Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - MADRIGAL PHARMACEUTICALS, INC. | a2227746zex-23_1.htm |
| EX-31.1 - EX-31.1 - MADRIGAL PHARMACEUTICALS, INC. | a2227746zex-31_1.htm |
| EX-31.2 - EX-31.2 - MADRIGAL PHARMACEUTICALS, INC. | a2227746zex-31_2.htm |
| EX-32.1 - EX-32.1 - MADRIGAL PHARMACEUTICALS, INC. | a2227746zex-32_1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | ||
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2015 |
||
OR |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from to |
||
Commission file number: 001-33277
SYNTA PHARMACEUTICALS CORP.
(Exact name of registrant as specified in its charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
04-3508648 (I.R.S. Employer Identification No.) |
|
45 Hartwell Avenue Lexington, Massachusetts (Address of principal executive offices) |
02421 (Zip Code) |
Registrant's telephone number, including area code (781) 274-8200
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of each class | Name of each exchange on which registered | |
|---|---|---|
| Common Stock, $0.0001 Par Value Per Share | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate), computed by reference to the price at which the common stock was last sold on June 30, 2015, the last business day of the registrant's most recently completed second fiscal quarter, was $205,260,638.
As of March 10, 2016 the registrant had 137,806,441 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Annual Report on Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the registrant's Proxy Statement for the 2016 Annual Meeting of Stockholders.
Overview
Synta Pharmaceuticals Corp. is a company that has been historically focused on research, development and commercialization of novel oncology medicines that have the potential to change the lives of cancer patients. In October 2015, we announced the decision to terminate for futility the Phase 3 GALAXY-2 trial of our novel heat shock protein 90 (Hsp90) inhibitor, ganetespib, and docetaxel in the second-line treatment of patients with advanced non-small cell lung adenocarcinoma. Based on the review of a pre-planned interim analysis, the study's Independent Data Monitoring Committee (IDMC) concluded that the addition of ganetespib to docetaxel was unlikely to demonstrate a statistically significant improvement in overall survival, the primary endpoint of the study, compared to docetaxel alone.
We have also been evaluating several candidates from our proprietary Hsp90 inhibitor Drug Conjugate, or HDC, program, which leverages the preferential accumulation of Hsp90 inhibitors in tumors to selectively deliver a wide array of anti-cancer payloads. We are currently conducting preclinical studies for our first clinical candidate from the HDC program, STA-12-8666, in anticipation of potentially submitting an investigational new drug application, or IND, for STA-12-8666. While we have determined not to pursue an IND submission for STA-12-8666 in the immediate future, we may determine to do so at a later date.
Following termination of the GALAXY-2 trial in October 2015, we initiated a comprehensive review of our strategy. In November 2015, we committed to a restructuring that consisted primarily of a workforce reduction to better align our workforce to our revised operating plans, which included support of key ongoing ganetespib investigator-sponsored studies and continued effort on the development of candidates from the HDC program, in particular STA-12-8666. As announced in March 2016, in order to conserve cash while we continue to evaluate strategic alternatives to maximize value for stockholders, we committed to a further restructuring in February 2016 that consisted primarily of a workforce reduction of 23 positions, including 19 research and development positions, to a total of 10 remaining positions. In connection with this restructuring, we discontinued a substantial portion of our research and development activities.
We continue to conduct limited activities with respect to ganetespib and the drug candidates from the HDC program, including STA-12-8666. With the exception of ongoing clinical trials in ovarian cancer and sarcoma, we have undertaken efforts to wind down existing ganetespib investigator-sponsored clinical trials. We no longer anticipate expending material resources on any of our current drug candidates.
As previously announced in our Current Report on Form 8-K filed on March 1, 2016, reporting the restructuring of our workforce, we have been considering potential strategic alternatives to enhance stockholder value. Such strategic alternatives include, but are not limited to, a sale of the company, a business combination or collaboration, joint development and partnership opportunities, a distribution of all or a significant amount of cash to stockholders, and liquidation of the company. We do not know if we will be successful in pursuing any strategic alternative or that any transaction will occur; however, we are committed to pursuing a strategic direction that our Board of Directors believes is in the best interests of our stockholders.
We currently do not have any drugs that are commercially available and none of our drug candidates have obtained the approval of the U.S. Food and Drug Administration, or FDA, or any similar foreign regulatory authority.
1
Our Oncology Drug Candidates
We have a clinical-stage drug candidate in oncology (ganetespib) and a novel, proprietary small molecule cancer drug development program (the HDC program).
Ganetespib (Hsp90 Inhibitor)
Summary
Ganetespib is a novel, potent, small molecule inhibitor of Hsp90, a molecular chaperone which is required for the proper folding and activation of many cancer-promoting proteins. Inhibition of Hsp90 by ganetespib leads to the simultaneous degradation of many of these client proteins and the subsequent death or cell cycle arrest of cancer cells dependent on those proteins. A number of Hsp90 client proteins are also involved in the resistance of cancer cells to other anti-cancer treatments, such as chemotherapy. The ability to reduce cancer-cell drug resistance suggests that the combination of ganetespib with chemotherapies or other anti-cancer agents may provide greater benefit than those agents administered alone. In preclinical studies, ganetespib has shown potent anti-cancer activity against a broad range of solid and hematologic cancers, both as a monotherapy and in combination with a variety of anti-cancer treatment approaches including chemotherapy, radiation, targeted therapy and immunotherapy.
Ganetespib Mechanism of Action
Hsp90 is required for the structural and functional maturation of numerous client proteins, many of which play critical roles in cell growth, differentiation and survival. Preclinical results have shown that ganetespib is a selective inhibitor of Hsp90. Relative to their normal counterparts, cancer cells are more reliant on the active Hsp90 complex. Recent published work has shown that cancer cells overexpress a modified form of Hsp90 that preferentially binds Hsp90 inhibitors. This preferential binding provides a possible explanation for the observed anticancer activity and lack of severe toxicity of Hsp90 inhibitors.
Ongoing Ganetespib Clinical Trials
We plan to continue to support the clinical trials in ovarian cancer and sarcoma described below by providing ganetespib drug supply and required safety and regulatory oversight until each of these respective studies conclude. We are also currently conducting limited preclinical activities with ganetespib.
GANNET53 Trial—Ganetespib in ovarian cancer
GANNET53, a Seventh Framework Programme (FP7) research project funded by the European Commission, is a pan-European randomized trial designed to evaluate the combination of ganetespib and paclitaxel vs. paclitaxel alone in over 200 patients with metastatic, predominantly p53 mutant, platinum-resistant ovarian cancer. Preclinical models have shown that mutant p53 is critical to the growth and proliferation of these cancers. Many mutations render p53 unable to fold appropriately, leaving the protein highly dependent on Hsp90 for stability. Inhibition of Hsp90 destroys the complex between Hsp90 and mutant p53, leading to the degradation of the protein and cancer cell death. We believe this hypothesized mechanism is further supported by results detailed in a July 2015 Nature publication, Improving survival by exploiting tumor dependence on stabilized mutant p53 for treatment, by E.M. Alexandrova, et al. Mice harboring mutant p53 treated with ganetespib had prolonged survival as compared to treated p53 null mice, and this activity is correlated with degradation of mutant p53 and tumor apoptosis. In the aggregate, we believe these data suggest the potential of mutated p53 to serve as a predictive biomarker for Hsp90 inhibitors such as ganetespib.
2
Hsp90 inhibition has also been shown to sensitize mutant p53 cancer cells to treatment with chemotherapies, as has been seen in preclinical studies evaluating ganetespib in other tumor types, supporting the planned trial design evaluating the combination of ganetespib and paclitaxel vs. paclitaxel alone.
Enrollment of the safety lead-in Phase 1 portion of GANNET53 in centers in Austria, Belgium, France, and Germany began in July 2014 and is now complete. Initial results from the Phase 1 portion were presented in June 2015 at the American Society of Clinical Oncology (ASCO) Annual Meeting, and these results demonstrated the feasibility and tolerability of combining ganetespib and paclitaxel in this treatment setting. In June 2015, we announced that the first patient was enrolled into the randomized Phase 2 portion of the trial.
We expect that enrollment in the Phase 2 portion of this trial will continue and be completed in 2017; however, as GANNET53 is an investigator-sponsored trial, we do not ultimately control the enrollment timeline for the study.
SARC 023—Ganetespib in Sarcoma
SARC 023, a clinical trial sponsored by the Sarcoma Alliance for Research through Collaboration (SARC), is an open label Phase 1/2 clinical trial of ganetespib in combination with the mTOR inhibitor sirolimus in patients with refractory sarcoma, including malignant peripheral nerve sheath tumors (MPNSTs). The Pediatric Subcommittee of the Oncologic Drugs Advisory Committee (ODAC) reviewed the design of SARC 023, as well as pre-clinical data demonstrating the scientific rationale for studying this combination in a clinical trial. The Phase 1 portion of the clinical trial, which is currently ongoing, is designed to assess the safety, tolerability, and maximum tolerated/recommended dose of the combination.
We expect completion of enrollment in the Phase 1 portion of this clinical trial to occur in 2017; however, as SARC 023 is an investigator-sponsored trial, we do not ultimately control the enrollment timeline for the study.
Other Ganetespib Clinical Trials
In light of the termination of the GALAXY-2 trial in October 2015, we have elected to no longer support investigator-sponsored clinical trials, other than GANNET53 and SARC 023, with the exception of ensuring drug supply of ganetespib to currently enrolled patients deemed to be receiving medical benefit and providing required safety and regulatory monitoring. Our expectation is that no additional patients will be enrolled on ganetespib containing treatment arms of clinical studies other than the ovarian cancer and sarcoma trials described above. Our intent is to wind down the ganetespib containing arms in all other remaining investigator-sponsored trials by mid-2016.
Included in these studies are the AML-LI (Less Intensive)-1 and AML-18 trials evaluating ganetespib in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), and the I-SPY 2 TRIAL (Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging And moL ecular Analysis 2) evaluating ganetespib in women with newly diagnosed, locally advanced breast cancer.
HDC Program
In September 2013, we announced the launch of a novel, proprietary small molecule cancer drug development program: the HDC program.
The HDC program is based on the observation that small molecule inhibitors of Hsp90 are retained in tumors for as much as 20 times longer than in blood or normal tissue. Preclinical experiments have shown that following intravenous administration in animals, ganetespib can persist in
3
tumor cells for over a week, while it is cleared from blood and normal tissues in a matter of hours. Similar results demonstrating this characteristic have been published by others using first-generation Hsp90 inhibitors such as 17-AAG and its derivatives, as well as other classes of Hsp90 inhibitors.
HDCs are drug candidates consisting of an Hsp90 inhibitor (targeting moiety) joined to an anti-cancer agent (payload) via a cleavable chemical linker optimized for controlled release of payload drug inside cancer cells. HDCs are small molecules that do not rely on cell surface antigens for targeting and internalization for cellular uptake. Upon cell entry, typically via small molecule uptake (passive diffusion and possibly active transport), HDCs can bind intracellular Hsp90 that is present in significant amounts in a wide range of cancers.
Upon systemic administration HDCs have the potential to achieve significantly higher concentrations of active anticancer drugs (payloads) in tumors than the concentrations achieved when such anticancer drugs are given in their original, unconjugated form. It is important to note that such high concentrations are sustained over prolonged periods of time, thus significantly increasing the exposure of tumors to the anticancer drug relative to the exposure that can be achieved when such anticancer drugs are given in their original, unconjugated form.
In October 2013, we announced the publication of the first key patent application covering our proprietary HDC technology, which includes composition of matter claims covering HDC compounds, methods for identifying therapeutically effective compounds, and methods of use against a wide range of diseases and conditions. Any resulting patent, if issued, would expire no earlier than 2034.
Our lead drug candidate from our HDC program is STA-12-8666, a conjugate of an Hsp90 inhibitor bound to SN-38, the highly potent active metabolite of the widely used chemotherapy irinotecan. We are currently conducting preclinical studies for STA-12-8666 in anticipation of potentially submitting an IND for STA-12-8666. As part of our strategic direction and in light of recent corporate developments, however, we have determined not to pursue an IND submission for STA-12-8666 at this time. We may, depending on a variety of corporate factors, determine to revisit our strategic direction and make such a submission in the future.
Other Programs
Elesclomol (Mitochondria-Targeting Agent)
In January 2016, we entered into an asset purchase agreement with a third party to further develop our drug candidate, elesclomol. We will no longer be performing research activities on this drug candidate and, as part of the arrangement, we will receive a minority interest and Board representation in the third party, payments based on achievement of certain development milestones and product royalties upon commercialization.
CRACM Ion Channel Inhibitors
In May 2014, we entered into a license arrangement for our CRACM program, including two lead candidates and the associated intellectual property portfolio, with PRCL Research Inc. (PRCL), a company funded by TVM Life Science Venture VII and the Fonds de Solidarité des Travailleurs du Québec, based in Montreal, Canada. PRCL's plans were to develop one of the two lead candidates licensed from us to proof-of-concept. We have recently been informed that PRCL has selected one of these candidates to move forward into IND enabling studies.
We hold a minority interest in PRCL and a seat on PRCL's Board of Directors. We are not required to provide any research funding or capital contributions to PRCL, and we are not required to perform any research activities related to these candidates. We are reimbursed by PRCL for intellectual property management costs in connection with the contributed intellectual property. If and when proof-of-concept is reached with either drug candidate, Eli Lilly and Company, which is an investor in TVM, will help manage the development program through one of its divisions and will have an option to acquire PRCL or its assets at the then fair value.
4
Manufacturing and Supply
We have historically relied on contract manufacturers to produce drug substances and drug products required for our clinical trials under current good manufacturing practices, or cGMP, with oversight by internal managers. We continue to provide limited ganetespib drug supply for the clinical trials in ovarian cancer and sarcoma described above. See "—Ongoing Ganetespib Clinical Trials."
Competition
The development and commercialization of new drugs is highly competitive. We will face competition with respect to all drug candidates we may develop or commercialize in the future from pharmaceutical and biotechnology companies worldwide. The key competitive factors affecting the success of any approved product will be its efficacy, safety profile, price, method of administration and level of promotional activity. The efficacy and safety profile of our drug candidates relative to competitors will depend upon the results of our clinical trials and experience with the approved product in the commercial marketplace. For risks associated with competition, see "Risks Related to Our Industry—Our market is subject to intense competition. If we are unable to compete effectively, our drug candidates may be rendered noncompetitive or obsolete" under "Risk Factors" below in Part I, Item 1A of this Annual Report on Form 10-K.
Patents and Proprietary Rights
Our success depends in part on our ability to obtain and maintain proprietary protection for our drug candidates, technology, and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions, and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
As of February 29, 2016, our complete patent portfolio had a total of 631 patents and patent applications filed worldwide, including specific patent filings with claims to the composition-of-matter of, and methods of use for, ganetespib and STA-12-8666. We own a total of 73 issued U.S. patents and 369 foreign patents.
Our Hsp90 inhibitor patent portfolio includes 544 domestic and international patents and patent applications. This portfolio covers ganetespib and structurally related analogs, pharmaceutical compositions comprising these compounds, and methods for treating different cancer types. Any U.S. or foreign patent that issues covering ganetespib will expire no earlier than 2025. The U.S. composition of matter patent claiming ganetespib will expire in 2027. We have also filed numerous U.S. and foreign patent applications covering our proprietary HDC program, including composition of matter claims for hundreds of compounds synthesized by us to date, methods for identifying therapeutically effective compounds, and methods of use against a wide range of diseases and conditions. Any resulting patent from our HDC program portfolio will expire no earlier than 2034.
Government Regulation
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, packaging, promotion, storage, advertising, distribution, marketing and export and import of products such as those we are developing. Our drug candidates must be approved by the FDA through the new drug application, or NDA, process before they may be legally marketed in the United States.
5
United States Government Regulation
NDA Approval Processes
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, or the FDCA, and implementing regulations. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include:
- •
- refusal to approve pending applications;
- •
- withdrawal of an approval;
- •
- imposition of a clinical hold;
- •
- warning letters;
- •
- product seizures;
- •
- total or partial suspension of production or distribution; or
- •
- injunctions, fines, civil penalties or criminal prosecution.
Any agency regulatory or judicial enforcement action could have a material adverse effect on us. The process of obtaining regulatory approvals and the subsequent substantial compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources.
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
- •
- completion of preclinical laboratory tests, formulation studies, animal studies conducted according to Good Laboratory Practices, or
GLPs, or other applicable regulations;
- •
- submission of an IND, which must become effective before human clinical trials may begin;
- •
- performance of adequate and well-controlled human clinical trials according to Good Clinical Practices, or GCPs, to establish the
safety and efficacy of the proposed drug for its intended use;
- •
- submission to the FDA of an NDA;
- •
- satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product candidate is produced to
assess compliance with current Good Manufacturing Practices, or cGMPs, and to assure that the facilities, methods and controls are adequate to preserve the drugs identity, strength, quality and
purity; and
- •
- FDA review and approval of the NDA.
Once a pharmaceutical candidate is identified for development, it enters the preclinical or nonclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. Some nonclinical testing may continue even after the IND is submitted. In addition to including the results of the preclinical studies, the IND will also include a protocol detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated if the first phase lends itself to an efficacy determination. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, specifically places the IND on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin. A clinical hold may occur at any
6
time during the life of an IND, and may affect one or more specific studies or all studies conducted under the IND.
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with GCPs. They must be conducted under protocols detailing the objectives of the trial, dosing procedures, research subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND, and progress reports detailing the status of the clinical trials must be submitted to the FDA annually. Sponsors also must timely report to FDA serious and unexpected adverse reactions, any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigation brochure, or any findings from other studies or animal or in vitro testing that suggest a significant risk in humans exposed to the drug. An institutional review board, or IRB, at each institution participating in the clinical trial must review and approve the protocol before a clinical trial commences at that institution and must also approve the information regarding the trial and the consent form that must be provided to each research subject or the subject's legal representative, monitor the study until completed and otherwise comply with IRB regulations.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
- •
- Phase 1. The drug is initially introduced into
healthy human subjects or patients with the disease and tested for safety, dosage tolerance, pharmacokinetics, pharmacodynamics, absorption, distribution metabolism, and elimination. In the case of
some products for severe or life-threatening diseases, especially when the product may be inherently too toxic to ethically administer to healthy volunteers, the initial human testing is often
conducted in patients.
- •
- Phase 2. Clinical trials are initiated in a
limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance
and optimal dosage.
- •
- Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide an adequate basis for product labeling.
Phase 1, Phase 2, and Phase 3 testing may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend a clinical trial at any time for a variety of reasons, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. In addition, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB's requirements, in accordance with the clinical protocol, or if the drug has been associated with unexpected serious harm to patients.
During the development of a new drug, sponsors are given an opportunity to meet with the FDA at certain points. These points are: prior to submission of an IND, at the end of Phase 1 or Phase 2, and before an NDA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and FDA to reach agreement on the next phase of development. Sponsors typically use the end of Phase 2 meeting to discuss their Phase 2 clinical results and present their plans for the pivotal Phase 3 clinical trial that they believe will support approval of the new drug. If a Phase 3 clinical trial is the subject of discussion at an end of Phase 2 meeting with the FDA, a sponsor may be able to request a Special Protocol Assessment, the purpose of which is to reach agreement with the FDA on the design of the Phase 3 clinical trial protocol design and analysis that will form the primary basis of an efficacy claim. If such an agreement is reached, it will be documented and made part of the administrative record, and it will be binding on the FDA unless
7
public health concerns unrecognized at the time of protocol assessment are evident, and may not be changed except under a few specific circumstances.
According to published guidance on the SPA process, a sponsor that meets the prerequisites may make a specific request for a SPA and provide information regarding the design and size of the proposed clinical trial. The FDA is supposed to evaluate the protocol within 45 days of the request to assess whether the proposed trial is adequate, and that evaluation may result in discussions and a request for additional information. A SPA request must be made before the proposed trial begins, and all open issues must be resolved before the trial begins.
On occasion, the FDA may suggest or the sponsor of a clinical trial may decide to use an independent data monitoring committee, or DMC, to provide advice regarding the continuing safety of trial subjects and the continuing validity and scientific merit of a trial. In 2006, the FDA published a final Guidance for Clinical Trial Sponsors on the Establishment and Operations of Clinical Trial Data Monitoring Committees in which it describes the types of situations in which the use of a DMC is appropriate and suggests how a DMC should be established and operated. DMCs evaluate data that may not be available to the sponsor during the course of the study to perform interim monitoring of clinical trials for safety and/or effectiveness and consider the impact of external information on the trial. They often make recommendations to the sponsor regarding the future conduct of the trial.
Concurrent with clinical trials, companies usually complete additional animal safety studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and the manufacturer must develop methods for testing the quality, purity and potency of the final drugs. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf-life.
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, results of chemical studies and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA is subject to the payment of user fees, but a waiver of such fees may be obtained under specified circumstances. The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. It may request additional information rather than accept a NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
Once the NDA is accepted for filing, the FDA begins an in-depth review. NDAs receive either standard or priority review. A drug representing a significant improvement in treatment, prevention or diagnosis of disease may receive priority review. The FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product's identity, strength, quality and purity. The FDA may refer the NDA to an advisory committee for review and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured and tested.
The FDA may require, as a condition of approval, restricted distribution and use, enhanced labeling, special packaging or labeling, expedited reporting of certain adverse events, pre-approval of
8
promotional materials, restrictions on direct-to-consumer advertising or commitments to conduct additional research post-approval. The FDA will issue a complete response letter if the agency decides not to approve the NDA in its present form. The complete response letter usually describes all of the specific deficiencies in the NDA identified by the FDA. If a complete response letter is issued, the applicant may either resubmit the NDA, addressing all of the deficiencies identified in the letter, or withdraw the application.
Satisfaction of FDA requirements or similar requirements of foreign regulatory authorities can take a considerable amount of time and the actual time required may vary substantially, based upon, among other things, the indication and the type, complexity and novelty of the product. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly requirements upon us. Success in early stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. Even if a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial application of the product. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Delays in obtaining, or failures to obtain, regulatory approvals for any drug candidate could substantially harm our business and cause our stock price to drop significantly. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, a company may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for medicines produced by biotechnology or those medicines intended to treat AIDS, cancer, neurodegenerative disorders, or diabetes and optional for those medicines which are highly innovative, provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for approval by one or more "concerned" member states based on an assessment of an application performed by one member state, known as the "reference" member state. Under the decentralized approval procedure, an applicant submits an application, or dossier, and related materials to the reference member state and concerned member states. The reference member state prepares a draft assessment and drafts of the related materials within 120 days after receipt of a valid application. Within 90 days of receiving the reference member state's assessment report, each concerned member state must decide whether to approve the assessment report and related materials. If a member state does not recognize the marketing authorization, the disputed points are eventually referred to the European Commission, whose decision is binding on all member states.
As in the United States, we may apply for designation of a product as an orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including up to
9
10 years of market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product.
Employees
As of the date of this filing, we have 10 full-time employees, principally in general and administrative functions. Our employees are not represented by any collective bargaining agreement.
Company History and Available Information
Our principal executive offices are located at 45 Hartwell Avenue, Lexington, Massachusetts 02421, and our telephone number is (781) 274-8200. Our website address is www.syntapharma.com . The information contained on our website is not incorporated by reference into, and does not form any part of, this Annual Report on Form 10-K. We have included our website address as a factual reference and do not intend it to be an active link to our website. Our trademarks include Synta Pharmaceuticals, our corporate logo and the GALAXY trial. Other service marks, trademarks and trade names appearing in this Annual Report on Form 10-K are the property of their respective owners. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to those reports, are available free of charge through the Investors section of our website as soon as reasonably practicable after such materials have been electronically filed with, or furnished to, the Securities and Exchange Commission, or the SEC. You may also read and copy any document we file at the SEC's Public Reference Room at 100 F Street N.E., Washington, D.C. 20549. Please call 1-800-SEC-0330 for further information on the operation of the Public Reference Room.
If any of the following risks occurs, our business, business prospects, financial condition, results of operations, or cash flows could be materially harmed.
Risks Relating to Our Evaluation of Strategic Alternatives and Our Business
Our exploration of strategic alternatives may not be successful.
We have implemented operating cost reductions and organizational restructurings, including a recent reduction in our workforce, to reduce overall cash burn. As previously announced in our Current Report on Form 8-K filed on March 1, 2016, reporting the restructuring of our workforce, we have been considering potential strategic alternatives to enhance stockholder value. Such strategic alternatives include, but are not limited to, a sale of the company, a business combination or collaboration, joint development and partnership opportunities, a distribution of all or a significant amount of cash to stockholders, and liquidation of the company. We do not know if we will be successful in pursuing any strategic alternative or that any transaction will occur; however, we are committed to pursuing a strategic direction that our Board of Directors believes is in the best interests of our stockholders.
We have incurred significant losses since our inception, and we expect to incur losses for the foreseeable future and may never reach profitability.
Since inception we have incurred significant operating losses and, as of December 31, 2015, we had an accumulated deficit of $706.2 million. We expect to continue to incur operating expenses and anticipate that we will continue to have losses in the foreseeable future as we evaluate and potentially act on strategic alternatives. Moreover, even if our Board determines to pursue a specific strategic alternative, we expect that significant expenses will be involved in implementing any such strategic path, which will further reduce our existing capital. We may never achieve or sustain profitability as a business.
10
Our future funding requirements will depend on many factors, including, but not limited to:
- •
- the completion of the ongoing investigator-sponsored clinical trials of ganetespib;
- •
- the costs involved in conducting preclinical activities for our ganetespib and HDC programs;
- •
- the costs of preparing, filing, and prosecuting patent applications and maintaining, enforcing and defending intellectual
property-related claims;
- •
- the extent to which we may elect to continue drug development activities in the future, if at all; and
- •
- the timing and completion of any transaction that may result from our ongoing strategic review.
If we decide to develop and commercialize our product candidates, we will need to obtain additional funding necessary to support our operations.
Although we have raised substantial funding to date, if we decide to further develop and commercialize our product candidates, we will require additional funding in order to complete clinical development and conduct the research and development and clinical and regulatory activities necessary to bring such drug candidates to market.
