Attached files
| file | filename |
|---|---|
| EX-31.2 - EX-31.2 - VERU INC. | c894-20150930xex312.htm |
| EX-31.1 - EX-31.1 - VERU INC. | c894-20150930xex311.htm |
| EX-23.1 - EX-23.1 - VERU INC. | c894-20150930xex231.htm |
| EX-10.29 - EX-10.29 - VERU INC. | c894-20150930ex1029e165b.htm |
| EX-10.9 - EX-10.9 - VERU INC. | c894-20150930ex1097330b1.htm |
| EX-10.24 - EX-10.24 - VERU INC. | c894-20150930ex102447ca7.htm |
| EX-32.1 - EX-32.1 - VERU INC. | c894-20150930xex321.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
|
|
|
|
|
☑ |
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended September 30, 2015
|
|
|
|
|
☐ |
|
TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 1-13602
The Female Health Company
(Name of registrant as specified in its charter)
|
|
|
|
|
Wisconsin |
|
39-1144397 |
|
|
|
|
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
|
|
|
|
|
515 N. State Street, Suite 2225, Chicago, Illinois |
|
60654 |
|
|
|
|
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code (312) 595-9123
Securities registered under Section 12(b) of the Act:
|
|
|
|
|
Title of each class |
|
Name of each exchange on which registered |
|
Common stock, $.01 par value |
|
NASDAQ Stock Market |
|
|
|
|
Securities registered under Section 12(g) of the Act:
None
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐ No ☑
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ☑ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
1
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer |
☐ |
|
|
Accelerated filer |
☑ |
|
|
|
|
|
|
|
|
Non-accelerated filer |
☐ |
(Do not check if a smaller reporting company) |
|
Smaller reporting company |
☐ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ☑
The aggregate market value of the voting stock held by non-affiliates of the registrant as of March 31, 2015, was approximately $75.0 million based on the per share closing price as of March 31, 2015 quoted on the NASDAQ Capital Market for the registrant’s common stock, which was $2.83.
There were 29,023,832 shares of the registrant’s common stock, $0.01 par value per share outstanding at November 27, 2015.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the Proxy Statement for the 2016 Annual Meeting of the Shareholders of the Registrant are incorporated by reference into Part III of this report.
As used in this report, the terms “we,” “us,” “our,” “The Female Health Company,” “FHC” and the “Company” mean The Female Health Company and its subsidiaries collectively, unless the context indicates another meaning, and the term "common stock" means shares of our common stock, par value of $0.01 per share.
2
THE FEMALE HEALTH COMPANY
FORM 10-K
September 30, 2015
|
PART I |
Page |
|||
|
|
5 |
|
||
|
|
12 |
|
||
|
|
15 |
|
||
|
|
16 |
|
||
|
|
16 |
|
||
|
|
16 |
|
||
|
|
|
|
|
|
|
PART II |
|
|
|
|
|
|
|
|
|
|
|
|
17 |
|
||
|
|
19 |
|
||
|
Management's Discussion and Analysis of Financial Condition and Results of Operations |
|
20 |
|
|
|
|
26 |
|
||
|
|
26 |
|
||
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
|
26 |
|
|
|
|
26 |
|
||
|
|
26 |
|
||
|
|
|
|
|
|
|
PART III |
|
|
|
|
|
|
|
|
|
|
|
|
28 |
|
||
|
|
28 |
|
||
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
|
28 |
|
|
|
Certain Relationships and Related Transactions, and Director Independence |
|
29 |
|
|
|
|
29 |
|
||
|
|
|
|
|
|
|
PART IV |
|
|
|
|
|
|
|
|
|
|
|
|
30 |
|
||
|
|
|
34 |
|
|
3
FORWARD-LOOKING STATEMENTS
Certain statements included in this Annual Report on Form 10-K which are not statements of historical fact are intended to be, and are hereby identified as, "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements can be identified by the use of forward-looking words or phrases such as "anticipate," "believe," "could," "expect," "intend," "may," "opportunity," "plan," "predict," "potential," "estimate," "will," "would" or the negative of these terms or other words of similar meaning. These statements are based upon the Company's current plans and strategies, and reflect the Company's current assessment of the risks and uncertainties related to its business, and are made as of the date of this report. The Company cautions readers that forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results, performance or achievements of the Company to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, but are not limited to, those described under the caption "Risk Factors" in Item 1A. of this report. The Company undertakes no obligation to make any revisions to the forward-looking statements contained in this report or to update them to reflect events or circumstances occurring after the date of this report.
4
PART I
General
The Female Health Company manufactures, markets, and sells the FC2 Female Condom (FC2). FC2 is the only currently available female-controlled product approved by the U.S. Food and Drug Administration (FDA) and cleared by the World Health Organization (WHO) for purchase by U.N. agencies that provides dual protection against unintended pregnancy and sexually transmitted infections (STIs), including HIV/AIDS. FC2 was approved by the FDA as a Class III medical device in 2009.
FC2’s primary usages are for disease prevention and family planning, and the public health sector is the Company’s main market. Within the public health sector, various organizations supply critical products such as FC2, at no cost or low cost, to those who need but cannot afford to buy such products for themselves.
The Company has a relatively small customer base, with a limited number of customers who generally purchase in large quantities. Over the past few years, significant customers have included large global agencies, such as the United Nations Population Fund (UNFPA) and the United States Agency for International Development (USAID), through its facilitator, John Snow, Inc., Sekunjalo Investments Corporation (PTY) Ltd (Sekunjalo), the Company’s distributor in the Republic of South Africa (RSA), and the Brazil Ministry of Health either through UNFPA or Semina Indústria e Comércio Ltda (Semina), the Company’s distributor in Brazil. Other customers include ministries of health or other governmental agencies, which either purchase directly or via in-country distributors, and non-governmental organizations (NGOs).
FC2 has been distributed in 144 countries. A significant number of countries with the highest demand potential are in the developing world. The incidence of HIV/AIDS, other STIs, and unwanted pregnancy in these countries represents a remarkable potential for significant sales of a product that benefits some of the world’s most underprivileged people. However, conditions in these countries can be volatile and result in unpredictable delays in program development, tender applications, and processing orders.
Purchasing patterns vary significantly from one customer to another, and may reflect factors other than simple demand. For example, some governmental agencies purchase through a formal procurement process in which a tender (request for bid) is issued for either a specific or a maximum unit quantity. Tenders also define the other elements required for a qualified bid submission (such as product specifications, regulatory approvals, clearance by WHO, unit pricing, and delivery timetable). Bidders have a limited period of time in which to submit bids. Bids are subjected to an evaluation process which is intended to conclude with a tender award to the successful bidder. The entire tender process, from publication to award, may take many months to complete. A tender award indicates acceptance of the bidder’s price rather than an order or guarantee of the purchase of any minimum number of units. Many governmental tenders are stated to be “up to” the maximum number of units, which gives the applicable government agency discretion to purchase less than the full maximum tender amount. Orders are placed after the tender is awarded; there are often no set dates for orders in the tender and there are no guarantees as to the timing or amount of actual orders or shipments. Orders received may vary from the amount of the tender award based on a number of factors including vendor supply capacity, quality inspections, and changes in demand. Administrative issues, politics, bureaucracy, process errors, changes in leadership, funding priorities, and/or other pressures may delay or derail the process and affect the purchasing patterns of public sector customers. As a result, the Company may experience significant quarter-to-quarter sales variations due to the timing and shipment of large orders.
The Company currently operates in one industry segment which includes the development, manufacture, and marketing of consumer health care products. Therefore, no segment data is disclosed in the Notes to the Consolidated Financial Statements contained in this report. Information regarding the Company's operations by geographic area is included in Note 10 in the Notes to the Consolidated Financial Statements contained in this report.
Company History
The female condom was invented by a Danish physician who obtained a U.S. patent for FC1, the Company’s first generation product, in 1988. The physician subsequently sold certain rights to the condom to Chartex Resources Limited (Chartex). In the years that followed, Chartex, with resources provided by a Danish entrepreneur and a nonprofit Danish foundation, developed the manufacturing processes and completed other activities associated with bringing the female condom to market in certain non-U.S. countries. The Wisconsin Pharmacal Company, Inc. (Wisconsin Pharmacal) owned certain rights to the female condom in the U.S., Canada, and Mexico. Wisconsin Pharmacal pursued the pre-clinical and clinical studies and overall development of the product, necessary for U.S. FDA approval and worldwide distribution of the product.
The Female Health Company is the successor to Wisconsin Pharmacal, a company which manufactured and marketed disparate specialty chemical and branded consumer products. Wisconsin Pharmacal was originally incorporated in 1971.
5
In fiscal 1995, the Company's Board of Directors approved a plan to complete a series of actions designed, in part, to maximize the potential of the Female Condom. First, the Company restructured and transferred the Wisconsin Pharmacal name and all of the assets and liabilities of the Company other than those related to the Female Condom to a newly formed, wholly-owned subsidiary of the Company, WPC Holdings, Inc. (Holdings). In January 1996, the Company sold Holdings to an unrelated third party. Then, in February 1996, the Company acquired Chartex. At the same time, the Company was renamed The Female Health Company. As a result of the sale of Holdings and the acquisition of Chartex, The Female Health Company evolved to its current state with its sole business consisting of the manufacture, marketing, and sale of the Female Condom.
The FDA approved FC1 for distribution in the U.S. in 1993 and approved the Company's U.K. FC1 manufacturing facility in 1994. FC1 was produced from a costly raw material, polyurethane, in a labor intensive manufacturing process in London, England. To expand women’s access to the female condom, increase sales volume, reduce costs, and significantly increase gross margin, the Company developed its second generation Female Condom, FC2, which was completed in 2005. The second generation product is made from a less costly raw material, a nitrile polymer. FC2’s production process is more efficient and less labor and capital intensive than that of FC1, making it less costly to produce. Its price is now approximately 30 percent less than FC1. FC2 is currently being produced at the Company’s facility in Selangor D.E., Malaysia. Production in London was discontinued with the final shipment of FC1 in October 2009. As a result of the successful development of FC2, the Company was able to both reduce the price to the public health sector and increase its gross margin.
FC2 was first marketed internationally in March 2007 and has been marketed in the U.S. since August 2009. In October 2009, the Company completed the transition from its first generation product, FC1, to its second generation product, FC2, and production of FC1 ceased. The Company retains ownership of certain world-wide rights, as well as various patents, regulatory approvals, and other intellectual property related to FC1.
FC2 was approved by the FDA as a Class III medical device on March 10, 2009. In addition to FDA approval, FC2 has been approved by other regulatory agencies, including the European Union, India, and Brazil. Based on a rigorous scientific review, WHO cleared FC2 for purchase by U.N. agencies in 2006.
Since FC2’s introduction in March 2007 through September 30, 2015, approximately 329 million FC2’s have been distributed in 144 countries. It is marketed to consumers through distributors, global public sector procurement organizations, and retailers in 16 countries. Since the first FDA approval in 1993 through September 30, 2015, the Company has manufactured and sold approximately 504 million Female Condoms (FC1 and FC2).
Strategy
The Company’s strategy is to fully develop global markets for FC2 for both contraception and STI prevention, including HIV/AIDS. Since the introduction of its first generation product, FC1, the Company has developed contacts and relationships with global public health sector organizations such as WHO, UNFPA, USAID, and the United Nations Joint Programme on HIV/AIDS (UNAIDS), country-specific health ministries, NGOs and commercial partners in various countries. The Company has representatives in various locations around the world to provide technical sales support and assist with its customers’ prevention and family planning programs.
In July 2014, the Company announced a new growth strategy with two key elements. The first element seeks to accelerate demand for FC2 by strengthening key customer relationships and creating greater awareness of FC2 in our current markets through increased sales and marketing efforts. The Company is currently evaluating the potential for FC2 in consumer markets in the U.S, to be followed by certain European and other markets. The Company believes its increased sales and marketing investment will accelerate global demand for FC2. The second element of the Company’s new growth strategy is product diversification. The Company is actively pursuing the potential acquisition of additional products, technologies, and businesses.
Products
Currently, there are only two FDA approved and marketed products that prevent the transmission of HIV/AIDS through sexual intercourse: the male condom and FC2. FC2 is currently the only FDA approved and marketed female-controlled product that prevents STIs, including HIV/AIDS. Used consistently and correctly, FC2 provides women dual protection against STIs, including HIV/AIDS, and unintended pregnancy. When used correctly the protection rates against unintended pregnancies are 95 percent for female condoms compared to 98 percent for male condoms according to the FDA. FC2 is not seen as directly competing with the male condom; it provides an alternative to either unprotected sex or male condom usage.
6
An economic analysis of the cost effectiveness of an FC2 HIV/AIDS prevention program conducted by Dr. David Holtgrave, the chairman of the Department of Health Behavior and Society at the Johns Hopkins Bloomberg School of Public Health, was featured in the March 26, 2012 issue of AIDS and Behavior. The study showed that the Washington, D.C. FC2 prevention program, a public-private partnership to provide and promote FC2, prevented enough HIV infections in the first year alone to save over $8 million in avoided future medical care costs (over and above the cost of approximately $445,000 for the program). This means that for every dollar spent on the program, there was a cost savings of nearly $20. In the article Dr. Holtgrave concluded, “These results clearly indicate that delivery of, and education about, Female Condoms is an effective HIV prevention intervention and an outstanding public health investment.” Washington, D.C. began its program in 2010 to fight a disease that is at epidemic levels. At least 3 percent of Washington, D.C. residents have HIV or AIDS, a prevalence rate that is the highest of any U.S. city.
In May 2014, a business case was published by Global Health Visions, LLC, commissioned by Rutgers WPF, the advocacy partner of the Universal Access to Female Condoms (UAFC) Joint Programme. Part of the publication was a study comparing total expected costs with total estimated economical benefits and it determined there was an excellent return on investment for female condoms in sub-Saharan Africa. For example, in Nigeria an investment of $1 offers a $3.20 return on investment to the country’s economy.
Numerous clinical and behavioral studies have been conducted regarding use of the female condom. Studies show that in many cultures, the female condom is found acceptable by women and their partners. Importantly, studies also show that when the female condom is made available as an option along with male condoms there is a significant increase in protected sex acts with a concurrent decrease in STIs. The increase in protected sex acts varies by country and averages between 10 percent and 35 percent.
FC2 has basically the same physical design, specifications, safety, and efficacy profile as FC1. Manufactured from a nitrile polymer formulation that is exclusive to the Company, FC2 is produced more economically than FC1, which was made from a more costly raw material, polyurethane. FC2 consists of a soft, loose fitting sheath and two rings: an external ring of rolled nitrile and a loose internal ring made of flexible polyurethane. FC2’s soft sheath lines the vagina, preventing skin-to-skin contact during intercourse. Its external ring remains outside the vagina, partially covering the external genitalia. The internal ring is used for insertion and helps keep the device in place during use.
FC2’s primary raw material, a nitrile polymer, offers a number of benefits over natural rubber latex, the raw material most commonly used in male condoms. FC2’s nitrile polymer is stronger than latex, reducing the probability that the female condom sheath will tear during use. Unlike latex, FC2’s nitrile polymer quickly transfers heat. FC2 can warm to body temperature immediately upon insertion, which may enhance the user’s sensation and pleasure. Unlike the male condom, FC2 may be inserted in advance of arousal, eliminating disruption during sexual intimacy. FC2 is also an alternative to latex sensitive users who are unable to use male condoms without irritation. For example, 7 percent to 20 percent of the individuals with significant exposure to latex rubber (i.e., health care workers) experience such irritation. To the Company's knowledge, there is no reported allergy to the nitrile polymer. FC2 is pre-lubricated, disposable, and recommended for use during a single sex act. FC2 is not reusable.
Global Market Potential
Because FC2 offers a woman dual protection against both unintended pregnancy and STIs, including HIV/AIDS, its market encompasses both family planning and disease prevention.
Disease Prevention. The first clinical evidence of AIDS was noted more than thirty years ago. Since then, HIV/AIDS has become the most devastating pandemic facing humankind in recorded history. In November 2009, WHO released statistics indicating that on a world-wide basis, HIV/AIDS is now the leading cause of death in women 15 to 44 years of age. According to WHO, in 2012 worldwide women comprised 50 percent of all the adults living with HIV and approximately 58 percent of all new adult cases of HIV/AIDS in Sub-Saharan Africa. In the United States the Centers for Disease Control and Prevention (CDC) and FDA both list heterosexual sex as the most common method of HIV transmission in women.
For sexually active couples, male condoms and FC2 are the only barrier methods approved by the FDA for preventing sexual transmission of HIV/AIDS. In recent years, scientists have sought to develop alternative means of preventing HIV/AIDS. Based on the complexities of such research, a viable prevention alternative is unlikely to be available in the foreseeable future. To date, it is clear that condoms, male and female, continue to play a key role in the prevention of STIs, including HIV/AIDS. UNAIDS has reported that, since the beginning of the HIV/AIDS epidemic, it is estimated that condoms have averted approximately 50 million new cases. FC2, when used consistently and correctly, gives a woman control over her sexual health by providing dual protection against STIs, including HIV/AIDS, and unintended pregnancy.
7
In the United States, the CDC continues to report that the HIV/AIDS epidemic is taking an increasing toll on women and girls. Women of color, particularly black women, have been especially hard hit. Women of color comprise both the majority of new HIV and AIDS cases among women and the majority of women living with the disease. In 2010, the CDC listed the rate of new HIV infection for black women as approximately 8 times the rate for white women in the United States. In 2010, in the United States, it estimated that one in 32 black women would be diagnosed with HIV in her lifetime, compared to the one in 526 incidence rate amongst white women.
The CDC estimates there are 20 million new cases of STIs that they track in the U.S. each year. It is also estimated that over 24,000 women each year in the U.S. lose the ability to conceive or carry a pregnancy to term due to undiagnosed or untreated STIs. In March 2008, the CDC announced that a study indicated 26 percent of female adolescents in the U.S. have at least one of the most common STIs. Led by the CDC’s Sara Forhan, the study is the first to examine the combined national prevalence of common STIs among adolescent women in the U.S. In addition to overall STI prevalence, the study found that by race, African American teenage girls had the highest prevalence, with an overall prevalence of 48 percent compared to 20 percent among both whites and Mexican Americans. Overall, approximately half of all the teens in the study reported ever having sex. Among these girls, the STI prevalence was 40 percent.
On November 29, 2012, in conjunction with World AIDS Day, U.S. Secretary of State Hillary Clinton, as part of the President’s Emergency Plan For AIDS Relief (PEPFAR), issued a blueprint for an AIDS Free Generation. In the blueprint it states that female condoms are unique in providing a female-controlled HIV prevention option and that PEPFAR will work with partner governments and other donors to promote female condoms wherever effective programs can build a sustained demand.
On December 3, 2013, donors pledged $12 billion, which includes $1.5 billion from the U.K. Government, over a 3 year period to the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Contraception. The feminization of HIV/AIDS has increased the relevance of FC2 for the prevention of unintended pregnancies as well as disease prevention. Unintended pregnancy may result in maternal and infant death, babies with HIV/AIDS, AIDS orphans, and increased health care costs.
On July 11, 2012, World Population Day, the U.K. Government and the Bill and Melinda Gates Foundation held a Summit on Family Planning in London, England (the London Summit). It was attended by public health officials, government officials, and private sector companies that supply contraceptives and related products. FHC was one of only fourteen companies, and the only condom manufacturer, invited to attend the London Summit. The primary goal of the London Summit was to increase access to contraceptives to an additional 120 million poor women in 69 developing countries by 2020.
