Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
[X] Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the fiscal year ended December 31, 2015
or
[ ] Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from ______ to ______
Commission file number - 1-11353
LABORATORY CORPORATION OF AMERICA HOLDINGS
(Exact name of registrant as specified in its charter)
Delaware | 13-3757370 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
358 South Main Street, | |
Burlington, North Carolina | 27215 |
(Address of principal executive offices) | (Zip Code) |
(Registrant's telephone number, including area code) 336-229-1127
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Name of exchange on which registered | |
Common Stock, $0.10 par value | New York Stock Exchange | |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark whether the registrant is well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [X] No [ ].
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes [ ] No [X].
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ].
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [X] No [ ].
1
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 232.405) is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [ ].
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “small reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer [X] | Accelerated Filer [ ] |
Non-accelerated filer [ ] (Do not check if a smaller reporting company) | Smaller reporting company [ ] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes [ ] No [X].
As of June 30, 2015, the aggregate market value of the common stock held by non-affiliates of the registrant was approximately $12.1 billion, based on the closing price on such date of the registrant’s common stock on the New York Stock Exchange.
Indicate the number of shares outstanding of each of the registrant's classes of common stock, as of the latest practicable date: 101.7 million shares as of February 23, 2016.
DOCUMENTS INCORPORATED BY REFERENCE
List hereunder the following documents if incorporated by reference and the Part of the Form 10-K into which the document is incorporated:
Portions of the Registrant’s Notice of Annual Meeting and Proxy Statement to be filed no later than 120 days following December 31, 2015 are incorporated by reference into Part III.
2
Index
Page | ||
Item 1. | ||
Item 1A. | ||
Item 1B. | ||
Item 2. | ||
Item 3. | ||
Item 4. | ||
Item 5. | ||
Item 6. | ||
Item 7. | ||
Item 7A. | ||
Item 8. | ||
Item 9. | ||
Item 9A. | ||
Item 9B. | ||
Item 10. | ||
Item 11. | ||
Item 12. | ||
Item 13. | ||
Item 14. | ||
Item 15. | ||
3
PART I
Item 1. | BUSINESS |
Laboratory Corporation of America® Holdings together with its subsidiaries (LabCorp® or the Company) is the world’s leading healthcare diagnostics company, providing comprehensive clinical laboratory services and end-to-end drug development support. The Company’s strategic vision is to improve health and improve lives by delivering world class diagnostic solutions, bringing innovative medicines to patients faster and developing technology-enabled solutions to change the way care is provided.
The Company, headquartered in Burlington, North Carolina, is a Delaware corporation and was incorporated in 1971 and was listed on the New York Stock Exchange under the ticker symbol LH in 1995. On February 19, 2015, the Company completed its acquisition of Covance Inc. (Acquisition), a global leader in drug development services, which significantly expanded the Company’s offering to the biopharmaceutical industry, increased the Company’s international presence and enhanced the Company’s financial growth opportunities.
The Company has more than 50,000 employees and serves more than 220,000 customers, including managed care organizations (MCOs), biopharmaceutical companies, governmental agencies, physicians, hospitals and health systems, employers, patients and consumers, food and nutritional companies and independent clinical laboratories. The Company believes that it generated more revenue from laboratory testing than any other company in the world in 2015.
The Company’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to those reports are made available free of charge through the Investor Relations section of the Company’s Website at www.labcorp.com as soon as reasonably practicable after such material is electronically filed with, or furnished to, the Securities and Exchange Commission (SEC). Additionally, the SEC maintains an Internet Website at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding issuers, including the Company, that file electronically with the SEC. The public may also read and copy any materials that the Company files with the SEC at the SEC's Public Reference Room at 100 F Street, NE, Washington, DC 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330.
The matters discussed in this “Business” section should be read in conjunction with the Consolidated Financial Statements found in Item 8 of Part II of this report, which include additional financial information about the Company, including financial information about geographic areas. This report includes forward-looking statements that involve risks or uncertainties. The Company’s results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risk factors described in Item 1A of Part I of this report and elsewhere. For more information about forward-looking statements, see “Forward Looking Statements” in Item 7.
Business Segments
The Company reports its business in two segments, LabCorp Diagnostics (LCD) and Covance Drug Development (CDD). For further financial information about these segments, including information for each of the last three fiscal years regarding revenue, operating income and other important information, see Note 20 to the Consolidated Financial Statements. In 2015, LCD and CDD contributed 72.9% and 27.1%, respectively, of net revenues to the Company.
LabCorp Diagnostics Segment
LCD is an independent clinical laboratory business that consists of a network of 39 primary laboratories and approximately 1,700 patient service centers (PSCs) along with a network of branches and STAT laboratories, which offer a focused menu of routine and frequently ordered tests with the ability to perform quickly and report the results to the physician.
With over 36,000 employees, LCD processes tests on approximately 500,000 patient specimens daily and has clinical laboratory locations throughout the U.S. and other countries including Canada, the U.K. and the United Arab Emirates. Its clients include physicians, hospitals, MCOs, governmental agencies, employers, and other independent clinical laboratories that do not have as wide a range of testing capabilities.
Clinical Laboratory Testing Industry
Laboratory tests and procedures are used generally by hospitals, physicians and other healthcare providers and commercial clients to assist in the diagnosis, monitoring and treatment of diseases and medical conditions through the examination of substances in blood, tissues and other specimens. The results of such tests can help in the evaluation of health, the detection of conditions or
4
pathogens and the selection of appropriate therapies. Clinical laboratory testing is generally categorized as either clinical pathology testing, which is performed on body fluids including blood, or anatomical pathology testing, in which a pathologist examines histologic or cytologic samples (i.e., tissue and other samples, including human cells). Clinical and anatomical pathology procedures are frequently ordered as part of regular physician office visits and hospital admissions in connection with the diagnosis and treatment of illnesses. Certain of these tests and procedures are used in the diagnosis and management of a wide variety of medical conditions such as cancer, infectious disease, endocrine disorders, cardiac disorders and genetic disease. It is estimated that although laboratory services account for less than 3% of total U.S. healthcare spending (and less than 2% of Medicare expenditures), the results of those tests influence approximately 70% of physician medical decisions.
The Company believes that in 2015, the U.S. clinical laboratory testing industry generated revenues of approximately $75.0 billion. The clinical laboratory industry consists primarily of three types of providers: hospital-based laboratories, physician-office laboratories and independent clinical and anatomical pathology laboratories, such as those operated by LCD. The clinical laboratory business is intensely competitive. The Centers for Medicare and Medicaid Services (CMS) of the Department of Health and Human Services (HHS) has estimated that in 2015 there were approximately 9,000 hospital-based laboratories, more than 122,000 physician-office laboratories and more than 6,000 independent clinical laboratories in the U.S. LCD competes with all of those laboratories.
LCD believes that healthcare providers selecting a laboratory often consider the following factors, among others:
• | accuracy, timeliness and consistency in reporting test results; |
• | reputation of the laboratory in the medical community or field of specialty; |
• | contractual relationships with MCOs; |
• | service capability and convenience offered by the laboratory; |
• | number and type of tests performed; |
• | connectivity solutions offered; and |
• | pricing of the laboratory’s services. |
LCD believes that consolidation in the clinical laboratory testing business will continue. In addition, LCD believes that it and the other large independent clinical laboratory testing companies will be able to increase their share of the overall clinical laboratory testing market due to a number of factors, including cost efficiencies afforded by large-scale automated testing, mergers and acquisitions of complementary businesses, reimbursement reductions and demands from health plans and other healthcare organizations for greater efficiency and large, integrated service networks. In addition, legal restrictions on physician referrals and their ownership of laboratories, as well as increased regulation of laboratories, are expected to contribute to the continuing consolidation of the industry.
Although testing for healthcare purposes and customers represents the most significant portion of clinical laboratory industry, clinical laboratories also perform testing for other purposes and customers, including employment and occupational testing, DNA testing to determine parentage and to assist in forensic investigations, environmental testing, veterinary testing, wellness testing, toxicology testing, pain management testing and nutritional analysis and food safety testing.
LCD Testing Operations, Services and Productivity
LCD has a network of primary testing laboratories, specialty testing laboratories, branches, PSCs and STAT laboratories. A branch is a central facility that collects specimens in a region for shipment to one of LCD’s laboratories for testing. A branch is also frequently used as a base for sales and distribution staff. Generally, a PSC is a facility maintained by LCD to serve the patients of physicians in a medical professional building or other strategic location. The PSC staff collect specimens for testing as requested by the physician. However, most patient specimens are collected in the customer's office by the customer's staff or in some cases by an LCD in-office phlebotomist. The specimens, and test request forms if the test order was not placed electronically, are collected from customer locations and, PSCs, and sent principally through LCD's in-house courier system (and, to a lesser extent, through independent couriers) to one of LCD's primary testing laboratories for testing. Some of LCD's PSCs also function as STAT labs.
Each specimen and related request form is checked for completeness and given a unique identification number. The unique identification number assigned to each specimen associates the results to the appropriate patient. The test request forms are sent to a data entry operator who enters the necessary testing and billing information. Once this information is entered into the software system, the tests are performed and the results are entered through an electronic data interchange interface or manually, depending upon the tests and the type of instrumentation involved. Most of LCD's automated testing equipment is connected to its information
5
systems. Most core testing is completed by early the next morning and test results are in most cases electronically delivered to clients via electronic interfaces, the LabCorp Beacon® platform, smart printers or personal computer-based products.
LCD maintains a constant focus on improving quality and productivity while lowering costs throughout all phases of its operations supported by LCD's technology, automation and facility rationalization initiatives. As part of an ongoing commitment to remain the most efficient and highest value provider of laboratory services, LCD has undertaken a comprehensive business process improvement initiative, referred to as Project LaunchPad, to reengineer its systems and processes to create a sustainable and more efficient business model, and improve the experience of all stakeholders. The Company expects this initiative to drive net savings in excess of $150.0 million between 2015 and 2017. In 2015, Project LaunchPad resulted in net savings of approximately $65.0 million.
LCD Testing Services
LCD offers a menu of over 4,700 tests. Several hundred of those tests are routinely used in general patient care by physicians to establish or support a diagnosis, to monitor treatment or to search for an otherwise undiagnosed condition. The most frequently requested of these tests include blood chemistry analyses, urinalyses, blood cell counts, thyroid tests, Pap tests, Hemoglobin A1C, PSA, STD tests (e.g. Ct, Ng, Tv, human immunodeficiency virus (HIV)), hepatitis C (HCV) tests, Vitamin D, microbiology cultures and procedures, and alcohol and other substance-abuse tests. LCD performs this core group of tests in its major laboratories using sophisticated and computerized instruments, with most results reported within 24 hours or less.
In addition, LCD provides a comprehensive range of specialty testing services in the areas of allergy, diagnostic genetics, women's health, cardiovascular disease, infectious disease, endocrinology, oncology, coagulation, pharmacogenetics, toxicology and pain management.
LCD also performs a range of other testing, including employment and occupational testing, DNA testing to determine parentage and to assist in forensic investigations, environmental testing, wellness testing, toxicology testing, pain management testing. LCD also provides services to the food, beverage, nutraceutical, animal feed, chemical and agrochemical industries, which include nutritional analysis and equivalency, nutritional content fact labels, microbiological and chemical contaminant safety analysis, product development expertise, sensory testing, pilot manufacturing, pesticide screening and stability testing.
LCD’s Specialty Testing Group performs esoteric testing, cancer diagnostics, and other complex procedures. LCD's specialty testing businesses and their areas of expertise are summarized in the chart below.

The Specialty Testing Group offers advanced methods and access to scientific expertise in the following disciplines:
Anatomic Pathology/Oncology. LCD offers advanced comprehensive tumor tissue analysis, including immunohistochemistry (IHC), cancer cytogenetics and fluorescence in situ hybridization (FISH) through its Dianon Pathology and Integrated Oncology specialty testing laboratories. Applications for molecular diagnostics continue to increase in oncology for both the analysis of leukemia as well as the assessment of solid tumors. In cancers such as colon and lung cancer, assays such as KRAS, BRAF and EGFR mutation analysis can help guide appropriate therapy choices for a given patient.
6
Cardiovascular Disease. LCD’s cardiovascular menu includes core cholesterol tests, expanded lipid profiles, a metabolic syndrome profile and tests for thrombosis and stroke. LCD also offers complete testing for monitoring disease progression and response to therapy, including the clinical decision support (CDS) reports available through Litholink.
Coagulation. LCD offers an extensive menu of tests for hemostasis and thrombosis, including bleeding profiles and screening tests, profiles for reproductive health, factor analysis, thrombin generation markers, and thrombotic risk evaluation.
Diagnostic Genetics. LCD offers cytogenetic, molecular cytogenetic, biochemical and molecular genetic tests. The biochemical genetics offerings include a variety of prenatal screening options including integrated and sequential prenatal assays and non-invasive prenatal testing (NIPT) for more sensitive and earlier assessment of risk for multiple fetal chromosomal aneuploidies (e.g., Down syndrome). LCD has expanded its cytogenetics offerings through the use of whole genome single-nucleotide polymorphism (SNP) microarray technology, which provides enhanced detection of subtle chromosomal changes associated with the etiology of mental retardation, developmental delay and autism. The molecular genetics services include multiplex analyses of a variety of disorders, gene sequencing applications for both somatic and germ-line alterations and whole exome sequencing. Through Integrated Genetics (formerly Genzyme Genetics), LCD provides the most comprehensive genetic test menu in the industry as well as approximately 140 genetic counselors and six medical geneticists to provide patients and their physicians with analysis, assessment and interpretation of genetics test results to help optimize patient decisions and outcomes.
Endocrinology. LCD is a leading provider of advanced hormone/steroid testing including comprehensive services for the endocrine specialist. LCD has expanded its menu in esoteric endocrine testing and has launched an initiative to develop steroid testing utilizing mass spectrometry technology. Mass spectrometry is used for detection of low levels of small molecule steroids including testosterone in women, children and hypogonadal men. LCD additionally offers endocrine related tests for genetic conditions including congenital adrenal hyperplasia, short stature, thyroid cancer, along with extensive age and gender-related reference intervals for those tests.
Infectious Disease. LCD provides complete HIV testing services including viral load measurements, genotyping and phenotyping and host genetic factors (e.g., HLA B*5701 test) that are important tools in managing and treating HIV infections. The addition of resistance tests, including PhenoSense®, PhenoSenseGT®, Trofile®, and GenoSure PRIme complement the existing HIV GenoSure® assay and provide an industry-leading, comprehensive portfolio of HIV resistance testing services. LCD also provides extensive testing services for HCV infections, including both viral load determinations and strain genotyping and host genetic factors (e.g., the IL-28B and HCV GenoSure NS3/4A tests). LCD continues to develop molecular assays for infectious disease.
Obstetrics/Gynecology. LCD offers a comprehensive menu of women's health testing, including NuSwab® high quality convenient STD testing, as well as liquid-based Pap testing with image-guided cervical cytology for improved cervical cancer detection, and out-of-the-vial Pap testing with options for human papillomavirus (HPV), chlamydia, and gonorrhea. LCD also offers tests and technologies that span the continuum of care for reproductive health, including maternal serum screening, prenatal diagnostics, ethnicity carrier screening, testing for causes of infertility or miscarriage and postnatal testing services.
Pharmacogenetics. LCD provides access to the latest tests in the emerging field of pharmacogenetics. These tests can help physicians understand how a patient will metabolize certain drugs, allowing them to select the most appropriate therapies or adjust dosing.
Identity. LCD provides forensic identity testing used in connection with criminal proceedings and parentage evaluation services that assist in determining parentage for child support enforcement proceedings and determining genetic relationships for immigration purposes. Parentage testing involves the evaluation of immunological and genetic markers in specimens obtained from the child, the mother and the alleged father. LCD also provides testing services in reconstruction cases, which assist in determining parentage without the presence of the parent in question.
Occupational Testing Services. LCD provides testing services for the detection of drug and alcohol abuse for private and government customers. These testing services are designed to produce forensic quality test results that satisfy the rigorous requirements of regulated and non-regulated workplace drug testing programs. LCD also provides other analytical testing and a variety of support services.
Chronic Disease Programs. Through Litholink, LCD uses a programmatic approach to the comprehensive treatment of chronic diseases, including kidney disease, cardiovascular disease, metabolic bone disease, diabetes, and offers CDS reports to both physicians and patients. LCD believes these chronic disease programs represent potential significant savings to the healthcare system by increasing the detection of early-stage diseases and effectively managing chronic disease conditions.
7
Development of New Tests
Advances in medicine continue to fundamentally change diagnostic testing. New tests are allowing clinical laboratories to provide unprecedented amounts of health-related information to physicians and patients. New molecular diagnostic tests that have been introduced over the past several years, including a gene-based test for HPV, HIV drug resistance assays, and molecular genetic testing for cystic fibrosis, have now become part of standard clinical practice. LCD continued its industry leadership in gene-based and esoteric testing in 2015, generating more than $2.0 billion in revenue from these testing services. As science continues to advance, LCD expects new testing technologies to emerge and therefore intends to continue to invest in advanced testing capabilities so that it can remain on the cutting edge of diagnostic laboratory testing. The Company has added, and expects to continue to add, new testing technologies and capabilities through a combination of internal development initiatives, technology licensing and partnership transactions, and selected business acquisitions. Through its sales force, LCD rapidly introduces new testing technologies to physician customers. This differentiation is important in the retention and growth of business.
In 2015, LCD continued its emphasis on scientific innovation and leadership with the introduction of significant test menu and automation enhancements. LCD is focused on the expansion of existing programs in molecular diagnostics as well as the introduction of new assay and assay platforms through licensing partnerships, acquisitions and internal development. Evidence of the commitment to the development of new diagnostics and applications for those diagnostics is evidenced by the annual publication of approximately 150 publications (e.g. articles, book chapters, books and abstracts) and presentations at scientific meetings and in presentations in academic medical center grand rounds and seminars. Examples of new tests and services introduced in 2015 include:
Cardiovascular Disease Risk Assessment - In September 2014, LabCorp acquired LipoScience, Inc. (LipoScience), and in 2015 its services were integrated into LCD's Burlington, NC laboratory, which now provides in-house testing for NMR LDL-particles, an advanced method for the assessment of cardiovascular risk.
Endocrine - LCD launched a mass spectroscopy based assay for thyroglobulin (in the presence of antibodies) and the Litholink CDS group developed a new Diabetes Patient Education report for patients with Type 2 Diabetes.
Infectious Diseases - LCD launched GenoSure Archive, the first laboratory test to help optimize antiretroviral drug regimens in virally suppressed HIV patients. LCD launched two new HCV drug resistance assays, HCV NS5A and NS5B, which expand LCD's portfolio of HCV resistance tests that already included HCV GenoSure NS3/4A, launched in 2011.
Breast Cancer Tests - LCD transitioned its suite of BRCA 1 and 2 tests, which identify gene mutations or alterations that signal an increased risk for several specific types of cancer, including breast cancer and ovarian cancer, to next-generation sequencing testing. In 2015, LCD entered into a cooperative agreement with Inserm, the French National Institute of Health and Medical Research Institution, and Quest Diagnostics to launch BRCA Share™, a novel datashare initiative to accelerate research on BRCA gene mutations.
Coagulation - In 2015, LCD introduced a mass spectroscopy based method serotonin release assay. This assay is important for diagnosing heparin-induced thrombocytopenia (HIT) and is the first non-radio labeled assay to be offered for such testing. This method shortens the turnaround time for reporting of results.
Obstetrics and Gynecology - In 2014, LCD launched the informaSeq Prenatal Test. This test is an advanced, non-invasive, next-generation sequencing prenatal screening test that can assess risk for multiple fetal chromosomal aneuploidies, or abnormalities in the number of chromosomes, from a single maternal blood draw. Through 2015, the test has been updated to include sex chromosome determination and LCD is preparing to launch additional screening for fetal fraction determination.
Immunotherapeutics and Oncology - In 2015, the Company played a pivotal role in the clinical trials that led to the approval of two immunotherapeutics for non-small cell lung cancer, and LCD was one of the first laboratories to offer the PD-L1 companion diagnostic and complementary diagnostic tests for those innovative therapeutics. The Company also made available the Lynch Syndrome cascade of tests for the diagnosis of inherited risk in colorectal cancer patients.
Phlebotomy - In 2015, LCD expanded access to AccuDraw®, its proprietary specimen collection and handling software) to all clients through the Company's website at www.LabCorp.com.
Genomic Testing - LCD introduced ExomeReveal, a whole exome sequencing testing service. Increasing evidence suggests that early genetic diagnosis can improve clinical outcomes, and ExomeReveal will provide genome-wide interpretation for children with serious childhood genetic diseases as well as additional diagnostic information for patients of any age. The 2015
8
launch of VistaSeq provides an assessment of inherited genetic mutations within a panel of 27 genes known to be associated with hereditary cancer syndromes.
LCD continues its collaboration with university, hospital and academic institutions such as Duke University, Johns Hopkins University, Boston University, Columbia University, Mt. Sinai Hospital and Yale University to license and commercialize new diagnostic tests.
LCD Technology-Enabled Solutions
LCD’s technology-enabled solutions include innovative decision support programs for chronic diseases, population health analytics tools, the LabCorp Beacon platform and Beacon LBS. These industry-leading, technology-enabled solutions are helping to improve health and improve lives by changing the way care is provided.
During 2015, LCD delivered more than 5 million enhanced CDS reports for chronic health conditions, including kidney disease, cardiovascular disease, metabolic bone disease and diabetes. LCD’s proprietary CDS reports integrate patient-specific diagnostic information and evidence-based healthcare content to help healthcare providers and patients better manage health. In addition, these decision support programs promote physician adherence to evidence-based treatment guidelines.
LCD also has new population health analytics programs in development to provide healthcare business intelligence tools to hospitals, physician practices, and accountable care organizations (ACOs). These tools are intended to assist customers in their compliance and reporting requirements with respect to efficient management of their productivity, quality and patient outcome metrics. LCD's robust rules engine maintains a large number of clinical quality measures that are highly customizable and support compliance with meaningful use and quality reporting requirements such as ACO standards, Joint Commission standards and the CMS Physician Quality Reporting System (PQRS). Real time clinical alerts highlight gaps in care for patients and patient populations.
The Company's centralized and proprietary LabCorp Beacon platform is a series of assets and functionalities that enhance the customer experience and provide an end-to-end lab solution. These assets and functionalities include:
• | Physician, patient and payer portals; |
• | Express electronic ordering for essentially all of LCD's brands and services; |
• | Integrated results viewing and enhanced reports; |
• | Lab analytics that provide one-click trending of patient, test and population data; |
• | CDS tools at the point of ordering and results; |
• | AccuDraw and LabCorp Touch systems that provide graphical, step-by-step guidance to help improve accuracy, workflow and turnaround time in the collection and processing of specimens at the point of care; |
• | Online appointment scheduling; |
• | LabCorp Beacon: Mobile, which provides solutions for market leading mobile devices; and |
• | Services-oriented architecture with rules-based engines, content aggregation and seamless integration with practice workflow. |
LCD's BeaconLBS business provides a technology-enabled solution that provides point-of-care decision support through interfaces with test ordering systems to assist physicians in lab and test selection. BeaconLBS helps physicians to order the appropriate test for the patient at the appropriate time. Physicians, patients, healthcare delivery systems and payers are expected to benefit from this innovation, which will improve the quality of lab services, support evidence-based guidelines for patient care, and more effectively manage trends without disrupting physician work flow. The BeaconLBS rules engine interfaces with payer policies for ordering, utilization, adjudication and payment.
In 2013, BeaconLBS signed an agreement with UnitedHealthcare® to implement the laboratory benefit management program in Florida utilizing BeaconLBS. UnitedHealthcare launched the laboratory benefit management program with BeaconLBS in Florida on October 1, 2014. In April 2015, BeaconLBS achieved its targeted implementation for UnitedHealthcare in Florida and LCD began recognizing revenue for providing this service.
Billing for Laboratory Services
Billing for laboratory services is a complicated process involving many payers such as MCOs, Medicare, Medicaid, physicians and physician groups, hospitals, patients and employer groups, all of which have different billing requirements. In addition, billing arrangements with third-party administrators may further complicate the billing process. Tests ordered by a physician may be billed to different payers depending on the medical benefits of a particular patient. Most testing services are billed to a party other
9
than the physician or other authorized person who ordered the test. A growing portion of the managed care fee-for-service revenues are derived from patients in the form of deductibles, coinsurance, non-covered tests and copayments.
LCD utilizes a centralized billing system in the collection of approximately 93.6% of its domestic revenue (88.4% of consolidated LCD revenue). This system generates bills to LCD customers based on payer type. Client billing is typically generated monthly, whereas patient and third-party billing are typically generated daily. Agings of accounts receivable are then monitored by billing personnel and re-bills and follow-up activities are conducted as necessary. Bad debt expense is recorded within selling, general and administrative expenses as a percentage of sales considered necessary to maintain the allowance for doubtful accounts at an appropriate level, based on LCD's experience with its accounts receivable. LCD writes off accounts against the allowance for doubtful accounts when accounts receivable are deemed to be uncollectible. For client billing, third party and managed care, accounts are written off when all reasonable collection efforts prove to be unsuccessful. Patient accounts are written off after the normal dunning cycle has occurred and the account has been transferred to a third-party collection agency.
A significant portion of LCD's bad debt expense is related to accounts receivable from patients who are unwilling or unable to pay. In 2015, LCD continued its focus on process and account management initiatives to reduce the negative impact of bad debt expense related to patient accounts receivable. As part of Project LaunchPad, LCD is identifying clients with high concentrations of write offs and implementing strategies to improve the financial performance of those accounts.
Another component of LCD’s bad debt expense is the result of non-credit related issues that slow the billing process, such as missing or incorrect billing information on test requisitions. LCD vigorously attempts to obtain any missing information or rectify any incorrect billing information received from the healthcare provider. However, LCD typically performs the requested tests and returns the test results regardless of whether billing information is incorrect or incomplete. LCD believes that this experience is similar to that of its primary competitors. LCD continues to focus on process initiatives aimed at reducing the impact of these non-credit related issues by reducing the number of requisitions received that are missing billing information or have incorrect information. This is accomplished through on-going identification of root-cause issues, deploying technology-enabled solutions, training provided to internal and external resources involved in the patient data capture process, and an emphasis on the use of electronic test ordering.
For the Company's operations in Ontario, Canada, the Ontario Ministry of Health and Long-Term Care (Ministry) determines who can establish a licensed community medical laboratory and caps the amount that each of these licensed laboratories can bill the government- sponsored healthcare plan. The Ontario government-sponsored healthcare plan covers the cost of clinical laboratory testing performed by the licensed laboratories. The provincial government discounts the annual testing volumes based on certain utilization discounts and establishes an annual maximum it will pay for all community laboratory tests. The agreed-upon reimbursement rates are subject to Ministry review at the end of each year and can be adjusted at the government's discretion based upon the actual volume and mix of testing services performed by the licensed providers in the province during the year. In 2015, the amount of the Company's capitated revenue derived from the Ontario government sponsored healthcare plan was CN$189.8 million.
Effect of Market Changes on the Clinical Laboratory Business
The delivery of, and reimbursement for, healthcare continues to change, impacting all stakeholders, including the clinical laboratory business. Medicare (which principally serves patients who are 65 and older), Medicaid (which principally serves low-income patients) and insurers have increased their efforts to control the cost, utilization and delivery of healthcare services. Measures to regulate healthcare delivery in general and clinical laboratories in particular have resulted in reduced prices, added costs and decreased utilization for the clinical laboratory industry by increasing complexity and adding new regulatory and administrative requirements. From time to time, government has also considered changes to the Medicare and Medicaid fee schedules, and LCD believes that pressure to reduce government reimbursement will continue.
Fees for most laboratory services reimbursed by Medicare are established in the Clinical Laboratory Fee Schedule (CLFS), and fees for other testing reimbursed by Medicare, primarily related to pathology, are covered by the Physician Fee Schedule (PFS). During 2015, approximately 12.3% of LCD’s revenue was reimbursed under the CLFS (12.6% in 2014), and approximately 0.9% was reimbursed under the PFS (1.0% in 2014). Over the past several years, LCD has experienced governmental pay reductions as a direct result of the Patient Protection and Affordable Care Act (ACA), the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) and the Achieving a Better Life Experience Act of 2014 (ABLE Act). In addition, the Protecting Access to Medicare Act (PAMA), which became law on April 1, 2014, is expected to result in a future net reduction in reimbursement revenue under the CLFS. These laws include provisions designed to control healthcare expenses reimbursed by government programs through a combination of reductions to fee schedules, incentives to providers to participate in alternative payment models such as risk-sharing and new methods to establish and adjust fees.
10
During 2013, government payment reductions and molecular pathology payment issues (largely driven by payer policy changes) reduced the Company’s net revenue by more than $100.0 million. The negative impact from these reimbursement challenges was largely sustained throughout 2014 and 2015. In addition to that reduction, in 2014, LCD experienced payments reductions from CLFS of $6.0 million and from PFS of $6.6 million. During 2015, LCD had also experienced a 0.25% payment reduction under the CLFS, equal to approximately $2.0 million in net revenue, which was offset by an increase in reimbursement from the PFS of approximately $2.1 million. During 2016, LCD will receive a 0.10% payment increase under the CLFS, representing approximately $0.76 million. Further, due to reductions in the PFS, LCD will also realize a $10.7 million reduction in net revenue over the two year period of 2016 and 2017. Due to provisions outlined in PAMA and the ABLE Act, the reduction in the 2016 PFS is limited in the first year of implementation. Accordingly, $2.4 million of the total $10.7 million reduction will be implemented in 2016, with the balance of $8.3 million to be realized as a reduction in 2017.
Under PAMA, beginning in 2017, CMS will be required to set and make adjustments to the CLFS using market-based information that reflects the scope of prices paid across the laboratory industry. On October 1, 2015, CMS issued a proposed rule to implement PAMA that would require applicable laboratories, including LCD, to begin reporting their test-specific private payer payment amounts to CMS during the first quarter of 2016. CMS intends to use that private market data to calculate weighted median prices for each test (based on applicable CPT codes) that would represent the new CLFS rates beginning in 2017, subject to certain phase-in limits. For 2017-2019, a test price cannot be reduced by more than 10.0% per year; for 2020-2022, a test price cannot be reduced by more than 15.0% per year. Reporting and pricing will occur every three years, or annually with respect to certain types of tests, to update the CLFS thereafter.
The American Clinical Laboratory Association (ACLA) and the laboratory community provided extensive comments on the proposed rule and will continue to work with CMS to try to ensure the final regulation accurately reflects the statutory language on applicable laboratories, and results in a reimbursement framework that reflects the broad scope of the laboratory market, encourages innovation, and maintains access to laboratory services for Medicare beneficiaries. The Company continues to evaluate the potential impact of the proposed rule, but since rulemaking to implement the provisions of PAMA has not yet been finalized, it is too early to assess the impact of PAMA.
In addition, market-based changes have affected and will continue to affect the clinical laboratory business. Reimbursement from commercial payors for diagnostic testing has shifted and will continue to shift away from traditional, fee-for-service model to alternatives including value-based, bundled pay-for-performance and other risk-sharing payment models.. The growth of the managed care sector and consolidation of MCOs present various challenges and opportunities to LCD and other clinical laboratories. In 2006, the Company signed a ten-year agreement with UnitedHealthcare to become its exclusive national laboratory. This agreement represented an industry first in terms of its length and exclusivity at a national level. In September 2011, the Company extended this agreement for an additional two years through the end of 2018. The various MCOs have different contracting philosophies, which are influenced by the design of their products. Some MCOs contract with a limited number of clinical laboratories and engage in direct negotiation of rates. Other MCOs adopt broader networks with generally uniform fee structures for participating clinical laboratories; in some cases, those fee structures are specific to independent clinical laboratories while the fees paid to hospital-based and physician-office laboratories may be different, and are typically higher. In addition, some MCOs use capitation to fix the cost of laboratory testing services for their enrollees. Under a capitated reimbursement mechanism, the clinical laboratory and the MCO agree to a per member, per month payment for all covered laboratory tests provided to MCO members during the month, regardless of the number or cost of the tests performed. For the year ended December 31, 2015, capitated contracts with MCOs accounted for approximately $219.9 million, or 3.5% of LCD's net revenues. LCD's ability to attract and retain MCO clients has become even more important as the impact of various healthcare reform initiatives continue, including expanded Health Insurance Exchanges and ACOs.
Despite the potential market changes discussed above, LCD believes that the volume of clinical laboratory testing will be positively influenced by several factors, including an expansion of Medicaid, managed care, and private insurance exchanges. In addition, LCD believe that increased knowledge of the human genome, and continued innovation in laboratory medicine, will continue to foster greater appreciation of the value of gene-based diagnostic assays. Additional factors that may lead to future volume growth include an increase in the number and types of tests that are readily available (due to advances in technology and increased cost efficiencies) for the diagnosis of disease, and the general aging of the U.S.
LCD believes its enhanced esoteric menu, geographic footprint and operating efficiency provide a strong platform for growth. In particular, LCD believes that it will benefit from the development of and increased interest in new companion and complementary diagnostics. Companion diagnostics are tests that must be used before a patient can be treated with a specific therapeutic, to help identify if the therapeutic will be effective or if it may cause adverse events. Complementary diagnostics are not required for determining who should receive the therapeutic, but can give physicians information about a patient’s potential response to a specific therapeutic or class of therapeutics. LCD and CDD are uniquely positioned to provide the full spectrum of support for the development and commercialization of companion and complementary diagnostics and their associated treatments.
11
The impact of these factors is expected to be partially offset by declines in volume as a result of increased controls over the utilization of laboratory services by Medicare, Medicaid, and other third-party payers, particularly MCOs. In addition, continued movement by patients into consumer driven health plans may have an impact on the utilization of laboratory testing.
Covance Drug Development Segment
CDD, a leader in drug development services, provides a range of drug development solutions on a worldwide basis, primarily to companies in the pharmaceutical and biotechnology industries. CDD has more than 14,000 employees worldwide and a global network of operations with offices in more than 30 countries. In addition, CDD supports trial activity in approximately 100 countries.
Drug Development Industry
Drug development services companies like CDD are also referred to as contract research organizations (CROs) and typically derive substantially all of their revenue from research and development (R&D) as well as marketing expenditures of the biopharmaceutical industry. CDD is the only CRO offering comprehensive global drug development services from preclinical research through all phases of clinical development and into commercialization. Outsourcing of R&D services from biopharmaceutical companies to CROs has significantly increased in the past, and is expected to continue increasing in the future, because of several factors, including: pressures to contain costs, limitations on internal R&D capacity, the need to reduce drug development timelines, customer demand for simultaneous research in multiple countries, stringent government regulation, and therapeutic and other expertise that customers lack internally. The investment and amount of time required to develop new drugs has been increasing, and these trends create opportunities for CDD and other CROs that can help make the drug development process more efficient.
The drug development industry has many participants ranging from hundreds of small providers to a limited number of full-service CROs with global capabilities. CDD competes against these small and full-service CROs, as well as in-house departments of pharmaceutical companies, and to a lesser extent, selected universities and teaching hospitals.
There is competition for customers on the basis of many factors, including: reputation for on-time quality performance; expertise and experience in specific areas, such as operations, technology, and therapeutic areas; scope of service offerings; strengths in various geographic markets; price; technological expertise and efficient drug development processes; ability to acquire, process, analyze and report data in a rapid and accurate manner; quality of relationships; ability to manage large-scale clinical trials domestically and internationally; quality of facilities; expertise and experience in market access services; and size and scale. CDD believes that it competes favorably in these areas.
Significant competitors for CDD's drug development services include Quintiles Transnational Holdings Inc., PAREXEL International Corporation, Pharmaceutical Product Development, LLC, ICON plc, INC Research Holdings, Inc. and Charles River Laboratories International, Inc., among others.
Early Development Services
CDD’s early development service offerings include research models, lead optimization, analytical services, safety assessment, and chemistry manufacturing and control (CMC) services for drug development. CDD also offers solution-based approaches to early development, including access to a team of experienced program development directors and project managers to help guide strategic decisions and manage molecule development in an integrated, streamlined manner. CDD's innovations in the preclinical area include technologies for enhanced client access to data, such as: StudyTracker, electronic animal identification, multimedia study reports and animal and test tube measures of induced cell proliferation or reproduction. StudyTracker®, an internet-based client access product, allows clients of toxicology, bioanalytical, metabolism and reproductive and developmental toxicology services to review study data and schedules on a near real-time basis. CDD has preclinical laboratories in the U.S., the U.K., Germany and China.
Research Models. CDD is an Association for Assessment and Accreditation of Laboratory Animal Care accredited provider of purpose-bred research models globally. Due to regulation by the U.S. Food and Drug Administration (FDA) and other foreign regulatory bodies, safety and efficacy testing on research models is required as part of the drug development process prior to testing in humans. CDD has a strong commitment to animal welfare, and has instituted progressive enrichment practices and rigorous health testing standards that exceed industry safeguard to protect the health of CDD's models. CDD is also committed to seeking out alternatives to the use of research models where possible. CDD's research models include standard lines as well as disease state and genetically altered models to accommodate clients’ needs. CDD offers purpose-bred specific pathogen
12
free (SPF) rabbits, canines, nonhuman primates, and other species, as well as blood and tissue products and surgical/technical services including telemetry. The purpose-bred research animals are provided to pharmaceutical and biotechnology companies, university research centers and CROs.
Lead Optimization. Lead optimization services are designed to enhance the connection between in vivo pharmacology and toxicity. Lead optimization services include non-GLP toxicology, in vivo pharmacology with model development and integrated safety and efficacy capabilities, nonclinical imaging, nonclinical pathology services, pharmacokinetic/toxicokinetic (PK/TK) analysis/reporting and immunology services.
Analytical Services. Bioanalytical testing services help determine the appropriate dose and frequency of drug application from late discovery evaluation through Phase III clinical testing on a full-scale, globally integrated basis. CDD’s analytical services offering includes liquid chromatography-mass spectroscopy (LC-MS) immunoanalytical solutions and specialty support, translational biomarker solutions, discovery bioanalysis, vaccine analysis, PK/TK analysis and reporting, and organic synthesis. In addition, CDD offers a growing list of validated, nonproprietary assays for hundreds of compounds, eliminating method development and validation time, and reducing program cost. CDD has dedicated lab facilities across three continents providing in vitro drug metabolism, in vivo radiolabeled absorption, distribution, metabolism and excretion (ADME) studies, metabolite identification/profiling and nonclinical PK screening in addition to radiosynthesis services. CDD also provides pharmaceutical chemistry services that determine the metabolic profile and bioavailability of drug candidates.
Safety Assessment. Safety assessment services include general, genetic, and immunotoxicology services, in addition to nonclinical pathology, safety pharmacology services and developmental and reproductive toxicology (DART) studies. CDD’s drug development services employ state-of-the-art technology and an integrated program for both large and small molecules with facilities across three continents. CDD's nonclinical pathology group is comprised of certified veterinary pathologists who provide critical insights and recommendations to help clients navigate the drug development process. CDD’s safety pharmacology services utilize Value Added Safety Pharmacology & Toxicology (VAST) to economically assess pharmacology endpoints during toxicology studies to minimize safety issues during the clinical phases. DART services help clients assess the birth defect risk for potential drug candidates.
Biopharm CMC Manufacturing. CMC offers packages supporting FDA Investigational New Drug Application and New Drug Application/Biologic License Application submissions, as well as programs to help CDD's clients meet acceptance criteria for the release of drug product for both biologics and small molecules. CMC provides well-coordinated capabilities and expertise operating within a global quality system framework to deliver robust, cost-effective solutions. Capabilities include safety, identity, strength, quality and purity assessments for biologics.
Early Phase Development Solutions. Early Phase Development Solutions (EPDS) offers clients access to a focused, multi-disciplinary team of experts that help craft integrated solutions to rapidly identify and develop lead drug candidates and reduce development challenges. EPDS provides clients with seamless integration of the complete array of CDD nonclinical services with a focus on scientific integrity and human subject safety. EPDS also offers an innovative parallel study approach for shorter proof of concept studies; this approach can increase clinical return on investment through application of medical, scientific and therapeutic expertise, along with patient stratification strategies.
Central Laboratory Services
Through its global network of central laboratories in the U.S., Switzerland, Belgium, Singapore and China, CDD provides central laboratory testing services to biotechnology and pharmaceutical customers. CDD also has an alliance for central laboratory services testing in Japan with BML, Inc., a leading Japanese laboratory testing company.
CDD’s capabilities provide clients the flexibility to conduct studies on a global basis. The data it provides can be combinable and result in global clinical trial reference ranges because CDD uses consistent laboratory equipment, methods, reagents and calibrators for studies where data is combined from different regions. Combinable data eliminates the cumbersome process of harmonizing results generated using different methods in different laboratories on different equipment. CDD also offers LabLink, an internet-based client access program that allows clients to review and query clinical trial lab data on a near real-time basis.
CDD operates the world’s largest automated clinical trial sample collection kit production line, located in Indianapolis, Ind. This facility supplies kits and supplies to investigator sites around the world, promoting global consistency in sample collection. Extensive automation in the kit production process enables kits to be produced with 5.5 sigma precision, while maintaining the scalability needed to meet increasing global demand. CDD has a biorepository facilities in Greenfield, Ind. and Kannapolis, N.C. dedicated to long-term storage of clinical trial specimens. These facilities are able to store a wide range of specimens, including plasma, serum, whole blood, peripheral blood, DNA and tissue.
13
Five of CDD's central laboratories are ISO 15189 certified to provide clients with the assurance that comes with a rigorous global standard. In addition, CDD has implemented a novel model for external lab selection and management that provides rigor and reduces internal resource drain for trial sponsors. The extended laboratory management solutions team focuses on managing all aspects of referral laboratory services, including vendor negotiations, governance, quality management, data services and contract services.
In addition to high volume safety testing, CDD offers a scientifically rich and diverse menu of specialty testing capabilities, spanning the clinical development continuum. These include applied genomics, next generation sequencing, anatomic pathology and histology, flow cytometry, clinical immunoassays and preclinical and exploratory biomarker development. CDD also offers differentiated capabilities and unparalled experience in companion and complementary diagnostic services to support the parallel development of a new medicine and its associated diagnostic assay. CDD’s dedicated team has helped develop more than two-thirds of all currently available FDA-approved companion diagnostics, and CDD was the exclusive laboratory to partner on clinical trials and regulatory submissions for three very important oncology companion diagnostics that received FDA approval in 2015. CDD can support both in vitro diagnostic (IVD) companion diagnostic development and laboratory-developed test (LDT) development. By combining CDD’s strength in central laboratory and early-stage clinical development with LCD’s strength in test commercialization, the Company is well positioned to offer comprehensive, end-to-end support for companion diagnostic development.
Clinical Development and Commercialization Services
CDD offers a comprehensive range of clinical trial services, including the full management of Phase I through IV clinical studies. CDD has extensive experience in all significant therapeutic areas, and provides the following core services either on an individual or aggregated basis to meet its clients’ needs: study design and modeling; coordination of study activities; trial logistics; monitoring of study site performance; clinical data management and biostatistical analysis; and medical writing and regulatory services.
CDD has extensive experience in designing and managing global clinical trials and regional clinical trial activities in North America, Europe, Latin America and the Asia Pacific region. These trials may be conducted separately or simultaneously as part of a multinational or global development plan. CDD can manage every aspect of clinical trials from clinical development plans and protocol design to new drug applications and other supporting services.
CDD provides clinical pharmacology services, including first-in-human trials, and early patient proof of concept studies of new pharmaceuticals at its five clinics located throughout the U.S. and Europe.
CDD offers a range of commercialization solutions, which include life cycle management and post-approval studies, which are typically conducted after a drug has successfully undergone clinical efficacy and safety testing and the New Drug Application has been submitted to the FDA and/or other regulatory bodies. CDD also offers market access solutions, including reimbursement consulting and hotlines, patient assistance programs, health economic and outcomes research services, observational studies, real world evidence and analytics services, and value communication services. Pharmaceutical and biotechnology companies purchase these services to serve patients in need of therapy and to help optimize their return on R&D investments.
CDD Technology-Enabled Solutions
CDD’s Xcellerate® informatics platform integrates multiple sources of data to deliver unique and timely information throughout the course of client studies. Xcellerate is a technology-enabled platform designed by CDD to help reduce the cost, time, complexity and risk associated with clinical trials. Key Xcellerate modules include Forecasting & Site Selection, Clinical Trial Management, Monitoring, and Insights. Xcellerate Forecasting & Site Selection enables clients to make more predictive choices to identify the optimal sites and investigators by drawing on the world’s largest proprietary clinical trial knowledge base. Xcellerate Clinical Trial Management provides near real time information on trial results and execution. Xcellerate Monitoring enables clients to proactively identify and mitigate risks by monitoring medical, statistical and operational data across the client’s portfolio of clinical trials. Xcellerate Insights supports analysis of a trial’s most recent operational metrics in a secure collaborative portal.
In addition to Xcellerate, CDD’s proprietary technology assets include CDD’s investigator database and analytic methodologies utilized to design and manage patient enrollment, site selection, and investigator selection to produce higher quality and faster clinical trials resulting in reduced costs and increased market potential for biopharmaceutical company clients. CDD and LCD are also collaborating to use LCD information to support clinical trial recruitment and post-trial monitoring.
14
Clients
The Company provides testing and drug development services to a broad range of healthcare providers, biopharmaceutical companies and other customers.
The primary client groups serviced by the Company include:
MCOs. The Company serves many MCOs. These organizations have different contracting philosophies, that are influenced by the design of the products. Some MCOs contract with a limited number of clinical laboratories and engage in direct negotiation of rates. Other MCOs adopt broader networks with generally uniform fee structures for participating clinical laboratories; in some cases, those fee structures are specific to independent clinical laboratories while the fees paid to hospital-based and physician-office laboratories may be different, and are typically higher. MCO’s also may offer Managed Medicare or Managed Medicaid plans. MCOs may also offer Managed care or Managed Medicaid plans. In addition, some MCOs use capitation to fix the cost of laboratory testing services for their enrollees. Under a capitated reimbursement mechanism, the clinical laboratory and the MCO agree to a per member, per month payment for all covered laboratory tests.
Pharmaceutical and Biotechnology Companies. The Company serves hundreds of biopharmaceutical companies, ranging from the world’s largest pharmaceutical and biotechnology companies to small and start-up organizations. Contracts with these institutions generally take the form of fee-for-service or fixed-price arrangements.
Independent Physicians and Physician Groups. Physicians requiring testing for their patients are one of the Company’s primary sources of requests for testing services. Fees for clinical laboratory testing services rendered for these physicians are billed either to the physician, the patient or the patient’s third-party payer, such as an insurance company, Medicare or Medicaid. Billings are typically on a fee-for-service basis. If the billings are to the physician, they are based on a customer fee schedule and are subject to negotiation. Otherwise, the patient or third-party payer is billed at the Company's patient fee schedule, subject to third-party payer contract terms and negotiation by physicians on behalf of their patients. Patient sales are recorded at the Company’s patient fee schedule, net of any discounts negotiated with physicians on behalf of their patients, or fees made available through charity care or an uninsured patient program. Revenues received from Medicare and Medicaid billings are based on government-set fee schedules and reimbursement rules.
Hospitals. The Company provides hospitals with services ranging from core and specialty testing to laboratory management services. Hospitals generally maintain an on-site laboratory to perform immediately needed testing for patients receiving care. However, they also refer less time-sensitive procedures, less frequently needed procedures and highly specialized procedures to outside facilities, including independent clinical laboratories and larger medical centers. The Company typically charges hospitals for any such tests on a fee-for-service basis that is derived from the Company’s client fee schedule. Fees for management services are typically billed monthly at contractual rates.
Other Clients. The Company serves other clients, including government agencies (in addition to government reimbursement programs such as Medicare and Medicaid), large employers, food manufacturers, other independent clinical laboratories that do not have the breadth of the Company’s testing capabilities and in jurisdictions where permitted, consumers who may order diagnostic testing directly. These clients typically pay on a negotiated fee-for-service basis or based on a set fee schedule.
Capital Allocation
The Company believes it has a strong track record of deploying capital to investments that enhance the Company's business and returning capital to shareholders.
Since 2010, the Company has invested net cash of approximately $5.8 billion and equity of $1.8 billion in strategic business acquisitions ($2.2 billion over the same period excluding the Acquisition). These acquisitions have significantly expanded the Company’s service offerings, expanded its customer and revenue mix, and strengthened and broadened the scope of its geographic presence. The Company continues to evaluate acquisition opportunities that leverage the Company’s core competencies, complement existing scientific and technological capabilities, increase the Company’s presence in key geographic areas, and meet or exceed the Company’s financial criteria.
On February 19, 2015, the Company completed the Acquisition, for $6,150.7 billion. Covance stockholders received $75.76 in cash and 0.2686 shares of the Company's common stock for each share of Covance common stock they owned immediately prior to the consummation of the Acquisition. Upon completion of the Acquisition, former Covance stockholders owned approximately 15.5% of the outstanding shares of the Company's stock.
15
Since 2003, the Company has repurchased approximately $5.9 billion in shares at an average price of approximately $69 per share. Following the announcement of the Acquisition, the Company suspended its share repurchases. The Company does not anticipate resuming its share repurchase activity until it approaches its targeted ratio of total debt to consolidated EBITDA of 2.5 to 1.0. However, the Company will continue to evaluate all opportunities for strategic deployment of capital in light of market conditions. During 2015, the Company repaid $500.0 million of its senior notes and $285.0 million of its term loan. In addition, the Company borrowed and repaid $460.0 million of debt within the year.
Since 2010, capital expenditures were $1.1 billion, representing approximately 3.0% of the Company’s total net revenue during the same period. The Company expects capital expenditures in 2016 to be approximately 3.0% of net revenues primarily in connection with projects to support growth in the Company's core businesses, projects related to Project LaunchPad and further Covance integration initiatives.
Seasonality
The Company experiences seasonality in both segments of its business. For example, testing volume generally declines during the year-end holiday period and other major holidays. Testing volume can also decline due to inclement weather, reducing net revenues and cash flows. CDD's operations are also impacted by changes in the global economy, exchange rate fluctuations, the progress of ongoing studies and the start-up of new studies, as well as the level of expenditures made by the pharmaceutical and biotechnology industries in R&D. Given the seasonality of the business, comparison of results for successive quarters may not accurately reflect trends or results for the full year.
Investments in Joint Venture Partnerships
The Company holds investments in three joint venture partnerships, two located in Alberta, Canada, and one located in Florence, South Carolina. These businesses primarily represent partnership agreements between the Company and other independent diagnostic laboratory investors. Under these agreements, all partners share in the profits and losses of the businesses in proportion to their respective ownership percentages. All partners are actively involved in the major business decisions made by each joint venture. The Company does not consolidate the results of these joint ventures. Effective June 30, 2015, the Company liquidated its interest in a joint venture partnership that had been located in Milwaukee, Wisconsin.
The first Canadian partnership is a leader in Occupational testing across Canada similar to LCD's U.S. Occupational testing services. The second Canadian partnership has a license to conduct diagnostic testing services in the province of Alberta. Substantially all of its revenue is received as reimbursement from the Alberta government's healthcare programs. In December 2013, Alberta Health Services (AHS), the Alberta government's healthcare program, issued a request for proposals for laboratory services that included the scope of services performed by the Canadian partnership. In October 2014, AHS informed the Canadian partnership that it had not been selected as the preferred proponent. In November 2014, the Canadian partnership submitted a vendor bid appeal upon the belief that there were significant flaws and failures in the conduct of the request for proposal process, which drove to a biased conclusion. AHS established a Vendor Bid Appeal Panel to hear the appeal, and the hearing was conducted in February 2015. In August 2015, AHS was directed to cancel the request for proposal process. Subsequently, the Canadian partnership entered into a one-year extension through March 31, 2017 of its existing contract with AHS. If the contract is not renewed after March 2017, then the Canadian partnership's revenues would decrease substantially and the carrying value of the Company's investment could potentially be impaired.
Sales, Marketing and Client Service
LCD offers its diagnostic services through a sales force focused on serving the specific needs of customers in different market segments. These market segments generally include Primary Care, Obstetrics-Gynecology, Specialty Medicine (e.g., Infectious Disease, Endocrinology, Gastroenterology and Rheumatology), Oncology and Hospitals. LCD competes primarily on the basis of quality of testing, breadth of menu, price, innovation of services, convenience and access points throughout the nation.
CDD’s global sales activities are conducted by sales personnel in North America, Europe, South America and the Asia Pacific region. The sales force provides client coverage across the biopharmaceutical industry for services including lead optimization, preclinical safety assessment, analytical services, clinical solutions, central laboratories and market access solutions. Client segments called upon include global and regional pharmaceutical and biotech companies and academic institutions. CDD positions itself as the company that delivers Solutions Made Real® to its clients, bringing high quality, innovation, scientific depth and the ability to help clients develop drugs along the entire continuum of development.
16
LCD's and CDD’s sales forces are compensated through a combination of salaries, commissions and bonuses at levels commensurate with each individual’s qualifications, performance and responsibilities. LCD's general sales force and CDD's sales team are responsible for both new sales and for customer retention and relationship building. LCD's general sales force is also supported by a team of clinical specialists who focus on selling esoteric testing and meeting the unique needs of the specialty medicine markets.
Information Systems
The Company is committed to developing and commercializing technology-enabled solutions to support its operations and change the way care is provided. The Company operates standard platforms for its core business services including laboratory diagnostics, drug development, billing, financial and reporting systems. These standard systems ensure consistency within our workflows and information as well as a high level of system availability and stability. The Company’s primary laboratory systems, including standardized support for molecular diagnostics, digital pathology and enhanced specialty laboratory solutions, is responsible for processing tests that generate approximately 93.6% of its domestic LCD revenue (approximately 88.4% of total LCD revenue) and 100% of its CDD central laboratory services revenue. The Company's centralized information systems are responsible for tremendous operational efficiencies, enabling the Company to achieve consistent, structured, and standardized operating results and superior patient care.
In addition, LCD and CDD each offer proprietary and industry-leading information systems, which are discussed in more detail in the sections dedicated to each of those Business Segments.
Quality
LCD and CDD have quality management programs designed to provide that comprehensive quality systems and processes that are appropriate for their respective businesses are in place. This includes licensing, credentialing, training and competency of professional and technical staff, and internal audits. In addition to the external inspections and proficiency testing programs that may be required by regulatory agencies, systems and procedures are in place to emphasize and monitor quality. Many of the Company’s laboratories, facilities and processes are subject to on-site regulatory evaluations, external proficiency testing programs, state surveys as applicable in the U.S. and the Company's own quality audit programs.
Quality also encompasses virtually all facets of the Company’s service, including turnaround time, client service, data integrity, patient satisfaction, and billing, as applicable. The Company’s quality assessment program includes measures that compare its current performance against desired performance goals. Using quality assessment techniques, the Company employs a variety of programs to monitor critical aspects of service to its clients and patients.
In addition, various groups within the Company, including the Company's supply chain management department, CDD's clinical trial services global vendor management department, CDD's central laboratory services expanded laboratory management services department, and CDD's project management staff, provide oversight to monitor and control vendor products and performance, and play an essential role in the Company’s approach to quality through improvements in automation.
LCD Customer Interaction. Processes to continually improve the customers’ experience with LCD are essential. Use of technology and improvements in workflow within LCD's PSCs are helping to reduce patient wait times by expediting the patient registration process (through LabCorp Patient Appointment Scheduling) and enhance the specimen collection process (through LabCorp Touch and AccuDraw).
Specimen Management. The use of logistics and specimen tracking technology allows the timely transportation, monitoring, and storage of specimens. The Company is continually improving its ability to timely collect, transport and track specimens from collection points to LCD locations.
