceling a portion of the indebtedness outstanding under the Platinum Loan Agreement equal to $4,781,333, the aggregate exercise price of the warrants. Pursuant to the Exercise Agreement, in lieu of common stock, Platinum received on exercise of the warrants 2,364.9 shares of the Company’s Series B, convertible into 7,733,223 shares of our common stock in the aggregate (3,270 shares of common stock per preferred share).
In March 2014, in connection with entering into the Oxford Loan Agreement (discussed below), we repaid all amounts outstanding under the GECC/MidCap Loan Agreement and entered into a second amendment to the Platinum Loan Agreement (the Second Platinum Amendment). Concurrent with the execution of the Second Platinum Amendment, the Company delivered an Amended and Restated Promissory Note (the Second Amended Platinum Note) to Platinum, which amended and restated the First Amended Platinum Note. The Second Amended Platinum Note adjusted the interest rate to the greater of (i) the United States prime rate as reported in The Wall Street Journal plus 6.75%, (ii) 10.0%, and (iii) the highest rate of interest then payable by the Company pursuant to the Oxford Loan Agreement plus 0.125%.
In May 2015, in connection with the execution of the CRG Loan Agreement, the Company amended the existing Platinum credit facility to allow this facility to remain in place in a subordinated role to the CRG Loan (the Third Platinum Amendment). Among other things, the Third Platinum Amendment (i) extends the term of the Platinum Loan Agreement until a date six months following the maturity date or earlier repayment of the CRG Term Loan; (ii) changes the interest rate to the greater of (a) the United States prime rate as reported in The Wall Street Journal plus 6.75%, (b) 10.0% and (c) the highest rate of interest then payable pursuant to the CRG Term Loan plus 0.125% (effective interest rate as of December 31, 2015 was 14.125%); (iii) requires such interest to compound monthly; and (iv) changes the provisions of the Platinum Loan Agreement governing Platinum’s right to convert advances into common stock of the Company. The Third Platinum Amendment provides for the conversion of all principal and interest outstanding under the Platinum Loan Agreement, but not until such time as the average daily volume weighted average price of the Company’s common stock for the ten preceding trading days exceeds $2.53 per share. The amendment became effective upon initial funding of the CRG Loan Agreement.
The Platinum Loan Agreement carries standard non-financial covenants typical for commercial loan agreements, many of which are similar to those contained in the CRG Loan Agreement, that impose significant requirements on us. Our ability to comply with these provisions may be affected by changes in our business condition or results of our operations, or other events beyond our control. The breach of any of these covenants would result in a default under the Platinum Loan Agreement, permitting Platinum to terminate our ability to obtain additional draws under the Platinum Loan Agreement and accelerate the maturity of the debt, subject to the limitations of the Subordination Agreement with CRG. Such actions by Platinum could materially adversely affect our operations, results of operations and financial condition, including causing us to substantially curtail our product development activities. As of the time of filing of this Report, we are in compliance with all covenants under the Platinum Loan Agreement.
The Platinum Loan Agreement, as amended, provides us with a credit facility of up to $50 million. We drew a total of $4.5 million and $4.0 million under the credit facility in each of the years ended December 31, 2015 and 2013. We did not make any draws under
43
the credit facility during the year ended December 31, 2014. In addition, $761,000 of interest was compounded and added to the balance of the Third Amended Platinum Note during the year ended December 31, 2015. As of December 31, 2015, the outstanding principal balance of the Third Amended Platinum Note was approximately $8.5 million, consisting of $7.7 million of draws and $761,000 of compounded interest, with $27.3 million still available under the credit facility. Based on the balance as of December 31, 2015, annual principal and interest payments on the Platinum credit facility are expected to be $0 in 2016 through 2020 and $19.2 million in 2021.
Under the terms of an Agreement dated March 14, 2016, among the Company and Platinum, including certain investment funds managed by it, Platinum has reaffirmed its obligations to make loans to the Company under the Platinum Loan Agreement. See Directors, Executive Officers and Corporate Governance – Directors.
Capital Royalty Partners II, L.P.
In May 2015, Navidea and its subsidiary Macrophage Therapeutics, Inc., as guarantor, executed a Term Loan Agreement (the CRG Loan Agreement) with Capital Royalty Partners II L.P. (CRG) in its capacity as a lender and as control agent for other affiliated lenders party to the CRG Loan Agreement (collectively, the Lenders) in which the Lenders agreed to make a term loan to the Company in the aggregate principal amount of $50 million (the CRG Term Loan), with an additional $10 million in loans to be made available upon the satisfaction of certain conditions stated in the CRG Loan Agreement. Closing and funding of the CRG Term Loan occurred on May 15, 2015 (the Effective Date). The principal balance of the CRG Term Loan will bear interest from the Effective Date at a per annum rate of interest equal to 14.0%. Through March 31, 2019, the Company has the option of paying (i) 10.00% of the per annum interest in cash and (ii) 4.00% of the per annum interest as compounded interest, added to the aggregate principal amount of the CRG Term Loan. During the year ended December 31, 2015, $1.3 million of interest was compounded and added to the balance of the CRG Term Loan. In addition, the Company began paying the cash portion of the interest in arrears on June 30, 2015. Principal is due in eight equal quarterly installments during the final two years of the term. All unpaid principal, and accrued and unpaid interest, is due and payable in full on March 31, 2021. As of December 31, 2015, the outstanding principal balance of the CRG Term Loan was $51.3 million including $1.3 million of compounded interest. Although we did not achieve the 2015 annual revenue target initially established under the CRG Loan Agreement, in December 2015 CRG agreed to a reduction of that target and we were able to meet that reduced target, thereby complying with the covenant, and as of December 31, 2015, we were in compliance with all covenants of the CRG Loan Agreement.
As of the time of filing this report, it appears likely that we will need to draw on the Platinum line of credit in order to maintain compliance with the $5 million liquidity covenant of the CRG Loan Agreement beginning in the second quarter of 2016. Our inability to meet the liquidity covenant would be an event of default under the CRG Loan Agreement. In addition, if we are unable to reach the 2016 annual Lymphoseek sales revenue target of $22.5 million, this would also be an event of default under the CRG Loan Agreement; however, potential shortfalls to this revenue covenant are curable by the Company depositing 2.5 times the amount of the shortfall in a bank account controlled by CRG. The events of default under the CRG Loan Agreement also include a failure of Platinum to perform its funding obligations under the Platinum Loan Agreement at any time as to which the Company had negative EBITDA for the most recent fiscal quarter, as a result either of Platinum’s repudiation of its obligations under the Platinum Loan Agreement, or the occurrence of an insolvency event with respect to Platinum. Our ability to comply with these covenants may be affected by changes in our business condition or results of operations, or other events beyond our control. An event of default would entitle CRG to accelerate the maturity of our indebtedness, increase the interest rate to the default rate of 18% per annum, and invoke other remedies available to it under the loan agreement and the related security agreement, including sale of the assets securing the debt, which could raise substantial doubt about the Company’s ability to continue as a going concern.
The majority of the proceeds from the CRG Note were used to repay all amounts outstanding under the Oxford Loan Agreement (discussed below). The remaining proceeds are being used to support the growth of the Company's Manocept technology and for general operating purposes. Based on the balance as of December 31, 2015 and assuming we continue to compound a portion of the interest through March 31, 2019, annual principal and interest payments on the CRG Term Loan are expected to be $5.3 million, $5.5 million, $5.7 million, $28.8 million, $32.9 million and $7.6 million in 2016, 2017, 2018, 2019, 2020 and 2021, respectively. Although we expect these obligations to be repaid primarily through cash flow from operations, it is possibly that we may need to consider other financing options including additional draws on the Platinum line of credit or raising capital from other sources.
Oxford Debt
In March 2014, we executed a Loan and Security Agreement (the Oxford Loan Agreement) with Oxford Finance, LLC (Oxford), providing for a loan to the Company of $30 million. Pursuant to the Oxford Loan Agreement, we issued Oxford: (1) Term Notes in the aggregate principal amount of $30 million, bearing interest at 8.5% (the Oxford Notes), and (2) Series KK warrants to purchase an aggregate of 391,032 shares of our common stock at an exercise price of $1.918 per share, expiring in March 2021 (the Series KK warrants). We began making monthly payments of interest only on April 1, 2014, and monthly payments of principal and interest beginning April 1, 2015. In May 2015, in connection with the consummation of the CRG Loan Agreement, the Company repaid all
44
amounts outstanding under the Oxford Loan Agreement. The payoff amount of $31.7 million included payments of $289,000 as a pre-payment fee and $2.4 million as an end-of-term final payment fee. The Series KK warrants remained outstanding as of December 31, 2015.
GECC/MidCap Debt
In June 2013, we executed a Loan and Security Agreement (the GECC/MidCap Loan Agreement) with General Electric Capital Corporation (GECC) and MidCap Financial SBIC, LP (MidCap), pursuant to which we issued GECC and MidCap: (1) Term Notes in the aggregate principal amount of $25,000,000 (the GECC/MidCap Notes), and (2) Series HH warrants to purchase an aggregate of 301,205 shares of our common stock at an exercise price of $2.49 per share, expiring in June 2023 (the Series HH warrants). In March 2014, in connection with the consummation of the Oxford Loan Agreement, we repaid all amounts outstanding under the GECC/MidCap Loan Agreement upon the receipt by GECC/MidCap of a payoff amount of $26.7 million, including $500,000 as a pre-payment fee and $1,000,000 as an end-of-term final payment fee. The Series HH warrants remained outstanding as of December 31, 2015.
Hercules Debt
In December 2011, we executed a Loan and Security Agreement (the Hercules Loan Agreement) with Hercules Technology II, L.P. (Hercules), pursuant to which we issued Hercules: (1) a Secured Term Promissory Note in the principal amount of $7,000,000 (the Hercules Note), and (2) Series GG warrants to purchase 333,333 shares of our common stock at an exercise price of $2.10 per share, expiring in December 2016 (the Series GG warrants). In June 2013, the Company used a portion of the proceeds from the GECC/MidCap Notes to pay the remaining $4.4 million of principal outstanding on the Hercules Note, as well as a $250,000 end-of-term fee and a $66,000 early payment penalty in accordance with the terms of the Hercules Loan Agreement. The Series GG warrants remained outstanding as of December 31, 2015.
2013 Public Offerings
During 2013, we completed two public offerings resulting in issuance of 3,642,389 shares of the Company’s common stock. The net proceeds to the Company were approximately $9.3 million after deducting expenses associated with the offerings. The 2013 public offerings were made pursuant to the Company’s existing effective shelf registration statement on Form S-3.
Additionally, in September 2013, we entered into a Securities Purchase Agreement with Crede CG III Ltd. (Crede) for a registered direct public offering of 10,563,381 shares of our common stock at a price of $2.84 per share for total gross proceeds of $30.0 million. The net proceeds to the Company upon issuance in 2013 were approximately $28.8 million after deducting expenses associated with the Securities Purchase Agreement, including placement agent fees of $999,000 (3.3% of the gross proceeds). In addition to the common stock, we issued Series JJ warrants to purchase 3,169,015 shares of our common stock at an exercise price of $3.83 per share, expiring in September 2016. In November 2014, Crede exchanged their Series JJ warrants for 3,843,223 shares of our common stock in accordance with the terms of the Series JJ warrant agreement.
Summary
Our future liquidity and capital requirements will depend on a number of factors, including our ability to achieve market acceptance of our products, our ability to comply with the covenants of our debt agreements, our ability to complete the development and commercialization of new products, our ability to monetize our investment in non-core technologies, our ability to obtain milestone or development funds from potential development and distribution partners, regulatory actions by the FDA and international regulatory bodies, the ability to procure required financial resources, and intellectual property protection.
Although we did not achieve the 2015 annual Lymphoseek sales revenue target of $11 million as initially established under the CRG Loan Agreement, in December 2015 CRG agreed to a reduction of that target to $10 million and we were able to meet that reduced target with Lymphoseek sales revenue of $10.3 million, thereby complying with the covenant. As of December 31, 2015, we were in compliance with all covenants of the CRG Loan Agreement. As of the time of filing this report, it appears likely that we will need to draw on the Platinum line of credit in order to maintain compliance with the $5 million liquidity covenant of the CRG Loan Agreement beginning in the second quarter of 2016. Our inability to meet the liquidity covenant would be an event of default under the CRG Loan Agreement. In addition, if we are unable to reach the 2016 annual Lymphoseek sales revenue target of $22.5 million, this would also be an event of default under the CRG Loan Agreement; however, potential shortfalls to this revenue covenant are curable by the Company depositing 2.5 times the amount of the shortfall in a bank account controlled by CRG. The events of default under the CRG Loan Agreement also include a failure of Platinum to perform its funding obligations under the Platinum Loan Agreement at any time as to which the Company had negative EBITDA for the most recent fiscal quarter, as a result either of Platinum’s repudiation of its obligations under the Platinum Loan Agreement, or the occurrence of an insolvency event with respect to
45
Platinum. Our ability to comply with these covenants may be affected by changes in our business condition or results of operations, or other events beyond our control. An event of default would entitle CRG to accelerate the maturity of our indebtedness, increase the interest rate to the default rate of 18% per annum, and invoke other remedies available to it under the loan agreement and the related security agreement, which could raise substantial doubt about the Company’s ability to continue as a going concern.
In May 2014, the Board of Directors made the decision to refocus the Company's resources to better align the funding of our pipeline programs with the expected growth in Lymphoseek revenue. This realignment primarily involved reducing our near-term support for our two neurological product candidates, NAV4694 and NAV5001, as we sought to secure a development partner or partners for these programs.
In April 2015, the Company entered into an agreement with Alseres to terminate the sub-license agreement dated July 31, 2012 for research, development and commercialization of NAV5001. Under the terms of this agreement, Navidea transferred all regulatory, clinical and manufacturing-related data related to NAV5001 to Alseres. Alseres agreed to reimburse Navidea for any incurred maintenance costs of the contract manufacturer retroactive to March 1, 2015. In addition, Navidea has supplied clinical support services for NAV5001 on a cost-plus reimbursement basis. However, to this point, Alseres has been unsuccessful in raising the funds necessary to restart the program and reimburse Navidea. As a result, we have taken steps to end our obligations under the agreement and notified Alseres that we consider them in breach of the agreement. We are in the process of trying to recover the funds we expended complying with our obligations under the termination agreement.
The Company is currently engaged in discussions related to the potential partnering or divestiture of NAV4694. We continue to have active interest from potential partners or acquirers; however, our negotiations have experienced delays due in large part to litigation brought by one of the potential partners (see Item 3 – Legal Proceedings). The Company believes the suit is without merit and has filed a motion to dismiss the action. While it is not possible to determine with any degree of certainty the ultimate outcome of this legal proceeding, including making a determination of liability, we believe that we have meritorious defenses with respect to the claims asserted against us and intend to vigorously defend our position.
The Company is also working to establish additional sources of non-dilutive funding, including collaborations and grant funding that can augment the balance sheet as the Company works to reduce spending to levels that can be increasingly offset by growing Lymphoseek revenue. In particular, substantial progress on the Manocept platform has resulted in several promising opportunities, including our R-NAV, LLC venture which began in July 2014, the formation of Macrophage Therapeutics, Inc. in January 2015, and Macrophage Therapeutics' research collaboration agreement with BIND Therapeutics, Inc. executed in June 2015.
We plan to focus our resources for 2016 primarily on increasing sales of Lymphoseek and development of products based on the Manocept platform. Although management believes that it will be able to achieve these objectives, they are subject to a number of variables beyond our control, including the nature and timing of any partnering opportunities, the ability to modify contractual commitments made in connection with these programs, and the timing and expense associated with suspension or alteration of clinical trials, and consequently we cannot assure you that we will be able to achieve our objective of bringing our expenses in line with our revenues, and we may need to seek additional debt or equity financing if we cannot achieve that objective in a timely manner.
As stated above, we believe that our current cash balance, in conjunction with projected revenue growth derived from sales of Lymphoseek and our Platinum line of credit, provides us with the foundation with which to build our business. Our capital position is further supported by our ability to control expenses, the potential for partnership funding, and the potential to access capital markets through our shelf registration (though we have no current intention to raise additional equity capital using the shelf registration) provide us with adequate financial resources to continue to fund our business plan. We continually monitor our cash flows and financial projections with the goal of reaching cash flow breakeven from operations as soon as possible. At this time, we expect to achieve cash flow breakeven from operations during the second half of 2016, and possibly as early as in the second quarter if the Company is successful in closing a transaction related to its NAV4694 product candidate. During 2015, we continued making limited investment in the NAV4694 clinical trial process based on our expectation that we will be successful in ultimately securing a partnership that will provide us some level of return on this investment which is incremental to the carrying costs we are presently incurring. However, we cannot assure you that Lymphoseek will generate our expected levels of sales and cash flow or that the partnership discussions in which we are engaged will yield the level of return we are anticipating. We will continue to evaluate our time lines, strategic needs, and balance sheet requirements. We cannot assure you that if we attempt to raise additional capital through debt, royalty, equity or otherwise, we will be successful in doing so on terms acceptable to the Company, or at all. We also cannot assure you that we will be able to gain access and/or be able to execute on securing new development opportunities, successfully obtain regulatory approval for and commercialize new products, achieve significant product revenues from our products, or achieve or sustain profitability in the future. See Risk Factors.
46
Recent Accounting Developments
In February 2015, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2015-02, Amendments to the Consolidation Analysis. ASU 2015-02 affects reporting entities that are required to evaluate whether they should consolidate certain legal entities. All legal entities are subject to reevaluation under the revised consolidation model. Specifically, the amendments: (i) modify the evaluation of whether limited partnerships and similar legal entities are variable interest entities (VIEs) or voting interest entities, (ii) eliminate the presumption that a general partner should consolidate a limited partnership, and (iii) affect the consolidation analysis of reporting entities that are involved with VIEs, particularly those that have fee arrangements and related party relationships. ASU 2015-02 is effective for public entities for fiscal years, and for interim periods within those fiscal years, beginning after December 15, 2015. The amendments may be applied using a modified retrospective approach or a full retrospective approach. Early adoption is permitted, including adoption in an interim period. The adoption of ASU 2015-02 is not expected to have a material effect on our consolidated financial statements.
In April 2015, the FASB issued ASU No. 2015-03, Simplifying the Presentation of Debt Issuance Costs. ASU 2015-03 requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability rather than as an asset. The recognition and measurement guidance for debt issuance costs are not affected by ASU 2015-03. ASU 2015-03 is effective for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. Early adoption is permitted. Entities must apply the amendments in ASU 2015-03 on a retrospective basis. During the second quarter of 2015, the Company elected early adoption of ASU 2015-03. The consolidated balance sheet as of December 31, 2014 has been adjusted to reflect retrospective application of the new method of presentation. Deferred debt issuance costs totaling $90,000 that were included in other assets as of December 31, 2014 were reclassified as discounts on notes payable, current, of $35,000 and discounts on notes payable, long term, of $55,000. We have reflected all debt issuance costs as a reduction of the debt on the balance sheets as of December 31, 2015 and 2014, and will continue to do so in future periods. The adoption of ASU 2015-03 had no impact on the consolidated statements of operations, stockholders' deficit or cash flows.
In July 2015, the FASB issued ASU No. 2015-11, Simplifying the Measurement of Inventory. ASU 2015-11 applies to all inventory that is measured using methods other than last-in, first-out or the retail inventory method, including inventory that is measured using first-in, first-out or average cost. ASU 2015-11 requires entities to measure inventory at the lower of cost and net realizable value, defined as the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. ASU 2015-11 is effective for public entities for fiscal years beginning after December 15, 2016, and interim periods with fiscal years beginning after December 15, 2017. The amendments in ASU 2015-11 should be applied prospectively with earlier application permitted as of the beginning of an interim or annual reporting period. We do not expect the adoption of ASU 2015-11 to have a material effect on our consolidated financial statements upon adoption.
In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers. ASU 2015-14 defers the effective date of ASU No. 2014-09 for all entities by one year. Public business entities should adopt the new revenue recognition standard for annual reporting periods beginning after December 15, 2017, including interim periods within that year. Early adoption is permitted only as of annual reporting periods beginning after December 15, 2016, including interim periods within that year. We are currently evaluating the potential impact that the adoption of ASU 2014-09 may have on our consolidated financial statements.
In November 2015, the FASB issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes. ASU 2015-17 eliminates the requirement to bifurcate deferred taxes between current and noncurrent on the balance sheet and requires that deferred tax assets and liabilities be classified as noncurrent on the balance sheet. ASU 2015-17 may be applied retrospectively or prospectively and early adoption is permitted. We early-adopted ASU 2015-17 as of December 31, 2015 and the statement of financial position as of this date reflects the revised classification of current deferred tax assets and liabilities as noncurrent. Adoption of ASU 2015-17 resulted in a retrospective reclassification between current deferred tax assets and noncurrent deferred tax assets.
In February 2016, the FASB issued ASU No. 2016-02, Leases. ASU 2016-02 establishes a right-of-use (ROU) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. While we are still evaluating the impact of our pending adoption of the new standard on our consolidated financial statements, we expect that upon adoption we will recognize ROU assets and lease liabilities and that the amounts could be material.
Critical Accounting Policies
Revenue Recognition. We currently generate revenue primarily from sales of Lymphoseek. Our standard shipping terms are FOB shipping point, and title and risk of loss passes to the customer upon delivery to a carrier for shipment. We generally recognize sales revenue related to sales of our products when the products are shipped. Our customers have no right to return products purchased in the ordinary course of business, however, we may allow returns in certain circumstances based on specific agreements.
47
We earn additional revenues based on a percentage of the actual net revenues achieved by Cardinal Health on sales to end customers made during each fiscal year. The amount we charge Cardinal Health related to end customer sales of Lymphoseek are subject to a retroactive annual adjustment. To the extent that we can reasonably estimate the end customer prices received by Cardinal Health, we record sales based upon these estimates at the time of sale. If we are unable to reasonably estimate end customer sales prices related to products sold, we record revenue related to these product sales at the minimum (i.e., floor) price provided for under our distribution agreement with Cardinal Health.
We also earn revenues related to our licensing and distribution agreements. The terms of these agreements may include payment to us of non-refundable upfront license fees, funding or reimbursement of research and development efforts, milestone payments if specified objectives are achieved, and/or royalties on product sales. We evaluate all deliverables within an arrangement to determine whether or not they provide value on a stand-alone basis. We recognize a contingent milestone payment as revenue in its entirety upon our achievement of a substantive milestone if the consideration earned from the achievement of the milestone (i) is consistent with performance required to achieve the milestone or the increase in value to the delivered item, (ii) relates solely to past performance and (iii) is reasonable relative to all of the other deliverables and payments within the arrangement.
We generate additional revenue from grants to support various product development initiatives. We generally recognize grant revenue when expenses reimbursable under the grants have been paid and payments under the grants become contractually due. Lastly, we recognize revenues from the provision of services to R-NAV and its subsidiaries.
Research and Development. Research and development (R&D) expenses include both internal R&D activities and external contracted services. Internal R&D activity expenses include salaries, benefits, and stock-based compensation, as well as travel, supplies, and other costs to support our R&D staff. External contracted services include clinical trial activities, chemistry, manufacturing and control-related activities, and regulatory costs. R&D expenses are charged to operations as incurred. We review and accrue R&D expenses based on services performed and rely upon estimates of those costs applicable to the stage of completion of each project.
Use of Estimates. The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. We base these estimates and assumptions upon historical experience and existing, known circumstances. Actual results could differ from those estimates. Specifically, management may make significant estimates in the following areas:
|
|
· |
Stock-Based Compensation. Stock-based payments to employees and directors, including grants of stock options and restricted stock, are recognized in the statements of operations based on their estimated fair values on the date of grant. The fair value of each option award is estimated on the date of grant using the Black-Scholes option pricing model to value share-based payments and the portion that is ultimately expected to vest is recognized as compensation expense over either (1) the requisite service period or (2) the estimated performance period. The determination of fair value using the Black-Scholes option pricing model is affected by our stock price as well as assumptions regarding a number of complex and subjective variables, including expected stock price volatility, risk-free interest rate, expected dividends and projected employee stock option behaviors. We estimate the expected term based on the contractual term of the awards and employees' exercise and expected post-vesting termination behavior. The restricted stock awards are valued based on the closing stock price on the date of grant and amortized ratably over the estimated life of the award. |
Since stock-based compensation is recognized only for those awards that are ultimately expected to vest, we have applied an estimated forfeiture rate to unvested awards for the purpose of calculating compensation cost. These estimates will be revised, if necessary, in future periods if actual forfeitures differ from estimates. Changes in forfeiture estimates impact compensation cost in the period in which the change in estimate occurs.
|
|
· |
Inventory Valuation. We record our inventory at the lower of cost (first-in, first-out method) or market. Our valuation reflects our estimates of excess and obsolete inventory as well as inventory with a carrying value in excess of its net realizable value. Write-offs are recorded when product is removed from saleable inventory. We review inventory on hand at least quarterly and record provisions for excess and obsolete inventory based on several factors, including current assessment of future product demand, anticipated release of new products into the market and product expiration. Our industry is characterized by rapid product development and frequent new product introductions. Regulations regarding use and shelf life, product recalls and variation in product utilization all impact the estimates related to excess and obsolete inventory. |
48
|
|
· |
Fair Value of Derivative Instruments. Derivative instruments embedded in contracts, to the extent not already a free-standing contract, are bifurcated and accounted for separately. All derivatives are recorded on the consolidated balance sheets at fair value in accordance with current accounting guidelines for such complex financial instruments. Unrealized gains and losses on the derivatives are classified in other expenses as a change in derivative liabilities in the consolidated statements of operations. We do not use derivative instruments for hedging of market risks or for trading or speculative purposes. |
Contractual Obligations and Commercial Commitments
The following table presents our contractual obligations and commercial commitments as of December 31, 2015.
|
|
|
Payments Due By Period |
|
|||||||||||||||||||||||||
|
Contractual Cash Obligations |
|
Total |
|
|
2016 |
|
|
2017 |
|
|
2018 |
|
|
2019 |
|
|
2020 |
|
|
Thereafter |
|
|||||||
|
Purchase obligations |
|
$ |
2,252,818 |
|
|
$ |
2,252,818 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
Capital lease obligation |
|
|
2,279 |
|
|
|
2,279 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Operating lease obligations |
|
|
1,979,703 |
|
|
|
271,831 |
|
|
|
277,946 |
|
|
|
284,247 |
|
|
|
290,734 |
|
|
|
297,405 |
|
|
|
557,540 |
|
|
Principal and interest on long-term debt (1) |
|
|
105,366,323 |
|
|
|
5,628,730 |
|
|
|
5,497,680 |
|
|
|
5,724,056 |
|
|
|
28,843,892 |
|
|
|
32,872,338 |
|
|
|
26,799,627 |
|
|
Total contractual cash obligations |
|
$ |
109,601,123 |
|
|
$ |
8,155,658 |
|
|
$ |
5,775,626 |
|
|
$ |
6,008,303 |
|
|
$ |
29,134,626 |
|
|
$ |
33,169,743 |
|
|
$ |
27,357,167 |
|
|
* |
This table does not include obligations such as license agreements, contracted services, or employment agreements as such obligations are dependent upon performance conditions. |
|
(1) |
The amounts in this table assume that the Company will choose to compound a portion of the interest as long as that option is available under the terms of the CRG Loan Agreement. |
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Interest Rate Risk. As of December 31, 2015, our $7.2 million in cash was primarily invested in interest-bearing money market accounts. Due to the low interest rates being realized on these accounts, we believe that a hypothetical 10% increase or decrease in market interest rates would not have a material impact on our consolidated financial position, results of operations or cash flows.
We also have exposure to changes in interest rates on our variable-rate debt obligations. As of December 31, 2015, the interest rate on certain of our debt obligations was the greater of: (a) the U.S. prime rate as reported in The Wall Street Journal plus 6.75%, (b) 10.0% and (c) the highest rate of interest payable pursuant to the CRG Term Loan plus 0.125% (effective interest rate as of December 31, 2015 was 14.125%). Based on the effective rate of our variable-rate borrowings, which totaled approximately $8.5 million at December 31, 2015, an immediate one percentage point increase or decrease in the U.S. prime rate would not affect our annual interest expense.
Foreign Currency Exchange Rate Risk. We do not currently have material foreign currency exposure related to our assets as the majority are denominated in U.S. currency and our foreign-currency based transaction exchange risk is not material. For the years ended December 31, 2015, 2014 and 2013, we recorded foreign currency transaction gains (losses) of approximately $41,000, $87,000 and $(8,000), respectively.
Equity Price Risk. We do not use derivative instruments for hedging of market risks or for trading or speculative purposes. Derivative instruments embedded in contracts, to the extent not already a free-standing contract, are bifurcated and accounted for separately. All derivatives are recorded on the consolidated balance sheet at fair value in accordance with current accounting guidelines for such complex financial instruments. The fair value of our warrant liabilities is determined using various inputs and assumptions, several of which are based on a survey of peer group companies since the warrants are exercisable for common stock of a non-public subsidiary company. As of December 31, 2015, we had approximately $63,000 of derivative liabilities recorded on our balance sheet related to outstanding MT warrants. Due to the relatively low valuation of the MT warrants, a hypothetical 50% change in our stock price would not have a material effect on the consolidated financial statements.
49
Item 8. Financial Statements and Supplementary Data
Our consolidated financial statements, and the related notes, together with the report of BDO USA, LLP dated March 23, 2016 are set forth at pages F-1 through F-31 attached hereto and incorporated herein by reference.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures designed to ensure that information required to be disclosed in reports filed under the Exchange Act is recorded, processed, summarized, and reported within the specified time periods. As a part of these controls, our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of December 31, 2015. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, including our principal executive and principal financial officers, as appropriate to allow timely decisions regarding required disclosure.
As described below, we identified a material weakness in the Company’s internal control over financial reporting. As a result, our CEO and CFO concluded that our disclosure controls were not effective as of December 31, 2015. In light of the identification of a material weakness in internal control over financial reporting, the Company has performed additional internal procedures, including validating the completeness and accuracy of the underlying data used for accounting for the subsidiary prior to filing this Annual Report on Form 10-K.
These additional procedures have allowed us to conclude that, notwithstanding the material weakness in our internal control over financial reporting, the consolidated financial statements included in this report fairly present, in all material respects, the Company’s consolidated financial position, results of operations and cash flows for the periods presented in conformity with accounting principles generally accepted in the United States of America.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, and includes those policies and procedures that:
|
|
· |
pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
|
|
· |
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles and that receipts and expenditures of the company are being made only in accordance with authorization of management and directors of the company; and |
|
|
|
· |
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements. |
|
Under the supervision and with the participation of our management, including the CEO and CFO, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2015 based upon the criteria set forth in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
50
As of December 31, 2015, we did not maintain effective monitoring and oversight of financial reporting of our subsidiary, Macrophage Therapeutics, Inc., by failing to ensure compliance with Company policies and procedures by the CEO and COO of our subsidiary regarding providing complete, accurate and timely financial information to the parent company. There were no material adjustments to our current or previously filed interim consolidated financial statements during the year ended December 31, 2015 as a result of this material weakness. However, management believes there is a reasonable possibility that this control deficiency, if uncorrected, could result in material misstatements in the annual or interim consolidated financial statements that would not be prevented or detected in a timely manner. Accordingly, we have determined that this control deficiency constitutes a material weakness.
Because of this material weakness, management concluded that we did not maintain effective internal control over financial reporting as of December 31, 2015, based on criteria described in Internal Control – Integrated Framework (2013) issued by COSO. BDO USA, LLP, our independent registered public accounting firm, has issued an attestation report covering our internal control over financial reporting, which begins on page 52.
Changes in Internal Control Over Financial Reporting
Except for the change noted above, during the quarter ended December 31, 2015, there were no changes in our internal control over financial reporting that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
51
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Navidea Biopharmaceuticals, Inc.
Dublin, Ohio
We have audited Navidea Biopharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Navidea Biopharmaceuticals, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Management’s Report on Internal Control Over Financial Reporting” included in Item 9A. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. A material weakness regarding management’s failure to design and maintain controls over the internal communication of timely, accurate and complete financial information between the Company’s subsidiary and the parent entity has been identified and described in management’s assessment. This material weakness was considered in determining the nature, timing, and extent of audit tests applied in our audit of the 2015 financial statements, and this report does not affect our report dated March 23, 2016 on those financial statements.
In our opinion, Navidea Biopharmaceuticals, Inc. did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2015 based on the COSO criteria. We do not express an opinion or any other form of assurance on management’s statements referring to any actions taken by the Company after the date of management’s assessment.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Navidea Biopharmaceuticals, Inc. as of December 31, 2015 and 2014, and the related consolidated statements of operations, stockholders’ deficit, and cash flows for each of the three years in the period ended December 31, 2015 and our report dated March 23, 2016 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
Chicago, Illinois
March 23, 2016
52
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Directors
Set forth below are the names and committee assignments of the persons who constitute our Board of Directors.
|
Name |
|
Age |
|
Committee(s) |
|
Brendan A. Ford |
|
57 |
|
Audit (Chairman); Compensation, Nominating and Governance |
|
Michael M. Goldberg, M.D. |
|
57 |
|
— |
|
Ricardo J. Gonzalez |
|
45 |
|
— |
|
Anton Gueth |
|
59 |
|
Audit; Compensation, Nominating and Governance (Chairman) |
|
Eric K. Rowinsky, M.D. |
|
59 |
|
Compensation, Nominating and Governance |
|
Gordon A. Troup |
|
62 |
|
Audit; Compensation, Nominating and Governance |
Under the terms of an Agreement (Agreement) dated March 14, 2016, among the Company and Platinum, including certain investment funds managed by it, the Company has agreed to elect two directors to fill vacancies in its Board of Directors. Dr. Mark Greene will serve for a term expiring at the 2017 annual meeting of stockholders, and Dr. Anthony Fiorino will serve for a term expiring at the 2018 annual meeting. The election of each director will take effect following the filing of this Report and upon the execution and delivery by the director of a director agreement and director questionnaire in the form requested by the Company. In addition, the Agreement provides that Brendan A. Ford will resign as a director of the Company immediately following the filing of this Report. Finally, the Agreement contains certain standstill provisions whereby the Platinum parties agreed to refrain for a period of 18 months from taking certain actions potentially affecting control of the Company.
