Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GENOCEA BIOSCIENCES, INC. | gnca-2015930x8kq3earningsr.htm |
| EX-99.1 - EXHIBIT 99.1 - GENOCEA BIOSCIENCES, INC. | exhibit991q3pressrelease.htm |

Q3 2015 Earnings Call November 5, 2015 1 Creating and advancing life- changing vaccines and immunotherapies Exhibit 99.2

This presentation contains “forward-looking” statements that are within the meaning of federal securities laws and are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities, potential market opportunities and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Forward- looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. Factors that may materially affect our results of operations include, among other things, those listed in our Annual Report on Form 10-K and other filings with the Securities and Exchange Commission (“SEC”). Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. You may get copies of our Annual Report on Form 10-K, Quarterly Report on Form 10-Q and our other SEC filings for free by visiting EDGAR on the SEC website at http://www.sec.gov. 2 Safe Harbor Statement

• GEN-003 Update • GEN-004 Update • ATLASTM Immuno-Oncology Strategy – Dana-Farber collaboration – Memorial Sloan Kettering collaboration – Epstein-Barr Virus program • Q3 2015 Financial Summary 3 Agenda

GEN-003 Update 4
• Improved impact on viral activity vs. Phase 1/2a • Durable clinical efficacy demonstrated across potential Phase 3 endpoints • Clear path to FDA end of Phase 2 meeting in Q4 2016 5 6-Month Phase 2 Efficacy Data Strengthens GEN-003 Value Proposition for Treatment of Genital Herpes

6 Three Significant Catalysts in Coming Quarters Q1 2016 Ph 2 – 12 Month Data Q4 2016 FDA End of Phase 2 Q2 2016 Ph 2b – Bridging • Potential to strengthen EoP2 package with confirmation of efficacy of Phase 3 material • Confirm Phase 3 trial design • Upside to value proposition if efficacy durable to 12 months • Read on booster timing

7 Potential Blockbuster Candidate with Phase 2 Efficacy Data vs. episodic treatment (~2/3 treated patients) • Reduce outbreaks • Reduce shedding to lower risk of transmission vs. chronic suppressive treatment (~1/3 treated patients) • Durable efficacy via novel mechanism • Orals reserved as rescue during outbreaks • Improved compliance & convenience Potential cornerstone treatment for genital herpes with >$1bn GNCA revenue opportunity in US alone Potential Advantages Over Oral Anti-Virals

GEN-004 Update 8

• Consistent reductions versus placebo in colonization rate and density but no statistical significance • Path to further development may require investigation of dose, adjuvant or trial population • Development suspended pending further review of data and expert consultation 9 GEN-004 Phase 2a Challenge Study Results

ATLASTM: Enabling New Immuno-oncology Therapies 10

• New therapies unleash T cells, but poor understanding of what T cells are targeting for efficacy • Breakthrough treatment advances, but with limitations: – Still not effective for many patients – Early signals of activity not always reliable – Significant toxicity • Potential targets of T cell response (T cell antigens) – Tumor-associated antigens (aberrantly-expressed self antigens): many identified; protective efficacy unproven – Neoantigens: patient-specific, predicted to be immunogenic1 11 Immuno-Oncology Transforming Cancer Treatment; Major Unmet Needs Remain 1 Gubin, Schreiber et al, Nature, vol 515, Nov 2015

• Finding the right T cell antigens may matter in cancer and infectious disease • GEN-003 efficacy reflects the power of the right T cell antigen • 2014 Dana-Farber collaboration established to apply ATLAS to cancer 12 Pursuing Immuno-Oncology Applications for ATLAS, a Natural Extension of Infectious Disease Expertise ATLAS may inform • T cell signatures of response and non- response • Cancer vaccine targets

• Neither immunogenicity nor immunodominance predict protective immunity1 • T cell antigens are only those peptides that T cells recognize and to which they strongly respond 13 Predicting T cell Antigens is Fraught with Challenges 1 Gilchuk, Joyce; Current Opinion in Immunology, June 2015 ATLAS solution: Don’t predict! • Find antigens eliciting the right T cell responses by association with clinically meaningful outcomes

