Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - EYEGATE PHARMACEUTICALS INC | v429127_8k.htm |
Exhibit 99.1

Eyegate Pharmaceuticals, Inc. Providing innovative products that enhance drug efficacy and patient compliance to improve vision Corporate Presentation January 2016

1 Forward Looking Statements Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s lead product EGP - 437, for the timing and outcome of the Company’s clinical trials, the potential approval to market EGP - 437, and the Company’s capital needs. Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation. Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful. The Company may consume more cash than it currently anticipates and faster than projected. Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates. If the FDA or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations. Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K filed with the SEC on March 31, 2015. The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law.

2 Company Overview ▪ Ophthalmology company (NASDAQ: EYEG) ▪ EyeGate ® II Delivery System: non - invasive delivery of therapeutics to front or back of eye ▪ Lead program: EGP - 437 (corticosteroid) delivered by system ▪ Licensed to Valeant Pharmaceuticals (Bausch + Lomb ) ▪ License Agreement for first indication (Uveitis) only ▪ Developing for other indications ▪ Macular Edema ▪ Post Cataract Surgery Inflammation ▪ Next Generation Delivery System: at home version

CONFIDENTIAL CONFIDENTIAL 3 Company Overview Ophthalmology: Drug Delivery Platform ▪ Drug: EGP - 437, a corticosteroid (Dexamethasone phosphate) ▪ First indication: non - infectious anterior uveitis ▪ 505(b)(2) NDA pathway ▪ Platform: EyeGate II ® Delivery System ▪ Proprietary, non - invasive delivery platform; >1,700 treatments performed to - date ▪ System expected to be approved through a 510(k) filing at time of drug NDA submission ▪ Easy to use: done by ophthalmologist or optometrist in <5 minutes ▪ Delivers small and large molecules to anterior or posterior of eye ▪ Significant patient and clinician advantages over drops or ocular injections

4 Unique Ophthalmic Delivery Platform

Ophthalmic Delivery Challenges Anterior Segment : Eye Drops Posterior Segment: Intravitreal Injections ▪ Protective layer and biological functions limit penetration of drug into tissues ▪ Frequent instillations required ▪ Extreme burden on patient: non - compliance ▪ S ight - threatening complications ▪ P otential for collateral damage ▪ Injections every 4 to 6 weeks ▪ Must be done by experienced ophthalmologist ▪ C ompanion required ▪ S ight - threatening complications 5
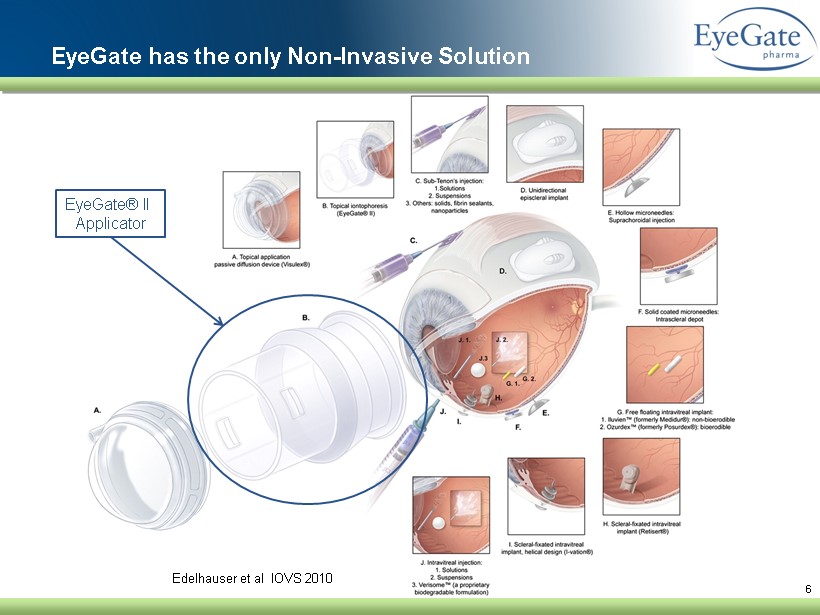
6 Edelhauser et al IOVS 2010 EyeGate has the only Non - Invasive Solution EyeGate® II Applicator
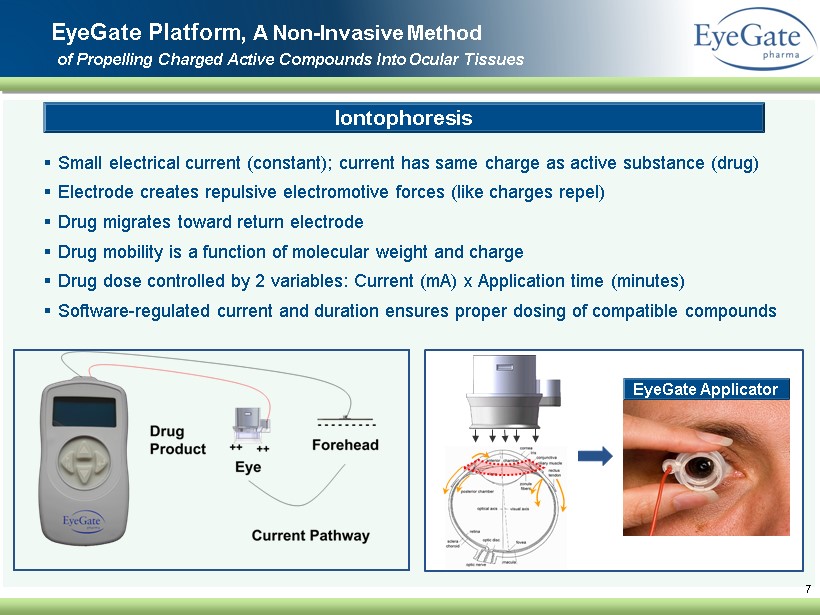
▪ Small electrical current (constant ); current has same charge as active substance (drug) ▪ Electrode creates repulsive electromotive forces (like charges repel) ▪ Drug migrates toward return electrode ▪ Drug mobility is a function of molecular weight and charge ▪ Drug dose controlled