Attached files
| file | filename |
|---|---|
| EX-32.1 - CERTIFICATION - Mawson Infrastructure Group Inc. | f10k2015ex32i_ophthalix.htm |
| EX-31.2 - CERTIFICATION - Mawson Infrastructure Group Inc. | f10k2015ex31ii_ophthalix.htm |
| EX-32.2 - CERTIFICATION - Mawson Infrastructure Group Inc. | f10k2015ex32ii_ophthalix.htm |
| EX-31.1 - CERTIFICATION - Mawson Infrastructure Group Inc. | f10k2015ex31i_ophthalix.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: December 31, 2015
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from _____________ to _____________
Commission File No. 000-52545
OphthaliX Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 88-0445167 | |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
10 Bareket St, Petach Tikva, Israel, 4951778
(Address of principal executive offices)
Issuer’s telephone number: +(972) 3-9241114
Securities Registered pursuant to Section 12(b) of the Act: None
Securities Registered pursuant to Section 12(g) of the Exchange Act: Common Stock, $.001 par Value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | ||
| Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company | ☒ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was $3,441,848 computed by reference to the average bid and asked price of the Common Stock as of the last business day of the registrant’s most recently completed second fiscal quarter.
At March 1, 2016, there were 10,441,251 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: NONE
TABLE OF CONTENTS
| PART I | ||
| Item 1 | Business | 3 |
| Item 1a | Risk Factors | 22 |
| Item 1b | Unresolved Staff Comments | 42 |
| Item 2 | Properties | 42 |
| Item 3 | Legal Proceedings | 42 |
| Item 4 | Mine Safety Disclosures | 42 |
| PART II | ||
| Item 5 | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 43 |
| Item 6 | Selected Financial Data | 43 |
| Item 7 | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 43 |
| Item 7a | Quantitative and Qualitative Disclosures About Market Risk | 46 |
| Item 8 | Financial Statements and Supplementary Data | F-1 |
| Item 9 | Changes In and Disagreements With Accountants on Accounting and Financial Disclosure | 47 |
| Item 9a | Controls and Procedures | 47 |
| Item 9b | Other Information | 48 |
| PART III | ||
| Item 10 | Directors, Executive Officers and Corporate Governance | 48 |
| Item 11 | Executive Compensation | 52 |
| Item 12 | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 54 |
| Item 13 | Certain Relationships and Related Transactions, Director Independence | 57 |
| Item 14 | Principal Accounting Fees and Services | 58 |
| PART IV | ||
| Item 15 | Exhibits, Financial Statement Schedules | 59 |
| SIGNATURES | 60 |
Throughout this report, unless otherwise designated, the terms “we,” “us,” “our,” “the Company” and “our company” refer to OphthaliX Inc., a Delaware corporation, and its Israeli subsidiary, Eyefite Ltd. All amounts in this report are in U.S. Dollars, unless otherwise indicated.
| - 2 - |
FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements about our expectations, beliefs or intentions regarding, among other things, our product development efforts, business, financial condition, results of operations, strategies or prospects. In addition, from time to time, we or our representatives have made or may make forward-looking statements, orally or in writing. Forward-looking statements can be identified by the use of forward-looking words such as “believe,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that these statements do not relate strictly to historical or current matters. These forward-looking statements may be included in, but are not limited to, various filings made by us with the United States Securities and Exchange Commission, or the SEC, press releases or oral statements made by or with the approval of one of our authorized executive officers. Forward-looking statements relate to anticipated or expected events, activities, trends or results as of the date they are made. Because forward-looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward-looking statements. Many factors could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including, but not limited to, those set forth in our most recent annual reports referenced below.
This report identifies important factors which could cause our actual results to differ materially from those indicated by the forward-looking statements, particularly those set forth under Item 1A. “Risk Factors”. Such risk factors are not necessarily all of the important factors that could cause actual results to differ materially from those expressed in any of our forward-looking statements. Given these uncertainties, readers are cautioned not to place undue reliance on such forward-looking statements.
All forward-looking statements attributable to us or persons acting on our behalf speak only as of the date of this report and are expressly qualified in their entirety by the cautionary statements included in this report. We undertake no obligations to update or revise forward-looking statements to reflect events or circumstances that arise after the date made or to reflect the occurrence of unanticipated events. In evaluating forward-looking statements, you should consider these risks and uncertainties.
PART I
ITEM 1. BUSINESS.
Business Overview
We are a clinical-stage biopharmaceutical company focused on developing therapeutic products for the treatment of ophthalmic disorders. We have in-licensed certain patents and patent applications protecting the use in the ophthalmic field of our current pipeline drug under development, a synthetic A3 adenosine receptor, or A3AR, agonist, CF101 (known generically as IB-MECA). CF101 is currently being developed by us to treat two ophthalmic indications: glaucoma and uveitis. We are currently conducting a Phase II trial with respect to the development of CF101 for the treatment of glaucoma or related syndromes of ocular hypertension.
CF101 is a highly-selective, orally bioavailable small molecule synthetic drug, which targets the A3AR. We believe that CF101 has a favorable safety profile and a potent anti-inflammatory activity, mediated via its capability to inhibit the production of inflammatory cytokines, such as TNF-α, MMPs, IL-1, and IL-6. This is mediated by activation of the A3AR, which is highly expressed in inflammatory tissues in contrast to normal tissues where expression levels of the receptor are very low. We believe that the anti-inflammatory and neuro-protective effects of CF101 make it a candidate for use in the treatment of ophthalmic indications.
We are focused on the development of CF101 for the treatment of glaucoma, with a Phase II study ongoing in Israel and Europe. This study is a randomized, double-masked, placebo-controlled, parallel-group study of the safety and efficacy of daily CF101 administered orally in subjects with elevated IOP. The objective of this study is to determine the safety and efficacy of oral CF101 in lowering IOP when administered BID for 16 weeks in subjects with elevated IOP. We have enrolled 44 subjects in the first segment of the study, randomized in a 3:1 ratio of CF101 1.0 mg treatment to the placebo. In 2015, we amended the ongoing Phase II study protocol and enrolled a further 44 subjects for the second segment, which was randomized in a 3:1 ratio of CF101 2.0 mg treatment to the placebo. This decision was based on certain positive data from the psoriasis Phase II/III study of CF101 by our majority stockholder and parent, Can-Fite BioPharma Ltd, or Can-Fite. As a result, there will not be an interim analysis and the full study data is expected to be announced in the second half of 2016.
| - 3 - |
If successful, the treatment of glaucoma with an oral drug has the potential to be a breakthrough treatment in resolving patient compliance issues with current topical treatments. A third-party validation for the utilization of A3 adenosine receptor agonists for lowering intraocular pressure and treating glaucoma has been published by Professor M. Francesca Cordeiro, a Professor of Glaucoma & Retinal Neuro-degeneration at the University College of London and Imperial College in London.
According to Market Scope the global glaucoma pharmaceutical market is expected to be nearly $6.1 billion in 2020 and according to GlobalData the global uveitis therapeutics market is expected to grow from $0.3 billion in 2010 to $1.6 billion by 2017. None of our product candidates have been approved for sale or marketing and, to date, there have been no commercial sales of any of our product candidates.
Our corporate strategy is to build a specialized ophthalmic company that develops and in-licenses drugs for the treatment of ophthalmic diseases. We intend to seek to obtain technologies that complement and expand our existing pipeline by entering into in-license or co-development agreements with academic institutions and biotechnology or pharmaceutical companies. We intend to commercialize our products through out-licensing arrangements with third parties who may perform any or all of the following tasks: completing development, securing regulatory approvals, manufacturing, marketing and sales. We do not intend to develop our own manufacturing facilities or sales forces.
CF101
CF101 is in development for the treatment of autoimmune-inflammatory diseases, psoriasis, rheumatoid arthritis and osteoarthritis, and the ophthalmic diseases, glaucoma and uveitis. In certain of our pharmacological studies, CF101 has also shown potential for development for the treatment of Crohn’s disease. While our majority stockholder and parent Can-Fite is developing CF101 for the treatment of autoimmune-inflammatory diseases, we have in-licensed from Can-Fite certain patents and patent applications for the development of CF101 for ophthalmic indications. In 2014, we decided to end the development of CF101 for the dry eye syndrome, or DES indication. This decision was based on a lack of correlation between patients' response to CF101 and over-expression of the drug target, the A3 adenosine receptor in this patient population.
CF101 is a highly-selective, orally bioavailable small molecule synthetic drug, which targets the A3AR. Based on clinical studies to date undertaken by Can-Fite and us, we believe that CF101 has a favorable safety profile and significant anti-inflammatory effects as a result of its capability to inhibit the production of inflammatory cytokines, such as TNF-α, IL-6 and IL-1, and chemokines, or small cytokines, such as MMPs, by signaling key proteins such as NF-кB and PKB/AKT. Overall, these up-stream events result in apoptosis of inflammatory cells. See Figure 1 below. CF101’s anti-inflammatory effect is mediated via the A3AR, which is highly expressed in inflammatory cells.
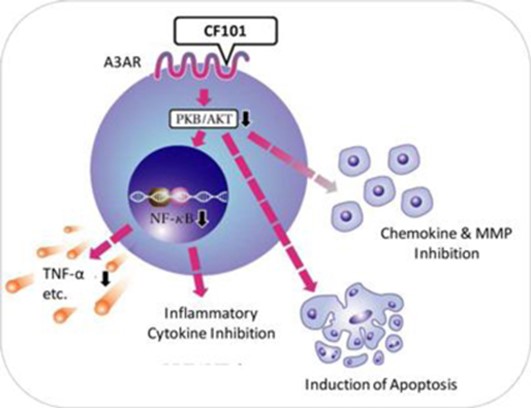
Figure 1: CF101 anti-inflammatory mechanism of action
| - 4 - |
CF101 – Pre-Clinical and Clinical Profile
Pre-Clinical Studies of CF101
The information below is based on the various studies conducted with CF101, including preclinical studies. All of the studies were conducted by Can-Fite and/or by Can-Fite’s partners or affiliates.
Pre-clinical studies are a set of experiments carried out in animals to show that a certain drug does not evoke toxicity. Based on the animal studies and safety data, one can approach the FDA and request permission to conduct a Phase I study in human beings.
The toxicity of CF101 has been evaluated following 28-day, 90-day, six-month and nine-month good laboratory practice repeated-dose toxicity studies in male and female mice (28-day, 90-day and six-month), dogs (single-dose only), and monkeys (28-day, 90-day and nine-month). Even though the dose of CF101 in these studies was escalated to an exposure that is many folds higher than the dose used in human clinical studies, no toxic side effects were identified.
Effects on cardiovascular parameters were evaluated in conscious instrumented monkeys and anesthetized dogs. These studies demonstrated no significant cardiovascular risk.
Genotoxicity studies were conducted in bacterial and mammalian mutation assays in vitro (i.e., laboratory) and in an in vivo (i.e., animal) mouse micronucleus assay. These studies were all negative, indicating no deleterious action on cellular genetic material.
Reproductive toxicology studies that we completed in mice and rabbits did not reveal evidence of negative effects on male or female fertility. In mouse teratology studies, or studies for abnormalities of physiological developments, craniofacial and skeletal abnormalities were observed at doses greater than 10 mg/kg; however, no such effects were observed at 3 mg/kg demonstrating the safety of the drug in this concentration range. Teratogenicity, or any developmental anomaly in a fetus, was not observed in rabbits given doses (greater than 13 mg/kg) that induced severe maternal toxicity in such rabbits.
Studies of P450 enzymes, or enzymes that participate in the metabolism of drugs, showed that CF101 caused no P450 enzyme inhibition, or increased drug activity, or induction, or reduced drug activity. Studies carried out with radiolabeled (C14) CF101 in rats showed that the drug is excreted essentially unchanged. These studies also showed that the drug is widely distributed in all body parts, except the central nervous system.
Clinical Studies of CF101
The information below is based on the various studies conducted with CF101, including clinical studies in patients with autoimmune inflammatory and non-ophthalmic related diseases. All of the studies were conducted by Can-Fite and/or by Can-Fite’s partners or affiliates.
Phase I Clinical Studies of CF101
CF101 has been studied comprehensively in normal volunteer trials to assess safety, pharmacokinetic metabolism and food interaction. Two Phase I studies in 40 healthy volunteers, single dose and repeated dose, indicated that CF101 is rapidly absorbed (reaching a maximal concentration within one to two hours) with a half-life of eight to nine hours. Some mild adverse events (principally, increased heart rate) were observed at doses higher than single doses of 10.0 mg and twice-daily doses of 5.0 mg. Such increase in heart rate was not accompanied by any change in QT intervals. The drug showed linear kinetics, in that the concentration that results from the dose is proportional to the dose and the rate of elimination of the drug is proportional to the concentration, and low inter-subject variability, meaning that the same dose of the drug does not produce large differences in pharmacological responses in different individuals. A fed-fast Phase I study (with and without food) demonstrated that food causes some attenuation in CF101 absorption; accordingly CF101 is administered to patients on an empty stomach. An additional Phase I study of the absorption, metabolism, excretion and mass balance of 4.0 mg (C14) CF101 was conducted in six healthy male subjects and demonstrated that CF101 was generally well-tolerated in this group.
Based on the findings from Phase I clinical studies, 4.0 mg BID, or twice daily, was selected as the upper limit for initial Phase II clinical trials.
| - 5 - |
Phase II, Phase II/III and Phase III Clinical Studies of CF101
CF101 has completed eight Phase II studies, one Phase II/III study and one Phase III study in psoriasis, rheumatoid arthritis or DES, in approximately 1,129 patients for an aggregate exposure of approximately 350 patient years. These studies indicate that CF101 has a favorable safety profile at doses up to 4.0 mg BID for up to 32 weeks. In these II studies, we did not observe a dose-response relationship between CF101 and adverse events. Moreover, we did not observe any clinically significant changes in vital signs, electrocardiograms, blood chemistry or hematology. CF101 given as a standalone therapy reached the primary endpoint in Phase II clinical studies in DES, as further detailed below. In addition to the ophthalmic indications, positive data were observed utilizing CF101 as a standalone drug in three Phase II clinical studies in psoriasis and rheumatoid arthritis. However, a Phase III study of CF101 for DES and two Phase IIb studies in rheumatoid arthritis utilizing CF101 in combination with methotrexate failed to reach the primary endpoint and a Phase II/III study of CF101 for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests CF101 as a potential systemic therapy for patients with moderate-severe psoriasis. Can-Fite continues to develop CF101 as a standalone therapy for auto-immune inflammatory indications including rheumatoid arthritis and psoriasis, in parallel to our development of CF101 for ophthalmic indications. Safety data from these studies will be shared between Can-Fite and the Company.
CF101 for the Treatment of Ophthalmic Indications
We are currently developing CF101 for the treatment of glaucoma and uveitis. In 2014, we decided to end the development of CF101 for the DES indication. This decision was based on a lack of correlation between patients' response to CF101 and over-expression of the drug target, the A3 adenosine receptor in this patient population. Set forth below are general descriptions of the ophthalmic diseases with respect to which CF101 has underwent, is currently undergoing, or is in preparation for clinical trials.
Glaucoma: Glaucoma is an eye disease in which the optic nerve is damaged. This optic nerve damage involves loss of retinal ganglion cells, or neurons located near the inner surface of the retina, in a characteristic pattern. There are many different subtypes of glaucoma, but they can all be considered to be a type of optic neuropathy. Raised intraocular pressure, or IOP, is the most important and only modifiable risk factor for glaucoma. However, some individuals may have high IOP for years and never develop optic nerve damage. This is known as ocular hypertension. Others may develop optic nerve damage at a relatively low IOP, and, thus, glaucoma. Untreated glaucoma can lead to permanent damage of the optic nerve and resultant visual field loss, which over time can progress to blindness.
Glaucoma can be roughly divided into two main categories, “open angle” and “closed angle” glaucoma. The angle refers to the area between the iris and cornea through which fluid must flow to exit the eye. The difficulty or inability of such fluid to exit the eye causes an acute increase of pressure and pain. Closed angle glaucoma can appear suddenly, is often painful and visual loss can progress quickly. However, the discomfort often leads patients to seek medical attention before permanent damage occurs. Open angle, chronic glaucoma tends to progress at a slower rate and patients may not notice they have lost vision until the disease has progressed significantly.
Uveitis: Uveitis is inflammation of the middle layer of the eye, or the uvea, caused by an immune reaction. Uveitis can be associated with auto-immune inflammatory diseases and various eye infections. Uveitis is a common cause of blindness. The most common form of uveitis is anterior uveitis, which involves inflammation in the front part of the eye. It is often called iritis because it usually only affects the iris, the colored part of the eye. The inflammation may be associated with autoimmune diseases, but most cases occur in healthy people. The disorder may affect only one eye and is most common in young and middle-aged people.
Posterior uveitis affects the back part of the uvea, and involves primarily the choroid, a layer of blood vessels and connective tissue in the middle part of the eye. This type of uveitis is called choroiditis. If the retina is also involved, it is called chorioretinitis. Anterior uveitis affects the front part of the uvea, and involves primarily the iris and the cilliary body. This type of uveitis is called iridocyclitis. These conditions may develop as a result of a body-wide, or systemic, infection or an autoimmune disease. Another form of uveitis is pars planitis. This inflammation affects the narrowed area, or the pars plana, between the iris, or colored part of the eye, and the choroid. Pars planitis usually occurs in young men and is generally not associated with any other disease. However, some evidence suggests it may be linked to Crohn’s disease and, possibly, multiple sclerosis.
CF101 for the Treatment of Glaucoma
We believe that the statistically significant decrease in IOP in the Phase II trial for DES, although observed in subjects without ocular hypertension, is clinically significant and indicates that CF101 may also have potential as a glaucoma therapy, as the main goal of glaucoma therapy is to reduce IOP. This finding led to a patent application for the use of CF101 for lowering IOP. It is our belief that this result, together with the neuro-protective and anti-inflammatory effects that have been demonstrated in our studies and the studies of others, warrants rapid progression into clinical study in this indication.
| - 6 - |
A Phase II study in patients with glaucoma or related syndromes of ocular hypertension is currently ongoing in Israel and Europe. This study is a randomized, double-masked, placebo-controlled, parallel-group study of the safety and efficacy of daily CF101 administered orally in subjects with elevated IOP. The objective of this study is to determine the safety and efficacy of oral CF101 in lowering IOP when administered BID for 16 weeks in subjects with elevated IOP. We have enrolled 44 subjects in the first segment of the study, randomized in a 3:1 ratio of CF101 1.0 mg treatment to the placebo. In 2015, we amended the ongoing Phase II study protocol and enrolled a further 44 subjects for the second segment, which was randomized in a 3:1 ratio of CF101 2.0 mg treatment to the placebo. This decision was based on certain positive data from the psoriasis Phase II/III study of CF101 by our majority stockholder and parent, Can-Fite BioPharma Ltd, or Can-Fite. As a result, there will not be an interim analysis and the full study data is expected to be announced in the second half of 2016. Neither we nor Can-Fite has filed an IND for this indication as CF101 for the treatment of glaucoma is not currently being clinically tested in the United States and there are no near-term plans to do so.
CF101 for the Treatment of Uveitis
Former pre-clinical pharmacology studies conducted by Can-Fite in collaboration with a worldwide leading laboratory in uveitis research at the National Eye Institute at the U.S National Institute of Health, or the NIH, under a Cooperative Research and Development Agreement, demonstrated that CF101 was effective in inhibiting the development of posterior uveitis in an experimental animal model. Additional preclinical studies conducted by the Company, showed that CF101 was effective in treating anterior uveitis in experimental animal models.
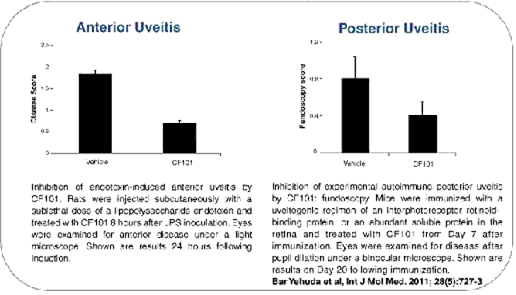
Figure 2: Inhibition of the development of posterior and anterior uveitis in experimental animal models
The efficacy of CF101 in treating both anterior and posterior uveitis in experimental animal models supports further testing of CF101 for the treatment of patients with either anterior or posterior uveitis. Can-Fite, together with the NIH, have applied for a patent for the use of CF101 for the treatment of uveitis. Can-Fite has licensed its share of this intellectual property to us and together we are in discussions with the NIH to obtain an exclusive license on the NIH’s share of this intellectual property. We designed a protocol for a Phase II uveitis study in Europe and Israel to investigate the efficacy and safety of CF101 in 45 patients with active, sight-threatening, noninfectious intermediate or posterior uveitis, who will be treated with either CF101 or a placebo for a period of six months. The primary endpoint of this study is the proportion of subjects whose vitreous haze score improves by two or more grades on the “Miami Scale” (Vitreous Haze: Miami Scale 2). We are currently reviewing our clinical development plans and intend to provide an update on the development for this indication at a later stage. Neither we nor Can-Fite has filed an IND for this indication as CF101 for the treatment of uveitis is not currently being clinically tested in the United States and there are no near-term plans to do so.
| - 7 - |
CF101 for the Treatment of Dry Eye Syndrome
Following positive results in a Phase II study, in December 2012, we initiated a Phase III DES trial, under an IND with the FDA which was conducted by the Company in the United States, Europe and Israel. DES is an eye disease caused by eye dryness, which, in turn, is caused by either decreased tear production or increased tear film evaporation. The randomized, double-masked Phase III clinical trial enrolled 237 patients with moderate-to-severe DES who were randomized to receive two oral doses of CF101 (0.1 and 1.0 mg) and a placebo, for a period of 24 weeks. The primary efficacy endpoint was complete clearing of corneal staining. In December 2013, we announced the results of this Phase III study of CF101 for the treatment of DES. In the study, CF101 did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints. Nonetheless, CF101 was found to be well tolerated. In 2014 we decided to end the development of CF101 for the DES indication. This decision was based on a lack of correlation between patients' response to CF101 and over-expression of the drug target, the A3 adenosine receptor in this patient population.
The License Agreement
On November 21, 2011, we entered into the License Agreement with Eyefite, our wholly-owned subsidiary, and Can-Fite according to which Can-Fite (i) granted to Eyefite a sole and exclusive worldwide license for the use of CF101, Can-Fite’s therapeutic drug candidate, solely in the field of ophthalmic diseases and (ii) assigned to Eyefite its rights, title and interest in and to any and all INDs, to CF101 in the ophthalmic field. The license granted to Eyefite allows Eyefite to sublicense its rights to CF101 to third parties, subject to the satisfaction of certain conditions. Pursuant to the License Agreement, Eyefite has sole responsibility for preparing and maintaining all regulatory documentation with respect to approvals of CF101 in the field of ophthalmic diseases and all approvals and related regulatory documentation shall be Eyefite’s sole and exclusive property. Under the License Agreement, Eyefite was required to assume responsibility for making payments to Can-Fite’s licensor, the NIH, pursuant to, and for the term of, a license agreement between Can-Fite and NIH for certain patent rights relating to CF101. In June 2015, Can-Fite’s license with NIH expired and as a result Eyefite is no longer obligated to make any payments to NIH in connection with Can-Fite’s now expired license with NIH (other than with respect to any accrued and unpaid payments to which NIH may be entitled to). Patent rights granted to Eyefite under the License Agreement by Can-Fite that are not NIH patents are free of any royalties and milestone payments.
The License Agreement will remain in effect until the expiration of the last of the patents licensed thereunder unless earlier terminated by one or both of the parties in accordance with the License Agreement. Can-Fite may terminate the License Agreement upon customary bankruptcy and insolvency events of Eyefite and upon Eyefite’s material breach of the License Agreement, upon 30 days’ prior written notice. Eyefite may terminate the License Agreement upon three months’ prior written notice for any reason and upon 30 days’ prior written notice for Can-Fite’s material breach of the License Agreement.
All inventions resulting from the development and commercialization of CF101 under the License Agreement belong to Can-Fite, whether such were invented solely by Can-Fite, solely by Eyefite or by both of entities. However, the License Agreement also grants Eyefite an exclusive license to use any such inventions in the field of ophthalmic diseases around the world for no additional consideration. Pursuant to the License Agreement, Can-Fite has the sole right to make elections with respect to patent term extension of or supplemental protection certificates with respect to the licensed Can-Fite patents and the sole right to seek and maintain any data exclusivity periods available for CF101. Also pursuant to the License Agreement, Can-Fite has retained the right to prosecute and maintain the patents licensed to us.
The Services Agreement
On November 21, 2011, EyeFite and Can-Fite entered into a Services Agreement pursuant to which Can-Fite manages, as an independent contractor, all activities relating to pre-clinical and clinical studies performed for the development of the ophthalmic indications of CF101. The Services Agreement will remain in force for an unlimited period of time unless earlier terminated as follows: (i) by either party upon six-months’ prior written notice to the other party; or (ii) at any time for cause by either EyeFite (which includes a breach of trust by Can-Fite, Can-Fite’s material breach of the Services Agreement or customary bankruptcy and insolvency events on the part of Can-Fite) or Can-Fite (which includes EyeFite’s material breach of the Services Agreement or the License Agreement, or customary bankruptcy and insolvency events on the part of EyeFite). As consideration for Can-Fite’s services pursuant to the Services Agreement, EyeFite must pay to Can-Fite (i) a services fee (consisting of all expenses and costs incurred by Can-Fite plus 15%, except in relation to patent payments which shall be treated on a pass through basis) and, (ii) additional fees equal to 2.5% of any revenues received by us (or any affiliate of ours including, Eyefite) for rights to CF101 from third-party sublicensees including up-front payments, developmental or commercial milestones, royalties on net sales and any similar payments, but not including payments to support or reimburse us for research, development, manufacturing or commercial expenses or for equity. We are required to pay the additional fees to Can-Fite within 30 days of receipt by us.
In February 2013, Can-Fite issued us a formal letter, which has been updated periodically (most recently in August 2015), stating that Can-Fite agrees to defer payments owed to it under the Services Agreement (under which Can-Fite manages, as an independent contractor, all activities relating to pre-clinical and clinical studies performed for the development of the ophthalmic indications of CF101) beginning on January 31, 2013 until the completion of fundraising by the Company sufficient to cover such deferred payments. As of December 31, 2015, the deferred payments to Can-Fite totaled approximately $3,690,000. In addition, in August 2015, Can-Fite issued a financial support letter pursuant to which it committed to cover any shortfall in our costs and expenses of the operations which are in excess of our available cash to finance our operations, including cash generated from any future sale of Can-Fite shares held by us. Both letters expire in October 2016 and any related balance bears interest at a rate of 3% per annum.
| - 8 - |
Seasonality
Our business and operations are generally not affected by seasonal fluctuations or factors.
Raw Materials and Suppliers
We believe that the raw materials that we require to manufacture CF101 are widely available from numerous suppliers and are generally considered to be generic industrial chemical supplies. We do not rely on a single or unique supplier for the current production of CF101.
Manufacturing
The relevant manufacturers of our drug products for our current clinical trials are compliant with both current Good Manufacturing Practices, or cGMP, and current Good Laboratory Practices, or cGLP. We anticipate that we will continue to rely on third parties to produce our drug products for clinical trials and commercialization. Can-Fite used the Chinese chemical manufacturer Chemspec International Limited, which produces APIs as well as other specialty chemicals, as the manufacturer of CF101.
There can be no assurance that our drug candidates, if approved, can be manufactured in sufficient commercial quantities, in compliance with regulatory requirements and at an acceptable cost. We and our contract manufacturers are, and will be, subject to extensive governmental regulation in connection with the manufacture of any pharmaceutical products or medical devices. We and our contract manufacturers must ensure that all of the processes, methods and equipment are compliant with cGMP and cGLP for drugs on an ongoing basis, as mandated by the FDA and other regulatory authorities, and conduct extensive audits of vendors, contract laboratories and suppliers.
Contract Research Organizations
Currently through Can-Fite under the Services Agreement, we outsource certain clinical development activities to contract research organizations, or CROs. Our clinical CROs are subject to guidelines from the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, which attempt to harmonize the FDA and the European Medicines Agency, or the EMA, regulations and guidelines. Also through Can-Fite under the Services Agreement, we create and implement the drug development plans and manage the CROs according to the specific requirements of the drug candidate under development. To the extent clinical research is conducted by Can-Fite or the CROs (or us in the future), compliance with certain federal regulations, including but not limited to 21 C.F.R. parts 50, 54, 56, 58 and 318, which pertain to, among other things, institutional review boards, informed consent, financial conflicts of interest by investigators, good laboratory practices and submitting IND applications, may be required.
