Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - POZEN INC /NC | a15-22599_1ex99d1.htm |
| 8-K - 8-K - POZEN INC /NC | a15-22599_18k.htm |
Exhibit 99.2
POZEN Corporate Overview 3Q 2015 Financial Results November 9, 2015
This presentation contains forward-looking statements under applicable securities laws, including, but not limited to, statements related to the anticipated consummation of the business combination transaction among Aralez, POZEN and Tribute and the timing and benefits thereof, the anticipated equity and debt financings and the closings thereof, the combined company's strategy, plans, objectives, expectations (financial or otherwise) and intentions, future financial results and growth potential, anticipated product portfolio, development programs and management structure, the proposed listing on the NASDAQ and TSX and other statements that are not historical facts. These forward-looking statements are based on POZEN's current assumptions and expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward looking statements as a result of these risks and uncertainties, which include, without limitation, risks related to the parties ability to complete the combination and anticipated equity and debt financings on the proposed terms and schedule the combined company meeting the listing requirements on the NASDAQ and TSX; risk that Aralez may be taxed as a U.S. resident corporation; risks associated with business combination transactions, such as the risk that the businesses will not be integrated successfully, that such integration may be more difficult, time-consuming or costly than expected or that the expected benefits of the transaction will not occur; risks related to future opportunities and plans for the combined company, including uncertainty of the expected financial performance and results of the combined company following completion of the proposed transaction; disruption from the proposed transaction, making it more difficult to conduct business as usual or maintain relationships with customers, employees or suppliers; the calculations of, and factors that may impact the calculations of, the acquisition price in connection with the proposed merger and the allocation of such acquisition price to the net assets acquired in accordance with applicable accounting rules and methodologies; and the possibility that if the combined company does not achieve the perceived benefits of the proposed transaction as rapidly or to the extent anticipated by financial analysts or investors, the market price of the combined company's shares could decline, as well as other risks related to POZEN's and Tribute’s business, including POZEN's inability to build, acquire or contract with a sales force of sufficient scale for the commercialization of YOSPRALA™ in a timely and cost-effective manner, the parties’ failure to successfully commercialize our product candidates; costs and delays in the development and/or FDA approval of our product candidates (including YOSPRALA), including as a result of the need to conduct additional studies or due to issues with third-party manufacturers, or the failure to obtain such approval of POZEN’s or Tribute’s product candidates for all expected indications, including as a result of changes in regulatory standards or the regulatory environment during the development period of any of its product candidates; the inability to maintain or enter into, and the risks resulting from POZEN’s dependence upon, collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution of any products, including its dependence on AstraZeneca and Horizon for the sales and marketing of VIMOVO®, POZEN’s dependence on Patheon for the manufacture of YOSPRALA 81/40 and YOSPRALA 325/40 ; the ability of POZEN and Tribute to protect their intellectual property and defend their patents; regulatory obligations and oversight; and those risks detailed from time-to-time under the caption "Risk Factors" and elsewhere in POZEN's SEC filings and reports, including in its Annual Report on Form 10-K for the year ended December 31, 2014 and any subsequent Quarterly Reports on Form 10-Q, in Tribute’s SEC filings and reports, including in its Annual Report on Form 10-K for the year ended December 31, 2014 and in the registration statement on Form S-4 filed by Aralez on July 20, 2015, as amended on August 19, 20156 and October 30, 2015. We undertake no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or changes in their expectations. This communication does not constitute an offer to sell, or the solicitation of an offer to sell, or the solicitation of an offer to subscribe for or buy, any securities nor shall there be any sale, issuance or transfer of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. Disclaimer 2
Topics to Cover Key Financial Highlights Aralez Strategic Rationale & Merger Status Fibricor® and YOSPRALA™ Commercialization Strategy Business Development Framework and Focus Short Term Corporate Priorities Question & Answer Session 3
$ millions Q3 2014 Q1 2015 Q2 2015 Q3 2015 Q3 vs. Q2 2015 Q3 vs. Q3 2014 VIMOVO® Royalty $5.5 $4.4 $5.2 $5.8 12% 5% Quarterly TRx (000’s)* 82.5 70.7 99.4 107.0 8% 30% License Fee $2.0 Total Revenue $7.5 $4.4 $5.2 $5.8 12% (23%) POZEN Revenue ROW VIMOVO Royalty rate increases from 6% to 10% in 2016 License fee = amortization of upfront payment from Sanofi in prior year 4 *Symphony Health Phast Monthly Rx Audit for US prescriptions only.
