Attached files
| file | filename |
|---|---|
| EX-32.1 - EXHIBIT 32.1 - Impax Laboratories, LLC | ex32-1.htm |
| EX-31.1 - EXHIBIT 31.1 - Impax Laboratories, LLC | ex31-1.htm |
| EX-21.1 - EXHIBIT 21.1 - Impax Laboratories, LLC | ex21-1.htm |
| EX-31.2 - EXHIBIT 31.2 - Impax Laboratories, LLC | ex31-2.htm |
| EX-23.1 - EXHIBIT 23.1 - Impax Laboratories, LLC | ex23-1.htm |
| EX-32.2 - EXHIBIT 32.2 - Impax Laboratories, LLC | ex32-2.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
(Mark One)
|
☒ |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
|
☐ |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission file number: 001-34263
|
Impax Laboratories, Inc. | ||
|
(Exact name of registrant as specified in its charter) | ||
|
Delaware |
65-0403311 | |
|
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
|
30831 Huntwood Avenue, Hayward, CA |
94544 | |
|
(Address of principal executive offices) |
(Zip Code) | |
Registrant’s telephone number, including area code:
(510) 476-2000
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Name of each exchange on which registered: | |
|
Common Stock, par value $0.01 per share |
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation of S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer ☒ |
Accelerated filer ☐ |
Non-accelerated filer ☐ |
Smaller reporting company ☐ |
(Do not check if a smaller reporting company)
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes ☐ No ☒
The aggregate market value of the registrant’s outstanding shares of common stock, other than shares held by persons who may be deemed affiliates of the registrant, computed by reference to the price at which the registrant’s common stock was last sold on The NASDAQ Stock Market LLC as of the last business day of the registrant’s most recently completed second fiscal quarter (June 30, 2015), was approximately $2,660,350,000.
As of February 12, 2016, there were 72,602,791 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain portions of the definitive proxy statement for the registrant’s Annual Meeting of Stockholders to be held on May 17, 2016 have been incorporated by reference into Part III of this Annual Report on Form 10-K.
TABLE OF CONTENTS
|
Forward-Looking Statements |
1 | |
|
PART I. |
||
|
Item 1. |
Business |
2 |
|
Item 1A. |
Risk Factors |
16 |
|
Item 1B. |
Unresolved Staff Comments |
37 |
|
Item 2. |
Properties |
38 |
|
Item 3. |
Legal Proceedings |
38 |
|
Item 4. |
Mine Safety Disclosures |
38 |
|
PART II. |
||
|
Item 5. |
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
39 |
|
Item 6. |
Selected Financial Data |
43 |
|
Item 7. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
44 |
|
Item 7A. |
Quantitative and Qualitative Disclosures about Market Risk |
67 |
|
Item 8. |
Financial Statements and Supplementary Data |
68 |
|
Item 9. |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
68 |
|
Item 9A. |
Controls and Procedures |
68 |
|
Item 9B. |
Other Information |
71 |
|
PART III. |
||
|
Item 10. |
Directors, Executive Officers and Corporate Governance |
72 |
|
Item 11. |
Executive Compensation |
72 |
|
Item 12. |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
72 |
|
Item 13. |
Certain Relationships and Related Transactions, and Director Independence |
72 |
|
Item 14. |
Principal Accounting Fees and Services |
72 |
|
PART IV. |
||
|
Item 15. |
Exhibits and Financial Statement Schedules |
73 |
|
SIGNATURES |
||
|
EXHIBIT INDEX |
||
Forward-Looking Statements
Statements included in this Annual Report on Form 10-K that do not relate to present or historical conditions are “forward-looking statements.” Such forward-looking statements involve risks and uncertainties that could cause results or outcomes to differ materially from those expressed in the forward-looking statements. Forward-looking statements may include statements relating to our plans, strategies, objectives, expectations and intentions. Words such as “believes,” “forecasts,” “intends,” “possible,” “estimates,” “anticipates,” and “plans” and similar expressions are intended to identify forward-looking statements. Our ability to predict results or the effect of events on our operating results is inherently uncertain. Forward-looking statements involve a number of risks, uncertainties and other factors that could cause actual results to differ materially from those discussed in this Annual Report on Form 10-K. Such risks and uncertainties include fluctuations in our revenues and operating income, our ability to successfully develop and commercialize pharmaceutical products in a timely manner, reductions or loss of business with any significant customer, the substantial portion of our total revenues derived from sales of a limited number of products, the impact of consolidation of our customer base, the impact of competition, our ability to sustain profitability and positive cash flows, any delays or unanticipated expenses in connection with the operation of our manufacturing facilities, the effect of foreign economic, political, legal and other risks on our operations abroad, the uncertainty of patent litigation and other legal proceedings, the increased government scrutiny on our agreements with brand pharmaceutical companies, product development risks and the difficulty of predicting FDA filings and approvals, consumer acceptance and demand for new pharmaceutical products, the impact of market perceptions of us and the safety and quality of our products, our determinations to discontinue the manufacture and distribution of certain products, our ability to achieve returns on our investments in research and development activities, changes to FDA approval requirements, our ability to successfully conduct clinical trials, our reliance on third parties to conduct clinical trials and testing, our lack of a license partner for commercialization of NUMIENT™ (IPX066) outside of the United States, impact of illegal distribution and sale by third parties of counterfeits or stolen products, the availability of raw materials and impact of interruptions in our supply chain, our policies regarding returns, rebates, allowances and chargebacks, the use of controlled substances in our products, the effect of current economic conditions on our industry, business, results of operations and financial condition, disruptions or failures in our information technology systems and network infrastructure caused by third party breaches or other events, our reliance on alliance and collaboration agreements, our reliance on licenses to proprietary technologies, our dependence on certain employees, our ability to comply with legal and regulatory requirements governing the healthcare industry, the regulatory environment, the effect of certain provisions in our government contracts, our ability to protect our intellectual property, exposure to product liability claims, risks relating to goodwill and intangibles, changes in tax regulations, our ability to manage our growth, including through potential acquisitions and investments, the risks related to our acquisitions of or investments in technologies, products or businesses, the restrictions imposed by our credit facility and indenture, our level of indebtedness and liabilities and the potential impact on cash flow available for operations, uncertainties involved in the preparation of our financial statements, our ability to maintain an effective system of internal control over financial reporting, the effect of terrorist attacks on our business, the location of our manufacturing and research and development facilities near earthquake fault lines, expansion of social media platforms and other risks described below in “Item 1A. Risk Factors.” You should not place undue reliance on forward-looking statements. Such statements speak only as to the date on which they are made, and we undertake no obligation to update or revise any forward-looking statement, regardless of future developments or availability of new information.
Rytary® is a registered trademark and NUMIENT™ is a trademark of Impax Laboratories, Inc. Other names are for informational purposes only and are used to identify companies and products and may be trademarks of their respective owners.
PART I.
Item 1. Business
Overview
We are a specialty pharmaceutical company applying formulation and development expertise, as well as our drug delivery technology, to the development, manufacture and marketing of bioequivalent pharmaceutical products, commonly referred to as “generics,” in addition to the development and marketing of branded products. We operate in two segments, referred to as “Impax Generics” and “Impax Specialty Pharma”. The Impax Generics division concentrates its efforts on the development, manufacture, sale and distribution of our generic products, which are the pharmaceutical and therapeutic equivalents of brand-name drug products and are usually marketed under their established nonproprietary drug names rather than by a brand name. The Impax Specialty Pharma division is engaged in the development of proprietary brand pharmaceutical products that we believe represent improvements to already-approved pharmaceutical products addressing the treatment of central nervous system (“CNS”) disorders. The Impax Specialty Pharma division is also engaged in the promotion, sale and distribution of several branded products, including our internally developed branded pharmaceutical product, Rytary®, an extended release oral capsule formulation of carbidopa-levodopa for the treatment of Parkinson’s disease (“PD”), post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication and/or manganese intoxication which was approved by the FDA in January 2015, and Zomig® (zolmitriptan) products, indicated for the treatment of migraine headaches, under the terms of a Distribution, License, Development and Supply Agreement (“AZ Agreement”) with AstraZeneca UK Limited (“AstraZeneca”). Each of the Impax Generics and Impax Specialty Pharma divisions also generates revenue from research and development services provided to unrelated third-party pharmaceutical entities. See “Item 15. Exhibits and Financial Statement Schedules — Note 23. Segment Information,” for financial information about our segments for the years ended December 31, 2015, 2014 and 2013.
On March 9, 2015, we completed our acquisition of Tower Holdings, Inc. (“Tower”), including its operating subsidiaries CorePharma LLC (“CorePharma”) and Amedra Pharmaceuticals LLC (“Amedra Pharmaceuticals”), and Lineage Therapeutics, Inc. (“Lineage”) for a purchase price of approximately $691.3 million, net of approximately $41.5 million of cash acquired and including the repayment of indebtedness of Tower and Lineage (the “Tower acquisition”). The privately-held companies specialized in the development, manufacture and commercialization of complex generic and branded pharmaceutical products. For additional information on the Tower acquisition, and the related financing of the acquisition, refer to “Item 15. Exhibits and Financial Statement Schedules - Note 2. Business Acquisitions” and “Note 14. Debt”.
In connection with the closing of the Tower acquisition, we renamed the operating and reporting structure of our two divisions into Impax Generics and Impax Specialty Pharma. Impax Generics includes our legacy Global Pharmaceuticals business as well as the acquired CorePharma and Lineage businesses. Impax Specialty Pharma includes the legacy Impax Pharmaceuticals business as well as the acquired Amedra Pharmaceuticals business.
Our Strategy
We plan to continue to expand our Impax Generics division by targeting complex solid oral and alternative dosage form Abbreviated New Drug Applications (“ANDAs”) with high revenue potential, including products with the potential to be first-to-file or first-to-market. Our products and product candidates are generally difficult to formulate and manufacture, providing certain competitive advantages. In addition to our product pipeline of 25 pending applications at the FDA as of February 16, 2016, we are continuing to evaluate and pursue external growth initiatives including acquisitions and partnerships.
The following information summarizes our generic pharmaceutical product development activities since inception through February 16, 2016:
|
● |
112 ANDAs approved by the U.S. Food and Drug Administration (“FDA”),including one tentatively approved (i.e. satisfying substantive FDA requirements but remaining subject to statutory restrictions). In addition, we have rights to market and/or share in profits to 14 approved ANDAs held by our third party alliance partners. The approved ANDAs (including those held by our partners) include generic versions of brand name pharmaceuticals such as Adderall®, Lamictal ODT®, Lofibra®, Opana ER® (NDA 021610) and Solaraze®. | |
|
● |
25 applications pending at the FDA that represent approximately $7.9 billion in 2015 U.S. product sales. | |
|
● |
A number of products in various stages of development for which applications have not yet been filed. |
A core component of our strategy includes an ongoing focus in our Impax Specialty Pharma division on proprietary brand-name pharmaceutical products to treat CNS disorders and other specialty segments. We believe that we have the research, development and formulation expertise to develop branded products that will deliver significant improvements over existing therapies. We plan to continue investing in our development pipeline, both internally and through acquisitions and partnerships primarily focused on late-stage and next generation product opportunities.
Impax Generics Division
In the generic pharmaceutical market, we focus our efforts on developing, manufacturing, selling and distributing complex solid dose and alternative dosage form products covering a broad range of therapeutic areas and having technically challenging drug-delivery mechanisms or unique product development formulations. We employ our technologies and formulation expertise to develop generic products that reproduce brand-name products’ physiological characteristics but do not infringe any valid patents relating to such brand-name products. Generic products contain the same active ingredient and are of the same route of administration, dosage form, strength and indication(s) as brand-name products already approved for use in the United States by the FDA. We generally focus our generic product development on brand-name products as to which the patents covering the active pharmaceutical ingredient have expired or are near expiration, and we employ our experience to develop bioequivalent versions of such brand-name products. We also develop, manufacture, sell and distribute specialty generic pharmaceuticals that we believe present certain competitive advantages, such as difficulty in raw materials sourcing, complex formulation or development characteristics or special handling requirements. We have generally obtained rights to our alternative dosage form products through third party alliance and collaboration agreements, such as through our partnership agreement with TOLMAR, Inc. (“Tolmar”).
We sell and distribute generic pharmaceutical products primarily through four sales channels:
|
● |
the “Impax Generics” sales channel: generic pharmaceutical prescription products we sell directly to wholesalers, large retail drug chains, and others; |
|
● |
the “Rx Partner” sales channel: generic prescription products sold through unrelated third-party pharmaceutical entities pursuant to alliance and collaboration agreements; |
|
● |
the “Private Label” sales channel: generic pharmaceutical over-the-counter (“OTC”) and prescription products we sell to unrelated third parties who in-turn sell the product under their own label; and |
|
● |
the “OTC Partner” sales channel: sales of generic pharmaceutical OTC products sold through unrelated third-party pharmaceutical companies pursuant to alliance, collaboration and supply agreements. |
As of February 16, 2016, we marketed 139 generic pharmaceutical products representing dosage variations of 55 different pharmaceutical compounds through our Impax Generics division, and five other generic pharmaceutical products, representing dosage variations of two different pharmaceutical compounds, through our alliance and collaboration agreement partners. As of February 16, 2016, our marketed generic products include, but are not limited to, authorized generic Adderall XR®, fenofibrate (generic to Lofibra®) and oxymorphone hydrochloride extended release tablets (AB rated to original OPANA® ER).
As of February 16, 2016, we had 25 applications pending at the FDA. The following table lists our publicly identified product applications pending at the FDA as of February 16, 2016:
|
Product |
Generic of | |
|
Colesevelam Tablets 625 mg |
Welchol® | |
|
Dutasteride/Tamsulosin Capsules 0.5 mg/0.4 mg |
Jalyn® | |
|
Fenofibric Acid DR Capsules 45 and 135 mg |
Trilipix® | |
|
Fentanyl Buccal Tablet 100, 200, 400, 600, 800 mcg |
Fentora® | |
|
Oxycodone ER Tablets (new formulation) 10, 15, 20, 30, 40, 60, 80 mg |
Oxycontin® | |
| Oxymorphone ER Tablets version 5, 7.5, 10, 15, 20, 30 and 40 mg (new formulation) | Opana® ER | |
|
Risedronate Sodium DR Tablets 35 mg |
Atelvia® | |
|
Sevelamer Carbonate Tablets 800 mg |
Renvela® | |
| Methylphenidate HCI ER Capsules | Metadate CD® |
Impax Specialty Pharma
The Impax Specialty Pharma division is primarily focused on the development and promotion through our specialty sales force of proprietary branded pharmaceutical products for the treatment of CNS disorders, which include migraine, multiple sclerosis, Parkinson’s disease and postherpetic neuralgia. We estimate there are approximately 16,000 neurologists in the United States. Historically, a concentrated number of these neurologists are responsible for writing the majority of neurology prescriptions. CNS is the largest therapeutic category in the United States with 2015 sales of about $72 billion, or 16.4% of the $437 billion U.S. prescription drug market. CNS product sales grew 2% in 2015, compared to 11% growth for the overall pharmaceutical market, while total CNS prescriptions increased 0.4%, slightly less than the overall pharmaceutical industry growth rate of 1.2%. (Source: IMS Health).
Our branded pharmaceutical product portfolio consists of commercial CNS products and development stage projects. Our internally developed branded pharmaceutical product, Rytary® (IPX066), an extended release oral capsule formulation of carbidopa-levodopa for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication and/or manganese intoxication, was approved by the FDA on January 7, 2015 and we began marketing the product in the United States in April 2015. During the fourth quarter of 2015, we received marketing authorization from the European Commission for NUMIENTTM (the brand name of IPX066 outside of the United States) and we are currently engaged in discussions with potential partners to market NUMIENTTM outside the United States.
Impax Specialty Pharma is also engaged in the sale and distribution of four other branded products; the more significant include Zomig® (zolmitriptan) products, indicated for the treatment of migraine headaches, under the terms of the AZ Agreement with AstraZeneca in the United States and in certain U.S. territories, and Albenza®, indicated for the treatment of tapeworm infections.
We have a couple of product candidates that are in varying stages of development and we currently intend to expand our portfolio of branded pharmaceutical products primarily through internal development and through licensing and acquisitions, with a focus on late-stage product opportunities.
Alliance and Collaboration Agreements
We have entered into several alliance and collaboration agreements with respect to certain of our products and services and may enter into similar agreements in the future. These agreements typically obligate us to deliver multiple goods and/or services over extended periods. Such deliverables include manufactured pharmaceutical products, exclusive and semi-exclusive marketing rights, distribution licenses, and research and development services. Our alliance and collaboration agreements often include milestones and provide for payments upon achievement of these milestones. For more information about the types of milestone events in our agreements and how we categorize them, see “Item 15. Exhibits and Financial Statement Schedules — Note 20. Alliance and Collaboration Agreements.”
Impax Generics Division – Alliance and Collaboration Agreements
License and Distribution Agreement with Shire
In January 2006, we entered into a License and Distribution Agreement with an affiliate of Shire Laboratories, Inc., which was subsequently amended (“Prior Shire Agreement”), under which we received a non-exclusive license to market and sell an authorized generic of Shire’s Adderall XR® product (“AG Product”) subject to certain conditions, but in any event by no later than January 1, 2010. We commenced sales of the AG Product in October 2009. On February 7, 2013, we entered into an Amended and Restated License and Distribution Agreement with Shire (the “Amended and Restated Shire Agreement”), which amended and restated the Prior Shire Agreement. The Amended and Restated Shire Agreement was entered into by the parties in connection with the settlement of our litigation with Shire relating to Shire’s supply of the AG Product to us under the Prior Shire Agreement. During 2013, we received a payment of $48,000,000 from Shire in connection with such litigation settlement, which was recorded in the first quarter of 2013 under the line item “Other Income” on our consolidated statement of operations.
Under the Amended and Restated Shire Agreement, Shire was required to supply the AG Product and we are responsible for marketing and selling the AG Product subject to the terms and conditions thereof until the earlier of (i) the first commercial sale of our generic equivalent product to Adderall XR® and (ii) September 30, 2014 (the “Supply Term”), subject to certain continuing obligations of the parties upon expiration or early termination of the Supply Term, including Shire’s obligation to deliver AG Products still owed to us as of the end of the Supply Term. We are required to pay a profit share to Shire on sales of the AG Product, of which we owed a profit share payable to Shire of $19,540,000, $21,089,000 and $20,406,000 on sales of the AG Product during the years ended December 31, 2015, 2014 and 2013, respectively, with a corresponding charge included in the cost of revenues line in our consolidated statement of operations. Although the Supply Term expired on September 30, 2014, we are permitted under the terms of the agreement to sell AG Products that we hold in our inventory or owed to us by Shire under the agreement until all such products are sold. We will continue to pay a profit share to Shire on sales of such products.
Development, Supply and Distribution Agreement with Tolmar, Inc.
In June 2012, we entered into a Development, Supply and Distribution Agreement with Tolmar (“Tolmar Agreement”). Under the terms of the Tolmar Agreement, Tolmar granted us an exclusive license to commercialize up to 11 generic topical prescription drug products, including ten then approved products and one product pending approval at the FDA, in the United States and its territories. Under the terms of the Tolmar Agreement, Tolmar is responsible for developing and manufacturing the products, and we are responsible for marketing and sale of the products. We are required to pay a profit share to Tolmar on sales of each product commercialized pursuant to the terms of the Tolmar Agreement. We owed a profit share payable to Tolmar of $77,683,000, $15,995,000 and $3,905,000 on sales of the topical products during the years ended December 31, 2015, 2014 and 2013, respectively, with a corresponding charge included in the Cost of Revenues line item on our consolidated statement of operations.
We paid Tolmar a $21,000,000 upfront payment upon signing of the agreement and pursuant to the terms of the agreement, are also required to make payments to Tolmar upon the achievement of certain specified milestone events. During the year ended December 31, 2012, we made a $1,000,000 milestone payment and during the fourth quarter ended December 31, 2013, we made a $12,000,000 payment to Tolmar, in each case upon Tolmar’s achievement of a regulatory milestone event. During the fourth quarter of 2014, we paid a $2,000,000 milestone payment to Tolmar related to the Diclofenac Sodium Gel 3% (Solaraze®) product pursuant to the Tolmar Agreement in accordance with the terms thereof. During the second quarter of 2015, we paid a $5.0 million milestone related to certain topical products pursuant to the Tolmar Agreement.
Product Acquisition Agreement with Teva Pharmaceuticals USA, Inc.
In August 2013, we, through our Amedra Pharmaceuticals subsidiary, entered into a product acquisition agreement (the “Teva Product Acquisition Agreement”) with Teva Pharmaceuticals USA, Inc. (“Teva”) pursuant to which we acquired the assets (including the ANDA and other regulatory materials) and related liabilities related to Teva’s mebendazole tablet product in all dosage forms (the “Mebendazole Tablet”). We have the potential to pay up to $3,500,000 in additional contingent milestone payments upon the achievement of predefined regulatory and commercialization milestones. We are also obligated to pay Teva a royalty payment based on net sales of the Mebendazole Tablet, including a specified annual minimum royalty payment, subject to customary reductions and the other terms and conditions set forth in the Teva Product Acquisition Agreement.
Rx Partner and OTC Partner Alliance Agreements
We have entered into alliance agreements with unrelated third-party pharmaceutical companies pursuant to which our partner distributes a specified product or products which we developed and, in some cases manufacture. Pursuant to these alliance agreements we typically receive payment on delivery of the product, and share in the resulting profits, or receive a royalty or receive other payments from our partners. Our alliance agreements are separated into two sales channels, the “Rx Partner” sales channel, for generic prescription products sold through our partners under their own label, and the “OTC Partner” sales channel, for sales of generic pharmaceutical OTC products sold through our partner under their own label. The revenue recognized and the percentage of gross revenue for each of the periods noted, for the Rx Partner and the OTC Partner alliance agreements, was as follows:
|
Year Ended December 31, |
||||||||||||||||||||||||
|
2015 |
2014 |
2013 | ||||||||||||||||||||||
| $’s in 000’s | ||||||||||||||||||||||||
|
Gross Revenue and % Gross Revenue |
||||||||||||||||||||||||
|
Rx Partner |
$ | 9,307 | 1 | % | $ | 14,114 | 1 | % | $ | 11,639 | 1 | % | ||||||||||||
|
OTC Partner |
$ | 1,744 | 1 | % | $ | 1,319 | 1 | % | $ | 1,173 | 1 | % | ||||||||||||
Rx Partner Alliance Agreement with Teva
We entered into a Strategic Alliance Agreement with Teva Pharmaceuticals Curacao N.V., a subsidiary of Teva Pharmaceutical Industries Limited, in June 2001, which was subsequently amended (“Teva Agreement”). The Teva Agreement commits us to develop and manufacture, and Teva to distribute, a specified number of controlled release generic pharmaceutical products (“generic products”), each for a 10-year period or such longer period as may be mutually agreed between the parties. As of December 31, 2015, we were supplying Teva with oxybutynin extended release tablets (Ditropan XL® 5, 10 and 15 mg extended release tablets) and have agreed to supply another product (currently under development) to Teva; the other products under the Teva Agreement have either been returned to us, are being manufactured by Teva at its election, were voluntarily withdrawn from the market or our obligations to supply such product had expired or were terminated in accordance with the agreement.
For more information about the Teva Agreement, see “Item 15. Exhibits and Financial Statement Schedules – Note 20. Alliance and Collaboration Agreements.”
OTC Partner Alliance Agreements
We have a Development, License and Supply Agreement with Pfizer, Inc., formerly Wyeth LLC (“Pfizer”) for a term of approximately 15 years, relating to our Loratadine and Pseudoephedrine Sulfate 5 mg/120 mg 12-hour Extended Release Tablets and Loratadine and Pseudoephedrine Sulfate 10 mg/240 mg 24-hour Extended Release Tablets for the OTC market. We previously developed the products and are currently only responsible for manufacturing the products, and Pfizer is responsible for marketing and sale. The agreement included payments by us upon achievement of development milestones, as well as royalties paid to us by Pfizer on its sales of the product. Pfizer launched this product in May 2003 as Alavert® D-12 Hour. In February 2005, the agreement was partially cancelled with respect to the 24-hour Extended Release Product due to lower than planned sales volume. In December 2011, we and Pfizer entered into an agreement with L. Perrigo Company (“Perrigo”), which was subsequently amended whereby the parties agreed that we would supply our Loratadine and Pseudoephedrine Sulfate 5 mg/120 mg 12-hour Extended Release Tablets to Perrigo in the United States and its territories. The agreements with Pfizer and Perrigo are no longer a core area of our business, and the over-the-counter pharmaceutical products we sell to Pfizer and Perrigo under the agreements are older products which are only sold to Pfizer and to Perrigo. As noted above, we are currently only required to manufacture the products under its agreements with Pfizer and Perrigo. We recognize profit share revenue in the period earned.
Research Partner Alliance Agreement
In November 2008, we entered into a Joint Development Agreement with Valeant Pharmaceuticals International, Inc., formerly Medicis Pharmaceutical Corporation (“Valeant”), providing for collaboration in the development of five dermatological products, including four of our generic products and one branded advanced form of Valeant’s SOLODYN® product. Valeant paid us an upfront fee of $40.0 million in December 2008. We have also received an aggregate of $15.0 million in milestone payments consisting of two $5.0 million milestone payments, paid by Valeant in March 2009 and September 2009, a $2.0 million milestone payment received in December 2009, and a $3.0 million milestone payment received in March 2011. We have the potential to receive up to an additional $8.0 million of contingent regulatory milestone payments as well as the potential to receive royalty payments from sales, if any, by Valeant of its advanced form SOLODYN® product under this agreement. We believe that all of the milestones under this agreement are substantive and expect to recognize the proceeds from these regulatory milestones as revenue when achieved. We do not expect to receive any of these additional milestone payments during the fiscal year ending December 31, 2016. To the extent we commercialize any of the four generic dermatology products covered by the agreement, we will pay to Valeant a gross profit share on sales of such products. We began selling one of the four dermatology products during the year ended December 31, 2011. During the three month period ended March 31, 2013, we extended the revenue recognition period for the Joint Development Agreement from the previous recognition period ending in November 2013 to December 2014 due to changes in the estimated timing of completion of certain research and development activities. All deferred revenue under the Joint Development Agreement was recognized as of December 31, 2014.
Impax Specialty Pharma – Alliance and Collaboration Agreements
Distribution, License, Development and Supply Agreement with AstraZeneca UK Limited
In January 2012, we entered into the AZ Agreement with AstraZeneca. Under the terms of the AZ Agreement, AstraZeneca granted to us an exclusive license to commercialize the tablet, orally disintegrating tablet and nasal spray formulations of Zomig® (zolmitriptan) products for the treatment of migraine headaches in the United States and in certain U.S. territories, except during an initial transition period when AstraZeneca fulfilled all orders of Zomig® products on our behalf and paid us the gross profit on such Zomig® product sales. We are obligated to fulfill certain minimum requirements with respect to the promotion of currently approved Zomig® products as well as other dosage strengths of such products approved by the FDA in the future. We may, but have no obligation to, develop and commercialize additional products containing zolmitriptan and additional indications for Zomig®, subject to certain restrictions as set forth in the AZ Agreement. We will be responsible for conducting clinical studies and preparing regulatory filings related to the development of any such additional products and would bear all related costs. In June 2015, the FDA approved the Zomig® nasal spray for use in pediatric patients 12 years of age or older for the acute treatment of migraine with or without aura. During the term of the AZ Agreement, AstraZeneca will continue to be the holder of the NDA for existing Zomig® products, as well as any future dosage strengths thereof approved by the FDA, and will be responsible for certain regulatory and quality-related activities for such Zomig® products. AstraZeneca will manufacture and supply Zomig® products to us and we will purchase our requirements of Zomig® products from AstraZeneca until a date determined in the AZ Agreement. Thereafter, AstraZeneca may terminate its supply obligations upon certain advance notice, in which case we would have the right to manufacture or have manufactured our own requirements for the applicable Zomig® product.
Under the terms of the AZ Agreement, AstraZeneca was required to make payments to us representing 100% of the gross profit on sales of AstraZeneca-labeled Zomig® products during the specified transition period. Under the terms of the AZ Agreement, we made quarterly payments totaling $130.0 million to AstraZeneca during the year ended December 31, 2012. Beginning in January 2013, we became obligated to pay AstraZeneca tiered royalty payments based on net sales of Zomig® products, depending on brand exclusivity and subject to customary reductions and other terms and conditions set forth in the AZ Agreement. We are also obligated to pay to AstraZeneca royalties after a certain specified date based on gross profit from sales of authorized generic versions of the Zomig® products subject to certain terms and conditions set forth in the AZ Agreement. In May 2013, our exclusivity period for branded Zomig® tablets and orally disintegrating tablets expired and we launched authorized generic versions of those products in the United States. We owed a royalty payable to AstraZeneca of $16,848,000, $14,262,000 and $36,113,000 for the years ended December 31, 2015, 2014 and 2013, respectively, with a corresponding charge included in the cost of revenues line on the consolidated statements of income.
Beginning after December 31, 2015, we may terminate the AZ Agreement for convenience upon specified notice. We may also terminate the AZ Agreement if certain of AstraZeneca’s annual manufacturing costs reflected in the supply price increase by more than a certain threshold. The AZ Agreement may also be terminated under certain other circumstances, including for material breach, as set forth in the AZ Agreement.
Development and Co-Promotion Agreement with Endo Pharmaceuticals Inc.
In June 2010, we entered into a Development and Co-Promotion Agreement (“Endo Agreement”) with Endo Pharmaceuticals Inc. ("Endo") under which we agreed to collaborate in the development and commercialization of a next-generation advanced form of our lead brand product candidate ("Endo Agreement Product"). The Endo Agreement was terminated upon mutual agreement by the parties effective December 23, 2015. Under the provisions of the Endo Agreement, in June 2010, Endo paid to us a $10.0 million upfront payment. Prior to termination of the agreement, we also had the potential to receive up to an additional $30.0 million of contingent milestone payments.
Agreement with DURECT Corporation
In 2014, we entered into an agreement with DURECT Corporation (“Durect”) granting us the exclusive worldwide rights to develop and commercialize DURECT’s investigational transdermal bupivacaine patch for the treatment of pain associated with post-herpetic neuralgia (PHN), which we refer to as IPX239. We paid Durect a $2,000,000 up-front payment upon signing of the agreement. We also have the potential to pay up to $61,000,000 in additional contingent milestone payments upon the achievement of predefined development and commercialization milestones. If IPX239 is commercialized, we would also be obligated to pay Durect a tiered royalty based on product sales.
Our Controlled-Release Technology
We have developed a number of different controlled-release delivery technologies which may be utilized with a variety of oral dosage forms and drugs. Controlled-release drug delivery technologies are designed to release drug dosages at specific times and in specific locations in the body and generally provide more consistent and appropriate drug levels in the bloodstream than immediate-release dosage forms. Controlled-release pharmaceuticals may improve drug efficacy, ensure greater patient compliance with the treatment regimen, reduce side effects or increase drug stability and be more patient friendly by reducing the number of times a drug must be taken.
We believe our controlled-release drug delivery technologies are flexible and can be applied to develop a variety of pharmaceutical products, both generic and branded. Our technologies utilize a variety of polymers and other materials to encapsulate or entrap the active pharmaceutical ingredients and to release them at varying rates or at predetermined locations in the gastrointestinal tract.
Competition
The pharmaceutical industry is highly competitive and is affected by new technologies, new developments, government regulations, health care legislation, availability of financing, and other factors. Many of our competitors have longer operating histories and substantially greater financial, research and development, marketing, and other resources than we have. We compete with numerous other companies that currently operate, or intend to operate, in the pharmaceutical industry, including companies that are engaged in the development of controlled-release drug delivery technologies and products, and other manufacturers that may decide to undertake development of such products. Our principal competitors in the generic pharmaceutical products market are Teva Pharmaceutical Industries Ltd., Allergan Inc., Mylan N.V., Sun Pharmaceutical Industries Ltd., Lannett Company, Inc., Lupin Pharmaceuticals, Inc., Endo International plc and Sandoz.
Due to our focus on relatively hard to replicate controlled-release products, competition in the generic pharmaceutical market is sometimes limited to those competitors who possess the appropriate drug delivery technology. The principal competitive factors in the generic pharmaceutical market are:
|
● |
the ability to introduce generic versions of products promptly after a patent expires; |
|
● |
price; |
|
● |
product quality; |
|
● |
customer service (including maintenance of inventories for timely delivery); and |
|
● |
the ability to identify and market niche products. |
In the brand-name pharmaceutical market, our principal competitors are pharmaceutical companies that are focused on Parkinson’s disease and other CNS disorders. In addition, with respect to products that we are developing internally and/or any additional products we may in-license from third parties, we expect that we will face increased competition from large pharmaceutical companies, drug delivery companies and other specialty pharmaceutical companies that have focused on the same disorders as our branded products.
A description of the competition we face from brand-name and generic pharmaceutical companies is included in “Item 1A. Risk Factors”.
Sales and Marketing
We market and sell our generic pharmaceutical prescription drug products within the continental United States and the Commonwealth of Puerto Rico. We have not made sales in any other jurisdictions over the last three fiscal years. We derive a substantial portion of our revenue from sales to a limited number of customers. The customer base for our products consists primarily of drug wholesalers, warehousing chain drug stores, mass merchandisers, and mail-order pharmacies. We market our products both directly, through our Impax Generics and Impax Specialty Pharma divisions, and indirectly through our Rx Partner and OTC Partner alliance and collaboration agreements. Together, our five major customers, McKesson Corporation, Cardinal Health, Amerisource-Bergen, CVS Caremark Corporation and N.C. Mutual, accounted for 89% of our gross revenue for the year ended December 31, 2015. These five customers individually accounted for 46%, 22%, 19%, 1% and 1%, respectively, of our total gross revenue for the year ended December 31, 2015. We do not have long-term contracts in effect with our five major customers. A reduction in or loss of business with any one of these customers, or any failure of a customer to pay us on a timely basis, would adversely affect our business.
Manufacturing and Distribution
We source our finished dosage form products from our own facilities in Hayward, California; Middlesex, New Jersey; and Taiwan. We also use several contract manufacturers for this purpose. During 2015, we restructured our packaging and distribution operations. As a result, we closed our Philadelphia packaging site and all of our company-wide distribution operations were outsourced to United Parcel Services (UPS).
We maintain an inventory of our products in connection with our obligations under our alliance and collaboration agreements. In addition, for products pending approval, we may produce batches for inventory in anticipation of the launch of the products. In the event that FDA approval is denied or delayed, we could be exposed to the risk of this inventory becoming obsolete.
Raw Materials
The active chemical raw materials essential to our business are generally readily available from multiple sources in the United States and throughout the world. Certain raw materials used in the manufacture of our products are, however, available from limited sources and, in some cases, a single source. Although we have not experienced any material delays in receipt of raw materials to date, any curtailment in the availability of such raw materials could result in production or other delays or, in the case of products for which only one raw material supplier exists or has been approved by the FDA, a material loss of sales with consequent adverse effects on our business and results of operations. Also, because raw material sources for pharmaceutical products must generally be identified and approved by regulatory authorities, changes in raw material suppliers may result in production delays, higher raw material costs, and loss of sales and customers. We obtain a portion of our raw materials from foreign suppliers, and our arrangements with such suppliers are subject to, among other risks, FDA approval, governmental clearances, export duties, political instability, and restrictions on the transfers of funds.
Those of our raw materials that are available from a limited number of suppliers include Bendroflumethiazide, Chloroquine Phosphate, Colestipol, Digoxin, Fenofibrate, Methyltestosterone, Nadolol and Pyridostigmine, all of which are active pharmaceutical ingredients. The manufacturers of several of these products are sole-source suppliers. Only a couple of our active ingredients are covered by long-term supply agreements and, although to date we have only experienced occasional interruptions in supplies, we cannot assure that we will continue to receive uninterrupted or adequate supplies of such raw materials.
Any inability to obtain raw materials on a timely basis, or any significant price increases not passed on to customers, could have a material adverse effect on our business, results of operations and financial condition.
Quality Control
We have in the past received a warning letter and Form 483 observations from the FDA regarding certain operations within our manufacturing network.
|
● |
In late May 2011, we received a warning letter from the FDA related to an on-site FDA inspection of our Hayward, California manufacturing facility citing deviations from current Good Manufacturing Practices (“cGMP”), which are extensive regulations governing manufacturing practices for finished pharmaceutical products and which establish requirements for manufacturing processes, stability testing, record keeping and quality standards and controls. | |
| ● |
During fiscal years 2012, 2013 and 2014, the FDA conducted cGMP inspections of our Hayward manufacturing facility and during the quarter ended June 30, 2015, the FDA conducted a cGMP and Pre-Approval Inspection at the facility. At the conclusion of each inspection, we received a Form 483. | |
| ● |
In July 2014, we received a Form 483 after the FDA conducted a cGMP and Pre-Approval Inspection of our Taiwan manufacturing facility. | |
| ● |
In March 2015, we received a Form 483 at our Horsham, Pennsylvania operations after the FDA conducted a pharmacovigilance inspection. |
During 2015, we successfully resolved the warning letter on our Hayward manufacturing facility and we also received the Establishment Inspection Reports (“EIRs”) for each of the aforementioned FDA inspections indicating acceptance of our corrective actions and closure by the FDA of each such inspection. Since our receipt of the 2015 notification from the FDA regarding closure of the warning letter, the FDA has approved a number of products that are manufactured at our Hayward facility.
We remain committed to continuing to improve our quality control and manufacturing practices. We cannot be assured, however, that the FDA will continue to be satisfied with our corrective actions and with our quality control and manufacturing systems and standards. If we receive any future FDA observations, we may be subject to regulatory action including, among others, monetary sanctions or penalties, product recalls or seizure, injunctions, total or partial suspension of production and/or distribution, and suspension or withdrawal of regulatory approvals. Further, other federal agencies, our customers and partners in our alliance, development, collaboration and other partnership agreements with respect to our products and services may take any such Form 483 observations or warning letters into account when considering the award of contracts or the continuation or extension of such partnership agreements. If we receive any future Form 483 observations or warning letters from the FDA, our business, consolidated results of operations and consolidated financial condition could be materially and adversely affected.
Research and Development
We conduct most of our research and development activities at our facilities in Hayward, California and Middlesex, NJ, with a staff of 157 employees as of December 31, 2015. In addition, we have outsourced a number of research and development projects to third-party laboratories.
We spent approximately $77.0 million, $78.6 million and $68.9 million on research and development activities during the years ended December 31, 2015, 2014 and 2013, respectively, as more fully set out in the tables below (in millions). The research and development expenses in the year ended December 31, 2015 included $6.4 million related to an impairment of acquired in-process research and development product rights, included in the line item “Other” below.
| (amounts in millions) | Impax | |||||||||||
|
Impax Generics |
Specialty Pharma |
Total Impax |
||||||||||
|
Year Ended December 31, 2015 |
||||||||||||
|
Clinical study expenses |
$ | 4.6 | $ | 0.8 | $ | 5.4 | ||||||
|
Personnel expenses |
28.6 | 10.0 | 38.6 | |||||||||
|
Experimental materials |
4.3 | - | 4.3 | |||||||||
|
Outside services |
5.8 | 4.5 | 10.3 | |||||||||
|
Facility expenses |
4.2 | .4 | 4.6 | |||||||||
|
Legal expenses |
0.4 | 0.2 | 0.6 | |||||||||
|
Other |
11.0 | 2.2 | 13.2 | |||||||||
|
Total |
$ | 58.9 | $ | 18.1 | $ | 77.0 | ||||||
|
Impax |
||||||||||||
|
Impax |
Specialty |
Total |
||||||||||
|
Generics |
Pharma |
Impax |
||||||||||
|
Year Ended December 31, 2014 |
||||||||||||
|
Clinical study expenses |
$ | 10.2 | $ | 7.6 | $ | 17.8 | ||||||
|
Personnel expenses |
17.1 | 17.7 | 34.8 | |||||||||
|
Experimental materials |
5.0 | 0.7 | 5.7 | |||||||||
|
Outside services |
2.7 | 4.9 | 7.6 | |||||||||
|
Facility expenses |
2.7 | 1.1 | 3.8 | |||||||||
|
Legal expenses |
0.3 | 0.5 | 0.8 | |||||||||
|
Other |
2.9 | 5.2 | 8.1 | |||||||||
|
Total |
$ | 40.9 | $ | 37.7 | $ | 78.6 | ||||||
|
Impax |
||||||||||||
|
Impax |
Specialty |
Total |
||||||||||
|
Generics |
Pharma |
Impax |
||||||||||
|
Year Ended December 31, 2013 |
||||||||||||
|
Clinical study expenses |
$ | 14.9 | $ | 6.0 | $ | 20.9 | ||||||
|
Personnel expenses |
14.9 | 15.7 | 30.6 | |||||||||
|
Experimental materials |
2.8 | 1.1 | 3.9 | |||||||||
|
Outside services |
2.4 | 1.4 | 3.8 | |||||||||
|
Facility expenses |
2.6 | 1.1 | 3.7 | |||||||||
|
Legal expenses |
0.9 | 0.4 | 1.3 | |||||||||
|
Other |
2.9 | 1.8 | 4.7 | |||||||||
|
Total |
$ | 41.4 | $ | 27.5 | $ | 68.9 | ||||||
We do not generally track research and development expense by individual product in either the Impax Generics division or the Impax Specialty Pharma division.
In the Impax Generics division, we focus our research and development efforts based on drug-delivery technology and on products that we believe may have certain competitive advantages, rather than on any particular therapeutic area. As of February 16, 2016, the Impax Generics division had 25 product applications pending with the FDA and another 18 products in development. Accordingly, we believe that our generic pipeline products will, in the aggregate, generate a significant amount of revenue for us in the future. However, while a generic product is still in development, we are unable to predict the level of commercial success that the product may ultimately achieve given the uncertainties relating to the successful and timely completion of bioequivalence studies, ANDA filing, receipt of marketing approval and resolution of any related patent litigation, as well as the amount of competition in the market at the time of product launch and thereafter and other factors detailed in “Item 1A. Risk Factors.” Additionally, we do not believe that any individual generic pipeline product is currently significant in terms of accrued or anticipated research and development expense given the large volume of products under development in the Impax Generics division, as detailed above. Further, on a per product basis, development costs for generic products tend to be significantly lower than for branded products, as the process for establishing bioequivalence is significantly less extensive than the standard clinical trial process. The regulatory approval process is significantly less onerous as well compared to the process for branded products.
In the Impax Specialty Pharma division, we currently market one internally developed branded pharmaceutical product, Rytary® (IPX066) for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication and/or manganese intoxication, which was approved by the FDA on January 7, 2015 and which we launched in the United States in April 2015. We also have a number of product candidates that are in varying stages of development. While we believe the pipeline products in this division are potentially viable, profitable product candidates for us, given the uncertainties relating to the successful completion of clinical trials, the FDA approval process for branded products, reimbursement levels, the amount of competition at the time of product launch and thereafter and other factors detailed in “Item 1A. Risk Factors,” such pipeline products are too early in the development process to be considered significant at this point in time.
Regulation
The manufacturing and distribution of pharmaceutical products are subject to extensive regulation by the federal government, primarily through the FDA and the Drug Enforcement Administration (“DEA”), and to a lesser extent by state and local governments. The Food, Drug, and Cosmetic Act, Controlled Substances Act and other federal statutes and regulations govern or influence the manufacture, labeling, testing, storage, record keeping, approval, advertising and promotion of our products. Facilities used in the manufacture, packaging, labeling and repackaging of pharmaceutical products must be registered with the FDA and are subject to FDA inspection to ensure that drug products are manufactured in accordance with current Good Manufacturing Practices. Noncompliance with applicable requirements can result in product recalls, seizure of products, injunctions, suspension of production, refusal of the government to enter into supply contracts or to approve drug applications, civil penalties and criminal fines, and disgorgement of profits.
FDA approval is required before any “new drug” may be marketed, including new formulations, strengths, dosage forms and generic versions of previously approved drugs. Generally, the following two types of applications are used to obtain FDA approval of a “new drug.”
New Drug Application (“NDA”). For a drug product containing an active ingredient not previously approved by the FDA, a prospective manufacturer must submit a complete application containing the results of clinical studies supporting the drug product’s safety and efficacy. An NDA is also required for a drug with a previously approved active ingredient if the drug will be used to treat an indication for which the drug was not previously approved or if the dosage form, strength or method of delivery is changed. The process required by the FDA before a pharmaceutical product may be approved for marketing in the U.S. generally involves the steps listed below, which could take from approximately three to more than ten years to complete.
|
● |
Laboratory and clinical tests; |
|
● |
Submission of an Investigational New Drug (“IND”) application, which must become effective before clinical studies may begin; |
|
● |
Adequate and well-controlled human clinical studies to establish the safety and efficacy of the proposed product for its intended use; |
|
● |
Submission of an NDA containing the results of the preclinical tests and clinical studies establishing the safety and efficacy of the proposed product for its intended use, as well as extensive data addressing such matters such as manufacturing and quality assurance; |
|
● |
Scale-up to commercial manufacturing; and |
|
● |
FDA approval of an NDA. |
As noted above, the submission of an NDA is not a guarantee that the FDA will find it complete and accept it for filing. The FDA reviews all NDAs submitted before it accepts them for filing. It may refuse to file the application and instead request additional information, in which case, the application must be resubmitted with the supplemental information. After the application is deemed filed by the FDA, FDA staff will review an NDA to determine, among other things, whether a product is safe and efficacious for its intended use.
If, after reviewing the NDA, the FDA determines that the application cannot be approved in its current form, the FDA sends the NDA applicant a Complete Response Letter identifying all outstanding deficiencies that preclude final approval. The FDA then halts its review until the applicant resubmits the NDA with new information designed to address the deficiencies. An applicant receiving a Complete Response Letter may resubmit the application with data and information addressing the FDA’s concerns or requirements, withdraw the application without prejudice to a subsequent submission of a related application or request a hearing on whether there are grounds for denying approval of the application. If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may require an applicant to conduct Phase 4 testing which involves clinical trials designed to further assess a drug’s safety and effectiveness after NDA approval, and may require surveillance programs to monitor the safety of approved products which have been commercialized. Once issued, the FDA may withdraw product approval if ongoing regulatory requirements are not met or if safety or efficacy questions are raised after the product reaches the market. The agency may also impose requirements that the NDA holder conduct new studies, make labeling changes, implement Risk Evaluation and Mitigation Strategies, and take other corrective measures.
Abbreviated New Drug Application (“ANDA”). For a generic version of an approved drug — a drug product that contains the same active ingredient as a drug previously approved by the FDA and is in the same dosage form and strength, utilizes the same method of delivery and will be used to treat the same indications as the approved product — the FDA requires only an abbreviated new drug application that ordinarily need not include clinical studies demonstrating safety and efficacy. An ANDA typically requires only data demonstrating that the generic formulation is bioequivalent to the previously approved “reference listed drug,” indicating that the rate of absorption and levels of concentration of the generic drug in the body do not show a significant difference from those of the reference listed drug. In July 2012, the Generic Drug Fee User Amendments of 2012 (“GDUFA”) was enacted into law. The GDUFA legislation implemented fees for new ANDA applications, Drug Master Files, product and establishment fees and a one-time fee for back-logged ANDA applications pending approval as of October 1, 2012. In return, the program is intended to provide faster and more predictable ANDA reviews by the FDA and increased inspections of drug facilities. Under GDUFA, generic product companies face significant penalties for failure to pay the new user fees, including rendering an ANDA application not “substantially complete” until the fee is paid. Prior to the implementation of GDUFA, the FDA took an average of approximately 30 months to approve an ANDA.
Under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the “Hatch-Waxman Act”, which established the procedures for obtaining approval of generic drugs, an ANDA filer must make certain patent certifications that can result in significant delays in obtaining FDA approval. If the applicant intends to challenge the validity or enforceability of an existing patent covering the reference listed drug or asserts that its drug does not infringe such patent, the applicant files a so called “Paragraph IV” certification and notifies the patent holder that it has done so, explaining the basis for its belief that the patent is not infringed or is invalid or unenforceable. If the patent holder initiates a patent infringement suit within 45 days after receipt of the Paragraph IV Certification, the FDA is automatically prevented from approving an ANDA until the earlier of 30 months after the date the Paragraph IV Certification is given to the patent holder, expiration of the patents involved in the certification, or when the infringement case is decided in the ANDA applicant’s favor. In addition, the first company to file an ANDA for a given drug containing a Paragraph IV certification can be awarded 180 days of market exclusivity following approval of its ANDA, during which the FDA may not approve any other ANDAs for that drug product.
During any period in which the FDA is required to withhold its approval of an ANDA due to a statutorily imposed non-approval period, the FDA may grant tentative approval to an applicant’s ANDA. A tentative approval reflects the FDA’s preliminary determination that a generic product satisfies the substantive requirements for approval, subject to the expiration of all statutorily imposed non-approval periods. A tentative approval does not allow the applicant to market the generic drug product.
The Hatch-Waxman Act contains additional provisions that can delay the launch of generic products. A five year marketing exclusivity period is provided for new chemical compounds, and a three year marketing exclusivity period is provided for approved applications containing new clinical investigations essential to an approval, such as a new indication for use, or new delivery technologies, or new dosage forms. The three year marketing exclusivity period applies to, among other things, the development of a novel drug delivery system, as well as a new use. In addition, companies can obtain six additional months of exclusivity if they perform pediatric studies of a reference listed drug product. The marketing exclusivity provisions apply to both patented and non-patented drug products. The Act also provides for patent term extensions to compensate for patent protection lost due to time taken in conducting FDA required clinical studies and during FDA review of NDAs.
The Generic Drug Enforcement Act of 1992 establishes penalties for wrongdoing in connection with the development or submission of an ANDA. In general, the FDA is authorized to temporarily bar companies, or temporarily or permanently bar individuals, from submitting or assisting in the submission of an ANDA, and to temporarily deny approval and suspend applications to market generic drugs under certain circumstances. In addition to debarment, the FDA has numerous discretionary disciplinary powers, including the authority to withdraw approval of an ANDA or to approve an ANDA under certain circumstances and to suspend the distribution of all drugs approved or developed in connection with certain wrongful conduct. The FDA may also withdraw product approval or take other correct measures if ongoing regulatory requirements are not met or if safety or efficacy questions are raised after the product reaches the market.
Other Regulatory Requirements
We are subject to the Maximum Allowable Cost Regulations, which limit reimbursements for certain generic prescription drugs under Medicare, Medicaid, and other programs to the lowest price at which these drugs are generally available. In many instances, only generic prescription drugs fall within the regulations’ limits. Generally, the pricing and promotion of, method of reimbursement and fixing of reimbursement levels for, and the reporting to federal and state agencies relating to drug products is under active review by federal, state and local governmental entities, as well as by private third-party reimbursers and individuals under whistleblower statutes. At present, the Justice Department and U.S. Attorneys Offices and State Attorneys General have initiated investigations, reviews, and litigation into industry-wide pharmaceutical pricing and promotional practices, and whistleblowers have filed qui tam suits. We cannot predict the results of those reviews, investigations, and litigation, or their impact on our business.
Virtually every state, as well as the District of Columbia, has enacted legislation permitting the substitution of equivalent generic prescription drugs for brand-name drugs where authorized or not prohibited by the prescribing physician, and some states mandate generic substitution in Medicaid programs.
In addition, numerous state and federal requirements exist for a variety of controlled substances, such as narcotics, that may be part of our product formulations. The DEA, which has authority similar to the FDA’s and may also pursue monetary penalties, and other federal and state regulatory agencies have far reaching authority.
The State of California requires that any manufacturer, wholesaler, retailer or other entity in California that sells, transfers, or otherwise furnishes certain so called precursor substances must have a permit issued by the California Department of Justice, Bureau of Narcotic Enforcement. The substances covered by this requirement include ephedrine, pseudoephedrine, norpseudoephedrine, and phenylpropanolamine, among others. The Bureau has authority to issue, suspend and revoke precursor permits, and a permit may be denied, revoked or suspended for various reasons, including (i) failure to maintain effective controls against diversion of precursors to unauthorized persons or entities; (ii) failure to comply with the Health and Safety Code provisions relating to precursor substances, or any regulations adopted thereunder; (iii) commission of any act which would demonstrate actual or potential unfitness to hold a permit in light of the public safety and welfare, which act is substantially related to the qualifications, functions or duties of the permit holder; or (iv) if any individual owner, manager, agent, representative or employee of the permit applicant/permit holder willfully violates any federal, state or local criminal statute, rule, or ordinance relating to the manufacture, maintenance, disposal, sale, transfer or furnishing of any precursor substances.
Patents, Trademarks and Licenses
We own or license a number of patents in the U.S. and other countries covering certain products and product candidates and have also developed brand names and trademarks for other products and product candidates. Generally, the brand pharmaceutical business relies upon patent protection to ensure market exclusivity for the life of the patent. We consider the overall protection of our patents, trademarks and license rights to be of material value and act to protect these rights from infringement. However, our business is not dependent upon any single patent, trademark or license.
In the branded pharmaceutical industry, the majority of an innovative product’s commercial value is usually realized during the period in which the product has market exclusivity. In the U.S. and some other countries, when market exclusivity expires and generic versions of a product are approved and marketed, there can often be very substantial and rapid declines in the branded product’s sales. The rate of this decline varies by country and by therapeutic category; however, following patent expiration, branded products often continue to have market viability based upon the goodwill of the product name, which typically benefits from trademark protection.
An innovator product’s market exclusivity is generally determined by two forms of intellectual property: patent rights held by the innovator company and any regulatory forms of exclusivity to which the innovator is entitled.
Patents are a key determinant of market exclusivity for most branded pharmaceuticals. Patents provide the innovator with the right to exclude others from practicing an invention related to the medicine. Patents may cover, among other things, the active ingredient(s), various uses of a drug product, pharmaceutical formulations, drug delivery mechanisms and processes for (or intermediates useful in) the manufacture of products. Protection for individual products extends for varying periods in accordance with the expiration dates of patents in the various countries. The protection afforded, which may also vary from country to country, depends upon the type of patent, its scope of coverage and the availability of meaningful legal remedies in the country.
Market exclusivity is also sometimes influenced by regulatory exclusivity rights. Many developed countries provide certain non-patent incentives for the development of medicines. For example, the U.S., the EU and Japan each provide for a minimum period of time after the approval of a new drug during which the regulatory agency may not rely upon the innovator’s data to approve a competitor’s generic copy. Regulatory exclusivity rights are also available in certain markets as incentives for research on new indications, on orphan drugs and on medicines useful in treating pediatric patients. Regulatory exclusivity rights are independent of any patent rights and can be particularly important when a drug lacks broad patent protection. However, most regulatory forms of exclusivity do not prevent a competitor from gaining regulatory approval prior to the expiration of regulatory data exclusivity on the basis of the competitor’s own safety and efficacy data on its drug, even when that drug is identical to that marketed by the innovator.