We have not yet generated any product revenue and may never do so. We cannot predict whether and to what extent we may continue drug development activities, if at all, and what our future cash needs may be for any such activities. We expect our $66.6 million in cash resources as of December 31, 2015, along with significantly lower operating expenses following the termination of the GALAXY-2 trial, subsequent restructurings in the fourth quarter of 2015 and the first quarter of 2016, and the discontinuation of a substantial portion of our research and development activities, will be sufficient to fund operations for at least the next twelve months. This estimate assumes no additional funding from new partnership agreements, equity financings or further sales under our at-the-market sales agreement, or the ATM Agreement, with Cowen and Company. The timing and nature of certain activities contemplated for the remainder of 2016 will be conducted subject to the availability of sufficient financial resources. We have an effective shelf registration statement on Form S-3 (File No. 333-206135) under which we have up to $300 million in securities available for future issuance, including up to $100 million in shares of common stock that we have reserved and that may be offered and sold under the ATM Agreement. However, pursuant to the instructions to Form S-3, we only have the ability to sell shares under the shelf registration statement, during any 12-month period, in an amount less than or equal to one-third of the aggregate market value of our common stock held by non-affiliates.
Our operating plans may change as a result of many factors currently unknown to us, and we may need additional funds sooner than planned. For instance, we cannot predict whether and to what extent we may continue drug development activities. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Raising additional capital may cause dilution to existing stockholders, restrict our operations or require us to relinquish rights.
We may seek the additional capital necessary to fund our operations through public or private equity offerings, collaboration agreements, debt financings, or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, existing stockholders' ownership interests will be diluted and the terms may include liquidation or other preferences that adversely affect their rights as a stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions such as
11
incurring additional debt, making capital expenditures, or declaring dividends. For example, the terms of our loan and security agreement with General Electric Capital Corporation subject us to certain negative covenants including a prohibition on declaring or paying dividends. If we raise additional funds through collaboration or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us.
We may experience difficulties, delays or unexpected costs and not achieve anticipated benefits and savings from our recently announced corporate restructuring plans, and our restructuring activities may adversely affect our business.
Following the termination for futility of the Phase 3 GALAXY-2 trial of ganetespib and to better align resources with our operational needs going forward, in November 2015, we announced the reduction of our workforce by approximately 60% to 33 full time employees. In February 2016, we committed to an additional reduction of our workforce from 33 full time employees to 10 full time employees and discontinued a substantial portion of our research and development activities. These reductions in force resulted in or will result in the loss of numerous long-term employees, the loss of institutional knowledge and expertise and the reallocation of certain job responsibilities, all of which could adversely affect operational efficiencies, employee performance and retention. To the extent that we are unable to effectively reallocate employee responsibilities, retain key employees, establish and maintain agreements with competent third-party contractors on terms that are acceptable to us, or effectively manage the work performed by any retained third-party contractors, our ability to advance our business or product candidates may be significantly impaired and our strategic goals and our financial results may be adversely affected.
Restructuring plans may yield unintended consequences, such as attrition beyond our intended reduction in workforce and reduced employee morale, which may cause our employees who were not affected by the reduction in workforce to seek alternate employment. Furthermore, employees whose positions will be eliminated in connection with these restructuring plans may seek future employment with our competitors. Although all our employees are required to sign a confidentiality agreement with us at the time of hire, we cannot assure you that the confidential nature of our proprietary information will be maintained in the course of such future employment. Additionally, as a result of our restructuring activities we may experience a loss of continuity, loss of accumulated knowledge and/or inefficiency during transitional periods. If we cannot successfully manage the transition of our restructured operations, we may be unsuccessful in executing our business strategy, which would have a material adverse effect on our financial condition and results of operations.
If we fail to continue to meet all applicable NASDAQ Global Market requirements and The NASDAQ Stock Market determines to delist our common stock, the delisting could adversely affect the market liquidity of our common stock, impair the value of your investment and harm our business.
Our common stock is currently listed on the NASDAQ Global Market. In order to maintain that listing, we must satisfy minimum financial and other requirements. On December 3, 2015, we received notice from the Listing Qualifications Department of the NASDAQ Stock Market, or NASDAQ, that our common stock had not met the $1.00 per share minimum bid price requirement for the last 30 consecutive business days pursuant to NASDAQ Listing Rule 5450(a)(1) and that, if we were unable to demonstrate compliance with this requirement during the applicable grace periods, our common stock would be delisted after that time. The notification letter stated that pursuant to NASDAQ Listing Rule 5810(c)(3)(A) we would be afforded 180 calendar days, or until May 31, 2016, to regain compliance with the minimum bid price requirement. In order to regain compliance, shares of our common stock must maintain a minimum closing bid price of at least $1.00 per share for a minimum of ten consecutive business days. If we do not regain compliance by May 31, 2016, NASDAQ will provide written notification to us that our common stock will be delisted. At that time, we may appeal
12
NASDAQ's delisting determination to a NASDAQ Listing Qualifications Panel. Alternatively, we may be eligible for an additional 180 day grace period if we satisfy all of the requirements, other than the minimum bid price requirement, for listing on the NASDAQ Capital Market set forth in NASDAQ Listing Rule 5505. The closing bid price of our common stock on the NASDAQ Global Market was $0.23 on March 10, 2016.
While we intend to engage in efforts to regain compliance, and thus maintain our listing, there can be no assurance that we will be able to regain compliance during the applicable time periods set forth above. If we fail to continue to meet all applicable NASDAQ Global Market requirements in the future and NASDAQ determines to delist our common stock, the delisting could substantially decrease trading in our common stock and adversely affect the market liquidity of our common stock; adversely affect our ability to obtain financing on acceptable terms, if at all, for the continuation of our operations; and harm our business. Additionally, the market price of our common stock may decline further and stockholders may lose some or all of their investment.
Any value which may be ascribed to Synta beyond our cash assets is currently dependent on the development of our HDC program, which, to date, has been limited.
The market capitalization of our company approximates our cash, cash equivalents and marketable securities. Therefore any value for our stockholders above these cash assets is currently dependent on the potential development of the HDC program. Our most advanced product candidate from the HDC program, STA-12-8666, is currently in early stages of development. We intend to continue to conduct preclinical studies for STA-12-8666, but given the timing of our current strategic process as described herein, we do not currently plan to submit an investigational new drug application or initiate clinical development. Given the current early stage nature of STA-12-8666 the value ascribed to Synta in a strategic transaction beyond its cash assets may be limited.
Our existing loan and security agreements contain affirmative and negative covenants that may restrict our business and financing activities. If we fail to comply with covenants in our loan and security agreements, we may be required to repay our indebtedness thereunder, which may have an adverse effect on our liquidity.
On September 30, 2010, we entered into a $15 million loan and security agreement with General Electric Capital Corporation, or GECC, and one other lender, which we refer to herein as the GECC Term Loan. In March 2013, we amended the GECC Term Loan, obtaining $12.9 million in additional loan funding and, as a result, increasing the principal balance to $22.5 million at March 31, 2013. The GECC Term Loan is secured by substantially all of our assets, except our intellectual property. We have, however, granted GECC a springing security interest in our intellectual property in the event that we are not in compliance with certain cash burn covenants set forth in the agreement. In addition, the GECC Term Loan contains restrictive covenants, including the requirement for us to receive prior written consent of GECC to enter into loans, other than up to $4.0 million of equipment financing, restrictions on the declaration or payment of dividends, restrictions on acquisitions, and customary default provisions that include material adverse events, as defined therein. Our failure to comply with these covenants may result in the declaration of an event of default that, if not cured or waived, may result in the acceleration of the maturity of indebtedness outstanding under the GECC Term Loan, which would require us to pay all amounts outstanding. If an event of default occurs, we may not be able to cure it within any applicable cure period, if at all. If the maturity of our indebtedness is accelerated, we may not have sufficient funds available for repayment or we may not have the ability to borrow or obtain sufficient funds to replace the accelerated indebtedness on terms acceptable to us or at all.
In March 2011, we entered into a $2.0 million loan and security agreement with Oxford Finance Corporation, or Oxford, which we refer to as the Oxford Term Loan. In December 2012, we entered into a loan modification agreement under which we may draw down up to an additional $0.6 million in
13
equipment financing until June 30, 2013, which has been fully utilized. The Oxford Term Loan is secured by certain laboratory and office equipment, furniture and fixtures. In connection with the Oxford Term Loan, Oxford and GECC entered into a Lien Subordination Agreement, whereby GECC granted Oxford a first priority perfected security interest in the loan collateral. The Oxford Term Loan contains restrictive covenants, including the requirement for us to receive the prior written consent of Oxford to enter into acquisitions in which we incur more than $2.0 million of related indebtedness, and customary default provisions that include material adverse events, as defined therein.
Our future success depends on our ability to retain our key executives.
The competition for qualified personnel in the biotechnology field is intense and we must retain and motivate highly qualified scientific personnel. We are highly dependent on Chen Schor, our President and Chief Executive Officer, Marc Schneebaum, our Chief Financial Officer, Wendy Rieder, our General Counsel, and certain other employees. All of the agreements with these individuals provide that employment is at-will and may be terminated by the employee at any time and without notice. The loss of the services of any of these persons might impede the achievement of our corporate objectives. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We do not maintain "key person" insurance on any of our employees. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
Risks Related to the Development and Regulatory Approval of Our Drug Candidates
Despite the failure of ganetespib in the Phase 3 GALAXY-2 trial in NSCLC, ganetespib is still in clinical development for other cancer indications. As a result, we must continue to supply ganetespib drug product and maintain our safety data reporting systems.
Following the failure of ganetespib in the GALAXY-2 clinical trial, we provided termination notice to most of the clinical trial sites where there were ongoing investigator-sponsored trials, or ISTs. However, we are contractually bound to continue supplying ganetespib drug product for two active and ongoing ISTs in ovarian cancer (GANNET53) and sarcoma (SARC 023). As a result, we must also maintain the safety data reporting systems required to collect adverse events and incur the costs associated therewith. In addition, we retain certain potential liability in the event that patients receiving ganetespib are harmed in connection with these ongoing trials.
We currently have only one product candidate in preclinical development. As we have closed our laboratory operations and no longer have the capability to discover new product candidates internally, we may not be able to overcome employee attrition without purchasing or relying on other sources for new product candidates.
Following two workforce reductions in November 2015 and February 2016, we have closed our laboratory operations and discontinued a substantial portion of our research and development activities. Following these actions, we do not have internal discovery and research capabilities to identify and discover new product candidates. We have no current plan to resume discovery or research activities. If in the future we were to resume these activities, we would need to recruit additional scientific and technical personnel and obtain access to laboratory facilities.
We currently have only one product candidate in preclinical trials and, without internal discovery and research, we will not be able to expand our pipeline with internal candidates. If we are unable to expand our portfolio of product candidates through acquisitions or in-licensing, which we may be unable to do on reasonable terms or at all, our business would be materially and adversely affected.
14
Our success may be largely dependent on the success of STA-12-8666 and any other HDC drug candidates that we may develop, and we cannot be certain that we will be able to obtain regulatory approval for or successfully commercialize any of these drug candidates.
If we do not enter into a strategic transaction, we anticipate that our success may be depend largely on the receipt of regulatory approval and successful commercialization of STA-12-8666 and any other HDC drug candidates we may develop. The future success of our drug candidates will depend on several factors, including the following:
- •
- our ability to recruit appropriate patients into our clinical trials and to complete the necessary preclinical studies and clinical
trials to support regulatory approval;
- •
- our ability to provide acceptable evidence of their safety and efficacy;
- •
- receipt of marketing approval from the U.S. Food and Drug Administration, or FDA, and any similar foreign regulatory authorities;
- •
- obtaining and maintaining commercial manufacturing arrangements with third-party manufacturers or establishing commercial-scale
manufacturing capabilities;
- •
- approval or use of competitive products in the indications for which we will market our drug candidates;
- •
- validation of the molecular targets or mechanisms of action of our drug candidates by us or by third parties;
- •
- approval of reimbursement in foreign countries with centralized health care; and
- •
- acceptance of any approved drug in the medical community and by patients and third-party payors.
Many of these factors are beyond our control. Accordingly, there can be no assurance that we will ever be able to generate revenues through the sale of an approved product or through strategic collaborations based on our products.
If we do not obtain the required regulatory approvals, we will be unable to market and sell our drug candidates.
Our drug candidates are subject to extensive governmental regulations relating to development, clinical trials, manufacturing, and commercialization. Performance of rigorous preclinical testing and clinical trials and an extensive regulatory review and approval process are required to be successfully completed in the United States and in many foreign jurisdictions before a new drug can be sold. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain, and subject to unanticipated delays. The time required to obtain approval by the FDA is unpredictable but typically exceeds five years following the commencement of clinical trials, depending upon the complexity of the drug candidate and the indication.
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including approval by the FDA. In connection with the clinical trials of our drug candidates, we face risks that:
- •
- the drug candidate may not prove to be safe and effective;
- •
- the dosing of the drug candidate in a particular clinical trial may not be optimal;
- •
- patients may die or suffer other adverse effects for reasons that may or may not be related to the drug candidate being tested;
- •
- the results may not confirm the positive results of earlier clinical trials or preclinical studies; and
15
- •
- the results may not meet the level of statistical significance or clinical benefit-to-risk ratio required by the FDA or other regulatory agencies for marketing approval.
Of the large number of drugs in development, only a small percentage result in the submission of a new drug application, or NDA, to the FDA and even fewer are approved for commercialization. Furthermore, even if we do receive regulatory approval to market a commercial product, any such approval may be subject to limitations on the indicated uses for which we may market the product.
Because our lead drug candidate is still in preclinical development, there is a high risk of failure, and we may never succeed in developing marketable products or generating product revenue.
We have no drug candidates that have received regulatory approval for commercial sale. We do not expect to have any commercial products on the market in the foreseeable future, if at all. We are exploring human diseases at the cellular level and attempting to develop drug candidates that intervene with cellular processes. Drug development is an uncertain process that involves trial and error, and we may fail at numerous stages along the way. Success in preclinical studies of a drug candidate may not be predictive of similar results in humans during clinical trials, and successful results from early or small clinical trials of a drug candidate may not be replicated in later and larger clinical trials. Accordingly, the results from preclinical studies may not be predictive of the results we may obtain in later stage clinical trials.
If clinical trials for our drug candidates are prolonged, delayed or suspended, we may be unable to commercialize our drug candidates on a timely basis, which would require us to incur additional costs and delay our receipt of any revenue from potential product sales.
We cannot predict whether we will encounter problems with any of our completed, ongoing or planned clinical trials that will cause us or any regulatory authority to delay or suspend those clinical trials or delay the analysis of data derived from them. A number of events, including any of the following, could delay the completion of our other ongoing and planned clinical trials and negatively impact our ability to obtain regulatory approval for, and to market and sell our drug candidates that are still in preclinical studies, including STA-12-8666 and any other HDC drug candidates that we may develop:
- •
- conditions imposed on us by the FDA or any foreign regulatory authority regarding the scope or design of our clinical trials;
- •
- delays in obtaining, or our inability to obtain, required approvals from institutional review boards or other reviewing entities at
clinical sites selected for participation in our clinical trials;
- •
- insufficient supply or deficient quality of our drug candidates or other materials necessary to conduct our clinical trials;
- •
- delays in obtaining regulatory agreement for the conduct of our clinical trials;
- •
- slower or lower than anticipated enrollment and retention rate of subjects in clinical trials;
- •
- negative or inconclusive results from clinical trials, or results that are inconsistent with earlier results, that necessitate
additional clinical trials (for example, due to patient-to-patient pharmacokinetic variability, or due to changes in patient management and outcomes);
- •
- serious and unexpected drug-related side effects experienced by patients in clinical trials; or
- •
- failure of our third-party contractors to comply with regulatory requirements or otherwise meet their contractual obligations to us.
Commercialization of our drug candidates may be delayed by the imposition of additional conditions on our clinical trials by the FDA or any foreign regulatory authority or the requirement of
16
additional supportive studies by the FDA or any foreign regulatory authority. In addition, clinical trials require sufficient patient enrollment, which is a function of many factors, including the size of the target patient population, the nature of the trial protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease, the conduct of other clinical trials that compete for the same patients as our clinical trials, and the eligibility criteria for our clinical trials. Our failure to enroll patients in our clinical trials could delay the completion of the clinical trial beyond our expectations. In addition, the FDA could require us to conduct clinical trials with a larger number of subjects than we have projected for any of our drug candidates. We may not be able to enroll a sufficient number of patients in a timely or cost-effective manner. Furthermore, enrolled patients may drop out of our clinical trials, which could impair the validity or statistical power of the clinical trials.
We do not know whether our clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, if at all. Delays in our clinical trials will result in increased development costs for our drug candidates. In addition, if our clinical trials are delayed, our competitors may be able to bring products to market before we do and the commercial viability of our drug candidates could be limited. If approved, we may not receive a package insert for any of our products that are competitive and differentiated, which may change our strategies with respect to how and when we commercialize any of our products.
If we inadvertently fail to comply with foreign regulatory requirements governing human clinical trials and marketing approval for drugs, we could be prevented from selling our drug candidates in foreign markets, which may adversely affect our operating results and financial condition.
The requirements governing the conduct of clinical trials, product licensing, pricing, and reimbursement for marketing our drug candidates outside the United States vary greatly from country to country and may require additional testing. We expect that our future clinical development of our drug candidates will involve a number of clinical trials in foreign jurisdictions, particularly in Europe. We have no experience in obtaining foreign regulatory approvals. The time required to obtain approvals outside the United States may differ from that required to obtain FDA approval. We may not obtain foreign regulatory approvals on a timely basis, if at all. Approval by the FDA does not guarantee approval by regulatory authorities in other countries, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other countries or by the FDA. Failure to comply with these regulatory requirements or obtain required approvals could impair our ability to develop foreign markets for our drug candidates and may have a material adverse effect on our results of operations and financial condition.
Our drug candidates will remain subject to ongoing regulatory review even if they receive marketing approval, and if we fail to comply with continuing regulations, we could lose these approvals and the sale of any approved commercial products could be suspended.
Even if we receive regulatory approval to market a particular drug candidate, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, and record keeping related to the product will remain subject to extensive regulatory requirements. If we fail to comply with the regulatory requirements of the FDA and other applicable domestic and foreign regulatory authorities or previously unknown problems with any approved commercial products, manufacturers, or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions, including:
- •
- restrictions on the products, manufacturers, or manufacturing processes;
- •
- warning letters;
- •
- civil or criminal penalties;
17
- •
- fines;
- •
- injunctions;
- •
- product seizures or detentions;
- •
- import bans;
- •
- suspension or withdrawal of regulatory approvals;
- •
- total or partial suspension of production; and
- •
- refusal to approve pending applications for marketing approval of new drug candidates or supplements to approved applications.
If side effects or toxicities increase or are identified during the time our drug candidates are in development or after they are approved and on the market, we may be required to perform lengthy additional clinical trials, change the labeling or limit the scope of indication of any such products, or withdraw any such products from the market, any of which would hinder or preclude our ability to generate revenues.
We have observed significant toxicities in preclinical animal studies of our clinical drug candidate, ganetespib. In clinical trials to date, we have not observed the serious liver and common ocular toxicities observed with first generation Hsp90 inhibitors.
We have observed a prolongation of the QTc interval in a Thorough QTc clinical study of ganetespib in healthy volunteers. This type of change in ECG tracings has been reported for a number of development-stage and approved oncology drugs, as well as drugs for other indications. The independent review also noted that the maximum mean change in QTcF (DDQTcF) from baseline of 21.5ms observed in the Thorough QTc clinical study places ganetespib in a zone of clinical ambiguity; it is not clear that this finding confers a substantial increased risk of torsades de pointes, a severe form of arrhythmia, in patients who are being treated with ganetespib for cancer. An independent review of the ganetespib clinical safety database in 2013 did not indicate an increased frequency or severity of cardiovascular adverse events in patients treated with ganetespib. We note that none of the 580 patients treated with ganetespib reviewed as part of this analysis had an adverse event of torsades de pointes reported. In addition, the independent review noted that the Thorough QTc study was conducted at a dose 33% higher than being evaluated in our ongoing combination studies; there was only one patient out of 45 that showed a QTc>450ms and no patients with a QTc>480ms; the number of outliers with change in QTc>30ms was low, only two subjects out of 45 (versus one subject in the placebo group, n=48); and there were no subjects with change in QTc>60ms. With enhanced ECG monitoring, we may find that the QTc prolongation effect of ganetespib treatment is more pronounced than we have observed to date. We may also find that the use of ganetespib in a larger number of patients may reveal an increase in the incidence or severity of cardiovascular adverse events.
Even if we are successful in obtaining regulatory approval for one or more of our drug candidates, as the drug is used in a larger patient population, if the incidence of side effects or toxicities increases or if other unacceptable effects are identified:
- •
- regulatory authorities may withdraw their approvals;
- •
- we may be required to reformulate any such products, conduct additional clinical trials, make changes in labeling of any such
products, or implement changes to or obtain new approvals of our or our contractors' manufacturing facilities;
- •
- we may experience a significant drop in the sales of the affected products;
- •
- our reputation in the marketplace may suffer; and
- •
- we may become the target of lawsuits, including class action suits.
18
Any of these events could harm or prevent sales of the affected products or could substantially increase the costs and expenses of commercializing and marketing any such products.
While we choose to test our drug candidates in specific clinical indications based in part on our understanding of their mechanisms of action, our understanding may be incorrect or incomplete and, therefore, our drugs may not be effective against the diseases tested in our clinical trials.
Our rationale for selecting the particular therapeutic indications for each of our drug candidates is based in part on our understanding of the mechanism of action of these drug candidates. However, our understanding of the drug candidate's mechanism of action may be incomplete or incorrect, or the mechanism may not be clinically relevant to the diseases treated. In such cases, our drug candidates may prove to be ineffective in the clinical trials for treating those diseases.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct our nonclinical safety assessment studies, and those third parties may not perform satisfactorily, including failing to meet established timelines for the completion of such clinical trials and studies.
We do not have the ability to independently conduct clinical trials and certain nonclinical safety assessment studies, particularly those studies conducted under Good Laboratory Practices, or GLP, for our drug candidates, and we rely on third parties such as contract research organizations, or CROs, medical institutions, and clinical investigators in the case of clinical trials, and CROs in the case of nonclinical safety assessment studies, to perform these functions. Our reliance on these third parties for clinical development activities reduces our control over these activities. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. To date, our CROs and other similar entities with which we are working have performed well; however, if these third parties do not successfully carry out their contractual duties, meet expected timelines, or comply with applicable regulatory requirements, we may be delayed in obtaining regulatory approvals for our drug candidates and may be delayed in our efforts to successfully commercialize our drug candidates for targeted diseases.
We have no manufacturing capacity and depend on third-party manufacturers to produce our drug supplies.
We do not currently operate manufacturing facilities or testing facilities for any of our preclinical drug candidates. We have limited experience in drug manufacturing, and we lack the resources and the capabilities to manufacture any of our drug candidates on a clinical or commercial scale. We continue to provide limited ganetespib drug supply for the clinical trials in ovarian cancer (GANNET53) and sarcoma (SARC 023). We still rely on third-party manufacturers to manufacture, test, supply, store, and distribute drug supplies for these clinical trials. Any performance failure on the part of our existing or future manufacturers could interrupt on-going clinical trials, delay clinical development or regulatory approval of our drug candidates or commercialization of any approved products, producing additional losses and depriving us of potential product revenue.
Our drug candidates require precise, high quality manufacturing. Failure by our contract manufacturers to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, delays or failures in testing or delivery, cost overruns, or other problems that could seriously hurt our business. Contract manufacturers may encounter difficulties involving production yields, quality control, and quality assurance. These manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state and foreign agencies to ensure strict compliance with current Good Manufacturing Practice regulations, or cGMPs, and other applicable U.S. and foreign government regulations and standards. We periodically audit our contract manufacturers responsible for supplying our clinical drug materials and have put quality agreements in place that we believe are appropriate for our materials. However, we do not have direct control over third party manufacturers' compliance with cGMPs and other standards and therefore, cannot provide assurance regarding such compliance.
19
If for some reason our contract manufacturers cannot perform as agreed, we may be unable to replace such third-party manufacturers in a timely manner and the production of our drug candidates would be interrupted, resulting in delays in clinical trials and additional costs. Switching manufacturers may be difficult because the number of potential manufacturers is limited and the FDA must approve any replacement manufacturer after our drug candidates are approved. Such approval would require new testing and compliance inspections. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our drug candidates after receipt of FDA approval. It may be difficult or impossible for us to find a replacement manufacturer on acceptable terms quickly, or at all.
We anticipate that we will continue to rely on third-party manufacturers if we are successful in obtaining marketing approval from the FDA and other regulatory agencies for any of our drug candidates.
To date, our drug candidates have been manufactured in relatively small quantities for preclinical testing and clinical trials by third-party manufacturers. If the FDA or other regulatory agencies approve any of our drug candidates for commercial sale, we expect that we would continue to rely, at least initially, on third-party manufacturers to produce commercial quantities of such approved drug candidates. These manufacturers may not be able to successfully increase the manufacturing capacity for any of our approved drug candidates in a timely or economic manner, or at all. Significant scale-up of manufacturing and increased production may require additional validation studies, which the FDA or other regulatory authorities must review and approve. If our third-party manufacturers are unable to successfully increase the manufacturing capacity for a drug candidate, or we are unable to establish our own manufacturing capabilities, the commercial launch of any approved products may be delayed or there may be a shortage in supply.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug candidates, we may be unable to generate product revenue.
We do not currently have an organization for the sales, marketing, and distribution of pharmaceutical products. In order to commercialize and market any of our products that may be approved by the FDA, we must build our sales, marketing, managerial, and other non-technical capabilities or make arrangements with third parties to perform these services. If we are unable to establish adequate sales, marketing, and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and we may not become profitable.
Risks Related to Our Intellectual Property
If our patent position does not adequately protect our drug candidates or any future products, others could compete against us more directly, which would harm our business.
Our success depends in part on our ability to obtain and maintain proprietary protection for our drug candidates, technology, and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions, and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities, as appropriate, to develop and maintain our proprietary position.
Our commercial success will depend in part on our ability to obtain additional patents and protect our existing patent position as well as our ability to maintain adequate protection of other intellectual property for our technologies, drug candidates, and any future products in the United States and other countries. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our
20
business and ability to achieve profitability. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated, or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies, drug candidates, and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
In addition, although we do not believe that any of the patents or patent applications that we currently license are material to our business, we may in the future license intellectual property that is material to us. In such cases, we may be dependent upon the licensors to obtain, maintain and enforce patent protection for the licensed intellectual property. These licensors may not successfully prosecute patent applications or may fail to maintain issued patents. The licensors may also determine not to pursue litigation against other companies that infringe the patents, or may pursue such litigation less aggressively than we would. If any of the foregoing occurs, and the terms of any such future license do not allow us to assume control of patent prosecution, maintenance and enforcement, any competitive advantage we may have due to the license may be diminished or eliminated.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
- •
- we or our licensors were the first to make the inventions covered by each of our pending patent applications;
- •
- we or our licensors were the first to file patent applications for these inventions;
- •
- others will not independently develop similar or alternative technologies or duplicate any of our technologies;
- •
- any of our or our licensors' pending patent applications will result in issued patents;
- •
- any of our or our licensors' patents will be valid or enforceable;
- •
- any patents issued to us or our licensors and collaborators will provide a basis for commercially viable products, will provide us
with any competitive advantages or will not be challenged by third parties;
- •
- we will develop additional proprietary technologies or drug candidates that are patentable; or
- •
- the patents of others will not have an adverse effect on our business.
