Attached files
| file | filename |
|---|---|
| EX-10.19 - EXHIBIT 10.19 - CAMBREX CORP | ex10-19.htm |
| EX-23 - EXHIBIT 23 - CAMBREX CORP | ex23.htm |
| EX-31.2 - EXHIBIT 31.2 - CAMBREX CORP | ex31-2.htm |
| EX-32 - EXHIBIT 32 - CAMBREX CORP | ex32.htm |
| EX-21 - EXHIBIT 21 - CAMBREX CORP | ex21.htm |
| EX-31.1 - EXHIBIT 31.1 - CAMBREX CORP | ex31-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2015
OR
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF
THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission file number 1-10638
CAMBREX CORPORATION
(Exact name of registrant as specified in its Charter)
|
Delaware |
|
22-2476135 |
|
(State or other jurisdiction of |
|
(I.R.S. Employer |
|
incorporation or organization) |
|
Identification No.) |
|
|
|
|
|
One Meadowlands Plaza, |
|
|
|
East Rutherford, New Jersey |
|
07073 |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant's telephone number, including area code: (201) 804-3000
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
Name of each exchange on which registered |
|
Common Stock, $.10 par value |
New York Stock Exchange |
Securities registered pursuant to Section 12 (g) of the Act: (None)
Indicate by check mark whether the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒. No ☐.
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐. No ☒.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒. No ☐.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☒. No ☐.
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer ☒ |
Accelerated filer ☐ |
Non-accelerated filer ☐ |
Smaller reporting company ☐ |
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐. No ☒.
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant was approximately $1,353,570,294 as of June 30, 2015.
As of January 29, 2016, there were 31,799,188 shares outstanding of the registrant's Common Stock, $.10 par value.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the Registrant’s definitive Proxy Statement for the 2016 Annual Meeting are incorporated by reference into Part III of this Report.
CAMBREX CORPORATION AND SUBSIDIARIES
INDEX TO ANNUAL REPORT ON FORM 10-K
For the Year Ended December 31, 2015
|
Item No. |
PART I |
Page No. |
|
|
|
|
|
1 |
Business |
4 |
|
1A |
Risk Factors |
9 |
|
1B |
Unresolved Staff Comments |
18 |
|
2 |
Properties |
19 |
|
3 |
Legal Proceedings |
19 |
|
4 |
Mine Safety Disclosures |
19 |
|
|
|
|
|
|
|
|
|
|
PART II |
|
|
|
|
|
|
5 |
Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
19 |
|
6 |
Selected Financial Data |
22 |
|
7 |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
23 |
|
7A |
Quantitative and Qualitative Disclosures about Market Risk |
36 |
|
8 |
Financial Statements and Supplementary Data |
36 |
|
9 |
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
75 |
|
9A |
Controls and Procedures |
75 |
| 9B | Other Information | 76 |
| PART III | ||
| 10 | Directors, Executive Officers and Corporate Governance | 77 |
| 11 | Executive Compensation | 78 |
| 12 | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 78 |
| 13 | Certain Relationships and Related Transactions, and Director Independence | 78 |
| 14 | Principal Accountant Fees and Services | 78 |
| PART IV | ||
| 15 | Exhibits and Financial Statement Schedules | 79 |
Forward-Looking Statements
This document contains and incorporates by reference forward-looking statements including statements regarding expected performance, including, but not limited to, the Company’s belief that cash flows from operations, along with funds available from the revolving line of credit, will be adequate to meet the operational and debt servicing needs of the Company, as well as other statements relating to expectations with respect to sales, the timing of orders, research and development expenditures, earnings per share, capital expenditures, the outcome of pending litigation (including environmental proceedings and remediation investigations) and related estimates of potential liability, acquisitions, divestitures, collaborations or other expansion opportunities. These statements may be identified by the fact that they use words such as “may,” “will,” “could,” “should,” “would,” “expect,” “anticipate,” “intend,” “estimate,” “believe” or similar expressions. Any forward-looking statements contained herein are based on current plans and expectations and involve risks and uncertainties that could cause actual outcomes and results to differ materially from current expectations. The factors described in Item 1A of Part I of this Annual Report on Form 10-K , captioned “Risk Factors,” or otherwise described in the Company’s filings with the Securities and Exchange Commission, provide examples of such risks and uncertainties that may cause the Company’s actual results to differ materially from the expectations the Company describes in its forward-looking statements, including, but not limited to, pharmaceutical outsourcing trends, competitive pricing or product developments, government legislation and regulations (particularly environmental issues), tax rates, interest rates, technology, manufacturing and legal issues, including the outcome of outstanding litigation, changes in foreign exchange rates, uncollectible receivables, the timing of orders, loss on disposition of assets, cancellation or delays in renewal of contracts, lack of suitable raw materials or packaging materials, the Company’s ability to receive regulatory approvals for its products and continued demand in the U.S. for late stage clinical products or the successful outcome of the Company’s investment in new products.
The forward-looking statements are based on the beliefs and assumptions of Company management and the information available to Company management as of the date of this report. The Company cautions investors not to place significant reliance on expectations regarding future results, levels of activity, performance, achievements or other forward-looking statements. The information contained in this Annual Report on Form 10-K is provided by the Company as of the date hereof, and, unless required by law, the Company does not undertake and specifically disclaims any obligation to update these forward-looking statements contained in this Annual Report on Form 10-K as a result of new information, future events or otherwise.
(dollars in thousands, except per share data)
PART I
Item 1 Business.
General
Cambrex Corporation (the "Company" or "Cambrex"), a Delaware corporation, began business in December 1981. Cambrex is a life sciences company that provides products and services that accelerate and improve the development and commercialization of new and generic therapeutics. The Company primarily supplies its products and services worldwide to innovator and generic pharmaceutical companies. The Company's overall strategy is to: grow its portfolio of custom development projects, especially those in the later stages of the clinical trial process; secure long-term supply agreements to produce active pharmaceutical ingredients (“APIs”) and intermediates for newly approved drug products; expand sales of products and projects based on its proprietary technologies; partner with generic drug companies to grow the Company’s extensive portfolio of generic APIs; and develop, or co-develop, with partners a portfolio of niche generic drug products in finished dosage form. The Company also seeks to demonstrate excellence in regulatory compliance, environmental, health and safety, and customer service. Cambrex has three operating segments, which are manufacturing facilities that have been aggregated as one reportable segment.
The Company uses a consistent business approach:
|
● |
Niche Market Focus: The Company participates in niche markets where significant technical expertise provides a competitive advantage and market differentiation. |
|
● |
Market Leadership: The Company secures leading market positions through excellent customer service, proprietary technologies, specialized capabilities and an outstanding regulatory record and leverages these capabilities across the market segments in which it participates. |
|
● |
New Products and Services: The Company continues to invest in research and development (“R&D”) in order to introduce new generic and controlled substance APIs, a portfolio of niche generic drug products in finished dosage form, and optimize manufacturing processes to accelerate revenue growth, provide a competitive advantage and maintain its leading market positions. |
|
● |
Operational Excellence: The Company maintains its commitment to continually improve productivity and customer service levels and maintains excellent quality and regulatory compliance systems. |
|
● |
Acquisition and Licensing: The Company may drive growth in strategic business segments through the prudent acquisition of businesses, products, product lines, technologies and capabilities to enhance the Company's position in its niche markets. |
Market Overview and Growth Drivers
The Company participates in markets that serve the healthcare industry. Customers include generic drug companies and companies that discover and commercialize small molecule human therapeutics using organic chemistry.
The aging western population, continued investment in healthcare research and drug development, growth in the world’s developing markets, and the necessity to develop therapeutics to address unmet needs drives business growth in life sciences companies. Aging "baby boomers" in the United States, Europe and Japan may provide an enormous healthcare opportunity. This group typically has more education, a higher socio-economic level and higher demands for healthcare services than previous generations.
(dollars in thousands, except per share data)
Demand for Cambrex products and services is dependent upon some of its customers’ continuing access to financial resources to advance their R&D projects for therapeutic candidates from the laboratory to the clinic, and eventually, to the patient. Healthcare investment comes from a variety of sources. Large pharmaceutical and biotechnology companies spend billions on drug discovery and development and billions more are spent by numerous smaller emerging pharmaceutical companies. Macro-economic conditions can have an impact on the availability of funding for the Company’s customers, especially many of the smaller companies that are often dependent upon venture capital and other private sources of funding.
Cambrex assists companies in developing robust processes for the manufacture of clinical and commercial quantities. Product testing, analytical methods and quality processes are integrated into the manufacturing process. Cambrex excels in the manufacture and testing of APIs and drug substances at laboratory, clinical and commercial scale and specializes in optimizing manufacturing processes.
Demand for outsourced services from pharmaceutical companies continues to grow. Large pharmaceutical companies outsource a portion of the development and manufacturing of intermediates and APIs to manage multiple internal priorities, access new technologies or additional capacity, preserve needed capital or ensure multiple sources of supply. Many emerging pharmaceutical and generic drug companies outsource all process development and manufacturing, and larger pharmaceutical companies typically outsource development and manufacturing. With large plants and product development resources in both Europe and the U.S., and large teams of professionals with substantial experience in the development, scale-up and operation of pharmaceutical manufacturing processes, Cambrex is particularly well positioned to assist drug companies with these much needed services for APIs.
New drugs are typically patented. When the patent expires, the drug may be manufactured and marketed in its generic form. Growth in the generic drug market is driven by the continuing stream of drug patents that will expire in the future and favorable market forces that encourage the use of generic pharmaceuticals as a more cost effective alternative to higher-priced branded drugs. In the United States, and many countries in Europe, governments and prescription benefit management companies provide incentives for generic substitution to reduce costs. Cambrex manufactures approximately 100 generic APIs, typically in relatively small quantities for use in niche therapeutics. The Company also continuously develops a portfolio of APIs for eventual commercial sale to generic drug companies upon future patent expiration.
The Company recently began developing a portfolio of finished dosage form generic drug products and expects to eventually file abbreviated New Drug Applications (“ANDAs”) in the U.S. and may make equivalent filings in other countries to market these products. Cambrex will work with formulation development, manufacturing and marketing partners and may fund all or a portion of the expenses necessary to bring these products to market. Given expected development and approval times, the Company does not expect to realize revenues from this initiative until 2018 at the earliest, although this could be sooner if the Company acquires products already being sold commercially.
The market for human therapeutics is regulated by the Food and Drug Administration (“FDA”) in the United States and other similar regulatory agencies throughout the world. These agencies oversee and regulate the development, manufacturing and commercialization processes for APIs and regulated intermediates. Excellent regulatory and quality systems as well as extensive experience in pharmaceutical fine chemical scale-up and manufacturing are essential to serve the industry and serve as a barrier to entry for potential new competitors.
Competitors from developing markets continually increase their capabilities in drug substance manufacturing and finished dosage form drugs. While overall global demand has been lifted by the rapid growth in certain developing markets, the presence of competitors within these markets, who have lower cost structures and competition in general, have resulted in downward pricing pressure throughout the pharmaceutical supply chain, and especially on generic APIs and early stage development services for clinical phase products. Pricing pressures, due to developing market competitors, on later stage clinical projects and supply arrangements for patented products has been limited to date, although these pressures may increase as competitors in developing markets improve their quality, regulatory and manufacturing systems to become more acceptable as suppliers to larger pharmaceutical companies. Cambrex regularly sources R&D services, raw materials and certain intermediates from developing market companies.
(dollars in thousands, except per share data)
Products
The Company uses its technical expertise in a wide range of chemical processes to meet the needs of its customers for high quality products and services for specialized applications.
The Company’s business is primarily comprised of the custom development and manufacture of pharmaceutical ingredients derived from organic chemistry. Products and services are supplied globally to innovator and generic drug companies. Products include APIs, pharmaceutical intermediates and, to a lesser extent, other fine chemicals.
The Company’s products and services are sold to a diverse group of several hundred customers, with one customer, Gilead Sciences, Inc., accounting for 34.5% of 2015 consolidated sales and 24.0% of 2014 consolidated sales. The Company’s products are sold through a combination of direct sales and independent agents. One API, an antiviral product, represented 32.1% and 22.9% of 2015 and 2014 consolidated sales, respectively.
The following table shows gross sales by geographic area:
|
2015 |
2014 |
2013 |
||||||||||
|
Europe |
$ | 280,593 | $ | 232,894 | $ | 210,463 | ||||||
|
North America |
127,024 | 117,477 | 86,974 | |||||||||
|
Asia |
14,024 | 12,865 | 13,800 | |||||||||
|
Other |
12,215 | 10,914 | 5,975 | |||||||||
|
Total |
$ | 433,856 | $ | 374,150 | $ | 317,212 | ||||||
Marketing and Distribution
Marketing generally requires significant cooperative effort among a highly trained sales and marketing staff, a scientific staff that can assess the technical fit and estimate manufacturing economics, manufacturing and engineering staff to scale up the chemical process and business unit management to determine the strategic and operational fit. The process to take a client's project from the clinical trial stage to a commercial, approved therapeutic may take from two to ten years. The Company uses sales agents in those areas where they are deemed to be more effective or economical than direct sales efforts, primarily to access generic API customers in markets outside the U.S. and Western Europe.
Raw Materials
The Company uses a wide array of raw materials in its businesses. For its products, the Company generally will attempt to have a primary and secondary supplier for its critical raw materials. Prices for these raw materials are generally stable, except for the petroleum-based solvents and certain other commodity materials, where prices can vary with market conditions.
Research and Development
The Company's R&D program is designed to increase the Company's competitiveness by improving its technology and developing processes for the manufacture of new products to meet customer requirements. The goals are to grow our portfolio of generic APIs, establish a portfolio of finished dosage form generic drug products, introduce innovative and proprietary products, improve manufacturing processes to reduce costs, improve quality and increase our capabilities to compete for business requiring significant technical expertise. R&D activities are performed at all of the Company's manufacturing facilities. As of December 31, 2015, 166 employees are at least partially involved in R&D activities worldwide.
(dollars in thousands, except per share data)
The Company spent $12,540, $13,075 and $10,387 in 2015, 2014 and 2013, respectively, on R&D efforts.
Patents and Trademarks
The Company has patent protection covering certain products, processes and services. In addition, the Company also relies on know-how and trade secrets (related to many of its manufacturing processes and techniques not generally known to other companies) for developing and maintaining its market position. The Company currently owns 22 issued patents and has 3 patent applications pending in the United States and owns over 190 patents and has over 100 patent applications pending in foreign countries covering various technologies. The Company seeks to protect its proprietary technology and prepares new patent applications as it develops new inventions.
The patent rights the Company considers most significant to its business are U.S. Patent Nos. 6,828,336 and 6,586,449 and 26 foreign counterparts which relate to its nicotine polacrilex resin products and methods of manufacturing, and expire on May 28, 2022.
The Company's products and services are sold around the world under trademarks that are owned by the Company. This includes Profarmaco, which is registered around the world as a word and design mark. Rights in this trademark will exist at least as long as the Company or its majority owned subsidiaries continue to use the trademark.
The Company has entered into a worldwide perpetual license agreement with Celgene Corporation and Celgro Corporation that gives the Company the exclusive rights to certain intellectual property, including know-how and technology, relating to the development and manufacture of chirally pure bulk APIs. This intellectual property is related to amphetamine salts currently sold by the Company. Under the terms of this agreement, the Company pays no royalties or fees related to its use of this intellectual property.
Competition
The Company has numerous primary API and advanced intermediate competitors throughout Western Europe and the United States and many more competitors within various product categories the Company serves, including a growing number of competitors in Asia, Eastern Europe and other low-cost areas. The Company believes that low cost providers have had the impact of driving prices down for many products and services for which the Company competes to provide, especially within the generic API market, and the Company anticipates that it will face increased competition from these providers in the future. It is expected that regulatory compliance, product quality, pricing, and logistics will determine the extent of the long term impact of these competitors in the primary markets that the Company serves. If the Company perceives significant competitive risk and a need for technical or financial commitment, it generally attempts to negotiate long term contracts or guarantees from its customers.
Environmental and Safety Regulations and Proceedings
Certain products manufactured by the Company involve the use, storage and transportation of toxic and hazardous materials. The Company's operations are subject to extensive laws and regulations relating to the storage, handling, emission, transportation and discharge of materials into the environment and the maintenance of safe working conditions. The Company maintains environmental and industrial safety and health compliance programs and training at its plants and believes that its manufacturing operations are in compliance with all applicable safety, health and environmental laws.
Prevailing legislation tends to hold companies primarily responsible for the proper disposal of its waste even after transfer to third party waste disposal facilities. Other future developments, such as increasingly strict environmental, safety and health laws and regulations, and enforcement policies, could result in substantial costs and liabilities to the Company and could subject the Company's handling, manufacture, use, reuse or disposal of substances or pollutants at its plants to more rigorous scrutiny than at present.
Known environmental matters that may result in liabilities to the Company and the related estimates and accruals are summarized in Note 20 to the Company’s consolidated financial statements.
(dollars in thousands, except per share data)
The Company’s policy is to comply with all legal requirements of applicable environmental, health and safety laws and regulations. The Company believes it is in compliance with such requirements and has adequate professional staff and systems in place to remain in compliance. In some cases, compliance can only be achieved by capital expenditures, and the Company made capital expenditures of $2,739, $3,733 and $3,554 in 2015, 2014 and 2013, respectively, for environmental projects. As the environmental proceedings in which the Company is involved progress from the remedial investigation and feasibility study stage to implementation of remedial measures, related capital and other expenditures may increase. The Company considers costs for environmental compliance to be a normal cost of doing business and includes such costs in pricing decisions.
Employees
At December 31, 2015, the Company had 1,228 employees worldwide (852 of whom were from international operations) compared with 1,117 employees at December 31, 2014 and 936 at December 31, 2013.
Non-U.S. production, administration, scientific and technical employees are represented by various local and national unions. The Company believes its labor relations are satisfactory.
Seasonality
The Company experiences some seasonality primarily due to planned plant shutdowns by the Company and certain customers in the third quarter. Operating results for any quarter, however, are not necessarily indicative of results for any future period. In particular, as a result of various factors including, but not limited to, acquisitions, plant shutdowns, and the timing of large contract revenue streams, the Company believes that period-to-period comparisons of its operating results should not be relied upon as an indication of future performance.
Export and International Sales
Export sales from the Company’s domestic operations in 2015, 2014 and 2013 amounted to $159,048, $101,101 and $86,850, respectively. Sales from international operations were $196,710, $187,415, and $164,010 in 2015, 2014 and 2013, respectively. Refer to Note 18 to the Company’s consolidated financial statements.
Additional Information
Cambrex Corporation was incorporated as a Delaware corporation in 1981. The Company’s principal office is located at One Meadowlands Plaza, East Rutherford, NJ 07073 and its telephone number is (201) 804-3000.
This Annual Report on Form 10-K, the Company’s Quarterly Reports on Form 10-Q, the Company’s Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act are made available free of charge on the Company’s website www.cambrex.com as soon as reasonably practicable after such material is electronically filed with, or furnished to, the SEC. The most recent certifications by the Company’s Chief Executive Officer and Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 are filed as exhibits to this Annual Report on Form 10-K. The Company also files with the New York Stock Exchange (“NYSE”) the Annual Chief Executive Officer Certification as required by Section 303A.12.(a) of the NYSE Listed Company Manual.
The following corporate governance documents are available free of charge on the Company’s website: the charters of its Audit, Regulatory Affairs, Compensation and Governance Committees, Corporate Governance Guidelines, Code of Business Conduct and Ethics and Independence Standards for Directors. These corporate governance documents are also available in print to any stockholder requesting a copy from the corporate secretary at the principal executive offices. Information contained on the website is not part of this report. The Company will also post on its website any amendments to or waivers of its Code of Business Conduct and Ethics that relate to its Chief Executive Officer, Chief Financial Officer and Principal Accounting Officer.
(dollars in thousands, except per share data)
|
Item 1A |
Risk Factors. |
Factors That May Affect Future Results
The following risk factors and other information included in this Annual Report on Form 10-K should be carefully considered, including the cautionary note under the heading “Forward-Looking Statements.” If any of the following risks manifests, the Company’s business, financial condition, operating results, cash flows and reputation could be materially adversely affected. The risks and uncertainties described below are not the only ones the Company faces. Additionally, risks and uncertainties not presently known to the Company or that it currently deems immaterial may also impair its business, financial condition, operating results and cash flows in the future.
Certain of the Company’s customers and suppliers comprise a significant percentage of the Company’s business and the loss of one or more of these customers or suppliers could have a material adverse effect on the Company’s financial position, results of operations and cash flows.
Sales to a relatively small number of customers have historically accounted for a significant percentage of the Company’s business. For example, one customer accounted for 34.5% of 2015 consolidated sales. Should this, or any other significant customer renegotiate on terms more favorable to them, or discontinue or decrease their usage of the Company’s products, the loss could have a material adverse effect on the Company’s financial position, results of operations and cash flow. The Company’s customers routinely attempt to reduce costs, including the costs of the Company’s products, as a result of macro-economic trends and various market dynamics specifically affecting the pharmaceuticals industry.
New technologies, competition or a reduction in demand for the Company’s products could reduce sales.
The markets for the Company’s products are competitive and price sensitive. The Company has numerous primary API and advanced intermediate competitors throughout Western Europe and the United States and many more competitors within various segments of the markets the Company serves, including a growing number of competitors in Asia, Eastern Europe and other low-cost areas. The Company’s competitors may lower prices on products in the future and the Company may, in certain cases, respond by lowering its prices. Conversely, failure to anticipate and respond to price competition may adversely impact the Company’s market share. In general, innovator pharmaceutical companies expect price declines over time and especially upon contract renewals. These price declines could have a significant negative impact on future profits. Competitors may develop new technologies or products, negatively impacting the Company. Several of the Company’s customers, especially those that buy its generic APIs and larger pharmaceutical companies that primarily sell patented products, have internal capabilities similar to the Company’s. If one or more of these customers replace the Company’s products with their own internal capabilities, demand for the Company’s products may decrease. In addition, demand for the Company’s products may weaken due to a reduction in R&D budgets, loss of distributors or other factors. A reduction in demand for the Company’s products, particularly the one product that represented 32.1% of sales in 2015, could impair profit margins and may have a material adverse effect on the Company’s financial position, results of operations and cash flow.
The Company’s failure to obtain new customer contracts or renew existing contracts may adversely affect its business.
The Company must continually renew existing customer contracts and win new contracts, which subjects the Company to potentially significant pricing pressures. In the event the Company is unable to replace these contracts timely or at all, or is forced to accept terms, including pricing terms, less favorable to the Company, the Company’s revenue may not be able to be sustained or may decline. In addition, certain of the Company’s long-term contracts may be cancelled or delayed by customers for any reason upon notice. Multiple cancellations, non-renewals, or renewals on less favorable terms to the Company of significant contracts could materially impact the Company’s business. While the Company’s preferred practice is to renegotiate new or extended agreements prior to expiration, if these contracts cannot be renewed or extended on terms acceptable to the Company or at all, the Company’s business, results of operations and financial condition could be materially adversely affected.
(dollars in thousands, except per share data)
Failure to obtain raw materials from third-party manufacturers could affect the Company’s ability to manufacture and deliver its products.
The Company relies on third-party manufacturers to supply many of its raw materials and intermediates. In addition, the Company has a single source for supplies of some raw materials to its products. Manufacturing problems may occur with these and other outside sources. Prolonged disruptions in the supply of any of the Company’s key raw materials, difficulty implementing replacement materials or new sources of supply, or a significant increase in the prices of raw materials could have a material adverse effect on the Company’s operating results, financial condition or cash flows. If a supplier provides the Company raw materials or other supplies that are deficient or defective or if a supplier fails to provide the Company such materials or supplies in a timely manner, the Company may have limited ability to find appropriate substitutes or otherwise meet required specifications and deadlines. Moreover, the Company could experience inventory shortages if it is required to use an alternative supplier on short notice, which also could lead to raw materials being purchased on less favorable terms than the Company has with its regular suppliers. If such problems occur, the Company may not be able to manufacture its products profitably or on time, which could harm the Company’s reputation and have a material adverse effect on the Company’s business.
Failure to obtain sufficient quota from the Drug Enforcement Administration ("DEA") could affect the Company’s ability to manufacture and deliver certain products.
The starting materials used in several of the Company's products and many of the Company's finished products are controlled substances and are regulated by the DEA. Consequently, their manufacture, shipment (including import and export), storage, sale and use are subject to a high degree of regulation. In particular, the DEA limits the manufacturing and distribution of certain starting materials and APIs manufactured by the Company and it must regularly apply for quota to obtain and manufacture these substances. As a result of these limitations, the Company may not be able to meet commercial demand for these substances, which could harm its relationship with customers and its reputation. If the Company’s DEA registration were revoked or suspended, or if any of the Company’s quota applications were rejected, the Company could no longer lawfully possess, manufacture or distribute controlled substances, which could have a material adverse effect on the Company’s business.
Disruptions to the Company’s or its customers’ manufacturing operations or supply chain could adversely affect its results.
Due to heavy reliance on manufacturing and related operations to produce and distribute the products the Company sells, the Company could be adversely affected by disruptions to these operations or its customers’ operations. The Company and its suppliers and customers operate in a highly regulated industry. Any violation of applicable regulations, failure to meet applicable manufacturing standards, or other actions by regulatory agencies, including, but not limited to, plant shutdowns or the removal of products from the market that eliminates or reduces the Company’s and its customer’s sales of products could negatively impact the Company’s business and reputation. In addition, a number of factors could cause production interruptions at the Company’s facilities, including equipment malfunctions, disruptions in the supply chain, facility contamination, labor problems, raw material shortages, natural disasters, disruption in utility services, fire, terrorist activities, human error or disruptions in the operations of the Company’s suppliers. Any significant disruption to those operations for these or any other reasons could adversely affect the Company’s sales and customer relationships. Any sustained reduction in the Company’s ability to provide products would negatively impact its sales growth expectations, cash flows and profitability.
Litigation may harm the Company or otherwise negatively impact its management and financial resources.
The Company’s business is subject to the risk of litigation by employees, customers, consumers, suppliers, stockholders or others through private actions, class actions, administrative proceedings, regulatory actions or other litigation. The outcome of litigation, particularly class action lawsuits and regulatory actions, is difficult to assess or quantify. Plaintiffs in these types of lawsuits may seek recovery of very large or indeterminate amounts, and the magnitude of the potential loss relating to such lawsuits may remain unknown for substantial periods of time. Complex or extended litigation could cause the Company to incur large expenditures and distract its management. The cost to defend current and future litigation may be significant. There may also be adverse publicity associated with litigation that could decrease customer acceptance of the Company’s products, regardless of whether the allegations are valid or whether the Company is ultimately found liable. Disputes from time to time with such companies or individuals are not uncommon, and the Company cannot be assured that it will always be able to resolve such disputes on terms favorable to the Company. As a result, litigation may adversely affect its business, financial condition and results of operations. In addition, certain contracts with our suppliers and customers contain provisions whereby the Company indemnifies, subject to certain limitations, the counterparty for damages suffered as a result of claims related to use of the Company’s products or facilities and other matters. Claims made under these provisions could be expensive to litigate and could result in significant payments.
(dollars in thousands, except per share data)
Refer to Note 20 to the Company’s consolidated financial statements for a discussion of the Company’s environmental and legal matters.
