Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Loxo Oncology, Inc. | a15-22425_28k.htm |
| EX-99.1 - EX-99.1 - Loxo Oncology, Inc. | a15-22425_2ex99d1.htm |
Exhibit 99.2
Clinical safety and activity from a Phase 1 study of LOXO-101, a selective TRKA/B/C inhibitor, in solid-tumor patients with NTRK gene fusions David S. Hong1, Marcia S. Brose2, Robert C. Doebele3, Alice T. Shaw4, Afshin Dowlati5, Todd M. Bauer6, Anna F. Farago4, Adriana Estrada-Bernal3, Anh T. Le3, Michael C. Cox7, Nisha Nanda7, Jennifer A. Low7, Howard A. Burris, III6 November 8, 2015 1 2015 AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics 1MD Anderson Cancer Center, Houston, TX 2University of Pennsylvania, Philadelphia, PA 3University of Colorado, Aurora, CO 4Massachusetts General Hospital, Boston, MA 5University Hospitals Case Medical Center, Cleveland, OH 6Sarah Cannon Research Institute, Tennessee Oncology, PLLC, Nashville, TN 7Loxo Oncology, South San Francisco, CA

Disclosures Author Disclosures David S. Hong Travel expenses supported by Loxo Oncology Marcia S. Brose None Robert C. Doebele Research grant from Loxo Oncology Alice T. Shaw None Afshin Dowlati None Todd M. Bauer None Anna F. Farago None Adriana Estrada-Bernal Research grant from Loxo Oncology Anh T. Le Research grant from Loxo Oncology Howard A. Burris, III None Michael C. Cox Nisha Nanda Jennifer A. Low Employees and shareholders of Loxo Oncology 2
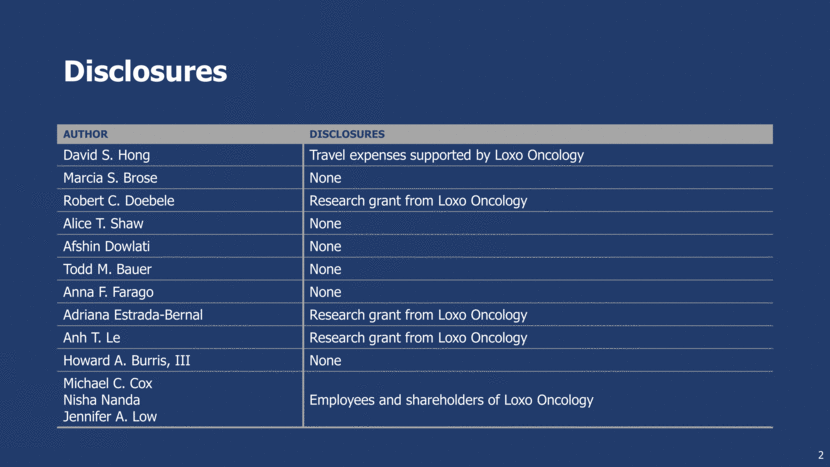