The Condom Market
The global public health sector market for male condoms is estimated to be greater than 8-10 billion units annually. The private sector market for male condoms is estimated at 10-15 billion units annually. The combined global male condom market (public and private sector) is estimated at a value of $4.5 billion annually. The female condom market represents a very small portion of the total global condom market.
Government Regulation
Female condoms as a group were classified by the FDA as a Class III medical device in 1989. Class III medical devices are deemed by the FDA to carry potential risks with use which must be tested prior to FDA approval, referred to as Premarket Approval (PMA), for sale in the U.S. As FC2 is a Class III medical device, prior to selling FC2 in the U.S., the Company was required to submit a PMA application containing technical information on the use of FC2, such as pre-clinical and clinical safety and efficacy studies, which was gathered together in a required format and content. The FC2 PMA was approved by the FDA as a Class III medical device in March 2009.
FC2 received the CE Mark which allows it to be marketed throughout the European Union. FC2 has also been approved by regulatory authorities in Brazil, India, Canada, and other jurisdictions.
8
In the U.S., FC2 is regulated by the FDA. Pursuant to section 515(a)(3) of the Safe Medical Amendments Act of 1990 (the SMA Act), the FDA may temporarily suspend approval and initiate withdrawal of the PMA if the FDA finds that FC2 is unsafe or ineffective, or on the basis of new information with respect to the device, which, when evaluated together with information available at the time of approval, indicates a lack of reasonable assurance that the device is safe or effective under the conditions of use prescribed, recommended, or suggested in the labeling. Failure to comply with the conditions of FDA approval invalidates the approval order. Commercial distribution of a device that is not in compliance with these conditions is a violation of the SMA Act. As an FDA approved medical device, the facilities in which FC2 is produced and tested are subject to periodic FDA inspection to ensure compliance with current Good Manufacturing Processes. The Company’s most recent FDA inspection was completed in September 2010.
The FDA’s approval order for FC2 includes conditions that relate to product labeling, including information on the package itself and instructions for use called a “package insert” which accompanies each product. The Company believes it is in compliance with the FDA approval order.
The Company’s facility may also be subject to inspection by UNFPA, USAID, International Organization for Standardization (ISO), and country specific ministries of health.
Significant Customers
Because FC2 provides dual protection against both STIs, including HIV/AIDS, and unintended pregnancy, it is an integral part of both HIV/AIDS prevention and family planning programs throughout the world. These programs are typically supplied by global public health sector buyers who purchase products for distribution, at low cost or no cost, to those who need but cannot afford to buy such products themselves. Within the global public health sector are large global agencies, such as UNFPA, USAID, DFID (the U.K.’s Department for International Development), and PSI (Population Services International), other social marketing groups, various government health agencies, and NGOs. The Company’s most significant customers are either global public health sector agencies, country specific ministries of health, or those who facilitate their purchases and/or distribution.
The Company's three largest customers in fiscal 2015 were the Brazil Ministry of Health (through Semina), UNFPA, and USAID. Semina accounted for 47 percent of unit sales in fiscal 2015 and less than 10 percent of unit sales in fiscal 2014 and 2013. UNFPA accounted for 18 percent of unit sales in fiscal 2015, 40 percent of unit sales in fiscal 2014, and 62 percent of unit sales in fiscal 2013. USAID accounted for 16 percent of unit sales in fiscal 2015, 17 percent of unit sales in fiscal 2014, and less than 10 percent of unit sales in fiscal 2013. Sekunjalo accounted for less than 10 percent of unit sales in fiscal 2015, 13 percent of unit sales in fiscal 2014, and less than 10 percent of unit sales in fiscal 2013. Azinor International Lda, a customer in Angola (Azinor), accounted for 11 percent of unit sales in fiscal 2014. No other single customer accounted for more than 10 percent of unit sales in fiscal 2015, 2014, or 2013. The Company considers its most significant customers to be UNFPA, USAID, Sekunjalo, and the Brazil Ministry of Health (either through UNFPA or Semina).
Commercial Markets – Direct to Consumers
The Company has distribution agreements and other arrangements with commercial partners which market to consumers through distributors and retailers in 16 countries, including the United States, Brazil, Spain, France, and the United Kingdom. These agreements are generally exclusive for a single country. Under these agreements, the Company sells FC2 to the distributor partners, who market and distribute the product to consumers in the established territory.
In the U.S., FHC initiated the FC2 College Health Mini-Grant Program in early 2013. The objective is to create awareness and sexual health knowledge that results in FC2 online/in-store retail purchases by young women and men. Education and training is the key content element for this program, similar to the public sector. College health and wellness centers were contacted and advised that they could apply to participate in the FC2 Program. During the pilot, FHC provided a mini-grant ($50-$500) and related education and training materials to help start or enhance an on-campus FC2 program. Grants were awarded based on a school’s intention to (1) raise awareness of FC2 on campus, (2) increase access to FC2 on campus, and (3) enhance students’ capacity to effectively and accurately use FC2. The pilot regions for The FC2 College Campus Program were determined through selection of the following four American College Health Associations Regional Affiliates: New England, New York, South, and South West College Health Associations. In total 30 colleges were chosen to receive grants for The FC2 College Campus Program, including Colgate University, Tulane University, and Duke University, plus student groups from institutions such as Boston College and University of Florida.
Due to the pilot program’s success, the program was implemented in 2014 with 20 schools chosen to receive grants between $500 and $1,000 along with related education and training materials. In 2015, 49 schools were chosen to receive an in kind donation of 300 FC2’s along with related education and training materials.
9
The Company is currently evaluating the potential for FC2 in consumer markets in the U.S., to be followed by certain European and other markets. Some recent changes in the market environment may represent an opportunity for the promotion of FC2 to consumers:
|
· |
FC2 is now reimbursable under the Affordable Care Act and most health plans. FC2 was registered and now has a UPC code to support reimbursement. |
|
· |
Increased public focus on preventing unwanted pregnancy and disease in young women. |
|
· |
The rise of social media in marketing to young women. |
|
· |
Increased online purchasing of condoms. It is estimated 33 percent of male condoms are purchased online. |
The Company believes the promotion of FC2 to consumers will be complementary to public sector marketing by increasing awareness of FC2.
An online store for direct-to-consumer purchases, ShopFemaleHealth.com, was launched in March 2015. Additionally, FC2 may now be purchased online through various ecommerce websites, including (but not limited to): Amazon.com, Walgreens.com, Drugstore.com, and MyQuestStore.com.
The Company has formed a medical advisory board to assist with determining the optimal approach to inform health care professionals of the benefits of FC2. Sampling and support information at gynecological practices is one tactic employed.
Relationships and Agreements with Public Health Sector Organizations
The Company’s customers are primarily large global agencies, NGOs, ministries of health, and other government agencies which purchase and distribute FC2 for use in HIV/AIDS prevention and family planning programs. The Company offers uniform, volume-based pricing to such agencies, rather than entering into long-term supply agreements.
In the U.S., FC2 is sold to city and state public health clinics as well as not-for-profit organizations such as Planned Parenthood. Municipal and state departments of health have been increasing access to FC2 within established condom programming. Chicago, Los Angeles, San Francisco, New York, and Washington, D.C. are all examples of cities with programs providing female and male condoms free of charge. In New York City, as of September 30, 2015, FC2 has been distributed to 1,760 locations.
The Company has encouraged growth in the U.S. through education and program development support. To make health professional education broadly available, the Company introduced its FC2 Online Training Program in March 2012.
The National Female Condom Coalition (NFCC) and UAFC sponsored the fourth annual Global Female Condom Day on September 16, 2015. The 2015 Global Female Condom Day drew greater attention and participation than in the previous years. Public events highlighting the need for access to female condoms and promoting their use in family planning and disease prevention were organized around the world and in the U.S., including events specifically initiated or co-sponsored by the Company.
Globally, the Company has a multilingual website that provides downloadable training and education information in English, Portuguese, Spanish, and French.
Outside of the U.S., training and education sessions were held in 10 countries, with an estimated 32,000 people participating in the sessions in 2015.
Employees
As of November 27, 2015, the Company had 180 full-time employees, including 11 located in the U.S., 13 in the U.K., 154 in Malaysia, and 2 in other countries to implement training and programs, and 1 part-time employee located in the U.S. None of the Company’s employees are represented by a labor union. The Company believes that its employee relations are good. In Malaysia, a significant proportion of direct labor is supplied by a contracted work force.
Environmental Regulation
The Company believes there are no material issues or material costs associated with the Company's compliance with environmental laws related to the manufacture and distribution of FC2. The Company has not incurred environmental expenses in fiscal 2015, 2014, or 2013, nor does it anticipate environmental expenses in the foreseeable future.
10
Raw Materials
The principal raw material used to produce FC2 is a nitrile polymer. While general nitrile formulations are available from a number of suppliers, the Company has chosen to work closely with the technical market leader in synthetic polymers to develop a grade ideally suited to the bio-compatibility and functional needs of a female condom. As a result, the Company relies on supply for its principal raw material from one supplier that could produce the raw material from multiple supply points within its organization.
Manufacturing Facilities
The Company leases production space in Selangor D.E., Malaysia for the production of FC2, which currently has manufacturing capacity of approximately 100 million units annually. In fiscal 2014 the Company added additional space, resulting in a total of 45,800 sq. ft. in the Company’s Malaysia facility, comprised of production and warehouse space and which provides sufficient space to add manufacturing capacity of up to an additional 100 million units annually. The Company will consider manufacturing in other locations as the demand for FC2 develops.
Competition
FC2 participates in the same market as male condoms; however, it is not seen as directly competing with male condoms. Rather, studies show that providing FC2 is additive in terms of prevention and choice. Male condoms cost less and have brand names that are more widely recognized than FC2. In addition, male condoms are generally manufactured and marketed by companies with significantly greater financial resources than the Company.
Other parties have developed and marketed female condoms. None of these female condoms marketed or under development by other parties have secured FDA approval. FDA approval is required to sell female condoms in the U.S. The Cupid female condom became the second female condom design to successfully complete the WHO prequalification process in July 2012 and be cleared for purchase by U.N. agencies. FC2 has also been competing with other female condoms in markets that do not require either FDA approval or WHO prequalification. We have experienced increasing competition in the global public sector, and competitors including Cupid received part of the last two South African tenders. Increasing competition in FC2’s markets may put pressure on pricing for FC2 or adversely affect sales of FC2, and some customers, particularly in the global public sector, may prioritize price over other features where FC2 may have an advantage. It is also possible that other female condoms may receive FDA approval or complete the WHO prequalification process, which would increase competition from other female condoms in FC2’s markets.
Patents and Trademarks
FC2 patents have been issued by the United States, Europe, Canada, Australia, South Africa, the People’s Republic of China, Japan, Mexico, Brazil, India and the African Regional Intellectual Property Organization (ARIPO), which includes Botswana, Gambia, Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Swaziland, Tanzania, Uganda, Zambia, and Zimbabwe. Further, the European patent for FC2 has been validated in the following countries: Austria, Belgium, Bulgaria, Switzerland, Republic of Cyprus, Czech Republic, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Italy, Luxembourg, Monaco, Netherlands, Portugal, Romania, Sweden, Slovenia, Slovakia, and Turkey. The patents cover the key aspects of FC2, including its overall design and manufacturing process. The patents have expiration dates in 2023 and 2024. In addition, patent applications for FC2 are pending in a number of other countries around the world. There can be no assurance that pending patent applications provide the Company with protection against copycat products entering markets during the pendency of the applications.
The Company has a registration for the trademark “FC2 Female Condom” in the United States. Furthermore, the Company has filed applications or secured registrations in 39 countries or jurisdictions around the world to protect the various names and symbols used in marketing FC2. In addition, the experience that has been gained through years of manufacturing its Female Condoms (FC1 and FC2) has allowed the Company to develop trade secrets and know-how, including certain proprietary production technologies, that further protect its competitive position.
Backlog
Unfilled product orders totaled $7,386,526 at November 27, 2015 and $9,848,220 at November 28, 2014. Unfilled orders materially fluctuate from quarter-to-quarter, and the amount at November 27, 2015 includes orders with requested delivery dates later in fiscal 2016. The Company expects current unfilled orders to be filled during fiscal 2016.
11
Available Information
The Company maintains a corporate website for investors at www.fhcinvestor.com and it makes available, free of charge, through this website its annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and amendments to those reports that the Company files with or furnishes to the Securities and Exchange Commission (SEC), as soon as reasonably practicable after it electronically files such material with, or furnishes it to, the SEC. Information on the Company's website is not part of this report.
You should carefully consider the risks described below, together with all of the other information included in this Annual Report and our other SEC filings, in considering our business and prospects. The risks described below are not the only risks we face. Additional risks that we do not yet know of or that we currently think are immaterial may also impair our business operations. If any of the events or circumstances described in the following risks occur, our business, financial condition, or results of operations could be materially adversely affected. In such cases, the trading price of our common stock could decline.
Our success is dependent upon the success of FC2.
At this time, we derive our revenues from sales of our only current product, FC2. The ultimate level of demand for FC2 is uncertain, and we may not be able to grow our business if demand for FC2 does not increase. We also depend on public sector agencies around the world to continue to include FC2 in their STI prevention and family planning programs, and on our commercial sector distribution partners to successfully market and distribute FC2. A decline in demand for FC2 would reduce our net revenues and profitability.
Our business may be affected by contracting risks with government and other international health agencies.
Our customers are primarily large international agencies and government health agencies which purchase and distribute FC2 for use in family planning and HIV/AIDS prevention programs. Sales to such agencies may be subject to government contracting risks, including the appropriations process and funding priorities, potential bureaucratic delays in awarding contracts under governmental tenders, process errors, politics or other pressures, and the risk that contracts may be subject to cancellation, delay, or restructuring. A governmental tender award indicates acceptance of the bidder’s price rather than an order or guarantee of the purchase of any minimum number of units. Many governmental tenders are stated to be “up to” the maximum number of units, which gives the applicable government agency discretion to purchase less than the full maximum tender amount. As a result, government agencies may order and purchase fewer units than the full maximum tender amount and there are no guarantees as to the timing or amount of actual orders or shipments under government tenders. Orders received may vary from the amount of the tender award based on a number of factors, including vendor supply capacity, quality inspections, and changes in demand. These contracting risks may cause significant quarter-to-quarter variations in our operating results and could adversely affect our net revenues and profitability. Budget issues, spending cuts, and global health spending priorities affecting government health agencies may also adversely affect demand for our product and our net revenues.
Competition from other products, including other female condoms, may have an adverse effect on our net revenues and profit margins.
We may be unable to compete successfully against current and future competitors, and competitive pressures could have a negative effect on our net revenues and profit margins. Other parties have developed and marketed female condoms, although only one such product has WHO pre-clearance and none of these female condoms have been approved by the FDA. FDA approval is required to sell female condoms in the U.S., and WHO pre-clearance is required to sell female condoms to U.N. agencies. FC2 has also been competing with other female condoms in markets that do not require either FDA approval or WHO prequalification. We have experienced increasing competition in the global public sector, and competitors received part of the last two South African tenders. Increasing competition in FC2’s markets may put pressure on pricing for FC2 or adversely affect sales of FC2, and some customers, particularly in the global public sector, may prioritize price over other features where FC2 may have an advantage. It is also possible that other companies will develop a female condom, and such companies could have greater financial resources and customer contacts than us. In addition, other contraceptive methods may compete with FC2 for funding and attention in the global public sector.
12
We may experience difficulties in implementing our growth initiatives.
We have announced new initiatives to increase our investment in sales and marketing activities. We may face a number of obstacles to successfully implement these initiatives, such as the costs associated with entering new markets or expanding current markets, retaining adequate numbers of effective sales and marketing personnel, developing and implementing effective marketing efforts, and establishing and maintaining appropriate regulatory compliance. We cannot assure you that we will be successful in implementing our growth strategies or that such strategies, even if implemented, will lead to the successful achievement of our objectives. Even if we are able to increase our sales as a result of our growth initiatives, we may not be able to achieve an adequate return on the amount we invest in these initiatives.
An inability to identify or complete future acquisitions could adversely affect our future growth.
As part of our growth initiatives, we intend to pursue acquisitions of new products, technologies, and/or businesses that are complementary to FC2 and enable us to leverage our competitive strengths. While we continue to evaluate potential acquisitions, we may not be able to identify and successfully negotiate suitable acquisitions, obtain financing for future acquisitions on satisfactory terms, obtain regulatory approval for acquisitions where required, or otherwise complete acquisitions in the future. An inability to identify or complete future acquisitions could limit our future growth.
We may experience difficulties in integrating strategic acquisitions.
The integration of acquired companies and their operations into our operations involves a number of risks, including:
|
· |
the acquired business may experience losses that could adversely affect our profitability; |
|
· |
unanticipated costs relating to the integration of acquired businesses may increase our expenses; |
|
· |
possible failure to accomplish the strategic objectives for an acquisition; |
|
· |
the loss of key personnel of the acquired business; |
|
· |
difficulties in achieving planned cost-savings and synergies may increase our expenses or decrease our net revenues; |
|
· |
diversion of management’s attention could impair their ability to effectively manage our business operations; |
|
· |
the acquired business may require significant expenditures for product development or regulatory approvals; |
|
· |
the acquired business may lack adequate internal controls or have other issues with its financial systems; |
|
· |
there may be regulatory compliance or other issues relating to the business practices of an acquired business; |
|
· |
we may record goodwill and nonamortizable intangible assets that are subject to impairment testing on a regular basis and potential impairment charges and we may also incur amortization expenses related to intangible assets; and |
|
· |
unanticipated management or operational problems or liabilities may adversely affect our profitability and financial condition. |
Additionally, we may borrow funds or issue equity to finance strategic acquisitions. Debt leverage resulting from future acquisitions could adversely affect our operating margins and limit our ability to capitalize on future business opportunities. Such borrowings may also be subject to fluctuations in interest rates. Equity issuances may dilute our existing shareholders and adversely affect the market price of our shares.
We depend on four major customers for a significant portion of our net revenues.
The Company's four largest customers currently are UNFPA, USAID, Sekunjalo and Semina. UNFPA accounted for 18 percent of unit sales in fiscal 2015, 40 percent of unit sales in fiscal 2014, and 62 percent of unit sales in fiscal 2013. USAID accounted for 16 percent of unit sales in fiscal 2015, 17 percent of unit sales in fiscal 2014, and less than 10 percent of unit sales in fiscal 2013. Sekunjalo accounted for less than 10 percent unit sales in fiscal 2015, 13 percent of unit sales in fiscal 2014, and less than 10 percent of unit sales in fiscal 2013. Semina accounted for 47 percent of unit sales in fiscal 2015, and less than 10 percent of unit sales in fiscal 2014 and 2013. An adverse change in our relationship with our largest customers could have a material adverse effect on our net revenues and profitability. In addition, we may have a concentration of accounts receivable with one or more of our largest customers, and a delay in payment by a large customer could have a material adverse effect on our cash flows and liquidity.
Since we sell product in foreign markets, we are subject to international business risks that could adversely affect our operating results.
Our international operations subject us to risks, including:
|
· |
economic and political instability; |
|
· |
changes in international regulatory requirements, import duties, or export restrictions, including limitations on the repatriation of earnings; |
|
· |
difficulties in staffing and managing foreign operations; |
13
|
· |
complications in complying with trade and foreign tax laws; |
|
· |
price controls and other restrictions on foreign currency; and |
|
· |
difficulties in our ability to enforce legal rights and remedies. |
Any of these risks might disrupt the supply of our products, increase our expenses or decrease our net revenues. The cost of compliance with trade and foreign tax laws increases our expenses, and actual or alleged violations of such laws could result in enforcement actions or financial penalties that could result in substantial costs.