Incidents related to hazardous materials could adversely affect the Company.
Portions of the Company’s operations require the controlled use of hazardous materials. Although the Company designs and implements safety procedures to comply with the standards prescribed by federal, state, and local regulations, the risk of accidental contamination of property, or injury to individuals from these materials, cannot be completely eliminated. In the event of accidental contamination of property or injury to individuals caused by these materials, the Company could be liable for damages which could adversely affect its business. Additionally, any incident could shut down the Company’s operations, which could have a material adverse effect on the business and results of operations of the Company.
The Company generates waste that must be transported to approved storage, treatment and disposal facilities. The transportation and disposal of such waste are required to meet applicable state and federal statutes and regulations. The handling of such waste potentially exposes the Company to environmental liability if, in the future, it is determined that the violation of statutes or regulations occurred.
The Company is also a party to several environmental remediation investigations and activities and, along with other companies, has been named a potentially responsible party (“PRP”) for certain waste disposal sites. The Company’s estimated reserve for environmental remediation is based on information currently available to it and may be subject to material adjustment in future periods as new facts or circumstances may indicate. Moreover, despite its efforts to comply with environmental laws, the Company may face significant remediation liabilities and additional legal proceedings concerning environmental matters, which could have a material adverse effect on the Company’s business.
It is the Company’s policy to record appropriate liabilities for environmental matters where remedial efforts are probable and the costs can be reasonably estimated. Such liabilities are based on the Company’s best estimate of the undiscounted future costs required to complete the remedial work. Environmental matters often span several years and frequently involve regulatory oversight or adjudication. Additionally, many remediation requirements are fluid and are likely to be affected by future technological, site and regulatory developments. Each of these matters is subject to various uncertainties, and it is possible that some of these liabilities will be materially higher than the Company has estimated.
In matters where the Company has been able to reasonably estimate its liability, the Company has accrued for the estimated costs associated with the study or remediation of applicable sites not owned by the Company and the Company's current and former operating sites. Reserves are adjusted periodically as remediation efforts progress or as additional technical, regulatory or legal information become available. In some jurisdictions environmental, health and safety regulations are still early in their development, and the Company cannot determine how these laws will be implemented and the impact of such regulation on the Company. Given the uncertainties regarding the status of laws, regulations, enforcement, policies, the impact of other PRPs, technology and information related to individual sites, the Company does not believe it is possible to currently develop an estimate of the range of reasonably possible environmental losses in excess of its reserves.
(dollars in thousands, except per share data)
Refer to Note 20 to the Company’s consolidated financial statements for a discussion of the Company’s environmental and legal matters.
Potential product liability claims, errors and omissions claims in connection with services the Company performs and potential liability under indemnification agreements between the Company and its officers and directors could adversely affect the Company.
The Company manufactures products intended for use by the public. These activities could expose the Company to risk of liability for personal injury or death to persons using such products. The Company seeks to reduce its potential liability through measures such as contractual indemnification provisions with customers (the scope of which may vary by customer, and the performances of which are not secured) and insurance maintained by customers. The Company could be materially and adversely affected if it were required to pay damages or incur defense costs in connection with a claim that is outside the scope of the indemnification agreements, if the indemnity, although applicable, is not performed in accordance with its terms or if the Company’s liability exceeds the amount of applicable insurance or indemnity. In addition, the Company could be held liable for errors and omissions in connection with the services it performs. The Company currently maintains product liability and errors and omissions insurance with respect to these risks. There can be no assurance, however, that the Company’s insurance coverage will be adequate or that insurance coverage will continue to be available on terms acceptable to the Company.
The Company also indemnifies its officers and directors for certain events or occurrences while the officer or director was serving at the Company’s request in such capacity. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited. Although the Company has a director and officer insurance policy that covers a portion of any potential exposure, the Company could be materially adversely affected if it were required to pay damages or incur legal costs in connection with a claim above its insurance limits.
Any claims beyond the Company’s insurance coverage limits, or that are otherwise not covered by the Company’s insurance, may result in substantial costs and a reduction in its available capital resources.
The Company maintains property insurance, employer’s liability insurance, product liability insurance, general liability insurance, business interruption insurance, and directors and officers liability insurance, among others. Although the Company maintains what it believes to be adequate insurance coverage, potential claims may exceed the amount of insurance coverage or may be excluded under the terms of the policy, which could cause an adverse effect on the Company’s business, financial condition and results from operations. Generally, the Company would be at risk for the loss of inventory that is not within customer specifications. These amounts could be significant. In addition, in the future the Company may not be able to obtain adequate insurance coverage or the Company may be required to pay higher premiums and accept higher deductibles in order to secure adequate insurance coverage.
The Company depends on key personnel and the loss of key personnel could harm the Company’s business and results of operations.
The Company depends on its ability to attract and retain qualified scientific and technical employees as well as a number of key executives. These employees may voluntarily terminate their employment with the Company at any time. There can be no assurance the Company will be able to retain key personnel, or to attract and retain additional qualified employees. The Company does not maintain key-man or similar policies covering any of its senior management or key personnel. The Company’s inability to attract and retain key personnel would have a material adverse effect on the Company’s business.
(dollars in thousands, except per share data)
The Company has and continues to make significant capital investments to its facilities to meet its potential future needs and, as a result, the Company depends on the success of attracting new and retaining existing customers’ business.
The Company has and continues to make substantial investments in all of its manufacturing facilities. As a result, the Company’s fixed costs have increased. If the Company is not able to utilize the facilities to capacity, its margins could be adversely affected.
The Company continues to expand its large-scale manufacturing capacity to support expected growth in the business. There can be no assurance that sales volumes will be sufficient to ensure the economical operation of this expanded capacity, in which case, the Company’s results of operations could be adversely affected.
Global growth is subject to a number of economic risks.
A reduction in the availability of debt or equity capital could adversely affect the ability of the Company’s customers to obtain financing for product development and could result in a decrease in, or cancellation of, orders for the Company’s products as well as impact the ability of the Company’s customers to make payments. The Company believes that cash flows from operations, along with funds available from a revolving line of credit, will be adequate to meet the operational and debt servicing needs of the Company, but if this does not continue to be the case the Company’s business may be materially adversely affected. There is a risk that the funds available to be drawn under the Company’s revolving line of credit may not be available in the event of the failure of one or more participant banks. Significant movements in the rate of exchange between the U.S. dollar and certain currencies, primarily the euro and Swedish krona, may also adversely affect the Company’s results.
If the Company acquires other businesses, it may be harmed by difficulties in integration and employee retention, unidentified liabilities of the acquired businesses, or obligations incurred in connection with financing the acquisition.
All acquisitions involve known and unknown risks that could adversely affect the Company’s future revenues and operating results. For example:
|
● |
The Company may fail to successfully integrate its acquisitions in accordance with its business strategy. |
|
● |
The initial rationale for the acquisition may not remain viable due to a variety of factors, including unforeseen regulatory changes and market dynamics after the acquisition, and this may result in a significant delay or reduction in the profitability of the acquisition. |
|
● |
Integration of acquisitions may divert management’s attention away from the Company’s primary product offerings, resulting in the loss of key customers or personnel, and may expose the Company to unanticipated liabilities. |
|
● |
The Company may not be able to retain the skilled employees and experienced management that may be necessary to operate the businesses it acquires. If the Company cannot retain such personnel, it may not be able to locate or hire new skilled employees and experienced management to replace them. |
|
● |
The Company may purchase a business that has contingent liabilities that include, among others, known or unknown environmental, patent or product liability claims. |
|
● |
The Company’s acquisition strategy may require it to obtain additional debt or equity financing, potentially resulting in a high level of debt obligations or significant dilution of ownership, or both. |
|
● |
The Company may purchase businesses located in jurisdictions where it does not have operations and as a result it may not be able to anticipate local regulations and the impact such regulations have on its business. |
Any indemnities or warranties obtained in connection with such acquisitions may not fully cover the ultimate actual liabilities the Company incurs due to limitations in scope, amount or duration, financial limitations of the indemnitor or warrantor or other reasons.
(dollars in thousands, except per share data)
As a result of acquiring businesses or entering into other significant transactions, the Company may experience significant charges to earnings for merger related expenses. If the Company is not able to successfully integrate the acquired business, it may affect the Company’s results of operations and the market price of its common stock. Furthermore, if the Company is unable to improve the operating margins of acquired businesses or operate them profitably, it may be unable to achieve its growth strategy.
In addition, if the Company makes one or more significant acquisitions in which the consideration includes equity shares or other securities or additional capital is raised through equity financings, equity interests in Cambrex may be significantly diluted and may result in a dilution of earnings per share. If the Company makes one or more significant acquisitions in which the consideration includes cash, it may be required to use a substantial portion of its available cash or incur a significant amount of debt or otherwise arrange additional funds to complete the acquisition, which may result in reduced liquidity, a decrease in its net income and a consequential reduction in its earnings per share.
The Company may be unable to effectuate a sale of Zenara in a timely manner or receive consideration in excess of the carrying value of the assets that are currently held for sale.
In the fourth quarter of 2015, the Company committed to a plan to sell Zenara. Although the Company expects a sale to be completed during 2016, it cannot provide any assurance that it will be successful in selling the assets or operations for a price in excess of the carrying value of the assets, which are currently classified as “held for sale.” For the year ended December 31, 2015, the Company recognized restructuring charges. In the event that the Company is unable to sell Zenara for a price at least equal to the remaining carrying value of the assets, then it will have to record additional charges, which could have an adverse effect on the Company’s financial position.
The Company has a significant amount of debt.
The Company has a $250,000 revolving credit facility of which $30,000 was outstanding at December 31, 2015. This facility expires in November 2016, and the Company may be unable to refinance its revolving credit facility on favorable terms. If the Company is unable to generate sufficient cash flow or otherwise obtain funds necessary to make required payments on the credit facility, it will be in default. This current debt arrangement requires the Company to comply with specified financial ratios. The Company’s ability to comply with these ratios may be affected by events beyond its control.
Even if the Company is able to meet its debt service obligations, the amount of debt it has could adversely affect the Company by limiting its ability to obtain any necessary financing in the future for working capital, capital expenditures, debt service requirements, or other purposes. It also may place the Company at a disadvantage relative to its competitors who may have lower levels of debt, while making it more vulnerable to a downturn in its business or the economy in general. It may also require the Company to use a substantial portion of its cash to pay principal and interest on its debt.
The Company’s liquidity, business, financial condition, results of operations and cash flows could be materially and adversely affected if the financial institutions which hold its funds fail.
The Company has significant funds held in bank deposits, money market funds and other accounts at certain financial institutions. A significant portion of the funds held in these accounts exceed insurable limits. In the normal course of business, the Company maintains cash balances with European Union banks ranging from the equivalent of $1,000 - $10,000. The Company routinely monitors the risks associated with these institutions and diversifies its exposure by maintaining smaller balances with multiple financial institutions. If any of the financial institutions where the Company has deposited funds were to fail, the Company may lose some or all of its deposited funds. Such a loss could have a material adverse effect on the Company’s liquidity, business, financial condition, results of operations and cash flows.
(dollars in thousands, except per share data)
The Company has significant inventories on hand.
The Company maintains significant inventories and has an allowance for slow-moving and obsolete inventory. Any significant unanticipated changes in future product demand or market conditions, including obsolescence or the uncertainty in the global market, could also have an impact on the value of inventory and adversely impact the Company’s results of operations.
International unrest or foreign currency fluctuations could adversely affect the Company’s results.
The Company’s international revenues, which include revenues from its non-U.S. subsidiaries and export sales from the U.S., represent the majority of its product revenues. The Company’s operations extend to numerous countries outside of the U.S.
There are a number of significant risks arising from the Company’s international operations, including:
|
● |
the possibility that nations or groups could boycott its products; |
|
● |
inflation, foreign currency exchange rates and the impact of shifts in the U.S. and local economies on those rates; |
|
● |
general economic decline or political unrest in the markets in which it operates; |
|
● |
geopolitical risks, terrorism, or acts of war or hostility; |
|
● |
compliance with local laws and regulations including laws restricting the inflow of capital or cash and unexpected changes in regulatory requirements; |
|
● |
difficulties and expenses of compliance with a wide variety of foreign laws and regulations; |
|
● |
longer accounts receivable cycles in certain foreign countries; |
|
● |
import and export licensing requirements; |
|
● |
government sanctions that may reduce or eliminate the Company’s ability to sell its products in certain countries; |
|
● |
the protection of the Company’s intellectual property and that of its customers. |
If the Company is unable to effectively manage these risks, it may not produce the revenues, earnings, or strategic benefits that it anticipates which could have a material adverse effect on the Company’s business.
A significant portion of the Company’s business is conducted in currencies other than the U.S. dollar, which is its reporting currency. The Company recognizes foreign currency gains or losses arising from its operations in the period incurred. As a result, currency fluctuations between the U.S. dollar and the currencies in which the Company does business have caused, and will continue to cause, foreign currency transaction gains and losses. The Company cannot predict the effects of exchange rate fluctuations upon its future operating results because of the number of currencies involved, the variability of currency exposures, and the potential volatility of currency exchange rates. The Company periodically engages in foreign exchange transactions to mitigate the impact of this volatility on its operations, but its strategies are short-term in nature and may not adequately protect its operating results from the full effects of exchange rate fluctuations.
Certain jurisdictions have experienced governmental corruption to some degree and, in some circumstances, anti-bribery laws may conflict with some local customs and practices. As a result of the Company’s policy to comply with the U.S. Foreign Corrupt Practices Act and similar anti-bribery laws, the Company may be at a competitive disadvantage to competitors that are not subject to, or do not comply with, such laws. Furthermore, while employees and agents must comply with these laws, the Company cannot be certain that internal policies and procedures will always prevent violations of these laws, despite a commitment to legal compliance and corporate ethics. Violations or mere allegations of such violations could have a material adverse effect on the Company’s business and reputation.
(dollars in thousands, except per share data)
The Company’s operating results may unexpectedly fluctuate in future periods.
The Company’s revenue and operating results can fluctuate on a quarterly basis. The operating results for a particular quarter may be higher or lower than expected as a result of a number of factors, including, but not limited to, the timing of contracts; the delay, cancellation or acceleration of a contract; seasonal slowdowns in different parts of the world; the timing of accounts receivable collections; pension contributions; changes in government regulations; and changes in exchange rates against the U.S. dollar. Because a high percentage of the Company’s costs are relatively fixed in the short term, such as the cost of maintaining facilities and compensating employees, any one of these factors could have a significant impact on the Company’s quarterly results. In some quarters, the Company’s revenue and operating results may be significantly lower than or higher than the expectations of securities analysts and investors due to any of the factors described above.
The possibility the Company will be unable to protect its technologies could affect its ability to compete.
The Company’s success depends to some degree upon its ability to develop proprietary products and technologies. However, the Company cannot be assured that patents will be granted on any of its patent applications. The Company also cannot be assured that the scope of any of its issued patents will be sufficiently broad to offer meaningful protection. The Company has patents issued in selected countries; therefore, third parties can make, use, and sell products covered by its patents in any country in which the Company does not have patent protection. In addition, the Company may be involved in patent litigation in the future. Issued patents or patents the Company licenses could be successfully challenged, invalidated or circumvented so that its patent rights would not create an effective competitive barrier. Although the Company intends to defend the validity of owned patents and use all appropriate methods to prevent their infringement, such efforts are expensive and time consuming, with no assurance of success. The ability to enforce patents depends on the laws of individual countries and each country’s practices regarding enforcement of intellectual property rights. The Company provides its customers the right to use its products under label licenses that are for research purposes only. These licenses could be contested, and the Company cannot be assured that it would either be aware of an unauthorized use or be able to enforce the restrictions in a cost-effective manner.
If a third party claimed an intellectual property right to technology the Company uses, the Company may need to discontinue an important product or product line, alter its products and processes, defend its right to use such technology in court or pay license fees. Although the Company may, under these circumstances, attempt to obtain a license to such intellectual property, it may not be able to do so on favorable terms, or at all. Additionally, if the Company’s products are found to infringe on a third party’s intellectual property, the Company may be required to pay damages for past infringement, and lose the ability to sell certain products or receive licensing revenues.
The Company also relies on trade secrets, unpatented proprietary know-how and continuing technological innovation that it seeks to protect, in part by confidentiality agreements with licensees, suppliers, employees and consultants. It is possible that these agreements will be breached and the Company will not have adequate remedies for any such breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Furthermore, the Company’s trade secrets and proprietary technology may otherwise become known or be independently developed by its competitors or the Company may not be able to maintain the confidentiality of information relating to such products.
Information technology systems could fail to perform adequately or the Company may fail to adequately protect such systems against data corruption, cyber-based attacks, or network security breaches.
The Company utilizes information technology networks and systems to process, transmit, and store electronic information. In particular, the Company depends on information technology infrastructure to effectively manage its business data, supply chain, logistics, accounting, and other business processes and electronic communications between employees, customers and suppliers. Ineffective allocation and management of the resources necessary to build and sustain an appropriate technology infrastructure could adversely affect the Company’s business. In addition, security breaches or system failures of this infrastructure can create system disruptions, shutdowns, or unauthorized disclosure of confidential information. Inability to prevent such breaches or failures, could disrupt the Company’s operations or cause financial damage or loss because of lost or misappropriated information.
(dollars in thousands, except per share data)
The Company may experience difficulties implementing its global enterprise resource planning system.
The Company is engaged in a multi-year implementation of a global enterprise resource planning system (“ERP”). The ERP is designed to accurately maintain the Company’s books and records and provide information important to the operation of the business to the Company’s management team. The Company’s ERP will continue to require significant investment of human and financial resources. In implementing the ERP, we may experience significant delays, increased costs and other difficulties. Any significant disruption or deficiency in the design and implementation of the ERP could adversely affect the Company’s ability to process orders, ship product, send invoices and track payments, fulfill contractual obligations or otherwise operate its business. Any issues with implementation could also cause the Company to fail to timely or accurately report its financial results. While the Company has invested significant resources in planning and project management, significant implementation issues may arise.
The Company could be subject to impairment charges in the future.
Under U.S. GAAP, the Company is required to evaluate goodwill for impairment at least annually. If the Company determines that the fair value is less than the carrying value, an impairment loss will be recorded in the Company’s statement of operations. The determination of fair value is a highly subjective exercise and can produce significantly different results based on the assumptions used and methodologies employed. If the Company’s projected long-term sales growth rate, profit margins or terminal rate are considerably lower or the assumed weighted average cost of capital is considerably higher, future testing may indicate impairment and the Company would have to record a non-cash goodwill impairment loss in its statement of operations.
Assessments by various tax authorities may be materially different than the Company has provided for and it may experience significant volatility in its annual and quarterly effective tax rate.
As a matter of course, the Company is regularly audited by federal, state, and foreign tax authorities. From time to time, these audits result in proposed assessments. In recent years, the Company utilized significant tax attributes such as domestic federal foreign tax credits to reduce U.S. cash taxes. While the Company believes that it has adequately provided for any taxes related to these items, and taxes related to all other aspects of its business, any such assessments or future settlements may be materially different than it has provided. Refer to Note 10 to the Company’s consolidated financial statements for a discussion of the Company’s income taxes.
The Company has deferred tax assets that it may not be able to use under certain circumstances.
If the Company is unable to generate future taxable income of sufficient amounts and type in certain jurisdictions, or if there is a significant change in tax rates or the time period within which taxable income is recognized, the Company could be required to increase its valuation allowances against its deferred tax assets resulting in an increase in its recorded tax expense and a potential adverse impact on future results.
Low investment performance by the Company’s defined benefit pension plan assets or other events including changes in regulations or actuarial assumptions may increase the Company’s pension expense, and may require the Company to fund a larger portion of its pension obligations, thus diverting funds from other potential uses.
The Company sponsors a defined benefit pension plan that covers certain eligible employees. The Company’s pension expense and required contributions to the pension plan are directly affected by changes in interest rates, the value of plan assets, the projected rate of return on plan assets, the actual rate of return on plan assets, and the actuarial assumptions used to measure the defined benefit pension plan obligations. If plan assets perform below the assumed rate of return used to determine pension expense, future pension expense will increase. The proportion of pension assets to liabilities, which is called the funded status, determines the level of contribution to the plan that is required by law. Changes in the plan’s funded status related to the value of assets or liabilities could increase the amount required to be funded. The Company cannot predict whether changing market or economic conditions, regulatory changes or other factors will further increase the Company’s pension funding obligations, diverting funds from other potential uses.
(dollars in thousands, except per share data)
Any significant change in government regulation of the drug development process could have a material adverse effect on the Company.
The manufacturing of pharmaceutical products is subject to extensive regulation by governmental authorities, including the FDA, the European Medicines Agency and comparable regulatory authorities in other countries. The process of obtaining regulatory approval to produce and market pharmaceutical products is rigorous, time-consuming, costly, and often unpredictable. The Company’s business, as well as its customers’ business depends in part on strict government regulation of the drug development process. Legislation may be introduced and enacted to modify regulations administered by the regulatory authorities governing the drug approval process. The Company may be unable to obtain requisite regulatory approvals on a timely basis for marketing and production of products. Conversely, any significant reduction in the scope of regulatory requirements or the introduction of simplified drug approval procedures could reduce barriers to entry which would increase competition and have a material adverse effect on the Company’s business.
Failure to comply with current Good Manufacturing Practices (“cGMP”) and other government regulations, as well as delays in obtaining regulatory approval by the Company or its customers could have a material adverse effect on the Company.
All facilities and manufacturing techniques used for manufacturing products for clinical use or for commercial sale in the U.S. must be operated in conformity with cGMP regulations as required by the FDA and other comparable regulatory authorities in other countries, and for certain products, the DEA. The Company’s facilities are subject to periodic regulatory and customer inspections to ensure compliance with cGMP and other requirements applicable to such products. A finding that the Company had materially violated these requirements could result in regulatory sanctions including, but not limited to, the regulatory agencies withholding approval of new drug applications or supplements and the denial of product entry into the U.S., or other countries, of products manufactured at non-compliant facilities, the loss of a customer contract, the disqualification of data for client submissions to regulatory authorities and a mandated closing of the Company’s facilities. Any such violations would have a material adverse effect on the Company’s business. The Company’s customers are typically subject to the same, or similar regulations and any such violations or other actions by regulatory agencies, including, but not limited to, plant shutdowns or product recalls that eliminate or reduce the Company’s sale of its products or services could negatively impact the Company’s business. In addition, the submission of new products to regulatory authorities for approval by the Company or its customers does not guarantee the approval to market the product will be granted. Each authority may impose its own requirements or delay or refuse to grant approval to the Company or customer even when the product has already been approved in another country. Products that have already been approved can be removed from the market by regulatory agencies for numerous reasons.
The overall level of late-stage clinical phase projects could decline and the outsourcing trends may decline, either of which could slow the Company’s growth.
The success of the Company’s business depends to a certain extent on the number of clinical phase contracts and the size of the contracts that it may obtain from pharmaceutical companies. A decline in the level of clinical phase projects or a slowing of the outsourcing trend could result in a diminished growth rate in the Company’s sales and adversely affect its business, financial condition and results of operations.
Item 1B Unresolved Staff Comments.
None.
(dollars in thousands, except per share data)
|
Item 2 |
Properties. |
Set forth below is information relating to manufacturing facilities owned by the Company as of December 31, 2015:
|
Location |
Acreage |
Operating Subsidiary |
Primary Product Lines Manufactured |
|
Charles City, Iowa |
57 acres |
Cambrex Charles City, Inc. |
APIs and Pharmaceutical Intermediates |
|
|
|
|
|
|
Karlskoga, Sweden |
42 acres |
Cambrex Karlskoga AB |
APIs and Pharmaceutical Intermediates |
|
|
|
|
|
|
Paullo (Milan), Italy |
12 acres |
Cambrex Profarmaco Milano S.r.l. |
APIs and Pharmaceutical Intermediates |
The Company’s corporate headquarters are located in East Rutherford, N.J.
|
Item 3 |
Legal Proceedings. |
See "Environmental and Safety Regulations and Proceedings" under Item 1 and Note 20 to the Company’s consolidated financial statements with respect to various proceedings involving the Company in connection with environmental matters. The Company is party to a number of other proceedings also discussed in Note 20 to the Company’s consolidated financial statements.
|
Item 4 |
Mine Safety Disclosures. |
None.
PART II
|
Item 5 |
Market for the Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
The Company’s common stock, $0.10 par value, is listed on the NYSE under the symbol CBM. The following table sets forth the closing high and low sales price of the common stock as reported on the NYSE:
|
2015 |
High |
Low |
||||||
|
First Quarter |
$ | 39.63 | $ | 21.34 | ||||
|
Second Quarter |
46.24 | 35.71 | ||||||
|
Third Quarter |
53.82 | 39.57 | ||||||
|
Fourth Quarter |
53.63 | 40.38 | ||||||
|
2014 |
High |
Low |
||||||
|
First Quarter |
$ | 22.18 | $ | 16.25 | ||||
|
Second Quarter |
22.14 | 17.55 | ||||||
|
Third Quarter |
22.67 | 18.68 | ||||||
|
Fourth Quarter |
24.12 | 16.33 | ||||||
As of January 29, 2016, there were 53 stockholders of record of the Company’s common stock. As of March 12, 2015, the most current report available, there were approximately 9,769 beneficial holders of the outstanding common stock of the Company.
The Company does not anticipate paying cash dividends in the foreseeable future. There were no cash dividends paid on our common stock during the past three fiscal years.
(dollars in thousands, except per share data)
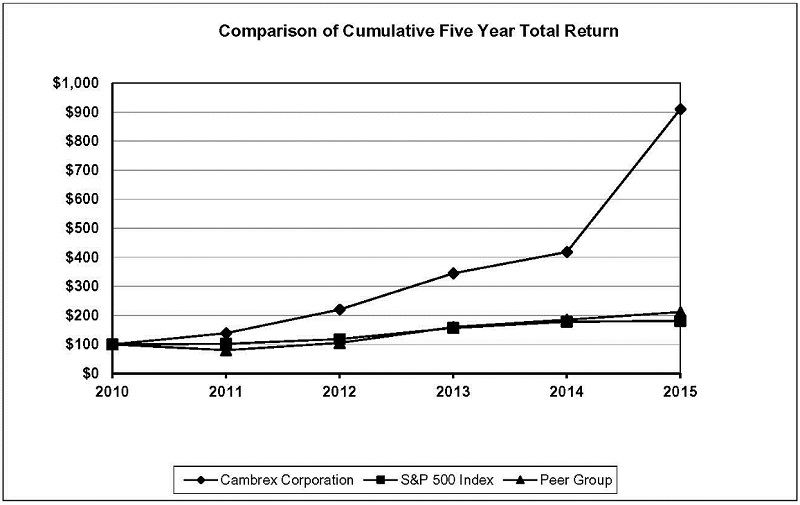
2015 Equity Compensation Table
The following table provides information as of December 31, 2015 with respect to shares of common stock that may be issued under the Company’s existing equity compensation plans.
|
Column (a) |
Column (b) |
Column (c) |
||||||||||
|
Plan category |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted average exercise price of outstanding options, warrants and rights |
Number of securities remaining for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
|
Equity compensation plans approved by security holders |
1,627,413 | $ | 19.21 | 1,763,030 | ||||||||
|
Equity compensation plans not approved by security holders |
4,500 | $ | 6.16 | - | ||||||||
|
Total |
1,631,913 | $ | 19.17 | 1,763,030 | ||||||||
The material features of the equity compensation plan under which equity securities are authorized for issuance that was adopted without stockholder approval are described below:
2000 Employee Performance Stock Option Plan
The 2000 Employee Stock Option Plan (the “2000 Plan”) was used to fund awards for Non-Executive Employees of the Company. The 2000 Plan is administered by the Compensation Committee of the Board of Directors, and that Committee may delegate responsibilities to others to assist in administering the 2000 Plan. The total number of shares of common stock which may be issued on exercise of stock options shall not exceed 500,000 shares, subject to adjustment in accordance with the 2000 Plan. No participant shall be granted options to purchase more than 100,000 shares of common stock in any twelve month period. The options were priced at fair market value on the date of grant and expire up to 10 years after the date of grant. If the employment of a participant terminates, other than as a result of death, disability or retirement, all unexercised awards shall be cancelled. In the event of death, disability or retirement, the options will expire one year from the date of the event. As of December 31, 2015 there were no shares remaining for future issuance under this plan.
