Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Endo International plc | d35988d8k.htm |
| EX-5.1 - EX-5.1 - Endo International plc | d35988dex51.htm |
| EX-99.1 - EX-99.1 - Endo International plc | d35988dex991.htm |
| EX-10.1 - EX-10.1 - Endo International plc | d35988dex101.htm |
 Endo International plc
Company Update
September 28, 2015
©2015 Endo Pharmaceuticals Inc. All rights reserved.
Exhibit 99.2 |
 Forward Looking Statements
©2015 Endo Pharmaceuticals Inc. All rights reserved.
1 This presentation contains information relating to the acquisition of Par by Endo that includes or is based on “forward-looking
statements” within the meaning of Section 27A of the
Securities Act of 1933, as amended, Section 21E of the Securities Exchange Act of 1934, as amended, and Canadian securities legislation. These statements include statements regarding the timing and the closing of the
transaction, the expected benefits of the transaction, the
expected accretion to earnings resulting from the transaction, expected product approvals and Endo’s plans to operate Par. Forward-looking statements include the information concerning our possible or assumed results
of operations. We have tried, whenever possible, to identify such
statements by words such as “believes,” “expects,” “anticipates,” “intends,” “estimates,” “plan,” “projected,” “forecast,” “will,” “may” or similar
expressions. We have based these forward-looking statements on our current expectations of future events. Because these statements reflect our current views concerning future events, these
forward-looking statements involve risks and uncertainties.
If underlying assumptions prove inaccurate or unknown, or unknown risks or uncertainties materialize, actual results could differ materially from those expressed in the forward-looking statements contained in this
presentation. Risks and uncertainties include, among other
things; that the FDA or other regulatory authorities do not approve any product(s) in the manner desired by Endo on a timely basis, or at all; that there is a material adverse change to Endo; that the integration of
Par’s business into Endo is not as successful as expected;
the failure of Endo to achieve the expected financial and commercial results from the transaction; other business effects, including effects of industry, economic or political conditions outside Endo’s control; transaction costs;
the outcome of litigation, actual or contingent liabilities; as
well as other cautionary statements contained elsewhere herein and in Endo’s periodic reports filed with the Securities and Exchange Commission (SEC) and with securities regulators in Canada on the System for Electronic
Document Analysis and Retrieval (SEDAR), including current
reports on Form 8-K, quarterly reports on Form 10-Q and annual reports on Form 10-K. We do not undertake any obligation to update our forward-looking statements after the date of this presentation for
any reason, even if new information becomes available or other
events occur in the future, except as may be required under applicable securities law. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not
consider this to be a complete discussion of all potential risks
or uncertainties. |
 Non-GAAP Financial Measures ©2015 Endo Pharmaceuticals Inc. All rights reserved. 2 This presentation refers to non-GAAP financial measures of Endo, including Adjusted EBITDA, adjusted diluted earnings per ordinary
share from continuing operations, adjusted gross margin, adjusted
operating expenses, adjusted interest expense and adjusted effective tax rates, and Adjusted EBITDA for Par Pharmaceutical Holdings, Inc. (Par), all of which are financial measures that are not prepared in
conformity with accounting principles generally accepted in the
United States (GAAP). We believe the presentation of Endo’s and Par’s non-GAAP financial measures provides useful supplementary information regarding operational performance because it enhances an investor's overall
understanding of the financial performance and prospects for
future core business activities by providing a basis for the comparison of results of core business operations between current, past and future periods. Management uses non-GAAP financial measures to prepare operating
budgets and forecasts and to measure performance against those
budgets and forecasts on a corporate and segment level. Endo also uses non- GAAP financial measures for evaluating management performance for compensation purposes. Reconciliations of projected adjusted
diluted earnings per share from continuing operations to the
nearest comparable GAAP amounts and Adjusted EBITDA to the nearest comparable GAAP amounts have been provided within the appendix at the end of this presentation. We have not provided a quantitative reconciliation of
other projected non-GAAP measures described above because not
all of the information necessary for quantitative reconciliation is available to us at this time without unreasonable efforts. This is due primarily to variability and difficulty in making accurate detailed forecasts and
projections. Accordingly, we do not believe that reconciling
information for such projected figures would be meaningful. Additional Information This presentation is provided for informational purposes only and is neither an offer to purchase nor a solicitation of an offer to sell
shares of Endo. Endo shareholders should read any filings
made by Endo with the SEC in connection with the proposed combination, as they will contain important information. Those documents, if and when filed, as well as Endo's other public filings with the SEC, may be obtained without
charge at the SEC’s website at www.sec.gov and at Endo's
website at endo.com. |
 Par Pharmaceutical Acquisition:
Compelling Strategic & Financial Rationale
Strategically expands product portfolio, R&D pipeline,
capabilities and long-term growth drivers
Adds extensive range of dosage forms and delivery systems
Focus on specialized, market leading products
Designed to accelerate Endo growth
Double-digit revenue growth over planning horizon, accretive to adjusted
diluted EPS, meaningful synergies, increased operating
margins Strong R&D pipeline capable of fueling long-term
organic growth Drives strategic expansion of overall corporate
profile, scope, and size, establishing a powerful platform for
future M&A Strong cash flow expected to lead to rapid
de-levering back to 3-4x net debt to EBITDA by
mid-2016 Aligned with Endo’s strategy of pursuing
accretive, value- creating growth opportunities
©2015 Endo Pharmaceuticals Inc. All rights reserved.
