Attached files
| file | filename |
|---|---|
| EX-3.2 - EX-3.2 - GALECTIN THERAPEUTICS INC | d20778dex32.htm |
| EX-31.2 - EX-31.2 - GALECTIN THERAPEUTICS INC | d20778dex312.htm |
| EX-32.2 - EX-32.2 - GALECTIN THERAPEUTICS INC | d20778dex322.htm |
| EX-23.1 - EX-23.1 - GALECTIN THERAPEUTICS INC | d20778dex231.htm |
| EX-23.2 - EX-23.2 - GALECTIN THERAPEUTICS INC | d20778dex232.htm |
| EX-21.1 - EX-21.1 - GALECTIN THERAPEUTICS INC | d20778dex211.htm |
| EX-32.1 - EX-32.1 - GALECTIN THERAPEUTICS INC | d20778dex321.htm |
| 10-K - FORM 10-K - GALECTIN THERAPEUTICS INC | d20778d10k.htm |
| EX-31.1 - EX-31.1 - GALECTIN THERAPEUTICS INC | d20778dex311.htm |
Exhibit 10.50
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT (INDICATED BY **) HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT.
Modification Number: 1
PROJECT ADDENDUM MODIFICATION
| Effective Date: | April 15, 2015 | Sponsor: | Galectin Therapeutics, Inc. | |||
| Protocol No: | GT-026 (Project #NASH-CX) |
Sponsor Contact: | Jack Callicutt | |||
| PPD Project Manager: | ** | Bus Dev Director: | ** | |||
The Project Addendum by and between PPD Development, L.P., a Delaware limited partnership and successor-in-interest to PPD Development, LLC, (“PPD”) and Galectin Therapeutics, Inc. (“Sponsor”) effective as of January 10, 2015 regarding the above-referenced Protocol shall be modified as follows:
General:
| • | The timeline is being extended by approximately seven (7) months, where the PPD end of Involvement date has been revised from 01 July 2017 to 01 February 2018. |
| • | Inflationary costs associated with this timeline extension have been captured as a line item in the study budget. |
| • | New activities have been added in North America (NA) to capture the additional labor needed for Protocol Amendment 1. |
| • | New activities have been added to capture the Direct and Pass Through Costs for Central Reader Site Evaluation, Interim Monitoring, and Site Closeout Visits for Hepatic Venous Pressure Gradient (HPVG) and Liver Biopsy. |
| • | New activity has been added to capture the labor associated with the mapping of raw data to the Study Data Tabulation Module (SDTM). |
| • | The Site Country Mix has been revised as detailed below: |
| Country |
Number of sites per Project Addendum |
Number of sites per Project Addendum Modification #1 | ||
| ** | ** | ** | ||
| ** | ** | ** |
| • | All tasks associated with **, where work has not already occurred, have been removed as the study has left the country entirely. |
| Estimated costs associated with this Project Addendum Modification: | $ 218,201.92 | Direct Fees | ||||
| $7,586,684.89 | Pass Through Costs | |||||
| $7,804,886.80 | Total Costs |
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
Page 1 of 31
Modification Number: 1
PROJECT ADDENDUM MODIFICATION
| Cumulative |
Budget NA |
Budget EMEA |
Total Budget | |||||||||
| TOTAL DIRECT COSTS |
$ | 9,059,751.07 | $ | 24,941.56 | $ | 9,084,692.63 | ||||||
| TOTAL PASS THROUGH COSTS |
$ | 13,293,084.54 | $ | 368,913.80 | $ | 13,661,998.34 | ||||||
| TOTAL STUDY COSTS |
$ | 22,352,835.61 | $ | 393,855.36 | $ | 22,746,690.97 | ||||||
| Prior |
||||||||||||
| TOTAL DIRECT COSTS |
$ | 8,842,785.02 | $ | 23,705.69 | $ | 8,866,490.71 | ||||||
| TOTAL PASS THROUGH COSTS |
$ | 5,738,284.26 | $ | 337,029.19 | $ | 6,075,313.45 | ||||||
| TOTAL STUDY COSTS |
$ | 14,581,069.28 | $ | 360,734.88 | $ | 14,941,804.16 | ||||||
| Incremental |
||||||||||||
| TOTAL DIRECT COSTS |
$ | 216,966.05 | $ | 1,235.87 | $ | 218,201.92 | ||||||
| TOTAL PASS THROUGH COSTS |
$ | 7,554,800.28 | $ | 31,884.61 | $ | 7,586,684.89 | ||||||
| TOTAL STUDY COSTS |
$ | 7,771,766.33 | $ | 33,120.47 | $ | 7,804,886.80 | ||||||
A revised estimated study timeline is attached hereto as Exhibit A and which replaces the estimated study timeline set forth in section 1.16 of Exhibit A to the Project Addendum.
A detailed explanation of the changes to the budget is attached hereto as Exhibit B. A revised Central Labs budget is attached hereto as Exhibit C. A revised study budget is attached hereto at Exhibit D, and which replaces the budget set forth in Exhibit B to the Project Addendum.
