Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Anacor Pharmaceuticals, Inc. | a16-1587_18k.htm |
| EX-99.2 - EX-99.2 - Anacor Pharmaceuticals, Inc. | a16-1587_1ex99d2.htm |
Exhibit 99.1
Investor Presentation January 2016

Cautionary Statements This presentation contains forward-looking statements, including statements regarding the results of our clinical trials, the safety and efficacy of our approved product and product development candidates, the timing of the approval of our product development candidates, the commercial success of KERYDIN and the timing and potential commercial success of our product development candidates. These statements constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. The words “may,” “might,” “will,” “should,” “estimate,” “project,” “plan,” “anticipate,” “expect,” “intend,” “outlook,” “believe” and other similar expressions are intended to identify forward-looking statements. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this presentation, and we undertake no obligation to update any forward-looking statement except as required by law. These forward-looking statements are based on estimates and assumptions by our management that, although believed to be reasonable, are inherently uncertain and subject to a number of risks and uncertainties. The following represent some, but not necessarily all, of the factors that could cause actual results to differ from historical results or those anticipated or predicted by our forward-looking statements: the successful commercialization of KERYDIN pursuant to our distribution and commercialization agreement with Sandoz Inc.; any issues, delays or failures arising as a result of our studies relating to crisaborole; the outcome, timing and cost of regulatory approvals, and content of approved labeling for our products, including any delay or failure by the U.S. Food and Drug Administration to approve crisaborole; our ability to timely and successfully launch, either alone or with a partner, crisaborole, if approved; the impact of general economic, industry, market or political conditions; and the other risks and uncertainties identified in our periodic filings, including our Annual Report on Form 10-K for the year ended December 31, 2014 and Quarterly Report on Form 10-Q for the quarter ended September 30, 2015. This presentation is intended for investor purposes only, and is not intended for promotional purposes.

A Biopharmaceutical Company Focused on Discovering, Developing, and Commercializing Novel Small Molecule Therapeutics Using Our Boron Chemistry Platform ®

Lead Assets * National Eczema Association and Decision Resources (2008) KERYDIN® Approved in July 2014 for the topical treatment of onychomycosis of the toenails Launched in the U.S. in September 2014 by PharmaDerm, the branded dermatology division of Sandoz, a Novartis Company 50% gross profit split $130M in total minimum gross profit split payments for 2016 and 2017 (i.e., $65M for 2016 and $65M for 2017) Crisaborole Topical Ointment, 2% (formerly AN2728) Target Product Profile – novel, non-steroidal, topical, anti-inflammatory PDE-4 inhibitor for the first-line treatment of mild-to-moderate atopic dermatitis NDA submitted January 2016 Market opportunity Atopic dermatitis affects ~18M-25M people in the U.S., including 8%-18% of children*
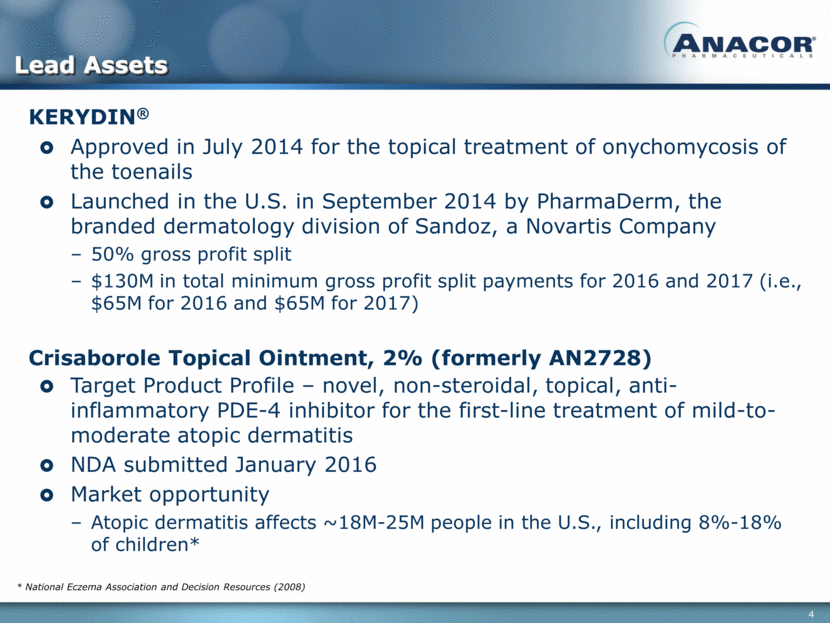
