Attached files
| file | filename |
|---|---|
| EX-21 - EX-21 - MusclePharm Corp | d103625dex21.htm |
| EX-31.1 - EX-31.1 - MusclePharm Corp | d103625dex311.htm |
| EX-31.2 - EX-31.2 - MusclePharm Corp | d103625dex312.htm |
| EX-32.2 - EX-32.2 - MusclePharm Corp | d103625dex322.htm |
| EX-32.1 - EX-32.1 - MusclePharm Corp | d103625dex321.htm |
| EX-10.17 - EX-10.17 - MusclePharm Corp | d103625dex1017.htm |
| EX-10.15 - EX-10.15 - MusclePharm Corp | d103625dex1015.htm |
| EX-10.14 - EX-10.14 - MusclePharm Corp | d103625dex1014.htm |
| EX-10.16 - EX-10.16 - MusclePharm Corp | d103625dex1016.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the Fiscal Year ended December 31, 2015
Commission File Number – 000-53166

MusclePharm Corporation
(Exact name of registrant as specified in its charter)
| Nevada | 77-0664193 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 4721 Ironton Street, Building A Denver, Colorado |
80239 | |
| (Address of principal executive offices) | (Zip code) | |
(303) 396-6100
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(g) of the Act:
Title of each class
Common Stock, Par Value $0.001 Per Share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. ¨ Yes x No
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. ¨ Yes x No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. x Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files) Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated file, an accelerated file, a non-accelerated filer, or a smaller reporting company. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | x | |||
| Non-accelerated filer | ¨ | Smaller reporting company | ¨ | |||
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ¨ Yes x No
Aggregate market value of the voting common stock held by non-affiliates of the registrant at June 30, 2015: $55,525,569
Number of shares of the registrant’s common stock outstanding at March 7, 2016: 13,933,785 excludes 875,621 common shares held in treasury.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the registrant’s Proxy Statement for the registrant’s 2016 Annual Meeting of Stockholders will be filed with the Commission within 120 days after the close of the registrant’s 2015 fiscal year and are incorporated by reference in Part III.
Table of Contents
Form 10-K
For the Year Ended December 31, 2015
TABLE OF CONTENTS
Table of Contents
Forward-Looking Statements
This Annual Report on Form 10-K contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this Annual Report on Form 10-K other than statements of historical fact, including statements regarding our future results of operations and financial position, our business strategy and plans, and our objectives for future operations, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including those described in Part I, Item 1A, “Risk Factors” in this Annual Report on Form 10-K. Moreover, new risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this Annual Report on Form 10-K may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements.
We undertake no obligation to revise or publicly release the results of any revision to these forward-looking statements, except as required by law. Given these risks and uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements.
| Item 1. | Business |
Overview
MusclePharm Corporation is a scientifically driven, performance lifestyle company that develops, manufactures, markets and distributes branded nutritional supplements. We offer a broad range of powders, capsules, tablets and gels. Our portfolio of recognized brands, including MusclePharm® Sport Series, and FitMiss®, are marketed and sold in more than 120 countries and available in over 50,000 retail outlets globally. These clinically-developed, scientifically-driven nutritional supplements are developed through a six-stage research process that utilizes the expertise of leading nutritional scientists, doctors and universities.
We compete in the global supplements market, and, during 2014, we opened offices in Dublin, Ireland. We continued our international expansion in 2015 and opened a subsidiary in Sydney, Australia.
MusclePharm has experienced growth across multiple nutritional supplement categories and geographies. We had net revenue of $166.9 million in 2015 and a five-year compound annual growth rate of 120%. For further information concerning our results of operations, see “Item 6. Selected Financial Data” and “Item 7. Management’s Discussion and Analysis of Financial Conditions and Results of Operations.”
As used in this Annual Report on Form 10-K, the terms “Company,” “we,” “our,” “MusclePharm” or “MP” refer to MusclePharm Corporation and its predecessors, subsidiaries and affiliates, unless the context indicates otherwise.
Products and Distribution
We focus the MusclePharm Brand product portfolio for athlete’s and active lifestyle use. We market our branded products in multiple performance and active lifestyle distribution channels that reach athletes of many types and demographics. Our goal is to serve the needs of all types of athletes, while fueling the engine of sport for all ages and genders. Our portfolio of brands targets every type of fitness enthusiast, from professional, combat sport, weight training, bodybuilding, running, basketball, soccer, cross fit, golf, tennis, volleyball and other active lifestyle activities.
1
Table of Contents
We place considerable emphasis on transparency, high-quality ingredients, innovation and science with our ingredients. Products are placed through rigorous 3rd party independent testing to ensure safe, quality ingredients that support an athletic lifestyle. Tests performed on products include: banned substance testing and protein verification.
MusclePharm® Sport Series – Scientifically-advanced, performance-driven supplements that cover the needs of athletes including their workout needs. This line of independent-tested products help fuel athletes safely by increasing strength, endurance, hydration, recovery, and overall athletic performance. MusclePharm Hybrid Series products like Assault, Amino1 and Combat Protein Powder contain ingredients that are designed to deliver performance. MusclePharm Core products, such as BCAA 3:1:2, CLA Core and Fish Oil, are designed to meet the day-in and day-out demands of athletes.
FitMiss® – Designed and formulated specifically for the active woman’s lifestyle utilizing ingredients that covers the range of busy women’s needs including weight loss, multi-vitamins, protein shakes, detox, skin care, and pre-workout energy mixes.
Sales and Marketing
Our core marketing strategy is to brand MusclePharm as the “must have” fitness brand for workout enthusiasts and elite athletes. We seek to be known as The Athletes Company®, run by athletes who create their products for other athletes both professional and enthusiast. We believe that our marketing strategy of sports endorsements and sponsorships coupled with product sampling with our retail partners is an optimal strategy to increase sales.
The MusclePharm brands are marketed across major global retail distribution channels – Specialty, International and Food, Drug, and Mass (FDM). Our three largest customers each accounted for more than 10% of our 2015 net revenue. For further information concerning our customer concentration, see Note 2 to the Consolidated Financial Statements.
Specialty Market: This channel is comprised of brick-and-mortar sales and e-commerce. Due to high competition within this market, we continually seek to respond to customer trends and shifts by adjusting the mix of existing product offerings, developing new innovative products and influencing preferences by extensive and strategic marketing.
International: We intend to focus on growing our international presence by continuing to offer new products, as well as improving the supply cycles and opening new distribution centers in select regions of the world to reduce tariffs, taxes and fees, as well as shipping time. Our principal international markets are Europe, the Middle East, Asia and South America.
During 2014, we established a subsidiary in Ireland to primarily market and distribute in the EU. During 2015, we established a subsidiary in Australia to market and distribute in Australia, New Zealand and the surrounding region.
FDM (Food, Drug, and Mass): This is a relatively new sales channel for MusclePharm that we intend to continue to grow by distribution expansion. We utilize the assistance of brokers to sell into this channel.
2
Table of Contents
Below is a table of net revenue by our major distribution channel:
| Year Ended December 31, | ||||||||||||||||||||||||
| 2015 | % of Total |
2014 | % of Total |
2013 | % of Total |
|||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Distribution Channel |
||||||||||||||||||||||||
| Specialty |
$ | 76,028 | 46 | % | $ | 73,847 | 42 | % | $ | 68,606 | 62 | % | ||||||||||||
| International |
46,260 | 28 | % | 66,407 | 37 | % | 34,113 | 31 | % | |||||||||||||||
| FDM |
35,657 | 21 | % | 24,790 | 14 | % | 8,159 | 7 | % | |||||||||||||||
| BioZone Net Revenue(1) |
8,913 | 5 | % | 12,345 | 7 | % | — | — | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 166,858 | 100 | % | $ | 177,389 | 100 | % | $ | 110,878 | 100 | % | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| (1) | Our wholly–owned subsidiary, BioZone Labs (“BioZone”), develops, manufactures and distributes over-the-counter drugs and preparations, cosmetics and nutritional supplements. We previously have announced that we are exploring the sale of BioZone; accordingly, we have presented BioZone separately from our major sales channels. |
Our advertising and promotions consist primarily of athletic endorsements and sponsorships, which includes both cash and stock-based compensation, promotional giveaways, trade show events, FDM distribution costs, and digital, print and media advertising. In connection with our restructuring plan commenced during the third quarter of 2015, we have begun negotiating or terminating a number of contracts with endorsers in a strategic shift away from such costly arrangements, and toward more cost effective brand partnerships as well as grass-roots marketing and advertising efforts.
We believe that influential brand partners such as MMA Team – Team Elevation, Olympian Jordan Burroughs, and other professional athletes help boost brand credibility by exposing our brand to millions of potential customers.
We believe that, along with our innovative products, providing superior, relevant and memorable brand experience drives our success. Our websites, including musclepharm.com, Arnold.com, fitmiss.com, and mpssi.com also provide additional educational information to consumers, customers, and healthcare professionals.
As noted above, we market our products globally. The following table sets forth revenue, net by customer geographic area based off shipping address (in thousands):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Revenue, net |
||||||||||||
| United States |
$ | 120,598 | $ | 110,514 | $ | 76,750 | ||||||
| International |
46,260 | 66,875 | 34,128 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue, net |
$ | 166,858 | $ | 177,389 | $ | 110,878 | ||||||
|
|
|
|
|
|
|
|||||||
Charitable Youth Sports Program
In March 2014, the Board of Directors of the Company approved and the Company established a charitable youth sports grant program (the “Program”) pursuant to which the Company will donate product, equipment and cash to organizations such as schools, sports teams and training facilities. The Company had tentatively established an annual budget of approximately $250,000 for the Program. The primary intent of the Program was to build MusclePharm brand awareness with youth athletes. The Company’s other business purposes in establishing the Program was to help needy organizations achieve their goals, promote the Company’s brand, help athletes
3
Table of Contents
develop stronger and better skills and to build the reputation of the Company as a contributor to the community. A committee formerly consisting of the Company’s former President, former Director of Team Development, and former Chief Operating Officer oversaw the Program. In 2014, the Company made an initial grant in the amount of approximately $250,000 to Arvada West High School and similar charitable contributions to other charitable sports organizations of approximately $30,000. The Company’s former Chief Executive Officer, Mr. Brad Pyatt, is a graduate of Arvada West High School and serves as a volunteer football coach. The Company did not make a charitable grant to Arvada West High School during 2015. The Company did make charitable grants to other youth sports organizations during 2015 totaling approximately $278,000. We expect this amount to decrease substantially in 2016 and any future grant will be approved by the Chief Executive Officer and Chief Financial Officer.
Product Research, Development and Quality Control
Science, product research and innovation are key factors to our success. Customers’ belief in the science and safety of our products is critical. Continued innovation in delivery techniques and ingredients, new product line extensions for existing products and new product offerings are important in order to create new market opportunities, meet consumer demand and strengthen consumer relationships. To support our research and development efforts, we invest in formulation, processing and packaging development, perform product quality and stability studies, complete product efficacy and safety studies, and conduct consumer market research to sample consumer opinions on product concepts, design, packaging, advertising and marketing campaigns.
MusclePharm’s lines of supplements are developed through a six-stage research process that utilizes the expertise of nutritional scientists, doctors and universities and strives to assure that each promote quality and safety for our customers.
MusclePharm is committed to science and sport being equal in our product development. We believe real-world applications are essential. The MP Sports Science Institute in Denver, Colorado is a state-of-the-art, 30,000 square-foot training and performance facility—the only professional facility in the world to use the ultrasound, Omega Wave, DEXA and Keiser performance equipment to gather cutting-edge feedback about our formulations. In addition, in our clinic, we can perform bone scans, blood work and ultrasounds to determine body composition as well as muscle and fat evaluations. Feedback and testing data helps our team strive to improve our products and allows MusclePharm to continue to be innovative. Active athlete testing also allows us to fine tune dosages, ensure proper ingredient combinations, and implement consumer safety measures.
Our quality control team follows detailed and supplier selection and certification processes, validation of raw material verification processes, analytical testing, process audits, and other quality control procedures. The quality management systems we employ also include a professionally equipped and staffed laboratory enabling finished goods testing for compliance to our specifications. Our products are also subject to extensive shelf life stability testing. We also use third party laboratories to routinely evaluate our internal testing processes and to supplement our internal testing procedures and capabilities.
We qualify ingredients, suppliers and facilities by performing site assessments and conducting on-going performance and process reviews. Dedicated quality teams regularly audit and assess manufacturing facilities for compliance Good Manufacturing Practices (“GMPs”), as regulated by the U.S. Food and Drug Administration (“FDA”) to ensure our compliance with all MusclePharm, regulatory and certification standards and requirements. To ensure overall consistency, our quality assurance team adheres to strict written procedures. From the raw ingredient stage to finished product stage, we monitor and perform quality control checks. Before distributing our products, we place our products under quarantine to test for environmental contaminants and verify that the finished product meets label claims. Once a product has successfully passed quality assurance testing and conforms to specifications for identity, purity, strength and composition, we then conduct testing with third-party laboratories for added label claim verification. Multi-level practices are part of our product development process to ensure athletes and our consumers receive what we believe to be the most scientifically-
4
Table of Contents
innovative and safe supplements on the market. Post-distribution, we have standard operating procedures in place for investigating and documenting any adverse events or product quality complaints.
We are committed to the process of having all of our products certified to be banned-substance-free before they are available to our athletes and consumers. We complete third party analytical testing on our products through Eurofins. We also have engaged an independent lab to validate our quality processes and conduct banned-substance testing on every branded MusclePharm product.
Manufacturing and Distribution
We have a strategic working relationship with multiple contract manufacturers. Currently, the majority of our products are produced by third party powder and capsule manufacturing facilities located in Tennessee, New York, Utah and our subsidiary, BioZone, in California. Certain of our vendors supply in excess of 10% of our products. In particular, one of our third-party suppliers, Capstone, was responsible for 59% of our manufacturing spend in 2015. We have undertaken an evaluation of our agreements with Capstone, and at this time cannot anticipate whether Capstone will continue to be a principal vendor to us. See Note 2 to the consolidated Financial Statements for a discussion of these vendors. Once a product is manufactured, it is sent to one of our distribution centers. Our main distribution center is located in Spring Hill, Tennessee, and we have a second distribution center in Pittsburg, California. We also utilize contract manufacturers to ship products directly to our customers. All of our manufacturing and distribution facilities are designed and operated to meet the current GMPs as promulgated by the FDA.
Our manufacturing process generally consists of the following operations: (i) qualifying ingredients for products, (ii) testing of all raw ingredients, (iii) measuring ingredients for inclusion in production, (iv) granulating, blending and grinding ingredients into a mixture with a homogeneous consistency, (v) encapsulating or filling the blended mixture into the appropriate dosage form using either automatic or semiautomatic equipment, and (vi) testing finished products prior to distribution.
We maintain and operate a system that integrates with distribution, warehousing and quality control, providing real-time lot and quality tracking of raw materials, work in progress and finished goods.
We employ a supply chain staff that works with sales, marketing, product development and quality control personnel to create our products.
Our Competitors
The nutritional supplements market is very competitive and the range of products is diverse. Competitors use price, shelf space and store placement, brand and product recognition, new product introductions and raw materials to create market share.
Our range of competitors includes numerous nutritional supplement companies that are highly fragmented in terms of geographic market coverage, distribution channels and product categories. In addition, large pharmaceutical companies and packaged food and beverage companies compete with us in the nutritional supplement market. Many of these companies have greater financial and distribution resources available to them than MusclePharm and many of these companies compete through vertical integration. Private label entities have gained a foothold in many nutrition categories and also are direct competitors. A few of these are private label entities have become market leaders. Our principal competitors are Optimum Nutrition, Inc., Cellucor, and Quest Nutrition, LLC.
In this industry, most of the companies are privately held. With respect to retailer sales, we cannot fully gauge their sizes and our relative ranking. We believe that retailers look to partner with suppliers who demonstrate brand awareness, market intelligence, customer service and science. We believe we are competitive in all of these areas.
5
Table of Contents
Government Regulation
The formulation, manufacturing, packaging, labeling, advertising, distribution and sale of each of our major product groups are subject to regulation by one or more governmental agencies. The most active of these is the FDA, which regulates our products under the Federal Food, Drug and Cosmetic Act (“FDCA”) and regulations promulgated thereunder. The FDCA defines the terms “food” and “dietary supplement” and sets forth various conditions that, unless complied with, may constitute adulteration or misbranding of such products. The FDCA has been adjusted several times with respect to dietary supplements, most recently by the Nutrition Labeling and Education Act of 1990 (the “NLEA”) and the Dietary Supplement Health and Education Act of 1994.
FDA regulations relating specifically to foods and dietary supplements for human use are set forth in Title 21 of the Code of Federal Regulations. These regulations include basic labeling requirements for both foods and dietary supplements. Additionally, FDA regulations require us to meet relevant good manufacturing practice regulations for the preparation, packaging and storage of our food and dietary supplements.
Our business practices and products are also regulated by the Federal Trade Commission (“FTC”), the Consumer Product Safety Commission, the United States Department of Agriculture (“USDA”) and the Environmental Protection Agency. Our activities, including our direct selling distribution activities, are also regulated by various agencies of the states, localities and foreign countries in which our products are sold.
In foreign markets, prior to commencing operations and prior to making or permitting sales of our products in the market, we may be required to obtain an approval, license or certification from the country’s ministry of health or comparable agency. Prior to entering a new market in which a formal approval, license or certificate is required, we work extensively with local consultants and authorities in order to obtain the requisite approvals. We must also comply with product labeling and packaging regulations that vary from country to country. Our failure to comply with these regulations can result in a product being removed from sale in a particular market, either temporarily or permanently.
Intellectual Property
We regard our trademarks and other proprietary rights as valuable assets and believe that protecting our key trademarks is crucial to the continued successful implementation of our business strategy of building strong brand name recognition. Since we regard our intellectual property as a crucial element of our business with significant value in the marketing of our products, our policy is to pursue registrations for our trademarks associated with our products.
We have over 140 trademark applications in the United States, 51 of which are currently registered with the United States Patent and Trademark Office for our MusclePharm brands and certain of our products and slogans.
We also have filed for protection of various marks throughout the world and are committed to a significant long-term strategy to build and protect the MusclePharm brand globally. We have applied for the “MusclePharm” mark in 18 countries, including the United States. The mark has been granted final trademark registration in 16 of those countries.
Seasonality
Our business does not typically experience seasonal variations but revenue may fluctuate based upon promotions. During 2015, revenue fluctuated mainly due to issues in supply chain, which limited availability of inventory. Additionally, we experienced periods of mis-match of inventory on hand relative to customer sales orders.
6
Table of Contents
Employees
As of December 31, 2015, we had 248 total employees, of which 153 were full time. None of the employees are represented by a union. Management considers its relations with our employees to be good and to have been maintained in a normal and customary manner.
Corporate Information
Our principal executive offices are located at 4721 Ironton Street, Building A, Denver, Colorado 80239 and our telephone number is (303) 396-6100. We were incorporated in the State of Nevada in 2006. As used in this Annual Report on Form 10-K, the terms the “Company”, “we”, “our”, “MusclePharm”, or “MP” refer to MusclePharm Corporation and its predecessors, subsidiaries and affiliates, unless the context indicates otherwise. Our Internet addresses are www.musclepharm.com and www.musclepharmcorp.com. The information contained on to our websites is not incorporated herein.
Available Information
On company website is www.musclepharmcorp.com. We post the following filings as soon as reasonably practicable after they are electronically filed with or furnished to the Securities and Exchange Commission: our Annual Report on Form 10-K, our quarterly reports on Form 10-Q, our current reports on Form 8-K and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, as amended.
All such filings are available free of charge on the Investor Relations section of our website, or from the SEC’s website at www.sec.gov. Information on our website does not constitute part of this report. Also available on the Investor Relations section of our website are the charters of the committees of our Board of Directors, as well as our corporate governance guidelines and code of ethics. Copies of any of these documents will be provided in print to any shareholder who submits a request in writing to MusclePharm Investor Relations, 4721 Ironton Street, Building A, Denver, CO 80239. Additionally, our filings with the SEC may be read and copied at the SEC Public Reference Room at 100 F Street, NE, Washington, DC 20549. Information on the operation of the Public Reference Room may be obtained by calling 800-SEC-0330.
7
Table of Contents
| Item 1A. | Risk Factors |
Certain factors may have a material adverse effect on our business, financial condition and results of operations. You should consider carefully the risks and uncertainties described below, in addition to other information contained in this Annual Report on Form 10-K, including our consolidated financial statements and related notes. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties that we are unaware of, or that we currently believe are not material, may also become important factors that adversely affect our business. If any of the following risks actually occurs, our business, financial condition, results of operations and future prospects could be materially and adversely affected. In that event, the trading price of our common stock could decline, and you could lose part or all of your investment.
Risks Related to Our Business and Industry
Because our auditors have issued a going concern opinion, there is a substantial uncertainty that we will continue operations, in which case you could lose your investment.
Our auditors have issued a going concern opinion in their report on our financial statements for the fiscal year ended December 31, 2015. We have not established an ongoing source of revenue sufficient to cover our operating costs, and are dependent on obtaining adequate capital to continue operations, which raises substantial doubt as to our ability to continue as a going concern. Revenue was $166.9 million for the year ended December 31, 2015, cost of revenue was $109.9 million, operating expenses were $106.9 million and we incurred a net loss of $51.9 million. Our accumulated deficit as of December 31, 2015 was $147.5 million and we have had recurring losses from operations. We anticipate incurring additional losses until such time we can generate significant revenues, and/or reduce operating costs.
We have not yet established an ongoing source of revenue sufficient to cover our current level of operating costs for at least the next 12 months and allow us to continue as a going concern. Our ability to meet our total liabilities of $73.8 million as of December 31, 2015, and to continue as a going concern, is dependent on us obtaining adequate capital to fund operating losses until we become profitable. We can give no assurances that any additional capital that we are able to obtain, if any, will be sufficient to meet our needs, or that any such financing will be obtained on acceptable terms. If we are unable to obtain adequate capital, we could be forced to cease operations or substantially curtail our commercial activities. As of December 31, 2015, we had cash in the amount of $7.1 million and $20.0 million in working capital deficit.
As a result of our going concern qualification, there is an increased risk that you could lose the entire amount of your investment in our company.
We may not be able to obtain additional financing to fund our operations.
We believe we will need to raise additional funds in the future. There can be no assurance that sufficient debt or equity financing will be available at all or, if available, that such financing will be at terms and conditions acceptable to us. Should we fail to obtain additional debt financing or raise additional capital, we may not be able to achieve our longer term business objectives and may face other serious adverse consequences. If we raise additional funds by issuing equity securities or convertible debt, investors may experience significant dilution of their ownership interest, and the newly-issued securities may have rights senior to those of the holders of our common stock. If we raise additional funds by obtaining loans from third parties, the terms of those financing arrangements may include negative covenants or other restrictions on our business that could impair our operational flexibility and may require us to provide collateral to secure the loan. In addition, in a liquidation, debtholders will be entitled to repayment before any proceeds can be paid to our equityholders.
We may be unable to make payments on our debt.
If our cash flows and capital resources are insufficient to fund our interest and/or principal payments under our loans, we may be forced to take alternative measures to meet our cash requirements, including sales and
8
Table of Contents
disposition of our assets, including those used as collateral for the loans, which may have an adverse effect on our business and results of operations. If additional financing and/or replacement financing is not available when required or is not available on acceptable terms, we may be forced to cease operations or substantially curtail our commercial activities, or we may not be able to expand our business, develop or enhance our products, take advantage of business opportunities or respond to competitive pressures, which could result in lower revenue and reduce the competitiveness of our products.
We have recently undertaken a restructuring of our business. The restructuring may take longer than we anticipate or require us to incur a greater amount of charges than we currently estimate.
On August 24, 2015, our Board of Directors authorized us to undertake steps to commence a restructuring of our business and operations. In doing so, we closed certain facilities, reduced headcount, discontinued products, and terminated or renegotiated certain contracts. Although we expected the restructuring, workforce reduction and facility closures, product discontinuance and contract renegotiations and terminations to be completed by December 31, 2015, the restructuring has taken longer than anticipated, which could cause disruption to our business activities, diminish anticipated savings and/or result in increased restructuring costs, which would adversely affect our operating results and financial condition. We incurred approximately $21.2 million in restructuring and other charges during the year ended December 31, 2015 in connection with this restructuring, of which $9.4 million remains accrued for at year end.
If we fail to effectively manage our growth, our business and operating results could be harmed.
We have experienced and expect to continue to experience growth in our business both domestically and abroad, which has placed, and will continue to place, significant demands on our management, and our operational and financial infrastructure. To effectively manage this growth, we expect that we will need to continue to improve our operational, financial and management controls and our reporting systems and procedures. To accomplish these objectives, we may need to hire additional employees, make certain enhancements to our technology systems, make significant capital expenditures and utilize management resources. Failure to implement these proposed growth objectives could have a material adverse effect on our business and operating results.
We have a history of losses, and we may not be able to achieve profitability.
We have incurred a net loss in each year since our inception, including a net loss of $51.9 million, $13.8 million and $17.7 million in 2015, 2014, and 2013, respectively. As of December 31, 2015, and since our inception, we had incurred accumulated losses from operations of $147.5 million. For 2015 and 2014, our revenue was $166.9 million and $177.4 million, respectively, representing a decrease of 6% year-over-year. In future years, we may not be able to generate sufficient revenue to achieve profitability. We expect to continue to expend substantial financial and other resources on the following, which may increase our net losses:
| • | investing in research and development and the development of new products and product families, |
| • | expenses related to international expansion; |
| • | improving our infrastructure and hiring employees to support it; |
| • | sales and marketing expenses, including expansion of our salesforce; and |
| • | selling, general and administrative expenses, including information technology, legal, accounting, and other expenses. |
These investments may not result in increased revenue or growth of our business, which also could increase our net losses. If we fail to continue to grow our revenue at a rate greater than our expenses, our operating results and business will be harmed.
9
Table of Contents
Our operating results may fluctuate, which makes our results difficult to predict and could cause our results to fall short of expectations.
Our operating results may fluctuate as a result of a number of factors, many of which may be outside of our control. As a result, comparing our operating results on a period-to-period basis may not be meaningful, and you should not rely on our past results as an indication of our future performance. Our quarterly, year-to-date, and annual expenses as a percentage of our revenues may differ significantly from our historical or projected rates. Our operating results in future quarters may fall below expectations. Each of the following factors, as well as other factors, may affect our operating results:
| • | Our ability to deliver quality products in a timely manner in sufficient volumes; |
| • | Our ability to recognize product trends; |
| • | Our loss of one or more significant customers; |
| • | The introduction of successful new products by our competitors; and |
| • | Adverse media reports on the use or efficacy of nutritional supplements. |
Because our business is changing and evolving, our historical operating results may not be useful to you in predicting our future operating results.
The concentration of stock ownership with Mr. Drexler may influence the outcome of certain matters requiring stockholder approval.
As of December 31, 2015, Mr. Ryan Drexler, our Interim Chief Executive Officer, President, and Chairman, beneficially owned approximately 25% of our outstanding shares of common stock, including debt which is convertible into common stock at Mr. Drexler’s election. As a result, Mr. Drexler may be able to substantially influence the strategic direction of the Company and the outcome of matters requiring approval by our stockholders. Mr. Drexler’s interests, including his interests as a holder of a convertible secured promissory note for $6.0 million, may not be, at all times, the same as those of our other stockholders, and his control may delay, deter or prevent acts that may be favored by our other stockholders.
In connection with the Company entering into the convertible promissory note with Mr. Drexler, the Company granted Mr. Drexler the right to designate two directors to the Company’s Board of Directors. The Company agreed to take all actions necessary to permit such designation.
Our failure to respond appropriately to competitive challenges, changing consumer preferences and demand for new products could significantly harm our customer relationships and product sales.
The nutritional sports supplement industry is characterized by intense competition for product offerings and rapid and frequent changes in consumer demand. Our failure to predict accurately product trends could negatively impact our products and cause our revenues to decline.
Our success with any particular product offering (whether new or existing) depends upon a number of factors, including our ability to:
| • | Deliver quality products in a timely manner in sufficient volumes; |
| • | Accurately anticipate customer needs and forecast accurately to our manufacturers; |
| • | Differentiate our product offerings from those of our competitors; |
| • | Competitively price our products; and |
| • | Develop new products. |
Furthermore, products often have to be promoted heavily in stores or in the media to obtain visibility and consumer acceptance. Acquiring distribution for products is difficult and often expensive due to slotting and other promotional charges mandated by retailers. Products can take substantial periods of time to develop consumer awareness, consumer acceptance and sales volume. Accordingly, some products may fail to gain or maintain sufficient sales volume and as a result may have to be discontinued. In a highly competitive marketplace, it may be difficult to have retailer’s open stock-keeping units (SKU’s) for new products.
10
Table of Contents
Our industry is highly competitive, and our failure to compete effectively could adversely affect our market share, financial condition and future growth.
The nutritional supplement industry is highly competitive with respect to:
| • | Price; |
| • | Shelf space and store placement; |
| • | Brand and product recognition; |
| • | New product introductions; and |
| • | Raw materials. |
Most of our competitors are larger, more established companies and possess greater financial strength, personnel, distribution and other resources than we have. MusclePharm faces competition in the supplement market from a number of large nationally known manufacturers, private label brands and many smaller manufacturers.
Our industry is highly regulated. We may in the future incur increased compliance costs and/or incur substantial judgments, fines, legal fees and other costs.
Our industry is highly regulated. The manufacture, labeling and advertising for our products are regulated by various federal, state and local agencies as well as those of each foreign country to which we distribute. Our compliance costs may increase in the future, and those increases could be material. In addition, governmental authorities may commence regulatory or legal proceedings, which could restrict the permissible scope of our product claims or the ability to manufacture and sell our products in the future. For example, the FDA regulates our products to ensure that the products are not adulterated or misbranded. Failure to comply with FDA requirements may result in, among other things, injunctions, product withdrawals, recalls, product seizures, fines and criminal prosecutions. Our advertising is subject to regulation by the FTC under the Federal Trade Commission Act. In recent years, the FTC has initiated numerous investigations of dietary supplement and weight loss products and companies. Additionally, some states also permit advertising and labeling laws to be enforced by private attorney generals, who may seek relief for consumers, seek class action certifications, seek class wide damages and product recalls of products sold by us. Any of these types of adverse actions could have a material adverse effect on our business, financial condition and results of operations.
We rely on a limited number of customers for a substantial portion of our sales, and the loss of or material reduction in purchase volume by any of these customers would adversely affect our sales and operating results.
During 2015, our three largest customers, Costco, GNC and Bodybuilding.com, accounted for 41% of our net revenue. During 2014, our two largest customers, Costco and Bodybuilding.com, accounted for 29% of our net revenue. During 2013, our two largest customers, Bodybuilding.com and Europa, accounted for 35% of our net revenue. Net revenue is equal to our gross revenue less product discounts, customer rebates and incentives. The loss of any of our major customers, a significant reduction in purchases by any major customer, or any serious financial difficulty of a major customer is likely to have a material adverse effect on our sales and operating results.
Adverse publicity or consumer perception of our products and any similar products distributed by others could harm our reputation and adversely affect our sales.
We believe we are highly dependent upon positive consumer perceptions of the safety and quality of our products as well as similar products distributed by other sports nutrition supplement companies. Consumer perception of sports nutrition supplements and our products in particular can be substantially influenced by scientific research or findings, national media attention and other publicity about product use. Adverse publicity from these sources regarding the safety, quality or efficacy of nutritional supplements and our products could harm our reputation
11
Table of Contents
and results of operations. The mere publication of news articles or reports asserting that such products may be harmful or questioning their efficacy could have a material adverse effect on our business, financial condition and results of operations, regardless of whether such news articles or reports are scientifically supported or whether the claimed harmful effects would be present at the dosages recommended for such products.
We may be exposed to material product liability claims, which could increase our costs and adversely affect our reputation and business.
As a marketer and distributor of products designed for human consumption, we could be subject to product liability claims if the use of our products is alleged to have resulted in injury or undesired results. Our products consist of vitamins, minerals, herbs and other ingredients that are classified as dietary supplements and in most cases are not subject to pre-market regulatory approval in the United States or internationally. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur.
We have not had any significant product liability claims filed against us, but in the future we may be subject to various product liability claims, including due to tampering by unauthorized third parties, product contamination and claims that our products had inadequate instructions for use, or inadequate warnings concerning possible side effects and interactions with other substances. The cost of defense can be substantially higher than the cost of settlement even when claims are without merit. The high cost to defend or settle product liability claims could have a material adverse effect on our business and operating results and our insurance, if any, may not be adequate.
In addition, the perception of our products resulting from a product liability claim also could have a material adverse effect on our business and operating results.
Our insurance coverage or third party indemnification rights may not be sufficient to cover our legal claims or other losses that we may incur in the future.