Payment of such costs shall be made in accordance with the Revised Payment Schedule attached hereto as Exhibit E which shall replace the payment schedule set forth in Exhibit C to the Project Addendum.
All Exhibits attached hereto and incorporated herein by reference.
This Project Addendum Modification may be executed in counterparts, each of which shall be deemed an original and all of which together shall constitute one and the same instrument. Each party may execute this Project Addendum Modification by facsimile transmission or in Adobe Portable Document Format (PDF) sent by electronic mail. Facsimile or PDF signatures of authorized signatories of the parties will be deemed to be original signatures, will be valid and binding, and, upon delivery, will constitute due execution of this Project Addendum Modification.
Upon execution by the parties, this Project Addendum Modification will be made a part of the Project Addendum and incorporated by reference therein. Except as expressly provided herein or in any other mutual written agreement by the parties, all terms and conditions contained in the Project Addendum shall remain in full force and effect. In the event of any conflict between the terms of this Project Addendum Modification and the Project Addendum, the terms of this Project Addendum Modification shall govern and control. All capitalized terms used but not otherwise defined herein shall have the meanings ascribed to them in the Project Addendum.
SIGNATURES FOLLOW ON NEXT PAGE
Page 2 of 31
Modification Number: 1
PROJECT ADDENDUM MODIFICATION
ACCEPTED AND AGREED:
| PPD Development, L.P. By: PPD GP, LLC Its General Partner |
Galectin Therapeutics, Inc. | |||||||
| By: | /s/ Patti McNamara | By: | /s/ Peter G. Traber | |||||
| Name: | Patti McNamara | Name: | Peter G. Traber | |||||
| Title: | VP Finance | Title: | CEO and CMO | |||||
| Date: | March 11, 2016 | Date: | March 11, 2016 | |||||
Remainder of Page Intentionally Left Blank
Page 3 of 31
Modification Number: 1
PROJECT ADDENDUM MODIFICATION
Exhibit A
Revised Estimated Study Timeline
| Activity |
As of Project Addendum |
As of Project Addendum Modification #1 | ||
| Duration in Months | ||||
| Pre-Study Activities |
** | ** | ||
| Enrollment Period |
** | ** | ||
| Treatment Period |
** | ** | ||
| Close-Down Period |
** | ** | ||
| Total PPD Commitment |
** | ** | ||
Remainder of Page Intentionally Left Blank
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
Page 4 of 31
Modification Number: 1
PROJECT ADDENDUM MODIFICATION
Exhibit B
Detailed Explanation of Change
The Detailed Explanation of Change follows this cover page
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
Page 5 of 31
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION Modification #1
Cumulative Study Budget as of Project Addendum Incremental Budget as of Project Addendum Modification #1 Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Project Management Unit Type Unit Cost NA Total Hours NA # of Units NA Budget NA Total Hours NA ft of Units NA Budget NA Total Hours NA # of Units NA Budget NA
Justification
Develop Country Budget and Payment Schedule Template ** ** ** ** ** ** ** ** ** ** ** longer projected to be achieved, due to the study leaving,
Canada.
ICF Local Customized—Review and Approve ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the
study leaving Canada
ICF Local Customization—Review and Approve -Amendments ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected
to be achieved, due to the study leaving Canada.
Project Management—Start-up ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven
(7) month timeline extension.
Project Management—Enrollment ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven (7) month timeline
extension.
Project Management—Protocol Amendment 1 ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the additional labor needed for Protocol
Amendment 1.
** ** ** ** ** ** ** ** ** ** **
ICF Local Customization ** **
** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Management of Translation of
Protocol, Investigator Brochure, ICF & Technical Documents ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Review of Translation of ICF ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Client Teleconferences ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven (7) month timeline extension.
Internal Team Meetings ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven (7) month timeline extension.
Site Intelligence and Activation Management ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven (7) month timeline extension.
Hepatic Venous Pressure Gradient (HVPG) Vendor Site Evaluation Visit Prep/Follow-up ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated with Hepatic
Venous Pressure Gradient (HVPG) Site Evaluation Visits for Central Reader.
HVPG Vendor Site Evaluation Visit—Time On Site ** ** ** ** ** ** ** ** ** ** ** New
activity added to capture labor associated with HVPG Site Evaluation Visits for Central Reader.
HVPG Vendor Site Evaluation Visit—Travel ** ** ** ** ** ** **
** ** ** ** New activity added to capture labor associated with HVPG Site Evaluation Visits for Central Reader.
Subtotal ** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued:
17 February 2016
**Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with
respect to the omitted portions.