3 Creates shareholder value and drives benefits for patients & customers
Creates shareholder value and drives benefits for patients &
customers |
 4 Endo + Par: A Transformational Combination Company Long Term Growth Drivers Revenue (2014) Adj. EBITDA (2014) ~2.9bn ~1.3bn Strong performance from portfolio Attractive R&D pipeline Focus on specialized products with high adjusted gross margins Expansion of branded and generic portfolio and R&D pipeline Continued investment in M&A and licensing opportunities Operational synergies Double-digit growth Transformative M&A platform ~$1.2bn ~$434mn Pro forma 2014 Adj. EBITDA: ~$1.6bn Employees ~4,500 ~1,800 ~6,300 Pro forma 2014 Revenue: ~$4.2bn |
 5 Qualitest +Par: A Leader in Specialty Generics Business Generics (2014) Generics R&D Pipeline ~90 programs 6 ANDAs expected to be filed in 2015 220 programs 120 filed
ANDAs 100 programs in
development 2014 Revenue: ~$1.1bn (+56% from 2013) 2014 Revenue: ~$1.3bn (+19% from 2013) Pro forma 2014 Revenue: ~$2.4bn Employees Manufacturing Facilities Alabama North Carolina New York Connecticut California Michigan India ~1,750 ~1,800 ~3,550 Global Manufacturing and Supply Chain Operations ~300 programs ~2/3 in alternative
dosages >100 Para IV, FTF or FTM
20-30 new ANDAs / year ~ ~ |
 6 Addition of Par Generics Pipeline: Driving Near- and Long-Term Opportunities Total Pipeline contains 47 potential FTF / FTM opportunities and an opportunity of $42 Billion in market value* Anticipated Launches and Filings: 2016 – 2019 * Total Pipeline includes potential FTF/FTM opportunities not currently assumed to launch before
2019. Note: market value defined by IMS sales for 12 months ended June 30,
2015 8 FTF
46 Total 2016 & 2017 $8B market value $16B market value 12 FTF 66 Total 2018 & 2019 $4B market value $13B market value |
 7 2015-2016: Anticipated Product Launches Provide Near-Term Earnings Visibility Anticipated Launch Product Brand Market Value (~$mm, LTM) Competitive Landscape 4Q 2015 Dutasteride / Tamsulosin Jalyn® $90 FTF 1H 2016 Rivastigmine Patch* Exelon® $600 Multiple strengths July 2016 Rosuvastatin Tabs Crestor® $5,800 Has TA Nov 2016 Quetiapine ER Tabs* Seroquel® XR $1,300 Has TA on all 5 strengths; FTF on 4 (not 400mg) Dec 2016 Ezetimibe Tabs* Zetia® $2,000 Approved, FTF * Partnered Program Market Value based on IMS Sales Data for last 12 months ended June 30, 2015
Significant FTF launch opportunities in late 2016
drive sales growth in 2016 and 2017 |
 8 2017-2019: Robust Pipeline to Drive Long-Term Growth Selected Product Launches Brand Market Value (~$mm, LTM) Competitive Landscape Ciprofloxacin / Dexmethasone Ciprodex® $400 FTF Adapalene & Benzoyl Peroxide Gel 0.1-2.5% Epiduo® $350 Limited competition Amphetamine Salts ER Capsules Adderal® $900 Limited competition Sapropterin Dihydrochloride Tabs Kuvan® $100 FTF Everolimus Tabs Afinitor® $900 FTF (except 10mg) Abiraterone Tabs* Zytiga® $1,100 FTF; 250mg dose Methylphenidate HCl ER Tabs Concerta® $150 18, 27, 36 and 54mg doses Tolvaptan Tabs Samsca® $100 FTF Travoprost Z Travatan Z® $500 Limited competition >15 Potential FTF Launch Opportunities in 2017 - 2019 * Partnered Program Market Value based on IMS Sales Data for last 12 months ended June 30, 2015 |
 ©2015 Endo Pharmaceuticals Inc. All rights reserved.