We estimate the likely market exclusivity period for each of our branded products on a case-by-case basis. It is not possible to predict the length of market exclusivity for any of our branded products with certainty because of the complex interaction between patent and regulatory forms of exclusivity, and inherent uncertainties concerning patent litigation. There can be no assurance that a particular product will enjoy market exclusivity for the full period of time that we currently estimate or that the exclusivity will be limited to the estimate.
In addition to patents and regulatory forms of exclusivity, we also market products with trademarks. Trademarks have no effect on market exclusivity for a product, but are considered to have marketing value. Trademark protection continues in some countries as long as used; in other countries, as long as registered. Registration is for fixed terms and may be renewed indefinitely.
Environmental Laws
We are subject to comprehensive federal, state and local environmental laws and regulations that govern, among other things, air polluting emissions, waste water discharges, solid and hazardous waste disposal, and the remediation of contamination associated with current or past generation handling and disposal activities. We are subject periodically to environmental compliance reviews by various environmental regulatory agencies. While it is impossible to predict accurately the future costs associated with environmental compliance and potential remediation activities, compliance with environmental laws is not expected to require significant capital expenditures and has not had, and is not expected to have, a material adverse effect on our business, operations or financial condition.
Available Information
We maintain an Internet website at the following address: www.impaxlabs.com. We make available on or through our Internet website certain reports and amendments to those reports, as applicable, that we file with or furnish to the Securities and Exchange Commission (the “SEC”) in accordance with the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These include our Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K. Our website also includes our Code of Conduct and the charters of our Audit Committee, Nominating Committee, Compensation Committee and Compliance Committee of our Board of Directors. We make this information available on our website free of charge, as soon as reasonably practicable after we electronically file the information with, or furnish it to, the SEC. The contents of our website are not incorporated by reference in this Annual Report on Form 10-K and shall not be deemed “filed” under the Exchange Act.
Corporate and Other Information
We were incorporated in the State of Delaware in 1995. Our corporate headquarters are located at 30831 Huntwood Avenue, Hayward, California, 94544. We were formerly known as Global Pharmaceutical Corporation until December 14, 1999, when Impax Pharmaceuticals, Inc., a privately held drug delivery company, merged into Global Pharmaceutical Corporation and the name of the resulting entity was changed to Impax Laboratories, Inc.
Unless otherwise indicated, all product sales data and U.S. market size data in this Annual Report on Form 10-K are based on information obtained from IMS Health, unrelated third-party providers of prescription market data. We did not independently engage IMS Health to provide this information.
Employees
As of December 31, 2015, we had 1,290 full-time employees, of which 563 were in operations, 157 in research and development, 328 in the quality area, 181 in legal and administration, and 61 in sales and marketing. None of our employees are subject to collective bargaining agreements with labor unions, and we believe our employee relations are good.
Item 1A. Risk Factors
An investment in our common stock involves a high degree of risk. In deciding whether to invest in our common stock, you should consider carefully the following risk factors, as well as the other information included in this Annual Report on Form 10-K. The materialization of any of these risks could have a material adverse effect on our business, results of operations and financial condition. This Annual Report on Form 10-K contains forward looking statements that involve risks and uncertainties. Our actual results could differ materially from the results discussed in the forward looking statements. Factors that could cause or contribute to these differences include those discussed in this “Risk Factors” section. See “Forward-Looking Statements” on page 1 of this Annual Report on Form 10-K.
Risks Related to Our Business
Our revenues and operating income could fluctuate significantly.
Our revenues and operating results may vary significantly from year-to-year and quarter to quarter as well as in comparison to the corresponding quarter of the preceding year. Variations may result from, among other factors:
|
● |
the timing of FDA approvals we receive; |
|
● |
the timing of process validation, product launches, and market acceptance of such products launched; |
|
● |
changes in the amount we spend to research, develop, acquire, license or promote new products; |
|
● |
the outcome of our clinical trial programs; |
|
● |
serious or unexpected health or safety concerns with our products, the brand products we have genericized, or our product candidates; |
|
● |
the introduction of new products by others that render our products obsolete or noncompetitive; |
|
● |
the ability to maintain selling prices and gross margins on our products; |
|
● |
changes in our policies regarding returns, rebates, allowances and chargebacks for our products; |
|
● |
the outcome of our patent infringement litigation and other litigation matters and expenditures as a result of such litigation; |
|
● |
the ability to comply with complex governmental regulations which deal with many aspects of our business; |
|
● |
changes in coverage and reimbursement policies of health plans and other health insurers, including changes to Medicare, Medicaid and similar state programs; |
|
● |
increases in the cost of raw materials used to manufacture our products; |
|
● |
manufacturing and supply interruptions, including product rejections or recalls due to failure to comply with manufacturing specifications; |
|
● |
the ability of our license partner(s) to secure regulatory approval, gain market share, sales volume, and sales milestone levels; |
|
● |
timing of revenue recognition related to our alliance and collaboration agreements; |
|
● |
the ability to protect our intellectual property and avoid infringing the intellectual property of others; |
|
● |
our ability to manage our growth and integrate acquired businesses successfully; and |
|
● |
the addition or loss of customers. |
As an illustration, we earned significant revenues and gross profit from sales of our authorized generic Adderall XR® products during fiscal years 2012, 2013 and 2014 and from sales of our authorized generic Renvela® products during fiscal year 2014. With respect to our authorized generic Adderall XR® products, we were dependent on a third party pharmaceutical company to supply us with such products we market and sell through our Generics Division. Our supply agreement with such third party for the supply of generic Adderall XR® products expired on September 30, 2014. Although we continue to market and sell generic Adderall XR® products that we have in inventory, unless we are able to timely and successfully launch our corresponding generic product to Adderall XR®, we will not be able to continue to earn uninterrupted future revenue from the sale of such products after we exhaust our inventory. With respect to our generic Renvela® products, during the second quarter of fiscal year 2014, we were granted a license to sell an allotment of a specified number of bottles of the product under the terms of a third party settlement agreement and the sales from such product, a high margin product, contributed to significant revenue in our Generics Division during fiscal 2014. We did not receive any revenues from our authorized generic Renvela® products in fiscal year 2015, which contributed to reduced gross profit in our Generics Division during certain periods in 2015, as compared to the corresponding periods in the prior year.
In our branded products division, we earned significant revenues and gross profit from sales of our Impax-labeled Zomig® (zolmitriptan) tablet, orally disintegrating tablet and nasal spray formulation products that we began selling during the year ended December 31, 2012 pursuant to our Distribution, License, Development and Supply Agreement with AstraZeneca. In May 2013, our exclusivity period for branded Zomig® tablets and orally disintegrating tablets expired and we experienced diminution of our sales revenue and gross profit from those products as a result of generic competition. We launched authorized generic versions of those products in the United States. We continue to commercialize the branded Zomig® nasal spray which has U.S. patents expiring as late as May 2021, which patents have been challenged by an ANDA filer seeking to market a generic to Zomig® nasal spray. During fiscal year 2015, we began marketing Rytary®, our internally developed extended release oral capsule formulation of carbidopa-levodopa for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication and/or manganese intoxication for sale in the United States, which contributed to sales revenue in our branded products division during the year. We have patents covering Rytary® that expire as late as December 2028, which patents have been challenged by an ANDA filer seeking to market a generic version of Rytary®.
Any diminution of sales revenue and/or gross profit from such products or our other significant products due to existing or additional competition, product supply or any other reasons in future periods may materially and adversely affect our results of operations in such periods.
Due to the fluctuations in revenue and operating results due to factors discussed in greater detail below, our quarterly operating results are difficult to predict and may fluctuate significantly from period to period. We cannot predict with any certainty the timing or level of sales of our products in the future. As a result, period-to-period comparisons of our operating results should not be relied upon as indications of our future performance and any full-year financial forecast should not be relied upon as a guarantee of future performance for that year or for any given quarter within that year. If our quarterly sales or operating results fall below the expectations of investors or securities analysts, the value of our securities could decline substantially and our business, consolidated results of operations and consolidated financial condition could be materially and adversely affected.
We have in the past received a warning letter and Form 483 observations from the FDA which we have recently resolved. If we receive any future Form 483 observations or warning letters, however, our business, consolidated results of operations and consolidated financial condition could be materially and adversely affected.
We have in the past received a warning letter and Form 483 observations from the FDA regarding certain operations within our manufacturing network.
|
● |
In late May 2011, we received a warning letter from the FDA related to an on-site FDA inspection of our Hayward, California manufacturing facility citing deviations from current Good Manufacturing Practices (“cGMP”), which are extensive regulations governing manufacturing practices for finished pharmaceutical products and which establish requirements for manufacturing processes, stability testing, record keeping and quality standards and controls. |
|
● |
During fiscal years 2012, 2013 and 2014, the FDA conducted cGMP inspections of our Hayward manufacturing facility and during the quarter ended June 30, 2015, the FDA conducted a cGMP and Pre-Approval Inspection at the facility. At the conclusion of each inspection, we received a Form 483. |
|
● |
In July 2014, we received a Form 483 after the FDA conducted a cGMP and Pre-Approval Inspection of our Taiwan manufacturing facility. |
|
● |
In March 2015, we received a Form 483 at our Horsham, Pennsylvania operations after the FDA conducted a pharmacovigilance inspection. |
During 2015, we successfully resolved the warning letter on our Hayward manufacturing facility and we also received the Establishment Inspection Reports (“EIRs”) for each of the aforementioned FDA inspections indicating acceptance of our corrective actions and closure by the FDA of each such inspection. Since our receipt of the 2015 notification from the FDA regarding closure of the warning letter, the FDA has approved a number of products that are manufactured at our Hayward facility.
We remain committed to continuing to improve our quality control and manufacturing practices. We cannot be assured, however, that the FDA will continue to be satisfied with our corrective actions and with our quality control and manufacturing systems and standards. If we receive any future FDA observations, we may be subject to regulatory action including, among others, monetary sanctions or penalties, product recalls or seizure, injunctions, total or partial suspension of production and/or distribution, and suspension or withdrawal of regulatory approvals. Further, other federal agencies, our customers and partners in our alliance, development, collaboration and other partnership agreements with respect to our products and services may take any such Form 483 observations or warning letters into account when considering the award of contracts or the continuation or extension of such partnership agreements. If we receive any future Form 483 observations or warning letters from the FDA, our business, consolidated results of operations and consolidated financial condition could be materially and adversely affected.
Our continued growth is dependent on our ability to continue to successfully develop and commercialize new products in a timely manner.
Our financial results depend upon our ability to introduce and commercialize additional generic and branded products in a timely manner. In the generic pharmaceutical products market, revenue from newly launched generic products that we are the first to market is typically relatively high during the period immediately following launch and can be expected generally to decline over time. Revenue from generic drugs in general can also be expected to decline over time. Revenue from branded pharmaceutical products can be expected to decline as the result of entry of new competitors, particularly of companies producing generic versions of the branded products. Our continued growth is therefore dependent upon our ability to continue to successfully introduce and commercialize new generic and branded products.
As of February 16, 2016, we had 25 product applications pending at the FDA and 18 product candidates under development for generic versions of brand-name pharmaceuticals. In our branded products division, we have a few product candidates in various stages of development. The development and commercialization process for our products, particularly of our branded products, is time-consuming, costly and involves a high degree of business risk. The FDA and the regulatory authorities may not approve our products submitted to them or our other products under development. Additionally, we may not successfully complete our development efforts. Even if the FDA approves our products, we may not be able to market them successfully or profitably or, with respect to our generics products, we may not be able to market them at all if we do not prevail in the patent infringement litigation in which we are involved. Our future results of operations will depend significantly upon our ability to timely develop, receive FDA approval for, and market new pharmaceutical products or otherwise acquire new products.
A substantial portion of our total revenues is derived from sales to a limited number of customers.
We derive a substantial portion of our revenue from sales to a limited number of customers. In 2015, our five major customers, McKesson Corporation, Cardinal Health, Amerisource-Bergen, CVS Caremark Corporation and N.C. Mutual accounted for 46%, 22%, 19%, 1% and 1%, respectively, or an aggregate of 89%, of our gross revenue.
A reduction in, or loss of business with, any one of these customers, or any failure of a customer to pay us on a timely basis, would adversely affect our business.
A substantial portion of our total revenues is derived from sales of a limited number of products.
We derive a substantial portion of our revenue from sales of a limited number of products. In 2015, our top five products accounted for 17%, 12%, 8%, 7% and 6%, or an aggregate of 50%, of our product sales, net. The sale of our products can be significantly influenced by market conditions, as well as regulatory actions. We may experience decreases in the sale of our products in the future as a result of actions taken by our competitors, such as price reductions, or as a result of regulatory actions related to our products or to competing products, which could have a material impact on our results of operations. Actions which could be taken by our competitors, which may materially and adversely affect our business, results of operations and financial condition, may include, without limitation, pricing changes and entering or exiting the market for specific products.
Sales of our products may be adversely affected by the continuing consolidation of our customer base.
A significant proportion of our sales is made to relatively few retail drug chains, wholesalers, and managed care organizations. These customers are continuing to undergo significant consolidation. Such consolidation has provided and may continue to provide them with additional purchasing leverage, and consequently may increase the pricing pressures that we face. Additionally, the emergence of large buying groups representing independent retail pharmacies, and the prevalence and influence of managed care organizations and similar institutions, enable those groups to extract price discounts on our products.
Our net sales and quarterly growth comparisons may also be affected by fluctuations in the buying patterns of retail chains, major distributors and other trade buyers, whether resulting from pricing, wholesaler buying decisions or other factors. In addition, since such a significant portion of our revenues is derived from relatively few customers, any financial difficulties experienced by a single customer, or any delay in receiving payments from a single customer, could have a material adverse effect on our business, results of operations and financial condition.
We face intense competition from both brand-name and generic pharmaceutical companies.
The pharmaceutical industry is highly competitive and many of our competitors have longer operating histories and substantially greater financial, research and development, marketing, and other resources than we have. Further, the pharmaceutical industry has in recent years seen increased consolidation, resulting in larger competitors and placing further pressure on prices, development activities and customer retention. In addition, pharmaceutical manufacturers’ customer base consists of an increasingly limited number of large pharmaceutical wholesalers, chain drug stores that warehouse products, mass merchandisers and mail order pharmacies. Our competitors may be able to develop products competitive with or more effective or less expensive than our own for many reasons, including that they may have:
|
● |
proprietary processes or delivery systems; |
|
● |
greater resources in the area of research and development and marketing; |
|
● |
larger or more efficient production capabilities; |
|
● |
more expertise in a particular therapeutic area; |
|
● |
more expertise in preclinical testing and human clinical trials; |
|
● |
more experience in obtaining required regulatory approvals, including FDA approval; |
|
● |
more products; or |
|
● |
more experience in developing new drugs and financial resources, particularly with regard to brand manufacturers. |
In the generic products market, we face competition from other generic pharmaceutical companies, which may impact our selling price and revenues from such products. The FDA approval process often results in the FDA granting final approval to a number of ANDAs for a given product at the time a patent for a corresponding brand product or other market exclusivity expires. This often forces us to face immediate competition when we introduce a generic product into the market. As competition from other generic pharmaceutical companies intensifies, selling prices and gross profit margins often decline, which has been our experience with our existing products. Moreover, with respect to products for which we file a Paragraph IV certification, if we are not the first ANDA filer challenging a listed patent for a product, we are at a significant disadvantage to the competitor that first filed an ANDA for that product containing such a challenge, which is awarded 180 days of market exclusivity for the product. Conversely, in some cases when we are the first ANDA filer to challenge a listed patent, we may forfeit our 180 days of market exclusivity under certain circumstances. In that case, a competitor may obtain ANDA approval earlier than we obtain ANDA approval, in which case we will be at a disadvantage to such competitor. Accordingly, the level of market share, revenue and gross profit attributable to a particular generic product that we develop is generally related to the number of competitors in that product’s market and the timing of that product’s regulatory approval and launch, in relation to competing approvals and launches. Although we cannot assure, we strive to develop and introduce new products in a timely and cost effective manner to be competitive in our industry (see “Item 1 Business — Regulation”). Additionally, ANDA approvals often continue to be granted for a given product subsequent to the initial launch of the generic product. These circumstances generally result in significantly lower prices and reduced margins for generic products compared to brand products. New generic market entrants generally cause continued price and margin erosion over the generic product life cycle.
In addition to the competition we face from other generic pharmaceutical companies related to our generic products, we also face competition from brand-name pharmaceutical companies that may try to prevent, discourage or delay the use of generic versions through various measures, including introduction of new branded products, legislative initiatives, changing dosage forms or dosing regimens, regulatory processes, filing new patents or patent extensions, lawsuits, citizens’ petitions, and negative publicity prior to introduction of a generic product. In addition, brand-name competitors may lower their prices to compete with generic products, increase advertising, or launch, either through an affiliate or licensing arrangements with another company, an authorized generic at or near the time the first generic product is launched, reducing the generic product market exclusivity provided by the Hatch-Waxman Act.
Our principal competitors in the generic pharmaceutical products market are Teva Pharmaceutical Industries Ltd., Allergan, Inc., Mylan N.V., Sun Pharmaceutical Industries Ltd., Lannett Company, Inc., Lupin Pharmaceuticals, Inc., Endo International plc and Sandoz.
In the brand-name pharmaceutical market, our principal competitors are pharmaceutical companies that are focused on Parkinson’s disease and other CNS disorders. In addition, with respect to products that we are developing internally and/or any additional products we may in-license from third parties, we expect that we will face increased competition from large pharmaceutical companies, drug delivery companies and other specialty pharmaceutical companies that have focused on the same disorders as our branded products.
Any of the actions by our competitors as described above may significantly impact sales of our generic and branded products, which could have a material adverse effect on our business, results of operations and financial condition.
We have experienced operating losses and negative cash flow from operations in the past, and our future profitability is uncertain.
Although we have in recent years been profitable, we do not know whether our business will continue to be profitable or generate positive cash flow, and our ability to remain profitable or obtain positive cash flow is uncertain. To remain operational and profitable, we must, among other things:
|
● |
obtain FDA approval of our products; |
|
● |
successfully launch and market new products; |
|
● |
prevail in patent infringement litigation in which we are involved; |
|
● |
continue to generate or obtain sufficient capital on acceptable terms to fund our operations; and |
|
● |
comply with the many complex governmental regulations that deal with virtually every aspect of our business activities. |
Any delays or unanticipated expenses in connection with the operation of our limited number of facilities could have a material adverse effect on our business.
A substantial portion of our manufacturing capacity as well as our current production is attributable to our manufacturing facilities located in Hayward, California, Middlesex, New Jersey and Taiwan, R.O.C. and to certain third party suppliers. A significant disruption at any one of these facilities within our internal or third party supply chain, even on a short-term basis, whether due to an adverse quality or compliance observation, including a total or partial suspension of production and/or distribution by regulatory authorities, an act of God, civil or political unrest, or other events could impair our ability to produce and ship products to the market on a timely basis and could, among other consequences, subject us to exposure to claims from customers. Any of these events could have a material adverse effect on our business, results of operations and financial condition.
Our business is subject to the economic, political, legal and other risks of maintaining facilities and conducting clinical trials in foreign countries.
In 2010, we commenced shipment of commercial product from our new manufacturing facility in Taiwan, and we plan to increase our commercial manufacturing operations in Taiwan in the future. In addition, certain clinical trials for our product candidates are conducted at multiple sites in Europe. These foreign operations are subject to risks inherent in maintaining operations and doing business abroad, such as economic and political destabilization, international conflicts, restrictive actions by foreign governments, expropriation or nationalization of property, changes in laws and regulations, changes in regulatory requirements, the difficulty of effectively managing diverse global operations, adverse foreign tax or tariff laws, more limited intellectual property protection in certain foreign jurisdictions, and the threat posed by potential international disease pandemics in countries that do not have the resources necessary to deal with such outbreaks. Further, as our global operations require compliance with a complex set of foreign and U.S. laws and regulations, including data privacy requirements, labor relations laws, tax laws, anti-competition regulations, import and trade restrictions, export requirements, U.S. laws such as the Foreign Corrupt Practices Act of 1977, as amended, and local laws which also prohibit payments to governmental officials or certain payments or remunerations to customers, there is a risk that some provisions may be inadvertently breached. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers or our employees, and prohibitions on the conduct of our business. These foreign economic, political, legal and other risks could impact our operations and have an adverse effect on our business, results of operations and financial condition.
We are involved in various legal proceedings, including patent litigation that can delay or prevent our commercialization of generic products or accelerate generic competition for our branded products, all of which are uncertain, force us to incur substantial expense to defend and/or expose us to substantial liability.
Patent infringement litigation involves many complex technical and legal issues and its outcome is often difficult to predict, and the risk involved in doing so can be substantial. For generic product manufacturers, the potential consequences to such generic companies in the event of an unfavorable outcome include delaying generic launch until patent expiration and potential damages measured by the profits lost by the branded product manufacturer rather than the profits earned by the generic pharmaceutical company. For brand drug manufacturers, an unfavorable outcome may significantly accelerate generic competition ahead of patent expiration. Such litigation usually involves significant expense and can delay or prevent introduction or sale of our products. Our generic products division is routinely subject to patent infringement litigation brought by branded pharmaceutical manufacturers seeking to delay FDA approval to manufacture and market generic forms of their branded products. Likewise, our branded products division is currently involved in patent infringement litigation against generic drug manufacturers seeking FDA approval to market their generic drugs prior to expiration of patents covering our branded products.
We and/or our third party partners are routinely subject to patent infringement suits related to our Generics Division products, including as of February 16, 2016, one related to oxymorphone hydrochloride tablets. If this or any of our future patent litigation matters involving generic products are resolved unfavorably, we or our alliance or collaboration partners may be enjoined from manufacturing, developing or selling the generic product that is the subject of such litigation without a license from the other party. In addition, if we decide to market and sell generic products prior to the resolution of patent infringement suits, we could be held liable for lost profits if we are found to have infringed a valid patent, or liable for treble damages if we are found to have willfully infringed a valid patent. In our branded products division, as of February 16, 2016, we were involved in two patent infringement suits, one related to Zomig® nasal spray and the other related to Rytary®. If these patent litigation matters involving our branded products are resolved unfavorably, our Zomig® nasal spray product and/or Rytary® may face generic competition significantly earlier than the date of patent expirations for the products. We have incurred substantial expense to defend the foregoing patent litigation suits; during fiscal year 2015, we incurred costs of approximately $4.6 million in connection with our participation in the patent litigation matters described above, as well as for other matters that were resolved in 2015. Although it is not currently possible to quantify the liability we could incur if any of the above referenced patent litigation suits are decided against us, any unfavorable outcome on such matters could have a material adverse effect on our business, results of operations and financial condition.
In addition to patent infringement litigation claims, we are or may become a party to other litigation in the ordinary course of our business, including, among others, matters alleging product liability, other intellectual property rights infringement, violations of securities laws, employment discrimination or breach of commercial contract. A detailed description of our significant legal proceedings are described in “Item 15. Exhibits and Financial Statement Schedules – Note 22. Legal and Regulatory Matters.” In general, litigation claims can be expensive and time consuming to bring or defend against and could result in settlements or damages that could have a material adverse effect on our business, results of operations and financial condition.
Our agreements with brand pharmaceutical companies, which are important to our business, are facing increased government scrutiny in the United States, which may result in increased government actions and private litigation suits.
We are involved in numerous patent litigations in which we challenge the validity or enforceability of innovator companies’ listed patents and/or their applicability to our generic pharmaceutical products, as well as patent infringement litigation in which generic companies challenge the validity or enforceability of our patents and/or their applicability to their generic pharmaceutical products, and therefore settling patent litigations has been and is likely to continue to be an important part of our business. Parties to such settlement agreements in the United States, including us, are required by law to file them with the Federal Trade Commission (“FTC”) and the Antitrust Division of the Department of Justice for review. The FTC has publicly stated that, in its view, some of the brand - generic settlement agreements violate the antitrust laws and has brought actions against some brand and generic companies that have entered into such agreements. In June 2013, the U.S. Supreme Court in its decision in FTC v. Actavis determined that “reverse payment” settlement agreements between brand and generic companies could violate antitrust laws. The Supreme Court held that such settlement agreements are neither immune from antitrust attack nor presumptively illegal but rather should be analyzed under the “Rule of Reason.” It is currently uncertain the effect the Supreme Court’s decision will have on our existing settlement agreements or its impact on our ability to enter into such settlement agreements in the future or the terms thereof. The Supreme Court’s decision may result in heightened scrutiny from the FTC of such settlement agreements and we may become subject to increased FTC investigations or enforcement actions arising from such settlement agreements. Further, private plaintiffs, including direct and indirect purchasers of our products, may also become more active in bringing private litigation claims against us and other brand and generic pharmaceutical companies alleging that such settlement agreements violate antitrust laws.
In May 2012, we received a Civil Investigative Demand (“CID”) from the FTC concerning its investigation into the drug SOLODYN® and its generic equivalents. According to the FTC, the investigation was to determine whether we, along with Medicis Pharmaceutical Corporation (now a wholly owned subsidiary of Valeant Pharmaceuticals International, Inc.) and six other companies, had engaged or were engaged in unfair methods of competition in or affecting commerce by entering into agreements regarding SOLODYN® or its generic equivalents and/or engaging in other conduct regarding the sale or marketing of SOLODYN® or its generic equivalents. On November 6, 2015, the FTC issued a letter to us indicting that it had closed its investigation of the SOLODYN® agreements with no further action by the FTC. In February 2014, we received a CID from the FTC concerning its investigation into the drug Opana® ER and its generic equivalents. According to the FTC, the investigation relates to whether we and Endo Pharmaceuticals, Inc. have engaged or are engaged in unfair methods of competition in or affecting commerce by (i) entering into agreements regarding Opana® ER or its generic equivalents and/or (ii) engaging in other conduct regarding the regulatory filings, sale or marketing of Opana® ER or its generic equivalents. The FTC’s investigation related to Opana® ER currently remains open. To our knowledge, no proceedings by the FTC have been initiated against us at this time in the FTC’s investigation into Opana® ER; however, no assurance can be given as to the timing or outcome of such investigation.
Private plaintiffs have also filed class action complaints against us and other manufacturers of SOLODYN®, Opana® ER and their respective generic equivalents. A detailed description of the SOLODYN® and Opana® ER FTC investigations and class action suits are described in “Item 15. Exhibits and Financial Statement Schedules – Note 22. Legal and Regulatory Matters.” The defense of antitrust litigation investigation and claims are generally expensive and time consuming, and we can give no assurance as to the timing or outcome of such investigation or claims or of any future private litigation or government action alleging that one of our settlement agreements violates antitrust laws.
Our ability to develop or license, or otherwise acquire, and introduce new products on a timely basis in relation to our competitors’ product introductions involves inherent risks and uncertainties.
Product development is inherently risky, especially for new drugs for which safety and efficacy have not been established and the market is not yet proven. Likewise, product licensing involves inherent risks including uncertainties due to matters that may affect the achievement of milestones, as well as the possibility of contractual disagreements with regard to terms such as license scope or termination rights. The development and commercialization process, particularly with regard to new drugs, also requires substantial time, effort and financial resources. The process of obtaining FDA approval to manufacture and market new pharmaceutical products is rigorous, time consuming, costly and largely unpredictable. We, or a partner, may not be successful in obtaining FDA approval or in commercializing any of the products that we are developing or licensing.
Our approved products may not achieve expected levels of market acceptance.
Even if we are able to obtain regulatory approvals for our new products, the success of those products is dependent upon market acceptance. Levels of market acceptance for our new products could be affected by several factors, including:
|
● |
the availability of alternative products from our competitors; |
|
● |
the prices of our products relative to those of our competitors; |
|
● |
the timing of our market entry; |
|
● |
the ability to market our products effectively at the retail level; |
|
● |
the perception of patients and the healthcare community, including third-party payers, regarding the safety, efficacy and benefits of our drug products compared to those of competing products; and |
|
● |
the acceptance of our products by government and private formularies. |
Some of these factors are not within our control, and our products may not achieve expected levels of market acceptance. Additionally, continuing and increasingly sophisticated studies of the proper utilization, safety and efficacy of pharmaceutical products are being conducted by the industry, government agencies and others which can call into question the utilization, safety and efficacy of previously marketed products. In some cases, studies have resulted, and may in the future result, in the discontinuance of product marketing or other risk management programs such as the need for a patient registry.
Our business is highly dependent on market perceptions of us and the safety and quality of our products. Our business or products could be subject to negative publicity, which could have a material adverse effect on our business, results of operations and financial condition.
Market perceptions of our business are very important to us, especially market perceptions of the safety and quality of our products. If any of our products or similar products that other companies distribute are subject to market withdrawal or recall or are proven to be, or are claimed to be, harmful to consumers, then this could have a material adverse effect on our business, results of operations and financial condition. Also, because our business is dependent on market perceptions, negative publicity associated with product quality, illness or other adverse effects resulting from, or perceived to be resulting from, our products could have a material adverse impact on our business, results of operations and financial condition.
We may discontinue the manufacture and distribution of certain existing products, which may adversely impact our business, results of operations and financial condition.
We continually evaluate the performance of our products, and may determine that it is in our best interest to discontinue the manufacture and distribution of certain of our products. We cannot guarantee that we have correctly forecasted, or will correctly forecast in the future, the appropriate products to discontinue or that our decision to discontinue various products is prudent if market conditions change. In addition, we cannot assure you that the discontinuance of products will reduce our operating expenses or will not cause us to incur material charges associated with such a decision. Furthermore, the discontinuance of existing products entails various risks, including, in the event that we decide to sell the discontinued product, the risk that we will not be able to find a purchaser for such products or that the purchase price obtained will not be equal to at least the book value of the net assets for such products. Other risks include managing the expectations of, and maintaining good relations with, our customers who previously purchased products from our discontinued products, which could prevent us from selling other products to them in the future. Moreover, we may incur other significant liabilities and costs associated with our discontinuance of products, which could have a material adverse effect on our business, results of operations and financial condition.
We expend a significant amount of resources on research and development efforts that may not lead to successful product introductions or the recovery of our research and development expenditures.
We conduct research and development primarily to enable us to manufacture and market pharmaceuticals in accordance with FDA regulations. We spent approximately $77.0 million, $78.6 million and $68.9 million on research and development activities during the years ended December 31, 2015, 2014 and 2013, respectively. We are required to obtain FDA approval before marketing our drug products. The FDA approval process is costly and time consuming. Typically, research expenses related to the development of innovative products and the filing of NDAs are significantly greater than those expenses associated with ANDAs. As we continue to develop new products, our research expenses will likely increase. Because of the inherent risk associated with research and development efforts in our industry, our research and development expenditures may not result in the successful introduction of FDA-approved pharmaceuticals.
Our bioequivalence studies, other clinical studies and/or other data may not result in FDA approval to market our new drug products. While we believe that the FDA’s ANDA procedures will apply to our bioequivalent versions of branded drugs, these drugs may not be suitable for, or approved as part of, these abbreviated applications. In addition, even if our drug products are suitable for FDA approval by filing an ANDA, the abbreviated applications are costly and time consuming to complete. After we submit an NDA or ANDA, the FDA may require that we conduct additional studies, and as a result, we may be unable to reasonably determine the total research and development costs to develop a particular product. Also, for products pending approval, we may obtain raw materials or produce batches of inventory to be used in anticipation of the product’s launch. In the event that FDA approval is denied or delayed, we could be exposed to the risk of this inventory becoming obsolete. Finally, we cannot be certain that any investment made in developing products or product-delivery technologies will be recovered, even if we are successful in commercialization. To the extent that we expend significant resources on research and development efforts and are not able, ultimately, to introduce successful new products or new delivery technologies as a result of those efforts, we will be unable to recover those expenditures.
The time necessary to develop generic drugs may adversely affect whether, and the extent to which, we receive a return on our capital.
We generally begin our development activities for a new generic drug product several years in advance of the patent expiration date of the brand-name drug equivalent. The development process, including drug formulation, testing, and FDA review and approval, often takes three or more years. This process requires that we expend considerable capital to pursue activities that do not yield an immediate or near-term return. Also, because of the significant time necessary to develop a product, the actual market for a product at the time it is available for sale may be significantly less than the originally projected market for the product. If this were to occur, our potential return on our investment in developing the product, if approved for marketing by the FDA, would be adversely affected and we may never receive a return on our investment in the product. It is also possible for the manufacturer of the brand-name product for which we are developing a generic drug to obtain approvals from the FDA to switch the brand-name drug from the prescription market to the OTC market. If this were to occur, we would be prohibited from marketing our product other than as an OTC drug, in which case revenues could be substantially less than we anticipated.
Research and development efforts invested in our branded pharmaceutical products may not achieve expected results.
We invest increasingly significant resources to develop our branded products, both through our own efforts and through collaborations, in-licensing and acquisition of products from or with third parties. The development of proprietary branded drugs involves processes and expertise different from those used in the development of generic products, which increases the risks of failure that we face. For example, the time from discovery to commercial launch of a branded product can be 15 years or even longer, and involves multiple stages: not only intensive preclinical and clinical testing, but also highly complex, lengthy and expensive approval processes which can vary from country to country. The longer it takes to develop a product, the longer time it may take for us to recover our development costs and generate profits, if at all.
During each development stage, we may encounter obstacles that delay the process or approval and increase expenses, leading to significant risks that we will not achieve our goals and may be forced to abandon a potential product in which we have invested substantial amounts of time and money. These obstacles may include: preclinical failures; difficulty enrolling patients in clinical trials; delays in completing formulation and other work needed to support an application for approval; adverse reactions or other safety concerns arising during clinical testing; insufficient clinical trial data to support the safety or efficacy of the product candidate; and failure to obtain, or delays in obtaining, the required regulatory approvals for the product candidate or the facilities in which it is manufactured. For instance, during 2013, we discontinued our branded pharmaceutical development programs for the potential treatment of moderate to severe Restless Leg Syndrome (“RLS”) after the results from the study did not achieve the statistical criteria for its primary efficacy endpoints compared to placebo and our program for the potential treatment of epilepsy as a result of technical and competitive factors. As a result of the obstacles noted above, our investment in research and development of branded products can involve significant costs with no assurances of future revenues or profits.
Approvals for our new generic drug products may be delayed or become more difficult to obtain if the FDA institutes changes to its approval requirements.
The FDA may institute changes to its ANDA approval requirements, which may make it more difficult or expensive for us to obtain approval for our new generic products. For instance, in July 2012, the Generic Drug Fee User Amendments of 2012 (“GDUFA”) was enacted into law. The GDUFA legislation implemented fees for new ANDAs, Drug Master Files, product and establishment fees and a one-time fee for back-logged ANDAs pending approval as of October 1, 2012. In return, the program is intended to provide faster and more predictable ANDA reviews by the FDA and increased inspections of drug facilities. Under GDUFA, generic product companies face significant penalties for failure to pay the new user fees, including rendering an ANDA not “substantially complete” until the fee is paid. Any failure by us or our suppliers to pay the fees or to comply with the other provisions of GDFUA may impact or delay our ability to file ANDAs, obtain approvals for new generic products, generate revenues and thus may have a material adverse effect on our business, results of operations and financial condition.
In addition to the implementation of new fees and review procedures by the FDA, the FDA may also implement other changes that may directly affect some of our ANDA filings pending approval from the FDA, such as changes to guidance from the FDA regarding bioequivalency requirements for particular drugs. Such changes may cause our development of such generic drugs to be significantly more difficult or result in delays in FDA approval or result in our decision to abandon or terminate certain projects. Any changes in FDA requirements may make it more difficult for us to file ANDAs or obtain approval of our ANDAs and generate revenues and thus have a material adverse effect on our business, results of operations and financial condition.
The risks and uncertainties inherent in conducting clinical trials could delay or prevent the development and commercialization of our own branded products, which could have a material adverse effect on our business, results of operations and financial condition.
With respect to our branded products which do not qualify for the FDA’s abbreviated application procedures, we must demonstrate through clinical trials that these products are safe and effective for use. We have only limited experience in conducting and supervising clinical trials. The process of completing clinical trials and preparing an NDA may take several years and requires substantial resources. Our studies and filings may not result in FDA approval to market our new drug products and, if the FDA grants approval, we cannot predict the timing of any approval. There are substantial filing fees for NDAs that are not refundable if FDA approval is not obtained.
There are a number of risks and uncertainties associated with clinical trials. The results of clinical trials may not be indicative of results that would be obtained from large scale testing. Clinical trials are often conducted with patients having advanced stages of disease and, as a result, during the course of treatment these patients can die or suffer adverse medical effects for reasons that may not be related to the pharmaceutical agents being tested, but which nevertheless affect the clinical trial results. In addition, side effects experienced by the patients may cause delay of approval or limit the profile of an approved product. Moreover, our clinical trials may not demonstrate sufficient safety and efficacy to obtain approval from the FDA or foreign regulatory authorities. The FDA or foreign regulatory authorities may not agree with our assessment of the clinical data or they may interpret it differently. Such regulatory authorities may require additional or expanded clinical trials. Even if the FDA or foreign regulatory authorities approve certain products developed by us, there is no assurance that such regulatory authorities will not subject marketing of such products to certain limits on indicated use.
Failure can occur at any time during the clinical trial process and, in addition, the results from early clinical trials may not be predictive of results obtained in later and larger clinical trials, and product candidates in later clinical trials may fail to show the desired safety or efficacy despite having progressed successfully through earlier clinical testing. A number of companies in the pharmaceutical industry, including us, have suffered significant setbacks in clinical trials, even in advanced clinical trials after showing positive results in earlier clinical trials. For example, we had previously sought to develop an earlier product formulation containing carbidopa/levodopa for the treatment of Parkinson’s disease. Following completion of the clinical trials and submission of the NDA, the NDA was not approved due to the FDA’s concerns over product nomenclature and the potential for medication errors. In early 2013, we discontinued our branded pharmaceutical development program for IPX159, an oral controlled-release formulation for the potential treatment of moderate to severe RLS, after the results from the clinical study in patients did not achieve the statistical criteria for its primary efficacy endpoints compared to placebo. In the future, the completion of clinical trials for our product candidates may be delayed or halted for the reasons noted above in addition to many other reasons, including:
|
● |
delays in patient enrollment, and variability in the number and types of patients available for clinical trials; |
|
● |
regulators or institutional review boards may not allow us to commence or continue a clinical trial; |
|
● |
our inability, or the inability of our partners, to manufacture or obtain from third parties materials sufficient to complete our clinical trials; |
|
● |
delays or failure in reaching agreement on acceptable clinical trial contracts or clinical trial protocols with prospective clinical trial sites; |
|
● |
risks associated with trial design, which may result in a failure of the trial to show statistically significant results even if the product candidate is effective; |
|
● |
difficulty in maintaining contact with patients after treatment commences, resulting in incomplete data; |
|
● |
poor effectiveness of product candidates during clinical trials; |
|
● |
safety issues, including adverse events associated with product candidates; |
|
● |
the failure of patients to complete clinical trials due to adverse side effects, dissatisfaction with the product candidate, or other reasons; |
|
● |
governmental or regulatory delays or changes in regulatory requirements, policy and guidelines; and |
|
● |
varying interpretation of data by the FDA or foreign regulatory authorities. |
In addition, our product candidates could be subject to competition for clinical study sites and patients from other therapies under development which may delay the enrollment in or initiation of our clinical trials.
The FDA or foreign regulatory authorities may require us to conduct unanticipated additional clinical trials, which could result in additional expense and delays in bringing our product candidates to market. Any failure or delay in completing clinical trials for our product candidates would prevent or delay the commercialization of our product candidates. We cannot assure that our expenses related to clinical trials will lead to the development of brand-name drugs that will generate revenues in the near future. Delays or failure in the development and commercialization of our own branded products could have a material adverse effect on our business, results of operations and financial condition.
We rely on third parties to conduct clinical trials and testing for our product candidates, and if they do not properly and successfully perform their legal and regulatory obligations, as well as their contractual obligations to us, we may not be able to obtain regulatory approvals for our product candidates.
We design the clinical trials for our product candidates, but rely on contract research organizations and other third parties to assist us in managing, monitoring and otherwise carrying out these trials, including with respect to site selection, contract negotiation, analytical testing and data management. We do not control these third parties and, as a result, they may not treat our clinical studies as their highest priority, or in the manner in which we would prefer, which could result in delays.
Although we rely on third parties to conduct our clinical trials and related activities, we are responsible for confirming that each of our clinical trials is conducted in accordance with our general investigational plan and protocol. Moreover, the FDA and foreign regulatory agencies require us to comply with regulations and standards, commonly referred to as good clinical practices and good laboratory practices, for conducting, recording and reporting the results of clinical trials to ensure that the data and results are credible and accurate and that the trial participants are adequately protected. Our reliance on third parties does not relieve us of these responsibilities and requirements. The FDA enforces good clinical practices and good laboratory practices through periodic inspections of trial sponsors, principal investigators and trial sites. If we, our contract research organizations or our study sites fail to comply with applicable good clinical practices and good laboratory practices, the clinical data generated in our clinical trials may be deemed unreliable and the FDA may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, the FDA will determine that any of our clinical trials comply with good clinical practices and good laboratory practices. In addition, our clinical trials must be conducted with product manufactured under the FDA’s current Good Manufacturing Practices, or cGMP, regulations. Our failure or the failure of our contract manufacturers if any are involved in the process, to comply with these regulations may require us to repeat clinical trials, which would delay the regulatory approval process.
If third parties do not successfully carry out their duties under their agreements with us, if the quality or accuracy of the data they obtain is compromised due to failure to adhere to our clinical protocols or regulatory requirements, or if they otherwise fail to comply with clinical trial protocols or meet expected deadlines, our clinical trials may not meet regulatory requirements. If our clinical trials do not meet regulatory requirements or if these third parties need to be replaced, our clinical trials may be extended, delayed, suspended or terminated. If any of these events occur, we may not be able to obtain regulatory approval of our product candidates, which could have a material adverse effect on our business, results of operations and financial condition.
We currently do not have a license partner for commercialization of NUMIENT™ outside of the United States.
In November 2015, the European Commission granted marketing authorization for NUMIENT™ (IPX066) (referred to as Rytary® in the United States). The review of the NUMIENT™ application was conducted under the centralized licensing procedure as a therapeutic innovation, and the authorization is applicable in all 28 member states of the European Union, as well as Iceland, Liechtenstein and Norway. To date, we have not launched commercialization activities for NUMIENT™ outside of the United States and we do not currently have a license partner for the commercialization of the product outside of the United States. We previously were a party to a License, Development and Commercialization Agreement with Glaxo Group Limited (“GSK”) dated December 15, 2010 whereby GSK received an exclusive license to develop and commercialize IPX066 throughout the world, except in the United States and Taiwan, and certain follow-on products at the option of GSK. The License, Development and Commercialization Agreement with GSK was subsequently terminated and GSK’s rights to develop and commercialize the product outside the United States and Taiwan were transferred back to us in 2013. We are currently engaged in discussions with potential partners to market NUMIENT™ outside of the United States; however, no assurances can be made that we will find such a partner. If we are unsuccessful in entering into such third party collaboration arrangements for ex-United States commercialization activities of NUMIENT™, such failure could have a material adverse effect on our business, results of operations and financial condition.
The illegal distribution and sale by third parties of counterfeit versions of our products or of stolen products could have a negative impact on our reputation and a material adverse effect on our business, results of operations and financial condition.
Third parties could illegally distribute and sell counterfeit versions of our products, which do not meet the rigorous manufacturing and testing standards that our products undergo. Counterfeit products are frequently unsafe or ineffective, and can be life-threatening. Counterfeit medicines may contain harmful substances, the wrong dose of the active pharmaceutical ingredient or no active pharmaceutical ingredients at all. However, to distributors and users, counterfeit products may be visually indistinguishable from the authentic version.
Reports of adverse reactions to counterfeit drugs or increased levels of counterfeiting could materially affect patient confidence in the authentic product. It is possible that adverse events caused by unsafe counterfeit products will mistakenly be attributed to the authentic product. In addition, thefts of inventory at warehouses, plants or while in-transit, which are not properly stored and which are sold through unauthorized channels could adversely impact patient safety, our reputation and our business.
Public loss of confidence in the integrity of pharmaceutical products as a result of counterfeiting or theft could have a material adverse effect on our business, results of operations and financial condition.
We are dependent on a small number of suppliers for our raw materials that we use to manufacture our products and interruptions in our supply chain could materially and adversely affect our business.
We typically purchase the ingredients, other materials and supplies that we use in the manufacturing of our products, as well as certain finished products, from a small number of foreign and domestic suppliers. The FDA requires identification of raw material suppliers in applications for approval of drug products. If raw materials were unavailable from a specified supplier or the supplier was not in compliance with FDA or other applicable requirements, the FDA approval of a new supplier could delay the manufacture of the drug involved. As a result, there is no guarantee we will always have timely and sufficient access to a required raw material or other product. In addition, some materials used in our products are currently available from only one supplier or a limited number of suppliers. Generally, we would need as long as 18 months to find and qualify a new sole-source supplier. If we receive less than one year’s termination notice from a sole-source supplier that it intends to cease supplying raw materials, it could result in disruption of our ability to produce the drug involved. Further, a significant portion of our raw materials may be available only from foreign sources. Foreign sources can be subject to the special risks of doing business abroad, including:
|
● |
greater possibility for disruption due to transportation or communication problems; |
|
● |
the relative instability of some foreign governments and economies; |
|
● |
interim price volatility based on labor unrest, materials or equipment shortages, export duties, restrictions on the transfer of funds, or fluctuations in currency exchange rates; and |
|
● |
uncertainty regarding recourse to a dependable legal system for the enforcement of contracts and other rights. |
Those of our raw materials that are available from a limited number of suppliers include Bendroflumethiazide, Chloroquine Phosphate, Colestipol, Digoxin, Fenofibrate, Methyltestosterone, Nadolol and Pyridostigmin, all of which are active pharmaceutical ingredients. The manufacturers of several of these products are sole-source suppliers. Only a couple of our active ingredients are covered by long-term supply agreements and, although we have to date only experienced occasional interruptions in supplies, we cannot assure you that we will continue to receive uninterrupted or adequate supplies of such raw materials.
Many third-party suppliers are subject to governmental regulation and, accordingly, we are dependent on the regulatory compliance of these third parties. We also depend on the strength, enforceability and terms of our various contracts with these third-party suppliers. We also rely on complex shipping arrangements throughout the various facilities of our supply chain spectrum. Customs clearance and shipping by land, air or sea routes rely on and may be affected by factors that are not in our full control or are hard to predict.
Any inability to obtain raw materials on a timely basis, or any significant price increases which cannot be passed on to customers, could have a material adverse effect on our business, results of operations and financial condition.
Our policies regarding returns, rebates, allowances and chargebacks, and marketing programs adopted by wholesalers may reduce our revenues in future fiscal periods.
Based on industry practice, generic drug manufacturers have liberal return policies and have been willing to give customers post-sale inventory allowances. Under these arrangements, from time to time, we give our customers credits on our generic products that our customers hold in inventory after we have decreased the market prices of the same generic products due to competitive pricing. Therefore, if new competitors enter the marketplace and significantly lower the prices of any of their competing products, we would likely reduce the price of our product. As a result, we would be obligated to provide credits to our customers who are then holding inventories of such products, which could reduce sales revenue and gross margin for the period the credit is provided. Like our competitors, we also give credits for chargebacks to wholesalers that have contracts with us for their sales to hospitals, group purchasing organizations, pharmacies or other customers. A chargeback is the difference between the price the wholesaler pays and the price that the wholesaler’s end-customer pays for a product. Although we establish reserves based on our prior experience and our best estimates of the impact that these policies may have in subsequent periods, we cannot ensure that our reserves are adequate or that actual product returns, rebates, allowances and chargebacks will not exceed our estimates.
Certain of our products use controlled substances, the availability of which may be limited by the DEA and other regulatory agencies.
We utilize controlled substances in certain of our current products and products in development and therefore must meet the requirements of the Controlled Substances Act of 1970 and the related regulations administered by the DEA in the United States. These laws relate to the manufacture, shipment, storage, sale and use of controlled substances. The DEA and other regulatory agencies limit the availability of the active ingredients used in certain of our current products and products in development and, as a result, our procurement quota of these active ingredients may not be sufficient to meet commercial demand or complete clinical trials. We must annually apply to the DEA for procurement quota in order to obtain these substances. Any delay or refusal by the DEA in establishing our procurement quota for controlled substances could delay or stop our clinical trials or product launches, or could cause trade inventory disruptions for those products that have already been launched, which could have a material adverse effect on our business, results of operations and financial condition.
Unstable economic conditions may adversely affect our industry, business, results of operations and financial condition.
The global economy has undergone a period of significant volatility which has led to diminished credit availability, declines in consumer confidence and increases in unemployment rates. There remains caution about the stability of the U.S. economy, and we cannot assure that further deterioration in the financial markets will not occur. These economic conditions have resulted in, and could lead to further, reduced consumer spending related to healthcare in general and pharmaceutical products in particular.
In addition, we have exposure to many different industries and counterparties, including our partners under our alliance and collaboration agreements, suppliers of raw chemical materials, drug wholesalers and other customers that may be affected by an unstable economic environment. Any economic instability may affect these parties’ ability to fulfill their respective contractual obligations to us, cause them to limit or place burdensome conditions upon future transactions with us or drive us and our competitors to decrease prices, each of which could materially and adversely affect our business, results of operations and financial condition.
Furthermore, the capital and credit markets have experienced extreme volatility. Disruptions in the credit markets make it harder and more expensive to obtain funding. In the event current resources do not satisfy our needs, we may have to seek additional financing. The availability of additional financing will depend on a variety of factors such as market conditions and the general availability of credit. Future debt financing may not be available to us when required or may not be available on acceptable terms, and as a result we may be unable to grow our business, take advantage of business opportunities, or respond to competitive pressures.
We may be subject to disruptions or failures in our information technology systems and network infrastructures that could have a material adverse effect on our business.
We rely on the efficient and uninterrupted operation of complex information technology systems and network infrastructures to operate our business. We also hold data in various data center facilities upon which our business depends. A disruption, infiltration or failure of our information technology systems or any of our data centers as a result of software or hardware malfunctions, system implementations or upgrades, computer viruses, third-party security breaches, employee error, theft or misuse, malfeasance, power disruptions, natural disasters or accidents could cause breaches of data security, loss of intellectual property and critical data and the release and misappropriation of sensitive competitive information. Any of these events could result in the loss of key information, impair our production and supply chain processes, harm our competitive position, cause us to incur significant costs to remedy any damages and ultimately materially and adversely affect our business, results of operations and financial condition.
While we have implemented a number of protective measures, including firewalls, antivirus, patches, data encryption, log monitors, routine back-ups with offsite retention of storage media, system audits, data partitioning, routine password modifications and disaster recovery procedures, such measures may not be adequate or implemented properly to prevent or fully address the adverse effect of such events.
We may be adversely affected by alliance, collaboration, supply, or license and distribution agreements we enter into with other companies.
We have entered into several alliance, collaboration, supply or license and distribution agreements with respect to certain of our products and services and may enter into similar agreements in the future. These arrangements may require us to relinquish rights to certain of our technologies or product candidates, or to grant licenses on terms that ultimately may prove to be unfavorable to us. Relationships with alliance partners may also include risks due to regulatory requirements, incomplete marketplace information, inventories, and commercial strategies of our partners, and our agreements may be the subject of contractual disputes. If we or our partners are not successful in commercializing the products covered by the agreements, such commercial failure could adversely affect our business.
Pursuant to license and distribution agreements with unrelated third party pharmaceutical companies, we are dependent on such companies to supply us with product that we market and sell, and we may enter into similar agreements in the future. Any delay or interruption in the supply of product under such agreements could curtail or delay our product shipment and adversely affect our revenues, as well as jeopardize our relationships with our customers.
From time to time we may need to rely on licenses to proprietary technologies, which may be difficult or expensive to obtain.
We may need to obtain licenses to patents and other proprietary rights held by third parties to develop, manufacture and market products. If we are unable to timely obtain these licenses on commercially reasonable terms, our ability to commercially market our products may be inhibited or prevented, which could have a material adverse effect on our business, results of operations and financial condition.
We depend on qualified scientific and technical employees and are increasingly dependent on our direct sales force, and our limited resources may make it more difficult to attract and retain these personnel.
Because of the specialized scientific nature of our business, we are highly dependent upon our ability to continue to attract and retain qualified scientific and technical personnel. We are not aware of any pending, significant losses of scientific or technical personnel. Loss of the services of, or failure to recruit, key scientific and technical personnel, however, would be significantly detrimental to our product-development programs. As a result of our small size and limited financial and other resources, it may be difficult for us to attract and retain qualified officers and qualified scientific and technical personnel.
In addition, marketing of our branded products, such as the Zomig® products pursuant to our AZ Agreement with AstraZeneca and Rytary®, requires much greater use of a direct sales force compared to marketing of our generic products. Our ability to realize significant revenues from marketing and sales activities depends on our ability to attract and retain qualified sales personnel. Competition for qualified sales personnel is intense. Any failure to attract or retain qualified sales personnel could negatively impact our sales revenue and have a material adverse effect on our business, results of operations and financial condition.
We have entered into employment agreements with our executive officers and certain other key employees. Under the employment agreements, the employee may terminate his or her employment upon 60 days prior written notice to us. All of our other key personnel are employed on an at-will basis with no formal employment agreements. We do not maintain “Key Man” life insurance on any executives.
We are subject to significant costs and uncertainties related to compliance with the extensive regulations that govern the manufacturing, labeling, distribution, promotion and sale of pharmaceutical products as well as environmental, safety and health regulations.
The manufacturing, distribution, processing, formulation, packaging, labeling, promotion and sale of our products are subject to extensive regulation by federal agencies, including the FDA, DEA, FTC, Consumer Product Safety Commission and Environmental Protection Agency, among others. We are also subject to state and local laws, regulations and agencies in California, New Jersey, Pennsylvania and elsewhere, as well as the laws and regulations of Taiwan. Such regulations are also subject to change by the relevant federal, state and international agencies. For instance, beginning from January 1, 2015, manufacturers, wholesale distributors, and repackagers of certain prescription drugs are required to provide and capture certain product tracing information under the Drug Quality and Security Act (“DQSA”). Title II of the DQSA, referred to as the Drug Supply Chain Security Act, requires companies in certain prescription drugs’ chain of distribution to build electronic, interoperable systems to identify and trace the products as they are distributed in the United States. Compliance with the DQSA or any future federal or state electronic pedigree requirements may increase the Company's operational expenses and impose significant administrative burdens.