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1 Cumulative
Study Budget as of Project Addendum Incremental Budget as of Project Addendum Modification #1
Department/Activity Unit Type Unit Cost NA Total Hours NA # of Units
NA Budget NA Total Hours NA # of Units NA Budget NA Total Hours NA # of Units NA Budget NA Justification
Clinical Management
ICF Local Customization—Amendments ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Amendments—Management of Translation of Protocol, Investigator Brochure, ICF & Technical Documents ** ** ** ** ** ** ** ** ** ** ** Activity
decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Amendments—Review of Translation of ICF ** ** ** ** ** ** ** ** **
** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada-
Site Initiation Visits—Prep/Admin/Follow-up ** ** **
** ** ** ** ** ** ** ** Activity i increased due to additional unit needed, as sponsor requested an additional site.
Site Initiation Visits—Time on Site ** **
** ** ** ** ** ** ** ** ** Activity increased due to additional unit needed, as sponsor requested an additional site
Site Initiation Visits—Travel ** ** ** **
** ** ** ** ** ** ** Activity increased due to additional unit needed, as sponsor requested an additional site
Site Management ** ** ** ** ** ** ** ** ** ** **
Activity increased due to the seven (7) month timeline extension.
Annual Investigator File Audits ** ** ** ** ** ** ** ** ** ** ** Activity increased due to
Protocol Amendment 1.
Site Closeout Visits—Prep/Admin/Follow-up ** ** ** ** ** ** ** ** ** ** ** Activity increased due to additional unit needed, as sponsor
requested an additional site.
Site Closeout Visits—Time on Site ** ** ** ** ** ** ** ** ** ** ** Activity increased due to additional unit needed, as sponsor
requested an additional site.
Site Closeout Visits—Travel ** ** ** ** ** ** ** ** ** ** ** Activity increased due to additional unit needed, as sponsor
requested an additional site.
Unblended Investigator Meeting ** ** ** ** ** ** ** ** ** ** ** Activity decreased due to the inability to identify unblended
Clinical Research Associates (CRAs) in time to attend meeting.
CRA Team Meetings ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected
to be achieved.
Unblended CRA Team Meetings ** ** ** ** ** ** ** ** ** ** ** Activity increased due to Protocol Amendment 1.
Clinical Team Management ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven (7) month timeline extension.
Clinical Management—Protocol Amendment 1 ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the additional labor needed for Protocol Amendment 1
HVPG Vendor Interim Monitoring Visits—Prep/Follow-Up ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated with HVPG Interim Monitoring Visits (IMVs)
for Central Reader.
HVPG Vendor Interim Monitoring Visits—Time On Site ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated with
HVPG IMVs for Central Reader.
HVPG Vendor Interim Monitoring Visits—Travel ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated
with HVPG IMVs for Central Reader.
PPD Pmnnrfan; and Confidential
PPD BC#:
58004-01
Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budgetas of Project Addendum Modification #1 Cumulative Study
Budget as of Project Addendum Incremental Budget as of Project Addendum Modification #1
Department/Activity Unit Type Unit Cost NA Total Hours NA # of Units NA
Budget NA Total Hours NA # of Units NA Budget NA Total Hours NA # of Units NA Budget NA Justification
HVPG Vendor Site Closeout Visit—Prep/Follow-Up ** ** **
** ** ** ** ** ** ** ** New activity added to capture labor associated with HVPG Closeout Visits (COVs) for Central Reader.
HVPG Vendor Site Closeout
Visit—Time On Site ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated with HVPG COVs for Central Reader.
HVPG Vendor Site
Closeout Visit—Travel ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated with HVPG COVs for Central Reader.
Liver Biopsy Vendor
Interim Monitoring Visits -Prep/Follow-Up ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated with Liver Biopsy IMVs for Central Reader.
Liver Biopsy Vendor Interim Monitoring Visits—Time On S:te ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated with Liver Biopsy
IMVs for Central Reader.
Liver Biopsy Vendor Interim Monitoring Visits—Travel ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated
with Liver Biopsy IMVs for Central Reader.
Liver Biopsy Vendor Site Closeout Visits—Prep/Follow-Up ** ** ** ** ** ** ** ** ** ** ** New activity added to
capture labor associated with Liver Biopsy COVs for Central Reader.
Liver Biopsy Vendor Site Closeout Visits—Time On Site ** ** ** ** ** ** ** ** ** ** ** New
activity added to capture labor associated with Liver Biopsy COVs for Central Reader.
Liver Biopsy Vendor Site Closeout Visits—Travel ** ** ** ** ** ** ** **
** ** ** New activity added to capture labor associated with Liver Biopsy COVs for Central Reader.
Subtotal ** ** ** ** ** ** ** ** ** ** **
Identify, Select and Negotiate Contracts with Clinical Supply Vendors ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved.
Label Text Development ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved.
Label Text Translation Coordination ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Clinical Supply Forecasting ** ** ** ** ** ** ** ** ** ** ** Activity increased due to Protocol Amendment 1.
Monitor/Track Study Progress, Inventory Levels and Communication with Team/Sponsor ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven (7) month timeline
extension.
Depot Management ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven (7) month timeline extension.
Distribution Management ** ** ** ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved.
Investigators Meeting ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the labor associated with attending the Investigators Meeting.
Global Clinical Supplies—Protocol Amendment 1 ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the additional labor needed for Protocol Amendment 1.