Although third parties may challenge our rights to, or the scope or validity of our patents, to date we have not received any communications from third parties challenging our patents or patent applications covering our drug candidates.
We typically file for patent protection first on the composition-of-matter of our drug candidates and also claim their activities and methods for their production and use to the extent known at that time. As we learn more about the mechanisms of action and new methods of manufacture and use of these drug candidates, we generally file additional patent applications for these new inventions. Although our patents may prevent others from making, using, or selling similar products, they do not ensure that we will not infringe the patent rights of third parties. For example, because we sometimes identify the mechanism of action or molecular target of a given drug candidate after identifying its composition-of-matter and therapeutic use, we may not be aware until the mechanism or target is further elucidated that a third party has an issued or pending patent claiming biological activities or
21
targets that may cover our drug candidate. If such a patent exists or is granted in the future, we cannot provide assurances that a license will be available on commercially reasonable terms, or at all.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
Litigation or other proceedings or third-party claims of intellectual property infringement would require us to spend time and money and could prevent us from developing or commercializing our drug candidates.
Our commercial success will depend in part on not infringing upon the patents and proprietary rights of other parties and enforcing our own patents and proprietary rights against others. Certain of our research and development programs are in highly competitive fields in which numerous third parties have issued patents and patent applications with claims closely related to the subject matter of our programs. We are not currently aware of any litigation or other proceedings or claims by third parties that our drug candidates, technologies or methods infringe their intellectual property.
However, while it is our practice to conduct freedom to operate searches and analyses, we cannot guarantee that we have identified every patent or patent application that may be relevant to the research, development or commercialization of our drug candidates. In the case of patent applications, we assess the likelihood of claims in pending, third party patent applications being allowed which may interfere with our freedom to operate relative to our drug candidates. We cannot provide assurances that our assessments in this regard will be correct and that patent claims covering our drug candidates that were assessed a low likelihood of issuance by us will not issue to a third party in the future. Moreover, there can be no assurance that third parties will not assert against us patents that we believe are not infringed by us or are invalid.
In the event of a successful infringement action against us with respect to any third party patent rights, we may be required to:
- •
- pay substantial damages;
- •
- stop developing, commercializing, and selling the infringing drug candidates or approved products;
- •
- stop utilizing the infringing technologies and methods in our drug candidates or approved products;
- •
- develop non-infringing products, technologies, and methods; and
- •
- obtain one or more licenses from other parties, which could result in our paying substantial royalties or our granting of cross licenses to our technologies.
We may not be able to obtain licenses from other parties at a reasonable cost, or at all. If we are not able to obtain necessary licenses at a reasonable cost, or at all, we could encounter substantial delays in product introductions while we attempt to develop alternative technologies, methods, and products, which we may not be able to accomplish.
22
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers.
As is commonplace in our industry, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we have previously been subject to a claim by an alleged competitor that a prospective employee we sought to hire was bound by an ongoing non-competition obligation which prevented us from hiring this employee. We may be subject in the future to claims that our employees or prospective employees are subject to a continuing obligation to their former employers (such as non-competition or non-solicitation obligations) or claims that our employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
We may not be able to keep up with the rapid technological change in the biotechnology and pharmaceutical industries, which could make any future approved products obsolete and reduce our revenue.
Biotechnology and related pharmaceutical technologies have undergone and continue to be subject to rapid and significant change. Our future will depend in large part on our ability to maintain a competitive position with respect to these technologies. Our competitors may render our technologies obsolete by advances in existing technological approaches or the development of new or different approaches, potentially eliminating the advantages in our drug discovery process that we believe we derive from our research approach and proprietary technologies. In addition, any future products that we develop, including our clinical drug candidates, may become obsolete before we recover expenses incurred in developing those products, which may require that we raise additional funds to continue our operations.
Our market is subject to intense competition. If we are unable to compete effectively, our drug candidates may be rendered noncompetitive or obsolete.
We are engaged in segments of the pharmaceutical industry that are highly competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies, and other public and private research organizations are pursuing the development of novel drugs that target cancer. We face, and expect to continue to face, intense and increasing competition as new products enter the market and advanced technologies become available. In addition to currently approved drugs, there are a significant number of drugs that are currently under development and may become available in the future for the treatment of cancer. We would expect our drug candidates to compete with marketed drugs and potentially with drug candidates currently under development.
Many of our competitors have:
- •
- significantly greater financial, technical and human resources than we have and may be better equipped to discover, develop,
manufacture and commercialize drug candidates;
- •
- more extensive experience in preclinical testing and clinical trials, obtaining regulatory approvals and manufacturing and marketing
pharmaceutical products;
- •
- drug candidates that have been approved or are in late-stage clinical development; and/or
23
- •
- collaborative arrangements in our target markets with leading companies and research institutions.
Competitive products may render our products obsolete or noncompetitive before we can recover the expenses of developing and commercializing our drug candidates. Furthermore, the development of new treatment methods and/or the widespread adoption or increased utilization of any vaccine for the diseases we are targeting could render our drug candidates noncompetitive, obsolete or uneconomical. If we successfully develop and obtain approval for our drug candidates, we will face competition based on the safety and effectiveness of our drug candidates, the timing of their entry into the market in relation to competitive products in development, the availability and cost of supply, marketing and sales capabilities, reimbursement coverage, price, patent position and other factors. If we successfully develop drug candidates but those drug candidates do not achieve and maintain market acceptance, our business will not be successful.
If approved, STA-12-8666, may compete with the currently approved therapies for the treatment of cancers, and other cancer treatments under development. In particular, STA-12-8666 may compete with irinotecan and other novel formulations or approaches including antibody drug conjugates (ADCs) intended to improve the activity of irinotecan or its active metabolite SN-38. These include: Etirinotecan pegol, being developed by Nektar Therapeutics, MM-398, being developed by Merrimack Pharmaceuticals, CRLX101 being developed by Cerulean Pharma, NK012, being developed by Nippon Kayaku Co., HA-irinotecan being developed by Alchemia, IMMU-130 and IMMU-132, being developed by Immunomedics, BEL-0222, being developed by Belrose Pharma, PEG-SN-38 conjugate, being developed by Prolynx LLC, and IT-141, being developed by Intezyne, among others. In general, therapies from the HDC program, if approved, may compete with approved products and agents in development stemming from approaches that are designed to preferentially increase tumor exposure to an anticancer agent. These may include approved products and/or products that arise from various liposomal and nanoparticle delivery approaches and antibody drug conjugate (ADC) platforms, among others.
Our ability to compete successfully will depend largely on our ability to leverage our experience in drug discovery, development and commercialization to:
- •
- discover and develop on a timely-basis medicines that are superior to other products in the market;
- •
- attract high-quality scientific, product development, and commercial personnel;
- •
- obtain patent and/or proprietary protection for our medicines and technologies;
- •
- obtain required regulatory approvals;
- •
- selectively commercialize certain drug candidates in indications treated by specialist physicians; and
- •
- selectively partner with pharmaceutical companies in the development and commercialization of certain drug candidates.
Risks Related to Our Common Stock
Our stock price has been and is likely to continue to be volatile and the market price of our common stock may drop.
Prior to our February 2007 initial public offering, there was not a public market for our common stock. There is a limited history on which to gauge the volatility of our stock price; however, since our common stock began trading on The NASDAQ Global Market in February 2007, our stock price has fluctuated from a low of $0.29 to a high of $11.88. Furthermore, the stock market has experienced
24
significant volatility, particularly with respect to pharmaceutical, biotechnology, and other life sciences company stocks. The volatility of pharmaceutical, biotechnology, and other life sciences company stocks often does not relate to the operating performance of the companies represented by the stock. Some of the factors that may cause the market price of our common stock to fluctuate include:
- •
- results of clinical trials conducted by others on drugs that would compete with our drug candidates;
- •
- failure or delays in advancing STA-12-8666 or any other HDC drug candidates that we may develop, or other drug candidates that we may
discover or acquire in the future, into clinical trials;
- •
- results of clinical trials conducted by other pharmaceutical, biotechnology, and life sciences companies on drugs that would compete
with our drug candidates;
- •
- failure or discontinuation of any of our research programs;
- •
- potential for merger or acquisition;
- •
- key personnel changes;
- •
- issues in manufacturing our drug candidates or approved products;
- •
- regulatory developments or enforcement in the United States and foreign countries;
- •
- developments or disputes concerning patents or other proprietary rights;
- •
- introduction of technological innovations or new commercial products by us or our competitors;
- •
- changes in estimates or recommendations by securities analysts, if any cover our common stock;
- •
- public concern over our drug candidates or any approved products;
- •
- litigation;
- •
- general market conditions;
- •
- changes in the structure of healthcare payment systems;
- •
- failure of any of our drug candidates, if approved, to achieve commercial success;
- •
- economic and other external factors or other disasters or crises;
- •
- period-to-period fluctuations in our financial results; and
- •
- overall fluctuations in U.S. equity markets.
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In addition, in the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
Future sales of our common stock may cause our stock price to decline and impede our ability to raise capital.
Our directors and executive officers, together with their affiliates and related persons, beneficially own, in the aggregate, approximately 18.2% of our outstanding shares of common stock.
25
Sales into the public market by our officers, directors and their affiliates, or other major stockholders, of a substantial number of shares, or the expectation that such sales may occur, could significantly reduce the market price of our common stock. In addition, certain of our executive officers may establish predetermined selling plans under Rule 10b5-1 of the Securities Exchange Act of 1934, or the Exchange Act, for the purpose of effecting sales of common stock.
If any such sales occur, are expected to occur or a large number of our shares are sold in the public market, the trading price of our common stock could decline.
Insiders have substantial control over us which could delay or prevent a change in corporate control or result in the entrenchment of management and/or the board of directors.
Our directors and executive officers, together with their affiliates and related persons, beneficially own, in the aggregate, approximately 18.2% of our outstanding shares of common stock. These stockholders, if acting together, may have the ability to determine the outcome of matters submitted to our stockholders for approval, including the election and removal of directors and any merger, consolidation, or sale of all or substantially all of our assets. In addition, these persons, acting together, may have the ability to control the management and affairs of our company. Accordingly, this concentration of ownership may harm the market price of our common stock by:
- •
- delaying, deferring, or preventing a change in control;
- •
- entrenching our management and/or the board of directors;
- •
- impeding a merger, consolidation, takeover, or other business combination involving us; or
- •
- discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us.
Provisions of our charter, bylaws, and Delaware law may make an acquisition of us or a change in our management more difficult.
Certain provisions of our restated certificate of incorporation and restated bylaws could discourage, delay, or prevent a merger, acquisition, or other change in control that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. These provisions also could limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove our management.
These provisions:
- •
- allow the authorized number of directors to be changed only by resolution of our board of directors;
- •
- establish a classified board of directors, providing that not all members of the board of directors be elected at one time;
- •
- authorize our board of directors to issue without stockholder approval blank check preferred stock that, if issued, could operate as a
"poison pill" to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that is not approved by our board of directors;
- •
- require that stockholder actions must be effected at a duly called stockholder meeting and prohibit stockholder action by written consent;
26
- •
- establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be
acted on at stockholder meetings;
- •
- limit who may call stockholder meetings; and
- •
- require the approval of the holders of 80% of the outstanding shares of our capital stock entitled to vote in order to amend certain provisions of our restated certificate of incorporation and restated bylaws.
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or combining with us for a prescribed period of time.
We do not anticipate paying cash dividends, and accordingly, our stockholders must rely on stock appreciation for any return on their investment.
We currently intend to retain our future earnings, if any, to fund the development and growth of our business. In addition, we are currently prohibited from making a dividend payment under the terms of our loan and security agreement with GECC. As a result, capital appreciation, if any, of our common stock will be the sole source of gain on an investment in our common stock for the foreseeable future.
Item 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
Our operations are based primarily in Lexington, Massachusetts, which is located approximately 10 miles west of Boston, Massachusetts. We currently lease a total of 87,920 square feet of office and laboratory space, including 72,920 square feet in Lexington and 15,000 square feet in the neighboring town of Bedford, Massachusetts. We lease the following properties:
Location
|
Approximate Square Feet |
Use | Lease Expiration Date |
||||
|---|---|---|---|---|---|---|---|
45 Hartwell Avenue |
34,520 | Office and Laboratory | November 2016 | ||||
125 Hartwell Avenue |
38,400 | Office and Laboratory | November 2016 | ||||
45 - 47 Wiggins Avenue |
15,000 | Office and Laboratory | October 2016 | ||||
We are currently not a party to any material legal proceedings.
Item 4. MINE SAFETY DISCLOSURES
Not applicable.
27
Item 5. MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is traded on The NASDAQ Global Market under the symbol "SNTA." The following table sets forth the high and low sales prices of our common stock as quoted on The NASDAQ Global Market for the periods indicated.
2014:
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
First Quarter |
$ | 7.22 | $ | 4.07 | |||
Second Quarter |
4.60 | 3.91 | |||||
Third Quarter |
4.97 | 2.94 | |||||
Fourth Quarter |
3.44 | 2.54 | |||||
2015:
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
First Quarter |
$ | 2.98 | $ | 1.85 | |||
Second Quarter |
3.17 | 1.91 | |||||
Third Quarter |
2.37 | 1.57 | |||||
Fourth Quarter |
2.08 | 0.29 | |||||
Stockholders
As of March 10, 2016, there were approximately 40 stockholders of record of the 137,806,441 outstanding shares of our common stock.
Dividends
We have never paid or declared any cash dividends on our common stock and we are currently prohibited from making any dividend payment under the terms of our Loan and Security Agreement with General Electric Capital Corporation. We currently intend to retain all available funds and any future earnings to fund the development and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends will be at the discretion of our board of directors and will depend on our financial condition, results of operations, contractual restrictions, capital requirements, and other factors that our board of directors deems relevant.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
None.
28
Stock Performance Graph
The following graph compares the cumulative total stockholder return on our common stock from December 31, 2010 to December 31, 2015 with the cumulative total return of (i) the NASDAQ Composite Index and (ii) the NASDAQ Biotechnology Index. This graph assumes the investment of $100.00 on December 31, 2010 in our common stock, the NASDAQ Composite Index and the NASDAQ Biotechnology Index, and assumes any dividends are reinvested. We have not paid any dividends on our common stock, and we do not include dividends in the representation of our performance. The stock price performance on the graph below does not necessarily indicate future price performance.
COMPARISON OF CUMULATIVE TOTAL RETURN
SYNTA PHARMACEUTICALS CORP., NASDAQ COMPOSITE INDEX
AND NASDAQ BIOTECHNOLOGY INDEX
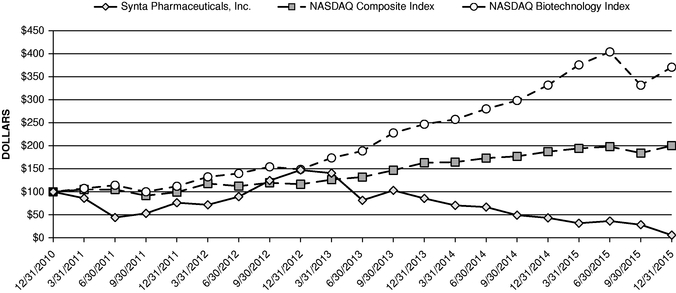
ASSUMES
$100 INVESTED ON DEC. 31, 2010
ASSUMES DIVIDEND REINVESTED
FISCAL YEAR ENDING DEC. 31, 2015
29
Item 6. SELECTED FINANCIAL DATA
The following table sets forth our selected consolidated financial data and has been derived from our audited consolidated financial statements. Consolidated balance sheets as of December 31, 2015 and 2014, as well as consolidated statements of operations for the years ended December 31, 2015, 2014 and 2013, and the reports thereon are included elsewhere in this Annual Report on Form 10-K. The information below should be read in conjunction with our audited consolidated financial statements (and notes thereon) and "Management's Discussion and Analysis of Financial Condition and Results of Operations," included below in Item 7.
| |
Years ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | 2012 | 2011 | |||||||||||
| |
(all amounts in thousands except per share data) |
|||||||||||||||
Consolidated Statement of Operations Data: |
||||||||||||||||
Revenues: |
||||||||||||||||
License and milestone revenue(1) |
$ | — | $ | — | $ | — | $ | — | $ | 6,731 | ||||||
Grant revenue |
— | — | — | 147 | 853 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total revenues |
— | — | — | 147 | 7,584 | |||||||||||
Operating expenses: |
||||||||||||||||
Research and development |
54,218 | 68,205 | 71,860 | 49,412 | 41,464 | |||||||||||
General and administrative |
13,392 | 15,746 | 15,699 | 11,676 | 11,552 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total operating expenses |
67,610 | 83,951 | 87,559 | 61,088 | 53,016 | |||||||||||
| | | | | | | | | | | | | | | | | |
Loss from operations |
(67,610 | ) | (83,951 | ) | (87,559 | ) | (60,941 | ) | (45,432 | ) | ||||||
Other expense, net |
(1,061 | ) | (2,210 | ) | (2,633 | ) | (1,849 | ) | (1,948 | ) | ||||||
| | | | | | | | | | | | | | | | | |
Net loss |
$ | (68,671 | ) | $ | (86,161 | ) | $ | (90,192 | ) | $ | (62,790 | ) | $ | (47,380 | ) | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
Net loss per common share: |
||||||||||||||||
Basic and diluted net loss per common share |
$ | (0.53 | ) | $ | (0.87 | ) | $ | (1.27 | ) | $ | (1.06 | ) | $ | (1.00 | ) | |
Basic and diluted weighted average number |
128,595 | 98,489 | 70,977 | 59,411 | 47,198 | |||||||||||
common shares outstanding |
||||||||||||||||
- (1)
- In December 2008, we entered into an agreement with Hoffman-La Roche (Roche) for our CRACM inhibitor program ("the Roche Agreement"). Roche provided written notification of termination in November 2011, resulting in accelerated recognition of $2.1 million of previously deferred revenue in the fourth quarter of 2011.
| |
As of December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | 2012 | 2011 | |||||||||||
Consolidated Balance Sheet Data: |
||||||||||||||||
Cash, cash equivalents and marketable securities |
$ | 66,574 | $ | 97,690 | $ | 91,476 | $ | 100,599 | $ | 39,725 | ||||||
Working capital |
49,987 | 68,457 | 60,034 | 77,899 | 25,138 | |||||||||||
Total assets |
68,195 | 100,675 | 95,203 | 103,017 | 42,324 | |||||||||||
Capital lease obligations, net of current portion |
— | 43 | 85 | 1 | 14 | |||||||||||
Term loans, current portion |
4,607 | 9,214 | 9,451 | 7,924 | 4,234 | |||||||||||
Term loans, net of current portion |
— | 4,607 | 13,820 | 4,464 | 12,388 | |||||||||||
Common stock and additional paid-in capital |
756,647 | 702,705 | 600,486 | 536,284 | 413,201 | |||||||||||
Accumulated deficit |
(706,244 | ) | (637,573 | ) | (551,412 | ) | (461,220 | ) | (398,430 | ) | ||||||
Total stockholders' equity |
50,407 | 65,136 | 49,091 | 75,066 | 14,774 | |||||||||||
30
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations.
This Management's Discussion and Analysis of Financial Condition and Results of Operations should be read together with the consolidated financial statements, related notes and other financial information included elsewhere in this Annual Report on Form 10-K.
Overview
Synta Pharmaceuticals Corp. is a company that has historically focused on the research, development and commercialization of novel oncology medicines that have the potential to change the lives of cancer patients. In October 2015, we announced the decision to terminate for futility the Phase 3 GALAXY-2 trial of our novel heat shock protein 90 (Hsp90) inhibitor, ganetespib, and docetaxel in the second-line treatment of patients with advanced non-small cell lung adenocarcinoma. Based on a review of a pre-planned interim analysis, the study's Independent Data Monitoring Committee (IDMC) concluded that the addition of ganetespib to docetaxel was unlikely to demonstrate a statistically significant improvement in the primary endpoint of overall survival compared to docetaxel alone.
We have also been evaluating several candidates from our proprietary Hsp90 inhibitor Drug Conjugate program, or HDC program, which leverages the preferential accumulation of Hsp90 inhibitors in tumors to selectively deliver a wide array of anti-cancer payloads. We are currently conducting studies for our first clinical candidate from the HDC program, STA-12-8666, in anticipation of potentially submitting an investigational new drug application, or IND, for STA-12-8666. While we have determined not to pursue an IND submission for STA-12-8666 in the immediate future, we may determine to do so at a later date.
We were incorporated in March 2000 and commenced operations in July 2001. Since that time, we were principally engaged in the discovery and development of novel drug candidates. As of December 31, 2015, we have raised an aggregate of approximately $868.9 million in cash proceeds to fund operations, including $665.9 million in net proceeds from private and public offerings of our equity, $30.5 million in gross proceeds from term loans and $167.2 million in non-refundable payments from partnering activities under prior collaborations, as well as $5.3 million from the exercise of common stock warrants and options. We have also generated funds from government grants, equipment lease financings and investment income.
We have historically devoted substantially all of our resources to the discovery and development of our drug candidates, as well as intellectual property prosecution. We currently do not have any drugs that are commercially available and none of our drug candidates have obtained approval of the U.S. Food and Drug Administration, or FDA, or any similar foreign regulatory authority. Since our inception, we have had no revenues from product sales. As of December 31, 2015, we had an accumulated deficit of $706.2 million.
Key Developments
Following the termination of the GALAXY-2 trial in October 2015, we initiated a comprehensive review of our strategy. In November 2015, we committed to a restructuring that consisted primarily of a workforce reduction to better align our workforce to our revised operating plans, which included support of key ongoing ganetespib investigator-sponsored studies and continued effort on development of candidates from the HDC program, in particular STA-12-8666. The restructuring was substantially completed during the fourth quarter of 2015. Cash payments in connection with the workforce reduction, comprised principally of severance, unused vacation payments, benefits continuation costs and outplacement services, were approximately $2.6 million of which approximately $1.3 million was paid during the fourth quarter of 2015. As of December 31, 2015, approximately $1.3 million was
31
accrued in remaining restructuring-related payments that are expected to be substantially paid during the first quarter of 2016.
As announced in March 2016, in order to conserve cash while we continue to evaluate business alternatives to maximize value for stockholders, we committed to an additional restructuring in February 2016 that consisted primarily of a workforce reduction of 23 positions, including 19 research and development positions, to a total of 10 remaining positions. In connection with this restructuring, we discontinued a substantial portion of our research and development activities. Cash payments in connection with the workforce reduction, comprised principally of severance, unused vacation payments, benefits continuation costs and outplacement services, will be approximately $1.5 million. We expect the restructuring to be substantially completed during the first quarter of 2016 and the majority of the related cash payments to be paid during the first half of 2016. Employees directly affected by the restructuring have received notification and will be provided with severance payments.
We continue to conduct limited activities with respect to ganetespib and the candidates in the HDC program, including STA-12-8666. With the exception of ongoing clinical trials in ovarian cancer and sarcoma, we have undertaken efforts to wind down existing ganetespib investigator-sponsored clinical trials. We have also entered into strategic agreements to out-license two of our prior drug candidates, elesclomol and our calcium release-activated calcium channel (CRACM) inhibitor program. However, we no longer anticipate expending material resources on any of our current drug candidates.
As previously announced in our Current Report on Form 8-K filed on March 1, 2016, reporting the restructuring of our workforce, we have been considering potential strategic alternatives to enhance stockholder value. Such strategic alternatives include, but are not limited to, a sale of the company, a business combination or collaboration, joint development and partnership opportunities, a distribution of all or a significant amount of cash to stockholders, and liquidation of the company. We do not know if we will be successful in pursuing any strategic alternative or that any transaction will occur; however, we are committed to pursuing a strategic direction that our Board of Directors believes is in the best interests of our stockholders.
We cannot predict whether and to what extent we may continue drug development activities, if at all, and what our future cash needs may be for any such activities. We expect our $66.6 million in cash resources as of December 31, 2015, along with significantly lower operating expenses following the termination of the GALAXY-2 trial, subsequent restructurings in the fourth quarter of 2015 and the first quarter of 2016, and the discontinuation of a substantial portion of our research and development activities will be sufficient to fund operations for at least the next twelve months.
On December 3, 2015, we received a notice from the Listing Qualifications Department of the NASDAQ Stock Market indicating that, for the last 30 consecutive business days, the bid price for our common stock had closed below the minimum $1.00 per share required for continued inclusion on The NASDAQ Global Market under NASDAQ Listing Rule 5450(a)(1). The notification letter states that pursuant to NASDAQ Listing Rule 5810(c)(3)(A) the Company will be afforded 180 calendar days, or until May 31, 2016, to regain compliance with the minimum bid price requirement. In order to regain compliance, shares of the Company's common stock must maintain a minimum bid closing price of at least $1.00 per share for a minimum of ten consecutive business days. If we do not regain compliance by May 31, 2016, we may transfer to The NASDAQ Capital Market in order to receive an additional 180-day compliance period to comply. In order to be eligible for the transfer and additional time, the Company will be required to meet the continued listing requirement for market value of publicly held shares and all of the initial listing requirements for The NASDAQ Capital Market, other than the minimum bid price requirement, and must notify NASDAQ in writing of its intention to cure the deficiency during the second compliance period. While we intend to engage in efforts to regain compliance, and thus maintain our listing, there can be no assurance that we will be able to regain compliance during the applicable time periods set forth.
32
Our Oncology Candidates
We have a clinical-stage drug candidate in oncology (ganetespib) and a novel, proprietary small molecule cancer drug development program (the HDC program).
Ganetespib (Hsp90 Inhibitor)
Ganetespib is a novel, potent, small molecule inhibitor of Hsp90, a molecular chaperone which is required for the proper folding and activation of many cancer-promoting proteins. Inhibition of Hsp90 by ganetespib leads to the simultaneous degradation of many of these client proteins and the subsequent death or cell cycle arrest of cancer cells dependent on those proteins. A number of Hsp90 client proteins are also involved in the resistance of cancer cells to other anti-cancer treatments, such as chemotherapy. The ability to reduce cancer-cell drug resistance suggests that the combination of ganetespib with chemotherapies or other anti-cancer agents may provide greater benefit than those agents administered alone. In preclinical studies, ganetespib has shown potent anti-cancer activity against a broad range of solid and hematologic cancers, both as a monotherapy and in combination with a variety of anti-cancer treatment approaches including chemotherapy, radiation, targeted therapy and immunotherapy.