Regulatory agencies such as the FDA regularly inspect our manufacturing facilities and the facilities of our third party suppliers. The failure of one of our facilities, or a facility of one of our third party suppliers, to comply with applicable laws and regulations may lead to breach of representations made to our customers or to regulatory or government action against us related to products made in that facility. As discussed above, we have in the past received a warning letter and Form 483 observations from the FDA regarding certain operations within our manufacturing network. During 2015, we successfully resolved the warning letter and we also received the EIRs for each of the FDA inspections resulting in the Form 483 indicating acceptance of our corrective actions and closure by the FDA of each inspection. Although we remain committed to continuing to improve our quality control and manufacturing practices, we cannot be assured that the FDA will continue to be satisfied with our corrective actions and with our quality control and manufacturing systems and standards. If we receive any future FDA observations, we may be subject to regulatory action including, among others, monetary sanctions or penalties, product recalls or seizure, injunctions, total or partial suspension of production and/or distribution, and suspension or withdrawal of regulatory approvals. Further, other federal agencies, our customers and partners in our alliance, development, collaboration and other partnership agreements with respect to our products and services may take any such Form 483 observations or warning letters into account when considering the award of contracts or the continuation or extension of such partnership agreements. If we receive any future Form 483 observations or warning letters from the FDA, our business, consolidated results of operations and consolidated financial condition could be materially and adversely affected.
With respect to environmental, safety and health laws and regulations, we cannot accurately predict the outcome or timing of future expenditures that we may be required to make in order to comply with such laws as they apply to our operations and facilities. We are also subject to potential liability for the remediation of contamination associated with both present and past hazardous waste generation, handling, and disposal activities. We are subject periodically to environmental compliance reviews by environmental, safety, and health regulatory agencies. Environmental laws are subject to change and we may become subject to stricter environmental standards in the future and face larger capital expenditures in order to comply with environmental laws.
Compliance with federal and state and local law regulations, including compliance with any newly enacted regulations, requires substantial expenditures of time, money and effort to ensure full technical compliance. Failure to comply with the FDA, EPA and other governmental regulations can result in fines, disgorgement, unanticipated compliance expenditures, recall or seizure of products, exposure to product liability claims, total or partial suspension of production or distribution, suspension of the FDA’s review of NDAs or ANDAs, enforcement actions, injunctions and civil or criminal prosecution, any of which could have a material and adverse effect on our business, results of operations and financial condition.
We may experience reductions in the levels of reimbursement for pharmaceutical products by governmental authorities, HMOs or other third-party payers. Any such reductions could have a material adverse effect on our business, results of operations and financial condition.
Various governmental authorities and private health insurers and other organizations, such as HMOs, provide reimbursement to consumers for the cost of certain pharmaceutical products. Demand for our products depends in part on the extent to which such reimbursement is available. In addition, third-party payers are attempting to control costs by limiting the level of reimbursement for medical products, including pharmaceuticals, and increasingly challenge the pricing of these products which may adversely affect the pricing of our products. Moreover, health care reform has been, and is expected to continue to be, an area of national and state focus, which could result in the adoption of measures that could adversely affect the pricing of pharmaceuticals or the amount of reimbursement available from third-party payers for our products.
Reporting and payment obligations under the Medicaid rebate program and other government programs are complex, and failure to comply could result in sanctions and penalties or we could be required to reimburse the government for underpayments, which could have a material adverse effect on our business.
Medicaid and other government reporting and payment obligations are highly complex and somewhat ambiguous. State attorneys general and the U.S. Department of Justice have brought suits or instituted investigations against a number of other pharmaceutical companies for failure to comply with Medicaid and other government reporting obligations. Our methodologies for making these calculations are complex and the judgments involved require us to make subjective decisions, such that these calculations are subject to the risk of errors. Government agencies may impose civil or criminal sanctions, including fines, penalties and possible exclusion from federal health care programs, including Medicaid and Medicare. Any such penalties or sanctions could have a material adverse effect on our business, results of operations and financial condition.
Legislative or regulatory programs that may influence prices of prescription drugs could have a material adverse effect on our business.
Current or future federal or state laws and regulations may influence the prices of drugs and, therefore, could adversely affect the prices that we receive for our products. Programs in existence in certain states seek to set prices of all drugs sold within those states through the regulation and administration of the sale of prescription drugs. Expansion of these programs, in particular, state Medicaid programs, or changes required in the way in which Medicaid rebates are calculated under such programs, could adversely affect the price we receive for our products and could have a material adverse effect on our business, results of operations and financial condition. Further, prescription drug prices have been the focus of increased scrutiny by the government, including certain state attorneys general, members of congress and the U.S. Department of Justice. Decreases in health care reimbursements or prices of our prescription drugs could limit our ability to sell our products or decrease our revenues, which could have a material adverse effect on our business, results of operations and financial condition.
Our failure to comply with the legal and regulatory requirements governing sales, marketing and pricing of our products may result in substantial fines, sanctions and restrictions on our business activities.
Our practices and activities related to the sales and marketing of our products, as well as the pricing of our products, are subject to extensive regulation under U.S. federal and state healthcare statutes and regulations intended to combat fraud and abuse to federal and state healthcare payment programs, such as Medicare and Medicaid, Tri-Care, CHAMPUS, and Department of Defense programs. These laws include the federal Anti-Kickback Statute, the federal False Claims Act, and similar state laws and implementing regulations. For example, the payment of any incentive to a healthcare provider to induce the recommendation of our product or the purchase of our products reimbursable under a federal or state program is prohibited under these laws. Likewise, knowingly presenting or causing to be presented a false claim for payment to a federal or state health care program would expose a company to sanctions and penalties. Similarly, the inaccurate reporting of prices leading to inflated reimbursement rates would also be considered a violation of these laws. The Physician Payment Sunshine Act enacted in 2010 imposes reporting and disclosure requirements on drug manufacturers for any “transfer of value” made or distributed to prescribers and other healthcare providers. Failure to submit this required information may result in significant civil monetary penalties. These laws and regulations are enforced by the U.S. Department of Justice, the U.S. Department of Health and Human Services, Office of Inspector General, state Medicaid Fraud Units and other state enforcement agencies.
Violations of the laws and regulations described above are punishable by criminal and civil sanctions, including substantial fines and penal sanctions, such as imprisonment. It is common for enforcement agencies to initiate investigations into sales and marketing practices, as well as pricing practices, regardless of merit. These types of investigations and any related litigation can result in: (i) large expenditures of cash for legal fees, payment for penalties, and compliance activities; (ii) limitations on operations; (iii) diversion of management resources; (iv) injury to our reputation; and (v) decreased demand for our products.
While we have developed corporate compliance programs based on what we believe to be current best practices, we cannot assure you that we or our employees or agents are or will be in compliance with all applicable federal or state regulations and laws. Further, the criteria for determining compliance are often complex and subject to change and interpretation. If we are in violation of any of these requirements or any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could include the imposition of significant criminal and civil fines and penalties, exclusion from federal healthcare programs or other sanctions, any which could have a material and adverse effect on our business, results of operations and financial condition.
We have entered into, and anticipate entering into, contracts with various U.S. government agencies. Unfavorable provisions in government contracts, some of which may be customary, may harm our business, results of operations and financial condition.
Government contracts customarily contain provisions that give the government substantial rights and remedies, many of which are not typically found in commercial contracts, including provisions that allow the government to:
|
● |
suspend or debar the contractor from doing business with the government or a specific government agency; |
|
● |
terminate existing contracts, in whole or in part, for any reason or no reason; |
|
● |
reduce the scope and value of contracts; |
|
● |
change certain terms and conditions in contracts; |
|
● |
claim rights to products, including intellectual property, developed under the contract; |
|
● |
take actions that result in a longer development timeline than expected; |
|
● |
direct the course of a development program in a manner not chosen by the government contractor; |
|
● |
audit and object to the contractor’s contract-related costs and fees, including allocated indirect costs; and |
|
● |
control and potentially prohibit the export of the contractor’s products. |
Generally, government contracts contain provisions permitting unilateral termination or modification, in whole or in part, at the government’s convenience. Under general principles of government contracting law, if the government terminates a contract for convenience, the terminated company may recover only its incurred or committed costs, settlement expenses and profit on work completed prior to the termination.
If the government terminates a contract for default, the defaulting company is entitled to recover costs incurred and associated profits on accepted items only and may be liable for excess costs incurred by the government in procuring undelivered items from another source. Some government contracts grant the government the right to use, for or on behalf of the U.S. government, any technologies developed by the contractor under the government contract. If we were to develop technology under a contract with such a provision, we might not be able to prohibit third parties, including our competitors, from using that technology in providing products and services to the government.
As a government contractor, we may also become subject to periodic audits and reviews. As part of any such audit or review, the government may review the adequacy of, and our compliance with, our internal control systems and policies, including those relating to our purchasing, property, compensation and/or management information systems. In addition, if an audit or review uncovers any improper or illegal activity, we may be subject to civil and criminal penalties and administrative sanctions, including termination of our contracts, forfeiture of profits, suspension of payments, fines and suspension or prohibition from doing business with the government. We could also suffer serious harm to our reputation if allegations of impropriety were made against us.
Legislative or regulatory reform of the healthcare system in the United States may harm our future business.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively commonly referred to as the “Healthcare Reform Act” may affect the operational results of companies in the pharmaceutical industry such as ours by imposing additional costs. Effective January 1, 2010, the Health Care Reform Act, amongst other changes, increased the minimum Medicaid drug rebates for pharmaceutical companies and revised the definition of “average manufacturer price” for reporting purposes, which may affect the amount of our Medicaid drug rebates to states. Beginning in 2011, the law also imposed a significant annual fee on companies that manufacture or import branded prescription drug products.
The Healthcare Reform Act contemplates the promulgation of significant future regulatory action which may also further affect our business. The Healthcare Reform Act and any further changes to health care laws or regulatory framework that reduce our revenues or increase our costs could also have a material adverse effect on our business, results of operations and financial condition.
We depend on our intellectual property, and our future success is dependent on our ability to protect our intellectual property and not infringe on the rights of others.
We believe intellectual property protection is important to our business and that our future success will depend, in part, on our ability to obtain patent protection, maintain trade secret protection and operate without infringing on the rights of others. We cannot assure you that:
|
● |
any of our future processes or products will be patentable; |
|
● |
our processes or products will not infringe upon the patents of third parties; or |
|
● |
we will have the resources to defend against charges of patent infringement by third parties or to protect our own rights against infringement by third parties. |
We rely on trade secrets and proprietary knowledge related to our products and technology which we generally seek to protect by confidentiality and non-disclosure agreements with employees, consultants, licensees and pharmaceutical companies. If these agreements are breached, we may not have adequate remedies for any breach, and our trade secrets may otherwise become known by our competitors.
We are subject to potential product liability claims that can result in substantial litigation costs and liability.
The design, development and manufacture of pharmaceutical products involve an inherent risk of product liability claims and associated adverse publicity. Product liability insurance coverage is expensive, difficult to obtain, and may not be available in the future on acceptable terms, or at all. Although we currently carry $50.0 million of such insurance, we believe that no reasonable amount of insurance can fully protect against all such risks because of the potential liability inherent in the business of producing pharmaceutical products for human consumption.
We face risks relating to our goodwill and intangibles.
At December 31, 2015, our goodwill, which includes goodwill generated as a result of the Tower acquisition, in addition to goodwill generated as a result of the December 1999 merger of Global Pharmaceuticals Corporation and Impax Pharmaceuticals, Inc., was approximately $210.2 million, or approximately 11% of our total assets. The carrying value of our intangible assets, composed of product rights, in-process research and development, and royalties was approximately $602.0 million, or approximately 31% of our total assets. The increase in carrying value of $576.9 million from the December 31, 2014 carrying value of $26.7 million was attributable to the Tower acquisition. We may never realize the value of our goodwill and intangibles. We regularly evaluate and will continue to regularly evaluate whether events or circumstances have occurred to indicate all, or a portion, of the carrying amount of goodwill or intangible assets may no longer be recoverable, in which case an impairment charge to earnings would become necessary. Goodwill and intangible assets are tested at least annually for impairment in accordance with Accounting Standards Codification (“ASC”) 350, Intangibles – Goodwill and Other. We perform this impairment testing during the fourth quarter of our fiscal year. During the three month period ended September 30, 2013, we recorded a $13.2 million impairment charge to cost of revenues for our Impax Generics division as a result of deteriorating market conditions. During the same period in 2013, we also recorded an intangible asset impairment charge of $0.8 million in research and development expenses as a result of a decision by management to withdraw one of our ANDAs and no longer seek FDA approval. The impairment charge represented the full carrying value of the ANDA. During the three month period ended March 31, 2014, as a result of a further decline in pricing, we revised our projections and performed an intangible asset impairment analysis. Based on the results of this analysis, we recorded a $2.9 million charge to cost of revenues for the Impax Generics division during 2014. As a result of our annual impairment testing performed during the fourth quarter of 2015, we recorded a total impairment charge related to our intangible assets of approximately $13.7 million, of which $7.3 million was recorded to cost of revenues and $6.4 million was recorded to research and development expenses on our Consolidated Statements of Income. The impairment charge was primarily attributable to deteriorating market conditions for two of the intangible assets acquired in the Tower acquisition. There was no impairment charge related to goodwill as a result of our annual testing in 2015. Any future acquisitions or investments in businesses could also result in an increase in goodwill, intangible assets and amortization expenses that could have a negative impact on our profitability. If the fair value of our goodwill or intangible assets is determined at some future date to be less than its recorded value, a change to earnings may be required. Any such charge or future determination requiring the write-off of a significant portion of the carrying value of our goodwill or intangible assets could have a material adverse effect on our business, results of operations and financial condition.
Changes in tax regulations and varying application and interpretations of these regulations could result in an increase in our existing and future tax liabilities.
We have potential tax exposures resulting from the varying application of statutes, regulations and interpretations, including exposures with respect to manufacturing, research and development, marketing, sales and distribution functions. Although our arrangements are based on accepted tax standards, tax authorities in various jurisdictions including the United States may disagree with and subsequently challenge the amount of profits taxed, which may increase our tax liabilities and could have a material adverse effect on our business, results of our operations and financial condition.
If we are unable to manage our growth, our business will suffer.
We have experienced rapid growth in the past several years and anticipate continued rapid expansion in the future. This growth has required us to expand, upgrade, and improve our administrative, operational, and management systems, internal controls and resources. We anticipate additional growth in connection with the expansion of our manufacturing operations, development of our brand-name products, and our marketing and sales efforts for the products we develop. Although we cannot assure you that we will, in fact, grow as we expect, if we fail to manage growth effectively or to develop a successful marketing approach, our business and financial results will be materially harmed. We may also seek to expand our business through complementary or strategic acquisitions of other businesses, products or assets, or through joint ventures, strategic agreements or other arrangements. Any such acquisitions, joint ventures or other business combinations may involve significant integration challenges, operational complexities and time consumption and require substantial resources and effort. It may also disrupt our ongoing businesses, which may adversely affect our relationships with customers, employees, regulators and others with whom we have business or other dealings. Further, if we are unable to realize synergies or other benefits expected to result from any acquisitions, joint ventures or other business combinations, or to generate additional revenue to offset any unanticipated inability to realize these expected synergies or benefits, our growth and ability to compete may be impaired, which would require us to focus additional resources on the integration of operations rather than other profitable areas of our business, and may otherwise cause a material adverse effect on our business, results of operations and financial condition.
We may make acquisitions of, or investments in, complementary technologies, businesses or products, which may be on terms that are not commercially advantageous, may require additional debt or equity financing, and may involve numerous risks, including the risks that we may be unable to integrate the acquired business successfully and that we may assume liabilities that adversely affect us.
We regularly review the potential acquisition of technologies, products, product rights and complementary businesses. We may choose to enter into such transactions at any time. Nonetheless, we cannot provide assurance that we will be able to identify suitable acquisition or investment candidates. To the extent that we do identify candidates that we believe to be suitable, we cannot provide assurance that we will be able to make such acquisitions or investments on commercially advantageous terms or at all. Further, there are a number of risks and uncertainties relating to closing such transactions. If such transactions are not completed for any reason, we will be subject to several risks, including the following: (i) the market price of shares of our common stock may reflect a market assumption that such transactions will occur, and a failure to complete such transactions could result in a negative perception by the market of us generally and a decline in the market price of our common stock; and (ii) many costs relating to the such transactions may be payable by us whether or not such transactions are completed.
If we make any acquisitions or investments, we may finance such acquisitions or investments through our cash reserves, debt financing, or by issuing additional equity securities, which could dilute the holdings of our then-existing stockholders. If we require financing, we cannot provide assurance that we will be able to obtain required financing when needed on acceptable terms or at all. Any such acquisitions or investments could also result in an increase in goodwill, intangible assets and amortization expenses that could ultimately negatively impact our profitability. If the fair value of our goodwill or intangible assets is determined at some future date to be less than its recorded value, a charge to earnings may be required. Further, our consolidated financial statements may also be impacted in future periods based on the accuracy of our valuations of any businesses we acquire. Such a charge to earnings or impact on our consolidated financial statements could be in amounts that are material to our business, results of operations and financial condition.
Additionally, acquisitions involve numerous risks, including difficulties in assimilating the personnel, operations and products of the acquired companies, the diversion of management’s attention from other business concerns, risks of entering markets in which we have limited or no prior experience, and the potential loss of key employees of the acquired company. There may be overlap between our products or customers and those of an acquired entity that may create conflicts in relationships or other commitments detrimental to the integrated businesses. If we are unable to successfully or timely integrate the operations of acquired companies with our business, we may incur unanticipated liabilities and be unable to realize the revenue growth, synergies and other anticipated benefits resulting from the acquisition, and our business, results of operations and financial condition could be materially and adversely affected.
As a result of acquiring businesses, we may incur significant transaction costs, including substantial fees for investment bankers, attorneys, accountants and financial printing. Any acquisition could result in our assumption of unknown and/or unexpected, perhaps material liabilities. Additionally, in any acquisition agreement, the negotiated representations, warranties and agreements of the selling parties may not entirely protect us, and liabilities resulting from any breaches could exceed negotiated indemnity limitations.
There are inherent uncertainties involved in estimates, judgments and assumptions used in the preparation of financial statements in accordance with GAAP. Any future changes in estimates, judgments and assumptions used or necessary revisions to prior estimates, judgments or assumptions could lead to a restatement of our results.
The consolidated financial statements included in this Annual Report on Form 10-K are prepared in accordance with GAAP. This involves making estimates, judgments and assumptions that affect reported amounts of assets (including intangible assets), liabilities, revenues, expenses and income. Estimates, judgments and assumptions are inherently subject to change in the future and any necessary revisions to prior estimates, judgments or assumptions could lead to a restatement. Any such changes could result in corresponding changes to the amounts of assets (including goodwill and other intangible assets), liabilities, revenues, expenses and income.
The terms of our senior secured credit facility and the indenture governing our 2.00% Convertible Senior Notes Due June 2022 impose financial and operating restrictions on us.
We have a $100.0 million senior secured revolving credit facility (the “Senior Secured Credit Facility”) pursuant to a credit agreement, dated as of August 4, 2015, by and among us, the lenders party thereto from time to time and Royal Bank of Canada, as administrative agent and collateral agent. We are also party to an indenture dated June 30, 2015 between us and Wilmington Trust, National Association (the “Indenture”) governing our 2.00% Convertible Senior Notes Due 2022 (the “Notes”). Our Senior Secured Credit Facility and Indenture contain a number of negative covenants that limit our ability to engage in activities. These covenants limit or restrict, among other things, our ability to:
|
● |
incur additional debt, guarantee other obligations or grant liens on our assets; |
|
● |
make certain loans or investments; |
|
● |
undertake certain acquisitions, mergers or consolidations, or dispose of assets; |
|
● |
make optional payments or modify certain debt instruments; |
|
● |
pay dividends or other payments on our capital stock, enter into arrangements that restrict our and our restricted subsidiaries’ ability to pay dividends or grant liens; or |
|
● |
engage in certain transactions with our affiliates. |
The terms of our Senior Secured Credit Facility also include a financial covenant which requires us to maintain a certain total net leverage ratio. These limitations and restrictions may adversely affect our ability to finance our future operations or capital needs or engage in other business activities that may be in our best interests. If we breach any of the covenants in our Senior Secured Credit Facility or Indenture, we may be in default and our borrowings under the facility and the Notes could be declared due and payable, including accrued interest and other fees, which could have a material adverse effect on our business, results of operations and financial condition.
Our level of indebtedness and liabilities could limit cash flow available for our operations, expose us to risks that could adversely affect our business, results of operations and financial condition and impair our ability to satisfy our obligations under our convertible notes and other debt instruments.
At December 31, 2015, our total consolidated liabilities were $860.1 million, including $600 million of outstanding convertible notes. We may also incur additional indebtedness to meet future financing needs. Our indebtedness could have significant negative consequences for our business, results of operations and financial condition, including:
|
● |
increasing our vulnerability to adverse economic and industry conditions; |
|
● |
limiting our ability to obtain additional financing; |
|
● |
requiring the dedication of a substantial portion of our cash flow from operations to service our indebtedness, thereby reducing the amount of our cash flow available for other purposes; |
|
● |
limiting our flexibility in planning for, or reacting to, changes in our business; |
|
● |
dilution experienced by our existing stockholders as a result of the conversion of the convertible notes into shares of common stock; and |
|
● |
placing us at a possible competitive disadvantage with less leveraged competitors and competitors that may have better access to capital resources. |
We cannot assure you that we will be able to continue to maintain sufficient cash reserves or continue to generate cash flow from operations at levels sufficient to permit us to pay principal, premium, if any, and interest on our indebtedness, or that our cash needs will not increase. If we are unable to generate sufficient cash flow or otherwise obtain funds necessary to make required payments, or if we fail to comply with the various requirements of our indebtedness then outstanding, we would be in default, which would permit the holders of the affected indebtedness to accelerate the maturity of such indebtedness and could cause defaults under our other indebtedness. Any default under any indebtedness could have a material adverse effect on our business, results of operations and financial condition.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results, timely file our periodic reports, maintain our reporting status or prevent fraud.
Our management or our independent registered public accounting firm may identify material weaknesses in our internal control over financial reporting in the future. The existence of internal control material weaknesses may result in current and potential stockholders and alliance and collaboration agreements’ partners losing confidence in our financial reporting, which could harm our business, the market price of our common stock, and our ability to retain our current, or obtain new, alliance and collaboration agreements’ partners.
In addition, the existence of material weaknesses in our internal control over financial reporting may affect our ability to timely file periodic reports under the Exchange Act. Although we remedied any past accounting issues and do not believe similar accounting problems are likely to recur, an internal control material weakness may develop in the future and affect our ability to timely file our periodic reports. The inability to timely file periodic reports under the Exchange Act could result in the SEC revoking the registration of our common stock, which would prohibit us from listing or having our stock quoted on any public market. This would have an adverse effect on our business and stock price by limiting the publicly available information regarding us and greatly reducing the ability of our stockholders to sell or trade our common stock.
Terrorist attacks and other acts of violence or war may adversely affect our business.
Terrorist attacks at or nearby our facilities in Hayward, California, Middlesex, New Jersey, or our manufacturing facility in Taiwan may negatively affect our operations. While we do not believe that we are more susceptible to such attacks than other companies, such attacks could directly affect our physical facilities or those of our suppliers or customers and could make the transportation of our products more difficult and more expensive and ultimately affect our sales.
We carry insurance coverage on our facilities of types and in amounts that we believe are in line with coverage customarily obtained by owners of similar properties. We continue to monitor the state of the insurance market in general and the scope and cost of coverage for acts of terrorism in particular, but we cannot anticipate what coverage will be available on commercially reasonable terms in future policy years. Currently, we carry terrorism insurance as part of our property and casualty and business interruption coverage. If we experience a loss that is uninsured or that exceeds policy limits, we could lose the capital invested in the damaged facilities, as well as the anticipated future net sales from those facilities.
Because of the location of our manufacturing and research and development facilities, our operations could be interrupted by an earthquake or be susceptible to climate changes.
Our corporate headquarters in California, manufacturing operations in California and Taiwan, and research and development activities related to process technologies are located near major earthquake fault lines. Although we have other facilities, we produce a substantial portion of our products at our California facility. A disruption at these California facilities due to an earthquake, other natural disaster, or due to climate changes, even on a short-term basis, could impair our ability to produce and ship products to the market on a timely basis. In addition, we could experience a destruction of facilities which would be costly to rebuild, or loss of life, all of which could materially adversely affect our business and results of operations.
We presently carry $10.0 million of earthquake coverage which covers all of our facilities on a worldwide basis. We carry an additional $40.0 million of earthquake coverage specifically for our California facilities. We believe the aggregate amount of earthquake coverage we currently carry is appropriate in light of the risks; however, the amount of our earthquake insurance coverage may not be sufficient to cover losses from earthquakes. We may discontinue some or all of this insurance coverage in the future if the cost of premiums exceeds the value of the coverage discounted for the risk of loss. If we experience a loss that is uninsured or that exceeds policy limits, we could lose the capital invested in the damaged facilities, as well as the anticipated future net sales from those facilities.
The expansion of social media platforms present new risks and challenges, which could cause a material adverse effect on our business, results of operations and financial condition.
The inappropriate use of certain media vehicles could cause brand damage or information leakage or could lead to legal implications from the improper collection and/or dissemination of personally identifiable information. In addition, negative posts or comments about us on any social networking website could seriously damage our reputation. Further, the disclosure of non-public company sensitive information through external media channels could lead to information loss as there might not be structured processes in place to secure and protect information. If our non-public sensitive information is disclosed or if our reputation is seriously damaged through social media, it could have a material adverse effect on our business, results of operations and financial condition.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
Our primary properties consist of various owned and leased facilities in California, Pennsylvania and New Jersey as well as a significant manufacturing facility that we own in Taiwan. The expiration dates of the lease agreements range between June 1, 2016 and January 31, 2020. Our properties are generally used to support the operations of both the Impax Generics division and the Impax Specialty Pharma division. The table below shows the square feet owned or leased by function at each location.
|
Location |
Owned |
Leased |
Total |
Function | |||||||||
|
Hayward, CA |
35,000 | -- | 35,000 |
Research & development | |||||||||
|
Hayward, CA |
50,000 | -- | 50,000 |
Manufacturing | |||||||||
|
Hayward, CA |
19,000 | -- | 19,000 |
Administration & lab | |||||||||
|
Hayward, CA |
50,400 | -- | 50,400 |
Warehouse | |||||||||
|
Hayward, CA |
13,300 | -- | 13,300 |
Manufacturing support | |||||||||
|
Hayward, CA |
-- | 76,180 | 76,180 |
Warehouse & lab | |||||||||
|
Hayward, CA |
-- | 45,000 | 45,000 |
Corporate offices | |||||||||
|
Hayward, CA |
-- | 88,677 | 88,677 |
Manufacturing & lab | |||||||||
|
California Properties |
167,700 | 209,857 | 377,557 | ||||||||||
|
Philadelphia, PA |
113,000 | -- | 113,000 |
Packaging & warehouse | |||||||||
|
Chalfont, PA |
-- | 44,000 | 44,000 |
Administration | |||||||||
|
Montgomeryville, PA |
-- | 40,000 | 40,000 |
Administration | |||||||||
|
Pennsylvania Properties |
113,000 | 84,000 | 197,000 | ||||||||||
|
Middlesex, NJ |
-- | 37,500 | 37,500 |
Manufacturing | |||||||||
|
Middlesex, NJ |
-- | 18,593 | 18,593 |
Packaging | |||||||||
|
Middlesex, NJ |
-- | 20,651 | 20,651 |
Research & development | |||||||||
|
Middlesex, NJ |
-- | 32,516 | 32,516 |
Administration | |||||||||
|
Bridgewater, NJ |
-- | 32,806 | 32,806 |
Administration | |||||||||
|
New Jersey Properties |
-- | 142,066 | 142,066 | ||||||||||
|
Taiwan |
397,917 | -- | 397,917 | Manufacturing * | |||||||||
|
Totals |
678,617 | 435,923 | 1,114,540 | ||||||||||
* This facility is on land that is leased from the state.
In our various facilities we maintain an extensive equipment base that includes new or recently reconditioned equipment for the manufacturing and packaging of compressed tablets, coated tablets and capsules. The manufacturing and research and development equipment includes mixers and blenders for capsules and tablets, automated capsule fillers, tablet presses, particle reduction, sifting equipment, and tablet coaters. The packaging equipment includes fillers, cottoners, cappers, and labelers. We also maintain two well equipped, modern laboratories used to perform all the required physical and chemical testing of our products. We also maintain a broad variety of material handling and cleaning, maintenance, and support equipment. We own substantially all of our manufacturing equipment and believe it is well maintained and suitable for its requirements.
We maintain property and casualty and business interruption insurance in amounts we believe are sufficient and consistent with practices for companies of comparable size and business.
Item 3. Legal Proceedings
Information pertaining to legal proceedings can be found in “Item 15. Exhibits and Financial Statement Schedules – Note 22. Legal and Regulatory Matters” and is incorporated by reference herein.
Item 4. Mine Safety Disclosures
Not applicable.
PART II.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Stock Price
Our common stock is traded on the NASDAQ Global Market under the symbol "IPXL". The following table sets forth the high and low sales prices for our common stock as reported by the NASDAQ Global Market, as follows:
|
Price Range per Share |
||||||||
|
High |
Low |
|||||||
|
Year Ending December 31, 2015 |
||||||||
|
First Quarter |
$ | 47.70 | $ | 29.76 | ||||
|
Second Quarter |
$ | 52.10 | $ | 42.25 | ||||
|
Third Quarter |
$ | 51.42 | $ | 31.85 | ||||
|
Fourth Quarter |
$ | 45.00 | $ | 31.83 | ||||
|
Year Ending December 31, 2014 |
||||||||
|
First Quarter |
$ | 28.50 | $ | 21.34 | ||||
|
Second Quarter |
$ | 30.74 | $ | 23.02 | ||||
|
Third Quarter |
$ | 31.04 | $ | 22.12 | ||||
|
Fourth Quarter |
$ | 33.05 | $ | 23.31 | ||||
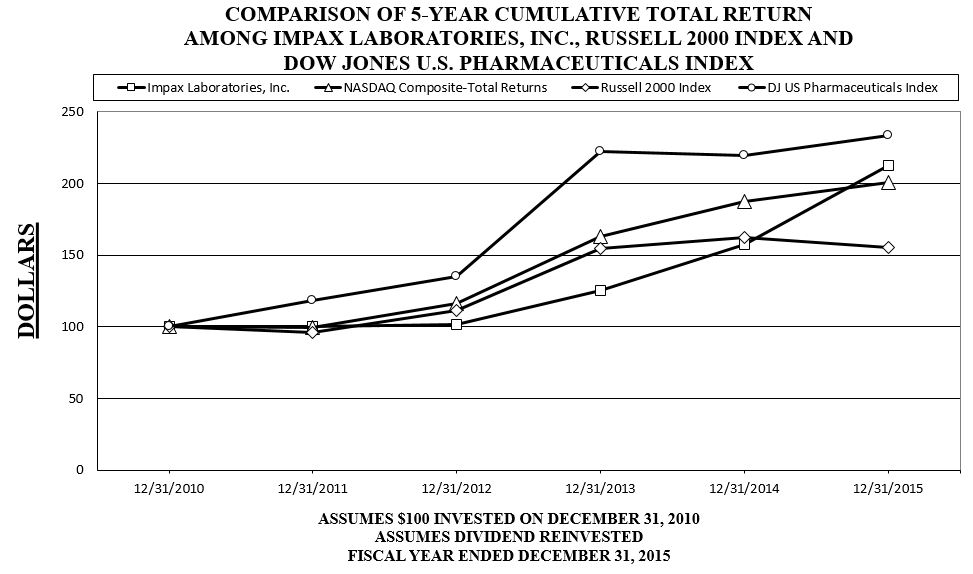
This performance graph shall not be deemed "soliciting material" or to be "filed" with the Securities and Exchange Commission for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, or otherwise subject to the liabilities under that Section, and shall not be deemed to be incorporated by reference into any filing of Impax Laboratories, Inc. under the Securities Act of 1933, as amended, or the Exchange Act.
Holders
As of February 12, 2016, there were approximately 220 holders of record of our common stock, solely based upon the count our transfer agent provided us as of that date.
Dividends
We have never paid cash dividends on our common stock and have no present plans to do so. Our current policy is to retain all earnings, if any, for use in the operation of our business. The payment of future cash dividends, if any, will be at the discretion of our Board of Directors and will be dependent upon our earnings, financial condition, capital requirements and other factors as our Board of Directors may deem relevant.
Special Meeting of Stockholders
On November 9, 2015, we filed a definitive proxy statement with the SEC related to our Special Meeting of Stockholders which was held on December 8, 2015, and where our stockholders approved our proposal to amend our Restated Certificate of Incorporation to increase the number of authorized shares of our common stock from 90 million shares to 150 million shares, which amendment has occurred.
Unregistered Sales of Securities
There were no sales of unregistered securities during the year ended December 31, 2015.
Purchases of Equity Securities by the Issuer
The following table provides information regarding the purchases of our equity securities by us during the quarter ended December 31, 2015.
|
Period |
Total Number of Shares (or Units) Purchased(1) |
Average Price Paid Per Share (or Unit) |
Total Number of Shares (or Units) Purchased as Part of Publicly Announced Plans or Programs |
Maximum Number (or Approximate Dollar Value) of Shares (or Units) that May Yet Be Purchased Under the Plans or Programs |
||||||||||||
|
October 1, 2015 to October 31, 2015 |
195,370 | $ | 35.92 | — | — | |||||||||||
|
November 1, 2015 to November 30, 2015 |
— | — | — | — | ||||||||||||
|
December 1, 2015 to December 31, 2015 |
— | — | — | — | ||||||||||||
|
Total |
195,370 | $ | 35.92 | — | — | |||||||||||
|
(1) |
Represents shares of our common stock that we accepted during the indicated periods as a tax withholding from certain of our employees in connection with the vesting of shares of restricted stock pursuant to the terms of our Second Amended and Restated 2002 Equity Incentive Plan (the “2002 Plan”). |
Equity Compensation Plans
The following table details information regarding our existing equity compensation plans as of December 31, 2015:
|
Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights |
Weighted Average Exercise Price of Outstanding Options, Warrants and Rights |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities reflected in |
||||||||||
|
Plan Category |
(a) |
(b) |
(c) |
|||||||||
|
Equity compensation plans approved by security holders |
2,405,371 | (1) | $ | 21.39 | 1,812,055 | |||||||
|
Equity compensation plans not approved by security holders |
--- | --- | 81,250 | (2) | ||||||||
|
Total: |
2,405,371 | $ | 21.39 | 1,893,305 | ||||||||
|
(1) |
Represents options issued pursuant to the 2002 Plan, and the Impax Laboratories, Inc. 1999 Equity Incentive Plan. |
|
(2) |
Represents 81,250 shares of common stock available for future issuance under the Impax Laboratories, Inc. 2001 Non-Qualified Employee Stock Purchase Plan. |
See “Item 15. Exhibits and Financial Statement Schedules — Note 18. Employee Benefit Plans” and “Note 17. Share-Based Compensation” for information concerning our employee benefit plans and equity compensation plans.
Item 6. Selected Financial Data
The following selected financial data should be read together with our consolidated financial statements and accompanying consolidated financial statement footnotes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” appearing elsewhere in this Annual Report on Form 10-K. The selected consolidated financial statement data in this section are not intended to replace our consolidated financial statements and the accompanying consolidated financial statement footnotes. Our historical consolidated financial results are not necessarily indicative of our future consolidated financial results.
The selected financial data set forth below are derived from our consolidated financial statements. The consolidated statements of operations data for the years ended December 31, 2015, 2014 and 2013 and the consolidated balance sheet data as of December 31, 2015 and 2014 are derived from our audited consolidated financial statements included elsewhere in this Annual Report on Form 10-K. These audited consolidated financial statements include, in the opinion of management, all adjustments necessary for the fair presentation of our financial position and results of operations for these periods.
|
(In thousands, except per share data) |
For the Years Ended December 31, |
|||||||||||||||||||
|
Statements of Income Data: |
2015 |
2014 |
2013 |
2012 |
2011 |
|||||||||||||||
|
Total revenues |
$ | 860,469 | $ | 596,049 | $ | 511,502 | $ | 581,692 | $ | 512,919 | ||||||||||
|
Research and development |
76,982 | 78,642 | 68,854 | 81,320 | 82,701 | |||||||||||||||
|
Total operating expenses |
282,836 | 223,837 | 205,687 | 199,562 | 158,684 | |||||||||||||||
|
Income (loss) from operations |
69,568 | 88,816 | (6,387 | ) | 82,992 | 99,611 | ||||||||||||||
|
Net income |
38,997 | 57,353 | 101,259 | 55,873 | 65,495 | |||||||||||||||
|
Net income per share — basic |
0.56 | 0.84 | 1.51 | 0.85 | 1.02 | |||||||||||||||
|
Net income per share — diluted |
0.54 | 0.81 | 1.47 | 0.82 | 0.97 | |||||||||||||||
|
(In thousands) |
As of December 31, |
|||||||||||||||||||
|
Balance Sheet Data: |
2015 |
2014 |
2013 |
2012 |
2011 |
|||||||||||||||
|
Cash, cash equivalents and short-term investments |
$ | 340,351 | $ | 414,856 | $ | 413,133 | $ | 298,918 | $ | 346,414 | ||||||||||
|
Working capital |
495,312 | 516,927 | 505,852 | 400,248 | 443,074 | |||||||||||||||
|
Total assets |
1,922,487 | 1,079,197 | 996,923 | 863,970 | 793,859 | |||||||||||||||
|
Long-term debt |
424,595 | -- | -- | -- | -- | |||||||||||||||
|
Total liabilities |
860,078 | 191,320 | 186,720 | 172,867 | 190,918 | |||||||||||||||
|
Retained earnings |
570,223 | 531,226 | 473,873 | 372,614 | 316,741 | |||||||||||||||
|
Total stockholders’ equity |
1,062,409 | 887,877 | 810,203 | 691,103 | 602,941 | |||||||||||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis, as well as other sections in this report, should be read in conjunction with the consolidated financial statements and related Notes to Consolidated Financial Statements included elsewhere herein. All references to years mean the relevant 12-month period ended December 31.
Overview
We are a specialty pharmaceutical company applying formulation and development expertise, as well as our drug delivery technology, to the development, manufacture and marketing of bioequivalent pharmaceutical products, commonly referred to as “generics,” in addition to the development and marketing of branded products. We operate in two segments, referred to as “Impax Generics” and “Impax Specialty Pharma”. Impax Generics concentrates its efforts on generic products, which are the pharmaceutical and therapeutic equivalents of brand-name drug products and are usually marketed under their established nonproprietary drug names rather than by a brand name. Impax Specialty Pharma utilizes its specialty sales force to market proprietary branded pharmaceutical products for the treatment of CNS disorders and other select specialty segments. Both Impax Generics and Impax Specialty Pharma generate revenue from research and development services provided to unrelated third-party pharmaceutical entities.
We plan to continue to expand Impax Generics through targeted ANDAs and a first-to-file and first-to-market strategy and to continue to evaluate and pursue external growth initiatives, including acquisitions and partnerships. We focus our efforts on a broad range of therapeutic areas including products that have technically challenging drug-delivery mechanisms or unique product formulations. We employ our technologies and formulation expertise to develop generic products that reproduce brand-name products’ physiological characteristics but do not infringe any valid patents relating to such brand-name products. We generally focus our generic product development on brand-name products as to which the patents covering the active pharmaceutical ingredient have expired or are near expiration, and we employ our proprietary formulation expertise to develop controlled-release technologies that do not infringe patents covering the brand-name products’ controlled-release technologies. We also develop, manufacture, sell and distribute specialty generic pharmaceuticals that we believe present one or more competitive advantages, such as difficulty in raw materials sourcing, complex formulation or development characteristics or special handling requirements. In addition to our focus on solid oral dosage products, we have expanded our generic pharmaceutical products portfolio to include alternative dosage form products, primarily through alliance and collaboration agreements with third parties. As of February 16, 2016, we marketed 139 generic pharmaceuticals, which represent dosage variations of 55 different pharmaceutical compounds through our Impax Generics division; another five of our generic pharmaceuticals representing dosage variations of two different pharmaceutical compounds are marketed by our alliance and collaboration agreement partners. As of February 16, 2016, we had 25 applications pending at the FDA and 18 other products in various stages of development for which applications have not yet been filed.
The Impax Generics division develops, manufactures, sells, and distributes generic pharmaceutical products primarily through the following sales channels:
|
● |
the “Impax Generics sales channel” for sales of generic prescription products we sell directly to wholesalers, large retail drug chains, and others; |
|
● |
the “Private Label Product sales channel” for generic pharmaceutical over-the-counter and prescription products we sell to unrelated third-party customers who in-turn sell the product to third parties under their own label; |
|
● |
the “Rx Partner sales channel” for generic prescription products sold through unrelated third-party pharmaceutical entities under their own label pursuant to alliance agreements; and |
|
● |
the “OTC Partner sales channel” for sales of generic pharmaceutical over-the-counter products sold through unrelated third-party pharmaceutical entities under their own label pursuant to alliance agreements. |
We sell our Impax Generics division products within the continental United States and the Commonwealth of Puerto Rico. We have no sales in foreign countries. Revenues from the Impax Generics sales channel and the Private Label Product sales channel are reported under the caption “Impax Generics sales, net” in our consolidated statements of income. We also generate revenue in our Impax Generics division from research and development services provided under a joint development agreement with another pharmaceutical company, and we report such revenue under the caption “Other Revenues” in “Item 15. Exhibits and Financial Statement Schedules – Note 24. Supplementary Financial Information.”
Impax Specialty Pharma is focused on developing proprietary branded pharmaceuticals products for the treatment of CNS disorders, which include migraine, multiple sclerosis, Parkinson’s disease and post herpetic neuralgia, as well as developing other select specialty products. Impax Specialty Pharma in involved in the promotion and sale of branded pharmaceutical products through our specialty sales force. We believe that we have the research, development and formulation expertise to develop branded products that will deliver significant improvements over existing therapies.
Our branded pharmaceutical product portfolio consists of commercial CNS and other select specialty products, as well as development stage projects. In February 2012, we licensed from AZ the exclusive U.S. commercial rights to Zomig® (zolmitriptan) tablet, orally disintegrating tablet and nasal spray formulations pursuant to the terms of the AZ Agreement and began sales of the Zomig® products under our label during the year ended December 31, 2012 through our specialty sales force. In May 2013, our exclusivity period for branded Zomig® tablets and orally disintegrating tablets expired and we launched authorized generic versions of those products in the United States. In June 2015, the FDA approved the Zomig® nasal spray for use in pediatric patients 12 years of age or older for the acute treatment of migraine with or without aura. Our branded products portfolio also includes Albenza® for invasive tapeworm infections, and two additional marketed products, all acquired in our acquisition of Tower and Lineage.
We currently market one internally developed branded pharmaceutical product, Rytary® (IPX066), for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication and/or manganese intoxication, which was approved by the FDA on January 7, 2015 and which we launched in the United States in April 2015. In November 2015, the European Commission granted marketing authorization for NUMIENT™ (IPX066) (referred to as Rytary® in the United States). The review of the NUMIENT™ application was conducted under the centralized licensing procedure as a therapeutic innovation, and the authorization is applicable in all 28 member states of the European Union, as well as Iceland, Liechtenstein and Norway.
We have entered into several alliance, collaboration or license and distribution agreements with respect to certain of our products and services and may enter into similar agreements in the future. These agreements may require us to relinquish rights to certain of our technologies or product candidates, or to grant licenses on terms which ultimately may prove to be unfavorable to us. Relationships with alliance and collaboration partners may also include risks due to the failure of a partner to perform under the agreement, incomplete marketplace information, inventories, development capabilities, regulatory compliance and commercial strategies of our partners and our agreements may be the subject of contractual disputes. For instance, we have historically experienced some disruptions in supply of certain products. If we suffer similar supply failures on our significant products in the future, or if we or our partners are not successful in commercializing the products covered by such alliance, collaboration or license and distribution agreements, our revenues and relationships with our customers may be materially adversely affected.
Critical Accounting Policies and Use of Estimates
The preparation of our consolidated financial statements in accordance with accounting principles generally accepted in the United States (“GAAP”) and the rules and regulations of the U.S. Securities & Exchange Commission (“SEC”) require the use of estimates and assumptions, based on complex judgments considered reasonable, and affect the reported amounts of assets and liabilities and disclosure of contingent assets and contingent liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. The most significant judgments are employed in estimates used in determining values of tangible and intangible assets, legal contingencies, tax assets and tax liabilities, fair value of share-based compensation related to equity incentive awards issued to employees and directors, and estimates used in applying the our revenue recognition policy including those related to accrued chargebacks, rebates, distribution service fees, product returns, Medicare, Medicaid, and other government rebate programs, shelf-stock adjustments, and the timing and amount of deferred and recognized revenue under the our several alliance and collaboration agreements. Actual results may differ from estimated results. Certain prior year amounts have been reclassified to conform to the presentation for the year ended December 31, 2015.
Although we believe our estimates and assumptions are reasonable when made, they are based upon information available to us at the time they are made. We periodically review the factors having an influence on our estimates and, if necessary, adjust such estimates. Although historically our estimates have generally been reasonably accurate, due to the risks and uncertainties involved in our business and evolving market conditions, and given the subjective element of the estimates made, actual results may differ from estimated results. This possibility may be greater than normal during times of pronounced economic volatility.
Impax Generics sales, net, and Impax Specialty Pharma sales, net. Revenue from the sale of products is recognized when title and risk of loss of the product is transferred to the customer and the sales price is fixed and determinable. Provisions for discounts, early payments, rebates, sales returns and distributor chargebacks under terms customary in the industry are provided for in the same period the related sales are recorded. We record estimated reductions to revenue at the time of the initial sale and these estimates are based on the sales terms, historical experience and trend analysis.
Gross to Net Sales Accruals. We base our sales returns allowance on estimated on-hand inventories at our customers, measured end-customer demand as reported by third-party sources, actual returns history and other factors, such as the trend experience for lots where product is still being returned or inventory centralization and rationalization initiatives conducted by major pharmacy chains, as applicable. If the historical data we use to calculate these estimates do not properly reflect future returns, then a change in the allowance would be made in the period in which such a determination is made and revenues in that period could be materially affected. Under this methodology, we track actual returns by individual production lots. Returns on closed lots, that is, lots no longer eligible for return credits, are analyzed to determine historical returns experience. Returns on open lots, that is, lots still eligible for return credits, are monitored and compared with historical return trend rates. Any changes from the historical trend rates are considered in determining the current sales return allowance.
Sales discount accruals are based on payment terms extended to customers.
Government rebate accruals are based on estimated payments due to governmental agencies for purchases made by third parties under various governmental programs. U.S. Medicaid rebate accruals are generally based on historical payment data and estimates of future Medicaid beneficiary utilization applied to the Medicaid unit rebate formula established by the Center for Medicaid and Medicare Services. The Medicaid rebate percentage was increased and extended to Medicaid Managed Care Organizations in March 2010. The accrual of the rebates associated with Medicaid Managed Care Organizations is calculated based on actual billings received from the states. We adjust the rebate accruals as more information becomes available and to reflect actual claims experience. Effective January 1, 2011, manufacturers of pharmaceutical products are responsible for 50% of the patient’s cost of branded prescription drugs related to the Medicare Part D Coverage Gap. In order to estimate the cost to us of this coverage gap responsibility, we analyze the historical invoices. This expense is recognized throughout the year as costs are incurred.
Rebates or administrative fees are offered to certain customers, group purchasing organizations and pharmacy benefit managers, consistent with pharmaceutical industry practices. Settlement of rebates and fees may generally occur from one to 15 months from the date of sale. We provide a provision for rebates at the time of sale based on contracted rates and historical redemption rates. Assumptions used to establish the provision include level of customer inventories, contract sales mix and average contract pricing. We regularly review the information related to these estimates and adjust the provision accordingly.
Chargeback accruals are based on the differentials between product acquisition prices paid by wholesalers and lower contract pricing paid by eligible customers.
Distributor service fee accruals are based on contractual fees to be paid to the wholesale distributor for services provided. TRICARE is a health care program of the U.S. Department of Defense Military Health System that provides civilian health benefits for military personnel, military retirees and their dependents. TRICARE rebate accruals are included in chargeback accruals and are based on estimated Department of Defense eligible sales multiplied by the TRICARE rebate formula.
A significant majority of our gross to net accruals are the result of chargebacks and rebates, with the majority of those programs having an accrual to payment cycle of approximately three months. In addition to this relatively short accrual to payment cycle, we receive monthly information from the wholesalers regarding their sales of our products and actual on hand inventory levels of our products. During the year ended December 31, 2015, the three large wholesalers account for approximately 98% of our chargebacks and approximately 77% of our indirect sales rebates. This enables us to execute accurate provisioning procedures. Consistent with the pharmaceutical industry, the accrual to payment cycle for returns is longer and can take several years depending on the expiration of the related products. However, returns represent the smallest gross to net adjustment. We have not experienced any significant changes in our estimates as it relates to our chargebacks, rebates or returns in each of the years in the three-year period ended December 31, 2015.
The following tables are roll-forwards of the activity in the reserves for the years ended December 31, 2015, 2014 and 2013 with an explanation for any significant changes in the accrual percentages:
|
(In thousands) |
As of December 31, |
|||||||||||
|
Chargeback Reserve |
2015 |
2014 |
2013 |
|||||||||
|
Beginning balance |
$ | 43,125 | $ | 37,066 | $ | 18,410 | ||||||
|
Acquired balances |
24,532 | --- | --- | |||||||||
|
Provision recorded during the period |
833,157 | 487,377 | 389,707 | |||||||||
|
Credits issued during the period |
(798,184 | ) | (481,318 | ) | (371,051 | ) | ||||||
|
Ending balance |
$ | 102,630 | $ | 43,125 | $ | 37,066 | ||||||
|
Provision as a percent of gross product sales: |
34 | % | 35 | % | 34 | % | ||||||
The aggregate provision for chargebacks, as a percent of gross product sales, decreased to 34% in 2015 from 35% in 2014 primarily as a result of product sales mix and inclusion of product sales from the Tower acquisition.
The aggregate provision for chargebacks, as a percent of gross product sales, increased to 35% in 2014 from 34% in 2013 primarily as a result of an increase in the estimated provision for chargebacks related to our authorized generic Trilipix® and branded Zomig® products during the year ended December 31, 2014 due to price erosion resulting from increased competition, partially offset by lower chargeback rates on our authorized generic Renvela® tablets and generic Solaraze®, both launched during 2014.
|
(In thousands) |
As of December 31, |
|||||||||||
|
Rebate Reserve |
2015 |
2014 |
2013 |
|||||||||
|
Beginning balance |
$ | 88,812 | $ | 88,449 | $ | 46,011 | ||||||
|
Acquired balance |
75,447 | --- | --- | |||||||||
|
Provision recorded during the period |
571,642 | 260,747 | 193,288 | |||||||||
|
Credits issued during the period |
(470,672 | ) | (260,384 | ) | (150,850 | ) | ||||||
|
Ending balance |
$ | 265,229 | $ | 88,812 | $ | 88,449 | ||||||
|
Provision as a percent of gross product sales: |
23 | % | 19 | % | 17 | % | ||||||
As noted in the table above, the provision for rebates, as a percent of gross product sales, increased from 19% during the year ended December 31, 2014 to 23% during the year ended December 31, 2015 as a result of product sales mix, the formation of alliances between major wholesalers and major retailers and the inclusion of product sales from the Tower acquisition which carry a higher rebate rate.
The provision for rebates, as a percent of gross product sales, increased from 17% during the year ended December 31, 2013 to 19% during the year ended December 31, 2014 as a result of product sales mix, as well as the impact of additional rebates resulting from the recent formation of new alliances between major wholesalers and major retailers.
|
(In thousands) |
As of December 31, |
|||||||||||
|
Returns Reserve |
2015 |
2014 |
2013 |
|||||||||
|
Beginning balance |
$ | 27,174 | $ | 28,089 | $ | 23,440 | ||||||
|
Acquired balances |
11,364 | --- | --- | |||||||||
|
Provision recorded during the period |
43,967 | 12,016 | 11,015 | |||||||||
|
Credits issued during the period |
(33,555 | ) | (12,931 | ) | (6,366 | ) | ||||||
|
Ending balance |
$ | 48,950 | $ | 27,174 | $ | 28,089 | ||||||
|
Provision as a percent of gross product sales: |
2 | % | 1 | % | 1 | % | ||||||
The provision for returns as a percent of gross product sales increased to 2% in 2015 compared to1% in 2014 as a result of the Tower acquisition whose products carry a higher historical returns experience, higher than anticipated returns volume resulting from the loss of exclusivity on certain Zomig® products and higher returns accruals due to price increases on certain generic products.
The provision for returns as a percent of gross product sales remained at 1% in 2014 compared to 2013.
Rx Partner and OTC Partner. Each of our Rx Partner and OTC Partner agreements contain multiple deliverables in the form of products, services and/or licenses over extended periods. Financial Accounting Standards Board (“FASB”) Accounting Standards CodificationTM (“ASC”) Topic 605-25 supplemented SAB 104 and provides guidance for accounting for such multiple-element revenue arrangements. With respect to our multiple-element revenue arrangements that are material to our financial results, we determine whether any or all of the elements of the arrangement should be separated into individual units of accounting under FASB ASC Topic 605-25. If separation into individual units of accounting is appropriate, we recognize revenue for each deliverable when the revenue recognition criteria specified by SAB 104 are achieved for the deliverable. If separation is not appropriate, we recognize revenue and related direct manufacturing costs over the estimated life of the agreement or our estimated expected period of performance using either the straight-line method or a modified proportional performance method.
The Rx Partners and OTC Partners agreements obligate us to deliver multiple goods and/or services over extended periods. Such deliverables include manufactured pharmaceutical products, exclusive and semi-exclusive marketing rights, distribution licenses, and research and development services. In exchange for these deliverables, we receive payments from our agreement partners for product shipments and research and development services, and may also receive other payments including royalty, profit sharing, upfront payments, and periodic milestone payments. Revenue received from our partners for product shipments under these agreements is generally not subject to deductions for chargebacks, rebates, product returns, and other pricing adjustments. Royalty and profit sharing amounts we receive under these agreements are calculated by the respective agreement partner, with such royalty and profit share amounts generally based upon estimates of net product sales or gross profit which include estimates of deductions for chargebacks, rebates, product returns, and other adjustments the alliance agreement partners may negotiate with their customers. We record the agreement partner's adjustments to such estimated amounts in the period the agreement partner reports the amounts to us.