We maintain insurance at what we believe are adequate levels for property, general product liability, product recall, directors and officer’s liability, and workers’ compensation to protect ourselves against potential loss exposures. In the future, insurance coverage may not be available at adequate levels or on adequate terms to cover potential losses, including on terms that meet our customer’s or manufacturer’s requirements. If insurance coverage is inadequate or unavailable, we may face claims that exceed coverage limits or that are not covered, which could increase our costs and adversely affect our operating results.
During 2014, we submitted insurance claims for coverage of our costs associated with the investigation by the SEC to our insurance carrier, which has denied payment of our claims. On February 12, 2015, we filed a complaint in the District Court, City and County of Denver, Colorado against Liberty Insurance Underwriters, Inc. (“Liberty”) claiming wrongful and unreasonable denial of coverage for the cost and expenses that we have incurred and continued to incur in connection with SEC investigation and related matters under our Directors and Officers insurance policies. We have commenced litigation against our insurer. The claims and the litigation may make it more difficult for us to obtain insurance at competitive prices or at all and our insurance costs may increase as a result.
We rely on skilled personnel and, if we are unable to retain or motivate key personnel or hire qualified personnel, we may not be able to grow effectively.
Our future success depends on our continuing ability to identify, hire, develop, motivate and retain skilled personnel for all areas of our organization, particularly sales and marketing. Competition in our industry for qualified employees is intense. In addition, our compensation arrangements, such as our bonus programs, may not always be successful in attracting new employees or retaining and motivating our existing employees.
12
Table of Contents
If we are unable to retain key management personnel, our ability to manage our business effectively and grow could be negatively impacted.
We believe our success depends in part on our ability to retain our key management personnel. Currently, we have executed employment agreements with our key management employees that extend through December 31, 2017 or beyond. On March 15, 2016, Mr. Brad Pyatt resigned as our Chief Executive Officer and President. In connection with Mr. Pyatt’s resignation, Mr. Ryan Drexler, the Executive Chairman of our Board of Directors, was appointed as our interim Chief Executive Officer, President and Chairman. Our Board of Directors has begun a search for a permanent replacement. There can be no assurance that we will be able to attract a qualified, permanent Chief Executive Officer who has the desired qualifications on acceptable terms, or at all. The loss or limitation of the services of any of our key management employees or the inability to additional qualified replacement personnel could have a material adverse effect on our business and results of operations.
Changes in the economies of the markets in which we do business may affect consumer demand for our products.
Consumer spending habits, including spending for our products, are affected by, among other things, prevailing economic conditions, levels of employment, fuel prices, changes in exchange rates, salaries and wages, the availability of consumer credit, consumer confidence and consumer perception of economic conditions. Economic slowdowns in the markets in which we do business and an uncertain economic outlook may adversely affect consumer spending habits, which may result in lower sales of our products in future periods. A prolonged global or regional economic downturn could have a material negative impact on our financial position, results of operation or cash flows.
We are exposed to fluctuations in currency exchange rates, which could negatively affect our results of operations.
Our consolidated results of operations and cash flows are subject to fluctuations due to changes in foreign currency exchange rates. For 2015, 28% of our revenues were from international sales. The majority of our revenue is denominated in U.S. Dollars, with the exception of Canada and Ireland, where we invoice primarily in local currencies. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in North America and Europe. Revenue resulting from selling in local currencies and costs incurred in local currencies are exposed to foreign currency exchange rate fluctuations that can affect our operating income. As exchange rates vary, our operating income may differ from expectations. To date, we have not entered into any hedging arrangements with respect to foreign currency risk or other derivative instruments.
Taxation and transfer pricing affect our operations.
As a U.S. company doing business in international markets, we are subject to foreign tax and intercompany pricing laws, including those relating to the flow of funds between our parent Company and our subsidiaries. These pricing laws are designed to ensure that appropriate levels of income and expense are reported by our U.S. and foreign entities, and that they are taxed appropriately. If regulators challenge our corporate structures, transfer pricing methodologies or intercompany transfers, our operations may be harmed, and our effective tax rate may increase. We are eligible to receive foreign tax credits in the United States for certain foreign taxes actually paid abroad. In the event any audits or assessments are concluded adversely to us, we may not be able to offset the consolidated effect of foreign income tax assessments through the use of U.S. foreign tax credits. Because the laws and regulations governing U.S. foreign tax credits are complex and subject to periodic legislative amendment, we cannot be sure that we would in fact be able to take advantage of any foreign tax credits in the future. The various customs, exchange control and transfer pricing laws are continually changing, and are subject to the interpretation of governmental agencies.
Despite our efforts to be aware of and to comply with such laws and changes to the interpretations thereof, there is a risk that we may not continue to operate in compliance with such laws. We may need to adjust our operating
13
Table of Contents
procedures in response to these interpretational changes, and such changes could have a material negative impact on our financial position, results of operation or cash flows.
Our intellectual property rights are valuable, and any inability to protect them could reduce the value of our products and brand.
We have invested significant resources to protect our brands and trademarks. However, we may be unable or unwilling to strictly enforce our intellectual property rights, including our brands and trademarks, from infringement. Our failure to enforce our intellectual property rights could diminish the value of our brands and product offerings and harm our business and future growth prospects.
We may be subject to intellectual property rights claims, which are costly to defend, could require us to pay damages and could limit our ability to sell some of our products.
Our industry is characterized by vigorous pursuit and protection of intellectual property rights, which has resulted in protracted and expensive litigation for several companies. Third parties may assert claims of misappropriation of trade secrets or infringement of intellectual property rights against us or against our end customers or partners for which we may be liable.
As our business expands, the number of products and competitors in our markets are expected to increase and product overlaps may occur and infringement claims may increase in number and significance. Intellectual property lawsuits are subject to inherent uncertainties due to the complexity of the technical issues involved, and we cannot be certain that we would be successful in defending ourselves against intellectual property claims. Further, many potential litigants have the capability to dedicate substantially greater resources than we can to enforce their intellectual property rights and to defend claims that may be brought against them. Furthermore, a successful claimant could secure a judgment that requires us to pay substantial damages or prevents us from distributing products or performing certain services.
An increase in product returns could negatively impact our operating results and profitability.
We permit the return of damaged or defective products and accept limited amounts of product returns in certain instances. While such returns from established customers have historically been nominal and within management’s expectations and the provisions established, future return rates may differ from those experienced in the past. Any significant increase in damaged or defective products or accepted returns could have a material adverse effect on our operating results for the period or periods in which such returns materialize.
We outsource a majority of our manufacturing and anticipate continued reliance on third-party manufacturers for the development and commercialization of most of our products.
Currently, we rely on third-party manufacturers to produce products required to meet substantially all of our sales needs. We plan to continue to rely upon contract manufacturers to produce commercial quantities of most of our products.
If our contract manufacturers fail to maintain high manufacturing standards and processes, it could harm our business. In the event of a natural disaster or business failure, including due to bankruptcy of a contract manufacturer, we may not be able to secure a replacement of our products on a timely or cost-effective basis, which could result in delays, additional costs and reduced revenues.
One of our third-party suppliers, Capstone, was responsible for 59% of our manufacturing spend in 2015, and others were responsible for 25% and 11%, respectively. Our supplier concentration exacerbates the risks described above.
14
Table of Contents
We may seek to enter into similar or different manufacturing arrangements in the future.
A shortage in the supply of key raw materials or a price increase could increase our costs or adversely affect our sales.
All of our raw materials for our products are obtained from third-party suppliers. Since all of the ingredients in our products are commonly used, we have not experienced any shortages or delays in obtaining raw materials. If circumstances changed, shortages could result in materially higher raw material prices or adversely affect our ability to have a product manufactured. Prices for our raw materials can and do fluctuate. Price increases from a supplier would directly affect our profitability if we are not able to pass price increases on to customers. Our inability to obtain adequate supplies of raw materials in a timely manner or a material increase in the price of our raw materials could have a material adverse effect on our business, financial condition and results of operations.
Our arrangements with Capstone may not have value to us.
We have undertaken an evaluation of the agreements with Capstone Nutrition (“Capstone”) and related agreements as well as a review of the performance of Capstone under the Manufacturing Agreement and prices for products. The Company is engaged in a dispute with Capstone, there can be no assurance that we have or will realize value from the Capstone arrangements nor that we will exercise the option agreement or that the warrant will have value to us in the future.
System and technology failures and obsolescence could harm our business.
Our business is highly dependent upon our information technology infrastructure (websites, supply chain, and ERP applications) to manage effectively and efficiently our operations, including order entry, customer billing, accurately tracking purchases and managing accounting, finance and inventory. The occurrences of natural disasters, security breaches or other unanticipated problems could result in interruptions in our day-to-day business that could adversely affect us.
Our share price has been and may continue to be volatile.
The market price of our common shares is subject to significant fluctuations in response to a multitude of factors, including variations in our quarterly operating results and financial condition. Factors other than our financial results that may affect our share price include, but are not limited to, market expectations of our performance, market perception or our industry, the activities of our managers, customers, and investors, and the level of perceived growth in the industry in which we participate, general trends in the markets for our products, general economic, business and political conditions in the countries and regions in which we conduct our business, and changes in government regulation affecting our business, many of which are not within our control.
We may, in the future, issue additional shares of common stock and/or preferred stock, which would reduce investors’ percent of ownership and may dilute our share value.
Our articles of incorporation, as amended, authorize the issuance of 100,000,000 shares of common stock and 10,000,000 shares of preferred stock, As of December 31, 2015, we do not have any outstanding shares of preferred stock. The future issuance of common stock and preferred stock may result in substantial dilution in the percentage of our common stock held by our then existing stockholders. The issuance of common stock for future services or acquisitions or other corporate actions may have the effect of diluting the value of the shares held by our investors, and might have an adverse effect on any trading market for our common stock.
15
Table of Contents
We may issue additional shares of preferred stock in the future that may adversely impact your rights as holders of our common stock.
Our Board of Directors has the authority to fix and determine the relative rights and preferences of our authorized but undesignated preferred stock, as well as the authority to issue shares of such preferred stock, without further stockholder approval. As a result, our Board of Directors could authorize the issuance of a series of preferred stock that would grant to holders preferred rights to our assets upon liquidation, the right to receive dividends before dividends are declared to holders of our common stock, and the right to the redemption of such preferred stock, together with a premium, prior to the redemption of the common stock. To the extent that we do issue such additional shares of preferred stock, your rights as holders of common stock could be impaired thereby, including, without limitation, dilution of your ownership interests in us. In addition, shares of preferred stock could be issued with terms calculated to delay or prevent a change in control or make removal of management more difficult, which may not be in your interest as a holder of common stock.
Our indebtedness may limit our operating flexibility.
As of the date hereof, we had a convertible secured promissory note with the Interim Chief Executive Officer, President and Chairman, Ryan Drexler, with an aggregate principle amount of $6.0 million.
Our indebtedness could have important consequences to us. For example, it could:
| • | make us more vulnerable to general adverse economic and industry conditions; |
| • | limit our ability to obtain additional financing for working capital, capital expenditures, acquisitions and other general corporate requirements; |
| • | require us to dedicate a portion of our cash flow from operations to payments on our debt, thereby reducing the availability of our cash flow for operations and other purposes; |
| • | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; and |
| • | place us at a competitive disadvantage compared to competitors that may have proportionately less debt and greater financial resources. |
In addition, our ability to pay or refinance our debt depends on our successful financial and operating performance, cash flows and capital resources, which in turn depend upon prevailing economic conditions and certain financial, business and other factors, many of which are beyond our control. These factors include, among others:
| • | economic and demand factors affecting our industry; |
| • | pricing pressures; |
| • | increased operating costs; |
| • | competitive conditions; and |
| • | other operating difficulties. |
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay capital expenditures, sell material assets or operations, seek to obtain additional capital, or restructure our debt. There is no assurance we will be able to access capital on terms that would be acceptable. In the event that we are required to dispose of material assets or operations to meet our debt service and other obligations, the value realized on such assets or operations will depend on market conditions and the availability of buyers. Accordingly, any such sale may not, among other things, be for a sufficient dollar amount. The convertible promissory note is secured by a security interest in all of our operating company’s inventories, receivables and proceeds from those items. The foregoing encumbrances may limit our ability to dispose of material assets or operations. We also may not be able to restructure our indebtedness on favorable economic terms, if at all.
16
Table of Contents
We may incur additional indebtedness in the future. Our incurrence of additional indebtedness would intensify the risks described above.
For a description of our indebtedness see “Item 7. Management’s Discussion and Analysis of Financial Conditions and Results of Operations–Liquidity and Capital Resources.”
Our convertible promissory note agreement contains covenants that limit our ability to incur additional debt and grant liens on assets.
Our convertible promissory note agreement that we have entered into with Ryan Drexler, Interim Chief Executive Officer, President and Chairman, contains restrictive covenants that limit our ability to, among other things, incur additional debt and grant liens on assets. If we fail to comply with the restrictions in such convertible promissory note agreement, a default may allow Mr. Drexler to accelerate the related debt and to exercise his remedies under the agreement, which includes the right to declare the principal amount of that debt, together with accrued and unpaid interest and other related amounts, immediately due and payable, and to exercise any remedies he may have to foreclose on assets that are subject to liens securing that debt.
For additional details regarding our indebtedness, see Note 9 to our Consolidated Financial Statements.
If we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure controls and procedures, or, if we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our stock price could decline significantly and raising capital could be more difficult.
If we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure controls and procedures, or, if we discover material weaknesses and other deficiencies in our internal control and accounting procedures, our business could be adversely impacted. Continued effective internal controls are necessary for us to produce reliable financial reports and are important to helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed and investors could lose confidence in our reported financial information. In the past, we have discovered significant deficiencies in our internal controls and procedures with respect to perquisites, related party transactions and certain other matters that did not amount to material weaknesses. While we have implemented new controls regarding these deficiencies, we may not have rectified all of these matters and may continue to face internal control failures resulting in potential legal or regulatory issues if we fail to take corrective actions.
Our common stock is quoted on the OTCQB which may have an unfavorable impact on our stock price and liquidity.
Our common stock is quoted on the OTCQB Marketplace (“OTCQB”). The OTCQB is an automated quotation service operated by OTC Markets, LLC. The quotation of our shares on the OTCQB may result in a less liquid market available for existing and potential stockholders to trade shares of our common stock, in part because of the inability or unwillingness of certain investors to acquire shares of common stock not traded on a national securities exchange, and could depress the trading price of our common stock and have a long-term adverse impact on our ability to raise capital in the future.
Nevada corporation laws limit the personal liability of corporate directors and officers and require indemnification under certain circumstances.
Section 78.138(7) of the Nevada Revised Statutes provides that, subject to certain very limited statutory exceptions or unless the articles of incorporation provide for greater individual liability, a director or officer of a Nevada corporation is not individually liable to the corporation or its stockholders for any damages as a result of any act or failure to act in his or her capacity as a director or officer, unless it is proven that the act or failure to act constituted a breach of his or her fiduciary duties as a director or officer and such breach involved intentional
17
Table of Contents
misconduct, fraud or a knowing violation of law. We have not included in our articles of incorporation any provision intended to provide for greater liability as contemplated by this statutory provision.
In addition, Section 78.7502(3) of the Nevada Revised Statutes provides that to the extent a director or officer of a Nevada corporation has been successful on the merits or otherwise in the defense of certain actions, suits or proceedings (which may include certain stockholder derivative actions), the corporation shall indemnify such director or officer against expenses (including attorneys’ fees) actually and reasonably incurred by such director or officer in connection therewith.
Our articles of incorporation, our amended and restated by-laws and Nevada law could deter a change of our management, which could discourage or delay offers to acquire us.
Certain provisions of Nevada law and of our articles of incorporation, and by-laws, as amended, could discourage or make it more difficult to accomplish a proxy contest or other change in our management or the acquisition of control by a holder of a substantial amount of our voting stock. It is possible that these provisions could make it more difficult to accomplish, or could deter transactions that stockholders may otherwise consider to be in their best interests or in our best interests. These provisions include:
| • | requiring stockholders who wish to request a special meeting of the stockholders to disclose certain specified information in such request and to deliver such request in a specific way within a certain timeframe, which may inhibit or deter stockholders from requesting special meetings of the stockholders; |
| • | requiring that stockholders who wish to act by written consent request a record date from us for such action and such request must include disclosure of certain specified information, which may inhibit or deter stockholders from acting by written consent; |
| • | establishing the Board as the sole entity to fill vacancies of the Board of Directors, which lengthens the time needed to elect a new majority of the board; |
| • | establishing a two-thirds majority vote of the stockholders to remove a director from the Board of Directors, as opposed to a simple majority, which lengthens the time needed to elect a new majority of the board; |
| • | establishing that any person who acquires equity in us shall be deemed to have notice and consented to the forum selection provision of our Bylaws requiring actions to be brought only in New York, which may inhibit or deter stockholders actions (i) on behalf of us, (ii) asserting claims of breach of fiduciary duty by officers or directors of us, or (iii) arising out of the Nevada Revised Statutes; and |
| • | establishing more detailed disclosure in any stockholder’s advance notice to nominate a new member of the Board, including specified information regarding such nominee, which may inhibit or deter such nomination and lengthen the time needed to elect a new majority of the board. |
| Item 1B. | Unresolved Staff Comments |
None.
18
Table of Contents
| Item 2. | Properties |
As of December 31, 2015, we leased office facilities across the U.S., Canada and Ireland totaling approximately 200,000 square feet, including approximately 30,000 square feet for our corporate headquarters and MP Sports Science Center in Denver, Colorado. We do not own any real property. Our office space and locations as of December 31, 2015 can be seen in the below table.
| Location |
Function |
Approximate Square Feet |
Expiration Date of Lease |
Monthly Rent |
||||||||||||
| Denver, CO |
Company Headquarters, MP Sports Science Center | 30,302 | December 31, 2020 | $ | 10,500 | |||||||||||
| Denver, CO(1) |
Clinical study testing | 496 | — |
$ | 1,033 | |||||||||||
| Denver, CO |
Administration and Finance | 10,300 | June 30, 2020 | $ | 7,717 | |||||||||||
| Hamilton, Ontario, Canada(1) |
MP Canada subsidiary, including warehouse, distribution, and sales | 10,000 | — |
CAD | 8,333 | |||||||||||
| Miami, FL(1) |
Sales, product development, and strategy | 1,450 | — |
$ | 3,730 | |||||||||||
| Franklin, TN |
Warehouse and distribution | 152,562 | August 31, 2015 | $ | 25,833 | |||||||||||
| Spring Hill, TN |
Warehouse and distribution | 52,740 | June 30, 2020 | $ | 18,652 | |||||||||||
| Boise, ID(1) |
Finance and sales | 14,376 | — |
$ | 8,000 | |||||||||||
| Boise, ID(1) |
Finance and sales | 9,600 | — |
$ | 6,233 | |||||||||||
| Columbus, OH(1) |
Social media and customer service center | 8,500 | — |
$ | 1,500 | |||||||||||
| Dublin, Ireland |
European sales and operations | 450 | December 31, 2016 | € | 1,800 | |||||||||||
| Pittsburg, CA |
Liquid, gel manufacturing and distribution | 101,511 | August 31, 2019 | $ | 32,426 | |||||||||||
| Pittsburg, CA |
Research and development quality control | 17,500 | February 28, 2029 | $ | 24,294 | |||||||||||
| (1) | These leased office facilities were part of our restructuring announced in August 2015. We have exited the facilities and recorded a restructuring liability of the estimated future payments related to these abandoned leaseholds. |
| Item 3. | Legal Proceedings |
The following is a summary of the more significant legal matters involving our company.
The Company is engaged in a dispute with Capstone concerning amounts allegedly owed under the Manufacturing Agreement. Capstone claims that it is owed approximately $22.0 million of outstanding accounts payable. The Company claims that Capstone owes the Company at least $13.5 million in losses caused by, among other things, Capstone’s failure to timely manufacture and supply the Company’s products. On February 12, 2016, Capstone commenced a mediation with the American Arbitration Association. As of the date of this report, the mediation has not yet been scheduled.
On January 15, 2016, ThermoLife International LLC (“ThermoLife”), a supplier of nitrates to MusclePharm, filed a complaint against the Company in Arizona state court. In its complaint, ThermoLife alleges that the Company failed to meet minimum purchase requirements contained in the parties’ supply agreement. On March 14, 2016, the Company filed an answer to ThermoLife’s complaint denying the allegations contained in the complaint, and a counterclaim alleging that ThermoLife breached its express warranty to MusclePharm because ThermoLife’s products were defective and could not be incorporated into the Company’s products. The action is pending.
As previously disclosed by us in a Current Report on Form 8-K filed with the SEC on January 6, 2016, on December 30, 2015, we accepted notice by Mr. Richard Estalella (“Estalella”) to terminate his employment as
19
Table of Contents
our President. Although Estalella sought to terminate his employment with us for “Good Reason,” as defined in Estalella’s employment agreement with us, we advised Estalella that we deemed his resignation to be without Good Reason.
On February 3, 2016, Estalella filed a complaint in Colorado state court against the Company and the Interim Chief Executive Officer, President and Chairman, alleging, among other things, that we breached the employment agreement, and seeking certain equitable relief and unspecified damages. The Company believes Estalella’s claims are without merit. Estalella remains a member of our Board of Directors.
The Company is engaged in a dispute with ETW Corp. (“ETW”) concerning the validity of, and payments allegedly owed under, amendments to an endorsement agreement with professional golfer Tiger Woods, (the “Endorsement Agreement”). ETW claims that the Company owes approximately $7.0 million under the Endorsement Agreement. The Company believes that it does not owe any amounts under the Endorsement Agreement and has demanded the return of payments previously made. The parties have agreed to mediate the dispute. The mediation has yet to be scheduled.
On October 27, 2015 Brian D. Gartner, derivatively and on behalf of MusclePharm Corporation, filed a verified shareholder derivative complaint in the 8th District Court, State of Nevada, Clark County (No. A-15-726810-B) alleging, among other things, breaches of fiduciary duty as members of the Board of Directors and/or executive officers of the Company against Brad Pyatt, Lawrence S. Meer, Donald W. Prosser, Richard Estalella, Jeremy R. Deluca, Michael J. Doron, Cory Gregory, L. Gary Davis, James J. Greenwell, John H. Bluher and Daniel J. McClory. Plaintiff alleges a series of accounting and disclosure failures resulted in the filing of materially false and misleading filings with the SEC from 2010 through July 2014 resulting in settlement with the SEC requiring payment of $700,000 of civil penalties. Plaintiff seeks various remedies, including interpretation of bylaws provisions, permanent injunctive relief, damages against defendants for breaches of their fiduciary duty, corporate governance changes to ensure the Company maintain proper internal controls and SEC reporting procedures, as well as costs and reasonable attorney’ fees, accountants’ and experts’ fees, costs and expenses. Individual defendants seek removal of the action to federal court and a scheduling stipulation is contemplated.
In July 2013, we received a formal order of investigation (the “Investigation”) from the Denver Regional Office of the SEC, which was investigating various areas of potential violation of the federal securities laws involving us and our management.
In September 2015, our proposal regarding final settlement of the Investigation was accepted and all aspects of the Investigation related to us were terminated. Without admitting or denying the SEC claims, we agreed to a payment of $700,000, which was previously accrued for, and $400,000 had already been paid into escrow. We also agreed to appoint an independent consultant, mutually acceptable to the Company and SEC, for a 12-month period to monitor the Company’s reporting practices and internal controls. We and the SEC agreed to the appointment of Chord Advisors, LLC.
During 2014, we submitted insurance claims for coverage of our costs associated with the Investigation to our insurance carrier which has denied payment of our claims. On February 12, 2015, we filed a complaint in the District Court, City and County of Denver, Colorado against Liberty claiming wrongful and unreasonable denial of coverage for the cost and expenses that we have incurred and continued to incur in connection with SEC investigation and related matters under our Directors and Officers insurance policies. We have commenced litigation against our insurer. The claims and the litigation may make it more difficult for us to obtain insurance at competitive prices or at all and our insurance costs may increase as a result.
| Item 4. | Mine Safety Disclosures |
None.
20
Table of Contents
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
The following table shows the reported high and low bid quotations per share for our common stock based on information provided by the OTCQB, based upon the closing price. Our common stock is quoted on the OTCQB under the symbol “MSLP”.
| High | Low | |||||||
| 2015 |
||||||||
| Fourth Quarter |
$ | 3.96 | $ | 2.00 | ||||
| Third Quarter |
$ | 6.19 | $ | 2.80 | ||||
| Second Quarter |
$ | 6.85 | $ | 4.05 | ||||
| First Quarter |
$ | 9.84 | $ | 3.88 | ||||
| 2014 |
||||||||
| Fourth Quarter |
$ | 14.04 | $ | 8.18 | ||||
| Third Quarter |
$ | 13.80 | $ | 10.91 | ||||
| Second Quarter |
$ | 12.15 | $ | 6.65 | ||||
| First Quarter |
$ | 9.20 | $ | 6.25 | ||||
| 2013 |
||||||||
| Fourth Quarter |
$ | 10.50 | $ | 7.30 | ||||
| Third Quarter |
$ | 13.10 | $ | 9.60 | ||||
| Second Quarter |
$ | 12.47 | $ | 8.06 | ||||
| First Quarter |
$ | 11.50 | $ | 3.90 | ||||
Quotations on the OTCQB reflect bid and ask quotations, may reflect inter-dealer prices, without retail markup, markdown or commission, and may not represent actual transactions.
Our transfer agent is Corporate Stock Transfer, Inc., which is located at 3200 Cherry Creek Drive South, Suite 430, Denver, Colorado 80209.
Holders of Record
As of March 7, 2016, there were approximately 341 holders of record of our common stock. This figure does not take into account those stockholders whose certificates are held in street name by brokers and other nominees.
Unregistered Sale of Securities
None.
Dividend Policy
We have never declared dividends on our common stock, and currently do not plan to declare dividends on shares of our common stock in the foreseeable future. We expect to retain our future earnings, if any, for use in the operation and expansion of our business. Subject to the foregoing, the payment of cash dividends in the future, if any, will be at the discretion of our Board of Directors and will depend upon such factors as restrictions in debt agreements, earnings levels, capital requirements, our overall financial condition and any other factors deemed relevant by our Board of Directors.
21
Table of Contents
Performance Graph
This performance graph shall not be deemed “soliciting material” or to be “filed” with the SEC for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (Exchange Act), or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any filing of MusclePharm Corporation under the Securities Act or the Exchange Act.
The following graph compares the performance of our common stock to the Standard & Poor’s 500 Stock Index (S&P 500 Index) and the Nasdaq Composite Index (NASDAQ Composite) from November 26, 2012 (the effective date of our 1 for 850 reverse stock split) through December 31, 2015. The comparison assumes $4.59 (the closing price of our common stock on November 26, 2012) was invested in our common stock and in each of the foregoing indices, and it assumes reinvestment of dividends, if any.
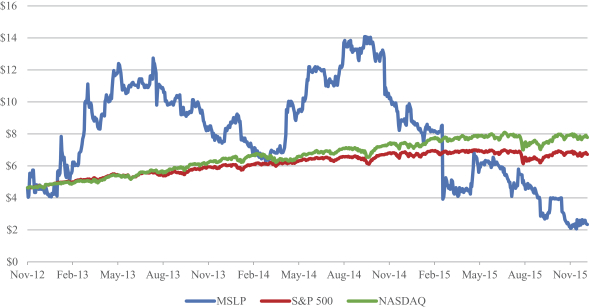
22
Table of Contents
| Item 6. | Selected Financial Data |
The consolidated statements of operations data for each of the years ended December 31, 2015, 2014 and 2013 and the consolidated balance sheets data as of December 31, 2015 and 2014 are derived from our audited consolidated financial statements included in Part II, Item 8, “Consolidated Financial Statements and Supplementary Data” of this Annual Report on Form 10-K. The consolidated statements of operations data for the years ended December 31, 2012 and 2011 and the consolidated balance sheets data as of December 31, 2013, 2012 and 2011 are derived from our audited consolidated financial statements that are not included in this Annual Report on Form 10-K. Our historical results are not necessarily indicative of our results in any future period.
You should read the following selected consolidated financial data in conjunction with Part II, Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included in Part II, Item 8, “Consolidated Financial Statements and Supplementary Data” of this Annual Report on Form 10-K.
| Year Ended December 31, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| (in thousands, except share and per share data) | ||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Revenue, net |
$ | 166,858 | $ | 177,389 | $ | 110,878 | $ | 67,055 | $ | 17,213 | ||||||||||
| Cost of revenue (1) |
109,927 | 121,379 | 77,686 | 52,727 | 14,845 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
56,931 | 56,010 | 33,192 | 14,328 | 2,368 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating expenses: |
||||||||||||||||||||
| Advertising and promotion |
26,985 | 28,053 | 15,535 | 8,430 | — | |||||||||||||||
| Salaries and benefits |
31,176 | 25,347 | 11,831 | 4,597 | — | |||||||||||||||
| Selling, general and administrative |
19,372 | 13,354 | 7,173 | 4,634 | 18,588 | |||||||||||||||
| Research and development |
4,251 | 3,997 | 1,119 | 278 | — | |||||||||||||||
| Professional fees |
6,801 | 4,635 | 11,831 | 5,125 | — | |||||||||||||||
| Restructuring and other charges |
18,293 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
106,878 | 75,386 | 47,489 | 23,064 | 18,588 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(49,947 | ) | (19,376 | ) | (14,297 | ) | (8,736 | ) | (16,220 | ) | ||||||||||
| Other (expense) income, net |
(1,806 | ) | 5,577 | (3,306 | ) | (10,217 | ) | (7,061 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss before provision for income taxes |
(51,753 | ) | (13,799 | ) | (17,603 | ) | (18,953 | ) | (23,281 | ) | ||||||||||
| Provision for income taxes |
105 | 33 | 115 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (51,858 | ) | $ | (13,832 | ) | $ | (17,718 | ) | $ | (18,953 | ) | $ | (23,281 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per share, basic and diluted |
$ | (3.81 | ) | $ | (1.25 | ) | $ | (2.46 | ) | $ | (13.00 | ) | $ | (70.30 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted-average shares used to compute net loss per share, basic and diluted |
13,621,255 | 11,038,761 | 7,193,784 | 1,458,757 | 331,158 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Cost of revenue for the year ended December 31, 2015 included restructuring charges of $2,942, which is comprised of i) $2,592 related to write-down of inventory, and ii) $350 related to purchase commitment of discontinued inventories not yet received by us, which remains accrued at December 31, 2015. |
| As of December 31, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Consolidated Balance Sheet Data: |
||||||||||||||||||||
| Cash |
$ | 7,081 | $ | 1,020 | $ | 5,412 | $ | — | $ | 660 | ||||||||||
| Working (deficit) capital |
(20,009 | ) | 3,587 | 12,158 | (11,571 | ) | (13,693 | ) | ||||||||||||
| Property and equipment, net |
6,693 | 7,805 | 2,614 | 1,356 | 908 | |||||||||||||||
| Total assets |
63,781 | 66,356 | 52,158 | 6,767 | 5,046 | |||||||||||||||
| Total indebtedness |
11,922 | 8,046 | 2,563 | 4,468 | 1,589 | |||||||||||||||
| Total liabilities |
73,849 | 42,976 | 32,423 | 16,525 | 18,017 | |||||||||||||||
| Total stockholders’ (deficit) equity |
(10,068 | ) | 23,380 | 19,735 | (9,758 | ) | (12,971 | ) | ||||||||||||
23
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes included elsewhere in this Annual Report on Form 10-K. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed below. See “Forward Looking Statements” and “Item 1A. Risk Factors.”
Overview
We are a scientifically driven, performance lifestyle company that develops, manufactures, markets and distributes branded nutritional supplements. We offer a broad range of powders, capsules, tablets and gels. Our portfolio of recognized brands, including MusclePharm® Sport Series, and FitMiss®, are marketed and sold in more than 120 countries and available in over 50,000 retail outlets globally. These clinically developed scientifically driven nutritional supplements are developed through a six-stage research process that utilizes the expertise of leading nutritional scientists, doctors and universities. We believe we are an innovator in the sports nutrition industry.
Our primary growth strategy is to:
| • | drive innovation, serve the needs of all athletes and fuel the engine of sport through new products and brand extensions; |
| • | increase our product distribution and sales through increased market penetrations both domestically and internationally; |
| • | increase our margins by negotiating reductions in the cost of our products; |
| • | continue to conduct additional testing of the safety and efficacy of our products and formulate new products to differentiate our products from our competition; and |
| • | increase awareness of our products through marketing and branding opportunities with cost effective brand partnerships and grass roots marketing and advertising. |
Restructuring Plan
On August 24, 2015, our Board of Directors approved a restructuring plan for the Company. The approved restructuring plan was designed to reduce costs and to better align our resources for profitable growth. Specifically, through December 31, 2015, the restructuring plan resulted in: 1) a reduction in our workforce; 2) abandoning certain leased facilities; 3) renegotiating or terminating a number of contracts with endorsers in a strategic shift away from such arrangements and towards more grass-roots marketing and advertising efforts; 4) discontinuing a number of SKU’s due to lower than expected revenue or margin performance and writing down inventory to estimated sales price; and 5) writing off certain assets. The Company completed additional reductions in workforce during the first quarter of 2016. Management is continuing to execute the approved restructuring plan, and as such, additional restructuring charges may be necessary.