Director Qualifications
The Board of Directors believes that individuals who serve on the Board should have demonstrated notable or significant achievements in their respective field; should possess the requisite intelligence, education and experience to make a significant contribution to the Board and bring a range of skills, diverse perspectives and backgrounds to its deliberations; and should have the highest ethical standards, a strong sense of professionalism and intense dedication to serving the interests of our stockholders. The following are qualifications, experience and skills for Board members which are important to our business and its future:
|
|
· |
General Management. Directors who have served in senior leadership positions are important to us as they bring experience and perspective in analyzing, shaping, and overseeing the execution of important operational and policy issues at a senior level. These directors’ insights and guidance, and their ability to assess and respond to situations encountered in serving on our Board of Directors, are enhanced by their leadership experience developed at businesses or organizations that operated on a global scale, faced significant competition, or involved other evolving business models. |
|
|
· |
Industry Knowledge. Because we are a pharmaceutical development company, education or experience in our industry, including medicine, pharmaceutical development, marketing, distribution, or the regulatory environment, is important because such experience assists our Directors in understanding and advising our Company. |
|
|
· |
Business Development/Strategic Planning. Directors who have a background in strategic planning, business development, strategic alliances, mergers and acquisitions, and teamwork and process improvement provide insight into developing and implementing strategies for growing our business. |
|
|
· |
Finance/Accounting/Control. Knowledge of capital markets, capital structure, financial control, audit, reporting, financial planning, and forecasting are important qualities of our directors because such qualities assist in understanding, advising, and overseeing our Company’s capital structure, financing and investing activities, financial reporting, and internal control of such activities. |
|
|
· |
Board Experience/Governance. Directors who have served on other public company boards can offer advice and insights with regard to the dynamics and operation of a board of directors, the relations of a board to the chief executive officer and other management personnel, the importance of particular agenda and oversight matters, and oversight of a changing mix of strategic, operational, and compliance-related matters. |
53
Set forth below is current biographical information about our directors, including the qualifications, experience and skills that make them suitable for service as a director. Each listed director’s respective experience and qualifications described below led the Compensation, Nominating and Governance Committee (CNG Committee) of our Board of Directors to conclude that such director is qualified to serve as a member of our Board of Directors.
Director whose term continues until immediately after this Annual Report on Form 10-K is filed:
Brendan A. Ford has served as a director of Navidea since July 2010. Since 2007, Mr. Ford has been a partner in Talisman Capital Partners, a private investment partnership focusing on middle-market companies. From 1991 through 2007, Mr. Ford served in various executive positions including Executive Vice President, Business Development and Corporate Strategy with Cardinal Health, Inc., primarily in capacities related to mergers, acquisitions and related strategic activities, and was involved in over $19 billion in acquisition and disposition transactions for Cardinal. Prior to his service with Cardinal Health, Mr. Ford practiced law with Baker and Hostetler from 1986 to 1991. From 1980 to 1983, Mr. Ford was employed by Touche Ross LLP as a certified public accountant. Mr. Ford has a B.S. in Business from Miami University, and a J.D. from The Ohio State University. Mr. Ford serves as a director and board committee member for several privately held companies. Under the terms of an Agreement dated March 14, 2016, among the Company and Platinum, Mr. Ford will resign as a director of the Company immediately following the filing of this Report.
Directors whose terms continue until the 2016 Annual Meeting:
Anton Gueth has served as a Director of Navidea since June 2015 and is Chairman of the Board of Directors. Mr. Gueth is currently Managing Director of EVOLUTION Life Science Partners. He is also the founder and Managing Partner of Gueth Consulting LLC, a consulting firm focusing on pharmaceutical and biotechnology clients in the areas of licensing, early stage financing and alliance management. Mr. Gueth was previously a Managing Director of Burrill Securities. Mr. Gueth’s career includes nearly 19 years with Eli Lilly and Company (Lilly), most recently as director of Alliance Management. He also served as General Manager of Lilly's African and Middle Eastern operations; Vice President of Financial Planning and Treasury of PCS Health Systems; as well as held other sales, marketing, operations and financial positions. He is currently a member of the board of directors of Antares Pharma. Mr. Gueth received his master’s degree in public affairs from Indiana University and an M.S. in agricultural economics from Justus Liebig University in Germany.
Eric K. Rowinsky, M.D. has served as a director of Navidea since July 2010. Dr. Rowinsky has served as Executive Chairman, President, and Head of the Scientific Advisory Board of RGenix, Inc, since June 2015. He has also been the Head of Research and Development, Executive Vice President, and Chief Medical Officer of Stemline Therapeutics, Inc. from 2012 to 2015, and was the Chief Executive Officer and Founder of Primrose Therapeutics from August 2010 to September 2011 at which time it was acquired by Stemline. From 2005 to 2009, he served as the Chief Medical Officer and Executive Vice President of Clinical Development and Regulatory Affairs of ImClone Systems Incorporated, a life sciences company, which was acquired by Eli Lilly. Prior to that, Dr. Rowinsky held several positions at the Cancer Therapy & Research Center’s Institute of Drug Development, including Director of the Institute, Director of Clinical Research and SBC Endowed Chair for Early Drug Development, and concurrently served as Clinical Professor of Medicine in the Division of Medical Oncology at the University of Texas Health Science Center at San Antonio. Dr. Rowinsky was an Associate Professor of Oncology at the Johns Hopkins University School of Medicine and on active staff at the Johns Hopkins School of Medicine from 1987 to 1996. Dr. Rowinsky is a member of the boards of directors of Biogen Idec, Inc., BIND Therapeutics, Inc., and Fortress Biosciences, Inc., publicly-held life sciences companies. He is also an Adjunct Professor of Medicine at New York University. Dr. Rowinsky has extensive research and drug development experience, oncology expertise, corporate strategy, and broad scientific and medical knowledge.
Directors whose terms continue until the 2017 Annual Meeting:
Michael M. Goldberg, M.D. has served as a director of Navidea since November 2013 and as Chief Executive Officer of Macrophage Therapeutics, Inc. since March 2015. Dr. Goldberg has served as a Managing Partner of Montaur Capital Partners since January 2007. Dr. Goldberg served as the Chief Executive Officer of Emisphere Technologies, Inc., from August 1990 to January 2007 and as its President from August 1990 to October 1995. He served as Vice President of The First Boston Corp., where he was a founding member of the Healthcare Banking Group. Dr. Goldberg is or has been a director of Alliqua, Inc., Echo Therapeutics, Inc., AngioLight, Inc., Urigen Pharmaceuticals, Inc., and Adventrx Pharmaceuticals, Inc. Dr. Goldberg received a B.S. degree from Rensselaer Polytechnic Institute, an M.D. from Albany Medical College of Union University in 1982, and an M.B.A. from Columbia University Graduate School of Business in 1985.
Ricardo J. Gonzalez joined Navidea in October 2014 and currently serves as our President and Chief Executive Officer. Prior to joining Navidea, Mr. Gonzalez held positions of increasing responsibility at Spectrum Pharmaceuticals, Inc. (Spectrum) from March
54
2008 to October 2014. While at Spectrum, Mr. Gonzalez led teams in the U.S. and abroad and played an active role in the evolution of the organization from a product development company to a global commercial enterprise. Most recently he was responsible for designing and leading the globalization and commercialization strategy for international markets including Europe, Asia, Middle East and Latin America. Of Mr. Gonzalez’s over 20 years of experience in the pharmaceutical industry, 14 years have been focused in specialty markets, including HIV/AIDS, Hematology and Oncology. Mr. Gonzalez’s prior experience includes roles in all aspects of product commercialization including sales, marketing, operations, distribution, managed markets, contracting, reimbursement, pricing and government affairs with several companies including Abraxis Oncology, Genzyme, Ligand Pharmaceuticals, Roche Laboratories and GlaxoSmithKline. Mr. Gonzalez earned his B.S. in Business Logistics from Penn State University.
Director whose term continues until the 2018 Annual Meeting:
Gordon A. Troup has served as a director of Navidea since July 2008. Mr. Troup served as President of the Nuclear Pharmacy Services business at Cardinal Health, Inc. (Cardinal Health), a multinational medical products and services company, from January 2003 until his retirement in December 2007. Mr. Troup joined Cardinal Health in 1990 and was appointed Group President of Pharmaceutical Distribution and Specialty Distribution Services in 1999. Prior to joining Cardinal Health, Mr. Troup was employed for 10 years by American Hospital Supply Corporation and for 3 years by Zellerbach Paper, a Mead Company. In July 2015 Mr. Troup retired as a partner, owner and Chairman of the Board of Scioto Properties, LLC, a provider of group homes to the developmentally disabled nationwide. Mr. Troup is currently Chairman of the Advisory Board of Guild Associates, Inc., a chemical engineering and research and development company serving the energy and military community. Mr. Troup is a member of several national healthcare trade organizations and is active in a number of not-for-profit organizations. Mr. Troup has a B.S. degree in Business Management from San Diego State University.
Executive Officers
In addition to Mr. Gonzalez, the following individuals are senior executive officers of Navidea and serve in the position(s) indicated below:
|
Name |
|
Age |
|
Position |
|
Frederick O. Cope, Ph.D. |
|
69 |
|
Senior Vice President and Chief Scientific Officer |
|
Thomas J. Klima |
|
44 |
|
Senior Vice President and Chief Commercial Officer |
|
Brent L. Larson |
|
52 |
|
Executive Vice President, Chief Financial Officer, Treasurer and Secretary |
|
William J. Regan |
|
64 |
|
Senior Vice President and Chief Compliance Officer |
Frederick O. Cope, Ph.D., F.A.C.N., C.N.S., has served as Senior Vice President and Chief Scientific Officer of Navidea since May 2013. Previous to that, Dr. Cope served as Senior Vice President, Pharmaceutical Research and Clinical Development of Navidea from July 2010 to May 2013 and as Vice President, Pharmaceutical Research and Clinical Development from February 2009 to July 2010. Prior to accepting his position with Navidea, Dr. Cope served as the Assistant Director for Research and Head of Program Research Development for The Ohio State University Comprehensive Cancer Center, The James Cancer Hospital and The Richard J. Solove Research Institute. Dr. Cope also served as head of the Cancer and AIDS product development and commercialization program for the ROSS/Abbott Laboratories division, and head of human and veterinary vaccine production and improvement group for Wyeth Laboratories. Dr. Cope served a fellowship in oncology at the McArdle Laboratory for Cancer Research at the University of Wisconsin and was the honored scientist in residence at the National Cancer Center Research Institute in Tokyo; he is the recipient of the Ernst W. Volwiler Research Award. Dr. Cope is also active in a number of professional and scientific organizations such as serving as an editorial reviewer for several professional journals, and as an advisor/director to the research program of Roswell Park Memorial Cancer Center. Dr. Cope received his B.Sc. from the Delaware Valley College of Science and Agriculture, his M.S. from Millersville University of Pennsylvania and his Ph.D. from the University of Connecticut.
Thomas J. Klima has served as Senior Vice President and Chief Commercial Officer of Navidea since January 2015. Before joining Navidea, Mr. Klima served as National Sales Director/Head of Sales of Algeta from September 2012 to April 2014, when Algeta was acquired by Bayer AG. Mr. Klima led oncology strategy for Bayer from April 2014 to December 2014. Prior to that, Mr. Klima was Senior Director, Marketing at Dendreon Corporation from September 2009 to September 2012. From February 2000 to September 2009, Mr. Klima held positions of increasing responsibility at Eli Lilly, most recently serving as Director, Marketing US Cymbalta Brand Team. Mr. Klima has a B.A. degree in Business Administration and Marketing from Western State College.
Brent L. Larson has served as Executive Vice President of Navidea since May 2013, as Senior Vice President from July 2010 to April 2013, as Chief Financial Officer and Treasurer since February 1999 and as Secretary since 2003. Prior to that, Mr. Larson served as our Vice President, Finance from July 1998 to July 2010 and as Controller from July 1996 to June 1998. Before joining Navidea, Mr. Larson was employed by Price Waterhouse LLP. Mr. Larson has a B.B.A. degree in accounting from Iowa State University of Science and Technology and is a Certified Public Accountant.
55
William J. Regan joined Navidea in October 2012 and currently serves as our Senior Vice President and Chief Compliance Officer. Prior to accepting his position with Navidea, Mr. Regan served as a consultant to Navidea from July 2011 to September 2012. As Principal of Regan Advisory Services (RAS) from September 2006 to September 2012, Mr. Regan consulted on all aspects of regulatory affairs within pharmaceutical, biotechnology and diagnostic imaging businesses, including PET diagnostic agents (cardiovascular, neurology, and oncology), contrast agents, and radiopharmaceuticals. Previous to RAS, Mr. Regan held roles of increasing responsibility in radiopharmaceutical manufacturing, quality assurance, pharmaceutical technology and regulatory affairs at Bristol-Myers Squibb (BMS). From September 2001 to August 2006, he served as global regulatory head for BMS’ Medical Imaging business where he was responsible for all regulatory aspects of the company’s in-market and pipeline products and led regulatory actions resulting in product approvals. Mr. Regan has led efforts to gain two major FDA label expansions for Lymphoseek and in addition has obtained EU regulatory approval for Lymphoseek during 2014. Mr. Regan has been an active member in the Society of Nuclear Medicine, Council on Radionuclides and Radiopharmaceuticals (CORAR), and Medical Imaging and Technology Alliance, and formerly served as the industry chair of the Regulatory and Clinical Practice committee on behalf of CORAR. Mr. Regan serves on the Mass Down Syndrome Congress Business Advisory Council and is a Managing Board Director for Turner Hill LLC. Mr. Regan holds a B.A. in Chemistry from Rutgers University.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 requires our officers and directors, and greater than 10% stockholders, to file reports of ownership and changes in ownership of our securities with the Securities and Exchange Commission. Copies of the reports are required by SEC regulation to be furnished to us. Based on our review of these reports and written representations from reporting persons, we believe that all reporting persons complied with all filing requirements during the fiscal year ended December 31, 2015, except for: Michael M. Goldberg, M.D. and Gordon A. Troup, who each had one late Form 4 filing related to stock issued in lieu of cash for payment of board retainers.
To the Company’s knowledge, Dr. Goldberg has not filed any report under Section 16(a) with respect to the securities of the Company that he as a right to acquire from Platinum. See Security Ownership of Principal Stockholders, Directors, Nominees and Executive Officers and Related Stockholder Matters.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to our directors, officers and all employees. The code of business conduct and ethics is posted on our website at www.navidea.com. The code of business conduct and ethics may also be obtained free of charge by writing to Navidea Biopharmaceuticals, Inc., Attn: Chief Financial Officer, 5600 Blazer Parkway, Suite 200, Dublin, Ohio 43017.
Corporate Governance
Our Board of Directors is responsible for establishing broad corporate policies and reviewing our overall performance rather than day-to-day operations. The primary responsibility of our Board is to oversee the management of Navidea and, in doing so, serve the best interests of the Company and our stockholders. Our Board selects, evaluates and provides for the succession of executive officers and, subject to stockholder election, directors. It reviews and approves corporate objectives and strategies, and evaluates significant policies and proposed major commitments of corporate resources. Our Board also participates in decisions that have a potential major economic impact on the Company. Management keeps our directors informed of Company activity through regular communication, including written reports and presentations at Board and committee meetings.
Board of Directors Meetings
Our Board of Directors held a total of 12 meetings in the fiscal year ended December 31, 2015, and each of the directors attended at least 75 percent of the aggregate number of meetings of the Board of Directors and committees (if any) on which he served. It is our policy that all directors attend the Annual Meeting of Stockholders. However, conflicts and unforeseen events may prevent the attendance of a director, or directors. All then-current members of our Board of Directors attended the 2015 Annual Meeting of Stockholders in person.
The Board of Directors maintains the following committees to assist it in its oversight responsibilities. The current membership of each committee is indicated in the list of directors set forth under “Board of Directors” above.
56
The Audit Committee of the Board of Directors selects our independent registered public accounting firm with whom the Audit Committee reviews the scope of audit and non-audit assignments and related fees, the accounting principles that we use in financial reporting, and the adequacy of our internal control procedures. The members of our Audit Committee are: Brendan A. Ford (Chairman), Anton Gueth, and Gordon A. Troup, each of whom is “independent” under Section 803A of the NYSE MKT Company Guide. The Board of Directors has determined that Brendan A. Ford meets the requirements of an “audit committee financial expert” as set forth in Section 407(d)(5) of Regulation S-K promulgated by the SEC. Under the terms of an Agreement dated March 14, 2016, among the Company and Platinum, Mr. Ford will resign as a director of the Company immediately following the filing of this Report. The Board is expected to appoint new Audit Committee members following Mr. Ford’s departure. The Audit Committee held nine meetings in the fiscal year ended December 31, 2015. The Board of Directors adopted a written Amended and Restated Audit Committee Charter on April 30, 2004. A copy of the Amended and Restated Audit Committee Charter is posted on the Company’s website at www.navidea.com.
Compensation, Nominating and Governance Committee
The CNG Committee of the Board of Directors discharges the Board’s responsibilities relating to the compensation of the Company's directors, executive officers and associates, identifies and recommends to the Board of Directors nominees for election to the Board, and assists the Board in the implementation of sound corporate governance principles and practices. With respect to its compensation functions, the CNG Committee evaluates and approves executive officer compensation and reviews and makes recommendations to the Board with respect to director compensation, including incentive or equity-based compensation plans; reviews and evaluates any discussion and analysis of executive officer and director compensation included in the Company’s annual report or proxy statement, and prepares and approves any report on executive officer and director compensation for inclusion in the Company’s annual report or proxy statement required by applicable rules and regulations; and monitors and evaluates, at the Committee’s discretion, matters relating to the compensation and benefits structure of the Company and such other domestic and foreign subsidiaries or affiliates, as it deems appropriate. The members of our CNG Committee are: Anton Gueth (Chairman), Brendan A. Ford, Eric K. Rowinsky, M.D., and Gordon A. Troup. Under the terms of an Agreement dated March 14, 2016, among the Company and Platinum, Mr. Ford will resign as a director of the Company immediately following the filing of this Report. The Board is expected to appoint new CNG Committee members following Mr. Ford’s departure. The CNG Committee held three meetings in the fiscal year ended December 31, 2015 to complement compensation-related discussions held by the full Board. The Board of Directors adopted a written Compensation, Nominating and Governance Committee Charter on February 26, 2009. A copy of the Compensation, Nominating and Governance Committee Charter is posted on the Company’s website at www.navidea.com.
Item 11. Executive Compensation
Compensation Discussion and Analysis
Overview of Compensation Program. The CNG Committee of the Board of Directors is responsible for establishing and implementing our compensation policies applicable to senior executives and monitoring our compensation practices. The CNG Committee seeks to ensure that our compensation plans are fair, reasonable and competitive. The CNG Committee is responsible for reviewing and approving all senior executive compensation, all awards under our cash bonus plan, and awards under our equity-based compensation plans.
Philosophy and Goals of Executive Compensation Plans. The CNG Committee’s philosophy for executive compensation is to:
|
|
· |
Pay for performance: The CNG Committee believes that our executives should be compensated based upon their ability to achieve specific operational and strategic results. Therefore, our compensation plans are designed to provide rewards for the individual’s contribution to our performance. |
|
|
· |
Pay commensurate with other companies categorized as value creators: The CNG Committee has set a goal that the Company should move towards compensation levels for senior executives that are, at a minimum, at the 40th to 50th percentile for similar executives in the workforce while taking into account current market conditions and Company performance. This allows us to attract, hire, reward and retain senior executives who formulate and execute our strategic plans and drive exceptional results. |
To ensure our programs are competitive, the CNG Committee periodically reviews compensation information of peer companies, national data and trends in executive compensation to help determine the appropriateness of our plans and compensation levels. These reviews, and the CNG Committee’s commitment to pay for performance, become the basis for the CNG Committee’s decisions on compensation plans and individual executive compensation payments.
57
The CNG Committee has approved a variety of programs that work together to provide a combination of basic compensation and strong incentives. While it is important for us to provide certain base level salaries and benefits to remain competitive, the CNG Committee’s objective is to provide compensation plans with incentive opportunities that motivate and reward executives for consistently achieving superior results. The CNG Committee designs our compensation plans to:
|
|
· |
Reward executives based upon overall company performance, their individual contributions and creation of stockholder value; |
|
|
· |
Encourage top performers to make a long-term commitment to our Company; and |
|
|
· |
Align executive incentive plans with the long-term interests of stockholders. |
The CNG Committee reviews competitive information and individual compensation levels at least annually. During the review process, the CNG Committee addresses the following questions:
|
|
· |
Do any existing compensation plans need to be adjusted to reflect changes in competitive practices, different market circumstances or changes to our strategic initiatives? |
|
|
· |
Should any existing compensation plans be eliminated or new plans be added to the executive compensation programs? |
|
|
· |
What are the compensation-related objectives for our compensation plans for the upcoming fiscal year? |
|
|
· |
Based upon individual performance, what compensation modifications should be made to provide incentives for senior executives to perform at superior levels? |
In addressing these questions, the CNG Committee considers input from management, outside compensation experts and published surveys of compensation levels and practices.
The CNG Committee does not believe that our compensation policies and practices for our employees give rise to risks that are reasonably likely to have a material adverse effect on the Company. As noted below, our incentive-based compensation is generally tied to Company financial performance (i.e., revenue or gross margin) or product development goals (i.e., clinical trial progress or regulatory milestones). The CNG Committee believes that the existence of these financial performance incentives creates a strong motivation for Company employees to contribute towards the achievement of strong, sustainable financial and development performance, and believes that the Company has a strong set of internal controls that minimize the risk that financial performance can be misstated in order to achieve incentive compensation payouts.
In addition to the aforementioned considerations, the CNG Committee also takes into account the outcome of stockholder advisory (“say-on-pay”) votes, taken every three years, on the compensation of our Chief Executive Officer, Chief Financial Officer, and our other three highest-paid executive officers (the Named Executive Officers). At the Annual Meeting of Stockholders held on July 17, 2014, approximately 74% of our stockholders voted in favor of the resolution relating to the compensation of our Named Executive Officers. The CNG Committee believes this affirmed stockholders’ support of the Company’s executive compensation program, and as such did not change its approach in 2015. The CNG Committee will continue to consider the results of future say-on-pay votes when making future compensation decisions for the executive officers.
Scope of Authority of the CNG Committee. The Board of Directors has authorized the CNG Committee to establish the compensation programs for all executive officers and to provide oversight for compliance with our compensation philosophy. The CNG Committee delegates the day-to-day administration of the compensation plans to management (except with respect to our executive officers), but retains responsibility for ensuring that the plan administration is consistent with the Company’s policies. Annually, the CNG Committee sets the compensation for our executive officers, including objectives and awards under incentive plans. Mr. Gonzalez provides input for the CNG Committee regarding the performance and appropriate compensation of the other officers. The CNG Committee gives considerable weight to Mr. Gonzalez’s evaluation of the other officers because of his direct knowledge of each officer’s performance and contributions. The CNG Committee also makes recommendations to the Board of Directors on appropriate compensation for the non-employee directors. In addition to overseeing the compensation of executive officers, the CNG Committee approves awards under short-term cash incentive and long-term equity-based compensation plans for all other employees. For more information on the CNG Committee’s role, see the CNG Committee’s charter, which can be found on our website at www.navidea.com.
Independent Compensation Expertise. The CNG Committee is authorized to retain independent experts to assist in evaluating executive compensation plans and in setting executive compensation levels. These experts provide information on trends and best practices so the CNG Committee can formulate ongoing plans for executive compensation. The CNG Committee retained Pearl Meyer & Partners (Pearl Meyer) as its independent expert to assist in the determination of the reasonableness and competitiveness of the executive compensation plans and senior executives’ individual compensation levels for fiscal 2015. No conflict of interest exists that would prevent Pearl Meyer from serving as independent consultant to the CNG Committee.
58
For fiscal 2015, Pearl Meyer performed a benchmark compensation review of our key executive positions, including our Named Executive Officers. Pearl Meyer utilized both published survey and proxy reported data from compensation peers, with market data aged to March 1, 2016 by an annualized rate of 3.0%, the expected pay increase in 2016 for executives in the life sciences industry.
In evaluating appropriate executive compensation, it is common practice to set targets at a point within the competitive marketplace. The CNG Committee sets its competitive compensation levels based upon its compensation philosophy. Following completion of the Pearl Meyer study for 2015, the CNG Committee noted that our overall executive compensation was, in aggregate, below the 25th percentile for an established peer group of companies.
Peer Group Companies. In addition to independent survey analysis, in 2015 the CNG Committee also reviewed the compensation levels at specific competitive benchmark companies. With input from management, the CNG Committee chose the peer companies because they operate within the biotechnology industry, have market capitalization between $100 million and $500 million, have similar business models to our Company or have comparable key executive positions. While the specific plans for these companies may or may not be used, it is helpful to review their compensation data to provide benchmarks for the overall compensation levels that will be used to attract, hire, retain and motivate our executives.
As competitors and similarly situated companies that compete for the same executive talent, the CNG Committee determined that the following peer group companies most closely matched the responsibilities and requirements of our executives:
|
Sangamo Biosciences, Inc. |
|
ArQule, Inc. |
|
Inovio Pharmaceuticals, Inc. |
|
Galena Biopharma, Inc. |
|
Geron Corporation |
|
Keryx Biopharmaceuticals, Inc. |
|
Rigel Pharmaceuticals, Inc. |
|
BioTime, Inc. |
|
OncoMed Pharmaceuticals, Inc. |
|
Omeros Corporation |
|
CTI BioPharma Corp. |
|
Immunomedics, Inc. |
|
Unilife Corporation |
|
Nymox Pharmaceutical Corporation |
Pearl Meyer and the CNG Committee used the publicly available compensation information for these companies to analyze our competitive position in the industry. Base salaries and short-term and long term incentive plans of the executives of these companies were reviewed to provide background and perspective in analyzing the compensation levels for our executives.
Specific Elements of Executive Compensation
Base Salary. Using information gathered by Pearl Meyer, peer company data, national surveys, general compensation trend information and recommendations from management, the CNG Committee approved the fiscal 2015 base salaries for our senior executives. Base salaries for senior executives are set using the CNG Committee’s philosophy that compensation should be competitive and based upon performance. Executives should expect that their base salaries, coupled with a cash bonus award, would provide them the opportunity to be compensated at or above the competitive market at the 40th to 50th percentile.
Based on competitive reviews of similar positions, industry salary trends, overall company results and individual performance, salary increases may be approved from time to time. The CNG Committee reviews and approves base salaries of all executive officers. In setting specific base salaries for fiscal 2015, the CNG Committee considered published proxy data for similar positions at peer group companies.
59
The following table shows the changes in base salaries for the Named Executive Officers that were approved for fiscal 2015 compared to the approved salaries for fiscal 2014:
|
Named Executive Officer |
|
Fiscal 2015 Base Salary(a) |
|
|
Fiscal 2014 Base Salary |
|
|
Change(b) |
|
|||
|
Ricardo J. Gonzalez |
|
$ |
375,000 |
|
|
$ |
375,000 |
|
|
|
0.0 |
% |
|
Frederick O. Cope, Ph.D. |
|
|
279,130 |
|
|
|
279,130 |
|
|
|
0.0 |
% |
|
Thomas J. Klima |
|
|
270,000 |
|
|
|
— |
|
|
N/A |
|
|
|
Brent L. Larson |
|
|
260,000 |
|
|
|
260,000 |
|
|
|
0.0 |
% |
|
Michael B. Tomblyn, M.D.(c) |
|
|
300,000 |
|
|
|
— |
|
|
N/A |
|
|
|
(a) |
The amount shown for fiscal 2015 is the approved annual salary of the Named Executive Officer in effect at the end of 2015. The actual amount paid to the Named Executive Officer during fiscal 2015 is shown under “Salary” in the Summary Compensation table below. |
|
(b) |
Based on the performance of the Company's stock during 2014, Mr. Gonzalez, Dr. Cope and Mr. Larson did not receive salary increases in 2015. |
|
(c) |
Dr. Tomblyn separated from the Company effective February 19, 2016. |
The CNG Committee has not approved any changes to base salaries of Named Executive Officers for fiscal 2016.
Short-Term Incentive Compensation. Our executive officers, along with all of our employees, are eligible to participate in our annual cash bonus program, which has four primary objectives:
|
|
· |
Attract, retain and motivate top-quality executives who can add significant value to the Company; |
|
|
· |
Create an incentive compensation opportunity that is an integral part of the employee’s total compensation program; |
|
|
· |
Reward participants’ contributions to the achievement of our business results; and |
|
|
· |
Provide an incentive for individuals to achieve corporate objectives that are tied to our strategic goals. |
The cash bonus compensation plan provides each participant with an opportunity to receive an annual cash bonus based on our Company’s performance during the fiscal year. Cash bonus targets for senior executives are determined as a percentage of base salary, based in part on published proxy data for similar positions at peer group companies. The following are the key provisions of the cash bonus compensation plan:
|
|
· |
The plan is administered by the CNG Committee, which has the power and authority to establish, adjust, pay or decline to pay the cash bonus for each participant, including the power and authority to increase or decrease the cash bonus otherwise payable to a participant. However, the Committee does not have the power to increase, or make adjustments that would have the effect of increasing, the cash bonus otherwise payable to any executive officer. The Committee has the right to delegate to the Chief Executive Officer its authority and responsibilities with respect to the cash bonuses payable to employees other than executive officers. |
|
|
· |
All Company employees are eligible to participate. |
|
|
· |
The CNG Committee is responsible for specifying the terms and conditions for earning cash bonuses, including establishing specific performance objectives. Cash bonuses payable to executive officers are intended to constitute “qualified performance-based compensation” for purposes of Section 162(m) of the Internal Revenue Code. Consequently, each cash bonus awarded to an executive officer must be conditioned on one or more specified “Performance Measures,” calculated on a consolidated basis. Possible Performance Measures include revenues; gross margin; operating income; net income; clinical trial progress; regulatory milestones; or any other performance objective approved by the CNG Committee. |
|
|
· |
As soon as reasonably practicable after the end of each fiscal year, the CNG Committee determines whether and to what extent each specified business performance objective has been achieved and the amount of the cash bonus to be paid to each participant. |
60
In March 2015, the CNG Committee established the fiscal 2015 targets and performance measures for all Company employees. For fiscal 2015, the cash bonus for each executive officer was a function of the designated target bonus amount and certain business performance objectives, weighted as a percentage of the total target amount. The business performance objectives established for fiscal 2015 were as follows:
|
|
· |
Achievement of a specified revenue amount from Lymphoseek sales during fiscal 2015, subject to a maximum 65% reduction of bonus if not achieved. |
|
|
· |
Adherence to corporate, departmental and project budgets to within a variance of +/- 5%, subject to a maximum 15% reduction of bonus if not achieved. |
|
|
· |
Initiation of enrollment in a clinical trial in support of Lymphoseek life cycle management in one of the three specified indications, subject to a maximum 5% reduction of bonus if not achieved. |
|
|
· |
Achievement of the following goals related to Macrophage Therapeutics, subject to a maximum 5% reduction of bonus if not achieved: |
|
|
o |
Form and engage the scientific advisory board to inform disease target selection; and |
|
|
o |
Establish a development plan based on disease targets. |
|
|
· |
Discretionary bonus, equal to 10% of the total bonus objective. |
For the Named Executive Officers, the following cash bonus targets were established for fiscal 2015:
|
Named Executive Officer |
|
Target Cash Bonus (% of Salary) |
|
|
Target Cash Bonus ($ Amount) |
|
||
|
Ricardo J. Gonzalez |
|
|
50.0 |
% |
|
|
187,500 |
|
|
Frederick O. Cope, Ph.D. |
|
|
35.0 |
% |
|
|
97,696 |
|
|
Thomas J. Klima |
|
|
35.0 |
% |
|
|
94,500 |
|
|
Brent L. Larson |
|
|
35.0 |
% |
|
|
91,000 |
|
|
Michael B. Tomblyn, M.D.(a) |
|
|
35.0 |
% |
|
|
105,000 |
|
|
(a) |
Dr. Tomblyn separated from the Company effective February 19, 2016, therefore no bonus will be awarded to Dr. Tomblyn for fiscal 2015. |
In February 2016, the CNG Committee determined the extent to which the Company’s executive officers achieved the goals during 2015. With respect to the first objective, (i) the Company generated only a portion of the specified target revenue amount from Lymphoseek sales during fiscal 2015, resulting in a reduction in the target bonus amount; (ii) adherence to corporate, departmental project budgets to within a variance of +/- 5% was achieved; (iii) initiation of enrollment in a clinical trial in support of Lymphoseek life cycle management in one of three specified indications was not achieved to the degree expected, resulting in a reduction in the target bonus amount; and (iv) the scientific advisory board for Macrophage Therapeutics was formed and engaged, and a development plan was established based on disease targets. With regard to the first objective, the specified revenue amount from Lymphoseek sales was not achieved as originally intended; however, the CNG Committee concluded that based on meeting the lower end of guidance for fiscal 2015, this component would be subject to a 39% reduction of the total bonus payout for executives and a 26% reduction of the total bonus payout for non-executives. With respect to the second objective, adherence to corporate, departmental and project budget goals evidenced successful achievement of that goal. With regard to the third objective, the CNG Committee concluded that this component would be subject to a further 2.5% reduction of the total bonus payout. With respect to the fourth objective, formation and engagement of the Macrophage Therapeutics scientific advisory board and establishment of a development plan based on disease targets evidenced successful achievement of that goal. With respect to the discretionary portion of the total bonus opportunity, the CNG Committee concluded that this component would be subject to a further 2.5% reduction of the total bonus payout for executives and a further 2% reduction of the total bonus payout for non-executives. After reviewing the business performance objectives and the related proposed payouts, the Board of Directors approved the total cash bonus payouts for non-executive employees of the Company. In an effort to conserve cash resources, the Board of Directors also determined that fifty percent of the 2015 bonus amount payable to certain executive officers would be paid in stock options in lieu of cash, calculated based on the Black-Scholes value of the options on the date of grant. As such, Dr. Cope, Mr. Klima and Mr. Larson were awarded options to purchase 58,510, 56,598 and 54,501, respectively, shares of common stock of the Company at an exercise price of $0.98 per share, vesting immediately upon the date of grant and expiring after ten years. The Board of Directors has not yet finalized what portion, if any, of Mr. Gonzalez’s 2015 bonus amount will be payable in equity.