TRK Fusions are Oncogenic and Signal Through Canonical Downstream Pathways Normal TRK Proteins Family of neurotrophin receptors TrkA (NTRK1) Pain, thermoregulation TrkB (NTRK2) Movement, memory, mood, appetite, body weight TrkC (NTRK3) Proprioception TRK Fusions Ligand binding domain replaced by 5’ fusion partner; highly expressed by promoter of 5’ fusion gene Ligand-independent activation 3 NTRK1/2/3 ERK AKT ERK AKT TrkA/B/C
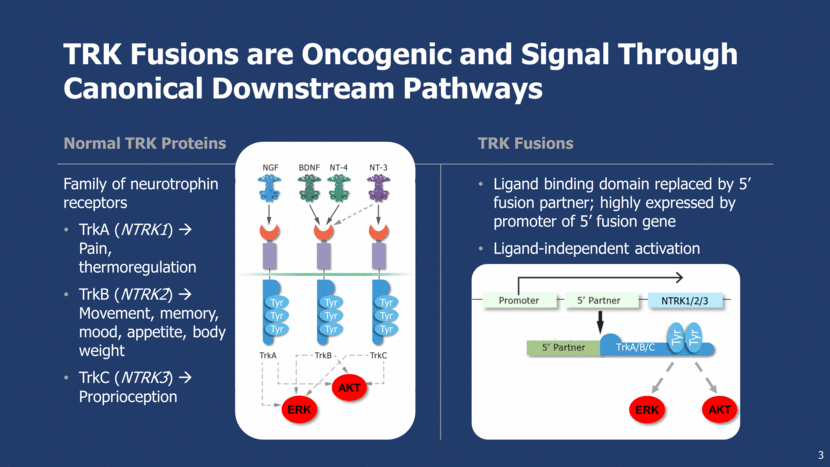
TRK Fusions Found in Diverse Cancer Histologies 4 TRK Fusion Frequency <5% 5–25% >75% CNS Astrocytoma Brain low-grade glioma Glioblastoma GI Colorectal cancer Cholangiocarcinoma Pancreatic cancer Head and neck Squamous cell carcinoma Lung Adenocarcinoma Large cell neuroendocrine Other Acute myeloid leukemia Breast invasive carcinoma Melanoma Sarcoma Congenital mesoblastic nephroma Papillary thyroid cancer Pontine glioma Spitz nevi Mammary analogue secretory carcinoma (MASC) of the salivary glands Secretory breast carcinoma Infantile fibrosarcoma
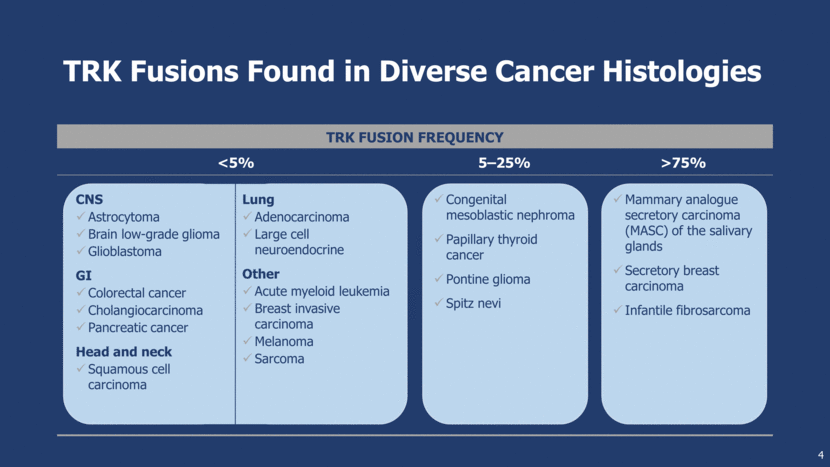
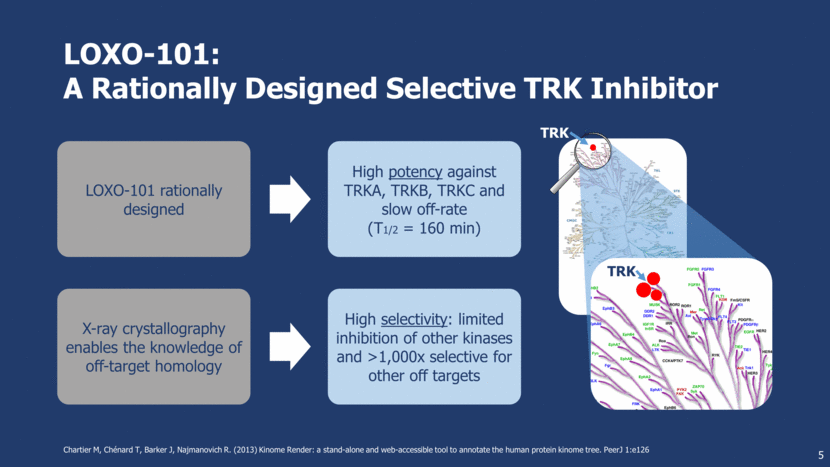
LOXO-101: A Rationally Designed Selective TRK Inhibitor 5 Chartier M, Chénard T, Barker J, Najmanovich R. (2013) Kinome Render: a stand-alone and web-accessible tool to annotate the human protein kinome tree. PeerJ 1:e126 TRK TRK LOXO-101 rationally designed High potency against TRKA, TRKB, TRKC and slow off-rate (T1/2 = 160 min) X-ray crystallography enables the knowledge of off-target homology High selectivity: limited inhibition of other kinases and >1,000x selective for other off targets