Pipeline of Key Internally Discovered Novel Boron-Based Compounds Discovery Research Phase 1 Phase 2 Phase 3 Commercial KERYDIN® (Onychomycosis of the toenails) Crisaborole (Mild-to-moderate atopic dermatitis) Crisaborole (Psoriasis) Next generation PDE-4 inhibitor Cytokine suppression program (related to inflammatory skin conditions) AN3365 AN5568(1) (HAT) Chagas Disease(2) Anti-fungal (topical) NDA submitted January 2016 Anti-inflammatories (topical) Approved July 2014 Gram-negative antibiotic (IV) Anti-parasitics (Oral) Pivotal Ph 2/3 to be initiated in 2016 DNDi responsible for clinical development Research funding provided by Wellcome Trust
[LOGO]
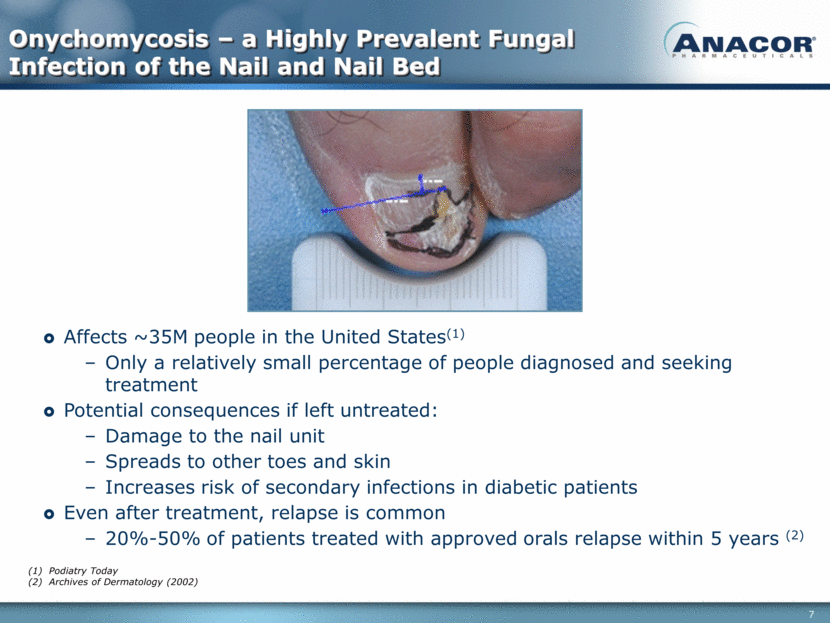
Onychomycosis – a Highly Prevalent Fungal Infection of the Nail and Nail Bed Affects ~35M people in the United States(1) Only a relatively small percentage of people diagnosed and seeking treatment Potential consequences if left untreated: Damage to the nail unit Spreads to other toes and skin Increases risk of secondary infections in diabetic patients Even after treatment, relapse is common 20%-50% of patients treated with approved orals relapse within 5 years (2) Podiatry Today Archives of Dermatology (2002)
PharmaDerm Established in topical branded dermatology market Experienced field force targets key potential KERYDIN prescribers Branded prescription product portfolio KERYDIN placed in the P1 sales position Summary of certain key financial terms Payments to Anacor $40M upfront in 3Q14 50% gross profit split $130M in total minimum gross profit split payments for 2016 and 2017 (i.e., $65M for 2016 and $65M for 2017) Increased commercial investment in KERYDIN in 2015 Included Sandoz expanded field force and a new multi-channel integrated marketing campaign Anacor has option to repurchase KERYDIN at the end of 2017 KERYDIN is Marketed and Distributed by PharmaDerm, the Branded Dermatology Division of Sandoz, a Novartis Company

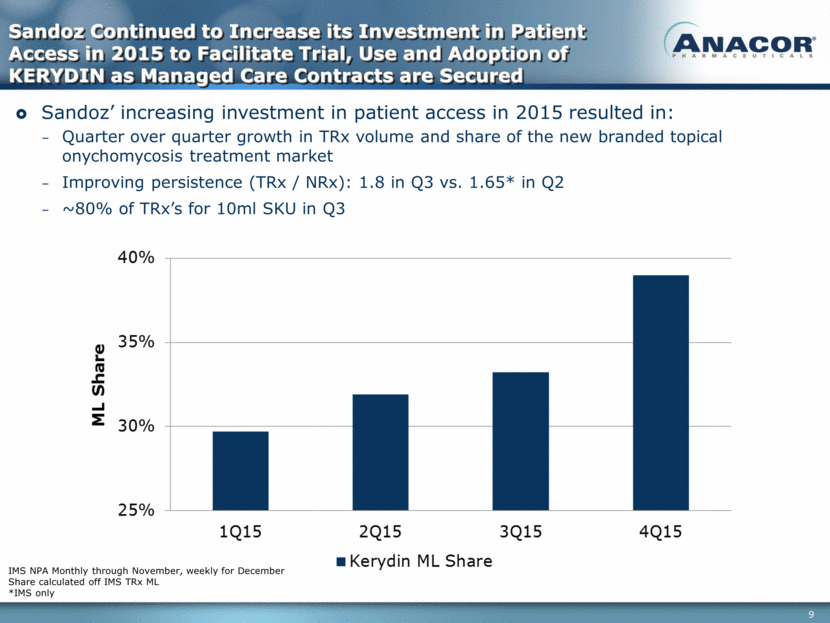
Sandoz Continued to Increase its Investment in Patient Access in 2015 to Facilitate Trial, Use and Adoption of KERYDIN as Managed Care Contracts are Secured IMS NPA Monthly through November, weekly for December Share calculated off IMS TRx ML *IMS only Sandoz’ increasing investment in patient access in 2015 resulted in: Quarter over quarter growth in TRx volume and share of the new branded topical onychomycosis treatment market Improving persistence (TRx / NRx): 1.8 in Q3 vs. 1.65* in Q2 ~80% of TRx’s for 10ml SKU in Q3
Sandoz Invested in Consumer Promotion and Access to Drive Prescription Volume New Interactive Website In-Office Advertisements & Education Materials Consumer Savings Program Search Engine Optimization Search Intercept Campaign

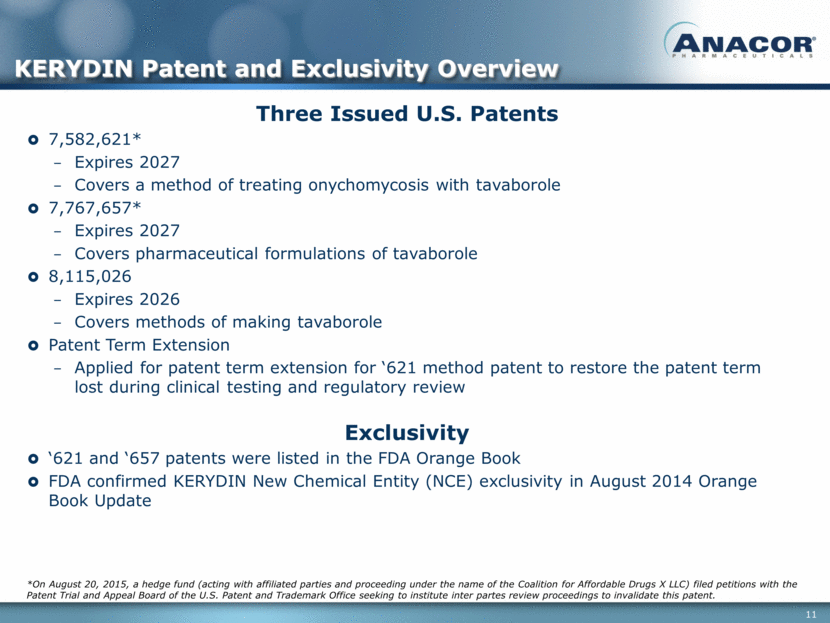
Three Issued U.S. Patents 7,582,621* Expires 2027 Covers a method of treating onychomycosis with tavaborole 7,767,657* Expires 2027 Covers pharmaceutical formulations of tavaborole 8,115,026 Expires 2026 Covers methods of making tavaborole Patent Term Extension Applied for patent term extension for ‘621 method patent to restore the patent term lost during clinical testing and regulatory review KERYDIN Patent and Exclusivity Overview Exclusivity ‘621 and ‘657 patents were listed in the FDA Orange Book FDA confirmed KERYDIN New Chemical Entity (NCE) exclusivity in August 2014 Orange Book Update *On August 20, 2015, a hedge fund (acting with affiliated parties and proceeding under the name of the Coalition for Affordable Drugs X LLC) filed petitions with the Patent Trial and Appeal Board of the U.S. Patent and Trademark Office seeking to institute inter partes review proceedings to invalidate this patent.