Quality Control. LCD regularly performs quality control testing by running quality control samples with known values at the same time patient samples are tested. Quality control test results are entered into LCD's computerized quality control database. In addition, results are continually monitored to detect potential analytical variances during testing. The real-time monitoring for any statistically and clinically significant analytical differences enables technologists and technicians to take immediate and appropriate corrective action prior to release of patient results. CDD operates a variety of quality control systems as appropriate for the type of work being conducted. These may include in-process and post-process quality control checks, use of control materials and reference standards, peer reviews, data review meetings, programmed data edit checks to detect variances and unusual data patterns, dual programming, and mock runs.
17
LCD Internal Proficiency Testing. LCD has an extensive internal proficiency testing program in which each laboratory receives samples to test. This internal proficiency program serves to test LCD's analytical and post-analytical phases of laboratory testing service including order entry, requisitioning systems, accuracy, precision of its testing protocols, and technologist/technician performance. This program supplements the external proficiency programs required by the laboratory accrediting agencies.
Accreditation. The Company participates in numerous externally-administered quality surveillance programs, including the College of American Pathology (CAP) program. CAP is an independent non-governmental organization of board-certified pathologists that offers an accreditation program to which laboratories voluntarily subscribe. CAP has been granted deemed status authority by CMS to inspect clinical laboratories to determine adherence to the Clinical Laboratory Improvement Amendments of 1988 (CLIA) standards. The CAP program involves both on-site inspections of the laboratory and participation in CAP's proficiency testing program for all categories in which the laboratory is accredited. A laboratory's receipt of accreditation by CAP satisfies the CMS requirement for certification. All of the Company's major diagnostic laboratories and CDD's Phase I clinical research units in Evansville, Ind. and Dallas, Texas are accredited by CAP.
The Company's forensic crime laboratory located in Lorton, Va. is accredited to ISO/IEC 17025:2005 by the American Society of Crime Laboratory Directors, Laboratory Accreditation Board (ASCLD/LAB) in the discipline of biology and categories of nuclear DNA, mitochondrial DNA, body fluid identification and individual characteristic database testing. Under the accreditation program managed by the ASCLD/LAB, a crime laboratory undergoes a comprehensive and in-depth inspection to demonstrate that its management, operations, employees, procedures and instruments, physical plant, and security and personnel safety procedures meet stringent quality standards.
The Company's full service forensic facilities in the U.K. are accredited to ISO/IEC 17025:2005 by the U.K. Accreditation Service in many areas of forensic analysis. These facilities provide crime scene investigative services, collecting samples for DNA analysis, mitochondrial DNA testing, microscopic analysis of tool marks and paint and other forms of forensic testing.
The Company has multiple labs that have received ISO 15189 accreditation. ISO 15189 is an international standard that recognizes the quality and technical competence of medical laboratories. The Company has fifteen (15) accredited laboratories in the U.S. In addition, the Company has six (6) laboratories accredited to this standard outside of the U.S., and the laboratory operated for CDD pursuant to an alliance with BML, Inc. also has this accreditation. The list below reflects the Company's labs that have achieved this accreditation and the year in which they achieved it.
LCD
• | Colorado Coagulation, Denver, Colorado - January, 2016 |
• | Dynacare-Gamma facility, Laval, Québec - March, 2015 |
• | LabCorp’s Regional Testing Facility, Dublin, Ohio - March, 2015 |
• | Endocrine Sciences, Calabasas, California - January, 2015 |
• | LabCorp's Regional Testing Facility, Dallas, Texas - April, 2014 |
• | LabCorp's Regional Testing Facility, Denver, Colorado - March, 2014 |
• | Integrated Genetics, Santa Fe, New Mexico - October, 2013 |
• | Integrated Genetics, Westborough, Massachusetts - September, 2013 |
• | Dynacare-Gamma facility, Montreal, Québec - June 2013 |
• | LabCorp's Regional Testing Facility, Phoenix, Arizona - April, 2013 |
• | LabCorp's Regional Testing Facility, Birmingham, Alabama - February, 2013 |
• | Integrated Oncology, Brentwood, Tenenssee - February, 2012 |
• | Viromed, Burlington, North Carolina - January, 2012 |
• | Center for Molecular Biology and Pathology (CMBP), Research Triangle Park, North Carolina - February, 2011 |
• | LabCorp's Regional Testing Facility, Tampa, Florida - January, 2010 |
• | Integrated Oncology, Phoenix, Arizona - September, 2009 |
CDD
• | Covance Central Laboratory Services Inc., Indianapolis, Indiana - August, 2015 |
• | BML Covance Central Laboratory, Tokyo, Japan - March, 2015 (Operated for CDD pursuant to an alliance with BML, Inc.) |
• | Covance Pharmaceutical Research and Development (Shanghai) Co. Ltd, Shanghai, China - March, 2015 |
• | Covance (Asia) Pte. Ltd., Singapore - June, 2014 |
• | Covance Central Laboratory Services SA, Geneva, Switzerland - October, 2013 |
18
Intellectual Property Rights
The Company relies on a combination of patents, trademarks, copyrights, trade secrets, nondisclosure and non-competition agreements to establish and protect its proprietary technology. The Company has filed and obtained numerous patents in the U.S. and abroad, and regularly files patent applications, when appropriate, to establish and protect its proprietary technology. From time to time, the Company also licenses U.S. and non-U.S. patents, patent applications, technology, trade secrets, know-how, copyrights or trademarks owned by others. The Company believes, however, that no single patent, technology, trademark, intellectual property asset or license is material to its business as a whole.
Employees
As of December 31, 2015, the Company had over 50,000 full-time equivalent employees worldwide, approximately 19.5% of whom were employed outside of the U.S. U.S. based subsidiaries of LCD have three collective bargaining agreements, which cover approximately 648 employees. Non-US based subsidiaries of CDD have 23 collective bargaining agreements, which cover approximately 951 employees.
The Company’s success is highly dependent on its ability to attract and retain qualified employees, and the Company believes that it has good working relationships with its employees.
Regulation and Reimbursement
General
Because the Company operates in a number of distinct operating environments and in a variety of locations worldwide, it is subject to numerous, and sometimes overlapping, regulatory environments. Both the clinical laboratory industry and the drug development business are subject to significant governmental regulation at the national, state and local levels. As described below, these regulations concern licensure and operation of clinical laboratories, claim submission and reimbursement for laboratory services, healthcare fraud and abuse, drug development services, security and confidentiality of health information, quality, and environmental and occupational safety.
Regulation of Clinical Laboratories
Virtually all clinical laboratories operating in the U.S. must be certified by the federal government or by a federally-approved accreditation agency. In most cases, that certification is regulated by CMS through CLIA. CLIA requires that applicable clinical laboratories meet quality assurance, quality control and personnel standards. Laboratories also must undergo proficiency testing and are subject to inspections.
Standards for testing under CLIA are based on the complexity of the tests performed by the laboratory, with tests classified as “high complexity,” “moderate complexity,” or “waived.” Laboratories performing high complexity testing are required to meet more stringent requirements than moderate complexity laboratories. Laboratories performing only waived tests, which are tests determined by the FDA to have a low potential for error and requiring little oversight, may apply for a certificate of waiver exempting them from most of the requirements of CLIA. All major and many smaller Company facilities hold CLIA certificates to perform high complexity testing. The Company's remaining smaller testing sites hold CLIA certificates to perform moderate complexity testing or a certificate of waiver. The sanctions for failure to comply with CLIA requirements include suspension, revocation or limitation of a laboratory's CLIA certificate, which is necessary to conduct business, cancellation or suspension of the laboratory's approval to receive Medicare and/or Medicaid reimbursement, as well as significant fines and/or criminal penalties. The loss or suspension of a CLIA certification, imposition of a fine or other penalties, or future changes in the CLIA law or regulations (or interpretation of the law or regulations) could have a material adverse effect on the Company.
The Company is also subject to state and local laboratory regulation. CLIA provides that a state may adopt laboratory regulations different from or more stringent than those under federal law, and a number of states have implemented their own laboratory regulatory requirements. State laws may require that laboratory personnel meet certain qualifications, specify certain quality controls, or require maintenance of certain records.
The Company believes that it is in compliance with all applicable laboratory requirements. The Company's laboratories have continuing programs to ensure that their operations meet all such regulatory requirements, but no assurances can be given that the Company's laboratories will pass all future licensure or certification inspections.
19
FDA Laws and Regulations
The FDA has regulatory responsibility over instruments, test kits, reagents and other devices used by clinical laboratories. The FDA has issued draft guidance regarding FDA regulation of LDTs. There are other regulatory and legislative proposals that would increase general FDA oversight of clinical laboratories and LDTs. The outcome and ultimate impact of such proposals on the business is difficult to predict at this time.
The FDA enforces U.S. laws and regulations that govern the development, testing, manufacturing, labeling, advertising, marketing, distribution and surveillance of diagnostic products, including many of the services and products offered by the Company and many of the client services and products that CDD works on. The FDA periodically inspects and reviews the manufacturing processes and product performance of diagnostic products. The FDA has the authority to take various administrative and legal actions for non-compliance such as fines, product suspensions, warning letters, recalls, injunctions and other civil and criminal sanctions. Other countries where the Company conducts business have similar agencies and laws with which the Company must also comply.
The operation of CDD’s preclinical laboratory facilities and clinical trial operations must conform at all times to good laboratory practice (GLP) and good clinical practice (GCP), respectively, as well as all other applicable standards and regulations. The preclinical and clinical studies that the Company conducts are subject to periodic inspections by the FDA as well as other drug regulatory agencies which may include, without limitation, the Medicines and Healthcare products Regulatory Agency in the U.K. (MHRA), the European Medicines Agency, the China Food and Drug Administration, and the Pharmaceuticals and Medical Devices Agency in Japan to determine compliance with GLP and GCP as well as other applicable standards and regulations. If the FDA determines during an inspection that the Company’s equipment, facilities, laboratories, operations, or processes do not comply with applicable FDA regulations and conditions of GLP and/or GCP, the FDA may issue a formal notice, which may be followed by a warning letter if observations are not addressed satisfactorily. Continued non-compliance may result in the FDA seeking civil, criminal or administrative sanctions and/or remedies against the Company, including suspension of its laboratory operations. Other countries where the Company conducts business have similar laws with which the Company must also comply.
Additionally, certain CDD services and activities, such as CMC services and manufacturing of investigational medicinal product for use in certain Phase I studies managed by CDD, must conform to current good manufacturing practice (cGMP). CDD is subject to periodic inspections by the FDA and the MHRA in order to assess, among other things, cGMP compliance. If the FDA or the MHRA identifies deficiencies during an inspection, it may issue a formal notice, which may be followed by a warning letter if observations are not addressed satisfactorily. Failure to maintain compliance with cGMP regulations and other applicable requirements of various regulatory agencies could result in fines, unanticipated compliance expenditures, suspension of manufacturing, enforcement actions, injunctions, or criminal prosecution. Other countries where the Company conducts business may have similar laws with which the Company may also be required to comply.
The Animal Welfare Act
The conduct of animal research at CDD’s facilities in the U.S. must be in compliance with the U.S. Animal Welfare Act (AWA), which governs the care and use of warm-blooded animals used for research in the U.S. other than laboratory rats, mice and chickens, and is enforced through periodic inspections by the U.S. Department of Agriculture (USDA). The AWA establishes facility standards regarding several aspects of animal welfare, including housing, ventilation, lighting, feeding and watering, handling, veterinary care, and recordkeeping. CDD complies with licensing and registration requirement standards set by the USDA and similar agencies in foreign jurisdictions such as the European Union and China for the care and use of regulated species. If the USDA determines that CDD’s equipment, facilities, laboratories or processes do not comply with applicable AWA standards, it may issue an inspection report documenting the deficiencies and setting deadlines for any required corrective actions. For continued noncompliance, the USDA may impose fines, suspend and/or revoke animal research licenses or confiscate research animals.
Payment for Clinical Laboratory Services
In 2015, LCD derived approximately 16.0% of its net revenue directly from the Medicare and Medicaid programs. In addition, LCD's other clinical laboratory testing business that is not directly related to Medicare or Medicaid nevertheless depends significantly on continued participation in these programs and in other government healthcare programs, in part because clients often want a single laboratory to perform all of their testing services. In recent years, both governmental and private sector payers have made efforts to contain or reduce healthcare costs, including reducing reimbursement for clinical laboratory services.
Reimbursement under the Medicare CLFS and PFS are capped at different rates in each Medicare carrier's jurisdiction. State Medicaid programs are prohibited from paying more than the Medicare fee schedule limit for clinical laboratory services furnished
20
to Medicaid recipient. Laboratories primarily bill and are reimbursed by Medicare and Medicaid directly for covered tests performed on behalf of Medicare and Medicaid beneficiaries; for beneficiaries that participate in Managed Medicare and Managed Medicaid plans, laboratory bills are submitted to and paid by MCOs who manage those plans.
As discussed previously in Item 1 of Part I, over the past several years LCD has experienced a series of reductions in payment from Medicare, and it expects to experience continued reductions through 2016 and 2017. However, rulemaking to implement PAMA has not yet been finalized, and the methods to be used by CMS to establish fees for laboratory services under the CLFS are not yet determined. Until those rules are final, the Company cannot assess the impact of further fee changes pursuant to PAMA.
Many pathology services performed by LCD are reimbursed by Medicare under the PFS. The PFS assigns relative value units to each procedure or service, and a conversion factor is applied to calculate the reimbursement. The PFS is also subject to adjustment on an annual basis. Such adjustments can impact both the conversion factor or relative value units. The Sustainable Growth Rate (SGR), the formula formerly used to calculate the fee schedule conversion factor, would have resulted in significant decreases in payment for most physician services for each year since 2003. However, Congress intervened repeatedly to prevent these payment reductions, and the conversion factor was increased or frozen for the subsequent year. MACRA permanently replaced the SGR formula and transitioned PFS reimbursement to a value-based payment system. MACRA retroactively avoided a 21.2% reduction in PFS reimbursement that had been scheduled for April 1, 2015, and provided for PFS conversion factor increases of 0.5% from July 1, 2015 to December 31, 2015, and 0.5% in each of years 2016-2019, followed by 0.0% updates for 2020-2025, and updates that vary based on participation in alternative payment models in subsequent years. These changes to the conversion factor may be offset by reductions to the relative value units, as was the case with the 2016 PFS reductions. In addition, rates will be adjusted under the new Merit-Based Incentive Payment System beginning in 2019. Approximately 0.9% of LCD's revenue is reimbursed under the PFS.
Because a significant portion of the Company's costs are relatively fixed, further payment reductions to Medicare, Medicaid and other government programs could have a direct adverse effect on the Company's net earnings and cash flows. The Company cannot predict whether changes will be implemented that will result in further payment reductions.
In addition to changes in reimbursement rates, LCD is also impacted by changes in coverage policies for laboratory tests. Congressional action in 1997 required HHS to adopt uniform coverage, administration and payment policies for many of the most commonly performed lab tests using a negotiated rulemaking process. The negotiated rulemaking committee established uniform policies limiting Medicare coverage for certain tests to patients with specified medical conditions or diagnoses, replacing local Medicare coverage policies which varied around the country. Since the final rules generally became effective in 2002, the use of uniform policies has improved LCD's ability to obtain necessary billing information in some cases. However, Medicare, Medicaid and private payer diagnosis code requirements and payment policies continue to negatively impact LCD's ability to be paid for some of the tests it performs. LCD also experienced delays in the pricing and implementation of new molecular pathology codes among various payers, including Medicaid, Medicare and commercial carriers. While some delays were expected, several non-commercial payers required an extended period of time to price key molecular codes and a number of those payers, mostly government entities, indicated that they would no longer pay for tests that they had previously covered. Further, several payers continue to require additional information to process claims or have implemented prior authorization policies. Many commercial payers were delayed in becoming aware of the impact of their claim edits and policies which impeded access to services that previously have been covered and reimbursed. These issues had a negative impact on revenue, revenue per requisition, margins and cash flows, which were largely sustained in the years 2013 through 2015, and are expected to have a continuing negative impact. Similarly, CLFS coding and billing changes related to toxicology and other procedures were implemented in 2015 and Palmetto implemented a revised Drugs of Abuse Local Coverage Policy which impacted the handling of such procedures for Medicaid and MCOs. The Company experienced delays in the pricing and implementation of the new toxicology codes, however, largely overcame issues related to price and margins through direct negotiation with the associated payers. Further coding and billing changes related to toxicology testing and other procedure types are to be implemented in 2016. The Company expects delays in the pricing and implementation of these new codes and it is unclear what impact will be experienced related to price and margins.
Future changes in national, state and local laws and regulations (or in the interpretation of current regulations) affecting government payment for clinical laboratory testing could have a material adverse effect on the Company. Based on currently available information, the Company is unable to predict what type of changes in legislation or regulations, if any, will occur.
Standard Electronic Transactions, Security and Confidentiality of Health Information and Other Personal Information
In the U.S., the Health Insurance Portability and Accountability Act of 1996 (HIPAA) was designed to address issues related to the security and confidentiality of health information. In an effort to improve the efficiency and effectiveness of the healthcare system by facilitating the electronic exchange of information in certain financial and administrative transactions, HIPAA regulations
21
were promulgated. These regulations apply to health plans, healthcare providers that conduct standard transactions electronically and healthcare clearinghouses (covered entities). Five such regulations have been finalized: (i) the Transactions and Code Sets Rule; (ii) the Privacy Rule; (iii) the Security Rule; (iv) the Standard Unique Employer Identifier Rule, which requires the use of a unique employer identifier in connection with certain electronic transactions; and (v) the National Provider Identifier Rule, which requires the use of a unique healthcare provider identifier in connection with certain electronic transactions.
The Company believes that it is in compliance in all material respects with the current Transactions and Code Sets Rule. The Company implemented Version 5010 of the HIPAA Transaction Standards and believes it has fully adopted the ICD-10-CM code set. The costs associated with ICD-10-CM Code Set were substantial, and failure of the Company, third party payers or physicians to apply the new code set could have had an adverse impact on reimbursement, days sales outstanding and cash collections in 2015 and forward. While to date the Company has not experienced any sustained disruption in receipts or indications of substantive reductions to reimbursement and net revenues related to the implementation of the ICD-10-CM code set, future application of restrictive clinical or payment policies could negatively impact the Company. The Company believes it is in compliance in all material respects with applicable laws and regulations for electronic funds transfers and remittance advice transactions.
The Privacy Rule regulates the use and disclosure of protected health information (PHI) by covered entities. It also sets forth certain rights that an individual has with respect to his or her PHI maintained by a covered entity, such as the right to access or amend certain records containing PHI or to request restrictions on the use or disclosure of PHI. The Privacy Rule requires covered entities to contractually bind third parties, known as business associates, in the event that they perform an activity or service for or on behalf of the covered entity that involves access to PHI. The Company believes that it is in compliance in all material respects with the requirements of the HIPAA Privacy Rule.
The Security Rule establishes requirements for safeguarding patient information that is electronically transmitted or electronically stored. The Company believes that it is in compliance in all material respects with the requirements of the HIPAA Security Rule.
The U.S. Health Information Technology for Economic and Clinical Health Act (HITECH), which was enacted in February 2009, strengthens and expands the HIPAA Privacy and Security Rules and their restrictions on use and disclosure of PHI. HITECH includes, but is not limited to, prohibitions on exchanging PHI for remuneration and additional restrictions on the use of PHI for marketing. HITECH also fundamentally changes a business associate’s obligations by imposing a number of Privacy Rule requirements and a majority of Security Rule provisions directly on business associates that were previously only directly applicable to covered entities. Moreover, HITECH requires covered entities to provide notice to individuals, HHS, and, as applicable, the media when unsecured PHI is breached, as that term is defined by HITECH. Business associates are similarly required to notify covered entities of a breach. The omnibus HIPAA regulation implementing most of the HITECH provisions was issued in January 2013 and made significant changes to the HIPAA Privacy, Security, Enforcement, and Breach Notification Rules. Compliance with most of the changes became required on September 23, 2013. The Company believes its policies and procedures are fully compliant with the HITECH requirements.
On February 6, 2014, CMS and HHS published final regulations that amended the HIPAA Privacy Rule to provide individuals (or their personal representatives) with the right to receive copies of their test reports from laboratories subject to HIPAA, or to request that copies of their test reports be transmitted to designated third parties. Previously laboratories that were CLIA-certified or CLIA-exempt were not subject to the provision in the Privacy Rule that provides individuals with the right of access to PHI. The HIPAA Privacy Rule amendment resulted in the preemption of a number of state laws that prohibit a laboratory from releasing a test report directly to the individual. The Company revised its policies and procedures to comply with these new access requirements and has updated its privacy notice to reflect individuals’ new access rights under this final rule.
The Standard Unique Employer Identifier Rule requires that employers have standard national numbers that identify them on standard transactions. The Employer Identification Number (also known as a Federal Tax Identification Number) issued by the Internal Revenue Service was selected as the identifier for employers and was adopted effective July 30, 2002. The Company believes it is in compliance with these requirements.
The administrative simplification provisions of HIPAA mandate the adoption of standard unique identifiers for healthcare providers. The intent of these provisions is to improve the efficiency and effectiveness of the electronic transmission of health information. The National Provider Identifier Rule requires that all HIPAA-covered healthcare providers, whether they are individuals or organizations, must obtain a National Provider Identifier (NPI) to identify themselves in standard HIPAA transactions. NPI replaces the unique provider identification number and other provider numbers previously assigned by payers and other entities - for the purpose of identifying providers in standard electronic transactions. The Company believes that it is in compliance with the HIPAA National Provider Identification Rule in all material respects.
22
The total cost associated with meeting the requirements of HIPAA and HITECH is not expected to be material to the Company’s operations or cash flows. However, future regulations and interpretations of HIPAA and HITECH could impose significant costs on the Company.
In addition to the HIPAA regulations described above, there are a number of other national, state and foreign laws regarding the confidentiality and security of medical information, some of which apply to clinical laboratories and CROs. These laws vary widely but they most commonly regulate or restrict the collection, use and disclosure of medical and financial information and other personal information. In some cases, state laws are more restrictive and, therefore, are not preempted by HIPAA. Penalties for violation of these laws may include sanctions against a laboratory's licensure, as well as civil and/or criminal penalties. Violations of the HIPAA provisions could result in civil and/or criminal penalties, including significant fines and up to 10 years in prison. HITECH also significantly strengthened HIPAA enforcement by increasing the civil penalty amounts that may be imposed, requiring HHS to conduct periodic audits to confirm compliance and authorizing state attorneys general to bring civil actions seeking either injunctions or damages in response to violations of the HIPAA privacy and security regulations that affect the privacy of state residents. Additionally, numerous other countries have similar laws governing the collection, use, disclosure and transmission of personal and/or patient information.
Fraud and Abuse Laws and Regulations
Existing U.S. laws governing federal healthcare programs, including Medicare and Medicaid, as well as similar state laws, impose a variety of broadly described fraud and abuse prohibitions on healthcare providers, including clinical laboratories. These laws are interpreted liberally and enforced aggressively by multiple government agencies, including the U.S. Department of Justice, HHS’ Office of Inspector General (OIG), and various state agencies. Historically, the clinical laboratory industry has been the focus of major governmental enforcement initiatives. The U.S. government's enforcement efforts have been increasing over the past decade, in part as a result of the enactment of HIPAA, which included several provisions related to fraud and abuse enforcement, including the establishment of a program to coordinate and fund U.S., state and local law enforcement efforts. The Deficit Reduction Act of 2005 also included new requirements directed at Medicaid fraud, including increased spending on enforcement and financial incentives for states to adopt false claims act provisions similar to the U.S. False Claims Act. Recent amendments to the False Claims Act, as well as other enhancements to the U.S. fraud and abuse laws enacted as part of the ACA, are widely expected to further increase fraud and abuse enforcement efforts. For example, the ACA established an obligation to report and refund overpayments from Medicare within 60 days of identification; failure to comply with this new requirement can give rise to additional liability under the False Claims Act and Civil Monetary Penalties statute. On February 16, 2012, CMS issued a proposed rule to establish regulations addressing the reporting and returning of overpayments. On February 11, 2016, CMS issued the final rule defining when an overpayment is identified and adopted a six-year lookback period. The rule is effective 30 days from the publication date.
The U.S. healthcare programs' Anti-Kickback Statute prohibits knowingly providing anything of value in return for, or to induce the referral of, Medicare, Medicaid or other U.S. healthcare program business. Violations can result in imprisonment, fines, penalties, and/or exclusion from participation in U.S. healthcare programs. The OIG has published “safe harbor” regulations which specify certain arrangements that are protected from prosecution under the Anti-Kickback Statute if all conditions of the relevant safe harbor are met. Failure to fit within a safe harbor does not necessarily constitute a violation of the Anti-Kickback Statute; rather, the arrangement would be subject to scrutiny by regulators and prosecutors and would be evaluated on a case by case basis. Many states have their own Medicaid anti-kickback laws and several states also have anti-kickback laws that apply to all payers (i.e., not just government healthcare programs).
From time to time, the OIG issues alerts and other guidance on certain practices in the healthcare industry that implicate the Anti-Kickback Statute or other fraud and abuse laws. Examples of such guidance documents particularly relevant to the Company and its operations follow.
In October 1994, the OIG issued a Special Fraud Alert on arrangements for the provision of clinical laboratory services. The Fraud Alert set forth a number of practices allegedly engaged in by some clinical laboratories and healthcare providers that raise issues under the U.S. fraud and abuse laws, including the Anti-Kickback Statute. These practices include: (i) providing employees to furnish valuable services for physicians (other than collecting patient specimens for testing) that are typically the responsibility of the physicians’ staff; (ii) offering certain laboratory services at prices below fair market value in return for referrals of other tests which are billed to Medicare at higher rates; (iii) providing free testing to physicians’ managed care patients in situations where the referring physicians benefit from such reduced laboratory utilization; (iv) providing free pick-up and disposal of bio-hazardous waste for physicians for items unrelated to a laboratory’s testing services; (v) providing general-use facsimile machines or computers to physicians that are not exclusively used in connection with the laboratory services; and (vi) providing free testing for healthcare providers, their families and their employees (i.e., so-called “professional courtesy” testing). The OIG emphasized in the Special Fraud Alert that when one purpose of such arrangements is to induce referrals of program-reimbursed laboratory
23
testing, both the clinical laboratory and the healthcare provider (e.g., physician) may be liable under the Anti-Kickback Statute, and may be subject to criminal prosecution and exclusion from participation in the Medicare and Medicaid programs. More recently, in June 2014, the OIG issued another Special Fraud Alert addressing compensation paid by laboratories to referring physicians for blood specimen processing and for submitting patient data to registries. This Special Fraud Alert reiterates the OIG's long-standing concerns about payments from laboratories to physicians in excess of the fair market value of the physician's services and payments that reflect the volume or value of referrals of federal U.S. program business.
Another issue the OIG has expressed concern about involves the provision of discounts on laboratory services billed to customers in return for the referral of U.S. healthcare program business. In a 1999 Advisory Opinion, the OIG concluded that a proposed arrangement whereby a laboratory would offer physicians significant discounts on non-U.S. healthcare program laboratory tests might violate the Anti-Kickback Statute. The OIG reasoned that the laboratory could be viewed as providing such discounts to the physician in exchange for referrals by the physician of business to be billed by the laboratory to Medicare at non-discounted rates. The OIG indicated that the arrangement would not qualify for protection under the discount safe harbor to the Anti-Kickback Statute because Medicare and Medicaid would not get the benefit of the discount. Similarly, in a 1999 correspondence, the OIG stated that if any direct or indirect link exists between a discount that a laboratory offers to a skilled nursing facility for tests covered under Medicare’s payments to the skilled nursing facility and the referral of tests billable by the laboratory under Medicare Part B, then the Anti-Kickback Statute would be implicated.
The OIG also has issued guidance regarding joint venture arrangements that may be viewed as suspect under the Anti-Kickback Statute. These documents have relevance to clinical laboratories that are part of (or are considering establishing) joint ventures with potential sources of U.S. healthcare program business. The first guidance document, which focused on investor referrals to such ventures was issued in 1989 and another concerning contractual joint ventures was issued in April 2003. Some of the elements of joint ventures that the OIG identified as “suspect” include: arrangements in which the capital invested by the physicians is disproportionately small and the return on investment is disproportionately large when compared to a typical investment; specific selection of investors who are in a position to make referrals to the venture; and arrangements in which one of the parties to the joint venture expands into a line of business that is dependent on referrals from the other party (sometimes called “shell” joint ventures). In a 2004 advisory opinion, the OIG expressed concern about a proposed joint venture in which a laboratory company would assist physician groups in establishing off-site pathology laboratories. The OIG indicated that the physicians' financial and business risk in the venture was minimal and that the physicians would contract out substantially all laboratory operations, committing very little in the way of financial, capital, or human resources. The OIG was unable to exclude the possibility that the arrangement was designed to permit the laboratory to pay the physician groups for their referrals, and, therefore, was unwilling to find that the arrangement fell within a safe harbor or had sufficient safeguards to protect against fraud or abuse.
Violations of other fraud and abuse laws can also result in exclusion from participation in U.S. healthcare programs, including Medicare and Medicaid. One basis for such exclusion is an individual or entity’s submission of claims to Medicare or Medicaid that are substantially in excess of that individual or entity’s usual charges for like items or services. In 2003, the OIG issued a notice of proposed rulemaking that would have defined the terms “usual charges” and “substantially in excess” in ways that might have required providers, including LCD, to either lower their charges to Medicare and Medicaid or increase charges to certain other payers to avoid the risk of exclusion. On June 18, 2007, however, the OIG withdrew the proposed rule, saying it preferred to continue evaluating billing patterns on a case-by-case basis. In its withdrawal notice, the OIG also said it “remains concerned about disparities in the amounts charged to Medicare and Medicaid when compared to private payers,” that it continues to believe its exclusion authority for excess charges “provides useful backstop protection for the public fisc from providers that routinely charge Medicare or Medicaid substantially more than their other customers” and that it will continue to use “all tools available … to address instances where Medicare or Medicaid are charged substantially more than other payers.” Thus, although the OIG did not proceed with its rulemaking, an enforcement action under this statutory exclusion basis is possible and, if pursued, could have an adverse effect on the Company. The enforcement by Medicaid officials of similar state law restrictions also could have a material adverse effect on the Company.
Under another U.S. statute, known as the Stark Law or “self-referral” prohibition, physicians who have a financial or a compensation relationship with a clinical laboratory may not, unless an exception applies, refer Medicare patients for testing to the laboratory, regardless of the intent of the parties. Similarly, laboratories may not bill Medicare for services furnished pursuant to a prohibited self-referral. There are several Stark Law exceptions that are relevant to arrangements involving clinical laboratories, including: i) fair market value compensation for the provision of items or services; ii) payments by physicians to a laboratory for clinical laboratory services; iii) an exception for certain ancillary services (including laboratory services) provided within the referring physician's own office, if certain criteria are satisfied; iv) physician investment in a company whose stock is traded on a public exchange and has stockholder equity exceeding $75.0 million; and v) certain space and equipment rental arrangements that are set at a fair market value rate and satisfy other requirements. All of the requirements of a Stark Law exception must be met in order for the exception to apply. Many states have their own self-referral laws as well, which in some cases apply to all patient referrals, not just Medicare.
24
There are a variety of other types of U.S. and state fraud and abuse laws, including laws prohibiting submission of false or fraudulent claims. The Company seeks to conduct its business in compliance with all U.S. and state fraud and abuse laws. The Company is unable to predict how these laws will be applied in the future, and no assurances can be given that its arrangements will not be subject to scrutiny under such laws. Sanctions for violations of these laws may include exclusion from participation in Medicare, Medicaid and other U.S. or state healthcare programs, significant criminal and civil fines and penalties, and loss of licensure. Any exclusion from participation in a U.S. healthcare program, or any loss of licensure, arising from any action by any U.S. or state regulatory or enforcement authority, would likely have a material adverse effect on the Company's business. In addition, any significant criminal or civil penalty resulting from such proceedings could have a material adverse effect on the Company's business.
Environmental, Health and Safety
The Company is subject to licensing and regulation under national, state and local laws and regulations relating to the protection of the environment, and human health and safety and laws and regulations relating to the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials. All Company laboratories are subject to applicable laws and regulations relating to biohazard disposal of all laboratory specimens and the Company generally utilizes outside vendors for disposal of such specimens. In addition, the U.S. Occupational Safety and Health Administration (OSHA) has established extensive requirements relating to workplace safety for healthcare employers, including clinical laboratories, whose workers may be exposed to blood-borne pathogens such as HIV, HCV and the hepatitis B virus (HCB). These regulations, among other things, require work practice controls, protective clothing and equipment, training, medical follow-up, vaccinations and other measures designed to minimize exposure to, and transmission of, blood-borne pathogens. Other countries where the Company conducts business have similar laws concerning the environment and human health and safety with which the Company must also comply.
In 2012, the OSHA Hazard Communication Standard was revised based on the adoption of the Globally Harmonized System (GHS) that provides criteria for the classification of chemical hazards. Updated copies of Safety Data Sheets for chemical products used across the Company were obtained prior to the effective date of June 1, 2015.
The Company seeks to comply with all relevant environmental and human health and safety laws and regulations. Failure to comply could subject the Company to various administrative and/or other enforcement actions.
Drug Testing
Drug testing for public sector employees is regulated by the U.S. Substance Abuse and Mental Health Services Administration (SAMHSA), which has established detailed performance and quality standards that laboratories must meet to be approved to perform drug testing on employees of U.S. government contractors and certain other entities. To the extent that the Company’s laboratories perform such testing, each must be certified as meeting SAMHSA standards. The Company’s laboratories in Research Triangle Park, N.C; Raritan, N.J.; Houston, Texas; Southaven, Miss; and St. Paul, Minn are all SAMHSA certified.
Controlled Substances
The use of controlled substances in testing for drugs of abuse is regulated by the U.S. Drug Enforcement Administration.
Compliance Program
The Company maintains a comprehensive, company-wide compliance program and continuously evaluates and monitors its compliance with all Medicare, Medicaid, FDA, and other rules and regulations in the U.S. and the other countries where the Company has operations. The objective of the Company’s compliance program is to develop, implement and update compliance safeguards as necessary. Emphasis is placed on developing compliance policies and guidelines, personnel training programs and various monitoring and audit procedures to achieve implementation and maintain compliance with all applicable rules and regulations.
The Company seeks to conduct its business in compliance with all statutes, regulations, and other requirements applicable to its clinical laboratory operations and drug development business. The clinical laboratory testing industry and drug development industries are, however, subject to extensive regulation, and many of these statutes and regulations have not been interpreted by the courts. There can be no assurance that applicable statutes and regulations will not be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that would adversely affect the Company. Potential sanctions for violation of these statutes and regulations include significant fines and the loss of various licenses, certificates, and authorizations, which could have a material adverse effect on the Company’s business.
25
Item 1A. | Risk Factors |
Investors should carefully consider all of the information set forth in this report, including the following risk factors, before deciding to invest in any of the Company’s securities. The risks below are not the only ones that the Company faces. Additional risks not presently known to the Company, or that the Company presently deems immaterial, may also negatively impact the Company. The Company’s business, consolidated financial condition, revenues, results of operations, profitability, reputation or cash flows could be materially impacted by any of these factors.
This report also includes forward-looking statements that involve risks or uncertainties. The Company’s results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks described below and elsewhere. See “Forward Looking Statements” in Item 7.
Changes in payer regulations or policies (or in the interpretation of current regulations or policies), insurance regulation or approvals or changes in other laws, regulations or policies may adversely affect governmental and third-party coverage and reimbursement for clinical laboratory testing and may have a material adverse effect upon the Company.
Government payers, such as Medicare and Medicaid, as well as insurers, including MCOs, have increased their efforts to control the cost, utilization and delivery of healthcare services. From time to time, Congress has considered and implemented changes in Medicare fee schedules in conjunction with budgetary legislation. Further reductions of reimbursement for Medicare and Medicaid services or changes in policy regarding coverage of tests or other requirements for payment, such as prior authorization or a physician or qualified practitioner’s signature on test requisitions, may be implemented from time to time. Reimbursement for pathology services performed by LCD is also subject to statutory and regulatory reduction. Reductions in the reimbursement rates and changes in payment policies of other third-party payers may occur as well. Such changes in the past have resulted in reduced payments as well as added costs and have decreased test utilization for the clinical laboratory industry by adding more complex new regulatory and administrative requirements. Further changes in third-party payer regulations or policies may have a material adverse impact on LCD's business. Actions by agencies regulating insurance or changes in other laws, regulations, or policies may also have a material adverse effect upon LCD's business.
The Company could face significant monetary damages and penalties and/or exclusion from the Medicare and Medicaid programs if it violates healthcare anti-fraud and abuse laws.
The Company is subject to extensive government regulation at the national, state and local levels. The Company’s failure to meet governmental requirements under these regulations, including those relating to billing practices and financial relationships with physicians and hospitals, could lead to civil and criminal penalties, exclusion from participation in Medicare and Medicaid and possible prohibitions or restrictions on the use of its laboratories. While the Company believes that it is in material compliance with all statutory and regulatory requirements, there is a risk that government authorities might take a contrary position. Such occurrences, regardless of their outcome, could damage the Company’s reputation and adversely affect important business relationships it has with third parties.
The Company’s business could be harmed from the loss or suspension of a license or imposition of a fine or penalties under, or future changes in, or interpretations of, the law or regulations of the Clinical Laboratory Improvement Act of 1967, and the Clinical Laboratory Improvement Amendments of 1988, or those of Medicare, Medicaid or other national, state or local agencies.
The clinical laboratory testing industry is subject to extensive regulation, and many of these statutes and regulations have not been interpreted by the courts. CLIA extends federal oversight to virtually all clinical laboratories operating in the U.S. by requiring that they be certified by the federal government or by a federally-approved accreditation agency. The sanction for failure to comply with CLIA requirements may be suspension, revocation or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as significant fines and/or criminal penalties. In addition, the Company is subject to regulation under state law. State laws may require that laboratories and/or laboratory personnel meet certain qualifications, specify certain quality controls or require maintenance of certain records.
Applicable statutes and regulations could be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that would adversely affect the Company's business. Potential sanctions for violation of these statutes and regulations include significant fines and the suspension or loss of various licenses, certificates and authorizations, which could have a material adverse effect on the Company’s business. In addition, compliance with future legislation could impose additional requirements on the Company which may be costly.
26
FDA regulation of diagnostic products and increased FDA regulation of LDTs could result in increased costs and the imposition of fines or penalties and have a material adverse effect upon the Company’s business.
The FDA has regulatory responsibility for instruments, test kits, reagents and other devices used by clinical laboratories. The FDA enforces laws and regulations that govern the development, testing, manufacturing, performance, labeling, advertising, marketing, distribution and surveillance of diagnostic products and regularly inspects and reviews the manufacturing processes and product performance of diagnostic products. LCD’s point-of-collection testing devices are subject to regulation by the FDA.
There are other regulatory and legislative proposals that would increase general FDA oversight of clinical laboratories and LDTs. On July 26, 2007, the FDA issued Draft Guidance for Industry, Clinical Laboratories, and FDA Staff: In Vitro Diagnostic Multivariate Index Assays. The guidance proposed certain changes to the agency's general past practice regarding the regulation of certain LDTs and announced that most devices deemed to be In Vitro Diagnostic Multivariate Index Assays (IVDMIAs) would either be Class II or Class III devices, although it is possible that an IVDMIA for a low-risk indication could be class I. Class II medical devices typically require FDA clearance or a premarket notification submission. Class III devices require the submission of an application for Premarket Approval (PMA). On October 3, 2014, the FDA published two additional draft guidance documents: Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs) which provides an overview of how FDA would regulate LDTs through a risk-based approach and FDA Notification and Medical Device Reporting for Laboratory Developed Tests which describes the process for clinical laboratories to notify the FDA of the LDTs they "manufacture" and describes the Medical Device Reporting (MDR) requirements for LDTs. On May 28, 2015, and October 22, 2015, the House Energy and Commerce Health Subcommittee released discussion drafts of a bill that would reform oversight of in vitro clinical tests (IVCTs), including both LDTs and test kits, under a new regulatory framework in which FDA would regulate IVCTs under a new category separate from medical devices, and regulation of laboratories by the Centers for Medicare and Medicaid Services (CMS) under the Clinical Laboratory Improvement Amendments (CLIA) would be modernized. On November 16, 2015, the FDA issued a report titled, The Public Health Evidence for FDA Oversight of Laboratory Developed Tests: 20 Case Studies (LDT Report). The LDT Report compiles 20 case studies involving LDTs where FDA alleges that non-compliance with FDA regulations led to serious issues, such as false positive or false-negative results, causing potential or actual harm to patients. On December 29, 2015, FDA published notice of its intent to finalize guidance on its policy for regulatory oversight of LDTs in 2016. The outcome and ultimate impact of such proposals on the business is difficult to predict at this time.
Current FDA regulation of the Company’s diagnostic products and potential future increased regulation of the Company’s LDTs could result in increased costs and administrative and legal actions for noncompliance, including warning letters, fines, penalties, product suspensions, product recalls, injunctions and other civil and criminal sanctions which could have a material adverse effect upon the Company.
Failure to comply with environmental, health and safety laws and regulations, including the Occupational Safety and Health Administration Act and the Needlestick Safety and Prevention Act, could result in fines and penalties and loss of licensure, and have a material adverse effect upon the Company’s business.
As previously discussed Item 1 of Part I of this report, the Company is subject to licensing and regulation under laws and regulations relating to the protection of the environment and human health and safety, including laws and regulations relating to the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials, as well as regulations relating to the safety and health of laboratory employees. Failure to comply with these laws and regulations could subject the Company to denial of the right to conduct business, fines, criminal penalties and/or other enforcement actions which would have a material adverse effect on its business. In addition, compliance with future legislation could impose additional requirements on the Company that may be costly.
Regulations requiring the use of standard transactions for healthcare services issued under HIPAA may negatively impact the Company’s profitability and cash flows.
Pursuant to HIPAA, the Secretary of HHS has issued regulations designed to improve the efficiency and effectiveness of the healthcare system by facilitating the electronic exchange of information in certain financial and administrative transactions while protecting the privacy and security of the information exchanged.
The HIPAA transaction standards are complex, and subject to differences in interpretation by payers. For instance, some payers may interpret the standards to require the Company to provide certain types of information, including demographic information not usually provided to the Company by physicians. In addition, new requirements for additional standard transactions, such as claims attachments, and the ICD-10-CM Code Set, could prove technically difficult, time-consuming or expensive to implement. As a result of inconsistent application of other transaction standards by payers or the Company’s inability to obtain certain billing information not usually provided to the Company by physicians, the Company could face increased costs and complexity, a temporary disruption in receipts and ongoing reductions in reimbursements and net revenues. The Company is working closely
27
with its payers to establish acceptable protocols for claim submission and with its trade association and an industry coalition to present issues and problems as they arise to the appropriate regulators and standards setting organizations.
Failure to maintain the security of customer-related information or compliance with security requirements could damage the Company’s reputation with customers, cause it to incur substantial additional costs and to become subject to litigation.
The Company receives certain personal and financial information about its customers. In addition, the Company depends upon the secure transmission of confidential information over public networks, including information permitting cashless payments. A compromise in the Company’s security systems that results in customer personal information being obtained by unauthorized persons or the Company’s failure to comply with security requirements for financial transactions could adversely affect the Company’s reputation with its customers and others, as well as the Company’s results of operations, financial condition and liquidity. It could also result in litigation against the Company and the imposition of fines and penalties.
Failure of the Company, third party payers or physicians to comply with the ICD-10-CM Code Set could negatively impact the Company's reimbursement, profitability and cash flow.
The Company believes that it is in compliance in all material respects with the current Transactions and Code Sets Rule. The Company implemented Version 5010 of the HIPAA Transaction Standards and believes it has fully adopted the ICD-10-CM Code Set. Clinical laboratories are typically required to submit healthcare claims with diagnosis codes to third party payers. The diagnosis codes must be obtained from the ordering physician. The failure of the Company, third party payers or physicians to apply the new code set could have an adverse impact on reimbursement, days sales outstanding and cash collections.
Failure to comply with privacy and security laws and regulations could result in fines, penalties and damage to the Company’s reputation with customers and have a material adverse effect upon the Company’s business.
The HIPAA privacy and security regulations, including the expanded requirements under HITECH, establish comprehensive standards with respect to the use and disclosure of PHI by covered entities, in addition to setting standards to protect the confidentiality, integrity and security of PHI. The regulations establish a complex regulatory framework on a variety of subjects, including:
• | the circumstances under which the use and disclosure of PHI are permitted or required without a specific authorization by the patient, including but not limited to treatment purposes, activities to obtain payments for the Company’s services, and its healthcare operations activities; |
• | a patient’s rights to access, amend and receive an accounting of certain disclosures of PHI; |
• | the content of notices of privacy practices for PHI; |
• | administrative, technical and physical safeguards required of entities that use or receive PHI; and |
• | the protection of computing systems maintaining electronic PHI. |
The Company has implemented policies and procedures designed to comply with the HIPAA privacy and security requirements as applicable. The privacy and security regulations establish a “floor” and do not supersede state laws that are more stringent. Therefore, the Company is required to comply with both additional federal privacy and security regulations and varying state privacy and security laws. In addition, for data transfers from and operations in other countries, the Company may also be required to comply with the data privacy and security laws of those other countries. HIPAA restricts the Company’s ability to use or disclose patient identifiable laboratory data, without patient authorization, for purposes other than payment, treatment or healthcare operations (as defined by HIPAA), except for disclosures for various public policy purposes and other permitted purposes outlined in the privacy regulations. HIPAA, as amended by HITECH, provides for significant fines and other penalties for wrongful use or disclosure of PHI in violation of the privacy and security regulations, including potential civil and criminal fines and penalties.
If the Company does not comply with existing or new laws and regulations related to protecting the privacy and security of personal or health information it could be subject to monetary fines, civil penalties or criminal sanctions. In addition, foreign, federal and state laws that protect the privacy and security of patient information may be subject to enforcement and interpretations by various governmental authorities and courts resulting in complex compliance issues. For example, the Company could incur damages under state laws pursuant to an action brought by a private party for the wrongful use or disclosure of health information or other personal information and in Europe both criminal and administrative sanctions are possible for violation of national implementations of the general data protection Directive 95/46/EC. In December 2015, the EU enacted a General Data Protection Regulation to replace Directive 95/46/EC, which is expected to take effect at some point in 2018, and which has enhanced penalties for noncompliance. The Company is evaluating its ability and the cost to comply with the new EU regulations, and as a result of that evaluation expects to make change to its business practices and incur additional costs.
28
Discontinuation or recalls of existing testing products; failure to develop, or acquire, licenses for new or improved testing technologies; or the Company’s customers using new technologies to perform their own tests could adversely affect the Company’s business.
From time to time, manufacturers discontinue or recall reagents, test kits or instruments used by the Company to perform laboratory testing. Such discontinuations or recalls could adversely affect the Company’s costs, testing volume and revenue.
The clinical laboratory industry is subject to changing technology and new product introductions. The Company’s success in maintaining a leadership position in genomic and other advanced testing technologies will depend, in part, on its ability to develop, acquire or license new and improved technologies on favorable terms and to obtain appropriate coverage and reimbursement for these technologies. The Company may not be able to negotiate acceptable licensing arrangements and it cannot be certain that such arrangements will yield commercially successful diagnostic tests. If the Company is unable to license these testing methods at competitive rates, its R&D costs may increase as a result. In addition, if the Company is unable to license new or improved technologies to expand its esoteric testing operations, its testing methods may become outdated when compared with the Company’s competition and testing volume and revenue may be materially and adversely affected.
In addition, advances in technology may lead to the development of more cost-effective technologies such as point-of-care testing equipment that can be operated by physicians or other healthcare providers in their offices or by patients themselves without requiring the services of freestanding clinical laboratories. Development of such technology and its use by the Company’s customers could reduce the demand for its laboratory testing services and negatively impact its revenues.
Currently, most clinical laboratory testing is categorized as “high” or “moderate” complexity, and thereby is subject to extensive and costly regulation under CLIA. The cost of compliance with CLIA makes it impractical for most physicians to operate clinical laboratories in their offices, and other laws limit the ability of physicians to have ownership in a laboratory and to refer tests to such a laboratory. Manufacturers of laboratory equipment and test kits could seek to increase their sales by marketing point-of-care laboratory equipment to physicians and by selling test kits approved for home or physician office use to both physicians and patients. Diagnostic tests approved for home use are automatically deemed to be “waived” tests under CLIA and may be performed in physician office laboratories as well as by patients in their homes with minimal regulatory oversight. Other tests meeting certain FDA criteria also may be classified as “waived” for CLIA purposes. The FDA has regulatory responsibility over instruments, test kits, reagents and other devices used by clinical laboratories and has taken responsibility from the Centers for Disease Control for classifying the complexity of tests for CLIA purposes. Increased approval of “waived” test kits could lead to increased testing by physicians in their offices or by patients at home, which could affect the Company’s market for laboratory testing services and negatively impact its revenues.
Healthcare reform and related products (e.g., Health Insurance Exchanges), changes in government payment and reimbursement systems, or changes in payer mix, including an increase in capitated reimbursement mechanisms and evolving delivery models, could have a material adverse impact on the Company's net revenues, profitability and cash flow.
LCD's testing services are billed to private patients, Medicare, Medicaid, commercial clients, MCOs and other insurance companies. Tests ordered by a physician may be billed to different payers depending on the medical insurance benefits of a particular patient. Most testing services are billed to a party other than the physician or other authorized person who ordered the test. Increases in the percentage of services billed to government and MCOs could have an adverse impact on the Company’s net revenues.
The various MCOs have different contracting philosophies, which are influenced by the design of the products they offer to their members. Some MCOs contract with a limited number of clinical laboratories and engage in direct negotiation of the rates reimbursed to participating laboratories. Other MCOs adopt broader networks with a largely uniform fee structure for participating clinical laboratories; in some cases, those fee structures are specific to independent clinical laboratories while the fees paid to hospital-based and physician-office laboratories may be different, and are typically higher. In addition, some MCOs have used capitation in an effort to fix the cost of laboratory testing services for their enrollees. Under a capitated reimbursement arrangement, the MCO makes a per member, per month payment for all covered laboratory tests rendered during the month for the members, regardless of the number or cost of the tests actually performed. Capitation shifts the risk of increased test utilization (and the underlying mix of testing services) to the clinical laboratory provider. The Company makes significant efforts to ensure that its services are adequately compensated in its capitated arrangements. For the year ended December 31, 2015, such capitated contracts accounted for approximately $219.9 million, or 2.6%, of the Company's net revenues.
The Company's ability to attract and retain MCOs is critical given the impact of healthcare reform, related products and expanded coverage (e.g., Health Insurance Exchanges and Medicaid Expansion) and evolving delivery models (e.g., ACOs).
29
A portion of the managed care fee-for-service revenues are collectible from patients in the form of deductibles, copayments and coinsurance. Collectibility may be impacted as patient cost-sharing increases.
In addition, Medicare and Medicaid and private insurers have increased their efforts to control the cost, utilization and delivery of healthcare services, including clinical laboratory services. Measures to regulate healthcare delivery in general, and clinical laboratories in particular, have resulted in reduced prices, added costs and decreased test utilization for the clinical laboratory industry by increasing complexity and adding new regulatory and administrative requirements. Pursuant to legislation passed in late 2003, the percentage of Medicare beneficiaries enrolled in Managed Medicare plans has increased. The percentage of Medicaid beneficiaries enrolled in Managed Medicaid plans has also increased, and is expected to continue to increase. Implementation of the ACA, the healthcare reform legislation passed in 2010, also may continue to affect coverage, reimbursement, and utilization of laboratory services, as well as administrative requirements.
The Company also experienced delays in the pricing and implementation of new molecular pathology codes among various payers, including Medicaid, Medicare and commercial carriers. While some delays were expected, several non-commercial payers required an extended period of time to price key molecular codes and a number of those payers, mostly government entities, indicated that they would no longer pay for tests that they had previously covered. Further, several payers continue to require additional information to process claims or have implemented prior authorization policies. Many commercial payers were delayed in becoming aware of the impact of their claim edits and policies, which impeded access to services that previously had been covered and reimbursed. These delays had a negative impact on 2014 revenue, revenue per requisition, margins and cash flows, which were largely sustained throughout 2015, and are expected to have a continuing negative impact. Similarly, CLFS coding and billing changes related to toxicology and other procedures were implemented in 2016 and Palmetto implemented a Drugs of Abuse Local Coverage Policy which impacted the handling of such procedures for Medicaid and MCOs. The Company experienced delays in the pricing and implementation of the new toxicology codes, however, largely overcame issues related to price and margins through direct negotiation with the associated payers. Further coding and billing changes related to toxicology and other procedure types are to be implemented in 2016. The Company expects delays in the pricing and implementation of these new codes and it is unclear what impact will be experienced related to price and margins.
The Company expects efforts to impose reduced reimbursement, more stringent payment policies and utilization and cost controls by government and other payers to continue. If LCD cannot offset additional reductions in the payments it receives for its services by reducing costs, increasing test volume and/or introducing new procedures, it could have a material adverse impact on the Company’s net revenues, profitability and cash flows. In 2014, Congress passed the PAMA, requiring Medicare to change the way payment rates are calculated for tests paid under the CLFS, and to base the payment on the weighted median of rates paid by private payers. On October 1, 2015, CMS published a proposed rule to implement PAMA. This rule proposes to require applicable laboratories, including LCD, to begin reporting their test-specific private payer payment amounts to CMS during the first quarter of 2016, which CMS would then use to calculate new CLFS rates that would be effective in 2017. The American Clinical Laboratory Association (ACLA) and the lab community provided extensive comments on the proposed rule and will continue to work with CMS to arrive at final regulation that accurately reflects the statutory language on applicable laboratories, and results in a reimbursement framework that reflects the broad scope of the laboratory market, encourages innovation, and maintains access to laboratory services for Medicare beneficiaries. The Company is continuing to evaluate the potential impact of the proposed rule.
As an employer, healthcare reform legislation also contains numerous regulations that will require the Company to implement significant process and record keeping changes to be in compliance. These changes increase the cost of providing healthcare coverage to employees and their families. Given the limited release of regulations to guide compliance, the exact impact to employers including the Company is uncertain.
In addition, implementation of healthcare reform legislation that adds costs could limit the profits that can be made from the development of new drugs. This could adversely affect R&D expenditures by pharmaceutical and biotechnology companies, which could in turn decrease the business opportunities available to CDD both in the U.S. and other countries. New laws or regulations may create a risk of liability, increase CDD costs or limit service offerings through CDD.
Changes in government regulation or in practices relating to the pharmaceutical industry could decrease the need for certain services that CDD provides.
CDD assists pharmaceutical and biotechnology companies in navigating the regulatory drug approval process. Changes in regulations such as a relaxation in regulatory requirements or the introduction of simplified drug approval procedures, or an increase in regulatory requirements that CDD has difficulty satisfying or that make its services less competitive, could eliminate or substantially reduce the demand for its services. Also, if government efforts to contain drug costs impact pharmaceutical and biotechnology company profits from new drugs, some of CDD’s customers may spend less, or reduce their growth in spending on R&D. If health insurers were to change their practices with respect to reimbursement for pharmaceutical products, some of
30
CDD’s customers may spend less, or reduce their growth in spending on R&D.
Failure to comply with the laws and regulations of the U.S. FDA and other drug regulatory agencies, such as the Medicines and Healthcare products Regulatory Agency in the U.K., the European Medicines Agency, the China Food and Drug Administration, and the Pharmaceuticals and Medical Devices Agency in Japan could result in sanctions and/or remedies against CDD and have a material adverse effect upon the Company.