Ongoing Ganetespib Clinical Trials
We expect to continue to support the clinical trials in ovarian cancer and sarcoma described below by providing ganetespib drug supply and required safety and regulatory oversight until each of these respective studies conclude.
GANNET53 Trial—Ganetespib in ovarian cancer
GANNET53, a Seventh Framework Programme (FP7) research project funded by the European Commission, is a pan-European randomized trial designed to evaluate the combination of ganetespib and paclitaxel vs. paclitaxel alone in over 200 patients with metastatic, predominantly p53 mutant, platinum-resistant ovarian cancer. Preclinical models have shown that mutant p53 is critical to the growth and proliferation of these cancers. Many mutations render p53 unable to fold appropriately, leaving the protein highly dependent on Hsp90 for stability. Inhibition of Hsp90 destroys the complex between Hsp90 and mutant p53, leading to the degradation of the protein and cancer cell death. We believe that mutated p53 may to serve as a predictive biomarker for Hsp90 inhibitors such as ganetespib.
Hsp90 inhibition has also been shown to sensitize mutant p53 cancer cells to treatment with chemotherapies, as has been seen in preclinical studies evaluating ganetespib in other tumor types, supporting the planned trial design evaluating the combination of ganetespib and paclitaxel vs. paclitaxel alone.
Enrollment of the safety lead-in Phase 1 portion of GANNET53 in centers in Austria, Belgium, France, and Germany began in July 2014 and is now complete. Initial results from the Phase 1 portion presented in June 2015 at the American Society of Clinical Oncology (ASCO) Annual Meeting demonstrated the feasibility and tolerability of combining ganetespib and paclitaxel in this treatment setting. In June 2015, we announced that the first patient was enrolled into the randomized Phase 2 portion of the trial.
We expect completion of enrollment in this trial to occur in 2017; however, as GANNET53 is an investigator-sponsored trial, we do not ultimately control the enrollment timeline for the study.
33
SARC 023—Ganetespib in Sarcoma
SARC 023, sponsored by the Sarcoma Alliance for Research through Collaboration (SARC), is an open label Phase 1/2 trial of ganetespib in combination with the mTOR inhibitor sirolimus in patients with refractory sarcoma, including malignant peripheral nerve sheath tumors (MPNSTs). The Pediatric Subcommittee of ODAC reviewed the design of SARC 023, as well as pre-clinical data demonstrating the scientific rationale for studying this combination in a clinical trial. The Phase 1 portion of the study, which is currently ongoing, is designed to assess the safety, tolerability, and maximum tolerated/recommended dose of the combination.
We expect completion of enrollment in this trial to occur in 2017; however, as SARC 023 is an investigator-sponsored trial, we do not ultimately control the enrollment timeline for the study.
Other Ganetespib Clinical Trials
In light of recent corporate developments, we have elected to no longer support other investigator-sponsored clinical trials with the exception of ensuring drug supply of ganetespib to currently enrolled patients deemed to be receiving medical benefit and providing required safety and regulatory monitoring. Our expectation is that no additional patients will be enrolled on ganetespib containing treatment arms of clinical studies other than the ovarian cancer and sarcoma trials described above. Our intent is to wind down the ganetespib containing arms in all other remaining investigator-sponsored trials by mid-2016.
Included in these studies are the AML-LI (Less Intensive)-1 and AML-18 trials evaluating ganetespib in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS), and the I-SPY 2 TRIAL (Investigation of Serial Studies to Predict Your Therapeutic Response with Imaging And moLecular Analysis 2) evaluating ganetespib in women with newly diagnosed, locally advanced breast cancer.
HDC Program
In September 2013, we announced the launch of a novel, proprietary small molecule cancer drug development program: the HDC program.
HDCs are drug candidates consisting of an Hsp90 inhibitor (targeting moiety) joined to an anti-cancer agent (payload) via a cleavable chemical linker optimized for controlled release of payload drug inside cancer cells. HDCs are small molecules that do not rely on cell surface antigens for targeting and internalization for cellular uptake. Upon cell entry, typically via small molecule uptake (passive diffusion and possibly active transporter), HDCs can bind intracellular Hsp90 that is present in significant amounts in a wide range of cancers.
Upon systemic administration HDCs have the potential to achieve significantly higher concentrations of active anticancer drugs (payloads) in tumors than the concentrations achieved when such anticancer drugs are given in their original, unconjugated form. It is important to note that such high concentrations are sustained over prolonged periods of time, thus significantly increasing the exposure of tumors to the anticancer drug relative to the exposure that can be achieved when such anticancer drugs are given in their original, unconjugated form.
In October 2013, we announced the publication of the first key patent application covering our proprietary HDC technology, which includes composition of matter claims covering HDC compounds, methods for identifying therapeutically effective compounds, and methods of use against a wide range of diseases and conditions. Any resulting patent, if issued, would expire no earlier than 2034.
34
Our lead drug candidate from our HDC Program is STA-12-8666, a conjugate of an Hsp90 inhibitor bound to SN-38, the highly potent active metabolite of the widely used chemotherapy irinotecan.
As part of our strategic overview and in light of recent corporate developments, however, we have decided not pursue an IND submission for STA-12-8666 at this time.
Other Candidates
Elesclomol (Mitochondria-Targeting Agent)
In January 2016, we entered into an asset purchase agreement with a third party to further develop our drug candidate, elesclomol. We will no longer be performing research activities on this drug candidate and, as part of the arrangement, we will receive a minority interest and Board representation in the third party, payments based on achievement of certain development milestones and product royalties upon commercialization.
CRACM Ion Channel Inhibitors
In May 2014, we entered into a license arrangement for our CRACM program, including two lead candidates and the associated intellectual property portfolio, with PRCL Research Inc. (PRCL), a company funded by TVM Life Science Venture VII and the Fonds de Solidarité des Travailleurs du Québec, based in Montreal, Canada. PRCL's plans were to develop one of the two lead candidates licensed from us to proof-of-concept. We have recently been informed that PRCL has selected one of these candidates to move forward into IND enabling studies.
We hold a minority interest in PRCL and a seat on PRCL's Board of Directors. We are not required to provide any research funding or capital contributions to PRCL, and we are not required to perform any research activities related to these candidates. We are reimbursed by PRCL for intellectual property management costs in connection with the contributed intellectual property. If and when proof-of-concept is reached with either drug candidate, Eli Lilly and Company, which is an investor in TVM, will help manage the development program through one of its divisions and will have an option to acquire PRCL or its assets at the then fair value.
Financial Operations Overview
Revenue
We have not yet generated any product revenue and may never do so. Our revenues to date have been generated primarily through our former collaboration and license agreements. The terms of these agreements included payment to us of upfront license fees, milestone payments, research and development cost sharing and royalties. We may seek to generate revenue from product sales and from future collaborative or strategic relationships. In the future, we expect any revenue we may generate will fluctuate from quarter-to-quarter as a result of the timing and amount of payments received and expenses incurred under future collaborations or strategic relationships, if consummated, and the amount and timing of payments we may receive upon the sale of our drug candidates, to the extent any are successfully commercialized.
Research and Development
Research and development expense consists of costs incurred in connection with developing and advancing our drug discovery technology and identifying and developing our drug candidates. We recognize research and development expenses as they are incurred.
35
Our research and development expenses have consisted primarily of:
- •
- internal costs associated with research, preclinical and clinical activities;
- •
- payments to third party contract research organizations, investigative sites and consultants in connection with our preclinical and
clinical development programs;
- •
- costs associated with drug formulation and supply of drugs for clinical trials;
- •
- personnel related expenses, including salaries, bonuses, stock-based compensation, benefits and travel; and
- •
- overhead expenses, including rent and maintenance of our facilities, and laboratory and other supplies.
We do not know if we will be successful in developing any of our drug candidates. Because we may seek to out-license or sell our drug candidates to one or more parties, we cannot forecast with any degree of certainty whether any of our drug candidates will be subject to future development or the timelines or capital requirements related to any such arrangement. Because of this uncertainty, the numerous risks and uncertainties related to drug development, clinical trials, regulatory requirements and product manufacturing, and the stage of development of our drug candidates, we are unable to accurately project total program-specific expenses through commercialization. The duration and cost of clinical trials may vary significantly over the life of a project as a result of unanticipated events arising during clinical development, including with respect to:
- •
- the number of clinical sites included in the trial;
- •
- the length of time required to enroll suitable subjects;
- •
- the number of subjects that ultimately participate in the trials; and
- •
- the efficacy and safety results of our clinical trials and the number of additional required clinical trials.
Our expenditures are subject to additional uncertainties, including the terms and timing of regulatory approvals. In addition, we may obtain unexpected or unfavorable results from future clinical trials. We may elect to discontinue, delay or modify clinical trials of some drug candidates or focus on others. A change in the outcome of any of the foregoing variables in the development of a drug candidate could mean a significant change in the costs and timing associated with the development of that drug candidate. For example, if the FDA or other regulatory authority were to require us to conduct clinical trials beyond those that we anticipate, or if we experience significant delays in any of our clinical trials, we would be required to expend significant additional financial resources and time on the completion of clinical development. Additionally, future commercial and regulatory factors beyond our control would evolve and therefore impact any potential future clinical development programs and plans over time.
We anticipate that overall research and development costs will decrease significantly for the foreseeable future as compared to prior periods due to the termination of the GALAXY-2 trial for futility, restructurings in the fourth quarter of 2015 and the first quarter of 2016, and the discontinuation of a substantial portion of our research and development activities for cash conservation purposes.
General and Administrative
General and administrative expense consists primarily of salaries, bonuses and related expenses for personnel in executive, finance, business and commercial development, investor and medical community relations, human resources and administrative functions. Other costs include stock-based compensation
36
costs, directors' and officers' liability insurance premiums, legal costs of pursuing patent protection of our intellectual property, fees for general legal, accounting, public-company requirements and compliance, and other professional services, as well as overhead-related costs not otherwise included in research and development.
We expect that general and administrative expense will increase in 2016 as compared to prior periods due to the re-allocation of facilities overhead costs that would otherwise be charged to research and development expense (until our facilities leases expire in the fourth quarter of 2016). This increase will be partially offset by lower personnel-related costs and stock compensation due to the restructurings in the fourth quarter of 2015 and the first quarter of 2016. In connection with the restructuring in February 2016, we discontinued a substantial portion of our research and development activities for cash conservation purposes.
Critical Accounting Policies and Estimates
Our management's discussion and analysis of our financial condition and results of operations are based on our financial statements which have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reported periods. We are required to make estimates and judgments with respect to contract research accruals, the recoverability of long-lived assets and measurement of stock-based compensation. We base our estimates on historical experience, known trends and events, and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources and the reported amounts of revenues and expenses. Actual results may differ from these estimates under different assumptions or conditions.
Revenue Recognition
Collaboration and License Agreements
Our principal source of revenue to date has been our former collaboration and license agreements, which included upfront license payments, development milestones, reimbursement of research and development costs, potential profit sharing payments, commercial and sales-based milestones and royalties. The accounting for collaboration and license agreements requires subjective analysis and requires management to make estimates and assumptions about whether deliverables within multiple-element arrangements are separable from the other aspects of the contractual arrangement into separate units of accounting and to determine the arrangement consideration to be allocated to each unit of accounting.
For multiple-element arrangements entered into or materially modified after January 1, 2011, we follow the provisions of Financial Accounting Standards Board (FASB) Accounting Standards Update (ASU) No. 2009-13—Multiple-deliverable Revenue Arrangements (ASU No. 2009-13). ASU No. 2009-13 amended certain provisions of Accounting Standards Codification (ASC) Topic 605—Revenue Recognition. This standard addresses the determination of the unit(s) of accounting for multiple-element arrangements and how an arrangement's consideration should be allocated to each unit of accounting.
Pursuant to this standard, each required deliverable is evaluated to determine if it qualifies as a separate unit of accounting. For us this determination includes an assessment as to whether the deliverable has "stand-alone value" to the customer separate from the undelivered elements. The arrangement's consideration is then allocated to each separate unit of accounting based on the relative selling price of each deliverable. The estimated selling price of each deliverable is determined using the
37
following hierarchy of values: (i) vendor-specific objective evidence of fair value, (ii) third-party evidence of selling price, or (iii) our best estimate of the selling price (BESP). The BESP reflects our best estimate of what the selling price would be if the deliverable was regularly sold by us on a stand-alone basis. We expect, in general, to use BESP for allocating consideration to each deliverable in future collaboration agreements. In general, the consideration allocated to each unit of accounting is then recognized as the related goods or services are delivered limited to the consideration not contingent upon future deliverables. We did not recognize any revenue related to collaboration and license agreements during the years ended December 31, 2015, 2014 and 2013.
We account for development milestones under collaboration and license agreements pursuant to ASU No. 2010-17 Milestone Method of Revenue Recognition (ASU No. 2010-17). ASU No. 2010-17 codified a method of revenue recognition that has been common practice. Under this method, contingent consideration from research and development activities that is earned upon the achievement of a substantive milestone is recognized in its entirety in the period in which the milestone is achieved. At the inception of each arrangement that includes milestone payments, we evaluate whether each milestone is substantive. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity's performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity's performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. We evaluate factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment. We do not have any ongoing collaboration and license agreements under which milestones may be achieved.
Royalty revenues are based upon a percentage of net sales. Royalties from the sales of products will be recorded on the accrual basis when results are reliably measurable, collectability is reasonably assured and all other revenue recognition criteria are met. Commercial and sales-based milestones, which are based upon the achievement of certain agreed-upon sales thresholds, will be recognized in the period in which the respective sales threshold is achieved and collectability is reasonably assured. We do not have any ongoing collaboration and license agreements under which royalties or commercial and sales-based milestones may be achieved.
Accrued Expenses and Accrued Contract Research Liabilities
As part of the process of preparing our financial statements, we are required to estimate accrued expenses. This process involves identifying services which have been performed on our behalf, and estimating the level of service performed and the associated cost incurred for such service as of each balance sheet date in our financial statements. Given our current business, the primary area of uncertainty concerning accruals which could have a material effect on our operating results is with respect to service fees paid to contract manufacturers in conjunction with the production of clinical drug supplies and to contract research organizations in connection with our preclinical studies and clinical trials. In connection with all of the foregoing service fees, our estimates are most affected by our understanding of the status and timing of services provided. The majority of our service providers, including contract research organizations, invoice us in arrears for services performed. In the event that we do not identify some costs which have begun to be incurred, or we under or over estimate the level of services performed or the costs of such services in a given period, our reported expenses for such period would be understated or overstated. We currently reflect the effects of any changes in estimates based on changes in facts and circumstances directly in our operations in the period such change becomes known. During the fourth quarter of 2015, we recorded a net reduction in accrued contract
38
research costs of approximately $2.9 million, principally as a result of the termination of the GALAXY-2 trial.
Our arrangements with contract research organizations in connection with clinical trials often provide for payment prior to commencing the project or based upon predetermined milestones throughout the period during which services are expected to be performed. We recognize expense relating to these arrangements based on the various services provided over the estimated time to completion. The date on which services commence, the level of services performed on or before a given date, and the cost of such services are often determined based on subjective judgments. We make these judgments based upon the facts and circumstances known to us based on the terms of the contract and our ongoing monitoring of service performance. During the years ended December 31, 2015, 2014 and 2013, we had arrangements with multiple contract research organizations whereby these organizations commit to performing services for us over multiple reporting periods. We recognize the expenses associated with these arrangements based on our expectation of the timing of the performance of components under these arrangements by these organizations. Generally, these components consist of the costs of setting up the trial, monitoring the trial, closing the trial and preparing the resulting data. Costs related to patient enrollment in clinical trials are accrued as patients are enrolled in the trial.
With respect to financial reporting periods presented in this Annual Report on Form 10-K, and based on our receipt of invoices from our third party providers, the timing of our actual costs incurred have not differed materially from our estimated timing of such costs. In light of the foregoing, we do not believe our practices for estimating future expenses and making judgments concerning the accrual of expenses are reasonably likely to change in the future. Except for the change in estimate recorded during the fourth quarter of 2015, there were no changes in our estimates and accruals for contract service fees that had a material net effect on our results of operations for the years ended December 31, 2015, 2014 and 2013, respectively.
Stock-Based Compensation
We recognize stock-based compensation expense based on the grant date fair value of stock options granted to employees, officers and directors. We use the Black- Scholes option pricing model to determine the grant date fair value as it is the most appropriate valuation method for our option grants. The Black-Scholes model requires inputs for risk-free interest rate, dividend yield, volatility and expected lives of the options. Expected volatility is based upon the weighted average historical volatility data of our common stock. The risk-free rate for periods within the expected life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant. The expected lives for options granted represent the period of time that options granted are expected to be outstanding. We use the simplified method for determining the expected lives of options. We estimate the forfeiture rate based on historical data. This analysis is re-evaluated at least annually and the forfeiture rate is adjusted as necessary.
For awards with graded vesting, we recognize compensation costs based on the grant date fair value of the award on a straight-line basis over the requisite service period, which is generally the vesting period.
Our net loss included stock-based compensation costs in the amount of $4.3 million, $7.4 million and $6.0 million for the years ended December 31, 2015, 2014 and 2013, respectively, and no income tax benefit related to our stock-based compensation arrangements for employee and non-employee awards. As of December 31, 2015, the total amount of unrecognized stock-based compensation expense was $7.0 million, which will be recognized over a weighted average period of 2.8 years.
39
Consolidated Results of Operations
Years Ended December 31, 2015, 2014 and 2013
Revenues
There were no revenues in each of 2015, 2014 and 2013.
Research and Development Expense
| |
Years Ended December 31, |
2015 / 2014 Comparison |
2014 / 2013 Comparison |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | $ | % | $ | % | |||||||||||||||
| |
(dollars in millions) |
|||||||||||||||||||||
Ganetespib |
$ | 43.3 | $ | 56.8 | $ | 64.5 | $ | (13.5 | ) | (24 | )% | $ | (7.7 | ) | (12 | )% | ||||||
STA-12-8666 |
6.5 | — | — | 6.5 | — | % | — | — | % | |||||||||||||
Elesclomol |
0.2 | 0.5 | 0.1 | (0.3 | ) | (60 | )% | 0.4 | 400 | % | ||||||||||||
CRACM |
0.1 | 0.3 | 0.9 | (0.2 | ) | (67 | )% | (0.6 | ) | (67 | )% | |||||||||||
Other early stage programs |
4.1 | 10.6 | 6.4 | (6.5 | ) | (61 | )% | 4.2 | 66 | % | ||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Total research and development |
$ | 54.2 | $ | 68.2 | $ | 71.9 | $ | (14.0 | ) | (21 | )% | $ | (3.7 | ) | (5 | )% | ||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Ganetespib
In 2015 as compared to 2014, costs incurred under our ganetespib program decreased by $13.5 million, including decreases of $3.4 million in personnel-related costs, related research supplies, operational and stock compensation resulting from a lower level of FTEs, and $10.1 million in external costs. Decreases in external costs principally resulted from lower costs incurred in 2015 in connection with the early termination of the GALAXY-2 trial in October 2015 for futility, and the close-outs of the GALAXY-1 trial, the ENCHANT-1 trial and other company-sponsored trials, as well as costs that were incurred in the first quarter of 2014 for validation manufacturing that were not incurred in 2015. These lower costs were partially offset by higher costs incurred in 2015 related to completion of enrollment in the I-SPY-2 trial that commenced in October 2014. We recorded a $2.9 million net reduction in accrued contract research costs during the fourth quarter of 2015, principally as a result of the termination of the GALAXY-2 trial. We anticipate that overall research and development costs in support of the ganetespib program will decrease significantly for the foreseeable future as compared to prior periods due to the termination of the GALAXY-2 trial for futility, restructurings in the fourth quarter of 2015 and the first quarter of 2016, and the discontinuation of a substantial portion of our research and development activities for cash conservation purposes.
In 2014 as compared to 2013, costs incurred under our ganetespib program decreased by $7.7 million, including decreases of $3.6 million in personnel-related costs, related research supplies, operational overhead and stock compensation, and $4.1 million in net decreases for external costs. Internal costs decreased principally due to available resources being allocated to our HDC program. External costs overall decreased due to lower costs incurred in 2014 related to the wind-down of the GALAXY-1 trial, the ENCHANT-1 trial and other company-sponsored trials, offset by the increase resulting from the advancement of the GALAXY-2 trial that commenced enrollment in April 2013 and the initiation of the I-SPY 2 breast cancer trial in October 2014. In addition, costs were incurred in 2013 for the conduct of NDA-supporting clinical pharmacology studies that were not incurred in 2014.
STA-12-8666
In 2015 as compared to 2014, costs incurred under our STA-12-8666 program increased by $6.5 million, including increases of $3.7 million for personnel-related costs, related research supplies, operational overhead and stock compensation, and $2.8 million for external costs. In the first quarter of
40
2015, we commenced pre-clinical development of our lead HDC candidate, STA-12-8666. Prior to commencing pre-clinical development in 2015, costs incurred under our STA-12-8666 program were reported under our early-stage programs. We anticipate that overall research and development costs in support of the STA-12-8666 program will decrease significantly for the foreseeable future as compared to prior periods due to the restructurings in the fourth quarter of 2015 and the first quarter of 2016, the discontinuation of a substantial portion of our research and development activities for cash conservation purposes and our decision not to pursue submitting an IND submission for STA-12-8666 in the immediate future.
Elesclomol
In 2015 as compared to 2014, costs incurred under our elesclomol program decreased by $0.3 million including decreases of $0.1 million for personnel-related costs, related research supplies, operational overhead and stock compensation, and $0.2 million for external costs.
In 2014 as compared to 2013, costs incurred under our elesclomol program increased by $0.4 million, principally due to increases of $0.1 million in personnel-related costs, related research supplies, operational overhead and stock compensation, and $0.3 million in external costs. These increases were principally related to the pace of the ongoing clinical trial in ovarian cancer.
CRACM
In 2015 as compared to 2014, costs incurred under our CRACM program decreased by $0.2 million in personnel-related costs, related research supplies, operational overhead and stock compensation. In May 2014, we entered into a license arrangement with PRCL under which we may conduct preclinical research activities in the future that would be reimbursed by PRCL.
In 2014 as compared to 2013, costs incurred under our CRACM program decreased by $0.6 million, principally due to decreases of $0.4 million in personnel-related costs, related research supplies, operational overhead and stock compensation, and $0.2 million in external costs. These decreases were the result of a lower investment in the CRACM program as we sought a corporate partner. In May 2014, we entered into a license arrangement with PRCL under which we are not required to perform any research activities.
Early-stage programs
In 2015 as compared to 2014, costs incurred under our early stage programs decreased by $6.5 million, including decreases of $6.2 million in personnel-related costs, related research supplies, operational overhead and stock compensation, and $0.3 million in external costs. In 2015, we advanced our lead HDC candidate, STA-12-8666, into IND-enabling studies and began reporting these costs separately under the STA-12-8666 program. We anticipate that overall research and development costs in support of the HDC discovery program will decrease significantly for the foreseeable future as compared to prior periods due to the restructurings in the fourth quarter of 2015 and the first quarter of 2016, and the discontinuation of a substantial portion of our research and development activities for cash conservation purposes.
In 2014 as compared to 2013, costs incurred under our early stage programs increased by $4.2 million, including increases of $4.1 million in personnel-related costs, related research supplies, operational overhead and stock compensation, and $0.1 million in external costs. These increases were principally the result of our investment in the HDC program that was announced in September 2013.
41
General and Administrative Expense
| |
Years Ended December 31, |
2015 / 2014 Comparison |
2014 / 2013 Comparison |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | $ | % | $ | % | |||||||||||||||
| |
(dollars in millions) |
|||||||||||||||||||||
General and administrative |
$ | 13.4 | $ | 15.7 | $ | 15.7 | $ | (2.3 | ) | (15 | )% | $ | — | — | % | |||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
In 2015 as compared to 2014, general and administrative expenses decreased by $2.3 million, including net decreases of $2.0 million in personnel-related costs, related overhead and stock compensation, and $0.3 million in external professional fees. The $2.0 million net decrease in internal costs was principally in connection with approximately $2.0 million in lower net compensation costs related to management changes in the position of President and Chief Executive Officer. In the first quarter of 2014, Safi Bahcall resigned and we recognized approximately $2.0 million in costs in connection with his separation agreement that were not incurred in 2015, including approximately $1.0 million in cash compensation being paid over two years and approximately $1.0 million in non-cash stock compensation expense related to the accelerated vesting and extended vesting period of certain of his stock options. We expect that general and administrative expense will increase in 2016 as compared to prior periods due to the re-allocation of facilities overhead costs that would otherwise be charged to research and development expense (until our facilities leases expire in the fourth quarter of 2016). This increase will be partially offset by lower personnel-related costs and stock compensation due to the restructurings in the fourth quarter of 2015 and the first quarter of 2016. In connection with the restructuring in February 2016, we discontinued a substantial portion of our research and development activities for cash conservation purposes.
In 2014 as compared to 2013, general and administrative expenses remained at a constant level, including $1.1 million in net decreases in external professional fees principally related to lower patent prosecution costs, offset by an increase of $1.1 million in personnel-related costs, related overhead and stock compensation. The net increase in internal costs was principally related to management changes in the position of President and Chief Executive Officer. In March 2014, Safi Bahcall, our former President and Chief Executive Officer, resigned and we entered into a separation agreement with him. In the first quarter of 2014, we recognized approximately $2.0 million in costs in connection with this separation agreement, including approximately $1.0 million in cash compensation to be paid over two years and approximately $1.0 million in non-cash stock compensation expense related to the accelerated vesting and extended vesting period of certain of his stock options. In August 2014, we announced the hiring of Anne Whitaker, our new President and Chief Executive Officer and entered into an employment contract with her. In the third quarter of 2014, we recognized approximately $0.4 million in related upfront cash compensation for a sign-on bonus and relocation allowance. These increases were partially offset by executive compensation that was incurred in 2013 that was not incurred in 2014 while we conducted a search for a new President and Chief Executive Officer following Safi Bahcall's departure.
Interest Expense, net
| |
Years Ended December 31, |
2015 / 2014 Comparison |
2014/ 2013 Comparison |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | $ | % | $ | % | |||||||||||||||
| |
(dollars in millions) |
|||||||||||||||||||||
Interest expense, net |
$ | 1.1 | $ | 2.2 | $ | 2.6 | $ | (1.1 | ) | (50 | )% | $ | (0.4 | ) | (15 | )% | ||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
In 2015 as compared to 2014, interest expense decreased due to principal payments under the GECC Term Loan and the original three-year $2.0 million loan under the Oxford Term Loan. Interest
42
expense will continue to decrease as a result of scheduled maturities of the GECC Term Loan and the Oxford Term Loan in June 2016 and July 2016, respectively.