(dollars in thousands, except per share data)
Comparison of Five-Year Cumulative Total Returns
The comparative stock performance graph below compares the five-year cumulative total stockholder return (assuming reinvestment of dividends, if any) from investing $100 on December 31, 2010, to the close of the last trading day of 2015, in each of (i) Cambrex common stock, (ii) the S&P 500 Index and (iii) an index of the Company’s peer group. The stock price performance reflected in the graph below is not necessarily indicative of future price performance.
|
|
The Company’s commercial activities are focused on manufacturing and marketing to customers concentrated in the Life Sciences Industry (including pharmaceutical chemicals and intermediates). Although the Company’s products are diverse, the Company believes that an index of its peer group based on its GICS code is a reasonable comparison group for the commercial activities on which it currently focuses. The peer group is for S&P GICS code 352030, Life Sciences Tools & Services, and is comprised of 52 companies as of December 31, 2015.
(dollars in thousands, except per share data)
|
Item 6 |
Selected Financial Data. |
The following selected consolidated financial data of the Company for each of the five years in the period through December 31, 2015 are derived from the audited financial statements. The consolidated financial statements of the Company as of December 31, 2015 and 2014 and for each of the years in the three year period ended December 31, 2015 and the reports of the independent registered public accounting firm are included elsewhere in this annual report. The data presented below should be read in conjunction with the financial statements of the Company, the notes to the financial statements and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere.
|
Years Ended December 31, |
||||||||||||||||||||
| 2015(1) | 2014(2) | 2013(3) | 2012(4) | 2011(5) | ||||||||||||||||
|
INCOME DATA: |
||||||||||||||||||||
|
Gross sales |
$ | 433,856 | $ | 374,150 | $ | 317,212 | $ | 277,931 | $ | 254,475 | ||||||||||
|
Net revenues |
433,326 | 374,613 | 318,176 | 276,501 | 255,653 | |||||||||||||||
|
Gross profit |
176,965 | 123,798 | 102,904 | 90,487 | 74,084 | |||||||||||||||
|
Selling, general and administrative expenses |
57,867 | 52,489 | 47,568 | 45,248 | 39,227 | |||||||||||||||
|
Research and development expenses |
12,540 | 13,075 | 10,387 | 9,544 | 11,037 | |||||||||||||||
|
Restructuring expenses |
15,573 | - | - | - | - | |||||||||||||||
|
Loss on voluntary pension settlement |
- | 7,170 | - | - | - | |||||||||||||||
|
Gain on sale of asset |
- | (1,234 | ) | (4,680 | ) | - | - | |||||||||||||
|
Operating profit |
90,985 | 52,298 | 49,629 | 35,695 | 23,820 | |||||||||||||||
|
Interest expense, net |
1,699 | 2,174 | 2,242 | 2,439 | 2,373 | |||||||||||||||
|
Equity in losses of partially-owned affiliates |
- | 4,623 | 2,262 | 1,766 | 1,621 | |||||||||||||||
|
Other (income)/expenses, net |
(279 | ) | (5 | ) | 118 | 122 | (111 | ) | ||||||||||||
|
Income before income taxes |
89,565 | 45,506 | 45,007 | 31,368 | 19,937 | |||||||||||||||
|
Provision/(benefit) for income taxes |
32,389 | (12,627 | ) | 14,732 | (31,861 | ) | 6,202 | |||||||||||||
|
Income from continuing operations |
57,176 | 58,133 | 30,275 | 63,229 | 13,735 | |||||||||||||||
|
Income/(loss) from discontinued operations, net of tax |
41 | (830 | ) | (4,360 | ) | (926 | ) | (2,767 | ) | |||||||||||
|
Net income |
57,217 | 57,303 | 25,915 | 62,303 | 10,968 | |||||||||||||||
|
EARNINGS PER SHARE DATA: |
||||||||||||||||||||
|
Earnings/(loss) per common share (basic): |
||||||||||||||||||||
|
Income from continuing operations |
$ | 1.82 | $ | 1.89 | $ | 1.00 | $ | 2.13 | $ | 0.46 | ||||||||||
|
Income/(loss) from discontinued operations, net of tax |
$ | 0.00 | $ | (0.03 | ) | $ | (0.14 | ) | $ | (0.03 | ) | $ | (0.09 | ) | ||||||
|
Net income |
$ | 1.82 | $ | 1.86 | $ | 0.86 | $ | 2.10 | $ | 0.37 | ||||||||||
|
Earnings/(loss) per common share (diluted): |
||||||||||||||||||||
|
Income from continuing operations |
$ | 1.76 | $ | 1.84 | $ | 0.98 | $ | 2.09 | $ | 0.46 | ||||||||||
|
Income/(loss) from discontinued operations, net of tax |
$ | 0.00 | $ | (0.03 | ) | $ | (0.14 | ) | $ | (0.03 | ) | $ | (0.09 | ) | ||||||
|
Net income |
$ | 1.76 | $ | 1.81 | $ | 0.84 | $ | 2.06 | $ | 0.37 | ||||||||||
|
Weighted average shares outstanding (in thousands): |
||||||||||||||||||||
|
Basic |
31,420 | 30,763 | 30,150 | 29,703 | 29,468 | |||||||||||||||
|
Diluted |
32,555 | 31,643 | 30,901 | 30,314 | 29,564 | |||||||||||||||
|
BALANCE SHEET DATA: (at end of period) |
||||||||||||||||||||
|
Working capital |
$ | 129,477 | $ | 125,172 | $ | 102,513 | $ | 60,018 | $ | 77,414 | ||||||||||
|
Total assets |
505,539 | 486,587 | 458,037 | 385,731 | 342,831 | |||||||||||||||
|
Long-term debt |
- | 60,000 | 79,250 | 64,000 | 98,000 | |||||||||||||||
|
Total stockholders' equity |
310,835 | 251,226 | 210,220 | 163,297 | 100,341 | |||||||||||||||
(dollars in thousands, except per share data)
|
(1) |
Income from continuing operations includes restructuring expenses of $15,573 and a tax benefit of $1,464 related to the decision to sell the finished dosage form facility in Hyderabad, India. Income from discontinued operations includes pre-tax income of $63, reduced by tax expense of $22, for environmental reimbursements related to sites of divested businesses. |
|
(2) |
Income from continuing operations includes a pre-tax gain on the sale of land of $1,234 reduced for tax expense of $387, a charge of $7,170 related to a voluntary lump sum pension settlement, a loss of $4,122 related to the purchase of the remaining shares in Zenara, a benefit of $26,902 for the release of a valuation allowance and a benefit of $3,948 for the settlement of tax disputes. Loss from discontinued operations includes pre-tax charges of $1,277, reduced for a tax benefit of $447, for environmental remediation related to sites of divested businesses. |
|
(3) |
Income from continuing operations includes a pre-tax gain on the sale of an office building of $4,680 reduced for tax expense of $1,470, and a tax benefit related to changes in tax laws of $1,155. Loss from discontinued operations includes pre-tax charges of $6,708, reduced for a tax benefit of $2,348, for environmental remediation related to sites of divested businesses. |
|
(4) |
Income from continuing operations includes the release of a valuation allowance on domestic deferred tax assets of $36,287 and the impact on deferred taxes of a statutory rate change of $1,328. Loss from discontinued operations includes pre-tax charges of $1,425, reduced for a tax benefit of $499, for environmental remediation related to sites of divested businesses. |
|
(5) |
Loss from discontinued operations includes pre-tax charges of $2,851 for environmental remediation, net of insurance proceeds, related to sites of divested businesses. |
|
Item 7 |
Management's Discussion and Analysis of Financial Condition and Results of Operations. |
Executive Overview
The Company’s business primarily consists of three manufacturing facilities. These facilities mainly manufacture APIs, pharmaceutical intermediates and, to a lesser extent, other fine chemicals. The Company also owns Zenara, a pharmaceutical company with final dosage form manufacturing capabilities based in India and is not material to the financial results. As of December 31, 2015, Zenara is held for sale.
The following significant events, which are explained in detail on the following pages, occurred during 2015:
|
● |
Gross sales in 2015 increased 16.0% to $433,856 from $374,150 in 2014. Foreign currency exchange unfavorably impacted sales 5.4%. |
|
● |
Operating profit increased 74.0% to $90,985 from $52,298 in 2014. Excluding Zenara related restructuring charges in 2015 and the loss on voluntary pension settlement and gain on sale of land in 2014, operating profit increased 83.0%. |
|
● |
The 2015 net cash balance was $13,974, an improvement of $28,456, compared to debt, net of cash of $14,482 in 2014. |
|
● |
Restructuring charges of $15,573 related to classifying Zenara as held for sale, and a related tax benefit of $1,464. |
Gross sales in 2015 of $433,856 were $59,706 or 16.0% higher than 2014. Excluding unfavorable foreign currency, sales increased 21.4% as a result of higher volumes. The volume increase was primarily due to higher sales of certain branded APIs, generic APIs and controlled substances.
Gross margins increased to 40.8% in 2015 compared to 33.1% in 2014. Current year gross margins included a 2.6% favorable impact from foreign currency versus 2014. Margins were positively impacted by plant efficiencies primarily driven by higher production volumes and favorable product mix.
(dollars in thousands, except per share data)
The Company reported income from continuing operations of $57,176, or $1.76 per diluted share in 2015, compared to $58,133 or $1.84 per diluted share in 2014. Excluding restructuring charges of $15,573 and a related tax benefit of $1,464 (discussed below), income from continuing operations was $71,285.
Critical Accounting Estimates
The Company’s critical accounting estimates are those that require the most subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. The Company bases its estimates on historical experience and on other assumptions that are deemed reasonable by management under each applicable circumstance. Actual results or amounts could differ from estimates and the differences could have a material impact on the consolidated financial statements. A discussion of the Company’s critical accounting policies, the underlying judgments and uncertainties affecting their application and the likelihood that materially different amounts would be reported under different conditions or using different assumptions, is as follows:
Revenue Recognition
Revenues are generally recognized when title to products and risk of loss are transferred to customers. Additional conditions for recognition of revenue are that collection of sales proceeds is reasonably assured and the Company has no further performance obligations.
Amounts billed in advance are recorded as deferred revenue or advance payments on the balance sheet. Since payments received are sometimes non-refundable, the termination of a contract by a customer prior to its completion could result in an immediate recognition of deferred revenue relating to payments already received but not previously recognized as revenue.
Sales terms to certain customers include rebates if certain conditions are met. Additionally, sales are generally made with a limited right of return under certain conditions. The Company estimates these rebates and returns at the time of sale based on the terms of agreements with customers and historical experience and estimated orders. The Company recognizes revenue net of these estimated costs which are classified as allowances and rebates.
The Company bills a portion of freight cost incurred on shipments to customers. Amounts billed to customers are recorded within net revenues. Freight costs are reflected in cost of goods sold.
Asset Valuations and Review for Potential Impairments
The review of long-lived assets, principally fixed assets and other amortizable intangibles, requires the Company to estimate the undiscounted future cash flows generated from these assets whenever events or changes in circumstances indicate that the carrying value may not be fully recoverable. If undiscounted cash flows are less than the carrying value, the long-lived assets are written down to fair value.
The review of the carrying value of goodwill and indefinite lived intangibles is conducted annually or whenever events or changes in circumstances indicate that the carrying value may not be fully recoverable utilizing a two-step process. In the first step, the fair value of the reporting units is determined using a discounted cash flow model and compared to the carrying value. If such analysis indicates that impairment may exist, the Company then estimates the fair value of the other assets and liabilities utilizing appraisals and discounted cash flow analyses to calculate an impairment charge.
The determination of fair value is judgmental and involves the use of significant estimates and assumptions, including projected future cash flows primarily based on operating plans, discount rates, determination of appropriate market comparables and perpetual growth rates. These estimates and assumptions could have a significant impact on whether or not an impairment charge is recognized and the magnitude of any such charge.
(dollars in thousands, except per share data)
Income Taxes
The Company applies the asset and liability method to accounting for income taxes. Deferred tax assets and liabilities are recognized for the expected future tax consequences of temporary differences between the financial statement carrying amounts and tax bases of assets and liabilities, and net operating loss (“NOL”) and tax credit carryovers, on a taxing jurisdiction basis using enacted tax rates in effect for the year in which the differences are expected to reverse or the NOLs or tax credit carryforwards are expected to be realized. The recoverability of deferred tax assets is dependent upon the Company’s assessment that it is more likely than not, considering both positive and negative evidence, that sufficient future taxable income of the appropriate type and in the appropriate taxable years will be generated in the relevant tax jurisdictions to utilize the deferred tax assets. This assessment takes into account the nature, frequency, and severity of any financial reporting losses, sources of future taxable income, and available prudent and feasible tax planning strategies. If, based on the weight of available evidence, it is more likely than not the deferred tax assets will not be realized, the Company records a valuation allowance against all or a portion of the deferred tax assets to adjust the balance to the amount considered more likely than not to be realized.
The Company has provided a valuation allowance against state items and foreign NOL carryovers. It is possible that changes in the assessment could result in the release of valuation allowance attributable to these items in the future, or the establishment of a valuation allowance against certain deferred tax assets for which the Company has no current reserves. The Company’s accounting for deferred taxes represents management’s best estimate of those future events. Changes in current estimates, due to unanticipated events, could have a material impact on the Company’s financial condition and results of operations.
The Company accounts for uncertain tax positions by applying the more likely than not threshold to recognition and de-recognition. Tax benefits from uncertain tax positions are recognized if it is more likely than not that the tax position will be sustained upon examination by taxing authorities with full knowledge of all relevant information, based on the technical merits of the position. The calculation of uncertain tax positions involves significant judgement in applying complex tax laws, and resolution of these matters in a manner inconsistent with management’s expectations could have a material impact on the Company’s financial condition and results of operations.
Environmental and Litigation Contingencies
The Company periodically assesses the potential liabilities related to any lawsuits or claims brought against it. See Note 20 to the Company’s consolidated financial statements for a discussion of the Company’s current environmental and litigation matters, reserves recorded and its position with respect to any related uncertainties. While it is typically very difficult to determine the timing and ultimate outcome of these actions, the Company uses its best judgment to determine if it is probable that the Company will incur an expense related to a settlement for such matters and whether a reasonable estimation of such probable loss, if any, can be made. If probable and estimable, the Company accrues for the costs of investigation, remediation, settlements and legal fees. Given the inherent uncertainty related to the eventual outcome of litigation and environmental matters, it is possible that all or some of these matters may be resolved for amounts materially different from any provisions that the Company may have made with respect to their resolution from time to time.
(dollars in thousands, except per share data)
Employee Benefit Plans
The Company provides a range of benefits to certain employees and retired employees, including pension benefits. The Company records annual amounts relating to these plans based on calculations, which include various actuarial assumptions, including discount rates, assumed rates of return and turnover rates. The Company reviews its actuarial assumptions on an annual basis and makes modifications to the assumptions based on current rates and trends when it is deemed appropriate to do so. The effect of the modifications is generally recorded and amortized over future periods. The Company believes that the assumptions utilized for recording obligations under its plans are reasonable.
The discount rate used to measure pension liabilities and costs is selected by projecting cash flows associated with plan obligations which are matched to a yield curve of high quality bonds. The Company then selects the single rate that produces the same present value as if each cash flow were discounted by the corresponding spot rate on the yield curve.
Results of Operations
2015 Compared to 2014
Gross sales in 2015 of $433,856 were $59,706 or 16.0% higher than 2014. Excluding unfavorable foreign currency, sales increased 21.4% as a result of higher volumes. The volume increase was primarily due to higher sales of certain branded APIs, generic APIs and controlled substances.
The Company’s products and services are sold to a diverse group of several hundred customers, with one customer accounting for 34.5% and 24.0% of 2015 and 2014 consolidated sales, respectively. The Company’s products are sold through a combination of direct sales and independent agents. One API, an antiviral product, represented 32.1% and 22.9% of 2015 and 2014 consolidated sales, respectively.
Gross profit in 2015 was $176,965 compared to $123,798 in 2014. Gross margins increased to 40.8% in 2015 compared to 33.1% in 2014. The 2015 gross margins included a 2.6% favorable impact from foreign currency versus 2014. Margins were positively impacted by plant efficiencies primarily driven by higher production volumes and favorable product mix.
Selling, general and administrative expenses were $57,867, or 13.3% of gross sales in 2015, compared to $52,489, or 14.0%, in 2014. The increase in administrative expenses is mainly due to higher personnel costs (approximately $5,000), costs related to the implementation of a new ERP system (approximately $1,000), pension (approximately $700), recruiting (approximately $400) and medical expenses (approximately $300). Sales and marketing expenses were also higher (approximately $2,200) mainly as a result of adding additional sales associates. Higher costs were partially offset by favorable foreign currency (approximately $5,100).
Research and development expenses were $12,540, or 2.9% of gross sales in 2015, compared to $13,075, or 3.5%, of gross sales in 2014. The decrease is primarily due to favorable foreign currency (approximately $1,300) partially offset by costs to develop new generic drug products (approximately $800).
Restructuring expenses relate to the decision to sell Zenara, which is classified as held for sale at December 31, 2015. Charges include the write off of goodwill and an amortizable intangible asset as well as adjusting Zenara’s assets and liabilities to reflect fair value. These charges totaled $15,573, the majority of which are non-cash expenses.
(dollars in thousands, except per share data)
Operating profit was $90,985 in 2015 compared to $52,298 in 2014. The increase in operating profit is primarily due to higher gross profit partially offset by higher operating expenses.
Net interest expense was $1,699 in 2015 compared to $2,174 in 2014. The decrease in net interest expense is attributed to higher capitalized interest as a result of large capital projects under construction in 2015, and a decrease in average borrowing and interest rates. The average interest rate on debt was 2.3% in 2015 versus 2.4% in 2014.
The Company recorded tax expense of $32,389 in 2015, resulting in an effective tax rate of 36.2%, compared to a benefit of $12,627 in 2014. Excluding the effects of the Zenara restructuring charges of $15,573 and the related tax benefit of $1,464, the effective tax rate was 32.2% in 2015. Excluding the effects of a benefit of $26,902 for domestic valuation allowance reversals, a benefit of $3,948 for tax audit settlements, a gain of $1,234 on the sale of land and related tax expense of $387, a loss of $7,170 on voluntary pension settlement, and a loss of $4,122 on the acquisition of Zenara shares, the effective tax rate was 32.1% in 2014.
During the fourth quarter of 2014, the Company entered into a final settlement with a tax authority, without any admission of fault or breach of laws, in order to avoid further litigation concerning intercompany transactions from 2003. The settlement required the Company to pay $1,487 in tax and interest during the fourth quarter of 2014 in full satisfaction of all liabilities for this matter, and in response the tax authority withdrew all pending litigation and renounced any outstanding claims. The settlement did not impose any penalties on the Company. Therefore, in the fourth quarter of 2014 the Company decreased its remaining reserve for unrecognized tax benefits for this matter by $4,137.
Income from continuing operations in 2015 was $57,176 or $1.76 per diluted share, versus $58,133, or $1.84 per diluted share in 2014. Income from continuing operations in 2014 includes a tax benefit of $26,902, or $0.85 per diluted share, resulting from the release of a valuation allowance on deferred tax assets.
2014 Compared to 2013
Gross sales in 2014 of $374,150 were $56,938 or 17.9% higher than 2013. Excluding foreign currency, sales increased 19.4% as a result of higher volumes (+19.7%) partially offset by slightly lower pricing (-0.3%). The volume increase was primarily due to higher sales of certain branded APIs, controlled substances and generic APIs. Also contributing to the increase in sales is the inclusion of Zenara’s results in the Company’s consolidated financial statements subsequent to the purchase of the remaining shares in Zenara during the second quarter of 2014. Zenara’s sales were $4,225 subsequent to the purchase.
The Company’s products and services are sold to a diverse group of several hundred customers, with one customer accounting for 24.0% and 18.3% of 2014 and 2013 consolidated sales, respectively. The Company’s products are sold through a combination of direct sales and independent agents. One API, an antiviral product, represented 22.9% of 2014 consolidated sales.
Gross profit in 2014 was $123,798 compared to $102,904 in 2013. Gross margins of 33.1% in 2014 were higher compared to 32.4% in 2013. The 2014 gross margin included a 0.6% favorable impact from foreign currency versus 2013. Excluding the foreign currency impact, gross margins were flat between years. Margins were positively impacted by favorable product mix and plant efficiencies mostly offset by higher production costs and lower pricing. The 2014 margins also benefited from the receipt of $1,900 from a business interruption insurance claim that covered 2014 and part of 2013.
Selling, general and administrative expenses of $52,489, or 14.0% of gross sales, in 2014 compared to $47,568, or 15.0%, in 2013. The increase in administrative expenses is mainly due to higher personnel (approximately $1,400) and medical expenses (approximately $700) as well as higher spending on costs related to completing the transaction to purchase the remaining shares in Zenara (approximately $500) and expenses related to due diligence on various acquisition opportunities (approximately $1,200). Sales and marketing expenses were also higher as a result of adding additional sales associates (approximately $1,400).
(dollars in thousands, except per share data)
Research and development expenses of $13,075 were 3.5% of gross sales in 2014, compared to $10,387 or 3.3% of gross sales in 2013. The increase is primarily due to increased headcount (approximately $1,800) and costs to develop a new generic drug product.
In the third quarter of 2014, the Company announced a program to offer a one-time option to elect to receive a voluntary lump-sum pension payout to certain former employees with deferred vested balances in the Company’s U.S. pension plan. As part of this voluntary lump-sum program, the Company settled $17,381 of pension obligations for the U.S. plan with an equal amount paid from plan assets. As a result, the Company recorded settlement losses of $7,170 reflecting the accelerated recognition of unamortized losses in the U.S. pension plan proportionate to the obligation that was settled. The loss on voluntary pension settlement is reflected as a separate line in the consolidated income statement with a corresponding balance sheet reduction in “Accumulated other comprehensive loss.”
Operating profit was $52,298 in 2014 compared to $49,629 in 2013. The increase in operating profit is primarily due to higher gross profit and a gain on the sale of land of $1,234 partially offset by the pension settlement charge discussed above, higher operating expenses and a benefit related to a gain on sale of an office building of $4,680 in 2013.
Net interest expense was $2,174 in 2014 compared to $2,242 in 2013. The decrease in net interest expense is attributed to a decrease in average borrowing partially offset by lower interest income and lower capitalized interest as a result of fewer large capital projects under construction in 2014. The average interest rate on debt was 2.4% in 2014 versus 2.3% in 2013.
Equity in losses of partially-owned affiliates was $4,623 and $2,262 in 2014 and 2013, respectively, primarily representing Zenara’s results. The Company’s portion of Zenara’s loss, prior to the purchase of the remaining shares in May 2014, was $458 and $1,956 for 2014 and 2013, respectively. These amounts include amortization expense of $333 and $882 for 2014 and 2013, respectively. In addition, 2014 includes a loss of $4,122 related to the purchase of the remaining shares in Zenara.
The Company recorded a tax benefit of $12,627 in 2014 compared to expense of $14,732 in 2013. The tax benefit for 2014 includes a benefit of $26,902 for a reversal of domestic valuation allowances. The reversal of the valuation allowance in 2014 resulted from the Company’s assessment of realizability of domestic federal foreign tax credit carryforwards due to expected future U.S. taxable income in amounts and type that support utilization of these credits. Excluding the effects of the valuation allowance reversal, tax audit settlements, the loss on voluntary pension settlement, the sale of the land, and the Zenara loss, the effective tax rate was 32.1% in 2014 compared to 32.7% in 2013. The lower effective tax rate in 2014 was mostly due to differences in the geographical mix of income.
During the fourth quarter of 2014, the Company entered into a final settlement with a tax authority, without any admission of fault or breach of laws, in order to avoid further litigation concerning intercompany transactions from 2003. The settlement required the Company to pay $1,487 in tax and interest during the fourth quarter of 2014 in full satisfaction of all liabilities for this matter, and in response the tax authority withdrew all pending litigation and renounced any outstanding claims. The settlement did not impose any penalties on the Company. Therefore, in the fourth quarter of 2014 the Company decreased its remaining reserve for unrecognized tax benefits for this matter by $4,137.
Income from continuing operations in 2014 was $58,133 or $1.84 per diluted share, versus $30,275, or $0.98 per diluted share in 2013. Income from continuing operations in 2014 includes a tax benefit of $26,902, or $0.85 per diluted share, resulting from the release of a valuation allowance on deferred tax assets.
(dollars in thousands, except per share data)
Liquidity and Capital Resources
During 2015, cash flows from operations provided $83,606, compared to $64,968 in the same period a year ago. The increase in cash flows from operations in 2015 compared to 2014 was largely due to higher net income after adjusting for non-cash items partially offset by increased inventory levels to support sales growth in 2016 and higher accounts receivable. Cash flows used in investing activities in 2015 of $60,239 mainly reflects cash flows related to capital expenditures of $62,491. Cash flows used in financing activities in 2015 of $22,739 mainly reflects the pay down of the Company’s debt partially offset by proceeds from stock options exercised. Debt, net of cash, decreased $28,456 during 2015 to a net cash balance of $13,974.
In November 2011, the Company entered into a $250,000 five-year Syndicated Senior Revolving Credit Facility (“Credit Facility”) which expires in November 2016. The Company pays interest on this Credit Facility at LIBOR plus 1.75% - 2.50% based upon certain financial measurements. The Credit Facility also includes financial covenants regarding interest coverage and leverage ratios. The Company was in compliance with all financial covenants at December 31, 2015. The Company anticipates renewing the credit facility prior to the November expiration.
In March 2012, the Company entered into an interest rate swap with a notional value of $60,000, at a fixed rate of 0.92%. This swap expired in September 2015. The Company’s strategy was to cover a portion of its outstanding floating rate debt with fixed interest rate protection. At December 31, 2015, the Company had floating rate debt of $30,000, none of which is fixed by an interest rate swap.
The 2015 and 2014 weighted average interest rates for long-term bank debt were 2.3% and 2.4%, respectively.
For 2016, capital expenditures are expected to be approximately $70,000 to $75,000.