Increases in the cost of raw materials, labor, and other costs used to manufacture our product could increase our cost of sales and reduce our gross margins.
We may experience increased costs of raw materials, including the nitrile polymer used in FC2, and increased labor costs. We may not be able to pass along such cost increases to our customers. As a result, an increase in the cost of raw materials, labor or other costs associated with manufacturing FC2 could increase our cost of sales and reduce our gross margins.
Currency exchange rate fluctuations could increase our expenses.
Because we manufacture FC2 in a leased facility located in Malaysia, a portion of our operating costs are denominated in a foreign currency. While a material portion of our future sales of FC2 are likely to be in foreign markets, all sales of FC2 are denominated in U.S. dollars. Manufacturing costs are subject to normal currency risks associated with fluctuations in the exchange rate of the Malaysian ringgit (MYR) relative to the U.S. dollar. Historically, we have not hedged our foreign currency risk.
We rely on a single facility to manufacture FC2, which subjects us to the risk of supply disruptions.
We manufacture FC2 in a single leased facility located in Malaysia. Difficulties encountered by this facility, such as fire, accident, natural disaster, or an outbreak of a contagious disease could halt or disrupt production at the facility, delay the completion of orders, or cause the cancellation of orders. Any of these risks could increase our expenses or reduce our net revenues.
Our product is subject to substantial government regulation, which exposes us to risks that we will be fined or exposed to civil or criminal liability, receive negative publicity, or be prevented from selling our product.
FC2 is subject to regulation by the FDA under the Food, Drug and Cosmetic Act, and by foreign regulatory agencies. Under the Food, Drug and Cosmetic Act, medical devices must receive FDA clearance before they can be sold. FDA regulations also require us to adhere to "Good Manufacturing Practices," which include testing, quality control, and documentation procedures. Our compliance with applicable regulatory requirements is monitored through periodic inspections by the FDA and foreign regulatory agencies. If we fail to comply with applicable regulations, we could:
|
· |
be fined or exposed to civil or criminal liability; |
|
· |
face suspensions of clearances, seizures, or recalls of products or operating restrictions; |
|
· |
receive negative publicity; or |
|
· |
be prohibited from selling our product in the U.S. or in foreign markets. |
Uncertainty and adverse changes in the general economic conditions may negatively affect our business.
If general economic conditions in the U.S. and other global markets in which we operate decline, or if consumers fear that economic conditions will decline, consumers may reduce expenditures for products such as our product. Adverse changes may occur as a result of adverse global or regional economic conditions, fluctuating oil prices, declining consumer confidence, unemployment, fluctuations in stock markets, contraction of credit availability, or other factors affecting economic conditions generally. These changes may negatively affect the sales of our product, increase the cost, and decrease the availability of financing, or increase costs associated with producing and distributing our product. In addition, a substantial portion of the sales of FC2 are made in the public market to government agencies, including USAID and other government agencies around the world. Worsening economic conditions as well as budget deficits and austerity measures may cause pressures on government budgets and result in a reduction in purchases of FC2 by governmental agencies. Sales of our product fluctuate, which causes our operating results to vary from quarter-to-quarter.
Sales of our product fluctuate based upon demand from our commercial partners and the public sector and the nature of government procurement processes. Historically, our net revenues and profitability have varied from quarter–to-quarter due to such buying patterns. Quarterly variations in operating results may cause us to fail to meet our earnings guidance or market expectations for our operating results and may tend to depress our stock price during such quarters.
14
Material adverse or unforeseen legal judgments, fines, penalties, or settlements could have an adverse impact on our profits and cash flows.
We may, from time to time, become a party to legal proceedings incidental to our business, including, but not limited to, alleged claims relating to product liability, environmental compliance, patent infringement, commercial disputes, and employment matters. Future litigation could require us to record reserves or make payments which could adversely affect our profits and cash flows. Even the successful defense of legal proceedings may cause us to incur substantial legal costs and may divert management's attention and resources away from our business.
Our success depends, in part, on our ability to protect our intellectual property.
We rely on our patented and other proprietary technology relating to FC2. The actions taken by us to protect our proprietary rights may not be adequate to prevent imitation of our product, processes, or technology. We cannot assure you that our proprietary technology will not become known to competitors, or that others will not independently develop a substantially equivalent or better female condom that does not infringe on our intellectual property rights, or will not challenge or assert rights in, and ownership of, our patents and other proprietary rights.
There are provisions in our charter documents, Wisconsin law, and change of control agreements with our officers that might prevent or delay a change in control of our company.
We are subject to a number of provisions in our charter documents, Wisconsin law, and change of control agreements that may discourage, delay, or prevent a merger or acquisition that a shareholder may consider favorable. These provisions include the following:
|
· |
the authority provided to our Board of Directors in our Amended and Restated Articles of Incorporation to issue preferred stock without further action by our shareholders; |
|
· |
change of control agreements we have entered into with three of our employees which provide for up to three years of compensation following a change of control as defined in the agreements; |
|
· |
the provision under Wisconsin law that permits shareholders to act by written consent only if such consent is unanimous; |
|
· |
the provision under Wisconsin law that requires for a corporation such as us, that was formed before January 1, 1973, the affirmative vote of the holders of at least two-thirds of the outstanding shares of our voting stock to approve an amendment to our articles of incorporation, a merger submitted to a vote of our shareholders, or a sale of substantially all of our assets; and |
|
· |
the Wisconsin control share acquisition statute and Wisconsin's "fair price" and "business combination" provisions which limit the ability of an acquiring person to engage in certain transactions or to exercise the full voting power of acquired shares under certain circumstances. |
The trading price of our common stock has been volatile, and investors in our common stock may experience substantial losses.
The trading price of our common stock has been volatile and may become volatile again in the future. The trading price of our common stock could decline or fluctuate in response to a variety of factors, including:
|
· |
our failure to meet market expectations for our performance; |
|
· |
the timing of announcements by us or our competitors concerning significant product developments, acquisitions, or financial performance; |
|
· |
fluctuation in our quarterly operating results; |
|
· |
substantial sales of our common stock; |
|
· |
general stock market conditions; or |
|
· |
other economic or external factors. |
You may be unable to sell your stock at or above your purchase price.
Item 1B. Unresolved Staff Comments
Not Applicable
15
Item 2. Properties
The Company leases approximately 5,100 square feet of office space at 515 North State Street, Suite 2225, Chicago, IL 60654. The lease expires October 31, 2016, although the Company has delivered notice of its intent to extend the term of the lease for an additional three year period ending October 31, 2019, provided that the Company has the right to rescind such lease extension upon receipt of the landlord’s notice of the new base rent for the extended term. The Company utilizes warehouse space and sales fulfillment services of an independent public warehouse located in Glendale Heights, IL for storage and distribution of FC2. In June 2010, the Company entered a new lease agreement for 6,400 square feet of office space located in London, England. The lease expires in June 2020. The Company manufactures and warehouses FC2 within a leased facility with 45,800 sq. ft. of production and warehouse space, in Selangor D.E., Malaysia. The FDA-approved manufacturing process is subject to periodic inspections by the FDA as well as the U.K. based “notified body”, which is responsible for CE and ISO accreditation. The lease currently has an expiration date of September 1, 2016 and is renewable at the option of the Company for an additional three year term. The Company’s Malaysian production capacity is approximately 100 million units annually.
The Company is not currently involved in any pending legal proceedings.
Item 4. Mine Safety Disclosures
Not Applicable
16
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Shares of our common stock trade on the NASDAQ Capital Market under the symbol "FHCO". The approximate number of record holders of our common stock at November 27, 2015 was 270. In January 2010, the Board of Directors adopted a quarterly cash dividend policy and declared the first cash dividend in the Company's history, which was paid in February 2010. In total, the Board has declared eighteen quarterly dividends, the most recent of which was paid in May 2014. All dividends have been paid from the Company's cash on hand. On July 14, 2014, the Company announced that its Board of Directors elected to suspend the payment of quarterly cash dividends in order to devote operating cash flows towards strategic growth initiatives. Under the Company's credit facility with Midland States Bank, dividends and share repurchases are permitted as long as after giving effect to the dividend or share repurchase the Company has a ratio of total liabilities to total stockholders' equity of not more than 1:1. Information regarding the high and low reported closing prices for our common stock and dividends paid on our common stock for the quarters indicated is set forth in the table below.
|
QUARTERS |
|||||||||||
|
FIRST |
SECOND |
THIRD |
FOURTH |
||||||||
|
2015 Fiscal Year |
|||||||||||
|
Price per common share – High |
$ |
4.59 |
$ |
3.92 |
$ |
3.33 |
$ |
1.78 | |||
|
Price per common share – Low |
$ |
3.32 |
$ |
2.80 |
$ |
1.80 |
$ |
1.32 | |||
|
Dividends paid |
$ |
— |
$ |
— |
$ |
— |
$ |
— |
|||
|
2014 Fiscal Year |
|||||||||||
|
Price per common share – High |
$ |
9.94 |
$ |
8.42 |
$ |
7.76 |
$ |
5.84 | |||
|
Price per common share – Low |
$ |
7.87 |
$ |
6.70 |
$ |
5.49 |
$ |
3.49 | |||
|
Dividends paid |
$ |
0.07 |
$ |
0.07 |
$ |
0.07 |
$ |
— |
|||
17
Performance Graph
The performance graph set forth below shows the value of an investment of $100 on September 30, 2010 in each of The Female Health Company, the NASDAQ Composite Index and NASDAQ Health Care Index. All values assume reinvestment of the pre-tax value of dividends paid by FHC and the companies included in the indices, and are calculated as of September 30 each year. The historical stock price performance of FHC is not necessarily indicative of future stock performance.
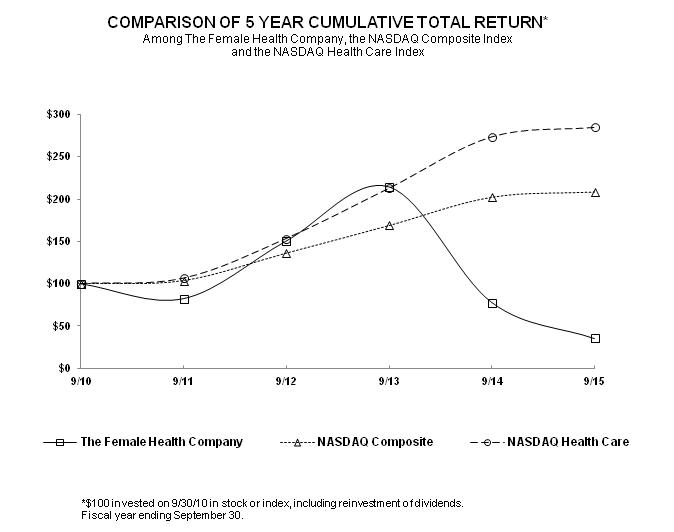
|
9/10 |
9/11 |
9/12 |
9/13 |
9/14 |
9/15 |
|
|
The Female Health Company |
100.00 | 82.42 | 150.44 | 214.34 | 77.79 | 35.22 |
|
NASDAQ Composite |
100.00 | 103.65 | 136.22 | 168.91 | 202.57 | 208.69 |
|
NASDAQ Health Care |
100.00 | 106.39 | 153.48 | 213.08 | 274.04 | 285.21 |
18
Item 6. Selected Financial Data
The data set forth below should be read in conjunction with Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements and Notes thereto appearing in this Annual Report on Form 10-K. The Consolidated Statement of Income Data for the years ended September 30, 2015, 2014, and 2013, and the Consolidated Balance Sheet Data as of September 30, 2015 and 2014, are derived from the Consolidated Financial Statements included elsewhere in this report. The Consolidated Statement of Income Data for the years ended September 30, 2012 and 2011, and the Consolidated Balance Sheet Data as of September 30, 2013, 2012 and 2011, are derived from Consolidated Financial Statements that are not included in this report. The historical results are not necessarily indicative of results to be expected for future periods.
|
Year ended September 30, |
||||||||||||||
|
Condensed Consolidated Statement of Income Data: |
2015 |
2014 |
2013 |
2012 |
2011 |
|||||||||
|
(In thousands, except per share data) |
||||||||||||||
|
Net revenues |
$ |
32,605 |
$ |
24,491 |
$ |
31,457 |
$ |
35,034 |
$ |
18,565 | ||||
|
Cost of sales |
13,635 | 11,370 | 13,953 | 14,413 | 8,700 | |||||||||
|
Gross profit |
18,970 | 13,121 | 17,504 | 20,621 | 9,865 | |||||||||
|
Operating expenses |
12,352 | 9,197 | 7,714 | 9,681 | 6,570 | |||||||||
|
Operating income |
6,618 | 3,924 | 9,790 | 10,940 | 3,295 | |||||||||
|
Non-operating income (expense) |
69 | 33 | 144 | (148) | (63) | |||||||||
|
Income before income taxes |
6,687 | 3,957 | 9,934 | 10,792 | 3,232 | |||||||||
|
Income tax expense (benefit) |
2,341 | 1,524 | (4,409) | (4,507) | (2,167) | |||||||||
|
Net income |
$ |
4,346 |
$ |
2,433 |
$ |
14,343 |
$ |
15,299 |
$ |
5,399 | ||||
|
Net income per basic common share outstanding |
$ |
0.15 |
$ |
0.09 |
$ |
0.51 |
$ |
0.55 |
$ |
0.20 | ||||
|
Basic weighted average common shares outstanding |
28,532 | 28,523 | 28,377 | 27,694 | 27,287 | |||||||||
|
Net income per diluted common share outstanding |
$ |
0.15 |
$ |
0.08 |
$ |
0.50 |
$ |
0.53 |
$ |
0.19 | ||||
|
Diluted weighted average common shares outstanding |
28,834 | 28,865 | 28,726 | 28,933 | 28,971 | |||||||||
|
Cash dividends declared per share |
$ |
— |
$ |
0.21 |
$ |
0.26 |
$ |
0.22 |
$ |
0.20 | ||||
|
Year ended September 30, |
||||||||||||||
|
Condensed Consolidated Balance Sheet Data: |
2015 |
2014 |
2013 |
2012 |
2011 |
|||||||||
|
(In thousands) |
||||||||||||||
|
Cash and cash equivalents |
$ |
4,106 |
$ |
5,796 |
$ |
8,922 |
$ |
5,296 |
$ |
4,318 | ||||
|
Working capital |
17,361 | 9,695 | 13,424 | 10,966 | 7,454 | |||||||||
|
Total assets |
37,472 | 31,673 | 35,170 | 30,446 | 19,443 | |||||||||
|
Accumulated deficit |
(27,996) | (32,342) | (28,715) | (35,594) | (44,697) | |||||||||
|
Long-term obligations |
15 | 39 | 67 | 174 | 209 | |||||||||
|
Total stockholders' equity |
$ |
33,133 |
$ |
28,065 |
$ |
31,403 |
$ |
24,218 |
$ |
16,753 | ||||
19
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations
Overview
The Company manufactures, markets and sells FC2. FC2 is the only currently available female-controlled product approved by the FDA that provides dual protection against unintended pregnancy and STIs, including HIV/AIDS.
FC2’s primary usages are for disease prevention and family planning, and the public health sector is the Company’s main market. Within the public health sector, various organizations supply critical products such as FC2, at no cost or low cost, to those who need but cannot afford to buy such products for themselves.
FC2 has been distributed in 144 countries. A significant number of countries with the highest demand potential are in the developing world. The incidence of HIV/AIDS, other STIs and unwanted pregnancy in these countries represents a remarkable potential for significant sales of a product that benefits some of the world’s most underprivileged people. However, conditions in these countries can be volatile and result in unpredictable delays in program development, tender applications and processing orders.
The Company has a relatively small customer base, with a limited number of customers who generally purchase in large quantities. Over the past few years, major customers have included large global agencies, such as UNFPA and USAID. Other customers include ministries of health or other governmental agencies, which either purchase directly or via in-country distributors, and NGOs.
Purchasing patterns vary significantly from one customer to another, and may reflect factors other than simple demand. For example, some governmental agencies purchase through a formal procurement process in which a tender (request for bid) is issued for either a specific or a maximum unit quantity. Tenders also define the other elements required for a qualified bid submission (such as product specifications, regulatory approvals, clearance by WHO, unit pricing and delivery timetable). Bidders have a limited period of time in which to submit bids. Bids are subjected to an evaluation process which is intended to conclude with a tender award to the successful bidder. The entire tender process, from publication to award, may take many months to complete. A tender award indicates acceptance of the bidder’s price rather than an order or guarantee of the purchase of any minimum number of units. Many governmental tenders are stated to be “up to” the maximum number of units, which gives the applicable government agency discretion to purchase less than the full maximum tender amount. Orders are placed after the tender is awarded; there are often no set dates for orders in the tender and there are no guarantees as to the timing or amount of actual orders or shipments. Orders received may vary from the amount of the tender award based on a number of factors including vendor supply capacity, quality inspections and changes in demand. Administrative issues, politics, bureaucracy, process errors, changes in leadership, funding priorities and/or other pressures may delay or derail the process and affect the purchasing patterns of public sector customers. As a result, the Company may experience significant quarter-to-quarter sales variations due to the timing and shipment of large orders.
During fiscal 2011, the Company’s unit shipments, revenues, and net income were adversely affected by bureaucratic delays and other timing issues involving the receipt and shipment of large orders from Brazil and RSA. Significant orders for both countries were received in the first quarter of fiscal 2012. The 20 million unit order received for shipment to Brazil which had been the largest order in the Company’s history. Receipt of these orders positively impacted fiscal 2012 and 2013 results.
In October 2014, the Company announced that Semina was awarded an exclusive contract under a public tender. The contract was valid through August 20, 2015, allowing the Brazil Ministry of Health to place orders against this tender at its discretion. Through the end of the contract, the Company received orders for 40 million units in fulfillment of the tender, 28 million of which were shipped during the year ended September 30, 2015.
Details of the quarterly unit sales for the last five fiscal years are as follows:
|
Period |
2015 |
2014 |
2013 |
2012 |
2011 |
|
October 1 – December 31 |
12,154,570 | 11,832,666 | 17,114,630 | 15,166,217 | 6,067,421 |
|
January 1 – March 31 |
20,760,519 | 7,298,968 | 16,675,035 | 13,945,320 | 8,905,099 |
|
April 1 – June 30 |
14,413,032 | 13,693,652 | 12,583,460 | 15,198,960 | 5,922,334 |
|
July 1 - September 30 |
13,687,462 | 9,697,341 | 8,386,800 | 17,339,500 | 11,977,716 |
|
Total |
61,015,583 | 42,522,627 | 54,759,925 | 61,649,997 | 32,872,570 |
Revenues. The Company's revenues are derived from sales of FC2, and are recognized upon shipment of the product to its customers.
