Attached files
| file | filename |
|---|---|
| 8-K - 8-K - POZEN INC /NC | a15-20272_18k.htm |
Exhibit 99.1
|
|
“Strategic Rationale and Vision for Aralez Pharmaceuticals” September 2015 |
|
|
This presentation contains forward-looking statements under applicable securities laws, including, but not limited to, statements related to the anticipated consummation of the business combination transaction among Aralez, POZEN and Tribute and the timing and benefits thereof, the anticipated equity and debt financings and the closings thereof, the combined company's strategy, plans, objectives, expectations (financial or otherwise) and intentions, future financial results and growth potential, anticipated product portfolio, development programs and management structure, the proposed listing on the NASDAQ and TSX and other statements that are not historical facts. These forward-looking statements are based on POZEN's current assumptions and expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward looking statements as a result of these risks and uncertainties, which include, without limitation, risks related to the parties ability to complete the combination and financings on the proposed terms and schedule; the parties ability to close the capital investment on the proposed terms and schedule; the combined company meeting the listing requiements on the NASDAQ and TSX; risk that Aralez may be taxed as a U.S. resident corporation; risks associated with business combination transactions, such as the risk that the businesses will not be integrated successfully, that such integration may be more difficult, time-consuming or costly than expected or that the expected benefits of the transaction will not occur; risks related to future opportunities and plans for the combined company, including uncertainty of the expected financial performance and results of the combined company following completion of the proposed transaction; disruption from the proposed transaction, making it more difficult to conduct business as usual or maintain relationships with customers, employees or suppliers; the calculations of, and factors that may impact the calculations of, the acquisition price in connection with the proposed merger and the allocation of such acquisition price to the net assets acquired in accordance with applicable accounting rules and methodologies; and the possibility that if the combined company does not achieve the perceived benefits of the proposed transaction as rapidly or to the extent anticipated by financial analysts or investors, the market price of the combined company's shares could decline, as well as other risks related to POZEN's and Tribute’s business, including POZEN's inability to build, acquire or contract with a sales force of sufficient scale for the commercialization of YOSPRALA™ in a timely and cost-effective manner, the parties’ failure to successfully commercialize our product candidates; costs and delays in the development and/or FDA approval of our product candidates (including YOSPRALA™), including as a result of the need to conduct additional studies or due to issues with