61
Long-Term Incentive Compensation. All Company employees are eligible to receive equity awards in the form of stock options or restricted stock. Equity instruments awarded under the Company’s equity-based compensation plan are based on the following criteria:
|
|
· |
Analysis of competitive information for comparable positions; |
|
|
· |
Evaluation of the value added to the Company by hiring or retaining specific employees; and |
|
|
· |
Each employee’s long-term potential contributions to our Company. |
Although equity awards may be made at any time as determined by the CNG Committee, they are generally made to all full-time employees once per year or on the recipient’s hire date in the case of new-hire grants.
The CNG Committee’s philosophy on equity awards is that equity-based compensation is an effective method to align the interests of stockholders and management and focus management’s attention on long-term results. When awarding equity-based compensation the CNG Committee considers the impact the participant can have on our overall performance, strategic direction, financial results and stockholder value. Therefore, equity awards are primarily based upon the participant’s position in the organization, competitive necessity and individual performance. Equity awards for senior executives are determined as a percentage of base salary, based on published proxy data for similar positions at peer group companies. Stock option awards have vesting schedules over several years to promote long-term performance and retention of the recipient, and restricted stock awards may include specific performance criteria for vesting or vest over a specified period of time.
Other Benefits and Perquisites. The Named Executive Officers participate in other benefit plans on the same terms as other employees. These plans include medical, dental, vision, disability and life insurance benefits, and our 401(k) retirement savings plan (the 401(k) Plan).
Our vacation policy allows employees to carry up to 40 hours of unused vacation time forward to the next fiscal year. Any unused vacation time in excess of the amount eligible for rollover is generally forfeited.
Our Named Executive Officers are considered “key employees” for purposes of IRC Section 125 Plan non-discrimination testing. Based on such non-discrimination testing, we determined that our Section 125 Plan was “top-heavy.” As such, our key employees are ineligible to participate in the Section 125 Plan and are unable to pay their portion of medical, dental, and vision premiums on a pre-tax basis. As a result, the Company reimburses its key employees an amount equal to the lost tax benefit.
We pay group life insurance premiums on behalf of all employees, including the Named Executive Officers. The benefit provides life insurance coverage at two times the employee’s annual salary plus $10,000, up to a maximum of $630,000.
We also pay group long-term disability insurance premiums on behalf of all employees, including the Named Executive Officers. The benefit provides long-term disability insurance coverage at 60% of the employee’s annual salary, up to a maximum of $10,000 per month, beginning 180 days after the date of disability and continuing through age 65.
401(k) Retirement Plan. All employees are given an opportunity to participate in our 401(k) Plan, following a new-hire waiting period. The 401(k) Plan allows participants to have pre-tax amounts withheld from their pay and provides for a discretionary employer matching contribution (currently, a 40% match up to 5% of salary in the form of our common stock). Participants may invest their contributions in various fund options, but are prohibited from investing their contributions in our common stock. Participants are immediately vested in both their contributions and Company matching contributions. The 401(k) Plan qualifies under section 401 of the Internal Revenue Code, which provides that employee and company contributions and income earned on contributions are not taxable to the employee until withdrawn from the Plan, and that we may deduct our contributions when made.
Employment Agreements
Our senior executive officers are generally employed under employment agreements which specify the terms of their employment such as base salary, benefits, paid time off, and post-employment benefits as shown in the tables below. Our employment agreements also specify that if a change in control occurs with respect to our Company and the employment of a senior executive officer is concurrently or subsequently terminated:
|
|
· |
by the Company without cause (cause is defined as any willful breach of a material duty by the senior executive officer in the course of his or her employment or willful and continued neglect of his or her duty as an employee); |
|
|
· |
by the expiration of the term of the employment agreement; or |
62
then, the senior executive officer would be paid a severance payment as disclosed in the tables below. For purposes of such employment agreements, a change in control includes:
|
|
· |
the acquisition, directly or indirectly, by a person (other than our Company, an employee benefit plan established by the Board of Directors, or a participant in a transaction approved by the Board of Directors for the principal purpose of raising additional capital) of beneficial ownership of 30% or more of our securities with voting power in the next meeting of holders of voting securities to elect the Directors; |
|
|
· |
a majority of the Directors elected at any meeting of the holders of our voting securities are persons who were not nominated by our then current Board of Directors or an authorized committee thereof; |
|
|
· |
our stockholders approve a merger or consolidation of our Company with another person, other than a merger or consolidation in which the holders of our voting securities outstanding immediately before such merger or consolidation continue to hold voting securities in the surviving or resulting corporation (in the same relative proportions to each other as existed before such event) comprising 80% or more of the voting power for all purposes of the surviving or resulting corporation; or |
|
|
· |
our stockholders approve a transfer of substantially all of our assets to another person other than a transfer to a transferee, 80% or more of the voting power of which is owned or controlled by us or by the holders of our voting securities outstanding immediately before such transfer in the same relative proportions to each other as existed before such event. |
Ricardo J. Gonzalez. Mr. Gonzalez is employed under a 36-month employment agreement effective through October 13, 2017. The employment agreement provides for an annual base salary of $375,000. For the calendar year ending December 31, 2015, the CNG Committee determined that the maximum bonus payment to Mr. Gonzalez would be $187,500.
Frederick O. Cope, Ph.D. Dr. Cope was employed under a 24-month employment agreement effective through December 31, 2014. The employment agreement provided for an annual base salary of $271,000. Effective May 1, 2013, Dr. Cope’s annual base salary was increased to $279,130. For the calendar year ending December 31, 2015, the CNG Committee determined that the maximum bonus payment to Dr. Cope would be $97,696. Although Dr. Cope's employment agreement expired on December 31, 2014, the terms of the agreement provide for continuation of certain terms of the employment agreement as long as Dr. Cope continues to be an employee of the Company following expiration of the agreement.
Thomas J. Klima. Mr. Klima is employed under a 24-month employment agreement effective through January 1, 2017. The employment agreement provides for an annual base salary of $270,000. For the calendar year ending December 31, 2015, the CNG Committee determined that the maximum bonus payment to Mr. Klima would be $94,500.
Brent L. Larson. Mr. Larson was employed under a 24-month employment agreement effective through December 31, 2014. The employment agreement provided for an annual base salary of $265,000. Effective May 1, 2013, Mr. Larson’s annual base salary was increased to $279,575. Effective January 1, 2014, Mr. Larson agreed to a reduction in his annual base salary to $260,000. For the calendar year ending December 31, 2015, the CNG Committee determined that the maximum bonus payment to Mr. Larson would be $91,000. Although Mr. Larson's employment agreement expired on December 31, 2014, the terms of the agreement provide for continuation of certain terms of the employment agreement as long as Mr. Larson continues to be an employee of the Company following expiration of the agreement.
Michael B. Tomblyn, M.D. Prior to his separation on February 19, 2016, Dr. Tomblyn was employed under a 24-month employment agreement effective through January 1, 2017. The employment agreement provided for an annual base salary of $300,000. For the calendar year ending December 31, 2015, the CNG Committee determined that the maximum bonus payment to Dr. Tomblyn would be $105,000.
Post-Employment Compensation
The following tables set forth the expected benefit to be received by each of our Named Executive Officers in the event of his termination resulting from various scenarios, assuming a termination date of December 31, 2015 and a stock price of $1.33, our closing stock price on December 31, 2015.
63
|
|
|
For Cause |
|
|
Resignation |
|
|
Death |
|
|
Disability |
|
|
End of Term |
|
|
Without Cause |
|
|
Change in Control |
|
|||||||
|
Cash payments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Severance (a) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
375,000 |
|
|
$ |
375,000 |
|
|
$ |
750,000 |
|
|
Disability supplement (b) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
185,100 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Paid time off (c) |
|
|
7,212 |
|
|
|
7,212 |
|
|
|
7,212 |
|
|
|
7,212 |
|
|
|
7,212 |
|
|
|
7,212 |
|
|
|
7,212 |
|
|
2015 401(k) match (d) |
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
Continuation of benefits (e) |
|
|
— |
|
|
|
— |
|
|
|
50,635 |
|
|
|
50,635 |
|
|
|
— |
|
|
|
50,635 |
|
|
|
50,635 |
|
|
Stock option vesting acceleration (f) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
49,000 |
|
|
|
49,000 |
|
|
|
49,000 |
|
|
Total |
|
$ |
12,512 |
|
|
$ |
12,512 |
|
|
$ |
63,147 |
|
|
$ |
248,247 |
|
|
$ |
436,512 |
|
|
$ |
487,147 |
|
|
$ |
862,147 |
|
|
(a) |
Severance amounts are pursuant to Mr. Gonzalez’s employment agreement. |
|
(b) |
During the first 6 months of disability, the Company will supplement disability insurance payments to Mr. Gonzalez to achieve 100% salary replacement. The Company’s short-term disability insurance policy currently pays $100 per week for a maximum of 24 weeks. |
|
(c) |
Amount represents the value of 40 hours of accrued but unused vacation time as of December 31, 2015. |
|
(d) |
Amount represents the value of 3,161 shares of Company stock which was accrued during 2015 as the Company’s 401(k) matching contribution but was unissued as of December 31, 2015. |
|
(e) |
Amount represents 22 months of medical, dental and vision insurance premiums at rates in effect at December 31, 2015. |
|
(f) |
Pursuant to Mr. Gonzalez’s stock option agreement, all unvested stock options outstanding will vest upon termination at the end of the term of his employment agreement, termination without cause, or a change in control. Amount represents the value of the stock at $1.33, the closing price of the Company’s stock on December 31, 2015, less the exercise price of the options. Amount does not include stock options with an exercise price higher than $1.33, the closing price of the Company’s stock on December 31, 2015. |
Frederick O. Cope, Ph.D.
|
|
|
For Cause |
|
|
Resignation |
|
|
Death |
|
|
Disability |
|
|
End of Term |
|
|
Without Cause |
|
|
Change in Control |
|
|||||||
|
Cash payments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Severance (a) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
245,000 |
|
|
$ |
245,000 |
|
|
$ |
367,500 |
|
|
Disability supplement (b) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
137,165 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Paid time off (c) |
|
|
5,368 |
|
|
|
5,368 |
|
|
|
5,368 |
|
|
|
5,368 |
|
|
|
5,368 |
|
|
|
5,368 |
|
|
|
5,368 |
|
|
2015 401(k) match (d) |
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
Continuation of benefits (e) |
|
|
— |
|
|
|
— |
|
|
|
19,238 |
|
|
|
19,238 |
|
|
|
— |
|
|
|
19,238 |
|
|
|
19,238 |
|
|
Stock option vesting acceleration (f) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Restricted stock vesting acceleration (g) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
66,450 |
|
|
Total |
|
$ |
10,668 |
|
|
$ |
10,668 |
|
|
$ |
29,906 |
|
|
$ |
167,071 |
|
|
$ |
255,668 |
|
|
$ |
274,906 |
|
|
$ |
463,856 |
|
|
(a) |
Severance amounts are pursuant to Dr. Cope’s employment agreement. |
|
(b) |
During the first 6 months of disability, the Company will supplement disability insurance payments to Dr. Cope to achieve 100% salary replacement. The Company’s short-term disability insurance policy currently pays $100 per week for a maximum of 24 weeks. |
|
(c) |
Amount represents the value of 40 hours of accrued but unused vacation time as of December 31, 2015. |
|
(d) |
Amount represents the value of 3,026 shares of Company stock which was accrued during 2015 as the Company’s 401(k) matching contribution but was unissued as of December 31, 2015. |
|
(e) |
Amount represents 12 months of medical, dental and vision insurance premiums at rates in effect at December 31, 2015. |
|
(f) |
Pursuant to Dr. Cope’s stock option agreements, all unvested stock options outstanding will vest upon termination at the end of the term of his employment agreement, termination without cause, or a change in control. Amount represents the value of the stock at $1.33, the closing price of the Company’s stock on December 31, 2015, less the exercise price of the options. Amount does not include stock options with an exercise price higher than $1.33, the closing price of the Company’s stock on December 31, 2015. |
|
(g) |
Pursuant to Dr. Cope’s restricted stock agreements, certain unvested restricted stock outstanding will vest upon a change in control. |
64
|
|
|
For Cause |
|
|
Resignation |
|
|
Death |
|
|
Disability |
|
|
End of Term |
|
|
Without Cause |
|
|
Change in Control |
|
|||||||
|
Cash payments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Severance (a) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
270,000 |
|
|
$ |
270,000 |
|
|
$ |
405,000 |
|
|
Disability supplement (b) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
132,600 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Paid time off (c) |
|
|
5,192 |
|
|
|
5,192 |
|
|
|
5,192 |
|
|
|
5,192 |
|
|
|
5,192 |
|
|
|
5,192 |
|
|
|
5,192 |
|
|
2015 401(k) match (d) |
|
|
1,804 |
|
|
|
1,804 |
|
|
|
1,804 |
|
|
|
1,804 |
|
|
|
1,804 |
|
|
|
1,804 |
|
|
|
1,804 |
|
|
Continuation of benefits (e) |
|
|
— |
|
|
|
— |
|
|
|
27,619 |
|
|
|
27,619 |
|
|
|
— |
|
|
|
41,429 |
|
|
|
27,619 |
|
|
Stock option vesting acceleration (f) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Restricted stock vesting acceleration (g) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
6,996 |
|
|
$ |
6,996 |
|
|
$ |
34,615 |
|
|
$ |
167,215 |
|
|
$ |
276,996 |
|
|
$ |
318,425 |
|
|
$ |
439,615 |
|
|
(a) |
Severance amounts are pursuant to Mr. Klima’s employment agreement. |
|
(b) |
During the first 6 months of disability, the Company will supplement disability insurance payments to Mr. Klima to achieve 100% salary replacement. The Company’s short-term disability insurance policy currently pays $100 per week for a maximum of 24 weeks. |
|
(c) |
Amount represents the value of 40 hours of accrued but unused vacation time as of December 31, 2015. |
|
(d) |
Amount represents the value of 1,050 shares of Company stock which was accrued during 2015 as the Company’s 401(k) matching contribution but was unissued as of December 31, 2015. |
|
(e) |
Amount represents 12 months of medical, dental and vision insurance premiums at rates in effect at December 31, 2015, except in the case of termination without cause, for which Mr. Klima’s employment agreement provides for continuation of benefits for 18 months. |
|
(f) |
Pursuant to Mr. Klima’s stock option agreements, all unvested stock options outstanding will vest upon termination at the end of the term of his employment agreement, termination without cause, or a change in control. Amount represents the value of the stock at $1.33, the closing price of the Company’s stock on December 31, 2015, less the exercise price of the options. Amount does not include stock options with an exercise price higher than $1.33, the closing price of the Company’s stock on December 31, 2015. |
|
(g) |
Mr. Klima’s restricted stock agreements do not include provisions for accelerated vesting. |
Brent L. Larson
|
|
|
For Cause |
|
|
Resignation |
|
|
Death |
|
|
Disability |
|
|
End of Term |
|
|
Without Cause |
|
|
Change in Control |
|
|||||||
|
Cash payments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Severance (a) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
207,000 |
|
|
$ |
207,000 |
|
|
$ |
310,500 |
|
|
Disability supplement (b) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
127,600 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Paid time off (c) |
|
|
5,000 |
|
|
|
5,000 |
|
|
|
5,000 |
|
|
|
5,000 |
|
|
|
5,000 |
|
|
|
5,000 |
|
|
|
5,000 |
|
|
2015 401(k) match (d) |
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
|
5,300 |
|
|
Continuation of benefits (e) |
|
|
— |
|
|
|
— |
|
|
|
27,619 |
|
|
|
27,619 |
|
|
|
— |
|
|
|
27,619 |
|
|
|
27,619 |
|
|
Stock option vesting acceleration (f) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
10,300 |
|
|
$ |
10,300 |
|
|
$ |
37,919 |
|
|
$ |
165,519 |
|
|
$ |
217,300 |
|
|
$ |
244,919 |
|
|
$ |
348,419 |
|
|
(a) |
Severance amounts are pursuant to Mr. Larson’s employment agreement. |
|
(b) |
During the first 6 months of disability, the Company will supplement disability insurance payments to Mr. Larson to achieve 100% salary replacement. The Company’s short-term disability insurance policy currently pays $100 per week for a maximum of 24 weeks. |
|
(c) |
Amount represents the value of 40 hours of accrued but unused vacation time as of December 31, 2015. |
|
(d) |
Amount represents the value of 2,966 shares of Company stock which was accrued during 2015 as the Company’s 401(k) matching contribution but was unissued as of December 31, 2015. |
|
(e) |
Amount represents 12 months of medical, dental and vision insurance premiums at rates in effect at December 31, 2015. |
|
(f) |
Pursuant to Mr. Larson’s stock option agreements, all unvested stock options outstanding will vest upon termination at the end of the term of his employment agreement, termination without cause, or a change in control. Amount represents the value of the stock at $1.33, the closing price of the Company’s stock on December 31, 2015, less the exercise price of the options. Amount does not include stock options with an exercise price higher than $1.33, the closing price of the Company’s stock on December 31, 2015. |
65
|
|
|
For Cause |
|
|
Resignation |
|
|
Death |
|
|
Disability |
|
|
End of Term |
|
|
Without Cause |
|
|
Change in Control |
|
|||||||
|
Cash payments: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Severance (a) |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
300,000 |
|
|
$ |
300,000 |
|
|
$ |
450,000 |
|
|
Disability supplement (b) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
147,600 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Paid time off (c) |
|
|
5,769 |
|
|
|
5,769 |
|
|
|
5,769 |
|
|
|
5,769 |
|
|
|
5,769 |
|
|
|
5,769 |
|
|
|
5,769 |
|
|
2015 401(k) match (d) |
|
|
4,543 |
|
|
|
4,543 |
|
|
|
4,543 |
|
|
|
4,543 |
|
|
|
4,543 |
|
|
|
4,543 |
|
|
|
4,543 |
|
|
Continuation of benefits (e) |
|
|
— |
|
|
|
— |
|
|
|
27,619 |
|
|
|
27,619 |
|
|
|
— |
|
|
|
41,429 |
|
|
|
27,619 |
|
|
Stock option vesting acceleration (f) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Restricted stock vesting acceleration (g) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Total |
|
$ |
10,312 |
|
|
$ |
10,312 |
|
|
$ |
37,931 |
|
|
$ |
185,531 |
|
|
$ |
310,312 |
|
|
$ |
351,741 |
|
|
$ |
487,931 |
|
|
(a) |
Severance amounts are pursuant to Dr. Tomblyn’s employment agreement. |
|
(b) |
During the first 6 months of disability, the Company will supplement disability insurance payments to Dr. Tomblyn to achieve 100% salary replacement. The Company’s short-term disability insurance policy currently pays $100 per week for a maximum of 24 weeks. |
|
(c) |
Amount represents the value of 40 hours of accrued but unused vacation time as of December 31, 2015. |
|
(d) |
Amount represents the value of 2,558 shares of Company stock which was accrued during 2015 as the Company’s 401(k) matching contribution but was unissued as of December 31, 2015. |
|
(e) |
Amount represents 12 months of medical, dental and vision insurance premiums at rates in effect at December 31, 2015, except in the case of termination without cause, for which Dr. Tomblyn’s employment agreement provides for continuation of benefits for 18 months. |
|
(f) |
Pursuant to Dr. Tomblyn’s stock option agreements, all unvested stock options outstanding will vest upon termination at the end of the term of his employment agreement, termination without cause, or a change in control. Amount represents the value of the stock at $1.33, the closing price of the Company’s stock on December 31, 2015, less the exercise price of the options. Amount does not include stock options with an exercise price higher than $1.33, the closing price of the Company’s stock on December 31, 2015. |
|
(g) |
Dr. Tomblyn’s restricted stock agreements do not include provisions for accelerated vesting. |
|
(h) |
Dr. Tomblyn separated from the Company effective February 19, 2016. |
Report of Compensation, Nominating and Governance Committee
The CNG Committee is responsible for establishing, reviewing and approving the Company’s compensation philosophy and policies, reviewing and making recommendations to the Board regarding forms of compensation provided to the Company’s directors and officers, reviewing and determining cash and equity awards for the Company’s officers and other employees, and administering the Company’s equity incentive plans.
In this context, the CNG Committee has reviewed and discussed with management the Compensation Discussion and Analysis included in this annual report on Form 10-K. In reliance on the review and discussions referred to above, the CNG Committee recommended to the Board, and the Board has approved, that the Compensation Discussion and Analysis be included in this annual report on Form 10-K for filing with the SEC.
|
|
|
The Compensation, Nominating |
|
|
|
and Governance Committee |
|
|
|
|
|
|
|
Anton Gueth (Chairman) |
|
|
|
Brendan A. Ford |
|
|
|
Eric K. Rowinsky, M.D. |
|
|
|
Gordon A. Troup |
Compensation, Nominating and Governance Committee Interlocks and Insider Participation
The current members of our CNG Committee are: Anton Gueth (Chairman), Brendan A. Ford, Eric K. Rowinsky, M.D., and Gordon A. Troup, and each served as a member of the CNG Committee during the last completed fiscal year. None of these individuals were at any time during the fiscal year ended December 31, 2015, or at any other time, an officer or employee of the Company. Under the terms of an Agreement dated March 14, 2016, among the Company and Platinum, Mr. Ford will resign as a director of the Company
66
immediately following the filing of this Report. The Board is expected to appoint new CNG Committee members following Mr. Ford’s departure.
No director who served on the CNG Committee during 2015 had any relationships requiring disclosure by the Company under the SEC’s rules requiring disclosure of certain relationships and related-party transactions. None of the Company’s executive officers served as a director or a member of a compensation committee (or other committee serving an equivalent function) of any other entity, the executive officers of which served as a director of the Company or member of the CNG Committee during 2015.
Summary Compensation Table
The following table sets forth certain information concerning the annual and long-term compensation of our Named Executive Officers for the last three fiscal years.
Summary Compensation Table for Fiscal 2015
|
Named Executive Officer |
|
Year |
|
Salary |
|
|
(a) Stock Awards |
|
|
(b) Option Awards |
|
|
(c) Non-Equity Incentive Plan Compensation |
|
|
(d) All Other Compensation |
|
|
Total Compensation |
|
||||||
|
Ricardo J. Gonzalez (e) |
|
2015 |
|
$ |
375,000 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
105,001 |
|
|
$ |
7,692 |
|
|
$ |
487,693 |
|
|
President and Chief Executive Officer |
|
2014 |
|
|
82,452 |
|
|
|
— |
|
|
|
768,247 |
|
|
|
— |
|
|
|
— |
|
|
|
850,699 |
|
|
|
|
2013 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frederick O. Cope, Ph.D. |
|
2015 |
|
$ |
279,130 |
|
|
$ |
— |
|
|
$ |
155,026 |
|
|
$ |
54,709 |
|
|
$ |
6,657 |
|
|
$ |
495,522 |
|
|
Senior Vice President and |
|
2014 |
|
|
279,130 |
|
|
|
— |
|
|
|
144,067 |
|
|
|
— |
|
|
|
6,173 |
|
|
|
429,370 |
|
|
Chief Scientific Officer |
|
2013 |
|
|
276,420 |
|
|
|
— |
|
|
|
265,888 |
|
|
|
50,813 |
|
|
|
6,179 |
|
|
|
599,300 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas J. Klima (f) |
|
2015 |
|
$ |
270,000 |
|
|
$ |
192,900 |
|
|
$ |
112,163 |
|
|
$ |
52,921 |
|
|
$ |
3,114 |
|
|
$ |
631,098 |
|
|
Senior Vice President and |
|
2014 |
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
||
|
Chief Commercial Officer |
|
2013 |
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Brent L. Larson |
|
2015 |
|
$ |
260,000 |
|
|
$ |
— |
|
|
$ |
144,499 |
|
|
$ |
50,960 |
|
|
$ |
7,692 |
|
|
$ |
463,151 |
|
|
Executive Vice President and |
|
2014 |
|
|
260,000 |
|
|
|
— |
|
|
|
134,318 |
|
|
|
— |
|
|
|
6,727 |
|
|
|
401,045 |
|
|
Chief Financial Officer |
|
2013 |
|
|
274,717 |
|
|
|
— |
|
|
|
260,387 |
|
|
|
51,013 |
|
|
|
6,847 |
|
|
|
592,964 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael B. Tomblyn, M.D. (g) |
|
2015 |
|
$ |
300,000 |
|
|
$ |
163,900 |
|
|
$ |
112,163 |
|
|
$ |
— |
|
|
$ |
6,246 |
|
|
$ |
582,309 |
|
|
Senior Vice President and |
|
2014 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Chief Medical Officer |
|
2013 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
Amount represents the aggregate grant date fair value in accordance with FASB ASC Topic 718. Assumptions made in the valuation of stock awards are disclosed in Note 1(e) of the Notes to the Consolidated Financial Statements in this Form 10-K. |
|
(b) |
Amount represents the aggregate grant date fair value in accordance with FASB ASC Topic 718. Assumptions made in the valuation of option awards are disclosed in Note 1(e) of the Notes to the Consolidated Financial Statements in this Form 10-K. |
|
(c) |
Amount represents the total incentive plan amounts which have been approved by the Board of Directors as of the date this filing, and are disclosed for the year in which they were earned (i.e., the year to which the service relates). The Board of Directors also determined that fifty percent of the 2015 amount payable to certain executive officers would be paid in stock options in lieu of cash, calculated based on the Black-Scholes value of the options on the date of grant. As such, Dr. Cope, Mr. Klima and Mr. Larson were awarded, respectively, options to purchase 58,510, 56,598 and 54,501 shares of common stock of the Company at an exercise price of $0.98 per share, vesting immediately upon the date of grant and expiring after ten years. Since these options represent incentive compensation earned in 2015, they are reported in this column, and not included in the column “Option Awards.” |
|
(d) |
Amount represents additional compensation as disclosed in the All Other Compensation table below. |
|
(e) |
Mr. Gonzalez commenced employment with the Company effective October 13, 2014. |
|
(f) |
Mr. Klima commence employment with the Company effective January 1, 2015. |
|
(g) |
Dr. Tomblyn commenced employment with the Company effective January 1, 2015 and separated from the Company effective February 19, 2016. |
67
The following table describes each component of the amounts shown in the “All Other Compensation” column in the Summary Compensation table above.
All Other Compensation Table for Fiscal 2015
|
Named Executive Officer |
|
Year |
|
(a) Reimbursement of Additional Tax Liability Related to Health Insurance Premiums |
|
|
(b) 401(k) Plan Employer Matching Contribution |
|
|
Total All Other Compensation |
|
|||
|
Ricardo J. Gonzalez |
|
2015 |
|
$ |
2,392 |
|
|
$ |
5,300 |
|
|
$ |
7,692 |
|
|
|
|
2014 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
2013 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frederick O. Cope, Ph.D. |
|
2015 |
|
$ |
1,357 |
|
|
$ |
5,300 |
|
|
$ |
6,657 |
|
|
|
|
2014 |
|
|
973 |
|
|
|
5,200 |
|
|
|
6,173 |
|
|
|
|
2013 |
|
|
1,179 |
|
|
|
5,000 |
|
|
|
6,179 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas J. Klima |
|
2015 |
|
$ |
1,310 |
|
|
$ |
1,804 |
|
|
$ |
3,114 |
|
|
|
|
2014 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
2013 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Brent L. Larson |
|
2015 |
|
$ |
2,392 |
|
|
$ |
5,300 |
|
|
$ |
7,692 |
|
|
|
|
2014 |
|
|
1,527 |
|
|
|
5,200 |
|
|
|
6,727 |
|
|
|
|
2013 |
|
|
1,847 |
|
|
|
5,000 |
|
|
|
6,847 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael B. Tomblyn, M.D. |
|
2015 |
|
$ |
1,703 |
|
|
$ |
4,543 |
|
|
$ |
6,246 |
|
|
|
|
2014 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
2013 |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
(a) |
Amount represents reimbursement of the lost tax benefit due to the ineligibility of our Named Executive Officers to pay their portion of medical, dental, and vision premiums on a pre-tax basis under our IRC Section 125 Plan. |
|
(b) |
Amount represents the value of the common stock contributed to the Named Executive Officer’s account in our 401(k) Plan as calculated on a quarterly basis. |
68
The following table sets forth certain information about plan-based awards that we made to the Named Executive Officers during fiscal 2015. For information about the plans under which these awards were granted, see the discussion under “Short-Term Incentive Compensation” and “Long-Term Incentive Compensation” in the “Compensation Discussion and Analysis” section above.
Grants of Plan-Based Awards Table for Fiscal 2015
|
|
|
|
|
Estimated Future Payouts Under Non-Equity Incentive Plan Awards (a) |
|
|
Estimated Future Payouts Under Equity Incentive Plan Awards |
|
|
All Other Stock Awards: Number of Shares of Stock |
|
|
All Other Option Awards: Number of Securities Underlying Options |
|
|
Exercise Price of Option Awards |
|
|
Grant Date Fair Value of Stock and Option Awards |
|
|
||||||||||||||
|
Named Executive Officer |
|
Grant Date |
|
Threshold |
|
|
Maximum |
|
|
Threshold |
|
|
Maximum |
|
|
All Other Stock Awards: Number of Shares of Stock |
|
|
All Other Option Awards: Number of Securities Underlying Options |
|
|
Exercise Price of Option Awards |
|
|
Grant Date Fair Value of Stock and Option Awards |
|
|
||||||||
|
Ricardo J. Gonzalez |
|
N/A |
|
$ |
— |
|
|
$ |
187,500 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
(a) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frederick O. Cope, Ph.D. |
|
N/A |
|
$ |
— |
|
|
$ |
97,696 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
(a) |
|
|
|
3/26/2015 |
|
$ |
— |
|
|
$ |
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
162,000 |
|
|
$ |
1.65 |
|
|
$ |
155,026 |
|
(b) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas J. Klima |
|
N/A |
|
$ |
— |
|
|
$ |
94,500 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
(a) |
|
|
|
1/1/2015 |
|
$ |
— |
|
|
$ |
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
100,000 |
|
|
$ |
1.89 |
|
|
$ |
112,163 |
|
(c) |
|
|
|
1/1/2015 |
|
$ |
— |
|
|
$ |
— |
|
|
|
— |
|
|
|
— |
|
|
|
100,000 |
|
|
|
— |
|
|
N/A |
|
|
$ |
192,900 |
|
(d) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Brent L. Larson |
|
N/A |
|
$ |
— |
|
|
$ |
91,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
(a) |
|
|
|
3/26/2015 |
|
$ |
— |
|
|
$ |
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
151,000 |
|
|
$ |
1.65 |
|
|
$ |
144,499 |
|
(b) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael B. Tomblyn, M.D. |
|
N/A |
|
$ |
— |
|
|
$ |
105,000 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
$ |
— |
|
|
$ |
— |
|
(a) |
|
|
|
1/1/2015 |
|
$ |
— |
|
|
$ |
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
100,000 |
|
|
$ |
1.89 |
|
|
$ |
112,163 |
|
(c) |
|
|
|
3/3/2015 |
|
$ |
— |
|
|
$ |
— |
|
|
|
— |
|
|
|
— |
|
|
|
100,000 |
|
|
|
— |
|
|
N/A |
|
|
$ |
163,900 |
|
(e) |
|
|
(a) |
The threshold amount reflects the fact that no cash bonus awards would have been payable if none of the specified business performance objectives were achieved. The maximum amount reflects the target cash bonus awards payable if all of the specified business performance objectives are achieved. For actual cash bonus award amounts, see the “Non-Equity Incentive Plan Compensation” column in the Summary Compensation table above. |
|
(b) |
These stock options vest as to one-third on each of the first three anniversaries of the date of grant, and expire on the tenth anniversary of the date of grant. If the employment of the Named Executive Officer with the Company is terminated due to a change in control or without cause before all of the stock options have vested, then pursuant to the terms of the Stock Option Award Agreements all stock options that have not vested at the effective date of the Named Executive Officer’s termination shall immediately vest and become exercisable. |
|
(c) |
These stock options vest as to one-fourth on each of the first four anniversaries of the date of grant, and expire on the tenth anniversary of the date of grant. If the employment of the Named Executive Officer with the Company is terminated due to a change in control or without cause before all of the stock options have vested, then pursuant to the terms of the Stock Option Award Agreements all stock options that have not vested at the effective date of the Named Executive Officer’s termination shall immediately vest and become exercisable. |
|
(d) |
These restricted stock awards vest upon achievement of certain goals as defined in the Restricted Stock Award Agreements. If the employment of the Named Executive Officer with the Company is terminated for any reason before all of the restricted stock awards have vested, then pursuant to the terms of the Restricted Stock Award Agreements all awards that have not vested at the effective date of the Named Executive Officer’s termination shall immediately be forfeited. |
|
(e) |
These restricted stock awards vest as to one-fourth on each of the first four anniversaries of the date of grant. If the employment of the Named Executive Officer with the Company is terminated for any reason before all of the restricted stock awards have vested, then pursuant to the terms of the Restricted Stock Award Agreements all awards that have not vested at the effective date of the Named Executive Officer’s termination shall immediately be forfeited. |
69
The following table presents certain information concerning outstanding equity awards held by the Named Executive Officers as of December 31, 2015.