Components of Results of Operations
Revenue
Revenues in 2015, 2014 and 2013 were $166.9 million, $177.4 million and $110.9 million, respectively, while our net losses were $51.9 million, $13.8 million and $17.7 million, respectively, for the same periods.
We derive our revenues through the sales of our various branded nutritional supplements. As discussed further in “—Critical Accounting Policies and Estimates—Revenue Recognition”, revenue is recognized when persuasive
24
Table of Contents
evidence of an arrangement exists, delivery has occurred, the price is fixed or determinable and collection is reasonably assured, which generally occurs upon shipment or delivery of the products. We record sales incentives as a direct reduction of revenue for various discounts provided to our customers consisting primarily of volume incentive rebates and advertising related credits. We accrue for sales discounts over the period they are earned. Sales discounts are a significant part of our marketing plan to our customers as they help drive increased sales and brand awareness with end users through promotions that we support through our distributors and re-sellers.
During 2015, our three largest customers, Costco, GNC and Bodybuilding.com, accounted for 41% of our net revenue. During 2014, our two largest customers, Costco and Bodybuilding.com, accounted for 29% of our net revenue. During 2013, our two largest customers, Bodybuilding.com and Europa, accounted for 35% of our net revenue.
Cost of Revenue and Gross Margin
Cost of revenue for MusclePharm products is directly related to the production, manufacturing and freight-in of the related products purchased from third party contract manufacturers. We mainly ship customer orders from our distribution centers in Spring Hill, Tennessee and Pittsburg, California. The facilities are operated with our equipment and employees, and we own the related inventory. We also use contract manufacturers to drop ship products directly to our customers.
In addition, BioZone, established in 2014 via an asset purchase agreement, manufactures products and, therefore, derives costs of revenue through the costs of raw materials, direct labor, freight-in and other supply and equipment expenses. We mainly ship BioZone customer orders from our distribution center in Pittsburg, California.
Our historical experience has been that, over the life cycle of a particular product, the cost of revenues as a percentage of total revenue has typically declined as a result of efficiencies and decreases in our product costs. The decrease in cost generally results from an increase in the volume purchased from manufacturing suppliers, as well as yield improvements and test enhancements.
Our gross profit fluctuates due to several factors, including sales incentives, new product introductions and upgrades to existing product lines, changes in customer and product mixes, the mix of product demand, shipment volumes, our product costs, pricing and inventory write downs. Cost of revenue is expected to decrease over time as a percentage of revenue due primarily to our focus on supply chain efficiency and negotiating better pricing with our manufacturers.
Operating Expenses
Advertising and Promotion
Our advertising and promotions consists primarily of athletic endorsements, and sponsorships which includes both cash and stock-based compensation, promotional giveaways, trade show events, FDM distribution costs, and digital, print, and media advertising.
Prior to our restructuring during the third quarter of 2015, advertising and promotions were a large part of both our growth strategy and brand awareness. We built strategic partnerships with sports athletes and fitness enthusiasts through endorsements, licensing, and co-branding agreements. Additionally, we have co-developed products with sports athletes and teams. We expect our advertising and promotion expenses to decrease in future periods as we seek to leverage existing brand recognition and move towards lower cost advertising outlets including social media.
Salaries and Benefits
Salaries and benefits consist primarily of salaries, bonuses, benefits and stock-based compensation. Personnel costs are a significant component of our operating expenses. During the third quarter of 2015, we executed a restructuring plan, resulting in a reduction in our workforce that continued through year-end. Salaries and benefits is expected to decrease due to a further reduction in headcount, limited headcount additions as well as future restricted stock awards, and a reduction in amortization of existing stock-based grants.
25
Table of Contents
Selling, General and Administrative
Our selling, general and administrative expenses consist primarily of freight out, depreciation, amortization, IT equipment and network, facilities related expenses, insurance, director’s fees, which include both cash and stock-based compensation, rental expenses related to equipment leases, supplies, legal settlement costs, and other corporate expenses. We expect our selling, general and administrative expenses to decrease due to our restructuring activities, as we have abandoned certain leased facilities and anticipate the disposal of our BioZone subsidiary.
Research and Development
Research and development expenses consist primarily of R&D personnel salaries, bonuses, benefits, and stock-based compensation, product quality control, which includes third-party testing, and research fees related to the development of new products. We expense research and development costs as incurred. Research and development is not a primary driver of our operating expenses and we expect research and development to decrease in future periods, as we have reduced the number of employees in this department.
Professional Fees
Professional fees consist primarily of legal fees, accounting and audit fees, consulting fees, which includes both cash and stock-based compensation, and investor relations costs. We expect our professional fees to increase in connection with our restructuring plan as we utilize professional service providers to defend ongoing and new legal matters and advise on corporate governance, internal controls, and process improvements.
Charitable Youth Sports Program
In March 2014, the Board of Directors of the Company approved and the Company established a charitable youth sports grant program (the “Program”) pursuant to which the Company will donate product, equipment and cash to organizations such as schools, sports teams and training facilities. The Company had tentatively established an annual budget of approximately $250,000 for the Program. The primary intent of the Program was to build MusclePharm brand awareness with youth athletes. The Company’s other business purposes in establishing the Program was to help needy organizations achieve their goals, promote the Company’s brand, help athletes develop stronger and better skills and to build the reputation of the Company as a contributor to the community. A committee formerly consisting of the Company’s former President, former Director of Team Development, and former Chief Operating Officer oversaw the Program. In 2014, the Company made an initial grant in the amount of approximately $250,000 to Arvada West High School and similar charitable contributions to other charitable sports organizations of approximately $30,000. The Company’s former Chief Executive Officer, Mr. Brad Pyatt, is a graduate of Arvada West High School and serves as a volunteer football coach. The Company did not make a charitable grant to Arvada West High School during 2015. The Company did make charitable grants to other youth sports organizations during 2015 totaling approximately $278,000. We expect this amount to decrease substantially in 2016 and any future grant will be approved by the Chief Executive Officer and Chief Financial Officer.
Other Income (Expense), Net
Other income (expense), net consists of interest, bargain purchase gain and contingent asset gain from a business acquisition, expenses related to the issuance and change in fair value of derivative liabilities, gains and losses on foreign currency transactions and purchased convertible notes, settlement of accounts payable and debt, foreign currency transactions and other miscellaneous expenses. The most significant of these consist of interest expense, the change in the fair value of our derivative liabilities and the bargain purchase gain and contingent asset gain from the business acquisition. Our derivative liabilities were related to embedded conversion features in certain shares of previously outstanding convertible preferred stock and warrants. These derivative instruments were no longer outstanding as of December 31, 2015 and 2014. However, prior to their settlement, they were marked to fair value each
26
Table of Contents
period with a corresponding gain or loss recognized through the consolidated statements of operations. Our bargain purchase gain and contingent asset gain was a one-time event related to the BioZone asset acquisition in January 2014. We do not expect to recognize any amounts from changes in fair value of derivative instruments or any bargain purchase gains going forward unless we enter into similar transactions again in the future. We do expect interest expense to increase in the near term, based on the interest rate on the convertible secured promissory note that we entered into in December 2015 and the Factor Agreement we entered into in January 2016.
Provision for Income Taxes
Provision for income taxes consists primarily of federal and state income taxes in the United States and income taxes in foreign jurisdictions in which we conduct business. Due to uncertainty as to the realization of benefits from our deferred tax assets, including net operating loss carry-forwards, research and development and other tax credits, we have a full valuation allowance reserved against such assets. We expect to maintain this full valuation allowance at least in the near term. Further, we are engaged with a Big Four accounting firm to advise us on our domestic and international tax strategy.
Results of Operations
The following table presents our historical operating results in dollars and as a percentage of revenues for the periods presented:
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| (in thousands) | ||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||
| Revenue, net |
$ | 166,858 | $ | 177,389 | $ | 110,878 | ||||||
| Cost of revenue (1) |
109,927 | 121,379 | 77,686 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
56,931 | 56,010 | 33,192 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Advertising and promotion |
26,985 | 28,053 | 15,535 | |||||||||
| Salaries and benefits |
31,176 | 25,347 | 11,831 | |||||||||
| Selling, general and administrative |
19,372 | 13,354 | 7,173 | |||||||||
| Research and development |
4,251 | 3,997 | 1,119 | |||||||||
| Professional fees |
6,801 | 4,635 | 11,831 | |||||||||
| Restructuring and other charges |
18,293 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
106,878 | 75,386 | 47,489 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(49,947 | ) | (19,376 | ) | (14,297 | ) | ||||||
| Other (expense) income, net |
(1,806 | ) | 5,577 | (3,306 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before provision for income taxes |
(51,753 | ) | (13,799 | ) | (17,603 | ) | ||||||
| Provision for income taxes |
105 | 33 | 115 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (51,858 | ) | $ | (13,832 | ) | $ | (17,718 | ) | |||
|
|
|
|
|
|
|
|||||||
| (1) | Cost of revenue for the year ended December 31, 2015 included restructuring charges of $2,942, which is comprised of i) $2,592 related to write-down of inventory, and ii) $350 related to purchase commitment of discontinued inventories not yet received by us, which remains accrued at December 31, 2015. |
27
Table of Contents
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Percentage of Revenue: |
||||||||||||
| Revenue, net |
100 | % | 100 | % | 100 | % | ||||||
| Cost of revenue |
66 | 68 | 70 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
34 | 32 | 30 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Advertising and promotion |
16 | 16 | 14 | |||||||||
| Salaries and benefits |
19 | 14 | 11 | |||||||||
| Selling, general and administrative |
12 | 8 | 6 | |||||||||
| Research and development |
2 | 2 | 1 | |||||||||
| Professional fees |
4 | 3 | 11 | |||||||||
| Restructuring and other charges |
11 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
64 | 43 | 43 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(30 | ) | (11 | ) | (13 | ) | ||||||
| Other (expense) income, net |
(1 | ) | 3 | (3 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net income loss before taxes |
(31 | ) | (8 | ) | (16 | ) | ||||||
| Provision for income taxes |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(31 | )% | (8 | )% | (16 | )% | ||||||
|
|
|
|
|
|
|
|||||||
Revenue
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Revenue, net |
$ | 166,858 | $ | 177,389 | $ | 110,878 | (6 | )% | 60 | % | ||||||||||
2015 compared to 2014. Revenue, net decreased $10.5 million or 6% to $166.9 million for the year ended December 31, 2015, compared to $177.4 million for the year ended December 31, 2014. Revenue, net for the year ended December 31, 2015 decreased due primarily to a misalignment of inventory on hand compared to customer sales orders, a reduction in international sales related to the strengthening of the US dollar and timing of the launch of the Arnold Schwarzenegger Series™ which was introduced late in 2013, resulting in significant initial sales during the first half of 2014 related to initial channel fill. Discounts and sales allowances increased to $29.5 million, or 15% of gross revenue, for the year ended December 31, 2015 from $28.2 million, or 14% of gross revenue, in 2014. The increase in discounts and allowances is primarily related to promotions of new product introductions and discounts and allowances on existing products with key customers.
2014 compared to 2013. Revenue, net increased 60% to $177.4 million in 2014, compared to $110.9 million in 2013. Revenue in 2014 increased due primarily to the introduction of two brands to our product portfolio as we continue to extend our brand and reach utilizing well known current and former athletes and moving into new and expanding markets. In particular, our Arnold Schwarzenegger Series™, which launched in the fourth quarter of 2013, resulted in an increase of $33.1 million from $16.5 million in 2013 to $49.6 million in 2014. In addition, our FitMiss® series, which launched in the third quarter of 2013, resulted in an increase of $6.9 million from $2.6 million in 2013 to $9.5 million in 2014. We also recognized an increase of $28.5 million due to sales of our existing products as we continued to execute our growth strategy that includes driving innovation, serving the needs of all athletes, fueling the engine of sport through new products, brand extensions, and increasing our product distribution and sales through increased market penetrations both domestically and internationally. We also acquired BioZone, which resulted in a $12.4 million increase of revenue in 2014 compared to zero in 2013.
28
Table of Contents
Discounts and sales allowances increased to $28.2 million, or 14%, of gross revenue in 2014 from $17.4 million, or 14%, of gross revenue in 2013. The decrease in discounts and allowances as a percent of gross revenue is a result of continued focus to define customer terms and allowances.
Cost of Revenue and Gross Profit
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Cost of revenue (1) |
$ | 109,927 | $ | 121,379 | $ | 77,686 | (9 | )% | 56 | % | ||||||||||
| (1) | Cost of revenue for the year ended December 31, 2015 included restructuring charges of $2,942, which is comprised of i) $2,592 related to write-down of inventory, and ii) $350 related to purchase commitment of discontinued inventories not yet received by us, which remains accrued at December 31, 2015. |
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Gross profit |
$ | 56,931 | $ | 56,010 | $ | 33,192 | 2 | % | 69 | % | ||||||||||
| Gross profit % |
34 | % | 32 | % | 30 | % | NA | NA | ||||||||||||
2015 compared to 2014. Costs of revenue decreased 9% to $109.9 million for the year ended December 31, 2015, compared to $121.4 million for the same period in 2014. Accordingly, gross profit for the year ended December 31, 2015 was $56.9 million, or 34% of revenue, compared to $56.0 million, or 32% of revenue in 2014. During the third and fourth quarter of 2015, we restructured our business, discontinuing a number of products and recognizing a restructuring charge of $2.9 million, including an inventory write down of $2.6 million and accrual of purchase commitment of discontinued inventories not yet received of $350,000, which is included as a component of cost of revenue. This inventory write down negatively impacted our gross profit margin by 2%. The gross profit margin, when excluding this inventory write down, increases to 36% as we continue to optimize our product cost with our contract manufacturing partners.
2014 compared to 2013. Costs of revenue increased 56% to $121.4 million for the year ended December 31, 2014, compared to $77.7 million in 2013. Accordingly, gross profit for 2014 was $56.0 million, or 32% of revenue, compared to $33.2 million, or 30% of revenue in 2013. Our cost of revenue increased consistent with the related revenue and the primary components of the increase were an $18.0 million increase from the Arnold Schwarzenegger Series™, a $3.0 million increase from the FitMiss series, and an $18.4 million increase from existing products related primarily to executing our growth strategy. In addition, we recognized an increase in cost of revenue in the amount of $5.8 million due to the inclusion of BioZone, which was acquired in the first quarter of 2014. The increase in gross profit was generally due to improved focus on process efficiencies throughout all departments including a decrease in spoilage, and the integration and continued improvement of enterprise resource planning (ERP) and reporting systems.
Operating Expenses
2015 compared to 2014. Operating expenses for the year ended December 31, 2015 were $106.9 million, compared to $75.4 million in 2014. These expenses primarily include costs for advertising and promotions, specifically tradeshow costs to generate visibility and connect with our customers and end-users, costs of strategic partnerships with athletes and sports teams, strategic advertising agreements to promote our brand, investing in our staffing needs in order to stay competitive in our industry, stock-based compensation, legal and litigation related fees, consulting costs, and other charges. Additionally, as a result of the restructuring plan commenced during the third quarter of 2015, we incurred restructuring costs of $18.3 million related to: 1) a reduction in our workforce; 2) abandoning certain leased facilities; and 3) renegotiating or terminating a number of contracts with endorsers in a strategic shift away from such arrangements and towards more grass-roots marketing and advertising efforts.
29
Table of Contents
2014 compared to 2013. Operating expenses for the year ended December 31, 2014 were $75.4 million, compared to $47.5 million in 2013. These expenses primarily included costs for advertising and promotions, specifically tradeshow costs to generate visibility and connect with our customers and end-users, costs of strategic partnerships with star athletes and strategic advertising agreements to promote our brand, investing in our staffing needs in order to stay competitive in our industry, developing and testing new products, stock-based compensation and other charges.
Advertising and Promotion
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Advertising and promotion |
$ | 26,985 | $ | 28,053 | $ | 15,535 | (4 | )% | 81 | % | ||||||||||
| Percentage of revenue |
16 | % | 16 | % | 14 | % | ||||||||||||||
2015 compared to 2014. Advertising and promotion expenses decreased 4% to $27.0 million for the year ended December 31, 2015, or 16% of revenue, compared to $28.1 million, or 16% of revenue, in 2014. Advertising and promotion expenses for the year ended December 31, 2015 included expenses related to strategic partnerships with athletes and sports teams. The expense associated with these partnerships decreased by $0.3 million as our agreement with the UFC expired during the fourth quarter of 2015 and we renegotiated or terminated a number of contracts as part of our restructuring activities. Additionally, our digital, print, and media advertising decreased by $2.3 million in 2015. This decrease was offset by an increase in trade shows and product giveaway costs of $1.2 million and an increase in outside sales costs of $0.6 million.
2014 compared to 2013. Advertising and promotion expenses increased 81% to $28.1 million for the year ended December 31, 2014, or 16% of revenue, compared to $15.5 million, or 14% of revenue, in 2013. Advertising and promotion expenses in 2014 included expenses related to strategic partnerships entered into with Tiger Woods and Johnny Manziel. These partnerships, along with our Arnold Schwarzenegger Series™ introduced in 2013, increased our strategic partnership stock expenses to $10.2 million in 2014, compared to $1.4 million in 2013.
Salaries and Benefits
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Salaries and benefits |
$ | 31,176 | $ | 25,347 | $ | 11,831 | 23 | % | 114 | % | ||||||||||
| Percentage of revenue |
19 | % | 14 | % | 11 | % | ||||||||||||||
2015 compared to 2014. Salaries and benefits increased 23% to $31.2 million, or 19% of revenue, for the year ended December 31, 2015 compared to $25.3 million, or 14% of revenue, in 2014. The increase was due to additional resources added to both our domestic operations and our foreign subsidiaries resulting in additional expense of $4.6 million as well as an increase in stock-based compensation charges of $1.1 million.
2014 compared to 2013. Salaries and benefits increased 114% to $25.3 million, or 14% of revenue, for the year ended December 31, 2014 compared to $11.8 million, or 11% of revenue, in 2013. The increase was due to additional resources added to both our domestic operations and our Canadian subsidiary. Another primary driver of the increase in salaries and benefits is due to an increase of $7.3 million in expenses related to restricted stock award grants.
30
Table of Contents
Selling, General and Administrative
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Selling, general and administrative |
$ | 19,372 | $ | 13,354 | $ | 7,173 | 45 | % | 86 | % | ||||||||||
| Percentage of revenue |
12 | % | 8 | % | 6 | % | ||||||||||||||
2015 compared to 2014. Selling, general and administrative expenses increased 45% to $19.4 million, or 12% of revenue, for the year ended December 31, 2015 compared to $13.4 million, or 8% of revenue, in 2014. The increase was due primarily to freight shipping expense of $3.1 million, depreciation and amortization of $1.1 million related to additional capital asset purchases during 2014 and 2015, equipment rentals and supplies of $0.6 million due to the Pittsburg, California distribution center being operational for all of 2015, IT equipment and network of $0.5 million as we continue to invest in infrastructural needs, stock-based compensation related to reimbursement of administrative costs related to an attempted debt financing and grant of restricted shares to Ryan Drexler, our Interim Chief Executive Officer, President and Chairman, in consideration for executing his guarantee with a banking institution of $0.4 million, additional corporate insurance premiums of $0.4 million, director fees of $0.4 million including both cash and stock-based compensation, and facility related expenses of $0.2 million. These increases were offset by a decrease in litigation and settlement expenses of $0.6 million mainly related to the SEC settlement which was accrued for in 2014.
2014 compared to 2013. Selling, general and administrative expenses increased 86% to $13.4 million, or 8% of revenue, for the year ended December 31, 2014 compared to $7.2 million, or 6% of revenue, in 2013. The increase was primarily due to amortization of intangible assets related to our acquisition of Biozone, depreciation of acquired capital assets, rent expense insurance and investments in infrastructure costs and freight out expense, as our selling, general and administrative costs are driven by our continued growth in all aspects of operations.
Research and Development
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Research and development |
$ | 4,251 | $ | 3,997 | $ | 1,119 | 6 | % | 257 | % | ||||||||||
| Percentage of revenue |
2 | % | 2 | % | 1 | % | ||||||||||||||
2015 compared to 2014. Research and development expenses increased 6% to $4.3 million, or 2% of revenue, for the year ended December 31, 2015 compared to $4.0 million, or 2% of revenue, in 2014. The increase was mainly due to increased personnel related costs of $0.6 million including both cash and stock-based compensation, offset by a decrease in depreciation expense of fixed assets used for research and development of $0.3 million.
2014 compared to 2013. Research and development expenses increased 257% to $4.0 million, or 2% of revenue, for the year ended December 31, 2014 compared to $1.1 million, or 1% of revenue, in 2013. The increase was due to a $0.7 million increase in quality control costs as we improved the quality of our product and the operations that go into formulating and developing the product. Other cost increases were due to an increase of $0.4 million related to researching fees as we continued to develop and test new products and a $0.4 million increase in depreciation expense allocated to research and development.
Professional Fees
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Professional fees |
$ | 6,801 | $ | 4,635 | $ | 11,831 | 47 | % | (61 | )% | ||||||||||
| Percentage of revenue |
4 | % | 3 | % | 11 | % | ||||||||||||||
31
Table of Contents
2015 compared to 2014. Professional fees increased 47% to $6.8 million, or 4% of revenue, for the year ended December 31, 2015, compared to $4.6 million, or 3% of revenue, in 2014. The primary reason for the increase in professional fees is due to additional consulting fees of $1.0 million, accounting consulting fees of $0.9 million, and investor relations of $0.6 million. These increases were offset by a decrease in legal fees of $0.5 million as we resolved the SEC investigation early in 2015.
2014 compared to 2013. Professional fees decreased 61% to $4.6 million for the year ended December 31, 2014, compared to $11.8 million in 2013. The primary reason for the decrease in professional fees is due to the $6.6 million investment advisory expenses in 2013, compared to zero in 2014. In addition, professional fees in 2014 included $2.3 million of legal fees due to the SEC investigation and expenses related to SOX 404(b) preparedness.
Restructuring and Other Charges
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||
| 2015 | 2014 | 2013 | ||||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Restructuring and other charges |
$ | 18,293 | $ | — | $ | — | 100 | % | — | % | ||||||||||
| Percentage of revenue |
11 | % | — | % | — | % | ||||||||||||||
2015 compared to 2014. For the year ended December 31, 2015, we incurred total restructuring and other charges of $21.2 million, of which $18.3 million was recorded in operating expenses. The restructuring charges to be paid in cash totaled $9.1 million, which are comprised of: (i) severance and termination benefit costs related to terminated employees of $1.3 million; (ii) charge of $7.0 million related to cancellation of certain contracts and sponsorship agreements; (iii) charge of $0.3 million related to purchase commitment of discontinued inventories not yet received; and (iv) costs associated with permanently vacating certain facilities of $0.5 million. Other non-cash charges associated with the restructuring totaled $9.5 million, which are comprised of: (i) stock-based compensation of terminated employees of $2.7 million; (ii) write-down of prepaid stock compensation related to terminated endorsement agreements of $5.4 million; (iii) write-down of prepaid asset related to terminated sponsorship agreements, discontinued products, and abandoned other agreements of $1.0 million; and (iv) write-off of long-lived assets related to the abandonment of certain lease facilities of $0.4 million. We anticipate incurring additional restructuring charges in 2016 to continue to move our business toward profitable operations.
2014 compared to 2013. There were no restructuring and other charges incurred in the years ending December 31, 2014 and 2013.
Other (Expense) Income, Net
The components of our other (expense) income, net consists of the following:
| Year Ended December 31, | 2014 to 2015 % Change |
2013 to 2014 % Change |
||||||||||||||||||||||
| 2015 | 2014 | 2013 |
|
|||||||||||||||||||||
| (in thousands) | ||||||||||||||||||||||||
| Interest income |
$ | — | $ | 223 | $ | 1,442 | (100 | )% | (85 | )% | ||||||||||||||
| Interest expense |
(779 | ) | (201 | ) | (783 | ) | 288 | % | (74 | )% | ||||||||||||||
| Derivative expense |
— | — | (97 | ) | — | % | (100 | )% | ||||||||||||||||
| Change in fair value of derivative liabilities |
— | 374 | (4,854 | ) | (100 | )% | (108 | )% | ||||||||||||||||
| Gain (loss) on settlement of accounts payable and debt |
— | 31 | 574 | (100 | )% | (95 | )% | |||||||||||||||||
| Gain (loss) on purchased convertible notes |
— | (386 | ) | 445 | (100 | )% | (187 | )% | ||||||||||||||||
| Bargain purchase gain and contingent asset gain on BioZone acquisition |
— | 5,265 | — | (100 | )% | 100 | % | |||||||||||||||||
| Foreign currency transaction gain (loss) |
(1,047 | ) | 19 | (31 | ) | * | (161 | )% | ||||||||||||||||
| Other |
20 | 252 | (2 | ) | (92 | )% | * | |||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Total other (expense) income, net |
$ | (1,806 | ) | $ | 5,577 | $ | (3,306 | ) | (132 | )% | (269 | )% | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| * | Not meaningful |
32
Table of Contents
2015 compared to 2014. Other (expense) income, net in 2015 was an expense of $1.8 million, compared to an income of $5.6 million in 2014. The significant fluctuations in other (expense) income, net were primarily related to the bargain purchase gain, foreign currency transaction gains and losses, and interest expense and interest income related to 2014.
2014 compared to 2013. Other (expense) income, net for the year ended December 31, 2014 was an income of $5.6 million, compared to an expense of $3.3 million in 2013. The significant fluctuations in other (expense) income, net were primarily related to the bargain purchase gain, changes in the derivative liabilities, gains and losses on accounts payable and debt settlements and on purchased convertible notes and interest.
Other (expense) income, net increased primarily due to a $5.3 million gain on the BioZone acquisition in 2014 and the $4.9 million decrease in the fair value of the derivative liabilities in 2013.
Summary of Unaudited Quarterly and Audited Annual Operations
The following tables set forth our unaudited quarterly and audited annual consolidated statements of income data in dollars and as a percentage of total revenue for each of the eight quarters and each year in the period ended December 31, 2015. We have prepared the quarterly consolidated statements of income data on a basis consistent with the audited consolidated financial statements included in Part II, Item 8, “Consolidated Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. In the opinion of management, the financial information reflects all adjustments, consisting only of normal recurring adjustments, which we consider necessary for a fair presentation of this data. This information should be read in conjunction with the audited consolidated financial statements and related notes included in Part II, Item 8, “Consolidated Financial Statements and Supplementary Data” in this Annual Report on Form 10-K. The results of historical periods are not necessarily indicative of the results of operations for any future period.
Quarterly Trends
We have historically experienced rapid growth, which has resulted in a historical increase in our revenue and a corresponding increase in our cost of revenue and operating expenses to support our growth. The historical increase in revenue was mainly due to an increase in product sales based upon customer demand, new products introductions and brand expansion. The slight decrease in revenue during 2015 was related to supply chain issues and mis-match of inventory on hand relative to customer sales orders. The increase in quarterly operating expenses was primarily due to the continued expansion of our infrastructure, athlete and sports team endorsements, increase in product promotion, expenses related to increases in employee headcount including stock-based compensation, and legal related fees.
33
Table of Contents
| Year Ended |
Three Months Ended | Year Ended |
Three Months Ended | |||||||||||||||||||||||||||||||||||||
| Dec 31, 2015 |
Dec 31, 2015 |
Sep 30, 2015 |
Jun 30, 2015 |
Mar 31, 2015 |
Dec 31, 2014 |
Dec 31, 2014 |
Sep 30, 2014 |
Jun 30, 2014 |
Mar 31, 2014 |
|||||||||||||||||||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||||||||||||||||||||||
| Revenue, net |
$ | 166,858 | $ | 41,078 | $ | 33,982 | $ | 50,476 | $ | 41,322 | $ | 177,389 | $ | 32,672 | $ | 47,768 | $ | 46,740 | $ | 50,209 | ||||||||||||||||||||
| Cost of revenue |
109,927 | 26,499 | 23,512 | 32,978 | 26,938 | 121,379 | 25,138 | 32,812 | 31,093 | 32,336 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Gross profit |
56,931 | 14,579 | 10,470 | 17,498 | 14,384 | 56,010 | 7,534 | 14,956 | 15,647 | 17,873 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Operating expenses |
||||||||||||||||||||||||||||||||||||||||
| Advertising and promotion |
26,985 | 4,382 | 7,093 | 8,285 | 7,225 | 28,053 | 8,057 | 7,749 | 5,919 | 6,328 | ||||||||||||||||||||||||||||||
| Salaries and benefits |
31,176 | 10,671 | 5,681 | 7,763 | 7,061 | 25,347 | 8,162 | 6,041 | 5,777 | 5,367 | ||||||||||||||||||||||||||||||
| Selling, general and administrative |
19,372 | 4,642 | 4,647 | 5,121 | 4,962 | 13,354 | 5,473 | 3,652 | 2,357 | 1,872 | ||||||||||||||||||||||||||||||
| Research and development |
4,251 | 928 | 1,437 | 921 | 965 | 3,997 | 1,001 | 735 | 1,164 | 1,097 | ||||||||||||||||||||||||||||||
| Professional fees |
6,801 | 1,302 | 1,980 | 2,064 | 1,455 | 4,635 | 1,241 | 1,316 | 1,293 | 785 | ||||||||||||||||||||||||||||||
| Restructuring and other charges |
18,293 | 1,643 | 16,650 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Total operating expenses |
106,878 | 23,568 | 37,488 | 24,154 | 21,668 | 75,386 | 23,934 | 19,493 | 16,510 | 15,449 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Income (loss) from operations |
(49,947 | ) | (8,989 | ) | (27,018 | ) | (6,656 | ) | (7,284 | ) | (19,376 | ) | (16,400 | ) | (4,537 | ) | (863 | ) | 2,424 | |||||||||||||||||||||
| Other (expense) income, net |
(1,806 | ) | (716 | ) | (559 | ) | (348 | ) | (183 | ) | 5,577 | 26 | 5,234 | (27 | ) | 344 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Income (loss) before provision for income taxes |
(51,753 | ) | (9,705 | ) | (27,577 | ) | (7,004 | ) | (7,467 | ) | (13,799 | ) | (16,374 | ) | 697 | (890 | ) | 2,768 | ||||||||||||||||||||||
| Provision for (benefit from) income taxes |
105 | 1 | 71 | 21 | 12 | 33 | (138 | ) | 94 | 45 | 32 | |||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Net income (loss) |
$ | (51,858 | ) | $ | (9,706 | ) | $ | (27,648 | ) | $ | (7,025 | ) | $ | (7,479 | ) | $ | (13,832 | ) | $ | (16,236 | ) | $ | 603 | $ | (935 | ) | $ | 2,736 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Earnings (net loss) per share |
||||||||||||||||||||||||||||||||||||||||
| Basic |
$ | (3.81 | ) | $ | (0.70 | ) | $ | (2.01 | ) | $ | (0.51 | ) | $ | (0.56 | ) | $ | (1.25 | ) | $ | (1.39 | ) | $ | 0.05 | $ | (0.09 | ) | $ | 0.27 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Diluted |
$ | (3.81 | ) | $ | (0.70 | ) | $ | (2.01 | ) | $ | (0.51 | ) | $ | (0.56 | ) | $ | (1.25 | ) | $ | (1.39 | ) | $ | 0.05 | $ | (0.09 | ) | $ | 0.23 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Percentage of Revenue: |
||||||||||||||||||||||||||||||||||||||||
| Revenue, net |
100 | % | 100 | % | 100 | % | 100 | % | 100 | % | 100 | % | 100 | % | 100 | % | 100 | % | 100 | % | ||||||||||||||||||||
| Cost of revenue |
66 | 65 | 69 | 65 | 65 | 68 | 77 | 69 | 67 | 64 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Gross profit |
34 | 35 | 31 | 35 | 35 | 32 | 23 | 31 | 33 | 36 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Operating expenses |
||||||||||||||||||||||||||||||||||||||||
| Advertising and promotion |
16 | 11 | 21 | 17 | 17 | 16 | 24 | 16 | 13 | 13 | ||||||||||||||||||||||||||||||
| Salaries and benefits |
19 | 26 | 17 | 15 | 17 | 14 | 25 | 13 | 12 | 11 | ||||||||||||||||||||||||||||||
| Selling, general and administrative |
12 | 11 | 13 | 10 | 12 | 8 | 17 | 8 | 5 | 4 | ||||||||||||||||||||||||||||||
| Research and development |
2 | 2 | 4 | 2 | 2 | 2 | 3 | 1 | 2 | 2 | ||||||||||||||||||||||||||||||
| Professional fees |
4 | 3 | 6 | 4 | 4 | 3 | 4 | 3 | 3 | 2 | ||||||||||||||||||||||||||||||
| Restructuring and other charges |
11 | 4 | 49 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Total operating expenses |
64 | 57 | 110 | 48 | 52 | 43 | 73 | 41 | 35 | 32 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Income (loss) from operations |
(30 | ) | (22 | ) | (79 | ) | (13 | ) | (17 | ) | (11 | ) | (50 | ) | (10 | ) | (2 | ) | 4 | |||||||||||||||||||||
| Other (expense) income, net |
(1 | ) | (2 | ) | (2 | ) | (1 | ) | (1 | ) | 3 | — | 11 | — | 1 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Income (loss) before provision for income taxes |
(31 | ) | (24 | ) | (81 | ) | (14 | ) | (18 | ) | (8 | ) | (50 | ) | 1 | (2 | ) | 5 | ||||||||||||||||||||||
| Provision for (benefit from) income taxes |
— | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Net income (loss) |
(31 | )% | (24 | )% | (81 | )% | (14 | )% | (18 | )% | (8 | )% | (50 | )% | 1 | % | (2 | )% | 5 | % | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
34
Table of Contents
Non-GAAP Adjusted EBITDA
In addition to disclosing financial results calculated in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”), this Annual Report on Form 10-K discloses Adjusted EBITDA, which is income (loss) from operations adjusted for income taxes, depreciation and amortization of property and equipment, amortization of intangible assets, provision for doubtful accounts, amortization of prepaid stock compensation, amortization of prepaid sponsorship fees, stock-based compensation, issuance of common stock warrants and restructuring and asset impairment charges. Management believes that these Non-GAAP measures provide investors with important additional perspectives into our ongoing business performance.