The operation of CDD's preclinical laboratory facilities and clinical trial operations must conform at all times to GLP and GCP as well as all other applicable standards and regulations, respectively, as further described in "Business" in Item 1 of this report.
Additionally, certain CDD services and activities must conform to cGMP as further described in "Business" in Item 1 of this report. Failure to maintain compliance with GLP, GCP, or cGMP regulations and other applicable requirements of various regulatory agencies could result in warning letters, fines, unanticipated compliance expenditures, suspension of manufacturing, civil, criminal or administrative sanctions and/or remedies against CDD, including suspension of its laboratory operations, which could have a material adverse effect upon the Company.
Increased competition, including price competition, could have a material adverse impact on the Company’s net revenues and profitability.
Both LCD and CDD operate in highly competitive industries. The clinical laboratory business is intensely competitive both in terms of price and service. Pricing of laboratory testing services is often one of the most significant factors used by healthcare providers and third-party payers in selecting a laboratory. As a result of significant consolidation in the clinical laboratory industry, larger clinical laboratory providers are able to increase cost efficiencies afforded by large-scale automated testing. This consolidation results in greater price competition. LCD may be unable to increase cost efficiencies sufficiently, if at all, and as a result, its net earnings and cash flows could be negatively impacted by such price competition. The Company may also face increased competition from companies that do not comply with existing laws or regulations or otherwise disregard compliance standards in the industry. Additionally, the Company may also face changes in fee schedules, competitive bidding for laboratory services or other actions or pressures reducing payment schedules as a result of increased or additional competition.
Competitors in the CRO industry range from small providers to full service global CROs. CDD’s main competition consists of in-house departments of pharmaceutical companies, full-service and functional CROs, and, to a lesser degree, universities and teaching hospitals. CDD competes on a variety of factors, including:
• | reputation for on-time quality performance and regulatory compliance; |
• | expertise and experience in specific areas; |
• | scope of service offerings; |
• | strengths in various geographic markets; |
• | price; |
• | technological expertise and efficiency of drug development processes; |
• | quality of facilities; |
• | ability to acquire, process, analyze and report data in a rapid and accurate manner; |
• | quality of relationships; |
• | ability to manage large-scale clinical trials both domestically and internationally; |
• | expertise and experience in market access services; and |
• | size and scale. |
CDD’s services have from time to time experienced periods of increased price competition which had an adverse effect on a segment's profitability and consolidated net revenues and net income.
There is competition among CROs for both clients and potential acquisition candidates. Additionally, entities considering entering the CRO industry will find few barriers to entry, thus further increasing possible competition. These competitive pressures may affect the attractiveness of CDD’s services and could adversely affect its financial results and the financial results of the Company.
A failure to obtain and retain new customers, a loss of existing customers or material contracts, a reduction in tests ordered or specimens submitted by existing customers, or the inability to retain existing and create new relationships with health systems could impact the Company’s ability to successfully grow its business.
To offset efforts by payers to reduce the cost and utilization of clinical laboratory services and to otherwise maintain and grow its business, the Company needs to obtain and retain new customers and business partners. In addition, a reduction in tests ordered
31
or specimens submitted by existing customers, a decrease in demand for the CDD's services from existing clients, or the loss of existing contracts, without offsetting growth in its customer base, could impact the Company's ability to successfully grow its business and could have a material adverse impact on the Company’s net revenues and profitability. The Company competes primarily on the basis of the quality of services, reporting and information systems, reputation in the medical community and the drug development industry, the pricing of services and ability to employ qualified personnel. The Company's failure to successfully compete on any of these factors could result in the loss of customers and a reduction in the Company's ability to expand its customer base.
In addition, as the broader healthcare industry trend of consolidation continues, including the acquisition of physician practices by health systems, relationships with hospital-based health systems and integrated delivery networks are becoming more important. LCD has a well-established base of relationships with those systems and networks, including collaborative agreements. LCD's inability to retain its existing relationships with those provider systems and networks and to create new relationships could impact its ability to successfully grow its business.
Continued and increased consolidation of managed care organizations, pharmaceutical companies, health systems, physicians and other customers could adversely affect the Company's business.
Many healthcare companies and providers, including MCOs, pharmaceutical companies, health systems and physician practices are consolidating through mergers, acquisitions, joint ventures and other types of transactions and collaborations. As the healthcare industry consolidates, competition to provide goods and services may become more intense. This competition and increased customer bargaining power may adversely affect the price and volume of the Company’s services.
LCD’s nutritional chemistry and food safety business exposes the Company to various risks, including liability for errors and omissions in work conducted for LCD clients.
LCD offers a range of product-development and product-integrity services to food and beverage manufacturers and retailers, industry organizations and academic institutions. LCD expects to expand its nutritional chemistry and food safety business by leveraging the Company’s expertise in microbiology and its infrastructure to enable testing to be performed close to the food source. LCD also is exploring the possibility of developing point of care testing for food safety. These business offerings and opportunities expose the Company to many of the same, or similar, risks that are applicable to other business activities of the Company, including with respect to the operations of its facilities and the application of applicable laws and regulations. The agricultural, food, beverage and dietary supplement industries are continuing to gain attention of governments and regulators around the world, and regulations and applicable laws have increased in recent years. For example, the Company’s customers will be subject to new nutrition labeling regulations and new food manufacturing requirements, including regulations issued under the Food Safety Modernization Act (FSMA). With these enhanced requirements on the Company’s clients, there is an increased risk that errors in or omissions from nutritional analysis and food safety tests conducted by the Company for its clients could result in liability for the Company under client contracts. If LCD determines to further expand its nutritional chemistry and food safety testing business in the future beyond what is currently anticipated, LCD could become subject to additional standards and regulations, including under the FSMA, and could face additional liabilities resulting from new and pending regulatory and other legal decisions.
Changes or disruption in services or supplies provided by third parties, including transportation, could adversely affect the Company’s business.
The Company depends on third parties to provide services critical to the Company’s business. The Company's laboratories and certain of the Company's other businesses are heavily reliant on air travel for transport of clinical trial and diagnostic testing supplies and specimens, research products, and people, and a significant disruption to the air travel system, or the Company's access to it, could have a material adverse effect on the Company's business. CDD depends on a limited number of suppliers for certain services and for certain animal populations. Disruptions to the continued supply of these services, products or animal populations may arise from export/import restrictions or embargoes, political or economic instability, adverse weather, natural disaster or other causes. Disruption of supply could have a material adverse effect on the Company’s business.
Damage or disruption to the Company’s facilities could adversely affect the Company’s business.
Many of the Company’s facilities would be difficult to replace in a short period of time. Any event that causes a disruption of the operation of these facilities might impact the Company's ability to provide service to customers and, therefore, could have a material adverse effect on the Company's financial condition, results of operations and cash flows.
32
The Company bears financial risk for contracts that are underpriced, for which there are cost overruns or that may be delayed or terminated or reduced in scope for reasons beyond the Company’s control.
The Company has many contracts that are structured as fixed-price for fixed-contracted services or fee-for-service with a cap. The Company bears the financial risk if these contracts are underpriced or if contract costs exceed estimates. Such underpricing or significant cost overruns could have a material adverse effect on the Company's business, results of operations, financial condition and cash flows.
Many of CDD’s contracts, in particular, provide for services on a fixed price or fee-for-service with a cap basis and they may be terminated or reduced in scope either immediately or upon notice. Cancellations may occur for a variety of reasons, including:
• | the failure of products to satisfy safety requirements; |
• | unexpected or undesired results of the products; |
• | insufficient patient enrollment; |
• | insufficient investigator recruitment; |
• | the client’s decision to terminate the development of a product or to end a particular study; and |
• | CDD’s failure to perform its duties properly under the contract. |
Although its contracts often entitle it to receive the costs of winding down the terminated projects, as well as all fees earned up to the time of termination, the loss, reduction in scope or delay of a large contract or the loss, delay or conclusion of multiple contracts could materially adversely affect CDD.
Contract research services in the drug development industry create liability risks.
In contracting to work on drug development trials and studies, CDD faces a range of potential liabilities, including:
• | errors or omissions that create harm to study volunteers during a trial or to consumers of a drug after the trial is completed and regulatory approval of the drug has been granted; |
• | general risks associated with clinical pharmacology facilities, including negative consequences from the administration of drugs to clinical trial participants or the professional malpractice of clinical pharmacology medical care providers; |
• | risks that animals in CDD’s breeding facilities may be infected with diseases that may be harmful and even lethal to themselves and humans despite preventive measures contained in CDD's business policies, including those for the quarantine and handling of imported animals; and |
• | errors and omissions during a trial that may undermine the usefulness of a trial or data from the trial or study or may delay the entry of a drug to the market. |
CDD also contracts with physicians, also referred to as investigators, to conduct the clinical trials to test new drugs on patients and volunteers. These tests can create a risk of liability for personal injury or death to volunteers, resulting from negative reactions to the drugs administered or from professional malpractice by third party investigators.
While CDD endeavors to include in its contracts provisions entitling it to be indemnified or entitling it to a limitation of liability, these provisions do not uniformly protect CDD against liability arising from certain of its own actions, such as negligence or misconduct. CDD could be materially and adversely affected if it were required to pay damages or bear the costs of defending any claim that is not covered by a contractual indemnification provision or in the event that a party who must indemnify it does not fulfill its indemnification obligations, or in the event that the damages and costs exceed CDD's insurance coverage. There can be no assurance that CDD will be able to maintain sufficient insurance coverage on terms acceptable to it.
Adverse results in material litigation matters could have a material adverse effect upon the Company’s business.
The Company may become subject in the ordinary course of business to material legal action related to, among other things, intellectual property disputes, professional liability and employee-related matters. The Company may also receive inquiries and requests for information from governmental agencies and bodies, including Medicare or Medicaid carriers, requesting comment and/or information on allegations of billing irregularities or billing and pricing arrangements that are brought to their attention through billing audits or third parties. Legal actions could result in substantial monetary damages as well as damage to the Company’s reputation with customers, which could have a material adverse effect upon its business.
The Company's quarterly operating results may vary.
The Company's operating results, particularly for CDD, may vary significantly from quarter to quarter and are influenced by factors over which the Company has little control such as:
• | changes in the general global economy; |
• | exchange rate fluctuations; |
• | the commencement, completion, delay or cancellation of large projects or groups of projects; |
• | the progress of ongoing projects; |
33
• | the timing of and charges associated with completed acquisitions or other events; and |
• | changes in the mix of the Company's services. |
The Company believes that operating results for any particular quarter are not necessarily a meaningful indication of future results. While fluctuations in the Company's quarterly operating results could negatively or positively affect the market price of the Company's common stock, these fluctuations may not be related to the Company's future overall operating performance.
CDD’s revenues depend on the pharmaceutical and biotechnology industries.
CDD’s revenues depend greatly on the expenditures made by the pharmaceutical and biotechnology industries in R&D. In some instances, companies in these industries are reliant on their ability to raise capital in order to fund their R&D projects. Accordingly, economic factors and industry trends affecting CDD’s clients in these industries may also affect CDD. If companies in these industries were to reduce the number of R&D projects they conduct or outsource, whether through inability to raise capital, industry trends, economic conditions or otherwise, CDD could be materially adversely affected.
Actions of animal rights activists may have an adverse effect on the Company.
CDD's early development services utilize animals in preclinical testing of the safety and efficacy of drugs, and also breed and sell animals for biomedical research. Such activities are required for the development of new medicines and medical devices under regulatory regimes in the U.S., Europe, Japan and other countries. Acts of vandalism and other acts by animal rights activists who object to the use of animals in drug development could have an adverse effect on the Company.
Animal populations may suffer diseases that can damage CDD's inventory, harm its reputation, result in decreased sales of research products or result in other liability.
It is important that research products be free of diseases, including infectious diseases. The presence of diseases can distort or compromise the quality of research results, cause loss of animals in CDD’s inventory, result in harm to humans or outside animal populations if the disease is not contained to animals in inventory, or result in other losses. Such results could harm CDD’s reputation or have an adverse effect on CDD's financial condition, results of operations, and cash flows.
Failure to conduct animal research in compliance with the Animal Welfare Act could result in sanctions and/or remedies against CDD and have a material adverse effect upon the Company.
The conduct of animal research at CDD’s facilities must be in compliance with the AWA, which governs the care and use of warm-blooded animals used for research in the U.S. other than laboratory rats, mice and chickens, and is enforced through periodic inspections by the USDA. The AWA establishes facility standards regarding several aspects of animal welfare, including housing, ventilation, lighting, feeding and watering, handling, veterinary care and recordkeeping. CDD complies with licensing and registration requirement standards set by the USDA and similar agencies in foreign jurisdictions such as the European Union and China for the care and use of regulated species. If the USDA determines that CDD’s equipment, facilities, laboratories or processes do not comply with applicable AWA standards, it may issue an inspection report documenting the deficiencies and setting deadlines for any required corrective actions. For continued noncompliance, the USDA may impose fines, suspend and/or revoke animal research licenses, or confiscate research animals. Other countries where CDD conducts business may have similar laws with which CDD must also comply.
An inability to attract and retain experienced and qualified personnel could adversely affect the Company’s business.
The loss of key management personnel or the inability to attract and retain experienced and qualified employees at the Company’s clinical laboratories and drug development facilities could adversely affect the business. The success of the Company is dependent in part on the efforts of key members of its management team. Success in maintaining the Company’s leadership position in genomic and other advanced testing technologies and in drug development will depend in part on the Company’s ability to attract and retain skilled research professionals. In addition, the success of the Company’s clinical laboratories also depends on employing and retaining qualified and experienced laboratory professionals, including specialists, who perform clinical laboratory testing services. In the future, if competition for the services of these professionals increases, the Company may not be able to continue to attract and retain individuals in its markets. The Company’s revenues and earnings could be adversely affected if a significant number of professionals terminate their relationship with the Company or become unable or unwilling to continue their employment.
Unionization of employees, union strikes, work stoppages or failure to comply with labor or employment laws could adversely affect the Company's operations and have a material effect upon the Company's business.
The Company is a party to collective bargaining agreements with various labor unions and is subject to employment and labor laws and unionization activity in the U.S. and other countries in which it conducts business. Disputes with regard to the terms of
34
these agreements, its potential inability to negotiate acceptable contracts with these unions, unionization activity, or a failure to comply with labor or employment laws could result in, among other things, labor unrest, strikes, work stoppages, slowdowns by the affected workers, fines and penalities. If any of these events were to occur, or other employees were to become unionized, the Company could experience a significant disruption of its operations or higher ongoing labor costs, either of which could have a material adverse effect upon the Company's business. Additionally, future labor agreements, or renegotiation of labor agreements or provisions of labor agreements, or changes in labor labor or employment laws, could compromise its service reliability and significantly increase its costs, which could have a material adverse impact upon the Company's business.
A significant increase in LCD's days sales outstanding could increase bad debt expense and have an adverse effect on the Company’s business including its cash flow.
Billing for laboratory services is a complex process. Laboratories bill many different payers including doctors, patients, hundreds of insurance companies, Medicare, Medicaid and employer groups, all of which have different billing requirements. In addition to billing complexities, LCD is experiencing increasing patient responsibility as a result of managed care fee-for-service plans which continue to increase deductibles, coinsurance and patient copayments. A material increase in LCD’s days sales outstanding level could have an adverse effect on the Company's business, including potentially increasing its bad debt rate and decreasing its cash flows.
Failure in the Company’s information technology systems or delays or failures in the development and implementation of the Company's LabCorp Beacon platform could significantly increase testing turn-around time or billing processes and otherwise disrupt the Company’s operations or customer relationships.
The Company’s laboratory operations and customer relationships depend, in part, on the continued performance of its information technology systems. Despite network security measures and other precautions the Company has taken, its information technology systems are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptions. In addition, the Company is in the process of integrating the information technology systems of its recently acquired subsidiaries, and the Company may experience system failures or interruptions as a result of this process. Sustained system failures or interruption of the Company’s systems in one or more of its operations could disrupt the Company’s ability to process laboratory requisitions, perform testing, provide test results or drug development data in a timely manner and/or bill the appropriate party. The Company is also continuing to enhance its LabCorp Beacon platform and could experience delays or deficiencies in the development process. Failure of the Company’s information technology systems could adversely affect the Company’s business, profitability and financial condition.
Operations may be disrupted and adversely impacted by the effects of natural disasters such as adverse weather and earthquakes, or acts of terrorism, or other criminal activities, or disease pandemics.
Such events may result in a temporary decline of testing volumes in both segments. In addition, such events may temporarily interrupt the Company’s ability to transport specimens, the Company's ability to register participants, the Company’s information technology systems, the Company’s ability to utilize certain laboratories, and/or the Company’s ability to receive material from its suppliers.
A significant deterioration in the economy could negatively impact testing volumes, cash collections and the availability of credit.
The Company’s operations are dependent upon ongoing demand for diagnostic testing services by patients, physicians, hospitals, MCOs and others. A significant downturn in the economy could negatively impact the demand for diagnostic testing as well as the ability of patients and other payers to pay for services ordered. In addition, uncertainty in the credit markets could reduce the availability of credit and impact the Company’s ability to meet its financing needs in the future.
Hardware and software failures, delays in the operation of computer and communications systems, the failure to implement system enhancements or cyber security breaches may harm the Company.
The Company's success depends on the efficient and uninterrupted operation of its computer and communications systems. A failure of the network or data gathering procedures could impede the processing of data, delivery of databases and services, client orders and day-to-day management of the business and could result in the corruption or loss of data. While certain operations have appropriate disaster recovery plans in place, there currently are not redundant facilities everywhere in the world to provide IT capacity in the event of a system failure. Despite any precautions the Company may take, damage from fire, floods, hurricanes, power loss, telecommunications failures, computer viruses, break-ins, cybersecurity breaches and similar events at the Company's various computer facilities could result in interruptions in the flow of data to the servers and from the servers to clients. In addition, any failure by the computer environment to provide required data communications capacity could result in interruptions in service.
35
In the event of a delay in the delivery of data, the Company could be required to transfer data collection operations to an alternative provider of server hosting services. Such a transfer could result in delays in the ability to deliver products and services to clients. Additionally, significant delays in the planned delivery of system enhancements, improvements and inadequate performance of the systems once they are completed could damage the Company's reputation and harm the business. Finally, long-term disruptions in the infrastructure caused by events such as natural disasters, the outbreak of war, the escalation of hostilities, acts of terrorism (particularly involving cities in which the Company has offices) and cybersecurity breaches could adversely affect the business. Although the Company carries property and business interruption insurance, the coverage may not be adequate to compensate for all losses that may occur.
Security breaches and unauthorized access to the Company or its customers’ data could harm the Company’s reputation and adversely affect its business.
Experienced computer programmers and hackers may be able to penetrate the Company’s layered security controls and misappropriate or compromise personal information or proprietary or confidential information, create system disruptions or cause shutdowns. They also may be able to develop and deploy viruses, worms and other malicious software programs that attack the Company’s systems or otherwise exploit any security vulnerabilities. Outside parties may also attempt to fraudulently induce employees to take actions, including the release of confidential or sensitive information or to make fraudulent payments, through illegal electronic spamming, phishing or other tactics. Although the Company believes that it has robust information security procedures and other safeguards in place, which are monitored and routinely tested internally and by external parties, because the techniques used to obtain unauthorized access, disable or degrade service, or sabotage systems change frequently and often are not recognized until launched against a target, the Company may be unable to anticipate all of these techniques or to implement adequate preventative measures. In addition, as cyber threats continue to evolve, the Company may be required to expend additional resources to continue to enhance the Company’s information security measures or to investigate and remediate any information security vulnerabilities. The Company’s remediation efforts may not be successful and could result in interruptions, delays or cessation of service. Breaches of the Company’s security measures and the unauthorized dissemination of personal information, proprietary or confidential information about the Company or its customers or other third-parties, could expose customers’ private information and could expose customers to the risk of financial or medical identity theft or expose the Company or other third-parties to a risk of loss or misuse of this information, result in litigation and potential liability for the Company, damage the Company’s brand and reputation or otherwise harm the Company’s business. Any of these disruptions or breaches of security could have a material adverse effect on the Company’s business, regulatory compliance, financial condition and results of operations.
Foreign currency exchange fluctuations and changes in reimbursement by foreign governments could have an adverse impact on the Company’s business.
The Company has business and operations outside the U.S. and CDD derives a large portion of its net revenues from international operations. Since the Company's consolidated financial statements are denominated in U.S. Dollars, fluctuations in exchange rates from period to period will have an impact on reported results. In addition, in certain circumstances, CDD may incur costs in one currency related to its services or products for which it is paid in a different currency. As a result, factors associated with international operations, including changes in foreign currency exchange rates, could significantly affect CDD's results of operations, financial condition and cash flows. Foreign currency exchange fluctuations and changes by foreign governments in reimbursement for LCD’s services could have an adverse impact on the Company’s business.
The Company's growing international operations could subject it to additional risks and expenses that could adversely impact the business or results of operations.
The expansion of the Company's international operations exposes it to risks from failure to comply with foreign laws and regulations that differ from those under which the Company operates in the U.S. In addition, the Company may be adversely affected by other risks of expanded operations in foreign countries, including export controls and trade regulations, changes in tax policies or other foreign laws, foreign labor and employee relations laws and regulations, restrictions on currency repatriation, judicial systems that less strictly enforce contractual rights, countries that provide less protection for intellectual property rights, and procedures and actions affecting approval, production, pricing, reimbursement and marketing of products and services. Further, international operations could subject the Company to additional expenses that the Company may not fully anticipate, including those related to enhanced time and resources necessary to comply with foreign laws and regulations, difficulty in collecting accounts receivable and longer collection periods, and difficulties and costs of staffing and managing foreign operations. In some countries, the Company's success will depend in part on its ability to form relationships with local partners. The Company's inability to identify appropriate partners or reach mutually satisfactory arrangements could adversely affect the business and operations.
36
A failure to identify and successfully close and integrate strategic acquisition targets could have a material adverse impact on the Company's business objectives and its net revenues and profitability.
Part of the Company's strategy involves deploying capital in investments that enhance the Company's business, which includes pursuing strategic acquisitions to strengthen the Company's scientific capabilities and enhance therapeutic expertise, enhance esoteric testing and global drug development capabilities and increase presence in key geographic areas. Since 2010, the Company has invested net cash of approximately $5.8 billion and equity of $1.8 billion in strategic business acquisitions ($2.2 billion over the same period excluding the acquisition of Covance). However, the Company cannot assure that it will be able to identify acquisition targets that are attractive to the Company or that are of a large enough size to have a meaningful impact on the Company's operating results. Furthermore, the successful closing and integration of a strategic acquisition entails numerous risks, including, among others:
• | failure to obtain regulatory clearance, including due to antitrust concerns; |
• | loss of key customers or employees; |
• | difficulty in consolidating redundant facilities and infrastructure and in standardizing information and other systems; |
• | unidentified regulatory problems; |
• | failure to maintain the quality of services that such companies have historically provided; |
• | coordination of geographically-separated facilities and workforces; and |
• | diversion of management's attention from the day-to-day business of the Company. |
The Company cannot assure that current or future acquisitions, if any, or any related integration efforts will be successful, or that the Company's business will not be adversely affected by any future acquisitions, including with respect to net revenues and profitability. Even if the Company is able to successfully integrate the operations of businesses that it may acquire in the future, the Company may not be able to realize the benefits that it expects from such acquisitions.
The Company’s substantially increased indebtedness could adversely affect the Company’s liquidity, results of operations and business.
At December 31, 2015, indebtedness on the Company's outstanding Senior Notes totaled approximately $5,525.0 million in aggregate principal. The Company is also a party to credit agreements relating to a $1.0 billion revolving credit facility and a term loan with a principal balance of $715.0 million as of December 31, 2015. Under the term loan facility and the revolving credit facility, the Company is subject to negative covenants limiting subsidiary indebtedness and certain other covenants typical for investment grade-rated borrowers, and the Company is required to maintain a leverage ratio that declines over time.
The Company’s increased indebtedness could adversely affect its business. In particular, it could increase the Company’s vulnerability to sustained, adverse macroeconomic weakness, limit its ability to obtain further financing and limit its ability to pursue certain operational and strategic opportunities.
The Company may also enter into additional transactions or credit facilities, including other long-term debt, which may increase its indebtedness and result in additional restrictions upon the business. In addition, major debt rating agencies regularly evaluate the Company's debt based on a number of factors. There can be no assurance that the Company will be able to maintain its existing debt ratings, and failure to do so could adversely affect the Company's cost of funds, liquidity and access to capital markets.
Failure to successfully integrate the business of Covance or to realize the expected benefits of the Acquisition could have a material adverse impact on the Company’s business, net revenues and profitability and the market price of its common stock.
If the Company fails to successfully complete the integration of Covance into its existing operations or is not able to achieve the anticipated benefits of the Acquisition and integration, its business and results of operations could be negatively affected. In addition, it is possible that the ongoing integration process could result in the loss of key employees, errors or delays in systems implementation, the disruption of the Company’s ongoing business, inconsistencies in standards, controls, procedures and policies, or disruptions in its relationships with suppliers and other parties with which it deals that could adversely affect the Company’s ability to maintain relationships with customers and employees or to achieve the anticipated benefits of the acquisition. Integration efforts could also place a significant burden on the Company's management, employees and internal resources, which could otherwise have been devoted to other business opportunities and improvements.
The ongoing success of the transaction will depend, in significant part, on the Company’s ability to realize the anticipated benefits from the acquisition, including the opportunity for revenue growth in development and commercialization of drugs and diagnostics, nutritional analysis and other areas, including a number of new business areas for the Company. Actual revenue growth may be
37
lower than the Company expects and may take longer to achieve than anticipated, and expenses may be higher than the Company expects. The Company has made certain assumptions relating to the Acquisition and integration that may prove to be materially inaccurate, including:
• | the Company’s assessments of the asset quality and value of Covance and its assets; |
• | projections of the business and CDD's future financial performance; |
• | timing and total costs of integrating a large number of processes, policies, procedures, operations, technologies and systems; |
• | the Company’s ability to realize synergies and the timeline for doing so; |
• | the Company’s ability to develop, maintain and deepen relationships with CDD's customers; and |
• | other financial and strategic risks of the Acquisition. |
If one or more of these assumptions are incorrect, it could have a material adverse effect on the Company’s business and operating results, and the value of its common stock may be adversely affected.
In addition, although CDD is subject to many of the same risks and uncertainties that the Company faces in its business, the acquisition of Covance also involves the Company entering new product and services areas, markets and industries, which presents risks resulting from the Company’s relative inexperience in these new areas. CDD’s business could react differently to economic and other external factors than the Company’s. The Company faces the risk that it will not be successful with these new products and services or in these new markets.
Item 1B. UNRESOLVED STAFF COMMENTS
None.
38
Item 2. PROPERTIES
The Company's corporate headquarter are located in Burlington, NC and include facilities that are both owned and leased.
LCD operates through a network of primary laboratories, branches, PSCs and STAT laboratories. The table below summarizes certain information as to LCD's principal operating and administrative facilities as of December 31, 2015.
Location | Nature of Occupancy |
Primary Facilities: | |
Birmingham, Alabama | Leased |
Phoenix, Arizona | Owned |
Prescott, Arizona | Leased |
Calabasas, California | Leased |
Los Angeles, California | Leased |
San Diego, California | Leased |
San Francisco, California | Leased |
Tustin, California | Leased |
Englewood, Colorado | Leased |
Shelton, Connecticut | Leased |
Hollywood, Florida | Leased |
Tampa, Florida | Leased |
Chicago, Illinois | Leased |
Itasca, Illinois | Leased |
Westborough, Massachusetts | Leased |
Battle Creek, Michigan | Owned |
Roseville, Minnesota | Leased |
St. Paul, Minnesota | Owned |
Ewing, New Jersey | Leased |
Raritan, New Jersey | Owned |
Santa Fe, New Mexico | Owned |
New York, New York | Leased |
Burlington, North Carolina (3) | Owned/Leased |
Raleigh, North Carolina | Leased |
Research Triangle Park, North Carolina | Leased |
Dublin, Ohio | Owned |
Oklahoma City, Oklahoma | Leased |
Brentwood, Tennessee | Leased |
Knoxville, Tennessee | Leased |
Austin, Texas | Leased |
Dallas, Texas | Leased |
Houston, Texas | Leased |
San Antonio, Texas | Leased |
Herndon, Virginia | Leased |
Lorton, Virginia | Leased |
Seattle, Washington | Leased |
Abingdon, United Kingdom | Leased |
39
CDD operates on a global scale. The table below summarizes certain information as to CDD's principal operating and administrative facilities as of December 31, 2015.
Location | Nature of Occupancy |
Primary Facilities: | |
Mechelen, Belgium | Leased |
Beijing, China | Leased |
Shanghai, China (2) | Owned/Leased |
Muenster, Germany | Owned |
Singapore | Leased |
Harrogate, United Kingdom | Owned |
Leeds, United Kingdom | Owned |
Maidenhead, United Kingdom | Leased |
Indianapolis, Indiana | Leased |
Greenfield, Indiana | Owned |
Cranford, New Jersey | Leased |
Princeton, New Jersey | Leased |
West Trenton, New Jersey | Leased |
Denver, Pennsylvania | Owned |
Alice, Texas | Owned |
Chantilly, Virginia | Leased |
Cumberland, Virginia | Owned |
Geneva, Switzerland | Leased |
Madison, Wisconsin | Owned |
All of the Company’s primary laboratory and drug development facilities have been built or improved for the single purpose of providing clinical laboratory testing or drug development services. The Company believes that these facilities are suitable and adequate and have sufficient production capacity for its currently foreseeable level of operations. The Company believes that if it were unable to renew a lease or if a lease were to be terminated on any of the facilities it presently leases, it could find alternate space at competitive market rates and readily relocate its operations to such new locations without material disruption to its operations.
Item 3. | LEGAL PROCEEDINGS |
The Company is involved from time to time in various claims and legal actions, including arbitrations, class actions, and other litigation (including those described in more detail below), arising in the ordinary course of business. Some of these actions involve claims that are substantial in amount. These matters include, but are not limited to, intellectual property disputes, commercial and contract disputes, professional liability, breach of contract, employee-related matters and inquiries, including subpoenas and other civil investigative demands, from governmental agencies and Medicare or Medicaid payers and MCOs reviewing billing practices or requesting comment on allegations of billing irregularities that are brought to their attention through billing audits or third parties. The Company receives civil investigative demands or other inquiries from various governmental bodies in the ordinary course of its business. Such inquiries can relate to the Company or other healthcare providers. The Company works cooperatively to respond to appropriate requests for information.
The Company also is named from time to time in suits brought under the qui tam provisions of the False Claims Act and comparable state laws. These suits typically allege that the Company has made false statements and/or certifications in connection with claims for payment from U.S. or state healthcare programs. The suits may remain under seal (hence, unknown to the Company) for some time while the government decides whether to intervene on behalf of the qui tam plaintiff. Such claims are an inevitable part of doing business in the healthcare field today.
The Company believes that it is in compliance in all material respects with all statutes, regulations and other requirements applicable to its clinical laboratory operations and drug development support services. The healthcare diagnostics and drug development industries are, however, subject to extensive regulation, and the courts have not interpreted many of the applicable statutes and regulations. There can be no assurance, therefore, that the applicable statutes and regulations will not be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that would adversely affect the Company. Potential sanctions for violation of these statutes and regulations include significant fines, the loss of various licenses, certificates and authorizations, and/or exclusion from participation in government programs.
40
Many of the current claims and legal actions against the Company are in preliminary stages, and many of these cases seek an indeterminate amount of damages. The Company records an aggregate legal reserve, which is determined using actuarial calculations based on historical loss rates and assessment of trends experienced in settlements and defense costs. In accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification Topic 450 “Contingencies”, the Company establishes reserves for judicial, regulatory, and arbitration matters outside the aggregate legal reserve if and when those matters present loss contingencies that are both probable and estimable and would exceed the aggregate legal reserve. When loss contingencies are not both probable and estimable, the Company does not establish separate reserves.
The Company is unable to estimate a range of reasonably probable loss for the proceedings described in more detail below in which damages either have not been specified or, in the Company's judgment, are unsupported and/or exaggerated and (i) the proceedings are in early stages; (ii) there is uncertainty as to the outcome of pending appeals or motions; (iii) there are significant factual issues to be resolved; and/or (iv) there are novel legal issues to be presented. For these proceedings, however, the Company does not believe, based on currently available information, that the outcomes will have a material adverse effect on the Company's financial condition, though the outcomes could be material to the Company's operating results for any particular period, depending, in part, upon the operating results for such period.
As reported, the Company reached a settlement in the previously disclosed lawsuit, California ex rel. Hunter Laboratories, LLC et al. v. Quest Diagnostics Incorporated, et al. (Hunter Labs Settlement Agreement), to avoid the uncertainty and costs associated with prolonged litigation. Pursuant to the executed Hunter Labs Settlement Agreement, the Company recorded a litigation settlement expense of $34.5 million in the second quarter of 2011 (net of a previously recorded reserve of $15.0 million) and paid the settlement amount of $49.5 million in the third quarter of 2011. The Company also agreed to certain reporting obligations regarding its pricing for a limited time period and, at the option of the Company in lieu of such reporting obligations, to provide Medi-Cal with a discount from Medi-Cal's otherwise applicable maximum reimbursement rate from November 1, 2011, through October 31, 2012. In June of 2012, the California legislature enacted Assembly Bill No. 1494, Section 9 of which directed the Department of Healthcare Services (DHCS) to establish new reimbursement rates for Medi-Cal clinical laboratory services that will be based on payments made to California clinical laboratories for similar services by other third-party payers. With stakeholder input, DHCS established data elements and a format for laboratories to report payment data from comparable third-party payers. Laboratories reported payment data to DHCS in the summer of 2013. On March 28, 2014, Assembly Bill No. 1124 extended the implementation deadline of new regulations until June 30, 2016. Assembly Bill No. 1494 provides that until the new rates are set through this process, Medi-Cal payments for clinical laboratory services will be reduced (in addition to a 10% payment reduction imposed by Assembly Bill No. 97 in 2011) by “up to 10 percent” for tests with dates of service on or after July 1, 2012, with a cap on payments set at 80% of the lowest maximum allowance established under the Medicare program. In April 2015, the Centers for Medicare and Medicaid Services (CMS) approved a 10% payment reduction under Assembly Bill No. 1494. This cut, which applied retroactively to the period of July 1, 2012 through June 30, 2015, is being implemented through recoupments from weekly reimbursements at a rate of 5% of the amount owed until paid in full. Under the terms of the Hunter Labs Settlement Agreement, the enactment of this California legislation terminates the Company's reporting obligations (or obligation to provide a discount in lieu of reporting) under that agreement. In December 2014, DHCS announced at a stakeholder meeting the results of its analysis of payment data reported by laboratories in 2013 and its proposed rate methodology, on which it solicited stakeholder comments. The Company objected to the proposal by DHCS to exclude from the new rate calculations data on payments from comparable third-party payers exceeding 80% of Medicare reimbursement amounts and its proposal to impose the 10% payment reduction enacted in Assembly Bill No. 97 after calculation of the new rates. In January 2015, after receiving stakeholder comments, DHCS instructed laboratories to submit 2014 payment data by March 27, 2015, which DHCS used (except for data on payment amounts exceeding 80% of Medicare reimbursement) to establish new rates. DHCS obtained CMS approval of a state plan amendment adopting the new rate methodology and rates effective July 1, 2015, and posted the new rates on the DHCS website. DHCS intends to apply the 10% payment reduction referenced in Assembly Bill No. 97 to the new rates. Due to an initial implementation delay, the new rates were to be implemented prospectively until November 1, 2015. A recoupment to account for differences between the new rates and the rates paid for the period of July 1, 2015 through October 31, 2015 was to be implemented through recoupments applied to each weekly reimbursement. Therefore, until the recoupment was paid in full, the Company was not to receive a weekly reimbursement. All retroactive recoupments were expected to be completed by February 2016. However, due to further implementation delays, the new rates have not yet been implemented, and no recoupment has yet occurred. DHCS has instructed laboratories to submit 2015 payment data by March 18, 2016, which will be used to establish new rates effective July 1, 2016. DHHS has not provided further direction regarding possible recoupments. Taken together, these changes are not expected to have a material impact on the Company's consolidated revenues or results of operations.
As previously reported, the Company responded to an October 2007 subpoena from the U.S. Department of Health & Human Services Office of Inspector General's regional office in New York. On August 17, 2011, the Southern District of New York unsealed a False Claims Act lawsuit, United States of America ex rel. NPT Associates v. Laboratory Corporation of America Holdings, which alleges that the Company offered UnitedHealthcare kickbacks in the form of discounts in return for Medicare business. The Plaintiff's Third Amended Complaint further alleges that the Company's billing practices violated the False Claims
41
Acts of fourteen states and the District of Columbia. The lawsuit seeks actual and treble damages and civil penalties for each alleged false claim, as well as recovery of costs, attorney's fees, and legal expenses. Neither the U.S. government nor any state government has intervened in the lawsuit. The Company's Motion to Dismiss was granted in October 2014 and Plaintiff was granted the right to replead. On January 11, 2016, Plaintiff filed a motion requesting leave to file an amended complaint under seal and to vacate the briefing schedule for the Company's Motion to Dismiss, while the government reviews the amended complaint. The Court granted the motion and vacated the briefing dates. Plaintiff then filed an amended complaint under seal. The Company will vigorously defend the lawsuit.
In addition, the Company has received various other subpoenas since 2007 related to Medicaid billing. In October 2009, the Company received a subpoena from the State of Michigan Department of Attorney General seeking documents related to its billing to Michigan Medicaid. In June 2010, the Company received a subpoena from the State of Florida Office of the Attorney General requesting documents related to its billing to Florida Medicaid. In October 2013, the Company received a civil investigative demand from the State of Texas Office of the Attorney General requesting documents related to its billing to Texas Medicaid. The Company is cooperating with these requests.
On November 4, 2013, the State of Florida through the Office of the Attorney General filed an Intervention Complaint in a False Claims Act lawsuit, State of Florida ex rel. Hunter Laboratories, LLC and Chris Riedel v. Quest Diagnostics Incorporated, et al. in the Circuit Court for the Second Judicial Circuit for Leon County. The Intervention Complaint, originally filed by a competitor laboratory, alleges that the Company overcharged Florida’s Medicaid program. The lawsuit seeks actual and treble damages and civil penalties for each alleged false claim, as well as recovery of costs, attorney’s fees, and legal expenses. The Company's Motion to Dismiss was denied in February 2015. The Company will vigorously defend the lawsuit.
On May 2, 2013, the Company was served with a False Claims Act lawsuit, State of Georgia ex rel. Hunter Laboratories, LLC and Chris Riedel v. Quest Diagnostics Incorporated, et al., filed in the State Court of Fulton County, Georgia. The lawsuit, filed by a competitor laboratory, alleges that the Company overcharged Georgia's Medicaid program. The State of Georgia filed a Notice of Declination on August 13, 2012, before the Company was served with the Complaint. The case was removed to the United States District Court for the Northern District of Georgia. The lawsuit seeks actual and treble damages and civil penalties for each alleged false claim, as well as recovery of costs, attorney's fees, and legal expenses. On March 14, 2014, the Company's Motion to Dismiss was granted. The Plaintiffs repled their complaint and the Company filed a Motion to Dismiss the First Amended Complaint. In May 2015, the Court dismissed the Plaintiffs' anti-kickback claim and remanded the remaining state law claims to the State Court of Fulton County. In July 2015, the Company filed a Motion to Dismiss these remaining claims. The Plaintiffs filed an opposition to the Company's Motion to Dismiss in August 2015. Also, the State of Georgia filed a brief as amicus curiae. The Company will vigorously defend the lawsuit.
On February 27, 2012, the Company was served with a False Claims Act lawsuit, United States ex rel. Margaret Brown v. Laboratory Corporation of America Holdings and Tri-State Clinical Laboratory Services, LLC, filed in the United States District Court for the Southern District of Ohio, Western Division. The Company owned 50% of Tri-State Clinical Laboratory Services, LLC, which was dissolved in June of 2011 pursuant to a voluntary petition filed under Chapter 7 of Title 11 of the United States Code. The lawsuit alleges that the defendants submitted false claims for payment for laboratory testing services performed as a result of financial relationships that violated the Stark and the Anti-Kickback Statute. The lawsuit seeks actual and treble damages and civil penalties for each alleged false claim, as well as recovery of costs, attorney's fees, and legal expenses. The U.S. government has not intervened in the lawsuit. The parties have reached a settlement in principle, but the Company will vigorously defend the lawsuit if the settlement is not finalized.
On June 7, 2012, the Company was served with a putative class action lawsuit, Yvonne Jansky v. Laboratory Corporation of America, et al., filed in the Superior Court of the State of California, County of San Francisco. The lawsuit alleges that the defendants committed unlawful and unfair business practices, and violated various other state laws by changing screening codes to diagnostic codes on laboratory test orders, thereby resulting in customers being responsible for co-payments and other debts. The lawsuit seeks injunctive relief, actual and punitive damages, as well as recovery of attorney's fees, and legal expenses. In June 2015, Plaintiff's Motion for Class Certification was denied. The Plaintiff has appealed the denial of class certification, and the trial court has stayed the case pending resolution of the appeal. The Company will vigorously defend the lawsuit.
On August 24, 2012, the Company was served with a putative class action lawsuit, Sandusky Wellness Center, LLC, et al. v. MEDTOX Scientific, Inc., et al., filed in the United States District Court for the District of Minnesota. The lawsuit alleges that on or about February 21, 2012, the defendants violated the U.S. Telephone Consumer Protection Act (TCPA) by sending unsolicited facsimiles to Plaintiff and more than 39 other recipients without the recipients' prior express invitation or permission. The lawsuit seeks the greater of actual damages or the sum of $0.0005 for each violation, subject to trebling under the TCPA, and injunctive relief. In September of 2014, Plaintiff’s Motion for Class Certification was denied. In January of 2015, the Company’s Motion
42
for Summary Judgment on the remaining individual claim was granted. Plaintiff has filed a notice of appeal. The Company will vigorously defend the lawsuit.
The Company was a defendant in two separate putative class action lawsuits, Christine Bohlander v. Laboratory Corporation of America, et al., and Jemuel Andres, et al. v. Laboratory Corporation of America Holdings, et. al., related to overtime pay. After the filing of the two lawsuits on July 8, 2013, the Bohlander lawsuit was consolidated into the Andres lawsuit and removed to the United States District Court for the Central District of California. In the consolidated lawsuit, the Plaintiffs alleged on behalf of similarly situated phlebotomists and couriers that the Company failed to pay overtime, failed to provide meal and rest breaks, and committed other violations of the California Labor Code. On May 28, 2015, the District Court issued a preliminary approval of the class action settlement and notice of the settlement terms has been sent to putative class members.
The Company is also a defendant in two additional putative class action lawsuits alleging similar claims to the Bohlander/Andres consolidated lawsuit. The lawsuit of Rachel Rabanes v. California Laboratory Sciences, LLC, et al., was filed in April 2014 in the Superior Court of California for the County of Los Angeles, and the lawsuit Rita Varsam v. Laboratory Corporation of America DBA LabCorp, was filed in June 2014 in the Superior Court of California for the County of San Diego. As a result of the Andres settlement, the Plaintiff in the Rabanes case dismissed her case in June 2015. The Varsam case was settled in November 2015.
On December 17, 2010, the Company was served with a lawsuit, Oliver Wuth, et al. v. Laboratory Corporation of America, et al., filed in the State Superior Court of King County, Washington. The lawsuit alleges that the Company was negligent in the handling of a prenatal genetic test order that allegedly resulted in the parents being given incorrect information. The matter was tried before a jury beginning on October 21, 2013. On December 10, 2013, the jury returned a verdict in in Plaintiffs’ favor in the amount of $50.0 million, with 50.0% of liability apportioned to the Company and 50.0% of liability apportioned to co-defendant Valley Medical Center. The Company filed post-judgment motions for a new trial, which were denied, and then appealed to the Court of Appeals of the State of Washington. The Appeals Court heard oral argument in June 2015. In August 2015, the Appeals Court affirmed the trial court's judgment. In September 2015, the Company filed a Petition for Review with the Supreme Court of the State of Washington. On March 1, 2016, the Supreme Court will decide whether it will review the merits of the case. The Company carries self-insurance reserves and excess liability insurance sufficient to cover the potential liability in this case.
On July 3, 2012, the Company was served with a lawsuit, John Wisekal, as Personal Representative of the Estate of Darien Wisekal v. Laboratory Corporation of America Holdings and Glenda C. Mixon, filed in the Circuit Court of the Fifteenth Judicial Circuit in and for Palm Beach County, Florida. The lawsuit alleges that the Company misread a Pap test. The case was removed to the United States District Court for the Southern District of Florida. The matter was tried before a jury beginning on April 1, 2014. On April 17, 2014, the jury returned a verdict in Plaintiff’s favor in the amount of $20.8 million, with non-economic damages reduced by 25% to account for the Plaintiff's negligence, for a final verdict of $15.8 million. The Company filed post-trial motions. On July 28, 2014, the Court granted the Company’s motion for remittitur and reduced the jury’s non-economic damages award to $5.0 million, reduced by 25.0% for the Plaintiff’s negligence. Accordingly, the total judgment is $4.4 million. The Plaintiff opposed the remittitur and the Court ordered a new trial on the issue of damages only. The trial was scheduled for January 2016, but did not go forward at that time. Pending the outcome of pre-trial motions, the trial date may be reset. The Company will vigorously defend this lawsuit.
On July 9, 2014, the Company was served with a putative class action lawsuit, Christopher W. Legg, et al. v. Laboratory Corporation of America, filed in the United States District Court for the Southern District of Florida. The Complaint alleges that the Company willfully violated the Fair and Accurate Credit Transactions Act by allegedly providing credit card expiration date information on an electronically printed credit card receipt. The lawsuit sought damages of not less than $0.0001 but not more than $0.01 per violation, and punitive damages, injunctive relief, and attorney’s fees. On November 4, 2015, the District Court issued preliminary approval of the class action settlement and notice of the settlement was sent to putative class members. On February 18, 2016, the District Court entered a final order approving the settlement.
On August 31, 2015, the Company was served with a putative class action lawsuit, Patty Davis v. Laboratory Corporation of America, et al., filed in the Circuit Court of the Thirteenth Judicial Circuit for Hillsborough County, Florida. The complaint alleges that the Company violated the Florida Consumer Collection Practices Act by billing patients who were collecting benefits under the Workers’ Compensation Statutes. The lawsuit seeks injunctive relief and actual and statutory damages, as well as recovery of attorney's fees and legal expenses. The Company will vigorously defend the lawsuit.
On September 24, 2014, LipoScience and the Company entered into a Merger Agreement pursuant to which the Company through its subsidiary, Bear Acquisition Corp, would acquire all of the outstanding shares of LipoScience at a purchase price of $5.25 per share in cash for each outstanding share of LipoScience (Merger). Prior to the closing of the Merger on November 20, 2014, purported stockholders of LipoScience filed four putative class action lawsuits against LipoScience, members of the
43
LipoScience board of directors, the Company and Bear Acquisition Corp. in the Delaware Court of Chancery and, with respect to one of the lawsuits, in the Superior Court of Wake County, North Carolina. The lawsuits alleged breach of fiduciary duty and/or other violations of state law arising out of the Merger. Each suit sought, among other things, injunctive relief enjoining the Merger. On October 23, 2014, the case in North Carolina was voluntarily dismissed without prejudice by the Plaintiff. On October 29, 2014, the Delaware Court of Chancery consolidated the remaining three actions under the caption In re LipoScience, Inc. Stockholder Litigation, Consolidated C.A. No. 10252-VCP. After limited discovery, the parties agreed on certain additional disclosures to the Company's definitive proxy statement filed on October 20, 2014, which were made in a supplement to the definitive proxy statement filed on November 10, 2014 (LipoScience Supplemental Disclosures). On November 30, 2015, the Court approved a stipulation under which Plaintiffs voluntarily dismissed the action without prejudice as to all Plaintiffs and any other putative class member. The Court retained jurisdiction solely for the purpose of adjudicating Plaintiffs' counsel's anticipated application for an award of attorneys' fees and reimbursement of expenses in connection with the LipoScience Supplemental Disclosures. The Company subsequently agreed to pay $0.2 million to Plaintiffs' counsel for attorney’s fees and expenses in full satisfaction of their claim for attorney’s fees and expenses in the action. The Court has not been asked to review, and will pass no judgment on, the payment of the attorney’s fees and expenses or their reasonableness. Contact information for counsel is Brian D. Long (BDL@rl-legal.com) for Plaintiffs and Raymond J. DiCamillo (dicamillo@rlf.com) for the Company.
On November 2, 2014, the Company entered into a definitive Merger Agreement to acquire Covance for $6,150.7 milion in cash and Company common stock (Merger). The Merger closed on February 19, 2015. Prior to the closing of the Merger, purported stockholders of Covance filed two putative class action lawsuits. One of the lawsuits, captioned Berk v Covance Inc., et al., C.A. No. 10440-VCL, was filed in the Delaware Court of Chancery on December 9, 2014. The other lawsuit, captioned Ojeda v. Herring et al., No. MER-C-92-14, was filed in the Superior Court of New Jersey, Chancery Division, Mercer County, New Jersey, on November 12, 2014. Both suits named as defendants Covance, members of the Covance board of directors, the Company and Neon Merger Sub, Inc., a wholly owned subsidiary of the Company that was merged out of existence in connection with the Merger. The lawsuits alleged breach of fiduciary duty and/or other violations of state law arising out of the Merger. Each suit sought, among other things, injunctive relief enjoining the merger. On January 21, 2015, the case in New Jersey was voluntarily dismissed without prejudice by the Plaintiff. After limited discovery, the parties agreed on certain additional disclosures to the Company's definitive proxy statement filed on November 26, 2014, which were made in a supplement to the definitive proxy statement filed on February 9, 2015 (Covance Supplemental Disclosures). On December 1, 2015, the Court approved a stipulation under which Plaintiffs voluntarily dismissed the action, with prejudice as to the named Plaintiff only. The Court retained jurisdiction solely for the purpose of adjudicating Plaintiffs' counsel's anticipated application for an award of attorney’s fees and reimbursement of expenses in connection with the Covance Supplemental Disclosures. The Company subsequently agreed to pay $0.2 million to Plaintiffs' counsel for attorney’s fees and expenses in full satisfaction of their claim for attorney’s fees and expenses in the action. The Court has not been asked to review, and will pass no judgment on, the payment of the attorneys' fees and expenses or their reasonableness.
In December 2014, the Company received a Civil Investigative Demand issued pursuant to the U.S. False Claims Act from the U.S. Attorney’s Office for South Carolina, which requests information regarding remuneration and services provided by the Company to physicians who also received draw and processing/handling fees from competitor laboratories Health Diagnostic Laboratory, Inc. and Singulex, Inc. The Company is cooperating with the request.
In March 2015, the Company received a subpoena from the Attorney General of the State of New York, which requested information regarding the Company’s relationship with Direct Laboratories LLC. The Company cooperated with the request, and the matter was settled in December 2015.
The Company holds an investment in a joint venture partnership located in Alberta, Canada. The Canadian partnership has a license to conduct diagnostic testing services in the province of Alberta. Substantially all of its revenue is received as reimbursement from the Alberta government's healthcare programs. In December 2013, Alberta Health Services (AHS), the Alberta government's healthcare program, issued a request for proposals for laboratory services that included the scope of services performed by the Canadian partnership. In October 2014, AHS informed the Canadian partnership that it had not been selected as the preferred proponent. In November 2014, the Canadian partnership submitted a vendor bid appeal upon the belief that there were significant flaws and failures in the conduct of the request for proposal process, which drove to a biased conclusion. AHS established a Vendor Bid Appeal Panel to hear the appeal, and the hearing was conducted in February 2015. In August 2015, AHS was directed to cancel the request for proposal process. Subsequently, the Canadian partnership entered into a one-year extension through March 31, 2017 of its existing contract with AHS. If the contract is not renewed after March 2017, then the Canadian partnership's revenues would decrease substantially and the carrying value of the Company's investment could potentially be impaired.
Under the Company's present insurance programs, coverage is obtained for catastrophic exposure as well as those risks required to be insured by law or contract. The Company is responsible for the uninsured portion of losses related primarily to general, professional and vehicle liability, certain medical costs and workers' compensation. The self-insured retentions are on a per
44
occurrence basis without any aggregate annual limit. Provisions for losses expected under these programs are recorded based upon the Company's estimates of the aggregated liability of claims incurred. At December 31, 2015, the Company had provided letters of credit aggregating approximately $45.4 million, primarily in connection with certain insurance programs. The Company’s availability under its Revolving Credit Facility is reduced by the amount of these letters of credit.
Item 4. | MINE SAFETY DISCLOSURES |
Not applicable.
PART II
Item 5. | MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS, AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
The Company's common stock, par value $0.10 per share (Common Stock), trades on the New York Stock Exchange (NYSE) under the symbol “LH.” The following table sets forth for the calendar periods indicated the high and low sales prices for the Common Stock reported on the NYSE Composite Tape.
High | Low | ||||||
Year Ended December 31, 2014 | |||||||
First Quarter | $ | 102.00 | $ | 87.25 | |||
Second Quarter | $ | 105.38 | $ | 95.12 | |||
Third Quarter | $ | 108.77 | $ | 101.55 | |||
Fourth Quarter | $ | 109.84 | $ | 95.61 | |||
Year Ended December 31, 2015 | |||||||
First Quarter | $ | 131.19 | $ | 108.73 | |||
Second Quarter | $ | 129.33 | $ | 116.00 | |||
Third Quarter | $ | 128.84 | $ | 105.77 | |||
Fourth Quarter | $ | 126.24 | $ | 107.30 | |||
Holders
On February 23, 2016, there were 101.7 million holders of record of the Common Stock.
Transfer Agent
The transfer agent for the Company's Common Stock is American Stock Transfer & Trust Company, Shareholder Services, 6201 Fifteenth Avenue, Brooklyn, NY 11219, telephone: 800-937-5449, website: www.amstock.com.
Dividends
The Company has not historically paid dividends on its Common Stock and does not presently anticipate paying any dividends on its Common Stock in the foreseeable future.
Common Stock Performance
The Company’s common stock is traded on the NYSE. The graph below shows the cumulative total return assuming an investment of $100 on December 31, 2010 in each of the Company’s common stock, the Standard & Poor’s (S&P) Composite-500 Stock Index and the S&P 500 healthcare Index (Peer Group) and assuming that all dividends were reinvested.