Outstanding Equity Awards Table at Fiscal 2015 Year-End
|
|
|
Option Awards |
|
Stock Awards |
||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity Incentive Plan Awards |
||||||||
|
|
|
Number of Securities Underlying Unexercised Options (#) |
|
|
Option Exercise Price |
|
|
Option Expiration Date |
|
Note |
|
Number of Shares of Stock that Have Not Vested |
|
Market Value of Shares of Stock that Have Not Vested |
|
Number of Unearned Shares |
|
|
Market Value of Unearned Shares (q) |
|
|
Note |
||||||||
|
Named Executive Officer |
|
Exercisable |
|
|
Unexercisable |
|
|
Option Exercise Price |
|
|
Option Expiration Date |
|
Note |
|
Number of Shares of Stock that Have Not Vested |
|
Market Value of Shares of Stock that Have Not Vested |
|
Number of Unearned Shares |
|
|
Market Value of Unearned Shares (q) |
|
|
Note |
|||||
|
Ricardo J. Gonzalez |
|
|
300,000 |
|
|
|
700,000 |
|
|
$ |
1.26 |
|
|
10/13/2024 |
|
(j) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Frederick O. Cope, |
|
|
50,000 |
|
|
|
— |
|
|
$ |
0.65 |
|
|
2/16/2019 |
|
(d) |
|
|
|
|
|
|
50,000 |
|
|
$ |
66,500 |
|
|
(m) |
|
Ph.D. |
|
|
75,000 |
|
|
|
— |
|
|
$ |
1.10 |
|
|
10/30/2019 |
|
(e) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
120,000 |
|
|
|
— |
|
|
$ |
1.90 |
|
|
12/21/2020 |
|
(f) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
95,250 |
|
|
|
31,750 |
|
|
$ |
3.28 |
|
|
2/17/2022 |
|
(g) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
72,500 |
|
|
|
72,500 |
|
|
$ |
3.08 |
|
|
2/15/2023 |
|
(h) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
33,250 |
|
|
|
99,750 |
|
|
$ |
1.77 |
|
|
1/28/2024 |
|
(i) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
162,000 |
|
|
$ |
1.65 |
|
|
3/26/2025 |
|
(l) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thomas J. Klima |
|
|
— |
|
|
|
100,000 |
|
|
$ |
1.89 |
|
|
1/1/2025 |
|
(k) |
|
|
|
|
|
|
50,000 |
|
|
$ |
66,500 |
|
|
(n) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Brent L. Larson |
|
|
50,000 |
|
|
|
— |
|
|
$ |
0.27 |
|
|
12/15/2016 |
|
(a) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
50,000 |
|
|
|
— |
|
|
$ |
0.362 |
|
|
1/3/2018 |
|
(b) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
25,000 |
|
|
|
— |
|
|
$ |
0.59 |
|
|
1/5/2019 |
|
(c) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
75,000 |
|
|
|
— |
|
|
$ |
1.10 |
|
|
10/30/2019 |
|
(e) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
95,000 |
|
|
|
— |
|
|
$ |
1.90 |
|
|
12/21/2020 |
|
(f) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
66,000 |
|
|
|
22,000 |
|
|
$ |
3.28 |
|
|
2/17/2022 |
|
(g) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
71,000 |
|
|
|
71,000 |
|
|
$ |
3.08 |
|
|
2/15/2023 |
|
(h) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31,000 |
|
|
|
93,000 |
|
|
$ |
1.77 |
|
|
1/28/2024 |
|
(i) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
151,000 |
|
|
$ |
1.65 |
|
|
3/26/2025 |
|
(l) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael B. Tomblyn, |
|
|
— |
|
|
|
100,000 |
|
|
$ |
1.89 |
|
|
1/1/2025 |
|
(k)(p) |
|
|
|
|
|
|
100,000 |
|
|
$ |
133,000 |
|
|
(o)(p) |
|
M.D. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a) |
Options were granted 12/15/2006 and vested as to one-third on each of the first three anniversaries of the date of grant. |
|
(b) |
Options were granted 1/3/2008 and vested as to one-third on each of the first three anniversaries of the date of grant. |
|
(c) |
Options were granted 1/5/2009 and vested as to one-third on each of the first three anniversaries of the date of grant. |
|
(d) |
Options were granted 2/16/2009 and vested as to one-third on each of the first three anniversaries of the date of grant. |
|
(e) |
Options were granted 10/30/2009 and vested as to one-third on each of the first three anniversaries of the date of grant. |
|
(f) |
Options were granted 12/21/2010 and vested as to one-fourth on each of the first four anniversaries of the date of grant. |
|
(g) |
Options were granted 2/17/2012 and vest as to one-fourth on each of the first four anniversaries of the date of grant. |
|
(h) |
Options were granted 2/15/2013 and vest as to one-fourth on each of the first four anniversaries of the date of grant. |
|
(i) |
Options were granted 1/28/2014 and vest as to one-fourth on each of the first four anniversaries of the date of grant. |
70
|
of 300,000 options will vest and become exercisable on or after the second anniversary of the date of grant, provided that the options will not be exercisable with respect to such shares unless and until the average closing price per share of the Company’s common stock for the ten trading days prior to exercise equals or exceeds $2.50 per share. The third tranche of 400,000 options will vest and become exercisable on or after the third anniversary of the date of grant, provided that the options will not be exercisable with respect to such shares unless and until the average closing price per share of the Company’s common stock for the ten trading days prior to exercise equals or exceeds $3.50 per share. |
|
(k) |
Options were granted 1/1/2015 and vest as to one-fourth on each of the first four anniversaries of the date of grant. |
|
(l) |
Options were granted 3/26/2015 and vest as to one-third on each of the first three anniversaries of the date of grant. |
|
(m) |
Restricted shares granted February 16, 2009. Pursuant to the terms of the restricted stock agreement between the Company and Dr. Cope, the restricted shares will vest upon the commencement of patient enrollment in a Phase 3 clinical trial in humans of NAV1800. All of the restricted shares vest upon the occurrence of a change in control as defined in Dr. Cope’s employment agreement. If the employment of Dr. Cope with the Company is terminated for reasons other than a change in control before all of the restricted shares have vested, then pursuant to the terms of the restricted stock agreement all restricted shares that have not vested at the effective date of Dr. Cope’s termination shall immediately be forfeited by Dr. Cope. |
|
(n) |
Restricted shares granted January 1, 2015. Pursuant to the terms of the restricted stock agreement between the Company and Mr. Klima, 25,000 of the restricted shares will vest upon achievement of $30 million in sales of Lymphoseek during 2016, and 25,000 of the restricted shares will vest upon achievement of $35 million in sales of Lymphoseek during 2016. Mr. Klima’s restricted stock agreements do not include provisions for accelerated vesting. If the employment of Mr. Klima with the Company is terminated before all of the restricted shares have vested, then pursuant to the terms of the restricted stock agreement all restricted shares that have not vested at the effective date of Mr. Klima’s termination shall immediately be forfeited by Mr. Klima. |
|
(o) |
Restricted shares granted March 3, 2015. Pursuant to the terms of the restricted stock agreement between the Company and Dr. Tomblyn, the restricted shares were to vest as to one-fourth on each of the first four anniversaries of the date of grant. Dr. Tomblyn’s restricted stock agreements do not include provisions for accelerated vesting. If the employment of Dr. Tomblyn with the Company is terminated before all of the restricted shares have vested, then pursuant to the terms of the restricted stock agreement all restricted shares that have not vested at the effective date of Dr. Tomblyn’s termination shall immediately be forfeited by Dr. Tomblyn. |
|
(p) |
Dr. Tomblyn separated from the Company effective February 19, 2016. All of Dr. Tomblyn’s stock options and restricted stock were forfeited as of the date of separation. |
|
(q) |
Estimated by reference to the closing market price of the Company’s common stock on December 31, 2015, pursuant to Instruction 3 to Item 402(p)(2) of Regulation S-K. The closing price of the Company’s common stock on December 31, 2015, was $1.33. |
Options Exercised and Stock Vested
The following table presents, with respect to the Named Executive Officers, certain information about option exercises and restricted stock vested during fiscal 2015.
Options Exercised and Stock Vested Table for Fiscal 2015
|
|
|
Option Awards |
|
|
Stock Awards |
|
|
|
||||||||||
|
Named Executive Officer |
|
Number of Shares Acquired on Exercise |
|
|
Value Realized on Exercise (a) |
|
|
Number of Shares Acquired on Vesting |
|
|
Value Realized on Vesting (a) |
|
|
Note |
||||
|
Ricardo J. Gonzalez |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
Frederick O. Cope, Ph.D. |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
Thomas J. Klima |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
Brent L. Larson |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
Michael B. Tomblyn, M.D. |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
(a) |
Computed using the fair market value of the stock on the date prior to or the date of exercise or vesting, as appropriate, in accordance with our normal practice. |
71
Compensation of Non-Employee Directors
Each non-employee director received an annual cash retainer of $50,000 during the fiscal year ended December 31, 2015. The Chairman of the Company’s Board of Directors received an additional annual retainer of $30,000, the Chairman of the Audit Committee received an additional annual retainer of $10,000, and the Chairman of the CNG Committee received an additional annual retainer of $7,500 for their services in those capacities during 2015. We also reimbursed non-employee directors for travel expenses for meetings attended during 2015.
Each non-employee director also received 22,000 shares of restricted stock during 2015 as a part of the Company’s annual stock incentive grants, in accordance with the provisions of the Navidea Biopharmaceuticals, Inc. 2014 Stock Incentive Plan. The restricted stock granted will vest on the first anniversary of the date of grant. The aggregate number of equity awards outstanding at February 29, 2016 for each Director is set forth in the footnotes to the beneficial ownership table provided in Part III, Item 12 of this Form 10-K. Directors who are also officers or employees of Navidea do not receive any compensation for their services as directors.
The following table sets forth certain information concerning the compensation of non-employee Directors for the fiscal year ended December 31, 2015.
|
Name |
|
(a) Fees Earned or Paid in Cash or Stock |
|
|
(b),(c) Option Awards |
|
|
(d),(e) Stock Awards |
|
|
All Other Compensation |
|
|
Total Compensation |
|
|||||
|
Peter F. Drake, Ph.D. (f) |
|
$ |
31,094 |
|
|
$ |
— |
|
|
$ |
36,300 |
|
|
$ |
— |
|
|
$ |
67,394 |
|
|
Brendan A. Ford |
|
|
61,148 |
|
|
|
— |
|
|
|
36,300 |
|
|
|
— |
|
|
|
97,448 |
|
|
Michael M. Goldberg, M.D. |
|
|
50,000 |
|
|
|
— |
|
|
|
36,300 |
|
|
|
— |
|
|
|
86,300 |
|
|
Anton Gueth (g) |
|
|
43,053 |
|
|
|
— |
|
|
|
39,960 |
|
|
|
— |
|
|
|
83,013 |
|
|
Perry A. Karsen (f) |
|
|
29,742 |
|
|
|
— |
|
|
|
36,300 |
|
|
|
— |
|
|
|
66,042 |
|
|
Eric K. Rowinsky, M.D. |
|
|
50,442 |
|
|
|
— |
|
|
|
36,300 |
|
|
|
— |
|
|
|
86,742 |
|
|
Gordon A. Troup |
|
|
71,223 |
|
|
|
— |
|
|
|
36,300 |
|
|
|
— |
|
|
|
107,523 |
|
|
(a) |
Amount represents fees earned during the fiscal year ended December 31, 2015 (i.e., the year to which the service relates). Quarterly retainers and meeting attendance fees are paid during the quarter following the quarter in which they are earned. |
|
(b) |
Amount represents the aggregate grant date fair value in accordance with FASB ASC Topic 718. Assumptions made in the valuation of stock option awards are disclosed in Note 1(e) of the Notes to the Consolidated Financial Statements in this Form 10-K. |
|
(c) |
At December 31, 2015, the non-employee directors held an aggregate of 93,764 options to purchase shares of common stock of the Company. Dr. Rowinsky held 73,764 options and Mr. Troup held 20,000 options. |
|
(d) |
Amount represents the aggregate grant date fair value in accordance with FASB ASC Topic 718. Assumptions made in the valuation of restricted stock awards are disclosed in Note 1(e) of the Notes to the Consolidated Financial Statements in this Form 10-K. |
|
(e) |
During the year ended December 31, 2015, the non-employee directors were issued an aggregate of 154,000 shares of restricted stock which vest as to 100% of the shares on the first anniversary of the date of grant. At December 31, 2015, the non-employee directors held an aggregate of 161,000 shares of unvested restricted stock. Messrs. Ford and Troup and Dr. Rowinsky each held 39,000 shares of unvested restricted stock, and Dr. Goldberg and Mr. Gueth each held 22,000 shares of unvested restricted stock. |
|
(f) |
Dr. Drake and Mr. Karsen retired from the Board effective July 16, 2015. |
|
(g) |
Mr. Gueth was appointed to the Board effective June 1, 2015. |
72
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plan Information
The following table sets forth additional information as of December 31, 2015, concerning shares of our common stock that may be issued upon the exercise of options and other rights under our existing equity compensation plans and arrangements, divided between plans approved by our stockholders and plans or arrangements not submitted to our stockholders for approval. The information includes the number of shares covered by, and the weighted average exercise price of, outstanding options and other rights and the number of shares remaining available for future grants excluding the shares to be issued upon exercise of outstanding options, warrants, and other rights.
|
|
|
(1) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
|
|
(2) Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights |
|
|
(3) Number of Securities Remaining Available for Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (1)) |
|
|||
|
Equity compensation plans approved by security holders (a) |
|
|
4,437,064 |
|
|
$ |
2.12 |
|
|
|
3,155,893 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity compensation plans not approved by security holders (b) |
|
|
1,000,000 |
|
|
|
1.26 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
|
5,437,064 |
|
|
$ |
1.96 |
|
|
|
3,155,893 |
|
|
(a) |
Our stockholders ratified the 2014 Stock Incentive Plan (the 2014 Plan) at the 2014 Annual Meeting of Stockholders held on July 17, 2014. The total number of shares available for awards under the 2014 Plan shall not exceed 5,000,000 shares, plus any shares subject to outstanding awards granted under prior plans and that expire or terminate for any reason. Although instruments are still outstanding under the Fourth Amended and Restated 2002 Stock Incentive Plan (the 2002 Plan), the plan has expired and no new grants may be made from it. The total number of securities to be issued upon exercise of outstanding options includes 1,390,325 issued under the 2014 Plan and 3,046,739 issued under the 2002 Plan. |
|
(b) |
The Board of Directors awarded 1,000,000 options to Mr. Gonzalez as an inducement to employment effective October 13, 2014. Pursuant to NYSE MKT rules, such inducement options do not require shareholder approval. |
73
Security Ownership of Principal Stockholders, Directors, Nominees and Executive Officers and Related Stockholder Matters
The following table sets forth, as of February 29, 2016, certain information with respect to the beneficial ownership of shares of our common stock by: (i) each person known to us to be the beneficial owner of more than 5% of our outstanding shares of common stock, (ii) each director or nominee for director of our Company, (iii) each of the Named Executive Officers (see “Executive Compensation – Summary Compensation Table”), and (iv) our directors and executive officers as a group.
|
Beneficial Owner |
|
Number of Shares Beneficially Owned (*) |
|
|
|
Percent of Class (**) |
|
|
||
|
Frederick O. Cope, Ph.D. |
|
|
748,949 |
|
(a) |
|
|
— |
|
(l) |
|
Brendan A. Ford |
|
|
239,950 |
|
(b) |
|
|
— |
|
(l) |
|
Michael M. Goldberg, M.D. |
|
|
331,572 |
|
(c) |
|
|
— |
|
(l) |
|
Ricardo J. Gonzalez |
|
|
300,000 |
|
(d) |
|
|
— |
|
(l) |
|
Anton Gueth |
|
|
16,918 |
|
(e) |
|
|
— |
|
(l) |
|
Thomas J. Klima |
|
|
81,598 |
|
(f) |
|
|
— |
|
(l) |
|
Brent L. Larson |
|
|
1,016,653 |
|
(g) |
|
|
— |
|
(l) |
|
Eric K. Rowinsky, M.D. |
|
|
259,484 |
|
(h) |
|
|
— |
|
(l) |
|
Gordon A. Troup |
|
|
257,568 |
|
(i) |
|
|
— |
|
(l) |
|
All directors and executive officers as a group (10 persons) |
|
|
3,628,950 |
|
(j)(m) |
|
|
2.3 |
% |
|
|
Platinum, including affiliates |
|
|
15,330,083 |
|
(k) |
|
|
9.9 |
% |
|
|
(*) |
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission which generally attribute beneficial ownership of securities to persons who possess sole or shared voting power and/or investment power with respect to those securities. Unless otherwise indicated, voting and investment power are exercised solely by the person named above or shared with members of such person’s household. |
|
(**) |
Percent of class is calculated on the basis of the number of shares outstanding on February 29, 2016, plus the number of shares the person has the right to acquire within 60 days of February 29, 2016. |
|
(a) |
This amount includes 659,760 shares issuable upon exercise of options which are exercisable within 60 days and 13,375 shares in Dr. Cope’s account in the 401(k) Plan, but it does not include 50,000 shares of unvested restricted stock and 210,750 shares issuable upon exercise of options which are not exercisable within 60 days. |
|
(b) |
This amount includes 22,000 shares of restricted stock which will vest within 60 days, but it does not include 17,000 shares of unvested restricted stock. |
|
(c) |
This amount includes 22,000 shares of restricted stock which will vest within 60 days. This amount does not include 5,411,850 shares of common stock that the Company believes Dr. Goldberg has the right to acquire from Platinum pursuant to an agreement effective March 28, 2014, and amended effective June 11,2015 (the Separation Agreement), which provides that Dr. Goldberg has sole voting and dispositive power over these securities. In addition, the Company believes that the Separation Agreement provides for the transfer to Dr. Goldberg of a 15% interest in the Platinum Loan Agreement, including any common stock issued upon exercise of the conversion rights therein. |
|
(d) |
This amount includes 300,000 shares issuable upon exercise of options which are exercisable within 60 days, but it does not include 700,000 shares issuable upon exercise of options which are not exercisable within 60 days. |
|
(e) |
This amount does not include 22,000 shares of unvested restricted stock. |
|
(f) |
This amount includes 81,598 shares issuable upon exercise of options which are exercisable within 60 days, but it does not include 50,000 shares of unvested restricted stock and 75,000 shares issuable upon exercise of options which are not exercisable within 60 days. |
|
(g) |
This amount includes 696,335 shares issuable upon exercise of options which are exercisable within 60 days and 98,081 shares in Mr. Larson’s account in the 401(k) Plan, but it does not include 198,166 shares issuable upon exercise of options which are not exercisable within 60 days. |
|
(h) |
This amount includes 22,000 shares of restricted stock which will vest within 60 days and 73,764 shares issuable upon exercise of options which are exercisable within 60 days, but it does not include 17,000 shares of unvested restricted stock. |
|
(i) |
This amount includes 22,000 shares of restricted stock which will vest within 60 days and 20,000 shares issuable upon exercise of options which are exercisable within 60 days, but it does not include 17,000 shares of unvested restricted stock. |
74
|
referenced in this disclosure include each director and named executive officer listed in the table as well as one additional executive officer who is not a named executive officer. |
|
(k) |
Based on information filed on Schedule 13D with the Securities and Exchange Commission on March 9, 2016. Platinum, 250 West 55th Street, 14th Floor, New York, NY 10019, holds 9,777,130 Series LL warrants, each warrant convertible into one share of our common stock at an exercise price of $0.01 per share. The Series LL Warrants provide that the holder may not exercise any portion of the warrants to the extent that such exercise would result in the holder and its affiliates together beneficially owning more than 9.99% of the outstanding shares of common stock, except on 61 days’ prior written notice to Navidea that the holder waives such limitation (the blocker). The number of shares beneficially owned by Platinum includes 4,365,280 shares of common stock issuable upon exercise of Series LL warrants, but does not include 5,411,850 additional shares of common stock issuable upon exercise of Series LL warrants that are subject to the blocker. |
|
(l) |
Less than one percent. |
|
(m) |
The address of all directors and executive officers is c/o Navidea Biopharmaceuticals, Inc., 5600 Blazer Parkway, Suite 200, Dublin, OH 43017. |
Item 13. Certain Relationships and Related Transactions, and Director Independence
Certain Relationships and Related Transactions
We adhere to our Code of Business Conduct and Ethics, which states that no director, officer or employee of Navidea should have any personal interest that is incompatible with the loyalty and responsibility owed to our Company. We adopted a written policy regarding related party transactions in December 2015. When considering whether to enter into or ratify a related party transaction, the Audit Committee considers a variety of factors including, but not limited to, the nature and type of the proposed transaction, the potential value of the proposed transaction, the impact on the actual or perceived independence of the related party and the potential value to the Company of entering into such a transaction. All proposed transactions with a potential value of greater than $120,000 are approved or ratified by the Audit Committee.
Effective November 13, 2013, the Company appointed Michael M. Goldberg, M.D., to serve on its Board of Directors. At the time of his appointment, Dr. Goldberg was Portfolio Manager of Platinum-Montaur Life Sciences, LLC, a Delaware limited liability company and Platinum affiliate.
Dr. Goldberg accepted his appointment to serve on the Company’s Board of Directors in accordance with the provisions of a Director Agreement, dated November 13, 2013, between the Company and Dr. Goldberg. Pursuant to the terms of the Director Agreement, Dr. Goldberg has acknowledged and agreed that, for as long as he is a member of the Board of Directors, he will not, directly or indirectly, have any power to direct or cause the direction of the voting or disposition of any securities of the Company directly or beneficially owned by Platinum or its affiliates. Dr. Goldberg has advised the Company that he is in the process of completing his withdrawal from any ownership or management position with Platinum, but has confirmed information obtained by the Company from Platinum in November 2015 that Dr. Goldberg entered into an agreement effective March 28, 2014, and amended effective June 11, 2015 (the Separation Agreement) which gives him the right to acquire 5,411,850 shares of Navidea common stock from Platinum and provides that Dr. Goldberg has sole voting and dispositive power over these shares. In addition, the Separation Agreement provides for the transfer to Dr. Goldberg of a 15% interest in the Platinum Loan Agreement, including any common stock issued upon exercise of the conversion rights therein.
SEC disclosure rules regarding transactions with related persons require the Company to provide information about transactions with Dr. Goldberg as a related person, even though Dr. Goldberg may not have been a related person at the time the Company entered into the transactions described below.
As of February 29, 2016, we believe that Platinum beneficially owned approximately 15,330,083 shares of our common stock, including 4,365,280 shares of our common stock issuable upon the exercise of Series LL Warrants, but excluding an additional 5,411,850 shares of our common stock issuable upon the exercise of Series LL Warrants.
In March 2015, Macrophage Therapeutics, Inc. (MT) entered into an agreement to sell up to 50 shares of its Series A Convertible Preferred Stock (MT Preferred Stock) and warrants to purchase up to 1,500 common shares of Macrophage Therapeutics (MT Common Stock) to Platinum and Dr. Michael Goldberg for a purchase price of $50,000 per share of MT Preferred Stock. On March 13, 2015, we announced that definitive agreements with the investors had been signed for the sale of the first tranche of 10 shares of MT Preferred Stock and warrants to purchase 300 shares of MT Common Stock to these investors, with gross proceeds to Macrophage Therapeutics of $500,000. Under the agreement, 40% of the MT Preferred Stock and warrants are committed to be purchased by Dr. Goldberg, and the balance by Platinum. The full 50 shares of MT Preferred Stock and warrants to be sold under the agreement are convertible into and exercisable for MT Common Stock representing an aggregate 1% interest on a fully converted and exercised basis.
In addition, we entered into an Exchange Agreement with the investors providing them an option to exchange their MT Preferred Stock for our common stock in the event that MT has not completed a public offering with gross proceeds to MT of at least $50 million by the second anniversary of the closing of the initial sale of MT Preferred Stock, at an exchange rate per share obtained by
75
dividing $50,000 by the greater of (i) 80% of the twenty-day volume weighted average price per share of our common stock on the second anniversary of the initial closing or (ii) $3.00. To the extent that the investors do not timely exercise their exchange right, we have the right to redeem their MT Preferred Stock for a price equal to $58,320 per share. We also granted MT an exclusive license for certain therapeutic applications of the Manocept technology.
In May 2015, in connection with the execution of the CRG Loan Agreement, the Company amended the existing Platinum credit facility to allow this facility to remain in place in a subordinated role to the CRG Loan (the Third Platinum Amendment). Among other things, the Third Platinum Amendment (i) extends the term of the Platinum Loan Agreement until a date six months following the maturity date or earlier repayment of the CRG Term Loan; (ii) changes the interest rate to the greater of (a) the United States prime rate as reported in The Wall Street Journal plus 6.75%, (b) 10.0% and (c) the highest rate of interest then payable pursuant to the CRG Term Loan plus 0.125% (effective interest rate as of December 31, 2015 was 14.125%); (iii) requires such interest to compound monthly; and (iv) changes the provisions of the Platinum Loan Agreement governing Platinum’s right to convert advances into common stock of the Company. The Third Platinum Amendment provides for the conversion of all principal and interest outstanding under the Platinum Loan Agreement, but not until such time as the average daily volume weighted average price of the Company’s common stock for the ten preceding trading days exceeds $2.53 per share. The amendment became effective upon initial funding of the CRG Loan Agreement. Navidea, Platinum, and CRG also entered into a Subordination Agreement, providing for subordination of the Company’s indebtedness under the Platinum Loan Agreement to the Company’s indebtedness under the CRG Loan Agreement, among other customary terms and conditions.
During 2015, the largest aggregate amount of principal outstanding under the Platinum credit facility was $8.5 million, and as of February 29, 2016, the amount of principal outstanding was $8.7 million, including $961,000 of compounded interest.
Director Independence
Our Board of Directors has adopted the definition of “independence” as described under the Sarbanes-Oxley Act of 2002 (Sarbanes-Oxley) Section 301, Rule 10A-3 under the Securities Exchange Act of 1934 (the Exchange Act) and Section 803A of the NYSE MKT Company Guide. Our Board of Directors has determined that Messrs. Ford, Gueth and Troup, and Dr. Rowinsky, meet the independence requirements.
Item 14. Principal Accountant Fees and Services
Audit Fees. The aggregate fees billed and expected to be billed for professional services rendered by BDO USA, LLP, primarily related to the audit of the Company’s annual consolidated financial statements for the 2015 fiscal year, the audit of the Company’s internal control over financial reporting as of December 31, 2015, and the reviews of the financial statements included in the Company’s Quarterly Reports on Form 10-Q for the 2015 fiscal year were $259,915 (including direct engagement expenses).
The aggregate fees billed for professional services rendered by BDO USA, LLP, primarily related to the audit of the Company’s annual consolidated financial statements for the 2014 fiscal year, the audit of the Company’s internal control over financial reporting as of December 31, 2014, the reviews of the financial statements included in the Company’s Quarterly Reports on Form 10-Q for the 2014 fiscal year, and consents related to the Company's registration statements during the 2014 fiscal year, were $249,500 (including direct engagement expenses).
Audit-Related Fees. No fees were billed by BDO USA, LLP for audit-related services for the 2015 or 2014 fiscal years.
Tax Fees. No fees were billed by BDO USA, LLP for tax-related services for the 2015 or 2014 fiscal years.
All Other Fees. No fees were billed by BDO USA, LLP for services other than the audit, audit-related and tax services for the 2015 or 2014 fiscal years.
Pre-Approval Policy. The Audit Committee is required to pre-approve all auditing services and permitted non-audit services (including the fees and terms thereof) to be performed for the Company by its independent auditor or other registered public accounting firm, subject to the de minimis exceptions for permitted non-audit services described in Section 10A(i)(1)(B) of the Securities Exchange Act of 1934 that are approved by the Audit Committee prior to completion of the audit. The Audit Committee, through the function of the Chairman, has given general pre-approval for 100% of specified audit, audit-related, tax and other services.