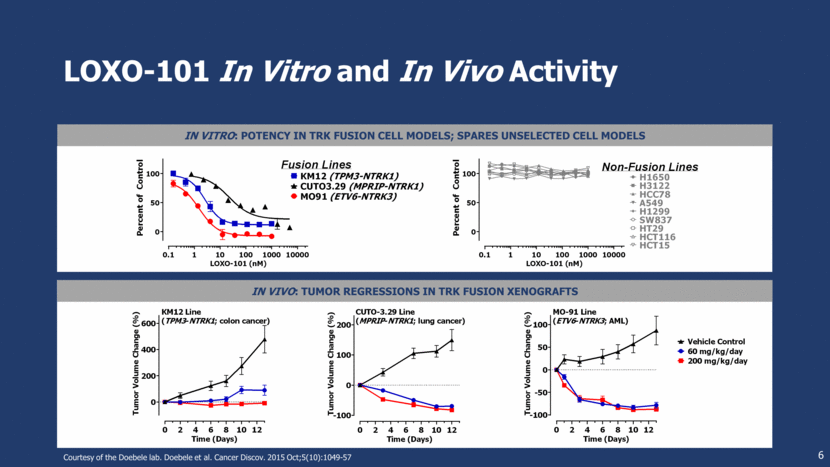
In Vivo: Tumor Regressions in TRK Fusion Xenografts LOXO-101 In Vitro and In Vivo Activity 6 In Vitro: Potency in TRK Fusion Cell Models; Spares Unselected Cell Models Courtesy of the Doebele lab. Doebele et al. Cancer Discov. 2015 Oct;5(10):1049-57 0 2 4 6 8 10 12 0 200 400 600 Time (Days) T u m o r V o l u m e C h a n g e ( % ) KM12 Line ( TPM3 - NTRK1 ; colon cancer) 0 2 4 6 8 10 12 -100 0 100 200 Time (Days) T u m o r V o l u m e C h a n g e ( % ) CUTO-3.29 Line ( MPRIP - NTRK1 ; lung cancer) 0.1 1 10 100 1000 10000 0 50 100 CUTO3.29 (MPRIP-NTRK1) KM12 (TPM3-NTRK1) MO91 (ETV6-NTRK3) LOXO-101 (nM) P e r c e n t o f C o n t r o l Fusion Lines 0.1 1 10 100 1000 10000 0 50 100 LOXO-101 (nM) P e r c e n t o f C o n t r o l H1650 H3122 HCC78 A549 H1299 SW837 HT29 HCT116 HCT15 Non-Fusion Lines 0 2 4 6 8 10 12 -100 -50 0 50 100 Time (Days) T u m o r V o l u m e C h a n g e ( % ) MO-91 Line ( ETV6 - NTRK3 ; AML) Vehicle Control 60 mg/kg/day 200 mg/kg/day
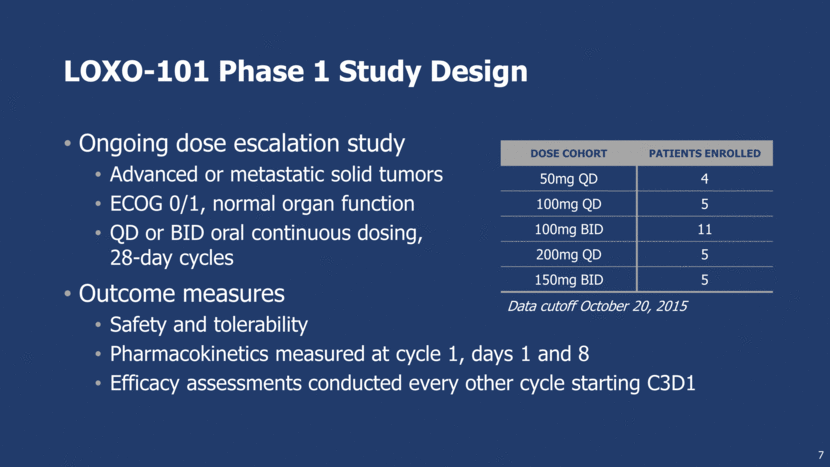
LOXO-101 Phase 1 Study Design Ongoing dose escalation study Advanced or metastatic solid tumors ECOG 0/1, normal organ function QD or BID oral continuous dosing, 28-day cycles Outcome measures Safety and tolerability Pharmacokinetics measured at cycle 1, days 1 and 8 Efficacy assessments conducted every other cycle starting C3D1 7 Dose Cohort Patients Enrolled 50mg QD 4 100mg QD 5 100mg BID 11 200mg QD 5 150mg BID 5 Data cutoff October 20, 2015