20
The Company’s most significant customers are either global public health sector agencies or those who facilitate their purchases and/or distribution of FC2 for use in HIV/AIDS prevention and/or family planning. The Company's four largest customers currently are UNFPA, USAID, Sekunjalo and Semina. UNFPA accounted for 18 percent of unit sales in fiscal 2015, 40 percent of unit sales in fiscal 2014, and 62 percent of unit sales in fiscal 2013. USAID accounted for 16 percent of unit sales in fiscal 2015, 17 percent of unit sales in fiscal 2014, and less than 10 percent of unit sales in fiscal 2013. Sekunjalo accounted for less than 10 percent of unit sales in fiscal 2015, 13 percent of unit sales in fiscal 2014, and less than 10 percent of unit sales in fiscal 2013. Semina accounted for 47 percent of unit sales in fiscal 2015 and less than 10 percent of unit sales in fiscal 2014 and 2013. Azinor accounted for 11 percent of unit sales in fiscal 2014. No other single customer accounted for more than 10 percent of unit sales in fiscal 2015, 2014, or 2013. We sell to the Brazil Ministry of Health either through UNFPA or Semina. In the U.S., FC2 is sold to city and state public health clinics as well as to not-for-profit organizations such as Planned Parenthood.
Because the Company manufactures FC2 in a leased facility located in Malaysia, a portion of the Company's operating costs are denominated in foreign currencies. While a material portion of the Company's future sales are likely to be in foreign markets, all sales are denominated in the U.S. dollar. Effective October 1, 2009, the Company’s U.K. and Malaysia subsidiaries adopted the U.S. dollar as their functional currency, further reducing the Company’s foreign currency risk.
Expenses. The Company manufactures FC2 at its facility located in Selangor D.E., Malaysia. The Company's cost of sales consists primarily of direct material costs, direct labor costs and indirect production and distribution costs. Direct material costs include raw materials used to make FC2, principally a nitrile polymer. Indirect production costs include logistics, quality control and maintenance expenses, as well as costs for electricity and other utilities. All of the key components for the manufacture of FC2 are essentially available from either multiple sources or multiple locations within a source.
On April 1, 2015, a tariff exemption in Brazil for condoms was eliminated subjecting all shipments of FC2 clearing customs in Brazil on or after that date to a tariff. The Company agreed to share 50 percent of these tariff costs with Semina, its distributor in Brazil. The Company's share of these tariff costs was approximately $398,000 for the portion of the initial 25 million unit order received under the 2014 tender that was subject to the tariff. In August 2015, the Company announced it has received an additional order for 15 million units. The Company agreed to share 50 percent of the tariff costs with Semina for this additional order and will recognize the expense as the units are shipped.
The Company's operating expenses include costs for sales, marketing, education and training relating to FC2. During the London Summit, the Company announced a program to support the London Summit's goal to provide contraceptives to an additional 120 million women by 2020. This program includes a plan for the Company to invest up to $14 million over the period from 2013 through 2018 in reproductive health and HIV/AIDS prevention marketing, education and training in collaboration with global agencies. Such investment in marketing, education and training may increase the Company’s operating expenses in future periods, although the Company has not set a specific timetable for any such increased spending. In connection with the London Summit, the Company implemented a volume purchasing incentive program to award major public sector purchasers with FC2 equal to 5 percent of their total annual units purchased, at no-cost. The Company reserved for the no-cost product as a cost of sales, which impacted the Company’s gross margin. Effective January 1, 2015, the Company reduced the unit price to the major public sector purchasers to reflect the 5 percent no-cost product instead of awarding no-cost product.
Fiscal Year Ended September 30, 2015 Compared to Fiscal Year Ended September 30, 2014
Operating Highlights. The Company had net revenues of $32,604,865 during fiscal 2015, compared to $24,490,586 in fiscal 2014. The Company’s fiscal 2015 unit sales were 43 percent higher than fiscal 2014. The increase in unit sales and net revenues is primarily due to 28 million units shipped during fiscal 2015 under the 2014 Brazilian tender. The average sales price of FC2 decreased 7.2 percent in fiscal 2015 from fiscal 2014. Effective January 1, 2015, the unit price has been reduced for major public sector purchasers to replace the previous 5 percent no-cost product policy under the Company’s volume purchasing incentive program. The remaining decrease is primarily due to sales mix.
The Company used cash in operations of $1,548,697 in fiscal 2015 compared to $3,665,413 of cash generated from operations in fiscal 2014.
The Company had net income of $4,346,036, or $0.15 per diluted share, in fiscal 2015 compared to net income of $2,433,061, or $0.08 per diluted share, in fiscal 2014.
Results of Operations. The Company had net revenues of $32,604,865 and net income of $4,346,036, or $0.15 per diluted share, in fiscal 2015, compared to net revenues of $24,490,586 and net income of $2,433,061, or $0.08 per diluted share, in fiscal 2014. Net revenues increased $8,114,279, or 33 percent, in fiscal 2015 compared to the prior fiscal year.
Cost of sales increased $2,265,798, or 20 percent, to $13,634,906 in fiscal 2015 from $11,369,108 in fiscal 2014.
21
Gross profit increased $5,848,481, or 45 percent, to $18,969,959 in fiscal 2015 from $13,121,478 in fiscal 2014. Gross profit as a percentage of net revenues increased to 58 percent in fiscal 2015 from 54 percent in fiscal 2014. The increase reflects the favorable impact of changes in currency exchange rates slightly offset by higher costs associated with the increased unit sales.
Selling, general and administrative expenses increased $2,997,867, or 33 percent, to $12,131,737 in fiscal 2015 from $9,133,870 in fiscal 2014. Approximately 56 percent of the increased spending related to payments to our Brazilian distributor for ongoing programming related to the 2012 tender and for marketing and management fees related to the 2014 tender. $398,000 of the increase was for the Company’s share of tariff cost related expenses for the Brazilian tender. An accrual for fiscal year end incentive compensation, not incurred in the prior year period, was approximately 15 percent of the increase. Business development consulting costs associated with the portfolio diversification strategy was approximately 23 percent of the increase, minimal costs were incurred in the prior year period. These increased expenses were partially offset by a reduction of expenses relating to employee compensation.
Research and development expenses increased $214,240 to $219,815 in fiscal 2015 from $5,575 in fiscal 2014. The increase is primarily related to product enhancements.
Total operating expenses increased $3,153,986 to $12,351,552 in fiscal 2015 from $9,197,566 in fiscal 2014.
The Company's operating income increased $2,694,495, or 69 percent, to $6,618,407 in fiscal 2015 from $3,923,912 in fiscal 2014. The increase is primarily due to increased net revenues and improved gross margins partially offset by higher operating expenses.
The Company recorded non-operating income of $68,633 in fiscal 2015 compared to $33,279 in fiscal 2014. The impact of the foreign currency transactions was a gain of $58,483 in fiscal 2015 compared to a loss of $83,844 in fiscal 2014.
Income tax expense increased $816,874 to $2,341,004 in fiscal 2015 compared to income tax expense of $1,524,130 in fiscal 2014. The effective tax rate for fiscal 2015 and 2014 was 35.0 percent and 38.5 percent, respectively. The reduction in the effective tax rate is due to the mix of tax jurisdictions in which the Company recognized income before income taxes and the reduction in the Illinois state income tax rate, effective January 1, 2015, from 9.5 percent to 7.75 percent. The Company’s net operating loss (NOL) carryforwards will be utilized to reduce cash payments for income taxes based on the statutory rate in effect at the time of such utilization. Actual income taxes paid are reflected on the Company’s consolidated statements of cash flows. In fiscal 2015 the Company recorded income tax expense of $2,341,004, while due to the use of NOL carryforwards the Company made cash payments of $294,441 for income taxes.
Fiscal Year Ended September 30, 2014 Compared to Fiscal Year Ended September 30, 2013
Operating Highlights. The Company had net revenues of $24,490,586 during fiscal 2014, compared to $31,456,778 in fiscal 2013. The Company’s fiscal 2014 unit sales were 22 percent lower than fiscal 2013. The average sales price of FC2 increased 0.3 percent in fiscal 2014 from fiscal 2013.
The Company generated cash from operations of $3,663,596 in fiscal 2014 compared to $11,793,081 in fiscal 2013.
The Company had net income of $2,433,061, or $0.08 per diluted share, in fiscal 2014 compared to net income of $14,342,598, or $0.50 per diluted share, in fiscal 2013.
Results of Operations. The Company had net revenues of $24,490,586 and net income of $2,433,061, or $0.08 per diluted share, in fiscal 2014, compared to net revenues of $31,456,778 and net income of $14,342,598, or $0.50 per diluted share, in fiscal 2013. Net revenues decreased $6,966,192, or 22 percent, in fiscal 2014 compared to prior fiscal year. Results in fiscal 2013 were in part impacted by the receipt and shipment of Brazil orders delayed from fiscal 2011. The delayed orders were shipped in fiscal 2012 and 2013 and did not continue in fiscal 2014.
Cost of sales decreased $2,583,312, or 19 percent, to $11,369,108 in fiscal 2014 from $13,952,420 in fiscal 2013. The decrease is primarily due to a reduction in material costs due to lower unit sales partially offset by increased costs of quality control testing, a net increase in rent due to the leased warehouse facility and an increase in insurance costs.
Gross profit decreased $4,382,880, or 25 percent, to $13,121,478 in fiscal 2014 from $17,504,358 in fiscal 2013. Gross profit as a percentage of net revenues decreased to 54 percent in fiscal 2014 from 56 percent in fiscal 2013.
Advertising expenses decreased $163,597 to $58,121 in fiscal 2014 from $221,718 in fiscal 2013. The decrease is primarily due to a program launched in fiscal 2013 which did not recur in fiscal 2014.
22
Selling, general and administrative expenses increased $1,645,572, or 22 percent, to $9,133,870 in fiscal 2014 from $7,488,298 in fiscal 2013. The increase was primarily due to increased spending in sales, marketing, training and education. The majority of the increased spending relates to payments to our Brazilian distributor for programming related to the 2012 tender and for management fees for the recently announced 2014 tender.
Total operating expenses increased $1,482,805 to $9,197,566 in fiscal 2014 from $7,714,761 in fiscal 2013.
The Company's operating income decreased $5,865,685 to $3,923,912 in fiscal 2014 from $9,789,597 in fiscal 2013. The decrease is primarily due to lower unit sales and the increased spending in sales, marketing, training and education.
The Company recorded non-operating income of $33,279 in fiscal 2014 compared to $144,257 in fiscal 2013. The decrease is primarily due to the distribution upon demutualization of an insurance carrier received in fiscal 2013. The impact of the foreign currency transactions was a loss of $83,844 in fiscal 2014 compared to a loss of $101,288 in fiscal 2013.
Income tax expense increased $5,932,874 to $1,524,130 in fiscal 2014 compared to an income tax benefit of $4,408,744 in fiscal 2013. The increase is primarily due to the Company no longer recognizing an income tax benefit associated with reducing the Company’s valuation allowance on its deferred tax assets related to net operating loss carryforwards. During the year ended September 30, 2013, the valuation allowance on the Company’s deferred tax assets was fully realized and as a result the Company does not expect to recognize such tax benefits to any significant extent in its consolidated statements of income for periods after September 30, 2013. However the Company’s net operating loss carryforwards will be utilized to reduce cash payments for income taxes based on the statutory rate in effect at the time of such utilization. Actual income taxes paid are reflected on the Company’s consolidated statements of cash flows.
Liquidity and Sources of Capital
We generally fund our operations and working capital needs through cash generated from operations. Our operating activities used cash of $1.5 million in fiscal 2015, generated cash of $3.7 million in fiscal 2014, and generated cash of $11.8 million in fiscal 2013. The decrease of $5.2 million in cash generated from operating activities in fiscal 2015 as compared to fiscal 2014 was primarily due to an increase in accounts receivable of $11.2 million from $2.9 million at September 30, 2014 to $14.1 million at September 30, 2015. This increase is a result of orders received under the awarded Brazil 2014 tender. Semina’s accounts receivable balance represents 70 percent of the Company’s accounts receivable as September 30, 2015. Semina normally pays upon payment from the Brazilian Government; however due to economic issues in Brazil the government has been slower in paying vendors. In addition, total current liabilities increased $0.8 million, primarily due to $1.6 million owed to Semina related to the 2014 tender. In fiscal 2015, investing activities used cash of $0.1 million and financing activities used cash of $6,288. In fiscal 2014, investing activities used cash of $0.1 million and financing activities used cash of $6.7 million, most of which was used to pay quarterly cash dividends.
At September 30, 2015, the Company had working capital of $17.4 million and stockholders' equity of $33.1 million compared to working capital of $9.7 million and stockholders' equity of $28.1 million as of September 30, 2014.
Beginning February 16, 2010, through May 7, 2014, the Company paid a total of 18 consecutive dividends. The first 9 quarterly dividends were paid at a quarterly rate per share of $0.05 through February 9, 2012, 4 were paid at a quarterly rate per share of $0.06 from May 9, 2012 through February 6, 2013 and 5 were paid at a quarterly rate per share of $0.07 from May 8, 2013 through May 7, 2014. Cash dividends paid totaled $29.4 million during this period. The Company paid cash dividends of approximately $6.1 million and $7.5 million in fiscal 2014 and fiscal 2013, respectively. On July 14, 2014, the Company announced that its Board of Directors has elected to suspend the payment of quarterly cash dividends in order to devote operating cash flows towards strategic growth initiatives.
The Company believes its current cash position is adequate to fund operations of the Company in the next 12 months, although no assurances can be made that such cash will be adequate. If the Company needs additional cash, it may sell equity securities to raise additional capital and may borrow funds under its Midland States Bank credit facility.
23
On August 1, 2015, the Company entered into an amendment to the Second Amended and Restated Loan Agreement (as amended, the Loan Agreement) with Midland States Bank to extend the term of the Company’s revolving line of credit to August 1, 2016. The credit facility consists of a single revolving note for up to $2 million with Midland States Bank, with borrowings limited to a borrowing base determined based on 70 percent to 80 percent of eligible accounts receivable plus 50 percent of eligible inventory. Significant restrictive covenants in the Loan Agreement include prohibitions on any merger, consolidation or sale of all or a substantial portion of the Company’s assets and limits on the payment of dividends or the repurchase of shares. The Loan Agreement does not contain any financial covenants that require compliance with ratios or amounts. Dividends and share repurchases are permitted as long as after giving effect to the dividend or share repurchase the Company has a ratio of total liabilities to total stockholders’ equity of no more than 1:1. Borrowings on the revolving note bear interest at the national prime rate published by the Wall Street Journal (3.25 percent at September 30, 2015). The note is collateralized by substantially all of the assets of the Company. No amounts were outstanding under the revolving note at either September 30, 2015 or 2014.
As of November 27, 2015, the Company had approximately $3.9 million in cash, net trade accounts receivable of $14.6 million and current trade accounts payable of $0.5 million. Presently, the Company has no required debt service obligations.
The following table includes information relating to our contractual obligations as of September 30, 2015 in future fiscal years:
|
Contractual |
||||||||||||||||||||
|
Obligations |
Total |
2016 |
2017 |
2018 |
2019 |
2020 |
Thereafter |
|||||||||||||
|
Long-term debt |
$ |
- |
$ |
- |
$ |
- |
$ |
- |
$ |
- |
$ |
- |
$ |
- |
||||||
|
Capital lease obligations |
- |
- |
- |
- |
- |
- |
- |
|||||||||||||
|
Operating lease obligations |
839,972 | 371,528 | 131,032 | 123,002 | 122,491 | 91,919 |
— |
|||||||||||||
|
Purchase obligations |
- |
- |
- |
- |
- |
- |
- |
|||||||||||||
|
Other long-term obligations |
- |
- |
- |
- |
- |
- |
- |
|||||||||||||
|
Total |
$ |
839,972 |
$ |
371,528 |
$ |
131,032 |
$ |
123,002 |
$ |
122,491 |
$ |
91,919 |
$ |
— |
||||||
Critical Accounting Estimates
The preparation of financial statements requires management to make estimates and use assumptions that affect certain reported amounts and disclosures. Critical accounting estimates include the deferred income tax valuation allowance. Actual results may differ from those estimates.
The Company files separate income tax returns for its foreign subsidiaries. ASC Topic 740 requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial statements and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax assets are also provided for carryforwards for income tax purposes. In addition, the amount of any future tax benefits is reduced by a valuation allowance to the extent such benefits are not expected to be realized.
The Company accounts for income taxes using the liability method, which requires the recognition of deferred tax assets or liabilities for the tax-effected temporary differences between the financial reporting and tax bases of assets and liabilities, and for net operating loss and tax credit carryforwards.
The Company completes a detailed analysis of its deferred income tax valuation allowance on an annual basis or more frequently if information comes to our attention that would indicate that a revision to its estimates is necessary. In evaluating the Company’s ability to realize its deferred tax assets, management considers all available positive and negative evidence on a country by country basis, including past operating results and forecast of future taxable income. In determining future taxable income, management makes assumptions to forecast U.S. federal and state, U.K. and Malaysia operating income, the reversal of temporary differences, and the implementation of any feasible and prudent tax planning strategies. These assumptions require significant judgment regarding the forecasts of the future taxable income in each tax jurisdiction, and are consistent with the forecasts used to manage the Company’s business. It should be noted that the Company realized significant losses through 2005 on a consolidated basis. Since fiscal 2006, the Company has consistently generated taxable income on a consolidated basis, providing a reasonable future period in which the Company can reasonably expect to generate taxable income. In management’s analysis to determine the amount of the deferred tax asset to recognize, management projected future taxable income for each tax jurisdiction.
24
Although management uses the best information available, it is reasonably possible that the estimates used by the Company will be materially different from the actual results. These differences could have a material effect on the Company's future results of operations and financial condition.
Our effective tax rates have differed from the statutory rate primarily due to the tax impact of foreign operations, state taxes and reversal of the valuation allowance against the NOL carryforwards. Our future effective tax rates could be adversely affected by earnings being lower than anticipated in countries where we have lower statutory rates and higher than anticipated in countries where we have higher statutory rates, changes in the valuation of our deferred tax assets or liabilities, or changes in tax laws, regulations, and accounting principles. In addition, we are subject to the continuous examination of our income tax returns by the IRS and other tax authorities. We regularly assess the likelihood of adverse outcomes resulting from these examinations to determine the adequacy of our provision for income taxes.
Impact of Inflation and Changing Prices
Although the Company cannot accurately determine the precise effect of inflation, the Company has experienced increased costs of product, supplies, salaries and benefits, and increased general and administrative expenses. The Company has, where possible, increased selling prices to offset such increases in costs.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements as defined in Item 303(a) (4) of Regulation S-K.
25
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
The Company's exposure to market risk is limited to fluctuations in raw material commodity prices, particularly the nitrile polymer used to manufacture FC2, and foreign currency exchange rate risk associated with the Company's foreign operations. The Company does not utilize financial instruments for trading purposes or to hedge risk and holds no derivative financial instruments which would expose it to significant market risk. Effective October 1, 2009, the Company's U.K. subsidiary and Malaysia subsidiary each adopted the U.S. dollar as its functional currency. The consistent use of the U.S. dollar as the functional currency across the Company reduces its foreign currency risk and stabilizes its operating results. The Company’s distributors are subject to exchange rate risk as their orders are denominated in the U.S. dollars and they generally sell to their customers in the local country currency. If currency fluctuations have a material impact on a distributor it may ask the Company for pricing concessions or other financial accommodations. The Company currently has no significant exposure to interest rate risk. The Company has a line of credit with Midland States Bank, consisting of a revolving note for up to $2 million with borrowings limited to a percentage of eligible accounts receivable and eligible inventory. Outstanding borrowings under the line of credit will incur interest at a rate equal to the national prime rate published by the Wall Street Journal. As the Company has had no outstanding borrowings in the last five years, it currently has no significant exposure to market risk for changes in interest rates. Should the Company incur future borrowings under its line of credit, it would be subject to interest rate risk related to such borrowings.