76
Item 15. Exhibits, Financial Statement Schedules
The following documents are filed as part of this report:
|
|
(1) |
The following Financial Statements are included in this Annual Report on Form 10-K on the pages indicated below: |
|
|
(2) |
Financial statement schedules have been omitted because either they are not required or are not applicable or because the information required to be set forth therein is not material. |
77
|
Exhibit Number |
|
Exhibit Description |
|
|
|
|
|
3.1 |
|
Amended and Restated Certificate of Incorporation of Navidea Biopharmaceuticals, Inc., as corrected February 18, 1994, and amended June 27, 1994, July 25, 1995, June 3, 1996, March 17, 1999, May 9, 2000, June 13, 2003, July 29, 2004, June 22, 2005, November 20, 2006, December 26, 2007, April 30, 2009, July 27, 2009, August 2, 2010, January 5, 2012, and June 26, 2013)(filed as Exhibit 3.1 to the Company's Annual Report on Form 10-K filed March 14, 2014, and incorporated herein by reference). |
|
|
|
|
|
3.3 |
|
Amended and Restated By-Laws dated July 21, 1993, as amended July 18, 1995, May 30, 1996, July 26, 2007, and November 7, 2013 (filed as Exhibit 3.2 to the Company’s Quarterly Report on Form 10-Q filed November 12, 2013, and incorporated herein by reference). |
|
|
|
|
|
4.1 |
|
Amended and Restated Certificate of Designations, Voting Powers, Preferences, Limitations, Restrictions, and Relative Rights of Series B Cumulative Convertible Preferred Stock (incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed June 26, 2013). |
|
|
|
|
|
10.1 |
|
Navidea Biopharmaceuticals, Inc. 2014 Stock Incentive Plan, as amended March 3, 2015 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed May 11, 2015). ^ |
|
|
|
|
|
10.2 |
|
Form of Stock Option Agreement under the Navidea Biopharmaceuticals, Inc. 2014 Stock Incentive Plan (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed November 10, 2014). ^ |
|
|
|
|
|
10.3 |
|
Form of Restricted Stock Award and Agreement under the Navidea Biopharmaceuticals, Inc. 2014 Stock Incentive Plan (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed November 10, 2014). ^ |
|
|
|
|
|
10.4 |
|
Form of Employment Agreement between the Company and each of Dr. Frederick O. Cope and Mr. Brent L. Larson. This agreement is one of two substantially identical employment agreements and is accompanied by a schedule which identifies material details in which each individual agreement differs from the form filed herewith (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed January 7, 2013). ^ |
|
|
|
|
|
10.5 |
|
Schedule identifying material differences between the employment agreements incorporated by reference as Exhibit 10.4 to this Annual Report on Form 10-K (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed January 7, 2013). ^ |
|
|
|
|
|
10.6 |
|
Navidea Biopharmaceuticals, Inc. 2015 Cash Bonus Plan (incorporated by reference to the Company’s Current Report on Form 8-K filed April 1, 2014). ^ |
|
|
|
|
|
10.7 |
|
Supply and Distribution Agreement, dated November 15, 2007, between the Company and Cardinal Health 414, LLC (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed November 21, 2007). |
|
|
|
|
|
10.8 |
|
Manufacture and Supply Agreement, dated November 30, 2009, between the Company and Reliable Biopharmaceutical Corporation (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the Commission) (incorporated by reference to Exhibit 10.1 to the Company’s June 30, 2010 Form 10-Q). |
|
|
|
|
|
10.9 |
|
Loan Agreement, dated July 25, 2012, between the Company and Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed July 31, 2012). |
|
|
|
|
|
10.10 |
|
Amendment to Loan Agreement, dated June 25, 2013, between Navidea Biopharmaceuticals, Inc. and Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed June 28, 2013). |
|
|
|
|
|
10.11 |
|
Second Amendment to Loan Agreement, dated March 4, 2014, between Navidea Biopharmaceuticals, Inc. and Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed March 7, 2014). |
|
|
|
|
|
10.12 |
|
Promissory Note, dated July 25, 2012, made by Navidea Biopharmaceuticals, Inc. in favor of Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed July 31, 2012). |
|
|
|
|
78
|
Exhibit Number |
|
Exhibit Description |
|
|
Amended and Restated Promissory Note, dated June 25, 2013, made by Navidea Biopharmaceuticals, Inc. in favor of Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.6 to the Company’s Current Report on Form 8-K filed June 28, 2013). |
|
|
|
|
|
|
10.14 |
|
Second Amended and Restated Promissory Note, dated March 4, 2014, made by Navidea Biopharmaceuticals, Inc. in favor of Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed March 7, 2014). |
|
|
|
|
|
10.15 |
|
Asset Purchase Agreement, dated May 24, 2011, between Devicor Medical Products, Inc. and the Company (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the SEC) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed July 19, 2011). |
|
|
|
|
|
10.16 |
|
License Agreement, dated December 9, 2011, between AstraZeneca AB and the Company (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed April 11, 2012). |
|
|
|
|
|
10.17 |
|
Series GG Warrant to Purchase Common Stock of the Company issued to Hercules Technology II, L.P. on December 29, 2011 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed January 5, 2012). |
|
|
|
|
|
10.18 |
|
Director Agreement, dated November 13, 2013, by and between Navidea Biopharmaceuticals, Inc. and Michael M. Goldberg, M.D. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed November 19, 2013). |
|
|
|
|
|
10.19 |
|
Manufacturing Services Agreement, dated September 9, 2013, by and between Navidea Biopharmaceuticals, Inc. and OSO BioPharmaceuticals Manufacturing, LLC (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed September 12, 2013). |
|
|
|
|
|
10.20 |
|
Office Lease, dated August 29, 2013, by and between Navidea Biopharmaceuticals, Inc. and BRE/COH OH LLC (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed September 5, 2013) |
|
|
|
|
|
10.21 |
|
Manufacturing Services Agreement, dated August 16, 2013, by and between Navidea Biopharmaceuticals, Inc. and PETNET Solutions, Inc. (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed August 22, 2013). |
|
|
|
|
|
10.22 |
|
Series HH Warrant to purchase common stock of Navidea Biopharmaceuticals, Inc. issued to GE Capital Equity Investments, Inc., dated June 25, 2013 (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed June 28, 2013). |
|
|
|
|
|
10.23 |
|
Series HH Warrant to purchase common stock of Navidea Biopharmaceuticals, Inc. issued to MidCap Financial SBIC, LP, dated June 25, 2013 (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed June 28, 2013). |
|
|
|
|
|
10.24 |
|
NAV5001 Clinical Supply Agreement, dated May 10, 2013, by and between Nordion (Canada) Inc. and Navidea Biopharmaceuticals, Inc. (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed May 16, 2013). |
|
|
|
|
|
10.25 |
|
Form of Series KK Warrants to purchase common stock of Navidea Biopharmaceuticals, Inc. issued to Oxford Finance LLC on March 4, 2014 (incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K filed March 7, 2014). |
|
|
|
|
|
10.26 |
|
Second Amendment to Loan Agreement, dated March 4, 2014, between Navidea Biopharmaceuticals, Inc. and Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed March 7, 2014). |
|
|
|
|
79
|
Exhibit Number |
|
Exhibit Description |
|
|
Second Amended and Restated Promissory Note, dated March 4, 2014, made by Navidea Biopharmaceuticals, Inc. in favor of Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed March 7, 2014). |
|
|
|
|
|
|
10.28 |
|
Employment Agreement, dated April 21, 2014, by and between Navidea Biopharmaceuticals, Inc. and Cornelia B. Reininger, M.D., Ph.D. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed April 24, 2014). ^ |
|
|
|
|
|
10.29 |
|
Employment Agreement, dated April 23, 2014, by and between Navidea Biopharmaceuticals, Inc. and Mark J. Pykett, V.M.D., Ph.D. (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed April 24, 2014). ^ |
|
|
|
|
|
10.30 |
|
Separation Agreement and Release, effective May 30, 2014, between the Company and Mark J. Pykett (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed June 2, 2014). ^ |
|
|
|
|
|
10.31 |
|
Consulting Agreement, effective June 1, 2014, between the Company and Mark J. Pykett (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed June 2, 2014).^ |
|
|
|
|
|
10.32 |
|
Series A Preferred Unit Purchase Agreement, dated July 15, 2014, among the Company, R-NAV, LLC, and the other Purchasers named therein (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed July 16, 2014). |
|
|
|
|
|
10.33 |
|
Promissory Note, dated July 15, 2014, between the Company as Maker and R-NAV, LLC (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed July 16, 2014). |
|
|
|
|
|
10.34 |
|
Amended and Restated License Agreement, dated July 14, 2014, between the Company and the Regents of the University of California (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed August 11, 2014). |
|
|
|
|
|
10.35 |
|
Termination of License Agreement, dated July 14, 2014, between the Company and the Regents of the University of California (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed August 11, 2014). |
|
|
|
|
|
10.36 |
|
License Agreement, dated July 14, 2014, between the Company and the Regents of the University of California (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission) (incorporated by reference to Exhibit 10.3 to the Company’s Quarterly Report on Form 10-Q filed August 11, 2014). |
|
|
|
|
|
10.37 |
|
Employment Agreement, dated October 13, 2014, between Navidea Biopharmaceuticals, Inc. and Ricardo J. Gonzalez (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed October 15, 2014). ^ |
|
|
|
|
|
10.38 |
|
Stock Option Agreement, dated October 13, 2014, between Navidea Biopharmaceuticals, Inc. and Ricardo J. Gonzalez (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed October 15, 2014). ^ |
|
|
|
|
|
10.39 |
|
Securities Exchange Agreement, dated November 12, 2014, by and between Navidea Biopharmaceuticals, Inc. and Platinum Partners Value Arbitrage Fund, L.P. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed November 13, 2014). |
|
|
|
|
|
10.40 |
|
Employment Agreement between the Company and Thomas J. Klima, dated January 1, 2015 (incorporated by reference to Exhibit 10.1 to the Company’s Quarterly Report on Form 10-Q filed August 10, 2015).^ |
|
|
|
|
|
10.41 |
|
Employment Agreement between the Company and Michael Tomblyn, M.D., dated January 1, 2015 (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed August 10, 2015).^ |
|
|
|
|
|
10.42 |
|
Termination Agreement, dated April 21, 2015, by and between Navidea Biopharmaceuticals, Inc. and Alseres Pharmaceuticals, Inc. (portions of this Exhibit have been omitted pursuant to a request for confidential treatment and have been filed separately with the United States Securities and Exchange Commission) (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed April 27, 2015). |
|
|
|
|
|
10.43 |
|
Securities Exchange Agreement dated as of March 11, 2015 among Macrophage Therapeutics, Inc., Platinum-Montaur Life Sciences, LLC and Michael Goldberg, M.D. (incorporated by reference to Exhibit 10.2 to the Company’s Quarterly Report on Form 10-Q filed May 11, 2015). |
|
|
|
|
80
|
Exhibit Number |
|
Exhibit Description |
|
|
Term Loan Agreement, dated as of May 8, 2015, by and among Navidea Biopharmaceuticals, Inc., as borrower, Macrophage Therapeutics, Inc. as guarantor, and Capital Royalty Partners II L.P., Capital Royalty Partners II – Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as lenders (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed October 9, 2015). |
|
|
|
|
|
|
10.45 |
|
Security Agreement, dated as of May 15, 2015 among Navidea Biopharmaceuticals, Inc., as borrower, Macrophage Therapeutics, Inc. as guarantor, and Capital Royalty Partners II L.P., Capital Royalty Partners II – Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as lenders, and Capital Royalty Partners II L.P., as control agent (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed May 15, 2015). |
|
|
|
|
|
10.46 |
|
Subordination Agreement, dated as of May 8, 2015, among Platinum-Montaur Life Sciences, LLC, as subordinated creditor, Capital Royalty Partners II L.P., Capital Royalty Partners II – Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as senior creditors, and Capital Royalty Partners II L.P., as senior creditor agent, and consented to by Navidea Biopharmaceuticals, Inc. as borrower (incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed May 15, 2015). |
|
|
|
|
|
10.47 |
|
Third Amendment to Loan Agreement, dated as of May 8, 2015, by and between Navidea Biopharmaceuticals, Inc, as borrower, and Platinum-Montaur Life Sciences, LLC, as lender (incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed May 15, 2015). |
|
|
|
|
|
10.48 |
|
Third Amended and Restated Promissory Note, dated May 8, 2015, made by Navidea Biopharmaceuticals, Inc. in favor of Platinum-Montaur Life Sciences LLC (incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K filed May 15, 2015). |
|
|
|
|
|
10.49 |
|
Securities Exchange Agreement, dated as of August 20, 2015, among the Company, Montsant Partners LLC and Platinum Partners Value Arbitrage Fund, L.P. (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed August 26, 2015). |
|
|
|
|
|
10.50 |
|
Form of Series LL Warrant issued to Montsant Partners LLC and Platinum Partners Value Arbitrage Fund, L.P. (incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed August 26, 2015). |
|
|
|
|
|
10.51 |
|
Amendment 1 to Term Loan Agreement by and among Navidea Biopharmaceuticals, Inc., as borrower, and Capital Royalty Partners II L.P., Capital Royalty Partners II – Parallel Fund “A” L.P. and Parallel Investment Opportunities Partners II L.P., as lenders, dated as of December 23, 2015 (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed January 11, 2016). |
|
|
|
|
|
10.52 |
|
Agreement dated as of March 14, 2016 by and among the Company, Platinum Partners Value Arbitrage Fund L.P., Platinum Partners Liquid Opportunity Master Fund L.P., Platinum-Montaur Life Sciences, LLC, Platinum Management (NY) LLC, Platinum Liquid Opportunity Management (NY) LLC and Mark Nordlicht (incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed March 18, 2016). |
|
|
|
|
|
21.1 |
|
Subsidiaries of the registrant.* |
|
|
|
|
|
23.1 |
|
Consent of BDO USA, LLP.* |
|
|
|
|
|
24.1 |
|
Power of Attorney.* |
|
|
|
|
|
31.1 |
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* |
|
|
|
|
|
31.2 |
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* |
|
|
|
|
|
32.1 |
|
Certification of Chief Executive Officer of Periodic Financial Reports pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350.* |
|
|
|
|
|
32.2 |
|
Certification of Chief Financial Officer of Periodic Financial Reports pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350.* |
|
|
|
|
|
101.INS |
|
XBRL Instance Document * |
|
|
|
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document * |
|
|
|
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document * |
|
|
|
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document * |
|
|
|
|
81
|
Exhibit Number |
|
Exhibit Description |
|
|
XBRL Taxonomy Extension Label Linkbase Document * |
|
|
|
|
|
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document * |
|
^ |
Management contract or compensatory plan or arrangement. |
|
* |
Filed herewith. |
82
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Dated: March 23, 2016 |
|
|
|
|
NAVIDEA BIOPHARMACEUTICALS, INC. |
|
|
|
(the Company) |
|
|
|
|
|
|
|
By: |
/s/ Ricardo J. Gonzalez |
|
|
|
Ricardo J. Gonzalez |
|
|
|
President and Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Ricardo J. Gonzalez |
|
Director, President and Chief Executive Officer (principal executive officer) |
|
March 23, 2016 |
|
Ricardo J. Gonzalez |
|
|
|
|
|
|
|
|
|
|
|
/s/ Brent L. Larson* |
|
Executive Vice President and Chief Financial Officer (principal financial officer and principal accounting officer) |
|
March 23, 2016 |
|
Brent L. Larson |
|
|
|
|
|
|
|
|
|
|
|
/s/ Anton Gueth* |
|
Chairman, Director |
|
March 23, 2016 |
|
Anton Gueth |
|
|
|
|
|
|
|
|
|
|
|
/s/ Brendan A. Ford* |
|
Director |
|
March 23, 2016 |
|
Brendan A. Ford |
|
|
|
|
|
|
|
|
|
|
|
/s/ Michael M. Goldberg* |
|
Director |
|
March 23, 2016 |
|
Michael M. Goldberg |
|
|
|
|
|
|
|
|
|
|
|
/s/ Eric K. Rowinsky* |
|
Director |
|
March 23, 2016 |
|
Eric K. Rowinsky |
|
|
|
|
|
|
|
|
|
|
|
/s/ Gordon A. Troup* |
|
Director |
|
March 23, 2016 |
|
Gordon A. Troup |
|
|
|
|
|
*By: |
/s/ Ricardo J. Gonzalez |
|
|
Ricardo J. Gonzalez, Attorney-in-fact |
83
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
NAVIDEA BIOPHARMACEUTICALS, INC.
FORM 10-K ANNUAL REPORT
As of December 31, 2015 and 2014
and for Each of the
Three Years in the Period Ended
December 31, 2015
FINANCIAL STATEMENTS
NAVIDEA BIOPHARMACEUTICALS, INC. and SUBSIDIARIES
Index to Financial Statements
|
Consolidated Financial Statements of Navidea Biopharmaceuticals, Inc. |
|
|
|
|
|
F-2 |
|
|
|
|
|
Consolidated Balance Sheets as of December 31, 2015 and 2014 |
F-3 |
|
|
|
|
Consolidated Statements of Operations for the years ended December 31, 2015, 2014 and 2013 |
F-4 |
|
|
|
|
F-5 |
|
|
|
|
|
Consolidated Statements of Cash Flows for the years ended December 31, 2015, 2014 and 2013 |
F-6 |
|
|
|
|
F-7 |
F-1
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
Navidea Biopharmaceuticals, Inc.
Dublin, Ohio
We have audited the accompanying consolidated balance sheets of Navidea Biopharmaceuticals, Inc. as of December 31, 2015 and 2014 and the related consolidated statements of operations, stockholders’ deficit, and cash flows for each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and the significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Navidea Biopharmaceuticals, Inc. at December 31, 2015 and 2014, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Navidea Biopharmaceuticals, Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control−Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated March 23, 2016 expressed an adverse opinion thereon.
/s/ BDO USA, LLP
Chicago, Illinois
March 23, 2016
F-2
Navidea Biopharmaceuticals, Inc. and Subsidiaries
|
ASSETS |
|
December 31, 2015 |
|
|
December 31, 2014 |
|
||
|
Current assets: |
|
|
|
|
|
|
|
|
|
Cash |
|
$ |
7,166,260 |
|
|
$ |
5,479,006 |
|
|
Accounts and other receivables |
|
|
3,703,186 |
|
|
|
894,490 |
|
|
Inventory, net |
|
|
652,906 |
|
|
|
932,385 |
|
|
Prepaid expenses and other |
|
|
1,054,822 |
|
|
|
1,293,264 |
|
|
Total current assets |
|
|
12,577,174 |
|
|
|
8,599,145 |
|
|
Property and equipment |
|
|
3,871,035 |
|
|
|
4,124,028 |
|
|
Less accumulated depreciation and amortization |
|
|
1,943,427 |
|
|
|
1,614,320 |
|
|
|
|
|
1,927,608 |
|
|
|
2,509,708 |
|
|
Patents and trademarks |
|
|
233,596 |
|
|
|
219,558 |
|
|
Less accumulated amortization |
|
|
47,438 |
|
|
|
38,725 |
|
|
|
|
|
186,158 |
|
|
|
180,833 |
|
|
Investment in R-NAV, LLC |
|
|
— |
|
|
|
241,575 |
|
|
Other assets |
|
|
273,573 |
|
|
|
299,047 |
|
|
Total assets |
|
$ |
14,964,513 |
|
|
$ |
11,830,308 |
|
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT |
|
|
|
|
|
|
|
|
|
Current liabilities: |
|
|
|
|
|
|
|
|
|
Accounts payable |
|
$ |
1,767,523 |
|
|
$ |
1,477,499 |
|
|
Accrued liabilities and other |
|
|
3,038,713 |
|
|
|
3,234,120 |
|
|
Deferred revenue, current |
|
|
1,044,281 |
|
|
|
— |
|
|
Notes payable, current, net of discounts of $0 and $863,813, respectively |
|
|
333,333 |
|
|
|
4,348,678 |
|
|
Total current liabilities |
|
|
6,183,850 |
|
|
|
9,060,297 |
|
|
Deferred revenue |
|
|
192,728 |
|
|
|
— |
|
|
Notes payable, net of discounts of $2,033,506 and $1,585,882, respectively |
|
|
60,746,002 |
|
|
|
29,484,057 |
|
|
Other liabilities |
|
|
1,677,633 |
|
|
|
3,089,420 |
|
|
Total liabilities |
|
|
68,800,213 |
|
|
|
41,633,774 |
|
|
Stockholders’ deficit: |
|
|
|
|
|
|
|
|
|
Preferred stock; $.001 par value; 5,000,000 shares authorized; 0 and 4,519 Series B shares issued and outstanding at December 31, 2015 and 2014, respectively |
|
|
— |
|
|
|
4 |
|
|
Common stock; $.001 par value; 200,000,000 shares authorized; 155,649,665 and 150,200,259 shares issued and outstanding at December 31, 2015 and 2014, respectively |
|
|
155,650 |
|
|
|
150,200 |
|
|
Additional paid-in capital |
|
|
326,085,743 |
|
|
|
323,030,301 |
|
|
Accumulated deficit |
|
|
(380,546,651 |
) |
|
|
(352,983,971 |
) |
|
Total Navidea stockholders' deficit |
|
|
(54,305,258 |
) |
|
|
(29,803,466 |
) |
|
Noncontrolling interest |
|
|
469,558 |
|
|
|
— |
|
|
Total stockholders’ deficit |
|
|
(53,835,700 |
) |
|
|
(29,803,466 |
) |
|
Total liabilities and stockholders’ deficit |
|
$ |
14,964,513 |
|
|
$ |
11,830,308 |
|
See accompanying notes to consolidated financial statements.
F-3
Navidea Biopharmaceuticals, Inc. and Subsidiaries
Consolidated Statements of Operations
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Lymphoseek sales revenue |
|
$ |
10,254,352 |
|
|
$ |
4,233,953 |
|
|
$ |
614,423 |
|
|
Lymphoseek license revenue |
|
|
1,133,333 |
|
|
|
300,000 |
|
|
|
— |
|
|
Grant and other revenue |
|
|
1,861,622 |
|
|
|
1,740,896 |
|
|
|
516,207 |
|
|
Total revenue |
|
|
13,249,307 |
|
|
|
6,274,849 |
|
|
|
1,130,630 |
|
|
Cost of goods sold |
|
|
1,754,763 |
|
|
|
1,586,145 |
|
|
|
332,815 |
|
|
Gross profit |
|
|
11,494,544 |
|
|
|
4,688,704 |
|
|
|
797,815 |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
12,787,733 |
|
|
|
16,779,589 |
|
|
|
23,710,183 |
|
|
Selling, general and administrative |
|
|
17,257,329 |
|
|
|
15,542,071 |
|
|
|
15,525,946 |
|
|
Total operating expenses |
|
|
30,045,062 |
|
|
|
32,321,660 |
|
|
|
39,236,129 |
|
|
Loss from operations |
|
|
(18,550,518 |
) |
|
|
(27,632,956 |
) |
|
|
(38,438,314 |
) |
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net |
|
|
(6,873,736 |
) |
|
|
(3,690,068 |
) |
|
|
(2,759,942 |
) |
|
Equity in loss of R-NAV, LLC |
|
|
(305,253 |
) |
|
|
(523,809 |
) |
|
|
— |
|
|
Change in fair value of financial instruments |
|
|
(614,782 |
) |
|
|
(1,342,389 |
) |
|
|
(112,073 |
) |
|
Loss on extinguishment of debt |
|
|
(2,440,714 |
) |
|
|
(2,610,196 |
) |
|
|
(1,372,266 |
) |
|
Other, net |
|
|
26,808 |
|
|
|
72,749 |
|
|
|
(16,863 |
) |
|
Total other expense, net |
|
|
(10,207,677 |
) |
|
|
(8,093,713 |
) |
|
|
(4,261,144 |
) |
|
Loss before income taxes |
|
|
(28,758,195 |
) |
|
|
(35,726,669 |
) |
|
|
(42,699,458 |
) |
|
Benefit from income taxes |
|
|
436,051 |
|
|
|
— |
|
|
|
— |
|
|
Loss from continuing operations |
|
|
(28,322,144 |
) |
|
|
(35,726,669 |
) |
|
|
(42,699,458 |
) |
|
Income from discontinued operations, net of tax effect |
|
|
758,609 |
|
|
|
— |
|
|
|
— |
|
|
Net loss |
|
|
(27,563,535 |
) |
|
|
(35,726,669 |
) |
|
|
(42,699,458 |
) |
|
Less loss attributable to noncontrolling interest |
|
|
(855 |
) |
|
|
— |
|
|
|
— |
|
|
Deemed dividend on beneficial conversion feature of MT Preferred Stock |
|
|
(46,000 |
) |
|
|
— |
|
|
|
— |
|
|
Net loss attributable to common stockholders |
|
$ |
(27,608,680 |
) |
|
$ |
(35,726,669 |
) |
|
$ |
(42,699,458 |
) |
|
Income (loss) per common share (basic and diluted): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Continuing operations |
|
$ |
(0.19 |
) |
|
$ |
(0.24 |
) |
|
$ |
(0.35 |
) |
|
Discontinued operations |
|
$ |
0.01 |
|
|
$ |
- |
|
|
$ |
- |
|
|
Attributable to common stockholders |
|
$ |
(0.18 |
) |
|
$ |
(0.24 |
) |
|
$ |
(0.35 |
) |
|
Weighted average shares outstanding (basic and diluted) |
|
|
151,180,222 |
|
|
|
148,748,396 |
|
|
|
121,808,986 |
|
See accompanying notes to consolidated financial statements.
F-4
Navidea Biopharmaceuticals, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Deficit
|
|
Preferred Stock |
|
|
Common Stock |
|
|
Additional Paid-In |
|
|
Accumulated |
|
|
Non- controlling |
|
|
Total Stockholders' |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Deficit |
|
|
Interest |
|
|
Deficit |
|
||||||||
|
Balance, December 31, 2012 |
|
6,938 |
|
|
$ |
7 |
|
|
|
113,018,772 |
|
|
$ |
113,019 |
|
|
$ |
273,039,442 |
|
|
$ |
(274,557,844 |
) |
|
$ |
— |
|
|
$ |
(1,405,376 |
) |
|
Issued stock in connection with public offerings, net of costs |
|
— |
|
|
|
— |
|
|
|
14,205,770 |
|
|
|
14,205 |
|
|
|
38,117,267 |
|
|
|
— |
|
|
|
— |
|
|
|
38,131,472 |
|
|
Issued stock upon exercise of stock options, net |
|
— |
|
|
|
— |
|
|
|
39,649 |
|
|
|
40 |
|
|
|
(9,201 |
) |
|
|
— |
|
|
|
— |
|
|
|
(9,161 |
) |
|
Issued restricted stock |
|
— |
|
|
|
— |
|
|
|
73,500 |
|
|
|
74 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
74 |
|
|
Canceled stock to pay employee tax obligations |
|
— |
|
|
|
— |
|
|
|
(194,077 |
) |
|
|
(194 |
) |
|
|
(610,362 |
) |
|
|
— |
|
|
|
— |
|
|
|
(610,556 |
) |
|
Canceled forfeited restricted stock |
|
— |
|
|
|
— |
|
|
|
(29,250 |
) |
|
|
(29 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(29 |
) |
|
Issued stock upon exercise of warrants |
|
2,365 |
|
|
|
2 |
|
|
|
3,000,000 |
|
|
|
3,000 |
|
|
|
6,158,330 |
|
|
|
— |
|
|
|
— |
|
|
|
6,161,332 |
|
|
Issued stock to 401(k) plan |
|
— |
|
|
|
— |
|
|
|
22,126 |
|
|
|
22 |
|
|
|
66,755 |
|
|
|
— |
|
|
|
— |
|
|
|
66,777 |
|
|
Issued stock for payment of milestone fee |
|
— |
|
|
|
— |
|
|
|
100,000 |
|
|
|
100 |
|
|
|
165,900 |
|
|
|
— |
|
|
|
— |
|
|
|
166,000 |
|
|
Conversion of Series B Preferred Stock to common stock |
|
(1,738 |
) |
|
|
(1 |
) |
|
|
5,682,933 |
|
|
|
5,682 |
|
|
|
(5,681 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issued warrants in connection with debt issuance |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
967,115 |
|
|
|
— |
|
|
|
— |
|
|
|
967,115 |
|
|
Issued warrants in connection with public offering |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(7,686,046 |
) |
|
|
— |
|
|
|
— |
|
|
|
(7,686,046 |
) |
|
Stock compensation expense |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,908,269 |
|
|
|
— |
|
|
|
— |
|
|
|
2,908,269 |
|
|
Net loss |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(42,699,458 |
) |
|
|
— |
|
|
|
(42,699,458 |
) |
|
Balance, December 31, 2013 |
|
7,565 |
|
|
|
8 |
|
|
|
135,919,423 |
|
|
|
135,919 |
|
|
|
313,111,788 |
|
|
|
(317,257,302 |
) |
|
|
— |
|
|
|
(4,009,587 |
) |
|
Issued stock upon exercise of stock options, net |
|
— |
|
|
|
— |
|
|
|
299,360 |
|
|
|
300 |
|
|
|
(51,669 |
) |
|
|
— |
|
|
|
— |
|
|
|
(51,369 |
) |
|
Issued restricted stock |
|
— |
|
|
|
— |
|
|
|
380,250 |
|
|
|
380 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
380 |
|
|
Canceled stock to pay employee tax obligations |
|
— |
|
|
|
— |
|
|
|
(6,582 |
) |
|
|
(6 |
) |
|
|
(8,813 |
) |
|
|
— |
|
|
|
— |
|
|
|
(8,819 |
) |
|
Canceled forfeited restricted stock |
|
— |
|
|
|
— |
|
|
|
(300,000 |
) |
|
|
(300 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(300 |
) |
|
Issued stock to 401(k) plan |
|
— |
|
|
|
— |
|
|
|
36,455 |
|
|
|
36 |
|
|
|
100,007 |
|
|
|
— |
|
|
|
— |
|
|
|
100,043 |
|
|
Conversion of Series B Preferred Stock to common stock, net |
|
(3,046 |
) |
|
|
(4 |
) |
|
|
9,960,420 |
|
|
|
9,960 |
|
|
|
(9,956 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issued warrants in connection with debt issuance |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
464,991 |
|
|
|
— |
|
|
|
— |
|
|
|
464,991 |
|
|
Issued stock upon exchange of warrants |
|
— |
|
|
|
— |
|
|
|
3,843,223 |
|
|
|
3,843 |
|
|
|
7,682,930 |
|
|
|
— |
|
|
|
— |
|
|
|
7,686,773 |
|
|
Issued stock in payment of Board retainers |
|
— |
|
|
|
— |
|
|
|
67,710 |
|
|
|
68 |
|
|
|
89,307 |
|
|
|
— |
|
|
|
— |
|
|
|
89,375 |
|
|
Recovery of shareholder short swing profits |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
17,554 |
|
|
|
— |
|
|
|
— |
|
|
|
17,554 |
|
|
Stock compensation expense |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
1,634,162 |
|
|
|
— |
|
|
|
— |
|
|
|
1,634,162 |
|
|
Net loss |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(35,726,669 |
) |
|
|
— |
|
|
|
(35,726,669 |
) |
|
Balance, December 31, 2014 |
|
4,519 |
|
|
|
4 |
|
|
|
150,200,259 |
|
|
|
150,200 |
|
|
|
323,030,301 |
|
|
|
(352,983,971 |
) |
|
|
— |
|
|
|
(29,803,466 |
) |
|
Issued stock upon exercise of stock options, net |
|
— |
|
|
|
— |
|
|
|
124,238 |
|
|
|
124 |
|
|
|
54,206 |
|
|
|
— |
|
|
|
— |
|
|
|
54,330 |
|
|
Issued restricted stock |
|
— |
|
|
|
— |
|
|
|
354,000 |
|
|
|
354 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
354 |
|
|
Canceled forfeited restricted stock |
|
— |
|
|
|
— |
|
|
|
(158,000 |
) |
|
|
(158 |
) |
|
|
158 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Canceled stock to pay employee tax obligations |
|
— |
|
|
|
— |
|
|
|
(7,645 |
) |
|
|
(7 |
) |
|
|
(12,607 |
) |
|
|
— |
|
|
|
— |
|
|
|
(12,614 |
) |
|
Issued stock in payment of Board retainers |
|
— |
|
|
|
— |
|
|
|
90,977 |
|
|
|
91 |
|
|
|
172,878 |
|
|
|
— |
|
|
|
— |
|
|
|
172,969 |
|
|
Issued stock to 401(k) plan |
|
— |
|
|
|
— |
|
|
|
68,157 |
|
|
|
68 |
|
|
|
117,031 |
|
|
|
— |
|
|
|
— |
|
|
|
117,099 |
|
|
Exchanged Series B Preferred Stock for warrants |
|
(4,519 |
) |
|
|
(4 |
) |
|
|
— |
|
|
|
— |
|
|
|
4 |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Extension of warrant expiration date |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
149,615 |
|
|
|
— |
|
|
|
— |
|
|
|
149,615 |
|
|
Issued warrants in connection with advisory services agreement |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
256,450 |
|
|
|
— |
|
|
|
— |
|
|
|
256,450 |
|
|
Issued stock upon exercise of warrants |
|
— |
|
|
|
— |
|
|
|
4,977,679 |
|
|
|
4,978 |
|
|
|
(4,978 |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Stock compensation expense |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
2,368,685 |
|
|
|
— |
|
|
|
— |
|
|
|
2,368,685 |
|
|
Net loss |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(27,562,680 |
) |
|
|
(855 |
) |
|
|
(27,563,535 |
) |
|
Issuance of MT Preferred Stock, net of deemed dividend |
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(46,000 |
) |
|
|
— |
|
|
|
470,413 |
|
|
|
424,413 |
|
|
Balance, December 31, 2015 |
|
— |
|
|
$ |
0 |
|
|
|
155,649,665 |
|
|
$ |
155,650 |
|
|
$ |
326,085,743 |
|
|
$ |
(380,546,651 |
) |
|
$ |
469,558 |
|
|
$ |
(53,835,700 |
) |
See accompanying notes to consolidated financial statements.
F-5
Navidea Biopharmaceuticals, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
|
|
Year Ended December 31, |
|
|||||||||
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Net loss |
$ |
(27,563,535 |
) |
|
$ |
(35,726,669 |
) |
|
$ |
(42,699,458 |
) |
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization of property and equipment |
|
562,468 |
|
|
|
487,906 |
|
|
|
400,304 |
|
|
Amortization of patents and trademarks |
|
8,951 |
|
|
|
12,277 |
|
|
|
3,877 |
|
|
Loss on disposal and abandonment of assets |
|
33,184 |
|
|
|
31,794 |
|
|
|
1,160 |
|
|
Change in inventory reserve and other adjustments to inventory |
|
143,493 |
|
|
|
539,027 |
|
|
|
— |
|
|
Amortization of debt discount and issuance costs |
|
492,963 |
|
|
|
844,250 |
|
|
|
765,263 |
|
|
Compounded interest on notes payable |
|
2,048,960 |
|
|
|
— |
|
|
|
— |
|
|
Stock compensation expense |
|
2,368,685 |
|
|
|
1,634,162 |
|
|
|
2,908,269 |
|
|
Equity in loss of R-NAV, LLC |
|
305,253 |
|
|
|
523,809 |
|
|
|
— |
|
|
Change in fair value of financial instruments |
|
614,782 |
|
|
|
1,342,389 |
|
|
|
112,073 |
|
|
Loss on extinguishment of debt |
|
2,440,714 |
|
|
|
2,610,196 |
|
|
|
1,372,266 |
|
|
Issued stock to 401(k) plan for employer matching contributions |
|
117,099 |
|
|
|
100,043 |
|
|
|
66,777 |
|
|
Extension of warrant expiration date |
|
149,615 |
|
|
|
— |
|
|
|
— |
|
|
Issued warrants in connection with advisory services agreement |
|
256,450 |
|
|
|
— |
|
|
|
— |
|
|
Issuance of common stock for payment of milestone and sublicense fees |
|
— |
|
|
|
— |
|
|
|
166,000 |
|
|
Other |
|
109,292 |
|
|
|
50,718 |
|
|
|
4,432 |
|
|
Change in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Accounts and other receivables |
|
(2,808,696 |
) |
|
|
334,082 |
|
|
|
(1,042,280 |
) |
|
Inventory |
|
135,986 |
|
|
|
761,024 |
|
|
|
(1,934,936 |
) |
|
Prepaid expenses and other assets |
|
263,915 |
|
|
|
(476,860 |
) |
|
|
84,493 |
|
|
Accounts payable |
|
290,024 |
|
|
|
(944,850 |
) |
|
|
1,003,515 |
|
|
Accrued liabilities and other liabilities |
|
(282,642 |
) |
|
|
(1,250,047 |
) |
|
|
3,176,170 |
|
|
Deferred revenue |
|
1,237,009 |
|
|
|
— |
|
|
|
— |
|
|
Net cash used in operating activities |
|
(19,076,030 |
) |
|
|
(29,126,749 |
) |
|
|
(35,612,075 |
) |
|
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of equipment |
|
(39,001 |
) |
|
|
(1,114,448 |
) |
|
|
(1,239,666 |
) |
|
Proceeds from sales of equipment |
|
38,265 |
|
|
|
— |
|
|
|
— |
|
|
Patent and trademark costs |
|
(27,092 |
) |
|
|
(77,184 |
) |
|
|
(52,701 |
) |
|
Investment in R-NAV, LLC |
|
— |
|
|
|
(333,334 |
) |
|
|
— |
|
|
Net cash used in investing activities |
|
(27,828 |
) |
|
|
(1,524,966 |
) |
|
|
(1,292,367 |
) |
|
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of MT Preferred Stock and warrants |
|
500,000 |
|
|
|
— |
|
|
|
— |
|
|
Payment of issuance costs related to MT Preferred Stock and warrants |
|
(12,587 |
) |
|
|
— |
|
|
|
— |
|
|
Proceeds from issuance of common stock and short swing profits |
|
65,975 |
|
|
|
87,984 |
|
|
|
41,303,750 |
|
|
Payment of common stock issuance costs |
|
— |
|
|
|
— |
|
|
|
(1,752,932 |
) |
|
Payment of tax withholdings related to stock-based compensation |
|
(23,906 |
) |
|
|
(130,537 |
) |
|
|
(659,018 |
) |
|
Proceeds from notes payable |
|
54,500,000 |
|
|
|
30,000,000 |
|
|
|
29,000,000 |
|
|
Payment of debt-related costs |
|
(3,902,487 |
) |
|
|
(1,763,526 |
) |
|
|
(1,177,293 |
) |
|
Principal payments on notes payable |
|
(30,333,333 |
) |
|
|
(25,000,000 |
) |
|
|
(5,982,155 |
) |
|
Payments under capital leases |
|
(2,550 |
) |
|
|
(2,226 |
) |
|
|
(7,448 |
) |
|
Net cash provided by financing activities |
|
20,791,112 |
|
|
|
3,191,695 |
|
|
|
60,724,904 |
|
|
Net increase (decrease) in cash |
|
1,687,254 |
|
|
|
(27,460,020 |
) |
|
|
23,820,462 |
|
|
Cash, beginning of period |
|
5,479,006 |
|
|
|
32,939,026 |
|
|
|
9,118,564 |
|
|
Cash, end of period |
$ |
7,166,260 |
|
|
$ |
5,479,006 |
|
|
$ |
32,939,026 |
|
See accompanying notes to consolidated financial statements.
F-6
Notes to the Consolidated Financial Statements
|
1. |
Organization and Summary of Significant Accounting Policies |
|
|
a. |
Organization and Nature of Operations: Navidea Biopharmaceuticals, Inc. (Navidea, the Company, or we), a Delaware Corporation (NYSE MKT: NAVB), is a biopharmaceutical company focused on the development and commercialization of precision immunodiagnostic agents and immunotherapeutics. Navidea is developing multiple precision-targeted products and platforms including Manocept™ and NAV4694 to help identify the sites and pathways of undetected disease and enable better diagnostic accuracy, clinical decision-making, targeted treatment and, ultimately, patient care. |
Navidea’s Manocept platform is predicated on the ability to specifically target the CD206 mannose receptor expressed on activated macrophages. The Manocept platform serves as the molecular backbone of Lymphoseek® (technetium Tc 99m tilmanocept) injection, the first product developed by Navidea based on the platform. Lymphoseek is a novel, state-of-the-art, receptor-targeted, small-molecule radiopharmaceutical used in the evaluation of lymphatic basins that may have cancer involvement in patients. Lymphoseek is designed for the precise identification of lymph nodes that drain from a primary tumor, which have the highest probability of harboring cancer. Lymphoseek is approved by the U.S. Food and Drug Administration (FDA) for use in solid tumor cancers where lymphatic mapping is a component of surgical management and for guiding sentinel lymph node biopsy in patients with clinically node negative breast cancer, melanoma or squamous cell carcinoma of the oral cavity. Lymphoseek has also received European approval in imaging and intraoperative detection of sentinel lymph nodes in patients with melanoma, breast cancer or localized squamous cell carcinoma of the oral cavity.
Building on the success of Lymphoseek, the flexible and versatile Manocept platform acts as an engine for the design of purpose-built molecules offering the potential to be utilized across a range of diagnostic modalities, including single photon emission computed tomography (SPECT), positron emission tomography (PET), intra-operative and/or optical-fluorescence detection in a variety of disease states.