Contractual Obligations
At December 31, 2015, the Company’s contractual obligations with initial or remaining terms in excess of one year were as follows:
|
Total |
2016 |
2017 |
2018 |
2019 |
2020 |
2021+ |
||||||||||||||||||||||
|
Short term debt |
$ | 30,000 | $ | 30,000 | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||
|
Operating leases |
2,609 | 1,085 | 602 | 541 | 196 | 93 | 92 | |||||||||||||||||||||
|
Purchase obligations |
7,058 | 6,389 | 563 | 59 | 47 | - | - | |||||||||||||||||||||
|
Contractual cash obligations |
$ | 39,667 | $ | 37,474 | $ | 1,165 | $ | 600 | $ | 243 | $ | 93 | $ | 92 | ||||||||||||||
In addition to the contractual obligations listed above, the Company expects to contribute $759 in cash to its U.S. defined-benefit pension plan in 2016. It is possible that higher pension contributions could be required in 2017 and beyond. For the unfunded SERP and international pension plans, the Company expects to make benefit payments of approximately $1,300 for 2016 through 2018. The Company expects to make benefit payments of approximately $800 in 2019 and 2020 for the international pension plan. See Note 17 to the Company’s consolidated financial statements for details on the Company’s unfunded balance related to its pension plans. Also not included in the table above is $1,966 of uncertain tax positions due to uncertainties surrounding the timing of the obligation. See Note 10 to the Company’s consolidated financial statements for details on the Company’s tax positions. The Company may be required to make cash payments to remediate certain environmental sites at unknown future periods as discussed in Note 20 to the Company’s consolidated financial statements.
See Notes 11, 17, 19 and 20 to the Company’s consolidated financial statements for additional information regarding the Company’s debt, pension plans, and other commitments.
(dollars in thousands, except per share data)
The Company’s forecasted cash flow from future operations may be adversely affected by various factors including, but not limited to, declines in customer demand, increased competition, the deterioration in general economic and business conditions, interest rates, returns on assets within the Company’s domestic pension plan that are significantly below expected performance, tax audit payments, as well as other factors. See the Risk Factors section of this document for further explanation of factors that may negatively impact the Company’s cash flows. Any change in the current status of these factors could adversely impact the Company's ability to fund operating cash flow requirements.
Market Risks
Currency Risk Management
The Company's primary market risk relates to exposure to foreign currency exchange rate fluctuations on transactions entered into by international operations which are primarily denominated in the U.S. dollar, euro and Swedish krona. The Company may use foreign currency exchange forward contracts to mitigate the effect of short-term foreign exchange rate movements on the Company's operating results. The notional amount of the contracts outstanding as of December 31, 2015 was $9,322. The foreign exchange contracts have varying maturities with none exceeding twelve months.
With respect to the contracts outstanding at December 31, 2015, a 10% fluctuation of the local currency over a one-year period would cause approximately $931 pre-tax earnings to be at risk. These calculations do not include the impact of exchange gains or losses on the underlying positions that would offset the gains and losses of the derivative instrument.
Interest Rate Management
The Company employed a plan to mitigate interest rate risk by entering into an interest rate swap agreement. A swap is a contract to exchange floating rate for fixed interest payments periodically over the life of the agreement without the exchange of the underlying notional debt amount. The interest rate swap had a notional value of $60,000, at a fixed rate of 0.92%, which expired in September 2015. The Company’s strategy was to cover a portion of outstanding bank debt with interest rate protection.
Contingencies
The Company is subject to various investigations, claims and legal proceedings covering a wide range of matters that arise in the ordinary course of its business activities. The Company continually assesses known facts and circumstances as they pertain to applicable legal and environmental matters and evaluates the need for reserves and disclosures as deemed necessary based on these facts and circumstances. These matters, either individually or in the aggregate, could result in actual costs that are significantly higher than the Company’s current assessment and could have a material adverse effect on the Company's operating results and cash flows in future reporting periods. Based upon past experience, the Company believes that payments significantly in excess of current reserves, if required, would be made over an extended number of years.
Environmental
In connection with laws and regulations pertaining to the protection of the environment, the Company and its subsidiaries are a party to several environmental proceedings and remediation activities and along with other companies, have been named a potentially responsible party (“PRP”) for certain waste disposal sites ("Superfund sites"). Substantially all of the liabilities currently recorded on the Company’s balance sheet for environmental proceedings are associated with discontinued operations. The Company had insurance policies in place at certain of the discontinued operations for certain years that the Company believes should cover some portion of currently recorded liabilities or potential future liabilities.
(dollars in thousands, except per share data)
It is the Company’s policy to record appropriate liabilities for environmental matters where remedial efforts are probable and the costs can be reasonably estimated. Such liabilities are based on the Company’s estimate of the undiscounted future costs required to complete the remedial work. Each of these matters is subject to various uncertainties, and it is possible that some of these matters will be decided against the Company. The resolution of such matters often spans several years and frequently involves regulatory oversight or adjudication. Additionally, many remediation requirements are fluid and are likely to be affected by future technological, site and regulatory developments. It is not possible at this time for the Company to determine fully the effect of all asserted and unasserted claims on its consolidated financial condition, results of operations or liquidity; however, to the extent possible, where asserted and unasserted claims can be estimated and where such claims are considered probable, the Company would record a liability. Consequently, the ultimate liability with respect to such matters, as well as the timing of cash disbursements, is uncertain.
In matters where the Company is able to reasonably estimate the probable and estimable costs associated with environmental proceedings, the Company accrues for the estimated costs associated with the study and remediation of applicable sites. These reserves were $8,329 and $9,595 at December 31, 2015 and 2014, respectively. The decrease in the reserve includes payments of $2,257 and the impact of currency translation of $83, partially offset by adjustments to reserves of $1,074. The reserves are adjusted periodically as remediation efforts progress or as additional technical, regulatory or legal information becomes available. Given the uncertainties regarding the outcome of investigative and study activities, the status of laws, regulations, enforcement, policies, the impact of other PRPs, technology and information related to individual sites, the Company does not believe it is possible to currently develop an estimate of the range of reasonably possible environmental loss in excess of its reserves.
Bayonne
As a result of the sale of a Bayonne, New Jersey facility, the Company became obligated to investigate site conditions and conduct required remediation under the New Jersey Industrial Site Recovery Act. The Company intends to continue implementing a sampling plan at the property pursuant to the New Jersey Department of Environmental Protection’s (“NJDEP”) private oversight program. The results of the completed sampling, and any additional sampling deemed necessary, will be used to develop an estimate of the Company's future liability for remediation costs. As of December 31, 2015, the Company’s reserve was $432.
Clifton and Carlstadt
The Company has implemented a sampling and pilot program in Clifton, New Jersey pursuant to the NJDEP private oversight program. The results of the sampling and pilot program to date have been used to develop an estimate of the Company's future liability for remediation costs. As of December 31, 2015, the Company’s reserve was $1,090.
Additionally, the Company has implemented a sampling and pilot program in Carlstadt, New Jersey pursuant to the NJDEP private oversight program. The results of the sampling and pilot program to date have been used to develop an estimate of the Company's future liability for remediation costs. As of December 31, 2015, the Company’s reserve was $1,030.
Berry’s Creek
The Company received a notice from the United States Environmental Protection Agency (“USEPA”) that two subsidiaries of the Company are considered PRPs at the Berry’s Creek Study Area in New Jersey. These subsidiaries are among many other PRPs that were listed in the notice. Pursuant to the notice, the PRPs have been asked to perform a remedial investigation (“RI”) and feasibility study of the Berry’s Creek site. The Company has joined the group of PRPs and entered into an Administrative Settlement Agreement (“Agreement”) and Order on Consent with the USEPA agreeing to jointly conduct or fund an appropriate remedial investigation and feasibility study of the Berry’s Creek site with the other PRPs in the Agreement. The PRPs have engaged consultants to perform the work specified in the Agreement and develop a method to allocate related costs among the PRPs. As of December 31, 2015, the Company’s reserve was $64 to cover the current phase of investigation based on a tentative agreement on the allocation of the site investigation costs among the PRPs. Due to the very preliminary and uncertain nature of any estimates related to the method and costs of any remediation solution (not expected to be known prior to late 2018), the number of eventual PRPs, and their respective proportion of remediation costs, the Company’s liability cannot be reasonably estimated at this time; as such, no accrual is recorded for these potential future costs. The impact of the resolution of this matter on the Company’s results of operations in any future reporting period is not known.
(dollars in thousands, except per share data)
In July 2014, the Company received a notice from the U.S. Department of the Interior, U.S. Fish & Wildlife Service, regarding the Company’s potential liability for natural resource damages at the Berry’s Creek site and inviting the Company to participate in a cooperative assessment of natural resource damages. Most members of the Berry’s Creek PRP group received such notice letters, and the PRP Group coordinated a joint response, which was to decline participation in a cooperative assessment at this time, given existing investigation work at the site. The cost of any future assessment and the ultimate scope of natural resource damage liability are not yet known.
Maybrook Site
A subsidiary of Cambrex is named a PRP of a site in Hamptonburgh, New York by the USEPA in connection with the discharge, under appropriate permits, of wastewater at that site prior to Cambrex's acquisition in 1986. The PRPs implemented soil remediation which was completed in 2012 pending approval by the USEPA. The PRPs will continue implementing the ground water remediation at the site. As of December 31, 2015, the Company’s reserve was $322 to cover remaining ground water remediation and long-term monitoring.
Harriman Site
Subsidiaries of Cambrex and Pfizer are named as responsible parties for the Company’s former Harriman, New York production facility by the New York State Department of Environmental Conservation (“NYSDEC”). A final Record of Decision (“ROD”) describing the Harriman site remediation responsibilities for Pfizer and the Company was issued in 1997 (the “1997 ROD”) and incorporated into a federal court Consent Decree in 1998 (the “Consent Decree”). In December 2013, the Company, Pfizer and the NYSDEC entered into a federal court stipulation, which the court subsequently endorsed as a court order, resolving certain disputes with the NYSDEC about the scope of the obligations under the Consent Decree and the 1997 ROD, and requiring the Company and Pfizer to carry out an environmental investigation and study of certain areas of the Harriman Site.
Site clean-up work under the 1997 ROD, the Consent Decree and the 2013 stipulation is ongoing and is being jointly performed by Pfizer and the Company, with NYSDEC oversight. During 2014, Pfizer and the Company performed supplemental remedial investigation measures agreed to by the NYSDEC, and the findings were submitted to NYSDEC in a Supplemental RI Report and a Feasibility Study. In April 2015, the NYSDEC informed the Company and Pfizer by letter that the Supplemental RI Report was disapproved, and demanded that the Company and Pfizer perform additional environmental investigative work and revise certain aspects of that report. The Company and Pfizer are in discussions with the NYSDEC to address its written comments. As it is too soon to determine whether the discussion with NYSDEC will result in any significant changes to the Company’s responsibilities, no change to the reserve has been made. ELT Harriman, LLC ("ELT"), the current owner of the Harriman site, is conducting other investigation and remediation activities under a separate NYSDEC directive.
No final remedy for the site has been determined, which will follow further discussions with the NYSDEC. The Company estimates the range for its share of the liability at the site to be between $2,000 and $7,000. As of December 31, 2015, the Company’s reserve was $3,565. At this time, the Company is unable to provide an estimate of the ultimate investigative and remedial costs to the Company for any final remedy selected by the NYSDEC.
The Company intends to enforce all of its contractual rights to recover costs and for indemnification under a 2007 settlement agreement, and has filed such claims in an arbitration proceeding against ELT and the immediately preceding owner, Vertellus Specialties Holdings. ELT has filed counterclaims, and has threatened to file additional counterclaims, for contractual indemnification and for breach of the settlement agreement against the Company. Currently, the arbitration proceeding is stayed indefinitely.
(dollars in thousands, except per share data)
Scientific Chemical Processing (“SCP”) Superfund Site
A subsidiary of Cambrex was named a PRP of the SCP Superfund site, located in Carlstadt, New Jersey, along with approximately 130 other PRPs. The site is a former waste processing facility that accepted various waste for recovery and disposal including processing wastewater from this subsidiary. The PRPs are in the process of implementing a final remedy at the site. The SCP Superfund site has also been identified as a PRP in the Berry’s Creek Superfund site (see previous discussion). While the Company continues to dispute the methodology used by the PRP group to arrive at its interim allocation for cash contributions, the Company paid the funding requests in 2010 and 2014-2015. A final allocation of SCP Site costs (excluding Berry’s Creek costs) is expected to be finalized during 2016. As of December 31, 2015, the Company’s reserve was $934, of which approximately $598 is expected to be covered by insurance.
Newark Bay Complex
The USEPA and a private party group are evaluating remediation plans for the Passaic River, Newark Bay, Hackensack River, Arthur Kill, Kill Van Kull and adjacent waters (the “Newark Bay Complex”). Although the Company is not involved in the USEPA action, it continues to monitor developments related to the site due to its past involvement in a previously settled state action relating to the Newark Bay Complex. It is the Company’s understanding that the private party group and the USEPA have proposed remedies for the site with estimated costs ranging from $500 million to over a billion dollars. The USEPA is expected to select a remedy in the near future. Due to the uncertainty of the future scope and timing of any possible claims against the Company, no liability has been recorded.
The Company is involved in other related and unrelated environmental matters where the range of liability is not reasonably estimable at this time and it is not foreseeable when information will become available to provide a basis for adjusting or recording a reserve, should a reserve ultimately be required.
Litigation and Other Matters
Lorazepam and Clorazepate
In 1998, the Company and a subsidiary were named as defendants along with Mylan Laboratories, Inc. (“Mylan”) and Gyma Laboratories, Inc. (“Gyma”) in a proceeding instituted by the Federal Trade Commission in the United States District Court for the District of Columbia (the “District Court”). Suits were also commenced by several State Attorneys General and class action complaints by private plaintiffs in various state courts. The suits alleged violations of the Federal Trade Commission Act arising from exclusive license agreements between the Company and Mylan covering two APIs (Lorazepam and Clorazepate).
All cases have been resolved except for one brought by four health care insurers. In the remaining case, the District Court entered judgment after trial in 2008 against Mylan, Gyma and Cambrex in the total amount of $19,200, payable jointly and severally, and also a punitive damage award against each defendant in the amount of $16,709. In addition, at the time, the District Court ruled that the defendants were subject to a total of approximately $7,500 in prejudgment interest. The case is currently pending before the District Court following a January 2011 remand by the Court of Appeals. In July 2014, the District Court dismissed certain customers for which the plaintiffs were unable to establish jurisdiction and consequently, the plaintiffs currently have a motion pending before the District Court to reduce the damages award by a total of $9,600.
In 2003, Cambrex paid $12,415 to Mylan in exchange for a release and full indemnity against future costs or liabilities in related litigation brought by the purchasers of Lorazepam and Clorazepate, as well as potential future claims related to the ongoing matter. In the event of a final settlement or final judgment, Cambrex expects any payment required by the Company to be made by Mylan under the indemnity described above.
Other
The Company has commitments incident to the ordinary course of business including corporate guarantees of certain subsidiary obligations to the Company’s lenders related to financial assurance obligations under certain environmental laws for remediation; closure and third party liability requirements of certain of its subsidiaries and a former operating location; contract provisions for indemnification protecting its customers and suppliers against third party liability for the manufacture and sale of Company products that fail to meet product warranties and contract provisions for indemnification protecting licensees against intellectual property infringement related to licensed Company technology or processes.
(dollars in thousands, except per share data)
Additionally, as permitted under Delaware law, the Company indemnifies its officers, directors and employees for certain events or occurrences while the officer, director or employee is, or was, serving at the Company’s request in such capacity. The term of the indemnification period is for the officer's, director's or employee’s lifetime. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited; however, the Company has a director and officer insurance policy that covers a portion of any potential exposure. The Company currently believes the estimated fair value of its indemnification agreements is not material based on currently available information, and as such, the Company had no liabilities recorded for these agreements as of December 31, 2015.
Cambrex's subsidiaries are party to a number of other proceedings that are not considered material at this time.
Impact of Recent Accounting Pronouncements
Simplifying Balance Sheet Classification of Deferred Taxes
In November 2015 the FASB issued ASU 2015-07, which requires that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. This standard is effective for fiscal years beginning after December 15, 2016, including interim periods within that reporting period, and may be applied on a retrospective or prospective basis. The Company has elected to apply this new guidance early, as of December 31, 2015, on a retrospective basis to all periods presented. This change in accounting principle results in all deferred tax liabilities and assets being classified as noncurrent on the Company’s consolidated balance sheet. The reason for the change is to simplify the Company’s presentation of deferred income tax balances. The retrospective effects of the accounting change on the Company’s 2014 consolidated balance sheet is a reclassification of current deferred tax assets and current deferred tax liabilities to noncurrent, resulting in a net increase of $3,323 in noncurrent deferred tax assets and a net decrease of $296 in noncurrent deferred tax liabilities. The retrospective effects of the accounting change on the Company’s working capital for 2014, 2013, 2012, and 2011 presented in Item 6 selected financial data is a reclassification of current deferred tax assets and current deferred tax liabilities to noncurrent, resulting in a net decrease in working capital of $3,619, $2,776, $1,469, and $62, respectively.
Simplifying the Presentation of Debt Issuance Costs
In April 2015, the FASB issued ASU 2015-03 which requires that debt issuance costs related to a recognized debt liability be presented on the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts, rather than be presented as an asset. In August 2015, the FASB issued ASU 2015-15, which amends the previously issued standard, to allow debt issuance costs related to a line-of-credit arrangement to continue to be reported as an asset. This standard is effective for fiscal years beginning after December 15, 2015, including interim periods within that reporting period, and must be applied on a retrospective basis. This pronouncement will not have a material impact on the Company’s financial position or results of operations.
Simplifying the Measurement of Inventory
In July 2015, the FASB issued ASU 2015-11 which requires that inventory be measured at the lower of cost and net realizable value, which eliminates the other two options that currently exist for market, replacement cost and net realizable value less an approximately normal profit margin. This standard is effective for fiscal years beginning after December 15, 2016, including interim periods within that reporting period. The Company is currently evaluating the new guidance to determine the impact, if any, it will have on its consolidated financial statements.
(dollars in thousands, except per share data)
Revenue from Contracts with Customers
In May 2014, the FASB issued ASU 2014-09 that introduces a new five-step revenue recognition model in which an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This ASU also requires disclosures sufficient to enable users to understand the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers, including qualitative and quantitative disclosures about contracts with customers, significant judgments and changes in judgments, and assets recognized from the costs to obtain or fulfill a contract. This standard is effective for fiscal years beginning after December 15, 2017, including interim periods within that reporting period. The Company is currently evaluating the new guidance to determine the impact, if any, it will have on its consolidated financial statements.
Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity
In April 2014, the FASB issued ASU 2014-08, which includes amendments that change the requirements for reporting discontinued operations and require additional disclosures about discontinued operations. Under the new guidance, only disposals representing a strategic shift in operations - that is, a major effect on the organization's operations and financial results - should be presented as discontinued operations. Additionally, the ASU requires expanded disclosures about discontinued operations that will provide financial statement users with more information about the assets, liabilities, income and expenses of discontinued operations. This update was effective in the first quarter of 2015. This pronouncement did not have an impact on the Company’s financial position or results of operations.
(dollars in thousands, except per share data)
|
Item 7A |
Quantitative and Qualitative Disclosures about Market Risk. |
The information required in this section can be found in the “Market Risks” section of Item 7 on page 30 of this Form 10-K.
|
Item 8 |
Financial Statements and Supplementary Data. |
The following consolidated financial statements and selected quarterly financial data of the Company are filed under this item:
|
|
Page Number (in this Report) |
|
Reports of Independent Registered Public Accounting Firm |
37 |
|
Consolidated Balance Sheets as of December 31, 2015 and 2014 |
39 |
|
Consolidated Income Statements for the Years Ended December 31, 2015, 2014 and 2013 |
40 |
|
Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2015, 2014 and 2013 |
41 |
|
Consolidated Statements of Stockholders' Equity for the Years Ended December 31, 2015, 2014 and 2013 |
42 |
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2015, 2014 and 2013 |
43 |
|
Notes to Consolidated Financial Statements |
44 |
| Selected Quarterly Financial and Supplementary Data (unaudited) | 74 |
The financial statement schedules are filed pursuant to Item 15 of this report.
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of Cambrex Corporation,
We have audited the accompanying consolidated balance sheets of Cambrex Corporation as of December 31, 2015 and 2014 and the related consolidated statements of income, comprehensive income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2015. In connection with our audits of the financial statements, we have also audited the financial statement schedules listed in the accompanying index. These financial statements and schedules are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and schedules based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements and schedules. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Cambrex Corporation at December 31, 2015 and 2014, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America.
Also, in our opinion, the financial statement schedules, when considered in relation to the basic consolidated financial statements taken as a whole, present fairly, in all material respects, the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Cambrex Corporation’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated February 9, 2016 expressed an unqualified opinion thereon.
/s/ BDO USA, LLP
Woodbridge, NJ
February 9, 2016
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders of Cambrex Corporation,
We have audited Cambrex Corporation’s internal control over financial reporting as of December 31, 2015, based on criteria established in Internal Control – Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Cambrex Corporation’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying “Item 9A, Management’s Report on Internal Control Over Financial Reporting.” Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Cambrex Corporation maintained, in all material respects, effective internal control over financial reporting as of December 31, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Cambrex Corporation as of December 31, 2015 and 2014, and the related consolidated statements of income, comprehensive income, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2015 and our report dated February 9, 2016 expressed an unqualified opinion thereon.
/s/ BDO USA LLP
Woodbridge, NJ
February 9, 2016
CAMBREX CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(dollars in thousands, except per share data)
|
December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
ASSETS |
||||||||
|
Current assets: |
||||||||
|
Cash and cash equivalents |
$ | 43,974 | $ | 45,518 | ||||
|
Trade receivables, less allowances of $304 and $346 at respective dates |
90,920 | 77,124 | ||||||
|
Other receivables |
7,278 | 10,610 | ||||||
|
Inventories, net |
109,920 | 85,630 | ||||||
|
Prepaid expenses and other current assets |
7,187 | 4,880 | ||||||
|
Total current assets |
259,279 | 223,762 | ||||||
|
Property, plant and equipment, net |
186,487 | 163,567 | ||||||
|
Goodwill |
32,063 | 43,912 | ||||||
|
Intangible assets, net |
6,691 | 8,902 | ||||||
|
Deferred income taxes |
19,259 | 41,747 | ||||||
|
Other non-current assets |
1,760 | 4,697 | ||||||
|
Total assets |
$ | 505,539 | $ | 486,587 | ||||
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
||||||||
|
Current liabilities: |
||||||||
|
Accounts payable |
$ | 39,257 | $ | 43,670 | ||||
|
Deferred revenue and advance payments |
16,298 | 14,095 | ||||||
|
Accrued expenses and other current liabilities |
44,247 | 40,825 | ||||||
|
Short-term debt |
30,000 | - | ||||||
|
Total current liabilities |
129,802 | 98,590 | ||||||
|
Long-term debt |
- | 60,000 | ||||||
|
Deferred income taxes |
7,735 | 10,249 | ||||||
|
Accrued pension benefits |
42,661 | 50,949 | ||||||
|
Other non-current liabilities |
14,506 | 15,573 | ||||||
|
Total liabilities |
194,704 | 235,361 | ||||||
|
Commitments and contingencies (see Notes 19 and 20) |
||||||||
|
Stockholders' equity: |
||||||||
|
Common Stock, $.10 par value; authorized 100,000,000 issued 33,528,915 and 32,836,930 shares at respective dates |
3,353 | 3,284 | ||||||
|
Additional paid-in capital |
131,980 | 119,265 | ||||||
|
Retained earnings |
245,698 | 188,481 | ||||||
|
Treasury stock, at cost, 1,729,727 and 1,738,624 shares at respective dates |
(14,747 | ) | (14,823 | ) | ||||
|
Accumulated other comprehensive loss |
(55,449 | ) | (44,981 | ) | ||||
|
Total stockholders' equity |
310,835 | 251,226 | ||||||
|
Total liabilities and stockholders' equity |
$ | 505,539 | $ | 486,587 | ||||
See accompanying notes to consolidated financial statements.
CAMBREX CORPORATION AND SUBSIDIARIES
CONSOLIDATED INCOME STATEMENTS
(dollars in thousands, except per share data)
|
Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Gross Sales |
$ | 433,856 | $ | 374,150 | $ | 317,212 | ||||||
|
Commissions, allowances and rebates |
1,949 | 2,306 | 1,351 | |||||||||
|
Net sales |
431,907 | 371,844 | 315,861 | |||||||||
|
Other revenues |
1,419 | 2,769 | 2,315 | |||||||||
|
Net revenues |
433,326 | 374,613 | 318,176 | |||||||||
|
Cost of goods sold |
256,361 | 250,815 | 215,272 | |||||||||
|
Gross profit |
176,965 | 123,798 | 102,904 | |||||||||
|
Selling, general and administrative expenses |
57,867 | 52,489 | 47,568 | |||||||||
|
Research and development expenses |
12,540 | 13,075 | 10,387 | |||||||||
|
Restructuring expenses |
15,573 | - | - | |||||||||
|
Operating expenses |
85,980 | 65,564 | 57,955 | |||||||||
|
Loss on voluntary pension settlement |
- | 7,170 | - | |||||||||
|
Gain on sale of asset |
- | (1,234 | ) | (4,680 | ) | |||||||
|
Operating profit |
90,985 | 52,298 | 49,629 | |||||||||
|
Other expenses/(income) |
||||||||||||
|
Interest expense, net |
1,699 | 2,174 | 2,242 | |||||||||
|
Equity in losses of partially-owned affiliates |
- | 4,623 | 2,262 | |||||||||
|
Other (income)/expenses, net |
(279 | ) | (5 | ) | 118 | |||||||
|
Income before income taxes |
89,565 | 45,506 | 45,007 | |||||||||
|
Provision/(benefit) for income taxes |
32,389 | (12,627 | ) | 14,732 | ||||||||
|
Income from continuing operations |
57,176 | 58,133 | 30,275 | |||||||||
|
Income/(loss) from discontinued operations, net of tax |
41 | (830 | ) | (4,360 | ) | |||||||
|
Net income |
$ | 57,217 | $ | 57,303 | $ | 25,915 | ||||||
|
Basic earnings per share |
||||||||||||
|
Income from continuing operations |
$ | 1.82 | $ | 1.89 | $ | 1.00 | ||||||
|
Income/(loss) from discontinued operations, net of tax |
$ | 0.00 | $ | (0.03 | ) | $ | (0.14 | ) | ||||
|
Net income |
$ | 1.82 | $ | 1.86 | $ | 0.86 | ||||||
|
Diluted earnings per share |
||||||||||||
|
Income from continuing operations |
$ | 1.76 | $ | 1.84 | $ | 0.98 | ||||||
|
Income/(loss) from discontinued operations, net of tax |
$ | 0.00 | $ | (0.03 | ) | $ | (0.14 | ) | ||||
|
Net income |
$ | 1.76 | $ | 1.81 | $ | 0.84 | ||||||
|
Weighted average shares outstanding: |
||||||||||||
|
Basic weighted average shares outstanding |
31,420 | 30,763 | 30,150 | |||||||||
|
Effect of dilutive stock based compensation |
1,135 | 880 | 751 | |||||||||
|
Diluted weighted average shares outstanding |
32,555 | 31,643 | 30,901 | |||||||||
See accompanying notes to consolidated financial statements.
