Attached files
As filed with the Securities and Exchange Commission on February 12, 2016
Registration No.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
REDWOOD SCIENTIFIC TECHNOLOGIES, INC
A Nevada Corporation
| 41-3165559 | ||||
| (State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
250 W. First Street,
Suite 310
Claremont, CA 91711
Tel: 310-693-5401
Copies to:
Louis Taubman, Esq.
Hunter Taubman Fischer, LLC
1450 Broadway, Floor 26
New York, New York 10018
Approximate date of commencement of proposed sale to the public: From time to time after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ (Do not check if a smaller reporting company) | Smaller reporting company | ☒ |
CALCULATION OF REGISTRATION FEE
| Title of each class of securities to be registered | Amount to be registered (1) | Proposed maximum offering price per share (2) | Proposed maximum aggregate offering price | Amount of registration fee | ||||||||||||
| Common Stock (3) | 2,355,146 | 2.25 | 5,299,078 | 533.62 | ||||||||||||
| Common Stock underlying Warrants (4) | 1,707,127 | 2.25 | 3,841,035 | 386.79 | ||||||||||||
| Total | 4,062,273 | 2.25 | 9,140,114 | 920.41 | ||||||||||||
(1) Pursuant to Rule 416 and 457 of the Securities Act of 1933, as amended, the shares of common stock offered hereby also include such presently indeterminate number of shares of our common stock as shall be issued by the Registrant to the selling stockholders to prevent dilution resulting from stock splits, stock dividends or similar transactions.
(2) The offering price has been estimated solely for the purpose of computing the amount of the registration fee in accordance with Rule 457(a). Our common stock is not traded on any national exchange and in accordance with Rule 457, the offering price was determined by the price of the shares that were sold to our shareholders in the Offering. The price of $2.25 is a fixed price at which the selling security holders shall sell their shares until they are quoted on the OTC Bulletin Board, after which time they will be sold at prevailing market prices; we are currently in the process of applying to have our common stock quoted on the OTC Bulletin Board. There can be no assurance that our application for quotation will be approved.
(3) Includes: 1,529,445 shares of common stock underlying the Units issued in the Offering and 825,701 shares of common stock underlying $1,500,000 10% Secured Convertible Notes issued pursuant to the private placement we completed on March 2, 2015 (the "Private Placement").
(4) Includes: (i) 764,723 shares of common stock underlying the Warrants included in the Units; (ii) 761,421 shares of common stock underlying warrants issued pursuant to the secured convertible notes, including the shares of common stock issued pursuant to the anti-dilution provisions of such warrants; (iii) 180,983 shares of common stock underlying the warrants issued to the Placement Agents of the Offering.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission, acting pursuant to said section 8(a), may determine.
THE INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. THE SELLING STOCKHOLDERS MAY NOT SELL THESE SECURITIES PUBLICLY UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
SUBJECT TO COMPLETION, DATED FEBRUARY 12, 2016
PROSPECTUS
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
4,062,273 Shares of Common Stock
This prospectus relates to the sale by the selling shareholders listed on page 33 of up to 4,062,273 shares of our common stock
The selling stockholders named herein may sell common stock from time to time in the principal market on which the stock is traded at the prevailing market price, at prices related to such prevailing market price, in negotiated transactions or a combination of such methods of sale and pursuant to any other method permitted by applicable law. Unless all or a portion of the 1,707,127 shares of common stock issuable upon exercise of the Warrants, and to which this prospectus relates, are exercised, we will not receive any proceeds from the sales by the selling stockholders.
The number of shares of common stock which may be sold by each of the selling stockholders are subject under certain conditions to certain agreed upon limits described elsewhere in this prospectus.
THIS INVESTMENT INVOLVES A HIGH DEGREE OF RISK. YOU SHOULD PURCHASE SHARES ONLY IF YOU CAN AFFORD A COMPLETE LOSS OF YOUR INVESTMENT. SEE “RISK FACTORS” BEGINNING ON PAGE 6 FOR A DISCUSSION OF RISKS APPLICABLE TO US AND AN INVESTMENT IN OUR COMMON STOCK.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED THESE SECURITIES, OR DETERMINED IF THIS PROSPECTUS IS TRUTHFUL OR COMPLETE. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is February 12, 2016.
We have not authorized any person to give you any supplemental information or to make any representations for us. You should not rely upon any information about us that is not contained in this prospectus or in one of our public reports filed with the Securities and Exchange Commission (“SEC”) and incorporated into this prospectus. Information contained in this prospectus or in our public reports may become stale. You should not assume that the information contained in this prospectus, any prospectus supplement or the documents incorporated by reference are accurate as of any date other than their respective dates, regardless of the time of delivery of this prospectus or of any sale of the shares. Our business, financial condition, results of operations and prospects may have changed since those dates. The selling stockholders are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted.
All dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters.
The following summary is qualified in its entirety by the more detailed information appearing elsewhere in this S-1 Registration Statement. You should carefully read the entire Registration Statement and should consider, among other things, the matters set forth in ‘‘Risk Factors’’ before deciding to invest in the Units.
In this S-1 Registration Statement, unless the context otherwise requires, all references to “we,’’ ‘‘us,’’ ‘‘our,’’ and similar terms, and “Redwood,” “RST” and “the Company” refer to Redwood Scientific Technologies, Inc., a Nevada corporation and our wholly owned subsidiary Redwood Scientific Technologies, Inc., a California corporation.
Our Business
Redwood Scientific Technologies, Inc. was organized in January 2014 under the name Advanced Men's Institute Prolongz, LLC ("AMI") in the State of California and began selling its products in February 2014. In November 2014, AMI changed its name to Redwood Scientific Technologies, LLC. Immediately after the name change Redwood Scientific Technologies, LLC filed a statement of conversion whereby it converted from an LLC into a California corporation to become Redwood Scientific Technologies, Inc. This became the operating subsidiary to Redwood Scientific Technologies Inc., a Nevada Corporation (the “Company”), as a result of a share exchange on January 6, 2015.
The Company develops and markets consumer homeopathic drugs and supplements. The Company utilizes patent-pending, sublingual strip delivery technology that enhances bioavailability. Currently, the Company has four products for sale or about to reach the market: ProlongzTM (FDA Registration # 69308-000-01) is the first drug of its kind that addresses a specific sexual performance dysfunction in men and is the only drug that can be taken orally through a sublingual strip; TBX-FreeTM (FDA Registration # 69461-001-01) is the first FDA registered oral strip that helps smokers safely, quickly, and effectively eliminate the craving to smoke; Product-X: The first sublingual strip delivery medication for the treatment of erectile dysfunction; and Comfort-Time: a children's aspirin is the only oral strip pain reliever that quickly reduces inflammation and the pain without the risk of chocking. All the company’s products are sold either through a membership subscription model (a direct marketing program) or at any given number of retail locations. The Company develops and markets consumer homeopathic drugs and supplements. The Company utilizes patent-pending, sublingual strip delivery technology that enhances bioavailability.
The Company’s wholly owned subsidiary began operations in the first quarter of 2014 and the Company was incorporated in Nevada in December 2014. Through our subsidiary, we develop and market consumer OTC drugs and supplements that address unmet needs in the multi-billion-dollar health and wellness consumer markets. RST utilizes Patent-pending, sublingual strip delivery technology that enhances bioavailability.
The Company’s initial product, Prolongz, an Over-the-Counter drug, designed to treat premature ejaculation in men and management believes is the only such drug that can be taken orally through sublingual strip delivery. Prolongz has been registered with the FDA ‘# 69308-000-01’, allowing Redwood to elevate itself from its competitors. As of the date of this Registration Statement and based solely on our internal research, we believe that Prolongz is the only OTC drug registered with the FDA as useful treatment for premature ejaculation through our sublingual delivery mechanism.
In the fourth quarter 2015, the Company launched its smoking cessation product TBX-FreeTM (FDA #69461-001-01), the first FDA registered sublingual strip that helps smokers safely and quickly eliminate the craving to smoke. As of the date of this Registration Statement and based on our research, we believe that TBX-FreeTM is the only nicotine free OTC drug registered with the FDA as useful treatment for the cessation of cravings to smoke.
Redwood is on course to establish itself as the industry leader for both these products in their respective markets through its membership (subscription) model. The Company is aggressively trying to expand its customer population through heavy investments in national cable television commercials and infomercials which also drive consumer awareness and retail sales.
The Company is also in the development phase for other products that address health concerns for adults and children, prostate health, and other health areas, all through the use of Company’s patent pending sublingual delivery mechanism. All of Redwood’s products will be centered on the innovative sublingual strip technology that the Company has developed. These products may include a product for prostate health, women’s sexual health and others. The Company’s launch timeline for these additional products is set for 2016. It is intended that all of RST’s products, including those in the development pipeline will be submitted to the FDA registration process.
| 1 |
The Company retained separate FDA counsel who work directly with the FDA to ensure the Company’s compliance with federal and state regulatory authorities. The FDA counsel helps RST with the evaluation and development process for new products and shepherd’s the product through the regulatory process. The process taken is determined and dependent on how the products are to be marketed and their intended effects. Each FDA regulatory category brings with it certain benefits (regarding, for instance, the claims you can make related to the product) and challenges (regarding, for instance, pre-marketing clearance requirements).
RST recognizes the unmet potential for the treatment of sexual dysfunction which is part of the estimated $7B US homeopathic market which is growing at a rate of 3% per annum reaching $7.5B by 2017.
http://www.nutraceuticalsworld.com/issues/2013-07/view_industry-news/us-sales-of-homeopathic-herbal-remedies-reach-64-billion/.
According to researcher Euromonitor International (Euromonitor.com), the U.S. nicotine replacement therapy (“NRT”)
market accounts for nearly 40% of the $2.4 billion in global annual retail sales, which is growing at a constant annual growth
rate of 3%. Both the sexual dysfunction markets and the NRT markets represent significant opportunities for Redwood. Redwood’s
domestic and international expansion plans are underway as it is developing a distribution network to expand its market activities
in the United Kingdom, Central and South America for both these products.
Our History
On January 6, 2015, we entered into and consummated the transactions (the “Share Exchange”) contemplated under a Share Exchange Agreement (the “Share Exchange Agreement”) by and among us, Redwood Scientific Technologies, Inc., a California corporation (“Redwood California”) and Jason Cardiff (“Cardiff”), the sole shareholder of Redwood California who is also our Chief Executive Officer. Under the terms of the Share Exchange Agreement, Cardiff transferred all of his shares of Redwood California, which represented 100% of Redwood California’s shares outstanding, to the Company and Redwood California became a wholly-owned subsidiary of the Company. As part of the Share Exchange, Cardiff was issued 4,400,000 shares of Redwood Nevada’s common stock, par value $0.01 (the “Common Stock”), which represented 88% of the 5,000,000 issued and outstanding shares of the Company’s common stock immediately following the Share Exchange.
Redwood California, was organized in the State of California in January 2014 under the name Advanced Men’s Institute Prolongz, LLC. In early November 2014, the Company underwent a name change to operate under the legal name Redwood Scientific Technologies, LLC. Subsequently and immediately thereafter, Redwood Scientific Technologies, LLC filed a Statement of Conversion whereby Redwood Scientific Technologies, LLC converted from a limited liability company into a California corporation under that same name.
As a result of the Company’s acquisition of Redwood California, it became our wholly-owned subsidiary. All of our operations are conducted through Redwood California.
Recent Developments
Following our launch of Prolongz
We have received our second drug code from the FDA for TBX Free, our smoking cessation product, which is the only non-nicotine stop smoking product to be delivered by sub-lingual strip. We launched TBX Free in December 2015 on a nationwide television campaign and also have interest from several national retailers. TBX-FREE is the first FDA registered oral strip that helps smokers safely, quickly, and effectively eliminate the craving to smoke. Every TBX-FREE oral strip contains a precisely measured dose of cytisine; the active ingredient that reduces the urge to smoke. It is too early to assess the long term success of this product, however initial sales have exceeded expectations.
We have also filed for registration with the FDA of Sumnusent, an all-natural sleep aid that will also be delivered by our sub-lingual strip technology. Depending on registration, we are hopeful that we will be able to launch Sumnusent in the first quarter of fiscal 2016.
Risk Factors
The securities offered by this prospectus are speculative and involve a high degree of risks associated with our business. For more comprehensive discussion of these and other risk factors affecting us and our business, see the “Risk Factors” section beginning on page 6 of this prospectus.
For a more comprehensive discussion of these and other risk factors affecting us and our business, see the “Risk Factors” section beginning on page 9 of this prospectus.
The Unit Offering
On January 18, 2016, we completed a private offering (the “Offering”) of $3,441,252 Units, each Unit consisting of: (a) 1 share of the Company’s common stock, par value $0.01 per share (the “Common Stock”) and, (b) a warrant (the “Warrants”) to purchase that number of shares of Common Stock as is equal to 50% of the number of shares of Common Stock underlying the investor's Unit (such shares of common stock underlying the Warrants are hereinafter referred to as the “Warrant Shares,” the Warrants, the Warrant Shares and the Common Stock are sometimes collectively referred to as the “Securities”), at an initial exercise price of $2.75 per share (the “Exercise Price”). The Units were sold at $2.25 per Unit. Accordingly, pursuant to the Offering we issued a total of 1,529,445 shares of Common Stock and Warrants to purchase up to a total of 764,723 shares of Common Stock. (See “Description of Securities”)
| 2 |
If the Company issues Common Stock or any type of securities giving rights to Common Stock at a price below the Unit Price, Investors will be extended full ratchet anti-dilution protection through the issuance of additional common shares. Anti-dilution protection will not apply to shares (or options to purchase shares) issued to employees, directors and consultants as part of a pre-existing equity incentive plan or agreement adopted by the Company’s board up to an aggregate of 250,000 shares and other limited exceptions.
Additionally, if the Company has less than $20,000,000 in gross revenue or less than $1 in earnings before interest, taxes, depreciation and amortization (EBITDA) for the subsequent twelve months following the close of $6,500,000 in funding (including funds received from the Bridge Financing), the Company shall issue an additional 20% of the common stock and warrants underlying the Units issued pursuant to this Offering. In addition, the Company shall use reasonable best efforts to distribute preliminary financial statements for this twelve month period within 45 days after the twelve month period ends and audited financial statements if requested sixty days after written notice of the request, for purposes of determining whether the Company shall issue such additional Units. If the Company fails to use reasonable best efforts to distribute such financial statements containing the information necessary to calculate the Company’s Revenue, such failure shall result in (i) the investors receiving an additional 100% of the Units purchased in this Offering. Since the Company did not close with $6,500,000 in funding, we do not believe this clause is operative.
We also entered into a Registration Rights Agreement with the Investors pursuant to which we agreed to file this registration statement, by the 30th day following the close of the Offering, registering for resale all of the shares underlying the Units, including those underlying the Warrants, as well as a reasonable number of shares issuable under the Units and Warrants pursuant to potential adjustments that may occur pursuant thereto. If we fail to file the registration statement or keep it effective as per the terms of the Registration Rights Agreement, we shall pay each Investor liquidated damages in cash equal to 1% of such Investor’s purchase price to be incurred each month until the registration statement is filed or declared effective, as the case may be, but in no event more than 10%.
Copies of the Unit Purchase Agreement, Form of Warrant and Registration Rights Agreements for the Offering are incorporated herein by reference and are filed as Exhibits 10.1, 10.2 and 10.3, respectively, hereto. The description of the transactions contemplated by such agreements set forth herein do not purport to be complete and is qualified in its entirety by reference to the full text of the exhibits filed herewith and incorporated herein by reference.
In connection with the Offering, we paid $122,760 to our two placement agents for the Offering, which fees shall be apportioned by the placement agent among itself, other selling agents and broker-dealers which are members of FINRA and to pay related commissions to its associated persons who are qualified and eligible to accept such commission within the state or other jurisdiction in which the Units were sold and/or such commission is paid. The Placement Agents also received three year warrants to purchase an aggregate of up to 127,379 shares of our Common Stock (the “Placement Agent Warrants”). Additionally, one placement agent received a non-accountable expense fee equal to 2% of the total proceeds raised from the sale of the Units to their investors and all of the reasonable expenses such Placement Agent incurred in connection with the Offering, which amount equaled $7,500; we previously paid this placement agent $15,000 in legal fees and a due diligence fee of $2,500 following the closing of the Bridge Financing (as described below). We also paid $5,000 in out of pocket expenses to the other placement agent, as well as $5,000 for due diligence expenses and a 2% banking fee on gross funds such placement agent raised.
We also agreed to offer the Placement Agents the indemnification protections granted to the investors in the Offering as part of the agreement governing the registration of the securities sold to investors in the Offering, as a third party beneficiary to such provisions.
The private financing described herein was made pursuant to the exemption from the registration provisions of the Securities Act of 1933, as amended, provided by Sections 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated thereunder. The securities issued have not been registered under the Securities Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
The Prior Private Financing
On March 2, 2015, we completed a private offering (the “Bridge Financing”) of $1,500,000 Units consisting of: (i) a one (1) year secured 10% convertible note (individually a “Bridge Note” and collectively, the “Bridge Notes”) and (ii) warrants (the “Bridge Warrants”) to purchase 100% of the number of shares underlying the Bridge Notes, with thirty (30) accredited investors1. We received net proceeds of $1,399,400. The Bridge Notes are initially convertible at a conversion price of $2.00 per share (the “Conversion Price”) and the Bridge Warrants are initially exercisable at an exercise price of $2.50 per share (the “Exercise Price”); both the Conversion Price and Exercise Price are subject to adjustment and purchase price protection. In addition, the Bridge Notes are fully secured by the assets of the Company. Pursuant to the Bridge Financing, we issued an aggregate of $1,500,000 Convertible Bridge Notes and Bridge Warrants to purchase up to an aggregate of 761,421 shares of our Common Stock.
1 We held an initial closing on February 3, 2015, pursuant to which we received aggregate gross proceeds of $1,065,000; we received and closed on the balance on March 2, 2015
| 3 |
The securities offered in the Bridge Financing were sold pursuant to a Unit Purchase Agreement (the “Purchase Agreement”) by and among our company and the investors named in the Purchase Agreement (collectively, the “Investors”). Pursuant to the Purchase Agreement, we issued the Placement Agent warrants to purchase up to 53,604 shares of Common Stock, initially exercisable at $1.97 per share on a cashless basis (the “Placement Agent Bridge Warrants”). The Placement Agent Warrants are subject to adjustment and purchase price protection, but are not redeemable (See “Description of Securities, Placement Agent Warrants”).
Pursuant to the terms of the Bridge Notes, upon the Company’s receipt of at least $2,500,000 in additional financing, the outstanding balance of the Bridge Notes (principal plus all accrued interest up to the date of closing on at least $2,500,000) shall automatically convert into Common Stock at the “Discount Conversion Price” which is equal to the lesser of (i) the Conversion Price or (ii) a 12.5% discount to the price of the securities issued pursuant to such additional financing (the “Automatic Conversion Feature”)2. Accordingly, as of November 24, 2015 (“Conversion Date”), since we received an aggregate of $2,515,501.25 pursuant to the Unit Offering, the principal amount of the Bridge Notes automatically converted at a conversion price of $1.97 into 761,421 shares of Common Stock and the exercise price of the Bridge Warrants has been adjusted from $2.50 per share to $2.25 per share, resulting in the additional issuance of up to an aggregate of 11,421 shares of common stock upon the exercise of the Bridge Warrants after this Offering.
| Common Stock to be | Up to 4,062,273 consisting of: | |
| Offered by the Selling | ||
| Security holders | a) | 1,529,445 shares of the Company’s common stock, $0.01 par value per share (the “Common Stock”) underlying the Units; |
| b) | 764,723 shares of Common Stock underlying the Warrants; | |
|
c) | 825,701 shares of Common Stock underlying the Bridge Notes; |
| d) | 761,421 shares of Common Stock underlying the Bridge Warrants; | |
| e) | 127,379 shares of Common Stock underlying the Placement Agent Warrants; and, | |
| f) | 53,604 shares of Common Stock underlying the Placement Agent Bridge Warrants. | |
| Stock Exchange Symbol | N/A | |
2 The terms of the Bridge Note provide that the Automatic Conversion Feature shall be triggered by a financing transaction, in one or more closings (not including the Bridge Financing), which results in gross proceeds of at least $2,500,000 to the Company.
| 4 |
The following table summarizes the relevant financial data for our business and should be read in conjunction with our financial statements which are included elsewhere in this prospectus. The summary set forth below should be read together with our consolidated financial statements and the notes thereto, as well as “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” included elsewhere in this prospectus.
Consolidated Statement of Operations Data:
| For the nine months ended September 30 | ||||||||
| 2015 | 2014 | |||||||
| (in thousands) | ||||||||
| Net Revenues | $ | 5,143 | $ | 2,396 | ||||
| Gross profit | 4,109 | 1,685 | ||||||
| Comprehensive Profit (loss) | $ | (1,267 | ) | $ | 80 | |||
Consolidated Balance Sheet Data:
| As of September 30, 2015 | ||||
| Balance Sheet Data: | ||||
| Current assets | $ | 2,308 | ||
| Total assets | 2,382 | |||
| Total current liabilities, net of debt discounts | 1,419 | |||
| Total liabilities, net of debt discounts | 2,361 | |||
| Total stockholders’ equity (deficit) | $ | 21 | ||
NOTES REGARDING FORWARD-LOOKING STATEMENTS
The statements contained in this prospectus that are not purely historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act. These include statements about the Company’s expectations, beliefs, intentions or strategies for the future, which are indicated by words or phrases such as “anticipate,” “expect,” “intend,” “plan,” “will,” “the Company believes,” “management believes” and similar words or phrases. The forward-looking statements are based on the Company’s current expectations and are subject to certain risks, uncertainties and assumptions. The Company’s actual results could differ materially from results anticipated in these forward-looking statements. All forward-looking statements included in this document are based on information available to the Company on the date hereof, and the Company assumes no obligation to update any such forward-looking statements.
| 5 |
The Company’s limited operating history and lack of cash flow and profitability may continue which will affect our ability to remain in business.
The Company has a limited history of operations and has limited history of profitability. The Company completed the audit of its first year of operations ending December 31, 2014. Audited results of operation for the first fiscal year ended December 31, 2014, show that the Company’s gross revenues are $3,234,582, cost of goods sold $951,676, producing gross profit of $2,282,906; advertising and promotional expenses totaled $907,345, with all operating and general and administrative expenses totaling $1,095,415 for total expenses of $1,903,702, producing a profit before tax of $379,204.
For the nine month period ending September 30, 2015, the Company’s gross revenues are $5,143,405, costs of goods sold is $1,033,744, producing gross profit of $4,109,661; advertising and promotional expenses totaled $2,431,795 with all other general and administrative expenses totaling $465,824 for total expenses of $4,809,347, producing a before tax loss from operations of $1,699,246.
If we do not generate positive cash flow and maintain profitability, we may not be able to remain in business. The Company is also subject to business risks associated with new business enterprises. No assurance can be given as to the ability of the Company to operate and sustain profitability.
Due to our limited operating history, our ability to operate successfully is materially uncertain and our operations and prospects are subject to all risks inherent in a developing business enterprise. In investing in this Offering, potential Investors should be aware of the difficulties normally encountered by early stage companies and the high rate of failure of such enterprises. The likelihood of our viability and potential for revenue and profit generation must be considered in light of the significant problems, expenses, difficulties, complications and delays encountered in connection with the commercialization of our technologies and products in development. These potential problems include, but are not limited to:
| ● | products may never be developed or work as planned; | |
| ● | costs and expenses that may exceed current estimates; | |
| ● | our ability to generate sales; | |
| ● | our ability to attract and retain high quality personnel for sales and marketing; | |
| ● | competition of more established and better capitalized companies; and, | |
| ● | our ability to raise capital when needed to advance our business plans. |
Although we believe that the market for our products in development or planned is very large and growing, we can provide no assurances to Investors that we will be able to fully commercialize and market our products and services or generate any operating revenues or ever achieve profitable operations. If we cannot address the inherent risks facing our company, our intended business will likely fail and you will lose your entire investment.
