Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIO-PATH HOLDINGS INC | v428946_8k.htm |
Exhibit 99.1

“A New Paradigm in Antisense Drug Delivery” Corporate Presentation January 2016

This Presentation contains forward - looking statements with respect to business conducted by Bio - Path Holdings, Inc . By their nature, forward - looking statements and forecasts involve risks and uncertainties because they relate to events and depend on circumstances that will occur in the future . The Company does not undertake to update any forward - looking statements . There are a number of factors that could cause actual results and developments to differ materially and investors should use their own judgment to evaluate risks . Forward Looking Statements 2

Overview □ About Bio - Path Holdings □ Antisense and DNAbilize ™ Technology □ BP1001 (Liposomal Grb2) in the clinic □ Future plans and milestones □ Financial snapshot 3

□ Oncology focused pharmaceutical development company located in Houston, TX □ Established in 2008 with technology licensed from the University of Texas MD Anderson Cancer Center □ Listed on NASDAQ in March 2014 as BPTH □ Breakthrough science and cancer drugs ▪ DNAbilize ™ Technology is our proprietary antisense and delivery system that solves the antisense industry dilemma ▪ Demonstrated ability to deliver antisense DNA into target cells and down - regulate the protein □ F irst drug targeted to Grb2, BP1001 , is in Phase I/II trials for AML; Ph + CML ▪ Received Orphan Drug Designation for AML from the FDA ▪ R esults of Phase I/II combination testing very encouraging □ T riple negative and inflammatory breast cancer in preclinical stage □ Second target to Bcl2 (BP1002) in preparation for a clinical trial for follicular lymphoma Bio - Path Holdings 4

» Solving the antisense drug delivery challenge with DNAbilize ™ Technology » World’s leading cancer institution, MD Anderson, is significant shareholder Broad Vision, Focused Strategy » Liposomal delivery technology distributes antisense drugs throughout the human body by simple intravenous infusion Novel Mechanism of Action » Ability to apply delivery technology template to almost any protein target and expand outside cancer » Numerous new drug candidates and partners identified for development Versatile Delivery Technology » Initial two drug candidates have market potential of $4+ billion » Expanding pipeline with potential for licensing Significant Market Opportunity Investment Highlights 5

Core Organization Peter Nielsen Co - Founder, President, Chief Executive Officer and Chief Financial Officer • Officer and D irector since founding Company in 2007 Ulrich Mueller, PhD Chief Operating Officer • P reviously Vice President at the Fred Hutchinson Cancer Research Center • Former Managing Director Office of Technology Commercialization at MD Anderson Ana M. Tari, PhD & MBA Director, Preclinical Operations & Research • Key member of the research team that developed our liposomal delivery technology Tara Sadeghi, PhD Director, Clinical Operations • Over 24 years of drug development and clinical operations experience across all phases of clinical development (Phases I through III ) Suzanne Kennedy, PhD Director, Corporate Development • Over 15 years of marketing, business development, and research & development experience in the biotech industry Focus • Clinical team added to manage clinical trials and place new candidates into an IND, clinical trial • Expanding preclinical research and manufacturing capabilities 6