The U.S. GAAP measure most directly comparable to Adjusted EBITDA is income (loss) from operations. The Non–GAAP financial measure of Adjusted EBITDA should not be considered as an alternative to net income (loss). Adjusted EBITDA is not a presentation made in accordance with U.S. GAAP and has important limitations as an analytical tool and should not be considered in isolation or as a substitute for analysis of our results as reported under U.S. GAAP. Because Adjusted EBITDA excludes some, but not all, items that affect net earnings and is defined differently by different companies, our definition of Adjusted EBITDA may not be comparable to similarly titled measures of other companies.
Set forth below are reconciliations of Adjusted EBITDA to our reported GAAP net income (loss):
| Year Ended |
Three Months Ended | Year Ended |
Three Months Ended | |||||||||||||||||||||||||||||||||||||
| Dec 31, 2015 |
Dec 31, 2015 |
Sep 30, 2015 |
Jun 30, 2015 |
Mar 31, 2015 |
Dec 31, 2014 |
Dec 31, 2014 |
Sep 30, 2014 |
Jun 30, 2014 |
Mar 31, 2014 |
|||||||||||||||||||||||||||||||
| Net (loss) income |
$ | (51,858 | ) | $ | (9,706 | ) | $ | (27,648 | ) | $ | (7,025 | ) | $ | (7,479 | ) | $ | (13,832 | ) | $ | (16,236 | ) | $ | 603 | $ | (935 | ) | $ | 2,736 | ||||||||||||
| Non-GAAP adjustments: |
||||||||||||||||||||||||||||||||||||||||
| Stock-based compensation |
12,705 | 4,015 | 2,154 | 4,013 | 2,523 | 10,931 | 4,059 | 2,405 | 2,091 | 2,376 | ||||||||||||||||||||||||||||||
| Restructuring and asset impairment charges |
21,235 | 3,323 | 17,912 | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Amortization of prepaid sponsorship fees |
6,255 | 892 | 2,111 | 1,821 | 1,431 | 5,802 | 786 | 1,548 | 1,810 | 1,658 | ||||||||||||||||||||||||||||||
| Other (income) expense, net |
1,806 | 716 | 559 | 348 | 183 | (5,577 | ) | (26 | ) | (5,234 | ) | 27 | (344 | ) | ||||||||||||||||||||||||||
| Amortization of prepaid stock compensation |
3,901 | 703 | 962 | 1,127 | 1,109 | 3,716 | 1,134 | 1,004 | 783 | 795 | ||||||||||||||||||||||||||||||
| Depreciation and amortization of property and equipment |
1,760 | 430 | 492 | 456 | 382 | 1,285 | 335 | 303 | 333 | 314 | ||||||||||||||||||||||||||||||
| Amortization of intangible assets |
1,055 | 279 | 278 | 273 | 225 | 698 | 276 | (156 | ) | 293 | 285 | |||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of common stock to a related party for guaranty of debt |
80 | 80 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
| Provision for doubtful accounts |
219 | 51 | 70 | 68 | 30 | 201 | 37 | 24 | 64 | 76 | ||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of common stock warrants to third parties for services |
65 | 3 | 12 | 17 | 33 | 130 | 61 | 69 | — | — | ||||||||||||||||||||||||||||||
| Provision (benefit) for income taxes |
105 | 1 | 71 | 21 | 12 | 33 | (138 | ) | 94 | 45 | 32 | |||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
| Adjusted EBITDA |
$ | (2,672 | ) | $ | 787 | $ | (3,027 | ) | $ | 1,119 | $ | (1,551 | ) | $ | 3,387 | $ | (9,712 | ) | $ | 660 | $ | 4,511 | $ | 7,928 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||
Liquidity and Capital Resources
Since the inception of MusclePharm, other than cash from product sales, our primary source of cash has been from the sale of equity, issuance of convertible secured promissory notes and other short-term debt as discussed below. As of December 31, 2015, our cash balance was approximately $7.1 million, which consists primarily of cash on deposit with banks.
Our principal use of cash is to purchase inventory, pay for operating expenses, acquire capital assets and historically to repurchase outstanding shares of our capital stock. As of December 31, 2015, we had a deficit in working capital of $20.0 million, an accumulated deficit of $147.5 million and a total stockholders’ deficit of
35
Table of Contents
$10.1 million. As of December 31, 2015, we had outstanding borrowings of $3.0 million under our line of credit facility, $2.9 million under our term loan agreement, and $6.0 million under our convertible note.
Our management believes that the recently implemented restructuring, which includes a reduction in ongoing operating costs and expense controls, will enable us ultimately to be profitable; however, we may need to continue to raise capital in order to execute its business plan. There can be no assurance that such capital will be available on acceptable terms or at all.
Our net consolidated cash flows are as follows:
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| (in thousands) | ||||||||||||
| Consolidated Statements of Cash Flows Data: |
||||||||||||
| Net cash provided by (used in) operating activities |
$ | 5,492 | $ | (4,133 | ) | $ | (9,973 | ) | ||||
| Net cash used in investing activities |
(3,047 | ) | (1,600 | ) | (3,522 | ) | ||||||
| Net cash provided by financing activities |
3,722 | 1,393 | 18,913 | |||||||||
| Effect of exchange rate changes on cash |
(106 | ) | (52 | ) | (6 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash |
$ | 6,061 | $ | (4,392 | ) | $ | 5,412 | |||||
|
|
|
|
|
|
|
|||||||
Operating Activities
Our cash provided by operating activities is driven primarily by sales of our products and vendor provided credit. During the year ended December 31, 2015, our trade accounts payable increased $14.2 million. Our primary uses of cash from operating activities have been for inventory purchases, advertising and promotion expenses, personnel-related expenditures, manufacturing costs, professional fees, costs related to our facilities and legal related fees. Our cash flows from operating activities will continue to be affected principally by the results of operations and the extent to which we increase spending on personnel expenditures, sales and marketing activities, and our working capital requirements.
During the year ended December 31, 2015, cash provided by operating activities was $5.5 million, which differs from our net loss of $51.9 million primarily because of non-cash charges of $38.3 million, and a net change in our net operating assets and liabilities of $19.1 million. The non-cash charges primarily consisted of $9.5 million for non-cash restructuring and other charges, $2.6 million for inventory write down related to corporate restructuring, $12.7 million for stock-based compensation, $6.3 million for amortization of prepaid sponsorship and endorsement fees, $3.9 million for amortization of prepaid stock compensation, and $2.8 million for depreciation of our property and equipment and amortization of our intangible assets. The primary drivers of the changes in operating assets and liabilities were a $14.2 million increase in accounts payable primarily attributable to the timing of payments to vendors, a $5.9 million decrease in inventory primarily related to supply chain issues and our focus on aligning inventory with customer sales orders, a $5.4 million increase in accrued liabilities due primarily to the increase in accrued accounts payable for inventories received but not billed, and a $7.3 million increase in accrued restructuring charges, partially offset by a $6.8 million increase in prepaid sponsorship and endorsement fees due to an increase in advertising, a $5.6 million increase in accounts receivable primarily due to strong revenue growth in the fourth quarter of 2015, and a $2.2 million increase in prepaid expenses and other current assets due primarily to prepayment related to the manufacturing agreement with Capstone.
During the year ended December 31, 2014, cash used in operating activities was $4.1 million, primarily as a result of our net loss of $13.8 million and a $7.3 million net change in our operating assets and liabilities, which was partially offset by non-cash charges of $17.0 million. The net change in our operating assets and liabilities was primarily the result of higher accounts receivable balances and increased purchase of inventory, offset by
36
Table of Contents
higher accounts payable and increases in accrued expenses. The increase in accounts receivable of $2.6 million was primarily due to revenue growth in the year ended December 31, 2014. The increase in inventory of $4.5 million and accounts payable of $1.3 million was primarily due to our plan to stock up the inventory for anticipated growth in our business. The increase in accrued liabilities of $3.6 million was primarily due to the increase in royalty accruals for endorsements and higher accrued personnel costs due to growth in headcount. The net change in our operating assets and liabilities was also attributable to a $4.9 million increase in prepaid sponsorship and endorsement fees due to an increase in advertising. The non-cash charges primarily consisted of stock-based compensation of $10.9 million, amortization of prepaid sponsorship and endorsement fees of $5.8 million, amortization of prepaid stock compensation of $3.7 million, depreciation and amortization of our property and equipment and intangible assets of 2.0 million, partially offset by the bargain purchase gain and contingent asset gain on the BioZone acquisition of $5.3 million.
During the year ended December 31, 2013, cash used in operating activities was $10.0 million, primarily as a result of our net loss of $17.7 million and the net change in our operating assets and liabilities of $10.2 million, which was partially offset by non-cash charges of $18.0 million. The net change in our operating assets and liabilities was primarily the result of higher accounts receivable balances and increased purchases of inventory, offset by higher accounts payable and increases in accrued expenses. The increase in accounts receivable of $10.7 million was primarily due to revenue growth in year ended December 31, 2013. The increase in inventory of $15.5 million and accounts payable of $20.1 million was primarily due to our plan to stock up inventory for anticipated growth in our business. The increase in accrued liabilities of $2.3 million was primarily due to increase in royalty accruals for endorsements and higher accrued personnel costs due to growth in headcount. The net change in our operating assets and liabilities was also attributable to a $5.2 million increase in prepaid sponsorship and endorsement fees and a $0.8 million increase in prepaid giveaways due to an increase in advertising efforts through sponsorships, endorsements and giving away samples. The non-cash charges primarily consisted of amortization of prepaid stock compensation of $6.6 million, increase in fair value of derivative liabilities of $4.9 million, amortization of prepaid sponsorship and endorsement fees of $4.0 million, stock-based compensation of $3.1 million, and depreciation of our property and equipment of $0.7 million, partially offset by accretion of discount on purchased convertible notes of $1.4 million, and gain on settlement of accounts payable of $0.6 million.
Investing Activities
Cash used in investing activities of $3.0 million for the year ended December 31, 2015 was primarily due to cash payments of $0.9 million related to MusclePharm’s apparel rights acquisition, an investment in warrants to acquire shares in a contract manufacturer of $1.0 million related to our opportunity to acquire our contract manufacturer, Capstone, and the purchase of property and equipment of $1.5 million, partially offset by proceeds from the disposal of property and equipment of $0.5 million.
Cash used in investing activities was $1.6 million for the year ended December 31, 2014, primarily due to the purchase of property and equipment of $4.1 million, and the purchase of trademarks of $0.5 million, partially offset by decrease in the restricted cash balance of $2.5 million, and sales proceeds from a settlement of purchased convertible notes of $0.5 million.
Cash used in investing activities was $3.5 million for the year ended December 31, 2013, primarily due to an increase in the restricted cash balance of $2.5 million, purchases of convertible notes of $2.3 million, and the purchase of property and equipment of $1.9 million, partially offset by sales proceeds from a settlement of purchased convertible notes of $2.3 million, and repayments of notes receivable of $1.0 million.
37
Table of Contents
Financing Activities
Cash flows provided by financing activities of $3.7 million for the year ended December 31, 2015 was primarily due to repayment on our line of credit of $14.5 million and repayment of a term loan of $1.1 million, offset by proceeds from the issuance of our term loan of $4.0 million, draw down of our line of credit of $9.5 million, and proceeds from the issurance of our convertible note of $6.0 million.
Cash flows provided by financing activities was $1.4 million for the year ended December 31, 2014, primarily due to proceeds from our line of credit of $7.9 million, partially offset by the repurchase of common stock of $3.9 million, and a repayment on our line of credit of $2.5 million.
Cash flows provided by financing activities was $18.9 million for the year ended December 31, 2013, primarily due to proceeds from the issuance of preferred stock of $11.3 million, proceeds from the issuance of common stock and warrants of $10.6 million, and proceeds from our line of credit of $2.5 million, partially offset by repayments of debt of $4.4 million, and the repurchase of common stock of $1.0 million.
Indebtedness Agreements
In September 2014, we entered into a line of credit facility with ANB Bank for up to $8.0 million of borrowings. The line of credit originally renewed annually, matured in September 2017, and accrued interest at the prime rate plus 2%. The line of credit was secured by our inventory, accounts receivable, intangible assets and equipment. As of December 31, 2015, the outstanding borrowings under the line of credit were $3.0 million. We were not in compliance with certain financial covenants under the line of credit as of December 31, 2015, which limited further borrowings.
In February 2015, we entered into a $4.0 million term loan agreement with ANB Bank. The term loan carried a fixed interest rate of 5.25% per annum, was repayable in 36 equal monthly installments of principal and interest, and originally matured in February 2018. As of December 31, 2015, the outstanding borrowings under the term loan were $2.9 million. The term loan contained various events of default, including cross default provisions related to the line of credit, which could have required repayments of the term loan. We were not in compliance with certain financial covenants under the term loan as of December 31, 2015, and received various waivers from the lender during the period.
In October 2015, we entered into loan modification agreements with ANB Bank under the line of credit and term loan to: (i) change the maturity date of the loans to January 15, 2016, (ii) prohibit the loans to be declared in default prior to December 10, 2015, except for defaults resulting from failure to make timely payments, and (iii) delete certain financial covenants from the line of credit. In consideration for these modifications, Ryan Drexler, our Interim Chief Executive Officer, President and Chairman, and a family member, provided their individual guaranty for the remaining balance of the term loan and line of credit of $6.2 million. In consideration for executing his guaranty, we issued Ryan Drexler 28,571 shares of our common stock with a grant date fair value of $80,000 (based upon the closing price of common stock on the date of issuance).
In December 2015, we entered into a convertible secured promissory note agreement with Ryan Drexler, our Interim Chief Executive Officer, President and Chairman, under which he lended us $6.0 million. Proceeds from the note were used to fund working capital requirements. The convertible note is secured by all assets and properties of the Company and its subsidiaries, whether tangible or intangible. The convertible note carries an interest at 8% per annum, or 10% in the event of default. Both the principal and the interest under the convertible note are due in January 2017, unless converted earlier. The holder can convert the outstanding principal and accrued interest into shares of common stock for $2.30 per share at any time. We may prepay the convertible note at the aggregate principle amount therein plus accrued interest by giving the holder between 15 and 60 day-notice period, depending upon the specific circumstances, provided that the holder may convert the note during the notice period. We recorded the convertible note of $6.0 million as a liability in the balance sheet and also recorded a beneficial conversion feature of $52,000 as a debt discount upon issuance of the convertible note,
38
Table of Contents
which is being amortized over the term of the convertible debt using the effective interest method. The beneficial conversion feature was calculated based on the difference between the fair value of common stock and the effective conversion price of the convertible note. As of December 31, 2015, the convertible note had an outstanding principal balance of $6.0 million.
On January 11, 2016, we entered into a Purchase and Sale Agreement (the “Agreement”) with Prestige Capital Corporation (“Prestige”) pursuant to which we agreed to sell and assign and Prestige agreed to buy and accept, certain accounts receivable owing to us (“Accounts”). Under the terms of the Agreement, upon the receipt and acceptance of each assignment of Accounts, Prestige will pay us eighty percent (80%) of the net face amount of the assigned Accounts, up to a maximum total borrowings of $10.0 million outstanding at any time. In addition, we granted Prestige a continuing security interest in and lien upon all accounts receivable, inventory, fixed assets, general intangibles and other assets. The Agreement has an initial term of six month with options to extend. Prestige may cancel the Agreement with 30 day notice.
On January 13, 2016, we sold to Prestige accounts with an aggregate face amount of approximately $7.6 million and Prestige paid us approximately $6.2 million in cash. The proceeds from this factoring were utilized to pay off the existing line of credit and term loan with ANB Bank.
Contractual Obligations
Our principal commitments consist of obligations under operating leases for office and warehouse facilities, capital leases for manufacturing and warehouse equipment, debt, restructuring liability and non-cancelable endorsement and sponsorship agreements. The following table summarizes our commitments to settle contractual obligations in cash as of December 31, 2015:
| Payments Due by Period | ||||||||||||||||||||
| 1 Year | 2 to 3 Years | 4 to 5 Years | Thereafter | Total | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating lease obligations(1) |
$ | 1,064 | $ | 1,722 | $ | 1,139 | $ | 2,381 | $ | 6,306 | ||||||||||
| Capital lease obligations |
210 | 294 | 56 | — | 560 | |||||||||||||||
| Line of credit(2) |
3,000 | — | — | — | 3,000 | |||||||||||||||
| Term loan(2) |
2,949 | — | — | — | 2,949 | |||||||||||||||
| Convertible note |
— | 6,000 | — | — | 6,000 | |||||||||||||||
| Restructuring liability(3) |
9,140 | 185 | 94 | — | 9,419 | |||||||||||||||
| Other contractual obligations(4) |
7,954 | 9,992 | 10,152 | 6,667 | 34,765 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ |
24,317 |
|
$ | 18,193 | $ | 11,441 | $ | 9,048 | $ | 62,999 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | The amounts in the table above excluded $0.5 million in operating lease expenses, which were abandoned in conjunction with our restructuring plans and is included within the caption Restructuring liability. |
| (2) | Repaid in January 2016. |
| (3) | Restructuring liability includes restructuring charges of (i) $1.3 million related to severance and termination benefit costs related to the terminated employees, (ii) $7.0 million related to cancellation of certain contracts and sponsorship agreements, (iii) $350,000 related to purchase commitment of discontinued inventories not yet received by the Company, and (iv) $467,000 for costs associated with permanently vacating certain leased facilities. |
| (4) | Other contractual obligations consist of non-cancelable endorsement and sponsorship agreements. |
Going Concern Opinion
Our auditors have issued a going concern opinion in their report on our financial statements for the fiscal year ended December 31, 2015. We have not established an ongoing source of revenue sufficient to cover our operating costs and are dependent on obtaining adequate capital to continue operations, which raises substantial doubt as to our ability to continue as a going concern. See “Item 1A. Risk Factors—Because our auditors have issued a going concern opinion, there is a substantial uncertainty that we will continue operations, in which case you could lose your investment” and Note 1 to our Consolidated Financial Statements.
39
Table of Contents
Off-Balance Sheet Arrangements
We did not have any off-balance sheet arrangements as of December 31, 2015.
Critical Accounting Policies and Estimates
Our consolidated financial statements have been prepared in accordance with U.S. Generally Accepted Accounting Principles and form the basis for the following discussion and analysis on critical accounting policies and estimates. The preparation of the consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. We evaluate our estimates and assumptions on a regular basis. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results could differ from these estimates and those differences could have a material effect on our financial position and results of operations.
Prepaid Stock Compensation
Prepaid stock compensation represents amounts paid with restricted stock awards for future contractual benefits to be received. We record the fair value of these restricted stock awards to prepaid stock compensation and additional paid-in capital, upon issuance of the shares, and then amortize these costs to the consolidated statements of operations over the life of the contracts using the straight-line method.
Derivative Liabilities
Fair value accounting requires bifurcation of embedded derivative instruments, such as warrants or conversion features in equity instruments, and measurement of their fair value. In determining the appropriate fair value, we use Black-Scholes valuation model. Derivatives are adjusted to reflect estimated fair value at the end of each reporting period with any increase or decrease in the estimated fair value being recorded in other income (expense), net in the consolidated statements of operations. Once a derivative liability ceases to exist, any remaining estimated fair value is reclassified to additional paid-in capital if redeemed.
Share-Based Payments
Share-based compensation awards, including stock options and restricted stock awards, are recorded at estimated fair value on the awards’ grant date, based on estimated number of awards that are expected to vest. The grant date fair value is amortized on a straight line basis over the time in which the awards are expected to vest, or immediately if no vesting is required. Share-based compensation awards issued to non-employees for services are recorded at either the fair value of the services rendered or the fair value of the share-based payments whichever is more readily determinable. The fair value of restricted stock awards are based on the fair value of the stock underlying the awards on the grant date as there is no exercise price. The fair value of stock options is estimated using the Black-Scholes option-pricing model but have been insignificant during the periods included herein.
Revenue Recognition
Revenue is recognized when all of the following criteria are met:
| • | Persuasive evidence of an arrangement exists. Evidence of an arrangement consists of an order from our distributors, resellers or customers. |
| • | Delivery has occurred. Delivery is deemed to have occurred when title and risk of loss has transferred, either upon shipment of products to customers or upon delivery. |
| • | The fee is fixed or determinable. We assess whether the fee is fixed or determinable based on the terms associated with the transaction. |
40
Table of Contents
| • | Collection is reasonably assured. We assess collectability based on credit analysis and payment history. |
Our standard terms and conditions of sale do not allow for product returns. However, we grant an informal right of return to its customers. Estimates of expected future product returns are recognized at the time of sale based on analyses of historical return trends by customer type. Upon recognition, we reduce revenue and cost of revenue for the estimated return. Return rates can fluctuate over time, but are sufficiently predictable with established customers to allow us to estimate expected future product returns, and an accrual is recorded for future expected returns when the related revenue is recognized.
We offer sales incentives through various programs, consisting primarily of advertising related credits, volume incentive rebates and sales incentive reserves. We record advertising related credits with customers as a reduction to revenue as no identifiable benefit is received in exchange for credits claimed by the customer. Volume incentive rebates are provided to certain customers based on contractually agreed upon percentages once certain thresholds have been met. Sales incentive reserves are computed based on historical trending and budgeted discount percentages, which are typically based on historical discount rates with adjustments for any known changes, such as future promotions or one-time historical promotions that will not repeat for each customer. We record sales incentive reserves and volume rebate reserves as a reduction to revenue.
Intangible Assets
Acquired intangible assets are recorded at estimated fair value, net of accumulated amortization, and costs incurred in obtaining certain trademarks are capitalized, and are amortized over their related useful lives, using a straight-line basis consistent with the underlying expected future cash flows related to the specific intangible asset. Costs to renew or extend the life of intangible assets are capitalized and amortized over the remaining useful life of the asset. Amortization expenses are included as a component of selling, general and administrative expenses in the consolidated statements of operations.
Advertising and Promotion
Advertising and promotion expenses include digital and print advertising, trade show events, athletic endorsements and sponsorships, and promotional giveaways, and are expensed as incurred. Some of the contracts provide for contingent payments to endorsers or athletes based upon specific achievement in their sports (e.g. winning a championship). We record expense for these payments if and when the endorser achieves the specific achievement.
Income Taxes
Income taxes are accounted for using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial statement carrying amounts of existing assets and liabilities and their respective tax bases, operating loss and tax credit carry-forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. We recognize the effect of income tax positions only if those positions are more likely than not to be sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely to be realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs.
Recent Accounting Pronouncements
In February 2016, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-02, Leases (Topic 842), which supersedes Topic 840, Leases, (“ASU 2016-02”). The guidance in this new standard requires lessees to put most
41
Table of Contents
leases on their balance sheets but recognize expenses on their income statements in a manner similar to the current accounting and eliminates the current real estate-specific provisions for all entities. The guidance also modifies the classification criteria and the accounting for sales-type and direct financing leases for lessors. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years, with early adoption permitted. We are currently evaluating the impact of the adoption of ASU 2016-02.
In November 2015, the FASB issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes (“ASU 2015-17”), which requires that deferred tax assets and liabilities be classified as non-current in a classified statement of financial position. The accounting standard is effective, either prospectively to all deferred tax assets and liabilities or retrospectively to all periods presented, for annual periods beginning after December 15, 2016, and interim periods therein. Early adoption is permitted as of the beginning of an interim or annual reporting period. The Company early adopted this standard as of December 31, 2015 on a prospective basis.
In July 2015, the FASB issued ASU No. 2015-11, Inventory (Topic 330): Simplifying the Measurement of Inventory (“ASU 2015-11”), which simplifies the subsequent measurement of inventory by requiring inventory to be measured at the lower of cost and net realizable value. Net realizable value is the estimated selling price of inventory in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. ASU 2015-11 is effective for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. We are currently evaluating the impact of the adoption of ASU 2015-11.
In April 2015, the FASB issued ASU No. 2015-03, Interest — Imputation of Interest (Subtopic 835-30) — Simplifying the Presentation of Debt Issuance Costs (“ASU 2015-03”), which provides guidance on simplifying the presentation of debt issuance costs, requiring that debt issuance costs related to a recognized debt liability be presented in the consolidated balance sheets as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. In August 2015, the FASB issued ASU No. 2015-15, Interest – Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements — Amendments to SEC Paragraphs Pursuant to Staff Announcement at June 18, 2015 EITF Meeting (“ASU 2015-15”), which further clarifies ASU 2015-03 as it relates to presentation and subsequent measurement of debt issuance costs related to line-of-credit arrangements. ASU 2015-15 allows an entity deferring and presenting debt issuance costs related to line-of-credit arrangements as an asset and subsequently amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. Both ASU 2015-03 and ASU 2015-15 require retrospective adoption and are effective for financial statement periods beginning after December 15, 2015, and interim periods within those fiscal years, with early adoption permitted. We have not early adopted ASU 2015-03 or ASU 2015-15 and the adoption of these standards is not expected to have a material effect on its consolidated financial statements or disclosures.
In August 2014, the FASB issued ASU No. 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (“ASU 2014-15”). ASU 2014-15 explicitly requires management of all entities to evaluate whether there are conditions and events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the financial statements are issued (or available to be issued when applicable), and to provide related footnote disclosure in certain circumstances. ASU 2014-15 is effective for us for the first annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Earlier adoption is permitted. We have early adopted ASU 2014-15.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”), which provides guidance for revenue recognition. ASU 2014-09 affects any entity that either enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets and supersedes the revenue recognition requirements in Topic 605, Revenue Recognition, and most industry-specific guidance. This ASU also supersedes some cost guidance included in Subtopic 605-35, Revenue Recognition- Construction-Type and Production-Type Contracts. ASU 2014-09’s core principle is that a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the
42
Table of Contents
consideration to which a company expects to be entitled in exchange for those goods or services. In doing so, companies will need to use more judgment and make more estimates than under today’s guidance, including identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date (“ASU 2015-14”), which delays the effective date of ASU 2014-09 by one year. The FASB also agreed to allow entities to choose to adopt the standard as of the original effective date. As such, the updated standard will be effective for us in the first quarter of 2018, with the option to adopt it in the first quarter of 2017. We may adopt the new standard under the full retrospective approach or the modified retrospective approach. We are currently evaluating the method and impact the adoption of ASU 2014-09 will have on our consolidated financial statements and disclosures.
In April 2014, the FASB issued ASU No. 2014-08, Presentation of Financial Statements (Topic 205) and Property, Plant, and Equipment (Topic 360): Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity (“ASU 2014-08”). ASU 2014-08 changes the criteria for reporting a discontinued operation. Under the new pronouncement, a disposal of a part of an organization that has a major effect on its operations and financial results is a discontinued operation. We are required to adopt ASU 2014-08 prospectively for all disposals or components of its business classified as held for sale during fiscal periods beginning after December 15, 2014. We adopted ASU 2014-08 in 2015, and it did not have a material effect on our consolidated financial statements or disclosures.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
We are exposed to market risks related to changes to foreign currency exchange rates.
Foreign Currency Exchange Risk
We have foreign currency risks related to our revenue and operating expenses denominated in currencies other than the U.S. dollar, primarily the Canadian dollar and more recently the Euro. In general, we are a net receiver of currencies other than the U.S. dollar. Accordingly, changes in exchange rates, and in particular a strengthening of the U.S. dollar, will negatively affect our non-dollar denominated revenue and other operating results as expressed in U.S. dollars.
We have experienced and will continue to experience fluctuations in our net income as a result of transaction gains or losses related to revaluing certain current asset and current liability balances that are denominated in currencies other than the functional currency of the entities in which they are recorded. At this time, we have not entered into, but in the future we may enter into, derivatives or other financial instruments in an attempt to hedge our foreign currency exchange risk. It is difficult to predict the effect hedging activities would have on our results of operations. We recognized foreign currency loss of $1.0 million in 2015.
43
Table of Contents
| Item 8. | Financial Statements and Supplementary Data |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
44
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
MusclePharm Corporation
Denver, Colorado
We have audited the accompanying consolidated balance sheets of MusclePharm Corporation and subsidiaries (the “Company”) as of December 31, 2015 and 2014, and the related consolidated statements of operations, comprehensive loss, stockholders’ (deficit) equity, and cash flows for each of the years in the three-year period ended December 31, 2015. We have also audited the Company’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying management’s report on internal control over financial reporting. Our responsibility is to express an opinion on these consolidated financial statements and an opinion on the effectiveness of the Company’s internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the consolidated financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall consolidated financial statement presentation. Our audits of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of MusclePharm Corporation and subsidiaries as of December 31, 2015 and 2014, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, MusclePharm Corporation and subsidiaries maintained, in all material respects, effective internal control
45
Table of Contents
over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has not established an ongoing source of revenue sufficient to cover its operating costs and is dependent on obtaining adequate capital to continue operations, which raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ EKS&H LLLP
March 17, 2016
Denver, Colorado
46
Table of Contents
MusclePharm Corporation
(In thousands, except share and per share data)
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| ASSETS |
||||||||
| Current assets: |
||||||||
| Cash |
$ | 7,081 | $ | 1,020 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $347 and $159 as of December 31, 2015 and 2014 |
22,003 | 16,644 | ||||||
| Inventory |
12,549 | 21,069 | ||||||
| Prepaid giveaways |
307 | 1,228 | ||||||
| Prepaid stock compensation, current |
1,641 | 4,476 | ||||||
| Prepaid sponsorship and endorsement fees |
— | 238 | ||||||
| Prepaid expenses and other current assets |
3,698 | 1,742 | ||||||
|
|
|
|
|
|||||
| Total current assets |
47,279 | 46,417 | ||||||
| Property and equipment, net |
6,693 | 7,805 | ||||||
| Investments, long-term |
977 | — | ||||||
| Intangible assets, net |
8,652 | 7,074 | ||||||
| Prepaid stock compensation, long-term |
— | 4,952 | ||||||
| Other assets |
180 | 108 | ||||||
|
|
|
|
|
|||||
| TOTAL ASSETS |
$ | 63,781 | $ | 66,356 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 39,652 | $ | 27,761 | ||||
| Accrued liabilities |
12,526 | 7,023 | ||||||
| Accrued restructuring charges, current |
9,140 | — | ||||||
| Line of credit |
3,000 | 8,000 | ||||||
| Term loan |
2,949 | — | ||||||
| Other debt obligations |
21 | 46 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
67,288 | 42,830 | ||||||
| Convertible note with a related party, net of discount |
5,952 | — | ||||||
| Accrued restructuring charges, long-term |
279 | — | ||||||
| Other long-term liabilities |
330 | 146 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
|
73,849 |
|
42,976 | ||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 12) |
||||||||
| Stockholders’ (deficit) equity: |
||||||||
| Common stock, par value of $0.001 per share; 100,000,000 shares authorized as of December 31, 2015 and 2014; 14,664,161 and 13,996,007 shares issued as of December 31, 2015 and 2014; 13,788,540 and 13,120,386 shares outstanding as of December 31, 2015 and 2014 |
14 | 14 | ||||||
| Additional paid-in capital |
147,646 | 129,130 | ||||||
| Treasury stock, at cost; 875,621 shares as of December 31, 2015 and 2014 |
(10,039 | ) | (10,039 | ) | ||||
| Accumulated other comprehensive loss |
(172 | ) | (66 | ) | ||||
| Accumulated deficit |
(147,517 | ) | (95,659 | ) | ||||
|
|
|
|
|
|||||
| TOTAL STOCKHOLDERS’ (DEFICIT) EQUITY |
(10,068 | ) | 23,380 | |||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ (DEFICIT) EQUITY |
$ | 63,781 | $ | 66,356 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
47
Table of Contents
MusclePharm Corporation
Consolidated Statements of Operations
(In thousands, except share and per share data)
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Revenue, net |
$ | 166,858 | $ | 177,389 | $ | 110,878 | ||||||
| Cost of revenue (1) |
109,927 | 121,379 | 77,686 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
56,931 | 56,010 | 33,192 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating expenses: |
||||||||||||
| Advertising and promotion |
26,985 | 28,053 | 15,535 | |||||||||
| Salaries and benefits |
31,176 | 25,347 | 11,831 | |||||||||
| Selling, general and administrative |
19,372 | 13,354 | 7,173 | |||||||||
| Research and development |
4,251 | 3,997 | 1,119 | |||||||||
| Professional fees |
6,801 | 4,635 | 11,831 | |||||||||
| Restructuring and other charges |
18,293 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
106,878 | 75,386 | 47,489 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(49,947 | ) | (19,376 | ) | (14,297 | ) | ||||||
| Other (expense) income, net |
(1,806 | ) | 5,577 | (3,306 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before provision for income taxes |
(51,753 | ) | (13,799 | ) | (17,603 | ) | ||||||
| Provision for income taxes |
105 | 33 | 115 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (51,858 | ) | $ | (13,832 | ) | $ | (17,718 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share, basic and diluted |
$ | (3.81 | ) | $ | (1.25 | ) | $ | (2.46 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted-average shares used to compute net loss per share, basic and diluted |
13,621,255 | 11,038,761 | 7,193,784 | |||||||||
|
|
|
|
|
|
|
|||||||
| (1) | Cost of revenue for the year ended December 31, 2015 included restructuring charges of $2,942, which is comprised of i) $2,592 related to write-down of inventory, and ii) $350 related to purchase commitment of discontinued inventories not yet received by the Company, which remains accrued at December 31, 2015. |
The accompanying notes are an integral part of these consolidated financial statements.