If we fail to raise additional capital, our ability to implement our business model and strategy could be compromised.
We have limited capital resources and operations. To date, other than the Bridge Financing, our operations have been funded entirely from Jason Cardiff (our CEO and majority shareholder) and his affiliates, which funding is treated as contributions to capital. There is no minimum in this Offering. Notwithstanding the funds we received from the Offering, we expect to require substantial additional capital in the near future to further develop and market ProlongzTM and new products, although that cannot be guaranteed. The $4,941,252 we received from the Offering and Bridge Financing is only expected to provide us with working capital to maintain our planned level of operations through the fourth quarter of fiscal 2016 without significantly altering our business plan. (See “Use of Proceeds.”)
Based on the Company’s past and anticipated operating expenses, and without its planned investment in television advertising, we believe that the Company requires approximately $200,000 per month on an annualized basis for operating expenses to fund the costs associated with our financing activities, legal and accounting expenses, other general and administrative expenses, research and development, regulatory compliance, product development and maintenance, third party manufacturing fees, and compensation of executive management and our employees. Based on our current cash position, without additional financing we may not be able to pay our obligations past the fourth quarter of fiscal 2016. The monthly cash requirement for operating expenses does not include any extraordinary items or expenditures such as the planned expenditures on direct response marketing (television ad spend). Furthermore, we expect to incur additional costs associated with operating as a public company.
Accordingly, if we do not receive additional financing by the end of 2016 when we exhaust the funds received from the Offering, we will likely be unable to carry out our business plan. We currently do not have commitments for financing to meet our expected needs and we may not be able to obtain additional financing on terms acceptable to us, or at all. Even if we obtain financing for our near term operations and product development, we expect that we will require additional capital beyond the near term. If we are unable to raise capital when needed, our business, financial condition and results of operations would be materially adversely affected, and we could be forced to reduce or discontinue our operations.
| 6 |
Risks Related To Our Business
We are an early stage company with limited operating history, and we expect to incur net losses for the foreseeable future.
We have limited operating history and have generated limited revenue since our inception. We expect our research and development expenses to be significant for the foreseeable future. We expect to incur significant operating losses for the foreseeable future. These losses will have an adverse effect on our operations.
Our future success depends on the continued sales of our principal product.
Currently, our only proven saleable product is ProlongzTM. Accordingly, we depend on the continued acceptance of ProlongzTM by our customers and their continued renewal of our program. Our investments in and strategies used for our brand marketing are critical to achieve brand awareness with current consumers, educate potential new consumers and convert potential consumers into customers. However, there can be no assurance that ProlongzTM will continue to receive, maintain or increase market acceptance or that current customers will renew their program. The inability to successfully commercialize ProlongzTM in the future and/or expand its product distribution, for any reason, would have a material adverse effect on our financial condition, prospects and ability to continue operations. In December of 2015, we introduced our second FDA registered product called TBX-Free. TBX-FREE is the first FDA registered oral strip that helps smokers safely, quickly, and effectively eliminate the craving to smoke. TBX-FREE has the ability to help reduce smoking cravings. As a result, smokers may enjoy the freedom to live their lives without the powerful urge to light up cigarettes, cigars, and other tobacco or nicotine products. Every TBX-FREE oral strip contains a precisely measured dose of cytosine - the active ingredient that reduces the urge to smoke. It is too early to assess the long term success of this product, however initial sales have exceeded expectations.
Developing and increasing awareness of our brand is crucial to increasing our customer base and our revenues.
We believe that increasing awareness of Prolongz and TBX-Free brands will be critical to developing and expanding our customer base and our revenues. If we fail to advertise and market our products effectively, we may not succeed in maintaining or increasing awareness of our brands and we may lose customers and our revenues will decline. The delivery of quality products to our customers is also of utmost importance to maintaining and enhancing the reputation of our brand. If our customers do not perceive our products to be of high quality, demand for our products will decline, which could lead to a decline in revenues and an adverse effect on our financial condition.
Redwood depends on certain important employees, and the loss of any of those employees may harm our business.
The success of Redwood’s business will continue to be highly dependent upon Jason Cardiff, CEO, Jacques Poujade, Interim CFO, Stefan Galluppi, CTO, Eunjung Cardiff, Marketing Director and Anthony Raissen, Director of Retail Sales. The loss of services of any of these persons could have a materially adverse effect upon our business and development.
Our business is subject to significant competitive pressures.
The OTC healthcare product, life science, homeopathic drug market, and consumer product industries are highly competitive. Many of our competitors have substantially greater capital resources, technical staffs, facilities, marketing resources, product development, distribution and experience than we do. Our competitors may have certain advantages, including the ability to allocate greater resources for new product development, marketing and other purposes.
We believe that our ability to compete depends on a number of factors, including product quality and price, availability, speed to market, consumer marketing, reliability, credit terms, brand name recognition, delivery time and post-sale service and support, and new and existing product innovation and commercialization. There can be no assurance that we will be able to compete successfully in the future. If we are unable to compete effectively, our earnings may be significantly negatively impacted.
We will need to obtain additional capital to support long term product development and commercialization programs.
Our ability to achieve and sustain operating profitability depends in large part on our ability to commence, execute and complete new and existing product innovation and commercialization, and, if required, clinical programs to obtain regulatory approvals in the United States and elsewhere. We can give no assurance that we will be able to achieve such product innovation and commercialization, to obtain any required approvals or to achieve significant levels of sales.
The amount of capital that may be needed to complete product development initiatives will depend on many factors which may include but are not limited to (i) the cost involved in applying for and obtaining FDA, international regulatory or other technical approvals, (ii) whether we elect to establish partnering arrangements for development, sales, manufacturing and marketing of such products, (iii) the level of future sales of OTC related products, and expense levels for marketing efforts, and (iv) whether we can establish and maintain strategic arrangements for development, sales, manufacturing and marketing of our products.
| 7 |
Should research or commercialization activity progress on certain formulations, resulting expenditures may require substantial financial support. The current sales level of our ProlongzTM and our second product TBX-FreeTM may not generate all the funds we anticipate will be needed to support future product acquisition or development. Accordingly, in addition to funding from operations, we may in the short and long term seek to raise capital through the issuance of securities or to secure other financing sources to support our research, new product technologies, applications, licensing, commercialization and other development opportunities. If we obtain such funding through the issuance of equity securities, it would result in the dilution of current stockholders’ ownership in the Company. Any debt financing, if available, may include financial and other covenants that could restrict use of proceeds of such financing or impose other business and financial restrictions on us. In addition, we may consider alternative approaches such as licensing, joint venture, or partnership arrangements to provide long term capital. There can be no assurances that we will have access to the capital required to fund these aspects of our business on favorable terms or at all.
We currently derive our revenues primarily from direct response television marketing where we have approximately 14,900 subscribers purchasing the product on a monthly basis.
We currently rely on mainly two sales channels in order to derive revenues. The company’s main sales channel is Direct Sales using Direct Response Marketing such as commercials and infomercials broadcast on television. The customers are provided with call numbers during the infomercials. Once the customers dial in, an automated system collects the customers’ information (i.e. name, billing address, credit card information, etc.) and in turn sends an order form to the warehouse for fulfillment. The company’s secondary sales channel is its web-based system which is also advertised in the infomercial. Once accessing the company’s web site, customers can place their free-trial orders through the user-friendly, web-based platform via www.prolongz.com/www.tbxfree.com. There are no guarantees that the company will be able to continue to advertise on television because of such factors, as the cost to purchase “air time” may become too high and prohibitive, the levels of “response” from consumers decline, or whether the regulatory environment will continue to be amiable to such form of advertising and the claims made. The level of subscribers are significantly impacted by the company’s ad spend each month.
We are launching the retail store sales channel and in the very near term may have a concentration of sales to and accounts receivable from several large retail customers.
We are opening retail sales channels to expand our reach into the health and wellness market for our products. We will supply the company’s products to different retailers and utilize their networks of physical stores to promote and increase its sales. Our anticipated retail partners range from drug retailers to convenience stores, as well as grocery chains. We are currently selling product to General Nutrition Corporation throughout GNC’s network of 900+ retail locations in the US. Redwood’s products are also available at Circle K’s West Coast locations. These channel partners are an important part to the Company’s growth in the near future. The company is also working on developing its network of national drug retailers. The Company may or may not be successful in obtaining purchase orders from these retail chains. The success or not of obtaining these retail chains will significantly impact the Company’s projections and results of operations.
Although we are working on having a broad range of retail store chains and customers that includes many national chain, regional, specialty and local retail stores, our two largest retail customers did not account for a significant percentage of our sales, approximately 3% of total revenues from inception through November, 2015. These retail chain store customers however may be a significant percentage of sales in the future. In addition, although these retail chain customers did not comprise a significant accounts receivable balance at any month end, in the future they may represent a significant balance. We extend credit to retail store customers based upon an evaluation of their financial condition and credit history, and collateral is not generally required. If one or more of these large retail customers cannot pay, the write-off of their accounts receivable could have a material adverse effect on our operations and financial condition. The loss of sales to any one or more of these large retail customers would also have a material adverse effect on our financial condition, results of operations and cash flows.
If our outside suppliers and manufacturers fail to supply products in sufficient quantities and in a timely fashion, our business could suffer.
We are currently dependent on two manufacturers and a limited number of suppliers to make and supply all of our products. We do not have long-term agreements with our manufacturers or suppliers. Our profit margins and timely product delivery are dependent upon the ability of our outside suppliers and manufacturers to supply us with products in a timely and cost-efficient manner. Our ability to develop our business and enter new markets and sustain satisfactory levels of sales in each market depends upon the ability of our outside suppliers and manufacturers to produce the ingredients and products and to comply with all applicable regulations. The failure of our primary suppliers or manufacturers to supply ingredients or produce our products could adversely affect our business operations.
We do not have long-term contracts with suppliers, manufacturers and distributors and we are dependent on the services of these third parties.
We purchase all of our products from third-party suppliers and manufacturers pursuant to purchase orders, but without any long-term agreements. In the event that a current supplier or manufacturer is unable to meet our manufacturing and delivery requirements at some time in the future, we may suffer short-term interruptions of delivery of certain products while we establish an alternative source. We also rely on third-party carriers for product shipments, including shipments to and from our distribution facilities. We are therefore subject to the risks, including employee strikes and inclement weather, associated with our carrier’s ability to provide delivery services to meet our fulfillment and shipping needs. Failure to deliver products to our customers in a timely and accurate manner would harm our reputation and our business and results of operations.
| 8 |
If the outside contractors we currently use for production of our products fail to produce product in the volumes and quality that we require on a timely basis, we may be unable to meet demand for our products and may lose potential revenues.
We currently contract with specially equipped contractors to handle the large-scale mixing of the formulation components in our products. These external contractor relationships entail added costs and potential disruption to our finished goods schedule. These third-party contractors may encounter difficulties in production, including problems with quality control, quality assurance testing, shortages of qualified personnel, and compliance with federal, state and or other governmental regulations. Our contractors may not be able to expand capacity or to produce additional product requirements for us in the event that demands for our products increases. There can be no assurance that our contractors will be able to continue purchasing raw materials for our products from current suppliers or any other supplier on terms similar to current terms or at all. If these contractors were to encounter any of these difficulties, or experience any interruption in the availability of certain ingredients or significant increases in the prices paid for such materials, our ability to fulfill orders on a timely basis to our customers would be jeopardized. In the future, we intend to add the necessary industrial level mixing equipment to our current bottling facility to mix our own lubricant and lotion products, however, we cannot assure you when and if we will begin to mix such products in-house.
If we do not manage product inventory in an effective and efficient manner, our profitability could be adversely affected.
Many factors affect the efficient use and planning of product inventory, such as effectiveness of predicting demand, effectiveness of preparing manufacturing to meet demand, efficiently meeting product mix and product demand requirements and product expiration. We may be unable to manage our inventory efficiently, keep inventory within expected budget goals, or keep sufficient product on hand to meet demand. If we fail to manage inventory effectively, we may end up with unsold inventory that is past its expiration date and can no longer be sold. We periodically evaluate the composition of inventory and estimate an allowance to reduce inventory for slow moving, obsolete or damaged inventory. Our failure to manage inventory effectively may lead to increased costs and adversely affect our results of operations.
Retail customer’s strategic business plans may negatively influence the distribution of our products to consumer.
Changes in our retail and distribution customers strategic business plans including, but not limited to, (i) expansions, mergers, and/or consolidations, (ii) retail shelf space allocations for products within each outlet and in particular the cough/cold category in which we compete, (iii) changes in their private label assortment and (iv) product selections, distribution allocation, merchandising programs and retail pricing of our products as well as competitive products could affect the consumer sales of our products and could result in a material adverse effect to our business and financial condition.
Our products and potential new products are or may be subject to extensive governmental regulation.
Our business is regulated by various agencies, including federal and state agencies, where our products are sold. Governmental regulations in foreign countries where we manufacture our products, or plan to commence sales may prevent or delay entry into a market or prevent or delay the introduction, or require the reformulation of certain of our products. In addition, no prediction can be made as to whether new domestic or foreign legislation regulating our activities will be enacted. Any new legislation could have a material adverse effect on our business, financial condition and operations. Non-compliance with any applicable requirements may subject us or the manufacturers of our products to agency action, including warning letters, fines, product recalls, seizures and injunctions.
The manufacturing, processing, formulation, packaging, labeling and advertising of our products are subject to regulation by several federal agencies, including (i) the FDA, (ii) the Federal Trade Commission (“FTC”), (iii) the Consumer Product Safety Commission, (iv) the United States Department of Agriculture, (v) the United States Postal Service, (vi) the United States Environmental Protection Agency and (vii) the United States Occupational Safety and Health Administration.
In addition to OTC healthcare products and prescription drug products, the FDA regulates the safety, manufacturing, labeling and distribution of dietary supplements, including vitamins, minerals and herbs, food additives, food supplements, over-the-counter and prescription drugs and cosmetics. The FTC also has overlapping jurisdiction with the FDA to regulate the promotion and advertising of vitamins, over-the-counter drugs, cosmetics and foods. In addition, our products, ProlongzTM and TBX-FreeTM are considered homeopathic remedies, which are subject to standards established by the Homeopathic Pharmacopoeia of the United States (“HPUS”). HPUS sets the standards for source, composition and preparation of homeopathic remedies which are officially recognized under the Federal Food, Drug and Cosmetics Act, as amended.
| 9 |
Preclinical development, clinical trials, product manufacturing, labeling, distribution and marketing of potential new products are also subject to federal and state regulation in the United States and other countries. Clinical trials and product marketing and manufacturing are subject to the rigorous review and approval processes of the FDA and foreign regulatory authorities. To obtain approval of a new drug product, a company must demonstrate through adequate and well-controlled clinical trials that the drug product is safe and effective for its intended use. Obtaining FDA and other required regulatory approvals is lengthy and expensive. Typically, obtaining regulatory approval for pharmaceutical products requires substantial resources and takes several years. The length of this process depends on the type, complexity and novelty of the product and the nature of the disease or other indication to be treated. Preclinical studies must comply with FDA regulations. Clinical trials must also comply with FDA regulations to ensure safety of the human subjects in the trial and may require large numbers of test subjects, complex protocols and possibly lengthy follow-up periods. Consequently, satisfaction of government regulations may take several years: may cause delays in introducing potential new products for considerable periods of time and may require imposing costly procedures upon our activities. If regulatory approval of new products is not obtained in a timely manner or not at all, we could be materially adversely affected. Even if regulatory approval of new products is obtained, such approval may impose limitations on the indicated uses for which the products may be marketed which could also materially adversely affect our business, financial condition and future operations.
Consumers’ ability to successfully file a complaint against us and our products may be greater due to the homeopathic status of our products.
We market various OTC homeopathic drug products. Homeopathic drugs have a unique status under the FDCA because, unlike other drugs, FDA does not evaluate Homeopathic drugs for safety or efficacy prior to marketing. Instead, Homeopathic drugs must meet the standards of strength, quality, and purity set forth in the Homeopathic Pharmacopeia of the United States (“HPUS”). FDA has established a policy addressing the lawful sale of Homeopathic drugs under the FDC Act. See Compliance Policy Guide (“CPG”) 7132.15, “Conditions Under Which Homeopathic Drugs May Be Marketed,” CPG Manual § 400.400 (revised March 1995). Under this compliance policy, FDA generally exempts a homeopathic drug from regulation as a new drug if: the active ingredient is the subject of a HPUS monograph; the product does not include non-homeopathic active ingredients; the product is homeopathically prepared; the claims (indications) are consistent with homeopathic usage for the active ingredient(s) in the product, as described in a recognized “material medical” and the OTC homeopathic drug product is intended solely for self-limiting diseases amenable to self-diagnosis and treatment by consumers. CPG 7132.15. In contrast, the FTC treats Homeopathic drugs similar to other OTC drugs.
In recent years, state courts have concluded that, because Homeopathic drugs are not approved or marketed pursuant to an FDA regulation, claims against a manufacturer of a homeopathic drug are not preempted by the FDCA. Consequently, plaintiff's actions under state consumer protection laws for lack of substantiation have been allowed to proceed. Ignoring the unique character of homeopathic drug products, plaintiff's claims in these actions have been based on the evidence standard applied to conventional drugs. Generally, these actions involve claims for significant monetary damages.
The implementation of new regulations governing the marketing and sale of nutritional supplements could harm our business.
There has been an increasing movement in the U.S. and other jurisdictions to increase the regulation of supplements, which could impose additional restrictions or requirements on the marketing and sale of such products. For example, in the U.S., there has been a push to increase the FDA’s regulatory authority of nutritional supplements. Our business could be harmed if more restrictive legislation is successfully introduced and adopted in the future. Currently, our OTC healthcare product, life science, and homeopathic market product are not categorized as supplements. Nutritional supplement products are not subject to pre-market FDA approval. If regulations are adopted to require pre-market approval supplements or ingredients, our sale and release of new product could be delayed or inhibited. The adoption of similar laws in other countries in which we intend to expand sales of our OTC healthcare product could also harm our business. The FTC approved revisions to its Guides Concerning the Use of Endorsements and Testimonials in Advertising (“Guides”) in December 2009. The Guides state that advertisements that feature a consumer and his or her atypical experience with a product must clearly disclose the results that consumers generally can expect with such product. In addition, the Guides require disclosure of any material connections between an endorser and the company whose products he or she is endorsing. If we fail to comply with the Guides, the FTC could bring an enforcement action against us and we could be fined and/or forced to alter our marketing strategy.
Governmental regulation of Internet-based commerce and the collection and use of personal information may adversely affect the growth of our business or hinder our marketing efforts.
Any new law or regulation, or the application or interpretation of existing laws, regarding Internet-based commerce may decrease the growth in the use of the Internet-based commerce. Governmental regulation of Internet-based commerce continues to evolve in areas such as taxation, privacy, data protection, copyrights, patents, mobile communications and the provision of online payment services. We expect there will be an increasing number of laws and regulations implemented that govern Internet-based commerce, both in the United States and abroad. Unfavorable changes to regulations in these areas could harm our business.
| 10 |
The FTC has regulations regarding the collection and use of personal identifying information obtained from individuals when accessing websites, with particular emphasis on access by minors. In addition, other governmental authorities have regulations governing the collection and use of personal information that may be obtained from customers or visitors to websites. These regulations include requirements that procedures be established to disclose and notify users of our websites of our privacy and security policies, obtain consent from users for collection and use of personal information and provide users with the ability to access, correct or delete personal information stored by us. In addition, the FTC and other governmental authorities have made inquiries and begun investigations of companies’ practices with respect to their users’ personal information collection and dissemination practices to confirm these are consistent with stated privacy policies and to determine whether precautions are taken to secure consumer’s personal information. The FTC and certain state agencies also have made inquiries, and, in a number of situations, brought actions against companies to enforce the privacy policies of these companies, including policies relating to security of consumers' personal information. If we become a party to a similar investigation or become the subject of the FTC’s regulatory and enforcement efforts or those of other governmental bodies, our ability to collect demographic and personal information from users may be adversely affected, which could harm our marketing efforts.
Our third party manufacturers’ failure to comply with good manufacturing practices could harm our business operations.
All manufacturers and suppliers of OTC products must comply with applicable current good manufacturing practice, or cGMP, regulations for the manufacture of our nutritional supplement products, which are enforced by the FDA through its facilities inspection program. The FDA may conduct inspections of our third party manufacturers to assure they are in compliance with such regulations. These cGMP requirements include quality control, quality assurance and the maintenance of records and documentation, among other items. Our manufacturers may be unable to comply with these cGMP requirements and with other regulatory requirements. A failure to comply with these requirements may result in fines, product recalls or seizures and related publicity requirements, injunctions, total or partial suspension of production, civil penalties, warning or untitled letters, import or export bans or restrictions, and criminal prosecution and penalties. Any of these penalties could delay or prevent the promotion, marketing or sale of our products. If the safety of any products supplied to us is compromised due to a third party manufacturer’s failure to adhere to applicable laws or for other reasons, we may not be able to successfully sell our products. We cannot assure you that our third-party manufacturers will continue to reliably supply products to us at the levels of quality, or the quantities, we require, and in compliance with applicable laws and regulations, including cGMP requirements.
Our manufacturing operations are located in China, which exposes us to risks associated with doing business in that geographic area to include foreign currency risk.
Currently, Prolongz and TBX-Free are produced at a FDA-registered third party manufacturer in China. Our manufacturing operations in China could be adversely affected by changes in the interpretation and enforcement of legal standards, by strains on China's available labor pool, changes in labor costs and other employment dynamics, high turnover among Chinese employees, communications, trade, and other infrastructures, by natural disasters, by conflicts or disagreements between China and the United States, by labor unrest, and by other trade customs and practices that are dissimilar to those in the United States. Interpretation and enforcement of China's laws and regulations continue to evolve and we expect differences in interpretation and enforcement to continue in the foreseeable future.
Further, we may be exposed to fluctuations in the value of the local currency in the countries in which manufacturing occurs. Future appreciation of these local currencies could increase our component and other raw material costs. In addition, our labor costs could continue to rise as wage rates increase and the available labor pool declines. These conditions could adversely affect our gross margins and financial results.
In the event of a disruption of this facility, we would need to outsource to other third parties, at least temporarily, our manufacturing. While such secondary sources have been identified for our products, if we are unable to find other sources or there were a delay in the ramp-up for the production and distribution operations for some of our products, it could have a material adverse effect on our operations.
Our inability to find alternative sources for our manufacturing needs may have a material adverse effect on our operations and financial condition. In addition, the terms on which manufacturers and suppliers will make products and raw materials available to us could have a material effect on our success.
A deterioration in trade relations between U.S. and China could negatively impact our inventory production capacity.
The majority of components for our product line, including bottles, caps, boxes and labels, are purchased from manufacturers based in China. Any deterioration in relations between the U.S. and China could adversely affect our ability to continue obtaining such components from our current Chinese suppliers or cause delays in obtaining shipments from such suppliers. Delays in obtaining such items could cause delays in fulfilling orders which could negatively affect our reputation and business. While there are alternative domestic sources for the components that we currently buy from China, obtaining components from domestic suppliers could increase costs and negatively affect our results of operations.
| 11 |
Impediments to global shipping lanes can delay crucial deliveries and negatively impact our business, financial condition and results of operations.
Both our receipt of product packaging components and our shipment of finished goods depend heavily on ship cargo container delivery. Threats of dock workers’ strikes highlight our potential vulnerability to shipping interruption. Any shipment delays in obtaining our product packaging or shipping our finished products to our customers could negatively impact our business, financial condition and results of operations.
We are uncertain as to whether we can protect our proprietary rights.
The strength of our patent position and proprietary formulations and compounds may be important to our long-term success. We currently have applied for several U.S. patents in connection with ProlongzTM ; however there can be no assurance that these patents and proprietary formulations and compounds will effectively protect our products from duplication by others. In addition, we may not be able to afford the expense of any litigation which may be necessary to enforce our rights under any of the patents. Furthermore, there can be no assurance that third parties will not obtain access to or independently develop our technologies, know-how, ideas, concepts and documentation, which could have a material adverse effect on our financial condition.