Recent preclinical data being developed by the Company using tilmanocept linked to a therapeutic agent also suggest that tilmanocept’s binding affinity to CD206 receptors demonstrates the potential for this technology to be useful in treating diseases linked to the over-activation of macrophages. This includes various cancers as well as autoimmune, infectious, cardiovascular, and central nervous system diseases. Our efforts in this area were further supported by the January 2015 formation of Macrophage Therapeutics, Inc. (MT), a majority-owned subsidiary that was formed specifically to further explore therapeutic applications for the Manocept platform. Navidea owns 99.9% of MT.
Our focus on development of our proprietary Manocept platform technology further supports the 2014 decision by the Company’s Board of Directors to reduce our support for, while seeking to partner or out-license, our two neurological development programs, NAV4694 and NAV5001.
Other than Lymphoseek, none of the Company’s drug product candidates have been approved for sale in any market.
From our inception through August 2011, we also manufactured a line of gamma detection systems called the neoprobe® GDS system (the GDS Business). We sold the GDS Business to Devicor Medical Products, Inc. (Devicor) in August 2011. In exchange for the assets of the GDS Business, Devicor made net cash payments to us totaling $30.3 million, assumed certain liabilities of the Company associated with the GDS Business, and agreed to make royalty payments to us of up to an aggregate maximum amount of $20 million based on the net revenue attributable to the GDS Business through 2017. We recorded net income of $759,000 in 2015 related to royalty amounts earned based on 2015 GDS Business revenue. The royalty amount of $1.2 million was offset by $436,000 in estimated taxes which were allocated to discontinued operations, but were fully offset by the tax benefit from our net operating loss for 2015. We did not earn or receive any such royalty payments prior to 2015.
In December 2001, we acquired Cardiosonix Ltd. (Cardiosonix), an Israeli company with a blood flow measurement device product line in the early stages of commercialization. In August 2009, the Company’s Board of Directors decided to discontinue the operations and attempt to sell Cardiosonix. However, we were obligated to continue to service and support the Cardiosonix devices through 2013. The Company has not received significant expressions of interest in the Cardiosonix business and as such, we continue to wind down our activities in this area until a final shutdown of operations or a sale of the business unit is completed.
In 2005 we formed a new corporation, Cira Biosciences, Inc. (Cira Bio), to explore the development of patient-specific cellular therapies that have shown positive patient responses in a variety of clinical settings. Navidea owned 90% of the outstanding shares of Cira Bio with the remaining shares being held by the principals of Cira LLC. In October 2013, Cira Bio was dissolved in its entirety after several years of inactivity.
F-7
In July 2011, we established a European business unit, Navidea Biopharmaceuticals Limited, to address international development and commercialization needs for our technologies, including Lymphoseek. Navidea owns 100% of the outstanding shares of Navidea Biopharmaceuticals Limited.
|
|
b. |
Principles of Consolidation: Our consolidated financial statements include the accounts of Navidea and our wholly-owned subsidiaries, Navidea Biopharmaceuticals Limited and Cardiosonix Ltd, as well as those of our majority-owned subsidiary, MT. Our consolidated financial statements also include the accounts of our former wholly-owned subsidiary, Cira Bio, through the date of dissolution in October 2013. All significant inter-company accounts were eliminated in consolidation. Navidea's investment in R-NAV, LLC (R-NAV) is being accounted for using the equity method of accounting and is therefore not consolidated. |
|
|
c. |
Use of Estimates: The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. |
|
|
d. |
Financial Instruments and Fair Value: In accordance with current accounting standards, the fair value hierarchy prioritizes the inputs to valuation techniques used to measure fair value, giving the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). The three levels of the fair value hierarchy are described below: |
Level 1 – Unadjusted quoted prices in active markets that are accessible at the measurement date for identical, unrestricted assets or liabilities;
Level 2 – Quoted prices in markets that are not active or financial instruments for which all significant inputs are observable, either directly or indirectly; and
Level 3 – Prices or valuations that require inputs that are both significant to the fair value measurement and unobservable.
A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. In determining the appropriate levels, we perform a detailed analysis of the assets and liabilities whose fair value is measured on a recurring basis. At each reporting period, all assets and liabilities for which the fair value measurement is based on significant unobservable inputs or instruments which trade infrequently and therefore have little or no price transparency are classified as Level 3. See Note 3.
The following methods and assumptions were used to estimate the fair value of each class of financial instruments:
|
|
(1) |
Cash, accounts and other receivables, accounts payable, and accrued liabilities: The carrying amounts approximate fair value because of the short maturity of these instruments. |
|
|
(2) |
Notes payable: The carrying value of our debt at December 31, 2015 and 2014 primarily consists of the face amount of the notes less unamortized discounts. See Note 12. At December 31, 2015 and 2014, certain notes payable were also required to be recorded at fair value. The estimated fair value of our debt was calculated using a discounted cash flow analysis as well as a Monte Carlo simulation. These valuation methods include Level 3 inputs such as the estimated current market interest rate for similar instruments with similar creditworthiness. Unrealized gains and losses on the fair value of the debt are classified in other expenses as a change in the fair value of financial instruments in the consolidated statements of operations. At December 31, 2015 and 2014, the fair value of our notes payable is approximately $64.0 million and $37.9 million, respectively, compared to the carrying value of $61.1 million and $33.8 million, respectively. |
|
|
(3) |
Derivative liabilities: Derivative liabilities are related to certain outstanding warrants which are recorded at fair value. Derivative liabilities totaling $63,000 as of December 31, 2015 were included in other liabilities on the consolidated balance sheets. No derivative liabilities were outstanding as of December 31, 2014. The assumptions used to calculate fair value as of December 31, 2015 included volatility, a risk-free rate and expected dividends. In addition, we considered non-performance risk and determined that such risk is minimal. Unrealized gains and losses on the derivatives are classified in other expenses as a change in the fair value of financial instruments in the statements of operations. |
F-8
|
|
shares authorized under each plan are 12 million shares and 5 million shares, respectively. Although instruments are still outstanding under the 2002 Plan, the plan has expired and no new grants may be made from it. Under both plans, the exercise price of each option is greater than or equal to the closing market price of our common stock on the date of the grant. |
Stock options granted under the 2002 Plan and the 2014 Plan generally vest on an annual basis over one to four years. The stock options that were awarded as an employment inducement in connection with the appointment of our CEO will vest in three tranches based on certain service and market conditions as defined in the agreement. Outstanding stock options under the plans, if not exercised, generally expire ten years from their date of grant or up to 90 days following the date of an optionee’s separation from employment with the Company. We issue new shares of our common stock upon exercise of stock options.
Stock-based payments to employees and directors, including grants of stock options, are recognized in the consolidated statement of operations based on their estimated fair values. The fair value of each stock option award is estimated on the date of grant using the Black-Scholes option pricing model. Expected volatilities are based on the Company’s historical volatility, which management believes represents the most accurate basis for estimating expected future volatility under the current circumstances. Navidea uses historical data to estimate forfeiture rates. The expected term of stock options granted is based on the vesting period and the contractual life of the options. The risk-free rate is based on the U.S. Treasury yield in effect at the time of the grant. The assumptions used to calculate the fair value of stock option awards granted during the years ended December 31, 2015, 2014 and 2013 are noted in the following table:
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Expected volatility |
|
61%-64% |
|
|
61%-67% |
|
|
60%-71% |
|
|||
|
Weighted-average volatility |
|
|
62% |
|
|
|
65% |
|
|
|
65% |
|
|
Expected dividends |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Expected term (in years) |
|
5.1-6.3 |
|
|
5.3-7.4 |
|
|
5.0-6.2 |
|
|||
|
Risk-free rate |
|
1.5%-1.9% |
|
|
1.6%-2.0% |
|
|
1.0%-1.9% |
|
|||
The portion of the fair value of stock-based awards that is ultimately expected to vest is recognized as compensation expense over either (1) the requisite service period or (2) the estimated performance period. Restricted stock awards are valued based on the closing stock price on the date of grant and amortized ratably over the estimated life of the award. Restricted stock may vest based on the passage of time, or upon occurrence of a specific event or achievement of goals as defined in the grant agreements. In such cases, we record compensation expense related to grants of restricted stock based on management’s estimates of the probable dates of the vesting events. Stock-based awards that do not vest because the requisite service period is not met prior to termination result in reversal of previously recognized compensation cost. See Note 4.
|
|
f. |
Cash and Cash Equivalents: Cash equivalents are highly liquid instruments such as U.S. Treasury bills, bank certificates of deposit, corporate commercial paper and money market funds which have maturities of less than 3 months from the date of purchase. |
|
|
g. |
Accounts and Other Receivables: Accounts and other receivables are recorded net of an allowance for doubtful accounts. We estimate an allowance for doubtful accounts based on a review and assessment of specific accounts and other receivables and write off accounts when deemed uncollectible. See Note 6. |
|
|
h. |
Inventory: All components of inventory are valued at the lower of cost (first-in, first-out) or market. We adjust inventory to market value when the net realizable value is lower than the carrying cost of the inventory. Market value is determined based on estimated sales activity and margins. We estimate a reserve for obsolete inventory based on management’s judgment of probable future commercial use, which is based on an analysis of current inventory levels, estimated future sales and production rates, and estimated shelf lives. See Note 7. |
|
|
i. |
Property and Equipment: Property and equipment are stated at cost, less accumulated depreciation and amortization. Depreciation is generally computed using the straight-line method over the estimated useful lives of the depreciable assets. Depreciation and amortization related to equipment under capital leases and leasehold improvements is recognized over the shorter of the estimated useful life of the leased asset or the term of the lease. Maintenance and repairs are charged to expense as incurred, while renewals and improvements are capitalized. |
F-9
|
|
to have no recoverable value. We evaluate the potential alternative uses of all intangible assets, as well as the recoverability of the carrying values of intangible assets, on a recurring basis. |
|
|
k. |
Impairment or Disposal of Long-Lived Assets: Long-lived assets and certain identifiable intangibles are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to future undiscounted cash flows expected to be generated by the asset. If such assets are considered to be impaired, the impairment recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. No impairment was recognized during the years ended December 31, 2015, 2014 or 2013. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. |
|
|
l. |
Leases: Leases are categorized as either operating or capital leases at inception. Operating lease costs are recognized on a straight-line basis over the term of the lease. An asset and a corresponding liability for the capital lease obligation are established for the cost of capital leases. The capital lease obligation is amortized over the life of the lease. For build-to-suit leases, the Company establishes an asset and liability for the estimated construction costs incurred to the extent that it is involved in the construction of structural improvements or takes construction risk prior to the commencement of the lease. Upon occupancy of facilities under build-to-suit leases, the Company assesses whether these arrangements qualify for sales recognition under the sale-leaseback accounting guidance. If a lease does not meet the criteria to qualify for a sale-leaseback transaction, the established asset and liability remain on the Company's balance sheet. See Note 19. |
|
|
m. |
Derivative Instruments: Derivative instruments embedded in contracts, to the extent not already a free-standing contract, are bifurcated from the debt instrument and accounted for separately. All derivatives are recorded on the consolidated balance sheet at fair value in accordance with current accounting guidelines for such complex financial instruments. Derivative liabilities with expiration dates within one year are classified as current, while those with expiration dates in more than one year are classified as long term. We do not use derivative instruments for hedging of market risks or for trading or speculative purposes. |
|
|
n. |
Revenue Recognition: We currently generate revenue primarily from sales of Lymphoseek. Our standard shipping terms are FOB shipping point, and title and risk of loss passes to the customer upon delivery to a carrier for shipment. We generally recognize sales revenue related to sales of our products when the products are shipped. Our customers have no right to return products purchased in the ordinary course of business, however, we may allow returns in certain circumstances based on specific agreements. |
We earn additional revenues based on a percentage of the actual net revenues achieved by Cardinal Health on sales to end customers made during each fiscal year. The amount we charge Cardinal Health related to end customer sales of Lymphoseek are subject to a retroactive annual adjustment. To the extent that we can reasonably estimate the end-customer prices received by Cardinal Health, we record sales based upon these estimates at the time of sale. If we are unable to reasonably estimate end customer sales prices related to products sold, we record revenue related to these product sales at the minimum (i.e., floor) price provided for under our distribution agreement with Cardinal Health.
We also earn revenues related to our licensing and distribution agreements. The terms of these agreements may include payment to us of non-refundable upfront license fees, funding or reimbursement of research and development efforts, milestone payments if specified objectives are achieved, and/or royalties on product sales. We evaluate all deliverables within an arrangement to determine whether or not they provide value on a stand-alone basis. We recognize a contingent milestone payment as revenue in its entirety upon our achievement of a substantive milestone if the consideration earned from the achievement of the milestone (i) is consistent with performance required to achieve the milestone or the increase in value to the delivered item, (ii) relates solely to past performance and (iii) is reasonable relative to all of the other deliverables and payments within the arrangement. We received a non-refundable upfront cash payment of $2.0 million from SpePharm AG upon execution of the SpePharm License Agreement in March 2015. We have determined that the license and other non-contingent deliverables do not have stand-alone value because the license could not be deemed to be fully delivered for its intended purpose unless we perform our other obligations, including specified development work. Accordingly, they do not meet the separation criteria, resulting in these deliverables being considered a single unit of account. As a result, revenue relating to the upfront cash payment was deferred and is being recognized on a straight-line basis over the estimated obligation period of two years.
We generate additional revenue from grants to support various product development initiatives. We generally recognize grant revenue when expenses reimbursable under the grants have been paid and payments under the grants become contractually due. Lastly, we recognize revenues from the provision of services to R-NAV and its subsidiaries. See Note 10.
F-10
|
|
clinical trial activities, manufacturing and control-related activities, and regulatory costs. R&D expenses are charged to operations as incurred. We review and accrue R&D expenses based on services performed and rely upon estimates of those costs applicable to the stage of completion of each project. |
|
|
p. |
Income Taxes: Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating loss and tax credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Due to the uncertainty surrounding the realization of the deferred tax assets in future tax returns, all of the deferred tax assets have been fully offset by a valuation allowance at December 31, 2015 and 2014. |
Current accounting standards include guidance on the accounting for uncertainty in income taxes recognized in the financial statements. Such standards also prescribe a recognition threshold and measurement model for the financial statement recognition of a tax position taken, or expected to be taken, and provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure and transition. The Company believes that the ultimate deductibility of all tax positions is highly certain, although there is uncertainty about the timing of such deductibility. As a result, no liability for uncertain tax positions was recorded as of December 31, 2015 or 2014 and we do not expect any significant changes in the next twelve months. Should we need to accrue interest or penalties on uncertain tax positions, we would recognize the interest as interest expense and the penalties as a selling, general and administrative expense. As of December 31, 2015, tax years 2012-2015 remained subject to examination by federal and state tax authorities. See Note 16.
|
|
q. |
Change in Accounting Principle: In April 2015, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) No. 2015-03, Simplifying the Presentation of Debt Issuance Costs. ASU 2015-03 requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability rather than as an asset. The recognition and measurement guidance for debt issuance costs are not affected by ASU 2015-03. ASU 2015-03 is effective for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. Early adoption is permitted. Entities must apply the amendments in ASU 2015-03 on a retrospective basis. |
During the second quarter of 2015, the Company adopted ASU 2015-03. The consolidated balance sheet as of December 31, 2014 has been adjusted to reflect retrospective application of the new method of presentation. Deferred debt issuance costs totaling $90,000 that were included in other assets as of December 31, 2014 were reclassified as discounts on notes payable, current, of $35,000 and discounts on notes payable, long term, of $55,000. We have reflected all remaining unamortized costs as a reduction of the debt on the balance sheets as of December 31, 2015 and 2014, and will continue to do so in future periods. The adoption of ASU 2015-03 had no impact on the consolidated statements of operations, stockholders' deficit or cash flows.
In November 2015, the FASB issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes. ASU 2015-17 eliminates the requirement to bifurcate deferred taxes between current and noncurrent on the balance sheet and requires that deferred tax assets and liabilities be classified as noncurrent on the balance sheet. ASU 2015-17 may be applied retrospectively or prospectively and early adoption is permitted. We early-adopted ASU 2015-17 as of December 31, 2015 and the statement of financial position as of this date reflects the revised classification of current deferred tax assets and liabilities as noncurrent. Adoption of ASU 2015-17 resulted in a retrospective reclassification between current deferred tax assets and noncurrent deferred tax assets.
|
|
r. |
Recent Accounting Developments: In February 2015, the FASB issued ASU No. 2015-02, Amendments to the Consolidation Analysis. ASU 2015-02 affects reporting entities that are required to evaluate whether they should consolidate certain legal entities. All legal entities are subject to reevaluation under the revised consolidation model. Specifically, the amendments: (i) modify the evaluation of whether limited partnerships and similar legal entities are variable interest entities (VIEs) or voting interest entities, (ii) eliminate the presumption that a general partner should consolidate a limited partnership, and (iii) affect the consolidation analysis of reporting entities that are involved with VIEs, particularly those that have fee arrangements and related party relationships. ASU 2015-02 is effective for public entities for fiscal years, and for interim periods within those fiscal years, beginning after December 15, 2015. The amendments may be applied using a modified retrospective approach or a full retrospective approach. Early adoption is permitted, including adoption in an interim period. The adoption of ASU 2015-02 is not expected to have a material effect on our consolidated financial statements. |
In July 2015, the FASB issued ASU No. 2015-11, Simplifying the Measurement of Inventory. ASU 2015-11 applies to all inventory that is measured using methods other than last-in, first-out or the retail inventory method, including inventory
F-11
that is measured using first-in, first-out or average cost. ASU 2015-11 requires entities to measure inventory at the lower of cost and net realizable value, defined as the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. ASU 2015-11 is effective for public entities for fiscal years beginning after December 15, 2016, and interim periods with fiscal years beginning after December 15, 2017. The amendments in ASU 2015-11 should be applied prospectively with earlier application permitted as of the beginning of an interim or annual reporting period. We do not expect the adoption of ASU 2015-11 to have a material effect on our consolidated financial statements upon adoption.
In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers. ASU 2015-14 defers the effective date of ASU No. 2014-09 for all entities by one year. Public business entities should adopt the new revenue recognition standard for annual reporting periods beginning after December 15, 2017, including interim periods within that year. Early adoption is permitted only as of annual reporting periods beginning after December 15, 2016, including interim periods within that year. We are currently evaluating the potential impact that the adoption of ASU 2014-09 may have on our consolidated financial statements.
In February 2016, the FASB issued ASU No. 2016-02, Leases. ASU 2016-02 establishes a right-of-use (ROU) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. While we are still evaluating the impact of our pending adoption of the new standard on our consolidated financial statements, we expect that upon adoption we will recognize ROU assets and lease liabilities and that the amounts could be material.
|
2. |
Liquidity |
All of our material assets, except our intellectual property, have been pledged as collateral for our borrowings under the Term Loan Agreement (the CRG Loan Agreement) with Capital Royalty Partners II L.P. (CRG). In addition to the security interest in our assets, the CRG Loan Agreement carries covenants that impose significant requirements on us, including, among others, requirements that we (1) pay all principal, interest and other charges on the outstanding balance of the borrowed funds when due; (2) maintain liquidity of at least $5 million during the term of the CRG Loan Agreement; and (3) meet certain annual EBITDA or revenue targets ($22.5 million of Lymphoseek sales revenue in 2016) as defined in the CRG Loan Agreement. The events of default under the CRG Loan Agreement also include a failure of Platinum-Montaur Life Sciences LLC, an affiliate of Platinum Management (NY) LLC, Platinum Partners Value Arbitrage Fund L.P., Platinum Partners Liquid Opportunity Master Fund L.P., Platinum Liquid Opportunity Management (NY) LLC, and Montsant Partners LLC (collectively, Platinum) to perform its funding obligations under the Platinum Loan Agreement (as defined below) at any time as to which the Company had negative EBITDA for the most recent fiscal quarter, as a result either of Platinum’s repudiation of its obligations under the Platinum Loan Agreement, or the occurrence of an insolvency event with respect to Platinum. See Note 12.
We did not achieve the 2015 annual Lymphoseek sales revenue target of $11 million as initially established under the CRG Loan Agreement, but in December 2015 CRG agreed to a reduction of that target to $10 million and we were able to meet that reduced target with Lymphoseek sales revenue of $10.3 million, thereby complying with the covenant. It appears likely that we will need to draw on the Platinum line of credit in order to maintain compliance with the $5 million liquidity covenant of the CRG Loan Agreement beginning in the second quarter of 2016. Our inability to meet the liquidity covenant would be an event of default under the CRG Loan Agreement. In addition, if we are unable to reach the 2016 annual Lymphoseek sales revenue target of $22.5 million, this would also be an event of default under the CRG Loan Agreement; however, potential shortfalls to this revenue covenant are curable by the Company depositing 2.5 times the amount of the shortfall in a bank account controlled by CRG. The events of default under the CRG Loan Agreement also include a failure of Platinum to perform its funding obligations under the Platinum Loan Agreement at any time as to which the Company had negative EBITDA for the most recent fiscal quarter, as a result either of Platinum’s repudiation of its obligations under the Platinum Loan Agreement, or the occurrence of an insolvency event with respect to Platinum. Our ability to comply with these provisions may be affected by changes in our business condition or results of our operations, or other events beyond our control. An event of default would entitle CRG to accelerate the maturity of our indebtedness, increase the interest rate to the default rate of 18% per annum, and invoke other remedies available to it under the loan agreement and the related security agreement, which could raise substantial doubt about the Company’s ability to continue as a going concern.
In addition, our Loan Agreement with Platinum (the Platinum Loan Agreement) carries standard non-financial covenants typical for commercial loan agreements, many of which are similar to those contained in the CRG Loan Agreement, that impose significant requirements on us. Our ability to comply with these provisions may be affected by changes in our business condition or results of our operations, or other events beyond our control. The breach of any of these covenants would result in
F-12
a default under the Platinum Loan Agreement, permitting Platinum to terminate our ability to obtain additional draws under the Platinum Loan Agreement and accelerate the maturity of the debt, subject to the limitations of the Subordination Agreement with CRG. Such actions by Platinum could materially adversely affect our operations, results of operations and financial condition, including causing us to substantially curtail our product development activities. We are currently in compliance with all covenants under the Platinum Loan Agreement. See Note 12.
|
3. |
Fair Value Hierarchy |
Platinum has the right to convert all or any portion of the unpaid principal or unpaid interest accrued on all draws under the Platinum credit facility, under certain circumstances. Platinum’s debt instrument, including the embedded option to convert such debt into common stock, is recorded at fair value on the consolidated balance sheets. The estimated fair value of the Platinum notes payable is $11.5 million at December 31, 2015, and will continue to be measured on a recurring basis. See Note 12.
MT issued warrants to purchase MT Common Stock with certain characteristics including a net settlement provision that require the warrants to be accounted for as a derivative liability at fair value on the consolidated balance sheets. The estimated fair value of the MT warrants is $63,000 at December 31, 2015, and will continue to be measured on a recurring basis. See Notes 1(m) and 9.
The following tables set forth, by level, financial liabilities measured at fair value on a recurring basis:
|
Liabilities Measured at Fair Value on a Recurring Basis as of December 31, 2015 |
|
|||||||||||||||
|
|
|
Quoted Prices in Active Markets for Identical Assets and Liabilities |
|
|
Significant Other Observable Inputs |
|
|
Significant Unobservable Inputs (a)(b) |
|
|
Balance as of December 31, |
|
||||
|
Description |
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
|
2015 |
|
||||
|
Platinum notes payable |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
11,491,253 |
|
|
$ |
11,491,253 |
|
|
Liability related to MT warrants |
|
|
— |
|
|
|
— |
|
|
|
63,000 |
|
|
|
63,000 |
|
|
Liabilities Measured at Fair Value on a Recurring Basis as of December 31, 2014 |
|
|||||||||||||||
|
|
|
Quoted Prices in Active Markets for Identical Assets and Liabilities |
|
|
Significant Other Observable Inputs |
|
|
Significant Unobservable Inputs (a)(b) |
|
|
Balance as of December 31, |
|
||||
|
Description |
|
(Level 1) |
|
|
(Level 2) |
|
|
(Level 3) |
|
|
2014 |
|
||||
|
Platinum notes payable |
|
$ |
— |
|
|
$ |
— |
|
|
$ |
5,615,764 |
|
|
$ |
5,615,764 |
|
|
|
a. |
Valuation Processes-Level 3 Measurements: The Company utilizes third-party valuation services that use complex models such as Monte Carlo simulation to estimate the value of our financial liabilities. Each reporting period, the Company provides significant unobservable inputs to the third-party valuation experts based on current internal estimates and forecasts. |
|
|
|
|
b. |
Sensitivity Analysis-Level 3 Measurements: Changes in the Company’s current internal estimates and forecasts are likely to cause material changes in the fair value of certain liabilities. The significant unobservable inputs used in the fair value measurement of the liabilities include the amount and timing of future draws expected to be taken under the Platinum Loan Agreement based on current internal forecasts and management’s estimate of the likelihood of actually making those draws as opposed to obtaining other sources of financing. Significant increases (decreases) in any of the significant unobservable inputs would result in a higher (lower) fair value measurement. A change in one of the inputs would not necessarily result in a directionally similar change in the others. |
There were no Level 1 liabilities outstanding at any time during the years ended December 31, 2015 and 2014. There were no transfers in or out of our Level 2 liabilities during the years ended December 31, 2015 and 2014. Changes in the estimated fair value of our Level 3 liabilities relating to unrealized gains (losses) are recorded as changes in the fair value of financial instruments in the consolidated statements of operations. The change in the estimated fair value of our Level 3 liabilities during the years ended December 31, 2015 and 2014 was an increase of $615,000 and $1.3 million, respectively.
F-13
For the years ended December 31, 2015, 2014 and 2013, our total stock-based compensation expense was approximately $2.4 million, $1.6 million and $2.9 million, respectively. We have not recorded any income tax benefit related to stock-based compensation for the years ended December 31, 2015, 2014 and 2013.
A summary of the status of our stock options as of December 31, 2015, and changes during the year then ended, is presented below:
|
|
|
Year Ended December 31, 2015 |
|
|||||||||||
|
|
|
Number of Options |
|
|
Weighted Average Exercise Price |
|
|
Weighted Average Remaining Contractual Life |
|
Aggregate Intrinsic Value |
|
|||
|
Outstanding at beginning of year |
|
|
5,345,764 |
|
|
$ |
2.16 |
|
|
|
|
|
|
|
|
Granted |
|
|
1,365,400 |
|
|
|
1.67 |
|
|
|
|
|
|
|
|
Exercised |
|
|
(146,625 |
) |
|
|
0.61 |
|
|
|
|
|
|
|
|
Canceled and forfeited |
|
|
(1,081,725 |
) |
|
|
2.82 |
|
|
|
|
|
|
|
|
Expired |
|
|
(45,750 |
) |
|
|
0.39 |
|
|
|
|
|
|
|
|
Outstanding at end of year |
|
|
5,437,064 |
|
|
$ |
1.96 |
|
|
7.2 years |
|
$ |
301,969 |
|
|
Exercisable at end of year |
|
|
2,512,049 |
|
|
$ |
2.14 |
|
|
5.6 years |
|
$ |
251,889 |
|
The weighted average grant-date fair value of options granted in 2015, 2014, and 2013 was $1.67, $1.56 and $1.81, respectively. During 2015, 146,625 stock options with an aggregate intrinsic value of $144,000 were exercised in exchange for issuance of 124,238 shares of our common stock, resulting in gross proceeds of $66,000. During 2014, 468,000 stock options with an aggregate intrinsic value of $582,000 were exercised in exchange for issuance of 299,360 shares of our common stock, resulting in gross proceeds of $70,000. During 2013, 60,000 stock options with an aggregate intrinsic value of $126,000 were exercised in exchange for issuance of 39,649 shares of our common stock, resulting in gross proceeds of $39,000. In 2015, 2014, and 2013, the aggregate fair value of stock options vested during the year was $277,000, $4,000 and $36,000, respectively.
A summary of the status of our unvested restricted stock as of December 31, 2015, and changes during the year then ended, is presented below:
|
|
|
Year Ended December 31, 2015 |
|
|||||
|
|
|
Number of Shares |
|
|
Weighted Average Grant-Date Fair Value |
|
||
|
Unvested at beginning of year |
|
|
498,250 |
|
|
$ |
1.91 |
|
|
Granted |
|
|
354,000 |
|
|
|
1.73 |
|
|
Forfeited |
|
|
(108,000 |
) |
|
|
2.71 |
|
|
Expired |
|
|
(50,000 |
) |
|
|
1.93 |
|
|
Vested |
|
|
(333,250 |
) |
|
|
1.69 |
|
|
Unvested at end of year |
|
|
361,000 |
|
|
$ |
1.69 |
|
During 2015, 2014 and 2013, 333,250, 216,250 and 745,000 shares, respectively, of restricted stock vested with aggregate vesting date fair values of $511,000, $387,000 and $2.3 million, respectively.
During 2015, 120,000 shares of restricted stock held by non-employee directors with an aggregate fair value of $193,000 vested as scheduled according to the terms of the restricted stock agreements. Also during 2015, 193,250 shares of restricted stock held by employees with an aggregate fair value of $286,000 vested as scheduled according to the terms of the restricted stock agreements. During 2015, 27,000 shares of restricted stock held by employees with an aggregate fair value of $50,000 were forfeited in connection with their separation from employment. In April 2015, 20,000 shares of restricted stock held by an executive officer with an aggregate fair value of $32,000 vested upon reaching a milestone as defined by the terms of the restricted stock agreement. In May 2015, 20,000 shares of restricted stock held by an executive officer with an aggregate fair value of $25,000 were forfeited in connection with his separation from employment. In July 2015, 61,000 shares of restricted stock held by non-employee directors with an aggregate fair value of $107,000 were forfeited as a result of their departures from the Board.
F-14
During 2014, 61,250 shares of restricted stock held by non-employee directors with an aggregate fair value of $111,000 vested as scheduled according to the terms of the restricted stock agreements. Also during 2014, 40,000 shares of restricted stock held by executive officers with an aggregate fair value of $52,000 vested upon reaching certain milestones as defined by the terms of the restricted stock agreements. In March 2014, 100,000 shares of restricted stock with an aggregate fair value of $205,000 vested as scheduled according to the terms of the restricted stock agreement. In May 2014, 175,000 shares of restricted stock held by our former CEO with an aggregate fair value of $278,000 were forfeited in connection with his separation from employment. In September 2014, 125,000 shares of restricted stock held by our former CEO with an aggregate fair value of $166,000 were forfeited in connection with termination of his Consulting Agreement. In December 2014, 15,000 shares of restricted stock held by our former CEO with an aggregate fair value of $19,000 vested as scheduled in accordance with the terms of the restricted stock agreement.
In February 2013, 100,000 shares of restricted stock with an aggregate fair value of $310,000 vested as scheduled according to the terms of a restricted stock agreement. In March 2013, the Company received FDA approval to market Lymphoseek. As a result of the Lymphoseek approval, 560,000 shares of restricted stock vested with an aggregate fair value of $1.8 million. In April 2013, 85,000 shares of restricted stock held by non-employee directors with an aggregate fair value of $224,000 vested as scheduled according to the terms of the restricted stock agreements. In July 2013, 29,250 shares of restricted stock with an aggregate fair value of $83,000 were forfeited as a result of a non-employee director departing from the Board.
During 2015, 2014 and 2013, we paid minimum tax withholdings related to stock options exercised and restricted stock vested of $24,000, $131,000, and $659,000, respectively. As of December 31, 2015, there was approximately $1.1 million of total unrecognized compensation cost related to stock option and restricted stock awards, which we expect to recognize over remaining weighted average vesting terms of 1.6 years. See Note 1(e).
|
5. |
Earnings Per Share |
Basic earnings (loss) per share is calculated by dividing net income (loss) attributable to common stockholders by the weighted-average number of common shares and, except for periods with a loss from operations, participating securities outstanding during the period. Diluted earnings (loss) per share reflects additional common shares that would have been outstanding if dilutive potential common shares had been issued. Potential common shares that may be issued by the Company include convertible debt, convertible preferred stock, options and warrants.
The following table sets forth the calculation of basic and diluted earnings (loss) per share for the years ended December 31, 2015, 2014 and 2013:
|
|
|
Years Ended December 31, |
|
|||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||
|
Net loss |
|
$ |
(27,563,535 |
) |
|
$ |
(35,726,669 |
) |
|
$ |
(42,699,458 |
) |
|
Less loss attributable to noncontrolling interest |
|
|
(855 |
) |
|
|
— |
|
|
|
— |
|
|
Deemed dividend on beneficial conversion feature of MT Preferred Stock |
|
|
(46,000 |
) |
|
|
— |
|
|
|
— |
|
|
Net loss attributable to common stockholders |
|
$ |
(27,608,680 |
) |
|
$ |
(35,726,669 |
) |
|
$ |
(42,699,458 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average shares outstanding (basic and diluted) |
|
|
151,180,222 |
|
|
|
148,748,396 |
|
|
|
121,808,986 |
|
|
Loss per common share (basic and diluted) |
|
$ |
(0.18 |
) |
|
$ |
(0.24 |
) |
|
$ |
(0.35 |
) |
Diluted earnings (loss) per common share for the years ended December 31, 2015, 2014 and 2013 excludes the effects of 14.6 million, 19.0 million and 32.2 million common share equivalents, respectively, since such inclusion would be anti-dilutive. The excluded shares consist of common shares issuable upon exercise of outstanding stock options and warrants, and upon the conversion of convertible debt and convertible preferred stock.
The Company’s unvested stock awards contain nonforfeitable rights to dividends or dividend equivalents, whether paid or unpaid (referred to as “participating securities”). Therefore, the unvested stock awards are required to be included in the number of shares outstanding for both basic and diluted earnings per share calculations. However, due to our loss from continuing operations, 361,000, 498,250 and 634,250 shares of unvested restricted stock for the years ended December 31, 2015, 2014 and 2013, respectively, were excluded in determining basic and diluted loss per share because such inclusion would be anti-dilutive.