OTC Partner revenue is related to our alliance and collaboration agreement with Pfizer, Inc., formerly Wyeth LLC (“Pfizer”) and our supply agreement with L. Perrigo Company (“Perrigo”) with respect to the supply of over-the-counter pharmaceutical products. The OTC Partner sales channel is no longer a core area of our business, and the over-the-counter pharmaceutical products we sell through this sales channel are older products which are only sold to Pfizer and Perrigo. We are currently only required to manufacture the over-the-counter pharmaceutical products under our agreements with Pfizer and Perrigo. We recognize profit share revenue in the period earned.
Research Partner. We have entered into development agreements with unrelated third-party pharmaceutical companies under which we are collaborating in the development of five dermatological products, including four generic products and one branded dermatological product, and one branded CNS product. Under each of the development agreements, we received an upfront fee with the potential to receive additional milestone payments upon completion of contractually specified clinical and regulatory milestones. Additionally, we may also receive royalty payments from the sale, if any, of a successfully developed and commercialized branded product under one of the development agreements. We defer and recognize revenue received from the achievement of contingent research and development milestones in the period such payment is earned. We will recognize royalty fee income, if any, as current period revenue when earned.
Estimated Lives of Alliance and Collaboration Agreements. Because we may defer revenue we receive under our alliance agreements, and recognize it over the estimated life of the related agreement, or our expected period of performance, we are required to estimate the recognition period under each such agreement in order to determine the amount of revenue to be recognized in each period. Sometimes this estimate is based on the fixed term of the particular alliance agreement. In other cases the estimate may be based on more subjective factors as noted in the following paragraphs. While changes to the estimated recognition periods have been infrequent, such changes, should they occur, may have a significant impact on our consolidated financial statements.
As an illustration, the consideration received from the provision of research and development services under the Joint Development Agreement with Valeant Pharmaceuticals International, Inc. (“Valeant Agreement”), including the upfront fee and milestone payments received before January 1, 2011, have been initially deferred and are being recognized as revenue on a straight-line basis over our expected period of performance to provide research and development services under the Valeant Agreement. The completion of the final deliverable under the Valeant Agreement represents the end of our estimated expected period of performance, as we will have no further contractual obligation to perform research and development services under the Valeant Agreement, and therefore the earnings process will be complete. The expected period of performance was initially estimated to be a 48 month period, starting in December 2008, upon receipt of the $40.0 million upfront payment, and ending in November 2012. During the year ended December 31, 2012, we extended the end of the revenue recognition period for the Valeant Agreement from November 2012 to November 2013 and during the three month period ended March 31, 2013, we further extended the end of the revenue recognition period for the agreement from November 2013 to December 2014 due to changes in the estimated timing of completion of certain research and development activities under the agreement. All deferred revenue under the Valeant Agreement was completely recognized as of December 31, 2014.
Third-Party Research Agreements. In addition to our own research and development resources, we may use unrelated third-party vendors, including universities and independent research companies, to assist in our research and development activities. These vendors provide a range of research and development services to us, including clinical and bio-equivalency studies. We generally sign agreements with these vendors which establish the terms of each study performed by them, including, among other things, the technical specifications of the study, the payment schedule, and timing of work to be performed. Third-party researchers generally earn payments either upon the achievement of a milestone, or on a pre-determined date, as specified in each study agreement. We account for third-party research and development expenses as they are incurred according to the terms and conditions of the respective agreement for each study performed, with an accrued expense at each balance sheet date for estimated fees and charges incurred by us, but not yet billed to us. We monitor aggregate actual payments and compare them to the estimated provisions to assess the reasonableness of the accrued expense balance at each quarterly balance sheet date.
Share-Based Compensation. We recognize the grant date fair value of each option and restricted share over its vesting period. Options and restricted shares granted under the 2002 Plan generally vest over a three or four year period and generally have a term of ten years. We estimate the fair value of each stock option award on the grant date using the Black-Scholes-Merton option-pricing model, wherein expected volatility is based on historical volatility of our common stock. We base the expected term calculation on the “simplified” method described in SAB No. 107, Share-Based Payment and SAB No. 110, Share-Based Payment, because it provides a reasonable estimate in comparison to our actual experience. We base the risk-free interest rate on the U.S. Treasury yield in effect at the time of grant for an instrument with a maturity that is commensurate with the expected term of the stock options. The dividend yield is zero as we have never paid cash dividends on our common stock, and have no present intention to pay cash dividends. During the year ended December 31, 2014, we granted shares of restricted stock that vested upon the achievement of certain stock price performance criteria during the year ended December 31, 2015. We valued these awards using a Monte Carlo simulation.
Income Taxes. We are subject to U.S. federal, state and local income taxes, Netherlands income tax and Taiwan R.O.C. income taxes. We create a deferred tax asset, or a deferred tax liability, when we have temporary differences between the financial statement carrying values (GAAP) and the tax bases of our assets and liabilities.
Fair Value of Financial Instruments. Our deferred compensation liability is carried at the value of the amount owed to participants, and is derived from observable market data by reference to hypothetical investments. The carrying values of other financial assets and liabilities such as accounts receivable, accounts payable and accrued expenses approximate their fair values due to their short-term nature.
Contingencies. In the normal course of business, we are subject to loss contingencies, such as legal proceedings and claims arising out of our business, covering a wide range of matters, including, among others, patent litigation, stockholder lawsuits, and product and clinical trial liability. In accordance with FASB ASC Topic 450 - Contingencies, we record accrued loss contingencies when it is probable a liability will be incurred and the amount of loss can be reasonably estimated and we do not recognize gain contingencies until realized.
Intangible Assets
The following table identifies our preliminary allocations of the Tower acquisition purchase price to the intangible assets acquired in the acquisition by category:
|
Estimated Fair Value (in thousands) |
Weighted- Average Estimated Useful Life (in years) |
|||||||
|
Currently marketed product rights |
$ | 381,100 | 13 | |||||
|
Royalties |
80,800 | 12 | ||||||
|
In-process research and development |
170,700 | n/a | ||||||
|
Total intangible assets |
$ | 632,600 | 12 | |||||
The estimated fair value of the in-process research and development and identifiable intangible assets acquired in the Tower acquisition was determined using the “income approach,” which is a valuation technique that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each asset or product (including net revenues, cost of sales, research and development costs, selling and marketing costs and working capital/asset contributory asset charges), the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of each asset’s life cycle, the potential regulatory and commercial success risks, competitive trends impacting the asset and each cash flow stream as well as other factors. The discount rates used to arrive at the present value at the Tower acquisition date of currently marketed products was 15%. For in-process research and development, the discount rate used was 16% to reflect the internal rate of return and incremental commercial uncertainty in the cash flow projections. No assurances can be given that the underlying assumptions used to prepare the discounted cash flow analysis will not change. For these and other reasons, actual results may vary significantly from estimated results.
Goodwill. In accordance with FASB ASC Topic 350, "Goodwill and Other Intangibles", rather than recording periodic amortization of goodwill, goodwill is subject to an annual assessment for impairment by applying a fair-value-based test. Under FASB ASC Topic 350, if the fair value of the reporting unit exceeds the reporting unit’s carrying value, including goodwill, then goodwill is considered not impaired, making further analysis not required. We consider each of our Impax Generics division and Impax Specialty Pharma division operating segments to be a reporting unit, as this is the lowest level for each of which discrete financial information is available. We attribute approximately $60.2 million of goodwill to the Impax Specialty Pharma division and approximately $150.0 million of goodwill to the Impax Generics division. We concluded the carrying value of goodwill was not impaired as of December 31, 2015 and 2014, as the fair value of the Impax Specialty Pharma division and the Impax Generics division exceeded their respective carrying values at each date. We perform our annual goodwill impairment test in the fourth quarter of each year. We estimate the fair value of the Impax Specialty Pharma division and the Impax Generics division using a discounted cash flow model for both the reporting unit and the enterprise, as well as earnings and revenue multiples per common share outstanding for enterprise fair value. In addition, on a quarterly basis, we perform a review of our business operations to determine whether events or changes in circumstances have occurred that could have a material adverse effect on the estimated fair value of each reporting unit, and thus indicate a potential impairment of the goodwill carrying value. If such events or changes in circumstances were deemed to have occurred, we would perform an interim impairment analysis, which may include the preparation of a discounted cash flow model, or consultation with one or more valuation specialists, to analyze the impact, if any, on our assessment of the reporting unit’s fair value. To date, we have not deemed there to be any significant adverse changes in the legal, regulatory or business environment in which we conduct our operations.
Results of Operations - Year Ended December 31, 2015 Compared to Year Ended December 31, 2014
Overview:
The following table sets forth our summarized, consolidated results of operations for the years ended December 31, 2015 and 2014 (in thousands):
| Year Ended December 31, | ||||||||||||||||
| Increase/ | ||||||||||||||||
|
2015 |
2014 |
(Decrease) |
||||||||||||||
| $ | % | |||||||||||||||
|
Total revenues |
$ | 860,469 | $ | 596,049 | $ | 264,420 | 44 | % | ||||||||
|
Gross profit |
352,404 | 312,653 | 39,751 | 13 | % | |||||||||||
|
Income from operations |
69,568 | 88,816 | (19,248 | ) | (22 | )% | ||||||||||
|
Income before income taxes |
59,368 | 90,559 | (31,191 | ) | (34 | )% | ||||||||||
|
Provision for income taxes |
20,371 | 33,206 | (12,835 | ) | (39 | )% | ||||||||||
|
Net income |
$ | 38,997 | $ | 57,353 | $ | (18,356 | ) | (32 | )% | |||||||
Consolidated total revenues for the year ended December 31, 2015 increased by 44% or $264.5 million to $860.5 million, compared to $596.0 million for the year ended December 31, 2014. The increase in consolidated total revenues during 2015 was primarily due to the addition of product revenues from our acquisition of Tower and increased sales of diclofenac sodium gel and the addition of sales from Rytary® and 14 generic products launched in 2015. The year-over-year increase in revenue was partially offset by the absence of authorized generic Renvela® sales during 2015, for which there were sales during the prior year. Product volumes (including from acquisitions) increased revenues by approximately 36.0%, while product selling price decreased revenues by approximately 4%, in each case compared to the prior year. New product launches increased revenues by approximately 12% compared to the prior year.
Revenues from our Impax Generics division increased by $161.9 million during 2015, as compared to the prior year, driven primarily by the increase in revenues from the Tower acquisition and increased sales volume from diclofenac sodium gel, partially offset by the absence of sales of authorized generic Renvela® during 2015, for which there were sales during the prior year. Revenues from the Impax Specialty Pharma division increased by $102.6 million during 2015, as compared to the prior year, as a result of the launch of Rytary® as well as product volumes from the Tower acquired companies during 2015.
Net income for the year ended December 31, 2015 was $39.0 million, a decrease of $18.4 million as compared to $57.4 million for the year ended December 31, 2014. The decrease was primarily attributable to lower margins from product sales and higher operating expenses, in each case compared to the prior year. We also experienced higher amortization and impairment charges related to intangible assets, higher severance costs related to the restructuring of our packaging and distribution facilities announced on June 30, 2015 as well as costs related to the fair value of inventory resulting from the Tower acquisition. Such increased costs were partially offset by reduced Hayward facility remediation costs during 2015. In addition, we had a loss on debt extinguishment related to the repayment of our term loan with Barclays, as well as higher interest expense related to the Barclays term loan and to our convertible notes and a net loss on the change in the fair value of our derivatives in connection with the call spread overlay on our convertible notes, in each case during 2015 for which we did not have similar charges during the prior year period. The decrease in net income during 2015 was partially offset by the gain of $45.6 million from the sale of our rights to Daraprim® in 2015.
Impax Generics
The following table sets forth results of operations for the Impax Generics division for the years ended December 31, 2015 and 2014 (in thousands):
| Year Ended December 31, | ||||||||||||||||
| Increase/ | ||||||||||||||||
|
2015 |
2014 |
(Decrease) |
||||||||||||||
|
Revenues |
$ | % | ||||||||||||||
|
Impax Generics sales, net |
$ | 699,844 | $ | 528,512 | $ | 171,332 | 32 | % | ||||||||
|
Rx Partner |
9,307 | 14,114 | (4,807 | ) | (34 | )% | ||||||||||
|
Other Revenues |
1,781 | 6,456 | (4,675 | ) | (72 | )% | ||||||||||
|
Total revenues |
710,932 | 549,082 | 161,850 | 29 | % | |||||||||||
|
Cost of revenues |
450,045 | 260,459 | 189,586 | 73 | % | |||||||||||
|
Gross profit |
260,887 | 288,623 | (27,736 | ) | (10 | )% | ||||||||||
|
Operating expenses: |
||||||||||||||||
|
Research and development |
58,838 | 40,927 | 17,911 | 44 | % | |||||||||||
|
Patent litigation |
2,942 | 5,333 | (2,391 | ) | (45 | )% | ||||||||||
|
Selling, general and administrative |
29,641 | 17,144 | 12,497 | 73 | % | |||||||||||
|
Total operating expenses |
91,421 | 63,404 | 28,017 | 44 | % | |||||||||||
|
Income from operations |
$ | 169,466 | $ | 225,219 | $ | (55,753 | ) | (25 | )% | |||||||
Revenues
Total revenues for the Impax Generics division for the year ended December 31, 2015 were $710.9 million, an increase of 29% from the same period in 2014, principally resulting from the addition of product revenue from the Tower acquisition as well as increased sales from diclofenac sodium gel and 14 generic products launched during 2015 including Lamotrigine ODT, partially offset by the absence of revenues from sales of authorized generic Renvela® during 2015, for which there were sales during the prior year.
Cost of Revenues
Cost of revenues was $450.0 million for the year ended December 31, 2015, an increase of $189.5 million compared to cost of revenues of $260.5 million in the prior year. In addition to increased costs related to higher product sales, cost of revenues in the current period increased due to higher product amortization, intangible asset impairment charges, costs related to the step-up to fair value of inventory in connection with the Tower acquisition, as well as closing and severance costs related to the restructuring of our packaging and distribution operations announced on June 30, 2015. This increase was partially offset by lower remediation costs, as compared to the prior year.
Gross Profit
Gross profit for the year ended December 31, 2015 was $260.9 million, or approximately 37% of total revenues, as compared to $288.6 million, or approximately 53% of total revenues, in the prior year. The decline in gross profit and gross margin was primarily driven by product mix. Sales during 2014 of authorized generic Renvela®, a high margin product, for which we had no sales in 2015, were replaced by lower margin products from the Tower acquisition and sales of diclofenac sodium gel which carry a 50% profit share with our third party partner.
Research and Development Expenses
Total research and development expenses for the year ended December 31, 2015 were $58.8 million, an increase of 44%, as compared to the prior year. Generic research and development expenses increased in 2015 compared to the prior year, primarily due to the addition of research and development projects from the Tower acquisition including intangible asset impairment charges of $6.4 million.
Patent Litigation Expenses
Patent litigation expenses for the year ended December 31, 2015 were $2.9 million, a decrease of 45%, as compared to the prior year. The decrease in patent litigation expenses in 2015 of $2.4 million compared to the prior year was the result of legal activity related to several cases in the prior year for which there was no corresponding activity in 2015.
Selling, General and Administrative Expenses
Selling, general and administrative expenses for the year ended December 31, 2015 were $29.6 million, a 73% increase over the prior year. The increase during 2015 from the prior year was primarily the result of an increase in accounts receivable reserves and an increase in penalties paid to customers for delays or failures to supply product and the addition of the selling, general and administrative expenses from the Tower acquisition that were partially offset by lower personnel expense during 2015.
Impax Specialty Pharma
The following table sets forth results of operations for the Impax Specialty Pharma division for the years ended December 31, 2015 and 2014 (in thousands):
|
Year Ended December 31, |
||||||||||||||||
| Increase/ | ||||||||||||||||
|
2015 |
2014 |
(Decrease) |
||||||||||||||
|
Revenues |
$ | % | ||||||||||||||
|
Impax Product sales, net |
$ | 145,226 | $ | 45,938 | $ | 99,288 | * | |||||||||
|
Other Revenues |
4,311 | 1,029 | 3,282 | * | ||||||||||||
|
Total revenue |
149,537 | 46,967 | 102,570 | * | ||||||||||||
|
Cost of revenues |
58,020 | 22,937 | 35,083 | * | ||||||||||||
|
Gross profit |
91,517 | 24,030 | 67,487 | * | ||||||||||||
|
Operating expenses: |
||||||||||||||||
|
Research and development |
18,144 | 37,715 | (19,571 | ) | (52 | )% | ||||||||||
|
Patent litigation |
1,625 | 472 | 1,153 | * | ||||||||||||
|
Selling, general and administrative |
52,427 | 43,307 | 9,120 | 21 | % | |||||||||||
|
Total operating expenses |
72,196 | 81,494 | (9,298 | ) | (11 | )% | ||||||||||
|
Income (loss) from operations |
$ | 19,321 | $ | (57,464 | ) | $ | 76,785 | * | ||||||||
* Percentage exceeds 100%
Revenues
Total revenues for the Impax Specialty Pharma division were $149.5 million for the year ended December 31, 2015, an increase of $102.6 million compared to the year ended December 31, 2014, due to revenues from the launch of Rytary® and revenues from the Tower acquisition during 2015.
Cost of Revenues
Cost of revenues was $58.0 million for the year ended December 31, 2015, an increase of $35.1 million over the prior year. In addition to increased costs related to increased product sales, cost of revenues increased in 2015 due to higher amortization and costs related to the step-up to fair value of inventory, each incurred in connection with the Tower acquisition. This increase was partially offset by a reserve included in the cost of revenues during 2014 for pre-launch inventory related to Rytary® as a result of a Complete Response Letter received in 2014, for which there were no similar amounts included in cost of revenues in 2015.
Gross Profit
Gross profit for the year ended December 31, 2015 was $91.5 million or approximately 61% of total revenues, as compared to $24.0 million or approximately 51% of total revenues in the prior year. The revenue from Rytary® and revenue from the Tower acquisition were the primary drivers of the increase in gross profit compared to the prior year. This increase during 2015 was partially offset by a reserve included in the cost of revenues during 2014 for pre-launch inventory related to Rytary® as a result of a Complete Response Letter received in 2014, for which there were no similar amounts included in cost of revenues in 2015.
Research and Development Expenses
Total research and development expenses for the year ended December 31, 2015 were $18.1 million, a decrease of 52%, as compared to $37.7 million in the prior year. The decrease was primarily driven by a reduction in research and development expenses related to our branded initiatives and decreased costs related to reduced personnel compared to 2014 due to the restructuring and related reduction in workforce primarily in our research and development organization during the quarter ended December 31, 2014. In addition, research and development expense during 2014 included a $2.0 million upfront fee paid to Durect under an agreement to acquire the exclusive worldwide rights to develop and commercialize Durect’s investigational transdermal bupivacaine patch for the treatment of pain associated with post-herpetic neuralgia, for which there was no comparable payment made in 2015.
Patent Litigation Expenses
Patent litigation expenses for the year ended December 31, 2015 were $1.6 million, an increase of $1.1 million compared to the prior year amount of $0.5 million. The increase was the result of legal activity related to several cases in 2015 for which there was no corresponding activity in the prior year.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $52.4 million in the year ended December 31, 2015, an increase of $9.1 million as compared to $43.3 million in the prior year. The increase was primarily driven by an increase in advertising and promotion expenses to support the launch of Rytary® and related salesforce expansion as well as selling expenses from the Tower acquisition.
Corporate and other
The following table sets forth corporate general and administrative expenses, as well as other items of income and expense presented below income from operations for the years ended December 31, 2015 and 2014 (in thousands):
|
Year Ended December 31, |
||||||||||||||||
|
2015 |
2014 |
Increase/ (Decrease) |
||||||||||||||
| $ | % | |||||||||||||||
|
General and administrative expenses |
$ | 119,219 | $ | 78,939 | $ | 40,280 | 51 | % | ||||||||
|
Unallocated corporate expenses |
(119,219 | ) | (78,939 | ) | (40,280 | ) | (51 | )% | ||||||||
|
Interest income |
1,042 | 1,473 | (431 | ) | (29 | )% | ||||||||||
|
Interest expense |
(27,268 | ) | (43 | ) | (27,225 | ) | * | |||||||||
| Gain on sale of asset | 45,574 | --- | 45,574 | * | ||||||||||||
| Loss on debt extinguishment | (16,903 | ) | --- | (16,903 | ) | * | ||||||||||
| Net change in fair value of derivatives | (13,000 | ) | --- | (13,000 | ) | * | ||||||||||
| Other income, net | 355 | 313 | 42 | 13 | % | |||||||||||
|
Loss before income taxes |
(129,419 | ) | (77,196 | ) | (52,223 | ) | (68 | )% | ||||||||
|
Provision for income taxes |
$ | 20,371 | $ | 33,206 | $ | (12,835 | ) | (39 | )% | |||||||
* Percentage exceeds 100%
General and Administrative Expenses
General and administrative expenses for the year ended December 31, 2015 were $119.2 million, a $40.3 million increase, as compared to $78.9 million in the prior period. The increase was principally driven by higher business development expenses, the majority of which was transaction and/or integration activities related to the Tower acquisition, the inclusion of general and administrative expenses from the Tower acquisition, increased finance and information technology expenses as well as higher equity based compensation, in each case compared to the prior year. The Tower-related general and administrative expenses included $2.4 million in employee severance costs related to the acquisition.
Other Income, Net
Other income, net of $0.4 million in the year ended December 31, 2015, was consistent with the prior year, primarily related to our sale of an ANDA during 2015 for $1.0 million. Partially offsetting this income in 2015 was a fixed asset impairment, for which there was no corresponding charge in the prior year.
Loss on debt extinguishment
During the year ended December 31, 2015, we recognized a $16.9 million loss on the extinguishment of debt related to unamortized debt issuance costs upon the repayment of our term loan with Barclays, for which there was no comparable loss in the prior year.
Gain on sale of asset
During the year ended December 31, 2015, we recognized a gain of $45.6 million on the sale of our rights to Daraprim®, for which there was no comparable amount in the prior year.
Net change in fair value of derivatives
During the year ended December 31, 2015, we recognized a $13.0 million expense from the net change in the fair value of our derivative instruments related to our convertible senior notes at June 30, 2015 compared to December 8, 2015. We did not incur a corresponding charge in the prior year. See “Note 8. Derivatives” for additional information.
Interest Income
Interest income in the year ended December 31, 2015 was $1.0 million, a slight decrease from 2014.
Interest Expense
Interest expense in the year ended December 31, 2015 was $27.3 million, primarily related to interest expenses on debt issued in connection with the Tower acquisition as well as interest accrued on our outstanding convertible notes which were issued in 2015 and for which we did not incur similar expense during the prior year. Interest expense in 2015 also included $2.3 million in commitment fees incurred prior to the closing of the Tower acquisition, for which there were no corresponding fees in the prior year.
Income Taxes
During the year ended December 31, 2015, we recorded an aggregate tax provision of $20.4 million for U.S. domestic income taxes and for foreign income taxes, a decrease of $12.8 million compared to an aggregate tax provision of $33.2 million we recorded during the prior year. The decrease in the tax provision during 2015 compared to the prior year resulted from lower income before taxes in the year ended December 31, 2015. The effective tax rate decreased to 34% for the year ended December 31, 2015 compared to 37% for the year ended December 31, 2014. The 2015 effective tax rate was lower due to a change in the timing and mix of U.S. and foreign income. Other contributing factors to the rate fluctuation included favorable book-tax differences for federal tax benefits, including the R&D credit and domestic manufacturing deduction, on the Tower entities which we acquired in 2015.
Results of Operations - Year Ended December 31, 2014 Compared to Year Ended December 31, 2013
Overview
The following table sets forth our summarized, consolidated results of operations for the years ended December 31, 2014 and 2013 (in thousands):
|
Year Ended December 31, |
||||||||||||||||
|
Increase/ |
||||||||||||||||
|
2014 |
2013 |
(Decrease) |
||||||||||||||
| $ | % | |||||||||||||||
|
Total revenues |
$ | 596,049 | $ | 511,502 | $ | 84,547 | 17 | % | ||||||||
|
Gross profit |
312,653 | 199,300 | 113,353 | 57 | % | |||||||||||
|
Income (loss) from operations |
88,816 | (6,387 | ) | 95,203 | * | |||||||||||
|
Income before income taxes |
90,559 | 146,940 | (56,381 | ) | (38 | )% | ||||||||||
|
Provision for income taxes |
33,206 | 45,681 | (12,475 | ) | (27 | )% | ||||||||||
|
Net income |
$ | 57,353 | $ | 101,259 | $ | (43,906 | ) | (43 | )% | |||||||
* Percentage exceeds 100%
Consolidated total revenues for the year ended December 31, 2014 increased $84.5 million, or 17%, as compared to the year ended December 31, 2013. New product launches increased revenues during the year ended December 31, 2014 by $84.4 million, or 17%, compared to 2013, primarily related to sales of our authorized generic Renvela® tablets and generic Solaraze®. Decreased product volumes (excluding new product launches) decreased revenues during the year ended December 31, 2014 by $20.3 million, or 4%, compared to 2013, while selling price and product mix increased revenues by $20.4 million, or 4%, compared to 2013. Revenues from our Impax Generics division increased $150.7 million during the year ended December 31, 2014, as compared to 2013, driven primarily by sales of our authorized generic Renvela® tablets, in addition to higher sales of our Digoxin and generic Solaraze® products. Revenues from our Impax Specialty Pharma division decreased $66.2 million in 2014 as compared to 2013, as a result of a decline in sales of our Impax-labeled branded Zomig® products. In May 2013, our exclusivity period for branded Zomig® tablets and orally disintegrating tablets expired and we launched authorized generic versions of those products in the United States.
Net income for the year ended December 31, 2014 was $57.4 million, a decrease of $43.9 million as compared to $101.3 million for the year ended December 31, 2013. The decrease from 2013 was primarily attributable to a $102.0 million gain in connection with the settlement of litigation with Endo which we recorded as “Other Income” in the three month period ended March 31, 2013, as well as the receipt of a $48.0 million payment from Shire in the three month period ended March 31, 2013, in connection with the settlement of litigation and for which there were no corresponding gains or payments during the year ended December 31, 2014 and which were both partially offset by the tax consequences of the settlements. Also contributing to the change in net income was an increase in Impax Generics division revenue related to increased sales on several of our higher margin products, partially offset by a decline in sales of our Impax-labeled branded Zomig® products, as discussed above.
Impax Generics
The following table sets forth results of operations for the Impax Generics division for the years ended December 31, 2014 and 2013 (in thousands):
| Year Ended December 31, | ||||||||||||||||
|
Increase/ |
||||||||||||||||
|
2014 |
2013 |
(Decrease) |
||||||||||||||
|
Revenues |
$ | % | ||||||||||||||
|
Impax Generics sales, net |
$ | 528,512 | $ | 383,652 | $ | 144,860 | 38 | % | ||||||||
|
Rx Partner |
14,114 | 11,639 | 2,475 | 21 | % | |||||||||||
|
Other Revenues |
6,456 | 3,049 | 3,407 | * | ||||||||||||
|
Total revenues |
549,082 | 398,340 | 150,742 | 38 | % | |||||||||||
|
Cost of revenues |
260,459 | 253,836 | 6,623 | 3 | % | |||||||||||
|
Gross profit |
288,623 | 144,504 | 144,119 | 100 | % | |||||||||||
|
Operating expenses: |
||||||||||||||||
|
Research and development |
40,927 | 41,384 | (457 | ) | (1 | )% | ||||||||||
|
Patent litigation |
5,333 | 16,545 | (11,212 | ) | (68 | )% | ||||||||||
|
Selling, general and administrative |
17,144 | 17,684 | (540 | ) | (3 | )% | ||||||||||
|
Total operating expenses |
63,404 | 75,613 | (12,209 | ) | (16 | )% | ||||||||||
|
Income from operations |
$ | 225,219 | $ | 68,891 | $ | 156,328 | * | |||||||||
* Percentage exceeds 100%
Revenues
Total revenues for the Impax Generics division for the year ended December 31, 2014, were $549.1 million, an increase of 38% from 2013, resulting from increases in Impax Generics sales, net, and Other Revenues, as discussed below.
Impax Generics sales, net, were $528.5 million for the year ended December 31, 2014, an increase of 38% from 2013, primarily as a result of sales of our authorized generic Renvela® tablets launched during the three month period ended June 30, 2014, higher sales of our Digoxin products and the launch of our generic Solaraze® during the fourth quarter of 2013.
Rx Partner revenues were $14.1 million for the year ended December 31, 2014, an increase of 21% over the prior year resulting primarily from a $3.3 million profit share earned pursuant to our agreement with Perrigo related to the launch of generic Astepro®.
Other Revenues were $6.5 million for the year ended December 31, 2014, with the increase from the prior year primarily resulting from the receipt of a milestone payment pursuant to our agreement with a third-party pharmaceutical company.
Cost of Revenues
Cost of revenues was $260.5 million for the year ended December 31, 2014 and $253.8 million for the prior year, an increase of $6.6 million compared to the prior year. Cost of revenues increased due to increased revenue as discussed above. Such increases were partially offset by $10.3 of higher intangible asset impairment charges taken in the prior year, as discussed in “Note 12. Goodwill and Intangible Assets” and $13.0 million more in inventory reserves related to discontinued product and other reserves for pre-launch inventory taken during the year ended December 31, 2013.
Gross Profit
Gross profit for the year ended December 31, 2014 was $288.6 million, or approximately 53% of total revenues, as compared to $144.5 million, or approximately 36% of total revenues, in the prior year. Gross profit as a percent of total revenues increased in 2014 as compared to the prior year largely due to favorable product contribution from higher margin products, including Renvela®, as well as the other items noted above under Cost of Revenues.
Research and Development Expenses
Total research and development expenses for the year ended December 31, 2014 were $40.9 million, a decrease of 1%, as compared to the same period of the prior year. Generic research and development expenses decreased in 2014 compared to the prior year primarily due to lower external development spending of $5.8 million and a decrease in professional fees of $0.5 million. In addition, during 2013, we incurred an intangible impairment charge of $0.8 million, for which there was no comparable charge in 2014. These decreases were partially offset by an overall increase of $4.6 million associated with internal development spending and an increase in personnel costs of $2.0 million, which included $0.7 million of severance expense associated with the reduction in workforce primarily related to our research and development organization during the fourth quarter ended December 31, 2014.
Patent Litigation Expenses
Patent litigation expenses for the year ended December 31, 2014 were $5.3 million, a decrease of 68%, as compared to the prior year. The decrease in patent litigation expenses in 2014 of $11.2 million compared to the prior year was the result of legal activity related to several cases in 2013 for which there was no corresponding activity in 2014.
Selling, General and Administrative Expenses
Selling, general and administrative expenses for the year ended December 31, 2014 were $17.1 million, a 3% decrease over the prior year. The decrease from the prior year was primarily due to $2.9 million in lower customer claims relating to failure to supply products in accordance with their contractual terms. This decrease was partially offset by an increase in personnel expense of $2.5 million primarily related to severance expenses associated with the reduction in workforce primarily related to our research and development organization during the fourth quarter ended December 31, 2014.
Impax Specialty Pharma
The following table sets forth results of operations for the Impax Specialty Pharma division for the years ended December 31, 2014 and 2013 (in thousands):
|
Year Ended December 31, |
||||||||||||||||
|
Increase/ |
||||||||||||||||
|
2014 |
2013 |
(Decrease) |
||||||||||||||
|
Revenues |
$ | % | ||||||||||||||
|
Impax Product sales, net |
$ | 45,938 | $ | 111,900 | $ | (65,962 | ) | (59 | )% | |||||||
|
Other Revenues |
1,029 | 1,262 | (233 | ) | (18 | )% | ||||||||||
|
Total revenue |
46,967 | 113,162 | (66,195 | ) | (58 | )% | ||||||||||
|
Cost of revenues |
22,937 | 58,366 | (35,429 | ) | (61 | )% | ||||||||||
|
Gross profit |
24,030 | 54,796 | (30,766 | ) | (56 | )% | ||||||||||
|
Operating expenses: |
||||||||||||||||
|
Research and development |
38,187 | 27,470 | 10,717 | 39 | % | |||||||||||
|
Selling, general and administrative |
43,307 | 44,915 | (1,608 | ) | (4 | )% | ||||||||||
|
Total operating expenses |
81,494 | 72,385 | 9,109 | 13 | % | |||||||||||
|
Loss from operations |
$ | (57,464 | ) | $ | (17,589 | ) | $ | (39,875 | ) | * | ||||||
* Percentage exceeds 100%
Revenues
Total revenues for the Impax Specialty Pharma division were $47.0 million for the year ended December 31, 2014, a decrease of $66.2 million compared to the year ended December 31, 2013, due to lower sales of our Impax-labeled branded Zomig® products which we began selling during the year ended December 31, 2012. In May 2013, our exclusivity period for branded Zomig® tablets and orally disintegrating tablets expired and we launched authorized generic versions of those products in the United States. We continue to sell the branded Zomig® nasal spray.
Other Revenues for the year ended December 31, 2014 were relatively consistent with Other Revenues for the prior year, representing the recognition of an initial $10.0 million upfront payment we received in June 2010 under a Development and Co-Promotion Agreement with Endo Pharmaceuticals, Inc. During 2014, we recognized this upfront payment as revenue on a straight-line basis over our expected period of performance during the development period, which we estimated to be the 112 month period ending September 2019.
Cost of Revenues
Cost of revenues were $22.9 million for the year ended December 31, 2014, a decrease of $35.4 million over the prior year, commensurate with a reduction in revenues and lower amortization costs related to the loss of exclusivity on branded Zomig® tablets and orally disintegrating tablets in May 2013.
Gross Profit
Gross profit for the year ended December 31, 2014 was $24.0 million, a decrease of $30.8 million over the prior year, commensurate with a reduction in revenues and other costs noted above.
Research and Development Expenses
Total research and development expenses for the year ended December 31, 2014 were $38.2 million, an increase of 39%, as compared to $27.5 million in the prior year. The increase was primarily driven by an increase in research and development expenses related to our branded initiatives and increased personnel costs of $2.0 million, in each case compared to 2013. We also paid a $2.0 million upfront fee to Durect under an agreement to acquire the exclusive worldwide rights to develop and commercialize Durect’s investigational transdermal bupivacaine patch for the treatment of pain associated with PHN, referred to as IPX239, for which there was no corresponding charge in 2013.
Selling, General and Administrative Expenses
Selling, general and administrative expenses were $43.3 million in the year ended December 31, 2014, a decrease of $1.6 million as compared to $44.9 million in the prior year. The decrease compared to 2013 was primarily driven by a $1.4 million reduction in advertising and promotion expenses for Zomig® due to loss of exclusivity of branded Zomig® tablets and orally disintegrating tablets during 2013, a $1.0 million reduction in legal support of brand licensing initiatives and a $0.9 million expense due to a reduction in the sales force workforce. These reductions were partially offset by a $1.1 million increase in advertising and promotion expenses for pre-launch support for Rytary™ and increased personnel costs of $0.5 million during 2014.
Corporate and other
The following table sets forth corporate general and administrative expenses, as well as other items of income and expense presented below income from operations for the years ended December 31, 2014 and 2013 (in thousands):
|
Year Ended December 31, |
||||||||||||||||
|
Increase/ |
||||||||||||||||
|
2014 |
2013 |
(Decrease) |
||||||||||||||
| $ | % | |||||||||||||||
|
General and administrative expenses |
$ | 78,939 | $ | 57,689 | $ | 21,250 | 37 | % | ||||||||
|
Total operating expenses |
78,939 | 57,689 | 21,250 | 37 | % | |||||||||||
|
Loss from operations |
(78,939 | ) | (57,689 | ) | (21,250 | ) | (37 | )% | ||||||||
|
Other income (expense), net |
313 | 152,447 | (152,134 | ) | 100 | % | ||||||||||
|
Interest income |
1,473 | 1,299 | 174 | 13 | % | |||||||||||
|
Interest expense |
43 | 419 | (376 | ) | (90 | )% | ||||||||||
|
Income (loss) before income taxes |
(77,196 | ) | 95,638 | (172,834 | ) | * | ||||||||||
|
Provision for income taxes |
$ | 33,206 | $ | 45,681 | $ | (12,475 | ) | (27 | )% | |||||||
* Percentage exceeds 100%
General and Administrative Expenses
General and administrative expenses for the year ended December 31, 2014 were $78.9 million, a $21.3 million increase, as compared to $57.7 million in 2013. The increase was principally driven by increases in business development expenses of $10.2 million, information technology expenses of $3.8 million, legal expenses of $3.6 million, and net other costs of $3.5 million during 2014.
Other Income (Expense), Net
Other income, net of $0.3 million in the year ended December 31, 2014, a decrease of $152.1 million from the prior year, primarily due to a $102.0 million gain during 2013 in connection with the settlement of litigation with Endo which we recorded as Other Income in the three month period ended March 31, 2013, as well as a $48.0 million payment received from Shire in connection with the settlement of litigation in the three month period ended March 31, 2013, for which there were no comparable charges during 2014. In addition, we recorded a $3.0 million gain in connection with the settlement of litigation in Other Income during the three month period ended June 30, 2013, for which there was no comparable charge during 2014. Partially offsetting this income in the prior year period was a $0.9 million loss on disposal of software in the three month period ended March 31, 2013.
Interest Income
Interest income in the year ended December 31, 2014 was $1.5 million, a slight increase from 2013.
Interest Expense
Interest expense in the year ended December 31, 2014 was less than $0.1 million, a decrease of $0.4 million from 2013, resulting from a reduction in the interest related to our liability for uncertain tax positions.
Income Taxes
During the year ended December 31, 2014, we recorded an aggregate tax provision of $33.2 million for U.S. domestic income taxes and for foreign income taxes, a decrease of $12.5 million compared to an aggregate tax provision of $45.7 million we recorded during the prior year. The decrease in the tax provision during 2014 compared to the prior year resulted from lower income before taxes in the year ended December 31, 2014 as compared to the prior year. The effective tax rate increased to 36% for the year ended December 31, 2014 as compared to 31% for the year ended December 31, 2013. The 2013 effective tax rate was lower due to two years of the federal tax benefit being recorded as a result of the legislation’s enactment date. In addition, the 2014 effective tax rate was affected by losses incurred in certain zero rate jurisdictions, for which there were no corresponding costs in the prior year period.
Liquidity and Capital Resources
We generally fund our operations with cash from operations; however, we have used proceeds from the sale of debt and equity securities in the past. Our cash from operations consists primarily of the proceeds from the sales of our products and services.
We expect to incur significant operating expenses, including research and development activities and patent litigation expenses, for the foreseeable future. In addition, we are generally required to make cash expenditures to manufacture or acquire finished product inventory in advance of selling the finished product to our customers and collecting payment, which may result in significant periodic uses of cash. We believe our existing cash and cash equivalents, together with cash expected to be generated from operations, and our revolving line of credit will be sufficient to meet our financing requirements through the next 12 months. We may, however, seek additional financing through alliance, collaboration, and/or licensing agreements, as well as from the debt and equity capital markets to fund capital expenditures, research and development plans, potential acquisitions, and potential revenue shortfalls due to delays in new product introductions or otherwise. We cannot assure that such financing will be available on favorable terms, or at all.
Cash and Cash Equivalents
At December 31, 2015, we had $340.4 million in cash and cash equivalents, an increase of $125.5 million as compared to $214.9 million at December 31, 2014. As more fully discussed below, the increase in cash and cash equivalents during the year ended December 31, 2015 was driven by $521.4 million of net cash provided by financing activities and $71.9 million of net cash provided by operating activities, partially offset by net cash used in investing activities of $467.5 million.
Cash Flows
Year Ended December 31, 2015 Compared to Year Ended December 31, 2014.
Net cash provided by operating activities for the year ended December 31, 2015 was $71.9 million, an increase of $39.1 million as compared to the prior year $32.8 million net cash provided by operating activities. The significant factors contributing to the increased cash flow from operations during 2015 included increased net profit sharing accruals driven by the product sales mix experienced in the fourth quarter of 2015, increased amortization and impairment charges related to the Tower acquisition, increased share based compensation expense, as well as an add back for the write off of deferred financing costs which occurred in the second quarter of 2015. These increases were partially offset by reduced net income which was largely driven by the absence of sales of authorized generic Renvela® and a deduction for the gain on sale of our rights to Daraprim® during 2015.
Net cash used in investing activities for the year ended December 31, 2015 was $467.5 million as compared to $16.6 million for the prior year. The period over period increase in net cash used was due primarily to cash used to fund the Tower acquisition purchase price of $691.3 million (net of cash acquired in the acquisition), partially offset by a change in purchases of short term investments and a change in maturities of investments of $170.8 million with those cash proceeds used in part to fund the Tower acquisition. Net cash flow from investing activities also included $55.5 million in proceeds from the sale of our rights to Daraprim® during 2015.
Net cash provided by financing activities for the year ended December 31, 2015 was $521.4 million, representing an increase of $507.0 million as compared to the prior year $14.4 million of net cash provided by financing activities. The year-over-year increase in net cash provided by financing activities was due to the sales of our convertible notes and related bond hedge activities. Please refer to “Outstanding Debt Obligations” below for further details.
Cash Flows - Year Ended December 31, 2014 Compared to Year Ended December 31, 2013.
Net cash provided by operating activities for the year ended December 31, 2014 was $32.8 million, a decrease of $117.1 million as compared to the prior year $149.9 million net cash provided by operating activities. The period-over-period change in net cash provided by operating activities was driven by lower net income as a result of the payments received in connection with various legal settlements in 2013 as well as an increased investment in working capital. The increased net investment in working capital in 2014 compared to 2013 was largely driven by accounts receivable and inventory. The 2014 increase in accounts receivable was primarily due to increased sales in the fourth quarter of 2014 compared to the fourth quarter of 2013. The increase in accounts receivable in 2013 was due to timing of cash collections as we experienced some delays in payments. The 2014 increase in inventory was incurred to support the 2015 launch of Rytary™ as well as to support commercial activities on certain backordered products.
Net cash used in investing activities for the year ended December 31, 2014 was $16.6 million as compared to $115.9 million for the prior year. The decrease in cash used in investing activities during 2014 was due primarily due to a year-over-year increase in cash provided by net maturities of short-term investments of $109.4 million. This change was caused by the receipt of over $150 million in legal settlements in 2013 and our subsequent investment in short-term investments. Purchases of property, plant and equipment for the year ended December 31, 2014 were $29.9 million as compared to $32.8 million for the prior year, due to reduced spending on the Taiwan manufacturing facility, which was partially offset by increased remediation related projects.
Net cash provided by financing activities for the year ended December 31, 2014 was $14.4 million, representing an increase of $5.4 million as compared to the prior year $9.0 million of net cash provided by financing activities. The year-over-year increase in net cash provided by financing activities was due to a $2.9 million increase in the cash proceeds received from the exercise of stock options and contributions to the employee stock purchase plan and a $2.6 million increase in tax benefits related to the exercise of employee stock options.
Commitments and Contractual Obligations
Our contractual obligations as of December 31, 2015 were as follows (in thousands):
|
Payments Due by Period |
||||||||||||||||||||
|
Contractual Obligations |
Total |
Less Than 1 Year |
1-3 Years |
3-5 Years |
More Than 5 Years |
|||||||||||||||
|
Open Purchase Order Commitments |
$ | 67,091 | $ | 67,091 | $ | -- | $ | -- | $ | -- | ||||||||||
|
Operating Leases(a) |
22,756 | 5,797 | 9,026 | 3,476 | 4,457 | |||||||||||||||
|
Construction Contracts(b) |
262 | 262 | -- | -- | -- | |||||||||||||||
|
Total(c) |
$ | 90,109 | $ | 73,150 | $ | 9,026 | $ | 3,476 | $ | 4,457 | ||||||||||
|
(a) |
We lease office, warehouse, and laboratory facilities under non-cancelable operating leases with expiration dates through December 2026. We also lease certain equipment under various non-cancelable operating leases with various expiration dates through December 2018. |
|
(b) |
Construction contracts are related to ongoing expansion activities at our manufacturing facility in Taiwan. |
|
(c) |
Liabilities for uncertain tax positions FASB ASC Topic 740, Sub-topic 10, were excluded as we are not able to make a reasonably reliable estimate of the amount and period of related future payments. As of December 31, 2015, we had a $4.4 million provision for uncertain tax positions. |
Off-Balance Sheet Arrangements
We did not have any off-balance sheet arrangements as of December 31, 2015 and 2014.
Outstanding Debt Obligations
2% Convertible Senior Notes due June 2022
On June 30, 2015, we issued an aggregate principal amount of $600.0 million of 2.00% Convertible Senior Notes due June 2022 (the “Notes”) in a private placement offering, which are our senior unsecured obligations. The Notes were issued pursuant to an Indenture dated June 30, 2015 (the “Indenture”) between us and Wilmington Trust, N.A., as trustee. The Indenture includes customary covenants and sets forth certain events of default after which the Notes may be due and payable immediately. The Notes will mature on June 15, 2022, unless earlier redeemed, repurchased or converted. The Notes bear interest at a rate of 2.00% per year, and interest is payable semiannually in arrears on June 15 and December 15 of each year, beginning on December 15, 2015.
The conversion rate for the Notes is initially set at 15.7858 shares per $1,000 of principal amount, which is equivalent to an initial conversion price of $63.35 per share of our common stock. If a Make-Whole Fundamental Change (as defined in the Indenture) occurs or becomes effective prior to the maturity date and a holder elects to convert its Notes in connection with the Make-Whole Fundamental Change, we are obligated to increase the conversion rate for the Notes so surrendered by a number of additional shares of our common stock as prescribed in the Indenture. Additionally, the conversion rate is subject to adjustment in the event of an equity restructuring transaction such as a stock dividend, stock split, spinoff, rights offering, or recapitalization through a large, nonrecurring cash dividend (“standard antidilution provisions,” per ASC 815-40 – Contracts in Entity’s Own Equity).
The Notes are convertible at the option of the holders at any time prior to the close of business on the business day immediately preceding December 15, 2021 only under the following circumstances:
|
(i) |
If during any calendar quarter commencing after the quarter ending September 30, 2015 (and only during such calendar quarter) the last reported sale price of our common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter is greater than 130% of the conversion price on each applicable trading day; or | |
|
(ii) |
If during the five business day period after any 10 consecutive trading day period (the “measurement period”) in which the trading price per $1,000 of principal amount of Notes for each trading day of the measurement period was less than 98% of the product of the last report sale price of our common stock and the conversion rate on each such trading day; or | |
|
(iii) |
Upon the occurrence of corporate events specified in the Indenture. |
On or after December 15, 2021 until the close of business on the second scheduled trading day immediately preceding the maturity date, the holders may convert their Notes at any time, regardless of the foregoing circumstances. We may satisfy our conversion obligation by paying or delivering, as the case may be, cash, shares of our common stock, or a combination of cash and shares of our common stock, at our election and in the manner and subject to the terms and conditions provided in the Indenture.
Concurrently with the offering of the Notes and using a portion of the proceeds from the sale of the Notes, we entered into a series of convertible note hedge and warrant transactions (the “Note Hedge Transactions” and “Warrant Transactions”) which are designed to reduce the potential dilution to our stockholders and/or offset the cash payments we are required to make in excess of the principal amount upon conversion of the Notes. The Note Hedge Transactions and Warrant Transactions are separate transactions, in each case, entered into by us with a financial institution and are not part of the terms of the Notes. These transactions will not affect any holder’s rights under the Notes, and the holders of the Notes have no rights with respect to the Note Hedge Transactions and Warrant Transactions. See “Note 8. Derivatives” and “Note 15. Stockholders’ Equity” for additional information.
At the June 30, 2015 issuance date of the Notes, we did not have the necessary number of authorized but unissued shares of its common stock available to settle the conversion option of the Notes in shares of our common stock. Therefore, in accordance with guidance found in ASC 470-20 – Debt with Conversion and Other Options (“ASC 470-20”) and ASC 815-15 – Embedded Derivatives (“ASC 815-15”), the conversion option of the Notes was deemed an embedded derivative requiring bifurcation from the Notes (host contract) and separate accounting as a derivative liability. The fair value of the conversion option derivative liability at June 30, 2015 was $167.0 million, which was recorded as a reduction to the carrying value of the debt. This debt discount will be amortized to interest expense over the term of the debt using the effective interest method. Although we received stockholder approval on December 8, 2015 to amend our Restated Certificate of Incorporation to increase the number of authorized shares of our common stock (which amendment has occurred), the debt discount related to the conversion option derivative liability remains and continues to be amortized to interest expense. The effect of the increase in the authorized number of shares of our common stock on the derivative liability is discussed in “Note 8. Derivatives.”
In connection with the issuance of the Notes, we incurred approximately $18.7 million of debt issuance costs for banking, legal and accounting fees and other expenses during the year ended December 31, 2015. This was also recorded on our balance sheet as a reduction to the carrying value of the debt, in accordance with our early adoption of Accounting Standards Update (“ASU”) No. 2015-03 – Simplifying the Presentation of Debt Issuance Costs (“ASU 2015-03”), and will be amortized to interest expense over the term of the debt using the effective interest method.
For the year ended December 31, 2015, we recognized $16.3 million of interest expense related to the Notes, of which $6.0 million was cash and $10.3 million was non-cash accretion of the debt discounts recorded. As the Notes mature in 2022, such interest has been classified as long-term debt on our consolidated balance sheet, with a carrying value of approximately $424.6 million as of December 31, 2015.
Royal Bank of Canada $100.0 Million Revolver
On August 4, 2015, we entered into a senior secured revolving credit facility (the “Revolving Credit Facility”) of up to $100 million, pursuant to a credit agreement, by and among us, the lenders party thereto from time to time and Royal Bank of Canada, as administrative agent and collateral agent (the “Revolving Credit Facility Agreement”). The Revolving Credit Facility is available for working capital and other general corporate purposes. Borrowings under the Revolving Credit Facility will accrue interest at a rate equal to LIBOR or the base rate, plus an applicable margin. The applicable margin may be increased or reduced by 0.75% based on our total net leverage ratio. The Revolving Credit Facility will mature on August 4, 2020. No borrowings were drawn from the Revolving Credit Facility during the year ended December 31, 2015.
Loss on Early Extinguishment of Debt – Barclays $435.0 Million Term Loan
In connection with the acquisition of Tower during the first quarter of 2015, we entered into a $435.0 million senior secured term loan facility (the “Term Loan”) and a $50.0 million senior secured revolving credit facility (the “Barclays Revolver” and collectively with the Term Loan, the “Barclays Senior Secured Credit Facilities”), pursuant to a credit agreement, dated as of March 9, 2015, by and among us, the lenders party thereto from time to time and Barclays Bank PLC, as administrative and collateral agent (the “Barclays Credit Agreement”). In connection with the Barclays Senior Secured Credit Facilities, we incurred debt issuance costs for banking, legal and accounting fees and other expenses of approximately $17.8 million. Prior to repayment of the Term Loan on June 30, 2015, these debt issuance costs were to be amortized to interest expense over the term of the loan using the effective interest rate method.
On June 30, 2015, we used approximately $436.4 million of the proceeds from the sale of the Notes to repay the $435.0 million of principal and approximately $1.4 million of accrued interest due on the Term Loan under the Barclays Credit Agreement. In connection with this repayment of the loan, we recorded a loss on early extinguishment of debt of approximately $16.9 million related to the unamortized portion of the deferred debt issuance costs during the quarter ended June 30, 2015.
For the six months ended June 30, 2015, we incurred total interest expense on the Term Loan of approximately $10.7 million, of which $9.8 million was cash and $0.9 million was non-cash amortization of the deferred debt issuance costs. Included in the 2015 year-to-date cash interest expense of $15.8 million is approximately $2.3 million related to a ticking fee paid to Barclays during the first quarter of 2015, prior to the funding of the Senior Secured Credit Facilities on March 9, 2015, to lock in the financing terms from the lenders’ commitment of the Term Loan until the actual allocation of the loan occurred.
Recent Accounting Pronouncements
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers” (Topic 606) regarding the accounting for and disclosures of revenue recognition, with an effective date for annual and interim periods beginning after December 15, 2016. This update provides a single comprehensive model for accounting for revenue from contracts with customers. The model requires that revenue recognized reflect the actual consideration to which the entity expects to be entitled in exchange for the goods or services defined in the contract, including in situations with multiple performance obligations. In July 2015, the FASB issued ASU 2015-14, “Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date” which deferred the effective date, of the previously issued revenue recognition guidance, by one year. The guidance will be effective for annual and interim periods beginning after December 15, 2017. We are currently evaluating the effect that this guidance may have on our consolidated financial statements.
In April 2015, the FASB issued ASU 2015-03, “Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs”, which provided guidance on the presentation requirements for debt issuance costs and debt discount and premium. The update requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by the updated guidance. The updated guidance is effective for annual and interim periods beginning after December 15, 2015 and early adoption is permitted for financial statements that have not been previously issued. We adopted this guidance in the three month period ended March 31, 2015, and it had no effect on our results of operations.
In July 2015, the FASB issued ASU 2015-11, Inventory (Topic 330): “Simplifying the Measurement of Inventory”, with guidance regarding the accounting for and measurement of inventory. The update requires that inventory measured using first-in, first-out (FIFO) shall be measured at the lower of cost and net realizable value. When there is evidence that the net realizable value of inventory is lower than its cost, the difference shall be recognized as a loss in earnings in the period in which it occurs. The guidance will be effective for annual and interim periods beginning after December 15, 2016. We are currently evaluating the effect that this guidance may have on our consolidated financial statements.
In August 2015, the FASB issued ASU 2015-15, “Interest—Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements” with guidance on the presentation and measurement of debt issuance costs associated with line-of -credit arrangements. ASU 2015-03 previously issued in April 2015 did not address the presentation or subsequent measurement of debt issuance costs related to line-of-credit arrangements. ASU 2015-15 states that the debt issuance costs shall be presented as an asset and that such deferred debt issuance costs shall be amortized over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. We adopted this guidance in the three month period ended September 30, 2015, and it had no effect on our results of operations.
In September 2015, the FASB issued ASU 2015-16, Business Combinations (Topic 805): “Simplifying the Accounting for Measurement-Period Adjustments”, with guidance regarding the accounting for and disclosure of measurement-period adjustments that occur in periods after a business combination is consummated. This update requires that the acquirer recognize measurement-period adjustments in the reporting period in which they are determined and, as such, eliminates the previous requirement to retrospectively account for these adjustments. This update also requires an entity to present separately on the face of the income statement, or disclose in the notes, the amount recorded in the current-period income statement that would have been recorded in previous reporting periods if the adjustments had been recognized as of the acquisition date. The effective date for annual and interim periods begins after December 15, 2016. We are currently evaluating the effect that this guidance may have on our consolidated financial statements.