Although we believe that current and future products do not and will not infringe upon the patents or violate the proprietary rights of others, if any of our current or future products do infringe upon the patents or proprietary rights of others, we may have to modify the products or obtain an additional license for the manufacture and/or sale of such products. We could also be prohibited from selling the infringing products. If we were found to infringe on the proprietary rights of others, it is uncertain whether we would be able to take corrective actions in a timely manner, upon acceptable terms and conditions, or at all, and the failure to do so could have a material adverse effect upon our business, financial condition and operations.
We may in the future file patent litigation claims in the U.S. and foreign jurisdictions to protect our patent portfolio. If we are unsuccessful in these claims, our business, financial condition and results of operations could be adversely affected.
We may initiate litigation to assert claims of infringement, enforce our patents, protect our trade secrets or know-how, or determine the enforceability, scope and validity of the proprietary rights of others. Any lawsuits that we initiate could be expensive, time consuming and divert management’s attention from other business concerns. Furthermore, litigation may provoke third parties to assert claims against us and may put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not being issued.
In addition, we may not prevail in lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially valuable. The occurrence of any of these events may have a material adverse effect on our business, financial condition, and results of operations.
If our patents and other intellectual property rights do not adequately protect our products, we may lose market share to our competitors and be unable to operate our business profitably.
Patents and other proprietary rights may be essential to our business, and our ability to compete effectively with other companies depends on the proprietary nature of our technologies. We also rely upon trade secrets, know-how, continuing technological innovations and licensing opportunities to develop, maintain and strengthen our competitive position. We seek to protect these, in part, through confidentiality agreements with certain employees, consultants and other parties. We plan to pursue a policy of generally obtaining patent protection in both the United States and key foreign countries for patentable subject matter in our proprietary products and also attempt to review third-party patents and patent applications to the extent publicly available to develop an effective patent strategy, avoid infringement of third-party patents, identify licensing opportunities and monitor the patent claims of others. We expect our patent portfolio to initially include an exclusive license to one pending patent application. We cannot assure you that any pending or future patent applications will result in issued patents, that any current or future patents issued or licensed to us will not be challenged, invalidated or circumvented or that the rights granted thereunder will provide a competitive advantage to us or prevent competitors from entering markets that we currently serve. Any required license may not be available to us on acceptable terms, if at all. In addition, some licenses may be non-exclusive, and therefore our competitors may have access to the same technologies as we do. Furthermore, we may have to take legal action in the future to protect our trade secrets or know-how, or to defend them against claimed infringement of the rights of others. Any legal action of that type could be costly and time-consuming to us, and we cannot assure you that such actions will be successful. The invalidation of key patents or proprietary rights that we own or unsuccessful outcomes in lawsuits to protect our intellectual property may have a material adverse effect on our business, financial condition, and results of operations.
The laws of foreign countries may not protect our intellectual property rights to the same extent as the laws of the United States. If we cannot adequately protect our intellectual property rights in these foreign countries, our competitors may be able to compete more directly with us, which could adversely affect our competitive position and, as a result, our business, financial condition and results of operations.
| 12 |
Our existing product and potential new products expose us to potential product liability claims.
Our business results in exposure to an inherent risk of potential product liability claims, including claims for serious bodily injury or death caused by the sales of our existing product and the products which are being developed. These claims could lead to substantial damage awards. While we currently maintain product liability insurance, a successful claim brought against us in excess of, or outside of, existing insurance coverage could have a material adverse effect on our results of operations and financial condition. Claims against us, regardless of their merit or eventual outcome, may also have a material adverse effect on the consumer demand for our products.
The adult nature of some of our current product may prevent some companies from doing business with us.
Some companies we seek to provide products and services to us may be concerned that associating with our company due to the adult nature of our current product may prevent them from doing business with us. These companies may be reluctant to enter into or continue business relationships with us. We cannot assure you that we will be able to maintain our existing business relationships with the companies that currently provide services and products to us. We could be forced to enter into business arrangements on terms less favorable to us than we might otherwise obtain, which could lead to higher costs. If we are unable to maintain our existing business relationships or enter into business relationships with other product and service providers in the future, our business, financial condition and results of operations may be materially adversely affected.
If we experience product recalls, we may incur significant and unexpected costs, and our business reputation could be adversely affected.
We may be exposed to product recalls and adverse public relations if our products are alleged to cause injury or illness, or if we are alleged to have violated governmental regulations. A product recall could result in substantial and unexpected expenditures, which would reduce operating profit and cash flow. In addition, a product recall may require significant management attention. Product recalls may hurt the value of our brands and lead to decreased demand for our products. Product recalls also may lead to increased scrutiny by federal, state or international regulatory agencies of our operations and increased litigation and could have a material adverse effect on our business, results of operations, financial condition and cash flows.
We are highly dependent upon consumers' perception of the safety and quality of our products as well as similar products distributed by other companies in our industry; adverse publicity and negative public perception regarding particular ingredients or products or our industry in general could limit our ability to increase revenue and grow our business.
Decisions about purchasing made by consumers of our products may be affected by adverse publicity or negative public perception regarding particular ingredients or products or our industry in general. This negative public perception may include publicity regarding the legality or quality of particular ingredients or products in general or of other companies or our products or ingredients specifically. Negative public perception may also arise from regulatory investigations, regardless of whether those investigations involve us. We are highly dependent upon consumers' perception of the safety and quality of our products as well as similar products distributed by other companies. Thus, the mere publication of reports asserting that such products may be harmful could have a material adverse effect on us, regardless of whether these reports are scientifically supported. Publicity related to nutritional supplements and OTC healthcare and wellness products may also result in increased regulatory scrutiny of our industry and/or the healthy foods channel. Adverse publicity may have a material adverse effect on our business, financial condition, and results of operations. There can be no assurance of future favorable scientific results and media attention or of the absence of unfavorable or inconsistent findings.
We face intense competition from competitors that are larger, more established and that possess greater resources than we do, and if we are unable to compete effectively, we may be unable to maintain sufficient market share to sustain profitability.
Numerous manufacturers and retailers compete actively for consumers. There can be no assurance that we will be able to compete in this intensely competitive environment. In addition, nutritional supplements and OTC healthcare and wellness products can be purchased in a wide variety of channels of distribution. These channels include mass market retail stores and the Internet. Because these markets generally have low barriers to entry, additional competitors could enter the market at any time. Private label products of our customers also provide competition to our products. Additional national or international companies may seek in the future to enter or to increase their presence in the healthy foods channel or the vitamin, mineral supplement market, and OTC healthcare and wellness products. Increased competition in either or both could have a material adverse effect on us.
| 13 |
If our current and planned sales, marketing and servicing capabilities or entry into arrangements with third parties to sell and market our products and services adversely changes, our business may be harmed.
Currently, we sell our products to a few chain retail stores and through direct sales. We plan to distribute our products to more retail stores and and utilize various approaches for marketing and distribution of our products and services. If we develop our own marketing and sales capabilities, our sales force will be competing with the experienced and well-funded marketing and sales operations of our competitors. Developing a sales force is expensive and time-consuming and could delay or limit the success of any product launch. We may not be able to develop this capacity on a timely basis or at all. If we contract with third parties to market and sell our products and services, our revenues could be lower than if we directly marketed and sold our products and services. Furthermore, to the extent that we enter into co-promotion or other marketing and sales arrangements with other companies, any revenues received will depend on the skills and efforts of others, and we do not know whether these effors will be successful. Some of our future distributors may have products or product candidates that compete with ours, and they may have an incentive not to devote sufficient efforts to marketing our products. If we are unable to establish and maintain adequate sales, marketing and distribution capabilities, independently or with others, we may not be able to generate product revenue and may not become profitable.
There can be no assurance as to how much revenue our business will generate, and we may need substantial additional funding to support the business. We may be unable to raise capital when needed, which would force us to delay, reduce or eliminate our product development programs and acquisitions.
Even though we intend to begin our operations shortly after closing this offering, there is no assurance as to how much revenue the business will generate, and we may need to raise substantial additional capital to develop new products and services and establish sales operations or distribution relationships.
We believe that the maximum net proceeds from this offering will be sufficient to meet our anticipated cash requirements to execute our business plan through 2015. However, our future funding requirements will depend on many factors, including:
| ● | whether we are able to complete this offering; | |
| ● | the scope, rate of progress and cost of our development activities; | |
| ● | the cost of defending, in litigation or otherwise, any claims that we infringe third party patents or other intellectual property rights; | |
| ● | the cost and timing of regulatory approvals; | |
| ● | the cost and timing of establishing sales, marketing and distribution capabilities; | |
| ● | the cost of establishing clinical and commercial supplies of our product candidates and any products that we may develop; and, | |
| ● | the effect of competing technological and market developments. |
Until we can generate a sufficient amount of revenue, if ever, we expect to finance future cash needs through private equity offerings, debt financings, corporate collaborations, and licensing arrangements. If we raise additional funds by issuing equity securities, our stockholders may experience dilution. Any debt financing or additional equity that we raise may contain terms that are not favorable to our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish some rights to our technologies or our product candidates, or grant licenses on terms that are not favorable to the Company. If we are unable to raise adequate funds, we may have to liquidate some or all of our assets or delay, reduce the scope of or eliminate some or all of our development programs.
Failure to protect against security breaches and inappropriate use by Internet users could adversely affect our company.
We collect and retain a large amount of internal and customer data, including credit card numbers and other personally identifiable information of our customers, as well as personally identifiable information about our employees. The security of this data may potentially be breached due to a number of risks, including cyber-attack, system failure, human error, computer virus, or unauthorized or fraudulent use by customers or company employees. Any failure on our part to effectively prevent security breaches could significantly harm our business, reputation and results of operations and could expose us to lawsuits by state and federal consumer protection agencies, by governmental authorities in the jurisdictions in which we operate, and by consumers. Anyone who is able to circumvent our security measures could misappropriate proprietary information, including customer credit card and personal data, cause interruptions in our operations or damage our brand and reputation. Such breach of our security measures could involve the disclosure of personally identifiable information and could expose us to a material risk of litigation, liability or governmental enforcement proceedings. We cannot assure you that our systems are completely secure from security breaches or sabotage. We may be required to incur significant additional costs to protect against security breaches or to alleviate problems caused by such breaches. Any well-publicized compromise of our security or the security of any other Internet provider could deter people from purchasing our products, which could adversely affect our sales and results of operations.
Computer viruses may cause delays or other service interruptions, which may materially adversely affect our ability to operate our business and result in damage to our reputation. If a computer virus affecting the Internet in general is highly publicized or particularly damaging, our customers may not use the Internet or may be prevented from using the Internet to access our Wellness Store, which would have an adverse effect on sales of our products. The inadvertent transmission of computer viruses could also expose us to a material risk of loss or litigation and possible liability. The Company may be required to expend capital and resources to protect against or alleviate system failures or disruptions, which could negatively affect our results of operations.
| 14 |
Credit card and debit card fraud and other fraud could adversely affect our business.
Our consumer customers typically pay for their online orders with debit or credit cards. Our revenues and gross margins could decrease if we experienced significant credit card and debit card fraud. Failure to adequately detect and avoid fraudulent credit card and debit card transactions could cause us to lose our ability to accept credit cards or debit cards as forms of payment and/or result in charge-backs of the fraudulently charged amounts and/or significantly decrease revenues. Furthermore, widespread credit card and debit card fraud may lessen our customers’ willingness to purchase products, which could materially adversely affect our sales, financial condition and results of operations.
We have material weaknesses in our internal control over financial reporting which could adversely affect our ability to report our financial condition and results of operations accurately and on a timely basis.
We currently have material weaknesses in our internal control over financial reporting that could adversely impact our ability to provide timely and accurate financial information. We are developing remediation plans to strengthen our internal controls in response to the previously identified material weaknesses. If we are unsuccessful in developing, implementing or following our remediation plans, or fail to update our internal controls as our business evolves, we may not be able to timely or accurately report our financial condition, results of operations or cash flows or maintain effective disclosure controls and procedures. If we are unable to report financial information timely and accurately or to maintain effective disclosure controls and procedures, we could be subject to, among other things, regulatory or enforcement actions by the SEC, securities litigation and a general loss of investor confidence, any one of which could adversely affect our business prospects and the valuation of our common stock.
Furthermore, there are inherent limitations to the effectiveness of any system of controls and procedures, including the possibility of human error and the circumvention or overriding of the controls and procedures. We could face additional litigation exposure and a greater likelihood of an SEC enforcement regulatory action if further restatements were to occur or other accounting-related problems emerge.
Risks Related to Our Securities and
to This Offering
The outstanding warrants may adversely affect us in the future and cause dilution to existing stockholders.
Currently, there are outstanding warrants to purchase up to 1,707,127 shares of Common Stock. The terms of these warrants expire as early as January 2018 and as late December, 2018. The exercise price of these warrants range from $2.25 per share to $2.75 per share, subject to adjustment in certain circumstances. Exercise of the warrants may cause dilution in the interests of other shareholders as a result of the additional Common Stock that would be issued upon exercise. In addition, sales of the shares of our Common Stock issuable upon exercise of the warrants could have a depressive effect on the price of our stock, particularly if there is not a coinciding increase in demand by purchasers of our Common Stock. Further, the terms on which we may obtain additional financing during the period any of the warrants remain outstanding may be adversely affected by the existence of these warrants as well.
We may in the future issue additional shares of our Common Stock which would reduce investors’ ownership interests in the Company and which may dilute our share value.
Our Certificate of Incorporation, and any amendments thereto, authorize the issuance of 50,000,000 shares of Common Stock, par value $0.01 per share. The future issuance of all or part of our remaining authorized Common Stock may result in substantial dilution in the percentage of our Common Stock held by our then existing stockholders. Pursuant to the Bridge Financing and this Offering, additional shares of common stock may be issuable under certain conditions. If we sell securities at a price lower than the conversion price or exercise price of the Bridge Notes and Bridge Warrants, respectively, we will be required to issue additional shares pursuant to the anti-dilution provision provided therein; in fact, upon the closing of this Offering, the exercise price of the Bridge Warrants will be reduced and result in the potential issuance of up to an additional aggregate of 11,421 shares of common stock pursuant thereto. Additionally, if we do not meet certain revenue requirements, we will be required to issue additional shares of common stock (See, Terms of Offering and Description of Securities). We may value any Common Stock issued in the future on an arbitrary basis. The issuance of Common Stock for future services or acquisitions or other corporate actions may have the effect of diluting the value of the shares held by our investors, and might have an adverse effect on any trading market for our Common Stock.
Our charter documents may have anti-takeover effects that could prevent a change in control.
Our Certificate of Incorporation or our bylaws could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. We are authorized to issue up to 10,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. No preferred stock is currently outstanding. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Our certificate of incorporation and bylaws also contain provisions that could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. In particular, the articles of incorporation and bylaws, as applicable, among other things:
| ● | provide the board of directors with the ability to alter the bylaws without stockholder approval; and |
| ● | provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum. |
| 15 |
These provisions are expected to discourage certain types of coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of our company to first negotiate with its Board. These provisions may delay or prevent someone from acquiring or merging with us, which may cause the market price of our common stock to decline.
Absence of Public Market.
There has never been any established “public market” for shares of Common Stock of the Company, and no assurance can be given that any market for the Company's Common Stock will be developed or maintained. If a public market ever develops in the future, the sale of “unregistered” and “restricted” shares of Common Stock pursuant to Rule 144 of the Act, may adversely affect the market price, if any, for the Company's shares and could impair the Company's ability to raise capital through the sale of its equity securities. In order to qualify for the resale of Common Stock under Rule 144, certain holding periods must be met and either a legal opinion setting forth exemptions from the Act must be provided or a registration statement must be in effect relating to such Common Stock. Further, there is no assurance that Rule 144 will be applicable to the Company and investors may not be able to rely on its provisions now or in the future.
If a market for our Common Stock does not develop, shareholders may be unable to sell their shares.
We are currently a privately held company. A market for our Common Stock may never develop. While we intend to become a SEC reporting company and list our Common Stock on a national securities exchange or quotation system at such time as we meet the initial listing criteria, there is no guarantee that we will become an SEC reporting company or that our shares will be traded or, if traded, that a public market will materialize. If our Common Stock is not traded on a national securities exchange or quotation system or if a public market for our Common Stock does not develop, investors may not be able to re-sell their shares of Common Stock and may lose all of their investment.
The Company’s Common Stock may be deemed “penny stock”, which would make it more difficult for investors to sell their shares.
If a market develops and the price of the Company’s stock is below $5.00 per share, or the Company does not have $2,000,000 in net tangible assets, or is not listed on an exchange or on the NASDAQ National Market System, among other conditions, the Company’s shares may be subject to a rule promulgated by the SEC that imposes additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and institutional accredited investors. For transactions covered by the rule, the broker-dealer must make a special suitability determination for the Subscriber and receive the Subscriber’s written consent to the transaction prior to the sale. Furthermore, if the price of the Company’s stock is below $5.00, and does not meet the conditions set forth above, sales of the Company’s stock in the secondary market will be subject to certain additional new rules promulgated by the SEC. These rules generally require, among other things, that brokers engaged in secondary trading of stock provide customers with written disclosure documents, monthly statements of the market value of penny stocks, disclosure of the bid and asked prices, and disclosure of the compensation to the broker-dealers and disclosure of the sales person working for the broker-dealer. These rules and regulations may affect the ability of broker-dealers to sell the Company’s securities, thereby limiting the liquidity of the Company’s securities. They may also affect the ability of Subscribers in the Offering to resell their Securities in the secondary market.
If we do not timely file and have declared effective the registration statement to register the shares being offered by the selling stockholders named herein, we will be subject to liquidated damages.
Pursuant to the Offering and Bridge Financings, we entered into a registration rights agreement to register all of the shares being offered by the selling stockholders named in this prospectus. We are obligated to file this registration statement by the 30th day after the Offering closes and have this registration statement declared effective by the SEC no later than the earlier of (a) three (3) days after oral or written notice by the SEC that it may be declared effective or (b) the earlier of the ninetieth (90th) day following the later to occur of (i) the filing date or (ii) in the event the registration statement receives a “full review” by the Commission, the one hundred twentieth (120th) day following the filing date. We must keep this registration statement continuously effective until all of the securities covered by it have been sold pursuant to it or until all of the securities registered herein may be sold without registration under Rule 144 of the Securities Act. Although we believe that we and our advisors will be able to take all steps necessary to timely file and maintain the effectiveness of the registration statement, it may be impractical for us to respond to the SEC in a manner that permits us to have the registration statement declared effective in the time periods agreed. If we do not meet these timelines, then we shall pay each Investor liquidated damages in cash equal to 1% of such Investor’s purchase price to be incurred each month until the registration statement is filed or declared effective, as the case may be, but in no event more than 10%.
Our management team owns a significant percentage of our stock and will be able to exercise significant influence over our affairs.
Upon the closing of this offering the same group will continue to beneficially hold at least a majority of our voting stock. Even after this offering, these stockholders will be able to influence the composition of our board of directors, retain the voting power to approve all matters requiring stockholder approval and continue to have significant influence over our operations. The interests of these stockholders may be different from the interests of other stockholders on these matters. This concentration of ownership could also have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could reduce the value of our stock.
Failure to Pay Dividends.
Redwood has not paid cash dividends to its Common Shareholders in the past. There is no assurance that Redwood will pay dividends in the future and we do not intend to declare or pay cash dividends to our common shareholders in the foreseeable future. Earnings, if any, are expected to be retained to finance and expand its business.
| 16 |
SELECTED FINANCIAL INFORMATION
The selected financial information set forth below is derived from our statement of operations for the reviewed financial statements for our nine months ended September 30, 2015 and 2014, which appears elsewhere in this prospectus commencing on page 8 and which include footnotes required under generally accepted accounting principles. Prospective investors are urged to carefully review such detailed financial statements in their entirety.
| For the Nine Months Ended September 30, 2015 | For Nine Months Ended
September 30, 2014 | |||||||
| Sales, net | $ | 5,143,405 | $ | 2,396,730 | ||||
| Cost of sales | 1,033,744 | 712,121 | ||||||
| Gross profit | 4,109,661 | 1,684,609 | ||||||
| Operating expenses | ||||||||
| General and administrative expenses | 2,897,619 | 955,186 | ||||||
| Payroll, benefits and related | 540,039 | 465,000 | ||||||
| Rents and occupancy | 163,766 | 124,324 | ||||||
| Bad debt | 1,207,923 | 0 | ||||||
| Total operating expenses | 4,809,347 | 1,544,510 | ||||||
| Income (loss) from operations | (699,686 | ) | 140,099 | |||||
We have registered these shares because of registration rights granted to the Investors in our recent private financings and the other selling shareholders. We will not receive any proceeds upon the conversion of the Bridge Notes into shares of our common stock, however, we received net proceeds of approximately $4,941,252 from the initial sale of all of the Units issued pursuant to this Offering and the Bridge Financing and we could receive up to approximately $4,074,489, net of fees and expenses, from the exercise of the Warrants issued in connection with this financing and the Bridge Financing, when and if exercised. The net proceeds from the issuance of the Units and any proceeds received from the exercise of the Warrants have been and will be used as set forth in the table below.
| Use of Proceeds | Amount | Percentage | ||||||
| Key Hires and Working Capital | $ | 203,724 | 5.00 | % | ||||
| Direct Response Television Advertising | $ | 3,246,145 | 79.67 | % | ||||
| Transaction Fees | $ | 488,939 | 12.00 | % | ||||
| General Corporate Purposes | $ | 135,680 | 3.33 | % | ||||
| Total Use of Proceeds | $ | 4,074,489 | 100.00 | % | ||||
| 17 |
MARKET FOR OUR COMMON STOCK, DIVIDENDS AND
RELATED STOCKHOLDER INFORMATION
As of the date of this Statement, there is no public market for the Company’s common stock. This Registration Statement is a step toward creating a public market for our stock. However, there can be no assurance that a meaningful trading market will develop. The Company and its management make no representation about the present or future value of our common stock.
As of the date of this Registration Statement:
| 1. | There are 1,707,127 outstanding options or warrants to purchase, or other instruments convertible into, common equity of the Company; | |
| 2. | There are currently 0 shares of our common stock eligible for sale pursuant to Rule 144; and, | |
| 3. | Other than the stock registered under this Registration Statement, there is no stock that has been proposed to be publicly offered resulting in dilution to the current shareholders or that could have a material effect on the market price of our common equity. |
Record Holders. As of February 12, 2016, there were approximately 88 record holders of our common stock.
Dividend Policy. We have neither declared nor paid any cash dividends on either preferred or common stock. For the foreseeable future, we intend to retain any earnings to finance the development and expansion of our business and do not anticipate paying any cash dividends on our preferred or common stock. Any future determination to pay dividends will be at the discretion of the Board of Directors and will be dependent upon then existing conditions, including its financial condition, results of operations, capital requirements, contractual restrictions, business prospects, and other factors that the Board of Directors considers relevant
Securities Authorized for Issuance under Equity Compensation Plans. As of the date of this Registration Statement, the Company does not have any equity compensation plans or any individual compensation arrangements with respect to its common stock. However, the is formulating an Incentive Plan with a pool of up to 250,000 shares. The issuance of any of our common stock is within the discretion of our Board of Directors, which has the power to issue any or all of our authorized but unissued shares without stockholder approval.
| 18 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and result of operations contains forward-looking statements and involves numerous risks and uncertainties, including, but not limited to, those described in the "Risk Factors" section of the other reports we file with the Securities and Exchange Commission. Actual results may differ materially from those contained in any forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential” or “continue,” the negative of such terms or other comparable terminology. These statements are only predictions.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Moreover, neither we, nor any other person, assume responsibility for the accuracy and completeness of the forward-looking statements. We are under no obligation to update any of the forward-looking statements after the filing of this Quarterly Report to conform such statements to actual results or to changes in our expectations.
The following discussion and analysis of financial condition and results of operations relates to the operations and financial condition reported in the financial statements of Redwood Scientific Technologies, Inc. for the year ended December 31, 2014 and for the interim period ending September 30, 2015, and should be read in conjunction with such financial statements and related notes included in this report.