Subtotal ** ** ** ** ** ** ** ** ** ** **
IVRS
Investigational Product (IP) Accountability Module ** ** ** ** ** ** ** ** ** ** ** New activity added to capture labor associated with Investigational Product (IP) Accountability
Module.
Subtotal ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1 Cumulative
Study Budget as of Project Addendum Incremental Budget as of Project Addendum Modification #1
Department/Activity/ Unit Type Unit Cost NA Total Hours NA # of Units
NA Budget NA Total Hours NA # of Units NA Budget NA Total Hours NA # of Units NA Budget NA Justification
Data Management
Client Teleconferences ** ** ** ** ** ** Activity increased due to the seven (7) month timeline extension.
Internal Team Meetings ** ** Activity increased due to the seven (7) month timeline extension.
Quality Management ** ** ** Activity increased due to the seven (7) month timeline extension.
DM Project Management ** ** ** ** Activity increased due to the seven (7) month timeline extension.
Data Management—Protocol Amendment 1 ** ** ** ** ** ** New activity added to capture the additional labor needed for Protocol Amendment 1.
Subtotal ** ** ** ** ** **
Pharmacovigilance
Data Safety Monitoring Board Set-up Retired and rebuilt this activity as the new activity below in order increase the labor per unit due to Protocol Amendment.
Revised—Data Safety Monitoring Board Set-up ** ** ** New activity added per above in order to capture the labor associated with DSMB Set-up.
Analysis of Similar Events ** ** ** Activity decreased as unit is no longer projected to be achieved.
Development Safety Update Report (DSUR) Preparation ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Senior Medical Officer for Canada Maintenance—Monthly ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Senior Medical Officer Review of Canadian CTA ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Senior Medical Officer Review of Canadian CTA Amendment ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Data Safety Monitoring Board Face-to-Face Meeting Retired and rebuilt this activity as the new activity below in order increase the labor per unit due to Protocol
Amendment.
Revised—Data Safety Monitoring Board Face to Face Meeting New activity added per above in order to capture the labor associated with Data Safety
Monitoring Board (DSMB) Face to Face meetings.
Data Safety Monitoring Board Teleconference Meeting Retired and rebuilt this activity as the new activity below in
order increase the labor per unit due to Protocol Amendment.
Revised—Data Safety Monitoring Board Teleconference Meeting New activity added per above in order
to capture the labor associated with DUMB Teleconferences.
Pharmacovigilance Team Management ** ** ** ** ** Activity increased due to the seven (7) month
timeline extension.
PPD Pmnnrfan; and Confidential
PPD BC#: 58004-01
Date Issued: February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1 Cumulative
Study Budget as of Project Addendum Incremental Budget as of Project Addendum Modification #1_
Department/Activity Unit Type Unit Cost NA Total Hours NA # of Units
NA Budget NA Total Hours NA # of Units NA Budget NA Total Hours NA # of Units NA Budget NA Justification
Unblinded Medical Monitor ** ** ** ** ** ** ** ** New
activity added to capture labor associated with an Unblinded Medical Monitor.
PVG—Protocol Amendment 1 ** ** ** New activity added to capture the additional
labor needed for Protocol Amendment 1.
Subtotal ** ** ** **
Biostats Team
Management Activity increased due to the seven (7) month timeline extension.
Mapping Raw Data to Study Data Tabulation Model (SDTM) New activity added to
capture labor associated with the mapping of raw data to the Study Data Tabulation Module (SDTM).
Biostatistics—Protocol Amendment 1 ** ** ** ** ** New
activity added to capture the additional labor needed for Protocol Amendment 1.
Subtotal ** ** ** ** **
Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Review CRF ** ** ** ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved.
Medical Writing—Protocol Amendment 1 ** ** ** ** ** ** New activity added to capture the additional labor needed for Protocol Amendment 1.
Subtotal ** ** ** **
Information Governance & Compliance—Country File Set-up
Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Information Governance & Compliance—Country File
Archiving and Transfer ** ** ** ** ** Activity decreased as unit is no longer projected to be achieved, due to the study leaving Canada.
Clinical Supplies
OA—Project Agreements *. * *. ** » Activity decreased as unit is no longer projected to be achieved.
Clinical Supplies QA—Project Support „ **
** *. Activity decreased as unit is no longer projected to be achieved.
Subtotal ** ** ** **
Registration of Clinical Trials—Maintenance Activity increased due to the seven (7) month timeline extension.
** ** ** ** ** **
Pharmacokinetics—Protocol Amendment 1 New activity added to capture the
additional labor needed for Protocol Amendment 1.
Subtotal
Inflation: Added a
line item inflation adjustment to capture inflationary increases associated with the seven (7) month increase to the study duration.
Timeline Extension
Services Discount (CM1): ** ** ** ** PPD is providing a one-time discount associated with services performed in regards to the seven (7) month timeline extension.
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1 Cumulative
Study Budget as of Project Addendum Incremental Budget as of Project Addendum Modification #1
Department/Activity Unit Type Unit Cost NA Total Hours NA # of Units
NA Budget NA Total Hours NA # of Units NA Budget NA Total Hours NA # of Units NA Budget NA Justification
Pass Through Costs
Central Laboratory Fees ** ** ** ** ** ** ** ** ** ** ** Activity increased due to the seven (7) month timeline extension.