Crisaborole Topical Ointment, 2%

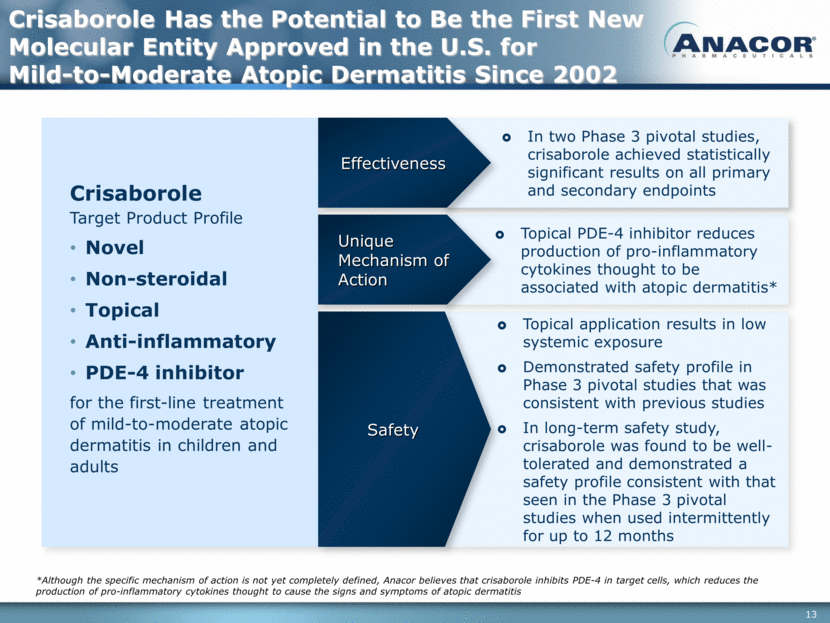
Topical application results in low systemic exposure Demonstrated safety profile in Phase 3 pivotal studies that was consistent with previous studies In long-term safety study, crisaborole was found to be well-tolerated and demonstrated a safety profile consistent with that seen in the Phase 3 pivotal studies when used intermittently for up to 12 months Safety Topical PDE-4 inhibitor reduces production of pro-inflammatory cytokines thought to be associated with atopic dermatitis* In two Phase 3 pivotal studies, crisaborole achieved statistically significant results on all primary and secondary endpoints Crisaborole Target Product Profile Novel Non-steroidal Topical Anti-inflammatory PDE-4 inhibitor for the first-line treatment of mild-to-moderate atopic dermatitis in children and adults Crisaborole Has the Potential to Be the First New Molecular Entity Approved in the U.S. for Mild-to-Moderate Atopic Dermatitis Since 2002 Unique Mechanism of Action Effectiveness *Although the specific mechanism of action is not yet completely defined, Anacor believes that crisaborole inhibits PDE-4 in target cells, which reduces the production of pro-inflammatory cytokines thought to cause the signs and symptoms of atopic dermatitis
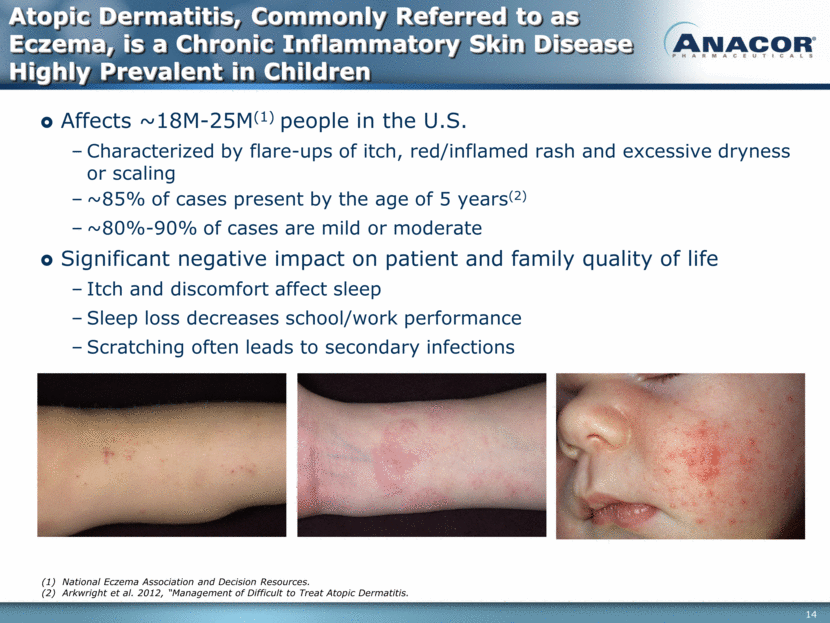
Atopic Dermatitis, Commonly Referred to as Eczema, is a Chronic Inflammatory Skin Disease Highly Prevalent in Children Affects ~18M-25M(1) people in the U.S. Characterized by flare-ups of itch, red/inflamed rash and excessive dryness or scaling ~85% of cases present by the age of 5 years(2) ~80%-90% of cases are mild or moderate Significant negative impact on patient and family quality of life Itch and discomfort affect sleep Sleep loss decreases school/work performance Scratching often leads to secondary infections National Eczema Association and Decision Resources. Arkwright et al. 2012, “Management of Difficult to Treat Atopic Dermatitis.