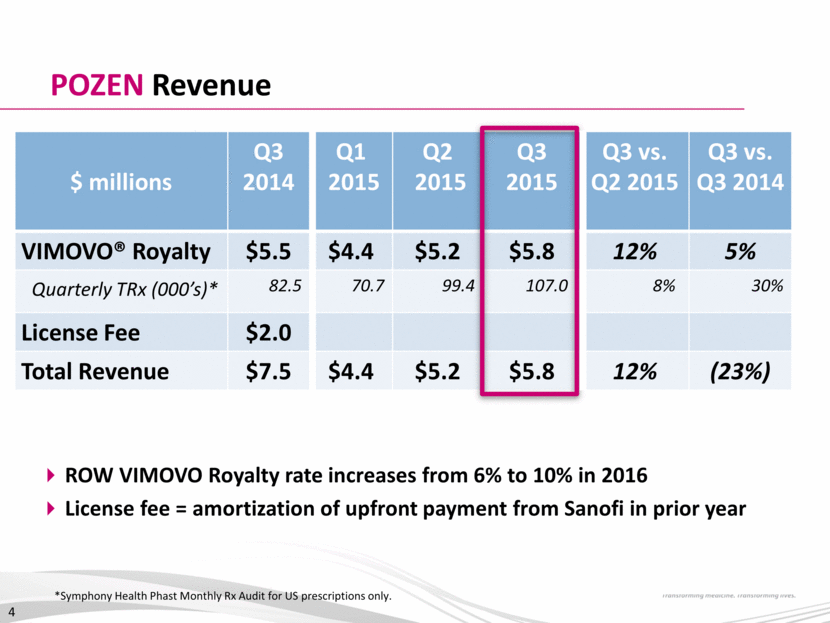
3Q 2015: Financial Results Amounts in $M Except Per Share Amounts 3Q 2014 2Q 2015 3Q 2015 Total Revenues $7.5 $5.2 $5.8 Operating Expenses: Research & Development 1.0 2.3 1.8 Commercial 0.0 0.7 2.4 G&A – ongoing 2.6 3.9 5.7 G&A - discrete(1) 0.0 13.6 4.1 Total Selling, General and Administrative 2.6 18.2 12.2 Other Income(2) 2.9 0.0 0.1 Income (Loss) Before Taxes 6.8 (15.3) (8.1) Income Tax (Expense) Benefit(3) 0.0 (1.0) 0.0 Net Income (Loss) $6.8 ($16.3) ($8.1) Net Income (Loss) Per Common Share $0.20 (fully diluted) ($0.50) (basic & fully diluted) ($0.25) (basic & fully diluted) G&A –discrete costs include transaction related costs ($3.0m) and severance / retention costs ($1.1m) Q3 2014 other income includes $2.4 million investment valuation of Pernix warrants 2015 taxes result from estimates of Alternative Minimum Tax in US on income from IP migration to Irish subsidiary 5
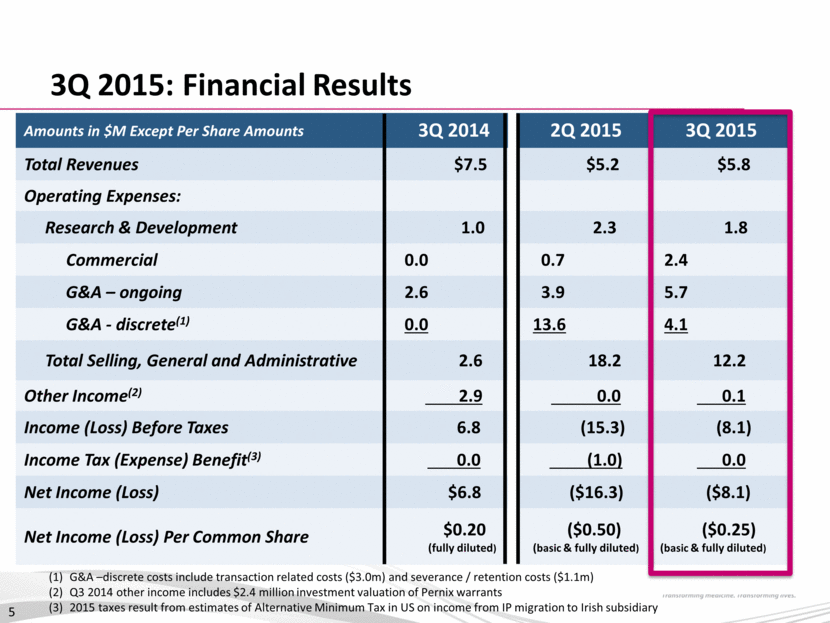
YTD: Financial Results Amounts in $M Except Per Share Amounts 9 Months Ended 3Q14 9 Months Ended 3Q15 Total Revenues(1) $22.5 $15.4 Operating Expenses: Research & Development 4.8 5.1 Commercial 0.2 3.5 G&A – ongoing 7.7 12.9 G&A - discrete(2) 0.0 17.3 Total Selling, General & Administrative 7.9 33.7 Other Income (Expense)(3) 2.9 (0.1) Income (Loss) Before Taxes 12.7 (23.5) Income Tax Expense (4) 0.0 (1.0) Net Income (Loss) $12.7 ($24.5) Net Income (Loss) Per Common Share $0.39 (fully diluted) ($0.75) (basic & fully diluted) (1) Q3 YTD 2014 includes $7 million from the amortization of an upfront fee from Sanofi (2) G&A – discrete includes transaction related costs ($8.1m) and severance / retention costs ($9.2m) (3) Q3 YTD 2014 other income includes $2.4 million investment valuation of Pernix warrants (4) Q3 YTD 2015 tax expense results from Alternative Minimum Tax in US on income from IP migration to Irish subsidiary 6