Marketing and Sales
We do not currently have any marketing or sales capabilities. We intend to license to, or enter into strategic alliances with, larger companies in the pharmaceutical business, which are equipped to market and/or sell our products, if any, through their well-developed marketing capabilities and distribution networks throughout the world.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our therapeutic candidates, technology and know-how, to operate without infringing the proprietary rights of others and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions and improvements that we believe are important to the development of our business. We also rely on trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary position.
| - 9 - |
Patents
Pursuant to the License Agreement, Eyefite has a field-of-use exclusive license under a patent portfolio owned or in-licensed by Can-Fite, or the Licensed IP. The Licensed IP includes patent and patent applications owned by Can-Fite or in-licensed by Can-Fite from others covering the manufacture of CF101 and certain other A3AR agonists and the use of CF101 and such other A3AR agonists for the treatment of a variety of ophthalmic diseases, including DES, glaucoma and uveitis. The field of use of the exclusive license to Eyefite includes the use of CF101 in the field of ophthalmology, or the Field. Eyefite is not aware of any issued or pending patent applications that could or would restrict or inhibit its ability to operate.
The patent portfolio for CF101 owned or in-licensed by Can-Fite and relating to the Field is described below and includes both issued patents and pending patent applications that were licensed by Can-Fite to Eyefite for the use within the Field:
| ● | A family of two patents which pertain to the use of A3AR agonists for the treatment of DES and granted in Japan and Mexico. The patents are set to expire in 2026; | |
| ● | a family of patents and pending patent applications which pertains to the use of A3AR agonists for the treatment or reduction of IOP. Such patents were granted in Australia, Israel, Japan and the United States. The patents are set to expire in 2030. The patent applications are pending in the EPO (designating all EPC member states), China, Canada, Mexico, South Korea and Hong Kong, each with a filing date of May 16, 2010 and a priority date of May 17, 2009. The patents that may be issued with respect to these pending patent applications would expire in 2030; and |
| ● | a family of patents and pending patent applications which pertain to the method for synthesizing CF101. Such patents were granted in China, India, Japan and Israel. These patents are set to expire in 2028. The patent applications are pending in the United States and the EPO (designating all EPC member states). Each patent application has a filing date of March 13, 2008 and a priority date of March 14, 2007. The patents that may be issued with respect to these pending applications would expire in 2028. |
We believe that our licensed patents provide broad and comprehensive coverage for the use of CF101 for the treatment of certain ophthalmic disorders. However, as a result of the termination of the NIH license agreement between Can-Fite and the NIH in June 2015 due to patent expiration, we no longer hold rights to a family of composition of matter patents relating to CF101 that were licensed from NIH. Nevertheless, because CF101 may be a new chemical entity (“NCE”), following approval of an NDA, we, if we are the first applicant to obtain NDA approval, may be entitled to five years of data exclusivity in the United States with resp ect to such NCEs. Analogous data and market exclusivity provisions, of varying duration, may be available in Europe and other foreign jurisdictions. We may also be entitled to the rights under Can-Fite’s pharmaceutical use issued patents with respect to CF101, which provide patent exclusivity within the ophthalmic field until the mid-2020s. While we believe that we may be able to protect our exclusivity in the ophthalmic field through such use patent portfolio and such period of exclusivity, the lack of composition of matter patent protection may diminish our ability to maintain a proprietary position for our intended uses of CF101. Moreover, we cannot be certain that we will be the first applicant to obtain an FDA approval for any indication of CF101 and we cannot be certain that we will be entitled to NCE exclusivity. In addition, Can-Fite has discontinued the prosecution of a family of pending patent applications under joint ownership of Can-Fite and NIH pertaining to the use of A3AR agonists for the treatment of uveitis. Such diminution of our proprietary position could have a material adverse effect on our business, results of operation and financial condition.
Pursuant to the License Agreement, Can-Fite has contractually agreed to be responsible for the preparation, filing, prosecution and maintenance of its intellectual property rights and our licensed rights. Can-Fite may also bring any action for infringement of its intellectual property rights which are licensed to us, to the extent that we do not bring any such action. However, the patent positions of biopharmaceutical companies, such as Can-Fite and ourselves, are generally uncertain and involve complex legal and factual questions. Can-Fite’s ability to maintain and solidify its proprietary position for the technology licensed to us will depend on its success in obtaining effective claims and enforcing those claims once granted. There is no certainty that any of Can-Fite’s pending patent applications or those pending patent applications that it licenses, including those licensed to us, will result in the issuance of any patents. The issued patents and those that may issue in the future, including those licensed to Can-Fite, may be challenged, narrowed, circumvented or found to be invalid or unenforceable, which could limit its ability to stop competitors from marketing related products or the length of term of patent protection that we may have for our products under the License Agreement. We cannot be certain that Can-Fite was the first to invent the inventions claimed in its owned or licensed patents or pending patent applications. In addition, our competitors may independently develop similar technologies or duplicate any technology developed by Can-Fite or us, and the rights granted under any issued patents may not provide us, as a licensee thereunder, with any meaningful competitive advantages against these competitors. Furthermore, because of the extensive time required for development, testing and regulatory review of a potential product, before any of our products can be commercialized, any related patent may expire or remain in force for only a short period following commercialization, thereby reducing any advantage of such patent. For more risks associated with the protection of our licensed intellectual property, see “Risk Factors—Risks Relating to Our Intellectual Property”.
| - 10 - |
Trade Secrets
We may rely, in some circumstances, on trade secrets to protect our technology. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements and assignment of inventions agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have confidence in these individuals, organizations and systems, such agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors or others.
Competition
The pharmaceutical industry is characterized by rapidly evolving technology, intense competition and a highly risky, costly and lengthy research and development process. Adequate protection of intellectual property, successful product development, adequate funding and retention of skilled, experienced and professional personnel are among the many factors critical to success in the pharmaceutical industry.
We believe that the characteristics of CF101, may position it well against the competition in the ophthalmic markets, where treatments, when available, often include frequent self-administered eye drops, which may be more difficult than taking pills and may result in less than the full dose of the drug actually entering the eye, have undesirable side effects and do not always treat the underlying cause of the disease.
On the other hand, other drugs on the market, new drugs under development (including drugs that are in more advanced stages of development in comparison to our drug pipeline) and additional drugs that were originally intended for other purposes, but were found effective for purposes targeted by us, may all be competitive to the current drug candidates in our pipeline. In fact, some of these drugs are well established and accepted among patients and physicians in their respective markets, can be efficiently produced and marketed, and are relatively safe. Moreover, other companies of various sizes engage in activities similar to ours. Most, if not all, of our competitors have substantially greater financial and other resources available to them. Competitors include companies with marketed products and/or an advanced research and development pipeline. The competitive landscape in the ophthalmic therapeutics field includes Novartis/Alcon, Allergan, Pfizer, Roche/Genentech, Merck (which acquired Inspire Pharmaceuticals), Santen (which acquired Novagali), Bausch & Lomb (which acquired ISTA Pharmaceuticals and was acquired by Valeant), GlaxoSmithKline, Sanofi-Aventis (which acquired Fovea) Shire (which acquired SARcode) and more.
| - 11 - |
Glaucoma
According to Datamonitor, as of 2010, 7 million people in the seven major markets suffered from glaucoma. Market Scope expects the global glaucoma pharmaceutical market to climb from about $4.7 billion in 2015 to nearly $6.1 billion in 2020 at a compounded annual rate of 5.1%. We expect that the number of people who suffer from glaucoma will increase as the population in each of the seven major markets ages.
The main drugs used to treat glaucoma include Xalatan®, Travatan® and Cosopt®. Xalatan® is recommended by the European Glaucoma Society and American Academy of Ophthalmologists as the first choice for the treatment of glaucoma. According to a Pfizer annual report, Xalatan®, which is marketed by Pfizer, is the leading drug used to treat glaucoma, and had global sales of approximately $0.6 billion in 2013 compared to $1.2 billion in 2011. Travatan® was first launched in the United States in 2001 and then Europe and the certain other markets in 2002. According to Evaluate Pharma, Travatan®, marketed by Alcon, experienced sales of approximately $600 million in 2010 and 2011. Travatan® is administered once each day, which ophthalmologists cite as a significant advantage over other drugs used to treat glaucoma. Cosopt® is the oldest combination therapy in the glaucoma market. Due to the expiration of patents covering Cosopt® in 2008, some ophthalmologists have begun to look to other brands or generic drugs in the treatment of glaucoma. Another leading company in this field is Allergan, which markets Lumigan®, Ganfort™, Alphagan®, and Combigan®, with over $1.0 billion in aggregate revenues in 2011. The glaucoma therapeutics market has witnessed major revenues depletion in the recent years due to a string of patent expirations, which started with the expiration of the Xalatan® patent.
Several therapies are in advanced clinical development for glaucoma. In addition, in 2012, the FDA approved tafluprost ophthalmic solution, Zioptan by Merck, the first preservative-free prostaglandin analog ophthalmic solution, or a solution derived from fatty acids, for the treatment of glaucoma.
While several anti-glaucoma drugs exist, the glaucoma therapeutics market has a high level of unmet need, which mainly arises from the lack of approved drugs targeting the disease’s progression. Many therapies approved provide only symptomatic relief. The therapies which are available for the treatment of glaucoma have shown low to moderate efficacy and safety profiles. Accordingly, there is a significant need for drugs that reduce IOP. In addition, part of the pathogenesis of glaucoma is damage to the optic nerve, so drugs that, in addition to lowering IOP, have a neuroprotective effect, would also satisfy an unmet need. Based on its toxicological profile, we believe that CF101 has the potential to have fewer side effects than existing drugs for the treatment of glaucoma. At the same time, CF101 offers the potential to act as a neuroprotective agent that prevents the death of retinal cells, as well as the potential to lower IOP. We also believe that CF101 will offer less frequent administration than most existing therapies.
Uveitis
According to Data Monitor, uveitis is estimated as the fifth or sixth leading cause of blindness in the United States. The incidence of uveitis worldwide varies from 14 to 52.4 per 100,000 people, while the overall prevalence around the world is reported as 0.73%. We estimate that there are approximately one million uveitis patients around the world. According to GlobalData, in 2010, the uveitis market was $0.32 billion and is estimated to reach $1.6 billion by 2017. The current treatments for uveitis include corticosteroids, anti-metabolites, T-cell inhibitors, alkylating agents and biological drugs, which often involve serious adverse side effects and lack of efficacy. Accordingly, we believe that a need exists for drugs used in the treatment of uveitis that are less toxic and more effective. There are currently several therapies in advance clinical development for anterior and posterior uveitis.
We believe that a need exists for drugs used for the treatment of uveitis that are less toxic and more effective than currently available therapies. Former pre-clinical pharmacology studies demonstrated that CF101 is effective in inhibiting the development of posterior and anterior uveitis and has a favorable safety profile in experimental animal models. We submitted a protocol for a Phase II study of uveitis. We are currently reviewing our clinical development plans and will provide an update at the development for this indication at a later stage.
Insurance
We maintain insurance for directors’ and officers’ liability with a coverage limit of $10.0 million in the aggregate for any and all losses arising out of any and all claims against our directors and officers, except for certain wrongful acts and certain claims arising out of securities offerings.
Our majority stockholder and parent, Can-Fite, which conducts our current clinical studies pursuant to the Services Agreement, maintains worldwide product and clinical trial liability insurance with a coverage limit of approximately $3.0 million with respect to the use of CF101 in clinical trials, including for indications other than ophthalmic diseases. Can-Fite also procures additional insurance coverage for each specific clinical trial it conducts, which includes coverage for a certain number of trial participants and varies based on the particular clinical trial. Certain of such policies are based on compliance with the Declaration of Helsinki, which is a set of ethical principles regarding human experimentation developed for the medical community by the World Medical Association and certain protocols from various health authorities throughout the world, some of which may be costly to comply with.
| - 12 - |
We believe that our insurance policies are adequate and customary for a business of our kind. However, because of the nature of our business, we cannot assure you that we will be able to maintain insurance on a commercially reasonable basis or at all, or that any future claims will not exceed our insurance coverage.
Environmental Matters
We and our agents, including Can-Fite pursuant to the Services Agreement, are subject to various environmental, health and safety laws and regulations, including those governing air emissions, water and wastewater discharges, noise emissions, the use, management and disposal of hazardous, radioactive and biological materials and wastes and the cleanup of contaminated sites. We believe that our business, operations and facilities, including those of our agents and service providers, such as Can-Fite, are being operated in compliance in all material respects with applicable environmental and health and safety laws and regulations. Can-Fite’s laboratory personnel in Israel have ongoing communication with the Israeli Ministry of Environmental Protection in order to verify compliance with relevant instructions and regulations. In addition, all laboratory personnel participate in instruction on the proper handling of chemicals, including hazardous substances before commencing employment, and during the course of their employment. In addition, all information with respect to any chemical substance is filed and stored as a Material Safety Data Sheet, as required by applicable environmental regulations. Based on information currently available to us, we do not expect environmental costs and contingencies to have a material adverse effect on us. The operation of testing facilities, however, entails risks in these areas. Significant expenditures could be required in the future if these facilities are required to comply with new or more stringent environmental or health and safety laws, regulations or requirements.
Research and Development
Research and development expenses for the year ended December 31, 2015 were approximately $812,000 compared to approximately $721,000 for the year ended December 31, 2014.
Government Regulation
We operate in a highly controlled regulatory environment. Stringent regulations establish requirements relating to analytical, toxicological and clinical standards and protocols in respect of the testing of pharmaceuticals. Regulations also cover research, development, manufacturing and reporting procedures, both pre- and post-approval. In many markets, especially in Europe, marketing and pricing strategies are subject to national legislation or administrative practices that include requirements to demonstrate not only the quality, safety and efficacy of a new product, but also its cost-effectiveness relating to other treatment options. Failure to comply with regulations can result in stringent sanctions, including product recalls, withdrawal of approvals, seizure of products and criminal prosecution.
Before obtaining regulatory approvals for the commercial sale of our product candidates, we must demonstrate through preclinical studies and clinical trials that our product candidates are safe and effective. Historically, the results from preclinical studies and early clinical trials often have not accurately predicted results of later clinical trials. In addition, a number of pharmaceutical products have shown promising results in clinical trials but subsequently failed to establish sufficient safety and efficacy results to obtain necessary regulatory approvals. We have incurred and will continue to incur substantial expense for, and devote a significant amount of time to, preclinical studies and clinical trials. Many factors can delay the commencement and rate of completion of clinical trials, including the inability to recruit patients at the expected rate, the inability to follow patients adequately after treatment, the failure to manufacture sufficient quantities of materials used for clinical trials, and the emergence of unforeseen safety issues and governmental and regulatory delays. If a product candidate fails to demonstrate safety and efficacy in clinical trials, this failure may delay development of other product candidates and hinder our ability to conduct related preclinical studies and clinical trials. Additionally, as a result of these failures, we may also be unable to obtain additional financing.
Governmental authorities in all major markets require that a new pharmaceutical product be approved or exempted from approval before it is marketed, and have established high standards for technical appraisal, which can result in an expensive and lengthy approval process. The time to obtain approval varies by country and some products are never approved. The lengthy process of conducting clinical trials, seeking approval and the subsequent compliance with applicable statutes and regulations, if approval is obtained, are very costly and require the expenditure of substantial resources.
| - 13 - |
A summary of the U.S., EU and Israeli regulatory processes follow below.
United States
In the United States, the Public Health Services Act and the Federal Food, Drug, and Cosmetic Act, as amended, and the regulations promulgated thereunder, and other federal and state statutes and regulations govern, among other things, the safety and effectiveness standards for our products and the raw materials and components used in the production of, testing, manufacture, labeling, storage, record keeping, approval, advertising and promotion of our products on a product-by-product basis.
Preclinical tests include in vitro and in vivo evaluation of the product candidate, its chemistry, formulation and stability, and animal studies to assess potential safety and efficacy. Certain preclinical tests must be conducted in compliance with good laboratory practice regulations. Violations of these regulations can, in some cases, lead to invalidation of the studies, requiring them to be replicated. After laboratory analysis and preclinical testing, a sponsor files an Investigational New Drug application, or IND, with the FDA to begin human testing. Typically, a manufacturer conducts a three-phase human clinical testing program which itself is subject to numerous laws and regulatory requirements, including adequate monitoring, reporting, record keeping and informed consent. In Phase I, small clinical trials are conducted to determine the safety and proper dose ranges of our product candidates. In Phase II, clinical trials are conducted to assess safety and gain preliminary evidence of the efficacy of our product candidates. In Phase III, clinical trials are conducted to provide sufficient data for the statistically valid evidence of safety and efficacy. The time and expense required for us to perform this clinical testing can vary and is substantial. We cannot be certain that we will successfully complete Phase I, Phase II or Phase III testing of our product candidates within any specific time period, if at all. Furthermore, the FDA, the Institutional Review Board responsible for approving and monitoring the clinical trials at a given site, the Data Safety Monitoring Board, where one is used, or we may suspend the clinical trials at any time on various grounds, including a finding that subjects or patients are exposed to unacceptable health risk.
If the clinical data from these clinical trials (Phases I, II and III) is deemed to support the safety and effectiveness of the candidate product for its intended use, then we may proceed to seek to file with the FDA an NDA seeking approval to market a new drug for one or more specified intended uses. We have not completed clinical trials for any candidate product for any intended use and therefore, we cannot ascertain whether the clinical data will support and justify filing an NDA. Nevertheless, if and when we are able to ascertain that the clinical data supports and justifies filing an NDA, we intend to make such appropriate filings.
The purpose of an NDA is to provide the FDA with sufficient information so that it can assess whether it ought to approve the candidate product for marketing for specific intended uses. The NDA normally contains, among other things, sections describing the chemistry, manufacturing, and controls, non-clinical pharmacology and toxicology, human pharmacokinetics and bioavailability, microbiology, the results of the clinical trials, and the proposed labeling which contains, among other things, the intended uses of the candidate product.
We cannot take any action to market any new drug or biologic product in the United States until our appropriate marketing application has been approved by the FDA. The FDA has substantial discretion over the approval process and may disagree with our interpretation of the data submitted. The process may be significantly extended by requests for additional information or clarification regarding information already provided. As part of this review, the FDA may refer the application to an appropriate advisory committee, typically a panel of clinicians. Satisfaction of these and other regulatory requirements typically takes several years, and the actual time required may vary substantially based upon the type, complexity and novelty of the product. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly procedures on our activities. We cannot be certain that the FDA or other regulatory agencies will approve any of our products on a timely basis, if at all. Success in preclinical or early stage clinical trials does not assure success in later-stage clinical trials. Even if a product receives regulatory approval, the approval may be significantly limited to specific indications or uses and these limitations may adversely affect the commercial viability of the product. Delays in obtaining, or failures to obtain regulatory approvals, would have a material adverse effect on our business.
Even after we obtain FDA approval, we may be required to conduct further clinical trials (i.e., Phase IV trials) and provide additional data on safety and effectiveness. We are also required to gain separate approval for the use of an approved product as a treatment for indications other than those initially approved. In addition, side effects or adverse events that are reported during clinical trials can delay, impede or prevent marketing approval. Similarly, adverse events that are reported after marketing approval can result in additional limitations being placed on the product’s use and, potentially, withdrawal of the product from the market. Any adverse event, either before or after marketing approval, can result in product liability claims against us.
| - 14 - |
In addition to regulating and auditing human clinical trials, the FDA regulates and inspects equipment, facilities, laboratories and processes used in the manufacturing and testing of such products prior to providing approval to market a product. If after receiving FDA approval, we make a material change in manufacturing equipment, location or process, additional regulatory review and approval may be required. We also must adhere to cGMP regulations and product-specific regulations enforced by the FDA through its facilities inspection program. The FDA also conducts regular, periodic visits to re-inspect our equipment, facilities, laboratories and processes following the initial approval. If, as a result of these inspections, the FDA determines that our equipment, facilities, laboratories or processes do not comply with applicable FDA regulations and conditions of product approval, the FDA may seek civil, criminal or administrative sanctions and/or remedies against us, including the suspension of our manufacturing operations.
We have currently received no approvals to market our products from the FDA or other foreign regulators.
We are also subject to various federal, state and international laws pertaining to health care “fraud and abuse,” including anti-kickback laws and false claims laws. The federal Anti-kickback law, which governs federal healthcare programs (e.g., Medicare, Medicaid), makes it illegal to solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug. Many states have similar laws that are not restricted to federal healthcare programs. Federal and state false claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment to third party payors (including Medicare and Medicaid), claims for reimbursement, including claims for the sale of drugs or services, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. If the government or a whistleblower were to allege that we violated these laws there could be a material adverse effect on us, including our stock price. Even an unsuccessful challenge could cause adverse publicity and be costly to respond to, which could have a materially adverse effect on our business, results of operations and financial condition. A finding of liability under these laws can have significant adverse financial implications for us and can result in payment of large penalties and possible exclusion from federal healthcare programs. We will consult counsel concerning the potential application of these and other laws to our business and our sales, marketing and other activities and will make good faith efforts to comply with them. However, given their broad reach and the increasing attention given by law enforcement authorities, we cannot assure you that some of our activities will not be challenged or deemed to violate some of these laws.
European Economic Area
Although we are not currently seeking regulatory approval in the EU, we may do so in the future. As such, a summary of the EU regulatory processes follows below.
A medicinal product may only be placed on the market in the European Economic Area, or EEA, composed of the 27 EU member states, plus Norway, Iceland and Lichtenstein, when a marketing authorization has been issued by the competent authority of a member state pursuant to Directive 2001/83/EC (as recently amended by Directive 2004/27/EC), or an authorization has been granted under the centralized procedure in accordance with Regulation (EC) No. 726/2004 or its predecessor, Regulation 2309/93. There are essentially three community procedures created under prevailing European pharmaceutical legislation that, if successfully completed, allow an applicant to place a medicinal product on the market in the EEA.
Centralized Procedure
Regulation 726/2004/EC now governs the centralized procedure when a marketing authorization is granted by the European Commission, acting in its capacity as the European Licensing Authority on the advice of the EMA. That authorization is valid throughout the entire community and directly or (as to Norway, Iceland and Liechtenstein) indirectly allows the applicant to place the product on the market in all member states of the EEA. The EMA is the administrative body responsible for coordinating the existing scientific resources available in the member states for evaluation, supervision and pharmacovigilance of medicinal products. Certain medicinal products, as described in the Annex to Regulation 726/2004, must be authorized centrally. These are products that are developed by means of a biotechnological process in accordance with Paragraph 1 to the Annex to the Regulation. Medicinal products for human use containing a new active substance for which the therapeutic indication is the treatment of acquired immune deficiency syndrome, or AIDS, cancer, neurodegenerative disorder or diabetes must also be authorized centrally. Starting on May 20, 2008, the mandatory centralized procedure was extended to autoimmune diseases and other immune dysfunctions and viral diseases. Finally, all medicinal products that are designated as orphan medicinal products pursuant to Regulation 141/2000 must be authorized under the centralized procedure. An applicant may also opt for assessment through the centralized procedure if it can show that the medicinal product constitutes a significant therapeutic, scientific or technical innovation or that the granting of authorization centrally is in the interests of patients at the community level. For each application submitted to the EMA for scientific assessment, the EMA is required to ensure that the opinion of the Committee for Medicinal Products for Human Use, or CHMP, is given within 210 days after receipt of a valid application. This 210 days period does not include the time that the applicant to answer any questions raised during the application procedure, the so-called ‘clock stop’ period. If the opinion is positive, the EMA is required to send the opinion to the European Commission, which is responsible for preparing the draft decision granting a marketing authorization. This draft decision may differ from the CHMP opinion, stating reasons for diverging for the CHMP opinion. The draft decision is sent to the applicant and the member states, after which the European Commission takes a final decision. If the initial opinion of the CHMP is negative, the applicant is afforded an opportunity to seek a re-examination of the opinion. The CHMP is required to re-examine its opinion within 60 days following receipt of the request by the applicant. All CHMP refusals and the reasons for refusal are made public on the EMA website. Without a centralized marketing authorization it is prohibited to place a medicinal product that must be authorized centrally on the market in the EU.
| - 15 - |
Mutual Recognition and Decentralized Procedures
With the exception of products that are authorized centrally, the competent authorities of the member states are responsible for granting marketing authorizations for medicinal products placed on their national markets. If the applicant for a marketing authorization intends to market the same medicinal product in more than one member state, the applicant may seek an authorization progressively in the community under the mutual recognition or decentralized procedure. Mutual recognition is used if the medicinal product has already been authorized in a member state. In this case, the holder of this marketing authorization requests the member state where the authorization has been granted to act as reference member state by preparing an updated assessment report that is then used to facilitate mutual recognition of the existing authorization in the other member states in which approval is sought (the so-called concerned member state(s)). The reference member state must prepare an updated assessment report within 90 days of receipt of a valid application. This report together with the approved Summary of Product Characteristics, or SmPC (which sets out the conditions of use of the product), and a labeling and package leaflet are sent to the concerned member states for their consideration. The concerned member states are required to approve the assessment report, the SmPC and the labeling and package leaflet within 90 days of receipt of these documents. The total procedural time is 180 days.
The decentralized procedure is used in cases where the medicinal product has not received a marketing authorization in the EU at the time of application. The applicant requests a member state of its choice to act as reference member state to prepare an assessment report that is then used to facilitate agreement with the concerned member states and the grant of a national marketing authorization in all of these member states. In this procedure, the reference member state must prepare, for consideration by the concerned member states, the draft assessment report, a draft SmPC and a draft of the labeling and package leaflet within 120 days after receipt of a valid application. As in the case of mutual recognition, the concerned member states are required to approve these documents within 90 days of their receipt.
For both mutual recognition and decentralized procedures, if a concerned member state objects to the grant of a marketing authorization on the grounds of a potential serious risk to public health, it may raise a reasoned objection with the reference member state. The points of disagreement are in the first instance referred to the Co-ordination Group on Mutual Recognition and Decentralized Procedures, or CMD, to reach an agreement within 60 days of the communication of the points of disagreement. If member states fail to reach an agreement, then the matter is referred to the EMA and CHMP for arbitration. The CHMP is required to deliver a reasoned opinion within 60 days of the date on which the matter is referred. The scientific opinion adopted by the CHMP forms the basis for a binding European Commission decision.
Irrespective of whether the medicinal product is assessed centrally, de-centrally or through a process of mutual recognition, the medicinal product must be manufactured in accordance with the principles of good manufacturing practice as set out in Directive 2003/94/EC and Volume 4 of the rules governing medicinal products in the European community. Moreover, community law requires the clinical results in support of clinical safety and efficacy based upon clinical trials conducted in the European community to be in compliance with the requirements of Directive 2001/20/EC, which implements good clinical practice in the conduct of clinical trials on medicinal products for human use. Clinical trials conducted outside the European community and used to support applications for marketing within the EU must have been conducted in a way consistent with the principles set out in Directive 2001/20/EC. The conduct of a clinical trial in the EU requires, pursuant to Directive 2001/20/EC, authorization by the relevant national competent authority where a trial takes place, and an ethics committee to have issued a favorable opinion in relation to the arrangements for the trial. It also requires that the sponsor of the trial, or a person authorized to act on his behalf in relation to the trial, be established in the community.
National Procedure
This procedure is available for medicinal products that do not fall within the scope of mandatory centralized authorization and are intended for use in only one EU member state. Specific procedures and timelines differ between member states, but the duration of the procedure is generally 210 days and based on a risk/efficacy assessment by the competent authority of the member state concerned, followed by determination of SmPC, package leaflet and label text/layout and subsequently grant of the marketing authorization. Marketing authorizations granted on this basis are not mutually recognized by other member states.
| - 16 - |
There are various types of applications for marketing authorizations:
Full Applications. A full application is one that is made under any of the community procedures described above and “stands alone” in the sense that it contains all of the particulars and information required by Article 8(3) of Directive 2001/83 (as amended) to allow the competent authority to assess the quality, safety and efficacy of the product and in particular the balance between benefit and risk. Article 8(3)(l) in particular refers to the need to present the results of the applicant’s research on (i) pharmaceutical (physical-chemical, biological or microbiological) tests, (ii) preclinical (toxicological and pharmacological) studies and (iii) clinical trials in humans. The nature of these tests, studies and trials is explained in more detail in Annex I to Directive 2001/83/EC. Full applications would be required for products containing new active substances not previously approved by the competent authority, but may also be made for other products.
Abridged Applications. Article 10 of Directive 2001/83/EC contains exemptions from the requirement that the applicant provide the results of its own preclinical and clinical research. There are three regulatory routes for an applicant to seek an exemption from providing such results, namely (i) cross-referral to an innovator’s results without consent of the innovator, (ii) well established use according to published literature and (iii) consent to refer to an existing dossier of research results filed by a previous applicant.