45
Comparison of Five Year Cumulative Total Return
12/2010 | 12/2011 | 12/2012 | 12/2013 | 12/2014 | 12/2015 | ||||||||||||||||||
Laboratory Corporation of America Holdings | $ | 100.00 | $ | 97.78 | $ | 98.52 | $ | 103.92 | $ | 122.73 | $ | 140.63 | |||||||||||
S&P 500 Index | $ | 100.00 | $ | 102.11 | $ | 118.45 | $ | 156.82 | $ | 178.29 | $ | 180.75 | |||||||||||
S&P 500 Health Care Index | $ | 100.00 | $ | 112.73 | $ | 132.90 | $ | 188.00 | $ | 235.63 | $ | 251.87 | |||||||||||
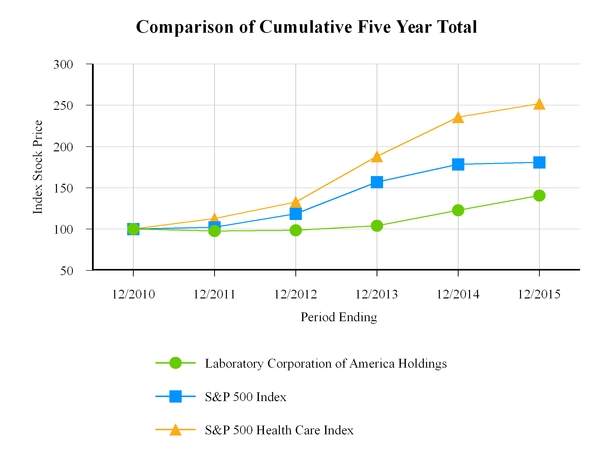
Issuer Purchases of Equity Securities
The Board of Directors has authorized the repurchase of specified amounts of the Company's common stock since 2007, including the Board of Director's authorization on February 10, 2012 to purchase up to $500.0 million of additional shares of the Company's common stock. As of December 31, 2015 and 2014, the Company had outstanding authorization from the Board of Directors to purchase up to $789.5 million of Company common stock. The repurchase authorization has no expiration date. Following the announcement of the acquisition of Covance Inc., the Company suspended its share repurchases. The Company does not anticipate resuming its share repurchase activity until it approaches its targeted leverage ratio of total debt to consolidated EBITDA of 2.5 to 1.0. However, the Company will continue to evaluate all opportunities for strategic deployment of capital in light of market conditions.
46
Item 6. | SELECTED FINANCIAL DATA |
The selected financial data presented below under the captions “Statement of Operations Data” and “Balance Sheet Data” as of and for the five-year period ended December 31, 2015 are derived from consolidated financial statements of the Company, which have been audited by an independent registered public accounting firm. This data should be read in conjunction with the accompanying notes, the Company's consolidated financial statements and the related notes thereto, and “Management's Discussion and Analysis of Financial Condition and Results of Operations,” all included elsewhere herein.
Year Ended December 31, | |||||||||||||||||||
(a) 2015 | (b) 2014 | (c) 2013 | (d) 2012 | (e) (f) 2011 | |||||||||||||||
(In millions, except per share amounts) | |||||||||||||||||||
Statement of Operations Data: | |||||||||||||||||||
Net revenues | $ | 8,505.7 | $ | 6,011.6 | $ | 5,808.3 | $ | 5,671.4 | $ | 5,542.3 | |||||||||
Gross profit | 2,903.3 | 2,203.1 | 2,223.2 | 2,249.7 | 2,274.7 | ||||||||||||||
Operating income | 1,002.9 | 910.4 | 990.9 | 1,023.5 | 948.4 | ||||||||||||||
Net earnings attributable to Laboratory | |||||||||||||||||||
Corporation of America Holdings | 436.9 | 511.2 | 573.8 | 583.1 | 519.7 | ||||||||||||||
Basic earnings per common share | $ | 4.42 | $ | 6.03 | $ | 6.36 | $ | 6.09 | $ | 5.20 | |||||||||
Diluted earnings per common share | $ | 4.34 | $ | 5.91 | $ | 6.25 | $ | 5.99 | $ | 5.11 | |||||||||
Basic weighted average common | |||||||||||||||||||
shares outstanding | 98.8 | 84.8 | 90.2 | 95.7 | 100.0 | ||||||||||||||
Diluted weighted average common | |||||||||||||||||||
shares outstanding | 100.6 | 86.4 | 91.8 | 97.4 | 101.8 | ||||||||||||||
Balance Sheet Data: | |||||||||||||||||||
Cash and cash equivalents, and | |||||||||||||||||||
short-term investments | $ | 716.4 | $ | 580.0 | $ | 404.0 | $ | 466.8 | $ | 159.3 | |||||||||
Goodwill and intangible assets, net | 9,551.2 | 4,575.2 | 4,594.8 | 4,569.4 | 4,302.5 | ||||||||||||||
Total assets | 14,221.7 | 7,301.8 | 6,965.9 | 6,795.0 | 6,111.8 | ||||||||||||||
Long-term obligations (g) | 6,417.0 | 3,029.8 | 3,000.4 | 2,655.0 | 2,221.0 | ||||||||||||||
Total shareholders' equity | 4,944.4 | 2,820.5 | 2,491.3 | 2,717.4 | 2,503.5 | ||||||||||||||
(a) | During 2015, the Company recorded net restructuring charges of $113.9. The charges were comprised of $59.2 in severance and other personnel costs and $55.8 in facility-related costs primarily associated with facility closures and general integration initiatives. These charges were offset by the reversal of previously established reserves of $1.1 in unused facility-related costs. |
(b) | During 2014, the Company recorded net restructuring charges of $17.8. The charges were comprised of $10.5 in severance and other personnel costs and $8.4 in facility-related costs primarily associated with facility closures and general integration initiatives. These charges were offset by the reversal of previously established reserves of $0.4 in unused severance and $0.7 in unused facility-related costs. |
(c) | During 2013, the Company recorded net restructuring charges of $21.8. The charges were comprised of $15.4 in severance and other personnel costs and $9.5 in facility-related costs primarily associated with facility closures and general integration initiatives. These charges were offset by the reversal of previously established reserves of $0.7 in unused severance and $2.4 in unused facility-related costs. |
(d) | During 2012, the Company recorded net restructuring charges of $25.3. The charges were comprised of $16.2 in severance and other personnel costs and $19.6 in facility-related costs primarily associated with the ongoing integration activities of Cellmark Forensics, Inc. (formerly Orchid Cellmark, Inc.) and the Integrated Genetics business (formerly Genzyme Genetics) and costs associated with the previously announced termination of an executive vice president. These charges were offset by the reversal of previously established reserves of $6.3 in unused severance and $4.2 in unused facility-related costs. As part of the Clearstone integration, the Company also recorded a $6.9 loss on the disposal of one of its European subsidiaries in Other, net under Other income (expenses) during 2012. In addition, the Company recorded $6.2 in accelerated amortization relating to the termination of a licensing agreement. |
(e) | During 2011, the Company recorded net restructuring charges of $44.6. Of this amount, $27.4 related to severance and other personnel costs, and $22.0 primarily related to facility-related costs associated with the ongoing integration |
47
of certain acquisitions including Genzyme Genetics and Westcliff Medical Laboratories, Inc. (Westcliff). These charges were offset by restructuring credits of $4.8 resulting from the reversal of unused severance and facility closure liabilities. In addition, the Company recorded fixed assets impairment charges of $18.9 primarily related to equipment, computer systems and leasehold improvements in closed facilities. The Company also recorded special charges of $14.8 related to the write-off of certain assets and liabilities related to an investment made in prior years, along with a $2.6 write-off of an uncollectible receivable from a past installment sale of one of the Company's lab operations.
(f) | Following the closing of its acquisition of Orchid in mid-December 2011, the Company recorded a net $2.8 loss on its divestiture of certain assets of Orchid's U.S. government paternity business, under the terms of the agreement reached with the U.S. Federal Trade Commission. This non-deductible loss on disposal was recorded in Other Income and Expense in the Company's Consolidated Statements of Operations and decreased net earnings for the twelve months ended December 31, 2011 by $2.8. |
(g) | Long-term obligations primarily include the Company’s zero-coupon convertible subordinated notes, 5.50% Senior Notes due 2013, 5.625% Senior Notes due 2015, 3.125% Senior Notes due 2016, 2.20% Senior Notes due 2017, 2.50% Senior Notes due 2018, 4.625% Senior Notes due 2020, 3.75% Senior Notes due 2022, 3.20% Senior Notes due 2022, 4.00% Senior Notes due 2023, 3.60% Senior Notes due 2025, 4.70% Senior Notes due 2045, term loan, revolving credit facility and other long-term obligations. The accreted balance of the zero-coupon convertible subordinated notes was $94.5, $93.9, $110.8, $130.0, and $135.5 at December 31, 2015, 2014, 2013, 2012, and 2011, respectively. The balance of the 5.50% Senior Notes, including principal and unamortized portion of a deferred gain on an interest rate swap agreement, was $0.0, $0.0, $0.0, $350.0, and $350.5 at December 31, 2015, 2014, 2013, 2012, and 2011, respectively. The principal balance of the 5.625% Senior Notes was $0.0 at December 31, 2015 and $250.0 at December 31, 2014, 2013, 2012, and 2011. The principal balance of the 3.125% Senior Notes was $325.0 at December 31, 2015, 2014, 2013, 2012, and 2011. The principal balance of the 4.625% Senior Notes was $600.0 at December 31, 2015, 2014, 2013, 2012, and 2011. The aggregate fair value of the fixed-to-variable interest rate swap on the 4.625% Senior Notes was $21.6 at December 31, 2015, $18.5 at December 31, 2014, and $0.0 for all other years presented. The principal balances of the 2.20% and 3.75% Senior Notes were $500.0 each at December 31, 2015, 2014, 2013 and 2012 and $0.0 for December 31, 2011. The principal balances of the 2.50% and 4.00% Senior Notes were $400.0 and $300.0, respectively, at December 31, 2015, 2014, and 2013 and $0.0 for all other years presented. The outstanding balance on the term loan was $715.0 at December 31, 2015 and $0.0 for all other years presented. The outstanding balance on the revolving credit facility was $0.0, $0.0, $0.0, $0.0, and $560.0 at December 31, 2015, 2014, 2013, 2012, and 2011, respectively. The remainder of other long-term obligations consisted primarily of capital leases and mortgages payable with balances of $55.5, $39.2, $14.6, $0.0, and $0.0 at December 31, 2015, 2014, 2013, 2012, and 2011, respectively. Long-term obligations exclude amounts due to affiliates. |
48
Item 7. | MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS (in millions) |
General
On February 19, 2015 (Acquisition Date), the Company completed its acquisition (Acquisition) of Covance Inc. (Covance), a leading drug development services company and a leader in nutritional analysis, for $6,150.7. The Acquisition contributed $2,209.7 to the Company's total net revenue from the Acquisition Date driving 36.7% year over year net growth. The Company expects the inclusion of Covance for a full twelve months in 2016 will provide an approximate 4.0% increase in revenue based upon Covance's pre-acquisition 2015 net revenues. Organic revenue growth in 2015, excluding currency, was 4.6%.
The Company achieved $45.0 in synergies in 2015 as a result of the Acquisition and expects annual cost synergies in excess of $100.0 to be fully realized within three years of closing the transaction. The Company's 2015 results were impacted by increased interest expense and historically lower margins from Covance. The Company is committed to maintaining its investment grade credit rating and plans to use its operating cash flows to meet its annual capital expenditure requirements and to reduce its overall debt leverage, while evaluating other strategic opportunities for deployment of its capital.
The Company has seen growth in the amount of its patient accounts receivable. A significant portion of the Company’s bad debt expense is related to LCD accounts receivable from patients. The Company believes its current allowance for doubtful accounts is sufficient to properly record its accounts receivable at their estimated net realizable value. Should the shift towards increased patient responsibility continue, the Company may need to increase its allowance for doubtful accounts and bad debt expense in future periods.
Prior to the first quarter of 2015, the chief operating decision maker (CODM) managed the operating results of the Company as two segments: clinical laboratory diagnostics and other. In connection with the Acquisition, the Company changed its operating segments to align with how the CODM evaluates financial information used to allocate resources and assess performance of the Company following the Acquisition. The segment information presented in the Company's consolidated financial statements has been conformed to present segments on this revised basis for all prior periods. Under the new organizational structure, the CODM manages the Company under two reportable segments: LabCorp Diagnostics (LCD) and Covance Drug Development (CDD). LCD includes the Company's legacy LabCorp business, and the Company's nutritional chemistry and food safety business, which were previously part of Covance, but excludes LabCorp’s legacy clinical trials testing business, which is now part of CDD. CDD includes the Covance legacy business, and LabCorp’s legacy clinical trials testing business, but excludes Covance's nutritional chemistry and food safety business, which are now part of LCD.
Seasonality
The Company experiences seasonality in both segments of its business. The majority of LCD's testing volume is dependent on patient visits to physician offices and other providers of healthcare. Testing volume in both segments generally declines during the year-end holiday periods and other major holidays. In addition, testing volume also declines due to inclement weather which may reduce net revenues and cash flows. CDD's operations are also impacted by changes in the global economy, exchange rate fluctuations, the progress of ongoing studies and the start-up of new studies, as well as the level of expenditures made by the pharmaceutical and biotechnology industries in research and development (R&D). Given the seasonality of the business, comparison of the results for successive quarters may not accurately reflect trends or results for the full year.
Results of Operations (amounts in millions)
Years ended December 31, 2015, 2014, and 2013
Net Revenues
Years Ended December 31, | Change | ||||||||||||||||
Net revenues | 2015 | 2014 | 2013 | 2015 | 2014 | ||||||||||||
LCD | $ | 6,199.3 | $ | 5,838.0 | $ | 5,669.4 | 6.2 | % | 3.0 | % | |||||||
CDD | 2,306.4 | 173.6 | 138.9 | 1,228.5 | % | 25.0 | % | ||||||||||
Total | $ | 8,505.7 | $ | 6,011.6 | $ | 5,808.3 | 41.5 | % | 3.5 | % | |||||||
The increase in net revenues for the year ended December 31, 2015 was driven primarily by the Acquisition along with strong organic volume growth in LCD and tuck-in acquisitions, price, and mix, partially offset by currency.
49
LCD net revenues for the year ended December 31, 2015 were $6,199.3, an increase of 6.2% over net revenues of $5,838.0 in the corresponding period in 2014. The increase in net revenues was driven by organic volume growth, measured by requisitions of 3.2%. Beacon LBS, the Company's technology-enabled solution providing point-of-care decision support, contributed 0.9%. The increase in net revenues was unfavorably impacted by (0.8%) of currency. Revenue per requisition favorably impacted revenue by 0.4%. In addition, acquisitions added 2.5% to net revenues.
CDD net revenues for the year ended December 31, 2015 were $2,306.4, an increase of 1,228.5% over net revenues of $173.6 in the corresponding period in 2014. The increase in net revenues was due to the Acquisition. Approximately 52.1% of CDD's net revenues are billed in currencies other than the U.S. dollar, with the Swiss franc, British pound, and the Euro representing approximately 66.8% of CDD's total currency exposure. The 2014 CDD net revenue amount represents LabCorp’s legacy clinical trials testing business.
The increase in LCD net revenues for the year ended December 31, 2014 was driven primarily by strong organic volume growth and the benefit of fold-in acquisitions, along with growth in the Company's managed care business and toxicology testing, partially offset by test and payer mix. During 2014, the impact of weather reduced LCD's revenues by an estimated $40.0. The increase in revenue per requisition in core testing is the result of the number of tests per requisition and a change in the mix of testing within that category. The decline in revenue per requisition in genomic and esoteric testing is primarily a result of a change in the mix of tests within those categories.
During 2013, the impact of weather, reduced LCD's revenues by an estimated $12.7, of which $5.3 occurred in the fourth quarter. The 2013 decline in revenue per requisition in genomic and esoteric testing is a result of a change in mix of genetic and histology testing. Histology revenue per requisition was also impacted by payment reductions on the Medicare physician fee schedule. Further, revenue per requisition also decreased due to delays in payments and denials of coverage for existing tests by some payers after implementation of new molecular pathology codes at the beginning of the year and the implementation of sequestration on April 1, 2013.
CDD net revenues for the year ended December 31, 2014 were $173.6, an increase of 25.0% over net revenues of $138.9 in the corresponding period in 2013. This increase in net revenues was driven by organic volume growth, measured by requisitions of 27.9%, offset by revenue per requisition of (2.9%).
Net Cost of Revenues
Years Ended December 31, | Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 | 2014 | |||||||||||||
Net cost of revenues | $ | 5,602.4 | $ | 3,808.5 | $ | 3,585.1 | 47.1 | % | 6.2 | % | |||||||
Cost of revenues as a % of net revenues | 65.9 | % | 63.4 | % | 61.7 | % | |||||||||||
Net cost of revenues (primarily laboratory and distribution costs) increased 47.1% in 2015 as compared with the 2014 primarily due to the Acquisition. Excluding acquisitions, net costs of revenues increased approximately 4.2% due to increased volume, measured by requisitions, and test mix changes. The increase in cost of sales as a percentage of net sales in 2014 as compared to 2013 is due to the increased number of tests per requisition. The increase in net cost of revenues in 2015 was favorably impacted by a net reduction of (0.8%) due to currency fluctuations.
Labor and testing supplies for the year ended December 31, 2015, comprise over 75.5% of the Company’s net cost of revenues. Net cost of revenues has increased over the three-year period ended December 31, 2015 primarily due to the impact of acquisitions, overall growth in the Company's volume and increases in labor.
Selling, General and Administrative Expenses
Years Ended December 31, | Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 | 2014 | |||||||||||||
Selling, general and administrative expenses | $ | 1,622.0 | $ | 1,198.2 | $ | 1,128.8 | 35.4 | % | 6.1 | % | |||||||
SG&A as a % of net revenues | 19.0 | % | 19.9 | % | 19.4 | % | |||||||||||
Selling, general and administrative expenses as a percentage of net revenues decreased to 19.0% in 2015 compared to 19.9% in 2014. The decrease in selling, general and administrative expenses as a percentage of net revenues is primarily due to the Acquisition and the impact of Project LaunchPad, LCD's comprehensive, enterprise-wide business process improvement initiative, and integration synergies. In addition, bad debt expense as a percentage of net revenues for LCD decreased to 4.3% of net revenues for that segment compared to 4.6% during 2014. This improvement in LCD's bad debt expense is a result of the segment's focus on improved cash collections and other related Project LaunchPad initiatives. As discussed in Restructuring and Other Special
50
Charges, the Company incurred $164.5 in one-time, non-recurring fees and expenses in 2015. The increase in selling, general and administrative expenses in 2015 was impacted by a net reduction of (0.8%) due to currency fluctuations.
Selling, general and administrative expenses as a percentage of net revenues increased to 19.9% in 2014 compared to 19.4% in 2013. The increase in selling, general and administrative expenses as a percentage of net revenues is partially due to $13.8 in fees related to acquisitions recorded in 2014. Additionally, bad debt expense increased to 4.6% of net revenues in 2014 as compared to 4.4% of net revenues in 2013. The Company also recorded $18.6 in consulting expenses (recorded in selling, general and administrative) relating to fees incurred as part of its business process improvement initiative as well as one-time CFO transition costs.
Amortization of Intangibles and Other Assets
Years Ended December 31, | Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 | 2014 | |||||||||||||
LCD | $ | 82.4 | $ | 75.5 | $ | 81.2 | 9.1 | % | (7.0 | )% | |||||||
CDD | 82.1 | 1.2 | 0.5 | 6,741.7 | % | 140.0 | % | ||||||||||
Amortization of intangibles and other assets | $ | 164.5 | $ | 76.7 | $ | 81.7 | 114.5 | % | (6.1 | )% | |||||||
The increase in amortization of intangibles and other assets in 2015 as compared to 2014 primarily reflect the net impact of the Acquisition.
The decrease in amortization of intangibles and other assets in 2014 as compared to 2013 primarily reflect the net impact of acquisitions closed during 2014 offset by adjustments to the fair value of deferred acquisition payments.
Restructuring and Other Special Charges
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Restructuring and other special charges | $ | 113.9 | $ | 17.8 | $ | 21.8 | |||||
During 2015, the Company recorded net restructuring and other special charges of $113.9; $39.2 within LCD and $74.7 within CDD. The charges were comprised of $59.2 related to severance and other personnel costs along with $55.8 in costs associated with facility closures and general integration initiatives. A substantial portion of these costs relate to the planned closure of two CDD operations that serviced a minimum volume contract that expired on October 31, 2015. These charges were offset by the reversal of previously established reserves of $1.1 in unused facility-related costs. Included within the facility-related charges noted above is a $26.7 asset impairment charge relating to CDD lab and customer service applications that will no longer be used.
The Company incurred additional legal and other costs of $5.7 relating to the wind-down of the minimum volume contract operations referred to in the previous paragraph. On February 9, 2016, the Company reached an agreement for the sale of the assets and business of one of these sites. As required by U.K. law, substantially all of the employees were transferred with the business. The Company also recorded $25.6 in consulting expenses relating to fees incurred as part of Project LaunchPad as well as Covance integration costs and employee compensation studies, along with $5.4 in short-term equity retention arrangements relating to the acquisition of Covance and $0.3 of accelerated equity compensation relating to the previously disclosed retirement of a Company executive (all recorded in selling, general and administrative expenses). During the fourth quarter, the Company paid $12.2 in settlement costs and litigation expenses related to the resolution of a U.S. federal court putative class action lawsuit. In addition, the Company incurred $3.0 of non-capitalized costs associated with the implementation of a major system as part of Project Launchpad.
During 2014, the Company recorded net restructuring charges of $17.8. The charges were comprised of $10.5 in severance and other personnel costs and $8.4 in facility-related costs primarily associated with general integration activities. These charges were offset by the reversal of previously established reserves of $0.4 in unused severance and $0.7 in unused facility-related costs.
In addition, during 2014, the Company recorded $18.6 in consulting expenses (recorded in selling, general and administrative expenses) relating to fees incurred as part of its business process improvement initiative as well as one-time CFO transition costs. The Company also recorded $10.8 of costs related to the Covance acquisition, of which $4.8 is included in selling, general and administrative expenses and $6.0 is included in interest expense.
During 2013, the Company recorded net restructuring charges of $21.8. The charges were comprised of $15.4 in severance and other personnel costs and $9.5 in facility-related costs primarily associated with the ongoing integration of Orchid and the
51
Integrated Genetics business (formerly Genzyme Genetics) and costs associated with the previously announced termination of an executive vice president. These charges were offset by the reversal of previously established reserves of $0.7 in unused severance and $2.4 in unused facility-related costs.
During 2014, the Company announced Project Launchpad, a comprehensive business process improvement initiative to re-engineer LCD's systems and processes to leverage technological advancements, create a sustainable and more efficient business model and improve the experience of all stakeholders. The Company expects this initiative to drive savings in excess of $150.0 over a three year period beginning in 2015, with associated one-time costs of approximately $30.0. The Company believes that any restructuring costs which may be incurred in future periods will be more than offset by subsequent savings realized from these potential actions and that any related restructuring charges will not have a material impact on the Company's operations or liquidity.
Interest Expense
Years Ended December 31, | Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 | 2014 | |||||||||||||
Interest expense | $ | 274.9 | $ | 109.5 | $ | 96.5 | 151.1 | % | 13.5 | % | |||||||
The increase in interest expense for 2015 as compared with 2014 is primarily due to the issuance of $3,900 in debt and other financing costs in connection with the Acquisition. Another component of the increase was a $37.4 make-whole payment that was required in connection with the prepayment of $250.0 Covance senior notes. In addition, the Company recorded $15.2 in interest expense relating to the deferred financing cost associated with the Company's previous credit agreement and a term loan bridge financing facility to complete the Acquisition. The bridge facility was repaid in March 2015.
The increase in interest expense for 2014 as compared with 2013 is primarily due to the issuance of $700.0 of senior notes in November 2013, reductions in borrowings under the Company's former Revolving Credit Facility, and Covance-related financing activities. This increase was also partially offset by a decrease in interest expense on the Company's senior notes due 2020 as a result of entering into a fixed to floating interest rate swap in the third quarter of 2013.
Equity Method Income
Years Ended December 31, | Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 | 2014 | |||||||||||||
Equity method income | $ | 10.0 | $ | 14.3 | $ | 16.9 | (30.1 | )% | (15.4 | )% | |||||||
Equity method income represents the Company's ownership share in joint venture partnerships along with equity investments in other companies in the healthcare industry. All of these partnerships reside within LCD. The decrease in income in 2015 was primarily due to liquidation of the Company's interest in one of the partnerships effective June 30, 2015.
The decrease in income in 2014 compared to 2013 is primarily the result of a decline in profitability of one of the Company's joint venture partnerships due to a challenging business climate in its market.
Other, net
Years Ended December 31, | Change | ||||||||||||||||
2015 | 2014 | 2013 | 2015 | 2014 | |||||||||||||
Other, net | $ | (7.8 | ) | $ | 10.4 | $ | 2.1 | (175.0 | )% | 395.2 | % | ||||||
Other, net represents the Company's gain on the sale of its investment in an equity security, partially offset by the impairment of other investments. The decrease in other, net was due to the dissolution of one of the Company's equity investments in 2015 as compared to a gain on the sale of investment of an equity investment in 2014.
Income Tax Expense
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Income tax expense | $ | 294.1 | $ | 314.1 | $ | 340.2 | |||||
Income tax expense as a % of income before tax | 40.2 | % | 38.0 | % | 37.2 | % | |||||
52
The effective rate for 2015 was unfavorably impacted by one-time restructuring and acquisition items, recording of additional uncertain tax reserves, and a decrease in the benefit recorded from releasing uncertain tax reserves. The 2015 rate was favorably impacted by CDD foreign earnings taxed at rates lower than the U.S. statutory rate.
The effective rate for 2014 was unfavorably impacted by the recording of a full valuation allowance for the write-down of two of the Company's investments.
The effective rate for 2013 was favorably impacted by the release of a capital loss valuation allowance and recording two years of a R&D tax credit. The American Taxpayer Relief Act of 2012 was enacted in early 2013 and reinstated the R&D tax credit for 2012 and extended the credit for calendar year 2013.
Liquidity, Capital Resources and Financial Position
The Company's strong cash-generating capability and financial condition typically have provided ready access to capital markets. The Company's principal source of liquidity is operating cash flow, supplemented by proceeds from debt offerings. The Company's senior unsecured revolving credit facility is further discussed in Note 11 (Debt) to the Company's Consolidated Financial Statements.
In summary the Company's cash flows were as follows:
For the Year Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Net cash provided by operating activities | $ | 982.4 | $ | 739.0 | $ | 818.7 | |||||
Net cash used for investing activities | (3,994.9 | ) | (350.1 | ) | (359.6 | ) | |||||
Net cash provided by (used in) financing activities | 3,184.6 | (200.6 | ) | (518.3 | ) | ||||||
Effect of exchange rate on changes in cash and cash equivalents | (35.7 | ) | (12.3 | ) | (3.6 | ) | |||||
Net change in cash and cash equivalents | $ | 136.4 | $ | 176.0 | $ | (62.8 | ) | ||||
Cash and Cash Equivalents
Cash and cash equivalents at December 31, 2015, 2014 and 2013 totaled $716.4, $580.0, and $404.0, respectively. Cash and cash equivalents consist of highly liquid instruments, such as commercial paper, time deposits and other money market investments, substantially all of which have original maturities of three months or less.
Cash Flows From Operating Activities
Net cash provided by operating activities for the year ended December 31, 2015 was $982.4 compared to $739.0 for the year ended December 31, 2014. The increase in cash provided by operations was due primarily to additional cash generated by CDD operations following the Acquisition as well as improved working capital. While there were lower net earnings due to the Acquisition, this included a number of one-time non-cash charges. The Company’s earnings were impacted by restructuring and special items of $113.9 during 2015, compared to $17.8 during the same period in 2014. During the first quarter of 2015, the Company's operating cash flows were reduced by $153.5 of cash payments in connection with the Acquisition.
Net cash provided by operating activities for the year ended December 31, 2014 was $739.0 compared to $818.7 for the year ended December 31, 2013. The decrease in cash flows from operating activities for the year ended December 31, 2014, as compared to the prior year, was primarily attributable to $62.8 lower net earnings. The Company’s lower net earnings were driven by a 2% decline in its operating margins due to changes in payer and test mix as well as increased acquisition and consulting costs. The Company’s cash collections improved by $36.4 compared to the prior year, representing improvements in collections efforts on the delays and denials of payments the Company experienced in 2013 as a result of new molecular pathology codes and government payment reductions. However, this improvement was overshadowed by an $82.0 million increase in the use of cash from accounts payable disbursements. During 2014, the Company’s interest payments increased $20.6 compared to 2013, reflecting the timing of interest payments on the $700.0 in new senior notes issued in November 2013. This was partially offset by a $17.4 decrease in net taxes paid during the year compared to 2013. In addition, the net impact of changes in noncash adjustments, primarily related to deferred taxes, depreciation and amortization, stock compensation and gain/loss on sale of assets contributed to the $79.7 decrease in operating cash flows for 2014 as compared to the prior year.
Cash Flows From Investing Activities
Net cash used in investing activities for the year ended December 31, 2015 was $3,994.9 as compared to $350.1 for the year ended December 31, 2014. The $3,644.8 increase in cash used in investing activities was primarily due to net cash paid for the
53
Acquisition of $3,607.4, ($4,388.2 cash paid, net of cash acquired of $780.8). Capital expenditures were $255.8 and $203.5 for the year ended December 31, 2015 and 2014, respectively. The Company expects capital expenditures of approximately 3.0% of revenue in 2016. The Company intends to continue to pursue acquisitions to fund growth and make important investments in its business, including in information technology, to improve efficiency and enable the execution of the Company's strategic vision. Such expenditures are expected to be funded by cash flow from operations, as well as borrowings under the Company's revolving credit facility or any successor facility as needed.
Net cash used in investing activities for the year ended December 31, 2014 was $350.1 as compared to $359.6 for the year ended December 31, 2013. The $9.5 decrease in cash used in investing activities for the year ended December 31, 2014, as compared to the prior year, was primarily the result of the $31.6 proceeds received from the sale of one of the company’s equity investments offset by cash outflows of $20.2 for additional equity investments.
Cash Flows From Financing Activities
Net cash provided by financing activities for the year ended December 31, 2015 was $3,184.6 compared to $200.6 net cash used in financing activities for the year ended December 31, 2014. The $3,385.2 increase in the cash provided by financing activities for 2015, as compared to the prior year, was primarily a result of $4,360.0 of financing proceeds for the Acquisition offset by repayments and debt issue costs of $781.7. The Company also repaid $500.0 of senior notes (including $250.0 of Covance Inc. senior notes) in 2015. The remainder of the period-over-period increase is primarily due to the suspension of share repurchases following the announcement of the Acquisition in the fourth quarter of 2014, compared to $269.0 of share repurchases for 2014.
As part of its financing of the Acquisition, the Company entered into a $1,000.0 term loan and $2,900.0 in debt securities consisting of $500.0 aggregate principal amount of 2.625% Senior Notes due 2020, $500.0 aggregate principal amount of 3.20% Senior Notes due 2022, $1,000.0 aggregate principal amount of 3.60% Senior Notes due 2025 and $900.0 aggregate principal amount of 4.70% Senior Notes due 2045. The term loan credit facility will mature five years after the closing date of the Acquisition and may be prepaid without penalty. The term loan balance at December 31, 2015 was $715.0.
On December 19, 2014, the Company also entered into an amendment and restatement of its existing senior revolving credit facility, which was originally entered into on December 21, 2011. The senior revolving credit facility consists of a five-year revolving facility in the principal amount of up to $1,000.0, with the option of increasing the facility by up to an additional $250.0, subject to the agreement of one or more new or existing lenders to provide such additional amounts and certain other customary conditions. The new revolving credit facility also provides for a subfacility of up to $100.0 for swing line borrowings and a subfacility of up to $125.0 for issuances of letters of credit. The new revolving credit facility is permitted to be used for general corporate purposes, including working capital, capital expenditures, funding of share repurchases and certain other payments, and acquisitions and other investments. There was $0.0 outstanding on the Company's revolving credit facility at December 31, 2015 and 2014.
On February 13, 2015, the Company entered into a 60-day cash bridge term loan credit facility in the principal amount of $400.0 for the purpose of financing a portion of the cash consideration and the fees and expenses in connection with the Acquisition. The 60-day cash bridge term loan credit facility was entered into under the terms set forth in the bridge facility commitment letter for the $400.0 60-day cash bridge tranche. The 60-day cash bridge term loan credit facility was advanced in full on February 19, 2015, the date of the Company’s completion of the Acquisition. The 60-day cash bridge term loan credit facility was repaid in March 2015.
Under the term loan credit facility and the new revolving credit facility, the Company is subject to negative covenants limiting subsidiary indebtedness and certain other covenants typical for investment grade-rated borrowers and the Company is required to maintain a leverage ratio that varies. Prior to the Acquisition Date, the leverage ratio was required to have been no greater than 3.75 to 1.00, calculated by excluding the $2,900.0 Acquisition Notes. From and after the Acquisition Date, the leverage ratio must be no greater than 4.75 to 1.00 with respect to the last day of each of the first four fiscal quarters ending on or after the closing date, 4.25 to 1.00 with respect to the last day of each of the fifth through eighth fiscal quarters ending after the closing date, and 3.75 to 1.00 with respect to the last day of each fiscal quarter ending thereafter. The Company was in compliance with all covenants under the credit facilities at December 31, 2015. As of December 31, 2015, the ratio of total debt to consolidated EBITDA was 3.6 to 1.0.
The term loan credit facility accrues interest at a per annum rate equal to, at the Company’s election, either a LIBOR rate plus a margin ranging from 1.125% to 2.00%, or a base rate determined according to a prime rate or federal funds rate plus a margin ranging from 0.125% to 1.00%. Advances under the new revolving credit facility will accrue interest at a per annum rate equal to, at the Company’s election, either a LIBOR rate plus a margin ranging from 1.00% to 1.60%, or a base rate determined according to a prime rate or federal funds rate plus a margin ranging from 0.00% to 0.60%. Fees are payable on outstanding letters of credit under the new revolving credit facility at a per annum rate equal to the applicable margin for LIBOR loans, and the Company is required to pay a facility fee on the aggregate commitments under the new revolving credit facility, at a per annum rate ranging
54
from 0.125% to 0.40%. The interest margin applicable to the credit facilities, and the facility fee and letter of credit fees payable under the new revolving credit facility, are based on the Company’s senior credit ratings as determined by Standard & Poor’s and Moody’s, which are currently BBB and Baa2, respectively.
As of December 31, 2015, the effective interest rate on the revolving credit facility was 1.5% and the effective interest rate on the term loan was 1.7%.
Net cash used for financing activities for the year ended December 31, 2014 was $200.6 compared to $518.3 for the year ended December 31, 2013. The $317.7 decrease in the cash used for financing activities for the year ended December 31, 2014, as compared to the prior year, was primarily a result of a $746.6 decrease in repurchases of common stock partially offset by a net increase of $328.5 in debt financing in 2013.
As of December 31, 2015, the Company provided letters of credit aggregating $45.4, primarily in connection with certain insurance programs. Letters of credit provided by the Company are issued under the Company's revolving credit facility and are renewed annually, around mid-year.
As of December 31, 2015, the Company had outstanding authorization from the Board of Directors to purchase up to $789.5 of Company common stock based on settled trades as of that date. Following the announcement of the Acquisition in the fourth quarter of 2014, the Company suspended its share repurchases. The Company does not anticipate resuming its share repurchase activity until it approaches its targeted leverage ratio of total debt to consolidated EBITDA of 2.5 to 1.0. However, the Company will continue to evaluate all opportunities for strategic deployment of capital in light of market conditions.
The Company had a $36.9 and $24.9 reserve for unrecognized income tax benefits, including interest and penalties as of December 31, 2015 and December 31, 2014, respectively. The Acquisition accounted for substantially all of the increase. Substantially all of these tax reserves are classified in other long-term liabilities in the Company's Consolidated Balance Sheets at December 31, 2015 and December 31, 2014.
On September 11, 2015, the Company announced that for the period of September 12, 2015 to March 11, 2016, the zero-coupon subordinated notes will accrue contingent cash interest at a rate of no less than 0.125% of the average market price of a zero-coupon subordinated note for the five trading days ended September 9, 2015, in addition to the continued accrual of the original issue discount.
On January 4, 2016, the Company announced that its zero-coupon subordinated notes may be converted into cash and common stock at the conversion rate of 13.4108 per $1,000 principal amount at maturity of the notes, subject to the terms of the zero-coupon subordinated notes and the Indenture, dated as of October 24, 2006 between the Company and The Bank of New York Mellon, as trustee and conversion agent. In order to exercise the option to convert all or a portion of the zero-coupon subordinated notes, holders are required to validly surrender their zero-coupon subordinated notes at any time during the calendar quarter beginning January 1, 2016, through the close of business on the last business day of the calendar quarter, which is 5:00 p.m., New York City time, on Thursday, March 31, 2016. If notices of conversion are received, the Company plans to settle the cash portion of the conversion obligation with cash on hand and/or borrowings under the new revolving credit facility.
Credit Ratings
The Company’s debt ratings of Baa2 from Moody’s and BBB from S&P’s contribute to its ability to access capital markets.
Contractual Cash Obligations | |||||||||||||||||||
Payments Due by Period | |||||||||||||||||||
2017- | 2019- | 2021 and | |||||||||||||||||
Total | 2016 | 2018 | 2020 | thereafter | |||||||||||||||
Operating lease obligations | $ | 346.0 | $ | 73.4 | $ | 108.0 | $ | 59.6 | $ | 105.0 | |||||||||
Contingent future licensing payments (a) | 18.2 | 3.4 | 7.2 | 4.4 | 3.2 | ||||||||||||||
Minimum royalty payments | 5.9 | 1.0 | 1.9 | 2.0 | 1.0 | ||||||||||||||
Purchase obligations | 60.0 | 29.5 | 28.4 | 2.1 | — | ||||||||||||||
Scheduled interest payments on Senior Notes | 2,148.2 | 197.7 | 364.0 | 326.4 | 1,260.1 | ||||||||||||||
Scheduled interest payments on Term Loan | 62.0 | 14.5 | 31.6 | 15.9 | — | ||||||||||||||
Long-term debt, other than revolving credit facility | 5,525.0 | 325.0 | 900.0 | 1,100.0 | 3,200.0 | ||||||||||||||
Total contractual cash obligations (b) and (c) | $ | 8,165.3 | $ | 644.5 | $ | 1,441.1 | $ | 1,510.4 | $ | 4,569.3 | |||||||||
55
(a) | Contingent future licensing payments will be made if certain events take place, such as the launch of a specific test, the transfer of certain technology, and when specified revenue milestones are met. |
(b) | The table does not include obligations under the Company’s pension and postretirement benefit plans, which are included in “Note 16 to Consolidated Financial Statements.” Benefits under the Company's postretirement medical plan are made when claims are submitted for payment, the timing of which is not practicable to estimate. |
(c) | The table does not include the Company’s reserves for unrecognized tax benefits. The Company had a $36.9 and $24.9 reserve for unrecognized tax benefits, including interest and penalties, at December 31, 2015 and 2014, respectively, which is included in “Note 13 to Consolidated Financial Statements.” Substantially all of these tax reserves are classified in other long-term liabilities in the Company’s Consolidated Balance Sheets at December 31, 2015 and 2014. |
Off-Balance Sheet Arrangements
The Company does not have transactions or relationships with “special purpose” entities, and the Company does not have any off-balance sheet financing other than normal operating leases and letters of credits.
Other Commercial Commitments
As of December 31, 2015, the Company provided letters of credit aggregating approximately $45.4, primarily in connection with certain insurance programs. Letters of credit provided by the Company are secured by the new Company’s Revolving Credit Facility and are renewed annually, around mid-year.
The contractual value of the noncontrolling interest put in the Company's Ontario subsidiary totaled $14.9 and $17.7 at December 31, 2015 and 2014, respectively, which have been classified as mezzanine equity in the Company's consolidated balance sheet.
Based on current and projected levels of operations, coupled with availability under its Revolving Credit Facility, the Company believes it has sufficient liquidity to meet both its anticipated short-term and long-term cash needs; however, the Company continually reassesses its liquidity position in light of market conditions and other relevant factors.
New Accounting Pronouncements
In April 2014, the FASB issued a new accounting standard on discontinued operations that significantly changed criteria for discontinued operations and disclosures for disposals. Under this new standard, to be a discontinued operation, a component or group of components must represent a strategic shift that has (or will have) a major effect on an entity's operations and financial results. Expanded disclosures for discontinued operations include more details about earnings and balance sheet accounts, total operating and investing cash flows, and cash flows resulting from continuing involvement. The Company has adopted the guidance of this new standard effective beginning in 2015 and will apply it prospectively to all new disposals of components and new classifications as held for sale. The adoption of this standard did not have a material impact on the consolidated financial statements.
In May 2014, the FASB issued the converged standard on revenue recognition with the objective of providing a single, comprehensive model for all contracts with customers to improve comparability in the financial statements of companies reporting using International Financial Reporting Standards and U.S. Generally Accepted Accounting Principles. The standard contains principles that an entity must apply to determine the measurement of revenue and timing of when it is recognized. The underlying principle is that an entity must recognize revenue to depict the transfer of goods or services to customers at an amount that the entity expects to be entitled to in exchange for those goods or services. An entity can apply the revenue standard retrospectively to each prior reporting period presented (full retrospective method) or retrospectively with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earnings. As originally issued, the new revenue recognition standard would be effective for the Company beginning January 1, 2017. On July 9, 2015, the FASB approved the proposal to defer the effective date of this standard by one year. The standard will be effective for the Company beginning January 1, 2018, with early adoption permitted for annual periods beginning after December 16, 2016. The Company is currently evaluating the expected impact of the standard.
In August 2014, the FASB issued a new accounting standard that explicitly requires management to assess an entity's ability to continue as a going concern, and to provide related financial statement footnote disclosures in certain circumstances. Under this standard, in connection with each annual and interim period, management must assess whether there is substantial doubt about an entity's ability to continue as a going concern within one year after the financial statements are issued (or available to be issued when applicable). Management shall consider relevant conditions and events that are known and reasonably knowable at such issuance date. Substantial doubt about an entity's ability to continue as a going concern exists if it is probable that the entity will
56
be unable to meet its obligations as they become due within one year after issuance date. Disclosures will be required if conditions or events give rise to substantial doubt. This standard is effective for the Company for the annual period ending after December 15, 2016, with early adoption permitted. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In April 2015, the FASB issued an update which requires debt issuance costs to be presented in the balance sheet as a direct deduction from the associated debt liability. This standard became effective for the Company beginning January 1, 2016. The new guidance applies on a retrospective basis. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In May 2015, the FASB issued a new accounting standard allowing entities to exclude investments measured at net asset value per share under the existing practical expedient from the fair value hierarchy. In addition, when the net asset value practical expedient is not applied to eligible investments, certain other disclosures are no longer required. The standard became effective for the Company beginning January 1, 2016. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In July 2015, the FASB issued a new accounting standard that requires an entity to measure inventory, except inventory that is measured using last-in, first-out (LIFO) or the retail inventory method, at the lower of cost and net realizable value. Net realizable value is the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The standard became effective for the Company beginning January 1, 2016. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In September 2015, the FASB issued a new accounting standard that eliminates the requirement to restate prior period financial statements for measurement period adjustments. The standard requires that the cumulative impact of a measurement period adjustment, including the impact on prior periods, be recognized in the reporting period in which the adjustment is identified. The standard became effective for the Company beginning January 1, 2016 and applies prospectively to measurement period adjustments that occur after the effective date. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In November 2015, the FASB issued a new accounting standard that requires the deferred tax liabilities and assets to be classified as noncurrent on the consolidated balance sheet. The standard will be effective for the Company beginning January 1, 2017, with early adoption permitted. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In January 2016, the FASB issued a new accounting standard that addresses certain aspects of recognition, measurement, presentation and disclosure of financial instruments. A financial instrument is defined as cash, evidence of ownership interest in a company or other entity, or a contract that both: (i) imposes on one entity a contractual obligation either to deliver cash or another financial instrument to a second entity or to exchange other financial instruments on potentially unfavorable terms with the second entity and (ii) conveys to that second entity a contractual right either to receive cash or another financial instruments from the first entity or to exchange other financial instruments on potentially favorable terms with the first entity. The standard will be effective for the Company beginning January 1, 2018, with early adoption permitted. The Company is evaluating the impact that this new guidance will have on the consolidated financial statements.
Critical Accounting Policies
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reported periods. While the Company believes these estimates are reasonable and consistent, they are by their very nature, estimates of amounts that will depend on future events. Accordingly, actual results could differ from these estimates. The Company’s Audit Committee periodically reviews the Company’s significant accounting policies. The Company’s critical accounting policies arise in conjunction with the following:
• | Revenue recognition and allowance for doubtful accounts; |
• | Pension expense; |
• | Accruals for self insurance reserves; |
• | Income taxes; and |
• | Goodwill and indefinite-lived assets. |
57
Revenue Recognition and Allowance for Doubtful Accounts
LCD recognizes revenue for services rendered when the testing process is complete and test results are reported to the ordering physician. The sales are generally billed to three types of payers – clients, patients and third parties such as MCOs, Medicare and Medicaid. For clients, sales are recorded on a fee-for-service basis at the Company’s client list price, less any negotiated discount. Patient sales are recorded at the Company’s patient fee schedule, net of any discounts negotiated with physicians on behalf of their patients, or fees made available through charity care or an uninsured patient program. LCD bills third-party payers in two ways: fee-for-service and capitated agreements. Fee-for-service third-party payers are billed at the Company's patient fee schedule amount, and third-party revenue is recorded net of contractual discounts. These discounts are recorded at the transaction level at the time of sale based on a fee schedule that is maintained for each third-party payer. The majority of the Company’s third-party sales are recorded using an actual or contracted fee schedule at the time of sale. For the remaining third-party sales, estimated fee schedules are maintained for each payer. Adjustments to the estimated payment amounts are recorded at the time of final collection and settlement of each transaction as an adjustment to revenue. These adjustments are not material to the Company’s results of operations in any period presented. The Company periodically adjusts these estimated fee schedules based upon historical payment trends. Under capitated agreements with MCOs, the Company recognizes revenue based on a negotiated monthly contractual rate for each member of the managed care plan regardless of the number or cost of services performed.
CDD recognizes revenue either as services are performed or products are delivered, depending on the nature of the work contracted. Historically, a majority of CDD’s net revenues have been earned under contracts that range in duration from a few months to a few years, but can extend in duration up to five years or longer. CDD also has committed minimum volume arrangements with certain clients. Underlying these arrangements are individual project contracts for the specific services to be provided. These arrangements enable CDD's clients to secure its services in exchange for which they commit to purchase an annual minimum dollar value of services. Under these types of arrangements, if the annual minimum dollar value of service commitment is not reached, the client is required to pay CDD for the shortfall. Progress towards the achievement of annual minimum dollar value of service commitments is monitored throughout the year. Annual minimum commitment shortfalls are not included in net revenues until the amount has been determined and agreed to by the client.
Service contracts generally take the form of fee-for-service or fixed-price arrangements subject to pricing adjustments based on changes in scope. In cases where performance spans multiple accounting periods, revenue is recognized as services are performed, measured on a proportional-performance basis, generally using output measures that are specific to the service provided. Examples of output measures in early development services, include among others, the number of slides read, or specimens prepared for preclinical laboratory services, or number of dosings or number of volunteers enrolled for clinical pharmacology. Examples of output measures in the clinical trials services, include among others, number of investigators enrolled, number of sites initiated, number of patients enrolled and number of monitoring visits completed. Revenue is determined by dividing the actual units of work completed by the total units of work required under the contract and multiplying that percentage by the total contract value. The total contract value, or total contractual payments, represents the aggregate contracted price for each of the agreed upon services to be provided. CDD does not have any contractual arrangements spanning multiple accounting periods where revenue is recognized on a proportional-performance basis under which the Company has earned more than an immaterial amount of performance-based revenue (i.e., potential additional revenue tied to specific deliverables or performance). Changes in the scope of work are common, especially under long-term contracts, and generally result in a change in contract value. Once the client has agreed to the changes in scope and renegotiated pricing terms, the contract value is amended with revenue recognized as described above. Estimates of costs to complete are made to provide, where appropriate, for losses expected on contracts. Costs are not deferred in anticipation of contracts being awarded, but instead are expensed as incurred.
Billing schedules and payment terms are generally negotiated on a contract-by-contract basis. In some cases, CDD bills the client for the total contract value in progress-based installments as certain non-contingent billing milestones are reached over the contract duration, such as, but not limited to, contract signing, initial dosing, investigator site initiation, patient enrollment or database lock. The term “billing milestone” relates only to a billing trigger in a contract whereby amounts become billable and payable in accordance with a negotiated predetermined billing schedule throughout the term of a project. These billing milestones are generally not performance-based (i.e., there is no potential additional consideration tied to specific deliverables or performance). In other cases, billing and payment terms are tied to the passage of time (e.g., monthly billings). In either case, the total contract value and aggregate amounts billed to the client would be the same at the end of the project. While CDD attempts to negotiate terms that provide for billing and payment of services prior or within close proximity to the provision of services, this is not always possible, and there are fluctuations in the levels of unbilled services and unearned revenue from period to period. While a project is ongoing, cash payments are not necessarily representative of aggregate revenue earned at any particular point in time, as revenues are recognized when services are provided, while amounts billed and paid are in accordance with the negotiated billing and payment terms.
58
In some cases, payments received are in excess of revenue recognized. For example, a contract invoicing schedule may provide for an upfront payment of 10% of the full contract value upon contract signing, but at the time of signing performance of services has not yet begun. Payments received in advance of services being provided are deferred as unearned revenue on the balance sheet. As the contracted services are subsequently performed and the associated revenue is recognized, the unearned revenue balance is reduced by the amount of revenue recognized during the period.
In other cases, services may be provided and revenue recognized before the client is invoiced. In these cases, revenue recognized will exceed amounts billed, and the difference, representing an unbilled receivable, is recorded for the amount that is currently unbillable to the customer pursuant to contractual terms. Once the client is invoiced, the unbilled services are reduced for the amount billed, and a corresponding account receivable is recorded. All unbilled services are billable to customers within one year from the respective balance sheet date.
Most contracts are terminable with or without cause by the client, either immediately or upon notice. These contracts often require payment to CDD of expenses to wind down the study or project, fees earned to date and, in some cases, a termination fee or a payment to CDD of some portion of the fees or profits that could have been earned by CDD under the contract if it had not been terminated early. Termination fees are included in net revenues when services are performed and realization is assured. In connection with the management of multi-site clinical trials, CDD pays on behalf of its customers fees to investigators, volunteers and certain out-of-pocket costs, for which it is reimbursed at cost, without mark-up or profit. Investigator fees are not reflected in net revenues or expenses where CDD acts in the capacity of an agent on behalf of the pharmaceutical company sponsor, passing through these costs without risk or reward to CDD. All other out-of-pocket costs are included in total revenues and expenses.
LCD has a formal process to estimate and review the collectibility of its receivables based on the period of time they have been outstanding. Bad debt expense is recorded within selling, general and administrative expenses as a percentage of sales considered necessary to maintain the allowance for doubtful accounts at an appropriate level. LCD’s process for determining the appropriate level of the allowance for doubtful accounts involves judgment and considers such factors as the age of the underlying receivables, historical and projected collection experience, and other external factors that could affect the collectibility of its receivables. Accounts are written off against the allowance for doubtful accounts based on LCD’s write-off policy (e.g., when they are deemed to be uncollectible). In the determination of the appropriate level of the allowance, accounts are progressively reserved based on the historical timing of cash collections relative to their respective aging categories within LCD’s receivables. These collection and reserve processes, along with the close monitoring of the billing process, help reduce the risks of material revisions to reserve estimates resulting from adverse changes in collection or reimbursement experience.
The following table presents the percentage of LCD’s net accounts receivable outstanding by aging category at December 31, 2015 and 2014:
Days Outstanding | 2015 | 2014 | |
0 – 30 | 46.8% | 48.4% | |
31 – 60 | 16.9% | 18.6% | |
61 – 90 | 12.1% | 11.9% | |
91 – 120 | 7.4% | 7.1% | |
121 – 150 | 4.4% | 3.8% | |
151 – 180 | 4.1% | 3.6% | |
181 – 270 | 7.1% | 5.7% | |
271 – 360 | 1.1% | 0.7% | |
Over 360 | 0.1% | 0.1% | |
The above table excludes the percentage of net accounts receivable outstanding by aging category for certain foreign operations, BeaconLBS, and the nutritional chemistry and food safety business. Combined these net accounts receivable balances comprise less than 6.7% of LCD's total net accounts receivable balances. The Company believes that including the agings of the accounts receivables for these would not be representative of the majority of accounts receivable by aging category for LCD. The majority of the accounts receivables for the foreign operations, BeaconLBS, and the nutritional chemistry and food safety business are generally paid within 30 to 60 days of billing.
CDD endeavors to assess and monitor the creditworthiness of its customers to which it grants credit terms in the ordinary course of business. CDD maintains a provision for doubtful accounts relating to amounts due that may not be collected. This bad debt provision is monitored on a monthly basis and adjusted as circumstances warrant. Since the recorded bad debt provision is based upon management's judgment, actual bad debt write-offs may be greater or less than the amount recorded. Historically, bad debt write-offs have not been material. The allowance for doubtful accounts amounted to $3.4 at December 31, 2015.
59
Pension Expense
The Company has a defined benefit retirement plan (Company Plan) and a non-qualified supplemental retirement plan (PEP). In October 2009, the Company received approval from its Board of Directors to freeze any additional service-based credits for any years of service after December 31, 2009 on the Company Plan and the PEP. Both plans have been closed to new participants. Employees participating in the Company Plan and the PEP no longer earn service-based credits, but continue to earn interest credits. In addition, effective January 1, 2010, all employees eligible for the defined contribution retirement plan (401K Plan) receive a minimum 3% non-elective contribution (NEC) concurrent with each payroll period. The 401K Plan also permits discretionary contributions by the Company of up to 1% and up to 3% of pay for eligible employees, based on service.
The Company Plan covers substantially all employees hired prior to December 31, 2009. The benefits to be paid under the Company Plan are based on years of credited service through December 31, 2009, interest credits and average compensation. The Company's policy is to fund the Company Plan with at least the minimum amount required by applicable regulations. The PEP covers the Company's senior management group. Prior to 2010, the PEP provided for the payment of the difference, if any, between the amount of any maximum limitation on annual benefit payments under the Employee Retirement Income Security Act of 1974 and the annual benefit that would be payable under the Company Plan but for such limitation. Effective January 1, 2010, employees participating in the PEP no longer earn service-based credits. The PEP is an unfunded plan.
In addition, as a result of the Acquisition, the Company also has a frozen non-qualified Supplemental Executive Retirement Plan (SERP). The SERP, which is not funded, is intended to provide retirement benefits for certain employees who were executive officers of Covance prior to the Acquisition. Benefit amounts are based upon years of service and compensation of the participating employees. The Company also sponsors two defined benefit pension plans for the benefit of its employees at two U.K. subsidiaries (U.K. Plans) and one defined benefit pension plan for the benefit of its employees at a German subsidiary (German Plan), all of which are legacy plans of previously acquired companies. Benefit amounts for all three plans are based upon years of service and compensation. The German Plan is unfunded while the U.K. Plans are funded. The Company’s funding policy for the U.K. Plans has been to contribute annually amounts at least equal to the local statutory funding requirements.
The Company's net pension cost is developed from actuarial valuations. Inherent in these valuations are key assumptions, including discount rates and expected return on plan assets, which are updated on an annual basis at the beginning of each year. The Company is required to consider current market conditions, including changes in interest rates, in making these assumptions. Changes in pension costs may occur in the future due to changes in these assumptions. The key assumptions used in accounting for the defined benefit retirement plans were a 4.0% discount rate and a 7.0% expected long-term rate of return on plan assets for the Company Plan and the PEP, a 3.8% discount rate for the SERP, a 2.5% discount rate and a 2.0% expected salary increase for the German plan, a 3.8% discount rate and a 3.6% expected salary increase for the U.K. Plans as of December 31, 2015.
Discount Rate
The Company evaluates several approaches toward setting the discount rate assumption that is used to value the benefit obligations of its retirement plans. At year-end, priority was given to use of the Towers Watson Bond:Link model, which simulates the purchase of investment-grade corporate bonds at current market yields with principal amounts and maturity dates closely matching the Company's projected cash disbursements from its plans. This completed model represents the yields to maturity that the Company could theoretically settle its plan obligations at year end. The weighted-average yield on the modeled bond portfolio is then used to form the discount rate assumption used for each retirement plan. A one percentage point decrease or increase in the discount rate would have resulted in a respective increase or decrease in 2015 retirement plan expense of $1.8 for the Company Plan and PEP. A one percentage point decrease or increase in the discount rate would have resulted in a respective increase or decrease in 2015 retirement plan expense of $1.0 for the U.K. Plans.