9 Measure Q3 2015 Revenues $720M - $740M Adjusted Interest Expenses** ~$95M Adjusted Effective Tax Rate 2% to 4% Weighted Average Diluted Shares Outstanding** 210M Q3 2015 Financial Guidance (Continuing Operations*) ** NOTE: as discussed around Q2 2015 results, the inclusion of pre-close financing
activities related to the acquisition of Par reduces Q3 Adjusted EPS by ~$0.23
cents * Continuing Operations includes Endo and Par and excludes
AMS Women’s Health |
 ©2015 Endo Pharmaceuticals Inc. All rights reserved.
10 Measure Prior 2015 Guidance Updated 2015 Guidance Revenues $2.90B - $3.00B $3.22B - $3.27B Adjusted Gross Margin 64% to 65% ~64% Adjusted Operating Expense to Revenue Ratio 23% to 24% ~21.5% Adjusted Interest Expenses ~$310M ~$375 Adjusted Effective Tax Rate 13% to 14% 9% to 10% Adjusted Diluted EPS $4.40 to $4.60 $4.50 to $4.60 Reported (GAAP) EPS $1.42 to $1.62 $1.81 to $1.91 Weighted Average Diluted Shares Outstanding ~180M ~201M 2015 Financial Guidance (Continuing Operations*) * Continuing Operations includes Endo and Par and excludes AMS Women’s Health |
 2016 Financial Guidance (Continuing Operations*)
©2015 Endo Pharmaceuticals Inc. All rights reserved.
11 In 2016, Endo is strongly positioned for: Double-digit revenue growth across our business Strong and rapid synergy capture from the Par acquisition Continued progression and execution of tax strategy Robust cash flow generation and continued, rapid de-levering enabling continued execution of strategic M&A 2016: Estimated Adjusted Diluted EPS of $5.85 to $6.15 2016: Estimated Adjusted Diluted EPS of $5.85 to $6.15 * Continuing Operations includes Endo and Par and excludes AMS Women’s Health |
 Appendix ©2015 Endo Pharmaceuticals Inc. All rights reserved. |
 Reconciliation of Non-GAAP Measures
Lower End of Range
Upper End of Range
Projected GAAP diluted earnings per ordinary share from continuing
operations $1.81
$1.91 Upfront and milestone-related payments to partners $0.06 $0.06 Amortization of commercial intangible assets and fair value inventory step-up
$3.06 $3.06 Acquisition related, integration and restructuring charges and certain excess costs that
will be eliminated pursuant to integration plans
$1.10 $1.10 Asset Impairment Charges $0.42 $0.42 Charges for litigation and other legal matters $0.11 $0.11 Interest expense adjustment for non-cash interest related to our 1.75% Convertible
Senior Subordinated Notes and other treasury related items
$0.01 $0.01 Tax effect of pre-tax adjustments at the applicable tax rates and certain other expected
cash tax savings as a result of acquisitions
($2.07) ($2.07) Projected Adjusted diluted earnings per ordinary share from continuing operations
$4.50 $4.60 The Company's guidance is being issued based on certain assumptions including:
©2015 Endo Pharmaceuticals Inc. All rights reserved.