Overview
Our Chief Executive Officer founded our operations in November 2013. Since first introducing its flagship product, Prolongz, our company has helped over 100,000 customers with challenges of premature ejaculation. The Company is based in Claremont, California. A second product TBX-Free was launched in December 2015. Prolongz and TBX-Free are marketed on national television as the world’s first sublingual mouth strip products for the treatment of premature ejaculation and smoking cessation respectively.
We specialize in the development of sublingual strip technologies that address health and wellness concerns of the consumer. The company initially launched, Prolongz a male sexual performance enhancement “Over-The-Counter” (“OTC”) drug. In December of 2015, the Company introduced its second FDA registered product called TBX-Free. TBX-FREE is the first FDA registered oral strip that helps smokers safely, quickly, and effectively eliminate the craving to smoke. We have been aggressively expanding our business throughout the United States through large investment in TV infomercials and Internet marketing of the product. The infomercials serve as a venue to raise awareness of the product’s health and wellness benefits, and as a lead generator producing thousands of phone calls and purchases. Once consumer awareness of the Prolongz and TBX-Free product is initiated through the Company’s infomercials, RST’s website www.prolongz.com and www.tbxfree.com then becomes the consumer’s source for more information regarding the product, its ingredients, benefits as well as feedback from other subscribers of Prolongz and TBX-Free. We also have an in-house team of customer representatives that cater to the customers’ needs. The Company takes great pride in its company’s products being the only Over-The-Counter drug registered with the FDA through sublingual strip technology..
Plan of Operations
The Company is actively engaged with introducing in the next six to twelve months its products to significant volume national retail chains such as Walmart, Walgreens, Rite Aid and Kroger. The Company has consistently ranked in the top three infomercials on the Jordan Whitney report throughout the year which will facilitate its launch with these retail chains.
Our mission is to be at the forefront of the fast-growing category of sublingual mouth strip as a method of delivering medication, homeopathic therapy, and other health and wellness products to the consumer.
With this mission in mind, we envision our company becoming the industry leader in sublingual strip delivery technology, starting with the male sexual performance enhancement product, and now the smoking cessation product. We will strive to command the largest share of the market while maintaining its price premium through continued Research and Development of health and wellness products that can be delivered through the sublingual strip delivery technology and advertised using the infomercial type marketing campaigns that develop customer awareness of the our brand.
| 19 |
Results of Operations for the Twelve-month Period Ended December 31, 2014 vs. December 31, 2013
The Company had no active operations for the period ended December 31, 2013.
REVENUES: Since inception and during the development stage, the Company reported $ 3,234,582 revenues from operations for the year ended December 31, 2014.
OPERATING EXPENSES: Total operating expenses were$1,903,702 for the year ended December 31, 2014. The operating expenses included general administrative expenses of $1,095,415, payroll benefits and related expenses of $646,420, and rent and occupancy expenses of $161,867. General and administrative expenses consisted primarily of advertising and media fees of $907,345 or 82.8% of general and administrative expenses.
NET GAIN: The Company incurred a net gain before taxes of $379,204 for the year ended December 31, 2014 based on revenues of $3,234,582, cost of goods sold of $951,676, and total operating expenses of $1,903,702.
Cash flow. Because the Company is “early stage” and launching with a minimum of capital, monitoring cash flow on a constant basis will be essential to growth.
Results of Operations for the Nine-month Period Ending September 30, 2015 vs. September 30, 2014
REVENUES: Net revenues increased $2,746,675 or 115% for the nine month period ending September 30, 2015 to $5,143,405, up from $2,396,730 over the same comparative period for the nine months ending September 30, 2014. Increased sales resulted from increased media expenditures of $1,442,744 to $2,274,231 for the nine month period ending September 30, 2015 up from $831,487 over the same comparative period ending September 30, 2014, an increase of 174%.
OPERATING EXPENSES: Gross margins increased 10% to 80% of sales up from 70% the same comparative period for the nine month period ending September 30, 2014. The latter translated into gross profits of $4,109,661 for the nine month period ending September 30, 2015 compared to $1,684,609 for the nine months ended September 30, 2014, an increase of $2,425,052 or 144%. As volumes increased throughout 2014, the company moved its manufacturing from Classic Imports in China to Dallian SML Health Products LTD resulting in significant cost savings. Cost saving were also achieved because of volume discounts resulting from higher volumes at a more efficient factory. Cost of printing of product boxes were also reduced by 50% resulting in costs staying approximately the same as the previous year period despite doubling of sales. Also included in cost of sales is fulfillment expense relating to computer generated answering service fees. These increased from $135,699 for the nine month period ending September 30, 2014 to $274,700 for the nine month period ended September 30, 2015, and increase of $139,001 an increase of 102% over the same comparative period. The increased answering fees are a result of increased sales generated from the automated service and are a variable cost.
Legal fees increased to $88,000 for the nine months ended September 30, 2015 from $5,000 for the nine months ended September 30, 2014 an increase of $83,000 or 1660%. The increase was in large part as a result of legal fees connected with corporate matters relating to organizational structure, and other matters totaling $48,000 and $30,000 in legal fees incurred relating to the Convertible Note Offering and the Private Placement Memorandum reflecting the company’s capital raise efforts undertaken in 2015. Fees paid to FDA counsel were $10,000 in 2015.
General and administrative expenses increased to $2,897,619 for the nine months ended September 30, 2015 up from $955,186 for the same comparative nine months ended September 30, 2014, an increase of $1,942,433 or 203%. As discussed above $1,455,227 of the increase (or 75% of the increase) is from increased media expenditures in the period January through September 2015 over the same comparative period the year before. Placement Agent fees, not incurred in 2014, totaled $100,154 (or 5.2% of the increase in G&A) in 2015. Increase in legal fees accounted for $83,000 (or 4.27% of the increase in G&A) in 2015 compared to the nine month comparative period in the previous year. Professional fees, including accounting fees increased to $87,190 for the nine months ended September 30, 2015 up from $33,652 for the nine month comparative period ending September 30, 2015. This represents 2.8% of the increase in 2015’s G&A. The latter accounts for 90% of the increase in 2015’s G&A over 2014. Provision for bad debt on uncollectable receivables increased a total of $1,208,000 for 2015 over the previous year’s period.
Payroll expense through the period September 30, 2014 was $465,000. The expense through September 30, 2015 is $540,000 an increase of $75,000 or 16%. The Company added an additional three customer service reps, in-house counsel for 7 months and in-house Controller during this period compared to the same comparative period ending September 30, 2014.
NET LOSS: The net loss from operations for the nine month period ending September 30, 2015 was $699,686 compared to a profit of $140,099 for the same nine month period ending September 30, 2014. Even though gross profits increased by $2,425,052, total operating expenses climbed 211% or $3,264,837 producing a loss of $699,686.
| 20 |
CONTINGENCIES
The Company may be subject to lawsuits, administrative proceedings, regulatory reviews or investigations associated with its business and other matters arising in the normal conduct of its business.
LIQUIDITY AND CAPITAL RESOURCES
Net cash used in operating activities was $1,918,316 resulted from a net loss after tax of ($1,266,934) a use of non-cash in derivative warrant liability of $98,348, a source of cash in amortization of debt discount of $998,344, a use of cash in deferred taxes of $305,936 and a use of non-cash in bad debt expense of $1,207,923, a use of cash in accounts receivable of $2,580,639, a use of cash in retention receivable of $4,998, a use of cash in inventory of $69,028, a use of cash in other assets of $1,500, a source of cash in accounts payable of $8,848, a source of cash in related party accounts payable of $85,606 and a source of cash in accrued liabilities of $234,722. This use of cash in operating activities was financed by cash proceeds from the unit offering as of September 30, 2015 of $1,063,751 less unit offering costs of $123,300, and from cash proceeds from the issuance of convertible note of $1,500,000 less convertible debt offering costs of $100,600 and payments on related party note payable of $12,500 producing an increase of cash for the period of $409,035.
We believe that anticipated cash flows from operations will be insufficient to satisfy our ongoing capital requirements. We are seeking financing in the form of equity capital in order to provide the necessary working capital. Our ability to meet our obligations and continue to operate as a going concern is highly dependent on our ability to obtain additional financing. We cannot predict whether this additional financing will be in the form of equity or debt, or be in another form. We may not be able to obtain the necessary additional capital on a timely basis, on acceptable terms, or at all. In any of these events, we may be unable to implement our current plans which circumstances would have a material adverse effect on our business, prospects, financial conditions and results of operations.
If we are not successful in generating sufficient liquidity from operations or in raising sufficient capital resources, on terms acceptable to us, this could have a material adverse effect on our business, results of operations liquidity and financial condition.
Off-Balance Sheet Arrangements
There are no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
CRITICAL ACCOUNTING POLICIES
Our significant accounting policies are disclosed in Note 1 of our Financial Statements included elsewhere in this Report.
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
During the past two years, there was no disagreement of the type described in paragraph (a)(1)(iv) or any reportable event as described in paragraph (a)(1)(v) of Item 304 of Regulation S-K that is required to be disclosed under this Item 9.
Product / Position – “Sublingual Strip Delivery Technology”
The Prolongz strip is a men's sexual dysfunction OTC drug ‘FDA # 69308-000-01 designed to treat premature ejaculation. The product is ingested via a dissolving strip that is placed on the tongue. The strip’s formula utilizes a blend of ingredients that once ingested react with the chemicals in the “host’s” body that control the 30 major components of sexual excitement. Instructions for using the product direct the “host” to consume one strip twice a day, each day, and once more before each sexual activity is undertaken. The Company’s strip is positioned as the first Over-The-Counter drug ‘FDA #69308-000-01 that addresses premature ejaculation in men”.. The product’s main ingredients include: (1) ginseng – a plant’s root that has long been used in eastern medicines as an aphrodisiac and stimulant as well as cure for sexual dysfunction in males; (2) Damiana – the plant is proven to improve sexual dysfunction in men by retraining the section of the male’s brain that allows control over the involuntary action of release during sexual excitement (http://www.ncbi.nlm.nih.gov/pubmed/10227074).
| 21 |
Drug Fact Panel per the FDA
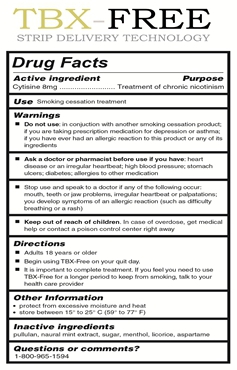 |
 In December of 2015, the Company introduced its second FDA registered product called TBX-Free. TBX-FreeTM (FDA Registration # 69461-001-01) is the first FDA registered oral strip that helps smokers safely, quickly, and effectively eliminate the craving to smoke. TBX-FREE has the ability to help reduce smoking cravings. As a result, smokers may enjoy the freedom to live their lives without the powerful urge to light up cigarettes, cigars, and other tobacco or nicotine products. Every TBX-FREE oral strip contains a precisely measured dose of cytisine; the active ingredient that reduces the urge to smoke. It is too early to assess the long term success of this product however initial sales have beat expectations. |
| 22 |
Thin-film drug delivery has emerged as an innovative alternative to the traditional pill form associated with prescription and over the counter medication. Similar in size, shape and thickness to a postage stamp, thin-film strips are typically designed for oral administration, with the user placing the strip on or under the tongue. These drug delivery options allow the medication to bypass the first pass metabolism thereby making the medication more bioavailable. As the strip dissolves, the drug can enter the blood stream enterically, buccally or sublingually. Evaluating the systemic transmucosal drug delivery, the buccal mucosa is the preferred region as compared to the sublingual mucosa3. The sublingual and buccal delivery of a drug via this patented film product has the potential to improve the onset of action, lower the dosing, and enhance the efficacy and safety profile of the medicament. Other benefits of the sublingual delivery mechanism are4:
| ● | All tablet dosage forms, soft gels and liquid formulations primarily enter the blood stream via the gastrointestinal tract, which subjects the drug to degradation from stomach acid, bile, digestive enzymes and other first-pass effects. As a result, such formulations often require higher doses and generally have a delayed onset of action/reaction. | |
| ● | Conversely, buccal and sublingual thin-film drug delivery mechanisms can avoid these issues and yield quicker onsets of action/reaction at lower doses. This is regulated by the FDA per Code of Federal Regulations under Title 21 “Food and Drugs” §330 (4-14-14 Edition). | |
| ● | Thin-film used in the sublingual delivery mechanism is more stable, durable and quicker dissolving than other conventional dosage forms. | |
| ● | Thin-film used in the sublingual delivery mechanism enables improved dosing accuracy relative to liquid formulations since every strip is manufactured to contain a precise amount of the drug. | |
| ● | Thin-film's ability to dissolve rapidly without the need for water provides an alternative to patients’ with swallowing disorders and to patients suffering from nausea, such as those patients receiving chemotherapy. | |
| ● | Sublingual film delivers a convenient, quick-dissolving therapeutic dose contained within an abuse-deterrent film matrix that cannot be crushed or injected by patients, and rapidly absorbs under the tongue to ensure compliance. | |
| ● | Sublingual film also allows 99% absorption rate compared to normal oral tablets that may have as low as a 5% absorption rate. |

| Figure 2 - Prolongz Strips | Figure 3 – TBX-FREE Strips |
Business Model – Continuity Subscription based Business Model
Presently, the Company’s flagship products are built upon a Continuity Subscription based Business Model, where consumers purchase the first month supply of ProlongzTM for $94.95 and are sold monthly refills at $69.95 and for TBX-FreeTM for $99.85 and $89.95 thereafter. RST solicits consumers to make an initial purchase through a free / deeply discounted offer through its infomercial campaigns. Once the customer completes the initial purchase, they receive a monthly shipment of the company’s products in the mail every 30 days. The Continuity Subscription based Business Model generates revenue on a month by month basis, similar to that of a health club membership; thus allowing Redwood to recoup the “customer acquisition cost” after just one month. The pricing model is a three step trial offer process: (1) The initial 15 day trial offer of the product, with a shipping fee of $4.95 to $9.95 (depending on the product) is charged for the 30 day supply; (2) If the customer does not cancel within the initial trial period, the customer is charged either $69.95 or $89.95 for the first month’s supply, depending on the product; (3) The customers are charged $69.95 or $89.95 for every month thereafter that they remain on the monthly subscription program.
3 Mucosal Delivery of Biopharmaceuticals Biology, Challenges and Strategies
https://books.google.com/books?id=puu3BAAAQBAJ&pg=PA151&lpg=PA151&dq=buccal+mucosa+is+preferred+region&
source=bl&ots=UL1SIQgc0o&sig=FQx0D9ysltCGMOocz15DW_Gw8g4&hl=en&sa=X ei=nQnIVPTUMunZsATt2IKwBA&
ved=0CCwQ6AEwAg#v=onepage&q=buccal%20mucosa%20is%20preferred%20region&f=false
4 See Footnote 1
| 23 |
After the initial trial period, our current retention rate is approximately 65% and we are focusing on how to maintain, or increase it. To that end, we are trying to implement a down-selling system strategy in which our sales representatives offer customers incentives to stay with the program instead of canceling their subscriptions. Incentives may include a reduction on the subscription or an addition complementary item to increase the value of their purchases.
The infomercials used by Company are in either the short form (30-minute duration) or long form (60-minute duration) shown on television and known as paid programming. The Company pre-purchases predetermined time slots on various network and non-network television stations across the United States. The infomercials describe the benefits of the use of the company’s products and include testimonials and witness statements from users of the product.
Retail Sales & Opportunities
Redwood has launched its retail sales channel through its partnership with General Nutrition Corporation (“GNC”). Prolongz is available throughout GNC’s 950+ corporate locations in the US.
Based upon verbal commitments, we expect that by mid fiscal 2016, the company’s products will be available on the shelves of Rite Aid Corporation and will be sold throughout Rite Aid’s 4,600 locations nationwide, allowing more direct access to consumers. The terms and total amount ordered are still under negotiation and therefore there is no guarantee if and when the order will be completed. The Company has also received verbal commitments with Walgreens to provide the product in 8,218 Walgreen locations nationwide by the mid 2016, but no formal agreements have been reached as of the date of this Registration Statement.
Another retail channel that Redwood is currently developing is the convenience store channel nationwide (known as “C-Stores”). The product, Prolongz, is already available throughout Circle K’s West Coast locations. As C-Stores are smaller in size but larger in total number of stores, Redwood will be able to develop a whole new retail distribution channel, increasing revenue. Circle K currently has over 7,300 locations worldwide, which may be very lucrative for Redwood and presents international distribution channel opportunities. Prolongz has developed a 4-day kit specifically for the convenience stores, allowing it to offer consumers a “trial experience” at the retail level.
Our Customers
Redwood is fully engaged in serving and providing its customers with the highest quality product. The Company has as its core value the importance of health and wellness in the lives of every human being and the Company has a core belief that it can play a big role in assisting its customers in addressing their health and wellness concerns. Today, the Company is focused on assisting male customers but it is preparing a full range of products for different health and wellness concerns for future launches that cater to both genders.
Market Analysis
Prolongz is a health and wellness product offering in the adult male sexual performance dysfunction 10 billion dollar market segment. Although the premature ejaculation (“PE”) market is not quantified, the pharmaceutical industry has found the erectile dysfunction (“ED”) market alone to be lucrative, with the top 3 companies posting sales of $3.1B in 2006. Varying estimates show the market growing at a rate of 3-5% per year. http://www.wikinvest.com/ wiki/Erectile_dysfunction. US global homeopathic market which is growing at a rate of 3% per annum reaching $7.5B by 2017 is not reported in these numbers dealing with market size
(http://www.nutraceuticalsworld.com/issues/2013-07/view_industry-news/us-sales-of-homeopathic-herbal-
remedies-reach-64-billion). Since Prolongz is taken sublingually, the Company has obtained a registration number from the FDA listing it as an OTC homeopathic drug. Redwood is capitalizing on the explosive growth in this market, capitalizing on significant profit opportunities for the Company, while helping men everywhere overcome their sexual limitations.
| 24 |
Pricing Model
Given Redwood’s established pricing model, the Company currently has a 80% gross profit margin on the product. The company’s products are priced using the value-based valuation system, where the customers know they are receiving a quality product for a premium price. The main value in the company’s products are that they are effective as a health and wellness remedy. The company’s products hold a premium product position due to their unique strip form delivery technology mechanism; the significant investment in marketing creating brand awareness and cognitive focus on the cure for the problem they treat.
Lifetime Analysis
Currently, both the company’s products are still in their product growth phase; we believe that we can quickly and significantly grow sales volumes through marketing campaigns allowing the Company to capture more market share. Redwood is in a good position to expand its customer base and convert them into loyal, regular and recurring consumers to the brand. The company’s products are in their prime to expand due to:
| ● | A scalable manufacturing and supply chain infrastructure; | |
| ● | First of its kind using a sublingual strip to address Premature Ejaculation in the $10+ Billion sexual dysfunction market; | |
| ● | First of its kind using a sublingual strip to address smoking cessation in the $2.4 billion nicotine replacement therapy market; | |
| ● | Its unique strip form “easy to use” delivery technology mechanism; and, | |
| ● | Its registration as an FDA homeopathic drug. |
Providing Our Product
Supplier
Redwood enjoys a close supplier relationship with an FDA-registered manufacturer in China that produces the product for Redwood. The Company’s ingredients are shipped from a Good Manufacturer Products (“GMP”) certified supplier in the United States to ensure the highest quality. The average lead-time per production batch ranges from five to seven days.
Redwood has been utilizing the “man on the ground” method, whereby the Company employs a manager in China to oversee all suppliers, oversee manufacturing, and shipping relationships as well as communications with the US office. This allows Redwood to maintain a close relationship through a local network, maintain the highest quality control for the product at all times, and lower other risk factors such as loss of communication due to language barrier or time zone differences.
| 25 |
Logistics
Once the product is manufactured in China, Redwood utilizes air shipment for all its Prolongz products through an air freight carrier, which takes approximately 3 days to arrive in the US at the earliest. It often takes approximately 12 to 14 days to clear all custom requirements before Redwood can ship the product to its warehouse for further distribution.
Redwood has also used DHL as another shipping service. This freight company only takes approximately 2 days for the products to arrive in the US. This is a faster method for Redwood to ship product since the service also covers all duty and custom clearance for the shipments. However, DHL costs Redwood almost twice as much per unit shipped to the US, but remains an option for the Company in the event rush shipments are needed.
Warehouse and Fulfillment
The warehouse is an integral part of Redwood’s supply chain as it serves as the central hub for all incoming and outgoing shipments. Once the shipment clears the Customs department, it will arrive at RST’s warehouse within 24 hours. The warehouse crew is responsible for unpacking the incoming as well as packing the outgoing shipments at the warehouse. All the orders are placed through a third party software service called “Ship Works”, which handles incoming orders. Ship Works enables the warehouse to be more efficient since it takes care of all aspects of processing the order, to include printing of shipping labels as well as updating inventory information and sending out tracking information to the customers via email and text alerts. The company’s warehouse crew then simply puts the labels on the packages and USPS and UPS handle the pick-up and delivery process.
Back Office
Another important part of Redwood’s supply chain is its back office operation. The office houses the customer representatives, whom receive calls, up-sell the product, and resolve customers’ inquiries. We strive to achieve additional revenues by selling additional products. The office does not involve directly with daily operations but rather provides support for all field operations. It also provides administrative support for the company such as bookkeeping and placing orders with the manufacturer.
Sales Channels
We rely mainly on two sales channels in order to drive revenues. The company’s main sales channel is Direct Sales using Direct Response Marketing such as commercials and infomercials broadcast on television. The customers are provided with call numbers during the infomercials. Once the customers dial in, an automated system collects the customers’ information (i.e. name, billing address, credit card information, etc.) and in turn sends an order form to the warehouse for fulfillment. The company’s secondary sales channel is its web-based system which is also advertised in the infomercial. Once accessing the Company’s web site, customers can place their free-trial orders through the user-friendly, web-based platform via www.prolongz.com and www.tbxfree.com.
Redwood is also opening the retail sales channel in order to expand its reach into the market and secure its position within. The company will be the supplier of its products to different retailers and utilize their networks of physical stores to promote and increase its sales. Redwood’s retail partners range from drug retailers to convenience stores as well as grocery chains. Redwood is currently providing products to General Nutrition Corporation throughout GNC’s network of 900+ retail locations in the US. This is a good relationship and opportunity for Redwood to gain nationwide recognition and increase revenue stream. Redwood is also available at Circle K’s West Coast locations. These channel partners are an important part to Redwood’s growth in the near future. We are also in the process of extending our reach toward all Circle K locations and negotiating with another nationally recognized convenience store.
Another channel that the company is developing is through the network of drug retailers, i.e. Walgreens and Rite Aid. The Company is working to produce commitments to have its products with Walgreens and RiteAid retailers to sell in stores during mid-2016.
Our expansion plans include launching Prolongz and TBX-Free internationally in the United Kingdom, Central and South America, but no formal agreements are in place to do so at this time.
Intellectual Property
Below is a list of our pending patents and registered trademarks:
| Patent | Trademark Serial # | Description | ||
| #62008368 | Premature Ejaculation Treatment Strips (Prolongz) |
| 26 |
| Serial Number | Reg. Number | Word Mark | ||||
| 1 | 86322301 | 4691599 | PROLONGZ = ADVANCED MEN'S INSTITUTE = "BETHE DRAGON" | |||
| 2 | 86336766 | 4682180 | PROLONGZ | |||
| 3 | 86326024 | 4681969 | "BE THE DRAGON" |
Regulations
Numerous federal, state and local regulations regulate nutritional supplements. The manufacturing, processing, formulation, packaging, labeling and advertising of our products are subject to regulation by several federal agencies, including (i) the FDA, (ii) the Federal Trade Commission (“FTC”), (iii) the Consumer Product Safety Commission, (iv) the United States Department of Agriculture, (v) the United States Postal Service, (vi) the United States Environmental Protection Agency and (vii) the United States Occupational Safety and Health Administration. Failure to comply with these regulations could have a material adverse effect on our business, financial condition and results of operations.