Return on Plan Assets
In establishing its expected return on plan assets assumption, the Company reviews its asset allocation and develops return assumptions based on different asset classes adjusting for plan operating expenses. Actual asset over/under performance compared to expected returns will respectively decrease/increase unrecognized loss. The change in the unrecognized loss will change amortization cost in upcoming periods. A one percentage point increase or decrease in the expected return on plan assets would have resulted in a corresponding change in 2015 pension expense of $2.6 for the Company Plan and the SERP. A one percentage point increase or decrease in the expected return on plan assets would have resulted in a corresponding change in 2015 pension expense of $2.0 for the U.K. Plans.
Net pension cost for 2015 was $12.0 as compared with $8.1 in 2014 and $11.0 in 2013. The increase in pension expense was due to increases in the amount of net amortization and deferral as a result of lower discount rates. The decrease in pension expense
60
in 2014 was due to decreases in the amount of net amortization and deferral as a result of higher discount rates. Projected pension expense for the Company Plan and the PEP is expected to increase to $12.9 in 2016 as a result of a lower assumed discount rate and changes in participant mortality tables. Projected pension expense for the SERP is expected to increase to $0.4 in 2016 as a result of a one-time curtailment gain in 2015 offset by an increase in the assumed discount rate. Projected pension expense for the Germany Plan and the United Kingdom Plans is expected to increase to $2.4 in 2016 as a result of a lower assumed discount rate and changes in participant mortality tables.
Further information on the Company’s defined benefit retirement plan is provided in Note 16 to the consolidated financial statements.
Accruals for Self-Insurance Reserves
Accruals for self-insurance reserves (including workers’ compensation, auto and employee medical) are determined based on a number of assumptions and factors, including historical payment trends and claims history, actuarial assumptions and current and estimated future economic conditions. These estimated liabilities are not discounted.
The Company is self-insured (up to certain limits) for professional liability claims arising in the normal course of business, generally related to the testing and reporting of laboratory test results. The Company maintains excess insurance which limits the Company’s maximum exposure on individual claims. The Company estimates a liability that represents the ultimate exposure for aggregate losses below those limits. The liability is discounted and is based on actuarial assumptions and factors for known and incurred but not reported claims, including the frequency and payment trends of historical claims.
If actual trends differ from these estimates, the financial results could be impacted. Historical trends have not differed materially from these estimates.
Income Taxes
The Company accounts for income taxes utilizing the asset and liability method. Under this method deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and for tax loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company does not recognize a tax benefit, unless the Company concludes that it is more likely than not that the benefit will be sustained on audit by the taxing authority based solely on the technical merits of the associated tax position. If the recognition threshold is met, the Company recognizes a tax benefit measured at the largest amount of the tax benefit that the Company believes is greater than 50% likely to be realized. The Company records interest and penalties in income tax expense.
Goodwill and Indefinite-Lived Assets
The Company assesses goodwill and indefinite-lived intangibles for impairment at least annually or whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. Following the Acquisition, the Company changed the timing of its annual impairment testing to the beginning of the fourth quarter. In accordance with the FASB updates to their authoritative guidance regarding goodwill and indefinite-lived intangible asset impairment testing, an entity is allowed to first assess qualitative factors as a basis for determining whether it is necessary to perform quantitative impairment testing. If an entity determines that it is not more likely than not that the estimated fair value of an asset is less than its carrying value, then no further testing is required. Otherwise, impairment testing must be performed in accordance with the original accounting standards. The updated FASB guidance also allows an entity to bypass the qualitative assessment for any reporting unit in its goodwill assessment and proceed directly to performing the first step of the two-step assessment. Similarly, a company can proceed directly to a quantitative assessment in the case of impairment testing for indefinite-lived intangible assets as well.
Step One of the goodwill impairment test includes the estimation of the fair value of each reporting unit as compared to the carrying value of the reporting unit. Reporting units are businesses with discrete financial information that is available and reviewed by management. The Company estimates the fair value of a reporting unit using both income-based and market-based valuation methods. The income-based approach is based on the reporting unit's forecasted future cash flows that are discounted to the present value using the reporting unit's weighted average cost of capital. For the market-based approach, the Company utilizes a number of factors such as publicly available information regarding the market capitalization of the Company as well as operating results, business plans, market multiples, and present value techniques. Based upon the range of estimated values developed from the income and market-based methods, the Company determines the estimated fair value for the reporting unit. If the estimated fair
61
value of the reporting unit exceeds the carrying value, the goodwill is not impaired and no further review is required. However, if the estimated fair value is less than the carrying value, the Company performs the second step of the goodwill impairment test to measure the amount of the impairment, if any. The second step involves a hypothetical allocation of the estimated fair value of the reporting unit to its tangible and intangible assets (excluding goodwill) and liabilities as if the reporting unit were newly acquired, which results in an implied fair value of the goodwill. The amount of the impairment charge is the excess of the recorded goodwill, if any, over the implied fair value of the goodwill.
The income-based fair value methodology requires management's assumptions and judgments regarding economic conditions in the markets in which the Company operates and conditions in the capital markets, many of which are outside of management's control. At the reporting unit level, fair value estimation requires management's assumptions and judgments regarding the effects of overall economic conditions on the specific reporting unit, along with assessment of the reporting unit's strategies and forecasts of future cash flows. Forecasts of individual reporting unit cash flows involve management's estimates and assumptions regarding:
• | Annual cash flows, on a debt-free basis, arising from future revenues and profitability, changes in working capital, capital spending and income taxes for at least a five-year forecast period. |
• | A terminal growth rate for years beyond the forecast period. The terminal growth rate is selected based on consideration of growth rates used in the forecast period, historical performance of the reporting unit and economic conditions. |
• | A discount rate that reflects the risks inherent in realizing the forecasted cash flows. A discount rate considers the risk-free rate of return on long-term treasury securities, the risk premium associated with investing in equity securities of comparable companies, the beta obtained from the comparable companies and the cost of debt for investment grade issuers. In addition, the discount rate may consider any company-specific risk in achieving the prospective financial information. |
Under the market-based fair value methodology, judgment is required in evaluating market multiples and recent transactions. Management believes that the assumptions used for its impairment tests are representative of those that would be used by market participants performing similar valuations of the reporting units.
Management concluded that no triggering events occurred during 2015 related to goodwill and indefinite-lived intangible assets. As such, management performed its annual goodwill and intangible asset impairment testing as of the beginning of the fourth quarter of 2015. The Company elected to perform the qualitative assessment for goodwill and intangible assets, with the exception of the indefinite-lived Canadian licenses, for all reporting units.
In this qualitative assessment, the Company considered relevant events and circumstances for each reporting unit, including (i) current year results, ii) financial performance versus management’s annual and five-year strategic plans, iii) changes in the reporting unit carrying value since prior year, (iv) industry and market conditions in which the reporting unit operates, (v) macroeconomic conditions, including discount rate changes, and (vi) changes in products or services offered by the reporting unit. If applicable, performance in recent years was compared to forecasts included in prior valuations. Based on the results of the qualitative assessment, the Company concluded that it was not more likely than not that the carrying values of the goodwill and intangible assets were greater than their fair values, and that further quantitative testing was not necessary.
It is possible that the Company's conclusions regarding impairment or recoverability of goodwill or intangible assets in any reporting unit could change in future periods. There can be no assurance that the estimates and assumptions used in the Company's goodwill and intangible asset impairment testing performed as of the beginning of the fourth quarter of 2015 will prove to be accurate predictions of the future, if, for example, (i) the businesses do not perform as projected, (ii) overall economic conditions in 2016 or future years vary from current assumptions (including changes in discount rates), (iii) business conditions or strategies for a specific reporting unit change from current assumptions, including loss of major customers, (iv) investors require higher rates of return on equity investments in the marketplace or (v) enterprise values of comparable publicly traded companies, or actual sales transactions of comparable companies, were to decline, resulting in lower multiples of revenues and EBITDA. A future impairment charge for goodwill or intangible assets could have a material effect on the Company's consolidated financial position and results of operations.
In 2015, the Company utilized an income approach to determine the fair value of its Canadian reporting unit and its indefinite-lived assets consisting of acquired Canadian licenses. Based upon the results of the quantitative assessment, the Company concluded that the fair value of the indefinite-lived Canadian licenses was greater than the carrying value.
62
FORWARD-LOOKING STATEMENTS
The Company has made in this report forward-looking statements concerning the Company’s operations, performance and financial condition, as well as its strategic objectives. Some of these forward-looking statements can be identified by the use of forward-looking words such as “believes”, “expects”, “may”, “will”, “should”, “seeks”, “approximately”, “intends”, “plans”, “estimates”, or “anticipates” or the negative of those words or other comparable terminology. Such forward-looking statements are subject to various risks and uncertainties and the Company claims the protection afforded by the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995. Actual results could differ materially from those currently anticipated due to a number of factors in addition to those discussed elsewhere herein, including in Item 1A Risk Factors, and in the Company’s other public filings:
1. | changes in U.S., state, local and third party payer regulations or policies or other future reforms in the healthcare system (or in the interpretation of current regulations), new insurance or payment systems, including state, regional or private insurance cooperatives (e.g., Health Insurance Exchanges), affecting governmental and third-party coverage or reimbursement for clinical laboratory testing; |
2. | significant monetary damages, fines, penalties, assessments, refunds, repayments, and/or exclusion from the Medicare and Medicaid programs, among other adverse consequences, resulting from interpretations of, or future changes in, laws and regulations, including laws and regulations of Medicare, Medicaid, the U.S. False Claims Act, interpretations of such laws and regulations by U.S. or state government agencies or investigations, audits, regulatory examinations, information requests and other inquiries by state or U.S. government agencies; |
3. | significant fines, penalties, costs and/or damage to the Company’s reputation arising from the failure to comply with U.S. and international privacy and security laws and regulations, including the Health Insurance Portability and Accountability Act of 1996, Health Information Technology for Economic and Clinical Health Act, U.S. state laws and regulations, and laws and regulations of the European Union and other countries; |
4. | loss or suspension of a license or imposition of a fine or penalties under, or future changes in, or interpretations of, the law or regulations of the Clinical Laboratory Improvement Act of 1967, and the Clinical Laboratory Improvement Amendments of 1988; |
5. | penalties or loss of license arising from the failure to comply with the U.S. Occupational Safety and Health Administration requirements and the U.S. Needlestick Safety and Prevention Act, or similar laws and regulations of U.S., state, local or international agencies; |
6. | fines, unanticipated compliance expenditures, suspension of manufacturing, enforcement actions, injunctions, or criminal prosecution arising from failure to maintain compliance with current good manufacturing practice (cGMP) regulations and other applicable requirements of various regulatory agencies; |
7. | sanctions or other remedies, including fines, unanticipated compliance expenditures, enforcement actions, injunctions or criminal prosecution arising from failure to comply with the Animal Welfare Act; |
8. | changes in testing guidelines or recommendations by government agencies, medical specialty societies and other authoritative bodies affecting the utilization of laboratory tests; |
9. | changes in government regulations or policies, including regulations and policies of the FDA, the U.S. Department of Agriculture or other U.S., state, local or international agencies, affecting the approval, availability of, and the selling and marketing of diagnostic tests or drugs or the conduct of drug development trials; |
10. | changes in government regulations pertaining to the pharmaceutical and biotechnology industries, changes in reimbursement of pharmaceutical products or reduced spending on R&D by pharmaceutical and biotechnology customers; |
11. | liabilities that result from the inability to comply with corporate governance requirements; |
12. | increased competition, including price competition, competitive bidding and/or changes or reductions to fee schedules and competition from companies that do not comply with existing laws or regulations or otherwise disregard compliance standards in the industry; |
13. | changes in payer mix, including an increase in capitated reimbursement mechanisms or the impact of a shift to consumer-driven health plans and adverse changes in payer reimbursement or payer coverage policies related to specific testing procedures or categories of testing; |
14. | failure to retain or attract MCO business as a result of changes in business models, including new risk based or network approaches, or other changes in strategy or business models by MCOs; |
63
15. | failure to obtain and retain new customers or a reduction in tests ordered, specimens submitted or services requested by existing customers; |
16. | difficulty in maintaining relationships with customers or retaining key employees as a result of uncertainty surrounding the integration of acquisitions and the resulting negative effects on the business of the Company; |
17. | consolidation of MCOs, pharmaceutical companies, health systems, physicians and other customers affecting pricing and sales; |
18. | failure to effectively develop and deploy system modifications or enhancements required in response to evolving market and business needs; |
19. | customers choosing to insource services that are or could be purchased from the Company; |
20. | failure to identify, successfully close and effectively integrate and/or manage newly acquired businesses; |
21. | inability to achieve the expected benefits and synergies of newly-acquired businesses, and impact on the Company's cash position, levels of indebtedness and stock price; |
22. | inability of the Company to meet expectations regarding accounting and tax treatments related to the Acquisition; |
23. | termination, loss, delay, reduction in scope or increased costs of contracts, including large contracts and multiple contracts; |
24. | liability arising from errors or omissions in the performance of contract research services or other contractual arrangements; |
25. | changes or disruption in services or supplies provided by third parties, including transportation; |
26. | damage or disruption to the Company's facilities; |
27. | damage to the Company's reputation, loss of business, harm from acts of animal rights extremists or potential liability arising from animal research activities; |
28. | adverse results in litigation matters; |
29. | inability to attract and retain experienced and qualified personnel; |
30. | failure to develop or acquire licenses for new or improved technologies, such as point-of-care testing and mobile health technologies, or potential use of new technologies by customers to perform their own tests; |
31. | substantial costs arising from the inability to commercialize newly licensed tests or technologies or to obtain appropriate coverage or reimbursement for such tests; |
32. | inability to obtain and maintain adequate patent and other proprietary rights for protection of the Company's products and services and successfully enforce the Company's proprietary rights; |
33. | scope, validity and enforceability of patents and other proprietary rights held by third parties that may impact the Company's ability to develop, perform, or market the Company's products or services or operate its business; |
34. | business interruption or other impact on the business due to adverse weather (including hurricanes), fires and/or other natural disasters, terrorism or other criminal acts, and/or widespread outbreak of influenza or other pandemic illness; |
35. | discontinuation or recalls of existing testing products; |
36. | a failure in the Company's information technology systems, including with respect to testing turnaround time and billing processes, or the failure to maintain the security of business information or systems or to protect against cyber security attacks, or delays or failures in the development and implementation of the Company’s LabCorp Beacon platform, any of which could result in a negative effect on the Company’s performance of services, a loss of business or increased costs, damages to the Company’s reputation, significant litigation exposure, an inability to meet required financial reporting deadlines, or the failure to meet future regulatory or customer information technology, data security and connectivity requirements; |
37. | business interruption, increased costs, and other adverse effects on the Company's operations due to the unionization of employees, union strikes, work stoppages, general labor unrest or failure to comply with labor or employment laws; |
38. | failure to maintain the Company's days sales outstanding and/or bad debt expense levels including negative impact on the Company's reimbursement, cash collections and profitability arising from the failure of the Company, third party payers or physicians to comply with the ICD-10-CM Code Set, which was effective October 1, 2015, or arising from unfavorable changes in third party payer policies in connection with the implementation of the ICD-10-CM Code Set; |
64
39. | impact on the Company's revenue, cash collections and the availability of credit for general liquidity or other financing needs arising from a significant deterioration in the economy or financial markets or in the Company's credit ratings by S&P's and/or Moody's; |
40. | changes in reimbursement by foreign governments and foreign currency fluctuations; |
41. | inability to obtain certain billing information not usually provided to the Company by physicians, resulting in increased costs and complexity, a temporary disruption in receipts and ongoing reductions in reimbursements and net revenues; |
42. | expenses and risks associated with international operations, including but not limited to compliance with the Foreign Corrupt Practices Act, the U.K. Bribery Act, and laws and regulations that differ from those of the U.S., and economic, political, legal and other operational risks associated with foreign markets; and |
43. | failure to achieve expected efficiencies and savings in connection with Project LaunchPad, LCD's comprehensive, enterprise-wide business process improvement initiative. |
Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK |
Market risk is the potential loss arising from adverse changes in market rates and prices, such as foreign currency exchange rates, interest rates and other relevant market rate or price changes. In the ordinary course of business, the Company is exposed to various market risks, including changes in foreign currency exchange and interest rates, and the Company regularly evaluates the exposure to such changes. The Company addresses its exposure to market risks, principally the market risks associated with changes in foreign currency exchange rates and interest rates, through a controlled program of risk management that includes, from time to time, the use of derivative financial instruments such as foreign currency forward contracts and interest rate swap agreements. Although, as set forth below, the Company’s zero-coupon subordinated notes contain features that are considered to be embedded derivative instruments, the Company does not hold or issue derivative financial instruments for trading purposes.
Foreign Currency Exchange Rates
Approximately 17.7% of the Company's net revenues for year ended December 31, 2015 and approximately 6.8% of those for year ended 2014 were denominated in currencies other than the U.S. Dollar. The Company's financial statements are reported in U.S. Dollars and, accordingly, fluctuations in exchange rates will affect the translation of revenues and expenses denominated in foreign currencies into U.S. Dollars for purposes of reporting the Company's consolidated financial results. In 2015, the most significant currency exchange rate exposures were to the Canadian Dollar, Swiss Franc, Euro and British Pound. In 2014, the most significant currency exposure was to the Canadian Dollar. Excluding the impacts from any outstanding or future hedging transactions, a hypothetical change of 10% in average exchange rates used to translate all foreign currencies to U.S. Dollars would have impacted income before income taxes for 2015 by approximately $9.6. Gross accumulated currency translation adjustments recorded as a separate component of shareholders’ equity were $370.7 and $89.5 at December 31, 2015 and 2014, respectively. The Company does not have significant operations in countries in which the economy is considered to be highly-inflationary.
The Company earns revenue from service contracts over a period of several months and, in some cases, over a period of several years. Accordingly, exchange rate fluctuations during this period may affect the Company's profitability with respect to such contracts. The Company is also subject to foreign currency transaction risk for fluctuations in exchange rates during the period of time between the consummation and cash settlement of transactions. The Company limits its foreign currency transaction risk through exchange rate fluctuation provisions stated in some of its contracts with customers, or it may hedge transaction risk with foreign currency forward contracts. At December 31, 2015, the Company had 4 open foreign exchange forward contracts relating to service contracts with various amounts maturing monthly through January 2016 with a notional value totaling approximately $93.1 million. At December 31, 2014, the Company had no open foreign exchange forward contracts.
Interest Rates
Some of the Company's debt is subject to interest at variable rates. As a result, fluctuations in interest rates affect the business. The Company attempts to manage interest rate risk and overall borrowing costs through an appropriate mix of fixed and variable rate debt including by the utilization of derivative financial instruments, primarily interest rate swaps.
Borrowings under the Company's term loan credit facility and revolving credit facility are subject to variable interest rates, unless fixed through interest rate swaps or other agreements. As of December 31, 2015, the Company had approximately $715.0 of unhedged variable rate debt from the term loan credit facility. As of December 31, 2014 the Company had no outstanding balance on its revolving credit facility and no term loan facility.
65
During the third quarter of 2013, the Company entered into two fixed-to-variable interest rate swap agreements for the 4.625% senior notes due 2020 with an aggregate notional amount of $600.0 and variable interest rates based on one-month LIBOR plus 2.298% to hedge against changes in the fair value of a portion of the Company's long term debt.
The Company’s zero-coupon subordinated notes contain the following two features that are considered to be embedded derivative instruments under authoritative guidance in connection with accounting for derivative instruments and hedging activities:
1) | The Company will pay contingent cash interest on the zero-coupon subordinated notes after September 11, 2006, if the average market price of the notes equals 120% or more of the sum of the issue price, accrued original issue discount and contingent additional principal, if any, for a specified measurement period. |
2) | Holders may surrender zero-coupon subordinated notes for conversion during any period in which the rating assigned to the zero-coupon subordinated notes by S&P’s Ratings Services is BB- or lower. |
Each quarter-point increase or decrease in the variable rate would result in the Company's interest expense changing by approximately $1.8 per year for the Company's unhedged variable rate debt.
Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Reference is made to the Index on Page F-1 of the Financial Report included herein.
Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not Applicable.
Item 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
As of the end of the period covered by this report, the Company carried out under the supervision and with the participation of the Company’s management, including the Company’s principal executive officer and principal financial officer, an evaluation of the effectiveness of the Company's disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended). Based upon this evaluation, the Company’s principal executive officer and principal financial officer concluded that the Company’s disclosure controls and procedures were effective as of the end of the period covered by this annual report.
Changes in Internal Control Over Financial Reporting
On February 19, 2015, the Company completed the Acquisition. The Company’s management has extended its oversight and monitoring processes that support internal control over financial reporting to include Covance's operations. The Company’s management is continuing to integrate the acquired operations of Covance into the Company’s overall internal control over financial reporting process. However, management has excluded Covance from its assessment of internal controls over financial reporting set forth below. There have been no other changes in the Company’s internal control over financial reporting (as defined in Exchange Act Rules 13a-15(f) and 15d-15(f)) during the fiscal year ended December 31, 2015 that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Report of Management on Internal Control Over Financial Reporting
The Company's management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934).
The internal control over financial reporting at the Company was designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the U.S. Internal control over financial reporting includes those policies and procedures that:
• | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
• | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the U.S.; |
66
• | provide reasonable assurance that receipts and expenditures of the Company are being made only in accordance with authorization of management and directors of the Company; and |
• | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of assets that could have a material effect on the consolidated financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements.
The Company's management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2015. Management based this assessment on criteria for effective internal control over financial reporting described in “Internal Control - Integrated Framework 2013” issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on this assessment, the Company's management determined that, as of December 31, 2015, the Company maintained effective internal control over financial reporting. Management reviewed the results of its assessment with the Audit Committee of the Company’s Board of Directors.
On February 19, 2015, the Company completed the acquisition of Covance Inc. As a result, management has excluded Covance Inc. from its assessment of internal control over financial reporting. Covance Inc. is a wholly-owned subsidiary whose total assets and total revenues represent 48% and 25%, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2015.
PricewaterhouseCoopers LLP, an independent registered public accounting firm, who audited and reported on the consolidated financial statements of the Company included in this annual report, also audited the effectiveness of the Company’s internal control over financial reporting as of December 31, 2015 as stated in its report, which is included herein immediately preceding the Company’s audited financial statements.
Item 9B. | OTHER INFORMATION |
On December 18, 2015, James T. Boyle, Jr., Executive Vice President and Chief Executive Officer of LabCorp Diagnostics, announced his intention to retire no later than July 1, 2016.
PART III
Item 10. | DIRECTORS, EXECUTIVE OFFICERS and CORPORATE GOVERNANCE |
Board of Directors
David P. King - Mr. King (59) has served as Chairman of the Board, President, and Chief Executive Officer of the Company since May 6, 2009; prior to that date he served as a director, President and Chief Executive Officer of the Company since January 1, 2007. Mr. King served as Executive Vice President and Chief Operating Officer from December 2005 to January 2007, as Executive Vice President of Strategic Planning and Corporate Development from January 2004 to December 2005 and was hired in September 2001 as Senior Vice President, General Counsel and Chief Compliance Officer. Prior to joining the Company, he was a partner with Hogan & Hartson LLP (now Hogan Lovells US LLP) in Baltimore, Maryland from 1992 to 2001. Mr. King was appointed to the board of directors of Cardinal Health, Inc. in 2011 and chairs its Human Resources and Compensation Committee. He also sits on the Boards of Directors of the Seattle Science Foundation, PATH, Inc., and the American Clinical Laboratory Association, and on the Board of Trustees of Durham Academy. Mr. King has over 10 years’ experience with the Company in a variety of roles of increasing responsibility in corporate operations, strategic planning, and corporate administration. Mr. King has a deep understanding of the clinical laboratory industry, business strategy, sales and marketing and executive management of the Company and its operations.
Kerrii B. Anderson1,4 - Mrs. Anderson (58) has served as a director of the Company since May 17, 2006. Ms. Anderson was Chief Executive Officer of Wendy’s International, Inc., a restaurant operating and franchising company, from April 2006 until September 2008, when the company was merged with Triarc. Ms. Anderson served as Executive Vice President and Chief Financial Officer of Wendy’s International from 2000 to 2006. Prior to this position, she was Chief Financial Officer, Senior Vice President of M/I Schottenstein Homes, Inc. from 1987 to 2000. Ms. Anderson served as the Chairwoman of the Board of Chiquita Brands International Inc. from October 2012 until the Company was sold on January 6, 2015, and was the Chairwoman of the Nominating and Corporate Governance Committee and a Member of the Audit Committee. She is also a director and a member of the Compensation Committee and Audit Committee of Worthington Industries, Inc. Ms. Anderson serves on the financial committee of Columbus Foundation, on the Board of Trustees of Ohio Health and on the Board of Trustees for Elon University, and she chairs
67
the Audit Committee for Elon. She also was a director of PF Chang’s China Bistro, Inc. from 2010 until June 2012 and Wendy’s International from 2006 until September 30, 2008. She has a strong record of leadership in operations and strategy. Ms. Anderson is also an audit committee financial expert as a result of her experience as CEO and CFO of Wendy’s International, Inc. Through her service on other public company boards, Ms. Anderson brings extensive financial, corporate governance and executive compensation experience to the Company’s Board.
Jean-Luc Bélingard2,3 - Mr. Bélingard (67) has served as a director of the Company since April 28, 1995. Since 2011, Mr. Bélingard has been Chairman of bioMérieux, the worldwide leader of the IVD microbiology segment. Mr. Bélingard also served as Chief Executive Officer of bioMérieux from July 2011 to April 2014. Mr. Bélingard retired as Chairman and Chief Executive Officer of Ipsen SA, a diversified French healthcare holding company, on November 22, 2010. He had served in that position since 2001. Prior to this position, Mr. Bélingard was Chief Executive Officer from 1999 to 2001 of bioMérieux-Pierre Fabre, a diversified French healthcare holding company, where his responsibilities included the management of that company’s worldwide pharmaceutical and cosmetic business. Mr. Bélingard is also a director of Stallergenes (France). Mr. Bélingard was also a director of Celera Corporation, a former division of Applera Corporation, from May 2008 to May 2010. Mr. Bélingard served as a director and member of the Audit Committee of Nicox (France) from December 2002 to August 2010. Mr. Bélingard was a director of Applera Corporation, from 1993 to June 2008. From 1990 to 1999, Mr. Bélingard was CEO of Roche Diagnostics and a member of the Hoffman-La Roche group Executive Committee. Mr. Bélingard’s long tenure at Roche, Ipsen and bioMérieux demonstrates his valuable business, leadership and management experience, including leading a large organization with global operations. He brings a strong strategic and operational background to the Company’s Board. He also brings an important international perspective to the Board’s deliberations. Mr. Bélingard has extensive corporate governance experience through his service on other public company boards.
D. Gary Gilliland, M.D., Ph.D.1,3 - Dr. Gilliland (61) has served as a director of the Company since April 1, 2014. Since January 2, 2015, Dr. Gilliland has served as President and Director of the NCI-designated Fred Hutchinson Cancer Research Center in Seattle, WA. Prior to that, he was the inaugural Vice Dean and Vice President for Precision Medicine at the University of Pennsylvania Perelman School of Medicine, where he was responsible for synthesizing research and clinical-care initiatives across all medical disciplines including cancer, heart and vascular medicine, neurosciences, genetics and pathology, in order to create a national model for the delivery of precise, personalized medicine. From 2009 until he joined Penn Medicine in October 2013, Dr. Gilliland was Senior Vice President of Merck Research Laboratories and Oncology Franchise Head. At Merck, Dr. Gilliland oversaw first-in-human studies, proof-of-concept trials, and Phase II/III registration trials, and managed all preclinical and clinical oncology licensing activities. Prior to joining Merck, Dr. Gilliland was a member of the faculty at Harvard Medical School for nearly 20 years, where he served as Professor of Medicine and a Professor of Stem Cell and Regenerative Biology. He was also an Investigator of the Howard Hughes Medical Institute from 1996 to 2009, Director of the Leukemia Program at the Dana-Farber/Harvard Cancer Center from 2002 to 2009, and Director of the Cancer Stem Cell Program of the Harvard Stem Cell Institute from 2004 to 2009. Dr. Gilliland has a Ph.D. in Microbiology from UCLA and an M.D. from UCSF. He is board-certified in Internal Medicine and had his Fellowship training in Hematology and Oncology, all at Harvard Medicine School. Dr. Gilliland’s expertise in cancer genetics and his experience working within medical communities ranging from academia to the pharmaceutical industry position him to provide a practical and balanced perspective to the Board.
Garheng Kong, M.D.2,4 - Dr. Kong (40) has served as a director of the Company since December 1, 2013. Dr. Kong is the Managing Partner of Sofinnova HealthQuest Capital, a healthcare focused investment firm, and was previously a general partner at Sofinnova Ventures, a position he held from 2010 to 2013. Before joining Sofinnova, Dr. Kong was a general partner from 2000 to 2010 at Intersouth Partners, a venture capital firm where he was a founding investor or board member for various life science ventures, several of which were acquired by large pharmaceutical companies. Prior to his investing career, Dr. Kong was employed by GlaxoSmithKline, McKinsey & Company, and TherOx. Dr. Kong has served on the board of directors of Histogenics Corporation, a public biotechnology company where he also serves as the Chairman of the Board, since 2012. Since November 2008, Dr. Kong has been the Chairman of the Board of Cempra Pharmaceuticals, where he has been a board member since September 2006. Dr. Kong has been on the board of Alimera Sciences since October 2012 and sits on the Duke University Medical Center Board of Visitors. Dr. Kong holds an M.D., Ph.D. in Biomedical Engineering and an M.B.A. from Duke University. Dr. Kong brings to the Board knowledge and experience in both the healthcare and finance fields based on his medical background and his work in life science-related venture capital.
Robert E. Mittelstaedt, Jr.2,4 - Mr. Mittelstaedt, Jr. (72) has served as a director of the Company since November 1996. Mr. Mittelstaedt is Dean Emeritus of the W. P. Carey School of Business at Arizona State University where he served as Dean and Professor of Management from 2004 to 2013. Prior to June 30, 2004, he was Vice Dean, Executive Education of The Wharton School, University of Pennsylvania. Mr. Mittelstaedt had served with The Wharton School since 1973, with the exception of the period from 1985 to 1989 when he founded, served as Chief Executive Officer, and subsequently sold Intellego, Inc., a company engaged in practice management, systems development, and service bureau billing operations in the medical industry. Mr. Mittelstaedt serves as a director and Compensation Committee chair of W. P. Carey, Inc. and also serves as a director and
68
Compensation Committee chair of Innovative Solutions & Support, Inc. Mr. Mittelstaedt brings to the Board experience as a recognized expert in business strategy, corporate governance and executive compensation issues. Mr. Mittelstaedt serves as the Board’s Lead Independent Director and brings a deep understanding of the role of the Board and its oversight of corporate governance and business strategy.
Peter M. Neupert1,4 - Mr. Neupert (59) has served as a director of the Company since January 2013. Mr. Neupert was an Operating Partner at Health Evolution Partners, a health only, middle market private equity firm, from January 2012 until June 2015. Prior to that, Mr. Neupert served as Corporate Vice President of the Microsoft Health Solutions Group from its formation in 2005 to January 2012. Mr. Neupert served on the President’s Information Technology Advisory Committee (PITAC), co-chairing the Health Information Technology subcommittee and helping to drive the “Revolutionizing Health Care Through Information Technology” report, published in June 2004. Mr. Neupert served as the founding President and Chief Executive Officer of drugstore.com from 1998 to 2001 and as Chairman of the Board of Directors through September 2004. Mr. Neupert is also a director of Clinithink Ltd, Freedom Innovations LLC, Adaptive Biotechnologies, Inc. and higi LLC. He served on the board of directors of QSI from August 2013 to January 2014. He serves as a trustee for the Fred Hutchinson Cancer Research Center and was an active member of the Institute of Medicine’s Roundtable on Value & Science-Driven Healthcare from 2007-2011. Mr. Neupert brings to the Board experience as a recognized expert in health information technology and perspective on how to grow shareholder value leveraging business strategies with technology. His prior experience as a public company CEO and board member of both private and public companies brings practical insight to the Board with respect to business strategy, corporate governance and emerging trends in healthcare. Mr. Neupert’s previous business experience also enables him to provide the Board with an understanding of businesses and services adjacent to the diagnostic testing industry.
Richelle Parham - Ms. Parham (48) has been a director of the Company since January 8, 2016. Ms. Parham has more than 20 years of experience at such companies as eBay, Visa Inc., Digitas, Inc. (now DigitasLBi) and Citibank, leading end-to-end marketing efforts including brand, performance and loyalty marketing. Ms. Parham was most recently the Chief Marketing Officer of eBay, and prior to that she served in senior marketing roles with Visa Inc. and Digitas, a world leading marketing agency. Ms. Parham was named to the Forbes 2014 “50 Most Influential CMOs in the World” and Fast Company’s “Most Creative People in Business 1000.” Ms. Parham has been a member of the Board of Directors of Scripps Network Interactive Inc. since May 2012, and a member of the Drexel University Board of Trustees since May 2014. She holds double Bachelor of Science degrees in Business Administration and Design Arts from Drexel University.
Adam H. Schechter2,3 - Mr. Schechter (51) has served as a director of the Company since April 1, 2013. Mr. Schechter is an Executive Vice President of Merck & Co., Inc. and has been since 2010 President of Merck’s Global Human Health Division, which includes the company’s worldwide pharmaceutical and vaccine businesses. He is a member of Merck’s executive committee and pharmaceutical and vaccines operating committee. Prior to becoming President, Global Human Health, Mr. Schechter served as President, Global Pharmaceutical Business from 2007 to 2010. Mr. Schechter’s extensive experience at Merck includes global and U.S.-focused leadership roles spanning sales, marketing, and managed markets, as well as business and product development. Mr. Schechter serves on the board of directors for the European Federation of Pharmaceutical Industries and Associations. He is a Board Member for Water.org and an Executive Board Member for the National Alliance for Hispanic Health. Mr. Schechter brings to the Board global business acumen and general management experience, as well as demonstrated success in leading large, innovation-focused organizations. Mr. Schechter’s deep knowledge of the pharmaceutical and healthcare industries and extensive experience collaborating with many of its key stakeholders to achieve patient-focused outcomes brings practical insight to the Board with respect to business strategies to service the changing healthcare environment.
R. Sanders Williams, M.D.1,3 - Dr. Williams, M.D. (66) has served as a director of the Company since May 16, 2007. Dr. Williams is President of The J. David Gladstone Institutes and Professor of Medicine at the University of California San Francisco. Prior to this appointment, Dr. Williams served Duke University between 2001 and 2010 as Dean of the School of Medicine, Senior Vice Chancellor, Senior Advisor for International Strategy, and founding Dean of the Duke-NUS Graduate Medical School Singapore. He has served previously as President of the Association of University Cardiologists, Chairman of the Research Committee of the American Heart Association, on the editorial boards of leading biomedical journals, on the Advisory Committee to the Director of the National Institutes of Health and on the Board of External Advisors of the National Heart, Lung and Blood Institute. Dr. Williams was a director of Bristol-Meyers Squibb Company from 2006 until May 2013 and has been a director of Amgen since October 2014. Dr. Williams is a member of the National Academy of Medicine, and a Fellow of the American Association for the Advancement of Science. His experience as a physician, biomedical scientist, and executive leader brings important perspective to his service to the Company as a director.
Committees:
1 Audit
2 Compensation
3 Quality and Compliance
69
4 Nominating and Corporate Governance
Management Team
David P. King - Mr. King serves as Chairman of the Board, President, and Chief Executive Officer. Refer to the biography above in the Board of Directors section.
Glenn A. Eisenberg - Mr. Eisenberg has served as Executive Vice President, Chief Financial Officer since June 2014. Mr. Eisenberg received his Bachelors of Arts degree from Tulane University in 1982 and his Master of Business Administration from Georgia State University in 1988. From 2002 until he joined the Company, he served as the Executive Vice President, Finance and Administration and Chief Financial Officer at The Timken Company, a $4.3 billion leading global manufacturer of highly engineered bearings and alloy steels and related products and services. Previously, he served as President and Chief Operating Officer of United Dominion Industries, now a subsidiary of SPX Corporation after working in several roles in finance, including Executive Vice President and Chief Financial Officer of United Dominion. Mr. Eisenberg served on the boards of directors of Family Dollar Stores Inc. until July 2015, where he chaired the Audit Committee, and Alpha Natural Resources Inc. until May 2015, where he was the lead independent director and chaired the Nominating and Corporate Governance Committee.
James T. Boyle, Jr. - Mr. Boyle has served as Executive Vice President and Chief Executive Officer of LabCorp Diagnostics since February 19, 2015. Prior to that, he served as Executive Vice President, Chief Operating Officer since October 2009. Prior to October 2009, Mr. Boyle was Senior Vice President, Managed Care since May 2006. In December of 2008, Mr. Boyle also assumed operating responsibility for the Company’s Occupational Testing/Employer Group Services in his then current role of Senior Vice President of Managed Care/OTS. Mr. Boyle previously held the position of Vice President of Managed Care from August 2004 to May 2006. Prior to that Mr. Boyle was the Director of Litigation and Assistant General Counsel from 1999 to 2004. Prior to joining the Company in 1999, Mr. Boyle was engaged in the private practice of law for more than 15 years, specializing in litigation. He currently serves as a member of the Seton Hall University Board of Regents.
Deborah L. Keller - Ms. Keller serves as the Chief Executive Officer of Covance Drug Development, a position she has held since August 2015. Prior to being named as the Chief Executive Officer in August 2015, Ms. Keller had served as the Executive Vice President, Covance and Group President, R&D Laboratories since November 2010, which includes Central Laboratory Services, Nonclinical Safety Assessment, Analytical Services, and Discovery and Translational Services. Ms. Keller holds a Bachelor of Science in chemistry and Bachelor of Business Administration in management & accounting from Nazareth College and a Masters in Business Administration from the University of Wisconsin-Madison. She is a board member for BioCrossroads, an organization that promotes Indiana’s life science industry, and serves on the advisory board of the Indiana University Kelley School of Business Life Sciences Program. A member of the American Association of Pharmaceutical Society and the Society of Toxicology, Ms. Keller was named one of FierceBiotech’s 10 Top Women in Biotech 2012.
Lance V. Berberian - Mr. Berberian has served as Senior Vice President, Chief Information Officer since February 2014. Prior to that, he served as Chief Information Officer at IDEXX Laboratories, a global leader in diagnostics and IT solutions for animal health and food and water quality, from May 2007 to January 2014. Mr. Berberian served as Chief Information Officer and President of Kellstrom Aerospace Defense, a fully integrated supply chain firm, from January 2000 to April 2007. He also served as Chief Information Officer of Interim Healthcare from September 1997 to January 2000.
Edward T. Dodson - Mr. Dodson has served as Senior Vice President, Chief Accounting Officer since June 2005. He also has served as the Principal Accounting Officer since December 2014. Mr. Dodson, who has been a Certified Public Accountant for 32 years, joined the Company in August 1997 as Vice President and Corporate Controller and became Senior Vice President in June 2001. Prior to joining the Company in 1997, Mr. Dodson was a senior manager in the audit and consulting practice of KPMG, LLP., where he worked for 17 years in that firm's Greensboro, NC and Brussels, Belgium offices.
F. Samuel Eberts III - Mr. Eberts has served as Senior Vice President, Chief Legal Officer, Secretary and Chief Compliance Officer since January 1, 2009. Prior to that time he served as Senior Vice President, General Counsel since August 2004. Prior to joining the Company, he was Vice President, Secretary, and General Counsel of Stepan Company. Before joining Stepan Company, he was Assistant General Counsel for Cardinal Health, Inc. from 1998 to 2001 and Associate General Counsel for Allegiance Healthcare Corporation (Allegiance Healthcare Corporation was purchased by Cardinal Health in 1998). Prior to that time, he was Chief Counsel of the Biotech North America division of Baxter International Inc.
Lisa J. Uthgenannt - Mrs. Uthgenannt has served as Chief Human Resources Officer since March 2015. Prior to that she served as Senior Vice President Human Resources for Covance since she joined Covance in November 2010. Prior to joining Covance, Ms. Uthgenannt held numerous leadership positions at Johnson & Johnson, in both medical devices and pharmaceutical businesses since 2000. In her last role as Vice President, Human Resources for the Comprehensive Care sector, she served as a key advisor
70
and executive coach to the Worldwide Chairman, helping to define the initial strategies and plans to advance the corporation’s comprehensive approach to chronic disease management. She also led the organization in designing and implementing a streamlined business model to increase product pipeline performance and accelerated growth opportunities.
Item 11. | EXECUTIVE COMPENSATION |
The information required by this item is incorporated by reference to information in the 2016 Proxy Statement under the captions “Executive Compensation” and “Director Compensation.”
Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
See “Note 14 to the Consolidated Financial Statements” for a discussion of the Company’s Stock Compensation Plans. Except for the above referenced footnote, the information called for by this item is incorporated by reference to information in the 2016 Proxy Statement under the captions “Security Ownership of Certain Beneficial Holders and Management,” “Compensation Discussion and Analysis” and “Executive Compensation.”
Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required by this item is incorporated by reference to information in the 2016 Proxy Statement under the captions “Board Independence” and “Related Party Transactions.”
Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required by this item is incorporated by reference to information in the 2016 Proxy Statement under the caption “Fees to Independent Registered Public Accounting Firm.”
71
PART IV
Item 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) List of documents filed as part of this report:
(1) | Consolidated Financial Statements and Report of Independent Registered Public Accounting Firm included herein: |
See Index on page F-1 | |
(2) | Financial Statement Schedules: |
See Index on page F-1 | |
All other schedules are omitted as they are inapplicable or the required information is furnished in the Consolidated Financial Statements or notes thereto. | |
(3) | Index to and List of Exhibits |
Exhibits 10.1 through 10.37 are management contracts or compensatory plans or arrangements.
72
2.1 | Asset Purchase Agreement, dated as of September 13, 2010, between the Company and Genzyme Corporation (incorporated herein by reference to Exhibit 2.1 to the Company's Current Report on Form 8-K filed on September 16, 2010). |
2.2 | Agreement and Plan of Merger, dated as of November 2, 2014, among the Company, Covance, Inc. and Neon Merger Sub, Inc. (incorporated herein by reference to Exhibit 2.1 to the Company's Current Report on Form 8-K filed on November 3, 2014). |
3.1 | Amended and Restated Certificate of Incorporation of the Company dated May 24, 2001 (incorporated herein by reference to Exhibit 3.1 to the Company's Registration Statement on Form S-3, filed with the Commission on October 19, 2001, File No. 333-71896). |
3.2 | Amended and Restated By-Laws of the Company dated March 25, 2008 (incorporated herein by reference to Exhibit 3.1 to the Company's current report on Form 8-K, filed with the Commission on March 31, 2008). |
4.1 | Specimen of the Company's Common Stock Certificate (incorporated herein by reference to Exhibit 4.1 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2001). |
4.2 | Registration Rights Agreement, dated as of January 28, 2003 between the Company and the Initial Purchasers (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K, filed with the Commission on February 3, 2003). |
4.3 | Indenture, dated as of January 31, 2003 between the Company and Wachovia Bank, National Association, as trustee (incorporated herein by reference to Exhibit 4.1 to the Company's Current Report on Form 8-K, filed with the Commission on February 3, 2003). |
4.4 | Indenture dated as of December 5, 2005, between the Company and The Bank of New York Trust Company, N.A., as trustee (Senior Debt Securities) (incorporated herein by reference to Exhibit 4.1 to the Company's Current Report on Form 8-K filed on December 14, 2005). |
4.5 | Indenture, dated as of October 23, 2006, between the Company and The Bank of New York, as trustee, including the Form of Global Note attached as Exhibit A thereto (incorporated herein by reference to Exhibit 4.1 to the Company's Current Report on Form 8-K filed on October 24, 2006). |
4.6 | Indenture, dated as of November 19, 2010, between the Company and U.S. Bank National Association, as trustee (incorporated herein by reference to Exhibit 4.1 to the Company's Current Report on Form 8-K filed on November 19, 2010). |
4.7 | First Supplemental Indenture, dated as of November 19, 2010, between the Company and U.S. Bank National Association, as trustee, including the form of the 2016 Notes (incorporated herein by reference to Exhibit 4.2 to the Company's Current Report on Form 8-K filed on November 19, 2010). |
4.8 | Second Supplemental Indenture, dated as of November 19, 2010, between the Company and U.S. Bank National Association, as trustee, including the form of the 2020 Notes (incorporated herein by reference to Exhibit 4.3 to the Company's Current Report on Form 8-K filed on November 19, 2010). |
4.9 | Third Supplemental Indenture, dated as of August 23, 2012, between the Company and U.S. Bank National Association, as trustee, including the form of the 2017 Notes (incorporated herein by reference to Exhibit 4.2 to the Company's Current Report on Form 8-K filed on August 23, 2012). |
4.10 | Fourth Supplemental Indenture, dated as of August 23, 2012, between the Company and U.S. Bank National Association, as trustee, including the form of the 2022 Notes (incorporated herein by reference to Exhibit 4.2 to the Company's Current Report on Form 8-K filed on August 23, 2012). |
4.11 | Fifth Supplemental Indenture, dated as of November 1, 2013, between the Company and U.S. Bank National Association, as trustee, including the form of the 2018 Notes (incorporated herein by reference to Exhibit 4.2 to the Company's Current Report on Form 8-K filed on November 1, 2013). |
4.12 | Sixth Supplemental Indenture, dated as of November 1, 2013, between the Company and U.S. Bank National Association, as trustee, including the form of the 2023 Notes (incorporated herein by reference to Exhibit 4.3 to the Company's Current Report on Form 8-K filed on November 1, 2013). |
4.13 | Seventh Supplemental Indenture, dated as of January 30, 2015, between the Company and U.S. Bank National Association, as trustee, including the form of the 2020 Notes (incorporated herein by reference to Exhibit 4.2 to the Company's Current Report on Form 8-K filed on January 30, 2015). |
4.14 | Eighth Supplemental Indenture, dated as of January 30, 2015, between the Company and U.S. Bank National Association, as trustee, including the form of the 2022 Notes (incorporated herein by reference to Exhibit 4.3 to the Company's Current Report on Form 8-K filed on January 30, 2015). |
4.15 | Ninth Supplemental Indenture, dated as of January 30, 2015, between the Company and U.S. Bank National Association, as trustee, including the form of the 2025 Notes (incorporated herein by reference to Exhibit 4.4 to the Company's Current Report on Form 8-K filed on January 30, 2015). |
4.16 | Tenth Supplemental Indenture, dated as of January 30, 2015, between the Company and U.S. Bank National Association, as trustee, including the form of the 2045 Notes (incorporated herein by reference to Exhibit 4.5 to the Company's Current Report on Form 8-K filed on January 30, 2015). |
10.1 | National Health Laboratories Incorporated Pension Equalization Plan (incorporated herein by reference to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 1992). |
73
10.2 | Laboratory Corporation of America Holdings amended and restated new Pension Equalization Plan (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the period ended September 30, 2004). |
10.3 | First Amendment to the Laboratory Corporation of America Holdings amended and restated new Pension Equalization Plan (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the period ended September 30, 2004). |
10.4 | Second Amendment to the Laboratory Corporation of America Holdings amended and restated new Pension Equalization Plan. (incorporated herein by reference to Exhibit 10.4 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2004). |
10.5 | National Health Laboratories 1988 Stock Option Plan, as amended (incorporated herein by reference to the Company's Registration Statement on Form S-1, filed with the Commission on July 9, 1990, File No. 33-35782). |
10.6 | National Health Laboratories 1994 Stock Option Plan (incorporated herein by reference to Exhibit 4.1 to the Company's Registration Statement on Form S-8, filed with the Commission on August 12, 1994, File No. 33-55065). |
10.7 | Laboratory Corporation of America Holdings Senior Executive Transition Policy (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the period ended June 30, 2004). |
10.8 | Laboratory Corporation of America Holdings 1995 Stock Plan for Non-Employee Directors (incorporated herein by reference Exhibit 4.c to the Company's Registration Statement on Form S-8, filed with the Commission on September 26, 1995, File No. 33-62913). |
10.9 | First Amendment to Laboratory Corporation of America Holdings 1995 Stock Plan for Non-Employee Directors (incorporated herein by reference to Annex II to the Company's Definitive Proxy Statement on Schedule 14A, filed with the Commission on June 6, 1997). |
10.10 | Second Amendment to the Laboratory Corporation of America Holdings 1995 Stock Plan for Non-Employee Directors (incorporated herein by reference to Annex I of the Company's Definitive Proxy Statement on Schedule 14A, filed with the Commission on April 25, 2001). |
10.11 | Laboratory Corporation of America Holdings 1997 Employee Stock Purchase Plan (incorporated herein by reference to Exhibit 99.1 of the Company's Registration Statement on Form S-8 filed with the Commission on December 13, 1996, File No. 333-17793). |
10.12 | Amendments to the Laboratory Corporation of America Holdings 1997 Employee Stock Purchase Plan (incorporated herein by reference to Exhibit 4.1 to the Company's Registration Statement on Form S-8, filed with the Commission on January 10, 2000, File No. 333-94331). |
10.13 | Amendments to the Laboratory Corporation of America Holdings 1997 Employee Stock Purchase Plan (incorporated herein by reference to Exhibit 4.2 to the Company's Registration Statement on Form S-8, filed with the Commission on May 26, 2004, File No. 333-115905). |
10.14 | Fourth Amendment to the Laboratory Corporation of America Holdings 1997 Employee Stock Purchase Plan (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed on May 2, 2012). |
10.15 | Laboratory Corporation of America Holdings Amended and Restated 1999 Stock Incentive Plan (incorporated herein by reference to Annex I to the Company's Definitive Proxy Statement on Schedule 14A filed with the Commission on May 3, 1999). |
10.16 | Laboratory Corporation of America Holdings 2000 Stock Incentive Plan (incorporated herein by reference to Exhibit 4.3 to the Company's Registration Statement on Form S-8, filed with the Commission on June 5, 2000, File No. 333-38608). |
10.17 | Laboratory Corporation of America Holdings 2000 Stock Incentive Plan as Amended and Restated April 3, 2002, (incorporated herein by reference to Exhibit 4.1 to the Company's Registration Statement on Form S-8, filed with the Commission on June 19, 2002, File No. 333-90764). |
10.18 | Dynacare Inc., Amended and Restated Employee Stock Option Plan (incorporated herein by reference to Exhibit 10.1 to the Company's Registration Statement on Form S-8, filed with the Commission on August 7, 2002, File No. 333-97745). |
10.19 | DIANON Systems, Inc. 1996 Stock Incentive Plan (incorporated herein by reference to Exhibit 10.1 the Company's Registration Statement on Form S-8, filed with the Commission on January 21, 2003, File No. 333-102602). |
10.20 | DIANON Systems, Inc. 1999 Stock Incentive Plan (incorporated herein by reference to Exhibit 10.1 the Company's Registration Statement on Form S-8, filed with the Commission on January 21, 2003, File No. 333-102602). |
10.21 | DIANON Systems, Inc. 2000 Stock Incentive Plan(incorporated herein by reference to Exhibit 10.1 to the Company's Registration Statement on Form S-8, filed with the Commission on January 21, 2003, File No. 333-102602). |
10.22 | DIANON Systems, Inc. 2001 Stock Incentive Plan (incorporated herein by reference Exhibit 10.1 to the Company's Registration Statement on Form S-8, filed with the Commission on January 21, 2003, File No. 333-102602). |
10.23 | UroCor, Inc. Second Amended and Restated 1992 Stock Option Plan (incorporated herein by reference Exhibit 10.5 to the Company's Registration Statement on Form S-8, filed with the Commission on January 21, 2003, File No. 333-102602). |
74
10.24 | Laboratory Corporation of America Holdings Deferred Compensation Plan (incorporated herein by reference to Exhibit 10.22 the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2004). |
10.25 | First Amendment to the Laboratory Corporation of America Holdings Deferred Compensation Plan (incorporated herein by reference to Exhibit 10.23 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2004). |
10.26 | Second Amendment to the Laboratory Corporation of America Holdings Deferred Compensation Plan (incorporated herein by reference to Exhibit 10.8 to the Company's Quarterly Report on Form 10-Q for the period ended June 30, 2005). |
10.27 | Third Amendment to the Laboratory Corporation of America Amended and Restated New Pension Equalization Plan (incorporated herein by reference Exhibit 10.28 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2006). |
10.28 | Third Amendment to the Laboratory Corporation of America Holdings Deferred Compensation Plan (incorporated herein by reference to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2006). |
10.29 | Fourth Amendment to the Laboratory Corporation of America Holdings Deferred Compensation Plan (incorporated herein by reference to Exhibit 10.34 to the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2007). |
10.30 | Consulting Agreement between Thomas P. Mac Mahon and the Company dated July 20, 2006 (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on July 21, 2006). |
10.31 | Laboratory Corporation of America Holdings 2008 Stock Incentive Plan (incorporated herein by reference to Annex III to the Company's Definitive Proxy Statement on Schedule 14A filed on March 28, 2008). |
10.32 | Amendment to Laboratory Corporation of America Holdings 2008 Stock Incentive Plan (incorporated herein by reference to Exhibit 10.2 to the Company's Current Report on Form 8-K filed on May 7, 2008). |
10.33 | Laboratory Corporation of America Holdings Amended and Restated Master Senior Executive Severance Plan (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the period ended March 31, 2009). |
10.34 | Laboratory Corporation of America Holdings Master Senior Executive Change in Control Severance Plan (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the period ended March 31, 2009). |
10.35 | First Amendment to the Laboratory Corporation of America Holdings Master Senior Executive Change in Control Severance Plan (incorporated herein by reference to Exhibit 10.1 to the Company's Quarterly Report on Form 10-Q for the period ended March 31, 2010). |
10.36 | Second Amendment to the Laboratory Corporation of America Holdings Master Senior Executive Change in Control Severance Plan (incorporated herein by reference to Exhibit 10.2 to the Company's Quarterly Report on Form 10-Q for the period ended March 31, 2010). |
10.37 | Laboratory Corporation of America Holdings 2012 Omnibus Incentive Plan (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on May, 2, 2012). |
10.38 | Bridge Facility Commitment Letter, dated as of November 2, 2014, by and among the Company, Bank of America, N.A., Merrill Lynch, Pierce, Fenner & Smith Incorporated, Wells Fargo Bank, National Association and Wells Fargo Securities, LLC (incorporated herein by reference to Exhibit 10.1 to the Company's Current Report on Form 8-K filed on November 3, 2014). |
10.39 | Amended and Restated Credit Agreement, dated as of December 19, 2014, (originally dated as of December 21, 2011) among the Company, Bank of America, N.A. as Administrative Agent, Swing Line Lender and L/C Issuer, Wells Fargo Bank, National Association as Syndication Agent and L/C Issuer, Credit Suisse AG, Cayman Islands Branch as Documentation Agent and L/C Issuer, the Bank of Tokyo-Mitsubishi UFJ, LTD., Barclays Bank PLC, Credit Suisse AG, Cayman Island Branch, KeyBank National Association, PNC Bank, National Association, TD Bank, N.A., and U.S. Bank National Association, as Documentation Agents, Merrill Lynch, Pierce, Fenner & Smith Incorporated, Wells Fargo Securities, LLC and Credit Suisse Securities (USA) LLC as Joint Lead Arrangers and Joint Book Managers, and the lenders named therein (incorporated herein by reference to Exhibit 10.39 to the Company's Annual Report on Form 10-K filed on February 26, 2015). |
10.40 | Term Loan Credit Agreement, dated as of December 19, 2014, among the Company, Bank of America, N.A., as Administrative Agent, Wells Fargo Bank, National Association, as Syndication Agent, the Bank of Tokyo-Mitsubishi UFJ, LTD., Barclays Bank PLC, Credit Suisse AG, Cayman Islands Branch, KeyBank National Association, PNC Bank, National Association, TD Bank, N.A. and U.S. Bank National Association, as Documentation Agents, Merrill Lynch, Pierce, Fenner & Smith Incorporated, Wells Fargo Securities, LLC and Credit Suisse Securities (USA) LLC as Joint Lead Arrangers and Joint Book Managers, and the lenders named therein (incorporated herein by reference to Exhibit 10.40 to the Company's Annual Report on Form 10-K filed on February 26, 2015). |
10.41 | Amendment No. 1, dated as of March 5, 2015, to the Term Loan Credit Agreement dated as of December 19, 2014, with Bank of America, N.A. (incorporated herein by reference to Exhibit 10.6 to the Company's Quarterly Report on Form 10-Q filed on May 4, 2015). |
12.1* | Ratio of earnings to fixed charges |
21* | List of Subsidiaries of the Company |
23.1* | Consent of PricewaterhouseCoopers LLP, an independent registered public accounting firm |
24.1* | Power of Attorney of Kerrii B. Anderson |
24.2* | Power of Attorney of Jean-Luc Bélingard |
24.3* | Power of Attorney of D. Gary Gilliland |
24.4* | Power of Attorney of Garheng Kong, M.D., Ph.D. |
24.5* | Power of Attorney of Robert E. Mittelstaedt, Jr. |
24.6* | Power of Attorney of Peter M. Neupert |
24.7* | Power of Attorney of Richelle Parham |
24.8* | Power of Attorney of Adam H. Schechter |
24.9* | Power of Attorney of R. Sanders Williams, M.D. |
31.1* | Certification by the Chief Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) |
31.2* | Certification by the Chief Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) |
32* | Written Statement of Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 (18 U.S.C. Section 1350) |
101.INS* | XBRL Instance Document |
101.SCH* | XBRL Taxonomy Extension Schema |
101.CAL* | XBRL Taxonomy Extension Calculation Linkbase |
101.DEF* | XBRL Taxonomy Extension Definition Linkbase |
101.LAB* | XBRL Taxonomy Extension Label Linkbase |
101.PRE* | XBRL Taxonomy Extension Presentation Linkbase |
* | Filed herewith |
75
76
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
LABORATORY CORPORATION OF AMERICA HOLDINGS
Registrant
By: | /s/ DAVID P. KING | ||
David P. King | |||
Chairman of the Board, President | |||
and Chief Executive Officer | |||
Dated: | February 29, 2016 | ||
77
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant on February 29, 2016 in the capacities indicated.