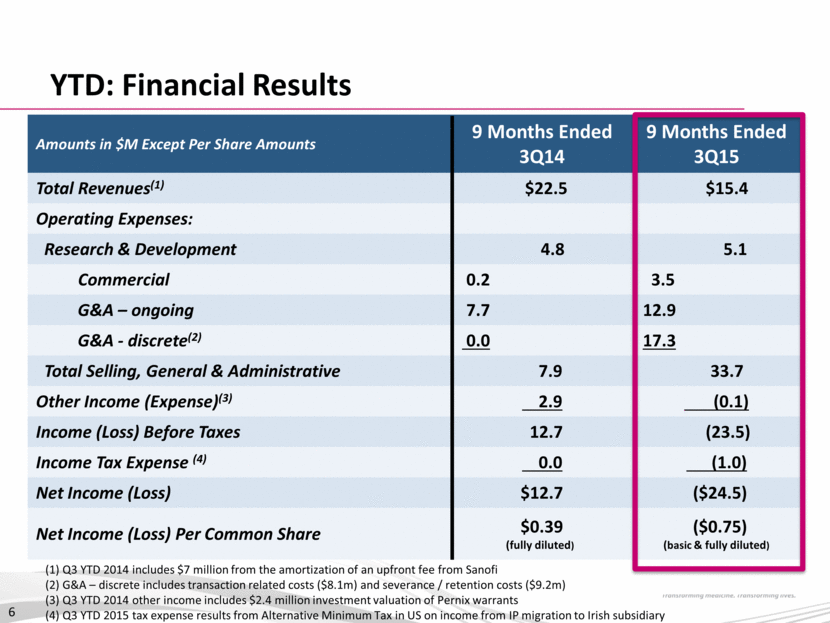
A Transformational Combination Strong Management with History of Success Diversified, Growing Revenue Base with North American Focus Strong Balance Sheet & Financial Position Irish Domicile Offers Financial & Competitive Advantage $150M at close ($75m equity/$75m convertible note) $200M committed credit facility (Led by Deerfield) 7
Aralez Status S-4 has been declared effective by the SEC Preparing for special shareholder meetings of POZEN and Tribute to be held December 10, 2015 and December 9, 2015, respectively; closing shortly thereafter Integration planning underway to ensure a smooth transition Upon close, company will immediately re-domicile to Ireland Aralez plans to trade on the NASDAQ (ticker ARLZ) and TSX (ticker ARZ) 8
Fibricor: Opportunity in Perspective Fibricor is a fenofibric acid formulation that is a lipid regulating agent indicated as adjunctive therapy to diet for treatment of severe hypertriglyceridemia (≥ 500 mg/dL), primary hypercholesterolemia or mixed dyslipidemia Product Profile Focused Commercialization Strategy Market & Product Opportunity Focus will be to maximize distribution for the short-term Preparing to recruit 20-25 person high quality sales force to promote Fibricor to key cardiologists following Tribute acquisition Grow product use moderately in the U.S. and develop “relationship springboard” ahead of YOSPRALA launch Fibricor is a small product that competes in the US$2.5 billion triglyceride lowering medication market Fibricor and its Authorized Generic version launched in October 2009, but have not been previously promoted 9
YOSPRALA: Significant Commercial Opportunity Once Approved by the FDA Market & Product Opportunity An estimated 26 MM* secondary prevention patients in US 70% take aspirin** Over 40% of physicians prescribe or recommend a GI agent when initiating aspirin therapy for secondary prevention