Cross-referral to Innovator’s Data
Articles 10(1) and 10(2)(b) of Directive 2001/83/EC provide the legal basis for an applicant to seek a marketing authorization on the basis that its product is a generic medicinal product (a copy) of a reference medicinal product that has already been authorized, in accordance with community provisions. A reference product is, in principle, an original product granted an authorization on the basis of a full dossier of particulars and information. This is the main exemption used by generic manufacturers for obtaining a marketing authorization for a copy product. The generic applicant is not required to provide the results of preclinical studies and of clinical trials if its product meets the definition of a generic medicinal product and the applicable regulatory results protection period for the results submitted by the innovator has expired. A generic medicinal product is defined as a medicinal product:
| ● | having the same qualitative and quantitative composition in active substance as the reference medicinal product; |
| ● | having the same pharmaceutical form as the reference medicinal product; and |
| ● | whose bioequivalence with the reference medicinal product has been demonstrated by appropriate bioavailability studies. |
| ● | Applications in respect of a generic medicinal product cannot be made before the expiry of the protection period. Where the reference product was granted a national marketing authorization pursuant to an application made before October 30, 2005, the protection period is either six years or 10 years, depending upon the election of the particular member state concerned. Where the reference product was granted a marketing authorization centrally, pursuant to an application made before November 20, 2005, the protection period is 10 years. For applications made after these dates, Regulation 726/2004 and amendments to Directive 2001/83/EC provide for a harmonized protection period regardless of the approval route utilized. The harmonized protection period is in total 10 years, including eight years of research data protection and two years of marketing protection. The effect is that the originator’s results can be the subject of a cross-referral application after eight years, but any resulting authorization cannot be exploited for a further two years. The rationale of this procedure is not that the competent authority does not have before it relevant tests and trials upon which to assess the efficacy and safety of the generic product, but that the relevant particulars can, if the research data protection period has expired, be found on the originator’s file and used for assessment of the generic medicinal product. The 10-year protection period can be extended to 11 years where, in the first eight years post-authorization, the holder of the authorization obtains approval for a new indication assessed as offering a significant clinical benefit in comparison with existing products. |
If the copy product does not meet the definition of a generic medicinal product or if certain types of changes occur in the active substance(s) or in the therapeutic indications, strength, pharmaceutical form or route of administration in relation to the reference medicinal product, Article 10(3) of Directive 2001/83/EC provides that the results of the appropriate preclinical studies or clinical trials must be provided by the applicant.
| - 17 - |
Well-established Medicinal Use
Under Article 10a of Directive 2001/83/EC, an applicant may, in substitution for the results of its own preclinical and clinical research, present detailed references to published literature demonstrating that the active substance(s) of a product have a well-established medicinal use within the community with recognized efficacy and an acceptable level of safety. The applicant is entitled to refer to a variety of different types of literature, including reports of clinical trials with the same active substance(s) and epidemiological studies that indicate that the constituent or constituents of the product have an acceptable safety/efficacy profile for a particular indication. However, use of the published literature exemption is restricted by stating that in no circumstances will constituents be treated as having a well-established use if they have been used for less than 10 years from the first systematic and documented use of the substance as a medicinal product in the EU. Even after 10 years’ systematic use, the threshold for well-established medicinal use might not be met. European pharmaceutical law requires the competent authorities to consider among other factors the period over which a substance has been used, the amount of patient use of the substance, the degree of scientific interest in the use of the substance (as reflected in the scientific literature) and the coherence (consistency) of all the scientific assessments made in the literature. For this reason, different substances may reach the threshold for well-established use after different periods, but the minimum period is 10 years. If the applicant seeks approval of an entirely new therapeutic use compared with that to which the published literature refers, additional preclinical and/or clinical results would have to be provided.
Informed Consent
Under Article 10c of Directive 2001/83/EC, following the grant of a marketing authorization the holder of such authorization may consent to a competent authority utilizing the pharmaceutical, preclinical and clinical documentation that it submitted to obtain approval for a medicinal product to assess a subsequent application relating to a medicinal product possessing the same qualitative and quantitative composition with respect to the active substances and the same pharmaceutical form.
Law Relating to Pediatric Research
Regulation (EC) 1901/2006 (as amended by Regulation (EC) 1902/2006) was adopted on December 12, 2006. This Regulation governs the development of medicinal products for human use in order to meet the specific therapeutic needs of the pediatric population. It requires any application for marketing authorization made after July 26, 2008 in respect of a product not authorized in the European Community on January 26, 2007 (the time the Regulation entered into force), to include the results of all studies performed and details of all information collected in compliance with a pediatric investigation plan agreed by the Pediatric Committee of the EMA, unless the product is subject to an agreed waiver or deferral or unless the product is excluded from the scope of Regulation 1902/2006 (generics, hybrid medicinal products, biosimilars, homeopathic and traditional (herbal) medicinal products and medicinal products containing one or more active substances of well-established medicinal use). Waivers can be granted in certain circumstances where pediatric studies are not required or desirable. Deferrals can be granted in certain circumstances where the initiation or completion of pediatric studies should be deferred until appropriate studies in adults have been performed. Moreover, this regulation imposes the same obligation from January 26, 2009 on an applicant seeking approval of a new indication, pharmaceutical form or route of administration for a product already authorized and still protected by a supplementary protection certificate granted under Regulation EC 469/2009 and its precursor (EEC) 1768/92 or by a patent that qualifies for the granting of such a supplementary protection certificate. The pediatric Regulation 1901/2006 also provides, subject to certain conditions, a reward for performing such pediatric studies, regardless of whether the pediatric results provided resulted in the grant of a pediatric indication. This reward comes in the form of an extension of six months to the supplementary protection certificate granted in respect of the product, unless the product is subject to orphan drug designation, in which case the 10-year market exclusivity period for such orphan products is extended to 12 years. If any of the non-centralized procedures for marketing authorization have been used, the six-month extension of the supplementary protection certificate is only granted if the medicinal product is authorized in all member states.
Post-authorization Obligations
In the pre-authorization phase the applicant must provide a detailed pharmacovigilance plan that it intends to implement post-authorization. An authorization to market a medicinal product in the EU carries with it an obligation to comply with many post-authorization organizational and behavioral regulations relating to the marketing and other activities of authorization holders. These include requirements relating to post-authorization efficacy studies, post-authorization safety studies, adverse event reporting and other pharmacovigilance requirements, advertising, packaging and labeling, patient package leaflets, distribution and wholesale dealing. The regulations frequently operate within a criminal law framework and failure to comply with the requirements may not only affect the authorization, but also can lead to financial and other sanctions levied on the company in question and responsible officers.
As a result of the currently on-going overhaul of EU pharmacovigilance legislation the financial and organizational burden on market authorization holders will increase significantly, such as the obligation to maintain a pharmacovigilance system master file that applies to all holders of marketing authorizations granted in accordance with Directive 2001/83/EC or Regulation (EC) No 726/2004. Marketing authorization holders must furthermore collect data on adverse events associated with use of the authorized product outside the scope of the authorization. Pharmacovigilance for biological products and medicines with a new active substance will be strengthened by subjecting their authorization to additional monitoring activities. The EU is currently in the process of issuing implementing regulations for the new pharmacovigilance framework.
| - 18 - |
Any authorization granted by member state authorities, which within three years of its granting is not followed by the actual placing on the market of the authorized product in the authorizing member state ceases to be valid. When an authorized product previously placed on the market in the authorizing member state is no longer actually present on the market for a period of three consecutive years, the authorization for that product shall cease to be valid. The same two three year periods apply to authorizations granted by the European Commission based on the centralized procedure.
Israel
In November 2011, Eyefite and Can-Fite entered into the Services Agreement, pursuant to which Can-Fite will manage, as an independent contractor, all activities relating to pre-clinical and clinical studies performed for the development of the ophthalmic indications of CF101. As such, in addition to regulations of other countries where we are seeking regulatory approval, Israeli government regulation will also govern the development of our product candidates.
Israel Ministry of the Environment — Toxin Permit
In accordance with the Israeli Dangerous Substance Law — 1993, the Ministry of the Environment may grant a permit in order to use toxic materials. Because Can-Fite utilizes toxic materials in the course of operation of their laboratories relating, in part, to our products, they were required to apply for a permit to use these materials. The current toxin permit held by Can-Fite will remain in effect until January 2017.
Other Licenses and Approvals
Can-Fite has a business license from the municipality of Petah-Tikva for a drug development research laboratory located at its offices in Petah Tikva, Israel. In order to obtain this license, Can-Fite also received approval from the Petah-Tikva Association of Towns Fire Department. Can-Fite’s business license is valid until December 2017. Can-Fite also has a radioactive materials or products containing radioactive materials license, which is valid until July, 2017.
In 2002, Can-Fite received approval from the National Council on Animal Experiments, approving it as an institution authorized to conduct experiments on animals.
Clinical Testing in Israel
In order to conduct clinical testing on humans in Israel, special authorization must first be obtained from the ethics committee and general manager of the institution in which the clinical studies are scheduled to be conducted, as required under the Guidelines for Clinical Trials in Human Subjects implemented pursuant to the Israeli Public Health Regulations (Clinical Trials in Human Subjects), as amended from time to time, and other applicable legislation. These regulations also require authorization from the Israeli Ministry of Health, except in certain circumstances, and in the case of genetic trials, special fertility trials and similar trials, an additional authorization of the overseeing institutional ethics committee. The institutional ethics committee must, among other things, evaluate the anticipated benefits that are likely to be derived from the project to determine if it justifies the risks and inconvenience to be inflicted on the human subjects, and the committee must ensure that adequate protection exists for the rights and safety of the participants as well as the accuracy of the information gathered in the course of the clinical testing. Since we intend to perform a portion of the clinical studies on certain of our therapeutic candidates in Israel, we will be required to obtain authorization from the ethics committee and general manager of each institution in which we intend to conduct our clinical trials, and in most cases, from the Israeli Ministry of Health.
Israel Ministry of Health
Israel’s Ministry of Health, which regulates medical testing, has adopted protocols that correspond, generally, to those of the FDA and the EMA, making it comparatively straightforward for studies conducted in Israel to satisfy FDA and the EMA requirements, thereby enabling medical technologies subjected to clinical trials in Israel to reach U.S. and EU commercial markets in an expedited fashion. Many members of Israel’s medical community have earned international prestige in their chosen fields of expertise and routinely collaborate, teach and lecture at leading medical centers throughout the world. Israel also has free trade agreements with the United States and the European Union.
| - 19 - |
Other Countries
In addition to regulations in the United States, the EU and Israel, we are subject to a variety of other regulations governing clinical trials and commercial sales and distribution of drugs in other countries. Whether or not our products receive approval from the FDA or EMA approval of such products must be obtained by the comparable regulatory authorities of countries other than the United States or European Union before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA or EMA approval. The requirements governing the conduct of clinical trials and product licensing vary greatly from country to country.
The requirements that we and our collaborators must satisfy to obtain regulatory approval by government agencies in other countries prior to commercialization of our products in such countries can be rigorous, costly and uncertain. In the European countries, Canada and Australia, regulatory requirements and approval processes are similar in principle to those in the United States. Additionally, depending on the type of drug for which approval is sought, there are currently two potential tracks for marketing approval in the European countries: mutual recognition and the centralized procedure. These review mechanisms may ultimately lead to approval in all European Union countries, but each method grants all participating countries some decision-making authority in product approval. Foreign governments also have stringent post-approval requirements including those relating to manufacture, labeling, reporting, record keeping and marketing. Failure to substantially comply with these on-going requirements could lead to government action against the product, us and/or its representatives.
Related Matters
From time to time, legislation is drafted, introduced and passed in governmental bodies that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA or EMA and other applicable regulatory bodies to which we are subject. In addition, regulations and guidance are often revised or reinterpreted by the national agency in ways that may significantly affect our business and our therapeutic candidates. It is impossible to predict whether such legislative changes will be enacted, whether FDA or EMA regulations, guidance or interpretations will change, or what the impact of such changes, if any, may be. We may need to adapt our business and therapeutic candidates and products to changes that occur in the future.
Employees
As of December 31, 2015, we had no full time or part-time employees.
Historical Background
We were originally incorporated in the State of Nevada on December 10, 1999, under the name Bridge Capital.com, Inc. Bridge Capital.com, Inc. was a nominally capitalized corporation that did not commence its operations until it changed its name to Denali Concrete Management, Inc., or Denali, in March 2001. Denali was a concrete placement company specializing in providing concrete improvements in the road construction industry and operated primarily in Anchorage, Alaska, placing curb and gutter, sidewalks and retaining walls for state, municipal and military projects. In December 2005, we ceased our principal business operations and focused our efforts on seeking a business opportunity.
We consummated our reverse acquisition, or the Transaction, of all the outstanding interests in Eyefite Ltd., a private company incorporated on June 27, 2011 under the laws of the State of Israel, or Eyefite, pursuant to an acquisition agreement dated November 21, 2011, or the Acquisition Agreement, by and between the Company and Can-Fite. The Transaction was accounted for as a reverse acquisition wherein Can-Fite was treated as the acquirer for accounting purposes. In connection with the completion of the Transaction on November 21, 2011, the following events occurred:
| ● | We entered into and completed a stock purchase agreement dated November 21, 2011,or the Stock Purchase Agreement, with Can-Fite, whereby Can-Fite purchased 8,000,000 shares of our common stock in exchange for all of the issued and outstanding ordinary shares of Eyefite. As a result of the consummation of the actions contemplated by the Stock Purchase Agreement, Eyefite became our wholly-owned subsidiary and Can-Fite became our majority stockholder and a parent. |
| ● | Eyefite and Can-Fite entered into a License Agreement dated November 21, 2011, or the License Agreement, pursuant to which Can-Fite granted to Eyefite a sole and exclusive worldwide license for the use of CF101, Can-Fite’s therapeutic drug candidate, or CF101, solely in the field of ophthalmic indications. See the “License Agreement” below. |
| - 20 - |
| ● | Eyefite and Can-Fite entered into a services agreement dated November 21, 2011, or the Services Agreement, pursuant to which Can-Fite manages, as an independent contractor, all activities relating to pre-clinical and clinical studies performed for the development of the ophthalmic indications of CF101. |
| ● | We issued a warrant, or the Warrant, to Can-Fite by which Can-Fite has the right, until the earlier of (a) the November 21, 2016 and (b) the closing of the acquisition of us by another entity, resulting in the exchange of our outstanding common shares such that our stockholders prior to such transaction own, directly or indirectly, less than 50% of the voting power of the surviving entity, to convert its right to the additional fee under the Services Agreement into 480,022 shares of our common stock (subject to adjustment in certain circumstances). The per share exercise price for the shares is $5.148. This Warrant may be exercised on either a cash or a cashless basis, provided that if the Warrant is exercised on a cashless basis, the Warrant must be exercised in whole, not in part. |
| ● | On November 21, 2011, we completed a private placement of shares of our common stock for gross proceeds of $3.3 million through the sale of 646,776 shares to third party investors and sold 466,139 shares of our common stock to Can-Fite in exchange for 714,922 ordinary shares of Can-Fite, valued at $2.4 million (as determined by reference to the previous trading day’s closing price for Can-Fite shares on the Tel Aviv Stock Exchange), and 97,112 shares to Can-Fite for gross proceeds of $500,000 (collectively, together with the shares issued to the investors, the “Financing”). In addition, we issued to Can-Fite and each of the other investors, for each four shares of the Company’s common stock purchased in the Financing, nine warrants valid for a period of five years from the closing of the Financing to acquire two shares of the Company for an exercise price of $7.74. |
| ● | On November 21, 2011, we repurchased 1,722,222 outstanding shares of our common stock from Mathew G. Rule, our sole officer and director at the time, for $7,750, or the Recapitalization. In addition, we issued 426,666 shares of common stock to certain investors for an aggregate of $97,000, which funds were used solely to retire the outstanding shares in the Recapitalization and to pay outstanding payables of the Company as of closing. These payments included satisfaction of a promissory note in the principal amount of $56,465 and payables to the transfer agent, accountants and Denali legal counsel at the time. |
| ● | In connection with the Transaction, the board of directors of the Company, or the Board, was expanded from one to three members, the sole prior director, Mathew G. Rule, resigned, and the following new directors were appointed: Dr. Pnina Fishman, Dr. Ilan Cohn and Guy Regev, each of whom was, and currently is, a director of Can-Fite. On February 2, 2012, the number of directors was increased to four persons and Dr. Roger Kornberg was appointed as the fourth director and on July 1, 2013, the number of directors was increased to five persons and Dr. Michael Belkin was appointed as the fifth director. |
Upon completion of the Transaction and after giving effect to the Financing, the Recapitalization, and the shares issued to raise funds to satisfy outstanding financial obligations existing prior to the closing of the Transaction, we had an aggregate of 10,441,251 issued and outstanding shares of common stock. Of these shares Can-Fite owned approximately 82% of our Company and assumed control of us.
With the completion of the Transaction, we, through our wholly owned subsidiary, became a clinical-stage biopharmaceutical company focused on developing therapeutic products for the treatment of ophthalmic diseases.
On January 25, 2012, we changed our name and trading symbol on the OTCQB to OphthaliX Inc. and OPLI, respectively. On February 6, 2012, Can-Fite, our majority stockholder owning 8,563,251 shares or approximately 82% of our issued and outstanding shares of common stock and voting rights, by written consent approved a change of the state of incorporation of the Company from the State of Nevada to the State of Delaware by merging the Company into OphthaliX Inc., a newly formed Delaware company, and approved an amendment to the Company’s Articles of Incorporation to change the capitalization of the Company by increasing the number of authorized shares of common stock, par value $.001 per share, from 50,000,000 to 100,000,000. The effective date for the approval of the change of domicile to the State of Delaware and the increase in the authorized shares of common stock was April 2, 2012.
On July 18, 2013, our stockholders approved a reverse stock split of one share for each four and one-half shares outstanding (1:4.5). The reverse split became effective as of the close of business on August 6, 2013.
| - 21 - |
ITEM 1A. RISK FACTORS.
Investing in us involves significant risks, including the risks described below. You should consult with your own financial and legal advisors and carefully consider the material risks described below, together with all of the other information in this Annual Report on Form 10-K. If any of the following risks actually occur, our business, financial condition and results of operations could suffer, and the trading price of our common stock could decline.
Risks Related to Our Financial Position and Capital Requirements
We have incurred operating losses since our inception and anticipate that we will continue to incur substantial operating losses for the foreseeable future.
We are a clinical stage biopharmaceutical company focused on developing therapeutic products for the treatment of ophthalmic disorders. Since November 2011, we have been focused on research and development activities with a view to developing our product candidate, CF101 for ophthalmic diseases. We have financed our operations primarily through the sale of equity securities and have incurred losses in each year since our inception. We have historically incurred substantial net losses, including net losses of approximately $1,477,000 in 2015 and $1,195,000 in 2014. At December 31, 2015, we had an accumulated deficit of approximately $8,773,000. We do not know whether or when we will become profitable. To date, we have not commercialized any products or generated any revenues from product sales and accordingly we do not have a revenue stream to support our cost structure. Our losses have resulted principally from costs incurred in development and discovery activities. We expect to continue to incur losses for the foreseeable future, and these losses will likely increase as we:
| ● | initiate and manage pre-clinical development and clinical trials for our current and new product candidates; |
| ● | seek regulatory approvals for our product candidates; |
| ● | implement internal systems and infrastructures; |
| ● | seek to license additional technologies to develop; |
| ● | hire management and other personnel; and |
| ● | move towards commercialization. |
If our product candidates fail in clinical trials or do not gain regulatory clearance or approval, or if our product candidates do not achieve market acceptance, we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations and cash flows. Moreover, our prospects must be considered in light of the risks and uncertainties encountered by an early-stage company and in highly regulated and competitive markets, such as the biopharmaceutical market, where regulatory approval and market acceptance of our products are uncertain. There can be no assurance that our efforts will ultimately be successful or result in revenues or profits.
| - 22 - |
We will need to raise additional capital to meet our business requirements in the future, and such capital raising may be costly or difficult to obtain and will dilute current shareholders’ ownership interests.
As of December 31, 2015, we had cash and cash equivalents of approximately $42,000. In February, 2013, we obtained a formal letter from Can-Fite agreeing to defer payments owed to it under the Services Agreement beginning on January 31, 2013 for the performance of the clinical trials of CF101 in ophthalmic indications until the completion of a fundraising by us. While Can-Fite has agreed in the past including as recently as August 2015 to extend the term of the deferral letter, there can be no assurance that Can-Fite will continue to extend the term of such deferral beyond October 2016 and the announcement in December 2013 that CF101 did not meet its Phase III DES trial end-points has had a material adverse effect on our ability to raise capital on acceptable terms. We have expended and believe that we will continue to expend substantial resources for the foreseeable future developing our product candidates. These expenditures will include costs associated with research and development, manufacturing, conducting preclinical experiments and clinical trials and obtaining regulatory approvals, as well as commercializing any products approved for sale. Because the outcome of our planned and anticipated clinical trials is highly uncertain, we cannot reasonably estimate the actual amounts necessary to successfully complete the development and commercialization of our product candidates. In addition, other unanticipated costs may arise. As a result of these and other factors currently unknown to us, we will require additional funds, through public or private equity or debt financings or other sources, such as strategic partnerships and alliances and licensing arrangements. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Our future capital requirements will depend on many factors, including the progress and results of our clinical trials, the duration and cost of discovery and preclinical development, and laboratory testing and clinical trials for our product candidates, the timing and outcome of regulatory review of our product candidates, the number and development requirements of other product candidates that we pursue, and the costs of activities, such as product marketing, sales, and distribution. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our anticipated clinical trials.
Our future capital requirements depend on many factors, including:
| ● | the failure to obtain regulatory approval or achieve commercial success of our product candidates; |
| ● | the results of our preclinical studies and clinical trials for our earlier stage product candidates, and any decisions to initiate clinical trials if supported by the preclinical results; |
| ● | the costs, timing and outcome of regulatory review of our product candidates that progress to clinical trials; |
| ● | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our issued patents and defending intellectual property-related claims; |
| ● | the cost of commercialization activities if any of our product candidates are approved for sale, including marketing, sales and distribution costs; |
| ● | the cost of manufacturing our product candidates and any products we successfully commercialize; |
| ● | the timing, receipt and amount of sales of, or royalties on, our future products, if any; |
| ● | the expenses needed to attract and retain skilled personnel; |
| ● | any product liability or other lawsuits related to our products; |
| ● | the extent to which we acquire or invest in businesses, products or technologies and other strategic relationships; and |
| ● | the costs of financing unanticipated working capital requirements and responding to competitive pressures. |
Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate preclinical studies, clinical trials or other research and development activities for one or more of our product candidates or delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our product candidates.
| - 23 - |
We may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition.
According to management estimates, liquidity resources as of December 31, 2015, together with the support letters from Can-Fite will be sufficient to maintain our operations for a period of less than twelve months which raises substantial doubt regarding our ability to continue as a going concern. If we do not continue as a going concern, investors could lose their entire investment.
According to management estimates, liquidity resources as of December 31, 2015, together with the support letters from Can-Fite described above, will be sufficient to maintain our operations at least through October 2016. Our inability to raise funds to conduct our research and development activities will have a severe negative impact on our ability to remain a viable company. These conditions raise substantial doubt about our ability to continue as a going concern. As such, the opinion of our independent registered public accounting firm on our audited financial statements as of and for the year ended December 31, 2015 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. This going concern opinion could materially limit our ability to raise additional funds through the issuance of debt or equity securities or otherwise. Future reports on our financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We may seek additional capital through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of existing shareholders will be diluted, and the terms may include liquidation or other preferences that adversely affect shareholder rights. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring debt, making capital expenditures or declaring dividends. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development or commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
If we fail to obtain necessary funds for our operations, we will be unable to maintain and improve our licensed technology, and we will be unable to develop and commercialize our products and technologies.
Our present and future capital requirements depend on many factors, including:
| ● | the level of research and development investment required to develop our product candidates, and maintain and improve our licensed technology position; |
| ● | the costs of obtaining or manufacturing product candidates for research and development and testing; |
| ● | the results of preclinical and clinical testing, which can be unpredictable in product candidate development; |
| ● | changes in product candidate development plans needed to address any difficulties that may arise in manufacturing, preclinical activities or clinical studies; |
| ● | our ability and willingness to enter into new agreements with strategic partners and the terms of these agreements; |
| ● | our success rate in preclinical and clinical efforts associated with milestones and royalties; |
| ● | the costs of investigating patents that might block us from developing potential product candidates; |
| ● | the costs of recruiting and retaining qualified personnel; |
| ● | the time and costs involved in obtaining regulatory approvals; |
| ● | the number of product candidates we pursue; |
| ● | our revenues, if any; |
| ● | the costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; and |
| ● | our need or decision to acquire or license complementary technologies or new platform or product candidate targets. |
If we are unable to obtain the funds necessary for our operations, we will be unable to maintain and improve our licensed technology, and we will be unable to develop and commercialize our products and technologies, which would materially and adversely affect our business, liquidity and results of operations.
| - 24 - |
Risks Related to our Business and Regulatory Matters
We have not yet commercialized any products or technologies, and we may never become profitable.
We have not yet commercialized any products or technologies, and we may never be able to do so. We do not know when or if we will complete any of our product development efforts, obtain regulatory approval for any product candidates incorporating our technologies or successfully commercialize any approved products. Even if we are successful in developing products that are approved for marketing, we will not be successful unless these products gain market acceptance for appropriate indications at favorable reimbursement rates. The degree of market acceptance of these products will depend on a number of factors, including:
| ● | the timing of regulatory approvals in the countries, and for the uses, we seek; | |
| ● | the competitive environment; | |
| ● | the establishment and demonstration in the medical community of the safety and clinical efficacy of our products and their potential advantages over existing therapeutic products; | |
| ● | our ability to enter into strategic agreements with pharmaceutical and biotechnology companies with strong marketing and sales capabilities; | |
| ● | the adequacy and success of distribution, sales and marketing efforts; and | |
| ● | the pricing and reimbursement policies of government and third-party payors, such as insurance companies, health maintenance organizations and other plan administrators. |
Physicians, patients, thirty-party payors or the medical community in general may be unwilling to accept, utilize or recommend, and in the case of third-party payors, cover any of our products or products incorporating our technologies. As a result, we are unable to predict the extent of future losses or the time required to achieve profitability, if at all. Even if we successfully develop one or more products that incorporate our technologies, we may not become profitable.
Our current pipeline is based on a single compound, the CF101 drug candidate. Failure to develop CF101 will have a material adverse effect on us. In the recent past, CF101 failed to reach the primary and secondary efficacy study end-points in a Phase III DES study.
Our current pipeline is based on the development of CF101 for the treatment of glaucoma and uveitis. As such, we are currently dependent on a single molecule for our potential commercial success, and any safety or efficacy problems or concern with CF101 could have a significant impact on our business. Failure to develop CF101 or any additional drug candidates which we may develop in the future, in whole or in part, would have a material adverse effect on us. We experienced the risks involved with the development of CF101 recently when we announced in December 2013 that CF101 did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints in a Phase III study of CF101. Nonetheless, CF101 was found to be well tolerated. In 2014 we decided to end the development of CF101 for the DES indication. This decision was based on a lack of correlation between patients' response to CF101 and over-expression of the drug target, the A3 adenosine receptor in this patient population. In addition, Can-Fite, which conducts our clinical trials, experienced the risks involved with conducting clinical trials, including but not limited to, increased expense and delay. For example, two Phase IIb studies in RA utilizing CF101 in combination with methotrexate, a generic drug commonly used for treating RA patients, or MTX, failed to reach their primary endpoints. Can-Fite believes that this may have been due to low A3AR expression in the subpopulation of RA patients that did not respond well to treatment with MTX. Because of their low A3AR expression, such patients also did not respond well to treatment with CF101. Can-Fite was not aware of this when it designed the studies. As such, Can-Fite conducted an additional Phase IIb RA trial of CF101 as a standalone therapy in patients with A3AR expression levels above a certain threshold, and positive results from this study were announced in December 2013. Furthermore, a Phase II/III study of CF101 for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests CF101 as a potential systemic therapy for patients with moderate-severe psoriasis.
We are currently completely dependent upon Can-Fite to service our operations and development activities. If, at the time we seek to establish our own operations, we are unable to attract and retain key personnel, it could adversely affect our ability to develop and market our products.
Because we currently rely on Can-Fite to manage, as an independent contractor, all activities relating to preclinical and clinical studies for the development of the ophthalmic indications of CF101, we cannot assure you that we will ever be able to manage these operations and activities on our own. We do not currently have the capability of managing all activities relating to the development of the ophthalmic indications of CF101. Accordingly, if Can-Fite ceases to provide such services, our business would likely be materially adversely affected. In the future, we are planning to take control of the development of the ophthalmic indications of CF101. In that event, our future success will depend in part on our ability, financially and otherwise, to identify, hire, and retain key personnel that can manage these operations. While we intend to attempt to attract and retain key personnel to the best of our ability, some of our competitors are likely to have greater resources and more experience than we do, making it difficult for us to compete successfully for key personnel. If we are ultimately unable to attract and retain key personnel, we likely would not be able to develop and market our products and would have to continue to rely upon Can-Fite to provide the necessary services. If we are unable to manage these operations and Can-Fite ceases to service our operations and development activities, our ability to develop and market our products, as well as our business, financial condition and results of operations, would be materially adversely affected.
| - 25 - |
Our product candidates are at various stages of clinical and preclinical development and may never be commercialized.