In November 2015, the FASB issued ASU 2015-17, “Balance Sheet Classification of Deferred Taxes”, which requires that deferred tax assets and liabilities be classified as noncurrent in a classified statement of financial position. This update is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years, and may be applied either prospectively to all deferred tax assets and liabilities or retrospectively to all periods presented. We elected to early-adopt this update on a retrospective basis, which resulted in $54.8 million of current deferred tax assets being reclassified to long-term as of December 31, 2014. The adoption of this update had no effect on our results of operations.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Our cash equivalents and short-term investments included a portfolio of high credit quality securities, including U.S. government securities, treasury bills, short-term commercial paper, and highly-rated money market funds. We had no short-term investments as of December 31, 2015 and the carrying value of the portfolio at December 31, 2014 approximated the market value of the related assets. As a result of the short-term investments at December 31, 2014, the portfolio was subject to interest rate risk. Based on the average duration of our investments as of December 31, 2014, an increase of one percentage point in interest rates would have resulted in an increase in interest income of approximately $2.4 million.
Financial instruments that potentially subject us to concentrations of credit risk consist principally of cash equivalents, short-term investments and accounts receivable. We limit our credit risk associated with cash equivalents and short-term investments by placing investments with high credit quality securities, including U.S. government securities, treasury bills, corporate debt, short-term commercial paper and highly-rated money market funds. We limit our credit risk with respect to accounts receivable by performing credit evaluations when deemed necessary. We do not require collateral to secure amounts owed to us by our customers.
As discussed above under “– Outstanding Debt Obligations,” we are party to the Revolving Credit Facility of up to $100 million, which is available for working capital and other general corporate purposes.
We also issued the Notes in a private placement offering on June 30, 2015, which are our senior unsecured obligations, as described above under “Outstanding Debt Obligations.”
Prior to June 30, 2015, we had no derivative assets or liabilities and did not engage in any hedging activities. As a result of our June 30, 2015 issuance of the Notes described above under “Outstanding Debt Obligations” and in “Item 15. Exhibits and Financial Statement Schedules - Note 14. Debt”, we entered into a series of convertible note hedge and warrant transactions (the “Note Hedge Transactions” and “Warrant Transactions”) which are designed to reduce the potential dilution to our stockholders and/or offset the cash payments we are required to make in excess of the principal amount upon conversion of the Notes. See “Item 15. Exhibits and Financial Statement Schedules - Note 8. Derivatives” for additional information.
We do not use derivative financial instruments or engage in hedging activities in our ordinary course of business and have no material foreign currency exchange exposure or commodity price risks. See “Item 15. Exhibits and Financial Statement Schedules – Note 23. Segment Information” for more information regarding the value of our investment in Impax Laboratories (Taiwan), Inc.
We do not believe that inflation has had a significant impact on our revenues or operations to date.
Item 8. Financial Statements and Supplementary Data
The consolidated financial statements and schedule listed in the Index to Financial Statements beginning on page F-1 are filed as part of this Annual Report on Form 10-K and incorporated by reference herein.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
Disclosure Controls and Procedures
We maintain disclosure controls and procedures (as defined in Rule 13a-15(e) of the Exchange Act) designed to ensure information required to be disclosed by us in reports we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Based upon this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures, as defined in Rule 13a-15(e) of the Exchange Act, were effective at the reasonable assurance level as of December 31, 2015.
Management Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f), to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles used in the United States (GAAP). Internal control over financial reporting includes those policies and procedures which (i) pertain to the maintenance of records, in reasonable detail, to accurately and fairly record the transactions and dispositions of our assets; (ii) provide reasonable assurance transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets which could have a material effect on the financial statements. Internal control over financial reporting includes the controls themselves, monitoring of those controls, internal audit practices, and actions taken to correct deficiencies as identified. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of internal control over financial reporting effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2015, the end of our fiscal year, and has reviewed the results of this assessment with the Audit Committee of our Board of Directors. Management based its assessment on criteria established in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Management’s assessment included evaluation of such elements as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies, and our overall control environment. Based on the assessment, management has concluded our internal control over financial reporting was effective as of the end of the fiscal year 2015 to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with GAAP.
The scope of management’s assessment of the effectiveness of its internal control over financial reporting as of December 31, 2015 included our consolidated operations except for the operations of Tower and Lineage, which we acquired in March 2015. Tower and Lineage represented 2% and 14% of our consolidated assets and consolidated revenues as of and for the year ended December 31, 2015.
The effectiveness of our internal control over financial reporting as of December 31, 2015 has been audited by KPMG LLP, an independent registered public accounting firm, as stated in their report which is included immediately below.
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Impax Laboratories, Inc.:
We have audited Impax Laboratories, Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Impax Laboratories, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Impax Laboratories, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
The scope of management’s assessment of the effectiveness of their internal control over financial reporting as of December 31, 2015 included Impax Laboratories, Inc.’s consolidated operations except for the operations of Tower Holdings, Inc. and Lineage Therapeutics Inc., which Impax Laboratories, Inc. acquired in March 2015. Tower Holdings, Inc. and Lineage Therapeutics Inc. represented 2% and 14% of Impax Laboratories, Inc. and subsidiaries’ consolidated assets and consolidated revenues as of and for the year ended December 31, 2015. Our audit of internal control over financial reporting of Impax Laboratories, Inc. also excluded an evaluation of the internal control over financial reporting of Tower Holdings, Inc. and Lineage Therapeutics Inc.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Impax Laboratories, Inc. and subsidiaries as of December 31, 2015 and 2014, and the related consolidated statements of income, comprehensive income, changes in stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2015, and our report dated February 22, 2016, expressed an unqualified opinion on those consolidated financial statements.
/s/ KPMG LLP
Philadelphia, Pennsylvania
February 22, 2016
Changes in Internal Control over Financial Reporting
During the quarter ended December 31, 2015, there were no changes in the Company’s internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) which materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Item 9B. Other Information
None.
PART III.
Item 10. Directors, Executive Officers and Corporate Governance
Code of Conduct
We have adopted a Code of Conduct, which was amended and restated effective February 16, 2016 (“Code of Conduct”). The Code of Conduct applies to all of our directors, employees, including our Chief Executive Officer, Chief Financial Officer and any other accounting officer, controller or persons performing similar functions, and contingent workers and business partners who perform work on our behalf. The Code of Conduct is available on our website (www.impaxlabs.com) and accessible via the “Investor Relations” page. Any amendments to, or waivers of, the Code of Conduct will be disclosed on our website within four business days following the date of such amendment or waiver.
Additional information required by this item is incorporated by reference to our definitive proxy statement for the Annual Meeting of Stockholders to be held on May 17, 2016 (“Proxy Statement”).
Item 11. Executive Compensation
The information required by this item is incorporated by reference to the Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The information required by this item is incorporated by reference to the Proxy Statement, except information concerning the equity compensation plans table which is set forth in “Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities” and which is incorporated herein by reference.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this item is incorporated by reference to the Proxy Statement.
Item 14. Principal Accounting Fees and Services
The information required by this item is incorporated by reference to the Proxy Statement.
PART IV.
Item 15. Exhibits and Financial Statement Schedules
(a)(1) Consolidated Financial Statements
The consolidated financial statements listed in the Index to Financial Statements beginning on page F-1 are filed as part of this Annual Report on Form 10-K.
(a)(2) Financial Statement Schedules
The financial statement schedule listed in the Index to Financial Statements on page F-1 is filed as part of this Annual Report on Form 10-K.
(a)(3) Exhibits
EXHIBIT INDEX
Exhibit No. Description of Document
|
2.1 |
Stock Purchase Agreement, dated as of October 8, 2014, by and among the Company, Tower Holdings, Inc. (“Tower”), Lineage Therapeutics Inc. (“Lineage”), Roundtable Healthcare Partners II, L.P., Roundtable Healthcare Investors II, L.P., the other stockholders of Tower and Lineage, the holders of options to purchase shares of Tower common stock and options to purchase shares of Lineage common stock, the holders of warrants to acquire shares of Tower common stock and warrants to acquire shares of Lineage common stock and, solely with respect to Section 8.3, Roundtable Healthcare Management II, LLC.(1) |
|
3.1.1 |
Certificate of Amendment of the Restated Certificate of Incorporation of the Company dated as of December 9, 2015.(2) |
|
3.1.2 |
Restated Certificate of Incorporation of the Company dated as of August 30, 2004.(3) |
|
|
|
|
3.1.3 |
Certificate of Designation of Series A Junior Participating Preferred Stock, as filed with the Secretary of State of Delaware on January 21, 2009.(4) |
|
3.2.1 |
Amendment No. 3 to Amended and Restated Bylaws of the Company, effective as of October 7, 2015.(5) |
|
3.2.2 |
Amendment No. 2 to Amended and Restated Bylaws of the Company, effective as of July 7, 2015.(5) |
|
3.2.3 |
Amendment No. 1 to Amended and Restated Bylaws of the Company, effective as of March 24, 2015.(5) |
|
3.2.4 |
Amended and Restated Bylaws of the Company, effective as of May 14, 2014.(5) |
|
4.1 |
Specimen of Common Stock Certificate.(6) |
|
|
|
|
4.2 |
Preferred Stock Rights Agreement, dated as of January 20, 2009, by and between the Company and StockTrans, Inc., as Rights Agent.(4) |
|
4.3 |
Indenture, dated as of June 30, 2015, between the Company, and Wilmington Trust, National Association, as trustee.(7) |
|
|
|
|
10.1 |
Credit Agreement, dated as of February 11, 2011, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association, as Administrative Agent.**(8) |
|
|
|
|
10.1.1 |
Amendment dated as of March 19, 2012 to the Credit Agreement, dated as of February 11, 2011, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association as Administrative Agent.(9) |
|
|
|
|
10.1.2 |
Second Amendment to Credit Agreement, dated as of January 10, 2013, to the Credit Agreement, dated as of February 11, 2011, as amended, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association, as Administrative Agent.(10) |
|
10.1.3 |
Third Amendment to Credit Agreement, dated as of February 20, 2014, to the Credit Agreement, dated as of February 11, 2011, as amended, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association, as Administrative Agent.(11) |
|
|
|
|
10.2 |
Security Agreement, dated as of February 11, 2011, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association, as Administrative Agent.(8) |
|
|
|
|
10.3 |
Letter Agreement, dated as of June 25, 2015, between RBC Capital Markets LLC and the Company regarding the Base Warrants.(7) |
|
10.4 |
Letter Agreement, dated as of June 25, 2015 between RBC Capital Markets LLC and the Company regarding the Base Call Option Transaction.(7) |
|
10.5 |
Letter Agreement, dated as of June 26, 2015, between RBC Capital Markets LLC and the Company regarding the Additional Warrants.(7) |
|
10.6 |
Letter Agreement, dated as of June 26, 2015, between RBC Capital Markets LLC and the Company regarding the Additional Call Option Transaction.(7) |
|
10.7 |
Credit Agreement, dated as of March 9, 2015, by and among the Company, the lenders party thereto from time to time and Barclays Bank PLC, as administrative agent and collateral agent.(12) |
|
10.8 |
Credit Agreement, dated as of August 4, 2015, by and among the Company, the lenders party thereto from time to time and Royal Bank of Canada, as administrative agent and collateral agent.(13) |
|
10.9.1 |
Impax Laboratories, Inc. 1999 Equity Incentive Plan.*(14) |
|
|
|
|
10.9.2 |
Form of Stock Option Grant under the Impax Laboratories, Inc. 1999 Equity Incentive Plan.*(14) |
|
|
|
|
10.10 |
Impax Laboratories, Inc. 2001 Non-Qualified Employee Stock Purchase Plan.*(6) |
|
|
|
|
10.11.1 |
Impax Laboratories, Inc. Second Amended and Restated 2002 Equity Incentive Plan.*(15) |
|
|
|
|
10.11.2 |
Form of Stock Option Agreement under the Impax Laboratories, Inc. Second Amended and Restated 2002 Equity Incentive Plan.*(16) |
|
|
|
|
10.11.3 |
Form of Restricted Stock (Stock Bonus) Agreement under the Impax Laboratories, Inc. Second Amended and Restated 2002 Equity Incentive Plan.*(16) |
|
|
|
|
10.12.1 |
Impax Laboratories, Inc. Executive Non-Qualified Deferred Compensation Plan, amended and restated effective January 1, 2008.*(17) |
|
|
|
|
10.12.2 |
Amendment to Impax Laboratories, Inc. Executive Non-Qualified Deferred Compensation Plan, effective as of January 1, 2009.* (17) |
|
|
|
|
10.13.1 |
Employment Agreement, dated as of January 1, 2010, between the Company and Larry Hsu, Ph.D.*(18) |
|
10.13.2 |
Separation Agreement, dated as of June 24, 2013, between the Company and Larry Hsu, Ph.D.*(19) |
|
10.13.3 |
Amendment, dated as of February 26, 2014, to the Separation Agreement by and between the Company and Larry Hsu, Ph.D., dated as of June 24, 2013.*(20) |
|
10.14.1 |
Employment Agreement, dated as of January 1, 2010, between the Company and Charles V. Hildenbrand.*(18) |
|
|
|
|
10.14.2 |
Confidential Separation and Release Agreement, dated as of July 5, 2011, between the Company and Charles V. Hildenbrand.*(21) |
|
10.15.1 |
Employment Agreement, dated as of January 1, 2010, between the Company and Arthur A. Koch, Jr.*(18) |
|
|
|
|
10.15.2 |
General Release and Waiver, effective as of July 17, 2012, between the Company and Arthur A. Koch, Jr.* (22) |
|
|
|
|
10.16.1 |
Employment Agreement, dated as of January 1, 2010, between the Company and Michael J. Nestor.*(18) |
|
10.16.2 |
Amendment, dated as of April 1, 2014, to the Employment Agreement, dated as of January 1, 2014, between the Company and Michael Nestor.*(23) |
|
|
|
|
10.17.1 |
Offer of Employment Letter, dated as of March 17, 2011, between the Company and Mark A. Schlossberg.*(24) |
|
|
|
|
10.17.2 |
Employment Agreement, dated as of May 2, 2011, between the Company and Mark A. Schlossberg.*(24) |
|
|
|
|
10.17.3 |
Amendment, dated as of April 1, 2014, to the Employment Agreement, dated as of May 2, 2011, between the Company and Mark A. Schlossberg.*(23) |
|
10.18.1 |
Offer of Employment Letter, dated as of August 18, 2011, between the Company and Carole Ben-Maimon, M.D.*(25) |
|
|
|
|
10.18.2 |
Employment Agreement, dated as of November 7, 2011, between the Company and Carole Ben-Maimon, M.D.*(26) |
|
10.18.3 |
Amendment dated, as of April 1, 2014, to the Employment Agreement, dated as of November 7, 2011, between the Company and Carole Ben-Maimon, M.D.*(23) |
|
10.18.4 |
Separation Agreement, dated as of October 22, 2014, between the Company and Carole Ben-Maimon, M.D.*(27) |
|
10.19.1 |
Employment Agreement, dated as of December 12, 2012, between the Company and Bryan M. Reasons.*(28) |
|
10.19.2 |
Amendment, dated as of April 1, 2014, to the Employment Agreement, dated as of December 12, 2012 between the Company and Bryan M. Reasons.*(23) |
|
10.20 |
Employment Agreement, dated as of April 21, 2014, by and between the Company and G. Frederick Wilkinson.*(29) |
|
10.21.1 |
Employment Agreement, dated as of November 28, 2011, by and between the Company and Jeffrey Nornhold.*(30) |
|
10.21.2 |
Amendment, dated as of April 1, 2014, to the Employment Agreement, dated as of November 28, 2011, by and between the Company and Jeffrey Nornhold.*(30) |
|
10.21.3 |
Letter Agreement, dated as of April 1, 2014, between the Company and Jeffrey Nornhold.*(30) |
|
10.22 |
Amended and Restated License and Distribution Agreement, dated as of February 7, 2013, between the Company and Shire LLC.**(31) |
|
|
|
|
10.23.1 |
Joint Development Agreement, dated as of November 26, 2008, between the Company and Medicis Pharmaceutical Corporation.**(8) |
|
|
|
|
10.23.2 |
Settlement Agreement, dated as of January 21, 2011, between the Company and Medicis Pharmaceutical Corporation.**(32) |
|
|
|
|
10.23.3 |
First Amendment, dated as of January 26, 2011, to the Joint Development Agreement, dated as of November 26, 2008, between the Company and Medicis Pharmaceutical Corporation.(24) |
|
10.24 |
Distribution, License, Development and Supply Agreement, dated as of January 31, 2012, between the Company and AstraZeneca UK Limited.**(33) |
|
|
|
|
11.1 |
Statement re computation of per share earnings (incorporated by reference to Note 16 to the Notes to Consolidated Financial Statements in this Annual Report on Form 10-K). |
|
21.1 |
Subsidiaries of the registrant. |
|
|
|
|
23.1 |
Consent of Independent Registered Public Accounting Firm. |
|
|
|
|
31.1 |
Certification of the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
31.2 |
Certification of the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
32.1 |
Certification of the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
32.2 |
Certification of the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
101 |
The following materials from the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets as of December 31, 2015 and 2014, (ii) Consolidated Statements of Operations for each of the three years in the period ended December 31, 2015, (iii) Consolidated Statements of Comprehensive Income for each of the three years in the period ended December 31, 2015, (iv) Consolidated Statements of Changes in Stockholders’ Equity for each of the three years in the period ended December 31, 2015, (v) Consolidated Statements of Cash Flows for each of the three years in the period ended December 31, 2016 and (vi) Notes to Consolidated Financial Statements for each of the three years in the period ended December 31, 2015. |
* Management contract, compensatory plan or arrangement.
** Confidential treatment granted for certain portions of this exhibit pursuant to Rule 24b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which portions are omitted and filed separately with the SEC.
(1) Incorporated by reference to the Company’s Current Report on Form 8-K filed on October 10, 2014.
(2) Incorporated by reference to the Company’s Current Report on Form 8-K filed on December 9, 2015.
(3) Incorporated by reference to Amendment No. 5 to the Company’s Registration Statement on Form 10 filed on December 23, 2008.
(4) Incorporated by reference to the Company’s Current Report on Form 8-K filed on January 22, 2009.
(5) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed on November 9, 2015.
(6) Incorporated by reference to the Company’s Registration Statement on Form 10 filed on October 10, 2008.
(7) Incorporated by reference to the Company’s Current Report on Form 8-K filed on June 30, 2015.
(8) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2011.
(9) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2012.
(10) Incorporated by reference to the Company’s Current Report on Form 8-K filed on January 10, 2013.
(11) Incorporated by reference to the Company’s Current Report on Form 8-K filed on February 25, 2014.
(12) Incorporated by reference to the Company’s Current Report on Form 8-K filed on March 12, 2015.
(13) Incorporated by reference to the Company’s Current Report on Form 8-K filed on August 5, 2015.
(14) Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008.
(15) Incorporated by reference to Appendix A to the Company’s Definitive Proxy Statement on Schedule 14A filed on April 15, 2013.
(16) Incorporated by reference to the Company’s Registration Statement on Form S-8 (file No. 333-189360) filed on June 14, 2013.
(17) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010.
(18) Incorporated by reference to the Company’s Current Report on Form 8-K filed on January 14, 2010.
(19) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2013.
(20) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2014.
(21) Incorporated by reference to the Company’s Current Report on Form 8-K filed on July 11, 2011.
(22) Incorporated by reference to the Company’s Current Report on Form 8-K filed on July 18, 2012.
(23) Incorporated by reference to the Company’s Current Report on Form 8-K filed on April 2, 2014.
(24) Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2011.
(25) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2011.
(26) Incorporated by reference to the Company’s Current Report on Form 8-K filed on November 9, 2011.
(27) Incorporated by reference to the Company’s Annual Report on Form 10-K filed on February 26, 2015.
(28) Incorporated by reference to the Company’s Current Report on Form 8-K filed on December 13, 2012.
(29) Incorporated by reference to the Company’s Current Report on Form 8-K filed on April 24, 2014.
(30) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014.
(31) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2013.
(32) Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012.
(33) Incorporated by reference to the Company’s Current Report on Form 8-K/A filed on April 2, 2012.
Impax Laboratories, Inc.
INDEX TO FINANCIAL STATEMENTS
|
Report of Independent Registered Public Accounting Firm |
F-2 |
|
Consolidated Balance Sheets as of December 31, 2015 and 2014 |
F-3 |
|
Consolidated Statements of Income for the years ended December 31, 2015, 2014 and 2013 |
F-4 |
|
Consolidated Statements of Comprehensive Income for the years ended December 31, 2015, 2014 and 2013 |
F-5 |
|
Consolidated Statements of Changes in Stockholders’ Equity for the years ended December 31, 2015, 2014 and 2013 |
F-6 |
|
Consolidated Statements of Cash Flows for the years ended December 31, 2015, 2014 and 2013 |
F-7 |
|
Notes to Consolidated Financial Statements |
F-8 |
|
Schedule II, Valuation and Qualifying Accounts |
S-1 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Impax Laboratories, Inc.
We have audited the accompanying consolidated balance sheets of Impax Laboratories, Inc. and subsidiaries as of December 31, 2015 and 2014, and the related consolidated statements of income, comprehensive income, changes in stockholders’ equity, and cash flows for each of the years in the three-year period ended December 31, 2015. In connection with our audits of the consolidated financial statements, we have also audited the related financial statement schedule. These consolidated financial statements and the related financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Impax Laboratories, Inc. and subsidiaries as of December 31, 2015 and 2014, and the results of their operations and their cash flows for each of the years in the three-year period ended December 31, 2015, in conformity with U.S. generally accepted accounting principles. Also in our opinion, the related financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Impax Laboratories, Inc.’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated February 22, 2016 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Our report dated February 22, 2016, on the effectiveness of internal control over financial reporting as of December 31, 2015, contains an explanatory paragraph that states that the scope of management’s assessment of their effectiveness of internal control over financial reporting as of December 31, 2015 included Impax Laboratories, Inc.’s consolidated operations except for the operations of Tower Holdings, Inc. and Lineage Therapeutics Inc., which Impax Laboratories, Inc. acquired in March 2015. Tower Holdings, Inc. and Lineage Therapeutics Inc. represented 2% and 14% of Impax Laboratories, Inc. and subsidiaries’ consolidated assets and consolidated revenues as of and for the year ended December 31, 2015. Our audit of internal control over financial reporting of Impax Laboratories, Inc. also excluded an evaluation of the internal control over financial reporting of Tower Holdings, Inc. and Lineage Therapeutics Inc.
/s/ KPMG LLP
Philadelphia, Pennsylvania
February 22, 2016
IMPAX LABORATORIES, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except share and per share data)
|
December 31, |
December 31, |
|||||||
|
2015 |
2014 | |||||||
|
Assets |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 340,351 | $ | 214,873 | ||||
|
Short-term investments |
-- | 199,983 | ||||||
|
Accounts receivable, net |
324,451 | 146,490 | ||||||
|
Inventory, net |
125,582 | 80,570 | ||||||
|
Prepaid expenses and other current assets |
31,689 | 33,710 | ||||||
|
Total current assets |
822,073 | 675,626 | ||||||
|
Property, plant and equipment, net |
214,156 | 188,169 | ||||||
|
Intangible assets, net |
602,020 | 26,711 | ||||||
|
Goodwill |
210,166 | 27,574 | ||||||
|
Deferred income taxes |
315 | 96,662 | ||||||
|
Other non-current assets |
73,757 | 64,455 | ||||||
|
Total assets |
$ | 1,922,487 | $ | 1,079,197 | ||||
|
Liabilities and Stockholders’ Equity |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 56,325 | $ | 31,976 | ||||
|
Accrued expenses |
204,711 | 110,470 | ||||||
|
Accrued profit sharing and royalty expenses |
65,725 | 15,346 | ||||||
|
Current portion of deferred revenue |
-- | 907 | ||||||
|
Total current liabilities |
326,761 | 158,699 | ||||||
|
Long-term debt, net |
424,595 | -- | ||||||
|
Deferred income taxes |
72,770 | -- | ||||||
|
Deferred revenue, net of current portion |
-- | 3,403 | ||||||
|
Other non-current liabilities |
35,952 | 29,218 | ||||||
|
Total liabilities |
860,078 | 191,320 | ||||||
|
Commitments and contingencies (Note 21 and Note 22) |
||||||||
|
Stockholders’ equity: |
||||||||
|
Preferred stock, $0.01 par value; 2,000,000 shares authorized; No issued or outstanding shares at December 31, 2015 and 2014 |
-- | -- | ||||||
|
Common stock, $0.01 par value; 150,000,000 shares authorized; 72,926,205 issued and 72,682,476 outstanding shares at December 31, 2015; 71,470,802 issued and 71,227,073 outstanding shares at December 31, 2014 |
729 | 714 | ||||||
|
Treasury stock at cost: 243,729 shares at December 31, 2015 and 2014 |
(2,157 | ) | (2,157 | ) | ||||
|
Additional paid-in capital |
504,077 | 364,103 | ||||||
|
Retained earnings |
570,223 | 531,226 | ||||||
|
Accumulated other comprehensive loss |
(10,463 | ) | (6,009 | ) | ||||
|
Total stockholders’ equity |
1,062,409 | 887,877 | ||||||
|
Total liabilities and stockholders’ equity |
$ | 1,922,487 | $ | 1,079,197 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
IMPAX LABORATORIES, INC.
CONSOLIDATED STATEMENTS OF INCOME
(In thousands, except share and per share data)
|
Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 | ||||||||||
|
Revenues: |
||||||||||||
|
Impax Generics, net |
$ | 710,932 | $ | 549,082 | $ | 398,340 | ||||||
|
Impax Specialty Pharma, net |
149,537 | 46,967 | 113,162 | |||||||||
|
Total revenues |
860,469 | 596,049 | 511,502 | |||||||||
|
Cost of revenues |
508,065 | 283,396 | 312,202 | |||||||||
|
Gross profit |
352,404 | 312,653 | 199,300 | |||||||||
|
Operating expenses: |
||||||||||||
|
Research and development |
76,982 | 78,642 | 68,854 | |||||||||
|
Patent litigation |
4,567 | 5,805 | 16,545 | |||||||||
|
Selling, general and administrative |
201,287 | 139,390 | 120,288 | |||||||||
|
Total operating expenses |
282,836 | 223,837 | 205,687 | |||||||||
|
Income (loss) from operations |
69,568 | 88,816 | (6,387 | ) | ||||||||
|
Other income (expense): |
||||||||||||
|
Interest expense |
(27,268 | ) | (43 | ) | (419 | ) | ||||||
|
Interest income |
1,042 | 1,473 | 1,299 | |||||||||
|
Gain on sale of asset |
45,574 | -- | -- | |||||||||
|
Loss on debt extinguishment |
(16,903 | ) | -- | -- | ||||||||
|
Net change in fair value of derivatives |
(13,000 | ) | -- | -- | ||||||||
|
Other, net |
355 | 313 | 152,447 | |||||||||
|
Income before income taxes |
59,368 | 90,559 | 146,940 | |||||||||
|
Provision for income taxes |
20,371 | 33,206 | 45,681 | |||||||||
|
Net income |
$ | 38,997 | $ | 57,353 | $ | 101,259 | ||||||
|
Net income per common share: |
||||||||||||
|
Basic |
$ | 0.56 | $ | 0.84 | $ | 1.51 | ||||||
|
Diluted |
$ | 0.54 | $ | 0.81 | $ | 1.47 | ||||||
|
Weighted-average common shares outstanding: |
||||||||||||
|
Basic |
69,640,417 | 68,185,552 | 66,921,181 | |||||||||
|
Diluted |
72,027,344 | 70,530,349 | 68,655,038 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
IMPAX LABORATORIES, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(In thousands)
|
Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Net income |
$ | 38,997 | $ | 57,353 | $ | 101,259 | ||||||
|
Other comprehensive loss component: |
||||||||||||
|
Currency translation adjustments |
(4,454 | ) | (7,149 | ) | (4,104 | ) | ||||||
|
Comprehensive income |
$ | 34,543 | $ | 50,204 | $ | 97,155 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
IMPAX LABORATORIES, INC.
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(In thousands)
| Accumulated | ||||||||||||||||||||||||||||
| Common Stock | Additional | Other | ||||||||||||||||||||||||||
| Number of | Par | Treasury | Paid-in | Retained | Comprehensive | |||||||||||||||||||||||
|
Shares |
Value |
Stock |
Capital |
Earnings |
Income (Loss) |
Total |
||||||||||||||||||||||
|
Balance, December 31, 2012 |
68,272 | $ | 685 | $ | (2,157 | ) | $ | 314,717 | $ | 372,614 | $ | 5,244 | $ | 691,103 | ||||||||||||||
|
Net income |
- | - | - | - | 101,259 | - | 101,259 | |||||||||||||||||||||
|
Other comprehensive loss: |
||||||||||||||||||||||||||||
|
Currency translation adjustment |
- | - | - | - | - | (4,104 | ) | (4,104 | ) | |||||||||||||||||||
|
Exercises of stock options, issuances of restricted stock and sales of common stock under ESPP |
1,412 | 14 | - | 3,538 | - | - | 3,552 | |||||||||||||||||||||
|
Share-based compensation |
- | - | - | 17,644 | - | - | 17,644 | |||||||||||||||||||||
|
Tax benefit related to exercises of stock options and vestings of restricted stock |
- | - | - | 749 | - | - | 749 | |||||||||||||||||||||
|
Balance, December 31, 2013 |
69,684 | $ | 699 | $ | (2,157 | ) | $ | 336,648 | $ | 473,873 | $ | 1,140 | $ | 810,203 | ||||||||||||||
|
Net income |
- | - | - | - | 57,353 | - | 57,353 | |||||||||||||||||||||
|
Other comprehensive loss: |
||||||||||||||||||||||||||||
|
Currency translation adjustment |
- | - | - | - | - | (7,149 | ) | (7,149 | ) | |||||||||||||||||||
|
Exercises of stock options, issuances of restricted stock and sales of common stock under ESPP |
1,544 | 15 | - | 3,255 | - | - | 3,270 | |||||||||||||||||||||
|
Share-based compensation |
- | - | - | 20,883 | - | - | 20,883 | |||||||||||||||||||||
|
Tax benefit related to exercises of stock options and vestings of restricted stock |
- | - | - | 3,317 | - | - | 3,317 | |||||||||||||||||||||
|
Balance, December 31, 2014 |
71,228 | $ | 714 | $ | (2,157 | ) | $ | 364,103 | $ | 531,226 | $ | (6,009 | ) | $ | 887,877 | |||||||||||||
|
Net income |
- | - | - | - | 38,997 | - | 38,997 | |||||||||||||||||||||
|
Other comprehensive loss: |
||||||||||||||||||||||||||||
|
Currency translation adjustment |
- | - | - | - | - | (4,454 | ) | (4,454 | ) | |||||||||||||||||||
|
Exercises of stock options, issuances of restricted stock and sales of common stock under ESPP |
1,698 | 15 | - | (3,533 | ) | - | - | (3,518 | ) | |||||||||||||||||||
|
Share-based compensation |
- | - | - | 28,613 | - | - | 28,613 | |||||||||||||||||||||
|
Sale of warrants |
- | - | - | 88,320 | - | - | 88,320 | |||||||||||||||||||||
|
Reclassification of derivatives to equity, net of related taxes |
- | - | - | 21,038 | - | - | 21,038 | |||||||||||||||||||||
|
Tax benefit related to exercises of stock options and vestings of restricted stock |
- | - | - | 5,536 | - | - | 5,536 | |||||||||||||||||||||
|
Balance, December 31, 2015 |
72,926 | $ | 729 | $ | (2,157 | ) | $ | 504,077 | $ | 570,223 | $ | (10,463 | ) | $ | 1,062,409 | |||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
IMPAX LABORATORIES, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 | ||||||||||
|
Cash flows from operating activities: |
||||||||||||
|
Net income |
$ | 38,997 | $ | 57,353 | $ | 101,259 | ||||||
|
Adjustments to reconcile net income to net cash provided by operating activities: |
||||||||||||
|
Depreciation and amortization |
68,637 | 34,026 | 36,006 | |||||||||
|
Non-cash interest expense |
11,230 | -- | -- | |||||||||
|
Share-based compensation expense |
28,613 | 20,883 | 17,644 | |||||||||
|
Tax benefit from employees’ exercises of stock options and vestings of restricted stock |
(5,536 | ) | (3,317 | ) | (749 | ) | ||||||
|
Deferred income taxes – net and uncertain tax positions |
(29,558 | ) | (11,810 | ) | (21,132 | ) | ||||||
|
Gain on sale of intangible asset |
(45,574 | ) | -- | -- | ||||||||
|
Loss on debt extinguishment |
16,903 | -- | -- | |||||||||
|
Net change in fair value of derivatives |
13,000 | -- | -- | |||||||||
|
Intangible asset impairment charges |
13,664 | 2,876 | 13,906 | |||||||||
|
Accrued profit sharing and royalty expense |
160,848 | 52,208 | 61,118 | |||||||||
|
Payments of profit sharing and royalty expense |
(116,542 | ) | (48,422 | ) | (54,494 | ) | ||||||
|
Provision for inventory reserves |
(8,179 | ) | 7,964 | 12,476 | ||||||||
|
Recognition of deferred revenue |
(4,310 | ) | (3,939 | ) | (4,390 | ) | ||||||
|
Other |
(81 | ) | 1,226 | (659 | ) | |||||||
|
Changes in certain assets and liabilities: |
||||||||||||
|
Accounts receivable |
(121,110 | ) | (33,497 | ) | (20,744 | ) | ||||||
|
Inventory |
(5,856 | ) | (24,302 | ) | 7,095 | |||||||
|
Prepaid expenses and other assets |
9,330 | (9,952 | ) | (7,646 | ) | |||||||
|
Accounts payable and accrued expenses |
48,106 | (8,980 | ) | 4,698 | ||||||||
|
Other liabilities |
(656 | ) | 500 | 5,552 | ||||||||
|
Net cash provided by operating activities |
71,926 | 32,817 | 149,940 | |||||||||
|
Cash flows from investing activities: |
||||||||||||
|
Payment for business acquisition, net of cash acquired |
(691,348 | ) | -- | -- | ||||||||
|
Proceeds from sale of assets acquired |
59,546 | -- | -- | |||||||||
|
Purchases of property, plant and equipment |
(25,199 | ) | (29,913 | ) | (32,785 | ) | ||||||
|
Payments for licensing agreements and acquisitions |
(5,850 | ) | (13,000 | ) | (12,000 | ) | ||||||
|
Investment in cash surrender value of insurance |
(4,750 | ) | (3,000 | ) | -- | |||||||
|
Maturities of short-term investments |
200,064 | 395,404 | 285,986 | |||||||||
|
Purchases of short-term investments |
-- | (366,092 | ) | (357,092 | ) | |||||||
|
Net cash used in investing activities |
(467,537 | ) | (16,601 | ) | (115,891 | ) | ||||||
|
Cash flows from financing activities: |
||||||||||||
|
Proceeds from sale of convertible notes |
600,000 | -- | -- | |||||||||
|
Proceeds from issuance of term loan |
435,000 | -- | -- | |||||||||
|
Repayment of term loan |
(435,000 | ) | -- | -- | ||||||||
|
Payment of deferred financing fees |
(36,941 | ) | -- | -- | ||||||||
|
Purchase of bond hedge derivative asset |
(147,000 | ) | -- | -- | ||||||||
|
Proceeds from sale of warrants |
88,320 | -- | -- | |||||||||
|
Tax benefit from employees’ exercises of stock options and vestings of restricted stock awards |
5,536 | 3,317 | 749 | |||||||||
|
Proceeds from exercises of stock options and ESPP |
11,472 | 11,097 | 8,213 | |||||||||
|
Net cash provided by financing activities |
521,387 | 14,414 | 8,962 | |||||||||
|
Effect of exchange rate changes on cash and cash equivalents |
(298 | ) | (369 | ) | (561 | ) | ||||||
|
Net increase in cash and cash equivalents |
125,478 | 30,261 | 42,450 | |||||||||
|
Cash and cash equivalents, beginning of year |
214,873 | 184,612 | 142,162 | |||||||||
|
Cash and cash equivalents, end of year |
$ | 340,351 | $ | 214,873 | $ | 184,612 | ||||||
|
Supplemental disclosure of cash flow information: |
||||||||||||
|
Cash paid for interest |
$ | 15,365 | $ | 17 | $ | 89 | ||||||
|
Cash paid for income taxes, net |
$ | 43,223 | $ | 72,174 | $ | 34,272 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
IMPAX LABORATORIES, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS
Impax Laboratories, Inc. (“Impax” or the “Company”) is a specialty pharmaceutical company that focuses on developing, manufacturing, marketing and distributing generic and branded pharmaceutical products. The Company has two reportable segments, referred to as “Impax Generics” and “Impax Specialty Pharma.” The Impax Generics division focuses on a broad range of therapeutic areas, including products having technically challenging drug-delivery mechanisms or unique product formulations. In addition to developing solid oral dosage products, the Impax Generic division’s portfolio includes alternative dosage form products, primarily through alliance and collaboration agreements with third parties. The Company’s Impax Specialty Pharma division is focused on the development and promotion, through the Company’s specialty sales force, of proprietary branded pharmaceutical products for the treatment of central nervous system (“CNS”) disorders and other select specialty segments. As described in detail below, in March 2015, the Company renamed its operating and reporting structure into its current structure; prior to such time, the Impax Generics division was referred to as “Global Pharmaceuticals” and the Impax Specialty Pharma division was referred to as “Impax Pharmaceuticals.”
Tower Acquisition
On March 9, 2015, Impax completed its acquisition of Tower Holdings, Inc. (“Tower”), including its operating subsidiaries CorePharma LLC (“CorePharma”) and Amedra Pharmaceuticals LLC (“Amedra Pharmaceuticals”), and Lineage Therapeutics Inc. (“Lineage”) for a purchase price of approximately $691.3 million, net of approximately $41.5 million of cash acquired and including the repayment of indebtedness of Tower and Lineage (the “Tower acquisition”). The privately-held companies specialized in the development, manufacture and commercialization of complex generic and branded pharmaceutical products. For additional information on the acquisition and the related financing of the acquisition, refer to “Note 2. Business Acquisition” and “Note 14. Debt.”
In connection with the Tower acquisition, the Company recorded an accrual for severance and related termination costs of $2.4 million during 2015 related to the elimination of approximately 10 positions at the acquired companies. As of December 31, 2015, $2.1 million has been paid and the Company currently expects the remainder of this balance to be paid by the first half of 2016.
Revised Operating and Reporting Structure
In connection with the closing of the Tower acquisition, Impax renamed the operating and reporting structure of its two divisions into Impax Generics and Impax Specialty Pharma. Impax Generics includes the Company’s legacy Global Pharmaceuticals business as well as the acquired CorePharma and Lineage businesses. Impax Specialty Pharma includes the legacy Impax Pharmaceuticals business as well as the acquired Amedra Pharmaceuticals business.
Impax Generics develops, manufactures, sells, and distributes generic pharmaceutical products primarily through the following four sales channels: the “Impax Generics” sales channel, for generic pharmaceutical prescription products the Company sells directly to wholesalers, large retail drug chains, and others; the “Private Label” sales channel, for generic pharmaceutical over-the-counter (“OTC”) and prescription products the Company sells to unrelated third-party customers who, in turn, sell the product to third parties under their own label; the “Rx Partner” sales channel, for generic prescription products sold through unrelated third-party pharmaceutical entities under their own label pursuant to alliance agreements; and the “OTC Partner” sales channel, for generic pharmaceutical OTC products sold through unrelated third-party pharmaceutical entities under their own labels pursuant to alliance and supply agreements. Revenues from the “Impax Generics” sales channel and the “Private Label” sales channel are reported under the caption “Impax Generics sales, net” in “Note 24. Supplementary Financial Information.” The Company also generates revenue in Impax Generics from research and development services provided under a joint development agreement with another unrelated third-party pharmaceutical company, and reports such revenue under the caption “Other Revenues” in “Note 24. Supplementary Financial Information.” The Company provides these services through the research and development group in Impax Generics. Revenues from the “OTC Partner” sales channel are also reported under the caption “Other Revenues” in “Note 24. Supplementary Financial Information.”
Impax Specialty Pharma is engaged in the development, sale and distribution of proprietary brand pharmaceutical products that the Company believes represent improvements to already-approved pharmaceutical products addressing CNS disorders and other select specialty segments. Impax Specialty Pharma currently has one internally developed branded pharmaceutical product, Rytary® (IPX066), an extended release oral capsule formulation of carbidopa-levodopa for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication and/or manganese intoxication, which was approved by the FDA on January 7, 2015 and which the Company began marketing in the United States (“U.S.”) in April 2015. The Company received marketing authorization from the European Commission for NUMIENT™ (the brand name of IPX066 outside of the United States) during the fourth quarter of fiscal year 2015. Impax Specialty Pharma is also engaged in the sale and distribution of four other branded products; the more significant include Zomig® (zolmitriptan) products, indicated for the treatment of migraine headaches, under the terms of a Distribution, License, Development and Supply Agreement (“AZ Agreement”) with AstraZeneca UK Limited (“AstraZeneca”) in the United States and in certain U.S. territories, and Albenza®, indicated for the treatment of tapeworm infections. Revenues from Impax-labeled branded products are reported under the caption “Impax Specialty Pharma sales, net” in “Note 24. Supplementary Financial Information.” Finally, the Company generates revenue in Impax Specialty Pharma from research and development services provided under a development and license agreement with another unrelated third-party pharmaceutical company, and reports such revenue under the caption “Other Revenues” in “Note 24. Supplementary Financial Information.” Impax Specialty Pharma also has a number of product candidates that are in varying stages of development. See “Note 23. Segment Information,” for financial information about our segments for the years ended December 31, 2015, 2014 and 2013.
The Company owns and/or leases facilities in California, Pennsylvania, New Jersey and Taiwan, Republic of China (“R.O.C.”). In California, the Company utilizes a combination of owned and leased facilities mainly located in Hayward. The Company’s primary properties in California consist of a leased office building used as the Company’s corporate headquarters, in addition to five properties it owns, including a research and development center facility and a manufacturing facility. Additionally, the Company leases two facilities in Hayward, utilized for additional research and development, equipment storage and quality assurance support. In Pennsylvania, the Company leases facilities in New Britain and Montgomeryville used for sales and marketing, finance, and administrative personnel. In addition, the Company owns a packaging plant in Philadelphia, PA that was closed in conjunction with the restructuring of packaging and distribution operations announced in June 2015 and, discussed below. In New Jersey, the Company leases manufacturing, packaging, research and development and warehousing facilities in Middlesex, New Jersey and office space in Bridgewater, New Jersey. Outside the United States, in Taiwan, R.O.C., the Company owns a manufacturing facility.
CEO Transition
On June 25, 2013, the Company announced that Dr. Larry Hsu planned to retire as President and Chief Executive Officer of Impax and on April 21, 2014, Dr. Hsu retired from those positions at Impax. Dr. Hsu subsequently resigned as a member of the Company’s Board of Directors, effective July 2, 2015. In connection with his retirement as the Company’s President and Chief Executive Officer, Dr. Hsu entered into a Separation Agreement with the Company dated June 24, 2013 (the “Separation Agreement”). Pursuant to the Separation Agreement, the Company provided Dr. Hsu with certain termination benefits and payments. The Company recorded $5.0 million in costs associated with Dr. Hsu’s retirement in the three month period ended June 30, 2013, comprised of $2.7 million of separation pay and benefits and $2.3 million of accelerated expense related to Dr. Hsu’s outstanding stock options and restricted stock. Refer to “Note 17. Share-based Compensation” for more information on the acceleration of Dr. Hsu’s equity awards.
Management Changes
During the three month period ended March 31, 2014, the Company announced a management change. The Company’s then Senior Vice President, Global Operations announced plans to retire and a Senior Vice President, Technical Operations was appointed. The Company’s then Senior Vice President, Global Operations subsequently retired from the Company in July 2014. In conjunction with the transition, the Company recorded $0.9 million in separation charges and accelerated share-based compensation expense in the six month period ended June 30, 2014.
On October 22, 2014, the Company announced that Carole S. Ben-Maimon, M.D., President of the Company’s Generics Division, informed the Company of her decision to retire from her position effective November 3, 2014. In connection with her retirement, Dr. Ben-Maimon entered into a Separation Agreement with the Company dated October 22, 2014 which provided Dr. Ben-Maimon with $1.9 million of certain termination benefits and payments that were recorded during the fourth quarter of 2014.
Workforce Reductions
On June 4, 2013, the Company committed to a reduction in the Company’s workforce, eliminating approximately 110 positions, with the majority of these positions at the Company’s Hayward, California manufacturing facility. The reduction in workforce is part of the Company’s efforts to streamline its operations in response to the need to reduce expenses and adapt to changing market conditions. The Company recorded an accrual for severance and related termination costs of $3.0 million in the three month period ended June 30, 2013 as a result of this workforce reduction. As of December 31, 2013, all accrued severance and related termination costs had been paid.
On October 30, 2014, the Company committed to a reduction in the Company’s workforce, eliminating approximately 41 positions, including 35 positions in the Company’s research and development (“R&D”) organization. The reduction in workforce is part of the Company’s reorganization of its R&D organizations by consolidating the product development and analytical functions of the generic and brand R&D organizations. The workforce reduction resulted in charges of $2.1 million for severance and related termination costs, which were recorded during the quarter ended December 31, 2014. As of December 31, 2015, all accrued severance and related termination costs had been paid.
Restructuring of Packaging and Distribution Operations
On June 30, 2015, the Company committed to a restructuring of its packaging and distribution operations. As a result of this restructuring, the Company closed its Philadelphia packaging site and all Company-wide distribution operations were outsourced to United Parcel Services (UPS). In conjunction with the restructuring, approximately 93 positions have been eliminated. The Company recorded an accrual for severance and related termination costs of $2.6 million in the three month period ended June 30, 2015. As of December 31, 2015, $0.9 million has been paid and the Company currently expects the remainder of this balance to be paid by December 31, 2016.
Restructuring of Technical Operations and R&D
In November 2015, management assessed the headcount in the technical operations and research and development groups, primarily as a result of the resolution of the warning letter at the Hayward facility. The Company eliminated 27 positions and recorded an accrual for severance and related termination costs of $2.5 million during the quarter ended December 31, 2015. As of December 31, 2015, $0.9 million has been paid and the Company currently expects the remainder of this balance to be paid by early 2017.
2. BUSINESS ACQUISITION
On March 9, 2015, the Company completed the Tower acquisition, which included the acquisition of all of the outstanding shares of common stock of Tower and Lineage, pursuant to the Stock Purchase Agreement dated as of October 8, 2014, by and among the Company, Tower, Lineage, Roundtable Healthcare Partners II, L.P., Roundtable Healthcare Investors II, L.P., and the other parties thereto, including holders of certain options and warrants to acquire the common stock of Tower or Lineage. In connection with the Tower acquisition, the options and warrants of Tower and Lineage that were outstanding at the time of the acquisition were cancelled. The total consideration paid for Tower and Lineage was approximately $691.3 million, net of approximately $41.5 million of cash acquired and including the repayment of indebtedness of Tower and Lineage. The Company incurred acquisition-related costs of $10.9 million, of which $6.7 million are included in selling, general and administrative expenses in the Company’s consolidated statement of income for the year ended December 31, 2015.
The Tower acquisition allows the Company to expand its commercialized generic and branded product portfolios. The Company also leverages its sales and marketing organization to promote the marketed products acquired.
Consideration
The Company has accounted for the Tower acquisition as a business combination under the acquisition method of accounting. The Company has preliminarily allocated the purchase price for the transaction based upon the estimated fair value of net assets acquired and liabilities assumed at the date of acquisition. Accordingly, the preliminary purchase price allocation described below is subject to change, as the Company expects to finalize the allocation of the purchase price upon the resolution of certain tax accounts that are based on the best estimates of management. The completion and filing of federal and state tax returns for the various purchased entities of Tower may result in adjustments to the carrying value of assets and liabilities. Any adjustments to the preliminary fair values will be made as soon as practicable but no later than one year from the March 9, 2015 acquisition date.
Recognition and Measurement of Assets Acquired and Liabilities Assumed at Fair Value
The following tables summarize the preliminary fair values of the tangible and identifiable intangible assets acquired and liabilities assumed at the acquisition date, net of cash acquired of approximately $41.5 million (in thousands):
|
Accounts receivable(1) |
$ | 56,851 | ||
|
Inventory |
31,259 | |||
|
Income tax receivable and other prepaid expenses |
11,690 | |||
|
Deferred income taxes |
24,508 | |||
|
Property, plant and equipment |
27,540 | |||
|
Intangible assets |
632,600 | |||
|
Assets held for sale |
4,000 | |||
|
Goodwill |
182,592 | |||
|
Other non-current assets |
3,844 | |||
|
Total assets assumed |
974,884 | |||
|
Current liabilities |
64,938 | |||
|
Other non-current liabilities |
7,799 | |||
|
Deferred tax liability |
210,799 | |||
|
Total liabilities assumed |
283,536 | |||
|
Cash paid, net of cash acquired |
$ | 691,348 |
|
(1) |
The accounts receivable acquired in the transaction had a fair value of approximately $57.0 million, including an allowance for doubtful accounts of approximately $9.0 million, which represents the Company’s best estimate on March 9, 2015 (the closing date of the transaction) of the contractual cash flows not expected to be collected by the acquired companies. |
Intangible Assets
The following table identifies the Company’s preliminary allocations of purchase price to the intangible assets acquired by category:
|
Estimated Fair Value (in thousands) |
Weighted- Average Estimated Useful Life (in years) |
|||||||
|
Currently marketed product rights |
$ | 381,100 | 13 | |||||
|
Royalties |
80,800 | 12 | ||||||
|
In-process research and development |
170,700 | n/a | ||||||
|
Total intangible assets |
$ | 632,600 | 12 | |||||
The estimated fair value of the in-process research and development and identifiable intangible assets was determined using the “income approach,” which is a valuation technique that provides an estimate of the fair value of an asset based on market participant expectations of the cash flows an asset would generate over its remaining useful life. Some of the more significant assumptions inherent in the development of those asset valuations include the estimated net cash flows for each year for each asset or product (including net revenues, cost of sales, research and development costs, selling and marketing costs and working capital/asset contributory asset charges), the appropriate discount rate to select in order to measure the risk inherent in each future cash flow stream, the assessment of each asset’s life cycle, the potential regulatory and commercial success risks, competitive trends impacting the asset and each cash flow stream as well as other factors. The discount rates used to arrive at the present value at the acquisition date of currently marketed products was 15%. For in-process research and development, the discount rate used was 16% to reflect the internal rate of return and incremental commercial uncertainty in the cash flow projections. No assurances can be given that the underlying assumptions used to prepare the discounted cash flow analysis will not change. For these and other reasons, actual results may vary significantly from estimated results.
Goodwill
The Company recorded approximately $182.6 million of goodwill in connection with the Tower acquisition, some of which will not be tax-deductible. Approximately $60.2 million of this goodwill was assigned to the Impax Specialty Pharma segment and approximately $122.4 million was assigned to the Impax Generics segment. Factors that contributed to the Company’s preliminary recognition of goodwill include the Company’s intent to expand its generic and branded pharmaceutical product portfolios and to acquire certain benefits from the Tower and Lineage product pipelines in addition to the anticipated synergies that the Company expects to generate from the acquisition.
Unaudited Pro Forma Results of Operations
The unaudited pro forma combined results of operations for the years ended December 31, 2015 and 2014 (assuming the closing of the acquisition of Tower and Lineage occurred on January 1, 2014) are as follows:
|
Year Ended December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
Total revenues |
$ | 892,906 | $ | 819,838 | ||||
|
Net income |
$ | 54,285 | $ | 30,838 | ||||
The pro forma results have been prepared for comparative purposes only and are not necessarily indicative of the actual results of operations had the closing of the Transaction taken place on January 1, 2014. Furthermore, the pro forma results do not purport to project the future results of operations of the Company.
The unaudited pro forma information reflects primarily the following adjustments:
|
• |
Adjustments to amortization expense related to identifiable intangible assets acquired. Net income for the year ended December 31, 2015 reflects the lack of amortization expense for an acquired intangible asset with a short remaining estimated useful life, which causes the asset to be fully amortized by the end of 2014 under the pro forma assumption that the acquisition took place January 1, 2014; |
|
• |
Adjustments to depreciation expense related to property, plant and equipment acquired; |
|
• |
Adjustments to interest expense to reflect the long-term debt held by Tower and Lineage paid out and eliminated at the closing and the Barclays Senior Secured Credit Facilities (described in detail in “Note 14. Debt” below); |
|
• |
Adjustments to cost of revenues related to the fair value adjustments in inventory sold including elimination of approximately $6 million for the year ended December 31, 2015 and additional costs of approximately $6 million for the year ended December 31, 2014; |
|
• |
Adjustments to selling, general and administrative expense related to severance and retention costs of approximately $3 million incurred as part of the Transaction. These costs were eliminated in the pro forma results for the year ended December 31, 2015 and included in the pro forma results for the year ended December 31, 2014; |
|
• |
Adjustments to selling, general and administrative expense related to transaction costs directly attributable to the Tower acquisition include the elimination of $12 million of charges in the year ended December 31, 2015 which have been included in the year ended December 31, 2014; and |
|
• |
Adjustments to reflect the elimination of approximately $2.3 million in commitment fees related to the $435 million term loan with Barclays that were incurred during the year ended December 31, 2015 and were included in the pro forma results for the year ended December 31, 2014. |
All of the above adjustments were adjusted for the applicable tax impact.
3. BASIS OF PRESENTATION
Principles of Consolidation
The consolidated financial statements of the Company include the accounts of the operating parent company, Impax Laboratories, Inc., its wholly owned subsidiaries, including Impax Laboratories (Taiwan), Inc., Impax Laboratories USA, LLC, ThoRx Laboratories, Inc., Impax International Holding, Inc., Impax Holdings, LLC, Impax Laboratories (Netherlands) B.V., Impax Laboratories (Netherlands) C.V., Lineage and Tower, including operating subsidiaries CorePharma, Amedra Pharmaceuticals, Mountain, LLC and Trail Services, Inc., in addition to an equity investment in Prohealth Biotech, Inc. (“Prohealth”), in which the Company held a 57.54% majority ownership interest at December 31, 2015. All significant intercompany accounts and transactions have been eliminated.
