Attached files
| file | filename |
|---|---|
| 8-K - 8-K - TESARO, Inc. | a15-21185_18k.htm |
Exhibit 99.1
Conference Call with Clients of Leerink Partners October 15, 2015

Safe Harbor Statement Statements made in this presentation about TESARO, Inc. that are not descriptions of historical facts are forward-looking statements reflecting the current beliefs and expectations of management. Forward-looking statements are sometimes identified by words such as “plan,” “may,” “will,” “expect,” and similar expressions referencing future events, conditions, or circumstances. Such forward-looking statements include statements regarding the expected WAC, net pricing, and length of patent protection for VARUBI. These forward-looking statements involve substantial risks and uncertainties that could cause actual future results, performance, or achievements to differ significantly from those expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, the uncertainties inherent in the execution of our planned commercial launch of VARUBI, actions by regulatory authorities, competitive pressures, and other matters that could affect the commercial launch and/or success of VARUBI. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see TESARO’s Annual Report on Form 10-K for the year ended December 31, 2014, and our preliminary prospectus supplement dated March 2, 2015. TESARO, Inc. undertakes no obligation to update or revise any forward-looking statement for any reason.

TESARO’s First Approved Product Approved by the U.S. FDA on September 1, 2015 Indicated in combination with other antiemetic agents in adults for the prevention of delayed CINV Profile includes: Single dose administration A long half-life and duration of action; sustained coverage No dose adjustment for dexamethasone, a CYP3A4 substrate Composition of matter protection until 2023 U.S. exclusivity into 2028 with expected Hatch-Waxman European data / market exclusivity of 8 + 2 (+1) years Launch is a significant milestone in TESARO’s evolution into an integrated biopharmaceutical company with strong development and commercial capabilities VARUBI™:

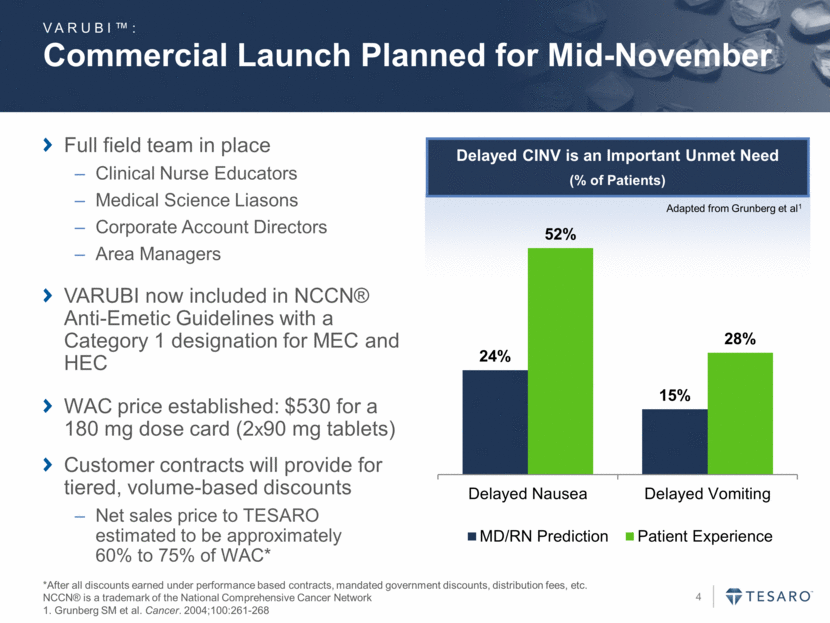
Commercial Launch Planned for Mid-November Full field team in place Clinical Nurse Educators Medical Science Liasons Corporate Account Directors Area Managers VARUBI now included in NCCN® Anti-Emetic Guidelines with a Category 1 designation for MEC and HEC WAC price established: $530 for a 180 mg dose card (2x90 mg tablets) Customer contracts will provide for tiered, volume-based discounts Net sales price to TESARO estimated to be approximately 60% to 75% of WAC* VARUBI™: *After all discounts earned under performance based contracts, mandated government discounts, distribution fees, etc. NCCN® is a trademark of the National Comprehensive Cancer Network 1. Grunberg SM et al. Cancer. 2004;100:261-268 Delayed CINV is an Important Unmet Need (% of Patients) Adapted from Grunberg et al1 24% 15% 52% 28% Delayed Nausea Delayed Vomiting MD/RN Prediction Patient Experience
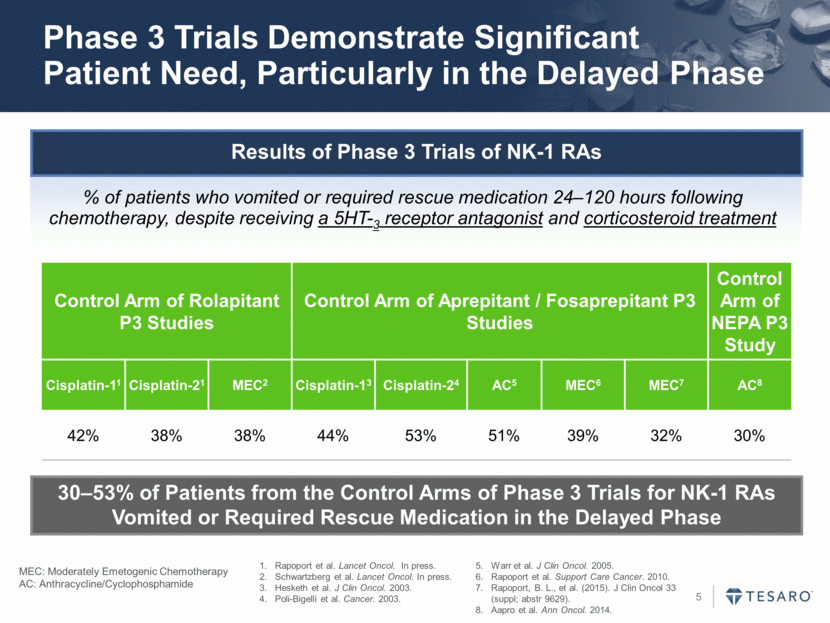
Phase 3 Trials Demonstrate Significant Patient Need, Particularly in the Delayed Phase Results of Phase 3 Trials of NK-1 RAs % of patients who vomited or required rescue medication 24–120 hours following chemotherapy, despite receiving a 5HT-3 receptor antagonist and corticosteroid treatment 30–53% of Patients from the Control Arms of Phase 3 Trials for NK-1 RAs Vomited or Required Rescue Medication in the Delayed Phase Control Arm of Rolapitant P3 Studies Control Arm of Aprepitant / Fosaprepitant P3 Studies Control Arm of NEPA P3 Study Cisplatin-11 Cisplatin-21 MEC2 Cisplatin-13 Cisplatin-24 AC5 MEC6 MEC7 AC8 42% 38% 38% 44% 53% 51% 39% 32% 30% Rapoport et al. Lancet Oncol. In press. Schwartzberg et al. Lancet Oncol. In press. Hesketh et al. J Clin Oncol. 