Item 8. Financial Statements and Supplementary Data
The response to this item is submitted in a separate section of this report. See “Index to Consolidated Financial Statements” for a list of the financial statements being filed herein.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
As of the end of the period covered by this report, the Company carried out an evaluation, under the supervision and with the participation of the Company's management, including the Company's Chief Executive Officer and the Company's Chief Financial Officer, of the effectiveness of the design and operation of the Company's disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended). Based on this evaluation, the Company's Chief Executive Officer and Chief Financial Officer concluded that the Company's disclosure controls and procedures were effective. It should be noted that in designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. The Company has designed its disclosure controls and procedures to reach a level of reasonable assurance of achieving desired control objectives and, based on the evaluation described above, the Company's Chief Executive Officer and Chief Financial Officer concluded that the Company's disclosure controls and procedures were effective at reaching that level of reasonable assurance.
Changes in Internal Control Over Financial Reporting
There was no change in the Company's internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended) during the Company's most recently completed fiscal quarter that has materially affected, or is reasonably likely to materially affect, the Company's internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting
The report of management required under this Item 9A is contained on page F-1 of this Annual Report on Form 10-K under the heading "Management's Report on Internal Control over Financial Reporting."
Report of Independent Registered Public Accounting Firm
The attestation report required under this Item 9A is contained on page F-2 of this Annual Report on Form 10-K under the heading "Report of Independent Registered Public Accounting Firm."
26
None.
27
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Information with respect to this item is incorporated herein by reference to the discussion under the headings “Proposal 1: Election of Directors,” “Executive Officers,” “Section 16(a) Beneficial Ownership Reporting Compliance,” “Corporate Governance Matters-Director Nominations” and “Audit Committee Matters – Audit Committee Financial Expert” in the Company’s Proxy Statement for the 2016 Annual Meeting of Shareholders, which will be filed with the SEC on or before January 28, 2016. Information regarding the Company’s Code of Business Ethics is incorporated herein by reference to the discussion under “Corporate Governance Matters –Code of Business Ethics” in the Company’s Proxy Statement for the 2016 Annual Meeting of Shareholders, which will be filed with the SEC on or before January 28, 2016.
The Audit Committee of the Company’s Board of Directors is an “audit committee” for purposes of Section 3(a)(58)(A) of the Securities Exchange Act of 1934. The members of the Audit Committee are Mary Margaret Frank (Chairperson), David R. Bethune and Andrew S. Love.
Item 11. Executive Compensation
Information with respect to this item is incorporated herein by reference to the discussion under the headings “Director Compensation and Benefits,” and “Executive Compensation” in the Company’s Proxy Statement for the 2016 Annual Meeting of Shareholders, which will be filed with the SEC on or before January 28, 2016. The information under the subsection "Executive Compensation – Compensation Committee Report" is not deemed to be "soliciting material" or to be "filed" with the SEC or subject to Regulation 14A under the Securities Exchange Act of 1934 or to be the liabilities of Section 18 of the Securities Exchange Act of 1934, and will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933 or the Securities Exchange Act of 1934, except to the extent it is specifically incorporated by reference into such a filing.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information with respect to this item is incorporated herein by reference to the discussion under the heading “Security Ownership” in the Company’s Proxy Statement for the 2016 Annual Meeting of Shareholders, which will be filed with the SEC on or before January 28, 2016.
Equity Compensation Plan Information
The following table summarizes share information, as of September 30, 2015, for the Company's equity compensation plans and arrangements. The plans and arrangements dated prior to July 2007 were not required to be approved by the Company's shareholders, and, accordingly, none of these plans or arrangements have been approved by the Company's shareholders. In March 2008, the Company’s shareholders approved the 2008 Stock Incentive Plan and authorized 2 million shares (subject to adjustment in the event of stock splits and other similar events) for issuance under the plan.
|
Shares Remaining |
||||||
|
Number of Shares To Be |
Weighted-Average |
Available For Future |
||||
|
Issued Upon Exercise Of |
Exercise Price Of |
Issuance Under Equity |
||||
|
Equity Plan Category |
Outstanding Options |
Outstanding Options |
Compensation Plans |
|||
|
Equity compensation plans approved by shareholders |
182,333 |
(1) |
$ |
2.86 | 712,115 | |
|
Equity compensation plans not approved by shareholders |
90,000 |
$ |
1.27 |
— |
||
|
Total |
272,333 |
$ |
2.33 | 712,115 | ||
______________
(1)Includes a right to receive 9,333 shares contingent on continued employment and rights to receive a total of 83,000 shares, or at a holder’s election cash based on the fair market value of the shares, contingent on continued employment or service.
The Company's equity compensation plans not approved by shareholders consists of the 1997 Stock Option Plan. Options granted under the 1997 Stock Option Plan are nonqualified stock options under the Internal Revenue Code. Options expire at such time as the Board of Directors determines, provided that no stock option may be exercised later than the tenth anniversary of the date of its grant. Options cannot be exercised until the vesting period, if any, specified by the Board of Directors. Options are not transferable other than by will or the laws of descent and distribution, and may be exercised during the life of the participant only by him or her. The option price per share is determined by the Board of Directors, but cannot be less than 100 percent of the fair market value of the
28
common stock on the date such option is granted. The 1997 Stock Option Plan expired as of December 31, 2006, thus no further shares can be issued under this plan.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Information with respect to this item is incorporated herein by reference to the discussion under the heading “Certain Relationships and Related Transactions” in the Company’s Proxy Statement for the 2016 Annual Meeting of Shareholders, which will be filed with the SEC on or before January 28, 2016. Information regarding director independence is incorporated by reference to the discussions under “Corporate Governance Matters – Director Independence” in the Company’s Proxy Statement for the 2016 Annual Meeting of Shareholders, which will be filed with the SEC on or before January 28, 2016.
Item 14. Principal Accountant Fees and Services.
Information with respect to this item is incorporated herein by reference to the discussion under the heading “Audit Committee Matters – Fees of Independent Registered Public Accounting Firm” in the Company’s Proxy Statement for the 2016 Annual Meeting of Shareholders, which will be filed with the SEC on or before January 28, 2016.
29
PART IV
Item 15. Exhibits and Financial Statement Schedules.
(a) The following documents are filed as part of this report:
1.Financial Statements
|
|
The following consolidated financial statements of the Company are included in Item 8 of this report: |
|
|
Report of Independent Registered Public Accounting Firm |
|
|
Consolidated Balance Sheets as of September 30, 2015 and 2014 |
|
|
|
|
|
Consolidated Statements of Income for the Years Ended September 30, 2015, 2014, and 2013 |
|
|
Consolidated Statements of Stockholders’ Equity for the Years Ended September 30, 2015, 2014, and 2013 |
|
|
Consolidated Statements of Cash Flows for the Years Ended September 30, 2015, 2014, and 2013 |
|
|
Notes to Consolidated Financial Statements |
2.Financial Statement Schedules
All schedules for which provision is made in the applicable accounting regulations of the SEC are not required under the related instructions, are inapplicable or the required information is shown in the financial statements or notes thereto, and therefore, have been omitted.
30
3.Exhibits
|
3.1 |
Amended and Restated Articles of Incorporation of the Company. (1) |
|
|
|
|
3.2 |
Articles of Amendment to the Amended and Restated Articles of Incorporation of the Company increasing the number of authorized shares of common stock to 27,000,000 shares. (2) |
|
|
|
|
3.3 |
Articles of Amendment to the Amended and Restated Articles of Incorporation of the Company increasing the number of authorized shares of common stock to 35,500,000 shares. (3) |
|
|
|
|
3.4 |
Articles of Amendment to the Amended and Restated Articles of Incorporation of the Company increasing the number of authorized shares of common stock to 38,500,000 shares. (4) |
|
|
|
|
3.5 |
Articles of Amendment to the Amended and Restated Articles of Incorporation of the Company designating the terms and preferences for the Class A Preferred Stock – Series 3. (5) |
|
|
|
|
3.6 |
Amended and Restated By-Laws of the Company. (6) |
|
|
|
|
4.1 |
Amended and Restated Articles of Incorporation, as amended (same as Exhibits 3.1, 3.2, 3.3, 3.4 and 3.5). |
|
|
|
|
4.2 |
Articles II, VII and XI of the Amended and Restated By-Laws of the Company (included in Exhibit 3.6). |
|
|
|
|
10.1 |
1997 Stock Option Plan, as amended. (7) |
|
|
|
|
10.2 |
Letter Agreement dated December 5, 2013 between the Company and Karen King. (8) |
|
|
|
|
10.3 |
Change of Control Agreement effective as of January 20, 2014, between the Company and Karen King. (8) |
|
|
|
|
10.4 |
Separation Agreement and General Release, dated as of July 10, 2015, among the Company, Karen King and certain directors of the Company. (9) |
|
|
|
|
10.5 |
Change of Control Agreement between the Company and Michele Greco dated November 9, 2012. (10) |
|
|
|
|
10.6 |
Letter Agreement dated November 9, 2012 between the Company and Michele Greco. (10) |
|
|
|
|
10.7 |
Consulting Agreement, dated as of January 1, 2013, between the Company and Donna Felch. (11) |
|
|
|
|
10.8 |
First Amendment to Consulting Agreement, dated as of October 1, 2014, between the Company and Donna Felch. (12) |
|
|
|
|
10.9 |
Letter Agreement dated August 27, 2015 terminating Consulting Agreement with Donna Felch. |
|
|
|
|
10.10 |
Letter Agreement dated November 18, 2014 terminating Amended and Restated Change of Control Agreement of O.B. Parrish. (12) |
|
|
|
|
10.11 |
Consulting Agreement, dated as of December 31, 2013, between the Company and Mary Ann Leeper. (13) |
|
|
|
|
10.12 |
Letter Agreement dated July 10, 2014 between the Company and Susan Ostrowski. (14) |
|
|
|
|
10.13 |
Change of Control Agreement effective as of July 10, 2014, between the Company and Susan Ostrowski. (14) |
|
|
|
|
10.14 |
Consultancy Agreement, dated as of September 15, 2014, between The Female Health Company (UK) PLC and Michael Pope. (15) |
|
|
|
|
10.15 |
Service Agreement, dated September 15, 2014, between The Female Health Company (UK) PLC and Martin Tayler. (15) |
|
|
|
|
10.16 |
Change of Control Agreement dated September 15, 2014 between the Company and Martin Tayler. (15) |
|
|
|
|
10.17 |
The Female Health Company 2008 Stock Incentive Plan. (16) |
|
|
|
|
10.18 |
Form of Nonstatutory Stock Option Grant Agreement for The Female Health Company 2008 Stock Incentive Plan. (17) |
|
|
|
31
|
10.19 |
Form of Restricted Stock Grant Agreement for The Female Health Company 2008 Stock Incentive Plan. (18) |
|
|
|
|
10.20 |
Second Amended and Restated Loan Agreement, dated as of August 1, 2011, between the Company and Heartland Bank. (19) |
|
|
|
|
10.21 |
First Amendment to Second Amended and Restated Loan Agreement, dated as of August 1, 2012, between the Company and Heartland Bank. (20) |
|
|
|
|
10.22 |
Second Amendment to Second Amended and Restated Loan Agreement, dated as of August 1, 2013, between the Company and Heartland Bank. (18) |
|
|
|
|
10.23 |
Third Amendment to Second Amended and Restated Loan Agreement, dated as of August 1, 2014, between the Company and Heartland Bank. (14) |
|
|
|
|
10.24 |
Fourth Amendment to Second Amended and Restated Loan Agreement, dated as of August 1, 2015, between the Company and Midland States Bank, successor-by-merger to Heartland Bank. |
|
|
|
|
10.25 |
Commercial Security Agreement, dated as of July 20, 2004, between the Company and Heartland Bank. (21) |
|
|
|
|
10.26 |
First Amendment to Commercial Security Agreement, dated as of July 1, 2010, between the Company and Heartland Bank. (22) |
|
|
|
|
10.27 |
Second Amendment to Commercial Security Agreement, dated as of August 1, 2011, between the Company and Heartland Bank. (19) |
|
|
|
|
10.28 |
Share Charge, dated as of August 30, 2011, between the Company and Heartland Bank. (23) |
|
|
|
|
10.29 |
Consulting Agreement, effective as of September 10, 2015, between the Company and SMI, Strategic Marketing & Consulting. |
|
|
|
|
21 |
Subsidiaries of Registrant. |
|
|
|
|
23.1 |
Consent of RSM US LLP. |
|
|
|
|
24.1 |
Power of Attorney (included as part of the signature page hereof). |
|
|
|
|
31.1 |
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
31.2 |
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
32.1 |
Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350 (Section 906 of the Sarbanes-Oxley Act of 2002. (24) |
|
|
|
|
101 |
The following materials from the Company's Annual Report on Form 10-K for the year ended September 30, 2015, formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Balance Sheets, (ii) Consolidated Statements of Income, (iii) Consolidated Statements of Stockholders’ Equity, (iv) Consolidated Statements of Cash Flows, and (v) the Notes to Consolidated Financial Statements. |
____________
|
(1) |
Incorporated herein by reference to the Company's Form SB-2 Registration Statement filed on October 19, 1999. |
|
|
|
|
(2) |
Incorporated herein by reference to the Company's Form SB-2 Registration Statement filed on September 21, 2000. |
|
|
|
|
(3) |
Incorporated herein by reference to the Company's Form SB-2 Registration Statement filed on September 6, 2002. |
|
|
|
|
(4) |
Incorporated herein by reference to the Company's March 31, 2003 Form 10-QSB. |
|
|
|
|
(5) |
Incorporated herein by reference to the Company's March 31, 2004 Form 10-QSB. |
|
|
|
|
(6) |
Incorporated herein by reference to the Company's Form 8-K filed on May 22, 2013. |
|
|
|
32
|
(7) |
Incorporated herein by reference to the Company's Form S-8 Registration Statement filed on March 26, 2010. |
|
|
|
|
(8) |
Incorporated herein by reference to the Company's Form 8-K filed on December 11, 2013. |
|
|
|
|
(9) |
Incorporated by reference to the Company’s Form 8-K filed on July 16, 2015. |
|
|
|
|
(10) |
Incorporated herein by reference to the Company's Form 8-K filed on November 9, 2012. |
|
|
|
|
(11) |
Incorporated herein by reference to the Company's Form 8-K filed on January 7, 2013. |
|
|
|
|
(12) |
Incorporated by reference to the Company’s December 31, 2014 Form 10-Q. |
|
|
|
|
(13) |
Incorporated herein by reference to the Company's December 31, 2013 Form 10-Q. |
|
|
|
|
(14) |
Incorporated by reference to the Company’s September 30, 2014 Form 10-K. |
|
|
|
|
(15) |
Incorporated herein by reference to the Company’s Form 8-K filed on September 16, 2014. |
|
|
|
|
(16) |
Incorporated herein by reference to the Company's Form 8-K filed on March 31, 2008. |
|
|
|
|
(17) |
Incorporated herein by reference to the Company's September 30, 2009 Form 10-K. |
|
|
|
|
(18) |
Incorporated herein by reference to the Company's September 30, 2013 Form 10-K. |
|
|
|
|
(19) |
Incorporated herein by reference to the Company's June 30, 2011 Form 10-Q. |
|
|
|
|
(20) |
Incorporated herein by reference to the Company's September 30, 2012 Form 10-K. |
|
|
|
|
(21) |
Incorporated herein by reference to the Company's March 31, 2010 Form 10-Q. |
|
|
|
|
(22) |
Incorporated herein by reference to the Company's June 30, 2010 Form 10-Q. |
|
|
|
|
(23) |
Incorporated herein by reference to the Company’s September 30, 2011 Form 10-K. |
|
|
|
|
(24) |
This certification is not "filed" for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or incorporated by reference into any filing under the Securities Exchange Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended. |
The response to this portion of Item 15 is submitted as a separate section of this report.
(c) Financial Statement Schedules
33
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Date: December 1, 2015 |
|
THE FEMALE HEALTH COMPANY |
||
|
|
|
|
|
|
|
|
|
BY: |
/s/ O.B. Parrish |
|
|
|
|
|
O.B. Parrish, Chairman and |
|
|
|
|
|
Chief Executive Officer |
|
|
|
|
|
|
|
|
|
|
BY: |
/s/ Michele Greco |
|
|
|
|
|
Michele Greco, Executive Vice President and |
|
|
|
|
|
Chief Financial Officer |
|
POWER OF ATTORNEY
Each person whose signature appears below hereby appoints O.B. Parrish and Michele Greco, and each of them individually, his true and lawful attorney-in-fact, with power to act with or without the other and with full power of substitution and resubstitution, in any and all capacities, to sign any or all amendments to the Form 10-K and file the same with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitutes, may lawfully cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
|
Signature |
Title |
Date |
|
|
|
|
|
/s/ O.B. Parrish |
Chairman and Chief Executive Officer |
December 1, 2015 |
|
O.B. Parrish |
(Principal Executive Officer) |
|
|
|
|
|
|
/s/ Michele Greco |
Executive Vice President and Chief Financial Officer |
December 1, 2015 |
|
Michele Greco |
(Principal Accounting and Financial Officer) |
|
|
|
|
|
|
/s/ William R. Gargiulo |
Secretary and Director |
December 1, 2015 |
|
William R. Gargiulo |
|
|
|
|
|
|
|
/s/ David R. Bethune |
Director |
December 1, 2015 |
|
David R. Bethune |
|
|
|
|
|
|
|
/s/ Donna Felch |
Director |
December 1, 2015 |
|
Donna Felch |
|
|
|
|
|
|
|
/s/ Mary Margaret Frank |
Director |
December 1, 2015 |
|
Mary Margaret Frank |
|
|
|
|
|
|
|
/s/ Andrew S. Love |
Director |
December 1, 2015 |
|
Andrew S. Love |
|
|
|
|
|
|
|
|
Director |
December 1, 2015 |
|
Sharon Meckes |
|
|
|
|
|
|
|
|
|
|
|
34
The Female Health Company
Index to Consolidated Financial Statements
|
Document |
Page No. |
|
Audited Consolidated Financial Statements. |
|
|
Management's Report on Internal Control over Financial Reporting. |
F-1 |
|
Report of RSM US LLP, Independent Registered Public Accounting Firm. |
F-2 |
|
Consolidated Balance Sheets as of September 30, 2015 and 2014. |
F-3 |
|
Consolidated Statements of Income for the years ended September 30, 2015, 2014, and 2013. |
F-4 |
|
F-5 through F-7 |
|
|
Consolidated Statements of Cash Flows for the years ended September 30, 2015, 2014 and 2013. |
F-8 |
|
F-9 through F-21 |
F-0
Management's Report on Internal Control over Financial Reporting
Management of the Company is responsible for establishing and maintaining effective internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended). The Company’s internal control over financial reporting is designed to provide reasonable assurance to the Company’s management and Board of Directors regarding the preparation and fair presentation of published financial statements. The Company’s internal control over financial reporting includes those policies and procedures that:
(i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the Company;
(ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and
(iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of the Company's internal control over financial reporting as of September 30, 2015. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework in 2013. Based on its assessment, management believes that, as of September 30, 2015, the Company's internal control over financial reporting was effective based on those criteria.
The effectiveness of our internal control over financial reporting as of September 30, 2015 has been audited by RSM US LLP, an independent registered public accounting firm, as stated in their report. See ”Report of Independent Registered Public Accounting Firm,” which appears on page F-2 of this report.