patients YOSPRALA has the opportunity to achieve mid-single digit market share in the secondary prevention patient population with a patient affordable pricing strategy Focused Commercialization Strategy After FDA approval, plan to launch YOSPRALA with ~110 sales professionals, targeting top 20% of secondary prevention specialist (CV and IM) physicians; Target ~14,000 HCPs initially, including 8,000 cardiologists “Invest into the opportunity” and expand to up to 300 sales professionals over time targeting ~40% of the defined market; Target ~35,000 HCPs, including 14,000 cardiologists 10 * American Heart Association Heart Disease and Stroke Statistics, 2015; ** Summit Market Research Consumer CV event/stroke & aspirin/PPI incidence check, February 2010, 1,000 patients interviewed; *** Praxis Yosprala Acceptance Study, April 2015, 403 physicians and 494 patients interviewed;
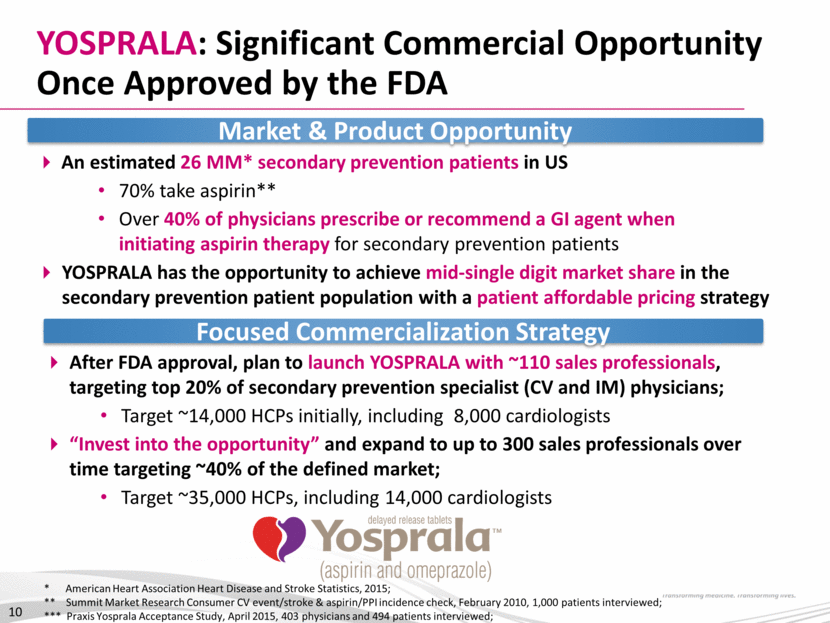
YOSPRALA: Proposed Positioning YOSPRALA has a coordinated delivery mechanism composed of an enteric-coated aspirin (325 mg or 81mg core), surrounded by immediate-release omeprazole (40 mg) for the secondary prevention of heart attack and stroke for patients at risk for an aspirin-induced ulcer Fixed-dose aspirin/PPI tablet designed to improve patient compliance to potential life-saving aspirin therapy, reducing the risk of recurrent CV events Combines the cardioprotection of EC aspirin with the gastroprotection of omeprazole Significantly reduces discontinuation rate due to upper GI events by up to 82% at six months compared to patients taking EC aspirin alone2 11 Miner et al. Aliment Pharmacol Ther. 2013 Relative reductions adapted from Whellan et al. Am Heart J 2014