Investigator Fees ** ** ** ** ** ** ** ** ** ** ** Activity increased due to Protocol Amendment
Site Closeout Visits—Travel ** ** ** ** ** ** ** ** ** ** ** Activity increased due to additional unit needed, as sponsor requested an additional site.
Site Initiation Visits—Travel ** ** ** ** ** ** ** ** ** ** ** Activity increased due to additional unit needed, as sponsor requested an additional site.
Central Reader—HVPG ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the costs projected through the duration of the study.
Central Reader—Liver Biopsy ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the costs projected through the duration of the study.
Data Safety Monitoring Board Member Honoraria ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the costs needed to include payments for three (3) members, which
will cover the contracted caps for each member.
Site Evaluation Visits—HVPG—Central Reader ** ** ** ** ** ** ** ** ** ** ** New activity added to capture
the costs needed to do a site evaluation visit for HVPG Central Reader.
Site Closeout Visits—HVPG—Central Reader ** ** ** ** ** ** ** ** ** ** ** New
activity added to capture the costs needed to do a site closeout visit for HVPG Central Reader.
Site Closeout Visits—Liver Biopsy—Central Reader ** ** **
** ** ** ** ** ** ** ** New activity added to capture the costs needed to do a site closeout visit for Liver Biopsy Central Reader.
Intenm Monitoring Visits-
HVPG—Central Reader ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the costs needed to do a interim monitoring visit for HVPG Central Reader.
Interim Monitoring Visits—Liver Biopsy—Central Reader ** ** ** ** ** ** ** ** ** ** ** New activity added to capture the costs needed to do a interim
monitoring visit for Liver Biopsy Central Reader.
Investigator Meeting Travel—Clinical Supplies Project Manager ** ** ** ** ** ** ** ** ** ** ** New activity
added to capture the cost for Clinical Supplies Project Manager to attend the Investigator Meeting.
Total Pass Throughs ** ** ** $7,554,800.28
Total Study Costs ** $7,771,786.33
Subtotals and Totals included in the Cumulative and Prior
sections are for reference to the budget grid only and do no represent totaling of the numbers included in this document. The Discrete Subtotals and Totals are representative of the numbers in this document.
PPD Proprietary and Confidential
PPD BC#: 58D04-01 Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1 Cumulative
Study Budget as of Project Addendum Incremental Budget as of Project Addendum Modification #1
Department/Activity Unit Type Unit Cost EMEA Total Hours EMEA # of
Units EMEA Budget EMEA Total Hours EMEA # of Units EMEA Budget EMEA Total Hours EMEA # of Units EMEA Budget EMEA Justification
Pharmacovigilance
Pharmacovigilance Team Management ** ** *. ** ** ** ** M Activity increased due to the seven (7) month timeline extension.
_Subtotal ** ** ** ** ** **
Total Direct Costs ** ** ** ** ** **
Inflation: Added a line item inflation adjustment to capture inflationary increases associated with the seven (7) month increase to the study duration.
Total Discounted Direct Costs ** ** ** ** ** **
Pass Through Costs
Central Labs Quote EMEA ** ** M ** m ** m Activity increased due to the seven (7) month timeline extension.
Total Pass Throughs ** ** ** ** ** **
Total Study Costs ** ** ** ** ** **
Subtotals and Totals included in the Cumulative an
d Prior sections are for reference to the
budget grid only and do not represent totaling of the numbers included in (his document. The Discrete Subtotals an
d Totals are representative of the numbers in this d
ocument.
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued: 17 February 2016
Modification Number: 1
PROJECT ADDENDUM MODIFICATION
Exhibit C
Revised Central Labs Budget
The Revised Central Labs Budget follows this cover page
Page 13 of 31
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PPD’s Central Lab, LLC Budget Estimate
PPD Central Labs
09-Jul-2015
Galectin Therapeutics, Inc.