December 1, 2015
F-1
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
The Female Health Company
We have audited the accompanying consolidated balance sheets of The Female Health Company as of September 30, 2015 and 2014, and the related consolidated statements of income, stockholders' equity, and cash flows for each of the three years in the period ended September 30, 2015. We also have audited The Female Health Company's internal control over financial reporting as of September 30, 2015, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013. The Female Health Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on these financial statements and an opinion on the Company's internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (b) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (c) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of The Female Health Company as of September 30, 2015 and 2014, and the results of its operations and its cash flows for each of the three years in the period ended September 30, 2015, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, The Female Health Company maintained, in all material respects, effective internal control over financial reporting as of September 30, 2015, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013.
/s/ RSM US LLP
Chicago, Illinois
December 1, 2015
F-2
THE FEMALE HEALTH COMPANY
SEPTEMBER 30, 2015 AND 2014
|
2015 |
2014 |
||||
|
ASSETS |
|||||
|
Current Assets |
|||||
|
Cash |
$ |
4,105,814 |
$ |
5,796,223 | |
|
Accounts receivable, net of allowance for doubtful accounts of $48,068 for 2015 and 2014 |
|
14,088,390 |
|
|
2,943,850 |
|
Income tax receivable |
21,251 |
— |
|||
|
Inventory, net |
1,745,180 | 2,983,447 | |||
|
Prepaid expenses and other current assets |
609,320 | 638,243 | |||
|
Deferred income taxes |
1,016,000 | 711,000 | |||
|
TOTAL CURRENT ASSETS |
21,585,955 | 13,072,763 | |||
|
Other assets |
136,766 | 166,084 | |||
|
PLANT AND EQUIPMENT |
|||||
|
Equipment, furniture and fixtures |
4,680,246 | 4,590,124 | |||
|
Leasehold improvements |
323,147 | 323,147 | |||
|
Less accumulated depreciation and amortization |
(3,763,403) | (3,310,964) | |||
|
Plant and equipment, net |
1,239,990 | 1,602,307 | |||
|
Deferred income taxes |
14,509,000 | 16,832,000 | |||
|
TOTAL ASSETS |
$ |
37,471,711 |
$ |
31,673,154 | |
|
LIABILITIES AND STOCKHOLDERS' EQUITY |
|||||
|
Current Liabilities |
|||||
|
Accounts payable |
$ |
1,077,349 |
$ |
1,124,859 | |
|
Accrued expenses and other current liabilities |
2,555,231 | 1,816,508 | |||
|
Accrued compensation |
592,428 | 436,843 | |||
|
TOTAL CURRENT LIABILITIES |
4,225,008 | 3,378,210 | |||
|
LONG-TERM LIABILITIES |
|||||
|
Deferred rent |
15,389 | 39,105 | |||
|
Deferred income taxes |
98,252 | 190,513 | |||
|
TOTAL LIABILITIES |
4,338,649 | 3,607,828 | |||
|
Commitments and Contingencies |
|||||
|
STOCKHOLDERS' EQUITY: |
|||||
|
Preferred stock; no shares issued and outstanding in 2015 or 2014. |
— |
— |
|||
|
Common Stock, par value $0.01 per share; authorized 38,500,000 shares; issued 31,192,536 and 30,958,669, and 29,008,832 and 28,775,215 shares outstanding in 2015 and 2014 respectively |
|
311,925 |
|
|
309,587 |
|
Additional paid-in-capital |
69,205,201 | 68,484,889 | |||
|
Accumulated other comprehensive loss |
(581,519) | (581,519) | |||
|
Accumulated deficit |
(27,995,940) | (32,341,976) | |||
|
Treasury stock, at cost |
(7,806,605) | (7,805,655) | |||
|
TOTAL STOCKHOLDERS' EQUITY |
33,133,062 | 28,065,326 | |||
|
TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY |
$ |
37,471,711 |
$ |
31,673,154 | |
|
See notes to consolidated financial statements. |
|||||
F-3
THE FEMALE HEALTH COMPANY
CONSOLIDATED STATEMENTS OF INCOME
YEARS ENDED SEPTEMBER 30, 2015, 2014 AND 2013
|
2015 |
2014 |
2013 |
||||||
|
Net revenues |
$ |
32,604,865 |
$ |
24,490,586 |
$ |
31,456,778 | ||
|
Cost of sales |
13,634,906 | 11,369,108 | 13,952,420 | |||||
|
Gross profit |
18,969,959 | 13,121,478 | 17,504,358 | |||||
|
Operating expenses: |
||||||||
|
Research and development |
219,815 | 5,575 | 4,745 | |||||
|
Advertising |
— |
58,121 | 221,718 | |||||
|
Selling, general, and administrative |
12,131,737 | 9,133,870 | 7,488,298 | |||||
|
Total operating expenses |
12,351,552 | 9,197,566 | 7,714,761 | |||||
|
Operating income |
6,618,407 | 3,923,912 | 9,789,597 | |||||
|
Non-operating income: |
||||||||
|
Interest and other income, net |
10,150 | 117,123 | 245,545 | |||||
|
Foreign currency transaction gain (loss) |
58,483 | (83,844) | (101,288) | |||||
|
Total non-operating income |
68,633 | 33,279 | 144,257 | |||||
|
Income before income taxes |
6,687,040 | 3,957,191 | 9,933,854 | |||||
|
Income tax expense (benefit) |
2,341,004 | 1,524,130 | (4,408,744) | |||||
|
Net income |
$ |
4,346,036 |
$ |
2,433,061 |
$ |
14,342,598 | ||
|
Net income per basic common share outstanding |
$ |
0.15 |
$ |
0.09 |
$ |
0.51 | ||
|
Basic weighted average common shares outstanding |
28,532,327 | 28,522,525 | 28,376,607 | |||||
|
Net income per diluted common share outstanding |
$ |
0.15 |
$ |
0.08 |
$ |
0.50 | ||
|
Diluted weighted average common shares outstanding |
28,917,048 | 28,865,384 | 28,726,478 | |||||
|
Cash dividends declared per common share |
$ |
— |
$ |
0.21 |
$ |
0.26 | ||
|
See notes to consolidated financial statements. |
||||||||
F-4
THE FEMALE HEALTH COMPANY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
YEARS ENDED SEPTEMBER 2015, 2014 and 2013
|
Accumulated |
||||||||||||||||||||||
|
Additional |
Other |
Treasury |
||||||||||||||||||||
|
Preferred |
Common Stock |
Paid-in |
Comprehensive |
Accumulated |
Stock |
|||||||||||||||||
|
Stock |
Shares |
Amount |
Capital |
Loss |
Deficit |
at Cost |
Total |
|||||||||||||||
|
Balance at September 30, 2012 |
$ |
— |
30,550,030 |
$ |
305,500 |
$ |
66,760,907 |
$ |
(581,519) |
$ |
(35,594,455) |
$ |
(6,672,243) |
$ |
24,218,190 | |||||||
|
Share-based compensation |
— |
73,176 | 731 | 700,288 |
— |
— |
— |
701,019 | ||||||||||||||
|
Issuance of 43,465 shares of common stock upon cashless exercise of 52,000 warrants |
— |
43,465 | 435 | (435) |
— |
— |
— |
— |
||||||||||||||
|
Issuance of 28,172 shares of common stock upon cashless exercise of 36,250 options |
— |
28,172 | 282 | (282) |
— |
— |
— |
— |
||||||||||||||
|
Stock repurchase – total 55,625 treasury shares |
— |
— |
— |
— |
— |
— |
(395,667) | (395,667) | ||||||||||||||
|
Common stock dividends |
— |
— |
— |
— |
— |
(7,463,183) |
— |
(7,463,183) | ||||||||||||||
|
Net income and comprehensive income |
— |
— |
— |
— |
— |
14,342,598 |
— |
14,342,598 | ||||||||||||||
|
Balance at September 30, 2013 |
$ |
— |
30,694,843 |
$ |
306,948 |
$ |
67,460,478 |
$ |
(581,519) |
$ |
(28,715,040) |
$ |
(7,067,910) |
$ |
31,402,957 | |||||||
|
See notes to consolidated financial statements. |
||||||||||||||||||||||
F-5
THE FEMALE HEALTH COMPANY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
YEARS ENDED SEPTEMBER 2015, 2014 and 2013
|
Accumulated |
||||||||||||||||||||||
|
Additional |
Other |
Treasury |
||||||||||||||||||||
|
Preferred |
Common Stock |
Paid-in |
Comprehensive |
Accumulated |
Stock |
|||||||||||||||||
|
Stock |
Shares |
Amount |
Capital |
Loss |
Deficit |
at Cost |
Total |
|||||||||||||||
|
Balance at September 30, 2013 (balance forward) |
$ |
— |
|
30,694,843 |
|
$ |
306,948 |
|
$ |
67,460,478 |
|
$ |
(581,519) |
|
$ |
(28,715,040) |
|
$ |
(7,067,910) |
|
$ |
31,402,957 |
|
Share-based compensation |
— |
216,863 | 2,169 | 907,281 |
— |
— |
— |
909,450 | ||||||||||||||
|
Issuance of 30,000 shares of common stock upon exercise of stock options |
|
— |
|
30,000 |
|
|
300 |
|
|
117,300 |
|
|
— |
|
|
— |
|
|
— |
|
|
117,600 |
|
Issuance of 16,963 shares of common stock upon cashless exercise of 30,000 options |
|
— |
|
16,963 |
|
|
170 |
|
|
(170) |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
Stock repurchase – total 169,000 treasury shares |
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
(737,745) |
|
|
(737,745) |
|
Common stock dividends |
— |
— |
— |
— |
— |
(6,059,997) |
— |
(6,059,997) | ||||||||||||||
|
Net income and comprehensive income |
|
— |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
2,433,061 |
|
|
— |
|
|
2,433,061 |
|
Balance at September 30, 2014 |
$ |
— |
30,958,669 |
$ |
309,587 |
$ |
68,484,889 |
$ |
(581,519) |
$ |
(32,341,976) |
$ |
(7,805,655) |
$ |
28,065,326 | |||||||
|
See notes to consolidated financial statements. |
||||||||||||||||||||||
F-6
THE FEMALE HEALTH COMPANY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
YEARS ENDED SEPTEMBER 2015, 2014 and 2013
|
Accumulated |
||||||||||||||||||||||
|
Additional |
Other |
Treasury |
||||||||||||||||||||
|
Preferred |
Common Stock |
Paid-in |
Comprehensive |
Accumulated |
Stock |
|||||||||||||||||
|
Stock |
Shares |
Amount |
Capital |
Loss |
Deficit |
at Cost |
Total |
|||||||||||||||
|
Balance at September 30, 2014 |
$ |
— |
30,958,669 |
$ |
309,587 |
$ |
68,484,889 |
$ |
(581,519) |
$ |
(32,341,976) |
$ |
(7,805,655) |
$ |
28,065,326 | |||||||
|
Share-based compensation |
— |
233,867 | 2,338 | 720,312 |
— |
— |
— |
722,650 | ||||||||||||||
|
Stock repurchase – total 250 treasury shares |
— |
— |
— |
— |
— |
— |
(950) | (950) | ||||||||||||||
|
Net income and comprehensive income |
— |
— |
— |
— |
— |
4,346,036 |
— |
4,346,036 | ||||||||||||||
|
Balance at September 30, 2015 |
$ |
— |
31,192,536 |
$ |
311,925 |
$ |
69,205,201 |
$ |
(581,519) |
$ |
(27,995,940) |
$ |
(7,806,605) |
$ |
33,133,062 | |||||||
|
See notes to consolidated financial statements. |
||||||||||||||||||||||
F-7
THE FEMALE HEALTH COMPANY
CONSOLIDATED STATEMENTS OF CASH FLOWS
YEARS ENDED SEPTEMBER 30, 2015, 2014 and 2013
|
2015 |
2014 |
2013 |
||||||
|
OPERATIONS |
||||||||
|
Net income |
$ |
4,346,036 |
$ |
2,433,061 |
$ |
14,342,598 | ||
|
Adjustments to reconcile net income to net cash (used in) provided by operating activities: |
||||||||
|
Depreciation and amortization |
494,258 | 589,343 | 556,304 | |||||
|
Provision for obsolete inventory |
173,634 | 37,603 | (6,662) | |||||
|
Provision for bad debts |
— |
38,068 | 3,180 | |||||
|
Share-based compensation |
489,689 | 858,615 | 727,609 | |||||
|
Deferred income taxes |
1,925,739 | 1,012,334 | (5,259,065) | |||||
|
Loss on disposal of fixed assets |
3,483 | 491 | 940 | |||||
|
Changes in operating assets and liabilities: |
||||||||
|
Accounts receivable |
(11,144,540) | (619,753) | 4,903,572 | |||||
|
Income tax receivable |
(21,251) | 78,440 | (51,071) | |||||
|
Inventories |
1,064,633 | (561,633) | (994,556) | |||||
|
Prepaid expenses and other assets |
58,241 | (151,656) | 93,933 | |||||
|
Accounts payable |
(47,510) | 220,810 | (871,278) | |||||
|
Accrued expenses and other current liabilities |
1,108,891 | (270,310) | (1,652,423) | |||||
|
Net cash (used in) provided by operating activities |
(1,548,697) | 3,665,413 | 11,793,081 | |||||
|
INVESTING ACTIVITIES |
||||||||
|
Capital expenditures |
(135,424) | (97,311) | (302,198) | |||||
|
Net cash used in investing activities |
(135,424) | (97,311) | (302,198) | |||||
|
FINANCING ACTIVITIES |
||||||||
|
Proceeds from exercise of stock options |
— |
117,600 |
— |
|||||
|
Purchases of common stock for treasury shares |
(950) | (737,745) | (395,667) | |||||
|
Dividends paid on common stock |
(5,338) | (6,074,164) | (7,468,248) | |||||
|
Net cash used in financing activities |
(6,288) | (6,694,309) | (7,863,915) | |||||
|
Net (decrease) increase in cash |
(1,690,409) | (3,126,207) | 3,626,968 | |||||
|
Cash at beginning of year |
5,796,223 | 8,922,430 | 5,295,462 | |||||
|
CASH AT END OF YEAR |
$ |
4,105,814 |
$ |
5,796,223 |
$ |
8,922,430 | ||
|
Supplemental Disclosure of Cash Flow Information: |
||||||||
|
Cash payments for income taxes |
294,441 | 773,041 | 345,657 | |||||
|
Schedule of noncash financing and investing activities: |
||||||||
|
Dividends payable |
— |
6,913 | 12,530 | |||||
|
Reduction of accrued expense upon issuance of shares |
255,577 | 311,515 | 200,088 | |||||
|
See notes to consolidated financial statements. |
||||||||
F-8
Note 1.Nature of Business and Significant Accounting Policies
Principles of consolidation and nature of operations: The consolidated financial statements include the accounts of the Company and its wholly owned subsidiary, The Female Health Company – UK, and its wholly owned subsidiaries, The Female Health Company - UK, plc and The Female Health Company (M) SDN.BHD. All significant intercompany transactions and accounts have been eliminated in consolidation. The Female Health Company (FHC or the Company) is currently engaged in the marketing, manufacture and distribution of a consumer health care product, the FC2 female condom (FC2). The Female Health Company - UK, is the holding company of The Female Health Company - UK, plc, which is located in a 6,400 sq. ft. leased office facility located in London, England (collectively the U.K. subsidiary). The Female Health Company (M) SDN.BHD leases a 45,800 sq. ft. manufacturing facility located in Selangor D.E., Malaysia (the Malaysia subsidiary).
FC2 has been distributed in either or both commercial (private sector) and public health sector markets in 144 countries. It is marketed to consumers through distributors, public health programs and retailers in 16 countries.
The Company's standard credit terms vary from 30 to 120 days, depending on the class of trade and customary terms within a territory, so accounts receivable is affected by the mix of purchasers within the period. As is typical in the Company's business, extended credit terms may occasionally be offered as a sales promotion or for certain sales. The Company has agreed to credit terms of up to 150 days with our distributor in the Republic of South Africa. For the most recent order of 15 million units under the Brazil tender, the Company has agreed to up to 360 day credit terms with our distributor in Brazil subject to earlier payment upon receipt of payment by the distributor from the Brazilian Government. For the past twelve months, the Company's average days’ sales outstanding was approximately 131 days. Over the past five years, the Company’s bad debt expense has been less than 0.03 percent of product sales.
Use of estimates: The preparation of financial statements requires management to make estimates and use assumptions that affect certain reported amounts and disclosures. Significant accounting estimates include the deferred income tax valuation allowance and the value of share-based compensation. Actual results may differ from those estimates.
Cash concentration: The Company’s cash is maintained primarily in three financial institutions, located in Clayton, Missouri, London, England and Kuala Lumpur, Malaysia, respectively.
Accounts receivable and concentration of credit risk: Accounts receivable are carried at original invoice amount less an estimate made for doubtful receivables based on a review of all outstanding amounts on a periodic basis. The components of accounts receivable consist of the following at September 30, 2015 and 2014:
|
2015 |
2014 |
|||||
|
Trade receivables |
$ |
13,975,905 |
$ |
2,814,558 | ||
|
Other receivables |
160,553 | 177,360 | ||||
|
Accounts receivable, gross |
14,136,458 | 2,991,918 | ||||
|
Less: allowance for doubtful accounts |
(48,068) | (48,068) | ||||
|
Accounts receivable, net |
$ |
14,088,390 |
$ |
2,943,850 | ||
The Company maintains an allowance for doubtful accounts for estimated losses resulting from the inability of its customers to make required payments on accounts receivable. Management determines the allowance for doubtful accounts by identifying troubled accounts and by using historical experience applied to an aging of accounts. Management also periodically evaluates individual customer receivables and considers a customer’s financial condition, credit history, and the current economic conditions. Accounts receivable are written-off when deemed uncollectible. The table below sets forth the components of the allowance for doubtful accounts for the years ended September 30:
|
Balance at |
Provision Charges |
Write offs/ |
Balance at |
||||||||
|
Year |
October 1 |
to Expenses |
Recoveries |
September 30 |
|||||||
|
2013 |
$ |
41,625 |
$ |
3,180 |
$ |
(31,625) |
$ |
13,180 | |||
|
2014 |
$ |
13,180 |
$ |
38,068 |
$ |
(3,180) |
$ |
48,068 | |||
|
2015 |
$ |
48,068 |
$ |
— |
$ |
— |
$ |
48,068 | |||
F-9
Recoveries of accounts receivable previously written-off are recorded when received. The Company’s customers are primarily large global agencies, non-government organizations, ministries of health and other governmental agencies which purchase and distribute the female condom for use in HIV/AIDS prevention and family planning programs. In fiscal year 2015, our significant customers were Semina Indústria e Comércio Ltda (Semina), United Nations Population Fund (UNFPA), and John Snow, Inc., facilitator of USAID I DELIVER project (USAID). In fiscal year 2014, our significant customers were UNFPA, USAID, Sekunjalo Investments Corporation (PTY) Ltd (Sekunjalo), and Azinor International Lda (Azinor). In fiscal year 2013, our significant customer was UNFPA. No other single customer accounted for more than 10 percent of unit sales during those periods.
|
Percentage of Unit Sales |
|||
|
Significant Customers |
2015 |
2014 |
2013 |
|
Semina |
47% |
* |
* |
|
UNFPA |
18% | 40% | 62% |
|
USAID |
16% | 17% |
* |
|
Sekunjalo |
* |
13% |
* |
|
Azinor |
* |
11% |
* |
|
Total Percentage of Unit Sales |
81% | 81% | 62% |
_____________________
* Less than 10 percent of unit sales.