In addition to OTC healthcare products and prescription drug products, the FDA regulates the safety, manufacturing, labeling and distribution of dietary supplements, including vitamins, minerals and herbs, food additives, food supplements, over-the-counter and prescription drugs and cosmetics. The FTC also has overlapping jurisdiction with the FDA to regulate the promotion and advertising of vitamins, over-the-counter drugs, cosmetics and foods. In addition, our ProlongzTM is considered a homeopathic remedy, which is subject to standards established by the Homeopathic Pharmacopoeia of the United States (“HPUS”). HPUS sets the standards for source, composition and preparation of homeopathic remedies which are officially recognized under the Federal Food, Drug and Cosmetics Act, as amended.
Preclinical development, clinical trials, product manufacturing, labeling, distribution and marketing of potential new products are also subject to federal and state regulation in the United States and other countries. Clinical trials and product marketing and manufacturing are subject to the rigorous review and approval processes of the FDA and foreign regulatory authorities. To obtain approval of a new drug product, a company must demonstrate through adequate and well-controlled clinical trials that the drug product is safe and effective for its intended use. Obtaining FDA and other required regulatory approvals is lengthy and expensive. Typically, obtaining regulatory approval for pharmaceutical products requires substantial resources and takes several years. The length of this process depends on the type, complexity and novelty of the product and the nature of the disease or other indication to be treated. Preclinical studies must comply with FDA regulations. Clinical trials must also comply with FDA regulations to ensure safety of the human subjects in the trial and may require large numbers of test subjects, complex protocols and possibly lengthy follow-up periods. Consequently, satisfaction of government regulations may take several years: may cause delays in introducing potential new products for considerable periods of time and may require imposing costly procedures upon our activities. If regulatory approval of new products is not obtained in a timely manner or not at all, we could be materially adversely affected. Even if regulatory approval of new products is obtained, such approval may impose limitations on the indicated uses for which the products may be marketed which could also materially adversely affect our business, financial condition and future operations.
Other Regulatory Factors. Political, economic and regulatory influences are fundamentally changing the healthcare industry in the United States. Congress, state legislatures and the private sector continue to review and assess alternative healthcare delivery and payment systems.
Employees
As of the date of this Registration Statement, we have 15 employees and various consultants. None of our employees are represented by a labor organization and we consider our relationship with our employees to be good. Each of our employees signs a Confidentiality and Proprietary Rights Agreement.
Legal Proceedings
We are currently not a party to any material legal or administrative proceedings and are not aware of any pending legal or administrative proceedings against us. We may from time to time become a party to various legal or administrative proceedings arising in the ordinary course of our business.
Property and Corporate Office
Our corporate offices are located at 250 W. 1st Street, Suite 310, Claremont, CA 91711. We rent our offices at the rate of $9,124.11 per month. We also rent a 1,597 warehouse at the rate of $1,350 per month; the warehouse is located in Claremont, CA.
| 27 |
Available Information
We are subject to the informational requirements of the Securities Exchange Act of 1934 and, in accordance therewith, file reports and other information with the SEC. Our reports and other information filed pursuant to the Securities Exchange Act of 1934 may be inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. Copies of such material can also be obtained from the Public Reference Room of the SEC at 100 F Street, N.E., Washington, D.C. 20549, at prescribed rates. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains a Web site that contains reports and other information regarding registrants that file electronically with the SEC. The address of the SEC’s Web site is http://www.sec.gov.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act of 1933 with respect to the common stock offered hereby. As permitted by the rules and regulations of the SEC, this prospectus, which is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement. Copies of the registration statement and the exhibits are on file with the SEC and may be obtained from the SEC’s Web site or upon payment of the fee prescribed by the SEC, or may be examined, without charge, at the offices of the SEC set forth above. For further information, reference is made to the registration statement and its exhibits. Information located on, or accessible through, our website is not incorporated into this filing unless this filing specifically indicates otherwise.
Directors And Executive Officers
Set forth below is information regarding our directors and executive officers as of the date of this Registration Statement. The Board is comprised of only one class. All of the directors will serve until the next annual meeting of shareholders and until their successors are elected and qualified, or until their earlier death, retirement, resignation or removal. Officers are elected by and serve at the discretion of the Board. To date we have not had an annual meeting. Other than the marital relationship between Jason Cardiff and Eunjung Cardiff, there are no family relationships between any of our directors or executive officers.
| Name | Age | Title | ||
| Jason Cardiff | 40 | Chief Executive Officer and President and Director | ||
| Jacques Poujade | 56 | Chief Financial Officer and Director | ||
| Eunjung Cardiff | 44 | Director | ||
| Jaci Warren | 58 | Director | ||
| Christine Hayes | 63 | Director | ||
| Kevin Harrington | 57 | Director | ||
| Patrick Moynihan | 47 | Director |
Jason Cardiff, President, CEO & Board Member: Since 2010, Mr. Cardiff served as Cigirex LLC’s CEO. Before that work, Mr. Cardiff founded First Choice Media, a private consulting company that ran marketing and placed all forms of media for national not-for-profit organizations while simultaneously finding success with his own national infomercial businesses and products. Jason Cardiff started his career at Mercedes Benz USA and was appointed the youngest retail Vice President at that time. Mr. Cardiff’s dedication and prior work experience qualify him to serve as our CEO. Mr. Cardiff attended Northern Arizona University.
Jacques Poujade, CFO & Board Member. Mr. Poujade has over thirty years of experience in the finance field. Prior to joining RST, Mr. Poujade was co-founder and CEO of First Allegiance Financial Corporation, which Jacques sold to CitiMortgage Services (“CHCO” – Nasdaq); he was co-founder, Managing Director and CFO of Lenders Direct Capital Corporation; since, 2007, he has served as the Managing Director and Chief Financial Officer for Tri-Emerald Financial Group, Inc. Mr. Poujade received his degree as a Chartered Accountant from the Canadian Order of Chartered Accountants, a Diploma in Public Accountancy, through the Faculty of Graduate Studies at McGill University in Montreal and a Bachelor of Commerce from Concordia University in Canada. Mr. Poujade’s accounting and financial knowledge and experience qualify him to serve as our CFO and a member of our board.
Eunjung Cardiff, Board Member, Marketing Director & Secretary. Mrs. Cardiff also supervises the Company’s research and development activities. Prior to joining RTI, Mrs. Cardiff worked at Grey Advertising. Thereafter, she opened and managed the New York branch of Cannella Response Television, the nation's largest direct response media placement agency. Mrs. Cardiff has a BS from Columbia University. Mrs. Cardiff’s management and advertising experience qualify her to serve as our Marketing Director and a member of our board.
| 28 |
Jaci Warren, Board Member. For the past five years, Mrs. Warren has been the owner/managing member of CilaJet, LLC. She is also the Vice President of AutoNation Financial, the largest Mercedes dealership managed $400 million in annual sales. She also serves on the Advisory Boards for BMW Financial, AutoNation Financial, and Volvo National Ad Committee. Mrs. Warren completed the General Dealership Management Academy program with the National Automotive Dealer Association. Her knowledge and experience regarding business management and product expansion qualify her to serve on our board.
Christine Hayes, Board Member. Mrs. Hayes has been the President and CEO of Rosemont Mortgage and Melrose Escrow, Inc. since 1992; she has also served on the board of both entities. Mrs. Hayes has 17 years of experience in operations management, including branch Management with Bank of America, including expertise in all aspects of lending. Mrs. Hayes received a BS in Business Management from California State Polytechnic University, Pomona. Mrs. Hayes’s knowledge and experience regarding business management and customer service qualify her to serve on our board.
Kevin Harrington, Board Member. Mr. Harrington has been involved in the infomercial industry since 1984. Mr. Harrington is an original investor shark on the ABC hit show “Shark Tank”, a reality television series produced by reality television producer Mark Burnett, which premiered August 9, 2009. In 2009, Mr. Harrington published a book entitled: “How to turn Ideas into Million-Dollar Products which chronicles his life and experiences in the direct response industry.” In 2008, Mr. Harrington formed TVGoods.com, LLC which was dissolved in 2009. From 1997 to 2008 Mr. Harrington served as CEO of Reliant International, LLC (formerly Reliant Interactive Media, LLC), a direct response marketing company. From 2007 to 2008 Mr. Harrington also served as CEO of ResponzeTV, PLC. From 1994 to 1997 Mr. Harrington served as CEO of HSN Direct, a joint venture Mr. Harrington formed with HSN, Inc. From 1988 to 1994 Mr. Harrington served as President of Quantum Marketing International, Ltd., an electronic retailing company. In 1991 Quantum Marketing International, Ltd. merged with National Media Corporation and renamed as Quantum International, Ltd. In 1984, Mr. Harrington produced one of the industry’s first 30 minute infomercials. Mr. Harrington was a co-founder of two global networking associations, the Entrepreneur's Organization (formerly the Young Entrepreneurs Organization) in 1997, and the Electronic Retailing Association in 2000.
Patrick Moynihan, Board Member. Mr. Moynihan is the founder of PayLock, Inc. a worldwide leader in vehicular-related debt collections and recovery, focusing primarily on the collection of unpaid tickets and violations for various municipalities throughout the United States with the City of New York being its biggest client. Since January 2015, he has worked as the Managing Director of Wholesale Direct Metals, Inc. Mr. Moynihan received his BA from Ithaca College.
Significant Employees
The following are employees who are not executive officers, but who are expected to make significant contributions to our business:
Stefan Galluppi, Chief Technology Officer. Mr. Galluppi oversees the entire information technology infrastructure at Redwood ranging from customer services software to customer relationship management systems as well as website development for Redwood. He also integrates Redwood’s software into the main infrastructure in order to streamline the ordering process and increase efficiencies and effectiveness between different functions at Redwood. Before joining RST, Mr. Galluppi worked as a licensed commodities futures Trader for a private equity firm, CQR Capital Management. Mr. Galluppi studied Computer Science at California State University, Long Beach.
Director Qualifications
We seek directors with established strong professional reputations and experience in areas relevant to the strategy and operations of our businesses. We also seek directors who possess the qualities of integrity and candor, who have strong analytical skills and who are willing to engage management and each other in a constructive and collaborative fashion, in addition to the ability and commitment to devote time and energy to service on the Board and its committees, as necessary. We believe that all of our directors meet the foregoing qualifications.
The Board believes that the leadership skills and other experience of the Board members described below, in addition to each person’s experience set forth above in their respective biographies, provide the Company with a range of perspectives and judgment necessary to guide our strategies and monitor our executives business execution.
Jason Cardiff, President, CEO & Board Member: Mr. Cardiff has vast experience and knowledge in running marketing companies and produced all forms of national media specializing in the infomercial space.
Jacques Poujade, CFO & Board Member. Mr. Poujade has over thirty years of experience in the finance field including five years with a professional audit firm, as well as considerable banking experience.
Eunjung Cardiff, Board Member, Marketing Director & Secretary. Mrs Cardiff has considerable experience in the advertising and purchase of direct media on television. Prior to joining RST, Mrs. Cardiff worked at Grey Advertising. Thereafter, she opened and managed the New York branch of Cannella Response Television, the nation's largest direct response media placement agency.
| 29 |
Jaci Warren, Board Member. Ms. Warren has considerable experience in finance and sales. For the past five years, Mrs. Warren has been the owner/managing member of CilaJet, LLC. She is also the Vice President of AutoNation Financial, the largest Mercedes dealership in the United States, having managed $400 million in annual sales. She also serves on the Advisory Boards for BMW Financial, AutoNation Financial, and Volvo National Ad Committee.
Christine Hayes, Board Member. Mrs. Hayes’s knowledge and experience regarding business management and customer service qualify her to serve on our board.
Kevin Harrington, Board Member. Mr. Harrington has been involved in the infomercial industry since 1984. Mr. Harrington is an original investor shark on the ABC hit show “Shark Tank”, a reality television series produced by reality television producer Mark Burnett, which premiered August 9, 2009. Mr. Harrington produced one of the industry’s first 30 minute infomercials.
Patrick Moynihan, Board Member. Mr. Moynihan has considerable skills in growing and managing businesses that interface directly with the consumer.
Involvement in Certain Legal Proceedings
To the best of the Company’s knowledge, none of the following events occurred during the past ten years that are material to an evaluation of the ability or integrity of any of our executive officers, directors or promoters:
(1) A petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing;
(2) Convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
(3) Subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities:
(i) Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
(ii) Engaging in any type of business practice; or
(iii) Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws;
(4) Subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in paragraph (3)(i) above, or to be associated with persons engaged in any such activity;
(5) Found by a court of competent jurisdiction in a civil action or by the Commission to have violated any Federal or State securities law, and the judgment in such civil action or finding by the Commission has not been subsequently reversed, suspended, or vacated;
(6) Found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
(7) Subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
(i) Any Federal or State securities or commodities law or regulation; or
(ii) Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
(iii) Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
| 30 |
(8) Subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization, any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
Executive Compensation
Since inception, in light of the Company’s cash position, none of our officers or directors received any compensation for their services as such. However, the Board resolved to pay the following compensation.
| Name | Title | Salary Payable | ||
| Jason Cardiff | CEO | $65,000 per annum plus 5% EBITDA not to exceed $300,000 per year | ||
| Jacques B. Poujade | CFO | $5,000 per month | ||
| Stefan Galluppi | CTO | $79,800 per annum plus 1% EBITDA not to exceed $60,000 per year | ||
| Eunjung Cardiff | Marketing Director, Director and Secretary | $65,000 per annum plus 2.5% EBITDA not to exceed $200,000 per year |
All of the persons listed above have agreed to defer their compensation until such time as we are cash flow positive; therefore, none of our officers have received any compensation as of the date of this Registration Statement. No retirement, pension, profit sharing, stock option or insurance programs or other similar programs have been adopted by the Company for the benefit of the Company’s employees as of the date of this Registration Statement. However, the Board is formulating an Incentive Plan with a pool of up to 250,000 shares.
Compensation Policies and Practices as They Relate to the Company’s Risk Management
We believe that our current compensation policies and practices for all employees, including executive officers, do not create risks that are reasonably likely to have a material adverse effect on us.
Employment Contracts
We have employment agreements with the following persons (collectively, the “Employees”), each of which are effective as of January 1, 2015 and have a term of twelve months, unless terminated earlier, to serve in the positions listed; each of these employment agreements shall continue on a monthly basis if not otherwise terminated or extended at the end of the initial 12 month term. Any future compensation will be determined by the Board of Directors, and, when established, our Compensation Committee.
| Jason Cardiff | Chief Executive Officer | |
| Eunjung Cardiff | Marketing Director | |
| Jacques Poujade | Chief Financial Officer | |
| Stefan Galluppi | Chief Technology Officer |
These employment agreements entitle each of the Employees to participate in the Company’s stock option plan, once created and to participate in any company profit sharing plans and benefit programs, if any. The agreements set forth the compensation payable to each of the Employees and provide that each of the Employees shall be reimbursed for reasonable company expenses. Under the respective agreements, Mr. Cardiff and Mrs. Cardiff are entitled to four weeks paid vacation; Messrs. Poujade and Galluppi are entitled to two weeks. The agreements also contain a non-solicitation clause and confidentiality clause.
The Company may terminate any of the employment agreements due to the inability of an Employee to perform his/her duties for more than 6 months, death or discharge for cause or without cause at any time. The Employees may resign for “good reason,” as such is defined in the agreement, with 30 days written notice to the Company after a 30 day cure period of such triggering event; if we waive an employee’s obligation to work during such time period and terminates the employment immediately, employee shall receive all salary owed at the end of such notice period.
If we terminate an Employee without cause or an Employee resigns for good reason, such Employee shall receive severance equal to his/her base salary for six months (Messrs. Poujade and Galluppi’s agreement provide for a severance payment of $100), in a lump sum no later than 60 days after his/her employment terminated, so long as such employee signed a general release of claims against the Company and its officers and directors; the Employee is also entitled to vested benefits in accordance with the termination provisions thereof in such circumstances. If employment is terminated for any reason other than Cause, death or disability, employee shall receive all vested shares and all shares set to vest on the next immediate vestment date; the remainder of shares to be vested are fortified. If an Employee is discharged for Cause or resigns without good reason, such Employee shall only receive his/her base salary for days worked through the termination date and any vested benefits as of such date.
Outstanding Equity Awards
There were no equity awards outstanding as of the end of the fiscal year ending December 31, 2015.
We are not a party to, nor are we proposed to be a party, to any transaction during the last fiscal year involving an amount exceeding $120,000 and in which a related person, as such term is defined by Item 404 of Regulation S-K, had or will have a direct or indirect material interest.
| 31 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth the number of shares of Common Stock beneficially owned by (i) each person who, as of the date hereof, was known by us to own beneficially more than five percent (5%) of our issued and outstanding common stock; (ii) each of the named Executive Officers; (iii) the individual Directors; and (iv) the Officers and Directors as a group.
Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. Shares of common stock that may be acquired by an individual or group within 60 days after the date of this Registration Statement, pursuant to the exercise of stock options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table.
Percentage ownership calculations for beneficial ownership are based on 7,508,167 shares of common stock outstanding as of February 10, 2016. Based on the $3,441,252 received in the Offering, percentage ownership calculations includes 1,529,445 shares of Common Stock underlying the Units and 825,701 shares of common stock underlying the $1,500,000 Bridge Notes, which by their terms, automatically converted into shares of common stock at the rate of $1.97 and includes $126,630 in interest that has accrued on the Notes through November 24, 2015, but excludes the following:
| 1. | 764,723 shares of Common Stock underlying the Warrants; | |
| 2. | 761,421 amount, including ratchet shares if issuable shares of common Stock underlying the Bridge Warrants, which are exercisable at an exercise price of $2.25; | |
| 3. | 53,604 shares of Common Stock underlying the warrants issued to the Placement Agent pursuant to the Bridge Financing; and, | |
| 4. | 127,379 shares of Common Stock underlying the warrants issuable to the Placement Agents pursuant to this Offering. |
Also not included or reflect in the table below and like the investors pursuant to this Offering, the holders of the Bridge Notes are entitled to additional shares if the Company has less than $20,000,000 in gross revenue or less than $1 in earnings before interest, taxes, depreciation and amortization (EBITDA) for the subsequent twelve months following the close of $6,500,000 in funding (including the $1,500,000 received from the Bridge Financing), in which case the Company shall issue an additional 20% of the common stock and warrants underlying the Bridge Notes and Bridge Warrants. If the Company fails to use reasonable best efforts to distribute the required financial statements containing the information necessary to calculate the Company’s revenue, such failure shall result in the Bridge Note holders receiving an additional 100% of the Units purchased in the Bridge Financing. $6,500,000 in funding was not attained therefore additional shares will not be issued.
Except as indicated in footnotes to this table, the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them, based on information provided to us by such stockholders. Unless otherwise indicated, the address for each director and executive officer listed is: c/o Redwood Scientific Technologies, Inc., 250 W. 1st Street, Claremont, CA.
| Name of Beneficial Owner | Amount of Shares Beneficially Owned | Percentage of Class | ||||||
| Jason Cardiff | 4,971,389 | (1) | 66.21 | % | ||||
| Jacques Poujade | 170,000 | 2.26 | % | |||||
| Eunjung Cardiff | 4,971,389 | (1) | 66.21 | % | ||||
| Jaci Warren | 45,000 | 0.59 | % | |||||
| Christine Hayes | 20,000 | 0.26 | % | |||||
| Kevin Harrington | - | |||||||
| Patrick Moynihan | - | |||||||
| All executive officers and directors as a group (7 persons) | 5,206,389 | 69.34 | % | |||||
* Less than 1%.
| 1) | These 4,971,389 shares are held in the name of Jurikel Family Trust, dated April 24, 2014, over which Mr. and Mrs. Cardiff serve as Trustees; however, Mrs. Cardiff does not maintain any voting control over the shares and disclaims beneficial ownership of same. |
| 32 |
Corporate Governance and Director Independence
Presently, we are not currently listed on a national securities exchange or in an inter-dealer quotation system and therefore are not required to comply with the director independence requirements of any securities exchange. In determining whether our directors are independent, however, we intend to comply with the rules of NASDAQ. The board of directors will also consult with counsel to ensure that the boards of director’s determinations are consistent with those rules and all relevant securities and other laws and regulations regarding the independence of directors, including those adopted under the Sarbanes-Oxley Act of 2002 with respect to the independence of audit committee members. Nasdaq Listing Rule 5605(a)(2)defines an “independent director” generally as a person other than an Executive Officer or employee of the Company or any other individual having a relationship which, in the opinion of the Company's board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Our Board of Directors has determined that 4 of the directors would qualify as “independent” as that term is defined by Nasdaq Listing Rule 5605(a)(2). Finally, we have determined that Jaci Warren, Christine Hayes, Kevin Harrington and Patrick Moynihan qualify as “independent” under Nasdaq Listing Rules applicable to board committees.
Due to our lack of operations and current size, we do not have an Audit Committee or any other separate committees. The Board will consider appointing members to each of the Committees at such time as a sufficient number of independent directors are appointed to the Board or as otherwise determined by the Board. Until such time, the full board of directors will undertake the duties of the audit committee, compensation committee and nominating committee.
A copy of our Code of Ethics is attached as Exhibit 14.1 which is incorporated herein by reference.
Other than as described below, none of the Selling Stockholders nor any of their affiliates has held any position or office with, been employed by or otherwise has had any material relationship with us or our affiliates during the three years prior to the date of this prospectus. Unless otherwise indicated below, none of the Selling Stockholders are broker-dealers or affiliates of a broker-dealer within the meaning of Section 3 of the Securities Exchange Act.
This prospectus relates to the offering and sale, from time to time, of up to 4,062,273 shares of our common stock held by the stockholders named in the table below.
The table below lists the selling stockholders and other information regarding the beneficial ownership (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder) of our common stock with respect to the securities held by each of the selling stockholders. The second column lists the number of shares of common stock beneficially owned by each of the selling stockholders as of February 10, 2016, including assuming exercise of the Bridge Notes, Warrants and/or Bridge Warrants held by each such selling stockholder on that date.
The third column lists the shares of common stock underlying the Units, the Warrants, the Bridge Notes and/or the Bridge Warrants being offered by this prospectus by the selling stockholders and does not take into account any limitations on conversion or exercise of the Warrants, Bridge Notes or Bridge Warrants, respectively set forth therein.
The fourth column assumes the sale of all of the shares offered by the selling stockholders pursuant to this prospectus. However, the selling stockholders may sell all, some or none of their shares in this offering. See “Plan of Distribution.”