by 2 variables: Current (mA) x Application time (minutes ) ▪ Software - regulated current and duration ensures proper dosing of compatible compounds 7 EyeGate Platform, A Non - Invasive Method of Propelling Charged A ctive C ompounds I nto O cular Tissues Iontophoresis EyeGate Applicator
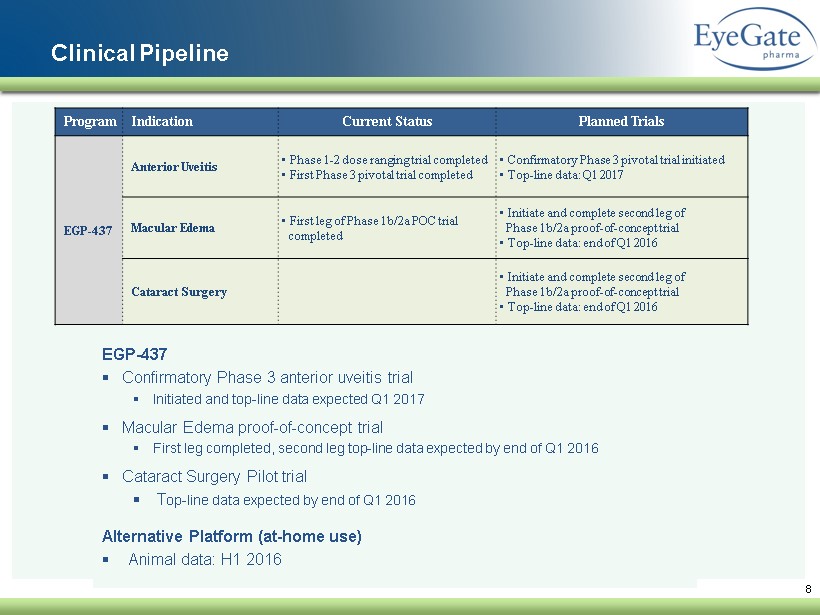
8 Clinical Pipeline EGP - 437 ▪ Confirmatory Phase 3 anterior uveitis trial ▪ Initiated and top - line data expected Q1 2017 ▪ Macular Edema proof - of - concept trial ▪ First leg completed, second leg top - line data expected by end of Q1 2016 ▪ Cataract Surgery Pilot trial ▪ T op - line data expected by end of Q1 2016 Alternative Platform (at - home use) ▪ Animal data: H1 2016 Program Indication Current Status Planned Trials EGP - 437 Anterior Uveitis • Phase 1 - 2 dose ranging trial completed • First Phase 3 pivotal trial completed • Confirmatory Phase 3 pivotal trial initiated • Top - line data: Q1 2017 Macular Edema • First leg of Phase 1b/2a POC trial completed • Initiate and complete second leg of Phase 1b/2a proof - of - concept trial • Top - line data: end of Q1 2016 Cataract Surgery • Initiate and complete second leg of Phase 1b/2a proof - of - concept trial • Top - line data: end of Q1 2016

9 EGP - 437 Anti - Inflammatory
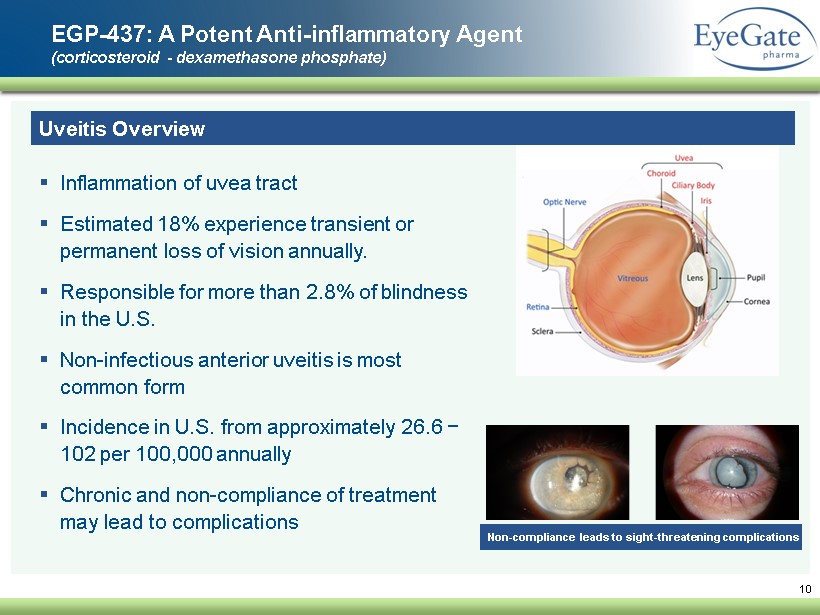
10 EGP - 437: A Potent A nti - inflammatory A gent (corticosteroid - dexamethasone phosphate) Uveitis Overview ▪ Inflammation of uvea tract ▪ E stimated 18% experience transient or permanent loss of vision annually. ▪ Responsible for more