GT-025
BC: 58004-01 Sc2 M1
Central Lab BC: 58004-02 mod #1
Budget Summary Total Charge (USD)
GT-025
Laboratory Testing **
Sample Management **
Kits and Supplies **
Clinical Trial Services Fees **
Direct Costs Estimate: **
_Logistics (Pass-Through) Estimate: **
Total Estimate: **
Original Contract (Rev 2)
Budget Summary Total Charge {USD) Differences
GT-025
Laboratory Testing ** **
Sample Management ** **
Kits and Supplies ** **
Clinical Trial Services Fees ** **
Direct Costs Estimate: ** **
Logistics (Pass-Through) Estimate: ** **
Total Estimate: ** **
Regional Budget Summary GT-025 NA & LA Region EMEA Region
AsiaPac Region China
Laboratory Testing ** ** ** **
Sample Management ** **
** **
Kits and Supplies ** ** ** **
Clinical Trial Services Fees ** ** ** **
Regional Direct Costs Estimate: ** ** ** **
Regional Logistics (Pass-Through)
Estimate: ** ** ** **
Regional Total Estimate: ** ** ** **
Countries Sites
Screened Subjects Enrolled Subjects Completed Subjects %
United States ** ** ** ** **
Canada ** ** ** ** **
North America ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PPD’s Central Labs, LLC Budget Estimate
# Patients: 260 260 26 156 156 156 156 156 156 156 156 156 156 156 156 156 156 16 0%
SV1 SV2
5V3 Ml NDV1 HDV2 Visit 2 V3 V4 NDV3 V7 VII V13 V20 V26 14-26 Day After 14 Days After NA&
Shipping Screen in g Random nation
LeOo ret -Dry Test i ng As sumptions Frequency ipment IWeek Screening Screening ^elM)’”” Week 13 Week 51 Week 53-55 Week 57
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
Sample Management |B| SV1 SV2 SV3 VI m NDV2 V>sit2 V, V4 N0V3 V7 v„ V20 V26 14-28 Dtiy After 14 Days After Toh NA&
?S3 Final Dots LATAM
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
**
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
**
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PPD’s Central Labs, LLC Budget Estimate
PPD
Estimate Subjects: US EU
M0D#1
NA & LATAM EMEA region
fx rate=EUR/USD 1.41 14
Laboratory Testing Assumptions UOM NAM A Unit
Charge (USD) Total Charge (USD) EU Unit Charge (Euro) No. Units Total Charge (EUR) Total Charge (USD)
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
Sample Management Assumptions UOM NA & LA Unit Charge
(USD) Total Charge (USD) EU Unit Charge (Euro) No. Units Total Charge (EUR) Total Charge (USD)
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
NA & LATAM region
fx rate=USD/EUR 0.7944 EMEA region
fx rate=EUR/USD 1.4144
Previous Total Charge (USD) Explanation Difference Previous Total
Charge (USD) Explanation Difference
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
Previous Total Charge (USD) Explanation Difference Previous Total Charge
(USD) Explanation Difference
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
PPD Proprietary and Confidentia
PPD BC#: 58004-01 Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PPD’s Central Labs, LLC Budget Estimate
PPD
Estimate Subjects:
Kits and Supplies Assumptions UOM NA & LA Unit Charge (USD) No. Units Total Charge (USD) EU Unit Charge (Euro) No. Units Total Charge (EUR) Total Charge (USD) Previous
Total Charge (USD) Explanation Difference Previous Total Charge (USD) Explanation Difference
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** **
**
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** **
** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** **
Clinical Trial Services Fees Assumptions UOM NA & LA Unit Charge (USD) No. Units Total Charge (USD) EU Unit Charge (Euro) NO. Units Total Charge (EUR) Total Charge (USD)
Previous Total Charge (USD) Explanation Difference Previous Total Charge (USD) Explanation Difference
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** **
**
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** **
** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PPD’s Central Labs, LLC Budget Estimate Exchange Rates: Global Logistics (Pass-Through}
Outbound, supplies to sites Inbound, Ambient/Refrig ., sites to PPD Inbound, Frozen, sites to PPD Total Charge
Country Sites Unit Type Courier Unit Cost (Euro) Unit Cost (USD) # of Units Sub-total Courier Unit Cost (Euro) Unit Cost (USD) #of Units Sub-total Courier Unit Cost (Euro) Unit
Cost (USD) # of Unite Sub-total Dry ice and shippers (USD) ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** * ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** ** Referral lab courier costs Frequency Courier Unit Type Unit
Cost (Euro) Unit Cost (USD) # of Units Su b-total Comments Total Charge (USD) TOTAL PASS THROUGH COSTS TOTAL PASS THROUGH COSTS NA/LA region (USD) MEA gion USD) AsiaPac region (USD) China (USD) Total Differences TOTAL PASS THROUGH COSTS NA/LA region
(USD) $31,287.91 EMEA region (USD) $0.00 AsiaPac region (USD) $0.00 China (USD) $0.00 $31,287.91
* Transportation costs do not include Saturday delivery charges,
taxes, tariffs, duties and fuel surcharge. This will be invoiced at the prevailing rate.
| * | Transportation fees are estimates only and based on primary cities. |
| * | Client will be invoiced based on actual fees incurred. |
* Laboratory kits may accommodate more than one patient visit/per inbound shipping box. For purposes of the estimate, 1 visit per inbound box
has been assumed as average.