We estimate that our costs and expenses of registering the shares listed herein for resale will be approximately $920.41.
| 33 |
| Name of Selling Stockholder | Number of Shares of Common Stock Owned Prior to Offering(1) | Maximum Number of Shares of Common Stock to be Sold Pursuant to this Prospectus (2) | Number of Shares of Common Stock Owned After Offering (3) | |||||||||
| 802 Investments LLC (4) | 165,668 | 165,668 | 0 | |||||||||
| Alan Talesnick (5) | 21,139 | 21,139 | 0 | |||||||||
| Allan Webber (6) | 30,000 | 30,000 | 0 | |||||||||
| Alva Terry Staples (7) | 54,683 | 54,683 | 0 | |||||||||
| Alva Terry Staples Family Ptnrshp (8) | 13,228 | 13,228 | 0 | |||||||||
| Andrew Iseman (9) | 75,000 | 75,000 | 0 | |||||||||
| Anthony J. Paratore (10) | 10,000 | 10,000 | 0 | |||||||||
| Chris Wrolstad (64) | 35,887 | 35,887 | 0 | |||||||||
| Chris Wrolstad Sep-IRA (11) | 16,500 | 16,500 | 0 | |||||||||
| Dan Diekel (12) | 26,424 | 26,424 | 0 | |||||||||
| Dan McGregor (13) | 15,000 | 15,000 | 0 | |||||||||
| Dan Perkins (14) | 31,704 | 31,704 | 0 | |||||||||
| David Charles (15) | 27,239 | 27,239 | 0 | |||||||||
| David Huebner (16) | 26,455 | 26,455 | 0 | |||||||||
| Dr. Zaki (17) | 60,000 | 60,000 | 0 | |||||||||
| Edward Sugar (18) | 16,667 | 16,667 | 0 | |||||||||
| Elle Ossello UTMA (19) | 10,597 | 10,597 | 0 | |||||||||
| Erik Manning (20) | 15,000 | 15,000 | 0 | |||||||||
| Evergreen Life (21) | 192,314 | 192,314 | 0 | |||||||||
| Five Votes LLC (22) | 52,875 | 52,875 | 0 | |||||||||
| Frank Barravechia (65) | 500 | 500 | 0 | |||||||||
| Generous Limestone LLC (23) | 52,841 | 52,841 | 0 | |||||||||
| Gerard McCabe (24) | 13,333 | 13,333 | 0 | |||||||||
| Gianna Ossello UTMA (25) | 10,597 | 10,597 | 0 | |||||||||
| Greg Erigero (26) | 30,898 | 30,898 | 0 | |||||||||
| Greg Erigero CUST Gina CA UTMA (27) | 10,600 | 10,600 | 0 | |||||||||
| Guy and Madeline Ossello (28) | 53,007 | 53,007 | 0 | |||||||||
| Guy Ossello IRA (29) | 79,501 | 79,501 | 0 | |||||||||
| Halen Capital Management (66) | 23,966 | 23,966 | 0 | |||||||||
| Jaci Warren (30) | 37,500 | 37,500 | 0 | |||||||||
| Jacob David Wiznitzer (31) | 47,333 | 47,333 | 0 | |||||||||
| Janice L. Paratore (32) | 10,000 | 10,000 | 0 | |||||||||
| Jean Pregerson (33) | 26,445 | 26,445 | 0 | |||||||||
| John Gaensbaur (34) | 27,232 | 27,232 | 0 | |||||||||
| John Kleinert (35) | 15,000 | 15,000 | 0 | |||||||||
| John Lemak IRA (36) | 83,333 | 83,333 | 0 | |||||||||
| Joseph M. Pankowski (37) | 7,500 | 7,500 | 0 | |||||||||
| Joseph Ransdell (67) | 6,811 | 6,811 | 0 | |||||||||
| JP Global, LLC (38) | 221,288 | 221,288 | 0 | |||||||||
| Juan Maio (39) | 15,000 | 15,000 | 0 | |||||||||
| Jurikel Family Trust 2 (40) | 875,834 | 875,834 | 0 | |||||||||
| Larry Helbringer (68) | 750 | 750 | 0 | |||||||||
| Leigh Severance (41) | 211,807 | 211,807 | 0 | |||||||||
| Leigh Severance profit sharing (42) | 211,724 | 211,724 | 0 | |||||||||
| Mark Hermann (43) | 26,455 | 26,455 | 0 | |||||||||
| Milan-Chase Cooper (44) | 133,333 | 133,333 | 0 | |||||||||
| Milan-Cooper (45) | 133,333 | 133,333 | 0 | |||||||||
| Milton Datsopoulos (46) | 75,000 | 75,000 | 0 | |||||||||
| Mohsen Khorassani (47) | 30,000 | 30,000 | 0 | |||||||||
| Nathan Halegua (48) | 15,000 | 15,000 | 0 | |||||||||
| Newport Coast Securities (69) | 2,806 | 2,806 | 0 | |||||||||
| Palisades Highlands Ptrs (49) | 53,007 | 53,007 | 0 | |||||||||
| Patrick O'Shaughnessy (50) | 66,667 | 66,667 | 0 | |||||||||
| 34 |
| Randy Curtis (51) | 15,000 | 15,000 | 0 | |||||||||
| Rare Space Inc (52) | 44,427 | 44,427 | 0 | |||||||||
| Reed Madison (70) | 48,609 | 48,609 | 0 | |||||||||
| Richard Rizzuto (71) | 1,555 | 1,555 | 0 | |||||||||
| Robert Schneiderman (53) | 15,000 | 15,000 | 0 | |||||||||
| Ruth Gruen/Marv Cohen (54) | 52,952 | 52,952 | 0 | |||||||||
| Scott Douglas (55) | 26,490 | 26,490 | 0 | |||||||||
| Stephen W. Dumford (56) | 7,500 | 7,500 | 0 | |||||||||
| Steve Ossello (57) | 84,979 | 84,979 | 0 | |||||||||
| Steve Ossello SEP IRA (58) | 26,465 | 26,465 | 0 | |||||||||
| Crystal Cascades (59) | 166,667 | 166,667 | 0 | |||||||||
| W&O Enterprises, LLC (60) | 21,181 | 21,181 | 0 | |||||||||
| William Gebhardt (61) | 15,000 | 15,000 | 0 | |||||||||
| WRS Capital (62) | 16,667 | 16,667 | 0 | |||||||||
| Ying Cui (63) | 15,000 | 15,000 | 0 |
(1) Unless otherwise noted, the Selling Stockholder became one of our shareholders pursuant to the 2015 Bridge Financing and 2015 Private Placement. Accordingly, prior to the Offering, the Selling Stockholder only owned shares of common stock and shares of common stock underlying the warrants received in the Bridge Financing and Private Placement (the “Securities”).
(2) This number represents all of the Securities that the Selling Stockholder received in the 2015 Bridge Financing and 2015 Private Placement, which we agreed to register in this Registration Statement pursuant to the Registration Rights Agreement we entered into in connection with the Bridge Financing and Private Placement.
(3) Since we do not have the ability to control how many, if any, of their shares each of the selling shareholders listed above will sell, we have assumed that the selling shareholders will sell all of the shares offered herein for purposes of determining how many shares they will own after the Offering and their percentage of ownership following the offering.
(4) Consists of 110,445 Shares of Common Stock and 55,223 Shares of Common Stock underlying Series B Warrants. The person having voting, dispositive or investment power over 802 Investments LLC is Juan M. Pinero Dagnery. The address for 802 Investments LLC is 53 Calle Palmeras Ste 802, San Juan, Puerto Rico, 00901.
(5) Consists of 10,987 Shares of Common Stock, 10,000 Shares of Common Stock underlying Series A Warrants and 152 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(6) Consists of 20,000 Shares of Common Stock and 10,000 Shares of Common Stock underlying Series B Warrants.
(7) Consists of 30,647 Shares of Common Stock, 18,750 Shares of Common Stock underlying Series A Warrants, 5,000 Shares of Common Stock underlying Series B Warrants and 285 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(8) Consists of 6,882 Shares of Common Stock and 6,250 Shares of Common Stock underlying the Bridge Notes and 95 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes. The person having voting, dispositive or investment power over the Alva Terry Staples Family Ptnrshp is Alva Terry Staples. The address for Alva Terry Staples Family Ptnrshp is 6705 E Dorado Pl, Greenwood Village, Colorado, 80111.
(9) Consists of 50,000 Shares of Common Stock and 25,000 Shares of Common Stock underlying Series B Warrants.
(10) Consists of 6,667 Shares of Stock and 3,333 Shares of Common Stock underlying Series B Warrants.
(11) Consists of 11,000 Shares of Common Stock and 5,500 Shares of Common Stock underlying Series B Warrants.
(12) Consists of 13,733 Shares of Common Stock and 12,500 Shares of Common Stock underlying Series B Warrants and 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(13) Consists of 10,000 Shares of Common Stock and 5,000 Shares of Common Stock underlying Series B Warrants.
(14) Consists of 16,476 Shares of Common Stock, 15,000 Shares of Common Stock underlying Series A Warrants and 228 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(15) Consists of 13,737 Shares of Common Stock, 12,500 Shares of Common Stock underlying Series A Warrants 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes and 812 warrants awarded as the placement agent.
(16) Consists of 13,765 Shares of Common Stock and 12,500 Shares of Common Stock underlying Series A Warrants and 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(17) Consists of 40,000 Share of Common Stock and 20,000 Shares of Common Stock underlying Series B Warrants.
(18) Consists of 11,111 Shares of Common Stock and 5,556 Shares of Common Stock underlying Series B Warrants.
(19) Consists of 5,521 Shares of Common Stock and 5,000 Shares of Common Stock underlying Series A Warrants and 76 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(20) Consists of 10,000 Shares of Common Stock and 5,000 Shares of Common Stock underlying Series B Warrants.
(21) Consists of 105,060 Shares of Common Stock and 75,000 Shares of Common Stock underlying Series A, 11,111 Shares of Common Stock underlying Series B Warrants and 1,142 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes. The person having voting, dispositive or investment power over Evergreen Life is Brian Landau. The address for Evergreen Life is 700 E. Palisade Ave, Englewood Cliffs, New Jersey, 07632.
| 35 |
(22) Consists of 27,495 Shares of Common Stock, 12,500 Shares of Common Stock underlying Series A Warrants, 12,500 Shares of Common Stock underlying Series B Warrants, and 380 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes. The person having voting, dispositive or investment power over Five Votes LLC is Jennifer Flink. The address for Five Votes LLC is 1755 Lake Cook Rd, Highland Park, Illinois, 60035.
(23) Consists of 27,460 Shares of Common Stock and 25,000 Shares of Common Stock underlying Series A Warrants and 380 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes. The person having voting, dispositive or investment power over Generous Limestone LLC is Robert Kalkstein. The address for Generous Limestone LLC is 53 Calle Palmeras 6th Floor, San Juan, Puerto Rico, 00901.
(24) Consists of 8,889 Shares of Common Stock and 4,444 Shares of common stock underlying Series B Warrants.
(25) Consists of 5,521 Shares of Common Stock and 5,000 Shares of Common Stock underlying Series A Warrants and 76 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(26) Consists of 18,284 Shares of Common Stock, 7,500 Shares of Common Stock underlying Series A Warrants, 5,000 Shares of Common Stock underlying Series B Warrants and 114 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(27) Consists of 5,524 Shares of Common Stock, 5,000 Shares of Common Stock underlying Series A Warrants and 76 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(28) Consists of 27,627 Shares of Common Stock, 25,000 Shares of Common Stock underlying Series A Warrants and 381 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(29) Consists of 41,430 Shares of Common Stock, 37,500 Shares of Common Stock underlying Series A Warrants and 571 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(30) Consists of 25,000 Shares of Common Stock and 12,500 Shares of Common Stock underlying Series B Warrants.
(31) Consists of 31,556 Shares of Common Stock and 15,778 Shares of Common Stock underlying Series B Warrants.
(32) Consists of 6,667 Shares of Stock and 3,333 Shares of Common Stock underlying Series B Warrants.
(33) Consists of 13,754 Shares of Common Stock, 12,500 Shares of Common Stock underlying Series A Warrants and 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(34) Consists of 13,730 Shares of Common Stock, 12,500 Share of Common Stock underlying Series A Warrants and 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes and 812 warrants awarded as placement agent.
(35) Consists of 10,000 Shares of stock and 5,000 Shares of Common Stock underlying Series B Warrants.
(36) Consists of 55,556 Shares of Common Stock and 27,778 Shares of Common Stock underlying Series B Warrants.
(37) Consists of 5,000 Shares of Common Stock and 2,500 Shares of Common Stock underlying Series B Warrants.
(38) Consists of 114,689 Shares of Common Stock, 105,000 Shares of Common Stock underlying Series A Warrants and 1,599 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes. The person having voting, dispositive or investment power over JP Global is Georgianne Ocasio. The address for JP Global is 53 Calle Palmeras 8th Floor, San Juan, Puerto Rico, 00901.
(39) Consists of 10,000 Shares of Common Stock and 5,000 Shares of Common Stock underlying Series B Warrants .
(40) Consists of 583,889 Shares of Common Stock and 291,945 Shares of Common Stock underlying Series B Warrants.
(41) Consists of 110,284 Shares of Common Stock, 100,000 Shares of Common Stock underlying Series A Warrants and 1,523 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(42) Consists of 110,201 Shares of Common Stock, 100,000 Shares of Common Stock underlying Series A Warrants and 1,523 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(43) Consists of 13,765 Shares of Common Stock, 12,500 Shares of Common Stock underlying Series A Warrants and 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(44) Consists of 88,889 Shares of Common Stock and 44,444 Shares of Common Stock underlying Series B Warrants. The person having voting, dispositive or investment power over Milan-Chase Cooper is Patrick O’Shaughnessy. The address for Milan-Chase Cooper is 1801 Broadway #920, Denver, Colorado. 80202.
(45) Consists of 88,889 Shares of Common Stock and 44,444 Shares of Common Stock underlying Series B Warrants. The person having voting, dispositive or investment power over Milan-Cooper is Patrick O’Shaughnessy. The address for Milan-Cooper is 1801 Broadway #920, Denver, Colorado. 80202.
(46) Consists of 50,000 Shares of Common Stock and 25,000 Shares of Common Stock underlying Series B Warrants.
(47) Consists of 20,000 Shares of Common Stock and 10,000 Shares of Common Stock underlying Series B Warrants.
(48) Consists of 10,000 Shares of Common Stock and 5,000 Shares of Common Stock underlying Series B Warrants.
(49) Consists of 27,627 Shares of Common Stock, 25,000 Shares of Common Stock underlying Series A Warrants and 381 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes. The person having voting, dispositive or investment power over Palisades Highlands Ptrs is Damon Geller. The address for Palisades Highlands Ptrs is 1544 Palisades Dr, Pacific Palisades, California. 90272.
(50) Consists of 44,444 Shares of Common Stock and 22,222 Shares of Common Stock underlying Series B Warrants.
(51) Consists of 10,000 Shares of Common Stock and 5,000 Shares of common stock underlying Series B Warrants.
(52) Consists of 25,737 Shares of Common Stock, 6,000 Shares of Common Stock underlying Series B Warrants, 12,500 Shares of Common Stock underlying Series A Warrants 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes. The person having voting, dispositive or investment power over Rare Space is Tom Grotewold. The address for Rare Space is 1331 17th St M-100, Denver, Colorado, 80202.
(53) Consists of 10,000 Shares of Common Stock and 5,000 Shares of Common Stock underlying Series B Warrants.
(54) Consists of 27,571 Shares of Common Stock, 25,000 Shares of Common Stock underlying Series A Warrants and 381 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
| 36 |
(55) Consists of 13,799 Shares of Common Stock, 12,500 Shares of Common Stock underlying Series A Warrants and 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(56) Consists of 5,000 Shares of Stock and 2,500 Shares of Common Stock underlying Series B Warrants.
(57) Consists of 13,813 Shares of Common Stock, 12,500 Shares of Common Stock underlying Series A Warrants and 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes and 19,267 Shares of Common Stock underlying Series A Warrants as placement agent and 39,208 Shares of Common Stock underlying Series B Warrants as placement agent.
(58) Consists of 13,775 Shares of Common Stock, 12,500 Shares of Common Stock underlying Series A Warrants and 190 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes.
(59) Consists of 111,111 Shares of Common Stock and 55,556 Shares of Common Stock underlying Series B Warrants. The person having voting, dispositive or investment power over Crystal Cascades is Tim Flaherty. The address for Crystal Cascades is P.O. Box 3080, Silverdale, Washington, 98383.
(60) Consists of 11,028 Shares of Common Stock, 10,000 Shares of Common Stock underlying Series A Warrants and 152 Shares of Common Stock underlying additional warrants due to anti-dilution provisions of the Bridge Notes. The person having voting, dispositive or investment power over W&O Enterprises is Chris Wrolstad. The address for W&O Enterprises is 1601 Arapahoe St Unit 4, Denver, Colorado, 80202.
(61) Consists of 10,000 Shares of Common Stock and 5,000 Shares of Common stock underlying Series B Warrants.
(62) Consists of 11,111 Shares of Common Stock and 5,556 Shares of Common stock underlying Series B Warrants. The person having voting, dispositive or investment power over WRS Capital is Will Schram. The address for WRS Capital is 4008 Lovees Lane, Dallas, Texas, 75225.
(63) Consists of 10,000 Shares of Common Stock and 5,000 Shares of Common Stock underlying Series B Warrants.
(64) Consists of 11,824 Shares of Common Stock underlying Series A Warrants and 24,063 Shares of Common Stock underlying Series B Warrants as placement agent.
(65) Consists of 500 Shares of Common Stock underlying Series B Warrants as placement agent.
(66) Consists of 23,966 Shares of Common Stock underlying Series B Warrants as placement agent. The person having voting, dispositive or investment power over Halen Capital Management is [ ]. The address for Halen Capital Management is 301 S Missouri Ave, Clearwater, Florida 33756.
(67) Consists of 6,811 Shares of Common Stock underlying Series B Warrants as placement agent.
(68) Consists of 750 Shares of Common Stock underlying Series B Warrants as placement agent.
(69) Consists of 2,806 Shares of Common Stock underlying Series B Warrants as placement agent. The person having voting, dispositive or investment power over Newport Coast Securities is [ ]. The address for Newport Coast Securities is 180 Maiden Lane, New York, New York, 10038.
(70) Consists of 16,016 Shares of Common Stock underlying Series A Warrants and 32,593 Shares of Common Stock underlying Series B Warrants as placement agent.
(71) Consists of 1,555 Shares of Common Stock underlying Series B Warrants as placement agent.
Except as otherwise indicated above or in the footnotes to the table, the selling stockholders have not held any position or office or had any material relationship with us or any of our subsidiaries within the past three years and possess sole voting and investment power with respect to the shares shown.
| 37 |
We will bear all fees and expenses incident to our obligation to register the shares of common stock.
The selling security holders and any of their pledgees, donees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock being offered under this prospectus on any stock exchange, market or trading facility on which shares of our common stock are traded or in private transactions. These sales may be at fixed or negotiated prices. The selling security holders may use any one or more of the following methods when disposing of shares:
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; | |
| ● | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; | |
| ● | purchases by a broker-dealer as principal and resales by the broker-dealer for its account; | |
| ● | an exchange distribution in accordance with the rules of the applicable exchange; | |
| ● | privately negotiated transactions; | |
| ● | to cover short sales made after the date that the registration statement of which this prospectus is a part is declared effective by the Commission; | |
| ● | broker-dealers may agree with the selling security holders to sell a specified number of such shares at a stipulated price per share; | |
| ● | a combination of any of these methods of sale; and | |
| ● | any other method permitted pursuant to applicable law. | |
| ● | The shares may also be sold under Rule 144 under the Securities Act of 1933, as amended (“Securities Act”), if available, rather than under this prospectus. The selling security holders have the sole and absolute discretion not to accept any purchase offer or make any sale of shares if they deem the purchase price to be unsatisfactory at any particular time. |
The selling security holders may pledge their shares to their brokers under the margin provisions of customer agreements. If a selling security holder defaults on a margin loan, the broker may, from time to time, offer and sell the pledged shares.
Broker-dealers engaged by
the selling security holders may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions
or discounts from the selling security holders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser)
in amounts to be negotiated, which commissions as to a particular broker or dealer may be in excess of customary commissions to
the extent permitted by applicable law.
If sales of shares offered under this prospectus are made to broker-dealers as principals, we would be required to file a post-effective amendment to the registration statement of which this prospectus is a part. In the post-effective amendment, we would be required to disclose the names of any participating broker-dealers and the compensation arrangements relating to such sales.
The selling security holders and any broker-dealers or agents that are involved in selling the shares offered under this prospectus may be deemed to be “underwriters” within the meaning of the Securities Act in connection with these sales. Commissions received by these broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Any broker-dealers or agents that are deemed to be underwriters may not sell shares offered under this prospectus unless and until we set forth the names of the underwriters and the material details of their underwriting arrangements in a supplement to this prospectus or, if required, in a replacement prospectus included in a post-effective amendment to the registration statement of which this prospectus is a part.
The selling security holders and any other persons participating in the sale or distribution of the shares offered under this prospectus will be subject to applicable provisions of the Exchange Act, and the rules and regulations under that act, including Regulation M. These provisions may restrict activities of, and limit the timing of purchases and sales of any of the shares by, the selling security holders or any other person. Furthermore, under Regulation M, persons engaged in a distribution of securities are prohibited from simultaneously engaging in market making and other activities with respect to those securities for a specified period of time prior to the commencement of such distributions, subject to specified exceptions or exemptions. All of these limitations may affect the marketability of the shares.
| 38 |
If any of the shares of common stock offered for sale pursuant to this prospectus are transferred other than pursuant to a sale under this prospectus, then subsequent holders could not use this prospectus until a post-effective amendment or prospectus supplement is filed, naming such holders. We offer no assurance as to whether any of the selling security holders will sell all or any portion of the shares offered under this prospectus.
We have agreed to pay all fees and expenses we incur incident to the registration of the shares being offered under this prospectus. However, each selling security holder and purchaser is responsible for paying any discounts, commissions and similar selling expenses they incur.
We and the selling security holders have agreed to indemnify one another against certain losses, damages and liabilities arising in connection with this prospectus, including liabilities under the Securities Act.
Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling stockholder will sell any or all of the shares of common stock registered pursuant to the registration statement, of which this prospectus forms a part.
Once sold under the registration statement, of which this prospectus forms a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
Pursuant to our Certificate of Incorporation, we are authorized to issue 50 million shares of common stock, $0.01 par value per share and 10 million shares of preferred stock, $0.01. As of the date of this Registration Statement, we have 7,508,167 shares of common stock outstanding and 88 stockholders of record; we do not have any shares of preferred stock issued or outstanding.
The following description of our capital stock and provisions of our articles of incorporation and bylaws are summaries and are qualified by reference to the articles of incorporation and bylaws.
Common Stock
Holders of Common Stock are entitled to cast one vote for each share on all matters submitted to a vote of shareholders. Voting for directors may be done cumulatively so long as the name of the candidate(s) a shareholder seeks to elect has been placed in nomination prior to the voting and such shareholder gave notice at the meeting prior to the voting of his/her intention to cumulate his votes; candidates receiving the highest number of votes up to the number of Directors to be elected shall be elected. The holders of Common Stock are entitled to receive ratably such dividends, if any, as may be declared by the Board out of funds legally available therefore. See “Dividend Policy.” Such holders do not have any preemptive or other rights to subscribe for additional shares. All holders of Common Stock are entitled to share ratably in any assets for distribution to shareholders upon the liquidation, dissolution or winding up of the Company. There are no conversion, redemption or sinking fund provisions applicable to the Common Stock. All outstanding shares of Common Stock are fully paid and non-assessable.
Preferred Stock
The Board of Directors is authorized, without further action by the shareholders, to issue, from time to time, up to 10,000,000 shares of preferred stock in one or more classes or series. Similarly, the Board of Directors will be authorized to fix or alter the designations, powers, preferences, and the number of shares which constitute each such class or series of preferred stock. Such designations, powers or preferences may include, without limitation, dividend rights (and whether dividends are cumulative), conversion rights, if any, voting rights (including the number of votes, if any, per share), redemption rights (including sinking fund provisions, if any), and liquidation preferences of any unissued shares or wholly unissued series of preferred stock. As of the date of this filing, we have not issued any of our preferred stock.
It is not possible to state the actual effect of any authorization of preferred stock upon the rights of holders of Common Stock until the Board of Directors determines the specific rights of the holders of any series of preferred stock. The Board of Director’s authority to issue preferred stock also provides a convenient vehicle in connection with possible acquisitions and other corporate purposes, but could have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting stock. Accordingly, the issuance of preferred stock may be used as an “anti-takeover” device without further action on the part of our stockholders, and may adversely affect the holders of the Common Stock.