In 2014 as compared to 2013, interest expense decreased due to the start of principal payments in January 2014 under the GECC Term Loan and the maturity in April 2014 of the original three-year $2.0 million loan under the Oxford Term Loan.
Liquidity and Capital Resources
Cash Flows
The following table provides information regarding our cash position, cash flows and capital expenditures for the years ended December 31, 2015, 2014 and 2013.
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
| |
(dollars in millions) |
|||||||||
Cash, cash equivalents and marketable securities |
$ | 66.6 | $ | 97.7 | $ | 91.5 | ||||
Working capital |
50.0 | 68.5 | 60.0 | |||||||
Cash flows (used in) provided by: |
||||||||||
Operating activities |
(71.4 | ) | (78.9 | ) | (77.4 | ) | ||||
Investing activities |
20.0 | (8.8 | ) | (24.7 | ) | |||||
Financing activities |
40.4 | 85.3 | 69.0 | |||||||
Capital expenditures (included in investing activities) |
(0.1 | ) | (0.1 | ) | (0.8 | ) | ||||
Our operating activities used cash of $71.4 million, $78.9 million and $77.4 million in 2015, 2014 and 2013, respectively. The use of cash in these periods principally resulted from our losses from operations, as adjusted for non-cash charges for depreciation and stock-based compensation, and changes in our working capital accounts.
In 2015, our investing activities provided cash of $20.0 million, including the sales and maturities of marketable securities in our investment portfolio in the amount of $137.2 million, offset by the purchases of marketable securities in the amount of $117.1 million and purchases of property and equipment in the amount of $0.1 million. In 2014, our investing activities used cash of $8.8 million, including the purchases of marketable securities in the amount of $93.8 million and purchases of property and equipment in the amount of $0.1 million, offset by maturities of marketable securities in our investment portfolio in the amount of $85.1 million. In 2013, our investing activities used cash of $24.7 million, including the purchases of marketable securities of $114.2 million and purchases of property and equipment of $0.8 million, offset by maturities of marketable securities in our investment portfolio in the amount of $90.3 million.
Our financing activities provided cash of $40.4 million, $85.3 million and $69.0 million in 2015, 2014 and 2013, respectively. In 2015, we raised approximately $49.6 million in net cash proceeds, including $41.9 million in net proceeds from the sale of 25,300,000 shares of our common stock in a public offering in April 2015 and $7.7 million in net proceeds from sales of 3,614,511 shares of our common stock under the at-the-market issuance sales agreements with MLV. In 2014, we raised approximately $94.8 million in net cash proceeds, including $89.0 million in net proceeds from sales of 21,692,753 shares of our common stock under at-the-market issuance sales agreements with MLV, $5.0 million in a registered direct offering of 1,250,000 shares of our common stock to an affiliate of a director who is our largest stockholder and $0.8 million from the exercise of common stock options. In 2013, we raised approximately $71.7 million in net cash proceeds, including $57.1 million in net proceeds from the sale of 16,100,000 shares of our common stock in a public offering in November 2013, $13.5 million in gross proceeds from additional funding under the GECC Term Loan and Oxford Term Loan and $1.1 million from the exercise of common stock options. We repaid $9.2 million,
43
$9.5 million and $2.6 million in principal payments in 2015, 2014 and 2013, respectively, in connection with the GECC Term Loan and the Oxford Term Loan. In January 2014, we began making 30 equal monthly payments of principal under the GECC Term Loan. During the period from July 2012 through March 2013, we made 9 equal monthly payments of principal under the GECC Term Loan. For the periods from April 2013 through December 2013 we made interest-only payments.
Contractual Obligations and Commitments
The following tables summarize our contractual obligations at December 31, 2015 and the effects such obligations are expected to have on our liquidity and cash flows in future periods (in millions).
Contractual Obligations (as of December 31, 2015)
|
Total | 2016 | 2017 through 2018 |
2019 through 2020 |
More than 5 years |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Operating and capital lease obligations |
$ | 2.1 | $ | 2.1 | $ | — | $ | — | $ | — | ||||||
GECC and Oxford Term Loans(1) |
5.5 | 5.5 | — | — | — | |||||||||||
Research and development contracts(2)(3) |
8.2 | 8.1 | 0.1 | — | — | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
$ | 15.8 | $ | 15.7 | $ | 0.1 | $ | — | $ | — | ||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Includes
scheduled interest payments and an exit fee of $788,000 due at the time of the final payment of the outstanding principal under the GECC Term Loan.
- (2)
- Research
and development contracts principally include contracts for human clinical studies, animal studies and clinical manufacturing. In the event a study
or manufacturing contract is terminated prior to the planned completion by mutual agreement between the contractor and us, the amount paid under such contracts may be less than the amounts presented.
- (3)
- Includes certain contract amendments entered into after December 31, 2015.
Amounts not included in the table of Contractual Obligations and Commitments
In July 2011, we entered into a co-development agreement with one of our clinical research organizations, or CRO, for the conduct of certain company-sponsored clinical trials. Under the co-development agreement, this CRO was performing clinical research services under a reduced fee structure in exchange for a share of licensing payments and commercial revenues, if any, up to a specified maximum payment, which is defined as a multiple of the fee reduction realized. The maximum amount of the service fee discount was realized in the year ended December 31, 2013.
In accordance with the termination provisions of the Roche Agreement, all rights to the CRACM licensed compounds under the agreement were returned to us. In May 2014, we entered into a license arrangement with PRCL Research, Inc for two lead candidates and the associated intellectual property portfolio. We may pay Roche a low single-digit royalty on any potential future sales of the licensed products.
In accordance with the termination provisions of the GSK Agreement, all rights to the elesclomol program were returned to us. In January 2016, we entered into an asset purchase agreement with a third party to further develop our drug candidate, elesclomol. We may pay GSK a low single-digit royalty on any potential future sales of elesclomol.
44
Under a license agreement, we may be obligated to pay up to an aggregate of $0.5 million if specified development milestones are met, as follows (in millions).
| Milestone | Amount | |||
|---|---|---|---|---|
Development-based milestones related to the conduct of clinical trials |
$ | 0.1 | ||
Development-based milestones related to regulatory submission and approval |
0.4 | |||
| | | | | |
Total |
$ | 0.5 | ||
| | | | | |
| | | | | |
| | | | | |
Public and Registered Direct Offerings
In April 2015, we raised approximately $44.3 million in gross proceeds from the sale of an aggregate 25,300,000 shares of our common stock in a public offering at a public offering price of $1.75 per share, including 3,300,000 shares upon the full exercise of the underwriters' option to purchase additional shares. Certain of our directors and their affiliates, including our largest stockholder, purchased an aggregate of 7,257,142 shares in this offering at the public offering price. The net offering proceeds to us were approximately $41.9 million after deducting underwriters' discounts, fees and commissions, and other offering expenses payable by us.
In April 2014, we sold 1,250,000 shares of our common stock at a purchase price of $4.01 per share in a registered direct offering to an affiliate of a director who is our largest stockholder. These shares were sold directly without a placement agent, underwriter, broker or dealer. The net proceeds to us were approximately $5.0 million after deducting offering expenses payable by the Company.
In November 2013, we raised approximately $60.4 million in gross proceeds from the sale of an aggregate 16,100,000 shares of our common stock in a public offering at a public offering price of $3.75 per share, including 14,000,000 shares in the initial offering and 2,100,000 shares upon the full exercise of the underwriters' option to purchase additional shares. Certain of our directors and their affiliates, including our largest stockholder, purchased an aggregate of 5,183,333 shares in this offering. The net offering proceeds to us were approximately $57.1 million after deducting underwriters' discounts, fees and commissions, and other offering expenses payable by us.
At-The-Market Issuance Sales Agreements
MLV & Co., LLC
We entered into at-the-market issuance sales agreements (May 2012, May 2014 and July 2014 Sales Agreements) with MLV & Co., LLC (MLV), pursuant to which we issued and sold shares of our common stock from time to time, at our option, through MLV as our sales agent. Sales of common stock through MLV were made pursuant to an "at-the-market" offering as defined in Rule 415 promulgated under the Securities Act of 1933, as amended, (the Securities Act). MLV used commercially reasonable efforts to sell the common stock based upon our instructions (including any price, time or size limits or other customary parameters or conditions that we imposed). All shares were sold pursuant to an effective shelf registration statement on Form S-3 (File No. 333-187242). We paid MLV a commission of up to 3% of the gross proceeds. The May 2012 and May 2014 Sales Agreements were terminated by us upon the sale of substantially all stock authorized for sale under each such agreements. In October 2015, we terminated the July 2014 Sales Agreement.
In March and April 2014, we sold an aggregate of 6,588,875 shares of common stock pursuant to the May 2012 Sales Agreement for an aggregate of approximately $28.0 million in gross proceeds at an average selling price of $4.25 per share. Net proceeds to us were approximately $27.3 million after deducting commissions and other transactions costs.
45
From May 2014 through July 2014, we sold an aggregate of 9,424,193 shares of common stock pursuant to the May 2014 Sales Agreement for an aggregate of approximately $40.0 million in gross proceeds at an average selling price of $4.24 per share. Net proceeds to us were approximately $39.2 million after deducting commissions and other transactions costs, including approximately $33.6 million from the sale of 8,060,244 shares in the second quarter of 2014 and approximately $5.6 million from the sale of 1,363,949 shares in July 2014.
In July 2014, we reserved up to $50 million under our shelf registration statement for issuance under the July 2014 Sales Agreement. In the third quarter of 2014, we sold an aggregate of 5,679,685 shares of common stock pursuant to the July 2014 Sales Agreement for an aggregate of approximately $23.0 million in gross proceeds at an average selling price of $4.05 per share. Net proceeds to us were approximately $22.5 million after deducting commissions and other transaction costs.
In the third quarter of 2015, we sold an aggregate of 3,614,511 shares of common stock pursuant to the July 2014 Sales Agreement for an aggregate of approximately $7.9 million in gross proceeds at an average selling price of $2.19 per share. Net proceeds to us were approximately $7.7 million after deducting commissions and other transaction costs.
Cowen and Company, LLC
In October 2015, we entered into an at-the-market issuance sales agreement (October 2015 Sales Agreement), with Cowen and Company, LLC (Cowen), pursuant to which we may issue and sell shares of our common stock, having an aggregate offering price of up to $100 million, from time to time, at our option, through Cowen as our sales agent. Sales of common stock through Cowen may be made by any method that is deemed an "at-the-market" offering as defined in Rule 415 promulgated under the Securities Act of 1933, as amended, including by means of ordinary brokers' transactions at market prices, in block transactions or as otherwise agreed by us and Cowen. Subject to the terms and conditions of the Sales Agreement, Cowen will use commercially reasonable efforts consistent with its normal trading and sales practices to sell the common stock based upon our instructions (including any price, time or size limits or other customary parameters or conditions we may impose). We are not obligated to make any sales of our common stock under the Sales Agreement. Any shares sold will be sold pursuant to an effective shelf registration statement on Form S-3 (file no. 333-206135). We will pay Cowen a commission of up to 3% of the gross proceeds. The October 2015 Sales Agreement may be terminated by us at any time upon 10 days' notice. No shares have been sold to-date under the October 2015 Sales Agreement.
Term Loans
General Electric Capital Corporation (GECC)
In March 2013, we amended our loan and security agreement entered into in September 2010 with GECC and one other lender, or the GECC Term Loan, and obtained $12.9 million in additional loan funding and, as a result, increased the principal balance to $22.5 million at March 31, 2013. Interest on the borrowings under the GECC Term Loan remains at the annual rate of 9.75%. We made interest-only payments for the period from April 2013 through December 2013. In January 2014, we began making 30 equal monthly payments of principal under the GECC Term Loan. During the period from July 2012 through March 2013, we made 9 equal monthly payments of principal under the GECC Term Loan. For the periods from April 2013 through December 2013 and prior to July 2012 we made interest-only payments. As of December 31, 2015, in accordance with the GECC Term Loan, $4.5 million in remaining principal payments is scheduled to be paid by June 2016, at which time we are obligated to pay an exit fee in the amount of $788,000. (See Note 9 of the accompanying consolidated financial statements.)
46
Oxford Finance Corporation (Oxford)
In March 2011, we entered into a loan and security agreement with Oxford and received $2.0 million in loan funding, and in December 2012, we entered into a loan modification agreement, as amended, under which we could elect to draw down up to an additional $0.6 million in equipment financing until June 30, 2013, which we collectively refer to herein as the Oxford Term Loan. As of June 30, 2013, the Company had fully utilized the $0.6 million in additional equipment financing. Interest on the borrowings under the Oxford Term Loan accrues at an annual rate of 13.35%. In May 2011, we began making 36 equal monthly payments of principal plus accrued interest on the initial $2.0 million outstanding balance that was fully paid in April 2014. We continue to make equal monthly payments of principal plus accrued interest on the $0.6 million in additional equipment financing. As of December 31, 2015, in accordance with the Oxford Term Loan, $107,000 in remaining principal payments is scheduled to be paid by July 2016. (See Note 9 of the accompanying condensed consolidated financial statements.)
Liquidity
Funding Requirements
Our future funding requirements will depend on many factors, including, but not limited to:
- •
- the completion of the ongoing investigator-sponsored clinical trials of ganetespib;
- •
- the costs involved in conducting preclinical activities for our ganetespib and HDC programs;
- •
- the costs of preparing, filing, and prosecuting patent applications and maintaining, enforcing and defending intellectual
property-related claims;
- •
- the extent to which we may elect to continue drug development activities in the future, if at all; and
- •
- the timing and completion of any transaction that may result from our ongoing strategy review.
As of December 31, 2015, we had $66.6 million in cash, cash equivalents and marketable securities, a decrease of $31.1 million from $97.7 million as of December 31, 2014. This decrease principally reflects $49.6 million that we raised in net cash proceeds, including $41.9 million in net proceeds from the sale of our common stock in a public offering and $7.7 million in net proceeds from sales of our common stock under the at-the-market issuance sales agreement with MLV, offset by cash used in operations and term loan principal payments as discussed under "Cash Flows" above.
We have not yet generated any product revenue and may never do so. We cannot predict whether and to what extent we may continue drug development activities, if at all, and what our future cash needs may be for any such activities. We expect our $66.6 million in cash resources as of December 31, 2015, along with significantly lower operating expenses following the termination of the GALAXY-2 trial, subsequent restructurings in the fourth quarter of 2015 and the first quarter of 2016, and the discontinuation of a substantial portion of our research and development activities will be sufficient to fund operations for at least the next twelve months. This estimate assumes no additional funding from new partnership agreements, equity financings or further sales under our ATM. The timing and nature of certain activities contemplated for the remainder of 2016 will be conducted subject to the availability of sufficient financial resources. We have an effective shelf registration statement on Form S-3 (File No. 333-206135) under which we have up to $300 million in securities available for future issuance, including up to $100 million in shares of common stock that we have reserved and that may be offered and sold under the October 2015 Sales Agreement with Cowen. However, pursuant to the instructions to Form S-3, we only have the ability to sell shares under the shelf registration statement, during any 12-month period, in an amount less than or equal to one-third of the aggregate market value of our common stock held by non-affiliates.
47
We may require significant additional funds earlier than we currently expect in order to conduct additional clinical trials and conduct additional preclinical and discovery activities. Because of the numerous risks and uncertainties associated with the development and commercialization of our drug candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with future research and development activities.
To the extent our capital resources are insufficient to meet our future operating and capital requirements, we will need to finance our future cash needs through public or private equity offerings, collaboration agreements, debt financings or licensing arrangements. However, additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. For example, if we raise additional funds by issuing equity securities or by selling convertible debt securities, further dilution to our existing stockholders may result. If we raise funds through collaboration agreements or licensing arrangements, we may be required to relinquish rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us.
If adequate funds are not available, we may be required to obtain funds through collaborators that may require us to relinquish rights to our technologies or drug candidates that we might otherwise seek to develop or commercialize independently. Conversely, we may elect to raise additional funds even before we need them if the conditions for raising capital are favorable due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements or relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities.
Tax Loss Carryforwards
For tax years through 2015 we performed analyses to determine if there were changes in ownership, as defined by Section 382 of the Internal Revenue Code that would limit our ability to utilize certain net operating loss and tax credit carryforwards. We determined that we experienced an ownership change, as defined by Section 382, in connection with our acquisition of Principia Associates, Inc. on September 20, 2002, but did not experience a change in ownership upon the effectiveness of our IPO, or any other equity offerings to date. As a result, the utilization of our federal tax net operating loss carryforwards generated prior to the ownership change is limited. As of December 31, 2015, we have net operating loss carryforwards for U.S. federal tax purposes of approximately $578.1 million, after excluding net operating losses that have expired unused as a result of Section 382 limitations, with the remainder expiring in varying amounts through 2035 unless utilized. As of December 31, 2015, we have state net operating loss carryforwards of approximately $321.9 million, which will expire through 2035 unless utilized. The net operating loss carryforwards include approximately $1.4 million of deductions related to the exercise of common stock options. This amount represents an excess tax benefit and has not been included in the gross deferred tax asset reflected for net operating losses. The utilization of these net operating loss carryforwards may be further limited if we experience future ownership changes as defined in Section 382 of the Internal Revenue Code.
Recent Accounting Pronouncements
Refer to Note 2, "Summary of Significant Accounting Policies," in the accompanying notes to the consolidated financial statements for a discussion of recent accounting pronouncements.
48
Certain Factors That May Affect Future Results of Operations
The Securities and Exchange Commission encourages companies to disclose forward- looking information so that investors can better understand a company's future prospects and make informed investment decisions. This Annual Report on Form 10-K contains such "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995.
Words such as "may," "anticipate," "estimate," "expects," "projects," "intends," "plans," "believes" and words and terms of similar substance used in connection with any discussion of future operating or financial performance, identify forward-looking statements. All forward-looking statements are management's present expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those described in the forward-looking statements. These risks include, but are not limited to those set forth under the heading "Risk Factors" contained in Item 1A of this Annual Report on Form 10-K.
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Annual Report on Form 10-K or in any document incorporated by reference might not occur. Stockholders are cautioned not to place undue reliance on the forward-looking statements, which speak only as of the date of this Annual Report on Form 10-K. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward- looking statements attributable to Synta or to any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Interest Rate Sensitivity. As of December 31, 2015, we had cash, cash equivalents and marketable securities of $66.6 million consisting of cash deposited in a highly rated financial institution in the United States and in a short-term U.S. Treasury money market fund, as well as high-grade corporate bonds and commercial paper. The primary objective of our investment activities is to preserve our capital for the purpose of funding operations and we do not enter into investments for trading or speculative purposes. We believe that we do not have material exposure to high-risk investments such as mortgage-backed securities, auction rate securities or other special investment vehicles within our money-market fund investments. We believe that we do not have any material exposure to changes in fair value as a result of changes in interest rates. Declines in interest rates, however, would reduce future investment income.
Capital Market Risk. We currently have no product revenues and depend on funds raised through other sources. One possible source of funding is through further equity offerings. Our ability to raise funds in this manner depends upon capital market forces affecting our stock price.
Item 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this Item 8 is included at the end of this Annual Report on Form 10-K beginning on page F-1.
Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
Not applicable.
49
Item 9A. CONTROLS AND PROCEDURES
1. Disclosure Controls and Procedures
Our principal executive officer and principal financial officer evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(c) and 15d-15(c) under the Securities Exchange Act of 1934, as amended) as of the end of the period covered by this Annual Report on Form 10-K. Based on the evaluation of our disclosure controls and procedures as of December 31, 2015, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC's rules and forms, and is accumulated and communicated to our management, including our principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
2. Internal Control Over Financial Reporting
- (a)
- Management's Annual Report on Internal Control Over Financial Reporting
Management's Annual Report On Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended. Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015. In making this assessment, it used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework (2013 framework). Based on our assessment we believe that, as of December 31, 2015, our internal control over financial reporting is effective at a reasonable assurance level based on those criteria.
Our independent registered public accounting firm has issued its report on the effectiveness of our internal control over financial reporting. This report appears below.
50
- (b)
- Attestation Report of the Registered Public Accounting Firm
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders of
Synta Pharmaceuticals Corp.
We have audited Synta Pharmaceuticals Corp.'s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the COSO criteria). Synta Pharmaceuticals Corp.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Synta Pharmaceuticals Corp. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Synta Pharmaceuticals Corp. as of December 31, 2015 and 2014 and the related consolidated statements of operations, comprehensive loss, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2015 and our report dated March 15, 2016 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston,
Massachusetts
March 15, 2016
51
- (c)
- Changes in Internal Controls Over Financial Reporting
There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during the fourth quarter of our last fiscal year that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Not applicable.
52
Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Management and Corporate Governance," "Section 16(a) Beneficial Ownership Reporting Compliance" and "Code of Conduct and Ethics" in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
We have adopted a code of conduct and ethics that applies to all of our directors, officers and employees. This code is publicly available on our website at www.syntapharma.com. Amendments to the code of conduct and ethics or any grant of a waiver from a provision of the code requiring disclosure under applicable Securities and Exchange Commission and The NASDAQ Stock Market rules will be disclosed in a Current Report on Form 8-K.
Item 11. EXECUTIVE COMPENSATION
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Compensation Discussion and Analysis," "Executive Officer and Director Compensation," "Management and Corporate Governance—Committees of the Board of Directors and Meetings" and "Compensation Committee Report" in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
Item 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Security Ownership of Certain Beneficial Owners and Management" and "Equity Compensation Plan Information" in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The response to this item is incorporated by reference from the discussion responsive thereto under the captions "Certain Relationships and Related Person Transactions," "Management and Corporate Governance—The Board of Directors" and "Management and Corporate Governance—Director Independence" in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
Item 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The response to this item is incorporated by reference from the discussion responsive thereto under the proposal captioned "Independent Registered Public Accounting Firm" in our Proxy Statement for the 2016 Annual Meeting of Stockholders.
53
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | |
Item 15(a) |
The following documents are filed as part of this Annual Report on Form 10-K: |
|
Item 15(a)(1) and (2) |
The Consolidated Financial Statements beginning on page F-1 are filed as part of this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto. |
|
Item 15(a)(3) |
Exhibits |
The following is a list of exhibits filed as part of this Annual Report on Form 10-K.