2003. Poli-Bigelli et al. Cancer. 2003. Warr et al. J Clin Oncol. 2005. Rapoport et al. Support Care Cancer. 2010. Rapoport, B. L., et al. (2015). J Clin Oncol 33 (suppl; abstr 9629). Aapro et al. Ann Oncol. 2014. MEC: Moderately Emetogenic Chemotherapy AC: Anthracycline/Cyclophosphamide
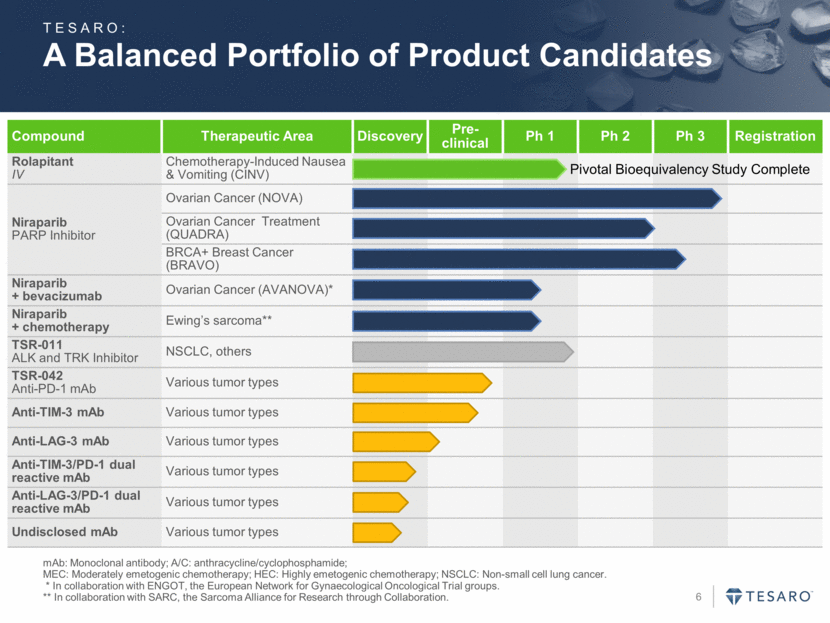
A Balanced Portfolio of Product Candidates mAb: Monoclonal antibody; A/C: anthracycline/cyclophosphamide; MEC: Moderately emetogenic chemotherapy; HEC: Highly emetogenic chemotherapy; NSCLC: Non-small cell lung cancer. * In collaboration with ENGOT, the European Network for Gynaecological Oncological Trial groups. ** In collaboration with SARC, the Sarcoma Alliance for Research through Collaboration. Compound Therapeutic Area Discovery Pre-clinical Ph 1 Ph 2 Ph 3 Registration Rolapitant IV Chemotherapy-Induced Nausea & Vomiting (CINV) Niraparib PARP Inhibitor Ovarian Cancer (NOVA) Ovarian Cancer Treatment (QUADRA) BRCA+ Breast Cancer (BRAVO) Niraparib + bevacizumab Ovarian Cancer (AVANOVA)* Niraparib + chemotherapy Ewing’s sarcoma** TSR-011 ALK and TRK Inhibitor NSCLC, others TSR-042 Anti-PD-1 mAb Various tumor types Anti-TIM-3 mAb Various tumor types Anti-LAG-3 mAb Various tumor types Anti-TIM-3/PD-1 dual reactive mAb Various tumor types Anti-LAG-3/PD-1 dual reactive mAb Various tumor types Undisclosed mAb Various tumor types TESARO: Pivotal Bioequivalency Study Complete
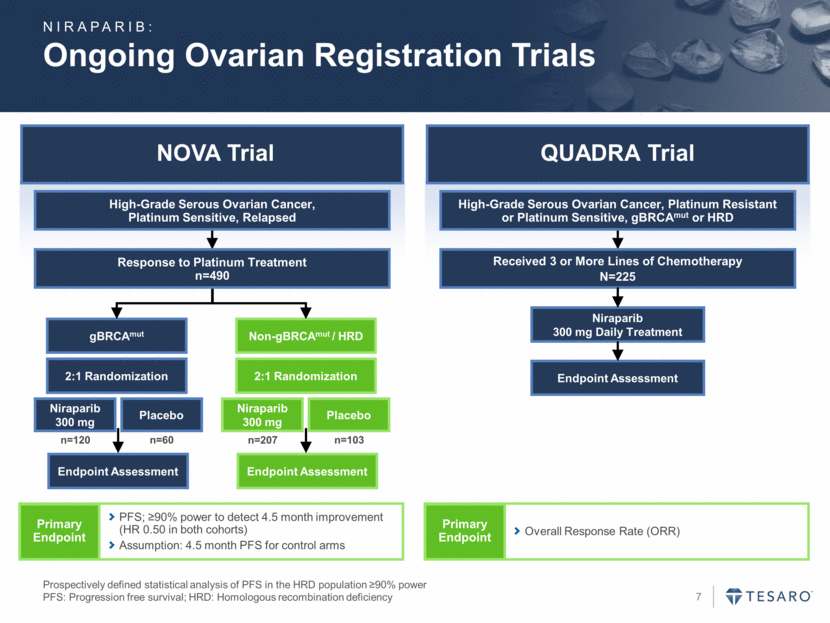
NOVA Trial QUADRA Trial Ongoing Ovarian Registration Trials Prospectively defined statistical analysis of PFS in the HRD population >90% power PFS: Progression free survival; HRD: Homologous recombination deficiency gBRCAmut Endpoint Assessment Niraparib 300 mg Placebo Non-gBRCAmut / HRD Endpoint Assessment Niraparib 300 mg Placebo 2:1 Randomization 2:1 Randomization n=120 n=60 n=207 n=103 Response to Platinum Treatment