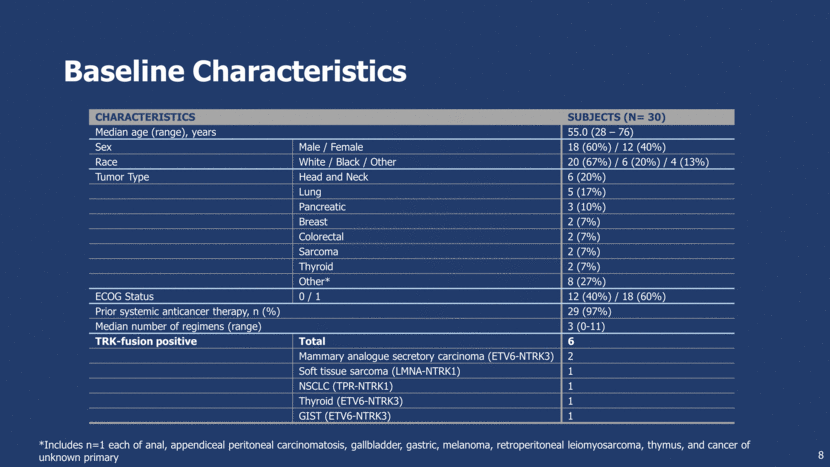
Baseline Characteristics characteristics Subjects (N= 30) Median age (range), years 55.0 (28 – 76) Sex Male / Female 18 (60%) / 12 (40%) Race White / Black / Other 20 (67%) / 6 (20%) / 4 (13%) Tumor Type Head and Neck 6 (20%) Lung 5 (17%) Pancreatic 3 (10%) Breast 2 (7%) Colorectal 2 (7%) Sarcoma 2 (7%) Thyroid 2 (7%) Other* 8 (27%) ECOG Status 0 / 1 12 (40%) / 18 (60%) Prior systemic anticancer therapy, n (%) 29 (97%) Median number of regimens (range) 3 (0-11) TRK-fusion positive Total 6 Mammary analogue secretory carcinoma (ETV6-NTRK3) 2 Soft tissue sarcoma (LMNA-NTRK1) 1 NSCLC (TPR-NTRK1) 1 Thyroid (ETV6-NTRK3) 1 GIST (ETV6-NTRK3) 1 8 *Includes n=1 each of anal, appendiceal peritoneal carcinomatosis, gallbladder, gastric, melanoma, retroperitoneal leiomyosarcoma, thymus, and cancer of unknown primary
RESULTS 9

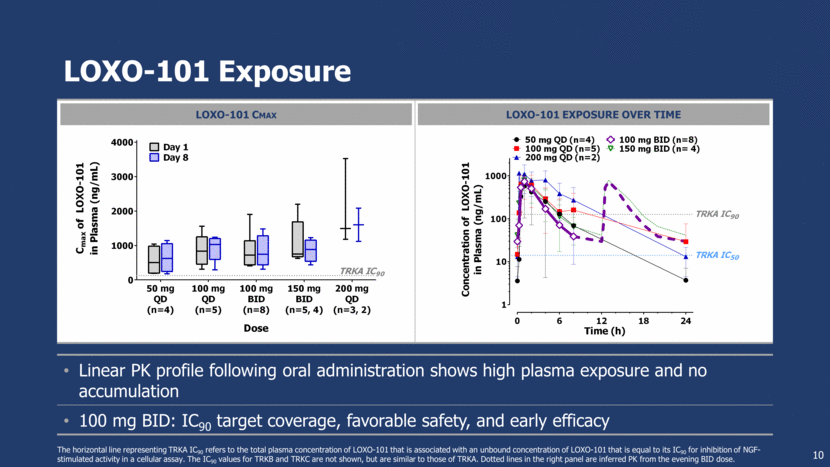
Linear PK profile following oral administration shows high plasma exposure and no accumulation 100 mg BID: IC90 target coverage, favorable safety, and early efficacy LOXO-101 Exposure 10 The horizontal line representing TRKA IC90 refers to the total plasma concentration of LOXO-101 that is associated with an unbound concentration of LOXO-101 that is equal to its IC90 for inhibition of NGF-stimulated activity in a cellular assay. The IC90 values for TRKB and TRKC are not shown, but are similar to those of TRKA. Dotted lines in the right panel