Jorge Cortes, M.D . Chairman • M.D . from la Facultad de Medicina , Universidad Nacional Autónoma de México • Jane and John Justin Distinguished Chair in Leukemia Research, Chief of the AML and CML sections , and Deputy Chair of the Department of Leukemia at The University of Texas MDACC • Has consulted leading pharmaceutical companies such as AstraZeneca on development of prenyltransferase inhibitors, GlaxoSmithKline on the use of topotecan in MDS and CMML, and Rhône - Poulenc Rorer on the use of PEG - Asparaginase in adult ALL. Amy P. Sing, M.D. Member, Bio - Path’s Board of Directors • M.D. from the Stanford University School of Medicine • Currently Senior Director of Medical Affairs at Genomic Health, Inc. • Former Senior Medical Director at Genentech, Inc., had an integral role in the Avastin ™ program • Former Senior Director of Medical and Regulatory Affairs at Seattle Genetics Recruiting additional members Scientific Advisory Board 7
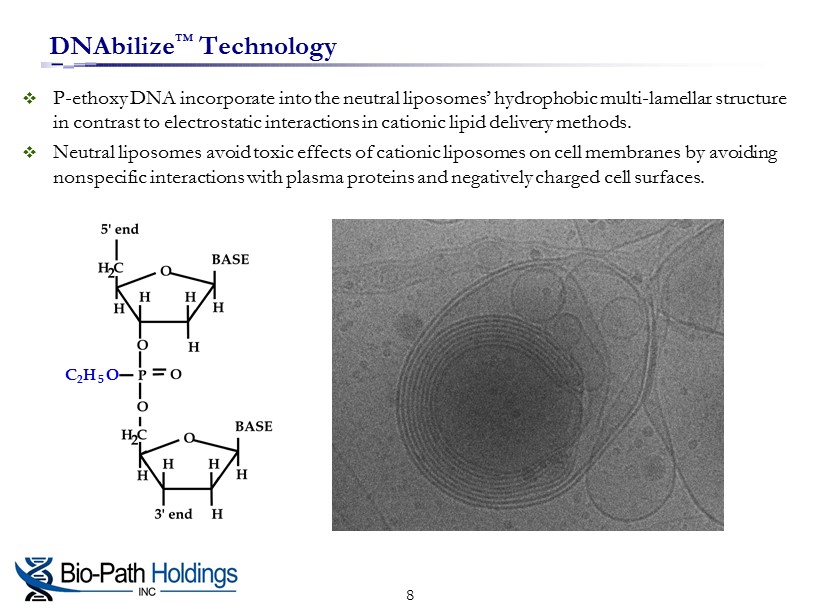
DNAbilize ™ Technology □ P - ethoxy DNA incorporate into the neutral liposomes’ hydrophobic multi - lamellar structure in contrast to electrostatic interactions in cationic lipid delivery methods. □ Neutral liposomes avoid toxic effects of cationic liposomes on cell membranes by avoiding nonspecific interactions with plasma proteins and negatively charged cell surfaces. C 2 H 5 O 8

DNAbilize ™ Antisense DNA: A Targeted Method for Treating Disease □ Antisense - molecules that interfere with the process of producing proteins inside cells (RNAi) » Does not use a toxic agent to kill cells, but instead blocks production of proteins » Advantage of specificity because it targets the disease - causing protein □ No toxicity - In numerous animal studies or human patients in BP1001 clinical trial » DNAbilize ™ liposome structure is similar to the cellular membrane » P - ethoxy DNA does not activate complement or inhibit the clotting cascade □ Systemic treatment - I.V. delivery to the main organs via blood flow □ High cellular uptake - liposome structure is similar to the cellular membrane □ Microscopic - sized liposomes - enable penetration into tumors for delivery of drug □ Proven target inhibition - demonstrated that DNAbilize ™ method inhibits target protein, proving delivery technology works 9

BP1001 in Clinical Testing □ Grb2 is a protein that bridges activated tyrosine kinases to the Ras signaling pathway □ Phase I clinical trial: » First 6 cohorts completed » P reliminary results show drug has been well tolerated in patients with AML, CML, and MDS with no signs of toxicity and signs of anti - leukemia activity □ Safety segment of P hase II (Phase Ib ) trial for Acute Myeloid Leukemia (AML) in combination with frontline therapy ongoing » Phase II efficacy trial planned to start early 2016 » Phase II trial in Chronic Myelogenous Leukemia (CML) planned □ Additional indications in triple negative and inflammatory breast cancers being developed and in other solid tumors 10

□ AML, CML, ALL & MDS Patients Refractory or Resistant to Current Therapies □ Dose escalating, treatment cycle 8 doses over 4 weeks Results through cohort 6 ( 90 mg/m 2 ) » Patients averaged 6 prior therapies » 15 of 20 evaluable patients’ blasts demonstrated anti - leukemia activity » 8 patients stabilized for extended treatments » 2 patients (010 and 014) transient improvement leukemia cutis lesions » D rug was well - tolerated □ Of the 18 evaluable with circulating blasts, 83% had a response to the drug □ Cohort 7 (Phase II safety segment) receiving LDAC + 60 mg/m 2 BP1001, 2 patients, 035 and 038 achieved complete remission . Summary of Phase I Monotherapy and Ib Combination Therapy Clinical Trial Results for BP1001 Complete remission Baseline Nadir Off-Tx 01 CML 93 82 97 <1 06 AML 15 2 5 5 07 MDS 8* 4* 6* 5 010** AML 23* 10* 10* 1 011 CML 24 7 50 1 014** AML 33 5 21 1 015 AML 51 31 72 1 020 AML 76 5 23 1 021 AML 71 43 74 2 022 AML 1 0 2 2 023 MDS NE* NE* NE* 1*** 024 MDS 0* 0* 2* 5 025 AML 10 3 19 2 026 AML 16 none 80 1 027 AML 93 92 97 1 028 AML 96 none 98 1 029 AML 33 7 27 1 030 AML 51 17 84 1 031 AML 17 NE 17 1 032 AML 24 NE 40 2 034 AML 66 ND ND 1 035 AML 17 2 ND 1 037 AML 25 25 ND 1 038 AML 23 2 ongoing 4 Peripheral or bone marrow (BM)* Blast % Cycles Completed Patients Diagnosis Cohort 7