CAMBREX CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
(dollars in thousands)
|
Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Net income |
$ | 57,217 | $ | 57,303 | $ | 25,915 | ||||||
|
Foreign currency translation adjustments: |
||||||||||||
|
Unrealized net change arising during the period |
(16,424 | ) | (25,800 | ) | 4,813 | |||||||
|
Reclassification adjustments for losses included in net income |
1,954 | 4,400 | - | |||||||||
|
Interest rate swap agreement: |
||||||||||||
|
Unrealized net losses |
(30 | ) | (152 | ) | (130 | ) | ||||||
|
Reclassification adjustments for losses included in net income |
333 | 465 | 444 | |||||||||
|
Income taxes |
(110 | ) | (109 | ) | (110 | ) | ||||||
|
Pension plans: |
||||||||||||
|
Actuarial gain/(loss) |
||||||||||||
|
Actuarial gain/(loss) arising during the period |
3,970 | (18,338 | ) | 13,651 | ||||||||
|
Amortization to net income of net actuarial loss |
1,295 | 802 | 1,339 | |||||||||
|
Prior service cost |
||||||||||||
|
Amortization to net income of net prior service cost |
52 | 50 | 50 | |||||||||
|
Lump-sum pension payout |
- | 7,170 | - | |||||||||
|
Income taxes |
(1,508 | ) | 5,493 | (4,928 | ) | |||||||
|
Comprehensive income |
$ | 46,749 | $ | 31,284 | $ | 41,044 | ||||||
See accompanying notes to consolidated financial statements.
CAMBREX CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY
(dollars in thousands, except per share data)
|
Common Stock |
||||||||||||||||||||||||||||
|
Shares Issued |
Par Value ($.10) |
Additional Paid-In Capital |
Retained Earnings |
Treasury Stock |
Accumulated Other Comprehensive Loss |
Total Stockholders' Equity |
||||||||||||||||||||||
|
Balance at December 31, 2012 |
31,704,230 | $ | 3,169 | $ | 104,173 | $ | 105,263 | $ | (15,217 | ) | $ | (34,091 | ) | $ | 163,297 | |||||||||||||
|
Net income |
25,915 | 25,915 | ||||||||||||||||||||||||||
|
Other comprehensive income |
15,129 | 15,129 | ||||||||||||||||||||||||||
|
Exercise of stock options |
536,565 | 54 | 3,170 | 3,224 | ||||||||||||||||||||||||
|
Vested restricted stock |
(534 | ) | 534 | - | ||||||||||||||||||||||||
|
Stock option expense |
1,994 | 1,994 | ||||||||||||||||||||||||||
|
Restricted stock expense |
427 | 427 | ||||||||||||||||||||||||||
|
Performance stock expense |
404 | 404 | ||||||||||||||||||||||||||
|
Share based compensation tax windfall |
131 | 131 | ||||||||||||||||||||||||||
|
Repurchase of shares |
(301 | ) | (301 | ) | ||||||||||||||||||||||||
|
Balance at December 31, 2013 |
32,240,795 | $ | 3,223 | $ | 109,765 | $ | 131,178 | $ | (14,984 | ) | $ | (18,962 | ) | $ | 210,220 | |||||||||||||
|
Net income |
57,303 | 57,303 | ||||||||||||||||||||||||||
|
Other comprehensive loss |
(26,019 | ) | (26,019 | ) | ||||||||||||||||||||||||
|
Exercise of stock options |
596,135 | 61 | 4,142 | 4,203 | ||||||||||||||||||||||||
|
Vested restricted stock |
(161 | ) | 161 | - | ||||||||||||||||||||||||
|
Stock option expense |
2,491 | 2,491 | ||||||||||||||||||||||||||
|
Restricted stock expense |
395 | 395 | ||||||||||||||||||||||||||
|
Performance stock expense |
2,116 | 2,116 | ||||||||||||||||||||||||||
|
Share based compensation tax windfall |
517 | 517 | ||||||||||||||||||||||||||
|
Balance at December 31, 2014 |
32,836,930 | $ | 3,284 | $ | 119,265 | $ | 188,481 | $ | (14,823 | ) | $ | (44,981 | ) | $ | 251,226 | |||||||||||||
|
Net income |
57,217 | 57,217 | ||||||||||||||||||||||||||
|
Other comprehensive loss |
(10,468 | ) | (10,468 | ) | ||||||||||||||||||||||||
|
Exercise of stock options |
691,985 | 69 | 5,747 | 5,816 | ||||||||||||||||||||||||
|
Vested restricted stock |
(76 | ) | 76 | - | ||||||||||||||||||||||||
|
Stock option expense |
2,975 | 2,975 | ||||||||||||||||||||||||||
|
Restricted stock expense |
353 | 353 | ||||||||||||||||||||||||||
|
Performance stock expense |
2,271 | 2,271 | ||||||||||||||||||||||||||
|
Share based compensation tax windfall |
1,445 | 1,445 | ||||||||||||||||||||||||||
|
Balance at December 31, 2015 |
33,528,915 | $ | 3,353 | $ | 131,980 | $ | 245,698 | $ | (14,747 | ) | $ | (55,449 | ) | $ | 310,835 | |||||||||||||
See accompanying notes to consolidated financial statements.
CAMBREX CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(dollars in thousands)
|
Years Ended December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Cash flows from operating activities: |
||||||||||||
|
Net income |
$ | 57,217 | $ | 57,303 | $ | 25,915 | ||||||
|
Adjustments to reconcile net income to cash flows: |
||||||||||||
|
Depreciation and amortization |
22,061 | 23,826 | 22,473 | |||||||||
|
Non-cash deferred revenue |
(12,372 | ) | (17,873 | ) | (10,093 | ) | ||||||
|
Restructuring expenses |
15,573 | - | - | |||||||||
|
Loss on voluntary pension settlement |
- | 7,170 | - | |||||||||
|
Increase in inventory reserve |
3,119 | 5,343 | 2,329 | |||||||||
|
Gain on sale of assets |
- | (1,099 | ) | (4,281 | ) | |||||||
|
Allowance for doubtful accounts |
(11 | ) | 61 | 433 | ||||||||
|
Stock based compensation |
5,599 | 5,002 | 2,825 | |||||||||
|
Deferred income tax provision |
19,736 | (16,025 | ) | 5,902 | ||||||||
|
Equity in losses of partially-owned affiliates |
- | 4,623 | 2,262 | |||||||||
|
Other |
(130 | ) | 523 | 348 | ||||||||
|
Changes in assets and liabilities: |
||||||||||||
|
Trade receivables |
(14,378 | ) | (8,955 | ) | (27,707 | ) | ||||||
|
Inventories |
(32,721 | ) | (9,062 | ) | (19,328 | ) | ||||||
|
Prepaid expenses and other current assets |
4,268 | (5,766 | ) | (2,651 | ) | |||||||
|
Accounts payable and other current liabilities |
10,334 | 12,369 | 10,107 | |||||||||
|
Deferred revenue and advance payments |
12,178 | 11,773 | 18,644 | |||||||||
|
Other non-current assets and liabilities |
(5,331 | ) | (2,387 | ) | 11,229 | |||||||
|
Discontinued operations: |
||||||||||||
|
Net cash used in discontinued operations |
(1,536 | ) | (1,858 | ) | (1,533 | ) | ||||||
|
Net cash provided by operating activities |
83,606 | 64,968 | 36,874 | |||||||||
|
Cash flows from investing activities: |
||||||||||||
|
Capital expenditures |
(62,491 | ) | (23,323 | ) | (57,320 | ) | ||||||
|
Proceeds from sale of assets |
2,308 | 2,355 | 2,378 | |||||||||
|
Advances to partially-owned affiliates |
- | (1,404 | ) | (1,655 | ) | |||||||
|
Acquisition of business and equity investment, net of cash acquired |
- | (2,426 | ) | - | ||||||||
|
Other |
(56 | ) | - | - | ||||||||
|
Net cash used in investing activities |
(60,239 | ) | (24,798 | ) | (56,597 | ) | ||||||
|
Cash flows from financing activities: |
||||||||||||
|
Long-term debt activity (including current portion): |
||||||||||||
|
Borrowings |
- | 25,750 | 70,950 | |||||||||
|
Repayments |
(30,000 | ) | (45,000 | ) | (55,700 | ) | ||||||
|
Proceeds from stock options exercised |
5,816 | 4,203 | 3,224 | |||||||||
|
Other |
1,445 | 522 | (301 | ) | ||||||||
|
Net cash (used in)/provided by financing activities |
(22,739 | ) | (14,525 | ) | 18,173 | |||||||
|
Effect of exchange rate changes on cash and cash equivalents |
(2,172 | ) | (2,872 | ) | 744 | |||||||
|
Net (decrease)/increase in cash and cash equivalents |
(1,544 | ) | 22,773 | (806 | ) | |||||||
|
Cash and cash equivalents at beginning of year |
45,518 | 22,745 | 23,551 | |||||||||
|
Cash and cash equivalents at end of year |
$ | 43,974 | $ | 45,518 | $ | 22,745 | ||||||
|
Supplemental disclosure: |
||||||||||||
|
Interest paid, net of capitalized interest |
$ | 1,340 | $ | 2,068 | $ | 2,325 | ||||||
|
Income taxes paid, net of refunds received |
$ | 5,731 | $ | 6,725 | $ | 7,211 | ||||||
See accompanying notes to consolidated financial statements.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(1) The Company
Cambrex Corporation and Subsidiaries (the “Company” or “Cambrex”) primarily provides products and services worldwide to pharmaceutical companies and generic drug companies. The Company is dedicated to accelerating its customers’ drug discovery, development and manufacturing processes for human therapeutics. The Company’s products consist of active pharmaceutical ingredients (“APIs”) and pharmaceutical intermediates produced under Food and Drug Administration current Good Manufacturing Practices for use in the production of prescription and over-the-counter drug products. Cambrex has three operating segments, which are manufacturing facilities that have been aggregated as one reportable segment.
(2) Summary of Significant Accounting Policies
Principles of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. Equity investments in which the Company exercises significant influence but does not control, are accounted for using the equity method. Certain reclassifications have been made to prior year amounts to conform to current year presentation and recent accounting pronouncements. All other significant intercompany balances and transactions have been eliminated in consolidation.
Cash Equivalents
Temporary cash investments with an original maturity of less than three months are considered cash equivalents. The carrying amounts approximate fair value.
Allowance for Doubtful Accounts
The Company maintains allowances for doubtful accounts relating to estimated losses resulting from customers being unable to make required payments. Allowances for doubtful accounts are based on historical experience and known factors regarding specific customers and the industries in which those customers operate. If the financial condition of the Company’s customers were to deteriorate, resulting in their inability to make payments, additional allowances would be required.
Concentrations of credit risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and cash equivalents and accounts receivable. In the normal course of business, the Company maintains cash balances with U.S. and European Union banks ranging from the equivalent of $1,000 - $10,000. The Company routinely monitors the risks associated with these institutions and diversifies its exposure by maintaining smaller balances with multiple financial institutions. Concentrations of credit risk with respect to accounts receivable are limited due to the Company's large number of customers and their dispersion throughout the world. At December 31, 2015 and 2014, the Company had receivables with one customer totaling nearly 51% and 38%, respectively, of overall accounts receivables. The Company does not consider this customer to pose any significant credit risk.
Derivative Instruments
Derivative financial instruments are periodically used by the Company primarily to mitigate a variety of working capital, investment and borrowing risks. The Company primarily uses foreign currency forward contracts to minimize foreign currency exchange rate risk associated with foreign currency transactions. Changes in the fair value on these forward contracts are recognized in earnings. The Company uses interest rate swap instruments only as hedges. As such, the differential to be paid or received in connection with these instruments is accrued and recognized in income as an adjustment to interest expense.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(2) Summary of Significant Accounting Policies (continued)
None of the foreign currency forward contracts entered into during 2015 and 2014 were designated for hedge accounting treatment.
Inventories
Inventories are stated at the lower of cost, determined on a first-in, first-out basis, or market. The determination of market value involves an assessment of numerous factors, including estimated selling prices. Reserves are recorded to reduce the carrying value for inventory determined to be damaged, obsolete or otherwise unsaleable.
Property, Plant and Equipment
Property, plant and equipment is stated at cost, net of accumulated depreciation. Plant and equipment are depreciated on a straight-line basis over the estimated useful lives for each applicable asset group as follows:
|
Buildings and improvements |
20 to 30 years, or term of lease if applicable |
|
Machinery and equipment |
7 to 15 years |
|
Furniture and fixtures |
5 to 7 years |
|
Computer hardware and software |
3 to 7 years |
Expenditures for additions, major renewals or betterments are capitalized and expenditures for maintenance and repairs are charged to income as incurred.
When assets are retired or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts, and any resulting gain or loss is reflected in cost of goods sold or operating expenses. Interest is capitalized in connection with the construction and acquisition of assets that are capitalized over longer periods of time for larger amounts. The capitalized interest is recorded as part of the cost of the asset to which it relates and is amortized over the asset’s estimated useful life. Total interest capitalized in connection with ongoing construction activities was $534 in 2015, $128 in 2014, and $298 in 2013.
Impairment of Goodwill
The Company reviews the carrying value of goodwill to determine whether impairment may exist on an annual basis or whenever it has reason to believe goodwill may not be recoverable. The annual impairment test of goodwill is performed during the fourth quarter of each fiscal year. The Company recorded a non-cash impairment charge at its Zenara facility for the year ended December 31, 2015. Refer to Note 7 to the Company’s consolidated financial statements for additional information on this impairment. For the years ended December 31, 2014 and 2013, the Company did not have an impairment.
Goodwill impairment is determined by the Company using a two-step process. The first step of the goodwill impairment test is to identify potential impairment by comparing the fair value of each reporting unit, determined using various valuation techniques, with the primary technique being a discounted cash flow analysis, to its carrying value. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is considered not impaired and the second step of the impairment test is unnecessary. If the carrying amount of a reporting unit exceeds its fair value, the second step of the goodwill impairment test is performed to measure the amount of the impairment loss, if any. The second step of the goodwill impairment test compares the implied fair value of the reporting unit’s goodwill with the carrying amount of that goodwill. If the carrying amount of the reporting unit’s goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(2) Summary of Significant Accounting Policies (continued)
Based upon the Company’s most recent analysis, the fair value of the reporting units substantially exceeded their carrying values.
Impairment of Long-Lived Assets
The Company assesses the impairment of its long-lived assets, including amortizable intangible assets, and property, plant and equipment, whenever economic events or changes in circumstances indicate that the carrying amounts of the assets may not be recoverable. Long lived assets are considered to be impaired when the sum of the undiscounted expected future operating cash flows is less than the carrying amounts of the related assets. If impaired, the assets are written down to fair market value.
The Company recorded a non-cash impairment charge on long-lived assets at Zenara for the year ended December 31, 2015. Refer to Note 7 to the Company’s consolidated financial statements for additional information on this impairment.
Revenue Recognition
Revenues are generally recognized when title to products and risk of loss are transferred to customers. Additional conditions for recognition of revenue are that collection of sales proceeds is reasonably assured and the Company has no further performance obligations.
Amounts billed in advance are recorded as deferred revenue and advance payments on the balance sheet. Since payments received are sometimes non-refundable, the termination of a contract by a customer prior to its completion could result in an immediate recognition of deferred revenue relating to payments already received but not previously recognized as revenue.
Sales terms to certain customers include rebates if certain conditions are met. Additionally, sales are generally made with a limited right of return under certain conditions. The Company estimates these rebates and returns at the time of sale based on the terms of agreements with customers and historical experience and estimated orders. The Company recognizes revenue net of these estimated costs which are classified as allowances and rebates.
The Company bills a portion of freight cost incurred on shipments to customers. Amounts billed to customers are recorded within net revenues. Freight costs are reflected in cost of goods sold.
Income Taxes
The Company and its eligible subsidiaries file a consolidated U.S. income tax return. Foreign subsidiaries are consolidated for financial reporting but are not eligible to be included in the consolidated U.S. income tax return, however the earnings of foreign subsidiaries are generally taxed by the U.S. when repatriated and such U.S. tax may be reduced or eliminated by federal foreign tax credits based on the foreign income and withholding taxes paid or accrued by the foreign subsidiary. Due in part to a continuing desire to limit credit and currency exposure for cash held in foreign currencies or in non-U.S. banks, the Company determined that it is likely that a portion of the undistributed earnings of its foreign subsidiaries will be repatriated to the U.S. in the future. Accordingly, the Company provides deferred taxes on certain undistributed foreign earnings. Subject to limitations, U.S. income tax on such foreign earnings, when actually repatriated, may be reduced or eliminated by unrecognized foreign tax credits that may be generated in connection with the repatriation or by existing foreign tax credit carryforwards or other tax attributes. The Company monitors available evidence and its plans for foreign earnings and expects to continue to provide deferred taxes based on the tax liability that would be due upon repatriation of amounts not considered permanently reinvested.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(2) Summary of Significant Accounting Policies (continued)
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant items subject to such estimates and assumptions include the valuation of inventory, accounts receivable, asset impairments, stock based compensation and deferred tax assets. Actual results could differ from those estimates.
Environmental Costs
The Company is subject to extensive and changing federal, state, local and foreign environmental laws and regulations, and has made provisions for the estimated financial impact of environmental activities. The Company’s policy is to accrue environmental related costs of a non-capital nature, including estimated litigation costs, when those costs are believed to be probable and can be reasonably estimated. The quantification of environmental exposures requires an assessment of many factors, including changing laws and regulations, advancements in environmental technologies, the quality of information available related to specific sites, the assessment stage of each site investigation, preliminary findings and the length of time involved in remediation or settlement. Such accruals are adjusted as further information develops or circumstances change. For certain matters, the Company expects to share costs with other parties. Recoveries of environmental remediation costs from other parties are recorded as assets when their receipt is deemed certain.
Foreign Currency
The functional currency of the Company's foreign subsidiaries is the applicable local currency. The translation of the applicable foreign currencies into U.S. dollars is performed for balance sheet accounts using current exchange rates in effect at the balance sheet date and for revenue and expense accounts and cash flows using average rates of exchange prevailing during the year. Adjustments resulting from the translation of foreign currency financial statements are accumulated in stockholders' equity until the entity is sold or substantially liquidated. Gains or losses relating to transactions of a long-term investment nature are also accumulated in stockholders' equity. Gains or losses resulting from third-party foreign currency transactions are included in the income statement as a component of other revenues in the consolidated income statement. Foreign currency net (losses)/gains were ($605), $186 and ($133) in 2015, 2014 and 2013, respectively.
Earnings per Common Share
All diluted earnings per share are computed on the basis of the weighted average shares of common stock outstanding plus common equivalent shares arising from the effect of dilutive stock options, equity-settled performance shares and restricted stock units, using the treasury stock method.
For the years ended December 31, 2015, 2014 and 2013, shares of 342,961, 940,038, and 916,350, respectively, were not included in the calculation of diluted shares outstanding because the effect would be anti-dilutive.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(2) Summary of Significant Accounting Policies (continued)
Comprehensive Income
Included within accumulated other comprehensive income (“AOCI”) for the Company are foreign currency translation adjustments, changes in the fair value related to the interest rate swap classified as cash flow hedges, net of related tax, and changes in the pensions, net of tax. Total comprehensive income/loss for the years ended December 31, 2015 and 2014 are included in the Statements of Comprehensive Income.
(3) Impact of Recently Issued Accounting Pronouncements
Simplifying Balance Sheet Classification of Deferred Taxes
In November 2015 the FASB issued ASU 2015-07, which requires that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. This standard is effective for fiscal years beginning after December 15, 2016, including interim periods within that reporting period, and may be applied on a retrospective or prospective basis. The Company has elected to apply this new guidance early, as of December 31, 2015, on a retrospective basis to all periods presented. This change in accounting principle results in all deferred tax liabilities and assets being classified as noncurrent on the Company’s consolidated balance sheet. The reason for the change is to simplify the Company’s presentation of deferred income tax balances. The retrospective effects of the accounting change on the Company’s 2014 consolidated balance sheet is a reclassification of current deferred tax assets and current deferred tax liabilities to noncurrent, resulting in a net increase of $3,323 in noncurrent deferred tax assets and a net decrease of $296 in noncurrent deferred tax liabilities. The retrospective effects of the accounting change on the Company’s working capital for 2014, 2013, 2012, and 2011 presented in Item 6 selected financial data is a reclassification of current deferred tax assets and current deferred tax liabilities to noncurrent, resulting in a net decrease in working capital of $3,619, $2,776, $1,469, and $62, respectively.
Simplifying the Presentation of Debt Issuance Costs
In April 2015, the FASB issued ASU 2015-03 which requires that debt issuance costs related to a recognized debt liability be presented on the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts, rather than be presented as an asset. In August 2015, the FASB issued ASU 2015-15, which amends the previously issued standard, to allow debt issuance costs related to a line-of-credit arrangement to continue to be reported as an asset. This standard is effective for fiscal years beginning after December 15, 2015, including interim periods within that reporting period, and must be applied on a retrospective basis. This pronouncement did not have a material impact on the Company’s financial position or results of operations.
Simplifying the Measurement of Inventory
In July 2015, the FASB issued ASU 2015-11 which requires that inventory be measured at the lower of cost and net realizable value, which eliminates the other two options that currently exist for market, replacement cost and net realizable value less an approximately normal profit margin. This standard is effective for fiscal years beginning after December 15, 2016, including interim periods within that reporting period. The Company is currently evaluating the new guidance to determine the impact, if any, it will have on its consolidated financial statements.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(3) Impact of Recently Issued Accounting Pronouncements (continued)
Revenue from Contracts with Customers
In May 2014, the FASB issued ASU 2014-09 that introduces a new five-step revenue recognition model in which an entity should recognize revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. This ASU also requires disclosures sufficient to enable users to understand the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers, including qualitative and quantitative disclosures about contracts with customers, significant judgments and changes in judgments, and assets recognized from the costs to obtain or fulfill a contract. This standard is effective for fiscal years beginning after December 15, 2017, including interim periods within that reporting period. The Company is currently evaluating the new guidance to determine the impact, if any, it will have on its consolidated financial statements.
Reporting Discontinued Operations and Disclosures of Disposals of Components of an Entity
In April 2014, the FASB issued ASU 2014-08, which includes amendments that change the requirements for reporting discontinued operations and require additional disclosures about discontinued operations. Under the new guidance, only disposals representing a strategic shift in operations - that is, a major effect on the organization's operations and financial results - should be presented as discontinued operations. Additionally, the ASU requires expanded disclosures about discontinued operations that will provide financial statement users with more information about the assets, liabilities, income and expenses of discontinued operations. This update was effective in the first quarter of 2015. This pronouncement did not have an impact on the Company’s financial position or results of operations.
(4) Net Inventories
Inventories are stated at the lower of cost, determined on a first-in, first-out basis, or market.
Net inventories consist of the following:
|
December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
Finished goods |
$ | 32,550 | $ | 24,200 | ||||
|
Work in process |
41,358 | 27,640 | ||||||
|
Raw materials |
30,830 | 28,558 | ||||||
|
Supplies |
5,182 | 5,232 | ||||||
|
Total |
$ | 109,920 | $ | 85,630 | ||||
The components of inventory stated above are net of reserves of $12,337 and $12,878 as of December 31, 2015 and 2014, respectively.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(5) Property, Plant and Equipment
Property, plant and equipment consist of the following:
|
December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
Land |
$ | 4,122 | $ | 4,117 | ||||
|
Buildings and improvements |
106,983 | 103,083 | ||||||
|
Machinery and equipment |
358,540 | 365,623 | ||||||
|
Furniture and fixtures |
1,734 | 1,821 | ||||||
|
Construction in progress |
39,813 | 20,085 | ||||||
|
Total |
511,192 | 494,729 | ||||||
|
Accumulated depreciation |
(324,705 | ) | (331,162 | ) | ||||
|
Net |
$ | 186,487 | $ | 163,567 | ||||
Depreciation expense was $21,196, $23,296 and $22,218 for the years ended December 31, 2015, 2014 and 2013, respectively. Total capital expenditures in 2015 and 2014 were $57,400 and $29,898, respectively.
For the year ended December 31, 2014, the Company recorded a gain on the sale of land of $1,234. For the year ended December 31, 2013, the Company recorded a gain on the sale of an office building of $4,680. The carrying values of the building and land were not material.
(6) Goodwill and Intangible Assets
The changes in the carrying amount of goodwill for the years ended December 31, 2015 and 2014 are as follows:
|
Balance as of December 31, 2013 |
$ | 38,670 | ||
|
Acquisition of business |
9,715 | |||
|
Translation effect |
(4,473 | ) | ||
|
Balance as of December 31, 2014 |
$ | 43,912 | ||
|
Impairment charge (see Note 7) |
(8,542 | ) | ||
|
Translation effect |
(3,307 | ) | ||
|
Balance as of December 31, 2015 |
$ | 32,063 |
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(6) Goodwill and Intangible Assets (continued)
Acquired intangible assets, which are amortized, consist of the following:
|
As of December 31, 2015 |
|||||||||||||||
|
Amortization Period (years) |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||||
|
Internal-use software |
3 |
- |
7 | $ | 4,147 | $ | (204 | ) | $ | 3,943 | |||||
|
Technology-based intangibles |
20 |
3,310 | (952 | ) | 2,358 | ||||||||||
|
Customer-related intangibles |
10 |
- |
15 | 642 | (252 | ) | 390 | ||||||||
| $ | 8,099 | $ | (1,408 | ) | $ | 6,691 | |||||||||
|
As of December 31, 2014 |
|||||||||||||||
|
Amortization Period (years) |
Gross Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||||
|
Technology-based intangibles |
10 |
- |
20 | $ | 8,228 | $ | (1,141 | ) | $ | 7,087 | |||||
|
Internal-use software |
7 |
1,332 | - | 1,332 | |||||||||||
|
Customer-related intangibles |
10 |
- |
15 | 716 | (233 | ) | 483 | ||||||||
| $ | 10,276 | $ | (1,374 | ) | $ | 8,902 | |||||||||
The change in the gross carrying amount in 2015 is mainly due to the implementation of a new enterprise resource planning (“ERP”) system, an impairment charge related to Zenara of $3,625 classified as technology-based intangibles, and the impact of foreign currency translation.
Beginning in 2014, the Company began implementing a new ERP system, as such, $2,639 and $1,332 has been capitalized and classified as internal-use software during the years ended December 31, 2015 and 2014, respectively.
Amortization expense amounted to $865, $530, and $255 for the years ended December 31, 2015, 2014 and 2013, respectively.
Amortization expense related to current intangible assets is expected to be approximately $806 for 2016, $845 for 2017 and 2018, $795 for 2019, and $783 for 2020.
(7) Restructuring Charges
In late October, the Board of Directors of the Company recommended that management evaluate strategic alternatives for Zenara Pharma due to a change in focus on higher growth initiatives as well as to reduce attention required by senior management to operate Zenara.
The Company determined that the sale of Zenara was the best option for its shareholders. As such, Cambrex management, with Board authority, committed to a plan to sell Zenara. The immaterial assets and liabilities of Zenara are included in prepaid expenses and other current assets and accrued expenses and other current liabilities on the Company’s balance sheet in the current period.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(7) Restructuring Charges (continued)
A long-lived asset classified as held for sale must be measured at the lower of its carrying amount or fair value less cost to sell. Prior to this measurement the Company assessed Zenara’s assets and liabilities as well as performed a goodwill and long-lived asset impairment assessment. These assessments were based on level 3 inputs and resulted in writing off all of Zenara’s goodwill of $8,542 and an amortizable intangible asset of $3,625 which are included in restructuring expenses on the income statement.