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3.1 | Restated Certificate of Incorporation of the Registrant. | S-1/A (Exhibit 3.2) |
1/23/07 | 333-138894 | |||||||
3.1.1 |
Certificate of Amendment to the Restated Certificate of Incorporation of Synta Pharmaceuticals Corp. |
8-K (Exhibit 3.1) |
6/17/13 |
001-33277 |
|||||||
3.2 |
Restated Bylaws of the Registrant. |
S-1/A (Exhibit 3.4) |
1/23/07 |
333-138894 |
|||||||
4.1 |
Form of Common Stock Certificate. |
S-1/A (Exhibit 4.1) |
2/5/07 |
333-138894 |
|||||||
4.2.1 |
Amended and Restated Investor Rights Agreement, dated December 13, 2002, by and among the Registrant and certain stockholders of the Registrant. |
S-1/A (Exhibit 4.2.1) |
12/1/06 |
333-138894 |
|||||||
4.2.2 |
First Amendment, dated January 11, 2005, to the Amended and Restated Investor Rights Agreement, dated December 13, 2002, by and among the Registrant and certain stockholders of the Registrant. |
S-1/A (Exhibit 4.2.2) |
12/1/06 |
333-138894 |
|||||||
4.2.3 |
Second Amendment, dated January 31, 2007, to the Amended and Restated Investor Rights Agreement, dated December 13, 2002, by and among the Registrant and certain stockholders of the Registrant. |
S-1/A (Exhibit 4.2.3) |
2/5/07 |
333-138894 |
|||||||
54
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4.2.4 | Third Amendment, dated November 30, 2011, to the Amended and Restated Investor Rights Agreement, dated December 13, 2002, by and among the Registrant and certain stockholders of the Registrant. | 8-K (Exhibit 10.1) |
12/1/11 | 001-33277 | |||||||
Lease Agreements |
|||||||||||
10.1 |
Duffy Hartwell Limited Partnership Commercial Lease, dated November 4, 1996, by and between Duffy Hartwell Limited Partnership and Shionogi BioResearch Corp., as amended by First Amendment to Commercial Lease, dated August 30, 2006. |
S-1/A (Exhibit 10.5) |
12/1/06 |
333-138894 |
|||||||
10.1.1 |
Second Amendment, dated May 27, 2008, to Commercial Lease by and between Duffy Hartwell LLC, as successor in interest to Duffy Hartwell Limited Partnership, and the Registrant, as successor in interest to Shionogi BioResearch Corp., dated November 4, 1996, as amended. |
10-Q (Exhibit 10.1) |
8/7/08 |
001-33277 |
|||||||
10.1.2 |
Third Amendment, dated April 19, 2011, to Commercial Lease by and between Duffy Hartwell LLC, as successor in interest to Duffy Hartwell Limited Partnership, and the Registrant, as successor in interest to Shionogi BioResearch Corp., dated November 4, 1996, as amended. |
8-K (Exhibit 10.1) |
4/22/11 |
001-33277 |
|||||||
10.2 |
Lease Agreement, dated as of June 9, 2011, by and between the Registrant and 125 Hartwell Trust. |
10-Q (Exhibit 10.3) |
8/4/11 |
001-33277 |
|||||||
55
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.3 | Pinnacle Properties Management, Inc. Standard Form Commercial Lease, dated May 31, 1999, by and between 6-8 Preston Court, L.L.C. and Asiana Pharmaceuticals Corporation, as amended by Amendment to Lease #1, dated July 31, 2000, Amendment to Lease #2, dated November 26, 2001, and Amendment to Lease #3, dated December 2003, and as assigned to the Registrant by Assignment and Assumption of Lease and Landlord's Consent, dated May 25, 2005, and Subordination, Non-Disturbance and Attornment Agreement, dated May 25, 2005. | S-1/A (Exhibit 10.8) |
12/1/06 | 333-138894 | |||||||
10.4 |
Lease Agreement, dated December 14, 2006, by and between ARE-MA Region No. 24, LLC and the Registrant. |
S-1/A (Exhibit 10.27) |
1/4/07 |
333-138894 |
|||||||
10.4.1 |
First Amendment, dated as of June 23, 2011, to Lease Agreement, dated December 14, 2006, by and between ARE-MA Region No. 24, LLC and the Registrant. |
10-Q (Exhibit 10.4) |
8/4/11 |
001-33277 |
|||||||
Credit Facilities, Loan and Equity Agreements |
|||||||||||
10.5 |
Common Stock Purchase Agreement, dated October 4, 2010, by and between the Registrant and Azimuth Opportunity Ltd. |
8-K (Exhibit 10.1) |
10/5/10 |
001-33277 |
|||||||
10.5.1 |
Amendment No. 1, dated August 19, 2011, to Common Stock Purchase Agreement, dated October 4, 2010, by and between Synta Pharmaceuticals Corp. and Azimuth Opportunity Ltd. |
8-K (Exhibit 10.1) |
8/19/11 |
001-33277 |
|||||||
10.6 |
Loan and Security Agreement, dated as of September 30, 2010, by and among the Registrant, Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
8-K (Exhibit 10.1.1) |
10/5/10 |
001-33277 |
|||||||
56
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.6.1 | First Amendment, dated as of November 9, 2010, to Loan and Security Agreement, dated as of September 30, 2010, by and among the Registrant, Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. | 10-K (Exhibit 10.11) |
3/11/11 | 001-33277 | |||||||
10.6.2 |
Second Amendment, dated as of March 3, 2011, to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among the Registrant, Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
10-Q (Exhibit 10.2) |
5/5/11 |
001-33277 |
|||||||
10.6.3 |
Third Amendment, dated as of July 1, 2011, to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among the Registrant, Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
10-Q (Exhibit 10.5) |
8/4/11 |
001-33277 |
|||||||
10.6.4 |
Fourth Amendment, dated as of January 23, 2012, to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among the Registrant, Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
10-K (Exhibit 10.6.4) |
2/22/11 |
001-33277 |
|||||||
10.6.5 |
Fifth Amendment, dated as of July 30, 2012, to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among the Registrant, Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
10-Q (Exhibit 10.2) |
8/2/12 |
001-33277 |
|||||||
57
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.6.6 | Sixth Amendment, dated as of December 6, 2012, to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among the Registrant, Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. | 10-K (Exhibit 10.6.6) |
3/14/13 | 001-33277 | |||||||
10.6.7 |
Seventh Amendment, dated as of December 14, 2012, to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among Synta Pharmaceuticals Corp., Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
8-K (Exhibit 10.1) |
12/20/12 |
001-33277 |
|||||||
10.6.8 |
Eighth Amendment to Loan and Security Agreement dated as of March 28, 2013 by and among the Company, Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
8-K (Exhibit 10.1.1) |
4/1/13 |
001-33277 |
|||||||
10.6.9 |
Ninth Amendment, dated as of November 25, 2013 to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among Synta Pharmaceuticals Corp., Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
8-K (Exhibit 10.1) |
12/2/13 |
001-33277 |
|||||||
10.6.10 |
Tenth Amendment, dated as of July 17, 2014 to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among Synta Pharmaceuticals Corp., Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. |
8-K (Exhibit 10.2) |
7/18/14 |
001-33277 |
|||||||
58
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.6.11 | Eleventh Amendment, dated as of July 10, 2015 to Loan and Security Agreement, dated as of September 30, 2010, as amended, by and among Synta Pharmaceuticals Corp., Synta Securities Corp., General Electric Capital Corporation, and MidCap Funding III, LLC. | 10-Q (Exhibit 10.1) |
11/5/15 | 001-33277 | |||||||
10.7 |
Amended and Restated Promissory Note issued by the Registrant to General Electric Capital Corporation. |
8-K (Exhibit 10.1.2) |
4/1/13 |
001-33277 |
|||||||
10.8 |
Promissory Note issued by the Registrant to MidCap Funding III, LLC. |
8-K (Exhibit 10.1.3) |
10/5/10 |
001/33277 |
|||||||
10.8.1 |
Amended and Restated Promissory Note issued by the Registrant to MidCap Funding III, LLC. |
8-K (Exhibit 10.1.3) |
4/1/13 |
001-33277 |
|||||||
10.9 |
Guaranty, dated as of September 30, 2010, by and among Synta Securities Corp. and General Electric Capital Corporation. |
8-K (Exhibit 10.1.4) |
10/5/10 |
001-33277 |
|||||||
10.10 |
Pledge Agreement, dated as of September 30, 2010, by and among the Registrant, Synta Securities Corp., and General Electric Capital Corporation. |
8-K (Exhibit 10.1.5) |
10/5/10 |
001-33277 |
|||||||
10.11 |
Form of Subscription Agreement, dated July 25, 2012, by and between the Registrant and each of the Purchasers participating in the Registrant's July Registered Direct Offering. |
8-K (Exhibit 10.1) |
7/26/12 |
001-33277 |
|||||||
10.12 |
Letter Agreement, dated May 7, 2014, by and between the Registrant and MLV & Co. LLC, terminating the At the Market Issuance Sales Agreement dated as of May 2, 2012. |
10-Q (Exhibit 10.4) |
5/8/14 |
001-33277 |
|||||||
10.13 |
Subscription Agreement, dated April 11, 2014, by and between the Registrant and KFO Holdings LLC. |
8-K (Exhibit 10.1) |
4/14/14 |
001-33277 |
|||||||
59
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.14 | New At-the-Market Issuance Sales Agreement, dated May 7, 2014, by and between the Registrant and MLV & Co. LLC. | 10-Q (Exhibit 10.3) |
5/8/14 | 001-33277 | |||||||
10.15 |
At the Market Issuance Sales Agreement, dated July 18, 2014, by and between the Registrant and MLV & Co. LLC. |
8-K (Exhibit 10.1) |
7/18/14 |
001-33277 |
|||||||
10.16 |
Sales Agreement, dated October 16, 2015, by and between the Registrant and Cowen and Company, LLC. |
8-K (Exhibit 10.1) |
10/16/2015 |
001-33277 |
|||||||
Agreements with Respect to Collaborations, Licenses, Research and Development |
|||||||||||
†10.17 |
Collaborative Development, Commercialization and License Agreement, dated October 8, 2007, by and between the Registrant and GlaxoSmithKline. |
10-K (Exhibit 10.24) |
3/20/08 |
001-33277 |
|||||||
†10.17.1 |
Amendment No. 1, dated June 27, 2008, to Collaborative Development, Commercialization and License Agreement, dated October 8, 2007, by and between the Registrant and GlaxoSmithKline. |
10-Q (Exhibit 10.4) |
8/7/08 |
001-33277 |
|||||||
Equity Compensation Plans |
|||||||||||
*10.18 |
2001 Stock Plan. |
S-1/A (Exhibit 10.1) |
12/1/06 |
333-138894 |
|||||||
*10.19 |
Amended and Restated 2006 Stock Plan. |
8-K (Exhibit 10.1) |
6/21/10 |
001-33277 |
|||||||
*10.20 |
Form of incentive stock option agreement under 2006 Stock Plan. |
S-1/A (Exhibit 10.2(a)) |
1/23/07 |
333-138894 |
|||||||
*10.21 |
Form of nonqualified stock option agreement under 2006 Stock Plan. |
S-1/A (Exhibit 10.2(b)) |
1/23/07 |
333-138894 |
|||||||
*10.22 |
Form of restricted stock agreement under 2006 Stock Plan. |
S-1/A (Exhibit 10.2(c)) |
1/23/07 |
333-138894 |
|||||||
*10.23 |
Form of nonqualified stock option agreement for directors under 2006 Stock Plan. |
S-1/A (Exhibit 10.2(d)) |
1/23/07 |
333-138894 |
|||||||
60
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| *10.24 | Form of restricted stock agreement for directors under 2006 Stock Plan. | S-1/A (Exhibit 10.2(e)) |
1/23/07 | 333-138894 | |||||||
*10.25 |
2015 Stock Plan. |
8-K (Exhibit 10.1) |
6/15/15 |
001-33277 |
|||||||
Agreements with Executive Officers and Directors |
|||||||||||
*10.26 |
Amended and Restated Director Compensation Policy, effective June 5, 2015. |
10-Q (Exhibit 10.1) |
8/6/15 |
001-33277 |
|||||||
*10.27 |
Non-Qualified Stock Option Agreement, dated February 27, 2008, by and between the Registrant and Keith R. Gollust. |
10-K (Exhibit 10.4) |
3/20/08 |
001-33277 |
|||||||
*10.28 |
Letter Agreement, dated January 14, 2003, by and between the Registrant and Wendy E. Rieder. |
S-1/A (Exhibit 10.18) |
12/1/06 |
333-138894 |
|||||||
*10.29 |
Letter Agreement, dated December 9, 2008, by and between the Registrant and Vojo Vukovic. |
10-K (Exhibit 10.29) |
3/11/10 |
001-33277 |
|||||||
*10.30 |
Executive Employment Agreement, dated August 1, 2014, between Synta Pharmaceuticals Corp. and Anne C. Whitaker. |
8-K (Exhibit 10.1) |
8/6/14 |
001-33277 |
|||||||
*10.31 |
Letter Agreement, dated December 3, 2014, between Synta Pharmaceuticals Corp. and Chen Schor. |
8-K (Exhibit 10.1) |
12/4/14 |
001-33277 |
|||||||
*10.32 |
Offer Letter Addendum, dated as of June 9, 2015, by and between the Registrant and Chen Schor. |
10-Q (Exhibit 10.3) |
8/6/15 |
001-33277 |
|||||||
*10.33 |
Letter Agreement, dated November 24, 2014, between Synta Pharmaceuticals Corp. and Marc R. Schneebaum |
8-K (Exhibit 10.3) |
12/4/14 |
001-33277 |
|||||||
*10.34 |
Separation Agreement between the Company and Dr. Bahcall, dated March 19, 2014. |
8-K (Exhibit 10.1) |
3/20/14 |
001-33277 |
|||||||
*10.35 |
Form of Severance and Change in Control Agreement between the Registrant and Vojo Vukovic. |
10-K (Exhibit 10.30) |
3/11/10 |
001-33277 |
|||||||
61
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| *10.36 | Form of Severance and Change in Control Agreement between the Registrant and each of Keith S. Ehrlich and Wendy E. Rieder. | 10-K (Exhibit 10.31) |
3/11/10 | 001-33277 | |||||||
*10.37 |
Severance and Change of Control Agreement, dated December 3, 2014, between Synta Pharmaceuticals Corp. and Chen Schor. |
8-K (Exhibit 10.2) |
12/4/14 |
001-33277 |
|||||||
*10.38 |
Severance and Change of Control Agreement, dated November 24, 2014, between Synta Pharmaceuticals Corp. and Marc R. Schneebaum. |
8-K (Exhibit 10.4) |
12/4/14 |
001-33277 |
|||||||
10.39 |
Form of Indemnification Agreement between the Registrant and its directors and executive officers. |
S-1/A (Exhibit 10.26) |
12/1/06 |
333-138894 |
|||||||
*10.40 |
Restricted Stock Agreement (outside of the Amended and Restated 2006 Stock Plan), dated September 2, 2014, between the Registrant and Anne C. Whitaker. |
10-Q (Exhibit 10.4) |
11/6/14 |
001-33277 |
|||||||
*10.41 |
Non-Qualified Stock Option Agreement (outside of the Amended and Restated 2006 Stock Plan), dated September 2, 2014, between the Registrant and Anne C. Whitaker. |
10-Q (Exhibit 10.5) |
11/6/14 |
001-33277 |
|||||||
*10.42 |
Non-Qualified Stock Option Agreement (outside of the Amended and Restated 2006 Stock Plan), dated December 8, 2014, between the Registrant and Chen Schor. |
10-K (Exhibit 10.45) |
3/12/15 |
001-33277 |
|||||||
*10.43 |
Non-Qualified Stock Option Agreement (outside of the Amended and Restated 2006 Stock Plan), dated December 8, 2014, between the Registrant and Marc Schneebaum. |
10-K (Exhibit 10.45) |
3/12/15 |
001-33277 |
|||||||
62
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| *10.44 | Restricted Stock Agreement (outside of the Amended and Restated 2006 Stock Plan), dated December 8, 2014, between the Registrant and Chen Schor. | 10-K (Exhibit 10.45) |
3/12/15 | 001-33277 | |||||||
*10.45 |
Restricted Stock Agreement (outside of the Amended and Restated 2006 Stock Plan), dated December 8, 2014, between the Registrant and Marc Schneebaum. |
10-K (Exhibit 10.45) |
3/12/15 |
001-33277 |
|||||||
*10.46 |
Separation Agreement between the Company and Keith Ehrlich, dated February 10, 2015. |
10-Q (Exhibit 10.1) |
5/7/15 |
001-33277 |
|||||||
*10.47 |
Separation Agreement between the Company and Arthur McMahon, dated April 13, 2015. |
10-Q (Exhibit 10.4) |
8/6/15 |
001-33277 |
|||||||
*10.48 |
Cash-Based Employee Retention and Incentive Bonus Plan. |
10-Q (Exhibit 10.1) |
5/7/15 |
001-33277 |
|||||||
21.1 |
List of Subsidiaries. |
10-K (Exhibit 21.1) |
3/11/14 |
001-33277 |
|||||||
23.1 |
Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm. |
X |
|||||||||
31.1 |
Certification of Principal Executive Officer under Section 302 of the Sarbanes-Oxley Act of 2002. |
X |
|||||||||
31.2 |
Certification of Principal Accounting and Financial Officer under Section 302 of the Sarbanes-Oxley Act of 2002. |
X |
|||||||||
32.1 |
Certification of the Principal Executive Officer and the Principal Accounting and Financial Officer under Section 906 of the Sarbanes-Oxley Act of 2002. |
X |
|||||||||
63
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 101 | The following materials from Synta Pharmaceuticals Corp.'s Annual Report on Form 10-K for the year ended December 31, 2015, formatted in XBRL (eXtensible Business Reporting Language): (i) the Consolidated Balance Sheets, (ii) the Consolidated Statements of Operations, (iii) the Consolidated Statements of Comprehensive Loss, (iv) the Consolidated Statements of Stockholders' Equity (Deficit) and Comprehensive Income (Loss), (v) the Consolidated Statements of Cash Flows, and (vi) Notes to Consolidated Financial Statements. | X | |||||||||
- *
- Management
contract, compensatory plan or arrangement.
- †
- Confidential portions of these documents have been filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment.
64
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| SYNTA PHARMACEUTICALS CORP. | ||||
Date: March 15, 2016 |
By: |
/s/ CHEN SCHOR Chen Schor President and Chief Executive Officer |
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated below and on the dates indicated.
Signatures
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ CHEN SCHOR Chen Schor |
President, Chief Executive Officer and Director (principal executive officer) | March 15, 2016 | ||
/s/ MARC R. SCHNEEBAUM Marc R. Schneebaum |
Senior Vice President, Chief Financial Officer (principal accounting and financial officer) |
March 15, 2016 |
||
/s/ KEITH R. GOLLUST Keith R. Gollust |
Chairman of the Board |
March 15, 2016 |
||
/s/ PAUL A. FRIEDMAN, M.D. Paul A. Friedman, M.D. |
Director |
March 15, 2016 |
||
/s/ BRUCE KOVNER Bruce Kovner |
Director |
March 15, 2016 |
||
/s/ DONALD W. KUFE, M.D. Donald W. Kufe, M.D. |
Director |
March 15, 2016 |
||
/s/ WILLIAM REARDON, C.P.A. William Reardon, C.P.A. |
Director |
March 15, 2016 |
65
Signatures
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ ROBERT N. WILSON Robert N. Wilson |
Director | March 15, 2016 | ||
/s/ SCOTT MORENSTEIN Scott Morenstein |
Director |
March 15, 2016 |
66
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
SYNTA PHARMACEUTICALS CORP.
Years ended December 31, 2015, 2014 and 2013
F-1
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders of
Synta Pharmaceuticals Corp.
We have audited the accompanying consolidated balance sheets of Synta Pharmaceuticals Corp. (the "Company") as of December 31, 2015 and 2014, and the related consolidated statements of operations, comprehensive loss, stockholders' equity and cash flows for each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Synta Pharmaceuticals Corp. at December 31, 2015 and 2014, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2015, in conformity with U.S. generally accepted accounting principles.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company's internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated March 15, 2016, expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Boston,
Massachusetts
March 15, 2016
F-2
SYNTA PHARMACEUTICALS CORP.
Consolidated Balance Sheets
(in thousands, except share and per share amounts)
| |
December 31, 2015 |
December 31, 2014 |
|||||
|---|---|---|---|---|---|---|---|
Assets |
|||||||
Current assets: |
|||||||
Cash and cash equivalents |
$ | 34,966 | $ | 46,024 | |||
Marketable securities |
31,608 | 51,666 | |||||
Prepaid expenses and other current assets |
1,201 | 1,656 | |||||
| | | | | | | | |
Total current assets |
67,775 | 99,346 | |||||
Property and equipment, net |
420 | 1,024 | |||||
Other assets |
— | 305 | |||||
| | | | | | | | |
Total assets |
$ | 68,195 | $ | 100,675 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Liabilities and Stockholders' Equity |
|||||||
Current liabilities: |
|||||||
Accounts payable |
$ | 1,299 | $ | 3,139 | |||
Accrued contract research costs |
6,863 | 12,317 | |||||
Other accrued liabilities |
4,976 | 6,177 | |||||
Current portion of capital lease obligations |
43 | 42 | |||||
Current portion of term loans |
4,607 | 9,214 | |||||
| | | | | | | | |
Total current liabilities |
17,788 | 30,889 | |||||
| | | | | | | | |
Long-term liabilities: |
|||||||
Capital lease obligations, net of current portion |
— | 43 | |||||
Term loans, net of current portion |
— | 4,607 | |||||
| | | | | | | | |
Total long-term liabilities |
— | 4,650 | |||||
| | | | | | | | |
Total liabilities |
17,788 | 35,539 | |||||
| | | | | | | | |
Commitments and contingencies (Note 12) |
|||||||
Stockholders' equity: |
|||||||
Preferred stock, par value $0.0001 per share Authorized: 5,000,000 shares at December 31, 2015 and 2014; no shares issued and outstanding at December 31, 2015 and 2014 |
— | — | |||||
Common stock, par value $0.0001 per share Authorized: 200,000,000 shares at December 31, 2015 and December 31, 2014; 137,788,584 and 109,120,670 shares issued and outstanding at December 31, 2015 and 2014, respectively |
14 | 11 | |||||
Additional paid-in-capital |
756,633 | 702,694 | |||||
Accumulated other comprehensive income |
4 | 4 | |||||
Accumulated deficit |
(706,244 | ) | (637,573 | ) | |||
| | | | | | | | |
Total stockholders' equity |
50,407 | 65,136 | |||||
| | | | | | | | |
Total liabilities and stockholders' equity |
$ | 68,195 | $ | 100,675 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See accompanying notes to consolidated financial statements.
F-3
SYNTA PHARMACEUTICALS CORP.
Consolidated Statements of Operations
(in thousands, except share and per share amounts)
| |
Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
Revenues: |
||||||||||
|
$ | — | $ | — | $ | — | ||||
Operating expenses: |
||||||||||
Research and development |
54,218 | 68,205 | 71,860 | |||||||
General and administrative |
13,392 | 15,746 | 15,699 | |||||||
| | | | | | | | | | | |
Total operating expenses |
67,610 | 83,951 | 87,559 | |||||||
| | | | | | | | | | | |
Loss from operations |
(67,610 | ) | (83,951 | ) | (87,559 | ) | ||||
Interest expense, net |
(1,061 | ) | (2,210 | ) | (2,633 | ) | ||||
| | | | | | | | | | | |
Net loss |
$ | (68,671 | ) | $ | (86,161 | ) | $ | (90,192 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Net loss per common share: |
||||||||||
Basic and diluted net loss per common share |
$ | (0.53 | ) | $ | (0.87 | ) | $ | (1.27 | ) | |
Basic and diluted weighted average number of common shares outstanding |
128,594,835 | 98,489,470 | 70,976,705 | |||||||
See accompanying notes to consolidated financial statements.
F-4
SYNTA PHARMACEUTICALS CORP.
Consolidated Statements of Comprehensive Loss
(in thousands)
| |
Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
Net loss |
$ | (68,671 | ) | $ | (86,161 | ) | $ | (90,192 | ) | |
Other comprehensive income (loss): |
||||||||||
Unrealized gain (loss) on available-for-sale securities |
— | (13 | ) | 15 | ||||||
| | | | | | | | | | | |
Comprehensive loss |
$ | (68,671 | ) | $ | (86,174 | ) | $ | (90,177 | ) | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
F-5
SYNTA PHARMACEUTICALS CORP.
Consolidated Statements of Stockholders' Equity
(in thousands, except share amounts)
| |
Common stock | |
Accumulated other comprehensive income (loss) |
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Additional paid-in Capital |
Accumulated deficit |
Total stockholders' equity |
||||||||||||||||
| |
Shares | Amount | |||||||||||||||||
Balance at December 31, 2012 |
68,930,082 | $ | 7 | $ | 536,277 | $ | 2 | $ | (461,220 | ) | $ | 75,066 | |||||||
Issuance of common shares in equity offering, excluding to related parties, net |
10,916,667 | 1 | 37,628 | — | — | 37,629 | |||||||||||||
Issuance of common shares to related parties |
5,183,333 | 1 | 19,436 | — | — | 19,437 | |||||||||||||
Issuance of restricted common shares |
140,000 | — | — | — | — | — | |||||||||||||
Forfeitures of restricted common shares |
(75,000 | ) | — | — | — | — | — | ||||||||||||
Exercise of stock options |
137,424 | — | 1,106 | — | — | 1,106 | |||||||||||||
Compensation expense related to stock options for services |
— | — | 6,030 | — | — | 6,030 | |||||||||||||
Unrealized gain on marketable securities |
— | — | — | 15 | — | 15 | |||||||||||||
Net loss |
— | — | — | — | (90,192 | ) | (90,192 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2013 |
85,232,506 | $ | 9 | $ | 600,477 | $ | 17 | $ | (551,412 | ) | $ | 49,091 | |||||||
Issuance of common shares in equity offering, excluding to related parties, net |
21,692,753 | 2 | 88,940 | — | — | 88,942 | |||||||||||||
Issuance of common shares to related parties |
1,250,000 | — | 4,992 | — | — | 4,992 | |||||||||||||
Issuance of restricted common shares |
764,022 | — | — | — | — | — | |||||||||||||
Forfeitures of restricted common shares |
(25,000 | ) | — | — | — | — | — | ||||||||||||
Exercise of stock options |
206,389 | — | 854 | — | — | 854 | |||||||||||||
Compensation expense related to stock options for services |
— | — | 7,431 | — | — | 7,431 | |||||||||||||
Unrealized loss on marketable securities |
— | — | — | (13 | ) | — | (13 | ) | |||||||||||
Net loss |
— | — | — | — | (86,161 | ) | (86,161 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2014 |
109,120,670 | $ | 11 | $ | 702,694 | $ | 4 | $ | (637,573 | ) | $ | 65,136 | |||||||
Issuance of common shares in equity offering, excluding to related parties, net |
21,657,369 | 2 | 36,940 | — | — | 36,942 | |||||||||||||
Issuance of common shares to related parties |
7,257,142 | 1 | 12,699 | — | — | 12,700 | |||||||||||||
Issuance of restricted common shares |
253,403 | — | — | — | — | — | |||||||||||||
Forfeitures of restricted common shares |
(500,000 | ) | — | — | — | — | — | ||||||||||||
Compensation expense related to stock options for services |
— | — | 4,300 | — | — | 4,300 | |||||||||||||
Net loss |
— | — | — | — | (68,671 | ) | (68,671 | ) | |||||||||||
| | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2015 |
137,788,584 | $ | 14 | $ | 756,633 | $ | 4 | $ | (706,244 | ) | $ | 50,407 | |||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
See accompanying notes to consolidated financial statements.
F-6
SYNTA PHARMACEUTICALS CORP.
Consolidated Statements of Cash Flows
(in thousands)
| |
Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
Cash flows from operating activities: |
||||||||||
Net loss |
$ | (68,671 | ) | $ | (86,161 | ) | $ | (90,192 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||
Stock-based compensation expense |
4,300 | 7,431 | 6,030 | |||||||
Depreciation and amortization |
662 | 673 | 516 | |||||||
Changes in operating assets and liabilities: |
||||||||||
Prepaid expenses and other current assets |
455 | 102 | 21 | |||||||
Other assets |
305 | 111 | (951 | ) | ||||||
Accounts payable |
(1,840 | ) | (3,450 | ) | 928 | |||||
Accrued contract research costs |
(5,454 | ) | 1,910 | 5,646 | ||||||
Other accrued liabilities |
(1,201 | ) | 459 | 591 | ||||||
| | | | | | | | | | | |
Net cash used in operating activities |
(71,444 | ) | (78,925 | ) | (77,411 | ) | ||||
| | | | | | | | | | | |
Cash flows from investing activities: |
||||||||||
Purchases of marketable securities |
(117,135 | ) | (93,845 | ) | (114,151 | ) | ||||
Maturities of marketable securities |
137,193 | 85,152 | 90,267 | |||||||
Purchases of property and equipment |
(58 | ) | (144 | ) | (769 | ) | ||||
| | | | | | | | | | | |
Net cash provided by (used) in investing activities |
20,000 | (8,837 | ) | (24,653 | ) | |||||
| | | | | | | | | | | |
Cash flows from financing activities: |
||||||||||
Proceeds from issuances of common stock, excluding to related parties, and exercise of common stock options, net of transaction costs |
36,942 | 89,796 | 38,735 | |||||||
Proceeds from the sale of common stock to related parties |
12,700 | 4,992 | 19,437 | |||||||
Proceeds from term loans |
— | — | 13,500 | |||||||
Payment of term loans |
(9,214 | ) | (9,450 | ) | (2,617 | ) | ||||
Payment of capital lease obligations |
(42 | ) | (42 | ) | (13 | ) | ||||
| | | | | | | | | | | |
Net cash provided by financing activities |
40,386 | 85,296 | 69,042 | |||||||
| | | | | | | | | | | |
Net decrease in cash and cash equivalents |
(11,058 | ) | (2,466 | ) | (33,022 | ) | ||||
Cash and cash equivalents at beginning of period |
46,024 | 48,490 | 81,512 | |||||||
| | | | | | | | | | | |
Cash and cash equivalents at end of period |
$ | 34,966 | $ | 46,024 | $ | 48,490 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Supplemental disclosure of cash flow information: |
||||||||||
Cash paid for interest |
$ | 957 | $ | 1,875 | $ | 2,512 | ||||
Assets acquired under capital lease |
— | — | $ | 126 | ||||||
See accompanying notes to consolidated financial statements.