YOSPRALA: Market Research Overview HCPs are Likely to Prescribe YOSPRALA HCPs state reduced pill burden for the patient is attractive1 Combined products ensure patients won’t forget to take second medication1 Automatic GI protection is helpful for patients who have had GI complaints related to Aspirin drug therapy1 As a result, PCPs/IMs & Cardiologists say they are likely to prescribe YOSPRALA for the appropriate patient population HCPs are Receptive to YOSPRALA Most HCPs see an appropriate role for YOSPRALA in their practices1,2, 3 Expected patient population: Patients with history of gastric problems or present with gastrointestinal (GI) complaints presumed related to Aspirin drug therapy2, 3 Patients Show Interest in YOSPRALA Patients state they will take YOSPRALA if it is recommended by their HCP and is affordable2, 3 For many HCPs and patients, cost to the patient will ultimately determine how much YOSPRALA is ultimately prescribed and filled2 12 1 Source: Qualitative Buying Process Research, September 2015, n=71 2 Source: Qualitative Positioning Research, August 2015, n=26 (PCP n=15), (CDs n=11) 3 Source: Qualitative Message Research, October 2015, n=15 Supporting Qualitative and Quantitative MR with Payers N=148; HCPs N=4000+ and Patients N= 2600+, 2008-2012, data on file
YOSPRALA API Aspirin Manufacturer Status YOSPRALA API primary aspirin manufacturer implementing its action plan to address FDA issues: will be subject to FDA re-inspection Steps also underway to qualify a second supplier of aspirin API Prelaunch Preparations and Tactical Activities Scaling up and building out commercial operations Completed scientific and payor advisory boards; input to guide launch preparations Completed messaging and positioning research; patient flow and pricing/forecasting research continues Creating payor value proposition materials and continuing key customer interactions to prepare for broad payor access for YOSPRALA Developing patient friendly programs to ensure availability and compliance Aralez presence at the American Heart Association Meeting YOSPRALA: Update 13