Our product candidates are at various stages of clinical development and may never be commercialized. The progress and results of any future pre-clinical testing or future clinical trials are uncertain, and the failure of our product candidates to receive regulatory approvals will have a material adverse effect on our business, operating results and financial condition to the extent we are unable to commercialize any products. None of our product candidates has received regulatory approval for commercial sale. In addition, we face the risks of failure inherent in developing therapeutic products. Our product candidates are not expected to be commercially available for several years, if at all.
In addition, our product candidates must satisfy rigorous standards of safety and efficacy before they can be approved by the U.S. Food and Drug Administration, or the FDA, the EMA and foreign regulatory authorities for commercial use. The FDA, EMA and foreign regulatory authorities have full discretion over this approval process. We will need to conduct significant additional research, involving testing in animals and in humans, before we can file applications for product approval. Typically, in the pharmaceutical industry, there is a high rate of attrition for product candidates in pre-clinical testing and clinical trials. Also, satisfying regulatory requirements typically takes many years, is dependent upon the type, complexity and novelty of the product and requires the expenditure of substantial resources. In addition, delays or rejections may be encountered based upon additional government regulation, including any changes in FDA policy, during the process of product development, clinical trials and regulatory reviews.
In order to receive FDA approval or approval from foreign regulatory authorities to market a product candidate or to distribute our products, we must demonstrate thorough pre-clinical testing and thorough human clinical trials that the product candidate is safe and effective for its intended uses (e.g., treatment of a specific condition in a specific way subject to contradictions and other limitations). Even if we comply with all FDA requests, the FDA may ultimately reject one or more of our new drug applications, or NDA, or grant approval for a narrowly intended use that is not commercially feasible. We might not obtain regulatory approval for our drug candidates in a timely manner, if at all. Failure to obtain FDA approval of any of our drug candidates in a timely manner or at all will severely undermine our business by reducing the number of salable products and, therefore, corresponding product revenues.
Results of earlier clinical trials may not be predictive of the results of later-stage clinical trials.
The results of preclinical studies and early clinical trials of product candidates may not be predictive of the results of later-stage clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy results despite having progressed thorough preclinical studies and initial clinical trials. For example, in December 2013, we announced top-line results of a Phase III study with CF101 for DES in which CF101 did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints. In addition, Can-Fite, which conducts our clinical trials, experienced the risks involved with conducting clinical trials, including but not limited to, increased expense and delay. For example, two Phase IIb studies in RA utilizing CF101 in combination with methotrexate, a generic drug commonly used for treating RA patients, or MTX, failed to reach their primary endpoints. Can-Fite believes that this may have been due to low A3AR expression in the subpopulation of RA patients that did not respond well to treatment with MTX. Because of their low A3AR expression, such patients also did not respond well to treatment with CF101. Can-Fite was not aware of this when it designed the studies. As such, Can-Fite conducted an additional Phase IIb RA trial of CF101 as a standalone therapy in patients with A3AR expression levels above a certain threshold, and positive results from this study were announced in December 2013. Furthermore, a Phase II/III study of CF101 for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests CF101 as a potential systemic therapy for patients with moderate-severe psoriasis. Many companies in the pharmaceutical industry have suffered significant setbacks in advanced clinical trials due to adverse safety profiles or lack of efficacy, notwithstanding promising results in earlier studies. Any delay in, or termination or suspension of, our clinical trials will delay the requisite filings with the FDA and, ultimately, our ability to commercialize our product candidates and generate product revenues. If the clinical trials do not support our product claims, the completion of development of such product candidates may be significantly delayed or abandoned, which will significantly impair our ability to generate product revenues and will materially adversely affect our results of operations.
This drug candidate development risk is heightened by any changes in the planned clinical trials compared to the completed clinical trials. As product candidates are developed from preclinical through early to late stage clinical trials towards approval and commercialization, it is customary that various aspects of the development program, such as manufacturing and methods of administration, are altered along the way in an effort to optimize processes and results. While these types of changes are common and are intended to optimize the product candidates for late stage clinical trials, approval and commercialization, such changes do carry the risk that they will not achieve these intended objectives.
Changes in our planned clinical trials or future clinical trials could cause our product candidates to perform differently, including causing toxicities, which could delay completion of our clinical trials, delay approval of our product candidates, and/or jeopardize our ability to commence product sales and generate revenues.
| - 26 - |
We might be unable to develop product candidates that will achieve commercial success in a timely and cost-effective manner, or ever.
Even if regulatory authorities approve our product candidates, they may not be commercially successful. Our product candidates may not be commercially successful because government agencies and other third-party payors may not cover the product or the coverage may be too limited to be commercially successful; physicians and others may not use or recommend our products, even following regulatory approval. A product approval, assuming one issues, may limit the uses for which the product may be distributed thereby adversely affecting the commercial viability of the product. Third parties may develop superior products or have proprietary rights that preclude us from marketing our products. We also expect that at least some of our product candidates will be expensive, if approved. Patient acceptance of and demand for any product candidates for which we obtain regulatory approval or license will depend largely on many factors, including but not limited to the extent, if any, of reimbursement of costs by government agencies and other third-party payors, pricing, the effectiveness of our marketing and distribution efforts, the safety and effectiveness of alternative products, and the prevalence and severity of side effects associated with our products. If physicians, government agencies and other third-party payors do not accept our products, we will not be able to generate significant revenue.
Clinical trials are very expensive, time-consuming and difficult to design and implement, and, as a result, we may suffer delays or suspensions in future trials which would have a material adverse effect on our ability to generate revenues.
Human clinical trials are very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Regulatory authorities, such as the FDA, may preclude clinical trials from proceeding. Additionally, the clinical trial process is time-consuming, failure can occur at any stage of the trials, and we may encounter problems that cause us to abandon or repeat clinical trials. The commencement and completion of clinical trials may be delayed by several factors, including:
| ● | unforeseen safety issues; | |
| ● | determination of dosing issues; | |
| ● | lack of effectiveness or efficacy during clinical trials; | |
| ● | failure of third party suppliers to perform final manufacturing steps for the drug substance; | |
| ● | slower than expected rates of patient recruitment and enrollment; |
| ● | lack of healthy volunteers and patients to conduct trials; | |
| ● | inability to monitor patients adequately during or after treatment; | |
| ● | failure of third party contract research organizations to properly implement or monitor the clinical trial protocols; | |
| ● | failure of institutional review boards to approve our clinical trial protocols; | |
| ● | inability or unwillingness of medical investigators and institutional review boards to follow our clinical trial protocols; and | |
| ● | lack of sufficient funding to finance the clinical trials. |
We have experienced the risks involved with conducting clinical trials, including but not limited to, increased expense and delay and failure to meet end points of the trial. For example, in December 2013, we announced top-line results of a Phase III study with CF 101 for DES in which CF101 did not meet the primary efficacy endpoint of complete clearing of corneal staining, nor the secondary efficacy endpoints. In addition, Can-Fite, which conducts our clinical trials, experienced the risks involved with conducting clinical trials, including but not limited to, increased expense and delay. For example, two Phase IIb studies in RA utilizing CF101 in combination with methotrexate, a generic drug commonly used for treating RA patients, or MTX, failed to reach their primary endpoints. Can-Fite believes that this may have been due to low A3AR expression in the subpopulation of RA patients that did not respond well to treatment with MTX. Because of their low A3AR expression, such patients also did not respond well to treatment with CF101. Can-Fite was not aware of this when it designed the studies. As such, Can-Fite conducted an additional phase IIb RA trial of CF101 as a standalone therapy in patients with A3AR expression levels above a certain threshold, and positive results from this study were announced in December 2013. Furthermore, a Phase II/III study of CF101 for psoriasis did not meet its primary endpoint although positive data from further analysis of the Phase II/III study suggests CF101 as a potential systemic therapy for patients with moderate-severe psoriasis.
| - 27 - |
In addition, we or regulatory authorities may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the regulatory authorities find deficiencies in our regulatory submissions or the conduct of these trials. Any suspension of clinical trials will delay possible regulatory approval, if any, and adversely impact our ability to develop products and generate revenue.
If we acquire or license additional technology or product candidates, we may incur a number of costs, may have integration difficulties and may experience other risks that could harm our business and results of operations.
We may acquire and license additional product candidates and technologies. Any product candidate or technology we license from others or acquire will likely require additional development efforts prior to commercial sale, including extensive pre-clinical or clinical testing, or both, and approval by the FDA and applicable foreign regulatory authorities, if any. All product candidates are prone to risks of failure inherent in pharmaceutical product development, including the possibility that the product candidate or product developed based on licensed technology will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot assure you that any product candidate that we develop based on acquired or licensed technology that is granted regulatory approval will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace. Moreover, integrating any newly acquired product candidates could be expensive and time-consuming. If we cannot effectively manage these aspects of our business strategy, our business may not succeed.
If the third parties upon whom we rely to manufacture our products are unable to timely manufacture our products in compliance with cGMP our business will be harmed.
We do not currently have the ability to manufacture the compounds that we need to conduct our clinical trials and, therefore, we rely upon, and intend to continue to rely upon, certain manufacturers to produce and supply our drug candidates (including the active pharmaceutical ingredients, or APIs) for use in clinical trials and for future sales. If such manufacturers are unable to manufacture the compounds in a timely fashion, our clinical development programs may be delayed which could have a detrimental effect on our business.
The manufacture of our product candidates is a chemical synthesis process and if one of our materials suppliers encounters problems manufacturing our products, our business could suffer.
The FDA and foreign regulators require manufacturers to register manufacturing facilities. The FDA and foreign regulators also inspect these facilities to confirm compliance with requirements that the FDA or foreign regulators establish. We do not intend to engage in the manufacture of our products other than for pre-clinical and clinical studies, but we or our materials suppliers may face manufacturing or quality control problems causing product production and shipment delays or a situation where we or the supplier may not be able to maintain compliance with the FDA’s or foreign regulators’ requirements necessary to continue manufacturing our drug substance. Drug manufacturers are subject to ongoing periodic unannounced inspections by the FDA, the U.S. Drug Enforcement Agency, or DEA, and corresponding foreign regulators to ensure strict compliance with requirements and other governmental regulations and corresponding foreign standards. Any failure to comply with DEA requirements or FDA or foreign regulatory requirements could adversely affect our clinical research activities and our ability to market and develop our product candidates.
We do not currently have sales, marketing or distribution capabilities or experience, and we are unable to effectively sell, market or distribute our product candidates now and we do not expect to be able to do so in the future. The failure to enter into agreements with third parties that are capable of performing these functions would have a material adverse effect on our business and results of operations.
We do not currently have and we do not expect to develop sales, marketing and distribution capabilities. If we are unable to enter into agreements with third parties to perform these functions, we will not be able to successfully market any of our platforms or product candidates. In order to successfully market any of our platform or product candidates, we must make arrangements with third parties to perform these services.
As we do not intend to develop a marketing and sales force with technical expertise and supporting distribution capabilities, we will be unable to market any of our product candidates directly. To promote any of our potential products through third parties, we will have to locate acceptable third parties for these functions and enter into agreements with them on acceptable terms, and we may not be able to do so. Any third-party arrangements we are able to enter into may result in lower revenues than we could achieve by directly marketing and selling our potential products. In addition, to the extent that we depend on third parties for marketing and distribution, any revenues we receive will depend upon the efforts of such third parties, as well as the terms of our agreements with such third parties, which cannot be predicted in most cases at this time. As a result, we might not be able to market and sell our products in the United States or overseas, which would have a material adverse effect on us.
| - 28 - |
We depend on key members of our management and key consultants and will need to add and retain additional leading experts. Failure to retain our management and consulting team and add additional leading experts could have a material adverse effect on our business, results of operations or financial condition.
We are highly dependent on our executive officers and other key management and technical personnel. Our failure to retain our Chief Executive Officer and Chairman, Pnina Fishman, Ph.D., who has developed much of the technology we utilize today, or any other key management and technical personnel, could have a material adverse effect on our future operations. Our success is also dependent on our ability to attract, retain and motivate highly trained technical, and management personnel, among others, to continue the development and commercialization of our current and future products.
We currently work mainly through external resources, including services via the Services Agreement with Can-Fite and the use of consultants. As such, our future success highly depends on our ability to attract, retain and motivate personnel, including contractors, required for the development, maintenance and expansion of our activities. There can be no assurance that we will be able to retain our existing personnel or attract additional qualified employees or consultants. The loss of key personnel or the inability to hire and retain additional qualified personnel in the future could have a material adverse effect on our business, financial condition and results of operation.
We face significant competition and continuous technological change, and developments by competitors may render our products or technologies obsolete or non-competitive. If we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may not ever be profitable.
We will compete against fully integrated pharmaceutical and biotechnology companies and smaller companies that are collaborating with larger pharmaceutical companies, academic institutions, government agencies and other public and private research organizations. In addition, many of these competitors, either alone or together with their collaborative partners, operate larger research and development programs than we do, and have substantially greater financial resources than we do, as well as significantly greater experience in:
| ● | developing drugs; | |
| ● | undertaking pre-clinical testing and human clinical trials; | |
| ● | obtaining FDA, addressing various regulatory matters and other regulatory approvals of drugs; | |
| ● | formulating and manufacturing drugs; and | |
| ● | launching, marketing and selling drugs. |
If our competitors develop and commercialize products faster than we do, or develop and commercialize products that are superior to our product candidates, our commercial opportunities will be reduced or eliminated. The extent to which any of our product candidates achieve market acceptance will depend on competitive factors, many of which are beyond our control. Competition in the biotechnology and biopharmaceutical industry is intense and has been accentuated by the rapid pace of technology development. Our competitors include large integrated pharmaceutical companies, biotechnology companies that currently have drug and target discovery efforts, universities, and public and private research institutions. Almost all of these entities have substantially greater research and development capabilities and financial, scientific, manufacturing, marketing and sales resources than we do. These organizations also compete with us to:
| ● | attract parties for acquisitions, joint ventures or other collaborations; | |
| ● | license proprietary technology that is competitive with the technology we are developing; | |
| ● | attract funding; and | |
| ● | attract and hire scientific talent and other qualified personnel. |
Our competitors may succeed in developing and commercializing products earlier and obtaining regulatory approvals from the FDA more rapidly than we do. Our competitors may also develop products or technologies that are superior to those we are developing, and render our product candidates or technologies obsolete or non-competitive. If we cannot successfully compete with new or existing products, our marketing and sales will suffer and we may not ever be profitable.
| - 29 - |
Our competitors currently include companies with marketed products and/or an advanced research and development pipeline. The competitive landscape in the ophthalmic therapeutics field includes Novartis/Alcon, Allergan, Pfizer, Roche/Genentech, Merck (which acquired Inspire Pharmaceuticals), Santen (which acquired Novagali), Bausch & Lomb (which acquired ISTA Pharmaceuticals and was recently acquired by Valeant), GlaxoSmithKline, Sanofi-Aventis (which acquired Fovea), Shire (which acquired SARcode) and more.
We may suffer losses from product liability claims if our product candidates cause harm to patients.
Any of our product candidates could cause adverse events. Although data from a pooled analysis by Can-Fite of 1035 patients treated with CF101, indicates that CF101 is safe and well tolerated at doses up to 4.0 mg administered twice daily for up to 32 weeks, there were incidences (albeit less than or equal to 5%) of adverse events in eight completed and fully analyzed trials in inflammatory disease. Such adverse events included nausea, diarrhea, constipation, common bacterial and viral syndromes (such as tonsillitis, otitis and respiratory and urinary tract infections), myalgia, arthralgia, dizziness, headache, palpitations and pruritus. Can-Fite observed an even lower incidence (less than or equal to 2%) of serious adverse events, including pancytopenia (although extensive evaluation suggests that such adverse event was associated with an inadvertent overdose of MTX), exacerbation of chronic obstructive lung disease and exacerbation of Parkinson’s Disease. Notwithstanding the foregoing, the placebo group in such studies had a higher incidence of overall adverse events than the pooled CF101 groups. No new safety concerns have been identified and no novel or unexpected safety concerns have appeared over 32 weeks of treatment in more recent Can-Fite trials.
There is also a risk that certain adverse events may not be observed in clinical trials, but may nonetheless occur in the future. If any of these adverse events occur, they may render our product candidates ineffective or harmful in some patients, and our sales would suffer, materially adversely affecting our business, financial condition and results of operations.
In addition, potential adverse events caused by our product candidates could lead to product liability lawsuits. If product liability lawsuits are successfully brought against us, we may incur substantial liabilities and may be required to limit the marketing and commercialization of our product candidates. Our business exposes us to potential product liability risks, which are inherent in the testing, manufacturing, marketing and sale of pharmaceutical products. We may not be able to avoid product liability claims. Product liability insurance for the pharmaceutical and biotechnology industries is generally expensive, if available at all. If, at any time, we are unable to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims, we may be unable to clinically test, market or commercialize our product candidates. A successful product liability claim brought against us in excess of our insurance coverage, if any, may cause us to incur substantial liabilities, and, as a result, our business, liquidity and results of operations would be materially adversely affected.
Our product candidates will remain subject to ongoing regulatory requirements even if they receive marketing approval, and if we fail to comply with these requirements, we could lose these approvals, and the sales of any approved commercial products could be suspended.
Even if we receive regulatory approval to market a particular product candidate, the product will remain subject to extensive regulatory requirements, including requirements relating to manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, distribution and recordkeeping. Even if regulatory approval of a product is granted, the approval may be subject to limitations on the uses for which the product may be marketed or the conditions of approval, or may contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product, which could negatively impact us or our collaboration partners by reducing revenues or increasing expenses, and cause the approved product candidate not to be commercially viable. In addition, as clinical experience with a drug expands after approval, typically because it is used by a greater number and more diverse group of patients after approval than during clinical trials, side effects and other problems may be observed after approval that were not seen or anticipated during pre-approval clinical trials or other studies. Any adverse effects observed after the approval and marketing of a product candidate could result in limitations on the use of or withdrawal of any approved products from the marketplace. Absence of long-term safety data may also limit the approved uses of our products, if any. If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, or previously unknown problems with any approved commercial products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions or other setbacks, including the following:
| ● | Restrictions on the products, manufacturers or manufacturing process; |
| ● | Warning letters; |
| - 30 - |
| ● | Civil or criminal penalties, fines and injunctions; | |
| ● | Product seizures or detentions; | |
| ● | Import or export bans or restrictions; | |
| ● | Voluntary or mandatory product recalls and related publicity requirements; | |
| ● | Suspension or withdrawal of regulatory approvals; | |
| ● | Total or partial suspension of production, and | |
| ● | Refusal to approve pending applications for marketing approval of new products or supplements to approved applications. |
If we or our collaborators are slow or unable to adapt to changes in existing regulatory requirements or adoption of new regulatory requirements or policies, marketing approval for our product candidates may be lost or cease to be achievable, resulting in decreased revenue from milestones, product sales or royalties, which would have a material adverse effect on our results of operations.
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology, including any cybersecurity incidents, could harm our ability to operate our business effectively.
Despite the implementation of security measures, our internal computer systems and those of third parties with which we contract are vulnerable to damage from cyber-attacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. System failures, accidents or security breaches could cause interruptions in our operations, and could result in a material disruption of our clinical activities and business operations, in addition to possibly requiring substantial expenditures of resources to remedy. The loss of clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our research and development programs and the development of our product candidates could be delayed.
We may encounter difficulties in managing our growth. Failure to manage our growth effectively will have a material adverse effect on our business, results of operations and financial condition.
We may not be able to successfully grow and expand. Successful implementation of our business plan will require management of growth, including potentially rapid and substantial growth, which will result in an increase in the level of responsibility for management personnel and place a strain on our human and capital resources. To manage growth effectively, we will be required to continue to implement and improve our operating and financial systems and controls to expand, train and manage our employee base. Our ability to manage our operations and growth effectively requires us to continue to expend funds to enhance our operational, financial and management controls, reporting systems and procedures and to attract and retain sufficient numbers of talented personnel. If we are unable to scale up and implement improvements to our control systems in an efficient or timely manner, or if we encounter deficiencies in existing systems and controls, then we will not be able to make available the products required to successfully commercialize our technology. Failure to attract and retain sufficient numbers of talented personnel will further strain our human resources and could impede our growth or result in ineffective growth. Moreover, the management, systems and controls currently in place or to be implemented may not be adequate for such growth, and the steps taken to hire personnel and to improve such systems and controls might not be sufficient. If we are unable to manage our growth effectively, it will have a material adverse effect on our business, results of operations and financial condition.
| - 31 - |
If we are unable to obtain adequate insurance, our financial condition could be adversely affected in the event of uninsured or inadequately insured loss or damage. Our ability to effectively recruit and retain qualified officers and directors could also be adversely affected if we experience difficulty in obtaining adequate directors’ and officers’ liability insurance.
We may not be able to obtain insurance policies on terms affordable to us that would adequately insure our business and property against damage, loss or claims by third parties. To the extent our business or property suffers any damages, losses or claims by third parties, which are not covered or adequately covered by insurance, our financial condition may be materially adversely affected.
We may be unable to maintain sufficient insurance as a public company to cover liability claims made against our officers and directors. If we are unable to adequately insure our officers and directors, we may not be able to retain or recruit qualified officers and directors to manage us.
Risks Related to Our Intellectual Property
Our ability to pursue the development of the ophthalmic indications of CF101 depends upon the continuation of our license from Can-Fite.
On November 21, 2011, we entered into a license agreement with EyeFite, our wholly-owned subsidiary, and Can-Fite, or the License Agreement, according to which Can-Fite (i) granted to EyeFite a sole and exclusive worldwide license for the use of CF101, Can-Fite’s therapeutic drug candidate, solely in the field of ophthalmic diseases and (ii) assigned to EyeFite its rights, title and interest in and to any and all INDs to CF101 in the ophthalmic field. The license granted to EyeFite allows EyeFite to sublicense its rights to CF101 to third parties, subject to the satisfaction of certain conditions. Pursuant to the License Agreement, EyeFite has sole responsibility for preparing and maintaining all regulatory documentation with respect to approvals of CF101 in the field of ophthalmic diseases and all approvals and related regulatory documentation shall be EyeFite’s sole and exclusive property. The License Agreement will remain in effect until the expiration of the last of the patents licensed thereunder unless earlier terminated by one of the parties in accordance with the License Agreement. Can-Fite may terminate the License Agreement upon certain bankruptcy and insolvency events of EyeFite and upon EyeFite’s material breach of the License Agreement, upon 30-days’ prior written notice. EyeFite may terminate the License Agreement upon three-months prior written notice for any reason and upon 30-days’ prior written notice for Can-Fite’s material breach of the License Agreement. All inventions resulting from the development and commercialization of CF101 under the License Agreement belong to Can-Fite, whether such were invented solely by Can-Fite, solely by EyeFite or by both entities. However, the License Agreement also grants EyeFite an exclusive license to use any such inventions in the field of ophthalmic diseases around the world for no additional consideration. If the License Agreement were terminated, we would lose our rights to develop and commercialize CF101 for the treatment of ophthalmic conditions, which would materially and adversely affect our business, results of operations and future prospects.
The termination of the NIH license agreement between Can-Fite and NIH due to patent expiration may diminish our proprietary position.
As a result of the termination of the NIH license agreement between Can-Fite and the NIH in June 2015 due to patent expiration, we no longer hold rights to a family of composition of matter patents relating to CF101 that were licensed from NIH. Nevertheless, because CF101 may be a new chemical entity, or NCE, following approval of an NDA, we, if we are the first applicant to obtain NDA approval, may be entitled to five years of data exclusivity in the United States with respect to such NCEs. Analogous data and market exclusivity provisions, of varying duration, may be available in Europe and other foreign jurisdictions. We may also be entitled to the rights under Can-Fite’s pharmaceutical use issued patents with respect to CF101, which provide patent exclusivity within the ophthalmic field until the mid-2020s. While we believe that we may be able to protect our exclusivity in the ophthalmic field through such use patent portfolio and such period of exclusivity, the lack of composition of matter patent protection may diminish our ability to maintain a proprietary position for our intended uses of CF101. Moreover, we cannot be certain that we will be the first applicant to obtain an FDA approval for any indication of CF101 and we cannot be certain that we will be entitled to NCE exclusivity. Without composition of matter patent protection on CF101 and without NCE exclusivity, we may need to rely primarily or entirely on the Can-Fite patents licensed to us for the methods of using CF101 in the treatment of certain ophthalmic disorders as protection against our competitors. In addition, Can-Fite has discontinued the prosecution of a family of pending patent applications under joint ownership of Can-Fite and NIH pertaining to the use of A3AR agonists for the treatment of uveitis. Such diminution of our proprietary position could have a material adverse effect on our business, results of operation and financial condition.
| - 32 - |
The failure to obtain or maintain patents, licensing agreements, including our current licensing agreements, and other intellectual property could impact our ability to compete effectively.
Our success, competitive position, and future revenues, if any, depend in part on our ability to obtain and successfully leverage intellectual property covering our products and product candidates, know-how, methods, processes, and other technologies, to protect our trade secrets, to prevent others from using our intellectual property and to operate without infringing the intellectual property rights of third parties.
The risks and uncertainties that we face with respect to our intellectual property rights include, but are not limited to, the following:
| ● | while the patents we license have been issued, any pending patent applications may not result in issued patents or may take longer than we expect to result in issued patents; | |
| ● | we may be subject to interference proceedings; | |
| ● | we may be subject to opposition proceedings in foreign countries; | |
| ● | any patents that are issued may not provide meaningful protection; | |
| ● | we may not be able to develop additional proprietary technologies that are patentable; | |
| ● | other companies may challenge patents licensed or issued to us or our customers; | |
| ● | other companies may independently develop similar or alternative technologies, or duplicate our technologies; | |
| ● | other companies may design around technologies we have licensed or developed; and | |
| ● | enforcement of patents is complex, uncertain and expensive. |
If patent rights covering our products and methods are not sufficiently broad, they may not provide us with any protection against competitors with similar products and technologies. Furthermore, if the United States Patent and Trademark Office, or the USPTO, or foreign patent offices issue patents to us or our licensors, others may challenge the patents or design around the patents, or the patent office or the courts may invalidate the patents. Thus, any patents we own or license from or to third parties may not provide any protection against our competitors.
We cannot be certain that patents will be issued as a result of any pending applications, and we cannot be certain that any of our issued patents, licensed from Can-Fite (or any other third-party in the future), will give us adequate protection from competing products. For example, issued patents, including the patents licensed by us, may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope.
In addition, since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain that we were the first to make our inventions or to file patent applications covering those inventions.
It is also possible that others may obtain issued patents that could prevent us from commercializing our products or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. As to those patents that we have licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
In addition to patents and patent applications, we depend upon trade secrets and proprietary know-how to protect our proprietary technology. We require our employees, consultants, advisors and collaborators to enter into confidentiality agreements that prohibit the disclosure of confidential information to any other parties. We require our employees and consultants to disclose and assign to us their ideas, developments, discoveries and inventions. These agreements may not, however, provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure.
Pursuant to the License Agreement, Can-Fite has the sole right to make elections with respect to patent term extension of or supplemental protection certificates with respect to the licensed Can-Fite patents and the sole right to seek and maintain any data exclusivity periods available for CF101. Also, pursuant to the License Agreement, Can-Fite has retained the right to prosecute and maintain the patents licensed to us. While Can-Fite is contractually obligated to us to obtain and maintain protection for those patent rights, and is required to keep us informed of all patent-related activities, we will be dependent upon Can-Fite for the prosecution and maintenance of our licensed patents. If Can-Fite determines not to protect the intellectual property rights that we license from them we may be unable defend such intellectual property rights on our own or we may have to undertake costly litigation to defend the intellectual property rights of Can-Fite ourselves. There can be no assurances that we will continue to have proprietary rights to any of the intellectual property that we license from Can-Fite or any other third party, or otherwise have the right to use through similar strategic relationships. Any loss or limitations on use with respect to our right to use such licensed intellectual property could have a material adverse effect on our business, results of operations and financial condition.
| - 33 - |
Costly litigation may be necessary to protect our intellectual property rights and we may be subject to claims alleging the violation of the intellectual property rights of others.
We may face significant expense and liability as a result of litigation or other proceedings relating to patents and other intellectual property rights of others. In the event that another party has also filed a patent application or been issued a patent relating to an invention or technology claimed by us in pending applications, we may be required to participate in an interference proceeding declared by the USPTO to determine priority of invention, which could result in substantial uncertainties and costs for us, even if the eventual outcome were favorable to us. We, or our licensors, also could be required to participate in interference proceedings involving issued patents and pending applications of another entity. An adverse outcome in an interference proceeding could require us to cease using the technology or to license rights from prevailing third parties.
The cost to us of any patent litigation or other proceeding relating to our licensed patents or patent applications, even if resolved in our favor, could be substantial. Our ability to enforce our patent protection could be limited by our financial resources, and may be subject to lengthy delays. If we are unable to effectively enforce our proprietary rights, or if we are found to infringe the rights of others, we may be in breach of our License Agreement.
