Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - BRAINSTORM CELL THERAPEUTICS INC. | v432184_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - BRAINSTORM CELL THERAPEUTICS INC. | v432184_ex31-2.htm |
| EX-10.53 - EXHIBIT 10.53 - BRAINSTORM CELL THERAPEUTICS INC. | v432184_ex10-53.htm |
| EX-21 - EXHIBIT 21 - BRAINSTORM CELL THERAPEUTICS INC. | v432184_ex21.htm |
| EX-32.2 - EXHIBIT 32.2 - BRAINSTORM CELL THERAPEUTICS INC. | v432184_ex32-2.htm |
| EX-31.1 - EXHIBIT 31.1 - BRAINSTORM CELL THERAPEUTICS INC. | v432184_ex31-1.htm |
| EX-23.1 - EXHIBIT 23.1 - BRAINSTORM CELL THERAPEUTICS INC. | v432184_ex23-1.htm |
| EX-10.12 - EXHIBIT 10.12 - BRAINSTORM CELL THERAPEUTICS INC. | v432184_ex10-12.htm |
| 10-K - FORM 10-K - BRAINSTORM CELL THERAPEUTICS INC. | v432184_10k.htm |
Exhibit 10.11
AMENDMENT NO. 2
TO SECOND AMENDED AND RESTATED RESEARCH AND LICENSE
AGREEMENT
This amendment no. 2 to the Second Amended and Restated Research and License Agreement (this “Amendment”) is entered into as of April 30, 2014 (the “Amendment Effective Date”), by and between Ramot at Tel Aviv University Ltd., a company organized under the laws of Israel with offices at Tel Aviv University, Tel Aviv, Israel (“Ramot”) and Brainstorm Cell Therapeutics Ltd., a limited liability company incorporated under the laws of Israel with offices at 12 Bazel Street, Petach Tikva, Israel 49170 (the “Company”).
WHEREAS, Ramot and Brainstorm Cell Therapeutics Inc. executed a Second Amended and Restated Research and License Agreement dated July 26, 2007, as amended on December 24, 2009 which was assigned from Brainstorm Cell Therapeutics Inc. to the Company on December 20, 2011 (the “Agreement”); and
WHEREAS, the Company and Ramot wish to have the TAU Team perform additional research under the Agreement as set forth herein.
NOW, THEREFORE, the parties hereto, intending to be legally bound, hereby agree as follows:
| 1. | Definitions. |
All terms used in this Amendment with an initial capital letter shall have the meanings ascribed to them in the Agreement, unless otherwised specified herein.
| 2. | New Research Period. |
Although the parties agreed that the Research Period ended on July 11, 2007, the parties wish to conduct additional research commencing on the Amendment Effective Date, for a period of 6 months (the “New Research Period”).
| 3. | New Research Plan. |
The new Research Plan is attached hereto as Exhibit A.
| 4. | New Research Funding. |
The Company shall fund the new Research performed during the New Research Period in the total amount of $25,000 plus value added tax, which shall be paid to Ramot as follows:
| - | $12,500 plus value added tax within 10 days of the Amendment Effective Date. |
| - | $12,500 plus value added tax within 60 days of the Amendment Effective Date. |
| 5. | Principal Investigator. |
For the purposes of Section 2 of the Agreement, the “Principal Investigator” shall be “Dr. Daniel Offen, or such other proncipal investigator who may replace him pursuant to Section 2.”
| 6. | General. |
Except for the terms revised herein, all other terms and conditions of the Agreement shall continue to apply and shall remain in full force and effect.
IN WITNESS HEREOF, the parties have caused this Amendment to be executed by their duly authorized representatives as of the date first written above.
| Ramot at Tel Aviv University, Ltd. | Brainstorm Cell Therapeutics Ltd. | |||
| By: | /s/ Shlomo Nimrodi | By: | /s/ Liat Sossover | |
| Name: | Shlomo Nimrodi | Name: | Liat Sossover | |
| Title: | CEO | Title: | CFO | |
| By: | /s/ Avi Nataneli |
| ||
| Name: | Avi Nataneli | |||
| Title: | Chief Financial Officer | |||
Dani Offen PhD
Neurotrophic factors secreting cells for the treatment of Autism Spectrum Disorders (ASD)
Rationale for the study
In the Neuroscience Laboratory, FMRC (Tel Aviv Uni.) we have vast experience using mesenchymal stem cells (MSCs) transplantation as a tool for treating brain diseases. In the current project, we plan to further explore this therapeutic strategy towards a clinical tool for the treatment of ASD by transplantation of MSC-NTF cells (Brainstorm protocol) to the brain through intraventricular transplantation in animal models relevant to autism.