The Company then compared the carrying amounts of the assets held for sale to their fair values. Accordingly, the Company recorded a charge of $1,269 for the difference between the net carrying value of these assets and the estimated fair value less cost to sell. Fair value less cost to sell was determined using the most current sales information available.
All the charges mentioned above, as well as a portion of certain retention bonuses, are included in restructuring expenses on the Company’s consolidated income statement for the year ended December 31, 2015.
The Company expects a sale to be completed during 2016.
(8) Partially-Owned Affiliates
In May 2014, the Company negotiated an accelerated purchase of Zenara for the remaining 49% for $2,680. As a result, the Company recorded an expense of $4,400 during 2014 representing the release of foreign currency translation adjustments previously recorded in other comprehensive income that were required to be recorded to the income statement as a result of the removal of the investment in partially-owned affiliate due to the full consolidation of Zenara as of the acquisition date.
(9) Accrued Expenses and Other Current Liabilities
The components of accrued expenses and other current liabilities are as follows:
|
December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
Salaries and employee benefits payable |
$ | 26,850 | $ | 26,893 | ||||
|
Taxes payable and related reserves |
8,469 | 5,231 | ||||||
|
Other |
8,928 | 8,701 | ||||||
|
Total |
$ | 44,247 | $ | 40,825 | ||||
(10) Income Taxes
Income before income taxes consists of the following:
|
December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Domestic |
$ | 71,323 | $ | 37,211 | $ | 23,068 | ||||||
|
International |
18,242 | 8,295 | 21,939 | |||||||||
|
Total |
$ | 89,565 | $ | 45,506 | $ | 45,007 | ||||||
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(10) Income Taxes (continued)
The provision for income taxes consist of the following provisions/(benefits):
|
December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Current: |
||||||||||||
|
Federal |
$ | 2,577 | $ | 2,572 | $ | 630 | ||||||
|
State |
- | - | 15 | |||||||||
|
International |
10,076 | 512 | 5,980 | |||||||||
| 12,653 | 3,084 | 6,625 | ||||||||||
|
Deferred: |
||||||||||||
|
Federal |
$ | 22,005 | $ | (14,965 | ) | $ | 7,014 | |||||
|
International |
(2,269 | ) | (746 | ) | 1,093 | |||||||
| 19,736 | (15,711 | ) | 8,107 | |||||||||
|
Total |
$ | 32,389 | $ | (12,627 | ) | $ | 14,732 | |||||
The provision/(benefit) for income taxes differs from the statutory federal income tax rate of 35% for 2015, 2014 and 2013 as follows:
|
December 31, |
||||||||||||
|
2015 |
2014 |
2013 |
||||||||||
|
Income tax provision at U.S federal statutory rate |
$ | 31,347 | $ | 15,927 | $ | 15,752 | ||||||
|
Effect of foreign income taxed at rates other than the U.S. federal statutory rate |
989 | 751 | 242 | |||||||||
|
Foreign income inclusions |
5,017 | 2,742 | - | |||||||||
|
Tax credits |
(4,685 | ) | (2,692 | ) | (250 | ) | ||||||
|
Changes in tax laws |
- | - | (1,155 | ) | ||||||||
|
Tax audit settlements |
- | (3,948 | ) | - | ||||||||
|
Net change in valuation allowance |
303 | (26,543 | ) | (97 | ) | |||||||
|
Permanent items and other |
(582 | ) | 1,136 | 240 | ||||||||
|
Total |
$ | 32,389 | $ | (12,627 | ) | $ | 14,732 | |||||
Foreign income inclusions represent distributions from foreign subsidiaries which give rise to newly recognized foreign tax credits. Tax audit settlements in 2014 included the final settlement of the European tax dispute concerning 2003 transactions. Net change in the valuation allowance in 2014 included the benefit of $26,902 for the remaining release of the domestic valuation allowance attributable to foreign tax credits, offset by $142 for state deferred taxes and $217 for foreign deferred taxes subject to valuation allowances.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(10) Income Taxes (continued)
The components of deferred tax assets and liabilities as of December 31, 2015 and 2014 relate to temporary differences and carryforwards as follows:
|
December 31, |
||||||||
|
2015 |
2014 |
|||||||
|
Deferred tax assets: |
||||||||
|
Inventory |
$ | 3,032 | $ | 3,313 | ||||
|
Foreign tax credit carryforwards |
3,990 | 19,925 | ||||||
|
Environmental |
2,795 | 2,993 | ||||||
|
Net operating loss carryforwards (foreign) |
1,191 | 3,381 | ||||||
|
Employee benefits |
14,978 | 18,879 | ||||||
|
Research & experimentation tax credit carryforwards |
- | 480 | ||||||
|
Alternative minimum tax credit carryforwards |
1,306 | 2,773 | ||||||
|
Property, plant and equipment |
4,188 | 3,898 | ||||||
|
Other |
6,251 | 5,605 | ||||||
|
Total gross deferred tax assets |
37,731 | 61,247 | ||||||
|
Valuation allowance |
(3,141 | ) | (5,053 | ) | ||||
|
Total deferred tax assets |
$ | 34,590 | $ | 56,194 | ||||
|
Deferred tax liabilities: |
||||||||
|
Property, plant and equipment |
(11,472 | ) | (9,649 | ) | ||||
|
Intangibles and other |
(8,192 | ) | (10,487 | ) | ||||
|
Unremitted foreign earnings |
(208 | ) | (726 | ) | ||||
|
Foreign tax allocation reserve |
(2,071 | ) | (1,856 | ) | ||||
|
Other |
(1,123 | ) | (1,978 | ) | ||||
|
Total deferred tax liabilities |
$ | (23,066 | ) | $ | (24,696 | ) | ||
|
Net deferred tax assets |
$ | 11,524 | $ | 31,498 | ||||
|
Classified as follows in the consolidated balance sheet: |
||||||||
|
Non-current deferred tax asset |
19,259 | 41,747 | ||||||
|
Non-current deferred tax liability |
(7,735 | ) | (10,249 | ) | ||||
| $ | 11,524 | $ | 31,498 | |||||
The Company elected to retrospectively apply recent accounting guidance requiring that all deferred tax balances be classified as noncurrent. Accordingly, all current deferred tax assets and current deferred tax liabilities have been classified as noncurrent for 2015 and the 2014 balance sheet has been reclassified to reflect retrospective treatment of this accounting standards update.
During 2014, the Company received updated customer projections that impact current and future years’ U.S. taxable income in amounts and type that supported full utilization of existing federal foreign tax credit carryforwards. As a result, the Company released $26,902 of valuation allowance against these foreign tax credits. The Company expects to maintain a domestic valuation allowance against state tax credits and deferred tax assets due to restrictive rules regarding realization. The Company expects to maintain a valuation allowance against certain foreign deferred tax assets, primarily NOL carryforwards, until such time as the Company attains an appropriate level of future profitability in the appropriate jurisdictions and is able to conclude that it is more likely than not that its foreign deferred tax assets are realizable.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(10) Income Taxes (continued)
The domestic valuation allowance for the years ended December 31, 2015, 2014 and 2013 decreased $381, $26,760 and $3, respectively. The 2015 decrease in the domestic valuation allowance is due to domestic state items. The 2014 decrease in the domestic valuation allowance was allocated as follows: the valuation allowance decreased $26,902 for the release of valuation allowance due to domestic profitability and increased $142 due to domestic state items. The 2013 decrease in the domestic valuation allowance is due to domestic state items.
The foreign valuation allowance for the years ended December 31, 2015, 2014 and 2013 decreased $1,531, increased $1,923, and decreased $48, respectively. The 2015 decrease in the foreign valuation allowance was allocated as follows: the valuation allowance increased $684 for foreign income and decreased $2,215 for deferred tax amounts, the reclass of Zenara valuation allowance for assets held for sale into other current liabilities, and currency translation adjustments included in other comprehensive income (“OCI”). The 2014 increase in the foreign valuation allowance was allocated as follows: the valuation allowance increased $217 for foreign income and increased $1,706 for deferred tax amounts and currency translation adjustments included in OCI. The 2013 decrease in the foreign valuation allowance was allocated as follows: the valuation allowance decreased $94 for foreign income and increased $46 for deferred tax amounts and currency translation adjustments included in OCI.
Under the tax laws of the various jurisdictions in which the Company operates, NOLs may be carried forward or back, subject to statutory limitations, to reduce taxable income in future or prior years. Foreign NOLs are approximately $12,716, of which $1,322 are attributable to NOLs acquired during 2010. NOLs in most foreign jurisdictions will carry forward indefinitely.
As of December 31, 2015, $3,990 of domestic federal foreign tax credits and $1,306 of alternative minimum tax credits are available as credits against future U.S. income taxes on worldwide income, subject to certain limitations. Under U.S. tax laws, domestic federal foreign tax credits will expire in 2016 and 2018, and the alternative minimum tax credit carryforwards have no expiration date.
In 2015 and 2014, the Company repatriated $9,850 and $5,442, respectively, of cash from its foreign subsidiaries in order to reduce its credit and currency exposure for cash held in foreign currencies or in non-U.S. banks and utilized the excess cash for debt reduction. Due in part to a continuing desire to limit credit and currency exposure related to cash held in foreign currencies or in non-U.S. banks, the Company determined that it is likely that a portion of the undistributed earnings of its foreign subsidiaries will be repatriated to the U.S. in the future. Accordingly, the Company has provided a deferred tax liability of $208 on certain undistributed foreign earnings as of December 31, 2015. Subject to limitations, U.S. income tax on such foreign earnings, when actually repatriated, may be reduced or eliminated by unrecognized foreign tax credits that may be generated in connection with the repatriation or by existing foreign tax credit carryforwards or other tax attributes. The Company monitors available evidence and its plans for foreign earnings and expects to continue to provide deferred taxes based on the tax liability that would be due upon repatriation of amounts not considered permanently reinvested.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(10) Income Taxes (continued)
The following table summarizes the activity related to the Company’s unrecognized tax benefits as of December 31, 2015, 2014 and 2013:
|
2015 |
2014 |
2013 |
||||||||||
|
Balance at January 1 |
$ | 1,643 | $ | 3,922 | $ | 3,967 | ||||||
|
Gross increases related to current period tax positions |
281 | 275 | 257 | |||||||||
|
Gross decreases related to prior period tax positions |
(52 | ) | (1,149 | ) | (427 | ) | ||||||
|
Expirations of statute of limitations for the assessment of taxes |
(241 | ) | (106 | ) | (37 | ) | ||||||
|
Settlements |
- | (1,113 | ) | - | ||||||||
|
Foreign currency translation |
(139 | ) | (186 | ) | 162 | |||||||
|
Balance at December 31 |
$ | 1,492 | $ | 1,643 | $ | 3,922 | ||||||
Of the total balance of unrecognized tax benefits at December 31, 2015, $1,492, if recognized, would affect the effective tax rate.
Gross interest and penalties at December 31, 2015, 2014 and 2013 of $475, $489 and $5,005, respectively, related to the above unrecognized tax benefits are not reflected in the table above. In 2015, 2014 and 2013, the Company accrued $58, $337 and $219, respectively, of interest and penalties in the income statement. Consistent with prior periods, the Company recognizes interest and penalties within its income tax provision.
Tax years 2012 and forward in the U.S. are open to examination by the IRS. The Company is also subject to examinations in its material non-U.S. jurisdictions for 2009 and later years.
The Company is also subject to audits in various states for various years in which it has filed income tax returns. Previous state audits have resulted in immaterial adjustments. In the majority of states where the Company files, the Company is subject to examination for tax years 2011 and forward.
During the fourth quarter of 2014, the Company entered into a final settlement with a tax authority, without any admission of fault or breach of laws, in order to avoid further litigation concerning intercompany transactions from 2003. The settlement required the Company to pay $1,487 in tax and interest during the fourth quarter of 2014 in full satisfaction of all liabilities for this matter, and in response the tax authority withdrew all pending litigation and renounced any outstanding claims. The settlement did not impose any penalties on the Company. Therefore, in the fourth quarter of 2014 the Company decreased its remaining reserve for unrecognized tax benefits for this matter by $4,137.
In the next twelve months, the Company may increase its reserve for unrecognized tax benefits for intercompany transactions and acquired tax attributes by approximately $500. This could affect the effective tax rate.
(11) Short-term and Long-term Debt
In November 2011, the Company entered into a $250,000 five-year Syndicated Senior Revolving Credit Facility (“Credit Facility”) which expires in November 2016. The Company pays interest on this Credit Facility at LIBOR plus 1.50% - 2.10% based upon certain financial measurements. The Credit Facility also includes financial covenants regarding interest coverage and leverage ratios. The Company was in compliance with all financial covenants at December 31, 2015. As of December 31, 2015, there was $30,000 outstanding on the Credit Facility which was reclassified to short-term debt in 2015. The 2015 and 2014 weighted average interest rate for long-term bank debt was 2.3% and 2.4%, respectively.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(12) Derivatives and Hedging Activities
The Company operates internationally and is exposed to fluctuations in foreign exchange rates and interest rates in the normal course of business. The Company, from time to time, uses derivatives to reduce exposure to market risks resulting from fluctuations in interest rates and foreign exchange rates.
All financial instruments involve market and credit risks. The Company is exposed to credit losses in the event of non-performance by the counterparties to the contracts. While there can be no assurance, the Company does not anticipate non-performance by these counterparties.
Foreign Currency Forward Contracts
The Company periodically enters into foreign currency forward contracts to protect against currency fluctuations of forecasted cash flows and existing balance sheet exposures at its foreign operations, as deemed appropriate. The Company may or may not elect to designate certain forward contracts for hedge accounting treatment.
For derivatives that are not designated for hedge accounting treatment, changes in the fair value are immediately recognized in earnings. This treatment has the potential to increase volatility of the Company’s earnings.
None of the foreign currency forward contracts entered into during 2015 or 2014 were designated for hedge accounting treatment. The notional amounts of the Company’s outstanding foreign exchange forward contracts were $9,322 and $3,632 at December 31, 2015 and 2014, respectively. There were no foreign currency forward contracts outstanding at December 31, 2013. The Company does not hold or purchase any foreign currency forward contracts for trading or speculative purposes and no contractual term is greater than twelve months.
The fair value of the Company’s foreign exchange forward contracts outstanding was immaterial at December 31, 2015.
Interest Rate Swap
The Company entered into an interest rate swap in March 2012 to reduce the impact of changes in interest rates on its floating rate debt. This swap expired in September 2015. The swap was a contract to exchange floating rate for fixed interest payments periodically over the life of the agreement without the exchange of the underlying notional debt amount.
The swap contract was designated as a cash flow hedge and, accordingly, changes in the fair value of this derivative were not recorded in earnings but were recorded each period in AOCI and reclassified into earnings as interest expense in the same period during which the hedged transaction affects earnings. The ineffective portion of the hedge was recognized in earnings and was immaterial to the Company's financial results.
The interest rate swap had a notional value of $60,000, at a fixed rate of 0.92%. The fair value of this swap was based on quoted market prices and was in a loss position of $304 and $616 at December 31, 2014 and 2013, respectively. This loss is reflected in the Company’s balance sheet under the caption “Accrued expenses and other current liabilities.”
Refer to Note 13 to the Company’s consolidated financial statements for the summary table containing the fair value of the Company’s financial instruments.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(13) Fair Value Measurements
U.S. GAAP establishes a valuation hierarchy for disclosure of the inputs to the valuations used to measure fair value. This hierarchy prioritizes the inputs into three broad levels as follows: Level 1 inputs are quoted prices (unadjusted) in active markets for identical assets or liabilities; Level 2 inputs are quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets in markets that are not active, inputs other than quoted prices that are observable for the asset or liability, including interest rates, yield curves and credit risks, or inputs that are derived principally from, or corroborated by, observable market data through correlation; Level 3 inputs are unobservable inputs based on the Company’s assumptions used to measure assets and liabilities at fair value. A financial asset or liability’s classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement.
The following table provides the assets and liabilities carried at fair value, measured on a recurring basis, as of December 31, 2014. The amounts were immaterial at December 31, 2015.
|
Fair Value Measurements at December 31, 2014 using: |
||||||||||||||||
|
Description |
Total |
Level 1 |
Level 2 |
Level 3 |
||||||||||||
|
Foreign currency forwards, liabilities |
$ | (102 | ) | $ | - | $ | (102 | ) | $ | - | ||||||
|
Interest rate swap, liabilities |
(304 | ) | - | (304 | ) | - | ||||||||||
|
Total |
$ | (406 | ) | $ | - | $ | (406 | ) | $ | - | ||||||
The fair value of the interest rate swap was estimated based on the present value of the difference between expected cash flows calculated at the contracted interest rate and the expected cash flows at current market interest rates using observable benchmarks for the LIBOR forward rates at the end of the period. The Company’s credit risk and its counterparties’ credit risks are also evaluated to estimate fair value.
The Company’s foreign currency forward contracts are measured at fair value using observable market inputs such as forward rates, the Company’s credit risk and its counterparties’ credit risks. Based on the Company’s continued ability to enter into forward contracts, the Company considers the markets for its fair value instruments to be active.
Based on these inputs, the Company’s interest rate swap and foreign currency forward contracts are classified within Level 2 of the valuation hierarchy.
The Company’s financial instruments also include cash and cash equivalents, accounts receivables and accounts payables. The carrying amount of these instruments approximates fair value because of their short-term nature. The carrying amount of the Company’s long-term debt approximates fair value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Refer to Note 12 to the Company’s consolidated financial statements for further disclosures on the Company’s financial instruments.
(14) Stockholders' Equity
The Company has two classes of common shares, Common Stock and Nonvoting Common Stock. Authorized shares of Common Stock were 100,000,000 at December 31, 2015 and 2014. Authorized shares of Nonvoting Common Stock were 730,746 at December 31, 2015 and 2014. Nonvoting Common Stock with a par value of $0.10 has equal rights with Common Stock, with the exception of voting power. Nonvoting Common Stock is convertible, share for share, into Common Stock, subject to any legal requirements applicable to holders restricting the extent to which they may own voting stock. As of December 31, 2015 and 2014, no shares of Nonvoting Common Stock were outstanding. The Company has authorized 5,000,000 shares of Series Preferred Stock, par value $0.10, issuable in series and with rights, powers and preferences as may be fixed by the Board of Directors. At December 31, 2015 and 2014, there was no preferred stock outstanding.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(14) Stockholders' Equity (continued)
The Company held treasury shares of 1,729,727 and 1,738,624 at December 31, 2015 and 2014, respectively, which are primarily used for issuance to employee compensation plans.
At December 31, 2015, there were 1,763,030 authorized shares of Common Stock reserved for issuance through equity compensation plans.
(15) Accumulated Other Comprehensive Loss
The following tables provide the changes in AOCI by component, net of tax, for the years ended December 31, 2015 and 2014:
|
Foreign Currency Translation Adjustments |
Interest Rate Swap |
Pension Plans |
Total |
|||||||||||||
|
Balance as of December 31, 2014 |
$ | (11,410 | ) | $ | (193 | ) | $ | (33,378 | ) | $ | (44,981 | ) | ||||
|
Other comprehensive (loss)/income before reclassifications |
(16,424 | ) | (23 | ) | 2,893 | (13,554 | ) | |||||||||
|
Amounts reclassified from accumulated other comprehensive loss |
1,954 | 216 | 916 | 3,086 | ||||||||||||
|
Net current-period other comprehensive (loss)/income |
(14,470 | ) | 193 | 3,809 | (10,468 | ) | ||||||||||
|
Balance as of December 31, 2015 |
$ | (25,880 | ) | $ | - | $ | (29,569 | ) | $ | (55,449 | ) | |||||
|
Foreign Currency Translation Adjustments |
Interest Rate Swap |
Pension Plans |
Total |
|||||||||||||
|
Balance as of December 31, 2013 |
$ | 9,990 | $ | (396 | ) | $ | (28,556 | ) | $ | (18,962 | ) | |||||
|
Other comprehensive loss before reclassifications |
(25,800 | ) | (99 | ) | (12,566 | ) | (38,465 | ) | ||||||||
|
Amounts reclassified from accumulated other comprehensive loss |
4,400 | 302 | 7,744 | 12,446 | ||||||||||||
|
Net current-period other comprehensive (loss)/income |
(21,400 | ) | 203 | (4,822 | ) | (26,019 | ) | |||||||||
|
Balance as of December 31, 2014 |
$ | (11,410 | ) | $ | (193 | ) | $ | (33,378 | ) | $ | (44,981 | ) | ||||
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(15) Accumulated Other Comprehensive Loss (continued)
The following tables provide the reclassifications out of AOCI by component for the years ended December 31, 2015 and 2014:
|
Details about AOCI Components |
Amount Reclassified from AOCI for the year ended December 31, 2015 |
Amount Reclassified from AOCI for the year ended December 31, 2014 |
||||||
|
Losses on cash flow hedge: |
||||||||
|
Interest rate swap |
$ | (333 | ) | $ | (465 | ) | ||
|
Tax benefit |
117 | 163 | ||||||
|
Net of tax |
$ | (216 | ) | $ | (302 | ) | ||
|
Amortization of defined benefit pension items: |
||||||||
|
Actuarial losses |
$ | (1,295 | ) | $ | (802 | ) | ||
|
Prior service costs |
(52 | ) | (50 | ) | ||||
|
Loss on voluntary settlement |
- | (7,170 | ) | |||||
|
Total before tax |
(1,347 | ) | (8,022 | ) | ||||
|
Tax benefit |
431 | 278 | ||||||
|
Net of tax |
$ | (916 | ) | $ | (7,744 | ) | ||
|
Foreign currency translation adjustment: |
||||||||
|
Release of currency translation adjustment |
$ | (1,954 | ) | $ | (4,400 | ) | ||
|
Net of tax |
$ | (1,954 | ) | $ | (4,400 | ) | ||
|
Total reclassification for the period |
$ | (3,086 | ) | $ | (12,446 | ) | ||
The interest rate swap is reflected in the Company’s income statement as interest expense. The Company recognizes net periodic pension cost, which includes amortization of actuarial losses and gains, and prior service costs in both selling, general and administrative expenses and cost of goods sold in its income statement depending on the functional area of the underlying employees included in the plan. The release of currency translation adjustments generated from Zenara’s balance sheet are reflected in the Company’s income statement as restructuring expenses in 2015 and equity in losses of partially-owned affiliates in 2014. The loss on voluntary pension settlement is reflected in the Company’s income statement as loss on voluntary pension settlement.
(16) Stock Based Compensation
The Company recognizes compensation cost for stock options awarded to employees based on their grant-date fair value. The value of each stock option is estimated on the date of grant using the Black-Scholes option-pricing model. The weighted-average fair value per share for the stock options granted to employees for the years ended December 31, 2015, 2014 and 2013 were $15.29, $7.15 and $7.97, respectively.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(16) Stock Based Compensation (continued)
The following assumptions were used in determining the fair value of stock options for grants issued in 2015, 2014 and 2013:
|
2015 |
2014 |
2013 | ||||||||||
|
Expected volatility |
41.84% | - | 58.25% | 35.14% | - | 46.32% | 44.17% | - | 71.58% | |||
| Expected term (years) | 1.25 | - | 6.83 | 1.75 | - | 4.75 | 1.25 | - | 4.75 | |||
|
Risk-free interest rate |
0.24% | - | 1.93% | 0.43% | - | 1.50% | 0.12% | - | 1.37% | |||
The Company does not have any publicly traded stock options; therefore, expected volatilities are based on historical volatility of the Company’s stock. The risk-free interest rate is based on the yield of a zero-coupon U.S. Treasury bond whose maturity period approximates the option’s expected term. The expected life assumption represents the weighted-average period of time that newly granted stock-based awards are expected to remain outstanding. The expected life is estimated by analyzing three components of historical grants with the same vesting schedules: (i) observed post-vesting forfeiture, (ii) observed exercise behavior, and (iii) expected exercise behavior. The expected time to early exercise is calculated by assuming that the options outstanding as of the valuation date will be exercised at the midpoint between the final vest date and the expiration date. If a grant is already fully vested, it is assumed the outstanding options exercise at the midpoint between the valuation date and the expiration date. The three components are then option-weighted to estimate expected life. The Company stratifies its employees as Board of Directors, Named Executives and all other employees, each with their own exercise behavior and thus, expected life.
For 2015, 2014, and 2013, the Company recorded $2,975, $2,491 and $1,994, respectively, in selling, general and administrative expenses for stock options. As of December 31, 2015, the total compensation cost related to unvested stock option awards granted to employees but not yet recognized was $8,756. The cost will be amortized on a straight-line basis over the remaining weighted-average vesting period of 2.7 years.
The following table is a summary of the Company’s stock option activity issued to employees and related information:
|
Weighted Average |
||||||||||||
|
Number of Shares |
Exercise Price |
Options Exercisable |
||||||||||
|
Outstanding at December 31, 2014 |
2,070,672 | $ | 12.08 | 971,797 | ||||||||
|
Granted |
333,601 | 39.76 | ||||||||||
|
Exercised |
(691,985 | ) | 8.40 | |||||||||
|
Forfeited or expired |
(80,375 | ) | 14.48 | |||||||||
|
Outstanding at December 31, 2015 |
1,631,913 | 19.17 | ||||||||||
|
Exercisable at December 31, 2015 |
709,255 | $ | 12.30 | |||||||||
The aggregate intrinsic value for all stock options exercised for the years ended December 31, 2015, 2014 and 2013 was $24,432, $8,910 and $5,017, respectively. The aggregate intrinsic values for all stock options outstanding and exercisable as of December 31, 2015 were $45,558 and $24,672, respectively.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(16) Stock Based Compensation (continued)
A summary of the Company’s nonvested stock options and restricted stock activity is presented below:
|
Nonvested Stock Options |
Nonvested Restricted Stock |
|||||||||||||||
|
Number of Shares |
Weighted- Average Grant- Date Fair Value |
Number of Shares |
Weighted- Average Grant- Date Fair Value |
|||||||||||||
|
Nonvested at December 31, 2014 |
1,098,875 | $ | 7.14 | - | $ | - | ||||||||||
|
Granted |
333,601 | 15.29 | 9,075 | 39.48 | ||||||||||||
|
Vested during period |
(429,443 | ) | 6.62 | (8,897 | ) | 39.34 | ||||||||||
|
Forfeited |
(80,375 | ) | 6.90 | - | - | |||||||||||
|
Nonvested at December 31, 2015 |
922,658 | $ | 10.35 | 178 | $ | 46.91 | ||||||||||
Members of the Cambrex Board of Directors currently participate in an incentive plan which rewards service with restricted stock units. Awards are made annually and vest over six months. On the six month anniversary of the grant, restrictions on sale or transfer are removed and shares are issued to the Directors. These awards are classified as equity awards.
For 2015, 2014, and 2013, the Company recorded $353, $395, and $427, respectively, in selling, general and administrative expenses for restricted stock units. As of December 31, 2015, total compensation cost related to unvested restricted stock not yet recognized was $6. The cost will be amortized on a straight-line basis over the remaining weighted-average vesting period of 0.3 years.