Foreign Currency Translation
The Company translates the assets and liabilities of the Taiwan dollar functional currency of its majority-owned affiliate Prohealth and its wholly-owned subsidiary Impax Laboratories (Taiwan), Inc. into the U.S. dollar reporting currency using exchange rates in effect at the end of each reporting period. The revenues and expenses of these entities are translated using an average of the rates in effect during the reporting period. Gains and losses from these translations are recorded as currency translation adjustments included in the consolidated statements of comprehensive income and the consolidated statements of changes in stockholders’ equity.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States (“GAAP”) and the rules and regulations of the U.S. Securities & Exchange Commission (“SEC”) requires the use of estimates and assumptions, based on complex judgments considered reasonable, and affect the reported amounts of assets and liabilities and disclosure of contingent assets and contingent liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. The most significant judgments are employed in estimates used in determining values of tangible and intangible assets, derivatives, legal contingencies, tax assets and tax liabilities, fair value of share-based compensation related to equity incentive awards issued to employees and directors, and estimates used in applying the Company’s revenue recognition policy, including those related to accrued chargebacks, rebates, product returns, Medicare, Medicaid, and other government rebate programs, shelf-stock adjustments, and the timing and amount of deferred and recognized revenue and deferred and amortized product manufacturing costs related to alliance and collaboration agreements. Actual results may differ from estimated results.
Reclassifications
Certain prior year amounts have been reclassified to conform to the presentation for the year ended December 31, 2015.
4. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Cash and Cash Equivalents
The Company considers all short-term investments with maturity of three months or less at the date of purchase to be cash equivalents. Cash and cash equivalents are stated at cost, which, for cash equivalents, approximates fair value due to their short-term maturity. The Company is potentially subject to financial instrument concentration of credit risk through its cash and cash equivalents. The Company maintains cash and cash equivalents with several major financial institutions. Such amounts frequently exceed Federal Deposit Insurance Corporation (“FDIC”) limits.
Short-Term Investments
Short-term investments represented investments in fixed rate financial instruments with maturities of greater than three months but less than 12 months at the time of purchase. The Company’s short-term investments were held in U.S. Treasury securities, corporate bonds, and high grade commercial paper, which are not insured by the FDIC. They were stated at amortized cost, which approximated fair value due to their short-term maturity, generally based upon observable market values of similar securities.
Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts for estimated losses resulting from amounts deemed to be uncollectible from its customers; these allowances are for specific amounts on certain accounts based on facts and circumstances determined on a case-by-case basis.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk are cash, cash equivalents, and accounts receivable. The Company limits its credit risk associated with cash and cash equivalents by placing its investments with high quality money market funds, corporate debt, and short-term commercial paper and in securities backed by the U.S. Government. The Company limits its credit risk with respect to accounts receivable by performing credit evaluations when deemed necessary. The Company does not require collateral to secure amounts owed to it by its customers.
The following tables present the percentage of total accounts receivable and gross revenues represented by the Company’s five largest customers as of and for the years ended December 31, 2015, 2014 and 2013:
|
Percent of Total Accounts Receivable |
2015 |
2014 |
2013 |
|||||||||
|
Customer #1 |
52.4 | % | 36.9 | % | 28.8 | % | ||||||
|
Customer #2 |
24.8 | % | 28.2 | % | 35.1 | % | ||||||
|
Customer #3 |
14.4 | % | 19.6 | % | 18.5 | % | ||||||
|
Customer #4 |
1.0 | % | 1.8 | % | 2.9 | % | ||||||
|
Customer #5 |
0.7 | % | 0.6 | % | 0.9 | % | ||||||
|
Customer #6 |
0.6 | % | 1.7 | % | 1.4 | % | ||||||
|
Customer #7 |
0.5 | % | 1.7 | % | 2.0 | % | ||||||
|
Total five largest customers |
94.4 | % | 90.5 | % | 89.6 | % | ||||||
|
Percent of Gross Revenues |
2015 |
2014 |
2013 |
|||||||||
|
Customer #1 |
45.6 | % | 36.0 | % | 30.6 | % | ||||||
|
Customer #2 |
21.7 | % | 20.7 | % | 25.1 | % | ||||||
|
Customer #3 |
18.8 | % | 19.3 | % | 20.3 | % | ||||||
|
Customer #4 |
1.4 | % | 2.5 | % | 2.5 | % | ||||||
|
Customer #5 |
1.1 | % | 1.8 | % | 1.8 | % | ||||||
|
Customer #6 |
0.4 | % | 1.9 | % | 0.3 | % | ||||||
|
Customer #7 |
-- | % | 1.5 | % | 2.4 | % | ||||||
|
Total five largest customers |
89.0 | % | 83.7 | % | 83.0 | % | ||||||
During the years ended December 31, 2015, 2014 and 2013, the Company’s top ten products accounted for 75%, 62% and 68%, respectively, of total Impax product sales, net. Refer to “Note 24. Supplemental Financial Information” for more information.
In July 2015, the Company received an unsolicited offer from Turing Pharmaceuticals AG (“Turing”) to purchase the U.S. rights to Daraprim®, one of the marketed products acquired in the Tower acquisition, as well as the active pharmaceutical ingredient for the product and the finished goods inventory on hand. Pursuant to the terms of the Asset Purchase Agreement between the Company and Turing dated August 7, 2015 (the “Turing APA”), the Company also granted a limited license to sell the existing Daraprim® product under the Company’s labeler code with the Company’s trade dress. The sale closed on August 7, 2015.
In accordance with the terms of the Turing APA, the Company received and is initially responsible for processing and paying (subject to reimbursement by Turing), all chargebacks and rebates resulting from utilization by Medicaid, Medicare and other federal, state and local governmental programs, health plans and other health care providers for product sold under the Company’s labeler code. Under the terms of the Turing APA, Turing is responsible for liabilities related to chargebacks and rebates that arise as a result of Turing’s marketing or selling related activities.
During the fourth quarter of 2015, the Company began receiving invoices for chargebacks from wholesalers and rebates from various state Medicaid agencies for Daraprim® purchases made by governmental agencies during the third quarter of 2015. As a result, the Company recorded a $40.6 million receivable representing an estimate for the third and fourth quarter 2015 reimbursement amounts owed by Turing to the Company. In addition, the Company recorded an accrued liability for the corresponding offsetting amount owed to wholesalers, Medicaid and other governmental agencies. The Company and Turing are currently in the process of finalizing the reimbursement amount owed by Turing to the Company for such chargebacks and rebates. If Turing for any reason does not, or is unable to, make such reimbursement payments to the Company, it could result in a material charge to the Company.
Inventory
Inventory is stated at the lower of cost or market. Cost is determined using a standard cost method, and the cost flow assumption is first in, first out (“FIFO”) flow of goods. Standard costs are revised annually, and significant variances between actual costs and standard costs are apportioned to inventory and cost of goods sold based upon inventory turnover. Costs include materials, labor, quality control, and production overhead. Inventory is adjusted for short-dated, unmarketable inventory equal to the difference between the cost of inventory and the estimated value based upon assumptions about future demand and market conditions. If actual market conditions are less favorable than those projected by the Company, additional inventory write-downs may be required. Consistent with industry practice, the Company may build pre-launch inventories of certain products which are pending required approval from the FDA and/or resolution of patent infringement litigation, when, in the Company’s assessment, such action is appropriate to prepare for the anticipated commercial launch and FDA approval is expected in the near term and/or the related litigation will be resolved in the Company’s favor. The Company accounts for all costs of idle facilities, excess freight and handling costs, and wasted materials (spoilage) as a current period charge in accordance with GAAP.
Property, Plant and Equipment
Property, plant and equipment are recorded at cost. Maintenance and repairs are charged to expense as incurred and costs of improvements and renewals are capitalized. Costs incurred in connection with the construction or major renovation of facilities, including interest directly related to such projects, are capitalized as construction in progress. Depreciation is recognized using the straight-line method based on the estimated useful lives of the related assets, which are generally 40 years for buildings, 10 to 15 years for building improvements, eight to 10 years for equipment, and four to 10 years for office furniture and equipment. Land and construction-in-progress are not depreciated.
Intangible Assets
The Company’s intangible assets include both indefinite lived and finite lived assets. Indefinite lived intangible assets are not amortized. In-process research and development assets acquired in a business combination are considered indefinite lived until the completion or abandonment of the associated research and development efforts. Finite lived intangible assets are amortized over the estimated useful life based on the pattern in which the economic benefits of the intangible asset are consumed or otherwise used up. If that pattern cannot be reliably determined, the straight-line amortization method is used. All of the Company’s intangible assets are tested for impairment at least annually during the fourth quarter of the fiscal year, or more often if indicators of impairment are present. Impairment testing requires management to estimate the future undiscounted cash flows of the finite lived intangible assets using assumptions believed to be reasonable, but which are unpredictable and inherently uncertain. Actual future cash flows may differ from the estimates used in the impairment testing. The Company recognizes an impairment loss when and to the extent that the estimated fair value of an intangible asset is less than its carrying value.
Goodwill
In accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification™ (“ASC”) Topic 350, "Goodwill and Other Intangibles", rather than recording periodic amortization, goodwill is subject to an annual assessment for impairment by applying a fair value based test. Under FASB ASC Topic 350, if the fair value of the reporting unit exceeds the reporting unit’s carrying value, including goodwill, then goodwill is considered not impaired, making further analysis not required. The Company considers the Impax Generics division and the Impax Specialty Pharma division operating segments to each be a reporting unit. The Company attributes $60.2 million of goodwill to the Impax Specialty Pharma division and $150.0 million of goodwill to the Impax Generics division.
The Company concluded the carrying value of goodwill was not impaired as of December 31, 2015 and 2014 as the fair value of the Impax Specialty Pharma division and the Impax Generics division exceeded their carrying value at each date. The Company performs its annual goodwill impairment test in the fourth quarter of each year. The Company estimated the fair value of the Impax Specialty Pharma division and the Impax Generics division using a discounted cash flow model for both the reporting unit and the enterprise. In addition, on a quarterly basis, the Company performs a review of its business operations to determine whether events or changes in circumstances have occurred which could have a material adverse effect on the estimated fair value of each reporting unit, and thus indicate a potential impairment of the goodwill carrying value. If such events or changes in circumstances were deemed to have occurred, the Company would perform an interim impairment analysis, which may include the preparation of a discounted cash flow model, or consultation with one or more valuation specialists, to determine the impact, if any, on the Company’s assessment of the reporting unit’s fair value. The Company has not to date deemed there to have been any significant adverse changes in the legal, regulatory, or general economic environment in which the Company conducts its business operations.
Derivatives
The Company generally does not use derivative instruments or engage in hedging activities in its ordinary course of business. Prior to June 30, 2015, the Company had no derivative assets or liabilities and did not engage in any hedging activities. As a result of the Company’s June 30, 2015 issuance of the convertible senior notes described in “Note 14. Debt”, the conversion option of the notes temporarily met the criteria for an embedded derivative liability which required bifurcation and separate accounting. Concurrently with the issuance of the notes, the Company entered into a series of convertible note hedge and warrant transactions which in combination are designed to reduce the potential dilution to the Company’s stockholders and/or offset the cash payments the Company is required to make in excess of the principal amount upon conversion of the notes. See “Note 8. Derivatives” and “Note 15. Stockholders’ Equity” for additional information regarding the note hedge transactions and warrant transactions. While the warrants sold were classified as equity and recorded in additional paid-in capital, the call options purchased were temporarily classified as a bond hedge derivative asset on the Company’s consolidated balance sheet. The Company engaged a third-party valuation firm with expertise in valuing financial instruments to determine the fair value of the bond hedge derivative asset and conversion option derivative liability at each reporting period. The Company’s interim consolidated balance sheets reflected the fair value of the derivative asset and liability as of the reporting date, and changes in the fair value were reflected in current period earnings, as appropriate. As result of the amendment to the Company’s Restated Certificate of Incorporation to increase the number of authorized shares of the Company's common stock discussed in “Note 15. Stockholders’ Equity,” both the derivative asset and liability were reclassified to additional paid-in capital. The Company had no derivative assets or liabilities and did not engage in any hedging activities as of December 31, 2015.
Contingencies
In the normal course of business, the Company is subject to loss contingencies, such as legal proceedings and claims arising out of its business, covering a wide range of matters, including, among others, patent litigation, stockholder lawsuits, and product and clinical trial liability. In accordance with FASB ASC Topic 450, "Contingencies", the Company records accruals for such loss contingencies when it is probable a liability will have been incurred and the amount of loss can be reasonably estimated. The Company, in accordance with FASB ASC Topic 450, does not recognize gain contingencies until realized. The Company records an accrual for legal costs in the period incurred. A discussion of contingencies is included in “Note 21. Commitments and Contingencies,” and “Note 22. Legal and Regulatory Matters” footnotes below.
Deferred Financing Costs
The Company capitalizes direct costs incurred to obtain debt financing and amortizes these costs to interest expense using the effective interest method over the term of the debt. These costs are recorded as a debt discount and the unamortized costs are netted against the related debt on the Company’s consolidated balance sheet. For line-of-credit arrangements with no outstanding borrowing, the costs incurred to obtain the credit facility are amortized to interest expense using the straight-line method over the term of the line-of-credit arrangement. The unamortized balance is included in other assets on the Company’s consolidated balance sheet..
Revenue Recognition
The Company recognizes revenue when the earnings process is complete, which under SEC Staff Accounting Bulletin No. 104, Topic No. 13, “Revenue Recognition” (“SAB 104”), is when revenue is realized or realizable and earned, there is persuasive evidence a revenue arrangement exists, delivery of goods or services has occurred, the sales price is fixed or determinable, and collectability is reasonably assured.
The Company accounts for material revenue arrangements which contain multiple deliverables in accordance with FASB ASC Topic 605-25, revenue recognition for arrangements with multiple elements, which addresses the determination of whether an arrangement involving multiple deliverables contains more than one unit of accounting. A delivered item within an arrangement is considered a separate unit of accounting only if both of the following criteria are met:
|
● |
the delivered item has value to the customer on a stand-alone basis; and |
|
● |
if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in the control of the vendor. |
Under FASB ASC Topic 605-25, if both of the criteria above are not met, then separate accounting for the individual deliverables is not appropriate. Revenue recognition for arrangements with multiple deliverables constituting a single unit of accounting is recognized generally over the greater of the term of the arrangement or the expected period of performance, either on a straight-line basis or on a modified proportional performance method.
The Company accounts for milestones related to research and development activities in accordance with FASB ASC Topic 605-28, milestone method of revenue recognition. FASB ASC Topic 605-28 allows for the recognition of consideration, which is contingent on the achievement of a substantive milestone, in its entirety in the period the milestone is achieved. A milestone is considered to be substantive if all of the following criteria are met: the milestone is commensurate with either: (1) the performance required to achieve the milestone, or (2) the enhancement of the value of the delivered items resulting from the performance required to achieve the milestone; the milestone relates solely to past performance; and, the milestone payment is reasonable relative to all of the deliverables and payment terms within the agreement.
Impax Generics revenues, net, and Impax Specialty Pharma revenues, net
The Impax Generics revenues, net and Impax Specialty Pharma revenues, net include revenue recognized related to shipments of generic and branded pharmaceutical products to the Company’s customers, primarily drug wholesalers and retail chains. Gross sales revenue is recognized at the time title and risk of loss passes to the customer, which is generally when product is received by the customer. Impax Generics and Impax Specialty Pharma revenue, net may include deductions from the gross sales price related to estimates for chargebacks, rebates, distribution service fees, returns, shelf-stock, and other pricing adjustments. The Company records an estimate for these deductions in the same period when revenue is recognized. A summary of each of these deductions is as follows:
Chargebacks
The Company has agreements establishing contract prices for certain products with certain indirect customers, such as managed care organizations, hospitals and government agencies who purchase products from drug wholesalers. The contract prices are lower than the prices the customer would otherwise pay to the wholesaler, and the price difference is referred to as a chargeback, which generally takes the form of a credit memo issued by the Company to reduce the invoiced gross selling price charged to the wholesaler. An estimated accrued provision for chargeback deductions is recognized at the time of product shipment. The primary factors considered when estimating the provision for chargebacks are the average historical chargeback credits given, the mix of products shipped, and the amount of inventory on hand at the major drug wholesalers with whom the Company does business. The Company also monitors actual chargebacks granted and compares them to the estimated provision for chargebacks to assess the reasonableness of the chargeback reserve at each quarterly balance sheet date.
Rebates
The Company maintains various rebate programs with its customers in an effort to maintain a competitive position in the marketplace and to promote sales and customer loyalty. The rebates generally take the form of a credit memo to reduce the invoiced gross selling price charged to a customer for products shipped. An estimated accrued provision for rebate deductions is recognized at the time of product shipment. The primary factors the Company considers when estimating the provision for rebates are the average historical experience of aggregate credits issued, the mix of products shipped and the historical relationship of rebates as a percentage of total gross product sales, the contract terms and conditions of the various rebate programs in effect at the time of shipment, and the amount of inventory on hand at the major drug wholesalers with whom the Company does business. The Company also monitors actual rebates granted and compares them to the estimated provision for rebates to assess the reasonableness of the rebate reserve at each quarterly balance sheet date.
Distribution Service Fees
The Company pays distribution service fees to several of its wholesaler customers related to sales of its Impax Products. The wholesalers are generally obligated to provide the Company with periodic outbound sales information as well as inventory levels of the Company’s Impax Products held in their warehouses. Additionally, the wholesalers have agreed to manage the variability of their purchases and inventory levels within specified days on hand limits. An accrued provision for distribution service fees is recognized at the time products are shipped to wholesalers.
Returns
The Company allows its customers to return product if approved by authorized personnel in writing or by telephone with the lot number and expiration date accompanying any request and if such products are returned within six months prior to or until twelve months following, the products’ expiration date. The Company estimates and recognizes an accrued provision for product returns as a percentage of gross sales based upon historical experience. The product return reserve is estimated using a historical lag period, which is the time between when the product is sold and when it is ultimately returned, and estimated return rates which may be adjusted based on various assumptions including changes to internal policies and procedures, changes in business practices, and commercial terms with customers, competitive position of each product, amount of inventory in the wholesaler supply chain, the introduction of new products, and changes in market sales information. The Company also considers other factors, including significant market changes which may impact future expected returns, and actual product returns. The Company monitors actual returns on a quarterly basis and may record specific provisions for returns it believes are not covered by historical percentages.
Shelf-Stock Adjustments
Based upon competitive market conditions, the Company may reduce the selling price of certain Impax Generics division products. The Company may issue a credit against the sales amount to a customer based upon their remaining inventory of the product in question, provided the customer agrees to continue to make future purchases of product from the Company. This type of customer credit is referred to as a shelf-stock adjustment, which is the difference between the sales price and the revised lower sales price, multiplied by an estimate of the number of product units on hand at a given date. Decreases in selling prices are discretionary decisions made by the Company in response to market conditions, including estimated launch dates of competing products and declines in market price. The Company records an estimate for shelf-stock adjustments in the period it agrees to grant such a credit memo to a customer.
Medicaid and Other Government Pricing Programs
As required by law, the Company provides a rebate on drugs dispensed under the Medicaid program, Medicare Part D, TRICARE, and other U.S. government pricing programs. The Company determines its estimated government rebate accrual primarily based on historical experience of claims submitted by the various states and other jurisdictions and any new information regarding changes in the various programs which may impact the Company’s estimate of government rebates. In determining the appropriate accrual amount, the Company considers historical payment rates and processing lag for outstanding claims and payments. The Company records estimates for government rebates as a deduction from gross sales, with a corresponding adjustment to accrued liabilities.
Cash Discounts
The Company offers cash discounts to its customers, generally 2% of the gross selling price, as an incentive for paying within invoice terms, which generally range from 30 to 90 days. An estimate of cash discounts is recorded in the same period when revenue is recognized.
Rx Partner and OTC Partner:
The Rx Partner and OTC Partner contracts include revenue recognized under alliance and collaboration agreements between the Company and unrelated third-party pharmaceutical companies. The Company has entered into these alliance agreements to develop marketing and/or distribution relationships with its partners to fully leverage its technology platform.
The Rx Partners and OTC Partners alliance agreements obligate the Company to deliver multiple goods and/or services over extended periods. Such deliverables include manufactured pharmaceutical products, exclusive and semi-exclusive marketing rights, distribution licenses, and research and development services. In exchange for these deliverables the Company receives payments from its agreement partners for product shipments and research and development services, and may also receive other payments including royalty, profit sharing, upfront, and periodic milestone payments. Revenue received from the alliance agreement partners for product shipments under these agreements is not subject to deductions for chargebacks, rebates, product returns, and other pricing adjustments. Royalty and profit sharing amounts the Company receives under these agreements are calculated by the respective agreement partner, with such royalty and profit share amounts generally based upon estimates of net product sales or gross profit which include estimates of deductions for chargebacks, rebates, product returns, and other adjustments the alliance agreement partners may negotiate with their respective customers. The Company records the agreement partner's adjustments to such estimated amounts in the period the agreement partner reports the amounts to the Company.
The Company applies the updated guidance of ASC 605-25 “Multiple Element Arrangements” to the Strategic Alliance Agreement, as amended with Teva Pharmaceuticals Curacao N.V., a subsidiary of Teva Pharmaceutical Industries Limited (“Teva Agreement”). The Company looks to the underlying delivery of goods and/or services which give rise to the payment of consideration under the Teva Agreement to determine the appropriate revenue recognition. The Company initially defers consideration received as a result of research and development-related activities performed under the Teva Agreement. The Company recognizes deferred revenue on a straight-line basis over the expected period of performance for such services. Consideration received as a result of the manufacture and delivery of products under the Teva Agreement is recognized at the time title and risk of loss passes to the customer which is generally when product is received by Teva. The Company recognizes profit share revenue in the period earned.
OTC Partner revenue is related to agreements with Pfizer, Inc., formerly Wyeth LLC (“Pfizer”) and L. Perrigo Company (“Perrigo”) with respect to the supply of over-the-counter pharmaceutical products. The OTC Partner sales channel is no longer a core area of the business, and the over-the-counter pharmaceutical products the Company sells through this sales channel are older products which are only sold to Pfizer and Perrigo. The Company is currently only required to manufacture the over-the-counter pharmaceutical products under its agreements with Pfizer and Perrigo. The Company recognizes profit share revenue in the period earned.
Research Partner:
The Research Partner contract includes revenue recognized under development agreements with unrelated third-party pharmaceutical companies. The development agreements generally obligate the Company to provide research and development services over multiple periods. In exchange for this service, the Company received upfront payments upon signing of each development agreement and is eligible to receive contingent milestone payments, based upon the achievement of contractually specified events. Additionally, the Company may also receive royalty payments from the sale, if any, of a successfully developed and commercialized product under one of these development agreements. The Company recognizes revenue received from the achievement of contingent research and development milestones in the period such payment is earned. Royalty fee income, if any, will be recognized as current period revenue when earned.
Shipping and Handling Fees and Costs
Shipping and handling fees related to sales transactions are recorded as selling expense. Shipping costs were $2,304,000, $2,382,000 and $1,890,000 for the years ended December 31, 2015, 2014 and 2013, respectively.
Research and Development Expenses
Research and development activities are expensed as incurred and consist of self-funded research and development costs and costs associated with work performed by other participants under collaborative research and development agreements.
Share-Based Compensation
The Company accounts for stock-based employee compensation arrangements in accordance with provisions of FASB ASC Topic 718 “Stock Compensation”. Under FASB ASC Topic 718, the Company recognizes the grant date fair value of stock-based employee compensation as expense on a straight-line basis over the vesting period of the grant. The Company uses the Black Scholes option pricing model to determine the grant date fair value of employee stock options; the fair value of restricted stock awards is equal to the closing price of the Company’s stock on the date such award was granted.
Income Taxes
The Company provides for income taxes using the asset and liability method as required by FASB ASC Topic 740, “Income Taxes”. This approach recognizes the amount of federal, state, local taxes, and foreign taxes payable or refundable for the current year, as well as deferred tax assets and liabilities for the future tax consequences of events recognized in the consolidated financial statements and income tax returns. Deferred income tax assets and liabilities are adjusted to recognize the effects of changes in tax laws or enacted tax rates in the period during which they are signed into law. Under FASB ASC Topic 740, a valuation allowance is required when it is more likely than not all or some portion of the deferred tax assets will not be realized through generating sufficient future taxable income.
FASB ASC Topic 740, Sub-topic 10 “Tax Positions”, defines the criterion an individual tax position must meet for any part of the benefit of the tax position to be recognized in financial statements prepared in conformity with generally accepted accounting principles. Under FASB ASC Topic 740, Sub-topic 10, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not the tax position will be sustained on examination by the taxing authorities, based solely on the technical merits of the tax position. The tax benefits recognized in the financial statements from such a tax position should be measured based on the largest benefit having a greater than 50% likelihood of being realized upon ultimate settlement with the tax authority. Additionally, FASB ASC Topic 740, Sub-topic 10 provides guidance on measurement, de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. In accordance with the disclosure requirements of FASB ASC Topic 740, Sub-topic 10, the Company’s policy on income statement classification of interest and penalties related to income tax obligations is to include such items as part of total interest expense and other expense, respectively.
Other Comprehensive Income
The Company follows the provisions of FASB ASC Topic 220, ”Comprehensive Income”, which establishes standards for the reporting and display of comprehensive income and its components. Comprehensive income is defined to include all changes in equity during a period except those resulting from investments by owners and distributions to owners. The Company recorded foreign currency translation gains and losses, which are reported as comprehensive income. Foreign currency translation losses for the years ended December 31, 2015, 2014 and 2013 were $4,454,000, $7,149,000 and $4,104,000 respectively.
5. RECENT ACCOUNTING PRONOUNCEMENTS
In May 2014, the FASB issued ASU 2014-09, “Revenue from Contracts with Customers” (Topic 606) regarding the accounting for and disclosures of revenue recognition, with an effective date for annual and interim periods beginning after December 15, 2016. This update provides a single comprehensive model for accounting for revenue from contracts with customers. The model requires that revenue recognized reflect the actual consideration to which the entity expects to be entitled in exchange for the goods or services defined in the contract, including in situations with multiple performance obligations. In July 2015, the FASB issued ASU 2015-14, “Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date”, which deferred the effective date, of the previously issued revenue recognition guidance by one year. The guidance will be effective for annual and interim periods beginning after December 15, 2017. The Company is currently evaluating the effect that this guidance may have on its consolidated financial statements.
In April 2015, the FASB issued ASU 2015-03, “Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs”, which provided guidance on the presentation requirements for debt issuance costs and debt discount and premium. The update requires that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by the updated guidance. The updated guidance is effective for annual and interim periods beginning after December 15, 2015 and early adoption is permitted for financial statements that have not been previously issued. The Company adopted this guidance during 2015, and it had no effect on the Company’s results of operations.
In July 2015, the FASB issued ASU 2015-11, Inventory (Topic 330): “Simplifying the Measurement of Inventory”, with guidance regarding the accounting for and measurement of inventory. The update requires that inventory measured using first-in, first-out (FIFO) shall be measured at the lower of cost and net realizable value. When there is evidence that the net realizable value of inventory is lower than its cost, the difference shall be recognized as a loss in earnings in the period in which it occurs. The guidance will be effective for annual and interim periods beginning after December 15, 2016. The Company is currently evaluating the effect that this guidance may have on its consolidated financial statements.
In August 2015, the FASB issued ASU 2015-15, “Interest—Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements”, with guidance on the presentation and measurement of debt issuance costs associated with line-of-credit arrangements. ASU 2015-03 previously issued in April 2015 did not address the presentation or subsequent measurement of debt issuance costs related to line-of-credit arrangements. ASU 2015-15 states that the SEC would not object to the deferral and presentation of debt issuance costs as an asset and subsequent amortization of debt issuance costs over the term of the line-of-credit arrangement, whether or not there are any outstanding borrowings on the line-of-credit arrangement. The Company adopted this guidance during 2015, and it had no effect on the Company’s results of operations.
In September 2015, the FASB issued ASU 2015-16, Business Combinations (Topic 805): “Simplifying the Accounting for Measurement-Period Adjustments”, with guidance regarding the accounting for and disclosure of measurement-period adjustments that occur in periods after a business combination is consummated. This update requires that the acquirer recognize measurement-period adjustments in the reporting period in which they are determined and, as such, eliminates the previous requirement to retrospectively account for these adjustments. This update also requires an entity to present separately on the face of the income statement, or disclose in the notes, the amount recorded in the current-period income statement that would have been recorded in previous reporting periods if the adjustments had been recognized as of the acquisition date. The effective date for annual and interim periods begins after December 15, 2015. The Company is currently evaluating the effect that this guidance may have on its consolidated financial statements.
In November 2015, the FASB issued ASU 2015-17, “Balance Sheet Classification of Deferred Taxes”, which requires that deferred tax assets and liabilities be classified as noncurrent in a classified statement of financial position, depending on the tax jurisdiction that they relate to. This update is effective for financial statements issued for fiscal years beginning after December 15, 2016, and interim periods within those fiscal years, and may be applied either prospectively to all deferred tax assets and liabilities or retrospectively to all periods presented. The Company elected to early-adopt this update on a retrospective basis, which resulted in $54.8 million of current deferred tax assets being reclassified to long-term as of December 31, 2014. The adoption of this update had no effect on the Company’s results of operations.
6. FAIR VALUE MEASUREMENT AND FINANCIAL INSTRUMENTS
The carrying values of cash equivalents, accounts receivable, prepaid expenses and other current assets, and accounts payable in the Company’s consolidated balance sheets approximated their fair values as of December 31, 2015 and 2014 due to their short-term nature.
Certain of the Company’s financial instruments are measured at fair value using a three-level hierarchy that prioritizes the inputs used to measure fair value. This hierarchy maximizes the use of observable inputs and minimizes the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
|
|
● |
Level 1 - Inputs are quoted prices for identical instruments in active markets. |
|
|
● |
Level 2 - Inputs are quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; or model-derived valuations whose inputs are observable or whose significant value drivers are observable. |
|
|
● |
Level 3 - Inputs are unobservable and reflect the Company's own assumptions, based on the best information available, including the Company's own data. |
The carrying amounts and fair values of the Company’s financial instruments at December 31, 2015 and 2014 are indicated below (in thousands):
|
As of December 31, 2015 |
||||||||||||||||||||
|
Fair Value Measurement Based on |
||||||||||||||||||||
|
Carrying Amount |
Fair Value |
Quoted Prices in Active Markets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||||||
|
Assets |
||||||||||||||||||||
|
Deferred compensation plan asset (1) |
$ | 30,726 | $ | 30,726 | $ | - | $ | 30,726 | $ | - | ||||||||||
|
Liabilities |
||||||||||||||||||||
|
2% convertible senior notes due June 2022 (2) |
$ | 600,000 | $ | 602,250 | $ | 602,250 | $ | - | $ | - | ||||||||||
|
Deferred compensation plan liability (1) |
$ | 25,581 | $ | 25,581 | $ | - | $ | 25,581 | $ | - | ||||||||||
|
As of December 31, 2014 |
||||||||||||||||||||
|
Fair Value Measurement Based on |
||||||||||||||||||||
|
Carrying Amount |
Fair Value |
Quoted Prices in Active Markets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
||||||||||||||||
|
Assets |
||||||||||||||||||||
|
Short-term investments |
$ | 199,983 | $ | 199,899 | $ | 199,899 | $ | - | $ | - | ||||||||||
|
Deferred compensation plan asset (1) |
$ | 29,241 | $ | 29,241 | $ | - | $ | 29,241 | $ | - | ||||||||||
|
Liabilities |
||||||||||||||||||||
|
Deferred compensation plan liability (1) |
$ | 25,837 | $ | 25,837 | $ | - | $ | 25,837 | $ | - | ||||||||||
|
|
(1) |
The deferred compensation plan liability is a non-current liability recorded at the value of the amount owed to the plan participants, with changes in value recognized as a compensation expense in the Company’s consolidated statements of income. The calculation of the deferred compensation obligation is derived from observable market data by reference to hypothetical investments selected by the participants and is included in the line items captioned “Other non-current liabilities” on the Company’s consolidated balance sheets. The Company invests in corporate-owned life insurance (“COLI”) policies, of which the cash surrender value is included in the line item captioned “Other non-current assets” on the Company’s consolidated balance sheets. |
| (2) |
The difference between the amount shown as the carrying value in the above table and the amount shown on the Company’s consolidated balance sheet at December 31, 2015 represents the unamortized discounts related to deferred debt issuance costs and the bifurcation of the conversion feature of the Notes. |
7. SHORT-TERM INVESTMENTS
Investments consist of commercial paper and corporate bonds. The Company’s policy was to invest in only high quality “AAA-rated” or investment-grade securities. Investments in debt securities were accounted for as “held-to-maturity” and were recorded at amortized cost, which approximates fair value, generally based upon observable market values of similar securities. The Company has historically held all investments in debt securities until maturity, and has the ability and intent to continue to do so. All of the Company’s investments had remaining contractual maturities of less than 12 months and were classified as short-term. Upon maturity, the Company used a specific identification method.
The Company had no short-term investments as of December 31, 2015. A summary of short-term investments as of December 31, 2014 is as follows (in thousands):
|
Gross |
Gross | |||||||||||||||
|
Amortized |
Unrecognized |
Unrecognized |
Fair | |||||||||||||
|
December 31, 2014 |
Cost |
Gains |
Losses |
Value |
||||||||||||
|
Commercial paper |
$ | 68,972 | $ | 17 | $ | -- | $ | 68,989 | ||||||||
|
Corporate bonds |
131,011 | -- | (101 | ) | 130,910 | |||||||||||
|
Total short-term investments |
$ | 199,983 | $ | 17 | $ | (101 | ) | $ | 199,899 | |||||||
8. DERIVATIVES
As discussed in “Note 14. Debt”, on June 30, 2015, the Company issued an aggregate principal amount of $600.0 million of 2.00% Convertible Senior Notes due June 2022 in a private placement offering (the “Notes”). Concurrently with the issuance of the Notes, the Company entered into convertible note hedge transactions with a financial institution (the “Note Hedge Transactions”), which are generally expected to reduce the potential dilution to the Company’s stockholders and/or offset the cash payments the Company is required to make in excess of the principal amount upon conversion of the Notes.
Derivative Asset
Pursuant to the Note Hedge Transactions, the Company purchased from the financial institution approximately 0.6 million call options on the Company’s common stock (the “Bond Hedge Derivative Asset”), for which it paid consideration of $147.0 million. Each call option entitles the Company to purchase 15.7858 shares of the Company’s common stock at an exercise price of $63.35 per share, is immediately exercisable, and has an expiration date of June 15, 2022, subject to earlier exercise.
The fair value of the Bond Hedge Derivative Asset at December 8, 2015 was $125.0 million, which was determined by a model-derived valuation utilizing Level 2 inputs which are observable or whose significant value drivers are observable. The following table summarizes the inputs and assumptions used in the Black-Scholes model to calculate the fair value of Bond Hedge Derivative Asset as of December 8, 2015:
|
Common stock price |
$42.56 | |||
|
Exercise price |
$63.35 | |||
|
Risk-free interest rate |
1.9% | |||
|
Volatility |
40% | |||
|
Dividend yield |
0% | |||
|
Remaining contractual term (in years) |
6.5 |
Derivative Liability
As of the June 30, 2015 issuance date of the Notes, the Company did not have the necessary number of authorized but unissued shares of its common stock available to share-settle the conversion option of the Notes. Therefore, in accordance with guidance found in ASC 470-20 and ASC 815-15, the conversion option of the Notes was deemed an embedded derivative that required bifurcation from the Notes (host contract) and separate accounting as a derivative liability. The Company recorded the $167.0 million fair value of the conversion option derivative liability as a debt discount at June 30, 2015 on the Company's consolidated balance sheet.
The fair value of the conversion option derivative liability at December 8, 2015 was $158.0 million, which was determined by a model-derived valuation utilizing Level 2 inputs which are observable or whose significant value drivers are observable. The following table summarizes the inputs and assumptions used in the binomial lattice model to calculate the fair value as of December 8, 2015:
|
Common stock price |
$42.56 | |||
|
Exercise price |
$63.35 | |||
|
Risk-free interest rate |
1.9% | |||
|
Volatility |
40% | |||
|
Annual coupon rate |
2% | |||
|
Remaining contractual term (in years) |
6.5 |
Reclassification to Additional Paid-in Capital
The Company held a Special Meeting of the Stockholders of the Company (the “Special Meeting”) on December 8, 2015, at which time the Company’s stockholders approved an amendment to the Restated Certificate of Incorporation to increase the number of authorized shares of the Company’s common stock, par value $0.01 per share, from 90,000,000 shares to 150,000,000 shares (the “Amendment”), which Amendment was subsequently filed with the Secretary of State of Delaware by the Company and effected. As a result of the increase in the authorized shares of common stock, both the derivative asset and the derivative liability met the derivative scope exception and were reclassified to additional paid-in capital, net of related taxes.
During the six month period ended December 31, 2015, the Company recognized in its consolidated statement of income $13.0 million of net expense related to the change in the fair value of the derivative instruments before the liability was reclassified to equity as described above.
9. ACCOUNTS RECEIVABLE
The composition of accounts receivable, net is as follows (in thousands):
|
December 31, |
December 31, |
|||||||
|
2015 |
2014 | |||||||
|
Gross accounts receivable |
$ | 738,730 | $ | 287,362 | ||||
|
Less: Rebate reserve |
(265,229 | ) | (88,812 | ) | ||||
|
Less: Chargeback reserve |
(102,630 | ) | (43,125 | ) | ||||
|
Less: Distribution services reserve |
(12,576 | ) | (1,331 | ) | ||||
|
Less: Discount reserve |
(18,657 | ) | (7,089 | ) | ||||
|
Less: Uncollectible accounts reserve |
(15,187 | ) | (515 | ) | ||||
|
Accounts receivable, net |
$ | 324,451 | $ | 146,490 | ||||
A roll-forward of the chargeback and rebate reserves activity for the years ended December 31, 2015, 2014 and 2013 is as follows (in thousands):
|
December 31, |
December 31, |
December 31, |
||||||||||
|
Rebate reserve |
2015 |
2014 |
2013 |
|||||||||
|
Beginning balance |
$ | 88,812 | $ | 88,449 | $ | 46,011 | ||||||
|
Acquired balances |
75,447 | -- | -- | |||||||||
|
Provision recorded during the period |
571,642 | 260,747 | 193,288 | |||||||||
|
Credits issued during the period |
(470,672 | ) | (260,384 | ) | (150,850 | ) | ||||||
|
Ending balance |
$ | 265,229 | $ | 88,812 | $ | 88,449 | ||||||
|
December 31, |
December 31, |
December 31, |
||||||||||
|
Chargeback reserve |
2015 |
2014 |
2013 |
|||||||||
|
Beginning balance |
$ | 43,125 | $ | 37,066 | $ | 18,410 | ||||||
|
Acquired balances |
24,532 | -- | -- | |||||||||
|
Provision recorded during the period |
833,157 | 487,377 | 389,707 | |||||||||
|
Credits issued during the period |
(798,184 | ) | (481,318 | ) | (371,051 | ) | ||||||
|
Ending balance |
$ | 102,630 | $ | 43,125 | $ | 37,066 | ||||||
10. INVENTORY
Inventory, net of carrying value reserves at December 31, 2015 and 2014 consisted of the following (in thousands):
|
December 31, |
December 31, |
|||||||
|
2015 |
2014 | |||||||
|
Raw materials |
$ | 52,366 | $ | 34,681 | ||||
|
Work in process |
4,417 | 2,447 | ||||||
|
Finished goods |
82,311 | 55,102 | ||||||
|
Total inventory |
139,094 | 92,230 | ||||||
|
Less: Non-current inventory |
13,512 | 11,660 | ||||||
|
Total inventory-current |
$ | 125,582 | $ | 80,570 | ||||
Inventory carrying value reserves were $24,136,000 and $25,639,000 at December 31, 2015 and 2014, respectively. During the three month period ended March 31, 2013, the Company decided to discontinue the manufacture and distribution of certain unprofitable products after the Company conducted a strategic review of its currently manufactured generic product portfolio. As a result of this decision, the Company recorded an inventory reserve of $6,700,000 related to the discontinued products. In addition, upon receipt of the Complete Response Letter from the FDA for Rytary™ in January 2013, the Company evaluated the impact of the expected delay of FDA approval on its ability to sell the associated inventory. The Company determined that a reserve of $5,000,000 was appropriate and recorded this amount in the three month period ended March 31, 2013. The Company subsequently received FDA approval for Rytary™ on January 7, 2015. During the three month period ended March 31, 2013, the Company also recorded a $6,400,000 reserve for pre-launch inventory of a product manufactured for another third-party pharmaceutical company due to the anticipated delayed launch of such product as a result of the warning letter related to the Company’s Hayward, California manufacturing facility.
The Company recognizes pre-launch inventories at the lower of its cost or the expected net selling price. Cost is determined using a standard cost method, which approximates actual cost, and assumes a FIFO flow of goods. Costs of unapproved products are the same as approved products and include materials, labor, quality control, and production overhead. When the Company concludes FDA approval is expected within approximately six months, the Company will generally begin to schedule manufacturing process validation studies as required by the FDA to demonstrate the production process can be scaled up to manufacture commercial batches. Consistent with industry practice, the Company may build quantities of pre-launch inventories of certain products pending required final FDA approval and/or resolution of patent infringement litigation, when, in the Company’s assessment, such action is appropriate to prepare for the anticipated commercial launch, FDA approval is expected in the near term, and/or the related litigation will be resolved in the Company’s favor. The capitalization of unapproved pre-launch inventory involves risks, including, among other items, FDA approval of product may not occur; approvals may require additional or different testing and/or specifications than used for unapproved inventory; and, in cases where the unapproved inventory is for a product subject to litigation, the litigation may not be resolved or settled in favor of the Company. If any of these risks were to materialize and the launch of the unapproved product delayed or prevented, then the net carrying value of unapproved inventory may be partially or fully reserved. Generally, the selling price of a generic pharmaceutical product is at discount from the corresponding brand product selling price. Typically, a generic drug is easily substituted for the corresponding brand product, and once a generic product is approved, the pre-launch inventory is typically sold within the next three months. If the market prices become lower than the product inventory carrying costs, then the pre-launch inventory value is reduced to such lower market value. If the inventory produced exceeds the estimated market acceptance of the generic product and becomes short-dated, a carrying value reserve will be recorded. In all cases, the carrying value of the Company's pre-launch product inventory is lower than the respective estimated net selling prices. The carrying value of unapproved inventory less reserves was $8,658,000 and $7,312,000 at December 31, 2015 and 2014, respectively.
To the extent inventory is not scheduled to be utilized in the manufacturing process and/or sold within twelve months of the balance sheet date, it is included as a component of other non-current assets. Amounts classified as non-current inventory consist of raw materials, net of valuation reserves. Raw materials generally have a shelf life of approximately three to five years, while finished goods generally have a shelf life of approximately two years.
11. PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment, net consisted of the following (in thousands):
|
December 31, |
December 31, |
|||||||
|
2015 |
2014 | |||||||
|
Land |
$ | 5,773 | $ | 5,773 | ||||
|
Buildings and improvements |
165,322 | 154,374 | ||||||
|
Equipment |
135,998 | 122,184 | ||||||
|
Office furniture and equipment |
14,548 | 12,623 | ||||||
|
Construction-in-progress |
25,659 | 9,404 | ||||||
|
Property, plant and equipment, gross |
$ | 347,300 | $ | 304,358 | ||||
|
Less: Accumulated depreciation |
(133,144 | ) | (116,189 | ) | ||||
|
Property, plant and equipment, net |
$ | 214,156 | $ | 188,169 | ||||
Depreciation expense was $25.5 million, $20.4 million and $16.8 million for the years ended December 31, 2015, 2014 and 2013, respectively.
Unpaid vendor invoices relating to purchases of property, plant and equipment of approximately $4.5 million, $1.9 million and $6.2 million which were accrued as of December 31, 2015, 2014 and 2013, respectively, are excluded from the purchase of property, plant, and equipment and the change in accounts payable and accrued expenses in the Company’s consolidated statements of cash flows for those respective years.
12. INTANGIBLE ASSETS AND GOODWILL
Intangible Assets
The Company’s finite lived intangible assets consist of marketed product rights and royalties received from product sales from the Company's third party partners. The Company’s indefinite lived intangible assets consist of acquired in-process research and development (IPR&D) product rights and acquired future royalty rights to be paid based on other companies’ net sales of products not yet approved. Amortization over the estimated useful life will commence at the time of the respective product’s launch. If FDA approval to market the product is not obtained, the Company will immediately expense the related capitalized cost. The following tables show the gross carrying values and accumulated amortization, where applicable, of the Company’s intangible assets by type for the Company's consolidated balance sheets presented (in thousands):
|
Gross |
||||||||||||
|
Carrying |
Accumulated |
Intangible |
||||||||||
|
December 31, 2015 |
Value |
Amortization |
Assets, Net |
|||||||||
|
Amortized intangible assets: |
||||||||||||
|
Marketed product rights |
$ | 458,676 | $ | (82,906 | ) | $ | 375,770 | |||||
|
Royalties |
2,200 | (189 | ) | 2,011 | ||||||||
| 460,876 | (83,095 | ) | 377,781 | |||||||||
|
Non-amortized intangible assets: |
||||||||||||
|
Acquired IPR&D product rights |
145,640 | - | 145,640 | |||||||||
|
Acquired future royalty rights |
78,600 | - | 78,600 | |||||||||
| 224,240 | - | 224,240 | ||||||||||
|
Total intangible assets |
$ | 685,115 | $ | (83,095 | ) | $ | 602,020 | |||||
|
Gross |
||||||||||||
|
Carrying |
Accumulated |
Intangible |
||||||||||
|
December 31, 2014 |
Value |
Amortization |
Assets, Net |
|||||||||
|
Amortized intangible assets: |
||||||||||||
|
Marketed product rights |
$ | 63,329 | $ | (43,118 | ) | $ | 20,211 | |||||
|
Non-amortized intangible assets: |
||||||||||||
|
Acquired IPR&D product rights |
6,500 | - | 6,500 | |||||||||
|
Total intangible assets |
$ | 69,829 | $ | (43,118 | ) | $ | 26,711 | |||||
As discussed in “Note 2. Business Acquisition,” the substantial increase in the Company’s intangible asset balances since December 31, 2014 was attributable to the Tower acquisition in March 2015.
In July 2015, the Company received an unsolicited offer from Turing Pharmaceuticals AG to purchase the U.S. rights to Daraprim®, one of the marketed products acquired in the Tower acquisition, as well as the active pharmaceutical ingredient for the product and the finished goods inventory on hand. The sale closed in August 2015, and the Company received proceeds of $55.5 million at the closing. The net book value of the Daraprim® product rights at the time of the sale was $9.3 million. The Company recognized a gain on the sale of the intangible asset of $45.6 million, net of expenses. Additionally in connection with the Tower acquisition, the Company acquired and then promptly resold at cost $4.0 million of product rights which were required by the FTC to be divested as part of the FTC’s approval of the Tower acquisition.
As a result of the annual intangible asset impairment testing performed during the fourth quarter of 2015, the Company recorded an impairment charge of $13.7 million, of which $7.3 million was recorded in cost of revenues and $6.4 million was recorded in research and development expenses in the Company’s consolidated statement of income. The impairment charge was generally attributable to deteriorating market conditions for a small number of marketed products or, for acquired IPR&D product rights, a delay to the anticipated product launch or an economic decision by management not to move forward with the development or marketing of a product. The Company recorded an impairment charge of $2.9 million to cost of revenues in the Company’s consolidated statement of income in 2014 as a result of continued severe price erosion on one of its market products, which price erosion began in 2013. The Company recorded total impairment charges of $13.9 million in 2013, of which $13.1 million was recorded in cost of revenues and $0.8 million was recorded in research and development expenses on the Company’s consolidated statement of income. These impairment charges related to one currently marketed product experiencing severe price erosion due to new competition and one IPR&D product for which the ANDA was withdrawn.
The Company recognized amortization expense of $40.2 million, $11.1 million and $13.1 million for the years ended December 31, 2015, 2014 and 2013, respectively, in cost of revenues in the consolidated statements of income presented. The following table shows the expected amortization of the Company’s finite lived intangible assets as of December 31, 2015 (in thousands):
|
Amortization |
||||
|
For the year ending December 31, |
Expense |
|||
|
2016 |
$ | 26,753 | ||
|
2017 |
27,527 | |||
|
2018 |
32,217 | |||
|
2019 |
38,863 | |||
|
2020 |
37,042 | |||
|
Thereafter |
215,379 | |||
| Total | $ | 377,781 | ||
Goodwill
Goodwill on the Company’s consolidated balance sheet at December 31, 2015 is the result of the 2015 Tower acquisition and the Company’s 1999 merger of Impax Pharmaceuticals, Inc. with Global Pharmaceuticals Corporation. Goodwill had a carrying value of $210.2 million and $27.6 million at December 31, 2015 and 2014, respectively, and the increase in the carrying value in 2015 was entirely attributable to the Tower acquisition. At December 31, 2015, the Company attributed $150.0 million and $60.2 million to the Impax Generics division and the Impax Specialty Pharma division, respectively. At December 31, 2014, the Company attributed the entire carrying amount of goodwill to the Impax Generics division. The Company concluded based on the results of the annual testing performed that the carrying value of goodwill was not impaired as of December 31, 2015 or 2014.
13. ACCRUED EXPENSES
The following table sets forth the Company’s accrued expenses (in thousands):
|
December 31, |
December 31, |
|||||||
|
2015 |
2014 | |||||||
|
Payroll-related expenses |
$ | 37,419 | $ | 33,812 | ||||
|
Product returns |
48,950 | 27,174 | ||||||
|
Accrued shelf stock |
6,619 | 1,852 | ||||||
|
Government rebates |
91,717 | 18,272 | ||||||
|
Legal and professional fees |
5,929 | 9,497 | ||||||
|
Income taxes payable |
830 | 40 | ||||||
|
Physician detailing sales force fees |
1,132 | 2,336 | ||||||
|
Litigation accrual |
-- | 12,750 | ||||||
|
Interest payable |
500 | -- | ||||||
|
Other |
11,615 | 4,737 | ||||||
|
Total accrued expenses |
$ | 204,711 | $ | 110,470 | ||||
Product Returns
The Company maintains a return policy to allow customers to return product within specified guidelines. The Company estimates a provision for product returns as a percentage of gross sales based upon historical experience for sales made through its Impax Generics and Impax Specialty Pharma sales channels. Sales of product under the Private Label, Rx Partner and OTC Partner alliance, collaboration and supply agreements are not subject to returns. A roll forward of the return reserve activity for the years ended December 31, 2015, 2014 and 2013 is as follows (in thousands):
|
December 31, |
December 31, |
December 31, |
||||||||||
|
Returns Reserve |
2015 |
2014 |
2013 |
|||||||||
|
Beginning balance |
$ | 27,174 | $ | 28,089 | $ | 23,440 | ||||||
|
Acquired balances |
11,364 | -- | -- | |||||||||
|
Provision related to sales recorded in the period |
43,967 | 12,016 | 11,015 | |||||||||
|
Credits issued during the period |
(33,555 | ) | (12,931 | ) | (6,366 | ) | ||||||
|
Ending balance |
$ | 48,950 | $ | 27,174 | $ | 28,089 | ||||||
14. DEBT
2% Convertible Senior Notes due June 2022
On June 30, 2015, the Company issued an aggregate principal amount of $600.0 million of 2.00% Convertible Senior Notes due June 2022 (the “Notes”) in a private placement offering, which are the Company’s senior unsecured obligations. The Notes were issued pursuant to an Indenture dated June 30, 2015 (the “Indenture”) between the Company and Wilmington Trust, N.A., as trustee. The Indenture includes customary covenants and sets forth certain events of default after which the Notes may be due and payable immediately. The Notes will mature on June 15, 2022, unless earlier redeemed, repurchased or converted. The Notes bear interest at a rate of 2.00% per year, and interest is payable semiannually in arrears on June 15 and December 15 of each year, beginning on December 15, 2015.
The conversion rate for the Notes is initially set at 15.7858 shares per $1,000 of principal amount, which is equivalent to an initial conversion price of $63.35 per share of the Company’s common stock. If a Make-Whole Fundamental Change (as defined in the Indenture) occurs or becomes effective prior to the maturity date and a holder elects to convert its Notes in connection with the Make-Whole Fundamental Change, the Company is obligated to increase the conversion rate for the Notes so surrendered by a number of additional shares of the Company’s common stock as prescribed in the Indenture. Additionally, the conversion rate is subject to adjustment in the event of an equity restructuring transaction such as a stock dividend, stock split, spinoff, rights offering, or recapitalization through a large, nonrecurring cash dividend (“standard antidilution provisions,” per ASC 815-40 – Contracts in Entity’s Own Equity).
The Notes are convertible at the option of the holders at any time prior to the close of business on the business day immediately preceding December 15, 2021 only under the following circumstances:
|
(i) |
If during any calendar quarter commencing after the quarter ending September 30, 2015 (and only during such calendar quarter) the last reported sale price of the Company’s common stock for at least 20 trading days (whether or not consecutive) during a period of 30 consecutive trading days ending on the last trading day of the immediately preceding calendar quarter is greater than 130% of the conversion price on each applicable trading day; or | |
|
(ii) |
If during the five business day period after any 10 consecutive trading day period (the “measurement period”) in which the trading price per $1,000 of principal amount of Notes for each trading day of the measurement period was less than 98% of the product of the last report sale price of the Company’s common stock and the conversion rate on each such trading day; or | |
|
(iii) |
Upon the occurrence of corporate events specified in the Indenture. |
On or after December 15, 2021 until the close of business on the second scheduled trading day immediately preceding the maturity date, the holders may convert their Notes at any time, regardless of the foregoing circumstances. The Company may satisfy its conversion obligation by paying or delivering, as the case may be, cash, shares of the Company’s common stock, or a combination of cash and shares of the Company’s common stock, at the Company’s election and in the manner and subject to the terms and conditions provided in the Indenture.
Concurrently with the offering of the Notes and using a portion of the proceeds from the sale of the Notes, the Company entered into a series of convertible note hedge and warrant transactions (the “Note Hedge Transactions” and “Warrant Transactions”) which are designed to reduce the potential dilution to the Company’s stockholders and/or offset the cash payments the Company is required to make in excess of the principal amount upon conversion of the Notes. The Note Hedge Transactions and Warrant Transactions are separate transactions, in each case, entered into by the Company with a financial institution and are not part of the terms of the Notes. These transactions will not affect any holder’s rights under the Notes, and the holders of the Notes have no rights with respect to the Note Hedge Transactions and Warrant Transactions. See “Note 8, Derivatives” and “Note 15, Stockholders’ Equity” for additional information.