than 2.8% of blindness in the U.S. ▪ Non - infectious anterior uveitis is most common form ▪ Incidence in U.S . from approximately 26.6 − 102 per 100,000 annually ▪ Chronic and non - compliance of treatment may lead to complications Non - compliance leads to sight - threatening complications
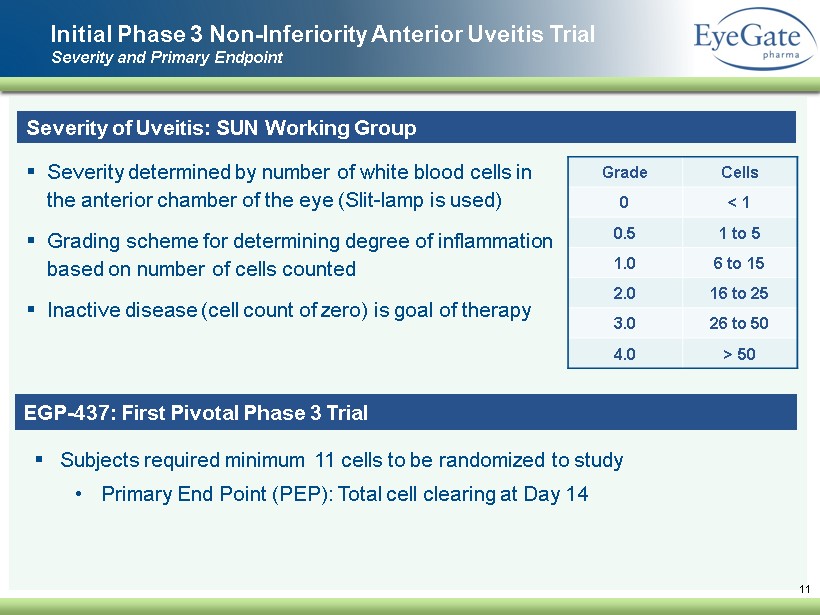
Initial Phase 3 Non - Inferiority A nterior U veitis Trial Severity and Primary Endpoint 11 Severity of Uveitis: SUN Working Group ▪ Severity determined by number of white blood cells in the anterior chamber of the eye (Slit - lamp is used) ▪ Grading scheme for determining degree of inflammation based on number of cells counted ▪ Inactive disease (cell count of zero) is goal of therapy Grade Cells 0 < 1 0.5 1 to 5 1.0 6 to 15 2.0 16 to 25 3.0 26 to 50 4.0 > 50 EGP - 437: First Pivotal Phase 3 Trial ▪ Subjects required minimum 11 cells to be randomized to study • Primary End Point (PEP): Total cell clearing at Day 14
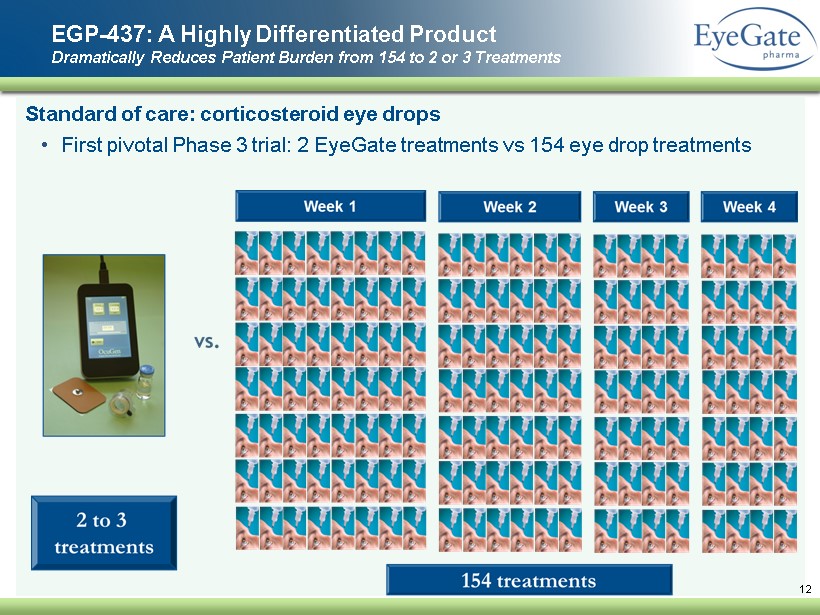
S tandard of care: corticosteroid eye drops • First pivotal Phase 3 trial: 2 EyeGate treatments vs 154 eye drop treatments EGP - 437: A Highly Differentiated Product Dramatically Reduces Patient Burden from 154 to 2 or 3 Treatments 12
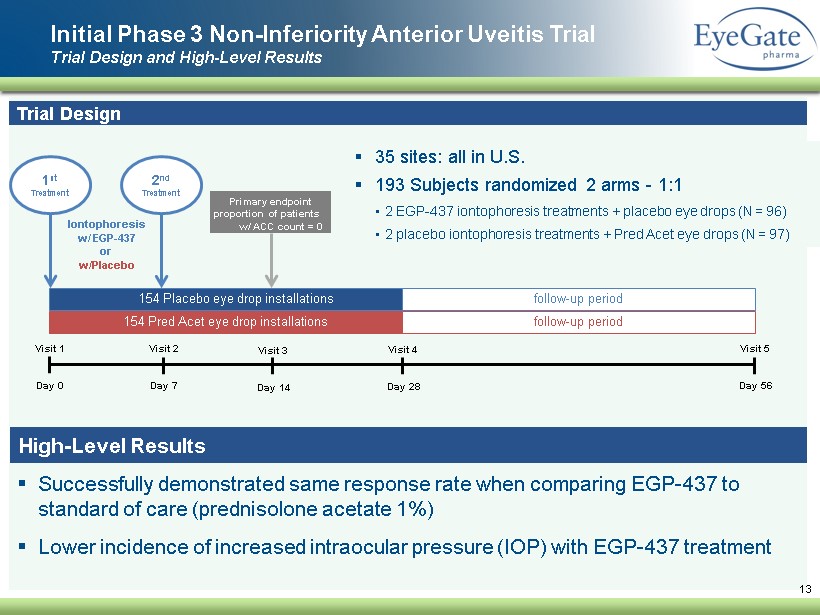
▪ Successfully demonstrated same response rate when comparing EGP - 437 to standard of care ( prednisolone acetate 1 %) ▪ Lower incidence of increased intraocular pressure (IOP ) with EGP - 437 treatment Initial Phase 3 Non - Inferiority Anterior Uveitis Trial Trial Design and High - Level Results 13 Trial Design ▪ 35 sites: all in U.S. ▪ 193 Subjects randomized 2 arms - 1:1 • 2 EGP - 437 iontophoresis treatments + placebo eye drops (N = 96) • 2 placebo iontophoresis treatments + Pred Acet eye drops (N = 97 ) Visit 1 Day 0 Visit 2 Day 7 Visit 3 Day 14 Visit 4 Day 28 Visit 5 Day 56 154 Pred Acet eye drop installations 154 Placebo eye drop installations vv follow - up period follow - up period 1 st Treatment Iontophoresis w/ EGP - 437 or w/Placebo Primary endpoint proportion of patients w/ ACC count = 0 2 nd Treatment High - Level Results
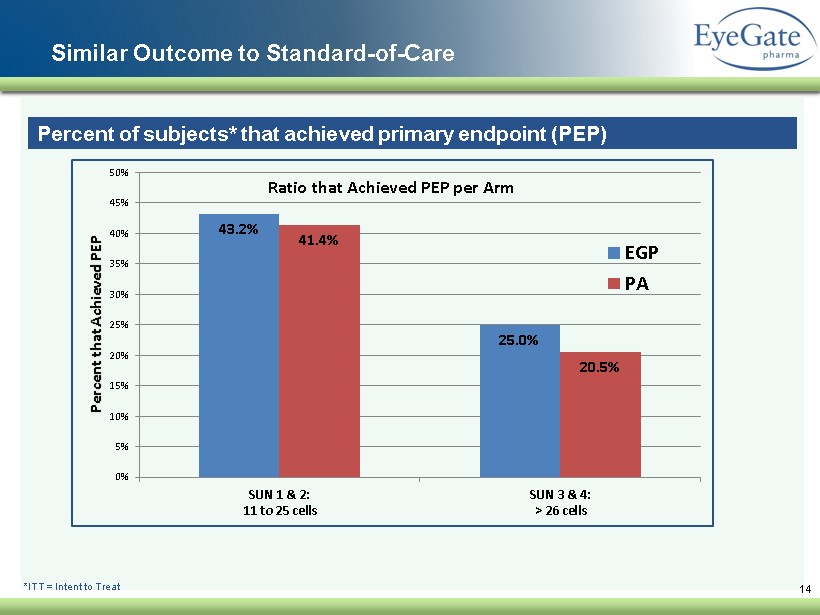
Similar Outcome to Standard - of - Care 14 Percent of subjects* that achieved primary endpoint (PEP) *ITT = Intent to Treat 43.2% 25.0% 41.4% 20.5% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% SUN 1 & 2: 11 to 25 cells SUN 3 & 4: > 26 cells Percent that Achieved PEP Ratio that Achieved PEP per Arm EGP PA
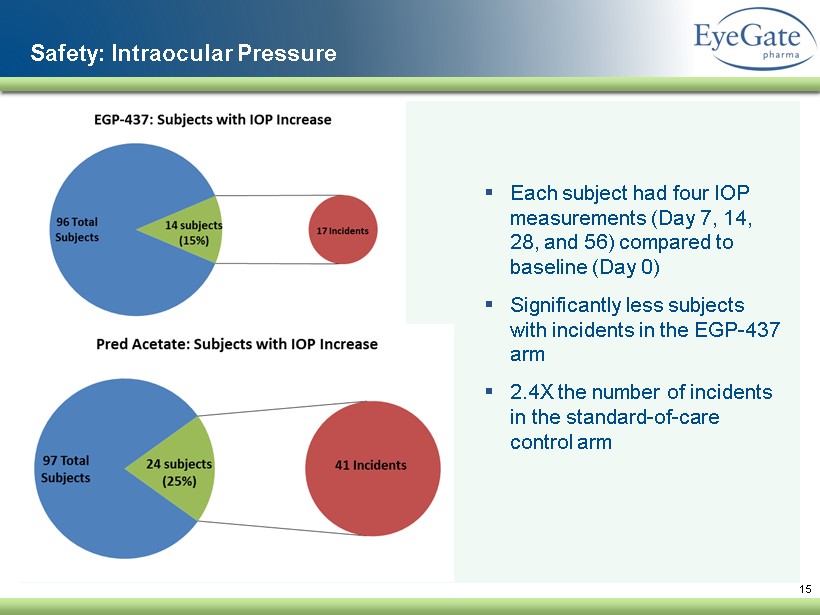
15 Safety: Intraocular Pressure ▪ Each subject had four IOP measurements (Day 7, 14, 28, and 56) compared to baseline (Day 0 ) ▪ Significantly less subjects with incidents in the EGP - 437 arm ▪ 2.4X the number of incidents in the standard - of - care control arm
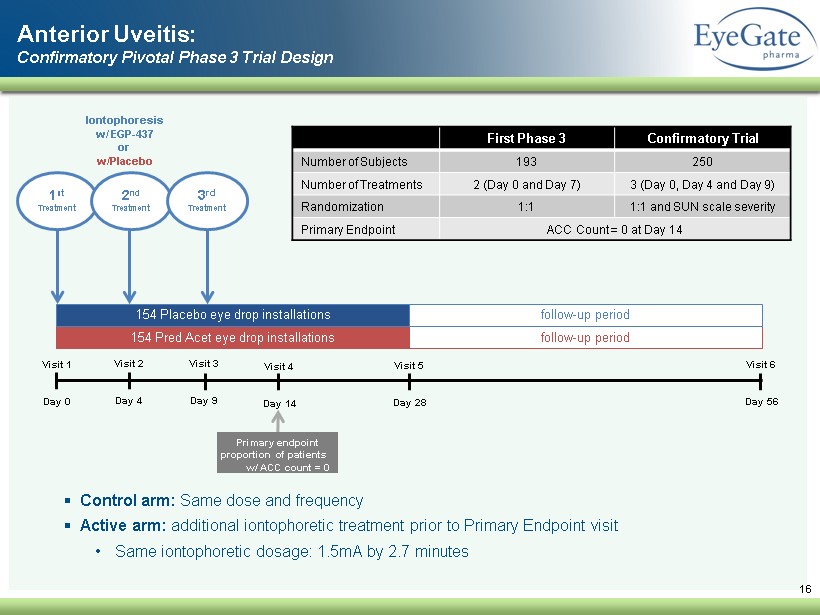
16 Visit 1 Day 0 Visit 3 Day 9 Visit 4 Day 14 Visit 5 Day 28 Visit 6 Day 56 154 Pred Acet eye drop installations 154 Placebo eye drop installations follow - up period follow - up period 1 st Treatment Iontophoresis w/ EGP - 437 or w/Placebo Primary endpoint proportion of patients w/ ACC count = 0 2 nd Treatment Anterior Uveitis: Confirmatory Pivotal Phase 3 Trial Design Visit 2 Day 4 3 rd Treatment ▪ Control arm: Same dose and frequency ▪ Active arm: additional iontophoretic treatment prior to Primary Endpoint visit • Same iontophoretic dosage: 1.5mA by 2.7 minutes First Phase 3 Confirmatory Trial Number of Subjects 193 250 Number of Treatments 2 (Day 0 and Day 7) 3 (Day 0, Day 4 and Day 9) Randomization 1:1 1:1 and SUN scale severity Primary Endpoint ACC Count = 0 at Day 14

▪ Abnormal thickening of macula associated with accumulation of excess fluid in extracellular space of neurosensory retina ▪ Considered leading cause of central vision loss in developed world ▪ Phase 1b / 2a clinical trial: First Leg ▪ Up to 20 patients with macular edema associated with Retinal Vein Occlusion, Diabetic Retinopathy or Post - Surgical (Cystoid) macular edema ▪ 3 treatments at 14.0 mA - min (3.5 mA) on Day 0, Day 4, and Day 9 ▪ Primary outcome: reduction in mean thickness on Day 4, Day 9, Day 14 ▪ Control: Ozurdex ® to subjects with no improvement at Day 14 and re - evaluated at Day 21 ▪ First Leg Completed 17 Macular Edema Trial Design
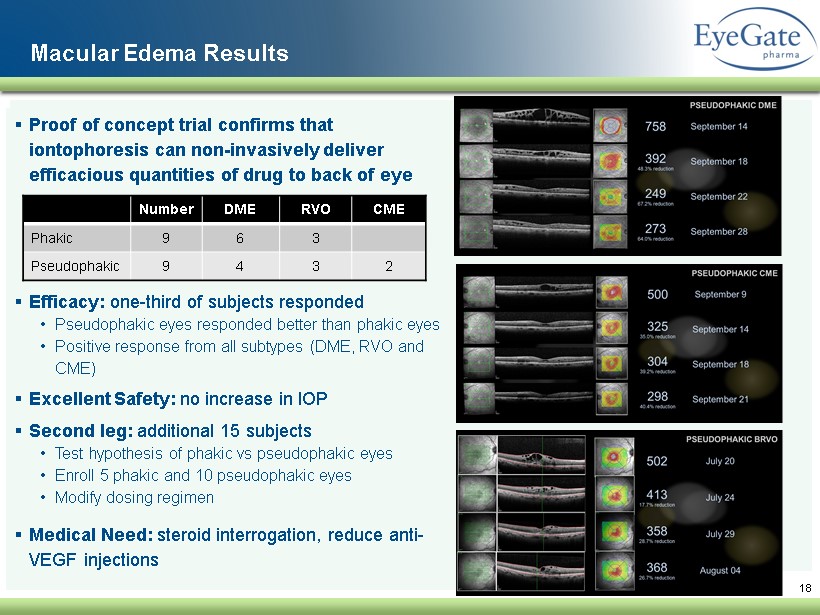
▪ Proof of concept trial confirms that iontophoresis can non - invasively deliver efficacious quantities of drug to back of eye ▪ Efficacy : one - third of subjects responded • Pseudophakic eyes responded better than phakic eyes • Positive response from all subtypes (DME, RVO and CME ) ▪ Excellent Safety: no increase in IOP ▪ Second leg: additional 15 subjects • Test hypothesis of phakic vs pseudophakic eyes • Enroll 5 phakic and 10 pseudophakic eyes • Modify dosing regimen ▪ Medical Need: steroid interrogation, reduce anti - VEGF injections 18 Macular Edema Results Number DME RVO CME Phakic 9 6 3 Pseudophakic 9 4 3 2

19 ▪ Valeant Pharmaceuticals – Bausch + Lomb (NYSE/TSX : VRX) ▪ Exclusive license to manufacture, sell, distribute and commercialize throughout the world for use in field of uveitis • Upfront cash payment and milestone payments • Royalties based on net sales : high single digits ▪ EyeGate responsible for completion of the development of anterior uveitis indication in U . S . ▪ Valeant responsible for development outside U . S . ▪ Valeant has right of last refusal for product outside field of uveitis • Must negotiate for access to additional indications ▪ EyeGate is developing EGP - 437 for additional indications ▪ Macular Edema ▪ Post Cataract Surgery Inflammation Licensing Agreement EG ® II Delivery System + EGP - 437

20 ▪ Combines drug vial and device disposables: • Ensures use of approved drug with applicator ▪ Shelf - life established at 24 months (drug and applicator) ▪ CPT Code: In addition to office reimbursement, reimbursement for performing treatment ▪ J - code: The kit (drug + disposables) will be billed under a J - code Payment that would be based on ASP ( price we establish) + x% for the kit Reimbursement Reimbursement: In - office treatment involves multiple code sets. Single - Use Kit