48
Table of Contents
Consolidated Statements of Comprehensive Loss
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Net loss |
$ | (51,858 | ) | $ | (13,832 | ) | $ | (17,718 | ) | |||
| Change in foreign currency translation adjustment |
(106 | ) | (52 | ) | (6 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (51,964 | ) | $ | (13,884 | ) | $ | (17,724 | ) | |||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
49
Table of Contents
MusclePharm Corporation
Consolidated Statements of Stockholders’ (Deficit) Equity
(In thousands, except share data)
| Series B Preferred Stock |
Series D Convertible Preferred Stock |
Common Stock | Additional Paid-In Capital |
Treasury Stock |
Accumulated Other Comprehensive Loss |
Accumulated Deficit |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||
| Balance – December 31, 2012 |
51 | $ | — | — | $ | — | 2,747,308 | $ | 3 | $ | 54,817 | $ | (461 | ) | $ | (8 | ) | $ | (64,109 | ) | $ | (9,758 | ) | |||||||||||||||||||||
| Issuance of preferred stock for cash |
— | — | 1,500,000 | 2 | — | — | 11,999 | — | — | — | 12,001 | |||||||||||||||||||||||||||||||||
| Issuance of common stock for: |
||||||||||||||||||||||||||||||||||||||||||||
| Cash |
— | — | — | — | 1,191,332 | 1 | 10,558 | — | — | — | 10,559 | |||||||||||||||||||||||||||||||||
| Contract settlement |
— | — | — | — | 25,000 | — | 256 | — | — | — | 256 | |||||||||||||||||||||||||||||||||
| Retirement of Series B preferred Stock |
(51 | ) | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||
| Treasury stock purchased |
— | — | — | — | (138,825 | ) | — | — | (1,037 | ) | — | — | (1,037 | ) | ||||||||||||||||||||||||||||||
| Reduction of additional paid-in capital attributable to value of conversion options on Series D offering |
— | — | — | — | — | — | (8,175 | ) | — | — | — | (8,175 | ) | |||||||||||||||||||||||||||||||
| Stock issuance costs |
— | — | — | — | — | — | (1,395 | ) | — | — | — | (1,395 | ) | |||||||||||||||||||||||||||||||
| Stock-based compensation |
— | — | — | — | 2,514,045 | 2 | 23,027 | — | — | — | 23,029 | |||||||||||||||||||||||||||||||||
| Reclassification of derivative liabilities to additional paid-in capital for conversion of Series D preferred stock |
— | — | (1,368,500 | ) | (2 | ) | 2,737,000 | 3 | 11,823 | — | — | — | 11,824 | |||||||||||||||||||||||||||||||
| Reclassification of derivative liabilities to additional paid-in capital upon contract settlement |
— | — | — | — | 13,630 | — | 155 | — | — | — | 155 | |||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (17,718 | ) | (17,718 | ) | |||||||||||||||||||||||||||||||
| Change in foreign currency translation adjustment |
— | — | — | — | — | — | — | — | (6 | ) | — | (6 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Balance – December 31, 2013 |
— | $ | — | 131,500 | $ | — | 9,089,490 | $ | 9 | $ | 103,065 | $ | (1,498 | ) | $ | (14 | ) | $ | (81,827 | ) | $ | 19,735 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Issuance of common stock for: |
|
— | ||||||||||||||||||||||||||||||||||||||||||
| Conversion of Series D convertible preferred stock |
— | — | (131,500 | ) | — | 263,000 | — | 773 | — | — | — | 773 | ||||||||||||||||||||||||||||||||
| BioZone acquisition |
— | — | — | — | 850,000 | 1 | 8,832 | (4,620 | ) | — | — | 4,213 | ||||||||||||||||||||||||||||||||
| Issuance of common stock warrants to third parties for services |
— | — | — | — | — | — | 130 | — | — | — | 130 | |||||||||||||||||||||||||||||||||
| Treasury stock purchased |
— | — | — | — | (355,700 | ) | — | — | (3,921 | ) | — | — | (3,921 | ) | ||||||||||||||||||||||||||||||
| Deferred stock compensation on restricted stock awards issued for endorsement agreements |
— | — | — | — | 476,853 | 1 | 5,402 | — | — | — | 5,403 | |||||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of restricted stock awards to employees, executives and directors |
— | — | — | — | 2,796,743 | 3 | 10,928 | — | — | — | 10,931 | |||||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (13,832 | ) | (13,832 | ) | |||||||||||||||||||||||||||||||
| Change in foreign currency translation adjustment |
— | — | — | — | — | — | — | — | (52 | ) | — | (52 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Balance – December 31, 2014 |
— | $ | — | — | $ | — | 13,120,386 | $ | 14 | $ | 129,130 | $ | (10,039 | ) | $ | (66 | ) | $ | (95,659 | ) | $ | 23,380 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
50
Table of Contents
MusclePharm Corporation
Consolidated Statements of Stockholders’ (Deficit) Equity (Continued)
(In thousands, except share data)
| Series B Preferred Stock |
Series D Convertible Preferred Stock |
Common Stock | Additional Paid-In Capital |
Treasury Stock |
Accumulated Other Comprehensive Loss |
Accumulated Deficit |
Total Stockholders’ Equity (Deficit) |
|||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||
| Balance – December 31, 2014 |
— | $ | — | — | $ | — | 13,120,386 | $ | 14 | $ | 129,130 | $ | (10,039 | ) | $ | (66 | ) | $ | (95,659 | ) | $ | 23,380 | ||||||||||||||||||||||
| Stock-based compensation related to issuance of common stock warrants to third parties for services |
— | — | — | — | — | — | 65 | — | — | — | 65 | |||||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of restricted stock awards to employees, executives and directors, net of cancellations |
— | — | — | — | 214,394 | — | 15,082 | — | — | — | 15,082 | |||||||||||||||||||||||||||||||||
| Stock issued in conjunction with product line expansion |
— | — | — | — | 150,000 | — | 1,198 | — | — | — | 1,198 | |||||||||||||||||||||||||||||||||
| Stock issued in conjunction with MusclePharm apparel rights acquisition |
— | — | — | — | 170,000 | — | 1,394 | — | — | — | 1,394 | |||||||||||||||||||||||||||||||||
| Stock issued in conjunction with attempted financing agreement |
— | — | — | — | 50,000 | — | 325 | — | — | — | 325 | |||||||||||||||||||||||||||||||||
| Stock issued in conjunction with non-employee consulting/endorsement agreement |
— | — | — | — | 55,189 | — | 320 | — | — | — | 320 | |||||||||||||||||||||||||||||||||
| Stock-based compensation related to issuance of common stock to a related party for guaranty of debt |
— | — | — | — | 28,571 | — | 80 | — | — | — | 80 | |||||||||||||||||||||||||||||||||
| Beneficial conversion feature related to convertible note |
— | — | — | — | — | — | 52 | — | — | — | 52 | |||||||||||||||||||||||||||||||||
| Change in foreign currency translation adjustment |
— | — | — | — | — | — | — | — | (106 | ) | — | (106 | ) | |||||||||||||||||||||||||||||||
| Net loss |
— | — | — | — | — | — | — | — | — | (51,858 | ) | (51,858 | ) | |||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
| Balance – December 31, 2015 |
— | $ | — | — | $ | — | 13,788,540 | $ | 14 | $ | 147,646 | $ | (10,039 | ) | $ | (172 | ) | $ | (147,517 | ) | $ | (10,068 | ) | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
51
Table of Contents
MusclePharm Corporation
Consolidated Statements of Cash Flows
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Cash flows from operating activities |
||||||||||||
| Net loss |
$ | (51,858 | ) | $ | (13,832 | ) | $ | (17,718 | ) | |||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||||||
| Depreciation of property and equipment |
1,760 | 1,285 | 709 | |||||||||
| Amortization of intangible assets |
1,055 | 698 | — | |||||||||
| Provision for doubtful accounts |
219 | 201 | 242 | |||||||||
| Non-cash restructuring and other charges |
9,494 | — | — | |||||||||
| Inventory write down related to corporate restructuring |
2,592 | — | — | |||||||||
| Amortization of prepaid stock compensation |
3,901 | 3,716 | 6,562 | |||||||||
| Amortization of prepaid sponsorship and endorsement fees |
6,255 | 5,802 | 4,011 | |||||||||
| Accretion of discount on purchased convertible notes |
— | (15 | ) | (1,409 | ) | |||||||
| Amortization of debt discount and issuance costs |
118 | 8 | 335 | |||||||||
| Stock-based compensation |
12,705 | 10,931 | 3,075 | |||||||||
| Stock-based compensation related to issuance of common stock to a related party for guaranty of debt |
80 | — | — | |||||||||
| Stock-based compensation related to issuance of common stock warrants to third parties for services |
65 | 130 | — | |||||||||
| Accretion of conversion option on debt security |
— | — | 2 | |||||||||
| Bargain purchase gain and contingent asset gain on BioZone acquisition |
— | (5,265 | ) | — | ||||||||
| Gain on settlement of accounts payable |
— | (31 | ) | (574 | ) | |||||||
| Loss on disposal of property and equipment |
16 | — | 11 | |||||||||
| Derivative expense |
— | — | 97 | |||||||||
| Change in fair value of derivative liabilities |
— | (374 | ) | 4,854 | ||||||||
| Unrealized loss on derivative assets |
— | — | 56 | |||||||||
| Realized gain on purchased convertible notes |
— | (96 | ) | (2 | ) | |||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
(5,578 | ) | (2,609 | ) | (10,681 | ) | ||||||
| Inventory |
5,928 | (4,466 | ) | (15,514 | ) | |||||||
| Prepaid giveaways |
921 | (50 | ) | (819 | ) | |||||||
| Prepaid sponsorship and endorsement fees |
(6,843 | ) | (4,895 | ) | (5,150 | ) | ||||||
| Prepaid expenses and other current assets |
(2,185 | ) | 2 | (405 | ) | |||||||
| Other assets |
(72 | ) | 36 | (19 | ) | |||||||
| Accounts payable |
14,221 | 1,329 | 20,105 | |||||||||
| Accrued liabilities |
5,399 | 3,628 | 2,275 | |||||||||
| Accrued restructuring charges |
7,299 | — | — | |||||||||
| Customer deposits |
— | (266 | ) | (70 | ) | |||||||
| Other long-term liabilities |
— | — | 54 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) operating activities |
5,492 | (4,133 | ) | (9,973 | ) | |||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
52
Table of Contents
MusclePharm Corporation
Consolidated Statements of Cash Flows (Continued)
(In thousands)
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Cash flows from investing activities |
||||||||||||
| Purchase of property and equipment |
(1,477 | ) | (4,108 | ) | (1,911 | ) | ||||||
| Purchase of convertible notes |
— | — | (2,274 | ) | ||||||||
| Sale proceeds from settlement of convertible notes |
— | 490 | 2,250 | |||||||||
| Change in restricted cash balance |
— | 2,500 | (2,491 | ) | ||||||||
| Proceeds from disposal of property and equipment |
519 | 2 | 18 | |||||||||
| Repayments of notes receivable |
— | — | 1,000 | |||||||||
| Purchase of MusclePharm apparel rights |
(850 | ) | — | — | ||||||||
| Purchase of trademarks |
(262 | ) | (484 | ) | (114 | ) | ||||||
| Investment in contract manufacturer |
(977 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in investing activities |
(3,047 | ) | (1,600 | ) | (3,522 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities |
||||||||||||
| Proceeds from line of credit |
$ | 9,507 | $ | 7,918 | $ | 2,492 | ||||||
| Payments on line of credit |
(14,507 | ) | (2,500 | ) | — | |||||||
| Repayments of term loan |
(1,051 | ) | — | — | ||||||||
| Repurchase of common stock |
— | (3,921 | ) | (1,037 | ) | |||||||
| Proceeds from issuance of term loan |
4,000 | — | — | |||||||||
| Issuance costs of term loan |
(40 | ) | — | — | ||||||||
| Proceeds from convertible note with a related party |
6,000 | — | — | |||||||||
| Repayments of other debt obligations |
(25 | ) | (17 | ) | (4,405 | ) | ||||||
| Repayment of capital lease obligations |
(162 | ) | (87 | ) | — | |||||||
| Proceeds from issuance of preferred stock, net of issuance cost |
— | — | 11,304 | |||||||||
| Proceeds from issuance of common stock and warrants, net of issuance cost |
— | — | 10,559 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
3,722 | 1,393 | 18,913 | |||||||||
|
|
|
|
|
|
|
|||||||
| Effect of exchange rate changes on cash |
(106 | ) | (52 | ) | (6 | ) | ||||||
| Net increase (decrease) in cash |
6,061 | (4,392 | ) | 5,412 | ||||||||
| Cash and cash equivalents, beginning of period |
1,020 | 5,412 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 7,081 | $ | 1,020 | $ | 5,412 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of cash flow information |
||||||||||||
| Cash paid for interest |
$ | 527 | $ | 158 | $ | 411 | ||||||
|
|
|
|
|
|
|
|||||||
| Cash paid for income taxes |
$ | 77 | $ | 301 | $ | 87 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosure of non-cash investing and financing activities |
||||||||||||
| Stock issued in conjunction with MusclePharm apparel rights acquisition |
$ | 1,394 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Stock issued for BioZone asset acquisition |
$ | — | $ | 8,833 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Stock issued for future services – third parties |
$ | — | $ | 5,403 | $ | 14,514 | ||||||
|
|
|
|
|
|
|
|||||||
| Stock issued to settle accounts payable, accrued liabilities and contracts |
$ | — | $ | — | $ | 5,544 | ||||||
|
|
|
|
|
|
|
|||||||
| Derivative liability on Series D offering |
$ | — | $ | — | $ | 8,175 | ||||||
|
|
|
|
|
|
|
|||||||
| Conversion of purchased convertible notes |
$ | — | $ | — | $ | 1,000 | ||||||
|
|
|
|
|
|
|
|||||||
| Common stock issued for board member compensation |
$ | — | $ | 115 | $ | 152 | ||||||
|
|
|
|
|
|
|
|||||||
| Reclassification of derivative liability to additional paid-in capital and warrant settlements |
$ | — | $ | 773 | $ | 11,979 | ||||||
|
|
|
|
|
|
|
|||||||
| Capital leases |
$ | 471 | $ | 148 | $ | 84 | ||||||
|
|
|
|
|
|
|
|||||||
| Purchase of property and equipment included in accounts payable and accrued liabilities |
$ | 48 | $ | 375 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Trademark registration included in accounts payable and accrued liabilities |
$ | 153 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
| Beneficial conversion feature related to convertible note |
$ | 52 | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of these consolidated financial statements.
53
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Note 1: Description of Business and Basis of Presentation
Description of Business
MusclePharm Corporation, or the Company, was incorporated in Nevada in 2006. The Company is a scientifically driven, performance lifestyle company that develops, manufactures, markets and distributes branded nutritional supplements. The Company is headquartered in Denver, Colorado and has the following wholly-owned operating subsidiaries: MusclePharm Canada Enterprises Corp (“MusclePharm Canada”), BioZone Laboratories, Inc. (“BioZone Labs”), MusclePharm Ireland Limited (“MusclePharm Ireland”) and MusclePharm Australia Pty Limited (“MusclePharm Australia”).
On August 24, 2015, the Board of Directors approved a restructuring plan for the Company. The approved restructuring plan was designed to reduce costs and to better align the Company’s resources for profitable growth. Specifically, during the quarters ended September 30, 2015 and December 31, 2015, the restructuring plan resulted in: 1) a reduction in the Company’s workforce; 2) the Company abandoning certain leased facilities; 3) the Company renegotiating or terminating a number of contracts with endorsers in a strategic shift away from such arrangements and towards more grass-roots marketing and advertising efforts; 4) the Company discontinuing a number of SKU’s and writing down inventory to estimated sales price; and 5) writing off certain assets. Management is continuing to execute on the approved restructuring plan, and as such, additional restructuring charges may be necessary. See Note 10 to the consolidated financial statements for further detail.
Management’s Plans with Respect to Liquidity and Capital Resources
The Company’s management believes the recently implemented restructuring, reduction in on-going operating costs and expense controls and the planned asset sale of BioZone Labs will create opportunities for the Company to be profitable. However, the Company may need to continue to raise capital. There can be no assurance that such capital will be available on acceptable terms or at all.
As of December 31, 2015, the Company had an accumulated deficit of $147.5 million and recurring losses from operations. The Company anticipates incurring additional losses until such time it can generate significant revenues and/or reduce operating costs. In September 2014, the Company borrowed $8.0 million under a line of credit. In February 2015, the Company entered into a term loan agreement and borrowed $4.0 million. In December 2015, the Company received an additional $6.0 million upon the issuance of a convertible note with a related party. As of December 31, 2015, the Company had approximately $7.1 million in cash and $20.0 million in working capital deficit.
The accompanying consolidated financial statements for the year ended December 31, 2015 were prepared on the basis of a going concern, which contemplates, among other things, the realization of assets and satisfaction of liabilities in the ordinary course of business. Accordingly, they do not give effect to adjustments that would be necessary should the Company be required to liquidate its assets. The Company has not established an ongoing source of revenue sufficient to cover its operating costs for at least the next 12 months and allow it to continue as a going concern. The ability of the Company to meet its total liabilities of $73.8 million at December 31, 2015, and to continue as a going concern is dependent on the Company obtaining adequate capital to fund operating losses until it becomes profitable. The Company can give no assurances that any additional capital that it is able to obtain, if any, will be sufficient to meet its needs, or that any such financing will be obtainable on acceptable terms. If the Company is unable to obtain adequate capital, it could be forced to cease operations or substantially curtail its commercial activities. These conditions raise substantial doubt as to the Company’s ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of these uncertainties.
54
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
Reclassifications
Certain prior period amounts have been reclassified to conform to the current period presentation.
Note 2: Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of MusclePharm Corporation and its wholly-owned subsidiaries. Acquisitions are included in the consolidated financial statements from the date of the acquisition. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported and disclosed in the consolidated financial statements and accompanying notes. Such estimates include, but are not limited to, allowance for doubtful accounts, revenue discounts and allowances, the valuation of inventory, the assessment of useful lives and recoverability of long-lived assets, likelihood and range of possible losses on contingencies, valuations of equity securities and intangible assets, fair value of derivatives, warrants and options, among others. Actual results could differ from those estimates.
Concentrations
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and accounts receivable. The Company minimizes its credit risk associated with cash by periodically evaluating the credit quality of its primary financial institution. The cash balance at times may exceed federally insured limits. Management believes the financial risk associated with these balances is minimal and has not experienced any losses to date.
Significant customers are those which represent more than 10% of the Company’s net revenue for each period presented, or the Company’s net accounts receivable balance as of each respective balance sheet date. For each significant customer, revenue as a percentage of total revenue and accounts receivable as a percentage of total net accounts receivable are as follows:
| Revenue, Net | Accounts Receivable, Net | |||||||||||||||||||
| Year Ended December 31, | As of December 31, | |||||||||||||||||||
| Customers |
2015 | 2014 | 2013 | 2015 | 2014 | |||||||||||||||
| Costco |
20 | % | 15 | % | * | 18 | % | 22 | % | |||||||||||
| Bodybuilding.com |
10 | % | 14 | % | 25 | % | * | 11 | % | |||||||||||
| GNC |
11 | % | * | * | 10 | % | * | |||||||||||||
| Europa |
* | * | 10 | % | 11 | % | * | |||||||||||||
| * | Represents less than 10% of revenue, net or accounts receivable, net. |
55
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
The Company uses a limited number of non-affiliated suppliers for contract manufacturing its products. The Company has quality control and manufacturing agreements in place with its primary manufacturers to ensure consistency in production and quality. The agreements ensure products are manufactured to the Company’s specifications and the contract manufacturers will bear the costs of recalled product due to defective manufacturing.
The Company had the following concentration of purchases with contract manufacturers for years ended December 31, 2015, 2014 and 2013:
| Year Ended December 31, | ||||||||||||
| Vendor |
2015 | 2014 | 2013 | |||||||||
| Capstone Nutrition |
59 | % | 44 | % | 67 | % | ||||||
| Nutra Blend |
25 | % | 50 | % | 32 | % | ||||||
| Bakery Barn |
11 | % | * | NA | ||||||||
| * | Represents less than 10% of purchases. |
Risk and Uncertainties
The Company operates in an industry that is subject to rapid change and intense competition. The Company’s operations are subject to significant risk and uncertainties including financial, operational, technological, regulatory and other risks, including the potential risk of business failure.
Cash
The Company considers all highly liquid investments purchased with an original maturity of three months or less at the date of purchase and money market accounts to be cash equivalents. As of December 31, 2015 and 2014, the Company had no cash equivalents and all cash amounts consisted of cash on deposit.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable represents trade obligations from customers that are subject to normal trade collection terms and are recorded at the invoiced amount, net of any allowance for doubtful accounts, and do not typically bear interest. The Company assesses the collectability of the accounts by taking into consideration the aging of accounts receivable, changes in customer credit worthiness, general market and economic conditions, and historical experience. Bad debt expenses are recorded as part of selling, general and administrative expenses in the consolidated statements of operations. The Company writes off the receivable balance against the allowance when management determines a balance is uncollectible. The Company also reviews its customer discounts and an accrual is made for discounts earned but not yet utilized at each period end.
The Company performs ongoing evaluations of its customers’ financial condition and generally does not require collateral. Some international customers are required to pay for their orders in advance of shipment.
56
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Accounts receivable consisted of the following as of December 31, 2015 and 2014 (in thousands):
| As of December 31, | ||||||||
| 2015 | 2014 | |||||||
| Accounts receivable |
$ | 26,057 | $ | 18,665 | ||||
| Less: allowance for discounts |
(3,707 | ) | (1,862 | ) | ||||
| Less: allowance for doubtful accounts |
(347 | ) | (159 | ) | ||||
|
|
|
|
|
|||||
| Accounts receivable, net |
$ | 22,003 | $ | 16,644 | ||||
|
|
|
|
|
|||||
The allowance for discount for the years ended December 31, 2015, 2014 and 2013 consisted of the following activity (in thousands):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Allowance for discount, beginning balance |
$ | 1,862 | $ | 1,060 | $ | 1,089 | ||||||
| Charges against revenues |
29,525 | 28,200 | 17,441 | |||||||||
| Utilization of sales return reserve |
(27,680 | ) | (27,398 | ) | (17,470 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Allowance for discount, ending balance |
$ | 3,707 | $ | 1,862 | $ | 1,060 | ||||||
|
|
|
|
|
|
|
|||||||
The allowance for doubtful accounts for the years ended December 31, 2015, 2014 and 2013 consisted of the following activity (in thousands):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Allowance for doubtful accounts, beginning balance |
$ | 159 | $ | 29 | $ | 25 | ||||||
| Charges to costs and expenses |
219 | 201 | 242 | |||||||||
| Recoveries |
— | — | 1 | |||||||||
| Deductions (write-offs) |
(27 | ) | (70 | ) | (239 | ) | ||||||
| Foreign currency translation adjustment |
(4 | ) | (1 | ) | — | |||||||
|
|
|
|
|
|
|
|||||||
| Allowance for doubtful accounts, ending balance |
$ | 347 | $ | 159 | $ | 29 | ||||||
|
|
|
|
|
|
|
|||||||
Purchased Convertible Notes & Issuer Warrants
The Company purchased convertible notes from unrelated public companies that it classified as trading securities which were carried at fair value with changes recognized through net loss. These purchased notes included warrants to purchase shares of the issuer’s common stock which were recorded as discounts against the carrying value of the related Notes based on their fair values upon issuance. See Notes 3 and 6 for further discussion of the Company’s purchased convertible notes and issuer warrants.
Inventory
MusclePharm products have historically been produced through third party manufacturers, and the cost of product inventory is recorded using actual cost on a first-in, first-out basis. BioZone products are manufactured in the Company’s production facilities in Pittsburg, CA, and the cost of inventory is recorded using a weighted average cost basis. Inventory is valued at the lower of cost or net realizable value. Adjustments to reduce the cost of inventory to its net realizable value are made, if required, and estimates are made for obsolescence, excess or
57
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
slow-moving inventories, non-conforming inventories and expired inventory. These estimates are based on management’s assessment of current future product demand, production plan, and market conditions.
Prepaid Giveaways
Prepaid giveaways represent non-inventory sample items which are given away to aid in promotion of the brand. Costs related to promotional giveaways are expensed as a component of advertising and promotion expenses in the consolidated statements of operations when the product is either given away at a promotional event or shipped to the customer.
Prepaid Stock Compensation
Prepaid stock compensation represents amounts paid with restricted stock awards for future contractual benefits to be received. The fair value of these restricted stock awards are recorded to prepaid stock compensation and additional paid-in capital, upon issuance of the shares, and then amortized to the consolidated statements of operations over the life of the contracts using the straight-line method. During the year ended December 31, 2015, in association with the restructuring, the Company wrote down $5.4 million of prepaid stock compensation related to terminated endorsement agreements.
Prepaid Sponsorship and Endorsement Fees
Prepaid sponsorship and endorsement fees represent fees paid in connection with Company sponsorships of certain events and trade shows as well as prepaid athlete endorsement fees, which are expensed over the period the fees are earned. A significant amount of the Company’s promotional expenses have resulted from payments under endorsement and sponsorship contracts. Accounting treatment for endorsement and sponsorship payments is based upon specific contract provisions. Generally, endorsement payments are expensed straight-line over the performance period(s) of the contract after giving recognition to periodic performance compliance provisions of the contract. Prepayments made under the contracts are included in either current or long-term prepaid expenses depending on the period for which the prepayment applies. During the year ended December 31, 2015, in connection with the restructuring, the Company wrote down $826,000 of prepaid sponsorship and endorsement fees related to terminated sponsorship agreements.
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of various payments the Company has made in advance for goods or services to be received in the future. These prepaid expenses include legal retainers, print advertising, insurance and service contracts requiring up-front payments. During the year ended December 31, 2015, in connection with the restructuring, the Company wrote down $155,000 of prepaid expense related to abandoned arrangements with certain vendors.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization is computed on a straight-line basis over the estimated useful lives of the respective assets or, in the case of leasehold improvements, the remaining lease term, if shorter. When assets are retired or otherwise disposed of, the assets and related accumulated depreciation are removed and the resulting gains or losses are recorded in the statements of operations. Repairs and maintenance costs are expensed as incurred.
58
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
The estimated useful lives of the property and equipment are as follows:
| Property and Equipment |
Estimated Useful Life | |
| Furniture, fixtures and equipment |
3 - 7 years | |
| Leasehold improvements |
Lesser of estimated useful life or remaining lease term | |
| Manufacturing and lab equipment |
3 - 5 years | |
| Vehicles |
3 - 5 years | |
| Displays |
5 years | |
| Website |
3 years |
Intangible Assets
Acquired intangible assets are recorded at estimated fair value, net of accumulated amortization, and costs incurred in obtaining certain trademarks are capitalized, and are amortized over their related useful lives, using a straight-line basis consistent with the underlying expected future cash flows related to the specific intangible asset. Costs to renew or extend the life of intangible assets are capitalized and amortized over the remaining useful life of the asset. Amortization expenses are included as a component of selling, general and administrative expenses in the consolidated statements of operations.
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment whenever events or changes in circumstances exist that indicate the carrying amount of an asset may not be recoverable. When indicators of impairment exist, an estimate of undiscounted future cash flows is used in measuring whether the carrying amount of the asset or related asset group is recoverable. Measurement of the amount of impairment, if any, is based upon the difference between the asset’s carrying value and estimated fair value. Based upon management’s analysis, the Company did not recognize any impairment charges on its long-lived assets during the years ended December 31, 2015, 2014 and 2013.
Issuance Costs and Debt Discount
The Company recognizes issuance costs related to the issuance of certain debt and equity instruments. Depending on the nature of the instrument, these costs are either carried as an asset on the balance sheet or recorded as a discount to the related debt or equity issuance. These costs are either amortized using the effective interest method over the life of the debt to interest expense, or not amortized if related to an equity issuance. If a conversion of the underlying debt occurs, a proportionate share of the unamortized cost or discount is immediately expensed.
Derivative Liabilities
Fair value accounting requires bifurcation of embedded derivative instruments, such as warrants or conversion features in equity instruments, and measurement of their fair value. In determining the appropriate fair value, the Company uses Black-Scholes valuation model. Derivatives are adjusted to reflect estimated fair value at the end of each reporting period with any increase or decrease in the estimated fair value being recorded in other income (expense), net in the consolidated statements of operations. Once a derivative liability ceases to exist, any remaining estimated fair value is reclassified to additional paid-in capital if redeemed.
59
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Revenue Recognition
Revenue is recognized when all of the following criteria are met:
| • | Persuasive evidence of an arrangement exists. Evidence of an arrangement consists of an order from the Company’s distributors, resellers or customers. |
| • | Delivery has occurred. Delivery is deemed to have occurred when title and risk of loss has transferred, either upon shipment of products to customers or upon delivery. |
| • | The fee is fixed or determinable. The Company assesses whether the fee is fixed or determinable based on the terms associated with the transaction. |
| • | Collection is reasonably assured. The Company assesses collectability based on credit analysis and payment history. |
The Company’s standard terms and conditions of sale do not allow for product returns. However, the Company grants an informal right of return to its customers. Estimates of expected future product returns are recognized at the time of sale based on analyses of historical return trends by customer type. Upon recognition, the Company reduces revenue and cost of revenue for the estimated return. Return rates can fluctuate over time, but are sufficiently predictable with established customers to allow the Company to estimate expected future product returns, and an accrual is recorded for future expected returns when the related revenue is recognized. Product returns incurred from established customers during the years ended December 31, 2015, 2014 and 2013 were insignificant.
The Company offers sales incentives through various programs, consisting primarily of advertising related credits, volume incentive rebates and sales incentive reserves. The Company records advertising related credits with customers as a reduction to revenue as no identifiable benefit is received in exchange for credits claimed by the customer. Volume incentive rebates are provided to certain customers based on contractually agreed upon percentages once certain thresholds have been met. Sales incentive reserves are computed based on historical trending and budgeted discount percentages, which are typically based on historical discount rates with adjustments for any known changes, such as future promotions or one-time historical promotions that will not repeat for each customer. The Company records sales incentive reserves and volume rebate reserves as a reduction to revenue.
During the years ended December 31, 2015, 2014 and 2013, the Company recorded discounts, and to a lesser degree, sales returns, totaling $29.5 million, $28.2 million and $17.4 million, which accounted for 15%, 14% and 14% of gross revenue in each period.
Cost of Revenue
Cost of revenue for MusclePharm, MusclePharm Canada and MusclePharm Ireland represents costs directly related to the production, manufacturing and freight-in of the Company’s products purchased from third party manufacturers. The Company ships customer orders from multiple locations. The facilities are operated with the Company’s equipment and employees, and inventory is owned by the Company. The Company also utilizes contract manufacturers to drop ship product directly to customers.