third-party manufacturers, or the failure to obtain such approval of POZEN’s product candidates for all expected indications, including as a result of changes in regulatory standards or the regulatory environment during the development period of any of its product candidates; the inability to maintain or enter into, and the risks resulting from POZEN’s dependence upon, collaboration or contractual arrangements necessary for the development, manufacture, commercialization, marketing, sales and distribution of any products, including its dependence on AstraZeneca and Horizon for the sales and marketing of VIMOVO®, POZEN’s dependence on Patheon for the manufacture of YOSPRALA™ 81/40 and YOSPRALA™ 325/40 ; the ability of POZEN and Tribute to protect their intellectual property and defend their patents; regulatory obligations and oversight; and those risks detailed from time-to-time under the caption "Risk Factors" and elsewhere in POZEN's SEC filings and reports, including in its Annual Report on Form 10-K for the year ended December 31, 2014 and Form 10-Q for the quarter ended June 30, 2015, in Tribute’s SEC filings and reports, including in its Annual Report on Form 10-K for the year ended December 31, 2014 and Form 10-Q for the quarter ended June 30, 2015, and in the registration statement on Form S-4 filed by Aralez on July 20, 2015, as amended August 19, 2015. We undertake no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or changes in their expectations. This communication does not constitute an offer to sell, or the solicitation of an offer to sell, or the solicitation of an offer to subscribe for or buy, any securities nor shall there be any sale, issuance or transfer of securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. Disclaimer |
|
|
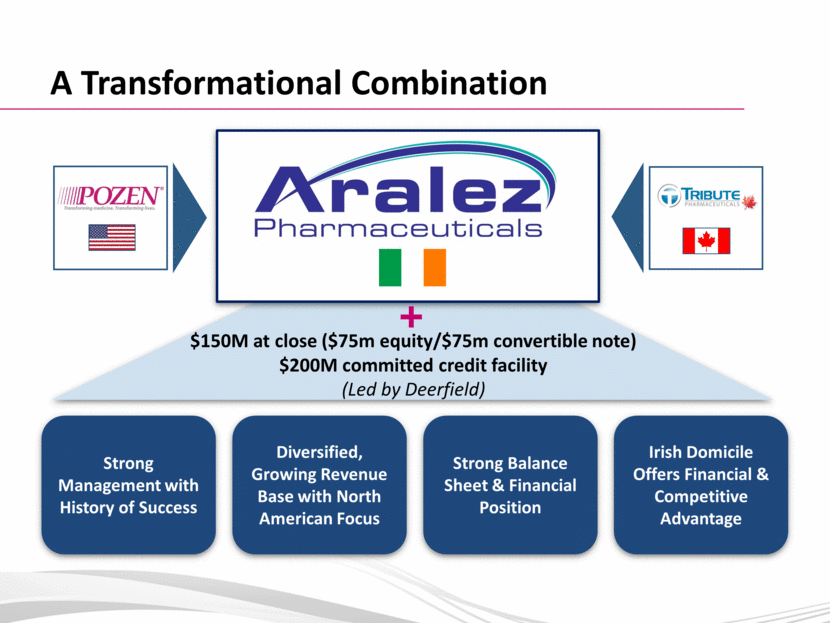
A Transformational Combination Strong Management with History of Success Diversified, Growing Revenue Base with North American Focus Strong Balance Sheet & Financial Position Irish Domicile Offers Financial & Competitive Advantage $150M at close ($75m equity/$75m convertible note) $200M committed credit facility (Led by Deerfield) |
|
|
POZEN is a US specialty pharmaceutical company that has developed multiple novel combination therapies for pain and cardiovascular diseases YOSPRALA™: Combination of delayed-release aspirin and immediate-release omeprazole for the secondary prevention of heart attack and stroke Awaiting FDA approval; expected US launch in 2016 Patent protection to 2023, with potential for extension to 2032 VIMOVO® (partnered): Combination of esomeprazole and naproxen for osteoarthritis Horizon Pharma (US) – 10% on US sales; annual minimum of $7.5M; Patented until 2031 AstraZeneca (ex-US) – 50+ countries; 6% on sales through YE 2015, increasing to 10% in 2016+ Royalties of $21.1M in 2014 Treximet®/MT400 (US partnered; ex-US POZEN): Combination of sumatriptan and naproxen sodium for acute migraine Pernix (US) – Royalty sold to CPPIB; receive 20% of CPPIB receipts starting April 2018 Treximet® – Adolescent Dose recently FDA approved POZEN: Focus on Cardiovascular & Pain Therapies |
|
|