A third party may claim that we are using inventions claimed by their patents and may go to court to stop us from engaging in our normal operations and activities, such as research, development and the sale of any future products. Such lawsuits are expensive and would consume time and other resources. There is a risk that the court will decide that we are infringing the third party’s patents and will order us to stop the activities claimed by the patents, redesign our products or processes to avoid infringement or obtain licenses (which may not be available on commercially reasonable terms). In addition, there is a risk that a court will order us to pay the other party damages for having infringed their patents.
Moreover, there is no guarantee that any prevailing patent owner would offer us a license so that we could continue to engage in activities claimed by the patent, or that such a license, if made available to us, could be acquired on commercially acceptable terms. In addition, third parties may, in the future, assert other intellectual property infringement claims against us with respect to our product candidates, technologies or other matters.
We rely on confidentiality agreements that could be breached and may be difficult to enforce, which could result in third parties using our intellectual property to compete against us.
Although we believe that we take reasonable steps to protect our intellectual property, including the use of agreements relating to the non-disclosure of confidential information to third parties, as well as agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees and consultants while we employ them, the agreements can be difficult and costly to enforce. Although we seek to obtain these types of agreements from our contractors, consultants, advisors and research collaborators, to the extent that employees and consultants utilize or independently develop intellectual property in connection with any of our projects, disputes may arise as to the intellectual property rights associated with our products. If a dispute arises, a court may determine that the right belongs to a third party. In addition, enforcement of our rights can be costly and unpredictable. We also rely on trade secrets and proprietary know-how that we seek to protect in part by confidentiality agreements with our employees, contractors, consultants, advisors or others. Despite the protective measures we employ, we still face the risk that:
| ● | these agreements may be breached; | |
| ● | these agreements may not provide adequate remedies for the applicable type of breach; | |
| ● | our trade secrets or proprietary know-how will otherwise become known; or | |
| ● | our competitors will independently develop similar technology or proprietary information. |
International patent protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we may have to expend substantial sums and management resources.
Patent law outside the United States is different than in the United States. Further, the laws of some foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States, if at all. A failure to obtain sufficient intellectual property protection in any foreign country could materially and adversely affect our business, results of operations and future prospects. Moreover, we may participate in opposition proceedings to determine the validity of our foreign patents or our competitors’ foreign patents, which could result in substantial costs and divert management’s resources and attention.
| - 34 - |
We license our patent rights from Can-Fite. Although most jurisdictions in which Can-Fite has applied for, intends to apply for, or has been issued patents have patent protection laws similar to those of the United States, some of them do not. For example, Can-Fite expects to do business in Brazil and India in the future. However, the Brazilian drug regulatory agency, ENVISA, has the authority to nullify patents on the basis of its perceived public interest and the Indian patent law does not allow patent protection for new uses of pharmaceuticals (many of Can-Fite’s current patent applications are of such nature). Additionally, due to uncertainty in patent protection law, Can-Fite has not filed applications in many countries where significant markets exist, including Indonesia, Pakistan, Russia, African countries and Taiwan.
Under current U.S. and Israeli law, we may not be able to enforce employees’ covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees.
We have entered into non-competition agreements with our key employees, in most cases within the framework of their employment agreements. These agreements prohibit our key employees, if they cease working for us, from competing directly with us or working for our competitors for a limited period. Under applicable U.S. and Israeli law, we may be unable to enforce these agreements. If we cannot enforce our non-competition agreements with our employees, then we may be unable to prevent our competitors from benefiting from the expertise of our former employees, which could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| ● | Others may be able to make compounds that are the same as or similar to our product candidates but that are not covered by the claims of the patents that we have exclusively licensed; | |
| ● | Our licensors or any future strategic partners might not have been the first to make the inventions covered by the issued patent or pending patent application that we have exclusively licensed; | |
| ● | Our licensors or any future strategic partners might not have been the first to file patent applications covering certain of our technology; | |
| ● | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights; | |
| ● | It is possible that any pending patent applications will not lead to issued patents; |
| ● | Issued patents that we have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors; | |
| ● | Our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; | |
| ● | We may not develop additional proprietary technologies that are patentable; and | |
| ● | The patents of others may have an adverse effect on our business. |
We may be subject to claims challenging the inventorship of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in our patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
| - 35 - |
Risks Related to Our Industry
We are subject to government regulations and we may experience delays in obtaining required regulatory approvals in the United States to market our proposed product candidates.
Various aspects of our operations are subject to federal, state or local laws, rules and regulations, any of which may change from time to time. Costs arising out of any regulatory developments could be time-consuming and expensive and could divert management resources and attention and, consequently, could adversely affect our business operations and financial performance.
Delays in regulatory approval, limitations in regulatory approval and withdrawals of regulatory approval may have a material adverse effect on us. If we experience significant delays in testing or receiving approvals or sign-offs to conduct clinical trials, our product development costs, or our ability to license product candidates, will increase. If the FDA grants regulatory approval to market a product, this approval will be limited to those disease states and conditions for which the product has demonstrated, through clinical trials, to be safe and effective. Any product approvals that we receive in the future could also include significant restrictions on the use or marketing of our products. Product approvals, if granted, can be withdrawn for failure to comply with regulatory requirements or upon the occurrence of adverse events following commercial introduction of the products. Failure to comply with applicable FDA or other applicable regulatory requirements may result in criminal prosecution, civil penalties, recall or seizure of products, total or partial suspension of production or injunction, as well as other regulatory action against our product candidates or us. If approval is withdrawn for a product, or if a product were seized or recalled, we would be unable to sell or license that product and our revenues would suffer. In addition, outside the United States, our ability to market any of our potential products is contingent upon receiving market application authorizations from the appropriate regulatory authorities and these foreign regulatory approval processes include all of the risks associated with the FDA approval process described above.
We expect the healthcare industry to face increased limitations on reimbursement as a result of healthcare reform, which could adversely affect third-party coverage of our products and how much or under what circumstances healthcare providers will prescribe or administer our products.
In both the United States and other countries, sales of our products will depend in part upon the availability of reimbursement from third-party payors, which include governmental authorities, managed care organizations and other private health insurers. Third-party payors are increasingly challenging the price and examining the cost effectiveness of medical products and services.
Increasing expenditures for healthcare have been the subject of considerable public attention in the United States. Both private and government entities are seeking ways to reduce or contain healthcare costs. Numerous proposals that would effect changes in the U.S. healthcare system have been introduced or proposed in Congress and in some state legislatures, including reducing reimbursement for prescription products and reducing the levels at which consumers and healthcare providers are reimbursed for purchases of pharmaceutical products.
In 2010, the United States Congress enacted the Patient Protection and Affordable Care Act of 2010 or, Affordable Care Act. The Affordable Care Act seeks to reduce the federal deficit and the rate of growth in health care spending through, among other things, stronger prevention and wellness measures, increased access to primary care, changes in health care delivery systems and the creation of health insurance exchanges. Enrollment in the health insurance exchanges began in October 2013. The Affordable Care Act requires the pharmaceutical industry to share in the costs of reform, by, among other things, increasing Medicaid rebates and expanding Medicaid rebates to cover Medicaid managed care programs. Other components of healthcare reform include funding of pharmaceutical costs for Medicare patients in excess of the prescription drug coverage limit and below the catastrophic coverage threshold. Under the Affordable Care Act, pharmaceutical companies are now obligated to fund 50% of the patient obligation for branded prescription pharmaceuticals in this gap, or “donut hole.” Additionally, commencing in 2011, an excise tax was levied against certain branded pharmaceutical products. The tax is specified by statute to be approximately $3 billion in 2012 through 2016, $3.5 billion in 2017, $4.2 billion in 2018, and $2.8 billion each year thereafter. The tax is to be apportioned to qualifying pharmaceutical companies based on an allocation of their governmental programs as a portion of total pharmaceutical government programs.
Although we cannot predict the full effect on our business of the implementation of existing legislation, including the Affordable Care Act or the enactment of additional legislation, we believe that legislation or regulations that reduce reimbursement for or restrict coverage of our products could adversely affect how much or under what circumstances healthcare providers will prescribe or administer our products. This could materially and adversely affect our business by reducing our ability to generate revenue, raise capital, obtain additional collaborators and market our products. In addition, we believe the increasing emphasis on managed care in the United States has and will continue to put pressure on the price and usage of pharmaceutical products, which may adversely impact product sales.
| - 36 - |
We are subject to federal anti-kickback laws and regulations. Our failure to comply with these laws and regulations could have adverse consequences to us.
There are extensive U.S. federal and state laws and regulations prohibiting fraud and abuse in the healthcare industry that can result in significant criminal and civil penalties. These federal laws include: the anti-kickback statute, which prohibits certain business practices and relationships, including the payment or receipt of remuneration for the referral of patients whose care will be paid by Medicare or other federal healthcare programs; the physician self-referral prohibition, commonly referred to as the Stark Law; the anti-inducement law, which prohibits providers from offering anything to a Medicare or Medicaid beneficiary to induce that beneficiary to use items or services covered by either program; the False Claims Act, which prohibits any person from knowingly presenting or causing to be presented false or fraudulent claims for payment by the federal government, including the Medicare and Medicaid programs; and the Civil Monetary Penalties Law, which authorizes the U.S. Department of Health and Human Services to impose civil penalties administratively for fraudulent or abusive acts.
Sanctions for violating these federal laws include criminal and civil penalties that range from punitive sanctions, damage assessments, money penalties, imprisonment, denial of Medicare and Medicaid payments, or exclusion from the Medicare and Medicaid programs, or both, and debarment. As federal and state budget pressures continue, federal and state administrative agencies may also continue to escalate investigation and enforcement efforts to root out waste and to control fraud and abuse in governmental healthcare programs. Private enforcement of healthcare fraud has also increased, due in large part to amendments to the civil False Claims Act in 1986 that were designed to encourage private persons to sue on behalf of the government. A violation of any of these federal and state fraud and abuse laws and regulations could have a material adverse effect on our liquidity and financial condition. An investigation into the use by physicians of any of our products once commercialized may dissuade physicians from either purchasing or using them, and could have a material adverse effect on our ability to commercialize those products.
Risks Relating to Ownership of Our Common Stock
With the exception of two of our directors, all of our current directors and officers serve as directors or officers of Can-Fite, and may have conflicts of interest in transactions or matters concerning us.
With the exception of two of our directors, all of our current directors and officers also serve as directors, officers or employees of Can-Fite. Dr. Fishman, our Chairman of the Board and Chief Executive Officer, is the Chief Executive Officer of Can-Fite and, Itay Weinstein, our Chief Financial Officer, is the Controller for Can-Fite and Messrs. Cohn and Regev, two of our directors, are also directors of Can-Fite. These individuals have certain fiduciary obligations to us and to Can-Fite. Such dual obligations may in the future result in a conflict of interest with respect to presenting certain business, financing or other opportunities to us or to Can-Fite. More specifically, Can-Fite is investigating CF101 for treatment of rheumatoid arthritis and psoriasis while we are investigating CF101 for treatment of various ophthalmic conditions. While EyeFite has sole responsibility for preparing regulatory filings with respect to approvals of CF101 for the treatment of ophthalmic conditions, Can-Fite manages all activities relating to clinical studies for the development of the ophthalmic indications of CF101 as well as those for psoriasis and rheumatoid arthritis. A conflict of interest may arise concerning the timing of the parties’ planned and ongoing clinical trials, new drug application filings and the parties’ opportunities for market or data exclusivity for CF101. In addition, they may be faced with decisions that could have different implications for us than they do for Can-Fite. Consequently, we cannot assure you that the members of our board and management would always act in our and our stockholders’ best interests in all situations where a conflict arises.
Failure to achieve and maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 could have a material adverse effect on our business, results of operation or financial condition. In addition, current and potential stockholders could lose confidence in our financial reporting, which could have a material adverse effect on the price of our common stock.
Effective internal controls are necessary for us to provide reliable financial reports and effectively prevent fraud. We are required to document and test our internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, which requires annual management assessments of the effectiveness of our internal controls over financial reporting. In addition, if we fail to maintain the adequacy of our internal controls, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404. Disclosing deficiencies or weaknesses in our internal controls, failing to remediate these deficiencies or weaknesses in a timely fashion or failing to achieve and maintain an effective internal control environment may cause investors to lose confidence in our reported financial information, which could have a material adverse effect on the price of our common stock. If we cannot provide reliable financial reports or prevent fraud, our operating results could be harmed.
| - 37 - |
As an “emerging growth company” under the JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements.
As an “emerging growth company” under the JOBS Act, we are permitted to, and intend to, rely on exemptions from certain disclosure requirements. We are an emerging growth company until the earliest of: (i) the last day of the fiscal year during which we had total annual gross revenues of $1 billion or more, (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement, (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt or (iv) the date on which we are deemed a “large accelerated issuer” as defined in Regulation S-K of the Securities Act. For so long as we remain an emerging growth company, we will not be required to:
| ● | have an auditor report on our internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; |
| ● | comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis); |
| ● | submit certain executive compensation matters to shareholders advisory votes pursuant to the “say on frequency” and “say on pay” provisions (requiring a non-binding shareholder vote to approve compensation of certain executive officers) and the “say on golden parachute” provisions (requiring a non-binding shareholder vote to approve golden parachute arrangements for certain executive officers in connection with mergers and certain other business combinations) of the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010; and |
| ● | include detailed compensation discussion and analysis in our filings under the Exchange Act, and instead may provide a reduced level of disclosure concerning executive compensation. |
Although we intend to rely on the exemptions provided in the JOBS Act, the exact implications of the JOBS Act for us are still subject to interpretations and guidance by the SEC and other regulatory agencies. In addition, as our business grows, we may no longer satisfy the conditions of an emerging growth company. We are currently evaluating and monitoring developments with respect to these new rules and we cannot assure you that we will be able to take advantage of all of the benefits from the JOBS Act.
In addition, as an “emerging growth company,” we may elect under the JOBS Act to delay adoption of new or revised accounting pronouncements applicable to public companies until such pronouncements are made applicable to private companies. Therefore, our financial statements may not be comparable to those of companies that comply with standards that are otherwise applicable to public companies.
We have not paid, and do not intend to pay, dividends on our common stock and therefore, unless our common stock appreciates in value, our investors may not benefit from holding our common stock.
We have not paid any cash dividends on our common stock since inception. We do not anticipate paying any cash dividends our common stock in the foreseeable future. As a result, investors in our common stock will not be able to benefit from owning our common stock unless the market price of our common stock becomes greater than the price paid for the stock by these investors.
The public trading market for our common stock is volatile and may result in higher spreads in stock prices, which may limit the ability of our investors to sell their shares at a profit, if at all.
Our common stock trades in the over-the-counter market and is quoted on the OTC Pink. The over-the-counter market for securities has historically experienced extreme price and volume fluctuations during certain periods. These broad market fluctuations may adversely affect the market price of our common stock and result in substantial losses to our investors. In addition, the spreads on stock traded through the over-the-counter market are generally unregulated and higher than on stock exchanges, which means that the difference between the price at which shares could be purchased by investors in the over-the-counter market compared to the price at which they could be subsequently sold would be greater than on these exchanges. Significant spreads between the bid and asked prices of the stock could continue during any period in which a sufficient volume of trading is unavailable or if the stock is quoted by an insignificant number of market makers. Historically our trading volume has been insufficient to significantly reduce this spread and we have had a limited number of market makers sufficient to affect this spread. These higher spreads could adversely affect investors who purchase the shares at the higher price at which the shares are sold, but subsequently sell the shares at the lower bid prices quoted by the brokers. Unless the bid price for the stock exceeds the price paid for the shares by the investor, plus brokerage commissions or charges, the investor could lose money on the sale. For higher spreads such as those on over-the-counter stocks, this is likely a much greater percentage of the price of the stock than for exchange listed stocks. There is no assurance that at the time an investor in our common stock wishes to sell the shares, the bid price will have sufficiently increased to create a profit on the sale.
| - 38 - |
We do not know whether a market for our common stock will be sustained or what the market price of our common stock will be and as a result it may be difficult for investors to sell their shares of our common stock.
Although our common stock trades on the OTC Pink, an active trading market for our shares may not be sustained. It may be difficult for investors to sell their shares without depressing the market price for the shares or at all. As a result of these and other factors, investors may not be able to sell their shares at or above the offering price or at all. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of common stock as consideration. If an active market for our common stock does not develop or is not sustained, it may be difficult to sell your common stock.
Our board can, without stockholder approval, cause preferred stock to be issued on terms that adversely affect common stockholders or which could be used to resist a potential take-over of the Company.
Under our certificate of incorporation, our board is authorized to issue up to 1,000,000 shares of preferred stock, none of which are issued and outstanding as of the date of this Annual Report on Form 10-K. Also, our board, without stockholder approval, may determine the price, rights, preferences, privileges and restrictions, including voting rights, of those shares. If the board causes shares of preferred stock to be issued, the rights of the holders of our common stock could be adversely affected. The board’s ability to determine the terms of preferred stock and to cause its issuance, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, could have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting stock. Preferred shares issued by the board could include voting rights, or even super voting rights, which could shift the ability to control the Company to the holders of the preferred stock. Preferred shares could also have conversion rights into shares of common stock at a discount to the market price of the common stock which could negatively affect the market for our common stock. In addition, preferred shares would have preference in the event of liquidation of the corporation, which means that the holders of preferred shares would be entitled to receive the net assets of the corporation distributed in liquidation before the common stock holders receive any distribution of the liquidated assets. We have no current plans to issue any shares of preferred stock.
The market price of our common stock may fluctuate significantly, which could result in substantial losses by our investors.
The market price of our common stock may fluctuate significantly in response to numerous factors, some of which are beyond our control, such as:
| ● | announcements of technological innovations, new products or product enhancements by us or others; |
| ● | announcements by us of significant strategic partnerships, out-licensing, in-licensing, joint ventures, acquisitions or capital commitments; |
| ● | expiration or terminations of licenses, research contracts or other collaboration agreements; |
| ● | public concern as to the safety of drugs we, our licensees or others develop; |
| ● | success of research and development projects; |
| ● | success in clinical and preclinical studies; |
| ● | developments concerning intellectual property rights or regulatory approvals; |
| ● | variations in our and our competitors’ results of operations; |
| ● | changes in earnings estimates or recommendations by securities analysts, if our common stock is covered by analysts; |
| ● | changes in government regulations or patent decisions; |
| ● | developments by our licensees; |
| ● | developments in the biotechnology industry; |
| ● | the results of product liability or intellectual property lawsuits; |
| ● | future issuances of common stock or other securities; |
| - 39 - |
| ● | the addition or departure of key personnel; |
| ● | announcements by us or our competitors of acquisitions, investments or strategic alliances; |
| ● | general market conditions, including the volatility of market prices for shares of biotechnology companies generally, and other factors, including factors unrelated to our operating performance; and |
| ● | the other factors described in this “Risk Factors” section. |
These factors and any corresponding price fluctuations may materially and adversely affect the market price of our common stock and result in substantial losses by our investors.
Further, the stock market in general, and the market for biotechnology companies in particular, has experienced extreme price and volume fluctuations in the past. Continued market fluctuations could result in extreme volatility in the price of our common stock, which could cause a decline in the value of our common stock. Price volatility of our common stock might be worse if the trading volume of our common stock is low. In the past, following periods of market volatility, stockholders have often instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and attention of management from our business, even if we are successful. Future sales of our common stock could also reduce the market price of such stock.
Moreover, the liquidity of our common stock is limited, not only in terms of the number of shares that can be bought and sold at a given price, but by delays in the timing of transactions and reduction in security analysts’ and the media’s coverage of us, if any. These factors may result in lower prices for our common stock than might otherwise be obtained and could also result in a larger spread between the bid and ask prices for our common stock. In addition, without a large float, our common stock is less liquid than the stock of companies with broader public ownership and, as a result, the trading prices of our common stock may be more volatile. In the absence of an active public trading market, an investor may be unable to liquidate its investment in our common stock. Trading of a relatively small volume of our common stock may have a greater impact on the trading price of our stock than would be the case if our public float were larger. We cannot predict the prices at which our common stock will trade in the future.
Some or all of the “restricted” shares of our common stock issued in connection with the Transaction or held by other of our stockholders may be offered from time to time in the open market pursuant to an effective registration statement or Rule 144 promulgated under Regulation D of the Securities Act, and these sales may have a depressive effect on the market for our common stock.
Because our common stock may be a “penny stock,” it may be more difficult for investors to sell shares of our common stock, and the market price of our common stock may be adversely affected.
Our common stock may be a “penny stock” if, among other things, the stock price is below $5.00 per share, it is not listed on a national securities exchange or it has not met certain net tangible asset or average revenue requirements. Broker-dealers who sell penny stocks must provide purchasers of these stocks with a standardized risk-disclosure document prepared by the SEC. This document provides information about penny stocks and the nature and level of risks involved in investing in the penny-stock market. A broker must also give a purchaser, orally or in writing, bid and offer quotations and information regarding broker and salesperson compensation, make a written determination that the penny stock is a suitable investment for the purchaser, and obtain the purchaser’s written agreement to the purchase. Broker-dealers must also provide customers that hold penny stock in their accounts with such broker-dealer a monthly statement containing price and market information relating to the penny stock. If a penny stock is sold to an investor in violation of the penny stock rules, the investor may be able to cancel its purchase and get its money back.
If applicable, the penny stock rules may make it difficult for investors to sell their shares of our common stock. Because of the rules and restrictions applicable to a penny stock, there is less trading in penny stocks and the market price of our common stock may be adversely affected. Also, many brokers choose not to participate in penny stock transactions. Accordingly, investors may not always be able to resell their shares of our common stock publicly at times and prices that they feel are appropriate.
| - 40 - |
Risks Related to our Operations in Israel
Potential political, economic and military instability in the state of Israel, where our senior management, our head executive office and Can-Fite’s research and development facilities are located, may adversely affect our results of operations.
Our head executive office and the facilities of Can-Fite, upon whom we rely for our initial research and development activities, as well as some of our planned clinical sites and suppliers are located in Israel. Our officers and most of our directors are residents of Israel. Accordingly, political, economic and military conditions in Israel and the surrounding region may directly affect our business and operations. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors. Any hostilities involving Israel or the interruption or curtailment of trade within Israel or between Israel and its trading partners could adversely affect our operations and results of operations and could make it more difficult for us to raise capital. During the summer of 2014 and the winter of 2012 and 2008, Israel was engaged in an armed conflict with Hamas, a militia group and political party operating in the Gaza Strip, and during the summer of 2006, Israel was engaged in an armed conflict with Hezbollah, a Lebanese Islamist Shiite militia group and political party. These conflicts involved missile strikes against civilian targets in various parts of Israel, and negatively affected business conditions in Israel. To date, Israel faces political tension with respect to its relationships with Turkey, Iran and other Arab neighbor countries. In addition, recent political uprisings and social unrest in various countries in the Middle East and North Africa are affecting the political stability of those countries. This instability may lead to deterioration of the political relationships that exist between Israel and these countries, and have raised concerns regarding security in the region and the potential for armed conflict. Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions and could harm our results of operations. For example, any major escalation in hostilities in the region could result in a portion of our employees and service providers being called up to perform military duty for an extended period of time. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements. Any future deterioration in the political and security situation in Israel will negatively impact our business.
Our commercial insurance does not cover losses that may occur as a result of events associated with the security situation in the Middle East. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions and could harm our results of operations.
Further, in the past, the State of Israel and Israeli companies have been subjected to an economic boycott. Several countries still restrict business with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition or the expansion of our business.
Our operations may be disrupted as a result of the obligation of Israeli citizens to perform military service.
Many Israeli citizens are obligated to perform one month, and in some cases more, of annual military reserve duty until they reach the age of 45 (or older, for reservists with certain occupations) and, in the event of a military conflict, may be called to active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will be military reserve duty call-ups in the future. Our operations could be disrupted by such call-ups. Such disruption could materially adversely affect our business, financial condition and results of operations.
Investors may have difficulties enforcing a U.S. judgment, including judgments based upon the civil liability provisions of the U.S. federal securities laws against us, Eyefite, and both companies’ executive officers and directors or asserting U.S. securities laws claims in Israel.
Eyefite’s directors and officers, as well as most of our directors and officers, are not residents of the United States and some of their, our and Eyefite’s assets are located outside the United States. Service of process upon Eyefite’s or our non-U.S. resident directors and officers and enforcement of judgments obtained in the United States against us or Eyefite, and some of our directors and executive officers may be difficult to obtain within the United States. The Company and Eyefite have been informed by their legal counsel in Israel that it may be difficult to assert claims under U.S. securities laws in original actions instituted in Israel or obtain a judgment based on the civil liability provisions of U.S. federal securities laws. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws against us or Eyefite or our respective officers and directors because Israel may not be the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel addressing the matters described above. Israeli courts might not enforce judgments rendered outside Israel, which may make it difficult to collect on judgments rendered against us, Eyefite and/or our respective officers and directors.
| - 41 - |
Moreover, among other reasons, including but not limited to, fraud, a lack of due process, a judgment which is at variance with another judgment that was given in the same matter and if a suit in the same matter between the same parties was pending before a court or tribunal in Israel, an Israeli court will not enforce a foreign judgment if it was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases) or if its enforcement is likely to prejudice the sovereignty or security of the State of Israel.
Because a certain portion of our expenses is incurred in currencies other than the US Dollar, our results of operations may be harmed by currency fluctuations and inflation.
Our reporting and functional currency is the U.S. Dollar, but some portion of our clinical trials and operations expenses are in NIS and Euro. As a result, we are exposed to some currency fluctuation risks. We may, in the future, decide to enter into currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rate of the currencies mentioned above in relation to the US Dollar. These measures, however, may not adequately protect us from adverse effects.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
None.
ITEM 2. PROPERTIES.
Our principal executive offices are located at 10 Bareket St, Petach Tikva, Israel, 4951778, which is also the office of Can-Fite, our majority stockholder and parent.
ITEM 3. LEGAL PROCEEDINGS.
Neither we nor our subsidiary, Eyefite, is a party to, nor is any of their property subject to, any legal proceedings which require disclosure pursuant to this item.
On June 29, 2015, Can-Fite, our majority stockholder and parent, received a lawsuit requesting recognition of this lawsuit as a class action. The lawsuit alleges, among other things, that Can-Fite misled the public with regard to disclosures concerning the efficacy its drug candidate, CF101. The claimant alleges that he suffered personal damages of over NIS 73,000, while also claiming that the shareholders of Can-Fite suffered damages of approximately NIS 125 million. Can-Fite has informed us that it strongly believes the lawsuit and its allegations to be baseless and without merit, and will vigorously defend this action.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
| - 42 - |
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our common stock is quoted on the OTC Pink under the symbol “OPLI.”
The following table sets forth the range of the high and low bid prices of the common stock for the periods indicated. The quotations reflect inter-dealer prices, without retail markup, markdown or commission, and may not represent actual transactions. Consequently, the information provided below may not be indicative of our common stock price under different conditions.
| 2015 | HIGH | LOW | ||||||
| First Quarter | 1.50 | 0.50 | ||||||
| Second Quarter | 1.90 | 0.70 | ||||||
| Third Quarter | 1.90 | 0.35 | ||||||
| Fourth Quarter | 0.99 | 0.99 | ||||||
| 2014 | HIGH | LOW | ||||||
| First Quarter | 2.90 | 0.55 | ||||||
| Second Quarter | 1.85 | 1.26 | ||||||
| Third Quarter | 1.75 | 1.01 | ||||||
| Fourth Quarter | 1.01 | 0.50 | ||||||
Holders
As of March 1, 2016, there were approximately 15 stockholders of record holding 10,441,251 shares of our common stock. This number does not include an indeterminate number of stockholders whose shares are held by brokers in street name. The holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. Holders of our common stock have no preemptive rights and no right to convert their common stock into any other securities. There are no redemption or sinking fund provisions applicable to our common stock.
Dividends
We have not paid, nor declared, any cash dividends since our inception and do not intend to declare or pay any such dividends in the foreseeable future. Our ability to pay cash dividends is subject to limitations imposed by state law.
ITEM 6. SELECTED FINANCIAL DATA.
As a “smaller reporting company”, we have elected not to provide the disclosure required by this item.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
Management’s Discussion and Analysis of Financial Condition and Results of Operations analyzes the major elements of our balance sheets, statements of income and cash flows. This section should be read in conjunction with the other sections of this Annual Report on Form 10-K for the year ended December 31, 2015 and our financial statements and accompanying notes to these financial statements. All amounts are in U.S. dollars and rounded.
Description of Business
We are a clinical-stage biopharmaceutical company focused on developing therapeutic products for the treatment of ophthalmic disorders. We have in-licensed certain patents and patent applications protecting the use in the ophthalmic field of our current pipeline drug under development, a synthetic A3 adenosine receptor, or A3AR, agonist, CF101 (known generically as IB-MECA). CF101 is currently being developed by us to treat two ophthalmic indications: glaucoma and uveitis. We are currently conducting a Phase II trial with respect to the development of CF101 for the treatment of glaucoma or related syndromes of ocular hypertension.
| - 43 - |
CF101 is a highly-selective, orally bioavailable small molecule synthetic drug, which targets the A3AR. We believe that CF101 has a favorable safety profile and a potent anti-inflammatory activity, mediated via its capability to inhibit the production of inflammatory cytokines, such as TNF-α, MMPs, IL-1, and IL-6. This is mediated by activation of the A3AR, which is highly expressed in inflammatory tissues in contrast to normal tissues where expression levels of the receptor are very low. We believe that the anti-inflammatory and neuro-protective effects of CF101 make it a candidate for use in the treatment of ophthalmic indications.