are inferred PK from the evening BID dose. LOXO-101 CMAX LOXO-101 EXPOSURE OVER TIME 0 6 12 18 24 1 10 100 1000 Time (h) C o n c e n t r a t i o n o f L O X O - 1 0 1 i n P l a s m a ( n g / m L ) TRKA IC 50 TRKA IC 90 50 mg QD (n=4) 100 mg QD (n=5) 100 mg BID (n=8) 150 mg BID (n= 4) 200 mg QD (n=2) 50 mg QD (n=4) 100 mg QD (n=5) 100 mg BID (n=8) 150 mg BID (n=5, 4) 200 mg QD (n=3, 2) 0 1000 2000 3000 4000 Dose C m a x o f L O X O - 1 0 1 i n P l a s m a ( n g / m L ) Day 1 Day 8 TRKA IC 90
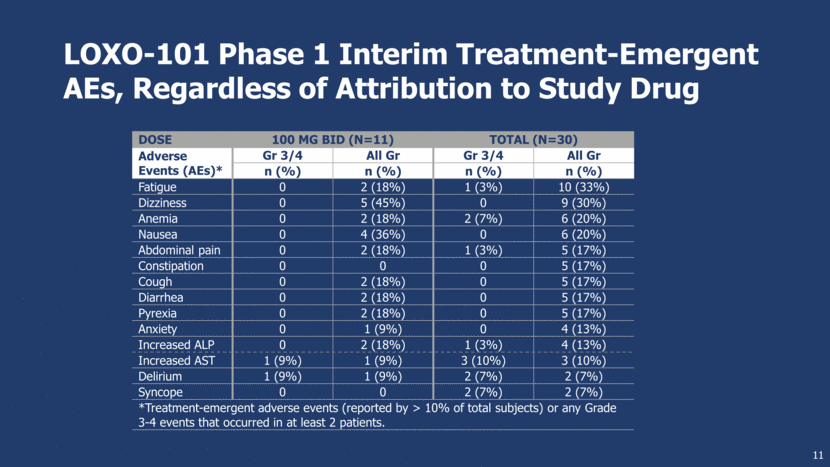
LOXO-101 Phase 1 Interim Treatment-Emergent AEs, Regardless of Attribution to Study Drug Dose 100 mg BID (n=11) Total (n=30) Adverse Events (AEs)* Gr 3/4 All Gr Gr 3/4 All Gr n (%) n (%) n (%) n (%) Fatigue 0 2 (18%) 1 (3%) 10 (33%) Dizziness 0 5 (45%) 0 9 (30%) Anemia 0 2 (18%) 2 (7%) 6 (20%) Nausea 0 4 (36%) 0 6 (20%) Abdominal pain 0 2 (18%) 1 (3%) 5 (17%) Constipation 0 0 0 5 (17%) Cough 0 2 (18%) 0 5 (17%) Diarrhea 0 2 (18%) 0 5 (17%) Pyrexia 0 2 (18%) 0 5 (17%) Anxiety 0 1 (9%) 0 4 (13%) Increased ALP 0 2 (18%) 1 (3%) 4 (13%) Increased AST 1 (9%) 1 (9%) 3 (10%) 3 (10%) Delirium 1 (9%) 1 (9%) 2 (7%) 2 (7%) Syncope 0 0 2 (7%) 2 (7%) *Treatment-emergent adverse events (reported by > 10% of total subjects) or any Grade 3-4 events that occurred in at least 2 patients. 11
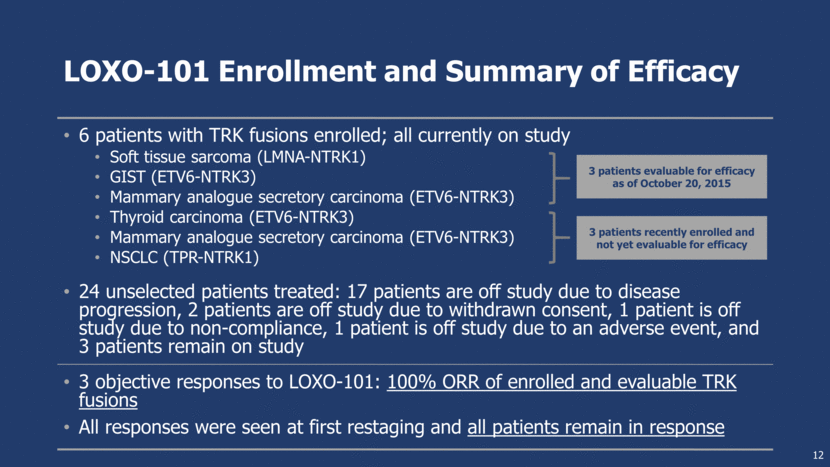