F-15
Accounts and other receivables at December 31, 2015 and 2014 consist of the following:
|
|
|
2015 |
|
|
2014 |
|
||
|
Trade |
|
$ |
2,498,087 |
|
|
$ |
816,544 |
|
|
Other |
|
|
1,205,099 |
|
|
$ |
77,946 |
|
|
|
|
$ |
3,703,186 |
|
|
$ |
894,490 |
|
At December 31, 2015 and 2014, approximately 67% and 91%, respectively, of net accounts and other receivables were due from Cardinal Health. In addition, at December 31, 2015, approximately 32% of net accounts and other receivables were due from Devicor related to royalty amounts earned based on 2015 GDS Business revenue. As of December 31, 2015 and 2014, there was no allowance for doubtful accounts. We do not believe we are exposed to significant credit risk related to Cardinal Health or Devicor based on the overall financial strength and credit worthiness of the entities. We believe that we have adequately addressed other credit risks in estimating the allowance for doubtful accounts.
|
7. |
Inventory |
The components of net inventory at December 31, 2015 and 2014, net of reserves of $345,000 and $539,000, respectively, are as follows:
|
|
|
2015 |
|
|
2014 |
|
||
|
Materials |
|
$ |
330,000 |
|
|
$ |
— |
|
|
Work-in-process |
|
|
392,457 |
|
|
|
1,034,476 |
|
|
Finished goods |
|
|
275,168 |
|
|
|
436,936 |
|
|
Reserves |
|
|
(344,719 |
) |
|
|
(539,027 |
) |
|
|
|
$ |
652,906 |
|
|
$ |
932,385 |
|
During 2015 and 2014, we capitalized $1.1 million and $110,000, respectively, of inventory costs associated with our Lymphoseek product. During 2015 and 2014, we utilized $446,000 and $198,000, respectively, of previously capitalized Lymphoseek inventory due to the consumption of the Lymphoseek material for product testing and development purposes.
During 2015, we wrote off $120,000 of materials related to production issues. During 2015 and 2014, we recorded obsolescence reserves of $52,000 and $539,000 of Lymphoseek inventory related to specific lots that expired or were nearing product expiry and therefore were no longer expected to be sold.
|
8. |
Property and Equipment |
The major classes of property and equipment are as follows:
|
|
|
Useful Life |
|
2015 |
|
|
2014 |
|
||
|
Production machinery and equipment |
|
5 years |
|
$ |
1,427,472 |
|
|
$ |
1,446,551 |
|
|
Other machinery and equipment, primarily computers and research equipment |
|
3 – 5 years |
|
|
421,318 |
|
|
|
602,974 |
|
|
Furniture and fixtures |
|
7 years |
|
|
648,131 |
|
|
|
670,567 |
|
|
Software |
|
3 years |
|
|
476,530 |
|
|
|
476,530 |
|
|
Leasehold improvements* |
|
Term of Lease |
|
|
897,584 |
|
|
|
927,406 |
|
|
|
|
|
|
$ |
3,871,035 |
|
|
$ |
4,124,028 |
|
|
|
* |
We amortize leasehold improvements over the term of the lease, which in all cases is shorter than the estimated useful life of the asset. |
Property and equipment includes $9,000 of equipment under capital leases with accumulated amortization of $7,000 and $5,000 at December 31, 2015 and 2014, respectively. During 2015, 2014 and 2013, we recorded $562,000, $488,000 and $400,000, respectively, of depreciation and amortization related to property and equipment.
|
9. |
Investment in Macrophage Therapeutics, Inc. |
In March 2015, MT, our previously wholly-owned subsidiary, entered into a Securities Purchase Agreement to sell up to 50 shares of its Series A Convertible Preferred Stock (MT Preferred Stock) and warrants to purchase up to 1,500 common shares of MT (MT Common Stock) to Platinum and Dr. Michael Goldberg (collectively, the Investors) for a purchase price of $50,000 per unit. A unit consists of one share of MT Preferred Stock and 30 warrants to purchase MT Common Stock. Under the agreement, 40% of the MT Preferred Stock and warrants are committed to be purchased by Dr. Goldberg, and the balance by
F-16
Platinum. The full 50 shares of MT Preferred Stock and warrants that may be sold under the agreement are convertible into, and exercisable for, MT Common Stock representing an aggregate 1% interest on a fully converted and exercised basis. Navidea owns the remainder of the MT Common Stock. On March 11, 2015, definitive agreements with the Investors were signed for the sale of the first 10 shares of MT Preferred Stock and warrants to purchase 300 shares of MT Common Stock to the Investors, with gross proceeds to MT of $500,000. The MT Common Stock held by parties other than Navidea is reflected on the consolidated balance sheets as a noncontrolling interest.
The warrants have certain characteristics including a net settlement provision that require the warrants to be accounted for as a derivative liability at fair value, with subsequent changes in fair value included in earnings. The fair value of the warrants was estimated to be $63,000 at issuance and at December 31, 2015. See Notes 1(m) and 3. In addition, the MT Preferred Stock includes a beneficial conversion feature. The conversion option was immediately convertible upon issuance, resulting in a deemed dividend of $46,000 related to the beneficial conversion feature. Finally, certain provisions of the Securities Purchase Agreement obligate the Investors to acquire the remaining MT Preferred Stock and related warrants for $2.0 million at the option of MT. The estimated relative fair value of this put option was $113,000 at issuance based on the Black-Scholes option pricing model and is classified within stockholders' equity.
In addition, we entered into a Securities Exchange Agreement with the Investors providing them an option to exchange their MT Preferred Stock for our common stock in the event that MT has not completed a public offering with gross proceeds to MT of at least $50 million by the second anniversary of the closing of the initial sale of MT Preferred Stock, at an exchange rate per share obtained by dividing $50,000 by the greater of (i) 80% of the twenty-day volume weighted average price per share of our common stock on the second anniversary of the initial closing or (ii) $3.00. To the extent that the Investors do not timely exercise their exchange right, MT has the right to redeem their MT Preferred Stock for a price equal to $58,320 per share. We also granted MT an exclusive license for certain therapeutic applications of the Manocept technology.
|
10. |
Investment in R-NAV, LLC |
In July 2014, Navidea formed a joint enterprise with Essex Woodlands-backed Rheumco, LLC, to develop and commercialize radiolabeled diagnostic and therapeutic products for rheumatologic and arthritic diseases. The joint enterprise, called R-NAV, LLC, combines Navidea’s proprietary Manocept CD206 macrophage targeting platform and Rheumco’s proprietary Tin-117m radioisotope technology to focus on leveraging the platforms across several indications with high unmet medical need, including the detection and treatment of RA and veterinary osteoarthritis.
Both Rheumco and Navidea have contributed licenses for intellectual property and technology to R-NAV in exchange for common units in R-NAV. The contributions of these licenses were recorded using the carryover basis. R-NAV was initially capitalized through a $4.0 million investment from third-party private investors, and the technology contributions from Rheumco and Navidea. Navidea committed an additional $1.0 million investment to be paid over three years, with $333,334 in cash contributed at inception and a promissory note in the principal amount of $666,666, payable in two equal installments on the first and second anniversaries of the transaction. In exchange for its capital and in-kind investment, the Company received 1,000,000 Series A preferred units of R-NAV (Series A Units). The Company will receive an additional 500,000 Series A Units for management and technical services associated with the programs described above to be performed by the Company for R-NAV pursuant to a services agreement. The Series A Units are convertible into common units at the option of the holder for a conversion price of $1 per unit, subject to broad-based weighted average anti-dilution rights.
Navidea initially owned approximately 33.7% of the combined entity. At December 31, 2015, Navidea owns approximately 27.3% of R-NAV. Joint oversight over certain aspects of R-NAV is shared between Navidea and the other investors; Navidea does not control the operations of R-NAV. Navidea has three-year call options to acquire, at its sole discretion, all of the equity of R-NAV’s Subsidiary 1A (focused on diagnosis of human RA) for $10.5 million prior to the launch of a Phase 3 clinical trial for its development program, and all of the equity of R-NAV’s Subsidiary 1B (focused on treatment of human RA) at fair value upon completion of radiochemistry and biodistribution studies for its development program.
Navidea's investment in R-NAV is being accounted for using the equity method of accounting. In accordance with current accounting guidance, the Company's contributions of cash and note payable totaling $1.0 million were allocated between the investment in R-NAV and the call option on Subsidiary 1A based on the relative fair values of the assets. As a result, we recorded an initial equity investment in R-NAV of $727,000 and a call option asset of $273,000 as non-current assets at the time of the initial investment. Navidea's equity in the loss of R-NAV was $305,253 and $523,809 for the years ended December 31, 2015 and 2014, respectively. The Company's obligation to provide $500,000 of in-kind services to R-NAV is being recognized as those services are provided. The Company provided $64,000 and $39,000 of in-kind services during the years ended December 31, 2015 and 2014, respectively. As of December 31, 2015, the Company has $398,000 of in-kind services remaining to provide under this obligation. A principal payment of $333,333 was made on the note payable to R-NAV in July 2015. As of December 31, 2015, the outstanding principal balance of the note payable to R-NAV was $333,333. The final payment of $333,333 is due in July 2016. See Note 12.
F-17
Accrued liabilities and other at December 31, 2015 and 2014 consist of the following:
|
|
|
2015 |
|
|
2014 |
|
||
|
Contracted services |
|
$ |
1,887,281 |
|
|
$ |
1,880,376 |
|
|
Compensation |
|
|
873,726 |
|
|
|
979,296 |
|
|
Royalties |
|
|
175,679 |
|
|
|
73,000 |
|
|
Interest |
|
|
478 |
|
|
|
213,457 |
|
|
Other |
|
|
101,549 |
|
|
|
87,991 |
|
|
|
|
$ |
3,038,713 |
|
|
$ |
3,234,120 |
|
|
12. |
Notes Payable |
Platinum
In July 2012, we entered into an agreement with Platinum to provide us with a credit facility of up to $50 million. Following the approval of Lymphoseek, Platinum was committed under the terms of the agreement to extend up to $35 million in debt financing to the Company. The agreement also provided for Platinum to extend an additional $15 million on terms to be negotiated. Through June 25, 2013, we drew a total of $8.0 million under the original facility.
In June 2013, in connection with entering into the GECC/MidCap Loan Agreement (discussed below), the Company and Platinum entered into an Amendment to the Platinum Loan Agreement (the First Platinum Amendment). Concurrent with the execution of the First Platinum Amendment, the Company delivered an Amended and Restated Promissory Note (the First Amended Platinum Note) to Platinum, which amended and restated the original promissory note issued to Platinum, in the principal amount of up to $35 million. The First Amended Platinum Note also adjusted the interest rate to the greater of (a) the U.S. Prime Rate as reported in the Wall Street Journal plus 6.75%; (b) 10%; or (c) the highest rate of interest then payable pursuant to the GECC/MidCap Loan Agreement plus 0.125%. In addition, the First Platinum Amendment granted Platinum the right, at Platinum’s option subject to certain conditions, to convert all or any portion of the unpaid principal or unpaid interest accrued on any future draw (the Conversion Amount), beginning on a date two years from the date the draw is advanced, into the number of shares of Navidea’s common stock computed by dividing the Conversion Amount by a conversion price equal to the lesser of (i) 90% of the lowest VWAP for the 10 trading days preceding the date of such conversion request, or (ii) the average VWAP for the 10 trading days preceding the date of such conversion request. The First Platinum Amendment also provided a conversion right on the same terms with respect to the amount of any mandatory repayment due following the Company achieving $2.0 million in cumulative revenues from sales or licensing of Lymphoseek.
In accordance with current accounting standards, the First Platinum Amendment was treated as an extinguishment of debt. The difference between the fair value of the new debt and the carrying value of the original Platinum loan balance was recorded as loss on extinguishment of $943,000. Platinum’s option to convert future draws into common stock was determined to meet the definition of a liability. The fair value of the new debt included the estimated fair value of the embedded conversion option.
Also in connection with the First Platinum Amendment, the Company and Platinum entered into a Warrant Exercise Agreement (Exercise Agreement), pursuant to which Platinum exercised its Series X Warrant and Series AA Warrant. The warrants were exercised on a cashless basis by canceling a portion of the indebtedness outstanding under the Platinum Loan Agreement equal to $4.8 million, the aggregate exercise price of the warrants. Pursuant to the Exercise Agreement, in lieu of common stock, Platinum received on exercise of the warrants 2,364.9 shares of the Company’s Series B Convertible Preferred Stock (the Series B Preferred Stock), convertible into 7,733,223 shares of our common stock in the aggregate (3,270 shares of common stock per preferred share).
In March 2014, in connection with entering into the Oxford Loan Agreement (discussed below), we repaid all amounts outstanding under the GECC/MidCap Loan agreement and entered into a second amendment to the Platinum Loan Agreement (the Second Platinum Amendment). Concurrent with the execution of the Second Platinum Amendment, the Company delivered an Amended and Restated Promissory Note (the Second Amended Platinum Note) to Platinum, which amended and restated the First Amended Platinum Note. The Second Amended Platinum Note adjusted the interest rate to the greater of (i) the United States prime rate as reported in The Wall Street Journal plus 6.75%, (ii) 10.0%, and (iii) the highest rate of interest then payable by the Company pursuant to the Oxford Loan Agreement plus 0.125%.
In May 2015, in connection with the execution of the CRG Loan Agreement (discussed below), the Company amended the existing Platinum credit facility to allow this facility to remain in place in a subordinated role to the CRG Loan (the Third Platinum Amendment). Among other things, the Third Platinum Amendment (i) extends the term of the Platinum Loan Agreement until a date six months following the maturity date or earlier repayment of the CRG Term Loan; (ii) changes the
F-18
interest rate to the greater of (a) the United States prime rate as reported in The Wall Street Journal plus 6.75%, (b) 10.0% and (c) the highest rate of interest then payable pursuant to the CRG Term Loan plus 0.125% (the effective interest rate at December 31, 2015 was 14.125%); (iii) requires such interest to compound monthly; and (iv) changes the provisions of the Platinum Loan Agreement governing Platinum’s right to convert advances into common stock of the Company. The Third Platinum Amendment provides for the conversion of all principal and interest outstanding under the Platinum Loan Agreement, but not until such time as the average daily volume weighted average price of the Company’s common stock for the ten preceding trading days exceeds $2.53 per share. The amendment became effective upon initial funding of the CRG Loan Agreement.
The net change in the estimated fair value of the Amended Platinum Notes was $(615,000), $(1.3) million and $(106,000), which was recorded as non-cash change in fair value of financial instruments during the years ended December 31, 2015, 2014 and 2013, respectively. The estimated fair value of the Amended Platinum Notes was $11.5 million as of December 31, 2015.
The Platinum Loan Agreement carries standard non-financial covenants typical for commercial loan agreements, many of which are similar to those contained in the CRG Loan Agreement, that impose significant requirements on us. Our ability to comply with these provisions may be affected by changes in our business condition or results of our operations, or other events beyond our control. The breach of any of these covenants would result in a default under the Platinum Loan Agreement, permitting Platinum to terminate our ability to obtain additional draws under the Platinum Loan Agreement and accelerate the maturity of the debt, subject to the limitations of the Subordination Agreement with CRG. Such actions by Platinum could materially adversely affect our operations, results of operations and financial condition, including causing us to substantially curtail our product development activities. As of December 31, 2015, we were in compliance with all covenants under the Platinum Loan Agreement.
The Platinum Loan Agreement, as amended, provides us with a credit facility of up to $50 million. We drew a total of $4.5 million and $4.0 million under the credit facility in each of the years ended December 31, 2015 and 2013. We did not make any draws under the credit facility during the year ended December 31, 2014. In addition, $761,000 of interest was compounded and added to the balance of the Third Amended Platinum Note during the year ended December 31, 2015. As of December 31, 2015, the remaining outstanding principal balance of the Amended Platinum Notes was approximately $8.5 million, consisting of $7.7 million of draws and $761,000 of compounded interest, with $27.3 million still available under the credit facility. In March 2016, Platinum reaffirmed its obligations to make loans to the Company under the Platinum Loan Agreement.
Capital Royalty Partners II, L.P.
In May 2015, Navidea and MT, as guarantor, executed a Term Loan Agreement (the CRG Loan Agreement) with Capital Royalty Partners II L.P. (CRG) in its capacity as a lender and as control agent for other affiliated lenders party to the CRG Loan Agreement (collectively, the Lenders) in which the Lenders agreed to make a term loan to the Company in the aggregate principal amount of $50 million (the CRG Term Loan), with an additional $10 million in loans to be made available upon the satisfaction of certain conditions stated in the CRG Loan Agreement. Closing and funding of the CRG Term Loan occurred on May 15, 2015 (the Effective Date). The principal balance of the CRG Term Loan will bear interest from the Effective Date at a per annum rate of interest equal to 14.0%. Through March 31, 2019, the Company has the option of paying (i) 10.00% of the per annum interest in cash and (ii) 4.00% of the per annum interest as compounded interest which is added to the aggregate principal amount of the CRG Term Loan. During 2015, $1.3 million of interest was compounded and added to the balance of the CRG Term Loan. In addition, the Company began paying the cash portion of the interest in arrears on June 30, 2015. Principal is due in eight equal quarterly installments during the final two years of the term. All unpaid principal, and accrued and unpaid interest, is due and payable in full on March 31, 2021. As of December 31, 2015, the outstanding principal balance of the CRG Term Loan was $51.3 million.
The Company may voluntarily prepay the CRG Term Loan in full, upon fifteen business days’ prior written notice to the Lenders, with a prepayment premium beginning at 5% and declining by 1% annually thereafter, with no premium being payable if prepayment occurs after the fifth year of the term. The CRG Term Loan required the payment on the borrowing date of a financing fee of $625,000 which was recorded as a discount to the debt. In addition, a facility fee of $1.0 million is payable at the end of the term or when the loan is repaid in full. A long-term liability has been recorded for the $1.0 million facility fee with a corresponding discount to the debt. The CRG Term Loan is collateralized by a security interest in substantially all of the Company's assets.
The CRG Loan Agreement requires that the Company adhere to certain affirmative and negative covenants, including financial reporting requirements and a prohibition against the incurrence of indebtedness, or creation of additional liens, other than as specifically permitted by the terms of the CRG Loan Agreement. The Lenders may accelerate the payment terms of the CRG Loan Agreement upon the occurrence of certain events of default set forth therein, which include the failure of the Company to make timely payments of amounts due under the CRG Loan Agreement, the failure of the Company to adhere to the covenants set forth in the CRG Loan Agreement, and the insolvency of the Company. The covenants of the CRG Loan Agreement include a covenant that the Company shall have EBITDA of no less than $5 million in each calendar year during the term or revenues from sales of Lymphoseek in each calendar year during the term of at least $11 million in 2015 and $22.5 million in 2016, with
F-19
the target minimum revenue increasing in each year thereafter until reaching $45 million in 2020. However, if the Company were to fail to meet the applicable minimum EBITDA or revenue target in any calendar year, the CRG Loan Agreement provides the Company a cure right if it raises 2.5 times the EBITDA or revenue shortfall in equity or subordinated debt and deposits such funds in a separate blocked account. Effective December 23, 2015, the CRG Loan Agreement was amended to reduce the required minimum net revenue of the Company during 2015 to $10 million. Additionally, the Company must maintain liquidity, defined as the balance of unencumbered cash and permitted cash equivalent investments, of at least $5 million during the term of the CRG Term Loan. The events of default under the CRG Loan Agreement also include a failure of Platinum to perform its funding obligations under the Platinum Loan Agreement at any time as to which the Company had negative EBITDA for the most recent fiscal quarter, as a result either of Platinum’s repudiation of its obligations under the Platinum Loan Agreement, or the occurrence of an insolvency event with respect to Platinum. As of December 31, 2015, we were in compliance with all applicable covenants of the CRG Loan Agreement.
In connection with the CRG Loan Agreement, the Company recorded a debt discount related to lender fees and other costs directly attributable to the CRG Loan Agreement totaling $2.2 million. The debt discount is being amortized as non-cash interest expense using the effective interest method over the term of the CRG Loan Agreement. As of December 31, 2015, the balance of the debt discount was $2.0 million.
Oxford Finance, LLC
In March 2014, we executed a Loan and Security Agreement (the Oxford Loan Agreement) with Oxford Finance, LLC (Oxford), providing for a loan to the Company of $30 million. Pursuant to the Oxford Loan Agreement, we issued Oxford: (1) Term Notes in the aggregate principal amount of $30 million, bearing interest at 8.5% (the Oxford Notes), and (2) Series KK warrants to purchase an aggregate of 391,032 shares of our common stock at an exercise price of $1.918 per share, expiring in March 2021 (the Series KK warrants). The Company recorded a debt discount related to the issuance of the Series KK Warrants and other fees to the lenders totaling $3.0 million. Debt issuance costs directly attributable to the Oxford Loan Agreement, totaling $120,000, were recorded as an additional debt discount on the consolidated balance sheet on the closing date. The debt discounts were being amortized as non-cash interest expense using the effective interest method over the term of the Oxford Loan Agreement. The final payment fee of $2.4 million was recorded in other non-current liabilities on the consolidated balance sheet on the closing date.
We began making monthly payments of interest only on April 1, 2014, and monthly payments of principal and interest beginning April 1, 2015. In May 2015, in connection with the consummation of the CRG Loan Agreement, the Company repaid all amounts outstanding under the Oxford Loan Agreement. The payoff amount of $31.7 million included payments of $289,000 as a pre-payment fee and $2.4 million as an end-of-term final payment fee. As of December 31, 2015, the Oxford Notes were no longer outstanding. The Series KK warrants remained outstanding as of December 31, 2015.
General Electric Capital Corporation/MidCap Financial SBIC, LP
In June 2013, we executed a Loan and Security Agreement (the GECC/MidCap Loan Agreement) with General Electric Capital Corporation (GECC) and MidCap Financial SBIC, LP (MidCap), pursuant to which we issued GECC and MidCap: (1) Term Notes in the aggregate principal amount of $25 million, bearing interest at 9.83%, (the GECC/MidCap Notes), and (2) Series HH warrants to purchase an aggregate of 301,205 shares of our common stock at an exercise price of $2.49 per share, expiring in June 2023 (the Series HH warrants). The GECC/MidCap Loan Agreement provided for an interest-only period beginning on June 25, 2013 and expiring on June 30, 2014. The principal and interest was to be repaid in 30 equal monthly installments, payable on the first of each month following the expiration of the interest-only period, and one final payment in an amount equal to the entire remaining principal balance of the GECC/MidCap Notes on the maturity date. The outstanding balance of the debt was due December 23, 2016. On the date upon which the outstanding principal amount of the loan was paid in full, the Company was required to pay a non-refundable end-of-term fee equal to 4.0% of the original principal amount of the loan.
The Company recorded a debt discount related to the issuance of the Series HH warrants and other fees to the lenders totaling $1.9 million. Debt issuance costs directly attributable to the GECC/MidCap Loan Agreement totaled $881,000. The debt discount and debt issuance costs were being amortized as non-cash interest expense using the effective interest method over the term of the GECC/MidCap Loan Agreement. The final payment fee of $1.0 million was recorded in other non-current liabilities on the consolidated balance sheet on the closing date.
In March 2014, in connection with the consummation of the Oxford Loan Agreement (discussed below), we repaid all amounts outstanding under the GECC/MidCap Notes for a payoff amount of $26.7 million, which included payments of $500,000 as a pre-payment fee and $1.0 million as an end-of-term final payment fee, resulting in a loss on extinguishment of $2.6 million. As of December 31, 2015, the GECC/MidCap Notes were no longer outstanding. The Series HH warrants remained outstanding as of December 31, 2015.
F-20
In December 2011, we executed a Loan and Security Agreement (the Hercules Loan Agreement) with Hercules Technology II, L.P. (Hercules), pursuant to which we issued Hercules: (1) a Secured Term Promissory Note in the principal amount of $7,000,000 (the Hercules Note), and (2) Series GG warrants to purchase 333,333 shares of our common stock at an exercise price of $2.10 per share, expiring in December 2016 (the Series GG warrants).
During 2012, we paid $1.3 million of principal payments on the Hercules Note. During the period from January 1, 2013 through June 24, 2013, we paid and additional $1.3 million of principal payments on the Hercules Note. On June 25, 2013, the Company used a portion of the proceeds from the GECC/MidCap Notes (discussed below) to pay the remaining $4.4 million of principal outstanding on the Hercules Note, as well as a $250,000 end-of-term fee and a $66,000 early payment penalty in accordance with the terms of the Hercules Loan Agreement. We recorded a loss on extinguishment of the Hercules Note of $429,000, consisting of the write-off of the remaining unamortized discount of $187,000 and unamortized debt issuance costs of $176,000, as well as the early payment penalty of $66,000. As of December 31, 2015, the Hercules Note was no longer outstanding. The Series GG warrants remained outstanding as of December 31, 2015.
R-NAV, LLC
In July 2014, in connection with entering into the R-NAV joint enterprise, Navidea executed a promissory note in the principal amount of $666,666, payable in two equal installments on July 15, 2015 and July 15, 2016, the first and second anniversaries of the R-NAV transaction. The note bears interest at the applicable federal rate, currently 0.31% per annum, compounded annually. A principal payment of $333,333 was made on the note payable to R-NAV in July 2015. As of December 31, 2015, the outstanding principal balance of the note payable to R-NAV was $333,333. The final payment of $333,333 is due in July 2016. See Note 10.
Summary
During the years ended December 31, 2015, 2014 and 2013, we recorded interest expense of $6.9 million, $3.7 million and $2.8 million, respectively, related to our notes payable. Of those amounts, $493,000, $844,000 and $765,000, respectively, was non-cash in nature related to amortization of the debt discounts and deferred financing costs related to our notes payable. An additional $2.0 million of this interest expense was compounded and added to the balance of our notes payable during 2015.
Annual principal maturities of our notes payable are $333,000, $0, $0, $21.9 million, $29.2 million, and $26.5 million in 2016, 2017, 2018, 2019, 2020 and 2021, respectively.
|
13. |
Preferred Stock |
As discussed in Note 12, in June 2013, the Company and Platinum entered into a Warrant Exercise Agreement, pursuant to which Platinum exercised its Series X warrant and Series AA warrant for 2,364.9 shares of the Company's Series B Preferred Stock, convertible into 7,733,223 shares of our common stock in the aggregate.
During 2013, Platinum converted 1,737.9 shares of the Series B Preferred Stock into 5,682,933 shares of our common stock under the terms of the Series B Preferred Stock. During 2014, Platinum converted 4,422 shares of the Series B Preferred Stock into 14,459,940 shares of our common stock under the terms of the Series B Preferred Stock. In November 2014, we entered into a second Securities Exchange Agreement with Platinum, pursuant to which Platinum exchanged 4,499,520 shares of our common stock owned by Platinum for 1,376 shares of our Series B Preferred Stock.
In August 2015, we entered into a Securities Exchange Agreement with two investment funds managed by Platinum to exchange the 4,519 shares of Series B Preferred Stock held by them for twenty-year warrants to purchase common stock of the Company (the Series LL warrants). The Series B Preferred Stock was convertible into common stock at a conversion rate of 3,270 shares of common stock per share of Series B Preferred Stock resulting in an aggregate number of shares of common stock into which the Series B Preferred Stock was convertible of 14,777,130 shares. The exercise price of the Series LL warrants is $0.01 per share, and the total number of shares of common stock for which the Series LL warrants are exercisable is 14,777,130 shares. The Series LL warrants contain cashless exercise provisions, and the other economic terms are comparable to those of the Series B Preferred Stock, except that there is no liquidation preference associated with the Series LL warrants or shares issuable on the exercise thereof. The Securities Exchange Agreement also contains certain provisions that prohibit the payment of dividends, distributions of common stock or issuances of common stock at effective prices less than $1.35. There was no other consideration paid or received for the exchange. No gain or loss was recognized in our consolidated financial statements as a result of the exchange. The exchange transaction was entered into in connection with the filing of an application to list the Company’s common stock on the Tel Aviv Stock Exchange (TASE) in order to comply with a listing requirement of the TASE requiring that listed companies have only one class of equity securities issued and outstanding. Following the exchange, the Company has no shares of preferred stock outstanding.
F-21
|
|
a. |
Common Stock Public Offerings: In February 2013, we completed a public offering of 1,542,389 shares of the Company’s common stock at a price of $3.10 per share (the February 2013 Offering). The net proceeds to the Company were approximately $4.5 million after deducting expenses associated with the February 2013 Offering. In April 2013, we completed another public offering of 2,100,000 shares of the Company’s common stock at a price of $2.43 per share (the April 2013 Offering). The net proceeds to the Company were approximately $4.8 million after deducting expenses associated with the April 2013 Offering. The February 2013 and April 2013 Offerings were underwritten by Ladenburg Thalmann & Co. Inc. and were made pursuant to the Company’s existing effective shelf registration statement on Form S-3. |
In September 2013, we entered into a Securities Purchase Agreement with Crede CG III Ltd. (Crede) for a registered direct public offering of 10,563,381 shares of our common stock at a price of $2.84 per share for total gross proceeds of $30.0 million. In addition to the common stock, we issued Series JJ warrants to purchase 3,169,015 shares of our common stock at an exercise price of $3.83 per share, expiring on September 24, 2016. The net proceeds to the Company were approximately $28.8 million after deducting expenses associated with the Securities Purchase Agreement, including placement agent fees of $999,000 (3.3% of the gross proceeds). The common stock, warrants, and shares of common stock underlying the warrants were issued pursuant to the Company's existing effective shelf registration statement on Form S-3.
|
|
b. |
Stock Warrants: At December 31, 2015, there are 11.7 million warrants outstanding to purchase our common stock. The warrants are exercisable at prices ranging from $0.01 to $3.04 per share with a weighted average exercise price per share of $0.38. |
The following table summarizes information about our outstanding warrants at December 31, 2015:
|
|
|
Exercise Price |
|
|
|
Number of Warrants |
|
|
Expiration Date |
||
|
Series BB |
|
$ |
2.00 |
|
|
|
|
300,000 |
|
|
July 2018 |
|
Series GG |
|
|
2.10 |
|
|
|
|
333,333 |
|
|
December 2016 |
|
Series HH |
|
|
2.49 |
|
|
|
|
301,205 |
|
|
June 2023 |
|
Series II |
|
|
3.04 |
|
|
|
|
275,000 |
|
|
June 2018 |
|
Series KK |
|
|
1.918 |
|
|
|
|
391,032 |
|
|
March 2021 |
|
Series LL |
|
|
0.01 |
|
|
|
|
9,777,130 |
|
|
August 2035 |
|
Series MM |
|
|
2.50 |
|
|
|
|
150,000 |
|
|
September 2019 |
|
Series MM |
|
|
2.50 |
|
|
|
|
150,000 |
|
|
October 2019 |
|
|
|
$ |
0.38 |
|
* |
|
|
11,677,700 |
|
|
|
|
|
* |
Weighted average exercise price. |
In addition, at December 31, 2015, there are 300 warrants outstanding to purchase MT Common Stock. The warrants are exercisable at $2,000 per share.
In March 2013, Platinum exercised 3,000,000 of their Series X warrants in exchange for the issuance of 3,000,000 shares of our common stock, resulting in gross proceeds of $1,380,000.
In June 2013, pursuant to the Exercise Agreement, Platinum exercised its Series X warrant and Series AA warrant for 2,364.9 shares of the Company’s Series B which were convertible into 7,733,223 shares of our common stock in the aggregate (3,270 shares of common stock per preferred share). The warrants were exercised on a cashless basis by canceling a portion of the indebtedness outstanding under the Platinum Loan Agreement equal to $4,781,333, the aggregate exercise price of the warrants.
Also in June 2013 and pursuant to the GECC/MidCap Loan Agreement, the Company issued to GECC/MidCap Series HH warrants to purchase an aggregate of 301,205 shares of our common stock at an exercise price of $2.49 per share, expiring in June 2023.
In addition, in June 2013 we issued five -year Series II warrants to purchase 275,000 shares of our common stock at an exercise price of $3.04 per share to an investment advisory firm in connection with the GECC transaction.
In September 2013, in connection with the Crede Securities Purchase Agreement, the Company issued to Crede Series JJ warrants to purchase 3,169,015 shares of our common stock at an exercise price of $3.83 per share, expiring in September 2016. Crede could exercise the Series JJ warrants at any time at a strike price of $3.83. The warrant agreement also provided for the potential exchange of the warrant into Navidea common stock for no additional consideration starting six months after the date of the Securities Purchase Agreement if, at the time of the exchange, the closing bid price for
F-22
Navidea’s common stock was below $3.83. The amount of shares issuable on exchange was based on dividing a Black-Scholes valuation of the warrants by the closing bid price for Navidea’s common stock on the date of the exchange. In November 2014, Crede exchanged their Series JJ warrants for 3,843,223 shares of our common stock in accordance with the terms of the Series JJ warrant agreement. As a result of the exchange of the Series JJ warrants, we reclassified $7.7 million in derivative liabilities related to those warrants to additional paid-in capital.
In March 2014, in connection with the Oxford Loan Agreement, the Company issued Series KK warrants to purchase an aggregate of 391,032 shares of our common stock at an exercise price of $1.918 per share, expiring in March 2021.
In July 2015, we extended the expiration date of our outstanding Series BB warrants by three years to July 2018. The modification of the Series BB warrant expiry resulted in recording a non-cash selling, general and administrative expense of approximately $150,000 during the third quarter of 2015.
In October 2015, 5,000,000 Series LL warrants were exercised on a cashless basis in exchange for the issuance of 4,977,679 shares of our common stock.
In September 2015, we issued four-year Series MM warrants to purchase 150,000 shares of our common stock at an exercise price of $2.50 per share pursuant to an advisory services agreement with Chardan Capital Markets, LLC (Chardan). In October 2015, we issued additional four-year Series MM warrants to purchase 150,000 shares of our common stock at an exercise price of $2.50 per share pursuant to the advisory services agreement with Chardan. The fair value of the warrants issued to Chardan of $256,000 was recorded as a non-cash selling, general and administrative expense during the third quarter of 2015.
|
|
c. |
Common Stock Reserved: As of December 31, 2015, we have reserved 24,365,285 shares of authorized common stock for the exercise of all outstanding stock options and warrants, and upon the conversion of convertible debt and convertible preferred stock. |
|
15. |
Reductions in Force |
In May 2014, the Company’s Board of Directors made the decision to refocus the Company's resources to better align the funding of our pipeline programs with the expected growth in Lymphoseek revenue. As a part of the realignment, the Company terminated a total of 11 employees, including the Chief Executive Officer, Dr. Mark J. Pykett.