As of the date of this Registration Statement, the Board does not have any plans to issue any shares of preferred stock or designate a class of such stock, but may do so in the future.
| 39 |
Warrants
Offering Warrants
The following is a list of the more significant material terms of the Warrants included in each Unit:
| a) | shall entitle the holder to purchase that number of shares of Common Stock (“Warrant Shares”) as shall be equal to 50% shares of Common Stock underlying his/her/its Unit; | |
| b) | shall be exercisable at $2.75 per whole share (“Exercise Price”); | |
| c) | shall be exercisable at any time after the Final Closing Date and shall expire on the third anniversary thereof; | |
| d) | may be exercised for cash or via a cashless exercise under certain circumstances as set forth in the Warrant agreement; | |
| e) | the Exercise Price applicable to any unexercised Warrants shall be subject to standard adjustments for stock splits and other customary changes in capital structure, to partial ratchet anti-dilution protection adjustments for issuances at less than the Exercise Price (subject to certain exempt issuances defined in the Warrant); | |
| f) | subject to 30 business days’ prior notice to the Warrant holders and provided an effective registration statement is in effect covering the Warrant Shares, the Company may call all, but not less than all, of the Warrants at $0.01 per share at any time after the closing price for the Common Stock exceeds 200% of the Unit Price for any 20 consecutive trading days and average daily volume during the same period exceeds 50,000 shares per day; and, | |
| g) | At any time this or any other registration statement covering the Warrant Shares is not effective, the Warrants shall be exercisable on a cashless. |
Bridge Warrants
The holders of the Bridge Notes received warrants to purchase 100% of the number of shares of Common Stock underlying the Bridge Notes (the “Bridge Warrants”). The exercise price of the Bridge Warrants shall be initially equal to $2.50 (“Bridge Exercise Price”), subject to adjustment, including a ratchet down to $2.25 per share upon the initial closing of this Offering. The Bridge Warrants shall have a three-year term and may be exercised at any time following the issuance thereof, unless otherwise earlier terminated as set forth therein. The Bridge Exercise Price applicable to any unexercised Bridge Warrants shall be subject to standard adjustments for stock splits and other customary changes in capital structure, to anti-dilution adjustments for issuances at less than the Exercise Price (subject to certain exempt issuances defined in the Bridge Warrant) and to underlying share adjustments in the event the Conversion Price of the Bridge Notes is adjusted under the anti-dilution provisions set forth therein. The Bridge Warrants may be exercised on a cashless basis. Subject to 30 business days’ prior notice to the holders of the Bridge Warrants, and provided an effective registration statement is in effect covering the Common Stock underlying the Bridge Warrants, all, but not less than all, of the Bridge Warrants will be callable by the Company at $0.01 per share at any time after the closing price for the Common Stock exceeds 250% of the Conversion Price for any 20 consecutive trading days and average daily volume during the same period exceeds 50,000 shares per day.
Placement Agent Warrants
The Placement Agents received warrants (the “Placement Agent Warrants”) pursuant to the Offering, with the same terms and conditions as those issued to the investors of the Offering, to purchase an aggregate of 127,379 shares; provided, however, that the Placement Agent Warrants are not redeemable and have an exercise price of $2.25 per share. The Placement Agent Warrants are exercisable for a period of three years. Additionally, the Placement Agents shall pay a warrant cost of $0.001 per share upon issuance of the Placement Agent Warrants.
Placement Agent Bridge Warrants
The Placement Agent received warrants (the “Placement Agent Bridge Warrants”) pursuant to the Bridge Financing with the same terms and conditions as those issued to the Bridge Note investors to purchase an aggregate of 53,604 shares; provided, however, that the Placement Agent Bridge Warrants are not redeemable and have an exercise price of $1.97 per share. The Placement Agent Bridge Warrants are exercisable for a period of three years. Additionally, the Placement Agent paid a warrant cost of $0.001 per share upon issuance of the Placement Agent Bridge Warrants.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by one of our directors, officers or controlling persons in the successful defense of any action, suit or proceeding) is asserted by that director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether that indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of that issue.
The consolidated financial statements of Redwood Scientific Technologies, Inc. at December 31, 2014, and for the year then ended, included in this Registration Statement have been audited by EKS&H LLLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
| 40 |

REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Consolidated
Financial Statements
and
Report
of Independent Registered Public Accounting Firm
December 31, 2014
| 41 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Table of Contents
| Page | |||
| Report of Independent Registered Public Accounting Firm | F-1 | ||
| Consolidated Financial Statements | |||
| Consolidated Balance Sheets | F-2 | ||
| Consolidated Statements of Operations | F-3 | ||
| Consolidated Statements of Stockholders’ Equity | F-4 | ||
| Consolidated Statements of Cash Flows | F-5 – F-6 | ||
| Notes to Consolidated Financial Statements | F-7 – F-19 |
| 42 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders
Redwood Scientific Technologies, Inc.
Claremont, California
We have audited the accompanying consolidated balance sheet of Redwood Scientific Technologies, Inc. (the "Company") as of December 31, 2014, and the related consolidated statements of operations, changes in stockholders' equity, and cash flows for the year then ended. The Company's management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall consolidated financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Redwood Scientific Technologies, Inc. as of December 31, 2014, and the results of their operations and their cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
DISCLAIMER OF OPINION ON CONSOLIDATED BALANCE SHEET AND STATEMENTS OF OPERATIONS, CHANGES IN STOCKHOLDERS' EQUITY, AND CASH FLOWS AND RELATED INFORMATION
Because we were not engaged to audit the consolidated balance sheet as of September 30, 2015 and the related consolidated statements of operations, changes in stockholders' equity, and cash flows for the periods from January 1, 2014 through September 30, 2014 and January 1, 2015 through September 30, 2015, we did not extend our auditing procedures to enable us to express an opinion on the results of operations, cash flows, and related notes to these consolidated financial statements for the periods from January 1, 2014 through September 30, 2014 and January 1, 2015 through September 30, 2015. Accordingly, we express no opinion on the results of operations, cash flows, and related information for the periods from January 1, 2014 through September 30, 2014 and January 1, 2015 through September 30, 2015.
EKS&H LLLP
February 12, 2016
Denver, Colorado
| F-1 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Consolidated Balance Sheets
| December 31, 2014 | September 30, 2015 | |||||||
| (Audited) | (Unaudited) | |||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash | $ | 3,018 | $ | 412,053 | ||||
| Accounts receivable, net | 42,628 | 1,415,344 | ||||||
| Retention receivable | 357,088 | 362,086 | ||||||
| Inventory | 30,960 | 99,988 | ||||||
| Other current assets | 16,858 | 18,358 | ||||||
| Total current assets | 450,552 | 2,307,829 | ||||||
| Non-current assets | ||||||||
| Deferred tax asset | - | 74,089 | ||||||
| Total assets | $ | 450,552 | $ | 2,381,918 | ||||
| Liabilities and Stockholders' Equity | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | 31,289 | $ | 40,137 | ||||
| Accounts payable- related party | - | 85,606 | ||||||
| Accrued liabilities | 18,943 | 253,665 | ||||||
| Income taxes payable | 165,427 | 39,051 | ||||||
| Note payable - related party | 12,500 | - | ||||||
| Convertible notes payable, net of unamortized debt discount of $499,172 | - | 1,000,828 | ||||||
| Total current liabilities | 228,159 | 1,419,287 | ||||||
| Non-current liabilities | ||||||||
| Derivative warrant liability | - | 941,509 | ||||||
| Total liabilities | 228,159 | 2,360,796 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders' equity | ||||||||
| Preferred stock, $0.01 par value; 10,000,000 shares authorized, no shares issued and outstanding | - | - | ||||||
| Common stock, $0.01 par value; 5,000,000 shares authorized, issued, and outstanding at December 31, 2014; 50,000,000 shares authorized and 5,472,778 shares issued and outstanding at September 30, 2015 (unaudited) | 50,000 | 54,728 | ||||||
| Additional paid-in capital | 207,073 | 1,268,008 | ||||||
| Accumulated deficit | (34,680 | ) | (1,301,614 | ) | ||||
| Total stockholders' equity | 222,393 | 21,122 | ||||||
| Total liabilities and stockholders' equity | $ | 450,552 | $ | 2,381,918 | ||||
See notes to consolidated financial statements.
| F-2 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Consolidated Statements of Operations
| For the Year Ended December 31, 2014 (Audited) | For the Period from January 1, 2015 through September 30, 2015 (Unaudited) | For the Period from January 1, 2014 through September 30, 2014 (Unaudited) | ||||||||||
| Sales, net | $ | 3,234,582 | $ | 5,143,405 | $ | 2,396,730 | ||||||
| Cost of sales | 951,676 | 1,033,744 | 712,121 | |||||||||
| Gross profit | 2,282,906 | 4,109,661 | 1,684,609 | |||||||||
| Operating expenses | ||||||||||||
| General and administrative expenses | 1,095,415 | 2,897,619 | 955,186 | |||||||||
| Payroll, benefits, and related | 646,420 | 540,039 | 465,000 | |||||||||
| Rents and occupancy | 161,867 | 163,766 | 124,324 | |||||||||
| Bad debts | - | 1,207,923 | - | |||||||||
| Total operating expenses | 1,903,702 | 4,809,347 | 1,544,510 | |||||||||
| Income (loss) from operations | 379,204 | (699,686 | ) | 140,099 | ||||||||
| Other (expense) income | ||||||||||||
| Interest expense | - | (1,097,908 | ) | - | ||||||||
| Derivative liability income | - | 98,348 | - | |||||||||
| Total other (expense) income | - | (999,560 | ) | - | ||||||||
| Income (loss) before distributions in lieu of income taxes | 379,204 | (1,699,246 | ) | 140,099 | ||||||||
| Income tax expense (benefit) | 165,427 | (432,312 | ) | 60,019 | ||||||||
| Net income (loss) | $ | 213,777 | $ | (1,266,934 | ) | $ | 80,080 | |||||
| Basic and diluted weighted average common shares outstanding | 5,000,000 | 5,198,635 | 5,000,000 | |||||||||
| Basic and diluted income (loss) per common share | $ | 0.04 | $ | (0.24 | ) | $ | 0.02 | |||||
See notes to consolidated financial statements.
| F-3 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Consolidated Statement of Changes in Stockholders' Equity
For the Year Ended December 31, 2014, and the Period from January 1, 2015 to September 30, 2015 (Unaudited)
| Additional | (Accumulated Deficit) | Total | ||||||||||||||||||
| Common Stock | Paid-In | Retained | Stockholders' | |||||||||||||||||
| Shares | Amount | Capital | Earnings | Equity | ||||||||||||||||
| Balance - January 1, 2014 | - | $ | - | $ | - | $ | - | $ | - | |||||||||||
| Contributions from stockholder in exchange for shares issued | 5,000,000 | 50,000 | 207,073 | - | 257,073 | |||||||||||||||
| Distributions to stockholder | - | - | - | (248,457 | ) | (248,457 | ) | |||||||||||||
| Net income | - | - | - | 213,777 | 213,777 | |||||||||||||||
| Balance - December 31, 2014 | 5,000,000 | 50,000 | 207,073 | (34,680 | ) | 222,393 | ||||||||||||||
| Transfer of shares to Redwood Nevada | (5,000,000 | ) | - | - | - | - | ||||||||||||||
| Issuance of shares from Redwood Nevada | 5,000,000 | - | - | - | - | |||||||||||||||
| Common stock issued with Unit offering | 472,778 | 4,728 | 1,059,023 | - | 1,063,751 | |||||||||||||||
| Warrants issued with common stock Unit offering | - | - | (224,968 | ) | - | (224,968 | ) | |||||||||||||
| Unit offering costs | - | - | (123,300 | ) | - | (123,300 | ) | |||||||||||||
| Beneficial conversion feature on convertible promissory notes | - | - | 582,027 | - | 582,027 | |||||||||||||||
| Beneficial conversion feature on convertible promissory notes - tax impact | - | - | (231,847 | ) | - | (231,847 | ) | |||||||||||||
| Net loss | - | - | - | (1,266,934 | ) | (1,266,934 | ) | |||||||||||||
| Balance - September 30, 2015 (unaudited) | 5,472,778 | $ | 54,728 | $ | 1,268,008 | $ | (1,301,614 | ) | $ | 21,122 | ||||||||||
See notes to consolidated financial statements.
| F-4 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Consolidated Statements of Cash Flows
| For the Year Ended December 31, 2014 | For the Period from January 1, 2015 through September 30, 2015 | For the Period from January 1, 2014 through September 30, 2014 | ||||||||||
| (Audited) | (Unaudited) | (Unaudited) | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Net income (loss) | $ | 213,777 | $ | (1,266,934 | ) | $ | 80,080 | |||||
| Adjustments to reconcile net income (loss) to net cash used in operating activities | ||||||||||||
| Change in derivative warrant liability | - | (98,348 | ) | - | ||||||||
| Amortization of debt discount | - | 998,344 | - | |||||||||
| Deferred taxes | - | (305,936 | ) | - | ||||||||
| Provision for bad debt | - | 1,207,923 | - | |||||||||
| Changes in assets and liabilities | ||||||||||||
| Accounts receivable, net | (42,628 | ) | (2,580,639 | ) | (56,815 | ) | ||||||
| Retention receivable | (357,088 | ) | (4,998 | ) | (285,235 | ) | ||||||
| Accounts receivable - related party | - | - | (61,166 | ) | ||||||||
| Inventory | (30,960 | ) | (69,028 | ) | (5,316 | ) | ||||||
| Other current assets | (16,858 | ) | (1,500 | ) | - | |||||||
| Accounts payable | 31,289 | 8,848 | 44,693 | |||||||||
| Accounts payable - related party | - | 85,606 | - | |||||||||
| Accrued liabilities | 18,943 | 234,722 | - | |||||||||
| Income taxes payable | 165,427 | (126,376 | ) | 60,019 | ||||||||
| (231,875 | ) | (651,382 | ) | (303,820 | ) | |||||||
| Net cash used in operating activities | (18,098 | ) | (1,918,316 | ) | (223,740 | ) | ||||||
| Cash flows from financing activities | ||||||||||||
| Cash proceeds from Unit offering | 257,073 | 1,063,751 | 257,073 | |||||||||
| Unit offering costs | - | (123,300 | ) | - | ||||||||
| Proceeds from issuance of convertible debt | - | 1,500,000 | - | |||||||||
| Convertible debt offering costs | - | (100,600 | ) | - | ||||||||
| Distributions to stockholders | (248,457 | ) | - | - | ||||||||
| Proceeds (payments) from note payable - related party | 12,500 | (12,500 | ) | - | ||||||||
| Net cash provided by financing activities | 21,116 | 2,327,351 | 257,073 | |||||||||
| Net increase in cash | 3,018 | 409,035 | 33,333 | |||||||||
| Cash - beginning of year | - | 3,018 | - | |||||||||
| Cash - end of year | $ | 3,018 | $ | 412,053 | $ | 33,333 | ||||||
(Continued on the following page)
See notes to consolidated financial statements.
| F-5 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Consolidated Statements of Cash Flows
(Continued from the previous page)
Supplemental disclosure of non-cash activity:
| During the period from January 1, 2015 through September 30, 2015 (unaudited), the Company recorded a beneficial conversion feature on convertible notes payable of $582,027. |
| During the period from January 1, 2015 through September 30, 2015 (unaudited), the Company recorded a derivative warrant liability of $814,889 in connection with the issuance of convertible notes - Warrants A. |
| During the period from January 1, 2015 through September 30, 2015 (unaudited), the Company recorded a derivative warrant liability of $224,968 in connection with the issuance of Units for common stock and Warrants B. |
See notes to consolidated financial statements.
| F-6 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 1 - Description of Business and Summary of Significant Accounting Policies
Redwood Scientific Technologies, Inc. ("Redwood California") was organized in January 2014 under the name Advanced Men's Institute Prolongz, LLC ("AMI") in the state of California and began selling its products in February 2014. In November 2014, AMI changed its name to Redwood Scientific Technologies, LLC. Immediately after the name change, Redwood Scientific Technologies, LLC filed a statement of conversion whereby it converted from a limited liability company into a California corporation to become Redwood Scientific Technologies, Inc.
In January 2015, Redwood California entered into and consummated the transactions (the "Share Exchange") contemplated under a Share Exchange Agreement by and among Redwood California and Jason Cardiff ("Cardiff"), the sole stockholder of Redwood California. Under the terms of the Share Exchange, the stockholder transferred all of his shares of Redwood California, which represented 100% of Redwood California's shares outstanding, to Redwood Scientific Technologies, Inc., a Nevada Corporation ("Redwood Nevada"), thereby becoming a wholly owned subsidiary of Redwood Nevada. As part of the Share Exchange, the stockholder was issued 4,387,500 shares of Redwood Nevada's common stock, par value $0.01, which represented 88% of the 5,000,000 issued and outstanding shares of Redwood Nevada's common stock immediately following the Share Exchange. As defined below, Redwood California, Redwood Nevada, and other entities together form the "Company." For accounting purposes, the Share Exchange was treated as a transfer between entities under common control and all assets and liabilities recorded at their carry amounts. As a result, the business and financial information included in the report includes the historical business and financial information of Redwood California.
The Company develops and markets consumer homeopathic drugs and supplements. The Company utilizes patent-pending, sublingual strip delivery technology that enhances bioavailability. Currently, the Company's only product for sale is ProlongzTM, which has been registered with and regulated by the Food and Drug Administration ("FDA") as an over-the-counter drug #079526408 product and is sold either through a membership subscription model (a direct marketing program) or at any given number of retail locations. Prolongz is the first drug of its kind that addresses a specific sexual performance dysfunction in men and is the only drug that can be taken orally through a sublingual strip.
The Company received its second drug code from the FDA for TBX Free (FDA Registration # 69461- 001-01), its smoking cessation product, which is the only non-nicotine stop-smoking product to be delivered by sublingual strip. The Company launched TBX Free in December 2015 on a nationwide television campaign. TBX-FREE is the first FDA-registered oral strip that helps smokers safely, quickly, and effectively eliminate the craving to smoke. Every TBX-FREE oral strip contains a precisely measured dose of cytisine, the active ingredient that reduces the urge to smoke.
The Company is also in the development phase for other products that address health concerns for adults, such as smoking cessation, prostate health, and aspirin regimens, all through the use of the Company's patent-pending sublingual delivery mechanism. All of the Company's products are centered on the delivery mechanism that results from the innovative sublingual strip technology that the Company has developed.
| F-7 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 1 - Description of Business and Summary of Significant Accounting Policies (continued)
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of Redwood Nevada and its wholly owned subsidiaries, Redwood California, Owl Enterprises, LLC, American Nutra Partners, LLC, Expo Supplements, LLC, Advanced Men's Institute Prolongz, LLC, Top Hill Shop LTD, Healthy Way Express, LLC, and Nature's Future, LLC. As described above, these entities are collectively referred to as the "Company." All intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash
The Company considers all highly liquid instruments purchased with an original maturity of three months or less to be cash equivalents. The Company continually monitors its positions with, and the credit quality of, the financial institutions with which it invests.
Retention Receivable
The Company has merchant processing agreements with various financial institutions. The retention receivable balances represent funds held in escrow by the institutions for credit card sales made by the Company. Each institution retains a percentage of cash collected from credit card sales made by the Company as an allowance for customer merchandise returns for a period of approximately six months after the initial sale.
Accounts Receivable
The Company records receivables for amounts due from customers in the ordinary course of business. The Company provides an allowance for doubtful accounts, which is based upon review of outstanding receivables, historical collection information, and existing economic conditions. As of December 31, 2014, the Company considers all accounts receivable fully collectible and has not recorded an allowance for doubtful accounts based on management's analysis. The Company has recorded an allowance for doubtful accounts based on management's analysis as of September 30, 2015 (unaudited) of $380,000. The entire accounts receivable balance as of December 31, 2014 is attributable to two customers.
| F-8 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 1 - Description of Business and Summary of Significant Accounting Policies (continued)
Inventory
Inventory consists of items purchased for resale and packaging supplies and is stated at the lower of cost or market, determined using the first-in, first-out method. The Company periodically reviews inventories in order to identify obsolete, slow-moving, or otherwise unsaleable items. If unsaleable items are observed, the Company will record a write-down to net realizable value in the period that impairment is first recognized.
Derivative Warrant Liability
Freestanding financial instruments that permit the holder to acquire shares that are either puttable by the holder or redeemable by the Company are required to be reported as liabilities in the consolidated financial statements. The issuer must present such liabilities on the balance sheet at their estimated fair values. Changes in fair values of the liabilities are calculated each reporting period, and any changes in values are recognized in earnings. The Company has determined that certain warrants issued to investors and placement agents, which are exercisable for shares of the Company's common stock, shall be classified as liabilities due to an anti-dilution protection clause of the underlying securities.
Fair Value Measurements
The Company's financial instruments include cash, accounts receivable, retention receivable, accounts payable, accrued liabilities, and warrant derivative liability. The carrying amount of cash, accounts receivable, retention receivable, accounts payable, and accrued liabilities approximate their fair value due to their short maturities.
The Company accounts for certain assets and liabilities that are required to be recorded at fair value under a framework for measuring fair value that requires enhanced disclosures about fair value measurements. Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. Fair value is determined for assets and liabilities and establishes a hierarchy for which these assets and liabilities must be grouped, based on significant levels of inputs as follows:
| Level 1: | Quoted prices in active markets for identical assets or liabilities; | |
| Level 2: | Quoted prices in active markets for similar assets and liabilities and inputs that are observable for the asset or liability; or | |
| Level 3: | Unobservable inputs in which there is little or no market data, which requires the reporting entity to develop its own assumptions. |
The determination of where assets and liabilities fall within this hierarchy is based upon the lowest level of input that is significant to the fair value measurement.
| F-9 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 1 - Description of Business and Summary of Significant Accounting Policies (continued)
Fair Value Measurements (continued)
The following table sets forth by level, within the fair value hierarchy, the Company's assets and liabilities measured at fair value on a recurring basis as of September 30, 2015 (unaudited):
| Description | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| Derivative warrant liability | $ | - | $ | - | $ | 941,509 | $ | 941,509 | ||||||||
The Company uses the income valuation technique to measure fair value of the warrant liability based on the Black-Scholes option pricing model and significant assumptions and inputs determined by the Company. For the period from January 1, 2015 through September 30, 2015 (unaudited), the Company estimated the fair value of the warrant liability using the Black-Scholes option pricing model with the following assumptions: a risk-free rate of 0.77% to 0.92%, a dividend yield of 0%, an expected volatility rate of 103%, and a contractual life of 2.3 to 3 years.
Due to the unobservable inputs needed to calculate the fair value of the derivative warrant liability (Notes 3 and 6), these liabilities are classified as Level 3 liabilities. The following is a reconciliation of the beginning and ending balances for assets measured at fair value on a recurring basis using significant unobservable inputs (Level 3) as of September 30, 2015:
| Derivative warrant liability | ||||
| Beginning balance | $ | - | ||
| Issuances | 1,039,857 | |||
| Change in fair value included in earnings | (98,348 | ) | ||
| Ending balance | $ | 941,509 | ||
There were no changes to the valuation methods of the warrant liabilities during the periods presented, and the quantitative inputs are detailed in Notes 3 and 6.
Revenue Recognition
The Company recognizes sales when merchandise has been delivered to the customer, no significant continuing obligations exist, and there is reasonable assurance regarding collectibility. Sales are recorded net of returns and include shipping and handling fees billed to customers.
Shipping and Handling
Shipping and handling costs are classified in cost of sales.
| F-10 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 1 - Description of Business and Summary of Significant Accounting Policies (continued)
Income Taxes
The Company recognizes deferred tax liabilities and assets based on the differences between the tax basis of assets and liabilities and their reported amounts in the consolidated financial statements that will result in taxable or deductible amounts in future years.
The Company evaluates its tax positions taken or expected to be taken in the course of preparing the Company's tax returns to determine whether the tax positions will more likely than not be sustained by the applicable tax authority. Tax positions not deemed to meet the more-likely-than-not threshold are not recorded as a tax benefit or expense in the current year. Interest and penalties, if applicable, are recorded in the period assessed as general and administrative expenses. However, no interest or penalties have been assessed as of December 31, 2014, September 30, 2014 (unaudited), and September 30, 2015 (unaudited).
Advertising Costs
The Company expenses advertising costs as incurred. Advertising expense for the year ended December 31, 2014 was $907,346 and for the periods from January 1, 2015 through September 30, 2015 (unaudited) and January 1, 2014 through September 30, 2014 (unaudited) was $2,431,795 and $847,923, respectively.