Semina’s accounts receivable balance represented 46 percent of current assets at September 30, 2015 and UNFPA’s accounts receivable balance represented 12 percent of current assets at September 30, 2014. No other single customer’s accounts receivable balance accounted for more than 10 percent of current assets at the end of those periods.
Inventory: Inventories are valued at the lower of cost or market. The cost is determined using the first-in, first-out (FIFO) method. Inventories are also written down for management’s estimates of product which will not sell prior to its expiration date. Write-downs of inventories establish a new cost basis which is not increased for future increases in the market value of inventories or changes in estimated obsolescence.
Foreign currency translation and operations: Effective October 1, 2009, the Company determined that there were significant changes in facts and circumstances, triggering an evaluation of its subsidiaries’ functional currency. The evaluation indicated that the U.S. dollar is the currency with the most significant influence upon the subsidiaries. Because all of the U.K. subsidiary's future sales and cash flows would be denominated in U.S. dollars following the October 2009 cessation of production of the Company’s first generation product, FC1, the U.K. subsidiary adopted the U.S. dollar as its functional currency effective October 1, 2009. As the Malaysia subsidiary is a direct and integral component of the U.K. parent’s operations, it, too, adopted the U.S. dollar as its functional currency as of October 1, 2009. The consistent use of the U.S. dollar as functional currency across the Company reduces its foreign currency risk and stabilizes its operating results. The Company recognized a foreign currency transaction gain of $58,483 and a foreign currency transaction loss of $83,844 and $101,288 for the years ended September 30, 2015, 2014, and 2013, respectively. The cumulative foreign currency translation loss included in accumulated other comprehensive loss was $581,519 as of September 30, 2015 and 2014. Assets located outside of the U.S. totaled approximately $10,000,000 and $12,000,000 at September 30, 2015 and 2014, respectively.
Equipment, furniture and fixtures: Depreciation and amortization are computed using primarily the straight-line method. Depreciation and amortization are computed over the estimated useful lives of the respective assets which range as follows:
|
Manufacturing equipment |
5 – 10 years |
|
Office equipment |
3 years |
|
Furniture and fixtures |
7 – 10 years |
Depreciation on leased assets is computed over the lesser of the remaining lease term or the estimated useful lives of the assets. Depreciation on leased assets is included with depreciation on owned assets.
F-10
Patents and trademarks: The costs for patents and trademarks are expensed when incurred. FC2 patents have been issued by the United States, Europe, Canada, Australia, South Africa, the People’s Republic of China, Japan, Mexico, Brazil, India and the African Regional Intellectual Property Organization (ARIPO), which includes Botswana, Gambia, Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Sierra Leone, Sudan, Swaziland, Tanzania, Uganda, Zambia, and Zimbabwe. Further, the European patent for FC2 has been validated in the following countries: Austria, Belgium, Bulgaria, Switzerland, Republic of Cyprus, Czech Republic, Germany, Denmark, Estonia, Spain, Finland, France, United Kingdom, Greece, Hungary, Ireland, Italy, Luxembourg, Monaco, Netherlands, Portugal, Romania, Sweden, Slovenia, Slovakia, and Turkey. The patents cover the key aspects of FC2, including its overall design and manufacturing process. The patents have expiration dates in 2023 and 2024. In addition, patent applications for FC2 are pending in a number of other countries around the world. There can be no assurance that pending patent applications provide the Company with protection against copycat products entering markets during the pendency of the applications.
The Company has a registration for the trademark “FC2 Female Condom” in the United States. Furthermore, the Company has filed applications or secured registrations in 39 countries or jurisdictions around the world to protect the various names and symbols used in marketing FC2. In addition, the experience that has been gained through years of manufacturing its Female Condoms (FC1 and FC2) has allowed the Company to develop trade secrets and know-how, including certain proprietary production technologies, that further protect its competitive position.
Financial instruments: The Company follows ASC Topic 820, Fair Value Measurements and Disclosures, which defines fair value, establishes a framework for measuring fair value, and expands disclosures about fair value measurements. The fair value framework requires the categorization of assets and liabilities into three levels based upon the assumptions (inputs) used to price the assets or liabilities. Level 1 provides the most reliable measure of fair value, whereas Level 3 generally requires significant management judgment.
The Company currently does not have any assets or liabilities measured at fair value on a recurring or non-recurring basis. Substantially all of the Company’s cash, as well as restricted cash, are held in demand deposits with three financial institutions. The Company has no financial instruments for which the carrying value is materially different than fair value.
Research and development costs: Research and development costs are expensed as incurred. The amount of costs expensed for the years ended September 30, 2015, 2014, and 2013 were $219,815, $5,575, and $4,745, respectively.
Restricted cash: Restricted cash relates to security provided to one of the Company’s U.K. banks for performance bonds issued in favor of customers. The Company has a facility of $250,000 for such performance bonds. Such security has been extended infrequently and only on occasions where it has been a contract term expressly stipulated as an absolute requirement by the customer or its provider of funds. The expiration of the bond is defined by the completion of the event such as, but not limited to, a period of time after the product has been distributed or expiration of the product shelf life. Restricted cash was $85,697 and $55,806 for the years ended September 30, 2015 and 2014, respectively, and is included in cash on the accompanying balance sheets.
Revenue recognition: The Company recognizes revenue from product sales when each of the following conditions has been met: an arrangement exists, delivery has occurred, there is a fixed price, and collectability is reasonably assured.
Share-based compensation: The Company accounts for stock-based compensation expense for equity awards exchanged for services over the vesting period based on the grant-date fair value. In many instances, the equity awards are issued upon the grant date subject to vesting periods. In certain instances, the equity awards provide for future issuance contingent on future continued employment or performance of services as of the issuance date.
Advertising: The Company's policy is to expense advertising costs as incurred. Advertising costs were $0, $58,121, and $221,718 for the years ended September 30, 2015, 2014, and 2013, respectively.
Income taxes: The Company files separate income tax returns for its foreign subsidiaries. ASC Topic 740 requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined based on the differences between the financial statements and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. Deferred tax assets are also provided for carryforwards for income tax purposes. In addition, the amount of any future tax benefits is reduced by a valuation allowance to the extent such benefits are not expected to be realized.
Earnings per share (EPS): Basic EPS is computed by dividing net income by the weighted average number of common shares outstanding for the period. Diluted EPS is computed by dividing net income by the weighted average number of common shares outstanding during the period after giving effect to all dilutive potential common shares that were outstanding during the period. Dilutive potential common shares consist of the incremental common shares issuable upon the exercise of stock options and unvested shares granted to employees and directors.
F-11
Other comprehensive income: Accounting principles generally require that recognized revenue, expenses, gains and losses be included in net income. Although certain changes in assets and liabilities, such as foreign currency translation adjustments, are reported as a separate component of the equity section of the accompanying consolidated balance sheets, these items, along with net income, are components of comprehensive income.
The U.S. parent company and its U.K. subsidiary routinely purchase inventory produced by its Malaysia subsidiary for sale to their respective customers. These intercompany trade accounts are eliminated in consolidation. The Company’s policy and intent is to settle the intercompany trade account on a current basis. Since the U.K. and Malaysia subsidiaries adopted the U.S. dollar as their functional currencies effective October 1, 2009, no foreign currency gains or losses from intercompany trade are recognized. In fiscal 2015, 2014, and 2013, comprehensive income is equivalent to the reported net income.
Reclassifications: Certain items in the 2014 and 2013 consolidated financial statements have been reclassified to conform to the 2015 presentation.
Note 2.Earnings per Share
Basic EPS is computed by dividing net income by the weighted average number of common shares outstanding for the period. Diluted EPS is computed by dividing net income by the weighted average number of common shares outstanding during the period after giving effect to all dilutive potential common shares that were outstanding during the period. Dilutive potential common shares consist of the incremental common shares issuable upon the exercise of stock options and unvested shares granted to employees and directors.
|
Year Ended September 30, |
||||||||
|
Denominator |
2015 |
2014 |
2013 |
|||||
|
Weighted average common shares outstanding - basic |
28,532,327 | 28,522,525 | 28,376,607 | |||||
|
Net effect of dilutive securities: |
||||||||
|
Options |
50,473 | 109,583 | 162,195 | |||||
|
Unvested restricted shares |
334,248 | 233,276 | 187,676 | |||||
|
Total net effect of dilutive securities |
384,721 | 342,859 | 349,871 | |||||
|
Weighted average common shares outstanding - diluted |
28,917,048 | 28,865,384 | 28,726,478 | |||||
|
Income per common share – basic |
$ |
0.15 |
$ |
0.09 |
$ |
0.51 | ||
|
Income per common share – diluted |
$ |
0.15 |
$ |
0.08 |
$ |
0.50 | ||
Options to purchase approximately 90,000 shares of common stock at an exercise price of $3.92 per share that were outstanding for the year end September 30, 2015, were not included in the computation of diluted net income per share because their effect was anti-dilutive. All other outstanding stock options were included in the computation of diluted net income per share for the years ended September 30, 2015, 2014, and 2013.
Note 3.Inventory
The components of inventory consist of the following at September 30, 2015 and 2014:
|
2015 |
2014 |
||||
|
Raw material |
$ |
839,179 |
$ |
1,091,703 | |
|
Work in process |
77,483 | 15,962 | |||
|
Finished goods |
868,270 | 1,936,655 | |||
|
Inventory, gross |
1,784,932 | 3,044,320 | |||
|
Less: inventory reserves |
(39,752) | (60,873) | |||
|
Inventory, net |
$ |
1,745,180 |
$ |
2,983,447 | |
F-12
The change in the inventory reserve for the years ended September 30 is as follows:
|
Balance at |
Charged to Costs |
Balance at |
|||||||||
|
Year |
October 1 |
and Expenses |
Write-offs |
September 30 |
|||||||
|
2013 |
$ |
49,810 |
$ |
(6,662) |
$ |
(2,015) |
$ |
41,133 | |||
|
2014 |
$ |
41,133 |
$ |
37,603 |
$ |
(17,863) |
$ |
60,873 | |||
|
2015 |
$ |
60,873 |
$ |
173,634 |
$ |
(194,755) |
$ |
39,752 | |||
Note 4.Revolving Line of Credit
On August 1, 2015, the Company entered into an amendment to the Second Amended and Restated Loan Agreement (as amended, the “Loan Agreement”) with Midland States Bank to extend the term of the Company’s revolving line of credit to August 1, 2016. The credit facility consists of a single revolving note for up to $2 million with Midland States Bank, with borrowings limited to a borrowing base determined based on 70 percent to 80 percent of eligible accounts receivable plus 50 percent of eligible inventory. Significant restrictive covenants in the Loan Agreement include prohibitions on any merger, consolidation or sale of all or a substantial portion of the Company’s assets and limits on the payment of dividends or the repurchase of shares. The Loan Agreement does not contain any financial covenants that require compliance with ratios or amounts. Dividends and share repurchases are permitted as long as after giving effect to the dividend or share repurchase the Company has a ratio of total liabilities to total stockholders’ equity of no more than 1:1. Borrowings on the revolving note bear interest at the national prime rate published by the Wall Street Journal (3.25 percent at September 30, 2015). The note is collateralized by substantially all of the assets of the Company. No amounts were outstanding under the revolving note at either September 30, 2015 or 2014.
Note 5.Operating Leases and Rental Expense
The Company’s corporate headquarters is located in approximately 5,100 square feet of office space located in Chicago, Illinois. On March 10, 2011, the Company signed a lease amendment, effective November 1, 2010, which extended the lease term for this office space for a five year period commencing on November 1, 2011 and ending on October 31, 2016. The lease amendment grants the Company a five month lease abatement beginning November 1, 2010, reduces base rent and provides a tenant improvement allowance. The lease requires escalating monthly payments ranging from $6,797 to $7,859, plus real estate taxes, utilities and maintenance expenses from April 1, 2011 to October 31, 2016. The Company has delivered notice of its intent to extend the term of the lease for an additional three year period ending October 31, 2019. The Company has the right to rescind such lease extension upon receipt of the landlord’s notice of the new base rent for the extended term.
The Company leases 6,400 square feet of office space located in London, England. The lease expires in June 2020. The lease requires quarterly payments of approximately $13,500 through December 2011 and quarterly payments of approximately $27,000 from January 2012 through June 2015. The lease stipulated that after 5 years (June 2015) the principal rent will be reviewed and adjusted to the higher of the principal rent immediately prior to the review date or the market rate. As of September 30, 2015, the lease has not been reviewed or adjusted and quarterly payments of approximately $27,000 will continue. Based on the terms of the lease agreement, the Company was also required to make a security deposit equivalent to six months’ rent (approximately $67,000).
The Company leases 45,800 square feet of manufacturing space in Selangor D.E., Malaysia under a lease that requires monthly payments of approximately $13,000 through September 2016 and may be renewed at the option of the Company for an additional three year term.
The Company also leases equipment under a number of lease agreements which expire at various dates through June 2020. The aggregate monthly rental was $616 at September 30, 2015. Details of operating lease expense, including real estate taxes and insurance, for the years ended September 30, 2015, 2014, and 2013 are as follows:
|
2015 |
2014 |
2013 |
||||||
|
Factory and office leases |
$ |
470,049 |
$ |
439,722 |
$ |
404,678 | ||
|
Other |
7,387 | 4,758 | 5,541 | |||||
|
Total |
$ |
477,436 |
$ |
444,480 |
$ |
410,219 | ||
F-13
Future minimum payments under leases consist of the following as of September 30, 2015:
|
Operating |
||
|
Leases |
||
|
2016 |
371,528 | |
|
2017 |
131,032 | |
|
2018 |
123,002 | |
|
2019 |
122,491 | |
|
2020 |
91,919 | |
|
Total minimum lease payments |
$ |
839,972 |
Note 6.Income Taxes
The Company accounts for income taxes using the liability method, which requires the recognition of deferred tax assets or liabilities for the tax-effected temporary differences between the financial reporting and tax bases of assets and liabilities, and for net operating loss and tax credit carryforwards.
The Company completes a detailed analysis of its deferred income tax valuation allowance on an annual basis or more frequently if information comes to our attention that would indicate that a revision to its estimates is necessary. In evaluating the Company’s ability to realize its deferred tax assets, management considers all available positive and negative evidence on a country by country basis, including past operating results and forecast of future taxable income. In determining future taxable income, management makes assumptions to forecast U.S. federal and state, U.K. and Malaysia operating income, the reversal of temporary differences, and the implementation of any feasible and prudent tax planning strategies. These assumptions require significant judgment regarding the forecasts of the future taxable income in each tax jurisdiction, and are consistent with the forecasts used to manage the Company’s
business. It should be noted that the Company realized significant losses through 2005 on a consolidated basis. Since fiscal year 2006, the Company has consistently generated taxable income on a consolidated basis, providing a reasonable future period in which the Company can reasonably expect to generate taxable income. In management’s analysis to determine the amount of the deferred tax asset to recognize, management projected future taxable income for each tax jurisdiction.
Although management uses the best information available, it is reasonably possible that the estimates used by the Company will be materially different from the actual results. These differences could have a material effect on the Company's future results of operations and financial condition.
Income before income taxes was taxed by the following jurisdictions for the years ended September 30, 2015, 2014, and 2013:
|
2015 |
2014 |
2013 |
||||||
|
Domestic |
$ |
4,524,499 |
$ |
2,837,835 |
$ |
7,461,329 | ||
|
Foreign |
2,162,541 | 1,119,356 | 2,472,525 | |||||
|
Total |
$ |
6,687,040 |
$ |
3,957,191 |
$ |
9,933,854 | ||
A reconciliation of income tax expense (benefit) and the amount computed by applying the statutory Federal income tax rate to income before income taxes for the years ended September 30, 2015, 2014, and 2013 is as follows:
|
2015 |
2014 |
2013 |
||||||
|
Income tax expense at statutory rates |
$ |
2,274,000 |
$ |
1,345,000 |
$ |
3,378,000 | ||
|
State income tax, net of federal benefits |
362,000 | 248,000 | 623,000 | |||||
|
Non-deductible expenses |
51,000 | (5,000) | 129,000 | |||||
|
Effect of lower foreign income tax rates |
(351,244) | (175,632) | (395,441) | |||||
|
Effect of change in U.K. tax rate |
— |
— |
(159,000) | |||||
|
Effect of reinvestment allowance - Malaysia |
— |
(9,000) | (75,000) | |||||
|
Effect of export allowance - Malaysia |
(85,000) |
— |
— |
|||||
|
Effect of change in Illinois tax rate |
202,000 |
— |
— |
|||||
|
Effect of conversion of charitable contribution to NOL |
(36,174) |
— |
— |
|||||
|
Other |
(59,578) | 56,762 |
— |
|||||
|
Change in valuation allowance |
(16,000) | 64,000 | (7,909,303) | |||||
|
Income tax expense (benefit) |
$ |
2,341,004 |
$ |
1,524,130 |
$ |
(4,408,744) | ||
F-14
As of September 30, 2015, the Company had federal and state net operating loss carryforwards of approximately $13,023,000 and $12,587,000, respectively, for income tax purposes expiring in years 2020 to 2027. The Company's U.K. subsidiary has U.K. net operating loss carryforwards of approximately $61,938,000 as of September 30, 2015, which can be carried forward indefinitely to be used to offset future U.K. taxable income.
The federal and state income tax expense (benefit) for the years ended September 30, 2015, 2014, and 2013 is summarized below:
|
2015 |
2014 |
2013 |
||||||
|
Deferred – U.S. |
$ |
1,856,000 |
$ |
561,000 |
$ |
(12,000) | ||
|
Deferred – U.K. |
162,000 | 496,000 | (5,288,000) | |||||
|
Deferred – Malaysia |
(92,261) | (44,666) | 40,935 | |||||
|
Subtotal |
1,925,739 | 1,012,334 | (5,259,065) | |||||
|
Current – U.S. |
83,606 | 219,000 | 625,606 | |||||
|
Current – Malaysia |
331,659 | 292,796 | 221,625 | |||||
|
Current - U.K. |
— |
— |
3,090 | |||||
|
Subtotal |
415,265 | 511,796 | 850,321 | |||||
|
Income tax expense (benefit) |
$ |
2,341,004 |
$ |
1,524,130 |
$ |
(4,408,744) | ||
Significant components of the Company's deferred tax assets and liabilities are as follows at September 30, 2015 and 2014:
|
Deferred Tax Assets |
2015 |
2014 |
|||
|
Federal net operating loss carryforwards |
$ |
4,428,000 |
$ |
5,871,000 | |
|
State net operating loss carryforwards |
644,000 | 1,067,000 | |||
|
AMT credit carryforward |
390,000 | 301,000 | |||
|
Foreign net operating loss carryforwards – U.K. |
12,388,000 | 12,574,000 | |||
|
Foreign capital allowance – U.K. |
114,000 | 110,000 | |||
|
Other, net - Malaysia |
13,097 | 30,153 | |||
|
Share-based compensation |
128,000 | 204,000 | |||
|
Other, net - U.S. |
8,000 | 7,000 | |||
|
Gross deferred tax assets |
18,113,097 | 20,164,153 | |||
|
Valuation allowance for deferred tax assets |
(2,575,000) | (2,591,000) | |||
|
Net deferred tax assets |
15,538,097 | 17,573,153 | |||
|
Deferred Tax Liabilities: |
|||||
|
Foreign capital allowance – Malaysia |
(111,349) | (220,666) | |||
|
Net deferred tax assets |
$ |
15,426,748 |
$ |
17,352,487 | |
The deferred tax amounts have been classified in the accompanying consolidated balance sheets at September 30 as follows:
|
2015 |
2014 |
||||
|
Current assets – U.S. |
$ |
854,000 |
$ |
711,000 | |
|
Current assets – U.K. |
162,000 |
— |
|||
|
Total current assets |
1,016,000 | 711,000 | |||
|
Long-term assets – U.S. |
4,740,000 | 6,739,000 | |||
|
Long-term assets – U.K |
9,769,000 | 10,093,000 | |||
|
Total long-term assets |
14,509,000 | 16,832,000 | |||
|
Long-term liability – Malaysia |
(98,252) | (190,513) | |||
|
$ |
15,426,748 |
$ |
17,352,487 | ||
F-15
The change in the valuation allowance for deferred tax assets for the years ended September 30 is as follows:
|
Balance at |
Charged to Costs |
Balance at |
|||||||||
|
Year |
October 1 |
and Expenses |
Deductions/Other |
September 30 |
|||||||
|
2013 |
$ |
12,600,000 |
$ |
(5,300,000) |
$ |
(5,153,000) |
$ |
2,147,000 | |||
|
2014 |
$ |
2,147,000 |
$ |
432,000 |
$ |
12,000 |
$ |
2,591,000 | |||
|
2015 |
$ |
2,591,000 |
$ |
(16,000) |
$ |
— |
$ |
2,575,000 | |||
The valuation allowance decreased by $16,000, increased by $444,000, and decreased by $10,453,000 for the years ended September 30, 2015, 2014, and 2013, respectively. Under the Internal Revenue Code, certain ownership changes, including the prior issuance of preferred stock, the public offering of common stock and the exercise of common stock warrants and options may subject the Company to annual limitations on the utilization of its net operating loss carryforward. Under the Inland Revenue statutes, certain triggering events may subject the Company to limitations on the utilization of its net operating loss carryforward in the U.K. As of September 30, 2015, management does not believe any limitations have occurred.