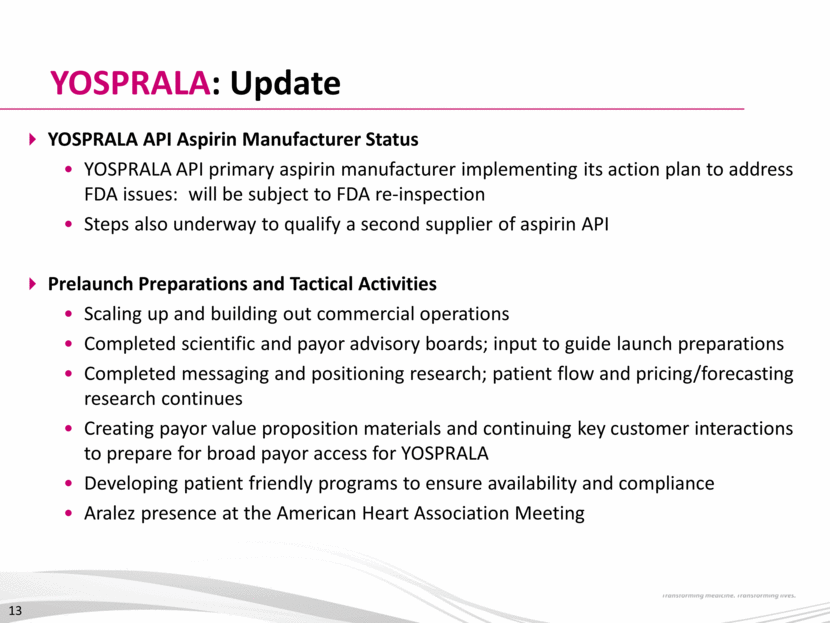
Aralez: Strong Projected Revenue Growth* $75M equity Robust balance sheet and demonstrated access to capital $75M convertible debt 2.5% with 32.5% premium $200M credit facility For future acquisitions * Figures reflect the addition of standalone projections and do not include any synergies or changes in business plan. Financing Concurrent with Transaction Close 14 $0 $50 $100 $150 $200 $250 2015E 2016E 2017E 2018E USD in millions Pozen Tribute
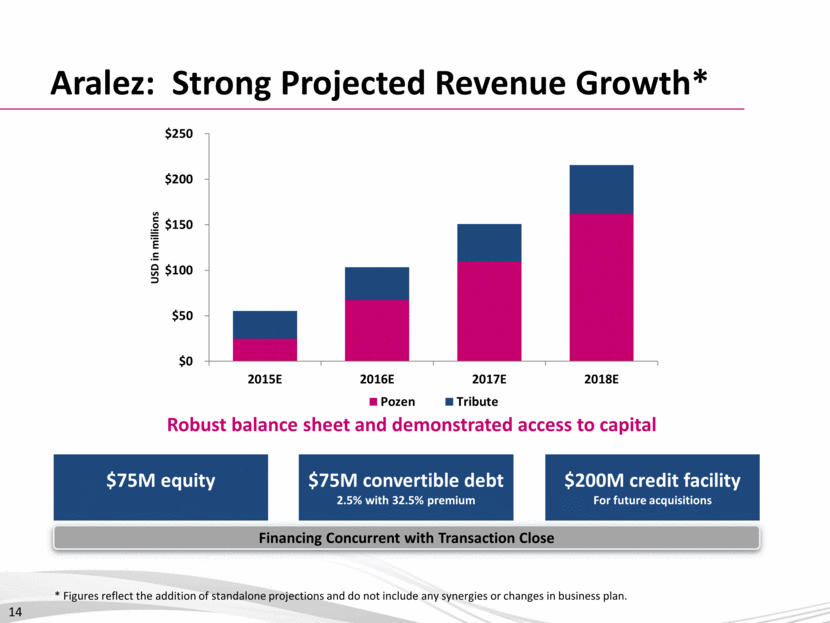
Business Development: Framework and Focus 15 Immediately EBITDA accretive* and near-term revenue generating products Approved products in Cardiovascular and Pain anchor areas Opportunistic approach to other specialty therapeutic areas meeting specified criteria US, Canada and ex-North American geographies Aligned, opportunistic or transformative M&A Focus & Priorities Management Team with Proven Success in Corporate Development Business Development Value Creation Strategy Competitive Position with Strong Financing and Tax-Efficient Structure * Excluding discrete items, including transaction related expenses and staff related costs.
Successfully close the Tribute Pharmaceuticals merger in December S-4 has been declared effective by the SEC Hold special shareholder meetings of POZEN and Tribute to be held on December 10, 2015 and December 9, 2015 respectively; closing shortly thereafter Integration planning underway to ensure a smooth transition Advance YOSPRALA to re-submission, prepare for anticipated launch in 2016 and execute a targeted, invest into the opportunity, commercialization strategy Prepare for Fibricor sales force deployment in early 2016 Actively assess and execute Business Development and M&A opportunities that are revenue generating and accretive Our Short Term Priorities 16
Q&A Session

POZEN Corporate Overview 3Q 2015 Financial Results November 9, 2015