* Drive-away and trans-shipment to international port of departure may apply. Applicable customs fees charged as pass through cost.
| * | Inbound transport costs are based on Weekday priority overnight shipments. |
* Outbound kits have standard transit time of 2-5 days. Overnight priority shipping provided with sponsor approval at additional shipping cost. PPD Proprietary and Confidential PPD BC#: 58004-01 Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PPD’s Central Labs, LLC Budget Estimate Terms and Conditions Study sst up will commence upon
written acceptance of Contract and Central Laboratory Specifications (CLS). The costs contained within this Proposal are valid for ** from date of proposal. The prices contained within this Proposal are estimates based upon information provided by
Sponsor. Cost will be revised if the Sponsor provides an amended protocol or updated information. *** no charge, regardless of number of users. All protocol materials will be archived for ** from the end of PPD involvement. Requests for protocol
materials will be shipped at the expense of the Sponsor/CRO. *PPD Global Central Labs requires ** from signed CLS for study initiation Study initiation is defined as the first Investigational site to receive specimen collection kits. Sponsor
requested changes ** will result in additional charges to be determined based upon the complexity of the revisions. *PPD Global Central Labs has one global database that supports all regions within the study. Global set-up fees will be invoiced upon
project
initiation once database set-up activities are complete. TRAINING/TRAVEL If requested to attend a Kick off meeting or Investigator meeting, ** Central Labs
presenter at one meeting including preparation, excluding travel expenses billed as pass through. This cost assumes a one day meeting with one day for travel. **. Sponsor request of technical attendees will be charged additional fees of Attendance
at the investigator meeting via WebEx will be charged **, *Site training via conference call for protocol specific laboratory procedures is available at sponsor’s request. This will be invoiced at **. •On site training visits to outline
protocol specific laboratory procedures at the sponsor’s request, will be invoiced at a rate of **. TRANSPORTATION MODIFICATIONS Any services requested by Sponsor (or sites) and not included in this cost estimate will be charged separately.
Services rendered will be invoiced as performed and a Contract Modification will be issued. Out of protocol testing will be invoiced per the unit price with an additional **” Project Management fee, per request. Additional charges will apply
for any off-cycle or expedited testing. Specimens requiring off-hour technician/processing time, will be invoiced with an added service charge of ** Any sample that is UTP (Unable To Perform) will be charged a Sample Handling fee. A sample
destruction fee will be invoiced for any sample that is required to be destroyed. This fee will be charged per sample destroyed. Expedited shipping fees will be applied at ** with less then notice, plus shipping costs. Additional label sets provided
at an additional fee of **. Additional requisition forms provided at an additional fee of*, Additional collection flow charts (CFC) provided at an additional fee of ** *Set up of additional sites will incur additional site initiation fees and other
applicable charges. Database modifications will be invoiced at ** Non-Standard Services for Data Management and Custom programming will be supplied upon request and billed at a programming rate of ** for services included but not limited to: a.
Custom data file formats b. Custom data management reports c. Data reconciliation requirements Returned kit fee of ** (break-down and disposal of kit contents) plus return shipping charges. Hard copy reports will be invoiced at **. Translation costs
reflect the average cost to translate a typical manual Translation costs for other documents besides the manual, will be charged ** Lab Manuals will be supplied to all sites upon initiation as part of the study set-up. Amended or revised manuals
will be supplied at ** If adjustments to kits are required, the kit tier may be revised and billed at the following rates: This budget for centrai laboratory services is based upon protocol requirements provided at the time of the RFP and is an
estimate only. PPD Central Labs will invoice Sponsor for actual services rendered and testing performed. Invoices may, therefore, differ from the Budget due to differences in actual services rendered versus those contained within this Budget. PPD
Proprietary and Confidential PPDBC#: 5S004-Q1 Date Issued: 17 February 2016 to the omitted portions. Kit Tier NA & LATAM EMEA AsiaPac ** ** ** ** ** ** ** ** ** ** **
Modification Number: 1
PROJECT ADDENDUM MODIFICATION
Exhibit D
Revised Study Budget
The Revised Study Budget follows this cover page
Page 20 of 31
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulativs Study Budget as of Project Addendum _Modification #1_ Cumulative
Study Budget as of Project Addendum Incremental Budget as of Project Addendum _Modification #1_