F-7
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements
(1) Nature of Business
Synta Pharmaceuticals Corp. (the Company) was incorporated in March 2000 and commenced operations in July 2001. The Company is a company that has historically focused on the research, development and commercialization of novel oncology medicines that have the potential to change the lives of cancer patients.
The Company is subject to risks common to emerging companies in the drug development and pharmaceutical industry including, but not limited to, uncertainty of product development and commercialization, lack of marketing and sales history, dependence on key personnel, uncertainty of market acceptance of products and product reimbursement, product liability, uncertain protection of proprietary technology, potential inability to raise additional financing and compliance with the U.S. Food and Drug Administration and other government regulations.
The Company has incurred significant operating losses since its inception and, as a result, as of December 31, 2015 had an accumulated deficit of $706.2 million. Operations have been funded principally through the sale of common stock and convertible preferred stock, capital leases, non-refundable payments under the former collaboration agreements with GlaxoSmithKline (GSK) and Hoffman-La Roche (Roche), and proceeds from term loans by General Electric Capital Corporation (GECC) and Oxford Finance Corporation (Oxford) (see Note 9).
In October 2015, the Company announced the decision to terminate for futility the Phase 3 GALAXY-2 trial of its novel heat shock protein 90 (Hsp90) inhibitor, ganetespib, and docetaxel in the second-line treatment of patients with advanced non-small cell lung adenocarcinoma, and initiated a comprehensive review of its strategy. In November 2015, the Company committed to a restructuring that consisted primarily of a workforce reduction of 45 positions, to a total of 33 positions, to better align its workforce to its revised operating plan.
As announced in March 2016, in order to conserve cash while the Company continues to evaluate business alternatives to maximize value for stockholders, the Company committed to an additional restructuring in February 2016 that consisted primarily of a workforce reduction of 23 positions, including 19 research and development positions, to a total of 10 remaining positions. In connection with this restructuring, the Company discontinued a substantial portion of its research and development activities and no longer anticipates expending material resources on any of its drug candidates.
As previously announced in the Company's Current Report on Form 8-K filed on March 1, 2016, reporting the restructuring of its workforce, the Company has been considering potential strategic alternatives to enhance stockholder value. Such strategic alternatives include, but are not limited to, a sale of the company, a business combination or collaboration, joint development and partnership opportunities, a distribution of all or a significant amount of cash to stockholders, and liquidation of the company. The Company does not know if it will be successful in pursuing any strategic alternative or that any transaction will occur; however, the Company is committed to pursuing a strategic direction that its Board of Directors believes is in the best interests of its stockholders.
The Company cannot predict whether and to what extent it may continue drug development activities, if at all, and what its future cash needs may be for any such activities. The Company expects its $66.6 million in cash resources as of December 31, 2015, along with significantly lower operating expenses following the termination of the GALAXY-2 trial, subsequent restructurings in the fourth quarter of 2015 and the first quarter of 2016, and the discontinuation of a substantial portion of the Company's research and development activities will be sufficient to fund operations for at least the next
F-8
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(1) Nature of Business (Continued)
twelve months. This estimate assumes no additional funding from new partnership agreements, equity financings or further sales under the Company's at-the-market-issuance sales agreement (ATM) with Cowen and Co. LLC (Cowen) (see Note 5). The timing and nature of certain activities contemplated for the remainder of 2016 will be conducted subject to the availability of sufficient financial resources.
The Company may require significant additional funds earlier than it currently expects in order to continue drug development activities and to continue to fund its operations. There can be no assurances, however, that additional funding will be available on favorable terms, or at all.
(2) Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the financial statements of the Company and its wholly owned subsidiaries. All significant intercompany balances and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect certain reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting periods. Significant items subject to such estimates and assumptions include contract research accruals, recoverability of long-lived assets and measurement of stock-based compensation. The Company bases its estimates on historical experience and various other assumptions that management believes to be reasonable under the circumstances. Changes in estimates are recorded in the period in which they become known. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase and an investment in a money market fund to be cash equivalents. Changes in the level of cash and cash equivalents may be affected by changes in investment portfolio maturities, as well as actual cash disbursements to fund operations.
The primary objective of the Company's investment activities is to preserve its capital for the purpose of funding operations and the Company does not enter into investments for trading or speculative purposes. The Company's cash is deposited in a highly rated financial institution in the United States. The Company invests in money market funds and high-grade, short-term commercial paper and corporate bonds, which management believes are subject to minimal credit and market risk. Declines in interest rates, however, would reduce future investment income.
Marketable Securities
Marketable securities consist of investments in high-grade corporate obligations, and government and government agency obligations that are classified as available-for-sale. Since these securities are available to fund current operations they are classified as current assets on the consolidated balance sheets.
F-9
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
The Company adjusts the cost of available-for-sale debt securities for amortization of premiums and accretion of discounts to maturity. The Company includes such amortization and accretion as a component of interest expense, net. Realized gains and losses and declines in value, if any, that the Company judges to be other-than-temporary on available-for-sale securities are reported as a component of interest expense, net. To determine whether an other-than-temporary impairment exists, the Company considers whether it intends to sell the debt security and, if the Company does not intend to sell the debt security, it considers available evidence to assess whether it is more likely than not that it will be required to sell the security before the recovery of its amortized cost basis. During the years ended December 31, 2015, 2014 and 2013, the Company determined it did not have any securities that were other-than-temporarily impaired.
Marketable securities are stated at fair value, including accrued interest, with their unrealized gains and losses included as a component of accumulated other comprehensive income or loss, which is a separate component of stockholders' equity. The fair value of these securities is based on quoted prices and observable inputs on a recurring basis. Realized gains and losses are determined on the specific identification method. During the years ended December 31, 2015, 2014 and 2013, the Company did not have any realized gains or losses on marketable securities.
Fair Value of Financial Instruments
The carrying amounts of the Company's financial instruments, which include cash equivalents, marketable securities and term loan obligations, approximate their fair values. The fair value of the Company's financial instruments reflects the amounts that would be received upon sale of an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value hierarchy has the following three levels:
Level 1—quoted prices in active markets for identical assets and liabilities.
Level 2—observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active.
Level 3—unobservable inputs that reflect the Company's own assumptions about the assumptions market participants would use in pricing the asset or liability.
Financial assets and liabilities are classified in their entirety within the fair value hierarchy based on the lowest level of input that is significant to the fair value measurement. The Company measures the fair value of its marketable securities by taking into consideration valuations obtained from third-party pricing sources. The pricing services utilize industry standard valuation models, including both income and market based approaches, for which all significant inputs are observable, either directly or indirectly, to estimate fair value. These inputs include reported trades of and broker-dealer quotes on the same or similar securities, issuer credit spreads, benchmark securities and other observable inputs. As of December 31, 2015, the Company's financial assets valued based on Level 1 inputs consisted of cash and cash equivalents in a money market fund and its financial assets valued based on Level 2 inputs consisted of high-grade corporate bonds and commercial paper. During the years ended December 31, 2015, 2014 and 2013, the Company did not have any transfers of financials assets between Levels 1 and 2. As of December 31, 2015, the Company did not have any financial liabilities that were recorded at fair value on the balance sheet. The disclosed fair value of the Company's term
F-10
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
loan obligations is determined using current applicable rates for similar instruments as of the balance sheet date. The carrying value of the Company's term loan obligations approximates fair value as the Company's interest rate yield is near current market rate yields. The disclosed fair value of the Company's term loan obligations is based on Level 3 inputs.
Property and Equipment
Property, equipment and software is carried at cost and depreciated using the straight-line method over the estimated useful lives of the related assets, which range from three to seven years. Leasehold improvements are amortized over the lesser of the lease term or estimated useful life. Repairs and maintenance costs are expensed as incurred.
Research and Development Costs
Research and development costs are expensed as incurred. Research and development costs are comprised of costs incurred in performing research and development activities, including internal costs for salaries, bonuses, benefits, facilities, research-related overhead and stock compensation, and external costs for payments to third party contract research organizations, investigative sites and consultants in connection with the Company's preclinical and clinical programs, costs associated with drug formulation and supply of drugs for clinical trials, and other external costs. During the fourth quarter of 2015, the Company revised its estimates of certain contract research costs incurred and recorded a net reduction in accrued contract research costs of approximately $2.9 million, principally as a result of the termination of the GALAXY-2 trial.
Patents
Costs to secure and defend patents are expensed as incurred and are classified as general and administrative expense in the Company's consolidated statements of operations. Patent expenses were approximately $1.1 million, $1.9 million, and $2.9 million for the years ended December 31, 2015, 2014 and 2013, respectively.
Income Taxes
The Company uses the liability method to account for income taxes. Deferred tax assets and liabilities are determined based on the expected future tax consequences of temporary differences between the Company's consolidated financial statement carrying amounts and the tax basis of assets and liabilities using enacted tax rates expected to be in effect in the years in which the differences are expected to reverse. A valuation allowance is provided to reduce the deferred tax assets to the amount that will more likely than not be realized.
As of December 31, 2015 and 2014, the Company had no items that were considered to be uncertain tax items or accrued interest or penalties related to uncertain tax positions. The tax years 2012 through 2015 remain open to examination by the major taxing jurisdictions to which the Company is subject.
F-11
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
Impairment of Long-Lived Assets
The Company assesses the potential impairments of its long-lived assets whenever events or changes in circumstances indicate that an asset's carrying value may not be recoverable. If the carrying value exceeds the undiscounted future cash flows estimated to result from the use and eventual disposition of the asset, the Company writes down the asset to its estimated fair value. Management believes that no long-lived assets were materially impaired as of December 31, 2015 and 2014.
Revenue Recognition
Collaboration and License Agreements
The Company's principal source of revenue to date has been its former collaboration and license agreements, which included upfront license payments, development milestones, reimbursement of research and development costs, potential profit sharing payments, commercial and sales-based milestones and royalties. The accounting for collaboration and license agreements requires subjective analysis and requires management to make estimates and assumptions about whether deliverables within multiple-element arrangements are separable from the other aspects of the contractual arrangement into separate units of accounting and to determine the arrangement consideration to be allocated to each unit of accounting.
For multiple-element arrangements entered into or materially modified after January 1, 2011, the Company follows the provisions of Financial Accounting Standards Board (FASB) Accounting Standards Update (ASU) No. 2009-13—Multiple-deliverable Revenue Arrangements (ASU No. 2009-13). ASU No. 2009-13 amended certain provisions of Accounting Standards Codification (ASC) Topic 605—Revenue Recognition. This standard addresses the determination of the unit(s) of accounting for multiple-element arrangements and how an arrangement's consideration should be allocated to each unit of accounting.
Pursuant to this standard, each required deliverable is evaluated to determine if it qualifies as a separate unit of accounting. For the Company this determination includes an assessment as to whether the deliverable has "stand-alone value" to the customer separate from the undelivered elements. The arrangement's consideration is then allocated to each separate unit of accounting based on the relative selling price of each deliverable. The estimated selling price of each deliverable is determined using the following hierarchy of values: (i) vendor-specific objective evidence of fair value, (ii) third-party evidence of selling price, or (iii) the Company's best estimate of the selling price (BESP). The BESP reflects the Company's best estimate of what the selling price would be if the deliverable was regularly sold by it on a stand-alone basis. The Company expects, in general, to use BESP for allocating consideration to each deliverable in future collaboration agreements. In general, the consideration allocated to each unit of accounting is then recognized as the related goods or services are delivered limited to the consideration not contingent upon future deliverables. The Company did not recognize any revenue related to collaboration and license agreements during the years ended December 31, 2015, 2014 and 2013.
The Company accounts for development milestones under collaboration and license agreements pursuant to ASU No. 2010-17 Milestone Method of Revenue Recognition (ASU No. 2010-17). ASU No. 2010-17 codified a method of revenue recognition that has been common practice. Under this method, contingent consideration from research and development activities that is earned upon the
F-12
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
achievement of a substantive milestone is recognized in its entirety in the period in which the milestone is achieved. At the inception of each arrangement that includes milestone payments, the Company evaluates whether each milestone is substantive. This evaluation includes an assessment of whether (a) the consideration is commensurate with either (1) the entity's performance to achieve the milestone, or (2) the enhancement of the value of the delivered item(s) as a result of a specific outcome resulting from the entity's performance to achieve the milestone, (b) the consideration relates solely to past performance and (c) the consideration is reasonable relative to all of the deliverables and payment terms within the arrangement. The Company evaluates factors such as the scientific, clinical, regulatory, commercial and other risks that must be overcome to achieve the respective milestone, the level of effort and investment required and whether the milestone consideration is reasonable relative to all deliverables and payment terms in the arrangement in making this assessment. The Company does not have any ongoing collaboration and license agreements under which milestones may be achieved.
Royalty revenues are based upon a percentage of net sales. Royalties from the sales of products will be recorded on the accrual basis when results are reliably measurable, collectability is reasonably assured and all other revenue recognition criteria are met. Commercial and sales-based milestones, which are based upon the achievement of certain agreed-upon sales thresholds, will be recognized in the period in which the respective sales threshold is achieved and collectability is reasonably assured. The Company does not have any ongoing collaboration and license agreements under which royalties or commercial and sales-based milestones may be achieved.
Stock-Based Compensation
The Company recognizes stock-based compensation expense based on the grant date fair value of stock options granted to employees, officers and directors. The Company uses the Black-Scholes option pricing model to determine the grant date fair value as management believes it is the most appropriate valuation method for its option grants. The Black-Scholes model requires inputs for risk-free interest rate, dividend yield, volatility and expected lives of the options. Expected volatility is based upon the weighted average historical volatility data of the Company's common stock. The risk-free rate for periods within the expected life of the option is based on the U.S. Treasury yield curve in effect at the time of the grant. The expected lives for options granted represent the period of time that options granted are expected to be outstanding. The Company uses the simplified method for determining the expected lives of options. The Company estimates the forfeiture rate based on historical data. This analysis is re-evaluated at least annually and the forfeiture rate is adjusted as necessary.
For awards with graded vesting, the Company recognizes compensation costs based on the grant date fair value of awards on a straight-line basis over the requisite service period, which is generally the vesting period.
Certain of the employee stock options granted by the Company are structured to qualify as incentive stock options (ISOs). Under current tax regulations, the Company does not receive a tax deduction for the issuance, exercise or disposition of ISOs if the employee meets certain holding requirements. If the employee does not meet the holding requirements, a disqualifying disposition occurs, at which time the Company may receive a tax deduction. The Company does not record tax benefits related to ISOs unless and until a disqualifying disposition is reported. In the event of a disqualifying disposition, the entire tax benefit is recorded as a reduction of income tax expense. The
F-13
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
Company has not recognized any income tax benefit for its share-based compensation arrangements due to the fact that the Company does not believe it is more likely than not it will realize the related deferred tax assets.
Comprehensive Loss
Comprehensive loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Changes in unrealized gains and losses on marketable securities represent the only difference between the Company's net loss and comprehensive loss.
Segment Reporting
Operating segments are determined based on the way management organizes its business for making operating decisions and assessing performance. The Company has a single operating segment, which is the discovery, development and commercialization of drug products.
Basic and Diluted Loss Per Common Share
Basic net loss per share is computed using the weighted average number of common shares outstanding during the period, excluding restricted stock that has been issued but is not yet vested. Diluted net loss per common share is computed using the weighted average number of common shares outstanding and the weighted average dilutive potential common shares outstanding using the treasury stock method. However, for the years ended December 31, 2015, 2014 and 2013, diluted net loss per share is the same as basic net loss per share as the inclusion of weighted average shares of unvested restricted common stock and common stock issuable upon the exercise of stock options would be anti-dilutive.
The following table summarizes outstanding securities not included in the computation of diluted net loss per common share as their inclusion would be anti-dilutive for the years ended December 31, 2015, 2014 and 2013, respectively:
| |
Years Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
Common stock options |
10,127,257 | 8,829,343 | 6,814,417 | |||||||
Unvested restricted stock |
426,706 | 744,514 | 45,000 | |||||||
Recent Accounting Pronouncements
In May 2014, the FASB issued ASU No. 2014-09,—Revenue from Contracts with Customers (Topic 606), which amends the guidance for accounting for revenue from contracts with customers. This ASU supersedes the revenue recognition requirements in ASC Topic 605, and creates a new Topic 606, Revenue from Contracts with Customers. This guidance was originally effective for fiscal years beginning after December 15, 2016, with early adoption not permitted. Two adoption methods are permitted: retrospectively to all prior reporting periods presented, with certain practical expedients permitted; or retrospectively with the cumulative effect of initially adopting the ASU recognized at the date of initial application. The FASB approved a one year deferral of the effective date of this standard to annual
F-14
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(2) Summary of Significant Accounting Policies (Continued)
periods beginning after December 15, 2017, along with an option to permit companies to early adopt the standard for annual periods beginning after December 15, 2016. The Company has not yet determined the date it plans to adopt ASU No. 2014-09, which adoption method it will utilize, or the effect that the adoption of this guidance will have on its consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15,—Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern. This ASU is intended to define management's responsibility to evaluate whether there is substantial doubt about an organization's ability to continue as a going concern within one year of the date of issuance of the entity's financial statements and to provide related footnote disclosures. This guidance is effective for fiscal years ending after December 15, 2016, with early application permitted. The adoption of this guidance may have an effect on the Company's disclosures in future periods.
(3) Cash, Cash Equivalents and Marketable Securities
A summary of cash, cash equivalents and available-for-sale marketable securities held by the Company as of December 31, 2015 and December 31, 2014 was as follows in thousands (see Note 2):
| |
December 31, 2015 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Cost | Unrealized gains |
Unrealized losses |
Fair value | |||||||||
| |
(in thousands) |
||||||||||||
Cash and cash equivalents: |
|||||||||||||
Cash and money market funds (Level 1) |
$ | 27,473 | $ | — | $ | — | $ | 27,473 | |||||
Corporate debt securities due within 3 months of date of purchase (Level 2) |
7,493 | — | — | 7,493 | |||||||||
| | | | | | | | | | | | | | |
Total cash and cash equivalents |
34,966 | — | — | 34,966 | |||||||||
Marketable securities: |
|||||||||||||
Corporate debt securities due within 1 year of date of purchase (Level 2) |
31,604 | 5 | (1 | ) | 31,608 | ||||||||
| | | | | | | | | | | | | | |
Total cash, cash equivalents and marketable securities |
$ | 66,570 | $ | 5 | $ | (1 | ) | $ | 66,574 | ||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| |
December 31, 2014 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Cost | Unrealized gains |
Unrealized losses |
Fair value | |||||||||
| |
(in thousands) |
||||||||||||
Cash and cash equivalents: |
|||||||||||||
Cash and money market funds (Level 1) |
$ | 45,004 | $ | — | $ | — | $ | 45,004 | |||||
Corporate debt securities due within 3 months of date of purchase (Level 2) |
1,020 | — | — | 1,020 | |||||||||
| | | | | | | | | | | | | | |
Total cash and cash equivalents |
46,024 | — | — | 46,024 | |||||||||
Marketable securities: |
|||||||||||||
Corporate debt securities due within 1 year of date of purchase (Level 2) |
51,662 | 12 | (8 | ) | 51,666 | ||||||||
| | | | | | | | | | | | | | |
Total cash, cash equivalents and marketable securities |
$ | 97,686 | $ | 12 | $ | (8 | ) | $ | 97,690 | ||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
F-15
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(4) Property and Equipment
Property and equipment as of December 31, 2015 and December 31, 2014 consisted of the following (in thousands):
| |
December 31, 2015 |
December 31, 2014 |
|||||
|---|---|---|---|---|---|---|---|
| |
(in thousands) |
||||||
Laboratory equipment |
$ | 12,217 | $ | 12,217 | |||
Leasehold improvements |
5,030 | 4,988 | |||||
Computers and software |
3,136 | 3,126 | |||||
Furniture and fixtures |
1,182 | 1,176 | |||||
| | | | | | | | |
|
21,565 | 21,507 | |||||
Less accumulated depreciation and amortization |
(21,145 | ) | (20,483 | ) | |||
| | | | | | | | |
|
$ | 420 | $ | 1,024 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Depreciation and amortization expenses of property and equipment, including equipment purchased under capital leases, were approximately $0.7 million, $0.7 million and $0.5 million for the years ended December 31, 2015, 2014 and 2013, respectively.
The net book value and accumulated amortization of equipment under capital lease was approximately $42,000 and $84,000 respectively, at December 31, 2015, and $84,000 and $42,000, respectively, at December 31, 2014.
(5) Stockholders' Equity
Common Stock
Each common stockholder is entitled to one vote for each share of common stock held. The common stock will vote together with all other classes and series of stock of the Company as a single class on all actions to be taken by the Company's stockholders. Each share of common stock is entitled to receive dividends, as and when declared by the Company's board of directors.
The Company has never declared cash dividends on its common stock and does not expect to do so in the foreseeable future.
Public and Registered Direct Offerings
In April 2015, the Company raised approximately $44.3 million in gross proceeds from the sale of an aggregate 25,300,000 shares of its common stock in a public offering at a public offering price of $1.75 per share, including 3,300,000 shares upon the full exercise of the underwriters' option to purchase additional shares. Certain of the Company's directors and their affiliates, including its largest stockholder, purchased an aggregate of 7,257,142 shares in this offering at the public offering price. The net offering proceeds to the Company were approximately $41.9 million after deducting underwriters' discounts, fees and commissions, and other offering expenses payable by the Company.
In April 2014, the Company sold 1,250,000 shares of its common stock at a purchase price of $4.01 per share in a registered direct offering to an affiliate of a director who is its largest stockholder. These shares were sold directly without a placement agent, underwriter, broker or dealer. The net proceeds to
F-16
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(5) Stockholders' Equity (Continued)
the Company were approximately $5.0 million after deducting offering expenses payable by the Company.
In November 2013, the Company raised approximately $60.4 million in gross proceeds from the sale of an aggregate 16,100,000 shares of its common stock in a public offering at a public offering price of $3.75 per share, including 14,000,000 shares in the initial offering and 2,100,000 shares upon the full exercise of the underwriters' option to purchase additional shares. Certain of the Company's directors and their affiliates, including its largest stockholder, purchased an aggregate of 5,183,333 shares in this offering. The net offering proceeds to the Company were approximately $57.1 million after deducting underwriters' discounts, fees and commissions, and other offering expenses payable by the Company.
At-The-Market Issuance Sales Agreements
MLV & Co. LLC
The Company entered into at-the-market issuance sales agreements (May 2012, May 2014 and July 2014 Sales Agreements) with MLV & Co. LLC (MLV), pursuant to which the Company issued and sold shares of its common stock from time to time, at the Company's option, through MLV as its sales agent. Sales of common stock through MLV were made pursuant to an "at-the-market" offering as defined in Rule 415 promulgated under the Securities Act of 1933, as amended. MLV used commercially reasonable efforts to sell the common stock based upon the Company's instructions (including price, time or size limits or other customary parameters or conditions the Company imposed). All shares were sold pursuant to an effective shelf registration statement on Form S-3 (File No. 333-187242). The Company paid MLV a commission of up to 3% of the gross proceeds. The May 2012 and May 2014 Sales Agreements were terminated by the Company upon the sale of substantially all stock authorized for sale under each such agreements. In October 2015, the Company terminated the July 2014 Sales Agreement.
In March and April 2014, the Company sold an aggregate of 6,588,875 shares of common stock pursuant to the May 2012 Sales Agreement for an aggregate of approximately $28.0 million in gross proceeds at an average selling price of $4.25 per share. Net proceeds to the Company were approximately $27.3 million after deducting commissions and other transactions costs.
From May 2014 through July 2014, the Company sold an aggregate of 9,424,193 shares of common stock pursuant to the May 2014 Sales Agreement for an aggregate of approximately $40.0 million in gross proceeds at an average selling price of $4.24 per share. Net proceeds to the Company were approximately $39.2 million after deducting commissions and other transactions costs, including approximately $33.6 million from the sale of 8,060,244 shares in the second quarter of 2014 and approximately $5.6 million from the sale of 1,363,949 shares in July 2014.
In July 2014, the Company reserved up to $50 million under its shelf registration statement for issuance under the July 2014 Sales Agreement. In the third quarter of 2014, the Company sold an aggregate of 5,679,685 shares of common stock pursuant to the July 2014 Sales Agreement for an aggregate of approximately $23.0 million in gross proceeds at an average selling price of $4.05 per share. Net proceeds to the Company were approximately $22.5 million after deducting commissions and other transaction costs.
F-17
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(5) Stockholders' Equity (Continued)
During the third quarter of 2015, the Company sold an aggregate of 3,614,511 shares of common stock pursuant to the July 2014 Sales Agreement for an aggregate of approximately $7.9 million in gross proceeds at an average selling price of $2.19 per share. Net proceeds to the Company were approximately $7.7 million after deducting commissions and other transaction costs.
Cowen and Co. LLC
In October 2015, the Company entered into an at-the-market issuance sales agreement (October 2015 Sales Agreement), with Cowen and Company, LLC (Cowen), pursuant to which the Company may issue and sell shares of its common stock, having an aggregate offering price of up to $100 million, from time to time, at the Company's option, through Cowen as its sales agent. Sales of common stock through Cowen may be made by any method that is deemed an "at-the-market" offering as defined in Rule 415 promulgated under the Securities Act of 1933, as amended, including by means of ordinary brokers' transactions at market prices, in block transactions or as otherwise agreed by the Company and Cowen. Subject to the terms and conditions of the Sales Agreement, Cowen will use commercially reasonable efforts consistent with its normal trading and sales practices to sell the common stock based upon the Company's instructions (including any price, time or size limits or other customary parameters or conditions the Company may impose). The Company is not obligated to make any sales of its common stock under the Sales Agreement. Any shares sold will be sold pursuant to an effective shelf registration statement on Form S-3 (file no. 333-206135). The Company will pay Cowen a commission of up to 3% of the gross proceeds. The October 2015 Sales Agreement may be terminated by the Company at any time upon 10 days' notice. No shares have been sold to-date under the October 2015 Sales Agreement.
(6) Stock-Based Compensation
The Company's 2006 Stock Plan provided for the grant of incentive stock options, non-statutory stock options and non-vested restricted stock to employees, officers, directors and consultants of the Company. In January 2015, the number of shares of common stock reserved for issuance under the 2006 Stock Plan was increased from 10,300,000 to 11,600,000 pursuant to an "evergreen" provision, which provides for an annual increase based on the lesser of 1,300,000 shares, 5% of the Company's then outstanding shares of common stock, or such other amount as the board of directors may determine. This increase was approved by the board of directors in December 2014. In June 2015, upon obtaining stockholder approval at its annual shareholder meeting, the Company implemented its new 2015 Stock Plan and reserved 8,741,000 shares of common stock for future issuance. In June 2015, the Company terminated its 2006 Stock Plan. The administration of these stock plans is under the general supervision of the compensation committee of the board of directors. The exercise price of the stock options is determined by the compensation committee of the board of directors, provided that incentive stock options are granted with an exercise price not less than fair market value of the common stock on the date of grant and expire no later than ten years from the date the option is granted. Options generally vest over four years. As of December 31, 2015, the Company had options outstanding to purchase 10,127,257 shares of its common stock, which includes options outstanding under its 2001 Stock Plan and 2006 Stock Plan that were terminated in March 2006 and June 2015, respectively. As of December 31, 2015, 8,067,115 shares were available for future issuance.