We are focused on the development of CF101 for the treatment of glaucoma, with a Phase II study ongoing in Israel and Europe. This study is a randomized, double-masked, placebo-controlled, parallel-group study of the safety and efficacy of daily CF101 administered orally in subjects with elevated IOP. The objective of this study is to determine the safety and efficacy of oral CF101 in lowering IOP when administered BID for 16 weeks in subjects with elevated IOP. We have enrolled 44 subjects in the first segment of the study, randomized in a 3:1 ratio of CF101 1.0 mg treatment to the placebo. In 2015, we amended the ongoing Phase II study protocol and enrolled a further 44 subjects for the second segment, which was randomized in a 3:1 ratio of CF101 2.0 mg treatment to the placebo. This decision was based on certain positive data from the psoriasis Phase II/III study of CF101 by our majority stockholder and parent, Can-Fite BioPharma Ltd, or Can-Fite. As a result, there will not be an interim analysis and the full study data is expected to be announced in the second half of 2016.
If successful, the treatment of glaucoma with an oral drug has the potential to be a breakthrough treatment in resolving patient compliance issues with current topical treatments. A third-party validation for the utilization of A3 adenosine receptor agonists for lowering intraocular pressure and treating glaucoma has been published by Professor M. Francesca Cordeiro, a Professor of Glaucoma & Retinal Neuro-degeneration at the University College of London and Imperial College in London.
According to Market Scope, the global glaucoma pharmaceutical market is expected to be nearly $6.1 billion in 2020 and according to GlobalData the global uveitis therapeutics market is expected to grow from $0.3 billion in 2010 to $1.6 billion by 2017. None of our product candidates have been approved for sale or marketing and, to date, there have been no commercial sales of any of our product candidates.
Our corporate strategy is to build a specialized ophthalmic company that develops and in-licenses drugs for the treatment of ophthalmic diseases. We intend to seek to obtain technologies that complement and expand our existing pipeline by entering into in-license or co-development agreements with academic institutions and biotechnology or pharmaceutical companies. We intend to commercialize our products through out-licensing arrangements with third parties who may perform any or all of the following tasks: completing development, securing regulatory approvals, manufacturing, marketing and sales. We do not intend to develop our own manufacturing facilities or sales forces.
Results of Operations -Year Ended December 31, 2015 Compared to the Year Ended December 31, 2014
| Year
Ended December 31, |
||||||||
| 2015 | 2014 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | 812,000 | $ | 721,000 | ||||
| General and administrative | 573,000 | 425,000 | ||||||
| Total operating costs | 1,385,000 | 1,146,000 | ||||||
| Financial expenses, net | 92,000 | 49,000 | ||||||
| Net loss | $ | 1,477,000 | $ | 1,195,000 | ||||
Revenues
We did not generate any revenues from operations. We had no revenues because we do not have any commercial products and we do not expect to have such products for several years, if at all.
| - 44 - |
Operating Expenses
Research and development expenses. Research and development expenses were $812,000 for the year ended December 31, 2015, compared to $721,000 for the year ended December 31, 2014, an increase of $91,000 or 13%. The increase in research and development expenses is primarily related to the increase in expenses related to our Phase II CF101 study for glaucoma in 2015 offset by completion of our Phase III study in DES during 2014 and a reduction in patent and licensing maintenance fees. Assuming we will have sufficient funding, we anticipate incurring significantly higher costs in the future if our Phase II CF101 study for glaucoma is successful.
General and administrative expenses. General and administrative expenses were $573,000 for the year ended December 31, 2015, compared to $425,000 for the year ended December 31, 2014, an increase of $148,000 or 35%. The increase in general and administrative expenses was primarily due to an increase in legal and professional expenses related to a proposed acquisition of Improved Vision Systems (I.V.S.) Ltd that was not completed.
Financial Expenses, Net
Financial expenses, net were $92,000 for the year ended December 31, 2015 compared to financial income, net of $49,000 for the year ended December 31, 2014, an increase of $43,000 or 88%. Financial expenses, net consisted primarily of interest expenses due to deferred payments to our majority shareholder and parent company, Can-Fite. The increase of financial expenses, net was mainly attributable to interest expenses due to deferred payments to Can-Fite.
Net Loss
As a result of the foregoing, we incurred a net loss of $1,477,000 for the year ended December 31, 2015 compared $1,195,000 for the year ended December 31, 2014, an increase of $282,000 or 24%.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations, and otherwise operate on an ongoing basis. Significant factors in the management of liquidity are funds generated by operations, levels of accounts receivable and accounts payable and capital expenditures.
As of December 31, 2015 and December 31, 2014, we had $42,000 and $9,000 in cash and cash equivalents, a working capital deficit of $3,281,000 and $1,691,000 and an accumulated deficit of $8,773,000 and $7,296,000, respectively. The increase in working capital deficit was primarily due to an increase in deferred payments to Can-Fite offset by the increase in the fair value of our investment in Can-Fite’s securities.
Net cash provided by in operating activities was approximately $33,000 for the year ended December 31, 2015, compared with net cash used in operating activities of approximately $413,000 for the year ended December 31, 2014. The increase in net cash used in operating activities was primarily related to the increase in debt to our parent company and the change in prepaid expenses.
There was no net cash used in investing activities and no net cash provided by financing activities for the years ended December 31, 2015 and 2014.
We have no current source of revenue to sustain our research and development activities, and we do not expect to generate revenue until, and unless, we commercialize any product candidate, which we do not expect to occur for several years. In February 2013, Can-Fite issued us a formal letter, which has been updated periodically (most recently in August 2015), stating that Can-Fite agrees to defer payments owed to it under the Services Agreement (under which Can-Fite manages, as an independent contractor, all activities relating to pre-clinical and clinical studies performed for the development of the ophthalmic indications of CF101) beginning on January 31, 2013 until the completion of fundraising by the Company sufficient to cover such deferred payments. As of December 31, 2015, the deferred payments to Can-Fite totaled approximately $3,690,000. In addition, in August 2015, Can-Fite issued a financial support letter pursuant to which it committed to cover any shortfall in our costs and expenses of the operations which are in excess of our available cash to finance our operations, including cash generated from any future sale of Can-Fite shares held by us. Both letters expire in October 2016 and any related balance bears interest at a rate of 3% per annum.
According to management estimates, liquidity resources as of December 31, 2015, together with the support letters from Can-Fite described above, will be sufficient to maintain our operations at least through October 2016. Our inability to raise funds to conduct our research and development activities will have a severe negative impact on our ability to remain a viable company. These conditions raise substantial doubt about our ability to continue as a going concern. As such, the opinion of our independent registered public accounting firm on our audited financial statements as of and for the year ended December 31, 2015 contains an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern. This going concern opinion could materially limit our ability to raise additional funds through the issuance of new debt or equity securities or otherwise. Future reports on our financial statements may include an explanatory paragraph with respect to our ability to continue as a going concern.
| - 45 - |
For a long term solution, we will need to seek additional capital for the purpose of further testing our products, managing our business and obtaining certifications necessary in order to market them. Developing drugs, conducting clinical trials and commercializing products is expensive and we will need to raise substantial additional funds to achieve our strategic objectives. Additional financing may not be available on acceptable terms, if at all. Our future capital requirements will depend on many factors, including:
| ● | the failure to obtain regulatory approval or achieve commercial success of our product candidates, which currently includes only CF101; | |
| ● | the level of research and development investment required to develop our product candidates, and maintain and improve our patented or licensed technology position; | |
| ● | the costs of obtaining or manufacturing product candidates for research and development and testing; | |
| ● | the results of preclinical and clinical testing, which can be unpredictable in product candidate development and any decision to initiate additional preclinical or clinical studies; | |
| ● | changes in product candidate development plans needed to address any difficulties that may arise in manufacturing, preclinical activities or clinical studies; | |
| ● | our success in establishing and effecting out-licensing agreements with strategic partners, the terms of these agreements and the success of these potential future licensees and partners in selling our products; | |
| ● | our success rate in preclinical and clinical efforts associated with milestones and royalties, if applicable; | |
| ● | the costs of investigating patents that might block us from developing potential product candidates; | |
| ● | the costs of recruiting and retaining qualified personnel; | |
| ● | the costs, timing and outcomes involved in obtaining regulatory approvals; | |
| ● | the number of product candidates we pursue in the future; | |
| ● | our revenues, if any; | |
| ● | the costs of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; | |
| ● | our need or decision to acquire or license complementary technologies or new platform or product candidate targets; and | |
| ● | the costs of financing unanticipated working capital requirements and responding to competitive pressures. |
We currently have no agreements, arrangements, or understandings with any person to obtain funds through bank loans, lines of credit, or any other sources, other than the formal letter from Can-Fite agreeing to defer payments owed to it under the services agreement. Until we can generate significant continuing revenues, we expect to satisfy our future cash needs through debt or equity financings, or by out-licensing our product candidate. We cannot be certain that additional funding will be available to us on acceptable terms, or at all. If funds are not available, we may be required to delay, reduce the scope of, or eliminate one or more of our research or development programs or our commercialization efforts.
We are addressing our liquidity issues by implementing initiatives to raise additional funds as well as other measures that we believe will allow the coverage of our anticipated budget deficit. Such initiatives may include monetizing of our assets, by intention to realize our remaining investment in Can-Fite’s shares.
As of the date of this report, we have no material capital commitments.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future material effect on our consolidated financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity capital expenditures or capital resources.
Critical Accounting Policies
Our significant accounting policies, which include management’s best estimates and judgments, are included in Note 2 to the financial statements for the year ended December 31, 2015 included in this Annual Report on Form 10-K for the year ended December 31, 2015.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
As a smaller reporting company, we have elected not to provide the disclosure required by this item.
| - 46 - |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
OPHTHALIX INC. AND ITS SUBSIDIARY
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2015
U.S. DOLLARS IN THOUSANDS
INDEX
| Page | ||
| Report of Independent Registered Public Accounting Firm | F-2 | |
| Consolidated Balance Sheets | F-3 | |
| Consolidated Statements of Comprehensive Loss | F-4 | |
| Consolidated Statements of Changes in Stockholders' Deficiency | F-5 | |
| Consolidated Statements of Cash Flows | F-6 | |
| Notes to Consolidated Financial Statements | F-7 - F-22 |
- - - - - - - - - - - - - - -
| F-1 |
 |
Kost Forer Gabbay & Kasierer 3 Aminadav St. Tel-Aviv 6706703, Israel |
Tel: +972-3-6232525 Fax: +972-3-5622555 ey.com |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
OPHTHALIX INC.
We have audited the accompanying consolidated balance sheets of OphthaliX Inc. and its subsidiary (the "Company") as of December 31, 2015 and 2014, and the related consolidated statement of comprehensive loss, changes in stockholders' deficiency and cash flows for each of the two years in the period ended December 31, 2015. These consolidated financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company's internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above, present fairly, in all material respects, the consolidated financial position of the Company and its subsidiary at December 31, 2015 and 2014, and the consolidated results of their operations and their cash flows for each of the two years in the period ended December 31, 2015, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1c to the consolidated financial statements, the Company has recurring losses from operations and has limited liquidity resources that raise substantial doubt about its ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1c. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| Tel-Aviv, Israel | KOST FORER GABBAY & KASIERER |
| March 2, 2016 | A Member of Ernst & Young Global |
| F-2 |
OPHTHALIX INC. AND ITS SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
U.S. dollars in thousands, except share and per share data
| December 31, | ||||||||
| 2015 | 2014 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 42 | $ | 9 | ||||
| Investment in Parent Company (Note 3) | 658 | 794 | ||||||
| Prepaid expenses | - | 209 | ||||||
| Total current assets | 700 | 1,012 | ||||||
| PROPERTY AND EQUIPMENT, NET | *)- | 1 | ||||||
| Total assets | $ | 700 | $ | 1,013 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIENCY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Parent Company (Note 9) | $ | 3,690 | $ | 2,457 | ||||
| Other accounts payable and accrued expenses | 291 | 246 | ||||||
| Total current liabilities | 3,981 | 2,703 | ||||||
| NON-CURRENT LIABILITIES: | ||||||||
| Derivative related to Service Agreement (Note 4) | *)- | *)- | ||||||
| STOCKHOLDERS' DEFICIENCY (Note 6): | ||||||||
| Share capital | ||||||||
| Preferred Stock - | ||||||||
| Authorized: 1,000,000 shares at December 31, 2015 and 2014, Issued and Outstanding: 0 shares at December 31, 2015 and 2014 | - | - | ||||||
| Common Stock of $0.001 par value - | ||||||||
| Authorized: 100,000,000 shares at December 31, 2015 and 2014, Issued and Outstanding: 10,441,251 shares at December 31, 2015 and 2014 | 10 | 10 | ||||||
| Additional paid-in capital | 5,516 | 5,494 | ||||||
| Accumulated other comprehensive income (loss) | (34 | ) | 102 | |||||
| Accumulated deficit | (8,773 | ) | (7,296 | ) | ||||
| Total stockholders' deficiency | (3,281 | ) | (1,690 | ) | ||||
| Total liabilities and stockholders' deficiency | $ | 700 | $ | 1,013 | ||||
*) Represents an amount lower than $1
The accompanying notes are an integral part of the consolidated financial statements.
| F-3 |
OPHTHALIX INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
U.S. dollars in thousands, except share and per share data
Year ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Operating expenses: | ||||||||
| Research and development | $ | 812 | $ | 721 | ||||
| General and administrative | 573 | 425 | ||||||
| Total operating expenses | 1,385 | 1,146 | ||||||
| Financial expenses, net (Note 8) | 92 | 49 | ||||||
| Net loss | 1,477 | 1,195 | ||||||
| Net loss per share: | ||||||||
| Basic and Diluted loss per share | $ | 0.14 | $ | 0.11 | ||||
| Weighted average number of shares of Common Stock used in computing basic and diluted net loss per share | 10,441,251 | 10,441,251 | ||||||
| Other comprehensive loss: | ||||||||
| Available-for-sale investments: | ||||||||
| Changes in net unrealized loss | 136 | 381 | ||||||
| Total other comprehensive loss | $ | 136 | $ | 381 | ||||
| Comprehensive loss | $ | 1,613 | $ | 1,576 | ||||
The accompanying notes are an integral part of the consolidated financial statements.
| F-4 |
OPHTHALIX INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' DEFICIENCY
U.S. dollars in thousands, except share and per share data
| Accumulated | ||||||||||||||||||||||||
| Shares of Common Stock | Additional paid-in | Accumulated | other comprehensive | Total stockholders' | ||||||||||||||||||||
| Number | Amount | capital | deficit | loss | deficiency | |||||||||||||||||||
| Balance as of January 1, 2014 | 10,441,251 | 10 | 5,469 | (6,101 | ) | 483 | (139 | ) | ||||||||||||||||
| Stock based compensation | - | - | 25 | - | - | 25 | ||||||||||||||||||
| Unrealized loss from investment in Parent Company | - | - | - | - | (381 | ) | (381 | ) | ||||||||||||||||
| Net loss | - | - | - | (1,195 | ) | - | (1,195 | ) | ||||||||||||||||
| Balance as of December 31, 2014 | 10,441,251 | 10 | 5,494 | (7,296 | ) | 102 | (1,690 | ) | ||||||||||||||||
| Stock based compensation | - | - | 22 | - | - | 22 | ||||||||||||||||||
| Unrealized loss from investment in Parent Company | - | - | - | - | (136 | ) | (136 | ) | ||||||||||||||||
| Net loss | - | - | - | (1,477 | ) | - | (1,477 | ) | ||||||||||||||||
| Balance as of December 31, 2015 | 10,441,251 | 10 | 5,516 | (8,773 | ) | (34 | ) | (3,281 | ) | |||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements
| F-5 |
OPHTHALIX INC. AND ITS SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
U.S. dollars in thousands, except share and per share data
Year ended December 31, | ||||||||
| 2015 | 2014 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | (1,477 | ) | $ | (1,195 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Decrease (increase) in prepaid expenses | 209 | (189 | ) | |||||
| Increase (decrease) in other accounts payable and accrued expenses | 45 | (17 | ) | |||||
| Increase in Parent Company balance | 1,233 | 970 | ||||||
| Change in fair value of the derivative related to Service Agreement | - | (7 | ) | |||||
| Stock based compensation | 22 | 25 | ||||||
| Depreciation | 1 | *)- | ||||||
| Net cash used in operating activities | 33 | (413 | ) | |||||
| Cash flows from investing activities: | ||||||||
| Purchase of property and equipment | (3 | ) | - | |||||
| Proceeds from sale of property and equipment | 3 | - | ||||||
| Net cash provided by investing activities | - | - | ||||||
| Cash flows from financing activities: | ||||||||
| Net cash provided by financing activities | - | - | ||||||
| Change in cash and cash equivalents | 33 | (413 | ) | |||||
| Cash and cash equivalents at the beginning of the year | 9 | 422 | ||||||
| Cash and cash equivalents at the end of the year | $ | 42 | $ | 9 | ||||
*) Represents an amount lower than $1
The accompanying notes are an integral part of the consolidated financial statements.
| F-6 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 1:- | GENERAL |
| a. | OphthaliX Inc. (the "Company" or "OphthaliX"), originally incorporated in the State of Nevada on December 10, 1999 under the name Bridge Capital.com Inc., was a nominally capitalized corporation that did not commence its operations until it changed its name to Denali Concrete Management Inc. ("Denali"), in March 2001. Denali was a concrete placement company specializing in providing concrete improvements in the road construction industry. In December 2005, the Company ceased its principal business operations and focused its efforts on seeking a business opportunity, becoming a public shell company in the U.S. |
Eye-Fite Ltd. ("Eye-Fite" or the "Subsidiary") was founded on June 27, 2011 in contemplation of the execution of a transaction between Can-Fite BioPharma Ltd. (the "Parent company" or "Can-Fite"), a public company in Israel and U.S, and the Company, as further detailed in Note 1b below (the “Reverse Recapitalization”). Can-Fite granted Eye-Fite an exclusive worldwide license for a therapeutic drug, CF101, solely in the field of ophthalmic diseases (the “License Agreement”). The Company conducts its research and development activities through Eye-Fite Ltd. See also Note 1b2.
Upon consummation of the Reverse Recapitalization, Denali changed its name to OphthaliX Inc. and also changed its corporate domicile from the state of Nevada to the state of Delaware.
| b. | Reverse Recapitalization and related arrangements: |
| 1. | Recapitalization: |
On November 21, 2011 (the "Closing Date"), Can-Fite purchased 8,000,000 shares of the Company’s common stock, par value $0.001 per share (the “Common Stock”), in exchange for all of the issued and outstanding ordinary shares of Eyefite pursuant to the terms of a stock purchase agreement (the “Purchase Agreement”). As a result, Eyefite became a wholly-owned subsidiary of the Company and Can-Fite became its majority stockholder and a parent.
Also on November 21, 2011, the Company issued a warrant to Can-Fite by which Can-Fite has the right, until the earlier of (a) the November 21, 2016 and (b) the closing of the acquisition of the Company by another entity, resulting in the exchange of the Company’s outstanding common shares such that its stockholders prior to such transaction own, directly or indirectly, less than 50% of the voting power of the surviving entity, to convert its right to the Additional Payment (as defined below) into 480,022 shares of Common Stock (subject to adjustment in certain circumstances). The per share exercise price for the shares is $5.148.
On November 21, 2011, the Company completed a private placement of shares of its common stock for gross proceeds of $3,330 through the sale of 646,776 shares of Common Stock to third party investors and sold 466,139 shares of Common Stock to Can-Fite in exchange for 714,922 ordinary shares of Can-Fite, valued at $2,400 and 97,112 shares to Can-Fite for gross proceeds of $500,000. In addition, the Company issued to Can-Fite and each of the other investors, for each four shares of the Company’s common stock purchased in the financing, nine warrants valid for a period of five years from the closing of the financing to acquire two shares of the Company for an exercise price of $7.74.
On June 17, 2013, the Company sold 268,095 Can-Fite ordinary shares for a total consideration of $511. As of December 31, 2015, the Company holds 446,827 Can-Fite ordinary shares, representing approximately 1.6% of Can-Fite's outstanding share capital.
The transaction was accounted for as a reverse recapitalization which is outside the scope ASC 805, Business Combinations. Under reverse capitalization accounting, Eye-Fite is considered the acquirer for accounting and financial reporting purposes, and acquired the assets and assumed the liabilities of the Company. Assets acquired and liabilities assumed are reported at their historical amounts. Consequently, the consolidated financial statements of the Company reflect the operations of the acquirer for accounting purposes together with a deemed issuance of shares, equivalent to the shares held by the former shareholders of the legal acquirer and a recapitalization of the equity of the accounting acquirer. These consolidated financial statements include the accounts of the Company since the effective date of the reverse capitalization and the accounts of Eye-Fite since inception.
| F-7 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 1:- | GENERAL (Cont.) |
| 2. | License and research and development services from Can-Fite: |
In connection with the consummation of the Reverse Recapitalization, the Company through EyeFite and Can-Fite entered into a license agreement, pursuant to which Can-Fite granted to EyeFite a sole and exclusive worldwide license for the use of CF101, solely in the field of ophthalmic diseases ("CF101").
In connection with the License Agreement, referred to above, Eye-Fite was obligated to make to the U.S. National Institutes of Health ("NIH"), with regard to the patents of which are included in the license to Eye-Fite, for as long as the license agreement between Can-Fite and NIH remains in effect, a nonrefundable minimum annual royalty fee and potential future royalties of 4.0% to 5.5% on net sales. In addition, the Company was obligated to make certain milestone payments to NIH ranging from $25 to $500 upon the achievement of various development milestones for each indication.
In June 2015, the license agreement between Can-Fite and NIH terminated due to patent expiration. As of December 31, 2015, the Company accrued an amount of $100 related to its DES Phase III clinical trial and $75 related to its Phase II glaucoma Phase II clinical trial. Eye-Fite will also be required to make payments of 20% of sublicensing revenues, excluding royalties and net of the required milestone payments. During 2015, the Company did not reach any milestone or generate revenue that would trigger additional payments to Can-Fite.
In addition, following the closing of the Reverse Recapitalization, an agreement was signed between Can-Fite, OphthaliX and Eye-Fite (the "Service Agreement"). According to the Service Agreement, Can-Fite will manage the research and development activities relating to pre-clinical and clinical studies for the development of the ophthalmic indications of CF101. According to the Service Agreement, in consideration for Can-Fite's services, Eye-Fite will pay to Can-Fite a service fee (consisting of all expenses and costs incurred by Can-Fite plus 15%).
As part of the Service Agreement, the Company is required to pay Can-Fite additional payments from future proceeds (due to commercialization or sublicense agreement) in relation to CF101 (the “Additional Payment”). Can-Fite has the right to exchange such future payments for additional shares of OphthaliX upon payment of an exercise price. For additional information refer to Note 4.
| c. | During the year ended December 31, 2015 and 2014, the Company incurred operating losses amounting to $1,385 and $1,146, respectively. The Company will be required to obtain additional liquidity resources in the near term in order to support the commercialization of its products and maintain its research and development activities. | |
| The Company is addressing its liquidity issues by implementing initiatives to raise additional funds as well as other measures that will allow it to cover its anticipated budget deficit. Such initiatives include a plan to monetize part or all of the Company's investment in Can-Fite's ordinary shares. |
| F-8 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 1:- | GENERAL (Cont.) |
In addition, in February 2013 Can-Fite issued to the Company a formal letter, which has been updated periodically (most recently in August 2015), stating that Can-Fite agrees to defer payments owing to it under the Services Agreement from January 31, 2013 for the performance of the clinical trials of CF101 in ophthalmic indications until the completion of fundraising by the Company sufficient to cover such deferred payments. Also, in August 2015, Can-Fite issued a financial support letter pursuant to which it committed to cover any shortfall in the costs and expenses of operations of the Company which are in excess of the Company's available cash to finance its operations, including cash generated from any future sale of Can-Fite shares held by the Company.
Both letters will expire in October 2016 and any related balance shall bear an interest at a rate of 3% per annum. As of December 31, 2015, the deferred payments to Can-Fite totaled $3,690 (also see Note 9).
There are no assurances, however, that the Company will be able to obtain an adequate level of financial resources that are required for the short and long-term development and commercialization of its product candidates. According to management estimates, liquidity resources as of December 31, 2015, together with the support letters from the Parent Company described above, will be sufficient to maintain the Company's operations at least through October 2016. The Company's inability to raise funds to conduct its research and development activities will have a severe negative impact on its ability to remain a viable company.
These conditions raise substantial doubt about the Company's ability to continue as a going concern. The accompanying financial statements do not include any adjustments to reflect the possible future effects on recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES |
The consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles ("U.S. GAAP").
| a. | Use of estimates: |
The preparation of financial statements, in conformity with U.S. GAAP, requires management to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Actual results could differ from those estimates.
| F-9 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| b. | Financial statements in U.S. dollars: |
The accompanying financial statements have been prepared in U.S. dollars, the functional and reporting currency of the Company.
Although the majority of the Company and its Subsidiary's operations are conducted in Israel, most of their expenses are in U.S dollar. Therefore, the Company's management believes that the U.S dollar is the functional currency of the primary economic environment in which the Company and its Subsidiary operate.
Transactions and balances denominated in U.S. dollars are presented at their original amounts. Monetary accounts maintained in currencies other than the U.S. dollar are re-measured into U.S. dollars in accordance with ASC 830-10, "Foreign Currency Matters". All transactions gains and losses of the re-measurement of monetary balance sheets items are reflected in the consolidated statements of comprehensive loss as financial income or expenses, as appropriate.
| c. | Principles of consolidation: |
The consolidated financial statements include the accounts of the Company and its Subsidiary. Intercompany transactions and balances have been eliminated upon consolidation.
| d. | Cash and cash equivalents: |
Cash equivalents include short-term highly liquid investments that are readily convertible to cash with original maturities of three months or less from time of deposit.
| e. | Prepaid expenses: |
Prepaid expenses are mainly composed of clinical trials drug-kits which expense based on a percentage of completion method of the related clinical trials.
| f. | Investment in Parent Company: |
The Company’s investment is its Parent Company’s securities are classified as available-for-sale carried at fair value, with unrealized gains and losses reported as a separate component of stockholders' equity under accumulated other comprehensive income in the consolidated balance sheets. Realized gains and losses on sales of available-for-sale securities are included as financials income, net in the consolidated statements of comprehensive loss.
| F-10 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
The Company recognizes an impairment charge when a decline in the fair value of its investments in securities is below the cost basis of such securities is judged to be other than temporary. Factors considered in making such a determination include the duration and severity of the impairment, the reason for the decline in value, the potential recovery period and the Company's intent to sell, including whether it is more likely than not that the Company will be required to sell the investment before recovery of cost basis.
For securities that are deemed other-than-temporarily impaired, an entity should recognize the difference between the cost basis of the impaired equity security and the fair value on the measurement date, as an other-than-temporarily impairment loss as part of financial income, net in the statement of comprehensive loss. The fair value on measurement date should be considered the equity security’s new cost basis. Unrealized gains and losses previously recorded through OCI, including the tax effects, should also be reversed.
The new cost basis should not be changed for subsequent increases in fair value. After an impairment loss is recognized for individual equity securities classified as available for sale, future increases or decreases in fair value (presuming no additional other-than-temporarily impairments exist) are included in OCI.
As of December 2015 and 2014 no impairment was recognized.
| g. | Property and equipment, net: |
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated by the straight-line method over the estimated useful lives of the assets at the following annual rates:
| % | |||||
| Computers, and peripheral equipment | 33 | ||||
| h. | Impairment of long-lived assets: |
The Company’s long-lived assets are reviewed for impairment in accordance with ASC 360, "Property, Plant and Equipment", whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the assets. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. As of December 31, 2015 and 2014, no impairment losses have been identified.
| i. | Research and development expenses: |
All research and development costs are charged to the consolidated statements of comprehensive loss, as incurred.
| j. | Accounting for stock-based compensation: |
The Company accounts for stock-based compensation in accordance with ASC 718, "Compensation - Stock Compensation" ("ASC 718"). ASC 718 requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the Company's consolidated statements of comprehensive loss.
| F-11 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
The Company recognizes compensation expenses for the value of its awards granted based on the accelerated recognition method over the requisite service period of each of the awards, net of estimated forfeitures. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The Company estimates the fair value of stock options granted using the binomial option pricing-model. The option-pricing model requires a number of assumptions, of which the most significant are the expected stock price volatility and the early exercise multiply. Expected volatility was calculated based upon historical volatilities of similar entities in the related sector index. The early exercise multiply is representing the value of the underlying stock as a multiple of the exercise price of the option which, if achieved, results in exercise of the option. The risk-free interest rate is based on the yield from U.S. treasury bonds with an equivalent term. The Company has historically not paid dividends and has no foreseeable plans to pay dividends.
| k. | Basic and diluted net loss per share: |
Basic net loss per share is computed based on the weighted average number of shares of Common Stock outstanding during each period. Diluted net loss per share is computed based on the weighted average number of shares of Common Stock outstanding during each period, plus dilutive potential Common Stock considered outstanding during the period, in accordance with ASC topic 260, "Earnings Per Share" ("ASC 260").