Signature | Title | |
/s/ DAVID P. KING | Chairman of the Board, President and Chief | |
David P. King | Executive Officer (Principal Executive Officer) | |
/s/ GLENN A. EISENBERG | Executive Vice President, Chief Financial | |
Glenn A. Eisenberg | Officer and Treasurer (Principal Financial Officer) | |
/s/ EDWARD T. DODSON | Chief Accounting Officer (Principal Accounting Officer) | |
Edward T. Dodson | ||
* | Director | |
Kerrii B. Anderson | ||
* | Director | |
Jean-Luc Bélingard | ||
* | Director | |
D. Gary Gilliland | ||
* | Director | |
Garheng Kong, M.D., Ph.D. | ||
* | Director | |
Robert E. Mittelstaedt, Jr. | ||
* | Director | |
Peter M. Neupert | ||
* | Director | |
Richelle Parham | ||
* | Director | |
Adam H. Schechter | ||
* | Director | |
R. Sanders Williams, M.D. | ||
* F. Samuel Eberts III, by his signing his name hereto, does hereby sign this report on behalf of the directors of the Registrant after whose typed names asterisks appear, pursuant to powers of attorney duly executed by such directors and filed with the Securities and Exchange Commission.
By: | /s/ F. SAMUEL EBERTS III | |
F. Samuel Eberts III | ||
Attorney-in-fact | ||
78
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
AND SCHEDULE
Page | |
Consolidated Financial Statements: | |
Financial Statement Schedule: | |
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of
Laboratory Corporation of America Holdings:
In our opinion, the consolidated financial statements listed in the accompanying index present fairly, in all material respects, the financial position of Laboratory Corporation of America Holdings and its subsidiaries at December 31, 2015 and 2014, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2015 in conformity with accounting principles generally accepted in the United States of America. In addition, in our opinion, the financial statement schedule listed in the accompanying index presents fairly, in all material respects, the information set forth therein when read in conjunction with the related consolidated financial statements. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control–Integrated Framework 2013 issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements and financial statement schedule, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the Report of Management on Internal Control Over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements, on the financial statement schedule, and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
As described in Item 9A Controls and Procedures, management has excluded Covance, Inc. from its assessment of internal control over financial reporting as of December 31, 2015 because it was acquired by the Company in a purchase business combination during 2015. We have also excluded Covance, Inc. from our audit of internal control over financial reporting. Covance, Inc. is a wholly-owned subsidiary whose total assets and total revenues represent 11% and 27%, respectively, of the related consolidated financial statement amounts as of and for the year ended December 31, 2015.
/s/ PricewaterhouseCoopers LLP
Charlotte, North Carolina
February 29, 2016
F-2
PART I – FINANCIAL INFORMATION
Item 1. Financial Information
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(In Millions)
December 31, 2015 | December 31, 2014 | ||||||
ASSETS | |||||||
Current assets: | |||||||
Cash and cash equivalents | $ | 716.4 | $ | 580.0 | |||
Accounts receivable, net of allowance for doubtful accounts of $217.0 and $211.6 at December 31, 2015 and 2014, respectively | 1,217.9 | 815.7 | |||||
Unbilled services | 156.6 | — | |||||
Supplies inventories | 191.0 | 139.5 | |||||
Prepaid expenses and other | 340.3 | 157.5 | |||||
Deferred income taxes | 40.8 | — | |||||
Total current assets | 2,663.0 | 1,692.7 | |||||
Property, plant and equipment, net | 1,747.4 | 786.5 | |||||
Goodwill, net | 6,227.7 | 3,099.4 | |||||
Intangible assets, net | 3,323.5 | 1,475.8 | |||||
Joint venture partnerships and equity method investments | 58.2 | 92.6 | |||||
Other assets, net | 201.9 | 154.8 | |||||
Total assets | $ | 14,221.7 | $ | 7,301.8 | |||
LIABILITIES AND SHAREHOLDERS’ EQUITY | |||||||
Current liabilities: | |||||||
Accounts payable | $ | 497.4 | $ | 282.3 | |||
Accrued expenses and other | 633.1 | 341.4 | |||||
Unearned revenue | 146.1 | — | |||||
Deferred income taxes | — | 5.5 | |||||
Short-term borrowings and current portion of long-term debt | 424.9 | 347.1 | |||||
Total current liabilities | 1,701.5 | 976.3 | |||||
Long-term debt, less current portion | 5,992.1 | 2,682.7 | |||||
Deferred income taxes and other tax liabilities | 1,245.7 | 530.4 | |||||
Other liabilities | 323.1 | 274.2 | |||||
Total liabilities | 9,262.4 | 4,463.6 | |||||
Commitments and contingent liabilities | |||||||
Noncontrolling interest | 14.9 | 17.7 | |||||
Shareholders’ equity | |||||||
Common stock, 101.3 and 84.6 shares outstanding at December 31, 2015 and 2014, respectively | 12.0 | 10.4 | |||||
Additional paid-in capital | 1,974.5 | — | |||||
Retained earnings | 4,223.0 | 3,786.1 | |||||
Less common stock held in treasury | (978.1 | ) | (965.5 | ) | |||
Accumulated other comprehensive income | (287.0 | ) | (10.5 | ) | |||
Total shareholders’ equity | 4,944.4 | 2,820.5 | |||||
Total liabilities and shareholders’ equity | $ | 14,221.7 | $ | 7,301.8 | |||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(In Millions, Except Per Share Data)
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Net revenues | $ | 8,505.7 | $ | 6,011.6 | $ | 5,808.3 | |||||
Reimbursable out-of-pocket expenses | 174.4 | — | — | ||||||||
Total revenues | 8,680.1 | 6,011.6 | 5,808.3 | ||||||||
Net cost of revenue | 5,602.4 | 3,808.5 | 3,585.1 | ||||||||
Reimbursable out-of-pocket expenses | 174.4 | — | — | ||||||||
Total cost of revenue | 5,776.8 | 3,808.5 | 3,585.1 | ||||||||
Gross profit | 2,903.3 | 2,203.1 | 2,223.2 | ||||||||
Selling, general and administrative expenses | 1,622.0 | 1,198.2 | 1,128.8 | ||||||||
Amortization of intangibles and other assets | 164.5 | 76.7 | 81.7 | ||||||||
Restructuring and other special charges | 113.9 | 17.8 | 21.8 | ||||||||
Operating income | 1,002.9 | 910.4 | 990.9 | ||||||||
Other income (expenses): | |||||||||||
Interest expense | (274.9 | ) | (109.5 | ) | (96.5 | ) | |||||
Equity method income, net | 10.0 | 14.3 | 16.9 | ||||||||
Investment income | 1.9 | 1.1 | 2.2 | ||||||||
Other, net | (7.8 | ) | 10.4 | 2.1 | |||||||
Earnings before income taxes | 732.1 | 826.7 | 915.6 | ||||||||
Provision for income taxes | 294.1 | 314.1 | 340.2 | ||||||||
Net earnings | 438.0 | 512.6 | 575.4 | ||||||||
Less: Net earnings attributable to the noncontrolling interest | (1.1 | ) | (1.4 | ) | (1.6 | ) | |||||
Net earnings attributable to Laboratory Corporation of America Holdings | $ | 436.9 | $ | 511.2 | $ | 573.8 | |||||
Basic earnings per common share | $ | 4.42 | $ | 6.03 | $ | 6.36 | |||||
Diluted earnings per common share | $ | 4.34 | $ | 5.91 | $ | 6.25 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE EARNINGS
(In Millions, Except Per Share Data)
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Net earnings | $ | 438.0 | $ | 512.6 | $ | 575.4 | |||||
Foreign currency translation adjustments | (370.7 | ) | (89.5 | ) | (63.2 | ) | |||||
Net benefit plan adjustments | 7.7 | (18.6 | ) | 42.1 | |||||||
Investment adjustments | (0.1 | ) | (16.3 | ) | 16.4 | ||||||
Other comprehensive loss before tax | (363.1 | ) | (124.4 | ) | (4.7 | ) | |||||
Provision for income tax related to items of comprehensive earnings | 86.6 | 47.7 | 1.5 | ||||||||
Other comprehensive loss, net of tax | (276.5 | ) | (76.7 | ) | (3.2 | ) | |||||
Comprehensive earnings | 161.5 | 435.9 | 572.2 | ||||||||
Less: Net earnings attributable to the noncontrolling interest | (1.1 | ) | (1.4 | ) | (1.6 | ) | |||||
Net comprehensive earnings attributable to Laboratory Corporation of America Holdings | $ | 160.4 | $ | 434.5 | $ | 570.6 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
(In Millions)
Common Stock | Additional Paid-in Capital | Retained Earnings | Treasury Stock | Accumulated Other Comprehensive Income (Loss) | Total Shareholders’ Equity | ||||||||||||||||||
BALANCE AT DECEMBER 31, 2012 | $ | 11.3 | $ | — | $ | 3,588.5 | $ | (951.8 | ) | $ | 69.4 | $ | 2,717.4 | ||||||||||
Net earnings attributable to Laboratory Corporation of America Holdings | — | — | 573.8 | — | — | 573.8 | |||||||||||||||||
Other comprehensive earnings, net of tax | — | — | — | — | (3.2 | ) | (3.2 | ) | |||||||||||||||
Issuance of common stock under employee stock plans | 0.2 | 173.8 | — | — | — | 174.0 | |||||||||||||||||
Surrender of restricted stock and performance share awards | — | — | — | (7.1 | ) | — | (7.1 | ) | |||||||||||||||
Conversion of zero-coupon convertible debt | — | 4.1 | — | — | — | 4.1 | |||||||||||||||||
Stock compensation | — | 37.3 | — | — | — | 37.3 | |||||||||||||||||
Income tax benefit from stock options exercised | — | 10.6 | — | — | — | 10.6 | |||||||||||||||||
Purchase of common stock | (1.0 | ) | (225.8 | ) | (788.8 | ) | — | — | (1,015.6 | ) | |||||||||||||
BALANCE AT DECEMBER 31, 2013 | 10.5 | $ | — | $ | 3,373.5 | $ | (958.9 | ) | $ | 66.2 | $ | 2,491.3 | |||||||||||
Net earnings attributable to Laboratory Corporation of America Holdings | — | — | 511.2 | — | — | 511.2 | |||||||||||||||||
Other comprehensive earnings, net of tax | — | — | — | — | (76.7 | ) | (76.7 | ) | |||||||||||||||
Issuance of common stock under employee stock plans | 0.2 | 114.6 | — | — | — | 114.8 | |||||||||||||||||
Surrender of restricted stock and performance share awards | — | — | — | (6.6 | ) | — | (6.6 | ) | |||||||||||||||
Conversion of zero-coupon convertible debt | — | 3.9 | — | — | — | 3.9 | |||||||||||||||||
Stock compensation | — | 45.7 | — | — | — | 45.7 | |||||||||||||||||
Income tax benefit from stock options exercised | — | 5.9 | — | — | — | 5.9 | |||||||||||||||||
Purchase of common stock | (0.3 | ) | (170.1 | ) | (98.6 | ) | — | — | (269.0 | ) | |||||||||||||
BALANCE AT DECEMBER 31, 2014 | 10.4 | $ | — | $ | 3,786.1 | $ | (965.5 | ) | $ | (10.5 | ) | $ | 2,820.5 | ||||||||||
Net earnings attributable to Laboratory Corporation of America Holdings | — | — | 436.9 | — | — | 436.9 | |||||||||||||||||
Other comprehensive earnings, net of tax | — | — | — | — | (276.5 | ) | (276.5 | ) | |||||||||||||||
Issuance of common stock for acquisition consideration | 1.5 | 1,761.0 | — | — | — | 1,762.5 | |||||||||||||||||
Issuance of common stock under employee stock plans | 0.1 | 98.8 | — | — | — | 98.9 | |||||||||||||||||
Surrender of restricted stock and performance share awards | — | — | — | (12.6 | ) | — | (12.6 | ) | |||||||||||||||
Conversion of zero-coupon convertible debt | — | 0.4 | — | — | — | 0.4 | |||||||||||||||||
Stock compensation | — | 102.1 | — | — | — | 102.1 | |||||||||||||||||
Income tax benefit from stock options exercised | — | 12.2 | — | — | — | 12.2 | |||||||||||||||||
BALANCE AT DECEMBER 31, 2015 | $ | 12.0 | $ | 1,974.5 | $ | 4,223.0 | $ | (978.1 | ) | $ | (287.0 | ) | $ | 4,944.4 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In Millions)
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
CASH FLOWS FROM OPERATING ACTIVITIES: | |||||||||||
Net earnings | $ | 438.0 | $ | 512.6 | $ | 575.4 | |||||
Adjustments to reconcile net earnings to net cash provided by operating activities: | |||||||||||
Depreciation and amortization | 457.8 | 245.5 | 230.1 | ||||||||
Stock compensation | 102.1 | 45.7 | 37.3 | ||||||||
Loss (gain) on sale of assets | 4.6 | (12.5 | ) | (3.9 | ) | ||||||
Accrued interest on zero-coupon subordinated notes | 2.0 | 2.0 | 2.3 | ||||||||
Cumulative earnings less than (in excess of) distributions from equity method investments | 0.1 | (5.8 | ) | (4.2 | ) | ||||||
Asset impairment | 39.7 | — | — | ||||||||
Deferred income taxes | (33.4 | ) | 27.7 | 56.2 | |||||||
Change in assets and liabilities (net of effects of acquisitions): | |||||||||||
Increase in accounts receivable (net) | (71.8 | ) | (31.1 | ) | (67.5 | ) | |||||
Increase in unbilled services | (16.9 | ) | — | — | |||||||
Increase in inventories | (0.2 | ) | (0.3 | ) | (15.3 | ) | |||||
Decrease (increase) in prepaid expenses and other | 62.3 | (12.9 | ) | (32.3 | ) | ||||||
Increase (decrease) in accounts payable | 30.7 | (21.2 | ) | 60.8 | |||||||
Increase in deferred revenue | 5.4 | — | — | ||||||||
Decrease in accrued expenses and other | (38.0 | ) | (10.7 | ) | (20.2 | ) | |||||
Net cash provided by operating activities | 982.4 | 739.0 | 818.7 | ||||||||
CASH FLOWS FROM INVESTING ACTIVITIES: | |||||||||||
Capital expenditures | (255.8 | ) | (203.5 | ) | (202.2 | ) | |||||
Proceeds from sale of assets | 0.6 | 1.4 | 1.1 | ||||||||
Proceeds from sale of investments | 8.0 | 31.6 | 7.5 | ||||||||
Acquisition of licensing technology | — | — | — | ||||||||
Investments in equity affiliates | (11.7 | ) | (20.2 | ) | (6.5 | ) | |||||
Acquisition of businesses, net of cash acquired | (3,736.0 | ) | (159.4 | ) | (159.5 | ) | |||||
Net cash used for investing activities | (3,994.9 | ) | (350.1 | ) | (359.6 | ) | |||||
CASH FLOWS FROM FINANCING ACTIVITIES: | |||||||||||
Proceeds from Senior Notes offerings | 2,900.0 | — | 700.0 | ||||||||
Proceeds from term loan | 1,000.0 | — | — | ||||||||
Payments on term loan | (285.0 | ) | — | — | |||||||
Proceeds from revolving credit facilities | 60.0 | — | 412.0 | ||||||||
Payments on revolving credit facilities | (60.0 | ) | — | (412.0 | ) | ||||||
Proceeds from bridge loan | 400.0 | — | — | ||||||||
Payments on bridge loan | (400.0 | ) | — | — | |||||||
Payments on Senior Notes | (500.0 | ) | — | — | |||||||
Payments on zero-coupon subordinated notes | (1.3 | ) | (18.9 | ) | (21.5 | ) | |||||
Payments on long-term debt | — | — | (350.0 | ) | |||||||
Payment of debt issuance costs | (36.7 | ) | (24.1 | ) | (9.3 | ) | |||||
Payments on long-term lease obligations | (4.3 | ) | (1.4 | ) | (0.4 | ) | |||||
Noncontrolling interest distributions | — | (1.2 | ) | (0.9 | ) | ||||||
Deferred payments on acquisitions | (0.1 | ) | (6.7 | ) | (5.6 | ) | |||||
Excess tax benefits from stock based compensation | 13.1 | 5.9 | 11.0 | ||||||||
Net proceeds from issuance of stock to employees | 98.9 | 114.8 | 174.0 | ||||||||
Purchase of common stock | — | (269.0 | ) | (1,015.6 | ) | ||||||
Net cash used for financing activities | 3,184.6 | (200.6 | ) | (518.3 | ) | ||||||
Effect of exchange rate changes on cash and cash equivalents | (35.7 | ) | (12.3 | ) | (3.6 | ) | |||||
Net increase (decrease) in cash and cash equivalents | 136.4 | 176.0 | (62.8 | ) | |||||||
Cash and cash equivalents at beginning of year | 580.0 | 404.0 | 466.8 | ||||||||
Cash and cash equivalents at end of year | $ | 716.4 | $ | 580.0 | $ | 404.0 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
1. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Financial Statement Presentation
Laboratory Corporation of America Holdings and its subsidiaries (Company) is the world’s leading healthcare diagnostics company, providing comprehensive clinical laboratory services and end-to-end drug development support. The Company’s strategic vision is to improve health and improve lives by delivering world class diagnostic solutions, bringing innovative medicines to patients faster, and changing the way care is provided through the deployment of technology-enabled solutions. The Company serves managed care organizations (MCOs), biopharmaceutical companies, governmental agencies, physicians, hospitals and health systems, employers, patients and consumers, food and nutritional companies and independent clinical laboratories. The Company believes that it generated more revenue from laboratory testing than any other company in the world in 2015.
The Company reports its business in two segments, LabCorp Diagnostics (LCD) and Covance Drug Development (CDD). For further financial information about these segments, including information for each of the last three fiscal years regarding revenue, operating income, and other important information, see Note 20 to the Consolidated Financial Statements. In 2015, LCD and CDD contributed 72.9% and 27.1%, respectively, of net revenues to the Company.
The consolidated financial statements include the accounts of the Company and its majority-owned subsidiaries for which it exercises control. Long-term investments in affiliated companies in which the Company exercises significant influence, but which it does not control, are accounted for using the equity method. Investments in which the Company does not exercise significant influence (generally, when the Company has an investment of less than 20% and no representation on the investee's board of directors) are accounted for using the cost method. All significant inter-company transactions and accounts have been eliminated. The Company does not have any variable interest entities or special purpose entities whose financial results are not included in the consolidated financial statements.
The financial statements of the Company's operating foreign subsidiaries are measured using the local currency as the functional currency. Assets and liabilities are translated at exchange rates as of the balance sheet date. Revenues and expenses are translated at average monthly exchange rates prevailing during the year. Resulting translation adjustments are included in “Accumulated other comprehensive income.”
Revenue Recognition
LCD recognizes revenue on the accrual basis at the time test results are reported, which approximates when services are provided. Services are provided to certain patients covered by various third-party payer programs including various MCOs, as well as the Medicare and Medicaid programs. Billings for services under third-party payer programs are included in sales net of allowances for contractual discounts and allowances for differences between the amounts billed and estimated program payment amounts. Adjustments to the estimated payment amounts based on final settlement with the programs are recorded upon settlement as an adjustment to revenue. In 2015, 2014 and 2013, approximately 16.0%, 15.0% and 16.0%, respectively, of LCD's revenues were derived directly from the Medicare and Medicaid programs. LCD has capitated agreements with certain MCO customers and recognizes related revenue based on a predetermined monthly contractual rate for each member of the managed care plan regardless of the number or cost of services provided by the Company. In 2015, 2014 and 2013, approximately 3.5%, 3.5% and 3.2%, respectively, of LCD's revenues were derived from such capitated agreements.
CDD recognizes revenue either as services are performed or products are delivered, depending on the nature of the work contracted. Historically, a majority of CDD’s net revenues have been earned under contracts that range in duration from a few months to a few years, but can extend in duration up to five years or longer. CDD also has committed minimum volume arrangements with certain clients. Underlying these arrangements are individual project contracts for the specific services to be provided. These arrangements enable CDD's clients to secure its services in exchange for which they commit to purchase an annual minimum dollar value of services. Under these types of arrangements, if the annual minimum dollar value of service commitment is not reached, the client is required to pay CDD for the shortfall. Progress towards the achievement of annual minimum dollar value of service commitments is monitored throughout the year. Annual minimum commitment shortfalls are not included in net revenues until the amount has been determined and agreed to by the client.
Service contracts generally take the form of fee-for-service or fixed-price arrangements subject to pricing adjustments based on changes in scope. In cases where performance spans multiple accounting periods, revenue is recognized as services are performed, measured on a proportional-performance basis, generally using output measures that are specific to the service provided.
F-8
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Examples of output measures in early development services, include among others, the number of slides read, or specimens prepared for preclinical laboratory services, or number of dosings or number of volunteers enrolled for clinical pharmacology. Examples of output measures in the clinical trials services, include among others, number of investigators enrolled, number of sites initiated, number of patients enrolled and number of monitoring visits completed. Revenue is determined by dividing the actual units of work completed by the total units of work required under the contract and multiplying that percentage by the total contract value. The total contract value, or total contractual payments, represents the aggregate contracted price for each of the agreed upon services to be provided. CDD does not have any contractual arrangements spanning multiple accounting periods where revenue is recognized on a proportional-performance basis under which the Company has earned more than an immaterial amount of performance-based revenue (i.e., potential additional revenue tied to specific deliverables or performance). Changes in the scope of work are common, especially under long-term contracts, and generally result in a change in contract value. Once the client has agreed to the changes in scope and renegotiated pricing terms, the contract value is amended with revenue recognized as described above. Estimates of costs to complete are made to provide, where appropriate, for losses expected on contracts. Costs are not deferred in anticipation of contracts being awarded, but instead are expensed as incurred.
Billing schedules and payment terms are generally negotiated on a contract-by-contract basis. In some cases, CDD bills the client for the total contract value in progress-based installments as certain non-contingent billing milestones are reached over the contract duration, such as, but not limited to, contract signing, initial dosing, investigator site initiation, patient enrollment or database lock. The term “billing milestone” relates only to a billing trigger in a contract whereby amounts become billable and payable in accordance with a negotiated predetermined billing schedule throughout the term of a project. These billing milestones are generally not performance-based (i.e., there is no potential additional consideration tied to specific deliverables or performance). In other cases, billing and payment terms are tied to the passage of time (e.g., monthly billings). In either case, the total contract value and aggregate amounts billed to the client would be the same at the end of the project. While CDD attempts to negotiate terms that provide for billing and payment of services prior or within close proximity to the provision of services, this is not always possible, and there are fluctuations in the levels of unbilled services and unearned revenue from period to period. While a project is ongoing, cash payments are not necessarily representative of aggregate revenue earned at any particular point in time, as revenues are recognized when services are provided, while amounts billed and paid are in accordance with the negotiated billing and payment terms.
In some cases, payments received are in excess of revenue recognized. For example, a contract invoicing schedule may provide for an upfront payment of 10% of the full contract value upon contract signing, but at the time of signing performance of services has not yet begun. Payments received in advance of services being provided are deferred as unearned revenue on the balance sheet. As the contracted services are subsequently performed and the associated revenue is recognized, the unearned revenue balance is reduced by the amount of revenue recognized during the period.
In other cases, services may be provided and revenue recognized before the client is invoiced. In these cases, revenue recognized will exceed amounts billed, and the difference, representing an unbilled receivable, is recorded for the amount that is currently unbillable to the customer pursuant to contractual terms. Once the client is invoiced, the unbilled services are reduced for the amount billed, and a corresponding account receivable is recorded. All unbilled services are billable to customers within one year from the respective balance sheet date.
Most contracts are terminable with or without cause by the client, either immediately or upon notice. These contracts often require payment to CDD of expenses to wind down the study or project, fees earned to date and, in some cases, a termination fee or a payment to CDD of some portion of the fees or profits that could have been earned by CDD under the contract if it had not been terminated early. Termination fees are included in net revenues when services are performed and realization is assured. In connection with the management of multi-site clinical trials, CDD pays on behalf of its customers fees to investigators, volunteers and certain out-of-pocket costs, for which it is reimbursed at cost, without mark-up or profit. Investigator fees are not reflected in net revenues or expenses where CDD acts in the capacity of an agent on behalf of the pharmaceutical company sponsor, passing through these costs without risk or reward to CDD. All other out-of-pocket costs are included in total revenues and expenses.
The Company's net revenues are comprised of the following:
Years Ended December 31, | |||||||||||
Total revenues | 2015 | 2014 | 2013 | ||||||||
LCD - net revenue | $ | 6,199.3 | $ | 5,838.0 | $ | 5,669.4 | |||||
CDD - net revenue | 2,306.4 | 173.6 | 138.9 | ||||||||
CDD - reimbursable out-of-pocket expenses | 174.4 | — | — | ||||||||
Total revenues | $ | 8,680.1 | $ | 6,011.6 | $ | 5,808.3 | |||||
F-9
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Reimbursable Out-of-Pocket Expenses
CDD pays on behalf of its customers certain out-of-pocket costs for which the Company is reimbursed at cost, without mark-up or profit. Out-of-pocket costs paid by CDD are reflected in operating expenses, while the reimbursements received are reflected in revenues in the consolidated statements of operations. CDD excludes from revenue and expense in the consolidated statements of operations fees paid to investigators and the associated reimbursement because CDD acts as an agent on behalf of the pharmaceutical company sponsors with regard to investigator payments.
Cost of Revenue
Cost of revenue includes direct labor and related benefit charges, other direct costs, shipping and handling fees, and an allocation of facility charges and information technology costs. Selling, general and administrative expenses consist primarily of administrative payroll and related benefit charges, advertising and promotional expenses, administrative travel and an allocation of facility charges and information technology costs. Cost of advertising is expensed as incurred.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reported periods. Significant estimates include the allowances for doubtful accounts, deferred tax assets, fair values and amortization lives for intangible assets, and accruals for self-insurance reserves and pensions. The allowance for doubtful accounts is determined based on historical collections trends, the aging of accounts, current economic conditions and regulatory changes. Actual results could differ from those estimates.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable.
The Company maintains cash and cash equivalents with various major financial institutions. The total cash balances on deposit that exceeded the balances insured by the Federal Deposit Insurance Commission, were approximately $243.6 at December 31, 2015. Cash equivalents at December 31, 2015, totaled $491.3, which includes amounts invested in money market funds, time deposits, municipal, treasury and government funds.
Substantially all of the Company’s accounts receivable are with companies in the healthcare industry and individuals. However, concentrations of credit risk are limited due to the number of the Company’s clients as well as their dispersion across many different geographic regions.
While the Company has receivables due from U.S. and state governmental agencies, the Company does not believe that such receivables represent a credit risk since the related healthcare programs are funded by U.S. and state governments, and payment is primarily dependent upon submitting appropriate documentation. Accounts receivable balances (gross) from Medicare and Medicaid were $112.0 and $135.1 at December 31, 2015 and 2014, respectively.
For the Company's operations in Ontario, Canada, the Ontario Ministry of Health and Long-Term Care (Ministry) determines who can establish a licensed community medical laboratory and caps the amount that each of these licensed laboratories can bill the government sponsored healthcare plan. The Ontario government-sponsored healthcare plan covers the cost of clinical laboratory testing performed by the licensed laboratories. The provincial government discounts the annual testing volumes based on certain utilization discounts and establishes an annual maximum it will pay for all community laboratory tests. The agreed-upon reimbursement rates are subject to Ministry review at the end of year and can be adjusted (at the government's discretion) based upon the actual volume and mix of test work performed by the licensed providers in the province during the year. The accounts receivable balances from the Ontario government sponsored healthcare plan were CN$21.3 and CN$22.4 at December 31, 2015 and 2014, respectively.
The portion of the Company's accounts receivable due from patients comprises the largest portion of credit risk. At December 31, 2015 and 2014, receivables due from patients represent approximately 18.8% and 27.1% of the Company's consolidated gross accounts receivable. The Company applies assumptions and judgments including historical collection experience for assessing collectibility and determining allowances for doubtful accounts for accounts receivable from patients.
F-10
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Earnings per Share
Basic earnings per share is computed by dividing net earnings attributable to Laboratory Corporation of America Holdings by the weighted average number of common shares outstanding. Diluted earnings per share is computed by dividing net earnings including the impact of dilutive adjustments by the weighted average number of common shares outstanding plus potentially dilutive shares, as if they had been issued at the earlier of the date of issuance or the beginning of the period presented. Potentially dilutive common shares result primarily from the Company’s outstanding stock options, restricted stock awards, performance share awards, and shares issuable upon conversion of zero-coupon subordinated notes.
The following represents a reconciliation of basic earnings per share to diluted earnings per share:
2015 | 2014 | 2013 | ||||||||||||||||||||||||||||||
Income | Shares | Per Share Amount | Income | Shares | Per Share Amount | Income | Shares | Per Share Amount | ||||||||||||||||||||||||
Basic earnings per share | $ | 436.9 | 98.8 | $ | 4.42 | $ | 511.2 | 84.8 | $ | 6.03 | $ | 573.8 | 90.2 | $ | 6.36 | |||||||||||||||||
Stock options | — | 1.2 | — | 1.1 | — | 1.1 | ||||||||||||||||||||||||||
Restricted stock awards and other | — | — | — | — | — | — | ||||||||||||||||||||||||||
Effect of convertible debt, net of tax | — | 0.6 | — | 0.5 | — | 0.5 | ||||||||||||||||||||||||||
Diluted earnings per share | $ | 436.9 | 100.6 | $ | 4.34 | $ | 511.2 | 86.4 | $ | 5.91 | $ | 573.8 | 91.8 | $ | 6.25 | |||||||||||||||||
The following table summarizes the potential common shares not included in the computation of diluted earnings per share because their impact would have been antidilutive:
Years Ended December 31, | |||||
2015 | 2014 | 2013 | |||
Stock options | — | — | 0.1 | ||
Stock Compensation Plans
The Company measures stock compensation cost for all equity awards at fair value on the date of grant and recognizes compensation expense over the service period for awards expected to vest. The fair value of restricted stock units and performance share awards is determined based on the number of shares granted and the quoted price of the Company’s common stock on the grant date. Such value is recognized as expense over the service period, net of estimated forfeitures. The estimation of equity awards that will ultimately vest requires judgment and the Company considers many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience. The cumulative effect on current and prior periods of a change in the estimated forfeiture rate is recognized as compensation expense in earnings in the period of the revision. Actual results and future estimates may differ substantially from the Company’s current estimates.
See Note 14 for assumptions used in calculating compensation expense for the Company’s stock compensation plans.
Cash Equivalents
Cash and cash equivalents consist of highly liquid instruments, such as commercial paper, time deposits, and other money market instruments, substantially all of which have original maturities of three months or less.
Inventories
Inventories, consisting primarily of purchased laboratory and client supplies and finished goods for CDD, are stated at the lower of cost (first-in, first-out) or market. Supplies accounted for $157.7 and $139.5 and finished goods accounted for $33.3 million and $0.0 million of total inventory at December 31, 2015 and 2014, respectively.
Prepaid Expenses and Other
In connection with the management of multi-site clinical trials, CDD pays on behalf of its customers certain out-of-pocket costs, for which the Company is reimbursed at cost, without markup or profit. Amounts receivable from customers in connection with such out-of-pocket pass-through costs are included in prepaid expenses and other in the accompanying consolidated balance sheets and totaled $92.5 at December 31, 2015.
F-11
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Also included in prepaid expenses and other current assets are assets held for sale. The Company records long-lived assets as held for sale when a plan to sell the asset has been initiated and all other held for sale criteria have been satisfied. Assets classified as held for sale of $72.4 as of December 31, 2015 are recorded in other current assets on the consolidated balance sheet at the lower of their carrying value or fair value less cost to sell. There were no assets held for sale as of December 31, 2014.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost. The cost of properties held under capital leases is equal to the lower of the net present value of the minimum lease payments or the fair value of the leased property at the inception of the lease. Depreciation and amortization expense is computed on all classes of assets based on their estimated useful lives, as indicated below, using the straight-line method.
Years | |||
Buildings and building improvements | 10 | - | 40 |
Machinery and equipment | 3 | - | 10 |
Furniture and fixtures | 5 | - | 10 |
Software | 3 | - | 10 |
Leasehold improvements and assets held under capital leases are amortized over the shorter of their estimated useful lives or the term of the related leases. Expenditures for repairs and maintenance are charged to operations as incurred. Retirements, sales and other disposals of assets are recorded by removing the cost and accumulated depreciation from the related accounts with any resulting gain or loss reflected in the consolidated statements of operations.
Capitalized Software Costs
The Company capitalizes purchased software which is ready for service and capitalizes software development costs incurred on significant projects starting from the time that the preliminary project stage is completed and the Company commits to funding a project until the project is substantially complete and the software is ready for its intended use. Capitalized costs include direct material and service costs and payroll and payroll-related costs. Research and development (R&D) costs and other computer software maintenance costs related to software development are expensed as incurred. Capitalized software costs are amortized using the straight-line method over the estimated useful life of the underlying system, generally five years.
Long-Lived Assets
The Company assesses goodwill and indefinite-lived intangibles for impairment at least annually or whenever events or changes in circumstances indicate that the carrying amount of such assets may not be recoverable. In 2015, the Company changed the timing of its annual impairment testing from the end of the year to the beginning of the fourth quarter. In accordance with the FASB updates to their authoritative guidance regarding goodwill and indefinite-lived intangible asset impairment testing, an entity is allowed to first assess qualitative factors as a basis for determining whether it is necessary to perform quantitative impairment testing. If an entity determines that it is not more likely than not that the estimated fair value of an asset is less than its carrying value, then no further testing is required. Otherwise, impairment testing must be performed in accordance with the original accounting standards. The updated FASB guidance also allows an entity to bypass the qualitative assessment for any reporting unit in its goodwill assessment and proceed directly to performing the first step of the two-step assessment. Similarly, a company can proceed directly to a quantitative assessment in the case of impairment testing for indefinite-lived intangible assets as well.
Step One of the goodwill impairment test includes the estimation of the fair value of each reporting unit as compared to the carrying value of the reporting unit. Reporting units are businesses with discrete financial information that is available and reviewed by management. The Company estimates the fair value of a reporting unit using both income-based and market-based valuation methods. The income-based approach is based on the reporting unit's forecasted future cash flows that are discounted to the present value using the reporting unit's weighted average cost of capital. For the market-based approach, the Company utilizes a number of factors such as publicly available information regarding the market capitalization of the Company as well as operating results, business plans, market multiples, and present value techniques. Based upon the range of estimated values developed from the income and market-based methods, the Company determines the estimated fair value for the reporting unit. If the estimated fair value of the reporting unit exceeds the carrying value, the goodwill is not impaired and no further review is required. However, if the estimated fair value is less than the carrying value, the Company performs the second step of the goodwill impairment test is performed to measure the amount of the impairment, if any. The second step involves a hypothetical allocation of the estimated fair value of the reporting unit to its tangible and intangible assets (excluding goodwill) and liabilities as if the reporting unit were newly acquired, which results in an implied fair value of the goodwill. The amount of the impairment charge is the excess of the recorded goodwill, if any, over the implied fair value of the goodwill.
F-12
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Management concluded that no triggering events occurred during 2015 related to goodwill and indefinite-lived intangible assets. As such, management performed its annual goodwill and intangible asset impairment testing as of the beginning of the fourth quarter of 2015. The Company elected to perform the qualitative assessment for goodwill and intangible assets, with the exception of the indefinite-lived Canadian licenses, for all reporting units.
In this qualitative assessment, the Company considered relevant events and circumstances for each reporting unit, including (i) current year results, ii) financial performance versus management’s annual and five-year strategic plans, iii) changes in the reporting unit carrying value since prior year, (iv) industry and market conditions in which the reporting unit operates, (v) macroeconomic conditions, including discount rate changes, and (vi) changes in products or services offered by the reporting unit. If applicable, performance in recent years was compared to forecasts included in prior valuations. Based on the results of the qualitative assessment, the Company concluded that it was not more likely than not that the carrying values of the goodwill and intangible assets were greater than their fair values, and that further quantitative testing was not necessary.
In 2015, the Company utilized an income approach to determine the fair value of its Canadian reporting unit and its indefinite-lived assets consisting of acquired Canadian licenses. Based upon the results of the quantitative assessment, the Company concluded that the fair value of the indefinite-lived Canadian licenses was greater than the carrying value.
There are inherent uncertainties related to the factors described above and judgment related to the Company's impairment assessments of goodwill and indefinite-lived intangibles. The assumptions underlying the impairment analyses may change in such a manner that impairment in value may occur in the future. Any such impairment will be recognized in the period in which it becomes known.
Long-lived assets, other than goodwill and indefinite-lived assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amounts may not be recoverable. Recoverability of assets to be held and used is determined by the Company at the level for which there are identifiable cash flows by comparison of the carrying amount of the assets to future undiscounted net cash flows before interest expense and income taxes expected to be generated by the assets. Impairment, if any, is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets (based on market prices in an active market or on discounted cash flows). Assets to be disposed of are reported at the lower of the carrying amount or fair value. The Company impaired long-lived assets of $39.7 and $0.0, for the years ended December 31, 2015 and 2014, respectively.
Intangible Assets
Intangible assets are amortized on a straight-line basis over the expected periods to be benefited, as set forth in the table below, such as legal life for patents and technology and contractual lives for non-compete agreements.
Years | |||
Customer relationships | 10 | - | 36 |
Patents, licenses and technology | 3 | - | 15 |
Non-compete agreements | 5 | - | 10 |
Trade names | 5 | - | 15 |
Debt Issuance Costs
The costs related to the issuance of debt are capitalized and amortized to interest expense over the terms of the related debt.
Professional Liability
The Company is self-insured (up to certain limits) for professional liability claims arising in the normal course of business, generally related to the testing and reporting of laboratory test results. The Company estimates a liability that represents the ultimate exposure for aggregate losses below those limits. The liability is discounted and is based on actuarial assumptions and factors for known and incurred but not reported claims, including the frequency and payment trends of historical claims.
Income Taxes
The Company accounts for income taxes utilizing the asset and liability method. Under this method deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and for tax loss carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences
F-13
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. The Company does not recognize a tax benefit unless the Company concludes that it is more likely than not that the benefit will be sustained on audit by the taxing authority based solely on the technical merits of the associated tax position. If the recognition threshold is met, the Company recognizes a tax benefit measured at the largest amount of the tax benefit that the Company believes is greater than 50% likely to be realized. The Company records interest and penalties in income tax expense.
Derivative Financial Instruments
Interest rate swap agreements, which have been used by the Company from time to time in the management of interest rate exposure, are accounted for at fair value. The Company’s zero-coupon subordinated notes contain two features that are considered to be embedded derivative instruments under authoritative guidance in connection with accounting for derivative instruments and hedging activities. The Company believes these embedded derivatives had no fair value at December 31, 2015 and 2014.
See Note 18 for the Company’s objectives in using derivative instruments and the effect of derivative instruments and related hedged items on the Company’s financial position, financial performance and cash flows.
Fair Value of Financial Instruments
Fair value measurements for financial assets and liabilities are determined based on the assumptions that a market participant would use in pricing an asset or liability. A three-tiered fair value hierarchy draws distinctions between market participant assumptions based on (i) observable inputs such as quoted prices in active markets (Level 1), (ii) inputs other than quoted prices in active markets that are observable either directly or indirectly (Level 2) and (iii) unobservable inputs that require the Company to use present value and other valuation techniques in the determination of fair value (Level 3).
Research and Development
The Company expenses R&D costs as incurred.
Foreign Currencies
For subsidiaries outside of the U.S. that operate in a local currency environment, income and expense items are translated to U.S. Dollars at the monthly average rates of exchange prevailing during the period, assets and liabilities are translated at period-end exchange rates and equity accounts are translated at historical exchange rates. Translation adjustments are accumulated in a separate component of shareholders’ equity in the consolidated balance sheets and are included in the determination of comprehensive income in the consolidated statements of comprehensive earnings and consolidated statements of changes in shareholders’ equity. Transaction gains and losses are included in the determination of net income in the consolidated statements of operations.
New Accounting Pronouncements
In April 2014, the FASB issued a new accounting standard on discontinued operations that significantly changed criteria for discontinued operations and disclosures for disposals. Under this new standard, to be a discontinued operation, a component or group of components must represent a strategic shift that has (or will have) a major effect on an entity's operations and financial results. Expanded disclosures for discontinued operations include more details about earnings and balance sheet accounts, total operating and investing cash flows, and cash flows resulting from continuing involvement. The Company has adopted the guidance of this new standard effective beginning of 2015 and will apply it prospectively to all new disposals of components and new classifications as held for sale. The adoption of this standard did not have a material impact on the consolidated financial statements.
In May 2014, the FASB issued the converged standard on revenue recognition with the objective of providing a single, comprehensive model for all contracts with customers to improve comparability in the financial statements of companies reporting using International Financial Reporting Standards and U.S. Generally Accepted Accounting Principles. The standard contains principles that an entity must apply to determine the measurement of revenue and timing of when it is recognized. The underlying principle is that an entity must recognize revenue to depict the transfer of goods or services to customers at an amount that the entity expects to be entitled to in exchange for those goods or services. An entity can apply the revenue standard retrospectively to each prior reporting period presented (full retrospective method) or retrospectively with the cumulative effect of initially applying the standard recognized at the date of initial application in retained earnings. As originally issued, the new revenue recognition standard would be effective for the Company beginning January 1, 2017. On July 9, 2015, the FASB approved the proposal to defer the effective date of this standard by one year. The standard will be effective for the Company beginning January 1, 2018,
F-14
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
with early adoption permitted for annual periods beginning after December 16, 2016. The Company is currently evaluating the expected impact of the standard.
In August 2014, the FASB issued a new accounting standard that explicitly requires management to assess an entity's ability to continue as a going concern, and to provide related financial statement footnote disclosures in certain circumstances. Under this standard, in connection with each annual and interim period, management must assess whether there is substantial doubt about an entity's ability to continue as a going concern within one year after the financial statements are issued (or available to be issued when applicable). Management shall consider relevant conditions and events that are known and reasonably knowable at such issuance date. Substantial doubt about an entity's ability to continue as a going concern exists if it is probable that the entity will be unable to meet its obligations as they become due within one year after issuance date. Disclosures will be required if conditions or events give rise to substantial doubt. This standard is effective for the Company for the annual period ending after December 15, 2016, with early adoption permitted. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In April 2015, the FASB issued an update which requires debt issuance costs to be presented in the balance sheet as a direct deduction from the associated debt liability. This standard became effective for the Company beginning January 1, 2016. The new guidance applies on a retrospective basis. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In May 2015, the FASB issued a new accounting standard allowing entities to exclude investments measured at net asset value per share under the existing practical expedient from the fair value hierarchy. In addition, when the net asset value practical expedient is not applied to eligible investments, certain other disclosures are no longer required. The standard became effective for the Company beginning January 1, 2016. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In July 2015, the FASB issued a new accounting standard that requires an entity to measure inventory, except inventory that is measured using last-in, first-out (LIFO) or the retail inventory method, at the lower of cost and net realizable value. Net realizable value is the estimated selling price in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. The standard became effective for the Company beginning January 1, 2016. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In September 2015, the FASB issued a new accounting standard that eliminates the requirement to restate prior period financial statements for measurement period adjustments. The standard requires that the cumulative impact of a measurement period adjustment, including the impact on prior periods, be recognized in the reporting period in which the adjustment is identified. The standard became effective for the Company beginning January 1, 2016 and applies prospectively to measurement period adjustments that occur after the effective date. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In November 2015, the FASB issued a new accounting standard that requires the deferred tax liabilities and assets to be classified as noncurrent on the consolidated balance sheet. The standard will be effective for the Company beginning January 1, 2017, with early adoption permitted. The adoption of this standard is not expected to have a material impact on the consolidated financial statements.
In January 2016, the FASB issued a new accounting standard that addresses certain aspects of recognition, measurement, presentation and disclosure of financial instruments. A financial instrument is defined as cash, evidence of ownership interest in a company or other entity, or a contract that both: (i) imposes on one entity a contractual obligation either to deliver cash or another financial instrument to a second entity or to exchange other financial instruments on potentially unfavorable terms with the second entity and (ii) conveys to that second entity a contractual right either to receive cash or another financial instruments from the first entity or to exchange other financial instruments on potentially favorable terms with the first entity. The standard will be effective for the Company beginning January 1, 2018, with early adoption permitted. The Company is evaluating the impact that this new guidance will have on the consolidated financial statements.
2. BUSINESS ACQUISITIONS
On February 19, 2015 (Acquisition Date), the Company completed its acquisition (Acquisition) of Covance Inc. (Covance), a leading drug development services company and a leader in nutritional analysis, for $6,150.7. The Company issued debt and common stock to fund the Acquisition. Covance stockholders received $75.76 in cash and 0.2686 shares of the Company's common stock for each share of Covance common stock they owned. The Company financed the Acquisition with 3,900.0 of debt, 15.3 shares of its common stock and $488.2 of available cash, $400.0 of which was derived from a bridge term loan credit facility. On
F-15
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
January 30, 2015, the Company issued $2,900.0 in debt securities, consisting of $500.0 aggregate principal amount of 2.625% Senior Notes due 2020, $500.0 aggregate principal amount of 3.20% Senior Notes due 2022, $1,000.0 aggregate principal amount of 3.60% Senior Notes due 2025 and $900.0 aggregate principal amount of 4.70% Senior Notes due 2045 (together, the Acquisition Notes). The Company also entered into a $1,000.0 term loan facility which was advanced in full on February 19, 2015. The term loan credit facility will mature five years after the closing date of the Acquisition and may be prepaid without penalty.
The Company has continued to finalize its purchase price allocation during the measurement period and obtained new fair value information related to the assets acquired and liabilities assumed of Covance, including the related lives of acquired assets. The facts and circumstances that existed at the date of acquisition, if known, would have affected the measurement of the amounts recognized at that date. In accordance with ASC 805, Business Combinations, measurement period adjustments are not included in current earnings, but recognized as of the date of the acquisition with a corresponding adjustment to goodwill resulting from the change in preliminary amounts. As a result, the Company adjusted the preliminary allocation of the purchase price initially recorded at the Acquisition Date to reflect these measurement period adjustments. While the purchase price allocation is substantially complete, it is still preliminary and subject to change. The areas of the purchase price that are not yet finalized relate primarily to the impact of finalizing deferred taxes and the final allocation of goodwill to the various foreign and domestic subsidiaries of Covance. Accordingly, adjustments may be made as additional information is obtained about the facts and circumstances that existed at the valuation date. The purchase price allocation and related push down to subsidiaries will be finalized in the first quarter of 2016. The impact of any such changes will be reflected in the opening balance sheet and the comparative periods will be revised to include the effect of the measurement period adjustments as if they had been completed on the Acquisition Date in accordance with ASC 805. Any adjustments impacting amounts allocable to international location as finalized will impact currency translation and therefore comprehensive income for the comparative periods as well.
The valuation of acquired assets and assumed liabilities at the date of Acquisition include the following:
Consideration Transferred | ||||||||||||
Stock consideration | $ | 1,762.5 | ||||||||||
Cash consideration | 4,388.2 | |||||||||||
$ | 6,150.7 | |||||||||||
Preliminary February 19, 2015 | Measurement Period Adjustments | Adjusted February 19, 2015 | ||||||||||
Net Assets Acquired | ||||||||||||
Cash and cash equivalents | $ | 780.8 | $ | — | $ | 780.8 | ||||||
Accounts receivable | 334.8 | — | 334.8 | |||||||||
Unbilled services | 138.7 | — | 138.7 | |||||||||
Inventories | 51.9 | — | 51.9 | |||||||||
Prepaid expenses and other | 261.4 | 86.5 | 347.9 | |||||||||
Deferred income taxes | 34.4 | 61.1 | 95.5 | |||||||||
Property, plant and equipment | 844.2 | 174.0 | 1,018.2 | |||||||||
Goodwill | 3,176.1 | (135.9 | ) | 3,040.2 | ||||||||
Customer relationships | 1,917.2 | (86.9 | ) | 1,830.3 | ||||||||
Trade names and trademarks | 289.4 | (18.9 | ) | 270.5 | ||||||||
Land use right | 4.9 | (4.9 | ) | — | ||||||||
Technology | — | 74.5 | 74.5 | |||||||||
Favorable leases | — | 5.5 | 5.5 | |||||||||
Other assets | 15.2 | (3.2 | ) | 12.0 | ||||||||
Total assets acquired | 7,849.0 | 151.8 | 8,000.8 | |||||||||
Accounts payable | 190.8 | — | 190.8 | |||||||||
Accrued expenses and other | 280.8 | — | 280.8 | |||||||||
Unearned revenue | 168.0 | — | 168.0 | |||||||||
Deferred income taxes | 730.2 | 122.3 | 852.5 | |||||||||
Senior Notes | 250.0 | — | 250.0 | |||||||||
Other liabilities | 78.5 | 29.5 | 108 | |||||||||
Total liabilities acquired | 1,698.3 | 151.8 | 1,850.1 | |||||||||
Net assets acquired | $ | 6,150.7 | $ | — | $ | 6,150.7 | ||||||
The amortization periods for intangible assets acquired are 28 years for customer relationships, 15 years for trade names and trademarks, 10 years for technology, and 8 years for favorable leases. The Company recorded certain measurement period
F-16
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
adjustments and certain classifications of expenses, including items associated with the allocation of stock compensation, from cost of revenue to selling, general and administrative expenses.
The Acquisition contributed $2,209.7 and $167.2 of revenue and operating income, respectively, during the year ended December 31, 2015.
Unaudited Pro Forma Information
The Company completed the Acquisition on February 19, 2015. Had the Acquisition been completed as of the beginning of 2014, the Company's pro forma results for 2015 and 2014 would have been as follows:
Year Ended December 31, | ||||||||
2015 | 2014 | |||||||
Total revenues | $ | 9,033.3 | $ | 8,711.2 | ||||
Operating income | 1,123.3 | 907.7 | ||||||
Net income attributable to Laboratory Corporation of America Holdings | 546.8 | 423.0 | ||||||
Earnings per share: | ||||||||
Basic | $ | 5.04 | $ | 4.22 | ||||
Diluted | $ | 5.02 | $ | 4.16 | ||||
The unaudited pro forma results reflect certain adjustments related to past operating performance, acquisition costs and acquisition accounting adjustments, such as increased amortization expense and decreased depreciation expense based on the estimated fair value of assets acquired, the impact of the Company’s new financing arrangements, and the related tax effects. The pro forma results include costs directly attributable to the Acquisition which are not expected to have a continuing impact on the combined company, such as transaction costs of $207.6; comprised of change in control, retention and severance arrangements of $26.8; acceleration of stock based compensation of $43.2 and related employer taxes of $9.4; legal, advisor and success fees of $75.6; write-off of bridge and other deferred financing fees of $15.2; and make-whole payments of $37.4 made in connection with the prepayment of Covance's existing private placement debt, all of which are included in the pro forma results of operations for the year ended December 31, 2014. The pro forma results do not include any anticipated synergies which may be achievable subsequent to the Acquisition. To produce the unaudited pro forma financial information, the Company adjusted Covance’s assets and liabilities to their estimated fair value based on a valuation as of the Acquisition Date. These pro forma results of operations have been prepared for comparative purposes only, and they do not purport to be indicative of the results of operations that actually would have resulted had the Acquisition occurred on the date indicated or that may result in the future.
During the year ended December 31, 2015, the Company also acquired various laboratories and related assets for approximately $128.6 in cash (net of cash acquired). The purchase consideration for these acquisitions has been allocated to the estimated fair market value of the net assets acquired, including approximately $17.4 in identifiable intangible assets (primarily customer relationships) and a residual amount of goodwill of approximately $68.4. These acquisitions were made primarily to extend the Company's geographic reach in important market areas and/or enhance the Company's scientific differentiation and esoteric testing capabilities. The Company's results would not have been materially different from its pro forma results had the Company's other 2015 acquisitions occurred at the beginning of 2014.
On November 20, 2014, the Company completed its acquisition of LipoScience, Inc. (LipoScience), a provider of specialized cardiovascular diagnostic laboratory tests based on nuclear magnetic resonance (NMR) technology, for a purchase price of $5.25 per share or a transaction value of $67.9 (net of cash acquired).
The LipoScience purchase consideration has been allocated to the estimated fair market value of the net assets acquired, including approximately $27.2 in identifiable intangible assets (primarily non-tax deductible customer relationships, technology and trade names and trademarks) with weighted-average useful lives of approximately 19.5 years; $9.4 in deferred tax liabilities (relating to identifiable intangible assets); and a residual amount of non-tax deductible goodwill of approximately $17.4.