13 Reconciliation of Projected GAAP Diluted Earnings Per Share from Continuing Operations to Adjusted Diluted Earnings
Per Share from Continuing Operations Guidance for the Year Ending December 31,
2015 •Certain of the above amounts are based on estimates and there can be no assurance that Endo will achieve these
results •Includes all completed business development transactions as of September 28, 2015
•Projected GAAP diluted earnings per ordinary share from continuing operations amounts do not include the impact of Par purchase accounting and other one-time charges associated with the closing of the Par transaction.
|
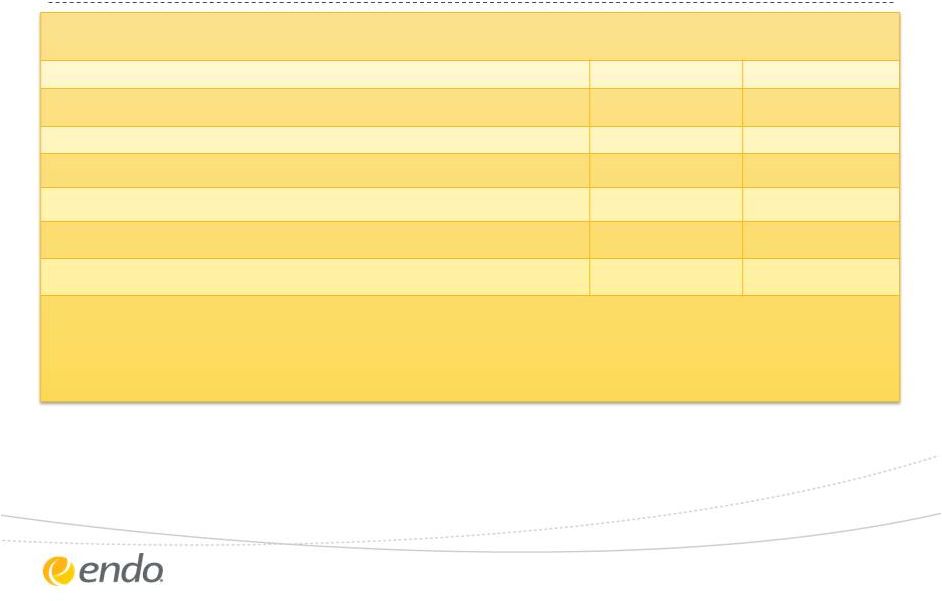 Reconciliation of Non-GAAP Measures
Lower End of Range
Upper End of Range
Projected GAAP diluted earnings per ordinary share from continuing
operations $2.98
$3.28 Upfront and milestone-related payments to partners $0.01 $0.01 Amortization of commercial intangible assets and fair value inventory step-up
$2.08 $2.08 Acquisition related, integration and restructuring charges $0.03 $0.03 Tax effect of pre-tax adjustments at the applicable tax rates and certain other expected
cash tax savings as a result of acquisitions
$0.75 $0.75 Projected Adjusted diluted earnings per ordinary share from continuing operations
$5.85 $6.15 The Company's guidance is being issued based on certain assumptions including:
©2015 Endo Pharmaceuticals Inc. All rights reserved.
14 Reconciliation of Projected GAAP Diluted Earnings Per Share from Continuing Operations to Adjusted Diluted Earnings Per
Share from Continuing Operations Guidance for the Year Ending December 31,
2016 •Certain of the above amounts are based on estimates and there can be no assurance that Endo will achieve these results •Includes
all
completed business development transactions as of September 28, 2015 •Projected
GAAP
diluted earnings per ordinary share from continuing operations amounts do not include the impact of Par purchase accounting and other one-time charges associated with the closing of the Par transaction. |
 Reconciliation of Non-GAAP Measures
©2015 Endo Pharmaceuticals Inc. All rights reserved.
15 Par Pharmaceuticals Adjusted EBITDA, Reconciliation Table for the Year Ending December 31, 2014 Statement of Operations Data: (Successor) (Unaudited) ($ in Thousands) ($105,517) 108,409 (72,993) and amortization 213,564 -up(a) 9,031 152,494 90,107 4,269 5,413 7,461 146,934 3,042 7,136 3,989 i) 8,678 4,000 281 $433,804 |
 Reconciliation of Non-GAAP Measures
©2015 Endo Pharmaceuticals Inc. All rights reserved.