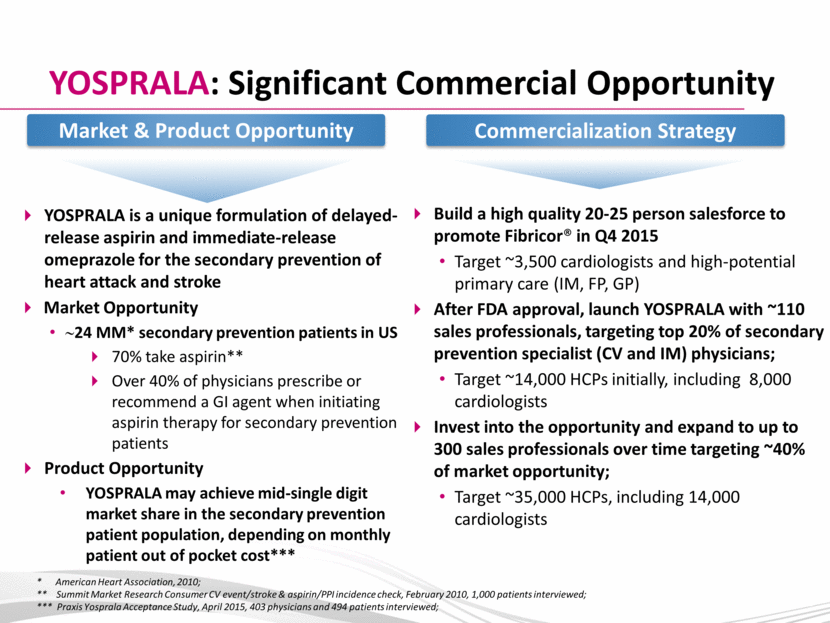
YOSPRALA: Significant Commercial Opportunity YOSPRALA is a unique formulation of delayed-release aspirin and immediate-release omeprazole for the secondary prevention of heart attack and stroke Market Opportunity ~24 MM* secondary prevention patients in US 70% take aspirin** Over 40% of physicians prescribe or recommend a GI agent when initiating aspirin therapy for secondary prevention patients Product Opportunity YOSPRALA may achieve mid-single digit market share in the secondary prevention patient population, depending on monthly patient out of pocket cost*** * American Heart Association, 2010; ** Summit Market Research Consumer CV event/stroke & aspirin/PPI incidence check, February 2010, 1,000 patients interviewed; *** Praxis Yosprala Acceptance Study, April 2015, 403 physicians and 494 patients interviewed; Build a high quality 20-25 person salesforce to promote Fibricor® in Q4 2015 Target ~3,500 cardiologists and high-potential primary care (IM, FP, GP) After FDA approval, launch YOSPRALA with ~110 sales professionals, targeting top 20% of secondary prevention specialist (CV and IM) physicians; Target ~14,000 HCPs initially, including 8,000 cardiologists Invest into the opportunity and expand to up to 300 sales professionals over time targeting ~40% of market opportunity; Target ~35,000 HCPs, including 14,000 cardiologists Market & Product Opportunity Commercialization Strategy |
|
|
YOSPRALA API Aspirin Manufacturer Status YOSPRALA API primary aspirin manufacturer executing against its action plan to address FDA issues: will be subject to FDA re-inspection Steps also being taken to qualify a second supplier of aspirin API Prelaunch Preparations Scaling up and building out commercial operations (managed care, marketing, operations) Planning 20-25 person sales force to promote Fibricor in Q4 ahead of anticipated YOSPRALA launch in 2016 Tactical Activities Conducting medical practitioner and payor advisory boards to guide launch preparations Conducting messaging, positioning, patient flow, and pricing research to inform tactics Creating payor value proposition materials and initiating key customer interactions to begin securing broad payor access for YOSPRALA Developing patient friendly programs to ensure availability and compliance YOSPRALA Update |
|
|
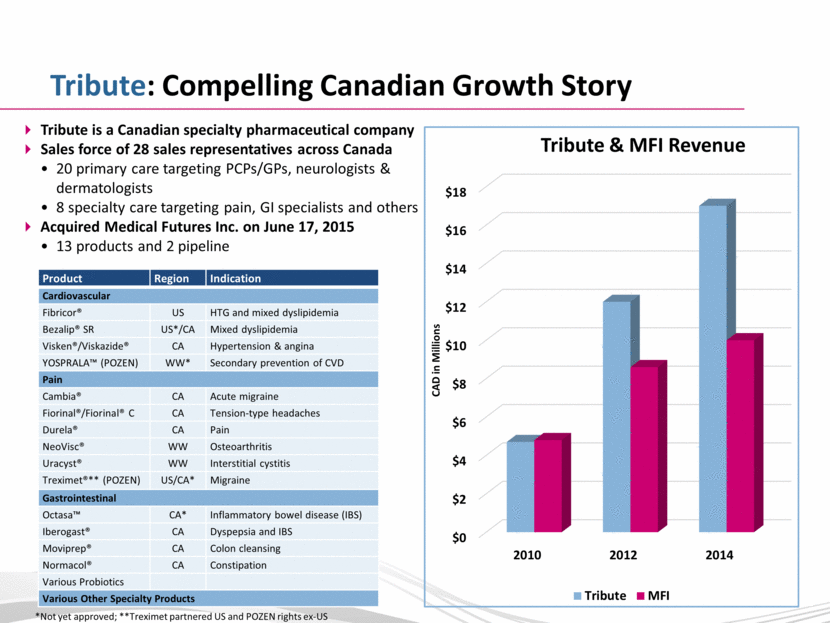
Tribute: Compelling Canadian Growth Story Tribute is a Canadian specialty pharmaceutical company Sales force of 28 sales representatives across Canada 20 primary care targeting PCPs/GPs, neurologists & dermatologists 8 specialty care targeting pain, GI specialists and others Acquired Medical Futures Inc. on June 17, 2015 13 products and 2 pipeline CAD in Millions *Not yet approved; **Treximet partnered US and POZEN rights ex-US Product Region Indication Cardiovascular Fibricor® US HTG and mixed dyslipidemia Bezalip® SR US*/CA Mixed dyslipidemia Visken®/Viskazide® CA Hypertension & angina YOSPRALA™ (POZEN) WW* Secondary prevention of CVD Pain Cambia® CA Acute migraine Fiorinal®/Fiorinal® C CA Tension-type headaches Durela® CA Pain NeoVisc® WW Osteoarthritis Uracyst® WW Interstitial cystitis Treximet®** (POZEN) US/CA* Migraine Gastrointestinal Octasa™ CA* Inflammatory bowel disease (IBS) Iberogast® CA Dyspepsia and IBS Moviprep® CA Colon cleansing Normacol® CA Constipation Various Probiotics Various Other Specialty Products |