During the year ended December 31, 2014, the Company also acquired various other laboratories and related assets for approximately $91.5 in cash (net of cash acquired). These acquisitions were made primarily to extend the Company's geographic reach in important market areas and/or enhance the Company's scientific differentiation and esoteric testing capabilities. The purchase consideration for these acquisitions has been allocated to the estimated fair market value of the net assets acquired, including approximately $22.0 in identifiable intangible assets (primarily customer relationships and non-compete agreements) and a residual amount of goodwill of approximately $63.4.
F-17
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
During the year ended December 31, 2013, the Company also acquired various other laboratories and related assets for approximately $159.5 in cash (net of cash acquired). These acquisitions were made primarily to extend the Company's geographic reach in important market areas and/or enhance the Company's scientific differentiation and esoteric testing capabilities.
Contingent consideration liabilities associated with the Company's business acquisitions are recorded at fair value based upon the estimated probability assessment of the earn-out criteria. Changes in the fair value of contingent consideration liabilities are recognized in earnings until the arrangement is settled.
3. RESTRUCTURING AND OTHER SPECIAL CHARGES
During 2015, the Company recorded net restructuring charges of $113.9; $39.2 within LCD and $74.7 within CDD. The charges were comprised of $59.2 in severance and other personnel costs and $55.8 in facility-related costs primarily associated with general integration activities. A substantial portion of these costs relate to the planned closure of two CDD operations that serviced a minimum volume contract that expired on October 31, 2015. The charges were offset by the reversal of previously established reserves of $1.1 in unused facility-related costs. Included within the facility-related charges noted above is a $26.7 asset impairment charge relating to CDD lab and customer service applications that will no longer be used.
In addition, during 2015, the Company recorded $25.6 in consulting expenses (recorded in selling, general and administrative expenses) relating to fees incurred as part of LCD's business process improvement initiative (Project LaunchPad) as well as one-time Covance integration costs and employee compensation studies, along with $5.4 in short-term equity retention arrangements relating to the acquisition of Covance and $0.3 of accelerated equity compensation relating to the previously disclosed retirement of a Company executive (all recorded in selling, general and administrative expenses). The Company also incurred $5.7 relating to the wind-down of the minimum volume contract operations referred to in the previous paragraph. On February 9, 2016, the Company reached an agreement for the sale of the assets and business of one of these sites. As required by U.K. law, substantially all of the employees were transferred with the business.
Additionally, the Company recorded $166.0 of deal costs related to the Acquisition, of which $113.4 is included in selling, general and administrative expenses and $52.6 is included in interest expense. During 2015, the Company also recorded a non-cash loss of $2.3, upon the dissolution of one of its equity investments, which is included in other, net expenses. During the fourth quarter, the Company paid $12.2 in settlement costs and litigation expenses related to the resolution of a U.S. court putative class action lawsuit. In addition, the Company incurred $3.0 of non-capitalized costs associated with the implementation of a major system as part of Project Launchpad.
During 2014, the Company recorded net restructuring charges of $17.8. The charges were comprised of $10.5 in severance and other personnel costs and $8.4 in facility-related costs primarily associated with general integration activities. These charges were offset by the reversal of previously established reserves of $0.4 in unused severance and $0.7 in unused facility related costs.
During 2013, the Company recorded net restructuring charges of $21.8. The charges were comprised of $15.4 in severance and other personnel costs and $9.5 in facility-related costs primarily associated with the ongoing integration of Orchid Cellmark, Inc. and the Integrated Genetics business (formerly Genzyme Genetics) and costs associated with the previously announced termination of an executive vice president. These charges were offset by the reversal of previously established reserves of $0.7 in unused severance and $2.4 in unused facility-related costs.
F-18
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
4. RESTRUCTURING RESERVES
The following represents the Company’s restructuring activities for the period indicated:
LCD | CDD | Total | |||||||||||||||||
Severance and Other Employee Costs | Lease and Other Facility Costs | Severance and Other Employee Costs | Lease and Other Facility Costs | ||||||||||||||||
Balance as of December 31, 2014 | $ | 0.4 | $ | 21.7 | $ | — | $ | — | $ | 22.1 | |||||||||
Restructuring charges | 9.7 | 29.4 | 48.2 | 26.6 | 113.9 | ||||||||||||||
Reduction of prior restructuring accruals | — | (1.1 | ) | — | — | (1.1 | ) | ||||||||||||
Cash payments and other adjustments | (10.0 | ) | (23.5 | ) | 3.3 | (25.5 | ) | (55.7 | ) | ||||||||||
Balance as of December 31, 2015 | $ | 0.1 | $ | 26.5 | $ | 51.5 | $ | 1.1 | $ | 79.2 | |||||||||
Current | $ | 58.6 | |||||||||||||||||
Non-current | 20.6 | ||||||||||||||||||
$ | 79.2 | ||||||||||||||||||
The non-current portion of the restructuring liabilities is expected to be paid out over 6 years. Cash payments and other adjustments includes the reclass of profit sharing, pension, and holiday accrual.
5. JOINT VENTURE PARTNERSHIPS AND EQUITY METHOD INVESTMENTS
At December 31, 2015 the Company had investments in the following unconsolidated joint venture partnerships and equity method investments:
Locations | Net Investment | Interest Owned | ||||
Joint Venture Partnerships: | ||||||
Alberta, Canada (2) | $ | 44.0 | 43.37 | % | ||
Florence, South Carolina | 10.4 | 49.00 | % | |||
Equity Method Investments: | ||||||
Various | 3.8 | various | ||||
The joint venture agreements that govern the conduct of business of these partnerships mandates unanimous agreement between partners on all major business decisions as well as providing other participating rights to each partner. The equity method investments represent the Company’s purchase of shares in clinical diagnostic companies. The investments are accounted for under the equity method of accounting as the Company does not have control of these investments. The Company has no material obligations or guarantees to, or in support of, these unconsolidated investments and their operations.
Effective June 30, 2015, the Company dissolved a joint venture partnership that had been located in Milwaukee, Wisconsin.
Condensed unconsolidated financial information for joint venture partnerships and equity method investments is shown in the following table.
As of December 31: | 2015 | 2014 | |||||
Current assets | $ | 22.6 | $ | 66.0 | |||
Other assets | 19.2 | 43.3 | |||||
Total assets | $ | 41.8 | $ | 109.3 | |||
Current liabilities | $ | 17.3 | $ | 28.2 | |||
Other liabilities | 1.3 | 1.4 | |||||
Total liabilities | 18.6 | 29.6 | |||||
Partners' equity | 23.2 | 79.7 | |||||
Total liabilities and partners’ equity | $ | 41.8 | $ | 109.3 | |||
For the period January 1 - December 31: | 2015 | 2014 | 2013 | ||||||||
Net revenues | $ | 213.7 | $ | 283.8 | $ | 255.2 | |||||
Gross profit | 54.3 | 81.3 | 84.1 | ||||||||
Net earnings | 20.1 | 31.0 | 37.7 | ||||||||
F-19
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
The Company’s recorded investment in one of its Alberta joint venture partnerships at December 31, 2015 includes $35.3 of value assigned to that partnership’s Canadian license (with an indefinite life and deductible for tax) to conduct diagnostic testing services in the province. This Canadian partnership has a license to conduct diagnostic testing services in the province of Alberta. Substantially all of its revenue is received as reimbursement from the Alberta government's healthcare programs. While the Canadian license provides the joint venture the ability to conduct diagnostic testing in Alberta, it does not guarantee that the provincial government will continue to reimburse diagnostic laboratory testing in future years at current levels. A decision by the provincial government to limit or reduce its reimbursement of laboratory diagnostic services would have a negative impact on the profits and cash flows the Company derives from this Canadian joint venture. In December 2013, Alberta Health Services (AHS), the Alberta government's healthcare program, issued a request for proposals for laboratory services that included the scope of services performed by the Canadian partnership. In October 2014, AHS informed the Canadian partnership that it had not been selected as the preferred proponent. In November 2014, the Canadian partnership submitted a vendor bid appeal. AHS established a Vendor Bid Appeal Panel to hear the appeal, and the hearing occurred in February 2015. In August 2015, AHS was directed to cancel the request for proposal process. Subsequently, the Canadian partnership entered into a one-year extension (through March 31, 2017) of its existing contract with AHS. If the contract is not renewed after March 2017, then the Canadian partnership's revenues would decrease substantially and the carrying value of the Company's investment could potentially be impaired.
6. ACCOUNTS RECEIVABLE, NET
December 31, 2015 | December 31, 2014 | ||||||
Gross accounts receivable | $ | 1,434.9 | $ | 1,027.3 | |||
Less allowance for doubtful accounts | (217.0 | ) | (211.6 | ) | |||
$ | 1,217.9 | $ | 815.7 | ||||
The provision for doubtful accounts was $265.4, $276.5 and $254.8 in 2015, 2014 and 2013 respectively.
7. PROPERTY, PLANT AND EQUIPMENT, NET
December 31, 2015 | December 31, 2014 | ||||||
Land | $ | 96.4 | $ | 29.3 | |||
Buildings and building improvements | 681.6 | 201.8 | |||||
Machinery and equipment | 944.2 | 719.8 | |||||
Software | 561.9 | 434.7 | |||||
Leasehold improvements | 271.6 | 200.1 | |||||
Furniture and fixtures | 70.1 | 57.7 | |||||
Construction in progress | 180.4 | 126.7 | |||||
Equipment and real estate under capital leases | 65.1 | 42.9 | |||||
2,871.3 | 1,813.0 | ||||||
Less accumulated depreciation and amortization of capital lease assets | (1,123.9 | ) | (1,026.5 | ) | |||
$ | 1,747.4 | $ | 786.5 | ||||
Depreciation expense and amortization of property, plant and equipment was $269.9, $157.6 and $144.7 for 2015, 2014 and 2013, respectively, including software depreciation of $66.1, $38.5, and $39.3 for 2015, 2014 and 2013, respectively.
F-20
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
8. GOODWILL AND INTANGIBLE ASSETS
The changes in the carrying amount of goodwill (net of accumulated amortization) for the years ended December 31, 2015 and 2014 are as follows:
LCD | CDD | Total | |||||||||||||||||||||
December 31, 2015 | December 31, 2014 | December 31, 2015 | December 31, 2014 | December 31, 2015 | December 31, 2014 | ||||||||||||||||||
Balance as of January 1 | $ | 2,988.9 | $ | 2,912.3 | $ | 110.5 | $ | 110.5 | $ | 3,099.4 | $ | 3,022.8 | |||||||||||
Goodwill acquired during the year | 161.6 | 81.8 | 2,979.5 | — | 3,141.1 | 81.8 | |||||||||||||||||
Foreign currency impact and other adjustments to goodwill | (12.8 | ) | (5.2 | ) | — | — | (12.8 | ) | (5.2 | ) | |||||||||||||
Balance at end of year | $ | 3,137.7 | $ | 2,988.9 | $ | 3,090.0 | $ | 110.5 | $ | 6,227.7 | $ | 3,099.4 | |||||||||||
As discussed in Footnote 2 (Business Acquisitions), the amount of the Acquisition goodwill has not yet been allocated to the underlying domestic and foreign subsidiaries of Covance.
The components of identifiable intangible assets are as follows:
December 31, 2015 | December 31, 2014 | ||||||||||||||||||||||
Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | Gross Carrying Amount | Accumulated Amortization | Net Carrying Amount | ||||||||||||||||||
Customer relationships | $ | 3,132.4 | $ | (724.7 | ) | $ | 2,407.7 | $ | 1,361.6 | $ | (606.8 | ) | $ | 754.8 | |||||||||
Patents, licenses and technology | 308.0 | (146.3 | ) | 161.7 | 125.9 | (95.9 | ) | 30.0 | |||||||||||||||
Non-compete agreements | 51.0 | (37.2 | ) | 13.8 | 45.6 | (31.7 | ) | 13.9 | |||||||||||||||
Trade names | 399.8 | (115.4 | ) | 284.4 | 133.3 | (91.6 | ) | 41.7 | |||||||||||||||
Favorable leases | 5.5 | (0.6 | ) | 4.9 | — | — | — | ||||||||||||||||
Canadian licenses | 451.0 | — | 451.0 | 635.4 | — | 635.4 | |||||||||||||||||
$ | 4,347.7 | $ | (1,024.2 | ) | $ | 3,323.5 | $ | 2,301.8 | $ | (826.0 | ) | $ | 1,475.8 | ||||||||||
A summary of amortizable intangible assets acquired during 2015, and their respective weighted average amortization periods are as follows:
Amount | Weighted Average Amortization Period | ||||
Customer relationships | $ | 1,855.0 | 29.2 | ||
Patents, licenses and technology | 41.2 | 10.0 | |||
Non-compete agreements | 4.3 | 4.9 | |||
Trade names | 270.5 | 15.0 | |||
Favorable leases | 5.5 | 8.0 | |||
2,176.5 | 27.0 | ||||
Amortization of intangible assets was $164.5, $76.7 and $81.7 in 2015, 2014 and 2013, respectively. The Company recorded earn-out and purchase accounting adjustments through amortization expense of $1.7, $10.4, and $5.8 in 2015, 2014 and 2013, respectively. Amortization expense of intangible assets is estimated to be $174.4 in fiscal 2016, $167.2 in fiscal 2017, $156.0 in fiscal 2018, $149.2 in fiscal 2019, $142.4 in fiscal 2020, and $2,017.3 thereafter.
F-21
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
9. ACCRUED EXPENSES AND OTHER
December 31, 2015 | December 31, 2014 | ||||||
Employee compensation and benefits | $ | 283.0 | $ | 183.9 | |||
Self-insurance reserves | 80.6 | 54.2 | |||||
Accrued taxes payable | 83.8 | 32.0 | |||||
Royalty and license fees payable | 7.2 | 8.2 | |||||
Restructuring reserves | 58.6 | 7.8 | |||||
Acquisition related reserves | 12.1 | 7.0 | |||||
Interest payable | 63.8 | 19.7 | |||||
Other | 44.0 | 28.6 | |||||
$ | 633.1 | $ | 341.4 | ||||
10. OTHER LIABILITIES
December 31, 2015 | December 31, 2014 | ||||||
Post-retirement benefit obligation | $ | 19.6 | $ | 26.7 | |||
Defined benefit plan obligation | 165.8 | 117.9 | |||||
Restructuring reserves | 20.6 | 14.3 | |||||
Self-insurance reserves | 10.0 | 34.9 | |||||
Acquisition related reserves | — | 2.2 | |||||
Deferred revenue | 2.5 | 3.4 | |||||
Deferred compensation plan obligation | 46.4 | 43.4 | |||||
Worker's compensation and auto | 28.6 | 23.1 | |||||
Other | 29.6 | 8.3 | |||||
$ | 323.1 | $ | 274.2 | ||||
11. DEBT
Short-term borrowings and current portion of long-term debt at December 31, 2015 and 2014 consisted of the following:
December 31, 2015 | December 31, 2014 | ||||||
Zero-coupon convertible subordinated notes | $ | 94.5 | $ | 93.9 | |||
5.625% Senior Notes due 2015 | — | 250.0 | |||||
3.125% Senior Notes due 2016 | 325.0 | — | |||||
Capital lease obligation | 5.4 | 3.2 | |||||
Total short-term borrowings and current portion of long-term debt | $ | 424.9 | $ | 347.1 | |||
Long-term debt at December 31, 2015 and 2014 consisted of the following:
F-22
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
December 31, 2015 | December 31, 2014 | ||||||
3.125% Senior Notes due 2016 | — | 325.0 | |||||
2.20% Senior Notes due 2017 | 500.0 | 500.0 | |||||
2.50% Senior Notes due 2018 | 400.0 | 400.0 | |||||
4.625% Senior Notes due 2020 | 621.6 | 618.5 | |||||
2.625% Senior Notes due 2020 | 500.0 | — | |||||
3.75% Senior Notes due 2022 | 500.0 | 500.0 | |||||
3.20% Senior Notes due 2022 | 500.0 | — | |||||
4.00% Senior Notes due 2023 | 300.0 | 300.0 | |||||
3.60% Senior Notes due 2025 | 1,000.0 | — | |||||
4.70% Senior Notes due 2045 | 900.0 | — | |||||
Term loan | 715.0 | — | |||||
Capital leases | 55.5 | 39.2 | |||||
Total long-term debt | $ | 5,992.1 | $ | 2,682.7 | |||
Credit Facilities
As part of its financing of the Acquisition, the Company entered into a $1,000.0 term loan. The term loan credit facility will mature five years after the closing date of the Acquisition and may be prepaid without penalty. The term loan balance at December 31, 2015 was $715.0.
On November 2, 2014, in connection with entering into the definitive merger agreement to acquire Covance (Merger Agreement), the Company entered into a bridge facility commitment letter. Under the bridge facility commitment letter, the lenders agreed to provide a $4,250.0 senior unsecured bridge term loan credit facility consisting of a $3,850.0 364-day unsecured debt bridge tranche and a $400.0 60-day unsecured cash bridge tranche for the purpose of financing all or a portion of the cash consideration and the fees and expenses in connection with the transactions contemplated by the Merger Agreement. The bridge facility was permitted to be drawn only in a single drawing on the closing date of the Acquisition.
On December 19, 2014, the Company entered into a five-year term loan credit facility in the principal amount of $1,000.0 for the purpose of financing a portion of the cash consideration and the fees and expenses in connection with the transactions contemplated by the Merger Agreement. Pursuant to the bridge facility commitment letter, upon the Company’s entry into the term loan credit facility, the $4,250.0 bridge facility was reduced to a $3,250.0 commitment, comprising a $2,850.0 364-day unsecured debt bridge tranche and a $400.0 60-day cash bridge tranche. The $1,000.0 of term loan commitments made under the term loan credit facility reduced the debt bridge tranche under the bridge facility dollar for dollar. The term loan credit facility was advanced in full on the Acquisition Date.
On December 19, 2014, the Company also entered into an amendment and restatement of its existing senior revolving credit facility, which was originally entered into on December 21, 2011. The senior revolving credit facility consists of a five-year revolving facility in the principal amount of up to $1,000.0, with the option of increasing the facility by up to an additional $250.0, subject to the agreement of one or more new or existing lenders to provide such additional amounts and certain other customary conditions. The new revolving credit facility also provides for a subfacility of up to $100.0 for swing line borrowings and a subfacility of up to $125.0 for issuances of letters of credit. The new revolving credit facility is permitted to be used for general corporate purposes, including working capital, capital expenditures, funding of share repurchases and certain other payments, and acquisitions and other investments. There were no balances outstanding on the Company's new Revolving Credit Facility at December 31, 2015 or on its former revolving credit facility at December 31, 2014.
On January 30, 2015, the Company issued the Acquisition Notes, which represent $2,900.0 in debt securities consisting of $500.0 aggregate principal amount of 2.625% Senior Notes due 2020, $500.0 aggregate principal amount of 3.20% Senior Notes due 2022, $1,000.0 aggregate principal amount of 3.60% Senior Notes due 2025 and $900.0 aggregate principal amount of 4.70% Senior Notes due 2045. Net proceeds from the offering of the Acquisition Notes were $2,870.2 after deducting underwriting discounts and other estimated expenses of the offering. Net proceeds were used to pay a portion of the cash consideration and the fees and expenses in connection with the Covance acquisition. Pursuant to the bridge facility commitment letter, upon the Company’s issuance of the Acquisition Notes the remaining $2,850.0 364-day unsecured debt bridge tranche under the senior unsecured bridge term loan credit facility was terminated.
F-23
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
On February 13, 2015, the Company entered into a 60-day cash bridge term loan credit facility in the principal amount of $400.0 for the purpose of financing a portion of the cash consideration and the fees and expenses in connection with the transactions contemplated by the Merger Agreement. The 60-day cash bridge term loan credit facility was entered into on the terms set forth in the bridge facility commitment letter for the $400.0 60-day cash bridge tranche. The 60-day cash bridge term loan credit facility was advanced in full on the Acquisition Date. On March 16, 2015, the Company elected to prepay the bridge facility without penalty.
Under the term loan facility and the new revolving credit facility, which have affirmative and negative covenants that are substantially identical, the Company is subject to negative covenants limiting subsidiary indebtedness and certain other covenants typical for investment grade-rated borrowers and the Company is required to maintain a leverage ratio that varies. Prior to the Acquisition Date, the leverage ratio was required to have been no greater than 3.75 to 1.0 calculated by excluding the $2,900.0 in total aggregate principal amount of the Company's senior notes issued for the purpose of funding the acquisition. From and after the acquisition closing date, the leverage ratio must be no greater than 4.75 to 1.0 with respect to the last day of each of the first four fiscal quarters ending on or after the closing date, 4.25 to 1.0 with respect to the last day of each of the fifth through eighth fiscal quarters ending after the closing date, and 3.75 to 1.0 with respect to the last day of each fiscal quarter ending thereafter. The Company was in compliance with all covenants in the term loan facility and the new revolving credit facility at December 31, 2015. As of December 31, 2015, the ratio of total debt to consolidated EBITDA was 3.6 to 1.0.
The term loan credit facility accrues interest at a per annum rate equal to, at the Company’s election, either a LIBOR rate plus a margin ranging from 1.125% to 2.00%, or a base rate determined according to a prime rate or federal funds rate plus a margin ranging from 0.125% to 1.00%. Advances under the new revolving credit facility will accrue interest at a per annum rate equal to, at the Company’s election, either a LIBOR rate plus a margin ranging from 1.00% to 1.60%, or a base rate determined according to a prime rate or federal funds rate plus a margin ranging from 0.00% to 0.60%. Fees are payable on outstanding letters of credit under the new revolving credit facility at a per annum rate equal to the applicable margin for LIBOR loans, and the Company is required to pay a facility fee on the aggregate commitments under the new revolving credit facility, at a per annum rate ranging from 0.125% to 0.40%. The interest margin applicable to the credit facilities, and the facility fee and letter of credit fees payable under the new revolving credit facility, are based on the Company’s senior credit ratings as determined by S&P’s and Moody’s, which are currently BBB and Baa2, respectively.
As of December 31, 2015, the effective interest rate on the new Revolving Credit Facility was 1.5%.
Zero-Coupon Convertible Subordinated Notes
The Company had $105.4 and $106.9 aggregate principal amount at maturity of zero-coupon convertible subordinated notes (the Notes) due 2021 outstanding at December 31, 2015 and 2014, respectively. The Notes, which are subordinate to the Company’s bank debt, were sold at an issue price of $671.65 per $1,000.0 principal amount at maturity (representing a yield to maturity of 2.0% per year). Each one thousand dollar principal amount at maturity of the Notes is convertible into 13.4108 shares of the Company’s common stock, subject to adjustment in certain circumstances, if one of the following conditions occurs:
1) | If the sales price of the Company’s common stock for at least 20 trading days in a period of 30 consecutive trading days ending on the last trading day of the preceding quarter reaches specified thresholds (beginning at 120% and declining 0.1282% per quarter until it reaches approximately 110% for the quarter beginning July 1, 2021 of the accreted conversion price per share of common stock on the last day of the preceding quarter). The accreted conversion price per share will equal the issue price of a note plus the accrued original issue discount and any accrued contingent additional principal, divided by the number of shares of common stock issuable upon conversion of a note on that day. The conversion trigger price for the fourth quarter of 2015 was $75.11. |
2) | If the credit rating assigned to the notes by S&P’s Ratings Services is at or below BB-. |
3) | If the Notes are called for redemption. |
4) | If specified corporate transactions have occurred (such as if the Company is party to a consolidation, merger or binding share exchange or a transfer of all or substantially all of its assets). |
The Company may redeem for cash all or a portion of the Notes at any time at specified redemption prices per one thousand dollar principal amount at maturity of the Notes.
The Company has registered the notes and the shares of common stock issuable upon conversion of the Notes with the Securities and Exchange Commission.
F-24
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
During 2015 and 2014, the Company settled notices to convert $1.5 and $21.9 aggregate principal amount at maturity of its zero-coupon subordinated notes with a conversion value of $1.3 and $28.7, respectively. The total cash used for these settlements was $1.3 and $18.9 and the Company also issued 0.0 and 0.1 additional shares of common stock, respectively. As a result of these conversions, in 2015 and 2014 the Company also reversed approximately $0.4 and $3.8, respectively, of deferred tax liability to reflect the tax benefit realized upon issuance of the shares.
On September 11, 2015, the Company announced that for the period of September 12, 2015 to March 11, 2016, the zero-coupon subordinated notes will accrue contingent cash interest at a rate of no less than 0.125% of the average market price of a zero-coupon subordinated note for the five trading days ended September 9, 2015, in addition to the continued accrual of the original issue discount.
On January 4, 2016, the Company announced that its zero-coupon subordinated notes may be converted into cash and common stock at the conversion rate of 13.4108 per $1,000.0 principal amount at maturity of the notes, subject to the terms of the zero-coupon subordinated notes and the Indenture, dated as of October 24, 2006 between the Company, and The Bank of New York Mellon as trustee and the conversion agent. In order to exercise the option to convert all or a portion of the zero-coupon subordinated notes, holders are required to validly surrender their zero-coupon subordinated notes at any time during the calendar quarter beginning January 1, 2016, through the close of business on the last business day of the calendar quarter, which is 5:00 p.m., New York City time, on Thursday, March 31, 2016. If notices of conversion are received, the Company plans to settle the cash portion of the conversion obligation with cash on hand and/or borrowings under the new revolving credit facility.
Senior Notes
On January 30, 2015, the Company issued the Acquisition Notes, which represent $2,900.0 in debt securities consisting of $500.0 aggregate principal amount of 2.625% Senior Notes due 2020, $500.0 aggregate principal amount of 3.20% Senior Notes due 2022, $1,000.0 aggregate principal amount of 3.60% Senior Notes due 2025 and $900.0 aggregate principal amount of 4.70% Senior Notes due 2045. Interest on these notes is payable semi-annually on February 1 and August 1 of each year, commencing on August 1, 2015. Net proceeds from the offering of the Acquisition Notes were $2,870.2 after deducting underwriting discounts and other estimated expenses of the offering. Net proceeds were used to pay a portion of the cash consideration and the fees and expenses in connection with the Covance acquisition.
On November 1, 2013, the Company issued $700.0 in new senior notes pursuant to the Company’s effective shelf registration on Form S-3. The senior notes consisted of $400.0 aggregate principal amount of 2.50% Senior Notes due 2018 and $300.0 aggregate principal amount of 4.00% Senior Notes due 2023. Interest on these notes is payable semi-annually on November 1 and May 1 of each year, commencing on May 1, 2014. The net proceeds were used to repay all of the outstanding borrowings under the Company’s Revolving Credit Facility and for general corporate purposes.
During the third quarter of 2013, the Company entered into two fixed-to-variable interest rate swap agreements for the 4.625% senior notes due 2020 with an aggregate notional amount of $600.0 and variable interest rates based on one-month LIBOR plus 2.298% to hedge against changes in the fair value of a portion of the Company's long term debt. These derivative financial instruments are accounted for as fair value hedges of the Senior Notes due 2020 outstanding at that time. These interest rate swaps are included in other long term assets or liabilities, as applicable, and added to the value of the senior notes, with an aggregate fair value of $21.6 at December 31, 2015.
The Senior Notes due 2017 and Senior Notes due 2022 bear interest at the rate of 2.20% per annum and 3.75% per annum, respectively, payable semi-annually on February 23 and August 23 of each year, commencing February 23, 2013.
The Senior Notes due 2015 bore interest at the rate of 5.625% per annum from December 14, 2005, and was payable semi-annually on June 15 and December 15.
The scheduled payments of long term debt and future minimum lease payments for capital leases at the end of 2015 are summarized as follows:
F-25
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Notes and Other | Capital Leases | Total | |||||||||
2016 | $ | 419.5 | $ | 11.9 | $ | 431.4 | |||||
2017 | 500.0 | 11.9 | 511.9 | ||||||||
2018 | 443.8 | 11.7 | 455.5 | ||||||||
2019 | 64.6 | 11.2 | 75.8 | ||||||||
2020 | 1,728.2 | 10.6 | 1,738.8 | ||||||||
Thereafter | 3,200.0 | 47.2 | 3,247.2 | ||||||||
6,356.1 | 104.5 | 6,460.6 | |||||||||
Less amounts representing interest | — | (43.6 | ) | (43.6 | ) | ||||||
Total long-term debt | 6,356.1 | 60.9 | 6,417 | ||||||||
Less current portion | (419.5 | ) | (5.4 | ) | (424.9 | ) | |||||
Long-term debt, due beyond one year | $ | 5,936.6 | $ | 55.5 | $ | 5,992.1 | |||||
12. PREFERRED STOCK AND COMMON SHAREHOLDERS’ EQUITY
The Company is authorized to issue up to 265.0 shares of common stock, par value $0.10 per share. The Company’s treasury shares are recorded at aggregate cost. Common shares issued and outstanding are summarized in the following table:
2015 | 2014 | ||||
Issued | 123.9 | 107.1 | |||
In treasury | (22.6 | ) | (22.5 | ) | |
Outstanding | 101.3 | 84.6 | |||
The Company is authorized to issue up to 30.0 shares of preferred stock, par value $0.10 per share. There were no preferred shares outstanding as of December 31, 2015 and 2014.
The changes in common shares issued and held in treasury are summarized below:
Common Shares Issued | ||||||||
2015 | 2014 | 2013 | ||||||
Common stock issued at January 1 | 107.1 | 108.1 | 115.8 | |||||
Common stock issued under employee stock plans | 1.5 | 1.6 | 2.6 | |||||
Common stock issued upon conversion of zero-coupon subordinated notes | — | 0.1 | 0.1 | |||||
Common stock issued in conjunction with the Acquisition | 15.3 | — | — | |||||
Retirement of common stock | — | (2.7 | ) | (10.4 | ) | |||
Common stock issued at December 31 | 123.9 | 107.1 | 108.1 | |||||
Common Shares Held in Treasury | ||||||||
2015 | 2014 | 2013 | ||||||
Common shares held in treasury at January 1 | 22.5 | 22.4 | 22.3 | |||||
Surrender of restricted stock and performance share awards | 0.1 | 0.1 | 0.1 | |||||
Common shares held in treasury at December 31 | 22.6 | 22.5 | 22.4 | |||||
Share Repurchase Program
As of December 31, 2015, the Company had outstanding authorization from the Board of Directors to purchase $789.5 of Company common stock based on settled trades as of these respective dates. The repurchase authorization has no expiration date. Following the announcement of the acquisition of Covance, the Company suspended its share repurchases. The Company does not anticipate resuming its share repurchase activity until it approaches its targeted leverage ratio of total debt to consolidated EBITDA of 2.5 to 1.0. However, the Company will continue to evaluate all opportunities for strategic deployment of capital in light of market conditions.
F-26
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Accumulated Other Comprehensive Earnings
The components of accumulated other comprehensive earnings are as follows:
Foreign Currency Translation Adjustments | Net Benefit Plan Adjustments | Unrealized Gains and Losses on Available for Sale Securities | Accumulated Other Comprehensive Earnings | ||||||||||||
Balance at December 31, 2012 | $ | 162.9 | $ | (93.5 | ) | — | $ | 69.4 | |||||||
Current year adjustments | (63.2 | ) | 31.6 | 16.4 | (15.2 | ) | |||||||||
Amounts reclassified from accumulated other comprehensive income (a) | — | 10.5 | — | 10.5 | |||||||||||
Tax effect of adjustments | 23.5 | (15.7 | ) | (6.3 | ) | 1.5 | |||||||||
Balance at December 31, 2013 | 123.2 | (67.1 | ) | 10.1 | 66.2 | ||||||||||
Current year adjustments | (89.5 | ) | (12.0 | ) | 2.0 | (99.5 | ) | ||||||||
Amounts reclassified from accumulated other comprehensive income (a) | — | (6.6 | ) | (18.3 | ) | (24.9 | ) | ||||||||
Tax effect of adjustments | 34.3 | 7.1 | 6.3 | 47.7 | |||||||||||
Balance at December 31, 2014 | 68.0 | (78.6 | ) | 0.1 | (10.5 | ) | |||||||||
Current year adjustments | (370.7 | ) | 19.0 | (0.1 | ) | (351.8 | ) | ||||||||
Amounts reclassified from accumulated other comprehensive income (a) (b) | — | (11.3 | ) | — | (11.3 | ) | |||||||||
Tax effect of adjustments | 90.1 | (3.5 | ) | — | 86.6 | ||||||||||
Balance at December 31, 2015 | $ | (212.6 | ) | $ | (74.4 | ) | $ | — | $ | (287.0 | ) | ||||
(a) The amortization of prior service cost is included in the computation of net periodic benefit cost. Refer to Note 16 Pension and Postretirement Plans for additional information regarding the Company's net periodic benefit cost.
(b) The realized gain from the sale of an available for sale investment and the other-than-temporary impairment on an available for sale investment are included in Other, net on the Consolidated Statement of Operations.
13. INCOME TAXES
The sources of income before taxes, classified between domestic and foreign entities are as follows:
Pre-tax income | 2015 | 2014 | 2013 | ||||||||
Domestic | $ | 599.6 | $ | 758.6 | $ | 844.2 | |||||
Foreign | 132.5 | 68.1 | 71.4 | ||||||||
Total pre-tax income | $ | 732.1 | $ | 826.7 | $ | 915.6 | |||||
The provisions (benefits) for income taxes in the accompanying consolidated statements of operations consist of the following:
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Current: | |||||||||||
Federal | $ | 218.3 | $ | 233.6 | $ | 231.6 | |||||
State | 39.8 | 30.1 | 29.9 | ||||||||
Foreign | 69.4 | 22.7 | 22.5 | ||||||||
$ | 327.5 | $ | 286.4 | $ | 284.0 | ||||||
Deferred: | |||||||||||
Federal | $ | (14.1 | ) | $ | 29.1 | $ | 55.2 | ||||
State | (4.2 | ) | 3.7 | 6.1 | |||||||
Foreign | (15.1 | ) | (5.1 | ) | (5.1 | ) | |||||
(33.4 | ) | 27.7 | 56.2 | ||||||||
$ | 294.1 | $ | 314.1 | $ | 340.2 | ||||||
F-27
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
A portion of the tax benefit associated with option exercises from stock plans reducing taxes currently payable is recorded through additional paid-in capital. The benefits recorded through additional paid-in capital are approximately $13.1, $5.9 and $10.6 in 2015, 2014 and 2013, respectively.
The effective tax rates on earnings before income taxes are reconciled to statutory U.S. income tax rates as follows:
Years Ended December 31, | ||||||||
2015 | 2014 | 2013 | ||||||
Statutory U.S. rate | 35.0 | % | 35.0 | % | 35.0 | % | ||
State and local income taxes, net of U.S. income tax effect | 3.2 | 2.7 | 2.6 | |||||
Foreign earnings taxes at lower rates than the statutory U.S. rate | (1.8 | ) | — | — | ||||
One-time restructuring and acquisition items | 2.7 | — | — | |||||
Other | 1.1 | 0.3 | (0.4 | ) | ||||
Effective rate | 40.2 | % | 38.0 | % | 37.2 | % | ||
The effective rate for 2015 was unfavorably impacted by one-time restructuring and acquisition items, recording of additional uncertain tax reserves, and a decrease in the benefit recorded from releasing uncertain tax reserves. The 2015 rate was favorably impacted by foreign earnings taxed at rates lower than the U.S. statutory rate.
The effective rate for 2014 was unfavorably impacted by the recording of a full valuation allowance for the write down of two of the Company's investments.
The effective rate for 2013 was favorably impacted by the release of the capital loss valuation allowance and recording two years of the R&D tax credit. The American Taxpayer Relief Act of 2012 was enacted in early 2013 and reinstated the R&D tax credit for 2012 and extended the credit for calendar year 2013.
The tax effects of temporary differences that give rise to significant portions of the deferred tax assets and deferred tax liabilities are as follows:
December 31, 2015 | December 31, 2014 | ||||||
Deferred tax assets: | |||||||
Accounts receivable | $ | 13.4 | $ | 12.9 | |||
Employee compensation and benefits | 140.6 | 110.7 | |||||
Self insurance reserves | 36.9 | 27.6 | |||||
Postretirement benefit obligation | 7.5 | 10.2 | |||||
Acquisition and restructuring reserves | 24.5 | 20.1 | |||||
Tax loss carryforwards | 98.3 | 69.1 | |||||
Other | 4.3 | 2.4 | |||||
325.5 | 253.0 | ||||||
Less: valuation allowance | (15.1 | ) | (17.1 | ) | |||
Net deferred tax assets | $ | 310.4 | $ | 235.9 | |||
Deferred tax liabilities: | |||||||
Deferred earnings | $ | (189.5 | ) | $ | (15.8 | ) | |
Intangible assets | (979.2 | ) | (496.3 | ) | |||
Property, plant and equipment | (211.9 | ) | (93.5 | ) | |||
Zero-coupon subordinated notes | (87.4 | ) | (92.8 | ) | |||
Currency translation adjustment | (47.3 | ) | (48.7 | ) | |||
Total gross deferred tax liabilities | (1,515.3 | ) | (747.1 | ) | |||
Net deferred tax liabilities | $ | (1,204.9 | ) | $ | (511.2 | ) | |
The valuation allowance decreased from $17.1 in 2014 to $15.1 in 2015. The decrease in the valuation allowance is primarily due to releasing an allowance for capital loss carryover as the Company recorded capital gain income during 2015.
The Company has foreign tax loss carryovers of $39.5 of which $27.4 has a full valuation allowance. Most of the foreign losses have an indefinite carryover. The Company has U.S. tax loss carryovers of approximately $245.0 expiring periodically
F-28
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
through 2035. The utilization of the tax loss carryovers is limited due to change of ownership rules. However, at this time the Company expects to fully utilize substantially all U.S. tax loss carryovers with the exception of approximately $3.9 for which a full valuation allowance has been provided. In addition to the net operating losses, the Company has a foreign capital loss carryover of $6.8. The loss has an indefinite life and has a full valuation allowance.
The unrecognized income tax benefits were $24.2 and $16.7 at December 31, 2015 and 2014, respectively. The increase in the reserve is primarily due to the beginning balances carried over in the Acquisition. It is anticipated that the amount of the unrecognized income tax benefits will change within the next twelve months; however, these changes are not expected to have a significant impact on the results of operations, cash flows or the financial position of the Company.
The Company recognizes interest and penalties related to unrecognized income tax benefits in income tax expense. Accrued interest and penalties related to uncertain tax positions totaled $12.7 and $8.2 as of December 31, 2015 and 2014, respectively. During the years ended December 31, 2015, 2014 and 2013, the Company recognized $1.8, $2.2 and $2.4, respectively, in interest and penalties expense, which was offset by a benefit of $2.2, $3.3 and $2.9, respectively.
The following table shows a reconciliation of the unrecognized income tax benefits from uncertain tax positions for the years ended December 31, 2015, 2014 and 2013:
2015 | 2014 | 2013 | |||||||||
Balance as of January 1 | $ | 16.7 | $ | 25.6 | $ | 36.4 | |||||
Increase in reserve for tax positions taken in the current year | 4.1 | — | 1.9 | ||||||||
Increase in reserve as a result of acquisition | 8.5 | — | — | ||||||||
Decrease in reserve as a result of settlements reached with tax authorities | — | — | (4.4 | ) | |||||||
Decrease in reserve as a result of lapses in the statute of limitations | (5.1 | ) | (8.9 | ) | (8.3 | ) | |||||
Balance as of December 31 | $ | 24.2 | $ | 16.7 | $ | 25.6 | |||||
As of December 31, 2015 and 2014, $24.2 and $16.7, respectively, is the approximate amount of unrecognized income tax benefits that, if recognized, would favorably affect the effective income tax rate in any future periods.
The Company has substantially concluded all U.S. federal income tax matters for years through 2011. Substantially all material state and local, and foreign income tax matters have been concluded through 2010 and 2004, respectively.
The Internal Revenue Service concluded the examination of the Company's 2010 and 2011 income tax returns during 2013. The Company was notified during the first quarter of 2016 that the 2014 income tax return is under examination. Canada Revenue Agency concluded its examination of 2009 and 2010 Canadian income tax returns during 2015. The Company has various state income tax examinations ongoing throughout the year. The Company believes adequate provisions have been recorded related to all open tax years.
The Company changed its method for accounting for foreign earnings at the beginning of 2015 as a result of the Acquisition. Prior to the Acquisition, the Company provided tax for substantially all of the foreign earnings. Other than certain amounts associated with the Acquisition, the Company considers its foreign earnings are now permanently invested outside of the U.S. The Company intends to leave its future generated unremitted foreign earnings invested indefinitely outside the U.S. given its expanded global profile. It is not practical to estimate the amount of additional tax that could be payable if accumulated earnings were remitted. Total unremitted foreign earnings for which income tax has not been provided is approximately $662.0 as of December 31, 2015.
14. STOCK COMPENSATION PLANS
Stock Incentive Plans
There are currently 10.2 shares authorized for issuance under the Laboratory Corporation of America Holdings 2012 Omnibus Incentive Plan and at December 31, 2015 there were 5.3 additional shares available for grant under the Plan. This Plan was approved by shareholders at the 2012 annual meeting. An additional 1.2 authorized shares were acquired with the Acquisition under the Covance 2014 Employee Equity Participation Plan and were utilized for the 2015 grant.
F-29
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Stock Options
The following table summarizes grants of non-qualified options made by the Company to officers, key employees, and non-employee directors under all plans. Stock options are generally granted at an exercise price equal to or greater than the fair market price per share on the date of grant. Also, for each grant, options vest ratably over a period of three years on the anniversaries of the grant date, subject to their earlier expiration or termination.
Changes in options outstanding under the plans for the period indicated were as follows:
Number of Options | Weighted-Average Exercise Price per Option | Weighted-Average Remaining Contractual Term | Aggregate Intrinsic Value | |||||||||
Outstanding at December 31, 2014 | 3.3 | $ | 81.07 | |||||||||
Granted | — | — | ||||||||||
Exercised | (1.0 | ) | 80.88 | |||||||||
Cancelled | — | — | ||||||||||
Outstanding at December 31, 2015 | 2.3 | $ | 81.14 | 5.0 | $ | 95.9 | ||||||
Vested and expected to vest at December 31, 2015 | 2.3 | $ | 81.14 | 5.0 | $ | 95.9 | ||||||
Exercisable at December 31, 2015 | 2.3 | $ | 81.14 | 5.0 | $ | 95.9 | ||||||
The aggregate intrinsic value in the table above represents the total pre-tax intrinsic value (the difference between the Company’s closing stock price on the last trading day of 2015 and the exercise price, multiplied by the number of in-the-money options) that would have been received by the option holders had all option holders exercised their options on December 31, 2015. The amount of intrinsic value will change based on the fair market value of the Company’s stock.
Cash received by the Company from option exercises, the actual tax benefit realized for the tax deductions and the aggregate intrinsic value of options exercised from option exercises under all share-based payment arrangements during the years ended December 31, 2015, 2014, and 2013 were as follows:
2015 | 2014 | 2013 | |||||||||
Cash received by the Company | $ | 82.6 | $ | 98.5 | $ | 158.0 | |||||
Tax benefits realized | $ | 16.2 | $ | 12.3 | $ | 21.3 | |||||
Aggregate intrinsic value | $ | 42.2 | $ | 32.1 | $ | 55.4 | |||||
The following table summarizes information concerning currently outstanding and exercisable options.
Options Outstanding | Options Exercisable | |||||||||
Range of Exercise Prices | Number Outstanding | Weighted Average | Number Exercisable | Weighted Average Exercise Price | ||||||
Remaining Contractual Life | Average Exercise Price | |||||||||
$ 6.80 - 59.37 | 0.0 | 0.3 | $59.37 | — | $59.37 | |||||
$59.38 - 67.60 | 0.2 | 3.1 | $60.35 | 0.2 | $60.35 | |||||
$67.61 - 75.63 | 0.6 | 3.7 | $71.63 | 0.6 | $71.63 | |||||
$75.64 - 80.37 | 0.1 | 2.2 | $79.59 | 0.1 | $79.59 | |||||
$80.38 - 98.49 | 1.4 | 5.8 | $87.48 | 1.4 | $87.48 | |||||
2.3 | 5.0 | $81.14 | 2.3 | $81.14 | ||||||
The following table shows the weighted average grant-date fair values of options issued during the respective year and the weighted average assumptions that the Company used to develop the fair value estimates:
F-30
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
2015 | 2014 | 2013 | |||
Fair value per option | N/A | N/A | N/A | ||
Valuation assumptions | |||||
Weighted average expected life (in years) | N/A | N/A | N/A | ||
Risk free interest rate | N/A | N/A | N/A | ||
Expected volatility | N/A | N/A | N/A | ||
Expected dividend yield | N/A | N/A | N/A | ||
The Black Scholes model incorporates assumptions to value stock-based awards. The risk-free interest rate for periods within the contractual life of the option is based on a zero-coupon U.S. government instrument over the contractual term of the equity instrument. Expected volatility of the Company’s stock is based on historical volatility of the Company’s stock. The Company uses historical data to calculate the expected life of the option. Groups of employees and non-employee directors that have similar exercise behavior with regard to option exercise timing and forfeiture rates are considered separately for valuation purposes. For 2015, 2014 and 2013, expense related to the Company’s stock option plan totaled $2.2, $6.9 and $14.5, respectively. The Company did not grant any options to employees during 2015 or 2014.
Restricted Stock, Restricted Stock Units and Performance Shares
The Company grants restricted stock, restricted stock units and performance shares (non-vested shares) to officers and key employees and grants restricted stock and restricted stock units to non-employee directors. Restricted stock and restricted stock units typically vest annually in equal one third increments beginning on the first anniversary of the grant. A performance share grant in 2013 represents a three-year award opportunity for the period 2013-2015, and if earned, vests fully (to the extent earned) in the first quarter of 2016. A performance share grant in 2014 represents a three-year award opportunity for the period of 2014-2016 and, if earned, vests fully (to the extent earned) in the first quarter of 2017. A performance share grant in 2015 represents a three-year award opportunity for the period of 2015-2017 and, if earned, vests fully (to the extent earned) in the first quarter of 2018. Performance share awards are subject to certain earnings per share, revenue, operating income, earnings before income taxes and total shareholder return targets, the achievement of which may increase or decrease the number of shares which the grantee earns and therefore receives upon vesting. Unearned restricted stock and performance share compensation is amortized to expense over the applicable vesting periods. For 2015, 2014 and 2013, total restricted stock, restricted stock unit and performance share compensation expense was $83.8, $34.8 and $19.3, respectively.
The following table shows a summary of non-vested shares for the year ended December 31, 2015:
Number of Shares | Weighted-Average Grant Date Fair Value | |||||
Non-vested at January 1, 2015 | 1.1 | $ | 91.30 | |||
Granted | 0.8 | 123.61 | ||||
Vested | (0.3 | ) | 95.44 | |||
Canceled | (0.1 | ) | 111.38 | |||
Non-vested at December 31, 2015 | 1.5 | $ | 109.00 | |||
As of December 31, 2015, there was $95.6 of total unrecognized compensation cost related to non-vested restricted stock, restricted stock unit and performance share-based compensation arrangements granted under the Company's stock incentive plans. That cost is expected to be recognized over a weighted average period of 1.9 years.
Employee Stock Purchase Plan
The Company has an employee stock purchase plan, begun in 1997 and amended in 1999, 2004, 2008 and 2012, with 6.3 shares of common stock authorized for issuance. The plan permits substantially all employees to purchase a limited number of shares of Company stock at 85% of market value. The Company issues shares to participating employees semi-annually in January and July of each year. Approximately 0.2 shares were purchased by eligible employees in 2015, 2014 and 2013, respectively. For 2015, 2014 and 2013, expense related to the Company’s employee stock purchase plan was $4.1, $4.0 and $3.5, respectively.
F-31
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
The Company uses the Black-Scholes model to calculate the fair value of the employee’s purchase right. The fair value of the employee’s purchase right and the assumptions used in its calculation are as follows:
2015 | 2014 | 2013 | |||||||||
Fair value of the employee’s purchase right | $ | 21.95 | $ | 19.48 | $ | 17.22 | |||||
Valuation assumptions | |||||||||||
Risk free interest rate | 0.3 | % | 0.1 | % | 0.1 | % | |||||
Expected volatility | 0.2 | 0.2 | 0.2 | ||||||||
Expected dividend yield | — | — | — | ||||||||
15. COMMITMENTS AND CONTINGENT LIABILITIES
The Company is involved from time to time in various claims and legal actions, including arbitrations, class actions, and other litigation (including those described in more detail below), arising in the ordinary course of business. Some of these actions involve claims that are substantial in amount. These matters include, but are not limited to, intellectual property disputes, professional liability, employee-related matters, and inquiries, including subpoenas and other civil investigative demands, from governmental agencies and Medicare or Medicaid payers and MCOs reviewing billing practices or requesting comment on allegations of billing irregularities that are brought to their attention through billing audits or third parties. The Company receives civil investigative demands or other inquiries from various governmental bodies in the ordinary course of its business. Such inquiries can relate to the Company or other healthcare providers. The Company works cooperatively to respond to appropriate requests for information.
The Company also is named from time to time in suits brought under the qui tam provisions of the False Claims Act and comparable state laws. These suits typically allege that the Company has made false statements and/or certifications in connection with claims for payment from U.S. or state healthcare programs. The suits may remain under seal (hence, unknown to the Company) for some time while the government decides whether to intervene on behalf of the qui tam plaintiff. Such claims are an inevitable part of doing business in the healthcare field today.
The Company believes that it is in compliance in all material respects with all statutes, regulations and other requirements applicable to its clinical laboratory operations and drug development support services. The healthcare diagnostics and drug development industries are, however, subject to extensive regulation, and the courts have not interpreted many of the applicable statutes and regulations. There can be no assurance, therefore, that the applicable statutes and regulations will not be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that would adversely affect the Company. Potential sanctions for violation of these statutes and regulations include significant fines, the loss of various licenses, certificates and authorizations, and/or exclusion from participation in government programs.
Many of the current claims and legal actions against the Company are in preliminary stages, and many of these cases seek an indeterminate amount of damages. The Company records an aggregate legal reserve, which is determined using actuarial calculations based on historical loss rates and assessment of trends experienced in settlements and defense costs. In accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification Topic 450 “Contingencies”, the Company establishes reserves for judicial, regulatory, and arbitration matters outside the aggregate legal reserve if and when those matters present loss contingencies that are both probable and estimable and would exceed the aggregate legal reserve. When loss contingencies are not both probable and estimable, the Company does not establish separate reserves.
The Company is unable to estimate a range of reasonably probable loss for the proceedings described in more detail below in which damages either have not been specified or, in the Company's judgment, are unsupported and/or exaggerated and (i) the proceedings are in early stages; (ii) there is uncertainty as to the outcome of pending appeals or motions; (iii) there are significant factual issues to be resolved; and/or (iv) there are novel legal issues to be presented. For these proceedings, however, the Company does not believe, based on currently available information, that the outcomes will have a material adverse effect on the Company's financial condition, though the outcomes could be material to the Company's operating results for any particular period, depending, in part, upon the operating results for such period.
As reported, the Company reached a settlement in the previously disclosed lawsuit, California ex rel. Hunter Laboratories, LLC et al. v. Quest Diagnostics Incorporated, et al. (Hunter Labs Settlement Agreement), to avoid the uncertainty and costs associated with prolonged litigation. Pursuant to the executed Hunter Labs Settlement Agreement, the Company recorded a litigation settlement expense of $34.5 in the second quarter of 2011 (net of a previously recorded reserve of $15.0) and paid the settlement amount of $49.5 in the third quarter of 2011. The Company also agreed to certain reporting obligations regarding its pricing for a limited time period and, at the option of the Company in lieu of such reporting obligations, to provide Medi-Cal with a discount
F-32
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
from Medi-Cal's otherwise applicable maximum reimbursement rate from November 1, 2011, through October 31, 2012. In June of 2012, the California legislature enacted Assembly Bill No. 1494, Section 9 of which directed the Department of Healthcare Services (DHCS) to establish new reimbursement rates for Medi-Cal clinical laboratory services that will be based on payments made to California clinical laboratories for similar services by other third-party payers. With stakeholder input, DHCS established data elements and a format for laboratories to report payment data from comparable third-party payers. Laboratories reported payment data to DHCS in the summer of 2013. On March 28, 2014, Assembly Bill No. 1124 extended the implementation deadline of new regulations until June 30, 2016. Assembly Bill No. 1494 provides that until the new rates are set through this process, Medi-Cal payments for clinical laboratory services will be reduced (in addition to a 10.0% payment reduction imposed by Assembly Bill No. 97 in 2011) by “up to 10 percent” for tests with dates of service on or after July 1, 2012, with a cap on payments set at 80.0% of the lowest maximum allowance established under the Medicare program. In April 2015, CMS approved a 10.0% payment reduction under Assembly Bill No. 1494. This cut, which applied retroactively to the period of July 1, 2012 through June 30, 2015, is being implemented through recoupments from weekly reimbursements at a rate of 5% of the amount owed until paid in full. Under the terms of the Hunter Labs Settlement Agreement, the enactment of this California legislation terminates the Company's reporting obligations (or obligation to provide a discount in lieu of reporting) under that agreement. In December 2014, DHCS announced at a stakeholder meeting the results of its analysis of payment data reported by laboratories in 2013 and its proposed rate methodology, on which it solicited stakeholder comments. The Company objected to the proposal by DHCS to exclude from the new rate calculations data on payments from comparable third-party payers exceeding 80.0% of Medicare reimbursement amounts and its proposal to impose the 10.0% payment reduction enacted in Assembly Bill No. 97 after calculation of the new rates. In January 2015, after receiving stakeholder comments, DHCS instructed laboratories to submit 2014 payment data by March 27, 2015, which DHCS used (except for data on payment amounts exceeding 80.0% of Medicare reimbursement) to establish new rates. DHCS obtained CMS approval of a state plan amendment adopting the new rate methodology and rates effective July 1, 2015, and posted the new rates on the DHCS website. DHCS intends to apply the 10.0% payment reduction referenced in Assembly Bill No. 97 to the new rates. Due to an initial implementation delay, the new rates were to be implemented prospectively until November 1, 2015. A recoupment to account for differences between the new rates and the rates paid for the period of July 1, 2015 through October 31, 2015 was to be implemented through recoupments applied to each weekly reimbursement. Therefore, until the recoupment was paid in full, the Company was not to receive a weekly reimbursement. All retroactive recoupments were expected to be completed by February 2016. However, due to further implementation delays, the new rates have not yet been implemented, and no recoupment has yet occurred. DHCS has instructed laboratories to submit 2015 payment data by March 18, 2016, which will be used to establish new rates effective July 1, 2016. DHCS has not provided further direction regarding possible recoupments. Taken together, these changes are not expected to have a material impact on the Company's consolidated revenues or results of operations.
As previously reported, the Company responded to an October 2007 subpoena from the U.S. Department of Health & Human Services Office of Inspector General's regional office in New York. On August 17, 2011, the Southern District of New York unsealed a False Claims Act lawsuit, United States of America ex rel. NPT Associates v. Laboratory Corporation of America Holdings, which alleges that the Company offered UnitedHealthcare kickbacks in the form of discounts in return for Medicare business. The Plaintiff's third amended complaint further alleges that the Company's billing practices violated the False Claims Acts of fourteen states and the District of Columbia. The lawsuit seeks actual and treble damages and civil penalties for each alleged false claim, as well as recovery of costs, attorney's fees, and legal expenses. Neither the U.S. government nor any state government has intervened in the lawsuit. The Company's Motion to Dismiss was granted in October 2014 and Plaintiff was granted the right to replead. On January 11, 2016, Plaintiff filed a motion requesting leave to file an amended complaint under seal and to vacate the briefing schedule for the Company's motion to dismiss, while the government reviews the amended complaint. The Court granted the motion and vacated the briefing dates. Plaintiff then filed an amended complaint under seal. The Company will vigorously defend the lawsuit.