Cost of revenue for products produced by Biozone Labs consist of raw material, direct labor, freight-in, supplies and equipment rental expenses. The Company primarily ships customer orders from its distribution center in Pittsburg, California.
60
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Advertising and Promotion
Advertising and promotion expenses include digital and print advertising, trade show events, athletic endorsements and sponsorships, and promotional giveaways, and are expensed as incurred. For major trade shows, the expenses are recognized within a calendar year over the period in which the Company recognizes revenue associated with sales generated at the trade show. Some of the contracts provide for contingent payments to endorsers or athletes based upon specific achievement in their sports (e.g. winning a championship). The Company records expense for these payments if and when the endorser achieves the specific achievement.
Share-Based Payments
Share-based compensation awards, including stock options and restricted stock awards, are recorded at estimated fair value on the awards’ grant date, based on estimated number of awards that are expected to vest. The grant date fair value is amortized on a straight line basis over the time in which the awards are expected to vest, or immediately if no vesting is required. Share-based compensation awards issued to non-employees for services are recorded at either the fair value of the services rendered or the fair value of the share-based payments whichever is more readily determinable. The fair value of restricted stock awards are based on the fair value of the stock underlying the awards on the grant date as there is no exercise price. The fair value of stock options is estimated using the Black-Scholes option-pricing model but have been insignificant during the periods included herein.
Foreign Currency
The functional currency of the Company’s foreign subsidiaries, MusclePharm Canada and MusclePharm Ireland, is the local currency. The assets and liabilities of the foreign subsidiaries are translated into U.S. dollars at exchange rates in effect at each balance sheet date. Revenue and expenses are translated at average exchange rates in effect during the year. Equity transactions are translated using historical exchange rates. The resulting translation adjustments are recorded to a separate component of accumulated other comprehensive income (loss) within stockholders’ equity.
Foreign currency gains and losses resulting from transactions denominated in a currency other than the functional currency are included in other income (expense), net in the accompanying consolidated statements of operations.
Comprehensive Income (Loss)
Comprehensive income (loss) is composed of two components: net income (loss) and other comprehensive income (loss). Other comprehensive income (loss) refers to revenue, expenses, gains, and losses that under GAAP are recorded as an element of stockholders’ equity, but are excluded from the Company’s net income (loss). The Company’s other comprehensive income (loss) is made up of foreign currency translation adjustments for all periods presented.
Segments
Management has determined that it currently operates in one segment. The Company’s chief operating decision maker reviews financial information on a consolidated basis, together with certain operating and performance measures principally to make decisions about how to allocate resources and to measure the Company’s performance.
Income Taxes
Income taxes are accounted for using the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the consolidated financial
61
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
statement carrying amounts of existing assets and liabilities and their respective tax bases, operating loss and tax credit carry-forwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company recognizes the effect of income tax positions only if those positions are more likely than not to be sustained. Recognized income tax positions are measured at the largest amount that is greater than 50% likely to be realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs.
The Company records interest and penalties related to unrecognized tax benefits in income tax expense. There were no interest or penalties related to unrecognized tax benefits for the years ended December 31, 2015, 2014 and 2013.
Recent Accounting Pronouncements
In February 2016, the Financial Accounting Standards Board (“FASB”) issued ASU No. 2016-02, Leases (Topic 842), which supersedes Topic 840, Leases, (“ASU 2016-02”). The guidance in this new standard requires lessees to put most leases on their balance sheets but recognize expenses on their income statements in a manner similar to the current accounting and eliminates the current real estate-specific provisions for all entities. The guidance also modifies the classification criteria and the accounting for sales-type and direct financing leases for lessors. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018, and interim periods within those fiscal years, with early adoption permitted. The Company is currently evaluating the impact of the adoption of ASU 2016-02.
In November 2015, the FASB issued ASU No. 2015-17, Balance Sheet Classification of Deferred Taxes (“ASU 2015-17”), which requires that deferred tax assets and liabilities be classified as non-current in a classified statement of financial position. The accounting standard is effective, either prospectively to all deferred tax assets and liabilities or retrospectively to all periods presented, for annual periods beginning after December 15, 2016, and interim periods therein. Early adoption is permitted as of the beginning of an interim or annual reporting period. The Company early adopted this standard as of December 31, 2015 on a prospective basis.
In July 2015, the FASB issued ASU No. 2015-11, Inventory (Topic 330): Simplifying the Measurement of Inventory (“ASU 2015-11”), which simplifies the subsequent measurement of inventory by requiring inventory to be measured at the lower of cost and net realizable value. Net realizable value is the estimated selling price of inventory in the ordinary course of business, less reasonably predictable costs of completion, disposal and transportation. ASU 2015-11 is effective for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years. The Company is currently evaluating the impact of the adoption of ASU 2015-11.
In April 2015, the FASB issued ASU No. 2015-03, Interest — Imputation of Interest (Subtopic 835-30) — Simplifying the Presentation of Debt Issuance Costs (“ASU 2015-03”), which provides guidance on simplifying the presentation of debt issuance costs, requiring that debt issuance costs related to a recognized debt liability be presented in the consolidated balance sheets as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. In August 2015, the FASB issued ASU No. 2015-15, Interest – Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements — Amendments to SEC Paragraphs Pursuant to Staff Announcement at June 18, 2015 EITF Meeting (“ASU 2015-15”), which further clarifies ASU 2015-03 as it relates to presentation and subsequent measurement of debt issuance costs related to line-of-credit arrangements. ASU 2015-15 allows an entity deferring and presenting debt issuance costs related to line-of-credit arrangements as an asset and
62
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
subsequently amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. Both ASU 2015-03 and ASU 2015-15 require retrospective adoption and are effective for financial statement periods beginning after December 15, 2015, and interim periods within those fiscal years, with early adoption permitted. The Company has not early adopted ASU 2015-03 or ASU 2015-15 and the adoption of these standards is not expected to have a material effect on its consolidated financial statements or disclosures.
In August 2014, the FASB issued ASU No. 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern (“ASU 2014-15”). ASU 2014-15 explicitly requires management of all entities to evaluate whether there are conditions and events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the financial statements are issued (or available to be issued when applicable), and to provide related footnote disclosure in certain circumstances. ASU 2014-15 is effective for the Company in the first annual period ending after December 15, 2016, and for annual periods and interim periods thereafter. Earlier adoption is permitted. The Company has early adopted ASU 2014-15.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”), which provides guidance for revenue recognition. ASU 2014-09 affects any entity that either enters into contracts with customers to transfer goods or services or enters into contracts for the transfer of nonfinancial assets and supersedes the revenue recognition requirements in Topic 605, Revenue Recognition, and most industry-specific guidance. This ASU also supersedes some cost guidance included in Subtopic 605-35, Revenue Recognition- Construction-Type and Production-Type Contracts. ASU 2014-09’s core principle is that a company will recognize revenue when it transfers promised goods or services to customers in an amount that reflects the consideration to which a company expects to be entitled in exchange for those goods or services. In doing so, companies will need to use more judgment and make more estimates than under today’s guidance, including identifying performance obligations in the contract, estimating the amount of variable consideration to include in the transaction price and allocating the transaction price to each separate performance obligation. In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date (“ASU 2015-14”), which delays the effective date of ASU 2014-09 by one year. The FASB also agreed to allow entities to choose to adopt the standard as of the original effective date. As such, the updated standard will be effective for us in the first quarter of 2018, with the option to adopt it in the first quarter of 2017. The Company may adopt the new standard under the full retrospective approach or the modified retrospective approach. The Company has not yet selected a transition method nor has determined the effect of ASU 2014-09 on its ongoing financial reporting.
In April 2014, the FASB issued ASU No. 2014-08, Presentation of Financial Statements (Topic 205) and Property, Plant, and Equipment (Topic 360): Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity (“ASU 2014-08”). ASU 2014-08 changes the criteria for reporting a discontinued operation. Under the new pronouncement, a disposal of a part of an organization that has a major effect on its operations and financial results is a discontinued operation. The Company is required to adopt ASU 2014-08 prospectively for all disposals or components of its business classified as held for sale during fiscal periods beginning after December 15, 2014. The Company adopted ASU 2014-08 in 2015, and it did not have a material effect on the Company’s consolidated financial statements or disclosures.
Note 3: Fair Value of Financial Instruments
GAAP defines fair value as the exchange price that would be received from selling an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company measures its financial assets and liabilities at fair value at each reporting period using a estimated fair value hierarchy which requires the Company to maximize
63
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s classification within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. Three levels of inputs may be used to measure fair value:
| • | Level 1 — Observable inputs are unadjusted quoted prices in active markets for identical assets or liabilities. |
| • | Level 2 — Observable inputs are quoted prices for similar assets and liabilities in active markets or inputs other than quoted prices which are observable for the assets or liabilities, either directly or indirectly through market corroboration, for substantially the full term of the financial instruments. |
| • | Level 3 — Unobservable inputs which are supported by little or no market activity and which are significant to the fair value of the assets or liabilities. These inputs are based on our own assumptions used to measure assets and liabilities at fair value and require significant management judgment or estimation. |
The determination of where assets and liabilities fall within this hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
Management believes the fair value of the line-of-credit and term loan approximates carrying value because the debt carries market rates of interest. The Company’s remaining financial instruments consisted primarily of accounts receivable, accounts payable, accrued liabilities, and accrued restructuring charges, all of which are short-term in nature with fair values approximating carrying value. As of December 31, 2015 and 2014, the Company held no assets or liabilities that required remeasurement at fair value on a recurring basis.
As of December 31, 2015 and 2014 the Company did not have any outstanding purchased convertible notes or related warrants. The following table summarizes the activity of the Company’s purchased convertible notes and related warrants during the years ended December 31, 2014 and 2013 (in thousands):
| BioZone Convertible Note |
BioZone Warrants |
Fuse Convertible Note |
Fuse Warrants |
Total | ||||||||||||||||
| Balance – December 31, 2012 |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Fair value of purchased convertible notes on purchase date |
1,955 | 1,248 | 275 | 175 | 3,653 | |||||||||||||||
| Premium on purchase date |
45 | — | — | — | 45 | |||||||||||||||
| Discount for value of issuer warrants and conversion option |
(1,248 | ) | — | (176 | ) | — | (1,424 | ) | ||||||||||||
| Accretion of discount |
1,248 | — | 161 | — | 1,409 | |||||||||||||||
| Conversion of principal |
(1,000 | ) | — | — | — | (1,000 | ) | |||||||||||||
| Repayments received |
(1,000 | ) | — | — | — | (1,000 | ) | |||||||||||||
| Sale of instruments |
— | (1,250 | ) | — | — | (1,250 | ) | |||||||||||||
| Realized gain on sale |
— | 2 | — | — | 2 | |||||||||||||||
| Unrealized loss |
— | — | — | (56 | ) | (56 | ) | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance – December 31, 2013 |
$ | — | $ | — | $ | 260 | $ | 119 | $ | 379 | ||||||||||
| Accretion of discount |
— | — | 15 | — | 15 | |||||||||||||||
| Repayments received |
— | — | (275 | ) | — | (275 | ) | |||||||||||||
| Sale of instruments |
— | — | — | (215 | ) | (215 | ) | |||||||||||||
| Realized gain on sale |
— | — | — | 96 | 96 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance – December 31, 2014 |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
64
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
As of December 31, 2015 and 2014, the Company did not have any outstanding derivative liabilities. The following table summarizes the activity of the Company’s financial liabilities marked to market during the years ended December 31, 2014 and 2013 (in thousands):
| Balance – December 31, 2012 |
$ | — | ||
| Fair value at the commitment date for equity instruments |
8,175 | |||
| Fair value at the commitment date for warrants issued |
97 | |||
| Fair value mark to market adjustment for equity instruments |
4,796 | |||
| Fair value mark to market adjustment for warrants |
58 | |||
| Conversion instruments exercised or settled |
(11,979 | ) | ||
|
|
|
|||
| Balance – December 31, 2013 |
1,147 | |||
| Fair value mark to market adjustment for equity instruments and warrants |
(374 | ) | ||
| Conversion instruments exercised |
(773 | ) | ||
|
|
|
|||
| Balance – December 31, 2014 |
$ | — | ||
|
|
|
Note 4: Acquisition
On January 2, 2014, the Company closed the transactions contemplated in the asset purchase agreement dated November 12, 2013 with BioZone Pharmaceuticals, Inc. (“BioZone”) (OTC: BZNE) and its subsidiaries, BioZone Laboratories, Inc., and Bakers Cummins Corporation (collectively, the “Seller”). At closing, the Company acquired substantially all of the operating assets of BioZone, including all assets associated with QuSomes, HyperSorb and EquaSomes drug delivery technologies and the name “Biozone”, “Biozone Laboratories” and similar names and domain names (excluding certain assets including cash on hand), for $7.1 million in MusclePharm common stock, net of an embedded derivative to repurchase common stock of $444,000 and a net contingent asset of $1.5 million.
The purchase price under the asset purchase agreement was 1,200,000 shares of the Company’s common stock of which 600,000 shares were issued to the seller and 600,000 shares were placed in escrow for a period of nine months to cover indemnification obligations. These 600,000 escrowed shares were also subject to repurchase from the escrow for $10.00 per share in cash which was accounted for as an embedded derivative. The initial 600,000 were issued to the seller upon closing and were subject to a lockup agreement which permits private sales (subject to the lockup and certain leak out provisions).
As of December 31, 2014, the Company completed the final fair value analysis of all assets and liabilities acquired. In October 2014, the Company sent a notice of claim to the seller and escrow agent for the shares being held in escrow. In October 2014, the Company received 350,000 shares from the escrow agent to settle the claim. Additionally, in October 2014, the Company exercised the repurchase option and acquired 250,000 shares of its common stock for $2.5 million. The total of these 600,000 shares are held in treasury stock as of December 31, 2014. In conjunction with the fair value analysis, the Company recognized a bargain purchase gain of $3.7 million, as the fair value of assets and liabilities acquired exceeded the total amount of consideration as BioZone was experiencing a distressed financial situation. After the return of shares held in escrow, the Company also recognized a $1.6 million gain as reimbursement of expenses and settlement of a contingent asset and liability related to one of the leased buildings that BioZone operates.
The bargain purchase gain and contingent asset gain are included as a component of other income (expense), net in the consolidated statements of operations.
65
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
The BioZone asset purchase is considered an acquisition of a business and was accounted for in accordance with accounting guidance for business combinations. The fair value of all identifiable tangible and intangible assets purchased in the acquisition was determined by the Company’s management. The following table summarizes the fair values of assets acquired and liabilities assumed (in thousands):
| Net Tangible Assets |
||||
| Current assets |
$ | 3,183 | ||
| Property and equipment |
1,859 | |||
| Liabilities assumed |
(1,379 | ) | ||
|
|
|
|||
| Total net tangible assets acquired |
3,663 | |||
|
|
|
|||
| Identified Intangible Assets |
||||
| Customer relationships |
3,130 | |||
| Technology |
2,158 | |||
| Brand |
1,776 | |||
| Non-compete agreements |
69 | |||
|
|
|
|||
| Total identified intangible assets acquired |
7,133 | |||
|
|
|
|||
| Bargain purchase gain |
(3,686 | ) | ||
|
|
|
|||
| Total purchase price allocation |
$ | 7,110 | ||
|
|
|
Supplemental Pro Forma Information for BioZone Acquisition
The consolidated statements of operations include the results of operations from BioZone since the acquisition date of January 2, 2014. The Company has determined that there were no significant transactions on January 1, 2014 and has therefore not presented the pro forma effects of the acquisition for the year ended December 31, 2014. Supplemental information on a pro forma basis is presented below for the BioZone acquisition as if the acquisition had occurred on January 1, 2013 (in thousands):
| Year Ended December 31, 2013 |
||||
| (Unaudited) | ||||
| Pro forma revenue, net |
$ | 119,120 | ||
| Pro forma loss from operations |
(19,031 | ) | ||
| Pro forma net loss |
$ | (22,576 | ) | |
The unaudited pro forma financial information combines the results of operations as if the BioZone acquisition had occurred as of January 1, 2013. The pro forma results include the acquisition accounting effects resulting from the acquisition such as the amortization charges from acquired intangible assets and acquisition-related transaction costs. The pro forma information presented does not purport to present what the actual results would have been had the acquisitions actually occurred on January 1, 2013, nor is the information intended to project results for any future period.
Note 5: Capstone Nutrition Agreements
The Company entered into a series of agreements with Capstone Nutrition (“Capstone”) effective March 2, 2015, including an amendment (the “Amendment”) to a Manufacturing Agreement dated November 27, 2013 (the “Manufacturing Agreement”). Pursuant to the Amendment, Capstone shall be the Company’s nonexclusive manufacturer of dietary supplements and food products sold or intended to be sold by the Company. The Amendment includes various agreements including amended pricing terms. The initial term ends January 1, 2022, and may be extended for three successive twenty-four month terms and includes renewal options.
66
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
The Company agreed to pay to Capstone a non-refundable sum of $2.5 million to be used by Capstone solely in connection with the expansion of its facility necessary to fulfill anticipated Company requirements under the Manufacturing Agreement and Amendment. The Company has paid Capstone $2.5 million as of December 31, 2015.
The Company and Capstone entered into a Class B Common Stock Warrant Purchase Agreement (“Warrant Agreement”) whereby the Company may purchase approximately 19.9% of Capstone’s parent company, INI Parent, Inc. (“INI”), on a fully-diluted basis as of March 2, 2015. Pursuant to the Warrant Agreement, INI issued to the Company a warrant (the “Warrant”) to purchase shares of INI’s Class B common stock, par value $0.001 per share at an exercise price of $0.01 per share (the “Warrant Shares”). The warrant may be exercised if the Company is in compliance with the terms and conditions of the Amendment.
The Company utilized the Black-Scholes valuation model to determine the value of the warrants and recorded an asset of $977,000, which was accounted for under the cost method and assessed for impairment. The warrant is included in the caption long-term investments within the consolidated balance sheet as of December 31, 2015. The Company also recorded $1.5 million of prepaid expenses and other assets on the consolidated balance sheet as of December 31, 2015, which is being amortized over the remaining life of the Manufacturing Agreement of 6.5 years.
The Company and INI also entered into an option agreement (the “Option Agreement”). Subject to additional provisions and conditions set forth in the Option Agreement, at any time on or prior to June 30, 2016, the Company shall have the right to purchase for cash all of the remaining outstanding shares of INI’s common stock not already owned by the Company after giving effect to the exercise of the Warrant, based on an aggregate enterprise value, equal to $200 million. The fair value of the option was deemed de minimus as of the transaction date.
The Company is engaged in a dispute with Capstone concerning amounts allegedly owed under the Manufacturing Agreement. Capstone claims that it is owed approximately $22.0 million of outstanding accounts payable. The Company claims that Capstone owes the Company at least $13.5 million in losses caused by, among other things, Capstone’s failure to timely manufacture and supply the Company’s products. On February 12, 2016, Capstone commenced a mediation with the American Arbitration Association. As of the date of this report, the mediation has not yet been scheduled.
Note 6: Purchased Convertible Notes and Issuer Warrants
BioZone Convertible Note
In August 2013, the Company purchased, for an aggregate purchase price of $2.0 million, a secured convertible promissory note from BioZone Pharmaceuticals, Inc. (“BioZone”) (OTC: BZNE) that matured one year from the date of issuance. The BioZone note bore interest at a rate of 10% per annum, was convertible at any time prior to the maturity date into 10,000,000 shares of BioZone common stock at the conversion rate of $0.20 per share, and contained warrants and certain put and call features discussed further below. The Company’s ability to convert the note into BioZone common stock was only restricted by a beneficial ownership limitation of 4.99% of the number of the common stock outstanding after giving effect to common stock issuable upon conversion.
In conjunction with the issuance of the BioZone convertible note, the Company received warrants to purchase up to 10,000,000 shares of BioZone common stock with an exercise price of $0.40 per share and an expiration date 10 years from issuance. The Company’s ability to exercise the warrant is limited by a beneficial ownership limitation of 4.99% of the number of the BioZone common shares outstanding after giving effect to the exercise
67
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
of the warrant. The fair value of the warrants was determined to be $1.2 million upon issuance which was recorded as a discount to the carrying value of the BioZone convertible note. In addition, a change of control put option was identified but not recorded as a derivative because the value was determined to be de minimis. The BioZone notes were also purchased at a premium of $45,000.
The Company classified the BioZone note as a Level 2 available-for-sale security, however it was only outstanding for two months during the year ended December 31, 2013. In addition, the Company engaged an independent third party firm to determine the fair value the note, warrants and embedded conversion features upon issuance and changes in fair value of the note were included as a component of other comprehensive income (loss) until the note was settled in October 2013 because the notes were considered to be available-for-sale. The $45,000 premium was netted against a discount of $1.2 million attributable to the BioZone warrants and was accreted to interest income over the stated maturity of the note.
In addition, the Company classified the BioZone warrant as a Level 2 fair value measurement and the fair value of the warrant was determined using a binomial lattice pricing model assuming an exercise price of $0.40 per share, contractual term of 10 years and a volatility of 70% upon issuance.
In October 2013, the Company converted the BioZone note as follows: principal in the amount of $1.0 million converted into 5,000,000 shares of BioZone’s common stock and principal of $1.0 million and accrued interest of $33,000 was repaid in cash to satisfy the remaining debt. All remaining amounts related to the note discount were recognized as interest income and the changes in fair value were recorded in net income (loss). All amounts carried in other comprehensive income (loss) related to this note were reclassified to net income (loss) upon its conversion and repayment. The Company recognized a total loss on the extinguishment of the BioZone note of $14,000. In November 2013, the Company entered into a sale agreement with several accredited investors to sell the BioZone warrants for an aggregate purchase price of $1.3 million. Accordingly, as of December 31, 2013, the BioZone notes and warrants were no longer owned.
Fuse Convertible Note
In November 2013, the Company purchased, for an aggregate purchase price of $200,000, a senior secured convertible promissory note from Fuse Science Inc. (“Fuse”) (OTC: DROP) that matured 90 days from the date of issuance. The Fuse note bore interest at a rate of 10% per annum, was convertible at any time prior to the maturity date into 3,076,923 shares of Fuse common stock at the conversion rate of $0.065 per share, and contained warrants and certain conversion features discussed further below. The Company’s ability to convert the note into Fuse common stock was only restricted by a beneficial ownership limitation of 9.99% of the number of the common stock outstanding after giving effect to common stock issuable upon conversion. In December 2013, the Company amended the Fuse note in order to purchase an additional $75,000 under the original terms of the note.
In conjunction with the issuance of the Fuse note, the Company received warrants to purchase up to 9,165,750 shares of Fuse common stock with an exercise price of $0.065 per share and an expiration dates of five years from the date of issuance. The fair value of the warrants was determined to be $175,000 upon issuance which was recorded as a discount to the carrying value of the Fuse convertible note. The conversion feature was determined to have a fair value of $2,000 upon issuance of the Fuse note.
The Company classified the Fuse note as a Level 2 trading security and used a Black-Scholes model to determine the fair value of the conversion option and warrants. Changes in the fair value of the Fuse note were included within other income (expense), net on the consolidated statements of operations. As of December 31, 2013, only discounts in the amount of $10,000 had not been fully accreted.
In January 2014, the Company renewed the $275,000 Fuse note providing for a new maturity date of January 3, 2019 and to update the conversion rate of the Fuse note to $0.02 per share, or convertible into 13,750,000 shares
68
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
of Fuse common stock. In addition, the Company recognized the conversion option of the convertible note as a derivative instrument with a fair value of $207,000, which was recorded as a discount against the note.
In April 2014, the Company entered into a security purchase agreement and sold the Fuse convertible note and warrants for an aggregate purchase price of $215,000.
Note 7: Balance Sheet Components
Inventory
On July 1, 2013, the Company terminated a Distribution Agreement dated November 17, 2010 with one of its key product manufacturers in which the manufacturer received and fulfilled customer sales orders for a majority of the Company’s products. In connection with the termination of the agreement, the Company purchased an aggregate $4.7 million of product inventory, and took over control of customer order fulfillment through the warehouse located at Franklin, Tennessee. In August 2014, the Company opened a second distribution center in Pittsburg, California. In July 2015, the Company moved the warehouse in Franklin, Tennessee to Spring Hill, Tennessee.
Inventory consisted of the following as of December 31, 2015 and 2014 (in thousands):
| As of December 31, | ||||||||
| 2015 | 2014 | |||||||
| Raw materials |
$ | 1,385 | $ | 1,169 | ||||
| Work-in-process |
22 | 101 | ||||||
| Finished goods |
11,142 | 19,799 | ||||||
|
|
|
|
|
|||||
| Inventory |
$ | 12,549 | $ | 21,069 | ||||
|
|
|
|
|
|||||
The Company writes down inventory for obsolete and slow moving inventory based on the age of the product as determined by the expiration date. Products within one year of their expiration dates are considered for write-off purposes. Historically, the Company has had minimal returns with established customers. Other than write-down of inventory during restructuring activities, the Company incurred insignificant inventory write-offs during the years ended December 31, 2015 and 2014.
As disclosed further in Note 10, the Company executed a restructuring plan in August 2015 and recorded a write-down of inventory related to discontinued products of $2.6 million, which was included in cost of revenue in the consolidated statement of operations. Inventory write downs are included as a component of cost of revenue in the accompanying consolidated statements of operations. Inventory write-downs, once established, are not reversed as they establish a new cost basis for the inventory.
Property and Equipment
Property and equipment consisted of the following as of December 31, 2015 and 2014 (in thousands):
| As of December 31, | ||||||||
| 2015 | 2014 | |||||||
| Furniture, fixtures and equipment |
$ | 3,621 | $ | 4,041 | ||||
| Leasehold improvements |
3,227 | 2,298 | ||||||
| Manufacturing and lab equipment |
1,659 | 1,388 | ||||||
| Vehicles |
1,146 | 470 | ||||||
| Displays |
483 | 488 | ||||||
| Website |
463 | 241 | ||||||
| Construction in process |
54 | 1,511 | ||||||
|
|
|
|
|
|||||
| Property and equipment, gross |
10,653 | 10,437 | ||||||
| Less: accumulated depreciation and amortization |
(3,960 | ) | (2,632 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 6,693 | $ | 7,805 | ||||
|
|
|
|
|
|||||
69
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Depreciation and amortization expense related to property and equipment was $1.8 million, $1.3 million and $709,000 for the years ended December 31, 2015, 2014 and 2013, which is included in the selling, general, and administrative in the consolidated statements of operations.
As disclosed further in Note 10, the Company executed a restructuring plan in August 2015 and wrote-off certain long-lived assets, primarily leasehold improvements, related to the abandonment of certain leased facilities. The write-off of long-lived assets of $406,000 is included as a component of restructuring and other charges in the accompanying consolidated statements of operations for the year ended December 31, 2015.
Intangible Assets
Intangible assets consist of the following (in thousands) and include the BioZone asset acquisition and MusclePharm’s apparel rights reacquired from Worldwide Apparel disclosed further in Note 15:
| As of December 31, 2015 | ||||||||||||||||
| Gross Value |
Accumulated Amortization |
Net Carrying Value |
Weighted Average Useful Lives (years) |
|||||||||||||
| Amortized intangible assets |
||||||||||||||||
| Customer relationships |
$ | 3,130 | $ | (417 | ) | $ | 2,713 | 15.0 | ||||||||
| Non-compete agreements |
69 | (69 | ) | — | 2.0 | |||||||||||
| Patents |
2,158 | (540 | ) | 1,618 | 8.0 | |||||||||||
| Trademarks |
933 | (133 | ) | 800 | 6.7 | |||||||||||
| Brand |
4,020 | (522 | ) | 3,498 | 10.5 | |||||||||||
| Domain name |
54 | (31 | ) | 23 | 5.0 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total intangible assets |
$ | 10,364 | $ | (1,712 | ) | $ | 8,652 | |||||||||
|
|
|
|
|
|
|
|||||||||||
| As of December 31, 2014 | ||||||||||||||||
| Gross Value |
Accumulated Amortization |
Net Carrying Value |
Weighted Average Useful Lives (years) |
|||||||||||||
| Amortized intangible assets |
||||||||||||||||
| Customer relationships |
$ | 3,130 | $ | (209 | ) | $ | 2,921 | 15.0 | ||||||||
| Non-compete agreements |
69 | (35 | ) | 34 | 2.0 | |||||||||||
| Patents |
2,211 | (293 | ) | 1,918 | 7.9 | |||||||||||
| Trademarks |
518 | (20 | ) | 498 | 4.5 | |||||||||||
| Brand |
1,776 | (118 | ) | 1,658 | 15.0 | |||||||||||
| Domain name |
68 | (23 | ) | 45 | 5.0 | |||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total intangible assets |
$ | 7,772 | $ | (698 | ) | $ | 7,074 | |||||||||
|
|
|
|
|
|
|
|||||||||||
Intangible amortization expense for the years ended December 31, 2015, 2014 and 2013 was $1.1 million, $698,000, and nil. In conjunction with the final valuation of the assets purchased from BioZone (see Note 4), the Company recognized a cumulative adjustment to amortization of intangible assets included in operating expenses on the consolidated statement of operations that resulted in a reduction of amortization expense of $430,000 for the year ended December 31, 2014.
70
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
As of December 31, 2015, the estimated future amortization expense of intangible assets is as follows (in thousands):
| Year Ending December 31, | ||||
| 2016 |
$ | 1,080 | ||
| 2017 |
1,071 | |||
| 2018 |
1,063 | |||
| 2019 |
1,061 | |||
| 2020 |
1,032 | |||
| Thereafter |
3,345 | |||
|
|
|
|||
| Total amortization expense |
$ | 8,652 | ||
|
|
|
|||
Note 8: Other (Expense) Income, Net
During the years ended December 31, 2015, 2014 and 2013, other (expense) income, net consists of the following (in thousands):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Other (expense) income, net |
||||||||||||
| Interest income |
$ | — | $ | 223 | $ | 1,442 | ||||||
| Interest expense |
(779 | ) | (201 | ) | (783 | ) | ||||||
| Derivative expense |
— | — | (97 | ) | ||||||||
| Change in fair value of derivative liabilities |
— | 374 | (4,854 | ) | ||||||||
| Gain on settlement of accounts payable and debt |
— | 31 | 574 | |||||||||
| Gain (loss) on purchased convertible notes |
— | (386 | ) | 445 | ||||||||
| Bargain purchase gain and contingent asset gain on BioZone acquisition |
— | 5,265 | — | |||||||||
| Foreign currency transaction gain (loss) |
(1,047 | ) | 19 | (31 | ) | |||||||
| Other |
20 | 252 | (2 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other (expense) income, net |
$ | (1,806 | ) | $ | 5,577 | $ | (3,306 | ) | ||||
|
|
|
|
|
|
|
|||||||
Note 9: Debt
As of December 31, 2015 and 2014, the Company’s debt consisted of the following (in thousands):
| As of December 31, | ||||||||
| 2015 | 2014 | |||||||
| Revolving line of credit |
$ | 3,000 | $ | 8,000 | ||||
| Term loan |
2,949 | — | ||||||
| Convertible note |
5,952 | — | ||||||
| Other |
21 | 46 | ||||||
|
|
|
|
|
|||||
| Total debt |
11,922 | 8,046 | ||||||
| Less: current portion |
(5,970 | ) | (8,046 | ) | ||||
|
|
|
|
|
|||||
| Long term debt |
$ | 5,952 | $ | — | ||||
|
|
|
|
|
|||||
71
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
In September 2014, the Company entered into a line of credit facility ANB Bank for up to $8.0 million of borrowings. The line of credit originally renewed annually, matured in September 2017, and accrued interest at the prime rate plus 2%. The line of credit was secured by our inventory, accounts receivable, intangible assets and equipment. As of December 31, 2015, the outstanding borrowings under the line of credit were $3.0 million. The Company was not in compliance with certain financial covenants under the line of credit as of December 31, 2015, which limited further borrowings.
In February 2015, the Company entered into a $4.0 million term loan agreement with ANB Bank. The term loan carried a fixed interest rate of 5.25% per annum, was repayable in 36 equal monthly installments of principal and interest, and originally matured in February 2018. The term loan contained various events of default, including cross default provisions related to the line of credit, which could have required repayments of the term loan. The Company was not in compliance with certain financial covenants under the term loan as of December 31, 2015, and received various waivers from the lender during the period. As of December 31, 2015, the outstanding borrowings under the term loan were $2.9 million.
On October 9, 2015, the Company entered into loan modification agreements with ANB Bank under the line of credit and term loan to: (i) change the maturity date of the loans to January 15, 2016, (ii) prohibit the loans to be declared in default prior to December 10, 2015, except for defaults resulting from failure to make timely payments, and (iii) delete certain financial covenants from the line of credit. In consideration for these modifications, Ryan Drexler, Interim Chief Executive Officer, President and Chairman, and a family member provided their individual guaranty for the remaining balance of the term loan and line of credit of $6.2 million. In consideration for executing his guaranty, the Company issued Ryan Drexler of Directors 28,571 shares of the Company’s common stock with a grant date fair value of $80,000 (based upon the closing price of common stock on the date of issuance).