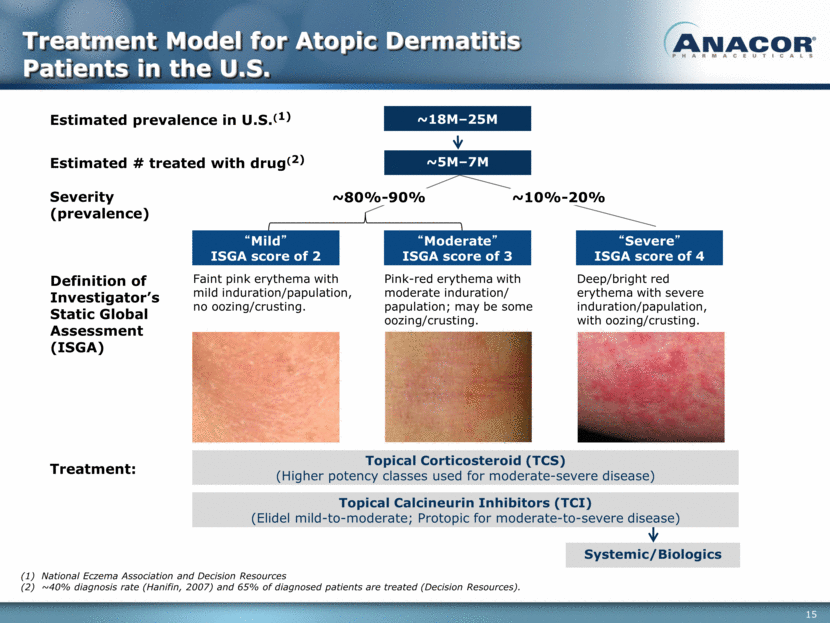
Treatment Model for Atopic Dermatitis Patients in the U.S. Systemic/Biologics “Severe” ISGA score of 4 “Moderate” ISGA score of 3 “Mild” ISGA score of 2 Topical Calcineurin Inhibitors (TCI) (Elidel mild-to-moderate; Protopic for moderate-to-severe disease) Severity (prevalence) Treatment: Topical Corticosteroid (TCS) (Higher potency classes used for moderate-severe disease) ~18M–25M Estimated prevalence in U.S.(1) Definition of Investigator’s Static Global Assessment (ISGA) Faint pink erythema with mild induration/papulation, no oozing/crusting. Pink-red erythema with moderate induration/ papulation; may be some oozing/crusting. Deep/bright red erythema with severe induration/papulation, with oozing/crusting. ~5M–7M Estimated # treated with drug(2) ~80%-90% ~10%-20% National Eczema Association and Decision Resources ~40% diagnosis rate (Hanifin, 2007) and 65% of diagnosed patients are treated (Decision Resources).
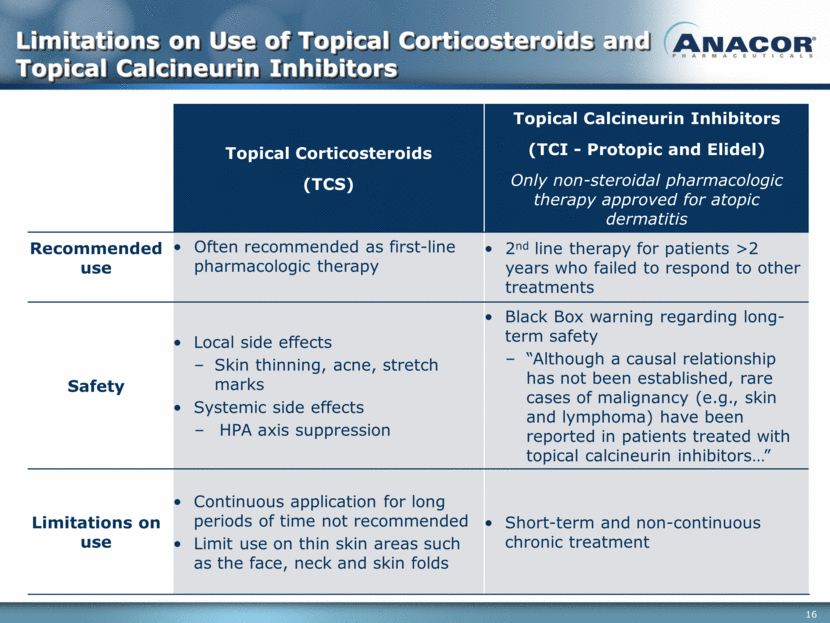
Limitations on Use of Topical Corticosteroids and Topical Calcineurin Inhibitors Topical Corticosteroids (TCS) Topical Calcineurin Inhibitors (TCI - Protopic and Elidel) Only non-steroidal pharmacologic therapy approved for atopic dermatitis Recommended use Often recommended as first-line pharmacologic therapy 2nd line therapy for patients >2 years who failed to respond to other treatments Safety Local side effects Skin thinning, acne, stretch marks Systemic side effects HPA axis suppression Black Box warning regarding long-term safety “Although a causal relationship has not been established, rare cases of malignancy (e.g., skin and lymphoma) have been reported in patients treated with topical calcineurin inhibitors ” Limitations on use Continuous application for long periods of time not recommended Limit use on thin skin areas such as the face, neck and skin folds Short-term and non-continuous chronic treatment
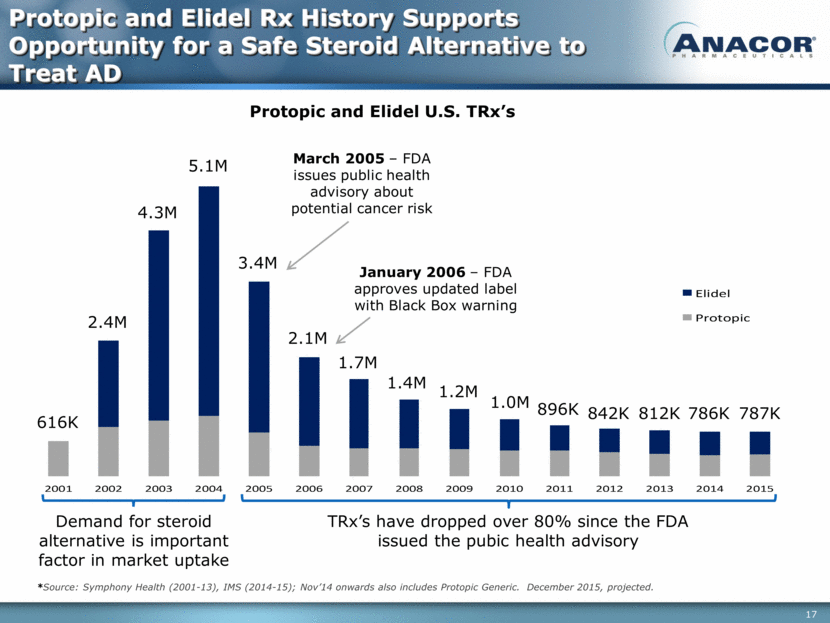
Protopic and Elidel U.S. TRx’s Demand for steroid alternative is important factor in market uptake TRx’s have dropped over 80% since the FDA issued the pubic health advisory March 2005 – FDA issues public health advisory about potential cancer risk 616K 2.4M 4.3M 5.1M 3.4M 2.1M 1.7M 1.4M 1.2M 1.0M 896K 842K 812K January 2006 – FDA approves updated label with Black Box warning 786K 787K Protopic and Elidel Rx History Supports Opportunity for a Safe Steroid Alternative to Treat AD *Source: Symphony Health (2001-13), IMS (2014-15); Nov’14 onwards also includes Protopic Generic. December 2015, projected. 2 0 0 1 2 0 0 2 2 0 0 3 2 0 0 4 2 0 0 5 2 0 0 6 2 0 0 7 2 0 0 8 2 0 0 9 2 0 1 0 2 0 1 1 2 0 1 2 2 0 1 3 2 0 1 4 2 0 1 5 E l i d e l P r o t o p i c