n=490 High-Grade Serous Ovarian Cancer, Platinum Sensitive, Relapsed Primary Endpoint PFS; >90% power to detect 4.5 month improvement (HR 0.50 in both cohorts) Assumption: 4.5 month PFS for control arms Received 3 or More Lines of Chemotherapy N=225 High-Grade Serous Ovarian Cancer, Platinum Resistant or Platinum Sensitive, gBRCAmut or HRD Niraparib 300 mg Daily Treatment Endpoint Assessment Primary Endpoint Overall Response Rate (ORR) NIRAPARIB:
Additional Potential Indications Currently Ongoing Trials BRAVO trial of niraparib for patients with gBRCAmut advanced/metastatic breast cancer treatment Niraparib + bevacizumab combination in patients with recurrent platinum-sensitive ovarian cancer Niraparib + enzalutamide combination in patients with metastatic castration-resistant prostate cancer Planned Trials PRIMA trial of niraparib for gBRCAmut and HRD+ patients with 1st line ovarian cancer Niraparib + pembrolizumab combination in patients with ovarian or triple-negative breast cancers Other tumor types to be announced NIRAPARIB:
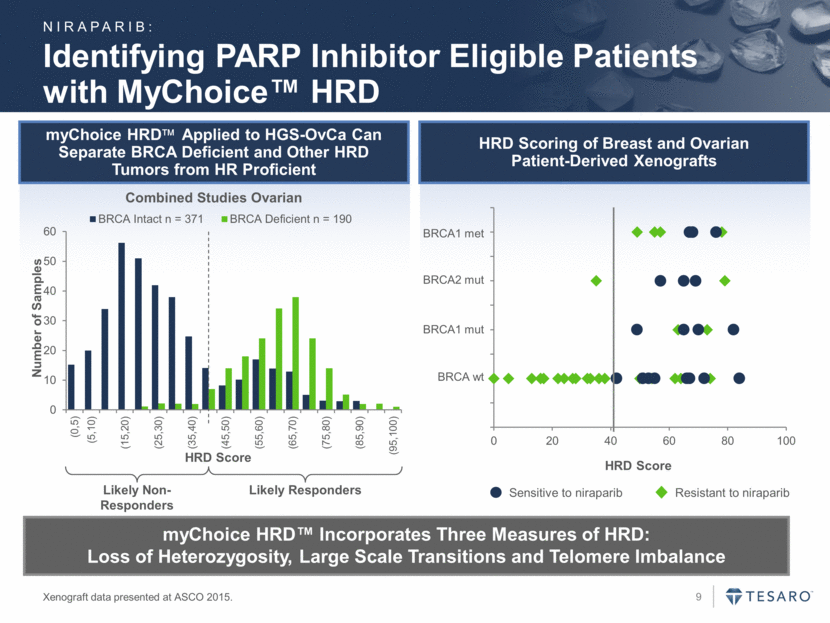
myChoice HRDApplied to HGS-OvCa Can Separate BRCA Deficient and Other HRD Tumors from HR Proficient HRD Scoring of Breast and Ovarian Patient-Derived Xenografts Identifying PARP Inhibitor Eligible Patients with MyChoice™ HRD Xenograft data presented at ASCO 2015. myChoice HRD™ Incorporates Three Measures of HRD: Loss of Heterozygosity, Large Scale Transitions and Telomere Imbalance Combined Studies Ovarian Likely Non-Responders Likely Responders NIRAPARIB: HRD Score BRCA wt BRCA1 mut BRCA2 mut BRCA1 met Resistant to niraparib Sensitive to niraparib 0 10 20 30 40 50 60 (0,5) (5,10) (15,20) (25,30) (35,40) (45,50) (55,60) (65,70) (75,80) (85,90) (95,100) Number of Samples HRD Score BRCA Intact n = 371 BRCA Deficient n = 190 0 20 40 60 80 100
[LOGO]

Conference Call with Clients of Leerink Partners October 15, 2015