At the June 30, 2015 issuance date of the Notes, the Company did not have the necessary number of authorized but unissued shares of its common available to settle the conversion option of the Notes in shares of the Company’s common stock. Therefore, in accordance with guidance found in ASC 470-20 – Debt with Conversion and Other Options (“ASC 470-20”) and ASC 815-15 – Embedded Derivatives (“ASC 815-15”), the conversion option of the Notes was deemed an embedded derivative requiring bifurcation from the Notes (host contract) and separate accounting as a derivative liability. The fair value of the conversion option derivative liability at June 30, 2015 was $167.0 million, which was recorded as a reduction to the carrying value of the debt. This debt discount will be amortized to interest expense over the term of the debt using the effective interest method. Although the Company received stockholder approval on December 8, 2015 to amend the Company’s Restated Certificate of Incorporation to increase the authorized number of shares of the Company’s common stock (which amendment has occurred), the debt discount remains and continues to be amortized to interest expense. The effect of the increase in the authorized share count on the derivative liability is discussed in “Note 8, Derivatives.”
In connection with the issuance of the Notes, the Company incurred approximately $18.7 million of debt issuance costs for banking, legal and accounting fees and other expenses. This amount was also recorded on the Company’s balance sheet as a reduction to the carrying value of the debt, in accordance with the Company’s early adoption of Accounting Standards Update (“ASU”) No. 2015-03 – Simplifying the Presentation of Debt Issuance Costs (“ASU 2015-03”), and will be amortized to interest expense over the term of the debt using the effective interest method.
For the year ended December 31, 2015, the Company recognized $16.3 million of interest expense related to the Notes, of which $6.0 million was cash and $10.3 million was non-cash accretion of the debt discounts recorded. As the Notes mature in 2022, they have been classified as long-term debt on the Company’s consolidated balance sheet, with a carrying value of approximately $424.6 million as of December 31, 2015.
Royal Bank of Canada $100.0 Million Revolver
On August 4, 2015, the Company entered into a senior secured revolving credit facility (the “Revolving Credit Facility”) of up to $100 million, pursuant to a credit agreement, by and among the Company, the lenders party thereto from time to time and Royal Bank of Canada, as administrative agent and collateral agent (the “Revolving Credit Facility Agreement”). The Revolving Credit Facility is available for working capital and other general corporate purposes. Borrowings under the Revolving Credit Facility will accrue interest at a rate equal to LIBOR or the base rate, plus an applicable margin. The applicable margin may be increased or reduced by 0.75% based on the Company’s total net leverage ratio. The Revolving Credit Facility will mature on August 4, 2020. No borrowings have been drawn from the Revolving Credit Facility during the year ended December 31, 2015.
Loss on Early Extinguishment of Debt – Barclays $435.0 Million Term Loan
In connection with the acquisition of Tower during the first quarter of 2015, the Company entered into a $435.0 million senior secured term loan facility (the “Term Loan”) and a $50.0 million senior secured revolving credit facility (the “Barclays Revolver” and collectively with the Term Loan, the “Barclays Senior Secured Credit Facilities”), pursuant to a credit agreement, dated as of March 9, 2015, by and among the Company, the lenders party thereto from time to time and Barclays Bank PLC, as administrative and collateral agent (the “Barclays Credit Agreement”). In connection with the Barclays Senior Secured Credit Facilities, the Company incurred debt issuance costs for banking, legal and accounting fees and other expenses of approximately $17.8 million. Prior to repayment of the Term Loan on June 30, 2015, these debt issuance costs were to be amortized to interest expense over the term of the loan using the effective interest rate method.
On June 30, 2015, the Company used approximately $436.4 million of the proceeds from the sale of the Notes to repay the $435.0 million of principal and approximately $1.4 million of accrued interest due on its Term Loan under the Barclays Credit Agreement. In connection with this repayment of the loan, the Company recorded a loss on early extinguishment of debt of approximately $16.9 million related to the unamortized portion of the deferred debt issuance costs during the quarter ended June 30, 2015.
For the six months ended June 30, 2015, the Company incurred total interest expense on the Term Loan of approximately $10.7 million, of which $9.8 million was cash and $0.9 million was non-cash amortization of the deferred debt issuance costs. Included in the 2015 year-to-date cash interest expense of $15.8 million is approximately $2.3 million related to a ticking fee paid to Barclays during the first quarter of 2015, prior to the funding of the Senior Secured Credit Facilities on March 9, 2015, to lock in the financing terms from the lenders’ commitment of the Term Loan until the actual allocation of the loan occurred.
15. STOCKHOLDERS’ EQUITY
Preferred Stock
Pursuant to its Restated Certificate of Incorporation (the “Certificate of Incorporation”), the Company is authorized to issue 2,000,000 shares of “blank check” preferred stock, $0.01 par value per share, which enables the Board of Directors, from time to time, to create one or more new series of preferred stock. Each series of preferred stock issued can have the rights, preferences, privileges and restrictions designated by the Board of Directors. The issuance of any new series of preferred stock could affect, among other things, the dividend, voting, and liquidation rights of the Company’s common stock. The Company had no preferred stock issued or outstanding as of December 31, 2015 or 2014.
Common Stock
A Special Meeting of the Stockholders of the Company (the “Special Meeting”) occurred on December 8, 2015, at which time the Company’s stockholders approved an amendment to the Restated Certificate of Incorporation to increase the number of authorized shares of the Company’s common stock, par value $0.01 per share, from 90,000,000 shares to 150,000,000 shares (the “Amendment”), which Amendment was subsequently filed with the Secretary of State of Delaware by the Company and effected. At December 31, 2015, the Company had 72,926,205 shares of its common stock issued and 72,682,476 shares of its common stock outstanding. In addition, the Company had reserved for issuance the following amounts of shares of its common stock for the purposes described below as of December 31, 2015 (in thousands):
|
Shares issued |
72,926 | |||
|
Stock options outstanding(1) |
2,405 | |||
|
Conversion of Notes payable(2) |
9,471 | |||
|
Warrants outstanding (see below) |
9,471 | |||
|
Total shares of common stock issued and reserved for issuance |
94,273 |
(1) See “Note 17. Share-based Compensation”
(2) See “Note 14. Debt”
Warrants
As discussed in “Note 14. Debt”, on June 30, 2015, the Company entered into a series of Note Hedge Transactions and Warrant Transactions with a financial institution which are designed to reduce the potential dilution to the Company’s stockholders and/or offset the cash payments the Company is required to make in excess of the principal amount upon conversion of the Notes. Pursuant to the Warrant Transactions, the Company sold to a financial institution 9.47 million warrants to purchase the Company’s common stock, for which it received proceeds of $88.3 million. The warrants have an exercise price of $81.277 per share (subject to adjustment), are immediately exercisable, and have an expiration date of September 15, 2022.
Additional paid-in capital
As a result of the Amendment and the increase in the number of authorized shares of the Company's common stock described above, both the derivative asset and the derivative liability met the derivative scope exception and were reclassified to additional paid-in capital. The net effect of the reclassification of the derivatives and the related tax effect was a $21 million increase in additional paid-in capital on the Company's consolidated balance sheet.
16. EARNINGS PER SHARE
The Company's basic earnings per common share (“EPS”) is computed by dividing net income available to the Company’s common stockholders (as presented on the consolidated statements of income) by the weighted-average number of shares of the Company’s common stock outstanding during the period. The Company’s restricted stock awards (non-vested shares) are issued and outstanding at the time of grant but are excluded from the Company’s computation of weighted-average shares outstanding in the determination of basic EPS until vesting occurs.
For purposes of calculating diluted EPS, the denominator includes both the weighted-average number of shares of common stock outstanding and the number of common stock equivalents if the inclusion of such common stock equivalents would be dilutive. Dilutive common stock equivalents potentially include warrants, stock options and non-vested restricted stock awards using the treasury stock method and the number of shares of common stock issuable upon conversion of the Company’s outstanding convertible notes payable. In the case of the Company’s outstanding convertible notes payable, the diluted EPS calculation is further affected by an add-back of interest expense, net of tax, to the numerator under the assumption that the interest would not have been incurred if the convertible notes had been converted into common stock.
The following is a reconciliation of basic and diluted net income per share of common stock for the three years ended December 31, 2015, 2014 and 2013 (in thousands, except per share amounts):
|
For the years ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Basic Earnings Per Common Share: |
||||||||||||
|
Net income |
$ | 38,997 | $ | 57,353 | $ | 101,259 | ||||||
|
Weighted-average common shares outstanding |
69,640 | 68,186 | 66,921 | |||||||||
|
Basic earnings per share |
$ | 0.56 | $ | 0.84 | $ | 1.51 | ||||||
|
Diluted Earnings Per Common Share: |
||||||||||||
|
Net income |
$ | 38,997 | $ | 57,353 | $ | 101,259 | ||||||
|
Add-back of interest expense on outstanding convertible notes payable, net of tax |
-- | (1) | -- | (2) | -- | (2) | ||||||
|
Adjusted net income |
$ | 38,997 | $ | 57,353 | $ | 101,259 | ||||||
|
Weighted-average common shares outstanding |
69,640 | 68,186 | 66,921 | |||||||||
|
Weighted-average incremental shares related to assumed exercise of warrants and stock options, vesting of non-vested shares and ESPP share issuance |
2,387 | (3) | 2,344 | 1,734 | ||||||||
|
Weighted-average incremental shares assuming conversion of outstanding notes payable |
-- | (1) | -- | (2) | -- | (2) | ||||||
|
Diluted weighted-average common shares outstanding |
72,027 | (4) | 70,530 | (5) | 68,655 | (6) | ||||||
|
Diluted net income per share |
$ | 0.54 | $ | 0.81 | $ | 1.47 | ||||||
|
(1) |
The numerator and denominator adjustments related to the Company’s convertible notes payable were excluded from the computation because the add-back of interest expense, net of tax, to the numerator had a greater effect on the quotient than the inclusion of the incremental shares assuming conversion of the convertible notes payable in the denominator, resulting in anti-dilution. |
|
(2) |
Not applicable to the period presented. |
|
(3) |
As of December 31, 2015, the approximately 9.47 million warrants outstanding have been excluded from the denominator of the diluted EPS computation under the treasury stock method because the exercise price of the warrants exceeds the average market price of the Company’s common stock for the period, so inclusion in the calculation would be anti-dilutive. |
|
(4) |
As of December 31, 2015, shares issuable but not included in the Company’s computation of diluted EPS, which could potentially dilute future earnings, include 9.47 million for warrants to purchase the Company’s common stock and 9.47 million shares for conversion of outstanding convertible senior notes payable. In addition, for the year ended December 31, 2015, the Company excluded from the computation as anti-dilutive 1,688,266 and 1,521,097 shares issuable upon the exercise of stock options and vesting of non-vested restricted stock awards, respectively. |
|
(5) |
For the year ended December 31, 2014, the Company excluded 946,288 stock options from the computation of diluted net income per common share as the effect of these options would have been anti-dilutive. |
|
(6) |
For the year ended December 31, 2013, the Company excluded 1,741,110 stock options from the computation of diluted net income per common share as the effect of these options would have been anti-dilutive. |
17. SHARE-BASED COMPENSATION
The Company recognizes the grant date fair value of each option and restricted share over its vesting period. Options and restricted shares granted under the Company’s Second Amended and Restated 2002 Equity Incentive Plan (“2002 Plan”) generally vest over a three or four year period and options have a term of ten years.
Impax Laboratories, Inc. 1999 Equity Incentive Plan
In October 2000, the Company’s stockholders approved an increase in the aggregate number of shares of common stock to be issued pursuant to the Company’s 1999 Equity Incentive Plan from 2,400,000 to 5,000,000 shares. Under the 1999 Equity Incentive Plan, 10,938, 30,438 and 44,937 stock options were outstanding at December 31, 2015, 2014 and 2013, respectively.
Impax Laboratories, Inc. Second Amended and Restated 2002 Equity Incentive Plan
Under the Company’s 2002 Plan, the aggregate number of shares of common stock for issuance pursuant to stock option grants and restricted stock awards was increased by the Company’s Board of Directors from 11,800,000 to 14,950,000 shares during 2013, which was approved by the Company’s stockholders. Under the 2002 Plan, stock options outstanding were 2,394,433, 3,006,367 and 3,720,593 at December 31, 2015, 2014 and 2013, respectively, and non-vested restricted stock awards outstanding were 2,146,498, 2,327,176 and 2,123,835 at December 31, 2015, 2014 and 2013, respectively.
The stock option activity for all of the Company’s equity compensation plans noted above is summarized as follows:
| Weighted- | ||||||||
| Average | ||||||||
| Exercise | ||||||||
| Number of Shares | Price | |||||||
|
Stock Options |
Under Option |
per Share |
||||||
|
Outstanding at December 31, 2012 |
4,177,221 | $ | 12.72 | |||||
|
Options granted |
506,000 | 18.06 | ||||||
|
Options exercised |
(814,177 | ) | 9.28 | |||||
|
Options forfeited |
(98,139 | ) | 19.51 | |||||
|
Outstanding at December 31, 2013 |
3,770,905 | 14.01 | ||||||
|
Options granted |
386,600 | 25.27 | ||||||
|
Options exercised |
(778,112 | ) | 13.76 | |||||
|
Options forfeited |
(337,213 | ) | 20.48 | |||||
|
Outstanding at December 31, 2014 |
3,042,180 | 14.78 | ||||||
|
Options granted |
406,950 | 41.27 | ||||||
|
Options exercised |
(1,042,198 | ) | 9.87 | |||||
|
Options forfeited |
(1,561 | ) | 16.7 | |||||
|
Outstanding at December 31, 2015 |
2,405,371 | 21.39 | ||||||
|
Options exercisable at December 31, 2015 |
1,645,568 | $ | 16.10 | |||||
As of December 31, 2015, stock options outstanding and exercisable had average remaining contractual lives of 6.97 years and 5.26 years, respectively. Also, as of December 31, 2015, stock options outstanding and exercisable each had aggregate intrinsic values of $52,370,000 and $48,873,000, respectively, and restricted stock awards outstanding had an aggregate intrinsic value of $91,784,000. As of December 31, 2015, the Company estimated 2,129,458 stock options and 1,900,280 restricted shares granted to employees which were vested or expected to vest.
The Company grants restricted stock to certain eligible employees as a component of its long-term incentive compensation program. The restricted stock award grants are made in accordance with the Company’s 2002 Plan and are issued and outstanding at the time of grant, though are subject to forfeiture if the vesting conditions are not met. A summary of the non-vested restricted stock awards is as follows:
|
Non-Vested |
Weighted- |
|||||||
|
Restricted |
Average |
|||||||
|
Stock |
Grant Date |
|||||||
| Restricted Stock Awards |
Awards |
Fair Value | ||||||
|
Non-vested at December 31, 2012 |
1,954,570 | $ | 20.97 | |||||
|
Granted |
1,032,924 | 19.92 | ||||||
|
Vested |
(617,302 | ) | 18.80 | |||||
|
Forfeited |
(246,357 | ) | 20.69 | |||||
|
Non-vested at December 31, 2013 |
2,123,835 | 21.13 | ||||||
|
Granted |
1,449,585 | 25.35 | ||||||
|
Vested |
(796,966 | ) | 21.36 | |||||
|
Forfeited |
(449,278 | ) | 21.47 | |||||
|
Non-vested at December 31, 2014 |
2,327,176 | 23.61 | ||||||
|
Granted |
973,742 | 45.40 | ||||||
|
Vested |
(930,159 | ) | 22.64 | |||||
|
Forfeited |
(224,261 | ) | 29.01 | |||||
|
Non-vested at December 31, 2015 |
2,146,498 | $ | 33.20 | |||||
Included in the 930,159 shares of restricted stock vested during the year ended December 31, 2015 are 370,449 shares with a weighted-average fair value of $40.48 per share that were withheld for minimum withholding tax purposes upon vesting of such awards from stockholders who elected to net share settle such tax withholding obligation.
As of December 31, 2015, the Company had 1,893,305 shares available for issuance of either stock options or restricted stock awards, including 1,515,134 shares from the 2002 Plan, 296,921 shares from the 1999 Plan, and 81,250 shares from the ESPP Plan.
As of December 31, 2015, the Company had total unrecognized share-based compensation expense, net of estimated forfeitures, of $63,014,000 related to all of its share-based awards, which will be recognized over a weighted average period of 2.15 years. The intrinsic value of options exercised during the years ended December 31, 2015, 2014 and 2013 was $33,043,000, $10,423,000 and $8,780,000, respectively. The total fair value of restricted shares which vested during the years ended December 31, 2015, 2014 and 2013 was $21,061,000, $16,959,000 and $11,604,000, respectively.
The Company estimated the fair value of each stock option award on the grant date using the Black-Scholes option pricing model with the following assumptions:
|
For the Years Ended December 31, |
||||||||||||||||||
|
2015 |
2014 |
2013 |
||||||||||||||||
|
Volatility (range) |
39.9 | - | 40.1% | 40.1% | - | 41.7% | 41.7% | |||||||||||
|
Volatility (weighted average) |
40.0% | 40.2% | 41.7% | |||||||||||||||
|
Risk-free interest rate (range) |
0.8 | - | 1.8% | 0.6% | - | 1.9% | 1.1% | - | 1.9% | |||||||||
|
Risk-free interest rate (weighted average) |
1.7% | 1.8% | 1.2% | |||||||||||||||
|
Dividend yield |
0% | 0% | 0% | |||||||||||||||
|
Expected life (years) |
6.18 | 6.07 | 6.19 | |||||||||||||||
|
Weighted average grant date fair value |
$17.08 | $10.45 | $7.54 | |||||||||||||||
The Company estimated the fair value of each stock option award on the grant date using the Black-Scholes option pricing model, wherein expected volatility is based on historical volatility of the Company’s common stock. The expected term calculation is based on the “simplified” method described in SAB No. 107, Share-Based Payment and SAB No. 110, Share-Based Payment, as the result of the simplified method provides a reasonable estimate in comparison to actual experience. The risk-free interest rate is based on the U.S. Treasury yield at the date of grant for an instrument with a maturity that is commensurate with the expected term of the stock options. The dividend yield of zero is based on the fact that the Company has never paid cash dividends on its common stock, and has no present intention to pay cash dividends. Options granted under each of the above plans generally vest from three to four years and have a term of ten years.
The amount of share-based compensation expense recognized by the Company is as follows (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Cost of revenues |
$ | 4,479 | $ | 2,494 | $ | 2,035 | ||||||
|
Research and development |
5,996 | 5,072 | 4,885 | |||||||||
|
Selling, general and administrative |
18,138 | 13,317 | 10,724 | |||||||||
|
Total |
$ | 28,613 | $ | 20,883 | $ | 17,644 | ||||||
In June 2013, the Company announced that Dr. Larry Hsu planned to retire as President and Chief Executive Officer of Impax and in April 2014, Dr. Hsu retired from those positions at Impax. Pursuant to his Separation Agreement, all option grants and restricted stock grants expected to vest in the 12 month period following his retirement date were accelerated and vested as of the retirement date. As a result, during the three month period ended June 30, 2013, the Company recorded $2.3 million of accelerated expense related to Dr. Hsu’s outstanding stock options and restricted stock.
On April 21, 2014, the Board of Directors of the Company announced that it had appointed Fred Wilkinson as the Company’s new President and Chief Executive Officer effective as of April 29, 2014. In accordance with Mr. Wilkinson’s employment agreement, the Company granted 150,000 shares of the Company’s restricted stock with a grant date fair value of $3.9 million, which vested as to one-third of the underlying shares on each of the first three six-month anniversaries of April 29, 2014, subject to Mr. Wilkinson’s continued employment with the Company on such vesting date. Further, Mr. Wilkinson also received an award of 375,000 shares of restricted stock that will vest in three tranches based upon continued service by Mr. Wilkinson to the Company and the achievement of certain performance criteria as set forth in his employment agreement. The Company valued these restricted stock awards using a Monte Carlo simulation and is recognizing the $7.6 million value of these awards over the longer of the derived or explicit service period, which is two years.
On October 22, 2014, the Company announced that Carole S. Ben-Maimon, M.D., President of the Company’s Impax Generics division, informed the Company of her decision to retire from her position effective November 3, 2014. Pursuant to her Separation Agreement, all option grants and restricted stock grants expected to vest in the 12 month period following her retirement date were accelerated and vested as of the retirement date. As a result, during the three month period ended December 31, 2014, the Company recorded $0.5 million of accelerated expense related to Dr. Ben-Maimon’s outstanding stock options and restricted stock.
The after tax impact of recognizing the share-based compensation expense related to FASB ASC Topic 718 on basic earnings per common share was $0.20, $0.20 and $0.19 for the years ended December 31, 2015, 2014 and 2013, respectively, and diluted earnings per common share was $0.20, $0.20 and $0.19 for the years ended December 31, 2015, 2014 and 2013, respectively. The Company recognized a deferred tax benefit of $9,150,000, $6,880,000 and $4,829,000 in the years ended December 31, 2015, 2014 and 2013, respectively, related to share-based compensation expense recorded for non-qualified employee stock options and restricted stock awards.
The Company’s policy is to issue new shares to satisfy stock option exercises and to grant restricted share awards. There were no modifications, other than discussed above, to any stock options during the years ended December 31, 2015, 2014 or 2013.
18. EMPLOYEE BENEFIT PLANS
401(k) Defined Contribution Plan
The Company sponsors a 401(k) defined contribution plan covering all employees. Participants are permitted to contribute up to 25% of their eligible annual pre-tax compensation up to established federal limits on aggregate participant contributions. Prior to January 1, 2015, the Company matched 50% of the employee contributions up to a maximum of 6% of employee compensation. Effective January 1, 2015, the Company updated its 401(k) policy to match 100% of the employee contributions up to a maximum of 5% of employee compensation. Discretionary profit-sharing contributions made by the Company, if any, are determined annually by the Board of Directors. Participants are 100% vested in discretionary profit-sharing and matching contributions made by the Company after three years of service, and are 25% and 50% vested after one and two years of service, respectively. There were $3,656,000, $1,615,000 and $1,501,000 in matching contributions and no discretionary profit-sharing contributions made under this plan for the years ended December 31, 2015, 2014 and 2013, respectively.
Employee Stock Purchase Plan
In February 2001, the Board of Directors approved the 2001 Non-Qualified Employee Stock Purchase Plan (“ESPP”), with a 500,000 share reservation. The purpose of the ESPP is to enhance employee interest in the success and progress of the Company by encouraging employee ownership of common stock of the Company. The ESPP provides the opportunity to purchase the Company’s common stock at a 15% discount to the market price through payroll deductions or lump sum cash investments. Under the ESPP plan, for the years ended December 31, 2015, 2014 and 2013, the Company sold shares of its common stock to its employees in the amount of 35,275, 35,350 and 39,748, respectively, for net proceeds of $1,184,000, $788,000 and $660,000, respectively.
Deferred Compensation Plan
In February 2002, the Board of Directors approved the Executive Non-Qualified Deferred Compensation Plan (“ENQDCP”) effective August 15, 2002 covering executive level employees of the Company as designated by the Board of Directors. Participants can defer up to 75% of their base salary and quarterly sales bonus and up to 100% of their annual performance based bonus. The Company matches 50% of employee deferrals up to 10% of base salary and bonus compensation. The maximum total match by the Company cannot exceed 5% of total base and bonus compensation. Participants are vested in the employer match contribution at 20% each year, with 100% vesting after five years of employment. Participants can earn a return on their deferred compensation based on hypothetical investments in investment funds. Changes in the market value of the participant deferrals and earnings thereon are reflected as an adjustment to the liability for deferred compensation with an offset to compensation expense. There were $1,098,000, $850,000 and $764,000 in matching contributions under the ENQDCP for the years ended December 31, 2015, 2014 and 2013, respectively.
The deferred compensation liability is a non-current liability recorded at the value of the amount owed to the ENQDCP participants, with changes in the value of such amounts recognized as a compensation expense in the consolidated statement of operations. The calculation of the deferred compensation obligation is derived from observable market data by reference to hypothetical investments selected by the participants and is included in the line item captioned “Other liabilities” on the consolidated balance sheets. The Company invests in corporate owned life insurance (“COLI”) policies, of which the cash surrender value is included in the line item captioned “Other assets” on the consolidated balance sheets. As of December 31, 2015 and 2014, the Company had a cash surrender value asset of $30,726,000 and $29,241,000, respectively, and a deferred compensation liability of $25,581,000 and $25,837,000, respectively, which approximated fair value. The asset representing the cash surrender value of the corporate owned life insurance and the deferred compensation liability are both Level 2 fair value measurements.
19. INCOME TAXES
The Company is subject to federal, state and local income taxes in the United States, and income taxes in Taiwan, R.O.C. and the Netherlands. The provision for income taxes is comprised of the following (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Current: |
||||||||||||
|
Federal taxes |
$ | 48,078 | $ | 42,635 | $ | 67,407 | ||||||
|
State taxes |
2,286 | 2,467 | 2,569 | |||||||||
|
Foreign taxes |
(442) | 832 | 742 | |||||||||
|
Total current tax expense |
49,922 | 45,934 | 70,718 | |||||||||
|
Deferred: |
||||||||||||
|
Federal taxes |
$ | (23,605 | ) | $ | (9,039 | ) | $ | (21,050 | ) | |||
|
State taxes |
(5,733 | ) | (3,597 | ) | (1,965 | ) | ||||||
|
Foreign taxes |
(213 | ) | (92 | ) | (2,022 | ) | ||||||
|
Total deferred tax (benefit) expense |
(29,551 | ) | (12,728 | ) | (25,037 | ) | ||||||
|
Provision for income taxes |
$ | 20,371 | $ | 33,206 | $ | 45,681 | ||||||
A reconciliation of the difference between the tax provision at the federal statutory rate and actual income taxes on income before income taxes, which includes federal, state, and other income taxes, is as follows (in thousands):
|
For the Years Ended December 31, |
||||||||||||||||||||||||
|
2015 |
2014 |
2013 | ||||||||||||||||||||||
|
Income before income taxes |
$ | 59,368 | $ | 90,559 | $ | 146,940 | ||||||||||||||||||
|
Tax provision at the federal statutory rate |
20,779 | 35.0 | % | 31,696 | 35.0 | % | 51,429 | 35.0 | % | |||||||||||||||
|
Increase (decrease) in tax rate resulting from: |
||||||||||||||||||||||||
|
Tax rate differential and permanent items on foreign income |
412 | 0.7 | % | 2,285 | 2.5 | % | 383 | 0.3 | % | |||||||||||||||
|
State income taxes, net of federal benefit |
365 | 0.6 | % | 887 | 1.0 | % | 1,616 | 1.1 | % | |||||||||||||||
|
State research and development credits |
(2,357 | ) | (4.0 | )% | (2,133 | ) | (2.4 | )% | (1,787 | ) | (1.2 | )% | ||||||||||||
|
Federal research and development credits |
(2,672 | ) | (4.5 | )% | (2,401 | ) | (2.6 | )% | (1,900 | ) | (1.3 | )% | ||||||||||||
|
Share-based compensation |
968 | 1.6 | % | 189 | 0.2 | % | 92 | 0.1 | % | |||||||||||||||
|
Executive compensation |
3,140 | 5.3 | % | 1,552 | 1.7 | % | 336 | 0.2 | % | |||||||||||||||
|
Domestic manufacturing deduction |
(1,422 | ) | (2.4 | )% | (679 | ) | (0.7 | )% | (1,666 | ) | (1.1 | )% | ||||||||||||
|
Other permanent book/tax differences |
2,003 | 3.4 | % | 170 | 0.2 | % | (967 | ) | (0.7 | )% | ||||||||||||||
|
Provision for uncertain tax positions |
184 | 0.3 | % | 952 | 1.1 | % | 1,718 | 1.1 | % | |||||||||||||||
|
Revision of prior years’ estimates |
859 | 1.5 | % | 664 | 0.7 | % | (1,150 | ) | (0.8 | )% | ||||||||||||||
|
Prior year Federal research and development credits |
-- | -- | % | --- | -- | % | (1,950 | ) | (1.3 | )% | ||||||||||||||
| Taiwan Rural Area Investment Tax Credit | (2,134 | ) | (3.6 | )% | --- | -- | % | --- | -- | % | ||||||||||||||
|
Other, net |
246 | 0.4 | % | 24 | 0.0 | % | (473 | ) | (0.3 | )% | ||||||||||||||
|
Provision for income taxes |
$ | 20,371 | 34.3 | % | $ | 33,206 | 36.7 | % | $ | 45,681 | 31.1 | % | ||||||||||||
On January 3, 2013, the research and development credit (the “R&D credit”) was reinstated retroactively as a part of The American Taxpayer Relief Act of 2012 for expenses paid or incurred from January 1, 2012 through December 31, 2012. Due to the fact that this legislation was not enacted prior to the Company’s 2012 year-end, no tax benefit related to potential R&D credits was reflected within the 2012 year-end tax provision. The 2012 R&D credit was reflected within the Company’s first quarter tax provision for the year ended December 31, 2013.
Deferred income taxes result from temporary differences between the financial statement carrying values and the tax bases of the Company’s assets and liabilities. Deferred tax assets principally result from deferred revenue related to certain of the Company’s alliance and collaboration agreements (see “Note 20. Alliance and Collaboration Agreements” below for a discussion of the Company's alliance and collaboration agreements), certain accruals and reserves currently not deductible for tax purposes, acquired product rights and intangibles, capitalized legal and share based compensation expense. Deferred tax liabilities principally result from the use of accelerated depreciation methods for income tax purposes. The components of the Company’s deferred tax assets and liabilities are as follows (in thousands):
|
December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
Deferred tax assets: |
||||||||
|
Deferred revenues |
$ | -- | $ | 1,550 | ||||
|
Accrued expenses |
83,414 | 46,206 | ||||||
|
Inventory reserves |
9,585 | 10,223 | ||||||
|
Net operating loss carryforwards |
38 | 46 | ||||||
|
Depreciation and amortization |
362 | 284 | ||||||
|
Acquired product rights and intangibles |
20,912 | 18,788 | ||||||
|
Capitalized legal fees |
7,352 | 12,829 | ||||||
|
R&D credit carryforwards |
6,149 | 4,331 | ||||||
|
Share based compensation expense |
5,471 | 4,397 | ||||||
|
Other |
389 | 1,048 | ||||||
|
Deferred tax assets |
$ | 133,672 | $ | 99,702 | ||||
|
Deferred tax liabilities: |
||||||||
|
Tax depreciation and amortization in excess of book amounts |
$ | 7,367 | $ | 1,028 | ||||
|
Acquired product rights and intangibles |
188,018 | -- | ||||||
|
Deferred manufacturing costs |
65 | 65 | ||||||
|
Derivative |
8,894 | -- | ||||||
|
Other |
1,783 | 1,947 | ||||||
|
Deferred tax liabilities |
$ | 206,127 | $ | 3,040 | ||||
|
Deferred tax assets (liabilities), net |
$ | (72,455 | ) | $ | 96,662 | |||
A rollforward of unrecognized tax benefits for the years ended December 31, 2015, 2014 and 2013 is as follows (in thousands):
|
For the Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Unrecognized tax benefits beginning of year |
$ | 6,517 | $ | 5,292 | $ | 2,920 | ||||||
|
Gross change for current year positions |
1,079 | 1,089 | 797 | |||||||||
|
Gross change for prior period positions |
(673 | ) | 310 | 1,575 | ||||||||
|
Gross change due to Tower acquisition |
1,037 | -- | -- | |||||||||
|
Decrease due to settlements and payments |
(2,280 | ) | (174 | ) | -- | |||||||
|
Unrecognized tax benefits end of year |
$ | 5,680 | $ | 6,517 | $ | 5,292 | ||||||
The amount of unrecognized tax benefits at December 31, 2015, 2014 and 2013 was $5.7 million, $6.5 million and $5.3 million respectively, of which $4.3 million, $5.0 million and $4.1 million would impact the Company’s effective tax rate, respectively, if recognized. The Company currently does not believe that the total amount of unrecognized tax benefits will increase or decrease significantly over the next 12 months. Interest expense related to income taxes is included in “Interest expense” on the consolidated statement of operations. Net interest expense related to unrecognized tax benefits for the year ended December 31, 2015 was $8,000 principally due to the settlement of the 2010-2011 California audit and the filing of a Tennessee Voluntary Disclosure Agreement for the years 2011-2014, compared to $5,000 in 2014. Accrued interest expense as of December 31, 2015 and 2014 was $589,000 and $597,000, respectively. Income tax penalties are included in “Other income (expense)” on the consolidated statements of operations. Accrued tax penalties of $598,000 were booked in 2015 related to the 2010-2011 California audit.
The Company is currently not under audit for its federal income tax. No provision has been made for U.S. federal deferred income taxes on accumulated earnings on foreign subsidiaries since it is the current intention of management to indefinitely reinvest the undistributed earnings in the foreign subsidiary.
20. ALLIANCE AND COLLABORATION AGREEMENTS
The Company has entered into several alliance, collaboration, license and distribution agreements, and similar agreements with respect to certain of its products and services, with unrelated third-party pharmaceutical companies. The statement of operations includes revenue recognized under agreements the Company has entered into to develop marketing and/or distribution relationships with its partners to fully leverage the technology platform of the Company or of such third party partners and revenue recognized under development agreements which generally obligate the Company to provide research and development services over multiple periods, and revenue recognized under a promotional services agreement which obligates the Company or the third party partner to provide research and development services over multiple periods.
The Company’s alliance and collaboration agreements often include milestones and provide for milestone payments upon achievement of these milestones. Generally, the milestone events contained in the Company’s alliance and collaboration agreements coincide with the progression of the Company’s or the partner’s products and technologies from pre-commercialization to commercialization.
The Company groups pre-commercialization milestones in its alliance and collaboration agreements into clinical and regulatory categories, each of which may include the following types of events:
Clinical Milestone Events:
|
● |
Designation of a development candidate. Following the designation of a development candidate, generally, IND-enabling animal studies for a new development candidate take 12 to 18 months to complete. |
|
● |
Initiation of a Phase I clinical trial. Generally, Phase I clinical trials take one to two years to complete. |
|
● |
Initiation or completion of a Phase II clinical trial. Generally, Phase II clinical trials take one to three years to complete. |
|
● |
Initiation or completion of a Phase III clinical trial. Generally, Phase III clinical trials take two to four years to complete. |
|
● |
Completion of a bioequivalence study. Generally, bioequivalence studies take three months to one year to complete. |
Regulatory Milestone Events:
|
● |
Filing or acceptance of regulatory applications for marketing approval such as a New Drug Application in the United States or Marketing Authorization Application in Europe. Generally, it takes six to 12 months to prepare and submit regulatory filings and approximately two months for a regulatory filing to be accepted for substantive review. |
|
● |
Marketing approval in a major market, such as the United States or Europe. Generally it takes one to three years after an application is submitted to obtain approval from the applicable regulatory agency. |
|
● |
Marketing approval in a major market, such as the United States or Europe for a new indication of an already-approved product. Generally it takes one to three years after an application for a new indication is submitted to obtain approval from the applicable regulatory agency. |
Commercialization milestones in the Company’s alliance and collaboration agreements may include the following types of events:
|
● |
First commercial sale in a particular market, such as in the United States or Europe. |
|
● |
Product sales in excess of a pre-specified threshold, such as annual sales exceeding $100 million. The amount of time to achieve this type of milestone depends on several factors including but not limited to the dollar amount of the threshold, the pricing of the product and the pace at which customers begin using the product. |
License and Distribution Agreement with Shire
In January 2006, the Company entered into a License and Distribution Agreement with an affiliate of Shire Laboratories, Inc., which was subsequently amended (“Prior Shire Agreement”), under which the Company received a non-exclusive license to market and sell an authorized generic of Shire’s Adderall XR® product (“AG Product”) subject to certain conditions, but in any event by no later than January 1, 2010. The Company commenced sales of the AG Product in October 2009. On February 7, 2013, the Company entered into an Amended and Restated License and Distribution Agreement with Shire (the “Amended and Restated Shire Agreement”), which amended and restated the Prior Shire Agreement. The Amended and Restated Shire Agreement was entered into by the parties in connection with the settlement of the Company’s litigation with Shire relating to Shire’s supply of the AG Product to the Company under the Prior Shire Agreement. During 2013, the Company received a payment of $48,000,000 from Shire in connection with such litigation settlement, which was recorded in the first quarter of 2013 under the line item “Other Income” on the consolidated statement of operations. Under the Amended and Restated Shire Agreement, Shire was required to supply the AG Product and Company was responsible for marketing and selling the AG Product subject to the terms and conditions thereof until the earlier of (i) the first commercial sale of the Company’s generic equivalent product to Adderall XR® and (ii) September 30, 2014 (the “Supply Term”), subject to certain continuing obligations of the parties upon expiration or early termination of the Supply Term, including Shire’s obligation to deliver AG Products still owed to the Company as of the end of the Supply Term. The Company is required to pay a profit share to Shire on sales of the AG Product, of which the Company owed a profit share payable to Shire of $19,540,000, $21,089,000 and $20,406,000 on sales of the AG Product during the years ended December 31, 2015, 2014 and 2013, respectively, with a corresponding charge included in the cost of revenues line in the consolidated statement of operations. Although the Supply Term expired on September 30, 2014, the Company was permitted to sell any AG Products in its inventory or owed to the Company by Shire under the Amended and Restated Shire Agreement until all such products are sold. The Company continued to pay a profit share to Shire on sales of such products during the year ended December 31, 2015.
Development, Supply and Distribution Agreement with TOLMAR, Inc.
In June 2012, the Company entered into the Tolmar Agreement with Tolmar. Under the terms of the Tolmar Agreement, Tolmar granted to the Company an exclusive license to commercialize up to 11 generic topical prescription drug products, including ten then approved products and one product pending approval at the FDA, in the United States and its territories. Under the terms of the Tolmar Agreement, Tolmar is responsible for developing and manufacturing the products, and the Company is responsible for marketing and sale of the products. The Company is required to pay a profit share to Tolmar on sales of each product commercialized pursuant to the terms of the Tolmar Agreement.
The Company paid Tolmar a $21 million upfront payment upon signing of the agreement and, pursuant to the terms of the agreement, is also required to make payments to Tolmar upon the achievement of certain specified milestone events. Such contingent milestone payments will initially be recognized in the period the triggering event occurs. Milestone payments which are contingent upon commercialization events will be accounted for as an additional cost of acquiring the product license rights. Milestone payments which are contingent upon regulatory approval events will be capitalized and amortized over the remaining estimated useful life of the approved product. During the year ended December 31, 2012, the Company made a $1.0 million milestone payment and, during the fourth quarter of 2013, the Company made a $12.0 million payment to Tolmar upon Tolmar’s achievement of a regulatory milestone event in accordance with the terms of pursuant to the Tolmar Agreement. The $21 million upfront payment for the Tolmar product rights has been allocated to the underlying topical products based upon the relative fair value of each product and will be amortized over the remaining estimated useful life of each underlying product, ranging from five to 12 years, starting upon commencement of commercialization activities by the Company during the second half of 2012. The amortization of the Tolmar product rights has been included as a component of cost of revenues on the consolidated statement of operations. The Company initially allocated $1.55 million of the upfront payment to two products which were in development and has recorded such amount as in-process research and development expense in its results of operations for the year ended December 31, 2012. The Company similarly recorded the $1,000,000 milestone paid in the year ended December 31, 2012 as a research and development expense. The Company is required to pay a profit share to Tolmar on sales of the topical products, of which the Company owed a profit share payable to Tolmar of $77,683,000, $15,995,000 and $3,905,000 during the years ended December 31, 2015, 2014 and 2013, respectively, with a corresponding charge included in the cost of revenues line in the Company’s consolidated statement of operations. During the fourth quarter of 2014, the Company paid a $2.0 million milestone related to the Diclofenac Sodium Gel 3% or Solaraze® product to Tolmar pursuant to the Tolmar Agreement. During the second quarter of 2015, the Company paid a $5.0 million milestone related to certain topical products pursuant to the Tolmar Agreement.
As discussed in “Note 12. Goodwill and Intangible Assets,” the Company recorded a $13.1 million intangible asset impairment charge to cost of revenues in the three month period ended September 30, 2013 related to the Tolmar product rights acquired under the Tolmar Agreement.
The Company entered into a Loan and Security Agreement with Tolmar in March 2012 (the “Tolmar Loan Agreement”), under which the Company agreed to lend to Tolmar one or more loans through December 31, 2014, in an aggregate amount not to exceed $15,000,000. As of December 31, 2015 and 2014, Tolmar owed the Company $15,000,000 under the Tolmar Loan Agreement, which is included in “Other Assets” on the consolidated balance sheets. The outstanding principal amount of, including any accrued and unpaid interest on, the loans under the Tolmar Loan Agreement are payable by Tolmar beginning from March 31, 2017 through March 31, 2020 or the maturity date, in accordance with the terms therein. Tolmar may prepay all or any portion of the outstanding balance of the loans prior to the maturity date without penalty or premium.
Strategic Alliance Agreement with Teva
The Company entered into a Strategic Alliance Agreement with Teva Pharmaceuticals Curacao N.V., a subsidiary of Teva Pharmaceutical Industries Limited, in June 2001, which was subsequently amended (“Teva Agreement”). The Teva Agreement commits the Company to develop and manufacture, and Teva to distribute, a specified number controlled release generic pharmaceutical products (“generic products”), each for a 10-year period. The Company is required to develop the products, obtain FDA approval to market the products, and manufacture the products for Teva. The revenue the Company earns from the sale of product under the Teva Agreement consists of Teva’s reimbursement of the Company’s manufacturing costs plus a profit share on Teva’s sales of the product to its customers. The Company invoices Teva for the manufacturing costs or products it ships to Teva and payment is due within 30 days. Teva has the right to determine all terms and conditions of the product sales to its customers. Within 30 days of the end of each calendar quarter, Teva is required to provide the Company with a report of its net sales and profits during the quarter and to pay the Company its share of the profits resulting from those sales. Net sales are Teva’s gross sales less discounts, rebates, chargebacks, returns, and other adjustments, all of which are based upon fixed percentages, except chargebacks, which are estimated by Teva and subject to a true-up reconciliation.
As of December 31, 2015, the Company was supplying Teva with oxybutynin extended release tablets (Ditropan XL® 5 mg, 10 mg and 15 mg extended release tablets) and has agreed to supply another product (currently under development) to Teva; the other products under the Teva Agreement have either been returned to the Company, are being manufactured by Teva at its election, were voluntarily withdrawn from the market the Company’s obligations to supply such product had expired or were terminated in accordance with the agreement.
OTC Partner Alliance Agreement
In June 2002, the Company entered into a Development, License and Supply Agreement with Pfizer, Inc., formerly Wyeth LLC (“Pfizer”), for a term of approximately 15 years, relating to the Company’s Loratadine and Pseudoephedrine Sulfate 5 mg/120 mg 12-hour Extended Release Tablets and Loratadine and Pseudoephedrine Sulfate 10 mg/240 mg 24-hour Extended Release Tablets for the OTC market. The Company previously developed the products, and is currently only responsible for manufacturing the products, and Pfizer is responsible for marketing and sale. The agreement included payments to the Company upon achievement of development milestones, as well as royalties paid to the Company by Pfizer on its sales of the product. Pfizer launched this product in May 2003 as Alavert® D-12 Hour. In February 2005, the agreement was partially cancelled with respect to the 24-hour Extended Release Product due to lower than planned sales volume. In December 2011, Pfizer and the Company entered into an agreement with L. Perrigo Company (“Perrigo”) whereby the parties agreed that the Company would supply the Company’s generic Claritin-D® 5 mg/120 mg 12-hour extended release product tablets to Perrigo in the United States and its territories. The agreements with Pfizer and Perrigo are no longer a core area of the Company’s business, and the over-the-counter pharmaceutical products the Company sells to Pfizer and Perrigo under the agreements are older products which are only sold to Pfizer and Perrigo. As noted above, the Company is currently only required to manufacture the products under its agreements with Pfizer and Perrigo. The Company recognizes profit share revenue in the period earned.
Agreements with Valeant Pharmaceuticals International, Inc.
In November 2008, the Company and Valeant Pharmaceuticals International, Inc., formerly Medicis Pharmaceutical Corporation (“Valeant”), entered into a Joint Development Agreement and a License and Settlement Agreement (“Joint Development Agreement”).
Joint Development Agreement
The Joint Development Agreement provides for the Company and Valeant to collaborate in the development of a total of five dermatology products, including four of the Company’s generic products and one branded advanced form of Valeant’s SOLODYN® product. Under the provisions of the Joint Development Agreement the Company received a $40 million upfront payment upon signing of the Joint Development Agreement in December 2008. The Company has also received an aggregate of $15,000,000 in milestone payments composed of two $5 million milestone payments, paid by Valeant in March 2009 and September 2009, a $2 million milestone payment paid by Valeant in December 2009, and a $3 million milestone payment paid by Valeant in March 2011. The Company has the potential to receive up to an aggregate of $8 million of additional contingent milestone payments, upon the achievement of certain specified regulatory events, each of which the Company believes to be substantive, as well as the potential to receive royalty payments from sales, if any, by Valeant of its advanced form SOLODYN® brand product. Finally, to the extent the Company commercializes any of its four generic dermatology products covered by the Joint Development Agreement, the Company will pay to Valeant a gross profit share on sales of such products. The Company began selling one of the four generic dermatology products during the year ended December 31, 2011. As of December 31, 2014, the full amount of deferred revenue under the Joint Development Agreement was recognized.
The Joint Development Agreement results in three items of revenue for the Company, as follows:
|
(1) |
Research & Development Services. Revenue received from the provision of research and development services including the $40,000,000 upfront payment and the $12,000,000 of milestone payments received prior to January 1, 2011, have been deferred and are being recognized on a straight-line basis over the expected period of performance of the research and development services. During the three month period ended March 31, 2013, the Company extended the revenue recognition period for the Joint Development Agreement from the previous recognition period ending in November 2013 to December 2014, due to changes in the estimated timing of completion of certain research and development activities. This change was made on a prospective basis, and resulted in a reduced periodic amount of revenue recognized in current and future periods. Revenue from the remaining $8,000,000 of contingent milestone payments, including the $3,000,000 received from Valeant in March 2011, will be recognized using the Milestone Method of accounting. Deferred revenue was recorded as a liability captioned “Deferred revenue.” Revenue recognized under the Joint Development Agreement is included in “Note 24. Supplementary Financial Information”, in the line item captioned “Other Revenues”. |
|
(2) |
Royalty Fees Earned — Valeant’s Sale of Advanced Form SOLODYN® (Brand) Product. Under the Joint Development Agreement, the Company granted Valeant a license for the advanced form of the SOLODYN® product, with the Company receiving royalty fee income under such license for a period ending eight years after the first commercial sale of the advanced form SOLODYN® product. Commercial sales of the new SOLODYN® product, if any, are expected to commence upon FDA approval of Valeant’s NDA. The royalty fee income, if any, from the new SOLODYN® product, will be recognized by the Company as current period revenue when earned. | |
| (3) | Accounting for Sales of the Company’s Four Generic Dermatology Products. Upon FDA approval of the Company’s ANDA for each of the four generic products covered by the Joint Development Agreement, the Company will have the right (but not the obligation) to begin manufacture and sale of its four generic dermatology products. The Company sells its manufactured generic products to all Impax Generics division customers in the ordinary course of business through its Impax Generics Product sales channel. The Company accounts for the sale, if any, of the generic products covered by the Joint Development Agreement as current period revenue according to the Company’s revenue recognition policy applicable to its Impax Generics products. To the extent the Company sells any of the four generic dermatology products covered by the Joint Development Agreement, the Company pays Valeant a gross profit share, with such profit share payments accounted for as a current period cost of revenues in the consolidated statement of operations. |
Development and Co-Promotion Agreement with Endo Pharmaceuticals Inc.
In June 2010, the Company and Endo Pharmaceuticals, Inc. ("Endo") entered into a Development and Co-Promotion Agreement (“Endo Agreement”) under which the Company and Endo agreed to collaborate in the development and commercialization of a next-generation advanced form of the Company’s lead branded product candidate ("Endo Agreement Product"). The Endo Agreement was terminated upon mutual agreement by the parties effective December 23, 2015. Under the provisions of the Endo Agreement, in June 2010, Endo paid to the Company a $10,000,000 upfront payment. Prior to termination of the agreement, the Company also had the potential to receive up to an additional $30 million of contingent milestone payments.
Prior to the termination of the Endo Agreement, the Company had recognized the $10 million upfront payment as revenue on a straight-line basis over a period of 112 months, which was the estimated expected period of performance of research and development activities under the Endo Agreement, commencing with the June 2010 effective date of the Endo Agreement and ending in September 2019, the estimated date of FDA approval of the Company's NDA for the Endo Agreement Product. The FDA approval of the Endo Agreement Product NDA represented the end of the Company’s expected period of performance, as the Company would have had no further contractual obligation to perform research and development activities under the Endo Agreement, and therefore the earnings process would have been completed on such date. Deferred revenue under the Endo Agreement was $0 and $4,310,000 as of December 31, 2015 and 2014, respectively. Revenue recognized under the Endo Agreement was reported in “Note 24. Supplementary Financial Information” in the line item captioned “Other Revenues”.
The Company and Endo also entered into a Settlement and License Agreement in June 2010 (the “Endo Settlement Agreement”) pursuant to which Endo agreed to make a payment to the Company should prescription sales of Opana® ER (as defined in the Endo Settlement Agreement) fall below a predetermined contractual threshold in the quarter immediately prior to the Company launching a generic version of Opana® ER. As a result of the Company’s launch of its generic version of Opana ER in January 2013 and Endo’s prescription sales of Opana ER during the fourth quarter of 2012, the Company recorded a $102,049,000 settlement gain during the three month period ended March 31, 2013, which is included in “Other Income” in the consolidated statement of operations. Payment of the $102,049,000 settlement was received from Endo in April 2013.
Distribution, License, Development and Supply Agreement with AstraZeneca UK Limited
In January 2012, the Company entered into the AZ Agreement with AstraZeneca. Under the terms of the AZ Agreement, AstraZeneca granted to the Company an exclusive license to commercialize the tablet, orally disintegrating tablet and nasal spray formulations of Zomig® (zolmitriptan) products for the treatment of migraine headaches in the United States and in certain U.S. territories, except during an initial transition period when AstraZeneca fulfilled all orders of Zomig® products on the Company’s behalf and AstraZeneca paid to the Company the gross profit on such Zomig® products. The Company is obligated to fulfill certain minimum requirements with respect to the promotion of currently approved Zomig® products as well as other dosage strengths of such products approved by the FDA in the future. The Company may, but has no obligation to, develop and commercialize additional products containing zolmitriptan and additional indications for Zomig®, subject to certain restrictions as set forth in the AZ Agreement. The Company will be responsible for conducting clinical studies and preparing regulatory filings related to the development of any such additional products and would bear all related costs. During the term of the AZ Agreement, AstraZeneca will continue to be the holder of the NDA for existing Zomig® products, as well as any future dosage strengths thereof approved by the FDA, and will be responsible for certain regulatory and quality-related activities for such Zomig® products. AstraZeneca will manufacture and supply Zomig® products to the Company and the Company will purchase its requirements of Zomig® products from AstraZeneca until a date determined in the AZ Agreement. Thereafter, AstraZeneca may terminate its supply obligations upon certain advance notice to the Company, in which case the Company would have the right to manufacture or have manufactured its own requirements for the applicable Zomig® product.
Under the terms of the AZ Agreement, AstraZeneca was required to make payments to the Company representing 100% of the gross profit on sales of AstraZeneca-labeled Zomig® products during the specified transition period. The Company received transition payments from AstraZeneca aggregating $43,564,000 during 2012. Beginning in January 2013, the Company was obligated to pay AstraZeneca tiered royalties on net sales of branded Zomig® products, depending on brand exclusivity and subject to customary reductions and other terms and conditions set forth in the AZ Agreement. The Company is also obligated to pay AstraZeneca royalties after a certain specified date based on gross profit from sales of authorized generic versions of the Zomig® products subject to certain terms and conditions set forth in the AZ Agreement. In May 2013, the Company’s exclusivity period for branded Zomig® tablets and orally disintegrating tablets expired and the Company launched authorized generic versions of those products in the United States. The Company owed a royalty payable to AstraZeneca of $16,848,000, $14,262,000 and $36,113,000 for the years ended December 31, 2015, 2014 and 2013, respectively, with a corresponding charge included in the cost of revenues line on the consolidated statements of income.
Agreement with DURECT Corporation
In 2014, the Company entered into an agreement with DURECT Corporation (“Durect”) granting the Company the exclusive worldwide rights to develop and commercialize DURECT’s investigational transdermal bupivacaine patch for the treatment of pain associated with post-herpetic neuralgia, referred to by the Company as IPX239. The Company paid Durect a $2 million up-front payment upon signing of the agreement which the Company recognized immediately as research and development expense. The Company has the potential to pay up to an aggregate of $61 million in additional contingent milestone payments upon the achievement of certain specified development and commercialization events under the agreement. If IPX239 is commercialized, the Company would also be required to pay a tiered royalty based on product sales.
Product Acquisition Agreement with Teva Pharmaceuticals USA, Inc.
In August 2013, the Company, through its Amedra Pharmaceuticals subsidiary, entered into a product acquisition agreement (the “Teva Product Acquisition Agreement”) with Teva Pharmaceuticals USA, Inc. (“Teva”) pursuant to which the Company acquired the assets (including the ANDA and other regulatory materials) and related liabilities related to Teva’s mebendazole tablet product in all dosage forms (the “Mebendazole Tablet”). The Company has the potential to pay up to $3,500,000 in additional contingent milestone payments upon the achievement of predefined regulatory and commercialization milestones. The Company is also obligated to pay Teva a royalty payment based on net sales of the Mebendazole Tablet, including a specified annual minimum royalty payment, subject to customary reductions and the other terms and conditions set forth in the Teva Product Acquisition Agreement.
21. COMMITMENTS AND CONTINGENCIES
Leases
The Company leases land, office, warehouse and laboratory facilities under non-cancelable operating leases expiring between March 2016 and December 2026. Rent expense for the years ended December 31, 2015, 2014 and 2013 was $4,146,000, $2,162,000 and $1,932,000, respectively. The Company recognizes rent expense on a straight-line basis over the lease period. The Company also leases certain equipment under various non-cancelable operating leases with various expiration dates between September 2016 and December 2018. Future minimum lease payments under the non-cancelable operating leases are as follows (in thousands):
|
Years ending December 31, |
||||
|
2016 |
$ | 5,797 | ||
|
2017 |
4,784 | |||
|
2018 |
4,242 | |||
|
2019 |
2,376 | |||
|
2020 |
1,100 | |||
|
Thereafter |
4,457 | |||
|
Total minimum lease payments |
$ | 22,756 |
Purchase Order Commitments
As of December 31, 2015, the Company had $67.1 million of open purchase order commitments, primarily for raw materials. The terms of these purchase order commitments are generally less than one year in duration.