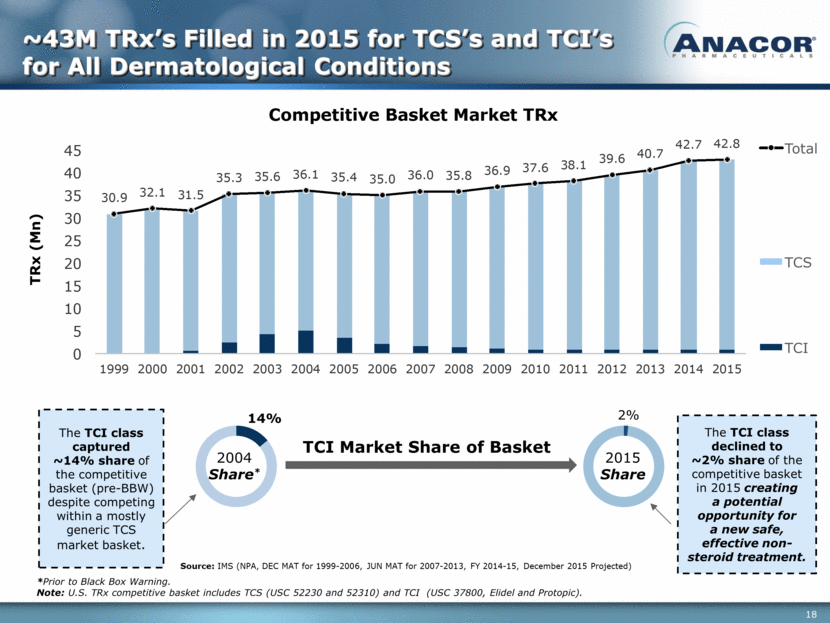
TCI Market Share of Basket TCI TCS Total TRx (Mn) 14% 2% 2004 Share* *Prior to Black Box Warning. Note: U.S. TRx competitive basket includes TCS (USC 52230 and 52310) and TCI (USC 37800, Elidel and Protopic). Source: IMS (NPA, DEC MAT for 1999-2006, JUN MAT for 2007-2013, FY 2014-15, December 2015 Projected) The TCI class captured ~14% share of the competitive basket (pre-BBW) despite competing within a mostly generic TCS market basket. 2015 Share The TCI class declined to ~2% share of the competitive basket in 2015 creating a potential opportunity for a new safe, effective non-steroid treatment. ~43M TRx’s Filled in 2015 for TCS’s and TCI’s for All Dermatological Conditions 30.9 32.1 31.5 35.3 35.6 36.1 35.4 35.0 36.0 35.8 36.9 37.6 38.1 39.6 40.7 42.7 42.8 0 5 10 15 20 25 30 35 40 45 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 C o m p e t i t i v e B as k e t M ar k e t T R x
Extensive research has been conducted resulting in validation of crisaborole’s commercial potential Analyzed over 15 years of data on historical prescription trends Performed comprehensive qualitative, quantitative and buying process research with physicians, patients and payers Consistent positive feedback on Target Product Profile among all stakeholders Physicians surveyed indicated they would treat ~30% - 60% of atopic dermatitis patients with a product meeting the target product profile Market research findings suggest high promotional sensitivity With pricing and contracting, a product meeting the target product profile is anticipated to receive favorable market access from payers Market Research Suggests Significant Market Opportunity Based on Target Product Profile Note: All Anacor market research was conducted on a double-blinded basis by an independent third party hired by Anacor.

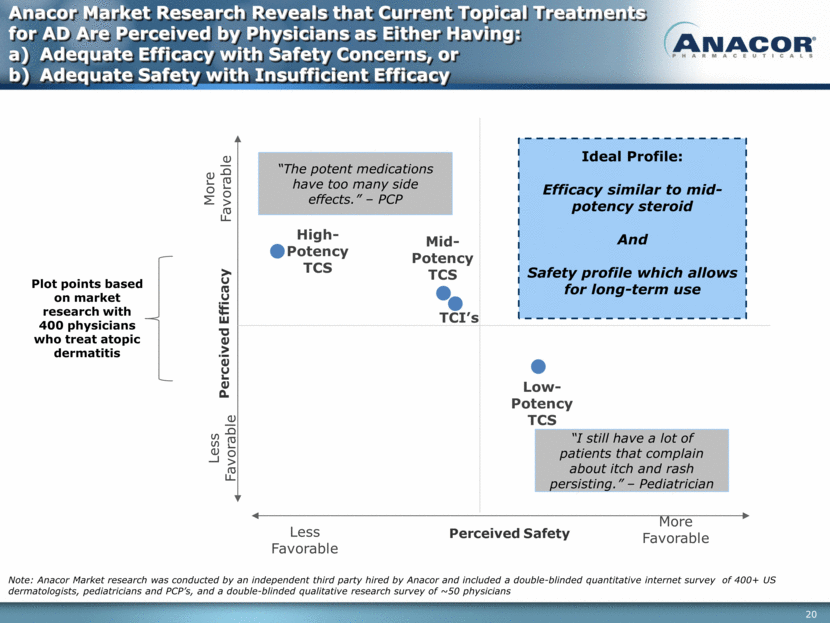
Anacor Market Research Reveals that Current Topical Treatments for AD Are Perceived by Physicians as Either Having: Adequate Efficacy with Safety Concerns, or Adequate Safety with Insufficient Efficacy Note: Anacor Market research was conducted by an independent third party hired by Anacor and included a double-blinded quantitative internet survey of 400+ US dermatologists, pediatricians and