□ Patient 002 : 32 year - old, Hispanic male with myeloid blast crisis of CML □ Prior therapies consist of: ― Gleevec ― Dasatinib ― Nilotinib ― DCC - 2036 ― Cytarabine/Fludarabine/ ― Dasatinib/Gemtuzumab ― PHA - 739358 ― Clofarabine/Dasatinib □ Patient taken off therapy, CNS disease was assumed (not confirmed) and intrathecal Ara - C was administered - patient succumbed due to disease progression prior to going back on treatment Response to Treatment for Patients 002 and 006 12 □ Extended Treatment: Patient 006 □ 54 year - old HIV+ male with AML transformed from Jak2 positive Polycythemia Vera □ 3 patients showed improvement and/or stable disease, received 5 treatment cycles over 5 months □ Patient 006 achieved stable disease and marked reduction in peripheral blasts

% Decrease of Target Grb2 Protein and pErk protein » Grb2 levels decreased 50% in 11 of 13 samples by end of treatment (EOT) » pErk levels decreased an average of 52% in 7 of 13 samples by EOT. NS 1 = no sample collected 2 Fewer cells used in analysis because sample had less cells

Results of Phase II Cohort 7 Combination LDAC + BP1001 Therapy □ S afety evaluation of combination l ow - dose cytarabine (LDAC) with BP1001 in refractory and relapsed patients □ Three (3) evaluable patients were treated twice a week for 4 weeks with 60 mg/m 2 of BP1001 and LDAC □ Total of 8 doses in combination with the standard regimen of LDAC » Two out of three patients achieved complete remission □ Cohort 8 patients being treated with 90 mg/m 2 of BP1001 in combination with frontline LDAC is ongoing Results were consistent with previous cohorts, showing BP1001 to be safe, well tolerated with significant anti - leukemia activity 14
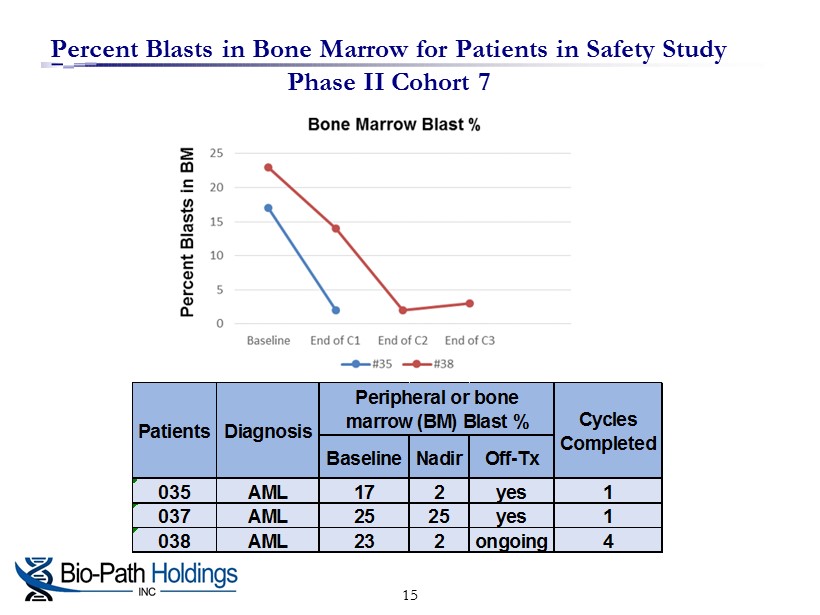
Percent Blasts in Bone Marrow for Patients in Safety Study Phase II Cohort 7 15 Baseline Nadir Off-Tx 035 AML 17 2 yes 1 037 AML 25 25 yes 1 038 AML 23 2 ongoing 4 Peripheral or bone marrow (BM) Blast % Cycles Completed Patients Diagnosis