The total weighted average number of shares related to the outstanding warrants and options excluded from the calculations of diluted net loss per share due to their anti-dilutive effect was 1,320,948 and 1,453,638 for the years ended December 31, 2015 and 2014, respectively.
| l. | Income taxes: |
The Company and its Subsidiary account for income taxes and uncertain tax positions in accordance with ASC 740, "Income Taxes" ("ASC 740"). ASC 740 prescribes the use of the liability method whereby deferred tax assets and liability account balances are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company and its subsidiary provide a full valuation allowance, to reduce deferred tax assets to the amounts that are more likely-than-not to be realized.
The Company implements a two-step approach to recognize and to measure uncertain tax positions in accordance with ASC 740.
| F-12 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
The first step is to evaluate the tax position taken or expected to be taken in a tax return by determining if the weight of available evidence indicates that it is more likely than not that, on an evaluation of the technical merits, the tax position will be sustained on audit, including resolution of any related appeals or litigation processes.
The second step is to measure the tax benefit as the largest amount that is more than 50% likely to be realized upon ultimate settlement. As of December 31, 2015 and 2014, no liability for unrecognized tax benefits was recorded as a result of the implementation of ASC 740.
| m. | Concentrations of credit risk: |
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents and the Company’s investment in Parent Company securities.
Cash and cash equivalents are deposited with a major bank in Israel. Such cash and cash equivalents and short-term bank deposits may be in excess of insured limits and are not insured in other jurisdictions. The Company's management believes that the financial institution that holds the Company's investments is an institution with high credit standing, and accordingly, minimal credit risk exists with respect to these investments.
| n. | Fair value of financial instruments: |
The Company adopted ASC 820, "Fair Value Measurements and Disclosures" ("ASC 820"). ASC 820 clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to release a liability in an orderly transaction between market participants.
As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering such assumptions, ASC 820 establishes a three-tier value hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value:
| Level 1 - | Observable input that reflects quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| F-13 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 2:- | SIGNIFICANT ACCOUNTING POLICIES (Cont.) |
| Level 2 - | Include other inputs that are based upon quoted prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active, and model-based valuation techniques for which all significant inputs are observable in the market or can be derived from observable market data. Where applicable, these models project future cash flows and discount the future amounts to a present value using market-based observable inputs including interest rate curves, foreign exchange rates, and credit ratings. | |
| Level 3 - | Unobservable inputs which are supported by little or no market activity. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
The carrying amounts of cash and cash equivalents, investment in Parent Company, prepaid expenses and other accounts payable and accrued expenses approximate their fair value due to the short-term maturity of such instruments. Derivative related to Service Agreement (see Note 4) is classified within Level 3 because it is valued using valuation techniques. Some of the inputs to these models are unobservable in the market and are significant.
| o. | Impact of recently issued Accounting Standards: |
In August 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, which defines management’s responsibility to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures if there is substantial doubt about its ability to continue as a going concern. The pronouncement is effective for annual reporting periods ending after December 15, 2016 with early adoption permitted. The Company is evaluating the effect, if any, that the adoption of this guidance will have on its financial statements.
In November 2015, the FASB issued ASU No. 2015-17, "Income Taxes – Balance Sheet Classification of Deferred Taxes” ("ASU 2015-17"). The purpose of the standard is to simplify the presentation of deferred taxes on a classified balance sheet. Under current GAAP, deferred income tax assets and liabilities are separated into current and noncurrent amounts in the balance sheet. The amendments in ASU 2015-17 require that all deferred tax assets and liabilities be classified as noncurrent in the balance sheet. ASU 2015-17 is effective for interim and annual periods beginning after December 15, 2017, and interim periods within annual periods beginning after December 15, 2018. Companies can adopt the guidance either prospectively or retrospectively. The Company does not expect the adoption of ASU 2015-17 to have a material impact on its consolidated financial statements or presentation.
| F-14 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 3: - | INVESTMENT IN PARENT COMPANY |
As previously discussed in Notes 1b1 and 2f, the Company currently owns 446,827 of Can-Fite’s ordinary shares, representing approximately 1.59% of Can-Fite's issued and outstanding share capital as of December 31, 2015.
As of December 31, 2015 and 2014 the fair value of the Company's investment in Parent Company shares amounted to $658 and $794, respectively (according to its quoted market price in the Tel-Aviv Stock Exchange). During the twelve month period ended December 31, 2015 and 2014, the related unrealized losses derive from the change in the fair value of the securities totaled $136 and $381, respectively.
| NOTE 4:- | DERIVATIVE RELATED TO SERVICE AGREEMENT |
On November 21, 2011 (the "Closing Date"), upon the consummation of the Reverse Recapitalization, and as part of the Service Agreement with Can-Fite (see Note 1b2), the Company is required to pay Can-Fite an Additional Payment of 2.5% from all future proceeds received by OphthaliX or any of its affiliates in relation to CF101. Can-Fite has the right (the "Exchange Right"), at any time from November 21, 2011 until the earlier of (a) the November 21, 2016 and (b) the closing of the acquisition of the Company by another entity, resulting in the exchange of the Company’s outstanding common shares such that its stockholders prior to such transaction own, directly or indirectly, less than 50% of the voting power of the surviving entity, to convert its right to the additional payment into 480,022 shares of Common Stock (subject to adjustment in certain circumstances). The exercise price for all the shares shall be an aggregate of US $2,500, equal to a per share exercise price of $5.148. The Exchange Right may be exercised on either a cash or a cashless basis, provided that if the warrant is exercised on a cashless basis, the warrant must be exercised in whole, not in part.
The Company's management applied ASC 815 to evaluate whether the Exchange Right (contingent call option to holders) instrument is a financial instrument that has the characteristics of a derivative. In particular, the Company's management also evaluated ASC 815-10-15-74(a) scope exception.
The Company's management concluded that the Exchange Right doesn't have fixed settlement provisions, and therefore, should be classified as a liability at inception. The Exchange Right is being re-measured at fair value each reporting period until the date of exercise or expiration with the change in value reported in the statements of comprehensive loss (as part of financial expenses, net).
As of December 31, 2015 and 2014 the fair value of the Derivative related to Service Agreement was immaterial.
| F-15 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 5:- | FAIR VALUE MEASUREMENTS |
In accordance with ASC 820, the Company measures its marketable securities and embedded derivatives at fair value. Marketable securities fair value is based on quoted prices for identical assets in active markets. These securities are classified within Level 1 and Level 2 on the fair value hierarchy. Derivatives are classified within Level 3 because they are valued using valuation techniques. Some of the inputs to these models are unobservable in the market and are significant.
The following table provides information by value level for financial assets and liabilities that are measured at fair value, as defined by ASC 820, on a recurring basis as of December 31, 2015 and 2014.
| December 31, 2015 | |||||||||||||||||
| Fair value measurements | |||||||||||||||||
| Description | Fair Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Investment in Parent Company | $ | 658 | $ | 658 | $ | - | $ | - | |||||||||
| Derivative related to Service Agreement | *)- | - | - | *)- | |||||||||||||
| Total Financial Assets, net | $ | 658 | $ | 658 | $ | - | $ | - | |||||||||
| December 31, 2014 | |||||||||||||||||
| Fair value measurements | |||||||||||||||||
| Description | Fair Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Investment in Parent Company | $ | 794 | $ | 794 | $ | - | $ | - | |||||||||
| Derivative related to Service Agreement | *)- | - | - | *)- | |||||||||||||
| Total Financial Assets, net | $ | 794 | $ | 794 | $ | - | $ | - | |||||||||
The following table presents the changes in Level 3 instruments measured on a recurring basis for the year ended December 31, 2015 and 2014. The Company's Level 3 instruments consist of Derivative (see Note 4).
Transaction in the value of Derivative related to Service Agreement:
| Balance at January 1, 2014 | $ | 7 | |||
| Change in fair value of derivatives | (7 | ) | |||
| Balance at December 31, 2014 | *)- | ||||
| Change in fair value of derivatives | - | ||||
| Balance at December 31, 2015 | $ | *)- |
*) Represents an amount lower than $1
| F-16 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 6:- | SHARE CAPITAL |
| a. | Shares of Common Stock: |
The shares of Common Stock represent the legal acquirer, meaning OphthaliX's share capital as of the transaction date.
Shares of Common Stock confer upon the holders the right to receive notice to participate and vote in the general meetings of the Company and the right to receive dividends, if declared.
On July 18, 2013 the Company's stockholders approved a reverse stock split of one share for each four and one-half shares outstanding (1:4.5) (the "Reverse Split"). The Reverse Split became effective as of the close of business on August 6, 2013. All shares of Common Stock, warrants, options, per share data and exercise prices included in these financial statements and notes for all periods presented have been retroactively adjusted to reflect the Reverse Split with respect to the Company's shares of Common Stock.
| b. | Warrants: |
In contemplation with the Reverse Recapitalization, it was agreed that for each four shares of Common Stock purchased by the investors and Can-Fite, they will be granted by the Company nine warrants to acquire two shares of Common Stock of the Company. The exercise price of the warrants is $7.74 per share of Common Stock. The warrants are exercisable for a period of five years from their date of grant. The warrants do not contain nonstandard anti-dilution provisions.
According to ASC 815-40-15 and 25 instructions, the Company's management evaluated whether the warrants are entitled to the scope exception in ASC 815-10-15-74 (as the warrants meet the definition of a derivative under ASC 815-10-15-83). Based on their straight forward terms (i.e., fixed exercise price, no down-round or other provisions that will preclude them from being considered indexed to the Company's own stock), the Company's management concluded that the warrants should be classified as equity at inception.
In contemplation of the transaction, the Company issued a total of 532,870 fully vested warrants to acquire 118,415 shares of Common Stock to consultants and brokers involved in the transaction. These warrants are exercisable upon the payment of $5.148 per share of Common Stock. As of December 31, 2015 and 2014 the intrinsic value of the Adviser Warrants is $0.
On November 12, 2012, the Board of Directors, complying with the undertaking taken as part of the recapitalization of the Company on November 21, 2011, formerly resolved to issue to certain investors and Can-Fite, 1,455,228 and 1,267,315 warrants to acquire 323,384 and 281,625 shares of Common Stock of the Company, respectively (the "Warrants"). The exercise price of such Warrants is $7.74 per share. The Warrants are exercisable for a period of five years from their date of grant and do not contain any non- standard anti-dilution provisions.
| F-17 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 6:- | SHARE CAPITAL (Cont.) |
| c. | Stock option plan and grant: |
In 2012, the Board of Directors approved the adoption of the 2012 Stock Incentive Plan (the "2012 Plan"). An Israeli annex was subsequently adopted in 2013 to comply with the requirements set by the Israeli law in general and in particular with the provisions of section 102 of the Israeli tax ordinance. Under the 2012 Plan and Israeli annex, the Company may grant its officers, directors, employees and consultants, Stock options, Restricted Stocks and Restricted Stock Units ("RSUs") of the Company. Each Stock option granted shall be exercisable at such times and terms and conditions as the Board of Directors may specify in the applicable option agreement, provided that no option will be granted with a term in excess of 10 years.
Upon the adoption of the 2012 Stock Option Plan the Company reserved for issuance 1,088,888 shares of Common Stock, $0.001 par value each. As of December 31, 2015 the Company has 971,388 shares of Common Stock available for future grant under the 2012 Plan.
In January 2012, the Company granted to one of its board members, options to purchase 52,222 shares of Common Stock of the Company at an exercise price of $9.00 per share. The options shall vest over a period of 36 months so long as he remains a director until fully vested.
In May 2013, the Company's Board of Directors approved an option grant, to purchase 13,055 shares of its Common Stock to the Company's Chief Financial Officer. The options have an exercise price of $9.00 per share and expire on May 29, 2023. Half of these options were fully vested at grant date, and the remaining options will vest over a period of three years on a quarterly basis for 12 consecutive quarters from the date of the grant.
Also in 2013, the Company granted one of its directors, options to purchase 52,222 shares of Common Stock of the Company at $6.638 per share. The options shall vest over a period of 12 consecutive quarters so long as he remains a director, until fully vested.
During the years ended December 31, 2015 and 2014, the Company did not grant any new stock options.
| F-18 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 6:- | SHARE CAPITAL (Cont.) |
The following table summarizes the activity of stock options:
| Shares Subject to Options Outstanding | |||||||||||||||||
| Description | Number of Shares | Weighted Average Exercise Price | Weighted- Average Remaining Contractual Term (in years) | Aggregate Intrinsic Value(1) | |||||||||||||
| Outstanding at the beginning and at the end of year | 117,500 | 7.96 | 7.8 | $ | - | ||||||||||||
| Exercisable at the end of the year | 107,708 | 8.05 | 6.8 | $ | - | ||||||||||||
| Vested and expected to be vested | 117,500 | 7.96 | 6.9 | $ | - | ||||||||||||
| (1) | The aggregate intrinsic value is calculated as the difference between the exercise price of the stock options and the closing price of the shares of the Company's Common Stock of $ 0.99 as of December 31, 2015. |
Stock-based compensation expenses recognized during the years ended December 31, 2015 and 2014 totaled to $22 and $25, respectively. As of December 31, 2015, there was $3 of unrecognized stock-based compensation expense all of which is related to stock options. This unrecognized compensation expense is expected to be recognized over a weighted-average period of approximately 0.3 years.
| NOTE 7:- | INCOME TAXES |
| a. | The Company and its Israeli Subsidiary are separately taxed under the domestic tax laws of the state of incorporation of each entity. |
| b. | Net operating losses carryforward: |
The Company is subject to U.S. income taxes. As of December 31, 2015, the Company has net operating loss carryforwards for federal income tax purposes of approximately $1,962 which expire in the years 2018 to 2035.
The Company has no operating loss carryforwards for state income tax purposes. Utilization of the U.S. net operating losses may be subject to substantial annual limitation due to the "change in ownership" provisions of the Internal Revenue Code of 1986 and similar state provisions. The annual limitation may result in the expiration of net operating losses before utilization.
| F-19 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 7:- | INCOME TAXES (Cont.) |
The Company's Subsidiary in Israel has estimated accumulated losses for tax purposes as of December 31, 2015, in the amount of approximately $2,698 which may be carried forward and offset against taxable income in the future for an indefinite period.
| c. | Loss before taxes is comprised as follows: |
| Year ended December 31, | |||||||||
| 2015 | 2014 | ||||||||
| Domestic | $ | 491 | $ | 367 | |||||
| Foreign (Israel) | 986 | 828 | |||||||
| $ | 1,477 | $ | 1,195 | ||||||
| d. | Deferred taxes: |
Deferred taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company and its subsidiary's' deferred tax assets are comprised of operating loss carryforward and other temporary differences.
Significant components of the Company and its subsidiaries deferred tax assets are as follows:
| December 31, | |||||||||
| 2015 | 2014 | ||||||||
| Operating loss carryforward | $ | 1,500 | $ | 1,268 | |||||
| Research and development expenses | 748 | 205 | |||||||
| Investment in Parent company | 337 | 282 | |||||||
| Deferred tax assets before valuation allowance | 2,585 | 2,755 | |||||||
| Valuation allowance | (2,585 | ) | (1,755 | ) | |||||
| Net deferred tax asset | $ | - | $ | - | |||||
The Company has provided valuation allowance in respect of deferred tax assets resulting from operating loss carryforward and other temporary differences.
Management currently believes that since the Company and its subsidiary have a history of losses it is more likely than not that the deferred tax regarding the loss carryforward and other temporary differences will not be realized in the foreseeable future.
| F-20 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 7:- | INCOME TAXES (Cont.) |
| e. | Tax rates applicable to the income of the Company: |
Corporate tax rate in Israel in 2014 and 2015 is 26.5%.
On January 4, 2016, the Israeli Parliament's Plenum approved by a second and third reading the Bill for Amending the Income Tax Ordinance (No. 217) (Reduction of Corporate Tax Rate), 2015, which consists of the reduction of the corporate tax rate from 26.5% to 25%. The Company estimates that the effect of the change in tax rates will result in a decrease in deferred tax balances as of December 31, 2015 in immaterial amounts.
The deferred tax balances included in the financial statements as of December 31, 2015 are calculated according to the tax rates that were substantially enacted as of the balance sheet date and therefore comply with the above changes, as applicable to the Company.
The corporate tax in the U.S. applying to a Company (incorporated in state of Delaware), consists of a progressive corporate tax at a rate of up to 35% plus state tax and local tax at rates depending on the state and the city in which the company manages its business. In the Company's estimation, it is subject to approximately a 40% tax rate.
| NOTE 8:- | FINANCE EXPENSES, NET |
Year ended December 31, | |||||||||
| 2015 | 2014 | ||||||||
| Bank fees | $ | 6 | $ | 4 | |||||
| Interest expenses | 86 | 52 | |||||||
| Revaluation of derivative related to service agreement | - | (7 | ) | ||||||
| Foreign currency translation adjustments | *)- | *)- | |||||||
| Finance expenses, net | $ | 92 | $ | 49 | |||||
*) Represents an amount lower than $ 1.
| F-21 |
OPHTHALIX INC. AND ITS SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
U.S. dollars in thousands, except share and per share data
| NOTE 9:- | RELATED PARTY TRANSACTIONS |
As of December 31, 2015 and 2014, and for the years then ended, the Company has several related party balances and transaction mainly in connection with the License Agreement with the Parent Company (refer to Notes 1b2 and 6). Details of the transactions with related parties are depicted in the following tables:
Transactions with related parties:
Year ended December 31, | |||||||||
| 2015 | 2014 | ||||||||
| Research and development expenses (1) | $ | 812 | $ | 701 | |||||
| General and administrative expenses (1) | $ | 29 | $ | 115 | |||||
| Finance expenses (income), net (1) | $ | 86 | $ | 45 | |||||
*) Represents an amount lower than $ 1.
Balances with Related Parties:
| December 31, | |||||||||
| 2015 | 2014 | ||||||||
| Parent Company (1) | $ | (3,690 | ) | $ | (2,457 | ) | |||
| Investment in Parent Company (2) | $ | 658 | $ | 794 | |||||
| Other account payables and accrued expenses (1) | $ | (175 | ) | $ | (175 | ) | |||
| Derivative related to Service Agreement (1) | $ | *) | $ | *) | |||||
| (1) | Related to Service Agreement (See Note 1b2). | |
| (2) | Related to Investment in Parent Company. See Notes 2f and Note 3. |
*) Represents an amount lower than $1.
- - - - - - - - - - - - - - - - - - -
| F-22 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief E Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a- 15(e) and 15d- 15(e) under the Securities Exchange Act of 1934, as amended (Exchange Act)), as of the end of the period covered by this Annual Report on Form 10-K. Based on such evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2015, our disclosure controls and procedures are designed at a reasonable assurance level and are effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the rules and forms of the Securities and Exchange Commission, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Management's Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of our company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed our internal control over financial reporting as of December 31, 2015, the end of our fiscal year. Management based its assessment on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included evaluation of such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies, and our overall control environment.
Based on our assessment, management has concluded that our internal control over financial reporting was effective, as of the end of the fiscal year, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with generally accepted accounting principles.
Changes in Internal Control
There were no changes in our internal control over financial reporting identified in management's evaluation pursuant to Rules 13a-15(d) or 15d-15(d) of the Exchange Act during the fourth quarter of 2015 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| - 47 - |
Limitations on Effectiveness of Controls and Procedures and Internal Control over Financial Reporting
In designing and evaluating the disclosure controls and procedures and internal control over financial reporting, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures and internal control over financial reporting must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors and Senior Management.
The following table sets forth the members of our executive officers and board of directors:
| Member | Position | Age | ||||
| Pnina Fishman, Ph.D. | Chairman and Chief Executive Officer | 67 | ||||
| Itay Weinstein. | Chief Financial Officer | 44 | ||||
| Ilan Cohn, Ph.D. | Director | 60 | ||||
| Guy Regev | Director | 47 | ||||
| Roger Kornberg, Ph.D. | Director | 68 | ||||
| Michael Belkin, Ph.D. | Director | 74 | ||||
Pnina Fishman, Ph.D. Pnina Fishman has served as our Chairman of the Board since November 2011 and our Chief Executive Officer since June 2014. She has also served as our Chief Executive Officer from November 2011 through December 2012. Dr. Fishman co-founded Can-Fite and has served as Can-Fite’s Chief Executive Officer and served on its board of directors since September 2005. Dr. Fishman is the scientific founder of Can-Fite and was previously a professor of Life Sciences and headed the Laboratory of Clinical and Tumor Immunology at the Felsenstein Medical Research Institute, Rabin Medical Center, Israel. Dr. Fishman has authored or co-authored over 150 publications and presented the findings of her research at many major scientific meetings. Her past managerial experience included seven years as Chief Executive Officer of Mor Research Application, the technology transfer arm of Clalit Health Services, the largest healthcare provider in Israel. Mor Research Application was also the first clinical research organization in Israel. Dr. Fishman currently also serves as a member of the board of directors of F.D Consulting Ltd., Ultratrend Ltd., and EyeFite Ltd.. Dr. Fishman holds a Ph.D. in Immunology from the Bar Ilan University in Ramat Gan, Israel. Dr. Fishman’s qualifications to serve on our board of directors include her senior management experience as Co-Founder and Chief Executive Officer of Can-Fite, her prior managerial experience and her scientific expertise in the field of life sciences.
Itay Weinstein. Itay Weinstein has served as our Chief Financial Officer since November, 2011. He is a Partner at Shimony C.P.A. and has been employed there since 1999. Mr. Weinstein is also controller for Can-Fite BioPharma Ltd. and has served as such since 2003. Prior to that, Mr. Weinstein served as auditor at Oren Horowitz. Mr. Weinstein holds a B.A. in economics and accounting from the Tel Aviv University, Israel, and has been a licensed CPA since 1999. Mr. Weinstein is a board member of Uno Management and Consulting Ltd.
| - 48 - |
Ilan Cohn, Ph.D. Ilan Cohn has served on our board since November 2011. He is a patent attorney and senior partner at the patent attorney firm Reinhold Cohn and Partners, where he has been an attorney since 1986. Dr. Cohn co-founded Can-Fite, served as its Chief Executive Officer until September 2004, served on our board of directors since 1994 and since May 30, 2013 serves as the Chairman of the Can-Fite board of directors.. Dr. Cohn holds a Ph.D. in biology and is a patent attorney with many years of experience in the biopharmaceutical field. He has served on the board of directors of a number of life science companies, including Discovery Laboratories Inc. (formerly Ansan Pharmaceuticals), a U.S. public company. Dr. Cohn has also been involved in the past in management of venture capital funds focused on investments in the life sciences industry. Dr. Cohn served a number of years as a co-chairman of the Biotech Committee of the US-Israeli Science and Technology Commission. Dr. Cohen is also currently a member of the board of directors of I.C.R.C Management Ltd, Famillion BVI Ltd. and Famillion Ltd. (a subsidiary of Famillion BVI Ltd.). Dr. Cohn holds a Ph.D. in Biology from the Hebrew University of Jerusalem. . Dr. Cohn’s qualifications to serve on our board of directors include his senior management experience as Co-Founder and former Chief Executive Officer of Can-Fite, his service on the boards of life sciences companies, including on the board of Can-Fite, and his knowledge of the industry as an investor and legal counsel to numerous companies.
Guy Regev. Guy Regev has over twelve years of experience in accounting, financial management and control and general management of commercial enterprises. He has served on our Board of Directors since November 2011. Mr. Regev has also been a director of Can-Fite since July 2011 and has served as a member of Can-Fite’s audit and compensation committees since February 2014. Mr. Regev is currently the Chief Executive Officer of Gaon Holdings Ltd, a publicly traded Israeli holding company traded on the TASE which focuses on three areas of operation - Cleantech / Water, Financial Services, Retail/Trading. Mr. Regev is currently also the Chief Executive Officer of Middle East Tube Company Ltd a publicly traded Israeli company traded on the TASE which focuses on steel pipe manufacturing and galvanization services. Mr. Regev was the Chief Executive Officer of Shaked Global Group Ltd, a privately-held equity investment firm that provides value added capital to environmental-related companies and technologies. Prior to joining Shaked, from 2001 to 2008, Mr. Regev was Vice President of Commercial Business at Housing & Construction Holding, or HCH, Israel’s largest infrastructure company. His duties included being responsible for the consolidation and financial recovery of various business units within HCH. Prior to that, Mr. Regev carried several roles within the group including as a Chief Financial Officer and later the Chief Executive Officer of Blue-Green Ltd., the environmental services subsidiary of HCH. Between 1999 and 2001, Mr. Regev was a manager at Deloitte & Touche, Israel. Mr. Regev holds an LLB degree in Law (Israel) and is a licensed attorney and has been a licensed CPA since 1999. Mr. Regev is also a director of The Green Way Ltd, Shtang Construction and Engineering Ltd, R.I.B.E. Consulting & Investment Ltd., Middle East Tube Company Ltd, Middle East Tube - Industries 2001 Ltd, Middle East Tubes - Galvanizing (1994) Ltd, I-Solar Greentech Ltd, Plassim Infrastructure Ltd, Plassim Advanced Solutions in Sanitation Ltd, Hakohav Valves Industries Metal (1987) Ltd, Metzerplas Agriculture Cooperative Ltd, B. Gaon Retail & Trading Ltd, Gaon Agro - Rimon Management Services Ltd, B. Gaon Business (2004) Ltd, Gaon Antan Investments Ltd, Or Asaf Investments Ltd, Hamashbir Holdings (1999) Ltd, and AHAVA Holdings LTD. Mr. Regev’s qualifications to serve on our board of directors include his managerial, accounting and financial experience and his service on the boards of companies including on the board of Can-Fite.
Roger Kornberg, Ph.D., Roger Kornberg has served on our board since February 6, 2012. He co-founded Cocrystal Discovery, Inc. in 2008 and serves as its Chief Scientist. Dr. Kornberg has been a Professor, Department of Structural Biology and Medicine for Stanford University since 1978. From 1984 to 1992, he served as the Chair of the Department of Structural Biology at Stanford and Professor of Harvard Medical School. He serves as the Chairman of Scientific Advisory Board at Cocrystal Discovery, Inc. He serves as a Member of the Board of Directors at Cocrystal Discovery, Inc. He has been a Director of Protalix BioTherapeutics, Inc. since February 7, 2008 and Teva Pharmaceutical Industries Ltd. Since July 17, 2007. he serves as a Member of Scientific Advisory Board at Pacific Biosciences, Inc. (alternately Pacific Biosciences of California, Inc.). He has been a Member of Scientific Advisory Board at Epiphany Biosciences, Inc. since February 15, 2007. He serves as a Member of Scientific Advisory Board at SuperGen Inc. (alternately Astex Pharmaceuticals, Inc.) and BioCancell Therapeutics Ltd. He serves as a Member of Advisory Board at Deloitte LLP and Deloitte & Touche LLP. Dr. Kornberg serves as a Member of Scientific Advisory Board at MDRNA, Inc. (alternately MDRNA Research, Inc.), a subsidiary of Nastech Pharmaceutical Company Inc. (alternately Marina Biotech, Inc.). He is a Member of the U.S. National Academy of Sciences, an honorary member of other academies and professional societies in the United States, Europe, and Japan and a fellow of the American Academy of Arts and Sciences. He is an author of over 200 published papers. He was awarded the 2006 Nobel Prize in Chemistry for his seminal studies of the molecular basis of eukaryotic transcription, the biological process by which genetic information from DNA is copied to RNA. His Nobel Prize-winning work included discovery of Mediator, a protein complex required to facilitate gene transcription, as well the solution of the three-dimensional crystal structure of RNA polymerase II, the most complex protein structure solved to date. In addition to the Nobel Prize, Dr. Kornberg has been honored for his work with the Eli Lilly Award, the Passano Award, the Ciba-Drew Award, the Gairdner International Award (shared with R. Roeder), the Hoppe-Seyler Lecture Award, the Harvey Prize from the Technion (Israel Institute of Technology), the ASBMB-Merck Award, the Welch Prize in 2001, the Pasarow Award in Cancer Research, the Le Grand Prix Charles-Leopold Mayer in 2002 and the 2005 Alfred P. Sloan Jr. Prize. He is a recipient of honorary degrees from universities in Europe and Israel, including the Hebrew University, where he is a Visiting Professor. Dr. Kornberg received a BA Degree from Harvard in 1967 and a Ph.D. in Chemistry from Stanford University in 1972 and did his postdoctoral studies with Aaron Klug and Francis Crick at the Medical Research Council (MRC) Laboratory of Molecular Biology in Cambridge (UK) where he discovered the Nucleosome. Dr. Kornberg’s qualifications to serve on our board of directors include his expertise in chemistry and medicine, his service on the boards of biotech and pharmaceutical companies, and his experience in the academic arena.
| - 49 - |
Michael Belkin, Ph.D. Michael Belkin has served on our board since July 1, 2013. Dr. Belkin is, and has been since 1980, a Professor, and since 2010, a Professor Emeritus, of Ophthalmology at Tel Aviv University in Tel Aviv, Israel, and the Director of the Ophthalmic Technologies Laboratory at the university’s Eye Research Institute at the Sheba Medical Center since 1997. Since 2006, Dr. Belkin has also served as Senior Consultant of the Eye Research Institute at the Singapore National Eye Institute. He was awarded a master’s degree in natural sciences by Cambridge University, England, and received a doctorate in medicine from the Hebrew University of Jerusalem. Dr. Belkin previously served as Director of Research, Development and Non-Conventional Warfare Medicine in the Israel Defense Forces Medical Corps. He was also the first full-time Director of the Tel Aviv University Eye Research Institute, Chairman of the Tel-Aviv University Department of Ophthalmology and the President of the Israel Society of Eye and Vision Research, of which he was one of the founders. Dr. Belkin is an author of over 250 scientific publications and 20 patents. He is an internationally recognized eye researcher and has received various research awards. His laboratory is dedicated to enabling the transfer of technologies from university-level research to clinical practice by providing expertise and facilities for laboratory, preclinical and clinical studies. He is an entrepreneur and advisor of several ophthalmic companies in the fields of lasers, optics, ophthalmic devices, pharmaceutics and biotechnology. One of his ideas, the ExPRESS miniature glaucoma shunt, is currently used worldwide. Dr. Belkin is an active, long-standing, member of the Association for Research in Vision and Ophthalmology (ARVO), the American Academy of Ophthalmology, the American Glaucoma Society and many others. He serves as chairman and member of various international and local scientific and professional committees as well as scientific journals’ editorial boards. Dr. Belkin’s qualifications to serve on our board of directors include his expertise in ophthalmic medical research, his medical experience, and his experience as an advisor to several ophthalmic companies.