PCP’s, and a double-blinded qualitative research survey of ~50 physicians Low-Potency TCS Mid-Potency TCS TCI’s High-Potency TCS More Favorable Less Favorable Less Favorable More Favorable Plot points based on market research with 400 physicians who treat atopic dermatitis Ideal Profile: Efficacy similar to mid-potency steroid And Safety profile which allows for long-term use “I still have a lot of patients that complain about itch and rash persisting.” – Pediatrician “The potent medications have too many side effects.” – PCP Perceived Efficacy Perceived Safety
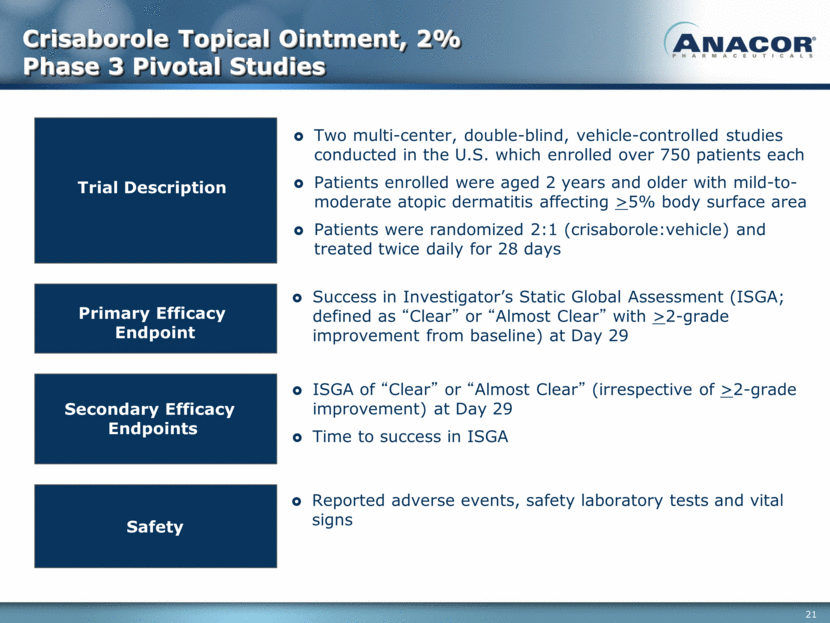
Crisaborole Topical Ointment, 2% Phase 3 Pivotal Studies Success in Investigator’s Static Global Assessment (ISGA; defined as “Clear” or “Almost Clear” with >2-grade improvement from baseline) at Day 29 Trial Description Primary Efficacy Endpoint Secondary Efficacy Endpoints Safety Two multi-center, double-blind, vehicle-controlled studies conducted in the U.S. which enrolled over 750 patients each Patients enrolled were aged 2 years and older with mild-to-moderate atopic dermatitis affecting >5% body surface area Patients were randomized 2:1 (crisaborole:vehicle) and treated twice daily for 28 days ISGA of “Clear” or “Almost Clear” (irrespective of >2-grade improvement) at Day 29 Time to success in ISGA Reported adverse events, safety laboratory tests and vital signs
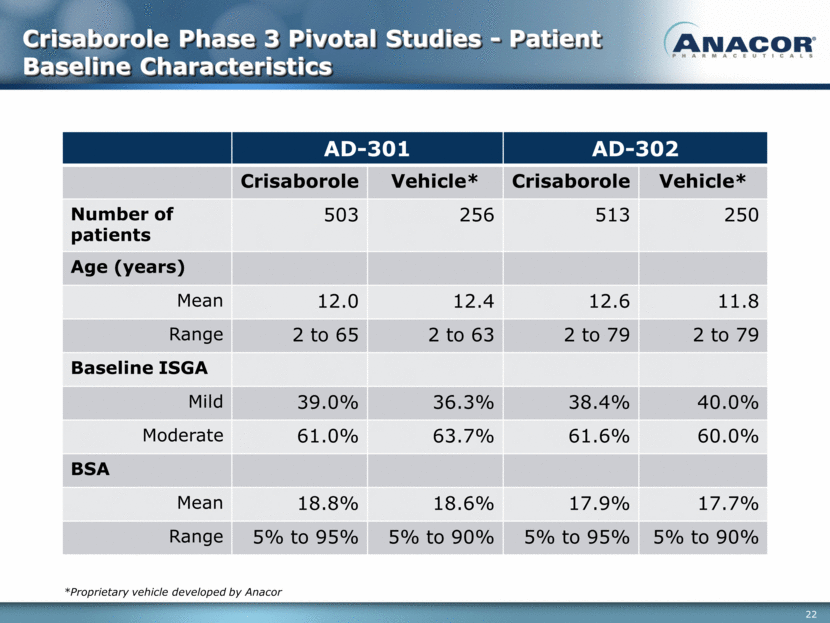
Crisaborole Phase 3 Pivotal Studies - Patient Baseline Characteristics AD-301 AD-302 Crisaborole Vehicle* Crisaborole Vehicle* Number of patients 503 256 513 250 Age (years) Mean 12.0 12.4 12.6 11.8 Range 2 to 65 2 to 63 2 to 79 2 to 79 Baseline ISGA Mild 39.0% 36.3% 38.4% 40.0% Moderate 61.0% 63.7% 61.6% 60.0% BSA Mean 18.8% 18.6% 17.9% 17.7% Range 5% to 95% 5% to 90% 5% to 95% 5% to 90% *Proprietary vehicle developed by Anacor
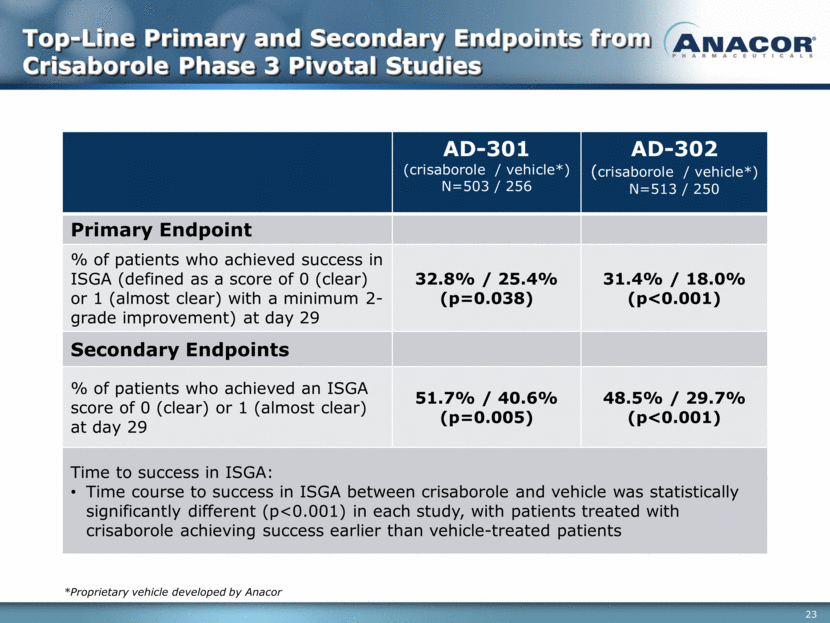
Top-Line Primary and Secondary Endpoints from Crisaborole Phase 3 Pivotal Studies AD-301 (crisaborole / vehicle*) N=503 / 256 AD-302 (crisaborole / vehicle*) N=513 / 250 Primary Endpoint % of patients who achieved success in ISGA (defined as a score of 0 (clear) or 1 (almost clear) with a minimum 2-grade improvement) at day 29 32.8% / 25.4% (p=0.038) 31.4% / 18.0% (p<0.001) Secondary Endpoints % of patients who achieved an ISGA score of 0 (clear) or 1 (almost clear) at day 29 51.7% / 40.6% (p=0.005) 48.5% / 29.7% (p<0.001) Time to success in ISGA: Time course to success in ISGA between crisaborole and vehicle was statistically significantly different (p<0.001) in each study, with patients treated with crisaborole achieving success earlier than vehicle-treated patients *Proprietary vehicle developed by Anacor