The Company has not recorded deferred income taxes applicable to undistributed earnings of foreign subsidiaries because it is the present intention of management to reinvest the undistributed earning indefinitely. Generally such earnings become subject to U.S. tax upon remittance of dividends and under certain other circumstances. It is not practicable to estimate the amount of deferred tax or such undistributed earnings.
ASC Topic 740 prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740 developed a two-step process to evaluate a tax position and also provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Company has not recorded a reserve for any tax positions for which the ultimate deductibility is highly certain but for which there is uncertainty about the timing of such deductibility.
The Company files tax returns in all appropriate jurisdictions, including foreign, U.S. Federal and Illinois and Virginia State tax returns. The following summarizes open tax years in the relevant jurisdictions:
|
· |
For the U.S., a tax return may be audited any time within 3 years from filing date. The U.S. open tax years are for fiscal years 2012 through 2014, which expire in years 2016 through 2018, respectively. |
|
· |
For Malaysia, a tax return may be audited any time within 5 years from filing date (7 months after the fiscal year end). The Malaysia open tax years are for 2010 through 2014, which expire on December 31, 2015 through 2019. |
|
· |
For the U.K., a tax return may be audited within 1 year from the later of: the filing date or the filing deadline (1 year after the end of the accounting period). The U.K. open tax year is for 2014, which expires in 2016. |
The fiscal year 2015 tax returns for each jurisdiction have not been filed as of the date of this filing. As of September 30, 2015 and 2014, the Company has no recorded liability for unrecognized tax benefits.
The Company recognizes interest and penalties related to uncertain tax positions as income tax expense as incurred. No expense for interest and penalties was recognized for the years ended September 30, 2015, 2014, and 2013.
Note 7.Equity and Share-based Payments
In March 2008, the Company’s shareholders approved the 2008 Stock Incentive Plan which is utilized to provide equity opportunities and performance–based incentives to attract, retain and motivate those persons who make (or are expected to make) important contributions to the Company. A total of 2 million shares are available for issuance under the plan. As of September 30, 2015, a total of 1,287,885 shares have been granted under the plan, of which 150,000 shares were in the form of stock options and the remainder were in the form of restricted stock or other share grants.
F-16
Stock Option Plans
Under the Company’s previous share-based long-term incentive compensation plan, the 1997 Stock Option Plan, the Company granted non-qualified stock options to employees. There are no shares available for grant under this plan which expired on December 31, 2006. Options issued under this plan expire 10 years after the date of grant and generally vested 1/36 per month, with full vesting after three years. Under the Company’s 2008 Stock Incentive Plan, options issued expire 10 years after the date of grant and vest 1/36 per month, with full vesting after three years. The Company did not grant any options during the years ended September 30, 2015, 2014, and 2013.
Compensation expense is recognized only for share-based payments expected to vest. The Company estimates forfeitures at the date of grant based on historical experience and future expectations. There was no stock compensation expense related to options for the years ended September 30, 2015, 2014, and 2013.
Option Activity
The following table summarizes the stock options outstanding and exercisable at September 30, 2015:
|
Weighted Average |
||||||||
|
Remaining |
Aggregate |
|||||||
|
Exercise Price |
Contractual Term |
Intrinsic |
||||||
|
Shares |
Per Share |
(years) |
Value |
|||||
|
Outstanding at September 30, 2012 |
276,250 |
$ |
2.57 | |||||
|
Granted |
— |
- |
||||||
|
Exercised |
(36,250) | 2.05 | ||||||
|
Forfeited |
— |
- |
||||||
|
Outstanding at September 30, 2013 |
240,000 |
$ |
2.64 | |||||
|
Granted |
— |
— |
||||||
|
Exercised |
(60,000) | 2.79 | ||||||
|
Forfeited |
— |
— |
||||||
|
Outstanding at September 30, 2014 |
180,000 |
$ |
2.60 | |||||
|
Granted |
— |
— |
||||||
|
Exercised |
— |
— |
||||||
|
Forfeited |
— |
— |
||||||
|
Outstanding at September 30, 2015 |
180,000 |
$ |
2.60 | 2.34 |
$ |
27,900 | ||
|
Exercisable on September 30, 2015 |
180,000 |
$ |
2.60 | 2.34 |
$ |
27,900 | ||
No stock options were exercised during the year ended September 30, 2015. During the year ended September 30, 2014, stock option holders exercised 60,000 stock options, 30,000 shares using the cashless exercise option available under the plan which entitled them to 16,963 shares of common stock and 30,000 shares using the cash exercise option available under the plan resulting in cash proceeds of $117,600. During the year ended September 30, 2013, stock option holders exercised 36,250 stock options, using the cashless exercise option available under the plan which entitled them to 28,172 shares of common stock.
The aggregate intrinsic value in the table above is before income taxes, based on the Company’s closing stock price of $1.58 on the last day of business for the period ended September 30, 2015. The total intrinsic value of options exercised during the years ended September 30, 2015, 2014, and 2013, was approximately $0, $154,000 and $272,000, respectively.
Restricted Stock
The Company issues restricted stock to employees, directors and consultants. Such issuances may have vesting periods that range from one to three years. In addition, the Company has issued stock awards to certain employees and directors that provide for future issuance contingent on continued employment or performance of services for periods that range from one to three years.
F-17
A summary of the non-vested stock activity for fiscal years 2015, 2014, and 2013 is summarized in the table below:
|
Weighted Average |
||||||
|
Grant -Date |
||||||
|
Shares |
Fair Value |
Vesting Period |
||||
|
Total Outstanding September 30, 2012 |
93,818 |
$ |
5.59 | |||
|
Stock Granted |
64,676 | 7.29 |
September 2013 - May 2016 |
|||
|
Vested |
(117,992) | 6.17 | ||||
|
Forfeited |
(7,000) | 5.80 | ||||
|
Total Outstanding September 30, 2013 |
33,502 |
$ |
6.80 | |||
|
Stock Granted |
213,576 | 7.80 |
September 2014 - December 2016 |
|||
|
Vested |
(105,393) | 8.15 | ||||
|
Forfeited |
(250) | 9.68 | ||||
|
Total Outstanding September 30, 2014 |
141,435 |
$ |
7.30 | |||
|
Stock Granted |
293,500 | 1.70 |
September 2015 - August 2018 |
|||
|
Vested |
(92,963) | 4.70 | ||||
|
Forfeited |
(58,250) | 7.36 | ||||
|
Total Outstanding September 30, 2015 |
283,722 |
$ |
2.31 | |||
The Company granted a total of 293,500, 213,576 and 64,676 shares of restricted stock or shares issuable pursuant to promises to issue shares of common stock during the years ended September 30, 2015, 2014, and 2013, respectively. The stock granted during the year ended September 30, 2015 includes rights to receive a total of 83,000 shares, or at a holder’s election cash based on the fair market value of the shares, contingent on continued employment or service. The fair value of the awards granted was approximately $499,000, $1,665,000 and $471,000 for the years ended September 30, 2015, 2014, and 2013, respectively. All such shares of restricted stock vest and all such shares must be issued pursuant to the vesting period noted, provided the grantee has not voluntarily terminated service or been terminated for cause prior to the vesting or issuance date. There were 58,250, 250 and 7,000 shares of restricted stock forfeited during the years ended September 30, 2015, 2014, and 2013, respectively.
The Company recognized the fair value of the restricted stock or promises to issue shares of common stock that vested during the fiscal year as share-based compensation expense of approximately $437,000, $859,000 and $728,000 for the years ended September 30, 2015, 2014, and 2013, respectively, $23,000, $256,000 and $227,000 of which was included in accrued expenses at year end since the related shares have not yet been issued at September 30, 2015, 2014, and 2013, respectively. For the year ended September 30, 2015, the Company issued a portion of the executive bonus in stock for a total share-based compensation expense of approximately $53,000. The share-based compensation expense was included in selling, general and administrative expenses for the respective periods. The Company recorded a tax benefit for stock-based compensation expenses of approximately $114,000, $204,000 and $0 for the years ended September 30, 2015, 2014, and 2013, respectively. The Company realized the tax benefit for stock-based compensation expenses, for the shares which vested, of approximately $190,000, $0 and $0 for the years ended September 30, 2015, 2014, and 2013, respectively. As of September 30, 2015, there was approximately $655,000, representing approximately 284,000 unvested shares, of total unrecognized compensation cost related to non-vested restricted stock compensation arrangements granted under the incentive plans. This unrecognized cost will be recognized over the weighted average period of the next 2.64 years.
Common Stock Purchase Warrants
The Company did not issue any common stock purchase warrants in fiscal year 2015, 2014, or 2013. In fiscal year 2013, a warrant holder exercised 52,000 warrants using the cashless exercise option available within the warrant agreements which entitled the warrant holder to 43,465 shares of common stock. There is no unrecognized compensation cost related to warrants as of September 30, 2015.
At September 30, 2015 and 2014, there were no outstanding warrants.
Preferred Stock
The Company has 5,000,000 shares designated as Class A Preferred Stock with a par value of $.01 per share. There are 1,040,000 shares of Class A Preferred Stock - Series 1 authorized; 1,500,000 shares of Class A Preferred Stock- Series 2 authorized; and 700,000 shares of Class A Preferred Stock - Series 3 authorized. There were no shares of Class A Preferred Stock of any series issued and outstanding in fiscal 2015 or 2014. The Company has 15,000 shares designated as Class B Preferred Stock with a par value of $.50 per share. There were no shares of Class B Preferred Stock issued and outstanding in fiscal 2015 or 2014.
F-18
Note 8.Stock Repurchase Program
The Company’s Stock Repurchase Program was announced on January 17, 2007. At initiation, the plan’s terms specified that up to 1,000,000 shares of its common stock could be purchased during the subsequent twelve months. Subsequently, the Board has amended the plan a number of times to both extend its term and increase the maximum number of shares which could be repurchased. Currently, the plan allows for a maximum repurchase of up to 3,000,000 shares through the period ending December 31, 2015. From the program’s onset through September 30, 2015, the total number of shares repurchased by the Company is 2,183,704. The Stock Repurchase Program authorizes purchases in privately negotiated transactions as well as in the open market. In October 2008, the Company's Board of Directors authorized repurchases in private transactions under the Stock Repurchase Program of shares issued under the Company's equity compensation plans to directors, employees and other service providers at the market price on the effective date of the repurchase request. Total repurchases under this provision currently are limited to an aggregate of 450,000 shares per calendar year and to a maximum of 50,000 shares annually per individual. Total repurchase transaction are as follows (in shares):
|
2015 |
2014 |
2013 |
|
|
Open market repurchase transactions |
- |
165,000 | 10,000 |
|
Private repurchase transactions |
250 | 4,000 | 45,625 |
|
Total repurchase transactions |
250 | 169,000 | 55,625 |
Total repurchase activity is as follows:
|
Issuer Purchases of Equity |
||||||
|
Securities: |
Details of Treasury Stock Purchases to Date through September 30, 2015: |
|||||
|
Total |
Average |
Aggregate Number |
Maximum Number |
|||
|
Number |
Price Paid |
of Shares Purchased |
of Shares that May |
|||
|
of Shares |
Per |
As Part of Publicly |
Yet be Purchased |
|||
|
Period |
Purchased |
Share |
Announced Program |
Under the Program |
||
|
January 1, 2007 – September 30, 2012 |
1,958,829 |
$ |
3.41 | 1,958,829 | 1,041,171 | |
|
October 1, 2012 – September 30, 2013 |
55,625 | 7.11 | 2,014,454 | 985,546 | ||
|
October 1, 2013 – September 30, 2014 |
169,000 | 4.37 | 2,183,454 | 816,546 | ||
|
October 1, 2014 – September 30, 2015 |
250 | 3.80 | 2,183,704 | 816,296 | ||
|
Total |
2,183,704 |
$ |
3.57 | 2,183,704 | 816,296 | |
Note 9.Employee Benefit Plan
The Company has a Simple Individual Retirement Account (IRA) plan for its employees. Employees are eligible to participate in the plan if their compensation reaches certain minimum levels and are allowed to contribute up to a maximum of $15,500 annual compensation to the plan. The Company has elected to match 100 percent of employee contributions to the plan up to a maximum of 3 percent of employee compensation for the years ended September 30, 2015, 2014, and 2013. Annual Company contributions were approximately $37,000, $31,000, and $29,000 for the years ended September 30, 2015, 2014, and 2013, respectively.
In March 2014, the Company elected to contribute 3 percent into the personal pension schemes of certain senior U.K. employees. Contributions for the years ended September 30, 2015 and 2014 were approximately $26,000 and $6,000, respectively. Pension contributions were not made for the certain senior U.K. employees for the year ended September 30, 2013.
Note 10.Industry Segments and Financial Information about Foreign and Domestic Operations
The Company currently operates in one industry segment which includes the development, manufacture and marketing of consumer health care products.
F-19
The Company operates in foreign and domestic regions. Information about the Company's operations by geographic area is as follows (in thousands).
|
Net Revenues to External Customers for |
Long-Lived Asset As Of |
||||||||||||||||
|
the Year Ended September 30, |
September 30, |
||||||||||||||||
|
2015 |
2014 |
2013 |
2015 |
2014 |
|||||||||||||
|
Brazil |
$ |
14,841 |
(1) |
$ |
* |
$ |
4,480 |
(1) |
$ |
- |
$ |
- |
|||||
|
Zimbabwe |
2,696 | 2,064 |
* |
- |
- |
||||||||||||
|
South Africa |
2,331 | 2,928 |
(1) |
5,421 |
(1) |
- |
- |
||||||||||
|
United States |
2,029 | 2,381 | 2,611 | 123 | 88 | ||||||||||||
|
Angola |
* |
2,477 |
(1) |
* |
- |
- |
|||||||||||
|
DR of Congo |
* |
2,185 | 2,467 |
- |
- |
||||||||||||
|
Tanzania |
* |
1,936 |
* |
- |
- |
||||||||||||
|
Nigeria |
* |
* |
2,879 |
- |
- |
||||||||||||
|
Uganda |
* |
* |
2,997 |
- |
- |
||||||||||||
|
Malaysia |
* |
* |
* |
1,134 | 1,528 | ||||||||||||
|
United Kingdom |
* |
* |
* |
120 | 152 | ||||||||||||
|
Other |
10,708 | 10,520 | 10,602 |
- |
- |
||||||||||||
|
Total |
$ |
32,605 |
$ |
24,491 |
$ |
31,457 |
$ |
1,377 |
$ |
1,768 | |||||||
* Less than 5 percent of total net revenues.
(1) Exceeds 10 percent of total net revenues.
Note 11.Contingent Liabilities
The testing, manufacturing and marketing of consumer products by the Company entail an inherent risk that product liability claims will be asserted against the Company. The Company maintains product liability insurance coverage for claims arising from the use of its products. The coverage amount is currently $10 million for FHC's consumer health care product.
Note 12.Dividends
Beginning February 16, 2010 through May 7, 2014, the Company paid 18 quarterly cash dividends. The first 9 were paid at a quarterly rate per share of $0.05 through February 9, 2012, 4 were paid at a quarterly rate per share of $0.06 from May 9, 2012 through February 6, 2013, and 5 were paid at a quarterly rate per share of $0.07 from May 8, 2013 through May 7, 2014. Cash dividends paid totaled $29.4 million through September 30, 2014. The Company paid cash dividends of approximately $6.1 million and $7.5 million in 2014 and 2013, respectively. On July 14, 2014, the Company announced that its Board of Directors elected to suspend the payment of quarterly cash dividends in order to devote operating cash flows towards strategic growth initiatives.
F-20
Note 13.Quarterly Financial Data (Unaudited)
|
First |
Second |
Third |
Fourth |
Year |
||||||||||
|
Quarter |
Quarter |
Quarter |
Quarter |
Ended |
||||||||||
|
2015 |
||||||||||||||
|
Net revenues |
$ |
6,659,206 |
$ |
10,977,467 |
$ |
7,813,207 |
$ |
7,154,985 |
$ |
32,604,865 | ||||
|
Gross profit |
3,819,673 | 6,394,107 | 4,632,535 | 4,123,644 | 18,969,959 | |||||||||
|
Operating expenses |
2,365,824 | 3,444,714 | 3,178,687 | 3,362,327 | 12,351,552 | |||||||||
|
Income tax expense |
670,430 | 1,306,445 | 284,900 | 79,229 | 2,341,004 | |||||||||
|
Net income |
804,917 | 1,667,574 | 1,170,974 | 702,571 | 4,346,036 | |||||||||
|
Net income per common share – basic |
0.03 | 0.06 | 0.04 | 0.02 | 0.15 | |||||||||
|
Net income per common share – diluted |
0.03 | 0.06 | 0.04 | 0.02 | 0.15 | |||||||||
|
2014 |
||||||||||||||
|
Net revenues |
$ |
6,690,195 |
$ |
4,346,223 |
$ |
7,900,055 |
$ |
5,554,113 |
$ |
24,490,586 | ||||
|
Gross profit |
3,678,494 | 2,385,941 | 4,170,270 | 2,886,773 | 13,121,478 | |||||||||
|
Operating expenses |
2,094,858 | 1,590,718 | 2,142,640 | 3,369,350 | 9,197,566 | |||||||||
|
Income tax expense |
98,875 | 504,898 | 851,321 | 69,036 | 1,524,130 | |||||||||
|
Net income (loss) |
1,464,603 | 375,081 | 1,159,498 | (566,121) | 2,433,061 | |||||||||
|
Net income (loss) per common share – basic |
0.05 | 0.01 | 0.04 | (0.02) | 0.09 | |||||||||
|
Net income (loss) per common share – diluted |
0.05 | 0.01 | 0.04 | (0.02) | 0.08 | |||||||||
F-21