In December, 2015, the Company entered into a convertible secured promissory note agreement with Ryan Drexler, Interim Chief Executive Officer, President and Chairman, under which he lended us $6.0 million. Proceeds from the note were used to fund working capital requirements. The convertible note is secured by all assets and properties of the Company and its subsidiaries whether tangible or intangible. The convertible note carries an interest at 8% per annum, or 10% in the event of default. Both the principal and the interest under the convertible note are due in January 2017, unless converted earlier. The holder can convert the outstanding principal and accrued interest into shares of common stock for $2.30 per share at any time. The Company may prepay the convertible note at the aggregate principal amount therein plus accrued interest by giving the holder between 15 and 60 day-notice, depending upon the specific circumstances, provided that the holder may convert the note during the notice period. The Company recorded the convertible note of $6.0 million as a liability in the balance sheet and also recorded a beneficial conversion feature of $52,000 as a debt discount upon issuance of the convertible note, which is being amortized over the term of the convertible debt using the effective interest method. The beneficial conversion feature was calculated based on the difference between the fair value of common stock and the effective conversion price of the convertible note. As of December 31, 2015, the convertible note had an outstanding principal balance of $6.0 million.
Other
Other debt primarily consists of debt in default as of December 31, 2015 and 2014 and is included as a component of short-term debt. Debt in default is related to convertible debt issued during the year ended December 31, 2012 and prior where the convertible debt was never converted to common stock or principal repaid. The Company is in the process of contacting the remaining debt holders and negotiating settlement of the debt.
72
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Note 10: Restructuring
As part of an effort to better focus and align the Company’s resources toward profitable growth, on August 24, 2015, the Board of Directors authorized the Company to undertake steps to commence a restructuring of the business and operations, which continued during the fourth quarter. The Company closed certain facilities, reduced headcount, discontinued products, and renegotiated certain contracts resulting in a restructuring and other charges of $21.2 million, of which $2.9 million was included in cost of revenue and $18.3 million was included in operating expenses in the accompanying consolidated statements of operations.
For the year ended December 31, 2015, restructuring charges of $9.1 million, to be paid in cash, were comprised primarily of: (i) $1.3 million related to severance and termination benefit costs related to terminated employees; (ii) $7.0 million related to cancellation of certain contracts and sponsorship agreements, which are payable through December 2016; (iii) $350,000 related to purchase commitment of discontinued inventories not yet received by the Company, which remains accrued at December 31, 2015; and (iv) $467,000 for costs associated with permanently vacating certain leased facilities.
The following table illustrates the provision of the restructuring charges and the accrued restructuring charges balance as of December 31, 2015 (in thousands):
| Employee Severance Costs |
Contract Termination Costs |
Purchase Commitment of Discontinued Inventories Not Yet Received |
Abandoned Leased Facilities |
Total | ||||||||||||||||
| Balance as of December 31, 2014 |
$ | — | $ | — | $ | — | $ | — | $ | — | ||||||||||
| Expensed |
1,353 | 6,979 | 350 | 467 | 9,149 | |||||||||||||||
| Cash payments |
(845 | ) | (949 | ) | — | (56 | ) | (1,850 | ) | |||||||||||
| Reclassification from accounts payable to cancellation of certain contracts and sponsorship agreements |
— | 2,120 | — | — | 2,120 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of December 31, 2015 |
$ | 508 | $ | 8,150 | $ | 350 | $ | 411 | $ | 9,419 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
As a result of the reduction in force, 657,310 shares of restricted common stock vested in accordance with the original stock grant terms and conditions and resulted in the recognition of employee stock-based compensation of $2.7 million. During the year ended December 31, 2015, in association with the restructuring, the Company recorded the following charges totaling $9.5 million (in thousands):
| Operating Expenses |
||||
| Employee stock-based compensation |
$ | 2,730 | ||
| Write-down of prepaid stock compensation related to terminated endorsement agreements |
5,377 | |||
| Write-down of prepaid assets related to terminated sponsorship agreements, discontinued products and abandoned other arrangements |
981 | |||
| Write-off of long-lived assets related to the abandonment of certain lease facilities |
406 | |||
|
|
|
|||
| Total other charges |
$ | 9,494 | ||
|
|
|
|||
73
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
The total future payments under the restructuring plan as of December 31, 2015 are as follows (in thousands):
| Year Ending December 31, | ||||||||||||||||||||||||
| Outstanding Payments |
2016 | 2017 | 2018 | 2019 | 2020 | Total | ||||||||||||||||||
| Contract termination costs |
$ | 8,150 | $ | — | $ | — | $ | — | $ | — | $ | 8,150 | ||||||||||||
| Purchase commitment of discontinued inventories not yet received |
350 | — | — | — | — | 350 | ||||||||||||||||||
| Employee severance costs |
508 | — | — | — | — | 508 | ||||||||||||||||||
| Abandoned leased facilities |
132 | 100 | 85 | 87 | 7 | 411 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total future payments |
$ | 9,140 | $ | 100 | $ | 85 | $ | 87 | $ | 7 | $ | 9,419 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Note 11: Derivative Liabilities
The Company identified various derivatives in the form of freestanding warrants and conversion features embedded within convertible preferred stock issued during the year ended December 31, 2013 as follows:
Embedded Conversion Feature
In January 2013, the Company sold 1,500,000 shares of Series D convertible preferred stock for aggregate gross proceeds of $12.0 million. The Series D convertible preferred stock contained an embedded derivative liability related to a conversion feature that was determined to be a derivative requiring bifurcation and separate accounting as a derivative liability. The related shares all converted to common stock during the years ended December 31, 2014 and 2013. Accordingly, the derivative liability was no longer outstanding as of December 31, 2015 and 2014. Upon elimination of the derivative liability, $773,000 was reclassified to additional paid-in capital in the consolidated balance sheets.
The fair value of the Series D embedded derivative was determined during the years ended December 31, 2014 and 2013 assuming the following:
| Commitment Date |
Re-measurement Date |
|||||||
| Expected term (in years) |
1 year | 1 year | ||||||
| Expected volatility |
120 | % | 47 | % | ||||
| Risk-free interest rate |
0.14 | % | 0.13 | % | ||||
| Dividend yield |
0 | % | 0 | % | ||||
Warrants
During the year ended December 31, 2013, the Company issued warrants to purchase 40,000 shares of common stock in conjunction with a consulting agreement. The Company did not issue any warrants during the years ended December 31, 2015 and 2014.
Derivatives Expense
In situations where the Company recorded the debt discount and initial value of derivative contracts associated with the convertible preferred stock issuance against the gross proceeds raised, any remaining value of the derivative that exceeded the gross proceeds of the offering was expensed immediately as derivative expense in other income (expense), net on the consolidated statements of operations.
74
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Note 12: Commitments and Contingencies
Operating Leases and Capital Leases
The Company leases office and warehouse facilities under operating leases which expire at various dates through 2029. The amounts reflected in the table below are for the aggregate future minimum lease payments under non-cancelable facility operating leases. Under lease agreements that contain escalating rent provisions, lease expense is recorded on a straight-line basis over the lease term. Rent expense for the years ended December 31, 2015, 2014 and 2013 amounted to $1.6 million, $1.3 million and $608,000.
As of December 31, 2015, future minimum lease payments are as follows (in thousands): (1)
| Year Ending December 31, |
||||
| 2016 |
$ | 1,064 | ||
| 2017 |
854 | |||
| 2018 |
868 | |||
| 2019 |
708 | |||
| 2020 |
431 | |||
| Thereafter |
2,381 | |||
|
|
|
|||
| Total minimum lease payments |
$ | 6,306 | ||
|
|
|
| (1) | The amounts in the table above excluded $0.5 million in operating lease expenses resulting from our restructuring plans expensed in 2015 (see Note 10). |
Capital Leases
The Company leases manufacturing and warehouse equipment under capital leases which expire at various dates through May 2019. As of December 31, 2015 and 2014, the Company had $865,000 and $356,000, respectively, in leased assets included in furniture, fixtures, and equipment and manufacturing and lab equipment balances of property and equipment in the consolidated balance sheets. The accumulated depreciation on leased assets as of December 31, 2015 and 2014 was $126,000 and $32,000, respectively. As of December 31, 2015 and 2014, short-term capital lease liabilities of $186,000 and $118,000, respectively are included as a component of current liabilities, and the long-term capital lease liabilities of $330,000 and $146,000 respectively are included as a component of long-term liabilities in the consolidated balance sheets.
In December 2014, the Company entered into a capital lease agreement providing for approximately $1.8 million in credit to lease up to 50 vehicles as part of a fleet lease program. As of December 31, 2015, the Company acquired 21 vehicles under the capital lease and the original costs and accumulated depreciation of leased assets are $670,000 and $90,000, respectively, which are included in vehicle balances of property and equipment in the consolidated balance sheets.
75
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
As of December 31, 2015, the Company’s future minimum lease payments under capital lease agreements are as follows (in thousands):
| Year Ending December 31, |
||||
| 2016 |
$ | 210 | ||
| 2017 |
165 | |||
| 2018 |
129 | |||
| 2019 |
56 | |||
|
|
|
|||
| Total minimum lease payments |
560 | |||
| Less amounts representing interest |
(44 | ) | ||
|
|
|
|||
| Present value of minimum lease payments |
$ | 516 | ||
|
|
|
Contingencies
In the normal course of business or otherwise, the Company may become involved in legal proceedings. The Company will accrue a liability for such matters when it is probable that a liability has been incurred and the amount can be reasonably estimated. When only a range of possible loss can be established, the most probable amount in the range is accrued. If no amount within this range is a better estimate than any other amount within the range, the minimum amount in the range is accrued. The accrual for a litigation loss contingency might include, for example, estimates of potential damages, outside legal fees and other directly related costs expected to be incurred. As of December 31, 2015, the Company was not involved in any material legal proceedings, with the exception of the lawsuit with a former executive. As of December 31, 2014, the Company was not involved in any material legal proceedings with the exception of the SEC Investigation discussed below.
Third-Party Manufacturer Dispute
The Company is engaged in a dispute with Capstone concerning amounts allegedly owed under the Manufacturing Agreement. Capstone claims that it is owed approximately $22.0 million in outstanding payables. The Company disputes Capstone’s claim, and claims that Capstone owes the Company at least $13.5 million in losses caused by, among other things, Capstone’s failure to timely manufacture and supply the Company’s products. On February 12, 2016, Capstone commenced a mediation with the American Arbitration Association. As of the date of this report, the mediation has not yet been scheduled.
Supplier Complaint
On January 15, 2016, ThermoLife International LLC (“ThermoLife”), a supplier of nitrates to MusclePharm, filed a complaint against the Company in Arizona state court. In its complaint, ThermoLife alleges that the Company failed to meet minimum purchase requirements contained in the parties’ supply agreement. On March 14, 2016, the Company filed an answer to ThermoLife’s complaint denying the allegations contained in the complaint, and a counterclaim alleging that ThermoLife breached its express warranty to MusclePharm because ThermoLife’s products were defective and could not be incorporated into the Company’s products. The action is pending.
Former Employee Lawsuit
On December 30, 2015, the Company accepted notice by Mr. Richard Estalella (“Estalella”) to terminate his employment as the Company’s President. Although Estalella sought to terminate his employment with the Company for “Good Reason,” as defined in Estalella’s employment agreement with the Company (the “Employment Agreement”), the Company advised Estalella that it deemed his resignation to be without Good Reason.
76
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
On February 3, 2016, Estalella filed a complaint in Colorado state court against the Company and Ryan Drexler, Interim Chief Executive Officer, President and Chairman, alleging, among other things, that the Company breached the Employment Agreement, and seeking certain equitable relief and unspecified damages. The Company believes Estalella’s claims are without merit. Estalella remains a member of the Company’s Board of Directors.
As of the date of this report, the Company has evaluated the potential outcome of this lawsuit and recorded the liability appropriately.
Endorser Dispute
The Company is engaged in a dispute with ETW Corp. (“ETW”) concerning the validity of, and payments allegedly owed under, an endorsement agreement with professional golfer Tiger Woods, and amendments thereto (the “Endorsement Agreement”). ETW claims that the Company owes approximately $7.0 million under the Endorsement Agreement. The Company believes that it does not owe any amounts under the Endorsement Agreement, and has demanded the return of payments previously made, as a result of, among other things, certain misrepresentations and omissions made by ETW and its representatives. The parties have agreed to mediate the dispute. The mediation has yet to be scheduled.
Shareholder Derivative Complaint
On October 27, 2015 Brian D. Gartner, derivatively and on behalf of MusclePharm Corporation, filed a verified shareholder derivative complaint in the 8th District Court, State of Nevada, Clark County (No. A-15-726810-B) alleging, among other things, breaches of fiduciary duty as members of the Board of Directors and/or executive officers of the Company against Brad Pyatt, Lawrence S. Meer, Donald W. Prosser, Richard Estalella, Jeremy R. Deluca, Michael J. Doron, Cory Gregory, L. Gary Davis, James J. Greenwell, John H. Bluher and Daniel J. McClory. Plaintiff alleges a series of accounting and disclosure failures resulted in the filing of materially false and misleading filings with the SEC from 2010 through July 2014 resulting in settlement with the SEC requiring payment of $700,000 of civil penalties. Plaintiff seeks various remedies, including interpretation of bylaws provisions, permanent injunctive relief, damages against defendants for breaches of their fiduciary duty, corporate governance changes to ensure the Company maintain proper internal controls and SEC reporting procedures, as well as costs and reasonable attorney’ fees, accountants’ and experts’ fees, costs and expenses. Individual defendants seek removal of the action to federal court and a scheduling stipulation is contemplated.
SEC Settlement
In September 2015, the Company’s proposal regarding final settlement of an SEC ongoing investigation was accepted and all aspects of the investigation related to the Company were terminated. The Company, without admitting or denying the SEC claims, agreed to a payment of $700,000 which was accrued for in 2015 and $400,000 had been paid into escrow. The Company also agreed to appointment of an independent consultant, mutually acceptable to the SEC and the Company, for a 12-month period to monitor the Company’s reporting practices and internal controls. The SEC and Company agreed to the appointment of Chord Advisors, LLC, a California consulting firm, as the monitor.
Insurance Carrier Lawsuit
On February 12, 2015, the Company filed a complaint in the District Court, City and County of Denver, Colorado against Liberty Insurance Underwriters, Inc. (“Liberty”) claiming wrongful and unreasonable denial of coverage for the cost and expenses that the Company has incurred and continued to incur in connection with the SEC investigation and related matters under the Company’s Directors and Officers insurance policies.
77
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Sponsorship and Endorsement Contract Liabilities
The Company has various non-cancelable endorsement and sponsorship agreements with terms expiring through 2022. The total value of future contractual payments as of December 31, 2015 was $34.8 million. The total future contractual payments are as follows (in thousands):
| Year Ending December 31, | ||||||||||||||||||||||||||||
| Outstanding Payments | 2016 | 2017 | 2018 | 2019 | 2020 | Thereafter | Total | |||||||||||||||||||||
| Endorsement |
$ | 2,852 | $ | 2,594 | $ | 2,500 | $ | 4,167 | $ | 5,000 | $ | 6,667 | $ | 23,780 | ||||||||||||||
| Sponsorship |
5,102 | 2,394 | 2,504 | 985 | — | — | 10,985 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Total |
$ | 7,954 | $ | 4,988 | $ | 5,004 | $ | 5,152 | $ | 5,000 | $ | 6,667 | $ | 34,765 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Note 13: Common Stock and Stockholders’ (Deficit) Equity
Common Stock
For the year ended December 31, 2015, the Company issued common stock including restricted stock awards, as follows (in thousands, except share and per share data):
| Transaction Type |
Quantity (Shares) |
Valuation ($) | Range of Value per Share |
|||||||||
| Stock issued to employees, executives and directors, net of cancellations |
214,394 | $ | 15,082 | $ | 2.80–8.60 | |||||||
| Stock issued in conjunction with product line expansion |
150,000 | 1,198 | 7.99 | |||||||||
| Stock issued in conjunction with MusclePharm apparel rights acquisition |
170,000 | 1,394 | 8.20 | |||||||||
| Stock issued in conjunction with attempted financing agreement |
50,000 | 325 | 6.49 | |||||||||
| Stock issued in conjunction with consulting/endorsement agreement |
55,189 | 320 | 5.30–5.85 | |||||||||
| Stock issued in conjunction with individual guaranty of debt |
28,571 | 80 | 2.80 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
668,154 | $ | 18,399 | $ | 2.80–8.60 | |||||||
|
|
|
|
|
|
|
|||||||
For the year ended December 31, 2014, the Company issued common stock including restricted stock awards, as follows (in thousands, except share and per share data):
| Transaction Type |
Quantity (Shares) |
Valuation ($) | Range of Value per Share |
|||||||||
| Conversion of series D preferred stock |
263,000 | $ | 773 | $ | 2.94 | |||||||
| BioZone acquisition (1) |
1,200,000 | 8,833 | 8.20 | |||||||||
| Restricted stock awards issued for endorsement agreements |
476,853 | 5,403 | 11.19–13.41 | |||||||||
| Stock-based compensation |
2,796,743 | 10,931 | 6.55–13.63 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
4,736,596 | $ | 25,940 | $ | 2.94–13.63 | |||||||
|
|
|
|
|
|
|
|||||||
| (1) | Subsequently reduced by 350,000 shares returned to treasury with a value of $4.6 million. |
78
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
The fair value of all stock issuances above is based upon either the quoted closing trading price on the date of issuance or the value of derivative instrument at the date of conversion.
Common stock outstanding as of December 31, 2015 and 2014 has been adjusted to include shares legally outstanding even if subject to future vesting.
Treasury Stock
No treasury stock transaction was incurred for the year ended December 31, 2015. The following table presents the Company’s treasury stock transactions for the years ended December 31, 2014 and 2013:
| Year Ended December 31, | ||||||||||||||||
| 2014 | 2013 | |||||||||||||||
| Number of Shares |
Weighted- Average Purchase Price |
Number of Shares |
Weighted- Average Purchase Price |
|||||||||||||
| Purchase of common stock in open market under the 2013 Stock Repurchase Plan |
105,700 | $ | 13.44 | 120,000 | $ | 7.78 | ||||||||||
| Settlement of common stock held in escrow during BioZone acquisition (1) |
350,000 | 13.20 | — | — | ||||||||||||
| Exercise of repurchase rights for common stock held in escrow during BioZone acquisition (1) |
250,000 | 10.00 | — | — | ||||||||||||
| Others |
— | — | 18,825 | 13.80 | ||||||||||||
|
|
|
|
|
|||||||||||||
| Total |
705,700 | $ | 12.10 | 138,825 | $ | 8.60 | ||||||||||
|
|
|
|
|
|||||||||||||
| (1) | Returned to treasury. |
For the year ended December 31, 2014, the Company repurchased 105,700 shares of its common stock for $1.4 million, or an average of $13.44 per share. This repurchase was completed under a stock repurchase plan approved by the Company’s Board of Directors on December 10, 2013, which allowed the Company to repurchase up to $5.0 million worth of common stock over a one year period. These repurchased shares are accounted for under the cost method and are included as a component of treasury stock in the consolidated balance sheets.
The Company received 350,000 shares released from escrow related to the BioZone asset purchase as described in Note 4. These shares were returned to the Company and are accounted for as treasury stock. In October 2014, the Company additionally exercised its option and acquired 250,000 shares at $10.00 per share related to the BioZone asset purchase. These shares were returned to the Company and are accounted for as treasury stock.
For the year ended December 31, 2013, the Company repurchased a total of 138,825 shares of common stock for $1.2 million, or an average of $8.60 per share. Of this amount, $1.0 million, or $7.47 per share was considered repurchase of securities and $156,000 was recorded as a loss on settlement and is included in gain on settlement of accounts payable in the consolidated statement of operations. Included in the repurchase of securities was 120,000 shares, or $934,000 of common stock repurchased by the Company as part of the stock repurchase plan described above.
79
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Note 14: Preferred Stock
In August 2011, the Company issued an aggregate of 51 shares of Series B preferred stock to two of its officers. The Company accounted for the share issuance at par value as there was no future economic value that could be associated with the issuance. In September 2013, the outstanding 51 shares of Series B preferred stock were returned to the Company and retired. Pursuant to the certificate of designation, these shares were added back to general preferred stock pool upon their surrender and are not available for reissuance as Series B preferred stock without a new designation.
In January 2013, the Board of Directors authorized for distribution up to 1,600,000 shares of Series D convertible preferred stock. In January and February 2013, the Company entered into purchase agreements with certain investors in connection with the offering, pursuant to which the Company sold 1,500,000 shares of Series D convertible preferred stock at $8.00 per share for aggregate gross proceeds of $12.0 million. The Series D convertible preferred stock was convertible into two shares of common stock at any time by the holders. For the year ended December 31, 2013, 1,368,500 shares of Series D convertible preferred stock converted into 2,737,000 shares of common stock. For the year ended December 31, 2014, the remaining 131,500 shares of Series D convertible preferred stock converted into 263,000 shares of common stock. The Series D convertible preferred stock contained an embedded derivative liability related to a conversion feature within the shares, which are discussed further in Note 11.
As of December 31, 2015 and 2014, there were no shares of preferred stock outstanding.
Note 15: Stock-Based Compensation
The Company’s stock-based compensation for the years ended December 31, 2015, 2014 and 2013 consist primarily of restricted stock awards and a de minimis amount related to stock options.
Stock Incentive Plans
Under its 2010 Stock Incentive Plan (the “2010 Plan”), the Company was able to grant incentive stock options, non-qualified stock options, restricted stock awards, restricted stock units and stock appreciation rights to key employees, directors, consultants, advisors and service providers of the Company or its subsidiaries. Shares of common stock purchased upon the exercise of a stock option must be paid for in full at the time of the exercise in cash or such other consideration determined by the Compensation Committee. Payment may include tendering shares of common stock or surrendering of a stock award, or a combination of methods. The 2010 Plan is administered by the Compensation Committee. The 2010 Plan may be amended by the Board of Directors or the Compensation Committee, without the approval of stockholders, but no such amendments may increase the number of shares issuable under the 2010 Plan or adversely affect any outstanding awards without the consent of the holders thereof. The total number of shares that may be issued under the 2010 Plan cannot exceed 5,883, subject to adjustment in the event of certain recapitalizations, reorganizations and similar transactions. The Company no longer grant stock awards under the 2010 Plan.
In 2015, the Board of Directors adopted the MusclePharm Corporation 2015 Incentive Compensation Plan (the “2015 Plan”), which replaced the 2010 Plan. The 2015 Plan provides for the issuance of incentive stock options, non-qualified stock options, restricted stock, stock appreciation rights, restricted stock units, dividend equivalent right, other share-based awards, and stock-based and cash-based awards that qualify as performance-based compensation under Section 162(m) of the Internal Revenue to employees, consultants and directors of the Company or its subsidiaries. The 2015 Plan is administered by the Board of Directors, unless the Board of Directors elects to delegate administration responsibilities to a committee (the “Committee”), and will continue
80
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
in effect until terminated by the Board of Directors. The 2015 Plan may be amended by the Board of Directors, without the approval of stockholders, but no such amendments may increase the number of shares available under the 2015 Plan or materially and adversely affect any outstanding awards without the consent of the holders thereof. The total number of shares that may be issued under the 2015 Plan cannot exceed 2,000,000, subject to adjustment in the event of certain changes in the capitalization of the Company.
The Committee determines the methods by which the exercise price of options is paid, including in cash or check, in shares, through a broker-dealer sale and remittance procedure and a net exercise arrangement. The Committee may allow a participant, provided that the participant is not an executive officer or member of the Board of Directors, to deliver an interest-bearing full recourse promissory note or through a third-party loan guaranteed by the Company in the amount of the exercise price and any associated withholding taxes. The Committee also determines the eligible individuals who will receive grants and the precise terms of the grants including accelerations or waivers of any restrictions, and the conditions under which such accelerated vesting or waivers occur, such as in connection with a participant’s death, subject to certain limitations in the case of performance-based awards that are intended to qualify as qualified performance-based compensation under Section 162(m) of the Internal Revenue Code.
The exercise price of stock options granted under the 2015 Plan may not be less or higher than 100% of the fair market value of a share of our common stock on the date of grant. Vesting is generally determined by the Compensation Committee within limits set forth in the 2015 Plan, except that no portion of an award may fully vest before the first anniversary of the grant date. A number of shares equal to 5% of the total number of shares reserved for issuance pursuant to awards granted under the 2015 Plan are not subject to this minimum vesting requirement. No stock option will be exercisable more than ten years after the date it is granted.
Section 162(m) of the Internal Revenue Code requires, among other things, that the maximum number of shares awarded to an individual during a specified period must be approved by the stockholders in order for the awards granted under the plan to be eligible for treatment as performance-based compensation that will not be subject to the $1 million limitation on tax deductibility for compensation paid to certain specified senior executives. In any calendar year, the maximum number of shares with respect to one or more awards that may be granted to any one participant during the year under the 2015 Plan is 350,000 shares, subject to adjustment in the event of specified capitalization events of our Company, and the maximum amount that may be paid in cash during any calendar year with respect to any award is $1.5 million. The shares subject to cancelled options will continue to count against the maximum number of shares with respect to which the option may be granted to a participant.
Stock Options
In April 2010, the Company issued stock options to purchase 3,260 shares of common stock under the 2010 Plan. These stock options have a contractual term of 5 years, and a grant date fair value of $631,000 which was expensed immediately as the stock options vested upon grant. The Company determined the fair value of the stock options using the Black-Scholes model. As of December 31, 2014, the Company had 472 stock options outstanding that were significantly underwater with an exercise price of $425 per share. These shares were not exercised and expired as of December 31, 2015.
81
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Restricted Stock Awards to Employees and Board Members
The Company’s stock-based compensation for the year ended December 31, 2015 consists primarily of restricted stock awards. The activity of restricted stock awards granted to employees, executives, and board members was as follows:
| Unvested Restricted Stock Awards | ||||||||
| Number of Shares |
Weighted- Average Grant Date Fair Value |
|||||||
| Unvested balance – December 31, 2012 |
129,413 | $ | 3.48 | |||||
| Granted |
1,569,363 | 10.97 | ||||||
| Vested |
(306,637 | ) | 9.95 | |||||
|
|
|
|||||||
| Unvested balance – December 31, 2013 |
1,392,139 | 10.50 | ||||||
| Granted |
1,404,604 | 12.47 | ||||||
| Vested |
(164,756 | ) | 6.33 | |||||
|
|
|
|||||||
| Unvested balance – December 31, 2014 |
2,631,987 | 11.67 | ||||||
|
|
|
|||||||
| Granted |
299,828 | 4.25 | ||||||
| Vested |
(1,805,816 | ) | 10.54 | |||||
| Cancelled |
(100,000 | ) | 4.29 | |||||
|
|
|
|||||||
| Unvested balance – December 31, 2015 |
1,025,999 | 12.34 | ||||||
|
|
|
|||||||
The total fair value of restricted stock awards granted to employees and board members for the years ended December 31, 2015, 2014 and 2013 was $1.3 million, $17.5 million and $17.2 million. As of December 31, 2015, the total unrecognized expense for unvested restricted stock awards, net of expected forfeitures, was $8.5 million, which is expected to be amortized over a weighted-average period of 3.0 years.
Restricted Stock Awards Related to Energy Drink Agreement
In January 2015, the Company entered into an energy drink agreement with Langer Juice and Creative Flavor Concepts to expand into a new product line. In connection with the agreement, the Company issued a total of 150,000 shares of its restricted common stock with trade restrictions for a period of three years. The restricted stock awards issued had a grant date fair value of approximately $1.2 million, which were initially included as a component of prepaid stock compensation and additional paid-in capital in the consolidated balance sheets upon issuance. The prepaid stock compensation was originally amortized over the performance period of ten years. In connection with the restructuring event disclosed further in Note 10, the Company discontinued this product and wrote-off the unamortized prepaid stock compensation of $1.1 million in August 2015.
Agreements with Worldwide Apparel, LLC – Muscle Pharm Apparel Rights
In February 2015, the Company entered into an agreement with Worldwide Apparel, LLC (“Worldwide”) to terminate Worldwide’s right to use MusclePharm’s brand images in apparel effective March 28, 2015. The brand rights were originally licensed in May 2011, and amended in March 2014 prior to the termination. The consideration related to the acquisition of the MusclePharm Apparel from Worldwide consists of cash consideration of $850,000 and 170,000 shares of MusclePharm common stock with an aggregated fair value of $1.4 million. The total cost of the MusclePharm apparel acquisition of $2.2 million is included in the caption brand within intangible assets, net, in the accompanying consolidated balance sheet, and is subject to amortization over a period of seven years.
82
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Restricted Stock Awards Issued Related to Attempted Financing Agreement
In May 2015, the Company negotiated the termination of an attempted financing agreement with a lending institution and issued 50,000 shares of its common stock. The fair value of the common stock was $325,000 based upon the closing price of common stock on the date of issuance, and was recorded in selling, general and administrative expense in the accompanying consolidated statement of operations.
Restricted Stock Awards Issued Related to Consulting/Endorsement Agreement
In May 2015, the Company entered into consulting and endorsement agreements with William Phillips. In connection with the endorsement agreements, the Company agreed to issue a total of 50,000 shares of its restricted common stock. The restricted common stock issued had a grant date fair value of $292,000, which was included as a component of prepaid stock compensation and additional paid-in capital in the consolidated balance sheets upon issuance. The prepaid stock compensation was originally amortized over the performance period of three years. In connection with the restructuring disclosed in Note 10, the Company terminated the consulting and endorsement agreements with William Phillips and wrote-off the unamortized prepaid stock compensation of $268,000.
In connection with the consulting agreement, the Company also agreed to issue restricted shares worth $25,000 (based upon the weighted average stock price during the 15-day-period prior to issuance) within 10 days after each subsequent three-month period term. In July 2015, the Company issued 5,189 shares of its common stock to William Phillips. The fair value of the common stock was $28,000 based upon the closing price of common stock on the date of issuance, and was recorded in advertising and promotion expense in the accompanying consolidated statement of operations. No additional common stock will be issued to William Phillips under this agreement.
Restricted Stock Awards Issued to Ryan Drexler, Interim Chief Executive Officer, President and Chairman, Related to Loan Modification
In October 2015, the Company entered into loan modification agreements with the banking institution under its line of credit and term loan to: (i) change the maturity date of the loans to January 15, 2016, (ii) prohibit the loans to be declared in default prior to December 10, 2015, except for defaults resulting from failure to make timely payments, and (iii) delete certain financial covenants from the line of credit. In consideration for these modifications, Ryan Drexler, Interim Chief Executive Officer, President and Chairman, and a family member, provided their individual guaranty for the remaining balance of the loans ($6.2 million). In consideration for executing his guaranty, the Company issued Ryan Drexler 28,571 shares of common stock with a grant date fair value of $80,000 (based upon the closing price of common stock on the date of issuance).
Restricted Stock Awards to Non-Employees
In July 2014, in connection with an Endorsement Agreement, the Company issued 446,853 shares of its restricted common stock to ETW Corp with an aggregate market value of $5.0 million (see Note 18). In September 2014, the Company entered into a consulting agreement with a third-party service provider and issued 30,000 shares of its restricted common stock with an aggregate market value of $402,000. These restricted stock awards granted to non-employees were initially included as a component of prepaid stock compensation and additional paid-in capital in the consolidated balance sheet upon issuance. The prepaid stock compensation was originally amortized over the performance period. In connection with the restructuring event disclosed further in Note 10, the Company wrote-off the unamortized prepaid stock compensation related to these restricted stock awards to non-employees of $3.8 million in August 2015.
83
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Note 16: Defined Contribution Plan
The Company established a 401(k) Plan (the “401(k) Plan”) for eligible employees of the Company. Generally, all employees of the Company who are at least twenty-one years of age and who have completed six months of service are eligible to participate in the 401(k) Plan. The 401(k) Plan is a defined contribution plan that provides that participants may make voluntary salary deferral contributions, on a pretax basis, of up to $18,000 for the year ended December 31, 2015 (subject to make-up contributions) in the form of voluntary payroll deductions. The Company may make discretionary contributions. For the years ended December 31, 2015, 2014 and 2013, the Company’s matching contribution was $250,000, $299,000 and $61,000, respectively.
Note 17: Net Loss per Share
Basic net loss per share is computed by dividing net loss for the period by the weighted average shares of common stock outstanding during each period. Diluted net loss per share is computed by dividing net loss for the period by the weighted average shares of common stock, common stock equivalents and potentially dilutive securities outstanding during each period. The Company uses the treasury stock method to determine whether there is a dilutive effect of outstanding option and warrant contracts.