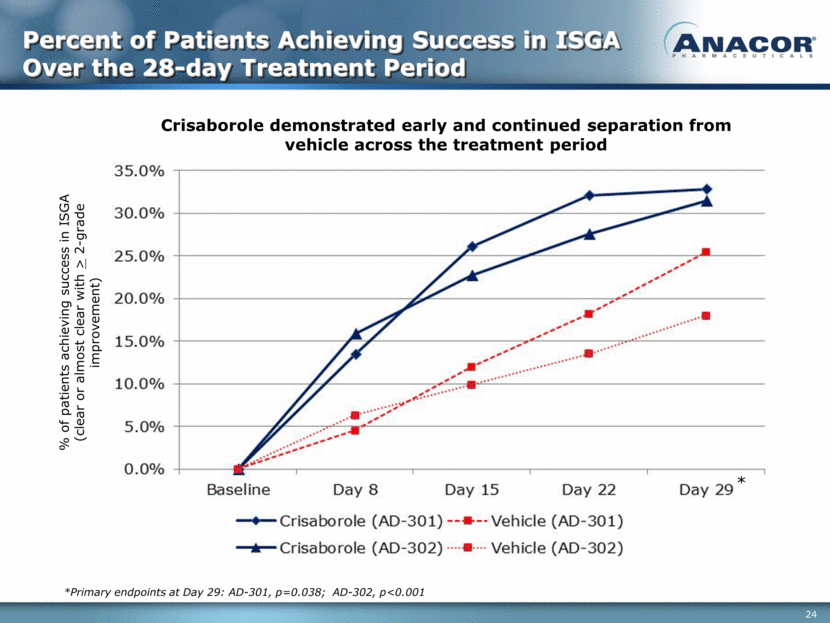
Percent of Patients Achieving Success in ISGA Over the 28-day Treatment Period % of patients achieving success in ISGA (clear or almost clear with > 2-grade improvement) Crisaborole demonstrated early and continued separation from vehicle across the treatment period *Primary endpoints at Day 29: AD-301, p=0.038; AD-302, p<0.001 *
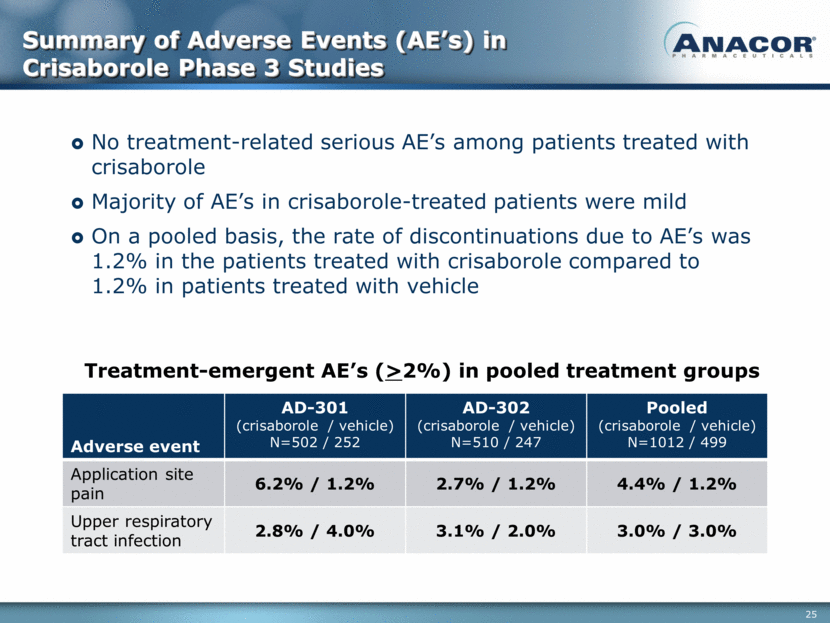
Summary of Adverse Events (AE’s) in Crisaborole Phase 3 Studies Adverse event AD-301 (crisaborole / vehicle) N=502 / 252 AD-302 (crisaborole / vehicle) N=510 / 247 Pooled (crisaborole / vehicle) N=1012 / 499 Application site pain 6.2% / 1.2% 2.7% / 1.2% 4.4% / 1.2% Upper respiratory tract infection 2.8% / 4.0% 3.1% / 2.0% 3.0% / 3.0% Treatment-emergent AE’s (>2%) in pooled treatment groups No treatment-related serious AE’s among patients treated with crisaborole Majority of AE’s in crisaborole-treated patients were mild On a pooled basis, the rate of discontinuations due to AE’s was 1.2% in the patients treated with crisaborole compared to 1.2% in patients treated with vehicle
Crisaborole is a leading product candidate in late-stage development for the topical treatment of mild-to-moderate atopic dermatitis in children and adults Subject to FDA approval, we expect limited branded competition at launch of crisaborole Significant unmet medical need Atopic dermatitis is estimated to affect 18M to 25M people in the U.S. (1) ~80% to 90% of cases are mild to moderate Significant negative impact on quality of life of patients and their families Currently, the most widely-used topical pharmacologic prescription therapies (corticosteroids and TCI’s) have limitations ~85% of cases present by the age of 5 years, so safety is an important factor in a treatment option(2) Highly concentrated core prescriber base: ~35K physicians (dermatologists, pediatricians and select PCP’s) ~350-400 person sales force to reach most productive prescribers With pricing and contracting, a product meeting the target product profile is anticipated to receive favorable market access from payers Crisaborole: The Opportunity Favorable market dynamics First mover National Eczema Association and Decision Resources. Arkwright et al. 2012, “Management of Difficult to Treat Atopic Dermatitis.