F-18
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(6) Stock-Based Compensation (Continued)
The following table summarizes stock option activity during the year ended December 31, 2015:
| |
Shares | Weighted average exercise price |
Weighted average remaining contractual life (years) |
Aggregate intrinsic value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Outstanding at January 1 |
8,829,343 | $ | 6.17 | ||||||||||
Options granted |
5,399,986 | 2.09 | |||||||||||
Options cancelled |
(4,102,072 | ) | 4.76 | ||||||||||
Options exercised |
— | — | |||||||||||
| | | | | | | | | | | | | | |
Outstanding at December 31 |
10,127,257 | $ | 4.56 | 5.29 | $ | — | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Exercisable at December 31 |
5,673,074 | $ | 6.17 | 2.09 | $ | — | |||||||
The aggregate intrinsic value of all options outstanding and exercisable represents the total pre-tax amount, net of the exercise price, which would have been received by option holders if all option holders had exercised all options with an exercise price lower than the closing stock price of $0.35 on December 31, 2015, which was the last trading day of the year. The total intrinsic value of options exercised during the years ended December 31, 2015, 2014 and 2013 was approximately $0, $435,000 and $385,000, respectively. The total cash received by the Company as a result of stock option exercises during 2015, 2014 and 2013 was $0, $0.9 million and $1.1 million, respectively. The weighted-average grant date fair values of options granted during the years ended December 31, 2015, 2014 and 2013 were $1.51, $4.00 and $7.28, respectively.
Non-Vested ("Restricted") Stock Awards with Service Conditions
The Company's share-based compensation plan provides for awards of restricted shares of common stock to employees, officers, directors and consultants to the Company. Restricted stock awards are subject to forfeiture if employment or service terminates during the prescribed retention period. Restricted shares vest over the service period. The total fair value of restricted stock that vested during the years ended December 31, 2015, 2014 and 2013 was $0.1 million, $0.1 million and $0.3 million, respectively. The following table summarizes unvested restricted share activity during the year ended December 31, 2015:
| |
Shares | Weighted average grant date fair value |
|||||
|---|---|---|---|---|---|---|---|
Outstanding at January 1 |
744,514 | $ | 3.65 | ||||
Vested |
(71,211 | ) | 2.72 | ||||
Granted |
253,403 | 2.32 | |||||
Forfeited |
(500,000 | ) | 4.00 | ||||
| | | | | | | | |
Outstanding at December 31 |
426,706 | $ | 2.61 | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
F-19
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(6) Stock-Based Compensation (Continued)
Stock-Based Compensation Expense
For the years ended December 31, 2015, 2014 and 2013, the fair value of each employee stock option award was estimated on the date of grant based on the fair value method using the Black-Scholes option pricing valuation model with the following weighted average assumptions:
| |
Years ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
Risk-free interest rate |
1.72 | % | 1.88 | % | 1.20 | % | ||||
Expected life in years |
6.25 years | 6.25 years | 6.25 years | |||||||
Volatility |
84 | % | 104 | % | 102 | % | ||||
Expected dividend yield |
— | — | — | |||||||
Stock-based compensation expense during the years ended December 31, 2015, 2014 and 2013 was as follows (in thousands):
| |
Years ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
Stock-based compensation expense by type of award: |
||||||||||
Employee stock options |
$ | 4,060 | $ | 7,076 | $ | 5,757 | ||||
Restricted stock |
240 | 355 | 273 | |||||||
| | | | | | | | | | | |
Total stock-based compensation expense |
$ | 4,300 | $ | 7,431 | $ | 6,030 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Effect of stock-based compensation expense by line item: |
||||||||||
Research and development |
$ | 2,530 | $ | 4,412 | $ | 3,220 | ||||
General and administrative |
1,770 | 3,019 | 2,810 | |||||||
| | | | | | | | | | | |
Total stock-based compensation expense included in net loss |
$ | 4,300 | $ | 7,431 | $ | 6,030 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Unrecognized stock-based compensation expense as of December 31, 2015 was as follows (dollars in thousands):
| |
Unrecognized stock compensation expense as of December 31, 2015 |
Weighted average remaining period (in years) |
|||||
|---|---|---|---|---|---|---|---|
Employee stock options |
$ | 6,087 | 2.98 | ||||
Restricted stock |
893 | 1.93 | |||||
| | | | | | | | |
Total |
$ | 6,980 | 2.84 | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
F-20
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
7) Other Accrued Liabilities
Other accrued liabilities as of December 31, 2015 and December 31, 2014 consisted of the following (in thousands):
| |
December 31, 2015 |
December 31, 2014 |
|||||
|---|---|---|---|---|---|---|---|
| |
(in thousands) |
||||||
Compensation and benefits |
$ | 3,072 | $ | 3,852 | |||
Professional fees |
867 | 1,285 | |||||
Other |
1,037 | 1,040 | |||||
| | | | | | | | |
|
$ | 4,976 | $ | 6,177 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
(8) Co-Development and License Agreements
Co-Development Agreement
In July 2011, the Company entered into a co-development agreement with a clinical research organization (CRO) for the conduct of certain company-sponsored clinical trials. Under the co-development agreement, this CRO was performing clinical research services under a reduced fee structure in exchange for a share of licensing payments and commercial revenues, if any, resulting from the product under development up to a specified maximum payment, which is defined as a multiple of the fee reduction realized. Research and development expenses were being recognized based on the reduced fee structure and expected payments will be recorded in the future if and when payment is probable. The maximum amount of the service fee discount was realized in the year ended December 31, 2013.
License Arrangement
In May 2014, the Company entered into a license arrangement for its CRACM program, including two lead candidates and the associated intellectual property portfolio, with PRCL Research Inc. (PRCL), a company funded by TVM Life Science Venture VII and the Fonds de Solidarité des Travailleurs du Québec, based in Montreal, Canada. PRCL plans to develop one of the two lead candidates licensed from the Company to proof-of-concept. Synta was recently informed that PRCL had selected one of these candidates to move forward into IND enabling studies. Synta was granted a minority interest in PRCL in exchange for its contribution of know-how and intellectual property and a seat on PRCL's Board of Directors. Synta is not required to provide any research funding or capital contributions to PRCL, and is not required to perform any research activities related to these candidates. Synta is reimbursed by PRCL for any ongoing intellectual property management costs in connection with the contributed intellectual property. If and when proof-of-concept is reached with either drug candidate, Eli Lilly and Company, which is an investor in TVM, will manage the development program through one of its divisions and will have an option to acquire PRCL or its assets at the then fair value.
F-21
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(9) Term Loans
General Electric Capital Corporation
In March 2013, the Company amended its loan and security agreement entered into in September 2010 with General Electric Capital Corporation (GECC) and another lender (the GECC Term Loan) and obtained $12.9 million in additional loan funding and, as a result, increased the principal balance to $22.5 million at March 31, 2013. This amendment was accounted for as a loan modification. Interest on the borrowings under the GECC Term Loan remains at the annual rate of 9.75%. In January 2014, the Company began making 30 equal monthly payments of principal plus accrued interest on the outstanding balance. During the period from July 2012 through March 2013, the Company made nine equal monthly payments of principal under the GECC Term Loan. For the periods from April 2013 through December 2013 and prior to July 2012 the Company made interest-only payments. As of December 31, 2015, in accordance with the GECC Term Loan, $4.5 million in remaining principal payments is scheduled to be paid by June 2016.
The Company has paid various transaction fees and expenses in connection with the GECC Term Loan, which are deferred and are being amortized as interest expense over the remaining term of the GECC Term Loan. In addition, the Company is obligated to pay an exit fee of $788,000 at the time of the final principal payment which is being accreted and expensed as interest over the remaining term of the GECC Term Loan. In the years ended December 31, 2015, 2014 and 2013, the Company recognized GECC Term Loan interest expense of $1.1 million, $2.1 million and $2.5 million, respectively, of which $0.2 million, $0.4 million and $0.6 million, respectively, was in connection with these transaction and exit fees and expenses. The Company may prepay the full amount of the GECC Term Loan, subject to prepayment premiums under certain circumstances. The Company did not issue any warrants in connection with the GECC Term Loan.
The GECC Term Loan is secured by substantially all of the Company's assets, except its intellectual property. The Company has granted GECC a springing security interest in its intellectual property in the event the Company is not in compliance with certain cash usage covenants, as defined therein. The GECC Term Loan contains restrictive covenants, including the requirement for the Company to receive the prior written consent of GECC to enter into loans, other than up to $4.0 million of equipment financing, restrictions on the declaration or payment of dividends, restrictions on acquisitions, and customary default provisions that include material adverse events, as defined therein. The Company has determined that the risk of subjective acceleration under the material adverse events clause is remote and therefore has classified the outstanding principal based on the timing of scheduled principal payments.
Oxford Finance Corporation
In March 2011, the Company entered into a loan and security agreement with Oxford Finance Corporation (Oxford) and received $2.0 million in loan funding, and in December 2012, the Company entered into a loan modification agreement, as amended, under which the Company could elect to draw down up to an additional $0.6 million in equipment financing until June 30, 2013 that would be payable in 36 equal monthly payments of principal plus accrued interest on the outstanding balance (collectively, the Oxford Term Loan). As of June 30, 2013, the Company had fully utilized the $0.6 million in additional equipment financing. Interest on the borrowings under the Oxford Term Loan accrues at an annual rate of 13.35%. In May 2011, the Company began making 36 equal monthly payments of principal plus accrued interest on the initial $2.0 million outstanding balance that was fully
F-22
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(9) Term Loans (Continued)
paid in April 2014. The Company continues to make equal monthly payments of principal plus accrued interest on the $0.6 million in additional equipment financing. As of December 31, 2015, in accordance with the Oxford Term Loan, $107,000 in remaining principal payments is scheduled to be paid by July 2016.
The Company recognized approximately $42,000, $59,000 and $127,000 in interest expense in the years ended December 31, 2015, 2014 and 2013, respectively, related to the outstanding principal under the Oxford Term Loan. In addition to the interest payable under the Oxford Term Loan, the Company paid approximately $108,000 of administrative and legal fees and expenses in connection with the Oxford Term Loan. These expenses have been deferred and are being expensed over the term of the Oxford Term Loan. The Company did not issue any warrants in connection with the Oxford Term Loan. The Company may prepay the Oxford Term Loan, subject to prepayment premiums under certain circumstances. Oxford has the right to require the Company to prepay the Oxford Term Loan if the Company prepays the full amount of the GECC Term Loan under certain circumstances.
The Oxford Term Loan is secured by certain laboratory and office equipment, furniture and fixtures. In connection with the Oxford Term Loan, Oxford and GECC entered into a Lien Subordination Agreement, whereby GECC granted Oxford a first priority perfected security interest in the loan collateral. The Oxford Term Loan contains restrictive covenants, including the requirement for the Company to receive the prior written consent of Oxford to enter into acquisitions in which the Company incurs more than $2.0 million of related indebtedness, and customary default provisions that include material adverse events, as defined therein. The Company has determined that the risk of subjective acceleration under the material adverse events clause is remote and therefore has classified the outstanding principal in current and long-term liabilities based on the timing of scheduled principal payments.
(10) Restructurings—November 2015 and February 2016
In October 2015, the Company announced its decision to terminate for futility its Phase 3 GALAXY-2 trial of ganetespib and docetaxel in the second-line treatment of patients with advanced non-small cell lung adenocarcinoma. Based on a review of a pre-planned interim analysis, the study's Independent Data Monitoring Committee concluded that the addition of ganetespib to docetaxel is unlikely to demonstrate a statistically significant improvement in the primary endpoint of overall survival compared to docetaxel alone.
In November 2015, following the termination of the GALAXY-2 trial, the Company committed to a restructuring that consisted primarily of a workforce reduction of 45 positions, to a total of 33 positions, to better align its workforce to its revised operating plan. The restructuring was substantially completed during the fourth quarter of 2015. Cash payments in connection with the workforce reduction, comprised principally of severance, unused vacation payments, benefits continuation costs and outplacement services, were approximately $2.6 million of which approximately $1.3 million was paid during the fourth quarter of 2015. As of December 31, 2015, approximately $1.3 million was accrued in remaining restructuring-related payments, including $1.2 million and $0.1 million that is expected to be paid in the first and second quarters of 2016, respectively.
In February 2016, in order to conserve cash while the Company continues to evaluate its strategies to maximize value for stockholders, the Company committed to an additional restructuring that consisted primarily of a workforce reduction of 23 positions, including 19 research and development
F-23
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(10) Restructurings—November 2015 and February 2016 (Continued)
positions, to a total of 10 positions. The Company estimates its cash payments in connection with the workforce reduction, comprised principally of severance, unused vacation payments, benefits continuation costs and outplacement services, will be approximately $1.5 million. The Company expects the restructuring to be substantially completed in the first quarter of 2016 and the majority of the related cash payments to be paid during the first half of 2016. Employees directly affected by the restructuring have received notification and will be provided with severance payments.
As a result of the restructuring in February 2016, the Company estimates it may incur an impairment charge for certain research laboratory equipment, computer equipment, and furniture and fixtures due to the fact that these assets may no longer be utilized. At this time, the Company is unable to estimate the amount of impairment costs as it is in the process of evaluating its facilities and equipment needs.
(11) Income Taxes
Differences between the actual tax provision (benefit) and the tax provision (benefit) computed using the United States federal income tax rate is as follows for the years ended December 31, 2015, 2014 and 2013, respectively:
| |
Years ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2015 | 2014 | 2013 | |||||||
| |
(in thousands) |
|||||||||
Benefit at statutory rate |
$ | (23,348 | ) | $ | (29,293 | ) | $ | (30,665 | ) | |
State taxes, net of federal benefit |
(2,569 | ) | (3,342 | ) | (3,735 | ) | ||||
State net operating loss expiration |
1,210 | — | 661 | |||||||
Stock-based compensation |
1,229 | 1,094 | 1,118 | |||||||
Tax credits |
(1,282 | ) | (1,720 | ) | (2,462 | ) | ||||
Foreign rate differential |
5,099 | 6,243 | 5,252 | |||||||
Other |
96 | 101 | (47 | ) | ||||||
Increase in valuation allowance |
19,565 | 26,917 | 29,878 | |||||||
| | | | | | | | | | | |
Income tax provision (benefit) |
$ | — | $ | — | $ | — | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-24
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(11) Income Taxes (Continued)
The effects of temporary differences that give rise to significant portions of deferred tax assets and deferred tax liabilities at December 31, 2015 and 2014, respectively, are presented below:
| |
2015 | 2014 | |||||
|---|---|---|---|---|---|---|---|
| |
(in thousands) |
||||||
Deferred tax assets: |
|||||||
Federal and state net operating loss carryforwards |
$ | 213,004 | $ | 194,091 | |||
Federal and state research and development credits |
22,520 | 21,401 | |||||
Depreciation and amortization |
2,321 | 2,423 | |||||
Deferred compensation |
7,884 | 7,643 | |||||
Other |
821 | 1,427 | |||||
| | | | | | | | |
Deferred tax assets |
246,550 | 226,985 | |||||
Less valuation allowance |
(246,550 | ) | (226,985 | ) | |||
| | | | | | | | |
Net deferred tax assets |
$ | — | $ | — | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The total valuation allowance increased by approximately $19.6 million, $26.9 million and $29.9 million in the years ended December 31, 2015, 2014 and 2013, respectively.
The Company has established valuation allowances against its deferred tax assets because management believes that, after considering all of the available objective evidence, both historical and prospective, the realization of the deferred tax assets does not meet the "more likely than not" criteria. The Company evaluates the need for a valuation allowance on a quarterly basis.
For tax years through 2015 the Company performed analyses to determine if there were changes in ownership, as defined by Section 382 of the Internal Revenue Code that would limit its ability to utilize certain net operating loss and tax credit carryforwards. The Company determined that it experienced an ownership change, as defined by Section 382, in connection with its acquisition of Principia Associates, Inc. on September 20, 2002, but did not experience a change in ownership upon the effectiveness of the Company's IPO, or any other equity offerings to date. As a result, the utilization of the Company's federal tax net operating loss carryforwards generated prior to the ownership change is limited. As of December 31, 2015, the Company has net operating loss carryforwards for U.S. federal tax purposes of approximately $578.1 million, after excluding net operating losses that have expired unused as a result of Section 382 limitations, with the remainder expiring in varying amounts through 2035 unless utilized. At December 31, 2015, the Company has state net operating loss carryforwards of approximately $321.9 million, which will expire through 2035 unless utilized. The net operating loss carryforwards include approximately $1.4 million of deductions related to the exercise of common stock options. This amount represents an excess tax benefit and has not been included in the gross deferred tax asset reflected for net operating losses. The utilization of these net operating loss carryforwards may be further limited if the Company experiences future ownership changes as defined in Section 382 of the Internal Revenue Code.
At December 31, 2015, the Company had approximately $17.8 million and $7.2 million, respectively, in federal and state research and development credits. Unless utilized, the federal credits will expire from 2016 through 2030, state research credits will expire from 2019 through 2030.
The Company is currently open to examination under the statute of limitations by the Internal Revenue Service and state jurisdictions for the tax years ended 2012 through 2015. Carryforward tax
F-25
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(11) Income Taxes (Continued)
attributes generated in years past may still be adjusted upon future examination if they have or will be used in a future period. The Company is currently not under examination by the Internal Revenue Service or any other jurisdictions for any tax years.
The Company does not consider any of its tax positions to be uncertain and accordingly there are no tax reserves for the years ended December 31, 2015, 2014 and 2013. The Company will recognize interest expense and penalties related to uncertain tax positions in income tax expense. The Company has not, as yet, conducted a study of its domestic research and development credit carryforwards. This study may result in an increase or decrease to the Company's research and development credit carryforwards; however, until a study is completed and any adjustment is known, no amounts are being presented as an uncertain tax position. A full valuation allowance has been provided against the Company's research and development credits, and if an adjustment is required, this adjustment would be offset by an adjustment to the valuation allowance. As a result, there would be no impact to the consolidated balance sheet, statement of operations and comprehensive loss or cash flows if an adjustment were required.
(12) Commitments and Contingencies
Leases
The Company leases its three research and office facilities under three non-cancelable and renewable operating leases with terms expiring in the fourth quarter of 2016. These lease agreements include customary provisions for rent increases, escalations for operating costs and renewals. As a result of the restructurings in November 2015 and in February 2016, and related events, the Company is evaluating possible early lease terminations for one or more of its office locations. The Company also leases equipment under various other non-cancellable operating leases. The Company recognizes rent expense on a straight-line basis over the non-cancelable term of the lease.
Future minimum payments, excluding operating costs and taxes, under the Company's capital and non-cancellable operating leases are approximately as follows (in thousands):
| |
Operating leases |
Capital leases |
|||||
|---|---|---|---|---|---|---|---|
Years ending December 31, |
|||||||
2016 |
$ | 1,995 | $ | 44 | |||
2017 |
25 | — | |||||
| | | | | | | | |
Total minimum payments |
$ | 2,020 | 44 | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Less: amount representing interest |
(1 | ) | |||||
| | | | | | | | |
Present value of minimum payments |
43 | ||||||
Less current portions of obligations |
(43 | ) | |||||
| | | | | | | | |
Long term obligation |
$ | — | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Rent expense under operating leases was approximately $2.4 million, $2.3 million and $2.2 million, for the years ended December 31, 2015, 2014 and 2013, respectively.
F-26
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(12) Commitments and Contingencies (Continued)
Guarantees
As permitted under Delaware law, the Company's Certificate of Incorporation and Bylaws provide that the Company will indemnify certain of its officers and directors for certain claims asserted against them in connection with their service as an officer or director. The maximum potential amount of future payments that the Company could be required to make under these indemnification provisions is unlimited. However, the Company has purchased a directors' and officers' liability insurance policy that reduces its monetary exposure and enables it to recover a portion of any future amounts paid. The Company believes the estimated fair value of these indemnification arrangements is minimal.
The Company customarily agrees in the ordinary course of its business to indemnification provisions in agreements with clinical trial investigators in its drug development programs, in sponsored research agreements with academic and not-for-profit institutions, in various comparable agreements involving parties performing services for the Company in the ordinary course of business, and in its real estate leases. The Company expects to agree to certain indemnification provisions in drug discovery and development collaboration agreements the Company may enter into. With respect to the Company's clinical trials and sponsored research agreements, these indemnification provisions typically apply to any claim asserted against the investigator or the investigator's institution relating to personal injury or property damage, violations of law or certain breaches of the Company's contractual obligations arising out of the research or clinical testing of the Company's compounds or drug candidates. With respect to lease agreements, the indemnification provisions typically apply to claims asserted against the landlord relating to personal injury or property damage caused by the Company, to violations of law by the Company or to certain breaches of the Company's contractual obligations. The indemnification provisions appearing in collaboration agreements are similar, but in addition provide some limited indemnification for the collaborator in the event of third-party claims alleging infringement of intellectual property rights. In each of the cases above, the term of these indemnification provisions generally survives the termination of the agreement, although the provision has the most relevance during the contract term and for a short period of time thereafter. The maximum potential amount of future payments that the Company could be required to make under these provisions is generally unlimited. The Company purchases insurance policies covering personal injury, property damage and general liability that reduce its exposure for indemnification and would enable it in many cases to recover a portion of any future amounts paid. The Company has never paid any material amounts to defend lawsuits or settle claims related to these indemnification provisions. Accordingly, the Company believes the estimated fair value of these indemnification arrangements is minimal.
(13) Related Party Transactions
In April 2015, the Company sold an aggregate of 7,257,142 shares of common stock to certain of the Company's directors and their affiliates, including its largest stockholder, at a purchase price of $1.75 per share in a public offering (see Note 5).
In April 2014, the Company sold 1,250,000 shares of its common stock at a purchase price of $4.01 per share in a registered direct offering to an affiliate of a director who is its largest stockholder (see Note 5).
In November 2013, the Company sold an aggregate of 5,183,333 shares of common stock to certain of the Company's directors and their affiliates, including its largest stockholder, at a purchase price of $3.75 per share in a public offering (see Note 5).
F-27
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(13) Related Party Transactions (Continued)
In January 2013, a director exercised an aggregate of 114,250 shares of common stock options that resulted in $1.0 million in proceeds to the Company.
(14) Retirement Plan
In 2003, the Company implemented a 401(k) retirement plan (the Synta 401(k) Plan) in which substantially all of its permanent employees are eligible to participate. Participants may contribute a percentage of their annual compensation to the plan, subject to statutory limitations. The Company may declare discretionary matching contributions to the Synta 401(k) Plan.
In April 2006, the Company began matching participants' contributions up to 50% of the first 6% of the employee's salary. The match is subject to a three-year equally graded vesting schedule and any forfeitures will be applied to reduce the Company's contributions. Company contributions for the years ended December 31, 2015, 2014 and 2013 were approximately $314,000, $359,000 and $413,000, respectively, subject to forfeitures.
(15) Quarterly Financial Data (unaudited)
The following tables present a summary of quarterly results of operations for 2015 and 2014:
| |
Three Months Ended | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
March 31, 2015 |
June 30, 2015 |
September 30, 2015 |
December 31, 2015 |
|||||||||
| |
(in thousands, except shares and per share data) |
||||||||||||
Revenues: |
|||||||||||||
Total revenues |
$ | — | $ | — | $ | — | $ | — | |||||
Operating expenses: |
|||||||||||||
Research and development |
16,182 | 16,377 | 14,413 | 7,246 | |||||||||
General and administrative |
4,150 | 3,127 | 2,981 | 3,134 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses |
20,332 | 19,504 | 17,394 | 10,380 | |||||||||
| | | | | | | | | | | | | | |
Loss from operations |
(20,332 | ) | (19,504 | ) | (17,394 | ) | (10,380 | ) | |||||
Interest expense, net |
(375 | ) | (296 | ) | (234 | ) | (156 | ) | |||||
| | | | | | | | | | | | | | |
Net loss |
$ | (20,707 | ) | $ | (19,800 | ) | $ | (17,628 | ) | $ | (10,536 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Basic and diluted net loss per common share |
$ | (0.19 | ) | $ | (0.15 | ) | $ | (0.13 | ) | $ | (0.08 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Basic and diluted weighted average number of common shares outstanding |
108,376,264 | 132,295,909 | 135,971,551 | 137,336,309 | |||||||||
F-28
SYNTA PHARMACEUTICALS CORP.
Notes to Consolidated Financial Statements (Continued)
(15) Quarterly Financial Data (unaudited) (Continued)
| |
Three Months Ended | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
March 31, 2014 |
June 30, 2014 |
September 30, 2014 |
December 31, 2014 |
|||||||||
| |
(in thousands, except shares and per share data) |
||||||||||||
Revenues: |
|||||||||||||
Total revenues |
$ | — | $ | — | $ | — | $ | — | |||||
Operating expenses: |
|||||||||||||
Research and development |
17,583 | 18,761 | 16,208 | 15,653 | |||||||||
General and administrative |
5,324 | 2,940 | 3,241 | 4,241 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses |
22,907 | 21,701 | 19,449 | 19,894 | |||||||||
| | | | | | | | | | | | | | |
Loss from operations |
(22,907 | ) | (21,701 | ) | (19,449 | ) | (19,894 | ) | |||||
Interest expense, net |
(650 | ) | (585 | ) | (517 | ) | (458 | ) | |||||
| | | | | | | | | | | | | | |
Net loss |
$ | (23,557 | ) | $ | (22,286 | ) | $ | (19,966 | ) | $ | (20,352 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Basic and diluted net loss per common share |
$ | (0.28 | ) | $ | (0.24 | ) | $ | (0.19 | ) | $ | (0.19 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Basic and diluted weighted average number of common shares outstanding |
85,438,127 | 94,046,278 | 105,774,949 | 108,366,504 | |||||||||
(16) Subsequent Event—Elesclomol (Mitochondria-Targeting Agent)
In January 2016, the Company entered into an asset purchase agreement with another party to further develop its drug candidate, elesclomol. The Company will no longer be performing research activities on this drug candidate and, as part of the arrangement, the Company will receive a minority interest and Board representation in the other party, payments based on achievement of certain development milestones and product royalties upon commercialization.
F-29