In a translational perspective, this technology will be presented as a novel therapy for ASD. Importantly, our preliminary study involved transplantation into the brain ventricles, which may be translatable to intrathecal transplantation in ASD patients.
Scientific background
Autism is a debilitating neuropsychiatric developmental condition affecting 1% of the population. Current available treatments offer no cure for patients affected with ASD. The mainstay of treatment includes behavioral interventions and the use of psychiatric medications to control symptoms often associated with ASD, such as aggression and agitation. Therefore, ASD research is focused on the search for novel therapeutic approaches.
To date, few studies have reported beneficial pharmacological interventions in ASD animal models which were described to improve some aspects of disease but not all the behavior features.
The difficulty of finding therapeutic agents capable of reducing the three symptoms (repetitive, cognitive and social behavior) emphasizes the beneficial potential of MSC implantation, since our preliminary experiments showed that MSCs-treated mice demonstrated improved function in all criteria tested. Mechanistically, stem cell based regenerative approaches have the potential to enhance repair to an ill-developing brain and perhaps be more effective in presenting a cure rather than pharmacological control of symptoms. Our previous studies have shown that MSCs transplantation indeed enhance the brain regenerative capacity (neurogenesis), a process that is known to be induced by the neurotrophic factors we plan to deliver in this study.
Preliminary results
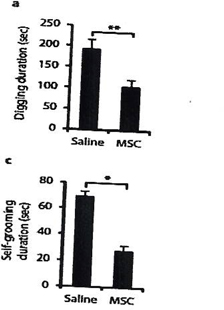
In a proof of concept study, we have shown that transplantation of MSCs diminishes core autistic symptoms in a mouse model relevant to autism (the BTBR mice) as manifested by the reduction of repetitive and stereotypical behavior (Figure 1) and improved cognitive flexibility (Figure 2) and social behavior (Figure 3). The behavioral effect of the cell therapy was accompanied by a significant increase in brain derived neurotrophic (BDNF) levels in the hippocampus of mice. Also, there was a significant enhancement of neurogenesis in the hippocampus of stem cells-treated mice.
Study aim: To evaluate the effect of transplantation of NTF-MSC compared to MSC on autistic behaviors of the BTBR mouse model of ASD
Specific aims:
| • | To evaluate transplantation outcome as observed in various behavioral tests representing core autistic behaviors including (1) repetitive behavior (2) social behavior (3) cognitive flexibility (2 months). |
| • | To evaluate the regenerative effect of transplantation on the brain as observed by measurements of neurotrophic factors levels in situ (one month). |
Dani Often PhD
We will include three treatment groups:
(1) intraventricular transplantation sham surgery (n=15)
(2) intraventricular transplantation human MSCs transplantation (n=15)
(3) intraventricular transplantation human MSC-NTFCs transplantation (n=15)
The cells will be provided by Brainstorm. ELISA tests will be performed in collaboration with Brainstorm.
Budget: $25,000
The costs include BTBR mice (45), cell transplantation, animal maintenance, behavioral tests, tissue analysis and Ramot overheads (35%)
 |
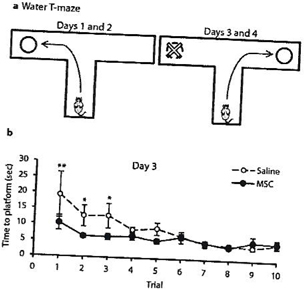 | |
| Figure. 1. MSCs transplantation reduce the repetitive behaviors (total duration of digging and self-grooming). | Figure. 2. Effect of MSCs transplantation on cognitive rigidity (time of reversal learning after the platform was moved to the other arm). |
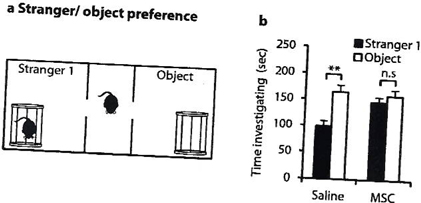
Figure. 3. MSCs transplantation improve sociability (sniffing durations in cage contained a stranger mouse).