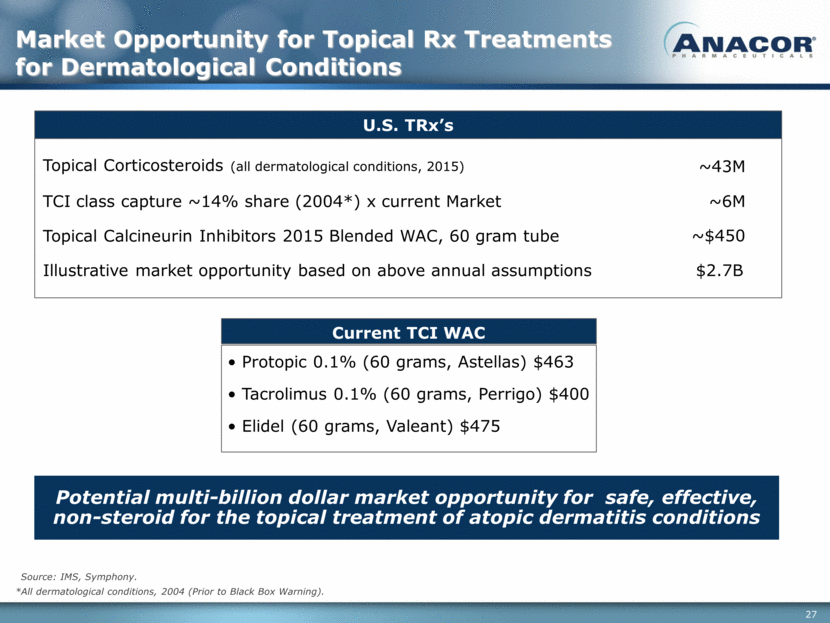
Source: IMS, Symphony. U.S. TRx’s Topical Corticosteroids (all dermatological conditions, 2015) ~43M TCI class capture ~14% share (2004*) x current Market ~6M Topical Calcineurin Inhibitors 2015 Blended WAC, 60 gram tube ~$450 Current TCI WAC Potential multi-billion dollar market opportunity for safe, effective, non-steroid for the topical treatment of atopic dermatitis conditions • Protopic 0.1% (60 grams, Astellas) $463 • Tacrolimus 0.1% (60 grams, Perrigo) $400 • Elidel (60 grams, Valeant) $475 *All dermatological conditions, 2004 (Prior to Black Box Warning). Market Opportunity for Topical Rx Treatments for Dermatological Conditions Illustrative market opportunity based on above annual assumptions $2.7B
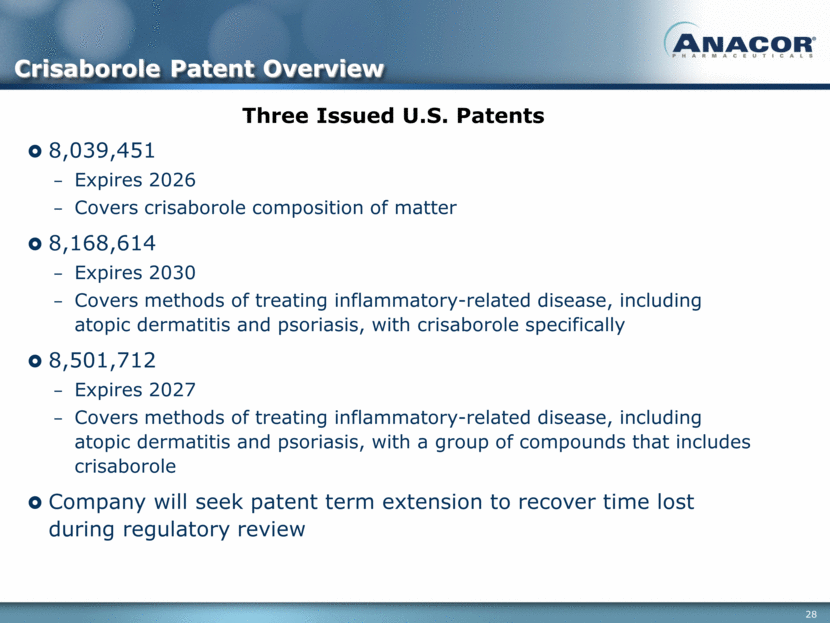
Three Issued U.S. Patents 8,039,451 Expires 2026 Covers crisaborole composition of matter 8,168,614 Expires 2030 Covers methods of treating inflammatory-related disease, including atopic dermatitis and psoriasis, with crisaborole specifically 8,501,712 Expires 2027 Covers methods of treating inflammatory-related disease, including atopic dermatitis and psoriasis, with a group of compounds that includes crisaborole Company will seek patent term extension to recover time lost during regulatory review Crisaborole Patent Overview
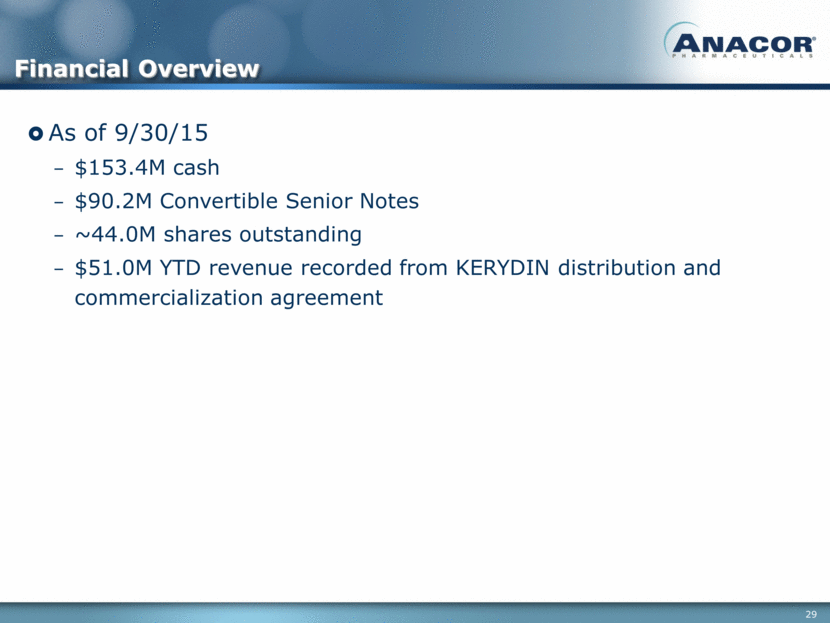
As of 9/30/15 $153.4M cash $90.2M Convertible Senior Notes ~44.0M shares outstanding $51.0M YTD revenue recorded from KERYDIN distribution and commercialization agreement Financial Overview
Thank you