ATLAS Enables Genocea to Identify Clinically Relevant T cell Antigens 14 RNANeon / Gritstone DNA Foundation Medicine Antigen Processing Antigen Presentation via MHC Protein Immatics / Immunocore DNA ATLAS Identifies Antigens of Optimal T cell Responses Tools Predicting Antigens Tools Predicting TCRs GENOCEA RNA T cell antigen TCR CD4+ or CD8+ T CellTumor Cell Tumor-associated peptide; not necessarily recognized by protective T cells Adaptive MHC I/II MHC I/II T cell Receptor
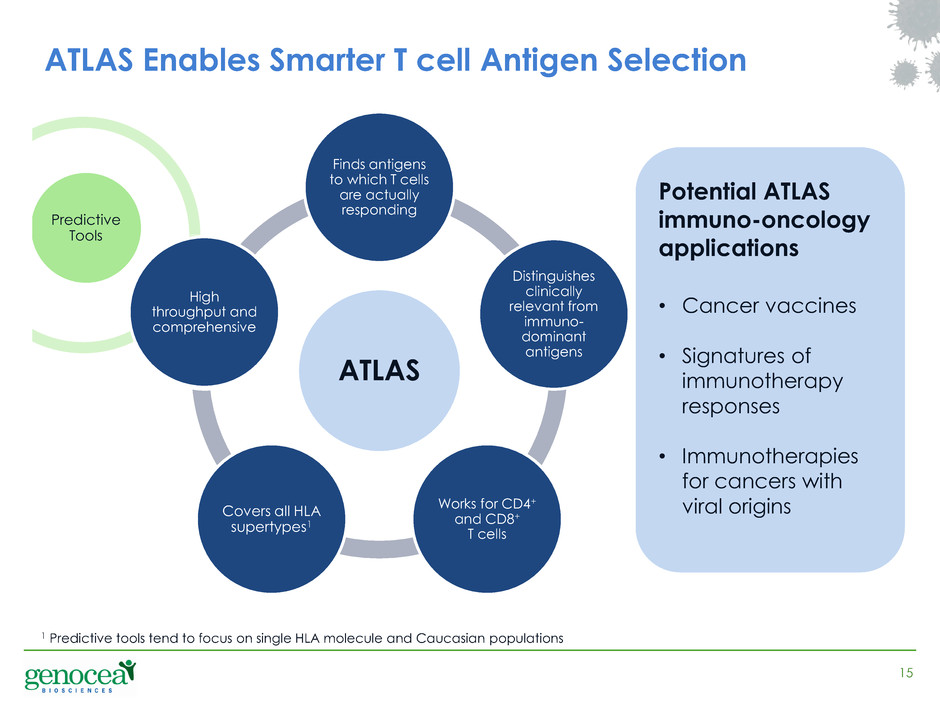
ATLAS Finds antigens to which T cells are actually responding Distinguishes clinically relevant from immuno- dominant antigens Works for CD4+ and CD8+ T cells Covers all HLA supertypes1 High throughput and comprehensive 15 ATLAS Enables Smarter T cell Antigen Selection Predictive Tools i t r t r si Potential ATLAS immuno-oncology applications • Cancer vaccines • Signatures of immunotherapy responses • Immunotherapies for cancers with viral origins 1 Predictive tools tend to focus on single HLA molecule and Caucasian populations