Election of Directors
Directors are elected to hold office until such director’s successor is elected and qualified or until such director’s earlier resignation or removal. Annual meetings of the stockholders, for the election of directors to succeed those whose terms expire, are to be held at such time each year as designated by the Board of Directors, which date shall be within 13 months of the last annual meeting of stockholders. Officers are elected by the Board of Directors, which is required to consider that subject at its first meeting after every annual meeting of stockholders. Each officer holds his office until his successor is elected and qualified or until his earlier resignation or removal.
There are no family relationships between any director, executive officer, or person nominated or chosen by the Company to become a director.
Legal Proceedings
During the past ten years there have been no events under any bankruptcy act, no criminal proceedings and no judgments, injunctions, orders or decrees material to the evaluation of the ability and integrity of any of our directors or executive officers.
| - 50 - |
We are not aware of any legal proceedings in which any director, officer or affiliate of ours, any owner of record or beneficially of more than five percent of any class of our voting securities, or any associate of any such director, officer, or affiliate of our company, or security holder is a party adverse to us or our subsidiary or has a material interest adverse to us or our subsidiary.
Code of Ethics
We have not adopted a code of ethics that applies to our officers, directors and employees but plan to do so during the fiscal year ending December 31, 2016.
Meetings and Committees of the Board of Directors
During 2015, the Board of Directors held six meetings and acted by unanimous written consent one time. All our directors attended at least 75% of the total number of meetings of the board of directors and committees (if any) on which they served that were held during 2015.
We currently have no committees organized but we plan to organize an audit committee, a compensation committee, and a nominating and corporate governance committee during the year ended December 31, 2016. Because of its small size, the Board carries out the duties of the committees. In selecting nominees for directorships, the Board considers a broad range of characteristics related to qualifications, background and diversity of nominees based on our current business needs. The Board has not adopted written guidelines regarding nominees for director. There have been no material changes to the procedures by which security holders may recommend nominees to our board of directors.
Board Leadership Structure and Role in Risk Oversight
In accordance with our Bylaws, the Board elects our officers, including our Chief Executive Officer, Chief Financial Officer, and such other officers as the Board may appoint from time to time. Dr. Pnina Fishman currently serves as our Chairman and Chief Executive Officer. The Board periodically considers whether changes to our overall leadership structure are appropriate.
Our Chairman is responsible for chairing meetings of the Board. Our Bylaws provide that if our Chairman is unable to preside at meetings of the Board, our Chief Executive Officer, if such officer is a director, shall preside at such meetings. Our Chairman is also responsible for chairing meetings of shareholders. In her absence, the Board may appoint another party to chair the shareholders’ meeting.
Risk is inherent with every business, and how well a business manages risk can ultimately determine its success. We face a number of risks, including strategic risks, enterprise risks, financial risks, regulatory risks, and others. Management is responsible for the day-to-day management of risks the company faces, while our board of directors, as a whole, has responsibility for the oversight of risk management. In its risk oversight role, our board of directors has the responsibility to satisfy itself that the risk management processes designed and implemented by management are adequate and functioning as designed.
The Board believes that full and open communication between management and the Board is essential for effective risk management and oversight. Senior management attends board meetings and is available to address any questions or concerns raised by our board of directors on risk management-related and any other matters. The Board receives presentations from senior management on strategic matters involving our operations and intends to hold regular strategic planning sessions with senior management to discuss strategies, key challenges, and risks and opportunities for our business.
Communications by Stockholders with Directors
We encourage stockholder communications to our board of directors and/or individual directors. Stockholders who wish to communicate with our board of directors or an individual director should send their communications to the care of Ronen Kantor, Secretary, OphthaliX Inc., 12 Abba Hillel Silver Road, Ramat Gan, Israel 52506. Mr. Kantor will maintain a log of such communications and will transmit as soon as practicable such communications to the Chairman of the Board or to the identified individual director(s), although communications that are abusive, in bad taste or that present safety or security concerns may be handled differently, as determined by Mr. Kantor.
| - 51 - |
Director Attendance at Annual Meetings
We will make every effort to schedule our annual meeting of stockholders at a time and date to accommodate attendance by directors taking into account the directors’ schedules. While all directors are encouraged to attend our annual meeting of stockholders, there is no formal policy as to their attendance at annual meetings of stockholders.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors, executive officers and persons who own more than 10% of our common stock to file with the SEC reports of ownership and changes in ownership of our common stock. Our directors, executive officers and greater than 10% beneficial owners of our common stock are required by SEC regulation to furnish us with copies of all Section 16(a) reports they file. Based solely upon information furnished to us and contained in reports filed with the SEC, we believe that all Section 16(a) reports of our directors, executive officers and greater than 10% beneficial owners were filed timely for the fiscal year ended December 31, 2015.
ITEM 11. EXECUTIVE COMPENSATION
Executive Compensation
The following table sets forth information concerning the annual compensation awarded to, earned by, or paid to the following named executive officers for all services rendered in all capacities to our company for the years ended December 31, 2015 and 2014.
| Name And Position | Year | Salary ($) (1) | Bonus
($) | Stock Awards ($) | Option Awards ($) | All Other Compensation ($) | Total ($) | |||||||||||||||
| Pnina Fishman, Ph.D. (2) | 2015 | — | — | — | — | — | — | |||||||||||||||
| Chairman and Chief Executive Officer | 2014 | — | — | — | — | — | — | |||||||||||||||
| Itay Weinstein (3) | 2015 | 18,000 | — | — | — | — | 18,000 | |||||||||||||||
| Chief Financial Officer | 2014 | 18,000 | — | — | — | — | 18,000 | |||||||||||||||
| (1) | Amounts shown represent consulting fees earned or paid during the fiscal year. |
| (2) | Dr. Fishman has served as our Chief Executive Officer since June 2, 2014. As Chief Executive Officer of Can-Fite, our majority shareholder and parent, Dr. Fishman is compensated by Can-Fite, for her services to us as Chairman of the Board and Chief Executive Officer. |
| (3) | We paid Mr. Weinstein $18,000 in each of 2014 and 2015 for his services as our Chief Financial Officer. Mr. Weinstein is also the controller of Can-Fite, our majority shareholder and parent, and is compensated by Can-Fite for services provided to Can-Fite. |
| - 52 - |
Executive Employment Agreements
We have not entered into any employment agreements with our named executive officers listed in the table above.
Outstanding Equity Awards at Fiscal Year-End
The following table provides information regarding all outstanding equity awards for our named executive officers as of December 31, 2015:
| Option Awards | ||||||||||||||||
| Number of | Number of | |||||||||||||||
| Securities | Securities | |||||||||||||||
| Underlying | Underlying | |||||||||||||||
| Unexercised | Unexercised | |||||||||||||||
| Options | Options | Option | Option | |||||||||||||
| (#) | (#) | Exercise Price | Expiration | |||||||||||||
| Name | Exercisable | Unexercisable | ($) | Date | ||||||||||||
| Itay Weinstein (1) | 11,967 | 1,088 | 9.00 | 5/29/23 | ||||||||||||
| (1) | These options were granted on May 9, 2013 and vest 50% upon grant and 50% on a quarterly basis over a three year period. |
Compensation of Directors
The following table provides information regarding the total compensation that we paid or awarded to our non-executive directors during the year ended December 31, 2015.
| Name | Fees Earned or Paid in Cash | Stock Awards | Option Awards | Nonqualified Deferred Compensation Earnings | All Other Compensation | Total | ||||||||||||||||||
| ($) | ($) | ($)(1) | ($) | ($) | ($) | |||||||||||||||||||
| Ilan Cohn, Ph.D.(2) | - | - | - | - | - | - | ||||||||||||||||||
| Guy Regev | - | - | - | - | - | - | ||||||||||||||||||
| Roger Kornberg, Ph.D.(3) | 3,000 | (4) | - | - | - | 3,000 | ||||||||||||||||||
| Michael Belkin, Ph.D.(3) | 4,500 | (5) | - | 20,000 | (6) | - | - | 24,500 | ||||||||||||||||
| (1) | Reflects the aggregate grant date fair value of option awards granted during the relevant fiscal year calculated in accordance with FASB ASC Topic 718. Amounts reported do not reflect amounts actually received by the director. |
| (2) | Reinhold Cohn and Partners, an Israeli partnership, of which Ilan Cohn, Ph.D. is a partner provides intellectual property legal services to us in the ordinary course of business. |
| (3) | In connection with the appointments of Drs. Kornberg and Belkin, we entered into agreements to pay director fees for attendance at board meetings or any committee of the board. Drs. Kornberg and Belkin will each receive US$2,000 for attendance in person at a meeting of the Board, US$750 for attendance by telephone at a meeting of the board, and US$750 for attendance at each meeting of any committee of the board. |
| - 53 - |
| (4) | Represents accrued but unpaid fees. |
| (5) | Includes $1,500 of accrued but unpaid fees. |
| (6) | On July 1, 2013, we granted to Dr. Belkin ten-year options to purchase 52,222 shares of our common stock at $6.63 per share. The options vest as follows: 4,351 on September 30, 2013 and 4,351 on the last day of each quarter thereafter so long as he remains a director until fully vested. The options were granted under our 2012 Stock Incentive Plan. |
We are currently considering the precise composition of our director compensation policy. We may adopt a policy of paying independent directors an annual retainer, stock options and a fee for attendance at board and committee meetings. We anticipate reimbursing each director for reasonable travel expenses related to such director’s attendance at board and committee meetings.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
The following table sets forth certain information concerning the ownership of our common stock as of March 1, 2016, of (i) each person who is known to us to be the beneficial owner of more than 5 percent of our common stock, without regard to any limitations on conversion or exercise of convertible securities or warrants; (ii) all directors and named executive officers; and (iii) our directors and executive officers as a group. The table also sets forth certain information concerning the ownership of ordinary shares of Can-Fite, our majority shareholder and parent, as of as of March 1, 2016, by (i) each of our directors and named executive officers, and (ii) our directors and executive officers as a group.
| Name and Address of Beneficial Owner | OphthaliX Common Stock Amount and Nature of Beneficial Ownership(1) | Percent of Class(1) | Can-Fite Ordinary Shares Amount and Nature of Beneficial Ownership(1) | Percent of Class(1) | ||||||||||||
| Pnina Fishman, Ph.D. Chairman of the Board and CEO | 14,567 | (2) | * | 599,603 | (3) | 2.1 | % | |||||||||
| Ilan Cohn, Ph.D. Director | -- | -- | 214,852 | (4) | * | |||||||||||
| Guy Regev Director | 56,786 | (5) | * | 54,240 | (6) | * | ||||||||||
| Roger Kornberg, Ph.D. Director | 52,222 | (7) | * | -- | -- | |||||||||||
| Michael Belkin, Ph.D. Director | 47,870 | (8) | * | -- | -- | |||||||||||
| Itay Weinstein CFO | 12,512 | (9) | * | 13,000 | (10) | * | ||||||||||
| Executive Officers and Directors as a Group (6 Persons) | 183,957 | 1.2 | % | 881,695 | 3.1 | % | ||||||||||
| Can-Fite BioPharma Ltd. 10 Bareket Street Kiryat Matalon P.O. Box 7537 Petah-Tikva 49170 Israel | 9,324,902 | (11) | 83.2 | % | -- | -- | ||||||||||
* Less than 1%
| - 54 - |
| (1) | This table is based upon information supplied by officers, directors and principal stockholders and is believed to be accurate. Unless otherwise indicated in the footnotes to this table, we believe that each of the stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. Shares of common stock subject to options, warrants, or other conversion privileges currently exercisable or convertible, or exercisable or convertible within 60 days of the date of this table, are deemed outstanding for computing the percentage of the person holding such option, warrant, or other convertible instrument but are not deemed outstanding for computing the percentage of any other person. Where more than one person has a beneficial ownership interest in the same shares, the sharing of beneficial ownership of these shares is designated in the footnotes to this table. At March 1, 2016, we had 10,441,251 common shares outstanding and Can-Fite had 27,672,901 ordinary shares outstanding (which excludes 446,827 ordinary shares held in treasury). |
| (2) | Represents (i) 9,812 ordinary shares, and (ii) warrants to purchase 4,855 shares of common stock at $7.74 per share expiring on November 20, 2016. |
| (3) | Represents (i) 263,433 ordinary shares, (ii) 7,570,761 unregistered options to purchase 302,830 ordinary shares, of which 4,890,761 options have an exercise price of NIS 0.50 per option and expire on August 23, 2016 and 2,680,000 options have an exercise price of NIS 0.644 per option and expire on January 13, 2021, and (iii) 33,340 unregistered options to purchase 33,340 ordinary shares at an exercise price of NIS 3.573 per option and expire on October 22, 2025. Excludes 166,660 unregistered options to purchase 166,660 ordinary shares that vest in more than 60 days from March 1, 2016. |
| (4) | Represents (i) 133,567 ordinary shares, (ii) 2,032,136 unregistered options to purchase 81,285 ordinary shares at an exercise price of NIS 1.247 per option and expiring on March 20, 2017. All such warrants and options are fully vested. |
| (5) | Includes 34,717 shares of common stock held in a brokerage account. |
| (6) | Represents (i) 24,240 ordinary shares, (ii) 250,000 registered warrants (Series 10) to purchase 10,000 ordinary shares at an exercise price of NIS 0.394 per warrant and expiring on October 31, 2016, (iii) 250,000 registered warrants (Series 11) to purchase 10,000 ordinary shares at an exercise price of NIS 0.392 per warrant and expiring on April 30, 2016, and (iv) 250,000 unregistered options are exercisable into 10,000 ordinary shares at an exercise price of NIS 0.60 per option and expire on May 2, 2023. All such warrants and options are fully vested. |
| (7) | Represents 52,222 shares of common stock issuable upon exercise of vested options. |
| (8) | Represents 47,870 shares of common stock issuable upon exercise of vested options. |
| (9) | Represents 12,512 shares of common stock issuable upon exercise of vested options. |
| (10) | Represents (i) 5,000 ordinary shares, and (ii) 200,000 registered warrants (Series 10) to purchase 8,000 ordinary shares at an exercise price of NIS 0.394 per warrant and expiring on October 31, 2016. |
| (11) | Includes 761,648 shares of common stock issuable to Can-Fite upon the exercise of warrants. |
Equity Compensation Plan Information
On February 6, 2012, our board of directors and shareholders adopted the 2012 Stock Incentive Plan, or the Plan. The purpose of the Plan is to advance the interests of our stockholders by enhancing the Company’s ability to attract, retain and motivate persons who are expected to make important contributions to us and by providing such persons with equity ownership opportunities and performance-based incentives that are intended to better align the interests of such persons with those of our stockholders.
| - 55 - |
There are 1,088,888 shares of common stock authorized for non-statutory and incentive stock options, restricted stock units, and stock grants under the Plan, which are subject to adjustment in the event of stock splits, stock dividends, and other situations.
The Plan is administered by our board of directors. The persons eligible to participate in the plan are as follows: all of our employees, officers and directors, as well as consultants and advisors to the Company (as such terms consultants and advisors are defined and interpreted for purposes of Form S-8 under the Securities Act of 1933, as amended, or any successor form) are eligible to be granted Awards under the Plan. Each person who is granted an Award under the Plan is deemed a “Participant.” “Award” means Options (as defined in Section 5(a)), “Restricted Stock” (as defined in Section 6(a)), “Restricted Stock Units” (as defined in Section 6(a)), “Other Stock-Based Awards” (as defined in Section 7(a)) and “Performance Awards” (as defined in Section 8(a)). follows: (a) our employees and any of our subsidiaries; (b) non-employee members of the board or non-employee members of our board of directors or any of our subsidiaries; and (c) consultants and other independent advisors who provide services to us or any of our subsidiaries.
The Plan will continue in effect until all of the stock available for grant or issuance has been acquired through exercise of options or grants of shares, or until February 6, 2022, whichever is earlier. The Plan may also be terminated in the event of certain corporate transactions such as a merger or consolidation or the sale, transfer or other disposition of all or substantially all of our assets.
On January 29, 2013, our board of directors conditionally approved the adoption of an annex (the “Annex”) to the Plan. Approval of the Annex by our board of directors was contingent upon the following: 1) 30 days elapsing since approval of the Annex by the Board of Directors, and 2) filing with Israeli income tax authorities (the “Tax Authorities”). On February 7, 2013, the Annex was filed with the Tax Authorities and on March 8, 2013, the Annex became effective.
The Annex applies only to grantees who are residents of the State of Israel at the date of grant or those who are deemed to be residents of the State of Israel for the payment of tax at the date of grant. U.S. tax rules and regulations will not apply to any grants to a grantee who is a resident of the State of Israel at the date of grant or those who are deemed to be residents of the State of Israel for the payment of tax at the date of grant.
The purpose of the approval and adoption of the Annex is to harmonize the terms and conditions of the Plan with applicable Israeli law and provide specific provisions regarding optionees who are subject to Section 102(a) of the Israeli Income Tax Ordinance (New Version), 5721-1961, or the Ordinance. The Annex is intended to promote our interests by providing present and future officers, other employees (including directors who are also our employees) and consultants with an incentive to enter into and continue in our employ and to acquire a proprietary interest in our long-term success. Our board of directors shall have the authority to determine additional persons which will be granted rights under the Annex.
Pursuant to the Annex, our board of directors is authorized to grant stock options to persons subject to the Ordinance. Our board of directors may grant to employees, officers, and directors options under Section 102 of the Ordinance, or 102 Options, and to consultants and other service providers options under Section 3(i) of the Ordinance, or 3(i) Options. Our board of directors may designate 102 Options as “Approved 102 Options,” for which the options and shares upon exercise must be held in trust and granted through a trustee, and as “Unapproved 102 Options,” for which the options and shares upon exercise do not have to be held in trust. As described further below, the type of option and duration of time the option and shares upon exercise are held in trust will determine the tax consequences to the participant. Of the Approved 102 Options, our board of directors may grant options as “Work Income Options,” for which the options and shares upon exercise must be held in trust for 12 months from the date of grant, or as “Capital Gain Options,” for which the options and shares upon exercise must be held in trust for 24 months from the date of grant. If the requirements of the Approved 102 Options are not met, the options are regarded as Unapproved 102 Options. 3(i) Options and the shares upon exercise may be held in trust as well, depending upon the agreement between our board of directors, optionee, and the trustee of the trust. Approved 102 Options which have been granted as "Capital Gains Options" enable the optionee to pay capital gains tax on such option provided the terms of the grant and Section 102 of the Ordinance have been met whilst all other option grants under the Annex are treated as regular income and are subject to the taxation applicable thereto. The trustee appointed under the Annex is required to qualify as a trustee under Section 102 of the Ordinance and shall hold any options granted under the Annex in trust for the respective holding periods as designated under the Annex and Section 102 of the Ordinance. The grant of options under the Annex requires the delivery of a grant notification letter to each optionee in which all the relevant terms and conditions of such grant are set out. The grant notification letter may include additional matters relating to the vesting of the options, exercise periods, events of termination of employment, etc. The Annex sets out that for as long as options or shares purchased pursuant to thereto are held by the trustee on behalf of the optionee, all rights of the optionee over the shares are personal, cannot be transferred, assigned, pledged or mortgaged, other than by will or laws of descent and distribution. The Annex shall be governed by and construed and enforced in accordance with the laws of the State of Delaware.
| - 56 - |
The following table summarizes information as of the close of business on December 31, 2015 concerning the Plan and other options outstanding.
| Plan category | Number of securities to be issued upon exercise of outstanding options (a) | Weighted-average exercise price of outstanding options (b) | Securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) (c) | |||||||||
| Equity compensation plans approved by security holders | 117,500 | $ | 7.96 | 971,388 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 117,500 | $ 7. 96 | 971,388 | |||||||||
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, DIRECTOR INDEPENDENCE.
Since January 1, 2015, there has not been, nor is there any proposed transaction where we were or will be a party in which the amount involved exceeded or will exceed $120,000 and in which any director, executive officer, holder of more than 5% of any class of our voting securities, or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
Executive Officers and Directors
We have made equity awards and paid compensation to our executive officers and certain of our directors as more fully described in Item 11.
Can-Fite License Agreement and Services Agreement
We have entered into a License Agreement and related Services Agreement as more fully described in Item 1.
| - 57 - |
Can-Fite Support Letters
In February 2013, Can-Fite issued us a formal letter, which has been updated periodically (most recently in August 2015 ), stating that Can-Fite agrees to defer payments owed to it under the Services Agreement (under which Can-Fite manages, as an independent contractor, all activities relating to pre-clinical and clinical studies performed for the development of the ophthalmic indications of CF101) beginning on January 31, 2013 until the completion of fundraising by the Company sufficient to cover such deferred payments. As of December 31, 2015, the deferred payments to Can-Fite totaled approximately $3,690,000. In addition, in August 2015, Can-Fite issued a financial support letter pursuant to which it committed to cover any shortfall in our costs and expenses of the operations which are in excess of our available cash to finance our operations, including cash generated from any future sale of Can-Fite shares held by us. Both letters expire in October 2016 and any related balance bears interest at a rate of 3% per annum.
Director Independence
Our securities are not listed on a national securities exchange or in an inter-dealer quotation system which has requirements that directors be independent. Therefore, we have adopted the independence standards of the NASDAQ to determine the independence of our directors and those directors serving on our committees. These standards provide that a person will be considered an independent director if he or she is not an officer of the company and is, in the view of the company’s board of directors, free of any relationship that would interfere with the exercise of independent judgment. Our board of directors has determined that Roger Kornberg and Michael Belkin meet this standard, and therefore, would be considered to be independent.
Can-Fite is our majority shareholder, beneficially owning 9,324,902 shares, or 83% of our common stock. Dr. Fishman, our Chief Executive Officer and Chairman, is also the Chief Executive Officer of Can-Fite and Messrs. Cohn and Regev, two of our directors, are also directors of Can-Fite. Barak Singer, our former Chief Executive Officer through June 2014, also served as Vice President, Business Development, of Can-Fite through July 2014. Itay Weinstein, our Chief Financial Officer, is the controller of Can-Fite.
Procedures for Approval of Related Party Transactions
Our board is charged with reviewing and approving all potential related party transactions. All such related party transactions must then be reported under applicable SEC rules. We have not adopted other procedures for review, or standards for approval, of such transactions, but instead review them on a case-by-case basis.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
Audit Fee
The aggregate fees billed for each of the last two fiscal years for professional services rendered by the principal accountant for the audit of our annual financial statement and review of financial statements included in our 10-Q reports and services normally provided by the accountant in connection with statutory and regulatory filings or engagements were $61,000 for the years ended 2015 and 2014.
Tax Fees
The aggregate fees billed in each of the last two fiscal years for professional services rendered by the principal accountant for tax compliance, tax advice, and tax planning were $4,000 for years ended 2015 and 2014.
All Other Fees
The aggregate fees billed in each of the last two fiscal years for products and services provided by the principal accountant, other than the services reported above, were $35,000 for fiscal year ended 2015 and $0 for fiscal year ended 2014.
We do not have an audit committee currently serving and as a result our board of directors performs the duties of an audit committee. Our board of directors will evaluate and approve in advance, the scope and cost of the engagement of an auditor before the auditor renders audit and non-audit services. We do not rely on pre-approval policies and procedures.
| - 58 - |
PART IV
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
| Exhibit Number | Description | |
| 2.1 | Acquisition Agreement, dated November 21, 2011, with Can-Fite Biopharma Ltd. (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on November 23, 2011) | |
| 2.2 | Agreement and Plan of Merger, dated February 24, 2012 (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on April 5, 2012) | |
| 2.3 | Delaware Certificate of Merger (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on April 5, 2012) | |
| 2.4 | Nevada Articles of Merger (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on April 5, 2012) | |
| 3.1 | Certificate of Incorporation (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on April 5, 2012) | |
| 3.2 | Certificate of Amendment to Certificate of Incorporation (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on July 18, 2013) | |
| 3.3 | Bylaws (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on May 10, 2013) | |
| 10.1 | Warrant dated November 21, 2011 to Can-Fite Biopharma Ltd. for 480,022 shares (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on November 23, 2011) | |
| 10.2 | Stock Purchase Agreement, dated November 21, 2011, with Can-Fite Biopharma Ltd. (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on November 23, 2011) | |
| 10.3 | License Agreement, dated November 21, 2011, between Can-Fite Biopharma Ltd. and Eyefite Ltd. (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on November 23, 2011) | |
| 10.4 | Services Agreement, dated November 21, 2011, by and among Can-Fite Biopharma Ltd., Eyefite Ltd. and the Company (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on November 23, 2011) | |
| 10.5 | 2012 Stock Incentive Plan (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on February 9, 2012) + | |
| 10.6 | 2012 Stock Incentive Plan, Annex A (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on March 8, 2013) + | |
| 10.7 | Agreement dated February 2, 2012, with Roger Kornberg (Incorporated by reference to Company’s Annual Report on Form 10-K filed with the SEC on March 30, 2012) | |
| 10.8 | Agreement dated July 1, 2013, with Michael Belkin (Incorporated by reference to Company’s Registration Statement on Form S-1 filed July 2, 2013) | |
| 21.1 | Subsidiaries (Incorporated by reference to Company’s Current Report on Form 8-K filed with the SEC on November 23, 2011) | |
| 31.1 | Certification of Chief Executive Officer pursuant to Sec. 302 of the Sarbanes-Oxley Act of 2002* | |
| 31.2 | Certification of Chief Financial Officer pursuant to Sec. 302 of the Sarbanes-Oxley Act of 2002* | |
| 32.1 | Certification of Chief Executive Officer pursuant to 18 U.S.C. SECTION 1350* | |
| 32.2 | Certification of Chief Financial Officer pursuant to 18 U.S.C. SECTION 1350* | |
| 101 | The following materials from OphthaliX, Inc.’s Annual Report on Form 10-K for the year ended December 31, 2015 are formatted in XBRL (eXtensible Business Reporting Language): (i) the Balance Sheets, (ii) the Statements of Comprehensive Loss, (iii) Statement of Changes in Shareholders' Equity (Deficiency), (iv) the Statements of Cash Flow, and (iv) Notes to Financial Statements. |
* Filed herewith
+ Management compensatory plan
| - 59 - |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| OphthaliX Inc. | ||
| Date: March 2, 2016 | By: | /s/ Pnina Fishman |
Pnina Fishman Chief
Executive Officer and Chairman | ||
| Date: March 2, 2016 | By: | /s/ Itay Weinstein |
Itay Weinstein Chief
Financial Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| NAME | TITLE | DATE | ||
| /s/ Pnina Fishman | ||||
| Pnina Fishman | Chairman and Chief Executive Officer | March 2, 2016 | ||
| /s/ Ilan Cohn | ||||
| Ilan Cohn | Director | March 2, 2016 | ||
| /s/ Guy Regev | ||||
| Guy Regev | Director | March 2, 2016 | ||
| /s/ Roger Kornberg | ||||
| Roger Kornberg | Director | March 2, 2016 | ||
| /s/ Michael Belkin | ||||
| Michael Belkin | Director | March 2, 2016 |
- 60 -