Research and Development
Research and development costs are expensed as incurred. Research and development costs for the year ended December 31, 2015 and the periods from January 1, 2015 through September 30, 2015 (unaudited) and January 1, 2014 through September 30, 2014 (unaudited) was approximately $48,000, $37,000 and $33,000 respectively, and is included in general and administrative expenses
Earnings per Share
Basic net income per common share is based on the weighted average number of common shares outstanding during the period. Diluted net income per common share is based on the weighted average number of common shares outstanding plus the dilutive effect of common share equivalents, which include outstanding warrants. As of December 31, 2014 and September 31, 2014 (unaudited), there were no dilutive common share equivalents outstanding. As of September 30, 2015 (unaudited), outstanding warrants were not included as their effect would be anti-dilutive.
| F-11 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 1 - Description of Business and Summary of Significant Accounting Policies (continued)
Recently Issued Accounting Pronouncements, Not Adopted as of September 30, 2015
In May 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2014-09 regarding Accounting Standards Codification ("ASC") Topic 606, Revenue from Contracts with Customers. The standard provides principles for recognizing revenue for the transfer of promised goods or services to customers with the consideration to which the entity expects to be entitled in exchange for those goods or services. In August 2015, the FASB issued ASU 2015-14, Revenue from Contracts with Customers: Deferral of the Effective Date, which deferred the effective date of the new revenue standard for periods beginning after December 15, 2017, with early adoption permitted. The Company is currently evaluating the new standard and its impact on the Company's consolidated financial statements.
In April 2015, the FASB issued ASU 2015-03, Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs, to simplify the presentation of debt issuance costs. The amendments in the update require that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct reduction of the carrying amount of the debt. Recognition and measurement of debt issuance costs were not affected by this amendment. In August 2015, FASB issued ASU 2015-15, Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements—Amendments to SEC Paragraphs Pursuant to Staff Announcement at June 18, 2015 EITF Meeting, which clarified that the SEC would not object to an entity deferring and presenting debt issuance costs as an asset and subsequently amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement. The amendments are effective for financial statements issued for fiscal years beginning after December 15, 2015. As of September 30, 2015, the Company early adopted this standard and recorded debt issuance costs as a reduction to the carrying amount of debt.
In July 2015, the FASB issued ASU 2015-11, Simplifying the Measurement of Inventory. ASU 2015- 11 clarifies that inventory should be held at the lower of cost or net realizable value. Net realizable value is defined as the estimated selling price, less the estimated costs to complete, dispose, and transport such inventory. ASU 2015-11 will be effective for fiscal years and interim periods beginning after December 15, 2016. ASU 2015-11 is required to be applied prospectively and early adoption is permitted. The Company is currently evaluating the new standard and its impact on the Company's consolidated financial statements.
In August 2014, the FASB issued ASU 2014-15, Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern. ASU 2014-15 is intended to define management's responsibility to evaluate whether there is substantial doubt about an organization's ability to continue as a going concern and to provide related footnote disclosures. The amendments in this ASU are effective for reporting periods beginning after December 15, 2016, with early adoption permitted. The Company is currently evaluating the new standard and its impact on the Company's consolidated financial statements
| F-12 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 1 - Description of Business and Summary of Significant Accounting Policies (continued)
Recently Issued Accounting Pronouncements, Not Adopted as of September 30, 2015 (continued)
In September 2015, the FASB issued ASU 2015-16, Business Combinations (Topic 805): Simplifying the Accounting for Measurement-Period Adjustments, which requires that an acquirer recognize adjustments to estimated amounts that are identified during the measurement period in the reporting period in which the adjustment amounts are determined. The amendments require that the acquirer record, in the same period's financial statements, the effect on earnings of changes in depreciation, amortization, or other income effects, if any, as a result of the change to the estimated amounts, calculated as if the accounting had been completed at the acquisition date. The amendments also require an entity to present separately on the face of the income statement or disclose in the notes the portion of the amount recorded in current-period earnings by line item that would have been recorded in previous reporting periods if the adjustment to the estimated amounts had been recognized as of the acquisition date. The amendment is effective for financial statements issued for fiscal years beginning after December 15, 2015 and early adoption is permitted. As of September 30, 2015, the Company has not entered into any business combinations.
In November 2015, the FASB issued ASU No. 2015-17 regarding ASC Topic 470, Income Taxes: Balance Sheet Classification of Deferred Taxes. The amendments in ASU 2015-17 eliminate the requirement to bifurcate deferred taxes between current and non-current on the balance sheet and require that deferred tax liabilities and assets be classified as non-current on the balance sheet. The amendments for ASU-2015-17 can be applied retrospectively or prospectively and early adoption is permitted. As of September 30, 2015 the Company early adopted this standard and recorded deferred tax assets as non-current on the balance sheet.
In January 2016, the FASB issued ASU 2016-01, Financial Instruments—Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities, which requires that all equity investments be measured at fair value with changes in the fair value recognized through net income (other than those accounted for under the equity method of accounting or those that result in consolidation of the investee). The amendments in this update also require an entity to present separately in other comprehensive income the portion of the total change in the fair value of a liability resulting from a change in the instrument-specific credit risk when the entity has elected to measure the liability at fair value in accordance with the fair value option for financial instruments. In addition, the amendments in this update eliminate the requirement to disclose the fair value of financial instruments measured at amortized cost for entities that are not public business entities and the requirement to disclose the method(s) and significant assumptions used to estimate the fair value that is required to be disclosed for financial instruments measured at amortized cost on the balance sheet for public business entities. The amendment is effective for financial statements issued for fiscal years beginning after December 15, 2017. Early adoption is not permitted. The Company is currently evaluating the new standard and its impact on the Company's consolidated financial statements.
| F-13 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 2 - Related Party Transactions
The Company shares its leased office and warehouse facilities with affiliated companies of the majority stockholder. The office and warehouse facilities' operating expenses are allocated on a pro rata basis of the square footage occupied by each company.
As of September 30, 2015, the Company had an account payable of $85,606 to Runaway Products, which purchases media for the Company and is a related entity to a stockholder of the Company.
Note 3 - Convertible Debt
In March 2015, Redwood Nevada issued convertible promissory notes (the "Notes") and warrants to investors (the "Debt Offering"). The Notes raised total proceeds of $1,500,000, bear interest at 10%, and are due one year from the issue date. The Notes are convertible into common stock at an initial conversion price of $2.00 per share. Pursuant to the terms of the Notes, upon Redwood Nevada's receipt of at least $2,500,000 in additional financing, the Notes shall automatically convert into common stock of Redwood Nevada at the lesser of the conversion price of $2.00 per share or a 12.5% discount to the price of the securities issued with the additional financing. If the Notes are not converted, the Notes and accrued interest will be repaid on the maturity date and the warrants shall terminate. The Notes are collateralized by substantially all assets of the Company. The warrants are exercisable at any time into common stock at an initial exercise price of $2.50 per share and shall terminate after three years. The holders of the Notes are entitled to an 8% bonus interest if a registration statement registering the shares of common stock underlying the Notes and warrants is not declared effective by the maturity date. In December 2015, the Company raised more than $2,500,000 in additional financing and, as such, the Notes were converted to common stock; this resulted in issuing additional warrants at a discounted conversion rate totaling 11,421 warrants.
At the date of issuance, the fair value of the warrants was determined to be $754,527 and has been recorded as a debt discount that is amortized to interest expense over the term of the debt. As of September 30, 2015 (unaudited), the unamortized balance of the debt discount was $251,509.
In connection with the issuance of the Notes, the Company additionally recorded a beneficial conversion feature of $582,027. The beneficial conversion feature has been recorded as a debt discount that is amortized to interest expense over the term of the debt. As of September 30, 2015 (unaudited), the unamortized balance of the debt discount was $194,009.
The Company did not give recognition to registration rights arrangements as management did not believe at issuance that a probable payment under the contingency would be required.
| F-14 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 3 - Convertible Debt (continued)
The Company paid the placement agents of the Debt Offering $100,600 and issued warrants to purchase 53,604 shares of common stock. The placement agent warrants have a term of three years with an exercise price of $2.00 per share. The warrants had a fair value at issue of $60,362, which, along with the $100,600 paid in cash, was recorded as debt issuance costs and amortized over the term of the debt. As of September 30, 2015 (unaudited), the unamortized balance of the debt discount was $53,654.
The Debt Offering includes a condition that, in the event the Company has less than $20,000,000 in gross revenue or less than $1 in earnings before interest, taxes, depreciation and amortization ("EBITDA") for the subsequent 12 months following the close of $6,500,000 in funding, the Company will be required to issue an additional 20% of the common stock and warrants underlying the debenture and pursuant to the Debt Offering. In addition, the Company shall use reasonable best efforts to distribute preliminary financial statements for this 12-month period within 45 days after the 12 month- ends and audited financial statements if requested 60 days after written notice of the request, for purposes of determining whether the Company shall issue such additional units. If the Company fails to use reasonable best efforts to distribute such financial statements containing the information necessary to calculate the Company's revenue, such failure shall result in the investors receiving an additional 100% of the units purchased in this Debt Offering. As of February 12, 2016, the $6,500,000 of funding has not been raised.
Note 4 - Income Taxes
The Company recognizes deferred tax liabilities and assets for the expected future tax consequences of events that have been included in the consolidated financial statements or tax returns. Deferred tax liabilities and assets are determined based on the differences between the financial statement and tax basis of assets and liabilities using the enacted tax rates in effect for the year in which the differences are expected to reverse. The measurement of deferred tax assets is reduced, if necessary, by the amount of any tax benefits that are not expected to be realized based on available evidence. The Company expects future taxable income and, therefore, believes it will recognize future benefits related to its deferred tax asset. The Company's effective tax rate differs from the statutory tax rate due to permanent differences and state tax provision, primarily related to amortization of debt discounts. Significant components of the company’s deferred tax assets and liabilities include allowance for bad debts.
| F-15 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 5 - Commitments and Contingencies
Operating Leases
The Company leases facilities, equipment, and vehicles under non-cancelable operating leases with affiliated companies of the majority stockholder. Rent expense for the year ended December 31, 2014 and the periods from January 1, 2015 through September 30, 2015 (unaudited) and January 1, 2014 through September 30, 2014 (unaudited) was $128,725, $100,154, and $124,324, respectively.
| Amount per | Company's | |||||||
| Lease | Allocated | |||||||
| Year Ending December 31, | Agreement | Amount | ||||||
| 2016 | $ | 110,000 | $ | 77,000 | ||||
Litigation
In the normal course of business, the Company is party to litigation from time to time. The Company maintains insurance to cover certain actions and believes that resolution of such litigation will not have a material adverse effect on the Company. While the outcome of these claims cannot be predicted with certainty, management does not believe that the outcome of any of these legal matters will have a material adverse effect on the Company's financial position or results of operations.
General Liability Insurance
The Company purchased general liability insurance to cover product liability of $2,000,000 per incident up to a maximum of $2,000,000 for the year ended December 31, 2014 and the periods from January 1, 2015 through September 30, 2015 (unaudited) and January 1, 2014 through September 30, 2014 (unaudited). The insurance policy expires in April 2016. In the opinion of the Company's management, the insurance coverage carried by the Company is adequate to materially protect the Company against such claims, if any, that may be brought against the Company.
Note 6 - Stockholders' Equity
The Company is authorized to issue up to 60,000,000 shares of stock, of which 50,000,000 is common stock with a par value of $0.01 per share and 10,000,000 is preferred stock with a par value of $0.01 per share. As of September 30, 2015 (unaudited), there were 5,472,778 shares of common stock outstanding.
During 2014, the founding stockholder contributed cash and certain intellectual property, which was recorded at cost totaling $257,073, in exchange for 5,000,000 shares of common stock.
In March 2015, Redwood Nevada began a private placement offering for up to a maximum of 2,222,222 units ("Units") at a purchase price of $2.25 per Unit (the "Unit offering"). Each Unit consists of one share of the Company's common stock and a warrant to purchase half that number of shares of common stock at an exercise price of $2.75 per share. As of September 30, 2015 (unaudited), 472,778 Units were issued for total gross proceeds of $1,063,751. The warrants issued to investors with the Unit offering were recorded at their fair value of $224,968 as additional offering costs.
| F-16 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 6 - Stockholders' Equity (continued)
Placement agents for the Unit offering receive up to 8% of gross proceeds sold by the placement agents and receive warrants for an amount equal to 8% of the number of shares of common stock underlying the Unit offering. As of September 30, 2015 (unaudited), the Company had accrued $123,300 of offering costs to be paid in cash and the estimated fair value of the warrants to be issued upon final closing of the Unit offering.
The Unit offering contains purchase price protection stating if the Company issues common stock or any type of securities giving rights to common stock at a price below the Unit price, investors will be extended full ratchet anti-dilution protection through the issuance of additional common shares. Anti- dilution protection will not apply to shares (or options to purchase shares) issued to employees, directors, and consultants as part of a pre-existing equity incentive plan or agreement adopted by the Company's board up to an aggregate of 250,000 shares and other limited exceptions.
The Unit investors are entitled to cash damages equal to 1% of an investor's purchase price per month if a registration statement registering the shares of common stock underlying the common stock and warrants is not filed within 90 days of the final closing of the Unit offering. The Company did not give recognition to registration rights arrangements as management did not believe at issuance that a probable payment under the contingency would be required.
The Unit offering contains a clause that, in the event the Company has less than $20,000,000 in gross revenue or less than $1 in EBITDA for the subsequent 12 months following the close of $6,500,000 in funding (including funds received from the bridge financing), the Company shall issue an additional 20% of the common stock and warrants underlying the Units issued pursuant to this Unit offering. In addition, the Company shall use reasonable best efforts to distribute preliminary financial statements for this 12-month period within 45 days after the 12-month period ends and audited financial statements if requested 60 days after written notice of the request, for purposes of determining whether the Company shall issue such additional Units. If the Company fails to use reasonable best efforts to distribute such financial statements containing the information necessary to calculate the Company's revenue, such failure shall result in the investors receiving an additional 100% of the Units purchased in this Unit offering. As of February 12, 2016, the $6,500,000 of funding has not been raised.
| F-17 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 6 - Stockholders' Equity (continued)
Warrants
Warrants A
As part of the Notes issued in March 2015, the Company issued warrants A ("Warrants A") to the Note holders. Warrants A are exercisable at any time into common stock at an initial exercise price of $2.50 per share and shall terminate after three years. The holders of the Notes are entitled to an 8% bonus interest if a registration statement registering the shares of common stock underlying the Notes and Warrants A are not declared effective by the maturity date. Warrants A may be exercised on a cashless basis. The exercise price applicable to any unexercised Warrants A shall be subject to standard adjustments for stock splits and other customary changes in capital structure, to partial ratchet anti- dilution protection adjustments for issuances at less than the exercise price (subject to certain exempt issuances defined in the Warrant); and subject to 30 business days' prior notice to the holders of the warrants, and provided an effective registration statement is in effect covering the common stock underlying Warrants A, all, but not less than all, of Warrants A will be callable by the Company at $0.01 per share at any time after the closing price if the Company's common stock exceeds 250% of the conversion price for any 20 consecutive trading days and average daily volume during the same period exceeds 50,000 shares per day.
Warrants B
As part of the Units issued during 2015, the Company issued warrants B ("Warrants B"), which are exercisable at any time after the final closing date at an initial exercise price of $2.75 per share, and shall expire on the third anniversary. Warrants B may be exercised for cash or via a cashless exercise under certain circumstances as set forth in the Warrant agreement.
Placement Agent Warrants
As part of the Debt Offering, the Company issued warrants to placement agents to purchase 53,604 shares of common stock. The placement agent warrants have a term of three years with an exercise price of $2.00 per share. The exercise price applicable to any unexercised placement agent warrants shall be subject to standard adjustments for stock splits and other customary changes in capital structure, to partial ratchet anti-dilution protection adjustments for issuances at less than the exercise price (subject to certain exempt issuances defined in the warrant); and subject to 30 business days' prior notice to the placement agent warrant holders and provided an effective registration statement is in effect covering the placement agent warrant shares, the Company may call all, but not less than all, of the placement agent warrants at $0.01 per share at any time after the closing price if the common stock exceeds 200% of the Unit price for any 20 consecutive trading days and average daily volume during the same period exceeds 50,000 shares per day.
| F-18 |
REDWOOD SCIENTIFIC TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
(Information with Respect to September 30, 2015 and 2014 is Unaudited)
Note 6 - Stockholders' Equity (continued)
Warrants (continued)
The following table presents the activity for warrants outstanding:
| Warrants A | Warrants B | Placement Agent Warrants | ||||||||||||||||||||||
| Weighted | Weighted | Weighted | ||||||||||||||||||||||
| Average | Average | Average | ||||||||||||||||||||||
| Exercise | Exercise | Exercise | ||||||||||||||||||||||
| Number | Price | Number | Price | Number | Price | |||||||||||||||||||
| Outstanding - December 31, 2014 | - | $ | - | - | $ | - | - | $ | - | |||||||||||||||
| Issued | 750,000 | 2.50 | 230,834 | 2.75 | 53,604 | 2.00 | ||||||||||||||||||
| Forfeited/canceled | - | - | - | - | - | - | ||||||||||||||||||
| Exercised | - | - | - | - | - | - | ||||||||||||||||||
| Outstanding - September 30, 2015 (unaudited) | 750,000 | $ | 2.50 | 230,834 | $ | 2.75 | 53,604 | $ | 2.00 | |||||||||||||||
All of the outstanding warrants are exercisable. Warrants A and placement agent warrants have a weighted average remaining contractual life of 2.33. Warrants B have a weighted average remaining contractual life of 3.00.
Note 7 - Subsequent Events
On February 12, 2016, the Company filed a Form S-1 filing with the United States Securities and Exchange Commission to register the shares, warrants, and placement agent warrants in connection with the Notes and the private placement memorandum.
Subsequent to September 30, 2015 (unaudited), the Company sold an additional 1,056,667 Units for $2,377,501. On January 18, 2016, the Company completed the private offering, which raised $3,441,252 from the sale of 1,609,445 Units. Accordingly, pursuant to the offering, the Company issued a total of 1,529,445 shares of common stock and warrants to purchase up to a total of 764,723 shares of common stock. In addition, the placement agents associated with the Units received $122,760 and 127,379 warrants to purchase shares of common stock.
| F-19 |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The following table sets forth the costs and expenses, other than underwriting discounts and commissions, to be paid by the Registrant in connection with the issuance and distribution of the common stock being registered. All amounts other than the SEC registration fee are estimates.
| SEC Registration Fee | $ | 920.41 | ||
| Printing and Engraving Expenses | $ | 0 | ||
| Legal Fees and Expenses | $ | 37,500 | ||
| Accounting Fees and Expenses | $ | 50,000 | ||
| Miscellaneous | $ | 0 | ||
| Total | $ | 88,420.41 |
*Estimated as permitted under Rule 511 of Regulation S-K
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Section 16 of our Bylaws provides that each person who was or is made a party or is threatened to be a party to or is involved in any action, suit or proceeding, whether civil, criminal, administrative or investigative ("proceeding"), by reason of the fact that he or she or a person for whom he or she is the legal representative is or was one of our directors or officers, employees or agents or is or was serving at our request as a director or officer, employee or agent of another corporation, or of a partnership, joint venture, trust or other enterprise, including service with respect to employee benefit plans, whether the basis of such proceeding is alleged action in an official capacity as a director, officer, employee or agent or in any other capacity while serving as a director, officer, employee or agent or in any other capacity while serving as a director, officer, employee or agent, shall be indemnified and held harmless to the fullest extent authorized by the Nevada Revised Statutes. Such right includes the right to be paid by the Company for expenses incurred in defending any such proceeding in advance of its final disposition; provided, however, that the payment of such expenses in advance of the final disposition of such proceeding, shall be made only upon delivery of an undertaking, by or on behalf of such director or officer, to repay all amounts so advanced if it should be determined ultimately that such officer or director is not entitled to be indemnified under our the laws.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the registrant, pursuant to the foregoing provisions, the Registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
During the past three years, we effected the following transactions in reliance upon exemptions from registration under the Securities Act as amended. Unless stated otherwise: (i) that each of the persons who received these unregistered securities had knowledge and experience in financial and business matters which allowed them to evaluate the merits and risk of the receipt of these securities, and that they were knowledgeable about our operations and financial condition; (ii) no underwriter participated in, nor did we pay any commissions or fees to any underwriter in connection with the transactions; (iii) the transactions did not involve a public offerings; and (iv) each certificate issued for these unregistered securities contained a legend stating that the securities have not been registered under the Act and setting forth the restrictions on the transferability and the sale of the securities.
On January 30, 2015, we issued $1,065,000 Series A Senior Convertible Redeemable Notes (the “Notes”) that are initially convertible into 587,415 shares of common stock and warrants to purchase up to an aggregate of 540,609 shares of our common stock, pursuant to a private financing.
On February 28, 2015 we issued $435,000additional Series A Senior Convertible Redeemable Notes that are initially convertible into 238,285 shares of common stock and warrants to purchase up to an aggregate of 220,812 shares of our common stock, pursuant to a private financing.
On January 18, 2016, we issued 1,529,445 Units, consisting of 1529,445 shares of Common Stock and Warrants to purchase up to a total of 764,723shares of Common Stock.
| II-1 |
Except as otherwise noted, all of the transactions listed above were made pursuant to the exemption from the registration provisions of the Securities Act of 1933, as amended, provided by Section 4(a)(2) of the Securities Act for sales not involving a public offering or Rule 506(b) of Regulation D promulgated by the SEC. The securities issued have not been registered under the Securities Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
EXHIBITS
The following exhibits are filed as part of this registration statement:
Exhibit Number |
Description: | |
| 3.1 | Certificate of Incorporation of Registrant | |
| 3.2 | Bylaws of the Company. | |
| 5.1 | Legal Opinion of Hunter Taubman Fischer, LLC | |
| 10.1 | Unit Purchase Agreement | |
| 10.2 | Form of Warrant | |
| 10.3 | Form of Registration Rights Agreement | |
| 10.4 | Unit Purchase Agreement for the Bridge Financing | |
| 10.5 | Form of Note | |
| 10.6 | Form of Warrant for the Bridge Financing | |
| 10.7 | Form of Registration Rights Agreement for the Bridge Financing | |
| 10.8 | Form of Security Agreement | |
| 10.9 | Employment Agreement between the Company and Jason Cardiff | |
| 10.10 | Employment Agreement between the Company and Jacques Poujade | |
| 10.11 | Employment Agreement between the Company and Eunjung Cardiff | |
| 10.12 | Employment Agreement between the Company and Stefan Galluppi | |
| 14.1 | Code of Ethics | |
| 21.1* | Subsidiaries of Redwood Scientific Technologies, Inc. | |
| 23.1 | Consent of Hunter, Taubman Fischer, LLC (included on Exhibit 5.1) | |
| 23.2 | Consent of EKS & LLLP |
* To be filed by amendment.
ITEM 17. UNDERTAKINGS
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made pursuant to this Registration Statement, a post-effective amendment to this registration statement:
(i) to include any prospectus required by Section 10(a)(3) of the Securities Act of 1933.
(ii) to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) to include any material information with respect to the plan of distribution not previously disclosed in this Registration Statement or any material change to such information in this Registration Statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new Registration Statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
| II-2 |
(4) The undersigned Registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the Registrant’s annual report pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(5) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions described in Item 15 above, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
| II-3 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Claremont, State of California on February 12, 2016.
| REDWOOD SCIENTIFIC TECHNOLOGIES, INC. | ||
| By: | /s/ Jason Cardiff | |
| Name: | Jason Cardiff | |
| Title: | CEO | |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Jason Cardiff | President, Chief Executive Officer, and Director | February 12, 2016 | ||
| Jason Cardiff | (Principal Executive Officer) | |||
| Chief Operating Officer, Chief Financial Officer and Secretary (Principal Financial Officer and Principal Accounting Officer) | February 12, 2016 | |||
| /s/ Jacques Poujade | ||||
| Jacques Poujade | Director | February 12, 2016 | ||
| /s/ Eunjung Cardiff | ||||
| Eunjung Cardiff | Director | February 12, 2016 | ||
| /s/ Jaci Warren | ||||
| Jaci Warren | Director | February 12, 2016 | ||
| /s/ Christine Hayes | ||||
| Christine Hayes | Director | February 12, 2016 | ||
| /s/ Kevin Harrington | ||||
| Kevin Harrington | Director | February 12, 2016 | ||
| /s/ Patrick Moynihan | ||||
| Patrick Moynihan | Director | February 12, 2016 |
II-4