The Company granted equity-settled performance shares (“PSs”) to certain executives. PS awards provide the recipient the right to receive a certain number of shares of the Company’s common stock in the future, which depends on the Company’s level of achievement of revenue and EBITDA growth as compared to the net revenue and EBITDA growth of the members of a specified peer group of companies over a three year period. The peer group consists of publicly-traded life sciences companies competing in the same industry as the Company. For 2015, 2014 and 2013, the Company recorded $2,271, $2,116 and $404, respectively, in selling, general and administrative expenses related to these PS awards.
The Company granted cash-settled performance share units (“PSUs”) to certain executives. PSU awards provide the recipient the right to receive the cash value of a certain number of shares of the Company’s common stock in the future, which depends on the Company’s level of achievement of revenue and EBITDA growth as compared to the net revenue and EBITDA growth of the members of a specified peer group of companies over a three year period. The peer group consists of publicly-traded life sciences companies competing in the same industry as the Company. As of December 31, 2015, there were no PSU awards outstanding. For 2014 and 2013, the Company recorded $445 and $2,620, respectively, in selling, general and administrative expenses for PSU awards. The decrease between 2014 and 2013 is primarily the result of the Company’s performance compared to the peer group partially offset by the Company’s higher share price.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(17) Retirement Plans
Domestic Pension Plan
The Company maintains a defined-benefit pension plan (“Domestic Pension Plan”) for certain salaried and certain hourly employees. The Company also has a Supplemental Executive Retirement Plan (“SERP”) for key executives. This plan is non-qualified and unfunded. Benefits accruing under both plans were frozen in 2007. In July 2008, the Board of Directors of the Company amended the SERP to allow for lump sum payments effective January 1, 2009. The lump sum value as of January 1, 2009 will be paid in 10 equal actuarial equivalent installments through 2018. It is the Company’s policy to contribute to the domestic pension plan to ensure adequate funds are available in the plan to make benefit payments to plan participants and beneficiaries when required.
In 2014, the Company announced a program to offer a one-time option to elect to receive a voluntary lump-sum pension payout to certain former employees with deferred vested balances in the Company’s U.S. pension plan. As part of this voluntary lump-sum program, the Company settled $17,381 of pension obligations for the U.S. plan with an equal amount paid from plan assets. As a result, the Company recorded settlement losses of $7,170 reflecting the accelerated recognition of unamortized losses in the U.S. pension plan proportionate to the obligation that was settled. The loss on voluntary pension settlement is reflected as a separate line in the consolidated income statement with a corresponding balance sheet reduction in accumulated other comprehensive loss.
International Pension Plans
A foreign subsidiary of the Company maintains a pension plan (“International Pension Plan”) for its employees that conforms to the common practice in that country. Based on local laws and customs, this plan is unfunded.
Savings Plan
Cambrex makes available to all domestic employees a savings plan. Employee contributions are matched in part by Cambrex. The cost of this plan amounted to $1,081, $941 and $731 in 2015, 2014 and 2013, respectively.
Other
The Company had a non-qualified Deferred Compensation Plan for Key Executives (“The Plan”). Under this Plan, officers and key employees may elect to defer all or any portion of their pre-tax earnings or elect to defer receipt of the Company’s stock which would otherwise have been issued upon the exercise of the Company’s options. As of December 31, 2015, the plan no longer had any deferred compensation. As such, as of December 31, 2015, the Company has no liabilities or assets recorded, and no Cambrex shares held in trust. Included within other liabilities at December 31, 2014 was $932, representing the Company’s obligation under the plan. The Company invested in certain mutual funds and as such, included within other assets at December 31, 2014 was $932, representing the fair value of these funds. The fair values of these mutual funds were based on quoted market prices in active markets (Level 1). The number of Cambrex shares held in trust under this plan as of December 31, 2014 was 49,121 and are included as a reduction of equity. The value of the shares held in trust and the corresponding liability of $1,062 at December 31, 2014 was also recorded in equity. The Plan was not funded by the Company, but the Company had established a Deferred Compensation Trust Fund which held the shares issued.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(17) Retirement Plans (continued)
The benefit obligations as of December 31, 2015 and 2014 are as follows:
|
Pension Plans |
||||||||||||||||||||||||
|
Domestic |
SERP |
International |
||||||||||||||||||||||
|
2015 |
2014 |
2015 |
2014 |
2015 |
2014 |
|||||||||||||||||||
|
Change in benefit obligation |
||||||||||||||||||||||||
|
Benefit obligation, beginning of year |
$ | 64,825 | $ | 70,709 | $ | 2,389 | $ | 3,086 | $ | 26,700 | $ | 25,627 | ||||||||||||
|
Service cost |
- | - | - | - | 778 | 674 | ||||||||||||||||||
|
Interest cost |
2,430 | 3,310 | 24 | 33 | 589 | 884 | ||||||||||||||||||
|
Actuarial (gain)/loss |
(5,263 | ) | 11,813 | (4 | ) | 2 | (2,397 | ) | 5,319 | |||||||||||||||
|
Benefits paid |
(3,436 | ) | (3,160 | ) | (609 | ) | (732 | ) | (697 | ) | (763 | ) | ||||||||||||
|
Currency translation effect |
- | - | - | - | (2,225 | ) | (5,041 | ) | ||||||||||||||||
|
Settlements |
- | (17,847 | ) | - | - | - | - | |||||||||||||||||
|
Benefit obligation, end of year |
$ | 58,556 | $ | 64,825 | $ | 1,800 | $ | 2,389 | $ | 22,748 | $ | 26,700 | ||||||||||||
The plan assets and funded status of the Domestic Pension Plan as of December 31, 2015 and 2014 are as follows:
|
2015 |
2014 |
|||||||
|
Change in plan assets |
||||||||
|
Fair value of plan assets, beginning of period |
$ | 41,665 | $ | 57,743 | ||||
|
Actual return on plan assets |
(806 | ) | 2,605 | |||||
|
Contributions |
1,712 | 2,324 | ||||||
|
Benefits paid |
(3,436 | ) | (3,160 | ) | ||||
|
Settlements |
- | (17,847 | ) | |||||
|
Fair value of plan assets, end of period |
$ | 39,135 | $ | 41,665 | ||||
|
Unfunded status |
(19,421 | ) | (23,160 | ) | ||||
|
Accrued benefit cost, end of period |
$ | (19,421 | ) | $ | (23,160 | ) | ||
The unfunded status of the SERP was $1,800 and $2,389 as of December 31, 2015 and 2014, respectively. The unfunded status of the International Pension Plan was $22,748 and $26,700 as of December 31, 2015 and 2014, respectively.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(17) Retirement Plans (continued)
The amounts recognized in AOCI as of December 31, 2015 and 2014 consist of the following:
|
Pension Plans |
||||||||||||||||||||||||
|
Domestic |
SERP |
International |
||||||||||||||||||||||
|
2015 |
2014 |
2015 |
2014 |
2015 |
2014 |
|||||||||||||||||||
|
Actuarial loss |
$ | 23,644 | $ | 26,042 | $ | 544 | $ | 702 | $ | 6,887 | $ | 9,597 | ||||||||||||
|
Prior service cost/(benefit) |
- | - | 115 | 172 | (12 | ) | (17 | ) | ||||||||||||||||
|
Total |
$ | 23,644 | $ | 26,042 | $ | 659 | $ | 874 | $ | 6,875 | $ | 9,580 | ||||||||||||
The components of net periodic benefit cost are as follows:
|
Pension Plans |
||||||||||||||||||||||||||||||||||||
|
Domestic |
SERP |
International |
||||||||||||||||||||||||||||||||||
|
2015 |
2014 |
2013 |
2015 |
2014 |
2013 |
2015 |
2014 |
2013 |
||||||||||||||||||||||||||||
|
Components of net periodic benefit cost |
||||||||||||||||||||||||||||||||||||
|
Service cost |
$ | - | $ | - | $ | - | $ | - | $ | - | $ | - | $ | 778 | $ | 674 | $ | 743 | ||||||||||||||||||
|
Interest cost |
2,430 | 3,310 | 3,057 | 24 | 33 | 41 | 589 | 884 | 658 | |||||||||||||||||||||||||||
|
Expected return on plan assets |
(2,870 | ) | (4,153 | ) | (3,826 | ) | - | - | - | - | - | - | ||||||||||||||||||||||||
|
Amortization of prior service cost/(benefit) |
- | - | - | 57 | 57 | 57 | (5 | ) | (7 | ) | (7 | ) | ||||||||||||||||||||||||
|
Recognized actuarial loss |
811 | 522 | 937 | 154 | 131 | 118 | 330 | 149 | 284 | |||||||||||||||||||||||||||
|
Settlement loss |
- | 7,170 | - | - | - | - | - | - | - | |||||||||||||||||||||||||||
|
Net periodic benefit cost |
$ | 371 | $ | 6,849 | $ | 168 | $ | 235 | $ | 221 | $ | 216 | $ | 1,692 | $ | 1,700 | $ | 1,678 | ||||||||||||||||||
The estimated amounts that will be amortized from AOCI into net periodic benefit cost in 2016 are as follows:
|
Pension Plans |
||||||||||||
|
Domestic |
SERP |
International |
||||||||||
|
Actuarial loss |
$ | 776 | $ | 182 | $ | 197 | ||||||
|
Prior service cost/(benefit) |
- | 57 | (5 | ) | ||||||||
|
Total |
$ | 776 | $ | 239 | $ | 192 | ||||||
Major assumptions used in determining the benefit obligations are presented in the following table:
|
2015 |
2014 |
|||||||
|
Discount rate: |
||||||||
|
Domestic Pension Plan |
4.20% | 3.85% | ||||||
|
SERP |
1.55% | 1.35% | ||||||
|
International Pension Plan |
3.35% | 2.40% | ||||||
|
Rate of compensation increase: |
||||||||
|
International Pension Plan |
2.55% | 2.20% | ||||||
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(17) Retirement Plans (continued)
Major assumptions used in determining the net benefit cost are presented in the following table:
|
2015 |
2014 |
2013 |
||||||||||
|
Discount rate: |
||||||||||||
|
Domestic Pension Plan |
3.85% | 4.80% | 3.90% | |||||||||
|
SERP |
1.35% | 1.40% | 1.35% | |||||||||
|
International Pension Plan |
2.40% | 3.70% | 3.40% | |||||||||
|
Expected return on plan assets: |
||||||||||||
|
Domestic Pension Plan |
7.00% | 7.25% | 7.25% | |||||||||
|
Rate of compensation increase: |
||||||||||||
|
International Pension Plan |
2.20% | 2.20% | 2.50% | |||||||||
In making its assumption for the long-term rate of return on plan assets, the Company has utilized historical rates earned on securities allocated consistently with its investments. The discount rate was selected by projecting cash flows associated with plan obligations, which were matched to a yield curve of high quality corporate bonds. The Company then selected the single rate that produced the same present value as if each cash flow were discounted by the corresponding spot rate on the yield curve.
The aggregate Accumulated Benefit Obligation (“ABO”) of $58,556 exceeds plan assets by $19,421 as of December 31, 2015 for the Domestic Pension Plan. The aggregate ABO is $21,764 for the International Pension Plan as of December 31, 2015. The International Pension Plan is unfunded.
The Company expects to contribute approximately $759 in cash to the Domestic Pension Plan in 2016. The Company does not expect to contribute cash to its International Pension Plan in 2016.
The following benefit payments are expected to be paid out of the plans:
|
Pension Plans |
|||||||||||||||
|
Domestic |
SERP |
International |
|||||||||||||
|
2016 |
$ | 3,355 | $ | 609 | $ | 698 | |||||||||
|
2017 |
$ | 3,380 | $ | 609 | $ | 713 | |||||||||
|
2018 |
$ | 3,359 | $ | 609 | $ | 766 | |||||||||
|
2019 |
$ | 3,430 | $ | - | $ | 814 | |||||||||
|
2020 |
$ | 3,497 | $ | - | $ | 829 | |||||||||
| 2021-2025 | $ | 17,743 | $ | - | $ | 4,824 | |||||||||
The investment objective for the Domestic Pension Plan’s assets is to achieve long-term growth with exposure to risk at an appropriate level. The Company invests in a diversified asset mix consisting of equities (domestic and international) and taxable fixed income securities. Assets are managed to obtain the highest total rate of return in keeping with a moderate level of risk. The target allocations for plan assets are 30% - 80% equity securities, 25% - 45% U.S. fixed income and 5% - 15% all other investments. Equity securities primarily include investments in large-cap and small-cap companies, U.S. fixed income securities including high quality corporate bonds, and U.S. government securities. Other types of investments include real asset funds, consisting primarily of investments in commodities, and Treasury Inflation-Protected Securities (“TIPS”).
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(17) Retirement Plans (continued)
The fair values of the Company’s pension plan assets by asset category are as follows:
|
Fair Value Measurements at December 31, 2015 using: |
||||||||||||||||
|
Asset Category |
Total |
(Level 1) |
(Level 2) |
(Level 3) |
||||||||||||
|
Equity securities: |
||||||||||||||||
|
U.S. companies |
$ | 13,547 | $ | - | $ | 13,547 | $ | - | ||||||||
|
International companies |
7,377 | - | 7,377 | - | ||||||||||||
|
U.S. fixed income |
13,375 | - | 11,207 | 2,168 | ||||||||||||
|
Commodities |
2,962 | - | 2,962 | - | ||||||||||||
|
TIPS |
1,874 | - | 1,874 | - | ||||||||||||
| $ | 39,135 | $ | - | $ | 36,967 | $ | 2,168 | |||||||||
|
Fair Value Measurements at December 31, 2014 using: |
||||||||||||||||
|
Asset Category |
Total |
(Level 1) |
(Level 2) |
(Level 3) |
||||||||||||
|
Equity securities: |
||||||||||||||||
|
U.S. companies |
$ | 14,513 | $ | - | $ | 14,513 | $ | - | ||||||||
|
International companies |
7,900 | - | 7,900 | - | ||||||||||||
|
U.S. fixed income |
14,093 | - | 11,964 | 2,129 | ||||||||||||
|
Commodities |
3,161 | - | 3,161 | - | ||||||||||||
|
TIPS |
1,998 | - | 1,998 | - | ||||||||||||
| $ | 41,665 | $ | - | $ | 39,536 | $ | 2,129 | |||||||||
The following table sets forth a summary of the changes in the fair value of the Domestic Plan’s Level 3 assets, which are annuity contracts with an insurance company, for the year ended December 31, 2015:
|
Group Annuity Contract |
||||
|
Balance at December 31, 2014 |
$ | 2,129 | ||
|
Net investment gain |
39 | |||
|
Balance at December 31, 2015 |
$ | 2,168 | ||
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(18) Foreign Operations and Sales
The following summarized data represents the gross sales and long lived assets for the Company’s domestic and foreign entities for 2015, 2014 and 2013:
|
Domestic |
Foreign |
Total |
||||||||||
|
2015 |
||||||||||||
|
Gross sales |
$ | 237,146 | $ | 196,710 | $ | 433,856 | ||||||
|
Long-lived assets |
93,142 | 132,099 | 225,241 | |||||||||
|
2014 |
||||||||||||
|
Gross sales |
$ | 186,735 | $ | 187,415 | $ | 374,150 | ||||||
|
Long-lived assets |
64,995 | 151,386 | 216,381 | |||||||||
|
2013 |
||||||||||||
|
Gross sales |
$ | 153,202 | $ | 164,010 | $ | 317,212 | ||||||
|
Long-lived assets |
59,496 | 155,151 | 214,647 | |||||||||
Export sales, included in domestic gross sales, in 2015, 2014 and 2013 amounted to $159,048, $101,101, and $86,850, respectively.
Sales to geographic areas consist of the following:
|
2015 |
2014 |
2013 |
||||||||||
|
Europe |
$ | 280,593 | $ | 232,894 | $ | 210,463 | ||||||
|
North America |
127,024 | 117,477 | 86,974 | |||||||||
|
Asia |
14,024 | 12,865 | 13,800 | |||||||||
|
Other |
12,215 | 10,914 | 5,975 | |||||||||
|
Total |
$ | 433,856 | $ | 374,150 | $ | 317,212 | ||||||
One customer accounted for 34.5%, 24.0% and 18.3% of 2015, 2014 and 2013 consolidated gross sales, respectively.
(19) Commitments
The Company has operating leases expiring on various dates through the year 2021. The leases are primarily for the rental of office space, office and laboratory equipment, and vehicles. At December 31, 2015, future minimum commitments under non-cancelable operating lease arrangements were as follows:
| Year ended December 31: | ||||
|
2016 |
$ | 1,085 | ||
|
2017 |
602 | |||
|
2018 |
541 | |||
|
2019 |
196 | |||
|
2020 |
93 | |||
|
2021 and thereafter |
92 | |||
|
Total commitments |
$ | 2,609 |
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(19) Commitments (continued)
Total operating lease expense was $1,048, $1,113, and $1,090 for the years ended December 31, 2015, 2014 and 2013, respectively.
The Company is party to several unconditional purchase obligations resulting from contracts that contain legally binding provisions with respect to quantities, pricing and timing of purchases. The Company’s purchase obligations mainly include commitments to purchase utilities. At December 31, 2015, future commitments under these obligations were as follows:
| Year ended December 31: | ||||
|
2016 |
$ | 6,389 | ||
|
2017 |
563 | |||
|
2018 |
59 | |||
|
2019 |
47 | |||
|
2020 |
- | |||
|
Total commitments |
$ | 7,058 |
(20) Contingencies
The Company is subject to various investigations, claims and legal proceedings covering a wide range of matters that arise in the ordinary course of its business activities. The Company continually assesses known facts and circumstances as they pertain to applicable legal and environmental matters and evaluates the need for reserves and disclosures as deemed necessary based on these facts and circumstances. These matters, either individually or in the aggregate, could result in actual costs that are significantly higher than the Company’s current assessment and could have a material adverse effect on the Company's operating results and cash flows in future reporting periods. Based upon past experience, the Company believes that payments significantly in excess of current reserves, if required, would be made over an extended number of years.
Environmental
In connection with laws and regulations pertaining to the protection of the environment, the Company and its subsidiaries are a party to several environmental proceedings and remediation activities and along with other companies, have been named a potentially responsible party (“PRP”) for certain waste disposal sites ("Superfund sites"). Substantially all of the liabilities currently recorded on the Company’s balance sheet for environmental proceedings are associated with discontinued operations. The Company had insurance policies in place at certain of the discontinued operations for certain years that the Company believes should cover some portion of currently recorded liabilities or potential future liabilities.
It is the Company’s policy to record appropriate liabilities for environmental matters where remedial efforts are probable and the costs can be reasonably estimated. Such liabilities are based on the Company’s estimate of the undiscounted future costs required to complete the remedial work. Each of these matters is subject to various uncertainties, and it is possible that some of these matters will be decided against the Company. The resolution of such matters often spans several years and frequently involves regulatory oversight or adjudication. Additionally, many remediation requirements are fluid and are likely to be affected by future technological, site and regulatory developments. It is not possible at this time for the Company to determine fully the effect of all asserted and unasserted claims on its consolidated financial condition, results of operations or liquidity; however, to the extent possible, where asserted and unasserted claims can be estimated and where such claims are considered probable, the Company would record a liability. Consequently, the ultimate liability with respect to such matters, as well as the timing of cash disbursements, is uncertain.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(20) Contingencies (continued)
In matters where the Company is able to reasonably estimate the probable and estimable costs associated with environmental proceedings, the Company accrues for the estimated costs associated with the study and remediation of applicable sites. These reserves were $8,329 and $9,595 at December 31, 2015 and 2014, respectively. The decrease in the reserve includes payments of $2,257 and the impact of currency translation of $83, partially offset by adjustments to reserves of $1,074. The reserves are adjusted periodically as remediation efforts progress or as additional technical, regulatory or legal information becomes available. Given the uncertainties regarding the outcome of investigative and study activities, the status of laws, regulations, enforcement, policies, the impact of other PRPs, technology and information related to individual sites, the Company does not believe it is possible to currently develop an estimate of the range of reasonably possible environmental loss in excess of its reserves.
Bayonne
As a result of the sale of a Bayonne, New Jersey facility, the Company became obligated to investigate site conditions and conduct required remediation under the New Jersey Industrial Site Recovery Act. The Company intends to continue implementing a sampling plan at the property pursuant to the New Jersey Department of Environmental Protection’s (“NJDEP”) private oversight program. The results of the completed sampling, and any additional sampling deemed necessary, will be used to develop an estimate of the Company's future liability for remediation costs. As of December 31, 2015, the Company’s reserve was $432.
Clifton and Carlstadt
The Company has implemented a sampling and pilot program in Clifton, New Jersey pursuant to the NJDEP private oversight program. The results of the sampling and pilot program to date have been used to develop an estimate of the Company's future liability for remediation costs. As of December 31, 2015, the Company’s reserve was $1,090.
Additionally, the Company has implemented a sampling and pilot program in Carlstadt, New Jersey pursuant to the NJDEP private oversight program. The results of the sampling and pilot program to date have been used to develop an estimate of the Company's future liability for remediation costs. As of December 31, 2015, the Company’s reserve was $1,030.
Berry’s Creek
The Company received a notice from the United States Environmental Protection Agency (“USEPA”) that two subsidiaries of the Company are considered PRPs at the Berry’s Creek Study Area in New Jersey. These subsidiaries are among many other PRPs that were listed in the notice. Pursuant to the notice, the PRPs have been asked to perform a remedial investigation (“RI”) and feasibility study of the Berry’s Creek site. The Company has joined the group of PRPs and entered into an Administrative Settlement Agreement (“Agreement”) and Order on Consent with the USEPA agreeing to jointly conduct or fund an appropriate remedial investigation and feasibility study of the Berry’s Creek site with the other PRPs in the Agreement. The PRPs have engaged consultants to perform the work specified in the Agreement and develop a method to allocate related costs among the PRPs. As of December 31, 2015, the Company’s reserve was $64 to cover the current phase of investigation based on a tentative agreement on the allocation of the site investigation costs among the PRPs. Due to the very preliminary and uncertain nature of any estimates related to the method and costs of any remediation solution (not expected to be known prior to late 2018), the number of eventual PRPs, and their respective proportion of remediation costs, the Company’s liability cannot be reasonably estimated at this time; as such, no accrual is recorded for these potential future costs. The impact of the resolution of this matter on the Company’s results of operations in any future reporting period is not known.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(20) Contingencies (continued)
In July 2014, the Company received a notice from the U.S. Department of the Interior, U.S. Fish & Wildlife Service, regarding the Company’s potential liability for natural resource damages at the Berry’s Creek site and inviting the Company to participate in a cooperative assessment of natural resource damages. Most members of the Berry’s Creek PRP group received such notice letters, and the PRP Group coordinated a joint response, which was to decline participation in a cooperative assessment at this time, given existing investigation work at the site. The cost of any future assessment and the ultimate scope of natural resource damage liability are not yet known.
Maybrook Site
A subsidiary of Cambrex is named a PRP of a site in Hamptonburgh, New York by the USEPA in connection with the discharge, under appropriate permits, of wastewater at that site prior to Cambrex's acquisition in 1986. The PRPs implemented soil remediation which was completed in 2012 pending approval by the USEPA. The PRPs will continue implementing the ground water remediation at the site. As of December 31, 2015, the Company’s reserve was $322 to cover remaining ground water remediation and long-term monitoring.
Harriman Site
Subsidiaries of Cambrex and Pfizer are named as responsible parties for the Company’s former Harriman, New York production facility by the New York State Department of Environmental Conservation (“NYSDEC”). A final Record of Decision (“ROD”) describing the Harriman site remediation responsibilities for Pfizer and the Company was issued in 1997 (the “1997 ROD”) and incorporated into a federal court Consent Decree in 1998 (the “Consent Decree”). In December 2013, the Company, Pfizer and the NYSDEC entered into a federal court stipulation, which the court subsequently endorsed as a court order, resolving certain disputes with the NYSDEC about the scope of the obligations under the Consent Decree and the 1997 ROD, and requiring the Company and Pfizer to carry out an environmental investigation and study of certain areas of the Harriman Site.
Site clean-up work under the 1997 ROD, the Consent Decree and the 2013 stipulation is ongoing and is being jointly performed by Pfizer and the Company, with NYSDEC oversight. During 2014, Pfizer and the Company performed supplemental remedial investigation measures agreed to by the NYSDEC, and the findings were submitted to NYSDEC in a Supplemental RI Report and a Feasibility Study. In April 2015, the NYSDEC informed the Company and Pfizer by letter that the Supplemental RI Report was disapproved, and demanded that the Company and Pfizer perform additional environmental investigative work and revise certain aspects of that report. The Company and Pfizer are in discussions with the NYSDEC to address its written comments. As it is too soon to determine whether the discussion with NYSDEC will result in any significant changes to the Company’s responsibilities, no change to the reserve has been made. ELT Harriman, LLC ("ELT"), the current owner of the Harriman site, is conducting other investigation and remediation activities under a separate NYSDEC directive.
No final remedy for the site has been determined, which will follow further discussions with the NYSDEC. The Company estimates the range for its share of the liability at the site to be between $2,000 and $7,000. As of December 31, 2015, the Company’s reserve was $3,565. At this time, the Company is unable to provide an estimate of the ultimate investigative and remedial costs to the Company for any final remedy selected by the NYSDEC.
The Company intends to enforce all of its contractual rights to recover costs and for indemnification under a 2007 settlement agreement, and has filed such claims in an arbitration proceeding against ELT and the immediately preceding owner, Vertellus Specialties Holdings. ELT has filed counterclaims, and has threatened to file additional counterclaims, for contractual indemnification and for breach of the settlement agreement against the Company. Currently, the arbitration proceeding is stayed indefinitely.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(20) Contingencies (continued)
Scientific Chemical Processing (“SCP”) Superfund Site
A subsidiary of Cambrex was named a PRP of the SCP Superfund site, located in Carlstadt, New Jersey, along with approximately 130 other PRPs. The site is a former waste processing facility that accepted various waste for recovery and disposal including processing wastewater from this subsidiary. The PRPs are in the process of implementing a final remedy at the site. The SCP Superfund site has also been identified as a PRP in the Berry’s Creek Superfund site (see previous discussion). While the Company continues to dispute the methodology used by the PRP group to arrive at its interim allocation for cash contributions, the Company paid the funding requests in 2010 and 2014-2015. A final allocation of SCP Site costs (excluding Berry’s Creek costs) is expected to be finalized during 2016. As of December 31, 2015, the Company’s reserve was $934, of which approximately $598 is expected to be covered by insurance.
Newark Bay Complex
The USEPA and a private party group are evaluating remediation plans for the Passaic River, Newark Bay, Hackensack River, Arthur Kill, Kill Van Kull and adjacent waters (the “Newark Bay Complex”). Although the Company is not involved in the USEPA action, it continues to monitor developments related to the site due to its past involvement in a previously settled state action relating to the Newark Bay Complex. It is the Company’s understanding that the private party group and the USEPA have proposed remedies for the site with estimated costs ranging from $500 million to over a billion dollars. The USEPA is expected to select a remedy in the near future. Due to the uncertainty of the future scope and timing of any possible claims against the Company, no liability has been recorded.