21 Strong Patent P ortfolio Ten families (73 patents granted) ▪ Eight belong to delivery system patent portfolio • 13 U.S. and 58 foreign patents granted • 3 U.S. and 16 foreign pending applications ▪ Two relate to drug compositions and treatments utilizing delivery system: • 1 U.S. and 1 foreign patent granted • 2 U.S. and 6 foreign applications * Granted patent protection until 2024, applications if granted extend this to 2029
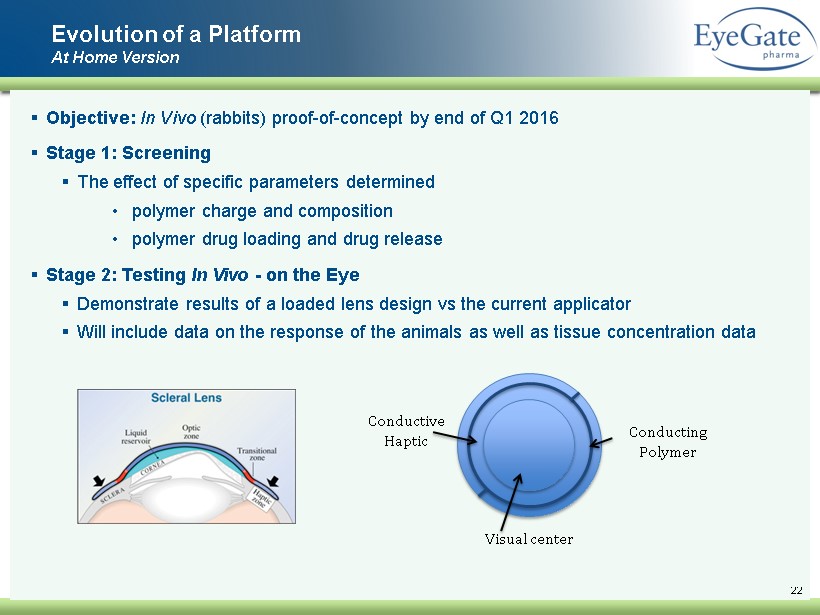
22 ▪ Objective: In Vivo (rabbits) proof - of - concept by end of Q1 2016 ▪ Stage 1: Screening ▪ The effect of specific parameters determined • polymer charge and composition • p olymer drug loading and drug release ▪ Stage 2: Testing In Vivo - on the Eye ▪ Demonstrate results of a loaded lens design vs the current applicator ▪ Will include data on the response of the animals as well as tissue concentration data Evolution of a Platform At Home Version Visual center Conductive Haptic Conducting Polymer

23 Investment Highlights ▪ Licensing deal signed with Valeant ▪ Exclusive, worldwide commercial and manufacturing rights for uveitis ▪ Upfront cash payment, milestone payments and royalties ▪ Phase 3 program with clear path to commercialization ▪ Confirmatory pivotal Phase 3 trial initiated ▪ Potent drug with proven safety profile when delivered by our system ▪ Mitigates corticosteroid side - effect, elevated IOP ▪ Macular Edema Trial Ongoing ▪ First clinical trial evaluating EyeGate® II delivery system in posterior of eye ▪ Alternative Platform Collaboration Initiated ▪ At - home non - invasive treatment for chronic diseases like macular degeneration