LOXO-101 Enrollment and Summary of Efficacy 6 patients with TRK fusions enrolled; all currently on study Soft tissue sarcoma (LMNA-NTRK1) GIST (ETV6-NTRK3) Mammary analogue secretory carcinoma (ETV6-NTRK3) Thyroid carcinoma (ETV6-NTRK3) Mammary analogue secretory carcinoma (ETV6-NTRK3) NSCLC (TPR-NTRK1) 24 unselected patients treated: 17 patients are off study due to disease progression, 2 patients are off study due to withdrawn consent, 1 patient is off study due to non-compliance, 1 patient is off study due to an adverse event, and 3 patients remain on study 3 objective responses to LOXO-101: 100% ORR of enrolled and evaluable TRK fusions All responses were seen at first restaging and all patients remain in response 12 3 patients evaluable for efficacy as of October 20, 2015 3 patients recently enrolled and not yet evaluable for efficacy
PATIENT CASES 13

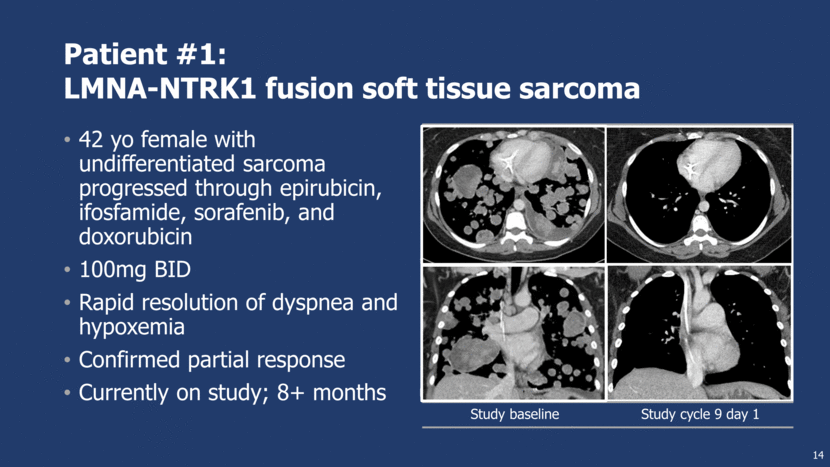
Patient #1: LMNA-NTRK1 fusion soft tissue sarcoma 42 yo female with undifferentiated sarcoma progressed through epirubicin, ifosfamide, sorafenib, and doxorubicin 100mg BID Rapid resolution of dyspnea and hypoxemia Confirmed partial response Currently on study; 8+ months Study baseline Study cycle 9 day 1 14
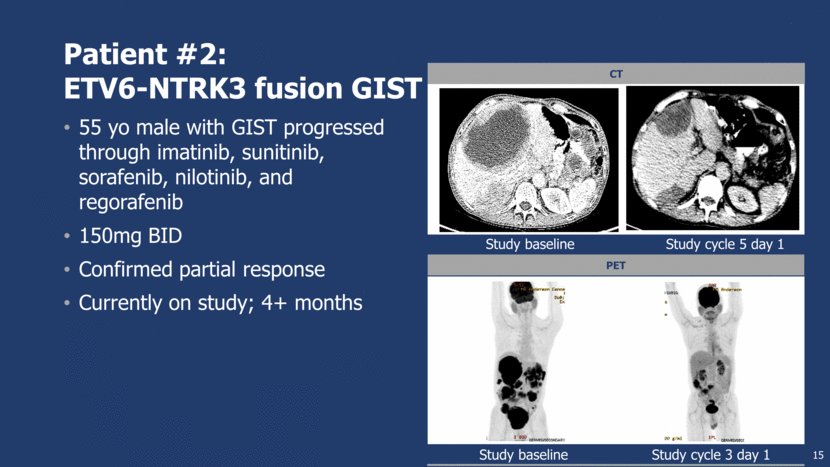
Patient #2: ETV6-NTRK3 fusion GIST 55 yo male with GIST progressed through imatinib, sunitinib, sorafenib, nilotinib, and regorafenib 150mg BID Confirmed partial response Currently on study; 4+ months 15 CT PET Study baseline Study cycle 5 day 1 Study baseline Study cycle 3 day 1
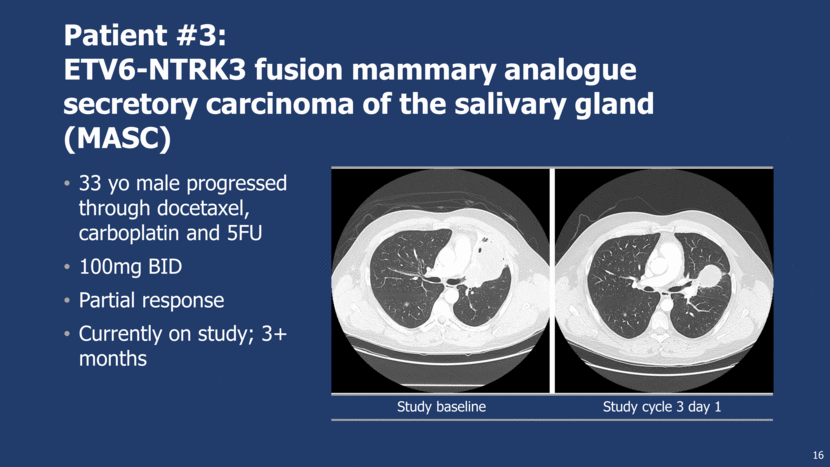