Effective May 30, 2014, the Company and Dr. Pykett entered into a Separation Agreement and Release. Following the termination date, Dr. Pykett was entitled to receive a $750,000 severance payment, payable in two equal installments on June 9, 2014, and January 2, 2015, respectively; a single payment for accrued vacation and personal days; and reimbursement for certain other expenses and fees. Certain of Dr. Pykett's equity awards terminated upon separation, while others were modified in conjunction with the Separation Agreement and the Consulting Agreement described below.
Effective June 1, 2014, the Company and Dr. Pykett entered into a Consulting Agreement pursuant to which Dr. Pykett was to serve as an independent consultant to the Company until December 31, 2014 with respect to clinical-regulatory activities, commercial activities, program management, and business development, among other services. Dr. Pykett was entitled to a consulting fee of $27,500 per month plus reimbursement of reasonable expenses. The Consulting Agreement also provided for a grant of 40,000 shares of restricted stock which were to vest upon certain service and performance conditions.
Dr. Pykett terminated the Consulting Agreement effective September 8, 2014. Certain of Dr. Pykett's equity awards were forfeited upon termination of the Consulting Agreement, while others vested on December 1, 2014 due to achievement of certain goals during the period of the Consulting Agreement, in accordance with the terms of the award agreements. The Company recognized expenses of $94,000 under the Consulting Agreement during the year ended December 31, 2014.
During the year ended December 31, 2014, the Company recognized approximately $557,000 of net expense as a result of the reduction in force, which included separation costs, incremental expense related to the modification of certain equity awards, and the reversal of stock compensation expense for certain equity awards for which the requisite service was not rendered.
The Company appointed Michael M. Goldberg, M.D., as interim Chief Executive Officer effective May 30, 2014. Dr. Goldberg currently serves as a member of the Board of Directors of the Company and did not receive any salary for his service as interim Chief Executive Officer, although the Company agreed to pay Montaur Capital Partners, LLC (Montaur), where Dr. Goldberg is principal, $15,000 per month to cover additional costs and resources Montaur expected to incur or redirect due to the unavailability of Dr. Goldberg's services resulting from his service as interim Chief Executive Officer of Navidea. During the year ended December 31, 2014, the Company paid Montaur a total of $53,000. Dr. Goldberg's service as interim Chief Executive Officer terminated with the appointment of Ricardo J. Gonzalez as the Company's Chief Executive Officer effective October 13, 2014.
F-23
In March 2015, the Company initiated a second reduction in force that included seven staff members and three executives. The executives continued as employees during transition periods of varying lengths, depending upon the nature and extent of responsibilities transitioned or wound down.
During the year ended December 31, 2015, the Company recognized approximately $1.3 million of net expense as a result of the reduction in force, which includes actual and estimated separation costs as well as the impact of accelerated vesting or forfeiture of certain equity awards resulting from the separation of $273,000.
The remaining accrued separation costs of $9,000 and $449,000 at December 31, 2015 and 2014, respectively, related to the Company's reductions in force represent the estimated cost of continuing healthcare coverage and separation payments, and are included in accrued liabilities and other on the consolidated balance sheets.
|
16. |
Income Taxes |
As of December 31, 2015 and 2014, our deferred tax assets were approximately $74.2 million and $63.2 million, respectively. The components of our deferred tax assets are summarized as follows:
|
|
|
As of December 31, |
|
|||||
|
|
|
2015 |
|
|
2014 |
|
||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards |
|
$ |
60,129,827 |
|
|
$ |
50,117,798 |
|
|
R&D credit carryforwards |
|
|
9,465,900 |
|
|
|
8,394,408 |
|
|
Stock compensation |
|
|
1,898,394 |
|
|
|
1,849,847 |
|
|
Intangibles |
|
|
1,921,934 |
|
|
|
1,931,193 |
|
|
Temporary differences |
|
|
801,002 |
|
|
|
910,354 |
|
|
Deferred tax assets before valuation allowance |
|
|
74,217,057 |
|
|
|
63,203,600 |
|
|
Valuation allowance |
|
|
(74,217,057 |
) |
|
|
(63,203,600 |
) |
|
Net deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
Current accounting standards require a valuation allowance against deferred tax assets if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets may not be realized. Due to the uncertainty surrounding the realization of these deferred tax assets in future tax returns, all of the deferred tax assets have been fully offset by a valuation allowance at December 31, 2015 and 2014.
In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities (including the impact of available carryback and carryforward periods), projected future taxable income, and tax-planning strategies in making this assessment. Based upon the level of historical taxable income and projections for future taxable income over the periods in which the deferred tax assets are deductible, management believes it is more likely than not that the Company will not realize the benefits of these deductible differences or tax carryforwards as of December 31, 2015.
As of December 31, 2015 and 2014, we had U.S. net operating loss carryforwards of approximately $177.6 million and $153.1 million, respectively. Of those amounts, $15.3 million and $15.3 million relates to stock-based compensation tax deductions in excess of book compensation expense (APIC NOLs) as of December 31, 2015 and 2014, respectively, that will be credited to additional paid-in capital when such deductions reduce taxes payable as determined on a "with-and-without" basis. Accordingly, these APIC NOLs will reduce federal taxes payable if realized in future periods, but NOLs related to such benefits are not included in the table above.
At December 31, 2015 and 2014, we had U.S. R&D credit carryforwards of approximately $9.1 million and $8.0 million, respectively.
F-24
There were no expirations of U.S. net operating loss carryforwards or R&D credit carryforwards during 2015 or 2014. The details of our U.S. net operating loss and federal R&D credit carryforward amounts and expiration dates are summarized as follows:
|
|
|
|
|
As of December 31, 2015 |
|
|||||
|
Generated |
|
Expiration |
|
U.S. Net Operating Loss Carryforwards |
|
|
U.S. R&D Credit Carryforwards |
|
||
|
1998 |
|
2018 |
|
$ |
17,142,781 |
|
|
$ |
1,173,387 |
|
|
1999 |
|
2019 |
|
|
— |
|
|
|
130,359 |
|
|
2000 |
|
2020 |
|
|
— |
|
|
|
71,713 |
|
|
2001 |
|
2021 |
|
|
— |
|
|
|
39,128 |
|
|
2002 |
|
2022 |
|
|
1,282,447 |
|
|
|
5,350 |
|
|
2003 |
|
2023 |
|
|
337,714 |
|
|
|
2,905 |
|
|
2004 |
|
2024 |
|
|
1,237,146 |
|
|
|
22,861 |
|
|
2005 |
|
2025 |
|
|
2,999,083 |
|
|
|
218,332 |
|
|
2006 |
|
2026 |
|
|
3,049,735 |
|
|
|
365,541 |
|
|
2007 |
|
2027 |
|
|
2,842,078 |
|
|
|
342,898 |
|
|
2008 |
|
2028 |
|
|
2,777,503 |
|
|
|
531,539 |
|
|
2009 |
|
2029 |
|
|
13,727,950 |
|
|
|
596,843 |
|
|
2010 |
|
2030 |
|
|
5,397,680 |
|
|
|
1,094,449 |
|
|
2011 |
|
2031 |
|
|
1,875,665 |
|
|
|
1,950,744 |
|
|
2012 |
|
2032 |
|
|
28,406,659 |
|
|
|
468,008 |
|
|
2013 |
|
2033 |
|
|
37,450,522 |
|
|
|
374,406 |
|
|
2014 |
|
2034 |
|
|
34,088,874 |
|
|
|
648,252 |
|
|
2015 |
|
2035 |
|
|
24,971,225 |
|
|
|
1,087,317 |
|
|
Total carryforwards |
|
$ |
177,587,062 |
|
|
$ |
9,124,032 |
|
||
As of December 31, 2015 and 2014, we also had state net operating loss carryforwards of approximately $24.7 million and $16.9 million, respectively. The state net operating loss carryforwards will begin expiring in 2032.
As of December 31, 2015 and 2014, Cardiosonix had tax loss carryforwards in Israel of approximately $7.6 million. Under current Israeli tax law, net operating loss carryforwards do not expire. Due to the uncertainty surrounding the realization of the related deferred tax assets in future tax returns, all of the deferred tax assets have been fully offset by a valuation allowance at December 31, 2015 and 2014.
Under Sections 382 and 383 of the IRC of 1986, as amended, the utilization of U.S. net operating loss and R&D tax credit carryforwards may be limited under the change in stock ownership rules of the IRC. The Company previously completed a Section 382 analysis in 2013 and does not believe a Section 382 ownership change has occurred since then that would impact utilization of the Company’s net operating loss and R&D tax credit carryforwards.
Reconciliations between the statutory federal income tax rate and our effective tax rate for continuing operations are as follows:
|
|
|
Years Ended December 31, |
|
|||||||||||||||||||||
|
|
|
2015 |
|
|
2014 |
|
|
2013 |
|
|||||||||||||||
|
|
|
Amount |
|
|
% |
|
|
Amount |
|
|
% |
|
|
Amount |
|
|
% |
|
||||||
|
Benefit at statutory rate |
|
$ |
(9,777,786 |
) |
|
|
(34.0 |
)% |
|
$ |
(12,147,068 |
) |
|
|
(34.0 |
)% |
|
$ |
(14,517,816 |
) |
|
|
(34.0 |
)% |
|
Adjustments to valuation allowance |
|
|
9,728,667 |
|
|
|
33.8 |
% |
|
|
12,925,380 |
|
|
|
36.2 |
% |
|
|
15,399,021 |
|
|
|
36.1 |
% |
|
Adjustments to R&D credit carryforwards |
|
|
(612,087 |
) |
|
|
(2.1 |
)% |
|
|
(340,886 |
) |
|
|
(1.0 |
)% |
|
|
(842,414 |
) |
|
|
(2.0 |
)% |
|
Disqualified debt interest |
|
|
438,007 |
|
|
|
1.5 |
% |
|
|
— |
|
|
|
0.0 |
% |
|
|
— |
|
|
|
0.0 |
% |
|
Permanent items and other |
|
|
(212,852 |
) |
|
|
(0.7 |
)% |
|
|
(437,426 |
) |
|
|
(1.2 |
)% |
|
|
(38,791 |
) |
|
|
(0.1 |
)% |
|
Benefit per financial statements |
|
$ |
(436,051 |
) |
|
|
|
|
|
$ |
— |
|
|
|
|
|
|
$ |
— |
|
|
|
|
|
F-25
We report information about our operating segments using the “management approach” in accordance with current accounting standards. This information is based on the way management organizes and reports the segments within the enterprise for making operating decisions and assessing performance. Our reportable segments are identified based on differences in products, services and markets served. There were no inter-segment sales. Prior to 2015, our products and development programs were all related to diagnostic substances. Our majority-owned subsidiary, Macrophage Therapeutics, Inc., was formed and received initial funding during the first quarter of 2015, which resulted in a re-evaluation of the Company's segment determination. We now manage our business based on two primary types of drug products: (i) diagnostic substances, including Lymphoseek and other diagnostic applications of our Manocept platform, our R-NAV subsidiary, NAV4694 and NAV5001 (license terminated in April 2015), and (ii) therapeutic development programs, including therapeutic applications of our Manocept platform and all development programs undertaken by Macrophage Therapeutics, Inc.
The information in the following tables is derived directly from each reportable segment’s financial reporting.
|
Year Ended December 31, 2015 |
|
Diagnostics |
|
|
Therapeutics |
|
|
Corporate |
|
|
Total |
|
||||
|
Lymphoseek sales revenue: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
United States (1) |
|
$ |
10,229,659 |
|
|
$ |
— |
|
|
$ |
— |
|
|
$ |
10,229,659 |
|
|
International |
|
|
24,693 |
|
|
|
— |
|
|
|
— |
|
|
|
24,693 |
|
|
Lymphoseek license revenue |
|
|
1,133,333 |
|
|
|
— |
|
|
|
— |
|
|
|
1,133,333 |
|
|
Grant and other revenue |
|
|
1,861,622 |
|
|
|
— |
|
|
|
— |
|
|
|
1,861,622 |
|
|
Total revenue |
|
|
13,249,307 |
|
|
|
— |
|
|
|
— |
|
|
|
13,249,307 |
|
|
Cost of goods sold, excluding depreciation and amortization |
|
|
1,654,800 |
|
|
|
— |
|
|
|
— |
|
|
|
1,654,800 |
|
|
Research and development expenses, excluding depreciation and amortization |
|
|
12,046,221 |
|
|
|
730,895 |
|
|
|
— |
|
|
|
12,777,116 |
|
|
Selling, general and administrative expenses, excluding depreciation and amortization (2) |
|
|
5,852,214 |
|
|
|
123,884 |
|
|
|
10,820,392 |
|
|
|
16,796,490 |
|
|
Depreciation and amortization (3) |
|
|
281,314 |
|
|
|
— |
|
|
|
290,105 |
|
|
|
571,419 |
|
|
Loss from operations (4) |
|
|
(6,585,242 |
) |
|
|
(854,779 |
) |
|
|
(11,110,497 |
) |
|
|
(18,550,518 |
) |
|
Other income (expense), excluding equity in the loss of R-NAV, LLC (5) |
|
|
— |
|
|
|
— |
|
|
|
(9,902,424 |
) |
|
|
(9,902,424 |
) |
|
Equity in the loss of R-NAV, LLC |
|
|
— |
|
|
|
— |
|
|
|
(305,253 |
) |
|
|
(305,253 |
) |
|
Benefit from income taxes |
|
|
— |
|
|
|
— |
|
|
|
436,051 |
|
|
|
436,051 |
|
|
Net loss from continuing operations |
|
|
(6,585,242 |
) |
|
|
(854,779 |
) |
|
|
(20,882,123 |
) |
|
|
(28,322,144 |
) |
|
Income from discontinued operations, net of tax effect (6) |
|
|
— |
|
|
|
— |
|
|
|
758,609 |
|
|
|
758,609 |
|
|
Net loss |
|
|
(6,585,242 |
) |
|
|
(854,779 |
) |
|
|
(20,123,514 |
) |
|
|
(27,563,535 |
) |
|
Total assets, net of depreciation and amortization: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
United States |
|
|
3,948,971 |
|
|
|
— |
|
|
|
10,603,863 |
|
|
|
14,552,834 |
|
|
International |
|
|
410,666 |
|
|
|
— |
|
|
|
1,013 |
|
|
|
411,679 |
|
|
Capital expenditures |
|
|
26,589 |
|
|
|
— |
|
|
|
12,412 |
|
|
|
39,001 |
|
|
|
(1) |
All sales to Cardinal Health are made in the United States; Cardinal distributes the product throughout the U.S. through its network of nuclear pharmacies. |
|
|
(2) |
General and administrative expenses, excluding depreciation and amortization, represent costs that relate to the general administration of the Company and as such are not currently allocated to our individual reportable segments. Marketing and selling expenses are allocated to our individual reportable segments. |
|
|
(3) |
Depreciation and amortization is reflected in cost of goods sold ($99,963 for the year ended December 31, 2015), research and development ($10,617 for the year ended December 31, 2015), and selling, general and administrative expenses ($460,839 for the year ended December 31, 2015). |
|
|
(4) |
Loss from operations does not reflect the allocation of certain selling, general and administrative expenses, excluding depreciation and amortization, to our individual reportable segments. |
|
|
(5) |
Amounts consist primarily of interest income, interest expense, changes in fair value of financial instruments, and losses on debt extinguishment, which are not currently allocated to our individual reportable segments. |
|
|
(6) |
Amount of contingent consideration recognized related to 2015 GDS Business revenue royalties pursuant to the 2011 sale of the GDS Business to Devicor, net of tax effect. See Note 1(a). |
F-26
|
|
a. |
Supply Agreements: In November 2009, we entered into a manufacture and supply agreement with Reliable Biopharmaceutical Corporation (Reliable) for the manufacture and supply of the Lymphoseek drug substance. The initial ten-year term of the agreement expires in November 2019, with options to extend the agreement for successive three-year terms. Either party has the right to terminate the agreement upon mutual written agreement, or upon material breach by the other party which is not cured within 60 days from the date of written notice of the breach. Total purchases under the manufacture and supply agreement were $225,000, $300,000, and $666,000 for the years ended December 31, 2015, 2014 and 2013. As of December 31, 2015, we have issued purchase orders under the manufacture and supply agreement with Reliable for $1.1 million of Lymphoseek drug substance for delivery through March 2016. |
In May 2013, we entered into a clinical supply agreement with Nordion (Canada), Inc. (Nordion) for the manufacture and supply of NAV5001 clinical trial material. The initial three-year term expires in May 2016. Navidea may terminate this agreement without cause or penalty upon 180 days prior written notice to Nordion. Upon such termination, Nordion will be entitled to retain all amounts paid by Navidea, and Navidea shall pay to Nordion any undisputed, unpaid amounts due or earned by Nordion. Either party may terminate upon material breach by the other party which is not cured within 30 days from the date of written notice of the breach. In August 2014, in connection with the Company’s decision to refocus its resources, the Nordion agreement was amended to provide for a suspension period during which the Company is to pay a monthly fee to maintain production space at Nordion’s facility until such time as manufacture may resume. Total purchases under the clinical supply agreement were $244,000, $505,000 and $771,000 for the years ended December 31, 2015, 2014 and 2013. As of December 31, 2015, we have issued purchase orders under the agreement with Nordion for approximately $150,000 to maintain production space in Nordion’s facility through June 2016.
In August 2013, we entered into a manufacturing services agreement with PETNET Solutions, Inc. (PETNET) for the manufacture and distribution of NAV4694. The initial three-year term of the agreement expires in August 2016. The agreement will automatically renew for additional one-year terms, unless either party gives written notice to the other at least 60 days prior to the end of the initial term. Either party may terminate upon material breach by the other party which is not cured within 30 days from the date of written notice of the breach. Total purchases under the manufacturing agreement were $855,000, $2.2 million and $1.4 million for the years ended December 31, 2015, 2014 and 2013. As of December 31, 2015, we have issued purchase orders under the agreement with PETNET for $132,000 of our products for delivery through February 2016.
In September 2013, we entered into a manufacturing services agreement with OSO BioPharmaceuticals Manufacturing, LLC (OsoBio) for contract pharmaceutical development, manufacturing, packaging and analytical services for Lymphoseek. The initial term of the agreement expires in December 2016, and automatically renews for additional two-year periods unless written notice is provided at least 12 months prior to the expiration of the initial term. Either party has the right to terminate the agreement upon mutual written agreement, or upon material breach by the other party which is not cured within 60 days from the date of written notice of the breach. During the term of agreement, OsoBio will be the primary supplier of the manufacturing services for Lymphoseek. In consideration for these services, the Company will pay a unit pricing fee. In addition, the Company will also pay OsoBio a fee for regulatory and other support services. Total purchases under the manufacturing services agreement were $472,000, $96,000 and $1.2 million for the years ended December 31, 2015, 2014 and 2013. As of December 31, 2015, we have issued purchase orders under the agreement with OsoBio for $680,000 of our products for delivery through March 2016.
Also in September 2013, we completed a service and supply master agreement with Gipharma S.r.l. (Gipharma) for process development, manufacturing and packaging of reduced-mass vials to be sold in the EU. The agreement has an initial term of three years and automatically renews for additional one-year periods unless written notice is provided at least six months prior to the expiration of the current term. Navidea may terminate the agreement for any reason by providing 60 days prior written notice. Either party may terminate the agreement upon material breach which is not cured within 30 days from the date of written notice of the breach, or upon written notice following the other party’s dissolution or cessation of normal business. In consideration for these services, the Company will pay fees as defined in the agreement. Total purchases under the service and supply master agreement were $677,000, $272,000 and $34,000 for the years ended December 31, 2015, 2014 and 2013, respectively. As of December 31, 2015, we have issued purchase orders under the agreement with Gipharma for $241,000 of services for delivery through January 2016.
F-27
|
|
consideration for the license rights, we agreed to pay UCSD a license issue fee of $25,000 and license maintenance fees of $25,000 per year. We also agreed to make payments to UCSD upon successfully reaching certain clinical, regulatory and cumulative sales milestones, and a royalty on net sales of licensed products subject to a $25,000 minimum annual royalty. In addition, we agreed to reimburse UCSD for all patent-related costs and to meet certain diligence targets. Total costs related to the UCSD license agreement for Lymphoseek were $777,000, $353,000 and $273,000 in 2015, 2014 and 2013, respectively. Royalties on net sales of Lymphoseek were recorded in cost of goods sold, license maintenance fees and patent-related costs were recorded in research and development expenses, and sublicense fees were recorded in selling, general and administrative expenses. |
In April 2008, we completed a second license agreement with UCSD for an expanded field of use allowing Lymphoseek to be developed as an optical or ultrasound agent. The license agreement was effective until the expiration date of the longest-lived underlying patent. Under the terms of the license agreement, UCSD granted us the exclusive rights to make, use, sell, offer for sale and import licensed products as defined in the agreement and to practice the defined licensed methods during the term of the agreement. We could also sublicense the patent rights, subject to certain sublicense terms as defined in the agreement. In consideration for the license rights, we agreed to pay UCSD a license issue fee of $25,000 and license maintenance fees of $25,000 per year. We also agreed to make payments to UCSD upon successfully reaching certain clinical, regulatory and cumulative sales milestones, and a royalty on net sales of licensed products subject to a $25,000 minimum annual royalty. In addition, we agreed to reimburse UCSD for all patent-related costs and to meet certain diligence targets. Total costs related to the UCSD license agreement for the use of Lymphoseek as an optical or ultrasound agent were $25,000 and $29,000 in 2014 and 2013, respectively, and were recorded in research and development expenses. The license agreement for the use of Lymphoseek as an optical or ultrasound agent was canceled in July 2014.
In July 2014, the Company replaced the license agreement for the use of Lymphoseek as an optical or ultrasound agent with an expanded license agreement for the exclusive world-wide rights to all diagnostic and therapeutic uses of tilmanocept (other than Lymphoseek). The license agreement is effective until the third anniversary of the expiration date of the longest-lived underlying patent. Under the terms of the license agreement, UCSD has granted us the exclusive rights to make, use, sell, offer for sale and import licensed products as defined in the agreement and to practice the defined licensed methods during the term of the agreement. We may also sublicense the patent rights, subject to certain sublicense terms as defined in the agreement. In consideration for the license rights, we agreed to pay UCSD a license issue fee of $25,000 and license maintenance fees of $25,000 per year. We also agreed to make payments to UCSD upon successfully reaching certain clinical, regulatory and cumulative sales milestones, and a royalty on net sales of licensed products subject to a $25,000 minimum annual royalty. In addition, we agreed to reimburse UCSD for all patent-related costs and to meet certain diligence targets. Total costs related to the UCSD license agreement for tilmanocept were $152,000 and $25,000 in 2015 and 2014, respectively, and were recorded in research and development expenses.
In December 2011, we executed a license agreement with AstraZeneca AB for NAV4694, a proprietary compound that is primarily intended for use in diagnosing Alzheimer’s disease and other central nervous system disorders. The license agreement is effective until the later of the tenth anniversary of the first commercial sale of NAV4694 or the expiration of the underlying patents. Under the terms of the license agreement, AstraZeneca granted us an exclusive worldwide royalty-bearing license for NAV4694 with the right to grant sublicenses. In consideration for the license rights, we paid AstraZeneca a license issue fee of $5.0 million upon execution of the agreement. We also agreed to pay AstraZeneca up to $6.5 million in contingent milestone payments based on the achievement of certain clinical development and regulatory filing milestones, and up to $11.0 million in contingent milestone payments due following receipt of certain regulatory approvals and the initiation of commercial sales of the licensed product. In addition, we agreed to pay AstraZeneca a royalty on net sales of licensed and sublicensed products. Total costs related to the AstraZeneca license agreement were $80,000, $81,000 and $5,000 in 2015, 2014 and 2013, respectively, and were recorded in research and development expenses.
In July 2012, we entered into an agreement with Alseres Pharmaceuticals, Inc. (Alseres) to sublicense NAV5001, an Iodine-123 radiolabeled imaging agent being developed as an aid in the diagnosis of Parkinson’s disease and other movement disorders, with a potential use as a diagnostic aid in dementia. Under the terms of the sublicense agreement, Alseres granted Navidea an exclusive, worldwide sublicense to research, develop and commercialize NAV5001. The terms of the agreement required Navidea to make a one-time sublicense execution payment to Alseres equal to (i) $175,000 in cash and (ii) 300,000 shares of our common stock. The sublicense agreement also provided for contingent milestone payments of up to $2.9 million, $2.5 million of which would have principally occurred at the time of product registration or upon commercial sales, and the issuance of up to an additional 1.15 million shares of Navidea common stock, 950,000 shares of which would have been issuable at the time of product registration or upon commercial sales. In addition, the sublicense terms anticipated royalties on annual net sales of the approved product which were consistent with industry-standard terms and certain sublicense extension fees, payable in cash and shares of common stock, in the event certain diligence milestones were not met. In April 2015, the Company entered into an agreement with Alseres to
F-28
terminate the Alseres sublicense agreement. Under the terms of this agreement, Navidea transferred all regulatory, clinical and manufacturing-related data related to NAV5001 to Alseres. Alseres agreed to reimburse Navidea for any incurred maintenance costs of the contract manufacturer retroactive to March 1, 2015. In addition, Navidea has supplied clinical support services for NAV5001 on a cost-plus reimbursement basis. However, to this point, Alseres has been unsuccessful in raising the funds necessary to restart the program and reimburse Navidea. As a result, we have taken steps to end our obligations under the agreement and notified Alseres that we consider them in breach of the agreement. We are in the process of trying to recover the funds we expended complying with our obligations under the termination agreement. Total costs related to the Alseres sublicense agreement were $5,000, $42,000 and $366,000 in 2015, 2014 and 2013, respectively, and were recorded in research and development expenses.
|
|
c. |
Employment Agreements: As of December 31, 2015, we have employment agreements with four of our senior officers. In addition, although certain employment agreements expired on or before December 31, 2015, the terms of the agreements provide for continuation of certain terms of the employment agreements as long as the senior officers continue to be employees of the Company following expiration of the agreements. The employment agreements contain termination and/or change in control provisions that would entitle each of the officers to 1.2 to 2.0 times their annual salaries, vest outstanding restricted stock and options to purchase common stock, and continue certain benefits if there is a termination without cause or change in control of the Company (as defined) and their employment terminates. As of December 31, 2015, our maximum contingent liability under these agreements in such an event is approximately $2.9 million. The employment agreements also provide for severance, disability and death benefits. |
|
19. |
Leases |
We lease certain office equipment under a capital lease which expires in 2016. We also lease office space in Ohio under an operating lease that expires in October 2022.
The future minimum lease payments for the years ending December 31 are as follows:
|
|
|
Capital Leases |
|
|
Operating Leases |
|
||
|
2016 |
|
$ |
2,279 |
|
|
$ |
271,831 |
|
|
2017 |
|
|
— |
|
|
|
277,946 |
|
|
2018 |
|
|
— |
|
|
|
284,247 |
|
|
2019 |
|
|
— |
|
|
|
290,734 |
|
|
2020 |
|
|
— |
|
|
|
297,405 |
|
|
Thereafter |
|
|
— |
|
|
|
557,540 |
|
|
|
|
|
2,279 |
|
|
$ |
1,979,703 |
|
|
Less amount representing interest |
|
|
125 |
|
|
|
|
|
|
Present value of net minimum lease payments |
|
|
2,154 |
|
|
|
|
|
|
Less current portion |
|
|
2,154 |
|
|
|
|
|
|
Capital lease obligations, excluding current portion |
|
$ |
— |
|
|
|
|
|
Total rental expense was $217,000, $357,000 and $400,000 for the years ended December 31, 2015, 2014 and 2013, respectively.
|
20. |
Employee Benefit Plan |
We maintain an employee benefit plan under Section 401(k) of the Internal Revenue Code. The plan allows employees to make contributions and we may, but are not obligated to, match a portion of the employee’s contribution with our common stock, up to a defined maximum. We also pay certain expenses related to maintaining the plan. We recorded expenses related to our 401(k) plan of $124,000, $125,000, and $104,000 during 2015, 2014 and 2013, respectively.
|
21. |
Supplemental Disclosure for Statements of Cash Flows |
During 2015, 2014 and 2013, we paid interest aggregating $4.6 million, $2.9 million and $1.9 million, respectively. During 2013, we issued 100,000 shares of our common stock valued at $166,000 as partial payment of a milestone fee. During 2015, 2014, and 2013, we issued 68,157, 36,455 and 22,126 shares of our common stock, respectively, as matching contributions to our 401(k) Plan which were valued at $117,000, $100,000 and $67,000, respectively. During 2015, 2014 and 2013, we recorded $1.0 million, $2.4 million and $1.0 million, respectively, of end-of-term fees associated with our notes payable to CRG, Oxford and GECC/MidCap.
F-29
During 2013, the Company and Platinum entered into an Exercise Agreement, pursuant to which Platinum exercised its Series X Warrant and Series AA Warrant for 2,364.9 shares of the Company’s Series B Preferred Stock. These warrants were exercised on a cashless basis by canceling a portion of the indebtedness outstanding under the Platinum Loan Agreement equal to $4.8 million, the aggregate exercise price of the warrants. Also during 2013, in conjunction with the GECC/MidCap Loan Agreement and the Crede Securities Purchase Agreement, we issued warrants with estimated fair values of $631,000 and $7.7 million, respectively.
During 2014, in connection with the Oxford Loan Agreement, we issued warrants with an estimated relative fair value of $465,000. Also during 2014, in connection with entering into the R-NAV joint enterprise, Navidea executed a promissory note in the principal amount of $666,666, payable in two equal installments on July 15, 2015 and July 15, 2016, the first and second anniversaries of the R-NAV transaction. See Note 10.
In connection with their initial investment in March 2015, the investors in MT were issued warrants that have been determined to be derivative liabilities with an estimated fair value of $63,000. A $46,000 deemed dividend related to the beneficial conversion feature within the MT Preferred Stock was also recorded at the time of the initial investment in MT.
|
22. |
Contingencies |
We are subject to legal proceedings and claims that arise in the ordinary course of business.
On August 12, 2015, a shareholder of the Company filed an action in the United States District Court for the Southern District of New York against two funds managed by Platinum alleging violations of Section 16(b) of the Securities Exchange Act in connection with purchases and sales of the Company’s common stock by the Platinum funds, and seeking disgorgement of the short-swing profits realized by the funds. Platinum has reported that they own more than five percent of the Company’s outstanding common stock. The Company is a nominal defendant in the action, no relief is sought against the Company, and a portion of any amount awarded to the plaintiff by judgment or settlement of the action will likely accrue to the benefit of the Company.
On August 31, 2015, Sinotau Pharmaceutical Group (Sinotau) filed a suit for damages, specific performance and injunctive relief against the Company in the United States District Court for the District of Massachusetts alleging breach of a letter of intent for licensing to Sinotau of the Company’s NAV4694 product candidate and technology. The Company believes the suit is without merit and has filed a motion to dismiss the action. While it is not possible to determine with any degree of certainty the ultimate outcome of this legal proceeding, including making a determination of liability, we believe that we have meritorious defenses with respect to the claims asserted against us and intend to vigorously defend our position.
In accordance with ASC Topic 450 - Contingencies, we make a provision for a liability when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated. Although the outcome of any litigation is uncertain, in our opinion, the amount of ultimate liability, if any, with respect to these actions will not materially affect our financial position.
F-30
Quarterly financial information for fiscal 2015 and 2014 are presented in the following table, in thousands, except per share data:
|
|
|
For the Quarter Ending |
|
|||||||||||||
|
|
|
March 31 |
|
|
June 30 |
|
|
September 30 |
|
|
December 31 |
|
||||
|
2015: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lymphoseek sales revenue |
|
$ |
1,835 |
|
|
$ |
1,964 |
|
|
$ |
2,953 |
|
|
$ |
3,503 |
|
|
Lymphoseek license revenue |
|
|
83 |
|
|
|
250 |
|
|
|
550 |
|
|
|
250 |
|
|
Grant and other revenue |
|
|
190 |
|
|
|
654 |
|
|
|
477 |
|
|
|
541 |
|
|
Gross profit |
|
|
1,659 |
|
|
|
2,535 |
|
|
|
3,522 |
|
|
|
3,778 |
|
|
Operating expenses |
|
|
9,475 |
|
|
|
6,346 |
|
|
|
7,845 |
|
|
|
6,379 |
|
|
Operating loss |
|
|
(7,816 |
) |
|
|
(3,811 |
) |
|
|
(4,323 |
) |
|
|
(2,601 |
) |
|
Net loss attributable to common stockholders |
|
|
(7,337 |
) |
|
|
(9,691 |
) |
|
|
(8,071 |
) |
|
|
(2,510 |
) |
|
Basic and diluted net loss per share (1) |
|
$ |
(0.05 |
) |
|
$ |
(0.06 |
) |
|
$ |
(0.05 |
) |
|
$ |
(0.02 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2014: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lymphoseek sales revenue |
|
$ |
627 |
|
|
$ |
1,046 |
|
|
$ |
1,101 |
|
|
$ |
1,460 |
|
|
Lymphoseek license revenue |
|
|
— |
|
|
|
— |
|
|
|
300 |
|
|
|
— |
|
|
Grant and other revenue |
|
|
125 |
|
|
|
28 |
|
|
|
849 |
|
|
|
738 |
|
|
Gross profit |
|
|
559 |
|
|
|
804 |
|
|
|
1,442 |
|
|
|
1,884 |
|
|
Operating expenses |
|
|
9,138 |
|
|
|
10,020 |
|
|
|
6,805 |
|
|
|
6,360 |
|
|
Operating loss |
|
|
(8,579 |
) |
|
|
(9,216 |
) |
|
|
(5,363 |
) |
|
|
(4,476 |
) |
|
Net loss attributable to common stockholders |
|
|
(11,741 |
) |
|
|
(10,222 |
) |
|
|
(6,899 |
) |
|
|
(6,865 |
) |
|
Basic and diluted net loss per share (1) |
|
$ |
(0.08 |
) |
|
$ |
(0.07 |
) |
|
$ |
(0.05 |
) |
|
$ |
(0.05 |
) |
|
|
(1) |
Net loss per share is computed independently for each of the quarters presented. Therefore the sum of the quarterly per-share calculations will not necessarily equal the annual per share calculation. |
F-31