In addition, the Company has received various other subpoenas since 2007 related to Medicaid billing. In October 2009, the Company received a subpoena from the State of Michigan Department of Attorney General seeking documents related to its billing to Michigan Medicaid. In June 2010, the Company received a subpoena from the State of Florida Office of the Attorney General requesting documents related to its billing to Florida Medicaid. In October 2013, the Company received a civil investigative demand from the State of Texas Office of the Attorney General requesting documents related to its billing to Texas Medicaid. The Company is cooperating with these requests.
On November 4, 2013, the State of Florida through the Office of the Attorney General filed an Intervention Complaint in a False Claims Act lawsuit, State of Florida ex rel. Hunter Laboratories, LLC and Chris Riedel v. Quest Diagnostics Incorporated, et al. in the Circuit Court for the Second Judicial Circuit for Leon County. The complaint, originally filed by a competitor laboratory, alleges that the Company overcharged Florida’s Medicaid program. The lawsuit seeks actual and treble damages and civil penalties
F-33
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
for each alleged false claim, as well as recovery of costs, attorney’s fees, and legal expenses. The Company's Motion to Dismiss was denied in February 2015. The Company will vigorously defend the lawsuit.
On May 2, 2013, the Company was served with a False Claims Act lawsuit, State of Georgia ex rel. Hunter Laboratories, LLC and Chris Riedel v. Quest Diagnostics Incorporated, et al., filed in the State Court of Fulton County, Georgia. The lawsuit, filed by a competitor laboratory, alleges that the Company overcharged Georgia's Medicaid program. The State of Georgia filed a notice of declination on August 13, 2012, before the Company was served with the Complaint. The case was removed to the United States District Court for the Northern District of Georgia. The lawsuit seeks actual and treble damages and civil penalties for each alleged false claim, as well as recovery of costs, attorney's fees, and legal expenses. On March 14, 2014, the Company's Motion to Dismiss was granted. The Plaintiffs repled their complaint and the Company filed a Motion to Dismiss the First Amended Complaint. In May 2015, the Court dismissed the Plaintiffs' anti-kickback claim and remanded the remaining state law claims to the State Court of Fulton County. In July 2015, the Company filed a Motion to Dismiss these remaining claims. The Plaintiffs filed an opposition to the Company's Motion to Dismiss in August 2015. Also, the State of Georgia filed a brief as amicus curiae. The Company will vigorously defend the lawsuit.
On February 27, 2012, the Company was served with a False Claims Act lawsuit, United States ex rel. Margaret Brown v. Laboratory Corporation of America Holdings and Tri-State Clinical Laboratory Services, LLC, filed in the United States District Court for the Southern District of Ohio, Western Division. The Company owned 50% of Tri-State Clinical Laboratory Services, LLC, which was dissolved in June of 2011 pursuant to a voluntary petition filed under Chapter 7 of Title 11 of the United States Code. The lawsuit alleges that the defendants submitted false claims for payment for laboratory testing services performed as a result of financial relationships that violated the Stark and Anti-Kickback Statute. The lawsuit seeks actual and treble damages and civil penalties for each alleged false claim, as well as recovery of costs, attorney's fees, and legal expenses. The U.S. government has not intervened in the lawsuit. The parties have reached a settlement in principle, but the Company will vigorously defend the lawsuit if the settlement is not finalized.
On June 7, 2012, the Company was served with a putative class action lawsuit, Yvonne Jansky v. Laboratory Corporation of America, et al., filed in the Superior Court of the State of California, County of San Francisco. The lawsuit alleges that the defendants committed unlawful and unfair business practices, and violated various other state laws by changing screening codes to diagnostic codes on laboratory test orders, thereby resulting in customers being responsible for co-payments and other debts. The lawsuit seeks injunctive relief, actual and punitive damages, as well as recovery of attorney's fees, and legal expenses. In June 2015, Plaintiff's Motion for class certification was denied. The Plaintiff has appealed the denial of class certification, and the trial court has stayed the case pending resolution of the appeal. The Company will vigorously defend the lawsuit.
On August 24, 2012, the Company was served with a putative class action lawsuit, Sandusky Wellness Center, LLC, et al. v. MEDTOX Scientific, Inc., et al., filed in the United States District Court for the District of Minnesota. The lawsuit alleges that on or about February 21, 2012, the defendants violated the U.S. Telephone Consumer Protection Act (TCPA) by sending unsolicited facsimiles to Plaintiff and more than 39 other recipients without the recipients' prior express invitation or permission. The lawsuit seeks the greater of actual damages or the sum of $0.0005 for each violation, subject to trebling under the TCPA, and injunctive relief. In September of 2014, Plaintiff’s Motion for Class Certification was denied. In January of 2015, the Company’s Motion for Summary Judgment on the remaining individual claim was granted. Plaintiff has filed a notice of appeal. The Company will vigorously defend the lawsuit.
The Company was a defendant in two separate putative class action lawsuits, Christine Bohlander v. Laboratory Corporation of America, et al., and Jemuel Andres, et al. v. Laboratory Corporation of America Holdings, et. al., related to overtime pay. After the filing of the two lawsuits on July 8, 2013, the Bohlander lawsuit was consolidated into the Andres lawsuit and removed to the United States District Court for the Central District of California. In the consolidated lawsuit, the Plaintiffs alleged on behalf of similarly situated phlebotomists and couriers that the Company failed to pay overtime, failed to provide meal and rest breaks, and committed other violations of the California Labor Code. On May 28, 2015, the District Court issued a preliminary approval of the class action settlement and notice of the settlement terms has been sent to putative class members.
The Company is also a defendant in two additional putative class action lawsuits alleging similar claims to the Bohlander/Andres consolidated lawsuit. The lawsuit of Rachel Rabanes v. California Laboratory Sciences, LLC, et al., was filed in April 2014 in the Superior Court of California for the County of Los Angeles, and the lawsuit Rita Varsam v. Laboratory Corporation of America DBA LabCorp, was filed in June 2014 in the Superior Court of California for the County of San Diego. As a result of the Andres settlement, the Plaintiff in the Rabanes case dismissed her case in June 2015. The Varsam case was settled in November 2015.
F-34
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
On December 17, 2010, the Company was served with a lawsuit, Oliver Wuth, et al. v. Laboratory Corporation of America, et al., filed in the State Superior Court of King County, Washington. The lawsuit alleges that the Company was negligent in the handling of a prenatal genetic test order that allegedly resulted in the parents being given incorrect information. The matter was tried before a jury beginning on October 21, 2013. On December 10, 2013, the jury returned a verdict in in plaintiffs’ favor in the amount of $50.0, with 50.0% of liability apportioned to the Company and 50.0% of liability apportioned to co-defendant Valley Medical Center. The Company filed post-judgment motions for a new trial, which were denied, and then appealed to the Court of Appeals of the State of Washington. The Appeals Court heard oral argument in June 2015. In August 2015, the Appeals Court affirmed the trial court's judgment. In September 2015, the Company filed a Petition for Review with the Supreme Court of the State of Washington. On March 1, 2016, the Supreme Court will decide whether it will review the merits of the case. The Company carries self-insurance reserves and excess liability insurance sufficient to cover the potential liability in this case.
On July 3, 2012, the Company was served with a lawsuit, John Wisekal, as Personal Representative of the Estate of Darien Wisekal v. Laboratory Corporation of America Holdings and Glenda C. Mixon, filed in the Circuit Court of the Fifteenth Judicial Circuit in and for Palm Beach County, Florida. The lawsuit alleges that the Company misread a Pap test. The case was removed to the United States District Court for the Southern District of Florida. The matter was tried before a jury beginning on April 1, 2014. On April 17, 2014, the jury returned a verdict in Plaintiff’s favor in the amount of $20.8, with non-economic damages reduced by 25.0% to account for the Plaintiff's negligence, for a final verdict of $15.8. The Company filed post-trial motions. On July 28, 2014, the Court granted the Company’s motion for remittitur and reduced the jury’s non-economic damages award to $5.0, reduced by 25.0% for the Plaintiff’s negligence. Accordingly, the total judgment is $4.4. The Plaintiff opposed the remittitur and the Court ordered a new trial on the issue of damages only. The trial was scheduled for January 2016, but did not go forward at that time. Pending the outcome of pre-trial motions, the trial date may be reset. The Company will vigorously defend this lawsuit.
On July 9, 2014, the Company was served with a putative class action lawsuit, Christopher W. Legg, et al. v. Laboratory Corporation of America, filed in the United States District Court for the Southern District of Florida. The complaint alleges that the Company willfully violated the Fair and Accurate Credit Transactions Act by allegedly providing credit card expiration date information on an electronically printed credit card receipt. The lawsuit sought damages of not less than $0.0001 but not more than $0.01 per violation, and punitive damages, injunctive relief, and attorney’s fees. On November 4, 2015, the District Court issued preliminary approval of the class action settlement and notice of the settlement was sent to putative class members. On February 18, 2016, the District Court entered a final order approving the settlement.
On August 31, 2015, the Company was served with a putative class action lawsuit, Patty Davis v. Laboratory Corporation of America, et al., filed in the Circuit Court of the Thirteenth Judicial Circuit for Hillsborough County, Florida. The complaint alleges that the Company violated the Florida Consumer Collection Practices Act by billing patients who were collecting benefits under the Workers’ Compensation Statutes. The lawsuit seeks injunctive relief and actual and statutory damages, as well as recovery of attorney's fees and legal expenses. The Company will vigorously defend the lawsuit.
On September 4, 2014, LipoScience, Inc. (LipoScience) and the Company entered into a Merger Agreement pursuant to which the Company through its subsidiary, Bear Acquisition Corp (Merger Sub), would acquire all of the outstanding shares of LipoScience at a purchase price of $5.25 per share in cash for each outstanding share of LipoScience (Merger). Prior to the consummation of the Merger on November 20, 2014, purported stockholders of LipoScience filed four putative class action lawsuits against LipoScience, members of the LipoScience board of directors, the Company and Bear Acquisition Corp., a wholly owned subsidiary of the Company, in the Delaware Court of Chancery and, with respect to one of the lawsuits, in the Superior Court of Wake County, North Carolina. The lawsuits alleged breach of fiduciary duty and/or other violations of state law arising out of the Merger. Each suit sought, among other things, injunctive relief enjoining the merger. On October 23, 2014, the case in North Carolina was voluntarily dismissed without prejudice by the Plaintiff. On October 29, 2014, the Delaware Court of Chancery consolidated the remaining three actions under the caption In re LipoScience, Inc. Stockholder Litigation, Consolidated C.A. No. 10252-VCP. After limited discovery, the parties agreed on certain additional disclosures to the Company's definitive proxy statement filed on October 20, 2014, which were made in a supplement to the definitive proxy statement filed on November 10, 2014 (LipoScience Supplemental Disclosures). On November 30, 2015, the Court approved a stipulation under which Plaintiffs voluntarily dismissed the action without prejudice as to all Plaintiffs and any other putative class member. The Court retained jurisdiction solely for the purpose of adjudicating Plaintiffs' counsel's anticipated application for an award of attorneys' fees and reimbursement of expenses in connection with the LipoScience Supplemental Disclosures. The Company subsequently agreed to pay $0.2 to Plaintiffs' counsel for attorney’s fees and expenses in full satisfaction of their claim for attorney’s fees and expenses in the action. The Court has not been asked to review, and will pass no judgment on, the payment of the attorney’s fees and expenses or their reasonableness. Contact information for counsel is Brian D. Long (BDL@rl-legal.com) for Plaintiffs and Raymond J. DiCamillo (dicamillo@rlf.com) for the Company.
F-35
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
On November 2, 2014, the Company entered into a definitive merger agreement to acquire Covance for $6,150.7 in cash and Company common stock. The transaction closed on February 19, 2015. Prior to the closing of the transaction, purported stockholders of Covance filed two putative class action lawsuits. One of the lawsuits, captioned Berk v Covance Inc., et al., C.A. No. 10440-VCL, was filed in the Delaware Court of Chancery on December 9, 2014. The other lawsuit, captioned Ojeda v. Herring et al., No. MER-C-92-14, was filed in the Superior Court of New Jersey, Chancery Division, Mercer County, New Jersey, on November 12, 2014. Both suits named as defendants Covance, members of the Covance board of directors, the Company and Neon Merger Sub, Inc., a wholly owned subsidiary of the Company that was merged out of existence in connection with the Acquisition. The lawsuits alleged breach of fiduciary duty and/or other violations of state law arising out of the proposed acquisition. Each suit sought, among other things, injunctive relief enjoining the merger. On January 21, 2015, the case in New Jersey was voluntarily dismissed without prejudice by the Plaintiff. After limited discovery, the parties agreed on certain additional disclosures to the Company's definitive proxy statement filed on November 26, 2014, which were made in a supplement to the definitive proxy statement filed on February 9, 2015 (Covance Supplemental Disclosures). On December 1, 2015, the Court approved a stipulation under which Plaintiffs voluntarily dismissed the action, with prejudice as to the named Plaintiff only. The Court retained jurisdiction solely for the purpose of adjudicating Plaintiffs' counsel's anticipated application for an award of attorney’s fees and reimbursement of expenses in connection with the Covance Supplemental Disclosures. The Company subsequently agreed to pay $0.2 to Plaintiff’s counsel for attorney’s fees and expenses in full satisfaction of their claim for attorney’s fees and expenses in the action. The Court has not been asked to review, and will pass no judgment on, the payment of the attorneys' fees and expenses or their reasonableness.
In December 2014, the Company received a Civil Investigative Demand issued pursuant to the U.S. False Claims Act from the U.S. Attorney’s Office for South Carolina, which requests information regarding remuneration and services provided by the Company to physicians who also received draw and processing/handling fees from competitor laboratories Health Diagnostic Laboratory, Inc. and Singulex, Inc. The Company is cooperating with the request.
In March 2015, the Company received a subpoena from the Attorney General of the State of New York, which requests information regarding the Company’s relationship with Direct Laboratories LLC. The Company cooperated with the request, and the matter was settled in December 2015.
The Company holds an investment in a joint venture partnership located in Alberta, Canada. The Canadian partnership has a license to conduct diagnostic testing services in the province of Alberta. Substantially all of its revenue is received as reimbursement from the Alberta government's healthcare programs. In December 2013, Alberta Health Services (AHS), the Alberta government's healthcare program, issued a request for proposals for laboratory services that included the scope of services performed by the Canadian partnership. In October 2014, AHS informed the Canadian partnership that it had not been selected as the preferred proponent. In November 2014, the Canadian partnership submitted a vendor bid appeal upon the belief that there were significant flaws and failures in the conduct of the request for proposal process, which drove to a biased conclusion. AHS established a Vendor Bid Appeal Panel to hear the appeal, and the hearing was conducted in February 2015. In August 2015, AHS was directed to cancel the request for proposal process. Subsequently, the Canadian partnership entered into a one-year extension through March 31, 2017 of its existing contract with AHS. If the contract is not renewed after March 2017, then the Canadian partnership's revenues would decrease substantially and the carrying value of the Company's investment could potentially be impaired.
Under the Company's present insurance programs, coverage is obtained for catastrophic exposure as well as those risks required to be insured by law or contract. The Company is responsible for the uninsured portion of losses related primarily to general, professional and vehicle liability, certain medical costs and workers' compensation. The self-insured retentions are on a per occurrence basis without any aggregate annual limit. Provisions for losses expected under these programs are recorded based upon the Company's estimates of the aggregated liability of claims incurred. At December 31, 2015, the Company had provided letters of credit aggregating approximately $45.4, primarily in connection with certain insurance programs. The Company’s availability under its Revolving Credit Facility is reduced by the amount of these letters of credit.
The Company leases various facilities and equipment under non-cancelable lease arrangements. Future minimum rental commitments for leases with non-cancelable terms of one year or more at December 31, 2015 are as follows:
F-36
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Operating | |||
2016 | $ | 73.4 | |
2017 | 63.8 | ||
2018 | 44.2 | ||
2019 | 31.4 | ||
2020 | 28.2 | ||
Thereafter | 105.0 | ||
Total minimum lease payments | 346.0 | ||
Less: | |||
Amounts included in restructuring and acquisition related accruals | (25.9 | ) | |
Non-cancelable sub-lease income | — | ||
Total minimum operating lease payments | $ | 320.1 | |
Rental expense, which includes rent for real estate, equipment and automobiles under operating leases, amounted to $287.1, $235.7 and $226.0 for the years ended December 31, 2015, 2014 and 2013, respectively.
16. PENSION AND POSTRETIREMENT PLANS
Pension Plans
The Company has a defined benefit retirement plan (Company Plan) and a nonqualified supplemental retirement plan (PEP). Both plans have been closed to new participants since December 31, 2009. Employees participating in the Company Plan and the PEP no longer earn service-based credits, but continue to earn interest credits. In addition, effective January 1, 2010, all employees eligible for the defined contribution retirement plan (401K Plan) receive a minimum 3% non-elective contribution (NEC) concurrent with each payroll period. Employees are not required to make a contribution to the 401K Plan to receive the NEC. The NEC is non-forfeitable and vests immediately. The 401K Plan also permits discretionary contributions by the Company of up to 1% and up to 3% of pay for eligible employees based on service.
The Company’s 401K Plan covers substantially all pre-Acquisition employees. Prior to 2010, Company contributions to the plan were based on a percentage of employee contributions. In 2013, 2012 and 2011, the Company made non-elective and discretionary contributions to the plan. In 2015, 2014, and 2013, non-elective and discretionary contributions were $43.3, $51.6 and $49.4, respectively. As a result of the Acquisition, the Company also incurred expense of $37.8 for the Covance 401K Plan during the year ended December 31, 2015. Under the Covance 401K Plan, which is available on a voluntary basis to substantially all US employees, the Company matches employee contributions up to a maximum Company contribution of 4.5%.
In addition, the Company Plan covers substantially all employees hired prior to December 31, 2009. The benefits to be paid under the Company Plan are based on years of credited service through December 31, 2009, interest credits and average compensation. The Company’s policy is to fund the Company Plan with at least the minimum amount required by applicable regulations. The Company made contributions to the Company Plan of $9.5, $12.4 and $8.4 in 2015, 2014 and 2013, respectively.
The PEP covers the Company’s senior management group. Prior to 2010, the PEP provided for the payment of the difference, if any, between the amount of any maximum limitation on annual benefit payments under the Employee Retirement Income Security Act of 1974 and the annual benefit that would be payable under the Company Plan but for such limitation. Effective January 1, 2010, employees participating in the PEP no longer earn service-based credits. The PEP is an unfunded plan.
Projected pension expense for the Company Plan and the PEP is expected to increase to $12.9 in 2016. This amount excludes any accelerated recognition of pension cost due to the total lump-sum payouts exceeding certain components of net periodic pension cost in a fiscal year. If such levels were to be met in 2016, the Company projects that it would result in additional pension expense of several million dollars. The actual amount would be determined in the fiscal quarter when the lump-sum payments cross the threshold and would be based upon the plan's funded status and actuarial assumptions in effect at that time.
The Company plans to make contributions of $12.7 to the Company Plan during 2016.
The effect on operations for both the Company Plan and the PEP are summarized as follows:
F-37
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Year ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Service cost for benefits earned | $ | 3.9 | $ | 3.4 | $ | 3.1 | |||||
Interest cost on benefit obligation | 15.1 | 16.4 | 14.7 | ||||||||
Expected return on plan assets | (18.3 | ) | (18.3 | ) | (17.3 | ) | |||||
Net amortization and deferral | 11.3 | 6.6 | 10.5 | ||||||||
Defined benefit plan costs | $ | 12.0 | $ | 8.1 | $ | 11.0 | |||||
Amounts included in accumulated other comprehensive earnings consist of unamortized net loss of $135.1. The accumulated other comprehensive earnings that are expected to be recognized as components of the defined benefit plan costs during 2016 are $10.5 related to amortization of the net loss.
A summary of the changes in the projected benefit obligations of the Company Plan and the PEP are summarized as follows:
2015 | 2014 | ||||||
Balance at January 1 | $ | 388.6 | $ | 349.7 | |||
Service cost | 3.9 | 3.4 | |||||
Interest cost | 15.1 | 16.4 | |||||
Actuarial (gain)/loss | (15.4 | ) | 47.1 | ||||
Benefits and administrative expenses paid | (29.1 | ) | (28.0 | ) | |||
Balance at December 31 | $ | 363.1 | $ | 388.6 | |||
The Accumulated Benefit Obligation was $363.1 and $388.6 at December 31, 2015 and 2014, respectively.
A summary of the changes in the fair value of plan assets follows:
2015 | 2014 | ||||||
Fair value of plan assets at beginning of year | $ | 269.1 | $ | 268.1 | |||
Actual return on plan assets | (5.2 | ) | 15.2 | ||||
Employer contributions | 11.0 | 13.8 | |||||
Benefits and administrative expenses paid | (29.1 | ) | (28.0 | ) | |||
Fair value of plan assets at end of year | $ | 245.8 | $ | 269.1 | |||
The net funded status of the Company Plan and the PEP at December 31:
Funded status | $ | 112.5 | $ | 119.6 | |||
Recorded as: | |||||||
Accrued expenses and other | $ | 1.9 | $ | 1.7 | |||
Other liabilities | 110.6 | 117.9 | |||||
$ | 112.5 | $ | 119.6 | ||||
Weighted average assumptions used in the accounting for the Company Plan and the PEP are summarized as follows:
2015 | 2014 | 2013 | ||||||
Discount rate | 4.0 | % | 4.8 | % | 4.8 | % | ||
Expected long term rate of return | 7.0 | % | 7.0 | % | 7.0 | % | ||
The Company also updated the mortality assumption to the RP-2014 Mortality Tables in 2015 which increased the Company's total projected obligation.
The Company maintains an investment policy for the management of the Company Plan’s assets. The objective of this policy is to build a portfolio designed to achieve a balance between investment return and asset protection by investing in indexed funds that are comprised of equities of high quality companies and in high quality fixed income securities which are broadly balanced and represent all market sectors. The target allocations for plan assets are 50% equity securities, 45% fixed income securities and 5% in other assets. Equity securities primarily include investments in large-cap, mid-cap and small-cap companies located in the
F-38
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
U.S. and to a lesser extent international equities in developed and emerging countries. Fixed income securities primarily include U.S. Treasury securities, mortgage-backed bonds and corporate bonds of companies from diversified industries. Other assets include investments in commodities. The weighted average expected long-term rate of return for the Company Plan’s assets is as follows:
Target Allocation | Weighted Average Expected Long-Term Rate of Return | ||||
Equity securities | 50.0 | % | 5.5 | % | |
Fixed income securities | 45.0 | % | 1.2 | % | |
Other assets | 5.0 | % | 0.3 | % | |
The fair values of the Company Plan’s assets at December 31, 2015 and 2014, by asset category are as follows:
Fair Value Measurements as of | |||||||||||||||
December 31, 2015 | |||||||||||||||
Fair Value as of December 31, 2015 | Using Fair Value Hierarchy | ||||||||||||||
Asset Category | Level 1 | Level 2 | Level 3 | ||||||||||||
Cash | $ | 6.1 | $ | 6.1 | $ | — | $ | — | |||||||
Equity securities: | |||||||||||||||
U.S. large cap - blend (a) | 55.1 | — | 55.1 | — | |||||||||||
U.S. mid cap - blend (b) | 21.0 | — | 21.0 | — | |||||||||||
U.S. small cap - blend (c) | 7.1 | — | 7.1 | — | |||||||||||
International equity - blend (d) | 35.7 | — | 35.7 | — | |||||||||||
Commodities index (e) | 11.8 | — | 11.8 | — | |||||||||||
Fixed income securities: | |||||||||||||||
U.S. fixed income (f) | 102.8 | — | 102.8 | — | |||||||||||
U.S inflation protection income (g) | 6.2 | — | 6.2 | — | |||||||||||
Total fair value of the Company Plan’s assets | $ | 245.8 | $ | 6.1 | $ | 239.7 | $ | — | |||||||
Fair Value Measurements as of | |||||||||||||||
December 31, 2014 | |||||||||||||||
Fair Value as of December 31, 2014 | Using Fair Value Hierarchy | ||||||||||||||
Asset Category | Level 1 | Level 2 | Level 3 | ||||||||||||
Cash | $ | 4.1 | $ | 4.1 | $ | — | $ | — | |||||||
Equity securities: | |||||||||||||||
U.S. large cap - blend (a) | 64.1 | — | 64.1 | — | |||||||||||
U.S. mid cap - blend (b) | 25.3 | — | 25.3 | — | |||||||||||
U.S. small cap - blend (c) | 7.9 | — | 7.9 | — | |||||||||||
International equity - blend (d) | 36.0 | — | 36.0 | — | |||||||||||
Commodities index (e) | 10.3 | — | 10.3 | — | |||||||||||
Fixed income securities: | |||||||||||||||
U.S. fixed income (f) | 115.1 | — | 115.1 | — | |||||||||||
U.S inflation protection income (g) | 6.3 | — | 6.3 | — | |||||||||||
Total fair value of the Company Plan’s assets | $ | 269.1 | $ | 4.1 | $ | 265.0 | $ | — | |||||||
a) | This category represents an equity index fund not actively managed that tracks the S&P 500 Index. |
b) | This category represents an equity index fund not actively managed that tracks the S&P mid-cap 400 Index. |
c) | This category represents an equity index fund not actively managed that tracks the Russell 2000 Index. |
d) | This category represents an equity index fund not actively managed that tracks the MSCI ACWI ex USA Index. |
e) | This category represents a commodities index fund not actively managed that tracks the Dow Jones - UBS Commodity Index. |
f) | This category primarily represents bond index funds not actively managed that track the Barclays Capital U.S. Aggregate Index as well as an actively managed strategy which utilizes the Barclays Capital U.S. Aggregate Bond Index as its primary prospectus benchmark. |
g) | This category primarily represents a bond index fund not actively managed that tracks the Barclays Capital U.S. TIPS Index. |
F-39
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
The following assumed benefit payments under the Company Plan and PEP, which were used in the calculation of projected benefit obligations, are expected to be paid as follows:
2016 | $ | 26.1 | |
2017 | 25.2 | ||
2018 | 24.7 | ||
2019 | 24.9 | ||
2020 | 24.7 | ||
Years 2021-2025 | 123.9 | ||
In addition to the PEP, as a result of the Acquisition, the Company also has a frozen non-qualified Supplemental Executive Retirement Plan (SERP). The SERP, which is not funded, is intended to provide retirement benefits for certain employees who were executive officers of Covance prior to the Acquisition. Benefit amounts are based upon years of service and compensation of the participating employees. The pension benefit obligation as of the Acquisition date was $32.8. For the 2015 period, the beginning of the year is February 19, 2015, and the net periodic pension cost is for the ten months ended December 31, 2015. The components of the net periodic pension cost are as follows:
Year Ended December 31, 2015 | |||
Service cost | $ | 0.2 | |
Interest cost | 0.9 | ||
Net amortization and deferral | — | ||
Curtailment gain | (0.7 | ) | |
Net periodic pension cost | $ | 0.4 | |
Assumptions used to determine defined benefit plan cost | |||
Discount rate | 3.3 | % | |
Expected return on assets | N/A | ||
The change in the projected benefit obligation, the funded status of the plan and a reconciliation of such funded status to the amounts reported in the consolidated balance sheet as of December 31, 2015 is as follows:
Balance at Acquisition Date | $ | 32.8 | |
Service cost | 0.2 | ||
Interest cost | 0.9 | ||
Actuarial loss | — | ||
Gross benefits paid | (2.3 | ) | |
Curtailments | (0.7 | ) | |
Balance at December 31, 2015 | 30.9 | ||
Funded status | $ | 30.9 | |
Recorded as: | |||
Accrued expenses and other | $ | 20.1 | |
Other liabilities | 10.8 | ||
$ | 30.9 | ||
The accumulated benefit obligation as of December 31, 2015 was $30.9.
The following assumed benefit payments under the SERP, which were used in the calculation of projected benefit obligations, are expected to be paid as follows:
F-40
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
2016 | $ | 20.2 | |
2017 | 5.4 | ||
2018 | 0.9 | ||
2019 | 0.1 | ||
2020 | 0.1 | ||
Years 2021-2025 | 3.4 | ||
As a result of the Acquisition, the Company sponsors two defined benefit pension plans for the benefit of its employees at two U.K. subsidiaries (U.K. Plans) and one defined benefit pension plan for the benefit of its employees at a German subsidiary (German Plan), all of which are legacy plans of previously acquired companies. Benefit amounts for all three plans are based upon years of service and compensation. The German Plan is unfunded while the U.K. Plans are funded. The Company’s funding policy has been to contribute annually amounts at least equal to the local statutory funding requirements.
U.K. Plans | German Plan | |||||||
Year Ended December 31, 2015 | Year Ended December 31, 2015 | |||||||
Service cost | $ | 4.1 | $ | 1.0 | ||||
Interest cost | 7.6 | 0.4 | ||||||
Expected return on plan assets | (10.7 | ) | — | |||||
Net amortization and deferral | — | — | ||||||
Expected participant contributions | (1.4 | ) | — | |||||
Defined benefit plan costs | $ | (0.4 | ) | $ | 1.4 | |||
Assumptions used to determine defined benefit plan cost: | ||||||||
Discount rate | 3.5 | % | 1.5 | % | ||||
Expected return on assets | 5.4 | % | N/A | |||||
Salary increases | 3.5 | % | 2.0 | % | ||||
The weighted average expected long-term rate of return on assets of the U.K Plans is based on the target asset allocation and the average rate of growth expected for the asset classes invested. The rate of expected growth is derived from a combination of historic returns, current market indicators, the expected risk premium for each asset class over the risk-free rate and the opinion of professional advisors.
The change in the projected benefit obligation and plan assets, the funded status of the plan and a reconciliation of such funded status to the amounts reported in the consolidated balance sheet as of December 31, 2015 is as follows:
Change in projected benefit obligation: | U.K. Plans | German Plan | ||||||
Balance at Acquisition Date | $ | 256.0 | $ | 29.4 | ||||
Service cost | 4.1 | 1.0 | ||||||
Interest cost | 7.6 | 0.4 | ||||||
Actuarial gain | (8.4 | ) | (5.8 | ) | ||||
Benefits paid | (4.5 | ) | (0.2 | ) | ||||
Foreign currency exchange rate changes | (8.3 | ) | (1.2 | ) | ||||
Balance at December 31, 2015 | $ | 246.5 | $ | 23.6 | ||||
Change in fair value of assets: | U.K. Plans | German Plan | ||||||
Balance at Acquisition Date | $ | 232.6 | $ | — | ||||
Company contributions | 6.6 | — | ||||||
Participant contributions | 1.4 | — | ||||||
Actual return on assets | (2.3 | ) | — | |||||
Benefits paid | (4.5 | ) | — | |||||
Foreign currency exchange rate changes | (7.6 | ) | — | |||||
Fair value of plan assets at December 31, 2015 | $ | 226.2 | $ | — | ||||
F-41
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
U.K. Plans | German Plan | |||||||
Funded status | $ | 20.3 | $ | 23.6 | ||||
Recorded as: | ||||||||
Accrued expenses and other | $ | — | $ | 0.2 | ||||
Other liabilities | 20.3 | 23.4 | ||||||
$ | 20.3 | $ | 23.6 | |||||
The Company contributed $6.6 in 2015 to the U.K. Plans and expected to contribute $17.1 in 2016. No contributions were made to the German plan during 2015, nor are any contributions expected to be made in 2016 since the plan is unfunded.
The accumulated benefit obligation for the U.K. Plans and the German Plan was $215.1 and $20.6 at December 31, 2015, respectively.
U.K. Plans | German Plan | |||||||
Net actuarial loss (gain) | $ | 4.8 | $ | (5.7 | ) | |||
Less: Tax benefit (expense) (deferred tax asset) | (0.9 | ) | 1.8 | |||||
Accumulated other comprehensive income impact | $ | 3.9 | $ | (3.9 | ) | |||
Assumptions used to determine benefit obligations: | ||||||||
Discount rate | 3.8 | % | 2.5 | % | ||||
Salary increases | 3.6 | % | 2.0 | % | ||||
There is no net actuarial loss for the U.K. Plans and German Plan required to be amortized from accumulated other comprehensive income into net periodic pension cost in 2016.
The investment policies for the U.K. Plans are set by the plan trustees, based upon the guidance of professional advisors and after consultation with the Company, taking into consideration the plans’ liabilities and future funding levels. The trustees have set the long-term investment policy largely in accordance with the asset allocation of a broadly diversified investment portfolio. Assets are generally invested within the target ranges as follows:
Equity securities | 60.0% | to | 70.0% | |
Debt securities | 10.0% | to | 15.0% | |
Annuities | 10.0% | to | 20.0% | |
Real estate | —% | to | 10.0% | |
Other | —% | to | 5.0% | |
The weighted average asset allocation of the U.K. Plans as of December 31, 2015 by asset category is as follows:
December 31, 2015 | ||
Equity securities | 68.0% | |
Debt securities | 13.0% | |
Annuities | 14.0% | |
Real estate | 5.0% | |
Investments are made in pooled investment funds. Pooled investment fund managers are regulated by the Financial Conduct Authority in the U.K. and operate under terms which contain restrictions on the way in which the portfolios are managed and require the managers to ensure that suitable internal operating procedures are in place. The trustees have set performance objectives for each fund manager and routinely monitor and assess the managers’ performance against such objectives. Annuities represent annuity buy-in insurance policies purchased by the plan trustees from large, financially sound insurers. The cash flows from the annuities are intended to match the plan’s obligations to specific groups of participants, typically those participants currently receiving benefits.
The fair value of the Company’s U.K. Plans' assets as of December 31, 2015, by asset category, are as follows:
F-42
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Fair Value Measurements as of | |||||||||||||||
December 31, 2015 | |||||||||||||||
December 31, 2015 | Using Fair Value Hierarchy | ||||||||||||||
Asset Category | Level 1 | Level 2 | Level 3 | ||||||||||||
Cash | $0.9 | $0.9 | $0.0 | $0.0 | |||||||||||
Mutual funds (a) | 193.3 | — | 193.3 | — | |||||||||||
Annuities (b) | 32.0 | — | — | 32.0 | |||||||||||
Total fair value of the Company Plan’s assets | $ | 226.2 | $ | 0.9 | $ | 193.3 | $ | 32.0 | |||||||
a) | Mutual funds represent pooled investment vehicles offered by investment managers, which are generally comprised of investments in equities, bonds, property and cash. The plans’ trustees hold units in these funds, the value of which is determined by the number of units held multiplied by the unit price calculated by the investment managers. That unit price is derived based on the market value of the securities that comprise the fund, which are determined by quoted prices in active markets. No element of the valuation is based on inputs made by the plans’ trustees. |
b) | Annuities represent annuity buy-in insurance policies, whereby the insurer pays the pension payments for the lifetime of the members covered. The annuities are assets of the plan and payments from the insurer are made to the plans’ trustees, who then use those proceeds to pay the pensioners. The cash flows from the annuities are intended to effectively match the payments to the pensioners covered by the policy. As such, these assets are valued actuarially based upon the value of the liabilities with which they are associated. As the valuation of these assets is judgmental, and there are no observable inputs associated with the valuation, these assets are classified as Level 3 in the fair value hierarchy. |
Expected future benefit payments are as follows:
U.K. Plans | German Plan | |||||||
2016 | $ | 3.7 | $ | 0.2 | ||||
2017 | 3.5 | 0.2 | ||||||
2018 | 4.3 | 0.3 | ||||||
2019 | 4.1 | 0.3 | ||||||
2020 | 5.3 | 0.5 | ||||||
Years 2021-2025 | 34.1 | 3.0 | ||||||
Post-employment retiree health and welfare plan
As a result of the Acquisition, the Company sponsors a post-employment retiree health and welfare plan for the benefit of eligible employees at certain U.S. subsidiaries who retire after satisfying service and age requirements. This plan is funded on a pay-as-you-go basis and the cost of providing these benefits is shared with the retirees. The net periodic post-retirement benefit cost for the year ended December 31, 2015 was $0.2, and the pension benefit obligation as of the Acquisition date was $6.3.
The components of net periodic post-retirement benefit cost for 2015 are as follows:
Year Ended December 31, 2015 | |||
Service cost | $ | — | |
Interest cost | 0.2 | ||
Net periodic post-retirement benefit cost | $ | 0.2 | |
Assumptions used to determine net periodic post-retirement benefit cost: | |||
Discount rate | 3.8 | % | |
Healthcare cost trend rate | 6.5 | % | |
The change in the projected post-retirement benefit obligation, the funded status of the plan and the reconciliation of such funded status to the amounts reported in the consolidated balance sheets as of December 31, 2015 is as follows:
F-43
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
2015 | |||
Balance at Acquisition Date | $ | 6.3 | |
Service cost | — | ||
Interest cost | 0.2 | ||
Participant contributions | 0.8 | ||
Actuarial gain | (0.7 | ) | |
Benefits paid | (1.4 | ) | |
Balance at December 31, 2015 | 5.2 | ||
2015 | |||
Funded status | $ | 5.2 | |
Recorded as: | |||
Accrued expenses and other | $ | 0.6 | |
Other liabilities | 4.6 | ||
$ | 5.2 | ||
The amounts recognized in accumulated other comprehensive income as of December 31, 2015 are as follows:
Year Ended December 31, 2015 | |||
Net actuarial loss | $ | 0.7 | |
Less: Deferred tax benefit | (0.3 | ) | |
Accumulated other comprehensive income impact | $ | 0.4 | |
Assumptions used to determine benefit obligation: | ||
Discount rate | 4.2 | % |
Healthcare cost trend rate | 7.0 | % |
A one percentage point (1%) increase or decrease in the assumed healthcare cost trend rate would not impact the net service and interest cost components of the net periodic post-retirement benefit cost or the post-retirement benefit obligation since future increases in plan costs are paid by participant contributions. The Company expects to contribute $0.6 to the post-employment retiree health and welfare plan in 2016.
Expected future gross benefit payments are as follows:
2016 | $ | 1.4 | |
2017 | 1.6 | ||
2018 | 1.5 | ||
2019 | 1.5 | ||
2020 | 1.4 | ||
2021 and thereafter | 6.6 | ||
Post-retirement Medical Plan
The Company assumed obligations under a subsidiary's post-retirement medical plan. Coverage under this plan is restricted to a limited number of existing employees of the subsidiary. This plan is unfunded and the Company’s policy is to fund benefits as claims are incurred. The effect on operations of the post-retirement medical plan is shown in the following table:
Year ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Service cost for benefits earned | $ | 0.1 | $ | 0.2 | $ | 0.4 | |||||
Interest cost on benefit obligation | 1.0 | 1.8 | 2.5 | ||||||||
Net amortization and deferral | (10.4 | ) | (7.9 | ) | 1.0 | ||||||
Post-retirement medical plan costs | $ | (9.3 | ) | $ | (5.9 | ) | $ | 3.9 | |||
F-44
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Amounts included in accumulated other comprehensive earnings consist of unamortized net loss of $9.3. The accumulated other comprehensive earnings that are expected to be recognized as components of the post-retirement medical plan costs during 2016 are $10.4 related to amortization of the net gain resulting from the shift of Medicare-eligible participants to private exchanges.
A summary of the changes in the accumulated post-retirement benefit obligation follows:
2015 | 2014 | ||||||
Balance at January 1 | $ | 28.9 | $ | 62.7 | |||
Service cost for benefits earned | 0.1 | 0.2 | |||||
Interest cost on benefit obligation | 1.0 | 1.8 | |||||
Participants contributions | — | 0.2 | |||||
Actuarial loss | (3.5 | ) | (7.2 | ) | |||
Benefits paid | (1.8 | ) | (2.5 | ) | |||
Plan amendment | (3.3 | ) | (26.3 | ) | |||
Balance at December 31 | $ | 21.4 | $ | 28.9 | |||
Recorded as: | |||||||
Accrued expenses and other | $ | 1.8 | $ | 2.2 | |||
Other liabilities | 19.6 | 26.7 | |||||
$ | 21.4 | $ | 28.9 | ||||
The weighted-average discount rates used in the calculation of the accumulated post-retirement benefit obligation were 4.4% and 4.0% as of December 31, 2015 and 2014, respectively. The healthcare cost trend rate was removed due to the expectation of future funding to be at the same level as the previous year's funding.
The following assumed benefit payments under the Company's post-retirement benefit plan, which reflect expected future service, as appropriate, and were used in the calculation of projected benefit obligations, are expected to be paid as follows:
2016 | $ | 1.8 | |
2017 | 1.7 | ||
2018 | 1.6 | ||
2019 | 1.6 | ||
2020 | 1.5 | ||
Years 2021-2025 | 7.1 | ||
Deferred Compensation Plan
In 2001, the Board approved the Deferred Compensation Plan (DCP) under which certain of the Company's executives, may elect to defer up to 100.0% of their annual cash incentive pay and/or up to 50.0% of their annual base salary and/or eligible commissions subject to annual limits established by the U.S. government. The DCP provides executives a tax efficient strategy for retirement savings and capital accumulation without significant cost to the Company. The Company makes no contributions to the DCP. Amounts deferred by a participant are credited to a bookkeeping account maintained on behalf of each participant, which is used for measurement and determination of amounts to be paid to a participant, or his or her designated beneficiary, pursuant to the terms of the DCP. The amounts accrued under this plan were $46.4 and $43.4 at December 31, 2015 and 2014, respectively. Deferred amounts are the Company's general unsecured obligations and are subject to claims by the Company's creditors. The Company's general assets may be used to fund obligations and pay DCP benefits.
F-45
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
17. FAIR VALUE MEASUREMENTS
The Company’s population of financial assets and liabilities subject to fair value measurements as of December 31, 2015 and 2014 are as follows:
Fair Value Measurements as of | |||||||||||||||
December 31, 2015 | |||||||||||||||
Fair Value as of December 31, 2015 | Using Fair Value Hierarchy | ||||||||||||||
Level 1 | Level 2 | Level 3 | |||||||||||||
Noncontrolling interest put | $ | 14.9 | $ | — | $ | 14.9 | $ | — | |||||||
Interest rate swap | 21.6 | — | 21.6 | — | |||||||||||
Cash surrender value of life insurance policies | 45.5 | — | 45.5 | — | |||||||||||
Deferred compensation liability | 46.4 | — | 46.4 | — | |||||||||||
Fair Value Measurements as of | |||||||||||||||
December 31, 2014 | |||||||||||||||
Fair Value as of December 31, 2014 | Using Fair Value Hierarchy | ||||||||||||||
Level 1 | Level 2 | Level 3 | |||||||||||||
Noncontrolling interest put | $ | 17.7 | $ | — | $ | 17.7 | $ | — | |||||||
Interest rate swap | 18.5 | — | 18.5 | — | |||||||||||
Cash surrender value of life insurance policies | 41.9 | — | 41.9 | — | |||||||||||
Deferred compensation liability | 43.4 | — | 43.4 | — | |||||||||||
The noncontrolling interest put is valued at its contractually determined value, which approximate fair value. During the year ended December 31, 2015, the carrying value of the noncontrolling interest put decreased by $2.8 consisting of a $2.0 decrease in the contractually determined value and a $0.8 decrease for foreign currency translation.
The Company offers certain employees the opportunity to participate in a DCP. A participant's deferrals are allocated by the participant to one or more of 16 measurement funds, which are indexed to externally managed funds. From time to time, to offset the cost of the growth in the participant's investment accounts, the Company purchases life insurance policies, with the Company named as beneficiary of the policies. Changes in the cash surrender value of the life insurance policies are based upon earnings and changes in the value of the underlying investments, which are typically invested in a similar manner to the participants' allocations. Changes in the fair value of the DCP obligation are derived using quoted prices in active markets based on the market price per unit multiplied by the number of units. The cash surrender value and the DCP obligations are classified within Level 2 because their inputs are derived principally from observable market data by correlation to the hypothetical investments.
The carrying amounts of cash and cash equivalents, accounts receivable, income taxes receivable, and accounts payable are considered to be representative of their respective fair values due to their short-term nature. The fair market value of the zero-coupon subordinated notes, based on market pricing, was approximately $177.1 and $155.6 as of December 31, 2015 and 2014, respectively. The fair market value of the senior notes, based on market pricing, was approximately $6,070.5 and $2,949.8 as of December 31, 2015 and 2014, respectively. The Company's note and debt instruments are considered level 2 instruments, as the fair market values of these instruments are determined using other observable inputs. The Company's investment in equity securities of $0.0 and $1.0 as of December 31, 2015 and 2014, respectively, is considered a Level 1 instrument, as the fair market value of this instrument is determined using observable inputs.
18. DERIVATIVE INSTRUMENTS AND HEDGING ACTIVITIES
The Company addresses its exposure to market risks, principally the market risk associated with changes in interest rates, through a controlled program of risk management that includes, from time to time, the use of derivative financial instruments such as interest rate swap agreements (see Interest Rate Swap section below). Although the Company’s zero-coupon subordinated notes contain features that are considered to be embedded derivative instruments (see Embedded Derivative section below), the Company does not hold or issue derivative financial instruments for trading purposes. The Company does not believe that its exposure to market risk is material to the Company’s financial position or results of operations.
F-46
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Interest Rate Swap
During the third quarter of 2013, the Company entered into two fixed-to-variable interest rate swap agreements for the 4.625% Senior Notes due 2020 with an aggregate notional amount of $600.0 and variable interest rates based on one-month LIBOR plus 2.298% to hedge against changes in the fair value of a portion of the Company's long term debt. These derivative financial instruments are accounted for as fair value hedges of the Senior Notes due 2020. These interest rate swaps are included in other long term assets or liabilities, as applicable, and added to the value of the senior notes, with an aggregate fair value of $21.6 at December 31, 2015. As the specific terms and notional amounts of the derivative financial instruments match those of the fixed-rate debt being hedged, the derivative instruments are assumed to be perfectly effective hedges and accordingly, there is no impact to the Company's consolidated statements of operations. Cash flows from the interest rate swaps are including in operating activities.
Embedded Derivatives Related to the Zero-Coupon Subordinated Notes
The Company’s zero-coupon subordinated notes contain the following two features that are considered to be embedded derivative instruments under authoritative guidance in connection with accounting for derivative instruments and hedging activities:
1) | The Company will pay contingent cash interest on the zero-coupon subordinated notes after September 11, 2006, if the average market price of the notes equals 120% or more of the sum of the issue price, accrued original issue discount and contingent additional principal, if any, for a specified measurement period. |
2) | Holders may surrender zero-coupon subordinated notes for conversion during any period in which the rating assigned to the zero-coupon subordinated notes by S&P’s Ratings Services is BB- or lower. |
The Company believes these embedded derivatives had no fair value at December 31, 2015 and 2014. These embedded derivatives also had no impact on the consolidated statements of operations for the years ended December 31, 2015, 2014 and 2013.
Derivatives Instruments
The Company periodically enters into foreign currency forward contracts, which are recognized as assets or liabilities at their fair value. These contracts do not qualify for hedge accounting and the changes in fair value are recorded directly to earnings. The contracts are short-term in nature and the fair value of these contracts is based on market prices for comparable contracts. The fair value of these contracts is not significant as of December 31, 2015.
19. SUPPLEMENTAL CASH FLOW INFORMATION
Years Ended December 31, | |||||||||||
2015 | 2014 | 2013 | |||||||||
Supplemental schedule of cash flow information: | |||||||||||
Cash paid during period for: | |||||||||||
Interest | $ | 166.1 | $ | 117.8 | $ | 97.2 | |||||
Income taxes, net of refunds | 249.4 | 284.1 | 301.5 | ||||||||
Disclosure of non-cash financing and investing activities: | |||||||||||
Surrender of restricted stock awards and performance shares | 12.6 | 6.6 | 7.1 | ||||||||
Conversion of zero-coupon convertible debt | 1.1 | 9.9 | 10.3 | ||||||||
Assets acquired under capital leases | 22.6 | 29.0 | 13.1 | ||||||||
Accrued property, plant and equipment | 4.3 | 6.2 | 9.1 | ||||||||
20. BUSINESS SEGMENT INFORMATION
The following table is a summary of segment information for the years ended December 31, 2015, 2014, and 2013. The “management approach” has been used to present the following segment information. This approach is based upon the way the management of the Company organizes segments within an enterprise for making operating decisions and assessing performance. Financial information is reported on the basis that it is used internally by the chief operating decision maker (CODM) for evaluating segment performance and deciding how to allocate resources to segments. The Company’s chief
F-47
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
executive officer has been identified as the CODM.
Prior to the first quarter of 2015, the CODM managed the operating results of the Company as two segments: clinical laboratory diagnostics and other. In connection with the Acquisition, the Company changed its operating segments to align with how the CODM evaluates financial information used to allocate resources and assess performance of the Company following the Acquisition. The segment information presented in these financial statements has been conformed to present segments on this revised basis for all prior periods. Under the new organizational structure, the CODM manages the Company under two segments: LCD and CDD. LCD includes the Company's legacy LabCorp business, and the Company's nutritional chemistry and food safety business, which were previously part of Covance, but excludes LabCorp’s legacy clinical trials testing business, which is now part of CDD. CDD includes Covance's legacy business, and LabCorp’s legacy clinical trials testing business, but excludes Covance's nutritional chemistry and food safety business, which are now part of LCD.
Segment asset information is not presented because it is not used by the CODM at the segment level. Operating earnings (loss) of each segment represents net revenues less directly identifiable expenses to arrive at operating income for the segment. General management and administrative corporate expenses are included in general corporate expenses below.
In the fourth quarter of 2015, the Company refined its methodology for how it allocates corporate expenses between the segments, which impacts segment results and has been applied to prior periods for comparative purposes.
2015 | 2014 | 2013 | ||||||||||
Net revenues: | ||||||||||||
LCD | $ | 6,199.3 | $ | 5,838.0 | $ | 5,669.4 | ||||||
CDD | 2,306.4 | 173.6 | 138.9 | |||||||||
Total net revenues | $ | 8,505.7 | $ | 6,011.6 | $ | 5,808.3 | ||||||
Operating earnings (loss): | ||||||||||||
LCD | $ | 1,061.4 | $ | 982.3 | $ | 1,061.8 | ||||||
CDD | 71.9 | 40.7 | 37.9 | |||||||||
General corporate expenses | (130.4 | ) | (112.6 | ) | (108.8 | ) | ||||||
Total operating income | 1,002.9 | 910.4 | 990.9 | |||||||||
Non-operating expenses, net | (270.8 | ) | (83.7 | ) | (75.3 | ) | ||||||
Earnings before income taxes | 732.1 | 826.7 | 915.6 | |||||||||
Provision for income taxes | 294.1 | 314.1 | 340.2 | |||||||||
Net earnings | 438.0 | 512.6 | 575.4 | |||||||||
Less: Net income attributable to noncontrolling interests | (1.1 | ) | (1.4 | ) | (1.6 | ) | ||||||
Net income attributable to Laboratory Corporation of America Holdings | $ | 436.9 | $ | 511.2 | $ | 573.8 | ||||||
2015 | 2014 | 2013 | ||||||||||
Depreciation and amortization | ||||||||||||
LCD | $ | 245.8 | $ | 184.8 | $ | 176.3 | ||||||
CDD | 184.4 | 5.3 | 4.1 | |||||||||
General corporate | 4.1 | 44.2 | 42.1 | |||||||||
Total depreciation and amortization | $ | 434.3 | $ | 234.3 | $ | 222.5 | ||||||
LCD | CDD | Total | ||||||||||
Geographic distribution of net revenues | ||||||||||||
US | $ | 5,847.3 | $ | 1,156.0 | $ | 7,003.3 | ||||||
Canada | 290.4 | — | 290.4 | |||||||||
United Kingdom | 46.2 | 235.5 | 281.7 | |||||||||
Switzerland | — | 359.7 | 359.7 | |||||||||
Other | 15.4 | 555.2 | 570.6 | |||||||||
Total net revenues | $ | 6,199.3 | $ | 2,306.4 | $ | 8,505.7 | ||||||
F-48
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
LCD | CDD | Total | ||||||||||
Geographic distribution of property, plant and equipment, net | ||||||||||||
U.S. | $ | 724.1 | $ | 687.8 | $ | 1,411.9 | ||||||
Canada | 45.1 | — | 45.1 | |||||||||
U.K. | 3.2 | 133.0 | 136.2 | |||||||||
Switzerland | — | 86.4 | 86.4 | |||||||||
Other | 3.3 | 64.5 | 67.8 | |||||||||
Total property, plant and equipment, net | $ | 775.7 | $ | 971.7 | $ | 1,747.4 | ||||||
21. QUARTERLY DATA (UNAUDITED)
The following is a summary of unaudited quarterly data:
Year ended December 31, 2015 | |||||||||||||||||||
1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | Full Year | |||||||||||||||
Net earnings | $ | 1,772.3 | $ | 2,218.7 | $ | 2,269.9 | $ | 2,244.8 | $ | 8,505.7 | |||||||||
Gross profit | 625.1 | 774.6 | 762.9 | 740.7 | 2,903.3 | ||||||||||||||
Net earnings attributable to Laboratory Corporation of America Holdings | 1.5 | 168.4 | 152.8 | 114.2 | 436.9 | ||||||||||||||
Basic earnings per common share | 0.02 | 1.67 | 1.51 | 1.13 | 4.42 | ||||||||||||||
Diluted earnings per common share | 0.02 | 1.64 | 1.49 | 1.11 | 4.34 | ||||||||||||||
The quarterly data above reflects all measurement period adjustments as of the Acquisition Date.
Year ended December 31, 2014 | |||||||||||||||||||
1st Quarter | 2nd Quarter | 3rd Quarter | 4th Quarter | Full Year | |||||||||||||||
Net earnings | $ | 1,430.7 | $ | 1,516.4 | $ | 1,551.8 | $ | 1,512.7 | $ | 6,011.6 | |||||||||
Gross profit | 516.8 | 568.6 | 571.2 | 546.5 | 2,203.1 | ||||||||||||||
Net earnings attributable to Laboratory Corporation of America Holdings | 113.1 | 141.3 | 137.2 | 119.6 | 511.2 | ||||||||||||||
Basic earnings per common share | 1.33 | 1.67 | 1.62 | 1.41 | 6.03 | ||||||||||||||
Diluted earnings per common share | 1.31 | 1.64 | 1.59 | 1.37 | 5.91 | ||||||||||||||
F-49
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Dollars and shares in millions, except per share data)
Schedule II
LABORATORY CORPORATION OF AMERICA HOLDINGS AND SUBSIDIARIES
VALUATION AND QUALIFYING ACCOUNTS AND RESERVES
Years Ended December 31, 2015, 2014 and 2013
(Dollars in millions)
Balance at beginning of year | Additions Charged to Costs and Expense | (1) Other (Deductions)Additions | Balance at end of year | ||||||||||||
Year ended December 31, 2015: | |||||||||||||||
Applied against asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 211.6 | $ | 265.4 | $ | (260.0 | ) | $ | 217.0 | ||||||
Valuation allowance-deferred tax assets | $ | 17.1 | $ | — | $ | (2.0 | ) | $ | 15.1 | ||||||
Year ended December 31, 2014: | |||||||||||||||
Applied against asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 198.3 | $ | 276.5 | $ | (263.2 | ) | $ | 211.6 | ||||||
Valuation allowance-deferred tax assets | $ | 16.5 | $ | 0.6 | $ | — | $ | 17.1 | |||||||
Year ended December 31, 2013: | |||||||||||||||
Applied against asset accounts: | |||||||||||||||
Allowance for doubtful accounts | $ | 191.5 | $ | 254.8 | $ | (248.0 | ) | $ | 198.3 | ||||||
Valuation allowance-deferred tax assets | $ | 18.4 | $ | 0.2 | $ | (2.1 | ) | $ | 16.5 | ||||||
(1) Other (Deductions) Additions consists primarily of write-offs of accounts receivable amounts.
F-50