Department/Activity Budget NA Budget EMEA Total Budget Budget NA
Budget EMEA Total Budget Budget NA Budget EMEA Total Budget
Project Management ** ** ** ** ** ** ** ** **
Study Start-Up ** ** ** ** ** ** ** ** **
Clinical Management ** ** ** ** ** ** ** ** **
Global Clinical Supplies ** ** ** ** ** ** ** ** **
IVRS ** ** ** ** ** ** **
** **
Data Management ** ** ** ** ** ** ** ** **
Pharmacovigilance ** ** **
** ** ** ** ** **
Biostatistics ** ** ** ** ** ** ** ** **
Medical Writing **
** ** ** ** ** ** ** **
Quality Assurance ** ** ** ** ** ** ** ** **
Regulatory Affairs ** ** ** ** ** ** ** ** **
Clinical Pharmacology (PK) **
** ** ** ** ** ** ** **
Electronic Data Capture ** ** ** ** ** ** ** ** **
TOTAL DIRECT COSTS ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
TOTAL DISCOUNTED DIRECT COSTS ** ** ** ** ** ** ** ** **
TOTAL PASS THROUGH COSTS ** ** ** **
** ** ** ** **
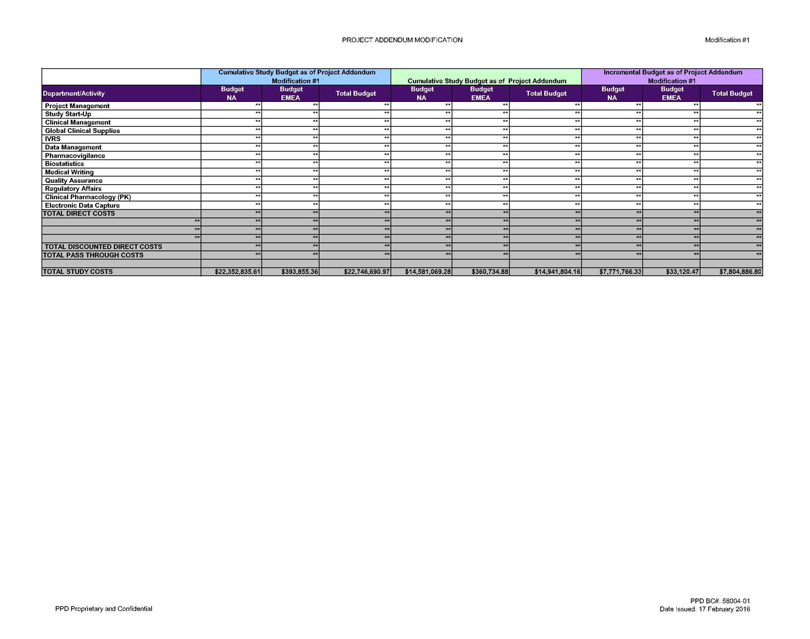
TOTAL STUDY COSTS $22,352,835.61 $393,855.36 $22,746,690.97 $14,581,069.28 $360,734.88 $14,941,804.16 $7,771,766.33 $33,120.47 $7,804,886.80
PPD Proprietary and Confidential
PPD BC#. 58004-01 Date Issued.
17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Unit Type Total Hours NA Unit Cost NA # of Units NA Budget NA Total Hours EMEA Unit Cost EMEA # of Units EMEA Budget EMEA Total Budget
Project Management
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Study Start-Up
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued. 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Unit Type Total Hours NA Unit Cost NA # of Units NA Budget NA Total Hours EMEA Unit Cost EMEA # of Units EMEA Budget EMEA Total Budget
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Clinical Management
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued. 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Unit Type Total Hours NA Unit Cost NA # of Units NA Budget NA Total Hours EMEA Unit Cost EMEA # of Units EMEA Budget EMEA Total Budget
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Global Clinical Supplies
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
IVRS
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued: 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Unit Type Total Hours NA Unit Cost NA # of Units NA Budget NA Total Hours EMEA Unit Cost EMEA # of Units EMEA Budget EMEA Total Budget
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Data Management
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Pharmacovigilance
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#. 58004-01 Date Issued. 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Unit Type Total Hours NA Unit Cost NA # of Units NA Budget NA Total Hours EMEA Unit Cost EMEA # of Units EMEA Budget EMEA Total Budget
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Biostatistics
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued. 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Unit Type Total Hours NA Unit Cost NA # of Units NA Budget NA Total Hours EMEA Unit Cost EMEA # of Units EMEA Budget EMEA Total Budget
*” ** ** ** ** ** ** ** **
** ** ** ** ** ** ** **
Medical Writinq
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Quality Assurance
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004 01 Date Issued. 17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Unit Type Total Hours NA Unit Cost NA # of Units NA Budget NA Total Hours EMEA Unit Cost EMEA # of Units EMEA Budget EMEA Total Budget
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Regulatory Affairs
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Clinical Pharmacoloqv (PK)
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Electronic Data Capture
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Total Direct Costs ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Total Discounted Direct Costs ** ** ** ** ** ** ** ** ** **
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued:
17 February 2016
|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PROJECT ADDENDUM MODIFICATION
Modification #1
Cumulative Study Budget as of Project Addendum Modification #1
Department/Activity Unit Type Total Hours NA Unit Cost NA # of Units NA Budget NA Total Hours EMEA Unit Cost EMEA # of Units EMEA Budget EMEA Total Budget
Pass Throuqh Costs
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
** ** ** ** ** ** ** ** ** ** **
Total Study Costs $22,352,835.61 $393,855.36 $22,746,690.97
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued: 17 February 2016
Modification Number: 1
PROJECT ADDENDUM MODIFICATION
Exhibit E
Revised Payment Schedule
The Revised Payment Schedule follows this cover page
Page 30 of 31

|
| ** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
PPD Payment Schedule
Sponsor: Galectin BC Number: 58004-01 Protocol :GT-026
Direct Costs:
Execution of Contract **
Monthly **
Project Management Fee **
** **
Milestones: **
** **
**
**
**
**
** **
**
** **
** **
** **
**
** **
** **
Total Direct Costs **
Indirect Costs: **
** **
Total Clinical Grants **
** **
Total Pass Through Costs **
Project Grand Total 22,746,690.97
** **
** **
| * | * ** |
PPD Proprietary and Confidential
PPD BC#: 58004-01 Date Issued: 17 February 2016