Study baseline Study cycle 3 day 1 Patient #3: ETV6-NTRK3 fusion mammary analogue secretory carcinoma of the salivary gland (MASC) 33 yo male progressed through docetaxel, carboplatin and 5FU 100mg BID Partial response Currently on study; 3+ months 16
Conclusions LOXO-101 is a purpose-built, oral, selective and potent TRK inhibitor with tumor regression in TRK-fusion preclinical xenograft models PK analyses demonstrate very high Cmax (mean levels >IC90) and linear PK with no significant drug accumulation LOXO-101 is well tolerated, with most common adverse events including Grade 1/2 fatigue, dizziness, anemia, and nausea; MTD not yet defined 6 TRK fusions enrolled from March 2015 to October 2015 Rapid RECIST responses observed in first 3 of 3 patients harboring TRK gene fusions All TRK fusion patients are currently on study without progression, with the longest patient at 8+ months Loxo Oncology has begun accruing a Phase 2 basket trial in patients with advanced or metastatic solid tumors with a TRK gene fusion 17
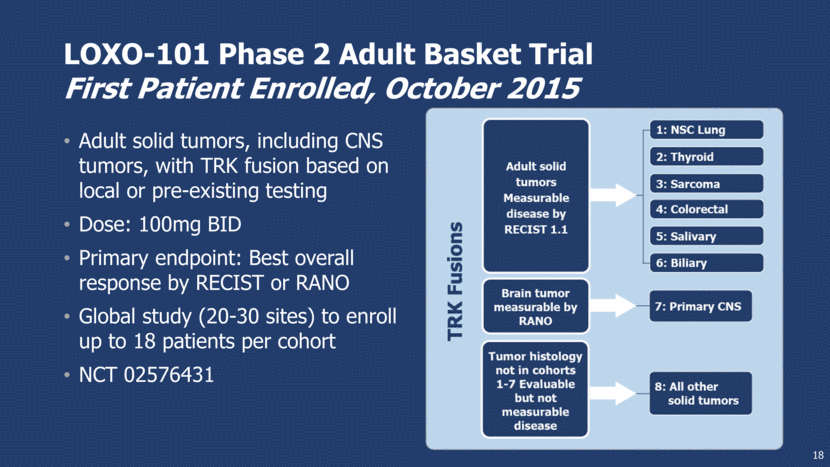
LOXO-101 Phase 2 Adult Basket Trial First Patient Enrolled, October 2015 Adult solid tumors, including CNS tumors, with TRK fusion based on local or pre-existing testing Dose: 100mg BID Primary endpoint: Best overall response by RECIST or RANO Global study (20-30 sites) to enroll up to 18 patients per cohort NCT 02576431 18
Acknowledgements LOXO-101 patients and their families and caregivers Co-investigators and study support staffs This trial sponsored and supported by Loxo Oncology 19