Plans For Phase II Clinical Trials of BP1001 □ The proposed clinical program is to evaluate BP1001 in AML in a Phase II clinical trial in combination with frontline therapy □ Currently completing the safety segment of the Phase II (Phase Ib) trial evaluating 2 cohorts at 2 dose levels, 3 patients per cohort, to test for any potential negative synergies of using BP1001 together with frontline therapy □ Phase II efficacy trial: » Planning to have approximately 54 patients with an interim analysis after 19 patients » If successful, the trial will be rolled into a pivotal trial for accelerated approval » Expected to be conducted at leading cancer centers in the US, including the MD Anderson Cancer Center » Primary endpoint for the study is the number of patients who achieve complete remission » Phase II trial in CML starting in Q1 of 2016 16

Recent Accomplishments □ Phase I monotherapy clinical trial completed through six cohorts with very promising results » 34 patients enrolled » Well tolerated drug with no toxicity » 18 evaluable patients with circulating blasts, 83% had a response » 8 of 21 evaluable patients stabilized for additional treatment □ BP1001 inhibits disease - causing protein in human patients with blood cancers □ BP1001 being developed for triple negative and inflammatory breast cancers □ Significant corporate development in 2015: » Phase II safety segment (Phase Ib) dosing for the combination therapy for BP1001 near completion with patients achieving complete remission » IND package for BP1002 (Liposomal Bcl2) in preparation to begin a clinical trial » Promising new targets in preclinical development » $25 million ATM financing in place 17

Upcoming Milestones □ Completion of safety segment of Phase II (Phase Ib) clinical trial □ Continued pre - clinical evaluation of BP1001 for inflammatory and triple negative breast cancer and if successful, rapid deployment into a Phase I clinical trial □ Exciting new drug candidates coming from our business development team » Expanding pipeline with new target drug candidates (lymphoma, pancreatic, brain) » New drug indication outside of cancer □ Value propositions for 2016: » Enrollment of first 19 patients in Phase II with an interim analysis to be completed with potential for switch to registration trial for accelerated approval » Initiation of safety segment for Phase II (Phase Ib) in CML for combination therapy » Demonstrated effectiveness of delivery technology (broad drug development, licensing implications) » Expanding pipeline and collaborations on new and creative drug candidates » Continued new manufacturing and target IP 18
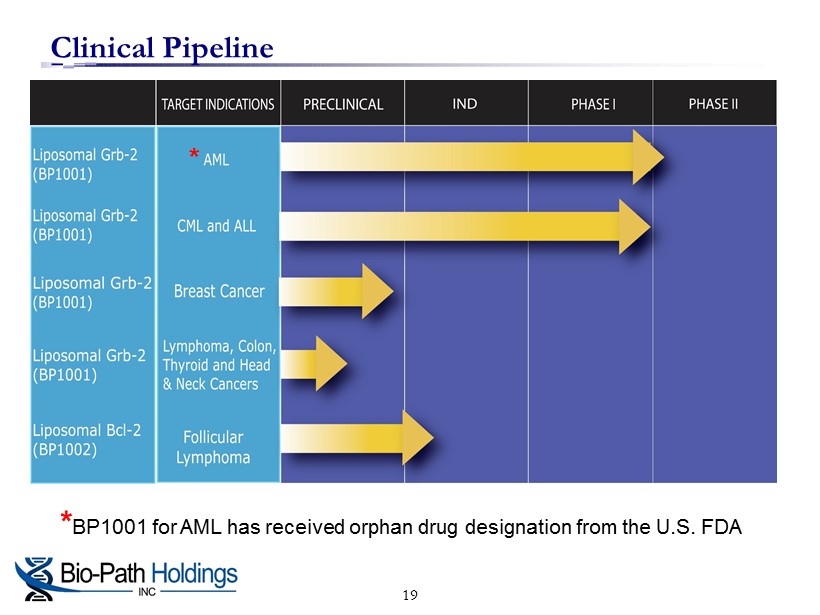
Clinical Pipeline * BP1001 for AML has received orphan drug designation from the U.S. FDA * 19

Ticker: NASDAQ: BPTH Shares: 89.8M shares outstanding Sept 30, 2015 Market Cap: approximately $130 MM Capital Raised: $30.6 MM Cash: $9.9 MM as of Sept 30, 2015 Burn rate: approximately $1,000,000 per quarter excluding the clinical trial costs Financing: $25 million ATM program in place Financial Snapshot 20