The Company is involved in other related and unrelated environmental matters where the range of liability is not reasonably estimable at this time and it is not foreseeable when information will become available to provide a basis for adjusting or recording a reserve, should a reserve ultimately be required.
Litigation and Other Matters
Lorazepam and Clorazepate
In 1998, the Company and a subsidiary were named as defendants along with Mylan Laboratories, Inc. (“Mylan”) and Gyma Laboratories, Inc. (“Gyma”) in a proceeding instituted by the Federal Trade Commission in the United States District Court for the District of Columbia (the “District Court”). Suits were also commenced by several State Attorneys General and class action complaints by private plaintiffs in various state courts. The suits alleged violations of the Federal Trade Commission Act arising from exclusive license agreements between the Company and Mylan covering two APIs (Lorazepam and Clorazepate).
All cases have been resolved except for one brought by four health care insurers. In the remaining case, the District Court entered judgment after trial in 2008 against Mylan, Gyma and Cambrex in the total amount of $19,200, payable jointly and severally, and also a punitive damage award against each defendant in the amount of $16,709. In addition, at the time, the District Court ruled that the defendants were subject to a total of approximately $7,500 in prejudgment interest. The case is currently pending before the District Court following a January 2011 remand by the Court of Appeals. In July 2014, the District Court dismissed certain customers for which the plaintiffs were unable to establish jurisdiction and consequently, the plaintiffs currently have a motion pending before the District Court to reduce the damages award by a total of $9,600.
CAMBREX CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands, except per share data)
(20) Contingencies (continued)
In 2003, Cambrex paid $12,415 to Mylan in exchange for a release and full indemnity against future costs or liabilities in related litigation brought by the purchasers of Lorazepam and Clorazepate, as well as potential future claims related to the ongoing matter. In the event of a final settlement or final judgment, Cambrex expects any payment required by the Company to be made by Mylan under the indemnity described above.
Other
The Company has commitments incident to the ordinary course of business including corporate guarantees of certain subsidiary obligations to the Company’s lenders related to financial assurance obligations under certain environmental laws for remediation; closure and third party liability requirements of certain of its subsidiaries and a former operating location; contract provisions for indemnification protecting its customers and suppliers against third party liability for the manufacture and sale of Company products that fail to meet product warranties and contract provisions for indemnification protecting licensees against intellectual property infringement related to licensed Company technology or processes.
Additionally, as permitted under Delaware law, the Company indemnifies its officers, directors and employees for certain events or occurrences while the officer, director or employee is, or was, serving at the Company’s request in such capacity. The term of the indemnification period is for the officer's, director's or employee’s lifetime. The maximum potential amount of future payments the Company could be required to make under these indemnification agreements is unlimited; however, the Company has a director and officer insurance policy that covers a portion of any potential exposure. The Company currently believes the estimated fair value of its indemnification agreements is not material based on currently available information, and as such, the Company had no liabilities recorded for these agreements as of December 31, 2015.
Cambrex's subsidiaries are party to a number of other proceedings that are not considered material at this time.
(21) Discontinued Operations
For all periods presented, financial results for discontinued operations relate to environmental investigation and remediation expenses for sites that were divested prior to December 31, 2014. For 2015, the Company recorded $41 as income from discontinued operations, net of tax. For 2014 and 2013, the Company recorded $830 and $4,360, respectively, as losses from discontinued operations, net of tax. As of December 31, 2015 and 2014 liabilities recorded on the Company’s balance sheet relating to discontinued operations were $8,209 and $8,647, respectively. At this time, we cannot reasonably estimate the period of time during which the involvement is expected to continue. Net cash used in discontinued operations was $1,536, $1,858, and $1,533 for 2015, 2014, and 2013, respectively. Refer to Note 20 to the Company’s consolidated financial statements for further disclosures on the Company’s environmental contingencies.
The following table is a reconciliation of the pre-tax income/(loss) from discontinued operations to the net income/(loss) from discontinued operations, as presented on the income statement:
|
2015 |
2014 |
2013 |
||||||||||
|
Pre-tax income/(loss) from discontinued operations |
$ | 63 | $ | (1,277 | ) | $ | (6,708 | ) | ||||
|
Income tax (expense)/benefit |
(22 | ) | 447 | 2,348 | ||||||||
|
Income/(loss) from discontinued operations, net of tax |
$ | 41 | $ | (830 | ) | $ | (4,360 | ) | ||||
CAMBREX CORPORATION AND SUBSIDIARIES
SELECTED QUARTERLY FINANCIAL AND SUPPLEMENTARY DATA - UNAUDITED
(in thousands, except share and per share data)
|
|
|
|
|
|||||||||||||
|
1st Quarter |
2nd Quarter |
3rd Quarter |
4th Quarter (1) |
|||||||||||||
|
2015 |
||||||||||||||||
|
Gross sales |
$ | 78,184 | $ | 106,379 | $ | 92,350 | $ | 156,943 | ||||||||
|
Net revenues |
77,525 | 106,635 | 92,979 | 156,187 | ||||||||||||
|
Gross profit |
29,079 | 45,945 | 35,680 | 66,261 | ||||||||||||
|
Income from continuing operations |
8,368 | 19,450 | 11,876 | 17,482 | ||||||||||||
|
Income/(loss) from discontinued operations (6) |
(375 | ) | 213 | (129 | ) | 332 | ||||||||||
|
Net income |
7,993 | 19,663 | 11,747 | 17,814 | ||||||||||||
|
Earnings per share of common stock: (7) |
||||||||||||||||
|
Basic |
0.26 | 0.63 | 0.37 | 0.56 | ||||||||||||
|
Diluted |
0.25 | 0.61 | 0.36 | 0.54 | ||||||||||||
|
Average shares: |
||||||||||||||||
|
Basic |
31,198 | 31,344 | 31,471 | 31,661 | ||||||||||||
|
Diluted |
32,158 | 32,440 | 32,593 | 32,784 | ||||||||||||
|
1st Quarter (2) |
2nd Quarter (3) |
3rd Quarter (4) |
4th Quarter (5) |
|||||||||||||
|
2014 |
||||||||||||||||
|
Gross sales |
$ | 66,192 | $ | 97,972 | $ | 81,145 | $ | 128,841 | ||||||||
|
Net revenues |
66,105 | 97,893 | 81,300 | 129,315 | ||||||||||||
|
Gross profit |
16,578 | 33,415 | 28,406 | 45,399 | ||||||||||||
|
Income from continuing operations |
1,166 | 19,827 | 8,882 | 28,258 | ||||||||||||
|
Loss from discontinued operations (6) |
(184 | ) | (160 | ) | (113 | ) | (373 | ) | ||||||||
|
Net income |
982 | 19,667 | 8,769 | 27,885 | ||||||||||||
|
Earnings per share of common stock: (7) |
||||||||||||||||
|
Basic |
0.03 | 0.64 | 0.28 | 0.90 | ||||||||||||
|
Diluted |
0.03 | 0.63 | 0.28 | 0.88 | ||||||||||||
|
Average shares: |
||||||||||||||||
|
Basic |
30,546 | 30,647 | 30,801 | 31,053 | ||||||||||||
|
Diluted |
31,408 | 31,428 | 31,599 | 31,803 | ||||||||||||
|
(1) |
Income from continuing operations includes restructuring expenses of $15,573 and a tax benefit of $1,464 related to the decision to sell our finished dosage form facility in Hyderabad, India. |
|
(2) |
Income from continuing operations includes the reversal of a valuation allowance on deferred tax assets of $198. |
|
(3) |
Income from continuing operations includes the reversal of a valuation allowance on deferred tax assets of $14,161 and a loss of $4,122 related to the purchase of the remaining shares in Zenara. |
|
(4) |
Income from continuing operations includes the reversal of a valuation allowance on deferred tax assets of $824. |
|
(5) |
Income from continuing operations includes a gain on sale of land of $1,234 and a corresponding tax expense of $387, a benefit of $11,719 for the reversal of a valuation allowance on deferred tax assets, a benefit of $3,948 for favorable audit settlements, and expense of $7,170 related to a voluntary lump sum pension settlement. |
|
(6) |
Discontinued operations include charges for environmental remediation related to sites of divested businesses. |
|
(7) |
Earnings per share calculations for each of the quarters are based on the weighted average number of shares outstanding for each period. As such, the sum of the quarters may not necessarily equal the earnings per share amount for the year. |
|
Item 9 |
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure. |
None.
|
Item 9A |
Controls and Procedures. |
Conclusion Regarding the Effectiveness of Disclosure Controls and Procedures
The Company maintains disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934 (“Exchange Act”) that are designed to ensure that information required to be disclosed in its reports filed or submitted under the Exchange Act is processed, recorded, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to the Company’s management, including the Company’s Chief Executive Officer and Chief Financial Officer, as appropriate, to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As required by SEC Rule 13a-15(b), the Company carried out an evaluation, under the supervision and with the participation of management, including the Company’s Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of the Company’s disclosure controls and procedures as of the end of the period covered by this Annual Report. Based on this evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that as of December 31, 2015, the disclosure controls and procedures are effective to ensure that information required to be disclosed by the Company in the reports filed or submitted under the Exchange Act are (i) recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and (ii) accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
Management's Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f). Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States, and include those policies and procedures that:
|
● |
Pertain to the maintenance of records, that in reasonable detail, accurately and fairly represent the transactions and dispositions of the assets of the Company, |
|
● |
Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and the Board of Directors of the Company, and |
|
● |
Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we carried out an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2015 based on the Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO"). Our management, including the Chief Executive Officer and Chief Financial Officer, concluded that based on its assessment, the Company’s internal control over financial reporting was effective as of December 31, 2015. Effectiveness of our internal control over financial reporting as of December 31, 2015 has been audited by BDO USA, LLP, an independent registered public accounting firm, as stated in their report which appears elsewhere herein.
Changes in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 or 15d-15 that occurred during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
|
Item 9B |
Other Information. |
None.
PART III
|
Item 10 |
Directors, Executive Officers and Corporate Governance. |
Executive Officers of the Registrant
The following table lists the officers of the Company:
|
Name |
Age |
Office | |
|
Steven M. Klosk (i) (ii) |
58 |
President, Chief Executive Officer | |
|
|
|
| |
|
Shawn P. Cavanagh (i) (ii) |
49 |
Executive Vice President and Chief Operating Officer | |
|
|
|
| |
|
James G. Farrell (ii) |
49 |
Vice President and Corporate Controller | |
|
|
|
| |
|
Samantha M. Hanley (i) (ii) |
38 |
Vice President, General Counsel, and Secretary | |
| Gregory P. Sargen (i) (ii) | 50 | Executive Vice President and Chief Financial Officer | |
|
|
|
| |
| (i) Executive Officer (ii) Corporate Officer | |||
The Company's corporate officers are appointed by the Board of Directors and serve at the Board's discretion.
Mr. Klosk joined Cambrex in October 1992 and has served as President and Chief Executive Officer since May 2008. He also became a member of the Board of Directors in May 2008. Mr. Klosk joined the Company as Vice President, Administration. He was appointed Executive Vice President, Administration in October 1996 and was promoted to the position of Executive Vice President, Administration and Chief Operating Officer for the Cambrex Pharma and Biopharmaceutical Business Unit in October 2003. In January 2005, Mr. Klosk assumed direct responsibility for the leadership of the Biopharmaceutical Business Unit as Chief Operating Officer. In August 2006, Mr. Klosk assumed the responsibility of the Pharma business as Executive Vice President and Chief Operating Officer – Biopharma & Pharma and in February 2007 was appointed to Executive Vice President, Chief Operating Officer and President, Pharmaceutical Products and Services. From 1988 until he joined Cambrex, Mr. Klosk was Vice President, Administration and Corporate Secretary for The Genlyte Group, Inc. From 1985 to 1988, he was Vice President, Administration for Lightolier, Inc., a subsidiary of The Genlyte Group, Inc. Mr. Klosk currently serves on the Board of Directors of Caladrius Biosciences, Inc., a publicly traded cell therapy company.
Mr. Cavanagh joined Cambrex in January 2011 and has served as Executive Vice President and Chief Operating Officer since he joined Cambrex. From 2007 to 2009 Mr. Cavanagh was employed with Lonza, which purchased Cambrex Bioproducts, most recently as President of Lonza Bioscience. From 1999 to 2007, Mr. Cavanagh worked for Cambrex Bioproducts. While at Cambrex Bioproducts, Mr. Cavanaugh held several positions of increasing responsibility including President of Cambrex Bioproducts. Prior to joining Cambrex Bioproducts, Mr. Cavanagh held various management and engineering positions with FMC Corporation.
Mr. Farrell joined Cambrex in September 2005 as Corporate Controller. He has served as Vice President and Corporate Controller since July 2007, except for a portion of 2008 when Mr. Farrell was employed by PDI, Inc. as Vice President and Corporate Controller/Interim Chief Financial Officer. From 1994 until 2005, he was with Ingersoll-Rand Company, most recently as Director, Accounting Policy, Procedures and External Reporting. Mr. Farrell was with Ernst & Young from 1988 to 1994, most recently as Audit Manager.
Ms. Hanley joined Cambrex in April 2009 and has served as Assistant General Counsel and Assistant Secretary since January 2013, and as Vice President, General Counsel and Secretary since February 2015. Ms. Hanley previously held the position of Senior Intellectual Property/Corporate Counsel and Assistant Secretary. Prior to joining Cambrex, Ms. Hanley worked at Alpharma Pharmaceuticals as Director of Intellectual Property and was an Associate with Lerner, David, Littenberg, Krumholtz & Mentlik, LLP, an intellectual property law firm.
Mr. Sargen joined Cambrex in February 2003 and has served as Vice President and Chief Financial Officer since February 2007 and Executive Vice President and Chief Financial Officer since January 2011. Mr. Sargen previously held the position of Vice President, Finance. Previously, he was with Exp@nets, Inc. from 1999 through 2002, serving in the roles of Executive Vice President, Finance/Chief Financial Officer and Vice President/Corporate Controller. From 1996 to 1998, he was with Fisher Scientific International’s Chemical Manufacturing Division, serving in the roles of Vice President, Finance and Controller. Mr. Sargen has also held various positions in finance, accounting and audit with Merck & Company, Inc., Heat and Control, Inc., and Deloitte & Touche.
The remaining information required by this item will be included in the 2016 Proxy Statement and is incorporated herein by reference.
|
Item 11 |
Executive Compensation. |
The remaining information required by this item will be included in the 2016 Proxy Statement and is incorporated herein by reference.
|
Item 12 |
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters. |
The remaining information required by this item will be included in the 2016 Proxy Statement and is incorporated herein by reference.
|
Item 13 |
Certain Relationships and Related Transactions and Director Independence. |
The remaining information required by this item will be included in the 2016 Proxy Statement and is incorporated herein by reference.
|
Item 14 |
Principal Accountant Fees and Services. |
The remaining information required by this item will be included in the 2016 Proxy Statement and is incorporated herein by reference.
PART IV
|
Item 15 |
Exhibits and Financial Statement Schedules. |
(a) 1. The following consolidated financial statements of the Company are filed as part of this report:
|
|
|
Page Number (in this report) |
| Financial Statements: | ||
|
Reports of Independent Registered Public Accounting Firm |
|
37 |
|
Consolidated Balance Sheets as of December 31, 2015 and 2014 |
|
39 |
|
Consolidated Income Statements for the Years Ended December 31, 2015, 2014 and 2013 |
|
40 |
| Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2015, 2014 and 2013 | 41 | |
| Consolidated Statements of Stockholders’ Equity for the Years Ended December 31, 2015, 2014 and 2013 | 42 | |
|
Consolidated Statements of Cash Flows for the Years Ended December 31, 2015, 2014 and 2013 |
|
43 |
| Notes to Consolidated Financial Statements | 44 | |
| Selected Quarterly Financial and Supplementary Data (unaudited) | 74 |
2. (i) The following schedule to the consolidated financial statements of the Company as filed herein and the Report of Independent Registered Public Accounting Firms are filed as part of this report.
|
|
|
Page Number (in this report) |
|
|
|
|
|
Schedule II – Valuation and Qualifying Accounts |
|
80 |
All other schedules are omitted because they are not applicable or not required or because the required information is included in the consolidated financial statements of the Company or the notes thereto.
3. The exhibits filed in this report are listed in the Exhibit Index on pages 82-85.
SCHEDULE II
CAMBREX CORPORATION
VALUATION AND QUALIFYING ACCOUNTS
FOR THE YEARS ENDED DECEMBER 31, 2015, 2014 and 2013
(dollars in thousands)
|
Column A |
Column B |
Column C |
Column D |
Column E |
||||||||||||||||
|
Additions |
||||||||||||||||||||
|
Balance Beginning of Year |
Charged/ (Credited) to Cost and Expenses |
Charged/ (Credited) to Other Accounts |
Deductions |
Balance End of Year |
||||||||||||||||
|
Description |
||||||||||||||||||||
|
Year ended December 31, 2015: |
||||||||||||||||||||
|
Doubtful trade receivables and returns and allowances |
$ | 346 | $ | (11 | ) | $ | (31 | ) | $ | - | $ | 304 | ||||||||
|
Deferred tax valuation allowance |
5,053 | 303 | (2,215 | ) | - | 3,141 | ||||||||||||||
|
Year ended December 31, 2014: |
||||||||||||||||||||
|
Doubtful trade receivables and returns and allowances |
$ | 1,058 | $ | 61 | $ | (130 | ) | $ | 643 | $ | 346 | |||||||||
|
Deferred tax valuation allowance |
29,890 | (26,543 | ) | 1,706 | - | 5,053 | ||||||||||||||
|
Year ended December 31, 2013: |
||||||||||||||||||||
|
Doubtful trade receivables and returns and allowances |
$ | 652 | $ | 433 | $ | 27 | $ | 54 | $ | 1,058 | ||||||||||
|
Deferred tax valuation allowance |
29,941 | (97 | ) | 46 | - | 29,890 | ||||||||||||||
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
CAMBREX CORPORATION |
| |
|
|
|
|
|
|
|
|
|
|
|
|
By: |
/s/ Gregory P. Sargen |
|
|
|
Gregory P. Sargen |
| |
|
|
Executive Vice President and Chief Financial Officer |
| |
| Date: February 9, 2016 | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | |||
|
|
|
|
| ||
| /s/ |
STEVEN M. KLOSK |
President and Chief Executive Officer, |
|
February 9, 2016 | |
| Steven M. Klosk |
and Director |
|
|||
|
|
|
||||
| /s/ | GREGORY P. SARGEN | Executive Vice President and Chief Financial | February 9, 2016 | ||
| Gregory P. Sargen | Officer (Principal Financial Officer | ||||
| and Accounting Officer) | |||||
| /s/ | SHLOMO YANAI | Chairman of the Board of Directors | February 9, 2016 | ||
| Shlomo Yanai | |||||
| /s/ | ROSINA B. DIXON | Director | February 9, 2016 | ||
| Rosina B. Dixon, M.D. | |||||
| /s/ | LOUIS J. GRABOWSKY | Director | February 9, 2016 | ||
| Louis J. Grabowsky | |||||
| /s/ | KATHRYN RUDIE HARRIGAN | Director | February 9, 2016 | ||
| Kathryn Rudie Harrigan, PhD | |||||
| /s/ | LEON J. HENDRIX, JR. | Director | February 9, 2016 | ||
| Leon J. Hendrix, Jr. | |||||
| /s/ | ILAN KAUFTHAL | Director | February 9, 2016 | ||
| Ilan Kaufthal | |||||
| /s/ | WILLIAM KORB | Director | February 9, 2016 | ||
| William Korb | |||||
| /s/ | PETER G. TOMBROS | Director | February 9, 2016 | ||
| Peter G. Tombros |
EXHIBIT INDEX
Exhibit No. Description
|
3.1 |
-- |
Restated Certificate of Incorporation of Cambrex Corporation.(O). |
|
3.2 |
-- |
By Laws of Cambrex Corporation, as amended.(U). |
|
4.1 |
-- |
Form of Certificate for shares of Common Stock of Cambrex Corporation.(C - Exhibit 4(a)). |
|
4.2 |
-- |
2009 Long-Term Incentive Plan (as amended and restated April 29, 2015).(T) | |
|
10.1 |
-- |
Form of Non-Employee Directors Stock Option Agreement.(F). | |
|
10.2 |
-- |
Form of Performance Share Agreement.(R). | |
|
10.3 |
-- |
Credit Agreement dated November 2, 2011 between Cambrex Corporation, the subsidiary borrowers party hereto, the subsidiary guarantors party hereto, the lenders party hereto and JP Morgan Chase Bank, N.A., as Administrative Agent.(J). | |
|
10.4 |
-- |
Settlement Agreement and Release and Environmental Escrow Agreement dated July 30, 2007, between Rutherford Chemicals LLC, Vertellus Specialties Holdings UK Ltd. (formerly Rutherford Chemicals UK Ltd.), Vertellus Specialties UK Ltd. (formerly Seal Sands Chemicals Ltd.), and Vertellus Specialties Holdings Corp. (formerly Rutherford Chemicals Holdings Corp.), and Cambrex Corporation, Nepera, Inc., CasChem Inc., Zeeland Chemicals, Inc., Nepcam, Inc., and Cambrex Ltd.(L). | |
|
10.5 |
-- |
Shawn P. Cavanagh Offer of Employment Letter.(M). | |
|
10.6 |
-- |
Employment Agreement dated January 17, 2011 between Cambrex Corporation and Shawn P. Cavanagh.(M). | |
|
10.7 |
-- |
Cambrex Corporation Savings Plan.(D). | |
|
10.8 |
-- |
Cambrex Corporation Supplemental Retirement Plan.(E). | |
|
10.9 |
-- |
Employment Agreement dated February 6, 2007 between Cambrex Corporation and Gregory P. Sargen.(K). | |
|
10.10 |
-- |
2001 Performance Stock Option Plan.(G). | |
|
10.11 |
-- |
2003 Performance Stock Option Plan.(G). | |
|
10.12 |
-- |
2004 Performance Incentive Plan.(H). | |
|
10.13 |
-- |
2004 Incentive Plan.(I). | |
|
10.14 |
-- |
Administrative Consent Order of the New Jersey Department of Environmental Protection to Cosan Chemical Corporation, dated September 16, 1985.(C – Exhibit 10(Q)). |
|
|
10.15 |
-- |
Form of Stock Option Agreement.(S). |
|
|
10.16 |
-- |
Form of Performance Share Unit Agreement.(Q). |
|
|
10.17 |
-- |
Executive Cash Incentive Plan.(P). |
|
|
10.18 |
-- |
2012 Equity Incentive Plan for Non-Employee Directors.(P). |
|
|
10.19 |
-- |
Gregory P. Sargen Offer of Employment Letter.(A). |
|
|
21 |
-- |
Subsidiaries of registrant.(A). |
|
|
23 |
-- |
Consent of BDO USA, LLP to the incorporation by reference of its report herein in Registration Statement Nos. 333-166260, 333-57404, 333-22017, 33-21374, 33-81782, 333-113612, 333-113613, 333-129473, 333-136529, 333-174124, 333-181053 and 333-190305 on Form S-8 of the registrant.(A). |
|
|
31.1 |
-- |
CEO Certification pursuant to Rule 13a – 14(a) and Rule 15d – 14(a) of the Securities Exchange Act, as amended.(A). |
|
|
31.2 |
-- |
CFO Certification pursuant to Rule 13a – 14(a) and Rule 15d – 14(a) of the Securities Exchange Act, as amended.(A). |
|
|
32 |
-- |
CEO and CFO Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.(B). |
|
|
101.INS |
-- |
XBRL Instance Document.(A)(N). |
|
|
101.SCH |
-- |
XBRL Taxonomy Extension Schema.(A)(N). |
|
|
101.CAL |
-- |
XBRL Taxonomy Extension Calculation Linkbase.(A)(N). |
|
|
101.DEF |
-- |
XBRL Taxonomy Extension Definition Linkbase.(A)(N). |
|
|
101.LAB |
-- |
XBRL Taxonomy Extension Label Linkbase.(A)(N). |
|
|
101.PRE |
-- |
XBRL Taxonomy Extension Presentation Linkbase.(A)(N). |
|
See legend on following page
EXHIBIT INDEX
|
(A) |
Filed herewith. |
|
(B) |
Furnished herewith. |
|
(C) |
Incorporated by reference to the indicated Exhibit to registrant's Registration Statement on Form S-1 (Registration No. 33-16419). |
|
(D) |
Incorporated by reference to registrant's Registration Statement on Form S-8 (Registration No. 33-81780) dated July 20, 1994. |
|
(E) |
Incorporated by reference to the registrant's Annual Report on Form 10-K for year end 1994 filed on March 24, 1995. |
|
(F) |
Incorporated by reference to the registrant’s Quarterly Report on Form 10-Q for the period ending March 31, 2013 filed on May 3, 2013. |
|
(G) |
Incorporated by reference to registrant’s Registration Statement on Form S-8 (Registration No. 333-113612) dated March 15, 2004. |
|
(H) |
Incorporated by reference to registrant’s Registration Statement on Form S-8 (Registration No. 333-113613) dated March 15, 2004. |
|
(I) |
Incorporated by reference to registrant’s Registration Statement on Form S-8 (Registration No. 333-129473) dated November 4, 2005. |
|
(J) |
Incorporated by reference to registrant’s Quarterly Report on Form 10-Q for the period ending September 30, 2011 filed on November 4, 2011. |
|
(K) |
Incorporated by reference to registrant’s Annual Report on Form 10-K for year end 2006 filed on March 15, 2007. |
|
(L) |
Incorporated by reference to registrant’s Quarterly Report on Form 10-Q for the period ending September 30, 2007 filed on November 2, 2007. |
|
(M) |
Incorporated by reference to the registrant’s Current Report on Form 8-K dated January 13, 2011. |
|
(N) |
Attached as Exhibit 101 to this report are the following formatted in XBRL (Extensible Business Reporting Language): (i) Consolidated Balance Sheets as of December 31, 2015 and 2014, (ii) Consolidated Income Statements for the years ended December 31, 2015, 2014 and 2013, (iii) Consolidated Statements of Comprehensive Income for the years ended December 31, 2015, 2014 and 2013, (iv) Consolidated Statements of Stockholders’ Equity for the years ended December 31, 2015, 2014 and 2013, (v) Consolidated Statement of Cash Flows for the years ended December 31, 2015, 2014 and 2013, and (vi) Notes to Consolidated Financial Statements. |
|
(O) |
Incorporated by reference to the registrant’s Current Report on Form 8-K dated April 30, 2012. |
|
(P) |
Incorporated by reference to the registrant’s Quarterly Report on Form 10-Q for the period ending March 31, 2012 filed May 4, 2012. |
|
(Q) |
Incorporated by reference to the registrant’s Quarterly Report on Form 10-Q for the period ending June 30, 2012 filed August 2, 2012. |
|
(R) |
Incorporated by reference to the registrant’s Annual Report on Form 10-K for year end 2012 filed on February 7, 2013. |
|
(S) |
Incorporated by reference to the registrant’s Quarterly Report on Form 10-Q for the period ending June 30, 2013 filed August 1, 2013 |
.
|
(T) |
Incorporated by reference to the registrant’s Registration Statement on Form S-8 (Registration No. 333-206045) dated August 3, 2015. |
|
(U) |
Incorporated by reference to the registrant’s Current Report on Form 8-K dated January 28, 2016. |
85