Taiwan Facility
The Company has entered into several contracts related to ongoing expansion activities at its Taiwan manufacturing facility. As of December 31, 2015, the Company had remaining obligations under these contracts of $0.3 million.
22. LEGAL AND REGULATORY MATTERS
Patent Litigation
There is substantial litigation in the pharmaceutical, biological, and biotechnology industries with respect to the manufacture, use, and sale of new products which are the subject of conflicting patent and intellectual property claims. One or more patents often cover the brand name products for which the Company is developing generic versions and the Company typically has patent rights covering the Company’s branded products.
Under federal law, when a drug developer files an ANDA for a generic drug seeking approval before expiration of a patent, which has been listed with the FDA as covering the brand name product, the developer must certify its product will not infringe the listed patent(s) and/or the listed patent is invalid or unenforceable (commonly referred to as a “Paragraph IV” certification). Notices of such certification must be provided to the patent holder, who may file a suit for patent infringement within 45 days of the patent holder’s receipt of such notice. If the patent holder files suit within the 45 day period, the FDA can review and approve the ANDA, but is prevented from granting final marketing approval of the product until a final judgment in the action has been rendered in favor of the generic drug developer, or 30 months from the date the notice was received, whichever is sooner. The Company’s generic products division is typically subject to patent infringement litigation brought by branded pharmaceutical manufacturers in connection with the Company’s Paragraph IV certifications seeking an order delaying the approval of the Company’s ANDA until expiration of the patent(s) at issue in the litigation. Likewise, the Company’s branded products division is currently involved in patent infringement litigation against generic drug manufacturers who have filed Paragraph IV certifications to market their generic drugs prior to expiration of the Company’s patents at issue in the litigation.
The uncertainties inherent in patent litigation make the outcome of such litigation difficult to predict. For the Company’s generic products division, the potential consequences in the event of an unfavorable outcome in such litigation include delaying launch of its generic products until patent expiration. If the Company were to launch its generic product prior to successful resolution of a patent litigation, the Company could be liable for potential damages measured by the profits lost by the branded product manufacturer rather than the profits earned by the Company if we are found to infringe a valid, enforceable patent. For the Company’s branded products division, an unfavorable outcome may significantly accelerate generic competition ahead of expiration of the patents covering the Company’s branded products. All such litigation typically involves significant expense.
The Company is generally responsible for all of the patent litigation fees and costs associated with current and future products not covered by its alliance and collaboration agreements. The Company has agreed to share legal expenses with respect to third-party and Company products under the terms of certain of the alliance and collaboration agreements. The Company records the costs of patent litigation as expense in the period when incurred for products it has developed, as well as for products which are the subject of an alliance or collaboration agreement with a third-party.
Although the outcome and costs of the asserted and unasserted claims is difficult to predict, the Company does not currently expect the ultimate liability, if any, for such matters to have a material adverse effect on its business, financial condition, results of operations, or cash flows.
Patent Infringement Litigation
Endo Pharmaceuticals Inc. and Grunenthal GmbH v. Impax Laboratories, Inc. and ThoRx Laboratories, Inc. (Oxymorphone hydrochloride); Endo Pharmaceuticals Inc. and Grunenthal GmbH v. Impax Laboratories, Inc. (Oxymorphone hydrochloride)
In November 2012, Endo Pharmaceuticals, Inc. and Grunenthal GmbH (collectively, “Endo”) filed suit against ThoRx Laboratories, Inc., a wholly owned subsidiary of the Company (“ThoRx”), and the Company in the U.S. District Court for the Southern District of New York alleging patent infringement based on the filing of ThoRx’s ANDA relating to Oxymorphone hydrochloride, Extended Release tablets, 5, 7.5, 10, 15, 20, 30 and 40 mg, generic to Opana ER®. In January 2013, Endo filed a separate suit against the Company in the U.S. District Court for the Southern District of New York alleging patent infringement based on the filing of the Company’s ANDA relating to the same products. ThoRx and the Company filed an answer and counterclaims to the November 2012 suit and the Company filed an answer and counterclaims with respect to the January 2013 suit. A bench trial was completed in April 2015. In August 2015, the Court entered judgment that the products described in the Company’s and ThoRx’s ANDAs would, if marketed, infringe certain claims of the patents asserted by Endo. The Court also found that the asserted claims of patents owned by Endo were not invalid, but that the asserted claims of patents owned by Grunenthal were invalid. As a result, the Court enjoined the Company and ThoRx from marketing their products until expiration of the Endo patents in 2023. Endo filed post-trial motions to correct and amend the judgment, which are currently pending. All parties have filed notices of appeal with respect to the Court's judgment. The appeals are deactivated until resolution of the post-trial motions.
In November 2014, Endo Pharmaceuticals Inc. and Mallinckrodt LLC filed suit against the Company in the U.S. District Court for the District of Delaware making additional allegations of patent infringement based on the filing of the Company’s Oxymorphone hydrochloride ANDA described above. Also in November 2014, Endo and Mallinckrodt filed a separate suit in the U.S. District Court for the District of Delaware making additional allegations of patent infringement based on the filing of ThoRx’s Oxymorphone hydrochloride ANDA described above. ThoRx and the Company filed an answer and counterclaim to those suits in which they are named as a defendant. ThoRx and the Company filed a motion to stay the litigation, which is pending.
Impax Laboratories Inc., et al. v. Lannett Holdings, Inc. and Lannett Company (Zomig®)
In July 2014, the Company filed suit against Lannett Holdings, Inc. and Lannett Company (collectively, “Lannett”) in the United States District Court for the District of Delaware, alleging patent infringement based on the filing of the Lannett ANDA relating to Zolmitriptan Nasal Spray, 5mg, generic to Zomig® Nasal Spray. Lannett filed an answer and counterclaims alleging non-infringement and invalidity in September 2014, and the Company filed an answer to the counterclaims in October 2014. Discovery is proceeding, and trial is set for September 6, 2016. A Markman hearing was held on November 16, 2015, and a claim construction order was issued on December 1, 2015. On July 28, 2015, Lannett filed petitions for Inter Partes Review (“IPR”) of U.S. Patent Nos. 6,750,237 and 7,220,767 related to the product in the U.S. Patent and Trademark Office before the Patent Trial and Appeal Board (“PTAB”). Patent owner filed its preliminary responses in these PTAB proceedings on November 4, 2015. In January 2016, the PTAB denied Lannett’s petitions, declining to institute IPR proceedings with respect to the patents.
Impax Laboratories Inc., et al. v. Actavis Laboratories, Inc. and Actavis Pharma Inc. (Rytary®)
In September 2015, the Company filed suit against Actavis Laboratories, Inc. and Actavis Pharma Inc. (collectively, “Actavis”) in the United States District Court for the District of New Jersey, alleging patent infringement based on the filing of the Actavis ANDA relating to carbidopa and levodopa extended release capsules, generic to Rytary®. Actavis filed an answer and counterclaims on November 19, 2015. Discovery is proceeding. A claim construction hearing is set for November/December 2016 and trial is scheduled for September 2017.
Shire LLC v. CorePharma LLC (Mixed Amphetamines)
In September 2014, Shire LLC (“Shire”) filed suit against CorePharma LLC, a wholly-owned subsidiary of the Company, in the United States District Court for the District of New Jersey alleging patent infringement based on the filing of CorePharma’s ANDA relating to dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine aspartate monohydrate, amphetamine sulfate extended-release capsules, 5 mg, 10 mg, 15 mg, 20 mg, 25 mg and 30 mg, generic to Adderall XR®. On November 14, 2014, CorePharma filed an answer and counterclaims. The case was settled and the Court dismissed the case on February 2, 2016.
Other Litigation Related to the Company’s Business
Civil Investigative Demand from the FTC (Minocycline Hydrochloride)
On May 2, 2012, the Company received a Civil Investigative Demand (“CID”) from the United States Federal Trade Commission (“FTC”) concerning its investigation into the drug SOLODYN® and its generic equivalents. According to the FTC, the investigation relates to whether Medicis Pharmaceutical Corporation, now a wholly owned subsidiary of Valeant Pharmaceuticals International, Inc. (“Medicis”), the Company, and six other companies have engaged or are engaged in unfair methods of competition in or affecting commerce by (i) entering into agreements regarding SOLODYN® or its generic equivalents and/or (ii) engaging in other conduct regarding the sale or marketing of SOLODYN® or its generic equivalents.
On November 6, 2015, the Company received a letter from FTC Secretary Donald S. Clark, informing the Company that the FTC has closed its investigation regarding the SOLODYN® agreements with no further action by the Commission.
Solodyn® Antitrust Class Actions
From July 2013 to January 2016, 18 complaints were filed as class actions on behalf of direct and indirect purchasers, as well as by certain direct purchasers, against manufacturers of the brand drug Solodyn® and its generic equivalents, including the Company.
On July 22, 2013, Plaintiff United Food and Commercial Workers Local 1776 & Participating Employers Health and Welfare Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On July 23, 2013, Plaintiff Rochester Drug Co-Operative, Inc., a direct purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On August 1, 2013, Plaintiff International Union of Operating Engineers Local 132 Health and Welfare Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Northern District of California on behalf of itself and others similarly situated. On August 29, 2013, this Plaintiff withdrew its complaint from the United States District Court for the Northern District of California, and on August 30, 2013, re-filed the same complaint in the United States Court for the Eastern District of Pennsylvania, on behalf of itself and others similarly situated.
On August 9, 2013, Plaintiff Local 274 Health & Welfare Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On August 12, 2013, Plaintiff Sheet Metal Workers Local No. 25 Health & Welfare Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On August 27, 2013, Plaintiff Fraternal Order of Police, Fort Lauderdale Lodge 31, Insurance Trust Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On August 29, 2013, Plaintiff Heather Morgan, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On August 30, 2013, Plaintiff Plumbers & Pipefitters Local 178 Health & Welfare Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On September 9, 2013, Plaintiff Ahold USA, Inc., a direct purchaser, filed a class action complaint in the United States District Court for the District of Massachusetts on behalf of itself and others similarly situated.
On September 24, 2013, Plaintiff City of Providence, Rhode Island, an indirect purchaser, filed a class action complaint in the United States District Court for the District of Arizona on behalf of itself and others similarly situated.
On October 2, 2013, Plaintiff International Union of Operating Engineers Stationary Engineers Local 39 Health & Welfare Trust Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the District of Massachusetts on behalf of itself and others similarly situated.
On October 7, 2013, Painters District Council No. 30 Health and Welfare Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the District of Massachusetts on behalf of itself and others similarly situated.
On October 25, 2013, Plaintiff Man-U Service Contract Trust Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On March 13, 2014, Plaintiff Allied Services Division Welfare Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the District of Massachusetts on behalf of itself and others similarly situated.
On March 19, 2014, Plaintiff NECA-IBEW Welfare Trust Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the District of Massachusetts on behalf of itself and others similarly situated.
On February 25, 2014, the United States Judicial Panel on Multidistrict Litigation ordered the pending actions transferred to the District of Massachusetts for coordinated pretrial proceedings, as In Re Solodyn (Minocycline Hydrochloride) Antitrust Litigation.
On March 26, 2015, Walgreen Co., The Kruger Co., Safeway Inc., HEB Grocery Company L.P., Albertson’s LLC, direct purchasers, filed a separate complaint in the United States District Court for the Middle District of Pennsylvania. On April 8, 2015, the Judicial Panel on Multi-District Litigation ordered the action be transferred to the District of Massachusetts, to be coordinated or consolidated with the coordinated proceedings. The original complaint filed by the plaintiffs asserted claims only against defendant Medicis. On October 5, 2015, the plaintiffs filed an amended complaint asserting claims against the Company and the other generic defendants.
On April 16, 2015, Rite Aid Corporation and Rite Aid Hdqtrs. Corp, direct purchasers, filed a separate complaint in the United States District Court for the Middle District of Pennsylvania. On May 1, 2015, the Judicial Panel on Multi-District Litigation ordered the action be transferred to the District of Massachusetts, to be coordinated or consolidated with the coordinated proceedings. The original complaint filed by the plaintiffs asserted claims only against defendant Medicis. On October 5, 2015, the plaintiffs filed an amended complaint asserting claims against the Company and the other generic defendants.
On January 25, 2016, CVS Pharmacy, Inc. filed a separate complaint in the United States District Court for the Middle District of Pennsylvania. The complaint asserts claims against the Company, Medicis, and the other generic defendants.
The consolidated amended complaints allege that Medicis engaged in anticompetitive schemes by, among other things, filing frivolous patent litigation lawsuits, submitting frivolous Citizen Petitions, and entering into anticompetitive settlement agreements with several generic manufacturers, including the Company, to delay generic competition of Solodyn® and in violation of state and federal antitrust laws. Plaintiffs seek, among other things, unspecified monetary damages and equitable relief, including disgorgement and restitution. On August 14, 2015, the Court granted in part and denied in part defendants’ motion to dismiss the consolidated amended complaints. Discovery is ongoing. No trial date has been scheduled.
Civil Investigative Demand from the FTC (Oxymorphone Hydrochloride)
On February 25, 2014, the Company received a CID from the FTC concerning its investigation into the drug Opana® ER and its generic equivalents. According to the FTC, the investigation relates to whether Endo Pharmaceuticals, Inc. (“Endo”) and the Company have engaged or are engaged in unfair methods of competition in or affecting commerce by (i) entering into agreements regarding Opana® ER or its generic equivalents and/or (ii) engaging in other conduct regarding the regulatory filings, sale or marketing of Opana® ER or its generic equivalents. The Company is cooperating with the FTC in producing documents, information and witnesses in response to the CID. To the knowledge of the Company, no proceedings by the FTC have been initiated against the Company at this time; however, no assurance can be given as to the timing or outcome of this investigation.
Opana ER® Antitrust Class Actions
From June 2014 to April 2015, 14 complaints were filed as class actions on behalf of direct and indirect purchasers, as well as by certain direct purchasers, against the manufacturer of the brand drug Opana ER® and the Company.
On June 4, 2014, Plaintiff Fraternal Order of Police, Miami Lodge 20, Insurance Trust Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On June 4, 2014, Plaintiff Rochester Drug Co-Operative, Inc., a direct purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On June 6, 2014, Plaintiff Value Drug Company, a direct purchaser, filed a class action complaint in the United States District Court for the Northern District of California on behalf of itself and others similarly situated. On June 26, 2014, this Plaintiff withdrew its complaint from the United States District Court for the Northern District of California, and on July 16, 2014, re-filed the same complaint in the United States District Court for the Northern District of Illinois, on behalf of itself and others similarly situated.
On June 19, 2014, Plaintiff Wisconsin Masons’ Health Care Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Northern District of Illinois on behalf of itself and others similarly situated.
On July 17, 2014, Plaintiff Massachusetts Bricklayers, an indirect purchaser, filed a class action complaint in the United States District Court for the Eastern District of Pennsylvania on behalf of itself and others similarly situated.
On August 11, 2014, Plaintiff Pennsylvania Employees Benefit Trust Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Northern District of Illinois on behalf of itself and others similarly situated.
On September 19, 2014, Plaintiff Meijer Inc., a direct purchaser, filed a class action complaint in the United States District Court for the Northern District of Illinois on behalf of itself and others similarly situated.
On October 3, 2014, Plaintiff International Union of Operating Engineers, Local 138 Welfare Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Northern District of Illinois on behalf of itself and others similarly situated.
On November 17, 2014, Louisiana Health Service & Indemnity Company d/b/a Blue Cross and Blue Shield of Louisiana, an indirect purchaser, filed a class action complaint in the United Stated District Court for the Middle District of Louisiana on behalf of itself and others similarly situated.
On December 19, 2014, Plaintiff Kim Mahaffay, an indirect purchaser, filed a class action complaint in the Superior Court of the State of California, Alameda County, on behalf of herself and others similarly situated. On January 27, 2015, the Defendants removed the action to the United States District Court for the Northern District of California.
On January 12, 2015, Plaintiff Plumbers & Pipefitters Local 178 Health & Welfare Trust Fund, an indirect purchaser, filed a class action complaint in the United States District Court for the Northern District of Illinois on behalf of itself and others similarly situated.
On December 12, 2014, the United States Judicial Panel on Multidistrict Litigation ordered the pending actions transferred to the Northern District of Illinois for coordinated pretrial proceedings, as In Re Opana ER Antitrust Litigation.
On March 26, 2015 Walgreen Co., The Kruger Co., Safeway Inc., HEB Grocery Company L.P., Albertson’s LLC, direct purchasers, filed a separate complaint in the United States District Court for the Northern District of Illinois.
On April 23, 2015, Rite Aid Corporation and Rite Aid Hdqtrs. Corp, direct purchasers, filed a separate complaint in the United States District Court for the Northern District of Illinois.
On February 1, 2016, CVS Pharmacy, Inc. filed a separate complaint in the United States District Court for the Northern District of Illinois.
In each case, the complaints allege that Endo engaged in an anticompetitive scheme by, among other things, entering into an anticompetitive settlement agreement with the Company to delay generic competition of Opana ER® and in violation of state and federal antitrust laws. Plaintiffs seek, among other things, unspecified monetary damages and equitable relief, including disgorgement and restitution. Consolidated amended complaints were filed on May 4, 2015. Defendants filed motions to dismiss the complaints on July 3, 2015.
On February 10, 2016, the Court denied defendants’ motion to dismiss the direct purchasers’ consolidated amended complaint. On the same date, the Court granted in part and denied in part defendants’ motion to dismiss the indirect purchasers’ consolidated amended complaint. In particular, the Court dismissed with prejudice the indirect purchasers’ claims under the state laws of Illinois, Puerto Rico, Rhode Island, Kansas and Mississippi and gave the indirect purchasers twenty-one (21) days to re-plead other state law claims. The Court has not ruled on defendants’ separate motion to dismiss the retailers’ complaints. Discovery has not commenced. No trial date has been scheduled.
Civil Investigation Demand from the Attorney General of the State of Alaska
On February 10, 2015, the Company received three CIDs from the Office of the Attorney General of the State of Alaska (“Alaska AG”) concerning its investigations into the drugs Adderall XR®, Effexor XR® and Opana® ER (each a “Product” and collectively, the “Products”) and their generic equivalents. According to the Alaska AG, the investigation is to determine whether the Company may have violated Alaskan state law by entering into settlement agreements with the respective brand name manufacturer for each of the foregoing Products that delayed generic entry of such Product into the marketplace. The Company has cooperated with the Alaska AG in producing documents and information in response to the CIDs. To the knowledge of the Company, no proceedings have been initiated against the Company at this time, however no assurance can be given as to the timing or outcome of this investigation.
United States Department of Justice Investigations
Previously on November 6, 2014, the Company disclosed that one of its sales representatives received a grand jury subpoena from the Antitrust Division of the United States Justice Department (the “Justice Department”). In connection with this same investigation, on March 13, 2015, the Company received a grand jury subpoena from the Justice Department requesting the production of information and documents regarding the sales, marketing, and pricing of certain generic prescription medications. In particular, the Justice Department’s investigation currently focuses on four generic medications: digoxin tablets, terbutaline sulfate tablets, prilocaine/lidocaine cream, and calcipotriene topical solution. The Company has been cooperating and intends to continue cooperating with the investigation. However, no assurance can be given as to the timing or outcome of the investigation.
Securities and Derivative Class Actions
On March 7, 2013 and April 8, 2013, two class action complaints were filed against the Company and certain current and former officers and directors of the Company in the United States District Court for the Northern District of California by Denis Mulligan, individually and on behalf of others similarly situated, and Haverhill Retirement System, individually and on behalf of others similarly situated, respectively (“Securities Class Actions”), alleging that the Company and those named officers and directors violated the federal securities law by making materially false and misleading statements and/or failed to disclose material adverse facts to the public in connection with manufacturing deficiencies at the Hayward, California manufacturing facility, including but not limited to the impact the deficiencies would have on the Company’s ability to gain approval from the FDA for the Company’s branded product candidate, Rytary® and its generic version of Concerta®. These two Securities Class Actions were subsequently consolidated, assigned to the same judge, and a lead plaintiff was chosen. The plaintiff’s consolidated amended complaint was filed on September 13, 2013. The Company filed a motion to dismiss the consolidated amended complaint on November 14, 2013. On April 18, 2014, the Court denied the Company’s motion to dismiss. On September 22, 2014, the Company, together with certain current and former officers and directors of the Company, agreed to settle this consolidated securities class action, without any admission or concession of wrongdoing or liability by the Company or the other defendants. Pursuant to the settlement, the Company paid $8.0 million for a full and complete release of all claims that were or could have been asserted against the Company or other defendants in this action. On January 16, 2015, the Court granted preliminary approval of the settlement and on July 23, 2015, the Court granted final approval of the settlement. The Company did not take any charges for the settlement as the settlement amount was paid for and covered by the Company’s insurance policies. The settlement did not resolve the related shareholder derivative litigations discussed below.
On March 19, 2013, Virender Singh, derivatively on behalf of the Company, filed a state court action against certain current and former officers and directors for breach of fiduciary duty and unjust enrichment in the Superior Court of the State of California County of Santa Clara, asserting similar allegations as those in the Securities Class Actions. On November 6, 2014, plaintiff Singh filed a First Amended Complaint, adding allegations similar to those in the Aruliah Class Action (as described below). The parties have agreed to a settlement in this matter and the court granted final approval of the settlement on October 13, 2015.
On September 24, 2014, Nicholas Karant, derivatively on behalf of the Company, filed an action against certain current and former officers and directors in the United States District Court for the Northern District of California, asserting similar allegations as those by Virender Singh. On June 25, 2015, the court dismissed Mr. Karant’s complaint without prejudice.
On August 13, 2014, a class action complaint was filed against the Company and certain current and former officers and directors of the Company in the United States District Court for the Northern District of California by Linus Aruliah, individually and on behalf of all others similarly situated (“Aruliah Class Action”). The complaint alleged that the Company and those named officers and directors violated the federal securities laws by making materially false and misleading statements and/or failed to disclose material adverse facts to the public in connection with manufacturing deficiencies at the Company’s Taiwan manufacturing facility, including but not limited to the impact the deficiencies would have on the Company’s ability to gain approval from the FDA for the Company’s then branded product candidate, Rytary® (which was subsequently approved by the FDA on January 7, 2015). On January 13, 2015, the Company, together with certain current and former officers and directors of the Company, agreed to settle this securities class action, without any admission or concession of wrongdoing or liability by the Company or the other defendants. Pursuant to the settlement, the Company paid $4.75 million for a full and complete release of all claims that were or could have been asserted against the Company or other defendants in this action. On June 22, 2015, the Court granted preliminary approval of the settlement. On December 21, 2015, the Court granted final approval of the settlement. The Company did not take any charges for the settlement as the settlement amount will be paid for and covered by the Company’s insurance policies.
On September 22, 2014, Randall Wickey, derivatively on behalf of the Company, filed an action against certain current and former officers and directors of the Company in the United States District Court for the Northern District of California, alleging breaches of fiduciary duty in connection with the Company’s response to various FDA notices and warnings regarding problems in the manufacturing and quality control processes at the Company’s Hayward, California and Taiwan manufacturing facilities. On November 10, 2014, International Union of Operating Engineers Local 478, derivatively on behalf of the Company, filed an action against certain current and former officers and directors of the Company in the United States District Court for the Northern District of California, asserting similar allegations as those by Randall Wickey. These two derivative actions were consolidated on February 5, 2015 and a consolidated complaint was filed on February 20, 2015. On October 6, 2015, the court dismissed the consolidated complaint with prejudice.
Attorney General of the State of Connecticut Interrogatories and Subpoena Duces Tecum
On July 14, 2014, the Company received a subpoena and interrogatories (the “Subpoena”) from the State of Connecticut Attorney General (“Connecticut AG”) concerning its investigation into sales of the Company’s generic product, digoxin. According to the Connecticut AG, the investigation is to determine whether anyone engaged in a contract, combination or conspiracy in restraint of trade or commerce which has the effect of (i) fixing, controlling or maintaining prices or (ii) allocating or dividing customers or territories relating to the sale of digoxin in violation of Connecticut state antitrust law. The Company intends to cooperate with the Connecticut AG in producing documents and information in response to the Subpoena. To the knowledge of the Company, no proceedings by the Connecticut AG have been initiated against the Company at this time, however no assurance can be given as to the timing or outcome of this investigation.
AWP Litigation
On December 30, 2015, Plumbers’ Local Union No. 690 Health Plan and others similarly situated filed a class action against several generic drug manufacturers, including the Company, in the Court of Common Pleas of Philadelphia County, First Judicial District of Pennsylvania, Civil Trial Division, alleging that the Company and others violated the law, including the Pennsylvania Unfair Trade Practices and Consumer Protection law, by inflating the Average Wholesale Price (“AWP”) of certain generic drugs.
On February 5, 2016, Delaware Valley Health Care Coalition filed suit asserting similar allegations against the same defendants in the same court.
23. SEGMENT INFORMATION
The Company has two reportable segments, Impax Generics and Impax Specialty Pharma. Impax Generics develops, manufactures, sells, and distributes generic pharmaceutical products, primarily through the following sales channels: the Impax Generics sales channel for sales of generic prescription products directly to wholesalers, large retail drug chains, and others; the Private Label Product sales channel for generic over-the-counter and prescription products sold to unrelated third-party customers who, in turn, sell the products under their own label; the Rx Partner sales channel for generic prescription products sold through unrelated third-party pharmaceutical entities under their own label pursuant to alliance agreements; and the OTC Partner sales channel for over-the-counter products sold through unrelated third-party pharmaceutical entities under their own labels pursuant to alliance and supply agreements. Revenues from the “Impax Generics” sales channel and the “Private Label” sales channel are reported under the caption “Impax Generics sales, net” in “Note 24. Supplementary Financial Information.” The Company also generates revenue in Impax Generics from research and development services provided under a joint development agreement with another unrelated third-party pharmaceutical company, and reports such revenue under the caption “Other Revenues” revenue in “Note 24. Supplementary Financial Information.” Revenues from the “OTC Partner” sales channel are also reported under the caption “Other Revenues” in “Note 24. Supplementary Financial Information.”
Impax Specialty Pharma is engaged in the development, sale and distribution of proprietary brand pharmaceutical products that the Company believes represent improvements to already-approved pharmaceutical products addressing central nervous system (“CNS”) disorders and other select specialty segments. Impax Specialty Pharma currently has one internally developed branded pharmaceutical product, Rytary® (IPX066), an extended release oral capsule formulation of carbidopa-levodopa for the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and parkinsonism that may follow carbon monoxide intoxication and/or manganese intoxication, which was approved by the FDA on January 7, 2015 and which the Company launched in April 2015. In November 2015, the European Commission granted marketing authorization for NUMIENT™ (IPX066) (referred to as Rytary® in the United States). The review of the NUMIENT™ application was conducted under the centralized licensing procedure as a therapeutic innovation, and authorization is applicable in all 28 member states of the European Union, as well as Iceland, Liechtenstein and Norway. Impax Specialty Pharma is also engaged in the sale and distribution of four other branded products including Zomig® (zolmitriptan) products, indicated for the treatment of migraine headaches, under the terms of the AZ Agreement with AstraZeneca in the United States and in certain U.S. territories, and Albenza®, indicated for the treatment of tapeworm infections. Revenues from Impax-labeled branded products are reported under the caption “Impax Specialty Pharma sales, net” in “Note 24. Supplementary Financial Information.” Finally, the Company generates revenue in Impax Specialty Pharma from research and development services provided under a development and license agreement with another unrelated third-party pharmaceutical company, and reports such revenue under the caption “Other Revenues” in “Note 24. Supplementary Financial Information.” Impax Specialty Pharma also has a number of product candidates that are in varying stages of development.
The Company’s chief operating decision maker evaluates the financial performance of the Company’s segments based upon segment income (loss) before income taxes. Items below income (loss) from operations are not reported by segment, since they are excluded from the measure of segment profitability reviewed by the Company’s chief operating decision maker. Additionally, general and administrative expenses, certain selling expenses, certain litigation settlements, and non-operating income and expenses are included in “Corporate and Other.” The Company does not report balance sheet information by segment since it is not reviewed by the Company’s chief operating decision maker. The accounting policies for the Company’s segments are the same as those described above in the discussion of "Revenue Recognition" and in “Note 4. Summary of Significant Accounting Policies.” The Company has no inter-segment revenue.
The tables below present segment information reconciled to total Company financial results, with segment operating income or loss including gross profit less direct research and development expenses, and direct selling expenses as well as any litigation settlements, to the extent specifically identified by segment (in thousands):
|
Impax |
||||||||||||||||
|
Impax |
Specialty |
Corporate |
Total |
|||||||||||||
|
Year Ended December 31, 2015 |
Generics |
Pharma |
and Other |
Company |
||||||||||||
|
Revenues, net |
$ | 710,932 | $ | 149,537 | $ | -- | $ | 860,469 | ||||||||
|
Cost of revenues |
450,045 | 58,020 | -- | 508,065 | ||||||||||||
|
Research and development |
58,838 | 18,144 | -- | 76,982 | ||||||||||||
|
Patent litigation |
2,942 | 1,625 | -- | 4,567 | ||||||||||||
|
Selling, general and administrative |
29,641 | 52,427 | 119,219 | 201,287 | ||||||||||||
|
Income (loss) before income taxes |
$ | 169,466 | $ | 19,321 | $ | (129,419 | ) | $ | 59,368 | |||||||
| Impax | ||||||||||||||||
|
Impax |
Specialty |
Corporate |
Total |
|||||||||||||
|
Year Ended December 31, 2014 |
Generics |
Pharma |
and Other |
Company |
||||||||||||
|
Revenues, net |
$ | 549,082 | $ | 46,967 | $ | -- | $ | 596,049 | ||||||||
|
Cost of revenues |
260,459 | 22,937 | -- | 283,396 | ||||||||||||
|
Research and development |
40,927 | 37,715 | -- | 78,642 | ||||||||||||
|
Patent litigation |
5,333 | 472 | -- | 5,808 | ||||||||||||
|
Selling, general and administrative |
17,144 | 43,307 | 78,939 | 139,390 | ||||||||||||
|
Income (loss) before income taxes |
$ | 225,219 | $ | (57,464 | ) | $ | (77,196 | ) | $ | 90,559 | ||||||
|
Impax |
Impax |
|||||||||||||||
|
Impax |
Specialty |
Corporate |
Total |
|||||||||||||
|
Year Ended December 31, 2013 |
Generics |
Pharma |
and Other |
Company |
||||||||||||
|
Revenues, net |
$ | 398,340 | $ | 113,162 | $ | -- | $ | 511,502 | ||||||||
|
Cost of revenues |
253,836 | 58,366 | -- | 312,202 | ||||||||||||
|
Research and development |
41,384 | 27,470 | -- | 68,854 | ||||||||||||
|
Patent litigation |
16,545 | -- | -- | 16,545 | ||||||||||||
|
Selling, general and administrative |
17,684 | 44,915 | 57,689 | 120,288 | ||||||||||||
|
Income (loss) before income taxes |
$ | 68,891 | $ | (17,589 | ) | $ | 95,638 | $ | 146,940 | |||||||
Foreign Operations
The Company’s wholly-owned subsidiary, Impax Laboratories (Taiwan) Inc., has constructed a facility in Taiwan which is utilized for manufacturing, research and development, warehouse, and administrative functions, with $131.6 and $126.4 of net carrying value of assets, composed principally of a building and equipment, included in the Company's consolidated balance sheets at December 31, 2015 and 2014, respectively.
24. SUPPLEMENTARY FINANCIAL INFORMATION (UNAUDITED)
Selected financial information for the quarterly periods noted is as follows (in thousands, except share and per share data):
|
2015 Quarters Ended |
||||||||||||||||
|
March 31 |
June 30 |
September 30 |
December 31 |
|||||||||||||
|
Revenue: |
||||||||||||||||
|
Impax Generics sales, gross |
$ | 355,321 | $ | 572,079 | $ | 565,261 | $ | 705,574 | ||||||||
|
Less: |
||||||||||||||||
|
Chargebacks |
126,607 | 228,977 | 212,588 | 239,920 | ||||||||||||
|
Rebates |
83,130 | 140,340 | 141,646 | 200,721 | ||||||||||||
|
Product returns |
6,427 | 7,528 | 6,276 | 8,888 | ||||||||||||
|
Other credits |
13,198 | 23,961 | 26,295 | 31,889 | ||||||||||||
|
Impax Generics sales, net |
125,959 | 171,273 | 178,456 | 224,156 | ||||||||||||
|
Rx Partner |
2,239 | 2,579 | 1,957 | 2,532 | ||||||||||||
|
Other Revenues |
543 | 827 | 253 | 158 | ||||||||||||
|
Impax Generics revenues, net |
128,741 | 174,679 | 180,666 | 226,846 | ||||||||||||
|
Impax Specialty Pharma sales, gross |
29,219 | 65,269 | 69,286 | 86,274 | ||||||||||||
|
Less: |
||||||||||||||||
|
Chargebacks |
5,561 | 4,452 | 5,893 | 9,159 | ||||||||||||
|
Rebates |
1,418 | 1,318 | 1,078 | 1,991 | ||||||||||||
|
Product returns |
2,620 | 6,763 | 2,824 | 2,641 | ||||||||||||
|
Other credits |
5,492 | 13,461 | 19,285 | 20,866 | ||||||||||||
|
Impax Specialty Pharma sales, net |
14,128 | 39,275 | 40,206 | 51,617 | ||||||||||||
|
Other Revenues |
227 | 228 | 227 | 3,629 | ||||||||||||
|
Impax Specialty Pharma revenues, net |
14,355 | 39,503 | 40,433 | 55,246 | ||||||||||||
|
Total net revenues |
143,096 | 214,182 | 221,099 | 282,092 | ||||||||||||
|
Gross profit |
59,234 | 84,851 | 93,549 | 114,770 | ||||||||||||
|
Net (loss) income |
$ | (6,333 | ) | $ | (1,852 | ) | $ | 35,755 | $ | 11,427 | ||||||
|
Net (loss) income per common share: |
||||||||||||||||
|
Basic |
$ | (0.09 | ) | $ | (0.03 | ) | $ | 0.51 | $ | 0.16 | ||||||
|
Diluted |
$ | (0.09 | ) | $ | (0.03 | ) | $ | 0.49 | $ | 0.16 | ||||||
|
Weighted-average common shares outstanding: |
||||||||||||||||
|
Basic |
68,967,875 | 69,338,789 | 69,820,348 | 70,416,757 | ||||||||||||
|
Diluted |
68,967,875 | 69,338,789 | 72,777,746 | 72,041,760 | ||||||||||||
Quarterly computations of net (loss) income per share amounts are made independently for each quarterly reporting period, and the sum of the per share amounts for the quarterly reporting periods may not equal the per share amounts for the year-to-date reporting period.
|
2014 Quarters Ended |
||||||||||||||||
|
March 31 |
June 30 |
September 30 |
December 31 |
|||||||||||||
|
Revenue: |
||||||||||||||||
|
Impax Generics sales, gross |
$ | 265,850 | $ | 375,269 | $ | 340,379 | $ | 322,707 | ||||||||
|
Less: |
||||||||||||||||
|
Chargebacks |
95,714 | 110,518 | 115,419 | 131,882 | ||||||||||||
|
Rebates |
52,054 | 74,079 | 64,442 | 66,685 | ||||||||||||
|
Product returns |
1,294 | 5,140 | 3,494 | 993 | ||||||||||||
|
Other credits |
10,671 | 21,571 | 13,449 | 8,288 | ||||||||||||
|
Impax Generics sales, net |
106,117 | 163,961 | 143,575 | 114,859 | ||||||||||||
|
Rx Partner |
2,435 | 9,204 | 1,447 | 1,028 | ||||||||||||
|
Other Revenues |
589 | 3,229 | 611 | 2,028 | ||||||||||||
|
Impax Generics revenues, net |
109,141 | 176,394 | 145,633 | 117,915 | ||||||||||||
|
Impax Specialty Pharma sales, gross |
20,643 | 24,375 | 23,840 | 23,348 | ||||||||||||
|
Less: |
||||||||||||||||
|
Chargebacks |
8,230 | 10,107 | 8,787 | 6,720 | ||||||||||||
|
Rebates |
1,070 | 938 | 469 | 1,010 | ||||||||||||
|
Product returns |
181 | 216 | 223 | 475 | ||||||||||||
|
Other credits |
1,853 | 1,654 | 2,261 | 2,074 | ||||||||||||
|
Impax Specialty Pharma sales, net |
9,309 | 11,460 | 12,100 | 13,069 | ||||||||||||
|
Other Revenues |
268 | 267 | 266 | 227 | ||||||||||||
|
Impax Specialty Pharma revenues, net |
9,577 | 11,727 | 12,366 | 13,296 | ||||||||||||
|
Total net revenues |
118,718 | 188,121 | 157,999 | 131,211 | ||||||||||||
|
Gross profit |
57,622 | 109,772 | 84,438 | 60,821 | ||||||||||||
|
Net income |
$ | 6,425 | $ | 35,071 | $ | 15,737 | $ | 120 | ||||||||
|
Net income per common share: |
||||||||||||||||
|
Basic |
$ | 0.09 | $ | 0.52 | $ | 0.23 | $ | 0.00 | ||||||||
|
Diluted |
$ | 0.09 | $ | 0.50 | $ | 0.22 | $ | 0.00 | ||||||||
|
Weighted-average common shares outstanding: |
||||||||||||||||
|
Basic |
67,702,296 | 68,095,159 | 68,254,327 | 68,678,779 | ||||||||||||
|
Diluted |
69,938,872 | 70,313,491 | 70,715,226 | 70,988,328 | ||||||||||||
Quarterly computations of net income per share amounts are made independently for each quarterly reporting period, and the sum of the per share amounts for the quarterly reporting periods may not equal the per share amounts for the year-to-date reporting period.
25. SUBSEQUENT EVENTS
On January 15, 2016, the Company announced that the FDA approved the Company's supplemental new drug application for EMVERM™ (mebendazole) 100 mg chewable tablets. EMVERM is indicated for the treatment of Enterobius vermicularis (pinworm), Trichuris trichiura (whipworm), Ascaris lumbricoides (common roundworm), Ancylostoma duodenale (common hookworm), Necator americanus (American hookworm) in single or mixed infections.
On February 17, 2016, the Company announced that the FDA approved the Company’s abbreviated new drug application for dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate and amphetamine sulfate (mixed salts of a single-entity amphetamine product) extended-release capsules, CII, 5 mg, 10 mg, 15 mg, 20 mg, 25 mg and 30 mg, a generic version of Adderall XR®.
SCHEDULE II, VALUATION AND QUALIFYING ACCOUNTS
(In thousands)
| Column A | Column B | Column C | Column D | Column E | ||||||||||||||||
| Balance at | Charge to | Charge to |
Balance at |
|||||||||||||||||
| Beginning of | Costs and | Other |
End of |
|||||||||||||||||
|
Description |
Period |
Expenses |
Accounts |
Deductions |
Period |
|||||||||||||||
|
For the Year Ended December 31, 2013: |
||||||||||||||||||||
|
Reserve for bad debts |
$ | 553 | --- | --- | (14 | ) | $ | 539 | ||||||||||||
|
For the Year Ended December 31, 2014: |
||||||||||||||||||||
|
Reserve for bad debts |
$ | 539 | --- | --- | (24 | ) | $ | 515 | ||||||||||||
|
For the Year Ended December 31, 2015: |
||||||||||||||||||||
|
Reserve for bad debts |
$ | 515 | 5,122 | 9,550* | --- | $ | 15,187 | |||||||||||||
* Represents reserve for bad debts acquired.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
IMPAX LABORATORIES, INC. |
| |
|
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Fred Wilkinson |
|
|
|
Name: |
Fred Wilkinson |
|
|
|
Title: |
President and Chief Executive Officer |
|
Date: February 22, 2016
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature |
Title |
Date | ||
| /s/ Fred Wilkinson |
President, Chief Executive Officer |
February 22, 2016 | ||
|
Fred Wilkinson |
(Principal Executive Officer) and Director |
|||
| /s/ Bryan M. Reasons |
Senior Vice President, Finance and |
February 22, 2016 | ||
|
Bryan M. Reasons |
Chief Financial Officer |
|||
|
(Principal Financial Officer and |
||||
|
Principal Accounting Officer) |
||||
| /s/ Robert L. Burr |
Chairman of the Board |
February 22, 2016 | ||
|
Robert L. Burr |
||||
| /s/ Leslie Z. Benet, Ph.D. |
Director |
February 22, 2016 | ||
|
Leslie Z. Benet, Ph.D. |
||||
| /s/ Allen Chao, Ph.D. |
Director |
February 22, 2016 | ||
|
Allen Chao, Ph.D. |
||||
| /s/ Nigel Ten Fleming, Ph.D. |
Director |
February 22, 2016 | ||
|
Nigel Ten Fleming, Ph.D. |
||||
| /s/ Janet S. Vergis |
Director |
February 22, 2016 | ||
|
Janet S. Vergis |
||||
| /s/ Michael Markbreiter |
Director |
February 22, 2016 | ||
|
Michael Markbreiter |
||||
| /s/ Peter R. Terreri |
Director |
February 22, 2016 | ||
|
Peter R. Terreri |
||||
| /s/ Mary K. Pendergast |
Director |
February 22, 2016 | ||
|
Mary K. Pendergast |
EXHIBIT INDEX
Exhibit No. Description of Document
|
2.1 |
Stock Purchase Agreement, dated as of October 8, 2014, by and among the Company, Tower Holdings, Inc. (“Tower”), Lineage Therapeutics Inc. (“Lineage”), Roundtable Healthcare Partners II, L.P., Roundtable Healthcare Investors II, L.P., the other stockholders of Tower and Lineage, the holders of options to purchase shares of Tower common stock and options to purchase shares of Lineage common stock, the holders of warrants to acquire shares of Tower common stock and warrants to acquire shares of Lineage common stock and, solely with respect to Section 8.3, Roundtable Healthcare Management II, LLC.(1) |
|
3.1.1 |
Certificate of Amendment of the Restated Certificate of Incorporation of the Company dated as of December 9, 2015.(2) |
|
3.1.2 |
Restated Certificate of Incorporation of the Company dated as of August 30, 2004.(3) |
|
|
|
|
3.1.3 |
Certificate of Designation of Series A Junior Participating Preferred Stock, as filed with the Secretary of State of Delaware on January 21, 2009.(4) |
|
3.2.1 |
Amendment No. 3 to Amended and Restated Bylaws of the Company, effective as of October 7, 2015.(5) |
|
3.2.2 |
Amendment No. 2 to Amended and Restated Bylaws of the Company, effective as of July 7, 2015.(5) |
|
3.2.3 |
Amendment No. 1 to Amended and Restated Bylaws of the Company, effective as of March 24, 2015.(5) |
|
3.2.4 |
Amended and Restated Bylaws of the Company, effective as of May 14, 2014.(5) |
|
4.1 |
Specimen of Common Stock Certificate.(6) |
|
|
|
|
4.2 |
Preferred Stock Rights Agreement, dated as of January 20, 2009, by and between the Company and StockTrans, Inc., as Rights Agent.(4) |
|
4.3 |
Indenture, dated as of June 30, 2015, between the Company, and Wilmington Trust, National Association, as trustee.(7) |
|
|
|
|
10.1 |
Credit Agreement, dated as of February 11, 2011, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association, as Administrative Agent.**(8) |
|
|
|
|
10.1.1 |
Amendment dated as of March 19, 2012 to the Credit Agreement, dated as of February 11, 2011, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association as Administrative Agent.(9) |
|
|
|
|
10.1.2 |
Second Amendment to Credit Agreement, dated as of January 10, 2013, to the Credit Agreement, dated as of February 11, 2011, as amended, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association, as Administrative Agent.(10) |
|
10.1.3 |
Third Amendment to Credit Agreement, dated as of February 20, 2014, to the Credit Agreement, dated as of February 11, 2011, as amended, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association, as Administrative Agent.(11) |
|
|
|
|
10.2 |
Security Agreement, dated as of February 11, 2011, by and among the Company, the Guarantors named therein, the Lenders named therein and Wells Fargo Bank, National Association, as Administrative Agent.(8) |
|
|
|
|
10.3 |
Letter Agreement, dated as of June 25, 2015, between RBC Capital Markets LLC and the Company regarding the Base Warrants.(7) |
|
10.4 |
Letter Agreement, dated as of June 25, 2015 between RBC Capital Markets LLC and the Company regarding the Base Call Option Transaction.(7) |
|
10.5 |
Letter Agreement, dated as of June 26, 2015, between RBC Capital Markets LLC and the Company regarding the Additional Warrants.(7) |
|
10.6 |
Letter Agreement, dated as of June 26, 2015, between RBC Capital Markets LLC and the Company regarding the Additional Call Option Transaction.(7) |
|
10.7 |
Credit Agreement, dated as of March 9, 2015, by and among the Company, the lenders party thereto from time to time and Barclays Bank PLC, as administrative agent and collateral agent.(12) |
|
10.8 |
Credit Agreement, dated as of August 4, 2015, by and among the Company, the lenders party thereto from time to time and Royal Bank of Canada, as administrative agent and collateral agent.(13) |
|
10.9.1 |
Impax Laboratories, Inc. 1999 Equity Incentive Plan.*(14) |
|
|
|
|
10.9.2 |
Form of Stock Option Grant under the Impax Laboratories, Inc. 1999 Equity Incentive Plan.*(14) |
|
|
|
|
10.10 |
Impax Laboratories, Inc. 2001 Non-Qualified Employee Stock Purchase Plan.*(6) |
|
|
|
|
10.11.1 |
Impax Laboratories, Inc. Second Amended and Restated 2002 Equity Incentive Plan.*(15) |
|
|
|
|
10.11.2 |
Form of Stock Option Agreement under the Impax Laboratories, Inc. Second Amended and Restated 2002 Equity Incentive Plan.*(16) |
|
|
|
|
10.11.3 |
Form of Restricted Stock (Stock Bonus) Agreement under the Impax Laboratories, Inc. Second Amended and Restated 2002 Equity Incentive Plan.*(16) |
|
|
|
|
10.12.1 |
Impax Laboratories, Inc. Executive Non-Qualified Deferred Compensation Plan, amended and restated effective January 1, 2008.*(17) |
|
|
|
|
10.12.2 |
Amendment to Impax Laboratories, Inc. Executive Non-Qualified Deferred Compensation Plan, effective as of January 1, 2009.* (17) |
|
|
|
|
10.13.1 |
Employment Agreement, dated as of January 1, 2010, between the Company and Larry Hsu, Ph.D.*(18) |
|
10.13.2 |
Separation Agreement, dated as of June 24, 2013, between the Company and Larry Hsu, Ph.D.*(19) |
|
10.13.3 |
Amendment, dated as of February 26, 2014, to the Separation Agreement by and between the Company and Larry Hsu, Ph.D., dated as of June 24, 2013.*(20) |
|
10.14.1 |
Employment Agreement, dated as of January 1, 2010, between the Company and Charles V. Hildenbrand.*(18) |
|
|
|
|
10.14.2 |
Confidential Separation and Release Agreement, dated as of July 5, 2011, between the Company and Charles V. Hildenbrand.*(21) |
|
| |
|
10.15.1 |
Employment Agreement, dated as of January 1, 2010, between the Company and Arthur A. Koch, Jr.*(18) |
|
|
|
|
10.15.2 |
General Release and Waiver, effective as of July 17, 2012, between the Company and Arthur A. Koch, Jr.* (22) |
|
|
|
|
10.16.1 |
Employment Agreement, dated as of January 1, 2010, between the Company and Michael J. Nestor.*(18) |
|
10.16.2 |
Amendment, dated as of April 1, 2014, to the Employment Agreement, dated as of January 1, 2014, between the Company and Michael Nestor.*(23) |
|
|
|
|
10.17.1 |
Offer of Employment Letter, dated as of March 17, 2011, between the Company and Mark A. Schlossberg.*(24) |
|
|
|
|
10.17.2 |
Employment Agreement, dated as of May 2, 2011, between the Company and Mark A. Schlossberg.*(24) |
|
|
|
|
10.17.3 |
Amendment, dated as of April 1, 2014, to the Employment Agreement, dated as of May 2, 2011, between the Company and Mark A. Schlossberg.*(23) |
|
10.18.1 |
Offer of Employment Letter, dated as of August 18, 2011, between the Company and Carole Ben-Maimon, M.D.*(25) |
|
10.18.2 |
Employment Agreement, dated as of November 7, 2011, between the Company and Carole Ben-Maimon, M.D.*(26) |
|
10.18.3 |
Amendment dated, as of April 1, 2014, to the Employment Agreement, dated as of November 7, 2011, between the Company and Carole Ben-Maimon, M.D.*(23) |
|
10.18.4 |
Separation Agreement, dated as of October 22, 2014, between the Company and Carole Ben-Maimon, M.D.*(27) |
|
10.19.1 |
Employment Agreement, dated as of December 12, 2012, between the Company and Bryan M. Reasons.*(28) |
|
10.19.2 |
Amendment, dated as of April 1, 2014, to the Employment Agreement, dated as of December 12, 2012 between the Company and Bryan M. Reasons.*(23) |
|
10.20 |
Employment Agreement, dated as of April 21, 2014, by and between the Company and G. Frederick Wilkinson.*(29) |
|
10.21.1 |
Employment Agreement, dated as of November 28, 2011, by and between the Company and Jeffrey Nornhold.*(30) |
|
10.21.2 |
Amendment, dated as of April 1, 2014, to the Employment Agreement, dated as of November 28, 2011, by and between the Company and Jeffrey Nornhold.*(30) |
|
10.21.3 |
Letter Agreement, dated as of April 1, 2014, between the Company and Jeffrey Nornhold.*(30) |
|
10.22 |
Amended and Restated License and Distribution Agreement, dated as of February 7, 2013, between the Company and Shire LLC.**(31) |
|
|
|
|
10.23.1 |
Joint Development Agreement, dated as of November 26, 2008, between the Company and Medicis Pharmaceutical Corporation.**(8) |
|
|
|
|
10.23.2 |
Settlement Agreement, dated as of January 21, 2011, between the Company and Medicis Pharmaceutical Corporation.**(32) |
|
|
|
|
10.23.3 |
First Amendment, dated as of January 26, 2011, to the Joint Development Agreement, dated as of November 26, 2008, between the Company and Medicis Pharmaceutical Corporation.(24) |
|
10.24 |
Distribution, License, Development and Supply Agreement, dated as of January 31, 2012, between the Company and AstraZeneca UK Limited.**(33) |
|
|
|
|
11.1 |
Statement re computation of per share earnings (incorporated by reference to Note 16 to the Notes to Consolidated Financial Statements in this Annual Report on Form 10-K). |
|
|
|
|
21.1 |
Subsidiaries of the registrant. |
|
|
|
|
23.1 |
Consent of Independent Registered Public Accounting Firm. |
|
|
|
|
31.1 |
Certification of the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
31.2 |
Certification of the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
32.1 |
Certification of the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
32.2 |
Certification of the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
101 |
The following materials from the Company’s Annual Report on Form 10-K for the year ended December 31, 2015, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets as of December 31, 2015 and 2014, (ii) Consolidated Statements of Operations for each of the three years in the period ended December 31, 2015, (iii) Consolidated Statements of Comprehensive Income for each of the three years in the period ended December 31, 2015, (iv) Consolidated Statements of Changes in Stockholders’ Equity for each of the three years in the period ended December 31, 2015, (v) Consolidated Statements of Cash Flows for each of the three years in the period ended December 31, 2016 and (vi) Notes to Consolidated Financial Statements for each of the three years in the period ended December 31, 2015. |
* Management contract, compensatory plan or arrangement.
** Confidential treatment granted for certain portions of this exhibit pursuant to Rule 24b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which portions are omitted and filed separately with the SEC.
(1) Incorporated by reference to the Company’s Current Report on Form 8-K filed on October 10, 2014.
(2) Incorporated by reference to the Company’s Current Report on Form 8-K filed on December 9, 2015.
(3) Incorporated by reference to Amendment No. 5 to the Company’s Registration Statement on Form 10 filed on December 23, 2008.
(4) Incorporated by reference to the Company’s Current Report on Form 8-K filed on January 22, 2009.
(5) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q filed on November 9, 2015.
(6) Incorporated by reference to the Company’s Registration Statement on Form 10 filed on October 10, 2008.
(7) Incorporated by reference to the Company’s Current Report on Form 8-K filed on June 30, 2015.
(8) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2011.
(9) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2012.
(10) Incorporated by reference to the Company’s Current Report on Form 8-K filed on January 10, 2013.
(11) Incorporated by reference to the Company’s Current Report on Form 8-K filed on February 25, 2014.
(12) Incorporated by reference to the Company’s Current Report on Form 8-K filed on March 12, 2015.
(13) Incorporated by reference to the Company’s Current Report on Form 8-K filed on August 5, 2015.
(14) Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008.
(15) Incorporated by reference to Appendix A to the Company’s Definitive Proxy Statement on Schedule 14A filed on April 15, 2013.
(16) Incorporated by reference to the Company’s Registration Statement on Form S-8 (file No. 333-189360) filed on June 14, 2013.
(17) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2010.
(18) Incorporated by reference to the Company’s Current Report on Form 8-K filed on January 14, 2010.
(19) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2013.
(20) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2014.
(21) Incorporated by reference to the Company’s Current Report on Form 8-K filed on July 11, 2011.
(22) Incorporated by reference to the Company’s Current Report on Form 8-K filed on July 18, 2012.
(23) Incorporated by reference to the Company’s Current Report on Form 8-K filed on April 2, 2014.
(24) Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2011.
(25) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2011.
(26) Incorporated by reference to the Company’s Current Report on Form 8-K filed on November 9, 2011.
(27) Incorporated by reference to the Company’s Annual Report on Form 10-K filed on February 26, 2015.
(28) Incorporated by reference to the Company’s Current Report on Form 8-K filed on December 13, 2012.
(29) Incorporated by reference to the Company’s Current Report on Form 8-K filed on April 24, 2014.
(30) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2014.
(31) Incorporated by reference to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2013.
(32) Incorporated by reference to the Company’s Annual Report on Form 10-K for the year ended December 31, 2012.
(33) Incorporated by reference to the Company’s Current Report on Form 8-K/A filed on April 2, 2012.