16 (a)
Represents the charge associated with acquisitions for acquired inventory which
was increased to its estimated selling price, less the cost of disposal and a reasonable profit allowance for the selling effort (the “inventory step-up”), as required under GAAP. The inventory step-up was
recognized into earnings based on normal inventory turns and
resulted in costs above standard post-acquisition costs. (b)
In 2014, we recorded an incremental provision of $91.0 million related to the
settlement of omeprazole/sodium bicarbonate patent litigation for $100.0 million. During 2014, we also received an arbitration award of approximately $0.9 million from a former partner related to a discontinued project. (c)
Consists of external legal costs incurred in conjunction with our defense of
the actions brought by various states and the Department of Justice (the “DOJ”) as it relates to the average wholesale price (“AWP”) litigation and the promotional practices of Par Specialty’s marketing of
Megace® ES. (d)
In 2014, subsequent to the Par Sterile acquisition, we eliminated 25 redundant
positions within Par Pharmaceutical and accrued severance and other employee-related costs for those employees affected by the workforce reduction. Additionally, due to a change in our product development strategy, we eliminated
36 redundant positions within our Irvine location and accrued
severance and other employee-related costs for these employees affected by the workforce reduction.
(e) Consists of
transaction-related expenses incurred in connection with the acquisition of Anchen Incorporated and its subsidiary Anchen Pharmaceuticals, Inc. (collectively, “Anchen”), Par Formulations and Par Sterile as well as transaction-related expenses incurred in connection with the Merger
and related transactions. (f)
During the year ended December 31, 2014 we recorded intangible asset
impairments totaling approximately $146.9 million related to an adjustment to the forecasted operating results for two in-process research and development (“IPR&D”) intangible asset groups and eight Par
Pharmaceutical segment products compared to their originally
forecasted operating results at date of acquisition, inclusive of one
discontinued product, one partially impaired product primarily due to the contract ending with the partner and a partially impaired IPR&D project from the Par Sterile acquisition due to an adverse court ruling pertaining to related patent
litigation. The estimated fair values of the assets were
determined by completing updated discounted cash flow models.
(g)
We recognized a loss on the sale of product rights of
$3.0 million during the fiscal year ended December 31, 2014, related to the sale of multiple ANDAs.
(h) In February 2014,
in conjunction with our acquisition of Par Sterile, we amended certain senior facilities. In accordance with the applicable accounting guidance for debt modifications and extinguishments, approximately $4.0 million of the existing unamortized deferred financing costs were written off in
connection with this repricing. (i)
Represents the non-cash expense associated with stock-based
compensation awards issued to various executive and non-executive employees.
(j)
In connection with the Merger and related transactions,
we entered into a management services agreement with an affiliate of TPG (the “Manager”) pursuant to such agreement, and in exchange for on-going consulting and management advisory services, the Manager receives an annual monitoring fee
paid quarterly equal to 1% of EBITDA as defined under the credit
agreement for the Senior Credit Facilities. There is an annual cap of $4.0 million for this fee. The Manager also receives reimbursement for out-of- pocket expenses incurred in connection with services provided pursuant to the agreement. We recorded an expense of $4.0 million for
consulting and management advisory service fees and
out-of-pocket expenses in the year ended December 31, 2014.
(k)
Other includes costs associated with our corporate
integrity agreement and additional pharmaceutical manufacturer’s fee charges recorded under PPACA due to final IRS regulations issued in 2014. |
 Reconciliation of Non-GAAP Measures
©2015 Endo Pharmaceuticals Inc. All rights reserved.
17 Endo International plc Adjusted EBITDA, Reconciliation Table for the Year Ending December 31, 2014 Statement of Operations Data: (Unaudited) ($ in Thousands) Net loss attributable to Endo International plc $ (721,319) Income tax (401,840) Interest expense, net 227,115 Depreciation and amortization 331,651 Inventory step-up 65,582 EBITDA (498,811) Other (income) expense, net (30,174) Loss on extinguishment of debt 31,817 Stock-based compensation 32,671 Asset impairment charges 22,542 Acquisition-related and integration items 85,534 Certain litigation-related charges, net 1,346,444 Upfront and milestone payments to partners 51,774 Cost reduction initiatives 29,525 Other charges 34,972 Discontinued operations, net of tax (5,677) Net income attributable to noncontrolling interests 3,135 Excise tax 54,300 Adjusted EBITDA $ 1,158,052 |
 ©2015 Endo Pharmaceuticals Inc. All rights reserved.
Endo International plc
Company Update
September 28, 2015 |