• Responders to therapy are different to non- responders – Greater breadth of T cell responses – Different characteristics of responses • ‘Decoy’ responses identified with no link to improved outcomes 16 Dana-Farber Collaboration Shows ATLAS Can Find Signatures of Checkpoint Inhibitor T cell Response Late-breaker poster presentation at Society for Immunotherapy of Cancer (SITC) on November 7th
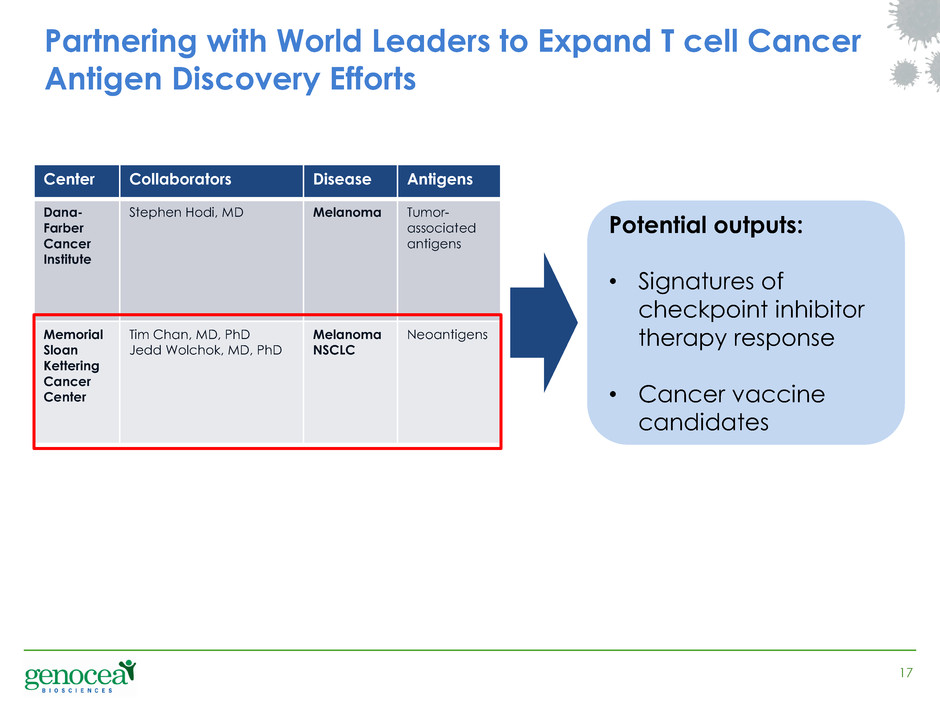
17 Partnering with World Leaders to Expand T cell Cancer Antigen Discovery Efforts Center Collaborators Disease Antigens Dana- Farber Cancer Institute Stephen Hodi, MD Melanoma Tumor- associated antigens Memorial Sloan Kettering Cancer Center Tim Chan, MD, PhD Jedd Wolchok, MD, PhD Melanoma NSCLC Neoantigens Potential outputs: • Signatures of checkpoint inhibitor therapy response • Cancer vaccine candidates

• Genocea well positioned – Picking the right T cell antigens likely central to vaccine efficacy – ATLAS may be the only platform able to identify clinically relevant T cell antigens, and has been proven to work – Significant vaccine discovery and development expertise – Flexibility to work with any adjuvant and/or delivery system to create cancer vaccines • Pursuing personalized cancer vaccines initially – Potential to incorporate common antigens over time – Expect faster path to clinic than for infectious disease 18 Therapeutic Development Strategy
• Therapeutic potential against cancers (and other diseases) with unmet needs: – Post-transplant lymphoproliferative disease – non-Hodgkin’s lymphoma – Nasopharyngeal carcinoma – Gastric carcinoma • Highly suited to ATLAS – T cells responses are crucial to protection – A large virus, making antigen prediction extremely challenging – EBV is a herpesvirus • Discovery research ongoing 19 Epstein-Barr Virus Immunotherapy Program Underway

• Personalized vaccines may enable expedited path to clinic • Response signatures may enable optimization of approved and in-development immunotherapies: – Patient prioritization / de-prioritization – Next generation targets and refinements • EBV: targeting disease with high unmet need and a path to differentiated product in Genocea’s herpesvirus area of expertise 20 ATLAS T cell Antigen Discovery Enables Multiple Paths to Value Creation

Q3 2015 Financial Summary 21

• Cash, cash equivalents and investments $112.5m sufficient to fund operating expenses and capex requirements into second half of 2017 – Oncology program fully funded • Q3 2015 vs. Q3 2014 – R&D expenses $6.1m (unchanged) – G&A expenses $3.6m ($0.8m increase) – Net loss $9.8m (0.6m increase) 22 Q3 2015 Financial Summary

Closing Remarks Q&A 23