|
|
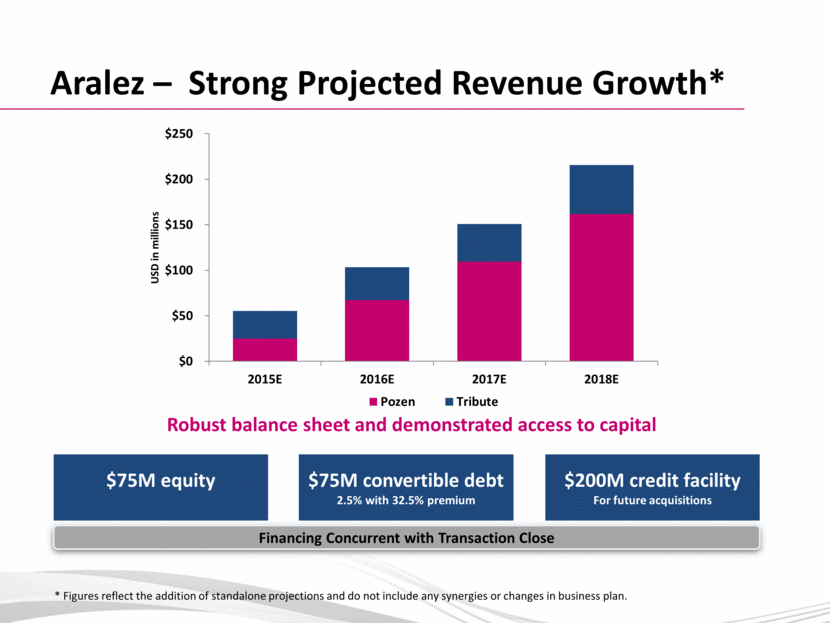
Aralez – Strong Projected Revenue Growth* $75M equity Robust balance sheet and demonstrated access to capital $75M convertible debt 2.5% with 32.5% premium $200M credit facility For future acquisitions * Figures reflect the addition of standalone projections and do not include any synergies or changes in business plan. Financing Concurrent with Transaction Close $0 $50 $100 $150 $200 $250 2015E 2016E 2017E 2018E USD in millions Pozen Tribute |
|
|
The Aralez Growth Strategy & Priorities Maximize the value of expanded portfolio & geographic footprint Build around a cardiovascular-based, North American-focused portfolio Aggressive approach to CD&L and M&A to drive growth supported by new platform Leverage platform for growth with tax-efficient structure Anchor therapeutic position in Cardiovascular supported by a Pain franchise Continue to leverage and invest in Cardiovascular, Pain and Dermatology platform in Canada to grow business Acquire low-risk, high potential, revenue generating products and/or companies in CV or Pain areas Seek product and M&A opportunities in other specialty areas in the U.S/ Canada that are revenue generating and accretive Maintain a lean, nimble and performance-orientated operating model with strong financial discipline Focus on growing shareholder value in the short, medium and long-term Ample liquidity to commercialize YOSPRALA and explore additional acquisition opportunities Grow Fibricor use in the U.S. and build springboard ahead of potential YOSPRALA approval and commercial launch Successfully launch YOSPRALA in the U.S. and grow use with cardiologists and high prescribing physicians Invest into the opportunity, build sales force, deploy managed care strategy and tactics to drive growth Plan to file for approval of YOSPRALA and MT400 (TREXIMET) in Canada in 2016; Plan to submit MAA regulatory application in Europe in 2016 for YOSPRALA 100/40mg dose that can be referenced in other countries around the world |
|
|
Advance YOSPRALA to submission and approval Focus on YOSPRALA prelaunch preparations & execute commercialization strategy Successfully close the Tribute Pharmaceuticals merger in the 4Q15 S4 remains subject to SEC review; In the process of responding to the SEC’s comments Finalize S-4 and go effective Clear HSR notification waiting period Prepare for special shareholder meeting Work on integration planning to help ensure a smooth transition Continue to actively assess BD&L product and M&A opportunities that are revenue generating and accretive Focus on product opportunities in Cardiovascular and Pain Remain opportunistic for other specialty areas in the U.S. and Canada Build out leadership team and infrastructure in a financially disciplined way Our Near Term Priorities |
|
|
“Strategic Rationale and Vision for Aralez Pharmaceuticals” September 2015 |