The following table sets forth the computation of the Company’s basic and diluted net loss per share for the periods presented (in thousands, except share and per share data):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Net loss |
$ | (51,858 | ) | $ | (13,832 | ) | $ | (17,718 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted-average common shares used in computing net loss per share, basic and diluted |
13,621,255 | 11,038,761 | 7,193,784 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss per share, basic and diluted |
$ | (3.81 | ) | $ | (1.25 | ) | $ | (2.46 | ) | |||
|
|
|
|
|
|
|
|||||||
The following securities were excluded from the computation of diluted net loss per share for the periods presented because including them would have been antidilutive:
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Stock options (exercise price - $425/share) |
— | 472 | 472 | |||||||||
| Warrants (exercise price - $4 - $1,275/share) |
100,000 | 100,089 | 263,089 | |||||||||
| Unvested restricted stock |
1,025,999 | 2,631,987 | 1,392,139 | |||||||||
| Convertible note (exercise price - $2.30/share) |
2,608,695 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total common stock equivalents |
3,734,694 | 2,732,548 | 1,655,700 | |||||||||
|
|
|
|
|
|
|
|||||||
84
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Note 18: Endorsement Agreements
Arnold Schwarzenegger
In July 2013, the Company entered into an Endorsement Licensing and Co-Branding Agreement by and among, the Company, Arnold Schwarzenegger, Marine MP, LLC, and Fitness Publications, Inc. Under the terms of the agreement, Mr. Schwarzenegger was co-developing a special Arnold Schwarzenegger product line being co-marketed under Mr. Schwarzenegger’s name and likeness.
In connection with this agreement, the Company also issued Marine MP, LLC fully vested restricted shares of common stock with an aggregate market value of $8.5 million. As of December 31, 2015 and 2014, the amount of unamortized stock compensation expense related to this agreement was $1.6 million and $4.5 million. The shares are being amortized over the original three-year term of the agreement. The current and non-current portions of this unamortized stock compensation are included as a component of prepaid stock compensation in the consolidated balance sheets.
In March 2016, the Company received a demand notice for the outstanding amounts due, which the Company had expensed and accrued for at December 31, 2015.
Tiger Woods
On July 1, 2014, the Company entered into an Endorsement Agreement with ETW Corp. Under the terms of the agreement, Tiger Woods agreed to endorse certain of the Company’s products and use a golf bag during all professional golf play which prominently displayed the MusclePharm name and logo.
In conjunction with this agreement, on July 3, 2014, the Company issued 446,853 shares of the Company’s restricted common stock to ETW Corp with an aggregate market value of $5.0 million. The shares were amortized over the original four-year term of the agreement. The current and non-current portions of the unamortized stock compensation were initially included as a component of prepaid stock compensation in the consolidated balance sheets. The amount of unamortized stock compensation expense of $3.5 million related to this agreement was written off in connection with the restructuring event disclosed further in Note 10.
The Company is engaged in a dispute with ETW Corp. (“ETW”) concerning the validity of, and payments allegedly owed under, amendments to an endorsement agreement with professional golfer Tiger Woods, (the “Endorsement Agreement”). ETW claims that the Company owes approximately $7.0 million under the Endorsement Agreement. The Company believes that it does not owe any amounts under the Endorsement Agreement and has demanded the return of payments previously made. The parties have agreed to mediate the dispute. The mediation has yet to be scheduled.
Johnny Manziel
On July 15, 2014, the Company entered into an Endorsement Agreement for the services of Johnny Manziel. As part of this agreement, the Company issued a warrant to purchase 100,000 shares of MusclePharm common stock at an exercise price of $11.90 per share. The warrants vest monthly over a period of 24 months beginning August 15, 2014, and have a five-year contractual term. For the years ended December 31, 2015 and 2014, the Company recognized stock-based compensation expense of $65,000 and $130,000, respectively, related to these warrants, which is included as a component of advertising and promotion expense in the consolidated statements of operations. The Company used the Black-Scholes model to determine the estimated fair value of the warrants, with the following assumptions: contractual life of five years, risk free interest rate of 1.7%, dividend yield of 0%, and expected volatility of 55%. In connection with the restructuring disclosed in Note 10, the Company
85
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
notified Johnny Manziel of its intention to terminate the endorsement agreement. As of December 31, 2015, 70,838 warrants were vested.
Note 19: Income Taxes
The components of loss before provision for income taxes for the years ended December 31, 2015 and 2014 are as follows (in thousands):
| Year Ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Domestic |
$ | (52,060 | ) | $ | (13,921 | ) | ||
| Foreign |
307 | 122 | ||||||
|
|
|
|
|
|||||
| Loss before provision for income taxes |
$ | (51,753 | ) | $ | (13,799 | ) | ||
|
|
|
|
|
|||||
Income taxes are provided for the tax effects of transactions reported in the financial statements and consist of taxes currently due. Deferred taxes relate to differences between the basis of assets and liabilities for financial and income tax reporting which will be either taxable or deductible when the assets or liabilities are recovered or settled.
As of December 31, 2015, the Company has a Federal net operating loss carry-forward of $81.4 million available to offset future taxable income. The Company has estimated state loss carry-forwards of $56.2 million. The Company also has federal and California research and development credit carryforwards of $0.5 million and $0.2 million, respectively, as of December 31, 2015. Utilization of net operating losses and R&D credits may be limited due to potential ownership changes under Section 382 of the Internal Revenue Code of 1986, as amended (the “Code”). These net operating loss carry-forwards and federal R&D credits have expiration dates starting in 2025 through 2035. The California R&D credits can be carried forward indefinitely.
Income taxes have not been provided on undistributed earnings of certain foreign subsidiaries in an aggregate amount of $0.9 million as of December 31, 2015 as the Company considers such earnings to be permanently reinvested outside the United States. The additional U.S. income tax that would arise on repatriation of the remaining undistributed earnings could be offset, in part, by foreign tax credits on such repatriation. However, it is impractical to estimate the amount of net income and withholding tax that might be payable.
The valuation allowance as of December 31, 2015 was $30.8 million. The net change in valuation allowance for the year ended December 31, 2015 was an increase of $18.3 million. In assessing the realizability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred income tax assets will not be realized. The ultimate realization of deferred income tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred income tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. Based on consideration of these items, management has determined that enough uncertainty exists relative to the realization of the deferred income tax asset balances to warrant the application of a full valuation allowance as of December 31, 2015.
86
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
The effects of temporary differences that gave rise to significant portions of deferred tax assets as of December 31, 2015 and 2014, are as follows (in thousands):
| As of December 31, | ||||||||
| 2015 | 2014 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 29,796 | $ | 16,224 | ||||
| Other |
5,142 | 771 | ||||||
|
|
|
|
|
|||||
| Gross deferred tax assets |
34,938 | 16,995 | ||||||
| Valuation allowance |
(30,834 | ) | (12,516 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
4,104 | 4,479 | ||||||
| Deferred tax liability |
||||||||
| Stock-based compensation |
(2,370 | ) | (2,688 | ) | ||||
| Intangibles |
(1,734 | ) | (1,791 | ) | ||||
|
|
|
|
|
|||||
| Gross deferred tax liabilities |
(4,104 | ) | (4,479 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
The Company incurred income tax expense of $105,000 and $33,000 for the years ended December 31, 2015 and 2014, respectively. Of the total tax provision, $12,000 and $26,000 is attributed to taxes for foreign operations.
The income tax provision for the years ended December 31, 2015, 2014 and 2013 includes the following (in thousands):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Current income tax expense: |
||||||||||||
| Federal |
$ | — | $ | — | $ | — | ||||||
| State |
93 | 7 | 10 | |||||||||
| Foreign |
12 | 26 | 105 | |||||||||
|
|
|
|
|
|
|
|||||||
| 105 | 33 | 115 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Deferred income tax provision: |
||||||||||||
| Federal |
— | — | — | |||||||||
| State |
— | — | ||||||||||
| Foreign |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| — | — | |||||||||||
| Provision for income taxes, net |
$ | 105 | $ | 33 | $ | 115 | ||||||
|
|
|
|
|
|
|
|||||||
87
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
The income tax provision differs from those computed using the statutory federal tax rate of 34% due to the following (in thousands):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Expected provision at statutory federal rate |
$ | (17,596 | ) | $ | (4,692 | ) | $ | (5,985 | ) | |||
| State tax — net of federal benefit |
74 | 5 | 757 | |||||||||
| Foreign income/losses taxed at different rates |
(43 | ) | (10 | ) | (30 | ) | ||||||
| Bargain purchase gain |
— | (1,790 | ) | — | ||||||||
| Derivative liability |
— | (127 | ) | 1,683 | ||||||||
| Stock-based compensation |
56 | 1,209 | 2,043 | |||||||||
| Other |
(125 | ) | (21 | ) | 438 | |||||||
| Change in valuation allowance |
17,739 | 5,459 | 1,209 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income tax expense |
$ | 105 | $ | 33 | $ | 115 | ||||||
|
|
|
|
|
|
|
|||||||
A reconciliation of the beginning and ending amount of unrecognized tax benefits (“UTB’s”) is as follows (in thousands):
| Gross UTB’s as of December 31, 2014 |
$ | — | ||
| Additions for tax positions taken in a prior year |
(66 | ) | ||
| Additions for tax positions taken in the current year |
(75 | ) | ||
|
|
|
|||
| Gross UTB’s as of December 31, 2015 |
$ | (141 | ) | |
|
|
|
If recognized, none of the Company’s unrecognized tax benefits as of December 31, 2015 would reduce its annual effective tax rate but would result in a corresponding adjustment to its deferred tax valuation allowance. As of December 31, 2015, the Company has not recorded a liability for potential interest or penalties. The Company also does not expect its unrecognized tax benefits to change significantly over the next 12 months.
By statute, all tax years are open to examination by the major taxing jurisdictions to which the Company is subject.
Note 20: Segments
The Company’s chief operating decision maker reviews financial information presented on a consolidated basis for purposes of allocating resources and evaluating financial performance. As such, the Company currently has a single reporting segment and operating unit structure. In addition, substantially all of the Company’s revenue and long-lived assets are attributable to operations in the U.S. for all the periods presented.
Revenue, net by geography is based on the company addresses of the customers. The following table sets forth revenue, net by geographic area (in thousands):
| Year Ended December 31, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Revenue, net |
||||||||||||
| United States |
$ | 120,598 | $ | 110,514 | $ | 76,750 | ||||||
| International |
46,260 | 66,875 | 34,128 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue, net |
$ | 166,858 | $ | 177,389 | $ | 110,878 | ||||||
|
|
|
|
|
|
|
|||||||
88
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Note 21: Related Party Transactions
Interim Chief Executive Officer, President and Chairman Debt Guarantee
In October 2015, the Company entered into loan modification agreements with ANB Bank under the line of credit and term loan to: (i) change the maturity date of the loans to January 15, 2016, (ii) prohibit the loans to be declared in default prior to December 10, 2015, except for defaults resulting from failure to make timely payments, and (iii) delete certain financial covenants from the line of credit. In consideration for these modifications, the Company’s Ryan Drexler, Interim Chief Executive Officer, President and Chairman and a family member, provided their individual guaranty for the remaining balance of the term loan and line credit of $6.2 million. In consideration for executing his guaranty, the Company issued Ryan Drexler 28,571 shares of common stock with a grant date fair value of $80,000 (based upon the closing price of common stock on the date of issuance).
Interim Chief Executive Officer, President and Chairman Convertible Secured Promissory Note Agreement
In December, 2015, the Company entered into a convertible secured promissory note agreement with Ryan Drexler, Interim Chief Executive Officer, President and Chairman under which he lended the Company $6.0 million. Proceeds from the note were used to fund working capital requirements. The convertible note is secured by all assets and properties of the Company and its subsidiaries whether tangible or intangible. The convertible note carries an interest at 8% per annum, or 10% in the event of default. Both the principal and the interest under the convertible note are due in January 2017, unless converted earlier. The holder can convert the outstanding principal and accrued interest into shares of common stock for $2.30 per share at any time. The Company may prepay the convertible note at the aggregate principal amount therein plus accrued interest by giving the holder between 15 and 60 day-notice, depending upon the specific circumstances, provided that the holder may to convert the note during the notice period. The Company recorded the convertible note of $6.0 million as a liability in the balance sheet and also recorded a beneficial conversion feature of $52,000 as a debt discount upon issuance of the convertible note, which is being amortized over the term of the convertible debt using the effective interest method. The beneficial conversion feature was calculated based on the difference between the fair value of common stock and the effective conversion price of the convertible note. As of December 31, 2015, the convertible note had an outstanding principal balance of $6.0 million.
In connection with the Company entering into the convertible promissory note with Mr. Drexler, the Company granted Mr. Drexler the right to designate two directors to the Company’s Board of Directors. The Company agreed to take all actions necessary to permit such designation.
Charitable Youth Sports Program
In March 2014, the Board of Directors of the Company approved and the Company established a charitable youth sports grant program (the “Program”) pursuant to which the Company will donate product, equipment and cash to organizations such as schools, sports teams and training facilities. The Company had tentatively established an annual budget of approximately $250,000 for the Program. The primary intent of the Program was to build MusclePharm brand awareness with youth athletes. The Company’s other business purposes in establishing the Program was to help needy organizations achieve their goals, promote the Company’s brand, help athletes develop stronger and better skills and to build the reputation of the Company as a contributor to the community. A committee formerly consisting of the Company’s former President, former Director of Team Development, and former Chief Operating Officer oversaw the Program. In 2014, the Company made an initial grant in the amount of approximately $250,000 to Arvada West High School and similar charitable contributions to other charitable sports organizations of approximately $30,000. The Company’s former Chief Executive Officer,
89
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Mr. Brad Pyatt, is a graduate of Arvada West High School and serves as a volunteer football coach. The Company did not make a charitable grant to Arvada West High School during 2015. The Company did make charitable grants to other youth sports organizations during 2015 totaling approximately $278,000. We expect this amount to decrease substantially in 2016 and any future grant will be approved by the Chief Executive Officer and Chief Financial Officer.
Sports Tickets
The Company maintains a luxury box at the Sports Authority Field in Denver, Colorado. Employees are able to attend Denver Bronco football games and utilize the luxury box. During 2015, our CEO donated tickets to a game to a youth football team which his family member is a participant. Additionally, other family members also attended the game. The total cost for the event was approximately $15,000.
Key Executive Life Insurance
For the year ended December 31, 2015, the Company purchased split dollar life insurance policies on certain key executives. In September 2015, the Company increased the coverage on one of the key executives. These policies provide a split of 50% of the death benefit proceeds to the Company and 50% to the officer’s designated beneficiaries.
Lease Agreement with Significant Shareholder
In October 2013, the Company entered into an Office Lease Agreement with Frost Real Estate Holdings, LLC, a Florida limited liability company owned by Dr. Phillip Frost, a significant shareholder. Pursuant to the lease, the Company rented 1,437 square feet of office space for an initial term of three years, with an option to renew the lease for an additional three-year term. This facility was closed in September 2015 and included in the Company’s restructuring plan. The remaining lease obligation through April 2017 for $77,000 was included in the restructuring expense. For the years ended December 31, 2015, 2014 and 2013, the Company incurred rent expense of $39,000, $54,000 and $13,000, respectively.
Lease Agreement with Former Employee
The Company leased office and warehouse facility in Hamilton, Ontario, Canada from 2017275 Ontario Inc., which is a company owned by Renzo Passaretti, VP and General Manager of MusclePharm Canada Enterprises Corp, the Company’s wholly-owned Canadian subsidiary. Mr. Passaretti separated from the Company on September 2, 2015. For the years ended December 31, 2015, 2014 and 2013, the Company paid rent of $83,000, $86,000 and $75,000, respectively. The lease was terminated in November 2015.
Business Relationship with Former Employee
Ryan DeLuca, the former Chief Executive Officer of Bodybuilding.com, is the brother of Jeremy DeLuca, MusclePharm’s former EVP, MusclePharm Brand and Global Business Development. The Company maintained a business relationship with Bodybuilding.com prior to hiring Mr. DeLuca. The Company does not offer preferential pricing of our products to Bodybuilding.com based on these relationships. Mr. DeLuca separated from MusclePharm on September 15, 2015. Net revenue from products sales to Bodybuilding.com were $16.9 million, $24.0 million and $29.8 million for the years ended December 31, 2015, 2014 and 2013, respectively. The Company had $1.5 million and $1.9 million in trade receivables with Bodybuilding.com as of December 31, 2015 and 2014, respectively. The Company purchased marketing services from Bodybuilding.com of $0.4 million and $1.4 million for the years ended December 31, 2015 and 2014, respectively.
90
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
Note 22: Subsequent Events
Agreement with Prestige Capital Corporation
On January 11, 2016, the Company entered into a Purchase and Sale Agreement (the “Agreement”) with Prestige Capital Corporation (“Prestige”) pursuant to which the Company agreed to sell and assign and Prestige agreed to buy and accept, certain accounts receivable owing to the Company (“Accounts”). Under the terms of the Agreement, upon the receipt and acceptance of each assignment of Accounts, Prestige will pay the Company eighty percent (80%) of the net face amount of the assigned Accounts, up to a maximum total borrowings of $10 million outstanding at any time. In addition, the Company granted Prestige a continuing security interest in and lien upon all accounts receivable, inventory, fixed assets, general intangibles and other assets. The Agreement has an initial term of six month with options to extend. Prestige may cancel the Agreement with 30 day notice.
On January 13, 2016, the Company sold to Prestige accounts with an aggregate face amount of approximately $7.6 million and Prestige paid to the Company approximately $6.2 million in cash. The proceeds from this factoring were utilized to pay off the existing line of credit and term loan with ANB Bank.
Employment Agreement of the Company’s Executive Chairman
On August 26, 2015, the Company’s Board of Directors appointed Ryan Drexler as the Company’s Executive Chairman. On February 11, 2016, the Company and Mr. Drexler entered into an employment agreement, approved by the Compensation Committee of the Company’s Board of Directors, setting forth the terms of Mr. Drexler’s employment as Executive Chairman of the Company (the “Employment Agreement”).
Pursuant to the terms of the Employment Agreement, Mr. Drexler will initially receive an annual base salary of $550,000, subject to annual adjustment by the Board of Directors. In addition, Mr. Drexler will be paid $250,000 on March 1, 2016 for his services starting on August 26, 2015, which was accrued at December 31, 2015. During his employment, Mr. Drexler will be eligible for certain target incentive bonuses, including an annual bonus of up to 200% of his base salary upon the achievement of certain performance targets, and a transaction bonus equal to 10% of the purchase price upon the occurrence of a qualified sale. Mr. Drexler will receive options to purchase shares of the Company’s common stock valued at $250,000 in addition to equity grants under the Company’s management incentive plan.
The term of the Employment Agreement is for a three year period commencing February 10, 2016, with an automatic renewal for successive one year periods unless terminated by either party with at least 3 months’ advanced written notice. Upon termination of his employment for any reason, Mr. Drexler will be entitled to: (i) base salary; (ii) reasonable expenses paid or incurred by Mr. Drexler in connection with and related to the performance of his duties and responsibilities for the Company; (iii) accrued but unused vacation time; (iv) annual bonuses or transaction bonus; and (v) vested equity awards, all of which were earned through the date of termination. Further, in the event of termination prior to expiration of his employment period, including due to his death or disability, but excluding termination by the Company for cause or by Mr. Drexler without good reason, and provided that he releases the Company and its affiliates from any releasable liability associated with the Employment Agreement within sixty (60) days following the termination and complies with his other obligations under the Employment Agreement, Mr. Drexler will remain eligible to receive the transaction bonus described below if a qualifying sale of the Company occurs before February 10, 2021.
Mr. Drexler’s bonuses and stock-based compensation (collectively, the “Clawback Benefits”) will be subject to certain clawback rights as follows: during the period that Mr. Drexler’s is employed by the Company, upon his termination and for a period of three years thereafter, if there is a restatement of any Company financial results
91
Table of Contents
MusclePharm Corporation
Notes to Consolidated Financial Statements
December 31, 2015, 2014 and 2013
from which any Clawback Benefits will have been determined, Mr. Drexler will repay the Clawback Benefits amounts paid in excess of the amounts that would have been paid based on the restatement of the Company’s financial information.
The Employment Agreement also contains customary confidentiality, non-competition and non-solicitation provisions in favor of the Company.
Additionally, the Employment Agreement provides that Mr. Drexler shall be eligible to receive a transaction bonus for certain qualifying business combinations. Mr. Drexler shall remain eligible to receive the transaction bonus if a qualifying sale of the Company occurs before the February 10, 2021; provided, that, on a termination without cause or a resignation for good reason, Mr. Drexler releases the Company and its affiliates from any releasable liability associated with the Employment Agreement (other than with respect to amounts not yet due) within sixty (60) days following the termination of employment and Mr. Drexler complies with his other obligations under the Employment Agreement.
Chief Executive Officer Resignation
On March 15, 2016, Mr. Brad Pyatt resigned from his position as the Company’s Chief Executive Officer and President, as a result of which his employment agreement was terminated. In connection with Mr. Pyatt’s resignation, Mr. Ryan Drexler, the Executive Chairman of our Board of Directors, was appointed interim Chief Executive Officer, President and Chairman.
92
Table of Contents
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures
We maintain “disclosure controls and procedures,” as such term is defined in Rule 13a-15(e) under the Exchange Act, that are designed to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognizes that disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Additionally, in designing disclosure controls and procedures, our management necessarily was required to apply judgment in evaluating the cost-benefit relationship of possible disclosure controls and procedures. Based on their evaluation at the end of the period covered by this Annual Report on Form 10-K, our chief executive officer and chief financial officer have concluded that our disclosure controls and procedures were effective at the reasonable assurance level as of December 31, 2015.
Management’s Report on Internal Control over Financial Reporting
The management of MusclePharm Corporation is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) and 15d-15(f) under the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements of external purposes in accordance with generally accepted accounting principles. Because of the inherent limitations of internal control over financial reporting, misstatements may not be prevented or detected on a timely basis. Also, projections of any evaluation of effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015 using criteria set forth in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission issued in 2013. Based on this assessment, our management determined that our internal control over financial reporting was effective as of December 31, 2015.
For the attestation report of the Company’s independent registered public accounting firm, please see “Report of Independent Registered Public Accounting Firm” on page 45 hereof.
Changes in Internal Control over Financial Reporting
Except as described herein, there have been no changes in our internal controls over financial reporting (as such term is defined in Rule 13a-15(f) and 15d-15(f) under the Securities Exchange Act) during the quarter ended December 31, 2015, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | Other Information |
Chief Executive Officer Resignation
On March 15, 2016, Mr. Brad Pyatt resigned from his position as the Company’s Chief Executive Officer and President, as a result of which his employment agreement was terminated. In connection with Mr. Pyatt’s resignation, Mr. Ryan Drexler, the Executive Chairman of our Board of Directors, was appointed as our interim Chief Executive Officer and President.
93
Table of Contents
| Item 10. | Directors, Executive Officers and Corporate Governance |
Our Board of Directors has adopted a Code of Conduct applicable to all officers, directors and employees, which is available on the Investor Relations portion of our website at www.ir.musclepharmcorp.com under “Investors—Corporate Governance.” We intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding amendment to, or waiver from, a provision of our Code of Ethics and by posting such information on the website address and location specified above.
The remaining information required by this item will be contained in our definitive Proxy Statement for our 2016 Annual Meeting of Stockholders, which will be filed not later than 120 days after the close of our fiscal year ended December 31, 2015 (the “Definitive Proxy Statement”) and is incorporated herein by reference.
| Item 11. | Executive Compensation |
The information required by this item will be contained in the Definitive Proxy Statement and is incorporated herein by reference.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item will be contained in the Definitive Proxy Statement and is incorporated herein by reference.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item will be contained in the Definitive Proxy Statement and is incorporated herein by reference.
| Item 14. | Principal Accountant Fees and Services |
The information required by this item will be contained in the Definitive Proxy Statement and is incorporated herein by reference.
94
Table of Contents
| Item 15. | Exhibits, Financial Statement Schedules |
| Incorporated by Reference | ||||||||||||||
| Exhibit |
Description |
Form | SEC File No. | Exhibit | Filing Date |
Filed Herewith |
Furnished Herewith | |||||||
| 3.1 | Articles of Incorporation of MusclePharm Corporation (successor to Tone In Twenty). | SB-2 | 333-147111 | 3.1 | November 2, 2007 | |||||||||
| 3.2 | Amendment to the Articles of Incorporation. | SB-2 | 333-147111 | 3.3 | November 2, 2007 | |||||||||
| 3.3 | Amendment to the Articles of Incorporation | 8-K | 000-53166 | 3.3 | February 24, 2010 | |||||||||
| 3.4 | Amendment to the Articles of Incorporation. | 10-Q | 000-53166 | 3.1 | May 23, 2011 | |||||||||
| 3.5 | Amendment to the Articles of Incorporation. | 8-K | 000-53166 | 3.1 | November 23, 2011 | |||||||||
| 3.6 | Amendment to the Articles of Incorporation. | 8-K | 000-53166 | 3.1 | January 27, 2012 | |||||||||
| 3.7 | Amendment to the Articles of Incorporation. | 8-K | 000-53166 | 3.1 | March 30, 2012 | |||||||||
| 3.8 | Certificate of Change. | 8-K | 000-53166 | 3.1 | November 28, 2012 | |||||||||
| 3.9 | Certificate of Amendment to Articles of Incorporation. | 8-K | 000-53166 | 3.2 | November 28, 2012 | |||||||||
| 3.10 | Certificate of Correction. | S-1/A | 333-184625 | 3.15 | December 26, 2012 | |||||||||
| 3.11 | Amended and Restated Bylaws | 8-K | 000-53166 | 3.1 | May 14, 2014 | |||||||||
| 3.12 | Amendment to the Amended and Restated By-Laws. | 10-Q | 000-53166 | 3.1 | May 11, 2015 | |||||||||
| 3.13 | Amendment to the Amended and Restated By-Laws. | 8-K | 000-53166 | 3.1 | September 1, 2015 | |||||||||
| 4.1 | Specimen of certificate for MusclePharm Corporation Common Stock. | S-1/A | 333-184625 | 4.4 | December 28, 2012 | |||||||||
| 4.2 | Form of Promissory Note, dated July 13, 2012, issued by MusclePharm Corporation in favor of TCA Global Credit Master Fund LP. | 8-K | 000-53166 | 4.1 | July 20, 2012 | |||||||||
| 4.3 | Form of Promissory Note. | 8-K | 000-53166 | 4.2 | December 10, 2012 | |||||||||
| 10.1 | Purchasing Agreement with General Nutrition Corporation dated December 16, 2009. | 8-K | 000-53166 | 10.2 | February 24, 2010 | |||||||||
95
Table of Contents
| Incorporated by Reference | ||||||||||||||
| Exhibit |
Description |
Form | SEC File No. | Exhibit | Filing Date |
Filed Herewith |
Furnished Herewith | |||||||
| 10.2 | Form of Registration Rights Agreement, dated July 13, 2012, between MusclePharm Corporation and TCA Global Credit Master Fund LP. | 8-K | 000-53166 | 10.1 | July 20, 2012 | |||||||||
| 10.3 | Form of Indemnification Agreement. | 8-K | 000-53166 | 10.1 | August 27, 2012 | |||||||||
| 10.4 | Endorsement Licensing and Co-Branding Agreement, dated July 26, 2013, by and among MusclePharm Corporation, Marine MP, LLC, and Fitness Publications, Inc. (1) |
10-K | 000-531666 | 10.37 | March 31, 2014 | |||||||||
| 10.5 | Separation and Release of Claims Agreement, dated April 8, 2014, between MusclePharm Corporation and L. Gary Davis. |
8-K | 000-53166 | 10.1 | April 14, 2014 | |||||||||
| 10.6 | Employment Agreement, dated April 29, 2015, between MusclePharm Corporation and John Price. |
8-K | 000-53166 | 10.1 | May 1, 2015 | |||||||||
| 10.7 | First Amendment to Manufacturing Agreement, dated March 2, 2015, between MusclePharm Corporation and Capstone Nutrition. |
10-Q | 000-53166 | 10.1 | May 11, 2015 | |||||||||
| 10.8 | Referral Agreement, dated March 2, 2015, between MusclePharm Corporation and Capstone Nutrition. |
10-Q | 000-53166 | 10.2 | May 11, 2015 | |||||||||
| 10.9 | Class B Stock Warrant Agreement, dated March 2, 2015, between MusclePharm Corporation and Capstone Nutrition. | 10-Q | 000-53166 | 10.3 | May 11, 2015 | |||||||||
| 10.10 | Option Agreement, dated March 2, 2015, between MusclePharm Corporation and Capstone Nutrition. | 10-Q | 000-53166 | 10.4 | May 11, 2015 | |||||||||
96
Table of Contents
| Incorporated by Reference | ||||||||||||||
| Exhibit |
Description |
Form | SEC File No. | Exhibit | Filing Date |
Filed Herewith |
Furnished Herewith | |||||||
| 10.11 | Confidentiality and Non-Disclosure Agreement, dated June 23, 2015, between MusclePharm Corporation and Consac, LLC, an affiliate of Ryan Drexler. | 10-Q | 000-53166 | 10.6 | August 10, 2015 | |||||||||
| 10.12 | Option Agreement, dated March 2, 2015, between MusclePharm Corporation and Capstone Nutrition. | 10-Q/A | 000-53166 | 10.4 | December 4, 2015 | |||||||||
| 10.13 | Executive Employment Agreement, dated February 11, 2016, between MusclePharm Corporation and Ryan Drexler. | 8-K | 000-53166 | 10.1 | February 16, 2016 | |||||||||
| 10.14 | Term Loan Modification Agreement, dated October 9, 2015, between MusclePharm Corporation and ANB Bank | 10-K | 000-53166 | 10.14 | March 17, 2016 | X | ||||||||
| 10.15 | Revolver Loan Modification Agreement, dated October 9, 2015, by MusclePharm Corporation and ANB Bank | 10-K | 000-53166 | 10.15 | March 17, 2016 | X | ||||||||
| 10.16 | Personal Guarantee, dated October 9, 2015, by Ryan Drexler in favor of ANB Bank | 10-K | 000-53166 | 10.16 | March 17, 2016 | X | ||||||||
| 10.17 | Personal Guarantee, dated October 9, 2015, by Jodi Drexler-Billet, the sibling of Ryan Drexler in favor of ANB Bank | 10-K | 000-53166 | 10.17 | March 17, 2016 | X | ||||||||
| 14.1 | Code of Ethics. | 8-K | 000-53166 | 14 | April 23, 2012 | |||||||||
| 14.2 | Corporate Governance Guidelines, adopted March 8, 2015. | 10-Q | 000-53166 | 99.1 | May 11, 2015 | |||||||||
| 21.1 | Subsidiaries of the Registrant. | X | ||||||||||||
| 31.1 | Certification of the Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | X | ||||||||||||
| 31.2 | Certification of the Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | X | ||||||||||||
97
Table of Contents
| Incorporated by Reference | ||||||||||||||
| Exhibit |
Description |
Form | SEC File No. | Exhibit | Filing |
Filed Herewith |
Furnished Herewith | |||||||
| 32.1 | Certification of the Chief Executive Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X | ||||||||||||
| 32.2 | Certification of the Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | X | ||||||||||||
| 101.INS | INS XBRL Instance Document. | |||||||||||||
| 101.SCH | SCH XBRL Schema Document. | |||||||||||||
| 101.CAL | CAL XBRL Calculation Linkbase Document. | |||||||||||||
| 101.DEF | DEF XBRL Definition Linkbase Document. | |||||||||||||
| 101.LAB | LAB XBRL Label Linkbase Document. | |||||||||||||
| 101.PRE | PRE XBRL Presentation Linkbase Document. | |||||||||||||
| (1) | An application for confidential treatment was submitted to the Securities and Exchange Commission in October 2013 with regards to the Endorsement Licensing and Co-Branding Agreement entered into among the Company, Marine MP, LLC and Fitness Publications, Inc. The attached Form represents such confidential treatment. |
98
Table of Contents
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| MUSCLEPHARM CORPORATION (the “Registrant”) | ||||||
| Dated: March 17, 2016 | By: | /s/ Ryan Drexler | ||||
| Interim Chief Executive Officer and President | ||||||
| (Interim Principal Executive Officer) | ||||||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Ryan Drexler |
Interim Chief Executive Officer, President and Chairman |
March 17, 2016 | ||
| Ryan Drexler |
(Interim Principal Executive Officer) | |||
| /s/ John Price |
Principal Financial Officer | March 17, 2016 | ||
| John Price |
(Principal Accounting Officer) | |||
| /s/ Michael J. Doron |
Director | March 17, 2016 | ||
| Michael J. Doron |
||||
| /s/ William Bush |
Director | March 17, 2016 | ||
| William Bush |
||||
| /s/ Stacey Jenkins |
Director | March 17, 2016 | ||
| Stacey Jenkins |
||||
| /s/ Noel Thompson |
Director | March 17, 2016 | ||
| Noel Thompson |
||||
| /s/ Richard F. Estalella |
Director | March 17, 2016 | ||
| Richard F. Estalella |
||||
99
